-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaTraumatic brain injury and the risk of dementia diagnosis: A nationwide cohort study
Using longitudinal, case-control, and sibling-matched analyses of nationwide data from Sweden, Peter Nordström and Anna Nordström describe the association between TBI and dementia, its time course, and the influence of familial factors.
Published in the journal: . PLoS Med 15(1): e32767. doi:10.1371/journal.pmed.1002496
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002496Summary
Using longitudinal, case-control, and sibling-matched analyses of nationwide data from Sweden, Peter Nordström and Anna Nordström describe the association between TBI and dementia, its time course, and the influence of familial factors.
Introduction
Traumatic brain injury (TBI) is a leading cause of death and disability in individuals aged <45 years in industrialized countries, and it is associated with developing a broad spectrum of pathophysiological symptoms, followed by long-term disability [1]. Accumulating evidence suggests that TBI is also associated with risk of developing dementia [2], a neurodegenerative disease with far-reaching social and medical implications.
Two meta-analyses of retrospective case-control studies have suggested that the risk of Alzheimer disease (AD) is doubled in men, but not in women, after TBI resulting in loss of consciousness [3,4]. Furthermore, a grading system for the risk of developing dementia based on TBI severity has been proposed for non-AD dementia [5] and for AD [6]. The retrospective MIRAGE study documented a fourfold increased risk of AD associated with TBI resulting in loss of consciousness and a twofold increased risk for TBI not resulting in loss of consciousness [6]. Other studies have yielded conflicting results, suggesting no association between previous TBI resulting in loss of consciousness and the development of AD or other types of dementia [7,8].
A previous study from our research group showed that the risk of developing young-onset dementia (YOD) after TBI was low for AD but strongly related to non-AD dementia, in a nationwide population-based cohort of 811,622 men and more than 30 years of follow-up [9]. Other researchers have suggested that a history of TBI accelerates the development of minimal cognitive impairment and AD; a retrospective study showed that the onset of dementia may occur ≥2 years earlier in individuals with TBI [10].
Thus, the details of how TBI is associated with the development, the time of onset, and the different types of dementia remain unclear. The aim of the present study was to examine whether different types of dementia diagnoses are associated with previous TBI and whether any observed association is time dependent, in a nationwide cohort.
Materials and methods
Materials
The cohort considered for inclusion in the present study included all men and women aged ≥50 years who lived in Sweden on December 31, 2005 (n = 3,329,360). Using data from Statistics Sweden (www.scb.se), information about early disability pension, civil status, and educational attainment in 2005 was linked to each individual in the cohort. From the total cohort, three component cohorts were formed. In the first retrospective cohort, individuals with TBI diagnoses and no prior diagnosis of dementia were each matched with two individuals without TBI during follow-up, based on birth year and sex. The baseline date for TBI cases and controls was the date of TBI. Controls who died or had a diagnosis of dementia prior to baseline were excluded. This procedure was repeated up to three times for each case. The National Patient Register was searched from 1964 through 2012, to identify prospective diagnoses of dementia.
The second cohort consisted of all full sibling pairs from the total cohort with discordant TBI status during follow-up. The baseline date for each pair was the date of TBI. Sibling pairs with death of the sibling without TBI or a diagnosis of dementia in at least one sibling before baseline were excluded. Prospective diagnoses of dementia from 1964 through 2012 were identified using the National Patient Register. The purpose of the sibling cohort was to adjust for potential uncontrolled confounding due to familial factors that would not be captured in the medical record.
In the third cohort, all subjects diagnosed with dementia during follow-up were matched with up to two controls with no dementia diagnosis during follow-up, based on birth year and sex. Controls who died prior to the date of dementia for the corresponding cases were excluded. This procedure was repeated up to three times for each case. The baseline date in this case-control cohort was the date of dementia for each case and corresponding controls. In this cohort, a retrospective search of the National Patient Register through 1964 was conducted to identify TBIs occurring before baseline. The purpose of this nested case-control cohort was to evaluate the results from the cohort study. The Regional Ethical Review Board in Umeå and the National Board of Health and Welfare approved this study. There was no written prospective research protocol for the analyses presented in the present study. However, the analyses presented were preplanned, with the exception of Fig 1, Fig 2 and Fig 3, which were constructed during the revision process in response to reviewer comments.
Fig. 1. The cumulative incidence of dementia diagnosis during follow-up in individuals with and without TBI at baseline (n = 491,252 individuals). 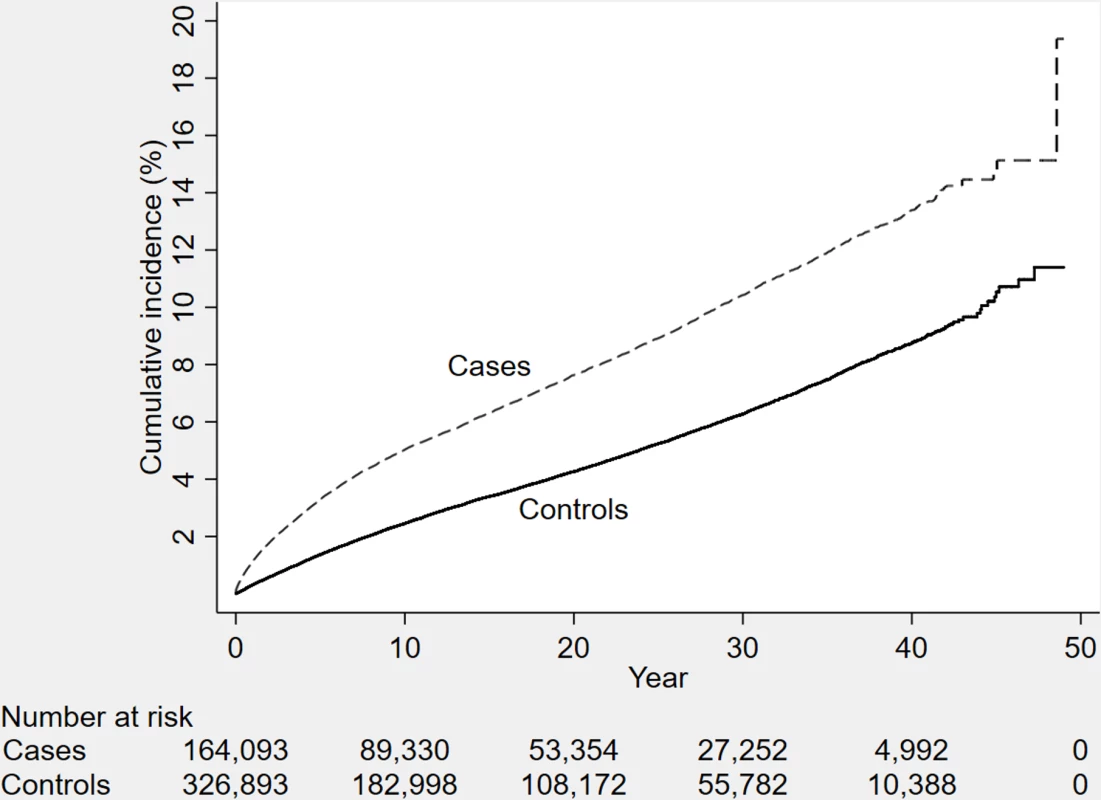
TBI, traumatic brain injury. Fig. 2. The risk of a dementia diagnosis in individuals with only one mild TBI (n = 108,463) and corresponding controls (n = 216,077), during follow-up. 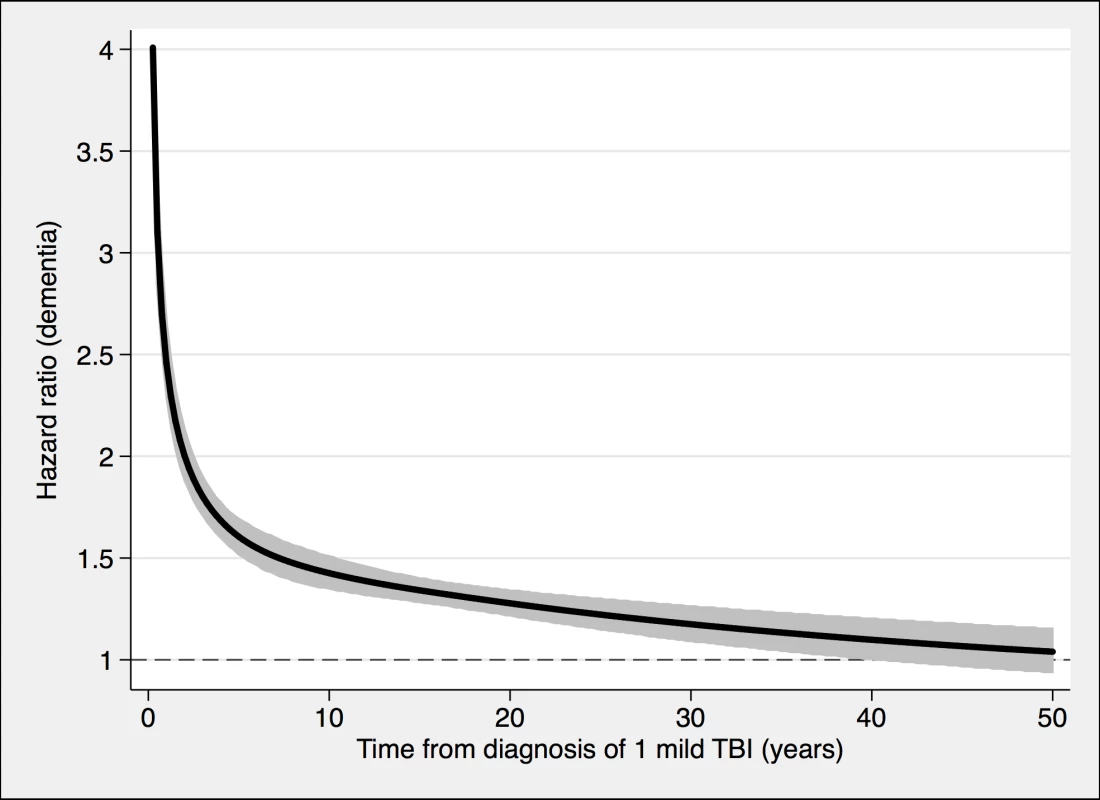
To model the effects, restricted cubic splines with four knots were used (resulting in three degrees of freedom), followed by fitting a proportional hazards model. TBI, traumatic brain injury. Fig. 3. The risk of a dementia diagnosis in individuals with at least one nonmild TBI (n = 39,374) and corresponding controls (n = 77,924), during follow-up. 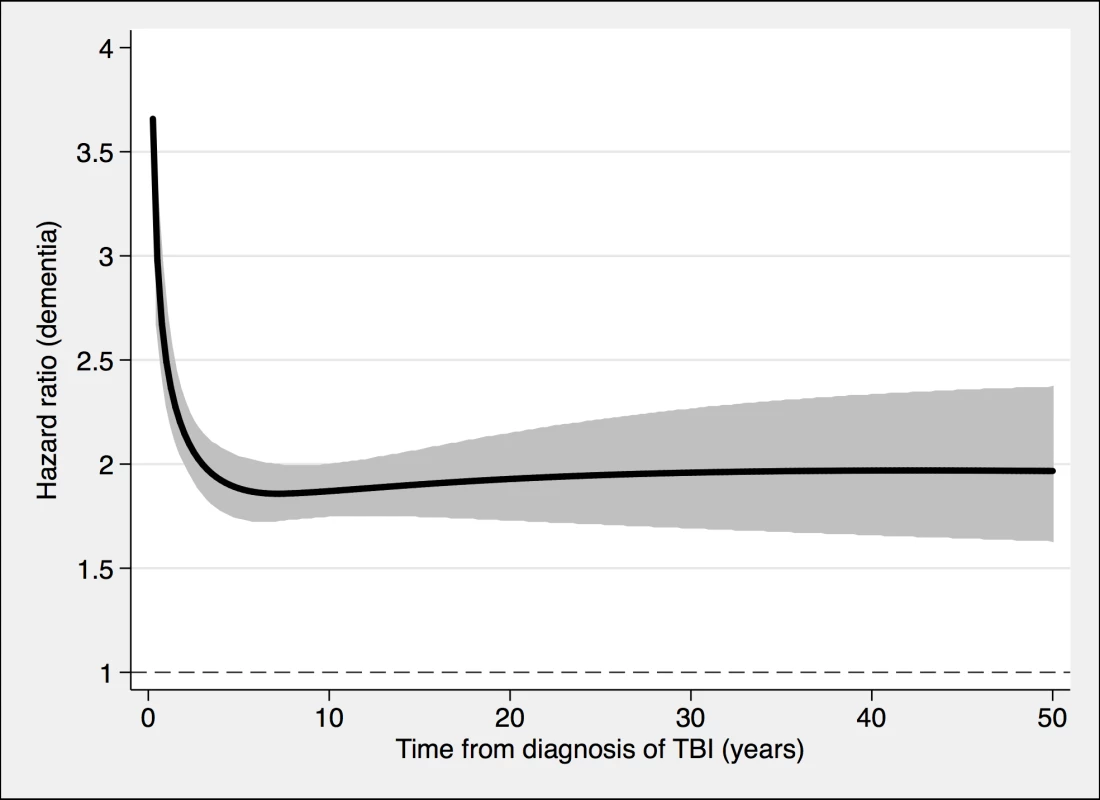
To model the effects, restricted cubic splines with four knots were used (resulting in three degrees of freedom), followed by fitting a proportional hazards model. TBI, traumatic brain injury. Diagnoses of dementia and TBI, death, and other covariates of interest
The Swedish National Patient Register (SNPR), controlled by the National Board of Health and Welfare, was searched through December 31, 2012, to identify diagnoses of dementia and TBI using appropriate International Classification of Disease (ICD; 8th, 9th, and 10th revisions) codes. Diagnoses of dementia were categorized as AD (ICD-10 code F00), vascular dementia (ICD-10 code F01), and dementia of unspecified type (ICD-10 code F039). For the diagnosis of dementia, the ICD-8 and ICD-9 code 290 was also included. TBI was coded as mild (ICD-10 code S060, ICD-8 and ICD-9 code 850) and more severe (ICD-10 code S06x, excluding S060, ICD-8 and ICD-9 code 851). A second TBI was defined as a new diagnosis recorded at least 6 months after the first diagnosis. Other diagnoses were selected based on known associations with the main exposure, outcome, or death; these included myocardial infarction, stroke, cancer, kidney failure, chronic pulmonary disease, atrial fibrillation, alcohol intoxication, depression, and diabetes. Diagnoses recorded in the SNPR have been validated, with positive predictive values of 85%–95% [11]. The SNPR has a national coverage rate of about 90% for inpatient care from 1970, and all specialized outpatient care has been included since 2001. Diagnoses of death were collected from the National Death Register, also controlled by the National Board of Health and Welfare.
Statistical analysis
To test whether the association between TBI and the risk of subsequent dementia was time dependent in the prospective cohort, we evaluated Schoenfeld’s residuals using the estat phtest command in the Stata software (version 12.1; StataCorp LP, TX). As the test indicated that the proportional hazards assumption was violated, the association between TBI and the risk of dementia was analyzed in intervals of follow-up using multivariable adjusted conditional logistic regression in all three cohorts. For this purpose, the clogit command in the Stata software was used to fit maximum likelihood (fixed-effect) models with the dichotomous dependent variable of interest, i.e., dementia in the retrospective cohort study and sibling cohort, and TBI in the case-control study. The likelihood was then calculated relative to each group, i.e., conditional likelihood was used. The first model was unadjusted, although adjusted for age and sex by design. The second model was additionally adjusted for age at baseline, civil status, education, early retirement pension, and 10 diagnoses at baseline (Table 1). To further illustrate the nonlinear association over time in the cohort study and sibling cohort, restricted cubic splines with four knots were used (resulting in three degrees of freedom), followed by fitting a proportional hazards model [12]. The Stata software and SPSS (version 23; IBM, NY) were used to fit the statistical models and graphically illustrate the results.
Tab. 1. Cohort characteristics at baseline. 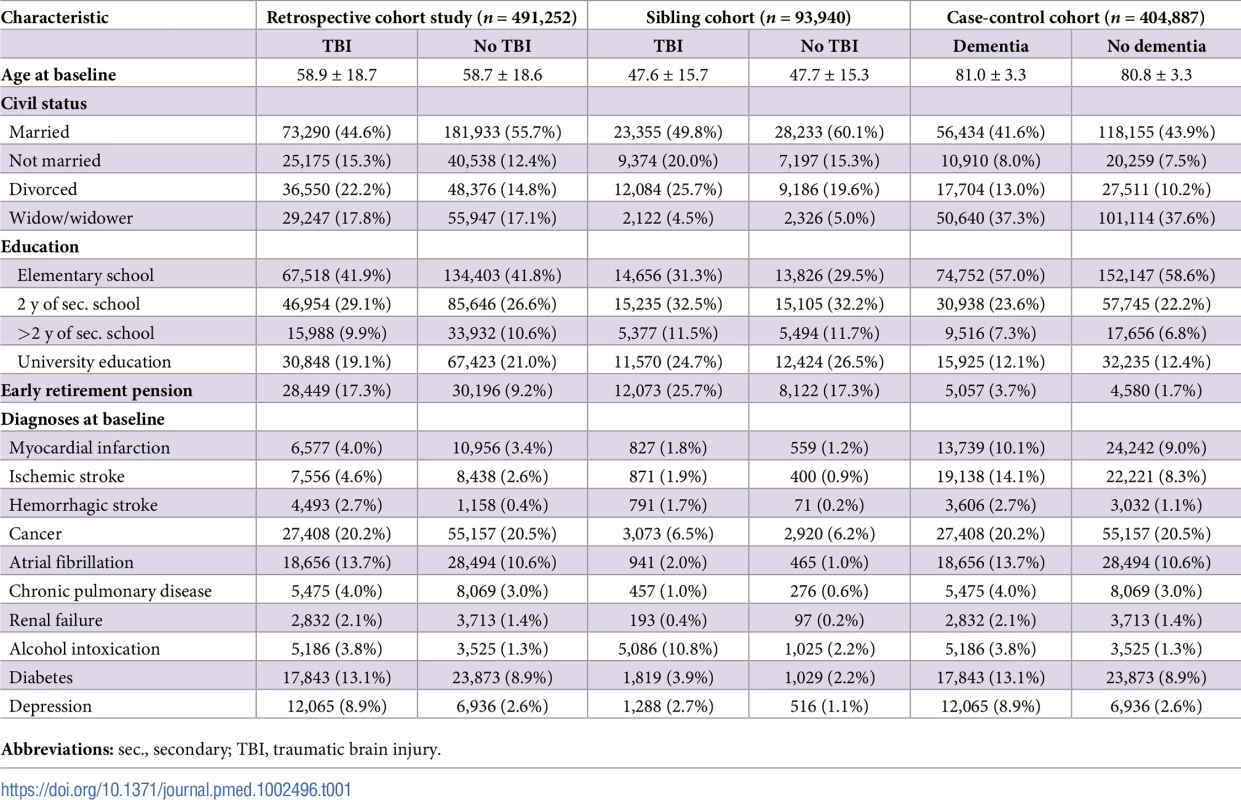
The first (prospective) cohort was matched according to TBI at baseline, and the risk of dementia was investigated during follow-up; the second cohort consisted of siblings with discordant TBI at baseline; the third (retrospective) cohort was matched according to dementia during follow-up, and diagnoses of TBI were investigated. Results
Baseline characteristics
Characteristics of the retrospective cohort study and the sibling cohort, in which the risk of dementia diagnoses after baseline was investigated, and characteristics of the case-control cohort, in which the risk of TBI before baseline was investigated, are presented in Table 1. In the retrospective cohort study, more individuals with than without TBI were divorced, received early retirement pensions, and had diagnoses of diabetes and depression. Similar characteristics were found in the sibling cohort, although the mean age at baseline was lower and diagnoses were less common. In the case-control study, individuals with dementia more often had myocardial infarction, stroke, atrial fibrillation, depression, and diabetes at baseline than did those without dementia.
Prospective risk of dementia diagnosis in the cohort study
During a mean follow-up period of 15.3 (range, 0–49) years, 21,963 individuals in the total cohort (6.3% of those diagnosed with TBI, 3.6% of the rest of the cohort) were diagnosed with dementia (fully adjusted odds ratio [aOR], 1.81; 95% confidence interval [CI], 1.75–1.86; Table 2, Fig 4). Other strong risk factors at baseline (p < 0.001 for all) included higher age (aOR, 1.13), early retirement pension (aOR, 3.10), alcohol intoxication (aOR, 1.75), and depression (aOR, 1.41). The association was similar in men (aOR, 1.88; 95% CI, 1.80–1.97) and in women (aOR, 1.75; 95% CI, 1.68–1.82). The risk of dementia diagnosis after TBI decreased rapidly the first year (Fig 4). Thus, the association between TBI and subsequent dementia was strongest in the first year after TBI (aOR, 3.52; 95% CI, 3.23–3.84; Table 2) but still increased more than 30 years after TBI (aOR, 1.25; 95% CI, 1.11–1.41; Table 2). The association between TBI and dementia was weaker for the outcome of AD (aOR, 1.58; 95% CI, 1.49–1.69) than for vascular dementia (aOR, 2.17; 95% CI, 2.02–2.32) and unspecified dementia (aOR, 1.78; 95% CI, 1.71–1.85). The risk of dementia diagnosis associated with one mild TBI and one more severe TBI both decreased rapidly the first years after TBI (Fig 2 and Fig 3, respectively). Overall, single mild TBI showed the weakest association with dementia (aOR, 1.63; 95% CI, 1.57–1.70), with stronger associations for more severe TBI (aOR, 2.06; 95% CI, 1.95–2.19) and multiple TBIs (aOR, 2.81; 95% CI, 2.51–3.15). The association between dementia and TBI was stronger for those diagnosed with dementia before the age of 65 years (aOR, 2.25; 95% CI, 1.91–2.65) than for those diagnosed after this age (aOR, 1.79; 95% CI, 1.74–1.85).
Fig. 4. Associations between TBI and the risk of dementia diagnosis during follow-up in 491,252 individuals. 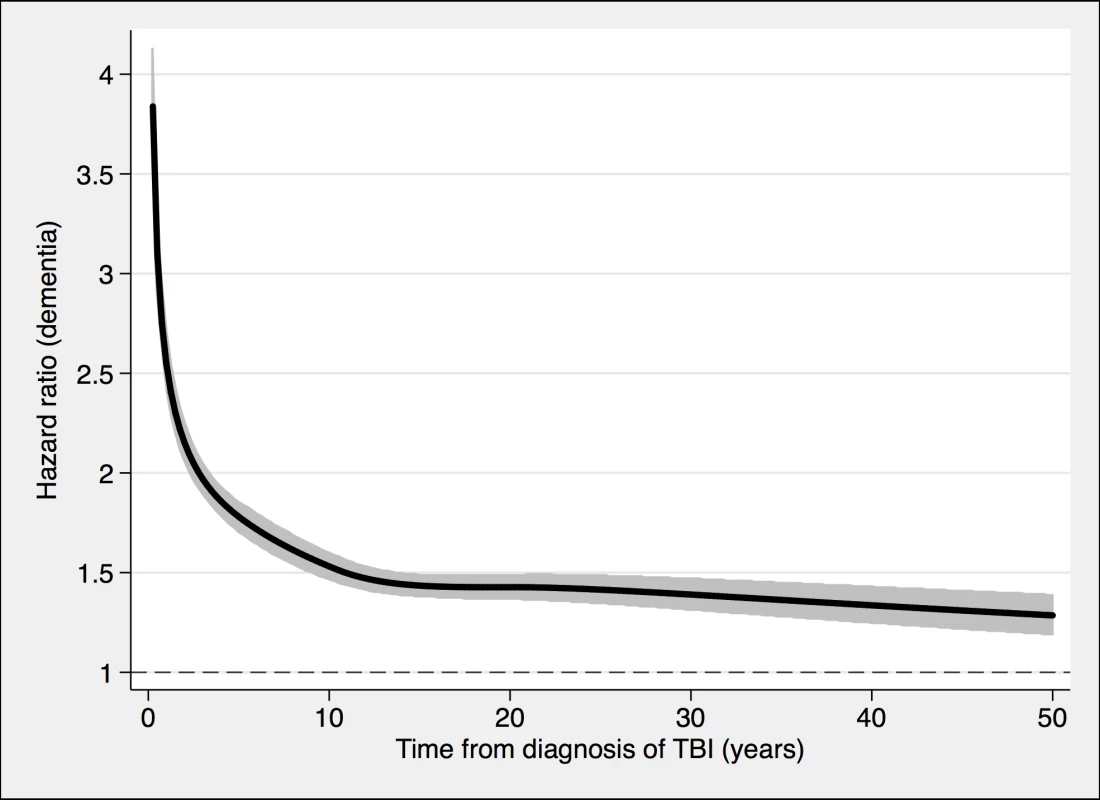
To model the effects, restricted cubic splines with four knots were used (resulting in three degrees of freedom), followed by fitting a proportional hazards model. TBI, traumatic brain injury. Tab. 2. Associations between TBI and the risk of dementia during follow-up in 491,252 individuals. 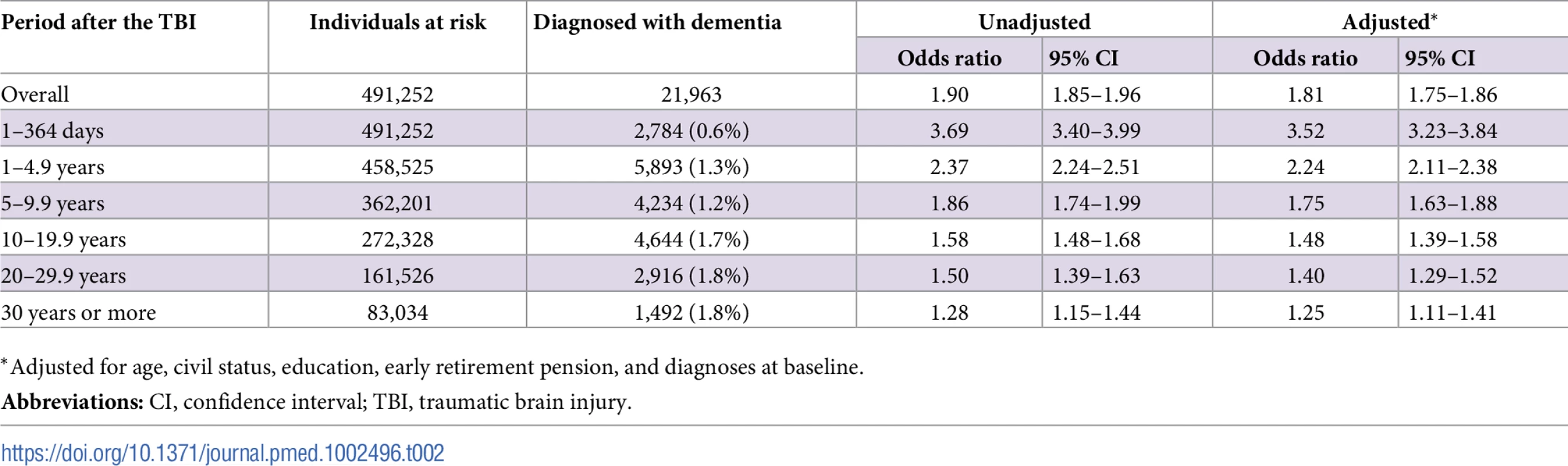
Odds ratios and 95% CIs were derived from conditional logistic regression analysis. Prospective risk of dementia diagnosis in the sibling cohort
During a mean follow-up period of 18.8 (range, 0–49) years, 1,204 individuals in the sibling cohort (1.8% of siblings with TBI at baseline, 0.8% of unaffected siblings) were diagnosed with dementia (aOR, 1.89; 95% CI, 1.62–2.21; Table 3 and Fig 5). The association between TBI and the risk of subsequent dementia was similar to that in the total cohort not consisting of siblings (Table 2). In fully adjusted models, the risk of dementia was highest in the first year after TBI (aOR, 6.09; 95% CI, 2.70–13.71; Table 3); it declined gradually but remained significant more than 10 years after TBI (aOR, 1.39; 95% CI, 1.12–1.72). The association was weaker for single mild TBI (aOR, 1.49; 95% CI, 1.23–1.80) than for single severe TBI (aOR, 3.31; 95% CI, 2.13–5.16) and multiple TBIs (aOR, 5.01; 95% CI, 2.37–10.61).
Fig. 5. Associations between TBI and a dementia diagnosis during follow-up in 46,970 sibling pairs discordant for TBI. 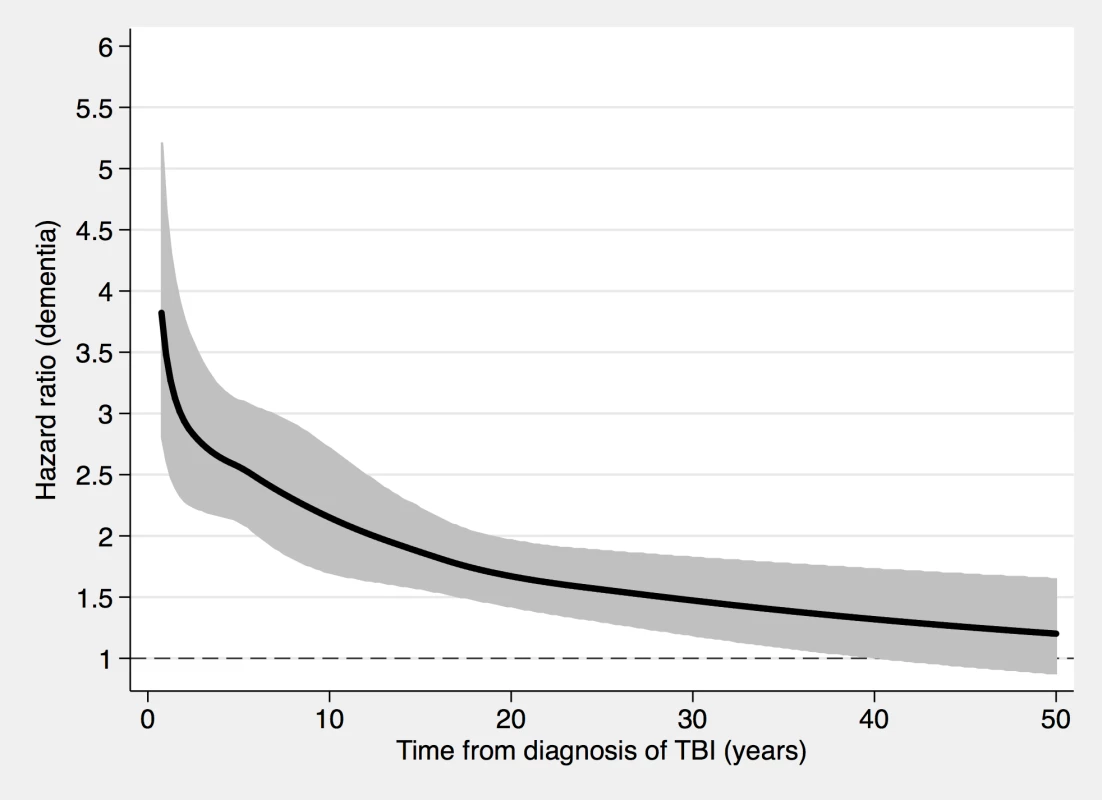
To model the effects, restricted cubic splines with four knots were used (resulting in three degrees of freedom), followed by fitting a proportional hazards model. TBI, traumatic brain injury. Tab. 3. Associations between TBI and the risk of dementia during follow-up in 46,970 full sibling pairs, discordant for TBI at baseline. 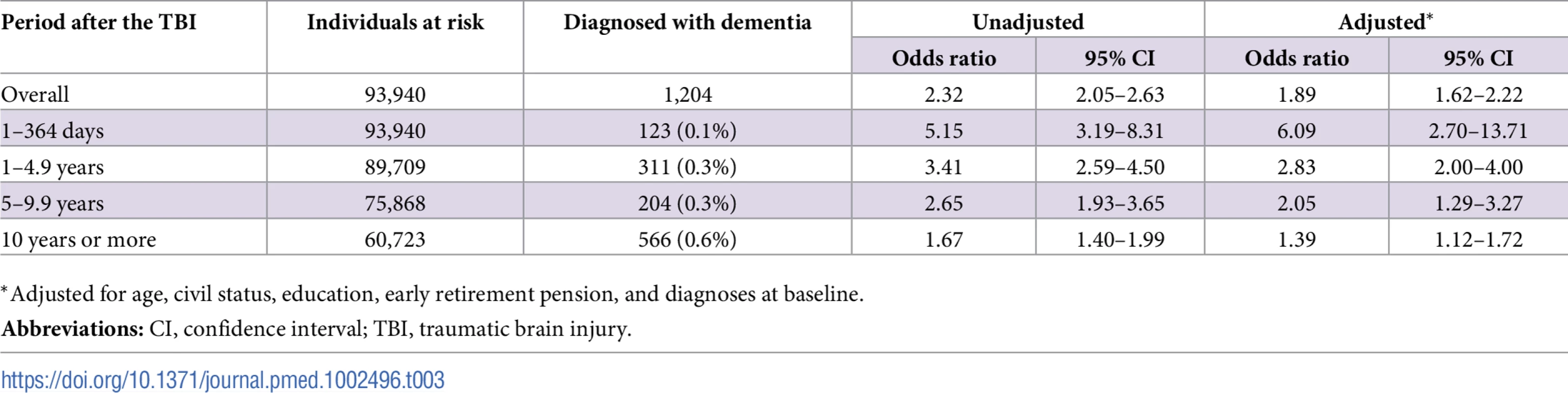
Odds ratios and 95% CIs were derived from conditional logistic regression analysis. Retrospective risk of TBI diagnosis in the case-control cohort
In total, 21,276 individuals in this cohort (7.7% of individuals with dementia, 4.0% of the rest of the cohort; p < 0.001) had a history of at least one TBI before baseline. After adjustment for all confounders, the risk of dementia was highest in the first year after TBI (OR, 3.89; 95% CI, 3.54–4.27; Table 4). This elevated risk was still significant 30 years after TBI in the unadjusted analysis (OR, 1.20; 95% CI, 1.09–1.32) but not after adjustment for all confounders (OR, 1.08; 95% CI, 0.97–1.19; Table 4). Overall, single mild TBI showed the weakest association with dementia (aOR, 1.59; 95% CI, 1.54–1.65), with stronger associations for more severe TBI (aOR, 1.95; 95% CI, 1.83–2.08) and multiple TBIs (aOR, 2.15; 95% CI, 1.93–2.40). The association was similar for diagnoses recorded before the age of 65 years (aOR, 1.75; 95% CI, 1.47–2.08) and after this age (aOR, 1.71; 95% CI, 1.65–1.76).
Tab. 4. Associations between dementia at baseline and previous TBI in the retrospective cohort (n = 404,887). 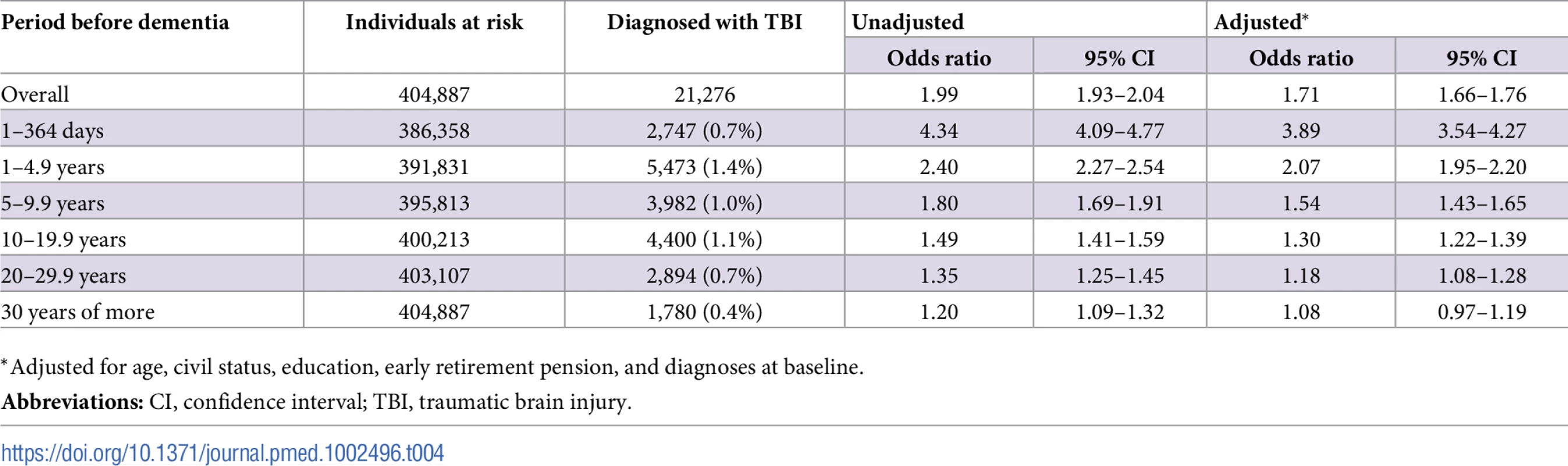
Odds ratios and 95% CIs were derived from conditional logistic regression analysis. Discussion
In the present nationwide cohort, with up to 50 years of follow-up, a clear association was observed between previous TBI and the risk of being diagnosed with dementia later in life. The risk of dementia was highest in the first years after TBI, but it was sustained more than 30 years thereafter. The association was also similar in a large cohort of full siblings and similar in men and in women. Finally, the risk of developing dementia appeared to have a dose–response relationship with regard to TBI severity and number of TBIs.
The link between TBI and developing dementia has been controversial for some time, due to the conflicting nature of available data. In the present study, the risk of dementia diagnosis was increased by about 80% during a mean follow-up period of 15 years for individuals diagnosed with TBI, compared with the rest of the cohort. The investigation of TBI as a risk factor for dementia entails the risk of reversed causality [13–15] or misdiagnosis due to post-concussive symptoms; thus, data from studies with short follow-up periods [16] should be interpreted with caution. In the aging population, dementia can be an underlying risk factor for accidents resulting in TBI, such as car accidents and injurious falls [17,18]. In the present study, the risk of being diagnosed with dementia in the first year after TBI was four to six times higher, compared with individuals with no TBI. Thereafter, this risk declined rapidly. The development of dementia, with impaired executive function and an increased risk of falling, likely began before the time of TBI in some individuals in these cohorts; thus, TBI may have been influenced by reduced cognitive function, with resulting reversed causality. Nevertheless, the significant association observed more than 30 years after TBI cannot be explained by reversed causality. Still, an unknown confounder may explain the increased risk of dementia also with longer follow-up. In a previous study, the strength of the association between YOD and TBI was reduced markedly after adjustment for confounders [9]. As in the present study, a previous TBI showed stronger associations with non-AD forms. To our knowledge, no previous prospective study with similar power and follow-up time has been reported, preventing direct comparisons to our data.
The relation of TBI severity to the risk of developing dementia is a matter of much debate. Evidence from previous studies is not conclusive; several studies have suggested that moderate to severe TBI is an important risk factor for subsequent dementia [4,6,19–21], but others have failed to confirm these results [14,22,23]. The lack of association could be due to limited statistical power, as severe and multiple TBIs are less common than single mild TBI, including in the present study. Data from the present study suggest a clear dose–response relationship, with single mild TBI showing a weaker association with dementia diagnosis than did more severe and multiple TBIs. In support, a recent nationwide Finnish study found that persons with more severe TBI were at increased risk of dementia, compared to those with mild TBI [24]. These graded associations may support the existence of a causal relationship. Another explanation could be that subjects with more severe or multiple TBIs more often have other risk factors for dementia, such as lower cognitive function before TBI [25].
The associations found between TBI and the risk of subsequent dementia diagnosis could also be influenced by familial factors, such as upbringing conditions, education, and genetic factors. To our knowledge, no previous population-based study with a long follow-up period has evaluated these potential influences. In the present study, we thus examined the association in about 47,000 full sibling pairs with discordant TBI status during follow-up. The risk of dementia diagnosis during follow-up was almost doubled in siblings with TBI compared with their counterparts without TBI, and it remained increased more than 10 years after TBI. These results are similar to those obtained for the other cohorts, suggesting that familial factors cannot explain the association between TBI and dementia.
The present study has several limitations that should be considered. Most importantly, no causal inference should be made based on observational data, although the time-dependent associations more than 30 years after TBI and the dose-dependent relationship according to TBI severity and number may support such causality. The strong association demonstrated for individuals with short follow-up is most likely subject to different forms of bias, e.g., reversed causality, as discussed previously. In addition, it is likely that individuals with TBI are subject to a more rigorous control from healthcare and relatives initially after TBI, increasing the chance of being diagnosed with dementia. The results of the present study are based on diagnoses made in specialist care; diagnoses made in primary care were not included, which may have affected the numbers of TBI and dementia diagnoses included in analyses. Furthermore, data were obtained from registers and diagnoses could not be confirmed clinically, although all diagnoses were recorded in the context of specialist healthcare. Nevertheless, a lower sensitivity with respect to these outcomes would, if anything, attenuate the associations found. The main strengths of the present study include the large body of recorded data covering a long period, which provided superior statistical power for the performance of reliable analyses, and a long follow-up period that was not subject to recall bias. The main results of the present study were also evaluated in a cohort of siblings, and the findings were consistent. Thus, this sensitivity analysis supported the validity of the main findings, and because the cohort was nationwide, the external validity of the results is likely to be high.
In summary, the findings of this study suggest the existence of a time - and dose-dependent risk of developing dementia more than 30 years after TBI. The association was stronger for more severe and multiple TBIs than for single mild TBI. The association was also present after adjustment for familial factors in a sibling analysis. Overall, the results may support a causal association between TBI and the risks of different types of dementia. However, given the observational study design, we cannot exclude the possibility that other factors explain the observed associations.
Supporting Information
Zdroje
1. Bruns J Jr., Hauser WA (2003) The epidemiology of traumatic brain injury: a review. Epilepsia 44 Suppl 10 : 2–10.
2. Shively S, Scher AI, Perl DP, Diaz-Arrastia R (2012) Dementia resulting from traumatic brain injury: what is the pathology? Arch Neurol 69 : 1245–1251. doi: 10.1001/archneurol.2011.3747 22776913
3. Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A (2003) Head injury as a risk factor for Alzheimer's disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry 74 : 857–862. 12810767
4. Mortimer JA, van Duijn CM, Chandra V, Fratiglioni L, Graves AB, et al. (1991) Head trauma as a risk factor for Alzheimer's disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol 20 Suppl 2: S28–35.
5. Lee YK, Hou SW, Lee CC, Hsu CY, Huang YS, et al. (2013) Increased risk of dementia in patients with mild traumatic brain injury: a nationwide cohort study. PLoS ONE 8: e62422. doi: 10.1371/journal.pone.0062422 23658727
6. Guo Z, Cupples LA, Kurz A, Auerbach SH, Volicer L, et al. (2000) Head injury and the risk of AD in the MIRAGE study. Neurology 54 : 1316–1323. 10746604
7. Crane PK, Gibbons LE, Dams-O'Connor K, Trittschuh E, Leverenz JB, et al. (2016) Association of Traumatic Brain Injury With Late-Life Neurodegenerative Conditions and Neuropathologic Findings. JAMA Neurol 73 : 1062–1069. doi: 10.1001/jamaneurol.2016.1948 27400367
8. Jellinger KA, Paulus W, Wrocklage C, Litvan I (2001) Traumatic brain injury as a risk factor for Alzheimer disease. Comparison of two retrospective autopsy cohorts with evaluation of ApoE genotype. BMC Neurol 1 : 3. 11504565
9. Nordstrom P, Michaelsson K, Gustafson Y, Nordstrom A (2014) Traumatic brain injury and young onset dementia: a nationwide cohort study. Ann Neurol 75 : 374–381. 24812697
10. Li W, Risacher SL, McAllister TW, Saykin AJ (2016) Traumatic brain injury and age at onset of cognitive impairment in older adults. J Neurol 263 : 1280–1285. doi: 10.1007/s00415-016-8093-4 27007484
11. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, et al. (2011) External review and validation of the Swedish national inpatient register. BMC public health 11 : 450. doi: 10.1186/1471-2458-11-450 21658213
12. Royston P, Parmar MK (2002) Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med 21 : 2175–2197. doi: 10.1002/sim.1203 12210632
13. Gardner RC, Burke JF, Nettiksimmons J, Kaup A, Barnes DE, et al. (2014) Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. JAMA Neurol 71 : 1490–1497. doi: 10.1001/jamaneurol.2014.2668 25347255
14. Mehta KM, Ott A, Kalmijn S, Slooter AJ, van Duijn CM, et al. (1999) Head trauma and risk of dementia and Alzheimer's disease: The Rotterdam Study. Neurology 53 : 1959–1962. 10599765
15. Rapoport M, Wolf U, Herrmann N, Kiss A, Shammi P, et al. (2008) Traumatic brain injury, Apolipoprotein E-epsilon4, and cognition in older adults: a two-year longitudinal study. J Neuropsychiatry Clin Neurosci 20 : 68–73. doi: 10.1176/jnp.2008.20.1.68 18305286
16. Carroll LJ, Cassidy JD, Peloso PM, Borg J, von Holst H, et al. (2004) Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med: 84–105. 15083873
17. Friedland RP, Koss E, Kumar A, Gaine S, Metzler D, et al. (1988) Motor vehicle crashes in dementia of the Alzheimer type. Ann Neurol 24 : 782–786. doi: 10.1002/ana.410240613 3207361
18. van Doorn C, Gruber-Baldini AL, Zimmerman S, Hebel JR, Port CL, et al. (2003) Dementia as a risk factor for falls and fall injuries among nursing home residents. J Am Geriatr Soc 51 : 1213–1218. 12919232
19. Molgaard CA, Stanford EP, Morton DJ, Ryden LA, Schubert KR, et al. (1990) Epidemiology of head trauma and neurocognitive impairment in a multi-ethnic population. Neuroepidemiology 9 : 233–242. 2087247
20. Salib E, Hillier V (1997) Head injury and the risk of Alzheimer's disease: a case control study. Int J Geriatr Psychiatry 12 : 363–368. 9152722
21. Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, et al. (2000) Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology 55 : 1158–1166. 11071494
22. Launer LJ, Andersen K, Dewey ME, Letenneur L, Ott A, et al. (1999) Rates and risk factors for dementia and Alzheimer's disease: results from EURODEM pooled analyses. EURODEM Incidence Research Group and Work Groups. European Studies of Dementia. Neurology 52 : 78–84. 9921852
23. Williams DB, Annegers JF, Kokmen E, O'Brien PC, Kurland LT (1991) Brain injury and neurologic sequelae: a cohort study of dementia, parkinsonism, and amyotrophic lateral sclerosis. Neurology 41 : 1554–1557. 1922795
24. Raj R, Kaprio J, Korja M, Mikkonen ED, Jousilahti P, et al. (2017) Risk of hospitalization with neurodegenerative disease after moderate-to-severe traumatic brain injury in the working-age population: A retrospective cohort study using the Finnish national health registries. PLoS Med 14: e1002316. doi: 10.1371/journal.pmed.1002316 28678790
25. Nordstrom A, Edin BB, Lindstrom S, Nordstrom P (2013) Cognitive function and other risk factors for mild traumatic brain injury in young men: nationwide cohort study. BMJ 346: f723. doi: 10.1136/bmj.f723 23482939
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2018 Číslo 1- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- What about drinking is associated with shorter life in poorer people?
- Life course socioeconomic position, alcohol drinking patterns in midlife, and cardiovascular mortality: Analysis of Norwegian population-based health surveys
- Pelvic inflammatory disease risk following negative results from chlamydia nucleic acid amplification tests (NAATs) versus non-NAATs in Denmark: A retrospective cohort
- Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: A population-based cohort study
- Sexually transmitted infections in the era of antiretroviral-based HIV prevention: Priorities for discovery research, implementation science, and community involvement
- Traumatic brain injury and the risk of dementia diagnosis: A nationwide cohort study
- Association between intake of less-healthy foods defined by the United Kingdom's nutrient profile model and cardiovascular disease: A population-based cohort study
- Progression of the first stage of spontaneous labour: A prospective cohort study in two sub-Saharan African countries
- The cost-effectiveness of alternative vaccination strategies for polyvalent meningococcal vaccines in Burkina Faso: A transmission dynamic modeling study
- PD-L1 checkpoint inhibition and anti-CTLA-4 whole tumor cell vaccination counter adaptive immune resistance: A mouse neuroblastoma model that mimics human disease
- Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: A Phase 1 open-label clinical trial in healthy adults
- Immune-related genetic enrichment in frontotemporal dementia: An analysis of genome-wide association studies
- Long-term trends in mortality and AIDS-defining events after combination ART initiation among children and adolescents with perinatal HIV infection in 17 middle- and high-income countries in Europe and Thailand: A cohort study
- What’s coming for health science and policy in 2018? Global experts look ahead in their field
- Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: A targeted metabolomics study
- Estimated mortality on HIV treatment among active patients and patients lost to follow-up in 4 provinces of Zambia: Findings from a multistage sampling-based survey
- From macro- to microfactors in health: Social science approaches in research on sexually transmitted infections
- The WHO 2016 verbal autopsy instrument: An international standard suitable for automated analysis by InterVA, InSilicoVA, and Tariff 2.0
- Long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: Systematic review and meta-analysis
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Traumatic brain injury and the risk of dementia diagnosis: A nationwide cohort study
- Pelvic inflammatory disease risk following negative results from chlamydia nucleic acid amplification tests (NAATs) versus non-NAATs in Denmark: A retrospective cohort
- PD-L1 checkpoint inhibition and anti-CTLA-4 whole tumor cell vaccination counter adaptive immune resistance: A mouse neuroblastoma model that mimics human disease
- Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: A Phase 1 open-label clinical trial in healthy adults
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



