-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaClosing the Gap between Knowledge and Clinical Application: Challenges for Genomic Translation
Despite early predictions and rapid progress in research, the introduction of personal genomics into clinical practice has been slow. Several factors contribute to this translational gap between knowledge and clinical application. The evidence available to support genetic test use is often limited, and implementation of new testing programs can be challenging. In addition, the heterogeneity of genomic risk information points to the need for strategies to select and deliver the information most appropriate for particular clinical needs. Accomplishing these tasks also requires recognition that some expectations for personal genomics are unrealistic, notably expectations concerning the clinical utility of genomic risk assessment for common complex diseases. Efforts are needed to improve the body of evidence addressing clinical outcomes for genomics, apply implementation science to personal genomics, and develop realistic goals for genomic risk assessment. In addition, translational research should emphasize the broader benefits of genomic knowledge, including applications of genomic research that provide clinical benefit outside the context of personal genomic risk.
Published in the journal: . PLoS Genet 11(2): e32767. doi:10.1371/journal.pgen.1004978
Category: Review
doi: https://doi.org/10.1371/journal.pgen.1004978Summary
Despite early predictions and rapid progress in research, the introduction of personal genomics into clinical practice has been slow. Several factors contribute to this translational gap between knowledge and clinical application. The evidence available to support genetic test use is often limited, and implementation of new testing programs can be challenging. In addition, the heterogeneity of genomic risk information points to the need for strategies to select and deliver the information most appropriate for particular clinical needs. Accomplishing these tasks also requires recognition that some expectations for personal genomics are unrealistic, notably expectations concerning the clinical utility of genomic risk assessment for common complex diseases. Efforts are needed to improve the body of evidence addressing clinical outcomes for genomics, apply implementation science to personal genomics, and develop realistic goals for genomic risk assessment. In addition, translational research should emphasize the broader benefits of genomic knowledge, including applications of genomic research that provide clinical benefit outside the context of personal genomic risk.
Introduction
Despite early predictions [1,2], genomic research has not (yet) created a new, more personalized medical care. Many reasons have been offered for this gap between expectations and reality. Some emphasize the evidence deficit: few genetic tests have been demonstrated to improve health outcomes [3,4]. Others point to the slow process of translation, calling for clinician education and decision support to expedite uptake of personal genomics [5,6]. Still others question the proposition that genomics will revolutionize medical care, arguing instead that expectations for personal genomics are inflated [7]. In this paper we explore these explanations and suggest that each offers insights for addressing the gap between genomic knowledge and clinical application.
Evidence
Many genetic tests have been marketed with scant evidence of clinical value. For example, a guidelines group evaluating CYP450 testing to inform use of selective serotonin reuptake inhibitors (SSRIs) for depression found no evidence that testing assisted decisions about drug use or improved patient outcomes [8]. Further, CYP450 genotypes were not clearly correlated with drug levels in people using SSRIs [8]. Clinicians are unlikely to embrace practice change when the evidence for benefit is so uncertain or incomplete.
But how much evidence is enough? The few tests that have moved rapidly into clinical practice suggest that evidence requirements vary. For example, clinical testing for BRCA mutations began within a few years of gene discovery, based on strong evidence of clinical validity—that is, evidence for a significant association between mutations in the BRCA1 and BRCA2 genes and risk of breast and ovarian cancer [9]—but without evidence of improved health outcomes after testing [10]. The likely explanation for this rapid translation is that clinicians valued a test that could identify which members of high-risk families had inherited the cancer risk. In this instance, clinical validity was sufficient to provide a test with a clear clinical purpose: to guide screening and prevention measures already in use for women at high risk [10].
Gene expression profiling of breast tumors [11] offers a more contested example. Gene expression assays can be used to identify women at low risk of recurrence, who might safely avoid adjuvant chemotherapy, and clinical studies document changes in chemotherapy recommendations with testing [12]. However, there are differences of opinion among expert groups about the evidence. Some consider the retrospective data establishing the clinical validity of gene expression profiling sufficient, while others argue that prospective clinical trials are still needed [13,14]. In fact, randomized controlled trials (RCTs) have played a pivotal role in the uptake of some genetic tests: an RCT demonstrating benefit was key to wide adoption of pharmacogenetic testing for the drug abacavir [15,16]. Conversely, recent RCTs with partially conflicting results have failed to resolve the debate about the value of pharmacogenetic testing for warfarin therapy [17–19].
The issue of adequate evidence is likely to become even more controversial as whole genome approaches are adopted. For example, success in the development of targeted therapies for some tumor mutations has led to increasing use of tumor genome analysis in oncology [20]. Yet tumors are often genetically heterogeneous and develop new genetic changes over time [20]; therefore, assessing appropriate uses and outcomes of this testing approach may require innovative analytic approaches.
These examples indicate that the evidence needed to justify clinical use of a new genetic test varies. For tests that meet a defined clinical need, the evidence requirements are likely to be obvious, and often may not involve RCTs, as BRCA testing illustrates. Where the purpose of testing is less clear, or the results difficult to interpret, the evidence requirements are likely to be more stringent, and experts may disagree on the evidence threshold. Expediting genomic translation therefore requires two efforts related to evidence: more clinical research focused on health outcomes, and consensus development concerning acceptable evidence thresholds for different uses of genomic information [21]. Evidence requirements will be particularly important—and challenging to define—as genomics moves from tests of individual genes to whole exome and whole genome testing.
Implementation Science
Although lack of evidence explains why some genetic tests are slow to enter clinical practice, it cannot explain the poor uptake of tests for which there is strong evidence of benefit. Documented barriers to appropriate genetic testing include lack of genetics knowledge among point-of-care physicians [22] and a dearth of useful (and useable) clinical decision support. In addition, patients may be concerned about the cost of testing and follow-up or have difficulty understanding complex testing protocols. Patient follow-up may be particularly challenging among socioeconomically disadvantaged populations [23]. As these barriers suggest, there is no single “correct” approach to implementation issues, because health systems vary in staffing, location, and capacity of the different clinical specialties involved. In particular, genetics resources (genetic counselors, medical geneticists, specialists with genetics expertise) vary and may dictate differing assignment of responsibilities in different health systems.
A case in point is universal tumor screening for Lynch Syndrome (LS) among patients with colorectal cancer. LS is a condition that confers a high lifetime risk of colorectal cancer and accounts for 2%–4% of colorectal cancers in the US [24]. The Evaluation of Genomic Applications in Practice and Prevention (EGAPP) program recommends screening for LS in individuals newly diagnosed with colorectal cancer to identify patients and family members at risk who will benefit from targeted screening and follow-up [25]. There are many approaches to LS screening, involving choices about the initial screening test, the application of family history criteria at different stages of screening, and the methods used to reach family members when a proband is diagnosed with LS. Successful screening therefore requires local planning to define the preferred screening approach, followed by systematic procedures for implementation of each step of the screening process [26,27]. Given this complexity, it is not surprising that universal LS screening is far from common, with significant variability in screening procedures and low rates of follow-up of patients and family members [23,27,28].
Implementation science, which focuses on identifying and overcoming barriers associated with deploying and tailoring new interventions, offers a means to address the gap between testing capability and practice. The Consolidated Framework for Implementation Research (CFIR), developed by health services researchers at the Veterans Administration, can be a particularly useful framework because it provides a model that can inform both the initial implementation approach and the subsequent analysis that identifies the barriers and facilitators of success that can be used to guide improvements [29]. As summarized in Table 1, CFIR recognizes several domains critical for successful deployment of a new medical service. The application of this framework to LS screening (Table 1) identifies many factors in implementation, and points to specific actions that institutions could take to improve uptake. For example, institutions need to be mindful of local capabilities and the need to coordinate LS screening across the different specialties of oncology, gastroenterology, and primary care. Some suggest the Electronic Medical Record as a way to standardize guideline adherence. However, not all organizations will have the information technology capabilities necessary for successful implementation [30,31]. Institutions may also need to consider the genetics knowledge of the clinicians ordering the tests and the commitment of institution administrators to implementation of a new standard of care. LS screening is likely to be launched successfully only if these issues are taken into account as screening procedures are adapted to local circumstances.
Tab. 1. Consolidated Framework for Implementation Research (CFIR) domains and Lynch Syndrome screening implementation. 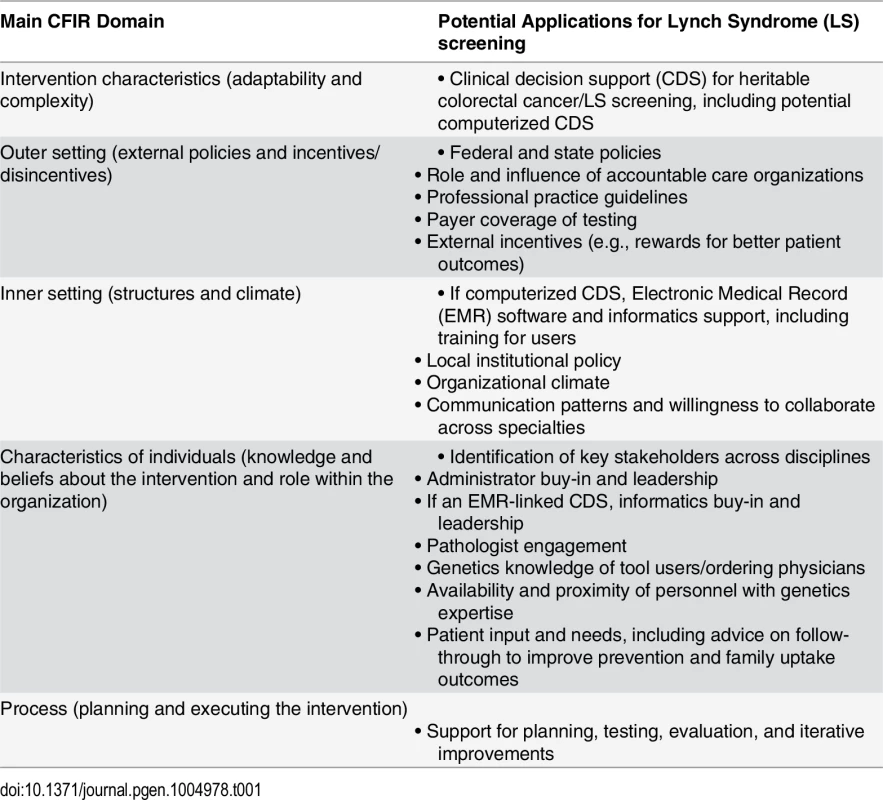
Planning, buy-in, and execution will take different forms for different applications of genomic medicine, but the systematic approaches suggested by the CFIR framework will remain relevant. With the move from programs based on single gene conditions such as LS to genome sequencing approaches, a wide range of implementation challenges will need to be considered, including efficient identification and referral of patients, informed consent procedures, laboratory quality measures, and the scope of secondary findings, unrelated to the clinical problem for which testing was done, to be assessed and delivered. To address these challenges, investments in implementation science will be an important priority for genomics.
Setting Expectations
As efforts to strengthen the evidence base and pursue implementation science are undertaken, there is a concurrent need to define realistic expectations for personal genomics. The health information incorporated in the human genome is complex and heterogeneous; its value varies according to both the nature of the information and the circumstances of the patient. Information about being a carrier for an X-linked or autosomal recessive disease, for example, is primarily of value for people of reproductive age, and only then if they choose to use such information in reproductive decision making. Similarly, cancer risk information may be highly valuable to a young adult but of little interest to an elderly patient with end stage heart disease. This heterogeneity points to a central challenge for genomic medicine: the need to define the genomic information that is useful in a particular clinical context.
For example, pharmacogenetic testing can improve the safety of abacavir treatment for HIV-AIDS, and thiopurine treatment for acute lymphoblastic leukemia [15,32]. In both cases, pharmacogenetic tests are relevant only in particular clinical circumstances and serve to identify the minority of patients who are at risk for severe adverse reactions, so that an alternative drug or dosage can be used. This information has high clinical utility and points to a future in which pharmacogenomic testing will improve the safety and efficacy of medication use. However, conventional drug choices work for most people; few need individualized adjustment. Similarly, the early benefits of whole genome sequencing have related to gene discovery for rare disorders [33,34] and improved diagnosis of individuals with rare phenotypes whose problems have eluded conventional work-up [35].
Although personal genomics is touted as the means to move from one-size-fits-all to a more individualized approach to health care [20,36], these examples, as well as LS and BRCA testing, suggest a different interpretation: genomic risk assessment identifies the minority of outliers who require a modification in treatment or prevention efforts. These successes of genomic medicine underscore, paradoxically, that one size often does fit most, if not all.
In other words, the benefits of genomic risk assessment are important, but have little to do with the health care needs of most people, most of the time. Universal recommendations for vaccinations, Pap testing, and blood pressure evaluation still apply in the era of genomics, and all of us will benefit from well-balanced diets, regular exercise, and avoidance of cigarettes, no matter what genetic predispositions we have. When individual adjustments to care are needed, they most often relate to comorbidities or social circumstances [37]—for example, individualizing an exercise program for a person who uses a wheelchair or adjusting Pap screening recommendations based on HPV status.
As a corollary, genomic risk prediction is likely to be least effective in addressing the population health burdens that matter most—those deriving from common complex diseases such as diabetes, heart disease, stroke, and cancer. Genetics contributes to risk for all these conditions. Rare outliers have high risk due to inheritance of highly penetrant mutations such as those causing LS. More commonly, genetics is only a modest contributor to risk.
For example, variation at over 40 loci is associated with likelihood of developing type 2 diabetes, but a few lifestyle factors account for the majority of risk [38–40]. A recent study evaluating diabetes risk in more than 25,000 individuals illustrates the key role of lifestyle. In this study, a genetic risk score had substantially less effect on absolute risk than obesity: among normal weight individuals, 10-year risk of type 2 diabetes ranged from 0.25% to 0.89% across genetic score quartiles, while for obese individuals the risk ranged from 4.22% to 7.99% [38].
The same general observation applies to virtually all other common diseases: although genetic variation contributes to differences in individual risk, lifestyle and other social determinants of health play a dominant role in health outcomes [41]. Variance in genetic risk for common complex diseases is modest compared to the effect of social factors. Thus, although the genomic risk profile of each individual is unique, most people’s genetic susceptibilities fall within a limited range, from a little below to a little above average [42]. This point is particularly important in considering health disparities. For most people, in the words of Thomas Frieden, head of the Centers for Disease Control and Prevention, “your longevity and health are more determined by your ZIP code than they are by your genetic code” [43]. While the heritability of many diseases is only partially defined [20,39], there is little reason to think that a more complete description of genetic contributors will change this fundamental reality [44].
Further, identifying risk for common diseases is generally not difficult: a variety of metrics, including weight, blood pressure, and biomarkers such as cholesterol and hemoglobin A1c, are available for this purpose. Assisting people to make behavioral changes to reduce their health risks is more difficult. Yet it has been estimated that hundreds of thousands of premature deaths could be avoided by reducing smoking, improving diet, and increasing activity levels [41]. As a corollary, public policies related to availability of healthy food, safe places to exercise, access to smoking cessation programs, and research on lifestyle modification are likely to be better long-term investments for improving public health than providing genetic screening for addressing common disease risks.
To be sure, genomic risk assessment is still of value, and opportunities to improve health outcomes through genomic screening are likely to increase over time. As the LS example illustrates, clinical translation of such discoveries will require both evidence for improved outcomes from screening and systematic efforts to implement screening programs. In addition to single gene conditions like LS, further research may point to ways in which genomic risk profiles can be used to identify outliers with high cumulative risk for complex diseases [42]. As this knowledge accumulates, there will be increasing justification for genome-scale screening to ensure that high-risk individuals are offered appropriate targeted care. In some instances, genomic risk profiling could provide benefits in cases not limited to high-risk conditions like LS: for example, a genomic risk profile for cancer could conceivably outperform family history as a means to identify individuals with moderately increased risk who are candidates for early breast, colorectal, or prostate cancer screening [45]. The potential harms of screening [46] and the many nongenetic contributors to risk suggest, however, that this type of genomic profiling will require robust evidence of benefit [47].
It remains the case that genomic discovery related to the major disease burdens of the population will yield many variants of small effect. For most such findings, there is no translational gap in personalized medicine to overcome, because the information lacks clinical value. Instead, there will be an increasing need for analytic, technical, and clinical strategies that pull from the genome the information that can improve health care, while avoiding the introduction of large amounts of poorly predictive and distracting health information into the medical record.
Conclusion: Moving Beyond Personal Genomes
Although most gene variants associated with common complex diseases will be poorly predictive and lack clinical utility for individual health care, they nevertheless represent highly valuable research findings. Every gene causally associated with disease is a marker for a biological pathway, potentially revealing unexpected mechanisms of disease, connections between different pathological processes, and interactions between biological pathways and environmental risk factors. Promising examples are proliferating at a rapid rate. For instance, genome wide association studies (GWAS) have clarified the importance of autophagy in the pathogenesis of autoimmune disease, identified new loci associated with disease, and pointed to shared pathways between inflammatory bowel disease susceptibility and host responses to mycobacterial infection [48]. In age-related macular degeneration, GWAS played a pivotal role in clarifying the importance of the complement system in pathophysiology of the disease [49], leading to new insights for therapy. Studies of the genetics of type 2 diabetes similarly provide insights about the relationship between this disorder and cardiovascular disease [50], the role of immune factors [51], and diverse contributors to insulin resistance [52]. These examples underscore the power of genome-scale research methods that do not rely on prior biological hypotheses for gene discovery, and point to the increasing potential for progress as researchers move from GWAS to whole genome studies [53].
Genomics is a source of an expanding set of molecular tools for other avenues of health research as well [20]. For example, there is a growing body of research demonstrating the powerful effect of epigenetic changes in gene expression as a source of disease risk [54]. These studies may provide new insights into social determinants of health and could conceivably inform social policies related to issues such as maternal and early childhood nutrition or other environmental exposures relevant to health.
Genomic research can also improve care by defining the genotypes of other organisms. In a recent widely publicized case, an infectious agent was identified and treated through the use of DNA-based diagnostics [55], pointing to the growing use of genomics in pathogen identification [56]. This use of genomics is an early indicator of an expanding role for the genomics of pathogenic and symbiotic organisms, with applications including the assessment (and potential amelioration) of the microbiome [57] and the use of genomics in vaccine development [56].
These examples provide only a glimpse of the potential benefits of genomic health research. However, they offer an important insight about closing the gap between genomic knowledge and clinical care: the task is not solely, or even primarily, one of learning how to use individual genomes in clinical care [58]. A person’s genotype undoubtedly offers useful information in some clinical circumstances, but much of the benefit will come from leveraging genomic knowledge to develop a more precise understanding of molecular physiology. Such efforts point to a different way in which the translational gap may be closed: by developing prevention and therapeutic strategies that provide benefit outside the context of genetic risk.
Zdroje
1. Subramanian G, Adams MD, Venter JC, Broder S (2001) Implications of the human genome for understanding human biology and medicine. JAMA 286 : 2296–2307. 11710896
2. Collins FS, McKusick VA (2001) Implications of the Human Genome Project for medical science. JAMA 285 : 540–544. 11176855
3. Hayes DF, Khoury MJ, Ransohoff D (2012) Why Hasn’t Genomic Testing Changed the Landscape in Clinical Oncology? Am Soc Clin Oncol Educ Book: e52–55. doi: 10.14694/EdBook_AM.2012.32.e52 24451831
4. Khoury MJ (2010) Dealing with the evidence dilemma in genomics and personalized medicine. Clin Pharmacol Ther 87 : 635–638. doi: 10.1038/clpt.2010.4 20485318
5. Korf BR, Berry AB, Limson M, Marian AJ, Murray MF, et al. (2014) Framework for development of physician competencies in genomic medicine: report of the Competencies Working Group of the Inter-Society Coordinating Committee for Physician Education in Genomics. Genet Med. 16 : 804–809. doi: 10.1038/gim.2014.35 24763287
6. Bell GC, Crews KR, Wilkinson MR, Haidar CE, Hicks JK, et al. (2014) Development and use of active clinical decision support for preemptive pharmacogenomics. J Am Med Inform Assoc 21: e93–99. doi: 10.1136/amiajnl-2013-001993 23978487
7. Chaufan C (2007) How much can a large population study on genes, environments, their interactions and common diseases contribute to the health of the American people? Soc Sci Med 65 : 1730–1741. 17618719
8. (2007) Recommendations from the EGAPP Working Group: testing for cytochrome P450 polymorphisms in adults with nonpsychotic depression treated with selective serotonin reuptake inhibitors. Genet Med 9 : 819–825. 18091431
9. Bansal A, Critchfield GC, Frank TS, Reid JE, Thomas A, et al. (2000) The predictive value of BRCA1 and BRCA2 mutation testing. Genet Test 4 : 45–48. 10794360
10. Burke W, Daly M, Garber J, Botkin J, Kahn MJ, et al. (1997) Recommendations for follow-up care of individuals with an inherited predisposition to cancer. II. BRCA1 and BRCA2. Cancer Genetics Studies Consortium. JAMA 277 : 997–1003. 9091675
11. Goncalves R, Bose R (2013) Using multigene tests to select treatment for early-stage breast cancer. J Natl Compr Canc Netw 11 : 174–182; quiz 182. 23411384
12. Carlson JJ, Roth JA (2013) The impact of the Oncotype Dx breast cancer assay in clinical practice: a systematic review and meta-analysis. Breast Cancer Res Treat 141 : 13–22. doi: 10.1007/s10549-013-2666-z 23974828
13. (2014) Aetna Clinical Policy Bulletin: Tumor Markers. http://www.aetna.com/cpb/medical/data/300_399/0352.html. Accessed 4 August 2014.
14. Azim HA Jr., Michiels S, Zagouri F, Delaloge S, Filipits M, et al. (2013) Utility of prognostic genomic tests in breast cancer practice: The IMPAKT 2012 Working Group Consensus Statement. Ann Oncol 24 : 647–654. doi: 10.1093/annonc/mds645 23337633
15. Mallal S, Phillips E, Carosi G, Molina JM, Workman C, et al. (2008) HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med 358 : 568–579. doi: 10.1056/NEJMoa0706135 18256392
16. Lai-Goldman M, Faruki H (2008) Abacavir hypersensitivity: a model system for pharmacogenetic test adoption. Genet Med 10 : 874–878. doi: 10.1097/GIM.0b013e31818de71c 19092439
17. Scott SA, Lubitz SA (2014) Warfarin pharmacogenetic trials: is there a future for pharmacogenetic-guided dosing? Pharmacogenomics 15 : 719–722. doi: 10.2217/pgs.14.18 24897277
18. Kimmel SE, French B, Kasner SE, Johnson JA, Anderson JL, et al. (2013) A pharmacogenetic versus a clinical algorithm for warfarin dosing. N Engl J Med 369 : 2283–2293. doi: 10.1056/NEJMoa1310669 24251361
19. Pirmohamed M, Burnside G, Eriksson N, Jorgensen AL, Toh CH, et al. (2013) A randomized trial of genotype-guided dosing of warfarin. N Engl J Med 369 : 2294–2303. doi: 10.1056/NEJMoa1311386 24251363
20. Topol EJ (2014) Individualized medicine from prewomb to tomb. Cell 157 : 241–253. doi: 10.1016/j.cell.2014.02.012 24679539
21. (2012) Genome-Based Diagnostics: Clarifying pathways to Clinical Use: Workshop Summary. Washington, DC: Institute of Medicine.
22. Harvey EK, Fogel CE, Peyrot M, Christensen KD, Terry SF, et al. (2007) Providers’ knowledge of genetics: A survey of 5915 individuals and families with genetic conditions. Genet Med 9 : 259–267. 17505202
23. Cragun D, Debate RD, Vadaparampil ST, Baldwin J, Hampel H, et al. (2014) Comparing universal Lynch syndrome tumor-screening programs to evaluate associations between implementation strategies and patient follow-through. Genet Med. 16 : 773–782. doi: 10.1038/gim.2014.31 24651603
24. Palomaki GE, McClain MR, Melillo S, Hampel HL, Thibodeau SN (2009) EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet Med 11 : 42–65. doi: 10.1097/GIM.0b013e31818fa2db 19125127
25. (2009) Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med 11 : 35–41. doi: 10.1097/GIM.0b013e31818fa2ff 19125126
26. Moreira L, Balaguer F, Lindor N, de la Chapelle A, Hampel H, et al. (2012) Identification of Lynch syndrome among patients with colorectal cancer. JAMA 308 : 1555–1565. doi: 10.1001/jama.2012.13088 23073952
27. Beamer LC, Grant ML, Espenschied CR, Blazer KR, Hampel HL, et al. (2012) Reflex immunohistochemistry and microsatellite instability testing of colorectal tumors for Lynch syndrome among US cancer programs and follow-up of abnormal results. J Clin Oncol 30 : 1058–1063. doi: 10.1200/JCO.2011.38.4719 22355048
28. Kidambi TD, Blanco A, Myers M, Conrad P, Loranger K, et al. (2014) Selective Versus Universal Screening for Lynch Syndrome: A Six-Year Clinical Experience. Dig Dis Sci. Epub ahead of print.
29. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, et al. (2009) Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 4 : 50. doi: 10.1186/1748-5908-4-50 19664226
30. Crews KR, Hicks JK, Pui CH, Relling MV, Evans WE (2012) Pharmacogenomics and individualized medicine: translating science into practice. Clin Pharmacol Ther 92 : 467–475. doi: 10.1038/clpt.2012.120 22948889
31. Ronquillo JG, Li C, Lester WT (2012) Genetic testing behavior and reporting patterns in electronic medical records for physicians trained in a primary care specialty or subspecialty. J Am Med Inform Assoc 19 : 570–574. doi: 10.1136/amiajnl-2011-000621 22511017
32. Relling MV, Gardner EE, Sandborn WJ, Schmiegelow K, Pui CH, et al. (2011) Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clin Pharmacol Ther 89 : 387–391. doi: 10.1038/clpt.2010.320 21270794
33. Yang Y, Muzny DM, Xia F, Niu Z, Person R, et al. (2014) Molecular findings among patients referred for clinical whole-exome sequencing. JAMA 312 : 1870–1879. doi: 10.1001/jama.2014.14601 25326635
34. Lee H, Deignan JL, Dorrani N, Strom SP, Kantarci S, et al. (2014) Clinical exome sequencing for genetic identification of rare Mendelian disorders. JAMA 312 : 1880–1887. doi: 10.1001/jama.2014.14604 25326637
35. Worthey EA, Mayer AN, Syverson GD, Helbling D, Bonacci BB, et al. (2011) Making a definitive diagnosis: successful clinical application of whole exome sequencing in a child with intractable inflammatory bowel disease. Genet Med 13 : 255–262. doi: 10.1097/GIM.0b013e3182088158 21173700
36. Patel CJ, Sivadas A, Tabassum R, Preeprem T, Zhao J, et al. (2013) Whole genome sequencing in support of wellness and health maintenance. Genome Med 5 : 58. doi: 10.1186/gm462 23806097
37. Burke W, Psaty BM (2007) Personalized medicine in the era of genomics. JAMA 298 : 1682–1684. 17925520
38. Langenberg C, Sharp SJ, Franks PW, Scott RA, Deloukas P, et al. (2014) Gene-lifestyle interaction and type 2 diabetes: the EPIC interact case-cohort study. PLoS Med 11: e1001647. doi: 10.1371/journal.pmed.1001647 24845081
39. Groop L, Pociot F (2014) Genetics of diabetes—are we missing the genes or the disease? Mol Cell Endocrinol 382 : 726–739. doi: 10.1016/j.mce.2013.04.002 23587769
40. Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, et al. (2001) Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 345 : 790–797. 11556298
41. Woolf SH (2007) Potential health and economic consequences of misplaced priorities. JAMA 297 : 523–526. 17284703
42. Khoury MJ (2 July 2014) Nobody is average but what to do about it? Genomics and Health Impact Blog. http://blogs.cdc.gov/genomics/2014/07/02/nobody-is-average/. Accessed 4 August 2014.
43. Weintraub K (1 May 2014) CDC: Lifespan more to do with geography than genetics. USA Today. http://www.usatoday.com/story/news/nation/2014/05/01/preventable-deaths-cdc/8570951/. Accessed 4 August 2014.
44. Roberts NJ, Vogelstein JT, Parmigiani G, Kinzler KW, Vogelstein B, et al. (2012) The predictive capacity of personal genome sequencing. Sci Transl Med 4 : 133ra 158.
45. Khoury MJ, Janssens AC, Ransohoff DF (2013) How can polygenic inheritance be used in population screening for common diseases? Genet Med 15 : 437–443. doi: 10.1038/gim.2012.182 23412608
46. Woolf SH, Harris R (2012) The harms of screening: new attention to an old concern. JAMA 307 : 565–566. doi: 10.1001/jama.2012.100 22318274
47. Khoury MJ, Gwinn ML, Glasgow RE, Kramer BS (2012) A population approach to precision medicine. Am J Prev Med 42 : 639–645. doi: 10.1016/j.amepre.2012.02.012 22608383
48. Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, et al. (2012) Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491 : 119–124. doi: 10.1038/nature11582 23128233
49. Schramm EC, Clark SJ, Triebwasser MP, Raychaudhuri S, Seddon JM, et al. (2014) Genetic variants in the complement system predisposing to age-related macular degeneration: A review. Mol Immunol 61 : 118–125 doi: 10.1016/j.molimm.2014.06.032 25034031
50. Yaghootkar H, Scott RA, White CC, Zhang W, Speliotes E, et al. (2014) Genetic evidence for a normal-weight “metabolically obese” phenotype linking insulin resistance, hypertension, coronary artery disease and type 2 diabetes. Diabetes 63 : 4369–4377. doi: 10.2337/db14-0318 25048195
51. Herder C, Nuotio ML, Shah S, Blankenberg S, Brunner EJ, et al. (2014) Genetic determinants of circulating interleukin-1 receptor antagonist levels and their association with glycemic traits. Diabetes 63 : 4343–4359. doi: 10.2337/db14-0731 24969107
52. Scott RA, Fall T, Pasko D, Barker A, Sharp SJ, et al. (2014) Common genetic variants highlight the role of insulin resistance and body fat distribution in type 2 diabetes, independently of obesity. Diabetes 63 : 4378–4387. doi: 10.2337/db14-0319 24947364
53. Visscher PM, Brown MA, McCarthy MI, Yang J (2012) Five years of GWAS discovery. Am J Hum Genet 90 : 7–24. doi: 10.1016/j.ajhg.2011.11.029 22243964
54. Bakulski KM, Fallin MD (2014) Epigenetic epidemiology: promises for public health research. Environ Mol Mutagen 55 : 171–183. doi: 10.1002/em.21850 24449392
55. Zimmer C (4 June 2014) In a first, test of DNA finds root of illness. New York Times. http://www.nytimes.com/2014/06/05/health/in-first-quick-dna-test-diagnoses-a-boys-illness.html?_r=0. Accessed 4 August 2014.
56. Fournier PE, Raoult D (2011) Prospects for the future using genomics and proteomics in clinical microbiology. Annu Rev Microbiol 65 : 169–188. doi: 10.1146/annurev-micro-090110-102922 21639792
57. Kinross JM, Darzi AW, Nicholson JK (2011) Gut microbiome-host interactions in health and disease. Genome Med 3 : 14. doi: 10.1186/gm228 21392406
58. Manolio TA, Chisholm RL, Ozenberger B, Roden DM, Williams MS, et al. (2013) Implementing genomic medicine in the clinic: the future is here. Genet Med 15 : 258–267. doi: 10.1038/gim.2012.157 23306799
Štítky
Genetika Reprodukční medicína
Článek 2014 Reviewer Thank YouČlánek Discovery of CTCF-Sensitive Cis-Spliced Fusion RNAs between Adjacent Genes in Human Prostate CellsČlánek K-homology Nuclear Ribonucleoproteins Regulate Floral Organ Identity and Determinacy in ArabidopsisČlánek A Nitric Oxide Regulated Small RNA Controls Expression of Genes Involved in Redox Homeostasis inČlánek Contribution of the Two Genes Encoding Histone Variant H3.3 to Viability and Fertility in MiceČlánek The Genetic Architecture of the Genome-Wide Transcriptional Response to ER Stress in the Mouse
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 2
-
Všechny články tohoto čísla
- 2014 Reviewer Thank You
- Systematic Cell-Based Phenotyping of Missense Alleles Empowers Rare Variant Association Studies: A Case for and Myocardial Infarction
- African Glucose-6-Phosphate Dehydrogenase Alleles Associated with Protection from Severe Malaria in Heterozygous Females in Tanzania
- Genomics of Divergence along a Continuum of Parapatric Population Differentiation
- microRNAs Regulate Cell-to-Cell Variability of Endogenous Target Gene Expression in Developing Mouse Thymocytes
- A Rolling Circle Replication Mechanism Produces Multimeric Lariats of Mitochondrial DNA in
- Closing the Gap between Knowledge and Clinical Application: Challenges for Genomic Translation
- Partially Redundant Enhancers Cooperatively Maintain Mammalian Expression Above a Critical Functional Threshold
- Discovery of Transcription Factors and Regulatory Regions Driving Tumor Development by ATAC-seq and FAIRE-seq Open Chromatin Profiling
- Mutations in Result in Ocular Coloboma, Microcornea and Cataracts
- A Genome-Wide Hybrid Incompatibility Landscape between and
- Recurrent Evolution of Melanism in South American Felids
- Discovery of CTCF-Sensitive Cis-Spliced Fusion RNAs between Adjacent Genes in Human Prostate Cells
- Tissue Expression Pattern of PMK-2 p38 MAPK Is Established by the miR-58 Family in
- Essential Role for Endogenous siRNAs during Meiosis in Mouse Oocytes
- Matrix Metalloproteinase 2 Is Required for Ovulation and Corpus Luteum Formation in
- Evolutionary Signatures amongst Disease Genes Permit Novel Methods for Gene Prioritization and Construction of Informative Gene-Based Networks
- RR-1 Cuticular Protein TcCPR4 Is Required for Formation of Pore Canals in Rigid Cuticle
- GC-Content Evolution in Bacterial Genomes: The Biased Gene Conversion Hypothesis Expands
- Proteotoxic Stress Induces Phosphorylation of p62/SQSTM1 by ULK1 to Regulate Selective Autophagic Clearance of Protein Aggregates
- K-homology Nuclear Ribonucleoproteins Regulate Floral Organ Identity and Determinacy in Arabidopsis
- A Nitric Oxide Regulated Small RNA Controls Expression of Genes Involved in Redox Homeostasis in
- HYPER RECOMBINATION1 of the THO/TREX Complex Plays a Role in Controlling Transcription of the Gene in Arabidopsis
- Mitochondrial and Cytoplasmic ROS Have Opposing Effects on Lifespan
- Structured Observations Reveal Slow HIV-1 CTL Escape
- An Integrative Multi-scale Analysis of the Dynamic DNA Methylation Landscape in Aging
- Combining Natural Sequence Variation with High Throughput Mutational Data to Reveal Protein Interaction Sites
- Transhydrogenase Promotes the Robustness and Evolvability of Deficient in NADPH Production
- Regulators of Autophagosome Formation in Muscles
- Genomic Selection and Association Mapping in Rice (): Effect of Trait Genetic Architecture, Training Population Composition, Marker Number and Statistical Model on Accuracy of Rice Genomic Selection in Elite, Tropical Rice Breeding Lines
- Eye Selector Logic for a Coordinated Cell Cycle Exit
- Inflammation-Induced Cell Proliferation Potentiates DNA Damage-Induced Mutations
- The DNA Polymerase δ Has a Role in the Deposition of Transcriptionally Active Epigenetic Marks, Development and Flowering
- Contribution of the Two Genes Encoding Histone Variant H3.3 to Viability and Fertility in Mice
- Membrane Recognition and Dynamics of the RNA Degradosome
- P-TEFb, the Super Elongation Complex and Mediator Regulate a Subset of Non-paused Genes during Early Embryo Development
- is a Long Non-coding RNA in JNK Signaling in Epithelial Shape Changes during Drosophila Dorsal Closure
- A Pleiotropy-Informed Bayesian False Discovery Rate Adapted to a Shared Control Design Finds New Disease Associations From GWAS Summary Statistics
- Genome-wide Association Study Identifies Shared Risk Loci Common to Two Malignancies in Golden Retrievers
- and Hyperdrive Mechanisms (in Mouse Meiosis)
- Elevated In Vivo Levels of a Single Transcription Factor Directly Convert Satellite Glia into Oligodendrocyte-like Cells
- Systemic Delivery of MicroRNA-101 Potently Inhibits Hepatocellular Carcinoma by Repressing Multiple Targets
- Pooled Sequencing of 531 Genes in Inflammatory Bowel Disease Identifies an Associated Rare Variant in and Implicates Other Immune Related Genes
- Abscission Is Regulated by the ESCRT-III Protein Shrub in Germline Stem Cells
- Temperature Stress Mediates Decanalization and Dominance of Gene Expression in
- Transcriptome Wide Annotation of Eukaryotic RNase III Reactivity and Degradation Signals
- The Exosome Component Rrp6 Is Required for RNA Polymerase II Termination at Specific Targets of the Nrd1-Nab3 Pathway
- Sex-specific -regulatory Variation on the X Chromosome
- Regulation of Toll-like Receptor Signaling by the SF3a mRNA Splicing Complex
- Modeling of the Human Alveolar Rhabdomyosarcoma Chromosome Translocation in Mouse Myoblasts Using CRISPR-Cas9 Nuclease
- Asymmetry of the Budding Yeast Tem1 GTPase at Spindle Poles Is Required for Spindle Positioning But Not for Mitotic Exit
- TIM Binds Importin α1, and Acts as an Adapter to Transport PER to the Nucleus
- Antagonistic Roles for KNOX1 and KNOX2 Genes in Patterning the Land Plant Body Plan Following an Ancient Gene Duplication
- The Genetic Architecture of the Genome-Wide Transcriptional Response to ER Stress in the Mouse
- Fatty Acid Synthase Cooperates with Glyoxalase 1 to Protect against Sugar Toxicity
- Region-Specific Activation of mRNA Translation by Inhibition of Bruno-Mediated Repression
- An Essential Role of the Arginine Vasotocin System in Mate-Guarding Behaviors in Triadic Relationships of Medaka Fish ()
- Interaction between the tRNA-Binding and C-Terminal Domains of Yeast Gcn2 Regulates Kinase Activity In Vivo
- Hyper-Acetylation of Histone H3K56 Limits Break-Induced Replication by Inhibiting Extensive Repair Synthesis
- Prodomain Removal Enables Neto to Stabilize Glutamate Receptors at the Neuromuscular Junction
- Recent Selective Sweeps in North American Show Signatures of Soft Sweeps
- Identification and Functional Analysis of Healing Regulators in
- A Multi-Megabase Copy Number Gain Causes Maternal Transmission Ratio Distortion on Mouse Chromosome 2
- Drosophila Casein Kinase I Alpha Regulates Homolog Pairing and Genome Organization by Modulating Condensin II Subunit Cap-H2 Levels
- The Hippo Pathway Regulates Homeostatic Growth of Stem Cell Niche Precursors in the Ovary
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genomic Selection and Association Mapping in Rice (): Effect of Trait Genetic Architecture, Training Population Composition, Marker Number and Statistical Model on Accuracy of Rice Genomic Selection in Elite, Tropical Rice Breeding Lines
- Discovery of Transcription Factors and Regulatory Regions Driving Tumor Development by ATAC-seq and FAIRE-seq Open Chromatin Profiling
- Evolutionary Signatures amongst Disease Genes Permit Novel Methods for Gene Prioritization and Construction of Informative Gene-Based Networks
- Proteotoxic Stress Induces Phosphorylation of p62/SQSTM1 by ULK1 to Regulate Selective Autophagic Clearance of Protein Aggregates
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



