-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPolo, Greatwall, and Protein Phosphatase PP2A Jostle for Pole Position
article has not abstract
Published in the journal: . PLoS Genet 7(8): e32767. doi:10.1371/journal.pgen.1002213
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1002213Summary
article has not abstract
Commitment to mitosis is driven by activation of the Cdk1-Cyclin B protein kinase complex known as Mitosis Promoting Factor (MPF). MPF activation promotes downstream protein kinases that control the formation and function of the mitotic spindle. These kinases include members of the NIMA, Greatwall/Scant, Polo, and Aurora kinase families. Each kinase often phosphorylates multiple targets. Sophisticated dependency relationships enable a single kinase to promote distinct events at the same or successive stages of mitosis. Understanding mitosis means cataloguing each target for each kinase and deciphering the interplay between the ensuing pathways. Two papers in this issue of PLoS Genetics show that the ability of Greatwall/Scant kinase to generate an inhibitor of Protein Phosphatase 2A (PP2A) underpins an antagonistic interplay between Greatwall and Polo in Drosophila [1], [2].
Polo Kinase
The mitotic kinases Polo, Aurora, and Greatwall were identified through Drosophila genetics. “Polo” describes the circular profile of chromosomes associated with the monopolar spindles in polo mutants [3]. Humans have four Polo kinases. Drosophila Polo is considered to be analogous to mammalian Plk1 [4]. Plk1 participates in a multitude of functions ranging from MPF activation, through cohesin destruction at the metaphase-anaphase transition, to the timing and execution of cytokinesis. The defining feature of a Polo kinase is a Polo Box Domain (PBD) that docks Polo kinase to target proteins. In the majority of cases, Plk1's PBD binds to a phosphorylated motif in which the phosphorylation site matches the MPF consensus sequence. Thus, Polo must usually wait for targets to be phosphorylated by MPF before it can impose its authority [4].
Greatwall Kinase
greatwall mutants fail to correctly condense their chromosomes, leading to the naming of the kinase Greatwall as a protector of chromosome integrity [5]. While most closely related to NDR kinases, the presence of a large loop between kinase domains VII and VIII is a defining feature of Greatwall kinases [5]. Studies in cell-free Xenopus egg extracts demonstrate that Greatwall activity is critical to drive mitotic commitment [6]. Greatwall inhibits the PP2A-B55δ protein phosphatase complex [7], [8]. PP2A-B55δ activity oscillates as cells transit the cell cycle. It is high in interphase and low in mitosis [9], [10]. The activity of PP2A-B55δ counteracts MPF's efforts to promote and maintain the mitotic state [7], [8], [10]. PP2A-B55δ must therefore be switched off before a stable mitotic state can be achieved, making PP2A-B55δ inactivation an integral part of mitotic commitment [7], [8], [10], [11]. Once mitosis is complete, PP2A re-activation dephosphorylates MPF targets to drive mitotic exit [7], [8], [10]. Recent studies established that phosphorylation of the related molecules Endosulfine and Arpp19 by Xenopus Greatwall converts them into potent PP2A-B55δ inhibitors [12], [13]. Consequently, Greatwall activation upon mitotic commitment effectively locks the cell into the mitotic state.
Two further functions have been ascribed to Greatwall kinases: the modulation of RNA stability during G0 in budding yeast and, as discussed below, the antagonism of Polo kinase activity in Drosophila [14], [15]. Rim15, the budding yeast Greatwall kinase, phosphorylates yeast Endosulfine (and human Ensa and Arpp19) at the equivalent site to the Xenopus kinase, and yeast phospho-Endos subsequently binds components of a ribosome-associated protein complex to control mRNA stability [15]. Thus, it is plausible that phospho-Endos/Arrp19 may yet be found to target molecules other than PP2A in cell cycle control in other systems. The reduction in the protein levels of both Polo and the meiotic Cdc25 homologue Twine in Drosophila endosulfine (endos) mutants is consistent with altered translation in this system [16].
Drosophila Greatwall and Polo an Uneasy Pairing
The antagonistic relationship between Polo and Greatwall was revealed by a second-site mutant (Scant) that failed to complement the polo1 mutant with respect to embryonic viability [14]. Scant is a dominant, hyper-activating allele of gwl (denoted gwlscant) [17]. One component of this synthetic lethality may lie in the failed association of one centrosome with the prophase spindles in polo1/+ gwlscant/+ embryos. Increasing the polo:gwlscant ratio by duplication of polo+ suppressed this phenotype, while reducing this ratio by using a Polo inhibitor enhanced it [14], [17], [18]. Moreover, polo+ duplications restore fertility to polo1/+ gwlscant/+ females [17]. Taken together, these data demonstrate that the phenotype of the gwlscant mutant can be modulated by altering the dose of polo+.
Conservation of the Greatwall Control of PP2A Activity by Endos Phosphorylation
Rangone et al. demonstrate the ability of Drosphila endos mutants to phenocopy Scant intragenic supressors [1], [14]. They show the in vitro phosphorylation of Drosophila Endos by Drosophila Greatwall, supporting the view that polo1/+ gwlscant/+ embryos die because gwlscantgenerates excessive levels of phospho-Endos, which subsequently block PP2A activity. A reduction in the level of PP2A regulatory or catalytic subunits also reduces the fertility of polo1/+ gwlscant/+ females. The Drosophila and Xenopus stories mirror each other in two important respects. Of the four PP2A regulatory subunits, it is only the complex harbouring the B55 subunit (encoded by twins) that participates in Endos control [1]. Second, the introduction of Scant into Xenopus Greatwall increases its interphase activity and promotes premature commitment to mitosis [19].
Wang et al. derived similar conclusions from a very different starting point by systematically seeking deficiencies that enhanced the fertility defect of polo-compromised and Scant flies [2]. After realising that the strongest of the six hits they obtained corresponded to a twins deletion, they employed a genetic analysis to demonstrate that the Greatwall/Endos/PP2A relationship is conserved in Drosophila [2].
These two independent screens used opposite approaches (enhancers versus suppressors) to study Polo and Greatwall and found opposing components of the same regulatory network. The search for suppressors of gwlscant identified mutations in endos, i.e., mutations that increased PP2A activity. In contrast, the search for enhancers of the Scant phenotype identified PP2A and twins mutations that reduced it. More broadly speaking, the biochemical dissection of Xenopus extracts and genetic dissection of Drosophila come to remarkably consistent conclusions: the Greatwall/Endos/PP2A switch. The meiotic progression defects of twins and endos mutants and greatwall over-expressors suggest that these parallels extend to the control of the maintenance of the meiotic state in Drosophila [2], [16].
In Pursuit of Poles
The enhancement of the fertility defect of gwlscant by the same deletions that enhance polo in the Wang et al. study [2] both provides firm affirmation of the antagonism between Greatwall and Polo and poses the question “Why are polo mutants so sensitive to a reduction in PP2A levels?” The answer may be linked to the centrosome retention phenotype. Defective centrosome attachment is a common occurrence during the syncitial divisions of mitotic mutants, suggesting that it may simply be indicative of compromised spindle function (D. M. Glover, personal communication). However, the role this phenotype played in unlocking the Polo Greatwall/Endos/PP2A relationship suggests that the possibility of a functional link merits consideration.
Nuclear Envelope Integrity during Syncitial Divisions
The nuclear envelope remains largely intact throughout syncitial divisions of the early embryo. Limited fenestration at the spindle poles, beginning at pro-metaphase, enables the microtubules emanating from the centrosomes to capture kinetochores and form the central spindle [20]. These pores close following spindle disassembly. Newly duplicated prophase centrosomes migrate away from one another on the surface of the nuclear envelope to straddle the prophase nucleus just before fenestration grants them access to the nucleoplasm (Figure 1, WT).
Fig. 1. In wild-type syncitial embryos, the migration of centrosomes along the nuclear surface before their activation to nucleate microtubules co-incides with localised fenestration of the nuclear envelope beneath them. 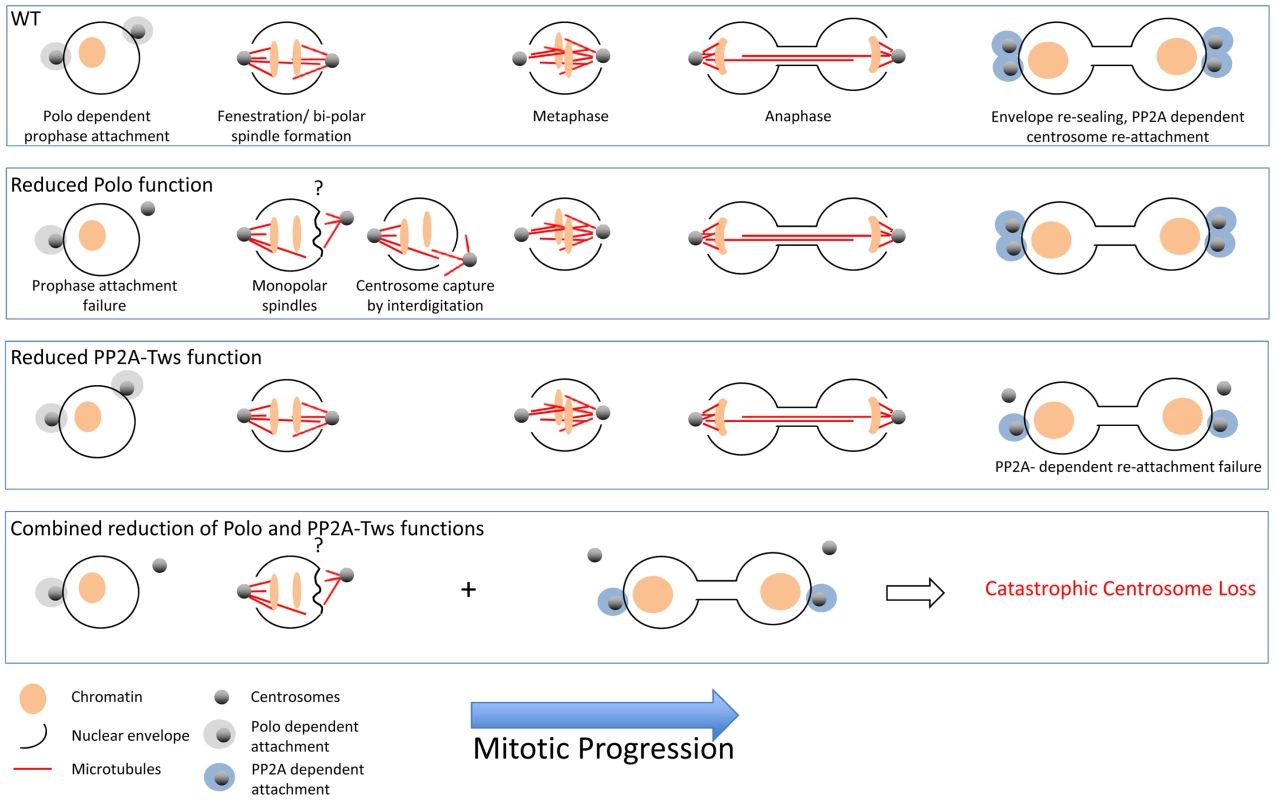
This pore remains open until completion of karyokinesis, whereupon the envelope re-seals and the centrosomes re-attach. Thus, the microtubules emanating from each centrosome are able to interdigitate and drive anaphase elongation, culminating in karyokinesis. As indicated in the appropriate panels, the association of the centrosomes fails during prophase in polo mutants and between anaphase and karyokinesis in PP2A-related mutants. The attachment is represented in the cartoons as a sphere around the centrosome to reflect our ignorance of the nature of the attachment regulated by these enzymes at each stage. The defect could be an inability to physically generate/recruit anchors on the centrosomes or an inability to activate anchors at the nuclear surface. For example, it is known that dynein is required for centrosome anchorage. Alternatively, the anchors may be activated at the centrosome and transported to the envelope along microtubules. The question mark above the monopolar spindle cartoon in the Polo reduction box reflects the uncertainty of the detail of the phenotype: fenestration or attachment? It is currently unclear whether the defect at this stage is truly an attachment defect. It could arise from a problem in generating the holes in the envelope to enable the microtubules emanating from the centrosome to meet one another and form the spindle. If the microtubules can not penetrate the envelope to establish a bipolar spindle, the force arising from polymerising microtubules repeatedly pushing against the intact envelope would push the centrosome away. This contrasts with the normal situation where the microtubule-driven forces arising from bipolar spindle formation would pull the two poles towards one another and keep the centrosomes attached. If fenestration is defective, then the “attachment” defect is rather a polo-directed fenestration deficiency. If so, then it is just the PP2A-related regulated events that represent a true attachment defect. Centrosome Detachment in polo Mutants
Reducing Polo activity can cause one of the two centrosomes to disassociate from the nuclear envelope around the time of fenestration [1], [2], [17], [18]. The pushing forces generated by microtubules emanating from this detached centrosome distort the nuclear envelope [2] (Figure 1, Polo deficiency). As similar distortions occur immediately before fenestration in wild-type embryos, the centrosome detachment phenotype may reflect a requirement for Polo to drive fenestration [20]. Alternatively, the problems in centrosome retention may lie in Polo's well-characterised role in promoting centrosome maturation [4]. The centrioles in the two centrosomes at either spindle pole are not of equivalent ages, making it possible that one is insufficiently mature to retain its grip on the nucleus when Polo activity is decreased. In this scenario, insufficient Polo leads to an inability to either recruit or activate anchors at either the centrosome or the nuclear envelope, or promote localised fenestration.
Cumulative Action of Greatwall and Polo
So how does the Greatwall/Endos/PP2A pathway impact centrosome retention? The name of the dominant Greatwall mutation, Scant (“Scott of the Antarctic”), holds the key: polo1/+ gwlscant/+ mutants have greater problems in retaining the association between the centrosome and the nuclear envelope (i.e., in finding the poles) than do polo1/+ +/+ mutants. Scant was named after British explorer Captain Scott, who set off on an unsuccessful mission to the South Pole.
As gwlscant is a hyperactive mutation, the enhancement of the detachment phenotype of polo mutants might indicate that PP2A assists Polo in promoting prophase attachment. However, this appears not to be the case, as PP2A mutants have no defect in prophase attachment [2]. Rather, PP2A single mutants display attachment defects at a later stage of mitosis; from late anaphase [2] (Figure 1, PP2A-Twins deficiency). In other words, PP2A-mediated dephosphorylation promotes centrosome docking to the envelope during mitotic exit. This timing is consistent with the distribution of Greatwall in the immediate vicinity of the nucleus throughout anaphase before nuclear import upon spindle dissolution [17]. If phospho-Endos is a short lived entity, removal of Greatwall from the vicinity of the envelope would generate a local burst of PP2A activity in the region where the centrosome binds the envelope.
Parallel or Sequential Pathways?
Are the two attachment phenotypes (Polo-driven association in prophase and PP2A-driven association during mitotic exit) connected (Figure 1, Polo/PP2A-Twins deficiency)? Wang et al. propose that they are not. Cells recover from the polo-dependent prophase centrosome loss by re-capturing the errant centrosome on their anaphase spindles [2]. The association of centrosomes with the envelopes of PP2A mutants before anaphase suggests that re-capture occurs in PP2A mutants as well [2]. However, they suggest that recovery becomes catastrophically challenging when detachment repeatedly occurs at distinct stages of the cycle in double mutants of polo and PP2A.
Alternatively, Rangone et al. provide plausible arguments for a functional link between the Polo and PP2A-driven dephosphorylation to promote centrosome attachment after mitotic exit. There are precedents in which Polo recruitment and subsequent function is driven by dephosphorylation or the dephosphorylated state. The structural component of the anaphase mid-zone, PRC1, is unable to recruit Polo until a Cdk phosphorylation site is dephosphorylated in anaphase [21]. Similarly, MAP205 must be dephosphorylated to bind and sequester Polo in interphase [18]. MAP205 normally binds Polo throughout interphase to contribute to its inactivation by keeping it away from targets; however, the key issue in the context of rising PP2A activity during mitotic exit is the antagonism between Cdk phosphorylation and the recruitment of Polo to dephosphorylated substrates.
Clearly, greater insight into the molecular basis of centrosome attachment is required before we can resolve these possibilities.
Perspectives
An overriding message from these studies is the power of Drosophila genetics to reveal the interplay between signalling networks. It is no accident that “cell cycle speak” has accumulated abstract names such as “Polo”, “Aurora”, “Scant”, and “Greatwall” at the heart of its everyday vocabulary. Drosophila genetics remains at the forefront of our attempts to piece together multiple regulator target relationships into a holistic view of the networks that constitute mitotic control. The focus and simplicity of the Scant screens in particular suggest that many insights into the Greatwall/Polo/PP2A axis will continue to emerge from this approach. In the immediate future, the attenuation of the phospho-Endos inhibitory signal is a particularly pressing objective for the field.
Zdroje
1. RangoneHWegelEGattMKYeungEFlowersA 2011 Suppression of Scant identifies Endos as a substrate of Greatwall kinase and a negative regulator of Protein Phosphatase 2A in mitosis. PloS Genet 7 e1002225 doi:10.1371/journal.pgen.1002225
2. WangPPinsonXArchambaultV 2011 PP2A-Twins is antagonized by Greatwall and collaborates with Polo for cell cycle progression and centrosome attachment to nuclei in Drosophila embryos. PLoS Genet 7 e1002227 doi:10.1371/journal.pgen.1002227
3. SunkelCEGloverDM 1988 Polo, a mitotic mutant of Drosophila displaying abnormal spindle poles. J Cell Sci 89 25 38
4. ArchambaultVGloverDM 2009 Polo-like kinases: conservation and divergence in their functions and regulation. Nat Rev Mol Cell Biol 10 265 275
5. YuJFlemingSLWilliamsBWilliamsEVLiZ 2004 Greatwall kinase: a nuclear protein required for proper chromosome condensation and mitotic progression in Drosophila. J Cell Biol 164 487 492
6. YuJZhaoYLiZGalasSGoldbergML 2006 Greatwall kinase participates in the Cdc2 autoregulatory loop in Xenopus egg extracts. Mol Cell 22 83 91
7. CastilhoPVWilliamsBCMochidaSZhaoYGoldbergML 2009 The M phase kinase Greatwall (Gwl) promotes inactivation of PP2A/B55delta, a phosphatase directed against CDK phosphosites. Mol Biol Cell 20 4777 4789
8. VigneronSBrioudesEBurgessALabbeJCLorcaT 2009 Greatwall maintains mitosis through regulation of PP2A. EMBO J 28 2786 2793
9. MochidaSHuntT 2007 Calcineurin is required to release Xenopus egg extracts from meiotic M phase. Nature 449 336 340
10. MochidaSIkeoSGannonJHuntT 2009 Regulated activity of PP2A-B55 delta is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. EMBO J 28 2777 2785
11. SchmitzMHHeldMJanssensVHutchinsJRHudeczO 2010 Live-cell imaging RNAi screen identifies PP2A-B55alpha and importin-beta1 as key mitotic exit regulators in human cells. Nat Cell Biol 12 886 893
12. MochidaSMaslenSSkehelMHuntT 2010 Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Science 330 1670 1673
13. Gharbi-AyachiALabbéJBurgessAVigneronSStrubJ 2010 The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science 330 1673 1677
14. WhitecooperHCarmenaMGonzalezCGloverDM 1996 Mutations in new cell cycle genes that fail to complement a multiply mutant third chromosome of Drosophila. Genetics 144 1097 1111
15. TalarekNCameroniEJaquenoudMLuoXBontronS 2010 Initiation of the TORC1-regulated G0 program requires Igo1/2, which license specific mRNAs to evade degradation via the 5′-3′ mRNA decay pathway. Mol Cell 38 345 355
16. Von StetinaJRTranguchSDeySKLeeLAChaB 2008 alpha-Endosulfine is a conserved protein required for oocyte meiotic maturation in Drosophila. Development 135 3697 3706
17. ArchambaultVZhaoXWhite-CooperHCarpenterATGloverDM 2007 Mutations in Drosophila Greatwall/Scant reveal its roles in mitosis and meiosis and interdependence with Polo kinase. PLoS Genet 3 e200 doi:10.1371/journal.pgen.0030200
18. ArchambaultVD'AvinoPPDeeryMJLilleyKSGloverDM 2008 Sequestration of Polo kinase to microtubules by phosphopriming-independent binding to Map205 is relieved by phosphorylation at a CDK site in mitosis. Genes Dev 22 2707 2720
19. YamamotoTBlake-HodekKWilliamsBLewellynAGoldbergM 2011 Regulation of Greatwall Kinase during Xenopus Oocyte Maturation. Mol Biol Cell 22 2157 2164
20. StafstromJStaehelinL 1984 Dynamics of the nuclear envelope and of nuclear pore complexes during mitosis in the Drosophila embryo. Eur J Cell Biol 34 179 189
21. NeefRGrunebergUKopajtichRLiXNiggE 2007 Choice of Plk1 docking partners during mitosis and cytokinesis is controlled by the activation state of Cdk1. Nat Cell Biol 9 436 444
Štítky
Genetika Reprodukční medicína
Článek The T-Box Factor MLS-1 Requires Groucho Co-Repressor Interaction for Uterine Muscle SpecificationČlánek B Chromosomes Have a Functional Effect on Female Sex Determination in Lake Victoria Cichlid FishesČlánek Distinct Cdk1 Requirements during Single-Strand Annealing, Noncrossover, and Crossover RecombinationČlánek Specification of Corpora Cardiaca Neuroendocrine Cells from Mesoderm Is Regulated by Notch SignalingČlánek Ongoing Phenotypic and Genomic Changes in Experimental Coevolution of RNA Bacteriophage Qβ and
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 8
-
Všechny články tohoto čísla
- Polo, Greatwall, and Protein Phosphatase PP2A Jostle for Pole Position
- Genome-Wide Association Analysis of Incident Coronary Heart Disease (CHD) in African Americans: A Short Report
- The T-Box Factor MLS-1 Requires Groucho Co-Repressor Interaction for Uterine Muscle Specification
- B Chromosomes Have a Functional Effect on Female Sex Determination in Lake Victoria Cichlid Fishes
- Analysis of DNA Methylation in a Three-Generation Family Reveals Widespread Genetic Influence on Epigenetic Regulation
- PP2A-Twins Is Antagonized by Greatwall and Collaborates with Polo for Cell Cycle Progression and Centrosome Attachment to Nuclei in Drosophila Embryos
- Discovery of Sexual Dimorphisms in Metabolic and Genetic Biomarkers
- Pervasive Sharing of Genetic Effects in Autoimmune Disease
- DNA Methylation and Histone Modifications Regulate Shoot Regeneration in by Modulating Expression and Auxin Signaling
- Mutations in and Reveal That Cartilage Matrix Controls Timing of Endochondral Ossification by Inhibiting Chondrocyte Maturation
- Variance of Gene Expression Identifies Altered Network Constraints in Neurological Disease
- Frequent Beneficial Mutations during Single-Colony Serial Transfer of
- Increased Gene Dosage Affects Genomic Stability Potentially Contributing to 17p13.3 Duplication Syndrome
- Distinct Cdk1 Requirements during Single-Strand Annealing, Noncrossover, and Crossover Recombination
- Hunger Artists: Yeast Adapted to Carbon Limitation Show Trade-Offs under Carbon Sufficiency
- Suppression of Scant Identifies Endos as a Substrate of Greatwall Kinase and a Negative Regulator of Protein Phosphatase 2A in Mitosis
- Temporal Dynamics of Host Molecular Responses Differentiate Symptomatic and Asymptomatic Influenza A Infection
- MK2-Dependent p38b Signalling Protects Hindgut Enterocytes against JNK-Induced Apoptosis under Chronic Stress
- Specification of Corpora Cardiaca Neuroendocrine Cells from Mesoderm Is Regulated by Notch Signaling
- Genome-Wide Gene-Environment Study Identifies Glutamate Receptor Gene as a Parkinson's Disease Modifier Gene via Interaction with Coffee
- Identification of Functional Toxin/Immunity Genes Linked to Contact-Dependent Growth Inhibition (CDI) and Rearrangement Hotspot (Rhs) Systems
- Genomic Analysis of the Necrotrophic Fungal Pathogens and
- Celsr3 Is Required for Normal Development of GABA Circuits in the Inner Retina
- Genetic Architecture of Aluminum Tolerance in Rice () Determined through Genome-Wide Association Analysis and QTL Mapping
- Predisposition to Cancer Caused by Genetic and Functional Defects of Mammalian
- Regulation of p53/CEP-1–Dependent Germ Cell Apoptosis by Ras/MAPK Signaling
- and but Not Interact in Genetic Models of Amyotrophic Lateral Sclerosis
- Gamma-Tubulin Is Required for Bipolar Spindle Assembly and for Proper Kinetochore Microtubule Attachments during Prometaphase I in Oocytes
- Ongoing Phenotypic and Genomic Changes in Experimental Coevolution of RNA Bacteriophage Qβ and
- Genetic Architecture of a Reinforced, Postmating, Reproductive Isolation Barrier between Species Indicates Evolution via Natural Selection
- -eQTLs Reveal That Independent Genetic Variants Associated with a Complex Phenotype Converge on Intermediate Genes, with a Major Role for the HLA
- The GATA Factor ELT-1 Works through the Cell Proliferation Regulator BRO-1 and the Fusogen EFF-1 to Maintain the Seam Stem-Like Fate
- and Control Optic Cup Regeneration in a Prototypic Eye
- A Comprehensive Map of Mobile Element Insertion Polymorphisms in Humans
- An EMT–Driven Alternative Splicing Program Occurs in Human Breast Cancer and Modulates Cellular Phenotype
- Evidence for Hitchhiking of Deleterious Mutations within the Human Genome
- A Broad Brush, Global Overview of Bacterial Sexuality
- Global Chromosomal Structural Instability in a Subpopulation of Starving Cells
- A Pre-mRNA–Associating Factor Links Endogenous siRNAs to Chromatin Regulation
- Glutamine Synthetase Is a Genetic Determinant of Cell Type–Specific Glutamine Independence in Breast Epithelia
- The Repertoire of ICE in Prokaryotes Underscores the Unity, Diversity, and Ubiquity of Conjugation
- Genome-Wide Association Analysis of Autoantibody Positivity in Type 1 Diabetes Cases
- Natural Polymorphism in BUL2 Links Cellular Amino Acid Availability with Chronological Aging and Telomere Maintenance in Yeast
- Chromosome Painting Reveals Asynaptic Full Alignment of Homologs and HIM-8–Dependent Remodeling of Chromosome Territories during Meiosis
- Ku Must Load Directly onto the Chromosome End in Order to Mediate Its Telomeric Functions
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- An EMT–Driven Alternative Splicing Program Occurs in Human Breast Cancer and Modulates Cellular Phenotype
- Chromosome Painting Reveals Asynaptic Full Alignment of Homologs and HIM-8–Dependent Remodeling of Chromosome Territories during Meiosis
- Discovery of Sexual Dimorphisms in Metabolic and Genetic Biomarkers
- Regulation of p53/CEP-1–Dependent Germ Cell Apoptosis by Ras/MAPK Signaling
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



