-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe cost-effectiveness of alternative vaccination strategies for polyvalent meningococcal vaccines in Burkina Faso: A transmission dynamic modeling study
Reza Yaesoubi and colleagues present a model that identifies health impacts and cost-effectiveness of different meningococcal vaccines and strategies in the meningitis belt in Africa.
Published in the journal: . PLoS Med 15(1): e32767. doi:10.1371/journal.pmed.1002495
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002495Summary
Reza Yaesoubi and colleagues present a model that identifies health impacts and cost-effectiveness of different meningococcal vaccines and strategies in the meningitis belt in Africa.
Introduction
N. meningitidis remains a major cause of morbidity and mortality in the meningitis belt, a region in sub-Saharan Africa extending from Senegal to Ethiopia, with an estimated population of 400 million people [1]. In this region, meningitis epidemics occur sporadically, resulting in tens of thousands of cases and imposing substantial economic costs to affected communities. Since the late 1970s, control of meningitis epidemics in the meningitis belt has relied on reactive vaccination campaigns using polysaccharide vaccines. These reactive campaigns are triggered once an outbreak surpasses an epidemic threshold defined by the World Health Organization (WHO). While timely implementation of this reactive strategy may blunt the severity of meningococcal epidemics [2–4], in many settings the impact of reactive vaccination campaigns is limited by delays in the diagnosis and reporting of meningitis cases and subsequent delays in the launch of these vaccination activities [5].
In December 2010, a new group A meningococcal conjugate vaccine, PsA-TT (MenAfriVac) [6], was introduced in Burkina Faso, Mali, and Niger, and within one month, almost 20 million individuals, ages 1–29 years, were vaccinated [7–9]. The introduction of MenAfriVac across the meningitis belt has been associated with a dramatic reduction of meningitis A cases and carriage during subsequent seasons [10–12]. While MenAfriVac has been successful in controlling group A disease and transmission, persistent threat from other meningococcal serogroups [13,14] has spurred development of polyvalent vaccines that target non-A serogroups, including C, Y, W, and X [15–17].
The advent of affordable polyvalent meningococcal vaccines offers the opportunity for more effective control and potential elimination of N. meningitidis epidemics in the meningitis belt, but the health impact and costs of alternative vaccination strategies are not yet clear [18]. While available polyvalent meningococcal polysaccharide (PMP) vaccines can only be used in reactive campaigns because they are poorly immunogenic in children under 2 years old [19,20] and induce a short period of immunity in adults [1,21], polyvalent meningococcal conjugate (PMC) vaccines are immunogenic among infants and can induce longer-term protection. Therefore, PMC vaccines can potentially replace MenAfriVac in the Expanded Program on Immunization (EPI) and can also be used in reactive and/or mass preventive vaccination campaigns. Consensus on how to best use these novel polyvalent meningococcal vaccines has not yet been achieved.
Here, we describe a mathematical transmission model that captures the key characteristics of meningococcal epidemics in Burkina Faso as well as the costs associated with routine, reactive, and preventive vaccination campaigns. We utilize this model to investigate the health effects and costs associated with alternative vaccination policies that can inform the use of PMP and PMC vaccines.
Methods
Model structure
We developed a stochastic compartmental model of meningococcal transmission to capture the essential characteristics of meningococcal epidemics within 55 districts of Burkina Faso. This level of spatial disaggregation by district is necessary to model reactive vaccination campaigns that are triggered within each district upon passing the WHO epidemic threshold of 10 cases per 100,000 population per week [22,23]. To allow for age-specific mixing patterns and targeting of vaccinations, the simulated population was stratified into relevant age groups. We used a gravity model to describe the mixing pattern of individuals residing in different districts (see S1 Text).
The adoption of MenAfriVac as part of the EPI is expected to eliminate serogroup A meningococcal epidemics in the meningitis belt by 2020 [10,11,24–26]. Our model therefore assumes that there is no circulation of serogroup A and aggregates all remaining serogroups covered in PMC and PMP vaccines (Fig 1). Upon infection, individuals become asymptomatic carriers, but only a small portion of these infections lead to meningitis. Individuals with active disease and individuals with asymptomatic carriage contribute to the force of infection. Because the duration of carriage is relatively short [27], we assume that the probability of superinfection during carriage is negligible. Individuals who recover from active disease and carriers who clear carriage will remain temporarily immune to reinfection [27,28]. Details of our modeling approach are provided in S1 Text.
Fig. 1. Meningitis natural history and transmission dynamics, assuming a single aggregated circulating serogroup. 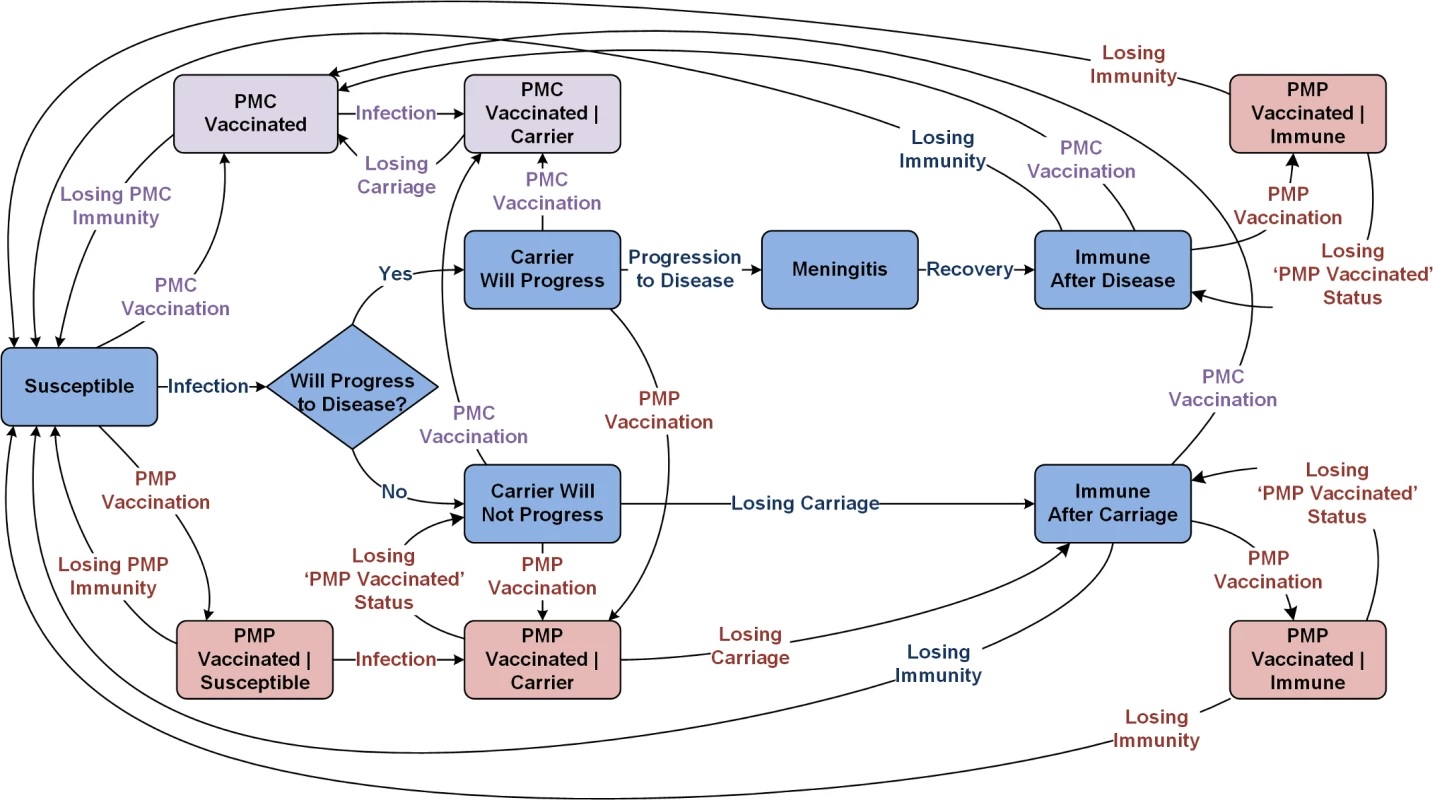
PMC, polyvalent meningococcal conjugate; PMP, polyvalent meningococcal polysaccharide. Modeling the impact of MenAfricVac vaccine
Because the immunity induced by MenAfriVac is serogroup specific, the possibility of strain replacement due to the rollout of MenAfriVac across the meningitis belt cannot yet be excluded [29,30]. Therefore, to account for the impact of MenAfriVac on future epidemics, we consider two extreme scenarios: (1) “with strain replacement” reflects the pessimistic assumption of essentially complete strain replacement and assumes that future epidemics will occur with similar frequency and magnitude after the introduction of MenAfriVac (Fig 2A) and (2) “without strain replacement” assumes that future serotype incidence will be similar to the past but with serogroup A excluded (i.e., potentially lower frequency and/or magnitude of epidemics). For the latter scenario (without strain replacement), we determined the weekly meningitis incidence using estimates for the proportion of confirmed meningitis cases during 2002–2015 due to non-serogroup A N. meningitidis (Fig 2B). Evaluation of vaccine strategies under these two extreme scenarios allows us to identify crude bounds on the performance of vaccine policies, given existing uncertainty about the degree to which strain replacement can be expected.
Fig. 2. (A) Weekly clinical meningitis cases in Burkina Faso reported between 2002 and 2015 (data were made available from the Ministry of Health, Burkina Faso). (B) Percentage of confirmed meningitis cases that are associated to Neisseria meningitis serogroup A, N. meningitidis non-A serogroups (including C, W, and X), and other pathogens (including Streptococcus pneumoniae and Haemophilus influenzae type b) from 2002–2015. 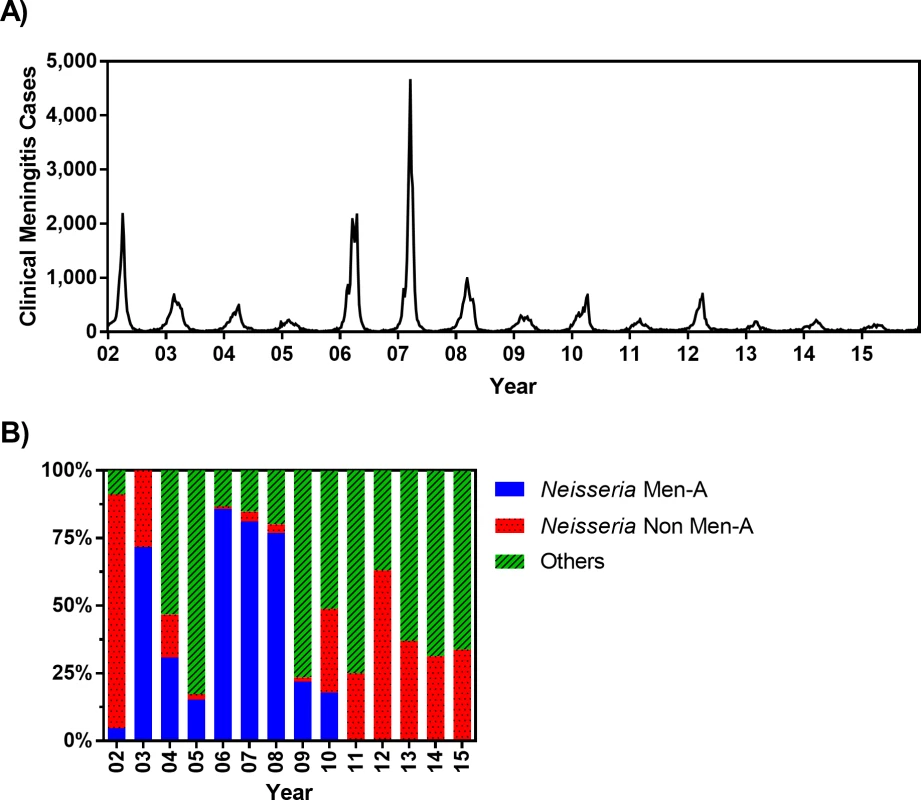
These estimates are obtained from WHO [31] (for 2002), WHO Enhanced Meningitis Bulletin (for 2003–2005), Burkina Faso Maladies Potentiel Épidémie (MPE) surveillance data (for 2006 and 2012–2015), and Novak et al. [11] (for 2007–2011) (see S1 Data). Men-A, meningitis serogroup A; Non Men-A, meningitis serogroups other than A. Data sources and model calibration
The age-specific mortality rate and life expectancy are informed by population data (S1 Text) [32,33]. The weekly clinical meningitis notification data are provided by Burkina Faso’s Ministry of Health (Fig 2A). Our model is calibrated against the age distribution of meningococcal meningitis incidence (Fig 3A) and average age-specific meningococcal carriage prevalence (Fig 3B). Because reactive campaigns are launched when the number of clinical meningitis cases exceeds the WHO epidemic threshold [22,23], the model must reproduce observed trends in clinical diagnoses (Fig 2A). To this end, we used the average, standard deviation, and periodicity (as characterized by Fourier analysis) of the time series of clinical diagnoses as additional calibration targets (Fig 3C–3E). Finally, Fig 3F demonstrates that the number of districts in which the epidemic threshold of 10 meningitis cases per 100,000 persons has been exceeded in our simulations is consistent with the epidemics observed between 2002 and 2015 in Burkina Faso.
Fig. 3. The proposed model matches the key characteristics of meningitis epidemics in Burkina Faso observed between 2002 and 2015. 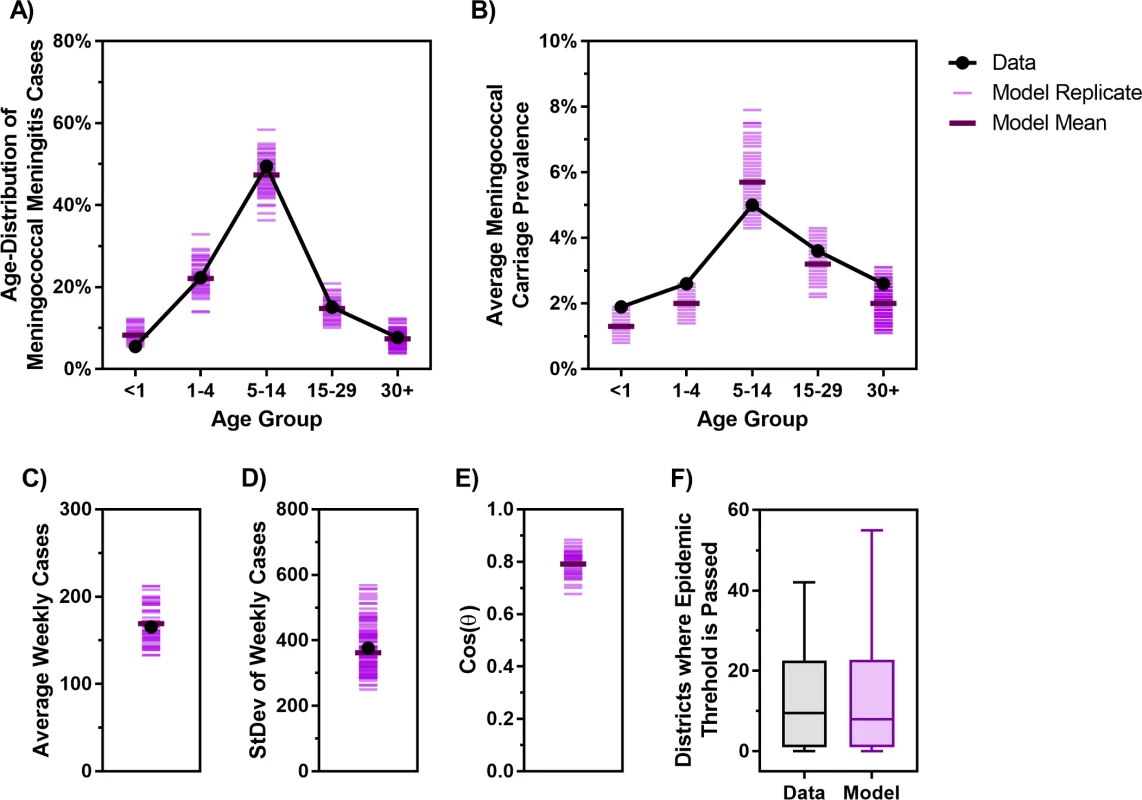
(A) Age distribution of probable meningococcal meningitis in Burkina Faso from 2007–2011 [11] versus the age distribution of cases generated by the model. (B) Estimated meningococcal carriage prevalence in different age groups from carriage survey studies in the African meningitis belt [34] versus the age-specific average carriage prevalence obtained from the model. (C–D) Average and standard deviation of weekly clinical meningitis cases observed from 2002–2015 versus those produced by the model. (E) Cosine of the angle (θ) between the vectors of Fourier amplitude for observed and simulated meningitis time series (cosine of 1 indicates total match in periodicity and cosine of 0 indicates no overlap between the significant periods of two time series; see S1 Text for additional details). (F) Observed (Data) and simulated (Model) number of districts in each year between 2002 and 2015 in which the threshold of 10 meningitis cases per 100,000 population was exceeded. Cos, cosine; StDev, standard deviation. Fig 4 displays the clinical meningitis time series from three simulated trajectories over 30 years produced by the calibrated model, in comparison with the clinical meningitis time series observed in Burkina Faso during 2002–2015. We emphasize that our goal is not to fit to the timing of past epidemics but instead to calibrate the model against the periodicity of past epidemics, in addition to calibration targets depicted in Fig 3. Details of our calibration approach are described in S1 Text.
Fig. 4. Comparing the clinical meningitis time series observed between 2002 and 2015 in Burkina Faso (black curve) with three simulated trajectories produced by the calibrated model (blue, green, and red curves). 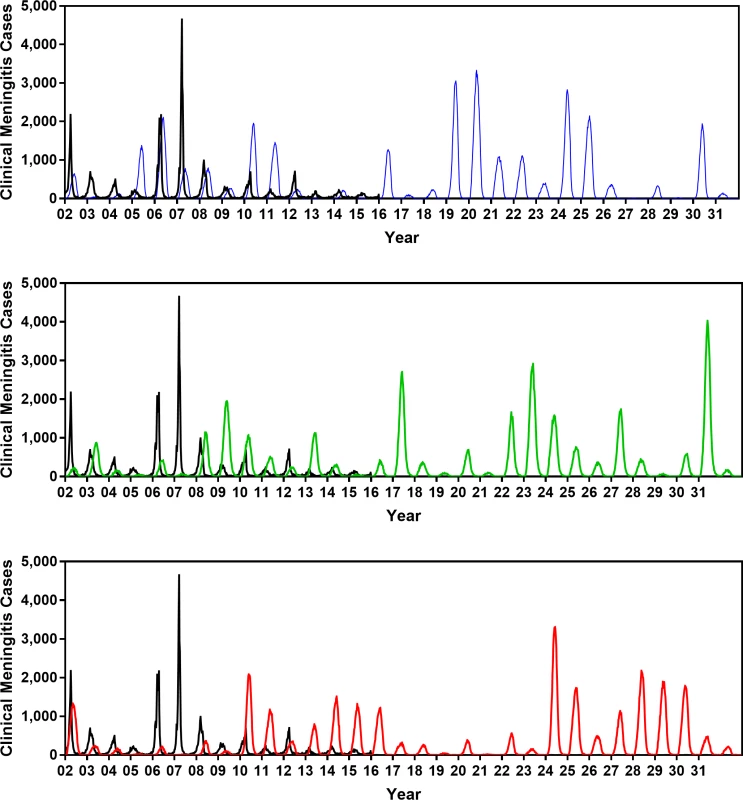
The periodicity at which simulated epidemics are occurring matches the periodicity of observed epidemics. Fig 3 shows that trajectories generated by our model also match other key properties of meningococcal epidemics in Burkina Faso (e.g., age distribution of cases, age-specific carriage prevalence, average weekly incidence, and number of districts in each year between 2002 and 2015 in which the threshold of 10 meningitis cases per 100,000 population was exceeded). Alternative vaccine policies
Even after the introduction of MenAfriVac in 2010, reactive strategies using PMP vaccine, alongside the EPI with MenAfriVac, remain important for responding to epidemics caused by non-A meningococci (Base strategy in Table 1) [35]. Because PMP vaccine is poorly immunogenic in infants and children younger than 2 years old [15], an alternative strategy is to use PMC vaccine in place of both MenAfriVac in EPI and PMP vaccines in reactive campaigns (Base Prime strategy in Table 1). Expanding PMC vaccine coverage to older age groups through preventive vaccination campaigns can both mitigate the risk of meningococcal epidemics by reducing the size of the at-risk population and can potentially lead to the elimination of meningitis (Prevention 1 and 2 strategies in Table 1). While the Base Prime strategy attempts to contain local outbreaks by implementing district-level reactive campaigns, the two Prevention strategies seek to reduce the population risk of infection through preventive campaigns implemented at a national level. We note that Base Prime can potentially lead to the same level of herd immunity as Prevention strategies over the long term, but this occurs only after the population has aged sufficiently such that all young adults were vaccinated through the EPI program or after outbreaks have occurred in a sufficient number of districts.
Tab. 1. Alternative vaccine strategies for employing polyvalent meningococcal vaccines (post 2020). 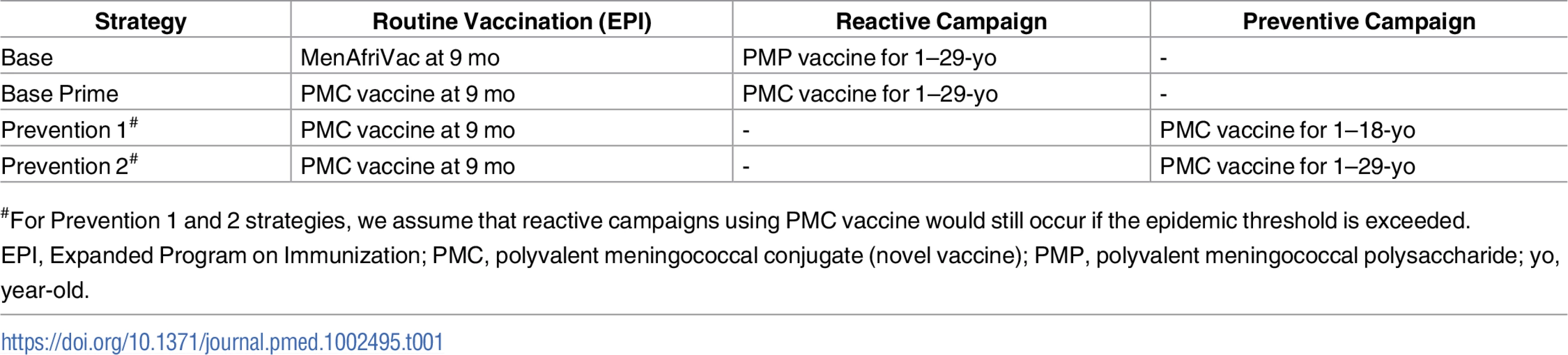
#For Prevention 1 and 2 strategies, we assume that reactive campaigns using PMC vaccine would still occur if the epidemic threshold is exceeded. Our model assumes that vaccinated individuals are protected against progression to invasive disease for a limited time (Fig 1), but the level and duration of protection differ for PMP and PMC vaccines. We assume that PMP - and PMC-vaccinated carriers still remain infectious and contribute to the force of infection. Because the duration of immunity provided by PMP vaccination is rather short (Table 2), we assume that PMP vaccination does not impact carriage - or disease-induced immunity. Upon vaccination with PMP, individuals with carriage - or disease-induced immunity move to PMP-Vaccinated compartments to prevent revaccination but will either return to the corresponding Immune compartments at the beginning of the following epidemic season or lose their carriage - or disease-induced immunity and join the Susceptible compartment.
Tab. 2. Vaccination assumptions. 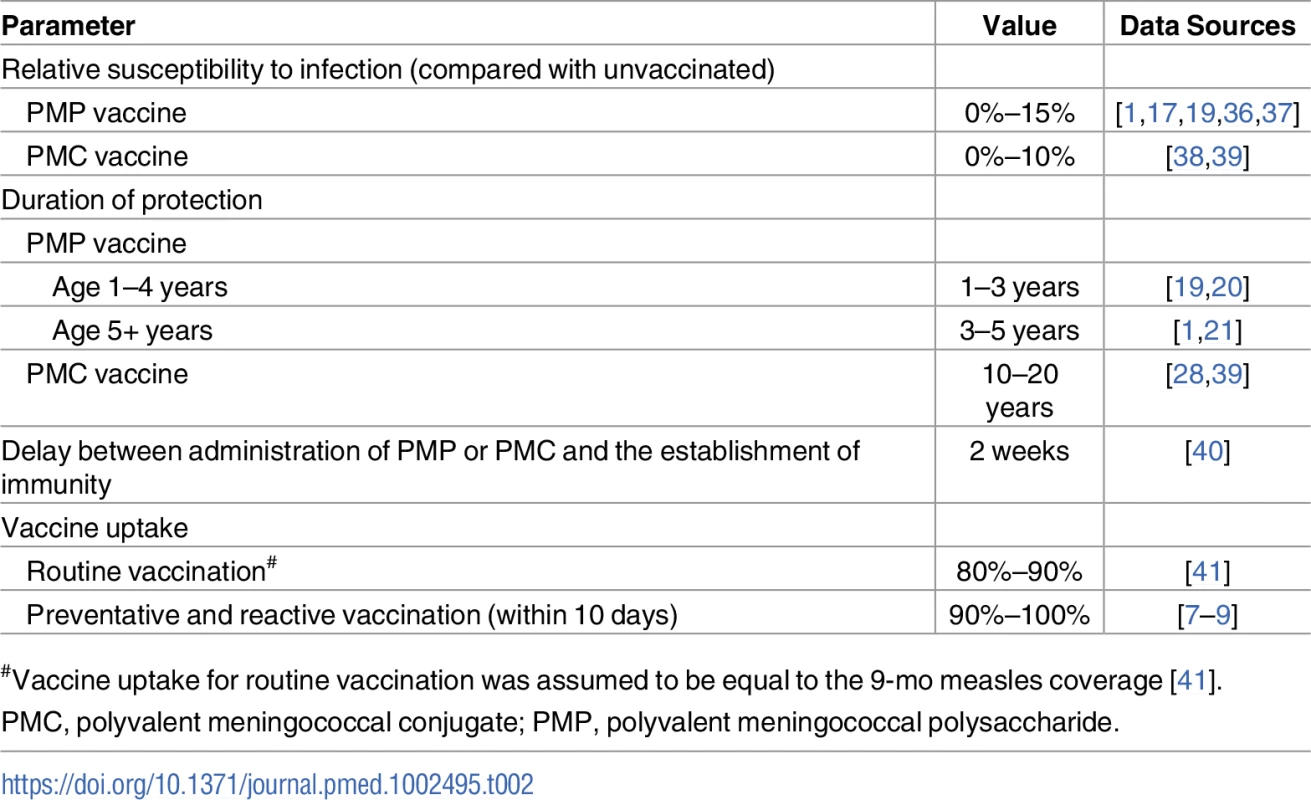
#Vaccine uptake for routine vaccination was assumed to be equal to the 9-mo measles coverage [41]. For the Base and Base Prime strategies, once an epidemic is declared in a district, a vaccine procurement order will be placed. For each district, we assume that the delay between exceeding the epidemic threshold and the initiation of a reactive vaccination campaign follows a discrete Uniform distribution [2, 10] weeks (Fig F in S1 Text). For Prevention strategies, a catch-up vaccination campaign is launched in November of the first simulation year and is completed before the start of the next epidemic season. During this period, PMC vaccine will be available to all individuals who are eligible for this catch-up vaccination.
Health and financial outcomes of vaccination strategies
The costs of vaccination programs in Burkina Faso are borne by the government and donors; hence, demonstration of affordability is essential for these programs to be considered in practice. We therefore take the payer’s perspective in estimating the costs associated with vaccine strategies. Our model accounts for costs incurred because of meningitis case management; care for patients who experience sequelae; and the operation costs of routine, reactive, and preventive vaccination campaigns. We assumed US$0.49 per MenAfriVac dose and US$4 per PMP vaccine dose [42]; as the price of the PMC vaccine is not yet determined, we allow this price to vary from US$4 to US$10 in sensitivity analyses. To measure the health outcome associated with each vaccine strategy, we use disability-adjusted life years (DALYs) [43]. Both costs (presented in the US dollars) and health outcomes are discounted at an annual rate of 3% to 2016. The details of the cost and DALY calculations are provided in S1 Text.
We followed the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) [44] to report the results of our cost-effectiveness analysis study (see S11 in S1 Text). All estimates from the model are presented as the average and 95% prediction intervals (the 2.5th and 97.5th percentiles) of 500 epidemic trajectories simulated over a 30-year period. The number of simulated trajectories was chosen such that the resulting prediction intervals were stable (i.e., the values of the 2.5th and 97.5th percentiles of estimates did not vary appreciably when additional trajectories were used). Costs and health effects of vaccinations strategies are presented with respect to the current WHO-recommended strategy of reactive vaccination campaigns using PMP vaccines (Base strategy in Table 1).
Results
Over a 30-year simulation period in Burkina Faso using the Base strategy of the EPI with MenAfriVac and reactive immunization with PMP vaccines, we project an annual average of 5,412 meningococcal cases (95% prediction interval: 105–16,550) with strain replacement and 1,642 (32–5,794) meningococcal cases without strain replacement. Compared to a counterfactual scenario in which reactive vaccination is not used, this represents an expected reduction of 45% (26%–62%) and 43% (22%–59%) in meningococcal incidence. The relatively modest impact of this strategy is attributable to (1) delays in the launch of reactive campaigns within districts upon crossing the epidemic threshold and (2) the short duration of immunity and lack of effect on carriage offered by PMP vaccines.
Vaccination strategies that use PMC vaccines could markedly reduce the expected annual number of meningitis cases (Fig 5), but they do not eliminate the possibility of meningitis outbreaks (Fig 6). The Prevention 2 strategy results in the most dramatic impact, averting 78% (63%–90%) of cases expected to occur under the Base strategy if strain replacement occurs and averting 87% (77%–93%) if no strain replacement occurs. Our model suggests that under strategies that utilize PMC vaccines, meningitis outbreaks may recur 10–15 years after the implementation of the first mass preventive campaign, when the immunity induced by PMC vaccines begins to wane in the adult population (Fig G in S1 Text).
Fig. 5. The expected percentage reduction in annual meningococcal cases over a 30-year simulation period for the vaccination strategies described in Table 1, compared to the Base strategy. 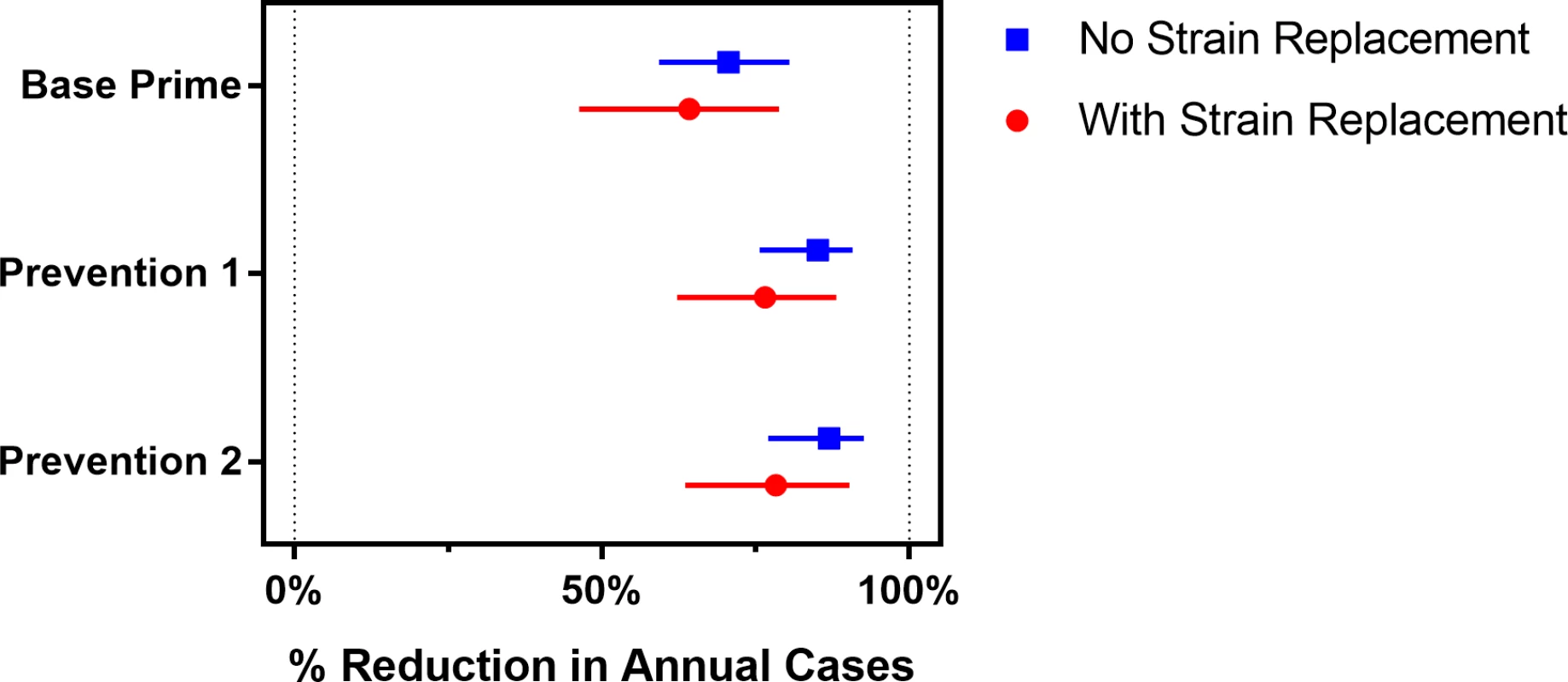
Bars represent the 95% prediction intervals. Fig. 6. Annual number of meningococcal cases for scenarios with and without strain replacement projected by the model under the vaccination strategies described in Table 1. 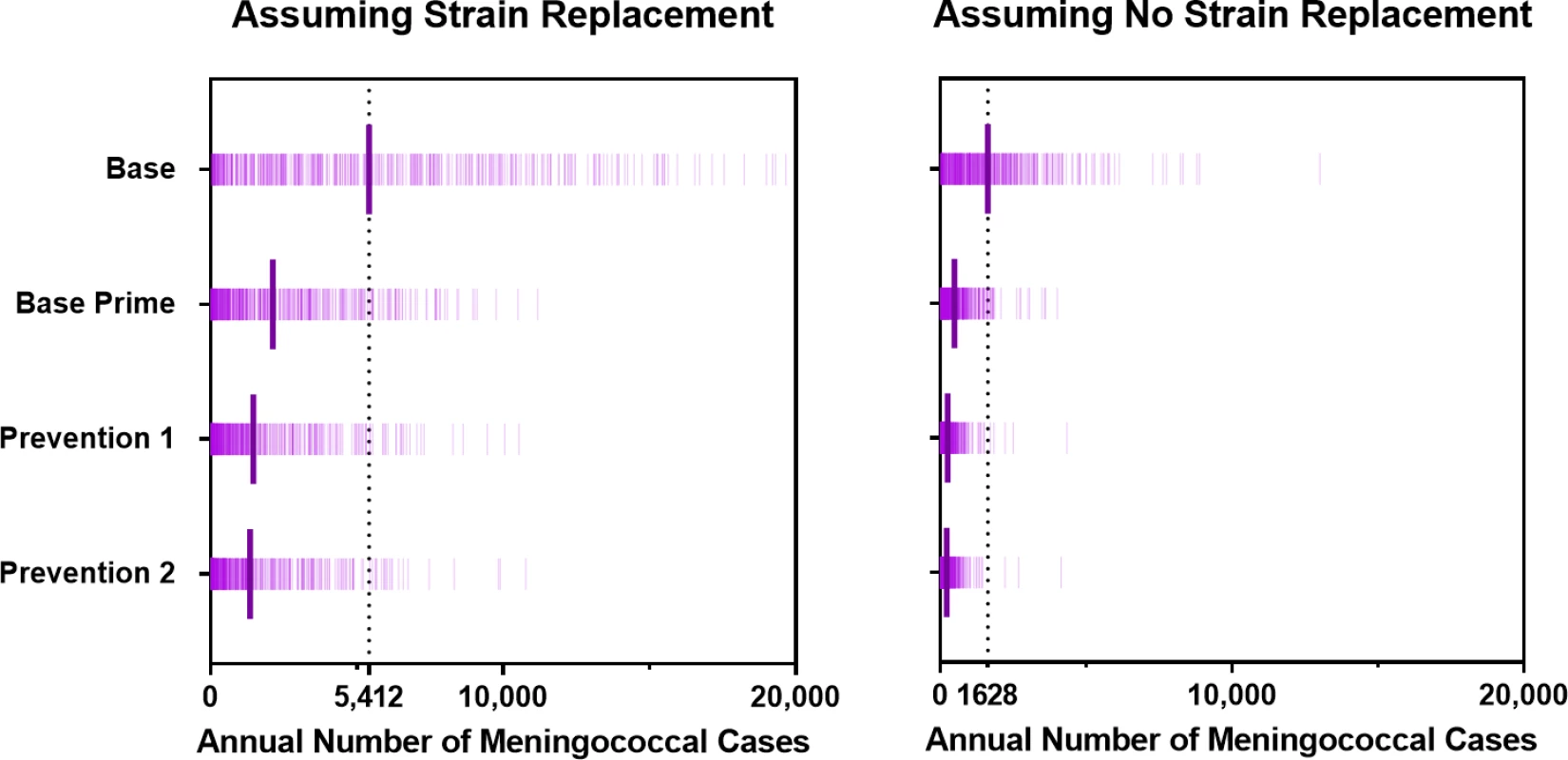
The long bars represent the average annual number of meningococcal cases expected under each strategy option. Fig 7 displays the expected number of vaccines required for each vaccination strategy. Under either strain-replacement assumption, the Base strategy has the highest expected annual consumption of total vaccine doses and the Base Prime strategy has the lowest expected annual consumption of vaccine doses. The wide prediction intervals for the estimated number of PMP vaccines used under the Base strategy are the result of the sporadic occurrence of meningococcal outbreaks that may trigger district-wide reactive campaigns. We also note that extending the projection horizon does not impact the estimated annual consumption of MenAfriVac, PMP, or PMC vaccines in routine programs, but it reduces the estimated annual consumption of PMC vaccines in reactive and mass preventive campaigns. This is because preventive campaigns are implemented a single time at the beginning of the projection period, and the outbreaks under the Base Prime strategy occur only in early years, when there is a pool of children and young adults who were born too early to receive PMC vaccine in their routine infant vaccination schedules.
Fig. 7. Expected number of vaccines used per year (over a 30-year simulation period) for scenarios with and without strain replacement. 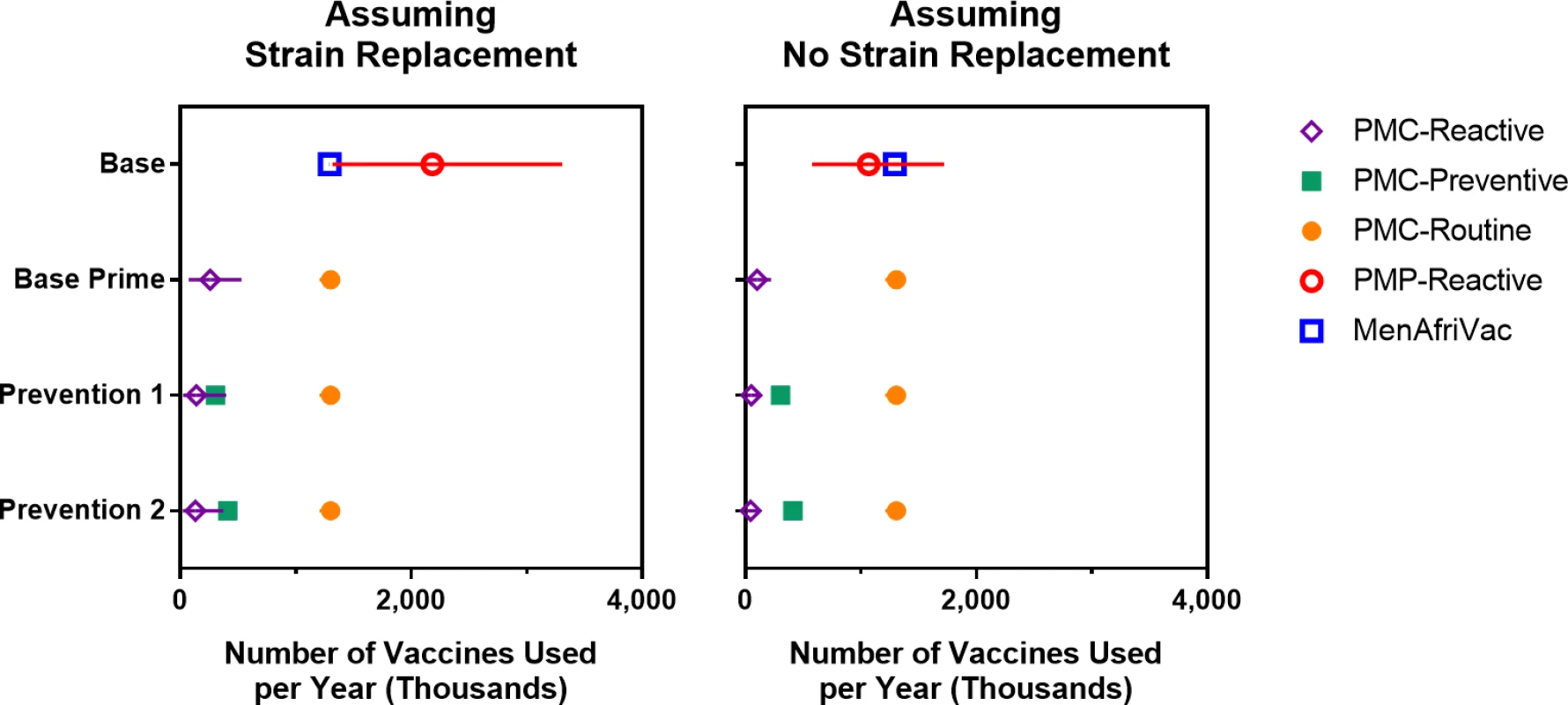
Error bars represents 95% projection intervals (error bars that are shorter than the width of symbols are not shown). PMC, polyvalent meningococcal conjugate; PMP, polyvalent meningococcal polysaccharide. For the scenario with strain replacement, the Base strategy is dominated by Base Prime, as the latter strategy is expected to cost less and reduce the population’s DALYs (Fig 8A and Table 3). If strain replacement does not occur, we estimate the incremental cost-effectiveness ratios (ICERs) for DALYs averted by Base Prime compared with Base at US$51 (−US$233–US$476). Per WHO recommendations, strategies that avert one DALY for less than the per capita gross domestic product (GDP) are considered “very cost-effective” and one DALY for less than three times the per capita GDP as “cost-effective” [45]. Hence, at the cost-effectiveness threshold of US$660, the per capita GDP of Burkina Faso in 2015, the Base Prime strategy is considered cost-effective with respect to the Base strategy under either strain-replacement scenario.
Fig. 8. Economic evaluation of vaccine strategies described in Table 1 for scenarios with and without strain replacement in which the price of PMP and PMC vaccines are US per dose (see S1 Text for sensitivity analysis to the vaccine prices). 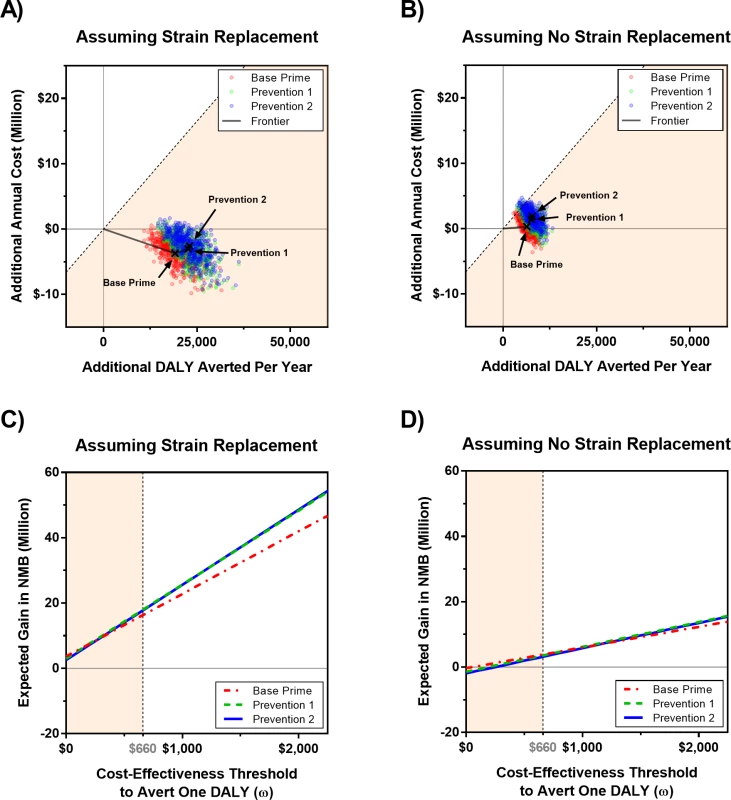
In cost-effectiveness panels (A) and (B), each dot represents the additional cost and DALYs averted in a simulated trajectory with respect to the Base strategy, which represents the current WHO policy that relies on reactive vaccination campaigns using PMP vaccines in districts in which the epidemic threshold is passed. The x’s represent the expected additional cost and DALYs averted with respect to the Base strategy. Panels (C) and (D) show the expected gain in NMB of a strategy with respect to the Base strategy for a given cost-effectiveness threshold, ω. The diagonal dashed line in panels (A) and (B) and the vertical dashed line in panels (C) and (D) represents the cost-effectiveness threshold of one per capita gross domestic product of Burkina Faso, which is estimated to be US0 in 2015 [48]. All costs and DALYs are discounted at rate 3% to year 2016. DALY, disability-adjusted life year; NMB, net monetary benefit; PMC, polyvalent meningococcal conjugate; PMP, polyvalent meningococcal polysaccharide. Tab. 3. Cost-effectiveness of vaccination strategies, assuming a vaccine cost of US per dose for PMP and PMC vaccines. 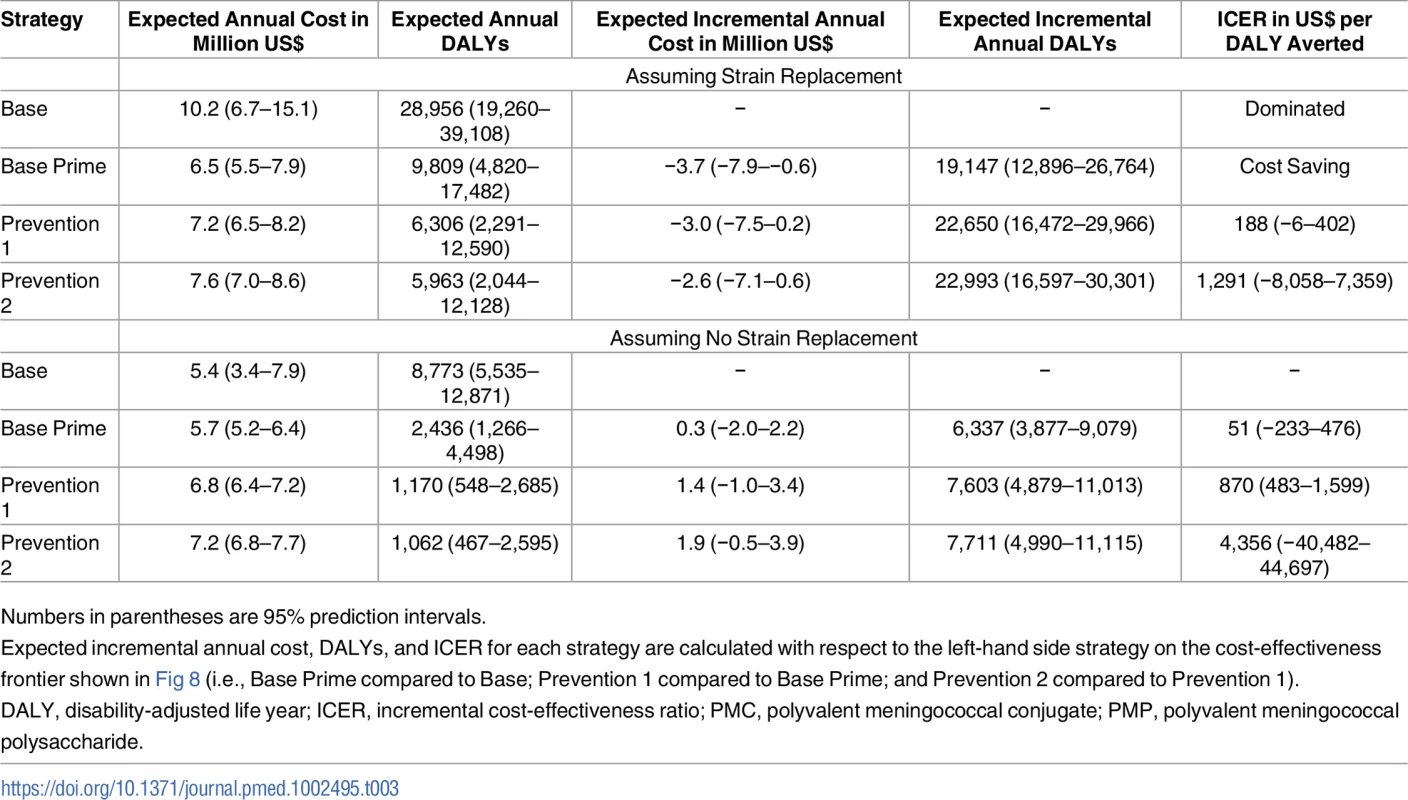
Numbers in parentheses are 95% prediction intervals. The ICER of Prevention 1 compared to Base Prime is estimated at US$188 (−US$6–US$402) and US$870 (US$483–US$1,599) per DALY averted for with and without strain replacement, respectively. These ICER estimates are below the cost-effectiveness threshold of US$1,980, three times the per capita GDP of Burkina Faso in 2015. Compared with the Base policy, strategies that use PMC vaccine (i.e., Base Prime and Preventions 1 and 2) are expected to be cost saving if strain replacement occurs and would cost US$51 (−US$236, US$490), US$188 (−US$97–US$626), and US$246 (−US$53–US$703) per DALY averted if strain replacement does not occur.
We also compare the performance of vaccination strategies in terms of their impact on the population’s net monetary benefit (NMB) [46,47] for varying values of cost-effectiveness threshold (ω). The expected gain in NMB of a strategy is calculated with respect to the Base strategy as ω × (additional DALYs averted by the strategy)–(additional cost of the strategy). Fig 8C and 8D confirms that strategies that use the PMC vaccine dominate Base and that Prevention 1 and 2 strategies demonstrate similar performance under both strain-replacement scenarios. As expected, the cost-effectiveness of the vaccine strategies varies between strain-replacement scenarios and all strategies—Base Prime, Prevention 1, and Prevention 2—present larger incremental benefit when strain A elimination is followed by complete strain replacement (Fig 8 and Table 3).
Our sensitivity analysis shows that reducing PMP vaccine price from US$4 to US$2 per dose does not change the conclusions about the comparative performance of these vaccination strategies (Fig H in S1 Text). While increasing the price of PMC vaccine from US$4 to US$10 per dose diminishes the cost-effectiveness of vaccination strategies that involve PMC vaccines, these strategies maintain their relative performance with respect to the Base policy (Fig I in S1 Text). If PMC vaccine price is US$10 per dose, we estimate an average cost per DALY averted by Base Prime and Prevention 1 and 2 strategies with respect to the Base strategy at US$257 (US$57–US$558), US$286 (US$84–US$546), and US$326 (US$117–US$602) when strain replacement occurs and at US$1,246 (US$631–US$2,336), US$1,369 (US$759–US$2,431), and US$1,488 (US$833–US$2,619) without strain replacement.
Discussion
While the currently recommended strategy for meningitis control in sub-Saharan Africa relies on reactive vaccination campaigns using PMP vaccines in districts where the epidemic threshold is passed, our model suggests that this approach will be outperformed by alternative policies using affordable PMC vaccines. The use of PMC vaccines in the EPI and in reactive vaccination programs could markedly reduce the public health burden of meningococcal epidemics but still leaves districts at substantial risk of sporadic outbreaks. The addition of nationwide catch-up vaccination campaigns to immunize 1–18-year-olds with PMC vaccines could prevent the majority of meningococcal cases. Our results suggest that this strategy is likely to be cost-effective (and potentially cost saving) with respect to the current WHO-recommended meningitis control strategy in sub-Saharan Africa once affordable PMC vaccine becomes available.
The introduction of MenAfriVac is expected to eliminate serogroup A meningitis in the meningitis belt [10,11,24–26], but little is known about the impact of MenAfriVac on the future non-A epidemics. As expected, benefits of additional vaccination interventions are highest when the elimination of serogroup A is followed by replacement by other circulating serogroups. While we do not know the likelihood or extent of serogroup replacement, our analysis shows that the comparative performance of the vaccination strategies we considered are not meaningfully altered by this source of uncertainty. Nevertheless, sustaining strong case-based surveillance in the post-MenAfriVac will facilitate more accurate estimates of the health impact and costs of these competing strategies.
Meningococcal outbreaks in the meningitis belt are sporadic and caused by different serogroups (mainly A, C, W, and X) [11,49], and the accurate prediction of future meningitis epidemics is challenged by the absence of data to characterize competition between these serogroups [50]. Most meningitis transmission models either describe the circulation of a single serogroup or two serogroups (e.g., vaccine type and non-vaccine type) [27,28,50–53]. In our study, we assume that polyvalent vaccines will offer protection against all meningococcal serogroups that can circulate in this setting, and therefore our model aggregates all serogroups into a single vaccine type serogroup. This simplification improves tractability but would compromise the insights derived from this model if there is differential effectiveness of the vaccine by serogroup or if there are complex between-serogroup interactions that influence the frequency and magnitude of future meningitis epidemics.
While our analysis is limited to Burkina Faso, the conclusions may well apply to other hyperendemic areas in the meningitis belt, including Mali, Niger, Chad, and Northern Nigeria, as the key characteristics of meningococcal epidemics in these regions (e.g., frequency of epidemics, age distribution of cases, and age-specific carriage prevalence) are similar to those in Burkina Faso [1,54]. However, additional studies are needed to confirm the generalizability of our conclusions to other settings.
This work suggests that there is a need to revisit the current WHO strategy for meningitis control in sub-Saharan Africa once affordable PMC vaccines become available. Our model-based results indicate that PMC vaccines can be used in a cost-effective manner to control meningococcal epidemics if adopted within the EPI and used in a catch-up preventive vaccination program.
Supporting Information
Zdroje
1. Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007;369(9580):2196–210. doi: 10.1016/S0140-6736(07)61016-2 17604802.
2. Ferrari MJ, Fermon F, Nackers F, Llosa A, Magone C, Grais RF. Time is (still) of the essence: quantifying the impact of emergency meningitis vaccination response in Katsina State, Nigeria. Int Health. 2014;6(4):282–90. doi: 10.1093/inthealth/ihu062 25193978; PubMed Central PMCID: PMC4311152.
3. Woods CW, Armstrong G, Sackey SO, Tetteh C, Bugri S, Perkins BA, et al. Emergency vaccination against epidemic meningitis in Ghana: implications for the control of meningococcal disease in West Africa. Lancet. 2000;355(9197):30–3. doi: 10.1016/S0140-6736(99)03366-8 10615888.
4. Parent du Chatelet I, Gessner BD, da Silva A. Comparison of cost-effectiveness of preventive and reactive mass immunization campaigns against meningococcal meningitis in West Africa: a theoretical modeling analysis. Vaccine. 2001;19(25–26):3420–31. 11348706.
5. Aguado MT, Jodar L, Granoff D, Rabinovich R, Ceccarini C, Perkin GW. From Epidemic Meningitis Vaccines for Africa to the Meningitis Vaccine Project. Clin Infect Dis. 2015;61 Suppl 5:S391–5. doi: 10.1093/cid/civ593 26553665.
6. Tiffay K, Jodar L, Kieny MP, Socquet M, LaForce FM. The Evolution of the Meningitis Vaccine Project. Clin Infect Dis. 2015;61 Suppl 5:S396–403. doi: 10.1093/cid/civ594 26553666.
7. Meyer SA, Kambou JL, Cohn A, Goodson JL, Flannery B, Medah I, et al. Serogroup A meningococcal conjugate (PsA-TT) vaccine coverage and measles vaccine coverage in Burkina Faso—implications for introduction of PsA-TT into the Expanded Programme on Immunization. Vaccine. 2015;33(12):1492–8. doi: 10.1016/j.vaccine.2015.01.043 25636915.
8. Centers for Disease Control and Prevention. Serogroup A Meningococcal Conjugate Vaccine Coverage After the First National Mass Immunization Campaign—Burkina Faso, 2011. Morbidity and Mortality Weekly Report. 2012;61(50):1022–24. 23254256
9. Djingarey MH, Barry R, Bonkoungou M, Tiendrebeogo S, Sebgo R, Kandolo D, et al. Effectively introducing a new meningococcal A conjugate vaccine in Africa: the Burkina Faso experience. Vaccine. 2012;30 Suppl 2:B40–5. doi: 10.1016/j.vaccine.2011.12.073 22607898.
10. Kristiansen PA, Diomande F, Ba AK, Sanou I, Ouedraogo AS, Ouedraogo R, et al. Impact of the serogroup A meningococcal conjugate vaccine, MenAfriVac, on carriage and herd immunity. Clin Infect Dis. 2013;56(3):354–63. doi: 10.1093/cid/cis892 23087396.
11. Novak RT, Kambou JL, Diomande FV, Tarbangdo TF, Ouedraogo-Traore R, Sangare L, et al. Serogroup A meningococcal conjugate vaccination in Burkina Faso: analysis of national surveillance data. Lancet Infect Dis. 2012;12(10):757–64. doi: 10.1016/S1473-3099(12)70168-8 22818241.
12. Gamougam K, Daugla DM, Toralta J, Ngadoua C, Fermon F, Page AL, et al. Continuing effectiveness of serogroup A meningococcal conjugate vaccine, Chad, 2013. Emerg Infect Dis. 2015;21(1):115–8. doi: 10.3201/eid2101.140256 25536336; PubMed Central PMCID: PMC4285275.
13. Cibrelus L, Medah I, Koussoube D, Yelbeogo D, Fernandez K, Lingani C, et al. Serogroup W Meningitis Outbreak at the Subdistrict Level, Burkina Faso, 2012. Emerg Infect Dis. 2015;21(11):2063–6. doi: 10.3201/eid2111.150304 26488128; PubMed Central PMCID: PMC4622241.
14. Sidikou F, Zaneidou M, Alkassoum I, Schwartz S, Issaka B, Obama R, et al. Emergence of epidemic Neisseria meningitidis serogroup C in Niger, 2015: an analysis of national surveillance data. Lancet Infect Dis. 2016. doi: 10.1016/S1473-3099(16)30253-5 27567107.
15. Girard MP, Preziosi MP, Aguado MT, Kieny MP. A review of vaccine research and development: meningococcal disease. Vaccine. 2006;24(22):4692–700. doi: 10.1016/j.vaccine.2006.03.034 16621189.
16. Ali A, Jafri RZ, Messonnier N, Tevi-Benissan C, Durrheim D, Eskola J, et al. Global practices of meningococcal vaccine use and impact on invasive disease. Pathog Glob Health. 2014;108(1):11–20. doi: 10.1179/2047773214Y.0000000126 24548156; PubMed Central PMCID: PMC4083163.
17. Soriano-Gabarro M, Toe L, Tiendrebeogo SR, Nelson CB, Dabal M, Djingarey MH, et al. Effectiveness of a trivalent serogroup A/C/W135 meningococcal polysaccharide vaccine in Burkina Faso, 2003. Vaccine. 2007;25 Suppl 1:A92–6. doi: 10.1016/j.vaccine.2007.04.048 17517451.
18. Dakar discussion group on priorities for research on epidemic meningococcal disease in A, Altmann D, Aseffa A, Bash M, Basta N, Borrow R, et al. Priorities for research on meningococcal disease and the impact of serogroup A vaccination in the African meningitis belt. Vaccine. 2013;31(11):1453–7. doi: 10.1016/j.vaccine.2012.12.035 23273967; PubMed Central PMCID: PMC3988351.
19. Centers for Disease Control and Prevention. Control and prevention of serogroup C meningococcal disease: evaluation and management of suspected outbreaks: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report: Recommendations and Reports. 1997;46(RR-5):11–21.
20. Reingold AL, Broome CV, Hightower AW, Ajello GW, Bolan GA, Adamsbaum C, et al. Age-specific differences in duration of clinical protection after vaccination with meningococcal polysaccharide A vaccine. Lancet. 1985;2(8447):114–8. 2862316.
21. Welte R, Trotter CL, Edmunds WJ, Postma MJ, Beutels P. The role of economic evaluation in vaccine decision making: focus on meningococcal group C conjugate vaccine. Pharmacoeconomics. 2005;23(9):855–74. 16153131.
22. World Health Organization. Meningitis Outbreak Response in Sub-Saharan Africa: WHO Guideline, Report No. WHO/HSE/PED/CED/14.5. Geneva: 2014.
23. World Health Organization. Detecting meningococcal meningitis epidemics in highly-endemic African countries. Weekly Epidemiology Record (WER). Weekly Epidemiology Record. 2000;38 : 306–9.
24. Daugla DM, Gami JP, Gamougam K, Naibei N, Mbainadji L, Narbe M, et al. Effect of a serogroup A meningococcal conjugate vaccine (PsA-TT) on serogroup A meningococcal meningitis and carriage in Chad: a community study. Lancet. 2014;383(9911):40–7. doi: 10.1016/S0140-6736(13)61612-8 24035220; PubMed Central PMCID: PMC3898950.
25. Paireau J, Mainassara HB, Jusot JF, Collard JM, Idi I, Moulia-Pelat JP, et al. Spatio-temporal factors associated with meningococcal meningitis annual incidence at the health centre level in Niger, 2004–2010. PLoS Negl Trop Dis. 2014;8(5):e2899. doi: 10.1371/journal.pntd.0002899 24852960; PubMed Central PMCID: PMC4031065.
26. Marc LaForce F, Ravenscroft N, Djingarey M, Viviani S. Epidemic meningitis due to Group A Neisseria meningitidis in the African meningitis belt: a persistent problem with an imminent solution. Vaccine. 2009;27 Suppl 2:B13–9. doi: 10.1016/j.vaccine.2009.04.062 19477559.
27. Irving TJ, Blyuss KB, Colijn C, Trotter CL. Modelling meningococcal meningitis in the African meningitis belt. Epidemiol Infect. 2012;140(5):897–905. doi: 10.1017/S0950268811001385 21781369.
28. Karachaliou A, Conlan AJ, Preziosi MP, Trotter CL. Modeling Long-term Vaccination Strategies With MenAfriVac in the African Meningitis Belt. Clin Infect Dis. 2015;61 Suppl 5:S594–600. doi: 10.1093/cid/civ508 26553693; PubMed Central PMCID: PMC4639487.
29. McIntyre PB, O'Brien KL, Greenwood B, van de Beek D. Effect of vaccines on bacterial meningitis worldwide. Lancet. 2012;380(9854):1703–11. WOS:000310951400033. doi: 10.1016/S0140-6736(12)61187-8 23141619
30. Greenwood B, Chiarot E, MacLennan CA, O'Ryan M. Can we defeat meningococcal disease in low and middle income countries? Vaccine. 2012;30 Suppl 2:B63–6. doi: 10.1016/j.vaccine.2011.12.063 22607901.
31. World Health Organization. Meningococcal disease, serogroup W135, Burkina Faso—Preliminary report, 2002. Weekly epidemiological record. 2002;18 : 141–56.
32. Central Intelligence Agency. The World Factbook: Central Intelligence Agency; 2015 [cited 2016 Aug 4]. Available from: https://www.cia.gov/library/publications/the-world-factbook/geos/uv.html.
33. World Health Organization. Life Tables by Country—Burkina Faso [updated 2015 Sep 15]. Available from: http://apps.who.int/gho/data/view.main.LT61990?lang=en.
34. MenAfriCar c. The Diversity of Meningococcal Carriage Across the African Meningitis Belt and the Impact of Vaccination With a Group A Meningococcal Conjugate Vaccine. J Infect Dis. 2015;212(8):1298–307. doi: 10.1093/infdis/jiv211 25858956; PubMed Central PMCID: PMC4577048.
35. World Health Organization. Meningitis outbreak response in sub-Saharan Africa—WHO guideline WHO/HSE/PED/CED/14.5. 2014.
36. Pinner RW, Onyango F, Perkins BA, Mirza NB, Ngacha DM, Reeves M, et al. Epidemic meningococcal disease in Nairobi, Kenya, 1989. The Kenya/Centers for Disease Control (CDC) Meningitis Study Group. J Infect Dis. 1992;166(2):359–64. 1634807.
37. Rondy M, Issifou D, Ibrahim AS, Maman Z, Kadade G, Omou H, et al. Vaccine Effectiveness of Polysaccharide Vaccines Against Clinical Meningitis—Niamey, Niger, June 2015. PLoS Curr. 2016;8. doi: 10.1371/currents.outbreaks.5d6e9c1d071a2088109c242771b68886 27158558; PubMed Central PMCID: PMC4846038.
38. Campbell JD, Edelman R, King JC Jr., Papa T, Ryall R, Rennels MB. Safety, reactogenicity, and immunogenicity of a tetravalent meningococcal polysaccharide-diphtheria toxoid conjugate vaccine given to healthy adults. J Infect Dis. 2002;186(12):1848–51. doi: 10.1086/345763 12447774.
39. Shepard CW, Ortega-Sanchez IR, Scott RD 2nd, Rosenstein NE, Team AB. Cost-effectiveness of conjugate meningococcal vaccination strategies in the United States. Pediatrics. 2005;115(5):1220–32. doi: 10.1542/peds.2004-2514 15867028.
40. de Voer RM, van der Klis FR, Engels CW, Schepp RM, van de Kassteele J, Sanders EA, et al. Kinetics of antibody responses after primary immunization with meningococcal serogroup C conjugate vaccine or secondary immunization with either conjugate or polysaccharide vaccine in adults. Vaccine. 2009;27(50):6974–82. doi: 10.1016/j.vaccine.2009.09.082 19800445.
41. World Health Organization. Immunization surveillance, assessment and monitory—Measles (MCV) immunization coverage among 1-year olds, 1980–2013 [September 2015]. [cited 2017 Dec 14]. Available from: http://gamapserver.who.int/gho/interactive_charts/immunization/mcv/atlas.html.
42. UNICEF Vaccine Price Data [cited 2017 Dec 14]. Available from: http://www.unicef.org/supply/index_57476.html.
43. World Health Organization. WHO methods and data sources for global burden of disease estimates 2000–2011. 2003.
44. Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231–50. doi: 10.1016/j.jval.2013.02.002 23538175.
45. Hutubessy R, Chisholm D, Edejer TT. Generalized cost-effectiveness analysis for national-level priority-setting in the health sector. Cost Eff Resour Alloc. 2003;1(1):8. doi: 10.1186/1478-7547-1-8 14687420; PubMed Central PMCID: PMC320499.
46. Stinnett AA, Mullahy J. Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making. 1998;18(2 Suppl):S68–80. doi: 10.1177/0272989X98018002S09 9566468.
47. Drummond MF, Sculpher MJ, Torrance GW, O'Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. 3rd ed: Oxford University Press; 2005.
48. World Development Indicators—Burkina Faso: The World Bank; 2016 [cited 2016 Aug 4]. Available from: http://databank.worldbank.org/data/reports.aspx?source=2&country=BFA.
49. Lingani C, Bergeron-Caron C, Stuart JM, Fernandez K, Djingarey MH, Ronveaux O, et al. Meningococcal Meningitis Surveillance in the African Meningitis Belt, 2004–2013. Clin Infect Dis. 2015;61 Suppl 5:S410–5. doi: 10.1093/cid/civ597 26553668.
50. Trotter CL, Gay NJ, Edmunds WJ. Dynamic models of meningococcal carriage, disease, and the impact of serogroup C conjugate vaccination. Am J Epidemiol. 2005;162(1):89–100. doi: 10.1093/aje/kwi160 15961591.
51. Tartof S, Cohn A, Tarbangdo F, Djingarey MH, Messonnier N, Clark TA, et al. Identifying optimal vaccination strategies for serogroup A Neisseria meningitidis conjugate vaccine in the African meningitis belt. PLoS ONE. 2013;8(5):e63605. doi: 10.1371/journal.pone.0063605 23671685; PubMed Central PMCID: PMC3650081.
52. Trotter CL, Edmunds WJ. Reassessing the cost-effectiveness of meningococcal serogroup C conjugate (MCC) vaccines using a transmission dynamic model. Med Decis Making. 2006;26(1):38–47. doi: 10.1177/0272989X05284109 16495199.
53. Christensen H, Hickman M, Edmunds WJ, Trotter CL. Introducing vaccination against serogroup B meningococcal disease: an economic and mathematical modelling study of potential impact. Vaccine. 2013;31(23):2638–46. doi: 10.1016/j.vaccine.2013.03.034 23566946; PubMed Central PMCID: PMC3743045.
54. Trotter CL, Greenwood BM. Meningococcal carriage in the African meningitis belt. Lancet Infect Dis. 2007;7(12):797–803. doi: 10.1016/S1473-3099(07)70288-8 18045562.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2018 Číslo 1- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- What about drinking is associated with shorter life in poorer people?
- Life course socioeconomic position, alcohol drinking patterns in midlife, and cardiovascular mortality: Analysis of Norwegian population-based health surveys
- Pelvic inflammatory disease risk following negative results from chlamydia nucleic acid amplification tests (NAATs) versus non-NAATs in Denmark: A retrospective cohort
- Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: A population-based cohort study
- Sexually transmitted infections in the era of antiretroviral-based HIV prevention: Priorities for discovery research, implementation science, and community involvement
- Traumatic brain injury and the risk of dementia diagnosis: A nationwide cohort study
- Association between intake of less-healthy foods defined by the United Kingdom's nutrient profile model and cardiovascular disease: A population-based cohort study
- Progression of the first stage of spontaneous labour: A prospective cohort study in two sub-Saharan African countries
- The cost-effectiveness of alternative vaccination strategies for polyvalent meningococcal vaccines in Burkina Faso: A transmission dynamic modeling study
- PD-L1 checkpoint inhibition and anti-CTLA-4 whole tumor cell vaccination counter adaptive immune resistance: A mouse neuroblastoma model that mimics human disease
- Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: A Phase 1 open-label clinical trial in healthy adults
- Immune-related genetic enrichment in frontotemporal dementia: An analysis of genome-wide association studies
- Long-term trends in mortality and AIDS-defining events after combination ART initiation among children and adolescents with perinatal HIV infection in 17 middle- and high-income countries in Europe and Thailand: A cohort study
- What’s coming for health science and policy in 2018? Global experts look ahead in their field
- Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: A targeted metabolomics study
- Estimated mortality on HIV treatment among active patients and patients lost to follow-up in 4 provinces of Zambia: Findings from a multistage sampling-based survey
- From macro- to microfactors in health: Social science approaches in research on sexually transmitted infections
- The WHO 2016 verbal autopsy instrument: An international standard suitable for automated analysis by InterVA, InSilicoVA, and Tariff 2.0
- Long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: Systematic review and meta-analysis
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Traumatic brain injury and the risk of dementia diagnosis: A nationwide cohort study
- Pelvic inflammatory disease risk following negative results from chlamydia nucleic acid amplification tests (NAATs) versus non-NAATs in Denmark: A retrospective cohort
- PD-L1 checkpoint inhibition and anti-CTLA-4 whole tumor cell vaccination counter adaptive immune resistance: A mouse neuroblastoma model that mimics human disease
- Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: A Phase 1 open-label clinical trial in healthy adults
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



