-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaIncreased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: A population-based cohort study
Krishnarajah Nirantharakumar and colleagues present results from a cohort of women with gestational diabetes and identify these as a target group for preventative interventions for cardiovascular disease and type 2 diabetes.
Published in the journal: . PLoS Med 15(1): e32767. doi:10.1371/journal.pmed.1002488
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002488Summary
Krishnarajah Nirantharakumar and colleagues present results from a cohort of women with gestational diabetes and identify these as a target group for preventative interventions for cardiovascular disease and type 2 diabetes.
Introduction
Gestational diabetes mellitus (GDM) is increasing, largely due to the obesity epidemic [1] and increasing maternal age [2]. Although inconsistencies exist across countries for screening for GDM [1] and diagnostic cutoff points for the oral glucose tolerance test [3], reported prevalences are 2%–6% for Europe [1], 7% for North America [4], and 1%–9% and 4%–24% for white British and South Asian (SA) women, respectively, in England and Southern Ireland [5], reflecting the higher 10%–20% prevalence in high-risk populations [2]. It is well accepted that the early identification and treatment of women with GDM reduces pregnancy and perinatal complications [1,6,7] and improves infant birth weights [8]. Women with GDM are also more likely to have markers for insulin resistance and beta cell dysfunction [9–13], particularly if overweight [14], and GDM is a well-established predictor for progression to type 2 diabetes and carries up to a 70% lifetime risk [15].
Although the association between GDM and type 2 diabetes is well established [15,16], onset of the latter following delivery is less well documented and understood in terms of underlying genetic and lifestyle factors [16]. Only 3 previous large population-based studies quantifying the increased risk of cardiovascular disease following delivery in women diagnosed with GDM were identified [17–19]. One Canadian retrospective study identified 8,191 women with GDM and age-matched them with 81,262 control women without GDM utilizing primary care records in Ontario [17]. One French study utilizing hospital records identified over 1.5 million women who delivered infants during 2007 and 2008 and included 62,958 women with GDM who were compared with all women without GDM who delivered a healthy infant during that period [18]. More recently, the North American Nurses’ Health Study II group reported on 5,992 nurses who self-reported a history of GDM from a total cohort of 89,479 [19].
All 3 studies reported an increase risk of cardiovascular events for women with GDM compared with women without GDM during the 12, 7, and 26 years of postpartum follow-up for the 3 studies, respectively. The increased risk for major cardiovascular events in people with type 2 diabetes in addition to other traditional risk factors [15,20] and at an early age [21] is well documented. Despite this, and the recommendation for annual screening for type 2 diabetes in women diagnosed with GDM [22] and evidence that lifestyle changes can improve outcomes [23], there is a paucity of reports on screening, and low rates have been reported [24,25]. The current National Institute for Health and Care Excellence (NICE) guidelines recommend screening for type 2 diabetes (between 6 and 13 weeks postpartum and an annual glycated hemoglobin [HbA1c] test) and lifestyle changes (weight control, diet, and exercise) for women diagnosed with GDM [26]. There is no recommendation to screen, identify, and actively manage cardiovascular risk factors (including hypertension, dyslipidemia, and smoking) in women diagnosed with GDM in the postpartum period in the current 2015 NICE guidelines [26].
The aim of this current study is to examine the risk of cardiovascular disease in women previously diagnosed with GDM in a population that is representative of all women diagnosed with GDM in the United Kingdom (UK). In addition, the proportion of women assessed for cardiovascular risk factors in the first 3 years postpartum in primary care will be documented. The results are expected to assist general practice in identifying and targeting cardiovascular risk factors in a group of relatively young women at high risk of long-term metabolic and cardiovascular disorders.
Methods
The study protocol was approved by the Scientific Review Committee (SRC Reference Number: 17THIN001) of the data provider, IQVIA.
Research design
A retrospective cohort study design was used to compare long-term cardiometabolic outcomes in women diagnosed with GDM and randomly matched pregnant control women not diagnosed with GDM, utilizing The Health Improvement Network (THIN) database—following the pre-analysis study plan (S1 Text). This database captures electronically recorded medical records in primary care and is designed to encourage research and improve healthcare delivery in the UK [27]. Over 675 general practices contribute to the THIN database, which captures about 6% (3.6 million) of the total registered population [27], is representative of the age structure of the UK population, and is made up predominantly of a white British, Welch, and Irish population (94% in 1991 although decreasing to 86% by 2011) [28]. Patient information is entered into the Vision patient record software, which uses Read code data (version 2) [29], rather than the World Health Organization International Classification of Diseases designed for hospital records. The Vision software also captures all British National Formulary drug prescription records [30]. General practices that had electronic medical record software for at least 1 year and had an acceptable mortality recording for at least 12 months were included in the analyses to ensure data quality and that all important covariates were recorded.
Study population
All records for women who became pregnant between 1 February 1990 and 15 May 2016 and were aged less than 50 years were accessed for possible inclusion in the study. Women diagnosed with GDM prior to delivery were identified and randomly matched with up to 4 pregnant control women without GDM by age and timing of entry of a code for pregnancy (up to 3 months).
The primary outcomes were the clinical diagnosis of coronary artery disease (ischemic heart disease [IHD]) and cerebrovascular disease (stroke or transient ischemic attack [TIA]). Secondary outcomes were cases of incident hypertension and type 2 diabetes. Outcomes were identified through clinical codes (S1 Data). Recording of diabetes, hypertension, and cardiovascular disease is considered accurate in UK primary care because there is a mandatory requirement for maintaining a register for these conditions, and incentive payments are made for identification and management of these cardiometabolic outcomes [31].
Women with a diagnosis of the outcome of interest prior to baseline (i.e., index date: at diagnosis of GDM for cases and confirmation of pregnancy for control women) were not included in the analysis for that outcome. All potential and available risk factors for all outcomes of interest (Townsend quintile, smoking, and body mass index [BMI]) and confounding variables (hypertension and lipid-lowering medication at baseline for IHD and stroke or TIA) were extracted for each of the women included in the study to adjust for confounding.
Patient involvement
Patients were not involved in the design of this research project, in conducting the study, or in preparing results or reports. In acknowledgment of the patients and general practices that contributed information to the THIN dataset, the published paper will be circulated via IQVIA to all current practices that contribute to the dataset.
Statistical analyses
Characteristics of women with GDM and matched control women in the cohort were reported using appropriate descriptive statistics (mean and median for continuous variables and proportions for categorical variables). Incidence rate ratios (IRRs) and 95% confidence intervals (CIs) were calculated using the Poisson regression model, offsetting the exposure for person-years of follow-up. Adjusted IRRs were constructed by including age, BMI, Townsend quintile (a measure of deprivation), and smoking status in the Poisson models for hypertension and diabetes outcomes. In addition to these covariates, baseline hypertension and lipid-lowering medication prescription were included in the models for cardiovascular disease outcomes. BMI (in kg/m2) was treated as a categorical variable and grouped into <25, 25 to 30, and >30 kg/m2, based on the World Health Organization BMI categories [32].
Missing data for BMI, Townsend quintile, and smoking were included in the regression model as a missing categorical variable. We did not include ethnicity in our primary analysis because of poor recording in the primary care setting (<50%). However, in a sensitivity analysis, we included the available recording of ethnicity along with a missing category in the model to assess its impact on findings. Statistical significance was set at 0.05. Cumulative incidence curves were generated utilizing the cumulative incidence function of the survival curves. In addition, we report on the proportions of women with GDM and control women who were screened in the subsequent 3 years postpartum for smoking, BMI, diabetes, hypertension, and dyslipidemia. All analyses were conducted using STATA 14.0 [33].
Results
A total of 9,118 women with GDM (based on an electronic code entry for GDM) were identified in the dataset. Table 1 outlines the demographic characteristics of the women diagnosed with GDM compared with pregnant control women (matched by age and timing of pregnancy) at baseline. Mean age at the time of delivery was 33 years and ranged from 14 to 47 years. A significantly greater proportion of women with GDM compared with controls were from economically deprived areas (Townsend quintile 4 or 5), were overweight or obese (BMI ≥ 25 kg/m2, 63% compared with 35%), and had been diagnosed with hypertension (including 2.5% prior to pregnancy), but women with GDM were less likely to be current smokers (16% versus 19%). The follow-up period varied from less than 1 to 25 years (median 2.9 years). There was a high proportion of SA women (17.1%) among those with a recording for ethnicity in the GDM cohort. Fig 1 outlines the sampling frame for the total number of pregnant women, those diagnosed with GDM, and control women matched by age and time of pregnancy.
Fig. 1. Flow diagram of the source population, women with gestation diabetes mellitus (GDM), and matched controls, including the proportion of women followed up in each group. 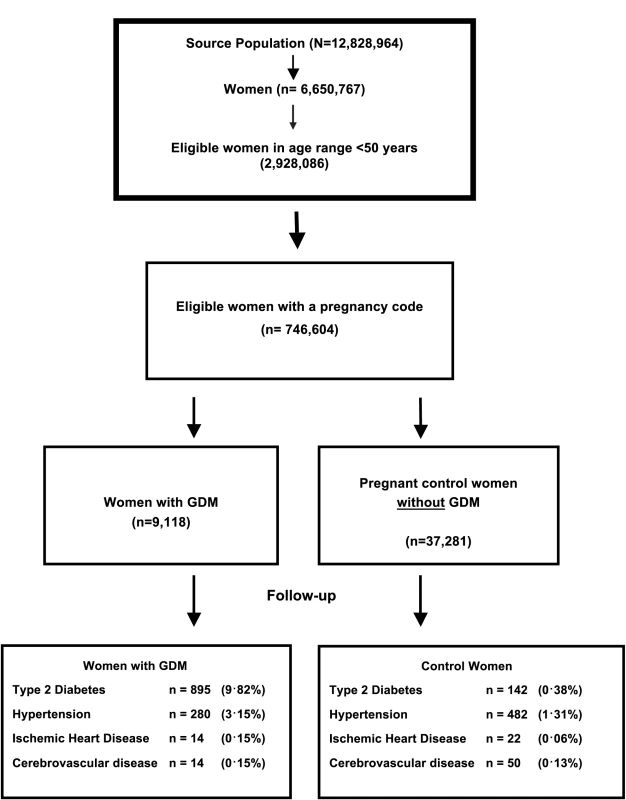
Tab. 1. Participant baseline characteristics. 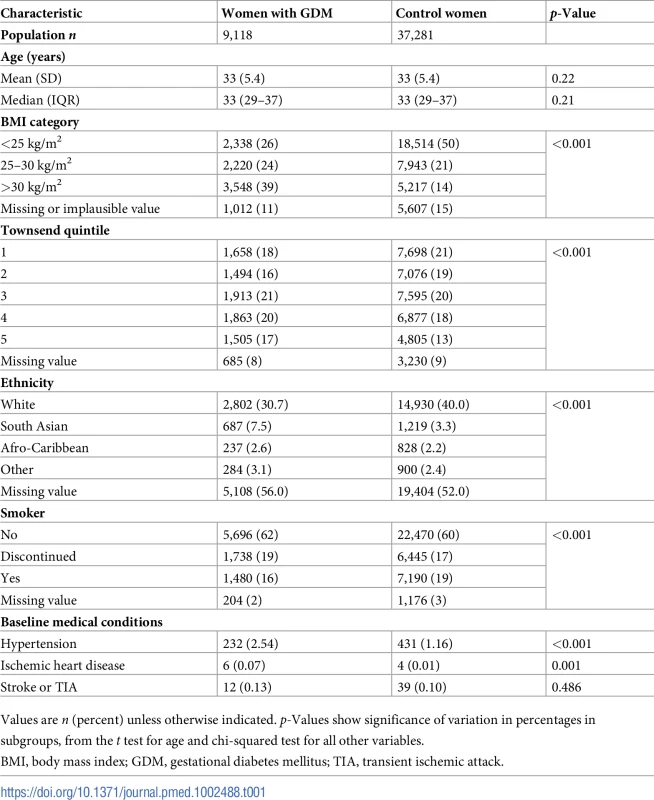
Values are n (percent) unless otherwise indicated. p-Values show significance of variation in percentages in subgroups, from the t test for age and chi-squared test for all other variables. Table 2 shows that women diagnosed with GDM were over 20 times more likely to develop type 2 diabetes (IRR = 21.96; 95% CI 18.31–26.34; p-value < 0.001) and had almost a 2-fold higher risk of developing hypertension (IRR = 1.85; 95% CI 1.59–2.16; p-value < 0.001) after adjusting for age, Townsend quintile, BMI, and smoking compared with control women. Further, after controlling for baseline lipid-lowering medication and hypertension in addition to the above covariates, women with GDM were more than 2.5 times more likely to develop IHD (IRR = 2.78; 95% CI 1.37–5.66; p-value = 0.005), but no increase in risk was found for cerebrovascular disease (IRR = 0.95; 95% CI 0.51–1.77; p-value = 0.87). Of the 14 women with GDM who developed IHD, only 5 also developed type 2 diabetes in the postpartum period, suggesting that the risk of cardiovascular disease is not always mediated through type 2 diabetes.
Tab. 2. Women with gestational diabetes mellitus (n = 9,118) who developed type 2 diabetes, hypertension, ischemic heart disease, and cerebrovascular disease in the postpartum period compared with control women (n = 37,281) who remained normoglycemic during pregnancy. 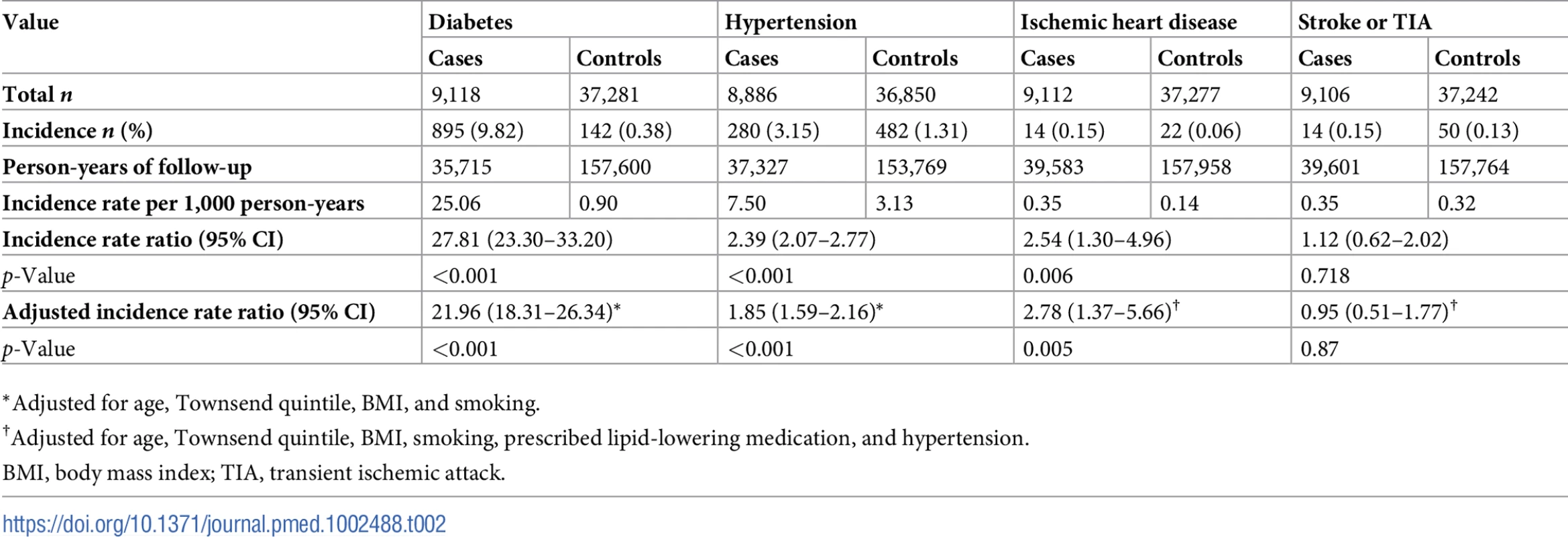
*Adjusted for age, Townsend quintile, BMI, and smoking. Fig 2 shows that the cumulative incidence of type 2 diabetes, hypertension, and IHD was higher for women with GDM compared with control women and that this difference persisted throughout the 25-year study period. The increased risk was specific for type 2 diabetes, hypertension, and IHD but not for stroke or TIA.
Fig. 2. Cumulative incidence of diabetes, hypertension, IHD, and stroke or TIA for women with GDM and control women. 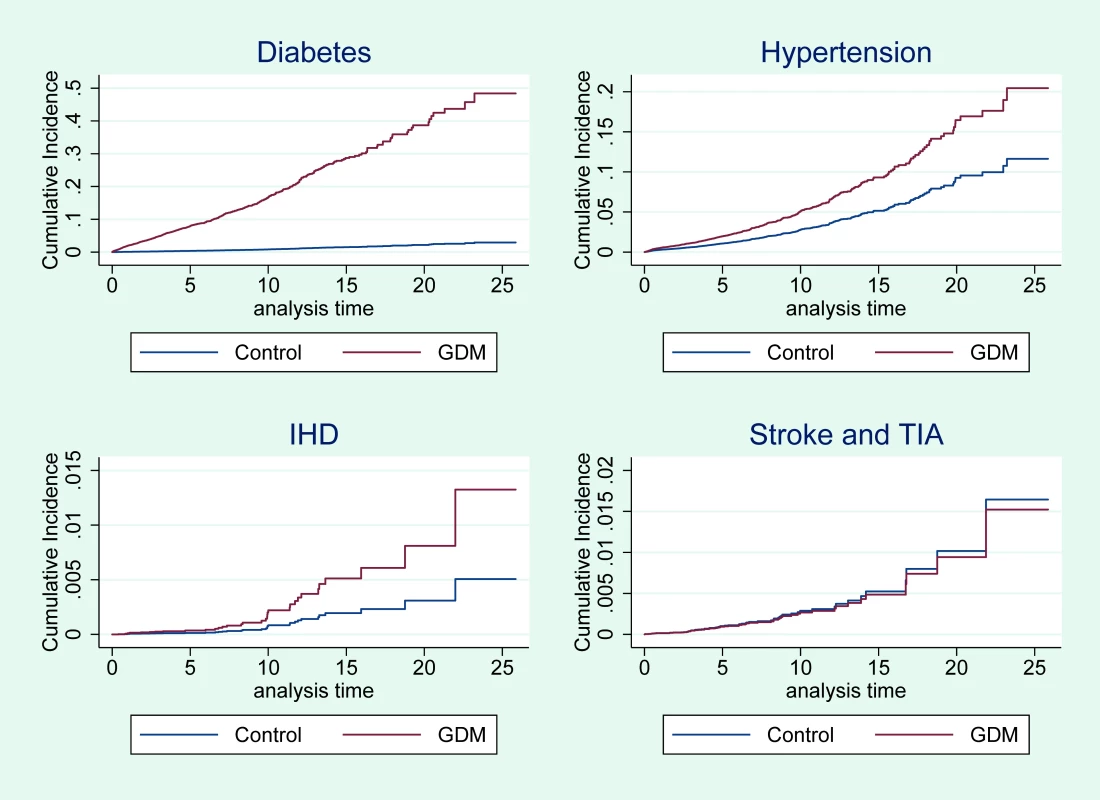
The analyses for diabetes and hypertension are adjusted for age, Townsend quintile, BMI, and smoking. The analyses for IHD and stroke or TIA are adjusted for the above covariates, prescribed lipid-lowering medication, and hypertension. BMI, body mass index; GDM, gestational diabetes mellitus; IHD, ischemic heart disease; TIA, transient ischemic attack. The sensitivity analysis that included ethnicity in the model did not significantly alter the effect sizes for any of the outcomes: IRR for type 2 diabetes = 21.08 (95% CI 17.57–25.30), IRR for hypertension = 1.82 (95% CI 1.56–2.13), IRR for IHD = 2.72 (95% CI 1.33–5.60), and IRR for stroke or TIA = 0.91 (95% CI 0.48–1.70). In an analysis restricted to women with GDM, SA women were twice as likely (IRR = 2.09; 95% CI 1.52–2.85) as white women to develop type 2 diabetes, and Afro-Caribbean (AC) women were 1.6 times more likely (IRR = 1.65; 95% CI 1.05–2.62). There was no increased risk for hypertension in AC women (IRR = 1.35; 95% CI 0.60–3.02) or SA women (IRR = 0.85; 95% CI 0.41–1.77) with GDM compared with white women with GDM. Ethnic subgroup analysis showed that white, AC, and SA women with GDM were at higher risk of developing type 2 diabetes than women without GDM, with IRR (95% CI) values of 35.2 (20.0–58.5), 22.15 (6.42–76.4) and 15.40 (6.54–36.25), respectively. Similar analyses for other outcomes were not possible due to the small number of outcomes among the relatively small number of women from the 2 minority ethnic groups.
Medical records for women with GDM showed that only 58% had some form of glycemic measurement in the first year following delivery (Table 3). Although 62% of women with GDM were tested after 1 January 2010, per the original 2008 NICE guideline publication recommending screening for type 2 diabetes [34], this proportion was not markedly different from the 53% tested prior to 2010. In the second and third year following delivery, the proportion with glycemic measurement decreased to less than 40%, and 24% of women did not have any glycemic measurement in the first 3 years postpartum. No difference was noted in the proportion of women with GDM who had blood pressure measured and recorded prior to and from 2010, with about 80% in the first year following delivery and declining to around 50% in the subsequent 2 years. BMI was recorded for almost half of women with GDM in the first year following delivery, decreasing to about a third in the subsequent 2 years, and did not change following the original guideline publication. Smoking status was recorded for 46% of women in the first year following delivery, and although this proportion declined in the second and third year, 73% had at least 1 record over this 3-year period. Lipid profiles were the most poorly recorded for women with GDM in the 3 years following delivery. Only 28% and 23% of women had any serum cholesterol or triglyceride level, respectively, recorded in this 3-year period.
Tab. 3. Follow-up screening for women with gestational diabetes mellitus (GDM, n = 9,118) and control women (n = 37,281). 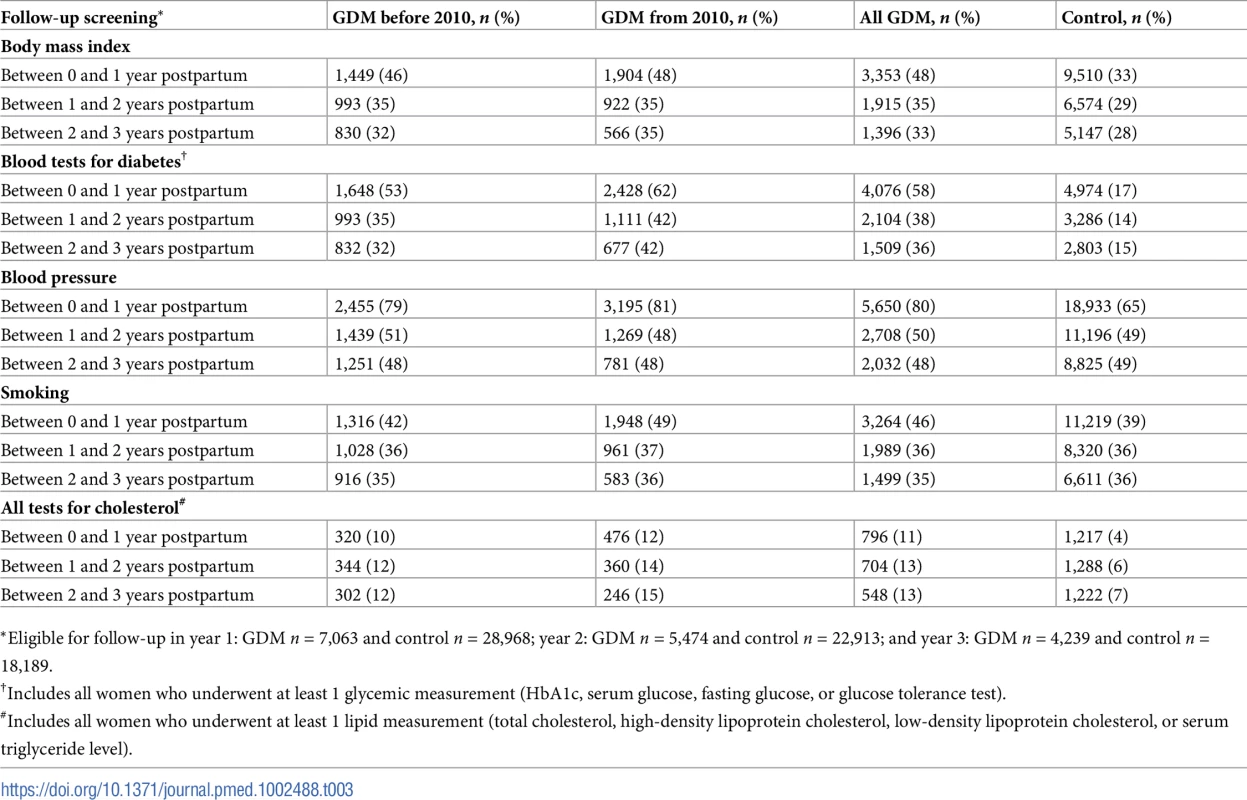
*Eligible for follow-up in year 1: GDM n = 7,063 and control n = 28,968; year 2: GDM n = 5,474 and control n = 22,913; and year 3: GDM n = 4,239 and control n = 18,189. In the control population, surveillance for risk factors in the postpartum period was comparatively lower than for women diagnosed with GDM across all assessments in year 1, and this difference persisted for type 2 diabetes and lipid measurements in years 2 and 3. In contrast, the proportion assessed for hypertension and smoking in years 2 and 3 did not differ between control women and women diagnosed with GDM. In a sensitivity analysis limited to patients who did not develop hypertension in the first year and were followed-up for more than 1 year, the increased risk for hypertension persisted (hazard ratio [HR] = 1.87; 95% CI 1.57–2.24), suggesting that surveillance bias did not affect this outcome.
Discussion
This is, to our knowledge, the first large population-based study in the UK that reports on the increased risk of cardiovascular disease in women diagnosed with GDM, and quantifies the high incidence of type 2 diabetes and hypertension for these women in the postpartum period. Women diagnosed with GDM were over 20 times more likely to develop type 2 diabetes, had almost twice the risk of developing hypertension, and were 2.8 times more likely to develop IHD in the postpartum period compared with control women. The increased risk persisted throughout the 25-year follow-up period. Despite the high risk of developing type 2 diabetes and cardiovascular disease, postpartum screening was poor, with less than 60% of women undergoing any type of screening test for diabetes in the 12 months following delivery and with the proportion declining to below 40% in the second year. Further, only half of the women with GDM had their blood pressure recorded in the second year following delivery. About a third had smoking status recorded, and very few women had lipids recorded, in the third year postpartum. The only improvement noted following publication of the 2008 NICE guidelines [34] was in any form of measurement for glycemia, and this improvement was only moderate.
Our findings are broadly consistent with the French study utilizing hospital records, the Canadian study utilizing primary care records, and the Nurses’ Health Study II using self-reported diagnosis of GDM. The French study reported a higher adjusted odds ratio (OR) for hypertension (2.72; 95% CI 2.58–2.88) than our study’s IRR, but a lower adjusted OR for cardiovascular outcomes that included angina pectoris (1.68; 95% CI 1.29–2.20) and myocardial infarction (1.92; 95% CI 1.36–2.71). Similar to our finding, the French study reported no effect for stroke [18]. The Canadian study reported an attenuated effect (HR = 2.09; 95% CI 1.19–3.67) for the development of coronary artery disease [17]. A significant but lower effect size was reported for the Nurses’ Health Study II for myocardial infarction (HR = 1.56; 95% CI 1.09–2.23) compared with our study, and no effect for stroke (HR = 1.22; 95% CI 0.80–1.86). In addition, a large Swedish case–control study also utilizing hospital records reported an increased risk of cardiovascular events (OR = 1.51; 95% CI 1.07–2.14) and hypertension (OR = 5.10; 95% CI 3.18–8.18) for women previously diagnosed with GDM compared with control women who had not had a cardiovascular event prior to the matched pregnancy and without a history of GDM [35]. Smaller studies have also shown associations between GDM and hypertension and cardiovascular disease in women with a family history of type 2 diabetes [36], and a higher risk of hypertension for Hispanic compared with white North American women diagnosed with GDM [37].
The increased incidence of type 2 diabetes for women with GDM found in our study is consistent with previous studies. Despite this, the incidence in this study was far higher than that reported in a review of observational studies (relative risk = 7.43, 95% CI 4.79–11.51) that also reported an increased risk after 5 years compared with the first 5 years postpartum [16]. However, the high incidence of type 2 diabetes in the first few years postpartum found in this current study was similar to findings in an older review that reported a higher cumulative incidence in the first 5 years postpartum than in subsequent years, after adjusting for cohort retention [15].
Historically, follow-up screening for type 2 diabetes in women diagnosed with GDM is poor in the postpartum period [38]. This study shows that fewer than 60% of women with diabetes in pregnancy were screened for type 2 diabetes, and far fewer had smoking status or lipids recorded, in the first year following delivery. Blood pressure recordings also decreased from 80% in the first year to 50% in the second year following delivery. The current 2015 NICE guidelines recommend annual screening for diabetes and lifestyle advice on “weight control, diet and exercise” for all women diagnosed with GDM [26]. However, there is no recommendation for screening and management of cardiovascular risk factors such as hypertension, dyslipidemia, or tobacco use following delivery in this group of high-risk women. In addition to enhancing early identification of type 2 diabetes, targeting this group also ensures that women know of their increased risk, which is important to them [39], and presents an opportunity to provide education and support for the lifestyle changes required to improve long-term outcomes [9,11], although there is currently a lack of evidence on exactly how to achieve this [40].
The study has several limitations. Our study captured women with GDM only if the condition was documented in the primary care medical records. Our estimates suggest we may have only captured around 49% of women with GDM in the THIN database (S1 Table). Selective documentation of women with more severe GDM may have resulted in an overestimation of the effect size, while any women with GDM misclassified in the control population may have resulted in an underestimation of the effect size. Though overall our findings are consistent with previous studies, the effect estimates for diabetes and IHD were higher than the estimates observed in other studies, while effect estimates were lower for hypertension and similar for stroke. Moreover, women with GDM may have underreported the use of tobacco during pregnancy as they were more likely to be overweight and to live in economically deprived areas, both of which are strongly associated with tobacco use in pregnancy [41]. A previous review of observational studies also found no association between smoking and GDM [42].
The timing and frequency of diabetes-related screening tests during and after pregnancy varied, potentially leading to a nondifferential error, with more women with GDM being diagnosed with diabetes and hypertension. Higher frequency of blood pressure measurements in the GDM population was noted only in year 1 of follow-up. When limiting our analysis to women without hypertension in the first year postpartum and with follow-up recordings beyond 1 year, the effect sizes remained the same for hypertension. Surveillance bias is less likely to affect outcomes that are symptomatic such as IHD and stroke. Further, there is limited information on baseline characteristics such as ethnicity and the number and order of pregnancies, limiting further in-depth analyses on factors that modify each of the outcomes. In particular, not sufficiently controlling for ethnicity might have resulted in an overestimation of the risk of type 2 diabetes in women with previous GDM, but this overestimation is likely to be small considering that the majority of women in the UK over the study period were white [28].
Additional limitations include the short median follow-up period, resulting in few women diagnosed with cardiovascular disease, and possible misclassification of outcome data related to the Read codes, which, although are ideally suited for general practice, are not always accurate. However, the outcomes are expected to be reported as part of the Quality and Outcomes Framework and have been shown to be reliable [43].
Despite these limitations, this study is, to our knowledge, the first UK and the largest population-based study of women with GDM utilizing primary care records to report on incidence of cardiovascular disease not requiring a hospital admission [17]. The findings add an important insight into the trajectory of the development of type 2 diabetes, hypertension, and cardiovascular disease in the early and later postpartum periods. Findings are consistent with previous reports on the risk of developing type 2 diabetes and cardiovascular disease. Furthermore, the findings report on a large population and identify an at-risk group of relatively young women ideally suited for targeting of risk factor management to improve long-term metabolic and cardiovascular outcomes. Targeting these high-risk women may also provide better value for money for prevention programs, as they are already known to general practice. While the value of preventing cardiovascular outcomes requires further studies, there is some evidence that targeting this subgroup of women may yield benefits in reducing conversion to type 2 diabetes [44].
Conclusion
Results showed that women diagnosed with GDM were significantly more likely to develop type 2 diabetes, hypertension, and IHD at a relatively young age compared with women without a previous diagnosis of GDM. The risk was greatest for type 2 diabetes in the first year following delivery and persisted for 25 years. Follow-up screening for type 2 diabetes was poor, with less than 60% of women with GDM undergoing screening in the first year following delivery, and the proportion decreased to less than 40% by the second year. Guideline recommendations for screening and management of hypertension, lipids, and smoking cessation are lacking and need to be reviewed.
Supporting Information
Zdroje
1. Buckley BS, Harreiter J, Damm P, Corcoy R, Chico A, Simmons D, et al. Gestational diabetes mellitus in Europe: prevalence, current screening practice and barriers to screening. A review. Diabet Med. 2012;29(7):844–54. doi: 10.1111/j.1464-5491.2011.03541.x 22150506
2. Galtier F. Definition, epidemiology, risk factors. Diabetes Metab. 2010;36(6 Pt 2):628–51. doi: 10.1016/j.diabet.2010.11.014 21163426
3. Agarwal MM, Dhatt GS, Othman Y. Gestational diabetes: differences between the current international diagnostic criteria and implications of switching to IADPSG. J Diabetes Complications. 2015;29(4):544–9. doi: 10.1016/j.jdiacomp.2015.03.006 25837380
4. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–90. doi: 10.2337/dc14-S081 24357215
5. Farrar D, Simmonds M, Griffin S, Duarte A, Lawlor DA, Sculpher M, et al. The identification and treatment of women with hyperglycaemia in pregnancy: an analysis of individual participant data, systematic reviews, meta-analyses and an economic evaluation. Health Technol Assess. 2016;20(86):1–348. doi: 10.3310/hta20860 27917777
6. Simmons D, Rowan J, Reid R, Campbell N, National GDM Working Party. Screening, diagnosis and services for women with gestational diabetes mellitus (GDM) in New Zealand: a technical report from the National GDM Technical Working Party. N Z Med J. 2008;121(1270):74–86. 18364758
7. Simmons D. Diabetes and obesity in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25(1):25–36. doi: 10.1016/j.bpobgyn.2010.10.006 21247811
8. Rowan JA, Budden A, Ivanova V, Hughes RC, Sadler LC. Women with an HbA of 41–49 mmol/mol (5.9–6.6%): a higher risk subgroup that may benefit from early pregnancy intervention. Diabet Med. 2016;33(1):25–31. doi: 10.1111/dme.12812 26031320
9. Kramer CK, Swaminathan B, Hanley AJ, Connelly PW, Sermer M, Zinman B, et al. Each degree of glucose intolerance in pregnancy predicts distinct trajectories of beta-cell function, insulin sensitivity, and glycemia in the first 3 years postpartum. Diabetes Care. 2014;37(12):3262–9. doi: 10.2337/dc14-1529 25231898
10. Sokup A, Ruszkowska-Ciastek B, Walentowicz-Sadlecka M, Grabiec M, Rosc D. Gestational diabetes mellitus worsens the profile of cardiometabolic risk markers and decrease indexes of beta-cell function independently of insulin resistance in nondiabetic women with a parental history of type 2 diabetes. J Diabetes Res. 2014;2014 : 743495. doi: 10.1155/2014/743495 25097861
11. Kim C. Maternal outcomes and follow-up after gestational diabetes mellitus. Diabet Med. 2014;31(3):292–301. doi: 10.1111/dme.12382 24341443
12. Li W, Zhang S, Liu H, Wang L, Zhang C, Leng J, et al. Different associations of diabetes with beta-cell dysfunction and insulin resistance among obese and nonobese Chinese women with prior gestational diabetes mellitus. Diabetes Care. 2014;37(9):2533–9. doi: 10.2337/dc14-0573 24914241
13. Kusunoki Y, Katsuno T, Nakae R, Watanabe K, Ochi F, Tokuda M, et al. Insulin resistance and beta-cell function influence postprandial blood glucose levels in Japanese patients with gestational diabetes mellitus. Gynecol Endocrinol. 2015;31(12):929–33. doi: 10.3109/09513590.2015.1075498 26288254
14. Lekva T, Bollerslev J, Godang K, Roland MC, Friis CM, Voldner N, et al. Beta-cell dysfunction in women with previous gestational diabetes is associated with visceral adipose tissue distribution. Eur J Endocrinol. 2015;173(1):63–70. doi: 10.1530/EJE-15-0153 25877991
15. Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25(10):1862–8. 12351492
16. Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373(9677):1773–9. doi: 10.1016/S0140-6736(09)60731-5 19465232
17. Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care. 2008;31(8):1668–9. doi: 10.2337/dc08-0706 18487472
18. Goueslard K, Cottenet J, Mariet AS, Giroud M, Cottin Y, Petit JM, et al. Early cardiovascular events in women with a history of gestational diabetes mellitus. Cardiovasc Diabetol. 2016;15 : 15. doi: 10.1186/s12933-016-0338-0 26817691
19. Tobias DK, Stuart JJ, Li S, Chavarro J, Rimm EB, Rich-Edwards J, et al. Association of history of gestational diabetes with long-term cardiovascular disease risk in a large prospective cohort of US women. JAMA Intern Med. 2017;177(12):1735–42. doi: 10.1001/jamainternmed.2017.2790 29049820
20. Emerging Risk Factors Collaboration, Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–22. doi: 10.1016/S0140-6736(10)60484-9 20609967
21. Keteepe-Arachi T, Sharma S. Underestimating risk in women delays diagnosis of CVD. Practitioner. 2016;260(1791):11–5. 27214974
22. Kalra B, Gupta Y, Kalra S. Gestational diabetes mellitus (GDM) follow up: as simple as ABCDE. Diabetes Res Clin Pract. 2015;107(2):e5–6. doi: 10.1016/j.diabres.2014.12.006 25624178
23. Smith BJ, Cinnadaio N, Cheung NW, Bauman A, Tapsell LC, van der Ploeg HP. Investigation of a lifestyle change strategy for high-risk women with a history of gestational diabetes. Diabetes Res Clin Pract. 2014;106(3):e60–3. doi: 10.1016/j.diabres.2014.09.035 25451910
24. Chang Y, Chen X, Cui H, Zhang Z, Cheng L. Follow-up of postpartum women with gestational diabetes mellitus (GDM). Diabetes Res Clin Pract. 2014;106(2):236–40. doi: 10.1016/j.diabres.2014.08.020 25271112
25. Paez KA, Eggleston EM, Griffey SJ, Farrar B, Smith J, Thompson J, et al. Understanding why some women with a history of gestational diabetes do not get tested for diabetes. Womens Health Issues. 2014;24(4):e373–9. doi: 10.1016/j.whi.2014.04.008 24981396
26. National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. London: National Institute for Health and Care Excellence; 2015 [cited 2017 Aug 4]. Available from: https://www.nice.org.uk/guidance/ng3/resources/diabetes-in-pregnancy-management-from-preconception-to-the-postnatal-period-51038446021.
27. University College London. THIN database. London: University College London; 2017 [cited 2017 Dec 3]. Available from: https://www.ucl.ac.uk/pcph/research-groups-themes/thin-pub/database.
28. Office for National Statistics. Ethnicity and national identity in England and Wales: 2011. Newport: Office for National Statistics; 2012 [cited 2017 Aug 4]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/culturalidentity/ethnicity/articles/ethnicityandnationalidentityinenglandandwales/2012-12-11.
29. Health and Social Care Information Centre. NHS Digital. 2017 [cited 2017 Oct 12]. Available from: https://www.gov.uk/government/organisations/health-and-social-care-information-centre.
30. British National Formulary. BNF publications. 2017 [cited 2017 Aug 4]. Available from: https://www.bnf.org/.
31. NHS Digital. Information and technology for better health and care. London: National Health Service; 2017 [cited 2017 Oct 24]. Available from: https://digital.nhs.uk/.
32. World Health Organization. Noncommunicable diseases global monitoring framework: indicator definitions and specifications. Geneva: World Health Organization; 2014 [cited 2017 Dec 4]. Available from: http://www.who.int/nmh/ncd-tools/indicators/GMF_Indicator_Definitions_FinalNOV2014.pdf?ua=1.
33. StataCorp. Stata Statistical Software. Release 14. College Station (Texas) StataCorp; 2015.
34. National Institute for Health and Care Excellence. Diabetes in pregnancy: management of diabetes and its complications from preconception to the postnatal period. London: National Institute for Health and Care Excellence; 2008.
35. Fadl H, Magnuson A, Ostlund I, Montgomery S, Hanson U, Schwarcz E. Gestational diabetes mellitus and later cardiovascular disease: a Swedish population based case-control study. BJOG. 2014;121(12):1530–6. doi: 10.1111/1471-0528.12754 24762194
36. Carr DB, Utzschneider KM, Hull RL, Tong J, Wallace TM, Kodama K, et al. Gestational diabetes mellitus increases the risk of cardiovascular disease in women with a family history of type 2 diabetes. Diabetes Care. 2006;29(9):2078–83. doi: 10.2337/dc05-2482 16936156
37. Bentley-Lewis R, Powe C, Ankers E, Wenger J, Ecker J, Thadhani R. Effect of race/ethnicity on hypertension risk subsequent to gestational diabetes mellitus. Am J Cardiol. 2014;113(8):1364–70. doi: 10.1016/j.amjcard.2014.01.411 24576544
38. Kim C, Tabaei BP, Burke R, McEwen LN, Lash RW, Johnson SL, et al. Missed opportunities for type 2 diabetes mellitus screening among women with a history of gestational diabetes mellitus. Am J Public Health. 2006;96(9):1643–8. doi: 10.2105/AJPH.2005.065722 16873752
39. Han S, Middleton PF, Bubner TK, Crowther CA. Women’s views on their diagnosis and management for borderline gestational diabetes mellitus. J Diabetes Res. 2015;2015 : 209215. doi: 10.1155/2015/209215 25785278
40. Hartling L, Dryden DM, Guthrie A, Muise M, Vandermeer B, Aktary WM, et al. Screening and diagnosing gestational diabetes mellitus. Evid Rep Technol Assess (Full Rep). 2012;210 : 1–327.
41. Vurbic D, Harder VS, Redner RR, Lopez AA, Phillips JK, Higgins ST. Co-occurring obesity and smoking among U.S. women of reproductive age: associations with educational attainment and health biomarkers and outcomes. Prev Med. 2015;80 : 60–6. doi: 10.1016/j.ypmed.2015.05.020 26051199
42. Wendland EM, Pinto ME, Duncan BB, Belizan JM, Schmidt MI. Cigarette smoking and risk of gestational diabetes: a systematic review of observational studies. BMC Pregnancy Childbirth. 2008;8 : 53. doi: 10.1186/1471-2393-8-53 19077324
43. Blak BT, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care. 2011;19(4):251–5. 22828580
44. Ratner RE, Christophi CA, Metzger BE, Dabelea D, Bennett PH, Pi-Sunyer X, et al. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab. 2008;93(12):4774–9. doi: 10.1210/jc.2008-0772 18826999
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2018 Číslo 1- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- What about drinking is associated with shorter life in poorer people?
- Life course socioeconomic position, alcohol drinking patterns in midlife, and cardiovascular mortality: Analysis of Norwegian population-based health surveys
- Pelvic inflammatory disease risk following negative results from chlamydia nucleic acid amplification tests (NAATs) versus non-NAATs in Denmark: A retrospective cohort
- Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: A population-based cohort study
- Sexually transmitted infections in the era of antiretroviral-based HIV prevention: Priorities for discovery research, implementation science, and community involvement
- Traumatic brain injury and the risk of dementia diagnosis: A nationwide cohort study
- Association between intake of less-healthy foods defined by the United Kingdom's nutrient profile model and cardiovascular disease: A population-based cohort study
- Progression of the first stage of spontaneous labour: A prospective cohort study in two sub-Saharan African countries
- The cost-effectiveness of alternative vaccination strategies for polyvalent meningococcal vaccines in Burkina Faso: A transmission dynamic modeling study
- PD-L1 checkpoint inhibition and anti-CTLA-4 whole tumor cell vaccination counter adaptive immune resistance: A mouse neuroblastoma model that mimics human disease
- Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: A Phase 1 open-label clinical trial in healthy adults
- Immune-related genetic enrichment in frontotemporal dementia: An analysis of genome-wide association studies
- Long-term trends in mortality and AIDS-defining events after combination ART initiation among children and adolescents with perinatal HIV infection in 17 middle- and high-income countries in Europe and Thailand: A cohort study
- What’s coming for health science and policy in 2018? Global experts look ahead in their field
- Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: A targeted metabolomics study
- Estimated mortality on HIV treatment among active patients and patients lost to follow-up in 4 provinces of Zambia: Findings from a multistage sampling-based survey
- From macro- to microfactors in health: Social science approaches in research on sexually transmitted infections
- The WHO 2016 verbal autopsy instrument: An international standard suitable for automated analysis by InterVA, InSilicoVA, and Tariff 2.0
- Long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: Systematic review and meta-analysis
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Traumatic brain injury and the risk of dementia diagnosis: A nationwide cohort study
- Pelvic inflammatory disease risk following negative results from chlamydia nucleic acid amplification tests (NAATs) versus non-NAATs in Denmark: A retrospective cohort
- PD-L1 checkpoint inhibition and anti-CTLA-4 whole tumor cell vaccination counter adaptive immune resistance: A mouse neuroblastoma model that mimics human disease
- Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: A Phase 1 open-label clinical trial in healthy adults
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



