-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAn Atypical Kinase under Balancing Selection Confers Broad-Spectrum Disease Resistance in Arabidopsis
The failure of gene-for-gene resistance traits to provide durable and broad-spectrum resistance in an agricultural context has led to the search for genes underlying quantitative resistance in plants. Such genes have been identified in only a few cases, all for fungal or nematode resistance, and encode diverse molecular functions. However, an understanding of the molecular mechanisms of quantitative resistance variation to other enemies and the associated evolutionary forces shaping this variation remain largely unknown. We report the identification, map-based cloning and functional validation of QRX3 (RKS1, Resistance related KinaSe 1), conferring broad-spectrum resistance to Xanthomonas campestris (Xc), a devastating worldwide bacterial vascular pathogen of crucifers. RKS1 encodes an atypical kinase that mediates a quantitative resistance mechanism in plants by restricting bacterial spread from the infection site. Nested Genome-Wide Association mapping revealed a major locus corresponding to an allelic series at RKS1 at the species level. An association between variation in resistance and RKS1 transcription was found using various transgenic lines as well as in natural accessions, suggesting that regulation of RKS1 expression is a major component of quantitative resistance to Xc. The co-existence of long lived RKS1 haplotypes in A. thaliana is shared with a variety of genes involved in pathogen recognition, suggesting common selective pressures. The identification of RKS1 constitutes a starting point for deciphering the mechanisms underlying broad spectrum quantitative disease resistance that is effective against a devastating and vascular crop pathogen. Because putative RKS1 orthologous have been found in other Brassica species, RKS1 provides an exciting opportunity for plant breeders to improve resistance to black rot in crops.
Published in the journal: . PLoS Genet 9(9): e32767. doi:10.1371/journal.pgen.1003766
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003766Summary
The failure of gene-for-gene resistance traits to provide durable and broad-spectrum resistance in an agricultural context has led to the search for genes underlying quantitative resistance in plants. Such genes have been identified in only a few cases, all for fungal or nematode resistance, and encode diverse molecular functions. However, an understanding of the molecular mechanisms of quantitative resistance variation to other enemies and the associated evolutionary forces shaping this variation remain largely unknown. We report the identification, map-based cloning and functional validation of QRX3 (RKS1, Resistance related KinaSe 1), conferring broad-spectrum resistance to Xanthomonas campestris (Xc), a devastating worldwide bacterial vascular pathogen of crucifers. RKS1 encodes an atypical kinase that mediates a quantitative resistance mechanism in plants by restricting bacterial spread from the infection site. Nested Genome-Wide Association mapping revealed a major locus corresponding to an allelic series at RKS1 at the species level. An association between variation in resistance and RKS1 transcription was found using various transgenic lines as well as in natural accessions, suggesting that regulation of RKS1 expression is a major component of quantitative resistance to Xc. The co-existence of long lived RKS1 haplotypes in A. thaliana is shared with a variety of genes involved in pathogen recognition, suggesting common selective pressures. The identification of RKS1 constitutes a starting point for deciphering the mechanisms underlying broad spectrum quantitative disease resistance that is effective against a devastating and vascular crop pathogen. Because putative RKS1 orthologous have been found in other Brassica species, RKS1 provides an exciting opportunity for plant breeders to improve resistance to black rot in crops.
Introduction
Pathogens are a threat for crops and natural plant populations. A major challenge in plant breeding and evolutionary biology is to identify the genetic and molecular bases for natural resistance variation in plant species. The identification of genes underlying natural resistance variation might have enormous practical implications by increasing crop yield and quality and gives fundamental insights in the prediction of evolutionary trajectories of natural populations. Disease resistance is constituted by an elaborate, multilayered system of defense [1], and substantial progress has been made in the understanding of plant specific disease resistance conferred by single R genes. However there is still very limited information about the genes underlying quantitative resistance, despite the fact that this form of resistance is much more prevalent in crops and natural plant populations than R gene specific resistance [2]. Such genes conferring partial resistance to pathogens have been identified only in few cases, all for fungal and nematode resistance, and encode diverse molecular functions underlying durable and broad-spectrum resistance [3]–[7].
How host plants achieve quantitative resistance against other major enemies remains largely unknown [8]–[9]. Biotrophic bacterial pathogens generally trigger extreme defense responses (cell death) that are typically mediated by R genes. Identification and understanding of alternative molecular mechanisms (i.e. quantitative resistance) to such enemies, is of high interest both on a basic and applied point of view. It is furthermore unknown whether quantitative resistance genes show hallmarks of selection similar to those for gene-for-gene resistance. R-genes often reveal a signature of balancing selection in natural plant species, i.e. long-lived polymorphism of R gene alleles [10]–[11]. Identifying the molecular mechanisms of quantitative resistance variation and understanding the evolutionary forces shaping variation in quantitative resistance should inform strategies for long-term broad-spectrum control of bacterial diseases.
Xanthomonas campestris pv. campestris (Xcc) is a biotrophic bacterium infecting the vascular system of plants. Xcc causes black rot disease, possibly the most important disease of crucifers [12]. It is also one of the most prevalent bacterial pathogens in natural populations of Arabidopsis thaliana [13]. Previous work on the Arabidopsis-Xcc interaction revealed that different sources of resistance and tolerance to Xcc exist in Arabidopsis [14]–[17], but until now no information is available about the molecular mechanisms underlying this resistance or tolerance.
By multiple approaches, we demonstrate that RKS1 is a quantitative resistance gene conferring broad-spectrum resistance to several Xanthomonas campestris races and pathovars. We show that RKS1 restricts bacterial spread from the infection site to the vascular system. This novel resistance mechanism in plants is associated with regulation of RKS1 expression. Moreover, we provide evidence that RKS1 allelic variation is a major component of quantitative resistance to Xc at the species level. Finally, a signature of balancing selection acting on RKS1 indicates that evolutionary stable broad-spectrum resistance to Xc may be achieved in natural populations of A. thaliana.
Results
A major QTL QRX3 confers resistance to the strain Xcc568
We subjected natural accessions of A. thaliana to infection by the Xanthomonas campestris pv. campestris (Xcc) strain 568. Substantial genetic variation for resistance to this strain was revealed by inoculation of a core collection of natural accessions of A. thaliana (Figure S1). To characterize the genetic basis of this variation, we carried out QTL (Quantitative Trait Locus) analysis using an F6 Recombinant Inbred Line (RIL) population of 115 lines derived from the accessions Columbia glabrous1 (Col-5) and Kashmir-1 (Kas-1) which exhibit contrasting phenotypes (Figures 1A and B) [18]. Three different experiments were conducted: a disease index was scored for both growth chamber and greenhouse grown 28-day old plants and in planta bacterial growth was measured using growth chamber grown plants. In each of those three independent experiments, the QTL analysis revealed one major QTL at the bottom of chromosome 3, QRX3 (Quantitative Resistance to Xcc568) that explained up to 53.7% of the phenotypic variance (Figure 1C, Table S1). Four minor QTLs were also detected in some experiments, on chromosomes 1 (QRX1.1 and QRX1.2), 2 (QRX2) and 5 (QRX5) (Table S1). The allelic additive effects of these QTLs were in the same direction, the Col-5 allele increasing resistance compared to the Kas-1 allele.
Fig. 1. Identification and mapping of the major QTL, QRX3, for resistance to Xcc. 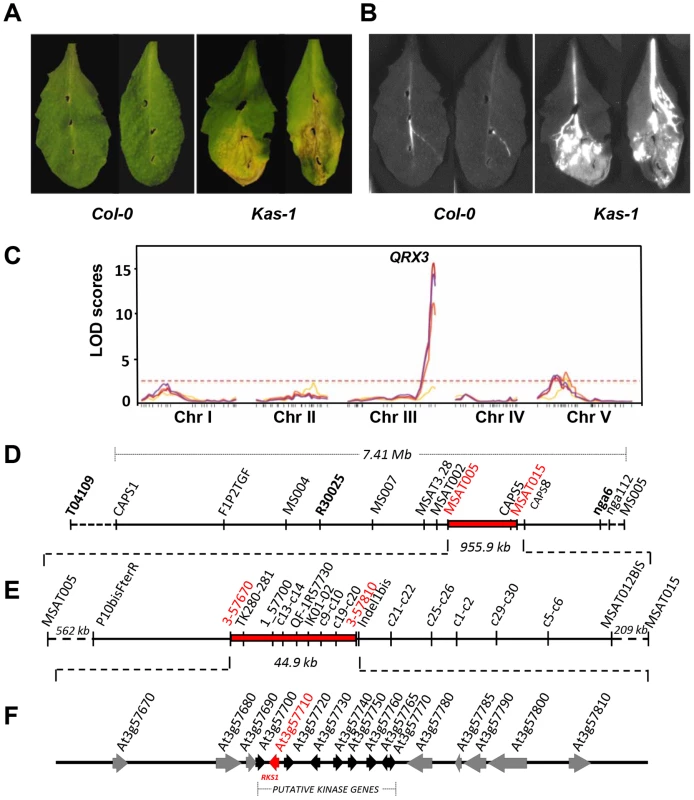
(A and B) Phenotype of susceptible (Kas-1) and resistant (Col-0) accessions (Col-5 and Col-0 (used here) show similar phenotypes) : (A) symptoms 7 days post-inoculation (dpi) and (B) bacterial invasion of leaf tissue using an Xcc568 reporter strain that carries the Photorhabdus luminescens lux operon. (C) QTL maps of resistance to Xcc in the Col-5 x Kas-1 recombinant inbred line population at four inoculation times: yellow, 3 dpi; orange, 5 dpi; red, 7 dpi and purple, 10 dpi. The horizontal dotted line represents the significance threshold for the LOD score (average = 2.50). (D to F) Map-based isolation of the QRX3 locus. (D) Genetic map of chromosome III is shown between markers T04109 and MS005 with the defined target interval for QRX3 (in red). (E) A number of additional markers and recombinant lines were used to reduce the QRX3 locus to a 44.9 kb region between the markers 3-57670 and 3-57810. (F) The corresponding physical interval contains 17 open reading frames (ORFs). Genes are represented by arrows. The black arrows correspond to a cluster of putative kinase genes, the red arrow corresponds to RKS1. The effect of QRX3 was validated in heterogeneous inbred families (HIFs); all homozygous HIF lines that carried the Kas-1 allele were susceptible to Xcc568 and all homozygous lines that carried the Col-5 allele were resistant, reflecting perfectly the parental phenotypes (Figure S2). These results confirm the quantitative contribution of this major locus to Xcc568 resistance. We thus selected QRX3 as a target for map-based cloning.
The At3g57710 (RKS1) gene identified by map-based cloning underlies QRX3
On the basis of three high-resolution populations, we reduced the target interval to a 44.9 kb region (Figure 1D and E, Tables S2 and S3), containing 17 predicted Open Reading Frames (ORFs) (Figure 1F). To identify the gene(s) implicated in resistance to Xcc568, one to six insertional mutants (T-DNA or transposon mutant lines) were selected for each gene within the narrowed region (Table S4). Of the 36 mutants characterized, only one (mutant 11/rks1-1) showed a susceptible phenotype, albeit less pronounced than the susceptible Kas-1 control line (Figures 2 and 3A). In rks1-1, the T-DNA insertion was located in the intergenic region between At3g57710 (herein termed RKS1, for Resistance-related KinaSe1) and At3g57720. These two genes encode putative protein kinases that belong to a cluster of putative kinases (spanning At3g57700 to At3g57770; Figure 1F). Complementation of rks1-1 with a 2.4 kb genomic fragment containing RKS1 from the resistant accession Col-0 (Figure S3) led to complete restoration of resistance (Figure 3A). These results were confirmed by evaluation of bacterial growth in planta, using a procedure adapted to the biology of the bacterial pathogen Xcc (Figure 3D), showing that RKS1 is involved in the resistance to bacterial colonization, a finding particularly meaningful as Xcc is a vascular pathogen.
Fig. 2. Phenotypic analysis of insertional mutants corresponding to genes of the QRX3 locus. 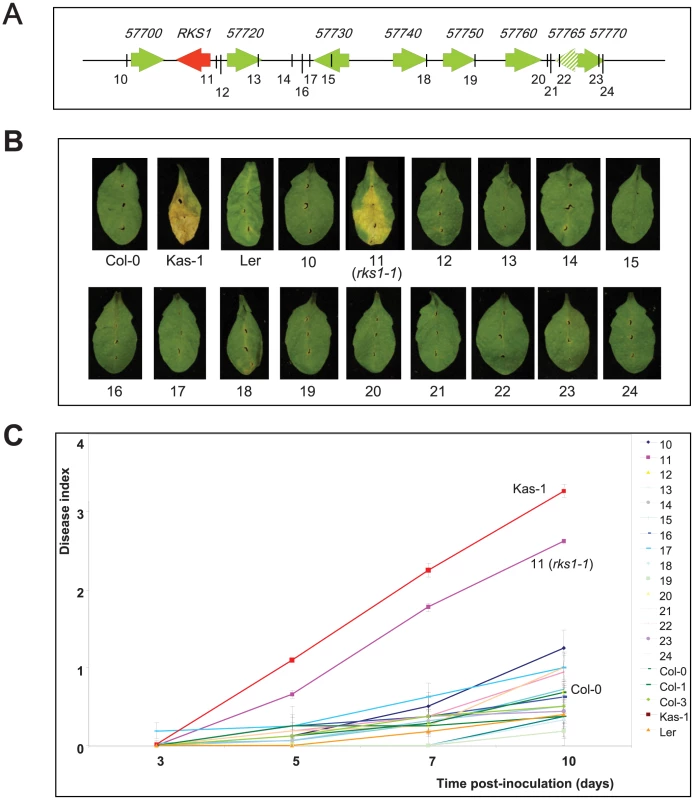
(A) Structure of the kinase cluster contained within the QRX3 locus and positions of the insertional mutations are indicated with vertical lines. (B) Disease symptoms were observed on leaves of mutant and wild-type plants, 10 days post-inoculation with a bacterial suspension adjusted to 2×108 cfu/mL. (C) Time course evaluation of disease index after inoculation with Xcc568 under the same conditions. Means and standard errors were calculated from 3–8 plants. Fig. 3. Genetic evidence that RKS1 is causal for QRX3 QTL. 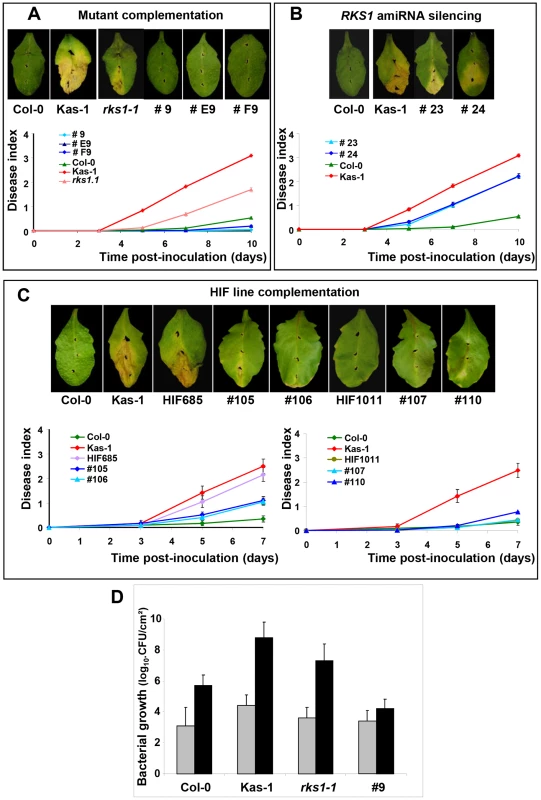
Disease symptoms were observed on leaves of wild-type plants, mutants, HIF lines or lines complemented with the RKS1 gene, at 7 (C) or 10 (A and B) days post-inoculation. Time course evaluation of our disease index was performed after inoculation with Xcc568 under the same conditions. (A) Mutant complementation (lines #9, #E9, #F9). (B) amiRNA silencing (lines #23 and #24). (C) HIF line complementation (lines #105 and #106 for the susceptible HIF (HIF685), lines #107 and #110 for the resistant HIF (HIF1011)). Means and standard errors were calculated for 16–60 plants (4–9 independent experiments). (D) Bacterial growth measurement (colony forming unit (CFU)/cm2 expressed in a log10 scale) in leaves of lines differing only by the presence of RKS1 gene (wild type (Col-0), rks1-1 mutant, and the complemented mutant line (#9)). The susceptible accession Kas-1 has been included as a positive control. Bacterial growth has been measured 0 (grey bars) and 7 (black bars) days after inoculation with Xcc strain 568. Data were collected from two independent experiments, each timepoint corresponds to 6 independent measurements, each on 3–5 individual plants (four leaves/plant). A gene silencing approach, via artificial microRNA (amiRNA), confirmed that RKS1 rather than At3g57720, is responsible for quantitative resistance to Xcc568. In particular, amiRNA lines for RKS1 in the resistant background Col-0 were susceptible to Xcc568, whereas silencing of At3g57720 had no effect on the resistant phenotype (Figure 3B, Figure S4). As expected, transformation of the original HIF (HIF685) containing the Kashmir-1 susceptible allele with the resistant (Col-0) allele of RKS1 restored a high level of resistance in the plants (Figure 3C left, Figure S3), whereas introduction of the same construct into the original resistant HIF containing the Columbia allele (HIF1011) did not lead to a change in the level of resistance (Figure 3C right, Figure S3). We also transformed the resistant accession Col-0 and resistant HIF1011 with the susceptible (Kas-1) allele of RKS1, and found a minor but significant increase in susceptibility (Figure S5). Taken together these data indicate that QRX3 can be explained by RKS1 allelic variation.
The RKS1 gene (At3g57710) encodes an atypical kinase
RKS1 encodes a predicted protein of 351 amino acids with an estimated molecular mass of 39.9 kDa (Figure 4A). BlastP analysis of the RKS1 sequence from Col-0 identified several hits corresponding to protein kinase-like or putative protein kinases. However, closer analysis of the RKS1 protein sequence suggests that RKS1 is an atypical kinase, since it lacks some critical domains in the kinase catalytic core that are essential for catalysis (Figure S6A). Only the His-Arg-Asp (HRD) motif, with the catalytic Asp residue that functions as a base acceptor to achieve proton transfer, is present in RKS1. In contrast, the glycine loop (GxGxxG), which binds and positions ATP, is not present in RKS1. Moreover, the presence of an Asp residue in the glycine loop has been reported to provide an acidic environment that inhibits ATP binding [19]. Second, the Val-Ala-Val-Lys (VAVK) motif, in which the Lys residue interacts with, anchors and orients the α and β phosphates of ATP, is not well conserved in RKS1. Third, the two tripeptide motifs in the activation segment [Asp-Phe-Gly (DFG) and Ala-Pro-Glu (APE)] are highly modified. In the DFG domain, only the Asp residue, responsible for chelating the Mg2+ ion that positions the phosphates for phosphotransfer [20] is present. Consistent with the absence of necessary domains for kinase activity, we were unable to detect any kinase activity displayed by RKS1 despite the wide range of experimental conditions tested: protein production and purification from E. coli or plant tissues; autophosphorylation assays or phosphorylation of a variety of substrates including MBP (myelin basic protein), casein and histone, in the presence of different ions (Ca2+, Mg2+, Mn2+…) and using buffers with different pH (from 5.5 to 9.5) (Figures S6B and C). Interestingly, atypical kinases (or pseudokinases) have been described as important regulators of signalling networks. Their mode of action is thought to involve acting as molecular scaffolds for assembly of multiprotein complexes or modulation of the activity of a catalytically active enzyme [21]–[22]. Sequencing of a 5kb region centered on RKS1 for Col-5 and Kas-1 revealed a single-SNP difference in the coding region resulting in an amino acid change in the activation segment relative to the catalytic kinase loop (Figure S6). Other polymorphisms were found in the 5′ and 3′ regulatory regions of RKS1 (Figure 4A). This raised the question of the level at which the Kas-1/Col-0 sequence change(s) affect(s) the observed phenotypic variation.
Fig. 4. RKS1 allelic forms and expression in susceptible and resistant accessions of Arabidopsis thaliana. 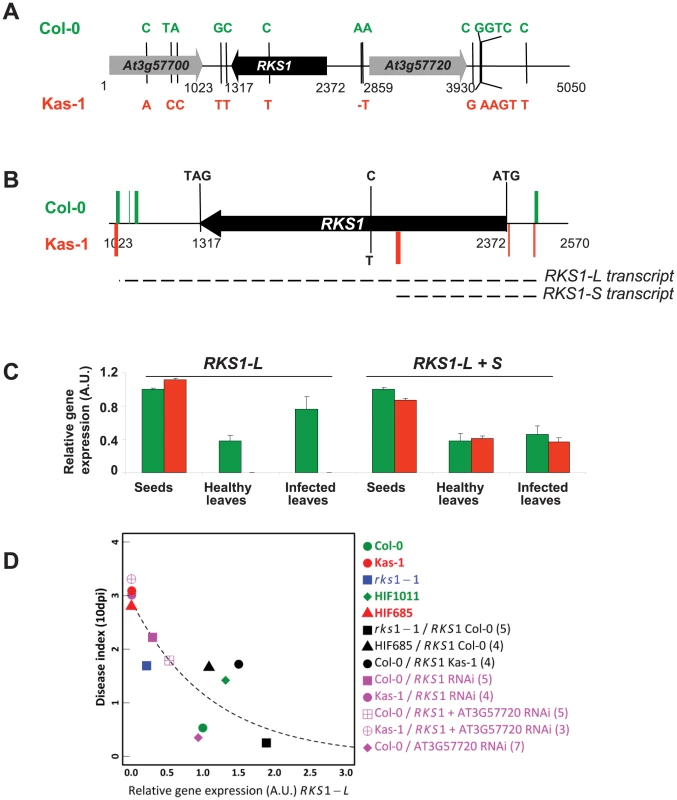
(A) Schematic representation of resistant (Col-0) and susceptible (Kas-1) RKS1 allele polymorphisms. Sequence changes in both alleles are indicated. (B) Schematic representation of the most frequent 5′ and 3′ ends of RKS1 transcripts found by 5′ and 3′ RACE experiments in resistant (Col-0, green) and susceptible (Kas-1, red) accessions. (C) RKS1 gene expression evaluated by Q-RT-PCR in germinating seeds and in leaves, healthy or inoculated with Xcc568, from the resistant accession (Col-0, green) and the susceptible accession (Kas-1, red). Different primers are used to evaluate long (L) and long+short (L+S) transcripts (Table S2) A.U.: arbitrary units. (D) Correlation between RKS1 gene expression after infection with Xcc568 and resistance phenotype. The dashed line indicates an exponentially decreasing function fitted on the median values of the 13 types of genetic line. Numbers in brackets indicate the number of representatives of each type of transgenic line. RKS1 expression in resistant and susceptible accessions
We next characterized the expression of RKS1 alleles in Kas-1 and Col-0 during development and in response to infection. Two different transcripts could be identified from 3′ and 5′ RACE experiments and cDNA sequencing. A long transcript (RKS1-L) is mainly found in the resistant accession Col-0, while the other short transcript (370 bp, RKS1-S) is associated with the susceptible accession (Kas-1) (Figures 4B and S7). While RKS1-L was expressed at high levels in germinating seeds and at lower levels in adult leaves (2.6 fold higher in seeds as compared to healthy leaves), it is differentially expressed in leaves. High levels of RKS1-L are observed in Col-0 whereas extremely low levels are detected in Kas-1 (Figure 4C)(RKS1-L expression is 235.1 fold higher in Col-0 as compared to Kas-1). Leaf infection by Xcc did not lead to a significant change in RKS1-L expression. These results suggest that RKS1 leaf expression levels might underlie the QRX3 QTL. While just a correlation, this hypothesis is reinforced by a significant nonlinear relationship between resistance and RKS1-L expression among the natural accessions Col-0 and Kas-1, HIFs, complemented and silenced lines (Figure 4D, Tables S5 and S6).
RKS1 confers broad-spectrum resistance
The species Xanthomonas campestris includes economically important bacteria with a broad range of hosts within Brassicaceae and Solanaceae, and include three main pathovars (campestris, raphani, incanae) [23]. For Xcc, 9 races (each including different strains) have been proposed by Fargier and Manceau [24] based on the reaction of different brassica species. RKS1-conferred resistance is effective not only against the strain Xcc568, belonging to race 3, but also against strains in each of four additional races (races 1, 5, 7 and 9; Figure 5A). In addition, by testing different strains from race 6, RKS1 was effective against one of 3 strains tested (Xcc4954). Interestingly, RKS1 was also found to confer resistance to additional pathovars of X. campestris: raphani, armoriaceae and incanae (Figure 5B). These results demonstrate that RKS1 confers broad spectrum resistance to Xanthomonas campestris including multiple strains belonging to most of the known races as well as other pathovars.
Fig. 5. RKS1 confers resistance to multiple strains and races and pathovars of Xcc. 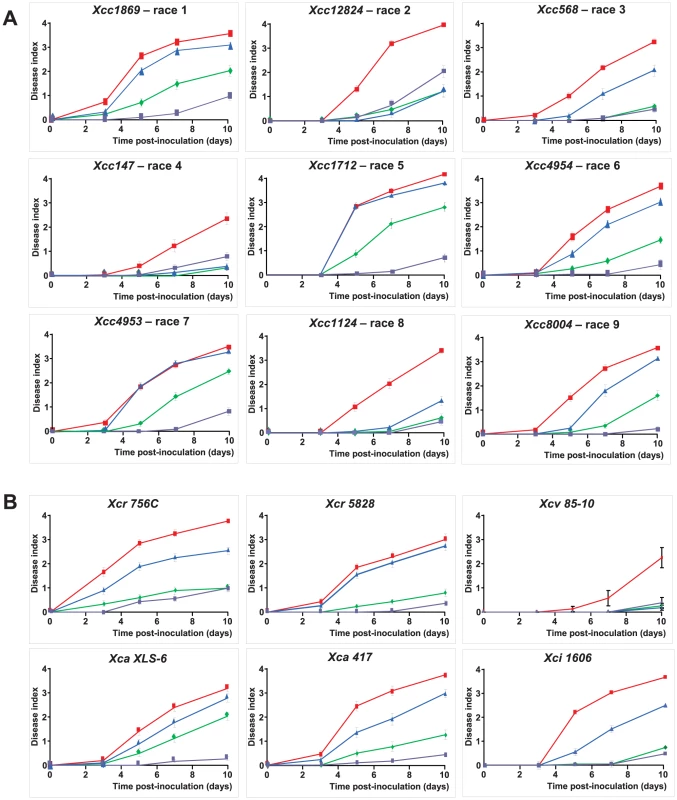
Time course evaluation of disease index in lines differing only by the presence of RKS1 gene (rks1-1 mutant (blue), complemented rks1-1 mutant (purple) and the parental lines Col-0 (green) and Kas-1 (red) after inoculation with different strains of (A) Xcc belonging to races as defined by Vicente et al. [49] and Fargier et al. [50], and of (B) Xc pathovars raphani (Xcr), armoriaceae (Xca), vesicatoria (Xcv) and incanae (Xci). Nested GWA mapping suggests an allelic series at RKS1
To investigate whether natural variation for quantitative resistance to Xcc568 is caused by RKS1 at the species level, we phenotyped 381 natural accessions that constitute a GWA (Genome Wide Association) mapping population in A. thaliana. In order to study natural variation for quantitative resistance to Xcc568 at different geographical scales, this set of accessions includes both worldwide accessions and French accessions corresponding to the French RegMap that contains two highly genetically polymorphic natural populations (MIB and TOU). We observed extensive phenotypic variation for a disease index, with a prevalence of resistant accessions (Figure 6A, Table S7) from a worldwide to a local scale (Table S7). GWA mapping revealed a unique large peak of association on chromosome 3 that overlaps with RKS1 (Figure 6B, Figures S8, S9 and S10), with the fourth most associated SNP (Single Nucleotide Polymorphism) corresponding to a single SNP in the 3′ region of RKS1 (i.e. SNP-3-21386192). However, even after splitting the accessions by the C/T polymorphism at this SNP, substantial phenotypic variation for resistance persists within both allelic groups (Figure 6A). While no obvious association peak was found within the more susceptible (S) allelic group SNP-3-21386192-T (Figure 6C), a unique peak of association was identified within the relatively resistant (R) allelic group SNP-3-21386192-C (Figure 6D, Figures S9, S10 and S11). The two most associated SNPs (i.e. SNP-3-21387206 and SNP-3-21387232) underlying this peak are located at the beginning of RKS1. Nested GWA mapping thus suggests a second susceptible allele SNP-3-21387232-T segregating within the R allelic group SNP-3-21386192-C that is also within or near RKS1. The three allelic groups explained 43.8% and 47.1% of natural variation in the local populations TOU (n = 71) and MIB (n = 50), respectively.
Fig. 6. The genetics of Xcc568 quantitative resistance at the species level identified by nested GWA mapping. 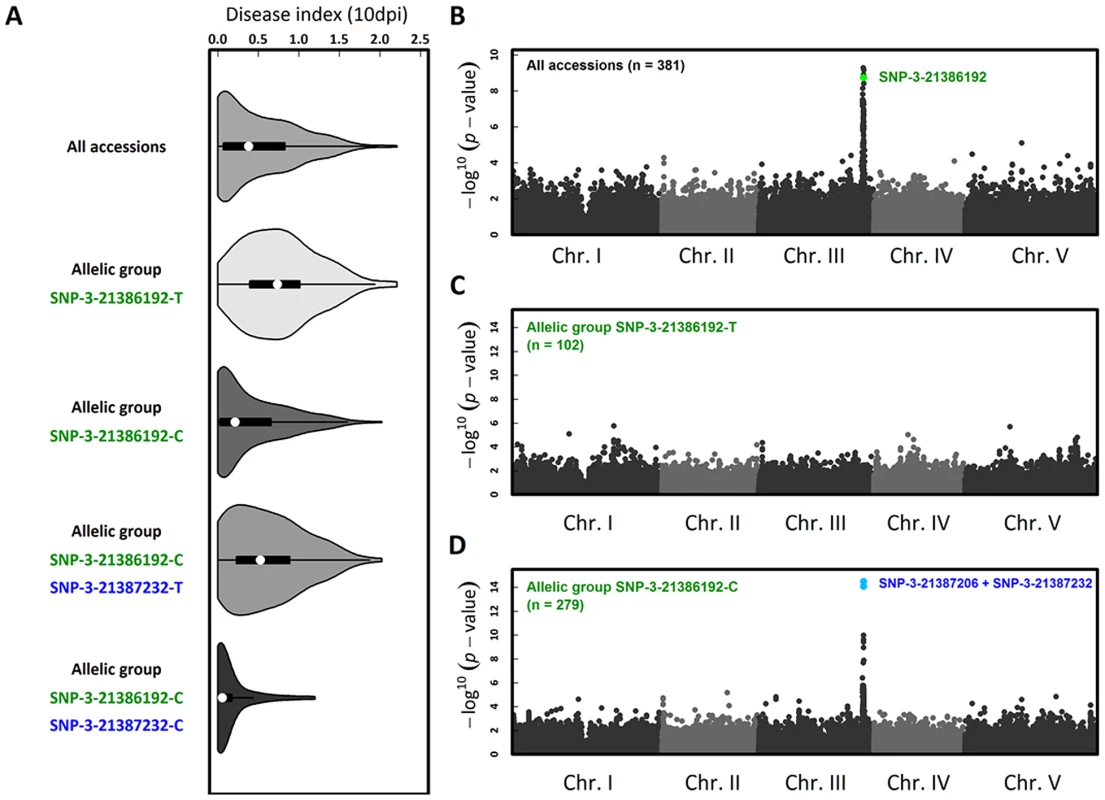
(A) Violin plots (i.e. box-and-whisker plot overlaid with a kernel density plot) of phenotypic variation of our disease index. Whole-genome scan of 214,051 SNPs for association with disease index at 10 dpi across (B) 381 accessions, (C) within the allelic group SNP-3-21386192-T and (D) within the allelic group SNP-3-21386192-C. The co-existence of long lived RKS1 haplotypes supports the ecological and evolutionary importance of RKS1
As for Col-0 and Kas-1 (Figure 4A), we sequenced a 5 kb region centered on RKS1 for 95 accessions (Table S8). Ninety-nine polymorphisms were revealed along the 5 kb region (Figure 7A). Interestingly, the region encompassing the first 186 bases of RKS1 and the intergenic region between RKS1 and At3g57720 defined two highly divergent haplotypes distinguished by 35 polymorphisms in complete linkage disequilibrium (LD, two non-synonymous and seven synonymous mutations in the first 186 bases of RKS1, 21 mutations and five indels (1 bp to 17 bp) in the intergenic region between RKS1 and At3g57720; Figures 7A and 7B). The most and less divergent haplotypes from A. lyrata and Brassica rapa (n = 51; Figure 7C and Figure S12) correspond to the resistant allele SNP-3-21387232-C and the second susceptible allele SNP-3-21387232-T detected by our approach of nested GWA mapping, respectively. Sequence analysis further revealed that our initial highly susceptible allelic group SNP-3-21386192-T (Figure 6A) is composed of two independent susceptible alleles within the R haplotype (n = 51; Figure 7C and Table S8). The first one corresponds to a stop codon at the fourth amino acid of RKS1 whereas the second one including Kas-1, contains several polymorphisms in complete LD that are scattered across the 5 kb region (Figure 4A, Table S8).
Fig. 7. Molecular evolutionary genetics. 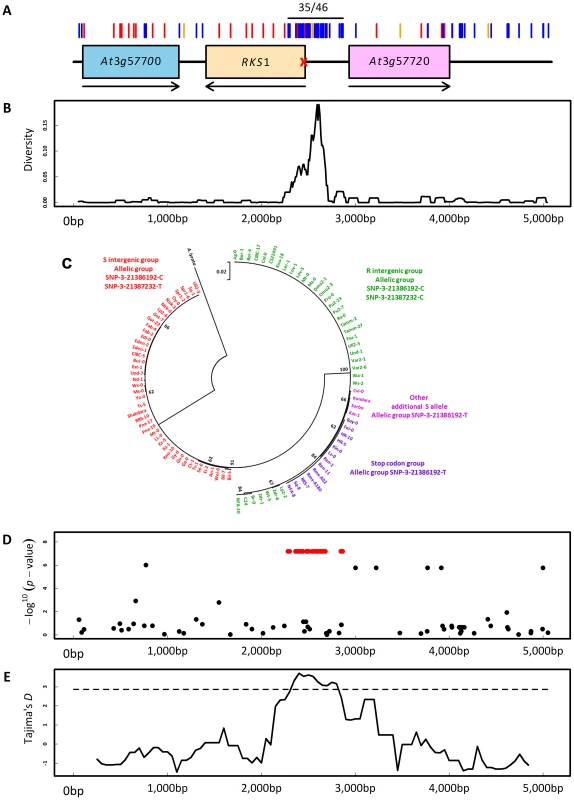
(A) Sequence diversity in the genomic region centered on RKS1. Red, blue and orange bars indicate non-synonymous mutations, silent mutations and indels, respectively. ‘35/46’ indicates the location of the 35 SNPs in complete linkage disequilibrium. The red cross indicates the position of the stop codon at the fourth amino-acid. Arrows indicate the orientation of the genes along the genome. (B) Observed nucleotide diversity between the two intergenic haplotypes. (C) Maximum Likelihood circular tree based on nucleotide variation of RKS1 and the intergenic region between RKS1 and At3g57720. (D) Scan for association with the relative gene expression of RKS1-L in the sequenced region centered on RKS1. Red points indicate the 35 SNPs in complete LD. The y-axis indicates the –log10 p-values using the EMMAX method. (E) Tajima's D values along the 5 kb genomic region centered on RKS1. The dashed line denotes the 1% significance level of an empirical distribution based on 876 short fragments obtained for the same set of accessions [63]. Consistent with the expression analysis above, our disease index was significantly negatively correlated with expression of RKS1-L among natural accessions (Figure S13, Tables S9 and S10). It was not associated with expression of At3g57720, used as a control (Figure S14). The R haplotype was strongly associated with a higher level of RKS1 expression as measured using either total mRNA or RKS1-L (Figure 7D, Figure S15). These results again suggest that regulation of RKS1 expression is a major component of quantitative resistance to Xcc568.
A signature of balancing selection acting on the two highly divergent haplotypes was suggested by significant positive values of Tajima's D (Figure 7E) and the presence of polymorphic populations across Europe (Figure S16A). In a survey of 476 accessions of the R haplotype, the second highly susceptible allele including Kas-1 is rare (n = 9) whereas the first highly susceptible allele associated with a stop codon is common throughout Western Europe (n = 162; Figure S16B).
Discussion
The understanding of the molecular mechanisms of quantitative resistance variation in general, and of quantitative resistance to biotrophic bacterial pathogens, remains largely unknown. Our work contributes to elucidating the molecular bases of quantitative resistance to the vascular pathogen Xanthomonas campestris, which is responsible for black rot, possibly the most important disease of crucifers worldwide. By analysing natural variation of resistance in Arabidopsis thaliana, we identified and cloned the gene RKS1 underlying a major QTL and conferring broad-spectrum resistance to Xc.
RKS1 confers quantitative resistance to Xcc
Resistance conferred by the RKS1 resistant allele is quantitative, as shown by different lines of evidence. First, RKS1 confers partial resistance to Xcc, and disease is only reduced (not absent) in presence of RKS1 alone. Second, levels of resistance of diverse accessions fit along a continuum measured by disease index (Figure S1). Third, RKS1 alone confers substantial resistance in the susceptible background, but stronger resistance is evident in combination with other QRX loci (other minor QTLs have been identified, each explaining 10 to 15% of the variability, Table S1). Fourth, introduction of an extra copy of the allele for susceptibility reduces resistance, as expected if the mechanism of action is quantitative (Figure S5). This latter observation is in good agreement with the intermediary level of resistance observed in heterozygous HIF lines (Figure S2), and can be explained by co-dominance of the two alleles. Molecularly, a possible explanation for this phenotype should consider, beyond the level of expression, the functionality of the two proteins, since a mutation is present in the Kas-1 coding region. The susceptible allele might act in competition with the resistant allele for interacting (directly or indirectly) with the same factors for triggering the resistance pathways, and might neutralize a part of the effect of the resistant allele. Another important feature of RKS1-conferred resistance is the absence of hypersensitive response (HR), as expected for a quantitative gene (for which the resistance response is less extreme than in the case of a gene-for-gene interaction). RKS1 is rather involved in the restriction of bacterial spread from the infection site (Figure 3), without visible cell death phenotype. This resistance mechanism may be particularly adapted to counteract the infection strategy of the vascular bacterial pathogen Xanthomonas.
As reported by Poland et al. and others [2], [25]–[26], quantitative resistance is not as well understood as qualitative resistance. It should also be mentioned that host resistance may not always be described as either qualitative or quantitative, and a gray zone may exist between these two categories [2]. For instance, it has been hypothesized that some forms of quantitative resistance may involve weak R genes [2]. Our results clearly indicate that this is not the case for RKS1. In favour of this assumption, the first cloned genes underlying QTLs are all structurally different from R-genes and do not encode LRR or similar protein motifs. They all encode diverse functions: Pi21 encodes a metal transport/detoxification protein involved in plant defense [5], LR34 encodes a putative ABC transporter [3], and Yr36, a kinase containing a putative START lipid binding domain [4]. The two recently cloned QTLs conferring resistance to nematodes, correspond for one of them, to a serine hydroxymethyltransferase [7], and for the other, to an aminoacid transporter, a α-SNAP protein and a WI12 (wound inducible domain) protein [6]. This is in agreement with the varied biological bases hypothesized for quantitative resistance [2]. RKS1, encoding an atypical kinase, represents a new category of function, putatively involved in signal transduction.
In summary, RKS1 appears to be a new quantitative gene conferring resistance to a vascular bacterial pathogen, X. campestris, for which the molecular bases were unknown, and that differs significantly in its phenotypic behaviour from R-gene mediated resistance.
RKS1 encodes an atypical kinase
Plants and animals sense invasion of potential pathogens using recognition receptors and launch cascades of immune responses that are critical for fitness and survival [27]. Protein kinases are involved in orchestrating these responses by integrating cell signaling networks. RKS1 encodes an atypical kinase, since (i) it lacks some critical domains in the kinase catalytic core that are essential for catalysis (Figure S6), and (ii) no kinase activity displayed by RKS1 could be detected despite the wide range of experimental conditions tested. This raises the question of the nature of RKS1 function: inactive pseudokinase or simply unusual active kinase? Even if three conserved kinase motifs are modified in RSK1, this does not demonstrate missing catalytic activity. Several kinases previously categorized as pseudo-kinases solely based on primary amino acid sequence analysis, have been later found to be active kinases [28]–[30]. A detailed functional study of RKS1 through different mutational analyses, including the effect of mutations in the conserved catalytic amino acids (HRD) on resistance, would be of high interest. If RKS1 reveals to be a pseudokinase, known to form complexes with active kinases [21], [22], it would be of special interest to identify members of the RKS1 complex. Interestingly, a type III effector (AvrAC) from Xcc has been recently shown to inhibit plant immunity by specifically targeting Arabidopsis BIK1 and RIPK, two receptor-like cytoplasmic kinases known to mediate immune signalling [31]. Implication of RKS1 in, or downstream of, this signalling pathway, possibly interacting with BIK1 and RIPK, constitutes an attractive hypothesis.
RKS1 confers broad-spectrum resistance to Xc
In the literature, broad-spectrum resistance refers in general to “resistance to multiple isolates/races of pathogens” [26], and quantitative resistance is generally presumed to be non-race specific, while R-mediated resistance is usually race-specific [25]. Although several lines of evidence challenge this dogma (e.g. some quantitative loci show race-specific resistance, while single resistance genes such as Rpg1 or mlo are associated with resistance to multiple isolates/races of pathogens [2]), a number of quantitative disease resistance genes/loci have been shown to confer broad-spectrum resistance. RFO1 confers resistance to three crucifer-specific fsp of Fusarium oxysporum [32], RB mediates resistance to all known races of Phythophthora infestans, the late blight pathogen [33]; the RCT1 gene from Medicago truncatula confers broad-spectrum resistance to races 1, 2 and 4 of Colletotrichum trifolii [34]; Wheat Yr36 provides high temperature-dependent QR to 8 stripe rust races (6 fully, 2 intermediary phenotype) [4]; Lr34 provides resistance to 2 rust diseases of wheat Puccinia striiformis and Puccinia triticina [3]. In this context, RKS1 belongs to this category of genes/loci, conferring resistance to different races and all pathovars of Xanthomonas campestris. However, similarly to other broad-spectrum resistance genes/QTLs, RKS1 does not confer resistance to other groups of pathogens, such as Ralstonia solanacearum (strain GMI1000), Sclerotinia sclerotiorum (S55 strain), Hyaloperonospora parasitica (strain Cala2), Erwinia carotovora (strain SCC1) or Alternaria brassicicola (data not shown). RKS1 cannot therefore be compared to genes like NDR1, EDS1 or WRKY33 that condition multiple disease resistance, and are involved in effector-triggered immunity (ETI) or PAMP triggered immunity (PTI) pathways. Although the resistance pathways involved in RKS1 mediated resistance are not known yet, we can hypothesize that this gene may be a common, downstream component of the signalling pathway triggered by numerous R genes involved in Xanthomonas resistance, controlling in part the resistance response mediated by these R genes. Alternatively, RKS1 may function in a resistance pathway depending on the recognition of a general signal/effector from Xc.
RKS1 expression level as a major component of resistance?
The phenotypic effect of a QTL might be due to one or more causal SNPs, which can be located either in the coding region or in the regulatory regions, affecting in this case the levels, timing and/or tissue-specificity of gene expression. In the case of the resistance QTLs recently cloned, the polymorphisms distinguishing the resistant allele from the susceptible one, are located in the coding region for most of them, with the exception of Yr36 which is absent in susceptible accessions, and Rhg1, for which resistance is conferred by copy number variation [4], [6].
In our study, we found a significant negative relationship between disease index and the expression of RKS1 long transcript using various transgenic lines as well as in natural accessions, suggesting an essential role of the regulation of RKS1 expression in mediating quantitative resistance to Xc. Interestingly, our nested GWA mapping approach suggests that the causal mutation(s) underlying the regulation of the expression of RKS1 long transcript might differ among alleles associated with susceptibility. First, while RKS1 total mRNA expression (assessed by RKS1-L+S quantitative RT PCR and in agreement with our characterization by RACE experiments) is similar between the resistant accession Col-0 and the susceptible accession Kas-1, RKS1 long and short transcripts are specifically expressed in leaves of the Col-0 and Kas-1, respectively (Figure 4), suggesting a post-transcriptional regulation of RKS1 in Kas-1. Different polymorphisms were found in the regulatory regions between these two accessions (Figure 4A), while only one was found in the coding region of RKS1. Second, the expression of both RKS1 total mRNA and RKS1 long transcript significantly differs among the two highly divergent haplotypes distinguished by 35 various polymorphisms (from point mutations to a 17 bp indel), in complete LD and located in the region encompassing the first 186 bases of RKS1 and the intergenic region between RKS1 and At3g57720 (Figure S13). However, either in the case of a potential post-transcriptional regulation of RKS1 in Kas-1 or in the case of RKS1 total mRNA and long transcript differential expression between two highly divergent haplotypes, the causal role of these polymorphisms is unknown. We cannot also exclude the possibility that the phenotype results from the additive effect or interaction of several polymorphisms. To get more insight into the determinism of RKS1 mediated resistance, the question of the role of the regulatory region will be addressed through complementation tests of the rks1-1 mutant with different constructs for instance. In addition, the mechanisms governing the transcriptional and post-transcriptional regulation of RKS1 through a complete and detailed functional analysis would also be the logical extension of our findings.
Alternative resistance mechanisms share common selective pressures
Like for a number of R genes [10]–[11], the ecological and evolutionary importance of RKS1 is further supported by a signature of balancing selection acting on two highly divergent haplotypes, and the presence of polymorphic populations across the native range of A. thaliana. While fitness cost of resistance is often considered as the main explanation for the maintenance of long-lived resistance polymorphisms in natural populations [35], two other non-exclusive hypotheses can be advanced to explain the coexistence of long-lived haplotypes. First, when evolutionary interactions are considered in a spatially realistic context (for example, metapopulations comprising multiple interacting populations and distance-dependent dispersal), theoretical work has shown that genetic polymorphisms in either host resistance or pathogen virulence genes can persist without the necessity of assuming differential fitness effects [36]–[38]. Second, alternative alleles may be maintained because each is beneficial in face of different enemies. For RPP13, a locus previously shown to have strong signatures of balancing selection in A. thaliana, different alleles encode different specificities to isolates of Hyaloperonospora parasitica [39]. Similarly, RPP8 contains alleles encoding resistance to alternative types of enemies [40]. In this context, no cost is expected when comparing fitness of alternative alleles in the absence of enemies.
Although identifying the opposing forces acting on RKS1 certainly deserves further investigation, the long-lived polymorphism associated with RKS1 indicates that evolutionary stable broad-spectrum resistance to Xc may be achieved in natural populations of A. thaliana. Whether such non-race-specific resistance is widespread across bacterial pathogens of A. thaliana remains an open question. Either way, because putative RKS1 orthologous have been found in other Brassica species (Figure S12), RKS1 provides an exciting opportunity for plant breeders to improve resistance to black rot in crops.
Materials and Methods
Plant materials
Plants were grown on Jiffy pots under controlled conditions [41]. To investigate natural variation of resistance to Xcc, we used 23 natural accessions of Arabidopsis thaliana including a core collection of 16 accessions (http://dbsgap.versailles.inra.fr/vnat/Fichier_collection/Rech_core_coll16.php) [42]. Then we used a set of RILs derived from the cross Kashmir (Kas-1) and Columbia gl1 (Col-5) [18], [43]. HIFs were obtained as previously described [44]–[45]. To perform Genome-Wide Association (GWA) mapping, a set of 384 natural accessions was used, including 179 worldwide accessions (WA), 188 French accessions (FA) as part of the French RegMap [46] and 17 accessions that are both WA and FA (Table S7). One hundred and twenty-one accessions from the French RegMap correspond to two natural populations from Burgundy. All the 384 natural accessions have been genotyped for 214,051 SNPs evenly spaced across the genome [46].
Bacterial material
The inoculation tests were done with the sequenced strain LMG568/ATCC33913 (Xcc568) [47] carrying the LUX operon of Photorhabdus luminescens [48] or different strains of Xcc belonging to races as defined by Vicente et al. [49] and Fargier et al. [50]. Broad spectrum resistance was estimated by inoculation tests with different pathovars of Xc, pathovars raphani (Xcr), armoriaceae (Xca), vesicatoria (Xcv) and incanae (Xci) [CIRM-CFBP collection, INRA, France]. All Xcc, Xcr, Xca, Xcv and Xci strains were grown on Kado medium [51]. Cultures of Xcc568 (LUX) were supplemented with 50 mg/mL rifampicin and 25 mg/mL kanamycin.
Phenotyping
The Col-5 x Kas-1 RIL population was evaluated by one experiment under greenhouse conditions, and two others in a growth chamber [41]. In these experiments, two blocks were designed and for each RIL two plants per block were evaluated for quantitative resistance to Xcc. For each experiment, each RIL was evaluated in a completely randomized block design.
Virulence of Xcc strains and other pathovars was tested on 28-day old plants after inoculation by piercing and scoring of the symptoms as described [52]. Each strain was tested in at least three separate experiments where Col-0 or Col-5 and Kas-1 were inoculated as controls.
In planta bacterial growth analysis (colony forming unit (CFU)/cm2 expressed in a log10 scale) was performed as described by Froidure et al. [53]. Due to the specificity of Xcc, a vascular bacteria, bacterial growth has been measured 0 and 7 days after inoculation by piercing with Xcc strain 568, at distance from the inoculation zone (at the tip of the inoculated leaves). Data were collected from two independent experiments, each time point corresponds to 6 independent measurements, each on 3–5 individual plants (four leaves/plant). At the inoculation site (basis of the inoculated leaves), bacterial growth was measured and found similar in the different lines (4.9±0.5 to 5.1±0.4 CFU/cm2 at T0; 8.9±0.5 to 9.2±0.6 CFU/cm2 at 7 dpi).
For data reported in Figure 3D, and according to parametric and non parametric tests, respectively ANOVA (df = 3; mean squares = 47.202 ; F = 53.887 ; P<0.001) and Kruskal-Wallis test (df = 3 ; Kruskal-Wallis test statistic = 34.136 ; P<0.001), the genetic background of the lines (Col-0, Kas-1, rks1-1 and complemented line) explained significantly (81%) the phenotypic variation observed at 7 dpi. The genetic background effect of lines analyzed by pairwise comparisons (Dwass-Steel-Chritchlow - Fligner test) was also significant with a p-value below 0.001 for all pairs, except for the couple mutant rks1-1 and Kas-1 with a marginal p-value of 0.051.
Genome-wide association mapping
An experiment with 1,760 plants was set up according to a completely randomized design involving four experimental blocks, each block being an independent randomization of one replicate per accession. Infected plants were placed in plastic mini-greenhouses, including two control accessions, Col-5 (resistant) and Kas-1 (susceptible), in the same positions within each mini-greenhouse. Mini greenhouses were placed in phytotrons (22°C, 9 h photoperiod, 100% humidity).
QTL fine mapping
QTL fine mapping was performed by developing HIFs of the RIL900, and by screening the individuals with the T04109, R30025 and nga6 markers that flank the QRX3 QTL. One individual, called HIF900-1 and heterozygous for these markers, was selfed. Three hundreds and seventy-six lines were screened with 12 additional markers located within the QRX3 interval of 7.41 Mb (Figure 1D). The region was then reduced to 4.6 Mb localized between MS004 and nga6 markers. To further refine the position of QRX3, 38 lines showing recombination events between these markers were phenotyped and screened for all markers available in a 4.6 Mb interval (Table S2). QRX3 was narrowed down to a 955.9 kb interval delimited by markers MSAT005 and MSAT015 (Figure 1E). Among these 38 recombinant lines, the HIF 900-1-368 was selected and its progeny was tested (1408 lines and 2 markers). QRX3 was mapped to a 44.9 kb region delimited by markers 3_57670 and Indel1bis (Figure 1E and Table S3). This region contains 17 predicted Open Reading Frames (ORFs) (Figure 1F).
Constructs and plant transformation
The plasmids used in this study were constructed with the Gateway technology (GW; Invitrogen) [53]. For complementation assays, genomic fragments containing an 811 bp non-coding region upstream of RKS1, RKS1 coding sequence (1056 bp) and 491 bp downstream of RKS1 were amplified by PCR using Col-0 genomic DNA with attB1-QFbis_57700 and attB2-QRter_57720 primers. The corresponding entry vector was recombined with the pAM-PAT-GW destination vector [54] to generate the complementation binary plasmid. amiRNAs for At3g57710 and At3g57720, alone and in combination (Table S2), were designed in the Col-0 background using the WMD online tool (http://wmd.weigelworld.org/) and the pRS300 vector as first PCR template.
Kinase activity assays
For construction, expression and purification of His-, GST - and MBP-tagged proteins in E. coli, RKS1 genes from Col-0 or Kas-1 were amplified from Arabidopsis genomic DNA using the attB1F-710 and attB2R-710 primers (Table S2). For production of RKS1 proteins in E. coli, three different tags were used to rule out the possibility that a given tag (and/or the protocol used for protein purification) may interfere with enzymatic activity. 3xHA-, GST-6xHis - and 6xHis-MBP-tagged proteins were generated by recombination of the corresponding pENTR constructs with pTH19-GW - 6His-3HA vector, pGEX-GST-GW-6His or pDEST-6-His-MBP-GW destination vectors, respectively. These constructs were introduced into the Rosetta strain of E. coli. Protein expression was induced and RKS1 proteins purified following standard protocols. For production of RKS1 proteins in N. benthamiana, corresponding pENTR constructs were recombined with pBin19 -35S-GW-3xFlag-TEV-3xHA and introduced into the C58C1 strain of Agrobacterium tumefaciens. Transient expression in N. benthamiana leaves was performed as described previously [53]. Soluble proteins were extracted according to Oh et al. [55] and RKS1 proteins affinity purified using anti-HA affinity matrix (Roche). Immunoprecipitated proteins were washed four times with extraction buffer and treated with TEV protease (Invitrogen) (0.1 U/µl) for 2 h at 16°C. For enzymatic assays, RKS1 autophosphorylation activity was tested as described [56]. In these assays, the kinase domain of the Arabidopsis BRI1 protein was used as a positive control and displayed kinase activity [55]. In the same conditions, the phosphorylation of histone (Sigma), casein (Sigma) or bovine Myelin Basic Protein (MBP, Upstate biotechnology, USA) as substrate was tested. Finally, in-gel kinase assays using casein, histone and MBP as substrate were performed following previously described protocols [19]–[20].
RNA isolation and Q-RT-PCR
Leaves from RNA extraction and Q-RT-PCR analysis were performed as described [53] using leaves from healthy plants or inoculated with the Xcc568 strain (28 hpi) [52]. A gene (At2g28390, SAND family) whose expression has been shown to be extremely stable under different physiological conditions [57] was used as a control. Average ΔCp was calculated from 4 independent experiments with 3 individual plants (3 leaves/plant). Data are expressed as fold induction of each point as compared to the wild type. RNA isolation from Arabidopsis seeds imbibed for 16 h was performed using a protocol adapted from http://cotton genome center.ucdavis.edu/protocols/RNA.
In order to study the relationship between disease index and expression level of RKS1 (or At3g57720) in different transgenic lines and natural accessions, linear and non-linear regressions were fitted using the ‘lm’ and ‘nls’ functions implemented in the R environment, respectively [58]. Model selection was based on a difference of three points in Akaike's information criterion (AIC), Bayesian information criterion (BIC) and AICc.
RACE assays
The 5′ and the 3′ ends of RKS1 mRNA accumulating in leaves were determined using the GeneRacer™ kit (Invitrogen, France) according to manufacturers' instructions. RNA from Col-0 and Kas-1 healthy and infected leaves (28 hpi) was prepared [53]. Products of three consecutive PCRs using primers shown in Table S2 were cloned in pGEM-T Easy vector (Promega Corporation) and sequenced.
Statistical analysis
For QTL mapping, data were analyzed for each block and each experiment. Adjusted means of disease scores (LSmeans) of RILs in blocks were estimated from variance analysis (ANOVA). Broad sense heritabilities (H2) were estimated from the mean square (MS) of ANOVA using the formula adapted from Gallais [59]. Variance analysis of in planta bacterial growth data was performed using PROC GLM of SAS with random effects. QTL analysis was done using the R-qtl package [60].
For GWA mapping, the following general linear model was used to analyze disease index (GLM procedure in SAS9.1, SAS Institute Inc., Cary, North Carolina, USA):
Where ‘μ’ is the overall mean; ‘block’ accounts for differences among the four experimental blocks; ‘accession’ corresponds to the 384 natural accessions; covCol-5 and covKas-1 are covariates accounting for mini-greenhouse effects; and ‘ε’ is the residual term. Normality of the residuals was not improved by transformation of the data. Least-square mean (LSmean) was obtained for each natural accession and was subsequently used for GWA mapping analyses. All the 384 accessions have been genotyped for 214,051 SNPs. In order to fine-map genomic regions associated with natural disease index variation, we ran a Wilcoxon rank-sum test [61] and a mixed-model approach implemented in the software EMMAX (Efficient Mixed-Model Association eXpedited [62]. The latter model includes a genetic kinship matrix as a covariate to control for population structure. The percentage of quantitative disease resistance explained by the three allelic groups detected by nested GWA mapping was estimated in two polymorphic natural populations MIB and TOU (Table S7).Sequencing of natural accessions and molecular evolutionary genetics analysis
Ninety-five natural accessions already sequenced for 876 short fragments of ∼500 bp [63] were sequenced for a 5,111 bp region centered on At3g57710 (i.e. RKS1), using five pairs of primers described in Table S2. Genbank accession numbers for the 5,111 bp sequences produced in this study are KF363545–KF363829.
The average number of nucleotide substitutions per site between the two intergenic haplotypes and the Tajima test were computed across the 5,085 bp region (window size = 100 bp, step size = 10 bp; window size = 500 bp, step = 50 bp, respectively) [64]. Note that nucleotide diversity and Tajima's D were estimated using nucleotide and insertion/deletion (indel) polymorphism data, the latter coded as single characters.
Supporting Information
Zdroje
1. JonesJD, DanglJL (2006) The plant immune system. Nature 444 : 323–329.
2. PolandJA, Balint-KurtiPJ, WisserRJ, PrattRC, NelsonRJ (2009) Shades of gray: the world of quantitative disease resistance. Trends Plant Sci 14 : 21–29.
3. KrattingerSG, LagudahES, SpielmeyerW, SinghRP, Huerta-EspinoJ, et al. (2009) A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 323 : 1360–1363.
4. FuD, UauyC, DistelfeldA, BlechiA, EpsteinL, et al. (2009) A kinase-START gene confers temperature-dependent resistance to wheat stripe rust. Science 323 : 1357–1360.
5. FukuokaS, SakaN, KogaH, OnoK, ShimizuT, et al. (2009) Loss of function of a proline-containing protein confers durable disease resistance in rice. Science 325 : 998–1001.
6. CookDE, LeeTG, GuoX, MelitoS, WangK, et al. (2012) Copy number variation of multiple genes at Rhg1 mediates nematode resistance in soybean. Science 338 : 1206–1209.
7. LiuS, KandothPK, WarrenSD, YeckelG, HeinzR, et al. (2012) A soybean cyst nematode resistance gene points to a new mechanism of plant resistance to pathogens. Nature 492 : 256–260.
8. KroymannJ, DonnerhackeS, SchnabelrauchD, Mitchell-OldsT (2003) Evolutionary dynamics of an Arabidopsis insect resistance quantitative trait locus. Proc Natl Acad Sci U S A 100 : 14587–14592.
9. TodescoM, BalasubramanianS, HuTT, TrawMB, HortonM, et al. (2010) Natural allelic variation underlying a major fitness trade-off in Arabidopsis thaliana. Nature 465 : 632–636.
10. BergelsonJ, KreitmanM, StahlEA, TianD (2001) Evolutionary dynamics of plant R-genes. Science 292 : 2281–2285.
11. BakkerEG, ToomajianC, KreitmanM, BergelsonJ (2006) A genome-wide survey of R gene polymorphisms in Arabidopsis. Plant Cell 18 : 1803–1818.
12. WilliamsPH (1980) Black rot: a continuing threat to world crucifers. Plant Disease 64 : 736–742.
13. KniskernJM, TrawMB, BergelsonJ (2007) Salicylic acid and jasmonic acid signaling defense pathways reduce natural bacterial diversity on Arabidopsis thaliana. Mol Plant Microbe Interact 20 : 1512–1522.
14. TsujiJ, SomervilleSC, HammerschmidtR (1991) Identification of a gene in Arabidopsis thaliana that controls resistance to Xanthomonas campestris pv. campestris. Physiol Mol Plant Pathol 38 : 57–65.
15. LummerzheimM, de OliveiraD, CastresanaC, MiguensFC, LouzadaE, et al. (1993) Identification of compatible and incompatible interactions between Arabidopsis thaliana and Xanthomonas campestris pv. campestris and characterization of the hypersensitive response. Mol Plant Microbe Interact 6 : 532–544.
16. BuellCR, SomervilleSC (1997) Use of Arabidopsis recombinant inbred lines reveals a monogenic and a novel digenic resistance mechanism to Xanthomonas campestris pv campestris. Plant J 12 : 21–29.
17. GodardF, LummerzheimM, SaindrenanP, BalaguéC, RobyD (2000) hxc2, an Arabidopsis mutant with an altered hypersensitive response to Xanthomonas campestris pv. campestris. Plant J 24 : 749–761.
18. WilsonIW, SchiffCL, HughesDE, SomervilleSC (2001) Quantitative trait loci analysis of powdery mildew disease resistance in the Arabidopsis thaliana accession kashmir-1. Genetics 158 : 1301–1309.
19. RomeisT, PiedrasP, ZhangS, KlessigDF, HirtH, JonesJD (1999) Rapid Avr9 - and Cf-9 -dependent activation of MAP kinases in tobacco cell cultures and leaves: convergence of resistance gene, elicitor, wound, and salicylate responses. Plant Cell 11 : 273–287.
20. RomeisT, PiedrasP, JonesJD (2000) Resistance gene-dependent activation of a calcium-dependent protein kinase in the plant defense response. Plant Cell 12 : 803–816.
21. ZeqirajE, van AaltenDM (2010) Pseudokinases-remnants of evolution or key allosteric regulators? Curr Opin Struct Biol 20 : 772–781.
22. BoudeauJ, Miranda-SaavedraD, BartonGJ, AlessiDR (2006) Emerging roles of pseudokinases. Trends Cell Biol 16 : 443–452.
23. VicenteJG, HolubEB (2013) Xanthomonas campestris pv. campestris (cause of black rot of crucifers) in the genomic era is still a worldwide threat to brassica crops. Mol Plant Pathol 14 : 2–18.
24. FargierE, ManceauC (2007) Pathogenicity assays restrict the species Xanthomonas campestris into three pathovars and reveal nine races within X. campestris pv. campestris. Plant Pathol 56 : 805–818.
25. KouY, WangS (2010) Broad-spectrum and durability: understanding of quantitative disease resistance. Curr Opin Plant Biol 13 : 181–185.
26. St ClairDA (2010) Quantitative resistance and quantitative resistance loci in breeding. Annu Rev Phytopathol 48 : 247–268.
27. BoudsocqM, WillmannMR, McCormackM, LeeH, ShanL, et al. (2010) Differential innate immune signalling via Ca(2+) sensor protein kinases. Nature 464 : 418–422.
28. EswaranJ, PatnaikD, FilippakopoulosP, WangF, SteinRL, et al. (2009) Structure and functional characterization of the atypical human kinase haspin. Proc Natl Acad Sci U S A 106 : 20198–20203.
29. TalyorSS, KornevAP (2010) Yet another “active” pseudokinase, Erb3. Proc Natl Acad Sci U S A 107 : 8047–8048.
30. ShiF, TelescoSE, LiuY, RadhakrishnanR, LemmonMA (2010) ErbB3/HER3 intracellular domain is competent to bind ATP and catalyze autophosphorylation. Proc Natl Acad Sci U S A 107 : 7692–7697.
31. FengF, YangF, RongW, WuX, ZhangJ, et al. (2012) A Xanthomonas uridine 5′-monophosphate transferase inhibits plant immune kinases. Nature 485 : 114–118.
32. DienerAC, AusubelFM (2005) RESISTANCE TO FUSARIUM OXYSPORUM 1, a dominant Arabidopsis disease-resistance gene, is not race specific. Genetics 171 : 305–321.
33. BhaskarPB, RaaschJA, KramerLC, NeumannP, WielgusSM, et al. (2008) Sgt1, but not Rar1, is essential for the RB-mediated broad-spectrum resistance to potato late blight. BMC Plant Biol 8 : 8.
34. YangS, GaoM, XuC, GaoJ, DeshpandeS, et al. (2008) Alfalfa benefits from Medicago truncatula: the RCT1 gene from M. truncatula confers broad-spectrum resistance to anthracnose in alfalfa. Proc Natl Acad Sci U S A 105 : 12164–12169.
35. Vila-AiubMM, NeveP, RouxF (2011) A unified approach to the estimation and interpretation of resistance costs in plants. Heredity 107 : 386–394.
36. GandonS, MichalakisY, EbertD (1996) Temporal variability and local adaptation. Trends Ecol Evol 11 : 431.
37. DamgaardC (1999) Coevolution of a plant host-pathogen gene-for-gene system in a metapopulation model without cost of resistance or cost of virulence. J Theor Biol 201 : 1–12.
38. ThrallPH, BurdonJJ, BeverJD (2002) Local adaptation in the Linum marginale-Melampsora lini host-pathogen interaction. Evolution 56 : 1340–1351.
39. Bittner-EddyPD, CruteIR, HolubEB, BeynonJL (2000) RPP13 is a simple locus in Arabidopsis thaliana for alleles that specify downy mildew resistance to different avirulence determinants in Peronospora parasitica. Plant J 21 : 177–188.
40. CooleyMB, PathiranaS, WuHJ, KachrooP, KlessigDF (2000) Members of the Arabidopsis HRT/RPP8 family of resistance genes confer resistance to both viral and oomycete pathogens. Plant Cell 12 : 663–676.
41. LacommeC, RobyD (1996) Molecular cloning of a sulfotransferase in Arabidopsis thaliana and regulation during development and in response to infection with pathogenic bacteria. Plant Mol Biol 30 : 995–1008.
42. McKhannHI, CamilleriC, BérardA, BataillonT, DavidJL, et al. (2004) Nested core collections maximizing genetic diversity in Arabidopsis thaliana. Plant J 38 : 193–202.
43. LiY, RoycewiczP, SmithE, BorevitzJO (2006) Genetics of local adaptation in the laboratory: flowering time quantitative trait loci under geographic and seasonal conditions in Arabidopsis. PLoS One 1: e105.
44. TuinstraR, EjetaG, GoldsbroughPB (1997) Heterogeneous inbred family (HIF) analysis: a method for developing near-isogenic lines that differ at quantitative trait loci. Theor Appl Genet 95 : 1005–1011.
45. LoudetO, GaudonV, TrubuilA, Daniel-VedeleF (2005) Quantitative trait loci controlling root growth and architecture in Arabidopsis thaliana confirmed by heterogeneous inbred family. Theor Appl Genet 110 : 742–753.
46. HortonMW, HancockAM, HuangYS, ToomajianC, AtwellS, et al. (2012) Genome-wide patterns of genetic variation in worldwide Arabidopsis thaliana accessions from the RegMap panel. Nat Genet 44 : 212–216.
47. da SilvaAC, FerroJA, ReinachFC, FarahCS, FurlanLR, et al. (2002) Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature 417 : 459–463.
48. WinsonMK, SwiftS, HillPJ, SimsCM, GriesmayrG, et al. (1998) Engineering the lux CDABE genes from Photorhabdus luminescens to provide a bioluminescent reporter for constitutive and promoter probe plasmids and mini-Tn5 constructs. FEMS Microbiol Lett 163 : 193–202.
49. VicenteJG, ConwayJ, RobertsSJ, TaylorJD (2001) Identification and Origin of Xanthomonas campestris pv. campestris Races and Related Pathovars. Phytopathol 91 : 492–499.
50. FargierE, Fischer-Le SauxM, ManceauC (2011) A multilocus sequence analysis of Xanthomonas campestris reveals a complex structure within crucifer-attacking pathovars of this species. Syst Appl Microbiol 34 : 156–165.
51. KadoCI, HeskettMG (1970) Selective media for isolation of Agrobacterium, Corynebacterium, Erwinia, Pseudomonas, and Xanthomonas. Phytopathol 60 : 969–976.
52. MeyerD, LauberE, RobyD, ArlatM, KrojT (2005) Optimization of pathogenicity assays to study the Arabidopsis thaliana-Xanthomonas campestris pv. campestris pathosystem. Mol Plant Pathol 6 : 327–333.
53. FroidureS, CanonneJ, DanielX, JauneauA, BrièreC, et al. (2010) AtsPLA2-alpha nuclear relocalization by the Arabidopsis transcription factor AtMYB30 leads to repression of the plant defense response. Proc Natl Acad Sci U S A 107 : 15281–15286.
54. BernouxM, TimmersT, JauneauA, BrièreC, de WitPJ, et al. (2008) RD19, an Arabidopsis cysteine protease required for RRS1-R-mediated resistance, is relocalized to the nucleus by the Ralstonia solanacearum PopP2 effector. Plant Cell 20 : 2252–2264.
55. OhMH, RayWK, HuberSC, AsaraJM, GageDA, ClouseSD (2000) Recombinant brassinosteroid insensitive 1 receptor-like kinase autophosphorylates on serine and threonine residues and phosphorylates a conserved peptide motif in vitro. Plant Physiol 124 : 751–766.
56. Klaus-HeisenD, NurissoA, Pietraszewska-BogielA, MbengueM, CamutS, et al. (2011) Structure-function similarities between a plant receptor-like kinase and the human interleukin-1 receptor-associated kinase-4. J Biol Chem 286 : 11202–11210.
57. CzechowskiT, StittM, AltmannT, UdvardiMK, ScheibleWR (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139 (1) 5–17.
58. R Development Core Team (2012) R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing.
59. Gallais A (1990) Théorie de la sélection en amélioration des plantes. Eds. Paris: Masson. 588 pp.
60. BromanKW, WuH, SenS, ChurchillGA (2003) R/qtl: QTL mapping in experimental crosses. Bioinformatics 19 : 889–890.
61. AtwellS, HuangYS, VilhjálmssonBJ, WillemsG, HortonM, et al. (2010) Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature 465 : 627–631.
62. KangHM, SulJH, ServiceSK, ZaitlenNA, KongSY, et al. (2010) Variance component model to account for sample structure in genome-wide association studies. Nat Genet 42 : 348–354.
63. NordborgM, HuTT, IshinoY, JhaveriJ, ToomajianC, et al. (2005) The pattern of polymorphism in Arabidopsis thaliana. PLoS Biol 3: e196.
64. LibradoP, RozasJ (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25 : 1451–1452.
65. HancockAM, BrachiB, FaureN, HortonMW, JarymowyczLB, et al. (2011) Adaptation to climate across the Arabidopsis thaliana genome. Science 334 : 83–86.
Štítky
Genetika Reprodukční medicína
Článek Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive SelectionČlánek Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand SkillČlánek Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage EvolvabilityČlánek Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation inČlánek Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 ModelČlánek Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period inČlánek VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival inČlánek Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2AČlánek A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding YeastČlánek Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on PhenotypeČlánek Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding SitesČlánek Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 9
-
Všechny články tohoto čísla
- The Pathway Gene Functions together with the -Dependent Isoprenoid Biosynthetic Pathway to Orchestrate Germ Cell Migration
- Take Off, Landing, and Fly Anesthesia
- Nucleosome Assembly Proteins Get SET to Defeat the Guardian of Chromosome Cohesion
- Whole-Exome Sequencing Reveals a Rapid Change in the Frequency of Rare Functional Variants in a Founding Population of Humans
- Evidence Is Evidence: An Interview with Mary-Claire King
- Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive Selection
- Convergent Transcription Induces Dynamic DNA Methylation at Loci
- Environmental Stresses Disrupt Telomere Length Homeostasis
- Ultra-Sensitive Sequencing Reveals an Age-Related Increase in Somatic Mitochondrial Mutations That Are Inconsistent with Oxidative Damage
- Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand Skill
- Genetic and Anatomical Basis of the Barrier Separating Wakefulness and Anesthetic-Induced Unresponsiveness
- The Locus, Exclusive to the Ambulacrarians, Encodes a Chromatin Insulator Binding Protein in the Sea Urchin Embryo
- Binding of NF-κB to Nucleosomes: Effect of Translational Positioning, Nucleosome Remodeling and Linker Histone H1
- Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage Evolvability
- Dynamics of DNA Methylation in Recent Human and Great Ape Evolution
- Functional Dissection of Regulatory Models Using Gene Expression Data of Deletion Mutants
- PAQR-2 Regulates Fatty Acid Desaturation during Cold Adaptation in
- N-alpha-terminal Acetylation of Histone H4 Regulates Arginine Methylation and Ribosomal DNA Silencing
- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation in
- miR-1/133a Clusters Cooperatively Specify the Cardiomyogenic Lineage by Adjustment of Myocardin Levels during Embryonic Heart Development
- Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 Model
- Genome-Wide Analysis of Genes and Their Association with Natural Variation in Drought Tolerance at Seedling Stage of L
- Deep Resequencing of GWAS Loci Identifies Rare Variants in , and That Are Associated with Ulcerative Colitis
- Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period in
- VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival in
- Analysis of Genes Reveals Redundant and Independent Functions in the Inner Ear
- Predicting the Risk of Rheumatoid Arthritis and Its Age of Onset through Modelling Genetic Risk Variants with Smoking
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
- A Shift to Organismal Stress Resistance in Programmed Cell Death Mutants
- Fragile Site Instability in Causes Loss of Heterozygosity by Mitotic Crossovers and Break-Induced Replication
- Tracking of Chromosome and Replisome Dynamics in Reveals a Novel Chromosome Arrangement
- The Condition-Dependent Transcriptional Landscape of
- Ago1 Interacts with RNA Polymerase II and Binds to the Promoters of Actively Transcribed Genes in Human Cancer Cells
- Nebula/DSCR1 Upregulation Delays Neurodegeneration and Protects against APP-Induced Axonal Transport Defects by Restoring Calcineurin and GSK-3β Signaling
- System-Wide Analysis Reveals a Complex Network of Tumor-Fibroblast Interactions Involved in Tumorigenicity
- Meta-Analysis of Genome-Wide Association Studies Identifies Six New Loci for Serum Calcium Concentrations
- and Are Required for Cellularization and Differentiation during Female Gametogenesis in
- Growth factor independent-1 Maintains Notch1-Dependent Transcriptional Programming of Lymphoid Precursors
- Whole Genome Sequencing Identifies a Deletion in Protein Phosphatase 2A That Affects Its Stability and Localization in
- An Alteration in ELMOD3, an Arl2 GTPase-Activating Protein, Is Associated with Hearing Impairment in Humans
- Genomic Identification of Founding Haplotypes Reveals the History of the Selfing Species
- Plasticity Regulators Modulate Specific Root Traits in Discrete Nitrogen Environments
- The IDD14, IDD15, and IDD16 Cooperatively Regulate Lateral Organ Morphogenesis and Gravitropism by Promoting Auxin Biosynthesis and Transport
- Stochastic Loss of Silencing of the Imprinted Allele, in a Mouse Model and Humans with Prader-Willi Syndrome, Has Functional Consequences
- The Prefoldin Complex Regulates Chromatin Dynamics during Transcription Elongation
- PKA Controls Calcium Influx into Motor Neurons during a Rhythmic Behavior
- A Pre-mRNA-Splicing Factor Is Required for RNA-Directed DNA Methylation in
- Cell-Type Specific Features of Circular RNA Expression
- The Uve1 Endonuclease Is Regulated by the White Collar Complex to Protect from UV Damage
- An Atypical Kinase under Balancing Selection Confers Broad-Spectrum Disease Resistance in Arabidopsis
- Genome-Wide Mutation Avalanches Induced in Diploid Yeast Cells by a Base Analog or an APOBEC Deaminase
- Extensive Divergence of Transcription Factor Binding in Embryos with Highly Conserved Gene Expression
- Bi-modal Distribution of the Second Messenger c-di-GMP Controls Cell Fate and Asymmetry during the Cell Cycle
- Cell Interactions and Patterned Intercalations Shape and Link Epithelial Tubes in
- A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding Yeast
- The Genome and Development-Dependent Transcriptomes of : A Window into Fungal Evolution
- SKN-1/Nrf, A New Unfolded Protein Response Factor?
- The Highly Prolific Phenotype of Lacaune Sheep Is Associated with an Ectopic Expression of the Gene within the Ovary
- Fusion of Large-Scale Genomic Knowledge and Frequency Data Computationally Prioritizes Variants in Epilepsy
- IL-17 Attenuates Degradation of ARE-mRNAs by Changing the Cooperation between AU-Binding Proteins and microRNA16
- An Enhancer Element Harboring Variants Associated with Systemic Lupus Erythematosus Engages the Promoter to Influence A20 Expression
- Genome Analysis of a Transmissible Lineage of Reveals Pathoadaptive Mutations and Distinct Evolutionary Paths of Hypermutators
- Type I-E CRISPR-Cas Systems Discriminate Target from Non-Target DNA through Base Pairing-Independent PAM Recognition
- Divergent Transcriptional Regulatory Logic at the Intersection of Tissue Growth and Developmental Patterning
- MEIOB Targets Single-Strand DNA and Is Necessary for Meiotic Recombination
- Transmission of Hypervirulence Traits via Sexual Reproduction within and between Lineages of the Human Fungal Pathogen
- Integration of the Unfolded Protein and Oxidative Stress Responses through SKN-1/Nrf
- Guanine Holes Are Prominent Targets for Mutation in Cancer and Inherited Disease
- Regulation of the Boundaries of Accessible Chromatin
- Natural Genetic Transformation Generates a Population of Merodiploids in
- Ablating Adult Neurogenesis in the Rat Has No Effect on Spatial Processing: Evidence from a Novel Pharmacogenetic Model
- Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on Phenotype
- The Molecular Mechanism of a -Regulatory Adaptation in Yeast
- Phenotypic and Genetic Consequences of Protein Damage
- Recent Acquisition of by Baka Pygmies
- Fatty Acid Taste Signals through the PLC Pathway in Sugar-Sensing Neurons
- A Critical Role for PDGFRα Signaling in Medial Nasal Process Development
- Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding Sites
- Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
- dTULP, the Homolog of Tubby, Regulates Transient Receptor Potential Channel Localization in Cilia
- Widespread Dysregulation of Peptide Hormone Release in Mice Lacking Adaptor Protein AP-3
- , a Direct Transcriptional Target, Modulates T-Box Factor Activity in Orofacial Clefting
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Recent Acquisition of by Baka Pygmies
- The Condition-Dependent Transcriptional Landscape of
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání




