-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaNebula/DSCR1 Upregulation Delays Neurodegeneration and Protects against APP-Induced Axonal Transport Defects by Restoring Calcineurin and GSK-3β Signaling
Post-mortem brains from Down syndrome (DS) and Alzheimer's disease (AD) patients show an upregulation of the Down syndrome critical region 1 protein (DSCR1), but its contribution to AD is not known. To gain insights into the role of DSCR1 in AD, we explored the functional interaction between DSCR1 and the amyloid precursor protein (APP), which is known to cause AD when duplicated or upregulated in DS. We find that the Drosophila homolog of DSCR1, Nebula, delays neurodegeneration and ameliorates axonal transport defects caused by APP overexpression. Live-imaging reveals that Nebula facilitates the transport of synaptic proteins and mitochondria affected by APP upregulation. Furthermore, we show that Nebula upregulation protects against axonal transport defects by restoring calcineurin and GSK-3β signaling altered by APP overexpression, thereby preserving cargo-motor interactions. As impaired transport of essential organelles caused by APP perturbation is thought to be an underlying cause of synaptic failure and neurodegeneration in AD, our findings imply that correcting calcineurin and GSK-3β signaling can prevent APP-induced pathologies. Our data further suggest that upregulation of Nebula/DSCR1 is neuroprotective in the presence of APP upregulation and provides evidence for calcineurin inhibition as a novel target for therapeutic intervention in preventing axonal transport impairments associated with AD.
Published in the journal: . PLoS Genet 9(9): e32767. doi:10.1371/journal.pgen.1003792
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003792Summary
Post-mortem brains from Down syndrome (DS) and Alzheimer's disease (AD) patients show an upregulation of the Down syndrome critical region 1 protein (DSCR1), but its contribution to AD is not known. To gain insights into the role of DSCR1 in AD, we explored the functional interaction between DSCR1 and the amyloid precursor protein (APP), which is known to cause AD when duplicated or upregulated in DS. We find that the Drosophila homolog of DSCR1, Nebula, delays neurodegeneration and ameliorates axonal transport defects caused by APP overexpression. Live-imaging reveals that Nebula facilitates the transport of synaptic proteins and mitochondria affected by APP upregulation. Furthermore, we show that Nebula upregulation protects against axonal transport defects by restoring calcineurin and GSK-3β signaling altered by APP overexpression, thereby preserving cargo-motor interactions. As impaired transport of essential organelles caused by APP perturbation is thought to be an underlying cause of synaptic failure and neurodegeneration in AD, our findings imply that correcting calcineurin and GSK-3β signaling can prevent APP-induced pathologies. Our data further suggest that upregulation of Nebula/DSCR1 is neuroprotective in the presence of APP upregulation and provides evidence for calcineurin inhibition as a novel target for therapeutic intervention in preventing axonal transport impairments associated with AD.
Introduction
Virtually all Down syndrome (DS) adults develop progressive neurodegeneration as seen in Alzheimer's disease (AD), and overexpression of the amyloid precursor protein (APP), a gene located on chromosome 21, is thought to contribute to AD in DS [1]–[3]. Consistently, duplication of a normal copy of APP is sufficient to cause familial AD [4], [5], confirming that it is a key gene in AD neuropathologies seen in DS. This well-known connection between AD and DS provides a unique opportunity to identify the genetic and molecular pathways contributing to AD. In addition to APP, another gene likely to play a crucial role in both AD and DS is the Down syndrome critical region 1 gene (DSCR1, also known as RCAN1). Intriguingly, post-mortem brains from AD patients show increased DSCR1 both at mRNA and protein levels [6]–[8]. Studies have also shown that oxidative stress and Aβ42 exposure can induce DSCR1 expression [8], [9]. DSCR1 is located on human chromosome 21 and encodes a highly conserved calcineurin inhibitor family called calcipressin [10]–[15]. DSCR1 has been implicated paradoxically in both promoting cell survival in response to oxidative stress and in inducing apoptosis [8], [9], [16], [17]. The role of DSCR1 in AD thus remains unclear and an important question is whether DSCR1 contributes to AD or plays a role in combating the toxic effects of APP overexpression.
To elucidate the role of DSCR1 in modulating APP-induced phenotypes, we used Drosophila as a model system, which has been used successfully to investigate various human neurodegenerative diseases including AD, Parkinson's, and polyglutamine-repeat diseases [18]–[27]. Overexpression of APP in both fly and mouse models have previously been shown to cause age-dependent neurodegeneration and axonal transport defects [28]–[31]. Furthermore, impaired transport of essential organelles and synaptic vesicles caused by APP perturbation is thought to be an underlying cause of synaptic failure and neurodegeneration in AD [32]–[34]. However, mechanisms for how APP induces transport defects remain unclear. Here, we show that Nebula, the fly homolog of DSCR1, delays neurodegeneration and reduces axonal transport defects caused by APP overexpression. We report that Nebula enhances anterograde and retrograde axonal trafficking as well as the delivery of synaptic proteins to the synaptic terminal. We find that APP upregulation elevates calcineurin activity and GSK-3β signaling, but Nebula co-upregulation corrects altered signaling to restore axonal transport. Together, our results indicate that Nebula/DSCR1 upregulation is neuroprotective in the presence of APP overexpression and further suggest that Nebula/DSCR1 upregulation may delay AD progression. In addition, our results for the first time link defective calcineurin signaling to altered axonal transport and imply that restoring calcineurin and GSK-3β signaling may be a feasible strategy for treating AD phenotypes caused by APP upregulation.
Results
Upregulation of Nebula Delays APP-Induced Neurodegeneration
To examine the role of DSCR1 in modulating APP-induced neurodegeneration and axonal transport defects, we generated transgenic flies containing UAS-APP (APP) in the presence or absence of UAS-nebula (nlat1) [15]. Targeted expression of human APP in the fly eyes using the Gmr-GAL4 driver caused age-dependent degeneration of the photoreceptor neurons, consistent with a previous report by Greeve et al [35]. As seen in Fig. 1A, staining with an antibody specific for the photoreceptor neurons (24B10) and antibody against the APP protein (6E10) revealed the presence of vacuoles in the retina (arrow). Surprisingly, overexpression of nebula together with APP (APP;nlat1) reduced neurodegeneration (as determined by calculating the fold change in the percentage of area lost), suggesting that Nebula upregulation is neuroprotective (Figs. 1A and 1B). By 45-days of age, flies expressing both nebula and APP started to show increased vacuole formation, but the extent of degeneration was significantly reduced compared to that of APP overexpression, further implying that Nebula delays the onset of neurodegeneration rather than completely preventing it.
Fig. 1. Nebula overexpression reduces APP-induced degeneration structurally and functionally. 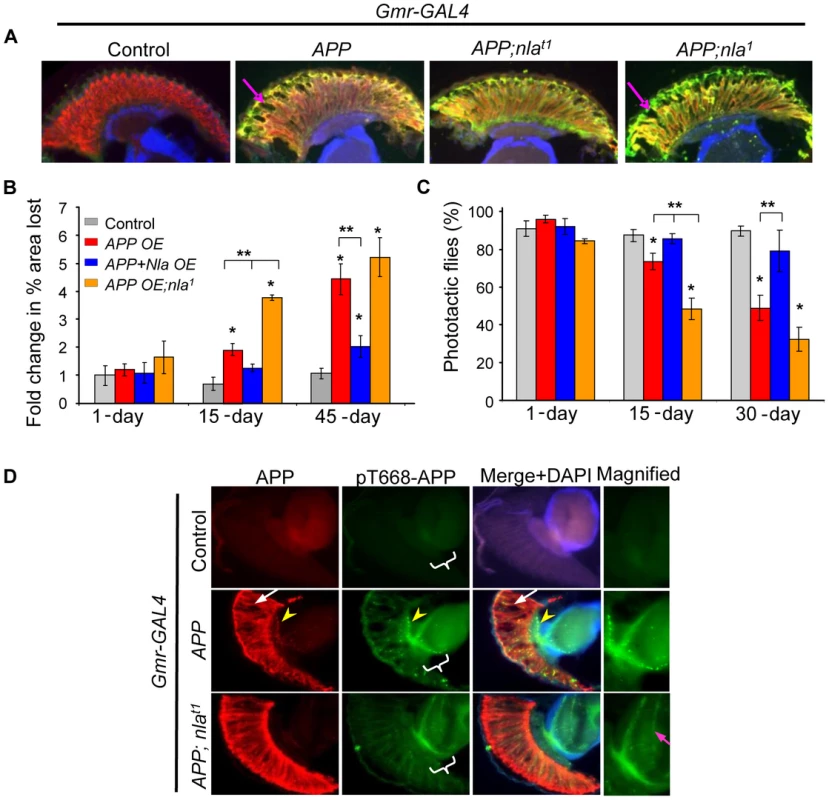
(A) Cryostat section of 15-day old flies. Neurodegeneration is seen as holes in the fly retina (arrow). Photoreceptor neurons were detected with mAb24B10 (red) and N-APP antibody (green). To normalize the number of transgenes found in different fly lines, control, APP overexpression (OE), or APP;nla1 flies also carry one copy of UAS-LacZ gene driven by Gmr-GAL4. (B) Fold change in % area lost. n>4 heads per genotype and age. (C) Percentage of flies that moved toward light. n = 3–4 separate tests, total >100 flies per genotype. All values are mean ± S.E.M, * p≤0.05 compared to control, ** P<0.05 compared to the indicated genotypes. (D) Sections of 45 day old fly heads stained with mAb6E10 (APP) and with antibody specific for pT668-APP. White arrow highlights vacuoles and yellow arrowhead points to aggregates. The medulla in which R7-8 terminate is magnified on the right. More pT668-APP is seen in the axon terminals of the photoreceptor neurons (highlighted by magenta arrow). To confirm that Nebula indeed protects against neurodegeneration caused by APP upregulation, we expressed APP in nla1, a previously characterized nla hypomorphic mutant [15]. Note that because nebula null alleles are lethal [15], nebula hypomorphs were examined. Fig. 1 shows that decreasing Nebula level enhanced APP-induced neurodegeneration in the retina (APP;nla1), thus highlighting the importance of endogenous Nebula protein in conferring neuroprotection. We did not detect significant neurodegeneration in nla1 mutant and nla overexpression flies even by 45 days of age (data not shown), indicating that APP is necessary for the observed phenotype. In addition, mitigation of photoreceptor degeneration by Nebula upregulation is not due to altered expression level of APP, since UAS-LacZ transgene was included to balance out the number of transgenes (we found Gmr-GAL4 is particularly sensitive to number of transgenes). The level of APP protein in each fly line is also further confirmed by staining with the 6E10 antibody (Fig. 1A) and Western blot analyses (Fig. S1). Comparable level of APP was detected in all transgenic lines, suggesting that rescue by nebula overexpression is not due to altered APP level.
We next determined if Nebula rescues functional defects in photoreceptor by measuring the ability of flies to see light. Flies are normally phototactic and will move toward light when placed in test tubes with light source on the opposite end [36]. We find that the severity of the vacuole phenotype was paralleled by impairments in phototactic behavior (Fig. 1C). Flies overexpressing APP showed age-dependent decline in phototaxis that is delayed by APP and nebula expression (Fig. 1C). Taken together, these results imply that nebula overexpression protects neurons structurally as well as functionally against the toxic effects of APP overexpression.
Nebula Upregulation Ameliorates APP-Induced Aggregate Formation in Axons
We also noticed that APP overexpression caused formation of APP aggregates in the photoreceptor axons as detected by 6E10 antibody (Fig. 1D; yellow arrow head). Previous studies have shown that APP phosphorylated on threonine 668 (pT668-APP) is preferentially transported in axons [37], we thus further monitored the distribution of pT668-APP. We found that overexpression of APP led to pT668-APP accumulations in the photoreceptor axons, whereas APP and nebula co-overexpression significantly enhanced the delivery of pT668-APP to synaptic terminals in the medulla (Fig. 1D). These results suggest that APP overexpression may lead to blocked transport that is alleviated by Nebula.
Axonal transport abnormalities are thought to precede the onset of AD [30], and APP overexpression has been shown to cause synaptic vesicle accumulations indicative of blocked axonal transport [28], [29]. We thus further investigated the role of Nebula in modulating APP-induced vesicle aggregation in larval motor axons, which is an excellent system for monitoring vesicle transport because of the long axons and stereotypical innervation of the neuromuscular junction (NMJ). As seen in Fig. 2A, APP overexpression in neurons using the Elav-GAL4 driver caused synaptic vesicle accumulation as detected by synaptotagmin staining in the motor axons, suggesting abnormal vesicle transport. Staining using the 4G8 antibody to detect APP revealed that APP aggregates frequently colocalized with synaptotagmin aggregates, implying that synaptotagmin and APP are either comparably inhibited by physical blockade within the nerve or that they are transported together as suggested by recent reports [38], [39]. Co-upregulation of Nebula and APP significantly prevented APP-induced synaptotagmin and APP accumulations. Decreasing Nebula by crossing it into nla1 background increased the number of synaptotagmin and APP aggregates slightly, although not significantly (Fig. 2B). As nla1 only reduces Nebula level by about 30% and that nla null alleles are lethal [15], we used RNAi strategy to further decrease Nebula level (Fig. S2). Figs. 2A–2B show that greater reduction in Nebula level using the UAS-nla-RNAi transgene (RNAi-nla) further exacerbated the APP-induced aggregation phenotype. To ensure that the observed rescue in phenotype is not due to altered APP overexpression, we monitored the level of neuronal APP protein, as well as Nebula, in different fly lines. As seen in Fig. S3, APP level was unaltered in flies containing different number of transgenes, and Nebula manipulations in APP overexpression background showed the expected changes. Similar results were obtained when performing western blot analyses using brains dissected from 3rd instar larvae (Fig. S4). Together, these results confirm that rescue of APP phenotype by Nebula is not due to altered APP expression. In addition, we examined the effect of altering Nebula levels alone on vesicle accumulation. Manipulations of Nebula levels alone did not cause synaptotagmin aggregate accumulation in nerves, suggesting the observed phenotype is APP-dependent (Figs. S5A and S5B).
Fig. 2. Nebula upregulation rescues APP-dependent aggregate accumulations in axons. 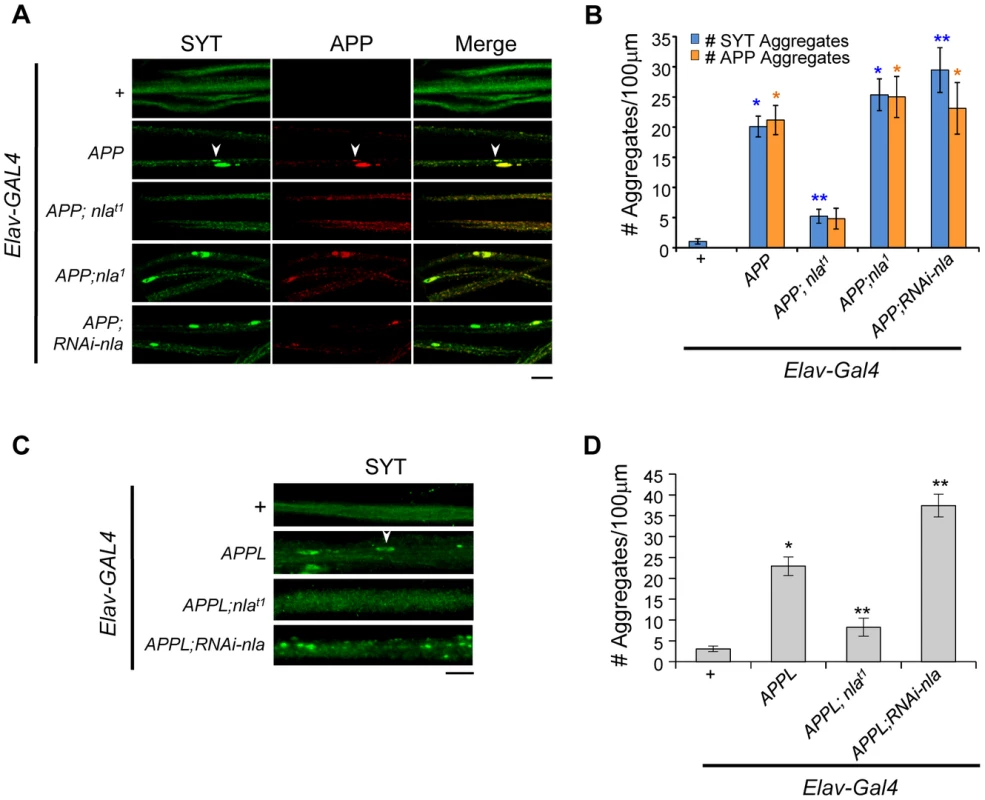
(A) Images showing 3rd instar segmental nerves stained with the indicated antibodies. Arrowhead points to an example of aggregate found in axon. APP and synaptotagmin aggregates frequently colocalize. (B) The number of synaptotagmin (SYT) and APP aggregates in axons. n≥10 experiments. * p≤0.05 compared to control, ** P≤0.05 compared to control and APP overexpression. (C) Synaptotagmin aggregates in the segmental nerves of 3rd instar larvae with APPL upregulation. (D) Quantification of the number of SYT aggregates. n≥4 independent experiments. All values represent mean ± S.E.M, * p≤0.05 compared to control, ** P≤0.05 compared to control and APPL overexpression. Scale bars = 10 µm. To verify that the synaptotagmin aggregate accumulation phenotype is not due to a non-specific effect of expressing human APP, we also monitored the effect of Nebula on modulating endogenous fly Appl gene function. Fig. 2C shows that upregulation of APPL in neurons also caused synaptotagmin accumulation in axons. Nebula co-upregulation significantly reduced the number of synaptotagmin aggregates, whereas Nebula reduction using RNAi significantly exacerbated the phenotype (Figs. 2C and 2D). Together, our results support earlier finding that mammalian APP and Drosophila APPL are functionally conserved [40], and further indicate that APP and APPL-induced axonal transport defects are regulated by Nebula in a similar fashion.
To determine to what degree aggregate accumulation corresponded to altered delivery of synaptic proteins to the synaptic terminal, we evaluated the levels of both synaptotagmin and APP in the NMJ. As demonstrated in Figs. 3A–3B, APP upregulation significantly reduced the level of average synaptotagmin intensity in the synapse while nebula co-overexpression enhanced the delivery of both synaptotagmin and APP to the synaptic terminal. This change is not due to altered overall synaptotagmin or APP levels (Figs. S4 and S5C). Note that the 4G8 antibody does not detect endogenous fly APPL; therefore, we normalized the level of APP delivered to the synapse to flies overexpressing APP and nebula. We found Nebula reduction did not further reduce the amount of synaptotagmin reaching the terminal (Fig. 3B), albeit it did increase the number of APP-induced aggregates in the axon (Fig. 2B). This result indicates that either retrograde transport of synaptotagmin is altered, or the increase in aggregate number has not yet reached a critical threshold for further impairment. In addition, although no detectable synaptotagmin aggregate was seen in flies with Nebula reduction alone, a decrease in synaptotagmin staining was detected in the synapse (Figs. S5B and S5D). This result suggests that Nebula itself may be required for reliable axonal transport.
Fig. 3. Nebula overexpression restores synaptotagmin level and enhances APP delivery to the synaptic terminals. 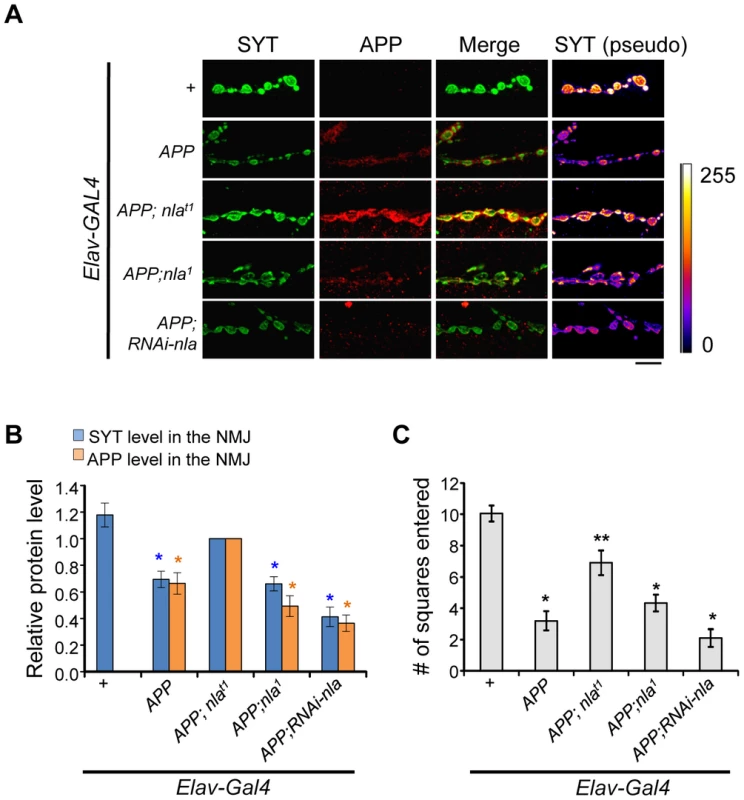
(A) Representative images of NMJ staining for the indicated genotypes. Scale bars = 10 µm. (B) Levels of SYT and APP in NMJ normalized to flies overexpressing APP;nlat1. n≥5 independent experiments. (C) Larval locomotor activity assay. n = 10 experiments. All values represent mean ± S.E.M, * p≤0.05 compared to control, ** P<0.05 compared to control and APP overexpression. We also examined the effect of abnormal aggregate accumulations and reduced delivery of synaptic proteins on locomotor behavior. Overexpression of APP dramatically impaired larval movement (Fig. 3C and Movie S1). Nebula co-overexpression significantly rescued this locomotor defect, in further support of the hypothesis that Nebula upregulation exerts beneficial effects on synaptic functions by alleviating abnormal aggregate accumulations. Note that further reduction of Nebula in APP overexpression background did not significantly worsen the locomotor defect of APP overexpressing larvae, perhaps due to a threshold effect. Reducing Nebula alone was sufficient to induce a mild defect in locomotor activity (Fig. S5E), suggesting delivery of synaptic proteins to the synaptic terminals is crucial for normal synaptic function.
Similar to APP overexpression, upregulation of APPL decreased the delivery of synaptotagmin to the synapse. APPL and Nebula co-upregulation showed a higher level of synaptotagmin in the NMJ, confirming Nebula interacts genetically with APPL to rescue impaired in transport (Fig. S6A and S6B). We also found that similar to RNAi-nla larvae, Appl null mutant (Appld) displayed a slight decrease in the level of synaptotagmin at the synapse independent of aggregate accumulation (Figs. S6B and S6C). Reducing Nebula in neurons of Appld larvae with the RNAi-nla transgene driven by the pan-neuronal nSyb-GAL4 driver (Appld; RANi-nla/nSyb-GAL4) did not further enhance the phenotype, suggesting that the two proteins act in the same pathway to modulate axonal transport.
While monitoring synaptotagmin levels at the NMJ, we also noticed that APP overexpression triggered changes in synaptic morphology as previously reported [41], [42]. Fig. 4 shows presynaptic terminals stained with HRP to outline the presynaptic terminals, which revealed an increase in the total number of boutons and satellite boutons brought upon by APP overexpression. Nebula co-upregulation also rescued APP-induced synapse proliferation phenotype, but not the number of satellite boutons (Fig. 4B and 4D). Manipulating levels of Nebula alone without APP did not influence bouton number or morphology, suggesting that the satellite bouton phenotype is dependent on the presence APP in the synapse. Since reducing Nebula levels alone decreased the delivery of synaptotagmin to the synaptic terminal without altering synaptic morphology, axonal transport problems are not secondary consequences of altered synaptic morphology. A plausible mechanism by which Nebula suppresses the APP-induced over-proliferation phenotype is that Nebula co-upregulation restores the delivery of proteins required for normal synaptic growth such as Fasciclin II (FasII), a cell adhesion molecule shown to influence synaptic morphology [43], [44]. Previous reports suggest that changes in FasII levels differentially affect synaptic growth [42]–[44], and that increasing FasII levels presynaptically can significantly suppress the increase in bouton number observed in APPL overexpression synapses [42]. We therefore quantified FasII levels in the NMJ (Fig. S7). We found that overexpression of APP reduced the level of FasII in the NMJ, whereas APP and nebula co-overexpression restored it (Fig. S7). While APP upregulation may play other roles in synapse formation, these results together with previous reports imply that depletion of FasII in the presynaptic terminal could partially contribute to the hyper-growth phenotype. Furthermore, our data reveal that Nebula upregulation is effective in protecting against multiple phenotypes caused by APP overexpression, including age dependent photoreceptor neurodegeneration, vesicle accumulations in axons, and changes in synaptic morphology.
Fig. 4. Nebula co-upregulation rescues synaptic bouton over-proliferation caused by APP. 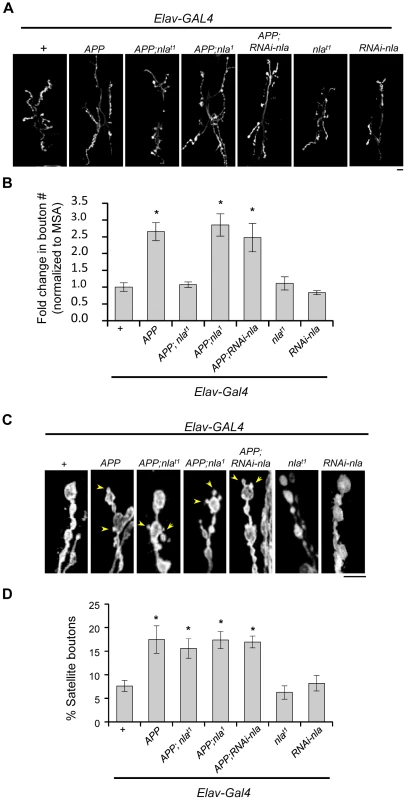
(A) Images representing the neuromuscular junction of 3rd instar larvae at segment A2 of muscle segment 6/7. HRP staining outlining synaptic boutons. Scale bar = 10 µm. (B) Quantification of the number of boutons normalized to the muscle surface area (MSA). (C) Magnified images demonstrating the presence of satellite bouton phenotype (arrowheads) in genotypes with APP upregulation. Scale bar = 5 µm. (D) Quantification of the percentage of boutons that are satellite. n≥6 experiments in (B) and (D). All values represent mean ± S.E.M, * p≤0.05 compared to control. Nebula Enhances Anterograde Transport of Amyloid Precursor Protein
To directly evaluate the effect of Nebula on APP transport and to determine whether the observed axonal aggregates correspond to defective axonal transport, we performed live-imaging of human APP tagged with yellow fluorescent protein (APP-YFP). APP-YFP vesicles in larval motor axons displayed movement in both the anterograde and retrograde directions over the 2-minute imaging period as represented by kymographs depicting distance traveled and time in the x - and y-directions, respectively (Fig. 5A). Nebula co-overexpression had a mild, but significant, effect on APP-YFP movement. Nebula co-upregulation increased the percentage of anterograde moving vesicles and resulted in reduced number of stationary APP-YFP; knockdown of Nebula using RNAi increased the number of stationary APP-YFP (Figs. 5A and 5B). Quantification of the average speed of APP-YFP movement revealed that overexpression of nebula also increased the speed of APP-YFP movement in both the anterograde and retrograde directions (Fig. 5C). Together, these results suggest that Nebula upregulation enhances the transport of APP, consistent with the decreased aggregate accumulations of APP in axons and increased APP staining in the NMJ when Nebula is co-expressed (Figs. 2A and 3A).
Fig. 5. Nebula upregulation enhances anterograde transport of APP-YFP in larval motor axons. 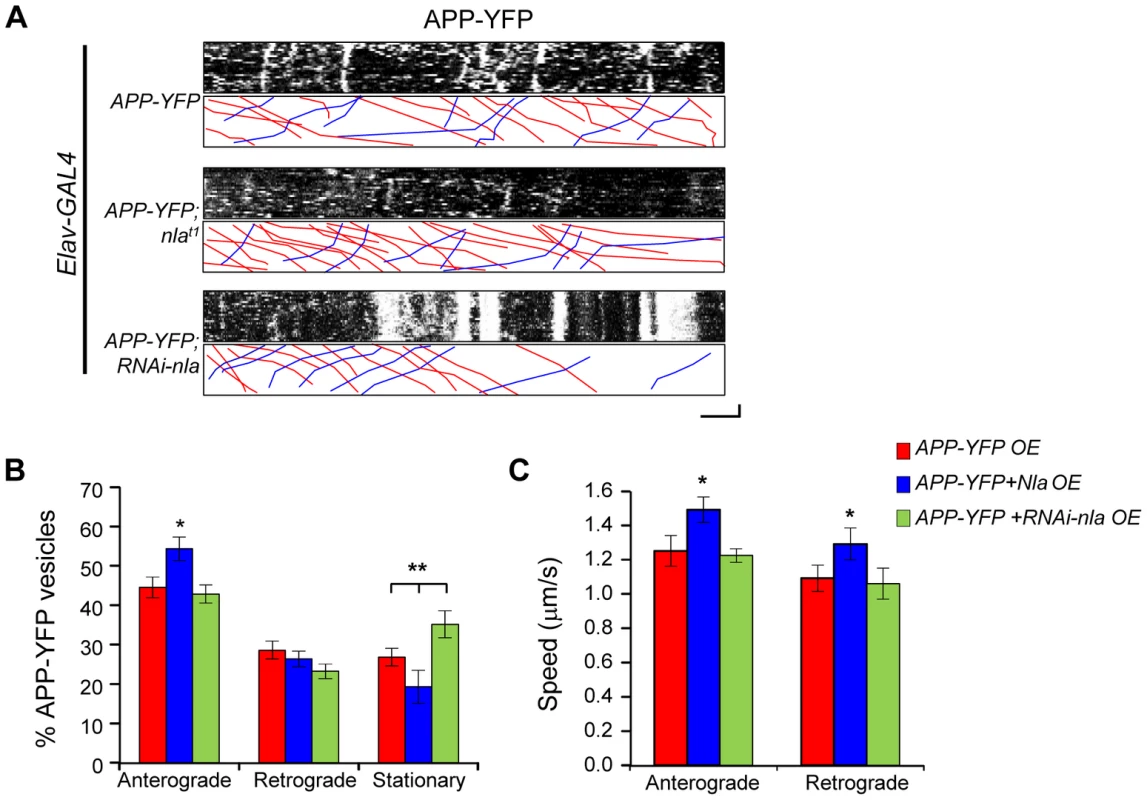
(A) Representative kymographs depicting trafficking of APP-YFP vesicles. Stationary vesicles are seen as vertical lines and anterograde movement is depicted as diagonal lines moving from left to right. Scale bars: 5 µm (X) and 30 seconds (Y). Red lines in the lower boxed region highlight anterograde moving vesicles. Blue lines depict retrograde moving vesicles. (B) Quantification of the percentage of anterograde and retrograde moving vesicles, as well as stationary vesicles in the indicated genotypes. nebula overexpression significantly increased the relative number of anterograde moving vesicles. (C) Speed of APP-YFP vesicles in the anterograde and retrograde directions. Nebula upregulation enhanced the speed in the anterograde and retrograde directions. All values represent mean ± S.E.M, * p≤0.05 compared to control, ** P<0.05 compared to the indicated genotypes. n≥6 independent experiments per genotype. Nebula Restores Anterograde and Retrograde Trafficking of Synaptic Vesicles and Mitochondria
To further confirm that Nebula facilitates synaptic vesicle movement in the presence of APP and to better assess the role of endogenous Nebula in regulating transport, we also monitored synaptotagmin movement in the motor axons of larvae expressing GFP-tagged synaptotagmin (GFP-SYT). We find the movement of GFP-SYT to be highly dynamic with anterograde, retrograde, and bi-directional movement (Fig. 6A). Overexpression of APP dramatically reduced the percentage of vesicles moving in both the anterograde and retrograde directions while nebula co-overexpression significantly facilitated synaptotagmin transport in both directions (Figs. 6A and 6B), albeit retrograde transport was more effectively restored by Nebula. Reducing Nebula using RNAi further diminished APP-induced synaptotagmin transport in both directions, confirming interaction between Nebula and APP. Reduction in the overall movement was also accompanied by a decrease in anterograde and retrograde velocity (Fig. 6C). Together, these results suggest that APP overexpression slows down the overall movement of vesicles, which may lead to accumulation of transported proteins. Nebula co-overexpression with APP partially restores the defect by increasing the movement and speed of transport in both the anterograde and retrograde directions.
Fig. 6. APP upregulation causes defective transport of synaptotagmin that is restored by Nebula co-upregulation. 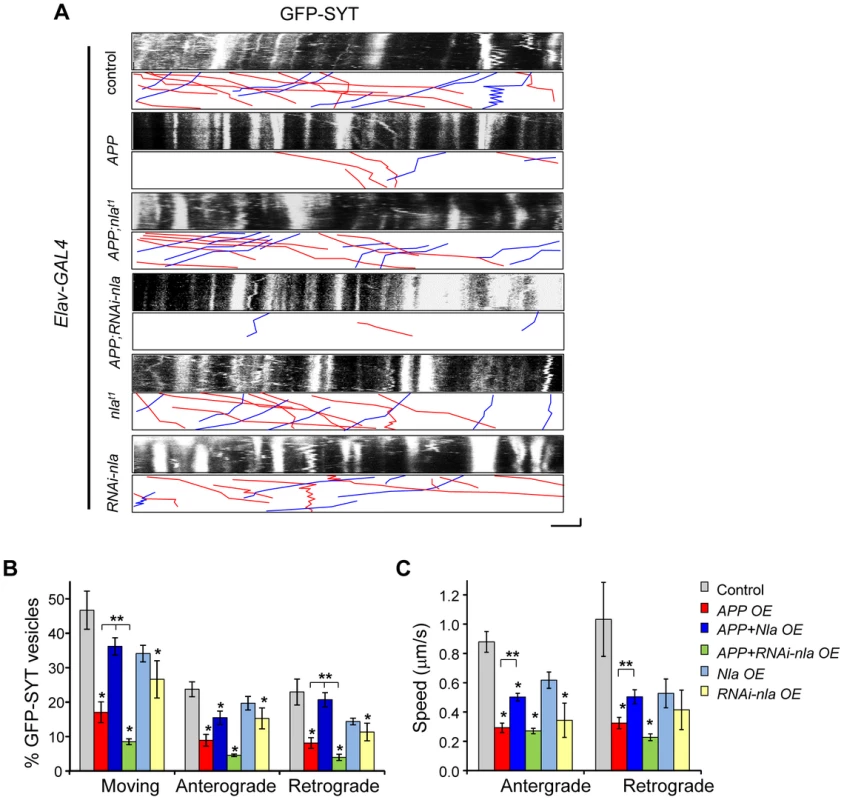
(A) Kymographs depicting movement of GFP-SYT. Scale bars: 5 µm (X) and 60 s (Y). Red lines: anterograde movement; blue lines: retrograde movement. (B) Quantification of the total number of moving vesicles, as well as relative movement in the anterograde and retrograde directions normalized to the total number of observed vesicles. APP overexpression (OE) caused significant defects in the movement of GFP-SYT. (C) Speed of GFP-SYT movement in the anterograde and retrograde directions. Values represent mean ± S.E.M, * p≤0.05 compared to control, ** P<0.05 compared to the indicated genotypes. n≥6 independent experiments per genotype. To understand the role of endogenous Nebula in axonal transport, we examined the effect of Nebula manipulations on GFP-SYT movement in the absence of APP overexpression. We find that Nebula upregulation alone did not significantly influence transport; decreasing Nebula through RNAi was sufficient to reduce the number of moving synaptotagmin vesicle in both directions, as well as the speed of anterograde transport (Fig. 6). This result is consistent with the decrease in synaptotagmin staining in the NMJ seen in static images, and further confirms that Nebula is required for efficient transport of synaptic proteins.
To further determine if general axonal transport is affected by APP and Nebula upregulation, we also monitored mitochondrial transport. Proper distribution of mitochondria is vital for normal cell functions and defects in mitochondrial transport can adversely affect cell survival [45]–[47]. Time-lapse live imaging was performed in larvae with GFP targeted to mitochondria (mito-GFP) for the indicated genotypes (Fig. 7). APP upregulation severely impaired the movement of mitochondria in both the anterograde and retrograde directions both in terms of percent in motion and the speed of movement (Figs. 7B and 7C). Nevertheless, the APP-induced mitochondrial transport defect was partially restored by Nebula co-upregulation (Fig. 7 and Movie S2), similar to what was observed for synaptic vesicle transport. Manipulations in the level of Nebula did not significantly alter the overall mitochondrial movement, except that nebula overexpression alone seemed to enhance both the proportion and the speed of mitochondria transported in the retrograde direction. This result is consistent with our observation that Nebula co-upregulation was more effective in restoring retrograde GFP-SYT transport. Together, our results suggest that Nebula influences general axonal transport that extends beyond synaptic proteins.
Fig. 7. Nebula alters APP-induced mitochondrial transport defects. 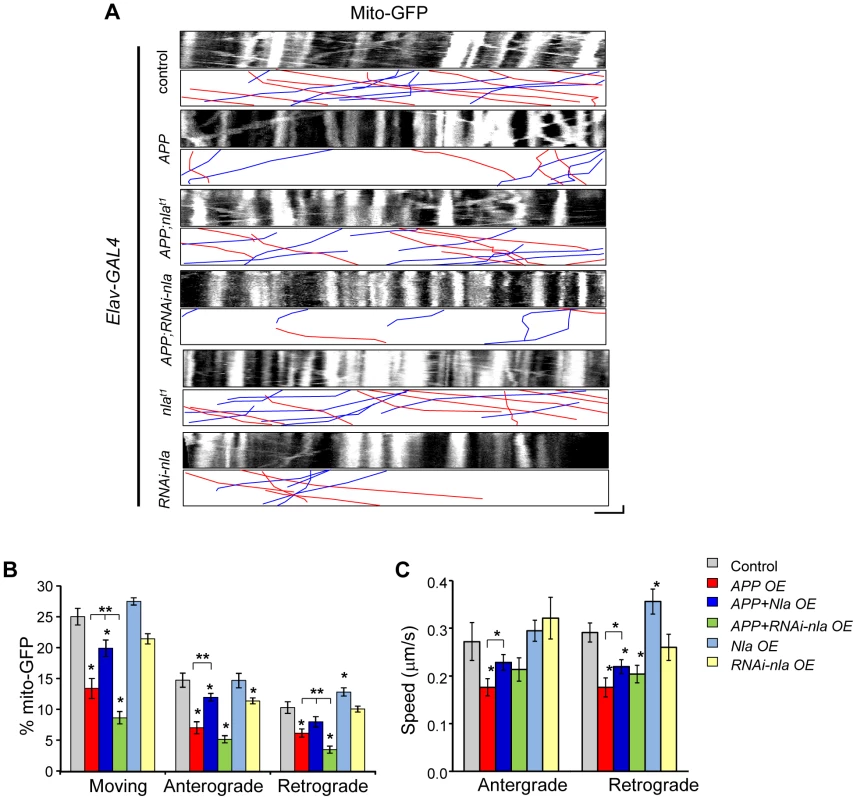
(A) Kymographs showing mito-GFP movement in motor axons. Scale bars: 5 µm (X) and 60 s (Y). Red lines: anterograde movement; blue lines: retrograde movement. (B) Quantification of the percent of mobile mitochondria, and mitochondria moving in the anterograde and retrograde directions. (C) Speed of mitochondrial movement. All values represent mean ± S.E.M, * p≤0.05 compared to control, ** P<0.05 compared to the indicated genotypes. n≥6 independent experiments per genotype. Mitochondria are dynamic organelles whose distribution is tightly regulated to meet the energy demands within the polarized neuron [45], [48]. We find that despite the decrease in mitochondrial movement in flies overexpressing APP, the distribution and density of mitochondria within the proximal axon where imaging was performed did not vary across genotypes (Fig. S8A). These results imply that impaired synaptic vesicle transport is not likely caused by local depletion of mitochondria within the axon. Furthermore, mitochondria did not accumulate near the site of synaptotagmin aggregate formation in the axons (Fig. S8B), suggesting that mitochondria are either able to move past the stalled synaptic vesicle accumulations or that mitochondria travel on other non-blocked microtubule tracks.
APP Overexpression Does Not Alter Microtubule Integrity
Despite increasing evidence linking defective trafficking of presynaptic proteins, mitochondria, and signaling molecules to neuropathologies of AD, mechanisms for how APP overexpression affects axonal transport remain unclear. We first tested the possibility that APP upregulation impairs axonal transport by influencing overall microtubule integrity. To this end, we stained the axonal nerves and NMJs with antibodies against acetylated tubulin, β-tubulin, and Futsch (Fig. 8). Acetylated tubulin is a marker for stable microtubules [49]; Futsch is a microtubule binding protein homolog to human MAP1B and is involved in maintaining microtubule integrity at presynaptic terminals during NMJ growth [50]. Our data revealed that APP overexpression did not cause fragmentation of microtubules as revealed by both acetylated tubulin and β-tubulin staining in the axons (Fig. 8A), and filamentous acetylated tubulin staining in the synaptic terminals across all genotypes (Fig. 8B). Note that in Fig. 8B, we also highlighted the presynaptic boutons by HRP staining (red), since acetylated tubulin in the muscles are also detected in the background. Western blot analyses of dissected larval brains further confirmed that the overall level of acetylated tubulin is not altered by APP overexpression (Fig. 8C). Closer examination of Futsch staining also did not reveal differences in overall microtubule integrity (Fig. 8D). Together, these results suggest that APP overexpression does not cause axonal transport problems by influencing microtubule stability, which is consistent with a recent report that showed normal microtubule stability and acetylated tubulin level in larvae overexpressing APP-YFP [51].
Fig. 8. APP overexpression does not alter gross microtubule structure in the axons or NMJ. 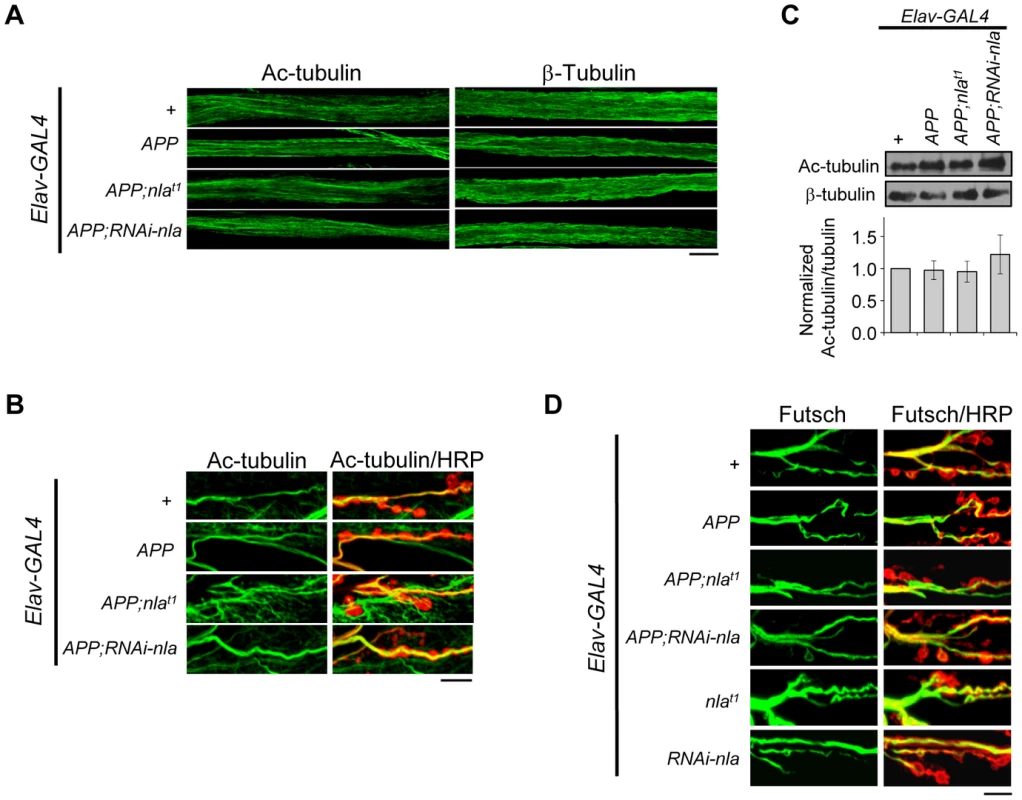
(A) Images of the larval segmental motor axons stained with acetylated tubulin (Ac-tubulin) or β-tubulin as indicated. Microtubule structural integrity was not influenced by APP overexpression. (B) Representative images of the larval neuromuscular junction at segment A2 of muscle 6/7 stained with Ac-tubulin (green) and HRP (red). Background Ac-tubulin signal is also detected in the muscle (outside the boundaries of HRP staining). (C) Western blot depicting Ac-tubulin levels for the indicated genotypes. Values were normalized to β-tubulin, which is used as loading control. Lower panel shows quantification of the relative protein level for the indicated transgene normalized to the control. Values represent mean ± SEM, n = 3 independent experiments. (D) Images of the 3rd instar larval NMJ at segment A2 of muscle 6/7 stained with Futsch (green) and HRP (red). All scale bars = 10 µm. Nebula Mitigates APP-Induced Phenotypes by Regulating Calcineurin and GSK-3β
Nebula encodes an inhibitor of calcineurin that is highly conserved across species [15], we therefore tested the hypothesis that calcineurin inhibition is an underlying mechanism for Nebula-mediated rescue of APP phenotypes. To this end, we genetically altered calcineurin activity in neurons using the UAS/GAL4 strategy. To elevate calcineurin activity, we expressed a constitutively active calcineurin (CaNAct) with its auto-inhibitory domain deleted (Figs. S9A and S9B). To reduce calcineurin activity, RNAi strategy against the calcineurin B gene (RNAi-CaNB), an obligatory subunit necessary for calcineurin activity, was used. We find that similar to Nebula upregulation, decreasing calcineurin using RNAi-CaNB in the presence of APP significantly reduced synaptotagmin aggregate accumulations and synaptic depletion, as well as restored larval locomotor behavior (Figs. S9C–E). Overexpression of CaNAct together with APP further exacerbated the APP-induced phenotypes (Figs. 9A and 9B), whereas co-overexpression of CaNAct and nebula diminished the ability of Nebula to protect against APP-induced transport defects. Similar to larvae with reduced levels of Nebula (RNAi-nla), larvae expressing CaNAct did not show aggregate accumulations in axons but displayed a reduced level of synaptotagmin staining in the synapse (Figs. S9D), indicating active calcineurin overexpression alone only has modest effect on axonal transport. As shown above, synaptotagmin aggregate accumulation in nerves and depletion in the synaptic terminals are reliable indicators of significant transport deficiencies; our results thus indicate that Nebula protects against APP-induced defects through inhibition of calcineurin. Furthermore, our data present for the first time that APP upregulation influences axonal transport through activation of calcineurin. This conclusion is further supported by direct measurement of calcineurin activity, in which we find that APP upregulation significantly elevated calcineurin activity but is further restored close to normal in flies overexpressing APP and nebula, or APP and RNAi-CaNB (Fig. 9C). Overexpression of APP, CaNAct, and nebula together showed an intermediate phenotype in both calcineurin activity and aggregate accumulations, suggesting that the severity of aggregate accumulation correlated with the level of calcineurin when APP is upregulated.
Fig. 9. Nebula modulates APP-dependent phenotypes by restoring calcineurin signaling. 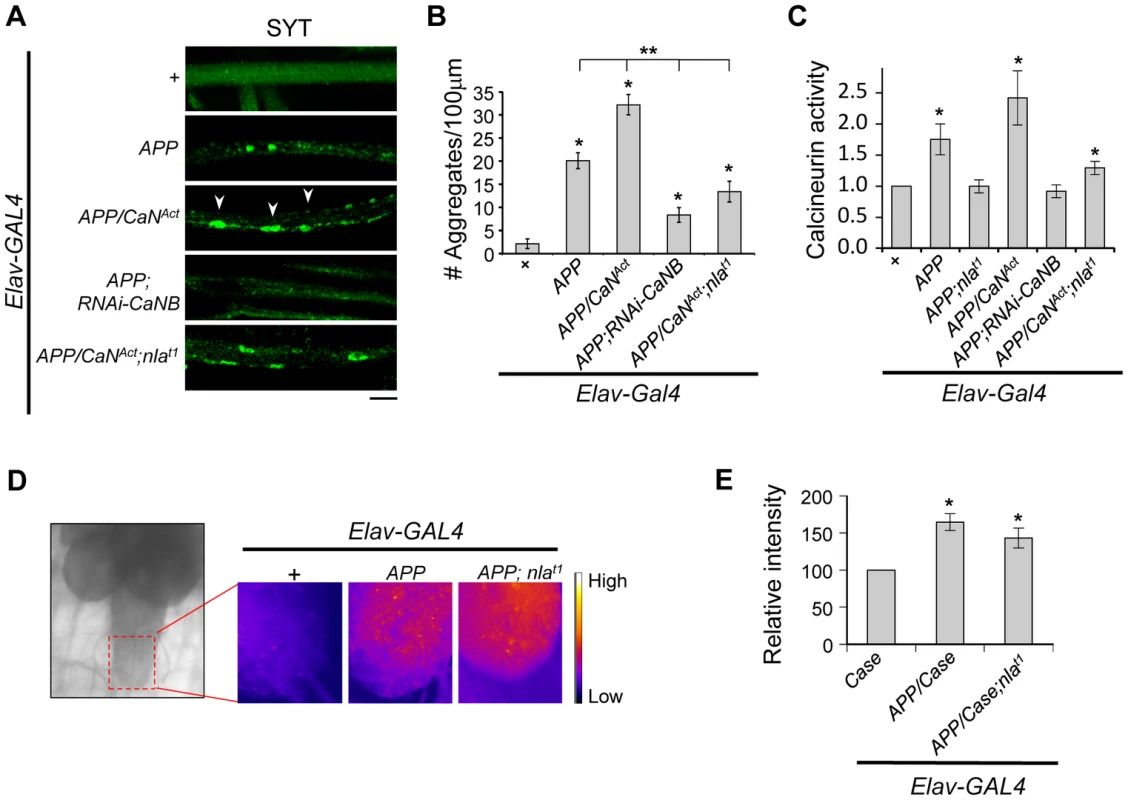
(A) Representative images of 3rd instar larval motor axons stained with synaptotagmin. Arrowheads point to examples of aggregates. Scale bar: 10 µm. (B) Quantification of the number of aggregates found in each genotype. (C) Calcineurin activity assay. n≥10 independent experiments per genotype for all experiments. (D) Image of 3rd instar brain highlighting the imaging area (left). Magnified images (right) depict the intensity of the genetically encoded fluorescent calcium sensor, Case12, across genotypes. (E) Quantification of the relative fluorescent intensity across genotypes. n = 6 experiments. All values represent mean ± S.E.M. * p≤0.05 compared to control and ** P<0.05 compared to the indicated genotypes. How does APP upregulation trigger calcineurin activation? Because calcineurin phosphatase activity is dependent on intracellular calcium concentration [52], we examined the possibility that APP overexpression elevates calcium levels. Using a genetically encoded fluorescent calcium sensor (Case12) previously shown to detect calcium with high sensitivity [53], [54], we compared Case12 signal across different genotypes. Fig. S10 shows that larval brain expressing Case12 displayed a significant increase in signal following application of calcimycin, a calcium ionophore, confirming that the Case12 construct can indeed detect increases in calcium. Overexpression of APP alone or overexpression of APP and nebula also caused a significant elevation in Case12 signal in the larval brain and the ventral ganglion (where the motor neuron cell bodies are located) as compared to the control (Figs. 9D and 9E). These data imply that an APP-mediated increase in calcium is triggering the increase in calcineurin activity. Furthermore, observations that co-overexpression of APP and nebula increased calcium while simultaneously restoring calcineurin activity indicate that Nebula is influencing axonal transport through calcineurin inhibition rather than acting at a step modulating calcium influx.
Mechanisms by which calcineurin regulates axonal transport are not well understood, but one potential pathway is through regulation of GSK-3β activity. Aberrant activation of GSK-3β has been associated with AD and calcineurin has been shown to activate GSK-3β through dephosphorylation of Ser9 of GSK-3β in vitro [55]–[58]. It was suggested that GSK-3β may negatively influence axonal transport by altering microtubule stability through hyperphosphorylation of tau, by inhibiting kinesin motor binding to the cargo through phosphorylation of the kinesin light chain (KLC), or by altering the kinesin motor activity [51], [59]–[61]. These previous findings led us to investigate the possibility that Nebula restores APP-dependent transport problems through calcineurin-mediated regulation of GSK-3β in vivo. The activity of GSK-3β is regulated by phosphorylation and dephosphorylation: dephosphorylation of Ser9 by a number of phosphatases including calcineurin is required to activate GSK-3β [56], [62], and phosphorylation at Tyr216 site is necessary to enhance GSK-3β activity [63], [64]. Interestingly, phosphorylation of GSK-3β at Ser9 can both inhibit GSK-3β activity and override the increase in activity even when phosphorylated at Tyr216 [65]. Because these phosphorylation sites are conserved between fly and human, we took advantage of phospho-specific antibodies to monitor GSK-3β activity. Western blot analyses using an antibody specific for phosphorylated Ser9 (pSer9) of GSK-3β revealed that APP upregulation indeed reduced the level of pSer9-GSK-3β while APP and Nebula co-upregulation partially restored the level to normal (Fig. 10A). This suggests APP upregulation leads to GSK-3β activation that is inhibited by Nebula upregulation.
Fig. 10. APP upregulation triggers changes in GSK-3β signaling downstream of calcineurin and affects synaptotagmin-kinesin interaction. 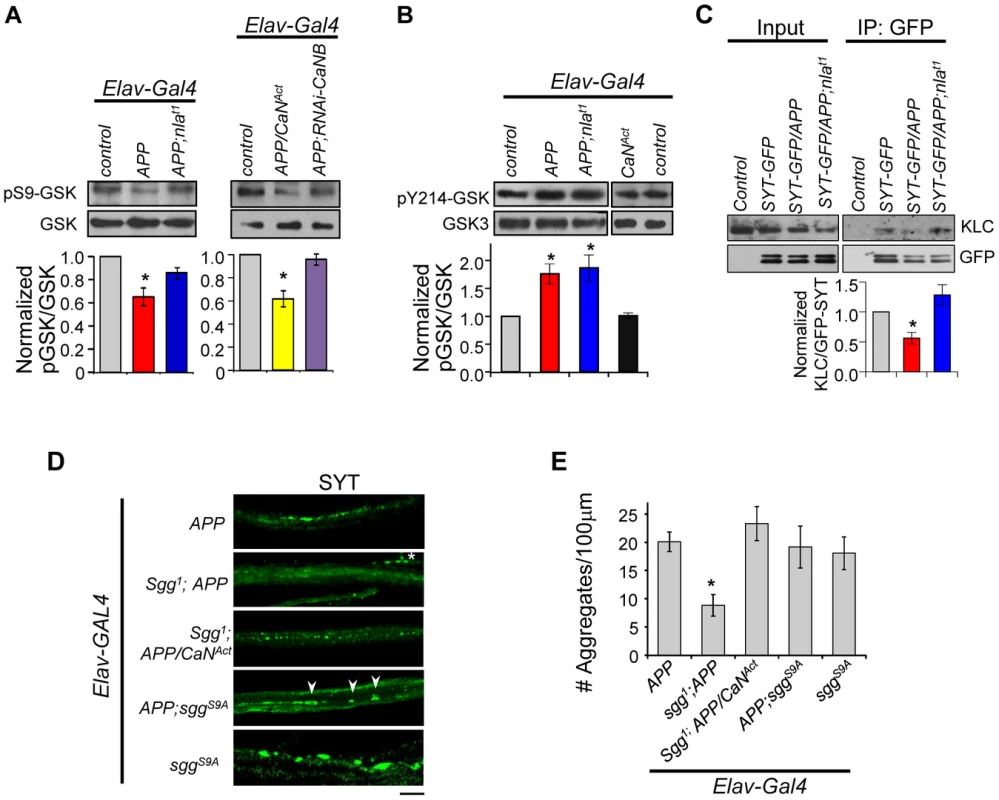
(A) Western blots performed using antibody specific for GSK-3β phosphorylated on Ser9 (pS9-GSK) and total GSK-3β. (B) Western blot using antibody specific for GSK phosphorylated at Tyr214 in Drosophila (pY214-GSK). For (A) and (B) quantification of the ratio of phosphorylated GSK and GSK-3β was normalized to control. n≥3 for each, * p<0.05. (C) Western blots showing reduced interaction between SYT-GFP and kinesin light chain (KLC). Control indicates parallel immunoprecipitation performed using flies that do not express SYT-GFP. n = 3, * p<0.05. (D) Images of 3rd instar larval motor axon stained with synaptotagmin. Arrowheads highlight aggregates and * indicates background staining coming from synaptotagmin in the NMJ. Scale bar: 10 µm. (E) Quantification of the number of aggregates found in each genotype. All values are mean ± S.E.M, * p≤0.05 compared to APP overexpression. n≥4 independent experiments per genotype. To verify that GSK-3β activation is due to calcineurin activation, we reduced calcineurin activity in APP overexpressing flies using RNAi-CaNB (Fig. 10A). We find that APP and RNAi-CaNB co-overexpression in neurons, which was sufficient to restore calcineurin activity, completely prevented GSK-3β dephosphorylation at Ser9 site. This result indicates that APP-induced GSK-3β dephosphorylation at Ser9 is dependent on calcineurin activation in vivo. Note that we did not detect enhanced GSK-3β dephosphorylation when APP is expressed together with constitutively active calcineurin (CaNAct), suggesting that calcineurin may in part directly influence transport through GSK-3β-independent pathways.
Our data strongly implicate activation of calcineurin and subsequent GSK-3β induction to be a mechanism underlying APP-induced aggregate phenotype. Because activation of calcineurin alone did not result in synaptotagmin aggregate accumulation, we further hypothesized that APP upregulation also enhances GSK-3β activity through phosphorylation at Tyr216. Western blot analyses show that the level of phosphorylated GSK-3β at Tyr214 (conserved Tyr216 site in Drosophila) is indeed elevated in flies overexpressing APP or APP and nebula (Fig. 10B). Overexpression of CaNAct alone, however, failed to induce phosphorylation at Tyr214, suggesting that phosphorylation of Tyr214 is not affected by calcineurin and dependent on the presence of APP. Together, our data demonstrate that in addition to activating GSK-3β by relieving inhibition through calcineurin, APP upregulation further enhances GSK-3β activity through phosphorylation at Y214 in fly.
Active GSK-3β had been shown to phosphorylate KLC, leading to detachment of the cargo from the motor [59], [66]. Since synaptotagmin transport was severely inhibited by APP overexpression, and that synaptotagmin transport can depend on kinesin 3 [67], [68] and kinesin 1 (both KLC and kinesin 1 heavy chain) [69]–[73], we tested the possibility that APP overexpression perturbs KLC and synaptotagmin interaction via immunoprecipitation. APP overexpression indeed reduced synaptotagmin (cargo) and KLC interaction while overexpression of APP and nebula preserved this interaction (Fig. 10C). These results suggest that Nebula is likely to restore APP-induced axonal transport defects by correcting GSK-3β signaling and stabilizing cargo-motor interaction.
Complex Interaction between Calcineurin and GSK-3β Signaling Regulates Axonal Transport
Having demonstrated that APP activates calcineurin signaling to regulate GSK-3β phosphorylation, we next examined if reducing GSK-3β can restore axonal transport. In the presence of APP upregulation, decreasing Shaggy (Sgg; fly homolog of GSK-3β) in flies with APP overexpression (sgg1;APP) resulted in significant suppression of the APP aggregate phenotype (Figs. 10D and 10E). This result is consistent with a recent report demonstrating mild enhancement of APP-YFP movement when GSK-3β is reduced [51]. Surprisingly, normal calcineurin activity was detected in these flies (1.00±0.16 fold of control for Sgg1;APP vs. 1.75±0.25 fold of control for APP). This result suggests the existence of feedback regulation of calcineurin activity and further implies that either a change in calcineurin activity or GSK-3β signaling could be responsible for the observed rescue. We therefore generated flies expressing APP and constitutively active calcineurin in sgg1 background (sgg1;APP/CaNAct). Note that we used the hypomorphic allele sgg1 because sgg null animals are lethal [74]. Consistent with GSK-3β being downstream of calcineurin, reducing Sgg diminished the effect of CaNAct in enhancing APP phenotype (Figs. 9B and 10E). We also expressed the constitutively active Sgg (sggS9A) together with APP, which surprisingly showed the same phenotype as APP overexpression. Calcineurin activity assay showed an unexpected decrease in calcineurin activity (0.74±0.06 fold of control) in these flies, suggesting that constitutive GSK-3β activation in the absence of calcineurin activation is sufficient to disrupt axonal transport potentially through phosphorylation of KLC. Interestingly, we find that overexpression of the constitutively active Sgg in neurons alone was sufficient to induce aggregate accumulation similar to flies with APP overexpression (Figs. 10D and 10E). Calcineurin activity assay revealed that these flies showed an increase in overall calcineurin activity (1.65±0.30 fold of control). This increase in calcineurin activity by active Sgg may be due to GSK dependent phosphorylation of Nebula, which has been shown to cause activation of calcineurin [75]. Since over-activation of calcineurin and GSK-3β pathway in the absence of APP upregulation fully replicated the aggregate accumulation phenotype, it suggests that abnormal activation of both the GSK-3β and calcineurin pathways are necessary for the severe axonal transport defect and aggregate accumulation phenotypes.
Discussion
We have demonstrated a novel role for Nebula, the Drosophila ortholog of DSCR1, in ameliorating axonal transport impairments associated with the upregulation of APP. We find that Nebula upregulation significantly delayed photoreceptor neurodegeneration and dramatically decreased the axonal “traffic jam” phenotype caused by APP overexpression. Reducing Nebula independent of APP was sufficient to trigger defects in axonal transport, suggesting that Nebula is normally required for reliable delivery of synaptic cargos, likely through calcineurin dependent pathway. We demonstrate for the first time that APP overexpression causes calcineurin-dependent activation of GSK-3β kinase in vivo, thus implicating altered calcineurin signaling as a novel mechanism regulating axonal transport (Fig. S11). We find that co-upregulation of Nebula preserved the vesicular cargo to molecular motor interaction, ameliorated axonal transport defects, and protected against locomotor deficits. As impaired transport of essential organelles and synaptic vesicles caused by perturbation of APP is thought to precede synaptic failure and neurodegeneration in AD, our findings further suggest that DSCR1 upregulation may be a neuroprotective mechanism used by neurons to combat the effects of APP upregulation and delay progression of AD.
Insights into Mechanisms Underlying APP-Induced Transport Defects
Although upregulation of APP had been shown to negatively influence axonal transport in mouse and fly models 28–31, mechanisms by which APP upregulation induces transport defects are poorly understood. Several hypotheses have been proposed, including titration of motor/adaptor by APP, impairments in mitochondrial bioenergetics, altered microtubule tracks, or aberrant activation of signaling pathways [76]. The motor/adaptor titration theory suggests that excessive APP-cargos titrates the available motors away from other organelles, thus resulting in defective transport of pre-synaptic vesicles [29]. Our finding that Nebula co-upregulation enhanced the movement and delivery of both synaptotagmin and APP to the synaptic terminal argues against this hypothesis. In addition, earlier finding suggest that Nebula upregulation alone impaired mitochondrial function and elevated ROS level [77], thus implying that Nebula is not likely to rescue APP-dependent phenotypes by selectively restoring mitochondrial bioenergetics. Furthermore, consistent with a recent report showing normal microtubule integrity in flies overexpressing either APP-YFP or activated GSK-3β [51], our data revealed normal gross microtubule structure in flies with APP overexpression. Together, these results suggest that changes in gross microtubule structure and stability is not a likely cause of APP-induced transport defects.
Instead, our results support the idea that Nebula facilitates axonal transport defects by correcting APP-mediated changes in phosphatase and kinase signaling pathways. First, we find that APP upregulation elevated intracellular calcium level and calcineurin activity, and that restoring calcineurin activity to normal suppressed the synaptotagmin aggregate accumulation in axons. The observed increase in calcium and calcineurin activity is consistent with reports of calcium dyshomeostasis and elevated calcineurin phosphatase activity found in AD brains [78]–[80], as well as reports demonstrating elevated neuronal calcium level due to APP overexpression and increased calcineurin activation in Tg2576 transgenic mice carrying the APPswe mutant allele [81], [82]. Second, APP upregulation resulted in calcineurin dependent dephosphorylation of GSK-3β at Ser9 site, a process thought to activate GSK-3β kinase [56]. APP upregulation also triggered calcineurin-independent phosphorylation at Tyr216 site, which has been shown to enhance GSK-3β activity [64], [65]. The kinase(s) that phosphorylates APP at Tyr216 is currently not well understood, it will be important to study how APP leads to Tyr216 phosphorylation in the future. Based on our results, we envision that APP overexpression ultimately leads to excessive calcineurin and GSK-3β activity, whereas nebula overexpression inhibits calcineurin to prevent activation of GSK-3β (Fig. S11). Our findings that nebula co-overexpression prevented GSK-3β activation and enhanced the transport of APP-YFP vesicles are consistent with a recent report by Weaver et al., in which they find decreasing GSK-3β in fly increased the speed of APP-YFP movement [51]. Furthermore, consistent with our result that APP upregulation triggers GSK-3β enhancement and severe axonal transport defect, Weaver et al. did not detect changes in GFP-synaptotagmin movement in the absence of APP upregulation.
Active GSK-3β has been shown to influence the transport of mitochondria and synaptic proteins including APP, although the exact mechanism may differ between different cargos and motors [51], [83], [84]. One mechanism proposed for GSK-3β-mediated regulation of axonal transport is through phosphorylation of KLC1, thereby disrupting axonal transport by decreasing the association of the anterograde molecular motor with its cargos [59]. Accordingly, we find that APP reduced KLC-synaptotagmin interaction while Nebula upregulation preserved it. Synaptotagmin transport in both the anterograde and retrograde directions were affected, consistent with previous reports showing that altering either the anterograde kinesin or retrograde dynein is sufficient affected transport in both directions [85], [86]. Our results also support work suggesting that synaptotagmin can be transported by the kinesin 1 motor complex in addition to the kinesin 3/imac motor [67]–[73]. As kinesin 1 is known to mediate the movement of both APP and mitochondria [37], [86]–[88], and that phosphorylation of KLC had been shown to inhibit mitochondrial transport [89], detachment of cargo-motor caused by GSK-3β mediated phosphorylation of KLC may lead to general axonal transport problems as reported here. However, GSK-3β activation may also perturb general axonal transport by influencing motor activity or binding of motors to the microtubule tract. Interestingly, increased levels of active GSK-3β and phosphorylated KLC and dynein intermediate chain (DIC), a component of the dynein retrograde complex, have been observed in the frontal complex of AD patients [90]. Genetic variability for KLC1 is thought to be a risk factor for early-onset of Alzheimer's disease [91]. There is also increasing evidence implicating GSK-3β in regulating transport by modulating kinesin activity and exacerbating neurodegeneration in AD through tau hyperphosphorylation [21], [51], [55]. It will be interesting to investigate if Nebula also modulates these processes in the future.
Calcineurin and GSK-3β Signaling Interactions
Although calcineurin had been shown to regulate many important cellular pathways, the link between altered calcineurin and axonal transport, especially in the context of AD, had not been established before. We show that calcineurin can regulate axonal transport through both GSK-3β independent and dependent pathways. This is supported by our observation that the severity of the aggregate phenotype was worse for flies expressing APP and active calcineurin than it was for flies expressing APP and active GSK-3β. These findings point to a role for calcineurin in influencing axonal transport directly, perhaps through dephosphorylation of motor or adaptor proteins. Our data also indicate that calcineurin in part modulates axonal transport through dephosphorylation of GSK-3β as discussed above; however, upregulation of APP is necessary for the induction of severe axonal transport problems, mainly by causing additional enhancement of GSK-3β signaling. GSK3 inhibition is widely discussed as a potential therapeutic intervention for AD, our results suggest that perhaps calcineurin is a more effective target for delaying degeneration by preserving axonal transport.
Implications for Delayed Progression of AD in DS
DSCR1 and APP are both located on chromosome 21 and upregulated in DS [4], [10]. Overexpression of DSCR1 alone had been contradictorily implicated in both conferring resistance to oxidative stress and in promoting apoptosis [8], [9], [16], [17]. Upregulation of Nebula/DSCR1 had also been shown to negatively impact learning and memory in fly and mouse models through altered calcineurin pathways [15], [92]. How could upregulation of DSCR1 be beneficial? We propose that DSCR1 upregulation in the presence of APP upregulation compensates for the altered calcineurin and GSK-3β signaling, shifting the delicate balance of kinase/phosphatase signaling pathways close to normal, therefore preserving axonal transport and delaying neurodegeneration. We also propose that axonal transport defects and synapse dysfunction caused by APP upregulation in our Drosophila model system occur prior to accumulation of amyloid plaques and severe neurodegeneration, similar to that described for a mouse model [30].
DS is characterized by the presence of AD neuropathologies early in life, but most DS individuals do not exhibit signs of dementia until decades later, indicating that there is a delayed progression of cognitive decline [2], [93]. The upregulation of DSCR1 may in fact activate compensatory cell signaling mechanisms that provide protection against APP-mediated oxidative stress, aberrant calcium, and altered calcineurin and GSK3-β activity.
Materials and Methods
Fly Stocks
Flies were cultured at 25°C on standard cornmeal, yeast, sugar, and agar medium under a 12 hour light and 12 hour dark cycle. The following fly lines were obtained from the Bloomington Drosophila Stock Center: Gmr-GAL4, UAS-APP695-N-myc (6700), sgg1/FM7a, UAS-sggS9A (Sgg constitutively active), UAS-nla-RNAi (27260), UAS-CaNB-RNAi (27307), UAS-syt.eGFP (6925), UAS-APP.YFP (32039), and UAS-mitoGFP. Elav-GAL4 stock was kindly provided by Dr. Feany (Harvard University), UAS-nlat1, and nla1 flies were reported previously [15]. UAS-ΔCaNAct construct (constitutively active calcineurin) was generated by deleting the autoinhibitory domain of the CaNA gene Pp2B-14D and subcloned into the pINDY6 vector similar to that described [94]. UAS-Case12 was generated by inserting Case12 (from Evrogen) into pINDY6 vector [53]. Transgenic flies were generated by standard germline transformation method [95].
Histology
Adult Drosophila of 0, 15, 30 and 45 days of age were collected, decapitated and had their proboscis removed. Heads were incubated in Mirsky's fixative for 30 minutes, washed with PBS, and post-fixed in 4% paraformaldehyde for 20 minutes. Fly heads were then transferred to 25% sucrose overnight at 4°C and were subsequently embedded in Tissue-Tek O.C.T Compound for cryostat sectioning (10 µm). Photoreceptor axons were immunostained with 24B10 (1∶10; Developmental Studies Hybridoma), Phosphorylated APP (1∶400; Sigma), and 4G8 (1∶500; Signet).
Phototaxis
Flies were placed in 2 clear round bottom test tubes joined at the opening. After allowing 2 minutes for the flies to acclimate to the tubes, flies were lightly tapped and the percentage of flies that moved toward light in horizontal position within 30 seconds was counted.
Immunocytochemistry
Wandering 3rd instar larvae were dissected in cold calcium-free dissection buffer and fixed with 4% paraformaldehyde in PBS for 25 minutes at room temperature (RT). Samples were blocked in 5% normal goat serum in PBS+0.1% triton for 1 hour at RT and then incubated with primary antibodies overnight at 4°C. Antibodies included synaptotagmin (1∶1,000; gift from H. Bellen) and mAb 4G8 (1∶1,000; Signet), β-tubulin (1∶1000; DSHB), acetylated tubulin (1∶500, Abcam), Cy3-conjugated HRP (1∶200, Jackson ImmunoResearch). Alexa-conjugated secondary antibodies were applied at 1∶500 and samples mounted in Pro -long Gold Antifade reagent (Invitrogen).
Static Image Acquisition and Quantification
Images of motor axons and synaptic terminals from NMJ 6/7 in segment A2 or A3 were captured in a z-series using Zeiss LSM5 scanning confocal. The number of aggregates was determined manually by counting the number of punctate staining with intensity above background and size greater than 0.2 µm2. For quantification of antibody staining intensities at the NMJ, dissected larvae were stained together using the same condition. Images were captured in a z-series and parameters were set to minimize saturation of pixel intensity. Intensity of Z-projected images was analyzed using ImageJ and fold changed calculated by comparing to the control.
Live-Imaging
Wandering 3rd instar larvae expressing APP-YFP or GFP-SYT in combination with other transgenes were dissected in calcium free dissection buffer: 128 mM NaCl, 1 mM EGTA, 4 mM MgCl2, 2 mM KCl, 5 mM HEPES, and 36 mM sucrose. Live imaging of GFP-SYT was done as described [96]. For imaging of mito-GFP, dissected larvae were bathed in HL-3 solution [97]. Time-lapse images were acquired at 5-s intervals using a Zeiss LSM5 confocal using minimum laser intensity to prevent photobleaching and damage to the tissues. Images were acquired for 5 minutes with a 63× lens and a zoom of 1.7. All live imaging experiments were completed within 15 minutes starting from the time of dissection in order to ensure health of the samples.
Live Imaging Analysis
The Manual tracking Plugin in ImageJ was used to track individual vesicle and mitochondria movement. At least 10 frames (>50 s) were used to calculate the average speed of movement. Percentage of movement was determined by counting the percentage of moving vesicles over the imaging period. A vesicle is labeled as moving if it moved in three consecutive frames (over a 15-s period) over a distance of at least 0.1 µm. Direction of movement is determined by direction of net displacement of the vesicle at the start of imaging. Average speed was determined by tracking a vesicle for an uninterrupted run in either the anterograde or retrograde direction. The total distance of movement was divided by the total duration of movement in a specific direction. Student's t-test was used to determine statistical significance.
Line Crossing Locomotor Assay
Deficits in larval locomotor behavior were assessed as described previously [98]. Briefly, larvae were washed with PBS and placed in 60 mm petri dish filled with 1% agarose. Using a moistened paint brush, 3rd instar larvae were collected and allowed to habituate for 30 seconds. The number 0.5 cm2 boxes entered was counted for a 60-s period.
Western Blots
Drosophila adults (1–2 days) were collected on dry ice. Heads were removed and homogenized in cold RIPA buffer. The brains of 3rd instar larvae were dissected and collected on dry ice. Equal amount of protein per genotype (10–20 µg) was run on SDS polyacrylamide gel and transferred to nitrocellulose membrane. Blocking for phosphorylated antibodies was performed using 5% BSA in PBS+0.1% tween (PBS-TW). Blocking for non-phosphorylated antibodies was done using 5% milk in PBS-TW for one hour at RT. Membranes were incubated with the following primary antibodies overnight at 4°C: N-APP (1∶5,000; Sigma), β-tubulin (1∶500; Developmental Studies Hybridoma Bank), Nebula (1∶7,000), Fasciclin II (1∶50 Developmental Studies Hybridoma), acetylated tubulin (1∶1,000, Cell Signaling), phospho-GSK3β Ser9 (1∶1000, Cell Signaling), phospho-GSK3β Tyr126 (1∶1000, Cell Signaling), and GSK3 α/β (1∶2,000, Cell Signaling). Secondary antibodies used were: anti-mouse Alexa 680 (Invitrogen), anti-rabbit Dylight 800 (Piercenet), anti-mouse coupled HRP or anti-rabbit coupled HRP. HRP signals were detected using ECL Reagents (GE Healthcare). Alexa 680 and Dylight 800 signals were detected using Odyssey Imaging system (LI-COR Biosciences). For reprobing, membranes were stripped using Reblot Plus strong antibody stripping solution (Millipore) and reprobed. NIH Image J software was used to measure signal intensity, and the fold change in specific protein level was normalized to a loading control and compared to the control flies.
Calcineurin Activity
Fly heads were collected over dry ice, decapitated, and homogenized in lysis buffer (10 mM Tris pH 7.5, 1 mM EDTA, 0.02% Sodium Azide). Calcineurin phosphatase activity was determined using the Ser/Threonine Phosphatase Assay Kit (Promega) following the manufacturer's protocol as done previously [15]. 5 µg of protein per genotype was used.
Immunoprecipitation
Flies heads were collected on dry ice by passing through molecular sieves and homogenized in lysis buffer (10 mM HEPES, 0.1 M NaCl, 1% NP-40, 2 mM EDTA, 50 mM NaF, 1 mM NA3VO4) plus Complete Mini protease inhibitor cocktail (Roche). Lysates were pre-cleared by incubating fly extract with magnetic A/G beads (Thermo Scientific) for 1 hour at 4°C. Pre-cleared extract was then used for IP using GFP antibody conjugated to magnetic beads (MBL International). Western blot analysis using an antibody against the kinesin light chain (1∶200; Novus Biologicals) was used to confirm interaction. To determine the efficiency of GFP pull down, an antibody against GFP (1∶1000, Abnova) was also used. To eliminate signal contamination from IgG, we used HRP conjugated TrueBlot anti-rabbit IgG (1∶1000, ebioscience) that is specific for native IgG as secondary antibody.
Supporting Information
Zdroje
1. WisniewskiKE, WisniewskiHM, WenGY (1985) Occurrence of neuropathological changes and dementia of Alzheimer's disease in Down's syndrome. Ann Neurol 17 : 278–282.
2. Epstein CJ (1995) Down syndrome (trisomy 21). In: Scriver CR B, AL, Sly WS, Vaile D, editor. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill, Inc. pp. 749–794.
3. ReevesRH, BaxterLL, RichtsmeierJT (2001) Too much of a good thing: mechanisms of gene action in Down syndrome. Trends Genet 17 : 83–88.
4. Rovelet-LecruxA, HannequinD, RauxG, Le MeurN, LaquerriereA, et al. (2006) APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet 38 : 24–26.
5. SleegersK, BrouwersN, GijselinckI, TheunsJ, GoossensD, et al. (2006) APP duplication is sufficient to cause early onset Alzheimer's dementia with cerebral amyloid angiopathy. Brain 129 : 2977–2983.
6. ErmakG, MorganTE, DaviesKJ (2001) Chronic overexpression of the calcineurin inhibitory gene DSCR1 (Adapt78) is associated with Alzheimer's disease. J Biol Chem 276 : 38787–38794.
7. CookCN, HejnaMJ, MagnusonDJ, LeeJM (2005) Expression of calcipressin1, an inhibitor of the phosphatase calcineurin, is altered with aging and Alzheimer's disease. J Alzheimers Dis 8 : 63–73.
8. SunX, WuY, ChenB, ZhangZ, ZhouW, et al. (2011) Regulator of calcineurin 1 (RCAN1) facilitates neuronal apoptosis through caspase-3 activation. J Biol Chem 286 : 9049–9062.
9. ErmakG, HarrisCD, DaviesKJ (2002) The DSCR1 (Adapt78) isoform 1 protein calcipressin 1 inhibits calcineurin and protects against acute calcium-mediated stress damage, including transient oxidative stress. FASEB J 16 : 814–824.
10. FuentesJJ, PritchardMA, PlanasAM, BoschA, FerrerI, et al. (1995) A new human gene from the Down syndrome critical region encodes a proline-rich protein highly expressed in fetal brain and heart. Hum Mol Genet 4 : 1935–1944.
11. FuentesJJ, GenescaL, KingsburyTJ, CunninghamKW, Perez-RibaM, et al. (2000) DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin-mediated signaling pathways. Hum Mol Genet 9 : 1681–1690.
12. GorlachJ, FoxDS, CutlerNS, CoxGM, PerfectJR, et al. (2000) Identification and characterization of a highly conserved calcineurin binding protein, CBP1/calcipressin, in Cryptococcus neoformans. EMBO J 19 : 3618–3629.
13. KingsburyTJ, CunninghamKW (2000) A conserved family of calcineurin regulators. Genes Dev 14 : 1595–1604.
14. RothermelB, VegaRB, YangJ, WuH, Bassel-DubyR, et al. (2000) A protein encoded within the Down syndrome critical region is enriched in striated muscles and inhibits calcineurin signaling. J Biol Chem 275 : 8719–8725.
15. ChangKT, ShiYJ, MinKT (2003) The Drosophila homolog of Down's syndrome critical region 1 gene regulates learning: implications for mental retardation. Proc Natl Acad Sci U S A 100 : 15794–15799.
16. PortaS, SerraSA, HuchM, ValverdeMA, LlorensF, et al. (2007) RCAN1 (DSCR1) increases neuronal susceptibility to oxidative stress: a potential pathogenic process in neurodegeneration. Hum Mol Genet 16 : 1039–1050.
17. SobradoM, RamirezBG, NeriaF, LizasoainI, ArbonesML, et al. (2012) Regulator of calcineurin 1 (Rcan1) has a protective role in brain ischemia/reperfusion injury. J Neuroinflammation 9 : 48.
18. WarrickJM, PaulsonHL, Gray-BoardGL, BuiQT, FischbeckKH, et al. (1998) Expanded polyglutamine protein forms nuclear inclusions and causes neural degeneration in Drosophila. Cell 93 : 939–949.
19. FeanyMB, BenderWW (2000) A Drosophila model of Parkinson's disease. Nature 404 : 394–398.
20. SteffanJS, BodaiL, PallosJ, PoelmanM, McCampbellA, et al. (2001) Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature 413 : 739–743.
21. JacksonGR, Wiedau-PazosM, SangTK, WagleN, BrownCA, et al. (2002) Human wild-type tau interacts with wingless pathway components and produces neurofibrillary pathology in Drosophila. Neuron 34 : 509–519.
22. ChenHK, Fernandez-FunezP, AcevedoSF, LamYC, KaytorMD, et al. (2003) Interaction of Akt-phosphorylated ataxin-1 with 14-3-3 mediates neurodegeneration in spinocerebellar ataxia type 1. Cell 113 : 457–468.
23. YangY, NishimuraI, ImaiY, TakahashiR, LuB (2003) Parkin suppresses dopaminergic neuron-selective neurotoxicity induced by Pael-R in Drosophila. Neuron 37 : 911–924.
24. IijimaK, LiuHP, ChiangAS, HearnSA, KonsolakiM, et al. (2004) Dissecting the pathological effects of human Abeta40 and Abeta42 in Drosophila: a potential model for Alzheimer's disease. Proc Natl Acad Sci U S A 101 : 6623–6628.
25. ClarkIE, DodsonMW, JiangC, CaoJH, HuhJR, et al. (2006) Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 441 : 1162–1166.
26. MuhammadA, FloresI, ZhangH, YuR, StaniszewskiA, et al. (2008) Retromer deficiency observed in Alzheimer's disease causes hippocampal dysfunction, neurodegeneration, and Abeta accumulation. Proc Natl Acad Sci U S A 105 : 7327–7332.
27. Carmine-SimmenK, ProctorT, TschapeJ, PoeckB, TriphanT, et al. (2009) Neurotoxic effects induced by the Drosophila amyloid-beta peptide suggest a conserved toxic function. Neurobiol Dis 33 : 274–281.
28. TorrojaL, ChuH, KotovskyI, WhiteK (1999) Neuronal overexpression of APPL, the Drosophila homologue of the amyloid precursor protein (APP), disrupts axonal transport. Curr Biol 9 : 489–492.
29. GunawardenaS, GoldsteinLS (2001) Disruption of axonal transport and neuronal viability by amyloid precursor protein mutations in Drosophila. Neuron 32 : 389–401.
30. StokinGB, LilloC, FalzoneTL, BruschRG, RockensteinE, et al. (2005) Axonopathy and transport deficits early in the pathogenesis of Alzheimer's disease. Science 307 : 1282–1288.
31. SalehiA, DelcroixJD, BelichenkoPV, ZhanK, WuC, et al. (2006) Increased App expression in a mouse model of Down's syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron 51 : 29–42.
32. StokinGB, GoldsteinLS (2006) Axonal transport and Alzheimer's disease. Annu Rev Biochem 75 : 607–627.
33. MorfiniGA, BurnsM, BinderLI, KanaanNM, LaPointeN, et al. (2009) Axonal transport defects in neurodegenerative diseases. J Neurosci 29 : 12776–12786.
34. KanaanNM, PiginoGF, BradyST, LazarovO, BinderLI, et al. (2013) Axonal degeneration in Alzheimer's disease: When signaling abnormalities meet the axonal transport system. Exp Neurol 246 : 44–53.
35. GreeveI, KretzschmarD, TschapeJA, BeynA, BrellingerC, et al. (2004) Age-dependent neurodegeneration and Alzheimer-amyloid plaque formation in transgenic Drosophila. The Journal of neuroscience : the official journal of the Society for Neuroscience 24 : 3899–3906.
36. BenzerS (1967) Behavioral mutants of Drosophila isolated by countercurrent distribution. Proc Natl Acad Sci USA 58 : 1112–1119.
37. MuresanZ, MuresanV (2005) Coordinated transport of phosphorylated amyloid-beta precursor protein and c-Jun NH2-terminal kinase-interacting protein-1. J Cell Biol 171 : 615–625.
38. GroemerTW, ThielCS, HoltM, RiedelD, HuaY, et al. (2011) Amyloid precursor protein is trafficked and secreted via synaptic vesicles. PLoS One 6: e18754.
39. KohliBM, PfliegerD, MuellerLN, CarbonettiG, AebersoldR, et al. (2012) Interactome of the amyloid precursor protein APP in brain reveals a protein network involved in synaptic vesicle turnover and a close association with Synaptotagmin-1. J Proteome Res 11 : 4075–4090.
40. LuoL, TullyT, WhiteK (1992) Human amyloid precursor protein ameliorates behavioral deficit of flies deleted for Appl gene. Neuron 9 : 595–605.
41. TorrojaL, PackardM, GorczycaM, WhiteK, BudnikV (1999) The Drosophila beta-amyloid precursor protein homolog promotes synapse differentiation at the neuromuscular junction. The Journal of neuroscience : the official journal of the Society for Neuroscience 19 : 7793–7803.
42. AshleyJ, PackardM, AtamanB, BudnikV (2005) Fasciclin II signals new synapse formation through amyloid precursor protein and the scaffolding protein dX11/Mint. The Journal of neuroscience : the official journal of the Society for Neuroscience 25 : 5943–5955.
43. SchusterCM, DavisGW, FetterRD, GoodmanCS (1996) Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron 17 : 641–654.
44. SchusterCM, DavisGW, FetterRD, GoodmanCS (1996) Genetic dissection of structural and functional components of synaptic plasticity. II. Fasciclin II controls presynaptic structural plasticity. Neuron 17 : 655–667.
45. MattsonMP, GleichmannM, ChengA (2008) Mitochondria in neuroplasticity and neurological disorders. Neuron 60 : 748–766.
46. Iijima-AndoK, HearnSA, ShentonC, GattA, ZhaoL, et al. (2009) Mitochondrial mislocalization underlies Abeta42-induced neuronal dysfunction in a Drosophila model of Alzheimer's disease. PLoS One 4: e8310.
47. Iijima-AndoK, SekiyaM, Maruko-OtakeA, OhtakeY, SuzukiE, et al. (2012) Loss of Axonal Mitochondria Promotes Tau-Mediated Neurodegeneration and Alzheimer's Disease-Related Tau Phosphorylation Via PAR-1. PLoS Genet 8: e1002918.
48. ShahpasandK, UemuraI, SaitoT, AsanoT, HataK, et al. (2012) Regulation of mitochondrial transport and inter-microtubule spacing by tau phosphorylation at the sites hyperphosphorylated in Alzheimer's disease. The Journal of neuroscience : the official journal of the Society for Neuroscience 32 : 2430–2441.
49. PipernoG, FullerMT (1985) Monoclonal antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J Cell Biol 101 : 2085–2094.
50. GodenaVK, RomanoG, RomanoM, AppocherC, KlimaR, et al. (2011) TDP-43 regulates Drosophila neuromuscular junctions growth by modulating Futsch/MAP1B levels and synaptic microtubules organization. PloS one 6: e17808.
51. WeaverC, LeidelC, SzpankowskiL, FarleyNM, ShubeitaGT, et al. (2013) Endogenous GSK-3/shaggy regulates bidirectional axonal transport of the amyloid precursor protein. Traffic 14 : 295–308.
52. KleeCB, CrouchTH, KrinksMH (1979) Calcineurin: a calcium - and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci U S A 76 : 6270–6273.
53. SouslovaEA, BelousovVV, LockJG, StrombladS, KasparovS, et al. (2007) Single fluorescent protein-based Ca2+ sensors with increased dynamic range. BMC Biotechnol 7 : 37.
54. ChangKT, NiescierRF, MinKT (2011) Mitochondrial matrix Ca2+ as an intrinsic signal regulating mitochondrial motility in axons. Proc Natl Acad Sci U S A 108 : 15456–15461.
55. HooperC, KillickR, LovestoneS (2008) The GSK3 hypothesis of Alzheimer's disease. Journal of neurochemistry 104 : 1433–1439.
56. KimY, LeeYI, SeoM, KimSY, LeeJE, et al. (2009) Calcineurin dephosphorylates glycogen synthase kinase-3 beta at serine-9 in neuroblast-derived cells. J Neurochem 111 : 344–354.
57. HernandezF, LucasJJ, AvilaJ (2012) GSK3 and Tau: Two Convergence Points in Alzheimer's Disease. J Alzheimers Dis 33 Suppl 1: S141–4.
58. Mondragon-RodriguezS, PerryG, ZhuX, MoreiraPI, WilliamsS (2012) Glycogen synthase kinase 3: a point of integration in Alzheimer's disease and a therapeutic target? Int J Alzheimers Dis 2012 : 276803.
59. MorfiniG, SzebenyiG, ElluruR, RatnerN, BradyST (2002) Glycogen synthase kinase 3 phosphorylates kinesin light chains and negatively regulates kinesin-based motility. EMBO J 21 : 281–293.
60. MudherA, ShepherdD, NewmanTA, MildrenP, JukesJP, et al. (2004) GSK-3beta inhibition reverses axonal transport defects and behavioural phenotypes in Drosophila. Mol Psychiatry 9 : 522–530.
61. SofolaO, KerrF, RogersI, KillickR, AugustinH, et al. (2010) Inhibition of GSK-3 ameliorates Abeta pathology in an adult-onset Drosophila model of Alzheimer's disease. PLoS Genet 6: e1001087.
62. LochheadPA, KinstrieR, SibbetG, RawjeeT, MorriceN, et al. (2006) A chaperone-dependent GSK3beta transitional intermediate mediates activation-loop autophosphorylation. Mol Cell 24 : 627–633.
63. PeineauS, BradleyC, TaghibiglouC, DohertyA, BortolottoZA, et al. (2008) The role of GSK-3 in synaptic plasticity. Br J Pharmacol 153 Suppl 1: S428–437.
64. HughesK, NikolakakiE, PlyteSE, TottyNF, WoodgettJR (1993) Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO J 12 : 803–808.
65. BhatRV, ShanleyJ, CorrellMP, FielesWE, KeithRA, et al. (2000) Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase-3beta in cellular and animal models of neuronal degeneration. Proc Natl Acad Sci U S A 97 : 11074–11079.
66. PiginoG, MorfiniG, PelsmanA, MattsonMP, BradyST, et al. (2003) Alzheimer's presenilin 1 mutations impair kinesin-based axonal transport. J Neurosci 23 : 4499–4508.
67. Pack-ChungE, KurshanPT, DickmanDK, SchwarzTL (2007) A Drosophila kinesin required for synaptic bouton formation and synaptic vesicle transport. Nat Neurosci 10 : 980–989.
68. BarkusRV, KlyachkoO, HoriuchiD, DicksonBJ, SaxtonWM (2008) Identification of an axonal kinesin-3 motor for fast anterograde vesicle transport that facilitates retrograde transport of neuropeptides. Mol Biol Cell 19 : 274–283.
69. HurdDD, SternM, SaxtonWM (1996) Mutation of the axonal transport motor kinesin enhances paralytic and suppresses Shaker in Drosophila. Genetics 142 : 195–204.
70. GindhartJGJr, DesaiCJ, BeushausenS, ZinnK, GoldsteinLS (1998) Kinesin light chains are essential for axonal transport in Drosophila. J Cell Biol 141 : 443–454.
71. ByrdDT, KawasakiM, WalcoffM, HisamotoN, MatsumotoK, et al. (2001) UNC-16, a JNK-signaling scaffold protein, regulates vesicle transport in C. elegans. Neuron 32 : 787–800.
72. TodaH, MochizukiH, FloresR (2008) UNC-51/ATG1 kinase regulates axonal transport by mediating motor-cargo assembly. Genes Dev 22 : 3292–3307.
73. HirokawaN, NodaY, TanakaY, NiwaS (2009) Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol 10 : 682–696.
74. RuelL, PantescoV, LutzY, SimpsonP, BourouisM (1993) Functional significance of a family of protein kinases encoded at the shaggy locus in Drosophila. EMBO J 12 : 1657–1669.
75. TakeoS, SwansonSK, NandananK, NakaiY, AigakiT, et al. (2012) Shaggy/glycogen synthase kinase 3beta and phosphorylation of Sarah/regulator of calcineurin are essential for completion of Drosophila female meiosis. Proc Natl Acad Sci U S A 109 : 6382–6389.
76. MitchellCS, LeeRH (2012) Cargo distributions differentiate pathological axonal transport impairments. J Theor Biol 300 : 277–291.
77. ChangKT, MinKT (2005) Drosophila melanogaster homolog of Down syndrome critical region 1 is critical for mitochondrial function. Nat Neurosci 8 : 1577–1585.
78. LaFerlaFM (2002) Calcium dyshomeostasis and intracellular signalling in Alzheimer's disease. Nat Rev Neurosci 3 : 862–872.
79. ReeseLC, TaglialatelaG (2011) A role for calcineurin in Alzheimer's disease. Curr Neuropharmacol 9 : 685–692.
80. GarwoodC, FaizullabhoyA, WhartonSB, IncePG, HeathP, et al. (2013) Calcium dysregulation in relation to Alzheimer-type pathology in the ageing brain. Neuropathol Appl Neurobiol [epub ahead of print doi:10.1111/nan.12033
81. D'AmelioM, CavallucciV, MiddeiS, MarchettiC, PacioniS, et al. (2011) Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer's disease. Nat Neurosci 14 : 69–76.
82. SantosSF, PierrotN, MorelN, GaillyP, SindicC, et al. (2009) Expression of human amyloid precursor protein in rat cortical neurons inhibits calcium oscillations. The Journal of neuroscience : the official journal of the Society for Neuroscience 29 : 4708–4718.
83. ChenS, OwensGC, CrossinKL, EdelmanDB (2007) Serotonin stimulates mitochondrial transport in hippocampal neurons. Mol Cell Neurosci 36 : 472–483.
84. MorelM, AutheletM, DedeckerR, BrionJP (2010) Glycogen synthase kinase-3beta and the p25 activator of cyclin dependent kinase 5 increase pausing of mitochondria in neurons. Neuroscience 167 : 1044–1056.
85. BradyST, PfisterKK, BloomGS (1990) A monoclonal antibody against kinesin inhibits both anterograde and retrograde fast axonal transport in squid axoplasm. Proc Natl Acad Sci U S A 87 : 1061–1065.
86. PillingAD, HoriuchiD, LivelyCM, SaxtonWM (2006) Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol Biol Cell 17 : 2057–2068.
87. ReisGF, YangG, SzpankowskiL, WeaverC, ShahSB, et al. (2012) Molecular motor function in axonal transport in vivo probed by genetic and computational analysis in Drosophila. Mol Biol Cell 23 : 1700–1714.
88. GunawardenaS, YangG, GoldsteinLS (2013) Presenilin controls kinesin-1 and dynein function during APP-vesicle transport in vivo. Hum Mol Genet [Epub ahead of print].
89. De VosK, SeverinF, Van HerrewegheF, VancompernolleK, GoossensV, et al. (2000) Tumor necrosis factor induces hyperphosphorylation of kinesin light chain and inhibits kinesin-mediated transport of mitochondria. J Cell Biol 149 : 1207–1214.
90. MorelM, HeraudC, NicaiseC, SuainV, BrionJP (2012) Levels of kinesin light chain and dynein intermediate chain are reduced in the frontal cortex in Alzheimer's disease: implications for axoplasmic transport. Acta neuropathologica 123 : 71–84.
91. AnderssonME, SjolanderA, AndreasenN, MinthonL, HanssonO, et al. (2007) Kinesin gene variability may affect tau phosphorylation in early Alzheimer's disease. International journal of molecular medicine 20 : 233–239.
92. DierssenM, ArqueG, McDonaldJ, AndreuN, Martinez-CueC, et al. (2011) Behavioral characterization of a mouse model overexpressing DSCR1/RCAN1. PLoS One 6: e17010.
93. CostaAC (2012) Alzheimer disease: Treatment of Alzheimer disease in Down syndrome. Nature reviews Neurology 8 : 182–184.
94. SullivanKM, RubinGM (2002) The Ca(2+)-calmodulin-activated protein phosphatase calcineurin negatively regulates EGF receptor signaling in Drosophila development. Genetics 161 : 183–193.
95. MontellC, JonesK, HafenE, RubinG (1985) Rescue of the Drosophila phototransduction mutation trp by germline transformation. Science 230 : 1040–1043.
96. KuznickiML, GunawardenaS (2010) In vivo visualization of synaptic vesicles within Drosophila larval segmental axons. J Vis Exp (44) pii: 2151.
97. LouieK, RussoGJ, SalkoffDB, WellingtonA, ZinsmaierKE (2008) Effects of imaging conditions on mitochondrial transport and length in larval motor axons of Drosophila. Comp Biochem Physiol A Mol Integr Physiol 151 : 159–172.
98. ChangKT, MinKT (2009) Upregulation of three Drosophila homologs of human chromosome 21 genes alters synaptic function: implications for Down syndrome. Proc Natl Acad Sci U S A 106 : 17117–17122.
Štítky
Genetika Reprodukční medicína
Článek Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive SelectionČlánek Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand SkillČlánek Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage EvolvabilityČlánek Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation inČlánek Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 ModelČlánek Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period inČlánek VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival inČlánek Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2AČlánek A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding YeastČlánek Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on PhenotypeČlánek Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding SitesČlánek Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 9
-
Všechny články tohoto čísla
- The Pathway Gene Functions together with the -Dependent Isoprenoid Biosynthetic Pathway to Orchestrate Germ Cell Migration
- Take Off, Landing, and Fly Anesthesia
- Nucleosome Assembly Proteins Get SET to Defeat the Guardian of Chromosome Cohesion
- Whole-Exome Sequencing Reveals a Rapid Change in the Frequency of Rare Functional Variants in a Founding Population of Humans
- Evidence Is Evidence: An Interview with Mary-Claire King
- Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive Selection
- Convergent Transcription Induces Dynamic DNA Methylation at Loci
- Environmental Stresses Disrupt Telomere Length Homeostasis
- Ultra-Sensitive Sequencing Reveals an Age-Related Increase in Somatic Mitochondrial Mutations That Are Inconsistent with Oxidative Damage
- Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand Skill
- Genetic and Anatomical Basis of the Barrier Separating Wakefulness and Anesthetic-Induced Unresponsiveness
- The Locus, Exclusive to the Ambulacrarians, Encodes a Chromatin Insulator Binding Protein in the Sea Urchin Embryo
- Binding of NF-κB to Nucleosomes: Effect of Translational Positioning, Nucleosome Remodeling and Linker Histone H1
- Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage Evolvability
- Dynamics of DNA Methylation in Recent Human and Great Ape Evolution
- Functional Dissection of Regulatory Models Using Gene Expression Data of Deletion Mutants
- PAQR-2 Regulates Fatty Acid Desaturation during Cold Adaptation in
- N-alpha-terminal Acetylation of Histone H4 Regulates Arginine Methylation and Ribosomal DNA Silencing
- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation in
- miR-1/133a Clusters Cooperatively Specify the Cardiomyogenic Lineage by Adjustment of Myocardin Levels during Embryonic Heart Development
- Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 Model
- Genome-Wide Analysis of Genes and Their Association with Natural Variation in Drought Tolerance at Seedling Stage of L
- Deep Resequencing of GWAS Loci Identifies Rare Variants in , and That Are Associated with Ulcerative Colitis
- Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period in
- VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival in
- Analysis of Genes Reveals Redundant and Independent Functions in the Inner Ear
- Predicting the Risk of Rheumatoid Arthritis and Its Age of Onset through Modelling Genetic Risk Variants with Smoking
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
- A Shift to Organismal Stress Resistance in Programmed Cell Death Mutants
- Fragile Site Instability in Causes Loss of Heterozygosity by Mitotic Crossovers and Break-Induced Replication
- Tracking of Chromosome and Replisome Dynamics in Reveals a Novel Chromosome Arrangement
- The Condition-Dependent Transcriptional Landscape of
- Ago1 Interacts with RNA Polymerase II and Binds to the Promoters of Actively Transcribed Genes in Human Cancer Cells
- Nebula/DSCR1 Upregulation Delays Neurodegeneration and Protects against APP-Induced Axonal Transport Defects by Restoring Calcineurin and GSK-3β Signaling
- System-Wide Analysis Reveals a Complex Network of Tumor-Fibroblast Interactions Involved in Tumorigenicity
- Meta-Analysis of Genome-Wide Association Studies Identifies Six New Loci for Serum Calcium Concentrations
- and Are Required for Cellularization and Differentiation during Female Gametogenesis in
- Growth factor independent-1 Maintains Notch1-Dependent Transcriptional Programming of Lymphoid Precursors
- Whole Genome Sequencing Identifies a Deletion in Protein Phosphatase 2A That Affects Its Stability and Localization in
- An Alteration in ELMOD3, an Arl2 GTPase-Activating Protein, Is Associated with Hearing Impairment in Humans
- Genomic Identification of Founding Haplotypes Reveals the History of the Selfing Species
- Plasticity Regulators Modulate Specific Root Traits in Discrete Nitrogen Environments
- The IDD14, IDD15, and IDD16 Cooperatively Regulate Lateral Organ Morphogenesis and Gravitropism by Promoting Auxin Biosynthesis and Transport
- Stochastic Loss of Silencing of the Imprinted Allele, in a Mouse Model and Humans with Prader-Willi Syndrome, Has Functional Consequences
- The Prefoldin Complex Regulates Chromatin Dynamics during Transcription Elongation
- PKA Controls Calcium Influx into Motor Neurons during a Rhythmic Behavior
- A Pre-mRNA-Splicing Factor Is Required for RNA-Directed DNA Methylation in
- Cell-Type Specific Features of Circular RNA Expression
- The Uve1 Endonuclease Is Regulated by the White Collar Complex to Protect from UV Damage
- An Atypical Kinase under Balancing Selection Confers Broad-Spectrum Disease Resistance in Arabidopsis
- Genome-Wide Mutation Avalanches Induced in Diploid Yeast Cells by a Base Analog or an APOBEC Deaminase
- Extensive Divergence of Transcription Factor Binding in Embryos with Highly Conserved Gene Expression
- Bi-modal Distribution of the Second Messenger c-di-GMP Controls Cell Fate and Asymmetry during the Cell Cycle
- Cell Interactions and Patterned Intercalations Shape and Link Epithelial Tubes in
- A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding Yeast
- The Genome and Development-Dependent Transcriptomes of : A Window into Fungal Evolution
- SKN-1/Nrf, A New Unfolded Protein Response Factor?
- The Highly Prolific Phenotype of Lacaune Sheep Is Associated with an Ectopic Expression of the Gene within the Ovary
- Fusion of Large-Scale Genomic Knowledge and Frequency Data Computationally Prioritizes Variants in Epilepsy
- IL-17 Attenuates Degradation of ARE-mRNAs by Changing the Cooperation between AU-Binding Proteins and microRNA16
- An Enhancer Element Harboring Variants Associated with Systemic Lupus Erythematosus Engages the Promoter to Influence A20 Expression
- Genome Analysis of a Transmissible Lineage of Reveals Pathoadaptive Mutations and Distinct Evolutionary Paths of Hypermutators
- Type I-E CRISPR-Cas Systems Discriminate Target from Non-Target DNA through Base Pairing-Independent PAM Recognition
- Divergent Transcriptional Regulatory Logic at the Intersection of Tissue Growth and Developmental Patterning
- MEIOB Targets Single-Strand DNA and Is Necessary for Meiotic Recombination
- Transmission of Hypervirulence Traits via Sexual Reproduction within and between Lineages of the Human Fungal Pathogen
- Integration of the Unfolded Protein and Oxidative Stress Responses through SKN-1/Nrf
- Guanine Holes Are Prominent Targets for Mutation in Cancer and Inherited Disease
- Regulation of the Boundaries of Accessible Chromatin
- Natural Genetic Transformation Generates a Population of Merodiploids in
- Ablating Adult Neurogenesis in the Rat Has No Effect on Spatial Processing: Evidence from a Novel Pharmacogenetic Model
- Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on Phenotype
- The Molecular Mechanism of a -Regulatory Adaptation in Yeast
- Phenotypic and Genetic Consequences of Protein Damage
- Recent Acquisition of by Baka Pygmies
- Fatty Acid Taste Signals through the PLC Pathway in Sugar-Sensing Neurons
- A Critical Role for PDGFRα Signaling in Medial Nasal Process Development
- Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding Sites
- Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
- dTULP, the Homolog of Tubby, Regulates Transient Receptor Potential Channel Localization in Cilia
- Widespread Dysregulation of Peptide Hormone Release in Mice Lacking Adaptor Protein AP-3
- , a Direct Transcriptional Target, Modulates T-Box Factor Activity in Orofacial Clefting
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Recent Acquisition of by Baka Pygmies
- The Condition-Dependent Transcriptional Landscape of
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



