-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaNutrient Availability as a Mechanism for Selection of Antibiotic Tolerant within the CF Airway
Microbes are subjected to selective pressures during chronic infections of host tissues. Pseudomonas aeruginosa isolates with inactivating mutations in the transcriptional regulator LasR are frequently selected within the airways of people with cystic fibrosis (CF), and infection with these isolates has been associated with poorer lung function outcomes. The mechanisms underlying selection for lasR mutation are unknown but have been postulated to involve the abundance of specific nutrients within CF airway secretions. We characterized lasR mutant P. aeruginosa strains and isolates to identify conditions found in CF airways that select for growth of lasR mutants. Relative to wild-type P. aeruginosa, lasR mutants exhibited a dramatic metabolic shift, including decreased oxygen consumption and increased nitrate utilization, that is predicted to confer increased fitness within the nutrient conditions known to occur in CF airways. This metabolic shift exhibited by lasR mutants conferred resistance to two antibiotics used frequently in CF care, tobramycin and ciprofloxacin, even under oxygen-dependent growth conditions, yet selection for these mutants in vitro did not require preceding antibiotic exposure. The selection for loss of LasR function in vivo, and the associated adverse clinical impact, could be due to increased bacterial growth in the oxygen-poor and nitrate-rich CF airway, and from the resulting resistance to therapeutic antibiotics. The metabolic similarities among diverse chronic infection-adapted bacteria suggest a common mode of adaptation and antibiotic resistance during chronic infection that is primarily driven by bacterial metabolic shifts in response to nutrient availability within host tissues.
Published in the journal: . PLoS Pathog 6(1): e32767. doi:10.1371/journal.ppat.1000712
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000712Summary
Microbes are subjected to selective pressures during chronic infections of host tissues. Pseudomonas aeruginosa isolates with inactivating mutations in the transcriptional regulator LasR are frequently selected within the airways of people with cystic fibrosis (CF), and infection with these isolates has been associated with poorer lung function outcomes. The mechanisms underlying selection for lasR mutation are unknown but have been postulated to involve the abundance of specific nutrients within CF airway secretions. We characterized lasR mutant P. aeruginosa strains and isolates to identify conditions found in CF airways that select for growth of lasR mutants. Relative to wild-type P. aeruginosa, lasR mutants exhibited a dramatic metabolic shift, including decreased oxygen consumption and increased nitrate utilization, that is predicted to confer increased fitness within the nutrient conditions known to occur in CF airways. This metabolic shift exhibited by lasR mutants conferred resistance to two antibiotics used frequently in CF care, tobramycin and ciprofloxacin, even under oxygen-dependent growth conditions, yet selection for these mutants in vitro did not require preceding antibiotic exposure. The selection for loss of LasR function in vivo, and the associated adverse clinical impact, could be due to increased bacterial growth in the oxygen-poor and nitrate-rich CF airway, and from the resulting resistance to therapeutic antibiotics. The metabolic similarities among diverse chronic infection-adapted bacteria suggest a common mode of adaptation and antibiotic resistance during chronic infection that is primarily driven by bacterial metabolic shifts in response to nutrient availability within host tissues.
Introduction
Microbes are subjected to selection in host environments during the course of chronic infections [1],[2],[3]. The characteristics selected may have profound impacts on disease outcomes, particularly if they confer increased microbial fitness or resistance to therapy. One example of this phenomenon is the adaptation of Pseudomonas aeruginosa within the airways of people with cystic fibrosis (CF). Diverse phenotypic changes have been observed among CF chronic P. aeruginosa infection isolates, including changes in several surface antigens [4],[5], altered antibiotic susceptibilities [6], and overproduction of the mucoid exopolysaccharide alginate [3]. P. aeruginosa CF adaptive changes have been associated with poor clinical outcomes [7],[8] and, in the case of mucoidy, a diminished likelihood of eradication by antibiotics [9].
Recently, several groups have described P. aeruginosa CF isolates with inactivating mutations in the gene lasR [2],[8],[10],[11],[12]. Genetic analyses demonstrated that these mutants emerged from existing, chronically-infecting lineages, as opposed to representing new infections, and that multiple lineages with independent lasR mutations occurred within individual patients, indicative of strong selective pressure against LasR function [2],[11]. lasR encodes a central regulator of the bacterial intercellular signaling system known as quorum sensing that requires the synthesis and recognition of P. aeruginosa small molecule products, including acyl-homoserine lactones (AHL). lasR mutant isolates occur in at least one-third of P. aeruginosa culture-positive individuals younger than 15 years attending CF clinics in Seattle [2],[8]. Among this population, lasR mutant isolates emerged relatively early during CF airway infection (on average 2 years before mucoidy), and were associated with worse lung function [8]. LasR inactivation conferred distinct phenotypic consequences, including distinctive colony morphology (autolysis and surface iridescent sheen) that facilitates the identification of mutant isolates, a growth advantage in specific amino acids abundant in CF secretions [13], and increased β-lactamase enzyme activity [2],[11]. These growth phenotypes suggested that selection may be due to exposure to antibiotics and nutrient availability within the CF airway. The latter possibility was further indicated by altered growth in specific nitrogen sources by lasR mutants compared with their wild-type counterparts [11].
AHL signaling was shown previously by transcriptional microarray [14] and enzymatic analyses [15],[16] to regulate the P. aeruginosa nitrogen metabolic pathway known as denitrification (Fig. 1A). However, the las system comprises only a portion of the complex AHL regulon, and the metabolic consequences of LasR inactivation were not defined previously. Previous evidence from a global physiological analysis of clinical isolates indicated that lasR mutation could confer a growth advantage in the denitrification substrates nitrate (NO3−) and nitrite (NO2−) [11], suggesting that lasR mutant P. aeruginosa cells may exhibit increased utilization of NO3− and NO2− as electron acceptors. Conversely, LasR inactivation conferred sensitivity to high concentrations of NO2− among these isolates [11], as would be predicted if lasR mutant cells avidly metabolize NO2− to nitric oxide (NO·), the chief toxic metabolic side-product of denitrification (Fig. 1A).
Fig. 1. Mutation in lasR increases denitrification, and leads to the buildup of the toxic metabolite NO·. 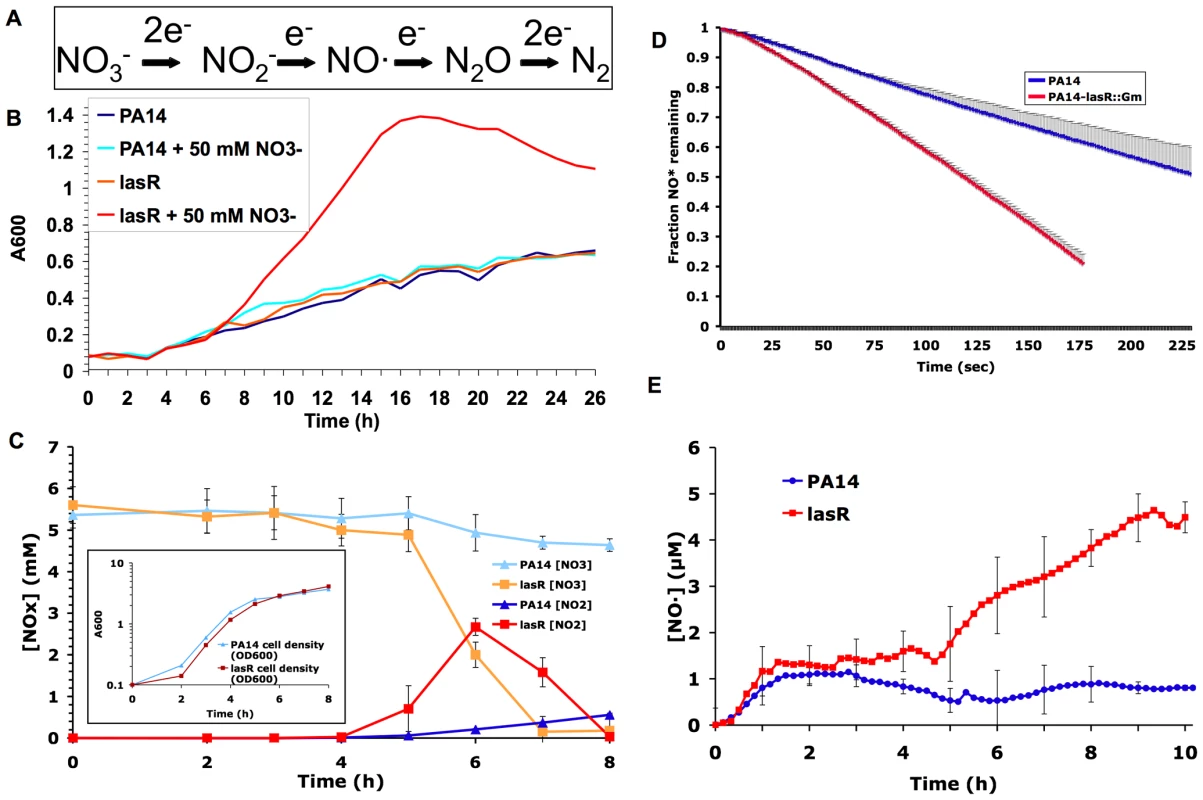
(A) The reactions that comprise the dissimilatory denitrification pathway, or the serial reduction of NO3− to nitrogen gas, in bacteria. (B) Growth of the wild-type P. aeruginosa strain PA14 and its derived lasR mutant PA14-lasR::Gm in a chemically defined medium (PN minimal medium) with and without NO3− supplementation, under low oxygen mass-transfer conditions. Results representative of three separate experiments. (C) Nitrate (NO3−) and nitrite (NO2−) concentrations in shaken cultures of the indicated strains grown with LB and 5 mM added nitrate. Results shown are the averages of technical triplicates ±s.d. and are representative of two separate experiments. Slopes for lines fit to each dataset between 4 and 6 minutes were significantly different with p<0.001 for [NO2−] and p<0.02 for [NO3−]. Also shown for reference (inset) are concurrent growth curves for each strain; results representative of two separate experiments. (D) Rates of degradation of added NO· from a mixture of NO· donors (DEANO and ProliNO) by cells pre-grown in a sealed chamber in LB prior to donor addition. Results shown are average ±s.d. for technical duplicates and are representative of two separate experiments. Slopes for lines fit to each dataset between 25 and 100 seconds were significantly different (p<0.02). (E) NO· concentrations in sealed, stirred LB cultures of the indicated strains during growth with 50 mM added NO3−. Results are average ±s.d. of three separate experiments. Slopes for lines fit to each dataset between 4 and 9 minutes were significantly different between wild-type and lasR mutant cultures (p<0.02). For all experiments shown, similar results were obtained in at least two separate experiments with the Patient 1 early isolate and its derived lasR mutant (described in Table 1), and complementation with the lasR gene on a plasmid restored wild-type phenotypes (not shown). The airways of people with CF are known to contain abundant concentrations of NO3− and other nitrogen species [17],[18], while the concentrations of NO· (an important antimicrobial component of host innate immunity) are usually significantly lower than in people without CF for as yet unknown reasons [19]. In addition, CF secretions infected with P. aeruginosa include areas with very low molecular oxygen tensions [20]. These conditions would tend to favor the use among infecting microbes of nitrogen oxides as electron acceptors at the expense of oxygen utilization [16],[21]. Thus, we hypothesized that lasR mutant P. aeruginosa cells have respiratory alterations favoring growth in the nitrogen and oxygen conditions characteristic of CF airways. Since many antibiotics work best under aerobic conditions [22],[23], such a metabolic shift could adversely affect susceptibility (and thus clinical response) to antibiotics. To test these hypotheses, we defined the consequences of lasR mutation with respect to nitrate and oxygen metabolism, as well as antibiotic susceptibility, in laboratory strains and CF clinical isolates of P. aeruginosa.
Results
lasR mutants have a growth advantage in NO3− and accumulate the toxic metabolite nitric oxide (NO·)
Given the evidence that lasR mutants may have a growth advantage in NO3− [11], we compared the growth of P. aeruginosa laboratory strain PA14 and clinical isolates carrying wild-type lasR alleles with their derived lasR mutant strains in the presence of various concentrations of NO3−. lasR mutants exhibited a substantial growth advantage in minimal medium with added NO3−. As shown in Fig. 1B for a lasR mutant with a gentamicin insertion cassette derived from PA14 (PA14-lasR::Gm), a growth advantage was detected in NO3− concentrations as low as 125 µM, well below the average NO3− concentrations recently measured in CF airway secretions [17],[18], and the advantage was more pronounced at higher NO3− concentrations (not shown). The average rate of lasR mutant growth (calculated as the slopes of lines fit to the datasets shown between 8 and 16 minutes) in 50 mM NO3− was increased ∼5-fold relative to wild-type. Similar results were obtained using Luria Broth, with PA14 with an unmarked lasR deletion (PA14ΔlasR), and with paired lasR wild-type and mutant clinical isolates (not shown). This analysis confirms and extends our previous finding that lasR mutations confer a growth advantage with nitrogen sources that are abundant in the CF airway, including NO3−, as well as with aromatic amino acids [11].
lasR mutant strains and isolates converted NO3− to NO2−, and degraded NO2−, at significantly higher rates than did wild-type strains and isolates (Fig. 1C). For example, the average rate of NO2− production by lasR mutant strains was ∼4.4-fold greater than by wild-type. In contrast, lasR mutant strains and isolates demonstrated a relatively modest spontaneous increase in NO· reduction relative to wild-type (Fig. 1D); slopes for lines fit to each dataset in Fig. 1D between 25 and 100 seconds demonstrated that lasR mutant cells had an NO· degradation rate only ∼1.8-fold greater than wild-type cells. These activities resulted in dramatically higher levels of NO· (Fig. 1E) in lasR mutant cultures that could not be explained by any concurrent difference in growth rates between lasR mutants and wild-type in added NO3− (compare Figs. 1B and 1E). The accumulation of NO·, a potent microbicide [24], in lasR mutant cultures would be predicted to result in cell death at very high cell densities (as observed with P. aeruginosa cells with mutations in the quorum sensing regulator rhlR [16]) and in increased susceptibility to exogenous NO· sources.
lasR mutation confers increased susceptibility to nitrosative stress, and selection requires a membrane-bound NO3− reductase
Because lasR mutant P. aeruginosa produces elevated levels of endogenous NO·, and bacterial cells possess a finite capacity for detoxifying NO· that can be exceeded by exposure to exogenous reactive nitrogen species (RNS) [25], we predicted that lasR mutants would also be more susceptible to the exogenous nitrosative stress presented by either NO· donors (which have relatively short aqueous half-lives [26]) or acidified NO2− (with substantially greater aqueous half-life [27]). Therefore, lasR mutant strains and isolates were tested for these susceptibility phenotypes. lasR mutants were more susceptible to growth inhibition by the addition of NO· donor compounds to liquid cultures (Fig. 2A), and by NO2− disks during growth on acidified agar medium (Fig. 2B). These results are in agreement with our previous phenotype array findings that lasR inactivation in clinical P. aeruginosa isolates confers increased susceptibility to high concentrations of NO2− in unbuffered liquid minimal medium [11]. Furthermore, analysis of clinical isolate pairs demonstrated that the impact of lasR mutation on NO2− susceptibility was similar to the effect demonstrated previously for mucoidy [27], as shown in Fig. 2B for one isolate pair (NCAMT0101-2 and -3).
Fig. 2. Mutation in lasR confers increased susceptibility to nitrosative stress, including acidified NO2−. 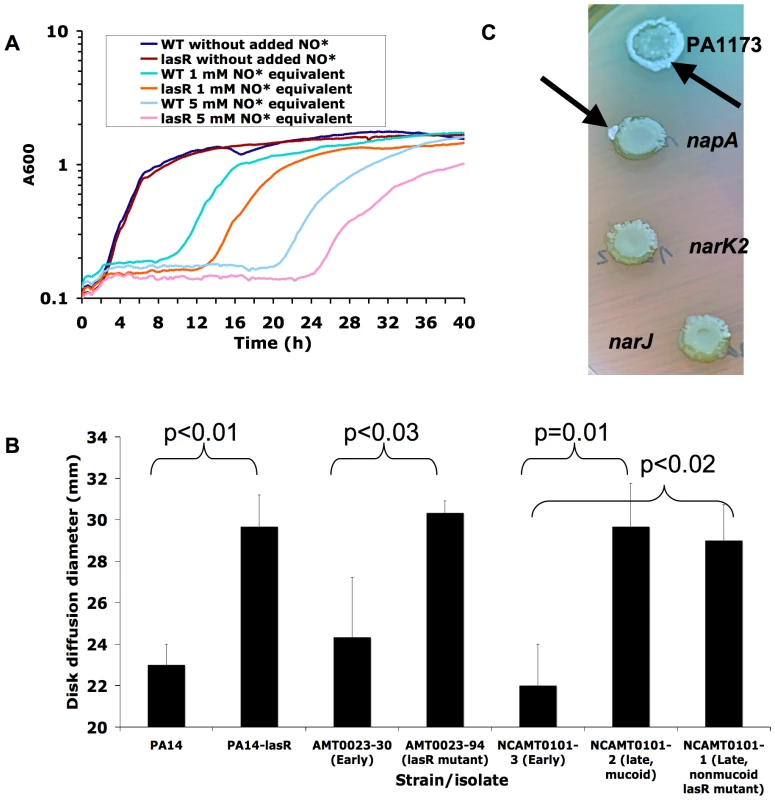
(A) Growth rate of PA14 versus PA14-lasR::Gm in LB in the presence and absence of two amounts of added NO· donor SPER-NO, transiently generating the indicated concentrations of NO·. Each result representative of at least two separate experiments. (B) Disk diffusion diameters of the indicated strains and isolates on LB agar buffered to pH 6.5, with disks containing 100 µmol of NaNO2, then incubated for 24 hours at 37°C under aerobic conditions. Average ±s.d. for triplicate experiments. Similar results were obtained with PA14ΔlasR, and complementation with a wild-type copy of lasR on a plasmid restored wild-type phenotypes to lasR mutants (not shown). (C) Spontaneous sectors displaying the lasR phenotype (metallic surface sheen and autolytic flattening, indicated by black arrows) arise during agar surface growth for 1 week of PA14-derived strains with transposon insertions in genes in the periplasmic NO3− reductase gene cluster (top, genes PA1173 and napA) but not from those with insertion in genes in the membrane-bound NO3− reductase gene cluster (bottom, genes narJ and narK2). Also visible around the lasR mutant sectors is the blue pigment pyocyanin, which is produced at higher levels by lasR mutant PA14 than by wild-type cells upon extended incubation [61]. Results representative of four separate experiments. Complementation with a wild-type copy of lasR on a plasmid restored wild-type phenotypes to lasR mutants isolated from sectors (not shown). P. aeruginosa encodes two NO3− reductases, one in the bacterial inner membrane and the other in the periplasm. It was found previously that, of these two, only the membrane-bound enzyme was required for anaerobic growth of P. aeruginosa [17]. Interestingly, we found that spontaneous lasR mutants did not emerge during extended growth on agar medium from strains with transposon insertions in genes encoding subunits of the membrane-bound NO3− reductase (narJ and narK2), while sectors displaying the characteristic lasR phenotype arose frequently among strains with similar mutations in genes encoding the periplasmic enzyme (PA1173 and napA) (Fig. 2C). Furthermore, the growth advantage in NO3− conferred by lasR mutation (Fig. 1B) was not observed in the absence of narK genes (narK1narK2lasR, data not shown). Thus, the membrane-bound NO3− reductase was required for both the growth advantage of lasR mutants in added NO3− and for rapid lasR mutant emergence in vitro. These results functionally link the growth advantage in NO3− conferred by a lasR mutation with the selection of these mutants, at least in vitro, and perhaps also in the NO3− -rich CF airway [17],[18].
Factors that detoxify NO· increase P. aeruginosa lasR mutant growth
The findings that lasR mutants overproduce the potent microbicide NO· (Fig. 1E), that they undergo autolysis at high cell density [11], and that lasR mutants exhibit increased growth inhibition by exogenous sources of NO· (Fig. 2A) suggested that factors that detoxify NO· could enrich for lasR mutant growth. This hypothesis is supported by the observation that the cell death observed when RhlR mutants are grown anaerobically as biofilms can be prevented by addition of an NO· scavenger [16]. Similarly, we found that P. aeruginosa lasR mutants growing near disks containing hemoglobin, which scavenges NO· stoichiometrically, also grew better than did cells farther away from the disk (Fig. 3A). These results suggest that the presence of an NO· “sink” such as hemoglobin increased growth of lasR mutant P. aeruginosa.
Fig. 3. lasR mutant P. aeruginosa growth is altered during co-culture with S. aureus, apparently due to detoxification of NO·. 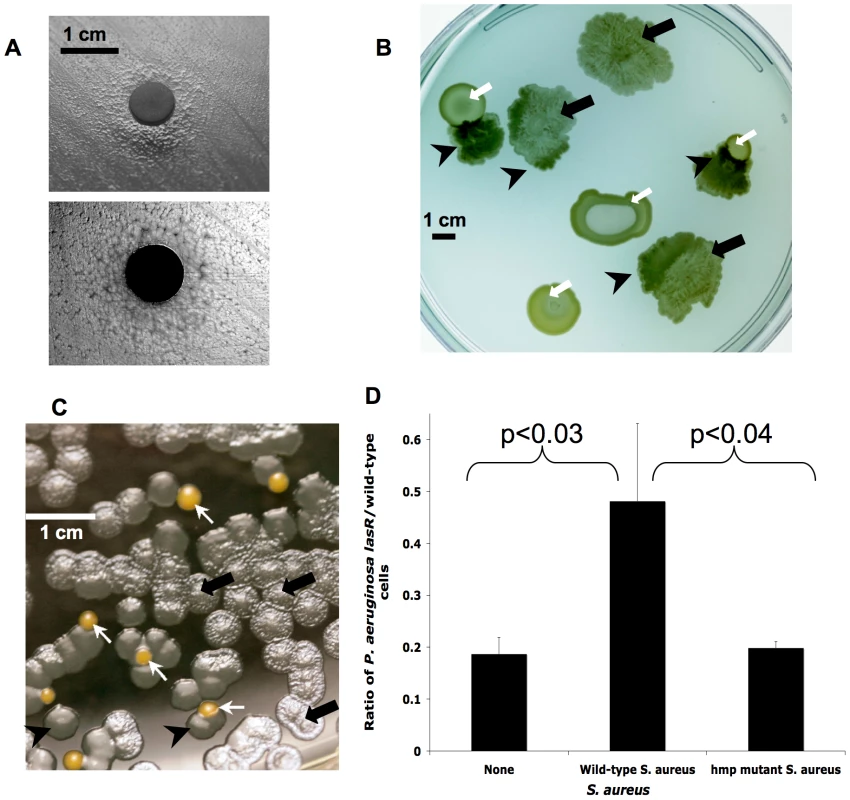
(A) Cells in a lawn of lasR mutant PA14 growing near a disk containing hemoglobin, which stoichiometrically scavenges NO· (as opposed to the catalytic effect of S. aureus colonies in B–C), do not display the autolysis of cells more distant from the disk, as shown in a photograph from above with illumination from above (upper) and below (lower). (B) Clinical isolates of lasR mutant P. aeruginosa (black arrows) and S. aureus (white arrows) grown together on LB agar with 400 µM added KNO3, with lasR colony autolysis and resulting translucency indicated through transillumination of the agar plate. Black arrows indicate areas of P. aeruginosa lysis and/or sheen, white arrows indicate colonies of S. aureus, and black arrowheads indicate where lasR colony autolysis is relieved in the presence of S. aureus. (C) Co-culture as in (B) except with laboratory strain PA14-lasR::Gm grown with wild-type S. aureus Newman strain (colored orange in silico for clarity) after inoculation at a cell ratio of 50∶1. Arrows as in (B). (D) Ratios of cell counts of P. aeruginosa lasR versus wild-type after inoculation of static cultures in liquid LB with 400 µM added KNO3 with equal numbers of each P. aeruginosa strain followed by growth for 48 hours in the presence and absence of equal cell numbers of the indicated S. aureus strains. Results are averages ±s.d. for triplicate counts and are representative of three separate experiments. Total final cell count was similar in each experiment. Some bacteria, including the gram-positive CF bacterial pathogen Staphylococcus aureus, are known to be relatively resistant to the effects of NO· as a result of efficient cellular detoxification mechanisms [24]. Furthermore, we found previously that the presence of live, but not dead, S. aureus decreased expression of a P. aeruginosa gene (fhp [25]) involved in NO· degradation [28], suggesting that S. aureus may detoxify NO· produced by P. aeruginosa. The catalytic effect of growing S. aureus cells would be predicted to be even more robust than that of the stoichiometric agent hemoglobin. Therefore, we compared the growth of lasR mutants and wild type bacteria in the presence and absence of S. aureus.
The CF pathogen S. aureus increases the growth of P. aeruginosa lasR mutants, apparently through NO· detoxification
When grown near S. aureus, lasR mutants exhibited wild-type growth phenotypes, as manifested by thicker colonies, using either clinical isolates or laboratory strains of each species (Figs. 3B–C). This phenotypic change did not require contact with S. aureus. Cell-free culture medium, cell sonicates, and organic extracts of S. aureus cultures did not exhibit the activity of S. aureus colonies, suggesting that S. aureus cell activity was required for this phenotypic change.
To further characterize the growth of lasR mutants and its modification by S. aureus, we inoculated static, liquid cultures with equal numbers of P. aeruginosa wild-type and lasR mutant cells in the presence or absence of wild-type or mutant S. aureus partially defective for NO· degradation (hmp mutants [24]), and measured the growth of each strain after incubation. As in previous experiments (e.g., Fig. 2A) [11], P. aeruginosa lasR mutants grown alone did not have a growth defect relative to wild-type strains and isolates in these nutrient conditions (not shown). We found that lasR mutant growth was enhanced by co-culture with wild-type S. aureus, but not by hmp mutant S. aureus (Fig. 3D). In addition, lasR mutant colonies growing on LB agar near colonies of hmp mutant S. aureus displayed substantially more autolysis than did lasR mutants growing near wild-type S. aureus (not shown), supporting the notion that S. aureus NO· detoxification is required to impede lasR P. aeruginosa colony autolysis. These results suggest that the presence of S. aureus, which commonly co-infects CF airways with P. aeruginosa [29], encourages the growth of lasR mutant P. aeruginosa by detoxifying NO·. This effect of S. aureus and other microbes could contribute to the relatively low tensions of NO· observed within CF airways [19], which would be predicted to further encourage the growth of lasR mutant P. aeruginosa by providing a mechanism to mitigate the toxic effects resulting from the shift to nitrate metabolism.
Oxygen utilization is diminished in lasR mutants, resulting in resistance to oxidative stress
Low molecular oxygen tension and abundant nitrogen oxides have been observed in CF secretions [18],[20]. Furthermore, deficiency in las signaling has been shown to result in decreased expression of cytochromes central to oxygen utilization [30]. Therefore, P. aeruginosa lasR mutants could have decreased utilization of oxygen as an electron acceptor. To test this hypothesis, we examined rates of oxygen utilization in liquid (Fig. 4A) and agar-grown (not shown) P. aeruginosa cultures. lasR mutant cultures exhibited oxygen consumption rates at approximately 40–50% those of wild-type cultures (determined by comparing slopes of lines fit to each dataset from 1–5 minutes in Fig. 4A). Aerobic metabolism generates toxic reactive oxygen species (ROS), including superoxide (O2−·) [31]. As lasR mutant cells exhibit decreased rates of oxygen utilization relative to wild-type cells (Fig. 4A), lasR mutant cells could consequently contain lower endogenous levels of ROS. Hydroethidine is a specific fluorescent indicator of intracellular O2−· [32]. Hydroethidine addition to air-grown agar (Fig. 4B) or liquid (not shown) cultures of lasR mutants yielded much lower cell fluorescence than did its addition to wild-type cultures. We demonstrated that cell permeability was equivalent in wild-type PA14 and lasR mutant cells using two established methods: one measuring uptake of ethidium bromide during efflux pump chemical blockade [33] and another based on the uptake of the fluorescent molecule NPN without efflux pump inactivation [34] (data not shown). These results indicate that intracellular O2−· concentrations are lower in lasR mutant cells than in wild-type cells. By analogy to the increased susceptibility of lasR mutants to exogenous nitrosative stress associated with higher endogenous NO· production (Figs. 1E and 2A–B), these results suggest that lasR mutant cells would be more resistant to exogenous sources of oxidative stress, including redox-cycling agents [31],[35].
Fig. 4. lasR mutant P. aeruginosa strains and isolates exhibit lower rates of oxygen utilization and resistance to paraquat, tobramycin and ciprofloxacin. 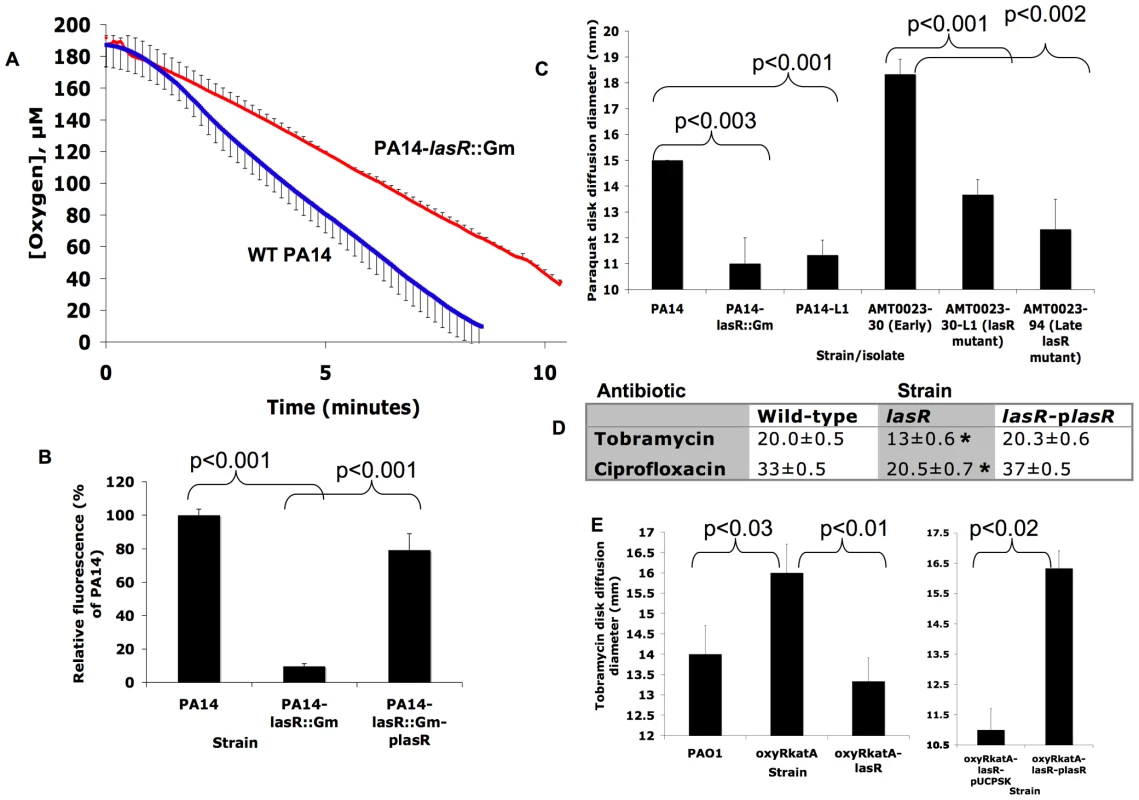
(A) Change in oxygen concentration during stirred incubation of washed cells of the indicated strains resuspended at equivalent cell densities in LB with 400 µM KNO3 at 37°C. Average of 3 experiments ±s.d.; results representative of 3 separate experiments. Slopes for lines fit to each dataset between 1 and 5 minutes were significantly different (p<0.04). Complementation of lasR mutants with a wild-type copy of lasR on a plasmid restored wild-type phenotypes (not shown). The difference was no longer statistically significant in the absence of added KNO3 (LB was shown previously to contain approximately 23 µM NO3− [71]; not shown). (B) Fluorescence yields generated by adding a saturated DMSO solution of hydroethidine (HE), a probe of superoxide concentration [32], for 5 minutes on lawns of the indicated strains (where plasR indicates complementation with a wild-type copy of lasR on a plasmid) grown on LB agar. Average ±s.d. of triplicates and representative of five separate experiments; similar results were obtained in liquid cultures and with clinical isolate pairs for Patient 1 (not shown). (C) Zone diameters of growth inhibition for the indicated clinical isolates and strains by disks containing 1 µmol of paraquat after 24 hours' incubation in air at 37°C on LB agar with 400 µM KNO3. Results shown are average ±s.d. for triplicates and are representative of >10 separate experiments. Complementation with a copy of lasR on a plasmid restored wild-type phenotypes to lasR mutants (data not shown). (D) As in (C), except with disks containing 3.75 µg of ciprofloxacin or 3 µg of tobramycin on MH agar and 400 µM KNO3 (the lasR mutant strain tested for tobramycin susceptibility was PA14-L1, which does not contain an engineered aminoglycoside resistance gene). Average ±s.d. for triplicates. *, p<0.001 compared both with wild-type and the complemented mutant. No decreases in susceptibility were noted with disks of control antibiotics: carbenicillin, tetracycline, aztreonam, and polymyxin. Results with the unmarked deletion strain PA14ΔlasR were similar to those with the lasR mutants shown for both (C) and (D). (E) Tobramycin disk diffusion diameters for experiments as in 5d except with the indicated strains. Experiment at right compares the oxyRkatA-lasR mutant carrying an empty plasmid vector with the same strain carrying the same plasmid but with a wild-type copy of lasR, and on agar media containing 300 µg/mL carbenicillin for plasmid maintenance. Similar results were observed for disks of ciprofloxacin (not shown). Results shown are averages ±s.d. for triplicates. To test this hypothesis, we measured the response of P. aeruginosa cultures to the redox-cycling agent paraquat, which reacts with intracellular oxygen to generate O2−· [35]. As shown in Fig. 4C, cultures of lasR mutants (in both laboratory strain and clinical isolate backgrounds) were more resistant to paraquat and, as with nitrite susceptibility (Fig. 2B, far right), this effect was present in lasR mutant clinical isolates after several years of infection (Fig. 4C, far right). Differences in susceptibility to exogenous hydrogen peroxide exhibited the same trend, but to a lesser extent (not shown). Thus, the susceptibility of lasR mutant P. aeruginosa to exogenous oxidative stress is altered, apparently due to lower endogenous production of ROS and higher residual capacity for detoxification. Polyacrylamide gel enzymatic activity assays [31] demonstrated that lasR mutant cells and their wild-type counterparts exhibited similar activities of superoxide dismutases, enzymes that degrade O2−· (data not shown), supporting the concept that the differences in endogenous O2−· levels, and susceptibility to paraquat, were due to differences in O2−· production rather than differences in O2−· degradation.
lasR mutants have a growth advantage under conditions of oxidative stress
The lower endogenous O2−· concentrations of lasR mutants indicate that they might have a growth advantage compared with wild-type cells when grown under conditions of oxidative stress. To test this hypothesis, agar-suspended cultures were inoculated with equal numbers of lasR and wild-type cells in the presence of paraquat, and then the density of each strain was determined in serial, thin culture slices. Using an oxygen microprobe, oxygen concentration within these cultures became undetectable within approximately 2 mm of depth below the surface after 24 hours of incubation (data not shown). This growth medium is a viscous gel, limiting the motility and sedimentation of cells and thus preserving two-dimensional culture structure, resulting in the establishment of a stable oxygen gradient. In this way, this culture may reproduce some aspects of CF respiratory secretions, which are relatively viscous compared to liquid cultures and exhibit oxygen gradients [20]. Furthermore, as ROS such as O2−· are side-products of oxygen-based respiration, ROS are produced at decreasing amounts with increased depth within the cultures. As shown in Fig. 5A, lasR cells greatly outcompeted wild-type cells at more superficial depths, where oxygen was detectable and O2−· could be produced upon paraquat exposure. This effect diminished with increasing depth and, therefore, with lower oxygen concentration. Thus, under growth conditions in which superoxide is generated, lasR mutants have a relative fitness advantage.
Fig. 5. Resistance of lasR mutant P. aeruginosa to tobramycin and ciprofloxacin is oxygen-dependent. 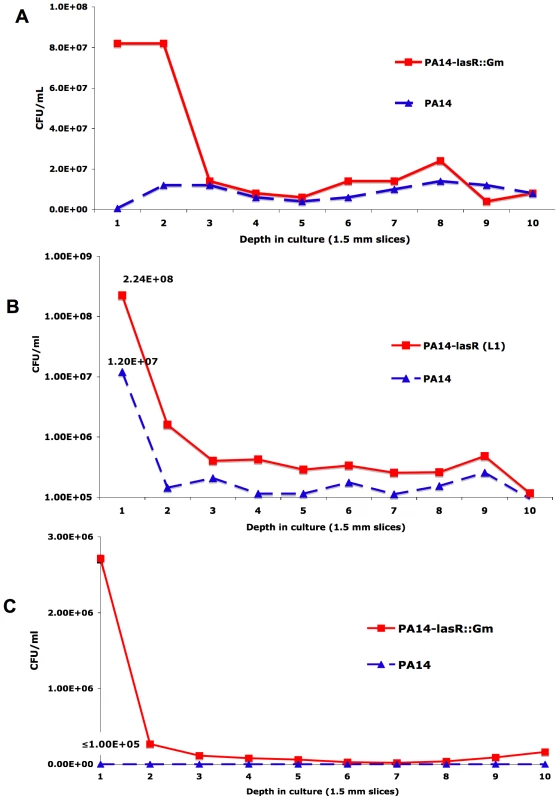
(A) Colony counts from serial slices of an agar-suspended culture with LB+400 µM KNO3 containing 5 mM paraquat inoculated with equal numbers of cells of PA14 and a derived lasR mutant (PA14-lasR::Gm) and incubated for 48 hours. (B) The same experiment as in (A), except with 1 µg/mL tobramycin instead of paraquat, and using PA14-L1 (because this lasR mutant lacks an aminoglycoside resistance cassette). (C) The same experiment as in (B), except with 0.25 µg/mL ciprofloxacin and with PA14-lasR::Gm. All results representative of at least 3 independent experiments. No differences in cell density were noted in the absence of antibiotics or paraquat under these conditions after 48 hours of growth (not shown), in agreement with liquid growth findings [11]. The shift to increased nitrate based metabolism by lasR mutants confers tolerance to antibiotics commonly used in CF treatment
One condition under which ROS are generated within bacterial cells is upon exposure to bactericidal antibiotics, including fluoroquinolones and aminoglycosides, under aerobic conditions [22],[36]. Bacterial killing by both classes of antibiotics has been shown to be attributable in part to induction of superoxide production [37]. In addition, efficient aminoglycoside uptake (and thus bacterial killing) requires aerobic electron transport [23]. Furthermore, a las-regulated P. aeruginosa exoproduct, the Pseudomonas quinolone signal, induces an oxidative stress response, increased cellular ROS, and increased susceptibility to fluoroquinolones in P. aeruginosa [38], functionally linking response to oxidative stress and susceptibility to fluoroquinolones. A relationship between fluoroquinolone susceptibility and oxidative stress is supported by work in other bacterial species [39], including the observation that spontaneous mutants in superoxide response regulators have been selected by exposure of both Escherichia coli and Salmonella enteritidis to fluoroquinolones [40]. Thus, we predicted that the lower oxygen utilization rates and increased resistance to sources of superoxide exhibited by lasR mutants would result in decreased susceptibility to the fluoroquinolone ciprofloxacin and the aminoglycoside tobramycin, both of which are used frequently to treat CF patients [41]. As shown in Fig. 4D, surface cultures on nitrate-containing agar medium of lasR mutants were less susceptible to disks containing these drugs. Agar-suspended cultures in the same medium demonstrated that these differences were oxygen-dependent (Figs. 5B–C), as with paraquat (Fig. 5A). These results suggest that, under these culture conditions, inactivating lasR mutation confers resistance to two of the antibiotics used most frequently in CF care, tobramycin and ciprofloxacin. To further investigate the relationship between oxidative stress and antibiotic resistance, we compared the susceptibilities to oxidative stress and antibiotics of strains of P. aeruginosa carrying the double mutation oxyRkatA, or the triple mutation oxyRkatAlasR. Strains null for oxyR and katA are defective for the defensive response to oxidative stress [36]; accordingly, the oxyRkatA mutant exhibited increased susceptibility to paraquat compared with wild-type (Fig. S1). However, the oxyRkatAlasR triple mutant was even more resistant to paraquat than was wild-type, confirming and extending the observation that a lasR mutation confers resistance to ROS (not shown). Similarly, the oxyRkatA mutant was more susceptible to tobramycin (Fig. 4E) (as shown for the oxyR single mutant and the aminoglycoside gentamicin [36]) and to ciprofloxacin (not shown) than was wild-type; as with paraquat, the oxyRkatAlasR triple mutant exhibited resistance to each of these drugs, an effect that was reversed by complementation with a wild-type copy of lasR on a plasmid (Fig. 4E and data not shown). These results indicate that lasR mutation confers resistance to these two antibiotics through its effects on respiratory activity and oxidative stress response. As lasR mutant strains and isolates also exhibit increased tolerance to some β-lactams due to increased β-lactamase activity [11], these results suggest that the emergence of lasR mutant isolates during chronic infections could adversely impact the clinical response to all three of the antibiotic classes used most commonly during standard CF treatment (β-lactams, fluoroquinolones, and aminoglycosides). The recent discovery [42] that increased bacterial production of NO· (which is increased by LasR inactivation, Fig. 1E) confers additional protection against a wide variety of antibiotics, including β-lactams, quinolones, and aminoglycosides, further supports this possibility.
Discussion
In this work, P. aeruginosa isolates with inactivating mutations in the AHL-responsive transcriptional regulator LasR exhibited a profound growth advantage with nitrogen substrates found in the CF airway. These differences are attributable to lasR-dependent increased utilization of nitrogen oxides and decreased utilization of oxygen. This metabolic shift results in an increase in the production of the RNS NO·, and a corresponding decrease in the ROS O2−·, the latter of which is associated with decreased susceptibility in our conditions to at least two antibiotics used frequently in treating CF lung infections. This growth advantage in conditions characteristic of CF airways, and the resulting antibiotic resistance, may explain the observed high prevalence of LasR mutants and the associated worse lung function of CF patients whose airways contain these mutants [8].
The metabolic changes that occur upon lasR inactivation would be predicted to favor growth of lasR mutants arising spontaneously in the CF airway due to the confluence of selective forces encountered in this environment. For example, the abundant NO3− and NO2− [17],[18] and low oxygen tensions [20] found in CF secretions, as well as the relatively low NO· levels [18], would provide optimal metabolic conditions for lasR mutant selection. As suggested previously [43], P. aeruginosa likely adapts to a continuum of different oxygen tensions, with variation in the relative ratio of oxygen and nitrate utilization. Inactivating mutations in lasR may confer advantages in a variety of these microenvironments found in the CF lung. Also contributing to the beneficial nature of this environment for lasR mutant growth is the presence of NO·-detoxifying microbes, such as S. aureus and perhaps anaerobic bacteria, the latter of which were recently found to occupy CF secretions at high densities [44]; it should be noted that, while contact of lasR mutant P. aeruginosa with wild-type P. aeruginosa was also shown previously to reverse autolysis and sheen [11], it is not yet clear whether the mechanism of this effect is similar to that of S. aureus. The availability of amino acids as nutrient sources in CF secretions [13] would provide an additional selective pressure for lasR mutant growth [11]. Similarly, lasR mutants are relatively resistant to sources of oxidative stress, including tobramycin and ciprofloxacin (Figs. 4D–E), two antibiotics that, along with ceftazidime (to which lasR mutants are also relatively tolerant due to augmented β-lactamase activity [11]), are among the antibiotics used most commonly in CF treatment [41]. Although other sources of ROS are present in CF airways, such as H2O2 from host cells [36], whether exogenously adding these molecules to P. aeruginosa effectively confers intracellular oxidative stress is not as clear as is is the effect of the above antibiotics [22],[23]. While the results presented here demonstrate that nutrient conditions (particularly relating to oxygen and nitrogen oxides) are sufficient to enrich for lasR mutant growth in vitro, the frequent treatment of CF patients with the above antibiotics likely provides additional selection for these mutants, resulting in a complex dynamic between the CF airway nutrient environment, P. aeruginosa adaptation, therapy, and pathophysiology. These ideas are summarized in the model in Fig. 6.
Fig. 6. A model for metabolic changes in CF-adapted lasR mutant isolates of P. aeruginosa. 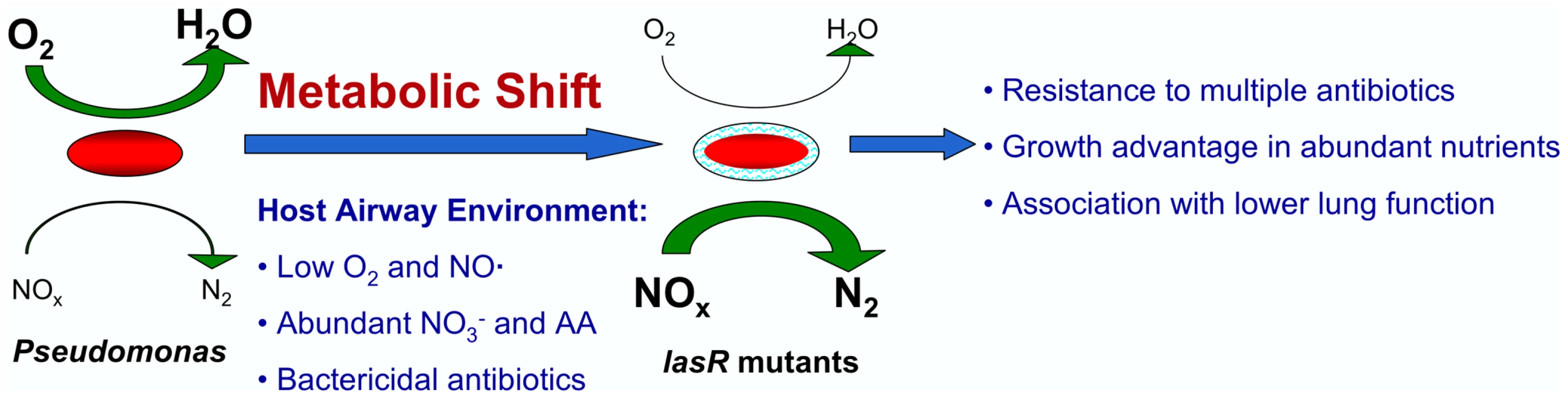
According to the model, patients are initially infected with environmental isolates carrying wild-type copies of the lasR gene (left). These isolates have relatively high utilization of oxygen (activities indicated by the sizes of the green arrows) and lower utilization of nitrogen oxides (NOx). Selective pressures encountered in the host, including abundant host NO3− and amino acids (AA), low host NO·, the presence of other bacterial species that metabolize NO·, reduced O2 concentrations, and treatment with β−lactams or antibiotics that generate ROS, favor the emergence of lasR mutant isolates with higher utilization of nitrates and lower utilization of oxygen. This metabolic shift confers a growth advantage in the nutrient conditions in the CF airway, including abundant NO3−, and relative resistance to the antibiotics used most frequently to treat CF patients. There are multiple therapeutic and pathophysiologic implications of the model in Fig. 6. For example, assuming that P. aeruginosa infection leads to airway inflammation, and thus to obstructive lung disease, as suggested by current models of CF pathogenesis [41], the growth advantage of lasR mutant cells within the CF airway would be predicted to render such mutants more pathogenic to CF patients by virtue of higher cell density and greater consequent inflammation. (It should be noted that while lasR mutant P. aeruginosa strains were shown to be less pathogenic in animal models of short-term respiratory infection [45], those models may not accurately reflect the pathogenic mechanisms of chronic CF airway infection, during which many “acute” virulence factors are not expressed [46]). This effect may contribute to the observed association between lasR mutant CF airway infection and worse lung function [8]. Furthermore, the clinical response to standard antibiotic therapy in patients infected with lasR mutants would be predicted to be poor relative to patients with wild type isolates, perhaps further contributing to the clinical impact and rendering eradication increasingly difficult [8]. Thus, the presence of lasR mutants in CF respiratory cultures may be of prognostic value, and aggressive, directed treatment of these mutants upon isolation (i.e., through the expanded use of monobactams, tetracyclines, or polymyxin in the case of lasR mutant infection) or with regimens that do not select for their growth may lead to improved outcomes.
While recent publications have shown that quorum sensing regulates the expression of denitrification genes [14],[15],[16] and oxygen metabolic genes [30] at the transcriptional level, the mechanism of the distinct metabolic behaviors of lasR mutant and wild-type cells is likely to be as complex as the quorum sensing system itself. In P. aeruginosa, quorum sensing involves at least three parallel signaling systems, at least four different signal receptors, and regulation by diverse environmental cues [14],[47],[48]. However, some mechanistic clues are evident from our results. Previously, we showed that the two-component metabolic regulatory system CbrAB contributes to the metabolic phenotypes of lasR mutant clinical isolates of P. aeruginosa [11]; mutants in this system have decreased capacities to use amino acids as nitrogen sources [49], and lasR mutant isolates have upregulated expression of the transcriptional metabolism regulator cbrB [11]. The current results also suggest an additional mechanism for the growth advantage of lasR mutant P. aeruginosa in specific amino acids (most markedly with phenylalanine, but also with other aromatic and branched-chain amino acids [11]). Many enzymes that metabolize amino acids are inactivated by reactive oxygen species (ROS), including the first enzyme in the phenylalanine catabolic pathway, phenylalanine hydroxylase [50],[51]. Therefore, cells with lower intracellular concentrations of ROS, such as lasR mutants (Fig. 4B), would be predicted to be better able to utilize amino acids such as phenylalanine as nutrient sources. Additionally, the las system is involved in regulating the levels and timing of production of a family of hydroxyalkylquinoline (HAQ) molecules [52], including the compounds 4-hydroxy-2-heptylquinoline (HHQ), the overproduction of which generates the sheen characteristic of lasR mutant colonies [11]; its N-oxide HQNO, which is a redox-cycling agent [29]; and the Pseudomonas quinolone signal (PQS) [52]. Exposure to PQS was shown to modify P. aeruginosa responses to reactive oxygen species and ciprofloxacin [38], suggesting a functional linkage between HAQs, oxidative stress responses, and susceptibility to fluoroquinolones. Therefore, these quinolines may regulate metabolic properties in both source and neighboring cells, and temporal differences in their production resulting from LasR inactivation may contribute to the observed metabolic changes.
Numerous explanations have been offered for the identification of lasR mutant P. aeruginosa in diverse clinical and experimental conditions [10],[11],[12],[53],[54],[55],[56],[57],[58],[59],[60]. For example, in experimental growth medium in which P. aeruginosa growth requires the production of lasR-regulated protease, lasR mutants emerge that “cheat” from the protease produced by wild-type strains [59],[60]. However, CF sputum is abundant in free amino acids [13] (upon which P. aeruginosa lasR mutants can grow without requiring protease [11],[59]), and it has been shown that both laboratory strains [61] and clinical isolates [12] may produce protease in the absence of a functional las system. Furthermore, lasR mutants are frequently isolated from CF sputum without detectable wild-type co-isolates [2],[11]. These findings suggest that cheating alone does not explain the high prevalence of lasR mutants among people with CF. Alternatively, it has been suggested that lasR mutants emerge due to physiological characteristics that confer relative fitness advantages in specific growth conditions [11],[56]. The current results support the hypothesis that lasR mutant P. aeruginosa have a growth advantage in nutrient and antibiotic conditions found in the CF airway (as summarized in Fig. 6). While it is unclear which of these forces, antibiotics or nutrients, predominates in vivo in selecting for inactivating lasR mutations, their combination would be predicted to exert powerful pressure against LasR function.
The hypothesis that P. aeruginosa adaptation to the CF airway is driven in large part by metabolic forces found in CF airway secretions is supported by findings with other adapted mutants from chronic infections. For example, mucoid P. aeruginosa strains and isolates also exhibit upregulated NO3− metabolism relative to non-mucoid P. aeruginosa and, as a result, are more susceptible than nonmucoid isolates to acidified NO2− [27]. Furthermore, the mucoid phenotype is promoted by hypoxia [20]. Similarly, P. aeruginosa isolates with another CF adaptation, mutations that upregulate the glucose-6-phosphate dehydrogenase gene zwf, confer resistance to oxidative stress and paraquat [62],[63]. The enrichment for lasR mutant P. aeruginosa, with growth advantages in CF airway conditions, by S. aureus is also reminiscent of the reverse interaction: the selection of S. aureus metabolic mutants, known as small-colony variants (SCVs), due to co-culture with wild-type P. aeruginosa [29]. S. aureus SCVs are defective for aerobic growth, are resistant to aminoglycoside antibiotics such as tobramycin, and frequently exhibit both increased expression of denitrification genes [64] and associated increased susceptibility to NO2− [65], much like lasR mutant P. aeruginosa. The symmetry of this S. aureus-P. aeruginosa relationship, in each direction favoring the growth of antibiotic-resistant, metabolic mutants with decreased aerobic activity, further suggests a common mechanism for selection during chronic CF infections, and perhaps during many other chronic infections, driven by the metabolic forces present in host tissues. In support of this hypothesis, the likelihood of persistent, latent infection by the respiratory pathogen Mycobacterium tuberculosis is thought to be determined in large part by the lung metabolic milieu, particularly the relative ambient concentrations of nitrogen and oxygen species [66]. Similarly, the pathogenic fungus Cryptococcus neoformans exhibits early metabolic adaptations in animal models of chronic pulmonary infection, including altered responses to nitrosative stress and superoxide [67]. As with M. tuberculosis [68], these findings support the concept that chronic CF airway infections with P. aeruginosa could be amenable to therapies that increase airway nitrosative stress. Such therapies could include inhaled NO2− [27] or L-arginine [19], two treatments already being examined as candidate CF treatments. Our results support the utility of these treatments both in preventing P. aeruginosa adaptive changes associated with advanced lung function decline [7],[8] and that may be attributable to current antibiotic regimens (Fig. 6), as well as in treating patients with advanced infection in which these adaptations have already occurred.
In summary, the nutrient conditions characteristic of the CF airway select for growth of lasR mutant P. aeruginosa, resulting in decreased susceptibility to antibiotics without the need for antibiotic exposure. Adaptation of many microbes to new environments during chronic infections may commonly result in metabolic changes that impact response to antibiotics. This scenario may be particularly relevant for opportunistic pathogens such as P. aeruginosa, many of which naturally occupy competitive and nutrient-poor environmental niches like soil and water, as they adapt to the specific nutrient conditions found in host environments such as the nitrogen-rich CF airway.
Materials and Methods
Bacteria
Table 1 lists the bacterial strains and isolates used in this work, except for the strains carrying transposon insertion mutations in nitrate metabolic genes, which were obtained from the PA14 transposon insertion mutant library [69]. The origins of all strains and isolates are described in the references provided in Table 1, except for the narK1K2 and narK1K2lasR mutants, described below.
Tab. 1. List of strains used in the described experiments. 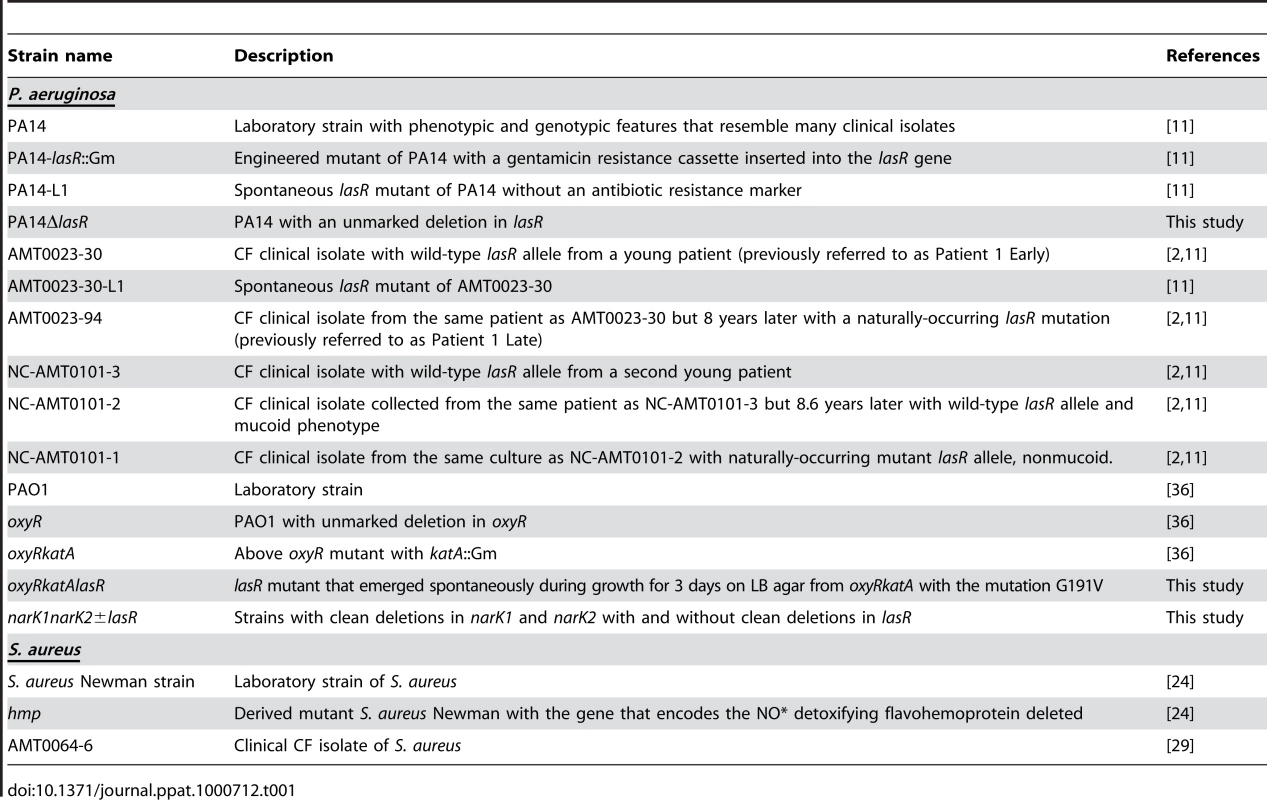
Mutant construction and plasmids
Each deletion in the lasR, narK1K2 and narK1K2lasR mutants was generated using allelic exchange with sacB-containing counterselectable gene replacement vectors using sucrose counterselection essentially as described [70]. Briefly, the lasR gene was entirely deleted from the chromosome except for the start and stop codon, using the plasmid sacB-based pEX18Gm for integration and excision. The narK1-narK2 genes, which are organized tandemly as an operon, were deleted as a one continuous stretch of DNA using identical methods, both in wild-type PA14 as well as the lasR mutant background. The deletion removed the narK coding sequence beginning from the 30th codon of narK1 until the 462nd codon of narK2, leaving the first 29 codons of narK1 and the last 7 codons of narK2 intact.
The plasmid pUCPSK-lasR was the kind gift of Eric Déziel and was used for complementation of lasR deficient strains and isolates as described [61].
Growth conditions and chemicals
Except where indicated, all cultures were inoculated from LB overnight cultures of bacteria or cells suspended from LB agar cultures. Liquid static cultures were grown in LB with 400 µM KNO3 (Sigma) except where indicated otherwise. Phosphate buffered LB agar was prepared as described [27]. Chemically defined PN medium was prepared as previously described [24], and consists of a phosphate buffer supplemented with a carbon source (glucose), nitrogen and sulfur sources [(NH4)2SO4 and MgSO4], amino acids, nucleic acid bases, and vitamins (thiamine, niacin, biotin, and pantothenic acid).
Chemicals
Hemoglobin, hydroethidine, tobramycin, paraquat (methylviologen dichloride hydrate), potassium nitrate, and sodium nitrite were obtained from Sigma. NO donors DEANO (DEA-NONOate) and ProliNO (Proli-NONOate) were purchased from AG Scientific (San Diego, CA) and SperNO was obtained from CalBiochem (San Diego, CA). Ciprofloxacin was from Biochemika/Sigma. Prepared antibiotic disks with tobramycin, kanamycin, gentamicin, carbenicillin, tetracycline, aztreonam, ceftazidime, and polymyxin B were from Becton Dickinson. Growth media and agar were from Becton Dickinson & Co.
Growth assays
Growth of cells in the indicated liquid media was measured optically using a BioScreen C Microbiology Microplate reader (Growth Curves USA, Piscataway, NJ) without shaking (except immediately prior to readings), a condition that limits oxygen mass-transfer. Assays to look for mutant sectors were performed by inoculating 10 µl drops of 1∶10-diluted overnight cultures on LB with 400 µM KNO3, followed by incubation at 37°C for 24 hours and then at room temperature for up to approximately 1 month thereafter.
Assays for denitrification activity and NO2− susceptibility
NO· was quantified using an ISO-NOPMC Mark II electrode (WPI Instruments, Fl) and dissolved oxygen was measured in parallel using a Clark-type electrode MLT1120 (ADI Instruments) with standard curves as per manufacturer instruction. Data from both probes were analyzed through an Analog Adapter MLT1122 (ADI Instruments). NO2− disk diffusion on acidified, buffered LB agar was performed as described [27], except that all incubations were performed with aerobic growth.
Assay for oxygen utilization
Respiration rates in liquid cultures were measured by resuspending PBS-washed cells in prewarmed LB with 400 µM KNO3 in a microrespiration system (Unisense AS, Denmark). Calibrations were performed according to manufacturer's instructions using air-purged and argon-purged growth medium.
Hydroethidine assay
Fluorescence after hydroethidine addition to lawns of cells during growth on LB agar (similar results were obtained with and without added NO3−) was measured using excitation/emission wavelengths of 396/570 nm [32], followed by photography and quantitation using NIH ImageJ software (NIH, Bethesda, Md, http://rsb.info.nih.gov/ij/).
Agar growth assay
Agar-suspended cultures were grown in 0.9% LB agar inoculated with equal cell numbers of all cell types - approximately 105 CFU of the indicated strains (resulting in a final cell density of approximately 2×103 CFU/mL), except when indicated otherwise - and with chemicals and antibiotics added as indicated. In each case, the prepared agar was inoculated with bacteria when the medium had cooled after autoclaving to approximately 37°C but before gelling. The medium was then poured into 10 mL syringes from which the port ends had been removed, leaving an open end, which was loosely covered for incubation. After incubation, the plunger of the syringe was depressed slowly, ejecting a cylinder of culture. Serial, 1.5 mm slices of culture were removed and added to 1 mL each of sterile PBS, and vortexed for 30 seconds before enumeration of cells from the resulting solution by plating.
Nitrogen metabolic assays
NO2− production was measured using the Griess Reagent System kit (Promega, Madison WI). Nitrate was quantified enzymatically using a commercially available reagent set (R-Biopharm, Marshall, MI). Rates of NO· degradation were determined as previously described [24]; briefly, five milliliter cultures in PN medium were grown by shaking at 37°C to an OD660≈0.4. Cells were then resuspended to 1×108 cfu ml−1 in 8 ml final volume. A two-hole rubber stopper sealed with Parafilm enclosed the cell suspension in an 8 ml glass vial with no gaseous headspace. Cells were stirred vigorously at 37°C as ProliNO was added through one open port to 1 µM. The resulting immediate release of approximately 2 µM NO· followed by the gradual decay of detectible signal was recorded and normalized to the fraction of initial [NO·]. Measurements were performed in triplicate for each strain tested. NO· susceptibility was determined by measuring the lag in growth after bacterial cultures in LB medium were supplemented with 0.5 or 2.5 mM SperNO (t1/2 = 39 min at 37°C).
Oxygen metabolism in deep-agar cultures
Deep-agar cultures inoculated with serial dilutions of P. aeruginosa lasR and wild-type cells in LB-0.9% agar with and without 400 µM KNO3 and with and without paraquat were grown overnight at 37°C. Oxygen concentrations were subsequently recorded using a microsensor setup (OX 10 oxygen microsensor, PA 2000 picoammeter, both from Unisense AS, Denmark) at 37°C in a preconditioned water bath. Data were recorded using SensorTrace Basic software (Unisense). The probe was advanced into the agar, and measurements taken, in 50 µm increments.
Statistics
Differences between experimental measurements were computed using unpaired, two-tailed Student's t-tests.
Supporting Information
Zdroje
1. GiannakisM
ChenSL
KaramSM
EngstrandL
GordonJI
2008 Helicobacter pylori evolution during progression from chronic atrophic gastritis to gastric cancer and its impact on gastric stem cells. Proc Natl Acad Sci U S A 105 4358 4363
2. SmithEE
BuckleyDG
WuZ
SaenphimmachakC
HoffmanLR
2006 Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci U S A 103 8487 8492
3. GovanJR
DereticV
1996 Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev 60 539 574
4. MahenthiralingamE
CampbellME
SpeertDP
1994 Nonmotility and phagocytic resistance of Pseudomonas aeruginosa isolates from chronically colonized patients with cystic fibrosis. Infect Immun 62 596 605
5. ErnstRK
AdamsKN
MoskowitzSM
KraigGM
KawasakiK
2006 The Pseudomonas aeruginosa lipid A deacylase: selection for expression and loss within the cystic fibrosis airway. J Bacteriol 188 191 201
6. BurnsJL
Van DalfsenJM
ShawarRM
OttoKL
GarberRL
1999 Effect of chronic intermittent administration of inhaled tobramycin on respiratory microbial flora in patients with cystic fibrosis. J Infect Dis 179 1190 1196
7. LiZ
KosorokMR
FarrellPM
LaxovaA
WestSE
2005 Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. Jama 293 581 588
8. HoffmanLR
KulasekaraHD
EmersonJ
HoustonLS
BurnsJL
2009 Pseudomonas aeruginosa lasR mutants are associated with cystic fibrosis lung disease progression. J Cyst Fibros 8 66 70
9. GibsonRL
EmersonJ
Mayer-HamblettN
BurnsJL
McNamaraS
2007 Duration of treatment effect after tobramycin solution for inhalation in young children with cystic fibrosis. Pediatr Pulmonol 42 610 623
10. SalunkheP
SmartCH
MorganJA
PanageaS
WalshawMJ
2005 A cystic fibrosis epidemic strain of Pseudomonas aeruginosa displays enhanced virulence and antimicrobial resistance. J Bacteriol 187 4908 4920
11. D'ArgenioDA
WuM
HoffmanLR
KulasekaraHD
DezielE
2007 Growth phenotypes of Pseudomonas aeruginosa lasR mutants adapted to the airways of cystic fibrosis patients. Mol Microbiol 64 512 533
12. TingpejP
SmithL
RoseB
ZhuH
ConibearT
2007 Phenotypic characterization of clonal and nonclonal Pseudomonas aeruginosa strains isolated from lungs of adults with cystic fibrosis. J Clin Microbiol 45 1697 1704
13. BarthAL
PittTL
1996 The high amino-acid content of sputum from cystic fibrosis patients promotes growth of auxotrophic Pseudomonas aeruginosa. J Med Microbiol 45 110 119
14. WagnerVE
BushnellD
PassadorL
BrooksAI
IglewskiBH
2003 Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J Bacteriol 185 2080 2095
15. ToyofukuM
NomuraN
FujiiT
TakayaN
MasedaH
2007 Quorum sensing regulates denitrification in Pseudomonas aeruginosa PAO1. J Bacteriol 189 4969 4972
16. YoonSS
HenniganRF
HilliardGM
OchsnerUA
ParvatiyarK
2002 Pseudomonas aeruginosa anaerobic respiration in biofilms: relationships to cystic fibrosis pathogenesis. Dev Cell 3 593 603
17. PalmerKL
BrownSA
WhiteleyM
2007 Membrane-bound nitrate reductase is required for anaerobic growth in cystic fibrosis sputum. J Bacteriol 189 4449 4455
18. GrasemannH
IoannidisI
TomkiewiczRP
de GrootH
RubinBK
1998 Nitric oxide metabolites in cystic fibrosis lung disease. Arch Dis Child 78 49 53
19. GrasemannH
KurtzF
RatjenF
2006 Inhaled L-arginine improves exhaled nitric oxide and pulmonary function in patients with cystic fibrosis. Am J Respir Crit Care Med 174 208 212
20. WorlitzschD
TarranR
UlrichM
SchwabU
CekiciA
2002 Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest 109 317 325
21. ChenF
XiaQ
JuLK
2006 Competition between oxygen and nitrate respirations in continuous culture of Pseudomonas aeruginosa performing aerobic denitrification. Biotechnol Bioeng 93 1069 1078
22. KohanskiMA
DwyerDJ
HayeteB
LawrenceCA
CollinsJJ
2007 A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130 797 810
23. BryanLE
1985 Antibiotic uptake and the cytoplasmic membrane. Antibiot Chemother 36 103 110
24. RichardsonAR
DunmanPM
FangFC
2006 The nitrosative stress response of Staphylococcus aureus is required for resistance to innate immunity. Mol Microbiol 61 927 939
25. AraiH
HayashiM
KuroiA
IshiiM
IgarashiY
2005 Transcriptional regulation of the flavohemoglobin gene for aerobic nitric oxide detoxification by the second nitric oxide-responsive regulator of Pseudomonas aeruginosa. J Bacteriol 187 3960 3968
26. RichardsonAR
LibbySJ
FangFC
2008 A nitric oxide-inducible lactate dehydrogenase enables Staphylococcus aureus to resist innate immunity. Science 319 1672 1676
27. YoonSS
CoakleyR
LauGW
LymarSV
GastonB
2006 Anaerobic killing of mucoid Pseudomonas aeruginosa by acidified nitrite derivatives under cystic fibrosis airway conditions. J Clin Invest 116 436 446
28. KlausenM
HoffmanLR
D'ArgenioDA
Tolker-NielsenT
MillerSI
2005 In vitro Interactions Between Pseudomonas aeruginosa and Staphylococcus aureus. Proceedings: 10th International Congress on Pseudomonas
29. HoffmanLR
DezielE
D'ArgenioDA
LepineF
EmersonJ
2006 Selection for Staphylococcus aureus small-colony variants due to growth in the presence of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 103 19890 19895
30. SchusterM
LostrohCP
OgiT
GreenbergEP
2003 Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J Bacteriol 185 2066 2079
31. HassettDJ
SchweizerHP
OhmanDE
1995 Pseudomonas aeruginosa sodA and sodB mutants defective in manganese - and iron-cofactored superoxide dismutase activity demonstrate the importance of the iron-cofactored form in aerobic metabolism. J Bacteriol 177 6330 6337
32. RobinsonKM
JanesMS
PeharM
MonetteJS
RossMF
2006 Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci U S A 103 15038 15043
33. BaderMW
NavarreWW
ShiauW
NikaidoH
FryeJG
2003 Regulation of Salmonella typhimurium virulence gene expression by cationic antimicrobial peptides. Mol Microbiol 50 219 230
34. ZhangL
DhillonP
YanH
FarmerS
HancockRE
2000 Interactions of bacterial cationic peptide antibiotics with outer and cytoplasmic membranes of Pseudomonas aeruginosa. Antimicrob Agents Chemother 44 3317 3321
35. HassettDJ
CharnigaL
BeanK
OhmanDE
CohenMS
1992 Response of Pseudomonas aeruginosa to pyocyanin: mechanisms of resistance, antioxidant defenses, and demonstration of a manganese-cofactored superoxide dismutase. Infect Immun 60 328 336
36. HassettDJ
AlsabbaghE
ParvatiyarK
HowellML
WilmottRW
2000 A protease-resistant catalase, KatA, released upon cell lysis during stationary phase is essential for aerobic survival of a Pseudomonas aeruginosa oxyR mutant at low cell densities. J Bacteriol 182 4557 4563
37. WangX
ZhaoX
2009 Contribution of oxidative damage to antimicrobial lethality. Antimicrob Agents Chemother 53 1395 1402
38. HausslerS
BeckerT
2008 The pseudomonas quinolone signal (PQS) balances life and death in Pseudomonas aeruginosa populations. PLoS Pathog 4 e1000166 doi:10.1371/journal.ppat.1000166
39. AlbesaI
BecerraMC
BattanPC
PaezPL
2004 Oxidative stress involved in the antibacterial action of different antibiotics. Biochem Biophys Res Commun 317 605 609
40. O'ReganE
QuinnT
PagesJM
McCuskerM
PiddockL
2009 Multiple regulatory pathways associated with high-level ciprofloxacin and multidrug resistance in Salmonella enterica serovar enteritidis: involvement of RamA and other global regulators. Antimicrob Agents Chemother 53 1080 1087
41. GibsonRL
BurnsJL
RamseyBW
2003 Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med 168 918 951
42. GusarovI
ShatalinK
StarodubtsevaM
NudlerE
2009 Endogenous nitric oxide protects bacteria against a wide spectrum of antibiotics. Science 325 1380 1384
43. Alvarez-OrtegaC
HarwoodCS
2007 Responses of Pseudomonas aeruginosa to low oxygen indicate that growth in the cystic fibrosis lung is by aerobic respiration. Mol Microbiol 65 153 165
44. TunneyMM
FieldTR
MoriartyTF
PatrickS
DoeringG
2008 Detection of anaerobic bacteria in high numbers in sputum from patients with cystic fibrosis. Am J Respir Crit Care Med 177 995 1001
45. WuH
SongZ
GivskovM
DoringG
WorlitzschD
2001 Pseudomonas aeruginosa mutations in lasI and rhlI quorum sensing systems result in milder chronic lung infection. Microbiology 147 1105 1113
46. TummlerB
BosshammerJ
BreitensteinS
BrockhausenI
GudowiusP
1997 Infections with Pseudomonas aeruginosa in patients with cystic fibrosis. Behring Inst Mitt 249 255
47. DiggleSP
CornelisP
WilliamsP
CamaraM
2006 4-quinolone signalling in Pseudomonas aeruginosa: old molecules, new perspectives. Int J Med Microbiol 296 83 91
48. LedghamF
VentreI
SosciaC
FoglinoM
SturgisJN
2003 Interactions of the quorum sensing regulator QscR: interaction with itself and the other regulators of Pseudomonas aeruginosa LasR and RhlR. Mol Microbiol 48 199 210
49. LiW
LuCD
2007 Regulation of carbon and nitrogen utilization by CbrAB and NtrBC two-component systems in Pseudomonas aeruginosa. J Bacteriol 189 5413 5420
50. BenovL
FridovichI
1999 Why superoxide imposes an aromatic amino acid auxotrophy on Escherichia coli. The transketolase connection. J Biol Chem 274 4202 4206
51. FinkRM
ElstnerEF
1984 Studies on the possible mechanism of inactivation of phenylalanine hydroxylase by destructive oxygen species. Z Naturforsch [C] 39 734 737
52. DezielE
LepineF
MilotS
HeJ
MindrinosMN
2004 Analysis of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs) reveals a role for 4-hydroxy-2-heptylquinoline in cell-to-cell communication. Proc Natl Acad Sci U S A 101 1339 1344
53. ZhuH
BandaraR
ConibearTC
ThuruthyilSJ
RiceSA
2004 Pseudomonas aeruginosa with lasI quorum-sensing deficiency during corneal infection. Invest Ophthalmol Vis Sci 45 1897 1903
54. FothergillJL
PanageaS
HartCA
WalshawMJ
PittTL
2007 Widespread pyocyanin over-production among isolates of a cystic fibrosis epidemic strain. BMC Microbiol 7 45
55. LujanAM
MoyanoAJ
SeguraI
ArgaranaCE
SmaniaAM
2007 Quorum-sensing-deficient (lasR) mutants emerge at high frequency from a Pseudomonas aeruginosa mutS strain. Microbiology 153 225 237
56. HeurlierK
DenervaudV
HaenniM
GuyL
KrishnapillaiV
2005 Quorum-sensing-negative (lasR) mutants of Pseudomonas aeruginosa avoid cell lysis and death. J Bacteriol 187 4875 4883
57. DenervaudV
TuQuocP
BlancD
Favre-BonteS
KrishnapillaiV
2004 Characterization of cell-to-cell signaling-deficient Pseudomonas aeruginosa strains colonizing intubated patients. J Clin Microbiol 42 554 562
58. CabrolS
OlliverA
PierGB
AndremontA
RuimyR
2003 Transcription of quorum-sensing system genes in clinical and environmental isolates of Pseudomonas aeruginosa. J Bacteriol 185 7222 7230
59. SandozKM
MitzimbergSM
SchusterM
2007 Social cheating in Pseudomonas aeruginosa quorum sensing. Proc Natl Acad Sci U S A 104 15876 15881
60. DiggleSP
GriffinAS
CampbellGS
WestSA
2007 Cooperation and conflict in quorum-sensing bacterial populations. Nature 450 411 414
61. DekimpeV
DezielE
2009 Revisiting the quorum-sensing hierarchy in Pseudomonas aeruginosa: the transcriptional regulator RhlR regulates LasR-specific factors. Microbiology 155 712 723
62. MaJF
HagerPW
HowellML
PhibbsPV
HassettDJ
1998 Cloning and characterization of the Pseudomonas aeruginosa zwf gene encoding glucose-6-phosphate dehydrogenase, an enzyme important in resistance to methyl viologen (paraquat). J Bacteriol 180 1741 1749
63. Silo-SuhL
SuhSJ
PhibbsPV
OhmanDE
2005 Adaptations of Pseudomonas aeruginosa to the cystic fibrosis lung environment can include deregulation of zwf, encoding glucose-6-phosphate dehydrogenase. J Bacteriol 187 7561 7568
64. KohlerC
von EiffC
LiebekeM
McNamaraPJ
LalkM
2008 A defect in menadione biosynthesis induces global changes in gene expression in Staphylococcus aureus. J Bacteriol 190 6351 6364
65. von EiffC
McNamaraP
BeckerK
BatesD
LeiXH
2006 Phenotype microarray profiling of Staphylococcus aureus menD and hemB mutants with the small-colony-variant phenotype. J Bacteriol 188 687 693
66. BoshoffHI
BarryCE3rd
2005 Tuberculosis - metabolism and respiration in the absence of growth. Nat Rev Microbiol 3 70 80
67. HuG
ChengPY
ShamA
PerfectJR
KronstadJW
2008 Metabolic adaptation in Cryptococcus neoformans during early murine pulmonary infection. Mol Microbiol
68. BrykR
GoldB
VenugopalA
SinghJ
SamyR
2008 Selective killing of nonreplicating mycobacteria. Cell Host Microbe 3 137 145
69. LiberatiNT
UrbachJM
MiyataS
LeeDG
DrenkardE
2006 An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc Natl Acad Sci U S A 103 2833 2838
70. HoangTT
Karkhoff-SchweizerRR
KutchmaAJ
SchweizerHP
1998 A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212 77 86
71. WeissB
2001 Endonuclease V of Escherichia coli prevents mutations from nitrosative deamination during nitrate/nitrite respiration. Mutat Res 461 301 309
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Polyoma Virus-Induced Osteosarcomas in Inbred Strains of Mice: Host Determinants of MetastasisČlánek Type I Interferon Induction Is Detrimental during Infection with the Whipple's Disease Bacterium,
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 1- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- CD8+ T Cell Control of HIV—A Known Unknown
- The Deadly Chytrid Fungus: A Story of an Emerging Pathogen
- Characterization of the Oral Fungal Microbiome (Mycobiome) in Healthy Individuals
- Polyoma Virus-Induced Osteosarcomas in Inbred Strains of Mice: Host Determinants of Metastasis
- Within-Host Evolution of in Four Cases of Acute Melioidosis
- The Type III Secretion Effector NleE Inhibits NF-κB Activation
- Protease-Sensitive Synthetic Prions
- Histone Deacetylases Play a Major Role in the Transcriptional Regulation of the Life Cycle
- Parasite-Derived Plasma Microparticles Contribute Significantly to Malaria Infection-Induced Inflammation through Potent Macrophage Stimulation
- β-Neurexin Is a Ligand for the MSCRAMM SdrC
- Structure of the HCMV UL16-MICB Complex Elucidates Select Binding of a Viral Immunoevasin to Diverse NKG2D Ligands
- Nutrient Availability as a Mechanism for Selection of Antibiotic Tolerant within the CF Airway
- Like Will to Like: Abundances of Closely Related Species Can Predict Susceptibility to Intestinal Colonization by Pathogenic and Commensal Bacteria
- Importance of the Collagen Adhesin Ace in Pathogenesis and Protection against Experimental Endocarditis
- N-glycan Core β-galactoside Confers Sensitivity towards Nematotoxic Fungal Galectin CGL2
- Two Plant Viral Suppressors of Silencing Require the Ethylene-Inducible Host Transcription Factor RAV2 to Block RNA Silencing
- A Small-Molecule Inhibitor of Motility Induces the Posttranslational Modification of Myosin Light Chain-1 and Inhibits Myosin Motor Activity
- Temporal Proteome and Lipidome Profiles Reveal Hepatitis C Virus-Associated Reprogramming of Hepatocellular Metabolism and Bioenergetics
- Marburg Virus Evades Interferon Responses by a Mechanism Distinct from Ebola Virus
- B Cell Activation by Outer Membrane Vesicles—A Novel Virulence Mechanism
- Killing a Killer: What Next for Smallpox?
- PPARγ Controls Dectin-1 Expression Required for Host Antifungal Defense against
- TRIM5α Modulates Immunodeficiency Virus Control in Rhesus Monkeys
- Immature Dengue Virus: A Veiled Pathogen?
- Panton-Valentine Leukocidin Is a Very Potent Cytotoxic Factor for Human Neutrophils
- In Vivo CD8+ T-Cell Suppression of SIV Viremia Is Not Mediated by CTL Clearance of Productively Infected Cells
- Placental Syncytiotrophoblast Constitutes a Major Barrier to Vertical Transmission of
- Type I Interferon Induction Is Detrimental during Infection with the Whipple's Disease Bacterium,
- The M/GP Glycoprotein Complex of Porcine Reproductive and Respiratory Syndrome Virus Binds the Sialoadhesin Receptor in a Sialic Acid-Dependent Manner
- Social Motility in African Trypanosomes
- Melanoma Differentiation-Associated Gene 5 (MDA5) Is Involved in the Innate Immune Response to Infection In Vivo
- Protection of Mice against Lethal Challenge with 2009 H1N1 Influenza A Virus by 1918-Like and Classical Swine H1N1 Based Vaccines
- Upregulation of xCT by KSHV-Encoded microRNAs Facilitates KSHV Dissemination and Persistence in an Environment of Oxidative Stress
- Persistent ER Stress Induces the Spliced Leader RNA Silencing Pathway (SLS), Leading to Programmed Cell Death in
- Evolutionary Trajectories of Beta-Lactamase CTX-M-1 Cluster Enzymes: Predicting Antibiotic Resistance
- Nucleoporin 153 Arrests the Nuclear Import of Hepatitis B Virus Capsids in the Nuclear Basket
- CD8+ Lymphocytes Control Viral Replication in SIVmac239-Infected Rhesus Macaques without Decreasing the Lifespan of Productively Infected Cells
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Panton-Valentine Leukocidin Is a Very Potent Cytotoxic Factor for Human Neutrophils
- CD8+ T Cell Control of HIV—A Known Unknown
- Polyoma Virus-Induced Osteosarcomas in Inbred Strains of Mice: Host Determinants of Metastasis
- The Deadly Chytrid Fungus: A Story of an Emerging Pathogen
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



