-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamapiRNAs Are Associated with Diverse Transgenerational Effects on Gene and Transposon Expression in a Hybrid Dysgenic Syndrome of .
Transposable elements (TEs) are selfish elements that copy themselves. More than half of the human genome is comprised of such elements. Studies in the fruit flies Drosophila melanogaster and D. virilis have been important in demonstrating a role for RNA silencing by PIWI-interacting RNAs (piRNAs) in protecting the genome against these harmful elements. These small RNAs are capable of recognizing TE mRNAs and mediating their destruction. They are also transmitted by the female germline to offspring in order to maintain a stable genome across generations. When males carrying a particular TE family are crossed with females lacking the element, the mother is unable to provide genome defense via complementary piRNAs that target the element. This leads to excess TE activation in the germline and sterility, a phenomenon known as hybrid dysgenesis. In this article we characterize the genomic landscape of TE destabilization that occurs in dysgenic crosses of D. virilis. We demonstrate that this mobilization is associated with an increased level of germline TE expression that persists through adulthood. In addition, we find that TE activation is associated with diverse effects on normal gene expression that are also mediated by piRNAs.
Published in the journal: . PLoS Genet 11(8): e32767. doi:10.1371/journal.pgen.1005332
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005332Summary
Transposable elements (TEs) are selfish elements that copy themselves. More than half of the human genome is comprised of such elements. Studies in the fruit flies Drosophila melanogaster and D. virilis have been important in demonstrating a role for RNA silencing by PIWI-interacting RNAs (piRNAs) in protecting the genome against these harmful elements. These small RNAs are capable of recognizing TE mRNAs and mediating their destruction. They are also transmitted by the female germline to offspring in order to maintain a stable genome across generations. When males carrying a particular TE family are crossed with females lacking the element, the mother is unable to provide genome defense via complementary piRNAs that target the element. This leads to excess TE activation in the germline and sterility, a phenomenon known as hybrid dysgenesis. In this article we characterize the genomic landscape of TE destabilization that occurs in dysgenic crosses of D. virilis. We demonstrate that this mobilization is associated with an increased level of germline TE expression that persists through adulthood. In addition, we find that TE activation is associated with diverse effects on normal gene expression that are also mediated by piRNAs.
Introduction
In sexually reproducing species, two unique haploid genomes join together in syngamy to establish each generation. This mixing of genomes introduces potentially advantageous variation under changing environmental conditions, but also provides a condition ripe for exploitation by selfish elements [1]. Because syngamy can introduce selfish elements to new genomes and recombination can separate selfish elements from their harmful consequences, selfish elements such as transposable elements (TEs) can proliferate [2,3]. This is exemplified by the P element in Drosophila melanogaster. Through a likely horizontal transfer event from the distant species D. willistoni, the P element invaded D. melanogaster less than 100 years ago and is now found in D. melanogaster world-wide [4,5].
Because TEs can be harmful and also drive a rapid accumulation of differences between species, they have been proposed to contribute to reproductive isolation. While their proliferative nature makes it very unlikely that TEs are drivers of speciation itself [6,7], TE misregulation has been observed in a variety of interspecific hybrids. For example, increased TE expression is observed in malformed backcrosses between recently diverged species of lake whitefish [8]. Similar observations have been made in species ranging from Arabidopsis [9] to wallaby [10,11]. Studies in Drosophila using interspecific crosses have been especially important for our understanding of TE control in hybrids. The results so far have been idiosyncratic. Interspecific hybrids between closely related members of the affinis, simulans, virilis and pseudoobscura groups of Drosophila show little evidence for increased transposition [6,7,12]. In contrast, increased transposition is observed in crosses between D. buzzatii and D. koepferae [13,14]. In the latter case, the increased rate of TE movement has been attributed to a form of genomic stress, though the nature of this stress is not clear. Additionally, interspecific hybrids between D. simulans and D. melanogaster (which are more distantly related compared to those in previous crosses examining this question [6,7]) do show increased expression of TEs [15] and this is attributed to adaptive divergence in components of the TE regulatory machinery. Since species may differ significantly both in TE profile and regulatory machinery protein function, it is challenging to determine how divergence in TE profile alone contributes to TE activation in interspecific hybrids.
For this reason, intraspecific syndromes of hybrid dysgenesis provide critical insight into the role that divergence in TE profile can play in determining TE activity across generations. Hybrid dysgenesis is defined as a syndrome of hybrid sterility [16,17] and germline damage that occurs in intraspecific crosses when the male carries one or more TE families absent in the female [18–20]. The dysgenesis phenomenon in Drosophila has provided crucial insight into mechanisms of host genome defense by small RNAs. This is because activation of TEs inherited solely through the Drosophila male germline can be explained by the fact that the maternal germline is the primary agent of transgenerational TE repression via PIWI-interacting RNAs (piRNAs) maternally loaded into the egg [21].
piRNAs are 23–30 nt RNAs found in complex with PIWI proteins and they play a crucial role in maintaining genome integrity via the repression of TEs. Many piRNAs are derived from TE fragments residing in distinct genomic regions known as piRNA clusters [22–24]. Anti-sense TE transcripts derived from these clusters are processed into piRNAs and, in complex with PIWI proteins, serve as guides to target resident TE transcripts for PIWI-mediated 'slicing' [25]. This system serves as a mechanism of genome defense because the proliferative nature of TEs can be inherently recognized by their tendency to transpose into piRNA clusters, whereby they serve as guides to recognize mRNAs of the same TE family. In the absence of the maternally provisioned piRNAs that target TE mRNAs for PIWI-mediated slicing, paternally inherited TEs become activated in the progeny germline. This has been demonstrated for the P-M and I-R systems of hybrid dysgenesis in D. melanogaster [24,26–28]. In the P-M system, P strains, but not M strains, carry active copies of the DNA transposon known as the P element. In the I-R system, I (Inducer) strains, but not R (Reactive) strains, carry active copies of the non-LTR retrotransposon I element. Dysgenesis arises when I or P strain males are mated, respectively, with R or M strain females lacking such elements. These females are unable to maternally provision matching piRNAs that target the activating element.
The P-M and I-R systems of dysgenesis represent cases in which the TE profile differs between strains with respect to only one family of inducing elements. In contrast, a third syndrome of hybrid dysgenesis in D. virilis represents a more complicated form of dysgenesis that appears to be caused by the mass action of multiple active elements abundant in one strain, but not another. Elements that likely contribute to this syndrome were first identified through direct analysis of induced lesions identified in the offspring of F1 progeny that escaped sterility from the dysgenic cross. The major driver of dysgenesis appears to be the Penelope element, the founding member of a clade of retroelements designated Penelope-like elements that are distinct from non-LTR and LTR retroelements [29]. Active copies of Penelope are abundant in the inducer strain (Strain 160) and only degenerate copies are present in the M/R-like reactive strain (Strain 9) [30]. Furthermore, expression of the Penelope element is elevated in the ovaries and testes of F1 dysgenic progeny that have escaped ablation of the gonads [31]. In addition to the Penelope element, three other elements (Helena, a non-LTR; Paris and Polyphemus, both DNA transposons [32,33]) are also more abundant in the 160 inducer strain, and these likely contribute to dysgenesis. A complex mode of hybrid dysgenesis, driven jointly by multiple elements, is supported by the fact that females of some "neutral" strains—capable of preventing dysgenesis when crossed with inducer males but also incapable of induction [34]–lack Penelope piRNAs in the their germline. If Penelope is the sole cause of paternal induction, it is difficult to explain how such strains could prevent induction when the female germline lacks Penelope piRNAs.
In light of this complexity, a second model for hybrid dysgenesis not directly driven by transposable elements in D. virilis has been proposed. A previous study showed that not only do inducer and reactive strains differ with respect to TE abundance, they also differ with respect to piRNA cluster activity [35]. In particular, small RNA sequencing in these two strains demonstrated that telomeric regions of the inducer strain exhibit uniquely strong piRNA cluster activity. Differences in telomeric cluster activity were proposed as potentially causative of dysgenesis. Here, we directly test this hypothesis by genetically assessing the contribution of inducer strain telomeres to hybrid sterility.
Because the 160 inducer strain and the 9 reactive strain of D. virilis differ with respect to multiple elements, the dysgenic syndrome in D. virilis may perhaps be more similar to that observed between species with respect to TE profiles, but with minimal divergence in protein coding function since it arises from an intraspecific cross. This syndrome may be considered an intermediate state between the P-M and I-R models and crosses between entirely different species that differ significantly at the genic level as well. Therefore, the dysgenic syndrome in D. virilis serves as a useful model for understanding the consequences of accumulating differences in TE profile between populations.
A fundamental question is how TE activation in dysgenic crosses influences the entire genomic TE landscape. Early studies of P element hybrid dysgenesis in D. melanogaster indicated downstream activation of additional TEs [36], but this interpretation was soon called into doubt [37]. Nonetheless, the syndrome of hybrid dysgenesis in D. virilis provides strong support for cascading germline activation of TEs because multiple TEs were found to transpose in the germline of dysgenic progeny [31,38]. While different TE families may contribute to the initial induction of dysgenesis in D. virilis, germline co-mobilization has been demonstrated by the transposition of TEs that are evenly distributed between the two strains, in contrast to Penelope, Helena and Paris, which are more abundant in inducer strain 160 [32,39,40]. Significantly, a recent study of the P element system indicates that the previous conclusion of no co-mobilization may have been premature [28]. In the face of P element activation, DNA damage can perturb piRNA biogenesis and this defective piRNA biogenesis is presumed to drive the mobilization of additional TEs. Thus, global TE mobilization may also be observed in syndromes of hybrid dysgenesis that are driven by a single element. Whether a similar mechanism caused by DNA damage explains co-mobilization in the D. virilis system is unknown.
To fully understand the mechanisms underlying TE activation in dysgenic crosses, the developmental context must be considered since co-mobilization may be induced at any point in the developing or aging germline. A critical feature of the P-M system is that the germline crisis ameliorates with age. In particular, as flies age to 21 days, fertility is partially restored and piRNA levels that target the P element are restored to the same level as non-dysgenic reciprocal females. Thus, even though P element derived piRNAs are not maternally inherited, de novo piRNA production from paternally inherited P insertions is evident. This de novo piRNA production also coincides with restored silencing of P element mRNA. Rescue of germline crisis is also proposed to be enhanced by movement of other elements into piRNA clusters [28].
Here we use the unique system of hybrid dysgenesis in D. virilis to define the landscape of TE expression that coincides with the initial activation of multiple TE families. Because analysis of piRNA production and TE expression can be confounded in atrophied gonads, we focus solely on germline tissues that have escaped complete gonadal atrophy. Specifically, we examine piRNA and TE expression in 0–2 hour old embryos laid by F1 females from non-dysgenic crosses, and F1 escaper females of the dysgenic cross. This represents an endpoint of the dysgenic crisis and also provides insight into how the effects of hybrid dysgenesis in females that escape sterility can be passed on to further generations. In contrast to the P-M system, which may resolve within the germline as flies age [28], the effects of dysgenesis on TE expression in the D. virilis system persist through adulthood. Because this occurs within an intraspecific cross, increased levels of persisting TE expression are not explained by divergence in the piRNA machinery.
By comparing 0–2 hour old embryos laid by genetically identical females derived from dysgenic versus non-dysgenic crosses, we show that germline activation of TEs is driven by a multi-layered mechanism. Diverse elements are activated corresponding to TE copy number asymmetry between strains but there is also corresponding activation of some TEs that are evenly distributed between strains. This state of chronic increased TE expression is maintained as flies age, suggesting a different mechanism underlying co-mobilization compared to the P-M system. Interestingly, increased and persistent TE expression in the germline of females of the dysgenic cross coincides with a shift in piRNA pools. This shift in piRNA pools is associated with increased abundance of piRNAs that target genes outside of piRNA clusters, leading to significant effects on non-TE gene expression. Finally, differences in the TE profile between strains coincide with different modes of trans-generational gene regulation by genic piRNAs that arise from polymorphic TE insertions. Overall, this work identifies multiple modes by which differences in the TE landscape between strains can influence patterns of TE and gene expression across generations via piRNAs.
Results
Genome wide asymmetry in TE abundance in a dysgenic cross of D. virilis
Previous studies identified Penelope to be the primary driver of dysgenesis in the D. virilis system because multiple active copies reside in the inducer strain 160, but only degenerate copies reside in the reactive strain 9. in situ hybridization has identified more than 45 euchromatic Penelope insertions in strain 160 and none in strain 9 [32]. In addition, the Helena elements (euchromatic insertions Strain 160 : 18; Strain 9 : 0) and Paris elements (euchromatic insertions Strain 160 : 26; Strain 9 : 0) were shown to be more abundant in inducer strain 160 [32]. Recently a third element, Polyphemus, was identified as more abundant in strain 160. Using genome sequence reads from strains 160 and 9, mapped to a D. virilis TE/repeat library (S1 Dataset), we identified additional factors more abundant in strain 160 and potentially contributing to dysgenesis. Consistent with previous results, Penelope and Polyphemus showed the largest excess in strain 160, validating this approach. Using a 3-fold cutoff as a threshold, we further validated our detection method by confirming that Helena and Paris copy numbers are enriched in strain 160 [32]. Overall, we identified eleven elements enriched in strain 160 and three elements enriched in strain 9. Of the eleven enriched in strain 160, two repeat sequences (258 and 1069; Fig 1A) show no apparent evidence of TE related coding capacity (S2 Dataset). Likewise, the three repeat sequences enriched in strain 9 show no apparent evidence of TE related coding capacity. In addition to the four TEs known to be overrepresented in copy number in strain 160, and the two putatively non-TE repeat sequences, we identify five additional elements enriched in strain 160 (Fig 1A). These are candidates for contributing to the dysgenic syndrome. It is important to note that in this comparison there is a form of ascertainment bias. Because strain 160 is more closely related to the reference strain, repeats entirely absent from the reference strain (but possibly present in strain 9) will be excluded.
Fig. 1. Multiple transposable elements are associated with induction of hybrid dysgenesis. 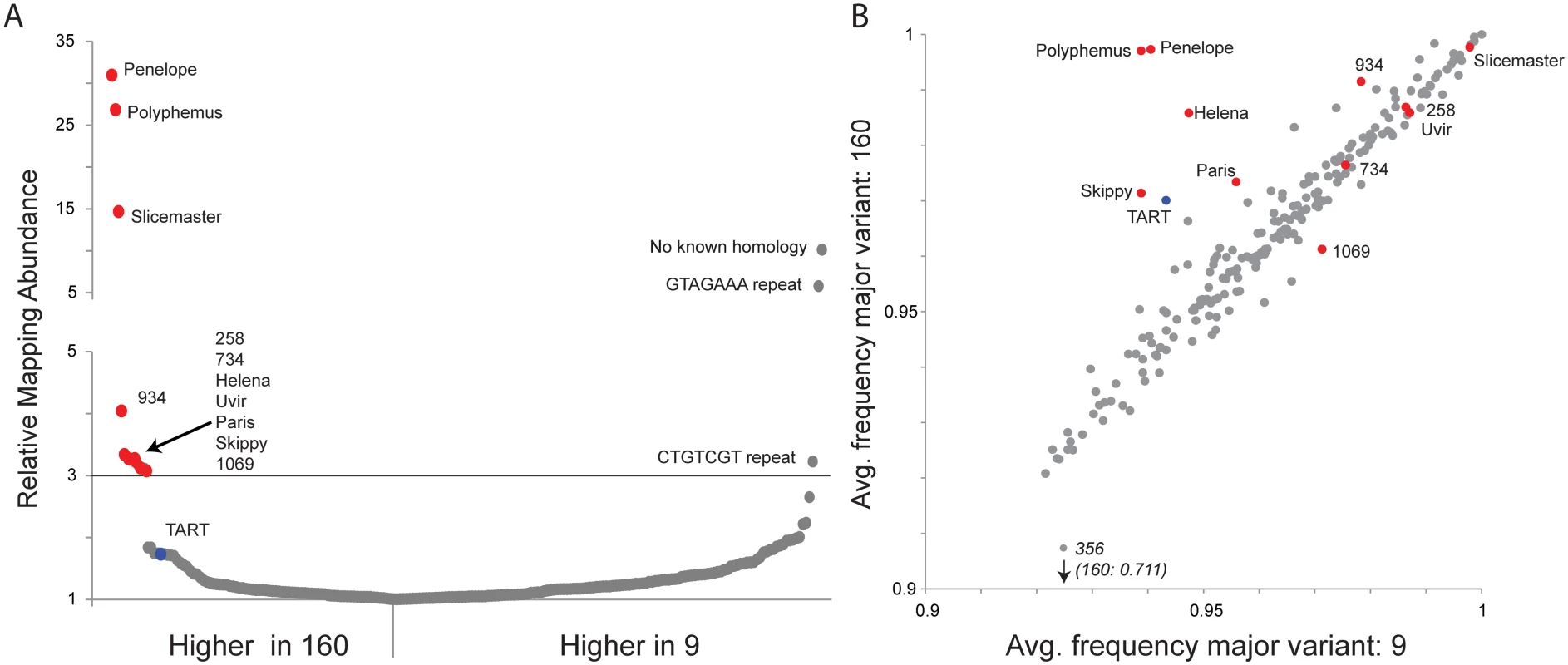
(A) Relative mapping abundance of single-end, 100 bp reads from strain 9 and strain 160 (normalized by reads mapping to the genome), to a consolidated repeat library. Eleven elements are in 3-fold excess in strain 160 and are indicated here and throughout with red. TART elements are about 1.7-fold in excess and are indicated here and throughout with blue. No apparent TEs were found in excess in strain 9. (B) Using piledriver (https://github.com/arq5x/piledriver) we assessed homogeneity within reads mapping to the TE library by determining the average frequency of the major variant in both strains. TEs in excess in strain 160 are either more homogenous in strain 160 or similarly aged between strains, with the exception of element 1069 which shows slightly more homogeneity in strain 9. Coincident with the excess of multiple elements in strain 160, we found that the telomeric TART elements exhibit higher mapping abundance in strain 160, albeit below the 3-fold enrichment threshold (Fig 1A). TART elements have functioned as telomeres for millions of years in Drosophila [41], and our result demonstrates a strain-specific increase in bulk abundance of this long term resident. Telomeric TE content is under piRNA control [42–44], and previous work has shown increased piRNA cluster activity in the telomeric regions of strain 160 compared to strain 9 [35]. Since reduced piRNA function can lead to increased telomeric TE activity, our observed TART excess in strain 160 may be a readout of compromised piRNA function in the inducer strain that is either a cause or consequence of TE excess. For these reasons, it was critical to test for a role of telomeric regions in the induction of dysgenesis. Overall, we found that diverse TE families are in excess in strain 160. This finding is consistent with the hypothesis that the invasion of the Penelope element itself into the reactive strain contributes to genome instability, possibly through the co-mobilization of other TEs within the strain [45].
TE age analysis identifies different modes for TE asymmetry between strains
Divergence in TE abundance between strains can result from different processes. For example, long-resident TEs may be in excess in one strain due to strain - or population-specific recent re-activation. By contrast, entirely new TEs may have invaded a species and have yet to spread equally throughout the genomes of the individuals within the population. The P element invasion in D. melanogaster is an example of the latter process [46]. It has recently invaded and is only present in natural populations/strains collected within the last seven decades.
To distinguish among alternative processes contributing to asymmetry in TE abundance between strains 160 and 9, we performed an age analysis of TE families using high sequence homogeneity within a TE family as an indicator of recent activation or invasion. A phylogenetic approach using full-length fragments is ideal for this purpose, but full-length TE assemblies are not available with short read sequencing technology. Therefore, we estimated relative TE family age by examining the sequence heterogeneity within mapping reads (Fig 1B) by considering the average frequency of the most common nucleotide variant, across all nucleotide variants within the mapping.
A young element that has recently invaded will show high similarity (higher homogeneity) among copies, nearing 1 for an average frequency of the major nucleotide variant. Older elements, with patterns of activation that occurred in the more distant past, accumulate sequence-level differences among insertions, which contribute to lower homogeneity. This accumulation of differences among multiple copies is evident by lower nucleotide frequencies of the most common variant. For a recent TE re-activation in only one strain, we expect higher sequence homogeneity within that strain but higher heterogeneity in the other strain, arising from degraded copies. For an element that has recently invaded a species and is present in both strains, but achieves greater copy number in one strain, we expect a similar level of sequence homogeneity in both strains. Finally, for an element that has recently invaded a species, but is entirely absent in one strain (similar to the P element in D. melanogaster), we expect higher homogeneity of reads in the carrying strain, but much higher heterogeneity in the naive strain, arising from sequence heterogeneity within the marginally mapping reads.
Our age analysis of TE families revealed two classes of TEs that are enriched in inducer strain 160 (Fig 1B). Consistent with previous analyses [30,33,47], one class includes Penelope, Polyphemus, Helena and Paris. In this class, we now also include Skippy and telomeric TART elements. Penelope and Polyphemus showed much higher homogeneity among copies in strain 160 compared to strain 9. We found the same pattern, albeit to a lesser extent, in Helena, Paris, Skippy and the telomeric TART elements. This pattern is highly consistent with recent activation of these elements from long-term resident status. A second class of elements exhibited a different pattern and included Slicemaster, Uvir, 258, 734 and 1069. Slicemaster, Uvir, 258 and 734 showed a pattern of nucleotide homogeneity consistent with similar age in both strains, while element 1069, appears to be slightly older in strain 160. In the case of elements like Slicemaster, which are very young (>99% nucleotide similarity), this can be explained by recent invasion of both strains but excess movement in strain 160, rather than re-activation in one strain from long-time resident status.
Increased germline TE expression in dysgenic females persists through adulthood
To determine the relationship between TE excess in strain 160 and TE expression in dysgenic progeny, we performed mRNA-seq from pooled 0–2 hour old embryos laid by F1 females of both the dysgenic (9 females X 160 males) and non-dysgenic (160 females X 9 males) directions of the cross. Notably, we did not measure TE expression in ovaries from F1 females because dysgenic ovaries are atrophied and expression analysis from these tissues is confounded by altered ratios of somatic and germline tissue. 0–2 hour old embryos laid by F1 mothers represent a sample of pure germline tissue, albeit lacking piRNAs and mRNAs residing solely in nurse cells that are not loaded into the egg [48]. This is because zygotic transcription in D. virilis, as measured with the early, zygotic fushi-tarazu (ftz) gene, begins after 2 hours [49]. Confirmation that embryos in these samples were collected prior to the onset of zygotic transcription was obtained by examining ftz expression in our RNA-seq dataset.
Full penetrance of dysgenesis, evidenced by fully atrophied gonads, is observed in approximately 50% of male and female progeny from 9 female X 160 male crosses. Therefore, embryos analyzed by mRNA-seq from the dysgenic cross were those laid by mothers that escaped full sterility. In contrast to other systems, hatch rates are normal in eggs laid by escaper females. For clarity, these tissues will be referred to as dysgenic, even though these tissues escaped complete atrophy. Sexual maturity in D. virilis occurs at about 5 days. To determine the dynamics of TE expression as flies aged, we analyzed mRNA-seq data from 0–2 hour old embryos laid by F1 mothers 12–16 days old, and 19–21 days old.
First, ignoring age effects, our mRNA-seq results indicated different modes of increased TE expression in the dysgenic germline (Fig 2A). Overall, we find 15 TEs that were differently expressed between dysgenic and non-dysgenic germlines (FDR<0.05). Of these, nine are significantly up in dysgenic and six are significantly up in reciprocal females, but the magnitude of increased expression in the dysgenic germline is significantly greater (Mann-Whitney U test comparing magnitudes of expression difference among differentially expressed TEs: U = 0, p<0.05). This is shown by the fact that all nine of the TEs with significantly increased expression in the dysgenic germline show more than a two-fold increase, but none of the six that are higher in expression in non-dysgenic progeny show this level of expression difference.
Fig. 2. Increased TE expression in the dysgenic germline persists through adulthood. 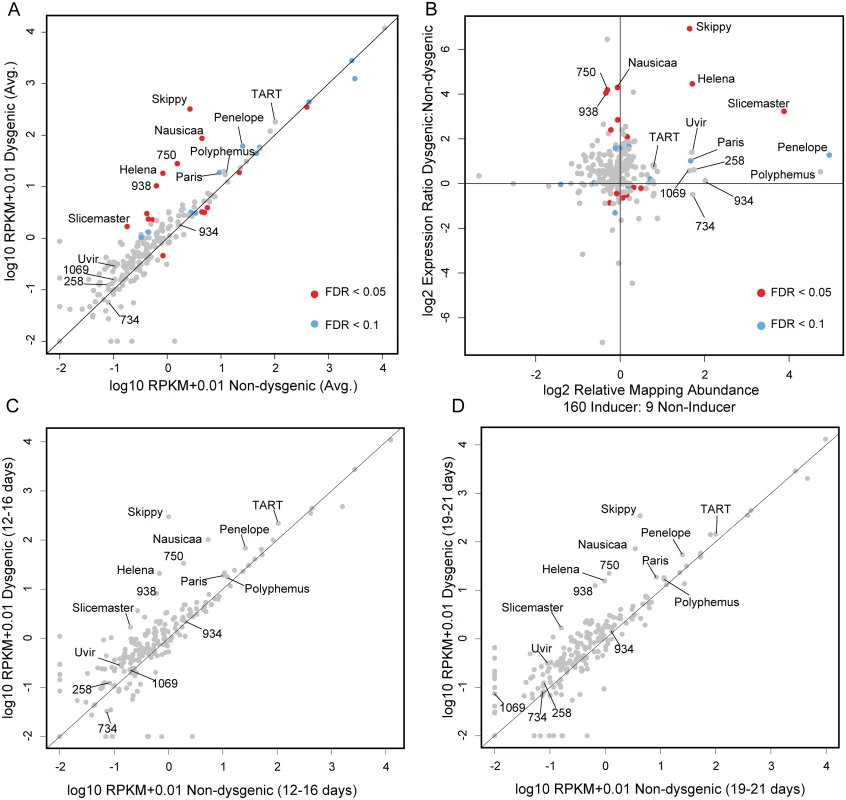
(A) RPKM+0.01 (log 10, average across both ages) for TEs, Dysgenic vs. Non-dysgenic germline. TEs that are in excess in 160 are more highly expressed, as well as many TEs that are not in excess. (B) Fold excess in expression (RPKM+0.01, log 2, average across both ages) vs. fold excess in abundance in strain 160. Nearly all TEs that are in excess in 160 show increased expression in the dysgenic germline (11/12). But multiple TEs that are equivalent in abundance between strains are also increased in expression. (C,D) Increased expression in the dysgenic germline is maintained as flies age. Note: Log scale obscures magnitude of difference for some TEs that demonstrate significant differences in expression identified due to low variation across replicates. TEs with excess abundance in the inducer strain showed increased expression in the dysgenic germline. There were high magnitude differences for some elements (Skippy and Helena; Fig 2A) but for others, the observed differences in expression between dysgenic and non-dysgenic germlines were more modest (Paris, Polyphemus and TART; Fig 2A.) Importantly, while an assemblage of TEs are more highly expressed in the dysgenic germline, many TEs are expressed at equal levels in dysgenic and non-dysgenic crosses. This is also seen for TE expression in the P-M system [28].
Overall, there is not a general rule that all elements more highly expressed in dysgenesis are higher in copy number in the inducer strain (Fig 2B). Ten of eleven elements that are more abundant in the inducer strain were expressed at higher levels in dysgenic progeny. However, five of the nine elements with significantly higher expression in the dysgenic germline are in slightly higher copy number in strain 9 (Fig 2B). This finding is consistent with the observed co-mobilization that was originally identified through genetic approaches—TEs with similar copy number between strains can be co-mobilized by dysgenesis. For example, the Ulysses element is in similar copy number between strains, mobilizes in dysgenesis and shows about a two-fold increase in expression in the dysgenic germline, though this expression difference was not significant. In contrast, the Telemac element, at near-equal abundance between strains 160 and 9, was one of the five expressed at slightly higher levels in the non-dysgenic germline.
Accounting for age of F1 mothers, we found that the observed patterns of increased TE expression in the dysgenic germline were maintained through adulthood (Fig 2C and 2D). For example, the Helena element, which is more abundant in the inducer strain, shows an approximate 30-fold higher expression in embryos laid by 12–16 day old mothers. And in embryos laid by 19–21 year old mothers, Helena maintains an approximately 15-fold higher expression. This persisting level of increased TE expression in the dysgenic germline stands in contrast to the P-M system, where by 21 days, P element expression equalizes between dysgenic and non-dysgenic females [28].
Maternal piRNA and siRNA deposition is an inconsistent predictor of TE expression in dysgenesis
To determine how maternal inheritance of piRNAs (defined as small RNAs, 23–30 nt, filtered against known non-piRNA classes) and also siRNAs (defined as small RNAs, 21 nt, filtered against known non-siRNA classes) might explain increased and persistent TE expression in the dysgenic germline we sequenced 18 to 30 nt RNAs from 0–2 hour old embryos laid by strain 9 and strain 160 mothers, and by F1 females from reciprocal crosses between the two strains. For F1 germline small RNAs, we collected embryos from the same pool of mothers used for mRNA-seq, but at intermediate maternal age (15–16 days old). This allowed us to determine whether the persistent differences in TE expression in the F1 germline of the dysgenic cross could be explained by a persistent defect in piRNA biogenesis.
A large number of TEs, including many with greater copy number in Strain 160, showed higher levels of maternally provisioned piRNA in strain 160 compared to strain 9 (Fig 3A). However, many TEs without large differences in copy number between strains also showed a more than 10-fold excess of maternally provisioned piRNA in the strain 160 genetic background (Fig 3A). Strikingly, despite the asymmetry in maternal provisioning observed in the strain 160 compared to the strain 9 background, piRNA differences are much less pronounced in the germlines of F1 individuals derived from the reciprocal 160 x 9 crosses (Fig 3B, non-overlapping 95% C.I.s for Pearson's correlation coefficient comparing to Fig 3A). This result is not consistent with global persistence of the maternally provisioned piRNA profile across generations. A significant exception to this is the Helena element, which maintains a higher level of piRNA in the non-dysgenic germline.
Fig. 3. TE expression as a function of piRNA and siRNA abundance in parental strains and progeny. 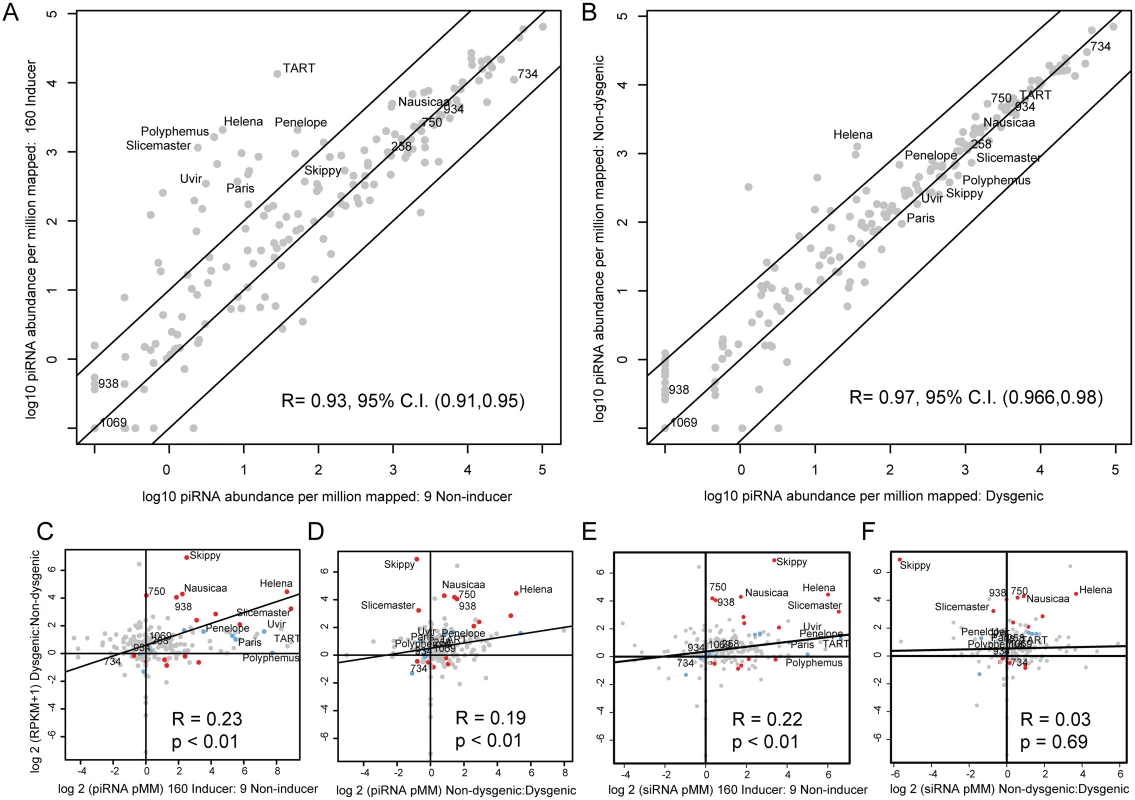
(A). Normalized (per 1 million mappers) piRNA abundance +0.1 (log 10) in the strain 9 germline vs. the strain 160 germline. A large number of TEs show increased piRNA expression in the strain 160 germline, especially TART and others enriched in abundance in strain 160. Diagonal lines indicate 10-fold levels of difference (B) Normalized (per 1 million mappers) piRNA abundance +0.1 (log 10) in the dysgenic germline vs. the non-dysgenic germline. piRNA abundances for many TEs with greater excess in strain 160 become similar in the dysgenic germline. A significant exception to this is the Helena element. Diagonal lines indicate 10-fold levels of difference. (C) TE piRNA excess in strain 160 vs. relative expression level in dysgenesis. TE piRNA asymmetry between 160 and 9 is not the sole determinant of increased expression in dysgenesis. Some elements, such as 750, are increased in expression in dysgenesis, despite similar piRNA abundances in 9 and 160. (D) TE piRNA excess in the non-dysgenic germline vs. relative TE expression level in dysgenesis. Elements such as Skippy and Slicemaster show equilibrated piRNA abundances, but excess expression in the dysgenic germline. (E) TE siRNA excess in strain 160 vs. relative TE expression level in dysgenesis. (F) TE siRNA excess in the non-dysgenic germline vs. relative TE expression level in dysgenesis. Fig 3C demonstrates the degree to which asymmetry in maternal provisioning predicts TE expression in reciprocal dysgenic and non-dysgenic progeny in the next generation. Many of the elements that are more abundant in strain 160 have greater piRNA abundance in the 160 female germline and also are expressed at higher levels in the germline of the dysgenic cross. This drives a significant positive correlation between the log 2 of the ratio of piRNA abundance between strains and relative TE expression levels. However, with Spearman's rho less than 0.2, this is not a strong relationship. Therefore, maternal provisioning of piRNA is only a modest predictor of TE expression in hybrid dysgenesis. For example, element 750 shows no difference in piRNA abundance between parental strains but is more highly expressed in the dysgenic germline. Additionally, a reduced level of TE piRNA abundance persisting in the dysgenic germline is positively associated with increased expression for many (Fig 3D, for example Helena), but not all elements. In contrast to Helena, Skippy and Slicemaster are both more highly expressed in the dysgenic germline even though both show higher levels of piRNA abundance in the dysgenic germline. Therefore, multiple mechanisms appear to explain increased TE expression that persists in the dysgenic germline.
We also examined the role that siRNAs had in predicting differences in TE expression between reciprocal progeny. Similar to the effect of piRNA provisioning, we found that differences in the maternal load of TE siRNAs were predictive of differential TE expression between dysgenic and non-dysgenic progeny (Fig 3E). Since differences in maternal piRNA and siRNA load are strongly correlated between the strains (S1 Fig), this is not surprising. Interestingly, in contrast to piRNA levels, zygotic siRNA levels were not predictive of differences in TE expression between reciprocal progeny (Fig 3F). Overall, maternal piRNA levels are predictive of F1 piRNA levels but maternal siRNA levels are not predictive of F1 siRNA levels (S1 Fig). Interestingly, maternal piRNA levels are in fact predictive of F1 siRNA levels (S1 Fig). These results suggest that while siRNA levels are poor predictors of TE expression differences, their biogenesis in F1 progeny may be coupled to piRNA abundance.
Increased and persisting dysgenic TE expression is not associated with a collapse of global piRNA biogenesis
Raw abundance measures of piRNAs ignore critical aspects of their biogenesis and recent studies have demonstrated that globally reduced signatures of robust piRNA biogenesis likely contribute to the mobilization of diverse TEs [15,28]. In contrast to interspecific crosses that show near complete collapse of the 23–30 nt small RNA pool, we found no evidence that piRNA biogenesis is skewed away from the 23–30 nt expectation based on the size distribution of small RNA reads (Fig 4A). For each TE, we estimated the percent ping-pong [24] as well as the normalized density of ping-pong pairs in the dysgenic and non-dysgenic germline. When we compared metrics directly (first column of heatmaps, Fig 4B) we found little evidence that piRNA biogenesis is grossly perturbed in the dysgenic cross, though a more sensitive comparison using normalized Z-scores indicated a modest reduction in piRNA abundance and density of ping-pong pairs (Fig 4B). This is observed in the Z-score heat maps (Fig 4B, second column of heatmaps) for abundance and ping-pong pair density. Both showed an excess of negative Z-scores for dysgenesis (p<0.0001, Wilcoxon Signed-Rank Test). Importantly, both ping-pong abundance and ping-pong pair density are normalized, proportional measures of abundance that are likely influenced by increases in the abundance of non-TE, genic piRNAs in the same library (see below).
Fig. 4. Signatures of piRNA biogenesis in the dysgenic germline show only modest defects. 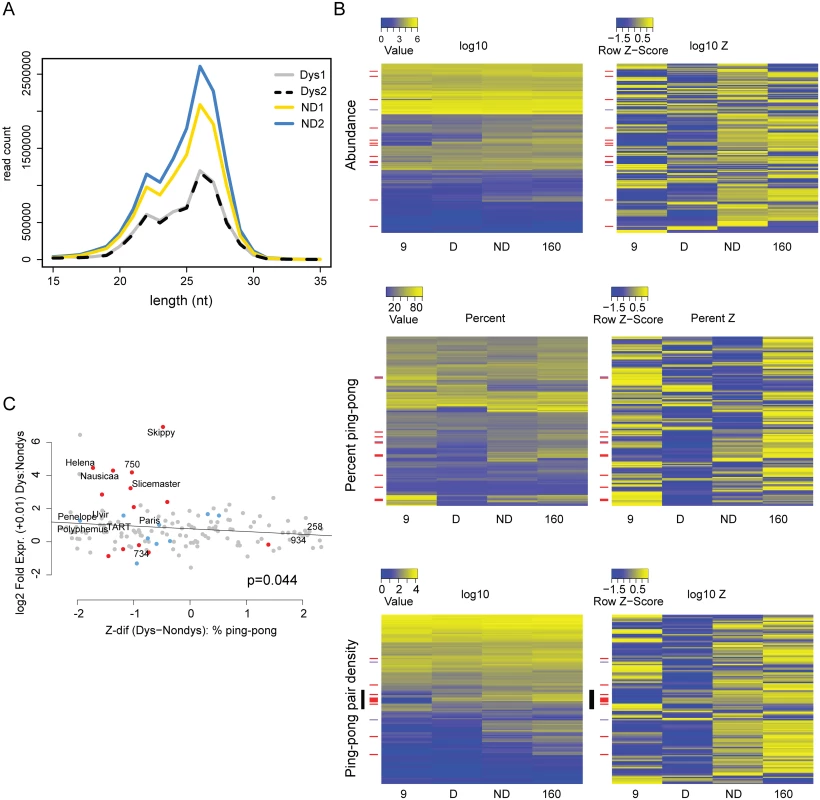
(A) Size distributions of small RNAs are similar between dysgenic and non-dysgenic germlines. Distribution of all small RNAs (not normalized) from four germline libraries (2 dysgenic, 2 non-dysgenic) filtered for tRNA, rRNA and snoRNA. (B) piRNA biogenesis signature heatmaps. TEs upregulated in the dysgenic germline (a difference of 5 RPKM or higher) are indicated with red bars. TEs upregulated in the non-dysgenic germline (a difference of 5 RPKM or higher) are indicated with purple. On the left are heatmaps for raw measures of abundance, the density of ping-pong pairs and percent ping-pong. On the right are heatmaps for the same metrics, but by row z-score. For raw measures, there are no globally discernible effects of dysgenesis on piRNA biogenesis. Row z-scores in dysgenesis do show lower values for abundance measures (abundance and ping-pong pair density), but not percent ping-pong (see text). (C) Fold excess in expression in dysgenesis vs. the difference in percent ping-pong Z-score between dysgenic and non-dysgenic germline. Of the top eight that are most differently expressed in dysgenesis, all have lower ping-pong z-scores in dysgenesis. In contrast to the piRNA abundance measures, we found no significant evidence for global ping-pong biogenesis disruption as measured by percent ping-pong. Note, for example, that many row Z-scores for percent ping-pong showed weaker Z-scores for non-dysgenic compared to dysgenic piRNA (compare upper and lower portions of the percent ping-pong Z-score heatmap, Fig 4B). A Wilcoxon Signed-Rank test also found no significant difference (p = 0.06) in percent ping-pong Z-score between dysgenesis and non-dysgenesis piRNA. If there is any tendency for perturbed piRNA biogenesis, it is not uniform across elements.
Overall, there is a significant relationship between the difference in the normalized density of ping-pong pairs between parents and the difference between dysgenic and non-dysgenic germlines (S2 Fig), demonstrating a role for maternal provisioning in establishing piRNA biogenesis in the next generation. In light of this, we found that transposable elements more highly expressed in dysgenesis (red bars, Fig 4B) are in excess among elements with a reduced percent ping-pong signature. In particular, we found a significant correlation between difference in percent ping-pong Z-score and fold TE expression between dysgenic and non-dysgenic germlines (p = 0.044, Fig 4C). This trend is driven by the top eight elements that show higher expression in dysgenesis and all have lower percent ping-pong Z-scores in dysgenesis (Fig 4C). These results do not support a model of global disruption in piRNA biogenesis maintained in adult flies. Rather, they support a model in which the persistence of higher expression for some TEs is driven by idiosyncratic defects in the restoration of piRNA biogenesis in aged females that occur on a TE-by-TE basis. Strikingly, for several TEs, signatures of piRNA biogenesis appear largely restored, despite increased expression in the dysgenic germline. For TEs that are increased in expression in the dysgenic germline, there appear to be multiple causes (Table 1), including, but not limited to, reduction in ping-pong pairs, suggesting multiple modes of TE derepression in hybrid dysgenesis.
Tab. 1. Properties of TEs significantly more expressed in the dysgenic germline by at last 2-fold (FDR<0.1). 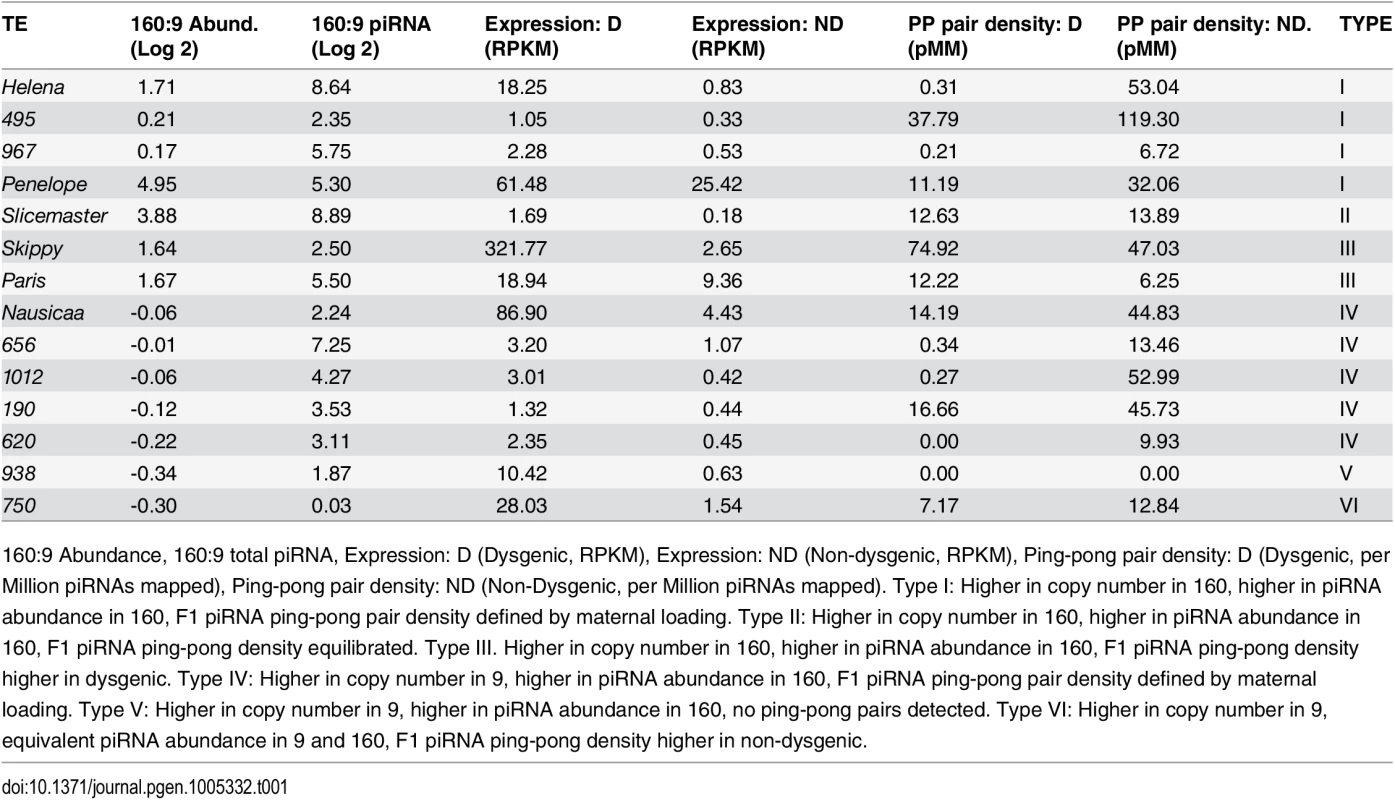
160:9 Abundance, 160:9 total piRNA, Expression: D (Dysgenic, RPKM), Expression: ND (Non-dysgenic, RPKM), Ping-pong pair density: D (Dysgenic, per Million piRNAs mapped), Ping-pong pair density: ND (Non-Dysgenic, per Million piRNAs mapped). Type I: Higher in copy number in 160, higher in piRNA abundance in 160, F1 piRNA ping-pong pair density defined by maternal loading. Type II: Higher in copy number in 160, higher in piRNA abundance in 160, F1 piRNA ping-pong density equilibrated. Type III. Higher in copy number in 160, higher in piRNA abundance in 160, F1 piRNA ping-pong density higher in dysgenic. Type IV: Higher in copy number in 9, higher in piRNA abundance in 160, F1 piRNA ping-pong pair density defined by maternal loading. Type V: Higher in copy number in 9, higher in piRNA abundance in 160, no ping-pong pairs detected. Type VI: Higher in copy number in 9, equivalent piRNA abundance in 9 and 160, F1 piRNA ping-pong density higher in non-dysgenic. To distinguish among the drivers of TE expression differences between the dysgenic and non-dysgenic germline, we used the leaps package in R to identify the single variable that was the best predictor within a multiple regression framework. We considered copy number difference between the two strains, differences in piRNA and siRNA abundance in parents and progeny, and differences in percent ping-pong. Differences in maternal piRNA abundance was selected as the single best predictor (R-squared: 0.054, p = 0.0005). Using an alternate approach to model selection using the AIC (stepAIC from the MASS package), the best fit model included only two variables: maternal siRNA abundance and piRNA abundance in the progeny (Multiple R-squared: 0.0674, p = 0.0005). Both of these variables are correlated with maternal piRNA abundance. Nonetheless, the selection of these two variables without including the maternal piRNA variable suggests that the influence of maternal piRNA abundance in the single variable model is jointly mediated by maternal siRNA pools and zygotic piRNA pools. It should be noted, however, the amount of variance explained by these models is low.
Many genes are differentially expressed in dysgenic and non-dysgenic germlines
We found that 267 genes are significantly upregulated, and 300 significantly downregulated in the dysgenic compared to non-dysgenic germline (FDR = 0.05). We performed GO analysis using the GOrilla program that identifies enriched ontologies in sorted lists [50]. Sorting by FDR value, we identified several interesting terms for genes down-regulated in dysgenesis. These include: reproductive process (FDR = 0.00015), chromatin organization (FDR = 0.0015) and gene silencing (FDR = 0.037) (S1 Table). Since chromatin marks and gene silencing are important for TE control, down-regulation of these genes may play a role in increased TE expression in the dysgenic germline. However, a large number of GO terms were identified in the set of down-regulated genes and GO analysis can be difficult to interpret [51].
Increased genic piRNA production in the dysgenic germline
Recent studies have demonstrated that in addition to TEs, genes may also be the target of piRNA silencing. Genic targeting by piRNAs can arise from neighboring TE insertions that drag flanking sequences into piRNA biogenesis and gene silencing in cis [52–57]. Previous work in the D. virilis system of dysgenesis identified a piRNA cluster overlapping the center divider (cdi) gene (Dvir\GJ14359) [35]. Global gene expression might be modulated if genic piRNA silencing were either attenuated or enhanced during dysgenesis. Therefore, we examined how the global landscape of genic piRNAs was influenced by the activation of diverse TEs in dysgenesis. For this analysis, we excluded genes that lacked hits to CDS regions. We also excluded genes that lacked orthologs in D. melanogaster to exclude TEs mis-annotated as genes.
In the dysgenic germline we found significant enrichment of piRNAs derived from genes. We identified 105 genes that had at least 5 piRNA per million mapping to genic CDS regions in either the parental strains or reciprocal F1 progeny. For these 105 genes, there was a significant excess of piRNAs in the dysgenic germline (Fig 5A; p-value < 0.0001, Wilcoxon-signed rank test). Genic piRNA production, relative to parental strains, is also higher in non-dysgenic progeny, indicating that this may arise from crosses between strains with divergent piRNA profiles. For some genes, the genic piRNAs were predominantly anti-sense, but the majority of genes were associated with primarily sense strand piRNAs (Fig 5B). Comparing genic piRNA density across introns and exons, we found that piRNAs from these genes are enriched on exons (Paired T-test across genes contrasting intronic and exonic density: Library 1: p = 0.0046, Library 2: p = 0.0024). This suggests that genic piRNA processing may occur in the cytoplasm. We examined genic piRNAs for piRNA biogenesis signatures: first position U bias and 10th position A bias. Results indicate that the genic piRNAs are primary piRNAs (S2 Table). From the entire set of 105 genes, focusing on 80 genes that primarily produce sense piRNAs, we found a signature of primary piRNA biogenesis and a very weak signature of secondary piRNA biogenesis (Library 1: U first position: 0.33, background U: 0.21, A 10th position: 0.25, background A: 0.22; Library 2: U first position: 0.33, background U: 0.21, A 10th position: 0.24, background A: 0.23).
Fig. 5. Genic piRNA targeting is increased in the dysgenic germline. 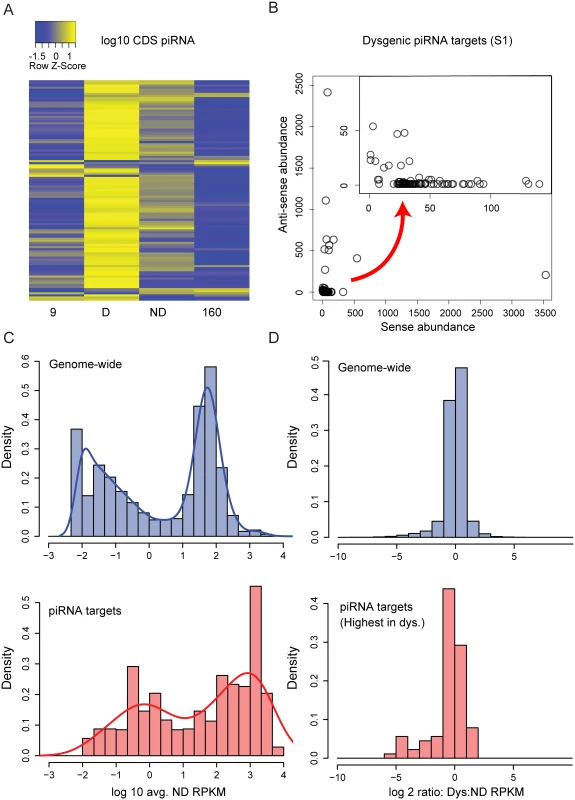
(A) log10 Z-score heat map of genic (CDS) piRNA density for D. melanogaster orthologs (above a threshold of 5 piRNAs per CDS per 1 million mapped in at least one of four columns). Of these 105 genes, there is an excess of genic piRNAs in the dysgenic germline (89 genes with greatest genic targeting in dysgenesis, P<0.001) (B) Sense vs. Anti-sense abundance for piRNAs in genic piRNA class for one library (Sample 1). Some CDS regions are predominantly the source of anti-sense piRNAs, but the majority are biased as a source of sense strand piRNA (C) Distribution of expression levels (log 10 RPKM+0.01) for all genes in the genome and piRNA target genes (expression levels from non-dysgenic germline). Genic piRNA targets are derived from more highly expressed genes (p < 0.001). (D) Of 105 genes, the 89 that show excess genic piRNA in dysgenesis are also more lowly expressed in dysgenesis. Shown is the distribution of expression ratios (dysgenic:non-dysgenic) for all genes and genes that are increased as a source of genic piRNAs in dysgenesis (p < 0.001). In addition, we looked for TE insertions within 2 kb upstream and downstream of these piRNA targeted genes. For the several genes that produced both sense and antisense piRNA, we often found evidence of either a TE in the reference genome or an indication of a nearby insertion in genomic mappings of one of the two strains. Not only did these genes have sense and antisense piRNA, but unique piRNA also mapped to intergenic regions around these genes, indicative of cluster spreading in cis. In contrast, genes with sense piRNA mapping only to exons usually showed no evidence of proximal TE insertions and piRNA did not map to intergenic regions. This supports the idea that these sense and exon-only piRNA are generated from processing of genic mRNA in the cytoplasm.
The 105 genes were also more highly expressed above the genome-wide background (Fig 5C, Wilcoxon rank sum test, p < 0.001). Among the genes with the greatest excess of piRNA abundance in the dysgenic germline, the primary piRNA biogenesis signature was strongest for the genes with expression level higher than 1000 RPKM (S2 Table). This indicates that these small RNAs are not simply degradation products of highly expressed genes. Importantly, the production of these genic sense piRNAs has an apparent effect on gene expression. Of the 105 genes, 89 were identified to show the highest piRNA abundance in the dysgenic germline. These 89 genes were more lowly expressed in the dysgenic germline compared to the non-dysgenic germline (Fig 5D, Wilcoxon rank sum test, p < 0.001). Among this set of 89 genes, there was a significant excess of genes more lowly expressed in dysgenesis compared to more highly expressed (p = 0.03, Sign Test). We attribute this to primary piRNA biogenesis from the sense strand since lower expression is observed for genes that are primarily targets of sense piRNA biogenesis (S3 Fig).
GO enrichment analysis using GOrilla indicated that these 105 genes are highly enriched for ribosomal proteins (FDR p-value = 1.9E-13, 19-fold enrichment; S3 Table). Seventeen of these eighteen ribosomal genes produce more piRNA in the dysgenic samples. These ribosomal piRNA targets are highly expressed, show a strong signature of primary biogenesis (S2 Table) and are not among the group that show differential mRNA expression between dysgenic and non-dysgenic samples. Notably, there is a gene with histone acetyltransferase activity, nejire (Dmel\nej), which has strong effects on TE expression upon knockdown in D. melanogaster [58] and is also orthologous to a piRNA target in our list (Dvir\GJ19060). Nejire produces more piRNA in the dysgenic samples than non-dysgenic, and has significantly lower mRNA levels in dysgenic versus non-dysgenic samples (p-adjusted value < 0.05).
Pericentric regions rather than telomeres influence dysgenesis
While the induction of hybrid dysgenesis in D. virilis has been attributed to TEs enriched in the inducer strain 160, it has been proposed that differences in telomeric cluster activity in the inducer strain may also contribute [35]. Therefore, we employed a genetic approach to determine whether identified telomeric clusters were causal of dysgenesis or, perhaps, simply consequences of excess telomeric TART activity identified in the inducer strain. In the previous study [35], two telomeric clusters specific to strain 160 were identified, one residing at the tip of the second chromosome and another residing at the tip of the sixth chromosome. We therefore tested whether chromosomes carrying telomeres from the inducer strain 160 were highly inductive, when transmitted paternally, or highly protective, when present in the female germline of the dysgenic cross.
We found that induction of dysgenesis is distributed across all chromosomes, with the exception of the dot sixth chromosome (Fig 6A). Therefore, strain 160 telomeres of the second and sixth chromosomes do not contribute uniquely to induction of dysgenesis. Using QTL analysis (Fig 6B) with special attention to telomeric and pericentric regions (motivated by the fact that these genomic compartments often contain TE-rich piRNA clusters[23,26]), we identified three genomic regions for which strain 160 variants at these positions significantly protected against F1 sterility when present in the mother (i.e., dysgenesis; Fig 6C). The genomic region with the most significant effect corresponded to the pericentric region of chromosome 5. The pericentric region of the X chromosome also explained a significant proportion of variation in protective ability, followed by a euchromatic region in the proximal arm region of chromosome 4. We tested for interactions between these loci and saw no evidence for synergism (p-value for all interactions >0.2). Our previous work also found chromosome 5 to be the most protective, followed by the X and then chromosome 4 [49]. Together with the results from this study, there is strong genetic evidence that pericentric, cluster-derived piRNAs play a role in the protection against dysgenesis in D. virilis. By contrast, variation in telomeric repeat abundance between strains does not explain variation in protection against dysgenesis. Thus, telomeric piRNA clusters and amplified TART elements are likely a result of TE destabilization in the inducer strain rather than a driver.
Fig. 6. Genetic analysis of zygotic induction and maternal repression of gonadal atrophy. 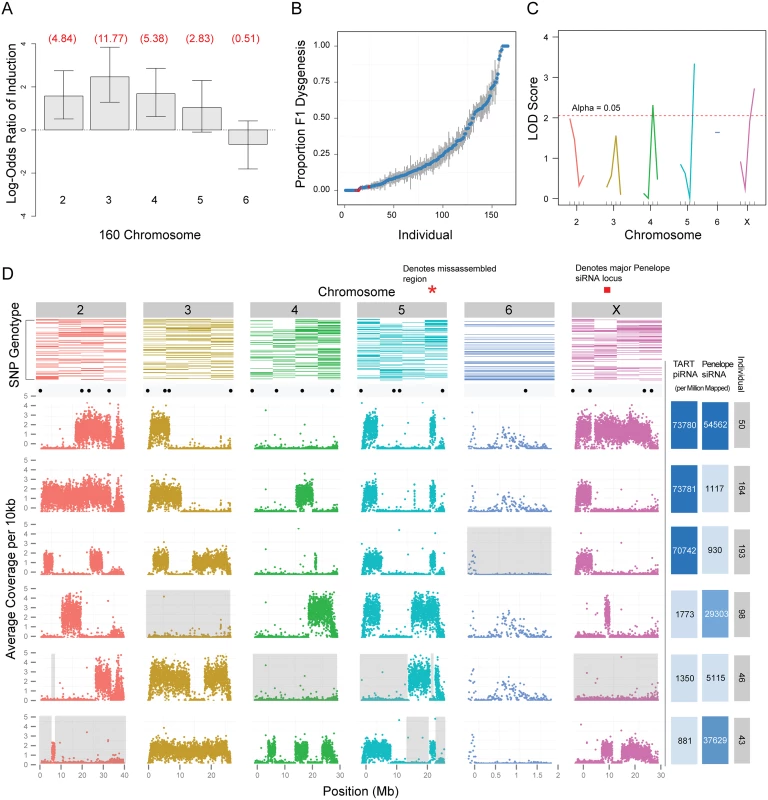
(A) Induction of sterility by 160 is broadly distributed across the genome, with the exception of chromosome 6 (the dot chromosome). Log odds ratios for probability of induction were estimated by crossing F1 males to strain 9, determining whether F2s had male gonadal atrophy and genotyping F2s to determine the chromosomes inherited from the father. Estimates were determined using a generalized linear model for logistic regression (binomial family with a logit link). Values in red are actual odds ratios. Whiskers are 95% confidence intervals. Chromosome 5 is significant at 0.1 level only. X chromosome is not scored because dysgenesis is scored in males and males do not inherit the X from their fathers (N = 92). (B) Scatterplot showing proportion of dysgenic testes (y axis) observed in the progeny of each F3 female individual (x axis). Red dots indicate F3 females that were selected for whole genome sequencing. (C) Single marker QTL analysis identified 3 putative QTLs: one flanking the centromeres of the 5th and X chromosomes and one of the tested euchromatic regions of the 4th chromosome. (D) Top row: Results from the genotyping assay. Colored rectangles represent the presence of strain 160 SNPs in individuals, ranked from top to bottom (most protective individuals on top). Scatterplots: sequencing results. Each dot represents the average number of base pairs that uniquely mapped to every 10kb of the 160 genome. Valleys indicate regions of strain 9 homozygosity. Black dots above scatterplots show the location of each SNP used for our genotyping assay. Grey background demonstrates that no region of the genome from 160 is necessary to protect against dysgenesis. Right-most columns: Number of piRNAs mapped to TART sequences, per million reads, for each F3 female individual. Color intensity is representative of TART piRNA abundance. Number of 21 nt endo-siRNAs mapped to Penelope sequences, per million 21 nt reads, for each F3 female individual. Color intensity is representative of Penelope endo-siRNA abundance. Red bar indicates position of one of several Penelope endo-siRNA loci on the X. No single region or piRNA pool is protective against dysgenesis
To determine precise regions of the 160 genome critical for protection in a dysgenic cross, we performed whole genome sequencing of the six most protective F1 females for which DNA was available (Fig 6B and 6D). We found that among the most protective F1 females there was no single genomic region consistently derived from strain 160. In addition, across all six mothers, we identified at least one mother homozygous for strain 9 at each position of the genome (excluding unassembled regions). Therefore, no single genomic region appears critical for protection against dysgenesis.
To determine whether piRNA from any particular TE enriched in 160 was dispensable for protection, we sequenced small RNAs from the individual pairs of ovaries of the six most protective F1 females. We first reasoned that the only TEs that are candidate inducers of dysgenesis are those for which piRNA abundance is greater in strain 160 than in strain 9. Among the 221 repeats within the TE library (S1 Dataset), there are 141 that meet this qualification. We further reasoned that if any female with full repressive ability had piRNA abundances for a TE that were similar to strain 9, we could rule out that TE as a driver of dysgenesis. Of the remaining 141 candidate TEs, 88 TEs have at least one protective female that has normalized piRNA abundance derived from that TE less than or equal to strain 9. Thus, we were left with 53 candidate contributing repeats. Using these criteria, we were unable to eliminate any of the elements 3-fold enriched from strain 160. Therefore, we are unable to conclude that maternal loading of piRNA corresponding to a single TE can mediate protection against the induction of dysgenesis.
piRNA sequence data from the six most protective F1 females suggests a minimal role for the TART element piRNAs as mediators of maternal protection. This is because the vast majority of TART piRNAs in the protective mothers are derived from the tip of the X chromosome from 160 (Fig 6D) and three of the six females that are most protective against dysgenesis lack the X 160 telomere allele. Notably, the telomeric region of the X has special silencing properties in D. melanogaster that may explain the excess of TART piRNAs derived from this one genomic region [59–63], but in this system this region plays no role in protection against dysgenesis.
Using small RNA sequence data from the six most protective F1 females we characterized the genetic basis for Penelope endo-siRNA production that we had previously shown to be derived from the X-chromosome [49]. We confirmed that endo-siRNAs are not abundant when the X-chromosome from strain 160 is lacking (See individual 46; Fig 6D). These data also demonstrated that Penelope endo-siRNAs are contributed by several loci on the X chromosome (compare individual 98 to 43 and individual 43 to 50; Fig 6D). However, as one individual (46) lacking the X chromosome is protective against dysgenesis, we can confirm that Penelope endo-siRNAs play a minimal role in mediating maternal protection.
Genic piRNAs influence gene expression across generations in multiple ways
Together, the above results support a model of hybrid dysgenesis driven by the mass action of multiple transposable elements. However, divergence in the TE repertoire between strains can also lead to divergence in the genic piRNA profile. This is because genic piRNAs can be produced when TE inserts flank genes [57].
Rozhkov et al. showed that strain 160 possesses a number of piRNA clusters absent in strain 9 [64]. The most well-characterized cluster is a telomeric cluster at the tip of chromosome 2 encompassing the gene center divider (cdi). This dual strand piRNA cluster was found to be present in strain 160, but absent in strain 9. We observed the same pattern in our divergent laboratory stocks (Fig 7). For a second cluster identified by Rozhkov et al., near the telomere of the 6th chromosome in strain 160, but absent in strain 9, we found the opposite pattern in our strains. This cluster in our strain 160 had 318 unique mappers per million mapped reads, whereas our strain 9 had 3,836 unique mappers per million mapped. The most parsimonious explanation for this is that this cluster was originally present in both lines, but independently lost in our strain 160 and Rozhkov et al.'s strain 9. Thus, some piRNA clusters may be prone to losing their activity over time.
Fig. 7. Germline and ovary genic cluster behavior across generations for D. virilis orthologs of center divider and oysgedart from D. melanogaster. 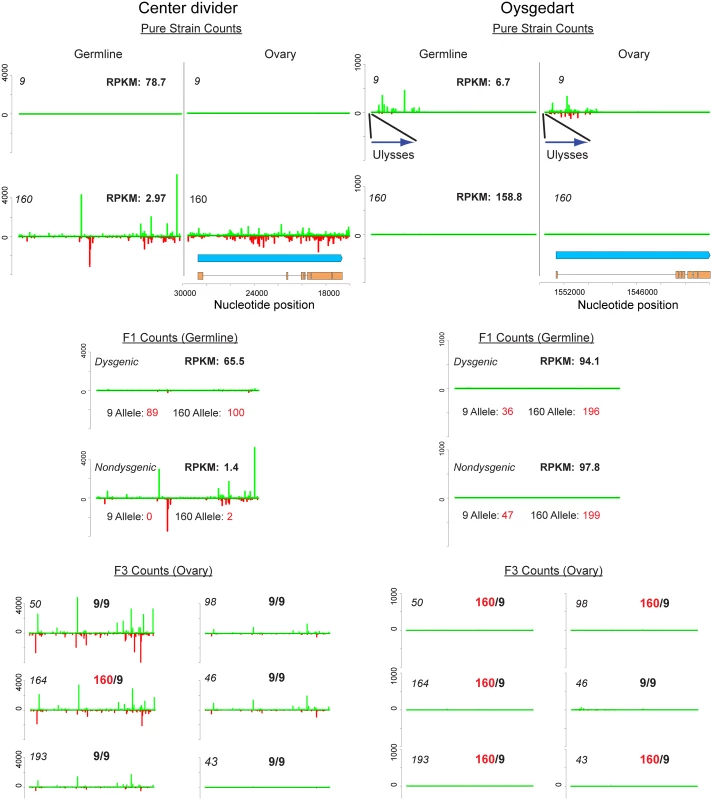
piRNA mapping densities are indicated. mRNA-seq RPKM for germline (0–2 H embryo) is also indicated. Allelism was determined by counting mRNA-seq reads based on SNPs that distinguish strain 9 and 160. Strain 160 cluster identity is maintained for cdi in non-dysgenic progeny in which strain 160 is the mother. This is correlated with silencing of both alleles in the non-dysgenic germline. In contrast, the cluster is not maintained in the dysgenic germline and both alleles are expressed. Somatic expression is not affected. Germline cluster identity for oysgedart (which in the germline is predominantly sense) is lost in progeny. In this case, expression is even between reciprocal progeny, but germline expression is lower from the 9 allele in both directions of the cross. For cluster behavior in F3 backcrosses, heterozygosity or homozygosity of the respective allele is indicated. Notice how cluster identity is maintained for cdi to varying degrees in individuals homozygous for the 9 allele. In contrast, cluster activity is absent in all progeny for oysgedart. In addition to cdi, we identified the oysgedart gene (Dvir\GJ17620) in strain 9 that displayed a novel mode of genic piRNA targeting. A Ulysses element insertion upstream of this gene in strain 9 is specifically associated with genic silencing and shunting oysgedart into piRNA biogenesis (Fig 7). 5' genic piRNAs derived from oysgedart are dual-strand in strain 9 ovaries (which includes somatic and germline tissue) but biased towards the sense strand in the germline.
Maternal deposition of piRNAs derived from a specific locus can mediate piRNA biogenesis at that locus in the next generation [65,66]. For cdi, a recent study demonstrated that maternal deposition leads to maintenance of piRNA biogenesis from the 160 cdi allele. In progeny of strain 160 mothers and strain 9 fathers, maternally deposited piRNAs derived from the cdi cluster also activate piRNA biogenesis and methylation of H3K9 from the strain 9 cdi allele [67].
We found that two modes of genic targeting by piRNAs result in contrasting effects on gene expression across generations. The first mode was found at the cdi locus (Fig 7). Here, maternal deposition of cdi piRNAs mediates silencing in the germline, but not the soma. cdi gene expression is highly reduced in the germline of strain 160 and the germline silencing of both alleles of cdi is maintained when transmitted maternally and made heterozygous in combination with the wild-type strain 9 allele. In contrast, when the cdi cluster allele is transmitted paternally, piRNA cluster activity is reduced and germline expression levels are maintained near the level of strain 9, with similar contributions from each allele. Critically, this asymmetry in gene expression is not observed in the soma. Carcasses of females from reciprocal directions of the cross showed similar levels of expression from both alleles (Dysgenic Carcass: 17.1 RPKM [9 Allele: 7 counts; 160 Allele: 9 counts] vs. Non-dysgenic Carcass: 23.4 RPKM [9 Allele: 5 counts; 160 Allele: 12 counts]) (Fig 7).
The second mode was found at the oysgedart locus (Fig 7). The oysgedart allele in strain 9 shows a novel mode of genic targeting by piRNA. Similar to cdi, we identified sense and anti-sense piRNAs in the ovary. However, germline piRNAs derived from oysgedart are primarily sense derived. Strikingly, in neither direction of the cross is this form of sense piRNA biogenesis maintained in progeny. Instead, the wild-type allele from strain 160 seems to function in trans to limit this mode of silencing. Thus, in both directions of the cross, the expression of oysgedart is maintained at equal levels, but only at about 60% of wildtype since the Ulysses insertion allele from strain 9 is expressed at a lower level. While sense piRNA biogenesis is turned off in reciprocal directions of the cross, expression of the 9 allele is reduced in cis. In neither direction of the cross does there appear a maternal effect on somatic expression for oysgedart. Even though expression of the 9 allele is reduced in the germline, it remains on in the soma of both dysgenic (RPKM: 62.0, [9 Allele: 21 counts; 160 Allele: 21 counts]) and non-dysgenic (RPKM: 80.9, [9 Allele: 29 counts; 160 Allele: 25 counts]) F1 females (Fig 7). This demonstrates that the allelic cis effects on local silencing by the Ulysses insertion are germline, not soma, specific (Fisher's exact test for difference in allele effects between soma and germline. Dysgenic: p<0.0001, Nondysgenic: p<0.0001). Interestingly, in contrast to cdi which has shown to be enriched for H3K9 methylation in strain 160 ovaries, we find no evidence that oysgedart is so enriched in strain 9 ovaries (S4 Table) [67].
We then tested how this mode of piRNA biogenesis is maintained in further generations. We found that the cdi piRNA cluster can cause heritable activation of piRNA biogenesis from the strain 9 allele, a process equivalent to paramutation. In F3 flies generated by backcrossing hybrid non-dysgenic females to strain 9 males (maintaining maternal transmission of the cluster) cluster behavior was maintained even when cdi was homozygous for the strain 9 allele (Fig 7). In one case, cluster behavior was lost. Because the cross scheme was maintained over two generations, we are unable to determine the generation in which the cluster activity was lost, however, it is clear that cdi can paramutate in one generation but that this paramutation is not robust across multiple generations (though see [66]). In the same F3 flies, we did not observe restoration of cluster maintenance for oysgedart.
Discussion
Due to their proliferative nature, differences in the TE landscape within populations and between species can accumulate rapidly. Since TEs are harmful, it has also been proposed that this divergence drives rapid evolution of the piRNA machinery itself [68–70]. Divergence at both levels is expected to greatly influence patterns of TE activity in crosses between individuals. Within a species, hybrid dysgenesis syndromes driven from a single element family reveal that maternally deposited piRNAs targeting the activating TE are critical for maintaining TE control and fertility. In contrast, crosses between species that differ with respect to both TE profile and the machinery of piRNA biogenesis show dramatic collapse of piRNA biogenesis and this can be attributed to divergence in the piRNA machinery rather than differences in the maternally deposited pool [15]. Previous studies, combined with results presented here, indicate that the D. virilis system represents a complex form of intraspecific hybrid dysgenesis involving more divergent TE profiles compared to other syndromes of hybrid dysgenesis. However, because it is an intraspecific cross, there is minimal divergence in the TE regulatory machinery. Therefore, the dysgenic syndrome in D. virilis can be considered an important model for understanding the dynamics of TE control at an intermediate stage in the divergence of TE profile within a single species.
Here we show that differences between genetically identical dysgenic and non-dysgenic individuals are manifested in multiple ways. When genomes from two strains of D. virilis are brought together, TEs that are more abundant in one genome become more highly expressed in the germline of the next generation. This difference in TE expression persists in the germline as flies age. Coincident with this, there is also a persistent increase in TE expression for several TEs that are evenly distributed between strains.
We identify multiple modes by which piRNAs modulate gene expression. First, many genes become off-targets for piRNA biogenesis, and their expression levels are reduced. The mechanism for this is unclear, but may be driven by the same mechanism that leads to idiosyncratic defects in piRNA biogenesis for some TEs. One possibility is that compromised piRNA function in the cytoplasm leads to a shift in the targets of primary piRNA biogenesis. A similar increase in sense genic piRNA abundance has been observed in rhino and uap56 mutants and this has been attributed to compromised specificity in piRNA processing [71]. Therefore, the increased genic piRNA abundance in the dysgenic germline may be a readout of compromised piRNA biogenesis and loss of specificity in the cytoplasm. Secondly, divergence in the TE profile between strains leads to differences in the pool of maternally deposited genic piRNAs. Depending on the nature of genic piRNAs, these can modulate gene expression in diverse ways across generations. For the cdi gene, maternally deposited piRNAs from both strands mediate gene silencing of both alleles in non-dysgenic progeny. Alleles of cdi share properties with imprinted genes since expression depends on which parent the allele is inherited from. However, cdi differs from canonical imprinting in that a silenced cdi allele is capable of silencing the other allele in trans when inherited maternally, similar to paramutations observed in maize, mice and recently in Drosophila melanogaster [66,72–74]. Imprinted genes can have a significant downstream effect on patterns of gene expression [75]. Even in Drosophila, where there is no evidence for DNA methylation, there are significant, albeit poorly understood, parent-of-origin allelic effects on global gene expression [76,77]. Such transgenerational effects on gene expression may contribute to large numbers of genes being differentially expressed between the dysgenic and non-dysgenic germline. In contrast to cdi, a Ulysses insertion upstream of the oysgedart gene seems to mediate only deposition of sense piRNAs. In this case, the wild-type non-insertion allele appears to resist transgenerational silencing and expression is maintained equally between reciprocal hybrids.
Since we identify a large number of differences in TE and genic expression that are mediated by piRNAs in diverse ways, it is difficult to distinguish between causal factors and downstream effects. However, our genetic analyses clearly demonstrate that both induction of and protection against dysgenesis is distributed across the genome. Since multiple TE families are in excess copy number in the inducer strain, the weight of evidence favors a complex mode of hybrid dysgenesis driven jointly by the mass action of multiple elements. This is supported by the fact that the regions of the genome that are most protective against dysgenesis are located in the pericentric regions, which are known to be critical sources of piRNAs [23,26], further supporting a model in which pericentric regions play a unique role in TE control [78].
Representing an intermediate state of TE divergence between single TE family dysgenesis syndromes and interspecific crosses, several observations are worth noting from the D. virilis system. First, in crosses between D. melanogaster and D. simulans, piRNA biogenesis is globally defective and this is attributed to the accumulation of incompatibilities that arise from rapid divergence in the protein sequence of the piRNA biogenesis machinery. In the D. virilis system where there is only divergence in the TE profile between strains, we see no such global collapse of piRNA biogenesis. In this sense, this is more similar to what is observed in the P-M system of dysgenesis.
However, there are some key distinctions between the P-M system, driven by a single element, and the D. virilis system of dysgenesis. In the P-M system, as flies age, piRNAs targeting the active element are restored and P element silencing is regained. We see very little restoration of silencing for many TEs that are most likely to contribute to dysgenesis. For example, the Helena element remains much more highly expressed in the dysgenic germline and this is also associated with failure to restore the piRNA pool targeting Helena. Therefore, despite the fact that these tissues (0–2 hour old embryos) have escaped the ablation event of dysgenesis, idiosyncratic defects in TE silencing persist and these are passed on to the next generation. Furthermore, increased TE expression is observed for some TEs that appear to have a restored level of piRNA biogenesis. Thus, a further defect in dysgenesis is a lack of effective silencing by a restored pool of piRNA. This suggests multiple mechanisms contribute to chronic increased TE expression in the dysgenic germline.
A long-standing question is what underlies TE co-mobilization in dysgenic syndromes in Drosophila. For many years, the D. virilis system was considered unique in that it was associated with increased movement of elements equally abundant between strains, the Ulysses element being the best example. Recent work by Theurkauf and colleagues suggest that co-mobilization in fact may be a general property of dysgenic syndromes [28]. By what mechanism does the movement of one element activate the movement of others? The current working model is that DNA damage from a moving element activates the DNA damage response within the germline. This, in turn, drives Chk-2 mediated phosphorylation and degradation of Vasa. Vasa is critical for nuage assembly and germline piRNA biogenesis [79]. In the absence of proper Vasa and germline piRNA function, resident transposons become activated, leading to co-mobilization.
This is a strong candidate for the mechanism of co-mobilization in the D. virilis system. A burst of TE mobilization in the germline at any time during development may drive movement of TEs found in equal copy number between strains via the DNA damage response. However, it is difficult to explain the persisting and idiosyncratic pattern of increased expression for some TEs in the dysgenic germline that is observed in aged females that have escaped sterility. In the P-M system, Vasa degradation is relieved as the aging dysgenic flies recover partial fertility and P element silencing is restored. In D. virilis, full silencing is not restored, even in the germline that has escaped ablation. Coinciding with increased levels of expression that persist for some TEs, we also note that there is a persisting increase in piRNAs that target genes. This is similar to observations in flies defective in rhino and uap56 [71]. We have shown that global defects in TE repression observed during hybrid dysgenesis share this feature. Strikingly, the genes that are piRNA targets are highly enriched for ribosomal proteins RNAs (FDR p-value: 1.9E-13, 19-fold enrichment; S3 Table). The mechanism by which increased genic piRNAs target ribosomal protein RNAs is unclear, however there are multiple lines of evidence that disrupted levels of ribosomal proteins can lead to increased levels of repeat expression [80]. For example, an early genome wide screen in C. elegans identified approximately 27 genes involved in transposon silencing, two of which were ribosomal proteins [81]. Disruption of the ribosome is known to trigger nucleolar stress and p53 activation [82]. Thus, nucleolar stress mediated by genic piRNAs may also contribute to persisting TE activation in the dysgenic germline.
Materials and Methods
Custom D. virilis TE library
Few annotated TEs are available for D. virilis. Therefore, we combined available annotated TE sequences with two computationally predicted libraries (generated with PILER [83] and REaS [84,85] ftp://ftp.flybase.net/genomes/aaa/transposable_elements/) to generate a manually curated library. Several annotations (Uvir, Helena, TART, Telemac) were improved by manual curation. Portions of the PILER library were also manually curated. Additional sequence from the Helena element was obtained by interrogating a de novo assembly of the strain 160 genome. Redundancy was removed from this combined library first by removing repeats with significant blastn hits between and within the PILER and annotated library, with priority to annotated and longer sequences. With this filtered set, further redundancy was removed by blasting this library with and between the REaS library.
Genome Sequencing and TE measurement from strain 9 and 160
Genome sequencing was performed on both reactive strains 9 (non-inducer) and strain 160 (inducer). As D. virilis has very high satellite content (more than 40%) [86], we elected to used wandering third instar larvae for our DNA source as previously described [87]. These tissues include polytene tissues that are underreplicated in heterochromatin, thereby reducing the number of satellite reads and enriching for euchromatic TE insertions that are expected to be the most active. Wandering 3rd larvae were collected from strain 9 and 160, rinsed with 50% bleach, and DNA was extracted. 100 bp paired end sequencing was performed on an Illumina GAII with 400 to 500 bp fragments. TE abundance estimation was performed with single ends that were trimmed by Sickle (https://github.com/najoshi/sickle), mapped to the TE library with BWA-MEM [88] and normalized by read numbers mapping to the reference. Homogeneity within mapped reads was measured using piledriver (github.com/arq5x/piledriver) and averaging the frequency of the major allele across all nucleotides within the mapping for each TE.
Estimation of zygotic effects of paternally inherited chromosomes
From the genome sequences of strain 9 and 160, restriction fragment length polymorphisms were identified that distinguish between the strains at two positions for each chromosome. F1 males were generated from a non-dysgenic cross and these males were crossed to strain 9. 96 F2 progeny were collected, (48 from each class, dysgenic or non-dysgenic) and genotyped for chromosomes inherited paternally with RFLPs. Log-odds ratios for the probability for being dysgenic with a given chromosome were estimated using a generalized linear model for logistic regression (binomial family with a logit link) in R. Some failed genotypes resulted in N = 92.
mRNA seq: Dysgenic and Non-dysgenic germline, Strains 9 and 160 germline, dysgenic and non-dysgenic soma
Dysgenic vs. non-dysgenic germline
RNA for sequencing was collected from embryos laid by F1 mothers from the dysgenic and non-dysgenic directions of the cross. Ovaries were not selected because dysgenic ovaries are often atrophied. Therefore, the germline tissue represented in this experiment is derived from mothers that have escaped germline ablation. Paternal effects on embryos that might occur when dysgenic females are mated with sterile dysgenic brothers were minimized by equally mixing males from reciprocal directions of the cross and allocating them in mating cages between reciprocal F1 females. This also ensured improved egg laying from dysgenic females. Overall, we collected four pools of 0–2 hour old embryos, aggregated across several days, from large population cages containing many hundreds of adults grown up simultaneously from multiple bottles. Females were maintained as continuously laying with a constant supply of yeast and grape plates and eggs were collected after 0–2 hour durations and flash frozen in liquid nitrogen. RNA was pooled from collections of 12 to 16 day old mothers and 19 to 21 day old mothers. From each age sample, two RNA-seq libraries were generated for single-end, 50 bp sequencing, for a total of four libraries per condition (dysgenic or non-dysgenic).
160 vs. 9 germline
The same strategy was employed for collection of 0–2 hour old eggs from strain 9 and 160. Here, pools were also aggregated over multiple collections from large cages of pure strain 9 and 160 from multiple grow up bottles. Pool were aggregated over 7 to 15 days (young) and 15 to 25 days (old) and split for two RNA seq libraries per pool with reciprocal barcodes. Results presented are average RPKM per library and allele counts were pooled across all libraries.
Dysgenic and Non-Dysgenic Soma: RNA for analysis from somatic gene expression was obtained from 3 dysgenic and 3 non-dysgenic crosses. From each cross, 10 females were collected and aged 3 to 9 days. RNA was collected from these pools of 10 females, with abdomens removed.
Analysis of mRNA seq data
For estimates of gene expression level, RPKM was used. For statistical analysis, mapped count data was used. Reads were quality trimmed at the 3' end (up to 16 bp) and reads with 2 bp of quality less than 20 were excluded using the Galaxy server. TE RPKM estimates were obtained by directly mapping with BWA to the annotated TE library and normalizing with known TE length and the number of reads that mapped to the reference genome. mRNA RPKM estimates were obtained using the RNA-seq tool in CLC. Fold analysis was performed by calculating RPKM(+0.01) ratios. We used DEseq2 [89] on RNAseq read count data to test for differential expression for both TEs and genes between dysgenic and non-dysgenic germ lines. Analysis of TEs and genes was performed separately. The model employed tested for treatment effects, age effects, and age x treatment interaction effects. While the same model was fit for genes and TEs, these models were run independently to account for possible differences in the mean/variance relationship between these groups. Genes with fewer than 40 total reads mapped across all samples were removed from the analyses prior to calling differential expression in order to reduce the loss of power due to multiple testing. Statistical significance was assessed using FDR, focusing on treatment effect only. GO analysis was performed with GOrilla[50] using D. melanogaster orthologs genes sorted by FDR value for the test of treatment effect.
Additional germline mRNA seq was performed using the same protocol for pure strain 9 and strain 160 (RPKMs averaged for cluster analysis across 2 libraries each for 7–15 day old females and 15–25 day old females). Allele counts were determined by direct counting within the RNA-seq mappings (summed across all library mappings) for a SNP known to distinguish the two strains within the transcripts for cdi and oysgedart. Somatic mRNA seq analysis was likewise performed (3 libraries per condition, each library from a 10 pooled female carcasses, RPKMs averaged across libraries and allele counts estimated as before).
Small RNA sequencing
All small RNA was size selected from 15% acrylamide, cut between an 18 bp oligo and the 30nt rRNA. Small RNA sequencing for dysgenic and non-dysgenic germline material was performed on embryos laid by the same mothers as for RNA seq, but at 15–16 days old, according to [90]. Small RNAs from strain 9 and strain 160 pooled ovaries were sequenced according to [91] with the oxidation reaction. Small RNAs from ovaries from individual F3 females was performed by Fasteris.
Small RNA Analysis
Reads were trimmed by removing adapters and filtered by size as piRNA (23-30nt) in CLC Genomics Workbench 7.0. Reads were then filtered by mapping to tRNA, ncRNA, miscellaneous RNA, and miRNA (including pre-miRNA) libraries from the D. virilis reference genome. The filtered 23–30 nt small RNA reads were mapped to our curated TE library with BWA.aln [92], using the default parameters. Reads were normalized by non-unique mappers to the D. virilis reference genome using BWA.aln defaults. Calculations for ping-pong percent [24] and density of piRNA pairs were done with the R package viRome (http://www.ark-genomics.org/bioinformatics/virome), with some modifications. For genic small RNA analysis, reads were mapped uniquely with BWA.aln to the D. virilis reference genome, using default parameters. Reads were normalized by non-unique mappers to the genome. BEDTools intersect [93] was utilized to count piRNA hits on genes and CDS sequences. Fastq reads specific to genic piRNA hits were extracted using Enve-omics (https://github.com/lmrodriguezr/enveomics) and FASTX-Toolkit (hannonlab.cshl.edu/fastx_toolkit/index.html) was used to process and analyze nucleotide bias for genic piRNA subsets. We did not perform DEseq2 analysis because estimation of the dispersion parameter with DEseq2 is unlikely to be robust with about 200 TEs, compared to standard mRNA-seq analysis that estimates the dispersion parameter using thousands of genes [89]. Percent ping-pong was defined as the percent of 23 to 30 nt mapping reads that had a corresponding read on the opposite strand with a 10 bp 5'-5' overlap. We also measured piRNA biogenesis by determining ping-pong pair density. This measure was obtained by counting all non-redundant ping-pong pairs (counting each read only once) per kb.
Genetic analysis of genomic regions from strain 160 that maternally protect against dysgenesis
To identify regions of the 160 genome that protect against dysgenesis when present in females, an F3 mapping/QTL experiment was performed. F3 females were generated by crossing 160 females to strain 9 males (a non-dysgenic cross), followed by two rounds of backcrossing to strain 9 males. This resulted in the production of F3 mothers for which strain 160 was the great-grandmother. All but the final cross was performed en masse. Dysgenic crosses were performed with >160 single 4 to 5 day old tester F3 females mated with three 4 to 5 day old strain 160 males. Adults were transferred to new vials daily and dysgenesis was estimated by counting the number of dysgenic testes in progeny over all testes counted (2 per male) across three broods. Females were then collected, ovaries removed (for small RNA sequencing by Fasteris, top protectors only) and carcasses retained for genomic DNA extraction. Genotyping was performed using the TaqMan Open Array platform on all females, with the exception of the top six females that had the strongest ability to protect against dysgenesis. SNPs distinguishing 160 and 9 chromosomes were chosen in pairs for redundancy, one pair at each telomere and pericentric region, as well as one or two euchromatic SNPs. Care was taken to avoid repeat sequences by screening with Blast against the reference and also using RepeatMasker. F3 Females were then genotyped for 160/9 heterozygosity, alongside pure strain 9 and 160 controls, by National Jewish Health. Single marker regression was carried out with RQTL after dropping individuals with missing genotype data from the analysis. 5000 permutations were done to find the significance threshold at alpha = 0.05. For the top 6 protectors, whole genome sequencing was performed (100 bp, paired-end) using the Nextera library prep protocol.
Genotyping by whole genome sequencing
Reference genome scaffolds from D. virilis were concatenated according to their supported positions and orientations on known Muller elements, with a large scaffold arbitrarily generated by concatenating scaffolds from unknown positions. Using this new "assembly" we mapped all strain 9 and strain 160 reads and generated two consensus genomes. A pseudo-diploid heterozygous genome was then assembled by placing these scaffolds into one file.
Paired-end reads from the top six protectors were mapped to the hybrid genome, using BWA’s default parameters, with the goal of inferring spans of heterozygosity for strain 160 by identifying reads that map uniquely, under high stringency, to the strain 160 haploid reference. The mapping output was piped into SAMtools for filtering by quality score (-q 42) and post-alignment processing (SAM to BAM conversion and indexing). A relatively high cutoff quality score was used in order to remove reads that could have mapped promiscuously. We were able to remove all reads that mapped to more than one locus/allele leaving us with reads that are specific to either strain 9 or 160. Spans of heterozygosity for strain 160 were visualized with a sliding window for read density along the 160 chromosomes within the psuedo-diploid reference genome.
Chromatin immunopreciptiation sequencing
Chromatin isolation and immunoprecipitation was performed using 200 pairs of ovaries from strain 9 and strain 160 according to the protocol described in Du and Elgin [52]. Immunoprecipitation (IP) was performed using anti-H3K9me2 (Abcam 1220) at 1 : 100. Input and IP DNA was used for SR50-bp Illumina sequencing. H3K9-me2 enrichment was estimated using the IP:Input ratio of reads (with duplicates removed) uniquely mapping to the cdi or oysgedart locus (normalized to the total number of reads mapping in the library).
Supporting Information
Zdroje
1. Hickey DA (1982) Selfish DNA: A sexually-transmitted nuclear parasite. Genetics 101 : 519–531. 6293914
2. Charlesworth B, Charlesworth D (1983) The population dynamics of transposable elements. Genetical Research 42 : 1–27.
3. Charlesworth B, Langley CH (1989) The population genetics of Drosophila transposable elements. Annual Review of Genetics 23 : 251–287. 2559652
4. Daniels SB, Peterson KR, Strausbaugh LD, Kidwell MG, Chovnick A (1990) Evidence for Horizontal Transmission of the P-Transposable Element between Drosophila Species. Genetics 124 : 339–355. 2155157
5. Anxolabehere D, Kidwell MG, Periquet G (1988) Molecular characteristics of diverse populations are consistent with the hypothesis of a recent invasion of Drosophila melanogaster by mobile P elements. Mol Biol Evol 5 : 252–269. 2838720
6. Coyne JA (1986) Meiotic segregation and male recombination in interspecific hybrids of Drosophila Genetics 114 : 485–494.
7. Coyne JA (1989) Mutation rates in hybrids between sibling species of Drosophila. Heredity (Edinb) 63 (Pt 2): 155–162.
8. Dion-Cote AM, Renaut S, Normandeau E, Bernatchez L (2014) RNA-seq reveals transcriptomic shock involving transposable elements reactivation in hybrids of young lake whitefish species. Mol Biol Evol 31 : 1188–1199. doi: 10.1093/molbev/msu069 24505119
9. Josefsson C, Dilkes B, Comai L (2006) Parent-dependent loss of gene silencing during interspecies hybridization. Current Biology 16 : 1322–1328. 16824920
10. O'Neill RJ, O'Neill MJ, Graves JA (1998) Undermethylation associated with retroelement activation and chromosome remodelling in an interspecific mammalian hybrid. Nature 393 : 68–72. 9590690
11. O'Neill RJ, O'Neill MJ, Graves JA (2002) Corrigendum:Undermethylation associated with retroelement activation and chromosome remodelling in an interspecific mammalian hybrid. Nature 420 : 106.
12. Hey J (1989) Speciation via hybrid dysgenesis: negative evidence from the Drosophila affinis subgroup. Genetica 78 : 97–104.
13. Vela D, Fontdevila A, Vieira C, Garcia Guerreiro MP (2014) A genome-wide survey of genetic instability by transposition in Drosophila hybrids. PLoS One 9: e88992. doi: 10.1371/journal.pone.0088992 24586475
14. Labrador M, Farre M, Utzet F, Fontdevila A (1999) Interspecific hybridization increases transposition rates of Osvaldo. Mol Biol Evol 16 : 931–937. 10406110
15. Kelleher ES, Edelman NB, Barbash DA (2012) Drosophila interspecific hybrids phenocopy piRNA-pathway mutants. PLoS Biol 10: e1001428. doi: 10.1371/journal.pbio.1001428 23189033
16. Kidwell MG, Novy JB (1979) Hybrid dysgenesis in Drosophila melanogaster—Sterility resulting from gonadal-dysgenesis in the P-M system. Genetics 92 : 1127–1140. 17248943
17. Kidwell MG, Kidwell JF, Sved JA (1977) Hybrid Dysgenesis in Drosophila melanogaster: A Syndrome of Aberrant Traits Including Mutation, Sterility and Male Recombination. Genetics 86 : 813–833. 17248751
18. Bingham PM, Kidwell MG, Rubin GM (1982) The molecular basis of P-M dysgenesis—The role of the P-element, a P-Strain-Specific Transposon Family. Cell 29 : 995–1004. 6295641
19. Bucheton A, Paro R, Sang HM, Pelisson A, Finnegan DJ (1984) The molecular basis of the I-R hybrid dysgenesis syndreom in Drosophila melanogaster—Identification, cloning and properties of the I-Factor. Cell 38 : 153–163. 6088060
20. Yannopoulos G, Stamatis N, Monastirioti M, Hatzopoulos P, Louis C (1987) hobo is responsible for the induction of hybrid dysgenesis by strains of Drosophila melanogater bearing the male recombination factor 23.5MRF. Cell 49 : 487–495. 3032457
21. Aravin AA, Lagos-Quintana M, Yalcin A, Zavolan M, Marks D, et al. (2003) The small RNA profile during Drosophila melanogaster development. Developmental Cell 5 : 337–350. 12919683
22. Aravin AA, Hannon GJ, Brennecke J (2007) The Piwi-piRNA pathway provides an adaptive defense in the transposon arms race. Science 318 : 761–764. 17975059
23. Brennecke J, Aravin AA, Stark A, Dus M, Kellis M, et al. (2007) Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell 128 : 1089–1103. 17346786
24. Brennecke J, Malone CD, Aravin AA, Sachidanandam R, Stark A, et al. (2008) An Epigenetic Role for Maternally Inherited piRNAs in Transposon Silencing. Science 322 : 1387–1392. doi: 10.1126/science.1165171 19039138
25. Gunawardane LS, Saito K, Nishida KM, Miyoshi K, Kawamura Y, et al. (2007) A slicer-mediated mechanism for repeat-associated siRNA 5 ' end formation in Drosophila. Science 315 : 1587–1590. 17322028
26. Grentzinger T, Armenise C, Brun C, Mugat B, Serrano V, et al. (2012) piRNA-mediated transgenerational inheritance of an acquired trait. Genome Res 22 : 1877–1888. doi: 10.1101/gr.136614.111 22555593
27. Chambeyron S, Popkova A, Payen-Groschene G, Brun C, Laouini D, et al. (2008) piRNA-mediated nuclear accumulation of retrotransposon transcripts in the Drosophila female germline. Proc Natl Acad Sci U S A 105 : 14964–14969. doi: 10.1073/pnas.0805943105 18809914
28. Khurana JS, Wang J, Xu J, Koppetsch BS, Thomson TC, et al. (2011) Adaptation to P Element Transposon Invasion in Drosophila melanogaster. Cell 147 : 1551–1563. doi: 10.1016/j.cell.2011.11.042 22196730
29. Evgen'ev MB, Arkhipova IR (2005) Penelope-like elements—a new class of retroelements: distribution, function and possible evolutionary significance. Cytogenet Genome Res 110 : 510–521. 16093704
30. Lyozin GT, Makarova KS, Velikodvorskaja VV, Zelentsova HS, Khechumian RR, et al. (2001) The structure and evolution of Penelope in the virilis species group of Drosophila: an ancient lineage of retroelements. Journal of Molecular Evolution 52 : 445–456. 11443348
31. Evgenev MB, Zelentsova H, Shostak N, Kozitsina M, Barskyi V, et al. (1997) Penelope, a new family of transposable elements and its possible role in hybrid dysgenesis in Drosophila virilis. Proceedings of the National Academy of Sciences of the United States of America 94 : 196–201. 8990185
32. Vieira J, Vieira CP, Hartl DL, Lozovskaya ER (1998) Factors contributing to the hybrid dysgenesis syndrome in Drosophila virilis. Genetical Research 71 : 109–117. 9717433
33. Blumenstiel JP (2014) Whole genome sequencing in Drosophila virilis identifies Polyphemus, a recently activated Tc1-like transposon with a possible role in hybrid dysgenesis. Mob DNA 5 : 6. doi: 10.1186/1759-8753-5-6 24555450
34. Rozhkov NV, Schostak NG, Zelentsova ES, Yushenova IA, Zatsepina OG, et al. (2013) Evolution and dynamics of small RNA response to a retroelement invasion in Drosophila. Mol Biol Evol 30 : 397–408. doi: 10.1093/molbev/mss241 23079419
35. Rozhkov NV, Aravin AA, Zelentsova ES, Schostak NG, Sachidanandam R, et al. (2010) Small RNA-based silencing strategies for transposons in the process of invading Drosophila species. Rna-a Publication of the Rna Society 16 : 1634–1645.
36. Gerasimova T, Mizrokhi L, Georgiev G (1984) Transposition bursts in genetically unstable Drosophila melanogaster. Nature 309 : 714–716.
37. Eggleston WB, Johnson-Schlitz DM, Engels WR (1988) P-M hybrid dysgenesis does not mobilize other transposable element families in D. melanogaster. Nature 331 : 368–370. 2829026
38. Petrov DA, Schutzman JL, Hartl DL, Lozovskaya ER (1995) Diverse transposable elements are mobilized in hybrid dysgenesis in Drosophila virilis Proceedings of the National Academy of Sciences of the United States of America 92 : 8050–8054. 7644536
39. Rozhkov NV, Zelentsova ES, Shostak NG, Evgen'ev MB (2011) Expression of Drosophila virilis retroelements and role of small RNAs in their intrastrain transposition. PLoS One 6: e21883. doi: 10.1371/journal.pone.0021883 21779346
40. Scheinker VS, Lozovskaya ER, Bishop JG, Corces VG, Evgenev MB (1990) A long terminal repeat-containing retrotransposon in mobilized during hybrid dysgenesis in Drosophila virilis. Proceedings of the National Academy of Sciences of the United States of America 87 : 9615–9619. 2175908
41. Casacuberta E, Pardue ML (2003) Transposon telomeres are widely distributed in the Drosophila genus: TART elements in the virilis group. Proc Natl Acad Sci U S A 100 : 3363–3368. 12626755
42. Savitsky M, Kwon D, Georgiev P, Kalmykova A, Gvozdev V (2006) Telomere elongation is under the control of the RNAi-based mechanism in the Drosophila germline. Genes & Development 20 : 345–354.
43. Shpiz S, Olovnikov I, Sergeeva A, Lavrov S, Abramov Y, et al. (2011) Mechanism of the piRNA-mediated silencing of Drosophila telomeric retrotransposons. Nucleic Acids Res 39 : 8703–8711. doi: 10.1093/nar/gkr552 21764773
44. Khurana JS, Xu J, Weng Z, Theurkauf WE (2010) Distinct functions for the Drosophila piRNA pathway in genome maintenance and telomere protection. PLoS Genet 6: e1001246. doi: 10.1371/journal.pgen.1001246 21179579
45. Evgen'ev MB (2013) What happens when Penelope comes?: An unusual retroelement invades a host species genome exploring different strategies. Mob Genet Elements 3: e24542. 23914310
46. Daniels SB, Peterson KR, Strausbaugh LD, Kidwell MG, Chovnick A (1990) Evidence for horizontal transmission of the P transposable element between Drosophila species. Genetics 124 : 339–355. 2155157
47. Morales-Hojas R, Vieira CP, Vieira J (2006) The evolutionary history of the transposable element Penelope in the Drosophila virilis group of species. J Mol Evol 63 : 262–273. 16830099
48. Chambeyron S, Popkova A, Payen-Groschene G, Brun C, Laouini D, et al. (2008) piRNA-mediated nuclear accumulation of retrotransposon transcripts in the Drosophila female germline. Proceedings of the National Academy of Sciences of the United States of America 105 : 14964–14969. doi: 10.1073/pnas.0805943105 18809914
49. Blumenstiel JP, Hartl DL (2005) Evidence for maternally transmitted small interfering RNA in the repression of transposition in Drosophila virilis. Proceedings of the National Academy of Sciences of the United States of America 102 : 15965–15970. 16247000
50. Eden E, Navon R, Steinfeld I, Lipson D, Yakhini Z (2009) GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics 10 : 48. doi: 10.1186/1471-2105-10-48 19192299
51. Pavlidis P, Jensen JD, Stephan W, Stamatakis A (2012) A critical assessment of storytelling: gene ontology categories and the importance of validating genomic scans. Mol Biol Evol 29 : 3237–3248. 22617950
52. Gu T, Elgin SC (2013) Maternal depletion of Piwi, a component of the RNAi system, impacts heterochromatin formation in Drosophila. PLoS Genet 9: e1003780. doi: 10.1371/journal.pgen.1003780 24068954
53. Haynes KA, Caudy AA, Collins L, Elgin SCR (2006) Element 1360 and RNAi components contribute to HP1-dependent silencing of a pericentric reporter. Current Biology 16 : 2222–2227. 17113386
54. Sentmanat MF, Elgin SC (2012) Ectopic assembly of heterochromatin in Drosophila melanogaster triggered by transposable elements. Proc Natl Acad Sci U S A 109 : 14104–14109. doi: 10.1073/pnas.1207036109 22891327
55. Sienski G, Donertas D, Brennecke J (2012) Transcriptional silencing of transposons by Piwi and maelstrom and its impact on chromatin state and gene expression. Cell 151 : 964–980. doi: 10.1016/j.cell.2012.10.040 23159368
56. Olovnikov I, Ryazansky S, Shpiz S, Lavrov S, Abramov Y, et al. (2013) De novo piRNA cluster formation in the Drosophila germ line triggered by transgenes containing a transcribed transposon fragment. Nucleic Acids Res 41 : 5757–5768. doi: 10.1093/nar/gkt310 23620285
57. Shpiz S, Ryazansky S, Olovnikov I, Abramov Y, Kalmykova A (2014) Euchromatic transposon insertions trigger production of novel Pi - and endo-siRNAs at the target sites in the drosophila germline. PLoS Genet 10: e1004138. doi: 10.1371/journal.pgen.1004138 24516406
58. Czech B, Preall JB, McGinn J, Hannon GJ (2013) A transcriptome-wide RNAi screen in the Drosophila ovary reveals factors of the germline piRNA pathway. Mol Cell 50 : 749–761. doi: 10.1016/j.molcel.2013.04.007 23665227
59. Ronsseray S, Josse T, Boivin A, Anxolabehere D (2003) Telomeric transgenes and trans-silencing in Drosophila. Genetica 117 : 327–335. 12723712
60. Ronsseray S, Marin L, Lehmann M, Anxolabehere D (1998) Repression of hybrid dysgenesis in Drosophila melanogaster by combinations of telomeric P-element reporters and naturally occurring P elements. Genetics 149 : 1857–1866. 9691042
61. Marin L, Lehmann M, Nouaud D, Izaabel H, Anxolabehere D, et al. (2000) P-element repression in Drosophila melanogaster by a naturally occurring defective telomeric P copy. Genetics 155 : 1841–1854. 10924479
62. Simmons MJ, Ragatz LM, Sinclair IR, Thorp MW, Buschette JT, et al. (2012) Maternal enhancement of cytotype regulation in Drosophila melanogaster by genetic interactions between telomeric P elements and non-telomeric transgenic P elements. Genet Res (Camb) 94 : 339–351.
63. Niemi JB, Raymond JD, Patrek R, Simmons MJ (2004) Establishment and maintenance of the P cytotype associated with telomeric P elements in Drosophila melanogaster. Genetics 166 : 255–264. 15020423
64. Rozhkov NV, Aravin AA, Zelentsova ES, Schostak NG, Sachidanandam R, et al. (2010) Small RNA-based silencing strategies for transposons in the process of invading Drosophila species. RNA 16 : 1634–1645. doi: 10.1261/rna.2217810 20581131
65. Le Thomas A, Stuwe E, Li S, Du J, Marinov G, et al. (2014) Transgenerationally inherited piRNAs trigger piRNA biogenesis by changing the chromatin of piRNA clusters and inducing precursor processing. Genes Dev 28 : 1667–1680. doi: 10.1101/gad.245514.114 25085419
66. de Vanssay A, Bouge AL, Boivin A, Hermant C, Teysset L, et al. (2012) Paramutation in Drosophila linked to emergence of a piRNA-producing locus. Nature 490 : 112–115. doi: 10.1038/nature11416 22922650
67. Le Thomas A, Marinov GK, Aravin AA (2014) A transgenerational process defines piRNA biogenesis in Drosophila virilis. Cell Rep 8 : 1617–1623. doi: 10.1016/j.celrep.2014.08.013 25199836
68. Obbard DJ, Gordon KHJ, Buck AH, Jiggins FM (2009) The evolution of RNAi as a defence against viruses and transposable elements. Philosophical Transactions of the Royal Society B-Biological Sciences 364 : 99–115.
69. Kolaczkowski B, Hupalo DN, Kern AD (2010) Recurrent Adaptation in RNA Interference Genes Across the Drosophila Phylogeny. Molecular Biology and Evolution 28 : 1033–1042. doi: 10.1093/molbev/msq284 20971974
70. Simkin A, Wong A, Poh YP, Theurkauf WE, Jensen JD (2013) Recurrent and recent selective sweeps in the piRNA pathway. Evolution 67 : 1081–1090. doi: 10.1111/evo.12011 23550757
71. Zhang F, Wang J, Xu J, Zhang Z, Koppetsch BS, et al. (2012) UAP56 couples piRNA clusters to the perinuclear transposon silencing machinery. Cell 151 : 871–884. doi: 10.1016/j.cell.2012.09.040 23141543
72. Arteaga-Vazquez M, Sidorenko L, Rabanal FA, Shrivistava R, Nobuta K, et al. (2010) RNA-mediated trans-communication can establish paramutation at the b1 locus in maize. Proc Natl Acad Sci U S A 107 : 12986–12991. doi: 10.1073/pnas.1007972107 20616013
73. Alleman M, Sidorenko L, McGinnis K, Seshadri V, Dorweiler JE, et al. (2006) An RNA-dependent RNA polymerase is required for paramutation in maize. Nature 442 : 295–298. 16855589
74. Chandler VL (2007) Paramutation: from maize to mice. Cell 128 : 641–645. 17320501
75. Mott R, Yuan W, Kaisaki P, Gan X, Cleak J, et al. (2014) The architecture of parent-of-origin effects in mice. Cell 156 : 332–342. doi: 10.1016/j.cell.2013.11.043 24439386
76. Gibson G, Riley-Berger R, Harshman L, Kopp A, Vacha S, et al. (2004) Extensive sex-specific nonadditivity of gene expression in Drosophila melanogaster. Genetics 167 : 1791–1799. 15342517
77. Wittkopp PJ, Haerum BK, Clark AG (2006) Parent-of-origin effects on mRNA expression in Drosophila melanogaster not caused by genomic imprinting. Genetics 173 : 1817–1821. 16702434
78. Bergman CM, Quesneville H, Anxolabehere D, Ashburner M (2006) Recurrent insertion and duplication generate networks of transposable element sequences in the Drosophila melanogaster genome. Genome Biol 7: R112. 17134480
79. Malone CD, Brennecke J, Dus M, Stark A, McCombie WR, et al. (2009) Specialized piRNA Pathways Act in Germline and Somatic Tissues of the Drosophila Ovary. Cell 137 : 522–535. doi: 10.1016/j.cell.2009.03.040 19395010
80. Komili S, Farny NG, Roth FP, Silver PA (2007) Functional specificity among ribosomal proteins regulates gene expression. Cell 131 : 557–571. 17981122
81. Vastenhouw NL, Fischer SE, Robert VJ, Thijssen KL, Fraser AG, et al. (2003) A genome-wide screen identifies 27 genes involved in transposon silencing in C. elegans. Curr Biol 13 : 1311–1316. 12906791
82. Zhang Y, Lu H (2009) Signaling to p53: ribosomal proteins find their way. Cancer Cell 16 : 369–377. doi: 10.1016/j.ccr.2009.09.024 19878869
83. Smith CD, Edgar RC, Yandell MD, Smith DR, Celniker SE, et al. (2007) Improved repeat identification and masking in Dipterans. Gene 389 : 1–9. 17137733
84. Li R, Ye J, Li S, Wang J, Han Y, et al. (2005) ReAS: Recovery of ancestral sequences for transposable elements from the unassembled reads of a whole genome shotgun. PLoS Comput Biol 1: e43. 16184192
85. Clark AG, Eisen MB, Smith DR, Bergman CM, Oliver B, et al. (2007) Evolution of genes and genomes on the Drosophila phylogeny. Nature 450 : 203–218. 17994087
86. Bosco G, Campbell P, Leiva-Neto JT, Markow TA (2007) Analysis of Drosophila species genome size and satellite DNA content reveals significant differences among strains as well as between species. Genetics 177 : 1277–1290. 18039867
87. Blumenstiel JP, Noll AC, Griffiths JA, Perera AG, Walton KN, et al. (2009) Identification of EMS-Induced Mutations in Drosophila melanogaster by Whole-Genome Sequencing. Genetics 182 : 25–32. doi: 10.1534/genetics.109.101998 19307605
88. LI H (2013) Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXivorg arXiv:1303.3997v2.
89. Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15 : 550. 25516281
90. Vigneault F, Ter-Ovanesyan D, Alon S, Eminaga S, D CC, et al. (2012) High-throughput multiplex sequencing of miRNA. Curr Protoc Hum Genet Chapter 11: Unit 11 12 11–10.
91. Li CJ, Vagin VV, Lee SH, Xu J, Ma SM, et al. (2009) Collapse of Germline piRNAs in the Absence of Argonaute3 Reveals Somatic piRNAs in Flies. Cell 137 : 509–521. doi: 10.1016/j.cell.2009.04.027 19395009
92. Li H, Durbin R (2010) Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26 : 589–595. doi: 10.1093/bioinformatics/btp698 20080505
93. Quinlan AR, Hall IM BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26 : 841–842. doi: 10.1093/bioinformatics/btq033 20110278
Štítky
Genetika Reprodukční medicína
Článek Loss and Gain of Natural Killer Cell Receptor Function in an African Hunter-Gatherer PopulationČlánek Let-7 Represses Carcinogenesis and a Stem Cell Phenotype in the Intestine via Regulation of Hmga2Článek Binding of Multiple Rap1 Proteins Stimulates Chromosome Breakage Induction during DNA ReplicationČlánek SLIRP Regulates the Rate of Mitochondrial Protein Synthesis and Protects LRPPRC from DegradationČlánek Protein Composition of Infectious Spores Reveals Novel Sexual Development and Germination Factors inČlánek The Formin Diaphanous Regulates Myoblast Fusion through Actin Polymerization and Arp2/3 RegulationČlánek Runx1 Transcription Factor Is Required for Myoblasts Proliferation during Muscle Regeneration
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 8
-
Všechny články tohoto čísla
- Putting the Brakes on Huntington Disease in a Mouse Experimental Model
- Identification of Driving Fusion Genes and Genomic Landscape of Medullary Thyroid Cancer
- Evidence for Retromutagenesis as a Mechanism for Adaptive Mutation in
- TSPO, a Mitochondrial Outer Membrane Protein, Controls Ethanol-Related Behaviors in
- Evidence for Lysosome Depletion and Impaired Autophagic Clearance in Hereditary Spastic Paraplegia Type SPG11
- Loss and Gain of Natural Killer Cell Receptor Function in an African Hunter-Gatherer Population
- Trans-Reactivation: A New Epigenetic Phenomenon Underlying Transcriptional Reactivation of Silenced Genes
- Early Developmental and Evolutionary Origins of Gene Body DNA Methylation Patterns in Mammalian Placentas
- Strong Selective Sweeps on the X Chromosome in the Human-Chimpanzee Ancestor Explain Its Low Divergence
- Dominance of Deleterious Alleles Controls the Response to a Population Bottleneck
- Transient 1a Induction Defines the Wound Epidermis during Zebrafish Fin Regeneration
- Systems Genetics Reveals the Functional Context of PCOS Loci and Identifies Genetic and Molecular Mechanisms of Disease Heterogeneity
- A Genome Scale Screen for Mutants with Delayed Exit from Mitosis: Ire1-Independent Induction of Autophagy Integrates ER Homeostasis into Mitotic Lifespan
- Non-synonymous FGD3 Variant as Positional Candidate for Disproportional Tall Stature Accounting for a Carcass Weight QTL () and Skeletal Dysplasia in Japanese Black Cattle
- The Relationship between Gene Network Structure and Expression Variation among Individuals and Species
- Calmodulin Methyltransferase Is Required for Growth, Muscle Strength, Somatosensory Development and Brain Function
- The Wnt Frizzled Receptor MOM-5 Regulates the UNC-5 Netrin Receptor through Small GTPase-Dependent Signaling to Determine the Polarity of Migrating Cells
- Nbs1 ChIP-Seq Identifies Off-Target DNA Double-Strand Breaks Induced by AID in Activated Splenic B Cells
- CCNYL1, but Not CCNY, Cooperates with CDK16 to Regulate Spermatogenesis in Mouse
- Evidence for a Common Origin of Blacksmiths and Cultivators in the Ethiopian Ari within the Last 4500 Years: Lessons for Clustering-Based Inference
- Of Fighting Flies, Mice, and Men: Are Some of the Molecular and Neuronal Mechanisms of Aggression Universal in the Animal Kingdom?
- Hypoxia and Temperature Regulated Morphogenesis in
- The Homeodomain Iroquois Proteins Control Cell Cycle Progression and Regulate the Size of Developmental Fields
- Evolution and Design Governing Signal Precision and Amplification in a Bacterial Chemosensory Pathway
- Rac1 Regulates Endometrial Secretory Function to Control Placental Development
- Let-7 Represses Carcinogenesis and a Stem Cell Phenotype in the Intestine via Regulation of Hmga2
- Functions as a Positive Regulator of Growth and Metabolism in
- The Nucleosome Acidic Patch Regulates the H2B K123 Monoubiquitylation Cascade and Transcription Elongation in
- Rhoptry Proteins ROP5 and ROP18 Are Major Murine Virulence Factors in Genetically Divergent South American Strains of
- Exon 7 Contributes to the Stable Localization of Xist RNA on the Inactive X-Chromosome
- Regulates Refractive Error and Myopia Development in Mice and Humans
- mTORC1 Prevents Preosteoblast Differentiation through the Notch Signaling Pathway
- Regulation of Gene Expression Patterns in Mosquito Reproduction
- Molecular Basis of Gene-Gene Interaction: Cyclic Cross-Regulation of Gene Expression and Post-GWAS Gene-Gene Interaction Involved in Atrial Fibrillation
- The Spalt Transcription Factors Generate the Transcriptional Landscape of the Wing Pouch Central Region
- Binding of Multiple Rap1 Proteins Stimulates Chromosome Breakage Induction during DNA Replication
- Functional Divergence in the Role of N-Linked Glycosylation in Smoothened Signaling
- YAP1 Exerts Its Transcriptional Control via TEAD-Mediated Activation of Enhancers
- Coordinated Evolution of Influenza A Surface Proteins
- The Evolutionary Potential of Phenotypic Mutations
- Genome-Wide Association and Trans-ethnic Meta-Analysis for Advanced Diabetic Kidney Disease: Family Investigation of Nephropathy and Diabetes (FIND)
- New Routes to Phylogeography: A Bayesian Structured Coalescent Approximation
- SLIRP Regulates the Rate of Mitochondrial Protein Synthesis and Protects LRPPRC from Degradation
- Satellite DNA Modulates Gene Expression in the Beetle after Heat Stress
- SHOEBOX Modulates Root Meristem Size in Rice through Dose-Dependent Effects of Gibberellins on Cell Elongation and Proliferation
- Reduced Crossover Interference and Increased ZMM-Independent Recombination in the Absence of Tel1/ATM
- Suppression of Somatic Expansion Delays the Onset of Pathophysiology in a Mouse Model of Huntington’s Disease
- Protein Composition of Infectious Spores Reveals Novel Sexual Development and Germination Factors in
- The Evolutionarily Conserved LIM Homeodomain Protein LIM-4/LHX6 Specifies the Terminal Identity of a Cholinergic and Peptidergic . Sensory/Inter/Motor Neuron-Type
- SmD1 Modulates the miRNA Pathway Independently of Its Pre-mRNA Splicing Function
- piRNAs Are Associated with Diverse Transgenerational Effects on Gene and Transposon Expression in a Hybrid Dysgenic Syndrome of .
- Retinoic Acid Signaling Regulates Differential Expression of the Tandemly-Duplicated Long Wavelength-Sensitive Cone Opsin Genes in Zebrafish
- The Formin Diaphanous Regulates Myoblast Fusion through Actin Polymerization and Arp2/3 Regulation
- Genome-Wide Analysis of PAPS1-Dependent Polyadenylation Identifies Novel Roles for Functionally Specialized Poly(A) Polymerases in
- Runx1 Transcription Factor Is Required for Myoblasts Proliferation during Muscle Regeneration
- Regulation of Mutagenic DNA Polymerase V Activation in Space and Time
- Variability of Gene Expression Identifies Transcriptional Regulators of Early Human Embryonic Development
- The Drosophila Gene Interacts Genetically with and Shows Female-Specific Effects of Divergence
- Functional Activation of the Flagellar Type III Secretion Export Apparatus
- Retrohoming of a Mobile Group II Intron in Human Cells Suggests How Eukaryotes Limit Group II Intron Proliferation
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Exon 7 Contributes to the Stable Localization of Xist RNA on the Inactive X-Chromosome
- YAP1 Exerts Its Transcriptional Control via TEAD-Mediated Activation of Enhancers
- SmD1 Modulates the miRNA Pathway Independently of Its Pre-mRNA Splicing Function
- Molecular Basis of Gene-Gene Interaction: Cyclic Cross-Regulation of Gene Expression and Post-GWAS Gene-Gene Interaction Involved in Atrial Fibrillation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



