-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaImmature Dengue Virus: A Veiled Pathogen?
Cells infected with dengue virus release a high proportion of immature prM-containing virions. In accordance, substantial levels of prM antibodies are found in sera of infected humans. Furthermore, it has been recently described that the rates of prM antibody responses are significantly higher in patients with secondary infection compared to those with primary infection. This suggests that immature dengue virus may play a role in disease pathogenesis. Interestingly, however, numerous functional studies have revealed that immature particles lack the ability to infect cells. In this report, we show that fully immature dengue particles become highly infectious upon interaction with prM antibodies. We demonstrate that prM antibodies facilitate efficient binding and cell entry of immature particles into Fc-receptor-expressing cells. In addition, enzymatic activity of furin is critical to render the internalized immature virus infectious. Together, these data suggest that during a secondary infection or primary infection of infants born to dengue-immune mothers, immature particles have the potential to be highly infectious and hence may contribute to the development of severe disease.
Published in the journal: . PLoS Pathog 6(1): e32767. doi:10.1371/journal.ppat.1000718
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000718Summary
Cells infected with dengue virus release a high proportion of immature prM-containing virions. In accordance, substantial levels of prM antibodies are found in sera of infected humans. Furthermore, it has been recently described that the rates of prM antibody responses are significantly higher in patients with secondary infection compared to those with primary infection. This suggests that immature dengue virus may play a role in disease pathogenesis. Interestingly, however, numerous functional studies have revealed that immature particles lack the ability to infect cells. In this report, we show that fully immature dengue particles become highly infectious upon interaction with prM antibodies. We demonstrate that prM antibodies facilitate efficient binding and cell entry of immature particles into Fc-receptor-expressing cells. In addition, enzymatic activity of furin is critical to render the internalized immature virus infectious. Together, these data suggest that during a secondary infection or primary infection of infants born to dengue-immune mothers, immature particles have the potential to be highly infectious and hence may contribute to the development of severe disease.
Introduction
Dengue virus (DENV) represents a major emerging arthropod-borne pathogen. There are four distinct serotypes of DENV which, according to WHO estimates, infect about 50-100 million individuals annually, mostly in the (sub)tropical regions of the world. While most DENV infections are asymptomatic or result in self-limited dengue fever (DF), an increasing number of patients present more severe, potentially fatal clinical manifestations, such as dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS). It is well established that a major risk factor for the development of DHF/DSS is secondary infection with a heterotypic virus serotype [1]–[3]. Also primary infection of infants born to dengue-immune mothers may lead to severe disease [1],[4],[5]. These observations have led to the hypothesis of antibody-dependent enhancement (ADE) of infection [3],[6],[7]. Increased disease severity appears to correlate with high circulating virus titers [3], [8]–[11], suggesting that antibodies directly influence the infectious properties of the virus. The molecular mechanisms by which antibodies enhance DENV infection however remain elusive.
DENV, as well as other major human pathogens like West Nile virus (WNV), yellow fever virus, and tick-borne encephalitis (TBEV) belong to the Flavivirus genus within the family Flaviviridae. Flaviviruses enter cells via clathrin-mediated endocytosis and fuse from within acidic endosomes, through which the viral genome gains access to the target cell cytoplasm[12]. Following RNA replication and protein translation, immature virions, which contain heterodimers of the transmembrane proteins E and a precursor form of M (prM), are assembled within the ER. Subsequently, the particles mature by passing through the Golgi and trans-Golgi network (TGN) [13]. In the acidic environment of the TGN, the virion undergoes a conformational change and the cellular endoprotease furin cleaves prM into M and a peptide (“pr”) that remains associated with the virion [14]. Upon release, the pr peptide dissociates from the virion, resulting in the formation of mature progeny virions.
Cells infected with DENV secrete high levels (∼30%) of prM-containing immature particles [15],[16] suggesting that cleavage of prM to M is not efficient. These DENV particles are released from infected cells as fully immature prM-containing particles and partially immature particles containing both prM and M proteins in the viral membrane [17]. Extensive functional analyses have revealed that fully immature flaviviruses lack the ability to infect cells, as the presence of uncleaved prM in the virion blocks the E glycoprotein from undergoing the pH-induced conformational changes that are required for membrane fusion [16], [18]–[22]. Although immature particles are therefore generally considered as irrelevant by-products of infected cells, the rates of prM antibody responses are significantly higher in patients with secondary infection compared to those with primary infection [23]. Furthermore, previous reports show that prM antibodies can enhance DENV infection. Enhancement of infection was observed for wild-type virus [24],[25], presumably due to the presence of uncleaved prM in these preparations, and with DENV particles containing high levels of prM generated from cells treated with chloroquine [26]. It is thus quite puzzling if indeed the presence of prM obstructs DENV infectivity, how immature particles contribute to disease pathogenesis and what role do anti-prM antibodies play in the enhancement of infection? The present study addresses these questions.
Results
Fully immature dengue virus particles becomes highly infectious in the presence of prM antibodies
First, we investigated if prM antibodies are able to render fully immature DENV infectious. To this end, immature DENV-2 strain 16681 particles were produced in furin-deficient LoVo cells. We have used this procedure before and showed that LoVo-derived particles have an average content of 94%±9% prM [16]. Furthermore, we demonstrated that the specific infectivity of LoVo-derived fully immature DENV is at least 10,000-fold reduced compared to that of wild-type virus on cells highly permissive to infection [16]. The infectious properties of fully immature DENV virions were determined in Fc-receptor-expressing K562 cells in the absence or presence of increasing concentrations of the 70–21 antibody. This is an IgG2a antibody that has been isolated from DENV-infected mice and is mapped to amino acids 53-67 of prM [25]. Antibodies recognizing this epitope are abundantly present in sera of DHF/DSS patients [16],[27]. K562 cells were infected with DENV at a multiplicity of 100 genome-containing particles per cell (MOG 100). The number of genome-containing particles (GCP) was determined by quantitative PCR analysis of reverse-transcribed viral RNA [15]. At 24–48 hours post-infection (hpi), cells were fixed and prepared for flow-cytometric analysis to determine the number of infected cells, measured on the basis of dengue E protein expression. We observed that 43 hpi is optimal for read-out as it represents a single round of infection together with a high mean fluorescence intensity per infected cell (Fig. S1).
In agreement with our previous study [15],[16], we observed that fully immature DENV particles are essentially non-infectious as the number of E-positive cells did not exceed the limit of detection (Fig. 1A). Remarkably however, substantial numbers of E-positive cells were observed upon infection of cells with fully immature particles opsonized with the anti-prM antibody (Fig. 1A). Subsequent titration of the cell supernatants at 43 hpi revealed that opsonization of immature DENV with anti-prM antibody dramatically enhanced (up to 30,000-fold) virus particle production (Fig. 1B). The results show that prM antibodies render essentially non-infectious immature DENV nearly as infectious as wild-type virus (Fig. 1B). Enhancement of immature DENV infectivity was seen in a broad antibody concentration range, even at conditions of high antibody excess.
Fig. 1. Immature DENV particles become highly infectious in the presence of anti-prM antibodies. 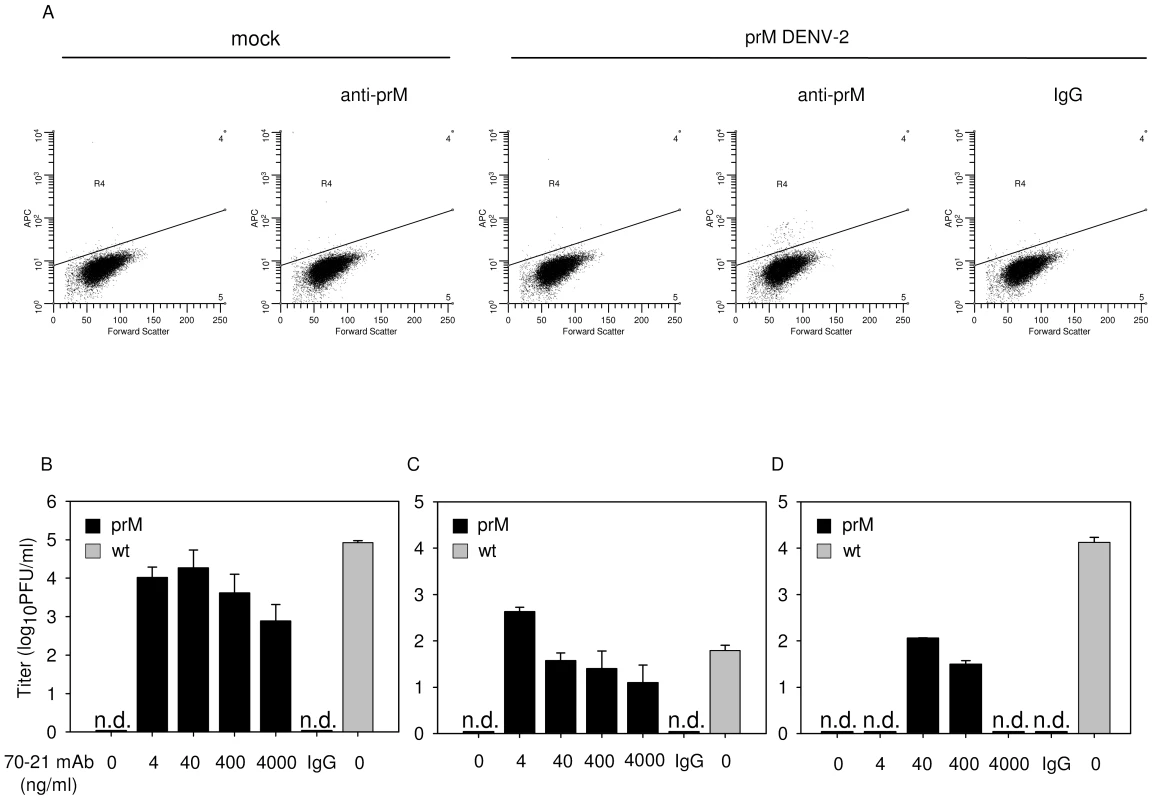
K562 cells were infected with immature (prM) or wild-type (wt) DENV-2 strain 16681 at MOG 100 in the presence or absence of anti-prM 70–21. (A) Representative read-out of the percentage of infected cells. Prior to infection, immature DENV particles were incubated with 40 ng/ml 70–21 antibody for 1 h at room temperature. At 43 hpi, the cells were fixed, stained intracellularly with Alexa-647-coupled anti-E antibody 3H5.1, and subjected to flow-cytometric analysis. Mock-infected cells and an IgG isotype control (murine IgG2a) antibody were used as controls. Titrations of the virus particles released at 43 hpi from infected K562 cells (B), U937 cells (C), and primary human PBMCs (D) were performed by plaque assay on BHK-15 cells. Data are expressed as means of at least three independent experiments. The error bars represent standard deviations (SD); (n.d.) denotes “not detectable”. To ensure that the observed high level of enhancement is not restricted to a single antibody we performed additional experiments with the murine IgG2a prM antibody 2H2 [28]. The results show that 2H2 stimulated the infectious properties of fully immature particles up to 1,000 fold (Fig. S2A). Although, this antibody has previously been shown to enhance DENV infectivity [26], the power of enhancement observed here is striking and demonstrates that prM antibodies render essentially non-infectious fully immature DENV highly infectious.
Subsequently, we investigated the enhancing properties of both prM antibodies in Fc-receptor-bearing human monocytic U937 cells and observed that the antibodies again significantly stimulate the infectivity of immature particles (Fig. 1C, S2B). Thereafter, we studied the infectious properties of immature DENV particles in primary human PBMCs, cells which are known to be involved in dengue pathogenesis. The results show that, also under these conditions, prM antibodies render fully immature particles infectious (Fig. 1D, S2C).
Antibodies against prM facilitate binding of immature dengue virus particles to FcγII-receptors on target cells
To better understand the mechanism by which prM antibodies trigger infectivity of immature DENV, we analyzed the distinct steps in the cell entry pathway of the virus. First, the binding of immature virions to K562 cells was determined by quantitative-PCR. In order to determine the number of bound GCP per cell, the amount of virus added per cell was increased 10-fold compared to the concentration used in the infectivity experiments. The results show that antibody-opsonized immature DENV binds approximately 30-fold more efficiently to cells than immature particles in the absence of antibody (Fig. 2A). Indeed, immature particles opsonized with anti-prM bound almost as efficiently to cells as wild-type DENV in the absence of antibody. Moreover, immature DENV particles failed to interact efficiently with baby hamster kidney cells (BHK-15), cells which are highly permissive for dengue infection (data not shown) suggesting that the observed lack of infectivity is partially related to the poor binding efficiency of immature particles to cells. It is likely that binding of virus-antibody complexes is mediated by direct interaction of the antibody with the Fc-receptor expressed on the cell surface. Indeed, treatment of cells with an anti-CD32 antibody to block FcγII-receptor interaction, or opsonization of particles with mAb70-21 F(ab')2 fragments severely reduced virus particle production upon infection of K562 cells with opsonized immature virions, whereas it had no effect on infection with wild-type virus (Fig. 2B). Although this antibody has been previously described to enhance the infectious properties of wild-type DENV in cells with or without Fc-receptors [25],[27], clearly in the case of immature particles interaction with the Fc-receptor is important for infectivity. Taken together, these data indicate that prM antibodies facilitate efficient interaction and cell entry of virus-immune complexes via the FcγII-receptor.
Fig. 2. Anti-prM antibody stimulates binding of immature DENV particles to cells through interaction with FcγIIR. 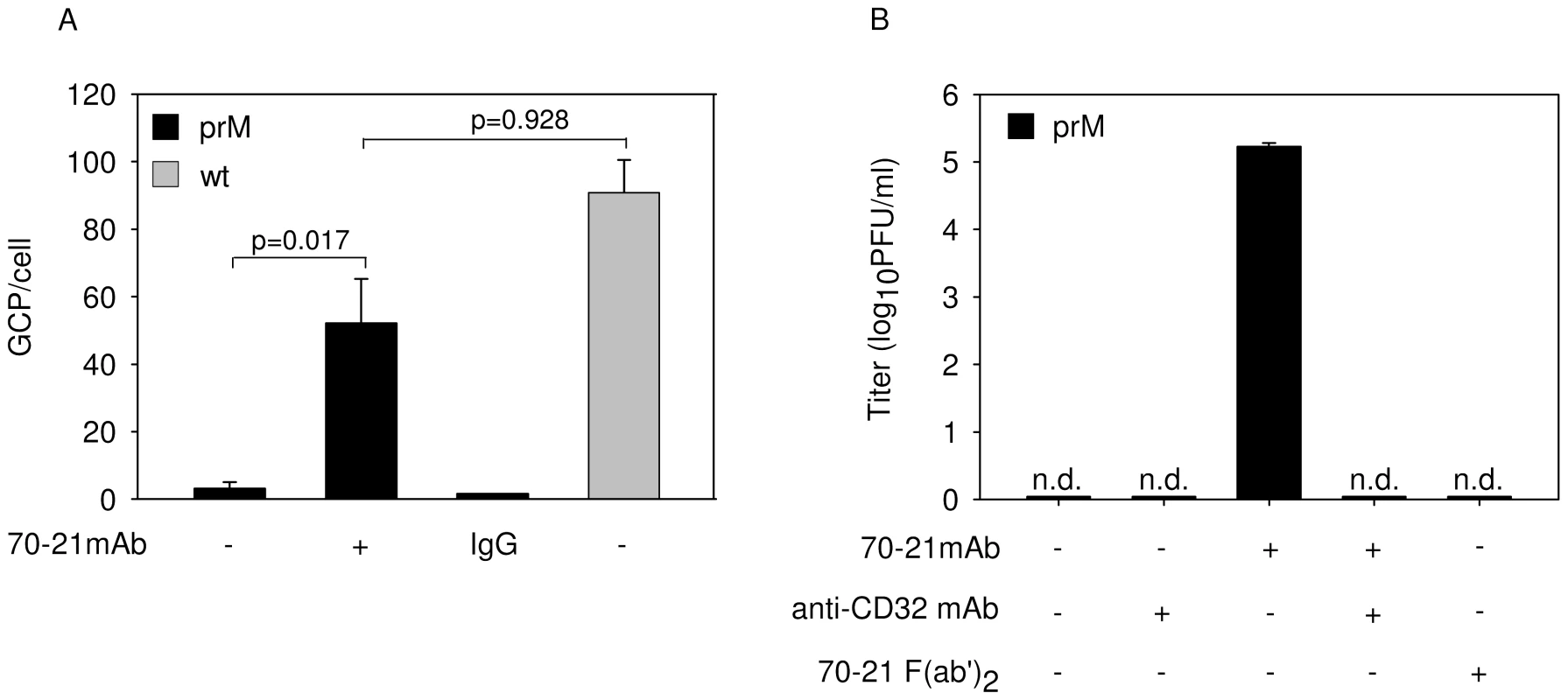
(A) Binding of immature and wild-type virions with and without prior opsonization to antibodies (40 ng/ml) to K562 cells. Virus-cell binding was measured after 1 h incubation at 4°C by q-PCR analysis. (B) Effect of anti-CD32 mAb and 70–21 F(ab')2 fragments on virus particle production. Virus particle production was determined as described in the legend to Figure 1B. Data are expressed as means and SD of three independent experiments. Two-tailed Student's t-tests were performed for statistical analysis of the data. Furin activity is critical in rendering immature particles infectious
Efficient FcγIIR-mediated cell entry does not however clarify what is the trigger for immature virions to become infectious, since the presence of prM has been shown to obstruct membrane fusion activity of the virus [14],[16],[18]. One could speculate that anti-prM antibody bound to immature virions induces a conformational change that would enable the E protein to trigger membrane fusion irrespective of the presence of prM. Another scenario might be that prM-containing virions mature upon cell entry since furin, although predominantly present in the TGN, also shuttles between early endosomes and the cell surface. To verify the potential involvement of furin during virus cell entry, we investigated the infectious properties of antibody-opsonized immature DENV in cells treated with furin inhibitor, decanoyl-L-arginyl-L-valyl-L-lysyl-L-arginyl-chloromethylketone (decRRVKR-CMK). In aqueous solution, decRRVKR-CMK has a half-life of 4–8 h [29] and therefore it is not expected to interfere with the maturation process of newly assembled virions within the infected cell. The results show that inhibition of furin activity completely abrogated virus particle production in cells infected with antibody-opsonized immature virions, whereas infection of cells with wild-type virus remained unaffected under these conditions (Fig. 3A).
Fig. 3. Immature DENV particles mature upon FcγIIR-mediated cell entry. 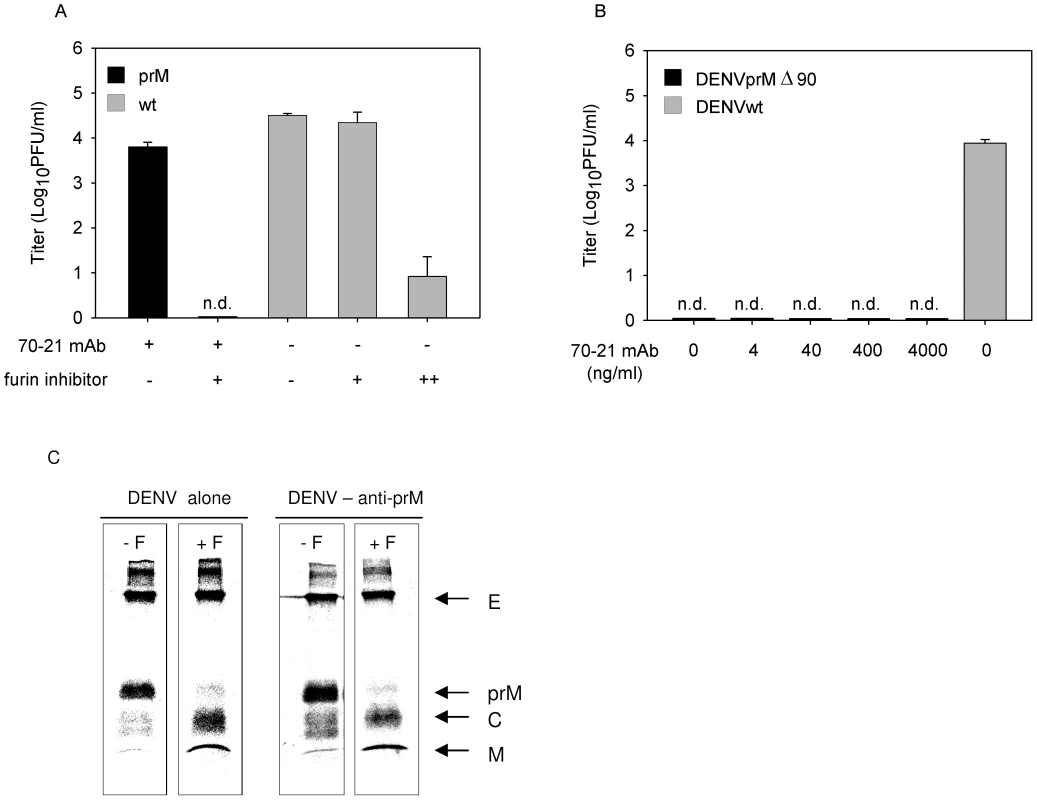
(A) K562 cells were infected with DENV or DENV-immune complexes in the presence or absence of furin inhibitor (25 µM). As a positive control for compound activity, wild-type DENV-infected cells were treated with an additional dose of furin inhibitor at 24 hpi to impede virus maturation and consequently the production of infectious virions (++). Virus particle production was determined as described in the legend to Figure 1B. Data are expressed as means and SD for three independent experiments; (n.d.) denotes “not detectable”. (B) K562 cells were infected with DENVprMΔ90 at MOG 100 in the presence of increasing concentrations of prM antibody 70–21. As control, the infectivity of wild-type virus (generated from the infectious clone pD2/IC-30P) at MOG 100 is shown. Virus production was assessed as described in the legend to Figure 1B. (C) prM antibodies do not affect cleavage maturation of DENV particles. Purified [35S]methionine-labeled immature particles with and without enhancing concentrations of anti prM mAb 70–21 were incubated with furin at pH 6 for 16 h. Next, viral protein composition was analyzed by non-reducing SDS-polyacrylamide gel electrophoresis. To further substantiate the role of furin in triggering viral infectivity, we generated a furin cleavage-deficient virus (pDENprMΔ90) by deletion of the lysine on the position 90 (87-R-R-E-K-R-91) within the furin recognition sequence. Subsequently, DENVprMΔ90 virus and wild-type DENV-2 16681 (generated from pD2/IC-30P) virus were produced by transfection of RNA transcripts derived from the cDNA plasmids into BHK-15 cells. Virus production was measured by determining the number of physical particles based on GCP and the number of infectious units as measured by plaque assay. The presence of physical particles was further evaluated in three-layer ELISA experiments, by coating plates with a similar number of genome-containing DENVprMΔ90 particles and LoVo-derived immature particles. Similar OD values were measured for DENVprMΔ90 and LoVo-derived viruses (data not shown), which confirms the presence of physical particles and suggests that the number of genome-containing particles is accurately determined. Subsequent titration studies revealed that the specific infectivity of DENVprMΔ90 mutant virus is reduced by a factor of 12.000 compared to that of wild-type virus (generated from pD2/IC-30P) and is comparable to LoVo-derived immature virus. Next, K562 cells were infected with DENVprMΔ90 mutant virus opsonized with increasing concentrations of prM antibody 70–21. Figure 3B shows that disruption of the furin-recognition motif within the prM protein of the virus abrogates the enhancing activity of the anti-prM-antibody, demonstrating that enzymatic cleavage of prM to M by furin is critical to render immature DENV infectious.
To address the question as to whether prM to M cleavage can occur upon interaction of immature DENV particles with antibodies, we incubated 35S-methionine-labeled immature particles in the absence and presence of antibodies with exogenous furin for 16 h at pH 6.0 [16]. Protein visualization was done by SDS-PAGE analysis. In agreement with previous studies, we observed that exogenous furin treatment induces efficient cleavage of prM to M (Fig. 3C). Importantly, we found that the presence of prM antibodies does not affect DENV maturation, as virtually complete cleavage of prM to M was observed (Fig. 3C).
Antibody-mediated entry of immature dengue virus particles does not lead to an increased production of virus particles per cell
It has been postulated that antibody-mediated entry of DENV leads to a higher production of virus particles per infected cell, a phenomenon often referred to as intrinsic ADE [30]. In this part of the study, we investigated whether prM-mediated entry of immature DENV supports intrinsic ADE. Since immature particles are essentially non-infectious in the absence of antibodies, we compared the production of prM-opsonized immature DENV particles with wild-type virus in K562 cells. For accurate comparison, we first searched for a condition that gives a similar percentage of infected cells. Infection of K562 cells with prM-opsonized immature DENV at a MOG of 100 leads to 0.53%+/−0.14 infected cells (Fig. 1, 4A). Comparable numbers of infected cells were detected for wild-type DENV at MOG 10 (Fig. 4A). Under these experimental conditions, no differences were observed in E protein expression and production of virus particles (Fig. 4B–C), which indicates that the presence of prM antibodies, while evidently stimulating the infectious properties of immature virions, has no enhancing effect on the number of progeny virions produced per cell.
Fig. 4. FcR-mediated entry of immature DENV does not enhance the number of progeny virions produced per cell. 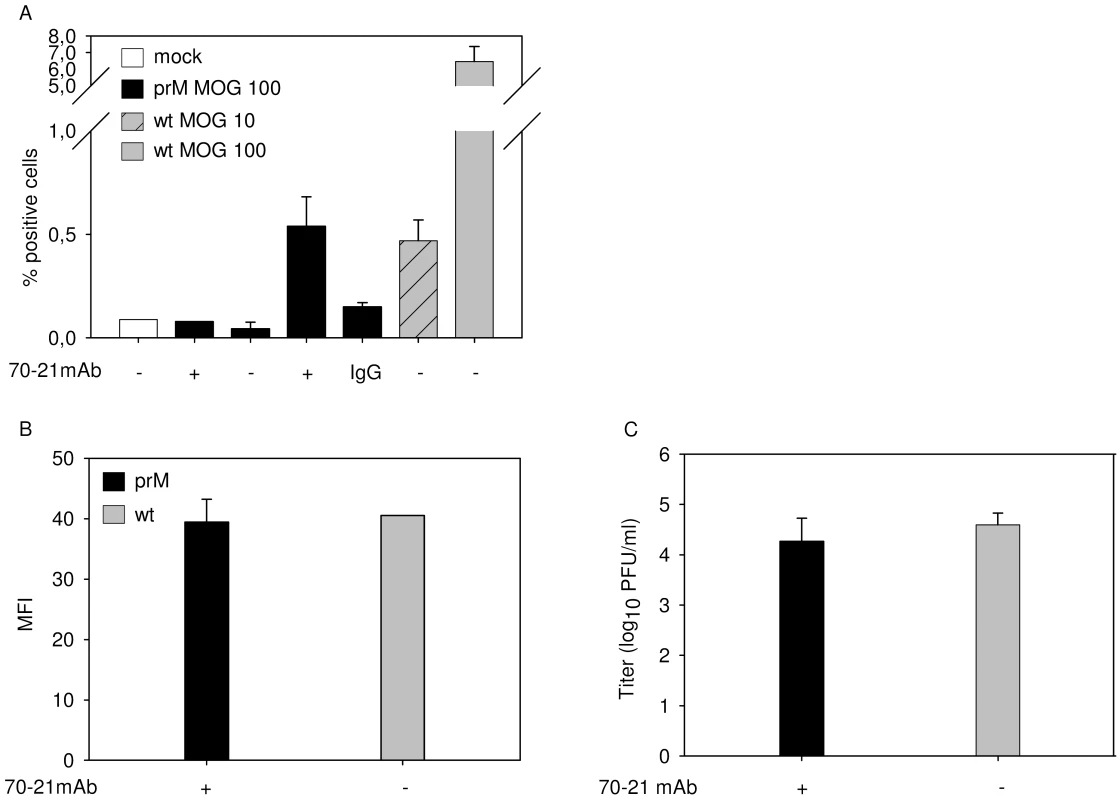
K562 cells were infected with immature (prM) DENV-2 at MOG 100 in the absence or presence of 40 ng/ml of 70–21 antibody and with wild-type virus at MOG 10 and 100. (A) At 43 hpi, the cells were fixed, stained intracellularly with Alexa-647-coupled anti-E antibody 3H5.1, and subjected to flow-cytometric analysis. Mock-infected cells and an irrelevant murine IgG2a antibody were used as controls. (B) Mean fluorescent intensity of cells infected with immature virus (MOG 100) and wild-type DENV (MOG 10) (C) Titration of the virus particles released at 43 hpi from infected K562 cells using plaque assay. Data are expressed as means of at least three independent experiments. The error bars represent standard deviations (SD). Antibody against prM enhances the infectivity of wild-type dengue virus in a furin-dependent manner
Given the high number of prM-containing particles in wild-type DENV preparations it is possible that prM antibodies also enhance the infectious properties of wild-type DENV. Indeed, in agreement with previous studies, opsonization of wild-type virus with prM antibodies results in a significant increase of viral infectivity (Fig. 5A-C, Fig. S3) [24],[25]. The level of enhancement is dependent on the cell type used and comparable to what has been described before for E antibodies [31],[32]. Enhancement of wild-type DENV infection was observed at higher antibody concentrations compared to that of immature particles. Although we do not completely understand these differences, we think that this may be related to the presence of structurally distinct immature virus particles (individual variations in prM/M content) in wild-type preparations [17]. Importantly, no enhancement of infection was observed in cells treated with furin inhibitor (Fig. 5D), demonstrating that furin activity in the target cells plays a vital role in triggering the infectious properties of antibody-opsonized immature particles in wild-type DENV preparations. Collectively, these results illustrate that prM antibodies enhance the infectious properties of prM-containing particles in wild-type DENV preparations and therefore may be important in disease pathogenesis.
Fig. 5. prM-specific antibody 70–21 enhances infectivity of wild-type DENV in a furin-dependent manner. 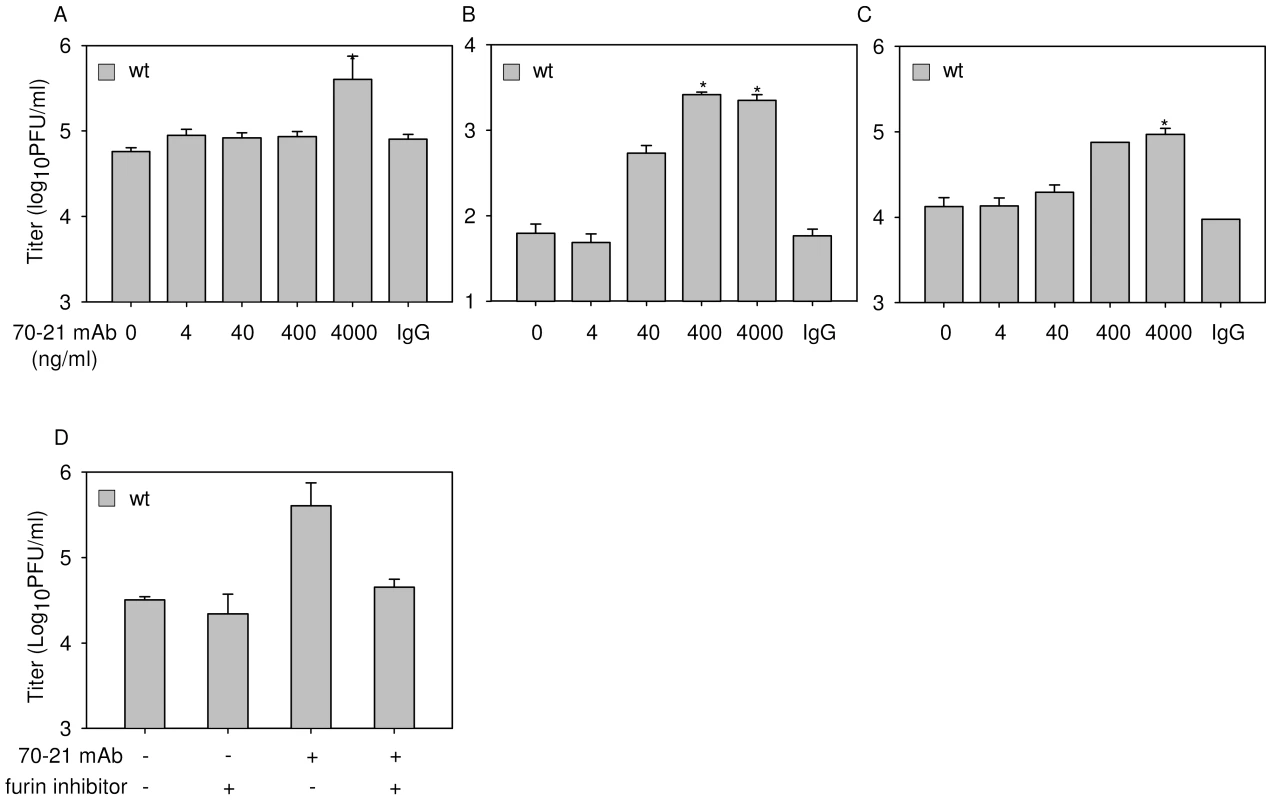
Cells were infected with wild-type (wt) DENV-2 at MOG 100 in the presence of increasing concentrations of anti-prM 70–21. Virus particle production was measured at 43 hpi by plaque assay on BHK-15 cells. (A) K562 cells, (B) U937 cells, (C) PBMCs. (D) Enhancement of infection is dependent on endogenous furin activity. K562 cells were infected with DENV with and without prior opsonization with 4000 ng/ml 70–21 in the presence or absence of furin inhibitor as described in the legend to Figure 3. Data are expressed as means of at least three independent experiments. The error bars represent standard deviations (SD); (n.d.) denotes “not detectable”; * denotes significance (p<0.05) analyzed using Two-tailed Student's t-tests. Dengue-immune sera enhances the infectious properties of immature dengue virus particles
As a first step towards elucidation of the implications of our findings in disease pathogenesis we evaluated the enhancing properties of 7 convalescent serum samples from patients infected with DENV-2. The infectious properties of immature particles opsonized with various dilutions of polyclonal sera were determined in U937 cells, since this cell line expresses both Fc receptors CD32 and CD64 on the cell surface. At 43 hr post-infection, the medium was harvested and the production of virus particles was measured by plaque assay. No plaques were found in the absence of sera and in the presence of DENV-naïve serum (Fig. 6). Convalescent sera from two distinct DENV-2 infected patients significantly enhanced the infectious properties of immature DENV particles at a 10,000 dilution (Fig. 6). Sera from two other patients enhanced the infectivity of immature particles to a minor extent as only a low number of plaques (average of 1.5 plaques) was observed. The three remaining patient sera did not show any effect on viral infectivity of immature particles as no viral plaques were observed. As expected, nearly all of the analyzed patient sera enhanced the infectious properties of wild-type virus particles (Fig. S4).
Fig. 6. Immature DENV becomes infectious in the presence of DENV-immune sera. 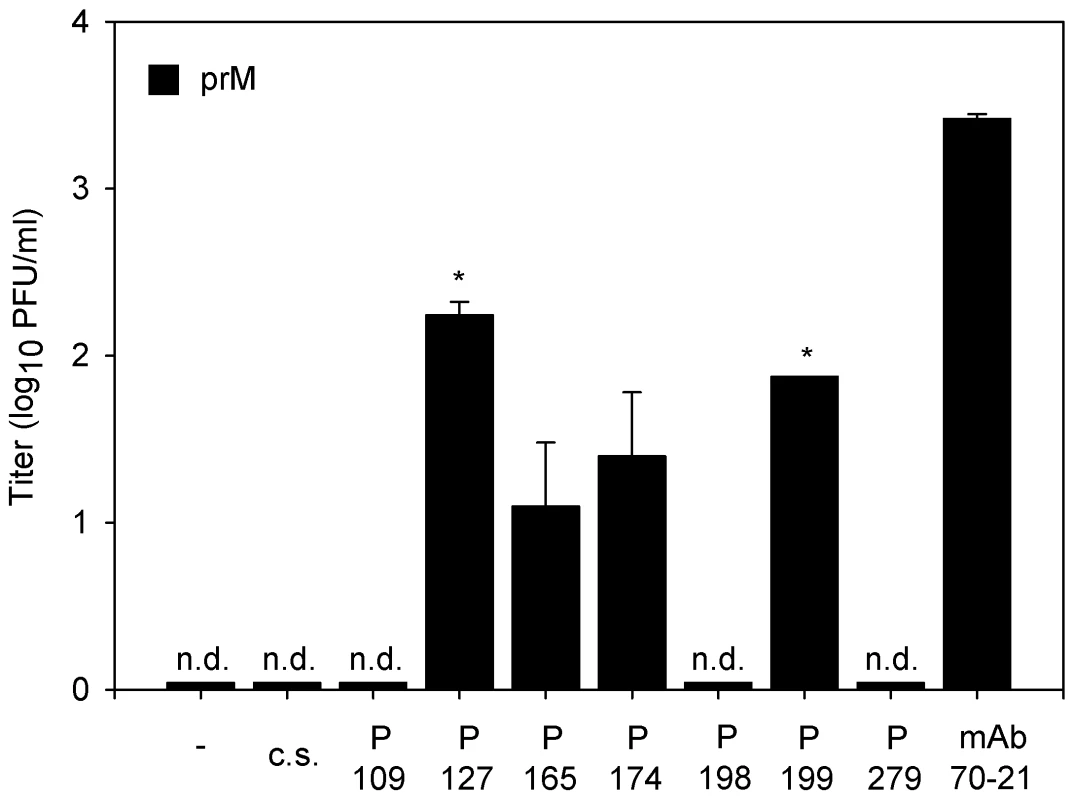
U937 cells were infected with immature (prM) DENV-2 at MOG 100 in the presence of 10-fold sequential dilutions of dengue patients sera. Virus particle production was measured at 43 hpi by plaque assay on BHK-15 cells. Sera of two patients showed significant enhancing activity towards immature DENV virions at a 104 sera dilution. The error bars represent standard deviations (SD); (c.s.) denotes “control serum”; (n.d.) denotes “not detectable”; * denotes significance (p<0.05) analyzed using Two-tailed Student's t-tests. Discussion
Multiple studies have shown that immature particles are non-infectious, the presence of prM obstructing the low-pH-induced conformational changes in the viral E glycoprotein required for membrane fusion of the virus [14],[16],[18],[21],[22],[33]. On the other hand, prM antibodies have been shown to enhance DENV infection [24],[25]. In this report, we show that the lack of infectivity of fully immature particles in the absence of antibodies is primarily related to inefficient binding of immature virions to the cell surface. If binding is facilitated through anti-prM antibodies, immature DENV particles become highly infectious presumably due to efficient intracellular processing of prM to M by the endoprotease furin.
Maturation upon entry has been previously reported for other enveloped viruses. Zhang and co-workers [34] showed that the infectivity of immature particles of Semliki Forest virus, an alphavirus, can be triggered by furin during viral endocytosis. It is likely that DENV maturation also occurs within acidic endosomes, since previous in vitro experiments have revealed that cleavage of immature particles by furin is dependent on the exposure of the virus to low pH [16]. We propose that the acidic conditions of the endosome, similar to those in the acidic TGN during processing of newly assembled virions, triggers an initial conformational change in the virion such that furin is able to cleave prM to M and the “pr” peptide. Interestingly, a recent report has shown that upon cleavage of prM a large fraction of pr peptide remains associated with the virion and that back-neutralization to pH 8.0 is required to release the pr peptide from the virion [14]. The authors interpreted this as a mechanism preventing newly assembled cleaved virions from undergoing membrane fusion in the acidic TGN. However, this notion is difficult to reconcile with our present observations, since virions that have matured within acidic endosomes of target cells do not return to neutral-pH conditions before initiating infection. One may speculate that the pr peptide stabilizes the E protein to such an extent that it survives the mildly acidic lumen of the TGN (∼pH 6.0), but is released at the more acidic pH of endosomes (∼pH 5.0) such that the E proteins have the capacity to rearrange to their fusion-active conformation. Another possibility is that upon cleavage of prM the pr peptide associates with the prM antibody instead of the E protein, thereby enabling the E proteins to adopt the fusion-active conformation.
The observed infectious potential of immature DENV virions in the presence of anti-prM antibodies may have important implications for our understanding of the processes involved in dengue pathogenesis. We speculate that in the early stages of a primary infection, before the appearance of virus-specific antibodies, immature virions would fail to penetrate host cells and therefore are of minor significance in disease development. On the other hand, during a secondary infection or primary infection of infants born to dengue-immune mothers, immature particles may become highly infectious due to the presence of anti-prM antibodies and hence may contribute to an increased dengue-infected cell mass and a high circulating virus titer, one of the preludes for the development of severe disease symptoms [3], [8]–[11]. Importantly, anti-prM antibodies may activate the infectious properties of a large population of virus particles, since we recently observed that a typical DENV-2 preparation of the prototype strain 16681 contains as much as 30% prM [16]. Taken together, our results suggest that immature DENV particles act as a veiled pathogen and can, like mature DENV contribute to the disease pathogenesis.
Variable levels of enhancement were seen with DENV-immune sera. As expected, virtually all of analyzed DENV-immune sera stimulated the infectivity of wild-type DENV. Interestingly, sera from 2 out of 7 patients significantly enhanced the infectious properties of immature particles. This suggests that individual patients develop different responses to prM. On the basis of these results, we believe that it is important to further investigate the antibody responses in DENV-infected patients and to unravel if patients with prM antibodies are more susceptible to develop severe disease. In this respect, it is interesting to note that the rates of prM antibody responses are significantly higher in patients experiencing a secondary infection compared to a primary infection [23]. Clearly, future clinical studies are required to obtain further evidence for the role of immature particles and prM antibodies in disease development.
Materials and Methods
Cells
Aedes albopictus C6/36 cells were maintained in minimal essential medium (Life Technologies) supplemented with 10% fetal bovine serum (FBS), 25 mM HEPES, 7.5% sodium bicarbonate, penicillin (100 U/ml), streptomycin (100 µg/ml), 200 mM glutamine and 100 µM nonessential amino acids at 28°C, 5% CO2. Baby Hamster Kidney-21 clone 15 cells (BHK-15) cells were cultured in DMEM (Life Technologies) containing 10% FBS, penicillin (100 U/ml), streptomycin (100 µg/ml), 10 mM HEPES, and 200 mM glutamine. Human adenocarcinoma LoVo cells were cultured in Ham's medium (Life Technologies) supplemented with 20% FBS at 37°C, 5% CO2. Human erythroleukemic K562 cells were maintained in DMEM supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (100 µg/ml) at 37°C, 5% CO2. Human leukemic monocyte lymphoma U937 cells were maintained in Iscove's modified Dulbecco's medium (GIBCO) supplemented with 10% FBS, 4 mM L-glutamine, penicillin (100 U/ml), and streptomycin (100 µg/ml) and adjusted to contain 1.5 g/l sodium bicarbonate, 10 mM HEPES and 1.0 mM sodium pyruvate (GIBCO). Cells were incubated at 37°C at 5% CO2. Human peripheral blood mononuclear cells (PBMCs) were maintained in RPMI 1640 medium supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (100 µg/ml). PBMCs were isolated from heparinized blood samples collected from healthy persons using standard density centrifugation procedures with Lymphoprep™ (AXIS-SHIELD). The PBMCs were used immediately after isolation or cryopreserved at −150°C. On the day of infection, the percentage of CD14+, CD19 - population within isolated PBMCs was determined (5%–10% depending on the blood donor) using cell surface markers CD-14 -FITC and CD19-R-PE purchased from commercial source (IQ Products).
Virus growth
DENV-2 strain 16681, kindly provided by dr. Claire Huang (Center for Disease Control and Prevention, USA), was propagated on C6/36 cells as described before [16]. Briefly, monolayer of C6/36 cells was infected at multiplicity of infection (MOI of 0.1). At 96 hpi, the medium was harvested, cleared from cellular debris by low-speed centrifugation, aliquoted, and stored at −80°C. Immature DENV particles were produced on LoVo cells as described previously [16]. Briefly, LoVo cells were infected at MOI 10. Virus inoculum was removed after 1.5 h and fresh medium was added after washing the cells twice with PBS. At 72 hpi, the medium containing the virus particles was harvested, cleared from cellular debris by low-speed centrifugation, aliquoted, and stored at −80°C. [35S] methionine-labeled immature virus was prepared, as described previously [16]. Briefly, cells were infected at a MOI of 10. At 2 hpi, 400 µCi of [35S]methionine (Amersham Biosciences) was added to 20 ml of medium and incubation was continued overnight. At 23 hpi, the medium was supplemented with an additional 200 µCi of radioactive label. At 72 hpi, the supernatant containing the viral particles was cleared from cell debris by low-speed centrifugation and the virions were pelletted and further purified on a discontinuous (20 and 55% w/v) Optiprep™ gradient (Axis-Shield) by ultracentrifugation. Virus was harvested from the gradient interface, aliquoted and stored at −80°C. Virus preparations were analyzed with respect to the infectious titer and the number of genome-containing particles, as described previously [15],[16].
The furin-cleavage mutant (pDENprMΔ90) was generated by deletion of the lysine codon within the furin-recognition site at position 90 of prM. The mutation was introduced in the DENV-2 16681 infectious cDNA clone (pD2/IC-30P) [35]. Briefly, two PCR fragments were generated using the following primers: forward primer A (5′-CTC AAC GAC AGG AGC ACG ATC AT - 3′) and reverse primer A (5′ - GAG TGC CAC TGA TCT TTC TCT TC-3′) and forward primer B (5′ - GAA GAG AAA Δ GAT CAG TGG CAC TCG TT-3′) and reverse B (5′-GTG TCA TTT CCG ACT GCA TGC TCT-3′). The PCR fragments were ligated, cut with SacI and Sph1, and ligated into pD2/IC-30P. The introduced deletion was confirmed by DNA sequence analysis using an automated capillary sequencing system (ABI). RNA transcripts were generated from pDENprMΔ90 and pD2/IC-30P using T7 RNA polymerase and transfected into BHK-15 cells cells by electroporation (Bio-Rad Gene Pulser apparatus; two pulses at 1.5 kV, 25 µF, and 200 Ω). At 12 hours post transfection (hpt) cells were washed extensively to remove remaining RNA copies. Virus preparations were harvested at 72 hpt, cleared from cellular debris by low-speed centrifugation, aliquoted, and stored at −80°C. Virus preparations were analyzed with respect to the infectious titer and the number of genome-containing particles, as described previously. The antigenic reactivity of DENVprMΔ90 was compared to LoVo-derived virus by standard three-layer ELISA. Briefly, microtiter ELISA plates (Greiner bio-one) were coated with 4×106 GCP of different virus preparations per well in 100 µl coating buffer, overnight. After blocking with 2% milk in coating buffer for 45 min, 100 µl of two-fold serial dilutions of anti-DENV mAbs were applied to the wells and incubated for 1.5 h, in triplicate. Subsequently, 100 µl of horseradish peroxidase-conjugated goat anti-mouse IgG-isotype antibody (Southern Biotech) was applied for 1 h. All incubations were performed at 37°C. Staining was performed using o-phenylene-diamine (OPD) (Eastman Kodak Company) and absorbance was read at 492 nm (A492) with an ELISA reader (Bio-tek Instruments, Inc.).
Patient sera
Convalescent sera from DENV-2 immune, hospitalized patients were kindly provided by dr. G. Comach (Biomed-UC, Lardidev, Maracay, Venezuela) and dr. T. Kochel (U.S. Naval Medical Research Center Detachment, Lima, Peru). All sera samples analyzed were obtained between 20–28 days following DENV-infection.
Infectivity assays
Virus or virus-antibody complexes were added to 2×105 K562 cells, at a multiplicity of 100 genome-containing particles (MOG) per cell. After 1.5 h incubation at 37°C, the inoculum was removed and fresh medium was added to the cells. At 24–48 hpi, the medium was harvested and virus production was analyzed by plaque assay on BHK-15 cells, as described previously [36]. To measure the number of infected cells, cells were fixed at 24–48 hpi, stained with 3H5-conjugated Alexa647, and analyzed using a FACS Calibur cytometer. For virus-antibody complex formation, virus particles were incubated for 1 h at 37°C with various dilutions of monoclonal prM antibody 70-21 and 2H2 in cell culture medium containing 2% FBS prior to the addition to cells. To investigate the involvement of the Fc receptor, mAb 70–21 F(ab')2 fragments produced by use of the immobilized pepsin (Pierce) were used. Alternatively, K562 cells were pretreated with 25 µg/ml of anti-FcγRII antibody (MCA1075PE, Serotec) for 1 h at 37°C, after which access antibody was removed by extensive washing. In furin blockage experiments, cells were treated with 25 µM of furin-specific inhibitor, decanoyl-L-arginyl-L-valyl-L-lysyl-L-arginyl-chloromethylketone (decRRVKR-CMK) (Calbiochem) prior and during virus infection. In control sample for the decRRVKR-CMK activity, additional 25 µM of the inhibitor was added to ensure blockage of the progeny virus maturation.
Binding assays
To determine the number of bound genome-containing particles per cell, virus or virus-antibody complexes were incubated with 2×105 K526 cells at MOG 1000 for 1 h at 4°C. Subsequently, cells were washed three times with ice-cold PBS containing MgCl2 and CaCl2 (Life Technologies) to remove unbound virus-antibody complexes. Then, viral RNA was extracted from the cells by use of the QIAamp Viral RNA mini Kit (QIAGEN). Thereafter, cDNA was synthesized from the viral RNA with reverse transcription-PCR (RT-PCR), copies of which were quantified using quantitative PCR [15].
In vitro furin cleavage assays
[35S]methionine-labeled immature particles or viral immune complexes were incubated with furin [New England BioLabs] for 16 h at pH 6.0, as described previously [16]. Following furin treatment samples were subjected to sodium dodecyl sulphate-polycrylamide gel electrophoresis (SDS-PAGE) analysis to visualize the protein composition.
Supporting Information
Zdroje
1. HalsteadSB
1970 Observations related to pathogenesis of dengue hemorrhagic fever. VI. Hypotheses and discussion. Yale J Biol Med 42 350 362
2. HalsteadSB
1988 Pathogenesis of dengue: challenges to molecular biology. Science 239 476 481
3. VaughnDW
GreenS
KalayanaroojS
InnisBL
NimmannityaS
2000 Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis 181 2 9
4. HalsteadSB
LanNT
MyintTT
ShweTN
NisalakA
2002 Dengue hemorrhagic fever in infants: research opportunities ignored. Emerg Infect Dis 8 1474 1479
5. KliksSC
NimmanityaS
NisalakA
BurkeDS
1988 Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg 38 411 419
6. HalsteadSB
2003 Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res 60 421 467
7. KliksSC
NisalakA
BrandtWE
WahlL
BurkeDS
1989 Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am J Trop Med Hyg 40 444 451
8. LibratyDH
YoungPR
PickeringD
EndyTP
KalayanaroojS
2002 High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis 186 1165 1168
9. LibratyDH
EndyTP
HoungHS
GreenS
KalayanaroojS
2002 Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J Infect Dis 185 1213 1221
10. WangWK
ChaoDY
KaoCL
WuHC
LiuYC
2003 High levels of plasma dengue viral load during defervescence in patients with dengue hemorrhagic fever: implications for pathogenesis. Virology 305 330 338
11. WangWK
ChenHL
YangCF
HsiehSC
JuanCC
2006 Slower rates of clearance of viral load and virus-containing immune complexes in patients with dengue hemorrhagic fever. Clin Infect Dis 43 1023 1030
12. van der SchaarHM
RustMJ
ChenC
vandE-M
WilschutJ
2008 Dissecting the cell entry pathway of dengue virus by single-particle tracking in living cells. PLoS Pathog 4 e1000244 doi:10.1371/journal.ppat.1000244
13. MackenzieJM
WestawayEG
2001 Assembly and maturation of the flavivirus Kunjin virus appear to occur in the rough endoplasmic reticulum and along the secretory pathway, respectively. J Virol 75 10787 10799
14. YuIM
ZhangW
HoldawayHA
LiL
KostyuchenkoVA
2008 Structure of the immature dengue virus at low pH primes proteolytic maturation. Science 319 1834 1837
15. van der SchaarHM
RustMJ
WaartsBL
vandE-M
KuhnRJ
2007 Characterization of the early events in dengue virus cell entry by biochemical assays and single-virus tracking. J Virol 81 12019 12028
16. ZybertIA
vandE-M
WilschutJ
SmitJM
2008 Functional importance of dengue virus maturation: infectious properties of immature virions. J Gen Virol 89 3047 3051
17. CherrierMV
KaufmannB
NybakkenGE
LokSM
WarrenJT
2009 Structural basis for the preferential recognition of immature flaviviruses by a fusion-loop antibody. EMBO J 28 3269 3276
18. ElshuberS
AllisonSL
HeinzFX
MandlCW
2003 Cleavage of protein prM is necessary for infection of BHK-21 cells by tick-borne encephalitis virus. J Gen Virol 84 183 191
19. ElshuberS
MandlCW
2005 Resuscitating mutations in a furin cleavage-deficient mutant of the flavivirus tick-borne encephalitis virus. J Virol 79 11813 11823
20. GuirakhooF
BolinRA
RoehrigJT
1992 The Murray Valley encephalitis virus prM protein confers acid resistance to virus particles and alters the expression of epitopes within the R2 domain of E glycoprotein. Virology 191 921 931
21. HeinzFX
StiasnyK
Puschner-AuerG
HolzmannH
AllisonSL
1994 Structural changes and functional control of the tick-borne encephalitis virus glycoprotein E by the heterodimeric association with protein prM. Virology 198 109 117
22. StadlerK
AllisonSL
SchalichJ
HeinzFX
1997 Proteolytic activation of tick-borne encephalitis virus by furin. J Virol 71 8475 8481
23. LaiCY
TsaiWY
LinSR
KaoCL
HuHP
2008 Antibodies to envelope glycoprotein of dengue virus during the natural course of infection are predominantly cross-reactive and recognize epitopes containing highly conserved residues at the fusion loop of domain II. J Virol 82 6631 6643
24. HuangKJ
YangYC
linYS
liuHS
YehTM
2005 Flow Cytometric Determination for Dengue Virus-Infected cells: Its application for Antibody-Dependent Enhancement study. Dengue Bulletin 29 142 150
25. HuangKJ
YangYC
LinYS
HuangJH
LiuHS
2006 The dual-specific binding of dengue virus and target cells for the antibody-dependent enhancement of dengue virus infection. J Immunol 176 2825 2832
26. RandolphVB
WinklerG
StollarV
1990 Acidotropic amines inhibit proteolytic processing of flavivirus prM protein. Virology 174 450 458
27. HuangKJ
linYS
HuangJH
liuHS
YehTM
2008 Anti-prM Antibody as an Autoantibody in Dengue Virus Infection. American Journal of Infectious Diseases 4 59 67
28. FalconarAK
1999 Identification of an epitope on the dengue virus membrane (M) protein defined by cross-protective monoclonal antibodies: design of an improved epitope sequence based on common determinants present in both envelope (E and M) proteins. Arch Virol 144 2313 2330
29. GartenW
HallenbergerS
OrtmannD
SchaferW
VeyM
1994 Processing of viral glycoproteins by the subtilisin-like endoprotease furin and its inhibition by specific peptidylchloroalkylketones. Biochimie 76 217 225
30. HalsteadSB
2007 Dengue. Lancet 370 1644 1652
31. BrandtWE
McCownJM
GentryMK
RussellPK
1982 Infection enhancement of dengue type 2 virus in the U-937 human monocyte cell line by antibodies to flavivirus cross-reactive determinants. Infect Immun 36 1036 1041
32. GoncalvezAP
EngleRE
StCM
PurcellRH
LaiCJ
2007 Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc Natl Acad Sci U S A 104 9422 9427
33. ZhangY
CorverJ
ChipmanPR
ZhangW
PletnevSV
2003 Structures of immature flavivirus particles. EMBO J 22 2604 2613
34. ZhangX
FugereM
DayR
KielianM
2003 Furin processing and proteolytic activation of Semliki Forest virus. J Virol 77 2981 2989
35. KinneyRM
ButrapetS
ChangGJ
TsuchiyaKR
RoehrigJT
1997 Construction of infectious cDNA clones for dengue 2 virus: strain 16681 and its attenuated vaccine derivative, strain PDK-53. Virology 230 300 308
36. DiamondMS
EdgilD
RobertsTG
LuB
HarrisE
2000 Infection of human cells by dengue virus is modulated by different cell types and viral strains. J Virol 74 7814 7823
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Polyoma Virus-Induced Osteosarcomas in Inbred Strains of Mice: Host Determinants of MetastasisČlánek Nutrient Availability as a Mechanism for Selection of Antibiotic Tolerant within the CF AirwayČlánek Type I Interferon Induction Is Detrimental during Infection with the Whipple's Disease Bacterium,
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 1- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- CD8+ T Cell Control of HIV—A Known Unknown
- The Deadly Chytrid Fungus: A Story of an Emerging Pathogen
- Characterization of the Oral Fungal Microbiome (Mycobiome) in Healthy Individuals
- Polyoma Virus-Induced Osteosarcomas in Inbred Strains of Mice: Host Determinants of Metastasis
- Within-Host Evolution of in Four Cases of Acute Melioidosis
- The Type III Secretion Effector NleE Inhibits NF-κB Activation
- Protease-Sensitive Synthetic Prions
- Histone Deacetylases Play a Major Role in the Transcriptional Regulation of the Life Cycle
- Parasite-Derived Plasma Microparticles Contribute Significantly to Malaria Infection-Induced Inflammation through Potent Macrophage Stimulation
- β-Neurexin Is a Ligand for the MSCRAMM SdrC
- Structure of the HCMV UL16-MICB Complex Elucidates Select Binding of a Viral Immunoevasin to Diverse NKG2D Ligands
- Nutrient Availability as a Mechanism for Selection of Antibiotic Tolerant within the CF Airway
- Like Will to Like: Abundances of Closely Related Species Can Predict Susceptibility to Intestinal Colonization by Pathogenic and Commensal Bacteria
- Importance of the Collagen Adhesin Ace in Pathogenesis and Protection against Experimental Endocarditis
- N-glycan Core β-galactoside Confers Sensitivity towards Nematotoxic Fungal Galectin CGL2
- Two Plant Viral Suppressors of Silencing Require the Ethylene-Inducible Host Transcription Factor RAV2 to Block RNA Silencing
- A Small-Molecule Inhibitor of Motility Induces the Posttranslational Modification of Myosin Light Chain-1 and Inhibits Myosin Motor Activity
- Temporal Proteome and Lipidome Profiles Reveal Hepatitis C Virus-Associated Reprogramming of Hepatocellular Metabolism and Bioenergetics
- Marburg Virus Evades Interferon Responses by a Mechanism Distinct from Ebola Virus
- B Cell Activation by Outer Membrane Vesicles—A Novel Virulence Mechanism
- Killing a Killer: What Next for Smallpox?
- PPARγ Controls Dectin-1 Expression Required for Host Antifungal Defense against
- TRIM5α Modulates Immunodeficiency Virus Control in Rhesus Monkeys
- Immature Dengue Virus: A Veiled Pathogen?
- Panton-Valentine Leukocidin Is a Very Potent Cytotoxic Factor for Human Neutrophils
- In Vivo CD8+ T-Cell Suppression of SIV Viremia Is Not Mediated by CTL Clearance of Productively Infected Cells
- Placental Syncytiotrophoblast Constitutes a Major Barrier to Vertical Transmission of
- Type I Interferon Induction Is Detrimental during Infection with the Whipple's Disease Bacterium,
- The M/GP Glycoprotein Complex of Porcine Reproductive and Respiratory Syndrome Virus Binds the Sialoadhesin Receptor in a Sialic Acid-Dependent Manner
- Social Motility in African Trypanosomes
- Melanoma Differentiation-Associated Gene 5 (MDA5) Is Involved in the Innate Immune Response to Infection In Vivo
- Protection of Mice against Lethal Challenge with 2009 H1N1 Influenza A Virus by 1918-Like and Classical Swine H1N1 Based Vaccines
- Upregulation of xCT by KSHV-Encoded microRNAs Facilitates KSHV Dissemination and Persistence in an Environment of Oxidative Stress
- Persistent ER Stress Induces the Spliced Leader RNA Silencing Pathway (SLS), Leading to Programmed Cell Death in
- Evolutionary Trajectories of Beta-Lactamase CTX-M-1 Cluster Enzymes: Predicting Antibiotic Resistance
- Nucleoporin 153 Arrests the Nuclear Import of Hepatitis B Virus Capsids in the Nuclear Basket
- CD8+ Lymphocytes Control Viral Replication in SIVmac239-Infected Rhesus Macaques without Decreasing the Lifespan of Productively Infected Cells
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Panton-Valentine Leukocidin Is a Very Potent Cytotoxic Factor for Human Neutrophils
- CD8+ T Cell Control of HIV—A Known Unknown
- Polyoma Virus-Induced Osteosarcomas in Inbred Strains of Mice: Host Determinants of Metastasis
- The Deadly Chytrid Fungus: A Story of an Emerging Pathogen
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



