-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Molecular Mechanism of a -Regulatory Adaptation in Yeast
Despite recent advances in our ability to detect adaptive evolution involving the cis-regulation of gene expression, our knowledge of the molecular mechanisms underlying these adaptations has lagged far behind. Across all model organisms, the causal mutations have been discovered for only a handful of gene expression adaptations, and even for these, mechanistic details (e.g. the trans-regulatory factors involved) have not been determined. We previously reported a polygenic gene expression adaptation involving down-regulation of the ergosterol biosynthesis pathway in the budding yeast Saccharomyces cerevisiae. Here we investigate the molecular mechanism of a cis-acting mutation affecting a member of this pathway, ERG28. We show that the causal mutation is a two-base deletion in the promoter of ERG28 that strongly reduces the binding of two transcription factors, Sok2 and Mot3, thus abolishing their regulation of ERG28. This down-regulation increases resistance to a widely used antifungal drug targeting ergosterol, similar to mutations disrupting this pathway in clinical yeast isolates. The identification of the causal genetic variant revealed that the selection likely occurred after the deletion was already present at high frequency in the population, rather than when it was a new mutation. These results provide a detailed view of the molecular mechanism of a cis-regulatory adaptation, and underscore the importance of this view to our understanding of evolution at the molecular level.
Published in the journal: . PLoS Genet 9(9): e32767. doi:10.1371/journal.pgen.1003813
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003813Summary
Despite recent advances in our ability to detect adaptive evolution involving the cis-regulation of gene expression, our knowledge of the molecular mechanisms underlying these adaptations has lagged far behind. Across all model organisms, the causal mutations have been discovered for only a handful of gene expression adaptations, and even for these, mechanistic details (e.g. the trans-regulatory factors involved) have not been determined. We previously reported a polygenic gene expression adaptation involving down-regulation of the ergosterol biosynthesis pathway in the budding yeast Saccharomyces cerevisiae. Here we investigate the molecular mechanism of a cis-acting mutation affecting a member of this pathway, ERG28. We show that the causal mutation is a two-base deletion in the promoter of ERG28 that strongly reduces the binding of two transcription factors, Sok2 and Mot3, thus abolishing their regulation of ERG28. This down-regulation increases resistance to a widely used antifungal drug targeting ergosterol, similar to mutations disrupting this pathway in clinical yeast isolates. The identification of the causal genetic variant revealed that the selection likely occurred after the deletion was already present at high frequency in the population, rather than when it was a new mutation. These results provide a detailed view of the molecular mechanism of a cis-regulatory adaptation, and underscore the importance of this view to our understanding of evolution at the molecular level.
Introduction
Evolutionary adaptation is the process that has given rise to the ubiquitous, yet remarkable, fit between all living organisms and their environments [1]. The origins of these adaptations at the molecular level have been a subject of great interest, with active debate surrounding the relative roles of two major classes of molecular mechanism: changes in protein sequences vs. changes in the expression levels/patterns of those proteins [2]–[5]. Until recently, the evidence cited in favor of both mechanisms was either anecdotal (involving studies of single genes) or theoretical in nature [2]–[4]. However, the advent of methods for characterizing gene expression adaptation genome-wide [6]–[9] (as well as methods for measuring cis-regulatory changes that may or may not be adaptive [10]–[11]) has paved the way for this question to be addressed in an unbiased, systematic fashion [5].
Although the distinction between protein sequence vs. gene expression regulation is important, it is only one of many levels at which molecular mechanisms can be distinguished. For example among cis-regulatory adaptations, mutations might act via alterations in transcription factor (TF) binding, nucleosome positioning, mRNA processing, binding of RNA-binding proteins, etc. As the field matures, it is likely that the distinctions between these more detailed mechanistic levels will be of increasingly greater interest, since only by investigating these mechanisms will we fully understand the nature of adaptation at the molecular level.
In order to investigate the molecular mechanism of an adaptation, it is generally necessary to first identify the causal mutation(s) (though see [12]). This prerequisite has been a significant bottleneck in studies of cis-regulatory adaptation. Because we cannot computationally predict the effects of most non-coding mutations, and such mutations can act at long distances from their target genes in many species (resulting in a large search space), only a handful of causal mutations underlying cis-regulatory adaptations have been reported. For example, large deletions of an enhancer driving the pelvic expression of the Pitx1 gene in sticklebacks have been found to result in adaptive pelvic reduction in freshwater populations [13]. In another case, five non-coding mutations at the ebony locus contributed to dark abdominal pigmentation found in high-altitude populations of Drosophila melanogaster [14] (although other examples exist where causal cis-regulatory mutations have been identified [15]–[17], these have not been shown to be adaptive). However even for these intensively studied cis-regulatory adaptations, and others where important factors such as fitness effects have been estimated [18], the molecular mechanisms by which the causal mutations act—e.g. which TFs and/or epigenetic states are affected by the mutations—remain unknown.
We previously reported a genome-wide scan for gene expression adaptation between two strains of the budding yeast Saccharomyces cerevisiae: a laboratory strain (BY4716, hereafter “BY”) and a vineyard strain (RM11-1a, hereafter “RM”) [8]. We found that over 200 genes had likely been subject to recent positive selection in these strains via reinforcing cis and trans-acting regulatory adaptations. Among these genes, there was a particularly strong enrichment of down-regulating mutations in one metabolic pathway: ergosterol biosynthesis. Ergosterol is an abundant lipid component of the fungal plasma membrane, and is of major biomedical importance, being targeted by numerous antifungal drugs [19]. Indeed, a common mechanism of resistance to ergosterol-targeting drugs (such as amphotericin B) is reducing ergosterol levels via disruption of this pathway [19]–[21]. We previously found that six genes within the pathway (underlined and red in Figure 1A) showed the strongest signs of selection, based on patterns of reinforcing cis/trans-regulatory mutations, as well as a population-genetic signature of selective sweeps in the genomes of multiple strains [8]. This represents the first known example of a polygenic gene expression adaptation, from any species. Here, we sought to gain a deeper understanding of this adaptation.
Fig. 1. A polygenic gene expression adaptation in the ergosterol biosynthesis (ERG) pathway. 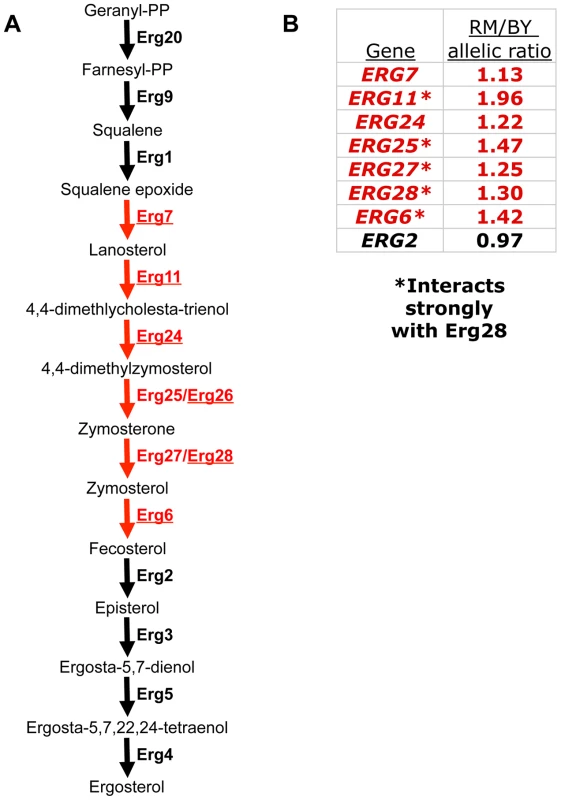
(A) The final steps of the ERG pathway. Eight genes whose down-regulation contributes to a polygenic gene expression adaptation are colored red; the six previously implicated genes [8] are underlined. Erg28 is shown next to its strongest interaction partner, Erg27. (B) Allelic bias of ERG genes, as measured by pyrosequencing in the RM/BY hybrid. The allelic bias indicates the magnitude of cis-regulatory divergence between RM and BY for each gene. Red color indicates genes that are part of the polygenic adaptation. Asterisks indicate those that interact strongly with Erg28 [24], all of which have stronger allelic bias than those that do not. Results
Further characterization of the ergosterol pathway adaptation
Because our initial identification of the polygenic gene expression adaptation within the ergosterol (ERG) biosynthesis pathway was based on expression data from genome-wide microarrays [22], we first sought to more precisely measure the cis-regulatory divergence at these loci. This divergence can be measured for any gene as the ratio of mRNA abundances of the two alleles present in a hybrid diploid: in the absence of cis-acting differences, the mRNA from the two alleles will be present in equal amounts (as they are in the genomic DNA), whereas they will be unequal in the presence of cis-regulatory divergence. To measure this ratio we employed pyrosequencing, a method that accurately quantifies allelic ratios at individual heterozygous sites [23].
Of the six genes we previously implicated, five were amenable to this approach (the sixth, ERG26, lacked any BY/RM sequence differences in its mRNA, so the alleles could not be distinguished). All five showed reproducible allelic imbalance in the expected direction (lower expression from the BY allele), with magnitudes ranging from 1.13-fold to 1.94-fold (Figure 1B). This result confirms that the “local eQTL” (genetic markers showing a statistical association with a nearby gene's expression level) previously mapped for these genes [22] likely represent cis-acting genetic variants.
To investigate if the polygenic adaptation extends beyond the six genes we originally identified, we also performed pyrosequencing on three additional ERG genes adjacent in the pathway to those already implicated: ERG25, ERG27, and ERG2 (allelic bias of ERG1, the other adjacent pathway member, could not be measured because it has no sequence differences between BY and RM). We found reproducible allelic bias in favor of RM for both ERG25 and ERG27, but not for ERG2 (Figure 1B). This suggests that the adaptive down-regulation extends to a total of at least eight genes, forming a contiguous block within the ERG pathway (Figure 1A, in red) that has been specifically targeted by natural selection.
Interestingly, in addition to the clear clustering of the down-regulated genes within the pathway, the genes with the strongest cis-regulatory differences correspond precisely to the core proteins in a stable complex organized by Erg28. Erg28 is the only known member of the ERG pathway lacking enzymatic activity; it is an endoplasmic reticulum transmembrane protein, highly conserved across eukaryotes (including humans), that acts as a scaffold promoting co-localization of ERG enzymes [24]–[26]. Erg28 physically interacts most strongly with Erg27 (and is thus shown next to Erg27 in Figure 1), but has also been found to interact strongly with itself and three other proteins: Erg25, Erg6, and Erg11; its other interactions are significantly weaker [24]. These five interacting proteins are not only all components of the polygenic adaptation (Figure 1), but are specifically those components with the strongest cis-acting down-regulation: all five have at least 1.25-fold differences between RM and BY alleles, while no other genes quite reach this threshold (Figure 1B). This pattern suggests that the precise magnitude of down-regulation may be influenced both by pathway position and by membership in the protein complex organized by Erg28.
Pinpointing a causal adaptive mutation
We decided to focus on ERG28 for further investigation. Not only is Erg28 the central member of the protein complex apparently targeted by natural selection, but sequence divergence in its promoter region was also minimal: there are only two sequence differences between BY and RM in the 590 bp upstream of the ERG28 transcription start site (TSS). These are one two-bp deletion (located in an 11 bp poly-A tract 112 bp upstream of the TSS, termed the AA112Δ allele), and one T/C SNP (229 bp upstream of the TSS, the T229C allele) (Figure 2A). Because promoters in S. cerevisiae are compact (generally <400 bp [27]), we decided to focus on these two candidate variants.
Fig. 2. Pinpointing the causal mutation affecting ERG28 cis-regulation. 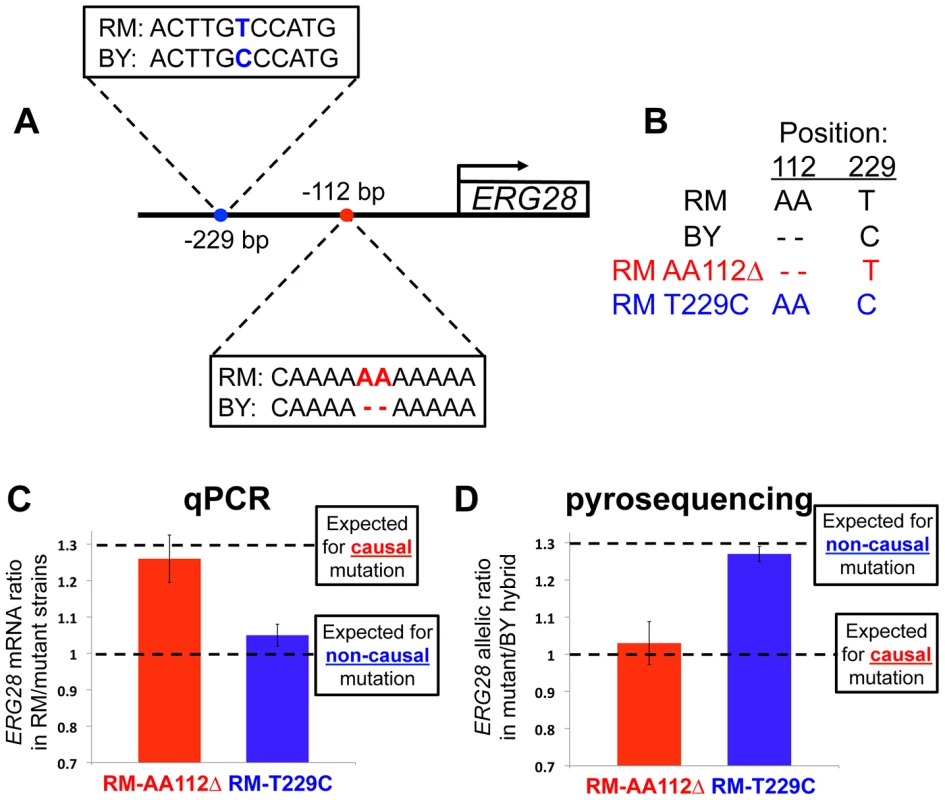
(A) Sequence divergence between RM and BY in the ERG28 promoter region. No other differences exist for 590 bp upstream of the gene, or in the 5′ UTR. (B) Genotypes at the two variable positions for RM, BY, and the two engineered strains. (C) The mRNA levels of ERG28 in each of the two engineered strains compared to wildtype RM, assayed by qPCR. The causal mutation is expected to result in a ∼1.30-fold difference, matching the allelic bias observed in the RM/BY hybrid (Figure 1B), whereas any non-causal mutation will not alter the RM expression level (∼1-fold change). (D) Allelic expression bias in hybrids between each engineered strain and BY, assayed by pyrosequencing. Any non-causal mutation will not alter the 1.30-fold RM/BY allelic bias, whereas the causal mutation is expected to be expressed at the same level as the BY allele (∼1-fold allelic bias). To definitively identify the mutation(s) underlying a cis-regulatory adaptation, the mutations must be individually tested for their effects on expression of the associated gene. Therefore we constructed allelic replacement strains in which individual BY variants were introduced into the RM genome. Using a method of in vivo site-directed mutagenesis known as delitto perfetto [28], we engineered strains that differed only by the desired mutation. We refer to the two resulting strains as RM AA112Δ and RM T229C (Figure 2b).
If a mutation can fully account for the 1.30-fold cis-acting difference between the RM/BY alleles of ERG28 (Figure 1B), and no additional mutations have any effect, then this mutation can be deemed causal. To test if this was the case for either of our candidate mutations, we measured the expression level of ERG28 in each strain, as well as in wild-type RM, by quantitative PCR (qPCR). While we found no effect of the T229C mutation (1.05-fold difference), we observed that the AA112Δ mutation led to a 1.26-fold decrease in mRNA level (Figure 2c), indistinguishable from the 1.30-fold change expected for the causal mutation(s).
To further test if the AA112Δ mutation could fully account for the RM/BY difference, we mated the RM AA112Δ strain with BY, and measured the allelic ratio of ERG28 mRNA in the resulting diploid strain. The causal mutation would be expected to reduce the 1.30-fold allelic difference to ∼1, while any non-causal mutation would have the same the 1.30-fold allelic imbalance found in the BY/RM hybrid. Consistent with the qPCR results, the RM AA112Δ/BY hybrid strain showed a 1.03-fold difference between alleles, while the RM T229C/BY hybrid showed a 1.27-fold difference (Figure 2d). Together, these results suggested that the AA112Δ mutation likely accounted for all, or nearly all, of the cis-acting divergence at ERG28 between RM and BY.
The molecular mechanism of the ERG28 cis-regulatory adaptation
We considered two potential mechanisms for how the AA112Δ mutation may be down-regulating transcription: nucleosome positioning and TF binding. Both processes are known to play important roles in determining rates of transcription initiation, and could potentially be affected by a 2-bp deletion.
Nucleosome positioning was an especially plausible mechanism because the 11-bp poly-A sequence in which the 2-bp deletion occurred is a strong nucleosome-disfavoring sequence [29]. Therefore we took advantage of published data on genome-wide nucleosome positions from BY and RM [30] to determine whether the nucleosome overlapping the deletion was affected. There was no significant difference between BY and RM in the nucleosomal occupancy or positioning at this location (nor was it differentially acetylated on histone H3 lysine 14 [30]), suggesting that nucleosome occupancy was not greatly affected by this deletion.
We therefore turned to TF binding as a second possible mechanism. Utilizing a published map of putative TF binding sites [31] we identified two highly conserved (across Saccharomyces sensu stricto) binding sites for the TFs Mot3 and Sok2, flanking the deletion (Figure 3a). Mot3 is a well-known repressor of ERG pathway genes, exerting its greatest effect in hypoxic or hyper-osmotic conditions [32]–[33], whereas Sok2 has not been previously linked to the ERG pathway to our knowledge. Neither binding site motif is directly affected by the 2-bp deletion; rather the only effect is on their spacing, reducing the distance between motif centers from 16 bp to 14.
Fig. 3. Determining the molecular mechanism of the causal mutation. 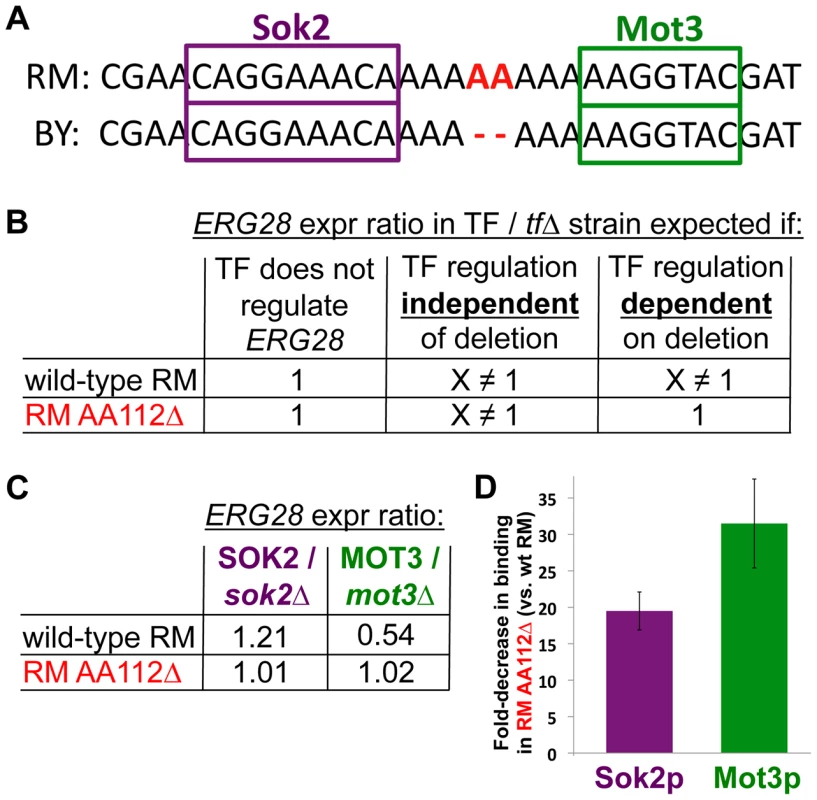
(A) Two predicted transcription factor (TF) binding sites flanking the deletion. (B) The expected fold-change in ERG28 expression level when deleting TFs under different scenarios. Left: if a TF does not regulate ERG28, its deletion should have no effect on ERG28 levels. Center: If a TF regulates ERG28 and acts independently of the two-base deletion, then deleting the TF should result in some fold-change X, which will be observed in both the wildtype RM and RM AA112Δ backgrounds. Right: If a TF regulates the wildtype ERG28 promoter, but the deletion abolishes this regulation, then the TF deletion may only affect ERG28 mRNA levels in the wildtype background (A fourth possible scenario, not shown, is where the TF only regulates ERG28 in RM AA112Δ). (C) qPCR data showing changes in ERG28 mRNA levels upon deleting either SOK2 or MOT3. In both cases, a difference is observed in the wildtype background (p = 7.5×10−5 for SOK2 and 5.0×10−3 for MOT3), but not the RM AA112Δ background (p = 0.28 for SOK2 and 0.67 for MOT3), consistent with the TF regulation being entirely abolished by the deletion. (D) Chromatin immunoprecipitation data showing the difference in binding for Sok2 and Mot3 to the ERG28 promoter in wildtype RM/RM AA112Δ. In both cases a significant decrease in binding is observed in RM AA112Δ. To test if the AA112Δ deletion may affect the regulation of ERG28 by either of these two TFs, we created knockout strains for each TF in both the wild-type RM and RM AA112Δ backgrounds. Several outcomes are possible (Figure 3b). First, if the TF does not regulate ERG28, then deleting it should have no effect in either genetic background. Second, if the TF does regulate ERG28 but is not affected by the AA112Δ deletion, then the effect of TF deletion should be equal in the two backgrounds. Finally, if the AA112Δ deletion is affecting the TF's regulation of ERG28, then the effect of TF deletion will depend on the background—for example, having an effect on ERG28 expression in wild-type RM but not in RM AA112Δ.
Consistent with Mot3's known role as a repressor of ERG pathway genes, we found that ERG28 was induced 1.85-fold in an RM mot3Δ strain compared with wild-type RM (Figure 3c). Likewise, Sok2 was found to be an activator of ERG28, with 1.21-fold lower expression in RM sok2Δ compared to wild-type RM. However neither TF had any measurable effect on ERG28 expression when deleted from the RM AA112Δ strain (Figure 3c). This suggests that although both TFs regulate ERG28 in RM, this regulation was abolished by the 2-bp deletion.
The effect of AA112Δ on regulation of ERG28 by Mot3 and Sok2 suggested that their binding to the promoter may be affected by the deletion. To investigate this, we performed chromatin immunoprecipitation (ChIP). Specifically, we HA-tagged both TFs in both wild-type RM and RM AA112Δ backgrounds, and quantified their binding to specific regions by quantitative PCR (qPCR). We found that for both factors, binding at the ERG28 promoter was reduced in RM AA112Δ, compared to wild-type RM: Sok2 showed ∼19-fold lower binding, while Mot3 had ∼31-fold lower binding (Figure 3d). This suggests that the loss of ERG28 regulation by these TFs in the AA112Δ background (Figure 3c) is likely due to their severely reduced binding.
Fitness effects of the ERG28 cis-regulatory adaptation
In order to investigate the phenotypic effects of the AA112Δ allele, we measured the growth rates of our engineered strains and RM in several environments (see Materials and Methods). While we did not observe any fitness advantage of the RM AA112Δ strain in most conditions (e.g. rich synthetic defined [SD] media; paired t-test p = 0.46 for RM AA112Δ vs. RM and p = 0.83 for RM T229C vs. RM; Figure 4a), we did find a growth advantage of this strain in the presence of the antifungal drug amphotericin B (Figure 4b). Specifically, RM AA112Δ had a 1.3% higher growth rate than RM when grown in the presence of the drug (p = 0.014), whereas RM T229C had no measurable difference from RM (p = 0.86). This suggests that the fitness benefit conferred by the AA112Δ allele is condition-specific.
Fig. 4. Fitness effect of the causal mutation. 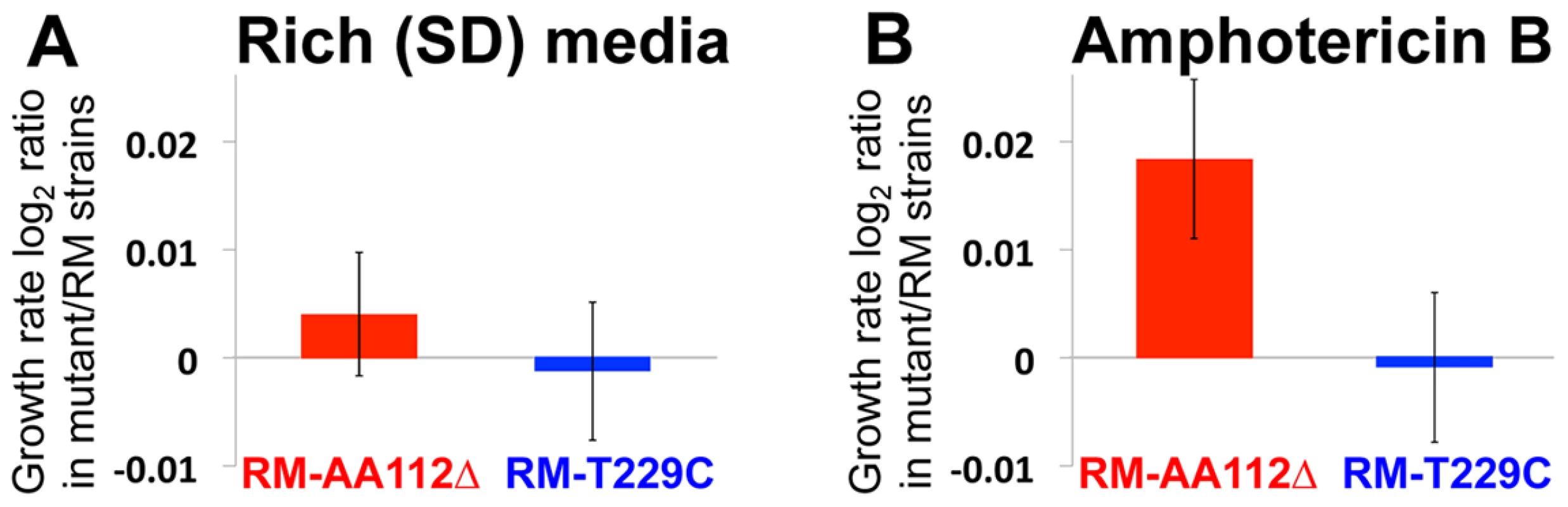
(A) In rich synthetic defined (SD) media, the RM AA112Δ strain and RM T229C strains show no significant difference from RM. (B) In the presence of the antifungal drug amphotericin B, the RM AA112Δ strain shows a growth rate advantage over RM, whereas the RM T229C strain shows no difference from RM. Bars represent the mean log2 ratios of log-phase growth rates from 48 replicate cultures, +/−1 S.E. Insights into the selection on ERG28 cis-regulation
Our identification of the AA112Δ allele as causal allows us to examine the distribution of this adaptive mutation across other yeast strains, in order to study its history. In particular, we wished to address the question of whether the selection occurred when the deletion was a new mutation that just recently arose (e.g. in the laboratory), or whether it was present as “standing variation” in S. cerevisiae for some time before the selection occurred. Population geneticists have theorized about the consequences of selection acting on pre-existing variation, as opposed to waiting for rare advantageous mutations to occur, but few clear examples exist [34]–[36].
To distinguish between these alternatives, we first examined the distribution of the AA112Δ allele across a set of 36 sequenced strains of S. cerevisiae [37]. The deletion is present in 12/36 sequenced strains (in addition to BY; Figure S1). These 12 strains are diverse in terms of both geography (from the Americas, Asia, Africa, and Europe) and lifestyle (lab strains, wild strains, sake strains, palm wine strains, and other fermentation strains). Furthermore they are genetically diverse, as evidenced by their lack of clustering within the S. cerevisiae phylogeny (Figure S1). This broad distribution across the species suggests that the AA112Δ allele is present at appreciable frequency in many populations of S. cerevisiae.
To further investigate this, we sequenced the ERG28 promoter in EM93, the wild strain that accounts for ∼88% of the BY genome [38]–[39]. Since EM93 is a diploid, we sequenced the promoter in the four spores from a single EM93 tetrad, in order to capture both alleles with no ambiguity. We found that the AA112Δ mutation was heterozygous within EM93, supporting our inference that it is commonly found in the wild. Together, these results suggest that the selection on ERG28 in the BY lineage [8] was likely acting on standing variation, as opposed to a new mutation. Because EM93 is heterozygous, we can infer the selective sweep most likely occurred in the descendants of EM93, after its introduction to the laboratory.
To attempt a similar analysis for the seven other ERG genes involved in this adaptation (Figure 1A), we sequenced their promoters in the same four EM93 spores. Because we do not know the causal variants, we performed this analysis at the level of promoter haplotypes (sets of co-occurring alleles). We found that for all seven genes, the complete BY promoter haplotype was either homozygous (for two genes, ERG25 and ERG26) or heterozygous (for five genes) in EM93, indicating that their cis-acting down-regulations were likely not due to new mutations occurring in the lab. Each of these BY haplotypes was also observed in between zero and six additional sequenced strains, indicating that some of the haplotypes are segregating at an appreciable frequency in S. cerevisiae. However the absence of a complete BY haplotype does not imply the absence of the causal BY variant, since most ERG promoter variants are not in perfect linkage disequilibrium with their neighboring variants. For example, although the AA112Δ variant was found in 12 strains (Figure S1), only five of these also had the T229C variant (and thus the complete BY promoter haplotype). This highlights the importance of identifying causal variants in order to study the evolutionary histories of specific adaptations.
Discussion
We have identified the causal mutation underlying a cis-regulatory adaptation that affects the ergosterol biosynthesis pathway in yeast, and characterized its molecular mechanism of action. The mutation, a 2 bp promoter deletion, reduces the expression of ERG28 by ∼1.3-fold. This effect is mediated by two TFs, Mot3 and Sok2, which bind immediately adjacent to the deletion; these TFs bind and regulate the wild-type RM ERG28 promoter, but not the ERG28 AA112Δ promoter.
Although it may seem surprising that a 2 bp deletion outside of TF binding sites can have such a strong effect on TF binding, it is consistent with previous work. First, most between-strain variation in the binding of the Ste12 TF in yeast cannot be linked to variation in any known TF motif, even when only considering those binding sites where occupancy was associated with nearby genetic markers [40]. Second, it was recently shown that changes in the positions of TF binding sites as small as 1–2 bp can result in substantial (>1.5-fold) effects on transcription [41]. Finally, minor changes in the copy number of very short tandem repeats in yeast promoters can also impact transcription [42].
It is also at first counterintuitive that decreased binding of a repressor (Mot3) could contribute to the down-regulation of ERG28 by AA112Δ, in particular since the repressive effects of Mot3 appear to be stronger than the activation by Sok2 (Figure 3C). We hypothesize that the AA112Δ mutation may have altered the TF binding landscape upstream of ERG28, not only for Mot3 and Sok2, but possibly for other TFs or their cofactors as well. The deletion's effect on transcription would then be determined by this altered landscape.
In addition to the focus on ERG28, our results also further characterize the polygenic ERG pathway adaptation as a whole. We found that two genes not implicated in our previous analysis of microarray data [8], ERG25 and ERG27, also show reduced expression from the BY allele (compared to RM). Moreover, our precise measurements of the cis-acting effect size for each ERG gene led us to an intriguing discovery: the five proteins that form the core of a complex at the ER membrane are also the five with the strongest cis-regulatory change. This pattern suggests an exquisite specificity of selection, in which the precise level of down-regulation is determined not only by position within the pathway, but also by membership in a particular protein complex.
While a handful of causal mutations underlying cis-regulatory adaptations in other model organisms have been previously reported [13]–[14], their molecular mechanisms are unknown. Compared to these, our knowledge of the ERG28 AA112Δ mutation is now relatively detailed, though still incomplete; for example, how the deletion disrupts binding has not been established. A plausible explanation is that Sok2 and Mot3 may bind cooperatively to the ERG28 promoter in wildtype RM; if this cooperativity is disrupted by the 2-bp deletion (which brings the binding sites ∼6.8 Å closer together and changes their relative angles by ∼70°), then neither factor would bind well to the AA112Δ promoter.
At the phenotypic level, we found that AA112Δ confers a condition-specific growth advantage in the presence of the antifungal drug amphotericin B. Because the AA112Δ mutation may also lead to a fitness advantage in other environments that were not tested, we cannot conclude whether amphotericin B is related to the specific selection pressure that gave rise to the ERG pathway adaptation in BY. However our results are quite consistent with previous observations that the down-regulation or inactivation of ERG pathway genes confers resistance to amphotericin B in diverse clinical yeast isolates [19]–[21]. Thus in addition to aiding our understanding of the molecular mechanisms of cis-regulatory adaptation, our results may shed light on potential mechanisms by which antifungal drug resistance can evolve.
Materials and Methods
Strain construction
We carried out all strain engineering in RM, as opposed to BY, because BY contains a very recent loss-of-function transposon insertion in the transcription factor HAP1, which alters the regulation of many ERG genes, including ERG28. Because this mutation was so recent (not even present in the very closely related lab strain W303 [8]), it must have happened after the ERG28 cis-regulatory adaptation, so the functional HAP1 in RM should more accurately reflect the original effects of any cis-regulatory mutations.
In vivo site-directed mutagenesis, known as delitto perfetto, was performed as described [27]. Briefly, the pCORE-UH cassette, containing K. lactis URA3 and hyg, was amplified using primers containing ∼70 bp of homology to the RM ERG28 promoter (Table S1). This PCR product was transformed into RM, and correct incorporation into the ERG28 promoter was verified by PCR. The site of incorporation was chosen in between the two candidate genetic variants, so that the same CORE cassette transformant could be used for engineering both mutations. The CORE cassette was then removed by separately transforming two PCR products from the BY ERG28 promoter, containing the desired mutation (either AA112Δ or T229C) as well as enough flanking DNA sequence (identical between RM and BY) to allow specific targeting of the PCR product. Because the efficiency of delitto perfetto is maximized when transforming longer DNA molecules, as well as double-stranded DNA [20], transforming long PCR products from BY (as opposed to shorter, single-stranded synthetic oligonucleotides) is a useful modification. Counter-selection of the resulting transformants on 5-FOA allowed isolation of successfully engineered strains that had replaced the CORE cassette with the desired mutation, which were then sequence-verified.
The complete coding regions of MOT3 and SOK2 were replaced with the hphMX6 antibiotic resistance gene via PCR-mediated gene disruption [43] in both RM and RM AA112Δ. Transformants were grown on hygromycin B, and verified by PCR. These two TFs were also HA-tagged at their C-termini via transformation of a PCR product including the HA tag, hphMX6, and flanking regions with 40 bp of homology to the targeted regions [43]. Transformants were grown on hygromycin B, and then verified by PCR and sequencing.
Table S2 lists all strains used in this work.
Growth conditions
With the exception of growth rate experiments (Figure 4), all strains were grown in standard YPD media at 30°C, and harvested in log-phase (OD600 ∼1) for either RNA extraction or chromatin immunoprecipitation.
RNA extraction and cDNA synthesis
We extracted total RNA with the Epicentre Biotechnologies RNA Purification kit, which includes a DNase treatment to remove contaminating genomic DNA. RNA concentration was quantified with a NanoDrop2000 spectrophotometer. For cDNA synthesis, total RNA samples were diluted to a concentration of 500 ng/µL. RNA was reverse transcribed into cDNA with SuperScript III RT (Invitrogen), following manufacturer protocols.
Pyrosequencing
Pyrosequencing was performed on a PyroMark Q24 (Qiagen), following manufacturer's protocols. Primers (Table S1) were designed to target individual SNPs in transcribed regions using the PyroMark Assay Design Software (Qiagen). Negative controls using no primers, or no cDNA template, were performed for each assay.
Quantitative PCR
cDNA was diluted 1∶100 prior to qPCR. qPCR was performed on an Eco Real-Time PCR machine (Illumina) following manufacturer's protocols. To quantify changes in ERG28 mRNA abundance, six control genes previously noted for their stability across conditions [44] were measured in each experiment: ACT1, TDH3, ALG9, TAF10, TFC1, and UBC6. All experiments were done in at least biological triplicate and technical duplicate. Experiments in Figure 3c were done in biological sextuplicate and technical quadruplicate. Data were analyzed using qBase Plus software (Biogazelle) [45].
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) was performed essentially as described [46]. Briefly, wildtype cells and cells expressing either Mot3-HA or Sok2-HA were grown to mid-log phase in 100 mL YPD. Cross-linking was performed by treating yeast with 1% formaldehyde for 15 minutes at 25°C. Chromatin was isolated from whole-cell extracts generated by spheroplasting and sheared by sonication. Immunoprecipitation was performed from 5 µg chromatin using mouse monoclonal anti-HA (Invitrogen, clone 5B1D10) and immune complexes were captured with Ultralink Immobilized Protein A/G resin (Pierce). Protein-DNA complexes were eluted with 1% SDS/0.1 M NaHCO3. Eluates were incubated at 65°C overnight to reverse cross-links and treated with proteinase K (Invitrogen) and RNAse A. DNA was phenol-chloroform extracted, ethanol-precipitated, and resuspended in water prior to qPCR.
ChIP DNA was amplified on an Eco Real-Time PCR machine (Illumina) following manufacturer's protocols. We quantified the abundance of the ERG28 promoter region containing the Mot3 and Sok2 binding sites, as well as part of the ACT1 coding region as a control to quantify the amount of DNA in each reaction. The concentration of ERG28 promoter DNA was normalized against this control before comparing across genetic backgrounds (RM vs. RM AA112Δ) for each TF.
Quantitative growth rate measurements
To perform quantitative growth rate measurements (Figure 4), we grew strains in 96-well plates and measured OD600 at 15-minute intervals using an automated plate reader (Tecan) until cultures reached saturation. Data shown in Figure 4 are the mean log2 ratios of the maximum log-phase growth rates (estimated by Magellan software, Tecan) for 48 replicate growth curves of each strain. Growth conditions were SD media alone or 0.8 ug/ml amphotericin B in SD media, both at room temperature (22°C). P-values were calculated using a paired t-test, pairing wells in the same row on each plate. Other conditions tested in an initial screening phase were hyperosmotic stress (NaCl or menadione) and temperature stress (heat/freezing).
Supporting Information
Zdroje
1. Darwin C (1876) On the Origin of Species by Means of Natural Selection: or, the Preservation of Favored Races in the Struggle for Life. New York, NY: D. Appleton and Co.
2. KingMC, WilsonAC (1975) Evolution at two levels in humans and chimpanzees. Science 188 : 107.
3. Prud'hommeB, GompelN, CarrollSB (2007) Emerging principles of regulatory evolution. Proc Natl Acad Sci USA 104 (Suppl 1) 8605–12.
4. HoekstraHE, CoyneJA (2007) The locus of evolution: Evo devo and the genetics of adaptation. Evolution 61 : 995–1016.
5. FraserHB (2013) Gene expression drives local adaptation in humans. Genome Research 23 : 1089.
6. FraserHB, LevyS, ChavanA, ShahHB, PerezJC, et al. (2012) Polygenic cis-regulatory adaptation in the evolution of yeast pathogenicity. Genome Research 22 : 1930.
7. FraserHB, BabakT, TsangJ, ZhouY, ZhangB, et al. (2011) Systematic detection of polygenic cis-regulatory evolution. PLoS Genetics 7: e1002023.
8. FraserHB, MosesA, SchadtEE (2010) Evidence for widespread adaptive evolution of gene expression in budding yeast. Proc Natl Acad Sci USA 107 : 2997.
9. FraserHB (2011) Genome-wide approaches to the study of adaptive gene expression evolution. Bioessays 33 : 469.
10. MartinHC, RoopJI, SchraiberJG, HsuTY, BremRB (2012) Evolution of a membrane protein regulon in Saccharomyces. Mol Biol Evol 29 : 1747–56.
11. TiroshI, ReikhavS, LevyAA, BarkaiN (2009) A yeast hybrid provides insight into the evolution of gene expression regulation. Science 324 : 659–62.
12. CrockerJ, TamoriY, ErivesA (2008) Evolution acts on enhancer organization to fine-tune gradient threshold readouts. PLoS Biol 6: e263.
13. ChanYF, MarksME, JonesFC, VillarrealGJr, ShapiroMD, et al. (2010) Adaptive evolution of pelvic reduction in sticklebacks by recurrent deletion of a Pitx1 enhancer. Science 327 : 302.
14. RebeizM, PoolJE, KassnerVA, AquadroCF, CarrollSB (2009) Stepwise modification of a modular enhancer underlies adaptation in a Drosophila population. Science 326 : 1663.
15. FrankelN, ErezyilmazDF, McGregorAP, WangS, PayreF, et al. (2011) Morphological evolution caused by many subtle-effect substitutions in regulatory DNA. Nature 474 : 598–603.
16. JeongS, RebeizM, AndolfattoP, WernerT, TrueJ, et al. (2008) The evolution of gene regulation underlies a morphological difference between two Drosophila sister species. Cell 132 : 783–93.
17. GerkeJ, LorenzK, CohenB (2009) Genetic interactions between transcription factors cause natural variation in yeast. Science 323 : 498–501.
18. LinnenCR, PohYP, PetersonBK, BarrettRD, LarsonJG, et al. (2013) Adaptive evolution of multiple traits through multiple mutations at a single gene. Science 339 : 1312–6.
19. MorschhäuserJ (2010) Regulation of multidrug resistance in pathogenic fungi. Fungal Genet and Biol 47 : 94–106.
20. YoungLY, HullCM, HeitmanJ (2003) Disruption of ergosterol biosynthesis confers resistance to amphotericin B in Candida lusitaniae. Antimicrob Agents Chemother 47 : 2717–24.
21. KellySL, LambDC, TaylorM, CorranAJ, BaldwinBC, et al. (1994) Resistance to amphotericin B associated with defective sterol Δ87 isomerase in a Cryptococcus neoformans strain from an AIDS patient. FEMS Microbiology Letters 122 : 39–42.
22. BremRB, KruglyakL (2005) The landscape of genetic complexity across 5,700 gene expression traits in yeast. Proc Natl Acad Sci USA 102 : 1572–1577.
23. WittkoppPJ (2011) Using pyrosequencing to measure allele-specific mRNA abundance and infer the effects of cis - and trans-regulatory differences. Methods Mol Biol 772 : 297–317.
24. MoC, BardM (2005) Erg28p is a key protein in the yeast sterol biosynthetic enzyme complex. J Lipid Res 46 : 1991–8.
25. MoC, ValachovicM, BardM (2004) The ERG28-encoded protein, Erg28p, interacts with both the sterol C-4 demethylation enzyme complex as well as the late biosynthetic protein, the C-24 sterol methyltransferase (Erg6p). Biochim Biophys Acta 1686 : 30–6.
26. GachotteD, EcksteinJ, BarbuchR, HughesT, RobertsC, et al. (2001) A novel gene conserved from yeast to humans is involved in sterol biosynthesis. J Lipid Res 42 : 150–4.
27. VentersBJ, WachiS, MavrichTN, AndersenBE, JenaP, et al. (2011) A comprehensive genomic binding map of gene and chromatin regulatory proteins in Saccharomyces. Mol Cell 41 : 480–92.
28. StoriciF, ResnickMA (2006) The delitto perfetto approach to in vivo site-directed mutagenesis and chromosome rearrangements with synthetic oligonucleotides in yeast. Methods Enzymol 409 : 329–45.
29. Raveh-SadkaT, LevoM, ShabiU, ShanyB, KerenL, et al. (2012) Manipulating nucleosome disfavoring sequences allows fine-tune regulation of gene expression in yeast. Nat Genet 44 : 743–50.
30. NagarajanM, VeyrierasJB, de DieuleveultM, BottinH, FehrmannS, et al. (2010) Natural single-nucleosome epi-polymorphisms in yeast. PLoS Genet 6: e1000913.
31. MacIsaacKD, WangT, GordonDB, GiffordDK, StormoGD, et al. (2006) An improved map of conserved regulatory sites for Saccharomyces cerevisiae. BMC Bioinformatics 7 : 113.
32. MontañésFM, Pascual-AhuirA, ProftM (2011) Repression of ergosterol biosynthesis is essential for stress resistance and is mediated by the Hog1 MAP kinase and the Mot3 and Rox1 transcription factors. Mol Microbiol 79 : 1008–23.
33. DaviesBS, RineJ (2006) A role for sterol levels in oxygen sensing in Saccharomyces cerevisiae. Genetics 174 : 191–201.
34. BarrettRD, SchluterD (2008) Adaptation from standing genetic variation. Trends Ecol Evol 23 : 38–44.
35. OrrHA, BetancourtAJ (2001) Haldane's sieve and adaptation from the standing genetic variation. Genetics 157 : 875–84.
36. HermissonJ, PenningsPS (2005) Soft sweeps: molecular population genetics of adaptation from standing genetic variation. Genetics 169 : 2335–52.
37. LitiG, CarterDM, MosesAM, WarringerJ, PartsL, et al. (2009) Population genomics of domestic and wild yeasts. Nature 458 : 337–341.
38. MortimerRK, JohnstonJR (1986) Genealogy of principal strains of the yeast genetic stock center. Genetics 113 : 35–43.
39. DimitrovLN, BremRB, KruglyakL, GottschlingDE (2009) Polymorphisms in multiple genes contribute to the spontaneous mitochondrial genome instability of Saccharomyces cerevisiae S288c strains. Genetics 183 : 365–83.
40. ZhengW, ZhaoH, ManceraE, SteinmetzLM, SnyderM (2010) Genetic analysis of variation in transcription factor binding in yeast. Nature 464 : 1187–91.
41. SharonE, KalmaY, SharpA, Raveh-SadkaT, LevoM, et al. (2012) Inferring gene regulatory logic from high-throughput measurements of thousands of systematically designed promoters. Nat Biotechnol 30 : 521–30.
42. GemayelR, VincesMD, LegendreM, VerstrepenKJ (2010) Variable tandem repeats accelerate evolution of coding and regulatory sequences. Annu Rev Genet 44 : 445–77.
43. Amberg DC, Burke DJ, Strathern JN (2005) Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
44. TesteMA, DuquenneM, FrançoisJM, ParrouJL (2009) Validation of reference genes for quantitative expression analysis by real-time RT-PCR in Saccharomyces cerevisiae. BMC Mol Biol 10 : 99.
45. HellemansJ, MortierG, De PaepeA, SpelemanF, VandesompeleJ (2007) qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 8: R19.
46. MeluhPB, BroachJR (1999) Immunological analysis of yeast chromatin. Methods Enzymol 304 : 414–430.
Štítky
Genetika Reprodukční medicína
Článek Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive SelectionČlánek Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand SkillČlánek Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage EvolvabilityČlánek Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation inČlánek Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 ModelČlánek Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period inČlánek VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival inČlánek Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2AČlánek A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding YeastČlánek Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on PhenotypeČlánek Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding SitesČlánek Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 9
-
Všechny články tohoto čísla
- The Pathway Gene Functions together with the -Dependent Isoprenoid Biosynthetic Pathway to Orchestrate Germ Cell Migration
- Take Off, Landing, and Fly Anesthesia
- Nucleosome Assembly Proteins Get SET to Defeat the Guardian of Chromosome Cohesion
- Whole-Exome Sequencing Reveals a Rapid Change in the Frequency of Rare Functional Variants in a Founding Population of Humans
- Evidence Is Evidence: An Interview with Mary-Claire King
- Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive Selection
- Convergent Transcription Induces Dynamic DNA Methylation at Loci
- Environmental Stresses Disrupt Telomere Length Homeostasis
- Ultra-Sensitive Sequencing Reveals an Age-Related Increase in Somatic Mitochondrial Mutations That Are Inconsistent with Oxidative Damage
- Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand Skill
- Genetic and Anatomical Basis of the Barrier Separating Wakefulness and Anesthetic-Induced Unresponsiveness
- The Locus, Exclusive to the Ambulacrarians, Encodes a Chromatin Insulator Binding Protein in the Sea Urchin Embryo
- Binding of NF-κB to Nucleosomes: Effect of Translational Positioning, Nucleosome Remodeling and Linker Histone H1
- Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage Evolvability
- Dynamics of DNA Methylation in Recent Human and Great Ape Evolution
- Functional Dissection of Regulatory Models Using Gene Expression Data of Deletion Mutants
- PAQR-2 Regulates Fatty Acid Desaturation during Cold Adaptation in
- N-alpha-terminal Acetylation of Histone H4 Regulates Arginine Methylation and Ribosomal DNA Silencing
- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation in
- miR-1/133a Clusters Cooperatively Specify the Cardiomyogenic Lineage by Adjustment of Myocardin Levels during Embryonic Heart Development
- Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 Model
- Genome-Wide Analysis of Genes and Their Association with Natural Variation in Drought Tolerance at Seedling Stage of L
- Deep Resequencing of GWAS Loci Identifies Rare Variants in , and That Are Associated with Ulcerative Colitis
- Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period in
- VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival in
- Analysis of Genes Reveals Redundant and Independent Functions in the Inner Ear
- Predicting the Risk of Rheumatoid Arthritis and Its Age of Onset through Modelling Genetic Risk Variants with Smoking
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
- A Shift to Organismal Stress Resistance in Programmed Cell Death Mutants
- Fragile Site Instability in Causes Loss of Heterozygosity by Mitotic Crossovers and Break-Induced Replication
- Tracking of Chromosome and Replisome Dynamics in Reveals a Novel Chromosome Arrangement
- The Condition-Dependent Transcriptional Landscape of
- Ago1 Interacts with RNA Polymerase II and Binds to the Promoters of Actively Transcribed Genes in Human Cancer Cells
- Nebula/DSCR1 Upregulation Delays Neurodegeneration and Protects against APP-Induced Axonal Transport Defects by Restoring Calcineurin and GSK-3β Signaling
- System-Wide Analysis Reveals a Complex Network of Tumor-Fibroblast Interactions Involved in Tumorigenicity
- Meta-Analysis of Genome-Wide Association Studies Identifies Six New Loci for Serum Calcium Concentrations
- and Are Required for Cellularization and Differentiation during Female Gametogenesis in
- Growth factor independent-1 Maintains Notch1-Dependent Transcriptional Programming of Lymphoid Precursors
- Whole Genome Sequencing Identifies a Deletion in Protein Phosphatase 2A That Affects Its Stability and Localization in
- An Alteration in ELMOD3, an Arl2 GTPase-Activating Protein, Is Associated with Hearing Impairment in Humans
- Genomic Identification of Founding Haplotypes Reveals the History of the Selfing Species
- Plasticity Regulators Modulate Specific Root Traits in Discrete Nitrogen Environments
- The IDD14, IDD15, and IDD16 Cooperatively Regulate Lateral Organ Morphogenesis and Gravitropism by Promoting Auxin Biosynthesis and Transport
- Stochastic Loss of Silencing of the Imprinted Allele, in a Mouse Model and Humans with Prader-Willi Syndrome, Has Functional Consequences
- The Prefoldin Complex Regulates Chromatin Dynamics during Transcription Elongation
- PKA Controls Calcium Influx into Motor Neurons during a Rhythmic Behavior
- A Pre-mRNA-Splicing Factor Is Required for RNA-Directed DNA Methylation in
- Cell-Type Specific Features of Circular RNA Expression
- The Uve1 Endonuclease Is Regulated by the White Collar Complex to Protect from UV Damage
- An Atypical Kinase under Balancing Selection Confers Broad-Spectrum Disease Resistance in Arabidopsis
- Genome-Wide Mutation Avalanches Induced in Diploid Yeast Cells by a Base Analog or an APOBEC Deaminase
- Extensive Divergence of Transcription Factor Binding in Embryos with Highly Conserved Gene Expression
- Bi-modal Distribution of the Second Messenger c-di-GMP Controls Cell Fate and Asymmetry during the Cell Cycle
- Cell Interactions and Patterned Intercalations Shape and Link Epithelial Tubes in
- A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding Yeast
- The Genome and Development-Dependent Transcriptomes of : A Window into Fungal Evolution
- SKN-1/Nrf, A New Unfolded Protein Response Factor?
- The Highly Prolific Phenotype of Lacaune Sheep Is Associated with an Ectopic Expression of the Gene within the Ovary
- Fusion of Large-Scale Genomic Knowledge and Frequency Data Computationally Prioritizes Variants in Epilepsy
- IL-17 Attenuates Degradation of ARE-mRNAs by Changing the Cooperation between AU-Binding Proteins and microRNA16
- An Enhancer Element Harboring Variants Associated with Systemic Lupus Erythematosus Engages the Promoter to Influence A20 Expression
- Genome Analysis of a Transmissible Lineage of Reveals Pathoadaptive Mutations and Distinct Evolutionary Paths of Hypermutators
- Type I-E CRISPR-Cas Systems Discriminate Target from Non-Target DNA through Base Pairing-Independent PAM Recognition
- Divergent Transcriptional Regulatory Logic at the Intersection of Tissue Growth and Developmental Patterning
- MEIOB Targets Single-Strand DNA and Is Necessary for Meiotic Recombination
- Transmission of Hypervirulence Traits via Sexual Reproduction within and between Lineages of the Human Fungal Pathogen
- Integration of the Unfolded Protein and Oxidative Stress Responses through SKN-1/Nrf
- Guanine Holes Are Prominent Targets for Mutation in Cancer and Inherited Disease
- Regulation of the Boundaries of Accessible Chromatin
- Natural Genetic Transformation Generates a Population of Merodiploids in
- Ablating Adult Neurogenesis in the Rat Has No Effect on Spatial Processing: Evidence from a Novel Pharmacogenetic Model
- Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on Phenotype
- The Molecular Mechanism of a -Regulatory Adaptation in Yeast
- Phenotypic and Genetic Consequences of Protein Damage
- Recent Acquisition of by Baka Pygmies
- Fatty Acid Taste Signals through the PLC Pathway in Sugar-Sensing Neurons
- A Critical Role for PDGFRα Signaling in Medial Nasal Process Development
- Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding Sites
- Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
- dTULP, the Homolog of Tubby, Regulates Transient Receptor Potential Channel Localization in Cilia
- Widespread Dysregulation of Peptide Hormone Release in Mice Lacking Adaptor Protein AP-3
- , a Direct Transcriptional Target, Modulates T-Box Factor Activity in Orofacial Clefting
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Recent Acquisition of by Baka Pygmies
- The Condition-Dependent Transcriptional Landscape of
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



