-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaBrg1 Loss Attenuates Aberrant Wnt-Signalling and Prevents Wnt-Dependent Tumourigenesis in the Murine Small Intestine
Aberrant Wnt signalling is responsible for the majority of colorectal cancers, the third leading cause of cancer-related mortality in the UK. However, no therapies directly targeting Wnt signalling are currently available. Using mouse models of intestinal cancer, we demonstrate that deleting chromatin remodelling factor Brg1 in the context of Apc-deficient small intestinal epithelium attenuates Wnt-driven gene expression changes and prevents adenoma formation, which results in extended animal survival. We also demonstrate that Brg1 loss impairs the small intestinal stem cell expansion associated with aberrant activation of Wnt signalling. These findings highlight Brg1 as a potential therapeutic target in Wnt-driven intestinal tumourigenesis and illustrate the viability of targeting the somatic stem cell as the ‘cell of origin’ of cancer, which might be particularly valuable in patients with known predisposition to cancer.
Published in the journal: . PLoS Genet 10(7): e32767. doi:10.1371/journal.pgen.1004453
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004453Summary
Aberrant Wnt signalling is responsible for the majority of colorectal cancers, the third leading cause of cancer-related mortality in the UK. However, no therapies directly targeting Wnt signalling are currently available. Using mouse models of intestinal cancer, we demonstrate that deleting chromatin remodelling factor Brg1 in the context of Apc-deficient small intestinal epithelium attenuates Wnt-driven gene expression changes and prevents adenoma formation, which results in extended animal survival. We also demonstrate that Brg1 loss impairs the small intestinal stem cell expansion associated with aberrant activation of Wnt signalling. These findings highlight Brg1 as a potential therapeutic target in Wnt-driven intestinal tumourigenesis and illustrate the viability of targeting the somatic stem cell as the ‘cell of origin’ of cancer, which might be particularly valuable in patients with known predisposition to cancer.
Introduction
More than 90% of colorectal cancers (CRC) are characterised by aberrant activation of the canonical Wnt/β-catenin pathway, which is proposed to play a major role in the initiation and progression of CRC [1]. Despite this clear link between deregulated Wnt signalling and disease, therapies which target the Wnt pathway remain limited [2]. There is, therefore, a substantial demand for novel approaches to inhibit the Wnt pathway, preferably downstream of common aberrations such as mutations in APC, AXIN2 or β-catenin. The emerging plethora of epigenetic factors involved in DNA methylation, histone modifications and chromatin remodelling represent a set of new and relatively unexplored opportunities for such therapeutic intervention [3].
Brahma-related gene 1 (BRG1) or SWI/SNF-related matrix-associated actin - dependent regulator of chromatin subfamily A member 4 (SMARCA4) is one of the two mutually exclusive ATPase subunits of the 2-MD family of SWItch/Sucrose Non-Fermentable (SWI/SNF) class of chromatin remodelling complexes. BRG1 has been implicated in a variety of biological processes, in both normal and neoplastic tissues [4], [5]. The majority of these studies, both in vitro and in vivo, suggest that BRG1 acts as a tumour suppressor. For example, it has been found to be mutated in numerous cancer cell lines and primary cancers [6]–[9]. In support of this, studies using Brg1 knock-out mouse models have shown that heterozygous loss of Brg1 increases susceptibility to both mammary gland and lung tumourigenesis [10], [11].
By contrast, BRG1 has been shown to interact with β-catenin and facilitate trans-activation of Wnt-dependent reporter assays and endogenous Wnt target genes in cancer cell lines [12], [13]. Therefore better understanding of the role of BRG1 in aberrant Wnt signalling may allow the development of novel Wnt intervention strategies.
We have recently reported that loss of Brg1 in the small intestinal epithelium results in depletion of the intestinal stem cell population [14]. In this study we aimed to investigate the functional interaction between Brg1 and the Wnt pathway by generating mice, which carried floxed alleles of both Apc [15] and Brg1 [16], [17] genes, thus placing Brg1 deficiency in the context of aberrant activation of the Wnt pathway.
Using three different conditional approaches, we find that additional loss of Brg1 from the small intestinal epithelium attenuates the Wnt-dependent phenotype resulting from Apc deletion.
Results
Brg1 loss attenuates aberrant Wnt signalling in the murine small intestinal epithelium
In order to assess the immediate consequences of Brg1 loss upon Wnt activation, we employed the (Tg(Vil-cre/ESR1)23Syr) transgene [18] driving expression of Cre-ERT2 recombinase under control of the Villin 1 promoter, further abbreviated as VillinCreERT2. To achieve high penetrance inactivation of targeted genes, VillinCreERT2+Apcfl/fl and VillinCreERT2+Apcfl/flBrgfl/fl mice along with VillinCreERT2− controls were induced with four daily injections of 80 mg/kg Tamoxifen. We sacrificed the mice 4 days after the first induction and collected samples of the small intestinal epithelium.
Immunohistochemical analysis of β-catenin and Brg1 expression in the jejunum epithelium at day 4 post induction revealed nuclear localisation of β-catenin in both VillinCreERT2+Apcfl/fl and VillinCreERT2+Apcfl/flBrgfl/fl mice as well as complete loss of Brg1 in double knock-out animals (Figure 1A). Upon histological inspection, the small intestinal epithelium of both VillinCreERT2+Apcfl/fl and VillinCreERT2+Apcfl/flBrgfl/fl mice displayed an aberrant proliferative and apoptotic response synonymous with Wnt pathway activation [19] (Figure 1A). Although no difference in crypt and villus length was observed between VillinCreERT2+Apcfl/fl and VillinCreERT2+Apcfl/flBrgfl/fl mice (Figure 1B, p>0.05, n≥4), quantitative analysis of other histological parameters such as apoptosis and mitosis levels as well as 5-bromo-2′-deoxyuridine (BrdU) incorporation 2 hours after a BrdU pulse showed significant differences between the experimental groups (Figure 1B–1C, Figure S1A–S1B). Quantification of cleaved Caspase3 positive cells showed reduced apoptosis in VillinCreERT2+Apcfl/flBrgfl/fl epithelium (1.85±0.51 and 1.0±0.40 Caspase3 positive cells per half-crypt, p = 0.04, n = 4, Figure 1B). This observation was supported by scoring of the number of apoptotic bodies in the jejunum of VillinCreERT2+Apcfl/fl and VillinCreERT2+Apcfl/flBrgfl/fl mice (4.09±0.34 and 2.67±0.45, p<0.01, n≥4, Figure S1A). Quantification of Ki67 positive cells revealed significantly reduced proliferation in the small intestine of double knock-out mice compared to their Apc-deficient counterparts (46.18 and 31.91 positive cells per half-crypt, pooled standard deviation 4.54, p = 0.0004, n≥4, Figure 1B). Consistent with this observation, scoring of BrdU positive cells 2 hours after labelling showed reduced BrdU incorporation in the jejunum of VillinCreERT2+Apcfl/flBrgfl/fl mice (32.51±6.38 and 23.91±0.99, p = 0.038, n≥4, Figure S1B). Cumulative distribution analysis of both Ki67 and BrdU positive cells revealed that the expansion of the proliferative compartment resulting from Apc deletion was attenuated by additional loss of Brg1 (Figure 1C and Figure S1C, for all comparisons Kolmogorov-Smirnov p<0.001).
Fig. 1. Brg1 loss attenuates the effects of Apc deletion in the small intestinal epithelium. 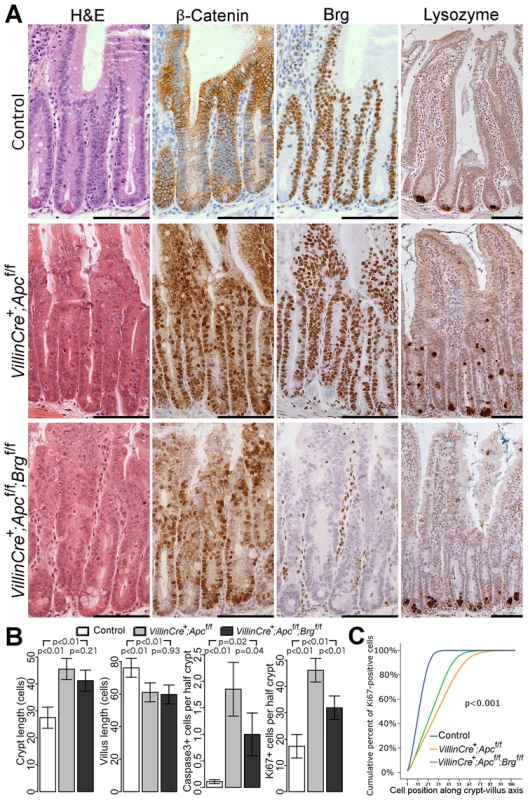
A. H&E, β-catenin and Brg1 staining of the small intestinal epithelium from control VillinCreERT2−, VillinCreERT2+Apcfl/fl and VillinCreERT2+Apcfl/flBrgfl/fl mice 4 days after high-dose induction revealed disturbed crypt architecture and nuclear localisation of β-catenin in both cohorts, as well as complete loss of Brg1 in VillinCreERT2+Apcfl/flBrgfl/fl epithelium. Lysozyme immunostaining showed mis-localisation of Paneth cells in VillinCreERT2+Apcfl/fl epithelium, which was restored to normal by inactivation of Brg1. Scale bars represent 100 µm. B. Scoring of the crypt and villus length revealed no differences between double knock-out mice (black) and VillinCreERT2+Apcfl/fl animals (grey). Scoring of the cleaved Caspase3 positive cells and Ki67 positive cells detected a decrease in both apoptosis and proliferation in VillinCreERT2+Apcfl/flBrgfl/fl mice compared to VillinCreERT2+Apcfl/fl animals. Data are shown as mean ± group's standard deviation for Caspase3 quantification and as mean ± pooled standard deviation otherwise, p value was calculated by means of t test not assuming equal variance for Caspase3 data and by means of one way ANOVA otherwise, p value was adjusted for multiple testing. For all comparisons n≥4. C. Analysis of cumulative frequency of Ki67 positive cells at each position along crypt-villus axis revealed expansion of the proliferative compartment in both double knock-out (green line) and VillinCreERT2+Apcfl/fl (orange line) mice compared to VillinCreERT2− controls (blue line). This expansion was less pronounced in double knock-out mice compared to VillinCreERT2+Apcfl/fl animals. For all pair wise comparisons Kolmogorov-Smirnov test p<0.001, n = 4. Interestingly, immunostaining with lysozyme antibody showed a difference in the distribution of Paneth cells between VillinCreERT2+Apcfl/flBrgfl/fl and VillinCreERT2+Apcfl/fl mice. Small intestinal epithelium of VillinCreERT2+Apcfl/fl animals displayed mislocalisation of Paneth cells throughout the crypt (Figure 1A, right panels), consistent with our earlier report of Apc loss [19]. Additional deletion of Brg1 in VillinCreERT2+Apcfl/flBrgfl/fl mice restored normal confinement of Paneth cells to the crypt base (Figure 1A, right panels). Quantitative analysis of Paneth cell number and position confirmed this observation as both number and distribution of Paneth cells in VillinCreERT2+Apcfl/flBrgfl/fl epithelium were indistinguishable from normal mucosa (Figure 2A–2B, for Paneth cell number p = 0.999, for Paneth cell position Kolmogorov-Smirnov p = 0.17).
Fig. 2. Brg1 loss attenuates Wnt target gene expression and prevents mislocalisation of Paneth cells and formation of aberrant organoids. 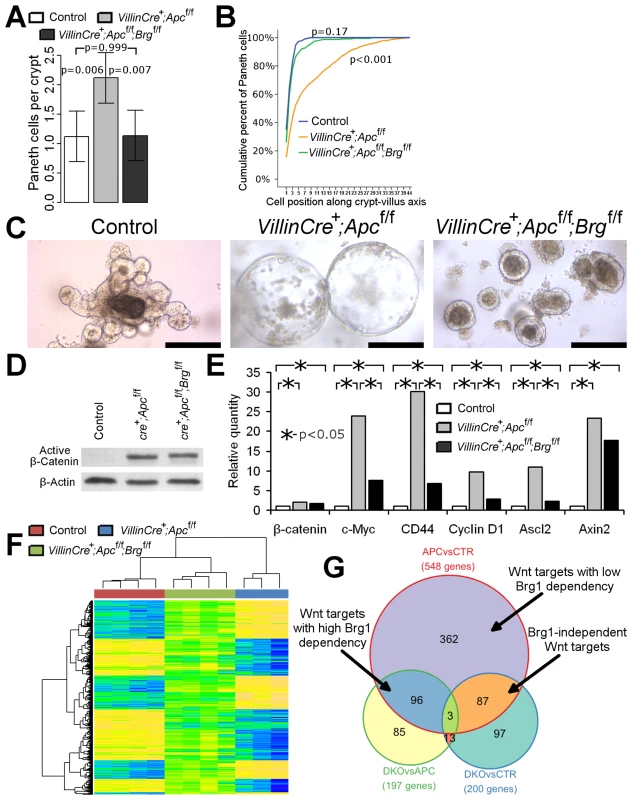
A. Scoring of the lysozyme positive cells revealed a significant increase in number of Paneth cells in VillinCreERT2+Apcfl/fl (grey bar) mice compared to double knock-out (black bar) and control (white bar) mice. No difference in Paneth cell numbers was observed between double knock-out and control epithelium. Data are shown as mean ± pooled standard deviation, p value was calculated by means of one way ANOVA, n = 4. B. Analysis of cumulative frequency of Paneth cells at each position along crypt-villus axis revealed their expansion into the upper portion of the crypt in VillinCreERT2+Apcfl/fl epithelium compared to double knock-out and control mice (Kolmogorov-Smirnov test p<0.001, n = 4). No difference in Paneth cell distribution was observed between double knock-out and control epithelium (Kolmogorov-Smirnov test p = 0.17, n = 4). C. Organoid culture of crypts derived from VillinCreERT2+Apcfl/fl epithelium produced large cystic organoids by day 6 of culture (day 9 post induction). Meanwhile, very few large cysts developed from VillinCreERT2+Apcfl/flBrgfl/fl crypts, with most crypts producing compact simple organoids. Crypts from control intestinal epithelium developed into complex organoid structures by the same time point. Scale bars represent 200 µm. D. Western-blotting analysis of total protein pooled from 3 different animals for each genotype did not reveal changes in the levels of activated β-catenin between VillinCreERT2+Apcfl/fl and VillinCreERT2+Apcfl/flBrgfl/fl epithelial samples. Substantially weaker signal was detected in the samples from VillinCreERT2− control animals. E. qRT-PCR analysis of total RNA extracted from the small intestinal epithelium revealed significantly increased expression of β-catenin and a range of Wnt target genes in VillinCreERT2+Apcfl/fl (grey bars) and VillinCreERT2+Apcfl/flBrgfl/fl (black bars) samples compared to control VillinCreERT2− (white bars) animals. Expression levels of c-Myc, CD44, CyclinD1 and Ascl2, but not β-catenin or Axin2 genes were found to be significantly lower in double knock-out epithelium compared to VillinCreERT2+Apcfl/fl samples. Mann-Whitney U-test was performed on ΔΔCt values, * - p<0.05, n≥4. F. Clustering analysis of genes differentially expressed between VillinCreERT2+Apcfl/fl and VillinCreERT2− control epithelium revealed that the same genes in VillinCreERT2+Apcfl/flBrgfl/fl epithelium largely assumed intermediate expression values between those of the other two groups. G. Venn diagram demonstrating overlap between pairwise comparisons of differentially expressed gene signatures within various cohorts. Unfortunately, high dose induction resulted in rapid health deterioration of both VillinCreERT2+Apcfl/flBrgfl/fl and VillinCreERT2+Apcfl/fl genotypes such that animals had to be killed by 4 days post induction. We therefore used the in vitro intestinal organoid system to investigate the fate of double knock-out small intestinal crypts beyond this time point. VillinCreERT2+Apcfl/fl and VillinCreERT2+Apcfl/flBrgfl/fl mice along with VillinCreERT2− controls were induced by 3 daily intraperitoneal injections of 3 mg Tamoxifen and sacrificed at day 3 post induction. Small intestinal crypts were isolated and placed in crypt organoid media lacking R-spondin (crypts from control animals were grown in presence of R-spondin). By day 6 of culture (day 9 post induction) small intestinal crypts from control animals became complex organoids (Figure 2C). At the same time, the majority of VillinCreERT2+Apcfl/fl crypts developed into large cystic structures (Figure 2C) consistent with a previous report of organoid culture under Wnt activated conditions [20]. In contrast, crypts derived from VillinCreERT2+Apcfl/flBrgfl/fl epithelium rarely developed into large cysts and instead the majority remained as small simple spherical organoids indicating that double knock-out crypts had limited proliferative capacity despite constitutive activation of Wnt signalling (Figure 2C).
Brg1 loss therefore attenuated the histological and physiological consequences of Apc loss, implicating a compromised Wnt pathway activity. Notably, we consistently observed fewer cells with nuclear β-catenin in the epithelium of double knock-out mice compared to the VillinCreERT2+Apcfl/fl animals. Since nuclear β-catenin is commonly used as a surrogate marker of β-catenin activation, we wished to investigate if Brg1 loss suppressed Wnt signalling upstream of β-catenin activation. Western-blotting analysis of ‘activated’ β-catenin (dephosphorylated on Ser37 or Thr41) levels in the intestinal epithelium at day 4 after high-dose induction did not reveal any differences between the two experimental groups (Figure 2D), suggesting that Brg1 deficiency was likely to suppress the Wnt pathway downstream of β-catenin activation.
In order to further investigate the consequences of Brg1 loss on the Wnt pathway, we assessed expression levels of known Wnt target genes in the small intestinal epithelium of VillinCreERT2+Apcfl/fl and double knock-out mice. qRT-PCR analysis of CD44, c-Myc, CyclinD1 and Ascl2 expression levels revealed significant down-regulation of these genes in VillinCreERT2+Apcfl/flBrgfl/fl mice compared to VillinCreERT2+Apcfl/fl epithelium (Figure 2E, p<0.05, n≥3). Notably, we did not observe a significant difference in expression levels of Axin2, which is commonly used as a readout of Wnt pathway activation [21], indicating possible differential recruitment of Brg1 to distinct Wnt target genes.
To further investigate the small intestine-specific role of Brg1 in the Wnt mediated transcriptional program, we performed genome-wide expression analysis of Apc-deficient and double knock-out (DKO) epithelium. VillinCreERT2+Apcfl/fl (n = 3) and VillinCreERT2+Apcfl/flBrgfl/fl (n = 4) mice along with VillinCreERT2− controls (n = 4) were induced with four daily injections of 80 mg/kg Tamoxifen and whole epithelial extract was harvested at day 4 post induction. RNA was extracted from epithelial samples, labelled and hybridised to Mouse Ref8 v2 Illumina array. To determine the baseline effect of Brg1 loss, we performed similar analyses on wild type and induced VillinCreERT2+Brgfl/fl samples, which are described in our earlier publication [14].
Analysis of gene expression between Wnt activated VillinCreERT2+Apcfl/fl and control epithelium (APCvsCTR gene set) detected 548 unique differentially expressed ‘Wnt target genes’ including a number of previously reported Wnt target genes, such as Cd44, Axin2, Lgr5, Tiam1, Ephb2, Efna4 and Sox9 [22] (Figure 2G, Table S1) with four of these genes found to be down-regulated in DKO epithelium compared to Apc-deficient samples (Table S2). Cluster analysis of genes differentially expressed between Apc-deficient and control epithelium revealed clustering of DKO mice with control samples (Figure 2F), indicating a greater similarity of the gene expression pattern between DKO and wild type samples compared to Apc-deficient intestine. Notably, the majority of gene expression values from DKO samples were intermediate between those of VillinCreERT2+Apcfl/fl and control samples (Figure 2F), indicating attenuation of the Wnt pathway transcriptional signature regardless of whether Wnt activation induced or suppressed gene expression. We also observed a strong negative correlation pattern when directions of gene expression changes between Apc-deficient and wild type epithelium were contrasted with changes between double knock-out and Apc-deficient mice (Figure S2A, ρ = −0.793, p<0.0001).
In order to evaluate how many Wnt target genes were attenuated by additional Brg1 loss we compared the overlap between the sets of differentially expressed genes (Figure 2G). We identified 197 unique differentially expressed genes between VillinCreERT2+Apcfl/fl and VillinCreERT2+Apcfl/flBrgfl/fl samples (DKOvsAPC set) (Table S2). Of these, half (99/197) appeared to be ‘Wnt targets’ defined as those present in APCvsCTR gene set (Figure 2G, Table S1), representing 18% (99/548) of the total number of genes that were deregulated following Apc loss (Table S4, Figure 2G). Since Brg1 loss was able to significantly attenuate deregulation of these Wnt targets, these genes could be designated as ‘highly dependent on Brg1’ (Table S7). Comparison of gene expression between VillinCreERT2+Apcfl/flBrgfl/fl and control samples (DKOvsCTR gene set) identified 200 unique differentially expressed genes (Figure 2G, Table S3). Comparison of this gene set to the APCvsCTR set revealed that 16% (87/548) of ‘Wnt target genes’ were present in both sets, but not in the DKOvsAPC set (Table S5). Since Brg1 loss failed to attenuate deregulation of these genes, they could be considered as ‘Brg1 independent Wnt targets’ (Table S7). Notably, Brg1 deficiency in double knock-out samples prevented deregulation of 84% (458/548) of Wnt target genes from their expression levels in control intestine. With the exception of the 99 highly Brg1 dependent Wnt targets these 66% (362/548) of genes could be designated as ‘Wnt targets with low Brg1 dependency’ (Table S7). This gene subset included such Wnt targets as Axin2, Ephb2 and Sox9. Overall, these data indicated that up to 84% of all Wnt target genes in the small intestinal epithelium are dependent to some extent on Brg1 for either their activation or suppression.
It could be argued that gene expression changes upon additional deletion of Brg1 in the Apc-deficient epithelium could arise from the global effect of Brg1 loss on gene expression rather than its specific relevance for the Wnt-driven transcriptional programme. To address this caveat we also analysed the genes differentially expressed between Brg1-deficient and wild type epithelium. Despite a drastic effect on epithelial homeostasis, Brg1 loss induced a relatively moderate perturbation in gene expression at day 4 post induction with 86 genes differentially expressed between Brg1-deficient and control epithelium (Table S8) [14]. Expectedly, a small proportion of the genes differentially expressed between Apc-deficient and double knock-out samples were also de-regulated between Brg1-deficient and wild type epithelium (23/197 genes (11.7%) Table S9, Figure S2B). This proportion was notably lower when Brg1 targets were compared to the list of Wnt targets with high Brg1 dependency (5/99 genes (5.1%), Table S9). Together, these observations strongly suggested that gene expression changes following Brg1 loss in the context of Apc deficiency were largely a result of a specific effect of Brg1 deficiency on the Wnt pathway transcriptional programme rather than an impact of Brg1 deletion on global gene expression.
16 genes which overlapped between the DKOvsCTR and DKOvsAPC sets (Table S6, Figure 2G) appeared deregulated by Brg1 deficiency regardless of Wnt activation and therefore most likely represented direct Brg1 targets independent of Wnt signalling. Most of these genes (11/16) were also present among genes differentially expressed between Brg1-deficient and control samples (Table S9, Figure S2C) [14].
Notably, one of the Wnt targets, whose expression was attenuated following additional loss of Brg1, was the proposed intestinal stem cell marker Lgr5 [23]. We therefore decided to explore the effects of Apc deletion and subsequent Brg1 loss on the expression of genes associated with the intestinal stem cell compartment. To this end we compared gene expression changes between our cohorts to the extensive stem cell signature. Using 3 distinct genome-wide expression platforms Muñoz et al. identified 510 genes that were preferentially expressed in the murine small intestinal Lgr5high cells [24]. We identified 460 of these stem cell-specific genes in our array, which corresponded to 721 probes (Table S10).
We then employed ROAST function [25] from the limma Bioconductor package [26] to determine if stem cell-specific genes were enriched among genes differentially expressed between our cohorts. Consistent with the reported stem cell expansion following aberrant activation of Wnt signalling [27]–[29], 50.5% of stem cell-specific genes were found to be up-regulated in VillinCreERT2+Apcfl/fl mice compared to the control cohort (p<0.0001). Double knock-out epithelium also displayed increased expression of stem cell-related genes compared to control samples, however only in 32.3% of genes (p = 0.0002). In contrast, 41.2% of all stem cell genes were suppressed in double knock-out epithelium compared to VillinCreERT2+Apcfl/fl mice (p<0.0001).
We also queried sets of differentially expressed genes between our cohorts for the presence of stem cell-specific genes. While this approach was less sensitive than using ROAST function, we observed a substantial number of stem cell signature genes in our differentially expressed gene sets (Tables S11, S12, S13). In line with the proposed Wnt-driven stem cell expansion [27]–[29], 10.6% (58/548) of all genes differentially expressed upon Apc deletion were found to belong to the stem cell signature. Of these 98% (57/58) were found to be up-regulated. In contrast, genes differentially expressed between double knock-out and Apc-deficient intestine contained 11.2% (22/197) of genes from the stem cell signature, all of which were down-regulated (Tables S11, S12, S13). Similar to the pattern of gene expression changes across all genes, stem cell related genes displayed strong negative correlation, when expression changes between Apc-deficient and wild type epithelium were contrasted with ones between double knock-out and Wnt activated intestine (Figure S2D, ρ = −0.859, p<0.0001).
Notably, the proposed Wnt-independent intestinal stem cell marker Olfm4 [27] was found to be down regulated in the double knock-out intestine when compared to both Apc-deficient and normal epithelium, indicating strong Brg1 control over Olfm4 expression.
Brg1 loss prevents Wnt-driven adenoma formation in the context of the murine small intestinal epithelium
The apparent requirement of Brg1 for the expression of Wnt target genes following aberrant Wnt activation in the small intestinal epithelium raises the possibility that Brg1 loss may also prevent Wnt-driven tumour development. To explore this possibility we assessed the effects of Brg1 loss on Wnt-driven adenoma formation by driving recombination of the floxed Apc and Brg1 alleles with the Tg(Cyp1a1-cre/ESR1)1Dwi transgene, further abbreviated as AhCreERT [30]. This transgene encodes Cre-ERT recombinase under the control of the Cyp1A promoter. In contrast to VillinCreERT2 recombinase, which is expressed in the entirety of the intestinal epithelium, AhCreERT transgene's expression is confined to the stem cell and early progenitor compartments. Additionally, AhCreERT recombinase requires exposure to both β-naphthoflavone and tamoxifen for its activation, which results in tighter control over its activity. We used this approach to inactivate Apc and Brg1 in the small intestinal epithelium at a lower frequency than above, thus extending animal survival and enabling us to analyse the long-term effects of Brg1 loss on Wnt-driven adenoma formation.
We induced 3 cohorts of mice (AhCreERT− controls, AhCreERT+Apcfl/fl and AhCreERT+Apcfl/flBrgfl/fl) with 5 bi-daily intraperitoneal injections of 80 mg/kg of β-naphthoflavone and 80 mg/kg of tamoxifen. Animals were aged and sacrificed either at day 10 post induction (n = 4) or when they displayed signs of terminal illness (n≥20).
Immunohistochemical analysis of β-catenin localisation in the small intestinal epithelium at day 10 revealed multiple aberrant foci with nuclear β-catenin in both AhCreERT+Apcfl/fl and double knock-out mice, indicating successful activation of the Wnt pathway in those lesions (Figure 3A, left and central). At the same time, Brg1 immunostaining revealed clusters of Brg1 negative cells in AhCreERT+Apcfl/flBrgfl/fl intestinal epithelium (Figure 3A, right). In contrast to the small intestine of VillinCreERT2+Apcfl/flBrgfl/fl mice, where the majority of cells after induction were deficient for Brg1 and displayed nuclear localisation of β-catenin, all the lesions with nuclear β-catenin in the AhCreERT+Apcfl/flBrgfl/fl intestine were Brg1-positive and we failed to detect any overlap between Brg1-deficient clusters and Wnt-activated lesions (Figure 3A, central and right). This observation suggested that, when driven by AhCreERT recombinase, Brg1 loss was incompatible with long term activation of the Wnt pathway and the development of aberrant crypt foci, consistent with inability of crypts from double knock-out epithelium to form aberrant organoids in vitro (Figure 2C).
Fig. 3. Brg1 loss is incompatible with Wnt-driven adenoma formation and improves animal survival upon Apc inactivation in the small intestine. 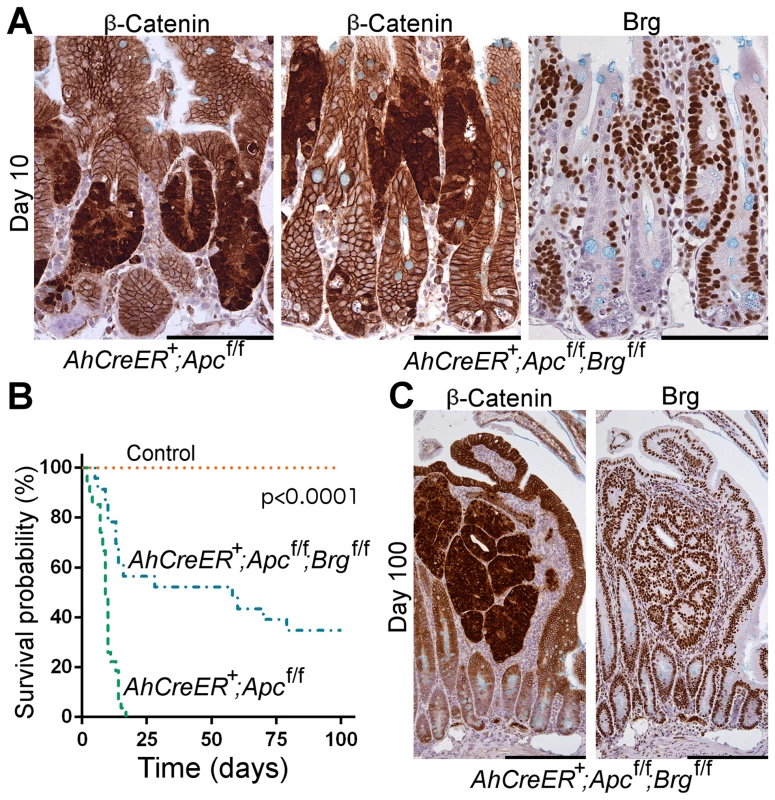
A. Immunohistochemical analysis of β-catenin revealed numerous lesions with nuclear localisation of β-catenin in the small intestine of AhCreERT+Apcfl/fl and AhCreERT+Apcfl/flBrgfl/fl mice 10 days post induction. Brg1 immunostaining of serial sections showed clusters of Brg1-deficient cells in AhCreERT+Apcfl/flBrgfl/fl intestine, but failed to detect any overlap between lesions with nuclear β-catenin and Brg1 negative cells. B. Survival analysis demonstrated significantly increased survival probability of AhCreERT+Apcfl/flBrgfl/fl mice (blue line, median survival 58 days) compared to AhCreERT+Apcfl/fl animals (green line, median survival 9 days). Log-rank test p<0.0001, n≥20. No control animals died within the timeframe of the experiment (n = 8). C. β-catenin immunostaining of the small intestinal epithelium of AhCreERT+Apcfl/flBrgfl/fl mice 100 days post induction revealed numerous micro adenomas enclosed within distended villi. Staining with Brg1 antibody showed that all the micro adenomas retained Brg1 expression. Scale bars represent 100 µm (A) and 200 µm (B). Analysis of the ageing cohorts revealed that combined deletion of Apc and Brg1 deletion provided a survival advantage in comparison to single Apc deletion (Figure 3B). Whilst all the AhCreERT+Apcfl/fl mice became terminally ill within 20 days post induction (median survival 9 days), the majority of AhCreERT+Apcfl/flBrgfl/fl mice survived substantially longer (median survival 58 days) with some mice surviving past 100 days (Figure 3B, Log-rank test p<0.0001, n≥20 for each cohort). No control animals (n = 8) developed signs of ill health within the timeframe of the experiment.
Histological inspection of the small intestine of the AhCreERT+Apcfl/flBrgfl/fl mice at late time points revealed numerous small lesions confined within the distended villi and identified as micro-adenomas (Figure 3C), as well as rare large adenomas. Immunohistochemical analysis of β-catenin expression showed that all of these lesions were positive for nuclear β-catenin, confirming aberrant Wnt signalling activation (Figure 3C, left). Notably, Brg1 staining demonstrated that all the lesions retained Brg1 expression (Figure 3C, right), indicating that they were likely to originate from cells that recombined at the Apc, but not at the Brg1 loci. Along with the apparent lack of double mutant lesions at day 10 post induction, this indicated that long-term progression of Wnt-driven neoplasia in the small intestine was incompatible with Brg1 deficiency. We therefore explored the possibility that Brg1 loss in the context of activated Wnt signalling could reduce tumour burden and thus improve animal survival. We induced AhCreERT+Apcfl/fl and AhCreERT+Apcfl/flBrgfl/fl mice with two bi-daily injections of 80 mg/kg β-naphthoflavone and 80 mg/kg tamoxifen to achieve attenuated recombination and harvested the small intestinal epithelium at day 40 post induction. We then scored the number of lesions with nuclear β-catenin normalised to the number of normal crypt units contained within the analysed region of the small intestinal epithelium. Quantitative analysis of the tumour burden from two independent experiments revealed a 2.92-fold decrease in the number of lesions in the double knock-out small intestinal epithelium compared to AhCreERT+Apcfl/fl animals (p = 0.026, n≥5) indicating reduced tumour burden upon Brg1 loss.
Stem cell-restricted Brg1 loss suppressed Wnt-driven tumourigenesis
Prevalence of micro-adenomas and lack of advanced adenomas in the small intestines of the AhCreERT+Apcfl/flBrgfl/fl mice as late as 100 days post induction indicated that this genetic environment favoured the development of lesions with a limited growth potential. Barker et al. [28] suggested that adenomas originating from intestinal stem cells had a higher tumourigenic potential compared to those derived from transit amplifying cells. To test whether stem cell-specific Brg1 loss could attenuate Wnt-driven adenoma formation we intercrossed mice expressing the GFP-IRES-CreERT2 knock-in allele under control of the Lgr5 promoter (further abbreviated as Lgr5-GFP-CreERT2) [23] with animals bearing targeted Apc and Brg1 alleles. Lgr5-GFP-CreERT2+Apcfl/fl and Lgr5-GFP-CreERT2+Apcfl/flBrgfl/fl mice were induced with four daily intraperitoneal injections of tamoxifen (initial dose of 3 mg was followed by three doses of 2 mg). Animals were aged for 420 days after induction or until they developed signs of ill health. Survival analysis of the cohorts revealed a substantial increase in survival of double knock-out mice compared to their Lgr5-GFP-CreERT2+Apcfl/fl counterparts (median survival 344 and 110 days post induction respectively, Figure 4A, Log-rank p<0.001, n = 14 for each cohort).
Fig. 4. Stem cell-specific Brg1 loss in the small intestine attenuates Wnt-driven adenoma formation and improves animal survival. 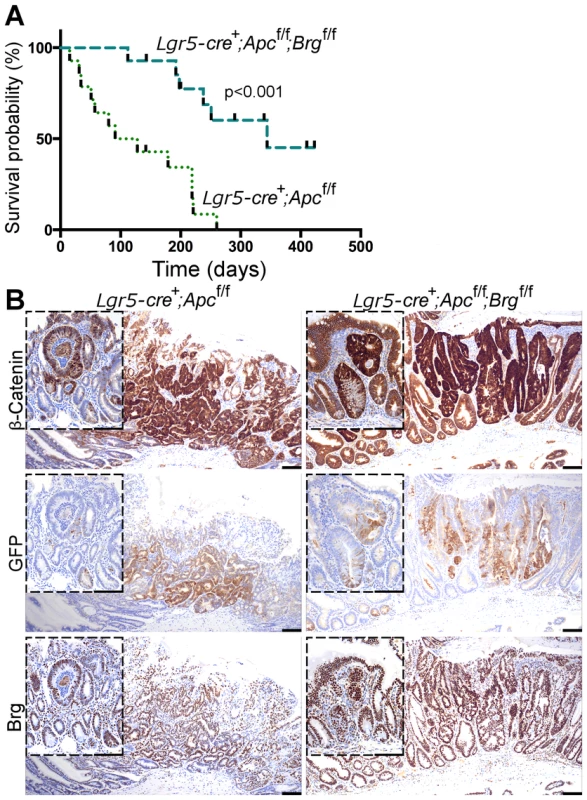
A. Survival analysis demonstrated significantly increased survival of Lgr5-GFP-CreERT2+Apcfl/flBrgfl/fl mice (median survival 344 days post induction) compared to Lgr5-GFP-CreERT2+Apcfl/fl animals (median survival 110 days). Log-rank test p<0.0001, n = 14. B. β-catenin immunostaining detected micro and macro adenomas with nuclear β-catenin in the distal small intestinal epithelium of Lgr5-GFP-CreERT2+Apcfl/fl and Lgr5-GFP-CreERT2+Apcfl/flBrgfl/fl mice at various time points. Both types of lesions were found to express GFP and Brg1 indicating their stem cell origin and selective elimination of Brg1-deficient tumours. Scale bars represent 100 µm. Immunohistochemical analysis of β-catenin expression in the small intestine of both Lgr5-GFP-CreERT2+Apcfl/fl and Lgr5-GFP-CreERT2+Apcfl/flBrgfl/fl mice revealed a mixture of micro and macro-adenomas with nuclear β-catenin at a range of time points (Figure 4B). Immunohistochemical analysis of GFP expression in small intestinal tumours detected GFP positive cells in both macro and micro adenomas, implying activity of the Lgr5-GFP-CreERT2 transgene and stem cell origin of both types of lesions (Figure 4B). This observation suggested that not all stem cell-derived adenomas were able to progress to advanced stages. Equally, we observed numerous micro adenomas devoid of GFP expression (not shown). These lesions were likely to arise from early progenitor cells, which might have lost self renewal ability, but retained some Cre-ERT2 recombinase expression.
Similar to the pattern observed in the small intestine of AhCreERT+Apcfl/flBrgfl/fl mice, adenomas in the small intestine of Lgr5-GFP-CreERT2+Apcfl/flBrgfl/fl mice retained Brg1 expression (Figure 4B) providing further support to the notion that Brg1 loss is incompatible with Wnt-driven adenoma formation.
Discussion
Brg1 acts as a positive regulator of Wnt signalling and is required for Wnt-driven adenoma formation in the small intestinal epithelium
We have demonstrated that inactivation of Brg1 resulted in reduced expression of Wnt target genes following activation of the canonical Wnt pathway via Apc deletion in the small intestinal epithelium. A number of studies have previously suggested that Brg1 facilitates trans-activation of Wnt target genes by activated β-catenin in cancer cell lines [12], [13], zebrafish [31] and during mammalian vascular development [32]. Our study provides the first evidence of the functional interaction between Brg1 and the Wnt pathway in the context of intestinal tumourigenesis using an in vivo system. Using transcriptome analysis, we identified sets of Wnt target genes which displayed differing levels of dependency on Brg1 function. Overall, we identified 548 genes that were deregulated upon Apc deletion in the small intestinal epithelium. Of these, 87 genes were Brg1 independent and 461 displayed a variable degree of dependency on Brg1 function, indicating that deregulation of the majority (85%) of Wnt responsive genes in the small intestinal epithelium depended (to differing degrees) on the presence of functional Brg1. This observation is in line with a previous report, which found a similar proportion (68%) of Wnt targets to rely on Brg1 for their response to Wnt activation in the HEK293T cell line [33].
Consistent with the requirement for Brg1 in the maintenance of the small intestinal stem cell population [14], we observed attenuated expression in a range of genes associated with the small intestinal stem cells following Brg1 loss in the context of aberrantly activated Wnt signalling. These genes included functionally validated stem cell markers such as Lgr5, Ascl2 and Olfm4, suggesting that Brg1 loss was able to impair the expansion of the ‘stem-like’ cell population characteristic of Wnt-driven intestinal tumourigenesis [27]–[29]. Furthermore, expression levels of Olfm4 in the small intestine of double knock-out mice were lower compared to that in both wild type and Apc-deficient epithelium, strongly suggesting a role of Brg1 in regulation of Olfm4 expression. Olfm4 is a secreted anti-apoptotic factor, which has been reported to be over-expressed in a variety of tumours [34]. Depletion of Olfm4 in gastric cancer cells has been reported to suppress proliferation and sensitise cancer cells to apoptosis [35], [36]. Olfm4 thus constitutes a potentially attractive therapeutic target, especially so in view of its secreted nature, which makes it a feasible target for monoclonal antibody therapies.
In addition to suppression of Wnt target genes, Brg1 loss also attenuated the physiological manifestations of Wnt activation in the intestinal epithelium, most notably the increased cell proliferation and mislocalisation of Paneth cells. Both Paneth cell quantity and position were restored to normal levels in the double knock-out epithelium, suggesting that Brg1 deletion was able to preserve physiological levels of EphB/Ephrin signalling in the context of Apc deficiency [37]. Consistent with this notion, our transcriptome analysis detected up-regulation of EphB2 expression in Apc-deficient intestine, but not in double knock-out epithelium, when compared to control samples. A similar effect of Wnt signalling attenuation on the Paneth cell mislocalisation has been previously demonstrated following loss of Mbd2 in the Apc-deficient small intestine [38]. Given the proposed role for Paneth cells as the intestinal stem cell niche [20], this effect of Brg1 loss on Paneth cell mislocalisation could contribute to the attenuated stem cell expansion in double knock-out intestine.
In the view of extensive role of Wnt signalling in tumourigenesis [1], Brg1 mediated modulation of the Wnt pathway may have implications for the development of novel therapeutic approaches. Here we report that loss of Brg1 in the context of Apc deletion improved animal survival by preventing the formation of double mutant adenomas. All adenomas observed in the small intestine of double knock-out mice retained Brg1 expression indicative of their origin from cells that had lost Apc but had escaped Brg1 deletion. This implies an absolute requirement of functional Brg1 for Wnt-mediated tumourigenesis in this tissue. A similar relationship between Brg1 inactivation and a loss of tumour suppressor Snf5 (Ini1) has been reported by Wang et al. [39]. Simultaneous inactivation of Brg1 and Snf5 under control of the T-cell lineage-specific Lck-Cre recombinase resulted in decreased tumour incidence and a longer disease onset. Similar to our observations, all the Snf5-deficient tumours, which developed in double-mutant animals, retained Brg1 expression [39].
Barker et al. [28] suggested a particular role for intestinal stem cells in Wnt-driven tumour initiation with non-stem cells giving rise to adenomas with limited growth capacity, while stem cell gene signature in human primary colorectal cancers was found to be associated with more aggressive phenotype [40]. In a similar fashion, Alcantara Llaguno et al. [41] reported neural stem cells as the cell of origin for malignant gliomas. Furthermore, a recent study has demonstrated a substantial survival advantage of genetic targeting of glioblastoma cancer stem cells using a neural stem cell marker Nestin [42]. Consistent with these reports, and in line with negative impact of Brg1 loss on long-term small intestinal stem cell survival, we observed a markedly improved survival upon stem cell-specific Brg1 deletion in the context of aberrant Wnt activation. Notably, the above study made use of stem cell-specific expression of the toxic thymidine kinase transgene to target the sub-population of cancer cells bearing normal stem cell markers [42]. In contrast to this, we report successful tumour suppression by targeting a gene required for the physiological small intestinal stem cell maintenance, a relationship, which to our knowledge has not been previously reported. It should be noted that mice with stem cell-specific Apc loss alone in the present report exhibited longer survival times compared to those in the study by Barker et al. [28], which could be attributed to increased levels of silencing of the Lgr5-GFP-CreERT2 transgene in our mice. Tumours in the small intestine of animals bearing the Lgr5-GFP-CreERT2 transgene and targeted Apc alleles were mainly detected in the distal third of the small intestine with fewer lesions in the proximal part and very few tumours in between. Given that biallelic Apc deletion would fall within ‘high pathological Wnt’ scenario described in Leedham et al. [43], this tumour distribution was consistent with the gradient of the Wnt signal and stem cell density in the murine intestine.
It should be noted that given the concurrency of Brg1 and Apc deletion, results presented in this report pertain to cancer prevention rather than therapy. Additionally, Brg1 loss-driven elimination of intestinal stem cells is likely to be a major contributing factor to attenuated tumour burden in double knock-out mice and may therefore obscure the effects of Brg1 deletion on non-stem tumour cells. Importantly, this toxicity of Brg1 loss in respect to the small intestinal stem cell homeostasis constitutes a potential serious caveat to the use of Brg1 as a therapeutic target. However our data from the previous report [14] as well as from AhCreERT+Apcfl/flBrgfl/fl and Lgr5-GFP-CreERT2+Apcfl/flBrgfl/fl models in the present study demonstrate that partial Brg1 loss is well tolerated by the small intestinal epithelium, which is gradually repopulated with wild type cells. At the same time, partial loss of Brg1 was sufficient to reduce the tumour burden, suggesting that a therapeutic window may exist that would allow targeting Brg1 in the intestinal polyps, while allowing repopulation of the normal intestinal epithelium. A strategy that would allow for the efficient Brg1 deletion in existing adenomas or the use of a Brg1 inhibitor would be required to further address the effects of Brg1 in non-stem cell portion of established Wnt-driven tumours, as well as the potential therapeutic window of targeting Brg1.
In summary, we demonstrate using mouse models of intestinal cancer that Brg1 is essential for Wnt-driven tumourigenesis in the murine small intestine with attenuation of Wnt target gene expression and elimination of transformed stem cells as two likely mechanisms. Brg1 therefore constitutes a potential therapeutic target in cancers with an aberrantly activated Wnt pathway. Combined with our earlier observation that Brg1 is essential for stem cell maintenance in the small intestinal epithelium under the physiological conditions, these data may serve as a proof of concept that targeting the somatic stem cell as a cancer initiating cell may provide a valuable therapeutic approach, especially in the context of predisposition to Wnt-driven carcinogenesis, such as in Familial Adenomatous Polyposis patients.
Materials and Methods
Experimental animals
All experiments were carried out in accordance with Animals (Scientific Procedures) Act 1986 under project licenses 30/2246 and 30/2737 issued by UK Home Office. The study was approved by the Cardiff University Research Ethics Committee. Mice were maintained on an outbred background and genotyped as described previously for targeted Apc allele [15], Cre-ERT and Cre-ERT2 transgenes [19], targeted Brg1 allele [16]. Detailed induction protocols and dissection procedures are described in Text S1.
Histological procedures
Detailed description of protocols for tissue fixation, processing, immunohistochemistry, and quantitative analysis of tissue sections is available in Text S1.
Statistical analysis of histological data
All statistical tests except survival and cumulative distribution analyses were carried out using R software [44]. Scoring data were tested for normality using Shapiro-Wilk test and for equal variance using Levene's test. Normally distributed data with equal variance were tested for difference between means using one-way ANOVA. Where appropriate, p values were adjusted for multiple testing using TukeyHSD function in R. In cases of unequal variance between groups the difference between those groups was tested using t-test not assuming equal variance. Unless otherwise specified, pooled standard deviation from one-way ANOVA was used to represent error bars. Positional data were analysed for differences in distribution with Kolmogorov-Smirnov Z-test in SPSS (version 16.0.2). Kaplan-Meier survival curve and Log-rank survival analysis were carried out using GraphPad Prism (version 6).
RNA extraction, quantitative RT-PCR and microarray analysis
Detailed description of the protocols and statistical methods used for RNA extraction, qRT-PCR and transcriptome analysis is available in Text S1. Microarray analysis was carried out using beadarray [45] and limma [26] packages from Bioconductor project. Microarray data for the study are publicly available from the GEO repository (http://www.ncbi.nlm.nih.gov/geo) under the series record GSE46129.
Protein extraction and western blot analysis
A detailed protocol for obtaining epithelial-enriched population of cells for protein extraction and western-blotting is provided in Text S1. Protein was extracted from the epithelium-enriched small intestinal samples, separated, transferred and probed as described previously [46]. The primary antibodies used: mouse anti-active β-catenin (1∶2000; Millipore) and mouse anti-β-actin (1∶5000; Sigma). Anti-mouse horseradish peroxidase conjugated secondary antibody (1∶3000; GE Healthcare) and ECL or ECL Plus reagents (Amersham Biosciences) were used to detect the signal according to the manufacturer's manual.
In vitro organoid culture
Small intestinal crypts from VillinCreERT2− controls, VillinCreERT2+Apcfl/fl and VillinCreERT2+Apcfl/flBrgfl/fl mice were isolated and cultured as described in Sato et al. [47] with minor adjustments. Detailed procedures are described in Text S1.
Supporting Information
Zdroje
1. GilesRH, van EsJH, CleversH (2003) Caught up in a Wnt storm: Wnt signaling in cancer. Biochimica et biophysica acta 1653 : 1–24.
2. GehrkeI, GandhirajanRK, KreuzerK-A (2009) Targeting the WNT/[beta]-catenin/TCF/LEF1 axis in solid and haematological cancers: Multiplicity of therapeutic options. European Journal of Cancer 45 : 2759–2767.
3. EllisL, AtadjaPW, JohnstoneRW (2009) Epigenetics in cancer: targeting chromatin modifications. Molecular Cancer Therapeutics 8 : 1409–1420.
4. KhavariPA, PetersonCL, TamkunJW, MendelDB, CrabtreeGR (1993) BRG1 contains a conserved domain of the SWI2/SNF2 family necessary for normal mitotic growth and transcription. Nature 366 : 170–174.
5. TrotterKW, ArcherTK (2008) The BRG1 transcriptional coregulator. Nuclear Receptor Signaling 6: e004.
6. BeckerTM, HaferkampS, DijkstraMK, ScurrLL, FraustoM, et al. (2009) The chromatin remodelling factor BRG1 is a novel binding partner of the tumor suppressor p16INK4a. Molecular Cancer 8 : 4.
7. MedinaPP, CarreteroJ, FragaMF, EstellerM, SidranskyD, et al. (2004) Genetic and Epigenetic screening for gene alterations of the chromatin-remodeling factor, SMARCA4/BRG1, in lung tumors. Genes, Chromosomes and Cancer 41 : 170–177.
8. ReismanDN, SciarrottaJ, WangW, FunkhouserWK, WeissmanBE (2003) Loss of BRG1/BRM in Human Lung Cancer Cell Lines and Primary Lung Cancers: Correlation with Poor Prognosis. Cancer Res 63 : 560–566.
9. WongAKC, ShanahanF, ChenY, LianL, HaP, et al. (2000) BRG1, a Component of the SWI-SNF Complex, Is Mutated in Multiple Human Tumor Cell Lines. Cancer Res 60 : 6171–6177.
10. BultmanSJ, HerschkowitzJI, GodfreyV, GebuhrTC, YanivM, et al. (2008) Characterization of mammary tumors from Brg1 heterozygous mice. Oncogene 27 : 460–468.
11. GlarosS, CirrincioneGM, PalancaA, MetzgerD, ReismanD (2008) Targeted Knockout of BRG1 Potentiates Lung Cancer Development. Cancer Res 68 : 3689–3696.
12. BarkerN, HurlstoneA, MusisiH, MilesA, BienzM, et al. (2001) The chromatin remodelling factor Brg-1 interacts with β-catenin to promote target gene activation. The EMBO Journal 20 : 4935–4943.
13. ParkJ-I, VenteicherAS, HongJY, ChoiJ, JunS, et al. (2009) Telomerase modulates Wnt signalling by association with target gene chromatin. Nature 460 : 66–72.
14. HolikAZ, KrzystyniakJ, YoungM, RichardsonK, JardéT, et al. (2013) Brg1 is required for stem cell maintenance in the murine intestinal epithelium in a tissue-specific manner. Stem Cells 31 : 2457–2466 doi:10.1002/stem.1498
15. ShibataH, ToyamaK, ShioyaH, ItoM, HirotaM, et al. (1997) Rapid Colorectal Adenoma Formation Initiated by Conditional Targeting of the Apc Gene. Science 278 : 120–123.
16. Sumi-IchinoseC, IchinoseH, MetzgerD, ChambonP (1997) SNF2beta-BRG1 is essential for the viability of F9 murine embryonal carcinoma cells. Molecular and Cellular Biology 17 : 5976–5986.
17. IndraAK, DupéV, BornertJ-M, MessaddeqN, YanivM, et al. (2005) Temporally controlled targeted somatic mutagenesis in embryonic surface ectoderm and fetal epidermal keratinocytes unveils two distinct developmental functions of BRG1 in limb morphogenesis and skin barrier formation. Development 132 : 4533–4544.
18. el MarjouF, JanssenK-P, ChangBH-J, LiM, HindieV, et al. (2004) Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis (New York, NY: 2000) 39 : 186–193.
19. SansomOJ, ReedKR, HayesAJ, IrelandH, BrinkmannH, et al. (2004) Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes & Development 18 : 1385–1390.
20. SatoT, van EsJH, SnippertHJ, StangeDE, VriesRG, et al. (2011) Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469 : 415–418.
21. JhoE-h, ZhangT, DomonC, JooC-K, FreundJ-N, et al. (2002) Wnt/{beta}-Catenin/Tcf Signaling Induces the Transcription of Axin2, a Negative Regulator of the Signaling Pathway. Mol Cell Biol 22 : 1172–1183.
22. Wnt Target genes | The Wnt Homepage (n.d.). Available: http://www.stanford.edu/group/nusselab/cgi-bin/wnt/target_genes. Accessed 25 September 2012.
23. BarkerN, van EsJH, KuipersJ, KujalaP, van den BornM, et al. (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449 : 1003–1007.
24. MuñozJ, StangeDE, SchepersAG, van de WeteringM, KooB-K, et al. (2012) The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent ‘+4’ cell markers. The EMBO journal 31 : 3079–3091.
25. WuD, LimE, VaillantF, Asselin-LabatM-L, VisvaderJE, et al. (2010) ROAST: rotation gene set tests for complex microarray experiments. Bioinformatics 26 : 2176–2182.
26. Smyth GK (2005) Limma: linear models for microarray data. Bioinformatics and Computational Biology Solutions Using {R} and Bioconductor. New York: Springer. pp. 397–420.
27. van der FlierLG, HaegebarthA, StangeDE, van de WeteringM, CleversH (2009) OLFM4 Is a Robust Marker for Stem Cells in Human Intestine and Marks a Subset of Colorectal Cancer Cells. Gastroenterology 137 : 15–17.
28. BarkerN, RidgwayRA, van EsJH, van de WeteringM, BegthelH, et al. (2009) Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457 : 608–611.
29. JubbAM, ChalasaniS, FrantzGD, SmitsR, GrabschHI, et al. (2006) Achaete-scute like 2 (ascl2) is a target of Wnt signalling and is upregulated in intestinal neoplasia. Oncogene 25 : 3445–3457.
30. KempR, IrelandH, ClaytonE, HoughtonC, HowardL, et al. (2004) Elimination of background recombination: somatic induction of Cre by combined transcriptional regulation and hormone binding affinity. Nucleic Acids Research 32: e92.
31. ErogluB, WangG, TuN, SunX, MivechiNF (2006) Critical role of Brg1 member of the SWI/SNF chromatin remodeling complex during neurogenesis and neural crest induction in zebrafish. Developmental Dynamics: An Official Publication of the American Association of Anatomists 235 : 2722–2735.
32. GriffinCT, CurtisCD, DavisRB, MuthukumarV, MagnusonT (2011) The chromatin-remodeling enzyme BRG1 modulates vascular Wnt signaling at two levels. Proceedings of the National Academy of Sciences 108 : 2282–2287.
33. MahmoudiT, BojSF, HatzisP, LiVSW, TaouatasN, et al. (2010) The Leukemia-Associated Mllt10/Af10-Dot1l Are Tcf4/β-Catenin Coactivators Essential for Intestinal Homeostasis. PLoS Biol 8: e1000539.
34. YuL, WangL, ChenS (2011) Olfactomedin 4, a novel marker for the differentiation and progression of gastrointestinal cancers. Neoplasma 58 : 9–13.
35. LiuR-h, YangM-h, XiangH, BaoL-m, YangH-a, et al. (2012) Depletion of OLFM4 gene inhibits cell growth and increases sensitization to hydrogen peroxide and tumor necrosis factor-alpha induced-apoptosis in gastric cancer cells. Journal of Biomedical Science 19 : 38.
36. OhH-K, TanAL-K, DasK, OoiC-H, DengN-T, et al. (2011) Genomic Loss of miR-486 Regulates Tumor Progression and the OLFM4 Antiapoptotic Factor in Gastric Cancer. Clinical Cancer Research 17 : 2657–2667.
37. BatlleE, HendersonJT, BeghtelH, van den BornMMW, SanchoE, et al. (2002) beta-Catenin and TCF Mediate Cell Positioning in the Intestinal Epithelium by Controlling the Expression of EphB/EphrinB. Cell 111 : 251–263.
38. PhesseTJ, ParryL, ReedKR, EwanKB, DaleTC, et al. (2008) Deficiency of Mbd2 Attenuates Wnt Signaling. Mol Cell Biol 28 : 6094–6103.
39. WangX, SansamCG, ThomCS, MetzgerD, EvansJA, et al. (2009) Oncogenesis caused by loss of the SNF5 tumor suppressor is dependent on activity of BRG1, the ATPase of the SWI/SNF chromatin remodeling complex. Cancer research 69 : 8094–8101.
40. Merlos-SuárezA, BarrigaFM, JungP, IglesiasM, CéspedesMV, et al. (2011) The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell stem cell 8 : 511–524.
41. Alcantara LlagunoS, ChenJ, KwonC-H, JacksonEL, LiY, et al. (2009) Malignant Astrocytomas Originate from Neural Stem/Progenitor Cells in a Somatic Tumor Suppressor Mouse Model. Cancer Cell 15 : 45–56.
42. ChenJ, LiY, YuT-S, McKayRM, BurnsDK, et al. (2012) A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 488 : 522–526.
43. LeedhamSJ, Rodenas-CuadradoP, HowarthK, LewisA, MallappaS, et al. (2013) A basal gradient of Wnt and stem-cell number influences regional tumour distribution in human and mouse intestinal tracts. Gut 62 : 83–93.
44. R Development Core Team (2008). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org.
45. DunningMJ, SmithML, RitchieME, TavaréS (2007) beadarray: R classes and methods for Illumina bead-based data. Bioinformatics 23 : 2183–2184.
46. MarshV, WintonDJ, WilliamsGT, DuboisN, TrumppA, et al. (2008) Epithelial Pten is dispensable for intestinal homeostasis but suppresses adenoma development and progression after Apc mutation. Nat Genet 40 : 1436–1444.
47. SatoT, VriesRG, SnippertHJ, van de WeteringM, BarkerN, et al. (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459 : 262–265.
Štítky
Genetika Reprodukční medicína
Článek Comparative Phylogenomics Uncovers the Impact of Symbiotic Associations on Host Genome EvolutionČlánek Distribution and Medical Impact of Loss-of-Function Variants in the Finnish Founder PopulationČlánek Common Transcriptional Mechanisms for Visual Photoreceptor Cell Differentiation among PancrustaceansČlánek Integrative Genomics Reveals Novel Molecular Pathways and Gene Networks for Coronary Artery DiseaseČlánek An ARID Domain-Containing Protein within Nuclear Bodies Is Required for Sperm Cell Formation inČlánek Knock-In Reporter Mice Demonstrate that DNA Repair by Non-homologous End Joining Declines with Age
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 7
-
Všechny články tohoto čísla
- Cuba: Exploring the History of Admixture and the Genetic Basis of Pigmentation Using Autosomal and Uniparental Markers
- Clonal Architecture of Secondary Acute Myeloid Leukemia Defined by Single-Cell Sequencing
- Mechanisms of Functional Variants That Impair Regulated Bicarbonate Permeation and Increase Risk for Pancreatitis but Not for Cystic Fibrosis
- Nucleosomes Shape DNA Polymorphism and Divergence
- Functional Diversification of Hsp40: Distinct J-Protein Functional Requirements for Two Prions Allow for Chaperone-Dependent Prion Selection
- Comparative Phylogenomics Uncovers the Impact of Symbiotic Associations on Host Genome Evolution
- Activation of the Immune System by Combinations of Common Alleles
- Age-Associated Sperm DNA Methylation Alterations: Possible Implications in Offspring Disease Susceptibility
- Muscle-Specific SIRT1 Gain-of-Function Increases Slow-Twitch Fibers and Ameliorates Pathophysiology in a Mouse Model of Duchenne Muscular Dystrophy
- MDRL lncRNA Regulates the Processing of miR-484 Primary Transcript by Targeting miR-361
- Hypersensitivity of Primordial Germ Cells to Compromised Replication-Associated DNA Repair Involves ATM-p53-p21 Signaling
- Intrapopulation Genome Size Variation in Reflects Life History Variation and Plasticity
- SlmA Antagonism of FtsZ Assembly Employs a Two-pronged Mechanism like MinCD
- Distribution and Medical Impact of Loss-of-Function Variants in the Finnish Founder Population
- Determinative Developmental Cell Lineages Are Robust to Cell Deaths
- DELLA Protein Degradation Is Controlled by a Type-One Protein Phosphatase, TOPP4
- Wnt Signaling Interacts with Bmp and Edn1 to Regulate Dorsal-Ventral Patterning and Growth of the Craniofacial Skeleton
- Common Transcriptional Mechanisms for Visual Photoreceptor Cell Differentiation among Pancrustaceans
- UVB Induces a Genome-Wide Acting Negative Regulatory Mechanism That Operates at the Level of Transcription Initiation in Human Cells
- The Nesprin Family Member ANC-1 Regulates Synapse Formation and Axon Termination by Functioning in a Pathway with RPM-1 and β-Catenin
- Combinatorial Interactions Are Required for the Efficient Recruitment of Pho Repressive Complex (PhoRC) to Polycomb Response Elements
- Recombination in the Human Pseudoautosomal Region PAR1
- Microsatellite Interruptions Stabilize Primate Genomes and Exist as Population-Specific Single Nucleotide Polymorphisms within Individual Human Genomes
- An Intronic microRNA Links Rb/E2F and EGFR Signaling
- An Essential Nonredundant Role for Mycobacterial DnaK in Native Protein Folding
- Integrative Genomics Reveals Novel Molecular Pathways and Gene Networks for Coronary Artery Disease
- The Genomic Landscape of the Ewing Sarcoma Family of Tumors Reveals Recurrent Mutation
- Evolution and Genetic Architecture of Chromatin Accessibility and Function in Yeast
- An ARID Domain-Containing Protein within Nuclear Bodies Is Required for Sperm Cell Formation in
- Stage-Dependent and Locus-Specific Role of Histone Demethylase Jumonji D3 (JMJD3) in the Embryonic Stages of Lung Development
- Genome Wide Association Identifies Common Variants at the Locus Influencing Plasma Cortisol and Corticosteroid Binding Globulin
- Regulation of Feto-Maternal Barrier by Matriptase- and PAR-2-Mediated Signaling Is Required for Placental Morphogenesis and Mouse Embryonic Survival
- Apomictic and Sexual Germline Development Differ with Respect to Cell Cycle, Transcriptional, Hormonal and Epigenetic Regulation
- Functional EF-Hands in Neuronal Calcium Sensor GCAP2 Determine Its Phosphorylation State and Subcellular Distribution , and Are Essential for Photoreceptor Cell Integrity
- Comparison of Methods to Account for Relatedness in Genome-Wide Association Studies with Family-Based Data
- Knock-In Reporter Mice Demonstrate that DNA Repair by Non-homologous End Joining Declines with Age
- Cis and Trans Effects of Human Genomic Variants on Gene Expression
- 8.2% of the Human Genome Is Constrained: Variation in Rates of Turnover across Functional Element Classes in the Human Lineage
- Novel Approach Identifies SNPs in and with Evidence for Parent-of-Origin Effect on Body Mass Index
- Hypoxia Adaptations in the Grey Wolf () from Qinghai-Tibet Plateau
- A Loss of Function Screen of Identified Genome-Wide Association Study Loci Reveals New Genes Controlling Hematopoiesis
- Unraveling Genetic Modifiers in the Mouse Model of Absence Epilepsy
- DNA Topoisomerase 1α Promotes Transcriptional Silencing of Transposable Elements through DNA Methylation and Histone Lysine 9 Dimethylation in
- The Coding and Noncoding Architecture of the Genome
- A Novel Locus Is Associated with Large Artery Atherosclerotic Stroke Using a Genome-Wide Age-at-Onset Informed Approach
- Brg1 Loss Attenuates Aberrant Wnt-Signalling and Prevents Wnt-Dependent Tumourigenesis in the Murine Small Intestine
- The PTK7-Related Transmembrane Proteins Off-track and Off-track 2 Are Co-receptors for Wnt2 Required for Male Fertility
- The Co-factor of LIM Domains (CLIM/LDB/NLI) Maintains Basal Mammary Epithelial Stem Cells and Promotes Breast Tumorigenesis
- Essential Genetic Interactors of Required for Spatial Sequestration and Asymmetrical Inheritance of Protein Aggregates
- Meiosis-Specific Cohesin Component, Is Essential for Maintaining Centromere Chromatid Cohesion, and Required for DNA Repair and Synapsis between Homologous Chromosomes
- Silencing Is Noisy: Population and Cell Level Noise in Telomere-Adjacent Genes Is Dependent on Telomere Position and Sir2
- The Two Cis-Acting Sites, and , Contribute to the Longitudinal Organisation of Chromosome I
- A Broadly Conserved G-Protein-Coupled Receptor Kinase Phosphorylation Mechanism Controls Smoothened Activity
- Requirements for Acute Burn and Chronic Surgical Wound Infection
- LIN-42, the PERIOD homolog, Negatively Regulates MicroRNA Transcription
- WAPL Is Essential for the Prophase Removal of Cohesin during Meiosis
- Expression in Planarian Neoblasts after Injury Controls Anterior Pole Regeneration
- Sox11 Is Required to Maintain Proper Levels of Hedgehog Signaling during Vertebrate Ocular Morphogenesis
- Accumulation of a Threonine Biosynthetic Intermediate Attenuates General Amino Acid Control by Accelerating Degradation of Gcn4 via Pho85 and Cdk8
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Wnt Signaling Interacts with Bmp and Edn1 to Regulate Dorsal-Ventral Patterning and Growth of the Craniofacial Skeleton
- Novel Approach Identifies SNPs in and with Evidence for Parent-of-Origin Effect on Body Mass Index
- Hypoxia Adaptations in the Grey Wolf () from Qinghai-Tibet Plateau
- DNA Topoisomerase 1α Promotes Transcriptional Silencing of Transposable Elements through DNA Methylation and Histone Lysine 9 Dimethylation in
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



