-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaTrans-Ancestral Studies Fine Map the SLE-Susceptibility Locus
We previously established an 80 kb haplotype upstream of TNFSF4 as a susceptibility locus in the autoimmune disease SLE. SLE-associated alleles at this locus are associated with inflammatory disorders, including atherosclerosis and ischaemic stroke. In Europeans, the TNFSF4 causal variants have remained elusive due to strong linkage disequilibrium exhibited by alleles spanning the region. Using a trans-ancestral approach to fine-map the locus, utilising 17,900 SLE and control subjects including Amerindian/Hispanics (1348 cases, 717 controls), African-Americans (AA) (1529, 2048) and better powered cohorts of Europeans and East Asians, we find strong association of risk alleles in all ethnicities; the AA association replicates in African-American Gullah (152,122). The best evidence of association comes from two adjacent markers: rs2205960-T (P = 1.71×10−34, OR = 1.43[1.26–1.60]) and rs1234317-T (P = 1.16×10−28, OR = 1.38[1.24–1.54]). Inference of fine-scale recombination rates for all populations tested finds the 80 kb risk and non-risk haplotypes in all except African-Americans. In this population the decay of recombination equates to an 11 kb risk haplotype, anchored in the 5′ region proximal to TNFSF4 and tagged by rs2205960-T after 1000 Genomes phase 1 (v3) imputation. Conditional regression analyses delineate the 5′ risk signal to rs2205960-T and the independent non-risk signal to rs1234314-C. Our case-only and SLE-control cohorts demonstrate robust association of rs2205960-T with autoantibody production. The rs2205960-T is predicted to form part of a decameric motif which binds NF-κBp65 with increased affinity compared to rs2205960-G. ChIP-seq data also indicate NF-κB interaction with the DNA sequence at this position in LCL cells. Our research suggests association of rs2205960-T with SLE across multiple groups and an independent non-risk signal at rs1234314-C. rs2205960-T is associated with autoantibody production and lymphopenia. Our data confirm a global signal at TNFSF4 and a role for the expressed product at multiple stages of lymphocyte dysregulation during SLE pathogenesis. We confirm the validity of trans-ancestral mapping in a complex trait.
Published in the journal: . PLoS Genet 9(7): e32767. doi:10.1371/journal.pgen.1003554
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003554Summary
We previously established an 80 kb haplotype upstream of TNFSF4 as a susceptibility locus in the autoimmune disease SLE. SLE-associated alleles at this locus are associated with inflammatory disorders, including atherosclerosis and ischaemic stroke. In Europeans, the TNFSF4 causal variants have remained elusive due to strong linkage disequilibrium exhibited by alleles spanning the region. Using a trans-ancestral approach to fine-map the locus, utilising 17,900 SLE and control subjects including Amerindian/Hispanics (1348 cases, 717 controls), African-Americans (AA) (1529, 2048) and better powered cohorts of Europeans and East Asians, we find strong association of risk alleles in all ethnicities; the AA association replicates in African-American Gullah (152,122). The best evidence of association comes from two adjacent markers: rs2205960-T (P = 1.71×10−34, OR = 1.43[1.26–1.60]) and rs1234317-T (P = 1.16×10−28, OR = 1.38[1.24–1.54]). Inference of fine-scale recombination rates for all populations tested finds the 80 kb risk and non-risk haplotypes in all except African-Americans. In this population the decay of recombination equates to an 11 kb risk haplotype, anchored in the 5′ region proximal to TNFSF4 and tagged by rs2205960-T after 1000 Genomes phase 1 (v3) imputation. Conditional regression analyses delineate the 5′ risk signal to rs2205960-T and the independent non-risk signal to rs1234314-C. Our case-only and SLE-control cohorts demonstrate robust association of rs2205960-T with autoantibody production. The rs2205960-T is predicted to form part of a decameric motif which binds NF-κBp65 with increased affinity compared to rs2205960-G. ChIP-seq data also indicate NF-κB interaction with the DNA sequence at this position in LCL cells. Our research suggests association of rs2205960-T with SLE across multiple groups and an independent non-risk signal at rs1234314-C. rs2205960-T is associated with autoantibody production and lymphopenia. Our data confirm a global signal at TNFSF4 and a role for the expressed product at multiple stages of lymphocyte dysregulation during SLE pathogenesis. We confirm the validity of trans-ancestral mapping in a complex trait.
Introduction
Tumour Necrosis Factor Superfamily (TNFS) members control wide-ranging facets of immunity when they interact with their complimentary TNF Receptors [1]. One of these, TNFSF4 (OX40L), uniquely binds its receptor, monomeric TNFRSF4 (OX40), on T lymphocytes to strongly activate NF-κB [2]. Several lines of evidence published over the last 15 years suggest the TNFSF4–TNFRSF4 interaction is required for the induction of anti-tumour immunity, allergy and autoimmunity [3]–[6] but also inhibits generation of adaptive T regulatory (TR1) cells [7]. The outcome is not limited to human disease; blockade of the TNFSF4-TNFRS4 interaction has ameliorative effects in animal models of T cell pathologies [8] including allergic and autoimmune manifestations [9]. Genetic variation at TNFSF4 has been associated with the autoimmune disease systemic lupus erythematosus (SLE), and other inflammatory conditions including atherosclerosis and ischaemic stroke.
SLE is the prototypic multi-system autoimmune disorder. High-affinity, pathogenic IgG autoantibodies to an array of nuclear antigens are a hallmark of pathogenesis and characterise the global perturbation of the immune system in SLE. Variation in at least 25 genetic loci with modest effect sizes are thought to explain the genetic component of SLE [10]. The strong genetic basis to disease is well-established and has been strengthened by the advent of GWAS, which has corroborated the association of immunologically relevant loci with SLE [11]–[13]. We have previously shown single nucleotide polymorphisms (SNPs) in the 5′ TNFSF4 region to be associated with lupus in European families and a case-control cohort [4]. The increased association of 5′ risk alleles with disease has been replicated in East Asian populations [14], [15], highlighting the genetic similarities at this locus in these ancestrally distinct populations. Multiple SLE risk-associated TNFSF4 variants are also associated with systemic sclerosis [16], primary Sjögren's syndrome [17] and myocardial infarction [18], [19].
A major obstacle in the identification of disease-specific causal variants at TNFSF4 in the European and East Asian SLE cohorts has been the strong linkage disequilibrium (R2>0.8) exhibited by genotyped TNFSF4 alleles, which has resulted in a high frequency extended haplotype associated with risk of disease instead of delineating causal variations at the locus [4]. It is probable that migration out of Africa involved many founder effects and bottlenecks to increase haplotype length in East Asian and European populations [20]. Hispanic and African-American populations are disproportionately affected by SLE [21] and health disparities in these groups show onset at a younger age [22].
Hispanic and African-American populations have genomes which reflect recent admixture on ancient substructures [16]. Hispanic cohorts have rich diversity of source ancestry with Southern European, Amerindian and West African contribution to the inherited genome and the forced diaspora of Africans to the Americas also resulted in gene flow and two-way admixture between previously reproductively isolated West African and European ancestral populations [23]. African populations today tend to have shorter haplotypes because they usually have ancestors who have experienced more recombination events without population bottlenecks or founder effects in emigrant populations [24]. Common shorter haplotypes are often subdivisions of the larger haplotypes found in non-Africans and so can be correlated to these [25]. In admixed populations, the genetic component attributable to the West African ancestral population would equate to a faster decay of LD. The breakdown of LD is therefore greater in African-Americans, because the major component (80% or more) of their genome is West-African, compared to Hispanics who have component estimates between 4–11% [23], [26].
We infer a fine-scale map of the recombination rate and location of hotspots within each entire population and in subgroups of interest. We have used principal components (PC)-based strategies to adjust for major ancestry before performing a high-resolution association study which utilises typed and probabilistic genotypes to map the TNFSF4 locus. By surveying common variants up to 1000 Genomes Phase 1(v3), we aim to identify common causal variation at TNFSF4 associated with SLE. Cross-comparison of associated risk haplotypes across four populations focuses these analyses. The AA association replicates in a smaller cohort of AA-Gullah [27]. Our haplotype analyses find informative recombinants in African-Americans and Europeans to resolve genetic variants associated with SLE at this locus.
These data are used to perform six case-control association studies and a trans-ancestral mapping experiment using in excess of 17900 subjects. We attempt to define causal variation at TNFSF4 in SLE susceptibility. In a complementary strategy we perform association analysis using TNFSF4 alleles and lupus phenotypes. We explore the mechanism by which TNFSF4 influences perturbation of the immune process in inflammatory disease. Finally, we interrogate risk alleles in terms of their influence on transcription factor binding using a bioinformatics approach. The research presented uses trans-ancestral mapping to inform this complex trait.
Results
To delineate the causal variation at TNFSF4, we genotyped SNPs in a 200 Kb section of chromosome 1q25.1 encompassing TNFSF4 (23.6 kb) and the 5′ region (150 kb). Population stratification bias and effects due to admixture were addressed using the approach of Namjou and colleagues [28]. We also genotyped 347 SNPs used by Halder [29] to correct for major ancestry in each population as identified by a PCA-based approach (Supplementary Figure S1). As outlined in methods, SNPs and individuals that failed quality control were filtered; pre - and post - imputation SNP counts and a description of the component sample sets is presented in Table 1.
Tab. 1. Population demographics and imputation reference data for SLE-control cohorts post QC filtering. 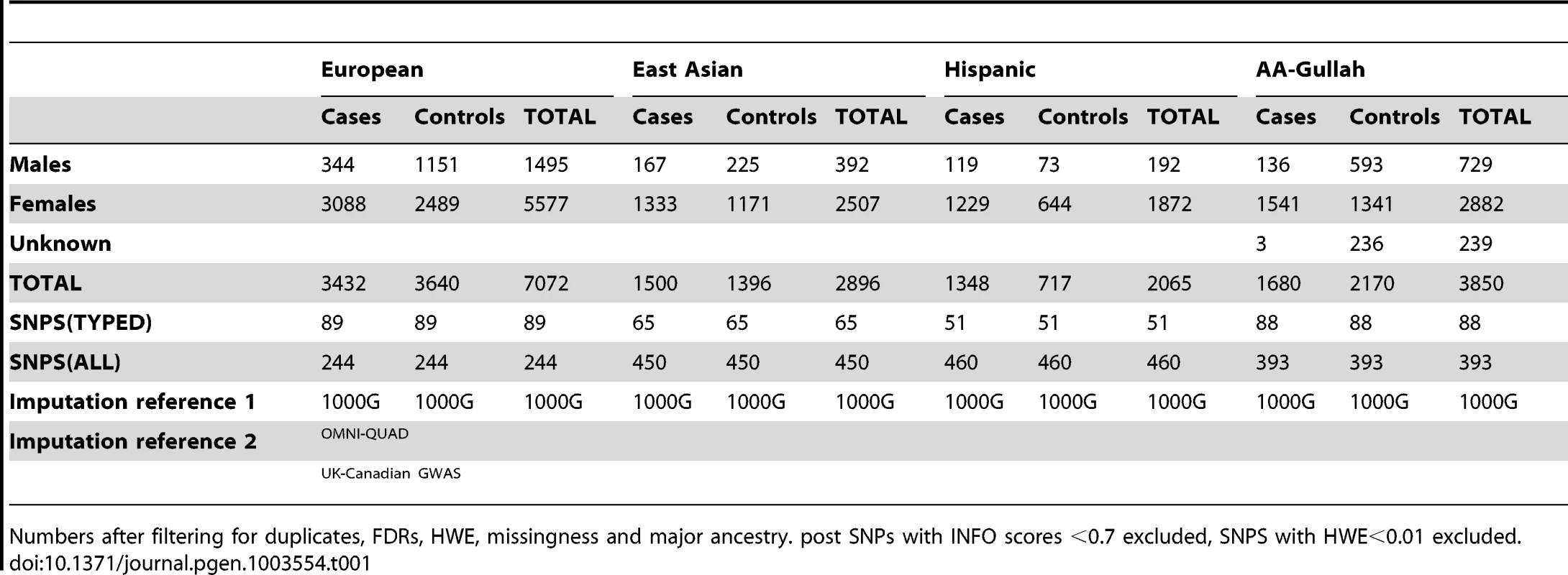
Numbers after filtering for duplicates, FDRs, HWE, missingness and major ancestry. post SNPs with INFO scores <0.7 excluded, SNPS with HWE<0.01 excluded. To directly compare genotyped SNPs we used the phased chromosomes of 1000 Genomes phase I integrated variant set v3 (March 2012, NCBI build37) (The 1000 Genomes Project Consortium) [30] and IMPUTE v2.2, together with second reference sets defined in Table 1. We imputed missing data and common variants (SNPs and INDELs) across the locus using MAF>1% in the imputation scaffold. As described in methods, we used a post imputation filter of HWE>0.01 and info >0.7 to include only genotyped and high quality imputed SNPs. The estimated concordance between imputed and true genotypes for the SNPs presented in this study is 0.95 for all cohorts. The final characteristics of all datasets are presented in Table 1.
Bayesian inference of fine-scale map of recombination rates and hotspot densities at TNFSF4
The European sex-averaged and female-only recombination maps generated by deCODE (http://www.decode.com/addendum/), are based on 15,257 and 8,850 directly observed recombinations, respectively. These maps have a resolution effective down to 10 kb and comparing them to the HapMap 3 and 1000 Genomes population-averaged maps [30], [31], we found differences at the TNFSF4 locus. Thus, we estimated background recombination rates in AA, East Asians, Europeans and Hispanics using a Bayesian composite-likelihood method. The inclusion of a hotspot model allowed sampling of hotspots from the Markov chain and inference of mean posterior hotspot densities from a threshold upwards of 0.25, giving a detection power of 50% and a false-discovery rate of 4% [32]. In Asians, Europeans and Hispanics the bulk of the recombination occurs in less than 5% of sequence (Figure 1 and Figure 2). An exception to this pattern is found in the African-American cohort, with increased recombination rate and higher density and proportion of hotspots across the locus (Figure 1, Figure 2). In all populations, peak recombination is at the 5′ boundary of the TNFSF4 gene and approximately 120 kb into the 5′ region. A difference in African-Americans is that recombination extends 30 kb from the TNFSF4 gene boundary into the 5′ region, whilst there is negligible recombination in this section in the other populations (Figure 1) and this is compatible with increased complexity of the genomic region in African-Americans.
Fig. 1. Fine scale maps of recombination rate inferred from East Asian, European, Hispanic and African-American control phased chromosomes. 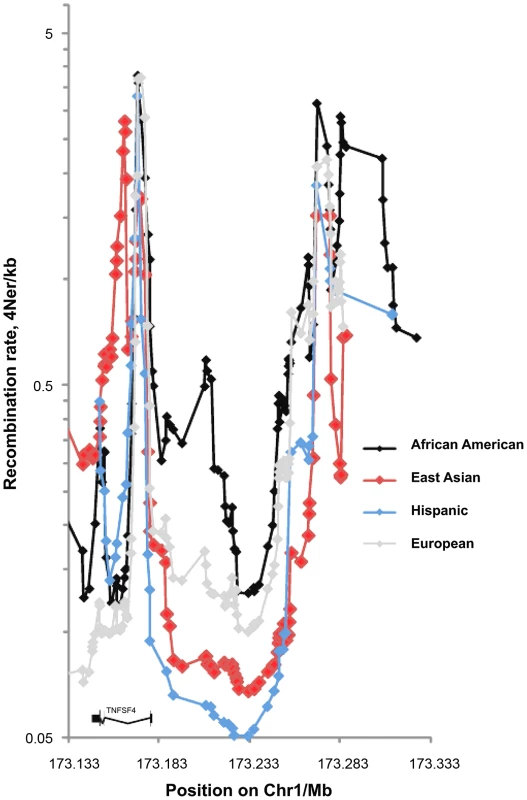
1568 randomly assigned chromosomes from each group were tested using Rhomap, from the LDHAT2.0 package. The fine-scale map of recombination rate (4Ner/kb) was inferred across 200 kb of chromosome 1q25 encompassing TNFSF4 gene and extended 5′ and 3′ regions. Fig. 2. Single marker association at TNFSF4 locus in A. East Asian, B. European, C. Hispanic, D. African-American SLE-control populations. 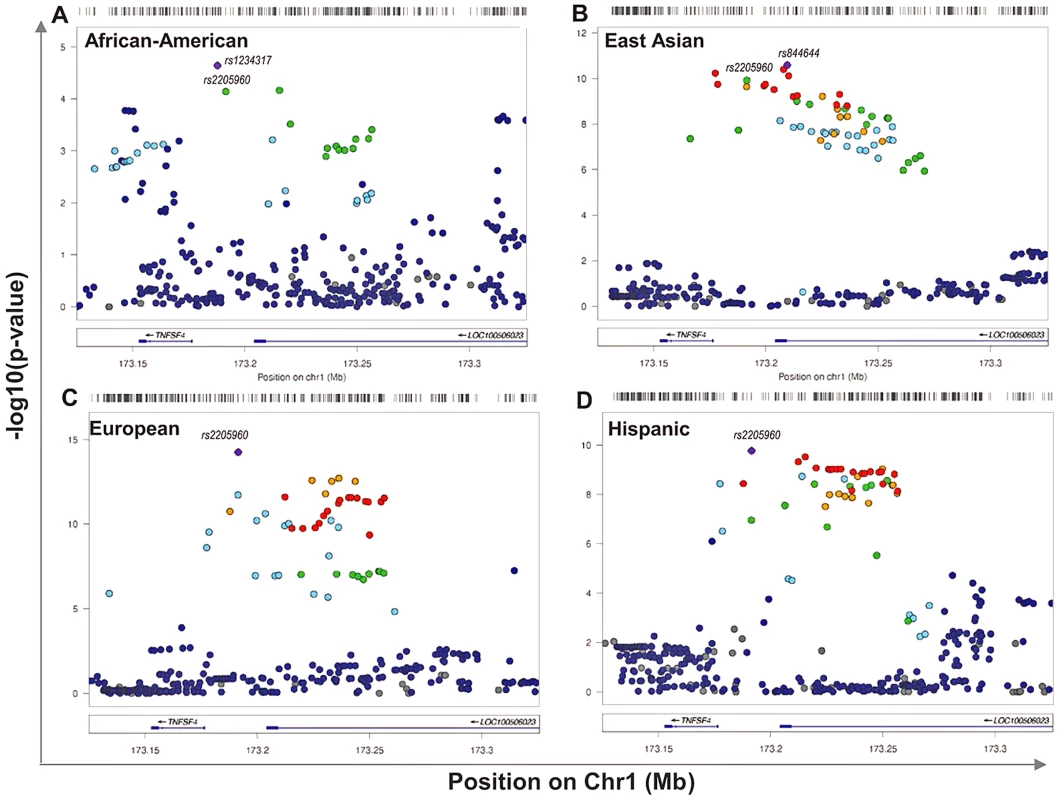
The strength of the association (-log10uncorrectedP) of markers across 240 kb of chromosome 1q25. This regional plot depicts TNFSF4 association with SLE versus chromosomal position (kb) in East Asians, Europeans, Hispanics and African-American populations. The most associated variants in each group are labelled, as is the best-associated meta-analysis SNP rs2205960. Markers are colour-coded for their correlation coefficient (r2) values according to the legend. Single marker association of 5′ TNFSF4 SNPs with SLE
The association data presented are for markers after imputation using the 1000 Genomes phase I integrated variant set v3 (March 2012, NCBI build37) [30]. The TNFSF4 locus is well established in SLE therefore we have presented uncorrected nominal p-values for variants. In East Asians, Europeans and Hispanics many strong associations (Pu 10−8<10−16) at TNFSF4 are detected. Multiple susceptibility alleles in the TNFSF4 5′ region are overrepresented in SLE cases (Table 2, Figure 2). In terms of single markers, best evidence of association with disease in Europeans is observed with rs2205960-T, 10 kb 5′ from the TNFSF4 gene (P = 5.61×10−15, OR = 1.34 (95%CI 1.25–1.44)). The T allele of rs2205960 also has strongest association with Hispanic SLE (P = 1.7×10−10, OR = 1.65 (95% 1.42–1.91)). In Europeans, an additional 15 SNPs reach genome-wide significance (P<5×10−8) most of these risk alleles also reach this level of significance in the East Asian and Hispanic cohorts (Table 2). Several 5′ risk alleles associated with disease in East Asians, Europeans and Hispanics are also associated in African-Americans and the 5′ association replicates in a small cohort of AA-Gullah (Supplementary Table S1), underpinning this gene as a global SLE susceptibility gene.
Tab. 2. Single marker association results for East Asian (As), European (Eur) and Hispanic (Hisp) SLE-control cohorts. 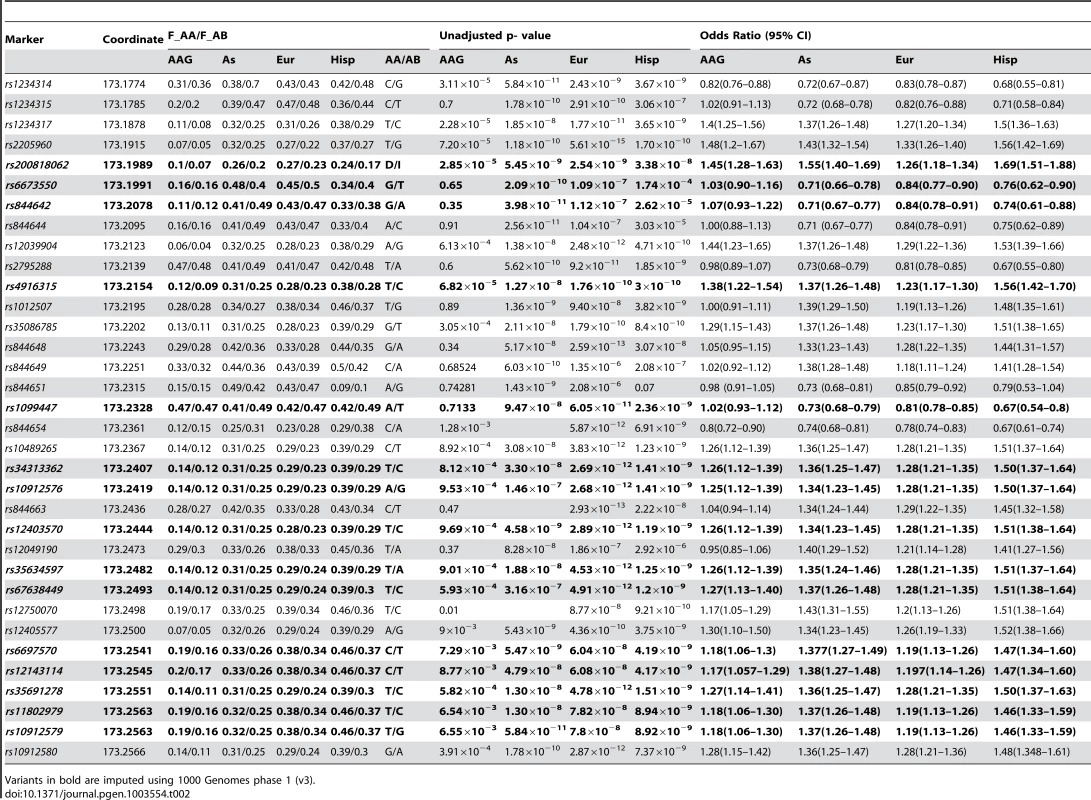
Variants in bold are imputed using 1000 Genomes phase 1 (v3). In African-Americans, the best evidence for the 5′ SNP association with disease are from rs1234317-T (P = 2.28×10−5, OR = 1.4 (95%CI 1.25–1.56)) and rs2205960-T (P = 7.2×10−5, OR = 1.48 (95%CI 1.22–1.67)) and rs1234314 –G (P = 3.11×10−5, OR = 1.22 (95%CI 1.13–1.32)). There is a trend for under-representation of the minor alleles of rs1234314-C, rs1234315-C, rs844642-G, rs844644-A, rs2795288-T and rs844654-A in SLE cases resulting in a flipped OR for these variants (Table 2).
Association of intragenic TNFSF4 single markers with SLE
Examining the genetic association between SNPs within the TNFSF4 gene and SLE we identify association of rs1234313-G, within intron1, with SLE in Asians (P = 4.37×10−8, OR = 1.38 (95%CI 1.32–1.44)), and Europeans (P = 1.11×10−5, OR = 1.15(95% CI 1.11–1.27). In both cohorts rs1234313-G is partitioned from other associated SNPs by a recombination hotspot at the TNFSF4 - 5′ boundary. However, correlation coefficient R2 values between this marker and risk-associated 5′ variants suggest strong correlation. We identify under representation of rs10798265-A in African-American SLE (P = 9.24×10−5, 0.84(95%CI 0.78–0.9)). There is suggestion of additional modest association signals (P<10−4) from a series of SNPs located at the TNFSF4 - 3′UTR boundary in the same cohort.
Imputation of typed bi-allelic indels
Imputation gave 257 common (>1% MAF) bi-allelic indels at the TNFSF4 locus, mostly neutral. The indels were included in the same imputation analysis and subject to the same QC as the SNPs and probabilistic genotypes incorporated into our association analyses. We identify a deletion at rs200818062 [-/G] to be associated with SLE in all groups tested. This indel is located 22.4 kb from the start site of the common transcript (Transcript 1) of TNFSF4 and is in strong LD with (R2>0.8) rs1234317 and rs2205960.
Best evidence meta-analysis
We used a logistic regression model fitted with an interaction term (effect) in the R statistical package to investigate cross-study heterogeneity. P-values for individual associated SNPs were generated using a likelihood-ratio test. We found no evidence of heterogeneity for the key risk - haplotype-tagging common variants which span the locus. Our null hypothesis - that all studies were evaluating the same effect size - held true for key variants associated with risk of SLE.
We combined the association data for variants across the 5′ TNFSF4 region in East Asians, Europeans, Hispanics and African-Americans, to more powerfully estimate the true effect size (Table 3). The average effect size across all datasets was computed using inverse variance weighting of each study. We find the 5′ association of TNFSF4 with SLE greatly reinforced. rs2205960-T, the most associated allele in Europeans and Hispanics, (P = 1.71×10−34, OR = 1.43[1.26–1.60]), and rs1234317-T (P = 1.16×10−28, OR = 1.38[1.24–1.54]) have the strongest combined association with disease (Table 3). These adjacent markers are separated by 3 kb of chromosome 1. Allele frequencies for rs2205960 for 1000 Genomes populations are presented in supplementary data.
Tab. 3. Best evidence meta-analysis of the association P value for TNFSF4 SNPs. 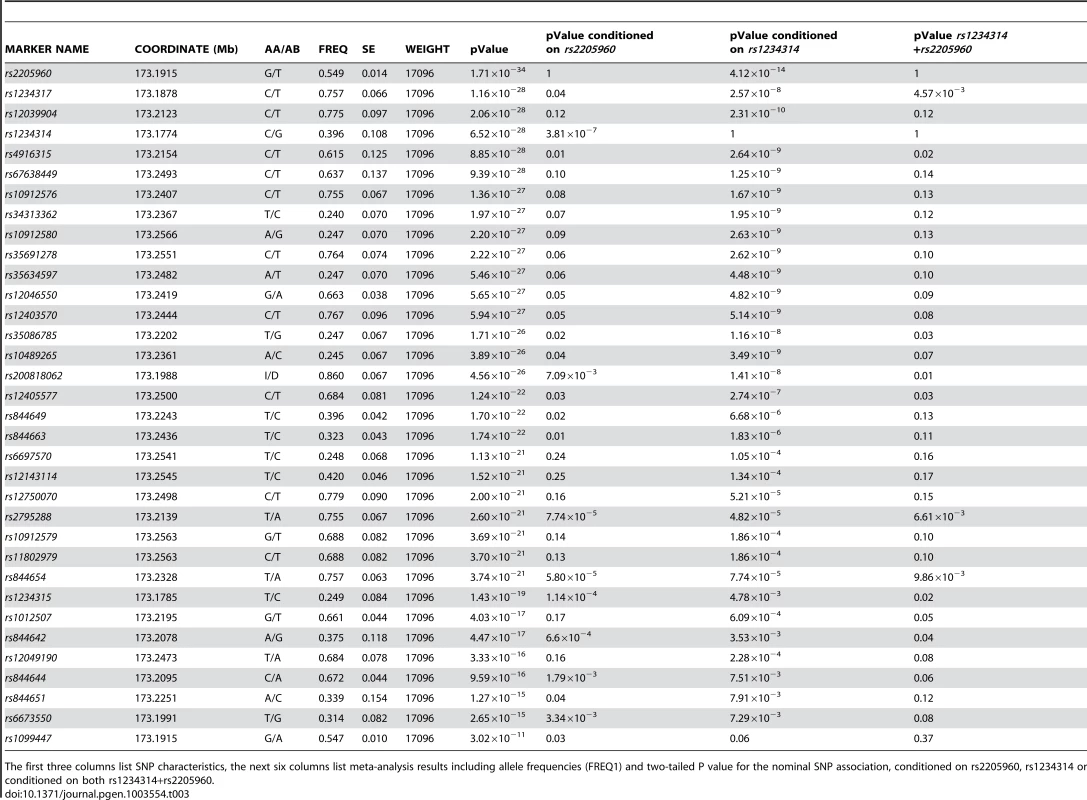
The first three columns list SNP characteristics, the next six columns list meta-analysis results including allele frequencies (FREQ1) and two-tailed P value for the nominal SNP association, conditioned on rs2205960, rs1234314 or conditioned on both rs1234314+rs2205960. Conditional regression analysis of 5′ single-markers
As expected, our 5′ association data suggest pairwise LD between markers is weakest in African Americans and strongest in Asians. In order to establish whether the signals identified by our trans-ancestral fine-mapping experiment represent causal variants, independent risk factors, or if we have simply found surrogate markers strongly correlated with causal variants, we conditioned the association data from each population with the marker which represented the best evidence of association.
In all populations, rs2205960-T, a risk-haplotype tag SNP with high p-value and effect size (Table 3, Figure 3) is associated with SLE; a similar trend is illustrated by the adjacent marker rs1234317-T. Conditioning on rs1234317 or rs2205960 we find the signal at rs1234317 is lost after conditioning for rs2205960, and this is consistent for across populations (Table 4). If the reverse analysis is performed and we condition on the presence of rs1234317, there is residual association at rs2205960 in Asians, Europeans and Hispanics (P = 9×10−4AS, P<10−4EU, P = 0.015Hi). In all apart from the AA group, conditioning on rs1234317 or rs2205960 leaves a residual signal at rs1234314. We included rs2205960, rs1234317 and rs1234314 in these analyses.
Fig. 3. LD plots at TNFSF4 locus in four populations. 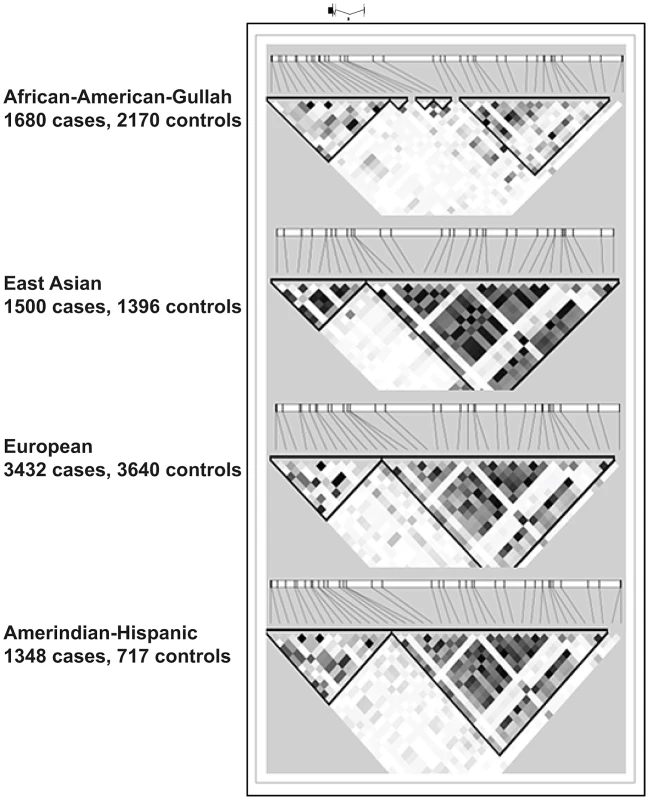
This section of chromosome 1q25.1 encompasses the TNFSF4 gene and upstream region as defined by custom algorithm in Haploview 4.2. The measure of LD was used to depict 57 SNPs common to all cohorts, post QC and 1000 genomes imputation. The pair-wise correlations between TNFSF4 markers is illustrated in these plots by the correlation coefficient R2(where r2 = 0 = no correlation, white; 0<R2<1, gradations of grey; R2 = 1 = complete correlation, black). The TNFSF4 gene is positioned above the plots relative to haplotype blocks (black triangles) and grey ticks indicate SNP locations to scale. Tab. 4. Conditional regression results for 5′TNFSF4 variants in four groups. 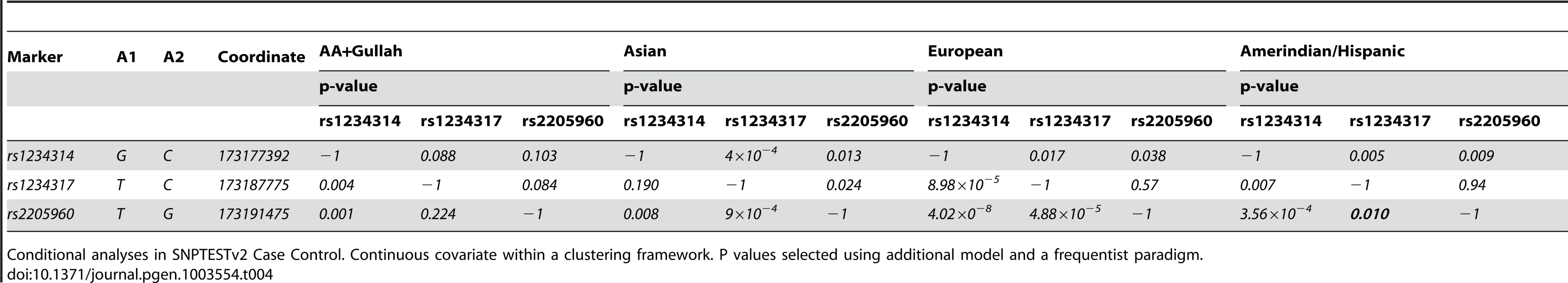
Conditional analyses in SNPTESTv2 Case Control. Continuous covariate within a clustering framework. P values selected using additional model and a frequentist paradigm. Conditional analysis of meta-analysis data
We find conditioning on the presence of rs1234317 or rs2205960, association of intron 1 markers tested for all groups is lost, confirming these as secondary to 5′ risk associations (Table 2). We also conditioned the meta-analysis association data on rs2205960 and found residual association at rs1234314 (P = 3.81×10−7), located at the TNFSF4-5′ boundary. The reverse analysis found increased residual association at rs2205960 (P = 4.12×10−14). These data suggest two independent signals with increased association and effect at rs2205960 compared to rs1234314 in SLE. Conditioning the meta-data on both rs1234314 and rs2205960 removed association at TNFSF4 (Table 3).
Modelling the pattern of inheritance in independent cohorts
In genotype-based analyses, the models that best fits the 5′ association of TNFSF4 with SLE are the additive/dominant models.
Haplotype bifurcation of TNFSF4 risk and non-risk haplotypes
Haplotypes significantly associated with risk of disease were identified for each population. To better visualise the breakdown of LD of associated haplotypes, we constructed bifurcation diagrams from phased genotypes for each cohort tested (Figure 4, risk). The plots illustrate the breakdown of linkage disequilibrium (LD) at increasing distances in both directions from rs1234314, the most proximal genotyped SNP located at the TNFSF4 gene-5′ boundary and which is used as the core variant in the figure (labelled, circular core from which haplotype branches). The location of rs1234317 and rs2205960, best-associated in the meta-analysis, are also marked onto the diagram. The thickness of the line in each plot corresponds to the number of samples with the haplotype, branches indicate breakdown of LD. For the risk haplotype, the lines are most robust in East Asians (Figure 4, risk), followed by Hispanics and Europeans, and least robust in African-Americans. We find branch junctions depicting breakdown of LD on the risk haplotype to be coincident with the section of the TNFSF4 locus encompassing rs1234317 and rs2205960.
Fig. 4. Haplotype Bifurcation Diagrams of TNFSF4risk and TNFSF4non-risk for Four Populations. 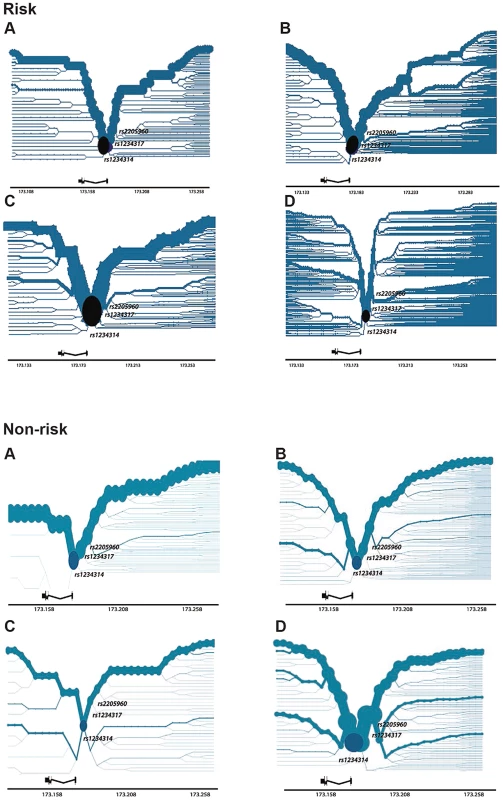
Plots are constructed using phased haplotypes for a. East Asians, b. Europeans, c. Hispanics and d. African-Americans and illustrate breakdown of LD with increasing distances from a core proximal TNFSF4 SNP and are approximately to scale. The core is located at the TNFSF4 gene-5′ boundary (black circle) and is selected as the most proximal 5′ marker, rs1234314, in each population. Gene location is depicted to scale; we have additionally labelled each plot to show the location of rs1234317 and rs2205960, the best-associated markers from the meta-analysis. The non-risk haplotype retains its thickness with distance from the core in the AA group, indicating long-range homozygosity (Figure 4, non-risk). Contrasting the recombination rate in risk and non-risk haplotype homozygotes finds increased recombination in the risk individuals (Supplementary Figure S3), supporting these bifurcation data.
Conservation of TNFSF4 haplotype structure at the TNFSF4 locus in ancestrally diverse populations
Significantly associated haplotypes are found in each population (Table 5). Low recombination and similar location of hotspots at the TNFSF4-5′ boundary in East Asians, Europeans, and Hispanics allow for the construction of near-identical haplotype blocks including risk and non-risk haplotypes (designated TNFSF4OR>1 and TNFSF4OR<1, respectively)(Figure 3) which extend approx. 80 kb into the TNFSF4 5′ region. Multiple associated risk alleles uniquely tag TNFSF4OR>1, the risk haplotype, which is overrepresented in SLE individuals in each population, whilst TNFSF4OR<1 is the most frequent haplotype for all cohorts tested but underrepresented in SLE individuals.
Tab. 5. <i>TNFSF4</i> haplotype association in four SLE-control populations. 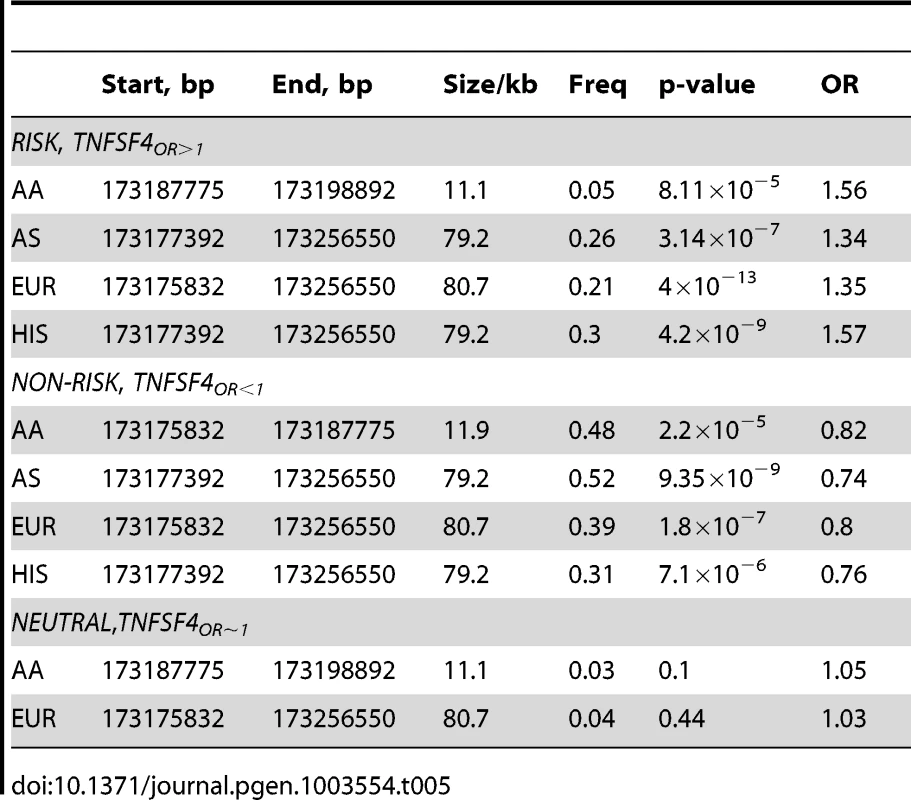
The risk haplotype found in East Asians, Europeans and Hispanics is fragmented in the African-American cohort; the most associated risk haplotype is 11 kb (P = 2.12×10−5, OR = 1.52). This haplotype block extends from rs1234317 to the bi-allelic indel rs200818062. Only one allele uniquely tags this haplotype, rs2205960-T, the associated alleles of rs1234317-T and rs200818062- are also found on a completely neutral haplotype. This haplotype block is separated from the adjacent distal block by R2 = 0.33. Haplotype association data for TNFSF4OR>1 and TNFSF4OR<1 are presented in Table 5.
In Asians, Europeans and Hispanics, the non-risk haplotype is tagged by rs1234314-C, rs1234315-C, rs844642-G, rs844644-A, rs2795288-T and rs844654-A. These variants have a flipped OR (Table 2) and there is residual signal at these variants after conditioning on risk-associated variants. In African-Americans, there is a signal at rs1234314-C. Conditioning our meta-analysis data on rs2205960-T there is residual association at each of these variants and the OR for the minor allele is flipped. The best-associated variant after conditioning on the risk signal is rs1234314-C. This variant is associated in all groups tested and resides at the TNFSF4-5′ boundary. Conditioning on rs1234314 and rs2205960 removes association at TNFSF4.
Conditional regression analysis of haplotypes
In all groups, we conditioned upon the presence of TNFSF4OR>1 and found residual association of TNFSF4OR<1. Reversing the analysis by conditioning on presence of TNFSF4OR<1 also finds residual association of TNFSF4OR>1. These analyses demonstrate that the observed signals in the TNFSF4 promoter region independently confer risk and protection against SLE.
Informative neutral haplotypes provide support for causal SNPs identified by conditional analysis
We found haplotypes in the European and AA cohorts which are tagged by the risk allele rs1234317-T but the non-risk allele rs2205960-G and not associated with disease. In African-Americans, the alleles of rs1234317-T and rs200818062- are found on a neutral haplotype, not associated with SLE. This haplotype block is separated from the adjacent distal block by a correlation coefficient value R2 = 0.33. These data support our conditional regression data which indicate rs2205960-T as the driver of the risk association.
Subphenotype analyses
Given TNFSF4 surface expression on a range of cell types which control immune functionality, one might expect TNFSF4 alleles to be associated with disease manifestations of SLE. Median (IQR) age at diagnosis, autoantibody production and renal disease were examined within SLE cases and against controls in each ancestral group. American College of Rheumatology (ACR) classification criteria [33] were additionally examined in East Asians, Europeans and Hispanics. Phenotypic subsets of SLE cases are less heterogenous than SLE per se and so may enrich for risk variants with increased effect size or prove informative for causal mechanism. Clinical characteristics of SLE individuals sorted by population are presented with case-only and phenotype-control association results (Supplementary Table S2).
Association of TNFSF4 markers with autoantibody production
Case-only analysis reveals association of TNFSF4 risk variants with autoantibody production in African-American, European and Hispanic SLE cohorts: Evidence of association of rs2205960-T with Anti-Sm autoantibodies in African-American cases (P = 5.1×10−3, OR = 1.57(95% CI 1.14–2.16) is reinforced by testing this variant against controls (P = 6.67×10−7, OR = 1.91(1.47–2.47)). We find this marker also segregates with Anti-Sm autoantibodies in European case-only and phenotype-control analyses. In Europeans the adjacent variant rs1234317-T is associated with Anti-Ro autoantibodies (P = 9.5×10−4, OR = 1.31(95% CI 1.12–1.54) and this is reinforced against controls (P = 9.5×10−8, OR = 1.52 (1.3–1.76)). In African-Americans analyses of 5′ variants against controls improves the significance of risk-haplotype-tagging variants with Anti-dsDNA autoantibodies (rs1234317-T, Pu = 5.36×10−6, OR = 1.68(95%CI 1.34–2.1.)) We find a transancestral trend of underrepresentation of TNFSF4 intron 1 alleles with autoantibody production (Hispanic P = 1.7×10−4, OR = 0.52(95% CI 0.36–0.73), European P = 2.5×10−3, OR = 0.81(0.7–0.93) and East Asian P = 3.6×10−2, OR = 0.7 (95% CI 0.5–0.98)). Conditional regression analysis of the best-associated marker in each population removes all evidence of association.
Association of TNFSF4 markers with age of onset
Examination within cases also reveals association of distal 5′ TNFSF4 alleles with age of onset (IQR) across all cohorts examined apart from East Asians (Supplementary Table S2). We classified the first and last quartile of age of onset into early and late onset in the analysis. Underrepresentation of distal 5′ TNFSF4 alleles in lupus individuals with early age of onset is found in AA (P = 9×10−4, OR = 0.69 (95% CI 0.56–0.86)), European (P = 1.43×10−3, OR = 0.78(0.68–0.91)) and Hispanic (P = 1.43×10−3, OR = 0.57(95% CI 0.41–0.81)) populations. Inverse square meta-analysis finds the marker with best evidence of association with this phenotype (rs844654-A, P = 8.7×10−6, Z score 4.45), 60 Kb from the TNFSF4 gene-5′ boundary.
Identification of 5′ end of putative TNFSF4 transcripts
To gain further insight into the transcriptional regulation of the TNFSF4 gene we analysed the 5′ ends of four putative TNFSF4 transcripts from the activated B lymphocytes of a European individual. We evaluated the mRNA predictions for TNFSF4 because the Gencode mRNA predictor annotates three TNFSF4 splice variants, whilst Aceview, which has increased sensitivity for the cDNA-supported transcriptome, annotates four mRNA splice variants [34]. To position our association data accurately against the TNFSF4 gene, we generated the 5′ ends of transcripts by 5′ RACE-PCR and found multiple transcripts which differ in their first exon usage (Figure 5) including a transcript for what is likely to be a soluble form of TNFSF4 (Figure 5), this transcript maps identically to a transcript found in the Ensembl and UCSC genome browsers, but is yet to be found translated. We have anchored our association data to the most abundant transcript (Transcript A, Figure 5) sequenced.
Fig. 5. Confirmation of TNFSF4 start site and splice variants. 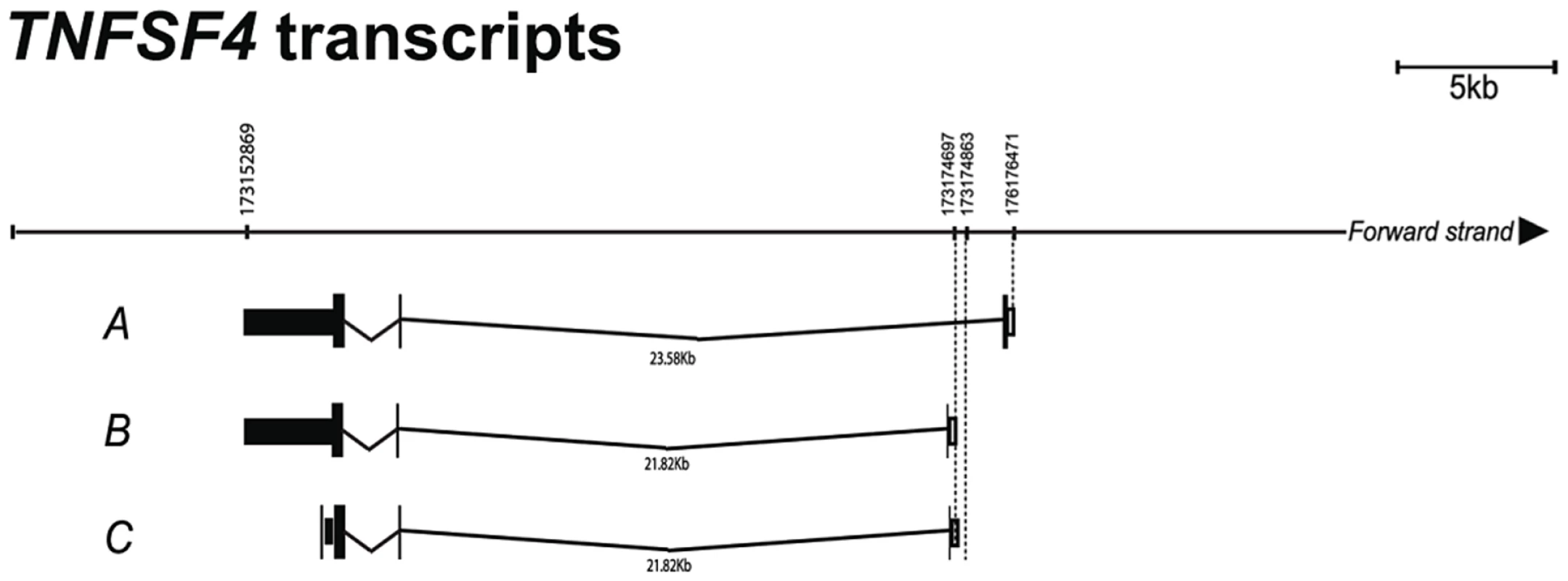
5′ RACE analysis was used to map the TNFSF4 transcription start site. Three out of four putative splice variants modelled by the Aceview tool in the UCSC genome browser were validated in a European individual. Splice variants a. and b. are protein coding, whilst variant c. is transcribed but not translated. Association of TNFSF4 variants with expression in LCLs
Expression profiling of common TNFSF4 variants was carried out in a cis eQTL study in LCL samples from 777 female TwinsUK participants [35]. Association of RNA expression with >2×106 SNPs was tested by two-step mixed model–based score test [35]. To characterize likely independent regulatory effects, the identified cis eQTLs were mapped to recombination hotspot intervals. For each gene, the most significant SNP per hotspot interval was selected, and LD filtering performed. The top-cis-eQTL in the LD bin, for the probe located at TNFSF4 (ILMN_2089875), was rs2205960 (P = 3.75×10−4).
Bioinformatic analysis
We examined the interaction of individual transcription factors (TFs) and other proteins with the DNA sequence at rs2205960. A decameric DNA sequence including the rs2205960 variant at the 8th position was predicted to bind to the NF-κB p65 protein (RELA) with high confidence. We investigated changing rs2205960 allele, from the minor (T) to major (G) allele and its impact on binding affinity of the motif for the target protein, p65. Using SELEX binding data and position weight matrix (PWM) profiles stored in the Jaspar core database [36], we found the DNA sequence with rs2205960-T at the 8th nucleotide position had a binding affinity of approximately 90% for NF-κB p65 (Figure 6). Altering the allele to rs2205960-G decreased the binding affinity for NF-κB p65 by over 10%, but highlighted degeneracy of the motif (Figure 6b). Binding of NF-κB at rs2205960 has been confirmed by genomewide ChIP-seq experiments in EBV - B cell lines as part of the ENCODE project (Figure 6c) [37]. These ChIP-seq data indicate that signal intensity for NF-κB at rs2205960 in a heterozygous (G/T) cell-line (GM12878) is double that for a non-risk homozygote (G/G) cell line (GM06990).
Fig. 6. SLE-associated rs2205960 predicted to be part of a decameric motif for NF-κB p65 (RELA). 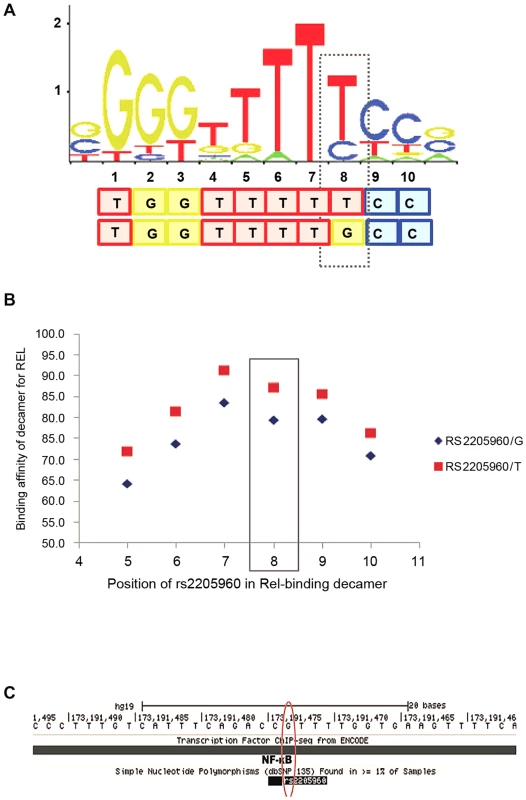
A. Degeneracy within the core 10-base motif is illustrated at all positions apart from position 7 which is non-degenerate by the stacked letters at each position. The relative height of each letter is proportional to its over-enrichment in the motif. A dashed line is boxed around rs2205960-T, this SLE-associated allele is predicted to form the 8th nucleotide in the motif. Predictions were made using the non-degenerate set of matrix profiles in the Jaspar Core database. B. Altering the rs2205960 allele from -T to -G decreases the binding affinity for NF-κB p65 by over 10%. C. Binding of NF-κB at rs2205960, suggested by genome-wide ChIP-seq ENCODE data. Profiles were generated for lymphoblatoid cell lines and stored in the UCSC genome database. We further examined the sequence encompassing rs1234314 for transcription factor binding. According to our conditional analysis, rs1234314 is the best-associated variant after conditioning on the risk-association. Furthermore this variant tags the non-risk haplotype. Scanning the data held in the Ensembl genome browser revealed rs1234314 to be part of a 400 bp segment which has repressed/low activity in LCL cells but with no such activity in a T cell line. The UCSC genome browser predicts rs1234314 to be located within a region associated with the H3K27Ac chromatin signature which is associated with active enhancers. Interrogating the sequence at rs1234314 with PWM binding data in the Jaspar core database gave no clear pattern of binding of either allele to the motif of a regulatory element.
Examining the sequence with rs1234317-T against PWM binding data stored in the Jaspar Core database finds it completes a TATATT-binding motif and this motif is disrupted in the presence of rs1234317-C. The ENCODE project does not highlight binding of a TBP protein at this variant. Genome-wide ChIP-seq data from the ENCODE project has data for LCLs which carry the T allele of rs1234317. For LCLs carrying the risk (T) allele, there are currently no regulatory features annotated at this position.
Discussion
We present a trans-ancestral fine-mapping association study of TNFSF4 in SLE. We have genotyped haplotype-tagging and proxy variants and major ancestry informative markers in 6 populations, including admixed groups, across 200 kb of 1q25 encompassing the TNFSF4 gene, and 5′ and 3′ regions. We also present a fine mapping association analysis of TNFSF4 SNPs in African-American SLE. Association testing of TNFSF4 variants revealed strong association of 5′ variants with disease in all cohorts (Tables 2–5) establishing it as a global lupus susceptibility gene. Resolution of the association signal was aided by increased recombination in the AA group (Figure 1), and by increased power from the large numbers in our European cohort. Maximal power was achieved testing with a genetic model concordant with the major underlying mode of inheritance of the 5′ TNFSF4 region in SLE, which is additive. Our study would suggest trans-ancestral mapping as a useful tool where linkage disequilibrium is an obstacle.
Testing most of the common polymorphisms at the locus allowed identification of additional candidate variants that might underlie association at TNFSF4. As expected, most high-frequency SNP probabilistic genotypes included in this study are present in dbSNP; especially in the TNFSF4 gene itself. Prior to QC filtering, the African-American population contributed the largest number of probabilistic genotypes at SNP loci. Although our ability to impute bi-allelic indels accurately from the 1000 Genomes Project resource is limited by FDR, it still increased power to detect association signals at a majority of common small indel sites accurately. In excess of 50% of the indels in the imputation scaffold were novel in all groups. We mention the bi-allelic deletion, rs200818062, which is in LD with our best-associated variant, rs2205960, and which is associated with SLE in all cohorts tested. Our AA and European data suggest this risk-associated deletion is found on a neutral haplotype which is not associated with disease. After QC filtering of imputed variants in these populations, our data suggests no new imputed variant better explained the risk signal than the typed SNP rs2205960-T.
A key limitation of this study is TNFSF4 imputation may have missed common variations located in the distal 5′ TNFSF4 region which could be causal. Accurate characterisation of variants remains challenging in low-complexity regions including the LINE element found in the distal 5′ section of this locus. As a result, variants in this region are systematically underrepresented in genetic association studies. Furthermore, an association signal may reside in the fraction of SNPs which have a lower imputation performance and were omitted using our info threshold of 0.7. This fraction is likely to include rare variants which are too infrequent to be imputed with confidence but which might have a large effect on risk. However, our data suggest the true causal variants are likely to be common (>5% frequency) and located in the proximal section of the 5′ region. The standard error of the beta coefficients for most imputed variants included in later analyses reflect high imputation certainty.
Mapping the alleles uniquely tagging the risk haplotype in each cohort has established the boundaries of risk and non-risk haplotypes in East Asians, Hispanics, and African-Americans and validated the haplotype boundaries previously defined in Europeans [4]. We avoided spurious associations through poor matching of cases with controls by the removal of outlying individuals (Supplementary Figures S1 and S2) and tested the association of risk alleles across all groups in this study.
Comparing recombination patterns in African-American individuals homozygous for the risk and non-risk haplotypes finds increased recombination in the risk-haplotype. Our results provide evidence for global association of rs2205960-T with SLE and assessment of the contribution of rs2205960-T to disease risk by conditional regression suggests that this allele drives the 5′ TNFSF4 association in African-Americans, Europeans and Hispanics. Increased decay of 5′ LD at TNFSF4 in AAs anchor the associated haplotype to the proximal 5′ region of TNFSF4. Examining the LD structure at TNFSF4 in African-Americans and Europeans validates our association data: Neutral haplotypes in these populations, recombinant between rs1234317, rs2205960 and rs200818062-, support our conditional regression results. Association testing within the Anti-Smith autoantibody-positive AA lupus subgroup strengthens the association P value and effect size of rs2205960-T and this trend replicates in Europeans (Table S2).
Curated and non-redundant profiles of SELEX binding experiments, stored in the JASPAR core database [36], suggest rs2205960-T would form the 8th nucleotide of a decameric motif with high binding affinity for NF-κB p65 (Figure 6). Altering the 8th nucleotide of the decamer to rs2205960-G reduces the binding affinity of this sequence for this NF-κB protein by approximately 10%, according to these data. ChIP-seq data generated for two Hapmap lymphoblastoid cell lines confirm binding of NF-κB at this location. ENCODE ChIP-seq data also suggest binding of the transcription factors BCL11a, MEF2a and BATF at rs2205960, albeit with lower signal intensity compared to NF-κB. These data suggest the genomic region encompassing rs2205960-T to have strong regulatory potential.
These data were generated for the ENCODE project [37], and establish that a positive signal for NF-κB binding is found at rs2205960 but not rs1234317. A signal is found in both cell lines and there is increased signal intensity in the risk/non-risk heterozygote compared to the non-risk homozygote cell line. These data suggest a mechanism by which rs2205960-T could increase gene expression, which may underlie the SLE risk.
Our data suggest a putative role for TNFSF4 in autoantibody generation, further clarifying the role of this gene in lupus pathogenesis. Correlation of rs1234317-T with the presence of anti-Ro autoantibodies in European cases is strengthened against controls. The Genomatix SNP analysis web tool predicts rs1234317-T to destroy the DNA binding site for the transcriptional repressor E4BP4, a transcription factor with a role in the survival of early B cell progenitors [38]. The DNA sequence encompassing either the C or T allele of rs1234317 was investigated for binding to this transcription factor using the curated set of binding profiles stored in the Jaspar core database. We could not confirm binding of the sequence with either allele to the E4BP4 repressor with these data. However, the T-allele of rs1234317 completes a TATATT consensus sequence for the TATA-Binding Protein (TBP). External sources of regulatory data stored in Ensembl and UCSC do not validate the binding of TBP or other members of the transcription initiation complex. The genomewide ChIP-seq data from the ENCODE project has data for LCLs which carry the T allele of rs1234317 associated with SLE risk. We would expect enrichment for TFs such as TBP, or marks of open chromatin, but there are currently no data for LCLs carrying the risk (T) allele. However binding of this factor is associated with transcription initiation and so this variant merits further investigation in Rho - autoantibody-positive subsets of SLE individuals.
Association of rs2205950-T with African-American lupus concurs with data published previously by our group establishing a 5′ TNFSF4 association with SLE in Northern Europeans [4]. The risk-associated variants rs2205960-T and rs1234317-T are strongly associated in the Minnesota cohort consistent with our results in four racial groups. In this previous study LD was a major obstacle in delineation of causal variation. Crucially we find association testing using a very large number of Europeans and the admixed AA group allow delineation of the signal through conditional analyses and the presence of neutral recombinant haplotypes. The African-American data presented does not validate data presented by Delgado-Vega and colleagues [39], suggesting rs12039904-T and rs1234317-T to explain the entire haplotypic effect at TNFSF4 with SLE. A possible explanation for the modest association of rs12039904-T in our African-American cohort is that it is monomorphic in West African populations such as the Yoruba from Ibadan, Nigeria. Our data find rs12039904-T a borderline rare allele in African-Americans and we find nominal allelic association of rs12039904-T with disease, conditional regression analyses of rs2205960 results in absence of an association signal at rs12039904 in all groups.
Sanchez and colleagues use TNFSF4 rs2205960 and single markers at 15 other lupus susceptibility loci to illustrate correlation of Amerindian ancestry with increased frequency of lupus risk alleles [40]. Delineation of rs2205960-T in the context of LD with adjacent markers isn't the aim of the Sanchez study, as a single SNP is typed at each locus. They find aggregation of deleterious alleles in Amerindian SLE individuals which are complemented by the increased effect sizes we find for associated TNFSF4 variants in Amerindians and Hispanics in this study.
In Asians, Europeans and Hispanics, the non-risk haplotype is tagged by rs1234314-C, rs1234315-C, rs844642-G, rs844644-A, rs2795288-T and rs844654-A. In African-Americans, there is a signal at rs1234314-C and a weaker signal at rs844654-A. Conditioning our meta-analysis data on rs2205960-T, the variant which is best-associated with risk in this study, there is residual association at each of these variants and the OR for the minor allele is flipped. The best-associated variant after conditioning on the risk signal is rs1234314-C. This variant is associated in all groups tested and resides at the TNFSF4-5′ boundary. Conditioning on rs1234314 and rs2205960 removes association at TNFSF4.
In summary, the data presented establish TNFSF4 as a global susceptibility gene in SLE. We have replicated and refined the 5′ association with disease and anchored risk and non-risk signals to the proximal TNFSF4 promoter region through our efforts in African-Americans, and in Europeans by virtue of increased power in this large cohort. Recombination at the locus in African-Americans, and the conditional regression strategies employed, focus the 5′ TNFSF4 association with disease to rs2205960-T. This variant uniquely tags the risk - haplotype in African-Americans and is strongly associated with disease in all groups tested. We find this marker segregates with autoantibody subsets in African-Americans, European and Amerindian/Hispanic groups. Furthermore, ChIP-Seq and bioinformatic data suggest that rs2205960-T sits within DNA that binds NF-κBp65 (RelA). This suggests that the risk allele would convey greater responsiveness of TNFSF4 expression to an NK-κB stimulus. Collectively, these data confirm cross-ancestral TNFSF4 association with SLE and suggest trans-ancestral mapping a useful strategy in complex traits.
Materials and Methods
Subjects
European samples held as part of the UK SLE and control collection held at Kings College London (KCL) were approved by 06/MRE02/009; additional AA samples from the CASSLE group were held at the University of Alabama at Birmingham (UAB) and approved by the UAB IRB. This study included over 17,900 SLE and control individuals of self-reported European, African-American (AA), AA-Gullah, East Asian and Hispanic/Amerindian ancestry. All cases fulfilled four or more of the 1997 ACR revised criteria for the classification of SLE and provided written informed consent. Samples were collected from multiple sites with Institutional Review Board (IRB) permission and processed at the Oklahoma Medical Research Foundation (OMRF) under guidance from the OMRF IRB.
Phenotypes
Clinical data on SLE manifestations in all subjects were obtained from medical record review performed at individual institutions, collected and processed at the OMRF, with additional phenotypic information from KCL, MUSC and UAB.
Genotyping and quality control
Genotyping was performed in two independent experiments on the Illumina iSelect platform at OMRF for combinations of haplotype tag SNPs and proxy variants capturing all common haplotypes, this meant we did not type all markers in all groups, marker selection was dictated by TNFSF4 locus architecture and included SNPs found to be associated in our previous European association study [4] and Hapmap phase 3 populations [31]. In all, 125 different SNPs in a 200 Kb region (chromosome 1, 171,400,000–171,600,000 NCBI build 36.3) encompassing the TNFSF4 gene and 5′ region were genotyped.
Population stratification bias and effects due to admixture were addressed by genotyping 347 genome-wide SNPs as used by Halder and colleagues [29] to correct for major ancestry. 20 Additional 1q25-specific ancestry markers were genotyped to correct for two-way admixture between Europeans and AAs. Within each population, Eigenstrat was used for principal components (PC) analysis and global ancestry estimates were additionally inferred by a combined Bayesian and sampling-theory approach (Admixmap). We spiked the African-American population with Yoruba, Tuscan and Northern/Western European Hapmap III individuals to cross-compare two-way admixed AAs with their founder populations (Supplementary Figure S1).
Markers with less than 90% genotyping efficiency were excluded from the analysis. Hardy-Weinberg Equilibrium (HWE) was assessed in control samples of each cohort. We included markers which deviated up to a P>0.01 threshold for HWE. We also included markers which had an acceptable HWE p-value in three of the four cohorts, if associated with SLE in multiple populations. Following filtering for duplicates, first-degree relatives, HWE, missingness and major ancestry, the non-imputed dataset comprised 111 TNFSF4 SNPs and 294 AIMs and 17900 samples prior to imputation (Table 1).
Imputation methods
Imputation of the genomic region from 173,112,930 to 173,349,886 (NCBI build 37) on chromosome 1q25.1 was performed using IMPUTE2.2 and the phased haplotypes from the 1000Genomes phase-1 integrated_v3 dataset [31]. Genotypes from our UK-Canadian GWAS (unpublished) were used as a second reference for the imputation of the European cohort (Table 1). Our aim was to fill missing gaps in the genotyping data and impute common markers (>1% MAF) missing between datasets to examine association at TNFSF4 and to better inform the structure of common haplotypes across the populations. We estimated concordance between imputed and true genotypes and Imputed SNPs were included in downstream analysis if SNP info scores exceeded 0.7 and a HWE>0.01. These criteria successfully filtered out all but the best-imputed SNPs.
Inference of population-specific recombination maps
We used FASTPHASE to phase 6272 unrelated control chromosomes (1568 from each population), randomly chosen after QC filtering. Rhomap from the LDhat2.0 package [32] was used to estimate population scale recombination rates in the presence of hotspots using pre-computed maximum likelihood tables in the analysis. Using the approach of Auton and colleagues, Rhomap was run for a total of 1,100,000 iterations including a burn-in of 100,000 iterations, the chain was sampled every 100 iterations after the burn-in. Each simulation incorporated 196 chromosomes meaning a total of 8 simulations were completed per group and the mean average recombination calculated between each pair of markers at the TNFSF4 locus. Simulations were executed in their entirety on 3 separate occasions to ensure there were no irregularities. The data did not change if we increased the parameters used. These analyses were then extended to infer recombination in phased chromosomes from African-American risk and non-risk homozygote individuals (Supplementary Figure S3).
Single marker association analyses
After QC filtering, single marker association and conditional data were generated using a case-control format and the continuous covariate function in SNPtestv2 under the additive model. We used a frequentist statistical paradigm and a probabilistic method for treating genotype uncertainty. Odds ratios (OR) with 95% confidence intervals (95% CI) were calculated using the beta statistic and 95% confidence intervals the SE. Data are represented as nominal uncorrected p-values.
Meta-analysis
We used a logistic regression model fitted with an interaction term (effect) in the R statistical package to investigate cross-study heterogeneity. P-values for individual associated SNPs were generated using the likelihood-ratio test. We found no evidence of cross-study heterogeneity for the key haplotype-tagging common variants which span the locus. These were rs1234314, rs1234317, rs2205960, rs12039904, and rs10912580. We have presented the results of a fixed-effects meta-.results for East Asians, Europeans and Hispanics and African-Americans to more powerfully estimate the true effect size (Table 3). The effect size across all datasets was computed using inverse variance weighting of each study.
Bifurcation
By using the Long Range Haplotype (LRH) test to look for common alleles with long-range linkage disequilibrium (LD), we were able to represent the breakdown of the risk haplotype, TNFSF4risk. TNFSF4risk was anchored by rs1234314 in all groups, a marker conveniently positioned at the boundary of the TNFSF4 gene and 5′ region. Haplotype bifurcation diagrams were generated in the program Sweep. Two SNPs which show best evidence of association after meta-analysis, rs1234317 and rs2205960, are marked on the scale of each bifurcation plot.
Haplotype association and conditional regression
Haplotypes in the TNFSF4 gene and 5′ region were generated using Haploview 4.2 using the custom algorithm, based on the R2 measure of linkage disequilibrium (LD). Markers and haplotypes with frequencies greater than 4% were included in the analyses. Haplotypes were anchored using tag SNP genotype data and boundaries were inferred using recombination data. SLE case-control association and step-wise conditional regression data for each haplotype was generated in PLINK, as were OR (95% CI) and the association is represented by nominal uncorrected p values.
Phenotypic association
Individuals with early age of SLE onset were classified using interquartile range and analysed using case–only format and case-control formats in SNPTest. Presence/absence of leukopenia and lymphopenia, anti-La, anti-Ro and anti-Sm autoantibody subsets, which are associated with SLE, together with renal disease, were analysed using both case-only and phenotype-control formats. Linear regression data of the most associated marker for each phenotype in each population was generated.
B cell isolation and cell stimulation
Peripheral blood mononuclear cells (PBMC) were isolated from 40 ml whole blood from a European individual using the ACCUSPIN System-Histopaque (Sigma-Aldrich). B lymphocytes, expressers of TNFSF4, were negatively selected using the Dynabeads Untouched Human B Cell kit (Invitrogen). Cell purity was assessed by FACS analysis of CD19-FITC-conjugated B cells and these were 97% pure. The cells were stimulated with 25 µg/ml anti-IgM-(Fab′)2, 0.1 µg CD40L and 0.1 µg enhancer of CD40L to upregulate TNFSF4. Upregulation of cell-surface TNFSF4 was assessed by FACS.
RNA isolation and 5′ rapid amplification of cDNA ends (RACE)
Total RNA was isolated using the TRIzol (Sigma) method from 5×106 B lymphocytes. 5′ ends of TNFSF4 transcripts were generated by the SMARTer RACE cDNA Amplification Kit (Clontech). Primer3 was used to design gene specific primers suitable for four alternative splicing variants predicted by the Aceview alternative splicing modelling tool [34]. During PCR a universal primer was added to the 5′ end of the cDNA. In combination with each transcript specific primer, cDNA was amplified up to the 5′ end as dictated by transcript sequence and in a positive control. In order to identify clones relevant for the TNFSF4 manuscript, we undertook colony hybridisation with a 32P-labelled probe specific for the 5′ region of TNFSF4 cDNA. Following colony selection, we cloned individual PCR products using the TOPO TA Cloning Kit (Invitrogen) in order to identify individual transcript isoforms. Bacterial cultures were mini-prepped as per manufactures instructions (QIAprep Spin Miniprep Kit, Qiagen). Samples were digested with EcoRI and different sized fragments sequenced and Blasted against transcript sequences.
Cis eQTL analysis
Genome-wide expression profiles stored in the Multiple Tissue Human Expression Resource (MuTHER) were available for download at http://www.muther.ac.uk/Data.html.
Bioinformatic analysis
Transcription factors (TFs) which are predicted to interact with DNA at the risk-associated TNFSF4 variants identified as part of this study were investigated in a sequence-specific manner. We analysed DNA-binding patterns at these locations using curated, non-redundant matrix profiles stored in the Jaspar core database [35]. In a complementary approach, putative risk loci were investigated using profiles derived from whole-genome ChIP-seq experiments on lymphoblastoid cell lines generated for the ENCODE project and stored in the Ensembl (http://www.ensembl.org/Homo_sapiens/encode.html), UCSC databases (http://genome.ucsc.edu/ENCODE/) and 1000genomes variant call format files downloaded from http://www.1000genomes.org/.
Ethics statement
In accordance with the Department of Health's Research Governance Framework for Health and Social Care, the research project titled ‘Genetics susceptibility of systemic lupus erythematosus (SLE)’ has received favourable approval from an ethics committee and approval from the Department of Research and Development prior to commencement.
Ethics number: 06/MRE02/009
Sponsor: Wellcome Trust
Funder: King's College London
End date: 31/03/2016
R&D Approval Date: 05/08/2011
Chief Investigator: Professor Timothy Vyse
Supporting Information
Zdroje
1. CroftM (2010) Control of immunity by the TNFR-related molecule OX40 (CD134). Annu Rev Immunol 28 : 57–78.
2. SoT, ChoiH, CroftM (2011) OX40 complexes with phosphoinositide 3-kinase and protein kinase B (PKB) to augment TCR-dependent PKB signalling. J Immunol 186 : 3547–55.
3. GriG, PiconeseS, FrossiB, ManfroiV, MerluzziS, et al. (2008) CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity 29 : 771–781.
4. Cunninghame GrahamDS, GrahamRR, MankuH, WongAK, WhittakerJC, et al. (2008) Polymorphism at the TNF Superfamily Gene OX40L Confers Susceptibility to Systemic Lupus Erythematosus. Nat Genet 40 : 83–9.
5. ZainiJ, AndariniS, TaharaM, SaijoY, IshiiN, et al. (2007) OX40 ligand expressed by DCs costimulates NKT and CD4+ Th cell antitumor immunity in mice. J Clin Invest 117 : 3330–3338.
6. SeshasayeeD, LeeWP, ZhouM, ShuJ, SutoE, et al. (2007) In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J Clin Invest 117 : 3868–3878.
7. ItoT, WangYH, DuramadO, HanabuchiS, PerngOA, et al. (2006) OX40 ligand shuts down IL-10-producing regulatory T cells. Proc Natl Acad Sci U S A 103 : 13138–13143.
8. CompaanDM, HymowitzSG (2006) The crystal structure of the costimulatory OX40-OX40L complex. Structure 14 : 1321–1330.
9. NoharaC, AkibaH, NakajimaA, InoueA, KohCS, et al. (2001) Amelioration of experimental autoimmune encephalomyelitis with anti-OX40 ligand monoclonal antibody: a critical role for OX40 ligand in migration, but not development, of pathogenic T cells. J Immunol 166 : 2108–2115.
10. RhodesB, VyseTJ (2008) The genetics of SLE: an update in the light of genome-wide association studies. Rheumatology. (Oxford) 47 : 1603–1611.
11. HomG, GrahamRR, ModrekB, TaylorKE, OrtmannW, et al. (2008) Association of systemic lupus erythematosus with C8orf13-BLK and ITGAM-ITGAX. N Engl J Med 358 : 900–909.
12. GrahamRR, CotsapasC, DaviesL, HackettR, LessardCJ, et al. (2008) Genetic variants near TNFAIP3 on 6q23 are associated with systemic lupus erythematosus. Nat Genet 40 : 1059–61.
13. HarleyJB, Alarcon-RiquelmeME, CriswellLA, JacobCO, KimberlyRP, et al. (2008) Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet 40 : 204–210.
14. HanJW, ZhengHF, CuiY, SunLD, YeDQ, et al. (2009) Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nat Genet 41 : 1234–1237.
15. YangW, ShenN, YeDQ, LiuQ, ZhangY, et al. (2010) Genome-wide association study in Asian populations identifies variants in ETS1 and WDFY4 associated with systemic lupus erythematosus. PLOS Genet 6: e1000841.
16. BrycK, VelezC, KarafetT, Moreno-EstradaA, ReynoldsA, et al. (2010) Colloquium paper: genome-wide patterns of population structure and admixture among Hispanic/Latino populations. Proc Natl Acad Sci U S A 107 : 8954–8961.
17. NordmarkG, KristjansdottirG, TheanderE, AppelS, ErikssonP, et al. (2011) Association of EBF1, FAM167A (C8orf13)-BLK and TNFSF4 gene variants with primary Sjögren's syndrome. Genes Immun 12 : 100–9.
18. WangX, RiaM, KelmensonPM, ErikssonP, HigginsDC, et al. (2005) Positional identification of TNFSF4, encoding OX40 ligand, as a gene that influences atherosclerosis susceptibility. Nat Genet 37 : 365–72.
19. RiaM, LagercrantzJ, SamnegårdA, BoquistS, HamstenA, et al. (2011) Common polymorphism in the promoter region of the TNFSF4 gene is associated with lower allele-specific expression and risk of myocardial infarction. PLOSOne 18: e17652.
20. FosterMW, SharpRR (2004) Beyond race: towards a whole-genome perspective on human populations and genetic variation. Nat Rev Genet 5 : 790–796.
21. MolinaJF, MolinaJ, GarciaC, GharaviAE, WilsonWA (1997) Ethnic differences in the clinical expression of systemic lupus erythematosus: a comparative study between African-Americans and Latin Americans. Lupus 6 : 63–67.
22. FernandezM, AlarconGS, Calvo-AlenJ, AndradeR, McGwinGJr, et al. (2007) A multiethnic, multicenter cohort of patients with systemic lupus erythematosus (SLE) as a model for the study of ethnic disparities in SLE. Arthritis Rheum 57 : 576–584.
23. WinklerCA, NelsonGW, SmithMW (2010) Admixture mapping comes of age. Annu Rev Genomics Hum Genet 11 : 65–89.
24. SebastianiP, LazarusR, WeissST, KunkelLM, KohaneIS (2003) Minimal haplotype tagging. Proc Natl Acad Sci U S A 100 : 9900–9905.
25. DalyMJ, RiouxJD, SchaffnerSF, HudsonTJ, LanderES (2001) High-resolution haplotype structure in the human genome. Nat Genet 29 : 229–232.
26. PriceAL, PattersonN, YuF, CoxDR, WaliszewskaA, et al. (2007) A genomewide admixture map for Latino populations. Am J Hum Genet 80 : 1024–1036.
27. KamenDL, BarronM, ParkerTM, ShaftmanSR, BrunerGR, et al. (2008) Autoantibody prevalence and lupus disease characteristics in a unique African American population. Arthritis Rheum 58 : 1237–47.
28. NamjouB, SestakAL, ArmstrongDL, ZidovetzkiR, KellyJA, et al. (2009) High-density genotyping of STAT4 reveals multiple haplotypic associations with systemic lupus erythematosus in different racial groups. Arthritis Rheum 60 : 1085–1095.
29. HalderI, ShriverM, ThomasM, FernandezJR, FrudakisT (2008) A panel of ancestry informative markers for estimating individual biogeographical ancestry and admixture from four continents: utility and applications. Hum Mutat 29 : 648–658.
30. The 1000 Genomes Project Consortium (2010) A map of human genome variation from population-scale sequencing. Nature 467 : 1061–73.
31. The International HapMap Consortium (2003) The International HapMap Project. Nature 426 : 789–796.
32. AutonA, McVeanG (2007) Recombination rate estimation in the presence of hotspots. Genome Res 17 : 1219–1227.
33. TanEM, CohenAS, FriesJF, MasiAT, McShaneDJ, et al. (1997) The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982; 25 : 1271–7. and Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus [letter]. Arthritis Rheu 40 : 1725.
34. Thierry-MiegD, Thierry-MiegJ (2006) AceView: a comprehensive cDNA-supported gene and transcripts annotation. Genome Biol 7 Suppl 1: S12–S14.
35. GrundbergE, SmallKS, HedmanÅK, NicaAC, BuilA, et al. (2012) Mapping cis - and trans-regulatory effects across multiple tissues in twins. Nat Genet 44 (10) 1084–9.
36. Portales-CasamarE, ThongjueaS, KwonAT, ArenillasD, ZhaoX, et al. (2010) JASPAR 2010: the greatly expanded open-access database of transcription factor binding profiles. Nucleic Acids Res 38: D105–10.
37. ENCODE Project Consortium (2012) BernsteinBE, BirneyE, DunhamI, GreenED, et al. (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489 : 57–74.
38. IkushimaS, InukaiT, InabaT, NimerSD, ClevelandJL, et al. (1997) Pivotal role for the NFIL3/E4BP4 transcription factor in interleukin 3-mediated survival of pro-B lymphocytes. Proc Natl Acad Sci U S A 94 : 2609–2614.
39. Delgado-VegaAM, AbelsonAK, SanchezE, WitteT, D'AlfonsoS, et al. (2009) Replication of the TNFSF4 (OX40L) promoter region association with systemic lupus erythematosus. Genes Immun 10 : 248–253.
40. SanchezE, WebbRD, RasmussenA, KellyJA, RibaL, et al. (2010) Genetically determined Amerindian ancestry correlates with increased frequency of risk alleles for systemic lupus erythematosus. Arthritis Rheum 12 : 3722–9.
Štítky
Genetika Reprodukční medicína
Článek Independent Evolution of Transcriptional Inactivation on Sex Chromosomes in Birds and MammalsČlánek The bHLH Subgroup IIId Factors Negatively Regulate Jasmonate-Mediated Plant Defense and DevelopmentČlánek Selective Pressures to Maintain Attachment Site Specificity of Integrative and Conjugative ElementsČlánek Reassembly of Nucleosomes at the Promoter Initiates Resilencing Following Decitabine ExposureČlánek Hepatocyte Growth Factor Signaling in Intrapancreatic Ductal Cells Drives Pancreatic Morphogenesis
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 7
-
Všechny články tohoto čísla
- An Solution for Crossover Formation
- Genome-Wide Association Mapping in Dogs Enables Identification of the Homeobox Gene, , as a Genetic Component of Neural Tube Defects in Humans
- Independent Evolution of Transcriptional Inactivation on Sex Chromosomes in Birds and Mammals
- Stepwise Activation of the ATR Signaling Pathway upon Increasing Replication Stress Impacts Fragile Site Integrity
- Genomic Analysis of Natural Selection and Phenotypic Variation in High-Altitude Mongolians
- Modification of tRNA by Elongator Is Essential for Efficient Translation of Stress mRNAs
- Role of CTCF Protein in Regulating Locus Transcription
- Gene Set Signature of Reversal Reaction Type I in Leprosy Patients
- Mapping of PARK2 and PACRG Overlapping Regulatory Region Reveals LD Structure and Functional Variants in Association with Leprosy in Unrelated Indian Population Groups
- Is Required for Formation of the Genital Ridge in Mice
- Monopolin Subunit Csm1 Associates with MIND Complex to Establish Monopolar Attachment of Sister Kinetochores at Meiosis I
- Recombination Dynamics of a Human Y-Chromosomal Palindrome: Rapid GC-Biased Gene Conversion, Multi-kilobase Conversion Tracts, and Rare Inversions
- Mechanisms of Protein Sequence Divergence and Incompatibility
- Histone Methyltransferase DOT1L Drives Recovery of Gene Expression after a Genotoxic Attack
- Female Behaviour Drives Expression and Evolution of Gustatory Receptors in Butterflies
- Combinatorial Regulation of Meiotic Holliday Junction Resolution in by HIM-6 (BLM) Helicase, SLX-4, and the SLX-1, MUS-81 and XPF-1 Nucleases
- The bHLH Subgroup IIId Factors Negatively Regulate Jasmonate-Mediated Plant Defense and Development
- The Role of Interruptions in polyQ in the Pathology of SCA1
- Dietary Restriction Induced Longevity Is Mediated by Nuclear Receptor NHR-62 in
- Fine Time Course Expression Analysis Identifies Cascades of Activation and Repression and Maps a Putative Regulator of Mammalian Sex Determination
- Genome-scale Co-evolutionary Inference Identifies Functions and Clients of Bacterial Hsp90
- Oxidative Stress and Replication-Independent DNA Breakage Induced by Arsenic in
- A Moonlighting Enzyme Links Cell Size with Central Metabolism
- Budding Yeast Greatwall and Endosulfines Control Activity and Spatial Regulation of PP2A for Timely Mitotic Progression
- The Conserved Intronic Cleavage and Polyadenylation Site of CstF-77 Gene Imparts Control of 3′ End Processing Activity through Feedback Autoregulation and by U1 snRNP
- The BTB-zinc Finger Transcription Factor Abrupt Acts as an Epithelial Oncogene in through Maintaining a Progenitor-like Cell State
- The Cohesion Protein SOLO Associates with SMC1 and Is Required for Synapsis, Recombination, Homolog Bias and Cohesion and Pairing of Centromeres in Drosophila Meiosis
- The RNA-binding Proteins FMR1, Rasputin and Caprin Act Together with the UBA Protein Lingerer to Restrict Tissue Growth in
- Pattern Dynamics in Adaxial-Abaxial Specific Gene Expression Are Modulated by a Plastid Retrograde Signal during Leaf Development
- A Network of HMG-box Transcription Factors Regulates Sexual Cycle in the Fungus
- Bacterial Adaptation through Loss of Function
- ENU-induced Mutation in the DNA-binding Domain of KLF3 Reveals Important Roles for KLF3 in Cardiovascular Development and Function in Mice
- Interplay between Structure-Specific Endonucleases for Crossover Control during Meiosis
- FGF Signalling Regulates Chromatin Organisation during Neural Differentiation via Mechanisms that Can Be Uncoupled from Transcription
- The Arabidopsis RNA Binding Protein with K Homology Motifs, SHINY1, Interacts with the C-terminal Domain Phosphatase-like 1 (CPL1) to Repress Stress-Inducible Gene Expression
- Selective Pressures to Maintain Attachment Site Specificity of Integrative and Conjugative Elements
- The Conserved ADAMTS-like Protein Lonely heart Mediates Matrix Formation and Cardiac Tissue Integrity
- The cGMP-Dependent Protein Kinase EGL-4 Regulates Nociceptive Behavioral Sensitivity
- RBM5 Is a Male Germ Cell Splicing Factor and Is Required for Spermatid Differentiation and Male Fertility
- Disease-Related Growth Factor and Embryonic Signaling Pathways Modulate an Enhancer of Expression at the 6q23.2 Coronary Heart Disease Locus
- Yeast Pol4 Promotes Tel1-Regulated Chromosomal Translocations
- A Dual Role for SOX10 in the Maintenance of the Postnatal Melanocyte Lineage and the Differentiation of Melanocyte Stem Cell Progenitors
- SLC26A4 Targeted to the Endolymphatic Sac Rescues Hearing and Balance in Mutant Mice
- Odoriferous Defensive Stink Gland Transcriptome to Identify Novel Genes Necessary for Quinone Synthesis in the Red Flour Beetle,
- Prediction of Complex Human Traits Using the Genomic Best Linear Unbiased Predictor
- Gene × Physical Activity Interactions in Obesity: Combined Analysis of 111,421 Individuals of European Ancestry
- Reassembly of Nucleosomes at the Promoter Initiates Resilencing Following Decitabine Exposure
- Exquisite Light Sensitivity of Cryptochrome
- miR-133a Regulates Adipocyte Browning In Vivo
- Strabismus Promotes Recruitment and Degradation of Farnesylated Prickle in Planar Polarity Specification
- Hepatocyte Growth Factor Signaling in Intrapancreatic Ductal Cells Drives Pancreatic Morphogenesis
- Is a Potential Tumor Suppressor Gene Commonly Inactivated by Epigenetic Mechanisms in Colorectal Cancer
- Joint Molecule Resolution Requires the Redundant Activities of MUS-81 and XPF-1 during Meiosis
- The Mating Competence of Geographically Diverse Strains in Their Natural and Unnatural Sand Fly Vectors
- Defective Repair of Oxidative Base Lesions by the DNA Glycosylase Nth1 Associates with Multiple Telomere Defects
- Effective Blocking of the Enhancer Requires Cooperation between Two Main Mechanisms Suggested for the Insulator Function
- Trans-Ancestral Studies Fine Map the SLE-Susceptibility Locus
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- SLC26A4 Targeted to the Endolymphatic Sac Rescues Hearing and Balance in Mutant Mice
- Bacterial Adaptation through Loss of Function
- The Cohesion Protein SOLO Associates with SMC1 and Is Required for Synapsis, Recombination, Homolog Bias and Cohesion and Pairing of Centromeres in Drosophila Meiosis
- Gene × Physical Activity Interactions in Obesity: Combined Analysis of 111,421 Individuals of European Ancestry
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



