-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPhosphoinositide Regulation of Integrin Trafficking Required for Muscle Attachment and Maintenance
Muscles must maintain cell compartmentalization when remodeled during development and use. How spatially restricted adhesions are regulated with muscle remodeling is largely unexplored. We show that the myotubularin (mtm) phosphoinositide phosphatase is required for integrin-mediated myofiber attachments in Drosophila melanogaster, and that mtm-depleted myofibers exhibit hallmarks of human XLMTM myopathy. Depletion of mtm leads to increased integrin turnover at the sarcolemma and an accumulation of integrin with PI(3)P on endosomal-related membrane inclusions, indicating a role for Mtm phosphatase activity in endocytic trafficking. The depletion of Class II, but not Class III, PI3-kinase rescued mtm-dependent defects, identifying an important pathway that regulates integrin recycling. Importantly, similar integrin localization defects found in human XLMTM myofibers signify conserved MTM1 function in muscle membrane trafficking. Our results indicate that regulation of distinct phosphoinositide pools plays a central role in maintaining cell compartmentalization and attachments during muscle remodeling, and they suggest involvement of Class II PI3-kinase in MTM-related disease.
Published in the journal: . PLoS Genet 7(2): e32767. doi:10.1371/journal.pgen.1001295
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001295Summary
Muscles must maintain cell compartmentalization when remodeled during development and use. How spatially restricted adhesions are regulated with muscle remodeling is largely unexplored. We show that the myotubularin (mtm) phosphoinositide phosphatase is required for integrin-mediated myofiber attachments in Drosophila melanogaster, and that mtm-depleted myofibers exhibit hallmarks of human XLMTM myopathy. Depletion of mtm leads to increased integrin turnover at the sarcolemma and an accumulation of integrin with PI(3)P on endosomal-related membrane inclusions, indicating a role for Mtm phosphatase activity in endocytic trafficking. The depletion of Class II, but not Class III, PI3-kinase rescued mtm-dependent defects, identifying an important pathway that regulates integrin recycling. Importantly, similar integrin localization defects found in human XLMTM myofibers signify conserved MTM1 function in muscle membrane trafficking. Our results indicate that regulation of distinct phosphoinositide pools plays a central role in maintaining cell compartmentalization and attachments during muscle remodeling, and they suggest involvement of Class II PI3-kinase in MTM-related disease.
Introduction
Myofibers are large, highly differentiated contractile cells that rely on strong extracellular attachments to preserve their integrity during force-generating muscle contractions. Myofiber attachments are mediated by integrin adhesion complexes (IACs) composed of α - and β - transmembrane heterodimers that associate with cytoskeletal bridging factors, similar to those found in non-muscle cells [1]. IACs are crucial at myotendinous junctions (MTJs), attaching the ends of myofibers to tendons. In addition, IACs concentrated at costameres associated with repeating sarcomeric Z-lines attach peripheral myofibrils to the extracellular matrix. IACs are known to be essential for invertebrate and vertebrate muscle cell attachments and organization [2], [3], but it is unclear how the critical pattern of spatially restricted adhesions is continuously maintained.
In non-muscle cells, integrin turnover through endocytic recycling has clear roles in localization of dynamic adhesion complexes that mediate cell migration and membrane remodeling in cytokinesis. Trafficking pathways that engage specific endocytic adaptors, protein kinases and Rab GTPases for internalization and recycling of specific integrins are emerging, as primarily understood in isolated cells [4]. In contrast, it is not clear how important regulated integrin turnover is in differentiated muscle, or how this turnover is regulated. In isolated myofibers, uptake of markers for endocytic recycling occurred in the vicinity of adhesion sites and trafficked to perinuclear compartments, distinct from a degradative pathway [5], suggesting common trafficking themes shared with non-muscle cells. Experiments using fluorescence recovery after photobleaching (FRAP) in intact flies recently provided the first observation of endocytosis-dependent, growth-regulated mobility of IAC proteins at MTJs [6], underscoring the significance of regulated endosomal integrin trafficking in muscles, as well.
Dynamic membrane compartment identity and functions are in part conveyed through phosphoinositides. Phosphoinositides exist as seven phosphorylated phosphatidylinositol forms interconverted by dedicated lipid kinases and phosphatases [7]. Different phosphoinositide forms can recruit specific binding proteins to distinct membranes in order to elicit spatiotemporal responses that include localized signaling, cytoskeletal reorganization, membrane deformation and trafficking. However, the complex cellular relationships in vivo are less defined.
The control of phosphoinositide balance by the Myotubularin (MTM) phosphoinositide phosphatases is both elaborate and crucial in metazoans. MTMs are encoded as a large family of genes (15 humans, 7 flies, 1 yeast) associated with human disease [8]. Mutations in human MTM1 lead to human X-linked myotubular (centronuclear) myopathy (XLMTM, OMIM #310400) characterized by centrally misplaced nuclei in hypotrophic myofibers [9], [10]. Disruption of related MTMR2 leads to Charcot-Marie-Tooth neuropathy (CMT4B1, OMIM #601382) with abnormal morphology and plasma membrane outfolds in myelinating Schwann cells [11]. Both MTM1 and MTMR2 partially localize to endosomal compartments and are attributed with PI(3)P and PI(3,5)P2 turnover [12]–[18]. However, it is not clear how disruption of MTMs and potential regulation of endosomal phosphoinositides might lead to the morphological defects found in MTM-related disease. The kinase(s) that coregulate the relevant phosphoinositide pool(s) for specific MTM functions in muscle have not been explored. Candidates include both Class II and Class III PI3-kinases (PI3KC2 and Vps34, respectively) that generate PI(3)P [19]. Vps34 has well established conserved roles at endosomal membranes. In contrast, PI3KC2 has less-understood roles mostly related with functions at the plasma membrane [18], [20]–[23].
Here we show that mtm (GenBank NM_078765), encoding the sole D. melanogaster homolog of human MTM1/MTMR2, acts with Class II Pi3K68D (GenBank NM_079304) to maintain attachments upon myofiber remodeling. We found that mtm controls β-integrin turnover and trafficking from perinuclear compartments to maintain spatially restricted adhesions at MTJs and costameres, reflecting a broad mtm requirement for integrin-mediated adhesion also needed in the wing. The defects discovered in flies were substantiated by observing similar integrin mislocalization in human XLMTM myopathy, suggesting a conserved MTM1 function in membrane trafficking and roles for integrin adhesions in maintenance of myofiber organization. Altogether, our results identify specific phosphoinositide regulation important for endocytic recycling and dynamic control of cell compartmentalization.
Results
Mtm is required to maintain myofibers during remodeling and for adult muscle function
Given the role for myotubularins in human myopathy, and our discovery of an mtm requirement in muscle essential for fly viability [18], we investigated the contribution of mtm-dependent phosphoinositide regulation to muscle cell function and compartmentalization. Loss of mtm function using either null alleles or muscle-directed RNAi had no visible effects on muscle in larvae, which remained mobile and exhibited normal body wall muscle formation, attachments and growth (Figure 1A and 1B–1B′, Figure S1A–S1C′). However, targeted RNAi depletion revealed muscle requirements for mtm at later developmental stages. Muscle-specific mtm knockdown, as indicated by protein depletion, showed either animal lethality (24B-GAL4) or developmental delay (DMef2-GAL4) around the stage of adult eclosion that was rescued by co-expression of either wildtype mtm or human MTMR2 (Figure 1A and 1C, Figure S1B).
Fig. 1. Mtm depletion leads to myofiber detachment and morphological defects common with human myotubular myopathy. 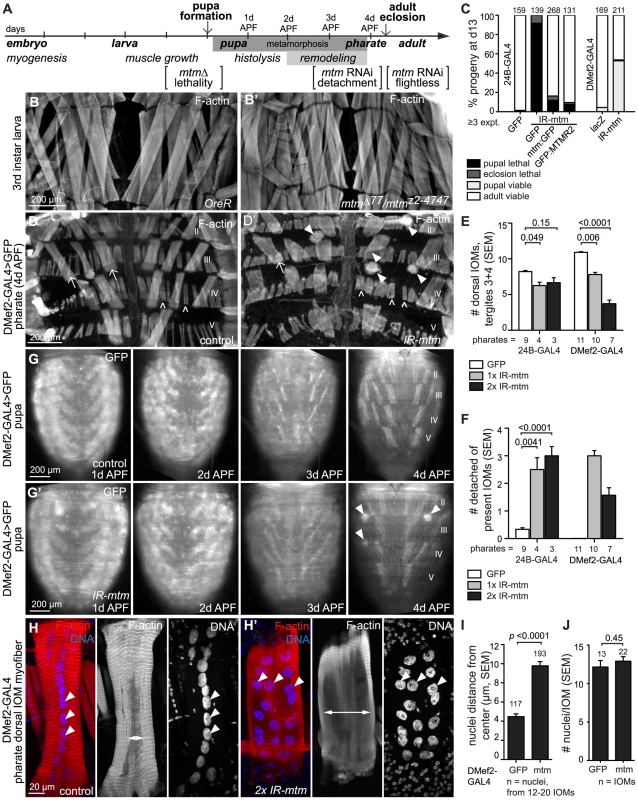
(A) Timeline of fly and muscle development, indicating stages of mtm requirements; days after puparium formation (APF). (B–B′) Normal body wall muscles in OreR and mtmΔ77/mtmz2-4747 third instar larvae. F-actin. (C) Percent viable and lethal progeny 13 days after egg lays with 24B-GAL4 and DMef2-GAL4 muscle-targeted mtm RNAi. (D–D′) Pharate dorsal abdominal muscles F-actin. Large IOMs (arrows) and smaller adult muscles (open arrowheads) span tergites (numerals). (D′) Detached IOMs (arrowheads) seen with mtm RNAi. (E–F) IOMs in filleted abdomens with 24B-GAL4 or DMef2-GAL4 expression of 1 or 2 copies of mtm RNAi hairpins. (E) Number IOMs present in tergites 3 and 4, including detached but present IOMs. (F) Number of present, visibly detached IOMs. (G–G′) Timelapse microscopy of GFP in IOMs imaged in same animals 1, 2, 3 and 4 days APF. (G′) With mtm RNAi, normal IOM formation (1d APF), survival upon histolysis of non-persistent muscles (2d APF), myofiber thinning (3d APF) and rethickening (4d APF) preceded detachment (4d APF, arrowheads). (H–H′) Individual IOMs. F-actin, red; DNA, blue. Projections, merged and nuclei images; central z-sections, F-actin. (H) Contractile myofibrils are normally tightly packed (double arrow) around linear aligned nuclei (arrowheads). (H′) With mtm RNAi, intact peripheral myofibrils surround expanded central area (double arrow) with unaligned nuclei (arrowheads). (I) Distance (µm) of nuclei from IOM midline. (J) Number nuclei per IOM. Scale bar 200 µm, except H–H′ 20 µm. Metamorphosis occurs inside a rigid pupal case that adult flies escape at eclosion with the help of muscle contractions, including supporting contractions from a subset of abdominal persistent larval muscles (PLMs) [24], [25]. The PLMs called dorsal temporary internal oblique muscles (IOMs) are large, individual, multinuceated myofibers that span abdominal segments (Figure 1D; Figure S1D). Consistent with defects in eclosion, there was a decrease in the number of both dorsal IOMs and ventral PLMs in mtm-depleted abdomens (Figure 1D′–1E; Figure S1D′–S1E). The remaining mtm-depleted myofibers were frequently detached and seen as rounded-up balls or as elongated fibers with one completely detached end (Figure 1D′, 1F; Figure S1D′, S1F), never observed in controls.
To explore the developmental requirement for an mtm muscle function, we first characterized myofibers using timelapse microscopy in intact animals. With mtm depletion, GFP-labeled IOMs were properly maintained during early pupal stages, when other larval muscles undergo developmentally regulated cell death (Figure 1A,1G–1G′, 2 days after pupal formation, APF). The mtm-depleted IOMs subsequently underwent normal myofiber thinning (3d APF) and rethickening (4d APF), indicative of developmental turnover and rebuilding of the contractile myofibrils [25]. While no detachment was observed in control animals (n = 19), IOM detachment occurred during remodeling in late pupal stages with mtm knockdown (n = 15) (Figure 1G′, 4d APF). Thus, mtm is not essential for IOM formation or survival, but is important for muscle attachments and maintenance upon remodeling.
To address whether mtm plays a role in other muscles, we examined different developmental stages and myofiber types. Although adult somatic muscles appeared to form normally with muscle-specific mtm knockdown (Figure 1D′ and not shown), 100.0±0.0% of the viable adult flies were flightless (versus 22.2±5.4% control; n = 10, ≥124 flies). Visceral muscles that normally migrate to ensheath the testis [26] were present but also disrupted with mtm muscle-depletion (Figure S1G–S1G′, 24B-GAL4). Taken together, mtm function appears dispensable for myogenesis, but is broadly required in both somatic and visceral muscles for myofiber remodeling, maintenance and function.
Mtm disruption models centronuclear myopathy, including T-tubule disorganization
Pathological hallmarks of XLMTM are small, rounded myofibers with nuclei displacement and disorganization of the perinuclear compartment [8]. In wildtype IOMs, myofibrils are normally tightly packed around centrally aligned nuclei following myofiber remodeling [25] (Figure 1H). In contrast, in mtm-depleted IOMs, central myofibrils were misaligned or absent around a normal number of centrally-displaced nuclei (2.1-fold increased nuclei distance from midline; Figure 1H′–1J). The nuclei were otherwise normal in size and morphology (Figure S2A–S2A′) and pharate adult IOMs were impermeable to propidium iodide staining (Figure S2B–S2C′), while ultrastructural analysis confirmed normal mitochondrial integrity (Figure S2D–S2D′), all indicating viability of mtm-depleted IOM cells. The peripheral myofibrils appeared normal (Figure S2E–S2H), suggesting that mtm is unlikely to function directly in sarcomere assembly.
We also found that transverse (T)-tubules were disrupted in mtm-depleted myofibers, consistent with defects recently described in vertebrate XLMTM [14], [27]. T-tubules are an extensive membrane network, continuous with the sarcolemma, which mediates excitation-contraction coupling throughout the myofiber interior. Although critical for force-generating contractions, there is little understanding of T-tubule biogenesis and structural regulation. We found that both the Amphiphysin (Amph) BAR-domain protein and Dlg1 membrane-associated guanylate kinase scaffold protein localize to T-tubules in wildtype abdominal myofibers (Figure S3A, S3B, S3C), as in flight muscles [28]. In mtm-depleted IOMs, although longitudinal elements of T-tubule membranes were present, lack of Amph and Dlg indicated that transversal membranes were specifically disorganized or absent (Figure S3A′, S3B′; 9.5% control versus 96.3% mtm RNAi with transversal membranes in <half of IOM; n≥21). These conclusions were confirmed by transmission electron microscopy (Figure S3D–S3D′). Altogether, the conserved mutant phenotypes and timing of onset suggests that mtm-depleted muscles in flies model hallmarks of XLMTM.
Mtm is required for βPS-integrin trafficking for adhesions in muscle and wing
Given the muscle detachment and myofibril misalignment observed in mtm mutant myofibers, we considered a possible defect in IACs at MTJs and costameres (Figure 2A–2C). We found that βPS-integrin, the single D. melanogaster β-integrin subunit encoded by mys (GenBank NM_080054), was dramatically mislocalized in mtm-depleted muscles (Figure 2B′). In contrast to wildtype muscle, βPS-integrin was absent at the ends of detached myofibers (Figure 2B″) and from costameres (Figure 2C′), consistent with detachment due to disruption of integrin adhesions. Although an intracellular pool of βPS-integrin protein was detected as small punctae within wildtype myofibers (Figure 2D), upon mtm knockdown, βPS-integrin became enriched along abnormal vacuolar inclusions within the myofiber center (Figure 2D′). Ultrastructural analyses revealed large, lucent membrane-bound compartments within the central regions of the mtm-depleted but not control myofibers (Figure 2E–2E″). Other proteins of the integrin adhesion complex, αPS2-integrin and Talin, were both detected at MTJs but not at the inclusions, suggesting βPS-integrin as a primary target of mtm function (Figure S4A–S4B′).
Fig. 2. Mtm is required for βPS-integrin flux from intracellular compartments and localization at sarcolemmal adhesions. 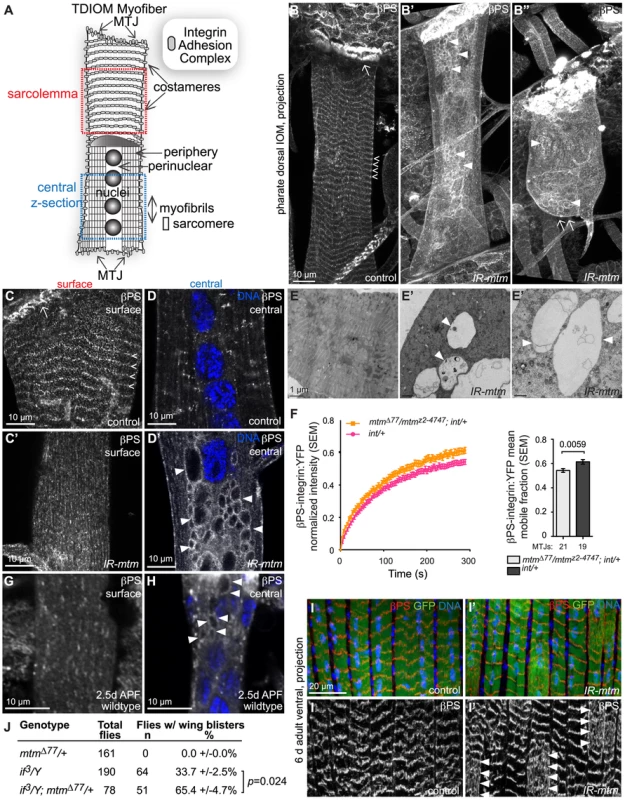
(A) Schematic of individual pharate IOM and regions imaged. MTJ, myotendinous junction. (B–B″) βPS-integrin in IOM z-projections. (B) βPS-integrin at MTJs (arrow) and costameres (open arrowheads) in control. (B′–B″) With mtm RNAi, βPS-integrin was absent from detached ends (B″, arrow) and costameres, and detected on abnormal inclusions (arrowheads). (C–C′) IOM sarcolemma highlighting βPS-integrin at costameres in control (C, open arrowheads), absent with mtm RNAi (C′). (D–D′) IOM central z-sections revealing βPS-integrin punctae in control (D), and accumulation on abnormal inclusions with mtm RNAi (D′, arrowheads). DNA, blue. (E–E′) Transmission electron microscopy of IOM cross-sections, showing densely packed central regions in control (E) and large lucent membrane compartments with mtm RNAi (E′, arrowheads). (F) Averaged FRAP recovery curves and mean mobile fraction for larval βPS-integrin:YFP (int/+) in wildtype background (pink) and trans-heterozygous null mtmΔ77/mtmz2-4747 (orange). (G) Little to no βPS-integrin present at the sarcolemma in wildtype pupal IOM, 2.5 days APF. (H) βPS-integrin on central inclusions (arrowheads) detected in wildtype pupal IOM, 2.5 days APF. DNA, blue. (I–I′) βPS-integrin (red, and single channel below) at costameres in z-projections of adult abdominal lateral transversal muscles (I), and sporadically absent from costameres and dispersed in regions of myofibers with mtm RNAi (I′) in 6 day old adult flies. GFP, green; DNA, blue. DMef2-GAL4. (J) Heterozygous mtmΔ77/+ enhanced frequency of adult wing blisters in hemizygous if3/Y flies. Scale bars 10 µm, except E–E′ 1 µm. To address the possible relationship between the appearance of membrane inclusions and muscle detachment, we examined βPS-integrin localization at earlier developmental stages. In mtm null or RNAi depleted larval muscles, normal integrin localization was detected at myofiber attachments, without any βPS-integrin-containing central inclusions (Figure S4C, S4C′). This indicates that appearance of inclusions coincides with detachment, and that mtm function is not needed for initial IAC formation. Although detected at the larval myofiber surface, βPS-integrin was not organized into uniform striations in either wildtype or mutant myofibers, as compared to the costameres observed in wildtype pharate adult IOMs. To address whether mtm function affects integrin trafficking prior to myofiber remodeling and detachment, we performed FRAP analysis of βPS-integrin:YFP along MTJs in intact larvae [6]. The mobile fraction of βPS-integrin:YFP was significantly increased in mtm mutant larval muscles (Figure 2F), indicating that mtm is required to stabilize sarcolemmal βPS-integrin localization, preceding myofiber remodeling.
To explore a basis for the sensitivity to mtm loss of function in pupal stages, we investigated integrin localization during IOM remodeling in metamorphosis. In wildtype myofibers at 2–3 days after pupal formation (APF), we discovered that there was a normal loss of integrin from the cell surface, along with detectable presence of integrin-marked inclusions (Figure 2G–2H). By 4 days APF, integrin was again predominantly absent in the myofiber center, with reappearance at costameres. This result reveals a normal redistribution of integrin that occurs with IOM remodeling, and suggests a distinct requirement for myofiber integrin regulation in pupal stages. To test a temporal requirement for mtm function specifically in pupal stages, we performed temperature shift experiments to induce conditional mtm knockdown. Due to the temperature sensitivity of the GAL4 transcription factor, flies with muscle-targeted mtm hairpin expression maintained normal myofiber attachments when raised continuously at 18°C with low GAL4 activity (Figure S4D). However, when flies were shifted during metamorphosis to 29°C for 1–2 days with increased GAL4 activity, the pharate adults then exhibited myofiber detachment (Figure S4D′–S4E, 0% versus 71% mtm pharates, respectively). Similarly, flies also carrying the temperature sensitive GAL80ts, an inhibitor of GAL4, raised continuously at 18°C did not exhibit integrin-containing inclusions (Figure S4F, 0%). In contrast, flies shifted to 29°C for 3 days (with shorter metamorphosis at higher temperatures), exhibited myofibers with integrin-containing inclusions (Figure S4F′–S4G, 58%). These results indicate a requirement for mtm function in pupal stages that is important for integrin localization at the cell surface following myofiber remodeling, and further supports a primary role for mtm in integrin trafficking.
The requirement for mtm function in myofiber remodeling during development raised the question whether there is a similar mtm requirement during cellular remodeling that may occur with ongoing adult muscle use, repair or ageing. We investigated integrin localization in adult abdominal myofibers, which are derived from a different developmental program from the persistent larval muscles. The long, thin adult ventral abdominal muscles, called lateral transversal muscles, normally exhibit a striated pattern of intense integrin localization at repeating costameres (Figure 2I). In contrast, integrin deviated from this pattern with a diffuse distribution in portions of mtm-depleted myofibers in both six and ten day old adult flies (Figure 2I′). This result points to an important role for mtm in the maintenance of integrin adhesions with ongoing muscle use in adult flies.
To test whether mtm function has a specific role for integrin localization in broader developmental contexts, we assayed function of integrin-mediated adhesions in the epithelial bilayer of the developing fly wing [1]. The low frequency of wing blisters resulting from a hypomorphic allele of αPS2-integrin, if3 [29], was dominantly enhanced when in combination with heterozygous mtm null alleles with reduced function (Figure 2J), similar to interactions seen with components known to be required for integrin adhesions [30]. The interaction in two different tissues suggests a specific and fundamental role for mtm in maintenance of integrin-mediated attachments.
Integrin adhesions and T-tubules have independent requirements for Mtm function
The disruption in mtm mutants of both IACs and T-tubules, and their normal proximity along the sarcolemma, raised the question whether a structural or functional relationship between the two compartments normally exists or is relevant to the abnormal membrane inclusions (Figure 3A). Moreover, in mtm-depleted myofibers, we noted that Dlg, similar to βPS-integrin, appeared along abnormal central inclusions (Figure 3B, 3B′). To characterize the membrane identity of the inclusions, we first tested whether βPS-integrin and Dlg or Amph co-localized, either in normal or mtm mutant muscles. In wildtype myofibers, Dlg and Amph were not detected at IACs. However, internal βPS-integrin frequently co-localized with Dlg (Figure 3C) and occasionally with Amph (Figure S5A, S5B, S5C) along apparent longitudinal elements of T-tubules. Upon mtm depletion, βPS-integrin extensively co-localized with Dlg and Amph on longitudinal T-tubules (Figure S5A, S5B′) and along the central inclusions (Figure 3C′, Figure S5B″), suggesting accumulation of a possible common precursor membrane or trafficking compartment in mtm-depleted muscles.
Fig. 3. Integrin adhesions are independent of T-tubules, but share an mtm function for maintained organization. 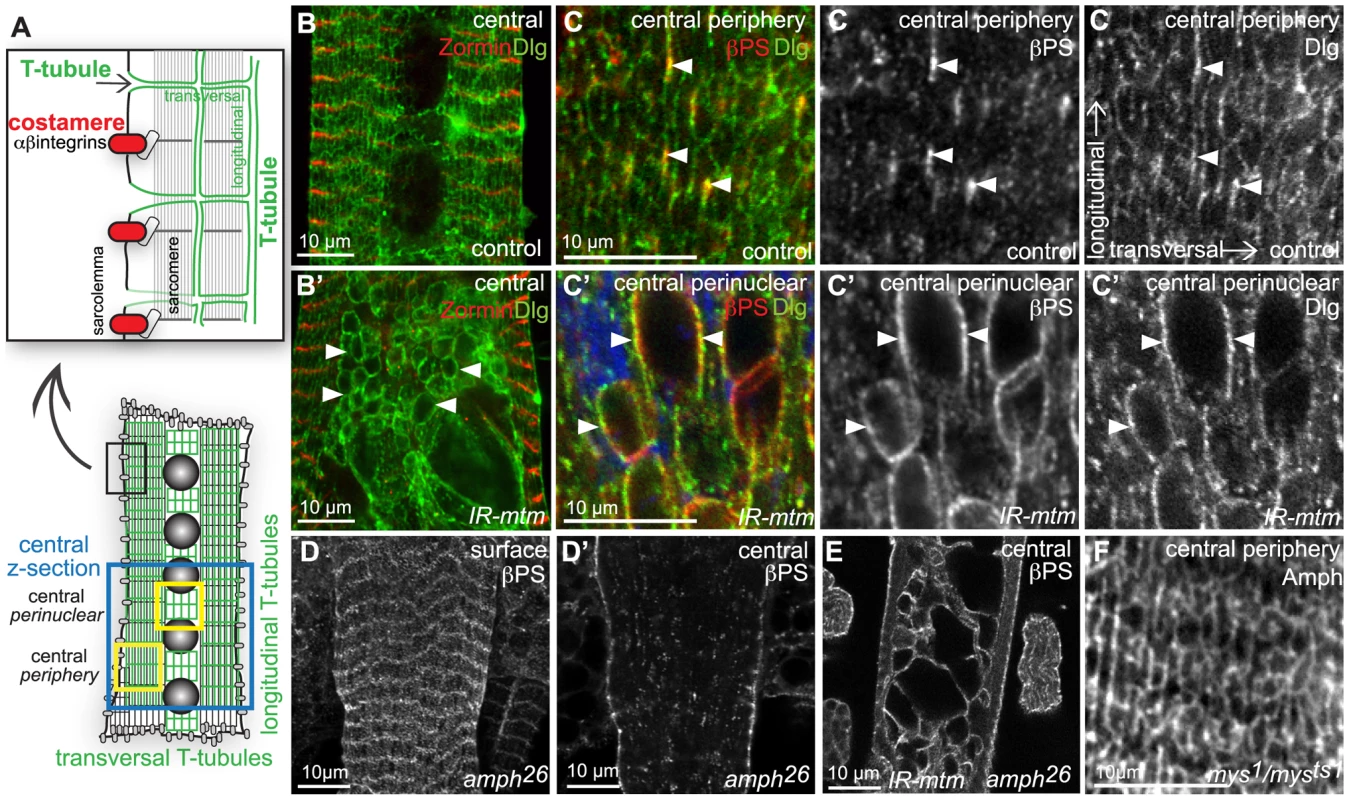
(A) IOM longitudinal section schematic of alternating sarcolemmal structures: T-tubule membranes (green) marked by Amph and Dlg; costameres (red) with IACs linked to Z-lines of peripheral myofibrils; z-section images from central, central periphery or central perinuclear regions, as shown. (B–B′) Dlg, green; Zormin, red. Dlg detected continuously on longitudinal and transversal T-tubules in control (B), but only on longitudinal tubules and on central inclusions with mtm RNAi (B′, arrowheads). (C–C′) βPS-integrin (red) and dlg1:GFP (green) partially co-localized at longitudinal T-tubules in control (C, open arrowheads), and on abnormal central inclusions with mtm RNAi (C′, arrowheads). Single channels, right. (D) Normal βPS-integrin localization at costameres and (D′) internal punctae in amph26 mutants that lack T-tubules. (E) Persistent βPS-integrin-inclusions with mtm RNAi in amph26 mutant. amph26, UAS-IR-mtm3.1/amph26; DMef2-GAL4/+. (F) Normal transverse tubule formation and Amph localization in mys1/mysts1 mutants reared at non-permissive temperature, with reduced βPS-integrin function. Scale bars 10 µm. We next considered whether there is functional co-dependence between integrin adhesions and T-tubules. In amph26 null mutants that lack T-tubules in IOMs (Figure S5D), as in adult flight muscles [28], we observed normal muscle attachments, normal βPS-integrin localization to costameres, and no βPS-integrin - or Dlg-inclusions (Figure 3D–3D′). This suggests that T-tubules are not required for βPS-integrin trafficking in the formation or maintenance of IACs, and that the inclusion defects in mtm mutants do not reflect a general consequence of failed T-tubule formation. Furthermore, we found βPS-integrin mislocalized to internal membrane inclusions upon mtm depletion in amph26 null mutants (Figure 3E), signifying that the abnormal inclusions are independent of transverse tubule membrane and possible misregulation of amph function. Conversely, transverse tubules were present normally in abdominal muscles with hypomorphic mys conditions that were pharate lethal (Figure 3F), suggesting that T-tubule organization does not require normal levels of βPS-integrin protein or IACs. The dramatic defects in both IACs and T-tubule organization upon mtm depletion therefore appear to reflect a requirement for two independent mtm functions.
PI(3)P role in Mtm-dependent muscle compartmentalization
The disrupted βPS-integrin localization together with the enlarged membrane inclusions suggested defective membrane trafficking in mtm mutant myofibers. Characterization of the central inclusions could point to a specific compartment or trafficking step that normally requires Mtm phosphatase activity in muscle remodeling. The inclusions did not noticeably contain markers of endoplasmic reticulum, the trans-Golgi network or autophagosomes (Figure S5E–S5E′ KDEL; Figure S5F–S5F′ PH-FAPP1; Figure S5G–S5G′ Atg8). In contrast, the majority of inclusions were decorated by the endosome-lysosomal marker, GFP:LAMP (Figure 4A–4A″). The inclusions were frequently colocalized with an indicator of early endosomes, GFP:Rab5 (Figure 4B–4B″), and infrequently by the Rab5 effector, Rbsn5 (Figure S5H–S5H′), but not an indicator of late endosome identity, GFP:Rab7 (Figure S5I–S5I′). Together, these results suggest a relationship between the inclusions and early endocytic traffic, and that mtm depletion disrupts endocytic traffic.
Fig. 4. Mtm depletion disrupts integrin trafficking at endosomal compartments. 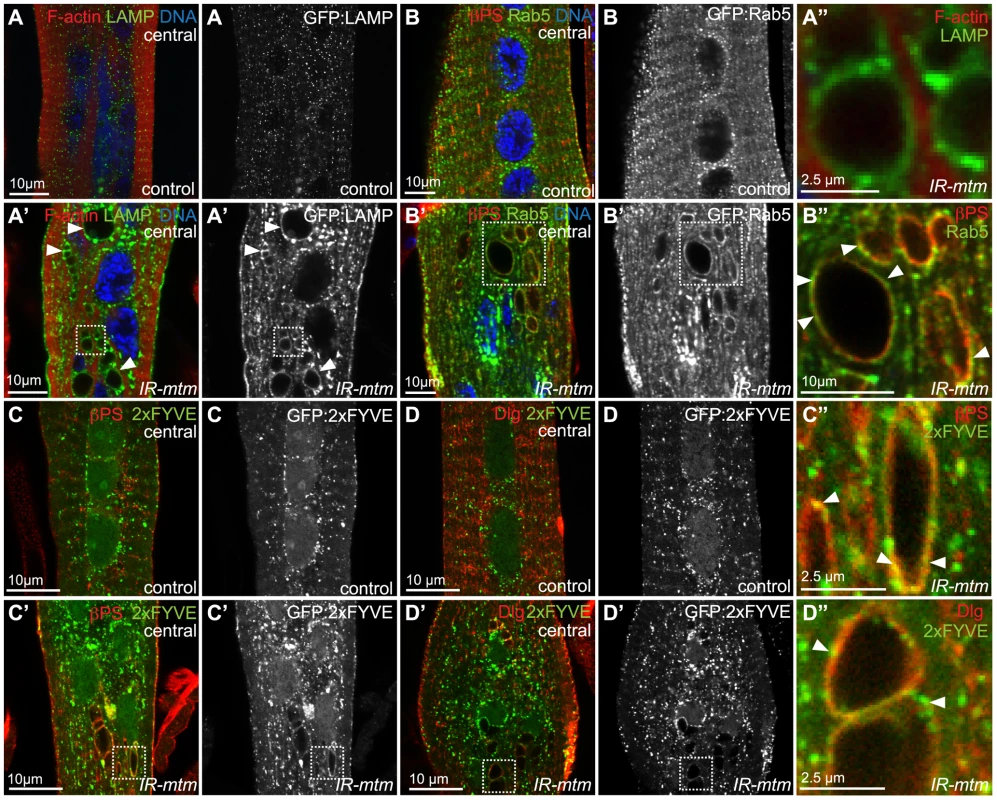
(A–A″) GFP:LAMP (green, and single channels) found as punctae throughout IOM controls (A) and localized to inclusions with mtm RNAi (A′, arrowheads; A″). F-actin, red; DNA, blue. (B) GFP:Rab5 (green, and single channels) found as punctae with normally little overlap with βPS-integrin (red) throughout IOM controls. (B′–B″) Rab5 partially co-localized with βPS-integrin on inclusions and accumulated at the plasma membrane and perinuclear with mtm RNAi. DNA, blue. (C–C″) PI(3)P detected by GFP:2xFYVE (green, and single channels) and βPS-integrin (red) exhibited little overlap in control (C), but co-localized on inclusions with mtm RNAi (C′–C″, arrowheads). (D–D″) PI(3)P detected by GFP:2xFYVE (green) and Dlg (red) exhibited little overlap in control (D), but co-localized on inclusions with mtm RNAi (D′–D″, arrowheads). Scale bars 10 µm, except zooms A″, C″, D″ 2.5 µm. PI(3)P is normally enriched at endosomal membranes. We have recently shown that the normal Mtm PI(3)P phosphatase activity promotes membrane efflux, effecting both endosomal homeostasis and cortical remodeling in macrophages [18]. We therefore explored PI(3)P distribution in muscle with respect to integrin adhesion and T-tubule compartments. In wildtype animals, muscle expression of the PI(3)P biosensor, GFP:2xFYVE, was detected along the sarcolemma and localized to punctae distributed throughout abdominal myofibers, with the greatest concentration in the perinuclear area without obvious overlap with βPS-integrin (Figure 4C) or Dlg (Figure 4D). Upon mtm-depletion, enlarged and more erratically positioned PI(3)P-containing compartments were detected (Figure 4C′, 4D′). In addition, GFP:2xFYVE co-localized with βPS-integrin (Figure 4C″) and with Dlg (Figure 4D″) along the abnormal inclusions in mtm-depleted myofibers, suggesting a possible role for Mtm phosphatase activity in PI(3)P turnover involved in integrin trafficking.
Pi3KC2 suppresses Mtm-related defects in βPS-integrin localization and muscle maintenance
To test if PI(3)P regulation is involved in mtm muscle functions, we investigated the contribution of Class II and III Pi3-kinases (Pi3K68D and Vps34, respectively), known to synthesize PI(3)P, to abdominal muscle maintenance. Muscle-targeted knockdown of Pi3K68D or expression of dominant negative kinase-dead Vps34-KD did not individually disrupt eclosion or animal viability. However, Pi3K68D depletion in combination with mtm RNAi was able to rescue the lethality and delayed development; in contrast, Vps34-KD expression enhanced lethality in combination with mtm depletion (Figure S6A, S6B). Neither Pi3K68D nor Vps34 knockdown rescued the loss of T-tubules with mtm-depletion (Figure S6C), and accordingly adult flies remained flightless (Figure S6D). These results indicate separable Pi3K68D-independent and dependent mtm muscle functions required for normal T-tubules and viability, respectively.
A similar functional relationship was seen between Pi3K68D and mtm for roles related to integrin adhesions, as with viability. Importantly, Pi3K68D, but not Vps34, depletion rescued muscle detachment (Figure 5A, 5B) and loss of βPS-integrin localization at costameres (Figure 5C, 5C′, 5D) that occurs with loss of mtm function. Consistent with rescue of the IACs, co-depletion of mtm and Pi3K68D, and not Vps34, also eliminated the βPS-integrin - and Dlg-containing membrane inclusions (Figure 5E, 5E′, 5F, Figure S6E), indicating a functional relationship between the abnormal central inclusions and IACs at the sarcolemma. The testis visceral muscle function was also restored to normal with Pi3K68D and mtm co-depletion, implicating turnover of integrin-mediated adhesions in the gonadal muscle. Altogether, these results signify that Pi3K68D function mediates mtm RNAi mutant defects in maintenance of IACs, and suggest that Pi3K68D may synthesize a PI(3)P subpool co-regulated by Mtm important for integrin trafficking and localization.
Fig. 5. Class II and Class III PI3-kinases affect mtm-dependent integrin adhesions differently. 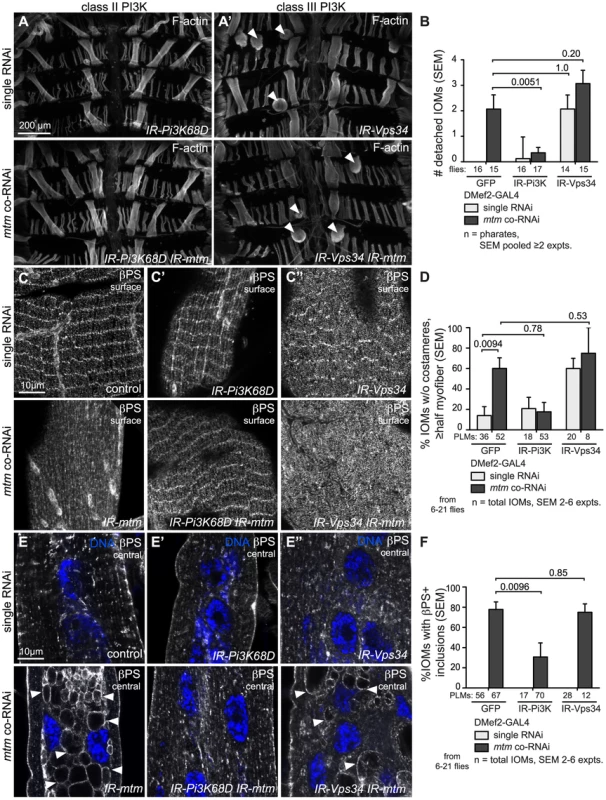
(A–A′) Pharate abdominal muscles, F-actin. (A) IR-Pi3K68D and (A′) IR-Vps34 single RNAi (top) and mtm co-RNAi (bottom). Arrowheads, detached IOMs. (B) Number of visibly detached IOMs. (C, C′, C″) Sarcolemmal βPS-integrin detected at costameres; (C) control, (C′) IR-Pi3K68D and (C″) IR-Vps34 in single RNAi (top) and mtm co-RNAi (bottom). Only Pi3K68D, mtm co-RNAi restored βPS-integrin at costameres. (D) Percentage IOMs that lack costameres ≥half of myofiber surface. (E, E′, E″) βPS-integrin central z-sections; (E) control, (E′) IR-Pi3K68D and (E″) IR-Vps34 single RNAi (top) and mtm co-RNAi (bottom). Only Pi3K68D, mtm co-RNAi reverted abnormal βPS-integrin-inclusions. (F) Percentage IOMs with βPS-integrin on inclusions. (B,D,F) IOMs in single RNAi (light bars) and mtm co-RNAi (dark bars) conditions. Scale bars 10 µm, except A–A′ 200 µm. Interestingly, muscle-targeted disruption of Vps34 shared with mtm depletion a similar staged semi-lethality (Figure S6A), IOM detachment (Figure 5A′, 5B) and loss of βPS-integrin at costameres (Figure 5C″, 5D), however, without inducing abnormal inclusions (Figure 5E″, 5F, Figure S6E). This points to possible shared or sequential roles for Vps34 and Mtm in phosphoinositide-mediated steps in IAC maintenance, distinct from trafficking points that involve antagonistic Pi3K68D and Mtm co-regulation. Vps34 is broadly attributed with roles in PI(3)P-regulated endocytosis and autophagy. We found that inhibition of autophagy upon depletion of the central regulator, Atg1, phenocopied the Vps34 integrin defects (Figure S6F, S6G, S6H), indicating an important role for autophagy in IOM remodeling. We tested whether the phenotypes associated with mtm phosphatase depletion are a result of increased PI(3)P-mediated autophagy. The myofiber detachment and integrin localization defects, including integrin-containing inclusions, persisted with co-depletion of Atg1 and mtm (Figure S6F′, S6G′, S6H′), indicating that autophagy is not responsible for the integrin-related defects upon mtm depletion.
Integrin is mislocalized in human myofibers with XLMTM myopathy
Given shared defects observed in mtm - and MTM1-disrupted myofibers in flies and human XLMTM, respectively (Figure 1H′, Figure S3A′–S3D′), we asked whether an mtm function required for integrin adhesions is also shared with MTM1 in human muscle. β1-integrin, the major β-integrin isoform found in vertebrate muscle, was detected along the myofiber sarcolemma in cross-sections of skeletal muscle from control subjects, as expected (Figure 6A). In contrast, β1-integrin localized throughout the perinuclear compartment of centronucleated myofibers in muscle from neonates with XLMTM (Figure 6B). The Dystroglycan adhesion complex (DAC) is a second complex localized to MTJs and costameres with key roles in muscle attachments, and mutations of DAC components are frequently associated with muscular dystrophy. Unlike integrin, the dystroglycan transmembrane protein exhibited only the expected peripheral staining along the sarcolemma in both control and XLMTM myofibers, without any abnormal centronuclear localization or inclusion (Figure 6C–6D). These results show that MTM1 is specifically required for normal β1-integrin localization in human myofibers, and suggests that disruption of integrin trafficking and adhesion complex function is important in XLMTM.
Fig. 6. β1-integrin is mislocalized to perinuclear inclusions in XLMTM myopathy. 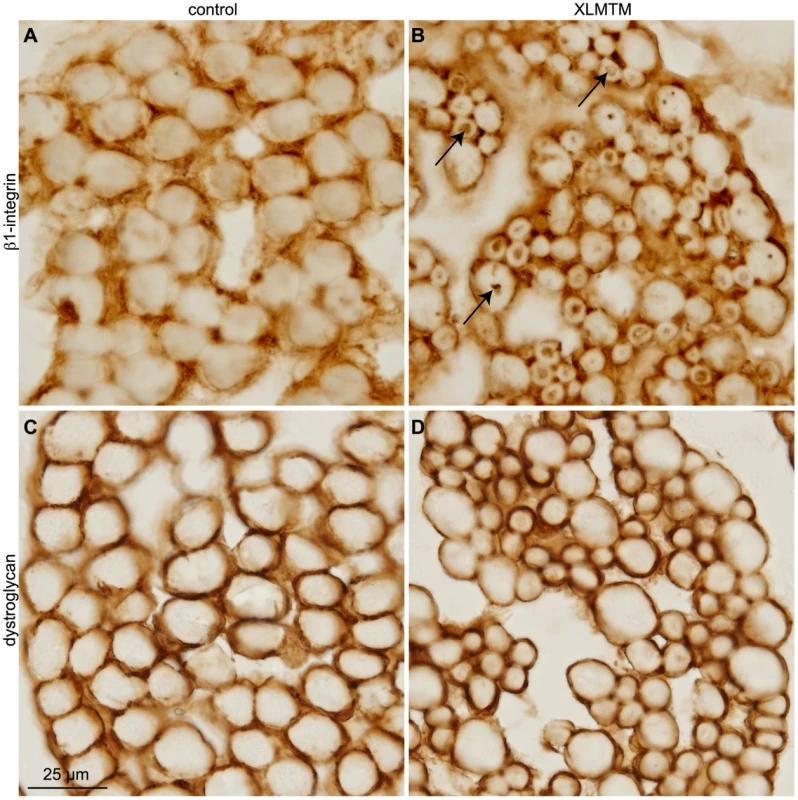
Cryosections from XLMTM (n = 3) and age-matched control human muscle biopsies were immunostained either with anti-β-dystroglycan or with anti-β1D-integrin. (A–B) β1D-integrin was found only along the sarcolemma in control muscle (A), but was mislocalized to the perinuclear compartment in XLMTM fibers (B, arrows). (C–D) In contrast, Dystroglycan was found normally distributed along the sarcolemma membrane in both control (C) and XLMTM (D) muscle. Scale bar, 25 µm. Discussion
We found that mtm regulates integrin adhesions in muscle and in the developing wing, and that integrin localization was disrupted in human XLMTM, pointing to a central role for Mtm/MTM1 in a trafficking pathway important for localization of β-integrin at the plasma membrane. It is well-established that integrin turnover contributes to cell motility, whereby targeted integrin recycling and reassembly of localized adhesions mediate polarized matrix attachments and signaling responses [4]. Our results reveal that regulated integrin turnover is also important for integrin adhesions in non-motile myofibers, after the establishment of attachments. Importantly, mtm disruption uncovered a demand for βPS-integrin trafficking in the maintenance of adhesions both at MTJs as well as at costameres, a less-understood adhesion site with putative roles in muscle integrity, mechanotransduction, and myofibril assembly [31], [32]. Although integrin was destabilized at larval MTJs in mtm mutants, the most severe consequences occurred later with specific loss of pupal or adult mtm function during developmental myofiber remodeling or adult muscle use, respectively. This is consistent with costamere sensitivity to integrin depletion in adult muscle [33] and the possibility that mtm similarly regulates integrin turnover with myofiber remodeling that occurs both in development and with demands in adult muscle growth, repair and aging.
In fly macrophages, Class II Pi3K68D and mtm co-depletion could revert both an imbalance in PI(3)P and defects in cortical remodeling that impaired macrophage shape and in vivo immune cell distribution [18]. Here, we found Pi3K68D disruption is also a specific and potent suppressor of integrin adhesion defects in mtm-depleted muscle. Despite distinct macrophage and myofiber morphology and function, a shared requirement for a PI3KC2/Mtm pathway highlights common functions during cellular remodeling. Loss of Mtm phosphatase activity could be considered a gain of function condition, analogous to ectopic kinase activity, leading to inappropriate phosphoinositide accumulation. In line with this, either mtm depletion (this study) or Pi3K68D overexpression [34] disrupted integrin adhesion in the fly wing, presumably through imbalanced responses to an accumulation of the same phosphoinositide pool. PI3KC2 and Mtm family members in vertebrates have been associated with antagonistic functions related to regulation of traffic to the plasma membrane. PI3KC2 isoforms are required to promote while overexpression of MTM1 impairs GLUT4 trafficking [20], [35] and integrin-mediated cell motility [21], [36]. Together, the observations point to a broad and conserved relationship for PI3KC2/Mtm co-regulation at the plasma membrane.
How might PI3KC2 and Mtm co-regulate integrin trafficking? One possibility is that the cycle of phosphoinositides co-regulated by PI3KC2/Mtm tunes the balance between endocytic-exocytic flux. The strong genetic interaction between mtm and Pi3K68D, in conjunction with Pi3KC2 ability to create PI(3)P in vivo [18], [20]–[23], supports the possibility that Pi3K68D could generate a PI(3)P substrate pool acted on by Mtm phosphatase. Alternatively, Pi3K68D could act more distantly on an interrelated phosphoinositide pool. We envision that Pi3K68D mediates early endocytic trafficking, tethering or sorting of integrin-containing vesicles. The integrin detected on large inclusions in mtm-depleted and XLMTM muscles in flies and humans, respectively, and evidence that mtm promotes membrane tubulation from PI(3)P compartments [18], [37], point to an Mtm/MTM1 role in membrane efflux for delivery of integrin to the plasma membrane. Mtm phosphatase could act to promote recycling or to negatively regulate retention, for example, through a PI(3)P-mediated fusion of integrin-containing vesicles with endosomes-lysosomes. An accumulation of β1-integrin on enlarged, perinuclear compartments has been observed with certain genetic manipulations in non-muscle cells. These results raise the possibility that normal Mtm phosphatase activity functions antagonistically to Rab21 GTPase or in concert with PKCε kinase, Rab11 and/or Arf6 GTPase, respectively, to control redelivery of β-integrin to the plasma membrane [38]–[40]. We found that class III PI3K, Vps34, also contributes to integrin localization upon myofiber remodeling, but with no effect on integrin-containing inclusions. A requirement for class III Pi3K could be at a shared step with the early endosomal Rab5 GTPase shown to be involved in integrin turnover at larval MTJs [6]. Thus, regulation of distinct PI(3)P pools is important for differential regulation of integrin endosomal trafficking, whereby Pi3KC2 and Mtm are dedicated to specific paired antagonistic functions.
We discovered that mtm is required in muscle for both integrin-mediated adhesions and T-tubule organization. The T-tubule requirement for mtm was similar to but not as severe as that for amph, the sole homolog of human AMPH2 that is also associated with centronuclear myopathy [41]. However, unlike mtm, null alleles of amph did not share a defect of myofiber detachment. Despite localization of βPS-integrin at T-tubules, and the dual requirements for mtm, we found that normal integrin adhesions and abnormal βPS-integrin localization on inclusions are independent of T-tubule organization. This suggests that mtm may serve a common function for integrin turnover and T-tubule formation at a shared precursor compartment, for example, at recycling endosomes, or alternatively, act independently at two distinct sites. β-integrin, Dlg and Amph are known to functionally interact at postsynaptic junctions [42]–[44], and MTMR2 has been shown to interact with Dlg1/SAP-97 and Dlg4/PSD-95 to promote postsynaptic function [45], [46]. Thus, the shared accumulation of βPS-integrin, Dlg and Amph on central membrane inclusions in mtm-depleted myofibers, and their elimination with Pi3K68D co-depletion, points to a possible role for a PI3KC2/Mtm pathway in endocytic recycling at neuromuscular junctions, as well as at MTJs.
Many of the defects observed in mtm mutant muscle parallel those associated with the human disease, XLMTM, demonstrating that the fly offers a tractable model for the cellular basis of centronuclear myopathy. Importantly, the discovery that mtm broadly regulates βPS-integrin turnover through endocytic trafficking led us to uncover a previously untested defect in β1-integrin localization in human XLMTM myofibers. Normal myofiber organization and function rely on integrin adhesions in vertebrate muscle [2], [3], [31], [47]. Thus, disruption of integrin regulation provides a basis for aspects of the severity of myofiber disorganization and dysfunction observed in XLMTM. The conservation between fly mtm and human MTM1 functions brings further significance to the potent interaction demonstrated between mtm and class II Pi3K68D for integrin regulation in flies. Whereas Class I and III PI3-kinases have been the focus of intense study as potential therapeutic targets of specific inhibitory compounds, the Class II PI3-kinases have received little attention. The knowledge of PI3KC2 contributions to specific MTM pathways is significant towards motivating similar studies for potential strategies addressing MTM-related disease.
Mtm is the single fly homolog related to both human MTM1 and MTMR2, and human MTMR2 expression was able to rescue integrin-related defects in mtm-depleted fly myofibers. An mtm pathway function in endocytic trafficking is therefore relevant to a more general understanding of the cell biological functions employed by MTM subfamily members. Mutations in MTMR2 associated with CMT4B neuropathy affect the morphology and function of myelinating Schwann cells [11], which like myofibers, share features of having an extensive plasma membrane and a reliance on integrin adhesions [48]. The regulation of integrin trafficking under the control of a conserved PI3KC2/Mtm pathway may be an important mechanism for controlling cell compartmentalization more broadly in different contexts, and relevant to different MTM-related human disease.
Materials and Methods
Ethics statement
Human samples were obtained and used as per institutional IRB accepted protocol.
Fly genetics
Flies were reared at 25°C, unless stated. Stocks used include: mtmΔ77, mtmz2-4747 and RNAi hairpins w; UAS-IR-mtm3-1 and w; UAS-IR-mtm3-5 interchangeably or in combination when “2x IR-mtm” noted, w; UAS-mtm:GFP7 and w; UAS-GFP:MTMR2 [18]; UAS-IR-Pi3K68Dv16240 and UAS-IR-Vps34v100296 (VDRC); UAS-Vps34-KDm8 [49]; How24B-GAL4, DMef2-GAL4, UAS-Dcr2; DMef2-GAL4, UAS-2xeGFPAH2, mys1 and mysts1 (Bloomington); if3 (F. Schöck); Ubi-βPS-integrin:YFP [6]; amph26 [28]; w, dlg1:GFPYC0005 (FlyTrap, [50]); UAS-GFP:LAMP [51]; UAS-GFP:Rab5 and UAS-GFP:myc:2xFYVE [52]; UAS-GFP:Rab7 [53]; UAS-mRFP:PH-FAPP1 (G. Polevoy and J. Brill). UAS-GFP:Atg8a [54] and Ubi-Talin:GFP [55]. To reduce βPS-integrin function, mys1/mysts1 flies raised at permissive 22°C were shifted to 29°C at pupation until lethality at pharate stage. To reduce mtm function specifically during pupation, UAS-EGFP/+; DMef2-GAL4, tubulin-Gal80ts/+ and UAS-EGFP, UAS-IR-mtm3.1/+; DMef2-GAL4, tubulin-Gal80ts/+ flies raised at permissive 18°C were shifted to 29°C at 2 days after pupation until pharate stage. To reduce mtm function in adults, UAS-Dcr2; Mef2-GAL4/UAS-EGFP and UAS-Dcr2; Mef2-GAL4/UAS-EGFP, UAS-IRmtm3.1 flies raised at permissive 18°C were shifted to 29°C at eclosion until adults were analyzed at 6 or 10 days old.
Muscle preparations and immunofluorescence
Staged pharate adults were removed from pupal case fastened to double-sided tape and pinned on a sylgard covered petri dish in dissecting buffer (5 mM HEPES, 128 mM NaCl, 2 mM KCl, 4 mM MgCl2, 36 mM sucrose, pH 7.2). Abdomens were opened with longitudinal and two lateral incisions, pinned flat, washed and fixed 30 min. (3.7% formaldehyde, 50 mM EGTA, PBS), washed, unpinned and blocked (0.3% bovine serum albuminum, 2% goat serum, 0.1% Triton, PBS), incubated with primary antibody overnight 4°C, washed (0.1% Triton PBS), reblocked and incubated overnight 4°C with Alexa-secondary antibodies (Molecular Probes), counterstained with phalloidin for F-actin and DNA as needed, and mounted in Fluorsave. Antibodies included Amph Ra29 (C. O'Kane), Dlg 4F3 and βPS-integrin CG.6G11 (Developmental Studies Hybridoma Bank), α-tubulin (Sigma-Aldrich), Zormin B1 (B. Bullard), muscle myosin (D. Kiehart), αPS2-integrin 7A10 [56], anti-Rabenosyn-5 [57] and KDEL (Babraham Institute). Propidium iodide (PI) staining was done on tissue. Pharate adult and adult abdomens were dissected in dissection buffer as above, washed once with PBS 0.2% BSA and maintained in 0.3 mg/ml PI final concentration in PBS 0.2% BSA throughout imaging. Images of abdomen fillets were taken with a Leica DMI 6000B inverted microscope using semi-apochromat 5× objective (N.A. 0.15), of live IOMs with a Zeiss Axiovert 200M using a LD-Plan NeoFluar 20× (N.A. 0.4) objective and of individual myofibers with FV1000 Olympus point scanning confocal using 60× Plan Apo N (N.A. 1.2) and 100× Plan Apo (N.A. 1.45) objectives. Exported TIFFs were handled by Adobe Photoshop or ImageJ software.
Timelapse microscopy
White pupae were positioned on double-sided tape on a coverslip, placed in a petri dish with water-soaked filter and incubated at 25°C. At each time point, the coverslip was flipped over for imaging on Leica DMI 6000B, as above.
FRAP
FRAP was carried out as described [6] in living 3rd instar larvae. Integrin:YFP was heterozygous (Int/+) in all experiments. A total of 21 and 19 individual FRAP experiments from multiple larvae were carried out for int/+ and mtmz2-4747/mtmΔ77;int/+ respectively.
Electron microscopy
Pharate adults dissected and pinned flat were fixed 1 hr. RT (0.1 M sodium phosphate, 3% paraformaldehyde, 2% glutaraldehyde, 2 mM sodium EGTA, 0.1 M sucrose, pH 7.2), postfixed 1 hr. (1% osmium tetroxide in 0.1 M cacodylate buffer) and stained 1 hr. (1% uranyl acetate). Abdomen fillets were embedded in epoxy resin and 70 nm sections were collected on Formvar and carbon-coated copper grids.
Viability and flight assays
To assay viability, pre-cleared vials were counted for surviving adults, dead adults post-eclosion on food and mid-eclosion, dead pharates and dead pupae at 13 or 17 days after egg laying. Flies 1–6 days old were tested for flight at least 24 hours after CO2 anesthesia, by releasing 12 females in a 2 L cylinder (50.8 cm high). Flies that landed below 0.6 L (14.6 cm) were scored flightless.
Human muscle studies
8 mM cryosections were obtained from muscle biopsies from 3 genetically confirmed cases of XLMTM and age matched cases without obvious histopathology (Carsten Bonnemann), per institutional IRB accepted protocol. Sections were stained using manufacturers instructions (NovoLink kit, Novocastra) and as previously described [14] using primary antibodies to beta1D integrin (Chemicon; 1∶25) and dystroglycan (Novocastra; 1∶20).
Statistical analyses
Visual quantification was made for number of IOMs in tergites 3 and 4, number of detached IOMs per abdomen, number of IOMs displaying βPS-integrin costameres or βPS-integrin - or Dlg-marked inclusions, and number of nuclei per IOM. ImageJ software was used to draw and measure nuclei distance to IOM midline and sarcomere length. CellProfiler was used to segment and quantify nuclei morphology. Statistical analysis in Prism software used to determine mean, standard error and Student's t-test, where possible.
Genotypes
Full genotypes used are as shown in Figure Legends and as follows:
Figure 1. (D,G,H,I,J) Control, UAS-EGFP/+; DMef2-GAL4/+. (D′,G′,H′,I,J) RNAi: UAS-EGFP, UAS-IR-mtm3.1/+; DMef2-GAL4/+. (C) 24B-GAL4. Control: UAS-EGFP/24B-GAL4. RNAi: UAS-EGFP, UAS-IR-mtm3.1/24B-GAL4. RNAi with mtm cDNA: UAS-mtm:EGFP, UAS-IR-mtm3.1/24B-GAL4. RNAi with human MTMR2 cDNA: UAS-EGFP:huMTMR2, UAS-IR-mtm3.1/24B-GAL4. DMef2-GAL4. Control: UAS-EGFP/+; DMef2-GAL4, UAS-LacZ/+. RNAi: UAS-EGFP/+; DMef2-GAL4, UAS-IR-mtm3.5/+. (E, F). 24B-GAL4: Control: UAS-EGFP/24B-GAL4. 1x RNAi: UAS-IR-mtm3.1/24B-GAL4. 2x RNAi: UAS-IR-mtm3.1/24B-GAL4; UAS-IR-mtm3.5/+. DMef2-GAL4. Control: UAS-EGFP/+; DMef2-GAL4/+. 1x RNAi: UAS-IR-mtm3.1/+; DMef2-GAL4/+. 2x RNAi: UAS-IR-mtm3.1/+; DMef2-GAL4/UAS-IR-mtm3.5.
Figure 2. (B,C,D,E) Control: UAS-EGFP/+; DMef2-GAL4/+. (B′,C′,D′,E′) RNAi: UAS-EGFP, UAS-IR-mtm3.1/+; DMef2-GAL4/+. (F) Control: Ubi-βPS-integrin:YFP/+. mutants: mtmΔ77/mtmz2-4747/Ubi-βPS-integrin:YFP. (G,H) dlg1:GFP. (I) Control: UAS-Dcr2; Mef2-GAL4/UAS-EGFP. RNAi: UAS-Dcr2; Mef2-GAL4/UAS-EGFP, UAS-IRmtm3.1.
Figure 3. (B,C) Control: UAS-EGFP/+; DMef2-GAL4/+. RNAi: UAS-EGFP, UAS-IR-mtm3.1/+; DMef2-GAL4/+. (D, D′) amph26. (E) amph26/amph26, UAS-IR-mtm3.1/+; DMef2-GAL4/+. (F) mys1/mysts1.
Figure 4. (A) Control: UAS-GFP:LAMP/+; DMef2-GAL4/+. (A′–A″) RNAi: UAS-GFP:LAMP, UAS-IR-mtm3.1/+; DMef2-GAL4/+. (B) Control: UAS-GFP:Rab5/+; DMef2-GAL4, UAS-lacZ/+. (B′–B″) RNAi: UAS-GFP:Rab5/+; DMef2-GAL4, UAS-IRmtm3.5/+. (C,D) Control: UAS-GFP:myc:2XFYVE/+; DMef2-GAL4/+. (C′,D′,C′,D″′) RNAi: UAS-GFP:myc:2XFYVE, UAS-IR-mtm3.1/+; DMef2-GAL4/+.
Figure 5. (B,C,D,E,F) Control: UAS-EGFP/+; DMef2-GAL4, UAS-lacZ/+. RNAi: UAS-EGFP/+; DMef2-GAL4, UAS-IR-mtm3.5/+. (A,A′,B,C′,C″D,E′,E″,F) Single RNAi: DMef2-GAL4, UAS-lacZ/UAS-IR-Pi3K68D or UAS-IR-Vps34/+; DMef2-GAL4, UAS-lacZ/+. mtm co-RNAi: DMef2-GAL4, UAS-IR-mtm3.5/UAS-IR-Pi3K68D or UAS-IR-Vps34/+; DMef2-GAL4, UAS-IR-mtm3.5/+.
Supporting Information
Zdroje
1. BrowerDL
2003 Platelets with wings: the maturation of Drosophila integrin biology. Curr Opin Cell Biol 15 607 613
2. MayerU
SaherG
FasslerR
BornemannA
EchtermeyerF
1997 Absence of integrin alpha 7 causes a novel form of muscular dystrophy. Nat Genet 17 318 323
3. VolkT
FesslerLI
FesslerJH
1990 A role for integrin in the formation of sarcomeric cytoarchitecture. Cell 63 525 536
4. CaswellPT
VadrevuS
NormanJC
2009 Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol 10 843 853
5. KaistoT
RahkilaP
MarjomakiV
PartonRG
MetsikkoK
1999 Endocytosis in skeletal muscle fibers. Exp Cell Res 253 551 560
6. YuanL
FairchildMJ
PerkinsAD
TanentzapfG
2010 Analysis of integrin turnover in fly myotendinous junctions. J Cell Sci 123 939 946
7. Di PaoloG
De CamilliP
2006 Phosphoinositides in cell regulation and membrane dynamics. Nature 443 651 657
8. NicotAS
LaporteJ
2008 Endosomal phosphoinositides and human diseases. Traffic 9 1240 1249
9. Buj-BelloA
LaugelV
MessaddeqN
ZahreddineH
LaporteJ
2002 The lipid phosphatase myotubularin is essential for skeletal muscle maintenance but not for myogenesis in mice. Proc Natl Acad Sci U S A 99 15060 15065
10. LaporteJ
HuLJ
KretzC
MandelJL
KioschisP
1996 A gene mutated in X-linked myotubular myopathy defines a new putative tyrosine phosphatase family conserved in yeast. Nat Genet 13 175 182
11. BolinoA
MugliaM
ConfortiFL
LeGuernE
SalihMA
2000 Charcot-Marie-Tooth type 4B is caused by mutations in the gene encoding myotubularin-related protein-2. Nat Genet 25 17 19
12. BergerP
BonneickS
WilliS
WymannM
SuterU
2002 Loss of phosphatase activity in myotubularin-related protein 2 is associated with Charcot-Marie-Tooth disease type 4B1. Hum Mol Genet 11 1569 1579
13. CaoC
BackerJM
LaporteJ
BedrickEJ
Wandinger-NessA
2008 Sequential actions of myotubularin lipid phosphatases regulate endosomal PI(3)P and growth factor receptor trafficking. Mol Biol Cell 19 3334 3346
14. DowlingJJ
VreedeAP
LowSE
GibbsEM
KuwadaJY
2009 Loss of myotubularin function results in T-tubule disorganization in zebrafish and human myotubular myopathy. PLoS Genet 5 e1000372 doi:10.1371/journal.pgen.1000372
15. KimSA
TaylorGS
TorgersenKM
DixonJE
2002 Myotubularin and MTMR2, phosphatidylinositol 3-phosphatases mutated in myotubular myopathy and type 4B Charcot-Marie-Tooth disease. J Biol Chem 277 4526 4531
16. TaylorGS
MaehamaT
DixonJE
2000 Inaugural article: myotubularin, a protein tyrosine phosphatase mutated in myotubular myopathy, dephosphorylates the lipid second messenger, phosphatidylinositol 3-phosphate. Proc Natl Acad Sci U S A 97 8910 8915
17. TsujitaK
ItohT
IjuinT
YamamotoA
ShishevaA
2004 Myotubularin regulates the function of the late endosome through the gram domain-phosphatidylinositol 3,5-bisphosphate interaction. J Biol Chem 279 13817 13824
18. VelichkovaM
JuanJ
KadandaleP
JeanS
RibeiroI
2010 Drosophila Mtm and class II PI3K coregulate a PI(3)P pool with cortical and endolysosomal functions. J Cell Biol 190 407 425
19. LindmoK
StenmarkH
2006 Regulation of membrane traffic by phosphoinositide 3-kinases. J Cell Sci 119 605 614
20. FalascaM
HughesWE
DominguezV
SalaG
FostiraF
2007 The role of phosphoinositide 3-kinase C2alpha in insulin signaling. J Biol Chem 282 28226 28236
21. MaffucciT
CookeFT
FosterFM
TraerCJ
FryMJ
2005 Class II phosphoinositide 3-kinase defines a novel signaling pathway in cell migration. J Cell Biol 169 789 799
22. SrivastavaS
DiL
ZhdanovaO
LiZ
VardhanaS
2009 The class II phosphatidylinositol 3 kinase C2beta is required for the activation of the K+ channel KCa3.1 and CD4 T-cells. Mol Biol Cell 20 3783 3791
23. WenPJ
OsborneSL
MorrowIC
PartonRG
DominJ
2008 Ca2+-regulated pool of phosphatidylinositol-3-phosphate produced by phosphatidylinositol 3-kinase C2alpha on neurosecretory vesicles. Mol Biol Cell 19 5593 5603
24. RoyS
VijayRaghavanK
1998 Patterning muscles using organizers: larval muscle templates and adult myoblasts actively interact to pattern the dorsal longitudinal flight muscles of Drosophila. J Cell Biol 141 1135 1145
25. WasserM
Bte OsmanZ
ChiaW
2007 EAST and Chromator control the destruction and remodeling of muscles during Drosophila metamorphosis. Dev Biol 307 380 393
26. KozopasKM
SamosCH
NusseR
1998 DWnt-2, a Drosophila Wnt gene required for the development of the male reproductive tract, specifies a sexually dimorphic cell fate. Genes Dev 12 1155 1165
27. Al-QusairiL
WeissN
ToussaintA
BerbeyC
MessaddeqN
2009 T-tubule disorganization and defective excitation-contraction coupling in muscle fibers lacking myotubularin lipid phosphatase. Proc Natl Acad Sci U S A 106 18763 18768
28. RazzaqA
RobinsonIM
McMahonHT
SkepperJN
SuY
2001 Amphiphysin is necessary for organization of the excitation-contraction coupling machinery of muscles, but not for synaptic vesicle endocytosis in Drosophila. Genes Dev 15 2967 2979
29. WilcoxM
DiAntonioA
LeptinM
1989 The function of PS integrins in Drosophila wing morphogenesis. Development 107 891 897
30. WalshEP
BrownNH
1998 A screen to identify Drosophila genes required for integrin-mediated adhesion. Genetics 150 791 805
31. ErvastiJM
2003 Costameres: the Achilles' heel of Herculean muscle. J Biol Chem 278 13591 13594
32. SparrowJC
SchockF
2009 The initial steps of myofibril assembly: integrins pave the way. Nat Rev Mol Cell Biol 10 293 298
33. PerkinsAD
EllisSJ
AsghariP
ShamsianA
MooreED
2010 Integrin-mediated adhesion maintains sarcomeric integrity. Dev Biol 338 15 27
34. MacDougallLK
GagouME
LeeversSJ
HafenE
WaterfieldMD
2004 Targeted expression of the class II phosphoinositide 3-kinase in Drosophila melanogaster reveals lipid kinase-dependent effects on patterning and interactions with receptor signaling pathways. Mol Cell Biol 24 796 808
35. ChaussadeC
PirolaL
BonnafousS
BlondeauF
Brenz-VercaS
2003 Expression of myotubularin by an adenoviral vector demonstrates its function as a phosphatidylinositol 3-phosphate [PtdIns(3)P] phosphatase in muscle cell lines: involvement of PtdIns(3)P in insulin-stimulated glucose transport. Mol Endocrinol 17 2448 2460
36. DominJ
HarperL
AubynD
WheelerM
FloreyO
2005 The class II phosphoinositide 3-kinase PI3K-C2beta regulates cell migration by a PtdIns3P dependent mechanism. J Cell Physiol 205 452 462
37. FiliN
CallejaV
WoscholskiR
ParkerPJ
LarijaniB
2006 Compartmental signal modulation: Endosomal phosphatidylinositol 3-phosphate controls endosome morphology and selective cargo sorting. Proc Natl Acad Sci U S A 103 15473 15478
38. IvaskaJ
WhelanRD
WatsonR
ParkerPJ
2002 PKC epsilon controls the traffic of beta1 integrins in motile cells. EMBO J 21 3608 3619
39. PellinenT
ArjonenA
VuoriluotoK
KallioK
FransenJA
2006 Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of beta1-integrins. J Cell Biol 173 767 780
40. PowelkaAM
SunJ
LiJ
GaoM
ShawLM
2004 Stimulation-dependent recycling of integrin beta1 regulated by ARF6 and Rab11. Traffic 5 20 36
41. NicotAS
ToussaintA
ToschV
KretzC
Wallgren-PetterssonC
2007 Mutations in amphiphysin 2 (BIN1) disrupt interaction with dynamin 2 and cause autosomal recessive centronuclear myopathy. Nat Genet 39 1134 1139
42. BeumerK
MatthiesHJ
BradshawA
BroadieK
2002 Integrins regulate DLG/FAS2 via a CaM kinase II-dependent pathway to mediate synapse elaboration and stabilization during postembryonic development. Development 129 3381 3391
43. MathewD
PopescuA
BudnikV
2003 Drosophila amphiphysin functions during synaptic Fasciclin II membrane cycling. J Neurosci 23 10710 10716
44. RegaladoMP
Terry-LorenzoRT
WaitesCL
GarnerCC
MalenkaRC
2006 Transsynaptic signaling by postsynaptic synapse-associated protein 97. J Neurosci 26 2343 2357
45. BolinoA
BolisA
PrevitaliSC
DinaG
BussiniS
2004 Disruption of Mtmr2 produces CMT4B1-like neuropathy with myelin outfolding and impaired spermatogenesis. J Cell Biol 167 711 721
46. LeeHW
KimY
HanK
KimH
KimE
2010 The phosphoinositide 3-phosphatase MTMR2 interacts with PSD-95 and maintains excitatory synapses by modulating endosomal traffic. J Neurosci 30 5508 5518
47. HayashiYK
ChouFL
EngvallE
OgawaM
MatsudaC
1998 Mutations in the integrin alpha7 gene cause congenital myopathy. Nat Genet 19 94 97
48. BarrosCS
NguyenT
SpencerKS
NishiyamaA
ColognatoH
2009 Beta1 integrins are required for normal CNS myelination and promote AKT-dependent myelin outgrowth. Development 136 2717 2724
49. JuhaszG
HillJH
YanY
SassM
BaehreckeEH
2008 The class III PI(3)K Vps34 promotes autophagy and endocytosis but not TOR signaling in Drosophila. J Cell Biol 181 655 666
50. Quinones-CoelloAT
PetrellaLN
AyersK
MelilloA
MazzalupoS
2007 Exploring strategies for protein trapping in Drosophila. Genetics 175 1089 1104
51. PulipparacharuvilS
AkbarMA
RayS
SevrioukovEA
HabermanAS
2005 Drosophila Vps16A is required for trafficking to lysosomes and biogenesis of pigment granules. J Cell Sci 118 3663 3673
52. WucherpfennigT
Wilsch-BrauningerM
Gonzalez-GaitanM
2003 Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J Cell Biol 161 609 624
53. EntchevEV
SchwabedissenA
Gonzalez-GaitanM
2000 Gradient formation of the TGF-beta homolog Dpp. Cell 103 981 991
54. ScottRC
SchuldinerO
NeufeldTP
2004 Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev Cell 7 167 178
55. TanentzapfG
BrownNH
2006 An interaction between integrin and the talin FERM domain mediates integrin activation but not linkage to the cytoskeleton. Nat Cell Biol 8 601 606
56. BogaertT
BrownN
WilcoxM
1987 The Drosophila PS2 antigen is an invertebrate integrin that, like the fibronectin receptor, becomes localized to muscle attachments. Cell 51 929 940
57. MorrisonHA
DionneH
RustenTE
BrechA
FisherWW
2008 Regulation of early endosomal entry by the Drosophila tumor suppressors Rabenosyn and Vps45. Mol Biol Cell 19 4167 4176
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 2
-
Všechny články tohoto čísla
- Break to Make a Connection
- A New Testing Strategy to Identify Rare Variants with Either Risk or Protective Effect on Disease
- Single-Tissue and Cross-Tissue Heritability of Gene Expression Via Identity-by-Descent in Related or Unrelated Individuals
- Pervasive Adaptive Protein Evolution Apparent in Diversity Patterns around Amino Acid Substitutions in
- The Architecture of Gene Regulatory Variation across Multiple Human Tissues: The MuTHER Study
- MiRNA Control of Vegetative Phase Change in Trees
- New Functions of Ctf18-RFC in Preserving Genome Stability outside Its Role in Sister Chromatid Cohesion
- Genome-Wide Association Studies of the PR Interval in African Americans
- Mapping of the Disease Locus and Identification of As a Candidate Gene in a Canine Model of Primary Open Angle Glaucoma
- Mapping a New Spontaneous Preterm Birth Susceptibility Gene, , Using Linkage, Haplotype Sharing, and Association Analysis
- A Population Genetic Approach to Mapping Neurological Disorder Genes Using Deep Resequencing
- and Genes Modulate the Switch between Attraction and Repulsion during Behavioral Phase Change in the Migratory Locust
- Targeted Sister Chromatid Cohesion by Sir2
- Correlated Evolution of Nearby Residues in Drosophilid Proteins
- Parallel Evolution of a Type IV Secretion System in Radiating Lineages of the Host-Restricted Bacterial Pathogen
- Lipophorin Receptors Mediate the Uptake of Neutral Lipids in Oocytes and Imaginal Disc Cells by an Endocytosis-Independent Mechanism
- Genome-Wide Association Study of Coronary Heart Disease and Its Risk Factors in 8,090 African Americans: The NHLBI CARe Project
- The Evolution of Host Specialization in the Vertebrate Gut Symbiont
- Genome-Wide Association of Familial Late-Onset Alzheimer's Disease Replicates and and Nominates in Interaction with
- Risk Alleles for Systemic Lupus Erythematosus in a Large Case-Control Collection and Associations with Clinical Subphenotypes
- Association between Common Variation at the Locus and Changes in Body Mass Index from Infancy to Late Childhood: The Complex Nature of Genetic Association through Growth and Development
- AID Induces Double-Strand Breaks at Immunoglobulin Switch Regions and Causing Chromosomal Translocations in Yeast THO Mutants
- A Study of CNVs As Trait-Associated Polymorphisms and As Expression Quantitative Trait Loci
- Whole-Genome Comparison Reveals Novel Genetic Elements That Characterize the Genome of Industrial Strains of
- Prevalence of Epistasis in the Evolution of Influenza A Surface Proteins
- Srf1 Is a Novel Regulator of Phospholipase D Activity and Is Essential to Buffer the Toxic Effects of C16:0 Platelet Activating Factor
- Two Frizzled Planar Cell Polarity Signals in the Wing Are Differentially Organized by the Fat/Dachsous Pathway
- Phosphoinositide Regulation of Integrin Trafficking Required for Muscle Attachment and Maintenance
- Pathogenic VCP/TER94 Alleles Are Dominant Actives and Contribute to Neurodegeneration by Altering Cellular ATP Level in a IBMPFD Model
- Meta-Analysis of Genome-Wide Association Studies in Celiac Disease and Rheumatoid Arthritis Identifies Fourteen Non-HLA Shared Loci
- A Genome-Wide Study of DNA Methylation Patterns and Gene Expression Levels in Multiple Human and Chimpanzee Tissues
- Nucleosomes Containing Methylated DNA Stabilize DNA Methyltransferases 3A/3B and Ensure Faithful Epigenetic Inheritance
- Mutations in Zebrafish Result in Adult-Onset Ocular Pathogenesis That Models Myopia and Other Risk Factors for Glaucoma
- [], the Prion Formed by the Chromatin Remodeling Factor Swi1, Is Highly Sensitive to Alterations in Hsp70 Chaperone System Activity
- Characterization of Transcriptome Remodeling during Cambium Formation Identifies and As Opposing Regulators of Secondary Growth
- The Cardiac Transcription Network Modulated by Gata4, Mef2a, Nkx2.5, Srf, Histone Modifications, and MicroRNAs
- Epistatic Interaction Maps Relative to Multiple Metabolic Phenotypes
- Quantitative Models of the Mechanisms That Control Genome-Wide Patterns of Transcription Factor Binding during Early Development
- Genome-Wide Transcript Profiling of Endosperm without Paternal Contribution Identifies Parent-of-Origin–Dependent Regulation of
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Meta-Analysis of Genome-Wide Association Studies in Celiac Disease and Rheumatoid Arthritis Identifies Fourteen Non-HLA Shared Loci
- MiRNA Control of Vegetative Phase Change in Trees
- Risk Alleles for Systemic Lupus Erythematosus in a Large Case-Control Collection and Associations with Clinical Subphenotypes
- The Cardiac Transcription Network Modulated by Gata4, Mef2a, Nkx2.5, Srf, Histone Modifications, and MicroRNAs
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



