-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaGenome Wide Association Studies Using a New Nonparametric Model Reveal the Genetic Architecture of 17 Agronomic Traits in an Enlarged Maize Association Panel
Genotype imputation has been used widely in the analysis of genome-wide association studies (GWAS) to boost power and fine-map associations. We developed a two-step data imputation method to meet the challenge of large proportion missing genotypes. GWAS have uncovered an extensive genetic architecture of complex quantitative traits using high-density SNP markers in maize in the past few years. Here, GWAS were carried out for 17 agronomic traits with a panel of 513 inbred lines applying both mixed linear model and a new method, the Anderson-Darling (A-D) test. We intend to show that the A-D test is a complement to current GWAS methods, especially for complex quantitative traits controlled by moderate effect loci or rare variations and with abnormal phenotype distribution. In addition, the traits associated QTL identified here provide a rich resource for maize genetics and breeding.
Published in the journal: . PLoS Genet 10(9): e32767. doi:10.1371/journal.pgen.1004573
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004573Summary
Genotype imputation has been used widely in the analysis of genome-wide association studies (GWAS) to boost power and fine-map associations. We developed a two-step data imputation method to meet the challenge of large proportion missing genotypes. GWAS have uncovered an extensive genetic architecture of complex quantitative traits using high-density SNP markers in maize in the past few years. Here, GWAS were carried out for 17 agronomic traits with a panel of 513 inbred lines applying both mixed linear model and a new method, the Anderson-Darling (A-D) test. We intend to show that the A-D test is a complement to current GWAS methods, especially for complex quantitative traits controlled by moderate effect loci or rare variations and with abnormal phenotype distribution. In addition, the traits associated QTL identified here provide a rich resource for maize genetics and breeding.
Introduction
Maize (Zea mays L.) is one of the most important food, feed and industrial crops globally. Grown extensively under different climate conditions across the world, maize shows an astonishing amount of phenotypic diversity [1]. Identifying the underlying natural allelic variations for the phenotypic diversity will have immense practical implications in maize molecular breeding for improving nutritional quality, yield potential, and stress tolerance.
With the rapid development of next generation sequencing and high-density marker genotyping techniques, there emerges tremendous interest in using association mapping to identify genes responsible for quantitative variation of complex traits [2]. The use of GWAS has been well demonstrated in model plants such as Arabidopsis [3] and rice [4]. In maize, we examined the genetic architecture of maize oil biosynthesis in 368 diverse maize inbred lines with over 1.06 million SNPs obtained from RNA sequencing and DNA array using the GWAS strategy [5]. Despite the great potential that GWAS has to pinpoint genetic polymorphisms underlying agriculturally important traits, false discoveries are a major concern and can be partially attributed to spurious associations caused by population structure and unequal relatedness among individuals in a given panel [6]. A number of statistical approaches have been proposed, among which the mixed linear model (MLM) is one of the popular methods that can eliminate the excess of low p values for most traits [6], [7]. However, Zhao et al. [8] performed GWAS using a NAÏVE model in each sub-population and MLM with inferred population structure as a fixed effect in the whole mapping panel of rice, and their results suggested that MLM may lead to false negatives by overcompensating for population structure and relatedness. To improve the MLM, some strategies to best utilize marker data have been proposed [9], [10]. The more we know about the genetics of a trait, the greater our power is to detect the rest of the genetic contribution. The problem is, of course, that we usually do not know what the causal loci are, and methods that try to identify them are prone to over-fitting [11]. Beló et al. [12] adopted the Kolmogorov–Smirnov (KS) test for association analysis in each subpopulation of the mapping panel and an allelic variant of fad2 associated with increased oleic acid level was successfully identified based on modest density markers. However, detailed instructions for the algorithm were not published. Most current GWAS methods lack the power to detect rare alleles and this has limited the application of GWAS, since rare alleles are common in maize diversity collections [1], [5]. Parametric tests of association are sensitive to SNPs with minor allele frequencies, which can artificially increase association scores. Balancing samples across population subdivisions can homogenize allele frequencies, elevating rates of globally rare variants that are common in certain subdivisions [5].
In this study, 513 diverse maize inbred lines [13], representing tropical/subtropical and temperate germplasm, were genotyped by MaizeSNP50 BeadChip containing 56,110 SNPs [14]. RNA sequencing (RNA-seq) was performed on 368 of these 513 lines and 556,809 high quality SNPs with a minor allelic frequency greater than 0.05 were obtained [5], [15], [16]. Seventeen agronomic traits were systematically phenotyped for the 513 lines under multiple environments and seasons (see Materials and Methods). The objectives of this research were (1) to explore an efficient imputation method to infer missing genotypes for the 145 inbreds that were only genotyped by SNP-chip (low density), not by RNA-seq (high density); (2) to develop a powerful statistical method for GWAS to identify robust QTL for complex agronomic traits in maize; and (3) to methodically analyze the underlying genetic architectures of the 17 agronomic traits in the diverse maize association mapping panel.
Results
Phenotypic variation for 17 agronomic traits
A brief description of each trait, its acronym, and evaluation methodology was summarized in Supplementary Table S1. All of the 17 traits in the 513 maize inbred lines were in accordance with a normal distribution (Figure S1A, S2, S3, S4, S5, S6, S7, S8, S9, S10, S11, S12, S13, S14, S15, S16, S17A). But the phenotype of each trait showed distinct differences among four subgroups (Figure S1B, S2, S3, S4, S5, S6, S7, S8, S9, S10, S11, S12, S13, S14, S15, S16, S17B). Analysis of Variance (ANOVA) showed that population structure explained 39.5% of phenotypic variation (PVE) for tassel main axis length, which was the highest among the 17 agronomic traits included in best linear unbiased prediction (Table S2), indicating vulnerability of this particular trait to the population structure and variable sensitivity of different traits to population structure. Furthermore, heritability (h2) was highest (0.683) for tassel main axis length (TMAL), while the lowest heritability (0.386) was observed for kernel number per row (KNPR) among the traits. Pair-wise Pearson's correlation coefficients of the 17 traits revealed that phenotypes within a category were more correlated. The values ranged from 0.001 between kernel width and plant height to 0.95 between days to anthesis and heading (Figure S18). These results indicated that all the tested lines possessed significant genetic variability and can be used for further genetic analyses.
SNP projection and imputation
The whole panel, 513 maize inbred lines, was genotyped using the MaizeSNP50 BeadChip containing 56,110 SNPs (Illumina). RNA sequencing was performed on immature seeds for 368 out of the 513 maize inbreds using 90-bp paired-end Illumina sequencing, resulting in 2,445.9 Gb of raw sequencing data. 556,809 high quality SNPs obtained by combining the two genotyping platforms (RNA-seq and SNP array) [5], [16] were used in the study. For the additional 145 maize lines, the genotype calls of unique loci from the integrated SNP data were projected based on regions of IBD to physical maps constructed using 56110 SNPs, and then high-density markers with more than 0.5 million SNPs were obtained for all the lines. Out of 56,110 SNPs from MaizeSNP50 data set, 49728 SNPs overlapped with the integrated SNPs data based on their physical positions (B73 RefGen_v2). The 49,728 common SNPs were regarded as core or frame markers for projection based on IBD regions. In order to evaluate the performance of IBD [17] based projection, training and validation datasets were established for chromosome 1, which had 7818 core markers from Illumina Maize SNP50 and 88581 SNPs from the integrated data set. The genotypes for one maize line with RNA-seq data in IBD regions were assigned to the matched target line without RNA-seq data for each SNP. The projection accuracy was calculated by comparing inferred genotypes of 368 lines with their real genotype obtained from RNA-seq. In addition, KNN algorithm [4] which infers a large number of missing genotypes generated from low-coverage genome sequencing was used to impute the missing genotypes of the unique loci from RNA-seq SNP data based on 49728 frame markers. Single method analysis, either IBD based projection or KNN algorithm, cannot achieve both optimal accuracy and coverage (see Materials and Methods). However, the combination of IBD based projection and KNN seemed effective to infer a large number of missing genotypes. In order to optimize the set of imputation parameters, a simulation was performed on chromosome 1 in 368 lines (Figure S19). The simulation result on chromosome 1 in 368 lines indicated that the missing rate was reduced from 91.6% (1–7,818/88,581) to 12.8%, with an accuracy rate 96.6% (Table S3). The optimal parameter combination (IBD: SNPs number≥150 in 5 Mb window size; KNN: w = 20, k = 6, p = −7, r = 1) was then used to impute the missing SNPs for the remaining 145 inbred lines, resulting in an 85.5% filling rate. Therefore, our approach combining SNP-chip data and RNA-seq SNP data with an effective projection procedure permits the quick construction of a high-density physical map and integration of SNPs from RNA-seq data set onto the whole population. This approach is also applicable to other genomes and genotyping data from different platforms for a variety of downstream analyses.
Statistical power of the imputation-based association test
The 368 maize inbreds with 556,809 SNPs genotyped by RNA-seq and Maize SNP50 array were defined as Data set 1. In addition, Data set 1 and 145 maize inbred lines with joint IBD-based projection and KNN imputed genotypes were defined as Data set 2 together. The 513 maize inbreds with Maize SNP50 array genotyped were defined as Data set 3. To evaluate the reliability of imputed genotypes for 145 inbred lines, GWAS was performed using MLM, with both Data sets 1, 2 and 3 focusing on kernel oil concentration, which has been thoroughly analyzed in our previous study [5].
For GWAS performed using MLM with both Data sets 1 to 3, a total of 26, 32 and 8 significant loci were identified in Data sets 1 to 3, respectively, at the Bonferroni-corrected threshold (−log P>5.74, α = 1,) (Figure 1). Almost all strong signals identified in data sets 1 and 3 were also identified in data set 2 (Table S4). More interestingly, we identified six additional significantly associated loci in dataset 2 (−log P>5.74, α = 1), including the phosphoinositide 3-kinases gene (PI3Ks) and the phosphatidylinositol transfer protein, which is known to be involved in the oil concentration trait [18] (Figure 1, Table 1, Table S4). This suggests that GWAS carried out using the imputed genotypes with a larger population (n = 513) increased the statistical power compared to the analyses of RNA-seq genotyped SNPs with the smaller population size (n = 368) or low density as DNA array SNPs with the same population size (n = 513).
Fig. 1. Comparison of mapping results for kernel oil concentration in two different data sets. 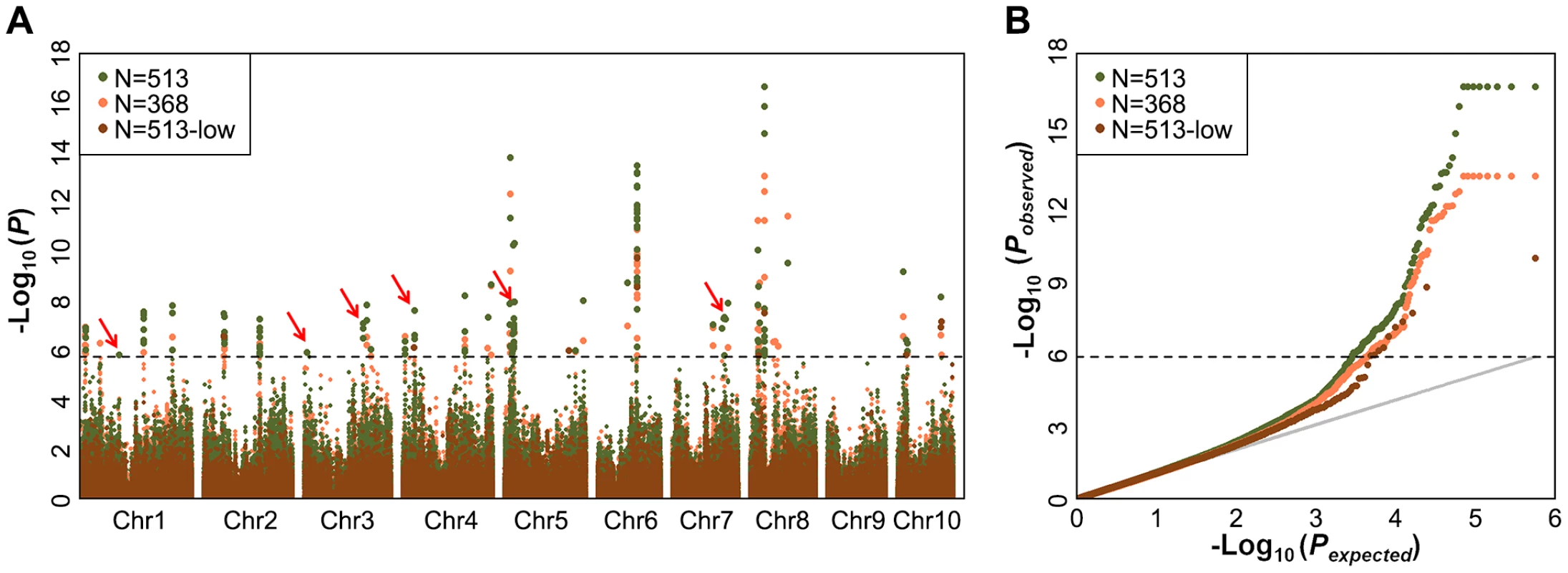
(A) Manhattan plots of mixed linear model conducted in data set 1 and 2, respectively (data set 1: n = 368, without imputed genotypic data; data set 2: n = 513, 145 lines with imputed genotypic data). The arrow and red boxes indicate the new loci that were not identified in previous study (Li et al, 2013); (B) Quantile-Quantile plots of p-values of mixed linear model conducted in data sets 1 and 2, respectively. Tab. 1. Additional SNPs and candidate genes significantly associated with oil concentration found using imputed genotype data. 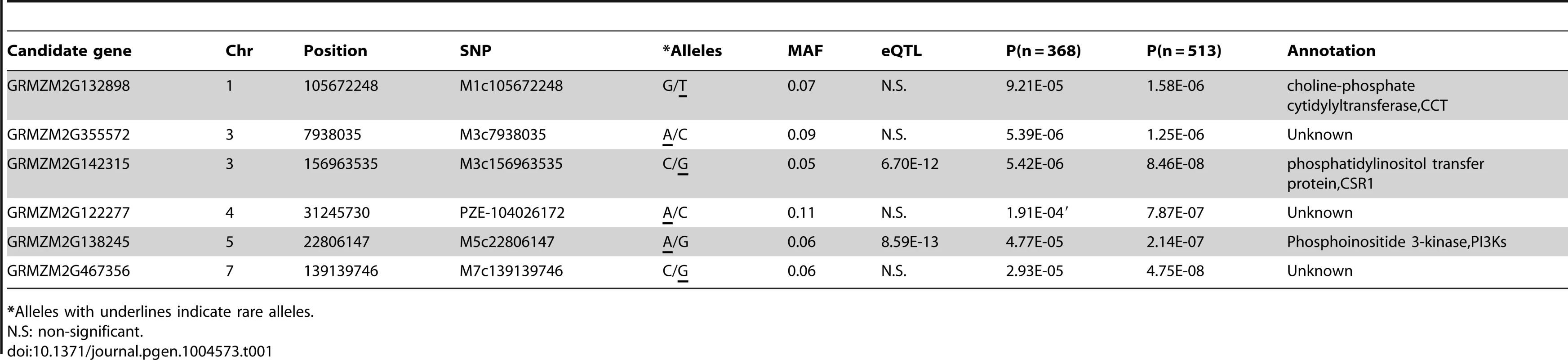
*Alleles with underlines indicate rare alleles. GWAS for 17 agronomic traits using MLM
GWAS for 17 agronomic traits using MLM was conducted with Data set 2 and the results are summarized in Figures S1C, D, S2, S3, S4, S5, S6, S7, S8, S9, S10, S11, S12, S13, S14, S15, S16, S17C, D. A total of 19 significant SNPs from 10 loci were identified for five traits (ear leaf width, ear length, kernel width, plant height and tassel main axis length) (Table 2). No significant SNP was found to be associated with the other 12 tested traits at the Bonferroni-corrected threshold (−log P>5.74, α = 1). If we set the Bonferrroni-corrected threshold as −log P>7.05 (α = 0.05), no SNPs were significant for all the 17 traits. It may be too strict to use 0.05/n as the cutoff since not all the markers are independent. One thousand permutation tests were conducted for three typical traits with different level of population structure (kernel width, ear height and days to heading) (Table S2). The results showed the cutoff value at α = 0.05 is quite similar (Table S5) with 0.05/n.
Tab. 2. Comparison of significant SNPs identified for 17 traits from MLM using imputed data (dataset 2). 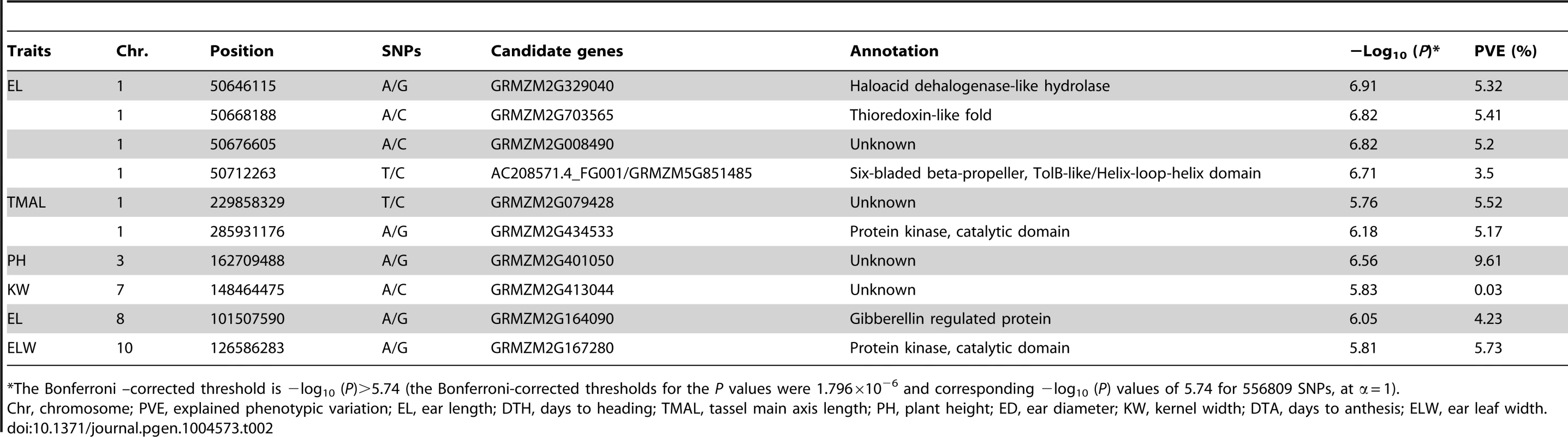
*The Bonferroni –corrected threshold is −log10 (P)>5.74 (the Bonferroni-corrected thresholds for the P values were 1.796×10−6 and corresponding −log10 (P) values of 5.74 for 556809 SNPs, at α = 1). Anderson-Darling (A-D) test, an alternative method for GWAS
The A-D test [19] is a nonparametric statistical method and a modification of the KS test [12], [20] that gives more weight to the tails of the distribution than the KS test. Since the identified loci were much less numerous than expected using the MLM method, the data set was reanalyzed using the A-D test. The same three traits (kernel width, ear height and days to heading) were used to perform 1000 time permutation tests to determine the cutoff values. The results showed the cut off value at α = 0.05 varied around the Bonferrroni-corrected threshold as 0.05/n (Table S5). To simplify the procedures, we used the uniform cutoff (−log P>7.05, α = 0.05) for further analysis. Flowering time is an important and well-studied trait, and many QTL or candidate genes have been identified [21], [22]. Recently, several studies have confirmed that ZmCCT is the gene underlying the major QTL affecting flowering time on chromosome 10 [22], [23]. Taking flowering time as an example, it provides a good opportunity to test whether A-D test is a feasible GWAS method for agronomic traits or not. Using the A-D test, we identified 30 loci associated with days to heading in Yunnan 2010. Around 20% of 30 loci were located within a QTL support interval reported in NAM population [22]. If the significant loci are randomly distributed in the genome, the probability by chance is equal to the ratio between the whole-length of QTL interval and the whole genome length (12%), which represents an almost twofold enrichment compared with the 12% expected by chance. A strong association (−log10 (P) = 7.59) was identified in 1.7 Kb upstream of ZmCCT (Figure 2A). Four other loci seem to be strong candidates including: one homologous gene (CIB1) [24] shown to be involved in the regulation of flowering time in Arabidopsis, two homologues containing CCT domain that was demonstrated as key photoperiod regulatory gene in plants [25], and one locus previously shown to affect flowering time in maize (Id1) [26] (Figure 2A). Using the MLM method, we were only able to identify the marginally significant association for ZmCCT (−log10 (P) = 5.64) and there were no strong signals in other genome regions (Figure 2B). Therefore, A-D test could be a more appropriate GWAS method for agronomic traits and we performed GWAS using the A-D test in each subpopulation of Data set 2 without controlling of population structure for all tested traits. The total number of unique SNPs significantly associated with the 17 traits was 678, of which 310 represented unique loci (Figure S1E, S2, S3, S4, S5, S6, S7, S8, S9, S10, S11, S12, S13, S14, S15, S16, S17E). The numbers of significant SNPs associated with different traits ranged from 1 (Cob Weight) to 35 (Tassel branch number and Days to silking). For plant height, a total of 107 loci were identified at the Bonferroni-corrected threshold (−log P>7.05, α = 0.05) (Table S6, S7). About 10% of the loci were detected to affect two or more different traits that were consistent with the observed correlations among the measured traits (Figure S18). There were 101, 71 and 171 loci detected in three subpopulations: SS (subpop-1), NSS (subpop-2) and TST (subpop-3), respectively. A reasonable number of spurious associations should be existed in the detected loci since the population structure is not properly addressed in each subpopulation. Genomic control [27]–[30] is a popular method to control the population stratification and cryptic relatedness that was applied to adjust A-D test statistic in each subpopulation in present study. In total, 19 loci were significantly associated with 13 traits at the Bonferroni-corrected threshold (−log P>5.74, α = 1) (Table S7).
Fig. 2. GWAS of the phenotype of days to heading in Yunnan 2010. 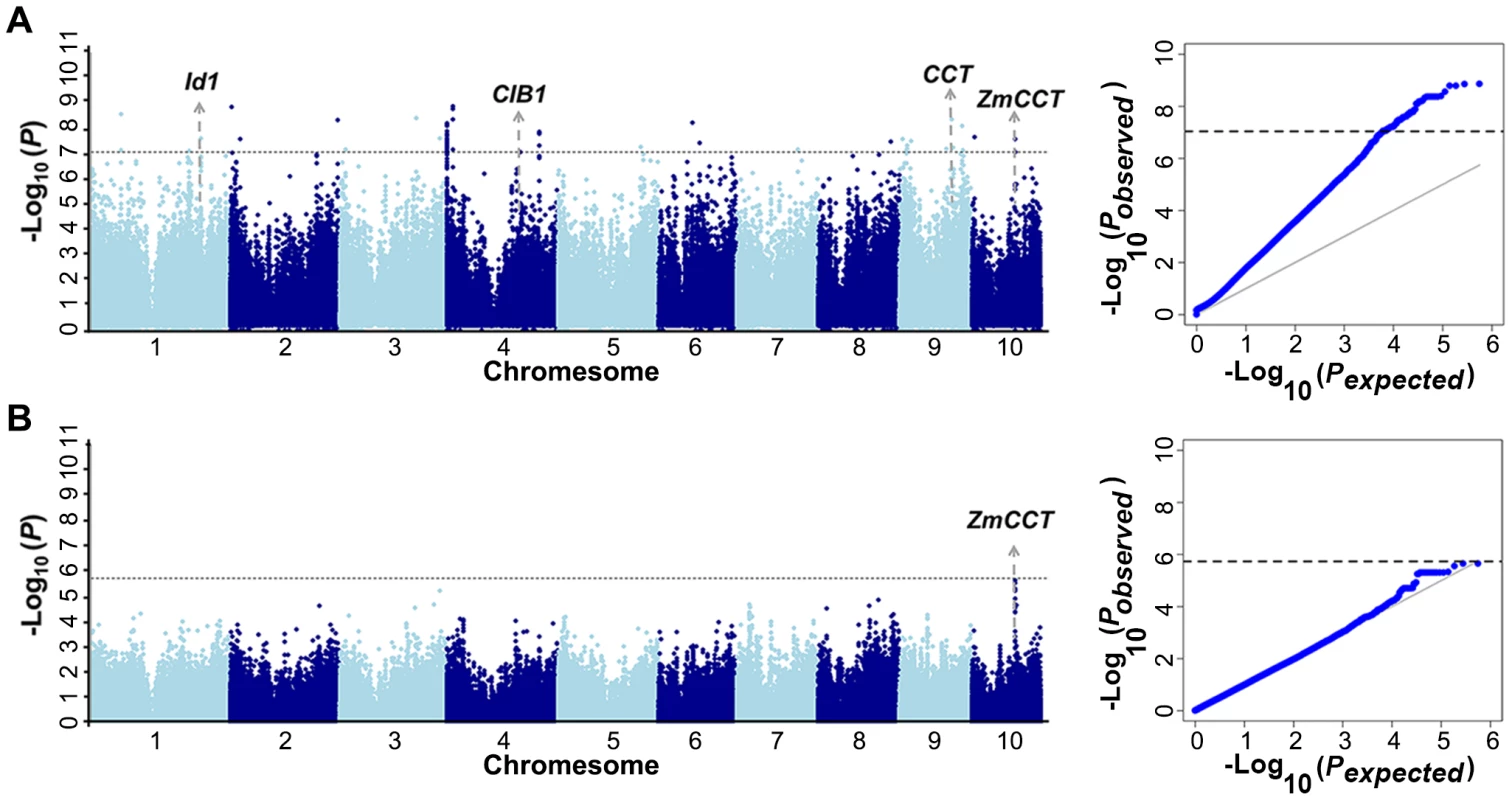
(A) GWAS result by Anderson–Darling test; (B) GWAS result by mixed linear model. To further examine the nature of statistically significant associations, we examined the phenotype distributions of individuals carrying each allele. Interestingly, some associations that differed in the width of the phenotype distribution but which had nearly identical trait means were found to be highly significant by the A-D test but not significant by MLM. Figure 3 illustrated significant loci for ear height with nearly identical trait means (Figure 3A) and significant loci for ear leaf width with an obvious shift of the means (Figure 3B). In total, 14.6% of significant loci identified by A-D test do not have an obvious shift of the mean between the two alleles (t-test, p>0.05) (Table S7). In this case the differences between distributions are real, but the corresponding genetic markers would not be useful in breeding if the objective is to change the phenotypic means.
Fig. 3. The nature of statistically significant associations. 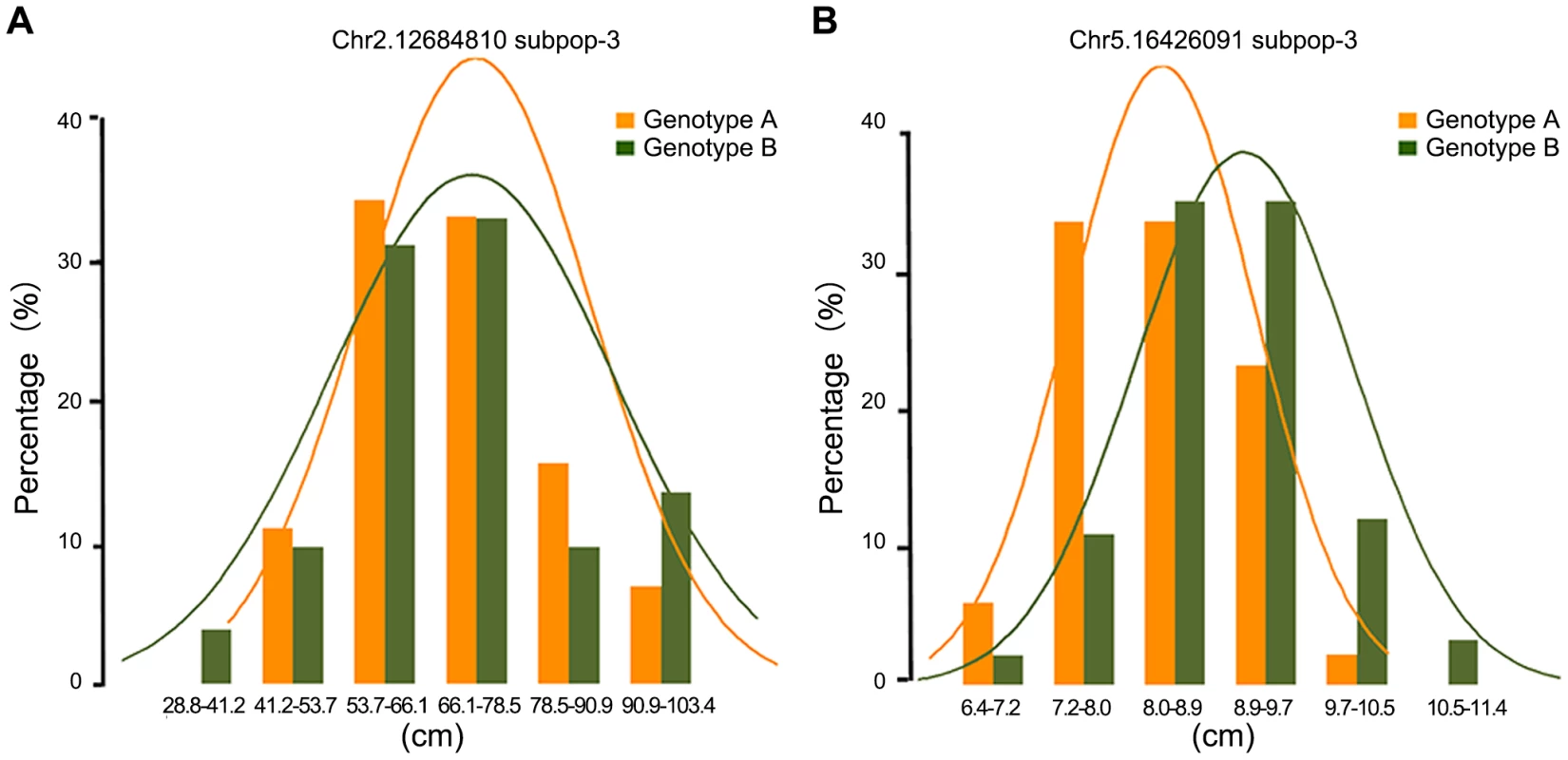
The illustration of associations those are highly significant by Anderson–Darling test, with nearly identical trait means for ear height (A) or with an obvious shift of the means for ear leaf width (B). Comparison of different association mapping methods based on simulated data
Causal allele frequencies and trait distributions are the main factors that affect association mapping efficiency [1], [31]. GWAS data were simulated by adding phenotypic effects to real genotypic data considering the population structure and epistasis from MaizeSNP50 BeadChip [13] under three scenarios: a normal distribution model, an abnormal distribution model caused by uncertain effectors like phenotyping errors and an abnormal distribution model caused by a larger effect QTN with rare alleles (Methods). We compared our noticed A-D test with three other mapping methods: Kruskal-Wallis (K-W) test and linear model (LM) which does not correct for population structure; MLM which corrects for population structure and kinship. Statistic power of the four methods were compared under the same level Type I error. For each method, QTNs were considered to be detected if their P value were below the threshold determined by 1,000 times permutation.
The results for the three simulation schemes are shown in Figure 4 and can be summarized as follows: First, MLM has more (Figure 4A, C, I) or similar (Figure 4G) power among the four methods for major QTNs in schemes 1 and 3. Second, regardless of the allele frequency, nonparametric methods usually have greater power than LM and MLM for moderate QTNs (Figure 4D–F, J–I). Third, A-D test is more powerful than K-W test in terms of QTNs with rare alleles (Figure 4J–I). Fourth, nonparametric methods are much more powerful than parametric methods in scheme 2 (Figure 4B, E, H, K) that the phenotype has an abnormal distribution model caused by uncertain effectors.
Fig. 4. Power comparisons in three simulation schemes for four different mapping methods: 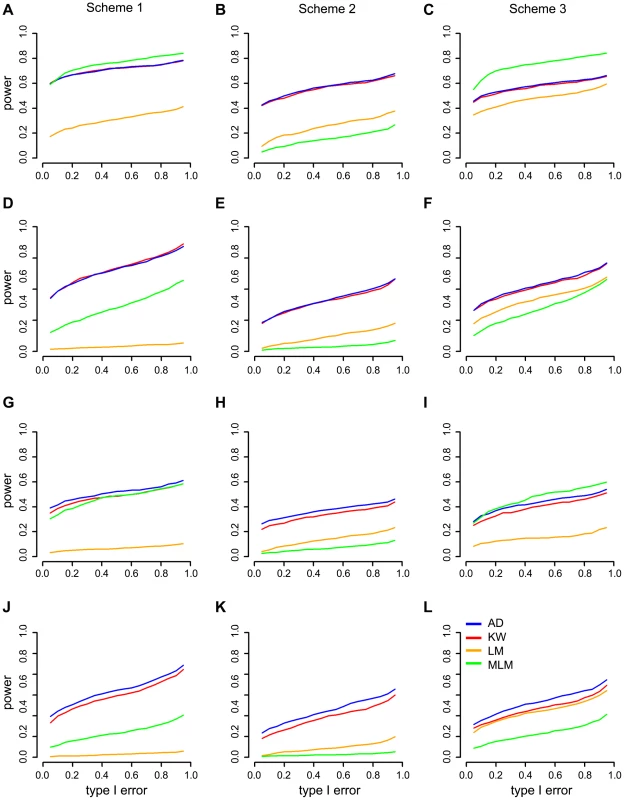
A-D, KW, MLM and LM. The “Power” was defined as the detection frequency in 500 repeats for a certain QTN. For the purpose of computing power, a causal SNP was considered to be detected only when the causal SNP was significant at a threshold from 1,000 times permutations. The power and type I error of major QTNs (A–C) and moderate QTNs (D–F) with common allele frequency. The power and type I error of major QTNs (G–I) and moderate QTNs (J–L) with rare allele frequency. A-D test: Anderson–Darling test; LM: linear model; K-W test: Kruskal-Wallis test; MLM: mixed linear model. Scheme 1, phenotypes with normal distribution; Scheme 2, phenotypes with abnormal distribution caused by uncertain effectors; Scheme 3, phenotypes with abnormal distribution caused by a larger effect QTN with rare allele frequency. Co-localization of QTL and candidate genes for agronomic traits
We compared our mapping results for 17 agronomic traits with QTL identified using different linkage segregation populations and with previously reported known genes. Loci identified by the A-D test which overlap with previously identified genes and loci mapped in biparental populations are summarized in Table S7. Considering the large confidence interval of previously reported QTL, 3 independent RIL populations genotyped with high-density SNP markers were used to conduct QTL analysis for 3 traits (kernel width, ear length and kernel number per row) of the 17 traits tested. For the compared traits, 9 loci (20% of the detected loci) identified by the A-D test were within the QTL confidence interval. One example is a major kernel width QTL which was mapped in chromosome 7 with BK/Yu8701 RILs and explains 18.7% of phenotypic variation (Figure 5B). Within the QTL interval, significant SNPs - kernel width association was detected (Figure 5A). Six candidate genes: GRMZM2G354539, GRMZM2G052893, GRMZM2G052817, GRMZM2G354525, GRMZM2G052610, and GRMZM2G052509 are found in the associated interval. An expression quantitative trait locus (eQTL) was detected for one (GRMZM2G052509, −log10 (P) = 10.16) of the six annotated genes, which can therefore be regarded as a candidate gene for further study. The second example of overlap included the SNP chr2.s_1972207(C/G) with −log10 (P) = 9.06and SNP chr2.s_1972176(C/G) with −log10 (P) = 8.49 which were both significantly associated with ear length, and a QTL affiliated with ear length identified in B73/By804 RIL near the associated peak (Figure 5D–F). SNP chr2.s_1972207 (C/G) and SNP chr2.s_1972176 (C/G) were the only two of the 36 SNPs within the gene GRMZM2G061877, which encodes a DHHC zinc finger domain containing protein, and both of them are in the CDS region. SNP chr2.s_1972176 (C/G) makes no difference to the translated protein sequence, while SNP chr2.s_1972207 (C/G) results in a change of Isoleucine to Methionine. Several zinc finger proteins that play important roles in maize inflorescence development, for instance transcription factors RA1, RA2 and RA3 in ramosa pathways [32], have been identified. In rice, a zinc finger transcription factor DST directly regulates OsCKX2 expression in the reproductive meristem leading to OsCKX2 regulated CK accumulation in the shoot apical meristem (SAM) and, therefore, controls the number of the reproductive organs; the dst mutant leads to lower plant height and longer rice panicle length [33]. These zinc finger genes are functioning as transcription factors. Since the DHHC protein domain product of GRMZM2G068177, which was strongly suggested as a candidate gene for the regulation of ear length, acts as an enzyme, this may suggest a novel function of zinc finger proteins in monocot reproductive organ development. However, further work is needed to test this hypothesis. The third example, one QTL located on chromosome 1 using K22/Dan340 RIL population which explains 11.8% of phenotypic variation for kernel number per row, also overlapped with significant association signals (Figure 5G–I) Four candidate genes: GRMZM2G088524, GRMZM2G022822, GRMZM2G108180 and GRMZM2G052666, located in a 200 kb window around the significant signals, were predicted.
Fig. 5. Co-localization of association peaks, QTL and well-annotated candidate genes. 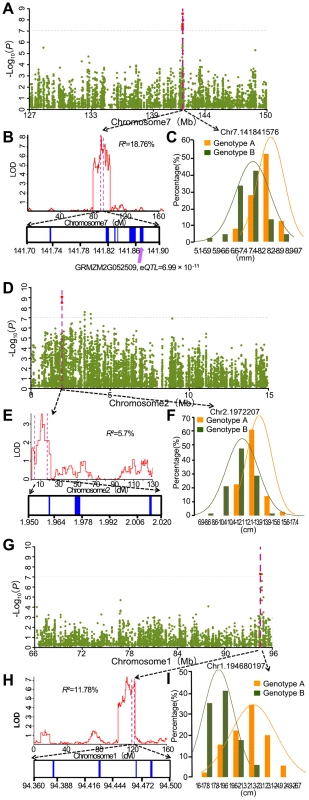
A. Significant association signals on chromosome 7 for kernel width; B. A major kernel width QTL (R2 = 18.7%) was mapped on chromosome 7 from 129 Mb to 149 Mb with BK/Yu8701 RILs and covered the significant association signals; C. The phenotype's frequency distribution histogram and normal distribution curve at the peak SNP of kernel width; D. Significant association signals on chromosome 2 for ear length; E. A major ear length QTL (R2 = 5.7%) was mapped on chromosome 2 in B73/By804 RILs and covered the significant association signals; F. The phenotype's frequency distribution histogram and normal distribution curve at the peak SNP of ear length; G. Significant association signals on chr1 for kernel number per row; H. A major kernel number per row QTL (R2 = 11.78%) was mapped on chr1 in K22/DAN340 RILs and covered the significant association signals; I. The frequency distribution histogram and normal distribution curve at the peak SNP of kernel number per row. Discussion
The genome-wide imputation of genotypes has attracted much attention given its broad applicability in the GWAS era. There are a number of methods for imputing missing genotypes, but many factors influence the accuracy of imputed genotypes [34], [35]. In this study, we proposed a two-step method combining IBD based projection and KNN algorithm to infer missing genotypes, resulting in 96.6% accuracy and 85.5% genome coverage in the tested samples. Considering that the missing genotypes consist of over 91.6% of our raw data set, this level of accuracy is acceptable. Compared with other methods [4], [17], [34], [35], the two-step method has its advantages. Imputation based only on IBD regions ensures high accuracy but a relatively low coverage rate. KNN algorithm has been proven to be a good strategy for sequencing data [4], however, it alone does not represent the true similarity of the inbred lines due to low density of frame markers and rapid LD decay in maize in our study. Therefore, we first used IBD based imputation to increase marker density, and then the KNN algorithm was used to infer the missing genotype, leading to high coverage rate and imputation accuracy. Imputation error is often caused by ignoring recombination and mutation within IBD regions. In addition, if an inbred line with low density markers share a region with two or more inbred lines with high density markers and the missing genotypes are inferred on the basis of only one of these lines, there is a high risk of error since the accuracy of the projection depends on the identity between the projected and chosen lines. Reanalysis of GWAS for kernel oil concentration revealed consistent results and a higher detection power. Six more associated loci were identified, most likely due to the increase in sample size. The implication is that mapping resolutions are enhanced by extracting moderately more information from the genome and expanding sample size.
The detection of loci controlling complex traits using GWAS has flourished and numerous statistical approaches for GWAS analysis in plants have recently been described [14], [36]–[40]. Linear statistical models like ANOVA, general linear model (GLM), and MLM establish significance cutoff by relying on the assumption that target traits have normal distribution. However, sometimes phenotype distribution in the moderate plant population is not normal in the tails that may be due to the population size, field experiment such as phenotyping errors, or genetic effects [31]. Based on our simulated data, nonparametric methods including A-D and KW tests usually have greater power than LM and MLM for abnormal phenotypes, rare alleles and moderate QTNs. It also implies that A-D and KW tests should perform well to detect the shifts of distribution as well as changes in the shape of distributions [31]. A-D test possesses advantages than K-W test in the detection of QTNs with rare alleles. However, MLM performs better than A-D and KW test for the major QTNs especially those with common alleles. However, we need to keep in mind that population structure of the studied samples is the key confounder for GWAS. In the measured 17 agronomic traits of present study, we observed the phenotypic variation explained by population structure ranged between 0.9% and 32.3% (Table S2). In the A-D test, we didn't account the confounding by population structure in the subpopulation that may lead to false-positive findings. Genomic control is a good alternative for controlling the statistic inflations [27]–[30], different inflation factors were observed in different traits and different subpopulations in present study (Table S7). We detected 19 loci significantly associated with 13 traits at the Bonferroni-corrected threshold (−log P>5.74, α = 1) (Table S7) using genomic-control () to adjust our real phenotype test statistic from A-D test. However, we also need to be careful that the adjusted −Log P might be over corrected, since A-D test has already controlled part of the population structure and genomic control method is affected significantly by the true association signals , even for the agronomic traits may involve a larger number of loci with small effects [21], [36], [37], [41]. And the influence of epistatic genetic effect to the genomic control is still not explored [27]–[30]. Another thing need to be noticed is testing within subpopulations (A-D test) and across the whole panel with controlling the population structure (MLM) are different. Testing within subpopulations changes allele frequencies of background alleles and therefore possibly changes the epistatic interactions that are mapped in an additive manner within subpopulations but were not mapped across populations.
In general, A-D test could be a good complement to current popular GWAS methods. As each method owning its own advantages, the preliminary understanding of the traits studied is needed for choosing GWAS methods or trying different GWAS methods would be helpful especially for those studies only few or none significant signals were identified by using only one method. In this study, we performed GWAS using both MLM and A-D test for 17 agronomic traits. In total, 18 overlapped regions were detected by the two approaches (Table S7). The A-D test also showed high concordance with previous studies in identifying a higher number of QTL related to agronomic traits.
Our noticed nonparametric statistical approach is robust with respect to non-normality, similarly to the KS test [12]. The KS test tends to be more sensitive around the median value and less sensitive at the extreme ends of the distribution. Thus, the KS test is not always appropriate for calculating the significance of data sets which differs at the tails of the probability distribution, while the median remains unchanged [31]. The A-D test improves upon the KS test because it has more sensitivity towards the tails of the pooled sample. More importantly, the performance of the A-D test for small samples is quite good, as demonstrated by numerous Monte Carlo simulations [19]. This means that, for complex traits, the A-D test can make a good use of SNPs that have minor allele frequency and keep detection ability to the relatively small effect loci. At the same time, it is important to recognize that there are always limitations to what can be achieved using statistics. It seems that A-D test does not work well for all traits. Interestingly, we identified 14.6% associations by A-D test that differed in the width of the phenotype distribution but which had nearly identical trait means (Figure 3A). In these cases the differences between distributions are real, but the corresponding genetic markers would not be useful in breeding if the objective is to change the phenotypic means. Instead, the associations appear to represent allelic differences in the apparent trait stability. Therefore, to confirm candidate loci, it is necessary to check both frequency distribution and normality of the distribution curves (Figure 3). Several studies in humans have confirmed that using multiple methods for statistical inference critically enables the interpretation of results and engenders stronger candidates for experimental follow-up [42].
We identified some genes affecting important agronomic traits in maize that are very good candidates for future detailed analysis, for allele mining to identify functional variation, and for marker development. As whole genome sequences become available for many crop species including maize, as well as for multiple genotypes of the same species through resequencing, along with cost-effective high-throughput genotyping systems and the next generation of sequencing technologies, GWAS becomes practical and its use in plant breeding will allow the manipulation of many traits at the whole-genome level. Association mapping using a set of global diverse breeding germplasm and high-throughput SNP markers, as shown in this study, provides high-resolution dissection of the genetic architecture of complex traits. This knowledge in turn will be useful not only for designing marker-assisted selection strategies but also for optimizing conventional breeding systems.
Materials and Methods
Plant material and phenotyping
A total of 513 maize lines with tropical, subtropical and temperate backgrounds representing the global maize diversity were employed for genome-wide association mapping in this study. All maize inbred lines have been well described in previous studies [13], [15] and the 513 maize lines were classified into four subgroups based on population structure Q matrix: Stiff stalk (SS) with 112 lines, Non-stiff stalk (NSS) with 116 lines, Tropical-subtropical (TST) with 258 lines, and an admixed group with 27 lines (detailed information also can be downloaded at (www. maizego.org/resource). A Randomized complete block design with one to two replications was used for field trials in five environments, including Ya'an (30°N, 103°E), Sanya (18°N, 109°E), Yunnan (25°N, 102°E) in 2009, Guangxi (23°N, 110°E) and Yunnan (25°N, 102°E) in 2010. A row length of 3 m was used for each line including 11 plants plot−1 with 25 cm plant to plant and 60 cm row to row distance. Five randomly selected plants were used for phenotypic data acquisition in each line and the mean data in each replication was used for phenotypic analysis. A total of 17 economically important traits were phenotyped (Table S1). These traits were divided into three categories: morphological attributes (plant height, ear height, ear leaf width and length, tassel main axis length, tassel branch number, and leaf number above ear), yield related traits (ear length and diameter, cob diameter, kernel number per row, 100-grain weight, cob weight and kernel width), and maturity traits (days to heading, anthesis, and silking). Best linear unbiased predictions (BLUP) for each line across five environments were calculated using the MIXED procedure in SAS (Release 9.1.3; SAS Institute, Cary, NC), and employed for evaluating trait variation in the association panel.
Imputation yield and accuracy
Imputation methods have not been developed to deal specifically with low density of SNP marker data. Of the available imputation models, identity by descent (IBD) based projection [17] and the k-nearest neighbor algorithm (KNN) [4] seemed to effectively infer a large number of missing genotypes. To assess the performance of IBD based projection, preliminary tests for chromosome 1 in 368 maize lines were conducted. We removed genotype data without frame SNPs and then compared the observed genotypes with those generated by projection. The number of IBD regions with consecutive SNPs for 368 lines varied from 1 to 285 on chromosome 1, and projection accuracy, defined as the percentage of correctly projected genotypes ranged from 74.8% to 99.1%, with an average of 92.6% (Table S3). The mean error ratio pooled over in 32,015 IBD regions on chromosome 1 for the 368 maize lines was also calculated (Figure S19A, B), and gradually declined with the increasing number of identical SNP and size in IBD regions. Preliminary testing suggested that IBD regions with 150 consecutive SNPs and a size of 5 Mb or more were highly conserved in maize and error rate for projection was well controlled within 5% (Figure S19A, B). The number of qualified IBD segments ranged from 0 to 20 for 368 lines and coverage rate, defined as the percentage of projected genotypes, accounted for 61.99% of genomic regions on chromosome 1, with projection accuracy increased from 92.59% to 96.62% on average (Table S3). Therefore, IBD based projection for regions with 150 consecutive SNPs and a size of 5 Mb were applied for integration of SNPs from RNA-seq data set onto the 145 additional maize inbred lines. Alternatively, the K-Nearest Neighbor (KNN) algorithm was also used to enrich the physical map of each line constructed by 56,110 SNPs from MaizeSNP50 chip by inferring the missing genotypes of the unique loci from RNA-seq SNP data. In the preliminary test, this method was efficient and the imputation accuracy and coverage rate for 368 lines were 97.48% and 75.35%, respectively (Table S3). The IBD based projection and KNN imputation revealed high inferred accuracy; however, the coverage rates were relatively low, with an average of 62% and 75%, respectively. In order to increase the coverage rate and keep high imputation accuracy, IBD based projection and KNN algorithm were combined to infer missing genotypes. The IBD method can provide more frame SNPs for the KNN algorithm, and simultaneously the KNN algorithm compensates for the weakness of the IBD method in coverage rate. About 38% of the genotypes were missing after prediction of IBD regions with 150 consecutive SNPs and 5 Mb size, and then the KNN algorithm was used to impute the missing data, resulting in 95.8% of accuracy for the missing data. The joint IBD based projection and KNN imputation of the genotypes of 368 lines increased coverage rate from 62% to 87.2%, with a total accuracy of 95.9% in the preliminary test. The projection accuracy was also affected by heterozygosity of each line, which increased from 95.88% to 96.60% after excluding 44 lines with more than 10% heterozygosity. The joint IBD based projection and KNN imputation that performed well in the preliminary test was used for the integration of SNPs from the high density SNPs data set onto 145 maize lines genotyped by 56110 SNPs. For 145 maize lines, 54.18% and 32.28% of loci across 10 chromosomes were inferred through IBD based projection and subsequent KNN imputation, respectively. As a result, 85.46% of loci for the whole maize genome were filled. The average density for the whole panel increased from 20 SNPs to more than 200 SNPs per Mb.
QTL mapping
The linkage analyses of ear length, kernel number per row, and kernel width were performed in three recombination inbred line (RIL) populations, BY804/B73 (197 individuals), K22/Dan340 (197 individuals), and BK/Yu8701 (165 individuals). All the RIL lines and their parents were genotyped using Maize SNP50 assays (Illumina) containing 56,110 SNPs [14]. The phenotype of BK/Yu8701 in Henan 2011 and BLUP value from 5 environments of BY804/B73 and K22/Dan340 were used. QTL mapping using the composite interval mapping method [43] was performed in the package QTL cartographer version 2.5 [44].
Statistical analysis and association mapping
ANOVA, correlation, and repeatability analyses for 17 agronomic traits were conducted using SAS software (Release 9.1.3; SAS Institute, Cary, NC). Heritability analysis and association analysis for the 17 agronomic traits in Data set 2 were conducted by MLM using TASSEL [45] software package. The observed p values from marker-trait associations were used to display Q-Q plots and Manhattan plots, using R. Permutation tests were used to determine the cutoff for GWAS. Considering the computation time, we only choose three typical traits with different population structure effects (kernel width, ear height and day of flowering time) as examples. The results showed that the cutoff values are similar with the Bonferroni correction. To simplify the procedures, we use the uniform Bonferroni-corrected thresholds at α = 1 and α = 0.05 as the cutoffs. When performing n tests, if the significance level for the entire series of tests is α, then each of the tests should have a probability of P = α/n. When the numbers of markers was 556809 SNPs, at α = 1 and α = 0.05, the Bonferroni-corrected thresholds for the p values were 1.796×10−6 and 8.95×10−8, with corresponding −log p values of 5.74 and 7.05, respectively. Regression estimator () of Genomic Control inflation factor was used [28]. Percentage of PVE by associated SNPs was calculated by ANOVA. Informative SNPs and candidate genes at the identified loci for the corresponding traits were from public maize genome data set B73 RefGen_v2.
Simulation study
To compare the power and FDR of A-D test, Kruskal-Wallis (K-W test) test, linear model (LM) and mixed linear model (MLM), three schemes with different phenotype distribution were simulated by considering the QTN effects and allele frequency.
Scheme 1 was used to simulate a normal distribution phenotype with the contribution of population structure, additive genetic effect, epistatic genetic effect and residual effect [6]. The population structure and epistasis explained 10% of the total phenotypic variation, respectively. The additive effect was the sum of all additive effects for 20 causal QTNs. For approaching the real genetic architecture, we set 20% major QTNs explaining 30% of the sum of all assigned genetic effect and 80% moderate QTNs explaining 70% of the sum of all assigned genetic effect. Half of major and moderate QTNs were rare alleles (MAF = 0.05–0.1) and half were common alleles (MAF = 0.25–0.45). Larger genetic effects were assigned to the rare alleles QTNs to ensure them could explain the same proportion of phenotypic variation as common alleles QTNs. The ratio of assigned genetic effects between rare alleles QTNs (at MAF = 0.075) and common alleles QTNs (at MAF = 0.35) was calculated based on . The genetic effect was assigned to all SNPs, one at a time [6]. The proportion of the additive effect was defined by narrow-sense heritability which is the proportion of additive variance over the total variance, and was examined. The residual effect followed a normal distribution and had a variance to satisfy the contributions from additive and epistatic effects at the designated level [6].
Scheme 2 was used to simulate an abnormal distribution phenotype with a long tail on one side. On the basis of scheme 1, 10% of lines were randomly selected and added an extra residual effect (1 to 6 fold standard deviation of the phenotype). All the others were same.
Scheme 3 was designed to simulate an abnormal distribution phenotype caused by a larger effect background rare QTN. The additive effect was still the sum of all additive effects for 20 causal QTNs. 1 background QTN, 3 major QTNs and 16 moderate QTNs explaining 25%, 20%, 60% of the sum of all assigned genetic effect respectively. The population structure effect, epistatic effect and residual effect were consistent with scheme 1.
Simulations of the phenotypes were repeated 500 times in all schemes. All simulated phenotypes had been analyzed with the four methods presented in the main text. 1,000 permutations had been done separately for the four methods to obtain the threshold at different type I error risk.
Anderson-Darling test
The Anderson-Darling two-sample procedure assumes that the two samples have a continuous distribution function and we are interested in testing the null hypothesis that the two phenotype samples divided by two alleles of one SNP have the same distribution, without specifying the nature of population:
The test procedure is as follows:
1. Calculate :
The computational formula for not adjusted for ties is,
and the corresponding adjusted for ties is, where:, indicates the two phenotype distribution function
k = 2; i = 1, 2
= data number in the ith sample; j = 1,2,…,
N = total number of two samples' individuals;
= data in the i sample and j observation within that sample
L = the number of unique data, where it will be less than n with tied data
z(j) = distinct values of all combined data ordered in ascendant way denoted z(1),z(2),…,z(L)
= number of values in the pooled sample equal to z(j)
= number of values in the combined samples less than z(j) plus one half of the number of values in the combined samples equal to z(j)
= number of values in the ith sample which are small than z(j) plus one half the number of values in this sample which are equal to z(j)
2. Calculate :
Under , the variance of is,
with: where:3. Calculate :
4. Refer to the upper α percentiles of the distribution table below, reject at significance level if exceeds the given point .If is outside the range of the table. Plotting the log-odds of versus , a strong linear pattern indicates that simple linear extrapolation should give good approximate p values.
where:URL. One R package (ADGWAS) for GWAS by Anderson-Darling test can be downloaded here: http://www.maizego.org/Resources.html
Supporting Information
Zdroje
1. YanJ, WarburtonM, CrouchJ (2011) Association mapping for enhancing maize (Zea mays L.) genetic improvement. Crop Sci 51 : 433–449.
2. ZhuC, GoreMA, BucklerES, YuJ (2008) Status and prospects of association Mapping in plants. Plant Genome 1 : 5–20.
3. AtwellS, HuangYS, VilhjálmssonBJ, WillemsG, HortonM, et al. (2010) Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature 465 : 627–631.
4. HuangX, WeiX, SangT, ZhaoQ, FengQ, et al. (2010) Genome-wide association studies of 14 agronomic traits in rice landraces. Nat Genet 42 : 961–967.
5. LiH, PengZ, YangX, WangW, FuJ, et al. (2013) Genome-wide association study dissects the genetic architecture of oil biosynthesis in maize kernels. Nat Genet 45 : 43–50.
6. ZhangZ, ErsozE, LaiCQ, TodhunterRJ, TiwariHK, et al. (2010) Mixed linear model approach adapted for genome-wide association studies. Nat Genet 42 : 355–360.
7. YuJ, PressoirG, BriggsWH, Vroh BiI, YamasakiM, et al. (2006) A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat Genet 38 : 203–208.
8. ZhaoK, TungCW, EizengaGC, WrightMH, AliML, et al. (2011) Genome-wide association mapping reveals a rich genetic architecture of complex traits in Oryza sativa. Nat Commun 2 : 467.
9. ListgartenJ, LippertC, KadieCM, DavidsonRI, EskinE, et al. (2012) Improved linear mixed models for genome-wide association studies. Nat Methods 9 : 525–526.
10. ChengR, ParkerCC, AbneyM, PalmerAA (2013) Practical considerations regarding the use of genotype and pedigree data to model relatedness in the context of genome-wide association studies. G3 (Bethesda) 3 : 1861–1867.
11. VilhjálmssonBJ, NordborgM (2013) The nature of confounding in genome-wide association studies. Nat Rev Genet 14 : 1–2.
12. BelóA, ZhengP, LuckS, ShenB, MeyerDJ, et al. (2008) Whole genome scan detects an allelic variant of fad2 associated with increased oleic acid levels in maize. Mol Genet Genomics 279 : 1–10.
13. YangX, GaoS, XuS, ZhangZ, PrasannaB, et al. (2011) Characterization of a global germplasm collection and its potential utilization for analysis of complex quantitative traits in maize. Mol Breed 28 : 511–526.
14. GanalMW, DurstewitzG, PolleyA, BérardA, BucklerES, et al. (2011) A large maize (Zea mays L.) SNP genotyping array: development and germplasm genotyping, and genetic mapping to compare with the B73 reference genome. PloS One 6: e28334.
15. LiQ, YangX, XuS, CaiY, ZhangD, et al. (2012) Genome-wide association studies identified three independent polymorphisms associated with α-tocopherol content in maize kernels. PloS One 7: e36807.
16. FuJ, ChengY, LinghuJ, YangX, KangL, et al. (2013) RNA sequencing reveals the complex regulatory network in maize kernel. Nat Commun 4 : 2832.
17. MoltkeI, AlbrechtsenA, HansenT, NielsenFC, NielsenR (2011) A method for detecting IBD regions simultaneously in multiple individuals—with applications to disease genetics. Genome Res 21 : 1168–1180.
18. Li-BeissonYH, ShorroshB, BeissonF, AnderssonMX, ArondelV, et al. (2010) Acyl-lipid metabolism. The Arabidopsis Book 8: e0133 doi/10.1199/tab.0133.
19. ScholzF, StephensM (1987) K-sample Anderson–Darling tests. J Am Stat Assoc 82 : 918–924.
20. Press WH, Flannery BP, Teudolsky SA, Vetterling WT (1992) Numerical recipes in C: the art of scientific computing (second edition). Cambridge University Press. Pp. 626–627.
21. BucklerES, HollandJB, BradburyPJ, AcharyaCB, BrownPJ, et al. (2009) The genetic architecture of maize flowering time. Science 325 : 714–718.
22. HungHY, ShannonLM, TianF, BradburyPJ, ChenC, et al. (2012) ZmCCT and the genetic basis of day-length adaptation underlying the postdomestication spread of maize. Proc Natl Acad Sci U S A 109 : 1913–1921.
23. YangQ, LiZ, LiW, KuL, WangC, et al. (2013) A CACTA-like transposable element in ZmCCT attenuated photoperiod sensitivity and accelerated the post-domestication spread of maize. Proc Natl Acad Sci U S A 110 : 16969–16974.
24. LiuH, YuX, LiK, KlejnotJ, YangH, et al. (2008) Photoexcited CRY2 Interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis. Science 322 : 1535–1539.
25. CockramJ, ThielT, SteuernagelB, SteinN, TaudienS, et al. (2012) Genome dynamics explain the evolution of flowering time CCT domain gene families in the Poaceae. PloS One 7: e45307.
26. ColasantiJ, TremblayR, WongAY, ConevaV, KozakiA, et al. (2006) The maize INDETERMINATE1 flowering time regulator defines a highly conserved zinc finger protein family inhigher plants. BMC Genomics 7 : 158.
27. DevlinB, RoederK (1999) Genomic control for association studies. Biometrics 55 : 997–1004.
28. YanTing, HouBo, YangYaning (2009) Correcting for cryptic relatedness by a regression-based genomic control method. BMC Genet 10 : 78.
29. ZhengG, FreidlinB, LiZ, GastwirthJL (2005) Genomic control for association studies under various genetic models. Biometrics 61 : 186–192.
30. TsepilovYA, RiedJS, StrauchK, GrallertH, van DuijnCM, et al. (2013) Development and application of genomic control methods for genome-wide association studies using non-additive models. PloS One 8: e81431.
31. Beló A, Luck SD (2010) Association mapping for the exploration of genetic diversity and identification of useful loci for plant breeding. In: Khalid M, Günter K,editors. The handbook of plant mutation screening: mining of natural and induced alleles. New York: John Wiley & Sons. Pp. 231–246.
32. GallavottiA, LongJA, StanfieldS, YangX, JacksonD, et al. (2010) The control of axillary meristem fate in the maize ramosa pathway. Development 137 : 2849–2856.
33. LiS, ZhaoB, YuanD, DuanM, QianQ, et al. (2013) Rice zinc finger protein DST enhances grain production through controlling Gn1a/OsCKX2 expression. Proc Natl Acad Sci U S A 110 : 3167–3172.
34. DaetwylerHD, WiggansGR, HayesBJ, WoolliamsJA, GoddardME (2011) Imputation of missing genotypes from sparse to high density using long-range phasing. Genetics 189 : 317–327.
35. HaoK, ChudinE, McElweeJ, SchadtE (2009) Accuracy of genome-wide imputation of untyped markers and impacts on statistical power for association studies. BMC Genet 10 : 27.
36. KumpKL, BradburyPJ, WisserRJ, BucklerES, et al. (2011) Genome-wide association study of quantitative resistance to southern leaf blight in the maize nested association mapping population. Nat Genet 43 : 163–168.
37. PolandJA, BradburyPJ, BucklerES, NelsonRJ (2011) Genome-wide nested association mapping of quantitative resistance to northern leaf blight in maize. Proc Natl Acad Sci U S A 108 : 6893–6898.
38. ZhaoK, AranzanaMJ, KimS, ListerC, ShindoC, et al. (2007) An Arabidopsis example of association mapping in structured samples. PloS Genet 3: e4.
39. StichB, MöhringJ, PiephoH-P, HeckenbergerM, BucklerES, MelchingerAE (2008) Comparison of mixed-model approaches for association mapping. Genetics 178 : 1745–1754.
40. StichB, MelchingerAE (2009) Comparison of mixed-model approaches for association mapping in rapeseed, potato, sugar beet, maize, and Arabidopsis. BMC Genomics 10 : 94.
41. TianF, BradburyPJ, BrownPJ, HungH, SunQ, et al. (2011) Genome-wide association study of leaf architecture in the maize nested association mapping population. Nat Genet 43 : 159–162.
42. StrangerBE, ForrestMS, ClarkAG, MinichielloMJ, DeutschS, et al. (2005) Genome-wide associations of gene expression variation in humans. PloS Genet 1: e78.
43. ZengZB (1994) Precision mapping of quantitative trait loci. Genetics 136 : 1457–1468.
44. Wang S, Basten CJ and Zeng ZB (2012). Windows QTL Cartographer 2.5. Department of Statistics, North Carolina State University, Raleigh, NC.
45. BradburyPJ, ZhangZ, KroonDE, CasstevensTM, RamdossY, et al. (2007) TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23 : 2633–2635.
Štítky
Genetika Reprodukční medicína
Článek An Evolutionarily Conserved Role for the Aryl Hydrocarbon Receptor in the Regulation of MovementČlánek Requirement for Drosophila SNMP1 for Rapid Activation and Termination of Pheromone-Induced ActivityČlánek Co-regulated Transcripts Associated to Cooperating eSNPs Define Bi-fan Motifs in Human Gene NetworksČlánek Identification of a Regulatory Variant That Binds FOXA1 and FOXA2 at the Type 2 Diabetes GWAS LocusČlánek tRNA Modifying Enzymes, NSUN2 and METTL1, Determine Sensitivity to 5-Fluorouracil in HeLa CellsČlánek Derlin-1 Regulates Mutant VCP-Linked Pathogenesis and Endoplasmic Reticulum Stress-Induced ApoptosisČlánek A Genetic Assay for Transcription Errors Reveals Multilayer Control of RNA Polymerase II FidelityČlánek The Proprotein Convertase KPC-1/Furin Controls Branching and Self-avoidance of Sensory Dendrites inČlánek Regulation of p53 and Rb Links the Alternative NF-κB Pathway to EZH2 Expression and Cell SenescenceČlánek BMPs Regulate Gene Expression in the Dorsal Neuroectoderm of and Vertebrates by Distinct MechanismsČlánek Unkempt Is Negatively Regulated by mTOR and Uncouples Neuronal Differentiation from Growth Control
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 9
-
Všechny články tohoto čísla
- Translational Regulation of the Post-Translational Circadian Mechanism
- An Evolutionarily Conserved Role for the Aryl Hydrocarbon Receptor in the Regulation of Movement
- Eliminating Both Canonical and Short-Patch Mismatch Repair in Suggests a New Meiotic Recombination Model
- Requirement for Drosophila SNMP1 for Rapid Activation and Termination of Pheromone-Induced Activity
- Co-regulated Transcripts Associated to Cooperating eSNPs Define Bi-fan Motifs in Human Gene Networks
- Targeted H3R26 Deimination Specifically Facilitates Estrogen Receptor Binding by Modifying Nucleosome Structure
- Role for Circadian Clock Genes in Seasonal Timing: Testing the Bünning Hypothesis
- The Tandem Repeats Enabling Reversible Switching between the Two Phases of β-Lactamase Substrate Spectrum
- The Association of the Vanin-1 N131S Variant with Blood Pressure Is Mediated by Endoplasmic Reticulum-Associated Degradation and Loss of Function
- Identification of a Regulatory Variant That Binds FOXA1 and FOXA2 at the Type 2 Diabetes GWAS Locus
- Regulation of Flowering by the Histone Mark Readers MRG1/2 via Interaction with CONSTANS to Modulate Expression
- The Actomyosin Machinery Is Required for Retinal Lumen Formation
- Plays a Conserved Role in Assembly of the Ciliary Motile Apparatus
- Hidden Diversity in Honey Bee Gut Symbionts Detected by Single-Cell Genomics
- Ribosome Rescue and Translation Termination at Non-Standard Stop Codons by ICT1 in Mammalian Mitochondria
- tRNA Modifying Enzymes, NSUN2 and METTL1, Determine Sensitivity to 5-Fluorouracil in HeLa Cells
- Causal Variation in Yeast Sporulation Tends to Reside in a Pathway Bottleneck
- Tissue-Specific RNA Expression Marks Distant-Acting Developmental Enhancers
- WC-1 Recruits SWI/SNF to Remodel and Initiate a Circadian Cycle
- Clonal Expansion of Early to Mid-Life Mitochondrial DNA Point Mutations Drives Mitochondrial Dysfunction during Human Ageing
- Methylation QTLs Are Associated with Coordinated Changes in Transcription Factor Binding, Histone Modifications, and Gene Expression Levels
- Differential Management of the Replication Terminus Regions of the Two Chromosomes during Cell Division
- Obesity-Linked Homologues and Establish Meal Frequency in
- Derlin-1 Regulates Mutant VCP-Linked Pathogenesis and Endoplasmic Reticulum Stress-Induced Apoptosis
- Stress-Induced Nuclear RNA Degradation Pathways Regulate Yeast Bromodomain Factor 2 to Promote Cell Survival
- The MAPK p38c Regulates Oxidative Stress and Lipid Homeostasis in the Intestine
- Widespread Genome Reorganization of an Obligate Virus Mutualist
- Trans-kingdom Cross-Talk: Small RNAs on the Move
- The Vip1 Inositol Polyphosphate Kinase Family Regulates Polarized Growth and Modulates the Microtubule Cytoskeleton in Fungi
- Myosin Vb Mediated Plasma Membrane Homeostasis Regulates Peridermal Cell Size and Maintains Tissue Homeostasis in the Zebrafish Epidermis
- GLD-4-Mediated Translational Activation Regulates the Size of the Proliferative Germ Cell Pool in the Adult Germ Line
- Genome Wide Association Studies Using a New Nonparametric Model Reveal the Genetic Architecture of 17 Agronomic Traits in an Enlarged Maize Association Panel
- Translational Regulation of the DOUBLETIME/CKIδ/ε Kinase by LARK Contributes to Circadian Period Modulation
- Positive Selection and Multiple Losses of the LINE-1-Derived Gene in Mammals Suggest a Dual Role in Genome Defense and Pluripotency
- Out of Balance: R-loops in Human Disease
- A Genetic Assay for Transcription Errors Reveals Multilayer Control of RNA Polymerase II Fidelity
- Altered Behavioral Performance and Live Imaging of Circuit-Specific Neural Deficiencies in a Zebrafish Model for Psychomotor Retardation
- Nipbl and Mediator Cooperatively Regulate Gene Expression to Control Limb Development
- Meta-analysis of Mutations in Autism Spectrum Disorders: A Gradient of Severity in Cognitive Impairments
- The Proprotein Convertase KPC-1/Furin Controls Branching and Self-avoidance of Sensory Dendrites in
- Hydroxymethylated Cytosines Are Associated with Elevated C to G Transversion Rates
- Memory and Fitness Optimization of Bacteria under Fluctuating Environments
- Regulation of p53 and Rb Links the Alternative NF-κB Pathway to EZH2 Expression and Cell Senescence
- Interspecific Tests of Allelism Reveal the Evolutionary Timing and Pattern of Accumulation of Reproductive Isolation Mutations
- PRO40 Is a Scaffold Protein of the Cell Wall Integrity Pathway, Linking the MAP Kinase Module to the Upstream Activator Protein Kinase C
- Low Levels of p53 Protein and Chromatin Silencing of p53 Target Genes Repress Apoptosis in Endocycling Cells
- SPDEF Inhibits Prostate Carcinogenesis by Disrupting a Positive Feedback Loop in Regulation of the Foxm1 Oncogene
- RRP6L1 and RRP6L2 Function in Silencing Regulation of Antisense RNA Synthesis
- BMPs Regulate Gene Expression in the Dorsal Neuroectoderm of and Vertebrates by Distinct Mechanisms
- Unkempt Is Negatively Regulated by mTOR and Uncouples Neuronal Differentiation from Growth Control
- Atkinesin-13A Modulates Cell-Wall Synthesis and Cell Expansion in via the THESEUS1 Pathway
- Dopamine Signaling Leads to Loss of Polycomb Repression and Aberrant Gene Activation in Experimental Parkinsonism
- Histone Methyltransferase MMSET/NSD2 Alters EZH2 Binding and Reprograms the Myeloma Epigenome through Global and Focal Changes in H3K36 and H3K27 Methylation
- Bipartite Recognition of DNA by TCF/Pangolin Is Remarkably Flexible and Contributes to Transcriptional Responsiveness and Tissue Specificity of Wingless Signaling
- The Olfactory Transcriptomes of Mice
- Muscular Dystrophy-Associated and Variants Disrupt Nuclear-Cytoskeletal Connections and Myonuclear Organization
- Interplay of dFOXO and Two ETS-Family Transcription Factors Determines Lifespan in
- Evidence for Widespread Positive and Negative Selection in Coding and Conserved Noncoding Regions of
- Genome-Wide Association Meta-analysis of Neuropathologic Features of Alzheimer's Disease and Related Dementias
- Rejuvenation of Meiotic Cohesion in Oocytes during Prophase I Is Required for Chiasma Maintenance and Accurate Chromosome Segregation
- Admixture in Latin America: Geographic Structure, Phenotypic Diversity and Self-Perception of Ancestry Based on 7,342 Individuals
- Local Effect of Enhancer of Zeste-Like Reveals Cooperation of Epigenetic and -Acting Determinants for Zygotic Genome Rearrangements
- Differential Responses to Wnt and PCP Disruption Predict Expression and Developmental Function of Conserved and Novel Genes in a Cnidarian
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Admixture in Latin America: Geographic Structure, Phenotypic Diversity and Self-Perception of Ancestry Based on 7,342 Individuals
- Nipbl and Mediator Cooperatively Regulate Gene Expression to Control Limb Development
- Genome Wide Association Studies Using a New Nonparametric Model Reveal the Genetic Architecture of 17 Agronomic Traits in an Enlarged Maize Association Panel
- Histone Methyltransferase MMSET/NSD2 Alters EZH2 Binding and Reprograms the Myeloma Epigenome through Global and Focal Changes in H3K36 and H3K27 Methylation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání














