-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaLong-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: Systematic review and meta-analysis
In a systematic review and meta-analysis, Sarah Stock exmines the long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies.
Published in the journal: . PLoS Med 15(1): e32767. doi:10.1371/journal.pmed.1002494
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002494Summary
In a systematic review and meta-analysis, Sarah Stock exmines the long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies.
Introduction
Rates of cesarean delivery continue to rise worldwide, with recent (2016) reported rates of 24.5% in Western Europe, 32% in North America, and 41% in South America [1,2]. In the presence of maternal or fetal complications, cesarean delivery can effectively reduce maternal and perinatal mortality and morbidity [2]; however, an increasing proportion of babies are delivered by cesarean when there is no medical or obstetric indication [3]. The short-term adverse associations of cesarean delivery for the mother, such as infection, haemorrhage, visceral injury, and venous thromboembolism, have been minimized to the point that cesarean delivery is considered as safe as vaginal delivery in high-income countries [4], though in low - and middle-income countries, there is an increased risk of adverse short-term maternal outcomes even with cesarean delivery without medical indication [1]. This notwithstanding, the long-term risks and benefits of cesarean delivery for mother, baby, and subsequent pregnancies are less frequently discussed with women, and there are few randomized controlled trials (RCTs) addressing the issue [5,6]. Systematic reviews of observational studies investigating the longer-term associations of cesarean delivery provide conflicting results on risks and benefits for mother and baby [7–13].
Maternal preferences are an important influence on decisions about mode of delivery. At present, evidence of longer-term complications of cesarean delivery has not been adequately synthesized to allow fully informed decisions about mode of delivery to be made. The aim of this systematic review and meta-analysis is to summarize the evidence about long-term risks and benefits of cesarean delivery for women, children, and the associations with future pregnancies.
Methods
We conducted a systematic review of literature according to the recommendations of the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) Guidelines for Meta-Analyses and Systematic Reviews of Observational Studies [14]. The study protocol was registered with the University of York Centre for Reviews and Dissemination International prospective register of systematic reviews (PROSPERO Record CRD42014007006, http://www.crd.york.ac.uk/PROSPERO/).
We developed and tested the search strategy in collaboration with a librarian experienced in literature searching. We searched Cumulative Index to Nursing and Allied Health Literature (CINAHL) and Cochrane library databases. The search terms are described in S1 Table; searches began 23 March 2014, and the last search was 25 May 2017. Additional studies were identified from reference lists of papers. After removal of duplicates, the abstracts were then screened for study inclusion criteria and full-text articles then assessed for eligibility.
We included RCTs and large (more than 1,000 participants) prospective cohort studies (including those with prospectively collected data analysed retrospectively) that assessed outcomes for women with term deliveries (>37 weeks gestation) after cesarean and vaginal delivery (exposures) with follow-up of greater than or equal to one year from the index delivery.
Two assessors (OEK and SJS) independently screened titles and abstracts of studies, then accessed and appraised full texts. Data were extracted onto the RevMan programme (version 5.3) (OEK and SJS). Where available, data for outcomes following operative vaginal delivery were included in the ‘vaginal delivery’ group. In order to detect bias and to grade the quality of studies, we used the Scottish Intercollegiate Guideline Network (SIGN) Methodology checklists for cohort studies and RCTs where appropriate and graded the studies as high quality with little or no risk of bias (++), acceptable with some flaws in the study with an associated risk of bias (+), or low quality with significant flaws (0) (OEK and SJS) [15]. As an additional assessment of bias and study quality, we used the Risk of Bias Assessment tool for Non-randomized Studies (RoBANS), which has shown moderate reliability and promising validity [16]. Studies were excluded if they did not provide sufficient information to assess methods or data analysis. Authors were contacted to clarify ambiguities in published results, in particular figures for outcomes in cesarean delivery and vaginal delivery groups [17–19]. Where there was disagreement over eligibility for inclusion or assessment of study quality, this was referred to a meeting of all authors.
We analysed the data in three groups of prespecified outcomes: maternal, childhood, and subsequent pregnancy outcomes. The primary outcome chosen for each database search was that which we felt patients would be most concerned about. As there were several other relevant outcomes for each database search, we added these as secondary outcomes (see Table 1).
Tab. 1. Primary and secondary outcomes. 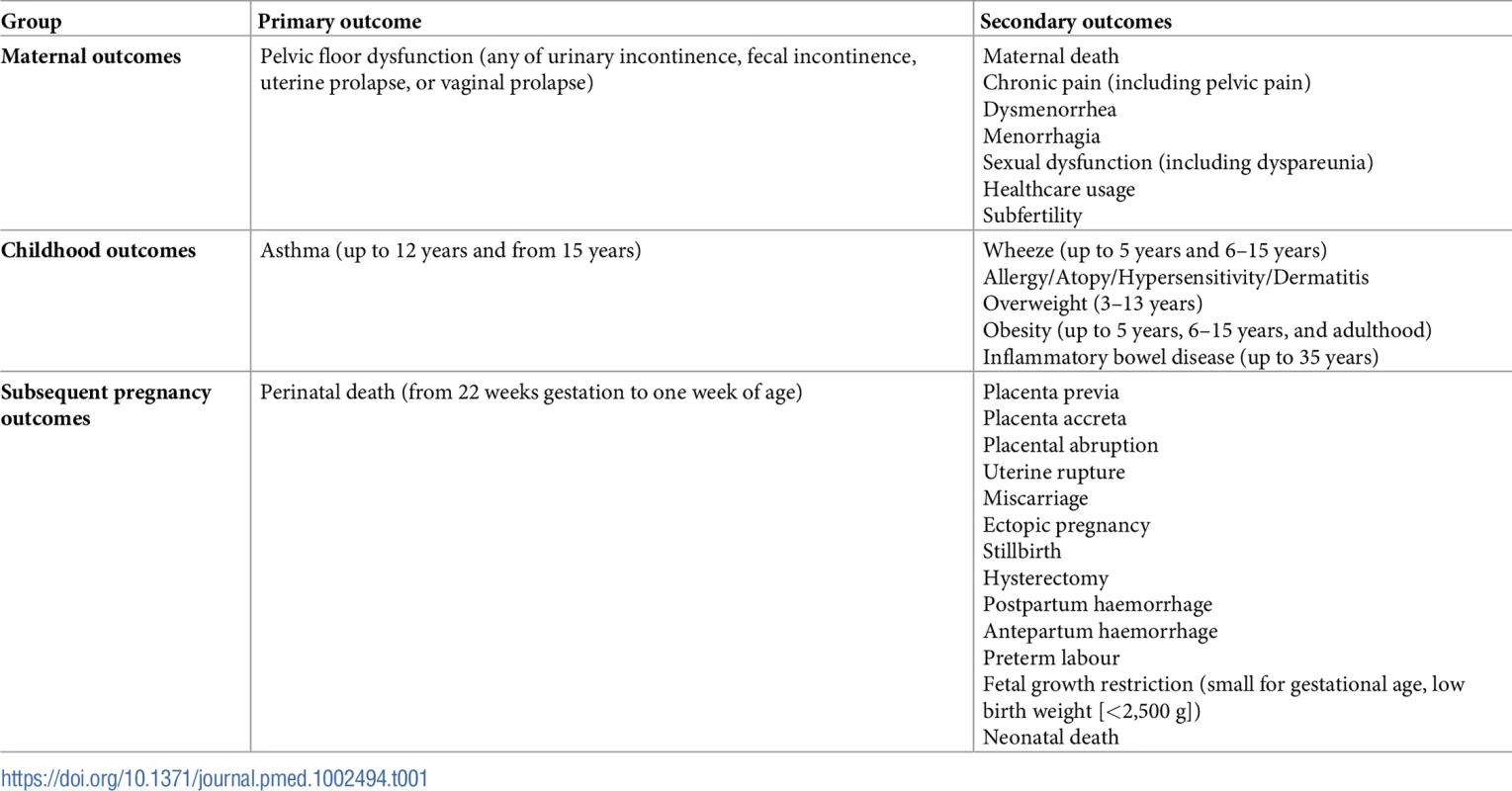
Table displaying the primary and secondary outcomes specified for database searches of maternal, childhood, and subsequent pregnancy outcomes. Results were pooled in a Mantel–Haenszel fixed effects meta-analysis with ORs, 95% confidence intervals, and two-sided p-values. Heterogeneity was assessed using the chi-squared and I-squared tests, with random effects models used when substantial heterogeneity was present, i.e., when I-squared exceeded 40%. Results were summarized in tables and illustrated using forest plots. Planned sensitivity analyses were by study quality, cohort size (>50,000), GDP of country of publication (top two thirds, bottom third of International Monetary Fund list), and study period (cohort pre-1980, post-1980) and were applied where appropriate. This study period cutoff was chosen as cesarean delivery rates and obstetric care have changed significantly since 1980.
Post hoc protocol changes to methods
Prior to analysis, we made the following changes to our methods from the published protocol. We clarified that the definition of ‘prospective cohort study’ included studies if data had been collected prospectively, even if analysis was retrospective. We changed the threshold of heterogeneity that we would use random effects meta-analysis from chi-squared test p-value <0.05 to the more conservative I2 > 40%. We added the RoBANS tool for the assessment of bias and study quality to the use of the SIGN checklist. In addition, at the data extraction stage, we made a decision to report both ‘small for gestational age’ and ‘low birth weight’ as secondary subsequent pregnancy outcomes in our analysis rather than ‘fetal growth restriction’ as specified in our protocol.
Results
Electronic searches provided 30,327 citations and hand-searching of references provided a further 57 papers. After exclusions, 80 studies were included (one RCT and 79 observational studies) (see flow diagrams in S1 Fig, S2 Fig and S3 Fig; of note, three of the 80 studies contributed to both the ‘maternal outcomes’ and ‘subsequent pregnancy outcomes’ meta-analyses and are included in both flowcharts; thus, the sum of all papers in flow diagrams is 83). For the purpose of combining estimates, the RCT was not meta-analysed with the observational studies, but the results were presented separately. Two independent reviewers assessed study quality. Several studies had high or unclear risk of detection bias through inadequate blinding of outcome assessments, and many had a high risk of attrition bias caused by the inadequate handling of incomplete outcome data. The majority of studies were of acceptable quality, and many were adjusted for multiple confounding factors. Of note, in the majority of studies, the adjusted ORs were not substantially different from the crude ORs. All studies were from high-income countries (top third of GDP list); 13 were hospital studies, and 67 were population studies (see S2 Table, S3 Table, S4 Table and S5 Table).
Results of meta-analyses are summarized in Table 2 and Figs 1–3.
Fig. 1. Modified forest plot of maternal outcomes meta-analyses. 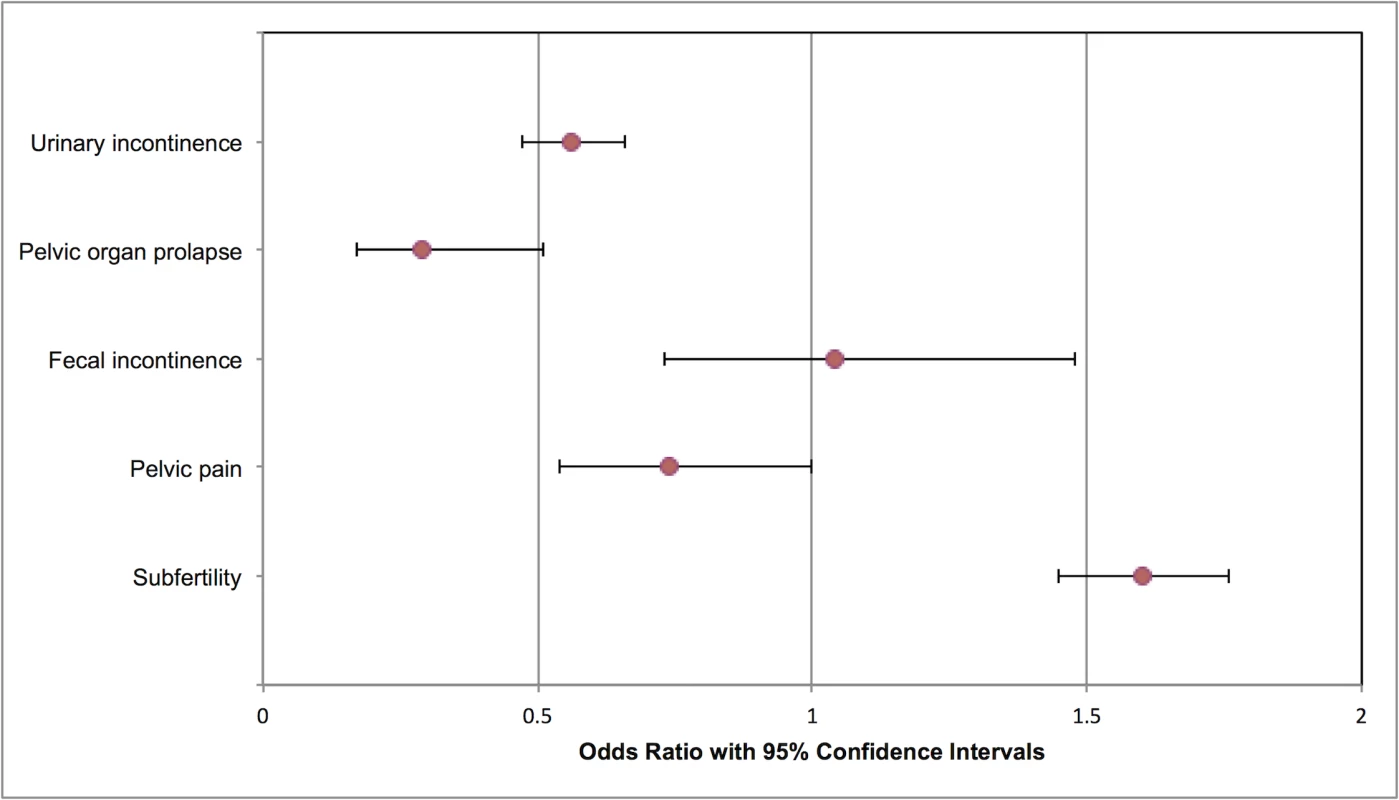
In addition to the meta-analyses shown, one RCT assessed dysmenorrhea and menorrhagia with no statistically significant associations. One RCT and one cohort study investigated sexual dysfunction, notably dyspareunia, with conflicting results. No studies investigated maternal death or healthcare usage. RCT, randomized controlled trial. Fig. 2. Modified forest plot of childhood outcomes meta-analyses. 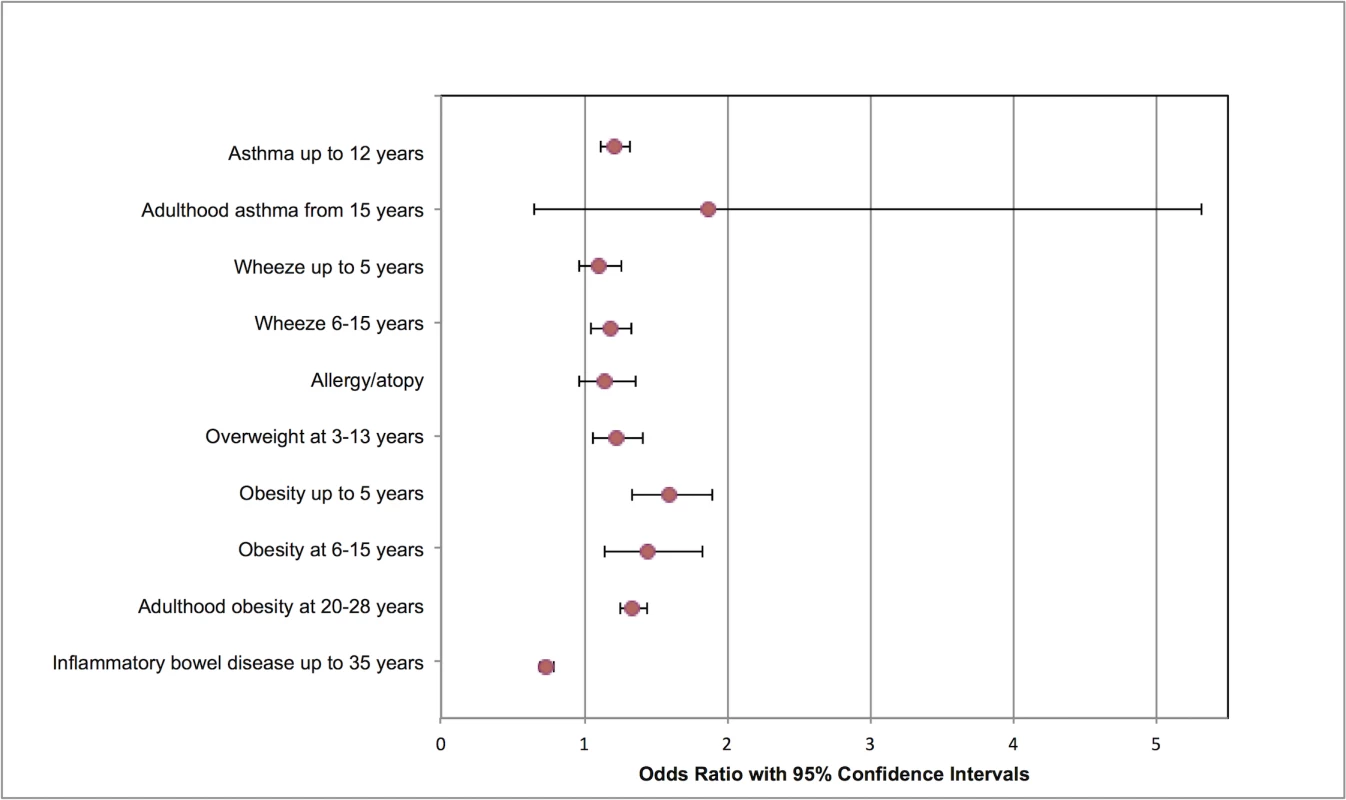
As studies had multiple cohorts and different follow-up periods, meta-analyses were divided according to age or duration of follow-up. Fig. 3. Modified forest plot of subsequent pregnancy outcomes meta-analyses. 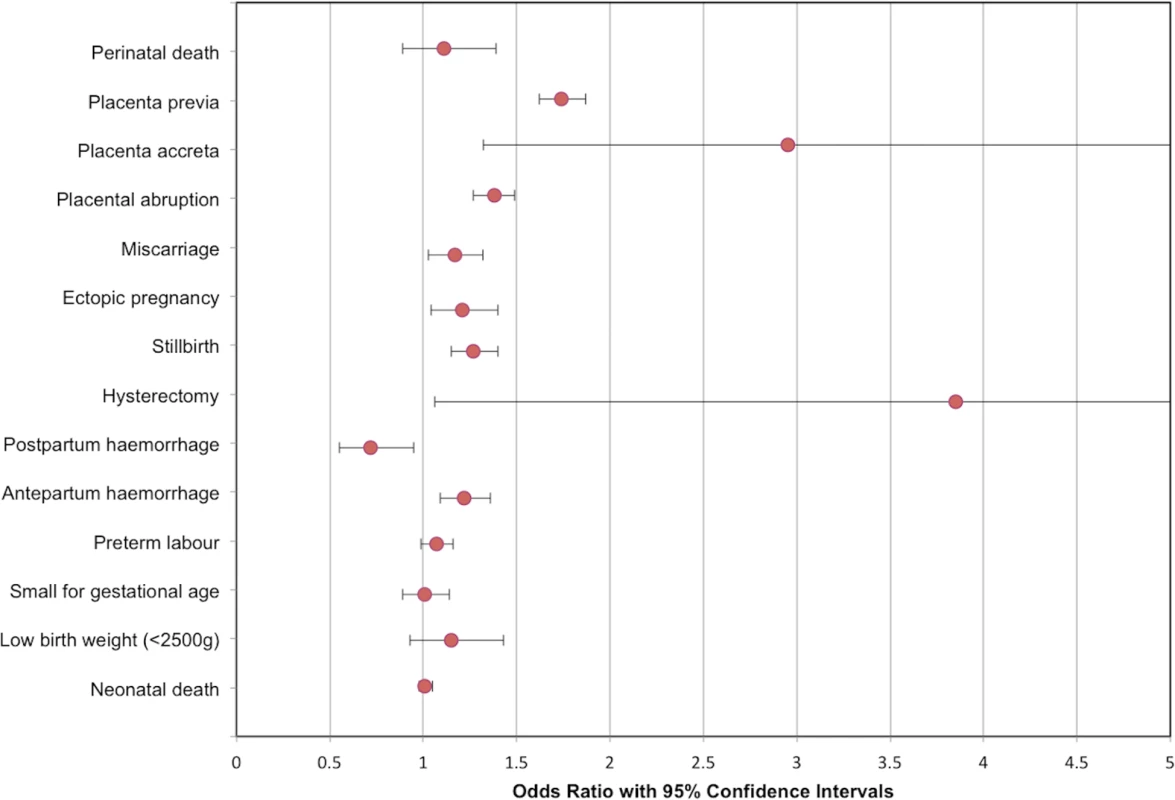
An additional outcome not included in this modified forest plot is uterine rupture, OR 25.81 (95% confidence intervals 10.96 to 60.76). OR, odds ratio. Tab. 2. Summary of meta-analyses. 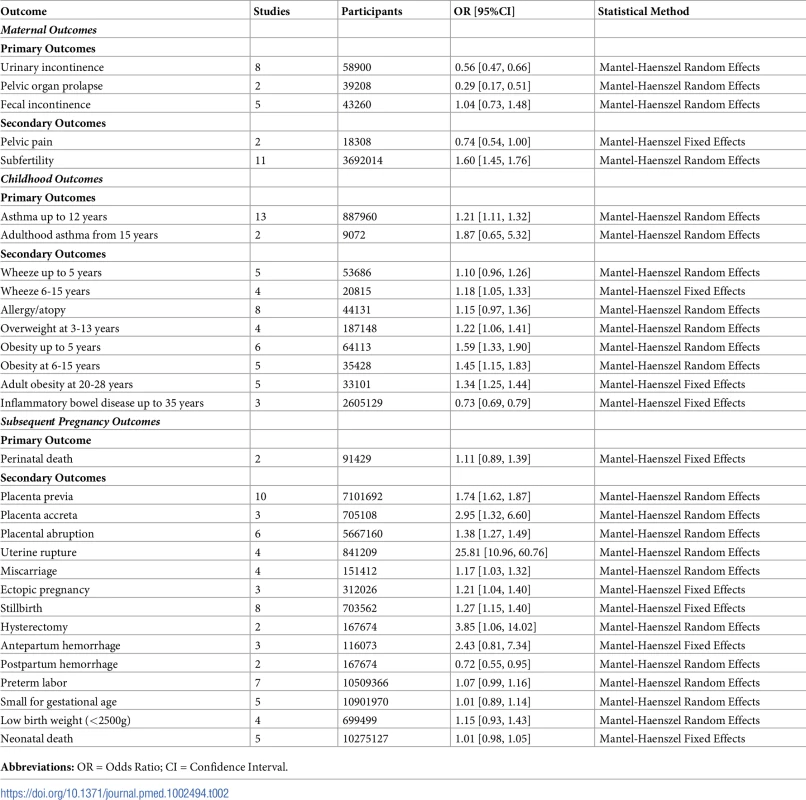
Table summarizing the meta-analyses performed detailing the number of studies, number of participants, effect estimate of each outcome and statistical method used. As studies had multiple cohorts and different follow-up periods, meta-analyses were divided according to age or duration of follow-up. Maternal outcomes
One RCT of 2,088 participants [5] and data from 23 reports of prospective cohort studies (total of 3,849,075 participants) were included [20–42] (see S2 Table for characteristics).
Primary outcome: Pelvic floor dysfunction
No studies reported ‘pelvic floor dysfunction’ as an outcome; therefore, the following individual outcomes were used: urinary incontinence, pelvic organ prolapse (to include uterine and/or vaginal prolapse), and fecal incontinence. The RCT did not demonstrate any statistically significant association of cesarean delivery with urinary incontinence (OR 0.78, 95% confidence intervals 0.56 to 1.08) or fecal incontinence (OR 3.07, 95% confidence intervals 0.90 to 10.49) [5]. In total, data from 11 manuscripts were eligible for meta-analysis, with follow-up ranging from 12 months postnatal to age 80 years [5,20,22,25–28,32,38,39,42,43]. Compared to vaginal delivery, cesarean delivery was associated with reduced odds of urinary incontinence (1,024/7,306 cesarean delivery versus 7,713/51,594 vaginal delivery; OR 0.56, 95% confidence intervals 0.47 to 0.66, p < 0.000011; I2 = 71%; 8 studies) (S4 Fig) [5,20,25,28,32,33,38,39,42]. Similar results were seen when sensitivity analysis was performed, excluding two low-quality studies [32,39] (955/6,883 cesarean delivery versus 7,129/49,319 vaginal delivery; OR 0.59, 95% confidence intervals 0.49 to 0.70, p < 0.00001; I2 = 72%; 6 studies).
Compared to vaginal delivery, cesarean delivery was associated with reduced odds of pelvic organ prolapse (116/4,898 cesarean delivery versus 2,055/34,310 vaginal delivery; OR 0.29, 95% confidence intervals 0.17 to 0.51, p = 0.005, I2 = 87%; 2 studies) (S5 Fig) [20,27]. There was no statistically significant difference in rates of fecal incontinence (234/6,449 cesarean delivery versus 705/36,811 vaginal delivery; OR 1.04, 95% confidence intervals 0.73 to 1.48, p = 0.82, I2 = 72%; 5 studies) (S6 Fig) [5,20,22,26,33,42]. Similar results were seen when sensitivity analysis was performed, excluding one low-quality study [22] (187/6,087 cesarean delivery versus 663/36,534 vaginal delivery; OR 1.09, 95% confidence intervals 0.71 to 1.67, p = 0.69, I2 = 77%; 4 studies).
Secondary outcomes: Menorrhagia and dysmenorrhea; chronic pain (including pelvic pain) and sexual dysfunction (including dyspareunia); and subfertility
Data from the one RCT showed no association between mode of delivery and heavy menstrual bleeding (menorrhagia) or painful menstrual bleeding (dysmenorrhea) [5].
Two studies investigated pelvic pain [21,42]. There was no statistically significant association of mode of delivery with pelvic pain (33/2,449 cesarean delivery versus 313/15,512 vaginal delivery; OR 0.74, 95% confidence intervals 0.54 to 1.00, p = 0.05, I2 = 0%) (S7 Fig).
When compared with vaginal delivery, cesarean delivery was associated with increased odds of dyspareunia in one cohort study (OR 1.49, 95% confidence intervals 1.11 to 2.00) [34], but there was no statistically significant effect demonstrated in the RCT (OR 0.96, 95% confidence intervals 0.61 to 1.50) [5].
There were no studies found investigating maternal death or healthcare usage as a long-term association of cesarean delivery.
Meta-analysis of 11 studies (3,692,014 women) showed an association between cesarean delivery and increased odds of subfertility when compared to vaginal delivery (246,096/567,155 previous cesarean delivery versus 995,022/3,124,859 previous vaginal delivery; OR 1.60, 95% confidence intervals 1.45 to 1.76, p < 0.00001) (S8 Fig) [23,24,29–31,35–37,40,41]. Between-study heterogeneity was high in this meta-analysis (I2 = 99%) due to the varying follow-up periods, varying cohort numbers, and study periods. Sensitivity analysis excluding four studies with <50,000 participants [29,30,35,36] did not alter these results (243,260/560,190 previous cesarean delivery versus 978,990/3,075,271 previous vaginal delivery; OR 1.64, 95% confidence intervals 1.46 to 1.84, p < 0.00001; I2 = 100%; 7 studies).
Childhood outcomes
Thirty-five manuscripts met the inclusion criteria (see S3 Table for characteristics) [17,19,44–76]. As studies had multiple cohorts and different follow-up periods, meta-analyses were divided according to age or duration of follow-up.
Primary outcome: Asthma
Meta-analysis of 13 studies (887,960 participants) [17,45,49,55,58,59,61,63,67,69,72,73,76] showed an association between cesarean delivery and increased odds of asthma in children aged up to 12 years compared to vaginal delivery (4,788/124,668 cesarean delivery versus 23,308/763,292 vaginal delivery; OR 1.21, 95% confidence intervals 1.11 to 1.32, p < 0.00001) (S9 Fig). There was significant heterogeneity between the studies (I2 = 75%). Planned sensitivity analysis excluding the single low-quality study [72] did not change findings (4,743/124,068 cesarean delivery versus 23,092/760,142 vaginal delivery; OR 1.22, 95% confidence intervals 1.11 to 1.33, p < 0.0001; I2 = 77%). Cesarean delivery was associated with increased risk of childhood asthma in another study that could not be included in the meta-analysis because results were not subdivided by duration of follow up [71]. Two studies (9,072 participants) investigated the development of adulthood asthma in children delivered by cesarean section (from 15 years) [74,75], and no statistically significant association between cesarean delivery and adulthood asthma was seen, although one of these studies was graded as low quality [74]; excluding this study changed the association to an increased odds of adulthood asthma following cesarean delivery (OR 3.31, 95% confidence intervals 1.81 to 6.05) (S10 Fig).
Secondary outcomes: Wheeze; hypersensitivity/dermatitis/allergy/atopy; overweight/obesity; and inflammatory bowel disease
There was no statistically significant association of mode of delivery with the development of childhood wheeze at up to 5 years [58,62,63,72], but at 6–15 years follow-up, cesarean delivery was associated with increased odds of wheeze in children when compared with those delivered vaginally (416/3,450 cesarean delivery versus 1,603/17,365 vaginal delivery; OR 1.18, 95% confidence intervals 1.05 to 1.33, p = 0.006, I2 = 0%) (S11 Fig, S12 Fig) [59,62,69,72]. Following sensitivy analysis, excluding two low-quality studies [59,72], there was no statistically significant association between mode of delivery and wheeze at this age (251/1,848 cesarean delivery versus 640/6,318 vaginal delivery; OR 1.14, 95% confidence intervals 0.97 to 1.34; p = 0.11, I2 = 0%).
Eight studies (n = 44,131) assessed allergies, hypersensitivity, dermatitis, or atopic conditions, evaluating a variety of outcomes [51,59,61,63,67,69,75,77]. In order to enable a meta-analysis, a single outcome from each study was chosen. All studies had follow-up of up to 8 years except one [75], which had 31 years follow-up. There was no statistically significant association between mode of delivery and odds of hypersensitivity/allergy/dermatitis/atopy in the meta-analysis (S13 Fig). There was moderate heterogeneity between the studies (I2 = 51%).
Compared with vaginal delivery, cesarean delivery was associated with increased odds of childhood overweight (3,221/39,866 cesarean delivery versus 9,792/147,282 vaginal delivery; OR 1.22, 95% confidence intervals 1.06 to 1.41, p = 0.007; 4 studies; I2 = 47%) [56,57,64,70]. In performing planned sensitivity analyses, we excluded one low-quality study [70], which did not alter results (3,191/39,721 cesarean delivery versus 9,587/145,740 vaginal delivery; OR 1.19, 95% confidence intervals 1.04 to 1.35; p = 0.01, I2 = 42%). Cesarean delivery was also associated with increased odds of childhood obesity at up to 5 years when compared with vaginal delivery (834/6,645 cesarean delivery versus 5,295/57,468 vaginal delivery; OR 1.59, 95% confidence intervals 1.33 to 1.90, p < 0.00001, I2 = 68%; 6 cohorts) [17,19,54,64], at 6–15 years (655/5,728 cesarean delivery versus 2,716/29,700 vaginal delivery; OR 1.45, 95% confidence intervals 1.15 to 1.83, p = 0.002, I2 = 63%; 5 cohorts) [19,44,53,64], and at 20–28 years (1,250/7,759 cesarean delivery versus 3,105/25,342 vaginal delivery; OR 1.34, 95% confidence intervals 1.25 to 1.44, p < 0.0001, I2 = 0%; 5 studies) [19,48,53,60,66] (S14 Fig, S15 Fig, S16 Fig, and S17 Fig).
In a meta-analysis of 3 studies, cesarean delivery was associated with reduced odds of inflammatory bowel disease when compared with vaginal delivery (878/319,164 cesarean delivery versus 7,806/2,285,965 vaginal delivery; OR 0.73, 95% confidence intervals 0.69 to 0.79, p < 0.00001, I2 = 0%) (S18 Fig) [10,17,68].
Subsequent pregnancy outcomes
There were 24 cohort studies assessing outcomes for pregnancy following cesarean delivery (see S4 Table for characteristics) [29,35,40,78–98].
Primary outcome: Perinatal death
The primary outcome of perinatal death (defined as the combination of stillbirth [as defined by the authors] and neonatal death [as defined by the authors]) was assessed in 2 studies (n = 91,429) [81,86,90,91,94,97]. There was no statistically significant association of mode of delivery with perinatal mortality (98/17,259 previous cesarean delivery versus 385/74,170 previous vaginal delivery; OR 1.11, 95% confidence intervals 0.89 to 1.39, p = 0.22; I2 = 34%) (S19 Fig).
Secondary outcomes
Women with previous cesarean delivery had increased odds of having placenta previa compared to women with a previous vaginal delivery (5,039/1,025,692 previous cesarean delivery versus 16,679/6,076,000 previous vaginal delivery; OR 1.74, 95% confidence intervals 1.62 to 1.87, p < 0.00001; I2 = 55%; 10 studies) (S20 Fig) [79,80,82,84–89,95]. Similar results were seen when prespecified sensitivity analysis was performed, omitting studies of <50,000 participants (OR 1.73, 95% confidence intervals 1.59 to 1.88, p < 0.00001; I2 = 68%) [80,85,86]. When pre-1980 cohorts were omitted, there was little impact on results (OR 1.77, 95% confidence intervals 1.62 to 1.94, p < 0.00001; I2 = 64%) [79,88,95].
Women with previous cesarean delivery also had increased odds of having placenta accreta compared to women with a previous vaginal delivery (44/66,241 previous cesarean delivery versus 188/638,867 previous vaginal delivery; OR 2.95, 95% confidence intervals 1.32 to 6.60, p = 0.008; I2 = 47%; 3 studies) (S21 Fig) [79,85,86,88,95]. In a sensitivity analysis excluding one study with a pre-1980 cohort [79], the association was no longer statistically significant (OR 5.32, 95% confidence intervals 0.67 to 44.26; p = 0.11, I2 = 68%).
When compared with women with previous vaginal delivery, women with a previous cesarean delivery also had increased odds of placental abruption (6,047/858,208 previous cesarean delivery versus 23,855/4,808,952 previous vaginal delivery; OR 1.38, 95% confidence intervals 1.27 to 1.49, p < 0.00001; I2 = 54%; 6 studies) [82,85–87,89,95] and uterine rupture (215/91,837 previous cesarean delivery versus 56/749,372 previous vaginal delivery; OR 25.81, 95% confidence intervals 10.96 to 60.76, p < 0.00001; I2 = 80%; 4 studies) (S22 Fig, S23 Fig) [79,85,86,97].
When compared with women with previous vaginal delivery, women with previous cesarean delivery had increased odds of miscarriage (2,060/19,106 previous cesarean delivery versus 12,663/132,306 previous vaginal delivery; OR 1.17, 95% confidence intervals 1.03 to 1.32, p = 0.01; I2 = 79%; 4 studies) [29,35,40,85], ectopic pregnancy (223/71,040 previous cesarean delivery versus 772/240,986 previous vaginal delivery; OR 1.21, 95% confidence intervals 1.04 to 1.40, p = 0.02; I2 = 0%; 3 studies) [35,78,85], and stillbirth (496/118,192 previous cesarean delivery versus 1,905/585,370 previous vaginal delivery; OR 1.27, 95% confidence intervals 1.15 to 1.40, p < 0.00001; I2 = 34%; 8 studies) (S24 Fig, S25 Fig, S26 Fig) [83,85,86,92,93,96–98].
Women with previous cesarean delivery had increased odds of hysterectomy (19/29,626 previous cesarean delivery versus 31/138,048 previous vaginal delivery; OR 3.85, 95% confidence intervals 1.06 to 14.02, p = 0.04; I2 = 69%; 2 studies) [85,97] and antepartum haemorrhage (413/17,259 previous cesarean delivery versus 1,237/74,170 previous vaginal delivery; OR 1.22, 95% confidence intervals 1.09 to 1.36, p = 0.0007; I2 = 0%; 2 studies) [86,90] but reduced odds of postpartum haemorrhage (1,087/29,626 previous cesarean delivery versus 7,455/138,048 previous vaginal delivery; OR 0.72, 95% confidence intervals 0.55 to 0.95, p = 0.02; I2 = 88%; 2 studies) [85,97] (S27 Fig, S28 Fig, S29 Fig). There was no statistically significant association between previous mode of delivery and preterm labour [85,86,90,91,94,97,98], small for gestational age [79,86,91,94,97], low birth weight (<2,500 g) [86,90,94,98] or neonatal death [81,86,91,94,97] (S30 Fig, S31 Fig, S32 Fig, S33 Fig).
Non-prespecified outcomes
Whilst searching for the outcomes defined in our protocol, we identified studies looking at the risk of additional outcomes, including childhood type 1 diabetes [17,99–102] and celiac disease [99,103]. These were not defined as outcome variables in our protocol, and we did not therefore systematically review the risks of these events. However, the results of these studies are summarized in S6 Table.
Discussion
This systematic review and meta-analysis has highlighted the long-term risks and benefits of cesarean delivery for mother, baby, and subsequent pregnancies when compared to vaginal delivery in term (>37 weeks gestation) pregnancies. We found that cesarean delivery is associated with reduced rates of urinary incontinence and pelvic organ prolapse but has adverse associations with fertility, future pregnancy outcome, future pregnancy complications, and long-term childhood outcomes.
We attempted to minimize bias in the review by adhering to a registered protocol and following the MOOSE guidelines [14]. We only included studies with a large number of participants. In order to minimize publication bias, the database searches were comprehensive, without language or date restrictions, and efforts were made to include unpublished data through contacting authors. However, as with all systematic reviews, publication bias is a possibility. Despite the strengths of this systematic review, we recognize that the associations are based on predominantly observational data, which itself may be vulnerable to bias.
We chose our outcomes a priori. Whilst this minimized bias, we have been unable to include some data from well-conducted prospective randomized trials. Examples include [6] and [104], both of which looked at neurodevelopmental outcomes at two years of age in children delivered by planned cesarean delivery versus planned vaginal delivery. Neither study demonstrated statistically significant differences in the two delivery groups; therefore, including these would not have substantially altered the conclusions of our review.
Two independent reviewers assessed study quality using two bias assessment tools that correlated well. Any bias was mainly due to attrition bias or detection bias. These biases are likely to have operated in different directions, with attrition bias reducing the observed difference between the treatment groups and detection bias magnifying it. Importantly, excluding studies of low quality did not change findings, suggesting that any bias will have had minimal effect. However, as with all meta-analyses of observational studies, some caution must be exercised in the interpretation of results. This is especially true in analyses where high levels of between-study heterogeneity were observed (pelvic organ prolapse, subfertility, placenta previa, uterine rupture, preterm labour), likely to reflect differences in the definitions of outcomes and confounders, follow-up times, and parity in cohorts, or where there the range of confidence intervals were very wide (placenta accreta, uterine rupture, hysterectomy, antepartum haemorrhage).
Observational studies of the risks and benefits of cesarean delivery have multiple potential confounding factors. The majority of included studies adjusted for at least some of these (S2 Table, S3 Table, S4 Table). Maternal age, parity, and BMI were commonly adjusted-for variables. Studies assessing childhood outcomes frequently also adjusted for birth weight, breastfeeding, maternal education, and maternal smoking. Studies assessing the association of cesarean delivery with subsequent pregnancy outcomes additionally adjusted for a range of maternal complications in previous pregnancy such as hypertension, diabetes and preterm labour. In this systematic review and summary meta-analysis of mainly observational data we were unable to adjust for confounding factors. However, it is worth noting that in the majority of studies included, multivariable analysis did not significantly alter findings of univariable analysis. Nevertheless, our findings must be interpreted with caution.
We were unable to analyse results by the indication for cesarean delivery or the category of cesarean delivery—planned (elective) or emergency. Nevertheless, several studies did assess outcomes by classification of cesarean delivery (elective or emergency) or timing of cesarean delivery (pre-labour, intrapartum, or second stage of labour) without significant changes in the ORs of complications [25,27,54,56,58,60,71]. Cesarean delivery rates varied depending on the country where the study was performed and the cohort dates; for example, the [75] study cohort in 1966 had a cesarean delivery rate of 5%. This may affect generalizability of the findings to modern practice, but temporal differences in obstetric practice are unavoidable in studies of long-term complications.
Although previous systematic reviews have assessed individual outcomes [8–12,101,105–109], we have found no other published reviews synthesizing the evidence for all long-term risks and benefits of cesarean delivery relating to mother, baby, and subsequent pregnancies. There is a lack of documented evidence about medium - to long-term outcomes in women and their babies after a planned cesarean delivery or a planned vaginal birth [4]. Therefore, the findings of this review will form a valuable and necessary addition to discussions about mode of delivery and consenting for planned cesarean delivery. Patients may attribute different weight to the outcomes; for example, some might prioritize minimizing the risk of stillbirth in a future pregnancy, while others might prioritize minimizing the risk of respiratory morbidity for their baby. The information included in this review will allow women (and their caregivers) to make more personally relevant decisions.
Although we cannot conclude that cesarean delivery causes certain outcomes, patients and clinicians should be aware that cesarean delivery is associated with long-term risks for the baby and for subsequent pregnancies and a reduced risk of urinary incontinence and pelvic organ prolapse for the mother. The significance that women attribute to these individual risks is likely to vary, but it is imperative that clinicians take care to ensure that women are made aware of any risk that they are likely to attach significance to. Women and clinicians thus should be aware of both the short - and long-term risks and benefits of cesarean delivery and discuss these when deciding on mode of delivery.
If the associations between cesarean delivery and outcomes were known to be causal, the key significant associations in this review could be summarized using ‘numbers needed to treat (NNT) for benefit or harm’. We have calculated the NNT for benefit and harm for each statistically significant outcome from the meta-analyses and displayed this in S7 Table. These are aimed to help put the risks and benefits of cesarean delivery into context and could be used as a basis for a tool to help counselling and consenting for cesarean delivery in the antenatal period, keeping in mind these figures are based on observational data. The estimates suggest that around 17 cesareans would be needed to prevent one case of urinary incontinence (NNT for benefit 17 95% CI 14,22), but for every 1,500 cesareans performed, there would be approximately nine additional cases of childhood asthma (NNT for harm 162 95% CI 107-308), and in subsequent pregnancies, an additional 166 women with subfertility (NNT for harm 9 95% CI 8-12), three women with placenta praevia (NNT for harm 494 95% CI 420, 589), two women with uterine rupture (NNT for harm 538 95% CI 224-1340), 21 miscarriages (NNT for harm 69 95% CI 37-386), and one stillbirth (NNT for harm 1144 95% CI 773-2059).
Conclusion
We have synthesised the evidence for the long-term risks and benefits of cesarean section. This information should help inform discussions about mode of delivery and may facilitate appropriate personalized delivery planning and shared decision-making. Further research into the long-term risks and benefits of cesarean delivery on maternal request will be beneficial. Whilst randomized trials might be the gold standard in this regard, one that addressed all relevant outcomes would have to be so large and with such a long follow-up so as to be likely to be unfeasible.
Supporting Information
Zdroje
1. Betran AP, Ye J, Moller AB, Zhang J, Gulmezoglu AM, Torloni MR. The increasing trend in caesarean section rates: Global, regional and national estimates: 1990–2014. PLoS ONE. 2016;11(2):e0148343. doi: 10.1371/journal.pone.0148343 26849801
2. Gibbons L, Belizan JM, Lauer JA, Betra AP, Merialdi M, Althabe F. The Global Numbers and Costs of Additionally Needed and Unnecessary Caesarean Sections Performed per Year: Overuse as a Barrier to Universal Coverage. World Health Report (2010). Background Paper, 30.
3. Thomas J, Paranjothy S. Royal College of Obstetricians and Gynaecologists Clinical Effectiveness Support Unit. National Sentinel Caesarean Section Audit Report. RCOG Press; 2001.
4. National Institute for Health and Clinical Excellence (2011) Caesarean Section (NICE Clinical Guideline 132). Available at: https://www.nice.org.uk/guidance/CG132 [Accessed 8th January 2018].
5. Hannah ME, Whyte H, Hannah WJ, Hewson S, Amankwah K, Cheng M, et al. Maternal outcomes at 2 years after planned cesarean section versus planned vaginal birth for breech presentation at term: The international randomized Term Breech Trial. Am J Obstet Gynecol. 2004;191(3):917–27. doi: 10.1016/j.ajog.2004.08.004 15467565
6. Whyte H, Hannah ME, Saigal S, Hannah WJ, Hewson S, Amankwah K, et al. Outcomes of children at 2 years after planned cesarean birth versus planned vaginal birth for breech presentation at term: the International Randomized Term Breech Trial. Am J Obstet Gynecol. 2004;191(3):864–71. doi: 10.1016/j.ajog.2004.06.056 15467555.
7. Fenner D. Anal incontinence: relationship to pregnancy, vaginal delivery, and cesarean section. Semin Perinatol. 2006;30(5):261–6. doi: 10.1053/j.semperi.2006.07.006 17011397.
8. Press JZ, Klein MC, Kaczorowski J, Liston RM, von Dadelszen P. Does Cesarean Section Reduce Postpartum Urinary Incontinence? A Systematic Review. BIRTH. 2007;34(3):228–37. doi: 10.1111/j.1523-536X.2007.00175.x 17718873
9. Thavagnanam S, Fleming J, Bromley A, Shields MD, Cardwell CR. A meta-analysis of the association between Caesarean section and childhood asthma. Clin Exp Allergy. 2008;38(4):629–33. doi: 10.1111/j.1365-2222.2007.02780.x 18352976.
10. Bager P, Wohlfahrt J, Westergaard T. Caesarean delivery and risk of atopy and allergic disease: meta-analyses. Clin Exp Allergy. 2008;38(4):634–42. doi: 10.1111/j.1365-2222.2008.02939.x 18266879.
11. Darmasseelane K, Hyde MJ, Santhakumaran S, Gale C, Modi N. Mode of delivery and offspring body mass index, overweight and obesity in adult life: a systematic review and meta-analysis. PLoS ONE. 2014;9(2):e87896. doi: 10.1371/journal.pone.0087896 24586295; PubMed Central PMCID: PMC3935836.
12. Li HT, Zhou YB, Liu JM. The impact of cesarean section on offspring overweight and obesity: a systematic review and meta-analysis. Int J Obes (Lond). 2013;37(7):893–9. doi: 10.1038/ijo.2012.195 23207407.
13. Koplin J, Allen K, Gurrin L, Osborne N, Tang ML, Dharmage S. Is caesarean delivery associated with sensitization to food allergens and IgE-mediated food allergy: a systematic review. Pediatr Allergy Immunol. 2008;19(8):682–7. doi: 10.1111/j.1399-3038.2008.00731.x 19076564.
14. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology—A proposal for reporting. JAMA. 2000;283(15):2008–12. doi: 10.1001/jama.283.15.2008 PubMed PMID: WOS:000086436600037.
15. Scottish Intercollegiate Guidelines Network (2014) Critical Appraisal: Notes and Checklists. Healthcare Improvement Scotland. Available at: http://www.sign.ac.uk/checklists-and-notes.html [Accessed 8th January 2018].
16. Kim SY, Park JE, Lee YJ, Seo HJ, Sheen SS, Hahn S, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013;66(4):408–14. doi: 10.1016/j.jclinepi.2012.09.016 23337781
17. Black M, Bhattacharya S, Philip S, Norman JE, McLernon DJ. Planned Cesarean Delivery at Term and Adverse Outcomes in Childhood Health. JAMA. 2015;314(21):2271–9. doi: 10.1001/jama.2015.16176 PubMed PMID: WOS:000365515700019. 26624826
18. Lin SL, Leung GM, Schooling CM. Mode of delivery and adiposity: Hong Kong's "Children of 1997" birth cohort. Ann Epidemiol. 2013;23(11):693–9. doi: 10.1016/j.annepidem.2013.06.090 PubMed PMID: WOS:000326137000005. 23880154
19. Barros FC, Matijasevich A, Hallal PC, Horta BL, Barros AJ, Menezes AB, et al. Cesarean section and risk of obesity in childhood, adolescence, and early adulthood: evidence from 3 Brazilian birth cohorts. Am J Clin Nutr. 2012;95(2):465–70. doi: 10.3945/ajcn.111.026401 22237058; PubMed Central PMCID: PMC3260073.
20. Abdel-Fattah M, Familusi A, Fielding S, Ford J, Bhattacharya S. Primary and repeat surgical treatment for female pelvic organ prolapse and incontinence in parous women in the UK: a register linkage study. BMJ Open. 2011;1:e000206. doi: 10.1136/bmjopen-2011-000206 22102637
21. Bjelland EK, Owe KM, Pingel R, Kristiansson P, Vangen S, Eberhard-Gran M. Pelvic pain after childbirth: a longitudinal population study. Pain. 2016;157(3):710–6. doi: 10.1097/j.pain.0000000000000427 PubMed PMID: WOS:000378258800022. 26588694
22. Brown SJ, Gartland D, Donath S, MacArthur C. Fecal Incontinence During the First 12 Months Postparum. Obstet Gynecol. 2012;119 : 240–9. doi: 10.1097/AOG.0b013e318242b1f7 22270274
23. Elvander C, Dahlberg J, Andersson G, Cnattingius S. Mode of delivery and the probability of subsequent childbearing: a population-based register study. BJOG. 2015;122(12):1593–600. doi: 10.1111/1471-0528.13021 PubMed PMID: WOS:000363729300032. 25135574
24. Fussing-Clausen C, Geirsson RT, Hansen T, Rasmussen S, Lidegaard O, Hedegaard M. Mode of delivery and subsequent reproductive patterns. A national follow-up study. Acta Obstet Gyn Scan. 2014;93(10):1034–41. doi: 10.1111/aogs.12469 PubMed PMID: WOS:000342582800012. 25138733
25. Gartland D, Donath S, MacArthur C, Brown SJ. The onset, recurrence and associated obstetric risk factors for urinary incontinence in the first 18 months after a first birth: an Australian nulliparous cohort study. BJOG. 2012;119(11):1361–9. doi: 10.1111/j.1471-0528.2012.03437.x 22827735.
26. Gyhagen M, Akervall S, Milsom I. Clustering of pelvic floor disorders 20 years after one vaginal or one cesarean birth. Int Urogynecol J. 2015;26(8):1115–21. doi: 10.1007/s00192-015-2663-3 PubMed PMID: WOS:000361229500005. 25708677
27. Gyhagen M, Bullarbo M, Nielsen TF, Milsom I. Prevalence and risk factors for pelvic organ prolapse 20 years after childbirth: a national cohort study in singleton primiparae after vaginal or caesarean delivery. BJOG. 2013;120(2):152–60. doi: 10.1111/1471-0528.12020 23121158.
28. Gyhagen M, Bullarbo M, Nielsen TF, Milsom I. The prevalence of urinary incontinence 20 years after childbirth: a national cohort study in singleton primiparae after vaginal or caesarean delivery. BJOG. 2013;120(2):144–51. doi: 10.1111/j.1471-0528.2012.03301.x 22413831.
29. Hall MH, Campbell D, Fraser C, Lemon J. Mode of delivery and future fertility. BJOG. 1989;96 : 1297–303. 2611168
30. Huttly SRA, Barros FC, Victora CG, Lombardi C, Vaughan JP. Subsequent pregnancies: Who has them and who wants them? Observations from an urban center in Southern Brazil. Rev Saude Publica. 1990;24(3):212–6.
31. Kjerulff KH, Zhu J, Weisman CS, Ananth CV. First birth Caesarean section and subsequent fertility: a population-based study in the USA, 2000–2008. Hum Reprod. 2013;28(12):3349–57. doi: 10.1093/humrep/det343 24021550; PubMed Central PMCID: PMC3829579.
32. Liang CC, Wu MP, Lin SJ, Lin YJ, Chang SD, Wang HH. Clinical impact of and contributing factors to urinary incontinence in women 5 years after first delivery. Int Urogynecol J. 2013;24(1):99–104. doi: 10.1007/s00192-012-1855-3 22777581.
33. MacArthur C, Glazener C, Lancashire R, Herbison P, Wilson D. Exclusive caesarean section delivery and subsequent urinary and faecal incontinence: a 12-year longitudinal study. BJOG. 2011;118(8):1001–7. doi: 10.1111/j.1471-0528.2011.02964.x 21477171
34. McDonald EA, Gartland D, Small R, Brown SJ. Dyspareunia and Childbirth: A Prospective Cohort Study. Obstet Gynecol Surv. 2015;70(5):319–20. PubMed PMID: WOS:000354725300013.
35. Mollison J, Porter M, Campbell D, Bhattacharya S. Primary mode of delivery and subsequent pregnancy. BJOG. 2005;112(8):1061–5. doi: 10.1111/j.1471-0528.2005.00651.x 16045518.
36. Murphy DJ, Stirrat GM, Heron J, Team AS. The relationship between Caesarean section and subfertility in a population-based sample of 14 541 pregnancies. Hum Reprod. 2002;17(7):1914–7. 12093860
37. O'Neill SM, Khashan AS, Henriksen TB, Kenny LC, Kearney PM, Mortensen PB, et al. Does a Caesarean section increase the time to a second live birth? A register-based cohort study. Hum Reprod. 2014;29(11):2560–8. doi: 10.1093/humrep/deu217 PubMed PMID: WOS:000344675700026. 25217610
38. Rortveit G, Daltveit AK, Hannestad YS, Hunskaar S, Study NE. Urinary incontinence after vaginal delivery or cesarean section. N Engl J Med. 2003;348(10):900–7. doi: 10.1056/NEJMoa021788 PubMed PMID: WOS:000181341100005. 12621134
39. Schytt E, Linkmark G, Waldenstrom U. Symptoms of stress incontinence 1 year after childbirth: prevalence and predictors in a national Swedish sample. Acta Obstet Gynecol Scand. 2004;83 : 928–36. doi: 10.1111/j.0001-6349.2004.00431.x 15453888
40. Smith GC, Wood AM, Pell JP, Dobbie R. First cesarean birth and subsequent fertility. Fertil Steril. 2006;85(1):90–5. Epub 2006/01/18. doi: S0015-0282(05)03433-3 [pii] doi: 10.1016/j.fertnstert.2005.07.1289 16412736.
41. Tollanes MC, Melve KK, Irgens LM, Skjaerven R. Reduced Fertility After Cesarean Delivery. Obstet Gynecol. 2007;110 : 1256–63. doi: 10.1097/01.AOG.0000292089.18717.9f 18055718
42. Woolhouse H, Perlen S, Gartland D, Brown SJ. Physical Health and Recovery in the First 18 Months Postpartum: Does Cesarean Section Reduce Long-Term Morbidity? BIRTH. 2012;39(3):221–9. doi: 10.1111/j.1523-536X.2012.00551.x 23281904
43. MacArthur C, Wilson D, Herbison P, Lancashire RJ, Hagen S, Toozs-Hobson P, et al. Urinary incontinence persisting after childbirth: extent, delivery history, and effects in a 12-year longitudinal cohort study. BJOG. 2016;123(6):1022–9. doi: 10.1111/1471-0528.13395 PubMed PMID: WOS:000374705100031. 25846816
44. Ajslev TA, Andersen CS, Gamborg M, Sorensen TI, Jess T. Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int J Obes. 2011;35(4):522–9. doi: 10.1038/ijo.2011.27 21386800.
45. Almqvist C, Cnattingius S, Lichtenstein P, Lundholm C. The impact of birth mode of delivery on childhood asthma and allergic diseases—a sibling study. Clin Exp Allergy. 2012;42(9):1369–76. doi: 10.1111/j.1365-2222.2012.04021.x 22925323; PubMed Central PMCID: PMC3564396.
46. Andersen V, Erichsen R, Froslev T, Sorensen HT, Ehrenstein V. Differential risk of ulcerative colitis and Crohn's disease among boys and girls after cesarean delivery. Inflamm Bowel Dis. 2013;19(1):E8–E10. doi: 10.1002/ibd.22841 22147542.
47. Bager P, Simonsen J, Nielsen NM, Frisch M. Cesarean section and offspringʼs risk of inflammatory bowel disease: A national cohort study. Inflamm Bowel Dis. 2012;18(5):857–62. doi: 10.1002/ibd.21805 21739532
48. Changzheng Y, Gaskins AJ, Blaine AI, Zhang C, Gillman MW, Missmer SA, et al. Association Between Cesarean Birth and Risk of Obesity in Offspring in Childhood, Adolescence, and Early Adulthood. JAMA Pediatrics. 2016;170(11):e162385. doi: 10.1001/jamapediatrics.2016.2385 27599167
49. Davidson R, Roberts SE, Wotton CJ, Goldacre MJ. Influence of maternal and perinatal factors on subsequent hospitalisation for asthma in children: evidence from the Oxford record linkage study. BMC Pulm Med. 2010;10. doi: Artn 14 doi: 10.1186/1471-2466-10-10 PubMed PMID: WOS:000208592700014.
50. Eggesbo M, Botten G, Stigum H, Nafstad P, Magnus P. Is delivery by cesarean section a risk factor for food allergy? J Allergy Clin Immunol. 2003;112(2):420–6. doi: 10.1067/mai.2003.1610 PubMed PMID: WOS:000184650600029. 12897751
51. Eggesbo M, Botten G, Stigum H, Samuelsen SO, Brunekreef B, Magnus P. Cesarean delivery and cow milk allergy/intolerance. Allergy. 2005;60(9):1172–3. doi: 10.1111/j.1398-9995.2005.00857.x 16076303.
52. Goldani HA, Bettiol H, Barbieri MA, Silva AA, Agranonik M, Morais MB, et al. Cesarean delivery is associated with an increased risk of obesity in adulthood in a Brazilian birth cohort study. Am J Clin Nutr. 2011;93(6):1344–7. doi: 10.3945/ajcn.110.010033 21508088.
53. Goldani MZ, Barbieri MA, Moura da Silva AA, Pereria Gutierrez MR, Bettiol H, Goldani HA. Cesarean section and increased body mass index in school children: two cohort studies from distinct socioeconomic background areas in Brazil. Nutr J. 2013;12.
54. Huh SY, Rifas-Shiman SL, Zera CA, Rich Edwards JW, Oken E, Weiss ST, et al. Delivery by caesarean section and risk of obesity in preschool age children: a prosepective cohort study. Arch Dis Child. 2012;97(610–616):610.
55. Kero J, Gissler M, Minna-Maija G, Kero P, Koskinen P, Hemminki E, et al. Mode of Delivery and Asthma—Is There a Connection? Pediatr Res. 2002;52(1):6–11. doi: 10.1203/00006450-200207000-00004 12084840
56. Li HT, Ye R, Pei L, Ren A, Zheng X, Liu JM. Caesarean delivery, caesarean delivery on maternal request and childhood overweight: a Chinese birth cohort study of 181 380 children. Pediatr Obes. 2014;9(1). Epub 2013 Mar 19.
57. Lin SL, Schooling CM, Leung GM. Mode of Delivery and Adiposity: Hong Kong's "Children of 1997" Birth Cohort. Am J Epidemiol. 2013;177:S8–S. PubMed PMID: WOS:000319870300031.
58. Magnus MC, Haberg SE, Stigum H, Nafstad P, London SJ, Vangen S, et al. Delivery by Cesarean section and early childhood respiratory symptoms and disorders: the Norwegian mother and child cohort study. Am J Epidemiol. 2011;174(11):1275–85. Epub 2011/11/01. doi: 10.1093/aje/kwr242 22038100; PubMed Central PMCID: PMC3254156.
59. Maitra A, Sherriff A, Strachan D, Team AS, Henderson J. Mode of delivery is not associated with asthma or atopy in childhood. Clin Exp Allergy. 2004;34 : 1349–55. doi: 10.1111/j.1365-2222.2004.02048.x 15347366
60. Mamun AA, Sutharsan R, O'Callaghan M, Williams G, Najman J, McIntyre HD, et al. Cesarean delivery and the long-term risk of offspring obesity. Obstet Gynecol. 2013;122(6):1176–83. doi: 10.1097/AOG.0000000000000016 24201680.
61. McKeever TM, Lewis SA, Smith C, Hubbard R. Mode of delivery and risk of developing allergic disease. J Allergy Clin Immunol. 2002;109(5):800–2. doi: 10.1067/mai.2002.124046 11994703
62. Menezes AM, Hallal PC, Matijasevich AM, Barros AJ, Horta BL, Araujo CL, et al. Caesarean sections and risk of wheezing in childhood and adolescence: data from two birth cohort studies in Brazil. Clin Exp Allergy. 2011;41(2):218–23. doi: 10.1111/j.1365-2222.2010.03611.x 20840395; PubMed Central PMCID: PMC3505367.
63. Negele K, Heinrick J, Borte M, von Berg A, Schaaf B, Lehmann I, et al. Mode of delivery and development of atopic disease during the first 2 years of life. Pediatr Allergy Immunol. 2004;15 : 48–54. 14998382
64. Pei Z, Heinrich J, Fuertes E, Flexeder C, Hoffmann B, Lehmann I, et al. Cesarean delivery and risk of childhood obesity. J Pediatr. 2014;164(5):1068–73 e2. doi: 10.1016/j.jpeds.2013.12.044 24508442.
65. Ponsonby AL, Catto-Smith AG, Pezic A, Dupuis S, Halliday J, Cameron D, et al. Association between early-life factors and risk of child-onset Crohn's disease among Victorian children born 1983–1998: a birth cohort study. Inflamm Bowel Dis. 2009;15(6):858–66. doi: 10.1002/ibd.20842 19107784.
66. Schooling CM, Mesquita DN, Barbieri MA, Goldani HAS, Cardoso VC, Goldani MZ, et al. Cesarean Section Is Associated with Increased Peripheral and Central Adiposity in Young Adulthood: Cohort Study. PLoS ONE. 2013;8(6):e66827. doi: 10.1371/journal.pone.0066827 23826150
67. Pyrhonen K, Nayha S, Hiltunen L, Laara E. Caesarean section and allergic manifestations: insufficient evidence of association found in population-based study of children aged 1 to 4 years. Acta Paediatr. 2013;102(10):982–9. doi: 10.1111/apa.12342 23826787.
68. Roberts SE, Wotton CJ, Williams JG, Griffith M, Goldacre MJ. Perinatal and early life risk factors for inflammatory bowel disease. World J Gastroenterol. 2011;17(6):743–9. doi: 10.3748/wjg.v17.i6.743 21390144; PubMed Central PMCID: PMC3042652.
69. Roduit C, Scholtens S, de Jongste JC, Wijga AH, Gerritsen J, Postma DS, et al. Ashtma at 8 years of age in children born by caesarean section. Thorax. 2009;64 : 107–13. doi: 10.1136/thx.2008.100875 19052046
70. Steur M, Smit HA, Schipper CMA, Scholtens S, Kerkhof M, de Jongste JC, et al. Predicting the risk of newborn children to become overweight later in childhood: the PIAMA birth cohort study. Int J Pediatr Obes. 2011;6:e170–e8. doi: 10.3109/17477166.2010.519389 20883125
71. Tollanes MC, Moster D, Daltveit AK, Irgens LM. Cesarean section and risk of severe childhood asthma: a population-based cohort study. J Pediatr. 2008;153(1):112–6. doi: 10.1016/j.jpeds.2008.01.029 18571547.
72. van Berkel AC, den Dekker HT, Jaddoe VWV, Reiss IK, Gaillard R, Hofman A, et al. Mode of delivery and childhood fractional exhaled nitric oxide, interrupter resistance and asthma: the Generation R study. Pediatr Allergy Immunol. 2015;26(4):330–6. doi: 10.1111/pai.12385 PubMed PMID: WOS:000355149800005. 25845270
73. van Nimwegen FA, Penders J, Stobberingh EE, Postma DS, Koppelman GH, Kerkhof M, et al. Mode and place of delivery, gastrointestinal microbiota, and their influence on asthma and atopy. J Allergy Clin Immunol. 2011;128(5):948–55 e1-3. doi: 10.1016/j.jaci.2011.07.027 21872915.
74. Werner A, Ramlau-Hansen CH, Jeppesen SK, Thulstrup AM, Olsen J. Caesarean delivery and risk of developing asthma in the offspring. Acta Paediatr. 2007;96(4):595–6. doi: 10.1111/j.1651-2227.2006.00150.x 17274805.
75. Xu B, Pekkanen J, Hartikainen AL, Jarvelin MR. Caesarean section and risk of asthma and allergy in adulthood. J Allergy Clin Immunol. 2001;107(4):732–3. doi: 10.1067/mai.2001.113048 11295666.
76. Xu B, Pekkanen J, Jarvelin MR. Obstetric Complications and Asthma in Childhood. J Asthma. 2000;37(7):589–94. 11059526
77. Eggesbø M, Botten G, Stigum H, Nafstad P, Magnus P. Is delivery by cesarean section a risk factor for food allergy? J Allergy Clin Immunol. 2003;112(2):420–6. doi: 10.1067/mai.2003.1610 12897751
78. Bowman ZS SK, Silver RM. Cesarean Delivery and Risk for Subsequent Ectopic Pregnancy. Am J Perinatol. 2015;32(9):815–20. doi: 10.1055/s-0034-1543952 25607224
79. Daltveit AK, Tollanes MC, Pihlstrom H, Irgens LM. Cesarean Delivery and Subsequent Pregnancies. Obstet Gynecol. 2008;111 : 1327–34. doi: 10.1097/AOG.0b013e3181744110 18515516
80. Downes KL HS, Sjaarda LA, et al. Previous prelabor or intrapartum cesarean delivery and risk of placenta previa. Am J Obstet Gynecol. 2015;212(5):669 e1-6. doi: 10.1016/j.ajog.2015.01.004 25576818
81. Galyean AM, Lagrew DC, Bush MC, Kurtzman JT. Previous cesarean section and the risk of postpartum maternal complications and adverse neonatal outcomes in future pregnancies. J Perinatol. 2009;29 : 726–30. doi: 10.1038/jp.2009.108 19626026
82. Getahun D, Oyelese Y, Salihu HM, Ananth CV. Previous cesarean delivery and risks of placenta previa and placental abruption. Obstet Gynecol. 2006;107(4):771–8. doi: 10.1097/01.AOG.0000206182.63788.80 16582111
83. Gray R, Quigley MA, Hockley C, Kurinczuk JJ, Goldacre M, Brocklehurst P. Caesarean delivery and risk of stillbirth in subsequent pregnancy: a retrospective cohort study in an English population. BJOG. 2007;114(3):264–70. doi: 10.1111/j.1471-0528.2006.01249.x 17261119.
84. Gurol-Urganci I, Cromwell DA, Edozien LC, Smith GC, Onwere C, Mahmood TA, et al. Risk of placenta previa in second birth after first birth cesarean section: a population-based study and meta-analysis. BMC Pregnancy Childbirth. 2011;11 : 95. doi: 10.1186/1471-2393-11-95 22103697; PubMed Central PMCID: PMC3247856.
85. Jackson S, Fleege L, Fridman M, Gregory K, Zelop C, Olsen J. Morbidity following primary cesarean delivery in the Danish National Birth Cohort. Am J Obstet Gynecol. 2012;206(2):139 e1-5. doi: 10.1016/j.ajog.2011.09.023 22051815.
86. Kennare R, Tucker G, Heard A, Chan A. Risks of Adverse Outcomes in the Next Birth After a First Cesarean Delivery. Obstet Gynecol. 2007;109 : 270–6. doi: 10.1097/01.AOG.0000250469.23047.73 17267823
87. Lydon-Rochelle M, Holt VL, Easterling TR, Martin DP. First-Birth Cesarean and Placental Abruption or Previa at Second Birth. Obstet Gynecol. 2001;97 : 765–9. 11339931
88. Rasmussen S, Albrechtsen S, Dalaker K. Obstetric history and the risk of placenta previa. Acta Obstet Gynecol Scand. 2000;79 : 502–7. 10857876
89. Yang Q, Wen SW, Oppenheimer L, Chen XK, Black D, Gao J, et al. Association of caesarean delivery for first birth with placenta praevia and placental abruption in second pregnancy. BJOG. 2007;114(5):609–13. doi: 10.1111/j.1471-0528.2007.01295.x 17355267.
90. Hemminki E, Shelley J, Gissler M. Mode of delivery and problems in subsequent births: a register-based study from Finland. Am J Obstet Gynecol. 2005;193(1):169–77. doi: 10.1016/j.ajog.2004.11.007 16021075.
91. Huang X, Lei J, Tan H, Walker M, Zhou J, Wen SW. Cesarean delivery for first pregnancy and neonatal morbidity and mortality in second pregnancy. Eur J Obstet Gynecol Reprod Biol. 2011;158 : 204–8.
92. Moraitis AA, Oliver-Williams C, Wood AM, Fleming M, Pell JP, Smith GCS. Previous caesarean delivery and the risk of unexplained stillbirth: retrospective cohort study and meta-analysis. BJOG. 2015;122(11):1467–74. doi: 10.1111/1471-0528.13461 PubMed PMID: WOS:000362752100009. 26033155
93. Osborne C, Ecker JL, Gauvreau K, Lieberman E. First birth cesarean and risk of antepartum fetal death in a subsequent pregnancy. J Midwifery Womens Health. 2012;57(1):12–7. doi: 10.1111/j.1542-2011.2011.00142.x 22251907.
94. Salihu HM, Bowen CM, Wilson RE, Marty PJ. The impact of previous cesarean section on the success of future fetal programming pattern. Arch Gynecol Obstet. 2011;284(2):319–26. doi: 10.1007/s00404-010-1665-0 20821225.
95. Salihu HM, Sharma PP, Kristensen S, Blot C, Alio AP, Ananth CV, et al. Risk of Stillbirth Following a Cesarean Delivery. Obstet Gynecol. 2006;107 : 383–90. doi: 10.1097/01.AOG.0000195103.46999.32 16449128
96. Smith GC, Pell JP, Dobbie R. Caesarean section and risk of unexplained stillbirth in subsequent pregnancy. Lancet. 2003;362(9398):1779–84. Epub 2003/12/05. doi: S0140673603148969 [pii]. 14654315.
97. Taylor LK, Simpson JM, Roberts CL, Olive EC, Henderson-Smart DJ. Risk of complications in a second pregnancy following caesarean section in the first pregnancy: a population-based study. Med J Aust. 2005;183 : 515–9. 16296964
98. Wood SL, Chen S, Ross S, Sauve R. The risk of unexplained antepartum stillbirth in second pregnancies following caesarean section in the first pregnancy. BJOG. 2008;115(6):726–31. doi: 10.1111/j.1471-0528.2008.01705.x 18410656
99. Adlercreutz EH, Svensson J, Hansen D, Buschard K, Lernmark A, Mortensen HB, et al. Prevalence of celiac disease autoimmunity in children with type 1 diabetes: regional variations across the Oresund strait between Denmark and southernmost Sweden. Pediatr Diabetes. 2015;16(7):504–9. doi: 10.1111/pedi.12200 PubMed PMID: WOS:000362550400004. 25131687
100. Algert CS, McElduff A, Morris JM, Roberts CL. Perinatal risk factors for early onset of Type 1 diabetes in a 2000–2005 birth cohort. Diabetic Med. 2009;26(12):1193–7. doi: 10.1111/j.1464-5491.2009.02878.x PubMed PMID: WOS:000272161900002. 20002469
101. Cardwell CR, Carson DJ, Patterson CC. Parental age at delivery, birth order, birth weight and gestational age are associated with the risk of childhood Type 1 diabetes: a UK regional retrospective cohort study. Diabetic Med. 2005;22(2):200–6. doi: 10.1111/j.1464-5491.2005.01369.x PubMed PMID: WOS:000226475700014. 15660739
102. Stene LC, Magnus P, Lie RT, Sovik O, Joner G, Norwegian Childhood Diabetes Study G. No association between preeclampsia or cesarean section and incidence of type 1 diabetes among children: a large, population-based cohort study. Pediatr Res. 2003;54(4):487–90. doi: 10.1203/01.PDR.0000081301.25600.5D 12815116.
103. Roberts SE, Williams JG, Meddings D, Davidson R, Goldacre MJ. Perinatal risk factors and coeliac disease in children and young adults: a record linkage study. Aliment Pharm Ther. 2009;29(2):222–31. doi: 10.1111/j.1365-2036.2008.03871.x PubMed PMID: WOS:000261781900009. 18945253
104. Asztalos EV, Hannah ME, Hutton EK, Willan AR, Allen AC, Armson BA, et al. Twin BIrth Study: 2-year neurodevelopmental follow-up of the randomized trial of planned cesarean or planned vaginal delivery for twin pregnancy. Am J Obstet Gynecol. 2016;214(3):371. doi: 10.1016/j.ajog.2015.12.051 26830380
105. Ananth CV, Smulian JC, Vintzileos AM. The association of placenta previa with history of cesarean delivery and abortion: a metaanalysis. Am J Obstet Gynecol. 1997;177(5):1071–8. 9396896.
106. Gurol-Urganci I, Bou-Antoun S, Lim CP, Cromwell DA, Mahmood TA, Templeton A, et al. Impact of Caesarean section on subsequent fertility: a systematic review and meta-analysis. Hum Reprod. 2013;28(7):1943–52. doi: 10.1093/humrep/det130 23644593.
107. O'Neill SM, Kearney PM, Kenny LC, Henriksen TB, Lutomski JE, Greene RA, et al. Caesarean delivery and subsequent pregnancy interval: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2013;13 : 165. doi: 10.1186/1471-2393-13-165 23981569; PubMed Central PMCID: PMCPMC3765853.
108. O'Neill SM, Kearney PM, Kenny LC, Khashan AS, Henriksen TB, Lutomski JE, et al. Caesarean delivery and subsequent stillbirth or miscarriage: systematic review and meta-analysis. PLoS ONE. 2013;8(1):e54588. doi: 10.1371/journal.pone.0054588 23372739; PubMed Central PMCID: PMCPMC3553078.
109. O'Neill SM, Khashan AS, Kenny LC, Greene RA, Henriksen TB, Lutomski JE, et al. Caesarean section and subsequent ectopic pregnancy: a systematic review and meta-analysis. BJOG. 2013;120(6):671–80. doi: 10.1111/1471-0528.12165 23398899.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2018 Číslo 1- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- What about drinking is associated with shorter life in poorer people?
- Life course socioeconomic position, alcohol drinking patterns in midlife, and cardiovascular mortality: Analysis of Norwegian population-based health surveys
- Pelvic inflammatory disease risk following negative results from chlamydia nucleic acid amplification tests (NAATs) versus non-NAATs in Denmark: A retrospective cohort
- Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: A population-based cohort study
- Sexually transmitted infections in the era of antiretroviral-based HIV prevention: Priorities for discovery research, implementation science, and community involvement
- Traumatic brain injury and the risk of dementia diagnosis: A nationwide cohort study
- Association between intake of less-healthy foods defined by the United Kingdom's nutrient profile model and cardiovascular disease: A population-based cohort study
- Progression of the first stage of spontaneous labour: A prospective cohort study in two sub-Saharan African countries
- The cost-effectiveness of alternative vaccination strategies for polyvalent meningococcal vaccines in Burkina Faso: A transmission dynamic modeling study
- PD-L1 checkpoint inhibition and anti-CTLA-4 whole tumor cell vaccination counter adaptive immune resistance: A mouse neuroblastoma model that mimics human disease
- Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: A Phase 1 open-label clinical trial in healthy adults
- Immune-related genetic enrichment in frontotemporal dementia: An analysis of genome-wide association studies
- Long-term trends in mortality and AIDS-defining events after combination ART initiation among children and adolescents with perinatal HIV infection in 17 middle- and high-income countries in Europe and Thailand: A cohort study
- What’s coming for health science and policy in 2018? Global experts look ahead in their field
- Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: A targeted metabolomics study
- Estimated mortality on HIV treatment among active patients and patients lost to follow-up in 4 provinces of Zambia: Findings from a multistage sampling-based survey
- From macro- to microfactors in health: Social science approaches in research on sexually transmitted infections
- The WHO 2016 verbal autopsy instrument: An international standard suitable for automated analysis by InterVA, InSilicoVA, and Tariff 2.0
- Long-term risks and benefits associated with cesarean delivery for mother, baby, and subsequent pregnancies: Systematic review and meta-analysis
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Traumatic brain injury and the risk of dementia diagnosis: A nationwide cohort study
- Pelvic inflammatory disease risk following negative results from chlamydia nucleic acid amplification tests (NAATs) versus non-NAATs in Denmark: A retrospective cohort
- PD-L1 checkpoint inhibition and anti-CTLA-4 whole tumor cell vaccination counter adaptive immune resistance: A mouse neuroblastoma model that mimics human disease
- Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: A Phase 1 open-label clinical trial in healthy adults
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



