-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaIdentification and Functional Characterization of Coding Variants Influencing Glycemic Traits Define an Effector Transcript at the Locus
Understanding how FI and FG levels are regulated is important because their derangement is a feature of T2D. Despite recent success from GWAS in identifying regions of the genome influencing glycemic traits, collectively these loci explain only a small proportion of trait variance. Unlocking the biological mechanisms driving these associations has been challenging because the vast majority of variants map to non-coding sequence, and the genes through which they exert their impact are largely unknown. In the current study, we sought to increase our understanding of the physiological pathways influencing both traits using exome-array genotyping in up to 33,231 non-diabetic individuals to identify coding variants and consequently genes associated with either FG or FI levels. We identified novel association signals for both traits including the receptor for GLP-1 agonists which are a widely used therapy for T2D. Furthermore, we identified coding variants at several GWAS loci which point to the genes underlying these association signals. Importantly, we found that multiple coding variants in G6PC2 result in a loss of protein function and lower fasting glucose levels.
Published in the journal: . PLoS Genet 11(1): e32767. doi:10.1371/journal.pgen.1004876
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004876Summary
Understanding how FI and FG levels are regulated is important because their derangement is a feature of T2D. Despite recent success from GWAS in identifying regions of the genome influencing glycemic traits, collectively these loci explain only a small proportion of trait variance. Unlocking the biological mechanisms driving these associations has been challenging because the vast majority of variants map to non-coding sequence, and the genes through which they exert their impact are largely unknown. In the current study, we sought to increase our understanding of the physiological pathways influencing both traits using exome-array genotyping in up to 33,231 non-diabetic individuals to identify coding variants and consequently genes associated with either FG or FI levels. We identified novel association signals for both traits including the receptor for GLP-1 agonists which are a widely used therapy for T2D. Furthermore, we identified coding variants at several GWAS loci which point to the genes underlying these association signals. Importantly, we found that multiple coding variants in G6PC2 result in a loss of protein function and lower fasting glucose levels.
Introduction
Large-scale GWAS of non-diabetic individuals have successfully identified > 60 loci associated with FG and FI levels, many of which are also implicated in susceptibility to T2D [1, 2, 3, 4]. Despite these successes, lead SNPs at GWAS loci have modest effects and cumulatively explain only a small proportion of the trait variance in non-diabetic individuals. By design, GWAS have focused predominantly on the interrogation of common variants, defined here to have MAF > 5%. Most of the identified variants are non-coding, complicating attempts to establish the molecular consequences of these GWAS loci. We therefore chose to extend discovery efforts to coding variants, particularly those of lower frequency that have not been well captured by GWAS genotyping and imputation. We aimed both to identify novel coding loci for FG and FI, and to evaluate the role of coding variants at known GWAS loci, thereby expecting to highlight causal transcripts and to facilitate characterization of the molecular mechanisms influencing glycemic traits and T2D susceptibility.
Results
We analyzed 33,231 (FG) and 30,825 (FI) non-diabetic individuals from 14 studies of European ancestry, all genotyped with the Illumina HumanExome BeadChip (see URLs). Characteristics of the contributing studies and study participants are summarized in S1-S2 Tables. Body mass index (BMI) adjustment has been shown to increase power to detect association with these glycemic traits [4], and in our study samples, BMI accounted for 6.1% and 24.6% of phenotypic variance of FG and FI, respectively. Consequently, within each study, we calculated residuals for both traits after adjustment for BMI and other study-specific covariates (S1 Table). Study-specific inverse-rank normalized residuals were tested for single-variant association using a linear mixed model to account for relatedness and fine-scale genetic population sub-structure [5]. We also repeated the analysis using the untransformed residuals to obtain allelic effect sizes. We then combined the association summary statistics across studies using fixed-effect meta-analysis. We restricted our single-variant analysis to 106,489 variants that pass quality-control and are polymorphic in more than one study. We declared a single-variant trait association as exome-wide significant at P < 5×10-7, corresponding to Bonferroni correction for the ~100,000 polymorphic variants. We also carried out gene-based meta-analysis [6, 7] by using the sequence kernel association test (SKAT) [8] and a frequency-weighted burden test [9] applying four alternate variant masks which combine functional annotation and allele frequency thresholds. Full details of the variant masks are provided in the Methods. Gene-based tests take into account overall variant-load within a specified locus and therefore may have greater power than single-variant tests to detect associations with multiple rare and low-frequency causal alleles. We note that this advantage is likely to be less in exome-array analysis compared with the more complete ascertainment of variants possible with exome sequencing [10]. We declared gene-based association as exome-wide significant at P < 2.5×10-6, corresponding to Bonferroni correction for ~20,000 protein-coding genes in the genome.
Coding variants influencing FG levels
Through single-variant analysis, we identified 12 coding variants (all of which were non-synonymous changes) associated with FG levels at exome-wide significance, two low-frequency and ten common (S3 Table). These variants mapped to seven loci, six of them previously implicated in FG regulation. The signals at known loci included previously-reported common coding variants driving GWAS signals at GCKR (p.Pro446Leu, MAF = 36.9%, P = 5.3×10−18) and SLC30A8 (p.Arg325Trp, MAF = 35.7%, P = 2.5×10−10) [2, 11]. Three additional common coding variants associated with FG (in C2orf16, GPN1, and SLC5A6) were in moderate linkage disequilibrium (LD; r2 = 0.2–0.4) with the p.Pro446Leu GCKR variant. Their associations were eliminated after conditioning on p.Pro446Leu, the known functional GWAS variant in this region [12, 13], indicating no causal role for these additional variants on FG regulation (S3 Table).
The sixth variant influencing FG levels at exome-wide significance was a low-frequency non-synonymous change which did not map to any previously known FG-influencing locus: p.Ala316Thr at GLP1R (MAF = 1.5%, P = 4.6×10−7; Table 1 and S1B Fig.). This variant showed modest association (P = 1.3×10−4) in a previous GWAS meta-analysis of FG [3], which partially overlaps with the present study, but now achieves exome-wide significance. The alanine residue at p.Ala316Thr is conserved across vertebrates, and the threonine substitution is predicted to be “possibly damaging” by in silico mutation analysis (S4 Table). GLP-1R (glucagon-like peptide 1 receptor) is the receptor for the incretin hormone glucagon-like peptide 1 (GLP1), which is released from enteroendocrine cells after food ingestion and potentiates insulin secretion. GLP1 receptor agonists are an established treatment for T2D [14].
Tab. 1. Coding variants associated with FG and FI levels at exome-wide significance. 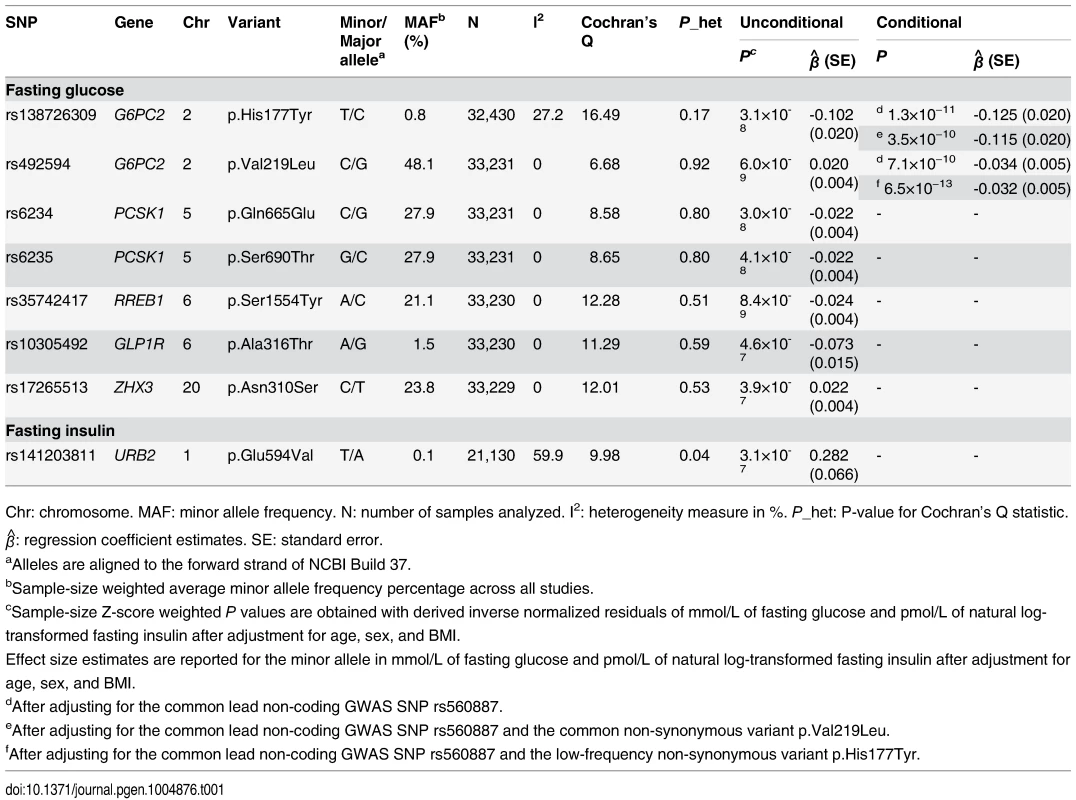
Chr: chromosome. MAF: minor allele frequency. N: number of samples analyzed. I2: heterogeneity measure in %. P_het: P-value for Cochran’s Q statistic. β^: regression coefficient estimates. SE: standard error. The six remaining coding variants all map to known FG-associated GWAS loci, but have not previously been implicated as playing a causal role. These include two variants in the islet-specific glucose-6-phosphatase catalytic subunit (G6PC2) gene at the G6PC2/ABCB11 locus: p.Val219Leu, a common variant (P = 6.0×10−9, MAF = 48.1%), and p.His177Tyr, a low-frequency variant (P = 3.1×10−8, MAF = 0.8%; S2A Fig.). These variants remained significantly associated (p.Val219Leu, Pconditional = 7.1×10−10 and p.His177Tyr, Pconditional = 1.3×10−11) with FG after conditioning on the intronic lead GWAS SNP, rs560887 (Table 1 and S2B Fig.). Conversely, conditioning on the coding variants did not completely abolish the association signal at the lead GWAS SNP (Punconditional = 6.4×10−78; Pconditional onHis177Tyr = 3.1×10−55, Pconditional onVal219Leu = 1.2×10−58, and Pconditional onHis177Tyr and Val219Leu = 2.1×10−83), confirming that the effect of the coding variants were largely independent of the lead GWAS SNP. Furthermore, the coding variants each remained associated with FG at exome-wide significance even after conditioning on both the lead GWAS SNP and the other coding variant, providing clear evidence of at least three association signals at this locus (Table 1 and S2C-S2D Fig.). These results are consistent with a recent study in Finnish individuals that reported a FG association signal at p.His177Tyr [15], but we extend that finding by demonstrating exome-wide significant association of multiple coding variants after conditioning on other associated variants in the region.
G6PC2 was also the only one of the 14,465 genes with multiple exome-array variants to demonstrate evidence of significant association with FG levels with any mask in gene-based tests (Table 2). In fact, for this gene we observed significant association with FG levels for all the masks encompassing multiple variants. These gene-based associations remained significant even after Bonferroni correction for testing of four different variant masks. Step-wise conditional analyses, adjusting for each variant included in the respective masks, revealed that the gene-based signals were primarily driven by two variants: p.His177Tyr and p.Tyr207Ser (S5 Table). The protein-truncating variant (PTV) p.Arg283*, which introduces a stop mutation in the last exon of the gene, showed no association with FG levels in single-variant or gene-based analyses. The most likely explanation is that this variant evades nonsense mediated decay (as is usual for PTV in the last exon) and that the truncated protein (missing only the terminal 72 amino acid residues) retains normal functional activity [16]. Due to its high allele frequency, and annotation as benign across multiple annotation algorithms, p.Val219Leu was not included in any of the gene-based variant masks (S6 Table). However, based on the single-variant it also has independent effects, with the result that we have three coding variants in G6PC2 (p.His177Tyr, p.Tyr207Ser, and p.Val219Leu) influencing FG levels. These three variants explain an additional 0.2% of the phenotypic variance in FG beyond that explained by the GWAS variant rs560887, bringing the total variance explained by this locus to 1.1%. However, we recognize that this estimate has been obtained in the discovery cohort and consequently might be inflated.
Tab. 2. G6PC2 gene-based association with FG levels using SKAT and BURDEN test 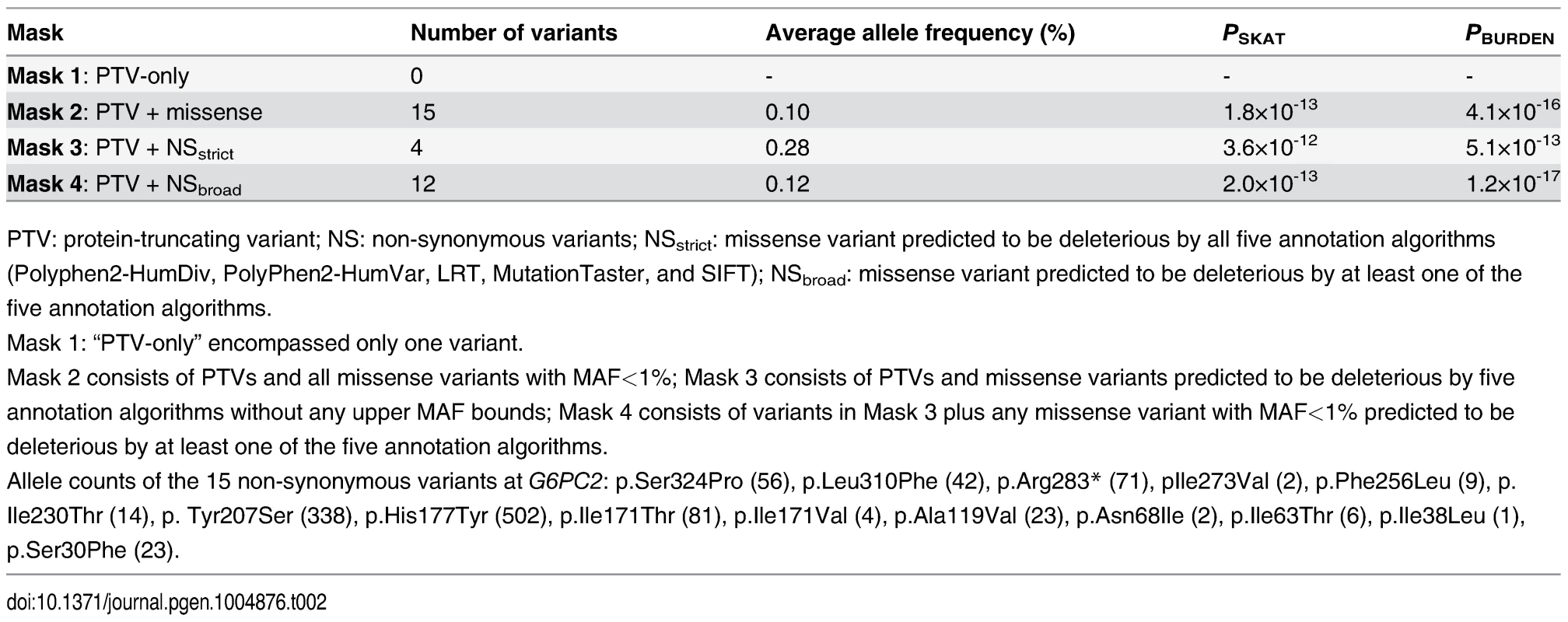
PTV: protein-truncating variant; NS: non-synonymous variants; NSstrict: missense variant predicted to be deleterious by all five annotation algorithms (Polyphen2-HumDiv, PolyPhen2-HumVar, LRT, MutationTaster, and SIFT); NSbroad: missense variant predicted to be deleterious by at least one of the five annotation algorithms. Both G6PC2 and ABCB11 have been considered strong biological candidate genes for glucose regulation, and advocated as potential effector transcripts at this GWAS locus [17, 18]. However, none of the 21 coding variants in ABCB11 that passed quality control (twelve rare, eight low-frequency, and one common) were significantly associated with FG levels, nor was there an aggregate association signal (P>0.05). Together, our genetic data provide compelling evidence that G6PC2 is an effector gene for FG regulation at this GWAS locus, with p.His177Tyr, p.Tyr207Ser, and p.Val219Leu as likely causal coding variants.
Loss of G6PC2 function leads to a reduction in FG levels
The phenotypic impact of these coding variants might, we reasoned, be influenced by the action of the non-coding GWAS variants on G6PC2 transcript regulation. Haplotype analysis of the four variants (rs560887, p.His177Tyr, p.Tyr207Ser, and p.Val219Leu) in a subset of 4,442 individuals revealed that the C allele encoding Leu219 was carried exclusively in cis with the glucose-raising allele at the GWAS SNP (Fig. 1). The estimated effect size of p.Val219Leu was considerably smaller (β^ =0.020 ± 0.004 mmol/L per allele) than rs560887 (β^ =0.070 ± 0.004 mmol/L per allele). These observations together explain the reversal in direction of effect of the Leu219 allele between conditional and unconditional analyses and indicate that, whilst Leu219 appears to have a glucose-raising effect in single-variant analyses, the molecular consequence of Leu219 is likely to be to lower glucose (Table 1). The minor alleles of the other two coding variants (p.His177Tyr, p.Tyr207Ser) also displayed glucose-lowering effects in conditional analyses. Given the role of G6PC2 in beta-cells we predicted that these would also be associated with reduced G6PC2 function, a hypothesis that we set out to test with a series of in vitro studies.
Fig. 1. Haplotypes of the lead non-coding GWAS SNP rs560887 and the three coding variants. 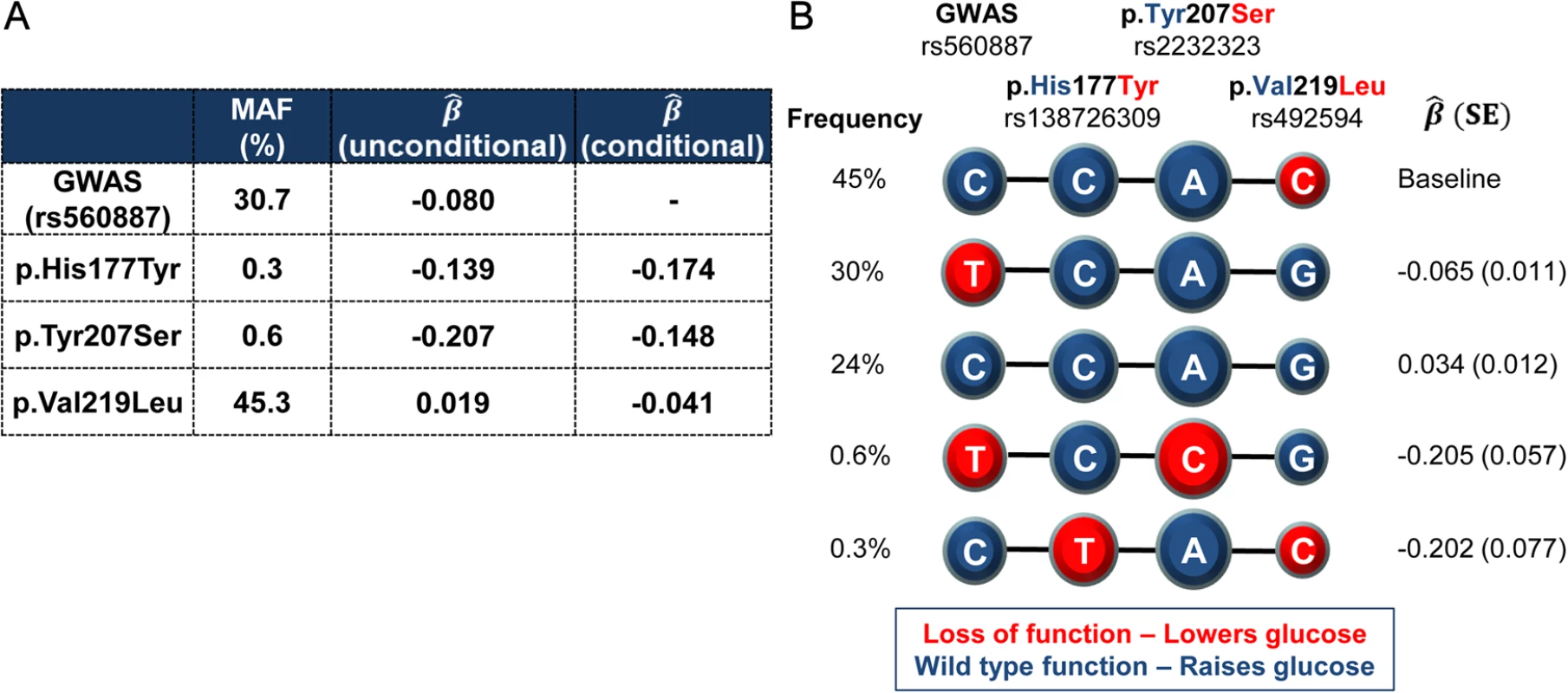
rs138726309 (p.His177Tyr), rs2232323 (p.Tyr207Ser), and rs492594 (p.Val219Leu), obtained from 4,442 unrelated individuals from the Oxford Biobank. (A) Percentage minor allele frequency (MAF) and effect size estimates (β^) of the four variants reported for the minor allele in mmol/L of FG after adjustment for age, sex, and BMI. (B) Haplotypes of the four associated variants in G6PC2 revealed that the glucose-lowering Leu219 allele was carried exclusively in cis with the glucose-raising allele at the GWAS SNP. Wild-type, glucose-raising alleles are circled in blue and the mutant, glucose-lowering alleles are circled in red. Diameter of the circle is proportional to the effect size estimates. Haplotype association was performed with FG derived residuals (after adjustment for age, sex, and BMI) using the most frequent haplotype as baseline. First, we generated recombinant FLAG-tagged G6PC2 constructs to investigate the impact of these variants on protein expression and subsequent cellular localization. Transient transfection of all three constructs showed a marked reduction in G6PC2 protein levels. In HEK293 cells, protein levels were decreased by 99% (p.His177Tyr), 100% (p.Tyr207Ser), and 49% (p.Val219Leu), when compared to wild type (defined for this study as the major allele for each of these variants) G6PC2 (Fig. 2A). Reduced protein expression was also confirmed in the rat pancreatic β-cell line INS-1E (76%, 97%, and 49% reduction, respectively; Fig. 2B). The functional impact of the variant proteins mirrored our genetic observations; the variant protein with the p.Val219Leu substitution, which has only a modest effect on FG, was present at higher levels in both HEK293 and INS-1E cells than the p.His177Tyr and p.Tyr207Ser proteins, both of which have larger impacts on FG levels.
Fig. 2. Functional characterization of wild type and variant G6PC2 proteins. 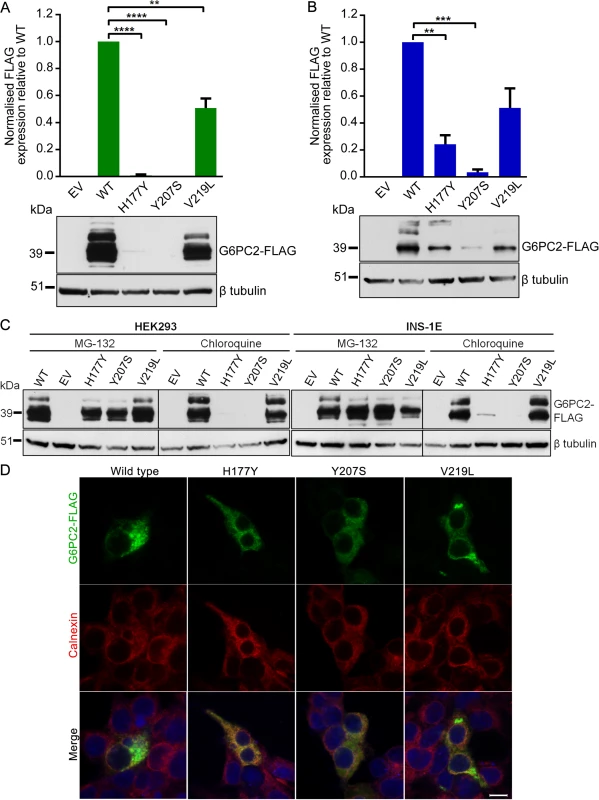
(A) Expression levels in HEK293 and (B) INS-1E cells were determined by western blot and densitometry analysis. The multiple bands on the western blot are likely to represent glycosylated G6PC2 protein products. Data are presented as mean ± standard error of the mean for at least three independent experiments. Significant differences are indicated as ** P<0.01; *** P<0.001; **** P<0.0001. EV, empty vector; WT, wild type. (C) Expression levels in HEK293 and INS-1E cells in the presence of proteasomal inhibitor MG-132 or lysosomal inhibitor chloroquine were determined by western blot. (D) Cellular localization in HEK293 cells was assessed by immunofluorescence microscopy. Cells were double immunostained for FLAG tag (green) and calnexin (red), and merged images with a DNA stain (blue) are shown. Images were taken with laser settings that were optimized separately for each sample. Scale bar, 10µm. Our functional results for p.His177Tyr and p.Tyr207Ser are concordant with data from the in silico mutation assessment tools SIFT and PolyPhen-2, which predicted these variants as deleterious (S4 Table). In contrast, the common p.Val219Leu variant was predicted to be benign, whereas in vitro characterization clearly shows a significant reduction in protein expression, demonstrating the importance of experimentally evaluating coding variants for functional consequences. The observed decrease in G6PC2 protein levels caused by the FG-associated variants is also directionally consistent with the evidence indicating that the non-coding GWAS SNP rs560887 may have effects on pre-mRNA splicing [19].
Next, we used specific inhibitors of the proteasomal and lysosomal pathways (MG-132 and chloroquine, respectively) to demonstrate that the three G6PC2 variant proteins with p.His177Tyr, p.Tyr207Ser, and p.Val219Leu substitutions were predominantly degraded through the ubiquitin-proteasome pathway, an important cellular mechanism for clearing misfolded proteins. Protein expression could be rescued in the presence of MG-132 but not chloroquine (Fig. 2C). We also evaluated the impact of the variants on cellular localization of G6PC2 to the endoplasmic reticulum (ER) using calnexin as an ER marker. The variant proteins displayed similar localization patterns to wild type G6PC2 (Fig. 2D). Our finding that loss of G6PC2 function leads to a reduction in FG levels in humans is consistent with rodent data, which show that G6pc2 knockout mice have a ~15% decrease in FG levels [20]. Hence, our data, linking genetic associations with reduced protein function, indicate that normal G6PC2 function is critical for glucose homeostasis and that these variants most likely impact FG levels through altered intracellular catalysis of glucose-6-phosphate to glucose and inorganic phosphate in pancreatic β-cells.
Impact of the functional G6PC2 variants on other related quantitative traits and disease outcomes
We evaluated the impact of these functional G6PC2 variants on other related quantitative traits and disease outcomes to gain further insights into the metabolic processes involved (S7 Table). None of the variants showed any evidence of association with FI levels (P>0.1) in 30,825 non-diabetic individuals analyzed in the present study. No association of the lead G6PC2 GWAS variant with T2D risk has previously been shown in Europeans [2, 21, 22]. However, in a meta-analysis of exome-array data from 28,344 T2D cases and 51,801 controls of European ancestry (including the 33,231 controls used in the present meta-analyses), the common coding variant, p.Val219Leu, showed modest association with T2D risk (P = 0.0011; odds ratio = 1.05, 95% confidence interval 1.02–1.06). The G allele encoding Val219, which displayed a glucose-raising effect, conferred an increased risk of disease. In contrast to the observations in FG single-variant analysis, the direction of effect on T2D at this variant was unchanged, even after conditioning on the GWAS variant. This is consistent with a small case-control study in 3,676 individuals of Chinese ancestry where a nominal association (P = 0.0062) with T2D risk was reported [23]. Our finding provides evidence that G6PC2 is critical not only for controlling FG levels in the physiological range, but that impairment of its function contributes to T2D pathogenesis. The effect on T2D risk is modest given the impact of the p.Val219Leu variant on FG levels but the effect size is similar to that reported for the common non-coding variant GWAS signal at GCK [2]. GCK encodes the glycolytic enzyme glucokinase which catalyzes the reverse reaction to G6PC2. These observations are consistent with defects in glucose sensing rather than β-cell function.
Likely effector transcripts at established FG GWAS loci
Of the 12 coding variants significantly influencing FG levels, we have discussed eight above. The four remaining signals mapped to three additional established FG-associated GWAS regions and support the candidacy of particular effector transcripts at these loci. Two map to PCSK1 (p.Gln665Glu, MAF = 27.9%, P = 3.0×10−8; p.Ser690Thr, MAF = 27.9%, P = 4.1×10−8), and have previously been proposed as likely causal variants at this GWAS locus because of strong LD with the lead non-coding GWAS SNP (rs4869272) in Europeans (r2 = 0.81) [3]. These two variants are in complete LD with each other (r2 = 1) and both have residual association signals after adjusting for the lead GWAS SNP (p.Gln665Glu, Pconditional = 8.1×10−3; p.Ser690Thr, Pconditional = 0.01) (Table 1 and S3 Table). Conversely, conditioning on the coding variants completely abolished the association signal at the GWAS SNP (Punconditional = 7.2×10−7; Pconditional onGln665Glu = 0.24, Pconditional onSer690Thr = 0.25, and Pconditional onGln665Glu and Ser690Thr = 0.34). Although we were unable to statistically distinguish between these two coding variants, there is suggestive in vitro evidence that the p.Gln665Glu variant may decrease PCSK1 activity whilst p.Ser690Thr behaved as wild-type [24], supporting the former as the most likely causal variant at the PCSK1 locus. PCSK1 encodes prohormone convertase 1/3 (PC1/3), a calcium-dependent serine endoprotease, which is essential for the conversion of a variety of prohormones, including proinsulin and proglucagon, to their bioactive forms.
The penultimate variant was in ZHX3 (p.Asn310Ser, MAF = 23.8%, P = 3.9×10−7) which maps to the TOP1 GWAS locus influencing FG levels [4]. Adjustment for p.Asn310Ser in a conditional analysis eliminated the association signal at the non-coding GWAS lead SNP rs6072275 (Punconditional = 2.5×10−5 and Pconditional = 0.33), supporting ZHX3 as the plausible causal gene at this locus (Table 1 and S3 Table). ZHX3 (zinc fingers and homeoboxes 3) encodes a transcriptional repressor which belongs to a protein family known to regulate gene expression in the kidney podocytes and plays roles in both lipoprotein metabolism and triglyceride regulation in mice [25, 26].
The final variant was in RREB1 (p.Ser1554Tyr, MAF = 21.1%, P = 8.4×10−9) which resides in the RREB1/SSR1 GWAS locus influencing FG levels [4] and T2D risk [27] (Table 1). We were unable to explore the relationship between this association signal and the lead FG (rs17762454) or T2D (rs9502570) GWAS SNPs at this locus as neither the variants themselves, nor a close proxy (r2>0.80), was present on the exome-array. Based on European haplotypes from the 1000 Genomes Project, the coding variant was more strongly correlated (r2 = 0.59) with rs9502570 as compared to rs17762454 (r2 = 0.08), which might indicate multiple FG association signals in this region. These data point to a likely functional role for RREB1 at this locus, although further studies are needed to confirm this hypothesis.
Coding variants influencing FI levels
Turning to the analysis of FI, we identified six non-synonymous variants associated at exome-wide significance (one rare and five common). These mapped to four loci, three of them previously implicated in FI regulation (S3 Table). The single novel FI - influencing locus was represented by a rare variant in URB2 (p.Glu594Val, MAF = 0.1%, P = 3.1×10−7; Table 1 and S1 Fig.). The variant allele was observed in individuals across Europe, including UK, Denmark, Finland, and Sweden, and genotype calls across all cohorts passed visual inspection for clustering accuracy. Heterozygous genotypes (n = 6) were 100% concordant for 3,999 individuals genotyped on the array that had also been exome sequenced (S8 Table). This variant has a relatively large effect, with each copy of the minor allele increasing FI level by 32%. URB2 encodes ribosome biogenesis 2 homolog, which interacts with nuclear lamins [28]. The precise mechanistic role of URB2 is still poorly understood, but conditions caused by defects in lamin genes (laminopathies), including familial partial lipodystrophy, can cause loss of adipose tissue, insulin resistance, and metabolic syndrome [29, 30].
The remaining five coding variants implicated in the FI analysis, included three previously-reported FI-associated common variants in GCKR (p.Pro446Leu, P = 8.1×10−11), PPARG (p.Pro12Ala, MAF = 14.7%, P = 1.3×10−7), and COBLL1 (p.Asn939Asp, MAF = 11.3%, P = 6.7×10−8) [3, 4]. The final two variants (in C2orf16 and GPN1) also mapped within the GCKR FG/FI-associated GWAS locus, and their associations were eliminated after conditioning on the GCKR p.Pro446Leu variant as seen in FG analysis (S3 Table).
Discussion
In the current study, we have examined the contribution of coding variants in influencing glycemic trait levels and identified significant associations at ten loci, highlighting multiple functional genes.
We have established a functional role for G6PC2 in FG homeostasis and have identified two novel loci associated with glycemic traits: GLP1R and URB2. In three additional GWAS regions for FG, we have identified potentially causal coding variants, highlighting likely effector transcripts (PCSK1, RREB1, and ZHX3). In line with earlier observations, very few of the FG - and FI-associated loci identified impact T2D risk [4]. The exception in this study is the p.Val219Leu G6PC2 variant, which showed a modest but directionally consistent association with T2D risk, in contrast to earlier reports suggesting that, paradoxically, glucose-raising alleles at this GWAS locus are protective against T2D [2].
Our results have a number of important implications for the design, analysis, and interpretation of association studies of coding variation. First, we have demonstrated that an appreciation of regional haplotypes is fundamental to understanding the relationship between genetic variation and gene function, phenotype, and disease. In this study, haplotype analysis was essential in resolving the apparent discrepancy between the observed phenotypic effect driven by the G6PC2 C allele at the variant encoding Leu219 and our in vitro data. From a clinical genomics perspective, this example illustrates how interpretation of single-variant analyses can be misleading and that the explicit phase relationships of variants are important for the correct interpretation of allelic effects. Furthermore, they highlight the importance of considering the possible interactions between regulatory and coding variants on protein levels [31]. The haplotype analysis also allows us to define specific regional diploid haplotypes that differ markedly in glucose levels. This is particularly relevant to efforts to follow up these variants in human data, whether for in vivo physiology, or for cellular studies on biopsies or patient derived stem cells.
Second, we have highlighted some limitations of widely used in silico prediction programs in accurately evaluating the impact of genomic variation on protein function. Earlier studies have shown that filtering functional variants based on in vitro data can substantially improve the power to detect causal genes [32, 33]. When such data are not available, in silico predictions can be used to help identify variants of functional significance [10, 34]. However, as we have demonstrated with the G6PC2 p.Val219Leu variant, current in silico predictions are not always accurate and should be validated with in vitro functional analysis wherever possible.
We find little support for a widespread impact of rare or low-frequency coding variants in accounting for known common SNP FG - and FI-associated GWAS signals. However, given that exome-array genotyping does not capture the complete repertoire of coding variation throughout the genome, exome sequencing is still required to achieve a complete assessment of their impact on glycemic traits at all associated loci. It is also likely that analysis of non-coding regulatory variation will be necessary to elucidate the causal genes and functional roles of association signals observed in prior GWAS. However, our study provides clear examples of how the analysis of coding variation can aid interpretation of GWAS findings where biology was previously unclear, and highlights the promise of this approach to provide insights into the pathophysiology of common complex disease.
Data availability
Summary statistics of single-variant and gene-based analyses are available at http://www.diagram-consortium.org/Mahajan_2014_ExomeChip/
Materials and Methods
Ethics statement
All human research was approved by the relevant institutional review boards, and conducted according to the Declaration of Helsinki and all patients provided written informed consent. FIN-D2D 2007, DPS, DR’s EXTRA, FINRISK 2007, FUSION, and METSIM were approved by the University of Michigan Health Sciences and Behavioral Sciences Institutional Review Board (ID: H03-00001613-R2). The Danish studies (Health 2006, Inter99, and Vejle Biobank) were approved by the local Ethical Committees of Capital Region (approval # H-3-2012-155, KA 98155 and KA-20060011) and Region of Southern Denmark (approval # S-20080097). The GoDARTS study was approved by EoS REC 09/S1402/44. The Twins UK study was approved by EC04/015. The OBB study was a approved by South Central, Oxford C, 08/H0606/107+5, IRAS project 136602. The PIVUS study is approved by 00–419 and ULSAM study by 251/90 and 2007/338. The PPP study was approved by the Committee On the Use of Humans as Experimental Subjects at MIT (IRB 0912003615).
Phenotypes
Study participants. FG was measured in mmol/L and FI in pmol/L. Individuals were excluded from the analysis if they had a physician diagnosis of diabetes, were on diabetes treatment (oral or insulin), or had a fasting plasma glucose concentration ≥7 mmol/L or ≥11.1 mmol/L following a 2-hour oral glucose tolerance test. Individual studies applied further sample exclusions, including for pregnancy, nonfasting measurements, and type 1 diabetes (S1 Table). Measures of fasting glucose made in whole blood were corrected to approximate plasma level by multiplying by 1.13 [35].
Trait transformations and adjustment. To achieve approximate normality of the traits within each study, FG and natural logarithm–transformed FI levels were adjusted for age, sex, BMI, and study specific covariates followed by inverse normalization of the residuals. Inverse normalized residuals were used as the dependent quantitative trait in genetic association models to calibrate type 1 error. Effect estimates were obtained using untransformed FG and natural logarithm–transformed FI levels after adjusting for age, sex, BMI, and study specific covariates.
Genotyping and quality control
The Illumina HumanExome Beadchip array was genotyped by individual studies. This custom array was designed to facilitate large-scale genotyping of 247,870 mostly rare (MAF<0.5%) and low-frequency (MAF 0.5–5%) protein altering variants selected from sequenced exomes and genomes of ~12,000 individuals (see URLs). Details of genotype calling and quality control are presented in S1 Table. To confirm the genotyping quality of all the variants discussed in the manuscript, we compared the genotype calls in 2,312 to 4,000 individuals for which we have both exome-array genotypes and exome-sequence data (S8 Table). The results are highly concordant for all variants (99% heterozygous genotype concordance and 100% concordance observed for non-reference homozygotes). Call rate and Hardy-Weinberg equilibrium p-values for each variant are provided in S9 Table.
Association analysis
Single-variant analysis. We tested single variants for association with FG - and FI-derived inverse normalized residuals assuming an additive genetic model using a linear mixed model to account for relatedness with EMMAX [5]. Study-specific QQ plots and genomic lambdas are shown in the S3 Fig. We repeated single-variant association analyses with untransformed FG and natural logarithm–transformed FI residuals to obtain effect estimates. We then combined the association summary statistics across studies by using a fixed-effects meta-analysis (sample size Z-score weighted) using METAL [36]. Genotype cluster plots of all variants described here were inspected visually in all studies.
Single-variant conditional analysis. Conditional analysis was performed to identify additional association signals at known or novel loci. The analysis included the genotypes of the lead variant(s) at the conditioning loci as covariate(s) in the regression analysis in EMMAX. We then performed meta-analysis of the association summary statistics across studies by using a fixed-effects meta-analysis (sample size Z-score weighted).
Gene-based analysis. For gene-based testing, we first computed single-variant score statistics and their covariance matrices for all polymorphic markers within each study. We then combined the single-variant score statistics from all studies using the Cochran-Mantel-Haenszel method and computed corresponding variance-covariance matrices centrally [6]. These variance-covariance matrices were used to compute gene-level statistics. We applied a frequency-weighted burden test which assumes variants have similar effect sizes and SKAT, a dispersion test that performs well when both protective and deleterious variants are present [8, 9]. Test-specific asymptotic distributions were used to evaluate significance. For gene-based analyses, we used only unrelated individuals (n = 32,223 for FG and n = 29,848 for FI) and included principal components as covariates to adjust for population structure. We generated four variant lists using frequency data and functional annotation. Variants were mapped to transcripts in Ensembl 66 (GRCh37.66). Using annotations from CHAoS v0.6.3, SnpEff v3.1, and VEP v2.7, we identified variants predicted to be protein-truncating (PTV; e.g. nonsense, frameshift, essential splice site) or protein-altering (e.g. missense, in-frame indel, non-essential splice site) in at least one mapped transcript (by at least one of the three algorithms) [37, 38]. We additionally used the procedure described by Purcell et al. to identify subsets of missense variants meeting “strict” or “broad” criteria for being deleterious, using annotation predictions from Polyphen2-HumDiv, PolyPhen2-HumVar, LRT, MutationTaster, and SIFT [10]. Masks 1 (“PTV-only”) and 3 (“PTV + NSstrict”) are restricted to variants with predicted major effect on protein function, and, as a result, disproportionately favor inclusion of rare variants, whilst masks 2 (“PTV + missense”) and 4 (“PTV + NSbroad”) are more permissive. In total, we tested 1,028, 14,465, 4,603, and 13,093 genes having at least two such variants in the above four categories respectively. For gene-based tests reaching exome-wide significance, if the conditional analysis showed that the signal was driven by a single variant, we required the variant to achieve exome-wide significance in the single-variant test as well.
Gene-based conditional analysis. We performed conditional gene-level analysis to evaluate whether rare or low-frequency variants associated with the trait in single-variant analysis could account for or were due to a gene-based test association signal [6]. The genotypes of the variant(s) at the conditioning locus were included as covariate(s) in this analysis.
Estimating phenotypic variance explained by SNPs. We used a subset of Finnish samples (FIN-D2D 2007, DPS, DR’s EXTRA, FINRISK 2007, and METSIM; n = 10,266) to calculate variance explained by G6PC2. We ran a model regressing BMI adjusted FG on the four G6PC2 variants: intronic GWAS lead SNP rs560887, p.His177Tyr (rs138726309), p.Tyr207Ser (rs2232323), and p.Val219Leu (rs492594), assuming an additive model (and adjusting for sex, age, age2, and study origin). A separate model was run excluding the GWAS SNP to determine the additional variance captured with the three coding variants.
Power calculations.We had >99.9% power to identify variants that explain >0.3% of the phenotypic variance and 80% power to detect coding variants that explain >0.1% of the phenotypic variance. To achieve >80% power for variants with MAF<0.05% would require effect sizes of at least 1 SD unit of residuals of mmol/L for FG and pmol/L for FI.
When we estimate power to detect association for aggregation tests, we make many assumptions: i) proportion of variants contributing to trait variation, ii) direction of effects, iii) number of variants aggregated, and iv) allele frequency distribution of the variants [34]. In this study, assuming 100% of the variants contribute to trait variation, we had >99.9% power to detect association for genes that explain >0.25% of the phenotypic variance and 80% to detect genes that explain >0.1% of the phenotypic variance using a burden test and >0.5% of the phenotypic variance using SKAT. In a less favorable scenario, for example assuming a gene explains 0.25% of the phenotypic variance, 25% of the variants contribute to trait variation, different directions of effect, 20 variants tested, and variants sampled from the reported allele frequency distribution in the exome-array design, power to detect association in this study may be near 0% for a burden test and 63% for SKAT [34].
Haplotype analysis. In 4,442 individuals from the Oxford Biobank, we used an expectation-maximization (EM) algorithm to obtain the posterior distribution of haplotypes consistent with the observed genotypes at four G6PC2 variants: intronic GWAS lead SNP rs560887, p.His177Tyr (rs138726309), p.Tyr207Ser (rs2232323), and p.Val219Leu (rs492594). Haplotype association with FG - and FI-derived residuals (after adjustment for age, sex, and BMI) was tested in a linear regression framework, as a function of haplotype dosage from posterior distribution, and including principal components as covariates to account for population structure using the most frequent haplotype as baseline.
In-silico mutation analysis. SIFT [39], PolyPhen-2 [40], and Condel [41] algorithms were used to predict the functional effects of the associated non-synonymous variants on protein function. Genomic Evolutionary Rate Profiling (GERP) [42] scores were calculated to indicate the degree of evolutionary conservation at a given human nucleotide based on multiple genomic sequence alignments and were measured as site-specific ‘rejected substitutions’: higher scores indicate greater conservation.
Functional studies
Site directed mutagenesis. Human G6PC2 cDNA (NM_021176.2) within a pCMV6-Entry vector (with a C-terminal FLAG-tag) was purchased from OriGene (RC211146). We generated non-synonymous variants in the G6PC2 coding sequence of the clone using Quikchange II Site-Directed Mutagenesis (Agilent). All mutations were verified by Sanger sequencing; in each case, only the desired nucleotide substitutions was introduced.
Western blot analyses. HEK293 and INS-1E 832–13 cells were transfected with each FLAG-tagged wild type or mutant G6PC2 construct using Lipofectamine 2000 (Invitrogen). In protein degradation assays, cells were treated with 10μM MG-132 (Calchembio) or 100μM chloroquine (Sigma) for 15h. At 48h after transfection, cells were collected and homogenized by sonication in lysis buffer. Total cellular protein was separated by 4–12% SDS-PAGE (Invitrogen) and transferred to nitrocellulose membranes. We determined G6PC2 expression by immunoblotting using a mouse monoclonal FLAG M2 antibody (Sigma, F1804). A rabbit polyclonal antibody against β tubulin (Santa Cruz, sc-9104) was used as a loading control. Secondary antibodies specific to mouse or rabbit IgG were obtained from Thermo Fisher Scientific. Protein bands were detected using the ECL reagent (Pierce Thermo Fisher Scientific). Western blots were quantified by densitometry analysis using ImageJ and paired t tests of densitometric data were carried out in GraphPad Prism.
Immunofluorescence. HEK293 cells were transfected with each FLAG-tagged G6PC2 construct using FuGene 6 transfection reagent (Promega) in 4-well chamber slides (BD Biosciences). After 48h, cells were fixed with 4% paraformaldehyde in PBS for 15 min. Cells were permeabilized with 0.05% Triton X-100 in PBS for 15 min, and blocked for 1 h with 10% BSA in PBS-Tween 20. We carried out double immunostaining of cells using FLAG M2 (Sigma, F1804) and calnexin (Santa Cruz, sc-11397) primary antibodies followed by anti-mouse fluorescein (Vector Labs) and anti-rabbit TRITC (Dako). DRAQ5 fluorescent probe (Thermo Fisher Scientific) was applied at 20μM as a far-red DNA stain. Slides were mounted with Vectashield mounting medium (Vector Labs) and visualized on a BioRad Radiance 2100 confocal microscope with a 60× 1.0 N.A. objective. Images were acquired with different laser settings that were optimized for each sample and therefore fluorescent intensities are not comparable across samples.
URLs
Exome-array design,
EPACTS,
METAL,
RareMETALS,
The 1000 Genomes Project,
Supporting Information
Zdroje
1. Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, et al. (2009) Variants in MTNR1B influence fasting glucose levels. Nat Genet 41 : 77–81. doi: 10.1038/ng.290 19060907
2. Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, et al. (2010) New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 42 : 105–116. doi: 10.1038/ng.520 20081858
3. Manning AK, Hivert M-F, Scott RA, Grimsby JL, Bouatia-Naji N, et al. (2012) A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat Genet 44 : 659–669. doi: 10.1038/ng.2274 22581228
4. Scott RA, Lagou V, Welch RP, Wheeler E, Montasser ME, et al. (2012) Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet 44 : 991–991005. doi: 10.1038/ng.2385 22885924
5. Kang HM, Sul JH, Service SK, Zaitlen NA, Kong S-Y, et al. (2010) Variance component model to account for sample structure in genome-wide association studies. Nat Genet 42 : 348–354. doi: 10.1038/ng.548 20208533
6. Liu DJ, Peloso GM, Zhan X, Holmen OL, Zawistowski M, et al. (2014) Meta-analysis of gene-level tests for rare variant association. Nat Genet 46 : 200–204. doi: 10.1038/ng.2852 24336170
7. Feng S, Liu D, Zhan X, Wing MK, Abecasis GR (2014) RAREMETAL: fast and powerful meta-analysis for rare variants. Bioinformatics. doi: 10.1093/bioinformatics/btu367 24894501
8. Wu MC, Lee S, Cai T, Li Y, Boehnke M, et al. (2011) Rare-variant association testing for sequencing data with the sequence kernel association test. Am J Hum Genet 89 : 82–93. doi: 10.1016/j.ajhg.2011.05.029 21737059
9. Magi R, Kumar A, Morris AP (2011) Assessing the impact of missing genotype data in rare variant association analysis. BMC Proc 5 Suppl 9. doi: 10.1186/1753-6561-5-S9-S107 22373025
10. Purcell SM, Moran JL, Fromer M, Ruderfer D, Solovieff N, et al. (2014) A polygenic burden of rare disruptive mutations in schizophrenia. Nature 506 : 185–190. doi: 10.1038/nature12975 24463508
11. Vaxillaire M, Cavalcanti-Proenca C, Dechaume A, Tichet J, Marre M, et al. (2008) The common P446L polymorphism in GCKR inversely modulates fasting glucose and triglyceride levels and reduces type 2 diabetes risk in the DESIR prospective general French population. Diabetes 57 : 2253–2257. doi: 10.2337/db07-1807 18556336
12. Beer NL, Tribble ND, McCulloch LJ, Roos C, Johnson PRV, et al. (2009) The P446L variant in GCKR associated with fasting plasma glucose and triglyceride levels exerts its effect through increased glucokinase activity in liver. Hum Mol Genet 18 : 4081–4088. doi: 10.1093/hmg/ddp357 19643913
13. Rees MG, Wincovitch S, Schultz J, Waterstradt R, Beer NL, et al. (2012) Cellular characterisation of the GCKR P446L variant associated with type 2 diabetes risk. Diabetologia 55 : 114–122. doi: 10.1007/s00125-011-2348-5 22038520
14. Drucker DJ, Nauck MA (2006) The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368 : 1696–1705. doi: 10.1016/S0140-6736(06)69705-5 17098089
15. Service SK, Teslovich TM, Fuchsberger C, Ramensky V, Yajnik P, et al. (2014) Re-sequencing expands our understanding of the phenotypic impact of variants at GWAS loci. PLoS genetics 10: e1004147. doi: 10.1371/journal.pgen.1004147 24497850
16. Khajavi M, Inoue K, Lupski JR. (2006) Nonsense-mediated mRNA decay modulates clinical outcome of genetic disease. Eur J Hum Genet 14 : 1074–81. doi: 10.1038/sj.ejhg.5201649 16757948
17. Chen W-M, Erdos MR, Jackson AU, Saxena R, Sanna S, et al. (2008) Variations in the G6PC2/ABCB11 genomic region are associated with fasting glucose levels. J Clin Invest 118 : 2620–2628. doi: 10.1172/JCI34566 18521185
18. Bouatia-Naji N, Rocheleau G, Van Lommel L, Lemaire K, Schuit F, et al. (2008) A polymorphism within the G6PC2 gene is associated with fasting plasma glucose levels. Science 320 : 1085–1088. doi: 10.1126/science.1156849 18451265
19. Baerenwald DA, Bonnefond A, Bouatia-Naji N, Flemming BP, Umunakwe OC, et al. (2013) Multiple functional polymorphisms in the G6PC2 gene contribute to the association with higher fasting plasma glucose levels. Diabetologia 56 : 1306–1316. doi: 10.1007/s00125-013-2875-3 23508304
20. Pound LD, Oeser JK, O’Brien TP, Wang Y, Faulman CJ, et al. (2013) G6PC2: a negative regulator of basal glucose-stimulated insulin secretion. Diabetes 62 : 1547–1556. doi: 10.2337/db12-1067 23274894
21. Morris AP, Voight BF, Teslovich TM, Ferreira T, Segre AV, et al. (2012) Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet 44 : 981–990. doi: 10.1038/ng.2383 22885922
22. Wang H, Liu L, Zhao J, Cui G, Chen C, et al. (2013) Large scale meta-analyses of fasting plasma glucose raising variants in GCK, GCKR, MTNR1B and G6PC2 and their impacts on type 2 diabetes mellitus risk. PLoS One 8: e67665. doi: 10.1371/journal.pone.0067665 23840762
23. Hu C, Zhang R, Wang C, Ma X, Fang Q, et al. (2009) A genetic variant of G6PC2 is associated with type 2 diabetes and fasting plasma glucose level in the Chinese population. Diabetologia 52 : 451–456. doi: 10.1007/s00125-008-1241-3 19082990
24. Pickett LA, Yourshaw M, Albornoz V, Chen Z, Solorzano-Vargas RS, et al. (2013) Functional consequences of a novel variant of PCSK1. PLoS One 8: e55065. doi: 10.1371/journal.pone.0055065 23383060
25. Liu G, Clement LC, Kanwar YS, Avila-Casado C, Chugh SS (2006) ZHX proteins regulate podocyte gene expression during the development of nephrotic syndrome. J Biol Chem 281 : 39681–39692. doi: 10.1074/jbc.M606664200 17056598
26. Gargalovic PS, Erbilgin A, Kohannim O, Pagnon J, Wang X, et al. (2010) Quantitative trait locus mapping and identification of Zhx2 as a novel regulator of plasma lipid metabolism. Circ Cardiovasc Genet 3 : 60–67. doi: 10.1161/CIRCGENETICS.109.902320 20160197
27. Mahajan A (2014) Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat Genet 46 : 234–244. doi: 10.1038/ng.2897 24509480
28. Vlcek S, Foisner R, Wilson KL (2004) Lco1 is a novel widely expressed lamin-binding protein in the nuclear interior. Exp Cell Res 298 : 499–511. doi: 10.1016/j.yexcr.2004.04.028 15265697
29. Hegele RA, Anderson CM, Wang J, Jones DC, Cao H (2000) Association between nuclear lamin A/C R482Q mutation and partial lipodystrophy with hyperinsulinemia, dyslipidemia, hypertension, and diabetes. Genome Res 10 : 652–658. doi: 10.1101/gr.10.5.652 10810087
30. Mesa JL, Loos RJF, Franks PW, Ong KK, Luan Ja, et al. (2007) Lamin A/C polymorphisms, type 2 diabetes, and the metabolic syndrome: case-control and quantitative trait studies. Diabetes 56 : 884–889. doi: 10.2337/db06-1055 17327461
31. Lappalainen T, Montgomery SB, Nica AC, Dermitzakis ET (2011) Epistatic selection between coding and regulatory variation in human evolution and disease. Am J Hum Genet 89 : 459–463. doi: 10.1016/j.ajhg.2011.08.004 21907014
32. Rees MG, Ng D, Ruppert S, Turner C, Beer NL, et al. (2012) Correlation of rare coding variants in the gene encoding human glucokinase regulatory protein with phenotypic, cellular, and kinetic outcomes. J Clin Invest 122 : 205–217. doi: 10.1172/JCI46425 22182842
33. Bonnefond A, Clement N, Fawcett K, Yengo L, Vaillant E, et al. (2012) Rare MTNR1B variants impairing melatonin receptor 1B function contribute to type 2 diabetes. Nat Genet 44 : 297–301. doi: 10.1038/ng.1053 22286214
34. Zuk O, Schaffner SF, Samocha K, Do R, Hechter E, et al. (2014) Searching for missing heritability: designing rare variant association studies. Proc Natl Acad Sci U S A 111 : 455–464. doi: 10.1073/pnas.1322563111 24443550
35. D’Orazio P, Burnett RW, Fogh-Andersen N, Jacobs E, Kuwa K, et al. (2005) Approved IFCC recommendation on reporting results for blood glucose (abbreviated). Clin Chem 51 : 1573–1576. doi: 10.1373/clinchem.2005.051979 16120945
36. Willer CJ, Li Y, Abecasis GR (2010) METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26 : 2190–2191. doi: 10.1093/bioinformatics/btq340 20616382
37. McLaren W, Pritchard B, Rios D, Chen Y, Flicek P, et al. (2010) Deriving the consequences of genomic variants with the Ensembl API and SNP Effect Predictor. Bioinformatics 26 : 2069–2070. doi: 10.1093/bioinformatics/btq330 20562413
38. Cingolani P, Platts A, Wang LL, Coon M, Nguyen T, et al. (2012) A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 6 : 80–92. doi: 10.4161/fly.19695
39. Kumar P, Henikoff S, Ng PC (2009) Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc 4 : 1073–1081. doi: 10.1038/nprot.2009.86 19561590
40. Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, et al. (2010) A method and server for predicting damaging missense mutations. Nat Methods 7 : 248–249. doi: 10.1038/nmeth0410-248 20354512
41. Gonzalez-Perez A, Lopez-Bigas N (2011) Improving the assessment of the outcome of nonsynonymous SNVs with a consensus deleteriousness score, Condel. Am J Hum Genet 88 : 440–449. doi: 10.1016/j.ajhg.2011.03.004 21457909
42. Cooper GM, Stone EA, Asimenos G, Green ED, Batzoglou S, et al. (2005) Distribution and intensity of constraint in mammalian genomic sequence. Genome Res 15 : 901–913. doi: 10.1101/gr.3577405 15965027
Štítky
Genetika Reprodukční medicína
Článek Phosphorylation of Elp1 by Hrr25 Is Required for Elongator-Dependent tRNA Modification in YeastČlánek Naturally Occurring Differences in CENH3 Affect Chromosome Segregation in Zygotic Mitosis of HybridsČlánek Insight in Genome-Wide Association of Metabolite Quantitative Traits by Exome Sequence AnalysesČlánek ALIX and ESCRT-III Coordinately Control Cytokinetic Abscission during Germline Stem Cell DivisionČlánek Deciphering the Genetic Programme Triggering Timely and Spatially-Regulated Chitin Deposition
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 1
-
Všechny články tohoto čísla
- The Combination of Random Mutagenesis and Sequencing Highlight the Role of Unexpected Genes in an Intractable Organism
- Ataxin-3, DNA Damage Repair, and SCA3 Cerebellar Degeneration: On the Path to Parsimony?
- α-Actinin-3: Why Gene Loss Is an Evolutionary Gain
- Origins of Context-Dependent Gene Repression by Capicua
- Transposable Elements Contribute to Activation of Maize Genes in Response to Abiotic Stress
- No Evidence for Association of Autism with Rare Heterozygous Point Mutations in Contactin-Associated Protein-Like 2 (), or in Other Contactin-Associated Proteins or Contactins
- Nur1 Dephosphorylation Confers Positive Feedback to Mitotic Exit Phosphatase Activation in Budding Yeast
- A Regulatory Hierarchy Controls the Dynamic Transcriptional Response to Extreme Oxidative Stress in Archaea
- Genetic Variants Modulating CRIPTO Serum Levels Identified by Genome-Wide Association Study in Cilento Isolates
- Small RNA Sequences Support a Host Genome Origin of Satellite RNA
- Phosphorylation of Elp1 by Hrr25 Is Required for Elongator-Dependent tRNA Modification in Yeast
- Genetic Mapping of MAPK-Mediated Complex Traits Across
- An AP Endonuclease Functions in Active DNA Demethylation and Gene Imprinting in
- Developmental Regulation of the Origin Recognition Complex
- End of the Beginning: Elongation and Termination Features of Alternative Modes of Chromosomal Replication Initiation in Bacteria
- Naturally Occurring Differences in CENH3 Affect Chromosome Segregation in Zygotic Mitosis of Hybrids
- Imputation of the Rare G84E Mutation and Cancer Risk in a Large Population-Based Cohort
- Polycomb Protein SCML2 Associates with USP7 and Counteracts Histone H2A Ubiquitination in the XY Chromatin during Male Meiosis
- A Genetic Strategy for Probing the Functional Diversity of Magnetosome Formation
- Interactions of Chromatin Context, Binding Site Sequence Content, and Sequence Evolution in Stress-Induced p53 Occupancy and Transactivation
- The Yeast La Related Protein Slf1p Is a Key Activator of Translation during the Oxidative Stress Response
- Integrative Analysis of DNA Methylation and Gene Expression Data Identifies as a Key Regulator of COPD
- Proteasomes, Sir2, and Hxk2 Form an Interconnected Aging Network That Impinges on the AMPK/Snf1-Regulated Transcriptional Repressor Mig1
- Functional Interplay between the 53BP1-Ortholog Rad9 and the Mre11 Complex Regulates Resection, End-Tethering and Repair of a Double-Strand Break
- Estrogenic Exposure Alters the Spermatogonial Stem Cells in the Developing Testis, Permanently Reducing Crossover Levels in the Adult
- Protein Poly(ADP-ribosyl)ation Regulates Immune Gene Expression and Defense Responses
- Sumoylation Influences DNA Break Repair Partly by Increasing the Solubility of a Conserved End Resection Protein
- A Discrete Transition Zone Organizes the Topological and Regulatory Autonomy of the Adjacent and Genes
- Elevated Mutation Rate during Meiosis in
- The Intersection of the Extrinsic Hedgehog and WNT/Wingless Signals with the Intrinsic Hox Code Underpins Branching Pattern and Tube Shape Diversity in the Airways
- MiR-24 Is Required for Hematopoietic Differentiation of Mouse Embryonic Stem Cells
- Tissue-Specific Effects of Genetic and Epigenetic Variation on Gene Regulation and Splicing
- Heterologous Aggregates Promote Prion Appearance via More than One Mechanism
- The Tumor Suppressor BCL7B Functions in the Wnt Signaling Pathway
- , A -Acting Locus that Controls Chromosome-Wide Replication Timing and Stability of Human Chromosome 15
- Regulating Maf1 Expression and Its Expanding Biological Functions
- A Polyubiquitin Chain Reaction: Parkin Recruitment to Damaged Mitochondria
- RecFOR Is Not Required for Pneumococcal Transformation but Together with XerS for Resolution of Chromosome Dimers Frequently Formed in the Process
- An Intracellular Transcriptomic Atlas of the Giant Coenocyte
- Insight in Genome-Wide Association of Metabolite Quantitative Traits by Exome Sequence Analyses
- The Role of the Mammalian DNA End-processing Enzyme Polynucleotide Kinase 3’-Phosphatase in Spinocerebellar Ataxia Type 3 Pathogenesis
- The Global Regulatory Architecture of Transcription during the Cell Cycle
- Identification and Functional Characterization of Coding Variants Influencing Glycemic Traits Define an Effector Transcript at the Locus
- Altered Ca Kinetics Associated with α-Actinin-3 Deficiency May Explain Positive Selection for Null Allele in Human Evolution
- Genetic Variation in the Nuclear and Organellar Genomes Modulates Stochastic Variation in the Metabolome, Growth, and Defense
- PRDM9 Drives Evolutionary Erosion of Hotspots in through Haplotype-Specific Initiation of Meiotic Recombination
- Transcriptional Control of an Essential Ribozyme in Reveals an Ancient Evolutionary Divide in Animals
- ALIX and ESCRT-III Coordinately Control Cytokinetic Abscission during Germline Stem Cell Division
- Century-scale Methylome Stability in a Recently Diverged Lineage
- A Re-examination of the Selection of the Sensory Organ Precursor of the Bristle Sensilla of
- Antagonistic Cross-Regulation between Sox9 and Sox10 Controls an Anti-tumorigenic Program in Melanoma
- A Dependent Pool of Phosphatidylinositol 4,5 Bisphosphate (PIP) Is Required for G-Protein Coupled Signal Transduction in Photoreceptors
- Deciphering the Genetic Programme Triggering Timely and Spatially-Regulated Chitin Deposition
- Aberrant Gene Expression in Humans
- Fascin1-Dependent Filopodia are Required for Directional Migration of a Subset of Neural Crest Cells
- The SWI2/SNF2 Chromatin Remodeler BRAHMA Regulates Polycomb Function during Vegetative Development and Directly Activates the Flowering Repressor Gene
- Evolutionary Constraint and Disease Associations of Post-Translational Modification Sites in Human Genomes
- A Truncated NLR Protein, TIR-NBS2, Is Required for Activated Defense Responses in the Mutant
- The Genetic and Mechanistic Basis for Variation in Gene Regulation
- Inactivation of PNKP by Mutant ATXN3 Triggers Apoptosis by Activating the DNA Damage-Response Pathway in SCA3
- DNA Damage Response Factors from Diverse Pathways, Including DNA Crosslink Repair, Mediate Alternative End Joining
- hnRNP K Coordinates Transcriptional Silencing by SETDB1 in Embryonic Stem Cells
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Global Regulatory Architecture of Transcription during the Cell Cycle
- A Truncated NLR Protein, TIR-NBS2, Is Required for Activated Defense Responses in the Mutant
- Proteasomes, Sir2, and Hxk2 Form an Interconnected Aging Network That Impinges on the AMPK/Snf1-Regulated Transcriptional Repressor Mig1
- The SWI2/SNF2 Chromatin Remodeler BRAHMA Regulates Polycomb Function during Vegetative Development and Directly Activates the Flowering Repressor Gene
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



