-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Combination of Random Mutagenesis and Sequencing Highlight the Role of Unexpected Genes in an Intractable Organism
article has not abstract
Published in the journal: . PLoS Genet 11(1): e32767. doi:10.1371/journal.pgen.1004895
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1004895Summary
article has not abstract
A large variety of organisms are capable of synthesizing hard matter in a process called biomineralization [1]. The transformation of a genetic blueprint into minerals such as, for example, calcium phosphate in bones and calcium carbonate in eggs or seashells provides a mechanical support for organismic growth and protection against predators, respectively. Iron oxides formed by fishes and birds provide them with magnetic properties used for magnetoreception and orientation [2], [3]. The biomineralization processes are remarkable for numerous reasons: organisms, contrary to engineers, have to form these biological materials with a limited subset of biologically available chemical elements and at physiological conditions. Still, these reduced means are not at the detriment of their function, which often surpasses man-made materials based on equivalent elements [4]. Therefore, understanding how biomineralizing organisms process chemical elements based on their genetic program is of primary interest. However, the biological mechanisms behind biomineralization have remained unclear, partly because of limited genetic knowledge: model organisms are limited to a few unicellular organisms [5], [6]. Therefore, the question has arisen of what genetic approach to use to get genetic information about the large majority of organisms that have remained intractable.
Magnetotactic Bacteria: Simply Microorganisms, but Not So Simple
The recent advances in sequencing techniques now offer the opportunity to bypass some of the restrictions associated with the unavailability of genetic systems to get novel insights into important microbial processes such as those associated with biomineralization. In their study, Rahn-Lee et al. [7] combine established genetic techniques (random mutagenesis) with modern sequencing platforms to understand magnetite biomineralization in the magnetotactic bacteria Desulfovibrio magneticus RS-1. Magnetotactic bacteria are microorganisms able to form intracellular magnetic nanoparticles made of the iron oxide magnetite (Fe3O4) or the iron sulfide greigite (Fe3S4) [8]. The nanoparticles together with their membrane envelope are called magnetosomes. They have strain-specific sizes and morphologies and are typically arranged in chains in order to form a magnetic dipole strong enough to passively orient the bacteria along the magnetic field lines of the Earth, a process called magnetotaxis [9].
The genomes of numerous strains have been sequenced [10]. However, genetic tools permitting the manipulation of the microorganisms are only available for two magnetospirilla strains: Magnetospirillum gryphiswaldense [11] and M. magneticum [12]. In these strains, the magnetosomes are formed and arranged thanks to a subset of genes called the magnetosome island [13], which, in particular, encompasses the mamAB, mamGFDC, mms6, and mamXY operons (Fig. 1A) [14], [15]. However, RS-1, the strain studied by Rahn-Lee et al. [7], forms elongated magnetosomes in contrast to magnetospirilla, which form cubooctaedral magnetosomes. Therefore, RS-1 would be a model organism to use to study the functional diversity of compartmentalization and biomineral formation in magnetotactic bacteria, providing tools were available to do so.
Fig. 1. Sketch of magnetite biomineralization and associated differences. 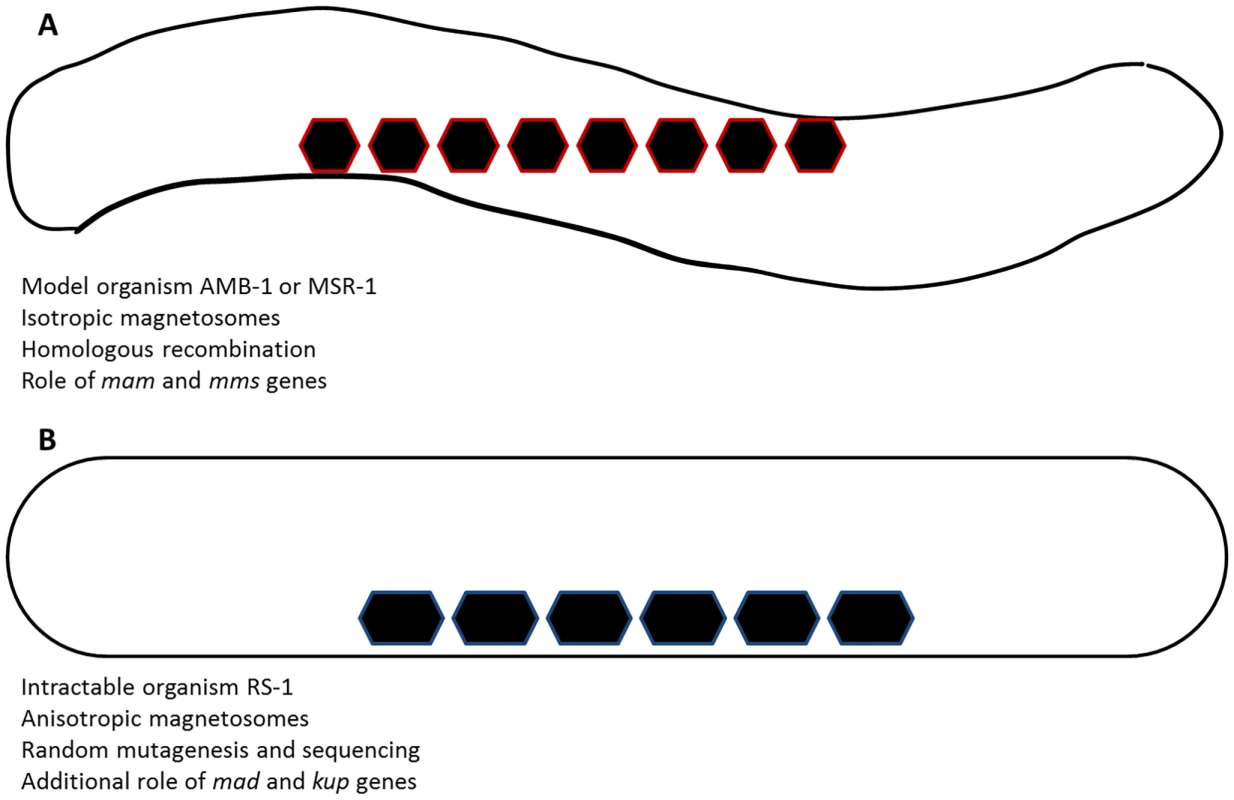
In the model organisms (magnetospirilla, AMB-1 or MSR-1), isotropic magnetosomes are produced. Genetic studies have highlighted the roles of mam and mms genes in the process (A). In turn, intractable organisms such as RS-1, where elongated magnetosomes are produced, could so far not be genetically studied. By random mutagenesis and whole-genome sequencing, Rahn-Lee et al. (2015) showed the additional role of kup and mad genes in the process, possibly in their morphology control of the nanoparticles (B). Getting Genetic Information in an Intractable Organism
The authors used random mutagenesis to generate nonmagnetic mutants and combined it with whole-genome re-sequencing to identify the mutated genes. In particular, Rahn-Lee et al. [7] first cultivated RS-1 in conditions where the microorganism no longer formed magnetosomes and then performed UV and chemical mutagenesis. Since screening a large number of colonies was impractical, they employed a two-step strategy that consisted of first selecting in liquid to increase the proportion of non-magnetic cells in the population and then only screening for single colonies of non-magnetic phenotypes [7]. These colonies of no - or low-magnetism were then analyzed by whole-genome sequencing to determine the causative genetic change. After the mutation for each strain was identified, the authors used PCR and Sanger sequencing to check for this change in the other strains isolated from the same outgrowth and analyzed those strains that were not clones by whole-genome sequencing to determine their mutation.
This approach led to the isolation of about 30 mutants, with mutations in genes shared amongst all magnetotactic bacteria, but also, more interestingly, with mad genes that are unique to the magnetotactic δ-proteo bacteria and even genes potentially unique to RS-1. The group of A. Komeili found that a potassium transporter (kup) is important for biomineralization of magnetite (Fig. 1B) [7], a surprising discovery since there is a priori no reason to expect the involvement of potassium in an iron oxide mineral. The authors, in addition, presented the first experimental proof of the involvement of mad genes in the control of the magnetosome morphology. This is an important confirmation since a bioinformatic study proposed earlier that these so-called mad genes could be responsible for the morphology control observed in some strains, since these genes are specifically found in magnetotactic δ-proteobacteria-forming elongated magnetosomes such as RS-1, and not in magnetospirilla [10].
In conclusion, the general methodology presented here will be of immediate relevance to other scientists working with fastidious and genetically intractable organisms, not limited to biomineralizing ones. In addition, the study delivers significant advancements for the understanding of biomineralization and its variety in prokaryotes by presenting the first genetic analysis of magnetotactic bacteria outside of the commonly studied α-proteobacteria. However, as random mutagenesis is stochastic and not directed, important genes might remain unprobed and, therefore, their role might possibly be overlooked by this method. Therefore, efforts in the development of genetic tools should not be abandoned. In addition, complementation of this approach by physical and chemical analytical techniques in the near future will enable the complete multidisciplinary understanding of biomineralization in different strains of magnetotactic bacteria.
Zdroje
1. LowenstamHA (1981) Minerals formed by organisms. Science 211 : 1126–1131.
2. Baeuerlein E (2007) The Biology of Biominerals Structure Formation; Baeuerlein E, editor. Weinheim: Wiley-VCH.
3. Baeuerlein E, Epple M (2007) Biomineralization in Medicine; Baeuerlein E, editor. Weinheim: Wiley-VCH.
4. FratzlP (2007) Biomimetic materials research: what can we really learn from nature's structural materials? J R Soc Interface 4 : 1–6.
5. ArmbrustEV, BergesJA, BowlerC, GreenBR, MartinezD, et al. (2004) The genome of the diatom Thalassiosira pseudonana: Ecology, evolution, and metabolism. Science 306 : 79–86.
6. MatsunagaT, OkamuraY, FukudaY, WahyudiAT, MuraseY, et al. (2005) Complete genome sequence of the facultative anaerobic magnetotactic bacterium Magnetospirillum sp strain AMB-1. DNA Res 12 : 157–166.
7. Rahn-LeeL, Byrne ME, ZhangM, Le SageD, Glenn DR, et al. (2015) A Genetic Strategy for Probing the Functional Diversity of Magnetosome Formation. PLoS Genet 11(1): e1004811 doi:10.1371/journal.pgen.1004811
8. FaivreD, SchülerD (2008) Magnetotactic Bacteria and Magnetosomes. Chem Rev 108 : 4875–4898.
9. Lefèvre CT, BennetM, LandauL, VachP, PignolD, et al. (2014) Diversity of Magneto-Aerotactic Behaviors and Oxygen Sensing Mechanisms in Cultured Magnetotactic Bacteria. Biophysical Journal 107 : 527–538.
10. Lefevre CT, TrubitsynD, AbreuF, KolinkoS, JoglerC, et al. (2013) Comparative genomic analysis of magnetotactic bacteria from the Deltaproteobacteria provides new insights into magnetite and greigite magnetosome genes required for magnetotaxis. Environ Microbiol 15 : 2712–2735.
11. SchultheissD, SchülerD (2003) Development of a genetic system for Magnetospirillum gryphiswaldense. Archives Microbiol 179 : 89–94.
12. MatsunagaT, NakamuraC, BurgessJG, SodeK (1992) Gene transfer in magnetic bacteria: transposon mutagenesis and cloning of genomic DNA fragments required for magnetosome synthesis. J Bacteriol 174 : 2748–2753.
13. UllrichS, KubeM, SchübbeS, ReinhardtR, SchülerD (2005) A Hypervariable 130-Kilobase Genomic Region of Magnetospirillum gryphiswaldense Comprises a Magnetosome Island Which Undergoes Frequent Rearrangements during Stationary Growth. J Bacteriol 187 : 7176–7184.
14. LohßeA, BorgS, RaschdorfO, KolinkoI, TompaE, et al. (2014) Genetic Dissection of the mamAB and mms6 Operons Reveals a Gene Set Essential for Magnetosome Biogenesis in Magnetospirillum gryphiswaldense. J Bacteriol 196 : 2658–2669.
15. MuratD, QuinlanA, ValiH, KomeiliA (2010) Comprehensive genetic dissection of the magnetosome gene island reveals the step-wise assembly of a prokaryotic organelle. Proc Natl Acad Sci USA 107 : 5593–5598.
Štítky
Genetika Reprodukční medicína
Článek Phosphorylation of Elp1 by Hrr25 Is Required for Elongator-Dependent tRNA Modification in YeastČlánek Naturally Occurring Differences in CENH3 Affect Chromosome Segregation in Zygotic Mitosis of HybridsČlánek Insight in Genome-Wide Association of Metabolite Quantitative Traits by Exome Sequence AnalysesČlánek ALIX and ESCRT-III Coordinately Control Cytokinetic Abscission during Germline Stem Cell DivisionČlánek Deciphering the Genetic Programme Triggering Timely and Spatially-Regulated Chitin Deposition
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 1
-
Všechny články tohoto čísla
- The Combination of Random Mutagenesis and Sequencing Highlight the Role of Unexpected Genes in an Intractable Organism
- Ataxin-3, DNA Damage Repair, and SCA3 Cerebellar Degeneration: On the Path to Parsimony?
- α-Actinin-3: Why Gene Loss Is an Evolutionary Gain
- Origins of Context-Dependent Gene Repression by Capicua
- Transposable Elements Contribute to Activation of Maize Genes in Response to Abiotic Stress
- No Evidence for Association of Autism with Rare Heterozygous Point Mutations in Contactin-Associated Protein-Like 2 (), or in Other Contactin-Associated Proteins or Contactins
- Nur1 Dephosphorylation Confers Positive Feedback to Mitotic Exit Phosphatase Activation in Budding Yeast
- A Regulatory Hierarchy Controls the Dynamic Transcriptional Response to Extreme Oxidative Stress in Archaea
- Genetic Variants Modulating CRIPTO Serum Levels Identified by Genome-Wide Association Study in Cilento Isolates
- Small RNA Sequences Support a Host Genome Origin of Satellite RNA
- Phosphorylation of Elp1 by Hrr25 Is Required for Elongator-Dependent tRNA Modification in Yeast
- Genetic Mapping of MAPK-Mediated Complex Traits Across
- An AP Endonuclease Functions in Active DNA Demethylation and Gene Imprinting in
- Developmental Regulation of the Origin Recognition Complex
- End of the Beginning: Elongation and Termination Features of Alternative Modes of Chromosomal Replication Initiation in Bacteria
- Naturally Occurring Differences in CENH3 Affect Chromosome Segregation in Zygotic Mitosis of Hybrids
- Imputation of the Rare G84E Mutation and Cancer Risk in a Large Population-Based Cohort
- Polycomb Protein SCML2 Associates with USP7 and Counteracts Histone H2A Ubiquitination in the XY Chromatin during Male Meiosis
- A Genetic Strategy for Probing the Functional Diversity of Magnetosome Formation
- Interactions of Chromatin Context, Binding Site Sequence Content, and Sequence Evolution in Stress-Induced p53 Occupancy and Transactivation
- The Yeast La Related Protein Slf1p Is a Key Activator of Translation during the Oxidative Stress Response
- Integrative Analysis of DNA Methylation and Gene Expression Data Identifies as a Key Regulator of COPD
- Proteasomes, Sir2, and Hxk2 Form an Interconnected Aging Network That Impinges on the AMPK/Snf1-Regulated Transcriptional Repressor Mig1
- Functional Interplay between the 53BP1-Ortholog Rad9 and the Mre11 Complex Regulates Resection, End-Tethering and Repair of a Double-Strand Break
- Estrogenic Exposure Alters the Spermatogonial Stem Cells in the Developing Testis, Permanently Reducing Crossover Levels in the Adult
- Protein Poly(ADP-ribosyl)ation Regulates Immune Gene Expression and Defense Responses
- Sumoylation Influences DNA Break Repair Partly by Increasing the Solubility of a Conserved End Resection Protein
- A Discrete Transition Zone Organizes the Topological and Regulatory Autonomy of the Adjacent and Genes
- Elevated Mutation Rate during Meiosis in
- The Intersection of the Extrinsic Hedgehog and WNT/Wingless Signals with the Intrinsic Hox Code Underpins Branching Pattern and Tube Shape Diversity in the Airways
- MiR-24 Is Required for Hematopoietic Differentiation of Mouse Embryonic Stem Cells
- Tissue-Specific Effects of Genetic and Epigenetic Variation on Gene Regulation and Splicing
- Heterologous Aggregates Promote Prion Appearance via More than One Mechanism
- The Tumor Suppressor BCL7B Functions in the Wnt Signaling Pathway
- , A -Acting Locus that Controls Chromosome-Wide Replication Timing and Stability of Human Chromosome 15
- Regulating Maf1 Expression and Its Expanding Biological Functions
- A Polyubiquitin Chain Reaction: Parkin Recruitment to Damaged Mitochondria
- RecFOR Is Not Required for Pneumococcal Transformation but Together with XerS for Resolution of Chromosome Dimers Frequently Formed in the Process
- An Intracellular Transcriptomic Atlas of the Giant Coenocyte
- Insight in Genome-Wide Association of Metabolite Quantitative Traits by Exome Sequence Analyses
- The Role of the Mammalian DNA End-processing Enzyme Polynucleotide Kinase 3’-Phosphatase in Spinocerebellar Ataxia Type 3 Pathogenesis
- The Global Regulatory Architecture of Transcription during the Cell Cycle
- Identification and Functional Characterization of Coding Variants Influencing Glycemic Traits Define an Effector Transcript at the Locus
- Altered Ca Kinetics Associated with α-Actinin-3 Deficiency May Explain Positive Selection for Null Allele in Human Evolution
- Genetic Variation in the Nuclear and Organellar Genomes Modulates Stochastic Variation in the Metabolome, Growth, and Defense
- PRDM9 Drives Evolutionary Erosion of Hotspots in through Haplotype-Specific Initiation of Meiotic Recombination
- Transcriptional Control of an Essential Ribozyme in Reveals an Ancient Evolutionary Divide in Animals
- ALIX and ESCRT-III Coordinately Control Cytokinetic Abscission during Germline Stem Cell Division
- Century-scale Methylome Stability in a Recently Diverged Lineage
- A Re-examination of the Selection of the Sensory Organ Precursor of the Bristle Sensilla of
- Antagonistic Cross-Regulation between Sox9 and Sox10 Controls an Anti-tumorigenic Program in Melanoma
- A Dependent Pool of Phosphatidylinositol 4,5 Bisphosphate (PIP) Is Required for G-Protein Coupled Signal Transduction in Photoreceptors
- Deciphering the Genetic Programme Triggering Timely and Spatially-Regulated Chitin Deposition
- Aberrant Gene Expression in Humans
- Fascin1-Dependent Filopodia are Required for Directional Migration of a Subset of Neural Crest Cells
- The SWI2/SNF2 Chromatin Remodeler BRAHMA Regulates Polycomb Function during Vegetative Development and Directly Activates the Flowering Repressor Gene
- Evolutionary Constraint and Disease Associations of Post-Translational Modification Sites in Human Genomes
- A Truncated NLR Protein, TIR-NBS2, Is Required for Activated Defense Responses in the Mutant
- The Genetic and Mechanistic Basis for Variation in Gene Regulation
- Inactivation of PNKP by Mutant ATXN3 Triggers Apoptosis by Activating the DNA Damage-Response Pathway in SCA3
- DNA Damage Response Factors from Diverse Pathways, Including DNA Crosslink Repair, Mediate Alternative End Joining
- hnRNP K Coordinates Transcriptional Silencing by SETDB1 in Embryonic Stem Cells
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Global Regulatory Architecture of Transcription during the Cell Cycle
- A Truncated NLR Protein, TIR-NBS2, Is Required for Activated Defense Responses in the Mutant
- Proteasomes, Sir2, and Hxk2 Form an Interconnected Aging Network That Impinges on the AMPK/Snf1-Regulated Transcriptional Repressor Mig1
- The SWI2/SNF2 Chromatin Remodeler BRAHMA Regulates Polycomb Function during Vegetative Development and Directly Activates the Flowering Repressor Gene
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



