-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaGlutamine Synthetase Is a Genetic Determinant of Cell Type–Specific Glutamine Independence in Breast Epithelia
Although significant variations in the metabolic profiles exist among different cells, little is understood in terms of genetic regulations of such cell type–specific metabolic phenotypes and nutrient requirements. While many cancer cells depend on exogenous glutamine for survival to justify the therapeutic targeting of glutamine metabolism, the mechanisms of glutamine dependence and likely response and resistance of such glutamine-targeting strategies among cancers are largely unknown. In this study, we have found a systematic variation in the glutamine dependence among breast tumor subtypes associated with mammary differentiation: basal - but not luminal-type breast cells are more glutamine-dependent and may be susceptible to glutamine-targeting therapeutics. Glutamine independence of luminal-type cells is associated mechanistically with lineage-specific expression of glutamine synthetase (GS). Luminal cells can also rescue basal cells in co-culture without glutamine, indicating a potential for glutamine symbiosis within breast ducts. The luminal-specific expression of GS is directly induced by GATA3 and represses glutaminase expression. Such distinct glutamine dependency and metabolic symbiosis is coupled with the acquisition of the GS and glutamine independence during the mammary differentiation program. Understanding the genetic circuitry governing distinct metabolic patterns is relevant to many symbiotic relationships among different cells and organisms. In addition, the ability of GS to predict patterns of glutamine metabolism and dependency among tumors is also crucial in the rational design and application of glutamine and other metabolic pathway targeted therapies.
Published in the journal: . PLoS Genet 7(8): e32767. doi:10.1371/journal.pgen.1002229
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002229Summary
Although significant variations in the metabolic profiles exist among different cells, little is understood in terms of genetic regulations of such cell type–specific metabolic phenotypes and nutrient requirements. While many cancer cells depend on exogenous glutamine for survival to justify the therapeutic targeting of glutamine metabolism, the mechanisms of glutamine dependence and likely response and resistance of such glutamine-targeting strategies among cancers are largely unknown. In this study, we have found a systematic variation in the glutamine dependence among breast tumor subtypes associated with mammary differentiation: basal - but not luminal-type breast cells are more glutamine-dependent and may be susceptible to glutamine-targeting therapeutics. Glutamine independence of luminal-type cells is associated mechanistically with lineage-specific expression of glutamine synthetase (GS). Luminal cells can also rescue basal cells in co-culture without glutamine, indicating a potential for glutamine symbiosis within breast ducts. The luminal-specific expression of GS is directly induced by GATA3 and represses glutaminase expression. Such distinct glutamine dependency and metabolic symbiosis is coupled with the acquisition of the GS and glutamine independence during the mammary differentiation program. Understanding the genetic circuitry governing distinct metabolic patterns is relevant to many symbiotic relationships among different cells and organisms. In addition, the ability of GS to predict patterns of glutamine metabolism and dependency among tumors is also crucial in the rational design and application of glutamine and other metabolic pathway targeted therapies.
Introduction
There are a large number of differentiated cell types in the human body. Even among the cells collectively known as fibroblasts [1], endothelial [2] and smooth muscle cells [3], gene expression analysis has identified an unexpected level of positional memory and topographic differentiation. Such functional specialization contributes to the phenotypic variations of many human diseases, including cancer. For example, gene expression analysis of breast cancers has identified five intrinsic subtypes (luminal A, luminal B, basal, HER2+, and normal-like) with unique clinical and histological properties [4], [5]. The classification nomenclature is based on the putative progenitor cell(s) for breast carcinogenesis with properties consistent with derivation from the basal and luminal epithelia arrested at specific differentiation stages or from different mature epithelial cells [4]–[7]. Importantly, these subtype-specific gene expression and phenotypic variations are also observed in many breast cancer cell lines with similar molecular phenotypes [8]–[11]. A number of studies have isolated the different populations of primary epithelial cells to investigate their relevant cellular origins and metabolic features for different breast cancer types [7], [12], [13]. Although the cellular origin of luminal and basal-like breast tumor has not been resolved [14], [15], cell lineage still appears to confer an important source of patterned heterogeneity to the disease.
Although gene expression analysis has yielded important insights into the cellular differentiation and various properties associated with tumors from different cell types, very little is known about the corresponding metabolic phenotypes and nutrient requirements. The processes of oncogenic transformation place energy demands on cancer cells to support proliferation, expansion, and invasion. Dysregulated tumor metabolism is a critical part of oncogenesis and may be targeted for therapeutic benefits [16], [17]. One prominent example of dysregulated tumor metabolism is “aerobic glycolysis” as recognized by Otto Warburg [18]. Most normal mammalian cells shift to glycolysis for energy generation when oxygen is inadequate for effective oxidative phosphorylation under hypoxia. But tumor cells tend to favor glycolysis even with the availability of oxygen, hence termed “aerobic glycolysis” [19]. Such preferential use of glycolysis leads to vigorous glucose uptake and explains the ability of the tracer glucose analog Fluorine-18 (F-18) FDG to image human cancers in FDG-PET. Such understanding of altered metabolism and nutrient requirement in cancer cells may allow us to exploit these differences for diagnostic and therapeutic benefits.
Another aspect of dysregulated tumor metabolism is manifested as altered requirements for amino acids. For example, patients with acute lymphocytic leukemia (ALL) benefit from asparaginase treatment as the leukemic cells require large amounts of exogenous asparagine due to a deficiency in this metabolic pathway [20]. Recently, evidence is also accumulating for the essential role of glutamine for cancer cells as a building block for protein synthesis, to supply cellular ATP, as a metabolic intermediate for nucleotide synthesis, and for its anti-oxidative capacity [21], [22]. Such glutamine dependence or addiction is reflected in the growth restriction and cell death in glutamine limiting conditions. The glutamine addiction is also critical for c-myc-mediated oncogenesis [23]–[25], linked with glucose requirement [26], and proposed as an attractive target for therapeutic intervention [22], [27].
The catabolism of glutamine is initiated by glutaminolysis mediated by two different subtypes of mitochondrial glutaminase (kidney or liver-type encoded by GLS or GLS2 respectively) to become glutamate [28]. The intracellular pool of glutamate is a versatile metabolic intermediate that connects with a wide variety of distinct biological processes including synthesis of the anti-oxidant glutathione, amino acid catabolism through transamination, and conversion to α-ketoglutarate as a substrate for the TCA cycle. This process of glutaminolysis by glutaminases has been shown to mediate signaling events [29], to be coupled with c-myc oncogenesis [25], and proposed as a critical step in targeting glutamine metabolism [24], [27]. In some cell types, glutamine can be generated from intracellular glutamate through glutamine synthetase (GS, encoded by GLUL, glutamate-ammonia ligase) catalyzing the reverse reaction of the glutaminases. This process is important for removal of ammonia or glutamate depending on the cellular context [30]. While glutaminase is known as an important regulator of glutamine requirement, few studies have focused on glutamine synthetase as a potential determinant of glutamine requirement. Although normal glutamine metabolism is well understood, the genetic parameters and mechanisms of variation in this key nutrient pathway among tumors are largely unknown.
Deprivation of glutamine and other amino acids triggers a canonical amino acid response (AAR) in most mammalian cells that is measurable by gene expression changes [31]. The free and uncharged t-RNA associated with glutamine deprivation activates a serine/threonine-protein kinase GCN2 which phosphorylates eIF2α and inhibits cap-dependent translation [32]. While reducing the global translation rate, eIF2α phosphorylation also preferentially increases the translation of ATF4 and other mRNAs [31]. The increased level of ATF4 protein triggers the AAR gene expression program characterized by the induction of XBP1 and DDIT3 as an adaptive response to amino acid deprivation. The importance of the AAR is demonstrated by the fact that deficiency of ATF4 compromises the AAR and renders cells susceptible to amino acid deprivation and oxidative stresses [33].
Through the analysis of how different breast cancer cells respond to glutamine deprivation, we have found a dramatic difference in the glutamine requirement among different breast cancer cells which tracks with the luminal versus basal type. These metabolic differences can be explained by cell-type specific expression of glutamine-metabolizing genes and enzymes likely acting in concert with cell type specific oncogenic programs. Therefore, we have provided a series of fundamental building blocks to understand how differentiation is coupled with distinct glutamine utilization in normal and neoplastic breast epithelia. Such an understanding will be relevant to both the mechanistic understanding of metabolic phenotypes and present insights into how best to select subsets of breast cancer patients most likely to benefit from glutamine-targeting therapies.
Results
Breast cancer cells exhibit subtype-specific phenotype of glutamine dependence
Many cancer cells require glutamine for survival and proliferation and thus exhibit a phenotype of “glutamine dependence” or “addiction” [22]. To determine whether such phenotypes could be also found in breast cancer cells, we tested how glutamine deprivation affected seven different breast cancer cell lines. Consistent with the idea of glutamine dependence, three cell lines (BT20, MDAMB231, and MDAMB157) had significantly reduced growth (MTT assay, Figure 1A) and prominent cell death (trypan blue exclusion assay, Figure 1B) upon glutamine deprivation for 48 h. Unexpectedly, glutamine deprivation had only modest effects on the growth and viability (Figure 1A, 1B) of the other four cell lines (T47D, BT474, MCF7, and MDAMB361) indicating relative glutamine independence. When we examined the properties associated with the distinct need for glutamine, we found the cell lines that exhibit glutamine dependence are all of the basal-type whereas the four lines that are more glutamine independent are luminal-type cells (Figure 1A, 1B) [34].
Fig. 1. Glutamine addiction phenotypes among different breast cancer cell lines. 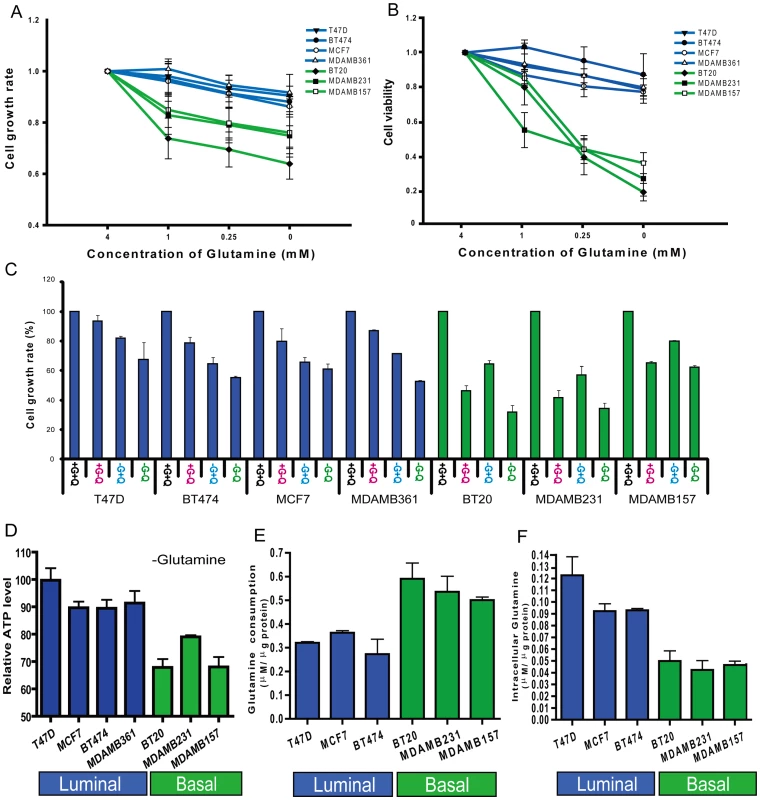
(A, B) The normalized cell growth (MTT assay) (A) and viability (trypan blue exclusion assay) (B) of seven indicated breast cancer cell lines (luminal-type: blue, basal-type: green) at different glutamine concentrations. (C) The normalized cell growth of the seven indicated cell lines under control (+G+Q), glutamine deficient (+G-Q), glucose deficient (-G+Q) and glucose/glutamine deficient condition (-G-Q). (D) The percentage of reduction in normalized cellular ATP of the indicated cell lines when cultured in glutamine deficient media for the indicated breast cancer cell lines. (E, F) The glutamine consumption (E) and the intracellular glutamine concentration (F) of the indicated breast cancer cell lines grown in regular media. As glucose and glutamine are two important energy sources for cancer cells we compared how deprivation of glutamine and glucose affected the growth of these breast cell lines. In the three basal-type cell lines, glutamine depletion had a stronger effect on cell growth than glucose depletion (Figure 1C). In contrast, glucose depletion had a more dramatic influence on cell growth than glutamine depletion in the four luminal cell lines (Figure 1C). These results suggested that there is a consistent variation in glutamine phenotype associated with cell lineage in breast cancers.
One important function of glutamine is to serve as an energy source in generating cellular ATP. To determine the relative importance of glutamine to ATP generation in the breast cancer cell lines, we measured ATP in cells grown in media containing either normal levels of glutamine (4mM) or no glutamine for 12 hours. Glutamine deprivation led to a much more significant reduction in ATP generation in the basal-type cells than the luminal-type breast cancer cell lines (Figure 1D). These results further support the concept that glutamine is a more important energy source in basal than luminal breast cell lines.
To further analyze glutamine metabolism among different cell types, we measured the consumption of glutamine in the medium and intracellular glutamine levels. When compared with luminal-type cells, the basal cell lines had significantly higher levels of glutamine consumption (Figure 1E) and lower intracellular glutamine concentrations (Figure 1F). Collectively, these data strongly support the concept of distinct glutamine metabolism and varying dependence for external glutamine between basal and luminal type breast cancer cells.
Differential expression of glutamine-metabolizing enzymes in the basal and luminal breast cancer cell lines
We hypothesized that such distinct glutamine dependence among basal and luminal breast cancer cell lines may be caused by variable expression of key enzymes involved in glutamine metabolism. Glutamine synthetase (GS encoded by GLUL – glutamate-ammonia ligase) and glutaminase (GLS – kidney form or GLS2 – liver form) mediate the opposite reaction in the reversible conversion between glutamate and glutamine. GS mediates the capture of an ammonia group by glutamate to synthesize glutamine, while glutaminase catalyzes the breakdown of glutamine to glutamate. We first examined the RNA expression of these genes in a microarray expression set [34] and found that the expression of GLUL (GS) was significantly higher in the luminal cell lines. In contrast, the expression of GLS (glutaminase, kidney) was higher in the basal lines. While lacking GLS expression, the luminal breast cell lines have a higher level of GLS2 (Figure 2A). We confirmed this cell-type specific differential mRNA expression of GLUL, GLS and GLS2 with real-time PCR (Figure 2B, 2C, and 2D). Differential expression was also found at the protein level as shown by the western blots for GLUL (GS) and GLS2 (in luminal cells) and GLS (in basal cells) (Figure 2E).
Fig. 2. Differential expression of genes encoding glutamine-metabolizing enzymes in the basal and luminal breast cancer cell lines. 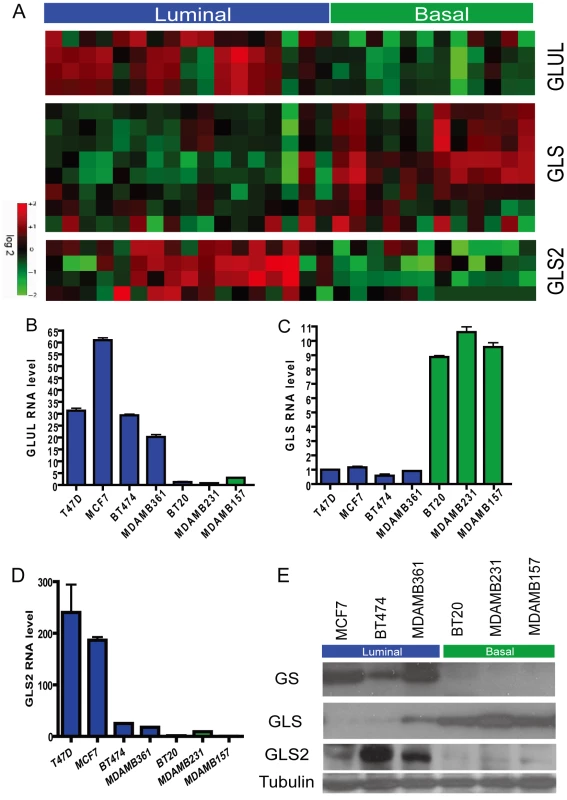
(A) The heatmap showing the expression levels of probesets for GLUL, GLS and GLS2 in the microarray data of indicated breast cancer cell lines known to be of luminal (blue) and basal (green) type. (B, C, D) The levels of mRNA expression of GLUL (B), GLS (C) and GLS2 (D) of the indicated luminal and breast cell lines determined with real-time PCR. (E) The levels of protein products of GLUL, GLS, and GLS2 in the indicated luminal and basal breast cell lines determined by Western blots. Differential expression of glutamine-metabolizing enzymes in primary human breast cancers
We next examined whether the expression patterns of GLUL, GLS and GLS2 found in luminal and basal cell lines were also reflected in the respective subtypes of primary human breast cancers. In a breast tumor expression dataset [35], we found significantly different expression levels of GLUL, GLS and GLS2 in the corresponding luminal (luminal A and B) and basal-types of breast tumors (Figure 3A). We also examined the expression levels of these three genes in the same dataset within the 5 intrinsic subtypes [35] and found significantly different expression between the luminal A and basal tumors (Figure S1). This concordance indicates the differential expression of GLUL, GLS and GLS2 in the luminal and basal-type cancer cell lines reflects similar systematic differences in primary breast tumors.
Fig. 3. Persistent differential expression of genes encoding glutamine-metabolizing enzymes in the basal and luminal breast tumors and primary epithelial cells. 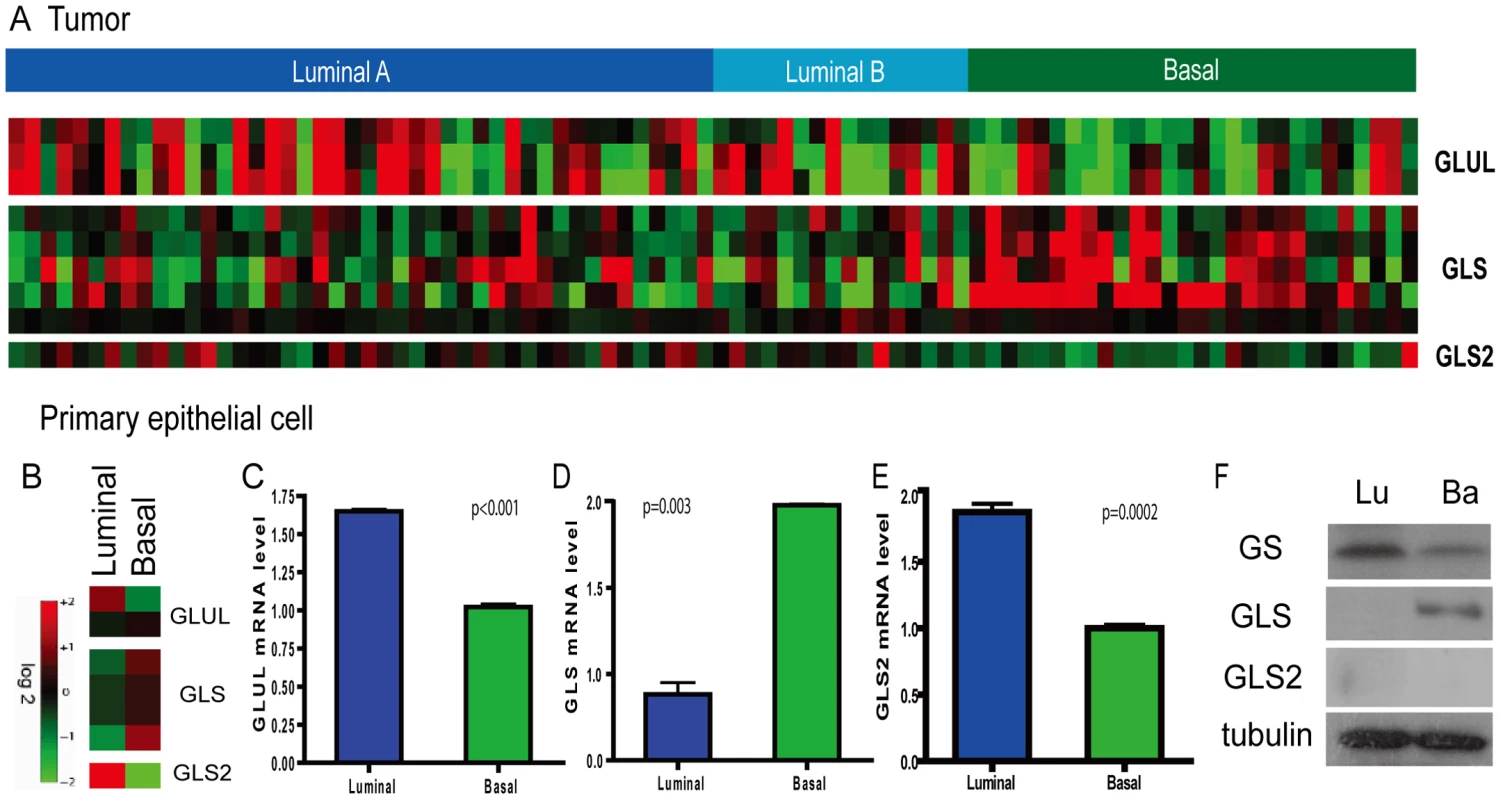
(A) The average expression levels were shown for the, GLS and GLS2 in the luminal and basal breast tumors. (B) The heatmap showing the expression levels of probesets for GLUL, GLS and GLS2 in the basal and luminal epithelial cells. (C, D, E) The expression levels determined by real-time PCR were shown for the GLUL (C), GLS (D), and GLS2 (E) in the primary luminal and basal breast epithelial cells. (F) The levels of protein products of GLUL and GLS in the basal and luminal breast epithelial cells by Western blots. Lineage-specific expression of glutamine-metabolizing enzymes in primary breast epithelia
To determine whether differential expression of genes driving glutamine metabolism is an intrinsic cell-lineage phenomenon in the breast, we examined their expression levels in normal non-transformed basal and luminal epithelial cells. Primary luminal and basal breast epithelial cells were separated based on surface expression of EPCAM (TACSTD1) from reduction mammoplasty specimens and gene expression levels were determined by microarray analysis [12]. Analysis of isogenic basal and luminal epithelial cells showed that the mRNA levels of GLUL, GLS and GLS2 exhibited similar cell-type specific expression in normal breast cells (Figure 3B). These findings were also confirmed by real-time PCR (Figure 3C–3E). In addition, expression of the GLUL (GS) and GLS proteins showed corresponding luminal and basal-specific expression patterns (Figure 3F). The level of GLS2 protein was below detection levels in both primary epithelial cells (Figure 3F). These results suggest that differential expression of glutamine metabolizing enzymes in cancers may be ascribed to systematic differences in cell lineage observed in normal basal and luminal epithelial cells.
Luminal-specific GLUL expression as a determinant of glutamine independence
Given the well-recognized glutamine dependency of many cancer cells, we investigated the roles of GLUL and GLS2 in the relative glutamine independence of the luminal-type cells. We first treated cells with a GS inhibitor (L-MS [36]) for 48 h and measured cell viability under glutamine deprivation. We found that L-MS reduced the survival of the luminal cell lines but had no statistically significant effect over glutamine starvation on all three tested basal cell lines (Figure 4A) indicating that GS is involved in the glutamine independence of the luminal cells.
Fig. 4. Contribution of luminal expression of GLUL and GATA3 to the glutamine-independence phenotype. 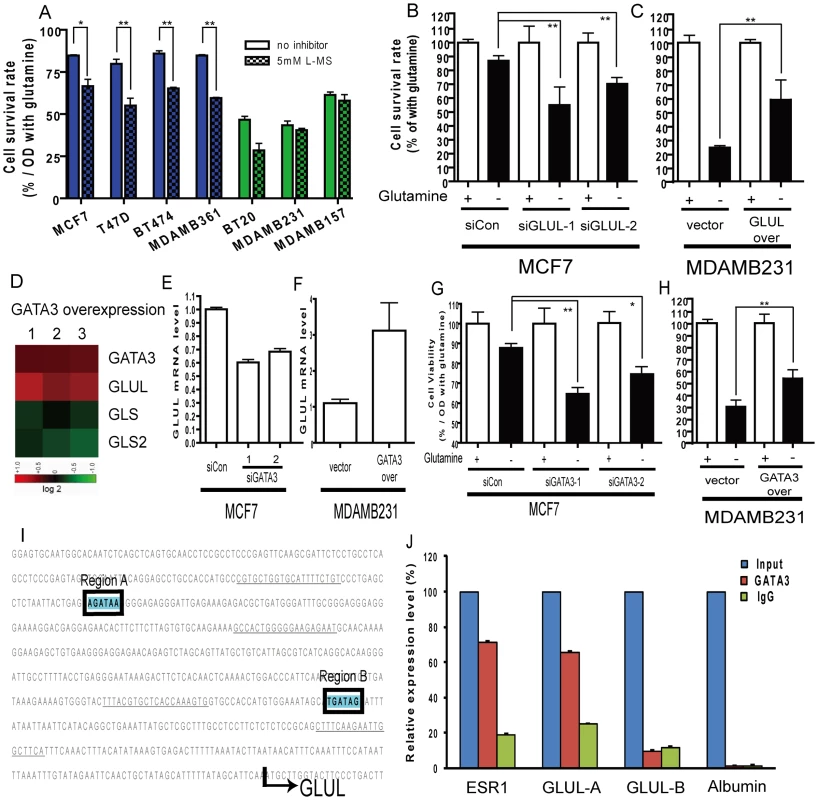
(A) The normalized cell survival of breast cancer cell lines (luminal-type: blue, basal-type: green) with or without treatment with GS inhibitor, L-MS, in the absence of glutamine. (B) The degree of cellular survival under glutamine deprivation for MCF7 (luminal cell) treated with either control or two siRNAs targeting GLUL. (C) The degree of cellular survival under glutamine deprivation for MDAMB231 (basal cell) transfected with either control vector or GLUL overexpression construct. (D) The changes of GLUL, GLS and GLS2 in the mouse mammary epithelia cells transfected with GATA3 from array analysis derived from an independent study [38]. (E) The levels of GLUL in MCF7 cells treated with either control or siRNA targeting GATA3. (F) The levels of GLUL in the MDAMB231 cells transfected with either control vectors or GATA3 expression constructs. (G) The relative survival under glutamine deprivation of MCF7 cells treated with control or two independent siRNAs targeting GATA3. (H) The cell survival rates shown in MDAMB231 cells with overexpression of control vector or GATA3 under glutamine deprivation. (I) The promoter regions of the GLUL with two potential binding sites of GATA3 are shown. (The sequences underlined indicate primer locations.) (J) The enrichment of different promoter regions of GLUL, ER (positive control) and albumin (negative control) which have been immunoprecipitated with GATA3 and control IgG antibodies. (All statistical comparisons: *p<0.05, **p<0.01) Next, we performed genetic experiments to examine the role of specific genes in the glutamine phenotype. Silencing of GLUL (encoding GS) in the luminal MCF7 line significantly reduced the RNA and protein expression of GS (Figure S2A and S2B) and led to a significant reduction in glutamine independence (Figure 4B). In contrast, similar silencing of GLS2 did not affect survival under glutamine deprivation (Figure S3). In addition, the ectopic overexpression of GLUL (verified in Figure S4A, S4B) in the basal MDAMB231 cells conferred partial glutamine independence by significantly increasing the cell survival under glutamine deprivation (Figure 4C). Taken together, these data suggest that GS expression significantly contributes to the differential glutamine phenotypes observed in breast cancer cell lines.
GATA3 regulates the luminal-specific expression of GS and glutamine independence
We next investigated potential regulatory mechanisms for the subtype-specific expression of glutamine metabolizing enzymes. During the differentiation of luminal epithelial cells, GATA3 is an important master regulatory transcription factor [37], [38]. The expression of GATA3 in luminal and basal cells is systematically different as previously noted [11], [39]. Using real-time PCR, we also demonstrated the cell-type specific expression of GATA3 mRNA in MCF7 (luminal) and MDAMB231 (basal) cells (Figure S5A). Re-analysis of microarray data of the overexpression of GATA3 in mouse breast epithelial cells [38] shows induction of GLUL and repression of GLS and GLS2 (Figure 4D). These data suggested a role for the lineage factor GATA3 in regulating the luminal and basal-specific expression of GLUL and GLS.
We directly tested the role of GATA3 in regulating the glutamine phenotype in breast cancer cell lines. The mRNA and protein levels of GATA3 could be effectively reduced by gene silencing through siRNAs (Figure S5B, S5C). Silencing of GATA3 in MCF7 cells led to significant reduction in GLUL at both the RNA and protein levels (Figure 4E, Figure S5C). Conversely, overexpression of GATA3 in the basal MDAMB231 line (Figure S5D) led to a significant upregulation of GLUL (Figure 4F, Figure S5E). Furthermore, the silencing of GATA3 in MCF7 cells reduced the survival under glutamine deprivation (Figure 4G, Figure S3), and overexpression of GATA3 in MDAMB231 cells increased the resistance to glutamine deprivation (Figure 4H), consistent with a direct role for GATA3 mediated GLUL expression in the glutamine independence of luminal breast cells. In addition, the glutamine independence caused by GLUL (Figure S6A) or GATA3 (Figure S6B) overexpression in MDAMB231 cells was also abolished with treatment of L-MS (GS inhibitor), indicating of the importance of the catalytic activities of GS.
GATA3 directly binds to the promoter of GLUL
Given the ability of GATA3 to increase the expression of GLUL, we examined the promoter region of GLUL and found two potential GATA3 binding sites at −524 to −518 bp (region A) and −200 to −194 bp (region B) upstream of the transcriptional start site (Figure 4I). We used chromatin immunoprecipitation (ChIP) to test whether GLUL may be a direct downstream target of GATA3 transactivation. Consistent with previous data [40], the promoters of ESR1 (estrogen receptor alpha), but not albumin, were enriched in the GATA3 ChIP samples. Of the two putative GATA3 binding sites in the GLUL promoter, the distal region A but not the more promoter proximal region B, was significantly enriched in the GATA3 ChIP samples (Figure 4J) indicating that GATA3 protein can directly bind to a regulatory region of GLUL suggesting that this gene is a target of the luminal transcription factor and further serving to explain the lineage specific requirement for glutamine.
Cell type–specific transcriptional responses to glutamine deprivation
The deprivation of amino acids in mammalian cells leads to the stabilization of the ATF4 (activating transcription factor 4) protein and resulting induction of a canonical gene expression program known as the amino acid response (AAR) [41]. The response includes the induction of XBP1 (X-box binding protein 1) and DDIT3 (DNA-damage-inducible transcript 3) which are essential for survival under amino acid deprivation [41]. Given the distinct growth and survival response of luminal and basal breast cells to glutamine deprivation, we used microarrays to compare their transcriptional responses on a global scale. Triplicate plates of MCF7 and MDAMB231 cells were cultured under both control (4 mM glutamine/Q4) and glutamine-depleted (no glutamine/Q0) conditions for 24 hours. RNA from each plate was interrogated with Affymetrix GeneChip U133-A2 arrays (results deposited in Gene Expression Omnibus (GSE26370)). Gene expression profiles of the 12 arrays were normalized by RMA and the transcriptional changes of glutamine deprivation in both cell types were derived by zero-transformation against the average expression levels of the control samples as performed previously [42]–[44]. Probes sets showing at least two fold changes in at least two samples (n = 405) were selected and arranged by hierarchical clustering according to similarities in expression patterns (Figure 5A). This analysis showed that glutamine deprivation induced a strong gene expression response in MDAMB231 (MB231) but less so in MCF7 cells (Figure 5A). We found that the canonical AAR genes were induced by glutamine deprivation only in MDAMB231 cells (Figure 5A). A previous study showed that glutamine deprivation inhibits cell growth by inducing the tumor suppressor gene TXNIP [29]. This gene was also induced only in the MDAMB231 line. We applied a published gene expression study of histidine deprivation [45] as training data and estimated the degree of AAR using a binary regression model. MDAMB231 but not the MCF7 line exhibited a significantly higher probability of AAR after glutamine deprivation using this approach (Figure 5B and 5C). The stronger amino acid response in the MDAMB231 cells was also confirmed by real-time PCR for XBP1 (Figure 5D) and DDIT3 (Figure 5E). These data provide further evidence that glutamine deprivation induces a much dramatic response in the basal cells and a weak response correlating with glutamine independence of the luminal cells.
Fig. 5. The transcriptional response of breast cancer cell lines to glutamine deprivation. 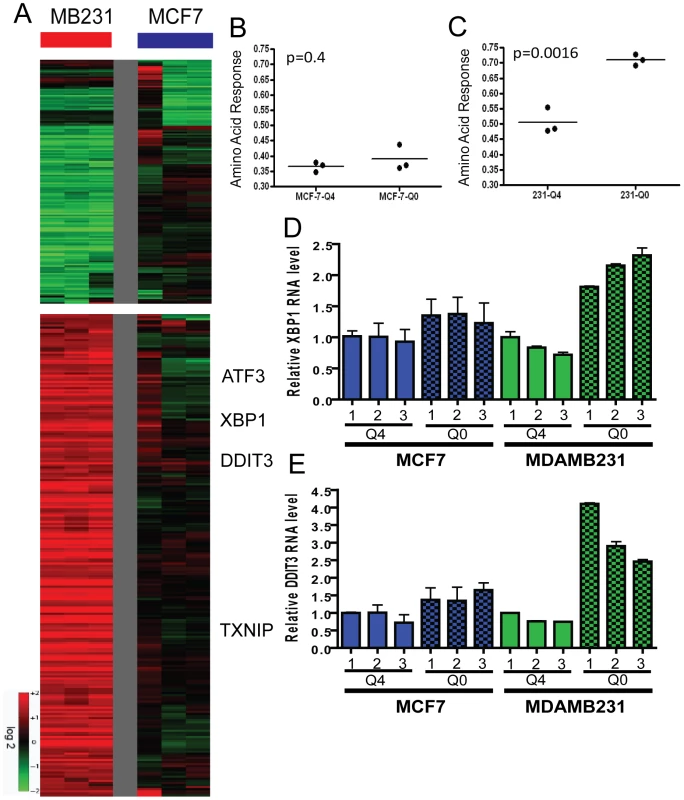
(A) The heatmap representing the transcriptional response of MCF7 (luminal) and MDAMB231 (basal) cells to glutamine deprivation. (B, C). The predictive probability of the amino acid response (AAR) are shown for the luminal (B, p = 0.4, unpaired t-test) and basal (C, p = 0.0016) cancer cell lines grown under normal (4 mM, Q4) and glutamine-deficient (0 mM, Q0) medium. (D, E) The expression of selected canonical amino acid response genes including XBP1 (D) and DDIT3 (E) in MCF7 and MDAMB231 under indicated concentrations of glutamine with real time RT-PCR. Potential glutamine symbiosis between luminal and basal types of cells
We examined how glutamine deprivation affected different glutamine-metabolizing enzymes and found that GS protein (Figure 6A), but not mRNA (Figure S7A), were significantly induced in MCF7 cells in a dosage-dependent manner. This translational regulation may be an adaptive response to compensate for reduced environmental levels of glutamine. To examine the role of GATA3 in the induction of GS during glutamine deprivation, we compared the GS protein levels under different glutamine levels in MCF-7 transfected with control or GATA3-targeting siRNA. We found that while the silencing of GATA3 reduced the GS levels, there was still significant protein induction during glutamine deprivation (Figure S7B). We also measured glutamine concentrations in glutamine deficient media used to culture MCF7 and MDAMB231 cells and found a significant increase in glutamine levels in medium cultured with MCF7 but not MDAMB231 cells (Figure 6B). Similarly, intracellular glutamine concentrations were increased only in MCF7 but not MDAMB231 cells under glutamine deprivation (Figure 6C). Therefore, the glutamine independence phenotype of luminal cells may be due to the capacity of these cells to synthesize glutamine from intracellular glutamate and other sources in the absence of external glutamine.
Fig. 6. Potential for glutamine symbiosis between luminal and basal cells. 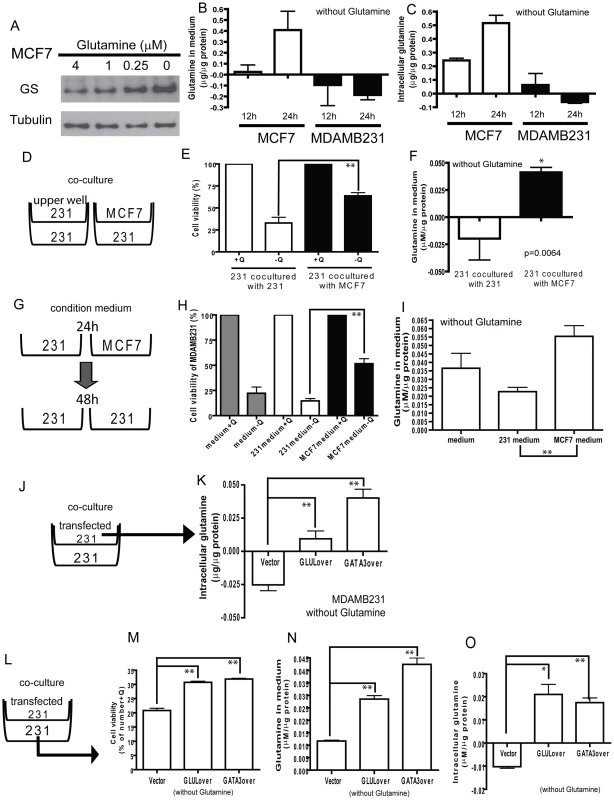
(A) The protein levels of GS in MCF7 under different concentrations of glutamine. (B, C) The changes of the levels of glutamine in medium (B) and intracellular glutamine concentrations (C) in MCF7 and MDAMB231 cells deprived of glutamine for 12 and 24 h. (D) A diagram illustrating the co-culture systems in E, F. (E, F) The cell viability (E) and the glutamine levels in medium (F) when used to propagate MDAMB231 cells co-cultured with MDAMB231 or MCF7. (G) A diagram illustrating the condition medium model for H, I. (H, I) The cell viability (H) and the glutamine in medium (I) in MDAMB231 cells cultured with fresh medium, MDAMB231 or MCF7 condition medium. (J) A diagram illustrating the model of K. (K) The intracellular glutamine in vector transfected MDAMB231 cells. (L) A diagram illustrating the model of co-culture system for M-O. (M-O) The cell viability (M), the glutamine in medium (N), and intracellular glutamine concentrations (O) in MDAMB231 co-cultured with transfected MDAMB231 cells. In normal breast ducts, luminal and basal cells are in close physical proximity. Because of the ability of luminal cells to synthesize glutamine and the requirement of basal cells for glutamine, we next tested the potential for glutamine symbiosis between these two cell types with transwell co-culturing experiments (Figure 6D–6F). We found that the viability of MDAMB231 cells under glutamine deficient media was significantly increased when MCF7 cells were used as a feeder layer (Figure 6E), consistent with observed higher extracellular glutamine levels (Figure 6F). Furthermore, conditioned medium from MCF7 cells was also able to support significantly the growth and viability of the MDAMB231 cells (Figure 6G–6I).
We showed above that increased levels of GLUL either by transfection with GLUL or GATA3 makes the MDAMB231 line more resistant to glutamine deprivation (Figure 4C and 4H). We next asked whether this was due to increased synthesis of the nutrient. Intracellular glutamine levels increased dramatically in MDAMB231 cells expressing either GLUL or GATA3 (5×104 cells in the upper well) (Figure 6J, 6K). The effects of GLUL and GATA3 overexpression in MDAMB231 cells on intracellular glutamine levels were blocked with L-MS treatment (Figure S8A). We also showed that the intracellular glutamine levels were reduced in MCF7 with siRNAs targeted to GLUL or GATA3 in medium with normal glutamine level (Q4) or no glutamine (Q0) (Figure S8B). Further, in the co-culture system (Figure 6L), MDAMB231 cells demonstrated increased viability when co-cultured with either GLUL or GATA3 expressing MDAMB231 cells (Figure 6M) and this correlated with both increased glutamine levels in the medium (Figure 6N) and intracellularly (Figure 6O). These data provide a consistent mechanistic picture of a gene expression program related to nutrient requirements and potential glutamine symbiosis.
GLUL repression of GLS contributes to the cell type–specific expression of glutamine-metabolizing enzymes
The expression of GLUL and GLS are inversely correlated in the luminal and basal types of primary breast cancers, cancer cell lines, and primary epithelial cells. This pattern of expression made us investigate whether cross-regulation exists between these two genes that encode enzymes mediating directly opposite chemical reactions. We first used siRNA to silence GLUL in MCF7 cells and observed an increase in GLS mRNA expression (Figure 7A). Further, ectopic over-expression of GLUL in MDAMB231 cells reduced GLS mRNA (Figure 7B). In contrast, similar silencing of GLS did not show any effect on GLUL levels (Figure 7C, 7D). The ability of GLUL overexpression in MDAMB231 to repress GLS was also seen at the protein level with a dose dependent decrease in GLS protein observed with increasing amounts of GS protein from varying levels of transfected GLUL (Figure 7E). These results indicated the ability of GLUL to repress the expression of GLS while GLS had no detectable effect on the level of GLUL.
Fig. 7. Repression of GLS by GLUL contributes to the polarized expression pattern. 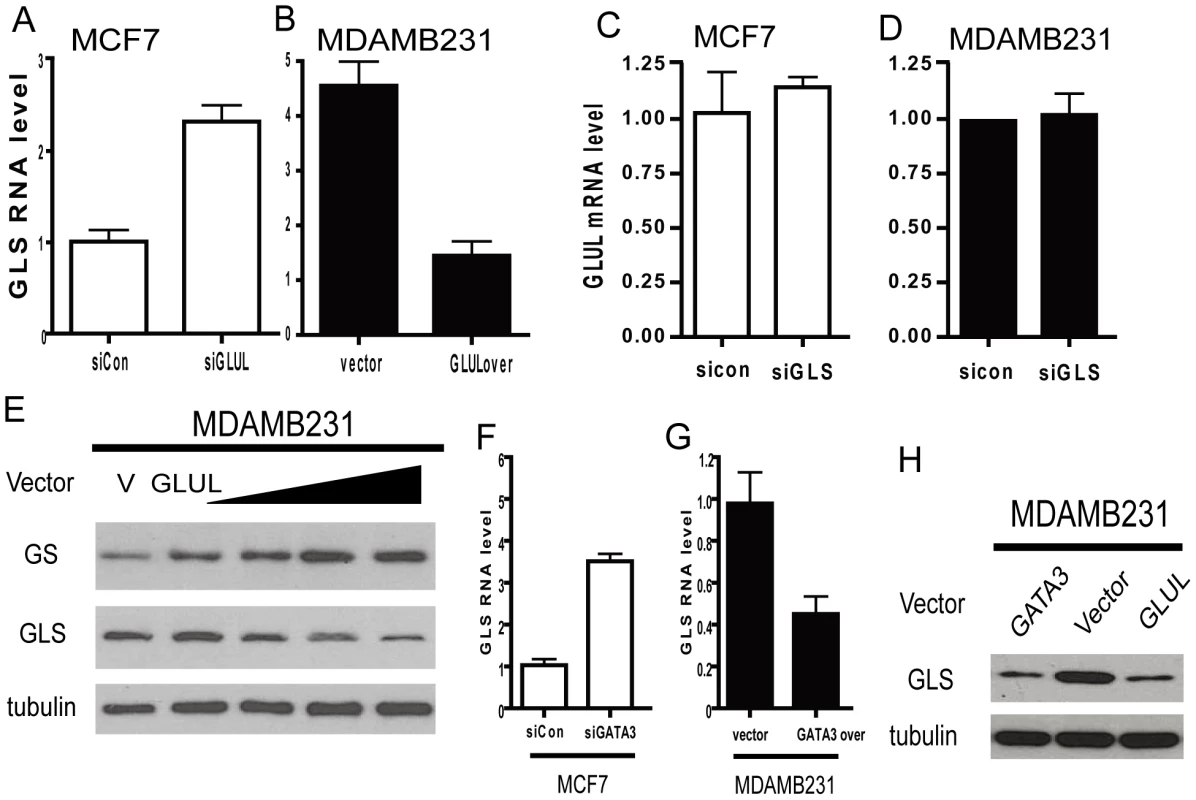
(A, B) The mRNA expression levels of GLS in the indicated MCF7 (luminal, empty) and MDAMB231 (basal, solid) when treated with indicated siRNAs or overexpression constructs. (C, D) The GLUL RNA expression levels in MCF7 (luminal, empty) and MDAMB231 (basal, solid) when treated with siRNA targeted to GLS. (E) GLUL and GLS protein expression levels in MDAMB231 treated with GLUL expression vector. (F, G) The expression of GLS mRNA in MCF7 (luminal, empty) and MDAMB231 (basal, solid) treated with indicated siRNAs or expression construct of GATA3. (H) The GLS protein expression levels in MDAMB231 cells with indicated expression constructs. Since GATA3 regulated the expression of GLUL, we tested the role of GATA3 in regulating GLS by silencing and overexpressing GATA3 in MCF7 and MDAMB231 cells, respectively. Silencing of GATA3 in MCF7 cells increased GLS expression (Figure 7F) and GATA3 overexpression in MDAMB231 significantly reduced the level of GLS (Figure 7G). These changes in GLS expression regulated by GATA3 were also detectable at the protein level compared with GLUL (Figure 7H). These results are also consistent with GATA3 overexpression in the mouse epithelial cells (Figure 4D) [38].
A proposed model for the regulation of glutamine dependence in breast cells
Based on the data presented, we propose that basal and luminal breast epithelial cells exhibit different patterns of glutamine metabolism (Figure 8). In the luminal cells, GATA3 triggers expression of GLUL and contributes to glutamine independence. Furthermore, GLUL has the ability to repress GLS which would also help to maintain the cell-type specific expression pattern and phenotype. Basal-specific expression of GLS may be maintained by the absence of GATA3 and higher activities of c-myc in the basal type cells [4], [46]. These findings suggest that glutamine deprivation may be a viable treatment strategy for basal-type breast cancers. In addition, the expression of GLUL in luminal type cancers correlates with the ability to synthesize glutamine from ammonia and glutamate, and therefore describes at the molecular level a type of cancer that is predicted to be more resistant to glutamine deprivation treatment.
Fig. 8. A model of glutamine metabolic regulation in different breast cells. 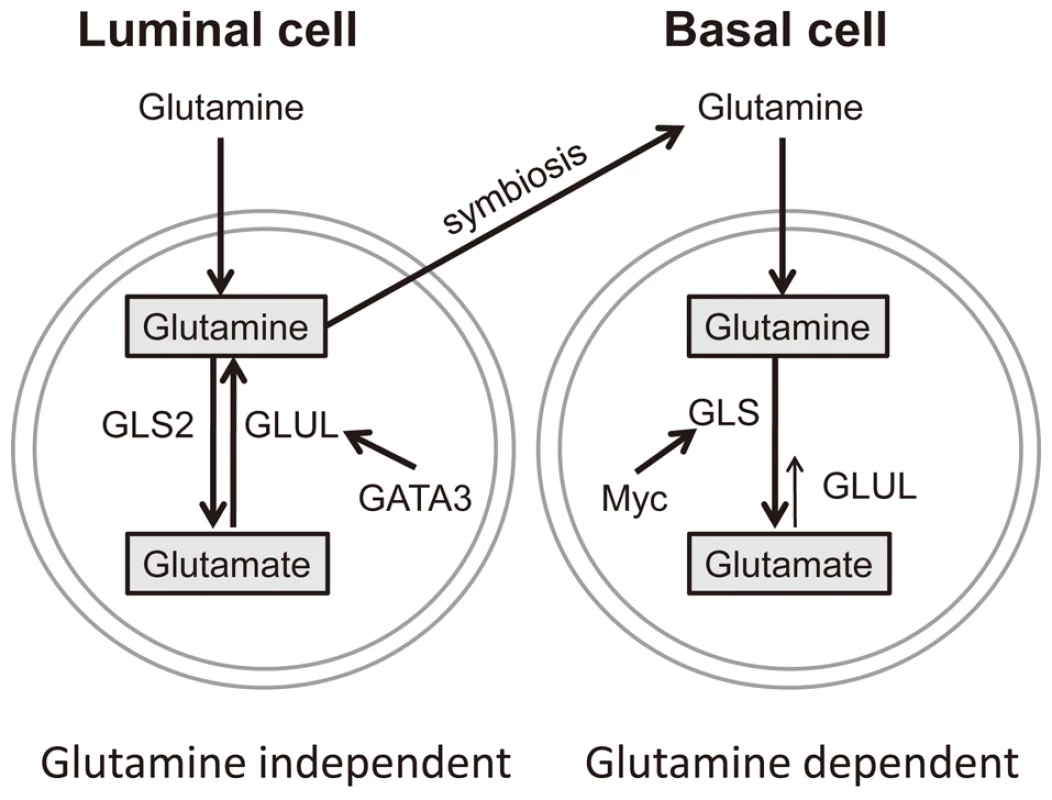
The regulatory mechanisms of glutamine metabolic enzymes based on data from luminal and basal breast cells. Discussion
While glutamine has been shown to be critical in many cancer types, its importance for breast cancers is not well defined. In this study, we found a cell lineage-specific variation in the response of basal and luminal breast cancer cells to glutamine deprivation. The basal-type breast cancer cells are dependent on glutamine and exhibit a phenotype of glutamine addiction. Such a phenotype of basal cells was previously reported to be sensitive to inhibitors of glutaminase [27], trans-amination by aspartate aminotransferase [47] and selective estrogen receptor modulators [48]. In contrast, the luminal-type breast cancer cells are much more glutamine-independent. We present a series of data which strongly suggest that this phenotypic difference is related to the luminal-specific expression of glutamine synthetase (GS encoded by the GLUL gene) which is in turn regulated by one of the key luminal transcription factors, GATA3. Further, GS itself represses the expression of glutaminase (GLS) to reinforce the metabolic pathway in the direction of glutamine synthesis in luminal breast cells and the potential for glutamine symbiosis with basal breast cells.
While variations in tumor metabolism can be caused by oncogenic events, our results highlight the importance of the differentiation status and cellular origins as a source of distinct metabolic patterns. Since differentiation state constitutes an important part of tumor heterogeneity, similar investigation into distinct metabolic needs may yield important information on how best to target tumor metabolism. As the induction of differentiation is an important component of some cancer therapeutics [49], such treatment-associated differentiation may also lead to changes in metabolic needs and may be exploited to enhance treatment efficacy. Similarly, the distinct nutrient requirements of tumor stem cells [50] may be used to target these unique populations which are more resistant to conventional cancer therapeutics.
Genetic regulation of the cell type–specific expression of glutamine-metabolizing enzymes
The distinct glutamine requirement and differential expression of glutamine-metabolizing enzymes among luminal and basal breast cancers are consistent with our understanding of the genetic circuitry governing breast cancer subtypes and regulation of these glutamine-metabolizing enzymes. For example, the higher GLS level and sensitivity to glutamine deprivation of basal-type breast cancer cells are consistent with a high level of c-myc activity in basal cells [51], [52] and the recently described role of c-myc in regulating GLS [24], [25]. The higher levels of GLS are also consistent with the susceptibility to growth inhibition by targeting this enzyme [27] and indicate the essential nature of this metabolic pathway in the basal cells. These results indicate that triple-negative basal-like breast tumors, with few current therapeutic options, are addicted to glutamine and may benefit from glutamine-targeting therapies [22], [27]. In contrast, the luminal specific expression of GLS2 may reflect the higher p53 (wild type) activity in luminal cells [4], [52] given the ability of p53 to regulate GLS2 [53], [54]. Our results suggest that GATA3 is directly involved in the transcriptional regulation of GLUL in luminal cells.
The spatial and cell-type expression of glutamine synthetase and glutaminase
The spatial and cell type specific expression of GLUL (GS) seen in our studies on breast epithelial cells is also observed in several other cellular contexts. In the brain, GS is expressed mainly in glial cells [55] and is responsible for the synthesis of glutamine from the uptake of glutamate secreted by adjacent neurons. Similar spatial division of glutamine degradation and synthesis also occurs in distinct patterns of GS and GLS expression in the liver [56]. Glutamine degradation by GLS occurs in the periportal cells where there is a high glutamine level from the digested nutrients in the gastrointestinal tract. In contrast, the expression of GLUL (GS) is restricted to zones of hepatocytes surrounding the central lobular vein with lower glutamine levels [56]. In the renal nephron, GLUL (GS) expression is restricted to the straight portion of the proximal tubules and plays an important role in the removal of ammonia [57]. Such physical separation of glutaminase and glutamine synthetase associated with differentiation and nutrient availability coordinate the glutamine synthesis and effective detoxification of ammonia and glutamate. Similar distinct glutamine metabolism in luminal and basal breast epithelial cells also appears to impact tumors derived from these different lineages and opens an additional window into the metabolic phenotypes of this heterogeneous disease. Therapeutic interventions based upon metabolic targets will need to incorporate these systematic differences between tumor subtypes.
Inter-cellular metabolic symbiosis
Under glutamine deprivation, the high mRNA levels of GLUL (GS) in luminal cells undergo further protein upregulation to provide glutamine and may also support the glutamine requirement in basal cells in physical proximity. Similar nutritional and metabolic interaction underlies many symbiotic relationships among different organisms and cell types, including the symbiotic nitrogen-fixing root nodules on legumes [58], the mutualistic symbiosis between bacteria and insects [59], and the glutamate-glutamine shuttle between neurons and astroglial cells in the brain [55], [60]. Interestingly, GS plays a critical role in all these diverse examples of metabolic symbiosis. In addition to the inter-cellular exchange of nutrients, the synthesis of glutamine from glutamate and ammonia by GS can also remove the potential toxicity from the accumulation of glutamate (neurotransmitter) and ammonia (nitrogen waste). Ammonia from glutaminolysis has been shown to act as a diffusible autocrine - and paracrine substance inducing autophagy [61]. Given the physical proximity between basal and luminal cells in breast ducts, such a reciprocal metabolic relationship may also be relevant under different environmental or growth conditions. When the tissue organization is disrupted in malignancy, glutamine dependence of the basal-type tumors may be exploited to treat this type of aggressive cancers.
The therapeutic implications
Our findings strongly suggest that there will be significant variation in response to glutamine-targeting therapies. Among breast cancers, systematic variation in the glutamine consumptive vs. synthetic behaviors seen in the basal and luminal tumors will directly influence this response. Similar heterogeneity may be important in other tumor types as well. Our data also provide evidence that glutamine-targeting therapeutics may be of special clinical utility for the triple-negative basal-like breast tumors with few therapeutic options. Many current glutamine-targeting therapeutics focus on glutaminase [25], [27], but the cell-type specific expression of GLS and GLS2 in different tumors indicates the importance of choosing compounds with intended specificity for particular glutaminase activities in the treated tumors. Since GS is a key genetic determinant of glutamine independence in luminal cells, the evaluation of the GS levels in tumors may be helpful in predicting response. In addition to the cell-autonomous variations in the GS expression and response to glutamine deprivation, the efficacy of glutamine-targeting therapies may also be affected by the ability of adjacent non-transformed cells to provide glutamine. It is important to note that GS activities have been reported in fibroblasts [62] and macrophages [63]. The availability of glutamine from other non-tumor cells or blood may reduce the efficacy of glutamine-targeting therapies. Thus, GS inhibition may be combined with glutamine-targeting therapies to further enhance efficacy and reduce resistance, similar to the use of GS inhibitors to sensitize cancer cells to L-asparaginase [64].
With the explosion of genomic data, we have obtained significant knowledge on how genetic dysregulation contributes to tumor heterogeneity in human cancers. Since dysregulated metabolism is an essential part of oncogenesis, similarly detailed knowledge of metabolic profiles may be of equal or greater importance in understanding and treating the disease [65], [66].
Materials and Methods
Cell culture
All breast cancer cells were cultured in DMEM with 4.5 g/L glucose, supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin in 5% CO2.
Primary luminal and basal cells were obtained from women undergoing breast reduction for non-malignant conditions and were separated by cell surface binding to the TACSD1 protein of the Ber-Ep4 antibody as described [12].
Cell viability assays
For the MTT assay, 2.5×103 cells in 100 µl of medium were seeded in a 96-well culture plate. After treatments, cell number was evaluated. In brief, 10 µl of MTT (Sigma M5655) (0.5 mg/ml) was added to each well, and then the plates were incubated at 37°C for 3 h. The formazan product was dissolved in DMSO, and the absorbance at 570 nm was measured using a microplate reader. To measure viability by direct counting, 2×104 cells were seeded in 12-well dishes and treated with medium containing different concentrations of glutamine for 48 h; the cells were collected and stained with 0.4% Trypan Blue. Cells excluding and taking up dye were counted on a hemocytometer under phase contrast microscopy. For glutamine synthetase inhibition, L-MS (L-Methionine-Sulfoximine, 5 mM, Sigma-Aldrich) was administered to cells for 48 h.
ATP level measurement
Cells (5×103/well of a 96 well dish) were treated with or without glutamine for 12 h and ATP content was measured in accordance with the protocol of the ATP-Lite luminescent ATP detection assay kit (Perkin-Elmer). Briefly, 100 µl of assay reagent was added to the wells and mixed for 10 min in the dark; intracellular ATP content was measured using a luminescence multi-label counter. The ATP levels were normalized based on cell counts measured by the MTT assay.
Determination of glutamine concentration
Cells (1×104/well) in a 24 well plate were cultured for 24 h in medium without phenol-red, medium was collected, and cells were lysed with RIPA buffer (Sigma-Aldrich). Concentrations of glutamine in the medium and in the cell lysate were determined with the glutamine/glutamate determination kit (GLN-1; Sigma-Aldrich). Each sample was divided into two parts; part 1 was measured with glutaminase for transferring the glutamine into glutamate, part 2 was measured directly. Samples were then dehydrogenized to α-ketoglutarate accompanied by reduction of NAD+ to NADH. The amount of NADH is proportional to the amount of glutamate and was measured using a spectrophotometer at 340 nm. A standard curve was determined for each experiment to calculate the concentration of glutamate in samples. Glutamine levels were calculated (part 1 minus part 2) and normalized to total protein levels. The glutamine level of normal culture medium was also measured, and the glutamine consumption was calculated as (glutamine in normal medium-glutamine in medium after culturing cells) and normalized to protein level.
Western blot analyses
Proteins were separated by 10–12% SDS–PAGE and transferred to Immobilon-P membranes (Millipore). Membranes were blocked with 5% skim milk, incubated with primary antibodies (GLUL, G2781, Sigma; GLS, ab60709, Abcam; GATA3, sc269, Santa Cruz; GLS2, ab91073, Abcam; tubulin, 2128, cell signaling), HRP-conjugated secondary antibody (Perkin-Elmer), and detected with the ECL Western blotting reagent (Amersham).
Microarray analysis
MCF7 and MDAMB231 cells were cultured in medium with or without glutamine for 24 h in triplicate. RNAs were collected with MirVana kit (Ambion) and hybridized to Affymetrix U133A2 arrays. Probe intensities were normalized by RMA and then the changes of expression by glutamine deprivation (0 mM glutamine/Q0) were derived by zero-transformation against the corresponding cells grown in glutamine containing medium (4 mM glutamine/Q4).
RNA interference and overexpression
Cells were transfected with non-targeting control or synthetic siRNAs targeting GLUL, GLS, GLS2 and GATA3 (Applied Biosystems) with lipofectamine 2000 (Invitrogen). For overexpression experiments, empty vector or overexpression constructs for GLUL or GATA3 (Origene) were transfected into cells with lipofectamine 2000 for 48 hours before the levels of indicated transcripts and proteins were examined by real-time RT-PCR and western blot.
Real-time RT-PCR
Total RNA was reverse-transcribed to cDNA with SuperScript II reverse transcription kit, then used for real-time PCR with Power SYBR Green PCR Mix (Applied Biosystems) and indicated primers (Table S1), and normalized to β-actin mRNA levels measured in parallel.
ChIP assay
10% formaldehyde solution was added to cells to crosslink DNA-protein complexes. Isolated nuclear chromatin extracts were sonicated and incubated overnight at 4°C with either anti-GATA3 (SC269, Santa Cruz) or normal mouse IgG (SC3878, Santa Cruz). This was followed by incubation with 20 ml of Protein G agarose beads (Roche) for 4 hours at 4°C. After extensive washing, DNA fragments were harvested by de-crosslinking the immunoprecipitates. Real time-PCR utilizing SYBR Green master mix (Applied Biosystems) was performed to check the enrichment of indicated promoter regions in pull-down samples using primers listed in Table S1 and normalized with albumin.
Co-culture and conditioned medium
For co-culture experiments, MDAMB231, MCF7, or transfected MDAMB231 cells were seeded in minicells (upper well/5×104 cells) with 0.4 µm pores (Millipore) and co-cultured with MDAMB231 (lower well/1×104 cells) for 12, 24 or 48 h in 24-well plates. For conditioned medium experiments, MDAMB231 or MCF7 cells (5×104) were seeded in a 24-well plate and incubated in medium with or without glutamine for 24 hours and then medium was transferred to new wells containing MDAMB231 cells (1×104). In 12 and 24 hours experiments, medium was collected; cells were washed by PBS and then lysed with 100 µl RIPA buffer. Glutamine concentration was measured with GLN-1 (Sigma). In 48 h experiments, cell numbers were counted by trypan blue exclusion assay.
Statistics
All experiments were expressed as mean ± standard deviation (SD) with t-test. Statistical significance was calculated by t test, considering p<0.05 (*) and p<0.01(**) as statistically significant.
Supporting Information
Zdroje
1. ChangHYChiJTDudoitSBondreCvan de RijnM 2002 Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci U S A 99 12877 12882
2. ChiJTChangHYHaraldsenGJahnsenFLTroyanskayaOG 2003 Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci U S A 100 10623 10628
3. ChiJTRodriguezEHWangZNuytenDSMukherjeeS 2007 Gene expression programs of human smooth muscle cells: tissue-specific differentiation and prognostic significance in breast cancers. PLoS Genet 3 e164 doi:10.1371/journal.pgen.0030164
4. PerouCMSorlieTEisenMBvan de RijnMJeffreySS 2000 Molecular portraits of human breast tumours. Nature 406 747 752
5. SorlieTPerouCMTibshiraniRAasTGeislerS 2001 Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 98 10869 10874
6. DairkeeSHPuettLHackettAJ 1988 Expression of basal and luminal epithelium-specific keratins in normal, benign, and malignant breast tissue. J Natl Cancer Inst 80 691 695
7. LimEVaillantFWuDForrestNCPalB 2009 Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med 15 907 913
8. RossDTPerouCM 2001 A comparison of gene expression signatures from breast tumors and breast tissue derived cell lines. Dis Markers 17 99 109
9. GatzaMLLucasJEBarryWTKimJWWangQ 2010 A pathway-based classification of human breast cancer. Proc Natl Acad Sci U S A 107 6994 6999
10. LacroixMLeclercqG 2004 Relevance of breast cancer cell lines as models for breast tumours: an update. Breast Cancer Res Treat 83 249 289
11. Charafe-JauffretEGinestierCMonvilleFFinettiPAdelaideJ 2006 Gene expression profiling of breast cell lines identifies potential new basal markers. Oncogene 25 2273 2284
12. HuperGMarksJR 2007 Isogenic normal basal and luminal mammary epithelial isolated by a novel method show a differential response to ionizing radiation. Cancer Res 67 2990 3001
13. GatzaMLKungHNBlackwellKLDewhirstMWMarksJR 2011 Analysis of tumor environmental response and oncogenic pathway activation identifies distinct basal and luminal features in HER2-related breast tumor subtypes. Breast Cancer Res 13 R62
14. Yalcin-OzuysalOBriskenC 2009 From normal cell types to malignant phenotypes. Breast Cancer Res 11 306
15. VisvaderJE 2009 Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev 23 2563 2577
16. HsuPPSabatiniDM 2008 Cancer cell metabolism: Warburg and beyond. Cell 134 703 707
17. TennantDADuranRVGottliebE 2010 Targeting metabolic transformation for cancer therapy. Nat Rev Cancer 10 267 277
18. WarburgO 1956 On respiratory impairment in cancer cells. Science 124 269 270
19. VaupelP 2004 Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol 14 198 206
20. ClavellLAGelberRDCohenHJHitchcock-BryanSCassadyJR 1986 Four-agent induction and intensive asparaginase therapy for treatment of childhood acute lymphoblastic leukemia. N Engl J Med 315 657 663
21. DeBerardinisRJChengT 2010 Q's next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 29 313 324
22. WiseDRThompsonCB 2010 Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci
23. YunevaMZamboniNOefnerPSachidanandamRLazebnikY 2007 Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol 178 93 105
24. WiseDRDeBerardinisRJMancusoASayedNZhangXY 2008 Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A 105 18782 18787
25. GaoPTchernyshyovIChangTCLeeYSKitaK 2009 c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 458 762 765
26. YangCSudderthJDangTBachooRMMcDonaldJG 2009 Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer Res 69 7986 7993
27. WangJBEricksonJWFujiRRamachandranSGaoP 2010 Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell 18 207 219
28. CurthoysNPWatfordM 1995 Regulation of glutaminase activity and glutamine metabolism. Annu Rev Nutr 15 133 159
29. KaadigeMRLooperREKamalanaadhanSAyerDE 2009 Glutamine-dependent anapleurosis dictates glucose uptake and cell growth by regulating MondoA transcriptional activity. Proc Natl Acad Sci U S A
30. NewsholmePProcopioJLimaMMPithon-CuriTCCuriR 2003 Glutamine and glutamate—their central role in cell metabolism and function. Cell Biochem Funct 21 1 9
31. KilbergMSPanYXChenHLeung-PinedaV 2005 Nutritional control of gene expression: how mammalian cells respond to amino acid limitation. Annu Rev Nutr 25 59 85
32. DongJQiuHGarcia-BarrioMAndersonJHinnebuschAG 2000 Uncharged tRNA activates GCN2 by displacing the protein kinase moiety from a bipartite tRNA-binding domain. Mol Cell 6 269 279
33. HardingHPZhangYZengHNovoaILuPD 2003 An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11 619 633
34. NeveRMChinKFridlyandJYehJBaehnerFL 2006 A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 10 515 527
35. ChinKDeVriesSFridlyandJSpellmanPTRoydasguptaR 2006 Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell 10 529 541
36. DeMarcoVDyessKStraussDWestCMNeuJ 1999 Inhibition of glutamine synthetase decreases proliferation of cultured rat intestinal epithelial cells. J Nutr 129 57 62
37. Asselin-LabatMLSutherlandKDBarkerHThomasRShackletonM 2007 Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol 9 201 209
38. Kouros-MehrHSlorachEMSternlichtMDWerbZ 2006 GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell 127 1041 1055
39. Kouros-MehrHBechisSKSlorachEMLittlepageLEEgebladM 2008 GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer Cell 13 141 152
40. EeckhouteJKeetonEKLupienMKrumSACarrollJS 2007 Positive cross-regulatory loop ties GATA-3 to estrogen receptor alpha expression in breast cancer. Cancer Res 67 6477 6483
41. KilbergMSShanJSuN 2009 ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol Metab 20 436 443
42. ChenJLLucasJESchroederTMoriSWuJ 2008 The genomic analysis of lactic acidosis and acidosis response in human cancers. PLoS Genet 4 e1000293 doi:10.1371/journal.pgen.1000293
43. ChenJLMerlDPetersonCWWuJLiuPY 2010 Lactic acidosis triggers starvation response with paradoxical induction of TXNIP through MondoA. PLoS Genet 6 e1001093 doi:10.1371/journal.pgen.1001093
44. ChiJTRodriguezEHWangZNuytenDSAMukherjeeS 2007 Gene Expression Programs of Human Smooth Muscle Cells: Tissue-Specific Differentiation and Prognostic Significance in Breast Cancers. PLoS Genet 3 e164 doi:10.1371/journal.pgen.0030164
45. ShanJLopezMCBakerHVKilbergMS 2010 Expression profiling after activation of the amino acid deprivation response in HepG2 human hepatoma cells. Physiol Genomics
46. BildAHYaoGChangJTWangQPottiA 2006 Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature 439 353 357
47. ThornburgJMNelsonKKClemBFLaneANArumugamS 2008 Targeting aspartate aminotransferase in breast cancer. Breast Cancer Res 10 R84
48. TodorovaVKKaufmannYLuoSSuzanne KlimbergV 2010 Tamoxifen and raloxifene suppress the proliferation of estrogen receptor-negative cells through inhibition of glutamine uptake. Cancer Chemother Pharmacol
49. ChangTHSzaboE 2000 Induction of differentiation and apoptosis by ligands of peroxisome proliferator-activated receptor gamma in non-small cell lung cancer. Cancer Res 60 1129 1138
50. LiZBaoSWuQWangHEylerC 2009 Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell 15 501 513
51. GrushkoTADignamJJDasSBlackwoodAMPerouCM 2004 MYC is amplified in BRCA1-associated breast cancers. Clin Cancer Res 10 499 507
52. BildAHParkerJSGustafsonAMAcharyaCRHoadleyKA 2009 An integration of complementary strategies for gene-expression analysis to reveal novel therapeutic opportunities for breast cancer. Breast Cancer Res 11 R55
53. HuWZhangCWuRSunYLevineA 2010 Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc Natl Acad Sci U S A 107 7455 7460
54. SuzukiSTanakaTPoyurovskyMVNaganoHMayamaT 2010 Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proc Natl Acad Sci U S A 107 7461 7466
55. Martinez-HernandezABellKPNorenbergMD 1977 Glutamine synthetase: glial localization in brain. Science 195 1356 1358
56. BrosnanMEBrosnanJT 2009 Hepatic glutamate metabolism: a tale of 2 hepatocytes. Am J Clin Nutr 90 857S 861S
57. BurchHBChoiSMcCarthyWZWongPYLowryOH 1978 The location of glutamine synthetase within the rat and rabbit nephron. Biochem Biophys Res Commun 82 498 505
58. MylonaPPawlowskiKBisselingT 1995 Symbiotic Nitrogen Fixation. Plant Cell 7 869 885
59. DouglasAE 1998 Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera. Annu Rev Entomol 43 17 37
60. DaikhinYYudkoffM 2000 Compartmentation of brain glutamate metabolism in neurons and glia. J Nutr 130 1026S 1031S
61. EngCHYuKLucasJWhiteEAbrahamRT 2010 Ammonia derived from glutaminolysis is a diffusible regulator of autophagy. Sci Signal 3 ra31
62. VermeulenTGorgBVoglTWolfMVargaG 2008 Glutamine synthetase is essential for proliferation of fetal skin fibroblasts. Archives of Biochemistry and Biophysics 478 96 102
63. GrasGPorcherayFSarnahBLeoneC 2006 The glutamate-glutamine cycle as an inducible, protective face of macrophage activation. Journal of Leukocyte Biology 80 1067 1075
64. TarditoSUggeriJBozzettiCBianchiMGRotoliBM 2007 The inhibition of glutamine synthetase sensitizes human sarcoma cells to L-asparaginase. Cancer Chemother Pharmacol 60 751 758
65. SreekumarAPoissonLMRajendiranTMKhanAPCaoQ 2009 Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature 457 910 914
66. DangLWhiteDWGrossSBennettBDBittingerMA 2010 Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 465 966
Štítky
Genetika Reprodukční medicína
Článek The T-Box Factor MLS-1 Requires Groucho Co-Repressor Interaction for Uterine Muscle SpecificationČlánek B Chromosomes Have a Functional Effect on Female Sex Determination in Lake Victoria Cichlid FishesČlánek Distinct Cdk1 Requirements during Single-Strand Annealing, Noncrossover, and Crossover RecombinationČlánek Specification of Corpora Cardiaca Neuroendocrine Cells from Mesoderm Is Regulated by Notch SignalingČlánek Ongoing Phenotypic and Genomic Changes in Experimental Coevolution of RNA Bacteriophage Qβ and
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 8
-
Všechny články tohoto čísla
- Polo, Greatwall, and Protein Phosphatase PP2A Jostle for Pole Position
- Genome-Wide Association Analysis of Incident Coronary Heart Disease (CHD) in African Americans: A Short Report
- The T-Box Factor MLS-1 Requires Groucho Co-Repressor Interaction for Uterine Muscle Specification
- B Chromosomes Have a Functional Effect on Female Sex Determination in Lake Victoria Cichlid Fishes
- Analysis of DNA Methylation in a Three-Generation Family Reveals Widespread Genetic Influence on Epigenetic Regulation
- PP2A-Twins Is Antagonized by Greatwall and Collaborates with Polo for Cell Cycle Progression and Centrosome Attachment to Nuclei in Drosophila Embryos
- Discovery of Sexual Dimorphisms in Metabolic and Genetic Biomarkers
- Pervasive Sharing of Genetic Effects in Autoimmune Disease
- DNA Methylation and Histone Modifications Regulate Shoot Regeneration in by Modulating Expression and Auxin Signaling
- Mutations in and Reveal That Cartilage Matrix Controls Timing of Endochondral Ossification by Inhibiting Chondrocyte Maturation
- Variance of Gene Expression Identifies Altered Network Constraints in Neurological Disease
- Frequent Beneficial Mutations during Single-Colony Serial Transfer of
- Increased Gene Dosage Affects Genomic Stability Potentially Contributing to 17p13.3 Duplication Syndrome
- Distinct Cdk1 Requirements during Single-Strand Annealing, Noncrossover, and Crossover Recombination
- Hunger Artists: Yeast Adapted to Carbon Limitation Show Trade-Offs under Carbon Sufficiency
- Suppression of Scant Identifies Endos as a Substrate of Greatwall Kinase and a Negative Regulator of Protein Phosphatase 2A in Mitosis
- Temporal Dynamics of Host Molecular Responses Differentiate Symptomatic and Asymptomatic Influenza A Infection
- MK2-Dependent p38b Signalling Protects Hindgut Enterocytes against JNK-Induced Apoptosis under Chronic Stress
- Specification of Corpora Cardiaca Neuroendocrine Cells from Mesoderm Is Regulated by Notch Signaling
- Genome-Wide Gene-Environment Study Identifies Glutamate Receptor Gene as a Parkinson's Disease Modifier Gene via Interaction with Coffee
- Identification of Functional Toxin/Immunity Genes Linked to Contact-Dependent Growth Inhibition (CDI) and Rearrangement Hotspot (Rhs) Systems
- Genomic Analysis of the Necrotrophic Fungal Pathogens and
- Celsr3 Is Required for Normal Development of GABA Circuits in the Inner Retina
- Genetic Architecture of Aluminum Tolerance in Rice () Determined through Genome-Wide Association Analysis and QTL Mapping
- Predisposition to Cancer Caused by Genetic and Functional Defects of Mammalian
- Regulation of p53/CEP-1–Dependent Germ Cell Apoptosis by Ras/MAPK Signaling
- and but Not Interact in Genetic Models of Amyotrophic Lateral Sclerosis
- Gamma-Tubulin Is Required for Bipolar Spindle Assembly and for Proper Kinetochore Microtubule Attachments during Prometaphase I in Oocytes
- Ongoing Phenotypic and Genomic Changes in Experimental Coevolution of RNA Bacteriophage Qβ and
- Genetic Architecture of a Reinforced, Postmating, Reproductive Isolation Barrier between Species Indicates Evolution via Natural Selection
- -eQTLs Reveal That Independent Genetic Variants Associated with a Complex Phenotype Converge on Intermediate Genes, with a Major Role for the HLA
- The GATA Factor ELT-1 Works through the Cell Proliferation Regulator BRO-1 and the Fusogen EFF-1 to Maintain the Seam Stem-Like Fate
- and Control Optic Cup Regeneration in a Prototypic Eye
- A Comprehensive Map of Mobile Element Insertion Polymorphisms in Humans
- An EMT–Driven Alternative Splicing Program Occurs in Human Breast Cancer and Modulates Cellular Phenotype
- Evidence for Hitchhiking of Deleterious Mutations within the Human Genome
- A Broad Brush, Global Overview of Bacterial Sexuality
- Global Chromosomal Structural Instability in a Subpopulation of Starving Cells
- A Pre-mRNA–Associating Factor Links Endogenous siRNAs to Chromatin Regulation
- Glutamine Synthetase Is a Genetic Determinant of Cell Type–Specific Glutamine Independence in Breast Epithelia
- The Repertoire of ICE in Prokaryotes Underscores the Unity, Diversity, and Ubiquity of Conjugation
- Genome-Wide Association Analysis of Autoantibody Positivity in Type 1 Diabetes Cases
- Natural Polymorphism in BUL2 Links Cellular Amino Acid Availability with Chronological Aging and Telomere Maintenance in Yeast
- Chromosome Painting Reveals Asynaptic Full Alignment of Homologs and HIM-8–Dependent Remodeling of Chromosome Territories during Meiosis
- Ku Must Load Directly onto the Chromosome End in Order to Mediate Its Telomeric Functions
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- An EMT–Driven Alternative Splicing Program Occurs in Human Breast Cancer and Modulates Cellular Phenotype
- Chromosome Painting Reveals Asynaptic Full Alignment of Homologs and HIM-8–Dependent Remodeling of Chromosome Territories during Meiosis
- Discovery of Sexual Dimorphisms in Metabolic and Genetic Biomarkers
- Regulation of p53/CEP-1–Dependent Germ Cell Apoptosis by Ras/MAPK Signaling
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



