-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaGenome-Wide Association Analysis of Autoantibody Positivity in Type 1 Diabetes Cases
The genetic basis of autoantibody production is largely unknown outside of associations located in the major histocompatibility complex (MHC) human leukocyte antigen (HLA) region. The aim of this study is the discovery of new genetic associations with autoantibody positivity using genome-wide association scan single nucleotide polymorphism (SNP) data in type 1 diabetes (T1D) patients with autoantibody measurements. We measured two anti-islet autoantibodies, glutamate decarboxylase (GADA, n = 2,506), insulinoma-associated antigen 2 (IA-2A, n = 2,498), antibodies to the autoimmune thyroid (Graves') disease (AITD) autoantigen thyroid peroxidase (TPOA, n = 8,300), and antibodies against gastric parietal cells (PCA, n = 4,328) that are associated with autoimmune gastritis. Two loci passed a stringent genome-wide significance level (p<10−10): 1q23/FCRL3 with IA-2A and 9q34/ABO with PCA. Eleven of 52 non-MHC T1D loci showed evidence of association with at least one autoantibody at a false discovery rate of 16%: 16p11/IL27-IA-2A, 2q24/IFIH1-IA-2A and PCA, 2q32/STAT4-TPOA, 10p15/IL2RA-GADA, 6q15/BACH2-TPOA, 21q22/UBASH3A-TPOA, 1p13/PTPN22-TPOA, 2q33/CTLA4-TPOA, 4q27/IL2/TPOA, 15q14/RASGRP1/TPOA, and 12q24/SH2B3-GADA and TPOA. Analysis of the TPOA-associated loci in 2,477 cases with Graves' disease identified two new AITD loci (BACH2 and UBASH3A).
Published in the journal: . PLoS Genet 7(8): e32767. doi:10.1371/journal.pgen.1002216
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002216Summary
The genetic basis of autoantibody production is largely unknown outside of associations located in the major histocompatibility complex (MHC) human leukocyte antigen (HLA) region. The aim of this study is the discovery of new genetic associations with autoantibody positivity using genome-wide association scan single nucleotide polymorphism (SNP) data in type 1 diabetes (T1D) patients with autoantibody measurements. We measured two anti-islet autoantibodies, glutamate decarboxylase (GADA, n = 2,506), insulinoma-associated antigen 2 (IA-2A, n = 2,498), antibodies to the autoimmune thyroid (Graves') disease (AITD) autoantigen thyroid peroxidase (TPOA, n = 8,300), and antibodies against gastric parietal cells (PCA, n = 4,328) that are associated with autoimmune gastritis. Two loci passed a stringent genome-wide significance level (p<10−10): 1q23/FCRL3 with IA-2A and 9q34/ABO with PCA. Eleven of 52 non-MHC T1D loci showed evidence of association with at least one autoantibody at a false discovery rate of 16%: 16p11/IL27-IA-2A, 2q24/IFIH1-IA-2A and PCA, 2q32/STAT4-TPOA, 10p15/IL2RA-GADA, 6q15/BACH2-TPOA, 21q22/UBASH3A-TPOA, 1p13/PTPN22-TPOA, 2q33/CTLA4-TPOA, 4q27/IL2/TPOA, 15q14/RASGRP1/TPOA, and 12q24/SH2B3-GADA and TPOA. Analysis of the TPOA-associated loci in 2,477 cases with Graves' disease identified two new AITD loci (BACH2 and UBASH3A).
Introduction
The presence of circulating antibodies to the body's own antigens, namely autoantibodies, is the major hallmark of autoimmunity, which can progress to the diagnosis of a variety of autoimmune diseases. Autoantibodies directed to antigens in the pancreatic islets, for example, glutamate decarboxylase (GADA) and islet antigen-2 (IA-2A), are characteristic of type 1 diabetes (T1D). The dynamics of T1D-associated autoantibodies in T1D patients are complex. They are detected prior to clinical diagnosis and often persist several years after diagnosis [1], but they can also disappear prior to T1D diagnosis [2], and, in general, decline from the time of diagnosis onwards. Antibodies are produced by B lymphocytes. The success of B cell depletion therapies in slowing beta-cell destruction in the mouse animal model [3] and more recently the positive effects of such therapies also reported in a clinical trial [4], demonstrate that B cells play a role in T1D pathogenesis. However, it is also generally accepted that anti-islet antibodies are not pathogenic themselves [5], in contrast, for example, to autoantibodies in systemic erythematosus lupus (SLE) [6]. The report of a T1D patient with a severe hereditary B cell deficiency [7], and the fact that in animal models of T1D the disease is transferable to healthy recipients by T cells but not by serum [8], are consistent with this view.
B cell maturation to autoantibody secreting state requires CD4 T helper cells to recognize human leukocyte antigen (HLA) class II molecules bound peptides on the surface of B cells and on other antigen-presenting cells [9]. Concordantly, candidate gene association studies have provided evidence for association of autoantibodies with HLA class II alleles [10], [11]. Outside of these HLA associations, relatively little is known about the genes associated with autoantibody production. However, we can hypothesize that there should be some overlap in the genes and their alleles that increase the risk of T1D with those that show association with autoantibody positivity. If autoantibody positivity per se is not a primary causal factor we should also observe T1D risk alleles that do not show evidence of association with the antibodies. We also predict that if a gene variant is associated with autoantibody positivity, then it becomes a strong candidate as a risk locus for the associated autoimmune disease. In the present report we illustrate that this strategy is successful with the identification of two new candidate genes for Graves' disease susceptibility, BACH2 and UBASH3A.
To investigate the genes involved in autoantibody production, we measured two T1D-associated anti-islet autoantibodies: glutamate decarboxylase (GADA, n = 2,506) and insulinoma-associated antigen 2 (IA-2A, n = 2,498) in plasma samples from T1D cases. In contrast with T1D, Graves' disease is known to be mediated by autoantibodies against the thyroid stimulating hormone receptor (TSHR), which leads to hypothyroidism. However, thyroid peroxidase autoantibodies (TPOA), which are detected in 75% of Graves' disease patients [12], are a sensitive and specific predictor of the disease. TPOA are also correlated with anti-TSHR autoantibodies and Hashimoto's thyroiditis [12]. This direct role of anti-TSHR antibodies in Graves' disease aetiology motivated the measurement, in the same collection of T1D cases, of TPOA (n = 8,300), which was an assay available at a significantly lower cost than the anti-TSHR autoantibody test. To further extend this analysis, we also measured autoantibodies directed against parietal cells (PCA, n = 4,328), a biomarker for autoimmune gastritis and pernicious anaemia. Eighty-six percent of pernicious anaemia patients are estimated to be PCA positive [13].
We combined these four autoantibody measurements (GADA, IA-2A, PCA and TPOA) with available genome-wide genotype data to carry out four distinct genome-wide association (GWA) scans for autoantibody positivity. Outside of the HLA region, we discovered the association of several genes with autoantibody positivity, including evidence for association of the ABO blood gene with autoimmunity and also, surprisingly, a strong association of the known autoimmunity gene, FCRL3, with IA-2A, but not with T1D. Associations with variants in the HLA region are the subject of a separate paper (Howson et al., Diabetes).
Results
In the 8,506 T1D samples the median age at venepuncture was 13 years, the median age at T1D diagnosis was 8 years and the median time between T1D diagnosis and venepuncture was 5 years. Thirty percent of the blood samples were taken within two years. The distribution of the four antibody measurements is shown in Figure S1. GADA and IA-2A -seropositive cases were at 50% and 59%, respectively (Table 1). Consistent with previous reports [1], time since diagnosis is negatively correlated with GADA and IA-2A positivity (Table 1), perhaps reflecting declining levels of beta-cell antigens and immuno-inflammatory activity following diagnosis [14]. We, therefore, included time since diagnosis (or disease duration) as a covariate in all statistical analysis. After controlling for time since diagnosis, early-onset T1D cases had lower frequencies of GADA and IA-2A (Table 1).
Tab. 1. Sample size and covariates correlated with autoantibody measurements. 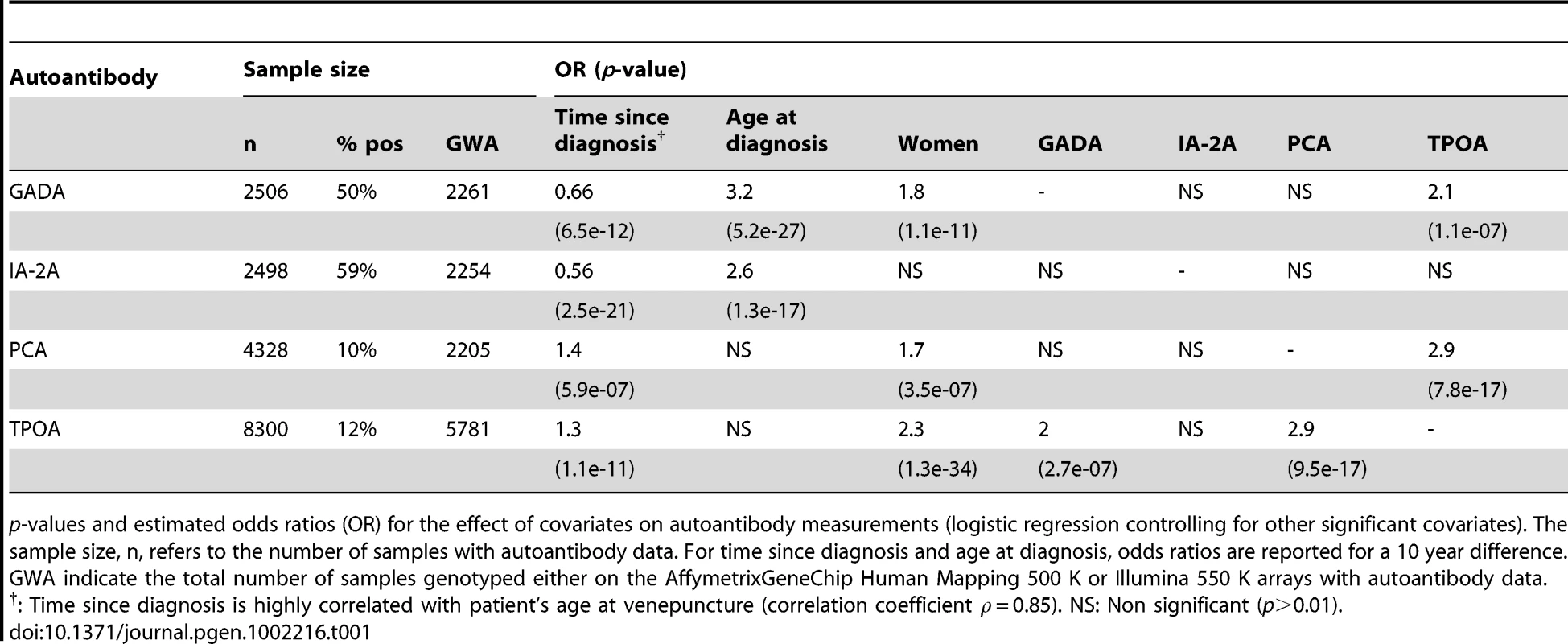
p-values and estimated odds ratios (OR) for the effect of covariates on autoantibody measurements (logistic regression controlling for other significant covariates). The sample size, n, refers to the number of samples with autoantibody data. For time since diagnosis and age at diagnosis, odds ratios are reported for a 10 year difference. GWA indicate the total number of samples genotyped either on the AffymetrixGeneChip Human Mapping 500 K or Illumina 550 K arrays with autoantibody data. PCA and TPOA frequencies in the T1D samples were 10% and 12%, respectively (Table 1). Age has been shown to have a major effect on TPOA frequency [15]. In our study, time since T1D diagnosis is positively correlated with PCA and TPOA positivity (Table 1), but because this covariate is strongly correlated with age at venepuncture (correlation coefficient 0.85), this observation is most likely a consequence of age. PCA, GADA and TPOA frequencies were higher in women, a result consistent with previous reports of elevated frequency of autoimmune diseases in women [16], [17]. Lastly, and after controlling for other covariates, significant positive correlations were observed between PCA and TPOA, and between TPOA and GADA (Table 1).
Analysis of the 1q23/FCRL1-FCRL3 region
We found two (non-MHC) associations using a genome-wide significance threshold of, p = 5×10−8. Firstly, we associated rs4971154 on chromosome 1q23 in exon 5 of the immune-regulatory receptor gene FCRL1 with IA-2A (p = 2.9×10−11, estimated odds ratio (OR) = 0.66 for the minor allele C, Table 2). Three other loci in this chromosome region have been associated with autoantibody and/or autoimmune diseases: rs7528684, located in the promoter region of the FCRL3 gene, was associated with rheumatoid arthritis (RA) and SLE risk, as well as frequency of cyclic citrullinated peptide autoantibodies (CCPA) in Japanese RA patients [18]. rs11264798 located in intron 8 of FCRL3, and rs10489678 in FCRL5, have been previously associated with Graves' disease [19].
Tab. 2. Autoantibody associations passing a genome-wide association significance threshold in the GWA scan (MHC excluded). 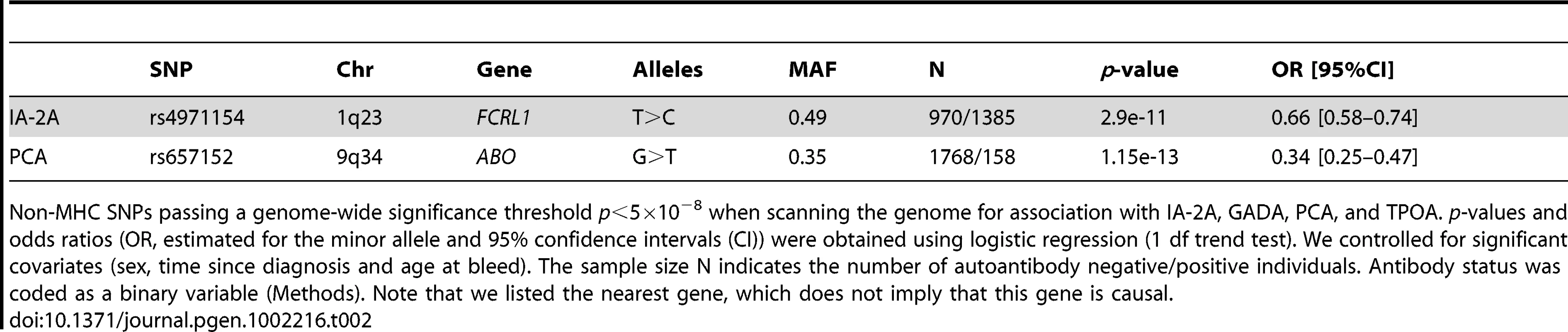
Non-MHC SNPs passing a genome-wide significance threshold p<5×10−8 when scanning the genome for association with IA-2A, GADA, PCA, and TPOA. p-values and odds ratios (OR, estimated for the minor allele and 95% confidence intervals (CI)) were obtained using logistic regression (1 df trend test). We controlled for significant covariates (sex, time since diagnosis and age at bleed). The sample size N indicates the number of autoantibody negative/positive individuals. Antibody status was coded as a binary variable (Methods). Note that we listed the nearest gene, which does not imply that this gene is causal. To understand the relationship between the TPOA, IA-2A, T1D and Graves' disease associations at the 1q23 locus, we genotyped rs4971154, rs7528684, rs11264798 and rs10489678 in the full T1D case-control collection (up to 10,596 controls and 8,506 T1D cases), as well as in 2,477 Graves' disease patients (the same cohort that was used in [19]). We used stepwise logistic regression to select the most associated SNP for each of the four traits. The alleles, minor allele frequencies and pairwise measures of LD are shown in Table S1. Association results for these SNPs with IA-2A and T1D are shown in Table 3. TPOA and Graves' results are shown in Table 4.
Tab. 3. T1D and IA-2A association results in the 1q23/FCRL3 gene region. 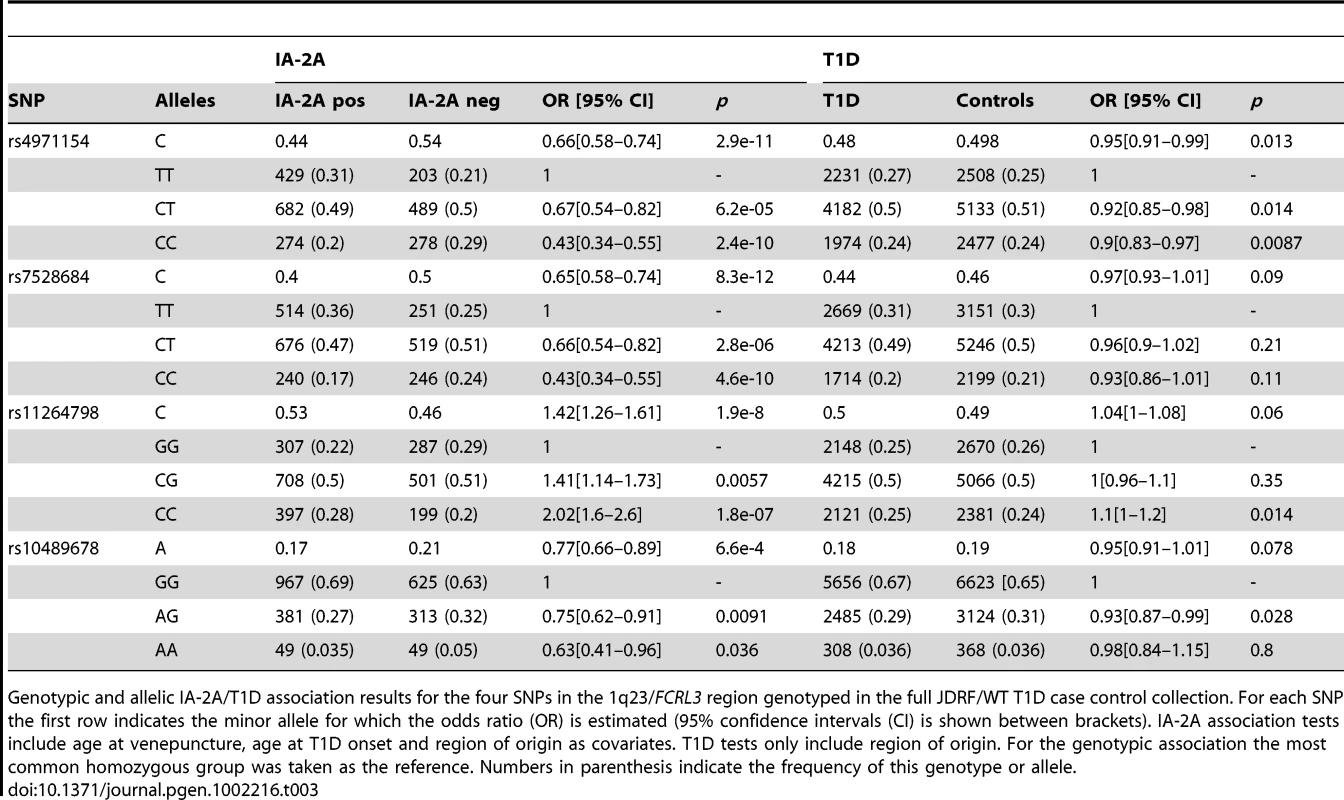
Genotypic and allelic IA-2A/T1D association results for the four SNPs in the 1q23/FCRL3 region genotyped in the full JDRF/WT T1D case control collection. For each SNP the first row indicates the minor allele for which the odds ratio (OR) is estimated (95% confidence intervals (CI) is shown between brackets). IA-2A association tests include age at venepuncture, age at T1D onset and region of origin as covariates. T1D tests only include region of origin. For the genotypic association the most common homozygous group was taken as the reference. Numbers in parenthesis indicate the frequency of this genotype or allele. Tab. 4. T1D, TPOA, and Graves' disease associations for SNPs genotyped in the Graves's disease cohort. 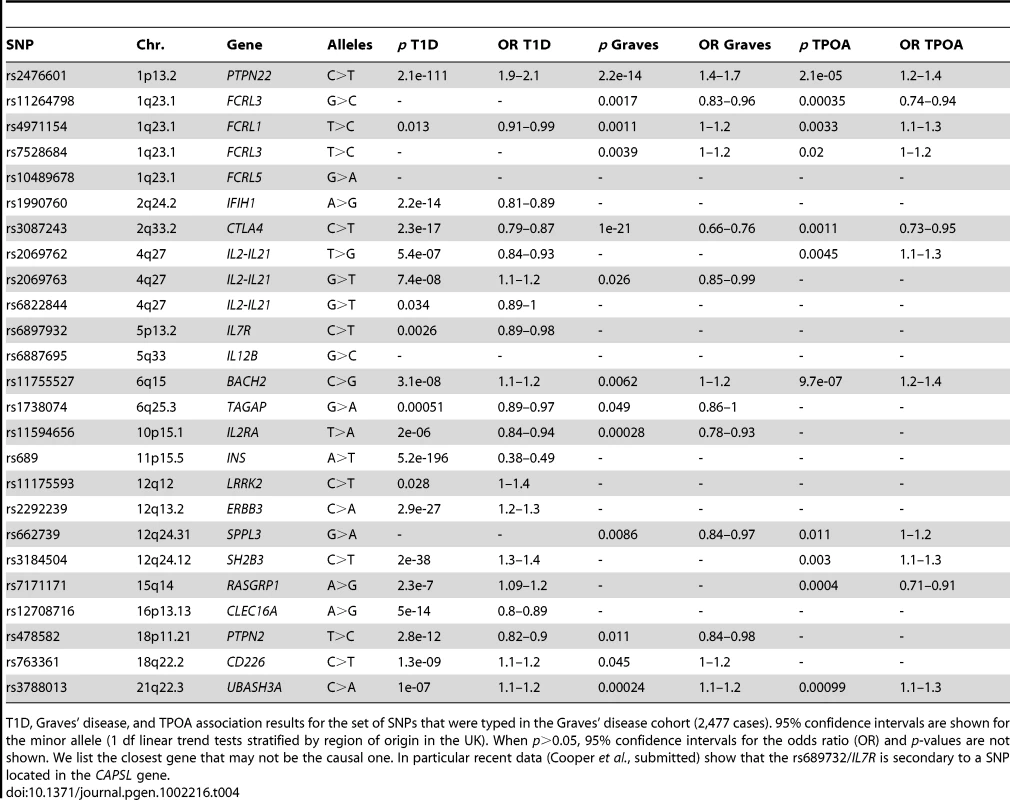
T1D, Graves' disease, and TPOA association results for the set of SNPs that were typed in the Graves' disease cohort (2,477 cases). 95% confidence intervals are shown for the minor allele (1 df linear trend tests stratified by region of origin in the UK). When p>0.05, 95% confidence intervals for the odds ratio (OR) and p-values are not shown. We list the closest gene that may not be the causal one. In particular recent data (Cooper et al., submitted) show that the rs689732/IL7R is secondary to a SNP located in the CAPSL gene. We found that the IA-2A association in T1D cases was fully accounted for by the FCRL3 SNP rs7528684 (p = 8.3×10−12, OR = 0.65 for the minor allele C, Table 3), which added to other single SNP models in the stepwise regression (p<0.01). rs7528684 was denoted as 169C→T in [18], where Kochi et al. reported that the RA and SLE risk allele rs7528684-C was also associated with increased FCRL3 expression and higher CCPA frequency in RA patients. In our European sample sets, the minor allele of rs7528684 is C and was strongly negatively associated with IA-2A positivity, a direction of effect opposed to the previously reported CCPA result. We checked this result thoroughly by resequencing both alleles in four selected samples and verified the initial data (Y. Kochi, personal communication), but found no errors. We also replicated the IA-2A/rs7528684 in 3,897 affected siblings from multiplex families (Methods) and found consistent evidence of IA-2A negative association (p = 7.6×10−9, OR = 0.69).
The TPOA and Graves' disease associations showed the same pattern of association, whereby a single (although different) SNP, rs11264798, alone explained both associations (p = 3.5×10−4 for TPOA and p = 1.7×10−3 for Graves' disease, Table 4). The SNP rs11264798 added to other single SNP models in a stepwise regression (p<0.01). The discrepancy between the TPOA and IA-2A results suggests that the causal variants underlying these two associations are distinct. We confirmed this hypothesis using a formal statistical test [20] (p = 0.004 against the null hypothesis of a single causal variant).
In contrast with the IA-2A and TPOA/Graves' disease result, there was no evidence for T1D case-control association in this region: p = 0.09 for rs7528684 and p = 0.06 for rs11264798 in 10,010 controls and 8,327 T1D cases, estimated OR = 0.97 and 1.04, respectively, for the minor alleles (Table 3). To evaluate this result further we genotyped both SNPs in a set of 8,038 T1D trios (3,598 multiplex families, see Methods), obtaining weak evidence of T1D association (p = 0.026 for rs7528684 and p = 0.04 for rs11264798, with relative risks 0.95 and 1.04, respectively). Taken together, these data suggest that the minor allele C of rs7528684 is very weakly protective for T1D (combined p = 0.0013, OR = 0.95) and the minor allele C of rs11264798 increases T1D risk very slightly (combined p = 0.0014, OR 1.04). The rs11264798-C minor allele is protective for TPOA, but is also in LD with the rs7528684-T major allele associated with IA-2A positivity.
PCA association at the 9q34/ABO locus
PCA positivity was associated with rs657152 (G>T) on chromosome 9q34, in intron 1 of the blood group gene ABO (p = 1.15×10−13, OR = 0.35 for the minor allele T, Table 2). rs657152-G is a marker for the ABO blood group O in Caucasian individuals (using blood group frequency estimates based on rs6872889 in [21], which is a proxy for rs657152, HapMap r2 = 0.93). We found a departure from the linear trend test assumption for this SNP (p = 0.02 when we compared a 2 degree-of-freedom genotype effect model to the standard 1 degree-of-freedom linear trend test). Taking the most common genotype GG as reference the estimated odds ratios were 0.26 for GT (95% CI: 0.17–0.39) and 0.23 for TT (95% CI: 0.11–0.49). Therefore in this dataset the observed PCA association is essentially a consequence of the elevated PCA frequency in the GG genotype group, which is closely correlated to the ABO blood group O.
The major allele G at this SNP, associated with higher PCA frequency in T1D cases, has also been associated with multiple traits including higher circulating levels of soluble intercellular adhesion molecule 1 (sICAM-1, [21]) and E-selectin [22], higher gastric ulcer risk [23] and lower pancreatic cancer risk [24], indicating that this blood group determinant enzyme has pleiotropic effects. We found no association of this SNP with T1D (p = 0.268 in 7,240 controls and 5,817 T1D cases). We investigated whether other sICAM associated SNPs in a different chromosome region (19p13, [21]) showed association with PCA (Table S2), but found no evidence supporting this.
At the FUT2 gene locus, the A allele of rs601338A>G (X143W/se428) prevents the secretion of ABO antigens in the gut and in saliva. The homozygous genotype AA has recently been associated with susceptibility for Crohn's disease [25], [26] and to T1D (DJS, JMMH, JAT, unpublished, www.t1dbase.org). We therefore tested a potential PCA association with this SNP. We found unconvincing evidence of PCA association: p = 0.077 linear trend test in 437 PCA positive and 3,697 PCA negative samples, estimated OR = 0.88 (95% CI: 0.77–1.01) for the G allele. Taking the AA non-secretor group as reference, a genotype association analysis suggests that the estimated ORs are not significantly different for the AG (OR = 0.81, 95% CI: 0.64–1.01) and GG (OR = 0.79, 95% CI: 0.796–1.05) genotype groups. Therefore, while the evidence is unconvincing for PCA, this recessive model of increased risk for non-secretor AA genotype group is consistent with the T1D and Crohn disease associations.
Autoantibody association at T1D loci
We then used additional genotyping data in the full T1D case collection to investigate autoantibody associations at 64 known T1D-associated SNPs in 52 distinct chromosome regions ([27], www.t1dbase.org). Owing to higher prior belief that these SNPs are autoantibody associated we used a less stringent threshold (p≤0.01) corresponding here to a false discovery rate of 16% (Benjamini-Hochberg estimation procedure [28]). We identified 13 independent associations, which included two SNPs associated with two distinct autoantibodies each (Table 5). The most significant finding was the TPOA association with the BACH2 T1D-associated SNP rs11755527 (C>G, p = 9.7×10−7, OR = 1.27). Eight out of these 13 associations involved TPOA. The association of TPOA with CTLA4 in T1D cases has been reported previously [16], and here, we extend support for this finding. We did not replicate a previously published GADA/PTPN22 interaction [29]. We also did not obtain evidence of association with any of the autoantibodies (p>0.05), including IA-2A and GADA, with INS, which shows the strongest association with T1D outside of the MHC region.
Tab. 5. Autoantibody associations at published T1D associated loci. 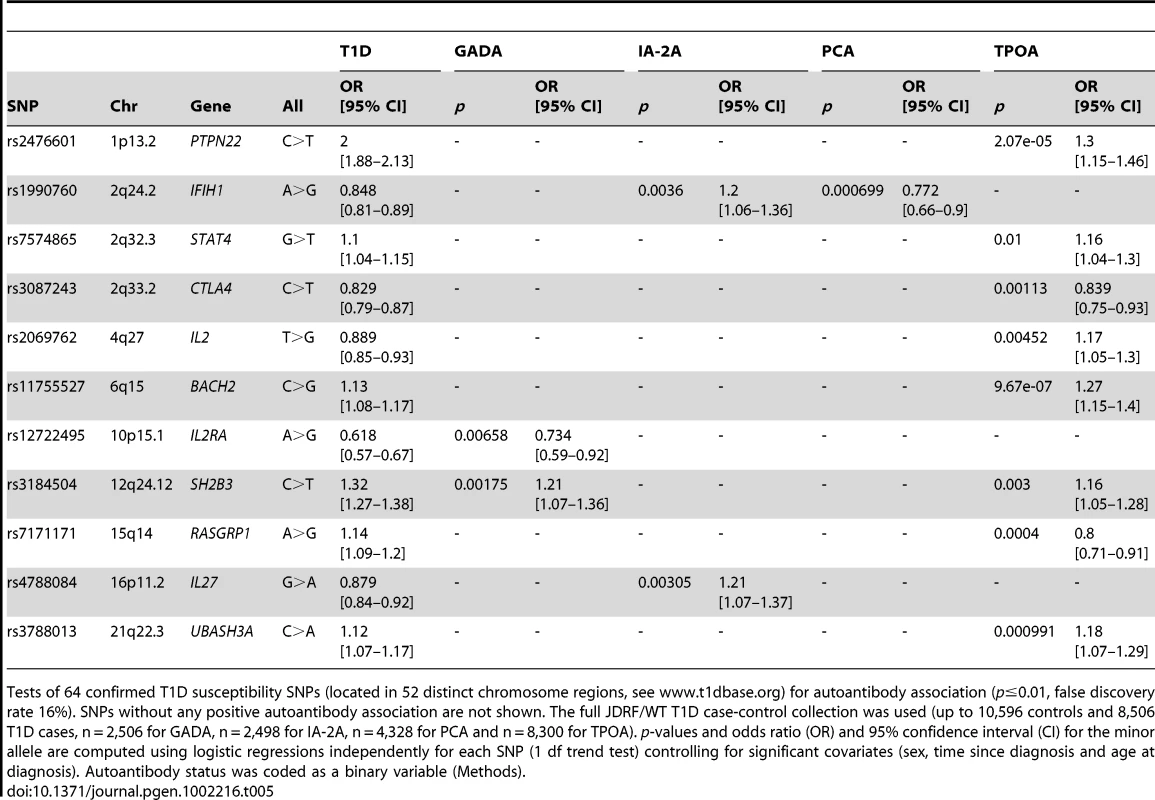
Tests of 64 confirmed T1D susceptibility SNPs (located in 52 distinct chromosome regions, see www.t1dbase.org) for autoantibody association (p≤0.01, false discovery rate 16%). SNPs without any positive autoantibody association are not shown. The full JDRF/WT T1D case-control collection was used (up to 10,596 controls and 8,506 T1D cases, n = 2,506 for GADA, n = 2,498 for IA-2A, n = 4,328 for PCA and n = 8,300 for TPOA). p-values and odds ratio (OR) and 95% confidence interval (CI) for the minor allele are computed using logistic regressions independently for each SNP (1 df trend test) controlling for significant covariates (sex, time since diagnosis and age at diagnosis). Autoantibody status was coded as a binary variable (Methods). To replicate both IA-2A associations in an independent collection, we genotyped the IL27 SNP rs4788084 and IFIH1 SNP rs1990760 in the 3,897 T1DGC affected sibling samples with IA-2A data (Methods). Both SNPs convincingly replicated the initial IA-2A results: two-tailed p = 0.0074, 8.4×10−4 for IFIH1/rs1990760, IL27/rs4788084, respectively, with the direction of effect consistent in both cases with the initial finding (estimated OR = 1.13 for IFIH1 and 1.17 for IL27).
Graves' disease association for newly identified TPOA loci
TPOA are commonly detected in Graves' disease patients and, therefore, the TPOA - associated SNPs located in the genes RASGRP1, UBASH3A and BACH2 that have not been previously tested for Graves' disease association are strong Graves' disease candidates. To investigate this hypothesis we genotyped these three SNPs in 2,477 Graves' disease cases (Table 4). We obtained p = 2.4×10−4, OR = 1.14 for the UBASH3A C>A SNP rs3788013 and p = 6.2×10−3, OR = 1.11 for the BACH2 C>G SNP rs11755527. For both SNPs the minor allele is the risk allele for Graves' disease and T1D and was associated with higher positivity for TPOA. No evidence of Graves' disease association was found for rs7171171 in RASGRP1 (p>0.05).
Analysis of additional autoimmune associated findings
Given the positive findings in T1D associated loci we extended our analysis to 135 SNPs in 100 autoimmune-associated loci [30], [31], [32]. Each SNP was tested for association with TPOA, GADA, PCA and IA-2A using p≤0.01 as a threshold (false discovery rate of 27%, Table S3). We found five additional associations: 2q37/PDCD1-IA-2A, 3p14.3/PXK-GADA, 5q33.3/IL12B-TPOA, 12q12/LRRK2-TPOA, 12q24.31/SPPL3-TPOA. We genotyped the full T1D case-control collection as well as the set of Graves' disease cases to validate the three TPOA findings, but none of them replicated for TPOA, or provided convincing evidence of Graves' disease or T1D association (p>0.05).
Discussion
The combination of genome-wide genotyping data with autoantibody measurements and case-control data for T1D and Graves' disease has enabled the discovery of several new genetic associations with autoantibody positivity and disease traits. We conclude that the nine loci (FCRL3, RASGRP1, SH2B3, STAT4, BACH2, UBASH3A, IL2, PTPN22, and CTLA4) associated with TPOA, which is not a T1D anti-islet autoantibody, may have general effects in adaptive immunity, in the complex interactions between antigen presenting cells and T cells leading to antibody-producing plasma B cells. Consistent with this general role in autoimmunity, six out of nine TPOA loci were associated with Graves' disease (except rs2069762 in IL2, rs3184504 in SH2B3 and rs7171171 in RASGRP, Table 5), including both newly identified Graves' disease loci BACH2 and UBASH3A. For these six TPOA/Graves' disease-associated SNPs, the Graves' disease risk allele is also the one associated with elevated frequency of TPOA. Lastly, four of the nine TPOA associated SNPs (in SH2B3, CTLA4, BACH2 and UBASH3A) have also been associated with celiac disease [33] (but not the SNPs in RASGRP1, STAT4, PTPN22, IL2 [20] and FCRL3 [33]). We note that the Graves' disease associated thyroglobulin (TG) gene region, was not associated with TPOA (p>0.01 at all SNPs within 300 kb of the TG gene). To further understand the association of genes with autoantibodies in T1D and in Graves' disease the measurement of TSHR autoantibodies will be informative.
After controlling for time since diagnosis we found that the patients' age at T1D diagnosis was positively correlated with the presence of GADA and IA-2A, such that patients diagnosed at a younger age are less likely to be IA-2A or GADA positive. This result is consistent with other studies that found that GADA positivity was associated with older age at diagnosis [34], [35]. Our finding suggests that earlier onset T1D involves pathogenesis directed towards autoantigens other than GADA/IA-2A, and/or beta-cell destruction is so profound in these children that they lose their autoantibodies very rapidly owing to extensive removal of islet antigens following T1D onset.
Owing to the fact that the plasma samples in this study were collected a median time of five years following diagnosis, with 30% within 2 years of diagnosis, we cannot exclude the possibility that the same GWA conducted using autoantibody data closer or prior to T1D onset might yield different results. Nevertheless, the prevalences of GADA and IA-2A in our study (Table 1) are consistent with previously reported measurements in paediatric patients at T1D diagnosis [35], [36], [37], [38]. The misclassification of autoantibody status in T1D cases may lower the statistical power but it is highly unlikely to generate false positive genetic associations. Hence, the convincing association results identified in this study (Table 2, Table 3, Table 4, Table 5) indicate that these measurements are valid.
Our estimated TPOA frequencies are consistent with a recent large scale study [17] which found 8.8% of T1D children aged less than 12 years to be TPOA positive. However, the strong effect of age, and the use in other studies of different assays with variable sensitivity, complicates a comparison of autoantibody frequency with healthy control groups. A previous study [39] found 2.6% of Finnish school children and 0.4% of Russian school children to be TPOA positive but another Swedish study [40] found 11.3% of 12 year old children to be TPOA positive. Our frequency of PCA positivity in plasma samples from T1D cases of 10% is comparable to previous reports of PCA frequencies in T1D diagnosed under age 30 years (9% in [41]), but higher in that the 2.2% in population controls aged 21–30 years [41].
Among the four autoantibodies we considered, the absence of correlations between IA-2A and GADA/PCA/TPOA (Table 1) suggests the involvement of distinct genes and pathways for IA-2A. Moreover, for the T1D-associated SNPs located in IFIH1 and IL27, the T1D risk allele is associated with reduced IA-2A positivity (Table 5). This result is different from the TPOA/T1D associations for which, in six out of eight cases, the T1D risk allele is also the allele associated with increased TPOA positivity (rs7171171 in RASGRP1 and rs2069762 in IL2 being the exceptions, see Table 5). This pattern has been reported previously between IA-2A and the T1D associated HLA-A*24 allele [36] and confirmed by our recent analyses (JMMH, JAT, Diabetes).
The 1q23/FCRL3 association data highlight the complexity of this autoimmune locus, which has previously been associated with SLE, RA and Graves' disease. Our results show that two distinct associations co-localize in this chromosome region. Firstly, the SNP rs7528684-C is associated with SLE and RA risk, CCPA positivity in RA patients, but is negatively associated with IA-2A positivity in T1D. These associations, which are in opposite directions for IA-2A compared to the other autoimmune traits, contrast with the consistency observed for the PTPN22 and CTLA4 variants, for which the risk allele is consistently the same across multiple autoimmune diseases (in particular Graves' disease, T1D and TPOA in T1D patients, see Table 4). Secondly, the SNP rs11264798-G is independently associated with Graves' disease, as well as with TPOA positivity in T1D patients. The three autoantibody associations in this chromosome region (with CCPA, IA-2A and TPOA) indicate that this locus is involved in the breakdown of self-tolerance and autoantibody production. On the other hand, the effect on T1D risk is not strong (combined case-control and family p = 0.001). Owing to the involvement of this region in multiple autoimmune disorders the prior belief that this locus is T1D associated is high. Therefore, the T1D association result could be real, but the effect size very small (estimated odds ratio 1.05). One explanation for these highly significant results in terms of IA-2A association is that the autoimmune disease-associated allele, C (of SNP rs7528684) is affecting anti-IA-2A T cells responses in a different way to autoantibody responses to this antigen [42]. The FCRL3 molecule could be affecting T regulatory cell development or function [43].
The ABO gene encodes a glycosyltransferase which is expressed in multiple human tissues. It could affect glycosylation, and therefore function or antigenicity of a wide range of molecules, in particular parietal cells antigens in the gastro-intestinal mucosal lining [44]. This blood group O, associated with increased PCA frequency, is also associated with increased frequency of gastric ulcers [23], a condition frequently caused by long-standing Helicobacter pylori infection. ABO blood groups are not associated with the presence of H. pylori [45] but the blood group O has been associated with increased inflammatory response to this bacterium [46]. A plausible hypothesis for the ABO-PCA association is that the inflammation caused by H. pylori can not only result in gastric ulcers, but can also initiate an autoimmune reaction directed against parietal cells. However, previous reports do not support an increased pernicious anaemia risk for individuals with the ABO blood group O [47], [48], [49], which indicates that the role of ABO in progression from PCA to pernicious anaemia is not straightforward.
Finally, we note that the majority of T1D regions did not associate with autoantibody positivity. Many of these chromosome regions contain genes of unknown function with no obvious candidate genes. It will be informative to continue to compare genetic associations from other diseases and traits (such as autoantibodies analysed here and other serum analytes, such as soluble CD25 [50]), to identify which of these newly-mapped, unexplored T1D loci are involved in certain pathways. Our current results place the candidate genes SH2B3, CTLA4, BACH2 and UBASH3A at the very heart of the immune response in the pathogenesis of both T1D and celiac disease.
Material and Methods
T1D cases and autoantibody measurements
9,381 T1D case samples (DNA and plasma) were available as part of the Juvenile Diabetes Research Foundation/Wellcome Trust Diabetes and Inflammation Laboratory type 1 diabetes case GRID (Genetic Resource Investigating Diabetes) collection (white British individuals diagnosed before age 17). Autoantibodies were measured in plasma for a subset of them (GADA, n = 2,506, IA-2A, n = 2,498, PCA, n = 4,328, TPOA, n = 8,300).
Presence of each of the autoantibodies (IA-2A, GADA) in the type 1 diabetes cases was tested using plasma stored at −80°C in aliquots. Autoantibodies to GAD and IA-2A were measured in the Department of Clinical Science at North Bristol, University of Bristol, using a radioimmunoassay [51]. GADA sensitivity was 86% and specificity 99%, while IA-2A sensitivity was 72% and specificity 93% in the Diabetes Antibody Standardization Program 2005 (23). Presence of GADA and IA-2A was taken as above 14 and 6 WHO Units/ml, respectively, which corresponds to the 97.5th percentile of the distribution of these autoantibodies in 2,860 school children from Oxford, UK [51].
TPOA and PCA were measured by the Department of Clinical Biochemistry, University of Cambridge with, respectively, a PLATO processor ELISA immunoassay (Phadia, Milton Keynes, UK) using recombinant TPO antigen standardised against the National Institute of Biological Standards and Controls standard serum 66/387 in which the positivity threshold for TPOA was 85 IU/ml, and ELISA manufactured by Phadia and analysed on a QIAGEN Plato III platform in which the threshold for presence of PCA was 10 U/ml.
Type 1 diabetes affected sib-pair families
To follow-up on a T1D association observed in the case-control data we genotyped 3,598 affected sib-pair families. The majority was available through the Type 1 Diabetes Genetics Consortium (T1DGC; http://www.t1dgc.org; http://www-gene.cimr.cam.ac.uk/todd/dna-refs.shtml). Of these T1DGC families: 237 were from the T1DGC Asia–Pacific region, 580 from T1DGC North-America and 1,103 from T1DGC-Europe. In addition, 354 families originated from the UK-Warren collection, 298 from HBDI (http://www.ndriresource.org/NDRI_Initiatives/HBDI/36/) and 1,026 from Finland. These samples were used for T1D association testing and IA-2A was the only autoantibody data available. As for the T1D case control samples, autoantibodies were measured on average several years after T1D diagnosis.
Graves' disease cases
To test for association with Graves' disease, a total of 2,477 unrelated white ethnic group, British Graves' disease patients were recruited as part of the autoimmune thyroid disease UK National Collection. Patients were recruited from centres across England and Wales including Birmingham, Bournemouth, Cambridge, Cardiff, Exeter, Leeds, Newcastle and Sheffield. All recruiting centres used standard clinical criteria to diagnose Graves' disease to avoid any clinical heterogeneity. These samples were solely used to test for Graves' disease association and no autoantibody data was available.
Control samples
To test for disease association (T1D and Graves' disease) control samples consisted of individuals from the British 1958 Birth Cohort and UK blood donors National Health Service Blood and Transplant [19]. Controls were matched to cases using place of recruitment for each of 12 geographical regions of Great Britain (Southern England, South-Western England, South-Eastern England, Eastern England, London, Midlands, Wales, North-Eastern England, North Midlands, East and West Ridings, Northern England, Scotland). All cases and controls were of self-reported white ethnicity. All DNA samples (T1D cases, Graves' cases and controls) were collected with approval from the relevant research ethics committee and written informed consent was obtained from the participants or their guardians. No autoantibody data were available for control samples.
Genotyping
Most of T1D cases with autoantibody data were genotyped previously using the Affymetrix 500K mapping array [19] or the Illumina 550K array [27]. We combined data from both arrays using an imputation procedure [27] to carry out a genome-wide scan for autoantibody association (n = 2,261 for GADA, n = 2,254 for IA-2A, n = 2,205 for PCA, n = 5,781 for TPOA).
Statistical analysis
Association between variants and autoantibodies were tested using regression models, treating positive autoantibody status as a binary outcome and using a one-degree-of-freedom trend test (log-scale additive disease model). Significant covariates were included (sex, time since diagnosis and age at bleed, age at diagnosis of T1D). Similar analysis was performed to test for T1D and Graves' disease association. Geographical region was included as a confounder in all logistic regression models. Statistical analyses were performed using the R statistical software.
Supporting Information
Zdroje
1. SavolaKSabbahEKulmalaPVähäsaloPIlonenJ 1998 Autoantibodies associated with Type I diabetes mellitus persist after diagnosis in children. Diabetologia 41 1293 1297
2. KnipMKorhonenSKulmalaPVeijolaRReunanenA 2010 Prediction of Type 1 Diabetes in the General Population. Diabetes Care 33 1206 1212
3. WongSWenLTangMRamanathanMVisintinI 2004 Investigation of the role of B-cells in type 1 diabetes in the NOD mouse. Diabetes 53 2581 2587
4. PescovitzMGreenbaumCKrause-SteinraufHBeckerDGitelmanS 2009 Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. The New England journal of medicine 361 2143 2152
5. SilveiraPGreyS 2006 B cells in the spotlight: innocent bystanders or major players in the pathogenesis of type 1 diabetes. Trends in endocrinology and metabolism: TEM 17 128 135
6. FlesherDTSunXBehrensTGrahamRCriswellL 2010 Recent advances in the genetics of systemic lupus erythematosus. Expert review of clinical immunology 6 461 479
7. MartinSWolf-EichbaumDDuinkerkenGScherbaumWKolbH 2001 Development of Type 1 Diabetes despite Severe Hereditary B-Cell Deficiency. New England Journal of Medicine 345 1036 1040
8. PetersenJMarshallMBækkeskovSHejnæsKHøier-MadsenM 1993 Transfer of Type 1 (insulin-dependent) diabetes mellitus associated autoimmunity to mice with severe combined immunodeficiency (SCID). Diabetologia 36 510 515
9. MillsDMCambierJC 2003 B lymphocyte activation during cognate interactions with CD4+ T lymphocytes: molecular dynamics and immunologic consequences. Seminars in immunology 15 325 329
10. WilliamsAJAitkenRJChandlerMAGillespieKMLampasonaV 2008 Autoantibodies to islet antigen-2 are associated with HLA-DRB1*07 and DRB1*09 haplotypes as well as DRB1*04 at onset of type 1 diabetes: the possible role of HLA-DQA in autoimmunity to IA-2. Diabetologia 51 1444 1448
11. GullstrandCWahlbergJIlonenJVaaralaOLudvigssonJ 2008 Progression to type 1 diabetes and autoantibody positivity in relation to HLA-risk genotypes in children participating in the ABIS study. Pediatric Diabetes 9 182 190
12. MariottiSCaturegliPPiccoloPBarbesinoGPincheraA 1990 Antithyroid peroxidase autoantibodies in thyroid diseases. The Journal of clinical endocrinology and metabolism 71 661 669
13. JeffriesGHSleisengerMH 1965 Studies of parietal cell antibody in pernicious anemia. The Journal of clinical investigation 44 2021 2028
14. WangXJiaSGeoffreyRAlemzadehRGhoshS 2008 Identification of a molecular signature in human type 1 diabetes mellitus using serum and functional genomics. Journal of immunology (Baltimore, Md : 1950) 180 1929 1937
15. HollowellJStaehlingNFlandersDHannonHGunterE 2002 Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). The Journal of clinical endocrinology and metabolism 87 489 499
16. HowsonDungerNutlandStevensWicker 2007 A type 1 diabetes subgroup with a female bias is characterised by failure in tolerance to thyroid peroxidase at an early age and a strong association with the cytotoxic T-lymphocyte-associated antigen-4 gene. Diabetologia 50 741 746
17. WarnckeKFrohlich-ReitererEThonAHoferSWiemannD 2010 Polyendocrinopathy in Children, Adolescents, and Young Adults With Type 1 Diabetes. Diabetes Care 33 2010 2012
18. KochiYYamadaRSuzukiAHarleyJShirasawaS 2005 A functional variant in FCRL3, encoding Fc receptor-like 3, is associated with rheumatoid arthritis and several autoimmunities. Nature Genetics 37 478 485
19. Wellcome Trust Case Control Consortium 2007 Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nature Genetics 39 1329 1337
20. PlagnolVSmythDJToddJAClaytonDG 2008 Statistical independence of the colocalized association signals for type 1 diabetes and RPS26 gene expression on chromosome 12q13. Biostatistics 10 327 334
21. ParéGChasmanDIKelloggMZeeRYRifaiN 2008 Novel association of ABO histo-blood group antigen with soluble ICAM-1: results of a genome-wide association study of 6,578 women. PLoS Genet 4 e1000118 doi:10.1371/journal.pgen.1000118
22. PatersonALopes-VirellaMWaggottDBorightAHosseiniM 2009 Genome-wide association identifies the ABO blood group as a major locus associated with serum levels of soluble E-selectin. Arteriosclerosis, thrombosis, and vascular biology 29 1958 1967
23. EdgrenGHjalgrimHRostgaardKNordaRWikmanA 2010 Risk of Gastric Cancer and Peptic Ulcers in Relation to ABO Blood Type: A Cohort Study. American Journal of Epidemiology 172 1280 1285
24. AmundadottirLKraftPStolzenberg-SolomonRFuchsCPetersenG 2009 Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nature Genetics 41 986 990
25. FrankeAMcGovernDBarrettJWangKRadford-SmithG 2010 Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nature Genetics 42 1118 1125
26. McGovernDJonesMTaylorKMarcianteKYanX 2010 Fucosyltransferase 2 (FUT2) non-secretor status is associated with Crohn's disease. Human Molecular Genetics 19 3468 3476
27. BarrettJClaytonDConcannonPAkolkarBCooperJ 2009 Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nature Genetics 41 703 707
28. BenjaminiYHochbergY 1995 Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 57 289 300
29. MaziarzMJanerMRoachJCHagopianWPalmerJP 2010 The association between the PTPN22 1858C>T variant and type 1 diabetes depends on HLA risk and GAD65 autoantibodies. Genes and Immunity 11 406 415
30. ZhernakovaAvan DiemenCWijmengaC 2009 Detecting shared pathogenesis from the shared genetics of immune-related diseases. Nature Review Genetics 10 43 55
31. GatevaVSandlingJHomGTaylorKChungS 2009 A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nature Genetics 41 1228 1233
32. Special Edition on Lupus Genetics 2002 Genes and Immunity 3
33. DuboisPTrynkaGFrankeLHuntKRomanosJ 2010 Multiple common variants for celiac disease influencing immune gene expression. Nature Genetics 42 295 302
34. VandewalleCLFalorniALernmarkAGoubertPDorchyH 1997 Associations of GAD65 - and IA-2 - autoantibodies with genetic risk markers in new-onset IDDM patients and their siblings. The Belgian Diabetes Registry. Diabetes care 20 1547 1552
35. GrahamJHagopianWKockumILiLSSanjeeviC 2002 Genetic effects on age-dependent onset and islet cell autoantibody markers in type 1 diabetes. Diabetes 51 1346 1355
36. QuH-QPolychronakosC 2009 The effect of the MHC locus on autoantibodies in type 1 diabetes. Journal of medical genetics
37. KnipMKukkoMKulmalaPVeijolaRSimellO 2002 Humoral beta-cell autoimmunity in relation to HLA-defined disease susceptibility in preclinical and clinical type 1 diabetes. American journal of medical genetics 115 48 54
38. KordonouriOHartmannRCharpentierNKnipMDanneT 2010 Genetic risk markers related to diabetes-associated autoantibodies in young patients with type 1 diabetes in berlin, Germany. Experimental and clinical endocrinology & diabetes 118 245 249
39. KondrashovaAViskariHHaapalaA-MSeiskariTKulmalaP 2008 Serological evidence of thyroid autoimmunity among schoolchildren in two different socioeconomic environments. The Journal of clinical endocrinology and metabolism 93 729 734
40. LindbergBCarlssonAEricssonUBKockumILernmarkA 1999 Prevalence of beta-cell and thyroid autoantibody positivity in schoolchildren during three-year follow-up. Autoimmunity 31 175 185
41. RoseNMacKayI 1998 The Autoimmune Diseases, third edition Academic Press
42. RoepBODuinkerkenGSchreuderGMKolbHde VriesRR 1996 HLA-associated inverse correlation between T cell and antibody responsiveness to islet autoantigen in recent-onset insulin-dependent diabetes mellitus. European journal of immunology 26 1285 1289
43. SwainsonLMoldJBajpaiUMcCuneJ 2010 Expression of the Autoimmune Susceptibility Gene FcRL3 on Human Regulatory T Cells Is Associated with Dysfunction and High Levels of Programmed Cell Death-1. The Journal of Immunology 184 3639 3647
44. LindénSMahdaviJSemino-MoraCOlsenCCarlstedtI 2008 Role of ABO Secretor Status in Mucosal Innate Immunity and H. pylori Infection. PLoS Path 4 e2 doi:10.1371/journal.ppat.0040002
45. UmlauftFKeeffeEBOffnerFWeissGFeichtingerH 1996 Helicobacter pylori infection and blood group antigens: lack of clinical association. The American journal of gastroenterology 91 2135 2138
46. AlkoutABlackwellCWeirD 2000 Increased Inflammatory Responses of Persons of Blood Group O to Helicobacter pylori. Journal of Infectious Diseases 181 1364 1369
47. RobertsJA 1957 Blood groups and susceptibility to disease: a review. British journal of preventive & social medicine 11 107 125
48. CallenderSLangmanMJMacleodINMosbechJNielsenKR 1971 ABO blood groups in patients with gastric carcinoma associated with pernicious anaemia. Gut 12 465 467
49. HoskinsLCLouxHABrittenAZamcheckN 1965 Distribution of ABO blood groups in patients with pernicious anemia, gastric carcinoma and gastric carcinoma associated with pernicious anemia. The New England journal of medicine 273 633 637
50. MaierLAndersonDSeversonCBaecher-AllanCHealyB 2009 Soluble IL-2RA levels in multiple sclerosis subjects and the effect of soluble IL-2RA on immune responses. Journal of immunology 182 1541 1547
51. MarciulionyteDWilliamsAJBingleyPJUrbonaiteBGaleEA 2001 A comparison of the prevalence of islet autoantibodies in children from two countries with differing incidence of diabetes. Diabetologia 44 16 21
Štítky
Genetika Reprodukční medicína
Článek The T-Box Factor MLS-1 Requires Groucho Co-Repressor Interaction for Uterine Muscle SpecificationČlánek B Chromosomes Have a Functional Effect on Female Sex Determination in Lake Victoria Cichlid FishesČlánek Distinct Cdk1 Requirements during Single-Strand Annealing, Noncrossover, and Crossover RecombinationČlánek Specification of Corpora Cardiaca Neuroendocrine Cells from Mesoderm Is Regulated by Notch SignalingČlánek Ongoing Phenotypic and Genomic Changes in Experimental Coevolution of RNA Bacteriophage Qβ and
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 8
-
Všechny články tohoto čísla
- Polo, Greatwall, and Protein Phosphatase PP2A Jostle for Pole Position
- Genome-Wide Association Analysis of Incident Coronary Heart Disease (CHD) in African Americans: A Short Report
- The T-Box Factor MLS-1 Requires Groucho Co-Repressor Interaction for Uterine Muscle Specification
- B Chromosomes Have a Functional Effect on Female Sex Determination in Lake Victoria Cichlid Fishes
- Analysis of DNA Methylation in a Three-Generation Family Reveals Widespread Genetic Influence on Epigenetic Regulation
- PP2A-Twins Is Antagonized by Greatwall and Collaborates with Polo for Cell Cycle Progression and Centrosome Attachment to Nuclei in Drosophila Embryos
- Discovery of Sexual Dimorphisms in Metabolic and Genetic Biomarkers
- Pervasive Sharing of Genetic Effects in Autoimmune Disease
- DNA Methylation and Histone Modifications Regulate Shoot Regeneration in by Modulating Expression and Auxin Signaling
- Mutations in and Reveal That Cartilage Matrix Controls Timing of Endochondral Ossification by Inhibiting Chondrocyte Maturation
- Variance of Gene Expression Identifies Altered Network Constraints in Neurological Disease
- Frequent Beneficial Mutations during Single-Colony Serial Transfer of
- Increased Gene Dosage Affects Genomic Stability Potentially Contributing to 17p13.3 Duplication Syndrome
- Distinct Cdk1 Requirements during Single-Strand Annealing, Noncrossover, and Crossover Recombination
- Hunger Artists: Yeast Adapted to Carbon Limitation Show Trade-Offs under Carbon Sufficiency
- Suppression of Scant Identifies Endos as a Substrate of Greatwall Kinase and a Negative Regulator of Protein Phosphatase 2A in Mitosis
- Temporal Dynamics of Host Molecular Responses Differentiate Symptomatic and Asymptomatic Influenza A Infection
- MK2-Dependent p38b Signalling Protects Hindgut Enterocytes against JNK-Induced Apoptosis under Chronic Stress
- Specification of Corpora Cardiaca Neuroendocrine Cells from Mesoderm Is Regulated by Notch Signaling
- Genome-Wide Gene-Environment Study Identifies Glutamate Receptor Gene as a Parkinson's Disease Modifier Gene via Interaction with Coffee
- Identification of Functional Toxin/Immunity Genes Linked to Contact-Dependent Growth Inhibition (CDI) and Rearrangement Hotspot (Rhs) Systems
- Genomic Analysis of the Necrotrophic Fungal Pathogens and
- Celsr3 Is Required for Normal Development of GABA Circuits in the Inner Retina
- Genetic Architecture of Aluminum Tolerance in Rice () Determined through Genome-Wide Association Analysis and QTL Mapping
- Predisposition to Cancer Caused by Genetic and Functional Defects of Mammalian
- Regulation of p53/CEP-1–Dependent Germ Cell Apoptosis by Ras/MAPK Signaling
- and but Not Interact in Genetic Models of Amyotrophic Lateral Sclerosis
- Gamma-Tubulin Is Required for Bipolar Spindle Assembly and for Proper Kinetochore Microtubule Attachments during Prometaphase I in Oocytes
- Ongoing Phenotypic and Genomic Changes in Experimental Coevolution of RNA Bacteriophage Qβ and
- Genetic Architecture of a Reinforced, Postmating, Reproductive Isolation Barrier between Species Indicates Evolution via Natural Selection
- -eQTLs Reveal That Independent Genetic Variants Associated with a Complex Phenotype Converge on Intermediate Genes, with a Major Role for the HLA
- The GATA Factor ELT-1 Works through the Cell Proliferation Regulator BRO-1 and the Fusogen EFF-1 to Maintain the Seam Stem-Like Fate
- and Control Optic Cup Regeneration in a Prototypic Eye
- A Comprehensive Map of Mobile Element Insertion Polymorphisms in Humans
- An EMT–Driven Alternative Splicing Program Occurs in Human Breast Cancer and Modulates Cellular Phenotype
- Evidence for Hitchhiking of Deleterious Mutations within the Human Genome
- A Broad Brush, Global Overview of Bacterial Sexuality
- Global Chromosomal Structural Instability in a Subpopulation of Starving Cells
- A Pre-mRNA–Associating Factor Links Endogenous siRNAs to Chromatin Regulation
- Glutamine Synthetase Is a Genetic Determinant of Cell Type–Specific Glutamine Independence in Breast Epithelia
- The Repertoire of ICE in Prokaryotes Underscores the Unity, Diversity, and Ubiquity of Conjugation
- Genome-Wide Association Analysis of Autoantibody Positivity in Type 1 Diabetes Cases
- Natural Polymorphism in BUL2 Links Cellular Amino Acid Availability with Chronological Aging and Telomere Maintenance in Yeast
- Chromosome Painting Reveals Asynaptic Full Alignment of Homologs and HIM-8–Dependent Remodeling of Chromosome Territories during Meiosis
- Ku Must Load Directly onto the Chromosome End in Order to Mediate Its Telomeric Functions
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- An EMT–Driven Alternative Splicing Program Occurs in Human Breast Cancer and Modulates Cellular Phenotype
- Chromosome Painting Reveals Asynaptic Full Alignment of Homologs and HIM-8–Dependent Remodeling of Chromosome Territories during Meiosis
- Discovery of Sexual Dimorphisms in Metabolic and Genetic Biomarkers
- Regulation of p53/CEP-1–Dependent Germ Cell Apoptosis by Ras/MAPK Signaling
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



