-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaTake Off, Landing, and Fly Anesthesia
article has not abstract
Published in the journal: . PLoS Genet 9(9): e32767. doi:10.1371/journal.pgen.1003788
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1003788Summary
article has not abstract
Anesthesia can be broken down into several components including unconsciousness, disconnection (unawareness of surgery and/or the environment), unresponsiveness, amnesia, and analgesia (absence of pain) [1]. Monitoring these components within clinical settings is challenging, although unresponsiveness, especially in the absence of neuromuscular blockade, or when a limb has been isolated from generalized paralysis, may be helpful. Unresponsiveness is therefore an appropriate anesthetic endpoint to study in both preclinical and clinical settings as it is well conserved across species and is assessable by observing behavior. While significant progress has been made through studies addressing molecular species [2], neural networks [3], neuroimaging [4], and electroencephalography (EEG) [5], the mechanisms of anesthesia-induced unresponsiveness, and its maintenance, remain elusive [1]. Pioneering work from Dr. Max B. Kelz's laboratory begins to shed light on the mechanisms involved in the induction into (“take off”) and emergence from (“landing”) anesthesia-induced unresponsiveness (referred to now as “anesthesia”; Figure 1).
Fig. 1. “Take off” and “landing” for fly anesthesia. 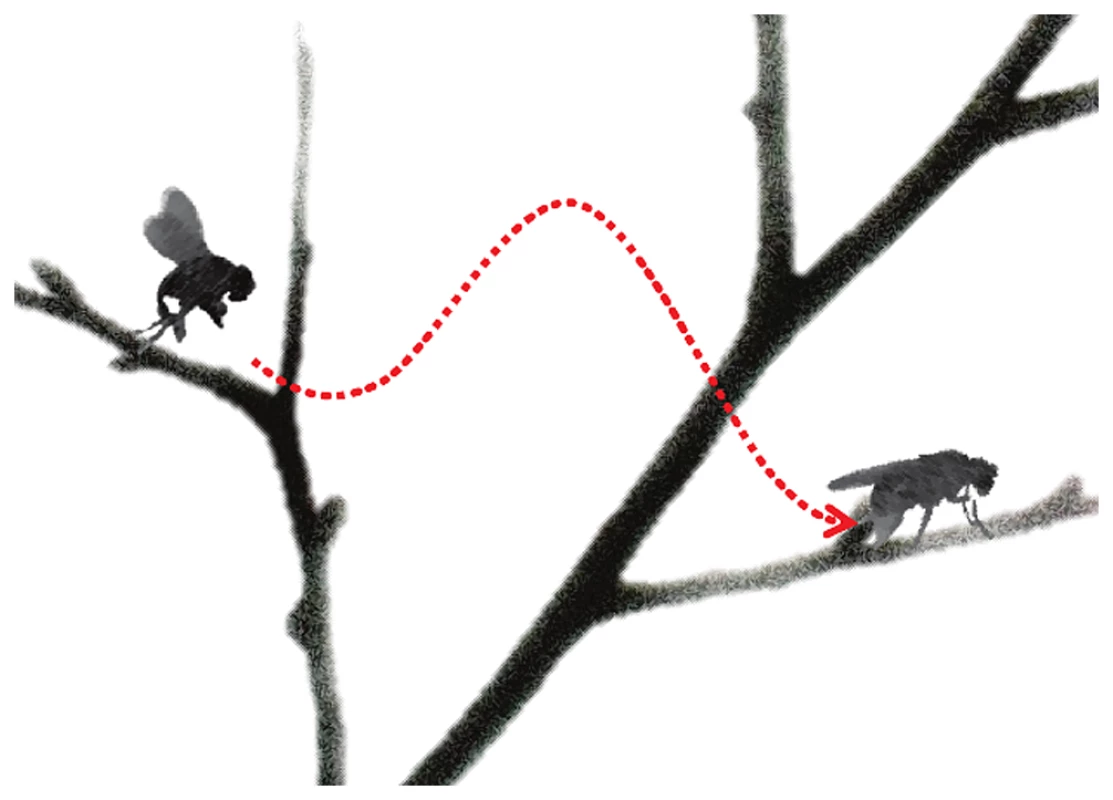
In this schematic, induction of anesthesia is represented by the fly taking off, with the height of the branch representing the drug dose. “Landing” (or emergence) from anesthesia occurs on a lower branch representing a lower drug dose than “take off”. The difference in height between the branches signifies neural inertia, the resistance in changing between anaesthetized and wakeful states. The parts of this figure are adapted from images by Antonia Foy (http://www.flickr.com/photos/antoniafoy/5542985500/) and John Tann (http://www.flickr.com/photos/31031835N08/8112956476/; http://www.flickr.com/photos/31031835N08/5387406710/), available on Flickr under a Creative Commons Attribution license. Classically, anesthetic recovery was considered to involve the reversal of events used in the induction; however, high-resolution EEG data revealed the presence of a hysteresis between the onset and offset of anesthesia that could not be explained in pharmacokinetic terms [5]. An analogy to flying is that landing a plane is not merely the reverse of taking off. Dr. Kelz has characterized the hysteresis as “neural inertia,” envisioning a resistance to change from anesthetized to awake states [6]; studies in his laboratory have demonstrated neural inertia to diverse anesthetic agents in mice and flies [6]–[8]. In mice, the role of the neuromodulators orexin [8] and noradrenaline [7] have been highlighted; in flies, mutation of the Shaker potassium channel [6] led to collapse of neural inertia. From this work the speculation arose that patients who lack neural inertia may be predisposed to anesthesia awareness [6]; others have suggested that increased neural inertia may protect against acute confusion (delirium) on “landing” from anesthesia by preventing patients connecting with their environment too early [1], [9].
In this issue of PLOS Genetics, Joiner et al. share their latest insights into the mechanisms of neural inertia based on a series of elegant fly studies [10] that examined both “take off” and “landing” from anesthesia. Four different mutations led to collapsed neural inertia, but two of these four mutations increased sensitivity to “take off” of anesthesia while the other two increased resistance to “take off” [10]. Furthermore, the differences in mechanisms of induction into, and emergence from, anesthesia were highlighted in studies showing that different mutations targeting glutamatergic signaling exerted different effects on induction and emergence from anesthesia [10]. Differential effects of two volatile anesthetics, halothane and isoflurane, on induction of anesthesia were also noted in some mutants, supporting the notion of discrete mechanisms of anesthesia for individual drugs [10]. Therefore, anesthesia “take off” and “landing” appear to have different neurobiology and henceforth should be considered separately.
Mechanistically, neural inertia is distinguished from arousal by Joiner et al., as hyperaroused mutants did not show altered neural inertia [10]. Further dissociations from arousal were supported by a lack of role for the circadian clock in influencing neural inertia (by studies conducted at different times of day) [10]. An interesting complexity occurs when studying sleep deprivation, however, as sleep-deprived flies showed increased neural inertia relative to rested controls [10]. The overlap between sleep and anesthetic mechanisms has been known for many years [3], but the insight that sleep deprivation did not affect induction of, but rather emergence from, anesthesia is particularly intriguing. While anesthetics converge on the sleep pathway to maintain the anesthetic state [2], [3], direct effects on higher corticothalamic centers (“top-down”) may dictate anesthetic induction [1], [11]. Hence, “take off” may depend on perturbation of corticothalamic activity, while effects on the sleep circuitry may contribute more significantly to the maintenance of, and “landing” from, anesthesia. The work of Joiner et al. adds to seminal discoveries that patients may not “fall asleep” into anesthesia, and whether they “wake up” (analogous to sleep) or emerge remains an exciting question.
Studies that seek to tease apart the neurobiologic correlate of “take off,” as distinct from either maintenance or “landing,” need to consider that passage into and through these states may have induced pharmacodynamics changes akin to tolerance. This problem arises because we cannot simply study arousal mechanisms without first inducing and maintaining the anesthetized state. Long-lasting changes are induced by the administration of general anesthetics [12]; in an analogous manner, changes induced by taking off and flying may affect the ability to land.
When considering the clinical relevance of the data reported by Joiner et al., we need to qualify our enthusiasm. Translating results from invertebrate systems to vertebrates, not to mention surgical patients with co-morbidities, raises several intriguing questions. Both orexin and norepinephrine are important neuromodulators of sleep and anesthesia sensitivity in vertebrates [6]–[8], but are untested in the fly. Mechanistically, it is also unclear how the neurobiology of unresponsiveness in Drosophila overlaps with human unresponsiveness [1]. Finally, anesthesia is provided to facilitate surgery, which itself has significant effects that could alter neural inertia (perhaps through noradrenergic effects on connectedness [1]).
Nonetheless, Joiner et al. have provided a new construct in which to consider the state of anesthesia [10]. In aggregate, the work from Kelz's laboratory provides support for their concept of neural inertia, through their careful dissection of the mechanisms of “take off” and “landing” from anesthesia, and for the dissociation of neural inertia from arousal. These findings may presage our understanding of how patients are induced into unresponsiveness, stay unresponsive during anesthesia, and subsequently reanimate. We support the authors' contention that understanding these mechanisms may lead to improvements in anesthesia as well as in conditions involving potentially pathological neural inertia, such as non-drug-induced comatose states. However, more work is required before the impact of these findings alter the perioperative care of surgical patients.
Zdroje
1. SandersRD, TononiG, LaureysS, SleighJW (2012) Unresponsiveness≠Unconsciousness Anesthesiology. 116 : 946–959.
2. FranksNP (2008) General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci 9 : 370–386.
3. NelsonLE, GuoTZ, LuJ, SaperCB, FranksNP, et al. (2002) The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat Neurosci 5 : 979–984.
4. AlkireMT, HudetzAG, TononiG (2008) Consciousness and anesthesia. Science 322 : 876–880.
5. Steyn-RossML, Steyn-RossDA, SleighJW (2004) Modelling general anaesthesia as a first-order phase transition in the cortex. Prog Biophys Mol Biol 85 : 369–385.
6. FriedmanEB, SunY, MooreJT, HungHT, MengQC, et al. (2010) A conserved behavioral state barrier impedes transitions between anesthetic-induced unconsciousness and wakefulness: evidence for neural inertia. PLoS One 5: e11903.
7. HuFY, HannaGM, WeiH, MardiniF, ThomasSA, et al. (2012) Hypnotic hypersensitivity to volatile anesthetics and dexmedetomidine in dopamine β-hydroxylase knockout mice. Anesthesiology 117 : 1006–7.
8. KelzMB, SunY, ChenJ, Cheng MengQ, MooreJT, et al. (2008) An essential role for orexins in emergence from general anesthesia. Proc Natl Acad Sci U S A 105 : 1309–1314.
9. SandersRD, MazeM (2012) Noradrenergic trespass in anesthetic and sedative states. Anesthesiology 117 : 945–947.
10. JoinerWJ, FriedmanEB, HungH-T, KohK, SowcikM, et al. (2013) Genetic and anatomical basis of the barrier separating wakefulness and anesthetic-induced unresponsiveness. PLoS Gen 9 (9) e1003605 doi:10.1371/journal.pgen.1003605
11. VellyLJ, ReyMF, BruderNJ, GouvitsosFA, WitjasT, et al. (2007) Differential dynamic of action on cortical and subcortical structures of anesthetic agents during induction of anesthesia. Anesthesiology 107 : 202–212.
12. SpissCK, SmithCM, TsujimotoG, HoffmanBB, MazeM (1985) Prolonged hyporesponsiveness of vascular smooth muscle contraction after halothane anesthesia in rabbits. Anaesth Analg 64 : 1–6.
Štítky
Genetika Reprodukční medicína
Článek Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive SelectionČlánek Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand SkillČlánek Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage EvolvabilityČlánek Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation inČlánek Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 ModelČlánek Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period inČlánek VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival inČlánek Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2AČlánek A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding YeastČlánek Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on PhenotypeČlánek Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding SitesČlánek Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 9
-
Všechny články tohoto čísla
- The Pathway Gene Functions together with the -Dependent Isoprenoid Biosynthetic Pathway to Orchestrate Germ Cell Migration
- Take Off, Landing, and Fly Anesthesia
- Nucleosome Assembly Proteins Get SET to Defeat the Guardian of Chromosome Cohesion
- Whole-Exome Sequencing Reveals a Rapid Change in the Frequency of Rare Functional Variants in a Founding Population of Humans
- Evidence Is Evidence: An Interview with Mary-Claire King
- Rapid Intrahost Evolution of Human Cytomegalovirus Is Shaped by Demography and Positive Selection
- Convergent Transcription Induces Dynamic DNA Methylation at Loci
- Environmental Stresses Disrupt Telomere Length Homeostasis
- Ultra-Sensitive Sequencing Reveals an Age-Related Increase in Somatic Mitochondrial Mutations That Are Inconsistent with Oxidative Damage
- Common Variants in Left/Right Asymmetry Genes and Pathways Are Associated with Relative Hand Skill
- Genetic and Anatomical Basis of the Barrier Separating Wakefulness and Anesthetic-Induced Unresponsiveness
- The Locus, Exclusive to the Ambulacrarians, Encodes a Chromatin Insulator Binding Protein in the Sea Urchin Embryo
- Binding of NF-κB to Nucleosomes: Effect of Translational Positioning, Nucleosome Remodeling and Linker Histone H1
- Manipulating or Superseding Host Recombination Functions: A Dilemma That Shapes Phage Evolvability
- Dynamics of DNA Methylation in Recent Human and Great Ape Evolution
- Functional Dissection of Regulatory Models Using Gene Expression Data of Deletion Mutants
- PAQR-2 Regulates Fatty Acid Desaturation during Cold Adaptation in
- N-alpha-terminal Acetylation of Histone H4 Regulates Arginine Methylation and Ribosomal DNA Silencing
- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Maternal Depletion of Piwi, a Component of the RNAi System, Impacts Heterochromatin Formation in
- miR-1/133a Clusters Cooperatively Specify the Cardiomyogenic Lineage by Adjustment of Myocardin Levels during Embryonic Heart Development
- Hsp104 Suppresses Polyglutamine-Induced Degeneration Post Onset in a Drosophila MJD/SCA3 Model
- Genome-Wide Analysis of Genes and Their Association with Natural Variation in Drought Tolerance at Seedling Stage of L
- Deep Resequencing of GWAS Loci Identifies Rare Variants in , and That Are Associated with Ulcerative Colitis
- Cooperative Interaction between Phosphorylation Sites on PERIOD Maintains Circadian Period in
- VAPB/ALS8 MSP Ligands Regulate Striated Muscle Energy Metabolism Critical for Adult Survival in
- Analysis of Genes Reveals Redundant and Independent Functions in the Inner Ear
- Predicting the Risk of Rheumatoid Arthritis and Its Age of Onset through Modelling Genetic Risk Variants with Smoking
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
- A Shift to Organismal Stress Resistance in Programmed Cell Death Mutants
- Fragile Site Instability in Causes Loss of Heterozygosity by Mitotic Crossovers and Break-Induced Replication
- Tracking of Chromosome and Replisome Dynamics in Reveals a Novel Chromosome Arrangement
- The Condition-Dependent Transcriptional Landscape of
- Ago1 Interacts with RNA Polymerase II and Binds to the Promoters of Actively Transcribed Genes in Human Cancer Cells
- Nebula/DSCR1 Upregulation Delays Neurodegeneration and Protects against APP-Induced Axonal Transport Defects by Restoring Calcineurin and GSK-3β Signaling
- System-Wide Analysis Reveals a Complex Network of Tumor-Fibroblast Interactions Involved in Tumorigenicity
- Meta-Analysis of Genome-Wide Association Studies Identifies Six New Loci for Serum Calcium Concentrations
- and Are Required for Cellularization and Differentiation during Female Gametogenesis in
- Growth factor independent-1 Maintains Notch1-Dependent Transcriptional Programming of Lymphoid Precursors
- Whole Genome Sequencing Identifies a Deletion in Protein Phosphatase 2A That Affects Its Stability and Localization in
- An Alteration in ELMOD3, an Arl2 GTPase-Activating Protein, Is Associated with Hearing Impairment in Humans
- Genomic Identification of Founding Haplotypes Reveals the History of the Selfing Species
- Plasticity Regulators Modulate Specific Root Traits in Discrete Nitrogen Environments
- The IDD14, IDD15, and IDD16 Cooperatively Regulate Lateral Organ Morphogenesis and Gravitropism by Promoting Auxin Biosynthesis and Transport
- Stochastic Loss of Silencing of the Imprinted Allele, in a Mouse Model and Humans with Prader-Willi Syndrome, Has Functional Consequences
- The Prefoldin Complex Regulates Chromatin Dynamics during Transcription Elongation
- PKA Controls Calcium Influx into Motor Neurons during a Rhythmic Behavior
- A Pre-mRNA-Splicing Factor Is Required for RNA-Directed DNA Methylation in
- Cell-Type Specific Features of Circular RNA Expression
- The Uve1 Endonuclease Is Regulated by the White Collar Complex to Protect from UV Damage
- An Atypical Kinase under Balancing Selection Confers Broad-Spectrum Disease Resistance in Arabidopsis
- Genome-Wide Mutation Avalanches Induced in Diploid Yeast Cells by a Base Analog or an APOBEC Deaminase
- Extensive Divergence of Transcription Factor Binding in Embryos with Highly Conserved Gene Expression
- Bi-modal Distribution of the Second Messenger c-di-GMP Controls Cell Fate and Asymmetry during the Cell Cycle
- Cell Interactions and Patterned Intercalations Shape and Link Epithelial Tubes in
- A Link between ORC-Origin Binding Mechanisms and Origin Activation Time Revealed in Budding Yeast
- The Genome and Development-Dependent Transcriptomes of : A Window into Fungal Evolution
- SKN-1/Nrf, A New Unfolded Protein Response Factor?
- The Highly Prolific Phenotype of Lacaune Sheep Is Associated with an Ectopic Expression of the Gene within the Ovary
- Fusion of Large-Scale Genomic Knowledge and Frequency Data Computationally Prioritizes Variants in Epilepsy
- IL-17 Attenuates Degradation of ARE-mRNAs by Changing the Cooperation between AU-Binding Proteins and microRNA16
- An Enhancer Element Harboring Variants Associated with Systemic Lupus Erythematosus Engages the Promoter to Influence A20 Expression
- Genome Analysis of a Transmissible Lineage of Reveals Pathoadaptive Mutations and Distinct Evolutionary Paths of Hypermutators
- Type I-E CRISPR-Cas Systems Discriminate Target from Non-Target DNA through Base Pairing-Independent PAM Recognition
- Divergent Transcriptional Regulatory Logic at the Intersection of Tissue Growth and Developmental Patterning
- MEIOB Targets Single-Strand DNA and Is Necessary for Meiotic Recombination
- Transmission of Hypervirulence Traits via Sexual Reproduction within and between Lineages of the Human Fungal Pathogen
- Integration of the Unfolded Protein and Oxidative Stress Responses through SKN-1/Nrf
- Guanine Holes Are Prominent Targets for Mutation in Cancer and Inherited Disease
- Regulation of the Boundaries of Accessible Chromatin
- Natural Genetic Transformation Generates a Population of Merodiploids in
- Ablating Adult Neurogenesis in the Rat Has No Effect on Spatial Processing: Evidence from a Novel Pharmacogenetic Model
- Genotype-Environment Interactions Reveal Causal Pathways That Mediate Genetic Effects on Phenotype
- The Molecular Mechanism of a -Regulatory Adaptation in Yeast
- Phenotypic and Genetic Consequences of Protein Damage
- Recent Acquisition of by Baka Pygmies
- Fatty Acid Taste Signals through the PLC Pathway in Sugar-Sensing Neurons
- A Critical Role for PDGFRα Signaling in Medial Nasal Process Development
- Chromatin-Specific Regulation of Mammalian rDNA Transcription by Clustered TTF-I Binding Sites
- Meiotic Recombination in Arabidopsis Is Catalysed by DMC1, with RAD51 Playing a Supporting Role
- dTULP, the Homolog of Tubby, Regulates Transient Receptor Potential Channel Localization in Cilia
- Widespread Dysregulation of Peptide Hormone Release in Mice Lacking Adaptor Protein AP-3
- , a Direct Transcriptional Target, Modulates T-Box Factor Activity in Orofacial Clefting
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Genome-Wide Systematic Analysis Reveals Different and Predictive Proliferation Expression Signatures of Cancerous vs. Non-Cancerous Cells
- Recent Acquisition of by Baka Pygmies
- The Condition-Dependent Transcriptional Landscape of
- Histone Chaperone NAP1 Mediates Sister Chromatid Resolution by Counteracting Protein Phosphatase 2A
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



