-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaNatural Genetic Transformation Generates a Population of Merodiploids in
Partial duplication of genetic material is prevalent in eukaryotes and provides potential for evolution of new traits. Prokaryotes, which are generally haploid in nature, can evolve new genes by partial chromosome duplication, known as merodiploidy. Little is known about merodiploid formation during genetic exchange processes, although merodiploids have been serendipitously observed in early studies of bacterial transformation. Natural bacterial transformation involves internalization of exogenous donor DNA and its subsequent integration into the recipient genome by homology. It contributes to the remarkable plasticity of the human pathogen Streptococcus pneumoniae through intra and interspecies genetic exchange. We report that lethal cassette transformation produced merodiploids possessing both intact and cassette-inactivated copies of the essential target gene, bordered by repeats (R) corresponding to incomplete copies of IS861. We show that merodiploidy is transiently stimulated by transformation, and only requires uptake of a ∼3-kb DNA fragment partly repeated in the chromosome. We propose and validate a model for merodiploid formation, providing evidence that tandem-duplication (TD) formation involves unequal crossing-over resulting from alternative pairing and interchromatid integration of R. This unequal crossing-over produces a chromosome dimer, resolution of which generates a chromosome with the TD and an abortive chromosome lacking the duplicated region. We document occurrence of TDs ranging from ∼100 to ∼900 kb in size at various chromosomal locations, including by self-transformation (transformation with recipient chromosomal DNA). We show that self-transformation produces a population containing many different merodiploid cells. Merodiploidy provides opportunities for evolution of new genetic traits via alteration of duplicated genes, unrestricted by functional selective pressure. Transient stimulation of a varied population of merodiploids by transformation, which can be triggered by stresses such as antibiotic treatment in S. pneumoniae, reinforces the plasticity potential of this bacterium and transformable species generally.
Published in the journal: . PLoS Genet 9(9): e32767. doi:10.1371/journal.pgen.1003819
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003819Summary
Partial duplication of genetic material is prevalent in eukaryotes and provides potential for evolution of new traits. Prokaryotes, which are generally haploid in nature, can evolve new genes by partial chromosome duplication, known as merodiploidy. Little is known about merodiploid formation during genetic exchange processes, although merodiploids have been serendipitously observed in early studies of bacterial transformation. Natural bacterial transformation involves internalization of exogenous donor DNA and its subsequent integration into the recipient genome by homology. It contributes to the remarkable plasticity of the human pathogen Streptococcus pneumoniae through intra and interspecies genetic exchange. We report that lethal cassette transformation produced merodiploids possessing both intact and cassette-inactivated copies of the essential target gene, bordered by repeats (R) corresponding to incomplete copies of IS861. We show that merodiploidy is transiently stimulated by transformation, and only requires uptake of a ∼3-kb DNA fragment partly repeated in the chromosome. We propose and validate a model for merodiploid formation, providing evidence that tandem-duplication (TD) formation involves unequal crossing-over resulting from alternative pairing and interchromatid integration of R. This unequal crossing-over produces a chromosome dimer, resolution of which generates a chromosome with the TD and an abortive chromosome lacking the duplicated region. We document occurrence of TDs ranging from ∼100 to ∼900 kb in size at various chromosomal locations, including by self-transformation (transformation with recipient chromosomal DNA). We show that self-transformation produces a population containing many different merodiploid cells. Merodiploidy provides opportunities for evolution of new genetic traits via alteration of duplicated genes, unrestricted by functional selective pressure. Transient stimulation of a varied population of merodiploids by transformation, which can be triggered by stresses such as antibiotic treatment in S. pneumoniae, reinforces the plasticity potential of this bacterium and transformable species generally.
Introduction
Partial duplications of genetic material provide potential for evolution of new traits in all kingdoms of life, with duplicated material able to evolve in the absence of functional selective pressure [1]. Such duplications are prevalent in eukaryotes, and were recently shown to be associated with susceptibility to diseases in the human population [2]. Duplications are also thought to provide genetic novelty in plants and yeast [3], [4]. Although diploidy favors gene evolution, prokaryotes are generally haploid in nature, possessing a single copy of most genetic material. Despite this, partial chromosomal duplications, known as merodiploids, allow prokaryotes to evolve new genes. Historically, merodiploidy in bacteria was first suggested in studies of binary encapsulation created by genetic transformation in Haemophilus influenzae [5] and Streptococcus pneumoniae [6], [7]. Subsequent studies have identified bacterial merodiploids, with various duplications uncovered in S. pneumoniae [8], [9], Bacillus subtilis [10], Escherichia coli [11] and Salmonella spp. [12], [13]. However, these studies frequently involved specific markers or chromosomal regions, and the structure and mechanisms of formation of these merodiploids have remained elusive. Extensive analysis conducted in Salmonella enterica led to the conclusion that in unselected laboratory cultures 0.005–3% of cells possess duplication of a specific gene [14], with the trade-off between their high formation rate and genetic instability resulting in a steady state after ∼30 generations [15]. However, while it is generally accepted that natural merodiploids occur at relatively high rates in bacteria [16], similar detailed information is not available for any other species.
The most common form of merodiploidy is tandem duplication (TD), where duplicated regions remain adjacent in the chromosome. TDs are generally thought to form spontaneously by homologous recombination between direct repeat sequences such as insertion sequences (IS) or rRNA sequences [17]–[19], an idea supported by the fact that mutation of the recombinase RecA abrogates >90% of TD formation in S. enterica [15]. Such homologous recombination events may involve unequal crossing over between different repeat regions of sister chromatids during replication, resulting in production of a TD with a hybrid repeat sequence separating duplicated regions [16]. However, in S. pneumoniae the site of spontaneous duplication within the capsule locus appeared to be random and sequences flanking the different duplications were unique [20]. A recent study in S. enterica also suggested that RecA-driven recombination may not be the sole mechanism for TD formation. Authors showed that most TDs formed on a replicative plasmid were dependent on an active transposase and conjugative apparatus, and suggested single-strand annealing between transposase-nicked sister chromosomes as an alternative mechanism of TD formation [21].
There is little information regarding TD formation during genetic transfer processes such as genetic transformation, conjugation and transduction. TDs formed by phage transduction have previously been reported but formation was dependent on transduction with non-natural junctions, defined as those not naturally present in the host genome, and therefore somewhat artificial [11], [12], [22]. Similarly, non-natural junctions were used to document formation of duplications during transformation in B. subtilis [23]. In the human pathogen S. pneumoniae, merodiploids spontaneously generated by transformation have been identified, with cells expressing two polysaccharide capsules isolated [6], [7]. However, the corresponding merodiploid structures have remained undefined and the mechanisms for their formation unknown.
The process of transformation involves internalization of single-stranded (ss) DNA fragments created from an exogenous double-stranded (ds) donor, and their integration into the recipient chromosome by homology. Here, we demonstrate that transformation of S. pneumoniae with homologous DNA generates TDs. Formation of these TDs does not require active transposases and is not dependent on non-natural donor junctions but only repeats present in both donor and host DNA. We establish that merodiploid formation involves alternative pairing of partly repeated (R) exogenous sequences resulting in interchromatid integration of internalized R ssDNA. These merodiploids produced by a genetic cross represent true merozygotes and occur throughout the genome. Transformation thus modulates the frequency of merodiploids in a pneumococcal population. These observations reveal a new, non-conservative facet of transformation in the major human pathogen S. pneumoniae, reinforcing the view that this species exhibits a great plasticity potential [24]. We discuss both the likelihood that the proposed model, relying on fundamental homologous recombination steps, applies to other transformable species, and the evolutionary potential offered by transformation-generated merodiploidy.
Results
A 107.4 kb merodiploid produced by lethal cassette transformation
During study of the essential pneumococcal gene codY, we identified two independent suppressing mutations allowing survival of otherwise lethal codY::trim mutant cells in the encapsulated strain D39 [25]. We attempted to recreate this genotype in laboratory strain R1502 by transformation with chromosomal DNA possessing the codY::trim cassette and the suppressing mutations. In reasonable agreement with our previous report [25], trimethoprim resistant (TrimR) transformants appeared with a frequency of ∼0.0020 relative to the rpsL41 reference marker (conferring resistance to streptomycin, SmR). However, a selected TrimR transformant (R2597) possessed neither suppressing mutation, but harboured both wildtype and codY::trim loci (Figure S1). This suggested that duplication of the codY region allowed R2597 to tolerate inactivation of one copy of codY. To identify the extent of the duplication, we sequenced the entire R2597 genome. Results showed that R2597 possesses a large duplication (Figure 1A), bordered by truncated IS861 sequences (Figure 1B and Figure S2A). This 107.4 kb duplication includes the codY region (Figure 1C). Sequence analysis revealed that total coverage of the duplicated region was lower than twice that of a strain lacking the duplication (Figure S2B) and trim sequence had lower coverage than codY sequence (Figure 1D) indicating that the codY::trim duplication was under-represented. This suggested instability of the duplicated material, which would be expected from a TD in the chromosome (Figure 2A) or a circular 107.4 kb extrachromosomal element (pop-out; Figure S3AB). Both of these should harbor an identical new junction (E-R2/1-A; Figure 2A and Figure S3AB), which was detected by PCR of R2597 (Figure 2A), sequencing of which revealed a chimeric IS861 made of parts of upstream (R1) and downstream (R2) IS861 sequences (Figure 2B).
Fig. 1. R2597 harbors a duplication of the codY chromosomal region. 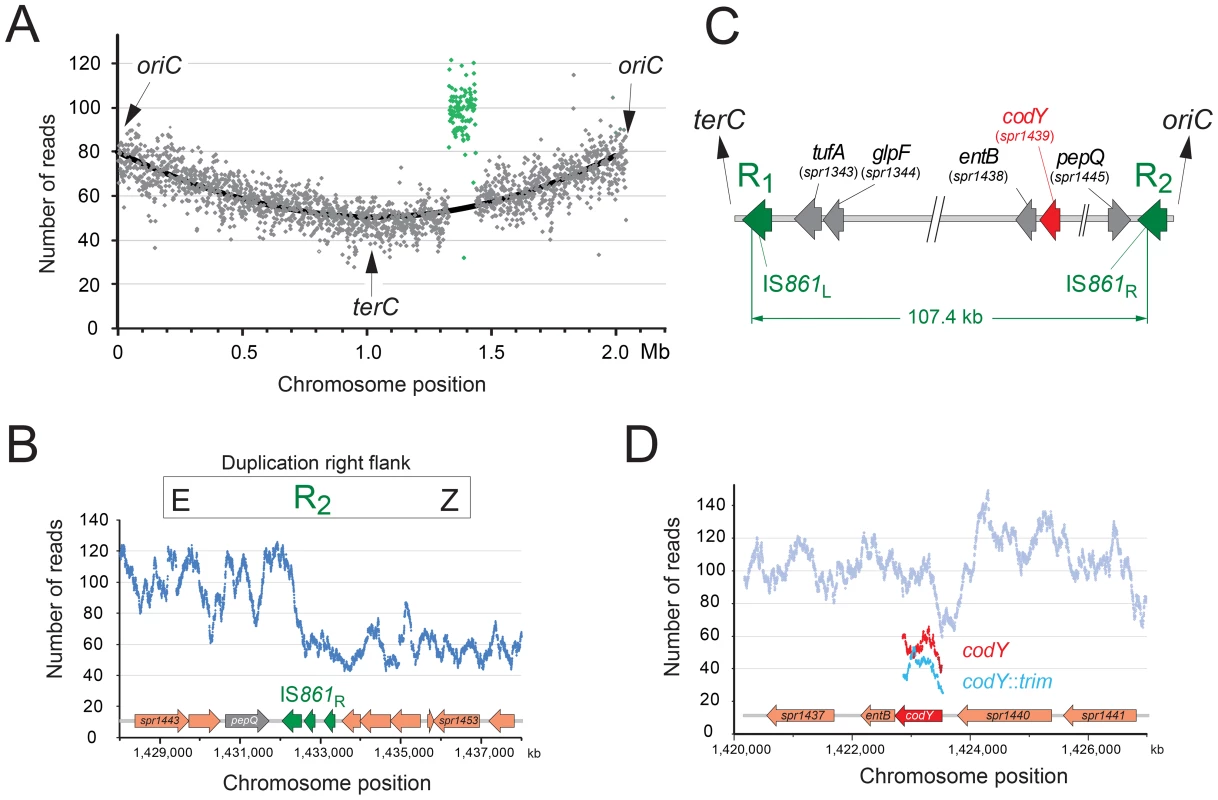
(A) Increased sequence coverage of a 107.4 kb region (green) containing codY suggests duplication. (B) Scale map of the duplication limits, bordered by IS861 sequences. (C) Coverage of the right hand limit of the duplication shows around 1/3 of the IS861R is duplicated. (D) Coverage of codY and trim sequences shows under-representation of trim. Fig. 2. The R2597 duplication is a TD in the chromosome. 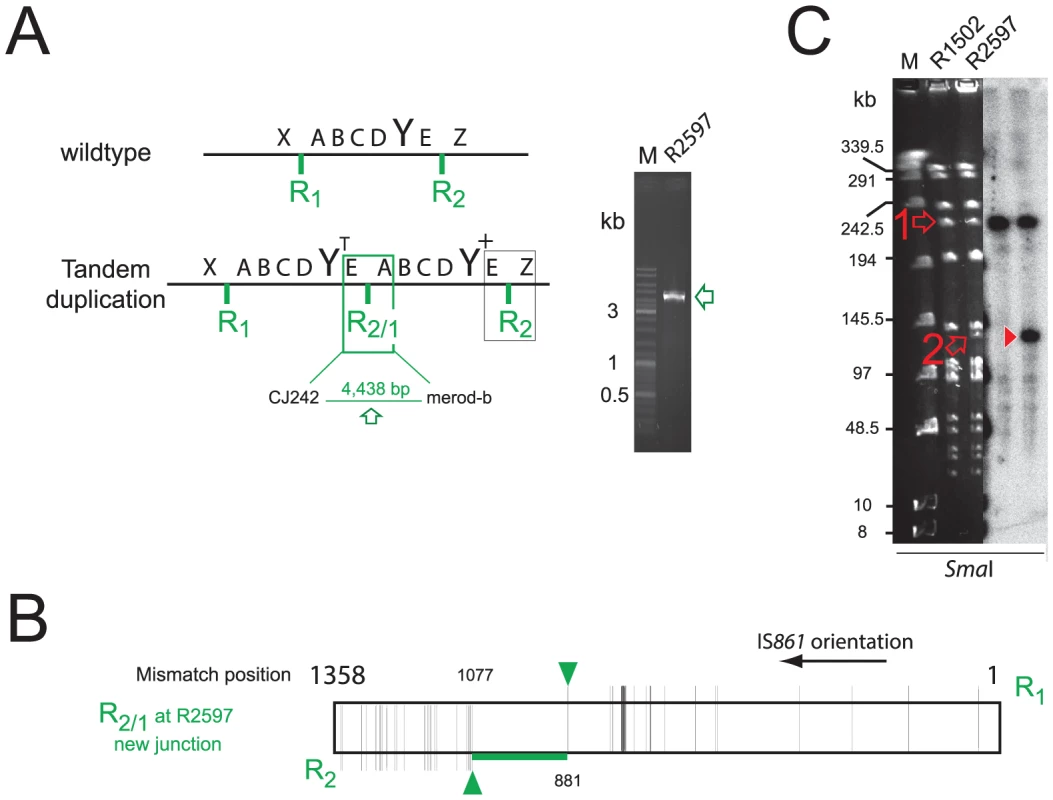
(A) Predicted R2597 chromosome structure. A–E, duplicated regions; X/Z, flanks; Y, codY; R1/R2 flanking IS861; R2/1, predicted hybrid IS861. PCR detected the predicted junction in R2597. Green rectangle, TD junction; black rectangle, sequence represented in Figure 1B. (B) Representation of the R2/1 hybrid at the TD junction in R2597 identified by sequencing. Mismatches are represented by vertical black lines, extending either above (R1) or below (R2) the diagram. Horizontal green line represents region within which R1 and R2 recombined. Green arrows represent limits of recombination between R1 and R2. (C) PFGE analysis of R2597. Open arrows, restriction fragments predicted in Table S1. Red arrowhead, merodiploid-specific fragment revealed by hybridization. Although the lanes shown were part of the same initial gel/membrane, lanes present between them have been removed to simplify comparison between lanes. To distinguish between a chromosomal TD and an extrachromosomal element, we analyzed R2597 by pulse-field gel electrophoresis (PFGE), and hybridization with codY and trim probes, as different restriction maps were predicted (Table S1). A ∼133-kb codY-positive SmaI fragment was detected in R2597 (Figure 2C). This band was predicted only for a 107.4 kb TD with a codY::trim/codY+ arrangement; trim-specific fragments predicted for this arrangement were also observed (Figure S3C). However, R2597 contained in addition a wildtype-like (224.6-kb) codY-positive SmaI fragment (Figure 2C). Together with the low band intensity of the ∼133-kb codY-positive fragment (Figure 2C), this revealed a mixed population with both non-TD (i.e., wildtype-like) and TD cells present in R2597 culture. Most likely, growth in absence of Trim prior to analyses resulted in partial loss of the duplication, explaining the under-representation observed in both genome sequencing and PFGE. We conclude that an unstable TD of 107.4 kb was created in R2597, rendering it merodiploid for codY and allowing mutation of one copy of the gene, and subsequent survival of otherwise lethal recombinants during TrimR selection.
Self-transformation stimulates merodiploid formation
R2597 was isolated following transformation with chromosomal DNA (containing codY::trim). To determine whether a sequence in the chromosomal DNA could stimulate merodiploid formation, we transformed cells with codY::trim (conferring trimethoprim resistance) or codY::spc (conferring spectinomycin resistance) PCR fragments in the presence or absence of isogenic chromosomal DNA. Co-transformation of chromosomal DNA stimulated formation of merodiploids between 6 and 8-fold (Figure 3A). PCR analysis of 10 clones recovered from the codY::trim transformation or the codY::trim plus R800 DNA transformation showed that both codY and trim sequences were present in all clones (Figure 3B). Out of 10 clones tested (5 from each transformation), 9 possessed the TD junction (Figure 3C). PFGE and hybridization analysis, and sequencing of the junction of clone 11 (R3023) confirmed the presence of a 107.4 kb TD but with a codY+/codY::trim arrangement instead of the opposite arrangement observed with R2597 (Figure 3DE, Figure S4A and S4C, and Table S1).
Fig. 3. Self-transformation generates merodiploids. 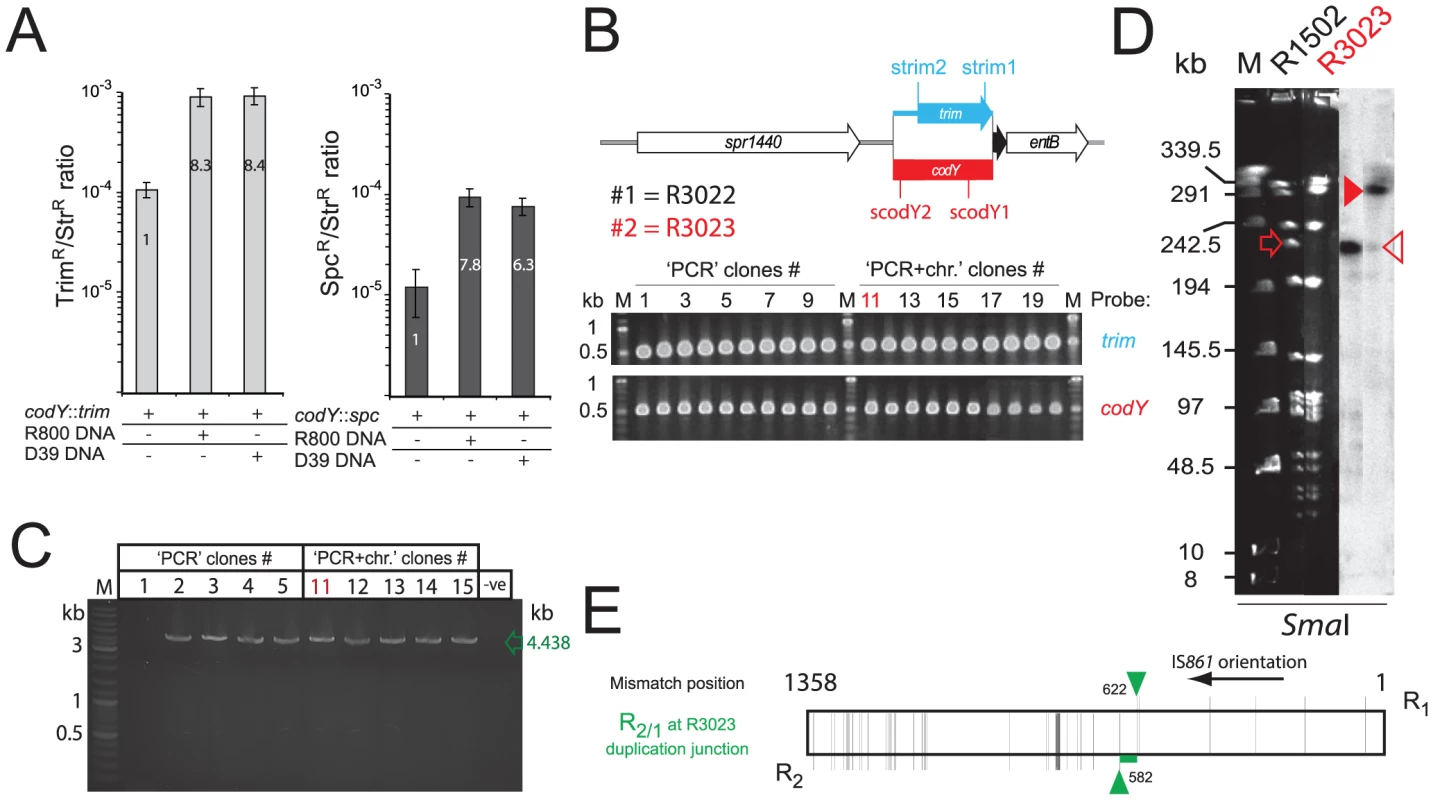
(A) Increased survival of lethal transformants after transformation with codY::trim (grey bars) or codY::spc (black bars) cassettes in presence of chromosomal DNA from R800 or D39. (B) Maps of codY+ and codY::trim loci, and detection of codY- (red, scodY1-scodY2 primers) and trim-specific (blue, strim1/strim2 primers) sequences by PCR in TrimR clones recovered from transformation with either codY::trim PCR alone (#1–10), or codY::trim PCR in presence of R800 chromosomal DNA (#11–20). (C) Detection of TD junction by PCR with CJ242/merod-b primers in clones #1–5 and #11–15 from Figure 3B. 9/10 of these clones possessed the TD junction. The one clone without the TD junction (clone #1, R3022) was found to possess an additive insertion of the codY::trim cassette in close proximity to the codY locus, creating a very small TD (See Figure S4B–E). (D) PFGE analysis of chromosomal DNA from clone #11 (R3023), digested by SmaI and hybridized with codY-specific probe. Open red arrow, expected wild-type codY ethidium bromide stained band; closed red arrowhead, R3023 codY fragment, expected for codY+/codY::trim merodiploid; open red arrowhead, wild-type codY band weakly present in R3023. Although the lanes shown were part of the same initial gel/membrane, lanes present between them have been removed to simplify comparison between lanes. (E) Representation of the R2/1 hybrid at the TD junction in R3023 identified by sequencing, layout as in Figure 2B. Analysis of clone 1 (R3022), lacking the TD junction, uncovered a small duplication of the codY locus with a codY+/codY::trim arrangement lacking flanking repeats (duplication of 3,276 bp, 1,422,105–1,425,381 on R6 genome, Figure S4B–E). Although the mechanisms of formation of such a duplication remain unclear, similar small duplications lacking flanking repeats have previously been documented in S. pneumoniae [20]. We confirmed formation of merodiploids independently of competence in pneumococcal cells by detecting the TD junction by PCR in a strain unable to develop competence (R1501; unpublished observations). These results suggested firstly that merodiploids occur at basal levels in a S. pneumoniae population, accounting for the appearance of transformants with only codY::trim or codY::spc PCR fragments as donor, and secondly that transformation transiently stimulated their formation. However, the alternative possibility that transformation was facilitating, by some ill-defined mechanism, the emergence of pre-existing merodiploids could not be formally excluded.
Transformation with R-NRf fragments triggers merodiploidy
Transformation-induced merodiploidy was investigated further with the aim of distinguishing between the two possibilities, duplication made by transformation or simple trapping of a pre-existing duplication. Since the identified TDs were flanked by IS861 repeat sequences (Figure 2A), and separated by a hybrid IS861 sequence (Figure 2B), we hypothesized that an alternative pairing event between donor DNA and host chromosome, followed by an unequal crossing over, could generate merodiploids during transformation. This would be dependent on a donor fragment containing R as well as associated non-repeated flanking sequence (NR-f). To test this, we amplified R-NRf PCR fragments representing R1-A and R2-Z (Figure 2A) and co-transformed cells with codY::trim and R1-A or R2-Z. Co-transformation increased the frequency of codY::trim ‘lethal cassette’ transformants up to 8-fold (Figure 4A) compared to transformation with codY::trim alone. All tested clones possessed the same TD, and sequencing of the junction of three of them identified three distinct R2/1 recombination junctions (Figure 4B). Identical transformation with similarly-sized PCR fragments lacking repeat sequences, AB, had no effect on frequency of lethal cassette transformants (Figure 4A). Furthermore, donor fragments containing R1 or R2 alone (i.e., without NRf) also had no effect (Figure 4C), showing that the R-NRf ‘hybrid’ structure of the donor fragment is key to merodiploid formation.
Fig. 4. R-NRf donor fragments promote merodiploid formation, allowing survival of otherwise lethal codY::trim transformants. 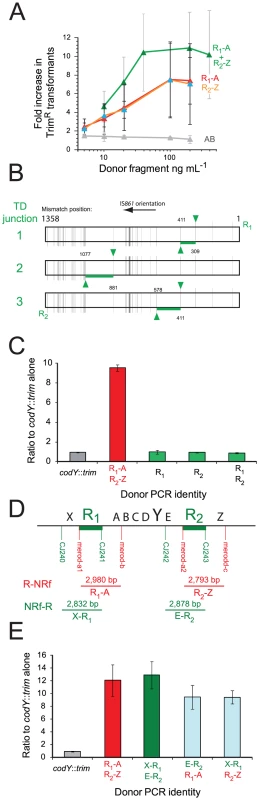
(A) Co-transformation of 100 ng mL−1 codY::trim with R1-A (red), R2-Z (orange), R1-A and R2-Z (green) or comparable non-R-NRf fragments (AB, grey). (B) R TD junction sequence of three independent clones. Layout as in Figure 2B. (C) Donor fragments with only repeats do not induce merodiploidy. DNA concentrations: 100 ng mL−1 for codY::trim cassette with 100 ng mL−1 of R1 or R2 PCR fragment; 50 ng mL−1 R1-A and 50 ng mL−1 R2-Z as positive control. PCR primers used: R1, merod-a1/CJ241; R2, merod-a2/CJ243. (D) Diagrammatic representation of the codY chromosomal region with location of primers used to produce PCR fragments assayed in transformation in combination with the codY::trim cassette (panel E). (E) Cooperativity requires two R-NRf fragments with same polarity. DNA concentrations: 100 ng mL−1 for codY::trim cassette; 50 ng mL−1 of each R-NRf fragment in each test, giving a total of 100 ng mL−1 fragment per test. Model for formation of merodiploids by transformation
Based on these observations, we elaborated a general model for merodiploid formation by transformation (Figure 5A), where alternative pairing of R-NRf donor fragments (red) leads to interchromatid integration, bridging the neosynthesized (blue) and parental (black) chromatids of a partially replicated chromosome. Alternative pairing is crucial to our model, occurring here by initial pairing of donor R1 with R2 in the host chromosome, displacing the neosynthesized strand, followed by pairing of flanking A with A on the sister chromatid (Figure 5A). Subsequent restoration of sister chromatid continuity (Figure 5B) results in unequal crossing-over, creating a chromosome dimer. Resolution of this dimer generates a merodiploid chromosome (possessing two copies of codY) and an abortive chromosome lacking codY (Figure 5D). Our results showed that R1-A and R2-Z were equally efficient at generating merodiploids (Figure 4A). We suggest that this is because R1-A can bridge chromatids associating loci 2 and 3 and R2-Z can bridge loci 1 and 4 (Figure 5A), the resulting recombinant chromosomes being indistinguishable.
Fig. 5. Model of merodiploid formation during pneumococcal transformation. 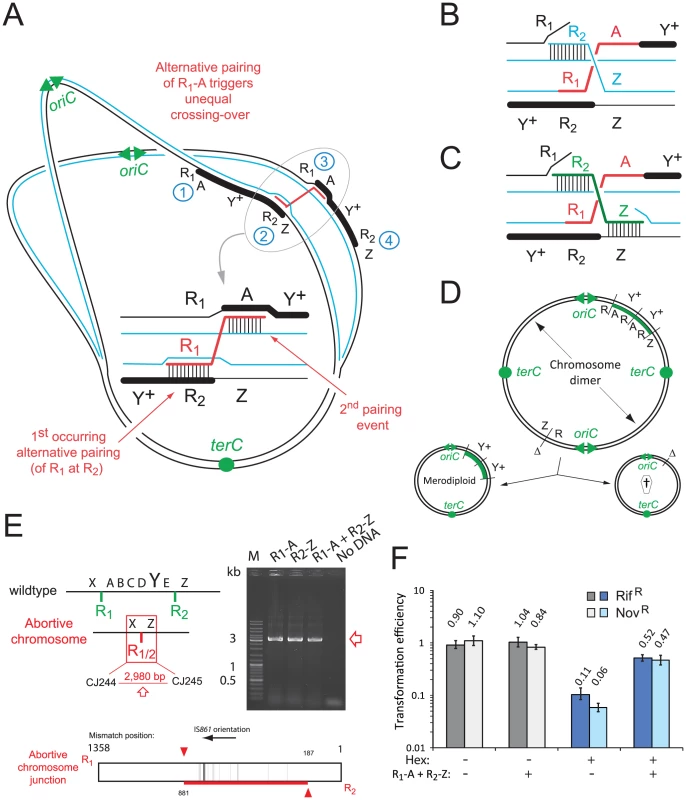
(A) Alternative pairing between R-NRf sequences triggers unequal crossing over. Letters as in Figure 1D. (B) Spontaneous restoration of complementary strand integrity. (C) Restoration of complementary strand integrity stimulated by R2-Z donor fragment. (D) Resolution of chromosome dimer to produce one tandem-duplicated chromosome and one abortive chromosome. oriC, origin of replication; terC, terminus of replication; Δ, deletion; †, abortive chromosome. (E) Predicted abortive chromosome structure. Layout as in Figure 2A. Red square; abortive chromosome junction. Detection of abortive chromosome junction by primers in Figure 3C. Diagram of R1/2 sequence at abortive chromosome junction amplified after R1-A transformation, determined by sequencing of PCR fragments. Vertical lines, R1/R2 mismatches; red horizontal line, mixed sequence .(F) Saturation of the Hex system establishes frequent alternative pairing of R-NRf fragment. Transformation efficiencies of RifR and NovR point mutations (carried by R304 chromosomal DNA) compared in hex− (R246) or hex+ (R800) recipients in the presence or absence of R-NRf PCR fragments. Transformation efficiencies normalized to SmR (rpsL41) point mutation, resistant to Hex. hex− recipient, shades of gray; hex+ recipient, shades of blue. The break in the complementary chromatid resulting from interchromatid integration of R1-A can be repaired spontaneously through invasion of the sister chromatid by the displaced R2 neosynthesized strand (blue) (Figure 5B). We also envisioned an alternative donor DNA-directed break repair mechanism involving interchromatid integration of donor R2-Z (Figure 5C). R2-Z fragment integration would require displacement of both the parental R1 strand (creating R1/2) and neosynthesized Z strand, bridging the gap between chromatids. Consistent with this model, transformation with both R1-A and R2-Z donor fragments increased the frequency of TrimR transformants by up to 10-fold, an increased efficiency compared to either fragment alone (Figure 4A). To determine whether observed cooperativity of donor fragments was dependent on polarity, we co-transformed cells with combinations of four donor fragments (R1-A, R2-Z, X-R1, E-R2; Figures 2A and 4D). Results showed that only donor fragments of the same polarity are able to cooperate to restore complementary strand integrity during merodiploid formation (Figure 4E). Thus, restoration of complementary strand integrity can occur either spontaneously or via interchromatid integration of an exogenous fragment, allowing chromosome dimer creation, and subsequent merodiploid formation (Figure 5D).
A predicted consequence of the repair of the complementary chromatid is the formation of an abortive chromosome, subsequent to the creation of the X-R1/2-Z junction (Figure 5E). We were able to detect this abortive chromosome junction by specific PCR on unselected cultures 30 minutes after transformation with R-NRf fragments (Figure 5E), showing that this junction is indeed produced. Analysis of the junction sequence produced by PCR on R1-A-transformed culture showed a long region of mixed sequence (Figure 5E), suggesting that a number of different alternative pairing events had occurred within the population of merodiploids produced.
Alternative pairing of R fragments is frequent
While sequencing of junctions provided evidence for alternative pairing of R segments (Figures 2B, 3E and 4B), the overall frequency of such events in the transformed population remained unknown. To further validate our model and obtain an estimate of this frequency, we took advantage of a previous observation that the generalized mismatch repair system (Hex) of S. pneumoniae can be saturated by excess mismatches [26], [27]. Hex operates on transformation intermediates, ejecting donor DNA from transformation heteroduplexes when 1–2 mismatches are present [28], [29]. A larger number of mismatches can result in saturation of Hex, rendering it unable to repair a single mismatch elsewhere in the genome. The R1 and R2 fragments share 96% sequence identity, with the 4% divergence a target for Hex if alternative pairing occurs. We therefore investigated the ability of these fragments to saturate Hex by comparing transformation efficiency of two Hex sensitive point mutations, rif23 and nov1 (conferring resistance to rifampicin, RifR, and to novobiocin, NovR, respectively) on chromosomal donor DNA with or without R-NRf fragments. Addition of R-NRf fragments resulted in a net increase in RifR and NovR transformation efficiencies in a hex+ recipient, indicative of escape from mismatch repair (Figure 5F). We conclude that Hex was saturated in >50% of cells, establishing that alternative pairing is a frequent event when a culture is transformed with a DNA fragment harboring a region repeated in the recipient chromosome.
Transformation does not select pre-existing structures but creates de novo merodiploids
Detection of abortive chromosome junctions on unselected cells shortly after transformation (Figure 5E) supported the idea of merodiploid formation by transformation irrespective of lethal selection. To provide further evidence for this, we detected TD and abortive chromosome junctions by PCR on transformed cultures at different times post-transformation with R-NRf fragments. The TD junction was readily detected in the transformed population as early as 10 min after DNA internalization (Figure 6A). Such timing is consistent with previous data indicating completion of ssDNA integration within ∼10 min after uptake [30]. This result confirmed that R-NRf fragments promote merodiploidy in the absence of the codY::trim lethal cassette and therefore independently of selection. Since self-transformation generated the same merodiploids as R-NRf transformation (Figure 3), TD junctions were expected to be present in cultures transformed with self DNA in the absence of selection. PCR on self-transformed cultures confirmed their presence (Figure 6C) supporting the view that transformation creates de novo merodiploids.
Fig. 6. Transformation does not select pre-existing structures but creates de novo merodiploids. 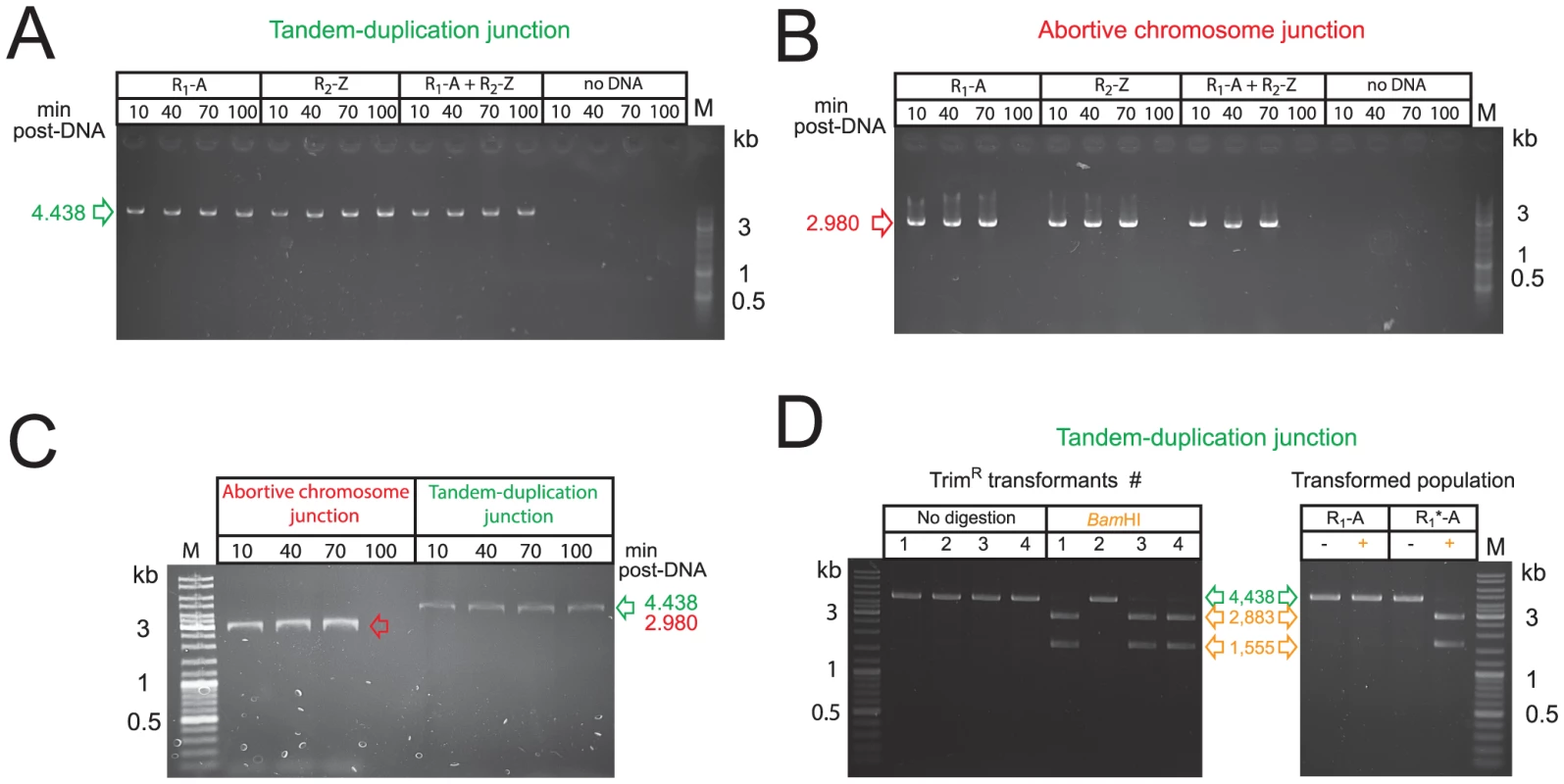
(A) Detection of tandem-duplication junction by PCR on cultures at different time points after transformation with R-NRf fragments alone. (B) Detection of abortive chromosome junction on same transformed cultures as in panel A. (C) Detection of tandem-duplication and abortive chromosome junctions by PCR on cultures at different time points after self-transformation. (D) Integration of donor R1*-A fragment during merodiploid formation. Right panel shows BamHI restriction of TD junction PCRs recovered from TrimR clones transformed with codY::trim and R1*-A PCR fragments. Undigested PCR shown in green, products of BamHI digestion shown in orange. Left panel shows BamHI digestion of TD junction PCR amplified from population transformed with R1-A or R1*-A. −, undigested PCR fragment; +, BamHI-digested PCR fragment. The abortive chromosome was detected by PCR on the same transformed cultures (Figure 6B). Similarly to TD junctions, abortive chromosome junctions were readily detected in cultures transformed with self DNA in the absence of selection (Figure 6C). Interestingly, the abortive chromosome junctions disappeared 100 minutes after uptake, presumably due to cell division, and loss of abortive cells, whereas TD junctions persisted (Figure 6B and 6C).
To definitely prove that the detected junctions were created by transformation and specifically depend upon physical integration of the exogenous R-NRf donor fragment, we mutated three bases at the 5′ end of R in R1-A to insert a restriction site (BamHI), creating R1*-A. We transformed cells with codY::trim and R1*-A PCR fragments, and recovered TrimR clones. TD junction PCRs of 3/4 tested clones were sensitive to BamHI restriction, confirming integration of donor R1*-A (Figure 6D, left panel). The single insensitive PCR fragment was presumably derived from a spontaneous TD. Furthermore, PCR fragments amplified from cultures transformed with R1*-A alone were sensitive to BamHI restriction, confirming integration of the R1*-A donor fragment in the absence of any selection (Figure 6D, right panel). Taken together, these results demonstrate that transformation does not simply select pre-existing merodiploids from a population but actively promotes formation of de novo merodiploids.
Transformation creates merodiploids throughout the genome
In order to determine whether creation of merodiploids by transformation is a general phenomenon, we investigated the triggering of merodiploidy at different chromosomal sites. We identified two other pairs of truncated IS repeat regions, each sharing >90% homology and separated respectively by 144.1 (site #2) and 210.6 (site #3) kb (Figure 7A and Figure S5AB). Transformation with R-NRf fragments corresponding to sites #2 and #3 triggered appropriate merodiploid formation, detected by PCR specific for the TD junctions (i.e., E-R4/3-A and E-R6/5-A; Figure S5CD) (Figure 7B). Sequencing of these junctions identified R4/3 and R6/5 mixed recombination sites (Figure S5EF).
Fig. 7. Transformation-triggered merodiploidy is a general process. 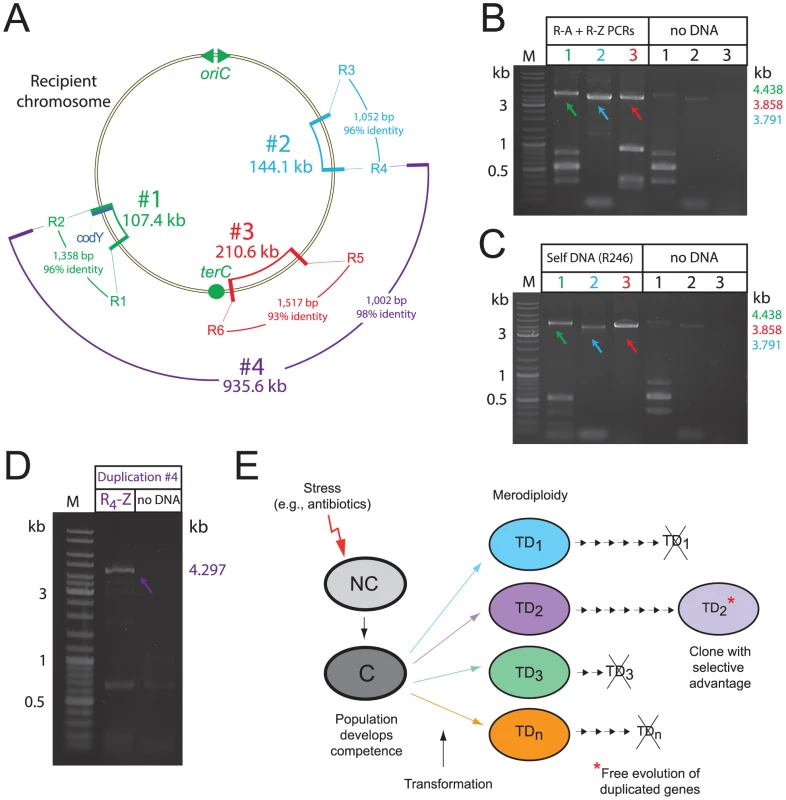
(A) Chromosomal location of generated TDs. Size, identity and homology of repeat sequences noted. (B) PCR detection of TD junctions in the transformed population 40 min after uptake of appropriate R-NRf fragments (R1-A+R2-Z; R3-A+R4-Z; R5-A+R6-Z). (C) PCR detection of TD junctions in the transformed population 40 min after self-transformation. PCRs carried out on same culture of transformed cells. Control PCRs without transforming DNA on the same cells as in Figure 5B. (D) Detection of TD #4 junction by PCR with primers in Figure S5G, PCR carried out on population transformed with R4-Z fragment. (E) Schematic representation of proposed evolutionary potential of merodiploidy triggered by transformation. Stresses such as antibiotics can induce competence in S. pneumoniae and other species [48]. NC, non-competent; C, competent; TD, tandem-duplication where numbers represent different merodiploids. Small arrows indicate subsequent cell divisions. Crossed-out TD indicates loss of duplication due to intrinsic instability. Self-transformation should also provide the recipient population with the R-NRf fragments required to produce any of the TDs shown in Figure 7A. PCR detection of junctions specific for site #1-3 TDs on the same self-transformed cells harvested 40 minutes after uptake of isogenic chromosomal DNA confirmed that all three TDs were created within the same transformed population (Figure 7C). We conclude that self-transformation is capable of simultaneously producing a large variety of merodiploids in a population.
Finally, to determine whether formation of a much larger merodiploid could be stimulated by transformation, we transformed cells with the R4-Z donor fragment, and selected TrimR transformants. R4 shares 98% identity with R2 over 1002 bp, and alternative pairing of donor R4 with chromosomal R2 should promote formation of a 935.6 kb merodiploid, duplicating codY and allowing insertion of codY::trim (Figure 7A). R4-Z increased transformation efficiency of codY::trim PCR 3-fold, suggesting merodiploid formation (data not shown). This was confirmed by transformation with only R4-Z and detection of the specific TD junction (E-R2/4-A; Figure S5G) by PCR on transformed culture (Figure 7D). Sequencing of this TD junction confirmed R2/4 recombination (Figure S5H). These results demonstrate that transformation-induced merodiploidy is a general process which can occur at many chromosomal locations, and that over 40% of the pneumococcal genome can be duplicated as the result of the uptake and processing of a single short R-NRf ssDNA fragment.
Discussion
Starting from a case study, the characterization of a merodiploid transformant obtained by mutating the essential pneumococcal gene codY [25], we provide evidence that merodiploidy promoted by self-transformation is a general phenomenon in the pneumococcus. Merodiploid formation relies on transformation with a donor DNA fragment consisting of R, a segment repeated in the recipient chromosome, flanked by NRf, its non-repeated flank. The mechanistic model proposed and validated in this study relies on alternative pairing of R (i.e., pairing at a secondary chromosomal copy) allowing subsequent homologous NRf pairing on the sister chromatid, which leads to interchromatid integration of the R-NRf exogenous fragment (Figure 5A–C). The resulting unequal crossing-over simultaneously creates a TD and generates a chromosome dimer, which upon resolution produces a chromosome with the TD and an abortive chromosome lacking the duplicated region (Figure 5D). Supporting this model, transformation with R-NRf fragments promoted predictable merodiploid formation (Figure 4A), with TD and abortive chromosome junctions identified (Figures 4B, 5E and 6). Furthermore, evidence for frequent alternative pairing of R was obtained (Figure 5F) and specific chromosomal integration of donor R was confirmed (Figure 6D).
Underlying mechanisms of merodiploid formation by transformation
Alternative pairing of R is a crucial step of our model (Figure 5A). RecA-directed homology search being random, R presumably has an equal chance of pairing with each chromosomal repeat and our data show that alternative pairing is frequent, occurring in >50% of cells (Figure 5F). Whilst pairing initiating at the ‘normal’ site (defined by its NRf) would result in RecA-driven synapsis extending into the NRf region, alternative pairing of R occurring first allows NRf to pair with its homologue in the sister chromatid, the second key feature of our model (Figure 5A). Such pairing is presumably facilitated by three-dimensional homology search [31] involving intersegmental contact sampling catalyzed by RecA [32]. Alternative pairing leading to interchromatid integration introduces a break in the complementary chromatid (Figure 5B). Restoration of complementary strand integrity, required to recover a double-stranded chromosome structure, can occur through alternative pairing and interchromatid integration of a second independently taken up R-NRf fragment (Figures 5C and 4A).
Restoration of complementary strand integrity can also occur spontaneously (Figure 5B). Owing to the conservation in nature of annealing of ssDNA displaced by recombinase-mediated strand exchange [33], annealing of the displaced R recipient strand to the sister chromatid R single strand by RecO could readily take place. Alternatively, this annealing could be catalyzed by the transformation-dedicated RecA loader, DprA, which has this ability [34]. Restoration of strand continuity produces a chromosome dimer which, upon resolution, generates both a merodiploid and an abortive chromosome (Figure 5D). Dimer resolution prior to division of the transformant could involve either site-specific recombination [35] or homologous recombination. In any case, merodiploidy triggered by transformation is a complex process associating both ssDNA integration (as a trigger) and subsequent dsDNA processing mechanisms. Formed by recombination during a genetic exchange process, these merodiploids are thus true merozygotes, defined as bacterial cells containing a second copy of part of the chromosome.
A single short donor fragment triggers >900 kb-long duplications
Remarkably, alternative pairing of a donor fragment as short as ∼3 kb triggers duplication of long chromosomal regions, ranging from the 107.4 kb TD observed in the codY::trim merodiploid R2597 (Figures 1 and 2) up to 935.6 kb (Figure 7A). This is reminiscent of an old observation that P1 phage transduction allowed the production of a transductant in which a duplication much larger than the quantity of P1 phage genetic material was produced [11]. The authors concluded that “…the requirements for transduction of the “condition of merodiploidy” appear to be the cotransduction of the (TD junction)…” and implicated “A mechanism whereby two recipient chromosomes interact with the transduced (junction)… to regenerate the TD …”. This outcome is in essence very similar to what we describe, although the mechanisms involved differ significantly. Merodiploid formation by transformation, involving internalization and processing of ssDNA rather than dsDNA, does not require uptake of a pre-existing TD junction but only a small R-NRf fragment. Interchromatid integration of this fragment is sufficient to trigger a cascade of recombination/repair events, ultimately leading to production of a merodiploid chromosome with a long TD. This process potentially occurs at many places in the pneumococcal chromosome, as demonstrated in Figure 7.
Efficiency of merodiploid formation by transformation
The frequency of duplications in a growing bacterial population is considered to range in Escherichia coli and Salmonella typhimurium from >10−2 to ∼10−4 [16], [17], and 0.005–3% of cells in an unselected laboratory culture of Salmonella enterica were estimated to contain duplication of a specified gene [14]. In S. pneumoniae, transformation frequencies with codY::trim PCR alone (Figure 3A) suggest that ∼0.05% of the recipient cells already possessed a codY duplication. We showed that 4 out of 5 transformants tested had the same TD junction as the transformation-generated merodiploids (Figure 3C). S. pneumoniae thus has basal levels of merodiploidy in noncompetent cells, and transformation can be viewed as a mechanism that transiently augments the formation of merodiploids in a population. For merodiploids generated by transformation with a R-NRf fragment, a rough estimate of the expected frequency of codY::trim transformants, assuming that every alternative pairing event promoted merodiploid formation (i.e., that each cell could accept the lethal cassette), indicates that the observed frequency is between 100 and 1000-fold lower than this expectation. This could result from a failure to restore complementary strand integrity and/or a reduced probability of interchromatid interactions, hence of interchromatid pairing of NRf, due to nucleoid organization and/or choreography [36]. Nevertheless, the isolation of the R2597 merodiploid after transformation with chromosomal DNA carrying the lethal codY::trim cassette and two independent suppressors, rather than a transformant having acquired the two suppressors, indicates that merodiploid formation is more frequent than simultaneous transformation by two independent mutations.
Merodiploidy and mutation of essential genes
The initial merodiploid in this study was identified via transformation with chromosomal DNA containing a lethal cassette, mutating the essential codY gene [25]. We have shown that both pre-existing and transformation-generated merodiploids allowed tolerance of the otherwise lethal codY::trim cassette. These observations show that the study of essential pneumococcal genes through inactivation by transformation carries its own potential drawback, as merodiploidy can obscure the results. This was illustrated when the clpX gene, encoding part of the Clp protease in S. pneumoniae, was shown to be an essential gene [37] after initial publication of a clpX mutant strain [38]. Authors suggested that mutation of clpX occurred in a merodiploid cell which maintained wild-type and mutated copies of the gene [37]. Coupled with our observations that a basal level of spontaneous merodiploid formation exists within a population and that transformation with self DNA can transiently increase the formation of merodiploids, these results suggest that great care should be taken when attempting to mutate genes with important roles in pneumococcal physiology, and that rigorous validation of successful mutation is essential.
Evolutionary potential of transformation-triggered merodiploidy
We have demonstrated that pneumococcal transformation increases the formation of merodiploids. It is thus particularly relevant that competence for genetic transformation is induced in response to antibiotics [39], constituting a pneumococcal SOS substitute [30]. This provides the pneumococcus with maximum potential for plasticity, including merodiploid formation, at a time when adaptation is crucial for survival. Interestingly, the increase in merodiploidy via transformation does not require the presence of non-self exogenous DNA but readily occurs with chromosomal DNA that could be released by daughter cells in the culture. Thus, the occurrence of fratricide within pneumococcal cultures [40] could provide the competent cells encountering adverse conditions with a transiently increased adaptive potential subsequent to transformation-generated gene duplication. The production of merodiploids by self-transformation thus constitutes a new, non-conservative facet of pneumococcal transformation in the sense that it creates novel junctions (the TD junction). In addition, the resulting gene duplications, allowing mutation of duplicated material without the constraints of selective pressure, are likely to be of great evolution potential [16]. We conclude that merodiploidy stimulated by transformation produces a wide variety of merodiploids within a population, maximising the adaptive potential of the transformed population in response to conditions of stress (Figure 7E).
Merodiploidy triggered by self-transformation, a mechanism shared by all transformable species?
Our validated model for merodiploid formation relies on canonical homologous recombination and repair steps. These are likely to be shared by any organism. This is supported by the fact that the vast majority of duplications (>90%) are RecA-dependent [15] and none of the steps leading to merodiploidy during pneumococcal transformation differ from those documented in E. coli or Salmonella species [11], [14], [17]. In light of the conservation of the transformation machinery, including the presence of the transformation-dedicated RecA loader DprA in transformable species [34], [41], it is likely that merodiploidy can be triggered by transformation in most species. The previous findings that chromosomal integration of foreign DNA linked, on one side, to a piece of DNA homologous to the recipient chromosome occurred with similar characteristics in species as phylogenetically distant as S. pneumoniae, Acinetobacter sp. and Pseudomonas stutzeri [42]–[44] further support the view that basic transformation mechanisms are conserved. It is therefore quite likely that merodiploidy promoted by self-transformation is a transient plasticity mechanism shared by all transformable species.
Materials and Methods
Bacterial strains, culture and transformation conditions
S. pneumoniae strains and primers are described in Table S2. CSP-induced transformation was performed as described previously [45], using precompetent cells treated at 37°C for 10 min with synthetic CSP1 (100 ng mL−1). After addition of transforming DNA, cells were incubated for 20 min at 30°C. Transformants were selected by plating on CAT-agar supplemented with 4% horse blood, followed by challenge with a 10 mL overlay containing kanamycin (250 µg mL−1), Nov (4 µg mL−1), Rif (2 µg mL−1), spectinomycin (100 µg mL−1), Sm (200 µg mL−1) or Trim (20 µg mL−1), after phenotypic expression for 120 min at 37°C.
Specifics of experiments for merodiploid formation with R-NRf PCR fragments, for creation and detection of IS861 TD and AC junctions, for detection of other TD junctions and for self-transformation are described in Text S1.
Whole-genome sequencing of D39 and D39ΔcodY
Roche 454 FLX whole genome sequencing was performed by LGC Genomics (Berlin, Germany) using genomic DNA isolated from mid-log cultures by the Genomic DNA kit (Qiagen). For each strain, a single read library and a 3-kb span paired-end library were generated and sequenced according to Roche standard protocols. A total of 451,127 reads (96,405 of which contained paired ends passing quality filtering) were obtained for R1502 (59-fold coverage), and 367,697 reads with 137,475 paired ends were obtained for R2597 (64-fold coverage).
Data from the sequencing runs were mapped to the reference R6 strain (Acc.no.: NC_003098) using Roche GsMapper [Release 2.3 (091027_1459)], and coverage data was extracted from the alignment results. Sequence coverage was defined as the number of times any given genomic base is represented in sequence reads. The loess function as implemented in the Loess R package was used to plot a smoothed line of the coverage as a function of genomic position.
PFGE and hybridization
PFGE analysis was based on a published protocol [46], [47]. Strains were grown in THY medium until OD550 0.2, and digestions carried out with ApaI, SacII and SmaI enzymes. To create hybridization probes, codY or trim PCR fragments were amplified with scodY1/scodY2 (D39) or strim1/strim2 (TD80) primer pairs.
Supporting Information
Zdroje
1. BergthorssonU, AnderssonDI, RothJR (2007) Ohno's dilemma: evolution of new genes under continuous selection. Proc Natl Acad Sci U S A 104 : 17004–17009.
2. ConradDF, PintoD, RedonR, FeukL, GokcumenO, et al. (2010) Origins and functional impact of copy number variation in the human genome. Nature 464 : 704–712.
3. FlagelLE, WendelJF (2009) Gene duplication and evolutionary novelty in plants. New Phytol 183 : 557–564.
4. HittingerCT, CarrollSB (2007) Gene duplication and the adaptive evolution of a classic genetic switch. Nature 449 : 677–681.
5. LeidyG, HahnE, AlexanderHE (1953) In vitro production of new types of Hemophilus influenzae. J Exp Med 97 : 467–482.
6. AustrianR, BernheimerHP (1959) Simultaneous production of two capsular polysaccharides by pneumococcus. I. Properties of a pneumococcus manifesting binary capsulation. J Exp Med 110 : 571–584.
7. BernheimerHP, WermundsenIE (1969) Unstable binary capsulated transformants in pneumococcus. J Bacteriol 98 : 1073–1079.
8. KashmiriSV, HotchkissRD (1975) Evidence of tandem duplication of genes in a merodiploid region of Pneumococcal mutants resistant to sulfonamide. Genetics 81 : 21–31.
9. RavinAW, TakahashiEA (1970) Merodiploid ribosomal loci arising by transformation and mutation in pneumococcus. J Bacteriol 101 : 38–52.
10. AuditC, AnagnostopoulosC (1973) Genetic studies relating to the production of transformed clones diploid in the tryptophan region of the Bacillus subtilis genome. J Bacteriol 114 : 18–27.
11. HillCW, SchifferD, BergP (1969) Transduction of merodiploidy: induced duplication of recipient genes. J Bacteriol 99 : 274–278.
12. AndersonRP, MillerCG, RothJR (1976) Tandem duplications of the histidine operon observed following generalized transduction in Salmonella typhimurium. J Mol Biol 105 : 201–218.
13. LehnerAF, HillCW (1980) Involvement of ribosomal ribonucleic acid operons in Salmonella typhimurium chromosomal rearrangements. J Bacteriol 143 : 492–498.
14. AndersonRP, RothJR (1977) Tandem genetic duplications in phage and bacteria. Annu Rev Microbiol 31 : 473–505.
15. ReamsAB, KofoidE, SavageauM, RothJR (2010) Duplication frequency in a population of Salmonella enterica rapidly approaches steady state with or without recombination. Genetics 184 : 1077–1094.
16. AnderssonDI, HughesD (2009) Gene amplification and adaptive evolution in bacteria. Annu Rev Genet 43 : 167–195.
17. AndersonP, RothJ (1981) Spontaneous tandem genetic duplications in Salmonella typhimurium arise by unequal recombination between rRNA (rrn) cistrons. Proc Natl Acad Sci U S A 78 : 3113–3117.
18. HaackKR, RothJR (1995) Recombination between chromosomal IS200 elements supports frequent duplication formation in Salmonella typhimurium. Genetics 141 : 1245–1252.
19. FloresM, MavinguiP, PerretX, BroughtonWJ, RomeroD, et al. (2000) Prediction, identification, and artificial selection of DNA rearrangements in Rhizobium: toward a natural genomic design. Proc Natl Acad Sci U S A 97 : 9138–9143.
20. WaiteRD, StruthersJK, DowsonCG (2001) Spontaneous sequence duplication within an open reading frame of the pneumococcal type 3 capsule locus causes high-frequency phase variation. Mol Microbiol 42 : 1223–1232.
21. ReamsAB, KofoidE, KugelbergE, RothJR (2012) Multiple pathways of duplication formation with and without recombination (RecA) in Salmonella enterica. Genetics 192 : 397–415.
22. HughesKT, RothJR (1985) Directed formation of deletions and duplications using Mud(Ap, lac). Genetics 109 : 263–282.
23. NiaudetB, JanniereL, EhrlichSD (1985) Integration of linear, heterologous DNA molecules into the Bacillus subtilis chromosome: mechanism and use in induction of predictable rearrangements. J Bacteriol 163 : 111–120.
24. ClaverysJP, PrudhommeM, Mortier-BarrièreI, MartinB (2000) Adaptation to the environment: Streptococcus pneumoniae, a paradigm for recombination-mediated genetic plasticity? Mol Microbiol 35 : 251–259.
25. CaymarisS, BootsmaHJ, MartinB, HermansPWM, PrudhommeM, et al. (2010) The global nutritional regulator CodY is an essential protein in the human pathogen Streptococcus pneumoniae. Mol Microbiol 78 : 344–360.
26. GuildWR, ShoemakerNB (1974) Intracellular competition for a mismatch recognition system and marker specific rescue of transforming DNA from inactivation by ultraviolet irradiation. Mol Gen Genet 128 : 291–300.
27. HumbertO, PrudhommeM, HakenbeckR, DowsonCG, ClaverysJP (1995) Homeologous recombination and mismatch repair during transformation in Streptococcus pneumoniae: saturation of the Hex mismatch repair system. Proc Natl Acad Sci USA 92 : 9052–9056.
28. ClaverysJP, LacksSA (1986) Heteroduplex deoxyribonucleic acid base mismatch repair in bacteria. Microbiol Rev 50 : 133–165.
29. ClaverysJP, MéjeanV, GascAM, SicardAM (1983) Mismatch repair in Streptococcus pneumoniae: relationship between base mismatches and transformation efficiencies. Proc Natl Acad Sci USA 80 : 5956–5960.
30. ClaverysJP, MartinB, PolardP (2009) The genetic transformation machinery: composition, localization and mechanism. FEMS Microbiol Rev 33 : 643–656.
31. GondaDK, RaddingCM (1986) The mechanism of the search for homology promoted by recA protein. Facilitated diffusion within nucleoprotein networks. J Biol Chem 261 : 13087–13096.
32. ForgetAL, KowalczykowskiSC (2012) Single-molecule imaging of DNA pairing by RecA reveals a three-dimensional homology search. Nature 482 : 423–427.
33. SugiyamaT, KantakeN, WuY, KowalczykowskiSC (2006) Rad52-mediated DNA annealing after Rad51-mediated DNA strand exchange promotes second ssDNA capture. EMBO J 25 : 5539–5548.
34. Mortier-BarrièreI, VeltenM, DupaigneP, MirouzeN, PiétrementO, et al. (2007) A key presynaptic role in transformation for a widespread bacterial protein: DprA conveys incoming ssDNA to RecA. Cell 130 : 824–836.
35. Le BourgeoisP, BugarelM, CampoN, veran-MingotML, LabonteJ, et al. (2007) The unconventional Xer recombination machinery of Streptococci/Lactococci. PLoS Genet 3: e117.
36. Reyes-LamotheR, NicolasE, SherrattDJ (2012) Chromosome replication and segregation in bacteria. Annu Rev Genet 46 : 121–143.
37. RobertsonGT, NgWL, GilmourR, WinklerME (2003) Essentiality of clpX, but not clpP, clpL, clpC, or clpE, in Streptococcus pneumoniae R6. J Bacteriol 185 : 2961–2966.
38. RobertsonGT, NgWL, FoleyJ, GilmourR, WinklerME (2002) Global transcriptional analysis of clpP mutations of type 2 Streptococcus pneumoniae and their effects on physiology and virulence. J Bacteriol 184 : 3508–3520.
39. PrudhommeM, AttaiechL, SanchezG, MartinB, ClaverysJP (2006) Antibiotic stress induces genetic transformability in the human pathogen Streptococcus pneumoniae. Science 313 : 89–92.
40. ClaverysJP, HåvarsteinLS (2007) Cannibalism and fratricide: mechanisms and raisons d'être. Nat Rev Microbiol 5 : 219–229.
41. Quevillon-CheruelS, CampoN, MirouzeN, Mortier-BarrièreI, BrooksMA, et al. (2012) Structure-function analysis of pneumococcal DprA protein reveals that dimerization is crucial for loading RecA recombinase onto DNA during transformation. Proc Natl Acad Sci USA 109: E2466–E2475.
42. PrudhommeM, LibanteV, ClaverysJP (2002) Homologous recombination at the border: insertion-deletions and the trapping of foreign DNA in Streptococcus pneumoniae. Proc Natl Acad Sci U S A 99 : 2100–2105.
43. De VriesJ, WackernagelW (2002) Integration of foreign DNA during natural transformation of Acinetobacter sp. by homology-facilitated illegitimate recombination. Proc Natl Acad Sci U S A 99 : 2094–2099.
44. MeierP, WackernagelW (2003) Mechanisms of homology-facilitated illegitimate recombination for foreign DNA acquisition in transformable Pseudomonas stutzeri. Mol Microbiol 48 : 1107–1118.
45. MartinB, PrudhommeM, AlloingG, GranadelC, ClaverysJP (2000) Cross-regulation of competence pheromone production and export in the early control of transformation in Streptococcus pneumoniae. Mol Microbiol 38 : 867–878.
46. Le BourgeoisP, MataM, RitzenthalerP (1989) Genome comparison of Lactococcus strains by pulsed-field gel electrophoresis. FEMS Microbiol Lett 59 : 65–69.
47. Le BourgeoisP, LautierM, van denBL, GassonMJ, RitzenthalerP (1995) Physical and genetic map of the Lactococcus lactis subsp. cremoris MG1363 chromosome: comparison with that of Lactococcus lactis subsp. lactis IL 1403 reveals a large genome inversion. J Bacteriol 177 : 2840–2850.
48. CharpentierX, PolardP, ClaverysJP (2012) Induction of competence for genetic transformation by antibiotics: convergent evolution of stress responses in distant bacterial species lacking SOS? Curr Op Microbiol 15 : 1–7.
Štítky
Genetika Reprodukční medicína
Článek Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive SelectionČlánek Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand SkillČlánek Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage EvolvabilityČlánek Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation inČlánek Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 ModelČlánek Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period inČlánek VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival inČlánek Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2AČlánek A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding YeastČlánek Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on PhenotypeČlánek Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding SitesČlánek Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 9
-
Všechny články tohoto čísla
- The Pathway Gene Functions together with the -Dependent Isoprenoid Biosynthetic Pathway to Orchestrate Germ Cell Migration
- Take Off, Landing, and Fly Anesthesia
- Nucleosome Assembly Proteins Get SET to Defeat the Guardian of Chromosome Cohesion
- Whole-Exome Sequencing Reveals a Rapid Change in the Frequency of Rare Functional Variants in a Founding Population of Humans
- Evidence Is Evidence: An Interview with Mary-Claire King
- Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive Selection
- Convergent Transcription Induces Dynamic DNA Methylation at Loci
- Environmental Stresses Disrupt Telomere Length Homeostasis
- Ultra-Sensitive Sequencing Reveals an Age-Related Increase in Somatic Mitochondrial Mutations That Are Inconsistent with Oxidative Damage
- Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand Skill
- Genetic and Anatomical Basis of the Barrier Separating Wakefulness and Anesthetic-Induced Unresponsiveness
- The Locus, Exclusive to the Ambulacrarians, Encodes a Chromatin Insulator Binding Protein in the Sea Urchin Embryo
- Binding of NF-κB to Nucleosomes: Effect of Translational Positioning, Nucleosome Remodeling and Linker Histone H1
- Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage Evolvability
- Dynamics of DNA Methylation in Recent Human and Great Ape Evolution
- Functional Dissection of Regulatory Models Using Gene Expression Data of Deletion Mutants
- PAQR-2 Regulates Fatty Acid Desaturation during Cold Adaptation in
- N-alpha-terminal Acetylation of Histone H4 Regulates Arginine Methylation and Ribosomal DNA Silencing
- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation in
- miR-1/133a Clusters Cooperatively Specify the Cardiomyogenic Lineage by Adjustment of Myocardin Levels during Embryonic Heart Development
- Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 Model
- Genome-Wide Analysis of Genes and Their Association with Natural Variation in Drought Tolerance at Seedling Stage of L
- Deep Resequencing of GWAS Loci Identifies Rare Variants in , and That Are Associated with Ulcerative Colitis
- Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period in
- VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival in
- Analysis of Genes Reveals Redundant and Independent Functions in the Inner Ear
- Predicting the Risk of Rheumatoid Arthritis and Its Age of Onset through Modelling Genetic Risk Variants with Smoking
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
- A Shift to Organismal Stress Resistance in Programmed Cell Death Mutants
- Fragile Site Instability in Causes Loss of Heterozygosity by Mitotic Crossovers and Break-Induced Replication
- Tracking of Chromosome and Replisome Dynamics in Reveals a Novel Chromosome Arrangement
- The Condition-Dependent Transcriptional Landscape of
- Ago1 Interacts with RNA Polymerase II and Binds to the Promoters of Actively Transcribed Genes in Human Cancer Cells
- Nebula/DSCR1 Upregulation Delays Neurodegeneration and Protects against APP-Induced Axonal Transport Defects by Restoring Calcineurin and GSK-3β Signaling
- System-Wide Analysis Reveals a Complex Network of Tumor-Fibroblast Interactions Involved in Tumorigenicity
- Meta-Analysis of Genome-Wide Association Studies Identifies Six New Loci for Serum Calcium Concentrations
- and Are Required for Cellularization and Differentiation during Female Gametogenesis in
- Growth factor independent-1 Maintains Notch1-Dependent Transcriptional Programming of Lymphoid Precursors
- Whole Genome Sequencing Identifies a Deletion in Protein Phosphatase 2A That Affects Its Stability and Localization in
- An Alteration in ELMOD3, an Arl2 GTPase-Activating Protein, Is Associated with Hearing Impairment in Humans
- Genomic Identification of Founding Haplotypes Reveals the History of the Selfing Species
- Plasticity Regulators Modulate Specific Root Traits in Discrete Nitrogen Environments
- The IDD14, IDD15, and IDD16 Cooperatively Regulate Lateral Organ Morphogenesis and Gravitropism by Promoting Auxin Biosynthesis and Transport
- Stochastic Loss of Silencing of the Imprinted Allele, in a Mouse Model and Humans with Prader-Willi Syndrome, Has Functional Consequences
- The Prefoldin Complex Regulates Chromatin Dynamics during Transcription Elongation
- PKA Controls Calcium Influx into Motor Neurons during a Rhythmic Behavior
- A Pre-mRNA-Splicing Factor Is Required for RNA-Directed DNA Methylation in
- Cell-Type Specific Features of Circular RNA Expression
- The Uve1 Endonuclease Is Regulated by the White Collar Complex to Protect from UV Damage
- An Atypical Kinase under Balancing Selection Confers Broad-Spectrum Disease Resistance in Arabidopsis
- Genome-Wide Mutation Avalanches Induced in Diploid Yeast Cells by a Base Analog or an APOBEC Deaminase
- Extensive Divergence of Transcription Factor Binding in Embryos with Highly Conserved Gene Expression
- Bi-modal Distribution of the Second Messenger c-di-GMP Controls Cell Fate and Asymmetry during the Cell Cycle
- Cell Interactions and Patterned Intercalations Shape and Link Epithelial Tubes in
- A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding Yeast
- The Genome and Development-Dependent Transcriptomes of : A Window into Fungal Evolution
- SKN-1/Nrf, A New Unfolded Protein Response Factor?
- The Highly Prolific Phenotype of Lacaune Sheep Is Associated with an Ectopic Expression of the Gene within the Ovary
- Fusion of Large-Scale Genomic Knowledge and Frequency Data Computationally Prioritizes Variants in Epilepsy
- IL-17 Attenuates Degradation of ARE-mRNAs by Changing the Cooperation between AU-Binding Proteins and microRNA16
- An Enhancer Element Harboring Variants Associated with Systemic Lupus Erythematosus Engages the Promoter to Influence A20 Expression
- Genome Analysis of a Transmissible Lineage of Reveals Pathoadaptive Mutations and Distinct Evolutionary Paths of Hypermutators
- Type I-E CRISPR-Cas Systems Discriminate Target from Non-Target DNA through Base Pairing-Independent PAM Recognition
- Divergent Transcriptional Regulatory Logic at the Intersection of Tissue Growth and Developmental Patterning
- MEIOB Targets Single-Strand DNA and Is Necessary for Meiotic Recombination
- Transmission of Hypervirulence Traits via Sexual Reproduction within and between Lineages of the Human Fungal Pathogen
- Integration of the Unfolded Protein and Oxidative Stress Responses through SKN-1/Nrf
- Guanine Holes Are Prominent Targets for Mutation in Cancer and Inherited Disease
- Regulation of the Boundaries of Accessible Chromatin
- Natural Genetic Transformation Generates a Population of Merodiploids in
- Ablating Adult Neurogenesis in the Rat Has No Effect on Spatial Processing: Evidence from a Novel Pharmacogenetic Model
- Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on Phenotype
- The Molecular Mechanism of a -Regulatory Adaptation in Yeast
- Phenotypic and Genetic Consequences of Protein Damage
- Recent Acquisition of by Baka Pygmies
- Fatty Acid Taste Signals through the PLC Pathway in Sugar-Sensing Neurons
- A Critical Role for PDGFRα Signaling in Medial Nasal Process Development
- Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding Sites
- Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
- dTULP, the Homolog of Tubby, Regulates Transient Receptor Potential Channel Localization in Cilia
- Widespread Dysregulation of Peptide Hormone Release in Mice Lacking Adaptor Protein AP-3
- , a Direct Transcriptional Target, Modulates T-Box Factor Activity in Orofacial Clefting
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Recent Acquisition of by Baka Pygmies
- The Condition-Dependent Transcriptional Landscape of
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



