-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
Reklama8.2% of the Human Genome Is Constrained: Variation in Rates of Turnover across Functional Element Classes in the Human Lineage
Nearly 99% of the human genome does not encode proteins, and while there recently has been extensive biochemical annotation of the remaining noncoding fraction, it remains unclear whether or not the bulk of these DNA sequences have important functional roles. By comparing the genome sequences of different species we identify genomic regions that have evolved unexpectedly slowly, a signature of natural selection upon functional sequence. Using a high resolution evolutionary approach to find sequence showing evolutionary signatures of functionality we estimate that a total of 8.2% (7.1–9.2%) of the human genome is presently functional, more than three times as much than is functional and shared between human and mouse. This implies that there is an abundance of sequences with short lived lineage-specific functionality. As expected, most of the sequence involved in this functional “turnover” is noncoding, while protein coding sequence is stably preserved over longer evolutionary timescales. More generally, we find that the rate of functional turnover varies significantly across categories of functional noncoding elements. Our results provide a pan-mammalian and whole genome perspective on how rapidly different classes of sequence have gained and lost functionality down the human lineage.
Published in the journal: . PLoS Genet 10(7): e32767. doi:10.1371/journal.pgen.1004525
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004525Summary
Nearly 99% of the human genome does not encode proteins, and while there recently has been extensive biochemical annotation of the remaining noncoding fraction, it remains unclear whether or not the bulk of these DNA sequences have important functional roles. By comparing the genome sequences of different species we identify genomic regions that have evolved unexpectedly slowly, a signature of natural selection upon functional sequence. Using a high resolution evolutionary approach to find sequence showing evolutionary signatures of functionality we estimate that a total of 8.2% (7.1–9.2%) of the human genome is presently functional, more than three times as much than is functional and shared between human and mouse. This implies that there is an abundance of sequences with short lived lineage-specific functionality. As expected, most of the sequence involved in this functional “turnover” is noncoding, while protein coding sequence is stably preserved over longer evolutionary timescales. More generally, we find that the rate of functional turnover varies significantly across categories of functional noncoding elements. Our results provide a pan-mammalian and whole genome perspective on how rapidly different classes of sequence have gained and lost functionality down the human lineage.
Introduction
“What proportion of the human genome is functional?” remains a contentious question [1]–[3]. In great part this reflects the use of definitions of ‘function’ that differ from the traditional definition that is based on fitness and selection (see e.g. [4] for a discussion). For instance, equating functionality with annotation by at least one of the ENCODE consortium's biochemical assays [5] results in approximately 80% of the human genome being labeled as functional [1], [6]. While this approach has the advantage of being empirical, it makes the definition of functionality dependent on the choice of experiments and details such as P value cutoffs. It is also questionable whether, for instance, introns should be classified as functional based merely on their transcription [2], [4].
By contrast, evolutionary studies often equate functionality with signatures of selection. While it is undisputed that many functional regions have evolved under complex selective regimes including selective sweeps [7] or ongoing balancing selection [8], [9], and it appears likely that loci exist where recent positive selection or reduction of constraint has decoupled deep evolutionary patterns from present functional status [10], [11], it is widely accepted that purifying selection persisting over long evolutionary times is a ubiquitous mode of evolution [12], [13]. While acknowledging the caveats, this justifies the definition of functional nucleotides used here, as those that are presently subject to purifying selection.
This is of course not useful as an operational definition, as selection cannot be measured instantaneously. Instead, most studies define functional sites as those subject to purifying selection between two (or more) particular species. Studies that follow this definition have estimated the proportion of functional nucleotides in the human genome, denoted as αsel [14], [15], between 3% and 15% ([3] and references therein, [16]). Since each species' lineage gains and loses functional elements over time, αsel needs to be understood in the context of divergence between species. The divergence influences the estimate of αsel in two ways. On the one hand, constrained sequence between closely related species, including lineage-specific constrained sequence, is harder to detect than more broadly conserved sequence because of a paucity of informative mutations, which reduces detection power. On the other hand, estimates of constraint between any two species will only include sequence that was present in their common ancestor and that has been constrained in the lineages leading up to both extant species' genomes, with the consequence that turnover of functional sequence leads to diminishing αsel estimates as the species divergence increases. Assuming that the first effect can be controlled for, higher estimates of sequence constraint that are obtained between more closely related species [15], [17] are thus indicative of the turnover of functional sequence [15]. Here we understand turnover to mean the loss or gain of purifying selection at a particular locus of the genome, when changes in the physical or genetic environment, or mutations at the locus itself, cause the locus to switch from being functional to being non-functional or vice versa.
Two previous studies have made quantitative estimates of the overall rate of turnover ([15], [17], reviewed in [3]). The estimate by Smith et al. (2004) [17] was derived from an analysis of point mutations in alignments across a 1.8 Mb genomic region. While a high rate of turnover was inferred, the authors emphasised the preliminary nature of their work as a consequence of the limited amount of data available to them at that time. Later, Meader et al. (2010) [15] performed genome-wide analysis with a neutral indel model (see [18], here referred to as NIM1) to estimate the fraction, termed αselIndel, of human sequence that was constrained with respect to insertions or deletion mutations (indels). This study also found a high rate of turnover, and estimated using two ad hoc heuristic approaches that 6.5–10% of the human genome is functional. Extrapolations using these data subsequently suggested that 10–15% of the human genome is presently functional [3].
NIM1 is a quantitative model describing the distribution of distances between neighbouring indels (intergap segments; IGSs) in neutrally evolving sequence, which provides an excellent description of the observed frequency of medium-sized IGSs. However, across whole genome alignments longer IGSs are strikingly overrepresented compared to this expectation under neutrality, presumably as a result of the presence of functional genomic segments under purifying selection in which indel mutations are unlikely to become fixed. By quantifying this overrepresentation it is possible to estimate αselIndel, the fraction of nucleotides contained within these functional segments. The model (which also accounts for G+C content and sex chromosome-dependent mutational biases) performs well for simulated data, and accurately identifies coding regions and ancestral repeats as highly conserved and neutrally evolving, respectively [15], [18]. However, some concerns about the model's derivation and the quality of whole-genome alignments we used were subsequently brought to our attention, which motivated us to initiate this study.
Here we present improved methods for the estimation of αselIndel and the inference of functional turnover, building on our previous approaches [15], [18]. We apply these improved approaches to pairwise alignments between the genomes of diverse eutherian mammals, and we estimate that 7.1–9.2% of the human genome is presently subject to purifying selection, equating to 220–286 Mb of constrained sequence. We also take advantage of the additional high-quality eutherian genome sequences that have become available since our previous study to provide improved estimates of the rate of turnover of functional sequence in these species. Improvements in biological and biochemical annotation of genomic sequence mean that we can investigate turnover rates within particular classes of functional elements, such as coding sequences, DNase 1 hypersensitivity sites (DNase HSs), transcription factor binding sites (TFBSs), enhancers, promoters, and long noncoding RNAs (lncRNAs). We find striking differences between the functional element classes; in particular constrained coding sequences are much more evolutionary stable than constrained noncoding sequences, and lncRNAs show the most rapid rate of turnover of all the noncoding element types.
Results
We developed three improvements for estimating αselIndel. First, we identified two issues in the original derivation of the NIM1 model, and found that corrections result in equal but opposite changes in the inferred αselIndel, so that these issues do not invalidate the original results (Text S1). To provide further assurance of the accuracy of the derivation we introduced a new likelihood neutral indel model (NIM2) that provides a partially independent validation of the revised NIM1 estimates (Text S2). Second, we find that earlier αselIndel estimates were upwardly biased as a consequence of poor quality alignments (Materials and Methods; Text S3; Figure S1; Figure S2). Third, we significantly extended the original simulation study, testing the influence of a wide range of modelling assumptions on the inferences. Results underscored the validity, accuracy and robustness of the model (Text S4; Text S5; Figure 1A; Figure S3).
Fig. 1. Evolutionary turnover of constrained sequence. 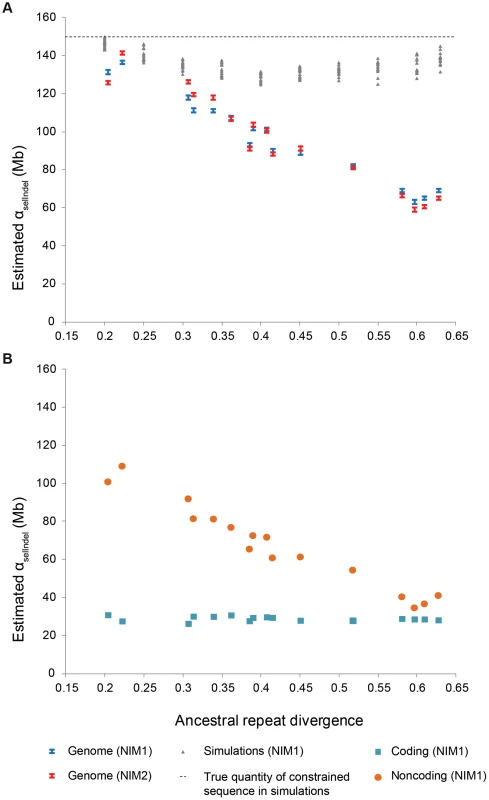
A. Quantity of constrained sequence (αselIndel) estimated by NIM1 (blue bars) and NIM2 (red bars) plotted against ancestral repeat divergence for different pairs of eutherian species genomes, with the simulated data (grey) shown under a non-turnover scenario. B. Coding sequence (blue squares) is seen to be broadly conserved, while constrained noncoding sequence (orange circles) shows a strong negative correlation between αselIndel and divergence, indicating rapid turnover. We applied the neutral indel model to estimate αselIndel on trimmed whole genome alignments between a wide range of eutherian species pairs for which high quality genome assemblies are available. Estimates of αselIndel (blue symbols in Figure 1A; Table 1) were largely concordant with the likelihood neutral indel model NIM2 (red symbols). Our new estimates are considerably reduced (by 10%–40%) compared to our previous αselIndel estimates (Table 1) [15]. These differences are largely attributable to alignment trimming. Previously we reported lower and higher bounds for αselIndel under two assumptions of the extent of clustering of functional sequence [15], but simulations indicate that the higher bound is irrelevant under all but unrealistically strong clustering. We therefore now report the lower bound only, and in addition provide 95% confidence intervals obtained from regression estimates and standard assumptions on error distributions. (Materials and Methods; Text S3).
Tab. 1. Estimated quantities of sequence constrained with respect to indels (αselIndel) between different species under different models. 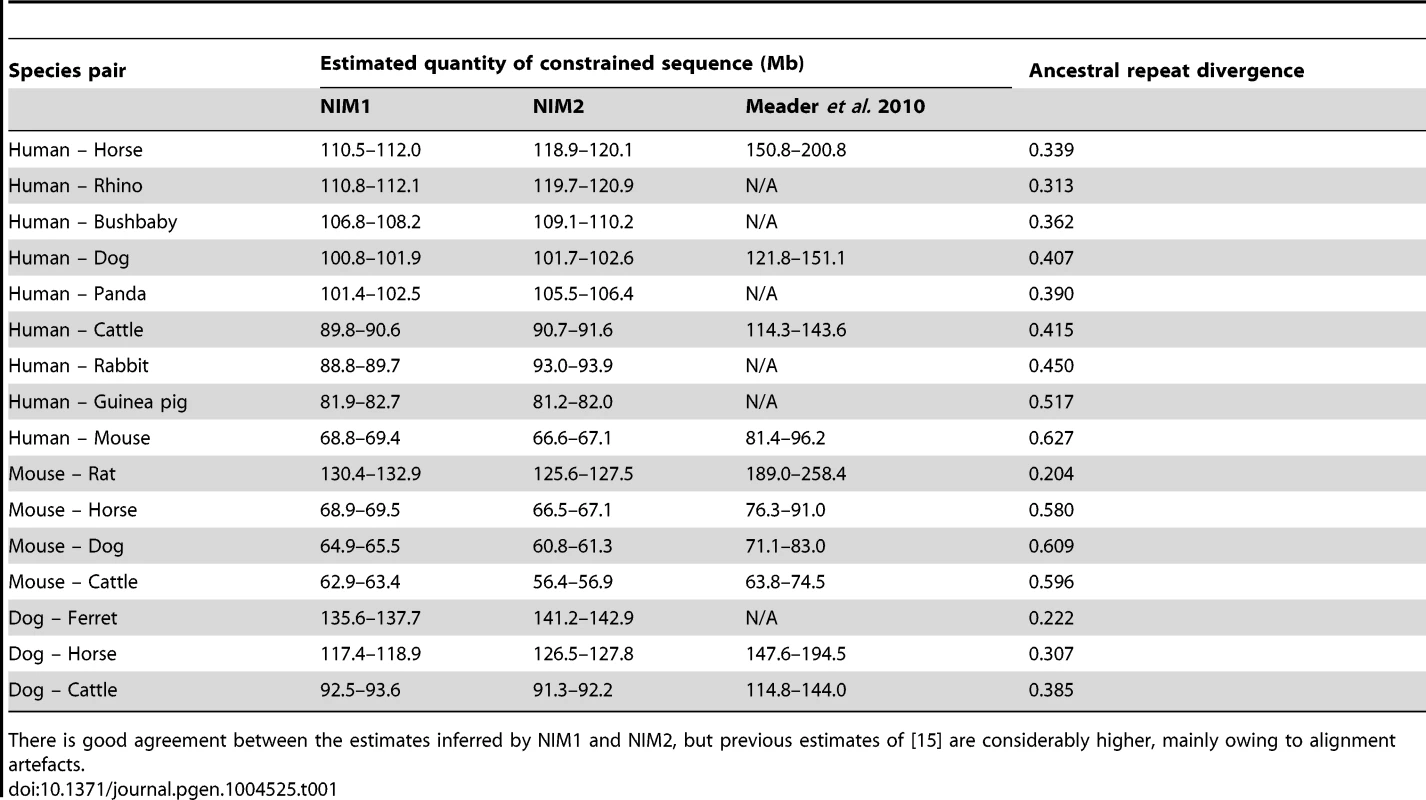
There is good agreement between the estimates inferred by NIM1 and NIM2, but previous estimates of [15] are considerably higher, mainly owing to alignment artefacts. Rapid turnover of functional sequence across eutherian evolution
We observe a strong negative correlation between estimates of αselIndel and the divergence of the two species being compared (Figure 1), consistent with substantial turnover of functional sequence and thus with earlier conclusions [15], [17], and inconsistent with simulation results under a scenario in which turnover is absent (Figure 1A).
To exclude the possibility that technical artefacts are driving this observation, we investigated ENCODE annotations in lineage-specific NIM1-constrained sequence. Specifically, we identified NIM1-constrained sequence that was not identified as pan-mammalian conserved by either the PhastCons [12] or GERP++ algorithms [19], and found that such sequence is enriched for biochemically annotated sequences (DNase HSs, TFBSs, and enhancers defined by the ENCODE consortium [5]) (Figure 2; Figure S4). This is expected if functional elements, including these ENCODE functional classes, have been subject to evolutionary turnover, but is not expected if technical artefacts were causing the observations in Figure 1. Furthermore, using low-frequency polymorphic indels from the 1000 Genomes project we could exclude the possibility that lower mutation rates in ENCODE functional regions were causing the observations. We therefore conclude that observations in Figure 1 reflect turnover of functional elements. A more detailed discussion on this issue is provided in Text S6 and Text S7.
Fig. 2. The overlap of constrained sequence with pan-mammalian conserved sequences. 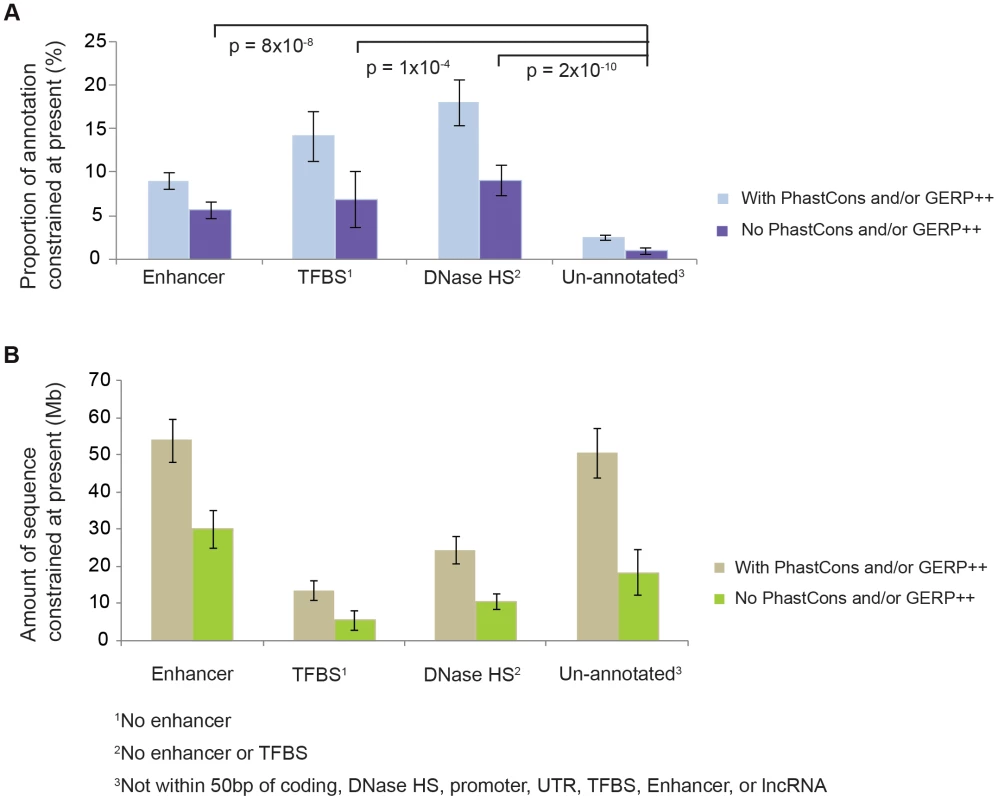
The proportions A., and quantities B., of constrained sequence at the present for different types of biochemically annotated and un-annotated sequences, with and without PhastCons or GERP++ conserved elements, estimated using linear extrapolations (Text S6, Text S7). The NIM1 has power to detect functional lineage-specific constrained sequence: NIM1 detects significantly higher fractions of linage-specific constrained sequence (defined as sequence identified by NIM1 but not annotated by PhastCons or GERP++ as being conserved across mammals) within 3 mutually exclusive classes of ENCODE biochemical annotations compared to sequence lacking such annotation; see Text S6 for details. A model for sequence turnover
To help describe and interpret the observations of turnover (Figure 1) we propose a time-homogeneous model for sequence turnover on a genomic scale. We apply this model to specific sequence classes, such as protein coding genes or TFBSs, allowing us to discuss the rates of turnover for particular types of functional element. The model assumes that within a particular functional class both the total amount a of functional sequence and the rate b of turnover per nucleotide (nt) are constant, and that the turnover rate is the same for all nts in a class. Under this model the total amount of functional nts in any class remains constant over time, but the amount that is currently functional and retains homology to functional nts in the ancestral species at divergence d (i.e., the amount that was constrained and has not turned over in the course of evolution to the present) is . We estimate the parameters a and b by fitting the model to observations using weighted linear regression (Materials and Methods). Instead of the rate parameter b, we, equivalently, often refer to the turnover half life, d1/2, which is defined as the divergence at which half the functional sequences in the class is expected to have turned over and is calculated as loge(2)/b. We express this divergence in time units corresponding to one expected nucleotide substitution per site in neutrally evolving sequence (‘divergence time’). To convert this divergence to years, we apply a substitution rate of 2.2×10−9 per site per year [20]. This will be a more appropriate value for the human lineage, on which we focus, than on rodent lineages whose per-year substitution rate are substantially higher.
The model is time-reversible, so that the same expression describes the amount of mutually constrained sequence between two extant species at divergence d, where d is calculated by adding the divergences along the two branches to their last common ancestor. Similarly, to convert d (in years) to the age of the most recent common ancestor, it should be divided by 2.
To calculate the divergence time we use ancestral repeats (ARs, sequence derived from transposable elements whose insertion predates the species' last common ancestor) as a proxy for neutrally evolving sequence, because they virtually all show the patterns of indel mutation expected under neutral evolution [18]. Our estimates of divergence using either ARs or synonymous sites as neutral proxy are concordant, hence our results are insensitive to the choice of putatively neutral sequence (Figure S5).
Different turnover rates for coding and noncoding sequence
We next used NIM1 to estimate the fraction of constrained sequence within coding and noncoding sequences (Materials and Methods). Within protein coding sequence selective constraint is pervasive, as expected (Figure 1B): 80–88% of human or mouse annotated coding sequence has been under selective constraint with respect to indels across eutherian evolution; slightly lower proportions were estimated under the NIM2 and for dog annotated coding sequences (Figure S6; Text S8).
In contrast to protein coding sequence, estimates for the extent of constraint in noncoding sequence show a pronounced drop-off with increasing divergence (orange filled circles in Figure 1B), an observation compatible with turnover occurring predominantly within the noncoding functional fraction of the genome. When applying the time-homogeneous turnover model to these data, we estimate the turnover rate parameter b for noncoding sequence at 2.48 turnover events per neutral substitution (2.26–2.71, 95% confidence interval), equivalent to a turnover half life d1/2 of 0.28 (0.25–0.31) in units of divergence time, or 127 My (116–139 My) in natural time units. The present estimate represents a slower turnover rate than a previous estimate of d1/2 = 0.19 (86 My) made by Ponting et al. (2011) [3] with data from Meader et al. (2010) [15].
We observe a low yet significantly non-zero rate of turnover in coding sequence, b = 0.24 (0.14–0.33) events per neutral substitution, corresponding to d1/2 = 2.9 (2.1–5.0), or in natural units 1300 My (950–2250 My). These estimates represent an average across the undoubtedly variable rates of turnover across different types of protein coding gene sequence. Nevertheless, under this simple model, we find that protein coding sequence is relatively evolutionarily stable, showing long-term conservation, so that assuming that protein coding sequences exhibit no turnover will often be justified (e.g. [3]). By contrast, present-day constrained noncoding sequence is less stable, being relatively rapidly gained and lost in a lineage-specific manner.
Constraint and turnover among classes of human constrained element
We next investigated whether various classes of functional element, identified in human primarily by the ENCODE project [5], exhibit contrasting levels of constraint, and whether these constrained element classes show a propensity to turn over at different rates. Of the functional classes we considered, promoters, untranslated regions (UTRs), DNAse HSs and TFBSs, enhancers and un-annotated sequences (defined as sequences not within 50 bp of ENCODE DNAse HSs, TFBS loci, lncRNAs from [21], Ensembl coding sequence, or UTRs) all show intermediate levels of turnover (Figure 3; Figure S7, Figure S8). LncRNA sequences show the highest level of turnover (Figure 3; Figure S8), and an even higher rate of turnover was inferred when the ENCODE-defined lncRNAs were used rather than the set from [21] (Figure S9). The fraction of sequence that the model inferred to be under present day constraint also varied across these categories, with intermediate fractions inferred for UTRs, DNAse HSs and TFBSs, and lower fractions for lncRNAs and enhancers. As expected, the lowest fractions were observed for un-annotated sequence; nevertheless, in absolute terms the amount of constrained sequence in this category is considerable (70 Mb, 45–85 Mb) (Figure 3). Constrained sequence in this category may represent lineage-specific functional sequences that were not identified by the ENCODE project, for instance because of their function in tissues or developmental stages not investigated by ENCODE. Finally, transposable element-derived sequences show very small amounts of constraint, and as a result our methods have little power to detect turnover in this class.
Fig. 3. Constraint and turnover for different classes of human functional element. 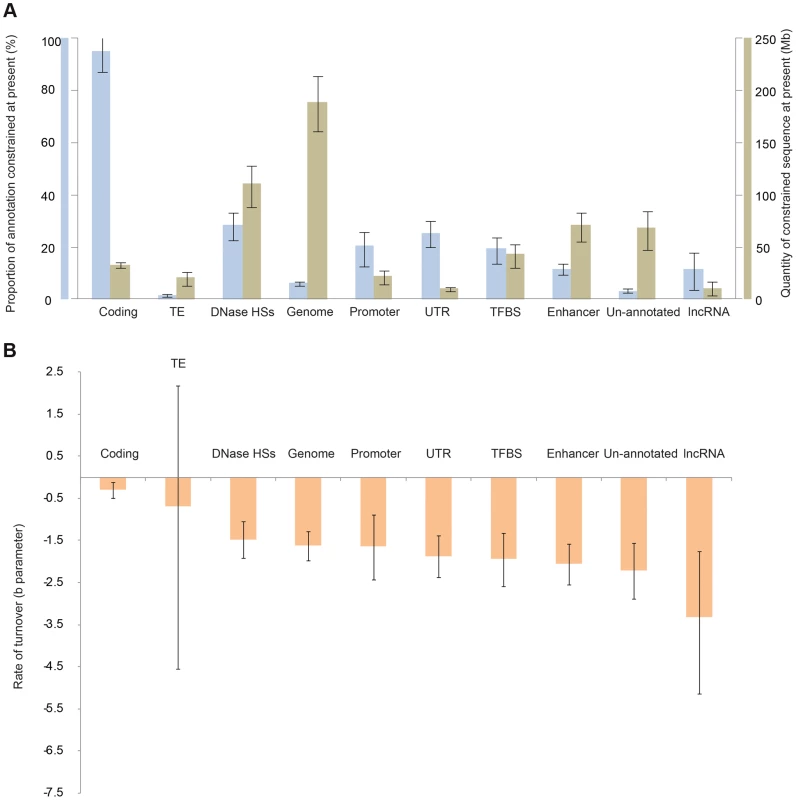
A. The total quantities of constrained sequence estimated for the present day by extrapolation for different element types. B. The estimated rate of turnover (b parameter) for different types of constrained element. Distribution of functional classes in present-day functional DNA
We next examined how constrained sequence in the human genome is distributed cumulatively for selected functional element categories. We do this by fitting the functional turnover model to the observed data and extrapolating to the present day. In this way we also infer the reciprocal quantities of sequence that, when comparing to another species or human ancestor at a particular divergence, are presently functional in human yet have lost (or not gained) constraint in the lineage leading to the ancestor or other species (Figure 4). We stress that this inference relies on the parsimonious yet not formally justified assumption that the total quantity of functional sequence in genomes remains constant over time and therefore across species, and within functional categories. With these caveats we estimate that 8.6 Mb (26%) of constrained coding sequence has lost constraint (and thus has turned over) since the divergence of humans from monotremes approximately 228 million year ago (AR divergence time 1.00), while 200 Mb (79%) of the constrained noncoding human genome is inferred to have lost constraint over the same period. DNAse HSs cover more indel constrained sequence at all divergence ranges than all other annotated noncoding sequence combined, implying that DNAse HSs are an abundant and informative biochemical marker of functionality outside protein coding regions. Enhancers also show a marked contribution towards the constrained human genome, while TFBSs, promoters, UTRs and lncRNAs contribute considerably less sequence once their overlap with other annotations is removed. Finally, about a quarter of sequence inferred to be presently under constraint is not present in any of the annotation categories we considered. In Figure 4 we sum up the quantities of constrained sequence estimated from independent NIM1 runs for different annotation types.
Fig. 4. Model-based inference of turnover by functional class. 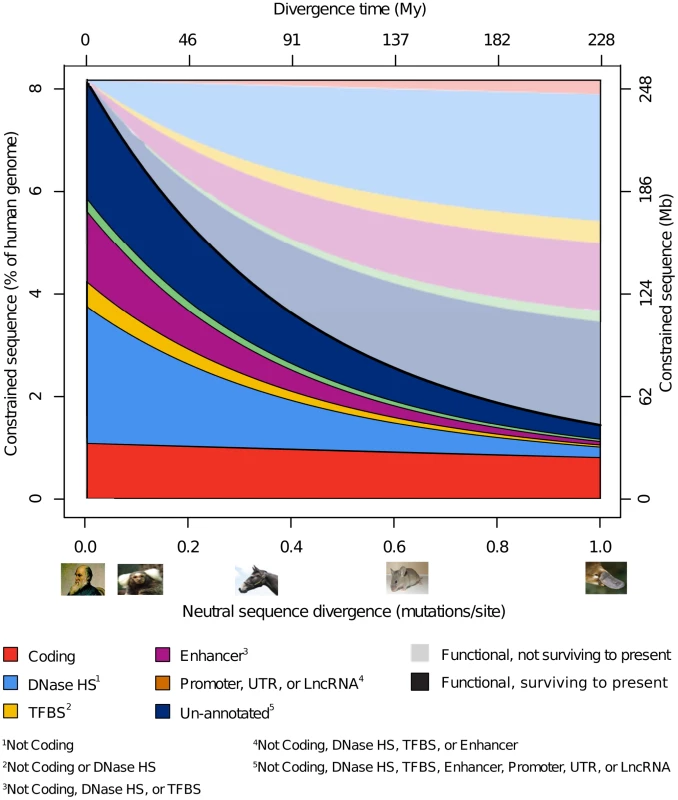
Schematic summary of the fraction of constrained sequence that has been retained (saturated colours) or turned over (pastel colours) in the human lineage over time (X-axis, divergence time) and how it has been distributed across various categories of functional element. In addition to showing the reduced quantity of preserved constrained sequence with increasing divergence, we infer the reciprocal quantity of sequence that is assumed to have been gained over human lineage evolution. For consistency this approach requires mutually exclusive annotation sets, in contrast to those used in Figure 3, making the results not directly comparable. Overlaps between the major different annotations are shown in Figure S10. 7.1–9.2% of human genomes is constrained at present
If we make the assumption that the exponential decay model of functional sequence applies outside of the range of divergences we examined, then by extrapolating back to zero divergence we can estimate the total proportion of human genomes that is under present-day purifying selection with respect to indels. We perform this extrapolation across different annotation sets (Table S6). Although there is some variation in these estimates, we quote the estimate derived separately across multiple different annotation categories, namely coding sequence, DNase HSs, TFBS, Enhancers, unannotated sequence, and other sequence (the latter consisting of promoter, UTR and lncRNA sequences). This is because this estimate allows the rate of turnover to vary across each annotation type, and thus is likely to be more accurate than the estimates that assume a single rate of turnover across the whole genome, or the whole noncoding genome. We therefore estimate that 8.2% of the human genome (253 Mb; 95% CI 7.1%–9.2%, 220–286 Mb) is presently under purifying selection with respect to indels.
Discussion
The question of what fraction of the human genome sequence are mutations preferentially purged owing to their deleterious effect has remained contentious ever since the first estimate was made in 2002 [22]. At that time it was not well appreciated that the amount of human constrained sequence that is also constrained in mouse is a minority (69 Mb; this study) of all human constrained sequence, owing to the relatively rapid gain and loss of functional sequence in their two lineages since their last common ancestor.
We find that NIM1-constrained sequence lacking evidence for pan-mammalian conservation is enriched for sequences with experimental evidence for biochemical activity, and we provide a detailed argument indicating that this is incompatible with the notion of technical artefacts causing the observed signature of turnover (Text S6). Extensive simulations indicating that estimates of constrained sequence are consistent across the divergence range we investigate further support this conclusion. Our estimate that 7.1–9.2% of human genomes is subject to contemporaneous selective constraint considerably exceeds previous estimates and falls short of others [3], [23]. We have shown that our method's previous estimates for specific species pairs, as well as the calculation that suggested 10–15% of the human genome is currently under negative selection were inflated [3], in large part owing to inaccuracies in whole genome alignments upon which our estimates were based. The problems associated with using whole-genome alignments could be circumvented entirely by instead using polymorphism data within a single species. However, this approach is technically highly challenging, and results have so far been controversial [16], [24], [25]; in addition this approach is not informative about functional turnover. Other published estimates [12], [18], [26] are lower because they, by design, were not sensitive to lineage-specific constrained sequence.
Our current estimates have their own particular caveats. While our results show that turnover is a real and substantial effect, simulations show that NIM1 underestimates the true amount of mutually constrained sequence to an extent that shows some dependence on the divergence. While simulations and theory indicate that point estimates of constraint remain conservative, the possibility of an upward bias in the inferred rate of turnover cannot be excluded, which in turn could lead to upwardly biased extrapolations of present-day constraint. In addition, the assumptions of the turnover model, in particular that all elements within a class are subject to the same rate of turnover, clearly are only approximately valid. These potential sources of error are not reflected in our confidence estimates (Table S6).
Our estimate that 7.1%–9.2% of the human genome is functional is around ten-fold lower than the quantity of sequence covered by the ENCODE defined elements [1], [5], [6]. This indicates that a large fraction of the sequence comprised by elements identified by ENCODE as having biochemical activity can be deleted without impacting on fitness. By contrast, the fraction of the human genome that is covered by coding exons, bound motifs and DNase1 footprints, all elements that are likely to contain a high fraction of nucleotides under selection, is 9%. While not all of the elements in these categories will be functional, and functional elements will exist outside of these categories, this figure is consistent with the proportion of sequence we estimate as being currently under the influence of selection.
As expected, turnover has occurred least in protein coding sequence, and thus has been most concentrated on noncoding sequence (Figure 4). For example, of the 43.5 Mb of sequence annotated by the ENCODE project as being within a human TFBS peak and that we find to be constrained (19.3% of the total extent of ENCODE TFBS peaks), only a third (30.6%; 13.3 Mb) is identified by NIM1 as being constrained in both human and mouse. A slightly higher proportion (45.6%; 19.8 Mb) is constrained in human and dog, presumably reflecting these species' lower divergence. These estimates are in good agreement with previous experimental findings: for instance 23–41% of TF binding events have been found to be conserved across human, dog and mouse for four liver TFs [27], while for two additional liver TFs, 7–14% of TF binding events are shared between human and mouse, and 15–20% between human and dog [28]. The phenomenon of turnover is well supported by both anecdotal evidence [27]–[29] and by broader studies of particular classes of elements, mostly TFBSs and enhancer elements [30]–[32]. The class of functional element inferred to turnover fastest was that of lncRNAs, again consistent with observations that most human lncRNAs are primate-specific and only 19% of lncRNAs are conserved over more than 90 My [33].
What our approach cannot clarify is to what extent the observed turnover at the sequence level amounts to different sequences encoding equivalent function [29], [30], or species-specific functional change [16], [31], [34]. Several lines of evidence, both from anecdotal [29] and broader [30], [31] studies of TFBSs, indicate that a large fraction of sequence changes involving TFBSs preserve function. For example, some deeply conserved transcription factors have species-specific binding sites in the vicinity of orthologous genes [27], [28] implying that despite their sequence divergence, the different DNA binding sites confer equivalent functions (on orthologous genes) in different lineages. Comprehensive studies of human and mouse embryonic heart enhancers found these to be weakly conserved [35], [36], despite human enhancers sequences largely driving expected tissue-specific expression in mouse embryonic heart tissue [36]. Another study found that two mammalian hypothalamic enhancers have no homolog across non-mammalian vertebrates, yet are still able to drive specific expression patterns in zebrafish neurons [37]. These findings are consistent with gene expression evolution being shaped predominantly by stabilizing selection on the expression level [38], while evolution on the sequence level may involve an interplay between fixation of weakly deleterious mutations through drift, and weak positive selection on compensatory mutations [39].
However, not all TFBS turnover events are neutral or nearly neutral on the level of gene expression, and the fraction of such events that change gene expression may be substantial [31]. More generally, lineage-specific sequence is clearly a likely substrate for lineage-specific biology [16], [34], although adaptations to pre-existing functional sequence remain an alternative plausible mode for creating species-specific change [40]. Nevertheless, the sheer ubiquity of sequence turnover, and the clear potential for substantial regulatory change resulting from it, suggests that many aspects of noncoding human biology will not be fully recapitulated by orthologous sequence in eutherian model organisms, including mouse. Thus, our findings could provide a more quantitative basis for assessing the relevance of model organisms to specific questions of human biology.
Materials and Methods
Sequence data
We restricted our analyses to genome assemblies that have been sequenced at relatively high coverage, not using for example the 2-fold coverage assemblies of mammalian genomes [41], to minimize the impact of sequencing and assembly errors. From the UCSC Genome Informatics website (http://genome.ucsc.edu/), we acquired softmasked versions of the following genome assemblies: human (hg19), mouse (mm10, mm9, and mm8), rat (rn5), cattle (bosTau7), dog (canFam2), horse (equCab2), guinea pig (cavPor3), rabbit (oryCun2), bushbaby (otoGar3), panda (ailMel1), and rhino (cerSim1). We also acquired a Ferret genome assembly (M_putorius_furo_v1) produced by the Broad Institute. We softmasked the ferret genome assembly using RepeatMasker with carnivore repeat libraries [42].
Alignment construction and trimming
When available, whole genome pairwise alignments were downloaded from the UCSC Genome Informatics website (http://genome.ucsc.edu). Otherwise, we constructed alignments following UCSC's protocol [43]. Initial alignments were constructed with LASTZ (http://www.bx.psu.edu/miller_lab/), a derivative of BLASTZ [44], and these alignments were subsequently chained and netted using tools from UCSC (Table S1 for alignment parameterisations).
We trimmed each of the whole genome alignments once we found that UCSC alignments contained a minority of poorly aligning sequence (Figure S1, Table S2). Each alignment was rescored to generate a new substitution matrix using a log-odds ratio approach as described previously [45]. We did not impose symmetry on the scoring matrixes with respect to strand or species. We then used the generated substitution matrix, with gap penalties derived from the original alignments, to discard (“trim”) the maximal non-positively scoring terminal segments of the alignment blocks and any non-positively scoring inter-gap segments. Trimming removes terminal and internal alignment segments that are more likely to have arisen under a model of independent evolution than of evolution from a common ancestor. Subsequent analyses were carried out following the discarding of all trimmed sequence. We also excluded alignments that were led by sequence not mapped to chromosomes. We did not exclude non-reciprocally aligning sequence or sequence that lay within known indel hotpot locations as we found removing such sequence had relatively small effects on estimates of αselIndel (Table S3).
An updated Neutral indel model 1 (NIM1)
The neutral indel model of Lunter et al. (2006) [18] (NIM1) estimates the genomic fraction (αselIndel) of sequence constrained with respect to indels between a species pair. The model examines the distribution of IGSs from a set of whole genome pairwise alignments using a regression approach over a range of medium IGS lengths to estimate the parameters of a predicted geometric distribution of IGSs in neutral sequence. αselIndel in bp is then estimated by summing up the quantity x - 2K over all the long IGSs inferred to be in excess of predictions under neutral evolution. Here where x is the length of the overrepresented IGS, and K is the estimated mean spacing between indels (“neutral overhang”). 20 equally populated G+C content bins are analysed separately to account, in part, for mutational variation that correlates with G+C content. The X chromosome is also analysed separately. A detailed description of the model is given in the original publications [15], [18]. However, two theoretical issues of the model have not been described previously. These are: (A) that thresholding biases the expected lengths of the neutral overhang and, (B) that neutral segments are depleted from the background distribution due to the presence of constrained segments, changing the expected neutral distribution of IGS lengths; resolution of the two issues is described in Text S1.
Our implementation of the NIM1 differs from that of the preceding studies in the manner in which we calculate the bounds of the estimates. The previous approaches constructed the upper and lower bound estimates based on the uncertainty in the degree of clustering of functional elements. The lower bound estimate was derived assuming that functional elements are unclustered (each overrepresented IGS contributes x - 2K bp towards the αselIndel estimate), while the upper bound was derived assuming a high degree of clustering (each overrepresented IGS contributes x - K bp). In our revised approach, we construct a 95% confidence interval around the lower x - 2K bp estimate. The impact of this change on αselIndel estimates can be seen in the simulation study (Table S5). We made this conservative modification to the NIM1 for five reasons: Firstly, the previous upper bound estimate assumes an unrealistically high degree of clustering of functional elements. Secondly, only our modified estimate is always conservative under all the simulation scenarios, whereas the previous implementation of the NIM1 sometimes overestimates the true value of αselIndel (Table S5). Thirdly, altering the clustering of functional elements in the simulations actually has only a minor effect on the estimated quantities of constrained sequence (Figure S11). Fourthly, in addition to the clustering of functional elements, other parameterisations also influenced αselIndel estimates (Table S5), yet the uncertainty in the values of these parameters was not also incorporated into the NIM1 estimate. Instead, we now choose to incorporate the full extent of uncertainty into the simulations. Finally, by providing a 95% confidence interval for the αselIndel estimate of NIM1, we have an estimate that is directly comparable to the NIM2 estimates.
Estimating the fraction of constraint in subsets of the genome
We have described above how NIM1 is used to estimate the fraction αselIndel of constrained bases within a genome G consisting largely of neutrally evolving sequence. To estimate αselIndel within a subset S⊆G that is not dominated by neutrally evolving sequence, for instance when estimating αselIndel within coding sequence, we instead estimate αselIndel within the subsets G and G\S; the difference between the resulting estimates is the estimate of αselIndel within S.
Estimating the neutral substitution rates
We extracted ancestral repeat (AR) alignments from the trimmed whole genome alignments using RepeatMasker annotations to identify transposable element and repeat-derived sequence [42]. We then calculated the substitution rate for the alignments using the HKY85 model applied in the PAML package BASEML [46]. We also estimated synonymous substitution rates (dS) across protein coding regions for some species pairs. Estimates of dS for a species pair were made by calculating the median dS of all one-to-one gene orthologs in the Ensembl Compara database with dS<1. Nucleotide substitution rates in AR sequences are very similar to estimates of the synonymous substitution rate (dS) (Figure S5), hence our results appear insensitive to the choice of neutral sequence standard.
Modelling turnover
The time-homogeneous turnover model makes the following assumptions: for a particular class of functional elements, both the total amount of functional sequence and the rate of turnover are constant in time, and the turnover rate (weighted by the length of the elements) is identical for all elements in the class. Specifically, within a class of functional sites comprising a nucleotides, in a small time interval dt a number a b dt of sites dispense with function, while an identical number gain function. Note that to arrive at this result we make an “infinite sites” assumption, namely that the genome can be considered infinitely large compared to a; otherwise one would need to account for reversions back to functionality of neutral but previously functional material. Fitting the data to this model under the assumption of independent normally distributed errors in the observations provides estimates and error bounds on parameters a and b.
Annotations
Coding sequence for human (hg19), mouse (mm10), and dog (canFam2) and UTR annotations for human (hg19) were obtained from Ensembl version 72 (http://www.ensembl.org/index.html). UTR sequence that overlapped coding sequence was not considered in the UTR analyses. Human (hg19) PhastCons conserved elements were taken from the vertebrate PhastConsElements46way track downloaded from UCSC Genome Informatics (http://genome.ucsc.edu/). Human (hg19) GERP++ conserved elements were downloaded from the Sidow laboratory website (http://mendel.stanford.edu/SidowLab/downloads/gerp/). Repetitive element annotations for all species were taken from RepeatMasker [42]. Other human (hg19) annotations were taken from the ENCODE data available at UCSC Genome Informatics (http://genome.ucsc.edu/ENCODE/). Specifically, the TFBS data and DNase HS data were acquired from the ENCODE clustered merged sets (wgEncodeRegTfbsClusteredV2.bed and wgEncodeRegDnaseClusteredV2.bed respectively). Promoter and enhancer elements were extracted from the ENCODE HMM Chromatin State segmentations tracks, and merged across these samples: wgEncodeBroadHmmGm12878HMM.bed.gz, wgEncodeBroadHmmH1hescHMM.bed.gz, wgEncodeBroadHmmHepg2HMM.bed.gz, wgEncodeBroadHmmHmecHMM.bed.gz, wgEncodeBroadHmmHsmmHMM.bed.gz, wgEncodeBroadHmmHuvecHMM.bed.gz, wgEncodeBroadHmmK562HMM.bed.gz, wgEncodeBroadHmmNhekHMM.bed.gz, and wgEncodeBroadHmmNhlfHMM.bed. We display the results from analysis of the set of Hangauer et al. (2013) [21] lncRNAs in Figure 3. We also used the smaller set of ENCODE lncRNAs in Figure S9.
Supporting Information
Zdroje
1. PennisiE (2012) Genomics. ENCODE project writes eulogy for junk DNA. Science 337 : 1159, 1161.
2. GraurD, ZhengY, PriceN, AzevedoRB, ZufallRA, et al. (2013) On the immortality of television sets: “function” in the human genome according to the evolution-free gospel of ENCODE. Genome Biol Evol 5 : 578–590.
3. PontingCP, HardisonRC (2011) What fraction of the human genome is functional? Genome Res 21 : 1769–1776.
4. DoolittleWF (2013) Is junk DNA bunk? A critique of ENCODE. Proc Natl Acad Sci U S A 110(14): 5294–300.
5. DunhamI, KundajeA, AldredSF, CollinsPJ, DavisCA, et al. (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489 : 57–74.
6. EckerJR, BickmoreWA, BarrosoI, PritchardJK, GiladY, et al. (2012) Genomics: ENCODE explained. Nature 489 : 52–55.
7. BersaglieriT, SabetiPC, PattersonN, VanderploegT, SchaffnerSF, et al. (2004) Genetic signatures of strong recent positive selection at the lactase gene. Am J Hum Genet 74 : 1111–1120.
8. TakahataN, SattaY, KleinJ (1992) Polymorphism and balancing selection at major histocompatibility complex loci. Genetics 130 : 925–938.
9. AllisonAC (1956) The sickle-cell and haemoglobin C genes in some African populations. Ann Hum Genet 21 : 67–89.
10. PollardKS, SalamaSR, KingB, KernAD, DreszerT, et al. (2006) Forces shaping the fastest evolving regions in the human genome. PLoS Genet 2: e168.
11. McLeanCY, RenoPL, PollenAA, BassanAI, CapelliniTD, et al. (2011) Human-specific loss of regulatory DNA and the evolution of human-specific traits. Nature 471 : 216–219.
12. SiepelA, BejeranoG, PedersenJS, HinrichsAS, HouM, et al. (2005) Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res 15 : 1034–1050.
13. Ureta-VidalA, EttwillerL, BirneyE (2003) Comparative genomics: genome-wide analysis in metazoan eukaryotes. Nat Rev Genet 4 : 251–262.
14. ChiaromonteF, WeberRJ, RoskinKM, DiekhansM, KentWJ, et al. (2003) The share of human genomic DNA under selection estimated from human-mouse genomic alignments. Cold Spring Harb Symp Quant Biol 68 : 245–254.
15. MeaderS, PontingCP, LunterG (2010) Massive turnover of functional sequence in human and other mammalian genomes. Genome Res 20 : 1335–1343.
16. WardLD, KellisM (2012) Evidence of abundant purifying selection in humans for recently acquired regulatory functions. Science 337 : 1675–1678.
17. SmithNG, BrandstromM, EllegrenH (2004) Evidence for turnover of functional noncoding DNA in mammalian genome evolution. Genomics 84 : 806–813.
18. LunterG, PontingCP, HeinJ (2006) Genome-wide identification of human functional DNA using a neutral indel model. PLoS Comput Biol 2: e5.
19. DavydovEV, GoodeDL, SirotaM, CooperGM, SidowA, et al. (2010) Identifying a high fraction of the human genome to be under selective constraint using GERP++. PLoS Comput Biol 6: e1001025.
20. KumarS, SubramanianS (2002) Mutation rates in mammalian genomes. Proc Natl Acad Sci U S A 99 : 803–808.
21. HangauerMJ, VaughnIW, McManusMT (2013) Pervasive Transcription of the Human Genome Produces Thousands of Previously Unidentified Long Intergenic Noncoding RNAs. PLoS Genet 9: e1003569.
22. WaterstonRH, Lindblad-TohK, BirneyE, RogersJ, AbrilJF, et al. (2002) Initial sequencing and comparative analysis of the mouse genome. Nature 420 : 520–562.
23. PontingCP, NellakerC, MeaderS (2011) Rapid turnover of functional sequence in human and other genomes. Annu Rev Genomics Hum Genet 12 : 275–299.
24. GreenP, EwingB (2013) Comment on “Evidence of abundant purifying selection in humans for recently acquired regulatory functions”. Science 340 : 682 discussion 682.
25. WardLD, KellisM (2013) Response to comment on “Evidence of abundant purifying selection in humans for recently acquired regulatory functions”. Science 340 : 682.
26. ThomasJW, TouchmanJW, BlakesleyRW, BouffardGG, Beckstrom-SternbergSM, et al. (2003) Comparative analyses of multi-species sequences from targeted genomic regions. Nature 424 : 788–793.
27. OdomDT, DowellRD, JacobsenES, GordonW, DanfordTW, et al. (2007) Tissue-specific transcriptional regulation has diverged significantly between human and mouse. Nat Genet 39 : 730–732.
28. SchmidtD, WilsonMD, BallesterB, SchwaliePC, BrownGD, et al. (2010) Five-vertebrate ChIP-seq reveals the evolutionary dynamics of transcription factor binding. Science 328 : 1036–1040.
29. LudwigMZ, BergmanC, PatelNH, KreitmanM (2000) Evidence for stabilizing selection in a eukaryotic enhancer element. Nature 403 : 564–567.
30. DermitzakisET, ClarkAG (2002) Evolution of transcription factor binding sites in Mammalian gene regulatory regions: conservation and turnover. Mol Biol Evol 19 : 1114–1121.
31. DonigerSW, FayJC (2007) Frequent gain and loss of functional transcription factor binding sites. PLoS Comput Biol 3: e99.
32. MosesAM, PollardDA, NixDA, IyerVN, LiXY, et al. (2006) Large-scale turnover of functional transcription factor binding sites in Drosophila. PLoS Comput Biol 2: e130.
33. NecsuleaA, SoumillonM, WarneforsM, LiechtiA, DaishT, et al. (2014) The evolution of lncRNA repertoires and expression patterns in tetrapods. Nature 505 : 635–640.
34. LoweCB, KellisM, SiepelA, RaneyBJ, ClampM, et al. (2011) Three periods of regulatory innovation during vertebrate evolution. Science 333 : 1019–1024.
35. BlowMJ, McCulleyDJ, LiZ, ZhangT, AkiyamaJA, et al. (2010) ChIP-Seq identification of weakly conserved heart enhancers. Nat Genet 42 : 806–810.
36. MayD, BlowMJ, KaplanT, McCulleyDJ, JensenBC, et al. (2012) Large-scale discovery of enhancers from human heart tissue. Nat Genet 44 : 89–93.
37. DomeneS, BumaschnyVF, de SouzaFS, FranchiniLF, NasifS, et al. (2013) Enhancer turnover and conserved regulatory function in vertebrate evolution. Philos Trans R Soc Lond B Biol Sci 368 : 20130027.
38. BrawandD, SoumillonM, NecsuleaA, JulienP, CsardiG, et al. (2011) The evolution of gene expression levels in mammalian organs. Nature 478 : 343–348.
39. ChaixR, SomelM, KreilDP, KhaitovichP, LunterGA (2008) Evolution of primate gene expression: drift and corrective sweeps? Genetics 180 : 1379–1389.
40. AmesRM, LovellSC (2011) Diversification at transcription factor binding sites within a species and the implications for environmental adaptation. Mol Biol Evol 28 : 3331–3344.
41. Lindblad-TohK, GarberM, ZukO, LinMF, ParkerBJ, et al. (2011) A high-resolution map of human evolutionary constraint using 29 mammals. Nature 478 : 476–482.
42. Smit AFA, Hubley R, Green P (1996–2010) RepeatMasker Open-3.0.
43. KentWJ, BaertschR, HinrichsA, MillerW, HausslerD (2003) Evolution's cauldron: duplication, deletion, and rearrangement in the mouse and human genomes. Proc Natl Acad Sci U S A 100 : 11484–11489.
44. SchwartzS, KentWJ, SmitA, ZhangZ, BaertschR, et al. (2003) Human-mouse alignments with BLASTZ. Genome Res 13 : 103–107.
45. ChiaromonteF, YapVB, MillerW (2002) Scoring pairwise genomic sequence alignments. Pac Symp Biocomput 115–126.
46. YangZ (2007) PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol 24 : 1586–1591.
Štítky
Genetika Reprodukční medicína
Článek Comparative Phylogenomics Uncovers the Impact of Symbiotic Associations on Host Genome EvolutionČlánek Distribution and Medical Impact of Loss-of-Function Variants in the Finnish Founder PopulationČlánek Common Transcriptional Mechanisms for Visual Photoreceptor Cell Differentiation among PancrustaceansČlánek Integrative Genomics Reveals Novel Molecular Pathways and Gene Networks for Coronary Artery DiseaseČlánek An ARID Domain-Containing Protein within Nuclear Bodies Is Required for Sperm Cell Formation inČlánek Knock-In Reporter Mice Demonstrate that DNA Repair by Non-homologous End Joining Declines with Age
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 7
-
Všechny články tohoto čísla
- Cuba: Exploring the History of Admixture and the Genetic Basis of Pigmentation Using Autosomal and Uniparental Markers
- Clonal Architecture of Secondary Acute Myeloid Leukemia Defined by Single-Cell Sequencing
- Mechanisms of Functional Variants That Impair Regulated Bicarbonate Permeation and Increase Risk for Pancreatitis but Not for Cystic Fibrosis
- Nucleosomes Shape DNA Polymorphism and Divergence
- Functional Diversification of Hsp40: Distinct J-Protein Functional Requirements for Two Prions Allow for Chaperone-Dependent Prion Selection
- Comparative Phylogenomics Uncovers the Impact of Symbiotic Associations on Host Genome Evolution
- Activation of the Immune System by Combinations of Common Alleles
- Age-Associated Sperm DNA Methylation Alterations: Possible Implications in Offspring Disease Susceptibility
- Muscle-Specific SIRT1 Gain-of-Function Increases Slow-Twitch Fibers and Ameliorates Pathophysiology in a Mouse Model of Duchenne Muscular Dystrophy
- MDRL lncRNA Regulates the Processing of miR-484 Primary Transcript by Targeting miR-361
- Hypersensitivity of Primordial Germ Cells to Compromised Replication-Associated DNA Repair Involves ATM-p53-p21 Signaling
- Intrapopulation Genome Size Variation in Reflects Life History Variation and Plasticity
- SlmA Antagonism of FtsZ Assembly Employs a Two-pronged Mechanism like MinCD
- Distribution and Medical Impact of Loss-of-Function Variants in the Finnish Founder Population
- Determinative Developmental Cell Lineages Are Robust to Cell Deaths
- DELLA Protein Degradation Is Controlled by a Type-One Protein Phosphatase, TOPP4
- Wnt Signaling Interacts with Bmp and Edn1 to Regulate Dorsal-Ventral Patterning and Growth of the Craniofacial Skeleton
- Common Transcriptional Mechanisms for Visual Photoreceptor Cell Differentiation among Pancrustaceans
- UVB Induces a Genome-Wide Acting Negative Regulatory Mechanism That Operates at the Level of Transcription Initiation in Human Cells
- The Nesprin Family Member ANC-1 Regulates Synapse Formation and Axon Termination by Functioning in a Pathway with RPM-1 and β-Catenin
- Combinatorial Interactions Are Required for the Efficient Recruitment of Pho Repressive Complex (PhoRC) to Polycomb Response Elements
- Recombination in the Human Pseudoautosomal Region PAR1
- Microsatellite Interruptions Stabilize Primate Genomes and Exist as Population-Specific Single Nucleotide Polymorphisms within Individual Human Genomes
- An Intronic microRNA Links Rb/E2F and EGFR Signaling
- An Essential Nonredundant Role for Mycobacterial DnaK in Native Protein Folding
- Integrative Genomics Reveals Novel Molecular Pathways and Gene Networks for Coronary Artery Disease
- The Genomic Landscape of the Ewing Sarcoma Family of Tumors Reveals Recurrent Mutation
- Evolution and Genetic Architecture of Chromatin Accessibility and Function in Yeast
- An ARID Domain-Containing Protein within Nuclear Bodies Is Required for Sperm Cell Formation in
- Stage-Dependent and Locus-Specific Role of Histone Demethylase Jumonji D3 (JMJD3) in the Embryonic Stages of Lung Development
- Genome Wide Association Identifies Common Variants at the Locus Influencing Plasma Cortisol and Corticosteroid Binding Globulin
- Regulation of Feto-Maternal Barrier by Matriptase- and PAR-2-Mediated Signaling Is Required for Placental Morphogenesis and Mouse Embryonic Survival
- Apomictic and Sexual Germline Development Differ with Respect to Cell Cycle, Transcriptional, Hormonal and Epigenetic Regulation
- Functional EF-Hands in Neuronal Calcium Sensor GCAP2 Determine Its Phosphorylation State and Subcellular Distribution , and Are Essential for Photoreceptor Cell Integrity
- Comparison of Methods to Account for Relatedness in Genome-Wide Association Studies with Family-Based Data
- Knock-In Reporter Mice Demonstrate that DNA Repair by Non-homologous End Joining Declines with Age
- Cis and Trans Effects of Human Genomic Variants on Gene Expression
- 8.2% of the Human Genome Is Constrained: Variation in Rates of Turnover across Functional Element Classes in the Human Lineage
- Novel Approach Identifies SNPs in and with Evidence for Parent-of-Origin Effect on Body Mass Index
- Hypoxia Adaptations in the Grey Wolf () from Qinghai-Tibet Plateau
- A Loss of Function Screen of Identified Genome-Wide Association Study Loci Reveals New Genes Controlling Hematopoiesis
- Unraveling Genetic Modifiers in the Mouse Model of Absence Epilepsy
- DNA Topoisomerase 1α Promotes Transcriptional Silencing of Transposable Elements through DNA Methylation and Histone Lysine 9 Dimethylation in
- The Coding and Noncoding Architecture of the Genome
- A Novel Locus Is Associated with Large Artery Atherosclerotic Stroke Using a Genome-Wide Age-at-Onset Informed Approach
- Brg1 Loss Attenuates Aberrant Wnt-Signalling and Prevents Wnt-Dependent Tumourigenesis in the Murine Small Intestine
- The PTK7-Related Transmembrane Proteins Off-track and Off-track 2 Are Co-receptors for Wnt2 Required for Male Fertility
- The Co-factor of LIM Domains (CLIM/LDB/NLI) Maintains Basal Mammary Epithelial Stem Cells and Promotes Breast Tumorigenesis
- Essential Genetic Interactors of Required for Spatial Sequestration and Asymmetrical Inheritance of Protein Aggregates
- Meiosis-Specific Cohesin Component, Is Essential for Maintaining Centromere Chromatid Cohesion, and Required for DNA Repair and Synapsis between Homologous Chromosomes
- Silencing Is Noisy: Population and Cell Level Noise in Telomere-Adjacent Genes Is Dependent on Telomere Position and Sir2
- The Two Cis-Acting Sites, and , Contribute to the Longitudinal Organisation of Chromosome I
- A Broadly Conserved G-Protein-Coupled Receptor Kinase Phosphorylation Mechanism Controls Smoothened Activity
- Requirements for Acute Burn and Chronic Surgical Wound Infection
- LIN-42, the PERIOD homolog, Negatively Regulates MicroRNA Transcription
- WAPL Is Essential for the Prophase Removal of Cohesin during Meiosis
- Expression in Planarian Neoblasts after Injury Controls Anterior Pole Regeneration
- Sox11 Is Required to Maintain Proper Levels of Hedgehog Signaling during Vertebrate Ocular Morphogenesis
- Accumulation of a Threonine Biosynthetic Intermediate Attenuates General Amino Acid Control by Accelerating Degradation of Gcn4 via Pho85 and Cdk8
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Wnt Signaling Interacts with Bmp and Edn1 to Regulate Dorsal-Ventral Patterning and Growth of the Craniofacial Skeleton
- Novel Approach Identifies SNPs in and with Evidence for Parent-of-Origin Effect on Body Mass Index
- Hypoxia Adaptations in the Grey Wolf () from Qinghai-Tibet Plateau
- DNA Topoisomerase 1α Promotes Transcriptional Silencing of Transposable Elements through DNA Methylation and Histone Lysine 9 Dimethylation in
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



