-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaOdoriferous Defensive Stink Gland Transcriptome to Identify Novel Genes Necessary for Quinone Synthesis in the Red Flour Beetle,
Chemical defense is one of the most important traits, which endow insects the ability to conquer a most diverse set of ecological environments. Chemical secretions are used for defense against anything from vertebrate or invertebrate predators to prokaryotic or eukaryotic parasites or food competitors. Tenebrionid beetles are especially prolific in this category, producing several varieties of substituted benzoquinone compounds. In order to get a better understanding of the genetic and molecular basis of defensive secretions, we performed RNA sequencing in a newly emerging insect model, the red flour beetle Tribolium castaneum (Coleoptera: Tenebrionidae). To detect genes that are highly and specifically expressed in the odoriferous gland tissues that secret defensive chemical compounds, we compared them to a control tissue, the anterior abdomen. 511 genes were identified in different subtraction groups. Of these, 77 genes were functionally analyzed by RNA interference (RNAi) to recognize induced gland alterations morphologically or changes in gland volatiles by gas chromatography-mass spectrometry. 29 genes (38%) presented strong visible phenotypes, while 67 genes (87%) showed alterations of at least one gland content. Three of these genes showing quinone-less (ql) phenotypes – Tcas-ql VTGl; Tcas-ql ARSB; Tcas-ql MRP – were isolated, molecularly characterized, their expression identified in both types of the secretory glandular cells, and their function determined by quantification of all main components after RNAi. In addition, microbe inhibition assays revealed that a quinone-free status is unable to impede bacterial or fungal growth. Phylogenetic analyses of these three genes indicate that they have evolved independently and specifically for chemical defense in beetles.
Published in the journal: . PLoS Genet 9(7): e32767. doi:10.1371/journal.pgen.1003596
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003596Summary
Chemical defense is one of the most important traits, which endow insects the ability to conquer a most diverse set of ecological environments. Chemical secretions are used for defense against anything from vertebrate or invertebrate predators to prokaryotic or eukaryotic parasites or food competitors. Tenebrionid beetles are especially prolific in this category, producing several varieties of substituted benzoquinone compounds. In order to get a better understanding of the genetic and molecular basis of defensive secretions, we performed RNA sequencing in a newly emerging insect model, the red flour beetle Tribolium castaneum (Coleoptera: Tenebrionidae). To detect genes that are highly and specifically expressed in the odoriferous gland tissues that secret defensive chemical compounds, we compared them to a control tissue, the anterior abdomen. 511 genes were identified in different subtraction groups. Of these, 77 genes were functionally analyzed by RNA interference (RNAi) to recognize induced gland alterations morphologically or changes in gland volatiles by gas chromatography-mass spectrometry. 29 genes (38%) presented strong visible phenotypes, while 67 genes (87%) showed alterations of at least one gland content. Three of these genes showing quinone-less (ql) phenotypes – Tcas-ql VTGl; Tcas-ql ARSB; Tcas-ql MRP – were isolated, molecularly characterized, their expression identified in both types of the secretory glandular cells, and their function determined by quantification of all main components after RNAi. In addition, microbe inhibition assays revealed that a quinone-free status is unable to impede bacterial or fungal growth. Phylogenetic analyses of these three genes indicate that they have evolved independently and specifically for chemical defense in beetles.
Introduction
Insects are among the most diverse group of animals on the planet and amazingly include more than a million described species, which is more than half of all known living organisms [1], [2]. Moreover, they have conquered almost every environment on earth. From a series of distinctive attributes that orchestrate together to endow them with the ability to live in a wide range of ecological environments, chemical defense is one of the most important traits [3]. Many chemical secretions have repellent or irritant properties [4], [5]. Tenebrionid beetles are especially prolific by producing several various substituted benzoquinone compounds [5]–[9]. Tribolium beetles (Coleoptera: Tenebrionidae) have dragged attentions of researchers to their particular secretions, since it was noted that their flour medium turns pink over time due to the secretion of a gaseous substance from adults [10], which also deleteriously affects the viscous and elastic properties of dough made from such infested flour [11].
Tribolium beetles possess for the purpose of chemical defense two pairs of specialized secretory organs – one in the prothorax and one in the posterior abdomen – termed odoriferous or stink glands [12]. The glands located in the prothorax are called prothoracic, thoracic, or anterior glands, while the other pair in the abdomen is referred to as abdominal, posterior, or pygidial glands. The fine structure of these glands revealed two types of secretory units composed of two slightly different types of cells with particular vesicular organelles (cell type 1 and cell type 2), tubules, reservoir, ducts and muscles [12]–[14]. At least four members of the genus Tribolium (T. anaphe, T. castaneum, T. confusum, and T. destructor) use the glands to produce the quinone derivatives 2-methoxybenzoquinone, ethyl-1,4-benzoquinone (EBQ), and methyl-1,4-benzoquinone (MBQ) [9], [12], [14]–[18]. However, only the latter two substances were detected in T. confusum [4], [6]. Besides benzoquinone derivatives, hydrocarbons were also reported as major secretion components. T. confusum secrets 1-pentadecene [19], 1,6-pentadecadiene and smaller amounts of 1-hexadecene, 1,6-hexadecadiene, hexadecatriene, 1-heptadecene, 1,8-heptadecadiene and heptadecatriene [20], [21]. In T. castaneum, 1-pentadecene and 1,6-pentadecadiene were identified, with the former as the main component [9], [18], plus two still unidentified hydrocarbons were found [18].
The red flour beetle, T. castaneum has been developed into a highly sophisticated genetic model organism [22] with plenty of genetic and genomic tools: reverse genetics based on systemic RNA interference [23], [24], forward genetics based on insertional mutagenesis [25], [26], transgene-based mis-expression systems [27], [28], as well as a fully annotated genome sequence [29]. Moreover, several mutants with odoriferous gland phenotypes, such as melanotic stink glands (msg, with both pairs of glands melanized) [30], tar (only prothoracic glands are darkly pigmented), and box (Abox, similar to tar, but only the abdominal glands are affected) [31] are known. For quinone biosynthesis, it has been reported in another quinone-producing tenebrionid beetle Eleodes longicollis that alkylated benzoquinones are formed by acetate condensation whereas the p-benzoquinones are generated from preformed aromatic rings of amino acids (tyrosine and phenylalanine) [5], [32], [33]. In the glandular secretory cells, p-quinones are in a form of phenolic ß-glucoside contained in the more apical part, which were then transferred to inner part of the gland and form active quinones by a series of enzymatic reactions [14]. The alkenes are biosynthesized from fatty acids by oxidative decarboxylation [21]. However, no data are available on the genes involved in these processes. In addition, arthropods have evolved mechanisms to reduce the autointoxicative effects of a variety of toxic compounds produced by themselves for defense purpose [5]. For example, a paradoxsomatid millipede, Oxidus gracilis, which produces toxic phenol from tyrosine, possesses the ability to rapidly convert the exogenous phenol to tyrosine by tyrosine phenol lyase [34]. Benzoquinones are highly reactive, unstable and also toxic. Besides the purpose of chemical defense, Tribolium beetles and other insects use them also as tanning agent and to sclerotize cuticles [35]–[37], which requires perfect handling and detoxication systems. Thus understanding of the mechanisms involved in the autodetoxication of the defensive compounds might provide inspirations to manage this cosmopolitan pest and potentially other coleopteran pests. Obviously, tenebrionids are protected from their own toxic secretions by cuticular linings both internally and externally [5]. Tribolium beetles have the ability to partition the secretion away from the somatic cells, firstly by producing the secretions in the cuticle-lined organelles [14] and then keeping them in storage sacs (reservoirs) that are formed from invaginations of the cuticle [12]. The newly emerged Tribolium adults lack the defensive secretions, implying the need for building up an adequate self-protective barrier [8]. Consequently, if such self-protection systems could be broken, the pests will harm themselves. Here, we present the first transcriptome data on beetle stink glands, which allowed us to identify three genes necessary for the production of defensive quinones.
Results
Stink gland transcriptome sequencing
The odoriferous defensive stink glands were dissected and identified by their special morphological structures, vesicular organelles [13], [14] (Figure S1). mRNA sequencing was performed in six stink gland samples and one control sample (anterior abdomen, where no odoriferous glands are located) on a next generation sequencing (NGS) platform. The abbreviations for the samples were, s1: sample 1, anterior abdomen from wild-type; s2: prothoracic glands from tar mutant; s3: wild-type male prothoracic glands; s4: wild-type female prothoracic glands; s5: wild-type male abdominal glands; s6: wild-type female abdominal glands. After sequencing, 27.8 to 29.7 million reads were obtained from each sample. About 50% of them were successfully mapped to the reference database, i.e. mRNAs of the Tribolium official gene set (OGS) in Beetlebase [22], [38]. And the average depths/coverages were in the range of 23.5 to 27.8. Moreover, ratios of covered region in reference varied from 52.5% to 68.0%. Detailed statistics are presented in Table 1. The mapping of the different transcriptomics samples as individual tracks to the newest Tribolium genome assembly is provided by the public iBeetle Genome Browser (http://bioinf.uni-greifswald.de/gb2/gbrowse/tcas).
Tab. 1. Statistics of transcriptome sequencing. 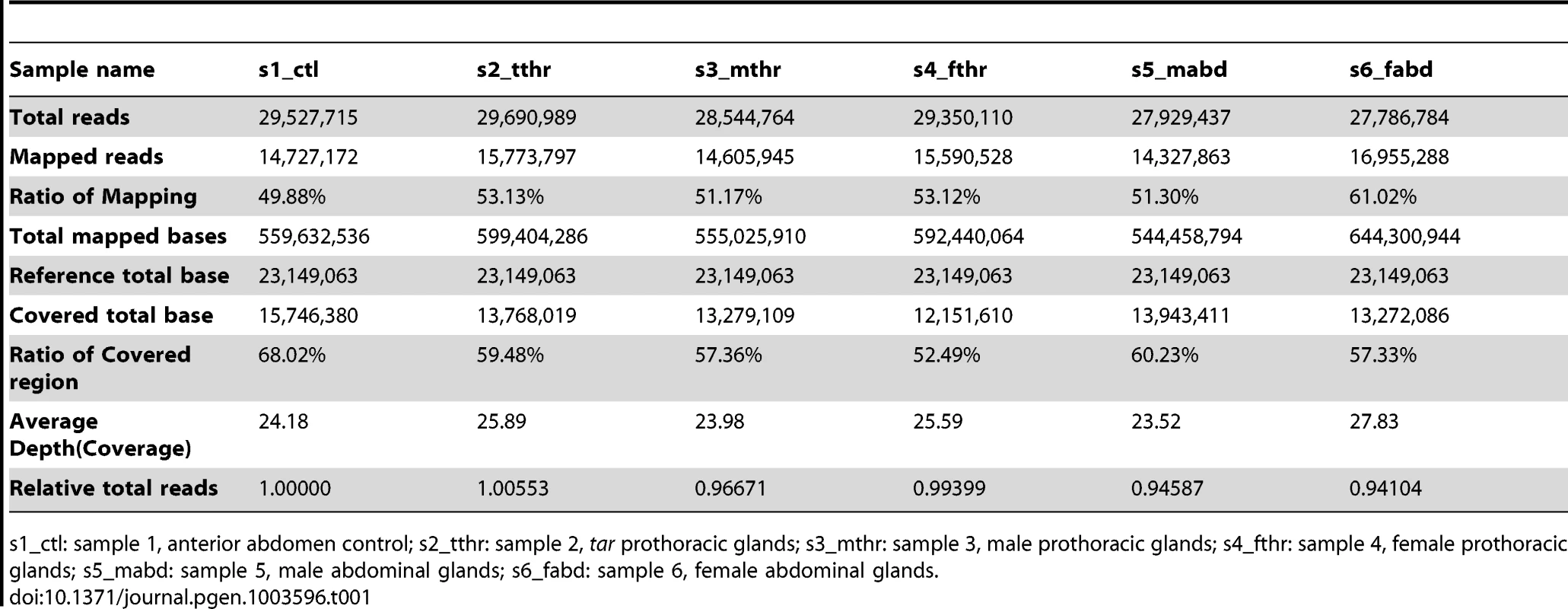
s1_ctl: sample 1, anterior abdomen control; s2_tthr: sample 2, tar prothoracic glands; s3_mthr: sample 3, male prothoracic glands; s4_fthr: sample 4, female prothoracic glands; s5_mabd: sample 5, male abdominal glands; s6_fabd: sample 6, female abdominal glands. mRNA-seq library subtractions
The constructed mRNA-seq libraries are presented in Dataset S1, which shows the reads and coverage (depth) of each gene in all the samples, respectively. Table 1 indicates that the relative total reads of all samples were quite close to each other. Therefore, the read number represented actual expression levels of all the Tribolium genes in various samples. In order to screen out the differentially or specifically expressed genes in odoriferous glands, ten different groups of subtractions were performed and in total 511 genes were identified (Figure 1; for detailed list and the subtractive conditions, see Dataset S2). There were 62 genes in Group 1, standing for the gland-specific genes, which were highly expressed in all wild-type gland samples [s3, s4, s5, s6] but not in the anterior abdomen control [s1]. Group 2 presented 23 thoracic gland-specific genes, which had higher reads in wild-type thoracic glands [s3 and s4] than in wild-type abdominal glands [s5 and s6 (and s1 control)]. Group 3 had 40 abdominal gland-specific genes [s5 and s6 against s3, s4 (and s1)]. For sex related subtractions, Group 4 offered zero male stink gland-specific genes [s3 and s5 against s4, s6 (and s1)], but Group 5 had four female stink gland-specific genes [s4 and s6 against s3 and s5]. There were zero male thoracic gland-specific genes in Group 6 [s3 against s4 and s1], yet 299 male abdominal gland-specific genes in Group 7 [s5 against s6 and s1]. Meanwhile, there were also zero female thoracic gland-specific genes in Group 8 [s4 against s3 and s1], but 39 female abdominal gland-specific genes in Group 9 [s6 against s5 and s1]. And Group 10 presented 44 genes which were either up or down regulated in prothoracic glands of tar mutants. The high number of genes identified in Group 7 is probably due to the contamination of the abdominal gland sample by male accessory glands, which are hard to separate from the abdominal glands.
Fig. 1. Odoriferous gland transcriptome screening result. 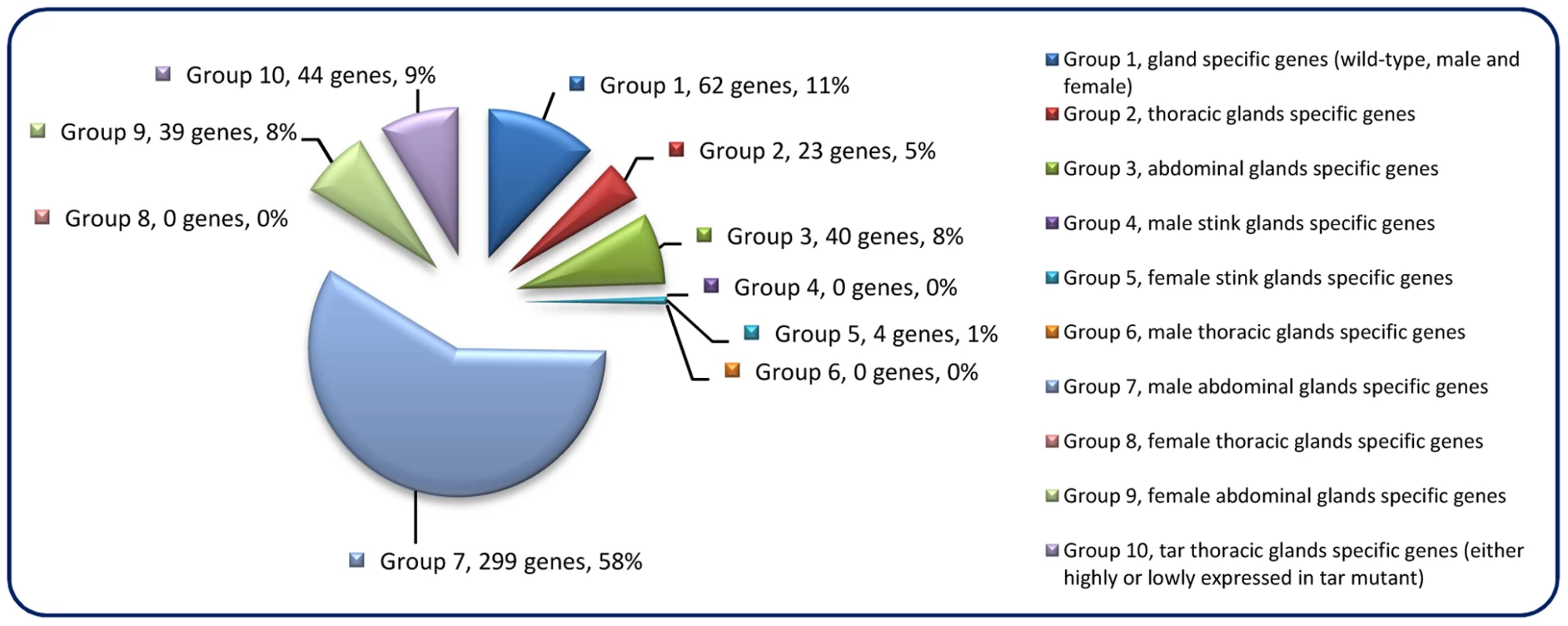
Each pie slice is indicated with the group name, the number of genes classified, and their percentage. Each group is signified as a specific non-overlapping subtraction group with each gene belonging to only one group. E.g. Group 1 consists of genes that are highly expressed in all glands but not specifically in one gland type or only in one sex. Gene ontology annotation
Gene ontology (GO) annotation allows meta-analyses of gene populations and associates the targeted genes to specific terms with hierarchical vocabularies describing three independent ontologies: biological process, molecular function, and cellular component [39]. Analyses were performed with 1451 genes abundant in the control, 1206 genes abundant in wild-type glands, and the 511 genes from subtraction Group 1 to Group 10 (The genes are listed in Dataset S2). Results (Figure 2A) showed that many genes were classified to metabolic and cellular processes in the GO term of biological process, and to catalytic activity and binding in molecular function. For the cellular component, most genes belonged to cell, macromolecular complex and organelle. These implied the existence of strong metabolisms in both gland and anterior abdomen. Moreover, similar trends were observed in separated analyses in the different subtraction groups (Figure 2B). Detailed GO results are presented in Dataset S3.
Fig. 2. GO annotation of odoriferous glands transcriptome data. 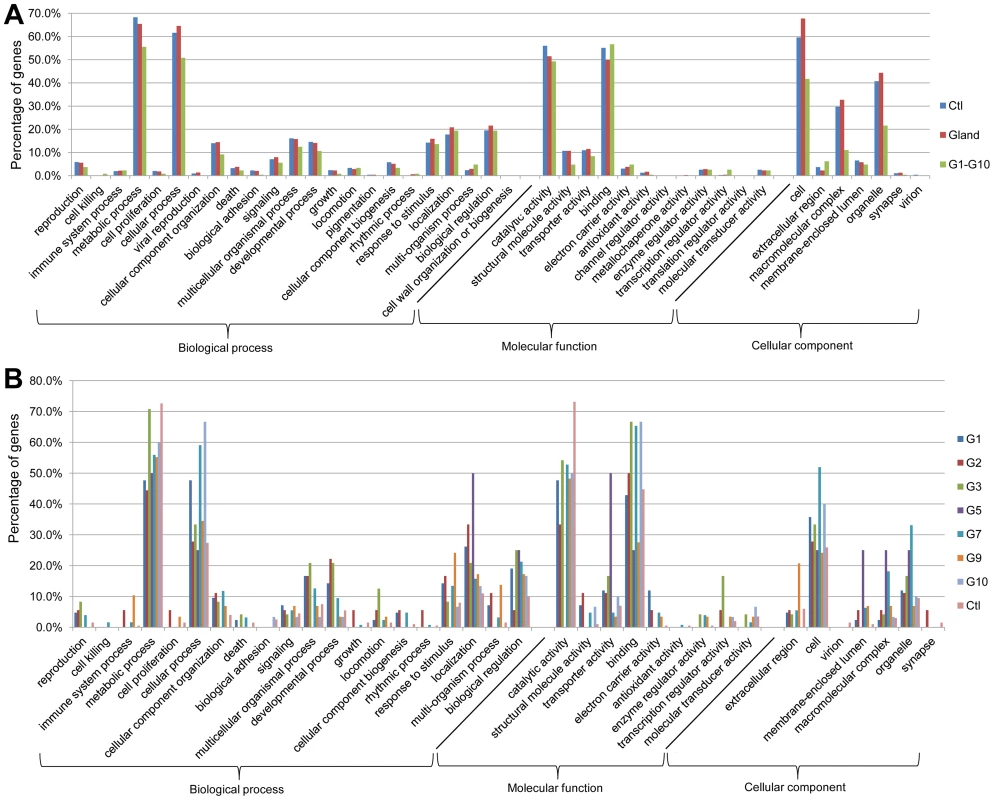
A, analyses of the genes abundant in control (Ctl; 1451 genes abundant in anterior abdomen transcriptome), wild-type glands (Glands; 1206 genes), and the genes identified in all ten subtraction groups (Figure 1) together (G1–G10; 511 genes). B, analyses of individual Group 1 to Group 10, respectively and the control (Ctl; 290 genes possessing at least 64 times higher reads in anterior abdomen than the wild-type gland samples). X-axis: different GO terms (level 2); Y-axis: percentage of the genes classified in each group. Transcriptomic exploration of candidate genes for quinone synthesis
Glucosidases, phenol oxidases, and peroxidases have been considered to be involved in the production of quinones in the odoriferous glands [14] and were annotated in the Tribolium genome [29]. In our stink gland transcriptome analysis, we have now explored these candidate genes for expression at the gland transcriptome level (Figure 3, details in Dataset S4). In total, 19 glucosidase, 14 phenol oxidase, and 18 peroxidase encoding genes were identified through blast searches and conserved domain confirmation. Transcriptomic explorations revealed that at least four glucosidase (TC000223, TC000537, TC002741, and TC011354), five phenol oxidase (TC000821, TC005376, TC006769, TC010489, and TC10490) and four peroxidase (TC010355, TC010362, TC012328 and TC014929) genes have increased reads in the gland samples, which confirms the importance of these three types of enzymes in defensive secretions and verifies the reliability of our transcriptome data.
Fig. 3. Annotated quinone synthesis-related genes and their relative gland transcriptome expression levels. 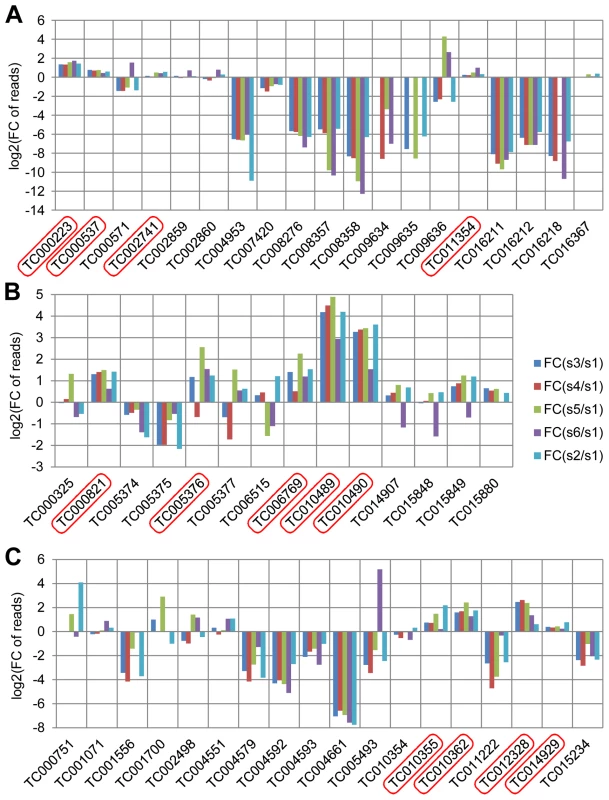
A, glucosidases, 19 genes; B, phenol oxidases, 14 genes; C, peroxidases, 18 genes were annotated. In all charts, along the X-axes the different genes are presented, while the Y-axes present log2[fold change of reads in glands against control]. Abbreviations: FC: fold change; s1: sample 1, anterior abdomen as a control; s3: sample 3, male prothoracic glands; s4: sample 4, female prothoracic glands; s5: sample 5, male abdominal glands; s6: sample 6, female abdominal glands; s2: sample 2, tar prothoracic glands. The genes with higher reads in gland samples are marked with red squares. Functional analysis of the most highly and gland-specifically expressed genes
In order to find novel gene functions involved in quinone synthesis, we functionally analyzed 77 genes from transcriptomic subtraction groups 1, 2, and 10 that were at least 64× higher expressed in the glands compared to the control tissue. RNAi of these genes resulted in various abnormal visible phenotypes (Figure 4). Additionally, gas chromatography–mass spectrometry (GC-MS) measurements revealed the alterations of different chemical components in both pairs of glands (an example of the chromatogram is depicted in Figure S2). The main components identified are listed in Table 2. Based on the extents of alterations of the chemicals, phenotypes were classified into six strengths (Figure 5A). 29 genes (38%, strength 1–3) showed strong changes, i.e. more than 75% reduction of at least one component, which were mostly accompanied with visible phenotypes (Figure 5B). In total, 67 of 77 genes (87%) showed alterations of at least one secreted chemical. Detailed descriptions on the phenotypic changes of all the 77 genes can be found in Dataset S5. In addition, gland cellular morphology was explored in all the 29 genes with strong phenotypes, but no visible abnormalities were observed in the secretory cells (data not shown).
Fig. 4. Visible morphological gland phenotypes after RNAi. 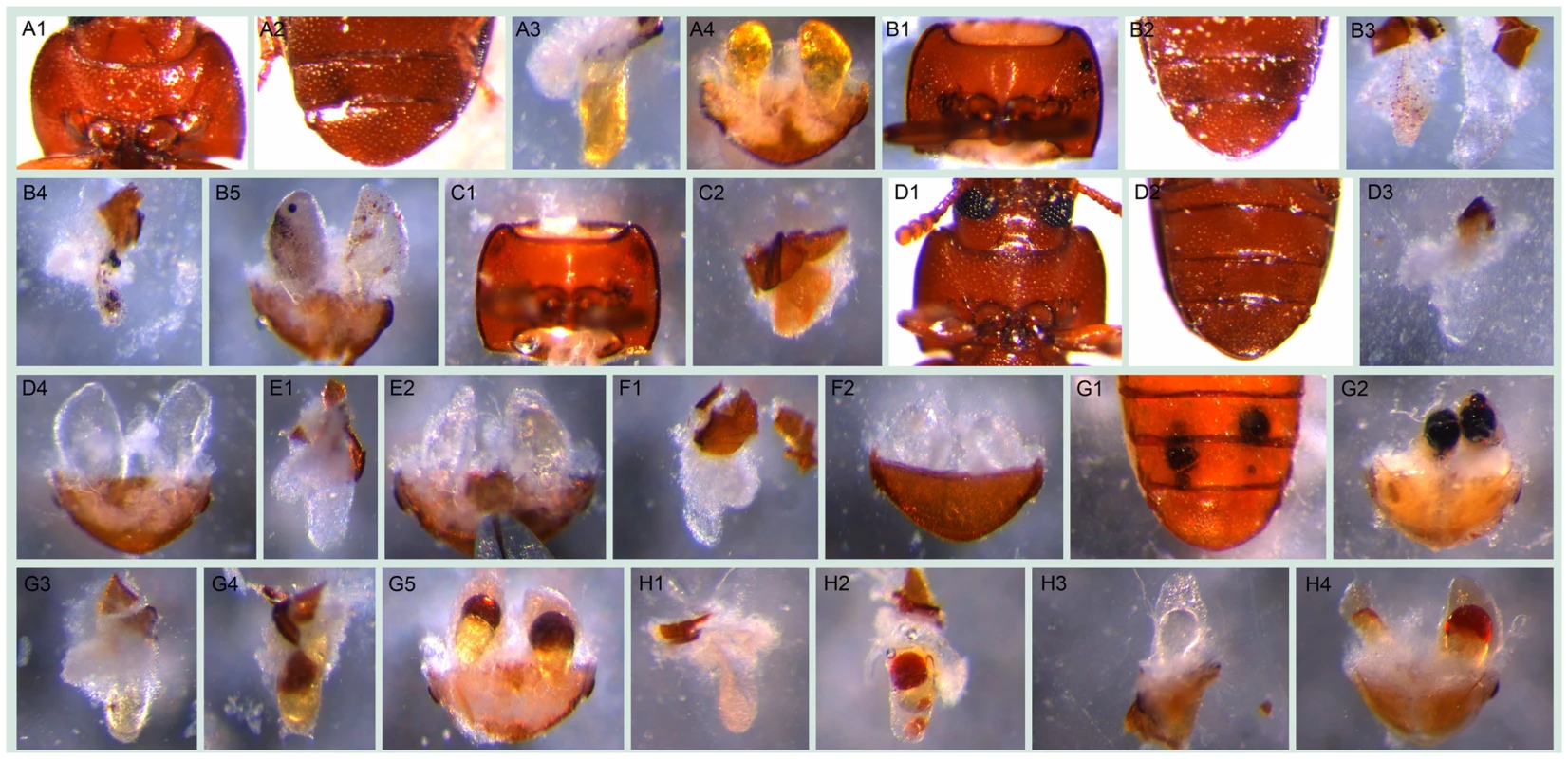
A1–A4, wild-type: A1, thr (darker regions on two sides); A2, abd (darker regions on the tip); A3, thrg reservoir filled with yellowish oily fluid ; A4, abdg; B1–B5, GT20 knock-down: B1, thr, visible melanized dots on both sides; B2, abd, darker gland regions; B3, thrg, left one, small localized brown dots, right one, tiny localized brown dots accompanied by colorless oily secretions; B4, thrg, localized melanized dots; B5, abdg, localized melanized (left) or brown (right) dots; C1–C2, GT47 knock-down: C1, thr; C2, thrg, condensed secretions, i.e. not as semitransparent as in wild-type and close to solid; D1–D4, GT62 knock-down: D1–D2, thr and abd with the gland regions almost invisible through cuticle; D3, thrg, very few colorless secretions, in some cases, empty-looking (not shown); D4, abdg, colorless secretions; E1–E2, GT39 knock-down, thrg (E1) and abdg (E2), colorless secretions; F1–F2, GT63 knock-down, thrg (F1) and abdg (F2, ventral view), colorless secretions; G1–G5, GT02 knock-down, at stage A10 localized melanized dots (G1, abd; G2, abdg), at stage 24 localized brown dots to different extent (G3 and G4, thrg, G5, abdg); H1–H4, GT25 knock-down, various phenotypes: H1 and H2, thrg, tiny (H1) or big (H2) localized brown dots; H3, left half abdg, oily colorless secretions; H4, abdg, localized brown dots. The beetles used for G3–G5 were from stage A24, all the others were at stage A10. The statistics of the visible phenotypes are in Figure 5B. Abbreviations used in this legend: stage A10, adults ten days; A24, twenty four days after eclosure; abd, abdominal tip, ventral view; abdg, dissected abdominal tip with abdominal glands, dorsal view; thr, prothorax, ventral view; thrg, dissected prothoracic gland(s), with the opening on top, the secretory cells are attached to the reservoir. Fig. 5. Phenotype classifications of 77 highly gland-specifically expressed genes by RNAi. 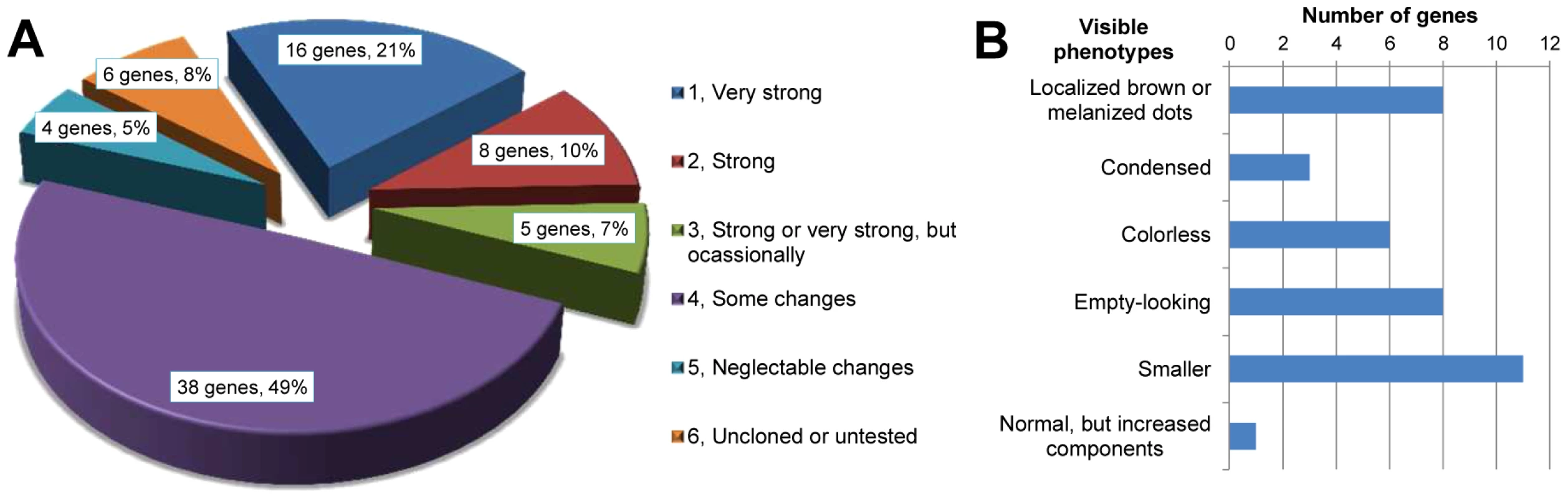
A, Description of phenotype strengths: 1, Very strong: at least one type of chemical was undetectable or less than 5% left in thoracic or/and abdominal glands; 2, Strong: at least one type of chemical was 75%–95% reduced or increased by more than 75% in thoracic or/and abdominal glands; 3, Strong or very strong but occasionally: phenotype was similar to 1 or 2 but not observed in all the injected beetles; 4, Some changes: at least one type of chemical was 25%–75% reduced or increased in thoracic or/and abdominal glands; 5, Neglectable changes: less than 25% reduction or increase in any type of chemical; 6, uncloned or untested. B, Strong and very strong gland phenotypes (strengths 1–3 in panel A) in details, some genes had more than one phenotype. Except for the last two classifications, examples are provided in Figure 4: localized brown or melanized dots (4B1-B5 and G2-G5); condensed (4C1-C2); colorless (4E1-F2); empty-looking (4D3). Tab. 2. Main gland volatiles identified by GC-MS. 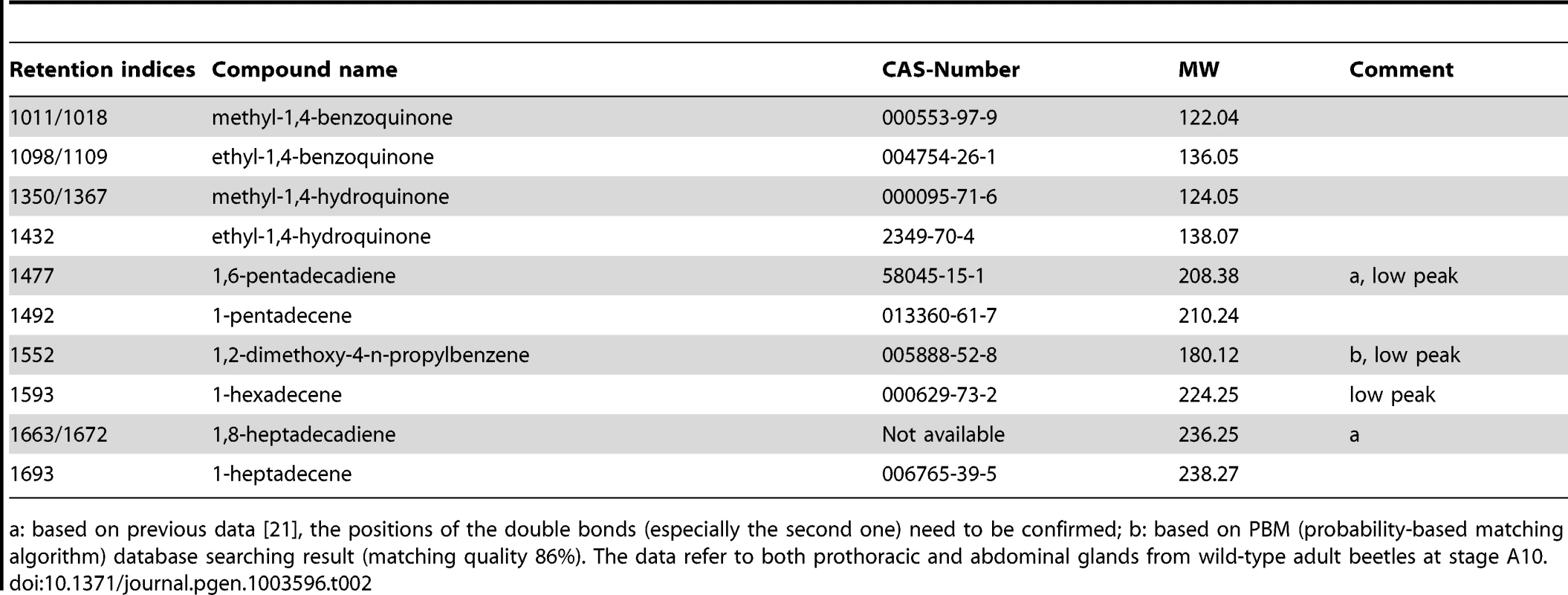
a: based on previous data [21], the positions of the double bonds (especially the second one) need to be confirmed; b: based on PBM (probability-based matching algorithm) database searching result (matching quality 86%). The data refer to both prothoracic and abdominal glands from wild-type adult beetles at stage A10. Quantification of volatile gland contents
Previous research has revealed the amount of different glandular components only on the whole beetle level [8], [9], [16], [18], [40]–[43]. In order to elucidate the chemical compositions of the volatiles in the different pairs of glands and the extent of reduction after gene knock-downs, three genes with strong quinone-less phenotypes were chosen from the 77 tested genes to quantify different glandular components. Wild-type and Enhanced green fluorescent protein (EGFP) double stranded RNA (dsRNA)-injected beetles were used as controls. Figure 6 shows the complete losses of all quinones in both pairs of glands in both females and males from the knock-down beetles of these three quinone-less genes (except for one GT39 dsRNA injected male out of sixteen injected males). In comparison, the alkenes were reduced to different extents. Statistical analyses revealed significant differences between wild-type and the knock-downs (Dataset S6), EGFP dsRNA injection surprisingly caused a few significant differences in alkenes compared to wild-type but not in quinones. Interestingly, all the alkenes in prothoracic glands of GT63 knock-downs were not statistically different from the wild-type, while only heptadecadiene and heptadecene in the abdominal glands showed the same trend. Sex differences were also analyzed (Dataset S6), which showed that most chemicals had no significant differences between males and females, except for all the alkenes in abdominal glands of GT39 knock-downs and in thoracic glands of GT62. In wild-type, only heptadecadiene showed a significant difference between different sexes while the other chemicals did not.
Fig. 6. Quantification of main volatile glandular chemicals by GC-MS in wild-type and novel quinone-less gene RNAi-knock-downs. 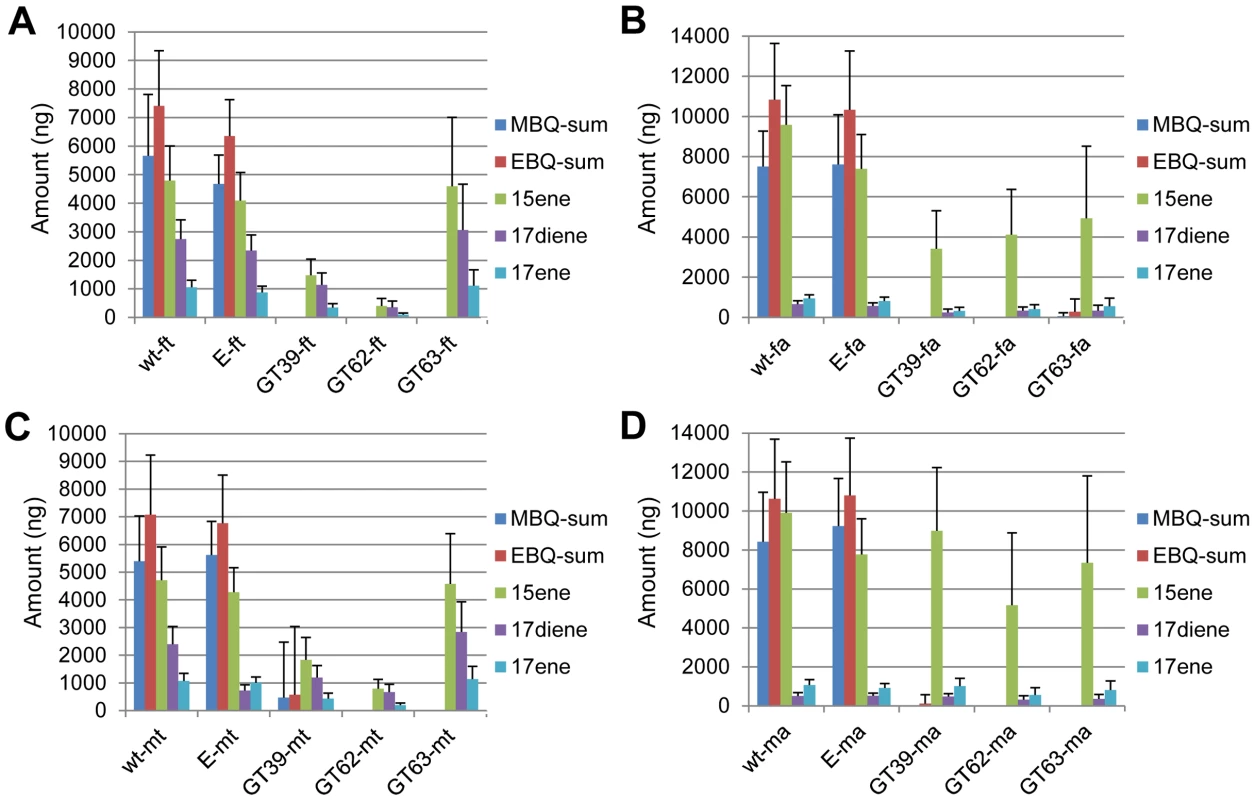
Comparisons in female thoracic glands (ft) (A), female abdominal glands (fa) (B), male thoracic glands (mt) (C), and male abdominal glands (ma) (D). Y-axis: amount in nanogram; X-axis: wild-type and different RNAi-knock-downs. Abbreviations: wt: wild-type; E: dsEGFP-injected control; GT39: Tcas-ql VTGl; GT62: Tcas-ql ARSB; GT63: Tcas-ql MRP; MBQ-sum: methyl-1,4-benzoquinone; EBQ-sum: ethyl-1,4-benzoquinone; 15ene: 1-pentadecene; 17diene: 1,8-heptadecadiene; 17ene: 1-heptadecene. The error bars indicate standard deviations at N = 15–30. Additionally, the amount of all the main components in wild-type A10 beetles is presented in Table 3 (details in Dataset S6). The prothoracic glands possess about 40% of either quinones or alkenes of all the stored secretions in the whole beetle, while abdominal glands have about 60%. But the molar ratios of quinones to alkenes are almost the same in both pairs of glands (thr, 2.60; abd, 2.70–2.74). And the molar ratios of MBQ to EBQ vary from 0.77 to 0.88 in different gland and sex levels. The only major dissimilarity between those two glands is the composition of distinct alkenes. The prothoracic glands have higher portions of 1-heptadecene (17ene) and 1,8-heptadecadiene (17diene), especially the former, but a lower portion of 1-pentadecene (15ene) (15ene: 17diene: 17ene = ∼60%: 28%: 12% in thr, ∼88%: 4%: 8% in abd).
Tab. 3. Quantification of the main volatiles in wild-type odoriferous stink glands. 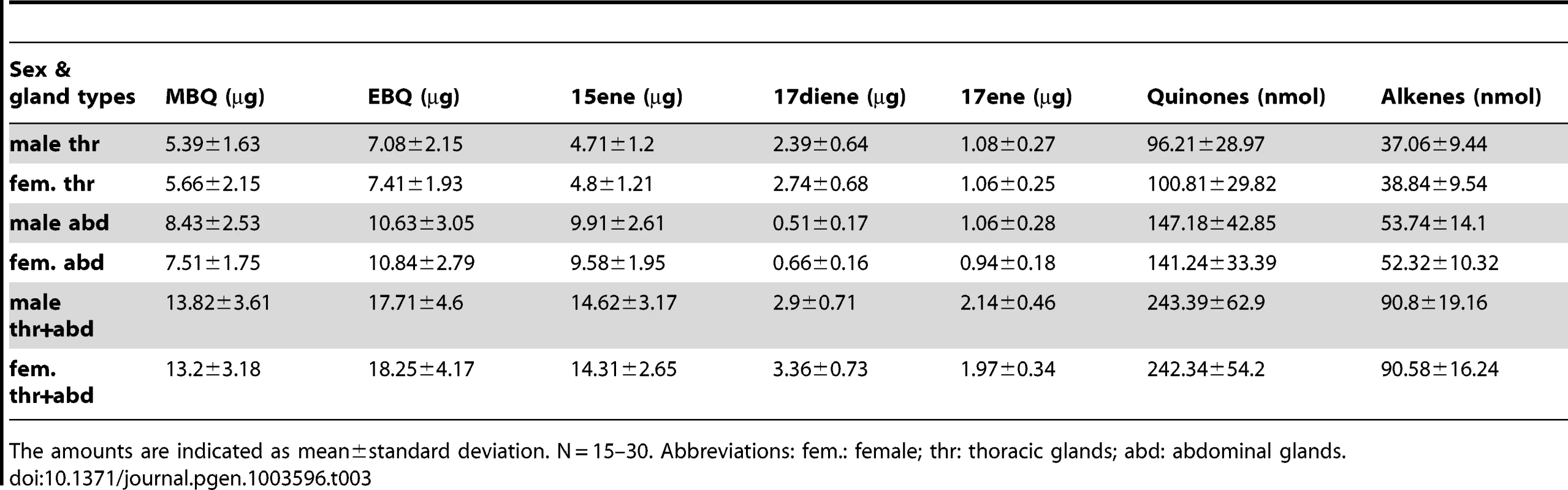
The amounts are indicated as mean±standard deviation. N = 15–30. Abbreviations: fem.: female; thr: thoracic glands; abd: abdominal glands. Full length cDNAs of the three quinone-less genes, GT39, GT62 and GT63, were cloned and the sequences submitted to GenBank (accession numbers JX569829, JX569830, and JX569831, respectively). Based on the phenotypes and their homology (see Dataset S7, S8, and S9 for respective protein sequences), GT39 has been designated as Tcas-quinone-less vitellogenin-like (Tcas-ql VTGl), GT62 as Tcas-quinone-less arylsulfatase b (Tcas-ql ARSB) and GT63 as Tcas-quinone-less multi-drug resistance protein (Tcas-ql MRP).
Phylogeny of the three newly identified quinone-less proteins
After quantification of the gland volatiles in these three gene knock-downs, their phylogeny was explored. In the phylogenetic tree (Figure 7A), Tcas-ql VTGl (GT39) was classified together with eight other Tribolium homologs, and 11 homologs were grouped in a branch close by, including proteins from Strongylocentrotus purpuratus, Danio rerio, Gallus gallus, Homo sapiens, Mus musculus, and Nasonia vitripennis (see Dataset S7 for all the sequences). Tcas-ql ARSB (GT62) was grouped together with two other Tribolium homologs (Figure 7B, see Dataset S8 for all the sequences), which were closest related to three Nasonia proteins, similarly to Tcas-ql MRP (GT63, Figure 7C, see Dataset S9 for all the sequences). Since all three quinone-less gene encoded proteins had several Tribolium homologs, we checked their homologs expression levels in the gland tanscriptome. The homologs were linked with corresponding GLEAN predictions and explored in the transcriptomic libraries (Dataset S10). It was shown (Figure 8) that no other gene was as highly expressed in the gland samples except for the three identified quinone-less genes, although some genes contained more than 16 times (2 to the power of 4, GI:91085475, Figure 8C) or 4 times (2 to the power of 2, GI:189236319, Figure 8B) higher reads in some cases. In conclusion, this indicates that all three quinone-less genes most probably have evolved independently and specifically for the quinone-based chemical defensive system in Tribolium.
Fig. 7. Phylogenetic trees of homologs of the three novel quinone-less proteins. 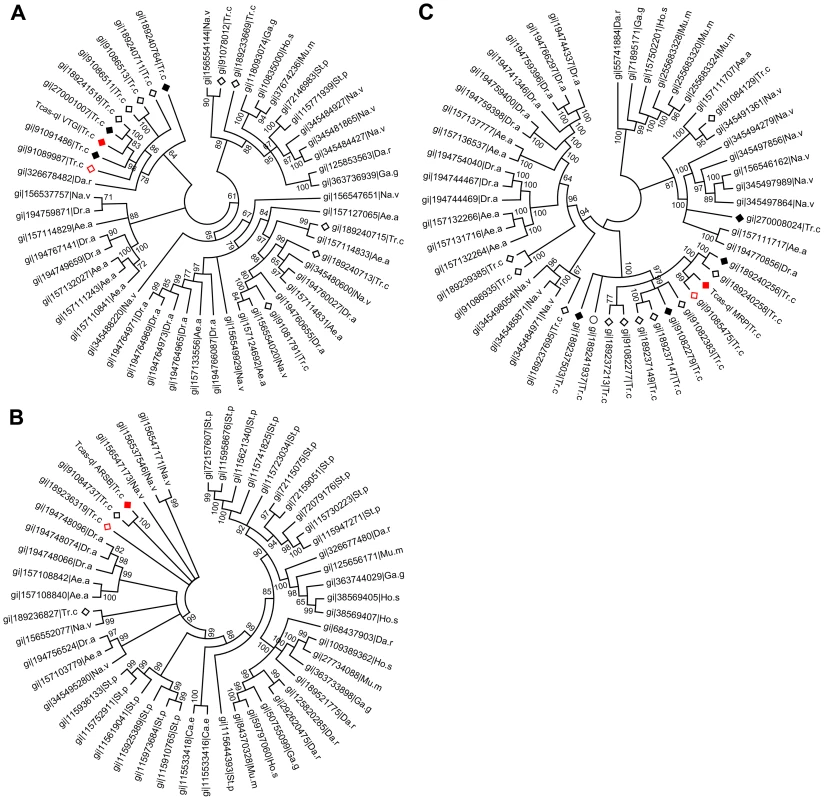
A, Tcas-ql VTGl (GT39); B, Tcas-ql ARSB (GT62); C, Tcas-ql MRP (GT63). All the Tribolium homologs were marked based on their relative gland expression levels (Figure 8). The solid red squares indicate each of the three quinone-less gene encoded proteins, the open red squares relatively high expression in gland samples, the solid black squares relatively high expression in the control sample, the open black squares the other Tribolium homologs, and an open black circle indicates a Tribolium homolog without OGS annotation. The numbers on the branching points are the statistical frequencies. Abbreviations of species names are: Ae.a, Aedes aegypti; Ca.e, Caenorhabditis elegans; Da.r, Danio rerio; Dr.a, Drosophila ananassae; Ga.g, Gallus gallus; Ho.s, Homo sapiens; Mu.m, Mus musculus; Na.v, Nasonia vitripennis; St.p, Strongylocentrotus purpuratus; Tr.c, Tribolium castaneum. Fig. 8. Relative transcriptomic gland expression levels of the Tribolium homologs of the three novel quinone-less genes. 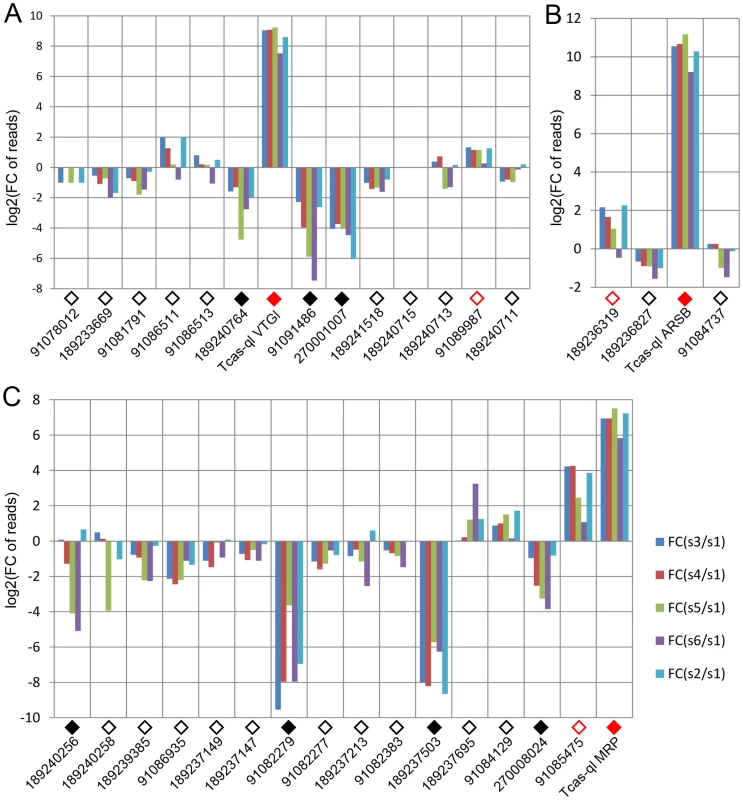
A, Tcas-ql VTGl (GT39) homologs. B, Tcas-ql ARSB (GT62) homologs. C, Tcas-ql MRP (GT63) homologs. In all charts, along the X-axes the different genes are presented (using the same GI numbers and expression level square codes as in Figure 7), while Y-axes present log2[fold change of reads in glands against control]. For abbreviations see Figure 3. Expression patterns of the quinone-less genes in gland tissue
The expression patterns of Tcas-ql VTGl (GT39), Tcas-ql ARSB (GT62) and Tcas-ql MRP (GT63) were explored in dissected odoriferous glands by gland whole mount fluorescent in situ hybridization (GWMFISH). All three genes showed strong expressions in both gland cell type 1 and cell type 2 [13], [14] of both pairs of glands (Figure 9), which confirms their involvement in Tribolium defensive secretion.
Fig. 9. Expression patterns of the three quinone-less genes. 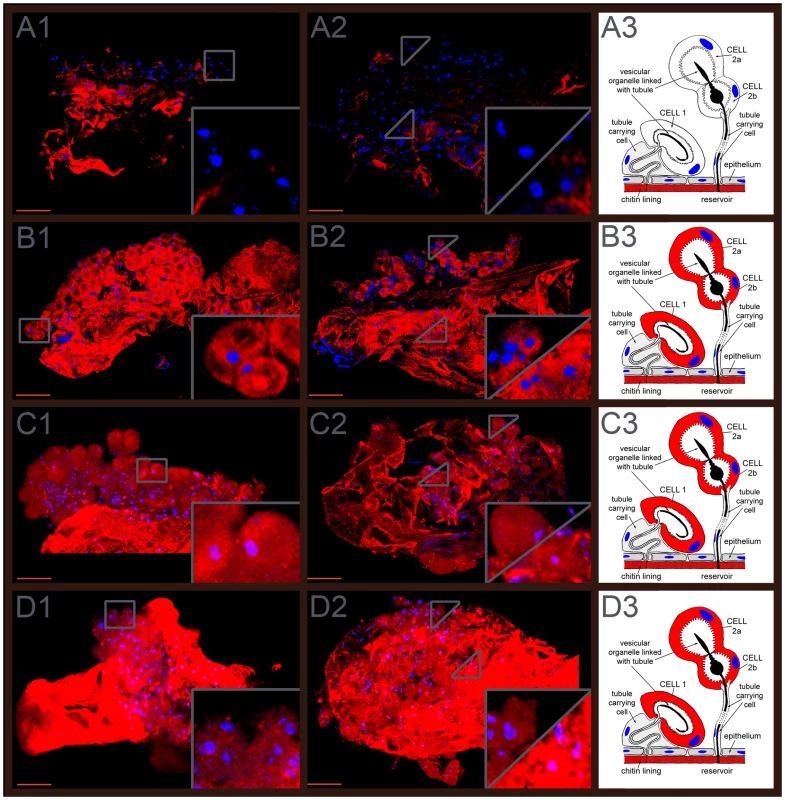
The first column (A1, B1, C1, and D1) shows prothoracic glands, the second (A2, B2, C2, D2) abdominal glands, and the third (A3, B3, C3, D3) gland schemes drawn based on own observations and previous depictions of Happ [14] and Eisner [13]. A1–A3, Tcas-ql VTGl (GT39) sense probes. B1–B3, Tcas-ql VTGl (GT39) antisense probes. C1–C3, Tcas-ql ARSB (GT62) antisense probes. D1–D3, Tcas-ql MRP (GT63) antisense probes. In the right lower corner of the panels in the first and second column, the expression was digitally magnified 4 times, with its original positions indicated by a square box or triangles within the same panel. Cell type 1 and cell type 2 were separately indicated in the abdominal glands, cell type 1 was zoomed in the right lower corner of the square, and cell type 2 in the left upper part of the square. Scale bars: 50 µm. Microbe inhibition assays
Microbe inhibition tests were carried out to identify the effect of losing quinones. It had been shown that beetle chemical secretions – especially the benzoquinones – can inhibit the growth of several microbes common to flour with artificial MBQ having the same effect [44], [45]. In our experiments, a fungus, Aspergillus niger, and a gram positive bacterium, Arthrobacter globiformis, were used to test the strength of the chemical defense mediated by beetle glands. As a control, we used RNAi against GT12 (detailed analysis on this gene will be presented elsewhere), representing a gene causing an alkene-less phenotype at knock-down, which is accompanied by only a slight reduction of quinones (see Dataset S5). The results (Figure 10) showed that wild-type beetle secretions could inhibit microbe growth in a certain area, but loss of quinones led to undetectable inhibitions (GT63), while an alkene-less state had only reduced inhibition effects (GT12).
Fig. 10. Microbe growth inhibition assays of wild-type and RNAi-knock-down glands. 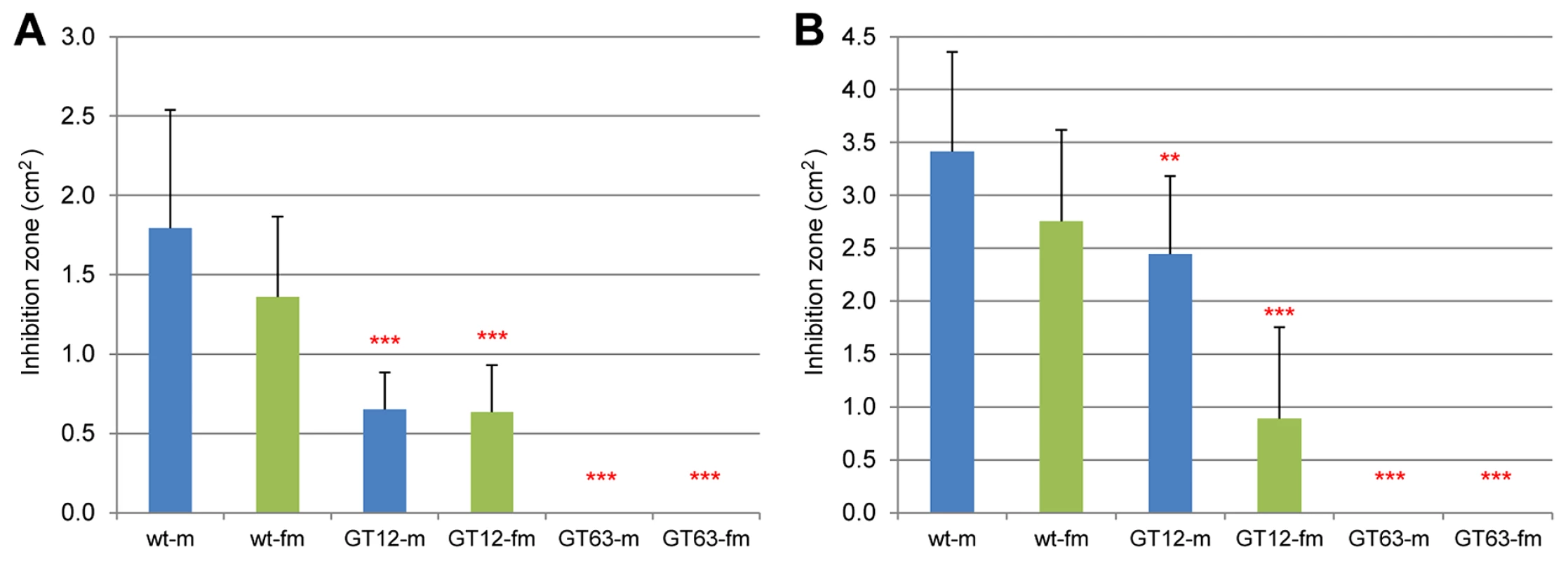
The fungus A. niger (A) and the bacterium A. globiformis (B) were analyzed for gland-mediated growth inhibition. Y-axes indicate the areas of respective inhibition zones (cm2). X-axes: sex-specific wild-type (wt) and different RNAi-knock-downs (m: male; fm: female; GT12: gene causing alkene-less phenotype; GT63: Tcas-ql MRP). Non-parametric comparisons were made between wild-type and knock-downs using Wilcoxon method, ***, P<0.001; **, 0.001<p<0.01. The error bars indicate standard deviations at N = 11–27. Phenol oxidase activity assays
Since chemical defense systems are responsible for defending the host from infection, we wanted to test, whether also a part of the innate immune system is affected directly or indirectly by the quinone-less phenotype. Thus, after RNAi-mediated knock-down of the quinone-less genes, phenol oxidase (PO) activities were measured as a general index of the melanization innate immune responses in invertebrates [46]. Compared to wild-type beetles, the three quinone-less gene knock-downs had significantly reduced levels of PO-activity both in females (Figure 11) and males (Figure S3), while buffer or EGFP dsRNA injected animals did not show significant changes. These data indicate that the extra-corporal chemical defense may be linked with the function of a part of the innate immune system in Tribolium.
Fig. 11. Phenol oxidase (PO) activity assays of wild-type and novel quinone-less gene RNAi knock-downs in females. 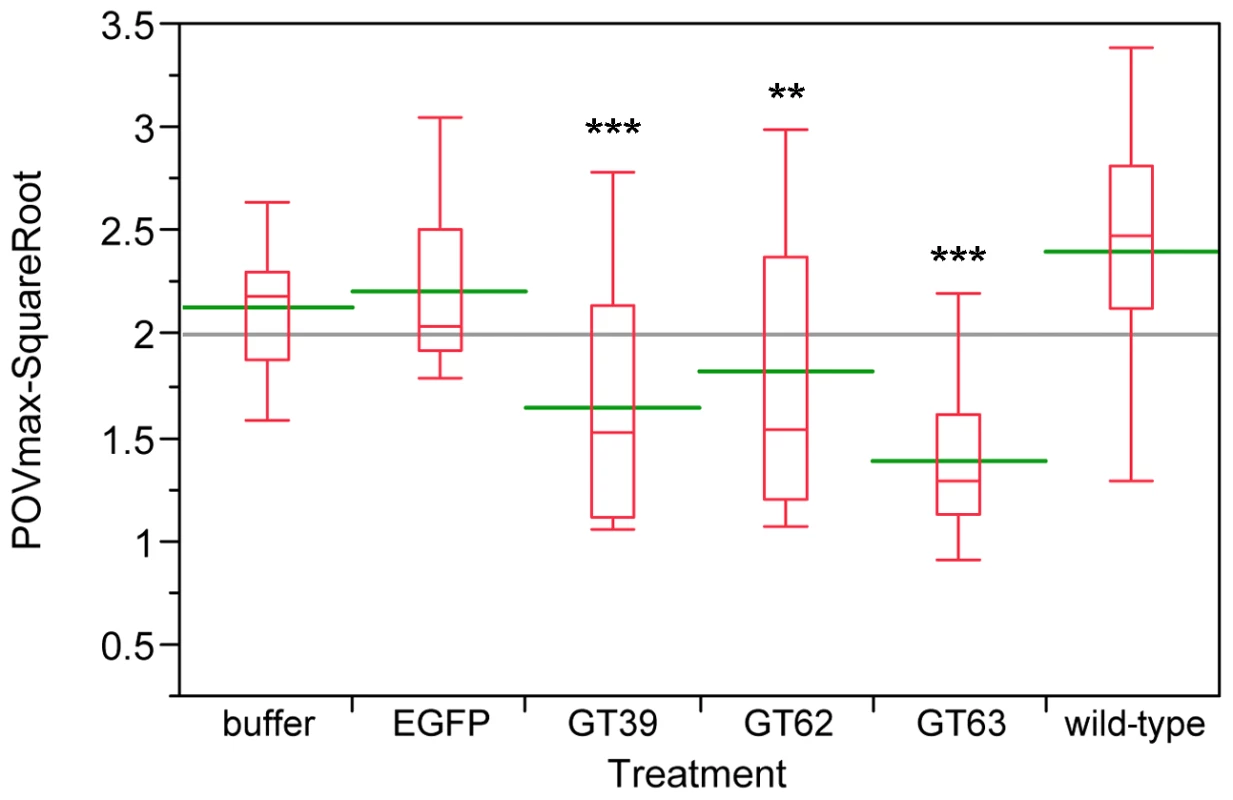
The Y-axis indicates the square root of PO Vmax, red boxes are boxplots, green lines represent the mean value, the gray line represents the grand mean, while the X-axis presents wild-type, control injections, and different RNAi-knock-downs (N = 12–25). Buffer: buffer-injection control; EGFP: dsEGFP-injection control; GT39: Tcas-ql VTGl; GT62: Tcas-ql ARSB; GT63: Tcas-ql MRP. The asterisks (*) mark the t-test results comparing to wild-type: ***: p<0.001; **: 0.001<p<0.01. Buffer- and EGFP-injected controls were not significantly different from wild-type. Discussion
Transcriptome sequencing was performed in odoriferous gland samples from Tribolium castaneum to identify novel gene functions in defensive quinone synthesis. In a next generation sequencing approach, 27.8–29.7 million reads were obtained, of which only 50 to 61% reads were successfully mapped to the Tribolium genome. Part of the low mapping ratio is probably explainable by the strain differences used for genome sequencing (inbred Georgia 2 strain) [29] and the San Bernardino strain we used for transcriptomics together with the strict mapping parameters. However, this did neither affect the comparison and subtraction between different samples, nor the library subtraction results, since all our wild-type samples have the same strain background and all samples were treated identically. Only the tar mutation is in different genetic background derived from sooty [47] and Maxillopedia-Dachs3 strains (mxpDachs3) [31]. However, the mapping ratio for this sample was similar to the others. Another reason for the low mapping ratio might be that the official gene set was from a combination of different automated gene prediction programs and annotation pipelines [29], which makes it possible that there were still unidentified genes in the Tribolium genome.
The comparisons we performed were between different tissues in wild-type and tar mutant. During the library subtractions, we chose a general cut-off of fold change at 64 times, which is much higher compared to many microarray analyses (mostly two times), but the number of the genes we got was reasonably high and seemed suitable to start to work with. Only subtraction Group 7 (male abdominal gland-specific genes), with 299 genes (Figure 1), showed an unusual high number, which might, however, be explained by the fact that the male accessory glands are hard to dissect away from the abdominal glands and many of the genes in Group 7 might actually be male accessory gland-specifically expressed genes. As these were not a topic of this study, these potentially interesting genes remain for future analysis.
GO annotations revealed that the glands have quite active metabolisms with many catalytic and binding related genes being highly expressed. The GO annotation rate of the genes, that had coverage of more than 50 in all the glands, was 70.1%, while the control had 78.8% (Dataset S3). This suggests that there are more orphan genes expressed in Tribolium odoriferous glands than in the control. In addition, only 53.6% of all the 511 genes from the subtractions were annotated, and surprisingly, Group 7 (male specific glands genes) had an annotation rate of only 42.5%, suggesting an even higher number of genes with unknown functions. We identified also some glucosidases, phenol oxidases and peroxidases highly transcribed in the glands, that are candidate enzymes to be involved in quinone biosynthesis. In conclusion, our transcriptome data have reliably detected candidate genes involved in quinone biosynthetic mechanisms of chemical defense in the red flour beetle.
Exploring the functions of a first batch of highly gland-specifically expressed genes by RNAi and GC-MS to potentially identify novel gene functions in quinone biosynthesis, 67 of 77 genes (87%) showed alterations of at least one secreted chemical, which not only confirmed their importance for semiochemical synthesis, but also signified the effectiveness of our transcriptome screening. Surprisingly, some genes with very high reads in the glands showed no big changes at the chemical level. For example, GT26 (TC007317), GT35 (TC010551) and GT41 (TC011337) from Group 1 had more than 441, 514 and 690 times higher reads respectively in all wild-type gland samples than control (Dataset S1), however, their knock-downs showed less than 75% reductions, or even neglectable changes (Dataset S5). We suggest that these genes might be involved in other biological processes indirectly related to chemical secretion. Additionally, in Group 10, the GT23 gene (TC006131) that had more than 126 times enriched reads in s2-tthr (tar prothoracic gland sample) compared to s3-mthr and s4-fthr (male and female prothoracic gland samples; Dataset S1), showed no changes in prothoracic glands, but slightly increased amounts of quinones and reduced amounts of alkenes in abdominal glands. Encoding the odorant binding protein 21 (OBP21, GI:270012767), GT23 might be involved in olfaction system. It is possible that the mutated tar somehow caused the mis-expression of this gene in a different type of tissue, or OBP21 belongs to the ubiquitous OBP type, such as encapsulin [48], which is probably involved in diverse physiological functions [49]. Moreover, GT23 showed about 10 times more reads in wild-type prothoracic glands than in the control sample. In addition, another OBP (GT76) and two chemosensory protein (GT30, GT77) encoding genes showed remarkable expression changes in the prothoracic glands of wild-type compared to tar mutants in the opposite direction. GT30, GT76, and GT77 are expressed at high levels in wild-type prothoracic glands, but their expression is strongly reduced in tar mutants. None of these four genes is expressed at significant levels in the abdominal glands, indicating a specific function for the anterior glands. However, no significant changes in volatile gland contents could be detected after RNAi knock-down of those genes.
During the morphological analyses, many abnormal glands were observed (Figure 4). However, besides the quantitative changes in the main glandular volatiles, GC-MS of the referred gene knock-downs showed no additional peak compared to wild-type, which indicates non-volatility of the accumulated brownish or black substances. The gland phenotypes could be explained as the knock-downs triggered the inhibition of chemical syntheses or the blocking of their transportation, or in some cases, the accumulation of intermediate substrates or unknown brown or black polymers. It was proposed for previously identified msg mutants that the black material is of high-molecular-weight and polymeric consisting of polymerized prematurely formed quinones due to the absence of the inhibitor in oxidation of hydroquinone [30], [50]. Moreover, the lack of specific amphipathic molecules – such as terminal alkenes – might cause the various phases (probably organic and aqueous) seen e.g. in Figure 4 G4, G5.
In the quantification part, the main glandular contents were quantified separately in both pairs of glands for the first time. Assuming that stage A10 at 32.5°C is equal to A12 at 30°C, which was predicted based on the Tribolium life parameter table [51], the MBQ and EBQ amounts we got (Dataset S6) in wild-type were 20–30% (females) and 40–70% (males) higher than the amounts detected by Unruh et al. [8]. This indicates that the dissection based extraction is much more accurate than the homogenization based method, since the latter may cause the loss of unstable chemicals during the crude preparation. Moreover, in our experiments, the males and females were not separated before harvesting, which is much closer to the natural conditions compared to the method Unruh et al. used [8]. In addition, more EBQ was detected in our tests (molar ratio of MBQ/EBQ: 0.81 in female, 0.87 in male), while the previously reported ratio was in the range of 0.59–0.61 [18], [42]. However, the weight ratio of quinones in the whole beetle (61.5%) was only a bit higher than 58.3% reported previously [18]. Interestingly, different hydrocarbon compositions were observed between the prothoracic and abdominal glands, which might reflect the dissimilar usage of their precursors, fatty acids [52], in distinct body parts and sexes. Furthermore, except for heptadecadiene in abdominal glands, all other components presented no significant differences between male and female at stage A10. Therefore, we propose that both sexes possess similar secretion levels in normal environment, and the higher level of benzoquinones in female observed before [8] was due to a different energy allocation when they produce no or less eggs as virgins, since reproduction (mating and egg production) could change the energy allocation and fitness in several other species [53]–[57].
After these first functional analyses of the complete set of highly gland-specifically expressed genes, three genes causing a quinone-less phenotype at knock-down were characterized further in our work. The quantitative data clearly showed the loss of quinones and the reduction of alkenes in both pairs of glands. Surprisingly, all the alkenes in Tcas-ql MRP (GT63) knock-downs showed no significant differences to wild-type in prothoracic glands but were significantly different in abdominal glands, while Tcas-ql VTGl (GT39) knock-downs caused significant differences to wild-type in almost all the comparisons except the three alkenes in the male abdominal glands. Tcas-ql ARSB (GT62) knock-downs showed significant changes to wild-type in all comparisons (Dataset S6). This implies that Tcas-ql MRP (GT63) has distinct functions in different glands, while Tcas-ql VTGl (GT39) and Tcas-ql ARSB (GT62) function diversely in both glands of the distinct sexes.
Potential molecular functions of these three genes in quinone synthesis are predicted on account of the homology and conserved domain analyses. Possessing a pancreatic lipase-like enzyme domain, Tcas-ql VTGl (GT39) is a vitellogenin like protein (31% identity). Vitellogenin is classified as a glycolipoprotein, having properties of a sugar, fat and protein, and belongs to a lipid transport protein family [58]. Some data in mealworm showed that a vitellogenin like protein (19.6% identity) could enhance the melanin synthetic process [59], in which o-quinones were produced in an intermediate step right before synthesizing eumelanin [60], [61]. Possibly, the benzoquinone production in odoriferous glands has similar pathways, in which Tcas-ql VTGl (GT39) regulates the related enzyme activity or reactions yet with more important roles, since almost no quinones were detected in its knock-down. Provided that common synthetic steps exist, the black material in msg and tar mutants might actually be composed of melanin or something alike.
Tcas-ql ARSB (GT62) is an arylsulfatase B (ARSB) protein (45% identity), which has a sulfatase domain responsible to hydrolyze sulfates in the body by breaking down large sugar molecules called glycosaminoglycans (GAGs). ARSB targets two GAGs in particular: dermatan sulfate and chondroitin sulfate. ARSB is located in lysosomes, compartments within cells that digest and recycle different types of molecules [62]. The deficiency of ARSB is the cause of mucopolysaccharidosis VI (MPS VI) which occurs in humans and cats, called also Maroteaux-Lamy syndrome. MPS VI is a progressive condition that causes many tissues and organs to enlarge and become inflamed or scarred, skeletal abnormalities are also common in this condition [63], [64]. Since lysosomes are the waste disposal system in the cell [65], a few possible functions of Tcas-ql ARSB (GT62) are proposed. Firstly, Tcas-ql ARSB (GT62) may have important roles in the detoxication of toxic substances in gland cells, whose knock-down leads to the initiation of an assumed feedback loop, then to the inhibitions of secretory chemical syntheses. Secondly, since different subcellular localization prediction tools gave distinct results (data not shown), Tcas-ql ARSB (GT62) may not be located in lysosomes but in the cytoplasm or elsewhere, functioning as an essential transporter for the intermediates involved in both quinone and alkene production, or as a key enzyme responsible for the activation of the newly translated transporters or other vital related proteins, or simply controlling the energy source of transportation, such as the pH gradient, or ion donators.
Tcas-ql MRP (GT63) belongs to ATP-binding cassette transporters, subfamily C (ABCC), which is also known as multidrug resistance-associated protein (MRP). Depending on ATP, the members of the MRP family can transport both hydrophobic uncharged molecules and water-soluble anionic compounds [66]. The latter includes the substrates conjugated with anions, such as glutathione, glucuronate or sulfate [67]. Considering our data, Tcas-ql MRP (GT63) might be an important transporter for the quinone precursors in all gland secretory cells, which also transports some alkene precursors in abdominal glands. And the transportations may occur from the hemolymph to glandular cells, or from the secreting cells to the vesicular compartments, where the toxicant-producing reactions are being segregated to [14].
Despite of these predictions on the functions of the three quinone-less gene encoded proteins based on their homologs, it should be noted that our phylogenic analyses show that they evolved independently and particularly for the chemical defense in the red flour beetle. Moreover, based on the GWMFISH results that confirm their expressions in the secretory units, all three genes play essential roles in producing the defensive quinones in the odoriferous glands of T. castaneum.
In chemical secretion of Tribolium and other quinone-producing arthropods, the quinones and the alkenes (hydrocarbons) have their respective roles. Quinones are toxic and quite reactive, therefore mainly responsible for the defense against pathogens, parasitoids, and predators [5]. Alkenes and mixtures of other organic solvents partition quinones to the hydrocarbon phase, help to spread them on the arthropod cuticle, and aid penetration of quinones through the cuticles of various enemies to improve the defensive effects [5], [68], [69]. Our inhibition assay results confirm these descriptions, since the quinone-less gene knock-downs (Tcas-ql MRP, GT63) showed no microbe inhibitions at all, whereas the alkene-less gene knock-down (GT12) presented only reduced inhibition effects.
In insects, innate immune responses include antimicrobial peptides, phagocytosis, nodulation, melanotic encapsulation and wound healing, which endow potential hosts the abilities to defend against pathogens and parasites [70]–[74]. The chemical defense system is also responsible for defending the host from infection by other organisms. Therefore, it was interesting to check how innate immunity was affected, when the chemical defense system is knocked-down. An oxidoreductase (PO) activity has been commonly assayed to provide a general index of melanization innate immune responses in invertebrates [46], since the activation of PO leads to the melanization reactions of invading pathogens, which is a major aspect of the innate immune system [75]. Therefore, PO activities were examined in quinone-less gene knock-downs compared to wild-type, buffer injected and EGFP dsRNA injected beetles. The obtained results indicate that the chemical defense may be linked with melanotic encapsulation innate immune responses. Thus the three quinone-less genes necessary for quinone biosynthesis in odoriferous glands might also be involved in the melanization cascade. In addition, the genomic annotation and expression analyses of the Tribolium POs showed that some POs are highly expressed in all gland samples but low in control, which implies that they might mainly function in the chemical defense system, and their preferred substrates are in the glands. However, whether they are involved in the melanin pathway is so far unknown. In case the POs are multifunctional, a linkage and crosstalk between these two systems would exist.
Materials and Methods
Transcriptome sequencing
Prothoracic and abdominal odoriferous glands were dissected separately from A10–A30 (reared at 32.5°C, 10–30 days after eclosion) adult beetles and stored in RNAlater solution (Ambion, Life Technologies GmbH, Darmstadt, Germany, Cat. No. AM7020) on ice. Males and females were separately prepared except for the prothoracic glands from tar mutants. About 500 beetles were used for each gland sample, while the anterior abdomen, where no glands are located, was taken as a control tissue. Then total RNA was extracted using RNAqueous-Micro Kit (Ambion, Life Technologies GmbH, Darmstadt, Germany, Cat. No. AM1931) and treated by DNase I. Transcriptome sequencing (mRNA-seq) was performed by Macrogen Inc. (Seoul, South Korea), on a next generation sequencing (NGS) platform (Illumina/Solexa Genome Analyzer IIx). After sequencing, reads (38 bp each) were mapped to the mRNAs of the official gene set (OGS) from Beetlebase 3.0 [22], [38] by Maq tool (http://maq.sourceforge.net/). The samples (s) were, s1: anterior abdomen; s2: prothoracic glands from tar mutant; s3: male prothoracic glands; s4: female prothoracic glands; s5: male abdominal glands; s6: female abdominal glands. Except of s2, all other tissues were from wild-type. Coverage (depth) is calculated as reads times 38 divided by specific length of gene transcript. The transcriptomic sequences derived from the different samples have also been mapped as individual tracks to the Tribolium genome (iBeetle Genome Browser: http://bioinf.uni-greifswald.de/gb2/gbrowse/tcas).
Gene ontology annotation and mRNA-seq library subtractions
The genes, which had coverage over 50 (about 2 times of the whole sequencing coverage), were regarded as abundant or richly expressed in either all wild-type gland samples or control. Their functionalities were explored by gene ontology (GO) annotation [39] using Blast2go [76], [77]. In order to screen gland-specific genes, statistical subtractions were carried out among different samples for various comparisons. In general, the cut-off for logarithm of fold change, with 2 as the base, was 6, which meant 64 times more reads in one sample than the other. The detailed subtraction conditions are presented in Dataset S2.
Transcriptomic exploration of candidate genes for quinone synthesis
Tribolium glucosidase, phenol oxidase and peroxidase were searched initially in protein database at the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/protein/). The obtained proteins were characterized based on conserved domains (CDD of NCBI, http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml) and probed back to the publicly accessible Tribolium genome at Beetlebase with blastp algorithm in order to be linked with OGS, avoid redundancies, and identify the homologs which were not covered by the previous searches. The newly identified proteins were then analyzed in CDD for confirmation.
Functional analysis of the most highly and gland-specifically expressed genes
To evaluate the subtraction results, 77 candidate genes were chosen from the gland transcriptome screening and functional analysis was performed by using RNA interference (RNAi) [78], [79]. An online tool, E-RNAi [80] was used to design fragments for double stranded RNA (dsRNA) synthesis with no or lowest off-target effects. Primers were designed by Primer Premier 5 [81] and listed in Dataset S5. Animals were injected with dsRNAs at mid pupal [79] or larval L5–L6 stage [82], and were checked at A10 and A24 (32.5°C) for morphological phenotypes on prothoracic and abdominal glands. Furthermore, both pairs of glands were dissected carefully and intact from one male and one female beetle and smashed in 100 µl methanol (Merck Millipore SupraSolv, Merck KGaA, Darmstadt, Germany, Cat. No. 106011), which represents a more specific gland volatile analysis than previously performed by Howard 1987 (whole beetle [17]) or Villaverde et al. 2007 (headspace [9]). Then the samples were stored at −20°C and measured within 24 hours. One microliter was loaded by a split injector into an Agilent gas chromatograph coupled with a mass spectrometer (GC-MS) (Detailed parameters are described in Text S1). The areas of the signals in chromatograms were calculated using the software MSD ChemStation D.02.00.275 (Agilent Technologies, Santa Clara, USA) under auto-integration mode. Then the data were compared between each candidate gene knock-down and the control. The phenotypes were grouped according to strengths of the alterations of the major components. For the three genes with quinone-less phenotypes, second independent dsRNA fragments, which had no overlaps with the first fragments, were designed with the same tools and used to confirm the phenotypes.
Quantification of volatile gland contents
In order to quantify different volatile components in the secretion, the following chemicals were obtained from commercial sources: methyl-1,4-benzoquinone (MBQ) (abcr GmbH & Co. KG, Karlsruhe, Germany, Cat. No. AB208176), 2-methylhydroquinone (MHQ) (abcr GmbH & Co. KG, Karlsruhe, Germany, Cat. No. AB132029), ethyl quinol (also called ethyl-1,4-hydroquinone: EHQ) (abcr GmbH & Co. KG, Karlsruhe, Germany, Cat. No. AB148997), 1-pentadecene (Fluka, SIGMA-ALDRICH Chemie GmbH, Munich, Germany, Cat. No. 76560) and 1-heptadecene (Fluka, SIGMA-ALDRICH Chemie GmbH, Munich, Germany, Cat. No. 51665). Then authentic standard solution series were made and a five-point calibration was performed by GC-MS. Based on the standard curves, the areas of the abundances from GC-MS were transformed to masses. Ethyl-1,4-benzoquinone (EBQ) and heptadecadiene, which were commercially unavailable, were calculated as equivalents based on the standard curve of EHQ and 1-heptadecene respectively. Quantification was carried out in wild-type, buffer injected, a dsRNA EGFP injected control, and three quinone-less gene knock-downs. After pupal RNAi, glands were prepared from A10 beetles (15–30 animals each sex). It was proposed that more than 80% of the glandular quinones are benzoquinones [8]. Because the small amounts of hydroquinones detected are precursors of benzoquinones [24], [64], the quantities of hydroquinones and benzoquinones were summed up and treated as secreted quinones. After quantification, statistical analyses were performed with software JMP 9.0.2 (SAS Institute, 2010) using student t-test for sex comparisons and a nonparametric method (Mann–Whitney–Wilcoxon) for group comparisons.
Phylogeny of the three novel quinone-less proteins
Full length cDNAs obtained from RACE reactions (see Text S1) were analyzed by the online tool ORF Finder (Open Reading Frame finder, http://www.ncbi.nlm.nih.gov/gorf/orfig.cgi) and translated to proteins. The amino acid sequences were submitted to NCBI to find the homologs through blastp search in Reference Proteins Database and the first fifty sequences were chosen, in which the Tribolium homologs were blasted again in Beetlebase (http://beetlebase.org/) to check redundancies and find the corresponding OGS numbers (listed in Dataset S10) in order to analyze their expressions at the glandular transcriptome level. Then all proteins (listed in Dataset S7, S8, and S9) were aligned by using MAFFT [83] and analyzed with FastTree [84] using maximum likelihood methods to construct dendrograms, which were displayed, marked and computed based on the branching frequencies (cut-off was 60%) using MEGA5 [85].
Gland whole mount fluorescent in situ hybridization
The protocol for gland whole mount fluorescent in situ hybridization (GWMFISH) was based on previous methods [86]–[90] with a few modifications. Details are described in Text S1. The only big difference was using the HNPP Fluorescent Detection Set (Roche Applied Science, Roche Diagnostics Deutschland GmbH, Mannheim, Germany, Cat. No. 11758888001) to detect the labeled probes after incubation of Anti-digoxigenin (DIG)-alkaline phosphatase (AP) Fab fragments (Roche Applied Science, Roche Diagnostics Deutschland GmbH, Mannheim, Germany, Cat. No. 11093274910; from sheep). Glands were counterstained with Hoechst 33342 (SIGMA-ALDRICH Chemie GmbH, Munich, Germany, Cat. No. B2261) prior to mounting and embedding in Aqua-Poly/Mount (Polyscience, Niles, Illinois, USA, Cat. No. 18606). The stainings were observed and captured with confocal laser scanning microscope Zeiss LSM780. 3D (3-dimensional) construction was performed using software ZEN2011 (Carl Zeiss MicroImaging GmbH, Oberkochen, Germany). Contrast and brightness were adjusted using Adobe Photoshop CS2.
Microbe inhibition assays
A fungus, Aspergillus niger, and a gram positive bacterium, Arthrobacter globiformis [91], were used to test the strength of the chemical defense. The A. niger strain was an isolate from old beetle cultures (GJ, unpublished), which was determined by the German collection of microorganisms and cell cultures (DSMZ) as Aspergillus niger, a common soil fungus also growing e.g. on bread and other food, known as ‘Black mold’. A. globiformis (from DSMZ, strain DSM20124) was another basic soil microbe and believed to have no contacts with Tribolium in nature. The culture media are listed in Text S1. Microbe lawns were made in the petri dishes using the method of [44], [92], by the modification that we put dissected abdominal glands to the holes on the lawn poked by a sterile glass pipet (one pair of glands per hole and breaking of the reservoirs in the holes) instead of freezing beetles on the plates. The plates were incubated at 25°C for 72 h (A. niger) or 28°C for 48 h (A. globiformis) respectively, and then the inhibition zones were photographed with a digital camera. The areas of the inhibition zones were measured with freeware ImageJ 1.44p.
Phenol oxidase activity assays
After RNAi, A10 beetles were harvested and frozen individually at −80°C in 150 µl Bis-Tris buffer (0.1 M, pH 7.5, sterile filtered. Bis-Tris: Fluka, SIGMA-ALDRICH Chemie GmbH, Munich, Germany, Cat. No. 14880) for at least 24 hours. To the frozen samples, a sterile steel ball (Ø 3 mm) was added each, and samples were homogenized using a GenoGrinder tissue homogenizer for 30 seconds at a speed of 1000 strokes per minute. After grinding, samples were placed on ice immediately before centrifuging three times at 6200 rpm 4°C (Eppendorf centrifuge 5810R) for five minutes to remove beetle debris. After each centrifugation step the supernatant was transferred to a new tube on ice before being centrifuged again. For measuring actual PO activity, a flat bottom 96well plate was prepared on ice with 50 µl sterile deionized water and 50 µl Bis-Tris buffer. In each well 20 µl of an individual sample extract was pipetted, or 20 µl Bis-Tris buffer when the well was serving as a blank. As PO activates the transfer of DOPA to Dopamine in insects [93], we added 50 µl L-DOPA (3,4-Dihydroxy-L-phenylalanine, SIGMA-ALDRICH Chemie GmbH, Munich, Germany, Cat. No. D9628; 4 mg/ml in Bis-Tris buffer, sterile filtered) into each well on ice. As the addition of substrate starts the reaction, plates needed to go to the Eon Microplate Spectrophotometer (Biotek Instruments, Inc., Bad Friedrichshall, Germany) immediately. Plates were read at 490 nm and 37°C with readings every two minutes for 90 minutes. After correcting the self-darkening of the substrate by subtracting the blanks, PO activity was estimated as Vmax of the linear phase of the reaction on every individual sample well (also compare with previous data [94]).
Supporting Information
Zdroje
1. Chapman AD (2009) Numbers of living species in Australia and the world. Second. Canberra, Australia: Australian Biological Resources Study (ABRS). 80 pp. Available:http://www.environment.gov.au/biodiversity/abrs/publications/other/species-numbers/2009/06-references.html. Accessed 9 August 2012.
2. Wilson EO (2006) Threats to Global Diversity. Available:http://www.globalchange.umich.edu/globalchange2/current/lectures/biodiversity/biodiversity.html. Accessed 9 August 2012.
3. Eisner T (1970) Chemical defense against predation in arthropods. In: Sondheimer E, Simeone JB, editors. Chemical Ecology. New York: Academic Press, Vol. Academic P. pp. 157–217.
4. EisnerT (1966) Beetle's spray discourages predators. Natural History 75 : 42–47.
5. Blum MS (1981) Chemical defenses of arthropods. United Kin. London: Academic Press. 562 pp. Available:http://www.cabdirect.org/abstracts/19820594729.htmljsessionid=6D34AB6A23BB5FBEB2313F77658399B7. Accessed 9 August 2012.
6. EisnerT, MeinwaldJ (1966) Defensive secretions of arthropods. Science 153 : 1341–1350 Available:http://www.sciencemag.org/content/153/3742/1341.short. Accessed 9 June 2012.
7. WeatherstonJ (1967) The chemistry of arthropod defensive substances. Quarterly Reviews, Chemical Society 21 : 287 Available:http://pubs.rsc.org/en/content/articlehtml/1967/qr/qr9672100287. Accessed 9 August 2012.
8. UnruhLM, XuR, KramerKJ (1998) Benzoquinone levels as a function of age and gender of the red flour beetle, Tribolium castaneum. Insect Biochemistry and Molecular Biology 28 : 969–977 Available:http://linkinghub.elsevier.com/retrieve/pii/S096517489800085X. Accessed 19 March 2012.
9. VillaverdeML, JuárezMP, MijailovskyS (2007) Detection of Tribolium castaneum (Herbst) volatile defensive secretions by solid phase microextraction–capillary gas chromatography (SPME-CGC). Journal of Stored Products Research 43 : 540–545 Available:http://dx.doi.org/10.1016/j.jspr.2007.03.003. Accessed 3 April 2012.
10. ChittendenFH (1896) Insects Affecting Cereals and Other Dry Vegetable Foods. Bulletin of the United States Department of Agriculture; Division of Entomology (New Series) 4 : 112–130.
11. PayneNM (1925) Some effects of Tribolium on flour. Journal of Economic Entomology 18 : 737–744 Available:http://www.ingentaconnect.com/content/esa/jee/1925/00000018/00000005/art00020.
12. RothLM (1943) Studies on the gaseous secretion of Tribolium confusum Duval. II. The odoriferous glands of Tribolium confusum. Annals of the Entomological Society of America 36 : 397–424 Available:http://www.ingentaconnect.com/content/esa/aesa/1943/00000036/00000003/art00011. Accessed 6 June 2012.
13. EisnerT, McHenryF, SalpeterMM (1964) Defense mechanisms of arthropods. XV. Morphology of the quinone-producing glands of a tenebrionid beetle (ELEODES longicollis lec.). Journal of morphology 115 : 355–399 Available:http://onlinelibrary.wiley.com/doi/10.1002/jmor.1051150304/abstract. Accessed 4 June 2012.
14. HappGM (1968) Quinone and hydrocarbon production in the defensive glands of Eleodes longicollis and Tribolium castaneum (Coleoptera, Tenebrionidae). Journal of insect physiology 14 : 1821–1837 Available:http://www.sciencedirect.com/science/article/pii/002219106890214X.
15. AlexanderP, BartonDHR (1943) The excretion of ethylquinone by the flour beetle. The Biochemical Journal 37 : 463–465 Available:http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1257939&tool=pmcentrez&rendertype=abstract. Accessed 26 March 2012.
16. LocontiJD, RothLM (1953) Composition of the odorous secretion of Tribolium castaneum. Annals of the Entomological Society of America 46 : 281–289 Available:http://www.ingentaconnect.com/content/esa/aesa/1953/00000046/00000002/art00011. Accessed 10 August 2012.
17. HowardRW (1987) Chemosystematic studies of the Triboliini (Coleoptera: Tenebrionidae): phylogenetic inferences from the defensive chemicals of eight Tribolium spp., Palorus ratzeburgi (Wissmann), and latheticus oryzae Waterhouse. Annals of the Entomological Society of America 80 : 398–405 Available:http://agris.fao.org/agris-search/search/display.do?f=1987/US/US87078.xmlUS8738541. Accessed 16 August 2012.
18. MarkarianH, FlorentineGJ, PraitJJJr (1978) Quinone production of some species of Tribolium. Journal of Insect Physiology 24 : 785–790 Available:http://www.sciencedirect.com/science/article/pii/0022191078900963. Accessed 10 August 2012.
19. Von EndtDW, WheelerJW (1971) 1-Pentadecene production in Tribolium confusum. Science 172 : 60–61 Available:http://www.sciencemag.org/content/172/3978/60.short. Accessed 19 March 2012.
20. SuzukiT, HuynhV, MutoT (1975) Hydrocarbon repellents isolated from Tribolium castaneum and T. confusum (Coleoptera: Tenebrionidae). Agricultural and Biological Chemistry 39 : 2207–2211 Available:http://agris.fao.org/agris-search/search/display.do?f=1976/JP/JP76004.xmlJP7601784.
21. GörgenG, FrößlC, BolandW, DettnerK (1990) Biosynthesis of 1-alkenes in the defensive secretions of Tribolium confusum (Tenebrionidae); stereochemical implications. Experientia 46 : 700–704 Available:http://www.springerlink.com/content/hx288200k15331hr/. Accessed 10 August 2012.
22. WangL, WangS, LiY, ParadesiMSR, BrownSJ (2007) BeetleBase: the model organism database for Tribolium castaneum. Nucleic acids research 35: D476–9 Available:http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1669707&tool=pmcentrez&rendertype=abstract. Accessed 9 May 2012.
23. BucherG, ScholtenJ, KlinglerM (2002) Parental RNAi in Tribolium (Coleoptera). Current biology: CB 12: R85–6 Available:http://www.ncbi.nlm.nih.gov/pubmed/11839285. Accessed 13 August 2012.
24. TomoyasuY, MillerSC, TomitaS, SchoppmeierM, GrossmannD, et al. (2008) Exploring systemic RNA interference in insects: a genome-wide survey for RNAi genes in Tribolium. Genome Biology 9: R10 Available:http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2395250&tool=pmcentrez&rendertype=abstract.
25. LorenzenMD, DoyunganZ, SavardJ, SnowK, CrumlyLR, et al. (2005) Genetic linkage maps of the red flour beetle, Tribolium castaneum, based on bacterial artificial chromosomes and expressed sequence tags. Genetics 170 : 741–747 Available:http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1450394&tool=pmcentrez&rendertype=abstract. Accessed 8 March 2012.
26. TraunerJ, SchinkoJ, LorenzenMD, ShippyTD, WimmerEA, et al. (2009) Large-scale insertional mutagenesis of a coleopteran stored grain pest, the red flour beetle Tribolium castaneum, identifies embryonic lethal mutations and enhancer traps. BMC biology 7 : 73 Available:http://www.biomedcentral.com/1741-7007/7/73. Accessed 5 March 2012.
27. SchinkoJB, WeberM, ViktorinovaI, KiupakisA, AverofM, et al. (2010) Functionality of the GAL4/UAS system in Tribolium requires the use of endogenous core promoters. BMC developmental biology 10 : 53 Available:http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2882914&tool=pmcentrez&rendertype=abstract. Accessed 27 October 2012.
28. SchinkoJB, HillebrandK, BucherG (2012) Heat shock-mediated misexpression of genes in the beetle Tribolium castaneum. Development genes and evolution 222 : 287–298 Available:http://www.ncbi.nlm.nih.gov/pubmed/22890852. Accessed 27 October 2012.
29. Tribolium Genome Sequencing Consortium (2008) RichardsS, GibbsRA, WeinstockGM, BrownSJ, et al. (2008) The genome of the model beetle and pest Tribolium castaneum. Nature 452 : 949–955 Available:http://dx.doi.org/10.1038/nature06784. Accessed 1 March 2012.
30. EngelhardtM, RapoportH, SokoloffA (1965) Odorous secretion of normal and mutant Tribolium confusum. Science (New York, NY) 150 : 632–633 Available:http://www.ncbi.nlm.nih.gov/pubmed/5837106. Accessed 13 August 2012.
31. BeemanRW, StuartJJ, HaasMS, FriesenKS (1992) Chromosome extraction and revision of linkage group 2 in Tribolium castaneum. The Journal of heredity 87 : 224–232 Available:http://www.ncbi.nlm.nih.gov/pubmed/8683098.
32. MeinwaldJ, KochKF, RogersJE, EisnerT (1966) Biosynthesis of Arthropod Secretions. III. Synthesis of Simple p-Benzoquinones in a Beetle (Eleodes longicollis). Journal of the American chemical society 88 : 1590–1592 Available:http://dx.doi.org/10.1021/ja00959a074. Accessed 9 June 2012.
33. Morgan ED (2004) Biosynthesis in Insects. Cambridge: Royal Society of Chemistry. 199 pp. Available:http://pubs.rsc.org/en/content/ebook/978-0-85404-691-1.
34. DuffeySS, BlumMS (1977) Phenol and guaiacol: Biosynthesis, detoxication, and function in a polydesmid millipede, Oxidus gracilis. Insect Biochemistry 7 : 57–65.
35. PryorMGM (1940) On the hardening of the cuticle of insects. Proc R Soc London Ser B128 : 393–407.
36. KramerKJ, KanostMR, HopkinsTL, JiangH, ZhuYC, et al. (2001) Oxidative conjugation of catechols with proteins in insect skeletal systems. Tetrahedron 57 : 385–392 Available:http://dx.doi.org/10.1016/S0040-4020(00)00949-2. Accessed 12 March 2013.
37. SudermanRJ, DittmerNT, KanostMR, KramerKJ (2006) Model reactions for insect cuticle sclerotization: cross-linking of recombinant cuticular proteins upon their laccase-catalyzed oxidative conjugation with catechols. Insect biochemistry and molecular biology 36 : 353–365 Available:http://www.ncbi.nlm.nih.gov/pubmed/16551549. Accessed 12 March 2013.
38. KimHS, MurphyT, XiaJ, CarageaD, ParkY, et al. (2010) BeetleBase in 2010: revisions to provide comprehensive genomic information for Tribolium castaneum. Nucleic acids research 38: D437–42 Available:http://nar.oxfordjournals.org/cgi/content/abstract/38/suppl_1/D437. Accessed 6 June 2012.
39. The Gene Ontology Consortium (2000) AshburnerM, BallCA, BlakeJA, BotsteinD, et al. (2000) Gene Ontology: tool for the unification of biology. Nature genetics 25 : 25–29 Available:http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3037419&tool=pmcentrez&rendertype=abstract. Accessed 8 March 2012.
40. LadishRK, LadishSK, HowePM (1967) Quinoid Secretions in Grain and Flour Beetles. Nature 215 : 939–940 Available:http://dx.doi.org/10.1038/215939a0. Accessed 16 August 2012.
41. WirtzRA, TaylorSL, SemeyHG (1978) Concentrations of substituted p-benzoquinones and 1-pentadecene in the flour beetles Tribolium madens (Charp.) and Tribolium Brevicornis (Lec.) (Coleoptera, Tenebrionidae). Comparative biochemistry and physiology 61 : 287–290 Available:http://www.sciencedirect.com/science/article/pii/0306449278900564.
42. PappasPW, WardropSM (1996) Quantification of benzoquinones in the flour beetles, Tribolium castaneum and Tribolium confusum. Preparative biochemistry & biotechnology 26 : 53–66 Available:http://www.ncbi.nlm.nih.gov/pubmed/8744422. Accessed 26 March 2012.
43. YezerskiA, GilmorTP, StevensL (2004) Genetic analysis of benzoquinone production in Tribolium confusum. Journal of chemical ecology 30 : 1035–1044 Available:http://www.ncbi.nlm.nih.gov/pubmed/15274446.
44. PrendevilleHR, StevensL (2002) Microbe inhibition by Tribolium flour beetles varies with beetle species, strain, sex, and microbe group. Journal of chemical ecology 28 : 1183–1190 Available:http://www.ncbi.nlm.nih.gov/pubmed/12184396.
45. YezerskiA, CicconeC, RozitskiJ, VolingavageB (2007) The effects of a naturally produced benzoquinone on microbes common to flour. Journal of chemical ecology 33 : 1217–1225 Available:http://www.ncbi.nlm.nih.gov/pubmed/17473960. Accessed 26 March 2012.
46. ArmitageS, Siva-JothyM (2005) Immune function responds to selection for cuticular colour in Tenebrio molitor. Heredity 94 : 650–656 Available:http://www.ncbi.nlm.nih.gov/pubmed/15815710. Accessed 20 March 2012.
47. Sokoloff A (1966) The genetics of Tribolium and related species. Advances in genetics, Supplement 1. New York: Academic Press. 212 pp.
48. LealWS (2005) Pheromone reception. Topics in Current Chemistry 240 : 1–36 Available:http://www.springerlink.com/index/WV1PY7HYC4861T2D.pdf. Accessed 22 August 2012.
49. PelletierJ, LealWS (2009) Genome analysis and expression patterns of odorant-binding proteins from the Southern House mosquito Culex pipiens quinquefasciatus. PloS one 4: e6237 Available:http://dx.plos.org/10.1371/journal.pone.0006237. Accessed 22 August 2012.
50. RothLM, EisnerT (1962) Chemical defenses of arthropods. Annual Review of Entomology 7 : 107–136 Available:http://www.annualreviews.org/doi/abs/10.1146/annurev.en.07.010162.000543? Accessed 9 August 2012.
51. Sokoloff A (1974) The Biology of Tribolium. Vol. 2. Oxford: Clarendon Press. 628 pp.
52. CavillGWK (1971) Chemistry of Some Insect Secretions. Journal and Proceedings of the Royal Society of New South Wales 103 : 109–118 Available:http://www.ncbi.nlm.nih.gov/pubmed/10440781.
53. FowlerK, PartridgeL (1989) A cost of mating in female fruitflies. Nature 338 : 760–761 Available:http://dx.doi.org/10.1038/338760a0. Accessed 11 November 2012.
54. ChapmanT, LiddleLF, KalbJM, WolfnerMF, PartridgeL (1995) Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature 373 : 241–244 Available:http://dx.doi.org/10.1038/373241a0. Accessed 6 November 2012.
55. KempDJ, RutowskiRL (2004) A survival cost to mating in a polyandrous butterfly, Colias eurytheme. Oikos 105 : 65–70 Available:http://doi.wiley.com/10.1111/j.0030-1299.2004.12874.x. Accessed 11 November 2012.
56. GilgMR, KruseKC (2003) Reproduction Decreases Life Span in the Giant Waterbug (Belostoma flumineum). The American Midland Naturalist 149 : 306–319 Available:http://dx.doi.org/10.1674/0003-0031(2003)149[0306:RDLSIT]2.0.CO;2. Accessed 11 November 2012.
57. RönnJ, KatvalaM, ArnqvistG (2006) The costs of mating and egg production in Callosobruchus seed beetles. Animal Behaviour 72 : 335–342 Available:http://dx.doi.org/10.1016/j.anbehav.2005.10.024. Accessed 11 November 2012.
58. TufailM, TakedaM (2009) Insect vitellogenin/lipophorin receptors: molecular structures, role in oogenesis, and regulatory mechanisms. Journal of insect physiology 55 : 87–103 Available:http://www.ncbi.nlm.nih.gov/pubmed/19071131. Accessed 14 October 2012.
59. LeeKM, LeeKY, ChoiHW, ChoMY, KwonTH, et al. (2000) Activated phenoloxidase from Tenebrio molitor larvae enhances the synthesis of melanin by using a vitellogenin-like protein in the presence of dopamine. European Journal of Biochemistry 267 : 3695–3703 Available:http://doi.wiley.com/10.1046/j.1432-1327.2000.01402.x. Accessed 28 May 2012.
60. HearingVJ (2011) Determination of melanin synthetic pathways. The Journal of investigative dermatology 131: E8–E11 Available:http://www.ncbi.nlm.nih.gov/pubmed/22094404. Accessed 30 October 2012.
61. EisenmanHC, CasadevallA (2012) Synthesis and assembly of fungal melanin. Applied microbiology and biotechnology 93 : 931–940 Available:http://www.ncbi.nlm.nih.gov/pubmed/22173481. Accessed 30 October 2012.
62. U.S. National Library of Medicine (2010) ARSB. Genetics Home Reference Available:http://ghr.nlm.nih.gov/gene/ARSB.
63. LitjensT, HopwoodJJ (2001) Mucopolysaccharidosis type VI: Structural and clinical implications of mutations in N-acetylgalactosamine-4-sulfatase. Human mutation 18 : 282–295 Available:http://www.ncbi.nlm.nih.gov/pubmed/11668612. Accessed 26 August 2012.
64. Neufeld E, Muenzer J (2001) The mucopolysaccharidoses. In: Scriver C, Beaudet A, Sly W, Valle D, editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill. pp. 3421–3452.
65. SaftigP, KlumpermanJ (2009) Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nature reviews Molecular cell biology 10 : 623–635 Available:http://www.ncbi.nlm.nih.gov/pubmed/19672277. Accessed 26 October 2012.
66. GlavinasH, KrajcsiP, CserepesJ, SarkadiB (2004) The role of ABC transporters in drug resistance, metabolism and toxicity. Current drug delivery 1 : 27–42 Available:http://www.ncbi.nlm.nih.gov/pubmed/16305368. Accessed 27 August 2012.
67. HomolyaL, VáradiA, SarkadiB (2003) Multidrug resistance-associated proteins: Export pumps for conjugates with glutathione, glucuronate or sulfate. BioFactors (Oxford, England) 17 : 103–114 Available:http://www.ncbi.nlm.nih.gov/pubmed/12897433. Accessed 23 August 2012.
68. PeschkeK, EisnerT (1987) Defensive secretion of the tenebrionid beetle,Blaps mucronata: Physical and chemical determinants of effectiveness. Journal of Comparative Physiology A 161 : 377–388 Available:http://www.springerlink.com/content/x06t6p8200072x86/. Accessed 9 June 2012.
69. DettnerK (1991) Solvent-dependent variability of effectiveness of quinone-defensive systems of Oxytelinae beetles (Coleoptera: Staphylinidae). Entomologia Generalis 15 : 275–292.
70. Ashida M, Brey P (1998) Recent advances on the research of the insect prophenoloxidase cascade. In: Brey P, Hultmark D, editors. Molecular mechanisms of immune responses in insects. London, UK: Chapman & Hall. pp. 135–172.
71. CereniusL, SöderhällK (2004) The prophenoloxidase-activating system in invertebrates. Immunological reviews 198 : 116–126 Available:http://www.ncbi.nlm.nih.gov/pubmed/15199959.
72. KanostMR, JiangH, YuX-Q (2004) Innate immune responses of a lepidopteran insect, Manduca sexta. Immunological reviews 198 : 97–105 Available:http://www.ncbi.nlm.nih.gov/pubmed/15199957. Accessed 31 July 2012.
73. MavrouliMD, TsakasS, TheodorouGL, LampropoulouM, MarmarasVJ (2005) MAP kinases mediate phagocytosis and melanization via prophenoloxidase activation in medfly hemocytes. Biochimica et biophysica acta 1744 : 145–156 Available:http://www.ncbi.nlm.nih.gov/pubmed/15921769. Accessed 31 July 2012.
74. VilcinskasA (2013) Evolutionary plasticity of insect immunity. Journal of insect physiology 59 : 123–129 Available:http://www.ncbi.nlm.nih.gov/pubmed/22985862. Accessed 11 March 2013.
75. SöderhällK, CereniusL (1998) Role of the prophenoloxidase-activating system in invertebrate immunity. Current Opinion in Immunology 10 : 23–28 Available:http://dx.doi.org/10.1016/S0952-7915(98)80026-5. Accessed 5 July 2012.
76. ConesaA, GötzS, García-GómezJM, TerolJ, TalónM, et al. (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21 : 3674–3676 Available:http://bioinformatics.oxfordjournals.org/cgi/content/abstract/21/18/3674. Accessed 9 March 2012.
77. GötzS, García-GómezJM, TerolJ, WilliamsTD, NagarajSH, et al. (2008) High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic acids research 36 : 3420–3435 Available:http://nar.oxfordjournals.org/cgi/content/abstract/36/10/3420. Accessed 3 March 2012.
78. HannonG (2002) RNA interference. Nature 418 : 24–26 Available:http://codex.cshl.org/publications/Hannon_2002_12110901.pdf. Accessed 1 June 2012.
79. PosnienN, SchinkoJ, GrossmannD, ShippyTD, KonopovaB, et al. (2009) RNAi in the red flour beetle (Tribolium). Cold Spring Harbor protocols 2009: pdb.prot5256 Available:http://www.ncbi.nlm.nih.gov/pubmed/20147232. Accessed 9 May 2012.
80. HornT, BoutrosM (2010) E-RNAi: a web application for the multi-species design of RNAi reagents–2010 update. Nucleic acids research 38: W332–9 Available:http://nar.oxfordjournals.org/cgi/content/abstract/38/suppl_2/W332. Accessed 12 March 2012.
81. LalithaS (2000) Primer Premier 5. Biotech Software & Internet Report 1 : 270–272 Available:http://online.liebertpub.com/doi/abs/10.1089%2F152791600459894.
82. TomoyasuY, DenellRE (2004) Larval RNAi in Tribolium (Coleoptera) for analyzing adult development. Development genes and evolution 214 : 575–578 Available:http://www.ncbi.nlm.nih.gov/pubmed/15365833. Accessed 2 April 2012.
83. KatohK, KumaK, TohH, MiyataT (2005) MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic acids research 33 : 511–518 Available:http://nar.oxfordjournals.org/cgi/content/abstract/33/2/511. Accessed 11 March 2012.
84. PriceMN, DehalPS, ArkinAP (2010) FastTree 2–approximately maximum-likelihood trees for large alignments. PloS one 5: e9490 Available:http://dx.plos.org/10.1371/journal.pone.0009490. Accessed 12 March 2012.
85. TamuraK, PetersonD, PetersonN, StecherG, NeiM, et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular biology and evolution 28 : 2731–2739 Available:http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3203626&tool=pmcentrez&rendertype=abstract. Accessed 29 February 2012.
86. FriedrichM, BenzerS (2000) Divergent decapentaplegic expression patterns in compound eye development and the evolution of insect metamorphosis. The Journal of experimental zoology 288 : 39–55 Available:http://www.ncbi.nlm.nih.gov/pubmed/10750052. Accessed 14 May 2012.
87. OsborneP, DeardenPK (2005) Non-radioactive in-situ hybridisation to honeybee embryos and ovaries. Apidologie 36 : 113–118 doi:10.1051/apido
88. SchinkoJ, PosnienN, KittelmannS, KoniszewskiN, BucherG (2009) Single and double whole-mount in situ hybridization in red flour beetle (Tribolium) embryos. Cold Spring Harbor protocols 2009: pdb.prot5258 Available:http://cshprotocols.cshlp.org/cgi/content/abstract/2009/8/pdb.prot5258. Accessed 8 June 2012.
89. SuzukiY, SquiresDC, RiddifordLM (2009) Larval leg integrity is maintained by Distal-less and is required for proper timing of metamorphosis in the flour beetle, Tribolium castaneum. Developmental biology 326 : 60–67 Available:http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2762819&tool=pmcentrez&rendertype=abstract. Accessed 7 June 2012.
90. AspJ, AbramssonA, BetsholtzC (2006) Nonradioactive in situ hybridization on frozen sections and whole mounts. Methods in molecular biology (Clifton, NJ) 326 : 89–102 Available:http://www.ncbi.nlm.nih.gov/pubmed/16780195.
91. ConnHJ, DimmickI (1947) Soil Bacteria Similar in Morphology to Mycobacterium and Corynebacterium. Journal of bacteriology 54 : 291–303 Available:http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=526554&tool=pmcentrez&rendertype=abstract. Accessed 7 June 2012.
92. FayeI, WyattGR (1980) The synthesis of antibacterial proteins in isolated fat body from Cecropia silkmoth pupae. Experientia 36 : 1325–1326 Available:http://www.ncbi.nlm.nih.gov/pubmed/7449923. Accessed 28 October 2012.
93. CereniusL, LeeBL, SöderhällK (2008) The proPO-system: pros and cons for its role in invertebrate immunity. Trends in immunology 29 : 263–271 Available:http://www.ncbi.nlm.nih.gov/pubmed/18457993. Accessed 17 July 2012.
94. RothO, JoopG, EggertH, HilbertJ, DanielJ, et al. (2010) Paternally derived immune priming for offspring in the red flour beetle, Tribolium castaneum. The Journal of animal ecology 79 : 403–413 Available:http://www.ncbi.nlm.nih.gov/pubmed/19840170. Accessed 30 March 2012.
Štítky
Genetika Reprodukční medicína
Článek Independent Evolution of Transcriptional Inactivation on Sex Chromosomes in Birds and MammalsČlánek The bHLH Subgroup IIId Factors Negatively Regulate Jasmonate-Mediated Plant Defense and DevelopmentČlánek Selective Pressures to Maintain Attachment Site Specificity of Integrative and Conjugative ElementsČlánek Reassembly of Nucleosomes at the Promoter Initiates Resilencing Following Decitabine ExposureČlánek Hepatocyte Growth Factor Signaling in Intrapancreatic Ductal Cells Drives Pancreatic Morphogenesis
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 7
-
Všechny články tohoto čísla
- An Solution for Crossover Formation
- Genome-Wide Association Mapping in Dogs Enables Identification of the Homeobox Gene, , as a Genetic Component of Neural Tube Defects in Humans
- Independent Evolution of Transcriptional Inactivation on Sex Chromosomes in Birds and Mammals
- Stepwise Activation of the ATR Signaling Pathway upon Increasing Replication Stress Impacts Fragile Site Integrity
- Genomic Analysis of Natural Selection and Phenotypic Variation in High-Altitude Mongolians
- Modification of tRNA by Elongator Is Essential for Efficient Translation of Stress mRNAs
- Role of CTCF Protein in Regulating Locus Transcription
- Gene Set Signature of Reversal Reaction Type I in Leprosy Patients
- Mapping of PARK2 and PACRG Overlapping Regulatory Region Reveals LD Structure and Functional Variants in Association with Leprosy in Unrelated Indian Population Groups
- Is Required for Formation of the Genital Ridge in Mice
- Monopolin Subunit Csm1 Associates with MIND Complex to Establish Monopolar Attachment of Sister Kinetochores at Meiosis I
- Recombination Dynamics of a Human Y-Chromosomal Palindrome: Rapid GC-Biased Gene Conversion, Multi-kilobase Conversion Tracts, and Rare Inversions
- Mechanisms of Protein Sequence Divergence and Incompatibility
- Histone Methyltransferase DOT1L Drives Recovery of Gene Expression after a Genotoxic Attack
- Female Behaviour Drives Expression and Evolution of Gustatory Receptors in Butterflies
- Combinatorial Regulation of Meiotic Holliday Junction Resolution in by HIM-6 (BLM) Helicase, SLX-4, and the SLX-1, MUS-81 and XPF-1 Nucleases
- The bHLH Subgroup IIId Factors Negatively Regulate Jasmonate-Mediated Plant Defense and Development
- The Role of Interruptions in polyQ in the Pathology of SCA1
- Dietary Restriction Induced Longevity Is Mediated by Nuclear Receptor NHR-62 in
- Fine Time Course Expression Analysis Identifies Cascades of Activation and Repression and Maps a Putative Regulator of Mammalian Sex Determination
- Genome-scale Co-evolutionary Inference Identifies Functions and Clients of Bacterial Hsp90
- Oxidative Stress and Replication-Independent DNA Breakage Induced by Arsenic in
- A Moonlighting Enzyme Links Cell Size with Central Metabolism
- Budding Yeast Greatwall and Endosulfines Control Activity and Spatial Regulation of PP2A for Timely Mitotic Progression
- The Conserved Intronic Cleavage and Polyadenylation Site of CstF-77 Gene Imparts Control of 3′ End Processing Activity through Feedback Autoregulation and by U1 snRNP
- The BTB-zinc Finger Transcription Factor Abrupt Acts as an Epithelial Oncogene in through Maintaining a Progenitor-like Cell State
- The Cohesion Protein SOLO Associates with SMC1 and Is Required for Synapsis, Recombination, Homolog Bias and Cohesion and Pairing of Centromeres in Drosophila Meiosis
- The RNA-binding Proteins FMR1, Rasputin and Caprin Act Together with the UBA Protein Lingerer to Restrict Tissue Growth in
- Pattern Dynamics in Adaxial-Abaxial Specific Gene Expression Are Modulated by a Plastid Retrograde Signal during Leaf Development
- A Network of HMG-box Transcription Factors Regulates Sexual Cycle in the Fungus
- Bacterial Adaptation through Loss of Function
- ENU-induced Mutation in the DNA-binding Domain of KLF3 Reveals Important Roles for KLF3 in Cardiovascular Development and Function in Mice
- Interplay between Structure-Specific Endonucleases for Crossover Control during Meiosis
- FGF Signalling Regulates Chromatin Organisation during Neural Differentiation via Mechanisms that Can Be Uncoupled from Transcription
- The Arabidopsis RNA Binding Protein with K Homology Motifs, SHINY1, Interacts with the C-terminal Domain Phosphatase-like 1 (CPL1) to Repress Stress-Inducible Gene Expression
- Selective Pressures to Maintain Attachment Site Specificity of Integrative and Conjugative Elements
- The Conserved ADAMTS-like Protein Lonely heart Mediates Matrix Formation and Cardiac Tissue Integrity
- The cGMP-Dependent Protein Kinase EGL-4 Regulates Nociceptive Behavioral Sensitivity
- RBM5 Is a Male Germ Cell Splicing Factor and Is Required for Spermatid Differentiation and Male Fertility
- Disease-Related Growth Factor and Embryonic Signaling Pathways Modulate an Enhancer of Expression at the 6q23.2 Coronary Heart Disease Locus
- Yeast Pol4 Promotes Tel1-Regulated Chromosomal Translocations
- A Dual Role for SOX10 in the Maintenance of the Postnatal Melanocyte Lineage and the Differentiation of Melanocyte Stem Cell Progenitors
- SLC26A4 Targeted to the Endolymphatic Sac Rescues Hearing and Balance in Mutant Mice
- Odoriferous Defensive Stink Gland Transcriptome to Identify Novel Genes Necessary for Quinone Synthesis in the Red Flour Beetle,
- Prediction of Complex Human Traits Using the Genomic Best Linear Unbiased Predictor
- Gene × Physical Activity Interactions in Obesity: Combined Analysis of 111,421 Individuals of European Ancestry
- Reassembly of Nucleosomes at the Promoter Initiates Resilencing Following Decitabine Exposure
- Exquisite Light Sensitivity of Cryptochrome
- miR-133a Regulates Adipocyte Browning In Vivo
- Strabismus Promotes Recruitment and Degradation of Farnesylated Prickle in Planar Polarity Specification
- Hepatocyte Growth Factor Signaling in Intrapancreatic Ductal Cells Drives Pancreatic Morphogenesis
- Is a Potential Tumor Suppressor Gene Commonly Inactivated by Epigenetic Mechanisms in Colorectal Cancer
- Joint Molecule Resolution Requires the Redundant Activities of MUS-81 and XPF-1 during Meiosis
- The Mating Competence of Geographically Diverse Strains in Their Natural and Unnatural Sand Fly Vectors
- Defective Repair of Oxidative Base Lesions by the DNA Glycosylase Nth1 Associates with Multiple Telomere Defects
- Effective Blocking of the Enhancer Requires Cooperation between Two Main Mechanisms Suggested for the Insulator Function
- Trans-Ancestral Studies Fine Map the SLE-Susceptibility Locus
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- SLC26A4 Targeted to the Endolymphatic Sac Rescues Hearing and Balance in Mutant Mice
- Bacterial Adaptation through Loss of Function
- The Cohesion Protein SOLO Associates with SMC1 and Is Required for Synapsis, Recombination, Homolog Bias and Cohesion and Pairing of Centromeres in Drosophila Meiosis
- Gene × Physical Activity Interactions in Obesity: Combined Analysis of 111,421 Individuals of European Ancestry
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



