-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAltered Behavioral Performance and Live Imaging of Circuit-Specific Neural Deficiencies in a Zebrafish Model for Psychomotor Retardation
In a wide range of brain disorders, mutations in specific genes cause alterations in the development and function of neural circuits that ultimately affect behavior. A major challenge is to uncover the mechanism and provide treatment which is capable of preventing brain damage. Allan-Herndon-Dudley syndrome (AHDS) is a severe psychomotor retardation characterized by intellectual disabilities, neurological impairment and abnormal thyroid hormone (TH) levels. Mutations in the TH transporter MCT8 are associated with AHDS. Mice that lack the MCT8 protein exhibited impaired TH levels, as is the case in human patients; however, they lack neurological defects. Here, we generated an mct8 mutant (mct8−/−) zebrafish, which exhibited neurological and behavioral deficiencies and mimics pathological conditions of AHDS patients. The zebrafish is a simple transparent vertebrate and its nervous system is conserved with mammals. Time-lapse live imaging of single axons and synapses, and video-tracking of behavior revealed deficiencies in neural circuit assembly, which are associated with disturbed sleep and altered locomotor activity. In addition, since the mct8−/ − larvae provides a highthroughput platform for testing therapeutic drugs, we showed that TH analogs can recover neurological deficiencies in an animal model for psychomotor retardation.
Published in the journal: . PLoS Genet 10(9): e32767. doi:10.1371/journal.pgen.1004615
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004615Summary
In a wide range of brain disorders, mutations in specific genes cause alterations in the development and function of neural circuits that ultimately affect behavior. A major challenge is to uncover the mechanism and provide treatment which is capable of preventing brain damage. Allan-Herndon-Dudley syndrome (AHDS) is a severe psychomotor retardation characterized by intellectual disabilities, neurological impairment and abnormal thyroid hormone (TH) levels. Mutations in the TH transporter MCT8 are associated with AHDS. Mice that lack the MCT8 protein exhibited impaired TH levels, as is the case in human patients; however, they lack neurological defects. Here, we generated an mct8 mutant (mct8−/−) zebrafish, which exhibited neurological and behavioral deficiencies and mimics pathological conditions of AHDS patients. The zebrafish is a simple transparent vertebrate and its nervous system is conserved with mammals. Time-lapse live imaging of single axons and synapses, and video-tracking of behavior revealed deficiencies in neural circuit assembly, which are associated with disturbed sleep and altered locomotor activity. In addition, since the mct8−/ − larvae provides a highthroughput platform for testing therapeutic drugs, we showed that TH analogs can recover neurological deficiencies in an animal model for psychomotor retardation.
Introduction
Circuit formation is a fundamental process in the development and operation of the nervous system. Deficiencies in neurogenesis and synaptic connectivity are thought to lie at the root of genetic mental-retardation syndromes [1], [2]. However, their mechanisms and treatment remain mostly indefinite due to the high complexity of brain networks. The Allan-Herndon-Dudley syndrome (AHDS) is a classic example of such a genetic neurological disorder. In AHDS, mutations in the monocarboxylate transporter 8 (mct8/slc16a2) gene, located on the X chromosome, produce severe psychomotor retardation in young males. AHDS is characterized by a combination of neurological impairments that include hypotonia, spastic paraplegia, lack of speech, and severe cognitive deficiency [3], [4]. In addition, since MCT8 is a thyroid-hormone (TH) transporter, AHDS patients exhibit endocrine alterations in their TH parameters, with decreased plasma concentration of the prohormone 3,5,3′,5′-triiodo-L-thyronine/thyroxine (T4) and increased concentration of the active form 3,5,3′-triiodo-L-thyronine (T3) [3], [4]. In all vertebrates, TH is an essential regulator of development, neurogenesis, growth, and metabolism [5]. In order to function, TH requires efficient transport across the cell membrane because T3 regulates gene transcription by binding to nuclear TH receptors (TRs) [5]. Accordingly, the underlying mechanism of AHDS is thought to involve a defect in the MCT8-dependent neuronal entry of T3, leading to impaired neurological development. However, as is often the case in other retardation syndromes, the location of the altered neuronal circuits and the nature of these deficiencies remain elusive, and adequate treatment is not available.
In humans and rodents, MCT8 is expressed in many tissues including the thyroid gland, the nervous and vascular systems [6]–[8]. In order to elucidate the pathophysiological mechanisms of AHDS, an MCT8 knockout (KO) mouse model was generated. These KO mice replicate the endocrine and metabolic abnormalities found in human patients [9]–[12]. However, they did not display any neurological or behavioral phenotypes. This can be explained by the pronounced expression of the anion transporting polypeptide 1C1 (OATP1C1), a specific T4 transporter, at the blood-brain barrier (BBB) in mice but not in humans, that can compensate for the loss of MCT8 [7], [13], [14]. Thus, development of an alternative animal model that lack MCT8 and demonstrates AHDS-like neurological phenotypes, is essential.
The zebrafish is a powerful model that combines invertebrate-like genetics with vertebrate brain structures, and its transparency allows the visualization of neural circuit dynamics in live animals [15]–[17]. In addition, the hypothalamic-pituitary-thyroid (HPT) axis is conserved in zebrafish [18], and zebrafish larvae have emerged as an attractive model for therapeutic drug screening [19]. In light of these advantages, we have recently isolated the zebrafish mct8 gene and promoter and showed that, as in humans, zebrafish mct8 is expressed primarily in the nervous and blood systems [20]. Importantly, zebrafish MCT8 mediates TH uptake in cell lines [21], and knock-down of MCT8 resulted in neurological abnormalities in zebrafish larvae [20].
In this study, in order to determine the function of MCT8 and the mechanisms of AHDS, we used the zinc-finger nuclease (ZFN)-genome editing system to establish an MCT8 mutant (mct8−/−) zebrafish model. Using gene quantification and localization assays, as well as time-lapse imaging of single neuronal circuits and synapses in live animals and video-tracking of behavior, we found altered expression of myelin-related genes, circuit-specific deficiencies in axon branching and synaptic density, and altered locomotor activity and sleep in mct8−/ − larvae. Strikingly, comparative pharmacological assays demonstrated that TH analogs can restore a portion of the neurological phenotypes. These findings suggest a neurological mechanism and treatment for AHDS and, potentially, other related psychomotor retardation disorders.
Results
Establishment of ZFN-mediated mct8 mutant zebrafish
In order to generate a zebrafish model for AHDS, we targeted a mutation into the genomic mct8 locus using custom-engineered ZFNs. A pair of ZFNs composed of 5 zinc-finger arrays, which match 15 bp at both sides of the HaeII cut site located on the first exon of the gene, were used (Fig. 1A). mRNA coding for each of the two ZFNs was injected into one-cell-stage wild-type (WT) embryos. To verify that the ZFNs system was efficient, on one day post-fertilization (dpf), genomic DNA was extracted from 20 of the injected embryos, and 234 bp genomic fragments that flanked the targeted sequence were amplified. In order to detect the mutation, PCR products were digested using HaeII restriction enzyme. An intact DNA fragment was shown in 18 out of the 20 embryos, indicating a mutated allele at the targeted HaeII restriction site (Fig. 1C), thus demonstrating the high efficiency of the method. The injected mosaic founder larvae (F0) were raised to adulthood and outcrossed with WT zebrafish. We screened eight F0 fish and found that four transferred the mutation to their F1 offspring. The F1 progeny of selected founder fish were raised to adulthood and 4 out of 6 F1 fish were identified as mutants using tail-clip and genotyping. Of the four F1 heterozygous-mutant (mct8+/−) fish, we selected one to establish our mutant zebrafish line. This mct8+/ − fish harbored a 7 bp deletion mutation that resulted in a frame shift and the incorporation of premature termination codon at amino acid 97, which led to a truncated protein (Fig. 1A, B). The selected F1 fish was outcrossed with WT fish and its progeny were raised to adulthood. The mct8+/ − F2 adults were intercrossed to produce homozygous mct8−/ − zebrafish. These mct8−/ − larvae were viable and fertile and the morphology of the larvae and adult appeared normal (Fig. 1D, E).
Fig. 1. Establishment of ZFN-mediated mct8 zebrafish mutant. 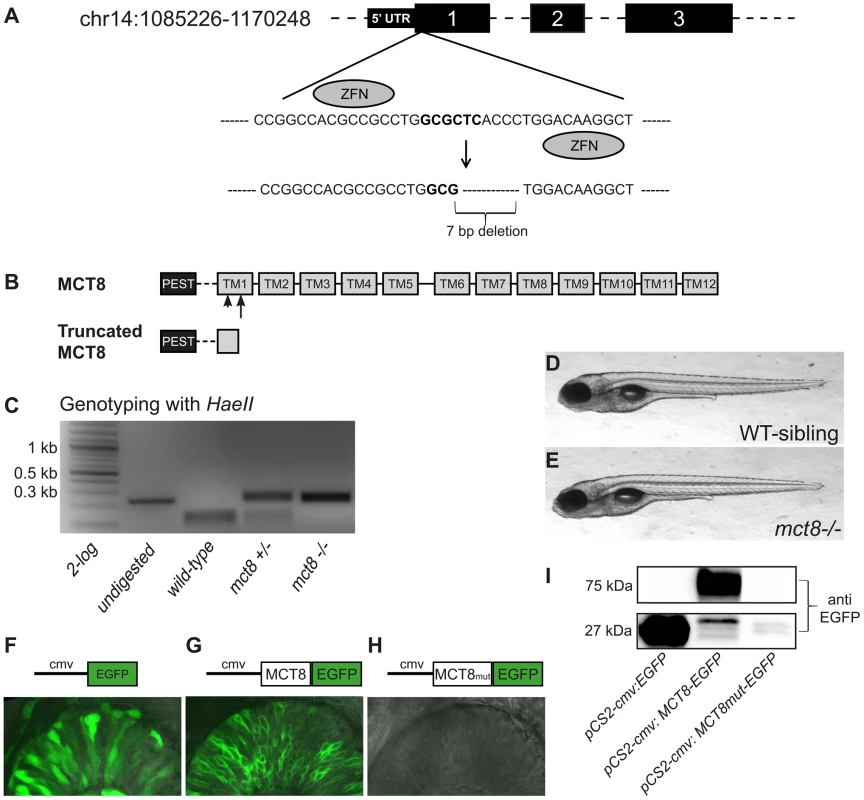
A. A pair of ZFNs was designed to target the HaeII restriction site (bold) within the first axon of the zebrafish mct8 gene located on chromosome 14. The targeted mutation resulted in a 7 bp deletion. B. The zebrafish MCT8 protein is schematically shown in the upper panel (TM – transmembrane domain). Arrowhead represent the location of the ZFN-mediated deletion and arrow represent the location of the premature stop codon. The truncated MCT8 protein is shown in the lower panel. C. Genotyping of WT, mct8+/−, and mct8−/− embryos. Genomic DNA was amplified, and the 234 bp PCR product (left lane) was digested with HaeII restriction enzyme. Complete digestion of WT DNA resulted in two short fragments of 104 bp and 130 bp. An intact 234 bp DNA fragment is shown in mct8−/− fish, confirming the introduction of a mutation at the target site. Heterozygous fish exhibit three DNA fragments, indicating both mutated and intact mct8 alleles. D, E. Lateral view of representative 6 dpf WT-sibling and mct8−/− larvae. F–H. Upper panel: schematic illustration of the pCS2-cmv:EGFP (F), pCS2-cmv:MCT8-EGFP (G) and pCS2-cmv:MCT8mut-EGFP (H) DNA constructs. Lower panel: lateral view of the eye in 30 hpf embryo-injected with EGFP (F), MCT8-EGFP (G) and MCT8mut-EGFP (H) mRNA. I. Western blotting using antibody against EGFP. The 27 kDa band represent EGFP and the 72 kDa band represent the fusion protein MCT8-EGFP. Notably, MCT8mut-EGFP protein was not detected. In order to confirm that the mutation eliminated MCT8 expression, transient expression studies were performed in larvae. The mct8 coding sequence was amplified from mct8−/− and their WT-siblings. Both mutated and WT coding sequences were fused upstream to EGFP and mRNA of EGFP and the two fusion proteins (MCT8-EGFP and MCT8mut-EGFP) were injected into one-cell-stage embryos. At 30 hours post fertilization (hpf), somatic and membrane-specific EGFP signal was observed in embryos injected with EGFP and MCT8-EGFP, respectively. The presence of a membrane pattern confirmed that the zebrafish MCT8 transporter is located in the cell membrane. As predicted, no EGFP expression was found in the MCT8mut-EGFP mRNA-injected embryos (Fig. 1F–H). These results were further confirmed using transfection of the three constructs into HEK293T cells followed by western blot. As expected, only in the pCS2-cmv:MCT8-EGFP transfected cells, detection with an antibody against EGFP revealed a 75 kDa band that corresponded to the size of the MCT8-EGFP fusion protein (Fig. 1I). This 75 kDa band was not detected in pCS2-cmv:mutMCT8-EGFP transfected cells. These in vivo and in vitro results show that the 7 bp deletion in the first exon of mct8 efficiently eliminates the MCT8 protein.
The expression of the TH-induced kruppel-like factor 9 and neurogranin genes is not altered in the mct8−/ − larvae
The main mechanism of action of TH is achieved through transcriptional regulation of an array of genes that control neurogenesis, cell growth, and metabolism [5]. Previous studies on mice demonstrated that hyperthyroidism and hypothyroidism in specific regions of the brain alter the expression of specific TH-induced genes, such as Kruppel-like factor 9 (klf9) and neurogranin (RC3/nrgn) [10], [22]. In order to study the spatial expression pattern and transcript levels of klf9 and nrgna in larvae, whole-mount in situ hybridization (ISH) and quantitative real-time PCR (qRT-PCR) assays were performed. Whole-mount ISH experiments showed that while klf9 is widely expressed in the CNS (Fig. 2A); nrgna is specifically expressed in discrete clusters of cells in the dorsal forebrain and hindbrain (Fig. 2B). These patterns of expression are in agreement with previous reports on mammals [10], [23]. To verify that T3 induces klf9 and nrgna expression in zebrafish, T3 (0.5 nM) was administered to embryos beginning at the one-cell stage and until 3 dpf. Transcript levels of klf9 and nrgna were measured using qRT-PCR at 3 dpf. Both klf9 and nrgna expression levels were increased by 49% (t = −2.643, df = 8, p<0.05, Fig. 2C) and 46% (t = −5.307, df = 8, p<0.05, Fig. 2C), respectively, under T3 administration. These results confirm that similar to mammals, klf9 and nrgna are T3-induced genes in zebrafish. To test whether T3 effect inside the cells is attenuated in the absence of a functional MCT8, mRNA levels of klf9 and nrnga were measured in mct8−/− and WT-sibling 3 dpf embryos. Although the expression levels of klf9 and nrnga were induced in response to T3 administration (Fig. 2C), their expression levels did not change in mct8−/− embryos (Fig. 2D). These results suggest that at 3 dpf, MCT8 does not affect the expression of key TH-induced genes.
Fig. 2. The expression of TH-induced and HPT-axis genes in mct8−/− embryos. 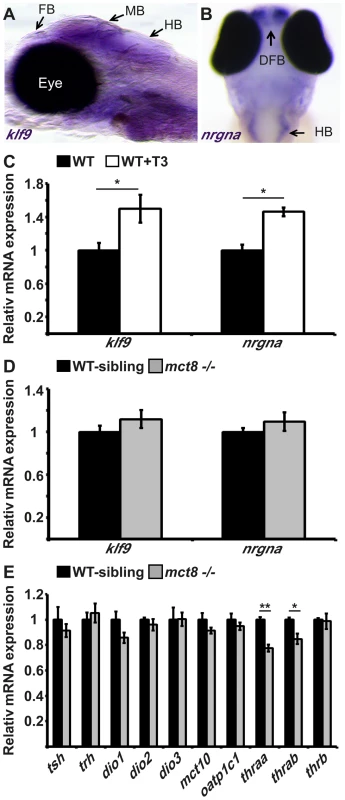
A. The expression pattern of klf9 in the forebrain (FB), midbrain (MB), and hindbrain (HB) of 6 dpf larvae (lateral view), as detected by whole-mount ISH. B. nrgna is predominantly expressed in the dorsal forebrain (DFB) and HB in 3 dpf embryo (dorsal view), as detected by whole-mount ISH. C. Relative mRNA expression levels of klf9 and nrgna in untreated and T3-treated WT embryos. D. Relative mRNA expression of klf9 and nrgna in 3 dpf mct8−/− and their WT-sibling embryos. E. Relative mRNA expression levels of tsh, trh, dio1, dio2, dio3, mct10, oatp1c1, thraa, thrab and thrb in 3 dpf mct8−/− and their WT-sibling embryos. Values represented as means±SEM (standard error of the mean). Statistical significance determined by t-test: two-sample assuming unequal variances followed by one-Sample Kolmogorov-Smirnov test. The hypothalamus-pituitary-thyroid axis is not affected by MCT8 elimination
MCT8 facilitates cellular influx and efflux of TH [24], [25], and elimination of this transporter in adult mice reduced TH levels in the brain, as shown by the expression of TH-related genes that are part of the hypothalamus-pituitary-thyroid (HPT) axis [26]. In zebrafish, the knock-down of MCT8, using morpholino-modified antisense oligonucleotides (MO), did not alter the expression of TH-related genes in 2 dpf embryos [20]. To quantify the effect of MCT8 elimination on transcript levels of HPT axis-related genes, we performed qRT-PCR on total mRNA extracted from 3 dpf mct8−/− and WT-sibling embryos. In agreement with previous results [20], the mRNA levels of thyrotropin-releasing hormone (trh), thyroid-stimulating hormone β (tshβ) and the three deiodinases (dio1, dio2, dio3), did not change in mct8−/− compared with their WT siblings (Fig. 2E). These findings raised the possibility that other TH transporters can compensate for the lack of MCT8, and balance TH transport. However, this suggested mechanism is unlikely because the expression patterns of mct10 and oatp1c1 generally do not overlap in zebrafish larvae [20]. Nevertheless, we quantified the expression of mct10 and oatp1c1 mRNA in 3 dpf mct8−/− and WT-sibling embryos. Similar to the expression results of TH-induced and HPT-axis genes, MCT8 elimination did not affect the expression of alternative TH transporters (Fig. 2E). These results suggest that at 3 dpf, right before the endogenous HPT axis becomes functional [18], MCT8 elimination does not affect the expression of HPT axis-related genes. However, these results do not rule out the possibility that TH parameters may be altered in specific tissues and at older developmental stages.
The expression of thyroid hormone receptor alpha is reduced in mct8−/ − embryos
TRs are nuclear ligand-inducible transcription factors that bind T3 and recognize specific DNA sequences, called TH-responsive elements (TREs), in the promoter of TH-induced genes [27]. Three genes encoding TRs are present in zebrafish: thyroid hormone receptor alpha a (thraa), thyroid hormone receptor alpha b (thrab) and thyroid hormone receptor beta (thrb). The two thra genes are weakly expressed in embryos and robustly expressed in adult ovaries and testes. At 3 dpf, thrb is expressed in the retina, midbrain and hindbrain [27]. To quantify the effect of MCT8 elimination on the transcript levels of TRs genes, qRT-PCR was performed on total mRNA extracted from 3 dpf mct8−/− and WT-sibling embryos. We found that mRNA levels of thraa and thrab were reduced by 23% (t = 6.65, df = 14, p<0.001) and 16% (t = 3.4, df = 12, p<0.05), respectively, in mct8−/− compared with their WT siblings (Fig. 2E). In contrast, transcript levels of thrb were not changed (Fig. 2E). These results suggest that at 3 dpf, loss of MCT8 does not affect the expression of TH-related genes, including thrb. However, it affects the levels of thraa and thrab. The reduction in expression of these two TH receptors may correlate with reduced TH levels inside the cells.
The expression of myelin-related genes is altered in mct8−/ − developing embryos
Brain magnetic resonance imaging (MRI) showed markedly delayed myelination and global lack of cerebral white matter in AHDS patients [28]–[30]. The cause of these myelin defects is not clear, and this phenotype was not replicated in MCT8 KO mice. Given the crucial role of TH in oligodendroglial development, maturation, and myelination [31], [32], we hypothesized that a lack of MCT8 alters myelination in the developing zebrafish embryos and that this effect is mediated by TH. Supporting this idea, in mammals, TRs are localized in glial cells expressing oligodendrocyte lineage transcription factor 2 (olig2), myelin basic protein (mbp), and myelin protein zero (p0) [33], [34]. The olig2 gene is specifically expressed in motor neurons and oligodendrocytes precursor cells in zebrafish and mammals [33], [35]. P0, a major structural protein of myelin [36], [37], and the MBP, which is the most abundant protein component of the myelin sheath [37], [38], are constitutively expressed in mature oligodendrocytes and Schwan cells in the zebrafish CNS and peripheral nervous system (PNS), and are well-established markers for myelination [37]. In mammals, the expression of mbp and other myelin-marker genes is induced by TH through binding to TRE present in their regulatory regions [33], [34], [39]. Similarly, in teleost fish, TRE is localized within the promoter of the mbp gene [40]. To test whether TRE is present in the promoters of olig2, p0, and mbp also in zebrafish, bioinformatics analysis was performed. In the 7 kb, 5 kb, and 2 kb promoters of olig2, p0, and mbp, respectively, consensus sequences of putative TRE were found approximately 500 bp upstream to the transcription start site (Fig. 3A). These results suggest that these proteins mediate the effect of TH on myelination.
Fig. 3. Altered expression of myelin-related genes in mct8−/− embryos is recovered by TH analogs. 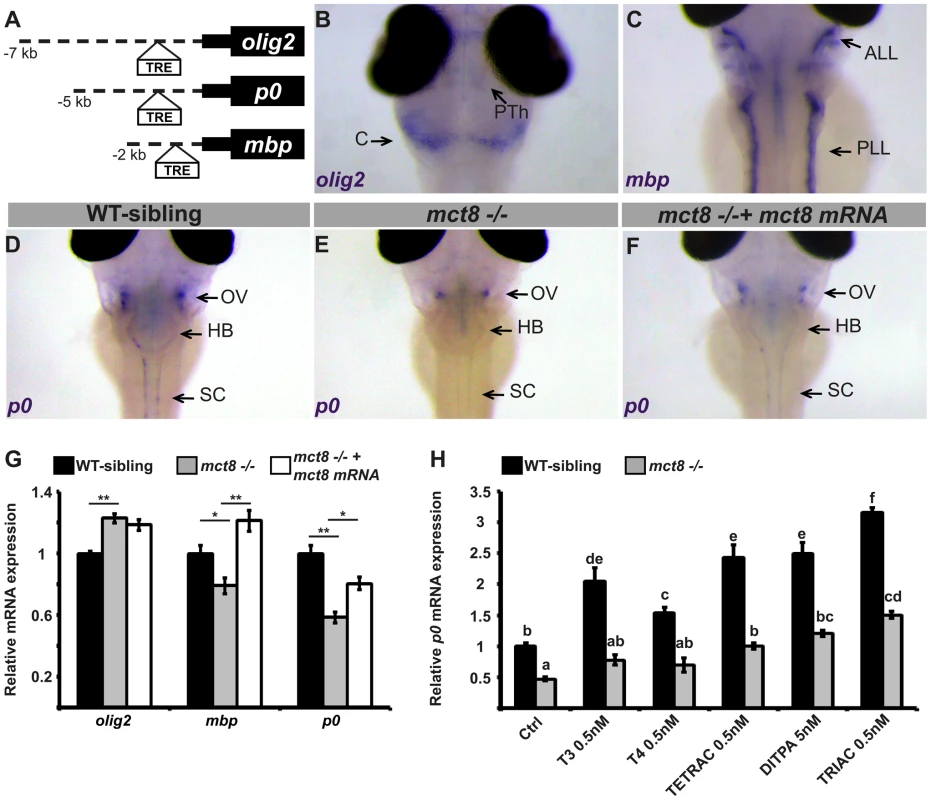
A. The location of putative thyroid response elements (TRE) in the 7, 5, and 2 kb promoter regions of the zebrafish olig2, p0, and mbp genes, respectively. B–F. Whole-mount ISH experiments detected the spatial mRNA expression in 3 dpf embryos (dorsal views) B. olig2 is predominantly expressed in the prethalamus (PTh) and cerebellum (C). C. mbp is expressed primarily in the anterior and posterior lateral line (ALL and PLL, respectively). D. p0 is primarily expressed in a cluster of cells above the otic vesicle (OV), the HB, and spinal cord (SC) in WT-sibling embryos. E. Low expression levels were detected above the otic vesicle in mct8−/− embryos. F. The expression pattern of p0 was recovered in mct8 mRNA-injected mct8−/− embryos. G. Relative mRNA expression of olig2, mbp, and p0 in 3 dpf WT-sibling, mct8−/−, and mct8 mRNA-injected mct8−/− embryos. Values represented as means ±SEM (standard error of the mean). Statistical significance determined by one-way ANOVA followed by a Tukey test (*p<0.05, **p<0.001). H. Relative mRNA expression of p0 in 3 dpf WT-sibling and mct8−/− embryos treated with 0.5 nM T3, 0.5 nM T4, 0.5 nM TETRAC, 5 nM DITPA, and 0.5 nM TRIAC compared to control (Ctrl, treated with 5×10−6 M NaOH) WT-sibling and mct8−/− embryos. Values represented as means ±SEM. Statistical significance determined by two-way ANOVA followed by a Tukey test. Different letters indicate significant difference. To evaluate the effect of MCT8 elimination on myelination, the spatial distribution of primary and differentiated glial cells was determined by whole-mount ISH. The expression of myelin-related genes is first detected at 2 dpf and the onset of myelination was reported to be at 3 dpf [41]. In 3 dpf embryos, olig2 was expressed mainly in precursor neural cells in the cerebellum, prethalamus, and spinal cord (Fig. 3B). The mbp gene was expressed in mature oligodendrocytes and Schwann cells in the hindbrain, the spinal cord, and in the anterior and posterior lateral line (Fig. 3C). The p0 was mainly expressed in mature oligodendrocytes in the lateral hindbrain, above the otic vesicle, and in the spinal cord (Fig. 3D). These gene expression patterns confirm previous observations in zebrafish embryos [42]–[44]. Notably, the expression pattern of p0 was markedly reduced above the otic vesicle and absent from the spinal cord in 3 dpf mct8−/− embryos (Fig. 3E). Next, transcript levels of olig2, mbp, and p0 were quantified by qRT-PCR in mct8−/− and WT-sibling embryos. At 3 dpf, olig2 mRNA levels were increased by 28% (F = 23.12, df = 2.27, p<0.001), mbp mRNA levels were reduced by 27% (F = 13.528, df = 2.27, p<0.05), and p0 mRNA levels were reduced by 33% (F = 22.308, df = 2.27, p<0.001) in mct8−/− compared with their WT siblings (Fig. 3G). These results suggest an increase in the number of precursor glial cells and a decrease in the number of mature glial cells in mct8−/− embryos. These findings also suggest deficient myelination in mct8−/− embryos. In order to confirm that the observed phenotype is specific to the loss of MCT8, rescue experiments were conducted. At the one-cell stage, mct8−/− embryos were injected with mct8 mRNA. At 3 dpf, the expression levels of mbp and p0 mRNA were increased by 42% (F = 13.528, df = 2.27, p<0.001) and 22% (F = 22.308, df = 2.27, p<0.05), respectively, in mct8 mRNA-injected mct8−/− compared with mct8−/− larvae (Fig. 3G). Furthermore, mct8 mRNA injection did not affect the normal morphology of the embryos but recovered the pattern of p0 expression (Fig. 3F). These results indicate that mct8 mRNA can rescue the expression levels of myelin-related genes in mct8−/− larvae, suggesting that the myelin phenotype is a specific result of MCT8 deficiency. Furthermore, alterations in the expression of these genes, which control oligodendrocyte differentiation and myelination [41] as well as axon growth and regeneration [45], suggest that deficiencies in myelination and circuit formation are the cause of altered behavioral performance in AHDS patients and could be a suitable target for therapeutic drugs.
Putative therapeutic drugs: TH analogs can restore the expression of myelin marker in mct8−/ − larvae
The therapeutic options for AHDS patients are limited. In order to exploit the zebrafish model, which is ideally suited for testing potential therapeutic molecules, we conducted a comparative pharmacological assay and evaluated the therapeutic potential of three TH analogs: 3,5,3′,5′-tetraiodothyroacetic acid (TETRAC/TA4), 3,3′,5-triiodothyroacetic acid (TRIAC/TA3), and 3,5-diiodothyropropionic acid (DITPA). In vitro and in vivo studies have demonstrated that these TH analogs can enter the cells independently of the presence of MCT8 [46], [47]. Furthermore, the effect of the T4 analog TETRAC, the T3 analog TRIAC, and the TH receptor agonist DITPA on TH-dependent gene expression and serum TH parameters, has been evaluated in MCT8-KO mice [48]–[50]. However, due to the lack of neurological symptoms, MCT8-KO mice could not be used to monitor the effect of putative drugs on neural recovery.
To monitor the putative therapeutic effect of TH analogs on the mechanism of myelination, mct8−/− and WT-sibling embryos were exposed to 0.5 nM T3, T4, TETRAC, TRIAC, and 5 nM DITPA. These component concentrations were chosen based on pre-calibration assays, where the highest dose that did not affect pigmentation and the general morphology of the embryos were selected (see Materials and Methods). Drugs were administered into the egg-water immediately after egg fertilization for three consecutive days. At 3 dpf, mRNA levels of p0 were quantified by qRT-PCR. This gene was selected because it exhibited the most significant reduction in expression in mct8−/ − embryos (Fig. 3D–G) and is co-localized with mct8 (Fig. S1). Similar to our previous results (Fig. 3G), the expression levels of p0 were reduced by 54% in mct8−/− compared with their WT-siblings (F = 50.533, df = 6.48, p<0.001, Fig. 3H). Remarkably, mRNA levels were increased and fully rescued in TETRAC-, DITPA-, and TRIAC-treated mct8−/− embryos compared with untreated control mct8−/− embryos (Ctrl: 0.46±0.02; TETRAC: 1.01±0.07; DITPA: 1.18±0.1; TRIAC: 1.5±0.07, p<0.001; Fig. 3H). In contrast, the expression levels of p0 in T3 - and T4-treated mct8−/− embryos were not changed compared with untreated control mct8−/− embryos, indicating that TH transport into the cells is altered (Fig. 3H). These results demonstrate that TH analogs can enter into the cells and bypass MCT8. Furthermore, the expression levels of p0 increased in WT-sibling embryos treated with both TH and TH analogs compared with untreated control WT-sibling embryos (ctrl: 1±0.05; T3 : 2.05±0.17; T4 : 1.5±0.08; TETRAC: 2.4±0.2; DITPA: 2.5±0.09; TRIAC: 3.15±0.2, p<0.001; Fig. 3H). These results were expected because THs are well known inducers of myelin-related processes [31]–[34]. Altogether, these results suggest that loss of MCT8 affects TH transport into the cells and that TH analogs can restore the expression of myelin-related genes. Furthermore, a similar drug mechanism may also be applied in AHDS patients.
MCT8 mutant exhibits reduced locomotor activity and altered responses to light and dark transitions
Deficiency in mobility and voluntary movements is a hallmark of AHDS, and includes the inability to sit, stand, or walk independently, as well as slow psychomotor reaction to sensory input [30]. These symptoms in humans together with the myelin-related phenotype we found in zebrafish, prompted us to test whether behavioral performance was altered in mct8−/− larvae. Using a video-tracking behavioral system that can monitor the locomotor activity of dozens of larvae simultaneously [51], [52], the rhythmic activity of mct8−/− (n = 144) and WT-sibling larvae (n = 144) was monitored during day and night. Under light/dark conditions (light: 14 h, dark: 10 h), both genotypes exhibited rhythmic activity that peaked during the day. Importantly, during day and night time, the locomotor activity of mct8−/ − larvae was reduced by 27% (t = 8.83, df = 286, p<0.001) and 21% (t = 7.13, df = 272, p<0.001), respectively, compared to their WT siblings (Fig. 4A, B), indicating reduced overall activity in mct8−/ − larvae. This reduction of overall activity was partially due to reduced ability to reach maximum velocity in mct8−/− larvae. During both day and night the maximum velocity per minute was reduced by 26% (t = 8.4, df = 286, p<0.001) and 24% (t = 9.58, df = 280, p<0.001), respectively, in mct8−/− compared to their WT sibling larvae (Fig. S2). Next, we analyzed the day/night transition states. Typically, larvae exhibit a burst of activity, followed by significant change in locomotor activity in response to day-to-night (zeitgeber time, ZT14) and night-to-day (ZT0) transitions. During the first hour following the day-to-night transition (ZT15), WT-sibling larvae reduced their activity and, in contrast, mct8−/ − larvae increased their activity compared with one hour before the transition (ZT14, mct8−/−: 0.7±0.1 cm/min, WT sibling: −0.16±0.15 cm/min; t = −4.8, df = 268, p<0.001; Fig. 4C). Similar comparison was made for the night-to-day transition (ZT1 vs. ZT0). While WT-sibling larvae exhibited the expected elevation in activity following the transition to day, mct8−/− larvae maintained relatively low activity levels (mct8−/−: 0.8±0.13 cm/min, WT sibling: 1.66±0.15 cm/min; t = 4.26, df = 285, p<0.001; Fig. 4C), indicating differential responses to day/night transitions.
Fig. 4. MCT8 mutant exhibits reduced locomotor activity and altered responses to light/dark transitions. 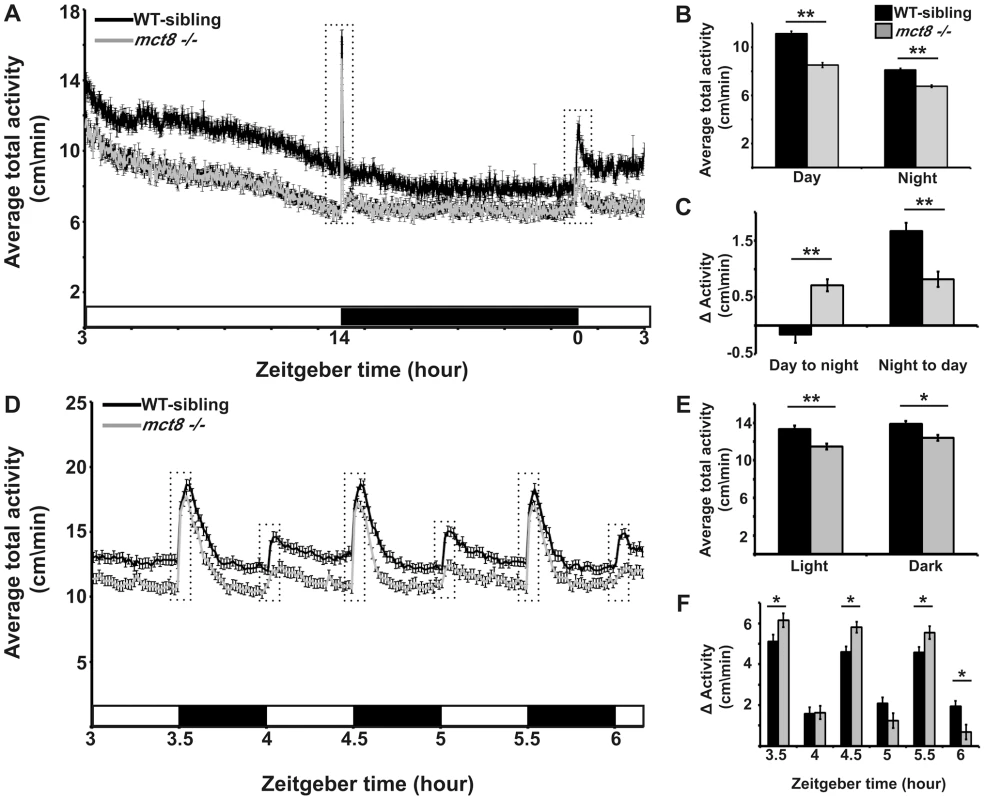
Locomotor activity recording was performed in 6 dpf mct8−/− larvae and their WT siblings throughout a daily cycle under a 14 h light/10 h dark cycle (A–C), or during 3 h of 30 min light/30 min dark intervals (D–F). White and black horizontal boxes represent light and dark periods, respectively. Average total activity of each genotype was measured as the average distance movement in 1 min (A and D). Dotted boxes represent 1 h and 5 min (in A and D, respectively) before and after the light-to-dark and dark-to-light transitions. The average total activity of each genotype was measured during day and night as well as during short light and dark periods (B and E, respectively). Differences in the average total activity of each genotype were calculated by comparing 1 h after and 1 h before the day-to-night and night-to-day transitions, as well as by comparing 5 min after and 5 min before light-to-dark and dark-to-light transitions (C and F, respectively). Values are represented as means±SEM (standard error of the mean). Statistical significance determined by t-test: two-sample assuming unequal variances (*p<0.05, ** p<0.001). The apparent altered response of mct8−/− larvae to day/night transitions could be affected by the circadian time, the light/dark transitions, or both. To test the response of mct8−/− larvae to light and dark stimuli, we exposed the larvae to three cycles of alternating 30 min periods of light and darkness during the day (ZT3–ZT6). Both mct8−/− (n = 139) and WT siblings (n = 139) responded to light and dark stimuli with robust changes in locomotor activity, as previously shown [51], [53]. Confirming our finding of reduced activity in mct8−/− larvae (Fig. 4A, B) during both light and dark periods, mct8−/− larvae were 14.2% (t = 4.26, df = 271, p<0.001) and 10.4% (t = 3.19, df = 272, p<0.05) less active compared with their WT siblings (Fig. 4D, E), respectively. Furthermore, analysis of the behavior during the light-to-dark transitions (comparison of the activity 5 min before and after the transition state) showed that the response to dark stimuli was increased in the mct8−/ − compared with the WT-sibling larvae (mct8−/−: 6.15±0.3, 5.8±0.26, 5.54±0.3 cm/min, WT-sibling: 5.1±0.3, 4.6±0.3, 4.6±0.26 cm/min, p<0.05; Fig. 4D, F). Notably, this tendency was repeated in all three light/dark cycles. These altered reactions to external stimuli are reversed when comparing the difference in activity between the light and dark phases flanking the dark-to-light transitions. In the third dark-to-light transition (ZT6), the response to light stimuli was decreased in the mct8−/ − compared with the WT-sibling larvae (mct8−/−: 0.7±0.35 cm/min, WT-sibling: 1.93±0.27 cm/min, p<0.05; Fig. 4D, F). These behavioral responses to light/dark transitions are in agreement with the behavioral responses found during the day/night transitions. Altogether, these results show reduced baseline locomotor activity and altered behavioral response to light/dark transitions in mct8−/ − larvae. These findings suggest that MCT8 is involved in the mechanism that regulates locomotor activity and behavioral response to external stimuli. The neurological basis for these deficient behaviors might be conserved between zebrafish and AHDS patients.
mct8−/ − larvae sleep more and their sleep is fragmented
The deficiencies in locomotor activity of zebrafish during the night and reports on abnormal sleep in AHDS patients (unpublished results) prompted us to characterize the sleep properties of mct8−/ − larvae. In the last decade, zebrafish have emerged as an attractive model to study sleep and sleep disorders [54]–[58]. In zebrafish larvae, at least one minute of immobility, which is associated with an increase in arousal threshold, is defined as sleep [51], [55]. To understand the effect of MCT8 elimination on sleep, we monitored sleep architecture in mct8−/− larvae (n = 144) and their WT siblings (n = 144) during day and night. As expected, both groups slept more during the night (Fig. 5A). However, sleep time was increased in mct8−/− larvae during the 24 h period. Specifically, during day and night, mct8−/− larvae slept 2.6 - and 1.8-fold more compared with their WT siblings, respectively (t = −9.01, df = 208, p<0.001; t = −8.82, df = 246, p<0.001, Fig. 5A). Furthermore, in order to study sleep consolidation, we monitored the number of transitions between wake and sleep states and sleep-bout length during day and night. In both strains, the number of sleep/wake transitions increased during the night compared with daytime. Of note, the number of transitions was higher by 2 and 1.4 fold during day and night, respectively, in the mct8−/− compared with the WT-sibling larvae (t = −6.16, df = 26, p<0.001; t = −6.3, df = 15, p<0.001, Fig. 5B). In addition, the number of transitions during the day in mct8−/− larvae was similar to the number of transitions during the night in WT siblings (Fig. 5B), suggesting night-like fragmented sleep in mct8−/− larvae during the day.
Fig. 5. Sleep architecture of mct8−/− larvae. 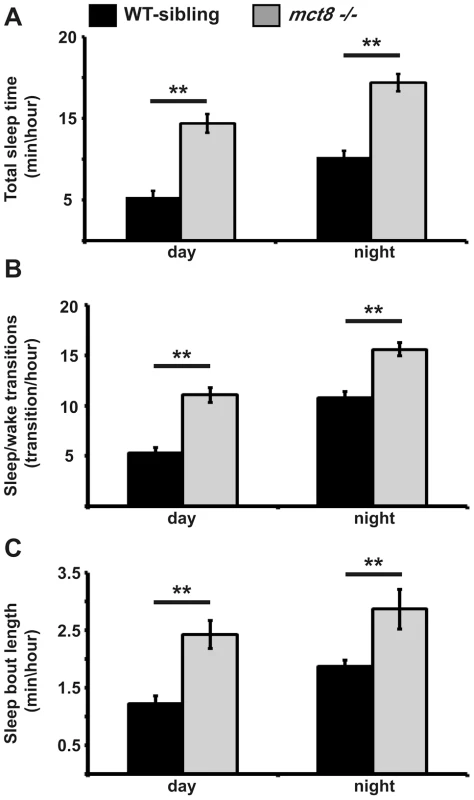
A–C. Recording of sleep was performed in 6 dpf mct8−/− and WT-sibling larvae during 24 h under a 14 h light/10 h dark cycle. Total sleep time (A), the number of sleep/wake transitions (B) and sleep-bout length (C) monitored in mct8−/− and WT-sibling larvae. Values are represented as means±SEM (standard error of the mean). Statistical significance was determined by t-test: two-sample assuming unequal variances (** p<0.001). In order to examine whether increased total sleep time reflects increased ability to maintain long sleep periods, sleep-bout length was analyzed. Sleep-bout length was higher by 2 and 1.5 fold during day and night, respectively, in the mct8−/− compared with the WT-sibling larvae (t = −6.2, df = 24, p<0.001; t = −8.4, df = 12, p<0.001, Fig. 5C). These results show that loss of MCT8 increases sleep time and sleep fragmentation during night and day. This is the first evidence of a sleep disorder in MCT8-deficient animals. Taking into account that TSH and TH are rhythmically secreted [59], [60] and their levels are altered in MCT8-KO mice and AHDS patients, sleep patterns should be further investigated in human patients.
MCT8 elimination does not affect the muscle structure
MCT8 deficiency in human patients affects muscle tone and locomotor activity. The cause for these deficiencies is thought to be neurological [3], [4]. To test whether the reduction in locomotor activity in mct8−/ − larvae is associated with altered muscle structure, the morphology and development of the fish muscles were studied. At 3 and 6 dpf, the expression pattern of myoD and F59, both well-established markers for muscle development [61], [62], was examined in mct8−/− and WT-sibling embryos. Confirming our previous finding in MCT8 morphants [20], the pattern of expression of myoD and F59 were similar in mct8−/ − and WT-sibling embryos (Fig. S3). The normal general morphology (Fig. 1D, E) and muscle organization (Fig. S3) observed in mct8−/− larvae suggest that altered neuronal circuits are the cause for the deficient behavior.
Loss of MCT8 reduces synaptic density in axonal arbors of the motor neuron
In the absence of adequately functioning MCT8, AHDS patients demonstrate severe cognitive deficiencies, low muscle tone, and dystonia, putatively reflecting the effect of TH deprivation on the CNS [3], [4]. Indeed, PAX8-KO mice, which do not produce endogenous THs, demonstrate severe deficiencies in brain development [48]. However, apparently due to a compensatory mechanism used by other TH transporters, no such brain damage was found in MCT8-KO mice [13], [63] and the neuropathological deficiencies of AHDS patients remain elusive. Taking into account the altered expression of myelin-related genes and deficient locomotor activity in mct8−/− zebrafish, we hypothesized that motoric and sensory neurological impairment might be found. To directly assess whether the development and plasticity of motor neurons are affected by loss of MCT8, we sought to image fluorescently labeled motor arbors in the trunk of mct8−/− live larvae. The huc pan-neural promoter is a well-established, robust tool for marking motor and sensory neurons and endogenous HUC co-localized with MCT8 in zebrafish larvae [20]; therefore, it was used to mark MCT8-expressing motor and sensory neurons [64], [65]. In order to confirm that single huc promoter-driven motor neurons express mct8, huc:GAL4 and uas:tRFP constructs were co-injected into tg(mct8:EGFP) one-cell-stage embryos. Next, single huc promoter-driven motor neurons were imaged in 2 dpf embryos. As expected, co-localization of mct8 and the huc pan-neural marker was detected in the motor neurons (Fig. 6A–C). We then tested the effect of MCT8 elimination on axon-arbor processing in single motor neurons. Transgenic tg(huc:GAL4Xuas:memYFP)/mct8+/− and mct8−/− zebrafish were crossed, and single motor neurons were imaged in live progeny at 3 and 6 dpf (Fig. 6D, E). Image analysis revealed that the total length of the arbor branches (Fig. 6F, G, and K) and the number of branches (Fig. 6F, G, and L) were similar in mct8−/− (3 dpf: n = 12, 6 dpf: n = 12) and mct8+/− larvae (3 dpf: n = 8, 6 dpf: n = 9). These results show that the structure and length of axon arbor in motor neurons are not affected by MCT8 elimination.
Fig. 6. Loss of MCT8 reduces synaptic density in axonal arbors of the motor neuron. 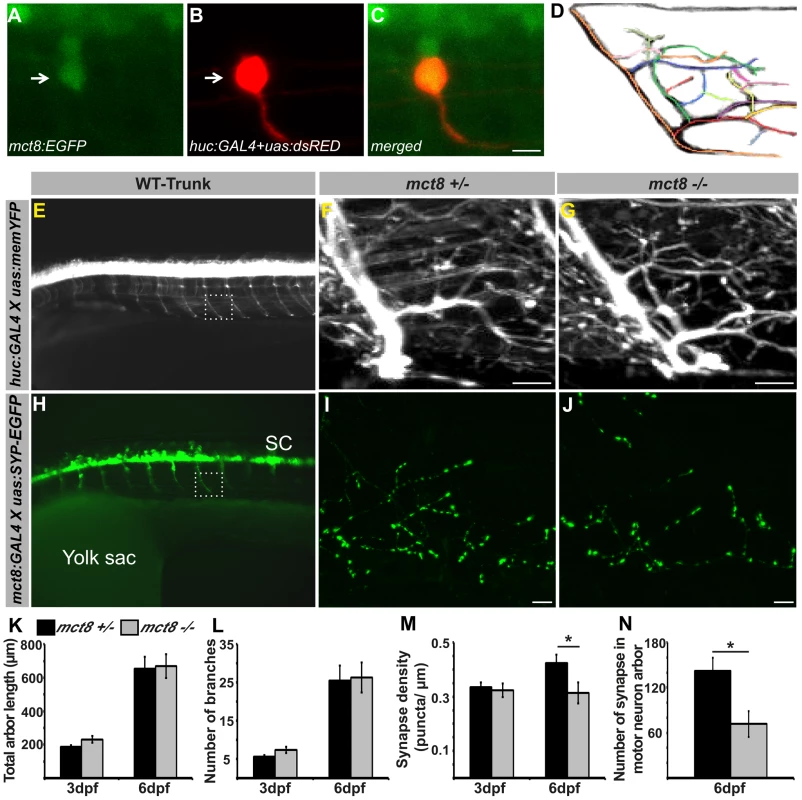
A–C. Confocal imaging of a 2 dpf live tg(mct8:EGFP) embryo co-injected with huc:GAL4 and uas:tRFP constructs revealed co-localization of mct8 (green) and the huc pan-neural marker (red) in a motor neuron. D. Schematic illustration of an axonal arbor in a motor neuron. Each color represents a single branch that was subjected to ImageJ software analysis. E. Lateral view of a 3 dpf tg(huc:GAL4Xuas:memYFP) embryo. memYFP expression driven by the huc promoter is observed in the spinal cord (SC) and in descending motor neurons. The dashed frame marks a single motor neuron that was selected for further comparative studies. High magnification of the framed area is shown in the trunk of 6 dpf tg(huc:GAL4Xuas:memYFP)/mct8+/− and tg(huc:GAL4Xuas:memYFP)/mct8−/− representative larvae (F and G, respectively). H. Lateral view of a 30 hpf tg(mct8:GAL4Xuas:SYP-EGFP) embryo. SYP-EGFP expression driven by the mct8 promoter is observed in the spinal cord (SC) and in descending motor neurons. In order to compare the number of synapses in mct8+/− and mct8−/− larvae, single motor-neuron arbors were selected (dashed frame). High magnification of the dashed frame is shown in 6 dpf tg(mct8:GAL4)/(uas:SYP-EGFP)/mct8+/− and tg(mct8:GAL4)/(uas:SYP-EGFP)/mct8−/− representative larvae (I and J, respectively). The total arbor length (K) and the number of branches (L) were measured in 3 and 6 dpf mct8+/− larvae and in 3 and 6 dpf mct8−/− larvae. M. Synapse density in the axons of the motor-neurons was measured along the last 50 µm of a single branch in 3 and 6 dpf mct8+/− larvae and in 3 and 6 dpf mct8−/− larvae. N. The total number of synapses was measured in the motor-neuron arbor of 6 dpf mct8+/− and mct8−/− larvae. Scale bar = 30 µm. Values represented as means±SEM (standard error of the mean). Statistical significance determined by t-test: Two-sample assuming unequal variances followed by one-sample Kolmogorov-Smirnov test, to assume normal distribution (*p<0.05). Since alteration in outgrowth and branching of motor neurons was not observed in mct8−/− larvae, we tested whether MCT8 is a regulator of structural synaptic dynamics. To visualize synapses on axon arbors in live fish, we labeled the neurons with the presynaptic protein synaptophysin (SYP) fused to enhanced green fluorescent protein (SYP-EGFP). This protein is a well-established synaptic marker in zebrafish [15] and was previously used to demonstrate rhythmic structural synaptic plasticity in the axons of hypocretin/orexin neurons [17]. In order to tag synapses in every circuit of interest, we established a stable transgenic line tg(uas:SYP-EGFP) expressing SYP-EGFP under the control of uas. In order to examine synapses in motor neurons, tg(mct8:GAL4) [20] and tg(uas:SYP-EGFP) were crossed and their progeny were imaged. In contrast to the wide expression of EGFP in tg(mct8:EGFP) larvae [20], in the double transgenic line EGFP was represented specifically in neurons and synapse structures in the axon fibers of 30 hpf embryos (Fig. 6H). Then tg(mct8:GAL4)/tg(uas:SYP-EGFP)/mct8−/− and tg(mct8:GAL4)/tg(uas:SYP-EGFP)/mct8+/− fish lines were generated. At 3 and 6 dpf, synapses in the axonal arbor of single motor neurons were imaged in both mct8 genotypes. While at 3 dpf, synaptic density was not changed (mct8+/−: n = 34, mct8−/−: n = 24, Fig. 6M), a reduction of 27% was found in 6 dpf tg(mct8:GAL4)/tg(uas:SYP-EGFP)/mct8−/− larvae (n = 20) compared with the control tg(mct8:GAL4)/tg(uas:SYP-EGFP)/mct8+/− larvae (n = 30, t = 2.309, df = 48, p<0.05, Fig. 6I, J, and M). In addition, a 50% reduction in the total number of SYP-EGFP puncta in the axonal arbor were found in tg(mct8:GAL4)/tg(uas:SYP-EGFP)/mct8−/− 6 dpf larvae (n = 17) compared with the control tg(mct8:GAL4)/tg(uas:SYP-EGFP)/mct8+/− 6 dpf larvae (n = 31, t = 2.639, df = 46, p<0.05, Fig. 6I, J, and N). These results show that loss of MCT8 decreases total synapse number and synaptic density in the axons of the motor neurons in 6 dpf larvae, while it does not impair axonal outgrowth and branching, suggesting that MCT8 affects structural synaptic changes and plays a crucial role in mediating neural signaling between the nervous system and the muscles.
MCT8 regulates axon branching in sensory neurons
The altered expression of myelin-related genes and the disrupted behavioral response to external stimuli in mct8−/− larvae suggest that in addition to the motor neurons, sensory neuronal circuits can be altered. In zebrafish larvae, the response to light and touch stimuli and initiation of the first escape response are mediated by the primary Rohon-Beard (RB) sensory neurons. These neurons are located in the dorsal spinal cord and have axons that project toward the hindbrain and the tail [66], [67] (Fig. 7A). In 2 dpf embryos, the RB neurons are fully mature while, at older ages, their axons are gradually abolished and the neurons differentiated to the dorsal root ganglia that have the same sensory functions as the RB neurons [66]. To verify that mct8 is expressed in RB neurons, double-ISH of mct8 and p2rx3.1, a marker for RB neurons [68], was performed. This staining showed that mct8 and p2rx3.1 are co-localized in RB neurons (Fig. 7B–D). In order to test the effect of MCT8 elimination on the pattern and spatial distribution of RB neurons, whole-mount ISH was performed using p2rx3.1 probe. At 33 hpf, no differences were found between mct8−/− and WT-sibling embryos (Fig. 7E and F). However, although the cell bodies of RB neurons were intact, an MCT8-dependent deficiency in axon outgrowth may be present.
Fig. 7. MCT8 regulates axon branching in the Rohon-Beard sensory neurons. 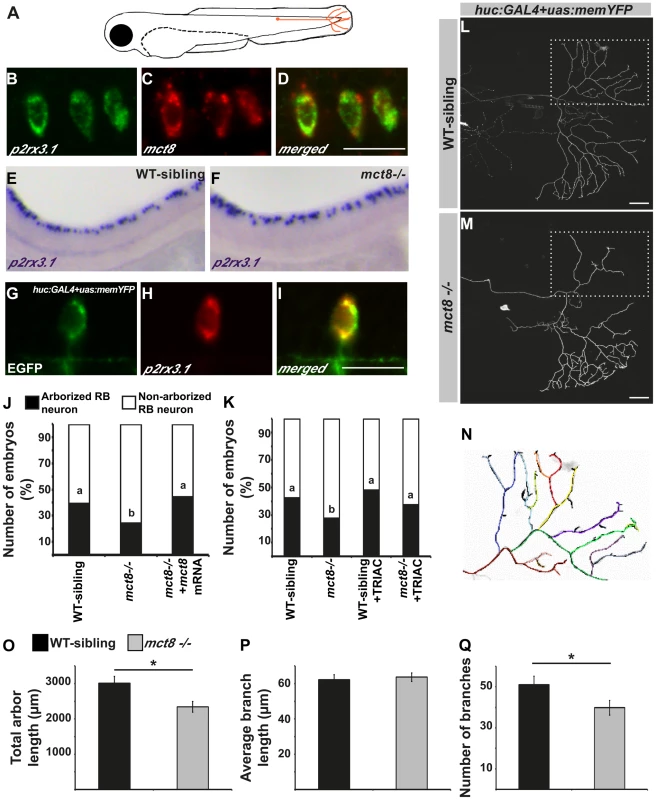
A. A representative scheme of the Rohon-Beard (RB) sensory neuron location in zebrafish larvae. B–D. Double fluorescent ISH in 33 hpf embryos revealed co-localization of p2rx3.1 (green) and mct8 (red) in RB cell bodies. E–F. Whole mount ISH showed the spatial expression of p2rx3.1 in the dorsal spinal cord of 2 dpf WT-sibling (E) and mct8−/− larvae (F). G–I. Whole-mount ISH and immunofluorescence revealed co-localization of EGFP (green) and p2rx3.1 (red) in the cell body of an RB neuron in 2 dpf huc:GAL4+uas:memYFP-injected embryos. J. The percentages of embryos that express memYFP in single arborized RB neurons in the tail (black bars), are shown in 2 dpf WT-sibling, mct8−/− and mct8 mRNA-injected mct8−/− embryos. Statistical significance was determined by the Chi square test. Different letters indicate significant difference. K. The percentages of embryos that express memYFP in single arborized RB neurons in the tail (black bars), are shown in 2 dpf WT-sibling, mct8−/−, WT-sibling treated with 0.5 nM TRIAC and mct8−/− treated with 0.5 nM TRIAC. Statistical significance was determined by the Chi square test. Different letters indicate significant difference. L, M. Lateral view of arborized RB-neuron that projects toward the tail in 2 dpf live mct8−/− and WT-sibling embryos, which are transiently expressed huc:GAL4 and uas:memYFP constructs. N. Schematic illustration of arborized RB sensory neuron. Each color represents a single branch that was subjected to ImageJ software analysis. Filopodia are colored in black. The total length (O), average length (P), and number of branches (Q) measured in mct8−/− and WT-sibling embryos. Scale bar = 30 µm. Values represented as means±SEM (standard error of the mean). Statistical significance determined by t-test: Two-sample assuming unequal variances followed by one-sample Kolmogorov-Smirnov test, to assume normal distribution (*p<0.05). In order to explore the specific effects of MCT8 elimination on the dynamics of RB-axon-arbor structure, we imaged single RB neurons in live embryos. The constructs huc:GAL4 and uas:memYFP were co-injected into mct8−/− and WT-sibling embryos. This transient expression resulted in mosaic memYFP expression in several types of neurons including p2rx3.1-positive RB-neurons (Fig. 7G–I). At 2 dpf, memYFP-positive embryos were sorted out and among the positive embryos 24% (n = 332) and 39% (n = 303) showed memYFP expression in single RB neuron that projected toward the tail in mct8−/− and WT-sibling embryos, respectively (χ2 = 20.337, df = 2, p<0.001, Fig. 7A, J). These results demonstrate that the number of mature RB neurons that contain axon arbor in the tail is reduced in mct8−/− embryos, and suggest that loss of MCT8 altered the development of the axonal arbor of RB neurons. To confirm that the observed differences are specific to MCT8 deficiency, we performed rescue experiments. The constructs huc:GAL4 and uas:memYFP were co-injected with and without mct8 mRNA into one-cell-stage mct8−/− embryos. At 2 dpf, the number of mct8 mRNA-injected embryos that showed memYFP expression in single arborized RB neurons increased to 44% (n = 52, χ2 = 20.337, df = 2, p<0.001, Fig. 7J), which is similar to the percentage of embryos observed in the WT-sibling group.
The MCT8-dependent arborization phenotype prompted us to test whether TH analogs can also rescue this neurological deficiency in mct8−/ − embryos. Two constructs, huc:GAL4 and uas:memYFP, were co-injected into one-cell-stage mct8−/− and WT-sibling embryos which were then raised in 0.5 nM TRIAC for two days. TRIAC was selected because it exhibited the most significant effect on the expression of p0 in WT-sibling and mct8−/ − embryos (Fig. 3H). Similar to previous observations (Fig. 7J), at 2 dpf, 27.5% (n = 109) and 42.8% (n = 66) of the positive embryos showed memYFP expression in single RB neuron that projected toward the tail in mct8−/− and WT-sibling embryos, respectively (χ2 = 4.1, df = 3, p<0.05, Fig. 7K). Importantly, the number of TRIAC-treated mct8−/− embryos that showed memYFP expression in single arborized RB neurons increased to 44% (n = 99, χ2 = 6.77, df = 3, p<0.05, Fig. 7K). In contrast, no significant differences were found in WT-sibling treated with TRIAC (n = 52). These results show that the TH analog can recover the development of axons in RB neurons of mct8−/− embryos.
To elucidate specific axonal deficiencies, we imaged and quantified the total arbor length, the number of branches, and the average branching length of single RB neurons in mct8−/− and WT-sibling embryos. Live imaging showed that total arbor length was reduced by 32% in mct8−/− (n = 34) compared with WT-sibling embryos (n = 23, t = 2.030, df = 55, p<0.05, Fig. 7L–O). This decrease in arbor length could be the result of a decrease in the number of branches per axon arbor, a decrease in branch length, or by a combination of both processes. Intriguingly, we found that the average length of a single branch was similar in both genotypes (Fig. 7L–N and P); however, the number of branches was reduced by 22% in mct8−/− (n = 34) compared with WT-sibling embryos (n = 23, t = 2.783, df = 55, p<0.05, Fig. 7L–N and Q). These results show that MCT8 is essential for the mechanism that controls RB-axon branching.
Filopodia dynamics in RB-axons is reduced in mct8−/ − embryos
The growth of RB-axon arbors is a highly dynamic process characterized by the formation of numerous transient and highly dynamic filopodia. Only a small fraction of the filopodia develops into stable branches in the mature arbor [69]. To understand the mechanism which regulates the reduction in the number of branches in the RB-axon arbor of mct8−/− embryos, the number of filopodia was quantified. First, to study the effect of T3 on filopodia dynamics in zebrafish larvae, T3 (0.5 nM) was administered to huc:GAL4/uas:memYFP injected embryos beginning at the one-cell stage and until 2 dpf. Under T3 administration, the number of filopodian branches per single axon arbor was increased by 25% (WT: n = 25, WT+TRIAC: n = 15, t = 2.06, df = 24, p<0.05, Fig. S4). These results confirm that similar to mammals [70], [71], TH induces filopodia dynamics in zebrafish. Then, the number of filopodian branches was quantified in WT-sibling and mct8−/− embryos. At 2 dpf, the number of filopodian branches in axon arbors was reduced by 30% in mct8−/− (n = 34) compared with their WT-sibling embryos (n = 23, t = 4.188, df = 43, p<0.05, Fig. 8A–C). In order to track filopodia dynamics in the axon arbors, time-lapse imaging was performed (Video S1). We quantified the number of total, new and lost filopodia per branch. New and lost filopodia were defined when they appeared or disappeared between frames (frame intervals: 15 min), respectively. During 135 minutes, the average number of new and lost filopodia was reduced by 95% and 94% in mct8−/− 2 dpf embryos, respectively (both genotypes: n = 10, t = 4.151, df = 9, p<0.001 and t = 4.453, df = 11, p<0.001, Fig. 8D, E). In addition, the average number of total filopodia per single branch was reduced by 78% in mct8−/− compared with WT-sibling 2 dpf embryos (t = 12.716, df = 14, p<0.001, Fig. 8D, E). These time-lapse imaging experiments show that the dynamic changes of filopodia formation and elimination are reduced in mct8−/− embryos. Altogether, these results suggest a mechanism by which reduction in the rate of filopodial turnover leads to a reduced number of stable filopodia that will develop into mature axon branches in mct8−/− larvae.
Fig. 8. MCT8 reduces filopodia dynamics in the axons of RB neurons. 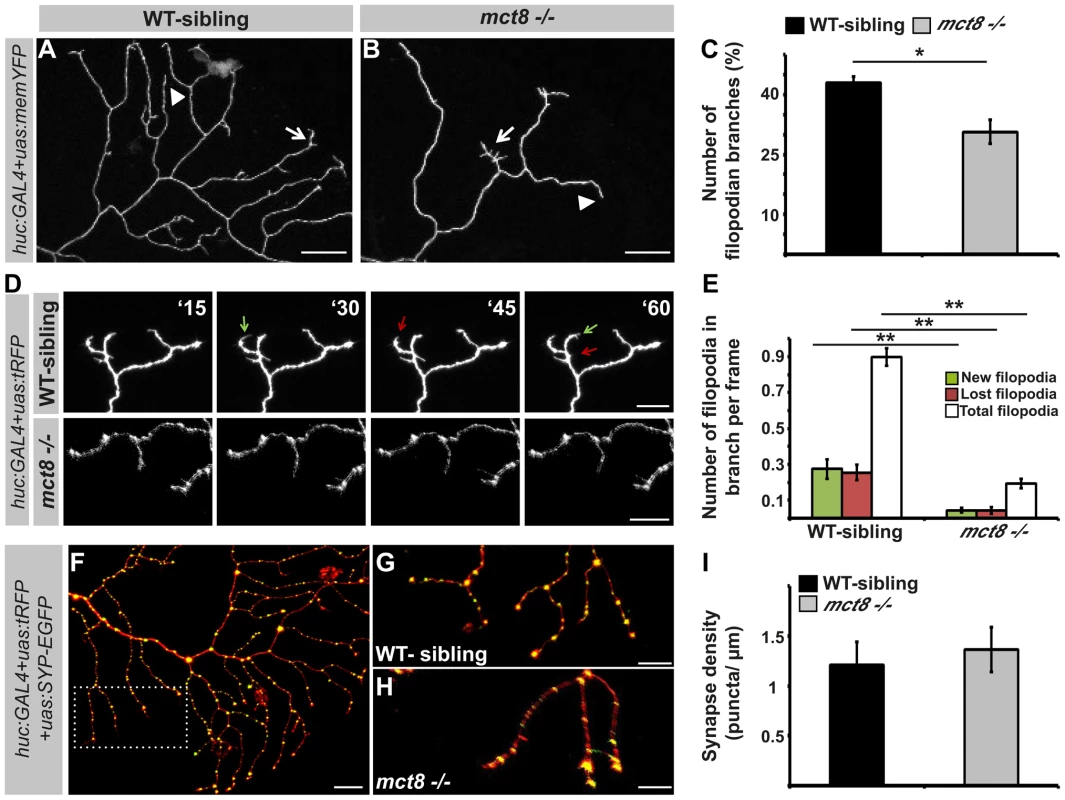
A–B. High magnification views of the dotted area shown in Fig. 7L and 7M, respectively. Arrows mark branches that contain filopodia and arrowheads mark branches that lack filopodia. C. Number of filopodian branches in mct8−/− and WT-sibling embryos. D. Time-lapse live imaging of axon arbor of RB sensory neuron (15 min intervals during 135 min). A representative series of images that were taken every 15 min in live mct8−/− and WT-sibling embryos is shown. Filopodia dynamics is defined as the number of new (green arrows) and lost (red arrows) filopodia per branch over time. E. Filopodia dynamics per branch during 150 min. F–H. Live imaging of synapses in the axons of the RB sensory neurons. F. Lateral view of axons and synapses marked with tRFP and SYP-EGFP, respectively. The dotted frame marks the area shown in high magnification in G and H. I. Synapse density in the RB-neuron arbor of mct8−/− and WT-sibling embryos measured along the last 50 µm of a single branch. Scale bar = 30 µm. Values represented as means ±SEM (standard error of the mean). Statistical significance determined by t-test: two-sample assuming unequal variances followed by one-sample Kolmogorov-Smirnov test to assume normal distribution (*p<0.05, ** p<0.001). The branching developmental defect might be associated with alteration in synaptic formation because, in other neuronal circuits, synaptogenesis guides the branching of axonal arbors in zebrafish [15]. Therefore, the huc:GAL4, uas:SYP-EGFP, and uas:tRFP constructs were co-injected into mct8−/− embryos and their WT siblings. At 2 dpf, synapse density was quantified in the axonal arbor of single RB neurons (Fig. 8F) in both genotypes. Unlike the alteration found in the motor neurons (Fig. 6I–J and M–N), synaptic density in the RB neurons was similar in both genotypes (mct8−/−: n = 8, WT-sibling: n = 8, Fig. 8G–I). Altogether, these results show that MCT8 regulates filopodial turnover and axon branching in sensory RB neurons.
Discussion
Elucidating the pathophysiological mechanisms underlying the inhibition of cognitive and motor activity in patients with psychomotor retardation will improve their therapeutic management. Psychomotor retardation AHDS is an inherited, X-linked, single-gene disorder. In the afflicted population, the mct8 gene is mutated, and the consequence in humans is altered TH levels and a diverse constellation of psychiatric and neurological symptoms [3], [4], [63]. Based on these symptoms, as well as on research on cell lines and MCT8-KO mice, it was suggested that the loss of MCT8 results in reduced transport of TH into the brain, thus TH signaling is altered and causes deficiencies in CNS development [72]. However, no neurological phenotype was found in MCT8-KO mice, and the mechanism and treatment of the disorder remained enigmatic. In the current research, we established and characterized a ZFN-based zebrafish mutant of MCT8. Transient transgensis and mct8 mRNA as well as pharmacological rescue experiments have shown that the ZFN-mediated mutation specifically and efficiently altered MCT8 function. Considering the methodological advantages of this transparent vertebrate, the mct8−/ − zebrafish provides a stable model that allows whole-brain analysis in live animals during all developmental stages. Using genetic manipulations, time-lapse live imaging, and video-tracking of behavior, we found alteration in the expression of myelin-related genes, circuit-specific alteration in circuit formation, and deficient behavior in mct8−/ − larvae. Comparative pharmacological assays showed that TH analogs can recover a portion of the neurological phenotypes. Thus, the zebrafish provides an attractive model to study the mechanisms and test possible treatments for AHDS specifically and psychomotor retardation in general.
The current explanation for the symptoms of AHDS suggests that diminished TH supply during critical stages of brain development alters the expression of the HPT-axis-related and TH-induced genes, and eventually leads to neurological and behavioral abnormalities [63], [73], [74]. Indeed, MCT8-KO mice replicated the abnormal thyroid parameters found in AHDS patients and showed increased serum T3 values and low T4 levels [63], [73], [74]. Furthermore, the level of expression of HPT-axis genes, such as tsh and dio2, and TH-induced genes, such as klf9 and nrgn, were altered [22], [48]. Intriguingly, these changes in gene expression were not associated with neurological impairments in the MCT8-KO mice [63]. In contrast, the expression levels of klf9, nrgna and HPT-axis-related genes were not changed in 3 dpf mct8−/ − zebrafish embryos; nevertheless, apparent neurological and behavioral deficiencies were found. A possible explanation to the lack of gene alteration in mct8−/ − zebrafish could be the relatively early developmental stage at which the embryos were sampled, when the negative TH feedback loop was still not apparent [18] and endogenous TH production was limited [75]. This unchanged TH status during the early stages of embryonic development might also be the case in MCT8-deficient mammals. Recently, multiphasic changes of thyroid levels and function were found in the perinatal MCT8-KO mice. While hypothyroidism exists in the brain of adult MCT8-KO mice [13], [22], TH levels were similar to those of WT mice on embryonic day 17 (E17). Unexpectedly, hyperthyroidism appeared at ages E18 and P0, one day prior to birth and on the day of birth, respectively [22]. Thus, we propose that at early stages of development, such as 3 dpf in zebrafish and approximately 6–7 weeks in a human fetus, MCT8 might have a TH-independent cellular function that induces neurological deficiencies in zebrafish and possibly in humans. Nevertheless, since MCT8 is a TH transporter in mammals and zebrafish [3], [21], [76], the involvement of TH signaling in the regulation of the neurological deficiencies should be further tested during several stages of zebrafish development. In addition, TH alteration may be a tissue - and even cell-specific condition in 3 dpf mct8−/ − embryos. Supporting this notion, we found that the expression of thraa and thrab is reduced in 3 dpf embryos suggesting reduced TH levels inside the cells. Furthermore, administration of T3 increased the expression of p0 in WT-sibling but did not recover the expression of p0 in mct8−/ − embryos, suggesting that elimination of MCT8 altered TH levels within the glial cells. Altogether, MCT8 regulates myelin-related gene expression and circuit formation in early developed zebrafish embryos and, putatively, also in human embryos, by an unknown function and by facilitates cellular influx and efflux of TH (Fig. 9).
Fig. 9. A proposed model for the mechanism underlying MCT8 deficiency. 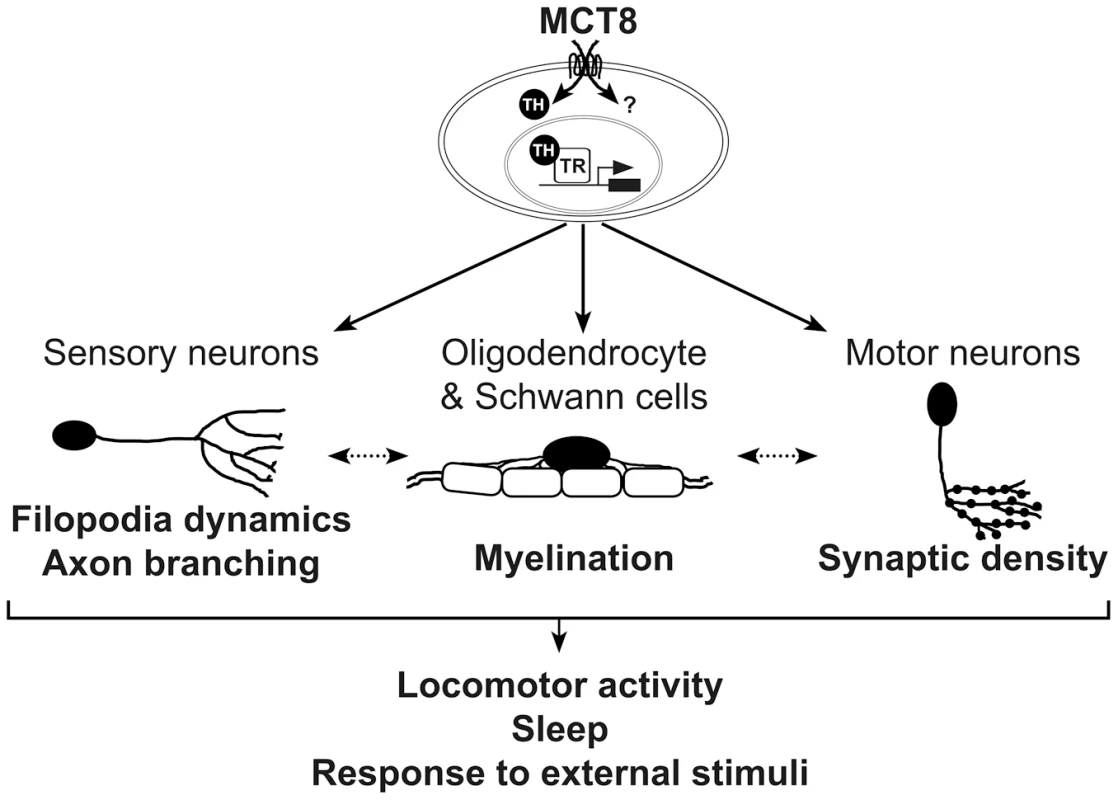
In zebrafish, MCT8 transports TH across the membrane of oligodendrocytes, Schwann cells, and neurons, and regulates gene expression through TRs. Other MCT8-dependent pathways can affect neuronal development, primarily at early embryonic developmental stages (represented by question mark). Loss of MCT8 affects myelination, filopodia dynamics, axon branching, and synaptic density. The deficiencies in axon processes and synapse numbers impaired the number of mature myelinated axons, or alternatively, deficiency in the development of glial cells alters filopodia dynamics, axon branching, and synaptic density (dotted arrow). The combined neural deficiencies alter behavioral performance, including locomotor activity, sleep and response to external stimuli. A portion of these MCT8-dependent deficiencies including the expression of myelin-related gene and axon outgrowth can be recovered by TH analogs. Similar mechanism may be applied in AHDS patients. Clinical observations showed that delayed myelination is a prominent feature in AHDS [28]–[30], [77], [78]. Loss of myelin sheaths produces a wide variety of neurological symptoms, including the slow progression of action potential and deficient axon elaboration [79]. TH signaling is known to promote oligodendrocyte development and myelin production, thus, lower myelination is a key phenotype in the hypothyroid brain [80]–[83]. We therefore sought to examine the effect of MCT8 deficiency on the expression levels of oligo2, p0, and mbp, as well as on tissue distribution of glial cells in developing embryos. The zebrafish CNS is rich in oligodendrocytes, which express orthologs of mammalian genes involved in myelin formation, such as olig2, p0, and mbp. We found that the expression of these myelin-related genes was altered in mct8−/ − embryos. These results suggest that loss of MCT8 enriched the number of neural precursor cells and delayed the development of mature oligodendrocytes and Schwann cells, and consequently, myelination. The mechanisms by which MCT8 regulates myelination could be explained by either direct effect on myelin-related genes, which results in impaired oligodendrocyte function, or indirect effect on axon maturation and processing that reduces the number of potentially myelinated axons [82], [84]. These MCT8-dependent myelin processes are likely partially regulated by TH signaling because TRs binding sites are located on the promoters of all three tested genes, and because TH induced the expression of p0 in WT-sibling but not in mct8−/ − embryos (Fig. 9).
Taking into account the deficiencies in behavioral performance and that deficient myelination affects axon processing [85], we examined the formation of specific neuronal circuits in mct8−/ − larvae. In zebrafish, like in other vertebrates, axons branch dynamically throughout pathfinding; branches are added and eliminated, and successive branches typically project toward the target zone [86]. In previous work, we used MO knockdown strategy to show that the transient reduction of MCT8 alters the organization of neural cells in the brain and spinal cord. In severe cases of MO-injected embryos, the morphology of the larvae was abnormal [20]. Similarly, the mct8−/ − larvae demonstrated altered neuronal development, but the general morphology was normal and the larvae were viable and fertile. This discrepancy is probably because of unspecific toxicity associated with the use of MO [87], which was undetectable in the ZFN-mediated stable mutant, further strengthening the use of the mutant methodological approach. Here, transgenesis and live-imaging enabled us to label specific sensory and motor neurons and measure different parameters of their arbor processes. Interestingly, we found that total arbor length was reduced because the number of mature branches was decreased. The reduction in the number of branches was linked to reduction in the number of filopodia. To understand the dynamics of filopodial plasticity, we performed time-lapse live imaging of single RB axon arbors. We found that the growth and branching of an axon arbor occurred by an iterative sequence of filopodial formation and elimination in both mct8−/ − and WT-sibling embryos. Importantly, the rate of filopodial turnover in the mct8−/ − was significantly reduced. Furthermore, filopodia number is increased under T3 administration. These results suggest that elimination of MCT8 stabilized filopodia dynamics, potentially through reduction in TH levels, which results in a reduced number of mature branches. Interestingly, the behavior and dynamics of axon growth in other zebrafish neuronal circuits, such as in the optic tectum, are reminiscent of the processes in RB axons [88], [89]. Thus MCT8 is likely involved in the mechanism that regulates axon arborization in the brain and spinal cord.
Ataxia and deficiencies in locomotor activity are prominent symptoms of AHDS patients [24], [90]. We therefore speculated that neuronal-circuit deficiencies occurred in the neuromuscular junction, particularly alterations in synaptic formation and plasticity. Using the presynaptic marker SYP-EGFP and live imaging, we quantified the synapse number. These experiments revealed reduction in synaptic density in the axons of the motor neurons. Synaptic morphology and number are closely linked to circuit function, and many psychiatric and neurological disorders, such as fragile X syndrome, are accompanied by alterations in synaptic connections [91], [92]. Hence, we suggest that reduced synaptic density in the motor neurons affects downstream behavioral performance of mct8−/ − larvae, and potentially also human patients. The mechanism by which MCT8 regulates synaptic density may be mediated by TH, which controls the number and activity of synapses [93], [94]. These findings reveal a critical role of MCT8 in the regulation of synaptic density in motor neurons, and can have important implications for understanding behavioral abnormalities in AHDS patients.
The altered expression of myelin genes and deficient circuit formations could be the cause for the altered behavioral performance of mct8−/ − larvae, and possibly also in humans. Indeed, AHDS patients exhibit severe hypotonia and develop a permanent severe mental and motor retardation, demonstrated by the inability to speak and walk independently [24], [90]. High-throughput video-tracking behavioral systems were used to show that the locomotor activity of mct8−/ − larvae was reduced during both day and night. This reduction is partially because of their inability to reach maximum velocity. In addition, mct8−/ − larvae demonstrate deficiency in their response to external light and dark stimuli. An intriguing explanation for this might involve both the motor and sensory neurons that regulate baseline locomotor activity and behavioral state transitions [95]. While reduced synaptic density in the motor neurons might inhibit baseline locomotor activity, altered formation of axon arbors in sensory neurons, such as RB neurons, might affect behavioral-state transitions. However, it is likely that other neurons, such as Mauthner cells [96], are also affected by MCT8 elimination and contribute to the behavioral deficiencies. Considering the diverse neurological and behavioral deficiencies exhibited by the mct8−/ − larvae, we suggest that MCT8 mediates locomotor activity and the response to external stimuli via regulation of neuronal processing and synaptogenesis in specific circuits (Fig. 9).
The deficiencies in locomotor activity during both day and night raised the possibility that these larvae also exhibit sleep difficulties. The zebrafish is a diurnal vertebrate that exhibits neurological and behavioral characteristics of mammalian sleep and wakefulness [51], [55]–[58], [97]. Here, we found that loss of MCT8 increased sleep time and the number and length of sleep episodes during both day and night. This phenotype was robust and might also be present in other models for MCT8 deficiency. To date, there has been no report that characterized the sleep pattern in AHDS patients. However, this issue was occasionally raised by the families of patients (unpublished results) and should be monitored routinely across the lifespans of patients.
The options for therapeutic treatment for AHDS patients are limited. Application of treatment immediately after birth and even earlier, during pregnancy, is expected to best prevent neurological deficiencies. To date, treatments have attempted to normalize serum TH levels. It is not anticipated that TH treatment will help because in the absence of MCT8, TH transport into the brain is impeded. A promising approach is the use of TH analogs that can activate TH receptors but are not dependent on MCT8 for cellular entry [46], [47]. Indeed, DITPA has been tested in adult MCT8-KO mouse and administered to several AHDS patients [49], [50]. In both rodents and humans, the peripheral state of hyperthyroidism improved. However, DITPA treatment did not lead to a significant improvement of neurological parameters in patients. This can be explained by the relatively advanced age of the patients at the beginning of the treatment or an alternative TH-independent role for MCT8. Other therapeutic options for AHDS patients are the TH analogs TETRAC and TRIAC. Recently, assays on MCT8-KO and double MCT8/PAX8-KO mice demonstrated the potency of TETRAC in replacing TH during brain development [48]. These pilot studies are promising; however, it is still unclear to what extent the three analogs can replace TH during all stages of brain development and, importantly, direct comparison of the drugs in the same animal model that exhibits neurological deficiencies, was not performed. Here, we administered TH and TH analogs to mct8−/ − embryos. Zebrafish provide an attractive system for high-throughput pharmacological screens because they can be easily treated with diverse drug concentrations at different time points, ranging from one-cell-stage to fully developed larvae. We found that at 3 dpf, all three TH analogs recovered the expression of p0, a key myelin-related gene, in mct8−/ − embryos. In contrast, TH administration did not restore the expression of p0. These results suggest that myelin-related deficiencies can be treated using TH analogs. Further research is needed to pinpoint the most advanced stage in development that enables efficient treatment. In addition, these pharmacological studies should be expanded to include more drugs and targeted phenotypes. Indeed, we also found that TRIAC positively affect axon outgrowth in RB neurons. This type of experiments, together with the overexpression of candidate genes, such as p0, that might bypass specific deficient neurological pathways, will not only advance our understanding of the mechanism of the disorder but might also provide future pharmacological and gene-therapy approaches to treat psychomotor retardation.
Our study demonstrates the feasibility of monitoring myelin-related processes, structural synaptic plasticity, behavior, and the development of single axon arbors longitudinally in live MCT8-deficient animals, with application to the study of AHDS and other psychomotor retardation disorders. We found that the neurological deficiencies diverge and are circuit-specific. They are probably not unique to neurons in the spinal cord, and additional live-imaging experiments within the transparent zebrafish brain are required to elucidate specific altered brain circuits. The neurological alterations are associated with reduced locomotor activity, altered locomotor response, and increased sleep. The cellular mechanism that regulates these neurological and behavioral deficiencies is likely involved the transport of TH, but other functions of MCT8 cannot be ruled out, particularly at early stages of development. In future studies, there is a need to further evaluate the role of TH signaling in specific tissues and during late developmental stages in mct8−/− larvae. The recently developed genome editing approaches including ZFN, transcription activator-like effector nucleases (TALENs) and clustered regulatory interspaced short palindromic repeat (CRISPR)/Cas9, which became a straightforward technology in zebrafish [98] will enable productive study on the function of TH-transporters and AHDS in late developmental stages and in adults. In addition, an important future direction is to understand the genetic, neuroanatomical and behavioral similarities and variations between zebrafish and mammals. The recent development of MCT8/OATP1C1 double KO mice, which demonstrated neurological and behavioral abnormalities [99] provides the opportunity to study the mechanisms underlying AHDS in two vertebrate models. Translating these findings into comparatively large-scale pharmacological screens, gene therapy and stem-cell therapy will hopefully lead to the finding of a suitable treatment for AHDS and other psychomotor retardation disorders.
Materials and Methods
Zebrafish husbandry
Adult zebrafish were raised and maintained in fully automated zebrafish housing systems (Aquazone, Israel; temperature 28±0.5°C, pH 7.0, conductivity 300 µS) under 14 h light/10 h dark cycles, and fed twice a day. Embryos were produced by natural spawning and raised in egg-water containing methylene blue (0.3 ppm) in a light-controlled incubator at 28±0.5°C, as previously described [51]. All animal protocols were reviewed and approved by the Bar-Ilan University Bioethics Committee.
Establishment of mct8 mutant line
Custom-designed ZFN plasmids and mRNA were commercially synthesized (Sigma-Aldrich, St. Louis, MO) to target a HaeII restriction site located in the first exon of the zebrafish mct8 gene. Each ZFN array was designed to recognize 15 bp sequence upstream and downstream to the HaeII restriction site (5′-CGGCCACGCCGCCTGgcgctcACCCTGGACAAGGCT-3′, Fig. 1A). Approximately 100 ng/µl of each ZFN mRNA was co-injected into one-cell-stage WT embryos. These mosaic embryos were raised to adulthood and out-crossed with WT fish in order to identify F0 founder fish. F1 heterozygous fish, which carry a 7 bp deletion mutation in the targeted site, was selected and out-crossed with WT fish. The F2 heterozygous progeny were inter-crossed to generate the homozygous mct8−/− line (Fig. 1A, B).
Mutation screens and genotyping were conducted as follows: genomic DNA was extracted from 1 dpf embryos or a clipped tail fin of adult fish, using the KAPA express extract Kit (Kapa Biosystems Inc., Boston, MA) according to the manufacturer's instructions. Genomic DNA was then amplified by PCR using the following primers: 5′-gaggagttcgaggagcagga-3′ and 5′ - caccagcatcatgtgcagaa-3′, and the 234 bp PCR product was digested with HaeII restriction enzyme. A digested PCR product was then run on 2% agarose gel. While complete digestion of WT DNA resulted in two short fragments of 104 bp and 130 bp, 234 bp PCR product was shown in mct8−/− fish, confirming the introduction of the mutation at the target site. When needed, this mutated DNA fragment was sequenced to confirm the presence of the 7 bp deletion. Heterozygous fish show three DNA fragments, indicating the presence of both mutated and WT mct8 alleles (Fig. 1C).
Cell culture and transient transfection
HEK293T Cell line were grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% heat-inactivated fetal bovine serum (FBS) and 1% nonessential amino acids (Biological Industries, Beth Haemek, Israel), and incubated at 37°C and 5% CO2. HEK293T cells were transfected with 10 µg of either pCS-cmv:MCT8-EGFP, pCS-cmv:MCT8mut-EGFP or pCS2-cmv:EGFP DNA constructs using the calcium phosphate method. The culture medium was changed 6 h after transfection and cells were harvested 24 h later.
Western blotting
Cells were lysed with RIPA buffer (20 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 0.5% sodium deoxycholate, 2 mM Na3VO4, 1 mM NaF and 10 mM β-glycerophosphate) and supplemented with complete protease inhibitor cocktail (Roche Applied Science, Penzberg, Germany). Lysates were incubated on ice for 20 min and centrifuged at max speed for 10 minutes at 4°C and the supernatants were obtained. Protein concentration was measured by Bradford analysis. A total of 30 µg protein extract per lane were separated on 10% SDS polyacrylamide gel. After electrophoresis, proteins were transferred to nitrocellulose membrane (BIO-RAD, Hercules, CA, USA) and membrane was blocked for 1 h with 5% skim milk in PBST. Next, the blots were incubated for 1 h at room temperature with 5% skim milk in PBST containing anti-GFP primary antibody (GFP (B-2): sc-9996, Santa Cruz Biotechnology, Dallas, TX, USA), diluted 1∶1000. Following three washes, membranes were incubated at room temperature for 1 hr with 5% skim milk in PBST containing the secondary antibody (goat anti-mouse IgG-HRP: sc-2005, Santa Cruz Biotechnology, Dallas, TX, USA), diluted 1∶4000. Signals were visualized by SuperSignal West Pico Chemiluminescent Substrate according to the manufacturer instructions (Thermo Fisher Scientific, Waltham, MA, USA).
Transgenic lines
Establishment of the tg(uas:SYP-EGFP) stable transgenic line was conducted using the Tol2 system [100]. In order to prepare the pT2-uas:SYP-EGFP construct, the upstream activation sequence (uas, kind gift of Prof. Philippe Mourrain, Stanford University) was double-digested with StuI and EcoRI, and ligated into a StuI/EcoRI-digested pT2-hcrt:SYP-EGFP vector [17], replacing the hcrt promoter.
To generate tg(huc:GAL4Xuas:memYFP) transgenic line on the background of mct8 mutation, tg(huc:GAL4Xuas:memYFP) transgenic line (kindly provided by Dr. Bettina Schmid, Ludwig-Maximilians University Munich, Germany) was crossed with mct8−/− zebrafish. Next, Transgenic tg(huc:GAL4Xuas:memYFP)/mct8+/− and mct8−/− zebrafish were crossed to produce the tg(huc:GAL4Xuas:memYFP)/mct8−/− and tg(huc:GAL4Xuas:memYFP)/mct8+/− lines.
To generate the tg(mct8:GAL4)/tg(uas:SYP-EGFP) double transgenic line on the background of the mct8 mutation, tg(mct8:GAL4) [20] and tg(uas:SYP-EGFP) were independently out-crossed with mct8−/− zebrafish. The resulting tg(mct8:GAL4)/mct8+/− and tg(uas:SYP-EGFP)/mct8+/− lines were crossed to produce the tg(mct8:GAL4)/tg(uas:SYP-EGFP)/mct8−/− and tg(mct8:GAL4)/tg(uas:SYP-EGFP)/mct8+/− fish lines.
DNA constructs and transient expression assays
Transient expression assays of the following DNA constructs, pT2-huc:Gal4-VP16 (kind gift of Prof. Thomas Misgeld, Technical University Munich, Germany), pT2-uas:tRFP (kind gift of Dr. Gordon Wang, Stanford University), uas:memYFP (kindly provided by Prof. Thomas Misgeld, Technical University Munich, Germany) and pT2-uas:SYP-EGFP were performed by microinjection of approximately 2 nl into one-cell-stage zebrafish zygotes, at a concentration of 20 ng/µl each, using micromanipulator and PV830 Pneumatic Pico Pump (World Precision Instruments, Sarasota, FL).
To prepare probes for whole mount ISH experiments, DNA fragments containing the coding region of the following genes: klf9 (NM_001128729.1), nrgna (ENSDART00000057910), mbp (AY860977.1), olig2 (NM_178100.1) and p0 (NM_194361.2) were PCR-amplified using the following primers: klf9: 5′ - atgacggacgtagatattgcagc-3′ and 5′-ttaaacaccagcagacatatg-3′; nrgna: 5′-atggactgtcgaaacgaagg-3′ and 5′-ctacttcggctcgcgttg-3′; mbp: 5′-atggccactgcaagcacctc-3′ and 5′tcagaagatggtgctccagcg-3′; p0: 5′-atgctgtccgtactggcact-3′ and 5′-tcagatacgctgtttttgctg-3′; olig2: 5′-atggactctgacacgagccgagtgt-3′ and 5′-tttgagtcactggtcagccgt-3′. All PCR products were cloned into a pCRII-TOPO vector (Invitrogen, Carlsbad, CA), linearized by NotI and served as a template to transcribe digoxigenin-labeled anti-sense RNA probes.
To prepare fusion constructs, WT and mutated mct8 coding sequences were amplified by PCR using the following primers, 5′-taaccggaattccgccaccatgcactcggaaagcgat-3′ and 5′-taaccgaccggttatgtgtgtctccatgtccg-3′, and subcloned into pCS2-cmv:EGFP vector [101] using EcoRI and AgeI. The resulting pCS2-cmv:MCT8-EGFP and pCS2-cmv:MCT8mut-EGFP fusion constructs and the pCS2-cmv:EGFP vector were linearized with XbaI restriction enzyme, and in vitro transcribed using the mMESSAGE mMACHINE SP6 kit (Ambion Inc., Austin, TX). Approximately 2 nl of 100 ng/µl mRNAs were microinjected into fertilized one-cell-stage embryo.
To prepare mct8 mRNA in vitro, the mct8 full coding sequence was PCR-amplified using a platinum taq DNA polymerase (Life Technologies, Grand Island, NY) and the following primers: 5′-cgcggatccatgcactcggaaagcgatgacaac-3′ and 5′-cgcactagttcatatgtgtgtctccatgtccgtg-3′. The PCR product was subcloned into pCS-TP vector [100] using BamHI and SpeI restriction enzymes. Following linearization with NotI, mct8 mRNA was in vitro transcribed using the mMESSAGE mMACHINE SP6 kit (Ambion Inc., Austin, TX), according to the manufacturer's instructions. Rescue experiments were conducted by the injection of approximately 2 nl volume of in vitro transcribed mct8 mRNA (100 ng/µl) to one-cell stage mct8−/− embryos.
Whole-mount ISH and immunohistochemistry assays
In both whole mount ISH and immunohistochemistry experiments, embryos and larvae were fixed in 4% paraformaldehyde overnight at 4°C, washed in PBST, and stored in 100% methanol. The location and level of mRNA expression were detected by whole-mount ISH, as described [51], [68]. Digoxigenin-labeled anti-sense RNA probes for klf9, nrgna, mbp, olig2 and p0 were generated from the vector templates described above using DIG RNA labeling kit (Roche, Indianapolis, IN), according to the manufacturer's instructions. Digoxigenin-labeled anti-sense RNA probes for myoD and p2rx3.1 are the same as those described previously [20], [68]. All probes were used at a concentration of 0.5–1 ng/µl.
For double fluorescence ISH, fluorescein-labeled anti-sense RNA probe for mct8 was transcribed from the vector template previously described [20], using Fluorescein RNA labeling kit (Roche, Indianapolis, IN). Digoxigenin-labeled p2rx3.1 and fluorescein-labeled mct8 anti-sense RNA probes (2 ng/µl) were simultaneously hybridized. Next, anti-fluorescein-POD antibody (Roche, Indianapolis, IN), diluted 1∶250, and anti-digoxigenin-AP antibody (Roche, Indianapolis, IN), diluted 1∶2500, were simultaneously incubated over-night at 4°C. p2rx3.1 mRNA was visualized using TSA Plus Fluorescein System (Perkin-Elmer, Waltham, MA). mct8 mRNA was subsequently visualized by an enzymatic reaction using Fast Red substrate (Roche, Indianapolis, IN).
Whole mount immunohistochemistry was carried out as previously described [20], using primary anti-MyHC for slow muscles antibody (F59, DSHB, USA; kind gift of Alon Daya and Prof. Stella Mitrani-Rosenbaum, Hebrew University of Jerusalem, Israel), diluted 1∶10, and a secondary goat anti-mouse Alexa Fluor 488 IgG (H+L) antibody (A-11029, Invitrogen, Carlsbad, CA), diluted 1∶250.
For double whole mount fluorescence immunohistochimestry-ISH labeling, ISH was conducted using Digoxigenin-labeled p2rx3.1 and p0 anti-sense RNA probes (2 ng/µl), following by detection using Fast Red substrate (Roche, Indianapolis, IN). Prior to counterstaining, larvae were washed 5 times in PBST, blocked with 20% goat serum diluted in PBST for 1 h at room temperature and incubated with rabbit anti-EGFP (SC-8334, Santa Cruz Biotechnology, Santa Cruz, CA) primer antibody, diluted 1∶250, in blocking buffer overnight at 4°C. Next, larvae were washed in PBST and blocked for 1 h. Anti-GFP antibodies were detected with a secondary goat anti-rabbit Alexa Fluor 488 IgG (H+L) antibody (A-11008, Invitrogen, Carlsbad, CA), diluted 1∶250.
Real-time PCR quantification assays
Relative mRNA quantification of klf9, nrgna, tsh, trh, dio1, dio2, dio3, mct10, oatp1c1, thraa, thrab, thrb, olig2, mbp and p0 was determined using qRT-PCR. Total RNA was extracted from 3 dpf embryos using the Direct-zol RNA MiniPrep kit (Zymo Research Corporation, Irvine, CA), according to the manufacturer's instructions. For each tested gene, a total of 5–15 biological samples were used. Each biological sample was contained a pool of 8–17 embryos. mRNA (1 µg) was reverse-transcribed using qScript cDNA SuperMix (Quanta BioSciences, Gaithersburg, MD). Relative transcript levels were determined by the 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). Triplicates of each cDNA sample were PCR-amplified using the PerfeCTa SYBR Green FastMix (Quanta BioSciences, Gaithersburg, MD) and the following specific primers: klf9: 5′-cgtttcatgtgcccactttg-3′ and 5′-tgtggatgttgaagagtgtcg-3′; nrgna: 5′-tggactgtcgaaacgaagg-3′ and 5′-accagcttgaatcttagcgg-3′; tsh: 5′-cccactgactacaccatctac-3′ and 5′-catcccctctgaacaataaaacg-3′; trh: 5′-gcagacccacagcatcag-3′ and 5′-caggccaagacgaacaca-3′; dio1: 5′-tgctttaattaccctggaccg-3′ and 5′-tgctgaagtccttgacaagc-3′; dio2: 5′-tggatgcctacaaacaggtg-3′ and 5′-ggcgatcaggagactcaaag-3′; dio3: 5′-ccagaagctggacttcttca-3′ and 5′-aagttgaggatcagcggtct-3′; mct10: 5′-tgtaacggctcggtgttc-3′ and 5′-aagatcatgcccatcgacag-3′; oatp1c1: 5′-tcatctcccaaggaaaacgag-3′ and 5′-agaaataggcgaaggacaagg-3′; thraa: 5′-ctgatgccatctttgatttggg-3′ and 5′-gtacatctcctgacacttctcg-3′; thrab: 5′-tctgatgccatcttcgacttg-3′ and 5′-gtacatctcctggcacttctc-3′; thrb: 5′-gctctggctcttatgacatgg-3′ and 5′-tcgctgatatctcgtgctttg-3′; olig2: 5′-cgagtgaactggaatagccttac-3′ and 5′-gctcgtgtcagagtccatg-3′; mbp: 5′-gaggagacaagaagagaaaggg -3′ and 5′-gaaatgcacgacagggttg-3′; p0: 5′-acctgtgatgccaagaacc-3′ and 5′-ttgccacaacgaggatca-3′; and β-actin: 5′-tgaatcccaaagccaacagag-3′ and 5′-ccagagtccatcacaataccag-3′. The relative quantification of each gene expression was normalized against β-actin mRNA expression levels and subjected to the ΔΔCT method [51].
Pharmacological assays
In all pharmacological assays one-cell stage embryos were placed in glass Petri dishes (50–80 embryos per dish) containing either a specific drug or 5×10−6 M NaOH diluted in zebrafish water for control groups. The exposure medium (25 ml per dish) was exchanged twice a day. Stock solutions of 100 µM T3, T4, TETRAC, TRIAC (Sigma-Aldrich, St. Louis, MO) and DITPA (Santa Cruz Biotechnology, TX), were prepared in 0.05 M–0.1 M NaOH and diluted in zebrafish water to the final administered concentrations. In order to choose the appropriate working dilution for each substance, a preliminary dose-dependent assay was performed using WT embryos. Four to five different concentrations in the range of 0.5 nM to 100 nM were tested for each substance. In addition, a control group of embryos was raised in 5×10−5 M NaOH, the highest NaOH concentration applied to the treated groups. During the experiments, embryos were screened for morphological developmental abnormalities, such as distorted body shape, pigmentation defects, and movement disabilities. The highest substance concentration that lacked morphological defects (0.5 nM for T3, T4, TETRAC, TRIAC and 5 nM for DITPA) was chosen as the working dilution for all pharmacological experiments.
Bioinformatical promoter analyses
Sequences of 7, 5, and 2 kb upstream to the 5′ UTR of olig2 [102], p0 [103], and mbp [104] genes, were analyzed using the University of California, Santa Cruz (UCSC) Genome Browser website. The prediction of TREs within the putative promoters was performed using the RXRA::VDR matrix of the Jaspar database tool (http://jaspar.genereg.net/). In general, TREs were identified as two or more hexamer (A/G)GGT(C/A)A consensus sequences arranged in tandem arrays [39], [40]. One putative site was predicted in the p0 and mbp promoters, and three putative sites were predicted in the oligo2 promoter; the one site that demonstrated the highest similarity to the consensus sequence was chosen.
Behavioral assays
At 6 dpf, mct8−/ − larvae and their WT siblings were placed, individually, in 48-well plates under 14 h light/10 h dark cycles. Larva-containing plates were placed in the Noldus DanioVision tracking system (Noldus Information Technology, Wageningen, Netherlands) and acclimated for one hour prior to activity recording. Light intensity in the tracking system was 70 LUX (25% in the operating software) for all experiments. To monitor rhythmic activity during a daily cycle, larvae were maintained under the same light-dark regime prior to the experiment. To monitor responses to light/dark transitions, larvae were subjected to 3 intervals of 30 min light/30 min darkness. For each experiment, live video-tracking and analysis were conducted for 3 independent assays, using the EthoVision XT 9 software (Noldus Information Technology, Wageningen, Netherlands), as previously described [51]. Data analyses of total activity, velocity and sleep were performed according to the threshold parameters described previously [51].
Imaging
Whole mount ISH-stained larvae were placed in 100% glycerol, and imaged from dorsal or lateral view using an epifluorescence stereomicroscope (Leica M165FC). Pictures were taken using Leica Application Suite imaging software version 3.7 (Leica, Wetzlar, Germany). In live imaging experiments, embryos and larvae were anesthetized with Tricaine (0.01%) and placed in low-melting-point agarose (1.0–2.0%) in a specially designed chamber filled with egg-water. Similar mounting protocol was used to image fixed embryos subjected to fluorescence ISH or immunohistochemistry. Confocal imaging was performed using a Zeiss LSM710 upright confocal microscope (Zeiss, Oberkochen, Germany). All images were processed using ImageJ (National Institutes of Health, Bethesda, MD) and Adobe Photoshop (San Jose, CA) software.
Calculation of total arbor length, number of branches, average branching length, and the number of filopodian branches in axon arbors of single motor neuron and RB neurons was performed using NeuronJ plugin in ImageJ software (National Institutes of Health, Bethesda, MD). Synaptic density was calculated by quantification of synapse number per 50 µm in the axonal arbor of single motor neuron or RB neurons, using ImageJ software (National Institutes of Health, Bethesda, MD).
In the time-lapse experiments, embryos were placed in a specially designed chamber with constant egg-water flow at a temperature of 28±0.5°C. Automatic imaging of several embryos was performed simultaneously using the ZEN 2011 software (Zeiss, Oberkochen, Germany), and up to 40 optical sections of 2 µm each were obtained for each embryo every 15 min, during 2.5 h.
Supporting Information
Zdroje
1. KaufmannWE, MoserHW (2000) Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex N Y N 1991 10 : 981–991.
2. RamockiMB, ZoghbiHY (2008) Failure of neuronal homeostasis results in common neuropsychiatric phenotypes. Nature 455 : 912–918 doi:10.1038/nature07457
3. BrockmannK, DumitrescuAM, BestTT, HanefeldF, RefetoffS (2005) X-linked paroxysmal dyskinesia and severe global retardation caused by defective MCT8 gene. J Neurol 252 : 663–666 doi:10.1007/s00415-005-0713-3
4. FriesemaECH, GruetersA, BiebermannH, KrudeH, von MoersA, et al. (2004) Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet 364 : 1435–1437 doi:10.1016/S0140-6736(04)17226-7
5. YenPM (2001) Physiological and molecular basis of thyroid hormone action. Physiol Rev 81 : 1097–1142.
6. PizzagalliF, HagenbuchB, StiegerB, KlenkU, FolkersG, et al. (2002) Identification of a novel human organic anion transporting polypeptide as a high affinity thyroxine transporter. Mol Endocrinol Baltim Md 16 : 2283–2296 doi:10.1210/me.2001-0309
7. RobertsLM, WoodfordK, ZhouM, BlackDS, HaggertyJE, et al. (2008) Expression of the thyroid hormone transporters monocarboxylate transporter-8 (SLC16A2) and organic ion transporter-14 (SLCO1C1) at the blood-brain barrier. Endocrinology 149 : 6251–6261 doi:10.1210/en.2008-0378
8. FriesemaECH, VisserTJ, BorgersAJ, KalsbeekA, SwaabDF, et al. (2012) Thyroid hormone transporters and deiodinases in the developing human hypothalamus. Eur J Endocrinol Eur Fed Endocr Soc 167 : 379–386 doi:10.1530/EJE-12-0177
9. DumitrescuAM, LiaoX-H, WeissRE, MillenK, RefetoffS (2006) Tissue-specific thyroid hormone deprivation and excess in monocarboxylate transporter (mct) 8-deficient mice. Endocrinology 147 : 4036–4043 doi:10.1210/en.2006-0390
10. TrajkovicM, VisserTJ, MittagJ, HornS, LukasJ, et al. (2007) Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J Clin Invest 117 : 627–635 doi:10.1172/JCI28253
11. Di CosmoC, LiaoX-H, YeH, FerraraAM, WeissRE, et al. (2013) Mct8-deficient mice have increased energy expenditure and reduced fat mass that is abrogated by normalization of serum t3 levels. Endocrinology 154 : 4885–4895 doi:10.1210/en.2013-1150
12. RodriguesTB, CeballosA, Grijota-MartínezC, NuñezB, RefetoffS, et al. (2013) Increased oxidative metabolism and neurotransmitter cycling in the brain of mice lacking the thyroid hormone transporter SLC16A2 (MCT8). PloS One 8: e74621 doi:10.1371/journal.pone.0074621
13. MayerlS, VisserTJ, DarrasVM, HornS, HeuerH (2012) Impact of Oatp1c1 deficiency on thyroid hormone metabolism and action in the mouse brain. Endocrinology 153 : 1528–1537 doi:10.1210/en.2011-1633
14. ItoK, UchidaY, OhtsukiS, AizawaS, KawakamiH, et al. (2011) Quantitative membrane protein expression at the blood-brain barrier of adult and younger cynomolgus monkeys. J Pharm Sci 100 : 3939–3950 doi:10.1002/jps.22487
15. MeyerMP, SmithSJ (2006) Evidence from in vivo imaging that synaptogenesis guides the growth and branching of axonal arbors by two distinct mechanisms. J Neurosci Off J Soc Neurosci 26 : 3604–3614 doi:10.1523/JNEUROSCI.0223-06.2006
16. NaumannEA, KampffAR, ProberDA, SchierAF, EngertF (2010) Monitoring neural activity with bioluminescence during natural behavior. Nat Neurosci 13 : 513–520 doi:10.1038/nn.2518
17. AppelbaumL, WangG, YokogawaT, SkariahGM, SmithSJ, et al. (2010) Circadian and homeostatic regulation of structural synaptic plasticity in hypocretin neurons. Neuron 68 : 87–98 doi:10.1016/j.neuron.2010.09.006
18. HeijlenM, HoubrechtsAM, DarrasVM (2013) Zebrafish as a model to study peripheral thyroid hormone metabolism in vertebrate development. Gen Comp Endocrinol 188 : 289–296 doi:10.1016/j.ygcen.2013.04.004
19. TamplinOJ, WhiteRM, JingL, KaufmanCK, LacadieSA, et al. (2012) Small molecule screening in zebrafish: swimming in potential drug therapies. Wiley Interdiscip Rev Dev Biol 1 : 459–468 doi:10.1002/wdev.37
20. VatineGD, ZadaD, Lerer-GoldshteinT, TovinA, MalkinsonG, et al. (2013) Zebrafish as a model for monocarboxyl transporter 8-deficiency. J Biol Chem 288 : 169–180 doi:10.1074/jbc.M112.413831
21. ArjonaFJ, de VriezeE, VisserTJ, FlikG, KlarenPHM (2011) Identification and functional characterization of zebrafish solute carrier Slc16a2 (Mct8) as a thyroid hormone membrane transporter. Endocrinology 152 : 5065–5073 doi:10.1210/en.2011-1166
22. FerraraAM, LiaoX-H, Gil-IbáñezP, MarcinkowskiT, BernalJ, et al. (2013) Changes in thyroid status during perinatal development of MCT8-deficient male mice. Endocrinology 154 : 2533–2541 doi:10.1210/en.2012-2031
23. TreichelD, BeckerMB, GrussP (2001) The novel transcription factor gene Sp5 exhibits a dynamic and highly restricted expression pattern during mouse embryogenesis. Mech Dev 101 : 175–179.
24. FriesemaECH, JansenJ, JachtenbergJ-W, VisserWE, KesterMHA, et al. (2008) Effective cellular uptake and efflux of thyroid hormone by human monocarboxylate transporter 10. Mol Endocrinol Baltim Md 22 : 1357–1369 doi:10.1210/me.2007-0112
25. KersseboomS, KremersG-J, FriesemaECH, VisserWE, KlootwijkW, et al. (2013) Mutations in MCT8 in patients with Allan-Herndon-Dudley-syndrome affecting its cellular distribution. Mol Endocrinol Baltim Md 27 : 801–813 doi:10.1210/me.2012-1356
26. Trajkovic-ArsicM, MüllerJ, DarrasVM, GrobaC, LeeS, et al. (2010) Impact of monocarboxylate transporter-8 deficiency on the hypothalamus-pituitary-thyroid axis in mice. Endocrinology 151 : 5053–5062 doi:10.1210/en.2010-0593
27. DarrasVM, Van HerckSLJ, HeijlenM, De GroefB (2011) Thyroid hormone receptors in two model species for vertebrate embryonic development: chicken and zebrafish. J Thyroid Res 2011 : 402320 doi:10.4061/2011/402320
28. GikaAD, SiddiquiA, HulseAJ, EdwardS, FallonP, et al. (2010) White matter abnormalities and dystonic motor disorder associated with mutations in the SLC16A2 gene. Dev Med Child Neurol 52 : 475–482 doi:10.1111/j.1469-8749.2009.03471.x
29. TondutiD, VanderverA, BerardinelliA, SchmidtJL, CollinsCD, et al. (2013) MCT8 deficiency: extrapyramidal symptoms and delayed myelination as prominent features. J Child Neurol 28 : 795–800 doi:10.1177/0883073812450944
30. HoldenKR, ZuñigaOF, MayMM, SuH, MolineroMR, et al. (2005) X-linked MCT8 gene mutations: characterization of the pediatric neurologic phenotype. J Child Neurol 20 : 852–857.
31. BarresBA, LazarMA, RaffMC (1994) A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Dev Camb Engl 120 : 1097–1108.
32. CalzaL, FernandezM, GiulianiA, AloeL, GiardinoL (2002) Thyroid hormone activates oligodendrocyte precursors and increases a myelin-forming protein and NGF content in the spinal cord during experimental allergic encephalomyelitis. Proc Natl Acad Sci U S A 99 : 3258–3263 doi:10.1073/pnas.052704499
33. HarsanL-A, SteibelJ, ZarembaA, AginA, SapinR, et al. (2008) Recovery from chronic demyelination by thyroid hormone therapy: myelinogenesis induction and assessment by diffusion tensor magnetic resonance imaging. J Neurosci Off J Soc Neurosci 28 : 14189–14201 doi:10.1523/JNEUROSCI.4453-08.2008
34. KnipperM, BandtlowC, GestwaL, KöpschallI, RohbockK, et al. (1998) Thyroid hormone affects Schwann cell and oligodendrocyte gene expression at the glial transition zone of the VIIIth nerve prior to cochlea function. Dev Camb Engl 125 : 3709–3718.
35. ParkH-C, MehtaA, RichardsonJS, AppelB (2002) olig2 is required for zebrafish primary motor neuron and oligodendrocyte development. Dev Biol 248 : 356–368.
36. PeiranoRI, GoerichDE, RiethmacherD, WegnerM (2000) Protein zero gene expression is regulated by the glial transcription factor Sox10. Mol Cell Biol 20 : 3198–3209.
37. BrösamleC, HalpernME (2002) Characterization of myelination in the developing zebrafish. Glia 39 : 47–57 doi:10.1002/glia.10088
38. ShineHD, ReadheadC, PopkoB, HoodL, SidmanRL (1992) Morphometric analysis of normal, mutant, and transgenic CNS: correlation of myelin basic protein expression to myelinogenesis. J Neurochem 58 : 342–349.
39. JeanninE, RobyrD, DesvergneB (1998) Transcriptional regulatory patterns of the myelin basic protein and malic enzyme genes by the thyroid hormone receptors alpha1 and beta1. J Biol Chem 273 : 24239–24248.
40. MarchandO, SafiR, EscrivaH, Van RompaeyE, PrunetP, et al. (2001) Molecular cloning and characterization of thyroid hormone receptors in teleost fish. J Mol Endocrinol 26 : 51–65.
41. BuckleyCE, MarguerieA, AldertonWK, FranklinRJM (2010) Temporal dynamics of myelination in the zebrafish spinal cord. Glia 58 : 802–812 doi:10.1002/glia.20964
42. FilippiA, JainokC, DrieverW (2012) Analysis of transcriptional codes for zebrafish dopaminergic neurons reveals essential functions of Arx and Isl1 in prethalamic dopaminergic neuron development. Dev Biol 369 : 133–149 doi:10.1016/j.ydbio.2012.06.010
43. SchebestaM, SerlucaFC (2009) olig1 Expression identifies developing oligodendrocytes in zebrafish and requires hedgehog and notch signaling. Dev Dyn Off Publ Am Assoc Anat 238 : 887–898 doi:10.1002/dvdy.21909
44. KazakovaN, LiH, MoraA, JessenKR, MirskyR, et al. (2006) A screen for mutations in zebrafish that affect myelin gene expression in Schwann cells and oligodendrocytes. Dev Biol 297 : 1–13 doi:10.1016/j.ydbio.2006.03.020
45. SchweitzerJ, BeckerT, BeckerCG, SchachnerM (2003) Expression of protein zero is increased in lesioned axon pathways in the central nervous system of adult zebrafish. Glia 41 : 301–317 doi:10.1002/glia.10192
46. MessierN, LangloisMF (2000) Triac regulation of transcription is T(3) receptor isoform - and response element-specific. Mol Cell Endocrinol 165 : 57–66.
47. VerhoevenFA, Van der PuttenHHAGM, HennemannG, LamersJMJ, VisserTJ, et al. (2002) Uptake of triiodothyronine and triiodothyroacetic acid in neonatal rat cardiomyocytes: effects of metabolites and analogs. J Endocrinol 173 : 247–255.
48. HornS, KersseboomS, MayerlS, MüllerJ, GrobaC, et al. (2013) Tetrac can replace thyroid hormone during brain development in mouse mutants deficient in the thyroid hormone transporter mct8. Endocrinology 154 : 968–979 doi:10.1210/en.2012-1628
49. Di CosmoC, LiaoX-H, DumitrescuAM, WeissRE, RefetoffS (2009) A thyroid hormone analog with reduced dependence on the monocarboxylate transporter 8 for tissue transport. Endocrinology 150 : 4450–4458 doi:10.1210/en.2009-0209
50. VergeCF, KonradD, CohenM, Di CosmoC, DumitrescuAM, et al. (2012) Diiodothyropropionic acid (DITPA) in the treatment of MCT8 deficiency. J Clin Endocrinol Metab 97 : 4515–4523 doi:10.1210/jc.2012-2556
51. ElbazI, Yelin-BekermanL, NicenboimJ, VatineG, AppelbaumL (2012) Genetic ablation of hypocretin neurons alters behavioral state transitions in zebrafish. J Neurosci Off J Soc Neurosci 32 : 12961–12972 doi:10.1523/JNEUROSCI.1284-12.2012
52. TovinA, AlonS, Ben-MosheZ, MracekP, VatineG, et al. (2012) Systematic identification of rhythmic genes reveals camk1gb as a new element in the circadian clockwork. PLoS Genet 8: e1003116 doi:10.1371/journal.pgen.1003116
53. EmranF, RihelJ, DowlingJE (2008) A behavioral assay to measure responsiveness of zebrafish to changes in light intensities. J Vis Exp JoVE doi:10.3791/923
54. ZhdanovaIV, WangSY, LeclairOU, DanilovaNP (2001) Melatonin promotes sleep-like state in zebrafish. Brain Res 903 : 263–268.
55. ProberDA, RihelJ, OnahAA, SungR-J, SchierAF (2006) Hypocretin/orexin overexpression induces an insomnia-like phenotype in zebrafish. J Neurosci Off J Soc Neurosci 26 : 13400–13410 doi:10.1523/JNEUROSCI.4332-06.2006
56. YokogawaT, MarinW, FaracoJ, PézeronG, AppelbaumL, et al. (2007) Characterization of sleep in zebrafish and insomnia in hypocretin receptor mutants. PLoS Biol 5: e277 doi:10.1371/journal.pbio.0050277
57. ZhdanovaIV (2011) Sleep and its regulation in zebrafish. Rev Neurosci 22 : 27–36 doi:10.1515/RNS.2011.005
58. ElbazI, FoulkesNS, GothilfY, AppelbaumL (2013) Circadian clocks, rhythmic synaptic plasticity and the sleep-wake cycle in zebrafish. Front Neural Circuits 7 : 9 doi:10.3389/fncir.2013.00009
59. Campos-BarrosA, MusaA, FlechnerA, HesseniusC, GaioU, et al. (1997) Evidence for circadian variations of thyroid hormone concentrations and type II 5′-iodothyronine deiodinase activity in the rat central nervous system. J Neurochem 68 : 795–803.
60. RussellW, HarrisonRF, SmithN, DarzyK, ShaletS, et al. (2008) Free triiodothyronine has a distinct circadian rhythm that is delayed but parallels thyrotropin levels. J Clin Endocrinol Metab 93 : 2300–2306 doi:10.1210/jc.2007-2674
61. DayaA, VatineGD, Becker-CohenM, Tal-GoldbergT, FriedmannA, et al. (2014) Gne depletion during zebrafish development impairs skeletal muscle structure and function. Hum Mol Genet 23 : 3349–3361 doi:10.1093/hmg/ddu045
62. WeinbergES, AllendeML, KellyCS, AbdelhamidA, MurakamiT, et al. (1996) Developmental regulation of zebrafish MyoD in wild-type, no tail and spadetail embryos. Dev Camb Engl 122 : 271–280.
63. HeuerH, VisserTJ (2013) The pathophysiological consequences of thyroid hormone transporter deficiencies: Insights from mouse models. Biochim Biophys Acta 1830 : 3974–3978 doi:10.1016/j.bbagen.2012.04.009
64. PaquetD, BhatR, SydowA, MandelkowE-M, BergS, et al. (2009) A zebrafish model of tauopathy allows in vivo imaging of neuronal cell death and drug evaluation. J Clin Invest 119 : 1382–1395 doi:10.1172/JCI37537
65. PlucińskaG, PaquetD, HruschaA, GodinhoL, HaassC, et al. (2012) In vivo imaging of disease-related mitochondrial dynamics in a vertebrate model system. J Neurosci Off J Soc Neurosci 32 : 16203–16212 doi:10.1523/JNEUROSCI.1327-12.2012
66. WilliamsJA, BarriosA, GatchalianC, RubinL, WilsonSW, et al. (2000) Programmed cell death in zebrafish rohon beard neurons is influenced by TrkC1/NT-3 signaling. Dev Biol 226 : 220–230 doi:10.1006/dbio.2000.9860
67. SagastiA, GuidoMR, RaibleDW, SchierAF (2005) Repulsive interactions shape the morphologies and functional arrangement of zebrafish peripheral sensory arbors. Curr Biol CB 15 : 804–814 doi:10.1016/j.cub.2005.03.048
68. AppelbaumL, SkariahG, MourrainP, MignotE (2007) Comparative expression of p2x receptors and ecto-nucleoside triphosphate diphosphohydrolase 3 in hypocretin and sensory neurons in zebrafish. Brain Res 1174 : 66–75 doi:10.1016/j.brainres.2007.06.103
69. WangF, WolfsonSN, GharibA, SagastiA (2012) LAR receptor tyrosine phosphatases and HSPGs guide peripheral sensory axons to the skin. Curr Biol CB 22 : 373–382 doi:10.1016/j.cub.2012.01.040
70. PorterfieldSP, HendrichCE (1993) The role of thyroid hormones in prenatal and neonatal neurological development–current perspectives. Endocr Rev 14 : 94–106 doi:10.1210/edrv-14-1-94
71. CayrouC, DenverRJ, PuymiratJ (2002) Suppression of the basic transcription element-binding protein in brain neuronal cultures inhibits thyroid hormone-induced neurite branching. Endocrinology 143 : 2242–2249 doi:10.1210/endo.143.6.8856
72. HeuerH, MaierMK, IdenS, MittagJ, FriesemaECH, et al. (2005) The monocarboxylate transporter 8 linked to human psychomotor retardation is highly expressed in thyroid hormone-sensitive neuron populations. Endocrinology 146 : 1701–1706 doi:10.1210/en.2004-1179
73. SchweizerU, KöhrleJ (2013) Function of thyroid hormone transporters in the central nervous system. Biochim Biophys Acta 1830 : 3965–3973 doi:10.1016/j.bbagen.2012.07.015
74. FuJ, RefetoffS, DumitrescuAM (2013) Inherited defects of thyroid hormone-cell-membrane transport: review of recent findings. Curr Opin Endocrinol Diabetes Obes 20 : 434–440 doi:10.1097/01.med.0000432531.03233.ad
75. ElsaliniOA, RohrKB (2003) Phenylthiourea disrupts thyroid function in developing zebrafish. Dev Genes Evol 212 : 593–598 doi:10.1007/s00427-002-0279-3
76. KinneA, KleinauG, HoefigCS, GrütersA, KöhrleJ, et al. (2010) Essential molecular determinants for thyroid hormone transport and first structural implications for monocarboxylate transporter 8. J Biol Chem 285 : 28054–28063 doi:10.1074/jbc.M110.129577
77. SijensPE, RödigerLA, MeinersLC, LunsingRJ (2008) 1H magnetic resonance spectroscopy in monocarboxylate transporter 8 gene deficiency. J Clin Endocrinol Metab 93 : 1854–1859 doi:10.1210/jc.2007-2441
78. NambaN, EtaniY, KitaokaT, NakamotoY, NakachoM, et al. (2008) Clinical phenotype and endocrinological investigations in a patient with a mutation in the MCT8 thyroid hormone transporter. Eur J Pediatr 167 : 785–791 doi:10.1007/s00431-007-0589-6
79. HartlineDK, ColmanDR (2007) Rapid conduction and the evolution of giant axons and myelinated fibers. Curr Biol CB 17: R29–35 doi:10.1016/j.cub.2006.11.042
80. MussaGC, MussaF, BrettoR, ZambelliMC, SilvestroL (2001) Influence of thyroid in nervous system growth. Minerva Pediatr 53 : 325–353.
81. DugasJC, IbrahimA, BarresBA (2012) The T3-induced gene KLF9 regulates oligodendrocyte differentiation and myelin regeneration. Mol Cell Neurosci 50 : 45–57 doi:10.1016/j.mcn.2012.03.007
82. BernalJ (2011) Thyroid hormone transport in developing brain. Curr Opin Endocrinol Diabetes Obes 18 : 295–299 doi:10.1097/MED.0b013e32834a78b3
83. Rodriguez-PeñaA, IbarrolaN, IñiguezMA, MuñozA, BernalJ (1993) Neonatal hypothyroidism affects the timely expression of myelin-associated glycoprotein in the rat brain. J Clin Invest 91 : 812–818 doi:10.1172/JCI116301
84. BernalJ, Guadaño-FerrazA, MorteB (2003) Perspectives in the study of thyroid hormone action on brain development and function. Thyroid Off J Am Thyroid Assoc 13 : 1005–1012 doi:10.1089/105072503770867174
85. ShermanDL, BrophyPJ (2005) Mechanisms of axon ensheathment and myelin growth. Nat Rev Neurosci 6 : 683–690 doi:10.1038/nrn1743
86. SimpsonHD, KitaEM, ScottEK, GoodhillGJ (2013) A quantitative analysis of branching, growth cone turning, and directed growth in zebrafish retinotectal axon guidance. J Comp Neurol 521 : 1409–1429 doi:10.1002/cne.23248
87. BedellVM, WestcotSE, EkkerSC (2011) Lessons from morpholino-based screening in zebrafish. Brief Funct Genomics 10 : 181–188 doi:10.1093/bfgp/elr021
88. NikolaouN, MeyerMP (2012) Imaging circuit formation in zebrafish. Dev Neurobiol 72 : 346–357 doi:10.1002/dneu.20874
89. KarlstromRO, TroweT, KlostermannS, BaierH, BrandM, et al. (1996) Zebrafish mutations affecting retinotectal axon pathfinding. Dev Camb Engl 123 : 427–438.
90. BocconeL, DessìV, MeloniA, LoudianosG (2013) Allan-Herndon-Dudley syndrome (AHDS) in two consecutive generations caused by a missense MCT8 gene mutation. Phenotypic variability with the presence of normal serum T3 levels. Eur J Med Genet 56 : 207–210 doi:10.1016/j.ejmg.2013.02.001
91. Van SpronsenM, HoogenraadCC (2010) Synapse pathology in psychiatric and neurologic disease. Curr Neurol Neurosci Rep 10 : 207–214 doi:10.1007/s11910-010-0104-8
92. WondolowskiJ, DickmanD (2013) Emerging links between homeostatic synaptic plasticity and neurological disease. Front Cell Neurosci 7 : 223 doi:10.3389/fncel.2013.00223
93. VincentJ, LegrandC, RabiéA, LegrandJ (1982) Effects of thyroid hormone on synaptogenesis in the molecular layer of the developing rat cerebellum. J Physiol (Paris) 78 : 729–738.
94. NunezJ, CeliFS, NgL, ForrestD (2008) Multigenic control of thyroid hormone functions in the nervous system. Mol Cell Endocrinol 287 : 1–12 doi:10.1016/j.mce.2008.03.006
95. LewisKE, EisenJS (2003) From cells to circuits: development of the zebrafish spinal cord. Prog Neurobiol 69 : 419–449.
96. TaborKM, BergeronSA, HorstickEJ, JordanDC, AhoV, et al. (2014) Direct activation of the Mauthner cell by electric field pulses drives ultra-rapid escape responses. J Neurophysiol doi:10.1152/jn.00228.2014
97. AppelbaumL, WangGX, MaroGS, MoriR, TovinA, et al. (2009) Sleep-wake regulation and hypocretin-melatonin interaction in zebrafish. Proc Natl Acad Sci U S A 106 : 21942–21947 doi:10.1073/pnas.906637106
98. GajT, GersbachCA, BarbasCF3rd (2013) ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol 31 : 397–405 doi:10.1016/j.tibtech.2013.04.004
99. MayerlS, MüllerJ, BauerR, RichertS, KassmannCM, et al. (2014) Transporters MCT8 and OATP1C1 maintain murine brain thyroid hormone homeostasis. J Clin Invest 124 : 1987–1999 doi:10.1172/JCI70324
100. KawakamiK, TakedaH, KawakamiN, KobayashiM, MatsudaN, et al. (2004) A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev Cell 7 : 133–144 doi:10.1016/j.devcel.2004.06.005
101. AppelbaumL, ToyamaR, DawidIB, KleinDC, BalerR, et al. (2004) Zebrafish serotonin-N-acetyltransferase-2 gene regulation: pineal-restrictive downstream module contains a functional E-box and three photoreceptor conserved elements. Mol Endocrinol Baltim Md 18 : 1210–1221 doi:10.1210/me.2003-0439
102. ShinJ, ParkH-C, TopczewskaJM, MawdsleyDJ, AppelB (2003) Neural cell fate analysis in zebrafish using olig2 BAC transgenics. Methods Cell Sci Off J Soc Vitro Biol 25 : 7–14 doi:10.1023/B:MICS.0000006847.09037.3a
103. YoshidaM, MacklinWB (2005) Oligodendrocyte development and myelination in GFP-transgenic zebrafish. J Neurosci Res 81 : 1–8 doi:10.1002/jnr.20516
104. JungS-H, KimS, ChungA-Y, KimH-T, SoJ-H, et al. (2010) Visualization of myelination in GFP-transgenic zebrafish. Dev Dyn Off Publ Am Assoc Anat 239 : 592–597 doi:10.1002/dvdy.22166
Štítky
Genetika Reprodukční medicína
Článek An Evolutionarily Conserved Role for the Aryl Hydrocarbon Receptor in the Regulation of MovementČlánek Requirement for Drosophila SNMP1 for Rapid Activation and Termination of Pheromone-Induced ActivityČlánek Co-regulated Transcripts Associated to Cooperating eSNPs Define Bi-fan Motifs in Human Gene NetworksČlánek Identification of a Regulatory Variant That Binds FOXA1 and FOXA2 at the Type 2 Diabetes GWAS LocusČlánek tRNA Modifying Enzymes, NSUN2 and METTL1, Determine Sensitivity to 5-Fluorouracil in HeLa CellsČlánek Derlin-1 Regulates Mutant VCP-Linked Pathogenesis and Endoplasmic Reticulum Stress-Induced ApoptosisČlánek A Genetic Assay for Transcription Errors Reveals Multilayer Control of RNA Polymerase II FidelityČlánek The Proprotein Convertase KPC-1/Furin Controls Branching and Self-avoidance of Sensory Dendrites inČlánek Regulation of p53 and Rb Links the Alternative NF-κB Pathway to EZH2 Expression and Cell SenescenceČlánek BMPs Regulate Gene Expression in the Dorsal Neuroectoderm of and Vertebrates by Distinct MechanismsČlánek Unkempt Is Negatively Regulated by mTOR and Uncouples Neuronal Differentiation from Growth Control
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 9
-
Všechny články tohoto čísla
- Translational Regulation of the Post-Translational Circadian Mechanism
- An Evolutionarily Conserved Role for the Aryl Hydrocarbon Receptor in the Regulation of Movement
- Eliminating Both Canonical and Short-Patch Mismatch Repair in Suggests a New Meiotic Recombination Model
- Requirement for Drosophila SNMP1 for Rapid Activation and Termination of Pheromone-Induced Activity
- Co-regulated Transcripts Associated to Cooperating eSNPs Define Bi-fan Motifs in Human Gene Networks
- Targeted H3R26 Deimination Specifically Facilitates Estrogen Receptor Binding by Modifying Nucleosome Structure
- Role for Circadian Clock Genes in Seasonal Timing: Testing the Bünning Hypothesis
- The Tandem Repeats Enabling Reversible Switching between the Two Phases of β-Lactamase Substrate Spectrum
- The Association of the Vanin-1 N131S Variant with Blood Pressure Is Mediated by Endoplasmic Reticulum-Associated Degradation and Loss of Function
- Identification of a Regulatory Variant That Binds FOXA1 and FOXA2 at the Type 2 Diabetes GWAS Locus
- Regulation of Flowering by the Histone Mark Readers MRG1/2 via Interaction with CONSTANS to Modulate Expression
- The Actomyosin Machinery Is Required for Retinal Lumen Formation
- Plays a Conserved Role in Assembly of the Ciliary Motile Apparatus
- Hidden Diversity in Honey Bee Gut Symbionts Detected by Single-Cell Genomics
- Ribosome Rescue and Translation Termination at Non-Standard Stop Codons by ICT1 in Mammalian Mitochondria
- tRNA Modifying Enzymes, NSUN2 and METTL1, Determine Sensitivity to 5-Fluorouracil in HeLa Cells
- Causal Variation in Yeast Sporulation Tends to Reside in a Pathway Bottleneck
- Tissue-Specific RNA Expression Marks Distant-Acting Developmental Enhancers
- WC-1 Recruits SWI/SNF to Remodel and Initiate a Circadian Cycle
- Clonal Expansion of Early to Mid-Life Mitochondrial DNA Point Mutations Drives Mitochondrial Dysfunction during Human Ageing
- Methylation QTLs Are Associated with Coordinated Changes in Transcription Factor Binding, Histone Modifications, and Gene Expression Levels
- Differential Management of the Replication Terminus Regions of the Two Chromosomes during Cell Division
- Obesity-Linked Homologues and Establish Meal Frequency in
- Derlin-1 Regulates Mutant VCP-Linked Pathogenesis and Endoplasmic Reticulum Stress-Induced Apoptosis
- Stress-Induced Nuclear RNA Degradation Pathways Regulate Yeast Bromodomain Factor 2 to Promote Cell Survival
- The MAPK p38c Regulates Oxidative Stress and Lipid Homeostasis in the Intestine
- Widespread Genome Reorganization of an Obligate Virus Mutualist
- Trans-kingdom Cross-Talk: Small RNAs on the Move
- The Vip1 Inositol Polyphosphate Kinase Family Regulates Polarized Growth and Modulates the Microtubule Cytoskeleton in Fungi
- Myosin Vb Mediated Plasma Membrane Homeostasis Regulates Peridermal Cell Size and Maintains Tissue Homeostasis in the Zebrafish Epidermis
- GLD-4-Mediated Translational Activation Regulates the Size of the Proliferative Germ Cell Pool in the Adult Germ Line
- Genome Wide Association Studies Using a New Nonparametric Model Reveal the Genetic Architecture of 17 Agronomic Traits in an Enlarged Maize Association Panel
- Translational Regulation of the DOUBLETIME/CKIδ/ε Kinase by LARK Contributes to Circadian Period Modulation
- Positive Selection and Multiple Losses of the LINE-1-Derived Gene in Mammals Suggest a Dual Role in Genome Defense and Pluripotency
- Out of Balance: R-loops in Human Disease
- A Genetic Assay for Transcription Errors Reveals Multilayer Control of RNA Polymerase II Fidelity
- Altered Behavioral Performance and Live Imaging of Circuit-Specific Neural Deficiencies in a Zebrafish Model for Psychomotor Retardation
- Nipbl and Mediator Cooperatively Regulate Gene Expression to Control Limb Development
- Meta-analysis of Mutations in Autism Spectrum Disorders: A Gradient of Severity in Cognitive Impairments
- The Proprotein Convertase KPC-1/Furin Controls Branching and Self-avoidance of Sensory Dendrites in
- Hydroxymethylated Cytosines Are Associated with Elevated C to G Transversion Rates
- Memory and Fitness Optimization of Bacteria under Fluctuating Environments
- Regulation of p53 and Rb Links the Alternative NF-κB Pathway to EZH2 Expression and Cell Senescence
- Interspecific Tests of Allelism Reveal the Evolutionary Timing and Pattern of Accumulation of Reproductive Isolation Mutations
- PRO40 Is a Scaffold Protein of the Cell Wall Integrity Pathway, Linking the MAP Kinase Module to the Upstream Activator Protein Kinase C
- Low Levels of p53 Protein and Chromatin Silencing of p53 Target Genes Repress Apoptosis in Endocycling Cells
- SPDEF Inhibits Prostate Carcinogenesis by Disrupting a Positive Feedback Loop in Regulation of the Foxm1 Oncogene
- RRP6L1 and RRP6L2 Function in Silencing Regulation of Antisense RNA Synthesis
- BMPs Regulate Gene Expression in the Dorsal Neuroectoderm of and Vertebrates by Distinct Mechanisms
- Unkempt Is Negatively Regulated by mTOR and Uncouples Neuronal Differentiation from Growth Control
- Atkinesin-13A Modulates Cell-Wall Synthesis and Cell Expansion in via the THESEUS1 Pathway
- Dopamine Signaling Leads to Loss of Polycomb Repression and Aberrant Gene Activation in Experimental Parkinsonism
- Histone Methyltransferase MMSET/NSD2 Alters EZH2 Binding and Reprograms the Myeloma Epigenome through Global and Focal Changes in H3K36 and H3K27 Methylation
- Bipartite Recognition of DNA by TCF/Pangolin Is Remarkably Flexible and Contributes to Transcriptional Responsiveness and Tissue Specificity of Wingless Signaling
- The Olfactory Transcriptomes of Mice
- Muscular Dystrophy-Associated and Variants Disrupt Nuclear-Cytoskeletal Connections and Myonuclear Organization
- Interplay of dFOXO and Two ETS-Family Transcription Factors Determines Lifespan in
- Evidence for Widespread Positive and Negative Selection in Coding and Conserved Noncoding Regions of
- Genome-Wide Association Meta-analysis of Neuropathologic Features of Alzheimer's Disease and Related Dementias
- Rejuvenation of Meiotic Cohesion in Oocytes during Prophase I Is Required for Chiasma Maintenance and Accurate Chromosome Segregation
- Admixture in Latin America: Geographic Structure, Phenotypic Diversity and Self-Perception of Ancestry Based on 7,342 Individuals
- Local Effect of Enhancer of Zeste-Like Reveals Cooperation of Epigenetic and -Acting Determinants for Zygotic Genome Rearrangements
- Differential Responses to Wnt and PCP Disruption Predict Expression and Developmental Function of Conserved and Novel Genes in a Cnidarian
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Admixture in Latin America: Geographic Structure, Phenotypic Diversity and Self-Perception of Ancestry Based on 7,342 Individuals
- Nipbl and Mediator Cooperatively Regulate Gene Expression to Control Limb Development
- Genome Wide Association Studies Using a New Nonparametric Model Reveal the Genetic Architecture of 17 Agronomic Traits in an Enlarged Maize Association Panel
- Histone Methyltransferase MMSET/NSD2 Alters EZH2 Binding and Reprograms the Myeloma Epigenome through Global and Focal Changes in H3K36 and H3K27 Methylation
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



