-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaUnraveling Genetic Modifiers in the Mouse Model of Absence Epilepsy
Absence seizures - also known as “petit-mal” - define a common form of epilepsy most prevalent in children, but also seen at other ages, and in related diseases such as juvenile myoclonic epilepsy. Absence seizures cause brief periods of unconsciousness, and are accompanied by characteristic abnormal brain waves called “spike-wave discharges” (SWD) due to their appearance in the electroencephalogram (EEG). Although few genes are known for human absence seizures, perhaps because the underlying genetics are complex, several laboratory rodent models exist, including one caused by mutation of a gene called Gria4. While studying Gria4, we noticed that a mouse strain called C3H can suppress or enhance the frequency and severity of Gria4-associated SWD in a perplexing manner; such effects are generally attributed to “modifier” genes. Here we identify a novel modifier – called “pecanex-like 2”, or Pcnxl2 for short – that reduces the severity of SWD in the C3H substrain in which the Gria4 mutation originally arose. This finding directed us to use of related substrains to locate additional modifiers, one of which has an even more profound effect on SWD episodes. Modifier genes, nature's way of controlling seizure severity, are promising targets for better understanding seizure mechanisms and potential new therapies in the future.
Published in the journal: . PLoS Genet 10(7): e32767. doi:10.1371/journal.pgen.1004454
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004454Summary
Absence seizures - also known as “petit-mal” - define a common form of epilepsy most prevalent in children, but also seen at other ages, and in related diseases such as juvenile myoclonic epilepsy. Absence seizures cause brief periods of unconsciousness, and are accompanied by characteristic abnormal brain waves called “spike-wave discharges” (SWD) due to their appearance in the electroencephalogram (EEG). Although few genes are known for human absence seizures, perhaps because the underlying genetics are complex, several laboratory rodent models exist, including one caused by mutation of a gene called Gria4. While studying Gria4, we noticed that a mouse strain called C3H can suppress or enhance the frequency and severity of Gria4-associated SWD in a perplexing manner; such effects are generally attributed to “modifier” genes. Here we identify a novel modifier – called “pecanex-like 2”, or Pcnxl2 for short – that reduces the severity of SWD in the C3H substrain in which the Gria4 mutation originally arose. This finding directed us to use of related substrains to locate additional modifiers, one of which has an even more profound effect on SWD episodes. Modifier genes, nature's way of controlling seizure severity, are promising targets for better understanding seizure mechanisms and potential new therapies in the future.
Introduction
Laboratory mouse strains are well known to vary in their susceptibility to convulsive seizures, including acute experimentally induced seizures [1], [2], [3], [4], [5], [6], and spontaneous seizures induced by genetic mutation [7], [8], [9], [10], [11], [12], [13]. Most of the known strain effects have been for convulsive seizures, where motor manifestations are obvious, including one modifier identified to date (Kcnv2 as a modifier of Scn2a1Q54 induced convulsive seizures [13]. There has been less attention to such effects in non-convulsive phenotypes, such as absence epilepsy, where the seizures lack a convulsive element. Nevertheless, strain differences were noted for three different genes that cause absence seizures when mutated – Scn8a [14]), Gabrg2 [15] and Gria4 [16], and for at least two the C3H strain generally worsens the absence seizure phenotype, compared with the relatively protective strain C57BL/6J (B6J).
For absence seizures caused by Gria4 mutation, the C3H strain has been a paradox. Each of three very closely-related C3H/He substrains has a similar level of spontaneous spike-wave discharges (SWD) - the distinctive electroencephalographic hallmark of absence seizures in human and in animal models - but only one substrain, C3H/HeJ (HeJ), carries a Gria4 mutation. This mutation is caused by an intracisternal A-particle retrotransposon (IAP) insertion in Gria4 [16], [17], [18]. Although genetic and later functional analysis proved that Gria4 is the cause of these seizures in HeJ [19], two other C3H/He substrains that lack this mutation still have appreciable SWD. At least one of these strains was shown to have a polygenic etiology, with no indication of any effect from proximal Chr 9 where Gria4 resides [18]. The further surprise was when HeJ mice were outcrossed to other strains - even to another C3H substrain, C3HeB/FeJ (FeJ), that does not have frequent SWD - about half of the next generation Gria4 deficient progeny had significantly higher SWD incidence than HeJ itself [16]. Together these results suggested the model whereby the HeJ substrain has both the initial seizure-causing Gria4 mutation, and also a mitigating or protective mutation, without which the seizures would be much more severe. The model also suggests that C3H strains in general have a high baseline susceptibility to absence seizures, compared with strains such as C57BL/6.
IAP insertions like the element in Gria4 are known to cause deleterious mutant phenotypes by reducing RNA expression of the target gene, and the vast majority of spontaneous IAP insertion mutations in mice have arisen in the C3H strain family (reviewed in [20]). While searching for genetic markers to distinguish C3H substrains for analysis of the putative HeJ suppressor, we discovered extensive IAP retrotransposition among C3H substrains. One of the HeJ substrain-private IAP insertions resides in the same chromosomal region as an epistatic modifier of Gria4 absence seizures mapped to Chr 8 [17]. Here we report that this Chr 8 IAP is inserted in the previously unstudied Pcnxl2 (pecanex-like 2) gene, affecting its RNA expression. We further use TALEN mutagenesis to create new Pcnxl2 alleles, confirming that Pcnxl2 loss of expression is responsible for mitigating Gria4 seizures of HeJ mice, accounting for the substrain difference. We also mapped modifiers that differ between C3H and B6J, and show that one – G4swdm1 – has a profound effect on seizure severity. The identification of the first absence seizure modifier Pcnxl2 provides significant traction to the complex genetics of absence seizures in the C3H strain family and possible new mechanisms for mitigating disease.
Results
Congenic strains highlight strain background effects on seizures
Prior studies of Gria4-deficient mice showed significant genetic background influence on the incidence and duration of spike-wave discharges (SWD) between C57BL/6J (B6J) and C3H strains [16], [17], whether the natural Gria4spkw1 allele (abbreviated hereafter as Gria4IAP) or the engineered knockout Gria4tm1Dgen (Gria4KO). While mapping the main phenotype to Gria4 in a backcross between C3H/HeJ (HeJ) and B6J, about half of the progeny were noted to have significantly more SWD than HeJ itself, and an epistatic modifier putatively due to this effect was mapped to distal Chr 8 [16], [17]. Interestingly, a similar phenomenon from crosses between HeJ and C3HeB/FeJ (FeJ) suggested this was due to a substrain difference [16], [17]. To confirm this, we backcrossed Gria4IAP allele from HeJ to FeJ and examined SWD in this congenic pair and compared to B6J-Gria4KO for reference. FeJ-Gria4IAP had significantly more frequent and longer SWD than HeJ or B6J-Gria4KO (Table 1). These results indicate that HeJ has a suppressor allele(s) not present in FeJ. It also suggest that further modifiers differ between FeJ and B6J strains.
Tab. 1. Genotypes and features of inbred and congenic strains used in this study. 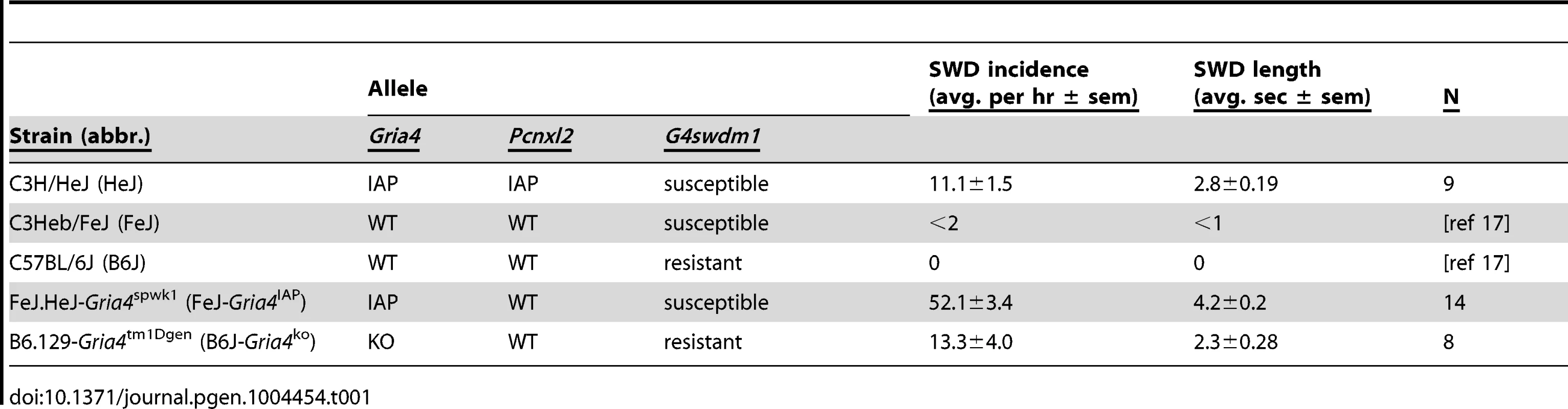
Extensive IAP insertion variation in C3H substrains
The HeJ suppressor is a challenge to pursue by classical recombination mapping because of the paucity of genetic markers between FeJ and HeJ substrains, which split into separate lines around 1950 [21]. However, C3H mice are known to have frequent spontaneous germline IAP retrotransposition, with de novo insertions accounting for a significant fraction of the spontaneous mutation load in C3H (see review by [20]). Moreover, almost all de novo IAP insertions, including Gria4IAP, are of a particular IAP subtype - IAP-1Δ1 - containing a characteristic in-frame 1.2 kb deletion of the gag-pol fusio gene [22]. Sequence similarity search of the B6J mouse genome assembly with an oligonucleotide sequence spanning the IAP-1Δ1 deletion detected approximately 200 independent genomic sites, whereas the same region from full-length IAP detected over 700 sites (data not shown), suggesting the feasibility of direct hybridization approaches to identify substrain-specific de novo IAP-1Δ1 insertions.
To determine whether C3H substrains vary significantly in IAP-1Δ1 content, we designed an oligonucleotide specific for the IAP-1Δ1 1.2 kb common deletion to examine proviral-host DNA junction fragments by direct dried gel hybridization and to subclone them by inverse PCR (Fig. 1A). Although it is unlikely that any visible ‘band’ in the direct hybridization represents a single IAP insertion, from the differential pattern – best observed in the 2 kb range - there are ample variation between four substrains examined; with HeJ appearing to have the highest load including at least 20 HeJ-specific bands evident (Fig. 1B). Inverse-PCR was used to identify and clone select IAP-1Δ1 – host junction fragments from FeJ and HeJ. A representative experiment to identify lower molecular weight junction fragments revealed a banding pattern reminiscent of the gel hybridization, and recapitulates the corresponding paucity of bands in this region in the FeJ strain (Fig. 1C). From this and other experiments, bands were excised, cloned and junction fragments sequenced, and PCR assays were developed ultimately leading to identification of at least 26 insertion-host junction fragments that are present in the HeJ substrain and absent from FeJ (Table 2). 9 are intergenic, but the majority are in introns of known or unclassified genes (Table 2). Most HeJ insertions we cloned were ultimately identified in the recent retrotransposon-mining of the whole genome sequence of HeJ and other mouse strains from the Sanger Mouse Genomes Project [23], although substrain specificity was not ascertained in that study.
Fig. 1. IAP-1Δ1 element diversity in C3H substrains. 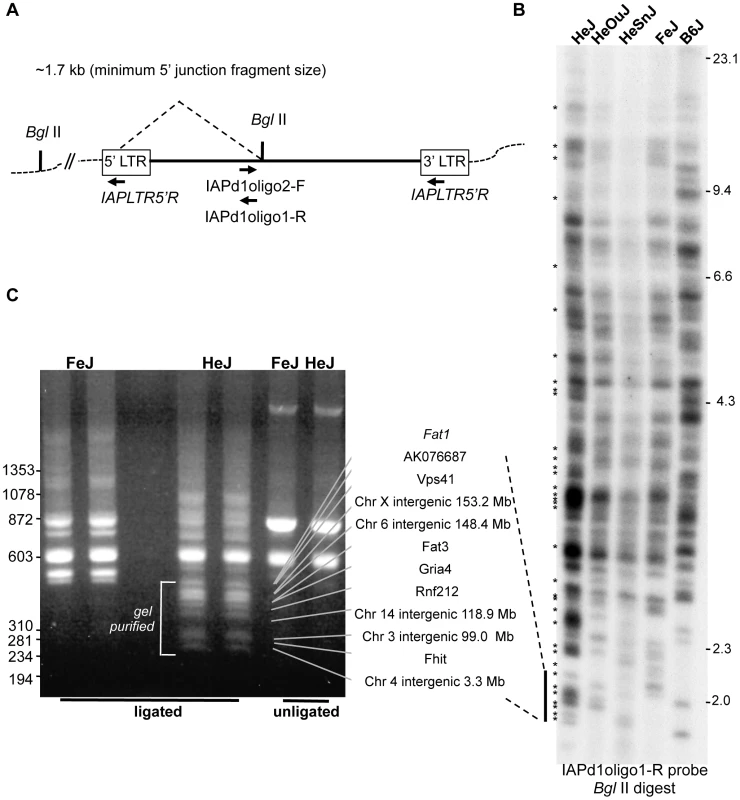
A. Strategy for detection of IAP-1Δ1 elements. For detection of host-IAP-1Δ1 5′ BglII-restricted junction fragments by dried gel hybridization, a 31-nt oligonucleotide hybridization probe (IAPd1oligo1-R) was designed to straddle the previously described 1.2 kb IAP-1Δ1 element common deletion [22]. For cloning of IAP-1Δ1 elements by inverse PCR, a complementary oligonucleotide (IAPd1oligo2F) was designed to pair with an oligonucleotide specific for the IAP LTR (IAPLTR5′) for amplification of circularized genomic BglII fragments. B. Dried gel detection of IAP-1Δ1 5′ junction fragments from C3H substrains (HeJ, HeOuJ, HeSnJ, FeJ) plus the outlier B6J. Bands estimated as unique to HeJ are indicated with asterisks on the left. C. Example of an inverse PCR experiment from HeJ and FeJ substrains showing BglII restricted genomic DNA (two lanes each), unligated controls (one lane each), and location of apparent HeJ-specific bands prepared. for cloning and Sanger sequencing. In additional experiments not shown, higher molecular weight BglII restricted fragments were similarly processed. Also shown with connecting lines are locus identities for specific bands after sequencing, the sizes of which correspond to the junction fragment lengths determined from the sequence, and dashed lines indicate the corresponding region from the dried gel, for the purpose of highlighting the similar banding pattern (N.b. the absolute sizes of fragments in panel C are smaller because the portion of the IAP genome between primers is expectedly absent from the inverse PCR product). Sizes of HindIII-digested bacteriophage lambda DNA are shown on the right, of panel B, and HaeIII-digested bacteriophage phi-X DNA markers are shown at the left of panel C. Tab. 2. C3H/HeJ IAP-1Δ1 insertions absent from C3HeB/FeJ. 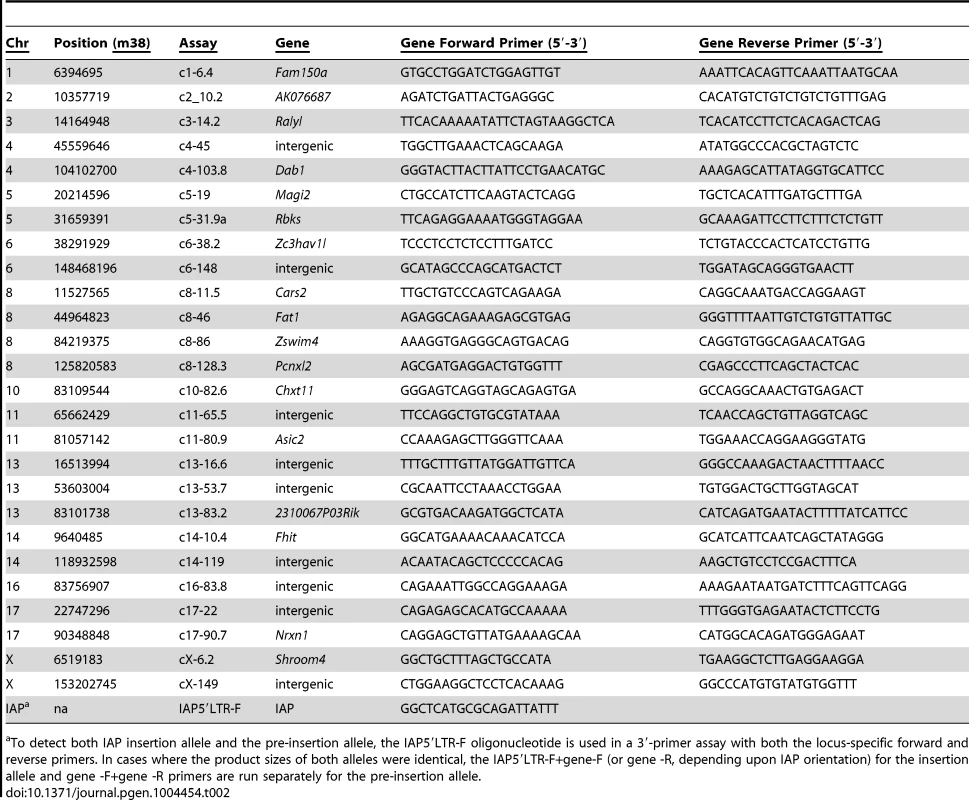
To detect both IAP insertion allele and the pre-insertion allele, the IAP5′LTR-F oligonucleotide is used in a 3′-primer assay with both the locus-specific forward and reverse primers. In cases where the product sizes of both alleles were identical, the IAP5′LTR-F+gene-F (or gene -R, depending upon IAP orientation) for the insertion allele and gene -F+gene -R primers are run separately for the pre-insertion allele. Reduced expression of Pcnxl2 due to a substrain-specific IAP insertion
One HeJ-specific IAP insertion resides at 128.3 Mb on Chr 8, in the same general region of the previously mapped epistatic modifier of Gria4 SWD [17]. This insertion was not seen in any of the 17 inbred mouse strains for which genome sequence is available [23], and the PCR assays used to identify it (Table 2) determined that it was also absent from C3HeB/FeJ (FeJ), C3H/HeOuJ and C3H/HeSnJ substrains (data not shown). This IAP is integrated in the 5′-LTR-3-′LTR orientation in intron 19 of Pcnxl2, the gene that encodes pecanex-like 2. Pcnxl2 is one of three mammalian paralogs of Drosophila melanogaster pecanex, a neurogenic gene about which little is known, but was recently suggested to be part of the notch signaling pathway in the endoplasmic reticulum [24], [25]. In adult mouse brain, Pcnxl2 is expressed highest in the hippocampal pyramidal cell layer, it is also expressed prominently in the area of cerebral cortex corresponding to layer V, and sparsely in the reticular thalamic nucleus (Allen Brain Atlas: see http://mouse.brain-map.org/experiment/show/70239051). As the latter two regions are key in abnormal cortico-thalamic oscillations associated with absence seizures generally and specifically in Gria4 mutants [19], Pcnxl2 is a suitable candidate for the suppressor.
To determine whether the IAP insertion affects Pcnxl2 gene expression, we initially examined Pcnxl2 transcript expression in brain from public datasets, comparing HeJ to other inbred strains. From inspecting publically available mouse strain hippocampal microarray (http://www.genenetwork.org) and in the Sanger Center's whole-brain RNAseq (ftp://ftp-mouse.sanger.ac.uk/current_rna_bams), we noted an HeJ-specific drop in relative expression or abundance of exons downstream of exon 19 (data not shown). To examine this between HeJ and FeJ substrains, by using quantitative RT-PCR we confirmed that Pcnxl2 expression across intron 19 is indeed lower in HeJ compared to FeJ (Table 3, compare sample IDs 1–2).
Tab. 3. Quantitative RT-PCR in Pcnxl2 mutants. 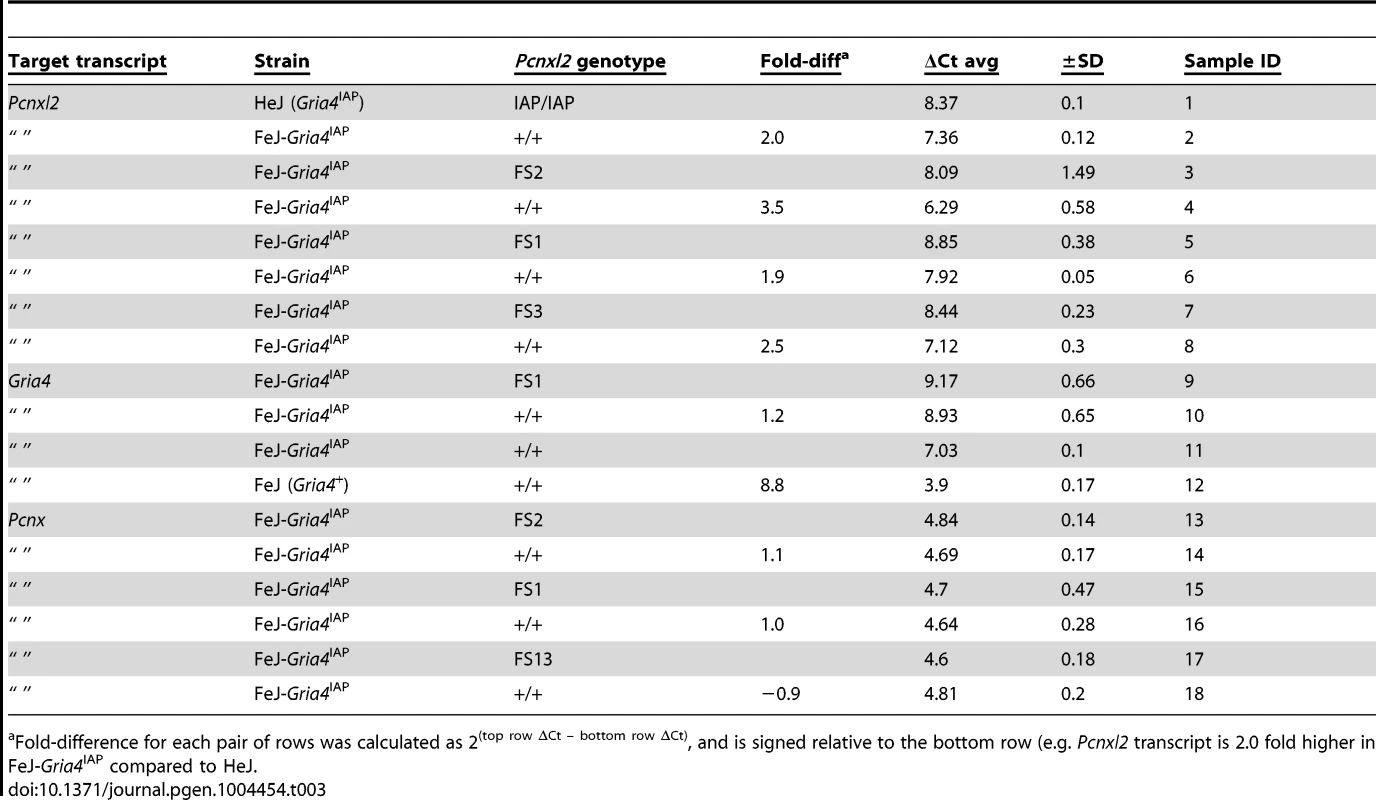
Fold-difference for each pair of rows was calculated as 2(top row ΔCt – bottom row ΔCt), and is signed relative to the bottom row (e.g. Pcnxl2 transcript is 2.0 fold higher in FeJ-Gria4IAP compared to HeJ. TALEN alleles confirm reduced Pcnxl2 expression modifies Gria4 absence seizures
To confirm that Pcnxl2 encodes the Gria4 SWD suppressor, we used TALEN mutagenesis [26] in the FeJ-Gria4IAP congenic strain to create Pcnxl2 frameshift mutations (Fig. 2A). Two Pcnxl2 exons were targeted separately: exon 16, roughly in the middle of the gene, and exon 29 nearer the 3′ end, the latter containing the so-called “pecanex” domain – a conserved domain of unknown function shared among pecanex paralogues. Three in 106 liveborn mice contained germline mutations and each created a frameshift allele that resulted in a premature translational stop codon (Figure 2).
Fig. 2. TALEN-induced frameshift alleles of Pcnxl2 and suppression of Gria4 SWD. 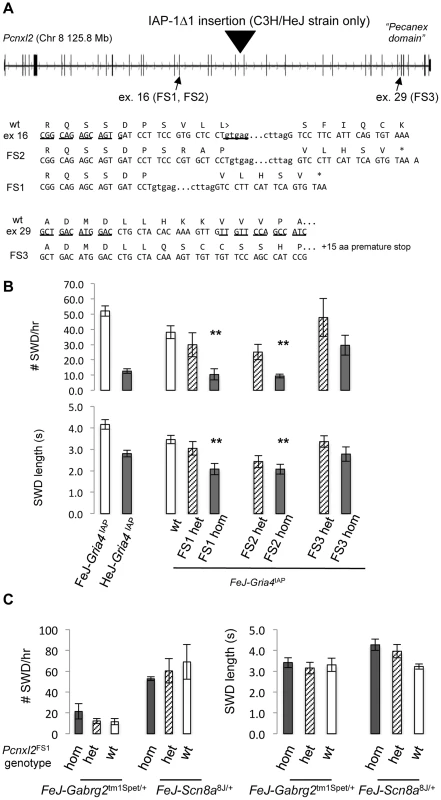
A. Exon-intron structure of mouse Pcnxl2 (from UCSC genome browser, version m38 mouse genome assembly), highlighting the position of the HeJ-specific IAP-1Δ1 insertion in intron 19, the two exons (exon 16 and exon 29, respectively) targeted for TALEN mutagenesis and the wildtype FeJ sequence of each together with the sequence of the respective frameshift alleles in TALEN mutations A+1 (FS2), A−11 (FS1), and B−2 (FS3). The asterisk indicates a premature translational stop codon in each mutant allele. B. Spike-wave discharge (SWD) incidence (top) and length (bottom) for parent FeJ-Gria4IAP congenic and HeJ-Gria4IAP inbred strain colonies, compared with littermate wildtype (wt), heterozygous or homozygous respective TALEN mutations created and maintained on the isogenic FeJ-Gria4IAP congenic strain background. Asterisks indicate where homozygous mutant genotypes were significantly different (p<0.01) from wildtype control. Sample sizes: FeJ-Gria4IAP (9), HeJ- Gria4IAP (18), wt (16), FS1 het (6), FS1 hom (6), FS2 het (9), FS2 hom (9), FS3 het (6), FS3 hom (6). Error bars are SEM. C. Spike-wave discharge (SWD) incidence (left) and length (right) for littermate TalA-11 genotypes (hom, het, wt) isogenic on the FeJ strain, in double mutant combination with Gabrg2tm1Spet or Scn8a8J/+ from a congenic FeJ strain background. None of the Pcnxl2 mutant allele homozygotes were significantly different from the other Pcnxl2 genotypes for either mutation or SWD measure. Sample sizes: Gabrg2tm1Spet (3 hom, 12 het, 2 wt); Scn8a8J/+ (2 hom, 4 het, 2 wt). To test the effect on SWD, EEG was examined in FeJ-Gria4IAP homozygotes carrying each of the three Pcnxl2 mutations (A−11, A+1 and B−2, hereafter referred to as FS1 – frameshift 1, FS2 and FS3, respectively). Pcnxl2FS1 and Pcnxl2FS2 mutations significantly lowered SWD incidence (Fig. 2B) and length (Fig. 2C), showing an additive effect across genotypes. The homozygotes were also slightly more suppressed than HeJ with its natural Pcnxl2IAP allele. An examination of Pcnxl2 transcripts by qPCR showed decreased (between 2 and 3.5-fold; Table 3, compare sample ID's 3–4, 5–6, 7–8) but not eliminated expression in each homozygous genotype; this is expected as frameshift mutations in middle exons often cause nonsense-mediated decay. However, the Pcnxl2FS3 allele did not decrease SWD as effectively as the others (Fig. 2B, C). Since commercial antisera to PCNXL2 are not effective in mouse brain (data not shown), we cannot know whether this is because a partial or truncated protein is still made and has some residual function, or whether nonsense-mediated decay was less complete. Regardless, the effect of at least two independent new Pcnxl2 alleles on SWD, combined with transcript reduction shows that Pcnxl2 encodes the suppressor of Gria4 SWD.
Pcnxl2 deficiency does not have a large effect on Scn8a8J or Gabrg2tm1Spet mutant SWD
To determine whether Pcnxl2 deficiency can affect SWD in other absence seizure mouse models, we examined double mutant genotypes between FeJ-Pcnxl2FS1 and either Scn8a8J [14], or Gabrg2tm1Spet [15], each of which encodes a dominant, SWD-causing missense mutation in the respective ion channel gene. Pcnxl2 deficiency had no significant effect on SWD on either of these mutants (Fig. 2C), suggesting that Pcnxl2 may be specific for mechanisms that involve Gria4. However, if Gria4-specific, the mechanism appears not to be on IAP mutagenesis itself, because there is no increase in Gria4 RNA expression in Gria4IAP, Pcnxl2FS1 double mutants (Table 3, compare sample IDs 9–10). We also note that the Pcnxl2 paralogue, Pcnx, is expressed widely in adult mouse brain, including likely overlap with Pcnxl2 (http://mouse.brain-map.org/experiment/show/73818801), suggesting the possibility of compensation. However, the Pcnx transcript is not altered in any of the three new Pcnxl2 frameshift alleles (Table 3, compare sample IDs 13–14, 15–16, 17–18), suggesting that if there is any compensation by this overlapping pecanex gene, it is not at the transcript level.
G4swdm1, an inter-strain modifier of Gria4 absence seizures
The fact that Gria4 mutant associated SWD are more pronounced when placed on the C3HeB/FeJ (FeJ) strain compared with B6J suggests additional modifiers, i.e. separate from Pcnxl2 whose genotype does not differ between B6J and FeJ (Table 1). To pursue these, we backcrossed (FeJ-Gria4IAP×B6J-Gria4KO)F1 animals to B6J - Gria4KO, creating 89 N2 mice segregating B6J vs. FeJ strain variants on a Gria4 mutant background (either homozygous Gria4KO or compound heterozygous Gria4KO/IAP) and scored their EEG for SWD incidence and length. The broad, almost continuous distribution of each raw SWD measure in this population suggests multiple factors (Fig. 3A, 3B insets). A genome-wide scan of the two SWD measures and their principal components revealed two highly significant regions (Fig. 3A, 3B): proximal Chr 8 (peak LOD, 3.4) and mid Chr 15 (peak LOD, 2.9). The Chr 15 region was significant for SWD length but negligible for incidence, and vice versa for Chr 8 (compare Fig. 3A with 3B), suggesting each region is primarily responsible for one of the two measures, at least in this cross. A region in mid Chr 3 was significant for SWD length only, and several suggestive peaks were observed for at least one SWD measure on Chrs 1, 2 and 5. A pairwise scan was done to look for pairwise epistatic interactions, but no significant interactions were observed (data not shown).
Fig. 3. Genome scans for SWD traits in Gria4 deficient backcross mice. 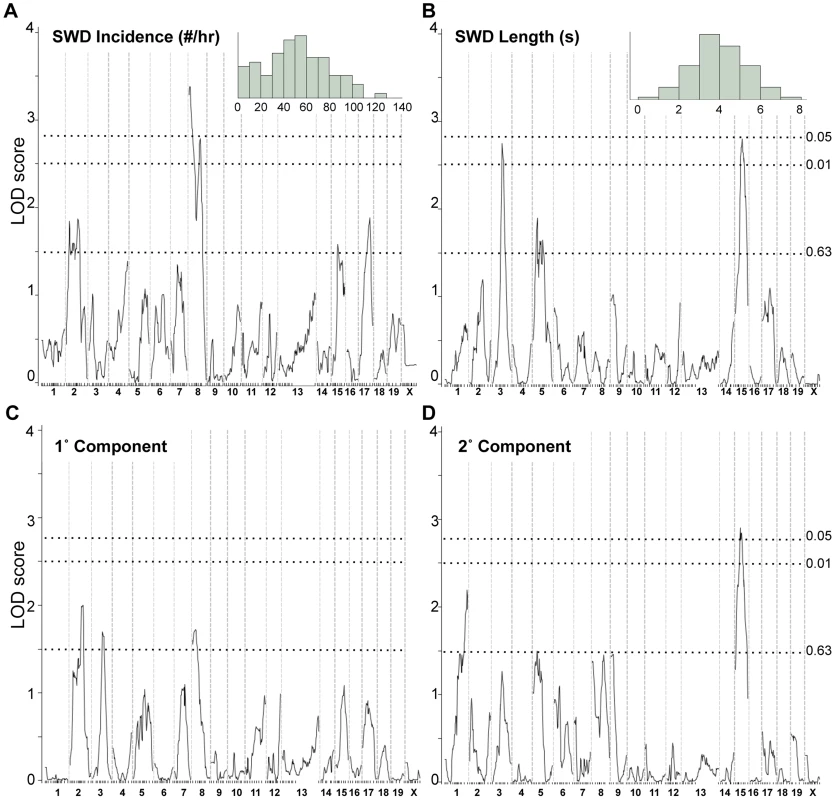
Shown are genome-wide interval mapping LOD score plots from 89 backcross Gria4 mutant mice for two observed spike-wave discharge (SWD) traits - SWD incidence (panel A), SWD length (panel B) - and their first two principal components (panels C and D). The insets of panels A and B show the frequency distribution of the respective raw traits, although for interval mapping all four traits were first rank- and quartile-normalized prior to analysis. The chromosomes are shown at the bottom, with tick marks indicating positions of individual markers within each chromosome. Dotted lines show positions of genome-wide significance thresholds determined from 1000 permutations. Because of the increased SWD length, we focused on validating the Chr 15 locus, now termed G4swdm1, for Gria4 spike-wave discharges modifier 1. We made a congenic strain in which the middle of Chr 15 was selectively bred from FeJ into B6J-Gria4KO, and N9F1 and N10F1 intercross mice were generated and tested. Highly significant effects were observed this time for both SWD length and incidence across the introgressed interval (Figure 4A, 4B). While G4swdm1 was initially detected as a dominant allele, the congenic intercross reveals additivity: FeJ homozygotes experience significantly longer and more frequent SWD (Figure 4C). Although SWD length was still the leading phenotype, in the B6J background the effect of G4swdm1 on SWD incidence was stronger than in the N2 population, likely reflecting additional genetic complexity. Indeed, several congenic individuals had SWD 15 s long (Fig. 4D); further, when the homozygous interval was placed together with the Gria4 mutant genotype on a (B6J×FeJ)F1 hybrid background, several very striking SWD, exceeding 40 s were observed (Figure 4D). The estimated 95% confidence interval (CI) for G4swdm1 is large, with the narrowed SWD length interval covering 27.2 Mb including 191 protein coding and 75 small RNA or unclassified genes (File S1), although the “bumpy” likelihood curve suggests the possibility of multiple modifiers. Among these genes are 27 that have 56 non-synonymous coding or potential splice altering SNPs or indels, between published C3H/HeJ and B6J genomes; further, comparison of FeJ exome sequence to HeJ revealed no similar coding variants (File S1). To gain additional evidence for candidacy, we examined gene expression by RNAseq, using somatosensory cortex and thalamus as tissue source, comparing parent strains B6J and FeJ to each other. In the SWD length 95% CI, of 93 mRNAs expressed, 15 had abundance differences (e.g. q<0.1; File S1). Most were modest (<5% change) but for Ly6a, one of several members of a cell membrane protein-encoding gene family, FeJ had a 21% transcript reduction in thalamus and 32% in cortex. Although Ly6a is best known for expression in lymphocytes, it is also expressed in brain and at least one Ly6a knockout allele has prenatal lethality [27]. Further refining the G4swdm1 critical interval, and more extensive testing of existing mutants or creation of others by mutagenesis is required before the correct G4swdm1 candidate(s) can be identified.
Fig. 4. SWD phenotypes conferred by G4swdm1, a Chr 15 strain modifier of Gria4 absence seizures. 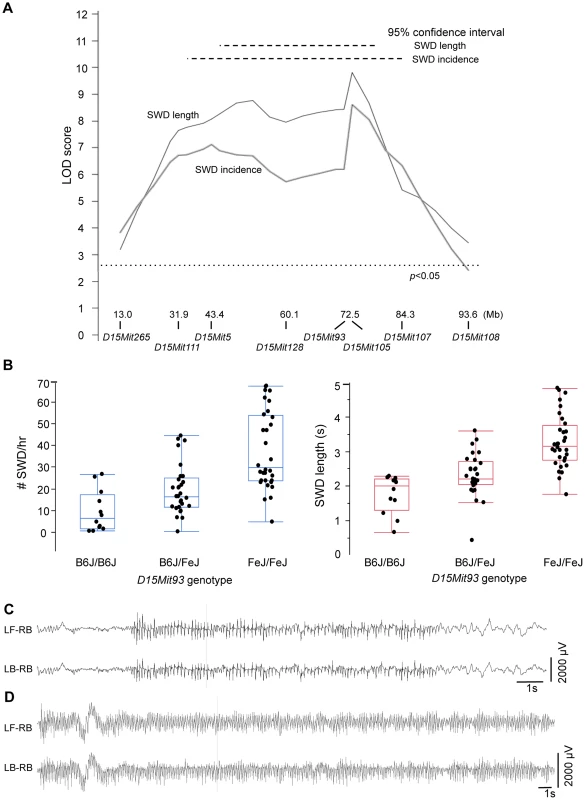
A. LOD score plot, similar to those in Figure 3, except only for Chr 15 in 84 N9F1 or N10F1 intercross congenic mice. At the bottom are Mb (mouse genome assembly m38) positions in of Chr 15 markers used for this analysis and the genome-wide significance threshold is also shown, as determined by 5000 permutations. B. For the marker D15Mit93 corresponding to the peak maximum likelihood location from panel A, quartile plots with data points for average SWD incidence or SWD length from individual mice, showing additivity for both traits. C. EEG from a Gria4KO/KO, G4swdm1Fe/FeJ double homozygote on the B6J congenic strain, showing a particularly long (15 second) SWD. D. EEG from a Gria4KO/IAP compound heterozygous, G4swdm1Fe/FeJ homozygous double mutant genotype on a (B6J.FeJ×FeJ)F1 hybrid background, showing a particularly long (46 second) SWD. For panels C and D, two of the six recorded channels are shown (LF-RB – left front, right back; LB-RB – left back, right back, corresponding to the electrode placement relative to Bregma and midline). 1 s: 1 second. Discussion
Here we unravel the genetic complexity of Gria4-deficiency absence seizure susceptibility in C3H mice. First, we show genetically that Pcnxl2 deficiency accounts for the unusual substrain difference in spike-wave discharges (SWD) in Gria4 mutants on C3H/HeJ (HeJ) compared to C3HeB/FeJ (FeJ). We determined that two new frameshift alleles, generated directly in FeJ-Gria4IAP by TALEN mutagenesis, confer SWD mitigation even more than the natural, presumably hypomorphic Pcnxl2 IAP insertion allele of HeJ. This insertion is of the same IAP-1Δ1 subtype that caused the original Gria4IAP mutation. Given increased incidence and severity of SWD in FeJ-Gria4IAP compared to HeJ-Gria4IAP, and the absence of the Pcnxl2 IAP from two other C3H/He substrains, we think it is reasonable to speculate that Gria4IAP conferred a selective disadvantage to the progenitors of HeJ mice, one that was later diminished upon fixation of the Pcnxl2IAP insertion. These findings also suggest that a number of apparent IAP element differences between C3H substrains, nominally 20% of the IAP-1Δ1 pool, remain a potentially powerful source of genome plasticity, which naturally would not be restricted to neurological phenotypes. Although it was suggested previously that most functional IAP insertions in mouse strains were lost because of deleterious effects, [23], clearly some remain functional – perhaps in the case of Pcnxl2 due to selective advantage such as the one we hypothesize.
The further phenotypic difference between FeJ and B6J strains highlights the existence of additional genetic influences on Gria4 associated absence seizures. To begin to dissect these, genome scans revealed several potential modifiers, affecting one or the other SWD measure. By using a congenic strain we validated one modifier locus – G4swdm1 on Chr 15 and observed its striking effect on SWD length; in F1 hybrid mutants, for example, we observed several SWD episodes lasting over 40 seconds. It is difficult to imagine that such frequent and contiguous states of neural hypersynchrony do not have a broader impact on behavior. Despite its clear effect, G4swdm1 accounts for only some of the phenotype difference between strains – perhaps 30% by comparing SWD length of B6J to F1 hybrid (e.g. as illustrated in Figure 4C to 4D). But when multiple interactions are likely, as in complex traits, any such estimates of effect are overly simplistic. Approaches to map such modifiers using conventional quantitative trait locus, especially in small crosses, are limited to loci that show significant main effects despite interactions, or to simple, pairwise interactions when they are quite strong. Novel computational approaches such as CAPE, which incorporates relationships between phenotypic features directly into the gene interaction model [28], may be required to parse more complex interactions in conventional cross designs.
Pcnxl2 is the first absence seizure modifier gene to be identified in any species, and as such it represents the first of what is likely to be many genetic interactions beneath the complexity of absence seizures. The predicted peptide structure of PCNXL2 is similar to that of other pecanex orthologues, with 8 putative transmembrane domains followed by a conserved so-called pecanex domain. But no known primary or predicted secondary structures have been identified that would predict further function. D. melanogaster pecanex (pcnx) localizes to the endoplasmic reticulum (ER) and functional genetic studies utilizing the protein unfolded response as a readout, suggest that it shows maternal inheritance and has a role in notch signaling [25]. From conservation among pecanex family members we might expect that Pcnxl2 is also expressed in the ER. Although much further discussion of function is merely speculation, if it is an ER protein one tempting possibility is a role in trafficking or in posttranslational modification of synaptic receptors such as Gria4 or other compensating ion channel receptors. The prominent Pcnxl2 expression in layer V of the cerebral cortex opens the door to the possibility that it is involved in mediating excitatory output from layer V pyramidal neurons to the reticular thalamus and thalamus. Whether any such function is the result of an acute Pcnxl2 role, or instead a role in circuit development, will require further studies, for example, the creation of a conditional allele to be induced at different ages.
Laboratory mouse strains are well known to vary in susceptibility to experimentally-induced partial or generalized tonic-clonic seizures and there are several well-characterized strain differences modifying the penetrance or severity of so-called monogenic seizure mutations (as discussed earlier) although only one such modifier gene has been identified to date, and interestingly this has also be implicated in human epilepsy [13]. The rate of human gene discovery is rapidly accelerating due to efficient high-throughput exome sequencing [29], but the new progress so far is for syndromic pediatric encephalopathies such as Lennox-Gastaut syndrome. The pace remains much slower for genetic generalized epilepsies, including absence epilepsy. With new mutagenesis technologies such as TALEN and CRISPR to more readily validate candidate genes in large intervals such as those defined in modifier or QTL mapping, the pursuit of modifiers still holds promise for unbiased discovery of new genes, pathways and future novel therapies for idiopathic disease.
Materials and Methods
Mouse strain care and sources
All mice were housed and procedures performed with approval of Institutional Animal Care and Use Committee (IACUC). All mice were obtained from The Jackson Laboratory, maintained in a room with a 14 h hour light on/10 h light off cycle, and given free access to LabChow meal and water.
C3H/HeJ (HeJ), C3HeB/FeJ (FeJ), C3H/HeOuJ, C3H/HeSnJ and C57BL/6J (B6J) inbred mouse strains were obtained from The Jackson Laboratory production colonies and subsequently maintained by sib-matings. C57BL/6J.129-Gria4KO congenic knockout mice were originally obtained from Deltagen, Inc, as previously described [16]. FeJ.HeJ-Gria4IAP congenic mice were generated by backcrossing the Gria4spkw1 mutation from its original strain, HeJ, to FeJ for 14 generations, bred to homozygosity and maintained by sib-matings. FeJ-Gabrg2tmSpet congenic mice were generated by backcrossing the Gabrg2tmSpet (also known as Gabrg2R43Q) knockout mutation from B6J.129-Gabrg2tmSpet congenic mice (originally obtained from Bionomics, LTD) successively for at least 20 generations to FeJ and maintained in the same way. FeJ.B6J-Scn8a8J congenic mice were generated by successive backcrossing of Scn8a8J (also known as Scn8aV929F) for at least 15 generations to FeJ and maintained in the same way, as described recently [30]. The 89 backcross mice used for genome-wide mapping were generated by mating (B6J.129-Gria4KO/KO×FeJ.HeJ-Gria4IAP/IAP)F1 hybrids to B6J.129-Gria4KO/KO congenic mice. The 84 B6J-FeJ-G4swdm1 congenic intercross mice were created by successively backcrossing the FeJ alleles for genetic markers in the critical interval on Chr15 from an N2 to the B6J.129 strain while also selecting for Gria4KO homozygotes, and then intercrossing at generation N9 or N10. The generation of Pcnxl2 mutants is described below.
TALEN mutagenesis
We contracted Transposagen Biopharmaceuticals, Inc. to design and construct plasmids for TALENs specific to exon 16 (TALEN A) and exon 29 (TALEN B) of Pcnxl2. Target sequences were as follows; TGAGCCGGCAGAGCAGTG and GTGAGTAGCTGTCCTGTA for TALEN A and TATTTGCTGACATGGAC and TTGTTCCAGCCATCCGAA for TALEN B. TALEN plasmids were linearized by PmeI endonuclease digestion. One microgram of linearized plasmid was used as a template for in vitro transcription using AmpliCap-Max T7 High Yield Message Maker Kit (CELLSCRIPT) according to the manufactures instruction. A poly(A) tail was added to the synthesized RNA with the A-Plus Poly(A) Polymerase Tailing Kit (CELLSCRIPT) according to the manufactures instruction. The poly(A) tailed capped RNA was purified by ammonium acetate precipitation, resuspended in RNase free water and the concentration determined by spectrophotometry. TALEN mRNA was diluted to 10 ng/υl in RNase free 1X TE (10 mM Tris-HCl, 1 mM EDTA, pH 7.5) immediately before microinjection into embryos obtained from superovulated FeJ.HeJ-Gria4IAP/IAP congenic mice. The genomic DNA made from the tail tip of 63 TALENA mutant founders was amplified with primers aFXTN (5′-CATCGTGGCTGTCGTAATTC -3′) and aRXTN (5′-CATAGCGTGGGAGAGAAAGA-3′). The product was purified and sequenced using primer aF2XTN (5′-GCACACACCACTCATTCATC-3′). The genomic DNA made from the tail tip of 43TALENB mutant founders was amplified with primers bFXTN (5′ - GCTTTGTAATGTGGGTTCTG-3′) and bRXTN (5′ - GGTTCTCTACTTCAGCCTATG-3′). The product was purified and sequenced using primer bF2XTN (5′-GAACTCGGGATCCATGTTTG -3′).
Genome scan, interval mapping
For the genome scan, genomic DNA was prepared from tail tips as previously described and sent to Kbioscience (currently LGC Genomics, LLC. Beverly, MA), using a custom single nucleotide polymorphisms (SNP) panel comprised of 187 roughly evenly spaced SNPs. Prior to interval mapping, the raw traits SWD incidence and SWD length were rank-ordered and normal quantile transformed, and from which principal components were derived using JMP software (SAS Institute); once obtained, both principal components were also rank - and normal-quantile transformed, also using tools in JMP. The computer program J/qtl [31] was then used for genome-wide interval mapping of the initial backcross, and for Chr 15 interval mapping of congenic intercross mice. To control for a modest effect (p<0.04) of Gria4KO/KO homozygous null vs Gria4KO/IAP compound heterozygous null/hypomorph genotypes segregating in the N2 cross, a marker linked to Gria4 on Chr 9 was used as a covariate. Sex-averaged genetic map coordinates for SNP markers were obtained from the Mouse Genome Informatics database at The Jackson Laboratory (http://informatics.jax.org). For interval mapping, the multiple imputation model was used and permutation shuffling employed to determine genome-wide significance thresholds.
Genotyping
C3H/HeJ-Pcnxl2IAP (IAP insertion in intron 19 of C3H/HeJ) was genotyped in standard PCR conditions and agarose gel electrophoresis using one assay for the wild-type allele (primers c8-128.3F 5′-AGCGATGAGGACTGTGGTTT-3′; c8-128.3R 5′-CGAGCCCTTCAGCTACTCAC-3′) and a second assay for the insertion allele (primers c8-128.3F 5′-AGCGATGAGGACTGTGGTTT-3′; IAPLTR5′R 5′-GGCTCATGCGCAGATTATTT-3′) giving a 364 bp product from the insertion allele and 432 bp product from the endogenous allele. The TALEN A+1 or frameshift 1 (FS2) allele was genotyped in standard PCR conditions at an annealing temperature of 64°C and agarose gel electrophoresis using one assay for the wild-type allele (primers Pcnxl2A1WF2 5′-GCAGAGCAGTGATCCTTCAG -3′; Pcnxl2A1WR2 5′-CCATAGCGTGGGAGAGAAAGAA -3′) and a second assay for the mutant allele (primers Pcnxl2A1MF2 5′-GCAGAGCAGTGATCCTTCAC-3′; Pcnxl2A1WR2 5′-CCATAGCGTGGGAGAGAAAGAA -3′) giving a 311 bp product for both alleles. TALEN B-2 or FS3 allele was genotyped in standard PCR conditions with an annealing temperature of 67°C and agarose gel electrophoresis using one assay for the wild-type allele (primers Pcnxl2BwtF 5′-TAGATGCTGGTAGGAGTGAAGA -3′; Pcnxl2BwtR 5′-GGCTGGAACAACAACTTTGTGT-3′) and a second assay for the mutant allele (primers Pcnxl2BwtF 5′-TAGATGCTGGTAGGAGTGAAGA -3′; Pcnxl2BmutR 5′-GGCTGGAACAACAACTTTGTAG-3′) giving a 228 bp product from the mutagenized allele and a 227 bp product from the wildtype allele. TALEN A-11 or FS1 allele was genotyped in standard PCR conditions at an annealing temperature of 55°C and agarose gel electrophoresis using primers Pcnxl2AF2 (5′ - CACTGTTCTCGGCCTTCTG-3′) and Pcnxl2AR (5′-AGACATGTGGACATGCGTTTA-3′) giving a 123 bp product from the mutagenized allele and a 134 bp product from the endogenous allele.
Detection and cloning of IAP-1Δ1 insertions from genomic DNA
For direct detection of IAP-1Δ1 insertions, dried gel hybridization was done essentially as previously described [32] except using an oligonucleotide probe that spans the 1.2 gag-pol common deletion of IAP-1Δ1 elements [22]. Briefly, 8 µg of high quality mouse genomic DNA (obtained from The Jackson Laboratory DNA Resource) was digested with restriction enzyme Bgl II, electrophoresed overnight on 0.8% Tris-borate EDTA agarose gels, EtBr-stained and imaged, denatured in NaOH, neutralized and dried for several hours on a flat slab gel dryer with minimal vacuum. 5′-32P radiolabeled 31-nt oligonucleotide probe (IAPd1oligo1-R; 5′ ATACCTCTTATCAGGTTCAGCAGAATAAGCTC-3′) was hybridized overnight, the dried gel was washed and imaged on x-ray film. For cloning of IAP-1Δ1-host junction fragments by inverse PCR: 1 µg of genomic DNA was digested with BglII, diluted, then 10 ng was ligated for 2 hrs at room-temperature using T4 DNA ligase, heat-inactivated, then circular product was amplified in a polymerase chain reaction (PCR) using an oligonucleotide that spanned the IAP-1Δ1 deletion (IAPd1oligo2F, 5′ - GAGCTTATTCTGCTGAACCTGATA-3′) paired with an oligonucleotide specific for the IAP LTR (IAPLTR5′, 5′ - GGCTCATGCGCAGATTATTT-′3). Amplified fragments were visualized by agarose gel electrophoresis (e.g. Figure 1C), excised and extracted from agarose using Qiagen minicolumns, cloned into Bluescript plasmid by T/A cloning (Invitrogen), transformed into a suitable E. coli K12 host, miniprepped and subjected to Sanger sequencing using T7 and Sp6 vector primers.
Quantitative RT-PCR
Total RNA was prepared from the whole brain of adult HeJ, FeJ, and FeJ-Gria4IAP and the hippocampus of adult FeJ - Gria4IAP either wildtype or carrying Pcnxl2 TALEN mutations with Trizol (Invitrogen) and treated with DNase I (Promega) under the manufacturer's suggested conditions. RNA (2 µg) was reverse transcribed with AMV reverse transcriptase (Promega). The cDNA was diluted 20-fold, and 2 µl was added to DyNAmo HS SYBR Green qPCR master mix (Thermo Scientific) with pairs of the following primers; beta-actinF (5′ - ATGCTCCCCGGGCTGTAT-3′) and beta-actinR (5′ - CATAGGAGTCCTTCTGACCCATTC-3′), Pcnxl2exon19F (5′-GGATCTCACATCCTGTGCTC -3′) and Pcnxl2exon20R (5′-CCACACGTAGAGTCTCTCAAAC -3′), Gria4exon15F (5′ - GGTGGCTTTGATAGAGTTCTGTTACA-3′) and Gria4exon16R (5′ - TCTTATGGCTTCGGAAAAAGTCA -3′), Pcnxexon35F (5′-GAACAGCTGGAAAGACTGGA-3′) and Pcnxexon36R (5′-CGATGTGGGACCTTGTACTT-3′). The PCR reactions were analyzed on an Applied Biosystems 7500 Real-Time PCR System. The PCR amplifications from three mice of each strain and/or genotype were run in triplicate. Amplification of the correct size products was confirmed by agarose gel electrophoresis. The ΔΔΧt method was adopted for the calculation of relative transcript levels.
mRNAseq
Somatosensory cortex or thalamus was dissected from wildtype or Scn8a8J/+ B6J and FeJ adult male mice in triplicate, and prepared for high-throughput sequencing on the Illumina HiSeq 2000. The Jackson Laboratory Gene Expression Service prepared mRNA sequencing libraries using the Illumina TruSeq methodology. Tissue was placed in RNALater (Qiagen, Inc, MD), RNA was extracted using TRIzol (Invitrogen, CA). For mRNA-Seq, mRNA was purified from total RNA using biotin tagged poly dT oligonucleotides and streptavidin coated magnetic beads followed by quality control using an Agilent Technologies 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). The mRNA was then amplified and double-stranded cDNA was generated by random priming. The ends of the fragmented DNA were converted into phosphorylated blunt ends. An ‘A’ base was added to the 3′ ends. Illumina-specific adaptors were ligated to the DNA fragments. Using magnetic bead technology, the ligated fragments were size selected and then a final PCR was performed to enrich the adapter-modified DNA fragments since only the DNA fragments with adaptors at both ends will amplify. The sequencing library was first validated using an Agilent Technologies 2100 Bioanalyzer to characterize DNA fragment sizes and concentration. The concentration of DNA fragments with the correct adapters on both sides was then determined using a quantitative PCR strategy, following the kit manufacturer's protocol (Kapa Biosystem, Cambridge, MA). Following library quantitation, libraries were diluted and pooled as necessary. Using the Illumina cBot, libraries were added to the flow cells and clusters were generated prior to 100 bp paired end sequencing on the Illumina HiSeq 2000 (Illumina, San Diego, CA, USA). During and after the sequencing run, sequence quality was monitored using the real time analysis (RTA) and sequence analysis viewer (SAV) software available by Illumina. Following sequencing, demultiplexed fastQ files were generated using the Illumina CASAVA software.
FastQ files were aligned to the C57BL/6J reference genome on a high performance computing cluster using Tophat (http://tophat.cbcb.umd.edu/) for the alignment and RSEM (http://deweylab.biostat.wisc.edu/rsem/) for isoform assembly and quantitation, except that frequency of reads per kilobase was normalized based on quartile instead of the total number of mapped reads. Further analysis was done in R (http://www.R- project.org) using ANOVA and linear modeling to test expression differences by strain, genotype and tissue, and FDR analysis was done in Microsoft Excel (Microsoft Corp).
EEG recording and analysis
Adult mice aged between 6 and 8 weeks were anesthetized with tribromoethanol (400 mg/kg i.p.). Small burr holes were drilled (1 mm anterior to the bregma and 2 mm posterior to the bregma) on both sides of the skull 2 mm lateral to the midline. Four teflon-coated silver wires were soldered onto the pins of a microconnector (Mouser electronics, Texas). The wires were placed between the dura and the brain and a dental cap was then applied. The mice were given a post-operative analgesic of carprofen (5 mg/kg subcutaneous) and allowed a minimum 48 h recovery period before recordings. Differential amplification recordings were recorded between all four electrode pairs, providing 6 channels for each subject. Mice were connected to the EEG Stellate Lamont Pro-36 programmable amplifier (Lamont Medical Instruments, Madison, WI) for a 2-hour period on 2 separate days, between the hours of 9 AM and 4 PM during the lights-on period. EEG data were recorded with Stellate Harmonie software (Stellate Systems, Inc., Montreal, Canada) into a database. SWD consist of adjacent, connected spike-wave (or wave-spike) complexes. Recordings were reviewed using low/hi bandpass filters at 0.3 Hz and 35 Hz respectively, and SWD episodes were scored blinded to genotype using the following criteria: at least 2 connected spike-wave complexes (typically spanning at least 0.5 seconds) with amplitudes at least two fold higher than background and observed concurrently in the majority of the 6 recording channels per mouse.
Supporting Information
Zdroje
1. KosobudAE, CrossSJ, CrabbeJC (1992) Neural sensitivity to pentylenetetrazol convulsions in inbred and selectively bred mice. Brain research 592 : 122–128.
2. KosobudAE, CrabbeJC (1990) Genetic correlations among inbred strain sensitivities to convulsions induced by 9 convulsant drugs. Brain research 526 : 8–16.
3. GoldenGT, SmithGG, FerraroTN, ReyesPF, KulpJK, et al. (1991) Strain differences in convulsive response to the excitotoxin kainic acid. Neuroreport 2 : 141–144.
4. FrankelWN, TaylorL, BeyerB, TempelBL, WhiteHS (2001) Electroconvulsive thresholds of inbred mouse strains. Genomics 74 : 306–312.
5. GoldenGT, FerraroTN, SmithGG, SnyderRL, JonesNL, et al. (2001) Acute cocaine-induced seizures: differential sensitivity of six inbred mouse strains. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 24 : 291–299.
6. FerraroTN, GoldenGT, SmithGG, DeMuthD, BuonoRJ, et al. (2002) Mouse strain variation in maximal electroshock seizure threshold. Brain research 936 : 82–86.
7. CoxGA, LutzCM, YangCL, BiemesderferD, BronsonRT, et al. (1997) Sodium/hydrogen exchanger gene defect in slow-wave epilepsy mutant mice. Cell 91 : 139–148.
8. KearneyJA, BuchnerDA, De HaanG, AdamskaM, LevinSI, et al. (2002) Molecular and pathological effects of a modifier gene on deficiency of the sodium channel Scn8a (Na(v)1.6). Human molecular genetics 11 : 2765–2775.
9. YangY, MahaffeyCL, BerubeN, MaddatuTP, CoxGA, et al. (2007) Complex seizure disorder caused by Brunol4 deficiency in mice. PLoS genetics 3: e124.
10. HawkinsNA, MartinMS, FrankelWN, KearneyJA, EscaygA (2011) Neuronal voltage-gated ion channels are genetic modifiers of generalized epilepsy with febrile seizures plus. Neurobiology of disease 41 : 655–660.
11. HawkinsNA, KearneyJA (2012) Confirmation of an epilepsy modifier locus on mouse chromosome 11 and candidate gene analysis by RNA-Seq. Genes, brain, and behavior 11 : 452–460.
12. BergrenSK, RutterED, KearneyJA (2009) Fine mapping of an epilepsy modifier gene on mouse Chromosome 19. Mammalian genome : official journal of the International Mammalian Genome Society 20 : 359–366.
13. JorgeBS, CampbellCM, MillerAR, RutterED, GurnettCA, et al. (2011) Voltage-gated potassium channel KCNV2 (Kv8.2) contributes to epilepsy susceptibility. Proceedings of the National Academy of Sciences of the United States of America 108 : 5443–5448.
14. PapaleLA, BeyerB, JonesJM, SharkeyLM, TufikS, et al. (2009) Heterozygous mutations of the voltage-gated sodium channel SCN8A are associated with spike-wave discharges and absence epilepsy in mice. Human molecular genetics 18 : 1633–1641.
15. TanHO, ReidCA, SingleFN, DaviesPJ, ChiuC, et al. (2007) Reduced cortical inhibition in a mouse model of familial childhood absence epilepsy. Proceedings of the National Academy of Sciences of the United States of America 104 : 17536–17541.
16. BeyerB, DeleuzeC, LettsVA, MahaffeyCL, BoumilRM, et al. (2008) Absence seizures in C3H/HeJ and knockout mice caused by mutation of the AMPA receptor subunit Gria4. Human molecular genetics 17 : 1738–1749.
17. FrankelWN, BeyerB, MaxwellCR, PretelS, LettsVA, et al. (2005) Development of a new genetic model for absence epilepsy: spike-wave seizures in C3H/He and backcross mice. The Journal of neuroscience : the official journal of the Society for Neuroscience 25 : 3452–3458.
18. TokudaS, BeyerBJ, FrankelWN (2009) Genetic complexity of absence seizures in substrains of C3H mice. Genes, brain, and behavior 8 : 283–289.
19. PazJT, BryantAS, PengK, FennoL, YizharO, et al. (2011) A new mode of corticothalamic transmission revealed in the Gria4(−/−) model of absence epilepsy. Nature neuroscience 14 : 1167–1173.
20. MaksakovaIA, RomanishMT, GagnierL, DunnCA, van de LagemaatLN, et al. (2006) Retroviral elements and their hosts: insertional mutagenesis in the mouse germ line. PLoS genetics 2: e2.
21. Laboratory TSoTJ (1997) Handbook on Genetically Standardized JAX® Mice; Fox RR, Witham BA, editors. Bar Harbor: The Jackson Laboratory.
22. IshiharaH, TanakaI, WanH, NojimaK, YoshidaK (2004) Retrotransposition of limited deletion type of intracisternal A-particle elements in the myeloid leukemia Clls of C3H/He mice. Journal of radiation research 45 : 25–32.
23. NellakerC, KeaneTM, YalcinB, WongK, AgamA, et al. (2012) The genomic landscape shaped by selection on transposable elements across 18 mouse strains. Genome biology 13: R45.
24. LaBonneSG, SunithaI, MahowaldAP (1989) Molecular genetics of pecanex, a maternal-effect neurogenic locus of Drosophila melanogaster that potentially encodes a large transmembrane protein. Developmental biology 136 : 1–16.
25. YamakawaT, YamadaK, SasamuraT, NakazawaN, KanaiM, et al. (2012) Deficient Notch signaling associated with neurogenic pecanex is compensated for by the unfolded protein response in Drosophila. Development 139 : 558–567.
26. CermakT, DoyleEL, ChristianM, WangL, ZhangY, et al. (2011) Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic acids research 39: e82.
27. StanfordWL, HaqueS, AlexanderR, LiuX, LatourAM, et al. (1997) Altered proliferative response by T lymphocytes of Ly-6A (Sca-1) null mice. The Journal of experimental medicine 186 : 705–717.
28. TylerAL, LuW, HendrickJJ, PhilipVM, CarterGW (2013) CAPE: an R package for combined analysis of pleiotropy and epistasis. PLoS computational biology 9: e1003270.
29. AllenAS, BerkovicSF, CossetteP, DelantyN, DlugosD, et al. (2013) De novo mutations in epileptic encephalopathies. Nature 501 : 217–221.
30. OlivaMK, McGarrTC, BeyerBJ, GazinaE, KaplanDI, et al. (2014) Physiological and genetic analysis of multiple sodium channel variants in a model of genetic absence epilepsy. Neurobiology of Disease
31. SmithR, SheppardK, DiPetrilloK, ChurchillG (2009) Quantitative trait locus analysis using J/qtl. Methods in molecular biology 573 : 175–188.
32. LuedersKK, FrankelWN, MietzJA, KuffEL (1993) Genomic mapping of intracisternal A-particle proviral elements. Mammalian genome : official journal of the International Mammalian Genome Society 4 : 69–77.
Štítky
Genetika Reprodukční medicína
Článek Comparative Phylogenomics Uncovers the Impact of Symbiotic Associations on Host Genome EvolutionČlánek Distribution and Medical Impact of Loss-of-Function Variants in the Finnish Founder PopulationČlánek Common Transcriptional Mechanisms for Visual Photoreceptor Cell Differentiation among PancrustaceansČlánek Integrative Genomics Reveals Novel Molecular Pathways and Gene Networks for Coronary Artery DiseaseČlánek An ARID Domain-Containing Protein within Nuclear Bodies Is Required for Sperm Cell Formation inČlánek Knock-In Reporter Mice Demonstrate that DNA Repair by Non-homologous End Joining Declines with Age
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 7
-
Všechny články tohoto čísla
- Cuba: Exploring the History of Admixture and the Genetic Basis of Pigmentation Using Autosomal and Uniparental Markers
- Clonal Architecture of Secondary Acute Myeloid Leukemia Defined by Single-Cell Sequencing
- Mechanisms of Functional Variants That Impair Regulated Bicarbonate Permeation and Increase Risk for Pancreatitis but Not for Cystic Fibrosis
- Nucleosomes Shape DNA Polymorphism and Divergence
- Functional Diversification of Hsp40: Distinct J-Protein Functional Requirements for Two Prions Allow for Chaperone-Dependent Prion Selection
- Comparative Phylogenomics Uncovers the Impact of Symbiotic Associations on Host Genome Evolution
- Activation of the Immune System by Combinations of Common Alleles
- Age-Associated Sperm DNA Methylation Alterations: Possible Implications in Offspring Disease Susceptibility
- Muscle-Specific SIRT1 Gain-of-Function Increases Slow-Twitch Fibers and Ameliorates Pathophysiology in a Mouse Model of Duchenne Muscular Dystrophy
- MDRL lncRNA Regulates the Processing of miR-484 Primary Transcript by Targeting miR-361
- Hypersensitivity of Primordial Germ Cells to Compromised Replication-Associated DNA Repair Involves ATM-p53-p21 Signaling
- Intrapopulation Genome Size Variation in Reflects Life History Variation and Plasticity
- SlmA Antagonism of FtsZ Assembly Employs a Two-pronged Mechanism like MinCD
- Distribution and Medical Impact of Loss-of-Function Variants in the Finnish Founder Population
- Determinative Developmental Cell Lineages Are Robust to Cell Deaths
- DELLA Protein Degradation Is Controlled by a Type-One Protein Phosphatase, TOPP4
- Wnt Signaling Interacts with Bmp and Edn1 to Regulate Dorsal-Ventral Patterning and Growth of the Craniofacial Skeleton
- Common Transcriptional Mechanisms for Visual Photoreceptor Cell Differentiation among Pancrustaceans
- UVB Induces a Genome-Wide Acting Negative Regulatory Mechanism That Operates at the Level of Transcription Initiation in Human Cells
- The Nesprin Family Member ANC-1 Regulates Synapse Formation and Axon Termination by Functioning in a Pathway with RPM-1 and β-Catenin
- Combinatorial Interactions Are Required for the Efficient Recruitment of Pho Repressive Complex (PhoRC) to Polycomb Response Elements
- Recombination in the Human Pseudoautosomal Region PAR1
- Microsatellite Interruptions Stabilize Primate Genomes and Exist as Population-Specific Single Nucleotide Polymorphisms within Individual Human Genomes
- An Intronic microRNA Links Rb/E2F and EGFR Signaling
- An Essential Nonredundant Role for Mycobacterial DnaK in Native Protein Folding
- Integrative Genomics Reveals Novel Molecular Pathways and Gene Networks for Coronary Artery Disease
- The Genomic Landscape of the Ewing Sarcoma Family of Tumors Reveals Recurrent Mutation
- Evolution and Genetic Architecture of Chromatin Accessibility and Function in Yeast
- An ARID Domain-Containing Protein within Nuclear Bodies Is Required for Sperm Cell Formation in
- Stage-Dependent and Locus-Specific Role of Histone Demethylase Jumonji D3 (JMJD3) in the Embryonic Stages of Lung Development
- Genome Wide Association Identifies Common Variants at the Locus Influencing Plasma Cortisol and Corticosteroid Binding Globulin
- Regulation of Feto-Maternal Barrier by Matriptase- and PAR-2-Mediated Signaling Is Required for Placental Morphogenesis and Mouse Embryonic Survival
- Apomictic and Sexual Germline Development Differ with Respect to Cell Cycle, Transcriptional, Hormonal and Epigenetic Regulation
- Functional EF-Hands in Neuronal Calcium Sensor GCAP2 Determine Its Phosphorylation State and Subcellular Distribution , and Are Essential for Photoreceptor Cell Integrity
- Comparison of Methods to Account for Relatedness in Genome-Wide Association Studies with Family-Based Data
- Knock-In Reporter Mice Demonstrate that DNA Repair by Non-homologous End Joining Declines with Age
- Cis and Trans Effects of Human Genomic Variants on Gene Expression
- 8.2% of the Human Genome Is Constrained: Variation in Rates of Turnover across Functional Element Classes in the Human Lineage
- Novel Approach Identifies SNPs in and with Evidence for Parent-of-Origin Effect on Body Mass Index
- Hypoxia Adaptations in the Grey Wolf () from Qinghai-Tibet Plateau
- A Loss of Function Screen of Identified Genome-Wide Association Study Loci Reveals New Genes Controlling Hematopoiesis
- Unraveling Genetic Modifiers in the Mouse Model of Absence Epilepsy
- DNA Topoisomerase 1α Promotes Transcriptional Silencing of Transposable Elements through DNA Methylation and Histone Lysine 9 Dimethylation in
- The Coding and Noncoding Architecture of the Genome
- A Novel Locus Is Associated with Large Artery Atherosclerotic Stroke Using a Genome-Wide Age-at-Onset Informed Approach
- Brg1 Loss Attenuates Aberrant Wnt-Signalling and Prevents Wnt-Dependent Tumourigenesis in the Murine Small Intestine
- The PTK7-Related Transmembrane Proteins Off-track and Off-track 2 Are Co-receptors for Wnt2 Required for Male Fertility
- The Co-factor of LIM Domains (CLIM/LDB/NLI) Maintains Basal Mammary Epithelial Stem Cells and Promotes Breast Tumorigenesis
- Essential Genetic Interactors of Required for Spatial Sequestration and Asymmetrical Inheritance of Protein Aggregates
- Meiosis-Specific Cohesin Component, Is Essential for Maintaining Centromere Chromatid Cohesion, and Required for DNA Repair and Synapsis between Homologous Chromosomes
- Silencing Is Noisy: Population and Cell Level Noise in Telomere-Adjacent Genes Is Dependent on Telomere Position and Sir2
- The Two Cis-Acting Sites, and , Contribute to the Longitudinal Organisation of Chromosome I
- A Broadly Conserved G-Protein-Coupled Receptor Kinase Phosphorylation Mechanism Controls Smoothened Activity
- Requirements for Acute Burn and Chronic Surgical Wound Infection
- LIN-42, the PERIOD homolog, Negatively Regulates MicroRNA Transcription
- WAPL Is Essential for the Prophase Removal of Cohesin during Meiosis
- Expression in Planarian Neoblasts after Injury Controls Anterior Pole Regeneration
- Sox11 Is Required to Maintain Proper Levels of Hedgehog Signaling during Vertebrate Ocular Morphogenesis
- Accumulation of a Threonine Biosynthetic Intermediate Attenuates General Amino Acid Control by Accelerating Degradation of Gcn4 via Pho85 and Cdk8
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Wnt Signaling Interacts with Bmp and Edn1 to Regulate Dorsal-Ventral Patterning and Growth of the Craniofacial Skeleton
- Novel Approach Identifies SNPs in and with Evidence for Parent-of-Origin Effect on Body Mass Index
- Hypoxia Adaptations in the Grey Wolf () from Qinghai-Tibet Plateau
- DNA Topoisomerase 1α Promotes Transcriptional Silencing of Transposable Elements through DNA Methylation and Histone Lysine 9 Dimethylation in
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



