-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaFemale Behaviour Drives Expression and Evolution of Gustatory Receptors in Butterflies
Secondary plant compounds are strong deterrents of insect oviposition and feeding, but may also be attractants for specialist herbivores. These insect-plant interactions are mediated by insect gustatory receptors (Grs) and olfactory receptors (Ors). An analysis of the reference genome of the butterfly Heliconius melpomene, which feeds on passion-flower vines (Passiflora spp.), together with whole-genome sequencing within the species and across the Heliconius phylogeny has permitted an unprecedented opportunity to study the patterns of gene duplication and copy-number variation (CNV) among these key sensory genes. We report in silico gene predictions of 73 Gr genes in the H. melpomene reference genome, including putative CO2, sugar, sugar alcohol, fructose, and bitter receptors. The majority of these Grs are the result of gene duplications since Heliconius shared a common ancestor with the monarch butterfly or the silkmoth. Among Grs but not Ors, CNVs are more common within species in those gene lineages that have also duplicated over this evolutionary time-scale, suggesting ongoing rapid gene family evolution. Deep sequencing (∼1 billion reads) of transcriptomes from proboscis and labial palps, antennae, and legs of adult H. melpomene males and females indicates that 67 of the predicted 73 Gr genes and 67 of the 70 predicted Or genes are expressed in these three tissues. Intriguingly, we find that one-third of all Grs show female-biased gene expression (n = 26) and nearly all of these (n = 21) are Heliconius-specific Grs. In fact, a significant excess of Grs that are expressed in female legs but not male legs are the result of recent gene duplication. This difference in Gr gene expression diversity between the sexes is accompanied by a striking sexual dimorphism in the abundance of gustatory sensilla on the forelegs of H. melpomene, suggesting that female oviposition behaviour drives the evolution of new gustatory receptors in butterfly genomes.
Published in the journal: . PLoS Genet 9(7): e32767. doi:10.1371/journal.pgen.1003620
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003620Summary
Secondary plant compounds are strong deterrents of insect oviposition and feeding, but may also be attractants for specialist herbivores. These insect-plant interactions are mediated by insect gustatory receptors (Grs) and olfactory receptors (Ors). An analysis of the reference genome of the butterfly Heliconius melpomene, which feeds on passion-flower vines (Passiflora spp.), together with whole-genome sequencing within the species and across the Heliconius phylogeny has permitted an unprecedented opportunity to study the patterns of gene duplication and copy-number variation (CNV) among these key sensory genes. We report in silico gene predictions of 73 Gr genes in the H. melpomene reference genome, including putative CO2, sugar, sugar alcohol, fructose, and bitter receptors. The majority of these Grs are the result of gene duplications since Heliconius shared a common ancestor with the monarch butterfly or the silkmoth. Among Grs but not Ors, CNVs are more common within species in those gene lineages that have also duplicated over this evolutionary time-scale, suggesting ongoing rapid gene family evolution. Deep sequencing (∼1 billion reads) of transcriptomes from proboscis and labial palps, antennae, and legs of adult H. melpomene males and females indicates that 67 of the predicted 73 Gr genes and 67 of the 70 predicted Or genes are expressed in these three tissues. Intriguingly, we find that one-third of all Grs show female-biased gene expression (n = 26) and nearly all of these (n = 21) are Heliconius-specific Grs. In fact, a significant excess of Grs that are expressed in female legs but not male legs are the result of recent gene duplication. This difference in Gr gene expression diversity between the sexes is accompanied by a striking sexual dimorphism in the abundance of gustatory sensilla on the forelegs of H. melpomene, suggesting that female oviposition behaviour drives the evolution of new gustatory receptors in butterfly genomes.
Introduction
Nearly 50 years ago Ehrlich and Raven proposed that butterflies and their host-plants co-evolve [1]. Based on field observations of egg-laying in adult female butterflies, feeding behavior of caterpillars, and studies of systematics and taxonomy of plants and butterflies themselves, they outlined a scenario in which plant lineages evolved novel defensive compounds which then permitted their radiation into novel ecological space. In turn, insect taxa evolved resistance to those chemical defences, permitting the adaptive radiation of insects to exploit the new plant niche. Ehrlich and Raven's theory of an evolutionary arms-race between insects and plants drew primarily from an examination of butterfly species richness and host-plant specialization. It did not specify the sensory mechanisms or genetic loci mediating these adaptive plant-insect interactions.
Insects possess gustatory hairs or contact chemosensilla derived from mechanosensory bristles, scattered along a variety of appendages [2]–[4]. In adult butterflies and moths, gustatory sensilla are found on the labial palps and proboscis (Figure 1), the legs (Figure 2A) [5], the antennae (Figure 2B) [6], [7], and the ovipositor [8], [9]. In adult Heliconius charithonia legs, the 5 tarsomeres of the male foreleg foretarsus are fused and lack chemosensory sensilla, while female foretarsi bear groups of trichoid sensilla (n = 70–90 sensilla/tarsus) associated with pairs of cuticular spines [10]. Each trichoid sensilla contains five receptor neurons. These sensilla are sensitive to compounds that may be broadly classified as phagostimulants (e.g., sugars and amino acids), which promote feeding behavior, or phagodeterrents (secondary plant compounds), which suppress it [11]; in adult females they may also modulate oviposition [12].
Fig. 1. Scanning electron micrographs of the proboscis of Heliconius butterflies. 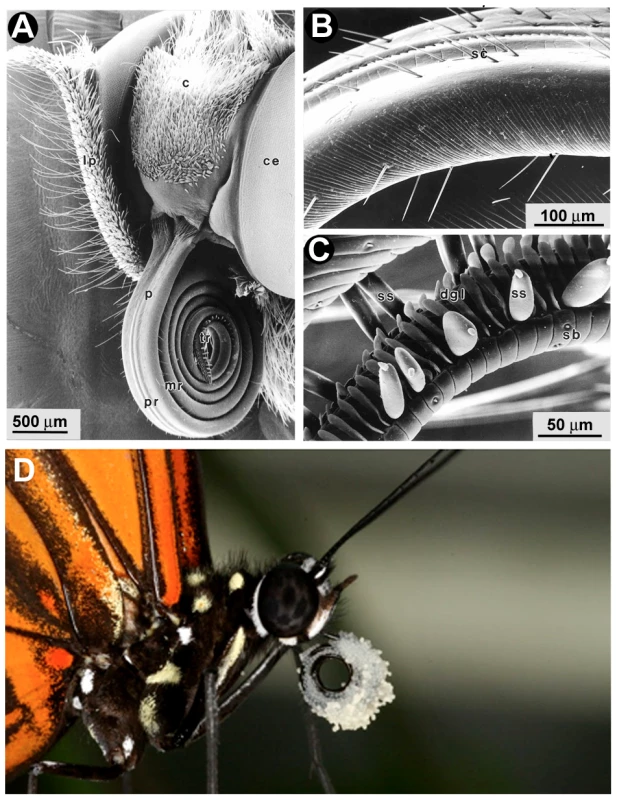
(A) The labial palps (lp) and proboscis (p) of the H. erato head contain gustatory sensilla. (B) The proximal portion of the H. melpomene proboscis has hair-like sensilla chaetica (sc). (C) The tip portion of the proboscis has specialized ridges for pollen collection along with sensilla styloconica (ss). Reproduced with permission [9]. (D) H. melpomene with a pollen-load. c, clypeus, ce, compound eye; pr, proximal region; mr, mid region; tr, tip region; dgl, dorsal galeal linking structures; sb, blunt-tipped sensilla. Fig. 2. Sexual dimorphism in H. melpomene chemosensory tissues. 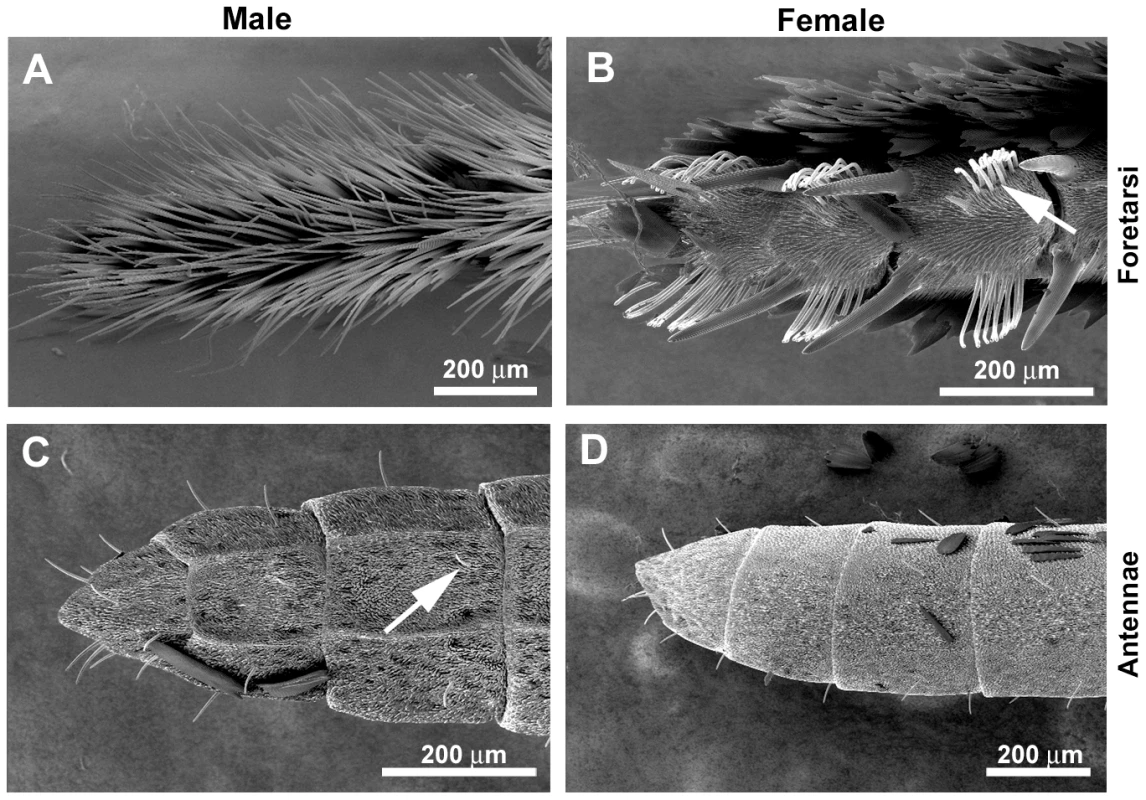
Scanning electron micrographs of adult legs showing a sexual dimorphism in gustatory (trichoid) sensilla. Foreleg foretarsi of a male (A) and a female (B). Four pairs of clumped taste sensilla are each found associated with a pair of cuticular spines on each female foot (only three are shown). Arrow indicates a clump of taste sensilla. Antennae of an adult male (C) and a female (D) showing individual gustatory sensilla (arrow). Genes for vision, taste and smell are likely to be crucial genomic loci underlying the spectacular diversity of butterfly-plant interactions. The availability of genomes for two butterfly species, the postman Heliconius melpomene (Nymphalidae) [13] and the monarch (Danaus plexippus) [14], as well as the silkmoth (Bombyx mori) [15], enables us to examine the evolutionary diversification of gustatory (Gr) and olfactory (Or) receptor genes that mediate insect-plant interactions. Each of these species feeds on hosts from different plant families. Silkmoth larvae feed on mulberry (Morus spp., Moraceae) and monarch larvae feed on milkweed (Asclepias spp., Apocynaceae). The larvae of Heliconius feed exclusively on passion flower vines, primarily in the genus Passiflora (Passifloraceae). In addition, adult Heliconius are notable for several derived traits such as augmented UV color vision [16], pollen feeding (Figure 1B) [17], [18], and the ability to sequester substances from their host plants that are toxic to vertebrate predators such as birds [19], [20].
In Drosophila melanogaster, the Gr gene family consists of 60 genes [21]–[24], several of which are alternatively spliced, yielding 68 predicted Gr transcripts [24]. One or more of these Gr proteins including possibly obligatory co-receptors [25]–[27] may be expressed in each gustatory receptor neuron [11]. Originally considered members of the G-protein-coupled receptor (GPCR) family, insect Grs have an inverted orientation in the membrane compared to the GPCR family of vertebrate Grs [28] and are part of the same superfamily as the insect Ors [21]. Signalling pathways for insect Grs may be both G-protein dependent [29], [30], [31] and G-protein independent [32]. For the vast majority of Drosophila Grs the specific compounds to which they are sensitive remain unknown. Nonetheless, several receptors for sugars [33]–[35], CO2 [26], [36], bitter substances [37]–[39] and plant-derived insecticides [25] have been identified in flies.
Knowledge of the Gr gene family for insects outside Drosophila is sparse and has primarily relied on the analyses of individual reference genomes. Expression studies are challenging, due to the very low expression of Grs in gustatory tissues [21], [23]. In addition, Grs and Ors typically have large introns, small exons and undergo fast sequence evolution, making their in silico identification using automated gene prediction algorithms from genomic sequences problematic. Thus, the large repertoire of Grs (and Ors) that have been examined in the reference genomes of the pea aphid [40], the honey bee [41], the red flour beetle Tribolium castaneum [42], the mosquitoes Aedes aegypti [43] and Anopheles gambiae [44], and several Drosophila spp. [45], [46] have required extensive manual curation. In Lepidoptera, a large insect group which includes ∼175,000 species, completely described Gr (and Or) gene models from genomes are rare and limited to B. mori [47], D. plexippus [14] and H. melpomene (Grs, this study; Ors, [13]). In other lepidopteran species, only fragmentary Gr data are available: five sequences in Spodoptera littoralis [48], three in Heliothis virescens [49], two in Manduca sexta [50], [51] and one in Papilio xuthus [52].
Adult females of each Heliconius species only lay eggs on a limited number of host plants [53], and therefore need to recognize different species from among the large and diverse Passifloraceae family, which also show a remarkable diversity of chemical defences [54]. The evolutionary arms race between Heliconius butterflies and their hosts led us to hypothesize that Heliconius Grs (and Ors) might be subject to rapid gene duplication and gene loss as well as copy-number variation (CNV). Recent work taking advantage of published Drosophila genomes has shown a relationship between host specialization and/or endemism and an increased rate of gene loss, as well as a positive relationship between genome size and gene duplication [46], [55]. Moreover, Drosophila Grs appear to be evolving under weaker purifying selection than Ors [55].
We previously used the reference genome sequence for H. melpomene to annotate three chemosensory gene families, encoding the chemosensory proteins (CSPs), the odorant-binding proteins (OBPs), and the olfactory receptors (Ors). This demonstrated a surprising diversity in these gene families. In particular there are more CSPs in the butterfly genomes than in any other insect genome sequenced to date [13]. We build on this work below by characterizing the Gr gene family in the reference H. melpomene melpomene genome and in two other lepidopteran species whose genomes have been sequenced, B. mori (Bombycidae) and D. plexippus (Nymphalidae), by performing in silico gene predictions and phylogenetic analysis. We then analyzed whole-genome sequences of twenty-seven individual butterflies, representing eleven species sampled across all major lineages of the Heliconius phylogeny and including sixteen individuals from two species, H. melpomene and its sister-species H. cydno. We also generated RNA-sequencing expression profiles of the proboscis and labial palps, antennae and legs of individual adult male and female butterflies of the sub-species H. melpomene rosina from Costa Rica (∼1 billion 100 bp reads). We used these data to address four major questions: Are different chemosensory modalities less prone to duplication and loss than others (e.g., taste vs. olfaction)? Is there evidence of lineage-specific differentiation of Gr (and Or) repertoires between genera, species and populations? What is the relationship between CNVs and the retention of paralogous genes over long-term evolutionary timescales? Are the life history differences between males and females reflected in the expression of Grs and Ors as well as in the retention of novel sensory genes in the genome?
We find higher turnover of the Grs than the Ors over longer evolutionary timescales, and evidence for both gene duplication and loss among a clade of intronless Grs between lepidopteran species and within the genus Heliconius. We also find for H. melpomene and its sister species, H. cydno, evidence of copy-number variation (CNVs) within their Gr and Or repertoires. Lastly, our RNA-sequencing suggests both tissue-specific and sex-specific differences in the diversity of expressed Grs and Ors, with female legs expressing a more diverse suite of Grs than male legs. Our data set revealing the expression of 67 of 73 predicted Gr genes and 67 of 70 predicted Or genes in adult H. melpomene butterflies is the most comprehensive profiling of these chemosensory gene families in Lepidoptera to date, and suggests how female host plant-seeking behaviour shapes the evolution of gustatory receptors in butterflies.
Results
Annotation of Grs in the reference genome of H. melpomene
In total, we manually annotated 86,870 bp of the H. melpomene melpomene reference genome (Table S1). Our 73 Gr gene models, consisted of 1–11 annotated exons, with the majority having three or four exons; six were intronless. We found genomic evidence (but not RNA-seq evidence) of possible alternative splicing of the last two exons of HmGr18, bringing the total number of predicted Grs to 74. Alternative splicing has not been previously described in the silkmoth B. mori [47], but is known to occur in most other insects examined, including D. melanogaster, Anopheles gambiae, Aedes aegypti and T. castaneum [24], [43], [44]. We also identified eleven new putative Grs in the monarch butterfly genome, DpGr48-56, DpGr66 and DpGr68 (Table S1) [14].
All but five of our gene models contained more than 330 encoded amino acids (AAs) while individual gene models ranged from 258–477 AAs. Several Gr genes contained internal stop codons (Table S1). In at least one case, we found RNA-seq evidence of an expressed pseudogene–HmGr61–with two in-frame stop codons. In other cases, the 5′ end of our assembled transcripts was not long enough to verify the internal stop codons in the genome assembly. The Grs are located on 33 distinct scaffolds, with 58 forming clusters of 2–8 genes on 18 scaffolds, distributed across 14 chromosomes.
Gene duplication and loss in a clade of putative bitter receptors
To study the patterns of gene duplication and loss more broadly across the Lepidoptera, we next examined the phylogenetic relationships of Grs from the three lepidopteran reference genomes [13]–[15]. Across the gene family phylogeny a large number of duplications among the putative ‘bitter’ gustatory receptors of Heliconius or Danaus have occurred, while the putative CO2 and sugar receptors are evolving more conservatively, with only single copies in the H. melpomene reference genome (see below)(black arcs, Figure 3). A majority (∼64%) of Gr genes found in the H. melpomene genome are the result of gene duplication since Heliconius shared a common ancestor with Danaus or Bombyx. This is in contrast to the more conserved pattern of evolution of the Ors (Figure 4) [13] where a majority (37 of 70 or 53%) of genes show a one-to-one orthologous relationship with either a gene in Danaus, in Bombyx or both.
Fig. 3. Phylogeny of the Grs identified in three lepidopteran genomes. 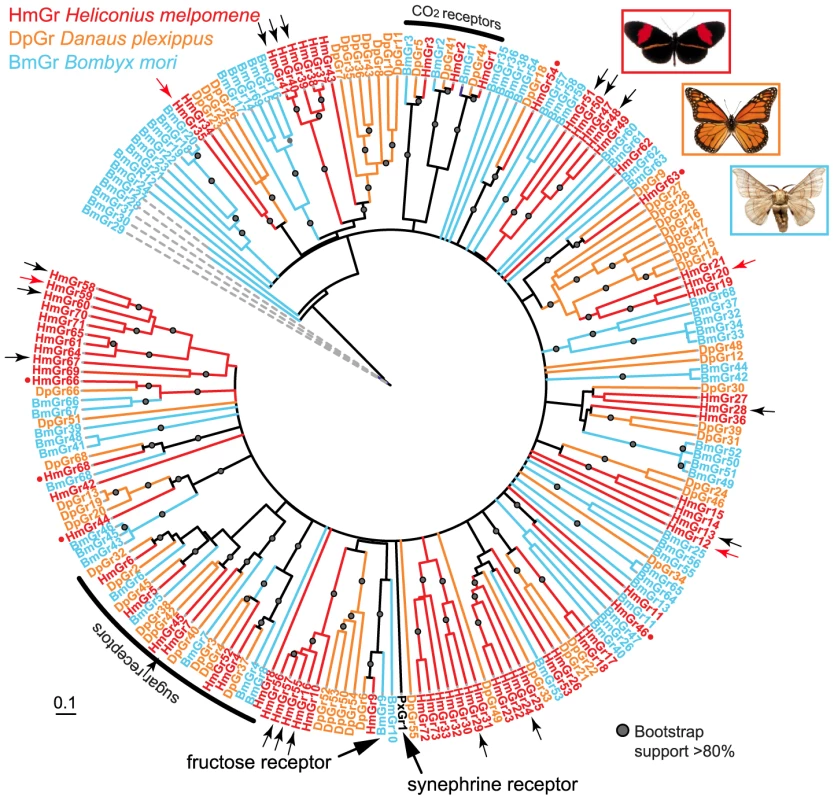
A maximum likelihood analysis of amino acid sequences was performed. Bootstrap support is out of 500 replicates. Putative CO2 and fructose receptors show a conserved 1-to-1 orthologous relationship in each of the three lepidopteran genomes, while putative sugar receptors of the monarch butterfly have duplicated twice. By contrast, numerous butterfly- or moth-specific gene duplications are evident among the remaining Grs, which are hypothesized to be bitter receptors. Small red dots indicate single-copy Heliconius Grs classified as conserved genes in the analyses shown in Table 1 and Table 2. Small black arrows indicate female-specific Grs expressed in adult H. melpomene legs. Small red arrows indicate Grs expressed in adult H. melpomene proboscis only. Bar indicates branch lengths in proportion to amino acid substitutions/site. Synephrine and fructose receptors are described in [52] and [32]. Bm = Bombyx mori, Hm = Heliconius melpomene, Dp = Danaus plexippus, Px = Papilio xuthus. Fig. 4. Phylogeny of the Ors identified in three lepidopteran genomes. 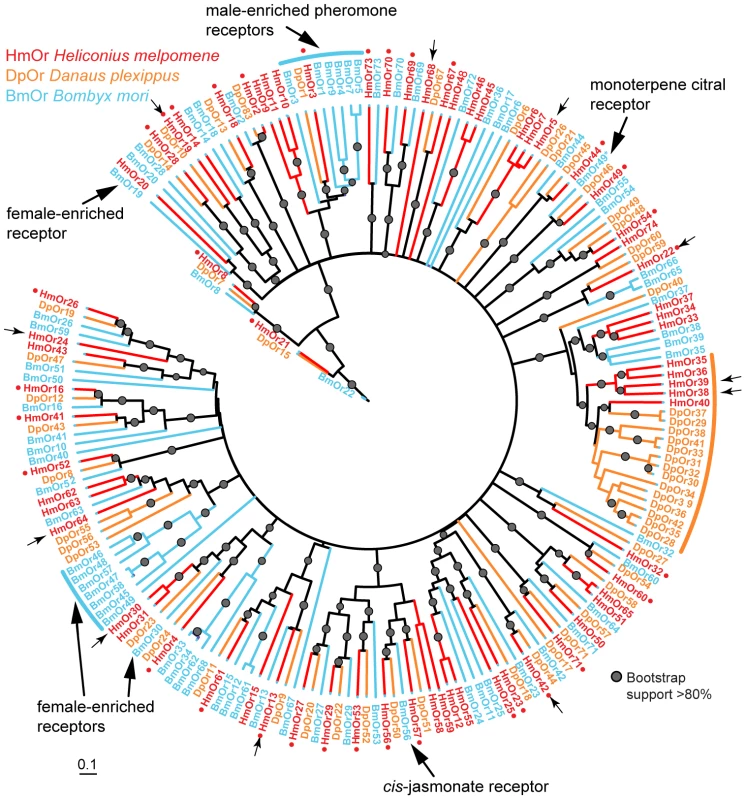
A maximum likelihood analysis of amino acid sequences was performed. Bootstrap support is out of 500 replicates. Fewer lineage-specific duplications are evident among the Ors compared to the Grs, with the exception of one large butterfly-specific expansion (orange arc). Small red dots indicate single-copy Heliconius Ors classified as conserved genes in the analyses shown in Table 1 and Table 2. Ors that are enriched in male or female adult B. mori antennae (blue and black arcs) are described in [91]; cis-jasmonate and monoterpene citral receptors are described in [92] and [93]. Phylogenetic tree reconstruction details are given in [13]. Bar indicates branch lengths in proportion to amino acid substitutions/site. Small arrows indicate female-specific Ors expressed in adult H. melpomene legs. Bm = Bombyx mori, Hm = Heliconius melpomene, Dp = Danaus plexippus. Within the genus Heliconius there is a great diversity of host plant preferences for different Passiflora species. To look at the relationship between gene duplication and loss over this shorter timescale, we focussed our efforts on a group of six intronless Grs, HmGr22-26 and Gr53, because it is only feasible to identify single-exon genes with high confidence, given that the Illumina whole-genome sequencing approach leads to poorly assembled genomes (Table S2). These genes are also of interest as some members of this group are very highly expressed. Notably HmGr22 is one of the most widely expressed genes in our adult H. melpomene transcriptomes, which was verified by reverse-transcriptase (RT)-PCR and sequencing of the PCR products (Figure 5A). In this regard HmGr22 resembles another intronless Gr, the silkmoth gene BmGr53, which is expressed in adult male and female antennae and larval antennae, maxilla, labrum, mandible, labium, thoracic leg, proleg and gut [32]. The remaining five intronless Grs have much more limited domains of expression in adult H. melpomene (see below). We searched for these genes in de novo assemblies of whole-genome Illumina sequences from eleven species across the Heliconius phylogeny. We investigate whether, as in Drosophila, a high turnover in putative bitter receptors is observed in species with host plant specializations or in species which are endemic and thus smaller in effective population size [46].
Fig. 5. HmGr22 expression in adults and intronless Grs from whole-genome sequence data across the Heliconius phylogeny. 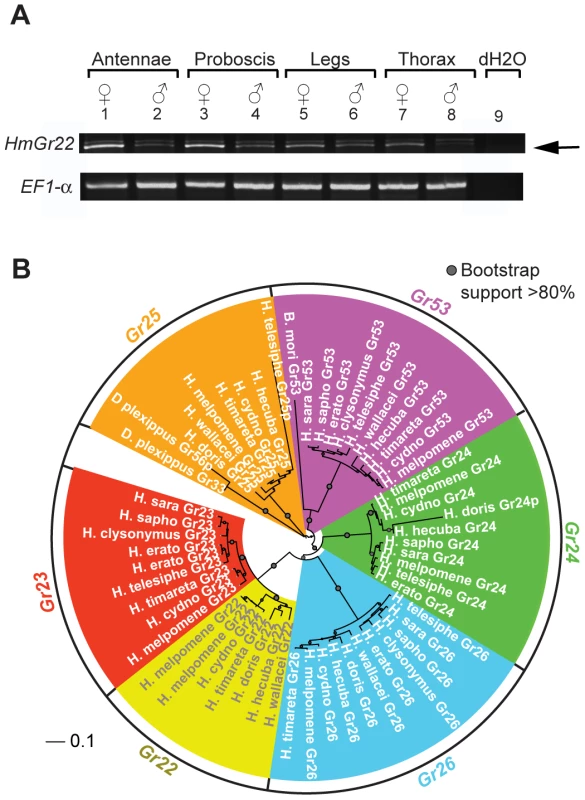
(A) Reverse-transcriptase PCR (RT-PCR) of adult H. melpomene tissues showing the expression of HmGr22 and elongation factor-1 alpha. Two products are evident from the Gr22 RT-PCR. The bottom RT-PCR product is HmGr22 (arrow) and the top RT-PCR product is 18 s rRNA, which was verified by Sanger sequencing. (B) Neighbor-joining tree showing the phylogenetic relationship between the forty-six intact Grs and four pseudogenes identified in the 13 lepidopteran genomes. Bootstrap support is out of 500 bootstrap replicates. Pseudogene sequences are indicated by a ‘p’ after the gene name. Although patterns of host plant use are complex within the genus, some notable host-plant shifts have occurred, leading to the prediction that gene loss may have occurred along more specialized lineages [46]. For example, H. doris unlike many Heliconius, tends to feed on large woody Passiflora that can support their highly gregarious larvae [53]. It also probably has a smaller effective population size than most other Heliconius species. From the 11 species studied, we identified a total of 44 intact or nearly intact intronless Grs, as well as three intronless pseudogenes (Genbank Accession Nos. KC313949-KC313997)(Table S2 and S3). We also identified one intact intronless Gr each in monarch and silkmoth and one intronless Gr pseudogene in monarch. Phylogenetic analysis indicates that six intact intronless Gr genes were present at the base of the genus Heliconius while the intronless Gr pseudogene in monarch was the result of duplication since Heliconius and monarch shared a common ancestor (Figure 5B, Figure 6). Subsequent to the radiation of the genus Heliconius, there have been a number of gene losses. Whereas all members of the melpomene clade (H. melpomene, H. cydno, H. timareta) retained genomic copies of all six genes, members of the erato clade (H. erato, H. clysonymus and H. telesiphe) and sara-sapho clade (H. sara and H. sapho) have lost their copies of Gr22 and Gr25. In addition, members of the so-called primitive clade (H. wallacei, H. hecuba, and H. doris) have lost Gr23, while H. doris and H. wallacei have apparently lost Gr24 independently (Figure 6). The woody plant specialist, H. doris, has retained the fewest intronless Grs, apparently also having lost its copy of Gr53, a pattern mirrored by Drosophila host plant specialists [46]. We have, however, no direct evidence that the intronless Grs are in fact involved in host plant discrimination so the observed patterns of loss may be better explained by other variables such as effective population size.
Fig. 6. Inferred patterns of intronless Gr gene gain and loss across the genus Heliconius. 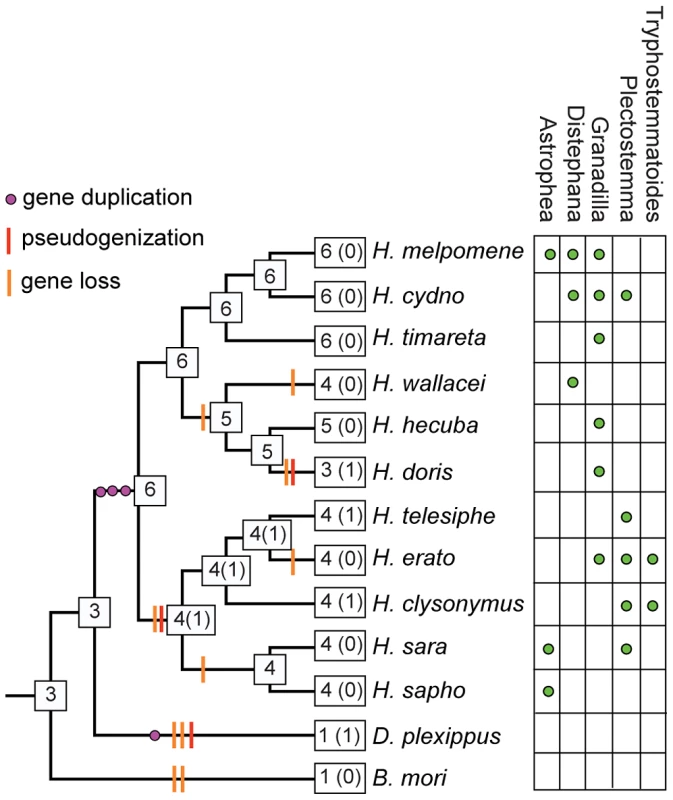
Estimates of the number of Gr loci (number of pseudogenes is indicated in parentheses) on internal nodes of the lepidopteran phylogeny and gene gain (purple dots), gene loss (orange slashes) and pseudogenisation events (red slashes) on each branch. Heliconius phylogeny is based on Beltran et al. (2007) [90]. Reconciliation of gene trees onto the species tree was performed in Notung using maximum likelihood gene family trees. Primary Passiflora host plant subgenera (green dots) affiliated with each Heliconius species [53]. No clear relationship exists between the number of known Passiflora subgenera used and the number of intronless Grs in a species, which are presumed to be putative bitter receptors, but whose ligands are not yet identified. The woody vine specialist, H. doris, with the smallest effective population size, has the fewest intact intronless Grs. CNVs occur frequently among paralogous gustatory receptor genes
We next tested whether the greater level of diversification of Grs as compared to Ors over long evolutionary timescales (compare Figure 3 and Figure 4), is similarly reflected in greater population level variation in Gr and Or duplicate genes. To test this hypothesis, we examined the incidence of CNVs among Grs and Ors that exist as single-copy genes in the reference H. melpomene genome with a one-to-one orthologous relationship with a gene in Danaus, Bombyx or both (conserved)(red dots, Figure 3 and 4), or as genes that are Heliconius-specific where no orthologue exists in either Danaus or Bombyx (non-conserved). We used whole genome resequence data (12 genomes) for three subspecies of H. melpomene (H. melpomene amaryllis, n = 4; H. melpomene aglaope, n = 4; and H. melpomene rosina, n = 4)(Figure 7, inset) and one sub-species of H. cydno (H. cydno chioneus, n = 4)(Table S4). We first mapped genomic resequence reads to the H. melpomene melpomene reference genome, and then searched for regions of abnormal coverage using CNVnator [56]. More than half of Gr loci showed presence of CNVs (37 out of 68 loci). However, there were noticeably fewer CNVs in Gr loci that evolve conservatively over the long-term, such as among the putative CO2 receptors, while there was an excess of CNVs in loci that show patterns of Heliconius-specific duplication (11.1% vs. 54.9%, respectively)(Fisher's Exact Test, two-tailed, P = 0.0004) (Table 1)(Figure 7). Intriguingly, many sugar receptor CNVs are sub-species specific; we observed fixed duplications relative to the reference genome in H. melpomene aglaope (HmGr4, Gr5, Gr6, Gr8, Gr45, Gr52) and H. melpomene amaryllis (Gr4, Gr5, Gr6, Gr7, Gr8, Gr45, Gr52), among genes that are found on different chromosomes (Table S5, Figure 7). Although the majority of CNVs are likely to be evolving neutrally, this raises the possibility of local adaptation within the species range around the detection of sugars. As expected given their long-term stability, Ors also show a lower incidence of CNVs (12 out of 67 loci), with no association between gene duplication and CNV incidence at least in H. melpomene (Table 1, Table S6). In H. cydno, a slight excess of Or CNVs was observed in loci that resulted in paralogous genes over longer evolutionary timescales (Fisher's Exact Test, two-tailed, P = 0.0475)(Table 1)(Figure 8).
Fig. 7. Copy-number variant (CNV) analysis of Grs in the H. melpomene genome. 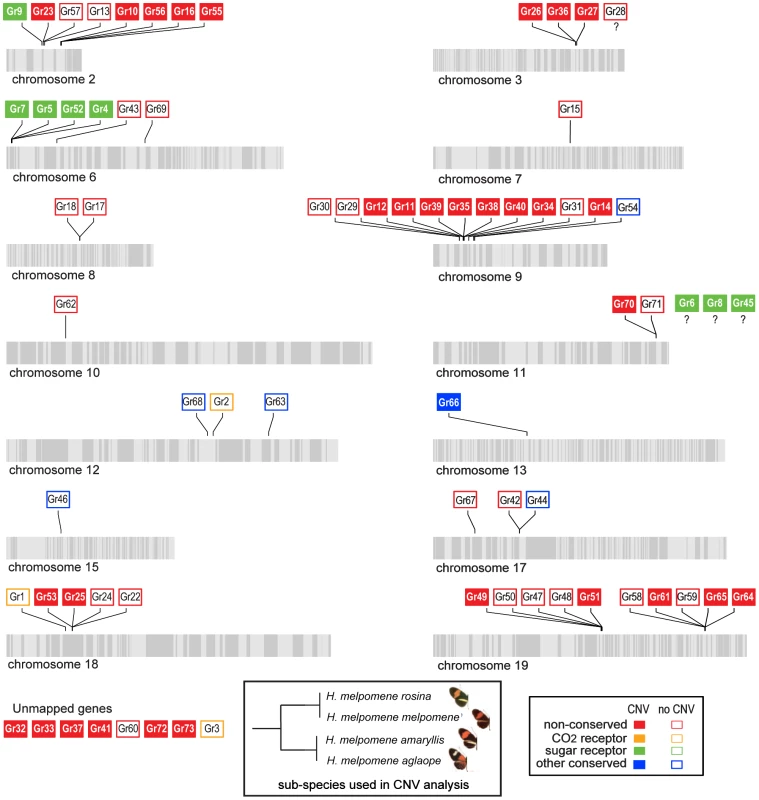
Scaffolds comprising each chromosome are indicated by alternating light and grey stripes. Grs without CNVs are indicated by open boxes and Grs with CNVs are indicated by closed boxes. Grs are classified as conserved if, in the H. melpomene reference genome, they have a one-to-one orthologous relationship with either a gene in Danaus, Bombyx or both (red dots, Figure 3). Grs are classified as non-conserved if they are duplicated in the H. melpomene reference genome or have no orthologue in either Danaus, Bombyx or both. Genes mapped to chromosomes but without precise locations are indicated by question marks. Scaffold arrangement is based on the published linkage map [13]. Fig. 8. Copy-number variant (CNV) analysis of Ors in the H. melpomene genome. 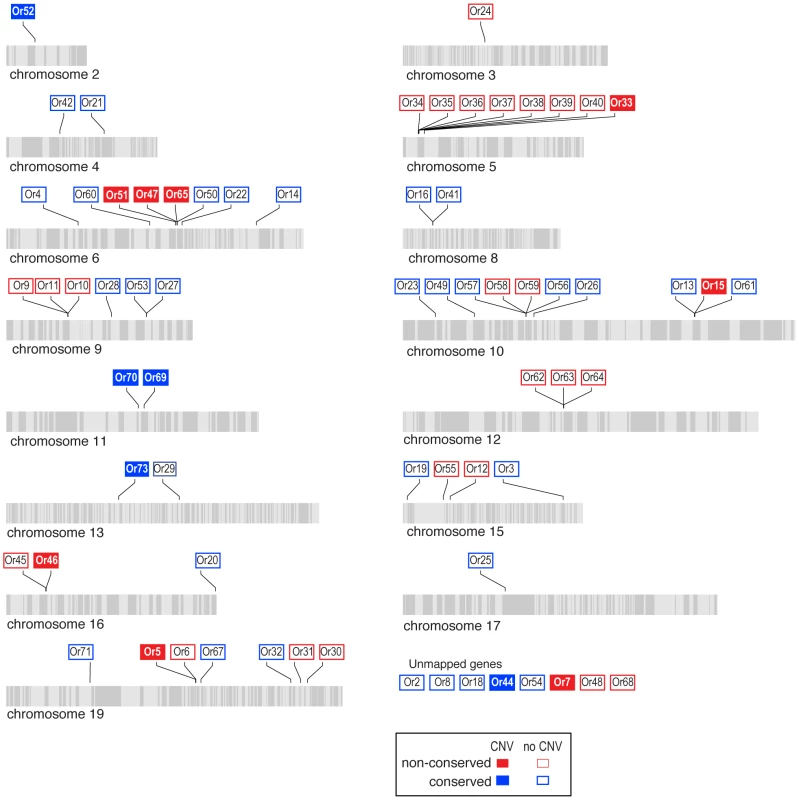
Scaffolds comprising each chromosome are indicated by alternating light and grey stripes. Ors without CNVs are indicated by open boxes and Ors with CNVs are indicated by closed boxes. The classification of Ors as being either conserved or non-conserved follows the same criteria as for the Grs. The eight genes for which the chromosome locality is not known are shown at the bottom. Tab. 1. Relationship between evolutionarily-conserved genes and copy-number variation (CNV). 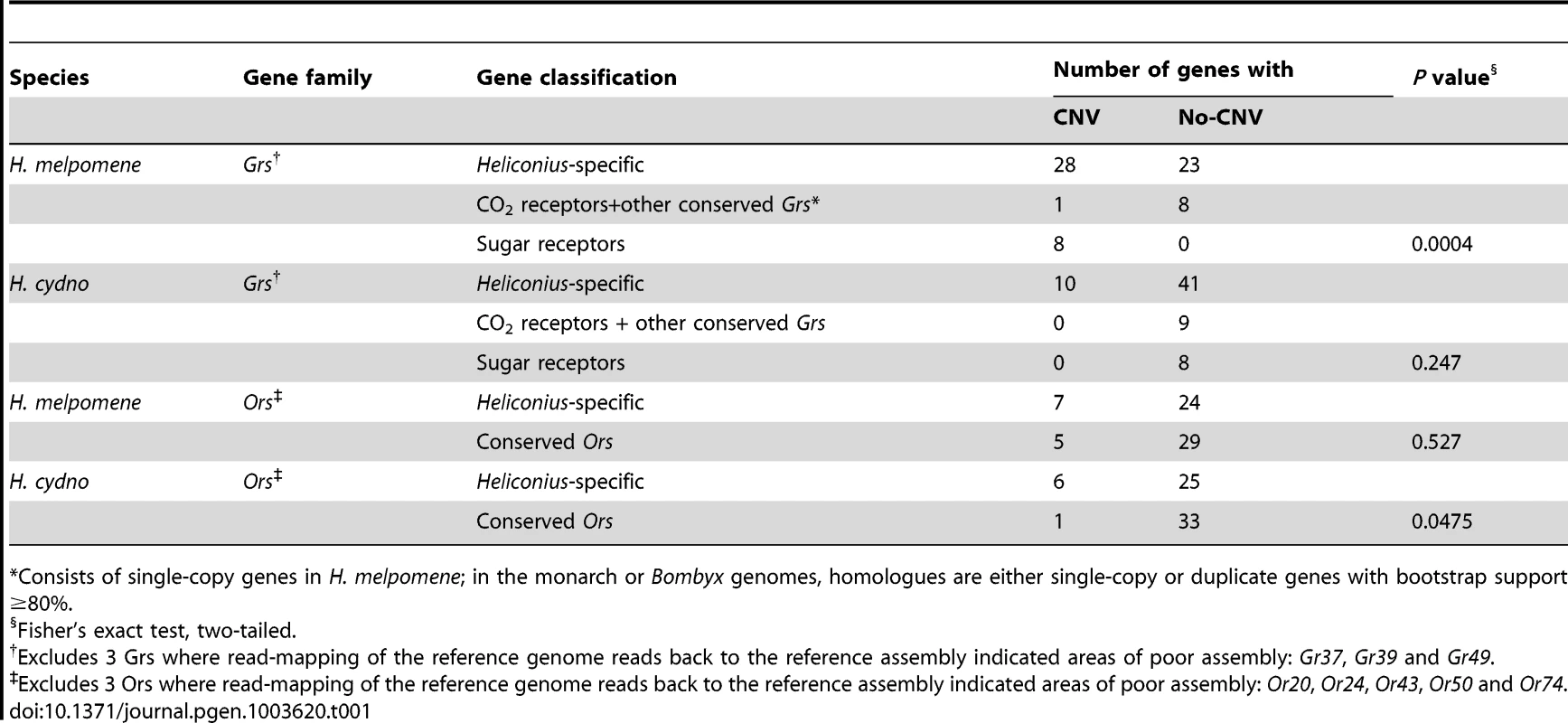
Consists of single-copy genes in H. melpomene; in the monarch or Bombyx genomes, homologues are either single-copy or duplicate genes with bootstrap support ≥80%. We have not experimentally verified the incidence of copy number variation in any of these genomes, and some of the regions identified as CNVs are likely to be false positives. To investigate the rate of false positives, we analysed resequence data from the reference genome itself and discovered 3 Gr and 3 Or CNVs, suggesting a false positive rate of around 4%. (We therefore excluded these loci from our statistical tests.) However, the fact that broad patterns of observed CNVs are consistent with the evolutionary patterns at deeper levels supports our conclusion that CNV, in the absence of strong purifying selection, is an important driver of gene family diversification. These results also provide a novel line of evidence that the butterfly Grs have a higher rate of evolutionary turnover as compared to Ors.
Sexually dimorphic gustatory sensilla in adult legs mirror Gr expression diversity
The life histories of adult male and female butterflies are similar with respect to the need to find food and potential mates except that adult females are under strong selection to identify suitable host plants for oviposition. To ascertain host-plant identity, female butterflies drum with their legs on the surface of leaves before laying eggs [10]. This behaviour presumably allows the female to taste oviposition stimulants. Consistent with this behaviour, adult nymphalid butterfly legs are known to contain gustatory sensilla [57], and it has been reported that while nymphalid butterfly females have clusters of gustatory sensilla on their foreleg foretarsi, males lack these entirely [10], [58]. Here we confirm this mostly anecdotal evidence for sexual dimorphism using scanning electron microscopy (SEM). The mid - and hindlegs of both male and female H. melpomene have similar numbers of individual gustatory sensilla along their entire lengths, but there is a striking difference in their abundance and distribution on the foretarsi of the female forelegs. Unlike males, females exhibit cuticular spines associated with gustatory (trichoid) sensillae (n∼80 sensilla/foretarsus for females; n = 0/foretarsus for males) (Figure 2A) [10].
We therefore hypothesized that the repertoire of expressed Gr and Or genes in H. melpomene legs might be more diverse in females as compared to males. Furthermore, if female-specific genes are used for assessment of potential host plants, then fast-evolving insect-host interactions might produce rapid duplication of these genes over evolutionary timescales. Accordingly, we examined the expression profiles of Grs and Ors in adult H. melpomene by RNA-sequencing of libraries prepared from mRNAs expressed in adult antennae, labial palps and proboscis, and legs from one deeply-sequenced male and female each of H. melpomene (6 libraries total)(Table S7 and S8). The number of 100 bp reads per individual library ranged from 17.4 to 25.9 million for paired-end sequencing or 74.8–103.9 million for single-end sequencing (Table S8). To confirm these findings, we subsequently made 12 individual libraries from two more males and two more females (Table S7). As coverage was uneven across these libraries, we analysed them by merging biological replicates by sex and tissue type, and then downsampling so that an equal number of reads was analyzed for each treatment. The number of 100 bp reads analyzed for paired-end sequencing ranged from 19.4 to 49.6 million (Table S8). After downsampling, we examined the expression levels of the widely-expressed elongation factor-1 alpha gene in each of the libraries as a control, and found a comparable level of expression between sexes within each tissue type (Table S8). By careful visual examination of the uniquely-mapped reads to our 143 reference Gr and Or sequences, we found evidence of Gr and Or expression in all three adult tissue-types, with both tissue-specific and sex-specific differences as detailed below (Figure 9, Tables S9, S10, S11, S12, S13, S14). In total, we found evidence for expression of 67 of 73 Grs and 67 of 70 Ors identified in the H. melpomene reference genome.
Fig. 9. Comparison of Gr and Or expression in male and female adult H. melpomene chemosensory tissues. 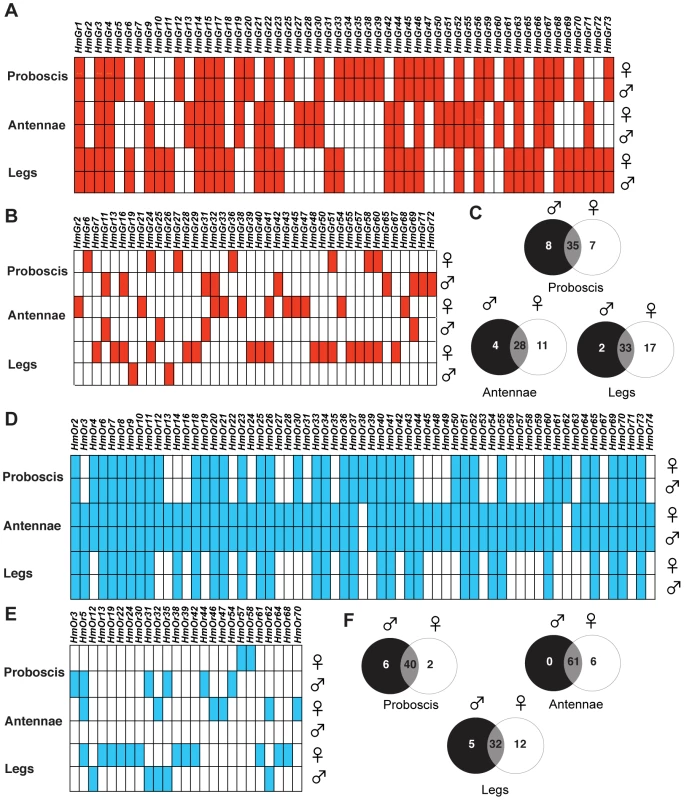
(A) The common set of Grs expressed in each tissue in both males and females. Red box indicates the presence of reads uniquely mapping to the coding region of each Gr gene model. To facilitate the visualization of tissue-specific expression found in both males and females, only Grs where both sexes show expression are indicated. Where only one sex or neither sex shows expression, the box is empty. (B) Grs showing sex-specific expression. To facilitate the visualization of sex-specific Grs, only Grs where one sex shows expression are indicated by a filled box. Grs which are expressed in both sexes or no sex are indicated by an empty box. (C) Venn diagram showing the number of uniquely expressed gustatory receptors in each transcriptome. (D) The common set of Ors expressed in each tissue in both males and females. Blue box indicates the presence of reads uniquely mapping to the coding region of each Or gene model. As above, only Ors where both sexes show expression are indicated. Where only one sex or neither sex show expression, the box is empty. (E) Ors showing sex-specific expression are indicated by a filled box. Ors which are expressed in both sexes or no sex are indicated by an empty box. (F) Venn diagram showing the number of uniquely expressed gustatory receptors in each transcriptome. The proboscis libraries also included both labial palps, the antennal libraries included both antennae, and the leg libraries included all six legs. Strikingly, the sexual dimorphism of gustatory sensilla we observed among the foreleg foretarsi is reflected in Gr gene expression patterns. A total of thirty-two Grs are expressed in both male and female H. melpomene leg transcriptomes including three CO2 receptors, HmGr1-3, four putative sugar receptors HmGr4, Gr6, Gr45 and Gr52 and a fructose receptor, HmGr9 (Figure 9A, Table S9, Supplementary Text). Many Grs showed sex-specific expression, however, with many more Grs in female (n = 46) as compared to male leg transcriptomes (n = 33)(Figure 9B, C).
In total 15 of these Grs expressed in female legs, HmGr10, Gr24, Gr26, Gr29, Gr40, Gr41, Gr48, Gr50, Gr51, Gr16, Gr55, Gr57, Gr58, Gr60 and Gr67, are the result of duplications since Heliconius and Danaus shared a common ancestor (Figure 3 small arrows, Figure 9B, Table S9). By contrast, only one of the three male-biased Grs, HmGr19, evolved as a result of recent duplication. There is an excess of Heliconius-specific Grs but not Ors (see below) that are expressed in female legs (Fisher's Exact Test, two-tailed, p = 0.019)(Table 2). Since male H. melpomene do not need to identify host-plants for oviposition, it seems likely that the 17 female-specific Grs in our leg transcriptomes are candidate receptors involved in mediating oviposition (Figure S1).
Tab. 2. An overabundance of Grs expressed in female legs are the result of Heliconius-specific duplication. 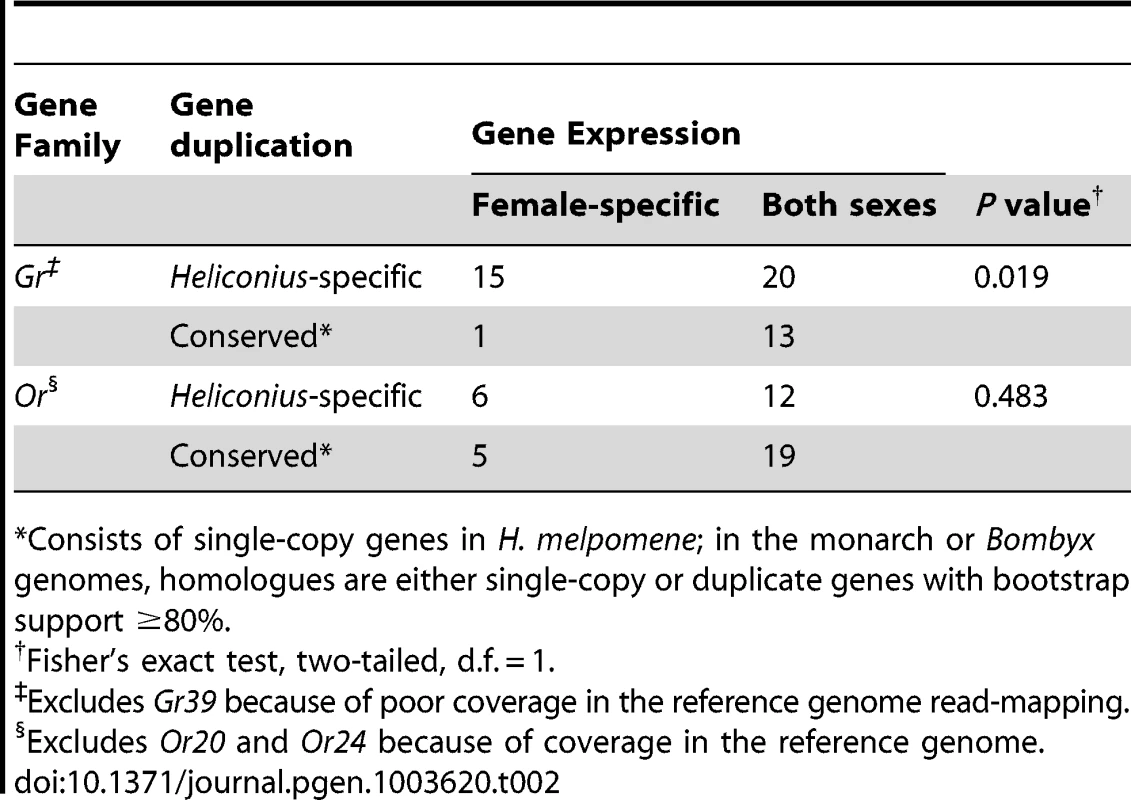
Consists of single-copy genes in H. melpomene; in the monarch or Bombyx genomes, homologues are either single-copy or duplicate genes with bootstrap support ≥80%. Female Gr expression is more diverse in antennae than male Gr expression
Besides using their antennae for olfaction, female nymphalid butterflies also taste a host plant by antennal tapping before oviposition. This tapping behaviour presumably allows the host plant chemicals to come into physical contact with gustatory sensilla on the antennae. We therefore examined whether there was any difference in the abundance of gustatory sensilla on the antennae of male and female H. melpomene. Using scanning electron microscopy, we found individual gustatory sensilla scattered along each antennae of both male and female H. melpomene but no obvious sexual dimorphism in their abundance or distribution (Figure 2B). We found 28 Grs expressed in both male and female H. melpomene antennae (Figure 9A, Table S10), including two sugar receptors, HmGr4 and HmGr52, a putative fructose receptor HmGr9 and two CO2 receptors, HmGr1 and Gr3. Besides the sugar and CO2 receptors noted, other conserved genes that are expressed in both male and female antennae include HmGr63, a candidate Gr co-receptor (see Text S1), and HmGr66, a candidate bitter receptor.
We also found 11 Grs expressed in female H. melpomene antennae that did not appear to be expressed in male antennae. Two of these, HmGr47 and Gr68, appeared in the top one-third of the most abundant female antennal Grs in terms of number of reads recovered from the individual butterfly transcriptome. In contrast, just four Grs were expressed in male antennae HmGr11, Gr25, Gr31, and Gr69 but not female antennae (Figure 9B, C, Table S10). Six of the female-biased Grs and two of the male-biased Grs (Gr31, Gr69) expressed in antennae are the result of duplication events since Heliconius and Danaus shared a common ancestor.
Candidate Heliconius gustatory receptors for nectar - and pollen-feeding
By contrast with the leg and antennal tissue, where more Grs are expressed in females compared to males, the labial palps and proboscis (Figure 1) transcriptomes contained the largest number of Grs (n = 35) expressed in both sexes (Figure 9A, C, Table S11). Five of the six candidate sugar receptors in the H. melpomene genome are expressed in both the male and the female transcriptomes along with two of the three conserved CO2 receptors, which may be used to assess floral quality [59] (Figure 3, Table S11). A majority (21 of 35) of Heliconius Grs expressed in both male and female labial palps and proboscis libraries have no existing ortholog in the silkmoth genome, apparently the result of gene loss in B. mori or gene duplication along the lineage leading to Heliconius (Figure 3). This may in part reflect the fact that adult silkmoths have lost the ability to feed. Interestingly, four Grs expressed in both male and female labial palps and proboscis transcriptomes could not be detected in male and female antennae and legs (HmGr12, Gr20, Gr35, and Gr59)(Figure 3, red arrows, Figure 9B). Some of these Grs might play a role in the pollen-feeding behaviour that is specific to Heliconius, and which involves preferences for particular species of flowers in the plant families Rubiaceae, Cucurbitaceae and Verbenaceae (see Discussion).
Widespread expression of Ors in H. melpomene antennae, proboscis and labial palps and legs
In addition to the Gr gene expression described above, we examined Or expression in the three adult tissues. The expression of Ors in antennal tissue has been widely studied in a variety of insects including Drosophila and some Lepidoptera [50], [60]. As expected, we observed that Or gene expression was high in the antennae. Unexpectedly, Or expression was about as prevalent as Gr expression in the proboscis and labial palps and leg transcriptomes (Figure 9D, E, F). In total across all three tissues profiled, we found evidence for the expression of nearly all predicted Or genes (67 of 70 genes)(Table S12, S13, S14) in the H. melpomene reference genome [13].
Discussion
Outside Drosophila, the study of sensory gene family evolution in insects has generally been limited to the comparison of a small number of phylogenetically distant reference genomes. Such studies have commonly involved a comparison of the size of gene families between taxa in order to document lineage-specific expansions (Figure 10), and the comparison of dN/dS ratios to identify branches subject to rapid evolution [61]. Here we have used a similar approach to annotate 73 Grs in the Heliconius melpomene reference genome. However, we have also demonstrated the power of next-generation sequencing to elucidate patterns of evolution and expression of these genes. These data have offered exciting new insights into a set of genes that show both rapid evolution and sex-specific expression patterns, suggesting that female oviposition behaviour drives the evolution of butterfly gustatory receptors.
Fig. 10. Insect chemosensory gene family repertoires. 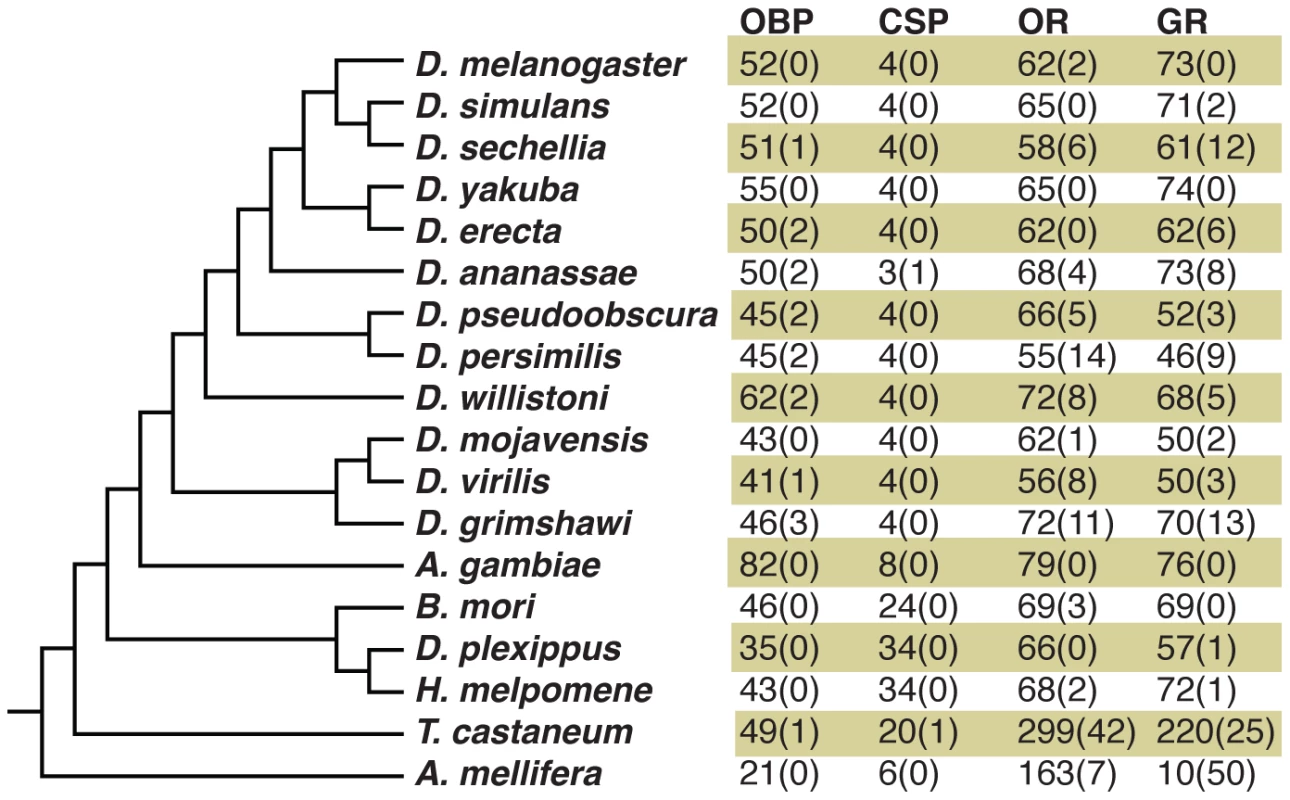
Numbers indicate intact genes and numbers in parentheses indicate pseudogenes. References are given in [13], [55], [94]. OBP = odorant binding protein; CSP = chemosensory protein; OR = olfactory receptor, GR = gustatory receptor. Previous work in other insects indicates that Grs are an important target for gene duplication and loss between species. Most notably, D. sechellia and D. erecta are host specialists, on Morinda citrifolia and Pandanus candelabrum respectively, while D. simulans is a generalist fly exploiting a broad array of rotting fruit [46]. Host specialization in the former species is associated with an acceleration of gene loss and increased rates of amino acid evolution at receptors that remain intact. Here we have used whole-genome Illumina sequencing of single diploid individuals to similarly document patterns of gene gain and loss across Heliconius. This method yields highly fragmented genome assemblies, but such assemblies have proven very informative, most notably for studying the evolution of the clade of single-exon bitter receptor genes. We identified three gene duplication events along the lineage leading to Heliconius, followed by eight independent instances of clade-specific pseudogenizations or losses of different members of the intronless Grs, Gr22-26 and Gr53, within Heliconius and one instance within Danaus plexippus (Figure 5 and Figure 6). In both Heliconius and Drosophila gene gain and loss appear to primarily affect Grs that are presumed to respond to bitter compounds (Figure 3). To verify whether this pattern holds within the genus Heliconius for the remaining gene family members with more complex intron-exon structure will require better genome assemblies for multiple Heliconius species (Table S2).
These patterns of rapid gene gain and loss are mirrored by within-population variation in copy number. From 16 resequenced genomes for H. melpomene and its sister species H. cydno, we have shown that CNVs occur more commonly among the Grs than the Ors (Figure 7, 8, Table 1). Within the Grs, the bitter receptors of H. melpomene represent a class of genes that are both highly prone to lineage-specific duplication and commonly subject to population-level copy number variation. These putative bitter receptor genes are also more likely to show female-specific expression, especially in the legs, which suggests a role in insect-host chemical interactions (Table 2, Figure 3, Figure S1).
In human genomes, a tendency for CNV-rich areas to display higher dN/dS ratios and yield paralogous genes has been noted [62], along with an enrichment of CNVs in genes involved in immune function and in the senses (specifically in Ors which are unrelated to the insect Ors) [63], [64]. It is also widely known that copy-number variation is an important source of disease-causing mutations in humans [64]. With the exception of insecticide resistance in insects [65], [66], the spectrum of naturally-occurring copy-number variants is only just starting to be explored in Drosophila [67], [68] and non-model systems. Our results demonstrate the great utility of high throughput sequencing to reveal the naturally-occurring spectrum of CNVs that underlie gene family expansions in non-model systems, in traits of ecological relevance.
Heliconius butterflies have complex relationships with their Passifloraceae host plants. Some species are host-specialist, feeding on only one or a few Passiflora species, others specialise on particular sub-genera within Passiflora, while others are generalists, albeit within this one host plant family (Figure 6) [53]. The Passifloraceae is extremely chemically diverse, most notably in their diversity of cyanogenic glycosides that protect the plant from herbivores. It seems likely that coevolution of the butterfly chemosensory and detoxification system on the one hand, with the plant biochemical defense on the other, has played an important role in the evolution of this chemical arsenal. In contrast to the research already carried out on the chemistry of the host plants [54], until recently almost nothing was known about the chemosensory system of Heliconius butterflies. All of these insect host-plant interactions are mediated primarily by adult female butterflies, which must correctly identify suitable host plants for oviposition [69], [70], or risk the survival of their offspring.
Expression data for Grs in the Lepidoptera have been limited until now–especially for adults–due to their low expression level. The largest previous study identified 14 Grs profiled in larval B. mori [32]. We have found evidence for adult expression for most (∼91%) of the 73 predicted Gr genes. This provides a marked contrast to the handful of gustatory receptors that have been identified from traditional expressed sequence tag (EST) projects in other Lepidoptera. Our methods may provide a greatly improved yield of expressed genes because we now have a set of well-annotated target Gr genes against which RNA-seq data can be mapped, together with a greater diversity of transcripts afforded by deep sequencing. Such methods have also permitted us to find widespread expression of their sister gene family, the Ors, in the adult chemosensory tissues examined (68 of 70 or 97% of predicted genes) (Figure 9).
Many of these Gr genes are likely to be involved in the detection of host plant attractants as well as toxic secondary metabolites and thus allow the discrimination of suitable hosts. Most notably, there were a large number of Heliconius-specific Grs with female-biased expression in both legs and antennae (Figure 9). As mentioned previously, these female-biased leg Grs (but not Ors) are also more likely to represent unique duplicates on the Heliconius lineage (Table 2). Female-biased Or expression, as quantified using RNA-seq data, has been reported for Ors expressed in the antennae of the adult mosquito, Anopheles gambiae [71]. Specifically, 22 Ors displayed enhanced expression in mosquito female antennae but not in male antennae. Since adult mosquito females but not males need to find hosts for a blood-meal, and adult butterfly females but not males need to find host plants for egg-laying, this suggests that host-seeking behaviour of female insects may be an important general driver of sensory gene evolution. Indirect evidence for the possible role of some of these Grs in Heliconius host plant detection comes from comparative studies of Grs mediating oviposition behaviour in swallowtail butterflies (Papilionidae). Papilio xuthus PxGr1 a member of the Gr subgroup that contains D. melanogaster Gr43a and HmGr9, has been characterized as a receptor for synephrine, which is an alkaloid found in citrus trees [52]. It is expressed in female P. xuthus tarsi and is necessary for the correct oviposition behavior of swallowtail butterflies [52]. Within the two clades most closely-related to PxGr1, are 9 butterfly-specific Grs: HmGr10, Gr16, Gr55, Gr56 and Gr57, and the newly-described DpGr16, Gr50, Gr52, and Gr54 (Figure 3). Four these Grs, HmGr16, Gr55, Gr56 and Gr57, result from Heliconius-specific gene duplications (i.e., no Danaus or Bombyx homologs). Grs55-57 are also in the top ten most highly expressed Grs in female legs. The identification of these sex-biased leg Grs has provided an important starting point for future ligand specificity studies combining heterologous expression, electrophysiology, RNAi [51], assays of the proboscis-extension reflex, and female oviposition behavior.
Lastly, the patterns of Gr gene expression among different tissues and sexes has permitted us to identify a number of Grs that are strong candidates for mediating the remarkable pollen feeding behaviour that is unique to Heliconius, among the butterflies. The Heliconius proboscis contains at least two types of gustatory sensilla, hair-like sensilla chaetica, and sensilla styloconica (Figure 1). Like other butterflies, Heliconius respond to varying amounts of sugars including sucrose present in floral nectar [72]. Unlike other moths and butterflies, Heliconius actively collect pollen with their proboscides, preferentially from Psychotria (Rubiaceae), Psiguria/Gurania (Cucurbitaceae) and Lantana (Verbenaceae) flowers [17], [18], [73]. Once a pollen load is collected (Figure 1D), the butterflies use a combination of mechanical shearing (coiling and uncoiling of the proboscis) and enzymatic activity (using proteases found in saliva) to release amino acids from the pollen [74]. The RNA-seq data we have collected for H. melpomene proboscis and labial palps should provide a useful resource for future studies examining the molecular basis of this unique digestive trait.
Pollen feeding in adult Heliconius has an important ecological function. Amino acids obtained from pollen are key resources used in male nuptial gifts and egg allocation [18], [75]–[77]. They also permit Heliconius adults to have exceptionally long lifespans. Pollen feeding behavior is not found outside the genus Heliconius, even in the sister genus Eueides, whose larvae share a preference for Passiflora host-plants with Heliconius. In the present study we have identified four Heliconius-specific Grs that are only expressed in the proboscis (HmGr12, Gr20, Gr35 and Gr59) but not in antennae or legs (Figure 9B), suggesting a role for these genes in pollen-feeding behaviour.
Taken together, the whole-genome and whole-transcriptome data suggest that Gr genes in particular are highly evolutionarily labile both on short and long evolutionary timescales, and begin to offer an insight into the likely molecular basis for the rapid coevolution observed between these butterflies and their host plants. Understanding the remarkable diversity underlying this ecological interaction at a molecular level has remained a challenge (but see [32], [52], [78], [79]). Thanks to technological innovations in sequencing, the genetic basis of taste and olfaction involved in host-plant adaptation in Heliconius is beginning to be uncovered.
Conclusions
We have shown that like the opsin visual receptors [80], the chemosensory superfamily composed of constituent Gr and Or families in Lepidoptera show rapid gene family evolution, with higher rates of copy-number variation and gene duplication among the Grs than the Ors, as well as gene losses in the Grs. In particular, there is a group of putative bitter receptors that show female-specific expression in the legs and that are especially prone to gene duplication, providing new material for sensory diversification in the insect-host plant arms race. We have also shown, for the first time, widespread expression of Ors in non-antennal tissues in a lepidopteran. With the most comprehensive data set on Gr and Or expression in butterflies to date we are one step closer to identifying the sensory and molecular genetic basis of the Heliconius-Passiflora co-evolutionary race that inspired Ehrlich and Raven in 1964.
Materials and Methods
Genome annotation
tBLASTn searches were conducted iteratively against the H. melpomene melpomene genome (version v1.1) and haplotype scaffolds [13] using B. mori [28], [47] and D. plexippus Grs [14] as input sequences. For these in silico gene predictions, intron-exon boundaries were identified by first translating the scaffold nucleotides in MEGA version 5 [81], searching for exons identified in the tBLASTn searches, then back translating to identify splice junctions. Intron sequences were then excised to verify that the remaining exonic sequences formed an in-frame coding sequence. Insect Grs are defined by a conserved C-terminal motif TYhhhhhQF, where ‘h’ is any hydrophobic amino acid [21]. We inspected our predicted protein sequences for this motif or variants thereof, specifically ‘S’, ‘M’ or ‘K’ instead of a ‘T’ or ‘L’, ‘T’ or ‘I’ instead of ‘F’. In the handful of cases where we were unable to find the last short exon that contains this motif, final assignment to the Gr gene family was based on using the predicted amino acid sequence as a search string for either tBLASTn or BLASTp against the nr/nt Genbank database. Gene annotations were submitted to the EnsemblMetazoa database http://metazoa.ensembl.org/Heliconius_melpomene/Info/Index as part of the H. melpomene v. 2 genome release (for GeneIDs see Table S1). Chromosomal assignments were based on published mapping of scaffolds in the H. melpomene melpomene reference genome [13].
Following amino acid alignment using ClustalW, preliminary phylogenetic trees were constructed in MEGA using neighbor-joining and pair-wise deletion to identify orthologous relationships with B. mori and D. plexippus Grs. Reciprocal tBLASTn searches against the B. mori and D. plexippus genomes as well as searches using the protein2genome module in EXONERATE [82] were then performed in order to search for ‘missing’ Grs in those genomes. Final phylogenetic analysis was performed using a maximum-likelihood (ML) algorithm and JTT model on an amino acid alignment that was inspected by eye and manually adjusted. These results were compared to a ML tree made from a Clustal-Omega alignment [83] and were found to be nearly identical. Once the initial H. melpomene Gr gene predictions were obtained, EXONERATE, Perl scripts and manual annotations in Apollo [84] were used to produce gff3 files for submission of the annotated H. melpomene genome scaffolds to EMBL-EBI.
RNA-sequencing
Butterfly pupae of H. melpomene rosina were obtained from Suministros Entomológicos Costarricenses, S.A., Costa Rica. Adult males and females were sexed and frozen at −80°C. Total RNAs were extracted separately from antennae, proboscis together with labial palps, and all six legs of three males and three females of H. melpomene using Trizol (Life Technologies, Grand Island, NY). A NucleoSpin RNA II kit (Macherey-Nagel, Bethlehem, PA) was used to purify total RNAs. Each total RNA sample was purified through one NucleoSpin RNA II column. Purified total RNA samples were quantified using a Qubit 2.0 Fluorometer (Life Technologies, Grand Island, NY). The quality of the RNA samples was checked using an Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA). 0.3–4.0 µg of purified total RNAs were used to make cDNA libraries. A TruSeq RNA sample prep kit (Illumina, San Diego, CA) was used to prepare 18 individual cDNA libraries. After being normalized according to their concentrations, the enriched individual libraries were pooled and then run on a 2% agarose gel. cDNA products ranging from 280 to 340 bp with an average of 310 bp were cut out and purified using a Geneclean III kit (MP Biomedicals, Solon, OH) to facilitate post-sequencing assembly. After being re-purified using Agencourt AMPure XP magnetic beads (Beckman Coulter Genomics, Danvers, MA), the cDNA pool was quantified using the Qubit 2.0 Fluorometer, and quality control-checked using the Agilent Bioanalyzer 2100. The cDNA pools were then normalized to 10 nM and run as either two paired-end or three single-end 100 bp runs on a HiSeq 2000 (Illumina, San Diego, CA) by the UCI Genomics High-Throughput Facility.
RNA-seq assembly and read mapping
mRNA sequences were demultiplexed, trimmed and sorted using Python and Perl scripts. A single de novo assembly of the combined libraries was performed using CLC Genomics Workbench 5 to check for missing exons in our gene models. The 73 corrected Gr gene models and 70 Or gene models were then used as an alignment reference to perform unique read mapping of each individual chemosensory transcriptome. To determine if an individual Gr or Or was expressed in a given tissue, each of the 1716 individual Gr and Or mapping alignments was inspected by eye for uniquely mapped reads, and any spuriously-mapped reads (i.e., reads <70 bp in length with indels or sequence mismatches at the ends) were discarded. As a control for potential differences in RNA preparation between samples, we also quantified the number of uniquely mapped fragments to the widely-expressed elongation factor 1-alpha (EF1α) gene transcript and calculated the Fragments Per Kilobase of transcript per Million mapped reads (FPKM) [85]. Illumina reads for each of the libraries were deposited as fastq files in the ArrayExpress archive under the accession number: E-TAB-1500 (Table S7).
Scanning electron microscopy
One week old adult H. melpomene rosina butterflies were sexed, frozen at −80°C, then dissected and mounted for imaging on an FEI/Philips XL30 FEG scanning electron microscope at UCI's Materials Characterization User Facility. Forelegs, middle legs, hindlegs and antennae were examined for the presence of gustatory sensilla.
Copy number variation analysis
We also examined resequenced genomes of twelve H. melpomene and four H. cydno individuals, including H. melpomene aglaope, H. melpomene amaryllis and H. melpomene rosina (Table S4), sequenced by The GenePool, University of Edinburgh, U.K. and the FAS Center, Harvard University, U.S.A., for evidence of copy-number variation (CNV) in the Grs and Ors using CNVnator [56]. These sequences were deposited in the European Nucleotide Archive (ENA) under accession number: ERP002440. The Illumina resequenced genomes were first mapped to the H. melpomene reference genome and the average read depth was calculated along a 100 bp sliding window. The output of CNVnator was parsed for candidate insertion and deletion variants, and those with estimated copy number of >2× were counted as potential duplications and <0.5× as potential deletions.
Whole-genome sequence assembly
The GenePool, University of Edinburgh, and the Oxford Genomics Centre, University of Oxford, U.K., produced whole genome 100 bp sequences from H. cydno, H. timareta, H. wallacei, H. doris, H. clysonymus, H. telesiphe, H. erato petiverana, H. sara and H. sapho using the Illumina Pipeline v. 1.5–1.7 with insert sizes ranging from 300 to 400 bp. We deposited sequences for H. sapho and H. sara in the Sequence Read Archive (SRA) under accession number ERP002444. We performed de novo assembly of the short reads using Abyss v. 1.2 [86] implemented in parallel at the School of Life Sciences, University of Cambridge, U.K. Based on previous results [87], recommendations estimated by the software, and comparison of N50 values in preliminary experiments, we chose a k-mer size of 31, a minimum number of pairs required n = 5 and the minimum mean k-mer coverage of a unitig c = 2 (full command: abyss-pe n = 5 k = 31 c = 2 in = ‘for.fastq rev.fastq’). In all assemblies, at least 96% of reads mapped back to the contigs. We created BLAST databases of these whole genome sequence assembly contigs (Table S2) in Geneious Pro v. 5.5.6. The lack of introns in the putative bitter receptor genes Gr22-26 and Gr53 permitted us to easily retrieve them from these BLAST databases. To confirm the identity and improve the quality of the sequences found, we mapped the reads to the assembled exon sequences in CLC Genomics Workbench v. 5.5.1, using the following conservative settings to prevent mis-mapping of paralogous sequences: mismatch, insertion and deletion cost of 3; length fraction and similarity fraction of 0.9. We then inspected all read-mappings by eye. Because the intronless Grs are closely related, we aligned the translated nucleotide sequences in MEGA using the ClustalW algorithm, and also inspected the alignment by eye. For all intronless Gr sequences except for the pseudogenes, sequence length was highly conserved (i.e., there were few indels). To illustrate the high substitution rate of the retrieved pseudogene sequences, we selected the neighbor-joining method for tree reconstruction and performed 500 bootstrap replicates.
Inferring gene duplications and losses
To infer the number of intronless Gr gene duplications and losses, we used the program Notung v. 2.6 [88], [89], which reconciles gene trees onto the species tree. The gene tree was made by a maximum likelihood analysis of 1074 nucleotide sites, aligned by Clustal-Omega, and 500 bootstrap replications. The species tree was derived from a phylogeny based on independent nuclear and mitochondrial DNA sequences [90].
RT-PCR
We verified the presence of HmGr22 in several adult tissues using reverse-transcriptase PCR and primers for HmGr22 (5′-CCATAATTTTGTCATCCT-3′ and 5′-GATTTCGAAATAAGGTCTGT-3′) and EF1alpha (5′-CGTTTCGAGGAAATCAAGAAGG-3′ and 5′-GACATCTTGTAAGGGAAGACGCAG 3′). RNA was extracted from fresh frozen specimens using Trizol and purified using the Nucleospin RNA II kit, which contains a DNAase-treatment step. RNA concentration was diluted to 12.5 µg/ml. Each 25 µl reaction had 2.5 µl 10× BD Advantage 2 PCR buffer, 2.5 µl dNTPs (2 mM), 0.5 µl (100 µM) forward and 0.5 µl reverse primer, 0.5 µl (1∶20 diluted) Stratagene Affinity Script Reverse Transcriptase, 0.5 µl 50× Advantage 2 Polymerase Mix, 17 µl H2O and 1 µl RNA. The PCR reaction consisted of 38 cycles of 95°C for 30 s, 55°C for 30 s, and 68°C for 55 s. The identity of the RT-PCR products was confirmed by Sanger sequencing.
Supporting Information
Zdroje
1. EhrlichPR, RavenPH (1964) Butterflies and plants: A study in coevolution. Evolution 18 : 586–608.
2. DethierVG (1937) Gustation and olfaction in lepidopterous larvæ. Biol Bull 72 : 7–23.
3. SchneiderD (1964) Insect antennae. Annu Rev Entomol 9 : 103–122.
4. SchoonhovenLM, DethierVM (1966) Sensory aspects of host-plant discrimination by lepidopterous larvae. Arch Neerl ZooI 16 : 497–530.
5. AndersonAL (1932) The sensitivity of the legs of common butterflies to sugars. J Exp Zool B Mol Dev Evol 63 : 235–259.
6. CalatayudP-A, ChimtawiM, TaubanD, Marion-PollR, RüBL, et al. (2006) Sexual dimorphism of antennal, tarsal and ovipositor chemosensilla in the African stemborer, Busseola fusca (Fuller) (Lepidoptera:Noctuidae). An Soc Entomol Fr 42 : 403–412.
7. JorgensenK, AlmaasTJ, Marion-PollF, MustapartaH (2007) Electrophysiological characterization of responses from gustatory receptor neurons of sensilla chaetica in the moth Heliothis virescens. Chem Senses 32 : 863–879.
8. Marion-PollFC, GuillauminD, MassonC (1992) Sexual dimorphism of tarsal receptors and sensory equipment of the ovipositor in the European corn borer, Ostrinia nubilalis. Cell Tissue Res 267 : 507–518.
9. KrennHW, PenzCM (1998) Mouthparts of Heliconius butterflies (Lepidoptera: Nymphalidae): A search for anatomical adaptations to pollen feeding behavior. Int J Insect Morphol Embryol 27 : 301–309.
10. RenouM (1983) Les récepteurs gustatifs du tarse antérieur de la femelle d'Heliconius charitonius (Lep.: Heliconiidae). Annls Soc Ent Fr (NS) 19 : 101–106.
11. ChapmanRF (2003) Contact chemoreception in feeding by phytophagous insects. Annu Rev Entomol 48 : 455–484.
12. MyersJ (1969) Distribution of foodplant chemoreceptors on the female Florida Queen butterfly, Danaus gilippus berenice (Nymphalidae). J Lepid Soc 23 : 196–198.
13. Heliconius Genome Consortium (2012) Butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature 487 : 94–98.
14. ZhanS, MerlinC, BooreJL, ReppertSM (2011) The monarch butterfly genome yields insights into long-distance migration. Cell 147 : 1171–1185.
15. International Silkworm Genome Consortium (2008) The genome of a lepidopteran model insect, the silkworm Bombyx mori. Insect Biochem Mol Biol 38 : 1036–1045.
16. BriscoeAD, BybeeSM, BernardGD, YuanF, Sison-MangusMP, et al. (2010) Positive selection of a duplicated UV-sensitive visual pigment coincides with wing pigment evolution in Heliconius butterflies. Proc Natl Acad Sci USA 107 : 3628–3633.
17. BoggsCL, SmileyJT, GilbertLE (1981) Patterns of pollen exploitation by Heliconius butterflies. Oecologia 48 : 284–289.
18. GilbertLE (1972) Pollen feeding and reproductive biology of Heliconius butterflies. Proc Natl Acad Sci USA 69 : 1403–1407.
19. Engler-ChaouatHS, GilbertLE (2007) De novo synthesis vs. sequestration: Negatively correlated metabolic traits and the evolution of host plant specialization in cyanogenic butterflies. J Chem Ecol 33 : 25–42.
20. BensonWW, BrownKS, GilbertLE (1975) Coevolution of plants and herbivores: Passion flower butterflies. Evolution 29 : 659–680.
21. ClynePJ, WarrCG, CarlsonJR (2000) Candidate taste receptors in Drosophila. Science 287 : 1830–1834.
22. DunipaceL, MeisterS, McNealyC, AmreinH (2001) Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system. Curr Biol 11 : 822–835.
23. ScottK, BradyRJr, CravchikA, MorozovP, RzhetskyA, et al. (2001) A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell 104 : 661–673.
24. RobertsonHM, WarrCG, CarlsonJR (2003) Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc Natl Acad Sci USA 100 : 14537–14542.
25. LeeY, KangMJ, ShimJ, CheongCU, MoonSJ, et al. (2012) Gustatory receptors required for avoiding the insecticide L-canavanine. J Neurosci 32 : 1429–1435.
26. JonesWD, CayirliogluP, KadowIG, VosshallLB (2007) Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature 445 : 86–90.
27. MontellC (2009) A taste of the Drosophila gustatory receptors. Curr Opin Neurobiol 19 : 345–353.
28. ZhangHJ, AndersonAR, TrowellSC, LuoAR, XiangZH, et al. (2011) Topological and functional characterization of an insect gustatory receptor. PLoS ONE 6: e24111.
29. IshimotoH, TakahashiK, UedaR, TanimuraT (2005) G-protein gamma subunit 1 is required for sugar reception in Drosophila. EMBO J 24 : 3259–3265.
30. KainP, BadshaF, HussainSM, NairA, HasanG, et al. (2010) Mutants in phospholipid signaling attenuate the behavioral response of adult Drosophila to trehalose. Chem Senses 35 : 663–673.
31. YaoCA, CarlsonJR (2010) Role of G-proteins in odor-sensing and CO2-sensing neurons in Drosophila. J Neurosci 30 : 4562–4572.
32. SatoK, TanakaK, TouharaK (2011) Sugar-regulated cation channel formed by an insect gustatory receptor. Proc Natl Acad Sci USA 108 : 11680–11685.
33. DahanukarA, FosterK, van der Goes van NatersWM, CarlsonJR (2001) A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nat Neurosci 4 : 1182–1186.
34. ChybS, DahanukarA, WickensA, CarlsonJR (2003) Drosophila Gr5a encodes a taste receptor tuned to trehalose. Proc Natl Acad Sci USA 100 Suppl 2 : 14526–14530.
35. SloneJ, DanielsJ, AmreinH (2007) Sugar receptors in Drosophila. Curr Biol 17 : 1809–1816.
36. KwonJY, DahanukarA, WeissLA, CarlsonJR (2007) The molecular basis of CO2 reception in Drosophila. Proc Natl Acad Sci USA 104 : 3574–3578.
37. MoonSJ, KottgenM, JiaoY, XuH, MontellC (2006) A taste receptor required for the caffeine response in vivo. Curr Biol 16 : 1812–1817.
38. LeeY, MoonSJ, MontellC (2009) Multiple gustatory receptors required for the caffeine response in Drosophila. Proc Natl Acad Sci USA 106 : 4495–4500.
39. WeissLA, DahanukarA, KwonJY, BanerjeeD, CarlsonJR (2011) The molecular and cellular basis of bitter taste in Drosophila. Neuron 69 : 258–272.
40. SmadjaC, ShiP, ButlinRK, RobertsonHM (2009) Large gene family expansions and adaptive evolution for odorant and gustatory receptors in the pea aphid, Acyrthosiphon pisum. Mol Biol Evol 26 : 2073–2086.
41. RobertsonHM, WannerKW (2006) The chemoreceptor superfamily in the honey bee, Apis mellifera: expansion of the odorant, but not gustatory, receptor family. Genome Res 16 : 1395–1403.
42. Abdel-LatiefM (2007) A family of chemoreceptors in Tribolium castaneum (Tenebrionidae: Coleoptera). PLoS ONE 2: e1319.
43. KentLB, WaldenKK, RobertsonHM (2008) The Gr family of candidate gustatory and olfactory receptors in the yellow-fever mosquito Aedes aegypti. Chem Senses 33 : 79–93.
44. HillCA, FoxAN, PittsRJ, KentLB, TanPL, et al. (2002) G protein-coupled receptors in Anopheles gambiae. Science 298 : 176–178.
45. ClarkAG, EisenMB, SmithDR, BergmanCM, OliverB, et al. (2007) Evolution of genes and genomes on the Drosophila phylogeny. Nature 450 : 203–218.
46. McBrideCS, ArguelloJR (2007) Five Drosophila genomes reveal nonneutral evolution and the signature of host specialization in the chemoreceptor superfamily. Genetics 177 : 1395–1416.
47. WannerKW, RobertsonHM (2008) The gustatory receptor family in the silkworm moth Bombyx mori is characterized by a large expansion of a single lineage of putative bitter receptors. Insect Mol Biol 17 : 621–629.
48. Jacquin-JolyE, LegeaiF, MontagneN, MonsempesC, FrancoisMC, et al. (2012) Candidate chemosensory genes in female antennae of the noctuid moth Spodoptera littoralis. Int J Biol Sci 8 : 1036–1050.
49. KriegerJ, RamingK, DewerYM, BetteS, ConzelmannS, et al. (2002) A divergent gene family encoding candidate olfactory receptors of the moth Heliothis virescens. Eur J Neurosci 16 : 619–628.
50. Grosse-WildeE, KueblerLS, BucksS, VogelH, WicherD, et al. (2011) Antennal transcriptome of Manduca sexta. Proc Natl Acad Sci USA 108 : 7449–7454.
51. HowlettN, DauberKL, ShuklaA, MortonB, GlendinningJI, et al. (2012) Identification of chemosensory receptor genes in Manduca sexta and knockdown by RNA interference. BMC Genomics 13 : 211.
52. OzakiK, RyudaM, YamadaA, UtoguchiA, IshimotoH, et al. (2011) A gustatory receptor involved in host plant recognition for oviposition of a swallowtail butterfly. Nat Commun 2 : 542.
53. BrownKS (1981) The biology of Heliconius and related genera. Annu Rev Entomol 26 : 427–456.
54. Spencer KC (1988) Chemical mediation of coevolution in the Passiflora-Heliconius interaction. In: Spencer KC, editor. Chemical mediation of coevolution. London: Academic Press. pp. 167–240.
55. GardinerA, BarkerD, ButlinRK, JordanWC, RitchieMG (2008) Drosophila chemoreceptor gene evolution: selection, specialization and genome size. Mol Ecol 17 : 1648–1657.
56. AbyzovA, UrbanAE, SnyderM, GersteinM (2011) CNVator: an approach to discover, genotype, and characterize typical and atypical CNVs from family and population genome sequencing. Genome Res 21 : 974–984.
57. OmuraH, HondaK, AsaokaK, InoueTA (2011) Divergent behavioral and electrophysiological taste responses in the mid-legs of adult butterflies, Vanessa indica and Argyreus hyperbius. J Insect Physiol 57 : 118–126.
58. FoxRM (1966) Forelegs of butterflies. I. Introduction: chemoreception. J Res Lepid 5 : 1–12.
59. ThomC, GuerensteinPG, MechaberWL, HildebrandJG (2004) Floral CO2 reveals flower profitability to moths. J Chem Ecol 30 : 1285–1288.
60. LiuY, GuS, ZhangY, GuoY, WangG (2012) Candidate olfaction genes identified within the Helicoverpa armiga antennae transcriptome. PLoS One 7: e48260.
61. LavagninoN, SerraF, ArbizaL, DopazoH, HassonE (2012) Evolutionary genomics of genes involved in olfactory behavior in the Drosophila melanogaster species subgroup. Evol Bioinform Online 8 : 89–104.
62. Schuster-BöcklerB, ConradD, BatemanA (2010) Dosage sensitivity shapes the evolution of copy-number varied regions. PLoS One 5: e9474.
63. BentonR, SachseS, MichnickSW, VosshallLB (2006) Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol 4: e20.
64. CooperGM, NickersonDA, EichlerEE (2007) Mutational and selective effects on copy-number variants in the human genome. Nat Genet 39: S22–S29.
65. PuineanAM, FosterSP, OpiphantL, DenholmI, FieldLM, et al. (2010) Amplification of a cytochrome P450 gene is associated with resistance to neonicotinoid insecticides in the aphid Myzus persicae. PLoS Genet 6: e1000999.
66. BariamiV, JonesCM, PoupardinR, VontasJ, RansonH (2012) Gene amplification, ABC transporters and cytochrome P450s: Unraveling the molecular basis of pyrethroid resistance in the dengue vector, Aedes aegypti. PLoS Negl Trop Dis 6: e1692.
67. SchmidtJM, GoodRT, AppletonB, SherrardJ, RaymantGC, et al. (2010) Copy number variation and transposable elements feature in recent, ongoing adaptation at the Cyp6g1 locus. PLoS Genet 6: e1000998.
68. SchriderDR, StevensK, CardeñoCM, LangleyCH, HahnMW (2011) Genome-wide analysis of regrogene polymorphism in Drosophila melanogaster. Genome Res 21 : 2087–2095.
69. BaurR, HaribalM, RenwickJAA, StädlerE (1998) Contact chemoreception related to host selection and oviposition behavior in the monarch butterfly, Danaus plexippus. Physiol Entomol 23 : 7–19.
70. ZaluckiMP, BrowerLP, MalcolmSB (1990) Oviposition by Danaus plexippus in relation to cardenolide content of three Asclepias species in the southeastern U.S.A. Ecol Entomol 15 : 231–240.
71. PittsRJ, RinkerDC, JonesPL, RokasA, ZwiebelLJ (2011) Transcriptome profiling of chemosensory appendages in the malaria vector Anopheles gambiae reveals tissue - and sex-specific signatures of odor coding. BMC Genomics 12 : 271.
72. BarpEA, SoaresGLG, GianiEJM, RodriguesD, MoreiraGRP (2011) Variation in nectar and pollen availability, sucrose preference and daily response in the use of flowers by Heliconius erato phyllis. J Insect Behav 24 : 200–219.
73. EstradaC, JigginsCD (2008) Interspecific sexual attraction because of convergence in warning colouration: is there a conflict between natural and sexual selection in mimetic species? J Evol Biol 21 : 749–760.
74. KrennHW, EberhardMJB, EberhardSH, HiklA-L, HuberW, et al. (2009) Mechanical damage to pollen aids nutrient acquisition in Heliconius butterflies (Nymphalidae). Arthropod-Plant Interact 3 : 203–208.
75. Dunlap-PiankaH, BoggsCL, GilbertLE (1977) Ovarian dynamics in Heliconiine butterflies: programmed senescence versus eternal youth. Science 197 : 487–490.
76. BoggsCL, GilbertLE (1979) Male contribution to egg production in butterflies: evidence for transfer of nutrients at mating. Science 206 : 83–84.
77. CardosoMZ, GilbertLE (2007) A male gift to its partner? Cyanogenic glycosides in the spermatophore of long wing butterflies (Heliconius). Naturwissenschaften 94 : 39–42.
78. ZhenY, AardemaML, MedinaEM, SchumerM, AndolfattoP (2012) Parallel molecular evolution in an herbivore community. Science 337 : 1634–1637.
79. CohenMB, SchulerMA, BerenbaumMR (1992) A host-inducible cytochrome P450 from a host-specific caterpillar—molecular cloning and evolution. Proc Natl Acad Sci USA 89 : 10920–10924.
80. FrentiuFD, BernardGD, Sison-MangusMP, BrowerAV, BriscoeAD (2007) Gene duplication is an evolutionary mechanism for expanding spectral diversity in the long-wavelength photopigments of butterflies. Mol Biol Evol 24 : 2016–2028.
81. TamuraK, PetersonD, PetersonN, StecherG, NeiM, et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28 : 2731–2739.
82. SlaterGSC, BirneyE (2005) Automated generation of heuristics for biological sequence comparison. BMC Bioinformatics 6 : 31.
83. SieversF, WilmA, DineenD, GibsonTJ, KarpusK, et al. (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7 Article Number 539.
84. LewisSE, SearleSM, HarrisN, GibsonM, LyerV, et al. (2002) Apollo: a sequence annotation editor. Genome Biol 3: RESEARCH0082.
85. TrapnellC, WilliamsBA, PerteaG, MortazaviA, KwanG, et al. (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28 : 511–515.
86. SimpsonJT, WongK, JackmanSD, ScheinJE, JonesSJ, et al. (2009) ABySS: a parallel assembler for short read sequence data. Genome Res 19 : 1117–1123.
87. SalzbergSL, PhillippyAM, ZiminA, PuiuD, MagocT, et al. (2012) GAGE: A critical evaluation of genome assemblies and assembly algorithms. Genome Res 22 : 557–567.
88. VernotB, StolzerM, GoldmanA, DurandD (2008) Reconciliation with non-binary species trees. J Comput Biol 15 : 981–1006.
89. DurandD, HalldorssonBV, VernotB (2006) A hybrid micro-macroevolutionary approach to gene tree reconstruction. J Comput Biol 13 : 320–335.
90. BeltranM, JigginsCD, BrowerAVZ, BerminghamE, MalletJ (2007) Do pollen feeding, pupal-mating and larval gregariousness have a single origin in Heliconius butterflies? Inferences from multilocus DNA sequence data. Biol J Linn Soc Lond 92 : 221–239.
91. WannerKW, AndersonAR, TrowellSC, TheilmannDA, RobertsonHM, et al. (2007) Female-biased expression of odourant receptor genes in the adult antennae of the silkworm, Bombyx mori. Insect Mol Biol 16 : 107–119.
92. TanakaK, UdaY, OnoY, TatsuroN, SuwaM, et al. (2009) Highly selective tuning of a silkworm olfactory receptor to a key mulberry leaf volatile. Curr Biol 19 : 881–890.
93. JordanMD, AndersonA, BegumD, CarraherC, AuthierA, et al. (2009) Odorant receptors from the light brown apple moth (Epiphyas postvittana) recognize important volatile compounds produced by plants. Chem Senses 34 : 383–394.
94. Sánchez-GraciaA, VieiraFG, RozasJ (2009) Molecular evolution of the major chemosensory gene families in insects. Heredity 103 : 208–216.
Štítky
Genetika Reprodukční medicína
Článek Independent Evolution of Transcriptional Inactivation on Sex Chromosomes in Birds and MammalsČlánek The bHLH Subgroup IIId Factors Negatively Regulate Jasmonate-Mediated Plant Defense and DevelopmentČlánek Selective Pressures to Maintain Attachment Site Specificity of Integrative and Conjugative ElementsČlánek Reassembly of Nucleosomes at the Promoter Initiates Resilencing Following Decitabine ExposureČlánek Hepatocyte Growth Factor Signaling in Intrapancreatic Ductal Cells Drives Pancreatic Morphogenesis
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 7
-
Všechny články tohoto čísla
- An Solution for Crossover Formation
- Genome-Wide Association Mapping in Dogs Enables Identification of the Homeobox Gene, , as a Genetic Component of Neural Tube Defects in Humans
- Independent Evolution of Transcriptional Inactivation on Sex Chromosomes in Birds and Mammals
- Stepwise Activation of the ATR Signaling Pathway upon Increasing Replication Stress Impacts Fragile Site Integrity
- Genomic Analysis of Natural Selection and Phenotypic Variation in High-Altitude Mongolians
- Modification of tRNA by Elongator Is Essential for Efficient Translation of Stress mRNAs
- Role of CTCF Protein in Regulating Locus Transcription
- Gene Set Signature of Reversal Reaction Type I in Leprosy Patients
- Mapping of PARK2 and PACRG Overlapping Regulatory Region Reveals LD Structure and Functional Variants in Association with Leprosy in Unrelated Indian Population Groups
- Is Required for Formation of the Genital Ridge in Mice
- Monopolin Subunit Csm1 Associates with MIND Complex to Establish Monopolar Attachment of Sister Kinetochores at Meiosis I
- Recombination Dynamics of a Human Y-Chromosomal Palindrome: Rapid GC-Biased Gene Conversion, Multi-kilobase Conversion Tracts, and Rare Inversions
- Mechanisms of Protein Sequence Divergence and Incompatibility
- Histone Methyltransferase DOT1L Drives Recovery of Gene Expression after a Genotoxic Attack
- Female Behaviour Drives Expression and Evolution of Gustatory Receptors in Butterflies
- Combinatorial Regulation of Meiotic Holliday Junction Resolution in by HIM-6 (BLM) Helicase, SLX-4, and the SLX-1, MUS-81 and XPF-1 Nucleases
- The bHLH Subgroup IIId Factors Negatively Regulate Jasmonate-Mediated Plant Defense and Development
- The Role of Interruptions in polyQ in the Pathology of SCA1
- Dietary Restriction Induced Longevity Is Mediated by Nuclear Receptor NHR-62 in
- Fine Time Course Expression Analysis Identifies Cascades of Activation and Repression and Maps a Putative Regulator of Mammalian Sex Determination
- Genome-scale Co-evolutionary Inference Identifies Functions and Clients of Bacterial Hsp90
- Oxidative Stress and Replication-Independent DNA Breakage Induced by Arsenic in
- A Moonlighting Enzyme Links Cell Size with Central Metabolism
- Budding Yeast Greatwall and Endosulfines Control Activity and Spatial Regulation of PP2A for Timely Mitotic Progression
- The Conserved Intronic Cleavage and Polyadenylation Site of CstF-77 Gene Imparts Control of 3′ End Processing Activity through Feedback Autoregulation and by U1 snRNP
- The BTB-zinc Finger Transcription Factor Abrupt Acts as an Epithelial Oncogene in through Maintaining a Progenitor-like Cell State
- The Cohesion Protein SOLO Associates with SMC1 and Is Required for Synapsis, Recombination, Homolog Bias and Cohesion and Pairing of Centromeres in Drosophila Meiosis
- The RNA-binding Proteins FMR1, Rasputin and Caprin Act Together with the UBA Protein Lingerer to Restrict Tissue Growth in
- Pattern Dynamics in Adaxial-Abaxial Specific Gene Expression Are Modulated by a Plastid Retrograde Signal during Leaf Development
- A Network of HMG-box Transcription Factors Regulates Sexual Cycle in the Fungus
- Bacterial Adaptation through Loss of Function
- ENU-induced Mutation in the DNA-binding Domain of KLF3 Reveals Important Roles for KLF3 in Cardiovascular Development and Function in Mice
- Interplay between Structure-Specific Endonucleases for Crossover Control during Meiosis
- FGF Signalling Regulates Chromatin Organisation during Neural Differentiation via Mechanisms that Can Be Uncoupled from Transcription
- The Arabidopsis RNA Binding Protein with K Homology Motifs, SHINY1, Interacts with the C-terminal Domain Phosphatase-like 1 (CPL1) to Repress Stress-Inducible Gene Expression
- Selective Pressures to Maintain Attachment Site Specificity of Integrative and Conjugative Elements
- The Conserved ADAMTS-like Protein Lonely heart Mediates Matrix Formation and Cardiac Tissue Integrity
- The cGMP-Dependent Protein Kinase EGL-4 Regulates Nociceptive Behavioral Sensitivity
- RBM5 Is a Male Germ Cell Splicing Factor and Is Required for Spermatid Differentiation and Male Fertility
- Disease-Related Growth Factor and Embryonic Signaling Pathways Modulate an Enhancer of Expression at the 6q23.2 Coronary Heart Disease Locus
- Yeast Pol4 Promotes Tel1-Regulated Chromosomal Translocations
- A Dual Role for SOX10 in the Maintenance of the Postnatal Melanocyte Lineage and the Differentiation of Melanocyte Stem Cell Progenitors
- SLC26A4 Targeted to the Endolymphatic Sac Rescues Hearing and Balance in Mutant Mice
- Odoriferous Defensive Stink Gland Transcriptome to Identify Novel Genes Necessary for Quinone Synthesis in the Red Flour Beetle,
- Prediction of Complex Human Traits Using the Genomic Best Linear Unbiased Predictor
- Gene × Physical Activity Interactions in Obesity: Combined Analysis of 111,421 Individuals of European Ancestry
- Reassembly of Nucleosomes at the Promoter Initiates Resilencing Following Decitabine Exposure
- Exquisite Light Sensitivity of Cryptochrome
- miR-133a Regulates Adipocyte Browning In Vivo
- Strabismus Promotes Recruitment and Degradation of Farnesylated Prickle in Planar Polarity Specification
- Hepatocyte Growth Factor Signaling in Intrapancreatic Ductal Cells Drives Pancreatic Morphogenesis
- Is a Potential Tumor Suppressor Gene Commonly Inactivated by Epigenetic Mechanisms in Colorectal Cancer
- Joint Molecule Resolution Requires the Redundant Activities of MUS-81 and XPF-1 during Meiosis
- The Mating Competence of Geographically Diverse Strains in Their Natural and Unnatural Sand Fly Vectors
- Defective Repair of Oxidative Base Lesions by the DNA Glycosylase Nth1 Associates with Multiple Telomere Defects
- Effective Blocking of the Enhancer Requires Cooperation between Two Main Mechanisms Suggested for the Insulator Function
- Trans-Ancestral Studies Fine Map the SLE-Susceptibility Locus
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- SLC26A4 Targeted to the Endolymphatic Sac Rescues Hearing and Balance in Mutant Mice
- Bacterial Adaptation through Loss of Function
- The Cohesion Protein SOLO Associates with SMC1 and Is Required for Synapsis, Recombination, Homolog Bias and Cohesion and Pairing of Centromeres in Drosophila Meiosis
- Gene × Physical Activity Interactions in Obesity: Combined Analysis of 111,421 Individuals of European Ancestry
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



