-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaSafety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: A Phase 1 open-label clinical trial in healthy adults
John Mascola and colleagues study the safety and pharmacokinetics of VRC01LS, a broadly neutralizing anti-HIV-1 antibody designed to have an extended half-life in serum, in healthy people.
Published in the journal: . PLoS Med 15(1): e32767. doi:10.1371/journal.pmed.1002493
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002493Summary
John Mascola and colleagues study the safety and pharmacokinetics of VRC01LS, a broadly neutralizing anti-HIV-1 antibody designed to have an extended half-life in serum, in healthy people.
Introduction
Advances in our understanding of the humoral immune responses against HIV-1 have led to the appreciation that broadly reactive neutralizing antibodies arise during the course of infection in some individuals [1–4]. Detailed studies of such donors, including the application of technologies to recover immunoglobulin genes from antigen-specific sorted or cultured B cells, have led to the isolation and characterization of numerous broadly neutralizing monoclonal antibodies (bnMAbs) targeting HIV-1 envelope glycoprotein (Env). These bnMAbs target distinct antigenic sites on the native HIV-1 Env trimer shared by multiple viral clades, including the CD4-binding site, the variable region 1 and 2 (V1V2) apex, the glycan-V3 region, the membrane-proximal external region (MPER), and the interface region between glycoprotein (gp)120 and gp41 [1–5]. Several human bnMAbs have been developed and tested in Phase I studies, including VRC01 and 3BNC117 to the CD4-binding site and 10–1074 to the glycan-V3 region [6–9]. The potency and breadth of the current generation of bnMAbs have led to interest in their clinical use both for prophylaxis and treatment of HIV-1 infection [10–12].
VRC01 is a member of a class of bnMAbs that target the CD4-binding site of HIV-1 Env, thereby disrupting viral entry into host cells [6,13–16]. VRC01 is capable of neutralizing 85%–90% of circulating HIV-1 isolates [17,18] and has a well-defined safety and tolerability profile, with known pharmacokinetic (PK) parameters in both HIV-infected [7] and uninfected [6] human populations. VRC01 is also currently being evaluated in an international Phase IIb adult HIV-1 prevention efficacy trial (clinicaltrials.gov NCT02568215, NCT 02716675). Notably, the trial is being conducted with VRC01 antibody infused intravenously, every 8 weeks.
Mutations to the constant (Fc) region of a monoclonal antibody (mAb) can be made to alter circulating half-life by taking advantage of the natural immunoglobulin G (IgG) homeostatic activities of the neonatal Fc receptor (FcRn). When IgG in the serum is endocytosed from the circulation by endothelial cells, the low pH endosomal environment promotes binding between the IgG Fc region and the FcRn. At physiological pH, bound IgG is re-released into the serum through an endosomal recycling mechanism, whereas unbound IgG is degraded within the cell [19,20]. The serum half-life is therefore closely related to the degree to which IgG binds to the FcRn [14]. In designing a mAb for which a longer half-life is desirable, an increased binding affinity to FcRn can be achieved through engineering amino acid substitutions into the FcRn binding site on the antibody. Changes to specific amino acids of the antibody Fc region are known to increase FcRn binding affinity, including Thr250, Met252, Ser254, Thr256, Thr307, Glu380, Met428, His433, and Asn434 [21–27]. Making multiple changes may have a synergistic effect. Motavizumab-YTE, an antibody to glycoprotein F of respiratory syncytial virus (RSV), is an example of an investigational mAb that underwent Fc modification in order to optimize FcRn binding, which resulted in an extended serum half-life [26,28]. This YTE change (M252Y/S254T/T256E) was engineered into another mAb against RSV, termed MEDI8897, and its half-life was extended in a similar manner as seen with motazvizumab [29].
VRC01LS is a human immunoglobulin G subclass 1 (IgG1) HIV-1 bnMAb that is identical to VRC01, with the exception of two amino acid changes (M428L and N434S) in the Fc region intended to extend serum half-life. The LS mutations result in enhanced IgG-FcRn binding but do not affect binding to the Fc-gamma receptor and thus do not impair Fc-mediated effector functions, such as antibody dependent cellular cytotoxicity (ADCC) [30]. A previously reported LS change to the mAb bevacizumab, targeting the vascular endothelial growth factor A, resulted in an 11-fold increase in FcRn binding affinity above the wild-type antibody [31]. We previously demonstrated that in nonhuman primates (NHPs), the LS mutation to VRC01 extended half-life in both serum and mucosal tissue compared to parental VRC01: its serum half-life was 2.5-fold greater and it persisted for up to 70 days in rectal tissues, whereas VRC01 was no longer detectable after 28 days [30]. Based on the results of these preclinical studies, the VRC01LS drug product was developed by the Vaccine Research Center (VRC), National Institute of Allergy and Infectious Diseases (NIAID) at the National Institutes of Health (NIH) for evaluation in humans. The VRC 606 Phase I clinical trial represents the first evaluation of the safety, tolerability, PK, and neutralizing potential of VRC01LS administration in healthy adults.
Methods
Study design and procedures
VRC 606 (clinicaltrials.gov NCT02599896) was a single-site Phase I open-label dose-escalation study examining the safety and PK parameters of VRC01LS in healthy, HIV-uninfected adults, aged 18–50 years. The study was conducted at the NIH Clinical Center by the VRC Clinical Trials Program, NIAID, NIH, Bethesda, Maryland. The Investigational New Drug application was sponsored by NIAID Division of AIDS. The protocol was reviewed and approved by the NIAID Institutional Review Board, and US Department of Health and Human Services guidelines for conducting clinical research were followed. All volunteers gave written informed consent prior to participation.
The dosages in the trial were determined based on previous trials with VRC01 [6,7]. Three groups received a single dose of intravenous (IV) VRC01LS (group 1 : 5 mg/kg, group 3 : 20 mg/kg, and group 4 : 40 mg/kg). One group received a single dose of subcutaneous (SC) VRC01LS (group 2 : 5 mg/kg). Two repeat dosing groups (group 5 : 5 mg/kg SC and group 6 : 20 mg/kg IV) received three product administrations 12 weeks apart (Fig 1). Groups 1, 2, and 5 were enrolled simultaneously. Groups 1 and 2 were block randomized in a 1 : 1 ratio using the electronic AdvantageEDC system (EMMES Corp, Rockville, MD). Sample size was consistent with a small Phase I trial design and calculated to capture adverse events (AEs).
Fig. 1. CONSORT flow diagram of the VRC 606 trial. 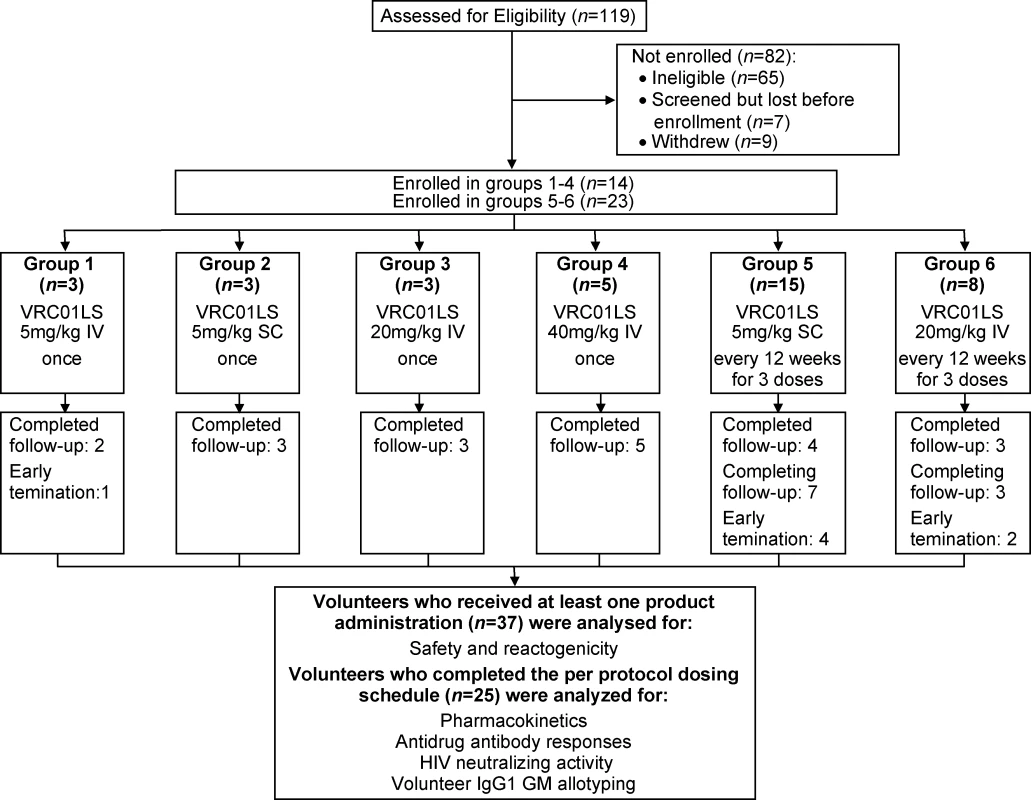
Thirty-seven volunteers were allocated to 6 groups. All volunteers who received at least one VRC01LS administration were analyzed for safety and reactogenicity. All volunteers who completed the per-protocol dosing schedule by the time of manuscript submission (n = 25) were additionally analyzed for pharmacokinetic parameters, serum neutralization activity, anti-drug antibodies, and IgG1 GM allotype. These 25 volunteers were all the volunteers in groups 1–4, the first six volunteers in group 5, and the first five volunteers in group 6. GM, genetic marker; IgG1, immunoglobulin G subclass 1; IV, intravenous; SC, subcutaneous; VRC, Vaccine Research Center. Interim safety reviews were conducted by the Protocol Safety Review Team at protocol-specified intervals for the two dose escalations. All product administrations were monitored by a study clinician. Safety laboratory tests were obtained prior to product administration, throughout the study, and at least 4 weeks post final product administration. Volunteers kept a diary card of solicited systemic symptoms for 3 days after each dose and clinicians objectively assessed the local site on the day of product administration, the day after product administration, and one week after product administration. All AEs were recorded through 56 days following product administration, while serious adverse events (SAEs) and new chronic medical conditions were recorded for the duration of the study. AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA) and severity was graded using Version 2.0 of the “DAIDS Table for Grading the Severity of Adult and Pediatric Adverse Events” (November 2014). Volunteers were followed for 24 weeks following their final study product administration.
Study product
VRC01 is a human IgG1 of allotype GM3. VRC01LS was modified from VRC01. The LS designation specifies methionine to leucine and asparagine to serine (M428L/N434S) changes within the C-terminus of the heavy chain constant region far outside of the antigen-binding site [31]. VRC01LS was produced in a Chinese hamster ovary (CHO) mammalian cell line under current Good Manufacturing Practice (cGMP) by VRC/NIAID/NIH at the VRC Pilot Plant operated under contract by the Vaccine Clinical Materials Program (VCMP), Leidos Biomedical Research, Inc., Frederick, MD. VRC01LS vials were filled at a concentration of 100 mg/mL. The formulation buffer was composed of 25 mM sodium citrate, 50 mM sodium chloride, and 150 mM L-arginine hydrochloride at pH 5.8. Vials were single use only and did not contain preservative.
A pharmacist prepared individual IV doses for volunteers by adding the calculated volume of VRC01LS needed to achieve the assigned mg/kg dose to a 103-mL bag of 0.9% sodium chloride. IV infusions were administered over 15–30 minutes using a volumetric pump. SC doses were administered by direct needle and syringe injection in the abdomen at a volume up to 2.5 mL per injection site.
Volunteer IgG1 GM allotyping
Volunteers were assessed for their genetic marker (GM) 3/17 allotypes to determine if allotype-specific effects influenced the PK of VRC01LS. IgG1 markers GM 3/f and 17/z (arginine to lysine) were determined as previously described [32] by a predesigned TaqMan genotyping assay from Applied Biosystems, Inc., employing the following primers and probes: forward primer: 5′ CCCAGACCTACATCTGCAACGTGA-3′, reverse primer: 5′ CTGCCCTGGACTGGGACTGCAT-3′, reporter 1 (GM 17-specific): VIC-CTCTCACCAACTTTCTTGT-NFQ, and reporter 2 (GM 3-specific): FAM-CTCTCACCAACTCTCTTGT-NFQ.
PK analysis
Quantification of VRC01LS concentrations was performed as previously described [6]. Briefly, VRC01LS serum concentration quantification was performed in 96 well plates on a Beckman Biomek based automation platform, utilizing the anti-idiotype mAb 5C9. Immulon-4HXB microtiter plates were coated with 5C9 overnight before blocking. Threefold dilutions were tested in duplicate wells covering the range of 1 : 100–1 : 24,300. Horseradish peroxidase-labeled goat antihuman antibody and 3,3′5,5′–tetramethylbenzidine (TMB substrate) were added for detection, and color development was halted through the addition of sulphuric acid. Plates were read at 450 nm within 30 minutes using a Molecular Devices Paradigm plate reader. Sample concentrations were quantitated using a four-parameter logistic curve regression of a standard curve of VRC01LS covering a range of 0.98–1,000 ng/ml.
PK analysis was performed using both noncompartmental and compartmental methods. Extensive PK samples were collected following IV and SC administration over 12 weeks after the first dose. PK results following the first dose in volunteers in multiple-dose groups 5 and 6 were combined with those from the single-dose groups. PK results for groups 5 and 6 following subsequent doses are still being collected and therefore are not included in this report. For the noncompartmental analysis, the area under the curve (AUC) for the VRC01LS concentration versus time curve was calculated over the first dose interval (weeks 0–12) using the trapezoidal method. The maximal concentration (Cmax) and time to maximal concentration (Tmax) were taken from the observed data. The PK data were also fit to a two-compartment model using the computer program NONMEM 7.3 (ICON, Dublin) to estimate clearance and the elimination half-life (t1/2β). Due to the small number of volunteers, population PK parameters were assumed to be proportional to body weight and no formal covariate assessment of population PK parameters was performed. Individual volunteer compartmental PK parameters were generated through a post hoc empiric Bayesian approach using the population VRC01LS parameter estimates and their variances.
Neutralizing antibody assay
Viral neutralization by trial participant serum samples were measured using single-round HIV-1 Env pseudovirus infection of TZM-bl target cells. Env-pseudotyped viruses were generated by transfection of 293T/17 cells with optimized ratios of envelope-expressing plasmid and backbone vector (pSG3DEnv). A panel of three viruses was tested for neutralizing activity spanning a range of 80% inhibitory concentrations (IC80) for bnMAb VRC01. Negative and positive controls were included in all assays. The tested viruses were Q23.17 (subtype A), PVO.04 (subtype B), and MW965.26 (subtype C). Serum neutralization assays were performed in 384-well plates using a Beckman Biomek liquid handling system, as previously described [33]. Experimental results were displayed as the serum dilution that produced 80% neutralization (ID80) against the viruses tested. Predicted ID80 values were calculated based on the concentration of VRC01LS present in the sera and the established inhibitory concentration (IC80) of the antibody against each virus.
Antidrug antibody analysis
A Meso Scale Discovery (MSD) electrochemiluminescence (ECL) homogenous bridging assay was developed to identify antidrug antibody (ADA) elicited by VRC01LS infusion, as previously described [6]. Briefly, diluted volunteer serum samples were incubated with equimolar amounts of SULFO-TAG–and biotin-labeled VRC01LS overnight, which bound any ADA present in the sera. Complexes resulting from ADA present in the volunteer serum were captured on a precoated streptavidin MSD plate and detected through the SUFO-TAG–labeled VRC01LS using an MSD sector imager 2400. An ECL threshold was established as previously described [34]. Values above the ECL unit threshold underwent an additional assay using the antigen-binding fragment (Fab) of VRC01 as the antigen in a sandwich ELISA format in order to determine whether the ECL result beyond threshold was due to background or genuine ADA against the mAb. Briefly, the VRC01 Fab was coated onto an MSD plate and serial dilutions of volunteer or control sera were analyzed. Data from this assay are represented as AUC for the dilution series and compared to healthy negative controls or ADA positive NHP sera.
Results
Study population
A total of 37 volunteers were enrolled in the study between November 16, 2015, and August 23, 2017 (Fig 1 and Table 1): 19 males (51%) and 18 females (49%). Mean volunteer weight was 74 kg, ranging from 46.5 to 96.5 kg. At the time of publication, 30 volunteers had completed their scheduled infusions and 20 had completed the protocol. One volunteer in group 1 completed study product administration but withdrew prior to study completion, citing issues with time commitment. Three volunteers withdrew from group 5. One group 5 volunteer who withdrew received two of three scheduled administrations and was discontinued from further product administration until a medical evaluation for a concurrent illness could be completed. Two group 5 volunteers withdrew after one scheduled administration: one cited injection site pain while the other had hyperpigmentation at the injection site, which later resolved. Two volunteers withdrew from group 6: one volunteer withdrew following one of three product infusions, citing problems with time commitment, and one volunteer was lost to follow-up after completing all three infusions prescribed by the protocol.
Tab. 1. Demographic characteristics of study participants. 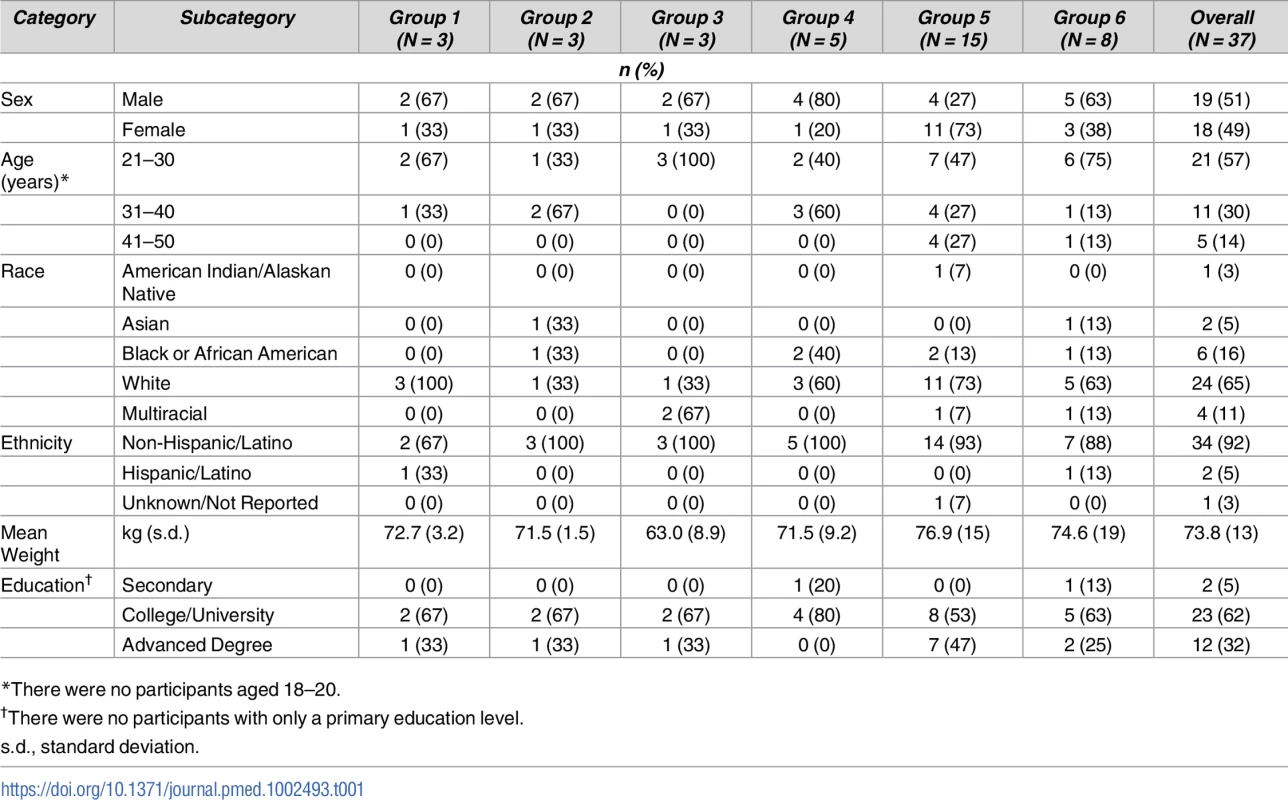
*There were no participants aged 18–20. Safety
To date, a total of 70 VRC01LS administrations have occurred during the trial. VRC01LS was safe and well tolerated, and there were no SAEs. Post administration symptoms were mild or moderate for both objective local and systemic reactogenicity, assessed at their prescribed time points (Tables 2 and 3).
Tab. 2. Maximum local reactogenicity up to day 7 after VRC01LS administration*. 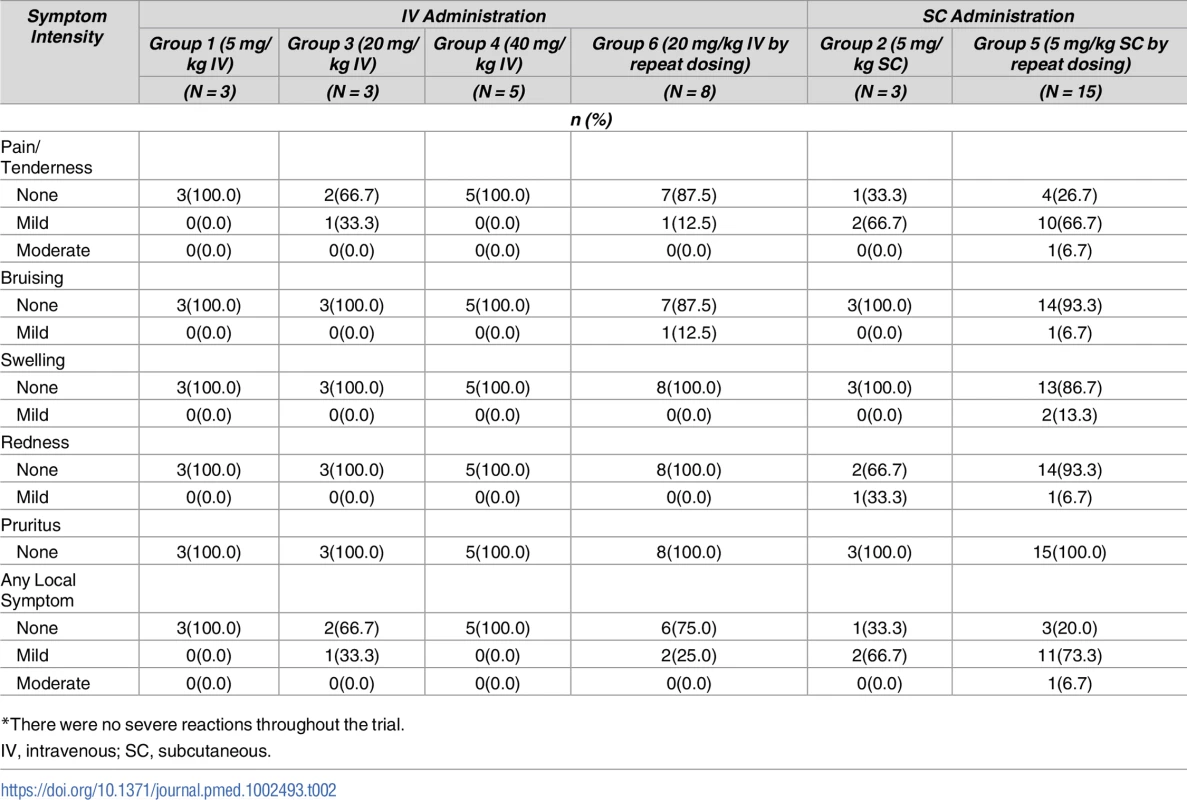
*There were no severe reactions throughout the trial. Tab. 3. Self-reported systemic reactogenicity up to 3 days after VRC01LS administration*. 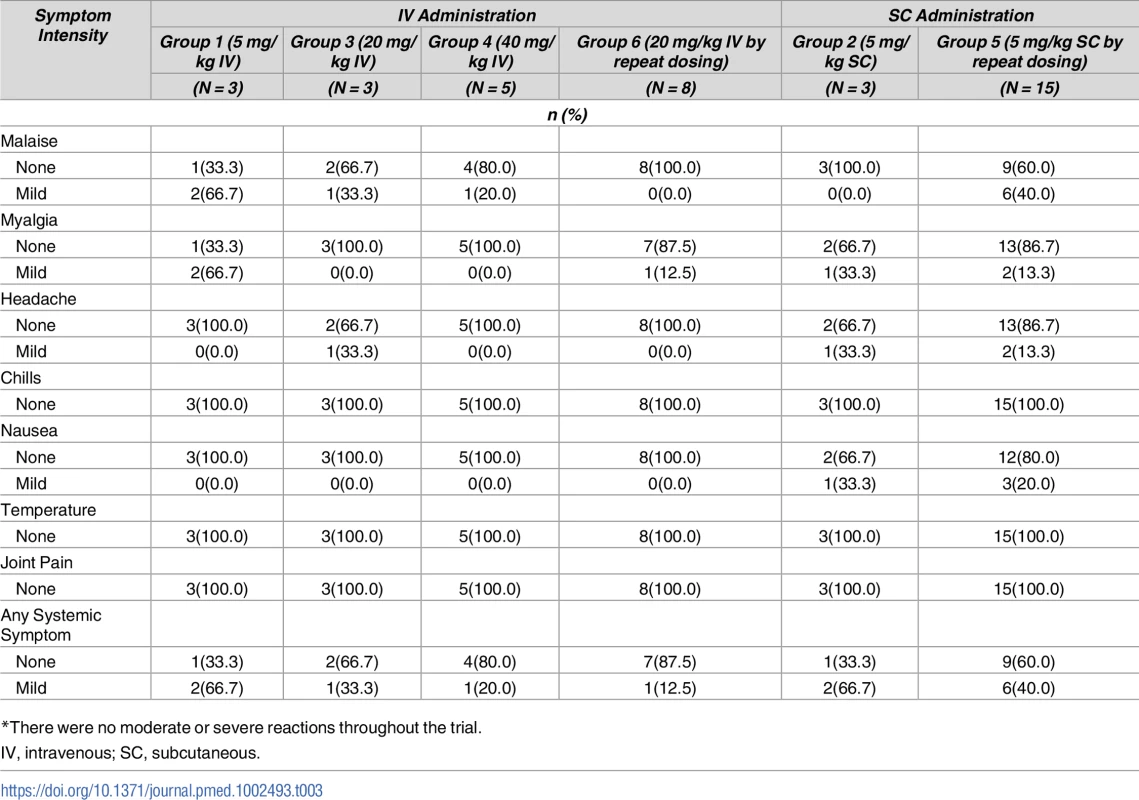
*There were no moderate or severe reactions throughout the trial. Six AEs were assessed as possibly related to VRC01LS administration and all were mild in severity. Two reports of diarrhea, one in group 1 and one in group 5, occurred on the day of VRC01LS administration and resolved the same day. One group 2 volunteer experienced lightheadedness on the day following administration, and symptoms resolved within 24 hours. One AE in group 5 pertained to an injection site reaction with hyperpigmentation that resolved 14 days post administration. Two AEs in group 5 were due to elevated alanine aminotransferase levels (56 and 69 IU/L) on day 14 post injection and both resolved within 15 days after onset. No volunteer had a positive HIV enzyme immunoassay (EIA) response during the study.
VRC01LS PK
Antibody serum concentrations for volunteers who received at least one administration of VRC01LS were analyzed to determine VRC01LS PK properties (Fig 2 and Table 4). A total of 25 volunteers had PK analysis, including the 16 volunteers who received at least one IV infusion and 9 volunteers who received at least one SC administration. Dose-dependent increases in Cmax and trough levels were observed. The mean (± SD) Cmax after one IV infusion were 246 ± 78, 1221 ± 397, and 2234 ± 548 μg/mL for 5 mg/kg, 20 mg/kg, and 40 mg/kg, respectively. Mean (± SD) 12-week serum trough concentrations were 40 ± 2.7, 180 ± 43, and 326 ± 35 μg/mL for these groups (Fig 3A and Table 4). Taking all IV administrations together, the overall VRC01LS clearance was 36 ± 8 mL/d with a t1/2β of 71 ± 18 days. The mean (± SD) PK parameters following one 5 mg/kg SC dose were Cmax of 69 ± 15 μg/mL, 12-week serum trough concentration of 25 ± 5 μg/mL, and t1/2β of 66 ± 24 days (Fig 3 and Table 4). Historical data showed that the t1/2 for the wild-type VRC01 is 15 days [6]; thus, VRC01LS displays a t1/2β more than 4-fold longer than VRC01. Serum VRC01 concentrations based on historical data are plotted alongside VRC01LS values in Fig 2 for comparison. Notably, serum concentrations 12 weeks after a single infusion of VRC01LS were higher than after two infusions (weeks 0, 4) of VRC01 (Fig 2). After IV or SC infusion of 5 mg/kg of VRC01LS, serum concentrations above 10 μg/mL were maintained for more than 20 weeks (Fig 2A and 2B). We were able to compare serum concentrations at 4 weeks post a single infusion for VRC01 and VRC01LS. Regardless of infusion dose or route of administration, VRC01LS serum concentrations were approximately 5-fold higher than corresponding levels of VRC01 (Fig 3B).
Fig. 2. Measurement of antibody serum concentration (μg/mL). 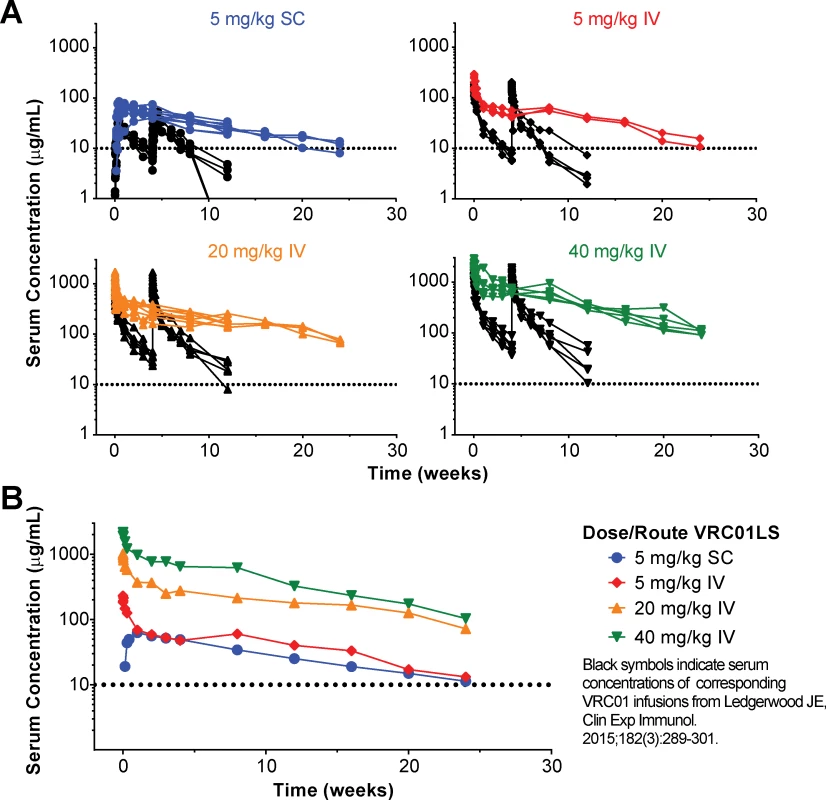
(A) Serum VRC01LS concentrations (colored plots) are shown from first measurement through week 24 after a single administration. The infusion dose and route are as specified in the legend. All values are the mean of duplicate samples run in different wells within the same plate. Previously published VRC01 concentrations based on historical data (black plots) after administration at weeks 0 and 4 are shown for comparison. (B) Geometric mean serum VRC01LS concentrations per group over time. The dotted line at 10 μg/mL on each graph is shown as a reference value. IV, intravenous; SC, subcutaneous. Fig. 3. Post infusion serum antibody concentrations and PK parameters by dose and route. 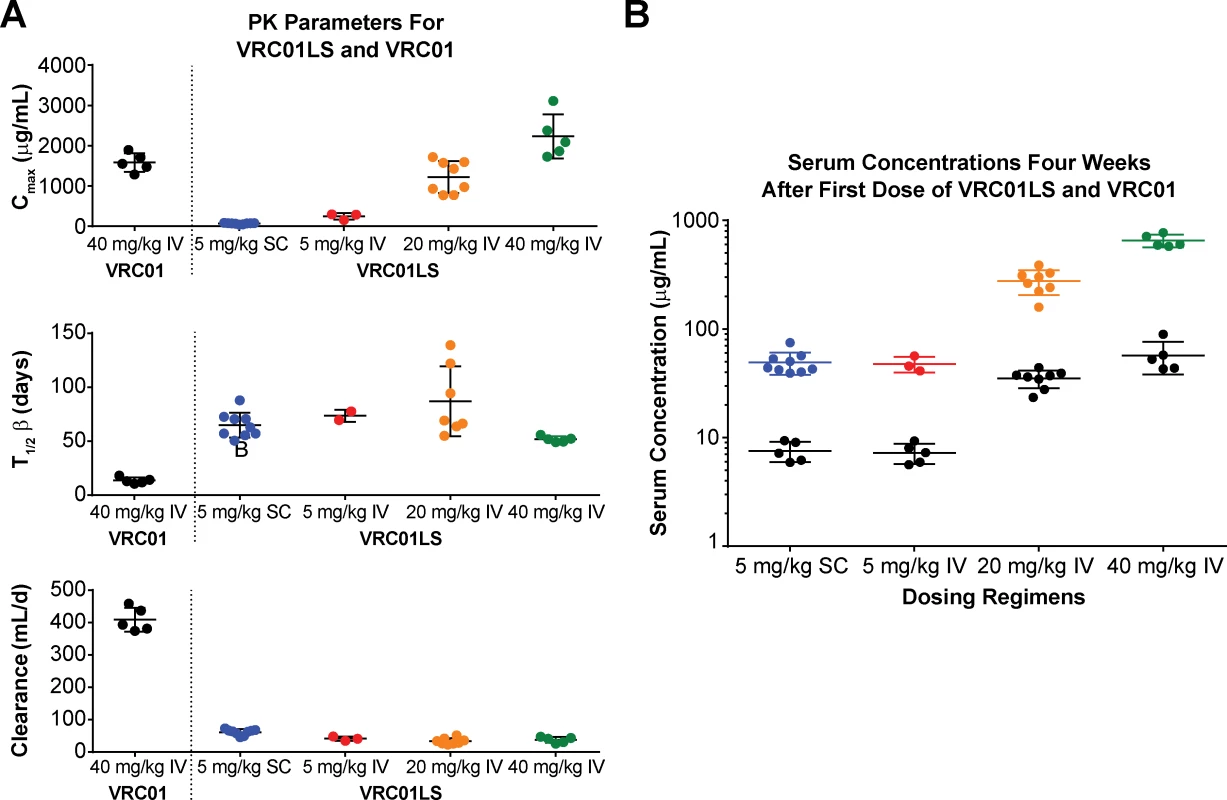
(A) Cmax (top panel) showing expected dose-dependent increases of VRC01LS, t1/2β (middle panel), and antibody clearance (bottom panel), showing similar values for doses and routes. Bars show mean values, with error bars indicating s.d. Previously published VRC01 data following 40 mg/kg infusion are shown for comparison. (B) VRC01LS and VRC01 serum concentrations 4 weeks after a single administration, as indicated on the x-axis. Each value is the mean of duplicate wells in the same plate. VRC01 data (black dots) were derived from historical data. Cmax, maximal concentration; IV, intravenous; PK, pharmacokinetic; SC, subcutaneous; s.d., standard deviation; t1/2β, elimination half-life. Tab. 4. VRC01LS mean PK parameter values. 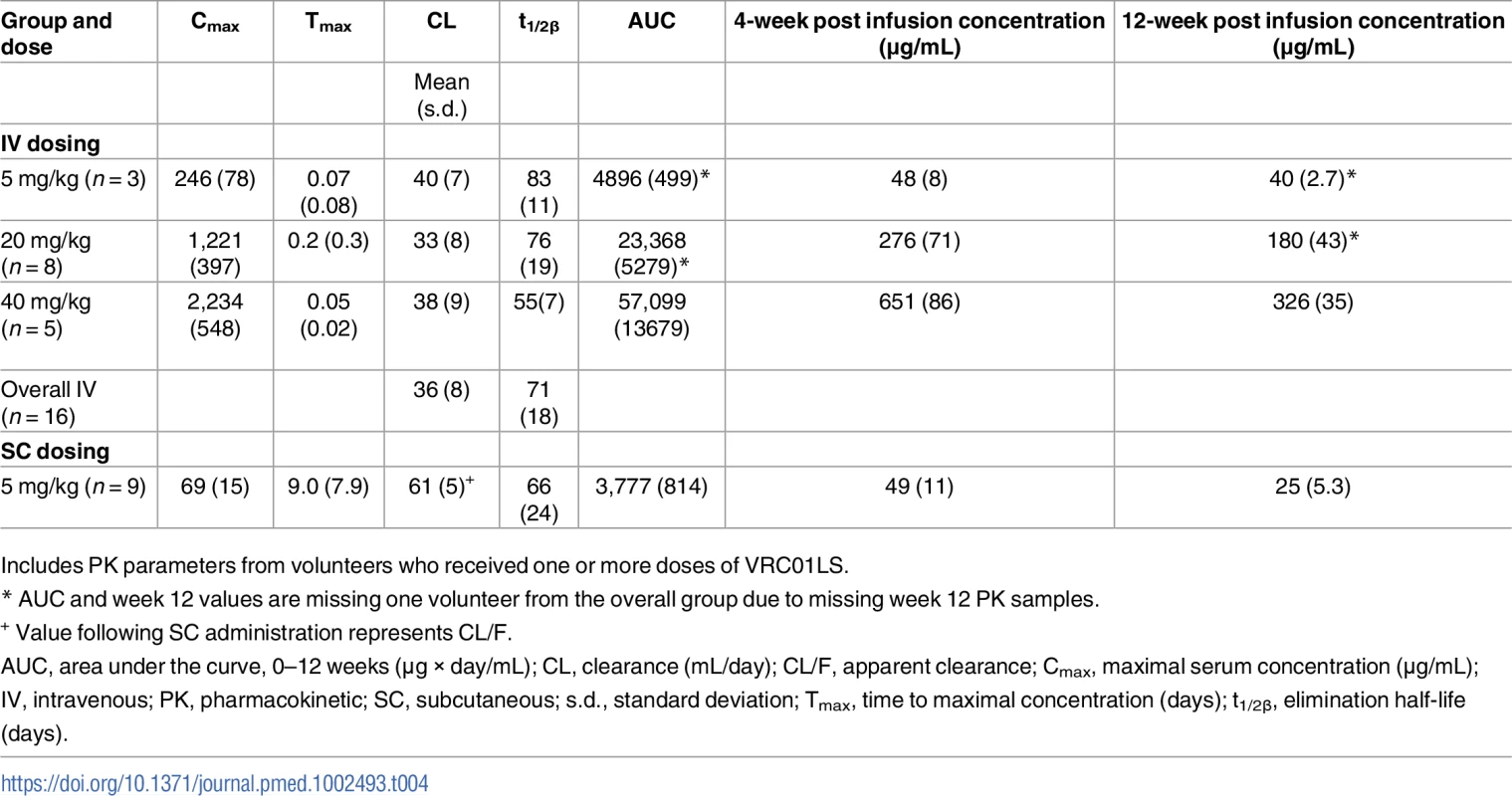
Includes PK parameters from volunteers who received one or more doses of VRC01LS. VRC01LS neutralizing activity is retained in serum after antibody infusion
Serum neutralization was assessed using a standardized neutralization assay and three Env-pseudoviral strains that differed in their sensitivity to neutralization by VRC01LS [17]. Over the course of the trial, serum inhibitory dilution neutralization titers (ID80) were assessed at 0, 2, and 84 days after a single VRC01LS infusion in groups 1–4 and at 0, 2, and 252 days after repeat doses in groups 5 and 6 (Fig 4). In all cases, the measured serum neutralization tracked closely with the predicted serum neutralization based on the measured concentration of VRC01LS (Fig 4A). As expected, serum neutralization activity increased with escalating VRC01LS doses. Thus, VRC01LS retains its expected neutralizing activity in serum, even after 36 weeks in serum. Confirmatory experiments compared serum with a known (measured) concentration of VRC01LS to the VRC01LS IgG, and the neutralization curves were similar (Fig 4B).
Fig. 4. Neutralizing ability of VRC01LS is maintained in serum after infusion. 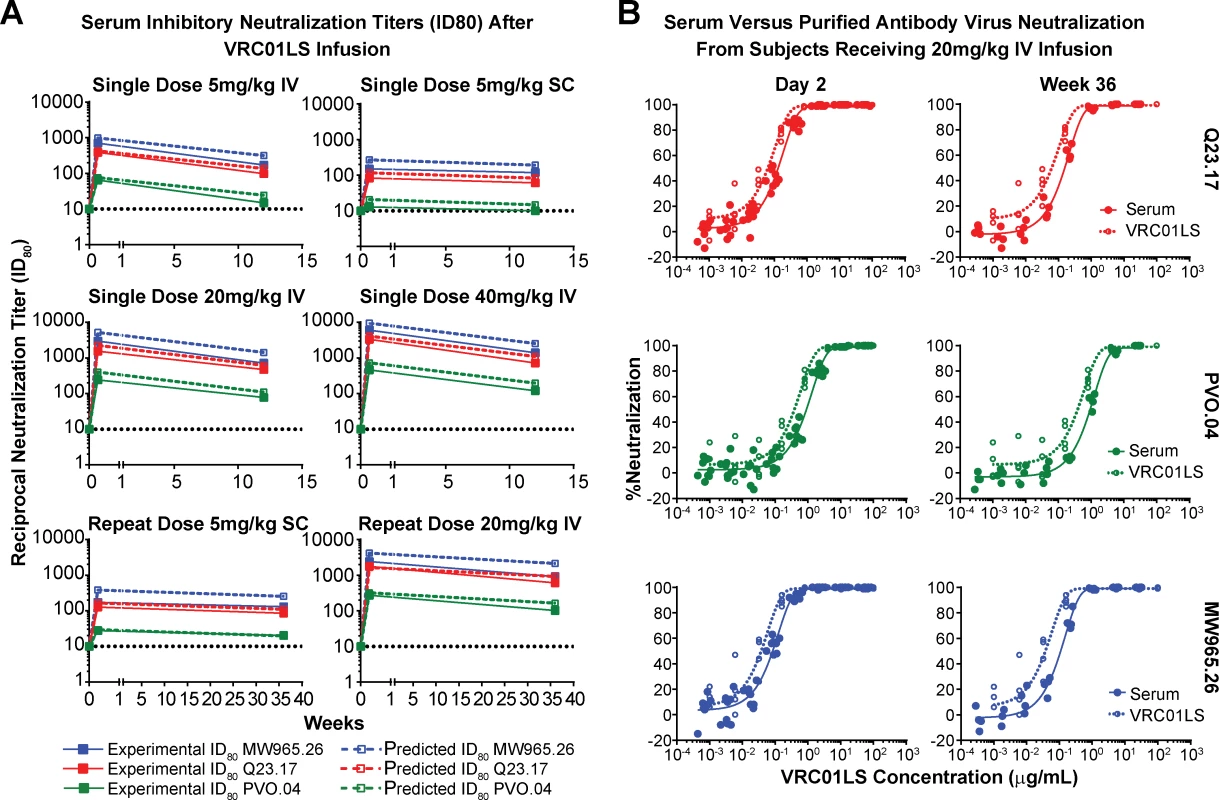
(A) Serum inhibitory neutralization titers (ID80) after VRC01LS infusion. Solid lines and closed squares indicate the reciprocal serum dilution that produces 80% virus neutralization for three viral strains. Dashed lines and open squares indicate predicted ID80 based on the measured amount of VRC01LS in the serum and the known inhibitory concentration (IC80) of VRC01LS for the viruses tested: MW965.26, 0.128 μg/mL; Q23.17, 0.298 μg/mL; and PVO.04, 1.66 μg/mL. All final time points were assessed 12 weeks after the final dose was administered. Values are the mean of duplicate wells run in the same plate. (B) Examples of serum and VRC01LS neutralization curves. Serum from day 2 and week 36 samples (closed circles) were used to generate neutralization curves (solid lines) that are compared to neutralization curves generated with uninfused VRC01LS IgG antibody (open circles and dashed lines) at different dilutions. The x-axis displays both the VRC01LS concentration acquired from serum and in vitro dilutions of uninfused antibody. IC80, 80% inhibitory concentration; ID80, dilution that produced 80% neutralization; IgG, immunoglobulin G; IV, intravenous; SC, subcutaneous. Lack of elicitation of anti-VRC01 antibodies in serum
Serum samples were tested for reactivity against VRC01LS and the isolated Fab region of VRC01. Using both assays, anti-VRC01LS antibody responses, also called ADA, were not detected in any volunteer at any time point during the trial (Fig 5). In addition, volunteers who received three infusions of VRC01LS showed no evidence of diminished peak or trough responses (Fig 5B).
Fig. 5. Evaluation of multiple administration of VRC01LS and assessment for anti-VRC01 antibodies. 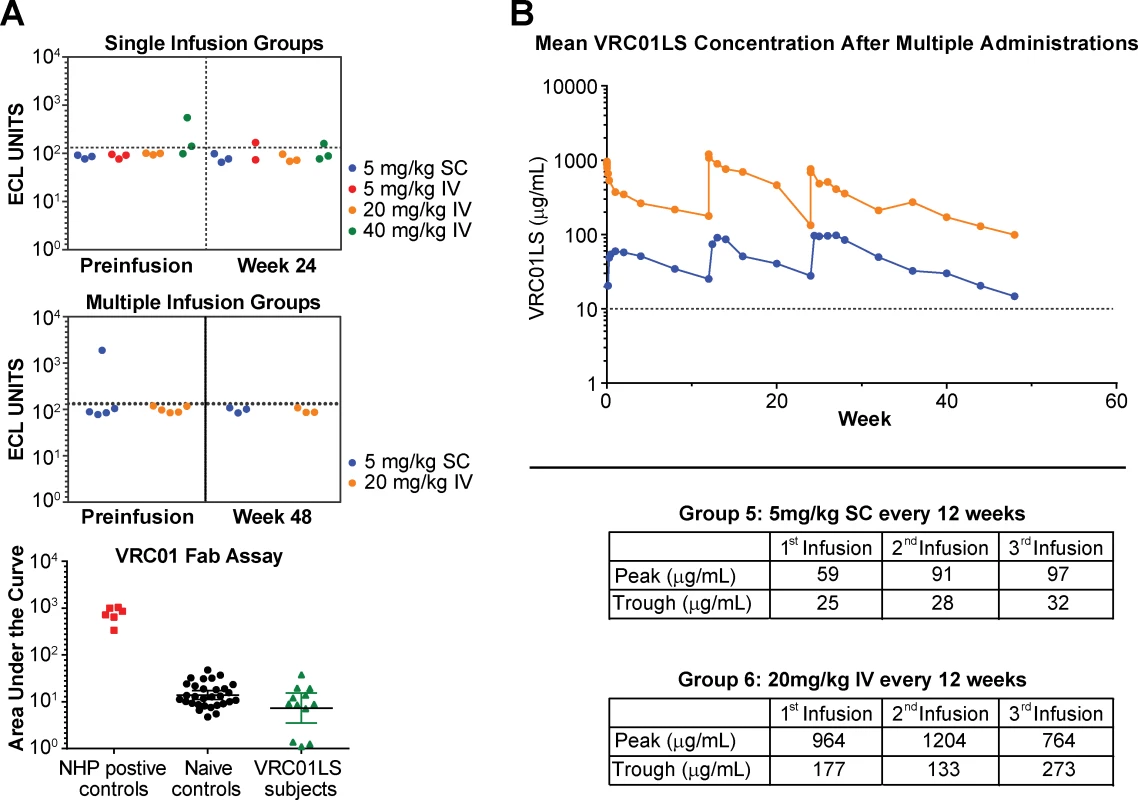
(A) ECL assay to assess for serum antibodies generated against VRC01. Data are shown for single (top panel) and multiple (middle panel) infusion groups. The few samples above the ECL cutoff value (dotted line) underwent additional analysis using VRC01 Fab as the antigen. These samples were compared to positive controls obtained from NHPs known to produce ADA against human antibodies, and negative controls obtained from humans who have never received VRC01 or VRC01LS (bottom panel). All samples were negative for ADA based on these assays. (B) Mean serum VRC01LS concentration following three administrations of 5 mg/kg SC (n = 6 volunteers, blue line) or 20 mg/kg IV (n = 5 volunteers, orange line). The table insert shows the values for peak concentration assessed within one day after administration and trough concentrations assessed 12 weeks after each administration. The dotted line at 10 μg/mL is shown as a reference value. ADA, antidrug antibody; ECL, electrochemiluminescence; Fab, antigen-binding fragment; IV, intravenous; NHP, nonhuman primate; SC, subcutaneous. Host volunteer IgG allotype
Study participants were assessed for their IgG1 allotype. Allotypes are designated GM3 or GM17, where GM refers to “genetic marker”, and volunteers can be homozygous or heterozygous. The majority of volunteers, 56%, were heterozygous GM 3/17, with 32% GM 3/3 homozygous and 12% GM 17/17 homozygous. There were no obvious correlations found between the GM allotype of volunteers and measured PK parameters.
Discussion
An expanded armamentarium of prevention and treatment methods is required to more adequately control the HIV-1 pandemic [35]. Mounting laboratory, preclinical, and clinical experience with HIV-1 bnMAbs has provided a greater understanding of their potency, breadth of reactivity, and potential to prevent or treat HIV-1 infection [6,9,36,37]. This experience is being acquired in tandem with two related international placebo-controlled Phase IIb efficacy trials assessing the impact of passive infusion of VRC01 on the risk of acquiring HIV-1 infection (clinicaltrials.gov NCT02716675, NCT02256631). In this Phase I study, we evaluated a variant of VRC01, designed to have an extended serum half-life, for safety and PK parameters. We observed that VRC01LS was safe and well tolerated and displayed a serum half-life more than four times longer than wild-type VRC01 as indicated by historical data [11,12]. The VRC01LS antibody retained its neutralizing activity in serum for the 48-week duration of this study, and no antibodies to VRC01LS were detected.
This Phase I study design includes 14 volunteers who received a single infusion of VRC01LS (at 5, 20, or 40 mg/kg) and 23 volunteers who received three infusions of VRC01LS 12 weeks apart. For PK analysis, we focused on analyzing the first VRC01LS infusion either from volunteers scheduled to receive only one infusion or from the first infusion from volunteers scheduled to receive multiple doses, in part because the unusually long half-life of this antibody, together with 12-week intervals between administration, require an extended observation time to accurately assess PK in the multiple-dose arms of the study. From this initial analysis, the half-life was 71 days—more than fourfold longer than the previously published wild-type VRC01 antibody half-life. This prolonged half-life was observed at each dose level and with SC administration. Although SC administration resulted in lower peak serum concentrations than IV administration, the serum concentration at week 4 was similar in the groups receiving 5 mg/kg IV and SC infusions. While the serum concentration needed to prevent HIV-1 infection in humans is not yet known, preclinical studies in NHPs suggest that levels above 10 μg/mL of VRC01 can be protective, although this value depends on several variables, including the virus used as part of the challenge experiment [11,30,37,38]. In this context, we observed that a dose of 5 mg/kg, which can be given by SC injection, produced serum levels above 10 μg/mL for up to 24 weeks.
Increased serum antibody concentration represents an important clinical metric when considering HIV-1 infection prevention potential but may not be the sole factor of protection. Tissue levels may also be important. While we did not assess tissue levels of VRC01LS in this study, such a Phase I clinical study is underway (clinicaltrials.gov NCT02797171). In NHPs, we previously demonstrated that the FcRn is present in mucosal tissue (both rectal and vaginal) and that tissue levels of VRC01LS persisted far longer than did those for VRC01 [30]. In an NHP challenge study directly comparing the protection afforded by a single IV infusion of VRC01 and VRC01LS, Gautam and colleagues showed that VRC01LS provided a significantly longer duration of protection. That study [39], as well as our Phase I study here, confirm that VRC01LS retains its full neutralizing activity in serum, even many weeks after infusion. Taken together, results from NHP challenge studies and data from this study offer evidence that VRC01LS produces an extended duration of physiologically relevant levels of antibody and represents a significant improvement over VRC01 in terms of HIV-1 prevention potential.
The PK results that we report from an LS mutation are comparable to PK alterations previously documented in antibodies with the YTE mutation. Motavizumab-YTE was first demonstrated to have a half-life three to four times longer than wild-type motavizumab in monkeys, which was later retained in studies involving humans [28]. MEDI8897, which also contains the YTE change, similarly has a half-life three to four times longer than wild-type MEDI8897 [29]. One distinguishing characteristic of the LS mutation compared to YTE, however, is the effect on binding to Fc-gamma. The YTE mutation reduces affinity for Fc-gamma and thus results in diminished ADCC [26,30]. The LS mutation does not significantly impact Fc-gamma binding and thus retains the Fc-mediated ADCC function of the antibody [30].
There are several potential limitations of the VRC 606 trial. It is a small study typical of Phase I trials, and therefore the conclusions drawn will require further prospective validation. This was the experience of early trials for VRC01, which have since shown similar results in expanded Phase I trials [40,41]. Additionally, this report focuses on data acquired after a single administration of VRC01LS. Data from multiple administration groups will require further reporting, and these data will also require replication in separate Phase I trials. Finally, the potential clinical impact of the extended half-life antibodies will depend on an efficacy signal established by Phase IIb efficacy trials of VRC01.
The increased durability of VRC01LS in serum could allow for administration at intervals of 3–6 months. This will depend, in part, on what is learned from an ongoing study (clinicaltrials.gov NCT02716675 and NCT02568215), in which VRC01 is being administered at either 10 or 30 mg/kg by IV infusion every 8 weeks. Notably, mAbs more potent than VRC01 are under development and, together with long half-life extension mutations, the goal of next-generation antibody products would be SC administration 2–4 times per year to maintain protective antibody levels. Such a goal may be met by using a combination of two mAbs or a single bi - or tri-specific antibody [42] to provide breadth of reactivity to the large majority of diverse HIV-1 strains. Notably, antibody prophylaxis during a finite period of risk, such as breastfeeding, would be faciliated with less frequent dosing. The extended duration would also simplify dosing regimens for persons with ongoing risk, such as HIV-discordant couples seeking to avoid sexual transmission. It would also provide advantages to groups who traditionally have difficulty with adherence to daily medication regimens. HIV-1 mAbs provided every three months could be more readily incorporated into existing care models for other extended-dosing injectable medications, such as women who receive medroxyprogesterone (Depo-Provera) for birth control. In summary, the present study suggests that if VRC01 is shown to be protective in ongoing efficacy trials, then VRC01LS or other long half-life mAbs with improved breadth and potency could provide improved efficacy with lower mAb amounts and less frequent dosing.
Supporting Information
Zdroje
1. Mascola JR, Haynes BF. HIV-1 neutralizing antibodies: understanding nature's pathways. Immunol Rev. 2013;254(1):225–44. Epub 2013/06/19. PubMed Central PMCID: PMC3738265. doi: 10.1111/imr.12075 23772623
2. Moir S, Fauci AS. B-cell responses to HIV infection. Immunol Rev. 2017;275(1):33–48. Epub 2017/01/31. doi: 10.1111/imr.12502 28133792; PubMed Central PMCID: PMC5300048.
3. McCoy LE, Burton DR. Identification and specificity of broadly neutralizing antibodies against HIV. Immunol Rev. 2017;275(1):11–20. Epub 2017/01/31. doi: 10.1111/imr.12484 28133814; PubMed Central PMCID: PMC5299474.
4. Wibmer CK, Moore PL, Morris L. HIV broadly neutralizing antibody targets. Curr Opin HIV AIDS. 2015;10(3):135–43. doi: 10.1097/COH.0000000000000153 25760932; PubMed Central PMCID: PMC4437463.
5. Julien JP, Lee PS, Wilson IA. Structural insights into key sites of vulnerability on HIV-1 Env and influenza HA. Immunol Rev. 2012;250(1):180–98. Epub 2012/10/11. doi: 10.1111/imr.12005 23046130; PubMed Central PMCID: PMC3479221.
6. Ledgerwood JE, Coates EE, Yamshchikov G, Saunders JG, Holman L, Enama ME, et al. Safety, pharmacokinetics and neutralization of the broadly neutralizing HIV-1 human monoclonal antibody VRC01 in healthy adults. Clin Exp Immunol. 2015;182(3):289–301. Epub 2015/09/04. doi: 10.1111/cei.12692 26332605; PubMed Central PMCID: PMC4636891.
7. Lynch RM, Boritz E, Coates EE, DeZure A, Madden P, Costner P, et al. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med. 2015;7(319):319ra206. Epub 2015/12/25. doi: 10.1126/scitranslmed.aad5752 26702094.
8. Caskey M, Klein F, Lorenzi JC, Seaman MS, West AP Jr., Buckley N, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522(7557):487–91. Epub 2015/04/10. doi: 10.1038/nature14411 25855300; PubMed Central PMCID: PMC4890714.
9. Caskey M, Schoofs T, Gruell H, Settler A, Karagounis T, Kreider EF, et al. Antibody 10–1074 suppresses viremia in HIV-1-infected individuals. Nat Med. 2017;23(2):185–91. Epub 2017/01/17. doi: 10.1038/nm.4268 28092665; PubMed Central PMCID: PMC5467219.
10. Kwong PD, Mascola JR, Nabel GJ. Broadly neutralizing antibodies and the search for an HIV-1 vaccine: the end of the beginning. Nat Rev Immunol. 2013;13(9):693–701. doi: 10.1038/nri3516 23969737.
11. Pegu A, Hessell AJ, Mascola JR, Haigwood NL. Use of broadly neutralizing antibodies for HIV-1 prevention. Immunol Rev. 2017;275(1):296–312. Epub 2017/01/31. doi: 10.1111/imr.12511 28133803; PubMed Central PMCID: PMC5314445.
12. Caskey M, Klein F, Nussenzweig MC. Broadly Neutralizing Antibodies for HIV-1 Prevention or Immunotherapy. N Engl J Med. 2016;375(21):2019–21. doi: 10.1056/NEJMp1613362 27959740.
13. Kwong PD, Mascola JR. Human antibodies that neutralize HIV-1: identification, structures, and B cell ontogenies. Immunity. 2012;37(3):412–25. Epub 2012/09/25. doi: 10.1016/j.immuni.2012.08.012 22999947; PubMed Central PMCID: PMC4706166.
14. Ward ES, Ober RJ. Chapter 4: Multitasking by exploitation of intracellular transport functions the many faces of FcRn. Adv Immunol. 2009;103 : 77–115. Epub 2009/09/17. doi: 10.1016/S0065-2776(09)03004-1 19755184; PubMed Central PMCID: PMC4485553.
15. Zhou T, Georgiev I, Wu X, Yang ZY, Dai K, Finzi A, et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010;329(5993):811–7. doi: 10.1126/science.1192819 20616231; PubMed Central PMCID: PMC2981354.
16. West AP Jr., Diskin R, Nussenzweig MC, Bjorkman PJ. Structural basis for germ-line gene usage of a potent class of antibodies targeting the CD4-binding site of HIV-1 gp120. Proc Natl Acad Sci U S A. 2012;109(30):E2083–90. doi: 10.1073/pnas.1208984109 22745174; PubMed Central PMCID: PMC3409792.
17. Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329(5993):856–61. doi: 10.1126/science.1187659 20616233; PubMed Central PMCID: PMC2965066.
18. Wagh K, Bhattacharya T, Williamson C, Robles A, Bayne M, Garrity J, et al. Optimal Combinations of Broadly Neutralizing Antibodies for Prevention and Treatment of HIV-1 Clade C Infection. PLoS Pathog. 2016;12(3):e1005520. Epub 2016/03/31. doi: 10.1371/journal.ppat.1005520 27028935; PubMed Central PMCID: PMC4814126.
19. Kuo TT, Aveson VG. Neonatal Fc receptor and IgG-based therapeutics. MAbs. 2011;3(5):422–30. doi: 10.4161/mabs.3.5.16983 22048693; PubMed Central PMCID: PMC3225846.
20. Ward ES, Devanaboyina SC, Ober RJ. Targeting FcRn for the modulation of antibody dynamics. Mol Immunol. 2015;67(2 Pt A):131–41. doi: 10.1016/j.molimm.2015.02.007 25766596; PubMed Central PMCID: PMC4529761.
21. Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs J, et al. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem. 2001;276(9):6591–604. Epub 2000/11/30. doi: 10.1074/jbc.M009483200 11096108.
22. Petkova SB, Akilesh S, Sproule TJ, Christianson GJ, Al Khabbaz H, Brown AC, et al. Enhanced half-life of genetically engineered human IgG1 antibodies in a humanized FcRn mouse model: potential application in humorally mediated autoimmune disease. Int Immunol. 2006;18(12):1759–69. Epub 2006/11/02. doi: 10.1093/intimm/dxl110 17077181.
23. Hinton PR, Johlfs MG, Xiong JM, Hanestad K, Ong KC, Bullock C, et al. Engineered human IgG antibodies with longer serum half-lives in primates. J Biol Chem. 2004;279(8):6213–6. Epub 2003/12/31. doi: 10.1074/jbc.C300470200 14699147.
24. Kamei DT, Lao BJ, Ricci MS, Deshpande R, Xu H, Tidor B, et al. Quantitative methods for developing Fc mutants with extended half-lives. Biotechnol Bioeng. 2005;92(6):748–60. Epub 2005/09/02. doi: 10.1002/bit.20624 16136591.
25. Vaccaro C, Zhou J, Ober RJ, Ward ES. Engineering the Fc region of immunoglobulin G to modulate in vivo antibody levels. Nat Biotechnol. 2005;23(10):1283–8. Epub 2005/09/28. doi: 10.1038/nbt1143 16186811.
26. Dall'Acqua WF, Kiener PA, Wu H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn). J Biol Chem. 2006;281(33):23514–24. Epub 2006/06/24. doi: 10.1074/jbc.M604292200 16793771.
27. Hinton PR, Xiong JM, Johlfs MG, Tang MT, Keller S, Tsurushita N. An engineered human IgG1 antibody with longer serum half-life. J Immunol. 2006;176(1):346–56. Epub 2005/12/21. PubMed 16365427.
28. Robbie GJ, Criste R, Dall'acqua WF, Jensen K, Patel NK, Losonsky GA, et al. A novel investigational Fc-modified humanized monoclonal antibody, motavizumab-YTE, has an extended half-life in healthy adults. Antimicrob Agents Chemother. 2013;57(12):6147–53. doi: 10.1128/AAC.01285-13 24080653; PubMed Central PMCID: PMC3837853.
29. Zhu Q, McLellan JS, Kallewaard NL, Ulbrandt ND, Palaszynski S, Zhang J, et al. A highly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants. Sci Transl Med. 2017;9(388). Epub 2017/05/05. doi: 10.1126/scitranslmed.aaj1928 28469033.
30. Ko SY, Pegu A, Rudicell RS, Yang ZY, Joyce MG, Chen X, et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature. 2014;514(7524):642–5. doi: 10.1038/nature13612 25119033; PubMed Central PMCID: PMC4433741.
31. Zalevsky J, Chamberlain AK, Horton HM, Karki S, Leung IW, Sproule TJ, et al. Enhanced antibody half-life improves in vivo activity. Nat Biotechnol. 2010;28(2):157–9. doi: 10.1038/nbt.1601 20081867; PubMed Central PMCID: PMC2855492.
32. Pandey JP, Namboodiri AM, Mohan S, Nietert PJ, Peterson L. Genetic markers of immunoglobulin G and immunity to cytomegalovirus in patients with breast cancer. Cell Immunol. 2017;312 : 67–70. Epub 2016/11/09. doi: 10.1016/j.cellimm.2016.11.003 27825564; PubMed Central PMCID: PMC5290197.
33. Sarzotti-Kelsoe M, Bailer RT, Turk E, Lin CL, Bilska M, Greene KM, et al. Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1. J Immunol Methods. 2014;409 : 131–46. doi: 10.1016/j.jim.2013.11.022 24291345; PubMed Central PMCID: PMC4040342.
34. Zhang L, Zhang JJ, Kubiak RJ, Yang H. Statistical methods and tool for cut point analysis in immunogenicity assays. J Immunol Methods. 2013;389(1–2):79–87. Epub 2013/01/12. doi: 10.1016/j.jim.2012.12.008 23305975.
35. Fauci AS, Marston HD. Ending AIDS—is an HIV vaccine necessary? N Engl J Med. 2014;370(6):495–8. doi: 10.1056/NEJMp1313771 24499210.
36. Scheid JF, Horwitz JA, Bar-On Y, Kreider EF, Lu CL, Lorenzi JC, et al. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature. 2016;535(7613):556–60. Epub 2016/06/25. doi: 10.1038/nature18929 27338952; PubMed Central PMCID: PMC5034582.
37. Rudicell RS, Kwon YD, Ko SY, Pegu A, Louder MK, Georgiev IS, et al. Enhanced potency of a broadly neutralizing HIV-1 antibody in vitro improves protection against lentiviral infection in vivo. J Virol. 2014;88(21):12669–82. Epub 2014/08/22. doi: 10.1128/JVI.02213-14 25142607; PubMed Central PMCID: PMC4248941.
38. Pegu A, Yang ZY, Boyington JC, Wu L, Ko SY, Schmidt SD, et al. Neutralizing antibodies to HIV-1 envelope protect more effectively in vivo than those to the CD4 receptor. Sci Transl Med. 2014;6(243):243ra88. doi: 10.1126/scitranslmed.3008992 24990883; PubMed Central PMCID: PMC4562469.
39. Gautam R, Nishimura Y, Pegu A, Nason MC, Klein F, Gazumyan A, et al. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature. 2016;533(7601):105–9. doi: 10.1038/nature17677 27120156; PubMed Central PMCID: PMC5127204.
40. Huang Y, Zhang L, Ledgerwood J, Grunenberg N, Bailer R, Isaacs A, et al. Population pharmacokinetics analysis of VRC01, an HIV-1 broadly neutralizing monoclonal antibody, in healthy adults. MAbs. 2017;9(5):792–800. Epub 2017/04/04. doi: 10.1080/19420862.2017.1311435 28368743; PubMed Central PMCID: PMC5524155.
41. Mayer KH, Seaton KE, Huang Y, Grunenberg N, Isaacs A, Allen M, et al. Safety, pharmacokinetics, and immunological activities of multiple intravenous or subcutaneous doses of an anti-HIV monoclonal antibody, VRC01, administered to HIV-uninfected adults: Results of a phase 1 randomized trial. PLoS Med. 2017;14(11):e1002435. Epub 2017/11/15. doi: 10.1371/journal.pmed.1002435 29136037.
42. Xu L, Pegu A, Rao E, Doria-Rose N, Beninga J, McKee K, et al. Trispecific broadly neutralizing HIV antibodies mediate potent SHIV protection in macaques. Science. 2017;358(6359):85–90. doi: 10.1126/science.aan8630 28931639.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2018 Číslo 1- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- What about drinking is associated with shorter life in poorer people?
- Life course socioeconomic position, alcohol drinking patterns in midlife, and cardiovascular mortality: Analysis of Norwegian population-based health surveys
- Pelvic inflammatory disease risk following negative results from chlamydia nucleic acid amplification tests (NAATs) versus non-NAATs in Denmark: A retrospective cohort
- Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: A population-based cohort study
- Sexually transmitted infections in the era of antiretroviral-based HIV prevention: Priorities for discovery research, implementation science, and community involvement
- Traumatic brain injury and the risk of dementia diagnosis: A nationwide cohort study
- Association between intake of less-healthy foods defined by the United Kingdom's nutrient profile model and cardiovascular disease: A population-based cohort study
- Progression of the first stage of spontaneous labour: A prospective cohort study in two sub-Saharan African countries
- The cost-effectiveness of alternative vaccination strategies for polyvalent meningococcal vaccines in Burkina Faso: A transmission dynamic modeling study
- PD-L1 checkpoint inhibition and anti-CTLA-4 whole tumor cell vaccination counter adaptive immune resistance: A mouse neuroblastoma model that mimics human disease
- Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: A Phase 1 open-label clinical trial in healthy adults
- Immune-related genetic enrichment in frontotemporal dementia: An analysis of genome-wide association studies
- Long-term trends in mortality and AIDS-defining events after combination ART initiation among children and adolescents with perinatal HIV infection in 17 middle- and high-income countries in Europe and Thailand: A cohort study
- What’s coming for health science and policy in 2018? Global experts look ahead in their field
- Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: A targeted metabolomics study
- Estimated mortality on HIV treatment among active patients and patients lost to follow-up in 4 provinces of Zambia: Findings from a multistage sampling-based survey
- From macro- to microfactors in health: Social science approaches in research on sexually transmitted infections
- The WHO 2016 verbal autopsy instrument: An international standard suitable for automated analysis by InterVA, InSilicoVA, and Tariff 2.0
- Long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: Systematic review and meta-analysis
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Traumatic brain injury and the risk of dementia diagnosis: A nationwide cohort study
- Pelvic inflammatory disease risk following negative results from chlamydia nucleic acid amplification tests (NAATs) versus non-NAATs in Denmark: A retrospective cohort
- PD-L1 checkpoint inhibition and anti-CTLA-4 whole tumor cell vaccination counter adaptive immune resistance: A mouse neuroblastoma model that mimics human disease
- Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: A Phase 1 open-label clinical trial in healthy adults
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



