-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe GATA Factor ELT-1 Works through the Cell Proliferation Regulator BRO-1 and the Fusogen EFF-1 to Maintain the Seam Stem-Like Fate
Seam cells in Caenorhabditis elegans provide a paradigm for the stem cell mode of division, with the ability to both self-renew and produce daughters that differentiate. The transcription factor RNT-1 and its DNA binding partner BRO-1 (homologues of the mammalian cancer-associated stem cell regulators RUNX and CBFβ, respectively) are known rate-limiting regulators of seam cell proliferation. Here, we show, using a combination of comparative genomics and DNA binding assays, that bro-1 expression is directly regulated by the GATA factor ELT-1. elt-1(RNAi) animals display similar seam cell lineage defects to bro-1 mutants, but have an additional phenotype in which seam cells lose their stem cell-like properties and differentiate inappropriately by fusing with the hyp7 epidermal syncytium. This phenotype is dependent on the fusogen EFF-1, which we show is repressed by ELT-1 in seam cells. Overall, our data suggest that ELT-1 has dual roles in the stem-like seam cells, acting both to promote proliferation and prevent differentiation.
Published in the journal: . PLoS Genet 7(8): e32767. doi:10.1371/journal.pgen.1002200
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002200Summary
Seam cells in Caenorhabditis elegans provide a paradigm for the stem cell mode of division, with the ability to both self-renew and produce daughters that differentiate. The transcription factor RNT-1 and its DNA binding partner BRO-1 (homologues of the mammalian cancer-associated stem cell regulators RUNX and CBFβ, respectively) are known rate-limiting regulators of seam cell proliferation. Here, we show, using a combination of comparative genomics and DNA binding assays, that bro-1 expression is directly regulated by the GATA factor ELT-1. elt-1(RNAi) animals display similar seam cell lineage defects to bro-1 mutants, but have an additional phenotype in which seam cells lose their stem cell-like properties and differentiate inappropriately by fusing with the hyp7 epidermal syncytium. This phenotype is dependent on the fusogen EFF-1, which we show is repressed by ELT-1 in seam cells. Overall, our data suggest that ELT-1 has dual roles in the stem-like seam cells, acting both to promote proliferation and prevent differentiation.
Introduction
The regulation of the decision between cell proliferation and differentiation is a key aspect of metazoan development, ensuring that the correct number and type of cells are present. The RUNX family of transcription factors (RUNX1, 2 and 3), together with their binding partner CBFβ, are key players in the control of stem cell proliferation in haematopoiesis [1]–[3], osteogenesis [4]–[6] and neurogenesis [7], [8]. Moreover, mutations in these genes are known to cause a variety of diseases, with both CBFβ and RUNX genes having the potential to act as either oncogenes or tumour suppressors, depending on the nature of the mutations and the context in which they act [9].
In the nematode worm, Caenorhabditis elegans, RUNX and CBFβ are each represented by a single gene, rnt-1 and bro-1 respectively. Reflecting their role in mammalian tissues, RNT-1 and BRO-1 are involved in the regulation of the division patterns of the stem cell-like seam cells [10]–[12]. The seam comprises a specialized epithelial tissue of lateral hypodermal cells located along each side of the worm and provides a tractable model system for studying the balance between proliferative and differentiative developmental decisions. The seam cells are considered stem cell-like because of their ability to both self-renew and to produce a variety of differentiated cell types; in addition to increasing the number of seam cells during some divisions, the lineage contributes cells to the hypodermis (an epithelial tissue), as well as giving rise to neurons and glial cells [13]. Seam cells do not self-renew throughout adult life, and have not yet been shown to reside in a classic “niche”, hence the qualification stem-like, but they do provide a useful simplified model for the stem cell mode of division throughout larval development that is well established [14]–[21].
The choice between the two very different developmental alternatives of proliferation and differentiation is intrinsically linked to the way in which the seam cells divide. Symmetrical divisions allow the expansion of the stem cell pool, as both daughters of the division retain the seam (stem cell-like) fate. Conversely, asymmetrical divisions initiate developmental pathways that result in the production of differentiated cell types. Whilst one daughter of the division (the posterior daughter, in the case of cells V1-6 in the seam lineage) retains the seam fate, the other loses its ability to proliferate further and instead proceeds to differentiate into one of a number of different cell types, most commonly assuming the hypodermal fate and fusing with the hyp7 syncytium. In this case, the differentiation event involves fusion with a different cell type, although seam cells can differentiate independently of fusion, for example taking on neuronal fates [13]. At the end of larval development, seam cells undergo homotypic fusion, producing the fully differentiated adult seam cell syncytium that secretes the alae [13]. The seam cells divide repeatedly throughout larval development, and the form that the division takes (symmetrical or asymmetrical) is critically dependent on the developmental stage of the worm. The nature of these stem cell-like divisions, and thus the fate of the daughter cells arising from them, therefore requires precise regulation both spatially and temporally.
In C. elegans, rnt-1 and bro-1 are rate-limiting regulators of seam cell divisions, with both genes being required for proliferation in this tissue. In rnt-1 and bro-1 mutants, the number of seam cells present in individuals is reduced due to division failures within the seam lineage [12]. Furthermore, over-expression of rnt-1 and bro-1 results in hyperplasia of the seam [10]. In this study, we have examined the mechanisms underlying the regulation of bro-1 expression. We identified a small (122 bp) conserved non-coding element (CNE) within the first intron of bro-1, which we show to be both necessary and sufficient for bro-1 expression in the seam. In order to identify direct upstream regulators of bro-1, a yeast one-hybrid screen was performed using this conserved element as bait. This, coupled with an in vitro binding assay, demonstrates that the GATA transcription factor ELT-1 directly regulates bro-1 through this CNE. The involvement of both GATA and RUNX/CBFβ factors in regulating a stem cell-like lineage in C. elegans thus mirrors the situation in mammalian systems, where the activities of these proteins are intricately linked with the specification of stem cell populations (for example haematopoietic stem cells [22]).
We then sought to further investigate the role of ELT-1 in seam cells. Analysis of the elt-1 RNAi phenotype suggests that ELT-1 has both bro-1-dependent and independent functions in the seam. ELT-1 functions through bro-1 to promote proliferation of seam cells and through eff-1 to repress inappropriate differentiation and fusion with the hypodermal syncytium. We also show that apical junction components themselves are important for the maintenance of seam cell fate, providing the correct contacts and therefore creating an environment in which cells are protected from differentiation signals emanating from surrounding tissues. Any disruption to the continuity of this environment contributes to the initiation of differentiation and loss of the seam fate.
Results
The first intron of bro-1 contains a highly conserved sequence element that is both necessary and sufficient for bro-1 expression in seam cells
Comparative sequence analysis revealed high levels of conservation between exonic regions of bro-1 in C. elegans and C. briggsae (73, 85 and 75% identity for exons 1, 2 and 3 respectively, compared to an average of 53% identity for introns 1 and 2). In contrast, we found no significant blocks of conservation in the 0.8 kb between bro-1 and the next upstream gene. However, a 122 bp region within the first intron was found to be highly conserved (3 species alignment shown in Figure 1B) (69% identity compared to 48% for the rest of the introns). We termed this the “bro-1 Conserved Non-coding Element” (bro-1 CNE).
Fig. 1. bro-1 contains a conserved non-coding element within the first intron. 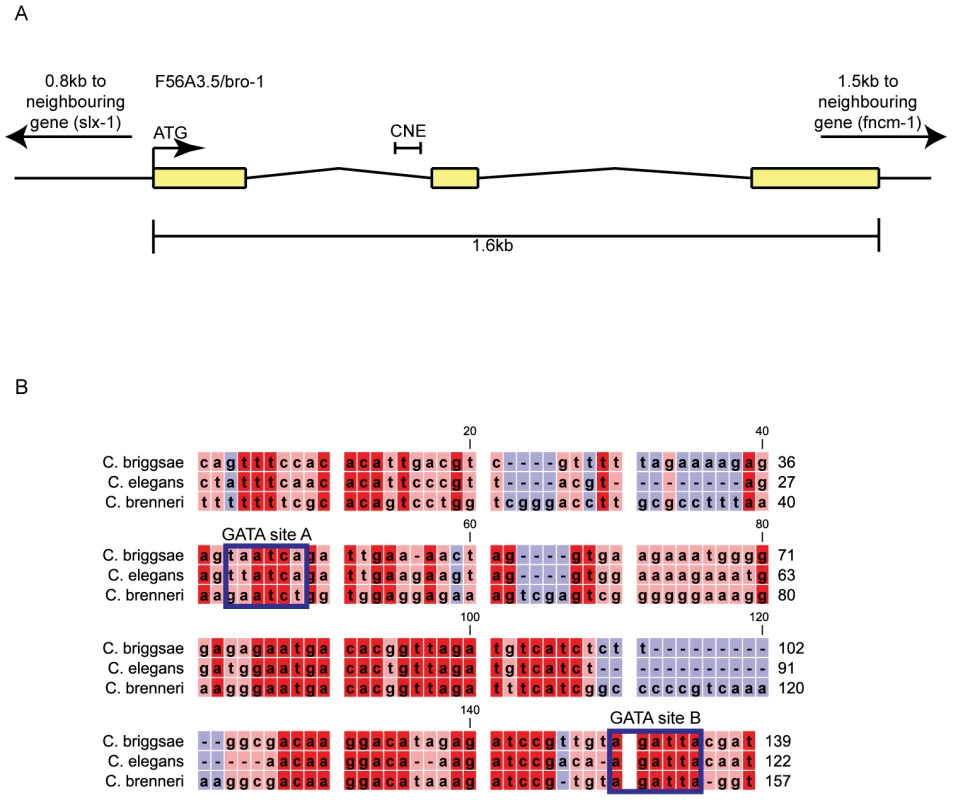
(A) Genomic structure of bro-1, showing the size and position of the CNE. (B) Alignment of the C. elegans bro-1 CNE with orthologues from C. briggsae and C. brenneri reveals high levels of sequence conservation at the 3′ end of intron 1. Conservation is still evident, though less pronounced, when C. remanei and C. japonica are included in the alignment (data not shown). Predicted GATA binding sites are labelled. Sequence alignments were performed using CLC Sequence Viewer v6.4 (CLC bio, Aarhus, Denmark). Red shading denotes that sequences are conserved across all 3 species of nematode; pink denotes conservation in 2 out of 3 species; blue denotes no conservation. Next, we tested the ability of the bro-1 CNE to act as an enhancer for cell-specific expression. When used in conjunction with the pes-10 minimal promoter (bro-1 CNE::gfp), the CNE was able to drive seam cell-specific GFP expression in worms (Figure 2A–2C). A vector containing a similar-sized fragment of a different, non-conserved region of intron 2 failed to drive any discernable GFP expression (data not shown). Furthermore, when the intergenic region between bro-1 and the nearest upstream gene was used, no GFP expression was evident (data not shown). To further characterise the importance of the CNE in regulating bro-1 expression, we first deleted the 122 bp region from the bro-1::dsRED2 construct [10]. The wild type construct (containing the full bro-1 genomic region) drives expression in seam cells and rescues the bro-1 mutant male tail phenotype caused by division failures in V and T lineage seam cells (Figure 2J). In contrast, the mutagenised bro-1::dsRED2 construct did not express in seam cells, and was unable to rescue the bro-1 male tail phenotype (Figure 2K). Secondly, we used the CNE (together with the pes-10 minimal promoter) to drive expression of bro-1 cDNA::gfp (see Text S1 and [23]). This construct drove seam cell expression, and rescued the bro-1 mutant phenotype (Figure 2L). Thus, the bro-1 CNE is both necessary and sufficient for bro-1 expression in seam cells.
Fig. 2. The bro-1 CNE is both necessary and sufficient for correct expression of bro-1. 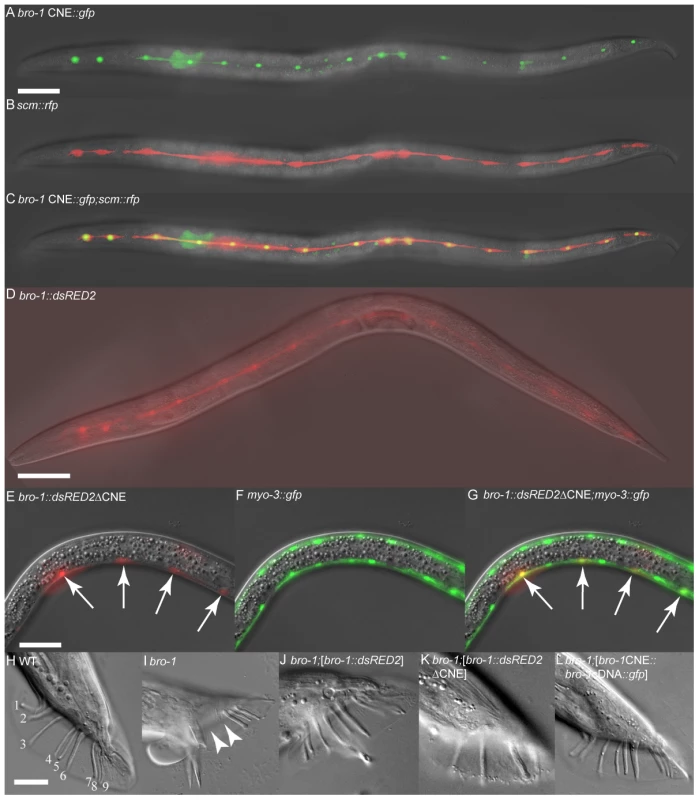
(A–C) Transgenic animal expressing scm::rfp [10] and bro-1 CNE::gfp. The co-localisation of these two reporters in seam cells demonstrates that the 122 bp CNE is sufficient to drive seam cell expression of GFP when present as a single copy upstream of the pes-10 minimal promoter in plasmid pPD107.94 (containing an NLS). RFP, driven by scm::rfp, localises to both the nucleus and cytoplasm of seam cells. A = bro-1 CNE::gfp only, B = scm::rfp only, C = merge. (D) bro-1::dsRED2 expression in the seam cells of a transgenic hermaphrodite. (E–G) Transgenic animal expressing the bro-1::dsRED2 construct with the CNE deleted and myo-3::gfp to mark body wall muscle cells. A = bro-1::dsRED2ΔCNE only, B = myo-3::gfp only, C = merge. Deletion of the CNE abolishes seam expression of bro-1 and results in high levels of expression in body wall muscle (arrows), where bro-1 is usually expressed only very weakly. dsRED2 expression is variably absent from some nuclei owing to mosaicism of the extrachromosomal array; the myo-3::gfp marker is integrated. Scale bars for A–G, 50 µm. (H) WT male tail with 18 sensory rays, 9 on each side (numbered 1–9). (I) bro-1 male tail, exhibiting missing rays (arrowheads). (J) bro-1 male tail, rescued by full length bro-1::dsRED2. (K) Transgenic bro-1 animal expressing bro-1::dsRED2, from which the CNE has been deleted. No rescue of the male tail phenotype is observed. (L) bro-1 male tail, rescued by bro-1 cDNA::gfp driven by the CNE. Scale bars for H–L, 20 µm. The GATA transcription factor ELT-1 interacts directly with the bro-1 CNE
Next, we used a yeast one-hybrid system to determine which transcription factors are able to bind to the bro-1 CNE. Three tandem copies of the CNE were used as bait and screened with a mixed stage C. elegans transcription factor cDNA library. Of 120 positive colonies, 110 contained the clone encoding the GATA factor ELT-1, demonstrating that ELT-1 protein binds to the bro-1 CNE in this assay.
The bioinformatics software Patch [24] revealed the presence of possible binding sites for GATA transcription factors in the CNE (Figure 1B), two of which are relatively well conserved across Caenorhabditis species [25], [26]. Site A matches the GATA consensus sequence WGATAR, while site B is slightly different (AGATTA), but matches the target sequence for the human GATA family member GATA6 [26]. To investigate the significance of these sites, both were deleted separately from the bro-1::dsRED2 rescuing construct. While deletion of site A had no effect on tail rescuing ability, deletion of site B abolished the ability of the construct to rescue bro-1 mutant tails (Figure 3A).
Fig. 3. Correct bro-1 expression depends on ELT-1 binding to GATA site B. 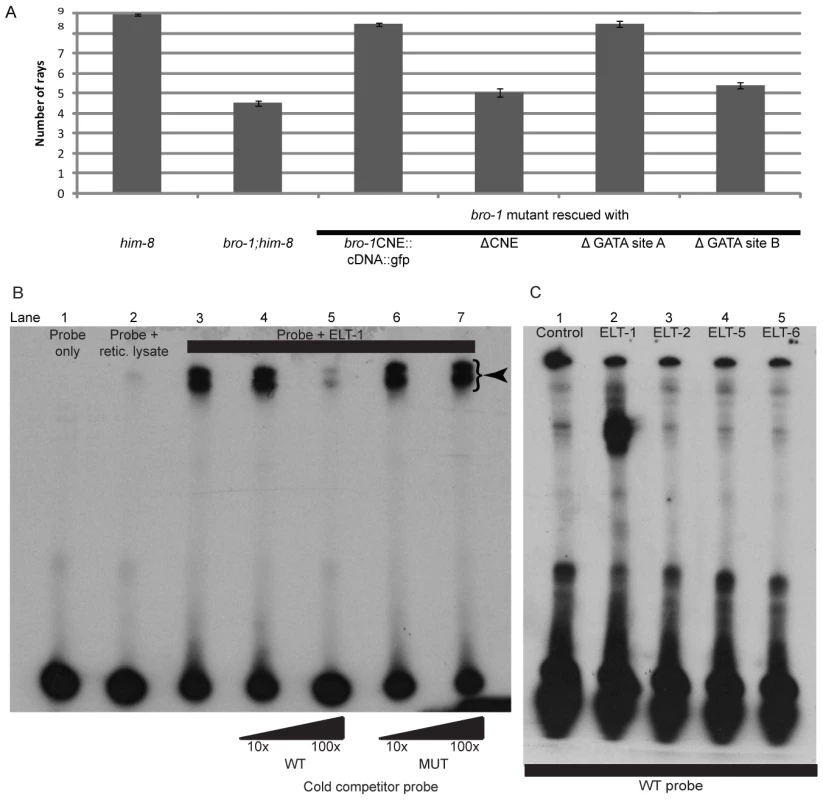
(A) Bar chart showing ray number in the following strains: him-8, bro-1;him-8, bro-1;him-8; bro-1CNE::bro-1cDNA::gfp, bro-1;him-8;bro-1ΔCNE::dsRED2, bro-1;him-8;bro-1CNEΔGATA site A::bro-1cDNA::gfp, bro-1;him-8;bro-1CNEΔGATA site B::bro-1cDNA::gfp. Deletion of GATA site A has no effect on the ability of the CNE::bro-1 cDNA construct to rescue the bro-1 mutant tail phenotype, while deletion of site B abolishes the rescuing ability of the construct. Error bars show SEM. (B) EMSA showing that in vitro translated ELT-1 shifts labelled probe (lane 3, DNA-protein complexes labelled with an arrowhead), which is not shifted on its own or in the presence of reticulocyte lysate only (lanes one and two respectively). Non-labelled WT cold competitor probe at 100× concentration (lane 5) significantly reduces the intensity of the shifted bands. Addition of mutated competitor probe has no such effect at 100× concentration (lane 7), demonstrating that the ELT-1-CNE interaction is dependent on GATA site B, which is altered in the mutated probe. (C) The interaction between ELT-1 and the bro-1 CNE is specific. Incubation of ELT-1 protein with labelled probe results in a shift (lane 2) relative to the control incubation (lane 1). Incubation of other GATA transcription factors (ELT-2, ELT-5 and ELT-6) with the labelled probe resulted in no shift. ELT-3 was not tested as in vitro transcription and translation proved problematic but, like ELT-2, ELT-3 is not expressed in the seam [61] so would not bind to bro-1 in seam cells, and was not detected amongst positive clones in the yeast one-hybrid screen, despite being present in the TF library used. ELT-1 specifically binds GATA site B within the bro-1 CNE
To confirm this interaction, an Electrophoretic Mobility Shift Assay (EMSA) was performed using in vitro translated ELT-1 protein and a portion of the bro-1 CNE containing GATA site B as a probe. ELT-1 protein was able to bind the CNE, resulting in a shift in the position of the labelled probe (Figure 3B). Cold competitor probe was able to compete with the identical labelled probe, resulting in a diminution in intensity of the shifted band. However, cold probe with a mutation in GATA site B (AGATTA to ATAGTA) was unable to compete with the wild type labelled probe, suggesting that ELT-1 protein interacts directly with GATA site B.
Other C. elegans members of the GATA family of transcription factors were tested for their ability to bind the bro-1 CNE using the same band shift assay; all failed to shift the labelled probe (Figure 3C). Taken together, these data suggest that amongst the members of the GATA family in C. elegans, ELT-1 alone mediates bro-1 transcriptional activity in the seam by direct binding to GATA site B within the CNE.
ELT-1 regulates bro-1 expression in vivo
The fact that bro-1 seam expression disappears when ELT-1 binding site B within the bro-1 regulatory region is deleted suggests that ELT-1 is an activator of bro-1 expression. To confirm this, we monitored bro-1::gfp expression in animals subjected to elt-1 RNAi and observed a decrease in signal intensity (data not shown). It was necessary to reduce the level of elt-1 expression by RNAi as elt-1 alleles are embryonic lethal; therefore only the effects of a small reduction in elt-1 expression could be analysed. To quantify this, we measured endogenous elt-1 and bro-1 transcript levels by qRT-PCR in animals surviving the elt-1 RNAi treatment. These animals (most of which exhibited seam defects) were found to have a 1.5 fold reduction in elt-1 transcript levels and a 1.7 fold decrease in bro-1 transcript levels, demonstrating that ELT-1 plays a major role in regulating bro-1 expression in vivo.
ELT-1-deficient worms, like bro-1 mutants, exhibit division failures in the seam lineage
ELT-1 has previously been reported to be essential for seam differentiation and maintenance, with elt-1(RNAi) animals having fewer seam cells as assayed by the seam cell marker scm::gfp [27]. Significantly, the average number of seam cells in elt-1(RNAi) animals (13.6 seam cells per side, ±0.3, n = 83) is very similar to that of bro-1 mutants (14.0 seam cells per side, ±0.3, n = 91) and significantly lower than animals fed control HT115 bacteria containing the empty feeding vector L4440 (15.8 seam cells per side, ±0.1, n = 30). The cellular basis of this phenotype has not been previously described, therefore lineage analysis was performed to elucidate the cellular mechanism of seam cell loss.
elt-1(RNAi) worms have variable seam division failures (Figure 4A). These defects were observed during both the symmetrical and asymmetrical divisions of L2, suggesting that the role of ELT-1 is not limited to one type of division. However, given the difficulties in pursuing the lineage analysis past L3 (these worms are very sick), we cannot exclude the possibility that the role of ELT-1 in regulating seam cell division is limited to the L2 stage. Division failures were not observed during the L1 asymmetric division, although embryonic developmental abnormalities often meant that the number of seam cells present at the start of L1 was lower than that of wild type worms. Nevertheless, the ability of the remaining seam cells to undergo the asymmetric L1 division parallels the situation in rnt-1 and bro-1 mutants, where division failures are restricted to the subsequent divisions. These division defects are distinct from the classic retarded heterochronic phenotypes, in which stage-specific stereotypical division “cassettes” occur at the wrong stage. In the case of lin-4, the L1 pattern of division is re-iterated at later stages, resulting in fewer seam cells overall [28]. In the case of elt-1(RNAi) and rnt-1/bro-1 mutants, the defects seen were not representative of stereotypical division patterns occurring at the wrong stage, but rather involved outright division failures, or occasionally symmetrisation towards the hypodermal fate.
Fig. 4. Reduction of elt-1 function causes V lineage division defects. 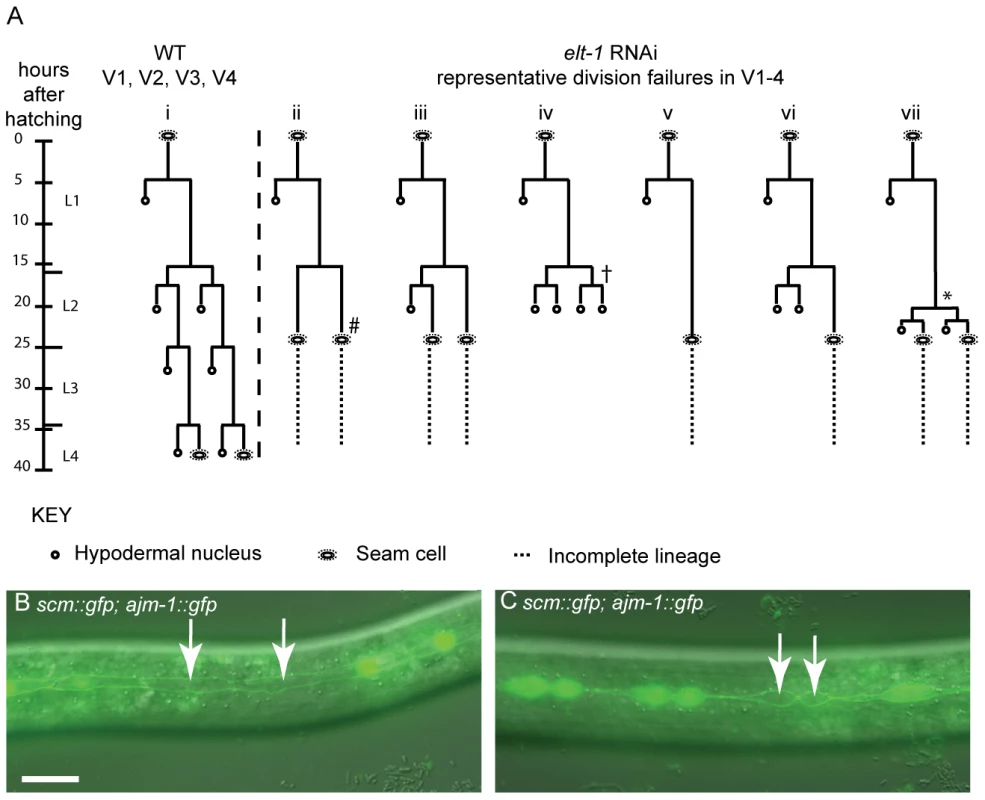
(A) Lineage traces of WT and elt-1(RNAi) hermaphrodites. In elt-1(RNAi) worms, seam cells often fail to divide but remain intact and retain their stereotypical seam cell ‘eye’ shape. One or more divisions may be missed, for example in lineage trace ii (#). There was no obvious bias for the V cell involved. Alternatively, certain V lineage seam cells sometimes undergo the symmetrical and asymmetrical divisions during L2, but significantly later relative to both other V cells and to the time of moulting. For example, whereas Vn.p cells should divide first symmetrically and then asymmetrically at the start of L2, these divisions have been observed to occur just before the L3 moult in elt-1(RNAi) animals, around 8 hours after they should have occurred (* in lineage trace vii). Similarly, the L2 symmetric and asymmetric divisions in V1-4 should take place slightly ahead of the comparable V6 divisions in WT worms. elt-1 RNAi, however, often delays these divisions such that they occur after V6p has divided symmetrically and then asymmetrically in L2. In addition to these proliferation defects, aberrant fate changes at division were also occasionally observed (approximately 10% of defects observed); instead of the division producing two seam cells or one seam daughter and one hypodermal daughter, both cells produced adopted the hypodermal fate, becoming smaller and rounder than normal seam cells, losing expression of the scm::gfp marker and failing to divide further (e.g. † marked on trace iv). This defect was also observed in rnt-1 and bro-1 animals [10]. Designation of the seam fate was based on cell shape, position and division potential, where possible, not scm::gfp expression. In no worms were L1 division defects observed. (B) and (C) elt-1(RNAi) animals carrying the scm::gfp and ajm-1::gfp transgenes. Seam cells often lose scm::gfp expression (white arrows). 52% of elt-1 (RNAi) worms were found to have one or more cells bounded by AJM-1::GFP but lacking SCM::GFP (n = 61) compared to 5% in control worms, fed on HT115 bacteria containing the empty L4440 RNAi feeding vector (n = 62). As can be seen, these cells retain their AJM-1::GFP boundary and seam morphology and were not observed to fuse with hyp7. The loss of SCM::GFP appears to be independent of the cells' stage in the cell cycle and can happen before (B) or after (C) division. Scale bar, 25 µm. To assess whether the division failures observed in elt-1(RNAi) worms were correlated with loss of seam fate, the integrated scm::gfp reporter wIs51 [29] was used as a seam cell marker. We observed that even worms which do not show gross morphological abnormalities, and which therefore can be followed by lineage analysis throughout development, frequently exhibit loss of scm::gfp from usually one or two seam cells from the late L1 stage onwards. While the seam cell remains clearly visible under DIC (retaining its distinctive eye-shaped seam morphology), GFP expression fades over 30–60 minutes until the cell shows no fluorescence at all (Figure 4B). To confirm the identity of these ‘seam’ cells that fail to express scm::gfp, cell boundaries were visualised using the ajm-1::gfp reporter (which marks the apical junctions between seam cells and the surrounding hyp7 syncytium, in which it is not expressed). These cells were always bounded by AJM-1::GFP, confirming their seam identity (as suggested by their morphology). Thus, when seam cells are counted in elt-1(RNAi) animals using scm::gfp, not all of the seam cells present will be observed. In addition to division failures, therefore, the loss of expression of the ‘seam’ marker scm::gfp also likely accounts for the apparent progressive loss of seam cells previously reported in elt-1(RNAi) worms [27].
Interestingly, a similar phenotype is observed in bro-1 and rnt-1 mutants (data not shown). However, while loss of this marker has been attributed to degeneration of seam cells [27], we have not observed this phenomenon in either rnt-1/bro-1 mutants or elt-1(RNAi) worms; seam cells retain their characteristic “eye” shape and position within the seam line, but may lose scm::gfp in these backgrounds. Given the prevalence of using scm::gfp as a marker of seam fate, we were surprised to observe that scm::gfp expression does not in fact seem to be tightly correlated with proliferative potential; lineage analysis revealed that cells that stop expressing scm::gfp often undergo the correct number of divisions at the appropriate times. Conversely, we observed division failures in seam cells strongly expressing scm::gfp. Taken together, this suggests that while scm::gfp may mark seam cells during normal development, it is not an infallible marker for seam cell fate, perhaps because particular elements of seam ‘fate’ are uncoupled in elt-1(RNAi) and rnt-1/bro-1 mutant animals.
Cells that remained in the seam line but failed to divide, always retained expression of ajm-1::gfp, regardless of whether or not they expressed scm::gfp (Figure 4B and 4C). This suggests that their failure to divide results not from a change of fate per se (from seam to hypodermis), but rather from a loss of proliferative ability. This is supported by dpy-7p::yfp expression, which marks cells that have adopted a hypodermal fate but is not expressed in seam cells; cells bounded by AJM-1::GFP never expressed dpy-7p::yfp, even when they fail to undergo scheduled divisions (data not shown).
ELT-1 works independently from BRO-1 to prevent seam cells fusing inappropriately with the hypodermis
Unlike the situation in bro-1 mutants, however, observation of elt-1(RNAi) animals carrying the integrated ajm-1::gfp construct revealed that some cells, at the same time as losing scm::gfp expression, lose AJM-1::GFP, used here as a marker of seam cell boundaries (data not shown and Figure 5A–5D). This may be predictive of inappropriate fusion with the hyp7 syncytium. Cells were never observed that lost ajm-1::gfp but retained scm::gfp. The ‘disintegration’ of the AJM-1::GFP continues until only vestigial traces are evident between the two seam cells on either side of the affected cell, as shown in Figure 5D.
Fig. 5. elt-1 RNAi causes division-independent seam to hypodermis fate transitions. 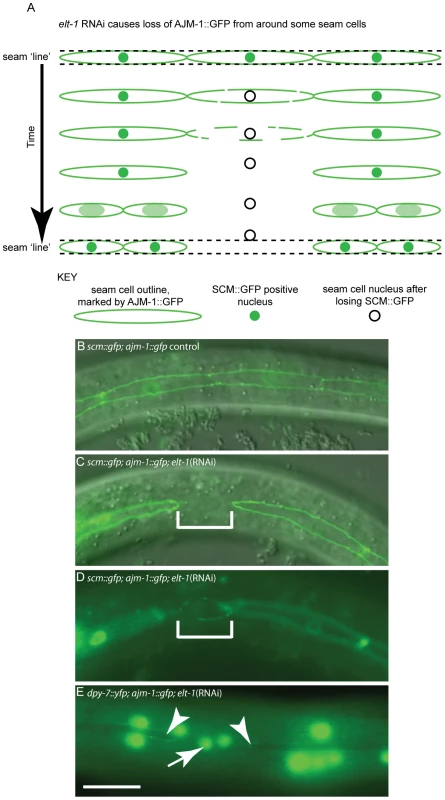
In addition to division defects, loss of seam fate was also observed between cell divisions following elt-1 knockdown. (A) Schematic diagram of AJM-1 loss from one cell in the V lineage, involving the gradual loss of AJM-1::GFP from around the cell until the cell is completely unbounded. SCM::GFP expression is always absent in such cells. Over a period of several hours, the cell becomes smaller and rounder and moves out of the line of the seam, into the hyp7 syncytium. Subsequently, these cells fail to divide, in contrast to cells that remain in the seam lineage and thus retain their stem fate. (B) Wild type seam lineage in animals expressing both ajm-1::gfp and scm::gfp, showing cells in a continuous line. (C) and (D) show the same cell in two different planes in an elt-1(RNAi) animal in which seam cells lose their AJM-1::GFP boundary (representative cell shown in bracketed region), changing shape and plane as they move into the hyp7 syncytium (change of shape and plane obvious in D). In order to quantify inappropriate fusion in these worms we scored animals with one or more breaks in the seam line. In elt-1(RNAi) animals 68% of animals had breaks in the seam (n = 44). In control animals (exposed to HT115 bacteria containing the empty RNAi feeding vector L4440) 1.8% of animals had breaks (n = 56). (E) Using worms expressing both AJM-1::GFP and dpy-7p::yfp, it is possible to show that cells which lose their AJM-1 boundary and move out of the seam lineage as a result of elt-1 RNAi, subsequently switch on dpy-7 expression (indicated by white arrow), a marker of hypodermal fate. Adjacent seam cells which retain AJM-1::GFP expression show no dpy-7 expression (white arrow heads). In the hours subsequent to losing ajm-1::gfp expression, we observed that the seam cell tends to become rounder and move out of the line of the seam, with both the morphological and positional changes being indicative of the acquisition of hypodermal fate. To test this, a strain carrying the integrated transgenes ajm-1::gfp and dpy-7::yfp was used. In several cases, cells were observed during lineage analysis losing ajm-1::gfp and then acquiring dpy-7::yfp expression. Gaps in ajm-1::gfp expression in the seam were correlated with the presence of dpy-7::yfp-expressing nuclei which had not yet, or only partially, moved out of the line of the seam (Figure 5E). Thus, elt-1 RNAi causes an additional phenotype not observed in rnt-1 or bro-1 mutants, whereby some seam cells differentiate inappropriately by fusing with the hypodermal syncytium.
Overall, our data suggests three distinct phenotypes observed in elt-1(RNAi) animals. The first, in common with rnt-1 and bro-1 mutants, involves division failure in the absence of a permanent change in cell fate (i.e. not involving fusion with the hypodermis). Secondly, loss of SCM::GFP is also observed in elt-1(RNAi) animals (as well as in rnt-1 and bro-1 mutants), but we found this to be independent of division failure. Thirdly, in elt-1(RNAi) animals but not in rnt-1/bro-1 mutants, we observed inappropriate adoption of the hypodermal fate as shown by the acquisition of DPY-7::YFP, preceded by loss of scm::gfp expression and of AJM-1::GFP from the apical junctions. While these cells always lose SCM::GFP, fusion with the hypodermis is not always the consequence of SCM::GFP loss. However, AJM-1::GFP is very tightly linked to the seam fate; loss of AJM-1::GFP is always coupled with fusion to the hyp7 syncytium and acquisition of the hypodermal fate.
The elt-1 (RNAi) fusion defect is dependent on EFF-1
If cells are lost from the seam in elt-1(RNAi) animals because of inappropriate fusion with the hyp7 syncytium, we would anticipate that ectopic eff-1 expression would be evident, as the fusogen EFF-1 is known to be required for this fusion event [30]. To test this, a transgenic strain carrying both an eff-1 transcriptional reporter, which normally expresses in dorsal and ventral hypodermis but not in seam cells, and ajm-1::mCherry was used. In elt-1(RNAi) animals, we observed ectopic eff-1p::gfp expression in seam cells that had lost their AJM-1 boundary (Figure 6A). Furthermore, we found that elt-1 RNAi induced fusion of seam cells with the hyp7 syncytium was suppressed in eff-1 mutants (Figure 5A, 5C, 5D and Figure 6C). Taken together, these data suggest that ELT-1 represses eff-1 expression in seam cells in order to prevent fusion of these cells with the surrounding hypodermal syncytium, thus maintaining their distinct stem cell-like fate.
Fig. 6. ELT-1 represses eff-1 expression in seam cells to preserve stem cell-like identity and maintain apical junction integrity. 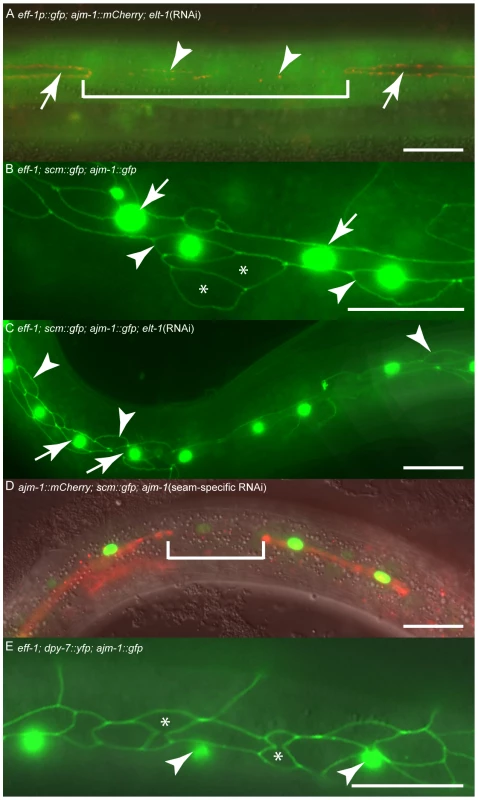
(A) Hermaphrodite carrying eff-1p::gfp and ajm-1::mCherry transgenes, subjected to elt-1 RNAi. Within the bracketed region a seam cell is in the process of losing ajm-1 expression, and only has vestiges of AJM-1::GFP remaining (arrowheads). This cell is now expressing eff-1p::gfp. In contrast, cells retaining their AJM-1 border never express eff-1p::gfp (white arrows). (B) eff-1 mutant hermaphrodite expressing scm::gfp and ajm-1::gfp. scm::gfp expression in the “true” seam is shown with white arrows. Anterior daughters of seam divisions sometimes retain expression of scm::gfp (white arrowheads), particularly where they remain in contact with the true seam (presumably these are the most recent daughters). Other anterior daughters do not express scm::gfp but retain their AJM-1 boundary (asterisks). In order to quantify inappropriate fusion in these worms we scored animals with one or more breaks in the seam line. In eff-1 mutants 3% of animals had breaks in the seam (n = 31). (C) eff-1 mutant hermaphrodite expressing scm::gfp and ajm-1::gfp, subjected to elt-1 RNAi. In these animals, no AJM-1 breakdown is observed, either around seam cells (white arrows) or around anterior daughters of asymmetric seam divisions (white arrowheads), demonstrating that the elt-1 (RNAi) induced fusion of seam cells with hyp7 is suppressed in eff-1 mutants. In order to quantify inappropriate fusion in these worms we scored animals with one or more breaks in the seam line. In eff-1 mutants subjected to elt-1 RNAi 0% of animals had breaks in the seam (n = 20), compared with 68% in elt-1(RNAi) animals alone (n = 44, Figure 5A, 5C, 5D). (D) Seam-specific RNAi strain hermaphrodite subjected to RNAi of ajm-1. In this experiment the RNAi deficient mutant rde-1 was used, rescued with rde-1 cDNA expressed under the control of the seam-specific SCM promoter, as described in detail in the Text S1. The bracketed cell has lost its AJM-1 border and the SCM::GFP has become much fainter. (E) eff-1 hermaphrodite carrying dpy-7p::yfp and ajm-1::gfp transgenes. Anterior daughters of seam divisions retain their AJM-1 borders but never express dpy-7 (asterisks). dpy-7 expression is only observed in the syncytial hypodermis surrounding these cells (white arrowheads). Scale bar, 25 µm. Seam cell membrane integrity, as marked by apical junctions, is required to maintain stem cell-like identity and sufficient to prevent differentiation
In order to address more directly the role of seam cell boundaries in determining the stem cell-like identity of seam cells, we knocked down the expression of components of apical junctions by RNAi. Given the essential functions of these proteins in a variety of cell types throughout development, it was necessary to use a seam-specific RNAi approach [31]. Knockdown of either ajm-1, let-413 or dlg-1 gave a similar phenotype, involving breaks in the AJM-1::GFP boundary, associated loss of scm::gfp expression and withdrawal from further division (Figure 6D; let-413 and dlg-1 data not shown). When ajm-1 was knocked down by RNAi, 66% of animals displayed breaks in the seam boundary as marked by ajm-1::mCherry (n = 50), whereas in controls not subjected to RNAi 5% of animals displayed breaks (n = 36) (the odd break in the seam would be expected due to mosaicism of the ajm-1::mCherry scm::gfp array). Therefore, seam cell membrane integrity, as marked by apical junctions, is essential for the maintenance of seam stem cell-like fate.
Next, we wanted to examine whether the presence of an intact boundary around the seam cells is sufficient to specify seam fate. This could happen in two ways: the boundary could either promote seam fate, or block differentiation signals from the surrounding environment. To test this, we used an eff-1 mutant in which anterior daughters retain their AJM-1::GFP marked boundary and remain in contact with the seam line instead of moving into the hypodermis. In other words, ectopic AJM-1::GFP boundaries are present in this strain. To assess whether these cells inappropriately retain the seam fate we monitored scm::gfp expression, as well as dpy-7::yfp expression. Firstly, we found that ectopic AJM-1::GFP bordered cells frequently do not express scm::gfp (Figure 6B), suggesting that these cells do not retain all aspects of seam fate. Perhaps this is not surprising, given that these cells are the anterior daughters of asymmetric seam divisions and would have already received the instruction to withdraw from further proliferation. However, we never observed dpy-7::yfp expression in these cells (Figure 6E), indicating that differentiation is repressed. This suggests that fusion of seam cells with hyp7 (mediated by EFF-1) is essential as a trigger for differentiation in this context. These cells are therefore in developmental ‘limbo’, being neither completely seam nor hypodermis. Thus, although the anterior daughters of asymmetric seam divisions are destined to differentiate at division, the nature of the differentiation event is not specified at this stage and requires further inputs; it is not until cell fusion and membrane breakdown occurs, as marked by the loss of apical junction boundaries, that the hypodermal fate is adopted.
Overall, therefore, seam cell boundaries may not be sufficient to specify all aspects of seam fate, but they are sufficient to prevent differentiation. Our data therefore suggest that the seam stem cell-like fate is retained by the blocking of “hypodermalizing” signals in cells that are bounded by apical junctions. ELT-1, therefore, plays dual roles in specifying the stem cell-like properties of the seam cells; on the one hand activating proliferative potential via bro-1, and on the other preventing inappropriate differentiation through the repression of eff-1 and consequent maintenance of seam boundaries (Figure 7).
Fig. 7. ELT-1 maintains the seam stem cell-like fate by promoting cell proliferation and preventing inappropriate differentiation. 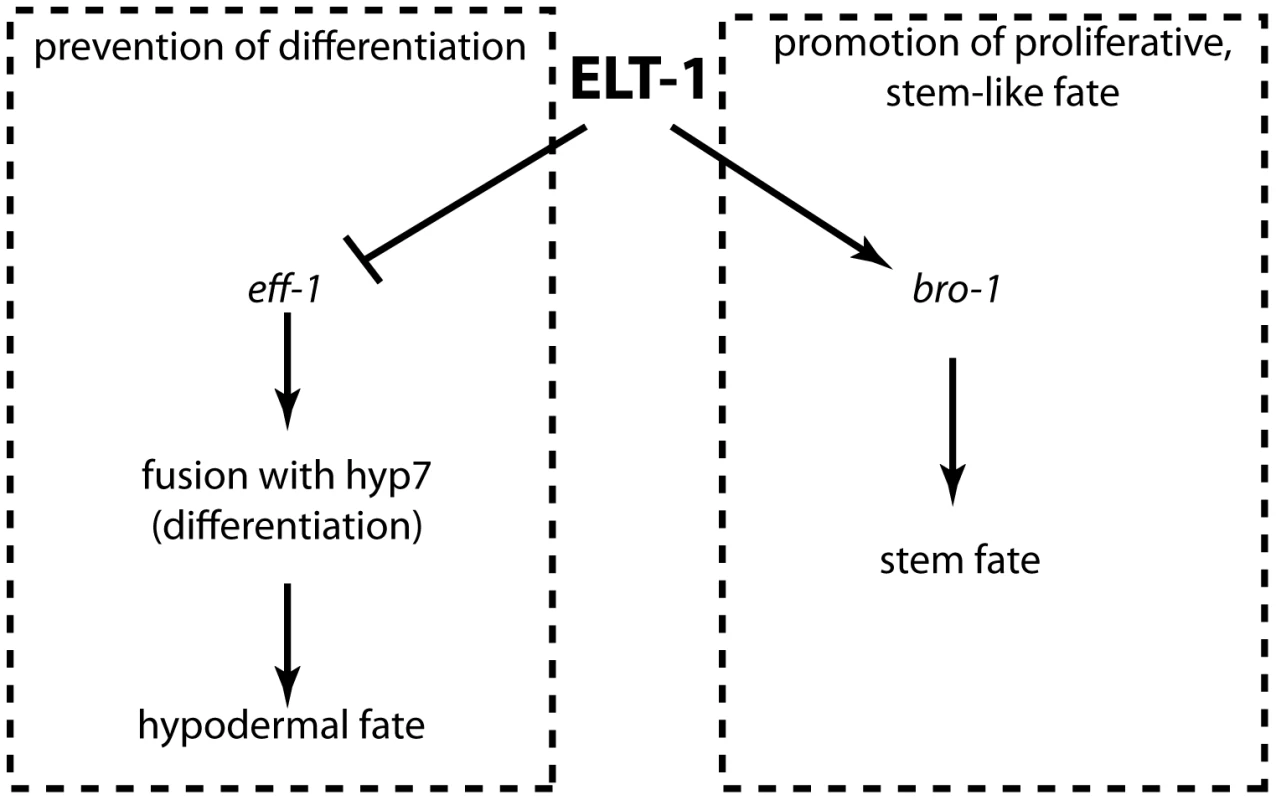
Model to illustrate the relationship between ELT-1, BRO-1 and EFF-1 in the maintenance of the seam stem cell-like fate. Direct transcriptional activation of bro-1 by ELT-1 promotes proliferation, while repression of eff-1 by ELT-1 (either directly or indirectly, for example through elt-5/6) represses fusion mediated by EFF-1, thereby preventing differentiation. Discussion
C. elegans seam cells are an excellent model for stem cell-like modes of division, sitting as they do at the crossroads between the developmental decision to proliferate (thus renewing the pool of pluripotent precursors), or to pursue a differentiation pathway towards one or more specialised cell types. We used comparative genomics coupled with a yeast one-hybrid screen and promoter deletion analysis to define upstream regulators of bro-1 expression; a gene known to be essential for seam cell proliferation. Surprisingly, we found that sequences necessary and sufficient for the expression of bro-1 lie exclusively within an intron. Our evidence adds strength to the concept that highly conserved non-coding sequences correlate with biologically important enhancer elements [32], [33]. Multiple lines of evidence suggest that ELT-1 binds to this intronic sequence; in a yeast one-hybrid screen using the CNE as bait, ELT-1 was identified in 92% of positive clones, whilst an EMSA band shift experiment not only confirmed this finding, but demonstrated that the putative GATA site towards the 3′ end of the CNE (GATA site B) is essential for ELT-1 binding, an observation confirmed by rescue experiments.
Taken together, evidence from our yeast one-hybrid screen, in vitro binding assay, rescue experiments and quantitative RT-PCR experiments suggest that ELT-1 is a direct upstream transcriptional regulator of bro-1. This model is supported by the similarities between elt-1 (RNAi) and bro-1 mutant phenotypes in terms of failures of seam cell divisions, and loss of scm::gfp expression. It therefore seems highly likely that elt-1 and bro-1 (as well as rnt-1) function within the same developmental pathway to promote seam cell proliferation. It is interesting to note that the L1 (asymmetric) division is robust and appears unaffected in both elt-1 RNAi and bro-1/rnt-1 mutant worms. This division is most likely regulated by a separate pathway. The involvement of both the CBFβ/RUNX complex and a GATA transcription factor in the regulation of the proliferation of the stem cell-like seam cells is reminiscent of the situation in Drosophila and in mammals, where GATA and RUNX factors work together to regulate blood cell formation [34]. Indeed, mammalian Runx1 is known to be transcriptionally activated by Gata2 [22]. Thus, the direct regulatory relationship between ELT-1 and bro-1 suggests yet another mode of interaction between members of these two gene families.
elt-1(RNAi) worms display a striking phenotype which is not observed in bro-1 mutant animals, however. This involves loss of the apical cell junction marker ajm-1::gfp from some seam cells, followed by movement of the cell out of the seam and subsequent differentiation into the hypodermal fate. It has previously been argued that seam cell phenotypes (loss of scm::gfp positive cells and gaps in ajm-1::gfp expression) in elt-1(RNAi) worms do not result from inappropriate fusion of the seam with the hypodermis, on the basis that scm::gfp-expressing cells are never observed in the hypodermal syncytium [27]. However, here we argue that cells do indeed fuse with hyp7 in elt-1(RNAi) animals, but first lose their scm::gfp and AJM-1 boundary, as well as changing their morphology as they undergo the transition from seam to hypodermal fate and begin to express hypodermal markers. Thus, we suggest that the breaks in AJM-1::GFP result not from degeneration of the seam cells but are the result of a transition between two cell fates; the proliferative seam fate, which is associated with the AJM-1::GFP boundary, and the differentiated syncytial hyp7 fate, which is not.
In terms of its bro-1-independent role in the seam, elt-1 appears to function upstream of EFF-1. The role of the fusogen eff-1 is critical in the seam [30] and is responsible for promoting the fusion of hypodermal seam daughters with the hyp7 syncytium by causing the formation of pores in the membrane [35]. Indeed, eff-1 over-expression has been shown to result in inappropriate fusion of seam cells with hyp7 [36]. This phenotype is strikingly similar to that seen in elt-1(RNAi) animals, and suggests that ELT-1 acts to repress eff-1 in the seam, thereby preventing seam cells from fusing with hyp7. Our finding that ectopic eff-1 expression is observed in elt-1(RNAi) animals confirms this.
Inappropriate fusion of the seam cells with the hypodermis has been reported previously; this phenomenon has been observed in embryos and newly hatched animals in which elt-5 and elt-6 (which act redundantly) had been knocked down by RNAi [29]. Moreover, similar to the observations described here for elt-1(RNAi) animals, fusion in the case of elt-5/elt-6 RNAi was accompanied by gradual dissolution of the AJM-1::GFP boundary around the seam cells [29]. We therefore suggest that there is a network of GATA factors acting to prevent inappropriate differentiation of seam cells throughout development. In addition, other transcription factors have been shown to regulate seam cell development, for example NHR-25, BAF-1 and CEH-16 as well as heterochronic regulators like LIN-14 and LIN-29 [14], [15], [28], [37]–[39]. The interactions between all these genes will be an interesting area for future study.
The apparent close relationship between the presence of intact apical junctions and the stem cell-like properties of seam cells is suggestive of similarities with stem cells in Drosophila. In the testes and ovaries of Drosophila, germline stem cells (GSCs) are retained in what has been termed a niche; the niche concept, introduced over 30 years ago [40], describes how stem cells can be maintained in a proliferative state by signals from a microenvironment, consisting of cells and the extracellular components they produce. The niche is essential for the stem properties of such cells and, as these cells move out of the niche, so they lose these properties in favour of differentiated cell fates. In this way, far from merely creating an inert environment in which the Drosophila GSCs reside, the cells around these stem cells provide cues which regulate the maintenance of the stem cell pool, physically anchor the GSCs to the niche, and even control the polarity of the stem cells, determining the positions of the daughters of GSC divisions relative to one another and to the niche. The mechanisms underlying the complex interactions between GSCs and their niche microenvironment involves extracellular signalling [41]–[45] as well as physical adhesion of stem cells to the niche [46], with the DE-cadherin and Armadillo/β-catenin apical junction complex being both important in recruiting GSCs to the niche and required for the maintenance of the stem cell pool. Loss of either of these proteins results in dramatic depletion of GSCs from the niche. Here, we show that C. elegans apical junction proteins are required to maintain the undifferentiated stem cell-like fate of the seam cells.
Perhaps there is something analogous to a seam stem cell “niche” in C. elegans, in the sense that cell contacts (marked by, and dependent on, apical junction proteins) are required to maintain a microenvironment in which the seam cells are prevented from differentiation. The importance of cellular contacts for seam development has previously been recognized. For example, the developmental fate of the V5 seam cell has been shown to be dependent on correct seam cell contacts either side [47], and proliferation of the seam cells has been shown to be perturbed when contacts between seam cells are not properly re-established following division [15]. Thus, both the proliferation and differentiation of the seam cells has been shown to be dependent on signals from the surrounding microenvironment, raising the question of whether they do in fact reside in a niche. In support of the niche concept, we find that cells that have withdrawn from the proliferation programme as a result of asymmetric division, but which have failed to fuse with the hyp7 syncytium (as a result of eff-1 mutation), do not express markers of differentiation like dpy-7. In other words, the boundary provides protection from differentiation signals. However, this notion of a niche has to remain speculative in the absence of defining the nature of these signals.
Intriguingly, the regulation we have discussed in seam cells, in which ELT-1 represses eff-1 in order to prevent fusion and differentiation of cells that have the proliferative fate, mirrors the situation in vulval precursor cells (VPCs). In the developing vulva, the 6 VPCs P3.p–P8.p are prevented from fusing with the hyp7 syncytium in early larval stages (in L3 this exclusion from hyp7 is limited to P5.p–P7.p) [48]. Fusion with hyp7 acts to limit the developmental potential of Pn.p cells (as it does with anterior seam daughters) and is restricted to those that flank the developing vulva [48]. LIN-39 acts to prevent this fusion by repressing eff-1 in P3.p–P8.p during L1 and in P5.p–P7.p during L3 [49]–[51]. In eff-1 mutants, unfused VPCs fail to differentiate into the hypodermal fate, retaining their AJM-1 boundary and at least some aspects of the vulval fate. These cells fail to proliferate, however, suggesting that other signals are required for the induction of normal vulval development. This is analogous to the situation with unfused seam cells in eff-1 mutants, which also fail to divide once they leave the seam line, remaining in developmental “limbo”. In both cases, however, fusion with hyp7 and associated breakdown of cell boundaries is required for cells to take on the differentiated hypodermal fate. In both the seam and the developing vulva, therefore, only those cells that are prevented from fusing with hyp7 (thereby retaining their boundaries) are protected from differentiation and retain further developmental and proliferative potential.
Overall, we present a model (Figure 7) in which the GATA factor ELT-1 plays important dual roles in maintaining the ‘stemness’ of the seam cells, by both promoting the proliferative fate and preventing differentiation. Firstly, ELT-1 acts directly through bro-1 to promote proliferation and self-renewal of the seam. Secondly, ELT-1 is essential for maintaining the integrity of the seam cell compartment, as marked by apical junctions. In fulfilling this latter role, ELT-1 works through EFF-1. When eff-1 is repressed, the boundaries around the seam cells are maintained, and thus differentiation is prevented. We also find that apical junction components themselves are important for maintaining seam cell fate, but are not arguing that ELT-1 acts directly on apical junction components. Indeed, as has been previously suggested, apical junction breakdown could be a relatively late event in the cell fusion process [35], [52]. Taken together, these data suggest that the seam cells reside in a microenvironment in which they are protected from differentiation by the boundary that separates them from the hyp7 syncytium. Thus, the seam microenvironment may satisfy the criteria of a niche in certain respects, protecting seam cells from influences that would otherwise trigger differentiation. The GATA factor ELT-1 works through bro-1 to promote seam cell proliferation and through eff-1 to maintain seam cells in the undifferentiated state.
Materials and Methods
Strains and maintenance of worms
All strains used were derived from the wild type N2 Bristol strain. Manipulations and maintenance of strains were performed as previously described [53]. Strains used are described in Table S1.
Lineage analysis and microscopy
Lineage analysis was performed as previously described [12]. For lineage analysis of elt-1(RNAi) animals, 3 µl of a freshly prepared solution of M9 and HT115 E. coli cells expressing the elt-1 dsRNA (scraped from a 2 mM IPTG NGM plate, on which they had been growing at 20°C for several days) was placed on the pad. For each slide, a single worm was transferred into this drop with an eyelash pick. A coverslip was then slowly lowered on top of the worm, and microscopy performed with Nomarski (DIC) optics and a 100× oil immersion objective (Zeiss). Photomicrographs were taken using a 63× Zeiss oil immersion objective and Axiovision software (Release 4.5).
RNAi
elt-1 knockdown by RNAi was performed as described previously [27], using an identical feeding construct to pPM88, named pAW565. The seam specific RNAi strategy is described in Text S1 and all other RNAi was performed by feeding as previously described [54].
Plasmid construction
Plasmids used in transgenic strains are described in Table S1 and detailed cloning strategies described in Text S1.
Construction of transgenic worms
Injections were performed as described previously [55] using the unc-119+ (pDP#MM016β) transformation marker [56]. Constructs were injected at 10–20 ng/µl.
Band shift experiments
cDNAs of elt-1, elt-2, elt-3, elt-5 and elt-6 were amplified from a mixed stage cDNA preparation using Phusion polymerase (Finnzymes). The PCR products were cloned into the pCR®-XL-TOPO vector (Invitrogen) and the TNT® Quick Coupled Transcription/Translation kit (Promega) was then used for in vitro transcription and translation. To make the labelled probes, oligonucleotides covering ‘GATA site B’ were synthesised (WT probes: CB204 gatccgacaagattacaatccacat, CB206 atgtggattgtaatcttgtcggatc; mutant probes: CB205 gatccgacaatagtacaatccacat; CB207 atgtggattgtactattgtcggatc), annealed by heating and gradual cooling, and labelled with [γ-32P] dATP (for hot WT probes) or without [γ -32P] dATP (for cold, competitor probes). The DNA binding reaction was carried out on ice for 30 minutes before the reaction mixture was loaded onto a 7% non-denaturing polyacrylamide gel and run at 4°C in 0.5×TBE.
Yeast one-hybrid screen
The entire 122 bp bro-1 CNE was used as bait in the yeast one-hybrid screen; three copies were inserted in the forward direction into the Clontech Matchmaker vectors pHisi-1 and pLacZi and integrated into yeast strain YM4271 [57] as described in Text S1. YM4271 [pbro-1HIS; pbro-1LAC] was then transformed with a mixed stage transcription factor cDNA library and plated onto –his, -leu, -ura 15 mM 3-AT SD plates. All colonies that grew within 3 days were assayed for lacZ expression [58], and after re-isolating plasmids and re-checking, positive clones were sequenced.
Quantitative RT-PCR analysis
qRT-PCR was performed on synchronised worms obtained by bleaching gravid animals and seeding the eggs onto elt-1 RNAi or L4440 control plates. Larvae were harvested after 1–2 days by washing with M9. RNA was extracted by the hot phenol method [59] and mRNA levels of elt-1/bro-1 and a normaliser (nuo-2, expressed in all seam cells) were assessed using SYBR-Green and a Qiagen Rotor-Gene Q machine. Expression levels were assayed by the 2−ΔΔCT method [60].
Supporting Information
Zdroje
1. WangQStacyTBinderMMarin-PadillaMSharpeAH 1996 Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc Natl Acad Sci U S A 93 3444 3449
2. OkudaTvan DeursenJHiebertSWGrosveldGDowningJR 1996 AML1, the Target of Multiple Chromosomal Translocations in Human Leukemia, Is Essential for Normal Fetal Liver Hematopoiesis. Cell 84 321 330
3. ChenMJYokomizoTZeiglerBMDzierzakESpeckNA 2009 Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature 457 887 891
4. OttoFThornellAPCromptonTDenzelAGilmourKC 1997 Cbfa1, a Candidate Gene for Cleidocranial Dysplasia Syndrome, Is Essential for Osteoblast Differentiation and Bone Development. Cell 89 765 771
5. KomoriTYagiHNomuraSYamaguchiASasakiK 1997 Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89 755 764
6. HassanMQGordonJARBelotiMMCroceCMvan WijnenAJ 2010 A network connecting Runx2, SATB2, and the miR-23a∼27a∼24-2 cluster regulates the osteoblast differentiation program. Proc Natl Acad Sci U S A 107 19879 19884
7. InoueKOzakiSShigaTItoKMasudaT 2002 Runx3 controls the axonal projection of proprioceptive dorsal root ganglion neurons. Nat Neurosci 5 946 954
8. LevanonDBettounDHarris-CerrutiCWoolfENegreanuV 2002 The Runx3 transcription factor regulates development and survival of TrkC dorsal root ganglia neurons. Embo Journal 21 3454 3463
9. BlythKCameronERNeilJC 2005 The RUNX genes: gain or loss of function in cancer. Nat Rev Cancer 5 376 387
10. KagoshimaHNimmoRSaadNTanakaJMiwaY 2007 The C. elegans CBF-beta homologue BRO-1 interacts with the RUNX factor, RNT-1, to promote stem cell proliferation and self-renewal. Development 134 3905 3915
11. XiaDZhangYHuangXSunYZhangH 2007 The C. elegans CBF-beta homolog, BRO-1, regulates the proliferation, differentiation and specification of the stem cell-like seam cell lineages. Dev Biol 309 259 272
12. NimmoRAntebiAWoollardA 2005 mab-2 encodes RNT-1, a C. elegans RUNX homologue essential for controlling cell proliferation in a stem cell-like developmental lineage. Development 132 5043 5054
13. SulstonJEHorvitzHR 1977 Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol 56 110 156
14. HuangXTianEXuYZhangH 2009 The C. elegans engrailed homolog ceh-16 regulates the self-renewal expansion division of stem cell-like seam cells. Dev Biol 333 337 347
15. SilhankovaMJindraMAsahinaM 2005 Nuclear receptor NHR-25 is required for cell-shape dynamics during epidermal differentiation in Caenorhabditis elegans. J Cell Sci 118 223 232
16. EisenmannDM 2011 C. elegans seam cells as stem cells: Wnt signaling and casein kinase I alpha regulate asymmetric cell divisions in an epidermal progenitor cell type. Cell Cycle 10 20 21
17. GleasonJEEisenmannDM 2010 Wnt signaling controls the stem cell-like asymmetric division of the epithelial seam cells during C. elegans larval development. Dev Biol 348 58 66
18. RenHZhangH 2010 Wnt signaling controls temporal identities of seam cells in Caenorhabditis elegans. Dev Biol 345 144 155
19. Albert HubbardEJ 2007 Caenorhabditis elegans germ line: A model for stem cell biology. Developmental Dynamics 236 3343 3357
20. NimmoRSlackF 2009 An elegant miRror: microRNAs in stem cells, developmental timing and cancer. Chromosoma 118 405 418
21. MizumotoKSawaH 2007 Two bs or not two bs: regulation of asymmetric division by β-catenin. Trends in Cell Biology 17 465 473
22. NottinghamWTJarrattABurgessMSpeckCLChengJ-F 2007 Runx1-mediated hematopoietic stem-cell emergence is controlled by a Gata/Ets/SCL-regulated enhancer. Blood 110 4188 4197
23. HobertO 2002 PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques 32 728 730
24. MatysVFrickeEGeffersRGosslingEHaubrockM 2003 TRANSFAC: transcriptional regulation, from patterns to profiles. Nucl Acids Res 31 374 378
25. EvansTReitmanMFelsenfeldG 1988b An erythrocyte-specific DNA binding factor recognizes a regulatory sequence common to all chicken globin genes. Proc Natl Acad Sci U S A 85 5976 5980
26. SakaiYNakagawaRSatoRMaedaM 1998 Selection of DNA Binding Sites for Human Transcriptional Regulator GATA-6. Biochem Biophys Res Comm 250 682 688
27. SmithJAMcGarrPGilleardJS 2005 The Caenorhabditis elegans GATA factor elt-1 is essential for differentiation and maintenance of hypodermal seam cells and for normal locomotion. J Cell Sci 118 5709 5719
28. AmbrosVHorvitzHR 1984 Heterochronic mutants of the nematode Caenorhabditis elegans. Science 226 409 416
29. KohKRothmanJH 2001 ELT-5 and ELT-6 are required continuously to regulate epidermal seam cell differentiation and cell fusion in C. elegans. Development 128 2867 2880
30. MohlerWAShemerGdel CampoJJValansiCOpoku-SerebuohE 2002 The Type I Membrane Protein EFF-1 Is Essential for Developmental Cell Fusion. Dev Cell 2 355 362
31. QadotaHInoueMHikitaTKoppenMHardinJD 2007 Establishment of a tissue-specific RNAi system in C. elegans. Gene 400 166 173
32. RuvinskyIRuvkunG 2003 Functional tests of enhancer conservation between distantly related species. Development 130 5133 5142
33. MarriSGuptaBP 2009 Dissection of lin-11 enhancer regions in Caenorhabditis elegans and other nematodes. Dev Biol 325 402 411
34. WaltzerLFerjouxGBatailleLHaenlinM 2003 Cooperation between the GATA and RUNX factors Serpent and Lozenge during Drosophila hematopoiesis. EMBO J 22 6516 6525
35. MohlerWASimskeJSWilliams-MassonEMHardinJDWhiteJG 1998 Dynamics and ultrastructure of developmental cell fusions in the Caenorhabditis elegans hypodermis. Current Biology 8 1087 1091
36. ShemerGSuissaMKolotuevINguyenKCQHallDH 2004 EFF-1 is sufficient to initiate and execute tissue-specific cell fusion in C. elegans. Cur Biol 14 1587 1591
37. ChenZEastburnDJHanM 2004 The Caenorhabditis elegans Nuclear Receptor Gene nhr-25 Regulates Epidermal Cell Development. Mol Cell Biol 24 7345 7358
38. MargalitANeufeldEFeinsteinNWilsonKLPodbilewiczB 2007 Barrier to autointegration factor blocks premature cell fusion and maintains adult muscle integrity in C. elegans. J Cell Biol 178 661 673
39. CassataGShemerGMorandiPDonhauserRPodbilewiczB 2005 ceh-16/engrailed patterns the embryonic epidermis of Caenorhabditis elegans. Development 132 739 749
40. SchofieldR 1978 The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 4 7 25
41. XieTSpradlingAC 2000 A niche maintaining germ line stem cells in the Drosophila ovary. Science 290 328 330
42. XieTSpradlingAC 1998 decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell 94 251 260
43. MatunisETranJGonczyPCaldwellKDiNardoS 1997 punt and schnurri regulate a somatically derived signal that restricts proliferation of committed progenitors in the germline. Development 124 4383 4391
44. KigerAAJonesDLSchulzCRogersMBFullerMT 2001 Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science 294 2542 2545
45. KaiTSSpradlingA 2003 An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proc Natl Acad Sci U S A 100 4633 4638
46. SongXZhuC-HDoanCXieT 2002 Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science 296 1855 1857
47. AustinJKenyonC 1994 Cell contact regulates neuroblast formation in the Caenorhabditis elegans lateral epidermis. Development 120 313 323
48. ClarkSGChisholmADHorvitzHR 1993 Control of cell fates in the central body region of C. elegans by the homeobox gene lin-39. Cell 74 43 55
49. Ch'ngQKenyonC 1999 egl-27 generates anteroposterior patterns of cell fusion in C. elegans by regulating Hox gene expression and Hox protein function. Development 126 3303 3312
50. GleasonJEKorswagenHCEisenmannDM 2002 Activation of Wnt signaling bypasses the requirement for RTK/Ras signaling during C. elegans vulval induction. Genes & Development 16 1281 1290
51. MaloofJNKenyonC 1998 The Hox gene lin-39 is required during C. elegans vulval induction to select the outcome of Ras signaling. Development 125 181 190
52. GattegnoTMittalAValansiCNguyenKCQHallDH 2007 Genetic Control of Fusion Pore Expansion in the Epidermis of Caenorhabditis elegans. Mol Biol Cell 18 1153 1166
53. SulstonJEHodgkinJ 1988 Methods. WoodWB The nematode Caenorhabditis elegans Cold Spring Harbor Laboratory Press 587 606
54. KamathRSAhringerJ 2003 Genome-wide RNAi screening in Caenorhabditis elegans. Methods RNA interference 30 313 321
55. MelloCFireA 1995 DNA transformation. Methods Cell Biol 48 451 482
56. MaduroMPilgrimD 1995 Identification and cloning of unc-119, a gene expressed in the Caenorhabditis elegans nervous system. Genetics 141 977 988
57. LiuJWilsonTEMilbrandtJJohnstonM 1993 Identifying DNA-Binding Sites and Analyzing DNA-Binding Domains Using a Yeast Selection System. Methods 5 125 137
58. BreedenLNasmythK 1985 Regulation of the yeast HO gene. Cold Spring Harb Symp Quant Biol 50 643 650
59. FurgerAMonksJProudfootNJ 2001 The retroviruses Human Immunodeficiency Virus Type 1 and Moloney Murine Leukemia Virus adopt radically different strategies to regulate promoter-proximal polyadenylation. J Virol 75 11735 11746
60. LivakKJSchmittgenTD 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25 402 408
61. GilleardJSShafiYBarryJDMcGheeJD 1999 ELT-3: A Caenorhabditis elegans GATA Factor Expressed in the Embryonic Epidermis during Morphogenesis. Dev Biol 208 265 280
Štítky
Genetika Reprodukční medicína
Článek The T-Box Factor MLS-1 Requires Groucho Co-Repressor Interaction for Uterine Muscle SpecificationČlánek B Chromosomes Have a Functional Effect on Female Sex Determination in Lake Victoria Cichlid FishesČlánek Distinct Cdk1 Requirements during Single-Strand Annealing, Noncrossover, and Crossover RecombinationČlánek Specification of Corpora Cardiaca Neuroendocrine Cells from Mesoderm Is Regulated by Notch SignalingČlánek Ongoing Phenotypic and Genomic Changes in Experimental Coevolution of RNA Bacteriophage Qβ and
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 8
-
Všechny články tohoto čísla
- Polo, Greatwall, and Protein Phosphatase PP2A Jostle for Pole Position
- Genome-Wide Association Analysis of Incident Coronary Heart Disease (CHD) in African Americans: A Short Report
- The T-Box Factor MLS-1 Requires Groucho Co-Repressor Interaction for Uterine Muscle Specification
- B Chromosomes Have a Functional Effect on Female Sex Determination in Lake Victoria Cichlid Fishes
- Analysis of DNA Methylation in a Three-Generation Family Reveals Widespread Genetic Influence on Epigenetic Regulation
- PP2A-Twins Is Antagonized by Greatwall and Collaborates with Polo for Cell Cycle Progression and Centrosome Attachment to Nuclei in Drosophila Embryos
- Discovery of Sexual Dimorphisms in Metabolic and Genetic Biomarkers
- Pervasive Sharing of Genetic Effects in Autoimmune Disease
- DNA Methylation and Histone Modifications Regulate Shoot Regeneration in by Modulating Expression and Auxin Signaling
- Mutations in and Reveal That Cartilage Matrix Controls Timing of Endochondral Ossification by Inhibiting Chondrocyte Maturation
- Variance of Gene Expression Identifies Altered Network Constraints in Neurological Disease
- Frequent Beneficial Mutations during Single-Colony Serial Transfer of
- Increased Gene Dosage Affects Genomic Stability Potentially Contributing to 17p13.3 Duplication Syndrome
- Distinct Cdk1 Requirements during Single-Strand Annealing, Noncrossover, and Crossover Recombination
- Hunger Artists: Yeast Adapted to Carbon Limitation Show Trade-Offs under Carbon Sufficiency
- Suppression of Scant Identifies Endos as a Substrate of Greatwall Kinase and a Negative Regulator of Protein Phosphatase 2A in Mitosis
- Temporal Dynamics of Host Molecular Responses Differentiate Symptomatic and Asymptomatic Influenza A Infection
- MK2-Dependent p38b Signalling Protects Hindgut Enterocytes against JNK-Induced Apoptosis under Chronic Stress
- Specification of Corpora Cardiaca Neuroendocrine Cells from Mesoderm Is Regulated by Notch Signaling
- Genome-Wide Gene-Environment Study Identifies Glutamate Receptor Gene as a Parkinson's Disease Modifier Gene via Interaction with Coffee
- Identification of Functional Toxin/Immunity Genes Linked to Contact-Dependent Growth Inhibition (CDI) and Rearrangement Hotspot (Rhs) Systems
- Genomic Analysis of the Necrotrophic Fungal Pathogens and
- Celsr3 Is Required for Normal Development of GABA Circuits in the Inner Retina
- Genetic Architecture of Aluminum Tolerance in Rice () Determined through Genome-Wide Association Analysis and QTL Mapping
- Predisposition to Cancer Caused by Genetic and Functional Defects of Mammalian
- Regulation of p53/CEP-1–Dependent Germ Cell Apoptosis by Ras/MAPK Signaling
- and but Not Interact in Genetic Models of Amyotrophic Lateral Sclerosis
- Gamma-Tubulin Is Required for Bipolar Spindle Assembly and for Proper Kinetochore Microtubule Attachments during Prometaphase I in Oocytes
- Ongoing Phenotypic and Genomic Changes in Experimental Coevolution of RNA Bacteriophage Qβ and
- Genetic Architecture of a Reinforced, Postmating, Reproductive Isolation Barrier between Species Indicates Evolution via Natural Selection
- -eQTLs Reveal That Independent Genetic Variants Associated with a Complex Phenotype Converge on Intermediate Genes, with a Major Role for the HLA
- The GATA Factor ELT-1 Works through the Cell Proliferation Regulator BRO-1 and the Fusogen EFF-1 to Maintain the Seam Stem-Like Fate
- and Control Optic Cup Regeneration in a Prototypic Eye
- A Comprehensive Map of Mobile Element Insertion Polymorphisms in Humans
- An EMT–Driven Alternative Splicing Program Occurs in Human Breast Cancer and Modulates Cellular Phenotype
- Evidence for Hitchhiking of Deleterious Mutations within the Human Genome
- A Broad Brush, Global Overview of Bacterial Sexuality
- Global Chromosomal Structural Instability in a Subpopulation of Starving Cells
- A Pre-mRNA–Associating Factor Links Endogenous siRNAs to Chromatin Regulation
- Glutamine Synthetase Is a Genetic Determinant of Cell Type–Specific Glutamine Independence in Breast Epithelia
- The Repertoire of ICE in Prokaryotes Underscores the Unity, Diversity, and Ubiquity of Conjugation
- Genome-Wide Association Analysis of Autoantibody Positivity in Type 1 Diabetes Cases
- Natural Polymorphism in BUL2 Links Cellular Amino Acid Availability with Chronological Aging and Telomere Maintenance in Yeast
- Chromosome Painting Reveals Asynaptic Full Alignment of Homologs and HIM-8–Dependent Remodeling of Chromosome Territories during Meiosis
- Ku Must Load Directly onto the Chromosome End in Order to Mediate Its Telomeric Functions
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- An EMT–Driven Alternative Splicing Program Occurs in Human Breast Cancer and Modulates Cellular Phenotype
- Chromosome Painting Reveals Asynaptic Full Alignment of Homologs and HIM-8–Dependent Remodeling of Chromosome Territories during Meiosis
- Discovery of Sexual Dimorphisms in Metabolic and Genetic Biomarkers
- Regulation of p53/CEP-1–Dependent Germ Cell Apoptosis by Ras/MAPK Signaling
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



