-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaLipophorin Receptors Mediate the Uptake of Neutral Lipids in Oocytes and Imaginal Disc Cells by an Endocytosis-Independent Mechanism
Lipids are constantly shuttled through the body to redistribute energy and metabolites between sites of absorption, storage, and catabolism in a complex homeostatic equilibrium. In Drosophila, lipids are transported through the hemolymph in the form of lipoprotein particles, known as lipophorins. The mechanisms by which cells interact with circulating lipophorins and acquire their lipidic cargo are poorly understood. We have found that lipophorin receptor 1 and 2 (lpr1 and lpr2), two partially redundant genes belonging to the Low Density Lipoprotein Receptor (LDLR) family, are essential for the efficient uptake and accumulation of neutral lipids by oocytes and cells of the imaginal discs. Females lacking the lpr2 gene lay eggs with low lipid content and have reduced fertility, revealing a central role for lpr2 in mediating Drosophila vitellogenesis. lpr1 and lpr2 are transcribed into multiple isoforms. Interestingly, only a subset of these isoforms containing a particular LDLR type A module mediate neutral lipid uptake. Expression of these isoforms induces the extracellular stabilization of lipophorins. Furthermore, our data indicate that endocytosis of the lipophorin receptors is not required to mediate the uptake of neutral lipids. These findings suggest a model where lipophorin receptors promote the extracellular lipolysis of lipophorins. This model is reminiscent of the lipolytic processing of triglyceride-rich lipoproteins that occurs at the mammalian capillary endothelium, suggesting an ancient role for LDLR–like proteins in this process.
Published in the journal: . PLoS Genet 7(2): e32767. doi:10.1371/journal.pgen.1001297
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001297Summary
Lipids are constantly shuttled through the body to redistribute energy and metabolites between sites of absorption, storage, and catabolism in a complex homeostatic equilibrium. In Drosophila, lipids are transported through the hemolymph in the form of lipoprotein particles, known as lipophorins. The mechanisms by which cells interact with circulating lipophorins and acquire their lipidic cargo are poorly understood. We have found that lipophorin receptor 1 and 2 (lpr1 and lpr2), two partially redundant genes belonging to the Low Density Lipoprotein Receptor (LDLR) family, are essential for the efficient uptake and accumulation of neutral lipids by oocytes and cells of the imaginal discs. Females lacking the lpr2 gene lay eggs with low lipid content and have reduced fertility, revealing a central role for lpr2 in mediating Drosophila vitellogenesis. lpr1 and lpr2 are transcribed into multiple isoforms. Interestingly, only a subset of these isoforms containing a particular LDLR type A module mediate neutral lipid uptake. Expression of these isoforms induces the extracellular stabilization of lipophorins. Furthermore, our data indicate that endocytosis of the lipophorin receptors is not required to mediate the uptake of neutral lipids. These findings suggest a model where lipophorin receptors promote the extracellular lipolysis of lipophorins. This model is reminiscent of the lipolytic processing of triglyceride-rich lipoproteins that occurs at the mammalian capillary endothelium, suggesting an ancient role for LDLR–like proteins in this process.
Introduction
Organisms need to tightly regulate the balance between energy intake, usage and storage. Imbalances in these processes are at the heart of several major human health problems such as obesity, cardiovascular disease and diabetes [1]. In recent years, the use of Drosophila and other genetically tractable model organisms has provided novel approaches and insights into the study of the mechanisms controlling energy balance. In particular, genetic screens have shown their potential for the identification of novel genes and regulatory mechanisms involved in the maintenance of lipid homeostasis. Importantly, despite the evolutionary distance separating humans from flies, many of the central pathways controlling metabolism are conserved (for reviews, see [2]–[6]). Despite these advances, several basic aspects of Drosophila lipid metabolism are still unknown. Here we focus on the mechanisms controlling the cellular uptake of neutral lipids.
Most metazoans accumulate triacylglycerol (TAG), a strongly hydrophobic molecule with a high energy content, as the main substrate for energy storage. Large amounts of TAG are stored in fat body cells, the Drosophila equivalent of adipocytes, but most other cell types also accumulate limited amounts of it as intracellular lipid droplets. Because of their hydrophobicity, the extracellular transport of lipids requires dedicated mechanisms to increase their solubility in extracellular fluids. In mammals, lipids are packed into several types of lipoprotein particles which contain a hydrophobic core of neutral lipids (mostly TAG and esterified cholesterol) surrounded by a monolayer of phospholipids. In addition, apolipoproteins stabilize and regulate these particles [7]. Similar lipoproteins, named lipophorins, are also found in insects [8], [9]. They share the same basic structure and play similar functions as mammalian lipoproteins. In Drosophila, apolipophorins are exclusively synthesized in the fat body [10], [11], where they are partially lipidated and released into the hemolymph. It has been suggested that lipophorins act as a reusable shuttle in lipid transport. Lipids, primarily diacylglycerol (DAG), derived from the digestion of food in the gut or from the mobilization of lipids in the fat body, are loaded onto pre-formed, circulating lipophorins, then transported through the body via the hemolymph and unloaded upon reaching peripheral tissues for use as a source of energy and phospholipids. During this cycling process, negligible degradation of apolipophorin occurs [8], [12].
In mammals, the Low Density Lipoprotein Receptor (LDLR) and other related proteins mediate endocytosis and the clearance of lipoproteins from plasma [13]. Similar proteins belonging to the LDLR family, known as lipophorin receptors, were subsequently identified in insects. They can bind to lipophorins and mediate their endocytosis both in cell culture systems and in vivo [14]–[16]. Because of these properties, it has been suggested that lipophorin receptors may play an important role in insect lipid metabolism [17].
Here, we examine the function of Drosophila lipophorin receptors in the uptake of neutral lipids. We show that this organism has two lipophorin receptor genes, the lipophorin receptor 1 (lpr1) and lpr2, which are translated into multiple, functionally diverse isoforms. lpr1 and lpr2 are required for neutral lipid uptake in imaginal disc cells and oocytes. Our results suggest a model where lpr1 and lpr2 promote the extracellular hydrolysis of neutral lipids contained in lipoprotein particles.
Results
Genomic organization and alternative splicing of Drosophila lipophorin receptor genes
A defining characteristic of the LDLR family of transmembrane receptors is the presence of an ectodomain containing specific combinations of three types of protein modules: LDL receptor type A modules (LA), also called complement-type repeats, EGF modules and YWTD β-propellers [18]. This structural definition was used to identify LDLR family members in the Drosophila genome. Of the seven genes identified (Figure S1), two are arranged in tandem and show a high degree of homology to insect lipophorin receptors. On this basis, we named then lipophorin receptor 1 (lpr1) and lpr2 (Figure 1A). The analysis of cDNA clones generated during large scale transcriptomic studies [19] suggested the existance of multiple isoforms for each gene. To further characterize the range of isoforms derived from lpr1 and lpr2, we obtained additional cDNAs from whole adult fly RNA, as well as from RNA purified from specific tissues. These cDNAs were genotyped by PCR and sequenced, leading to the identification of a total of 6 isoforms for lpr1 and 5 for lpr2 (Figure 1B). The different isoforms result from alternative splicing and from the use of two alternative promoters for each gene, that we named proximal and distal promoters, referring to their chromosomal position with respect to the centromere. The number and organization of exons are remarkably similar between the two genes, with lrp1 and lpr2 exons in equivalent positions coding for equivalent protein domains. Isoforms differ in three main characteristics: (1) the number and identity of LA modules; (2) the presence of an extended putative O-glycosylation region rich in serine and threonine next to the transmembrane domain and (3) the presence of an N-terminal domain with no homology to other proteins (non-conserved N-terminal domain, NCN) (Figure 1B). It is noteworthy that isoforms transcribed from the distal promoters are predicted, using PrediSi software [20], to have unusually long putative signal peptides: 68 and 88 amino acids for lpr1 and lpr2, respectively. Isoforms transcribed from the proximal promoters are predicted to have more typical signal peptides of 20 (lpr1) and 24 (lpr2) amino acids.
Fig. 1. Genomic organization of lpr1 and lpr2 genes. 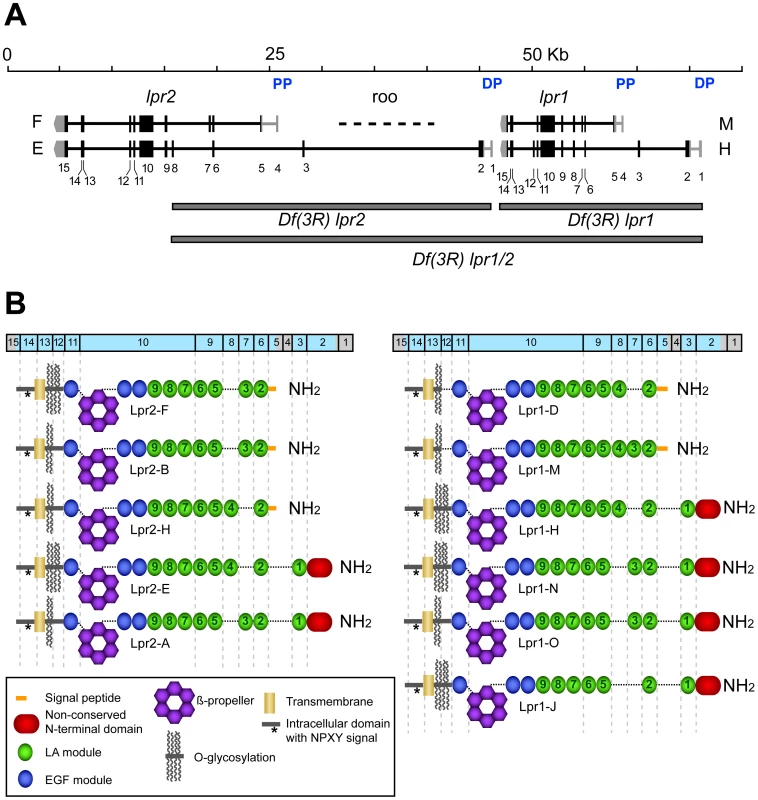
(A) lpr1 and lpr2 are transcribed as multiple isoforms. Each gene has two promoters, a distal promoter (DP) and a proximal promoter (PP). Only one isoform transcribed from each promoter is displayed in the figure. The complete list of isoforms is detailed in panel B. Exons are labeled from 1 to 15. We generated three deficiencies Df(3R)lpr1, Df(3R)lpr2 and Df(3R)lpr1/2, which are indicated as grey rectangles. A natural roo transposon is present in the second lpr2 intron. (B) Modular structure of Lpr1 and Lpr2 isoforms, showing the correspondence between protein domains and exons (blue box on top). The existence of all depicted isoforms is supported by the isolation of corresponding cDNAs. The UTR regions are marked in grey. lpr1 and lpr2 are required for the accumulation of lipid droplets in oocytes and imaginal disc cells
To examine the role of the lipophorin receptors in lipid metabolism, we first generated three small deletions in the lpr1-lpr2 genomic region (Figure 1A). Df(3R)lpr1 completely deletes lpr1; Df(3R)lpr2 deletes exons 1 to 8 of lpr2, including the promoters and translation initiation codons, while Df(3R)lpr1/2 affects both genes. The breakpoints for each deficiency were confirmed by PCR analysis. Even though several lpr2 exons are still present in Df(3R)lpr2 and Df(3R)lpr1/2 chromosomes, we did not detect Lpr2 protein expression in either deficiency using an antibody which recognizes Lpr2 intracellular domain, which is common to all Lpr2 isoforms (Figure S2C–S2F and not shown). This data strongly suggests that Df(3R)lpr2 is a null allele for lpr2 and Df(3R)lpr1/2 is a null allele for both lpr1 and lpr2. Flies with mutations in individual lipophorin receptor genes as well as the double mutant Df(3R)lpr1/2 were homozygous viable and displayed fertility phenotypes: Df(3R)lpr1 females laid eggs which hatched at rates similar to wild-type females (85.5% hatching rate for Df(3R)lpr1 compared to 87.5% for the wild-type stock Oregon R, n = 200). Df(3R)lpr2 females laid eggs but most of them failed to hatch (0.5% hatching rate; n = 200). Df(3R)lpr1/2 females were completely sterile, where the few eggs laid by young flies failed to hatch. These results indicate that lpr2, and to a lesser extent lpr1, are required for normal oogenesis. The Drosophila ovarian follicle is composed of a 16-cell germ-line cyst with one oocyte and 15 nurse cells, which is surrounded by somatic follicle cells. During vitellogenesis, the oocyte and nurse cells increase in volume and accumulate large amounts of yolk proteins and neutral lipids (Figure 2F–2I) that are captured from the surrounding hemolymph [21]. The Yolkless (Yl) receptor mediates the endocytic uptake of yolk proteins [22], [23]. However, no receptor involved in lipid uptake has been reported. To analyze whether lpr1 or lpr2 mediate lipid uptake during vitellogenesis, we first examined the lipid content of ovaries from wild-type and Df(3R)lpr2 females with the lipophilic nile red dye. Accumulation of neutral lipids was first visible in stage 9 wild-type egg chambers and reached a maximum by the end of vitellogenesis at stage 11 (Figure 2F–2I). A marked decrease in lipid droplets was observed in Df(3R)lpr2 egg chambers (Figure 2J, compare to Figure 2G. Figure S3A) or when this deficiency was combined with the double mutant Df(3R)lpr1/2 (not shown). Most of the embryos originating from Df(3R)lpr2 mutant oocytes could not complete embryogenesis and died at various stages of development, showing generalized apoptosis and pleiotropic phenotypes such as muscle detachment and nervous system malformations (not shown). A small number of Df(3R)lpr2 egg chambers exhibited higher lipid levels (Figure S3A). It seems likely that the few embryos that successfully hatched from Df(3R)lpr2 females (0.5%) were derived from these egg chambers.
Fig. 2. Lpr2 is required for the uptake of neutral lipids during Drosophila vitellogenesis. 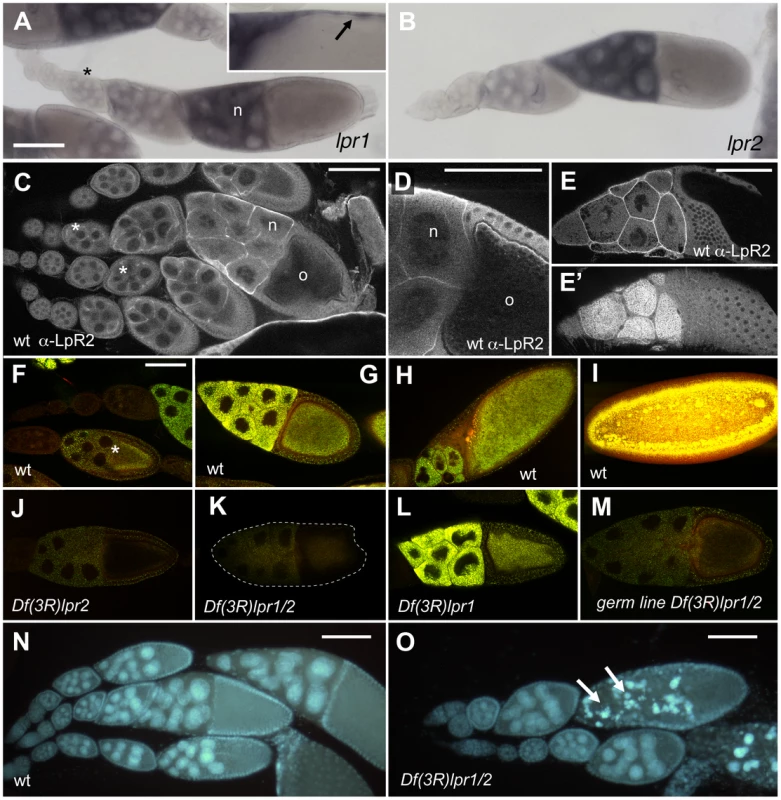
(A–B) lpr1 (A) and lpr2 (B) expression in wild-type ovarioles detected by in situ hybridization. Transcripts of both genes were first visible in the nurse cells (n) of stage 8 egg chambers (asterisk) and their levels increased thereafter. lrp1 transcripts were also detected in the follicle cells of mature egg chambers (A, arrow in inset). (C–E) Immunostaining showing Lpr2 protein localization during oogenesis. Lpr2 is first detected at low levels in stage 8 egg chambers, coinciding with the start of vitellogenesis (asterisks in C). (D) Magnification of a stage 10 egg chamber showing expression at the nurse cells (n) and oocyte (o) membranes. Maximal expression was detected at stage 11 egg chambers (E and E', two focal planes). (F–M) Nile red staining of egg chambers (F–H and J–M) and one blastoderm stage embryos (I) to reveal lipid droplets (yellow, nile red dye fluorescence was captured in the green and red channels). (F–I) Wild-type (wt) genotype. Neutral lipids start to accumulate at vitellogenic stages and reach a maximum in blastoderm embryos. Note that near the end of vitellogenesis, nurse cells degenerate and dump their content into the oocyte. (F) stage 9 (asterisk), (G) stage 10 and (H) stage 11 egg chambers. (J–M) stage 10 egg chambers of the indicated genotypes. Accumulation of neutral lipids is reduced in Df(3R)lpr2 (J), Df(3R)lpr1/2 (K, egg chamber outlined) and Df(3R)lpr1/2 germ-line clones (M) and is normal in Df(3R)lpr1 egg chambers (L). The Df(3R)lpr1/2 egg chamber shown in (K) was dissected from young females in which a few egg chambers in each ovary escaped degeneration at mid oogenesis. (N, O) Egg chambers of wild-type (N) and Df(3R)lpr1/2 (O) ovaries stained with DAPI to reveal the nuclei. Several stage 10 egg chambers display nuclear fragmentation in Df(3R)lpr1/2 females (arrows in O). Scale bars: 100 µm. (F–M) shown at the same magnification. We used a Lpr2-specific antibody to examine the distribution of Lpr2 in wild-type egg chambers, detecting Lpr2 protein at the membranes of nurse cells and oocytes in vitellogenic egg chambers (Figure 2C–2E). This expression was low at the beginning of vitellogenesis (stage 8, Figure 2C asterisk) and increased as the egg chamber matured, being maximal at stage 11 (Figure 2E). In situ hybridization detected a similar expression pattern for lpr2 transcripts (Figure 2B). Together, these results demonstrate that Lpr2 is the major receptor involved in the uptake of neutral lipids by nurse cells and oocytes. In contrast, our results indicate that lpr1 is not essential for this process since egg chambers from Df(3R)lpr1 females had normal amounts of neutral lipids (Figure 2L, Figure S3A). However, lpr1 appears to play some role in oogenesis since Df(3R)lpr1/2 females, which lack both receptors, exhibited stronger and qualitatively distinct phenotypes compared to Df(3R)lpr2 mutants. Ovaries from Df(3R)lpr1/2 females were severely reduced in size and contained abundant cellular debris. DAPI staining revealed condensation and fragmentation of the nurse cell nuclei at stages 9–10, indicating cell degeneration (Figure 2O, compare to Figure 2N. Figure S3B), a phenotype not observed in Df(3R)lpr2 females, explaining the egg laying phenotype described for these mutants earlier. Starvation and other adverse stimuli are known to activate an oogenesis checkpoint that results in the apoptosis of egg chambers at mid-oogenesis [24]. Thus, the degeneration of Df(3R)lpr1/2 ovaries probably results from activation of this checkpoint. As expected, the few non-degenerating stage 10 egg chambers that can be found in young Df(3R)lpr1/2 females had extremely low lipid content (Figure 2K). These results indicate that lpr1 has a partially redundant function during oogenesis, which was only revealed in the absence of lpr2. Accordingly, we detected lpr1 expression in nurse cells and follicle cells by in situ hybridization and RT-PCR (Figure 2A, Figure 5G) but not by antibody staining (not shown), suggesting that Lrp1 protein levels might be low. Oogenesis is a complex process regulated by hormonal signals that relay information about the nutritional status of the female and other stimuli [25]. Thus, it is conceivable that mutations in lpr1 and lpr2 could affect oogenesis, at least in part, by altering the hormonal or nutritional status of the female. To address this point, we eliminated lpr1 and lpr2 exclusively in the oocyte and nurse cells by generating Df(3R)lpr1/2 germ-line clones. The resulting females were sterile but laid abundant non-viable eggs, the egg chambers had low lipid content (Figure 2M) and no signs of degeneration were observed at mid oogenesis (not shown). Taken together, these experiments indicated that lpr2 and to a minor extent lpr1, are autonomously required in the oocyte and nurse cells to mediate lipid uptake during vitellogenesis. In addition, they suggest that somatic expression of the lipophorin receptors contributes to the regulation of the mid-oogenesis checkpoint.
To examine whether lpr1 or lpr2 are involved in lipid uptake in other tissues, we analyzed larval imaginal discs, an epithelial tissue known to accumulate abundant intracellular lipid droplets [26]. Wild-type wing imaginal discs displayed strong neutral lipid accumulation in the wing pouch region (Figure 3A). In contrast, Df(3R)lpr1/2 wing imaginal discs exhibited severely reduced levels of intracellular lipids droplets (Figure 3B), suggesting that lipophorin receptors might mediate neutral lipids uptake in imaginal disc cells in a similar way to the oocyte. Significantly, animals with single mutations in lpr1 or lpr2 did not exhibit this strong phenotype: Df(3R)lpr1 discs were undistinguishable from wild-type discs (Figure 3C) while Df(3R)lpr2 discs showed a mild reduction in lipid droplet content (Figure 3D), indicating that the functions of lpr1 and lpr2 are mostly redundant in this tissue. Consistent with a redundant function, we detected transcripts of both lpr1 and lpr2 in the wing pouch region by in situ hybridization (Figure 3E, 3F and [27]). High levels of Lpr2 protein were also detected in the wing pouch by immunostaining, with two stripes of lower expression along the antero-posterior and dorso-ventral compartment borders (Figure 3G, 3H).
Fig. 3. lpr1 and lpr2 are required for the uptake of neutral lipids by wing imaginal disc cells. 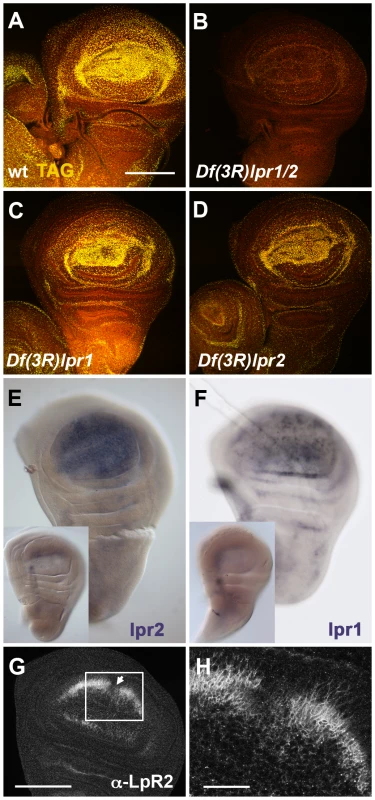
(A–D) Wild-type (A), Df(3R) lpr1/2 (B), Df(3R)lpr1 (C) and Df(3R)lpr2 (D) wing imaginal discs stained with nile red to reveal lipid droplets (yellow). In lpr1-, lpr2− double mutant there is a sharp decrease in lipid accumulation (B) whereas lpr2− discs display a mild reduction (D). (E, F) in situ hybridization to detect lpr2 (E) and lpr1 (F) mRNA expression in wild-type wing imaginal discs. Both genes are mainly expressed in the wing pouch area. Insets show a Df(3R)lpr1/2 disc (E) and a Df(3R)lpr1 disc (F) as negative controls. (G–H) Immunostaining with α-Lpr2 antibody detected expression in the wing pouch area of wild-type imaginal discs with minimal expression near the antero-posterior (arrow) and dorso-ventral compartment boundaries. (H) Magnification of the disc shown in (G). Lpr2 accumulates at baso-lateral membranes. Scale bars: 100 µm (A, G) and 25 µm (H). (A–F) shown at the same magnification. To examine whether Drosophila lipophorin receptors had an impact on total lipid content, we measured total TAG content in Df(3R)lpr1, Df(3R)lpr2 and Df(3R)lpr1/2 male flies under a normal diet as well as under starvation conditions. No differences were observed between the three genotypes and the wild-type control (Figure S4A–S4F and Text S1). The fat body stores most of the fly's TAG reserves and in agreement with our previous results, we did not observe differences in the number or size of lipid droplets in the fat body of Df(3R)lpr1/2 and control animals (Figure S4G–S4J), suggesting that TAG storage in the fat body is independent of the lipophorin receptors. Finally, since impaired neutral lipid uptake by peripheral tissues could have an impact on circulating lipid levels, we measured TAG content in the hemolymph of control, Df(3R)lpr1, Df(3R)lpr2 and the double mutant animals. We did not observe statistically significant differences between the four genotypes (Figure S4K and Text S1).
Only a subset of lipophorin receptor isoforms mediates the uptake of neutral lipids
lpr1 and lpr2 are transcribed as multiple isoforms (Figure 1B), raising the issue of whether they share similar properties regarding lipid uptake. To answer this question, we first compared the isoforms transcribed from the corresponding distal versus proximal promoters. We generated two HA-tagged transgenes that allowed controlled expression of Lpr2F isoform (UAS-lpr2F) and Lpr2E isoform (UAS-lpr2E) and examined their ability to rescue lipid uptake in Df(3R)lpr1/2 animals. Expression of UAS-lpr2E in the posterior compartment of the wing imaginal disc driven by en-gal4 completely rescued lipid accumulation in that compartment (Figure 4A). In contrast, expression of UAS-lpr2F did not rescue lipid uptake (Figure 4B). In a similar assay, we found that whereas Lpr1H isoform rescued lipid uptake, expression of Lpr1D did not (Figure 4C, 4D). Equivalent results were obtained when we examined the role of these isoforms during vitellogenesis. Germ-line expression of UASp-lpr1J or UASp-lpr2E, driven byV32-gal4, rescued oogenesis and fertility of Df(3R)lpr1/2 females. The amount of lipid droplets accumulated in nurse cells was similar to the wild-type (Figure 5C, 5E, compare to wild-type in Figure 2G) and the number of degenerating egg chambers was dramatically reduced (Figure 5D, 5F and Figure S3B). In contrast, expression of UASp-lpr2F did not rescue fertility, lipid uptake nor egg chamber degeneration (Figure 5A, 5B).
Fig. 4. Uptake of neutral lipids is mediated by a subset of lpr1 and lpr2 isoforms. 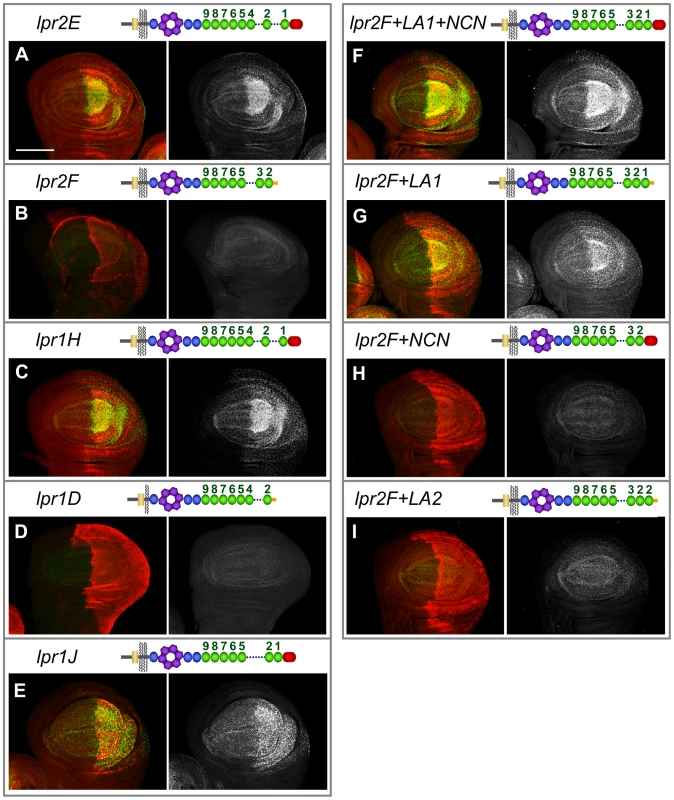
(A–I) Wing imaginal discs of Df(3R)lpr1/2 genotype in which the indicated lpr1 and lpr2 isoforms and chimeras were overexpressed in the posterior compartment using the en-gal4 driver. The relevant protein domains of the overexpressed isoforms are depicted in the drawings, which follow the code shown in Figure 1. Neutral lipids are shown in green and in grey in a separate channel. The overexpressed Lpr1 and Lpr2 proteins were detected by immunostaining using an antibody that recognizes the HA tag except in panel D in which α-Lpr1 was used. Lpr2E (A), Lpr1H (C), Lpr1J (E), Lpr2F+LA1+NCN (F) and Lpr2F+LA1 (G) proteins rescued lipid uptake in the posterior compartment whereas Lpr2F (B), Lpr1D (D), Lpr2F+NCN (H) and Lpr2F+LA2 (I) did not. Note that the presence of LA-1 defines the isoform ability to rescue neutral lipid uptake. Scale bar: 100 µm. All panels are shown at the same magnification. Fig. 5. Germ-line expression of Lpr1J or Lpr2E rescues the oogenesis phenotypes of lpr1-, lpr2− double mutant females. 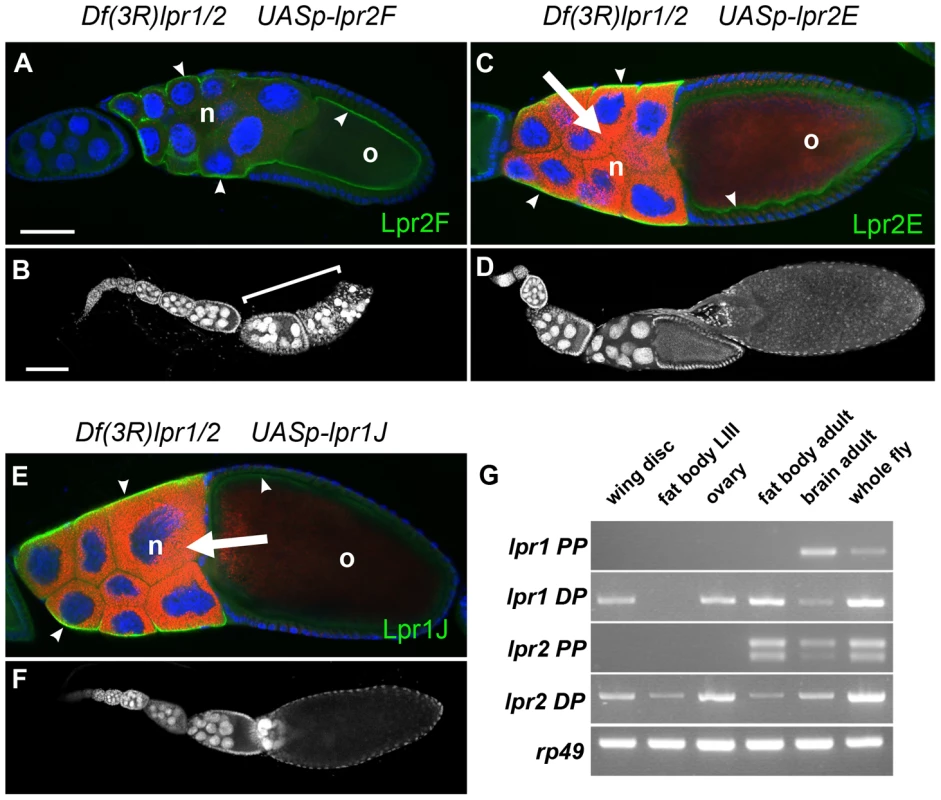
(A–F) Df(3R)lpr1/2 egg chambers overexpressing the lipid uptake-defective UASp-lpr2F isoform (A–B) or the lipid uptake-promoting UASp-lpr2E (C–D) or UASp-lpr1J (E–F) isoforms in the germ-line driven by V32-gal4. (A,C,E) Lpr2F (A), Lpr2E (C) and Lpr1J (E) proteins were detected in the nurse cells (n) and oocyte (o) membranes using an α-HA antibody (arrowheads, shown in green). Egg chambers were stained with the lipophilic nile red dye (red) and DAPI (blue) to mark nuclei. (B, D, F) Ovarioles containing multiple egg chambers stained with DAPI. Several degenerating egg chambers are labeled with a bracket in (B). Note that germ-line specific expression of UASp-lpr2E and UASp-lpr1J but not of UASp-lpr2F induced accumulation of neutral lipids in nurse cells (arrows in C and E) and rescued mid-oogenesis degeneration (D and F, compare also with the wild-type and Df(3R)lpr1/2 egg chambers in Figure 2). (G) Semiquantitative RT-PCR showing the relative abundance of lpr1 and lpr2 transcripts in several tissues, as indicated (wing imaginal discs, third instar larva fat body, ovary, adult fat body, adult brain and whole female fly). The oligo pairs used discriminated between isoforms transcribed from the proximal promoter (PP) and from distal promoter (DP). Analysis of ribosomal protein rp49 transcription was included as a control. Scale bars: (A, C and E) 50 µm, (B, D and F) 100 µm. The LA-1 module, present in all isoforms transcribed from the distal promoters, defines the capacity to mediate neutral lipid uptake
The surprising finding that only a subset of lpr1 and lpr2 isoforms tested were able to mediate lipid uptake prompted us to examine the essential protein domains required for this function. Lipid uptake-promoting isoforms Lpr1H and Lpr2E are both transcribed from the corresponding alternative distal promoters and contain a non-conserved N-terminal domain (NCN) adjacent to the LA module 1 (LA-1), encoded by exons 2 and 3 respectively. In contrast, these domains are not present in the lipid uptake-defective Lpr2F and Lpr1D isoforms (Figure 1B). Thus, a sequence critical for the uptake of neutral lipids may lie in this N-terminal region. However, given that Lpr1H and Lpr2E both contain eight LA modules whereas Lpr2F and Lpr1D have seven, it is also possible that the total number of LA modules is the key factor in defining lipid uptake function. To test the role of the NCN and LA-1, we made a chimeric protein identical to Lpr2F except for its signal peptide, which was replaced with the NCN and LA-1 domains from Lpr2E, generating UAS-lpr2F+LA1+NCN. Overexpression of this transgene in the posterior compartment of Df(3R)lpr1/2 wing imaginal discs completely rescued the accumulation of lipid droplets in this compartment (Figure 4F), demonstrating that this N-terminal segment was able to confer lipid uptake activity. To analyze whether NCN, LA-1 or both domains were required, we prepared two more transgenes. In UAS-lpr2F+LA1, the LA-1 domain from Lpr2E was inserted in between the signal peptide and LA-2 of Lpr2F. In UAS-lpr2F+NCN, the lpr2F signal peptide was replaced by the lpr2E NCN domain (Figure 4G, 4H). Using our rescue assay, only UAS-lpr2F+LA1 was able to mediate lipid uptake (Figure 4G, 4H). Thus, the NCN domain, even though present in all tested lipid uptake-promoting isoforms, was not essential for this function. These results suggested that the LA-1 module was critical for the uptake of neutral lipids. However, we still could not exclude the possibility that it is the number rather than the identity of LA modules that determines lipid uptake capacity. To analyze this possibility, we examined the Lpr1J isoform which is identical to the lipid uptake-promoting Lpr1H isoform except that it has seven LA modules due to the absence of LA-4. In our rescue assay, Lpr1J did mediate lipid uptake (Figure 4E), indicating that it is the presence of LA-1 and not the number of LA modules that defines the ability to mediate lipid uptake. Moreover, in an additional transgene we modified Lpr2F by introducing a tandem duplication of LA-2, so that the new protein (Lpr2F+LA2) had eight LA modules but still lacked LA-1. Lpr2+LA2 did not mediate lipid uptake, confirming our previous conclusion (Figure 4I). Taken together, these experiments indicate that the key property that distinguishes lipid uptake-promoting from lipid uptake-defective isoforms is the presence of the LA-1 domain. It should be noted that all isoforms containing LA-1 are transcribed from the distal promoters. Consistent with the lipid uptake phenotypes of Df(3R)lpr1/2 mutants in wing imaginal discs and ovaries, RT-PCR experiments showed that the predominant lpr1 and lpr2 isoforms expressed in these tissues were transcribed from the distal promoters and thus contained the LA-1 domain (Figure 5G).
Endocytosis is not required for the uptake of neutral lipids in the oocyte or imaginal discs
During the rescue experiments described in the previous sections, we realized that expression of UAS-lpr2E in the posterior compartment driven by en-gal4 not only autonomously rescued lipid uptake in that compartment but also promoted the formation of lipid droplets in a one to two cells wide region of anterior tissue abutting the anterior-posterior compartment border (Figure 6B, arrow). Analysis of confocal sections spanning the thickness of the imaginal disc confirmed that cells not expressing UAS-lpr2E but adjacent to expressing cells, accumulated higher levels of lipid droplets than more anteriorly located cells (Figure 6A, arrow). Similar results were obtained using a different Gal4 driver line to express UAS-lpr2E in the dorsal wing disc compartment (Figure S5C, S5D). To analyze whether non-autonomous lipid uptake could be detected in other tissues, we returned to the egg chamber because of its particular morphology. In the Drosophila egg chamber, the oocyte and nurse cells are surrounded by a closely associated somatic follicular epithelium. This allowed us to examine whether expression of the lipophorin receptors in the germ-line could non-autonomously direct lipid uptake in the follicular epithelium. Follicle cells of ovaries dissected from Df(3R)lpr1/2 females had very few lipid droplets (Figure 6C). However, after V32-gal4-mediated expression of UASp-lpr2E exclusively in the germ-line, we observed a remarkable increase in the number and size of lipid droplets in the follicular epithelium (Figure 6D). In fact, the rescue was similar to the one obtained by the expression of the lipid uptake-promoting UAS-lpr1J isoform directly in the follicle cells using the follicle cell driver CY2-gal4 (Figure 6E). These results indicated that Lpr2E can non-autonomously promote neutral lipid uptake in adjacent cells.
Fig. 6. Neutral lipid uptake does not require endocytosis. 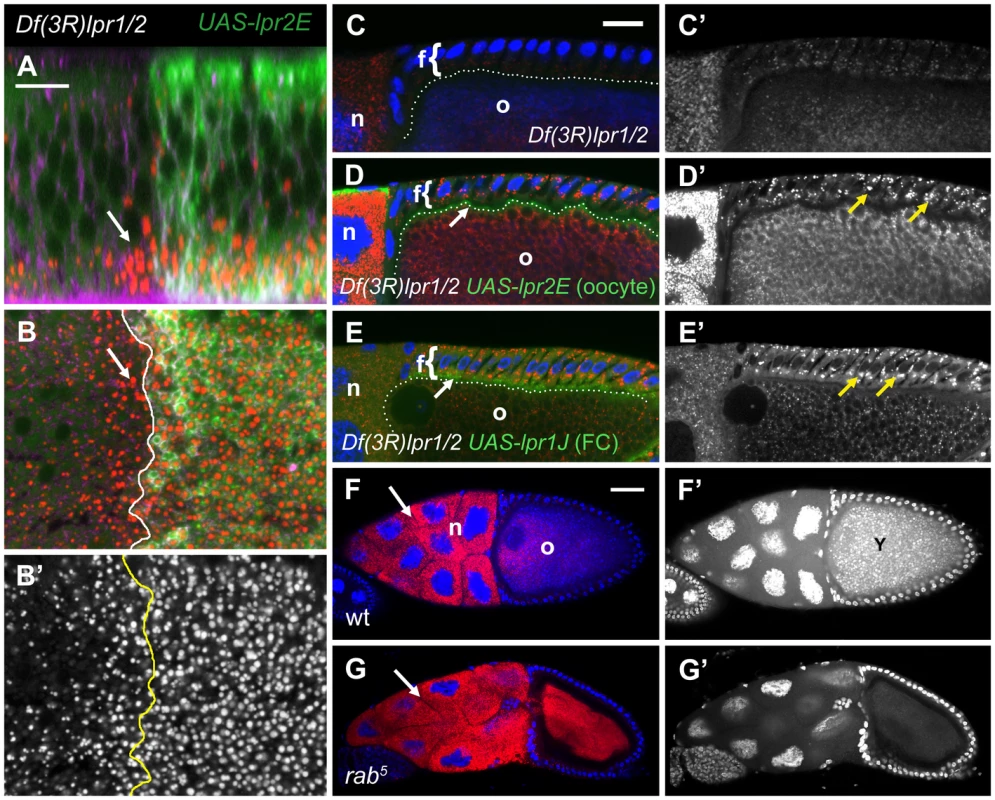
(A–B) Wing imaginal disc of Df(3R)lpr1/2 genotype expressing UAS-lpr2E in the posterior compartment driven by en-gal4. Neutral lipids were revealed by nile red staining (red). Lpr2E distribution was detected with α-HA antibody (green). Lipophorin distribution is shown in magenta to mark cell outlines. The wing imaginal disc is shown in a cross-section in (A), with the apical side at the top and the basal at the bottom. A basal section of the same disc is shown in (B). Note that Lpr2E expressing cells and two rows of adjacent non-expressing anterior cells (arrows) accumulate high levels of lipid droplets in a basal location. A line marks the limit of Lpr2E expression. Nile red staining is also shown in a separate panel (B'). (C–E) Detail of the somatic follicle epithelium (f) wrapping around the oocyte (o) in a stage 10 egg chamber dissected from a Df(3R)lpr1/2 female (C) and similar Df(3R)lpr1/2 mutant egg chambers overexpressing UASp-lpr2E in the germ-line driven by V32-gal4 (D) or UAS-lpr1J in the follicular epithelium driven by CY2-gal4 (E). Lpr2E and Lpr1J were detected with an α-HA antibody (green). The oocyte membrane was marked with a dotted line. Overexpressed proteins accumulated at the oocyte membrane (arrow in D) and at the apical region of the follicle cells (arrow in E). DAPI marks the cell nuclei (blue). Lipids are shown in grey as a separate channel (C', D' and E'). Note that expression of UASp-lpr2E in the nurse cells and oocyte induces an autonomous increase in lipid accumulation in these cells and also a non-autonomous rescue in the adjacent follicular epithelium (yellow arrows in D'). Expression of Lpr1J in the follicular epithelium rescues lipid accumulation in these cells to a similar extent (yellow arrows in E'). Note that the Df(3R)lpr1/2 egg chamber shown in (C) was dissected from young females in which a few egg chambers from each ovary escaped mid oogenesis degeneration. (F–G) Egg chambers stained with the lipophilic dye nile red (red) and DAPI (blue) to mark cell nuclei. (F) Wild-type genotype. Note the accumulation of lipids (arrow) in the nurse cells (n) and the auto-fluorescence of yolk proteins (Y) (in F') in the DAPI channel within the oocyte (o). (G) rab5 mutant egg chamber. Lipids accumulate in the nurse cells (arrow) similarly to the wild-type but no yolk proteins are present in the oocyte (G'), confirming that endocytosis is blocked in the rab5 genetic background. Scale bars: 10 µm (A–B), 20 µm (C–E), 100 µm (F–G). All members of the LDLR family are known to mediate endocytosis of ligands in clathrin coated vesicles [28]. In Drosophila, Lpr1 and Lpr2 have been shown to endocytose lipophorins when overexpressed in imaginal discs [11], [27]. Thus, a possible hypothesis for lipophorin receptor-mediated lipid uptake is that the receptors induce the endocytosis of lipophorin particles resulting in the catabolic hydrolysis of lipids in lysosomes. However, our finding that lipophorin receptors have non-autonomous activity appears to be in conflict with this simple hypothesis, prompting us to test the requirement of endocytosis for lipophorin receptor-mediated lipid uptake. We took advantage of the fact that the Drosophila oocyte is a well characterized model of endocytosis, and importantly, that the endocytic pathway can be blocked in the oocyte and nurse cells without affecting their survival [29], [30]. We generated germ-line clones of a null rab5 mutation, an essential mediator of endocytic vesicle formation and maturation [31]. As previously reported, rab52 mutant oocytes were unable to accumulate yolk proteins, demonstrating that the endocytic pathway was blocked [29], [30] (Figure 6G', compare to 6F'). More importantly, rab52 mutant egg chambers showed a normal accumulation of intracellular lipid droplets, indicating that the endocytosis of lipophorins is not required for neutral lipid uptake during vitellogenesis (Figure 6G, compare to 6F). Similarly, the AP-2 adaptor complex is not required for neutral lipid uptake (Figure S6 and Text S1). Together, these results suggest that in Drosophila, the neutral lipid uptake promoting activity of lipophorin receptors occurs extracellularly and that the targeting of lipophorins to lysosomes for their catabolic degradation is not required. In this scenario, a possible mechanism for lipophorin receptors to promote the uptake of neutral lipids would be by stabilizing lipophorins at the extracellular matrix. We tested this hypothesis by examining the extracellular distribution of lipophorins in Df(3R)lpr1/2 imaginal discs that overexpressed UAS-lpr2E in the posterior compartment. We observed increased accumulation of extracellular lipophorins at the basolateral membranes of cells expressing Lpr2E (Figure 7A, 7B, arrows). In contrast, similar expression of the lipid uptake-defective Lpr2F isoform had no visible effect on the extracellular distribution of lipophorin (Figure 7C, 7D). Similar results were obtained overexpressing UAS-lpr2E and UAS-lpr2F with additional gal4 drivers (Figure S7).
Fig. 7. Lpr2E stabilizes lipophorins in the extracellular space. 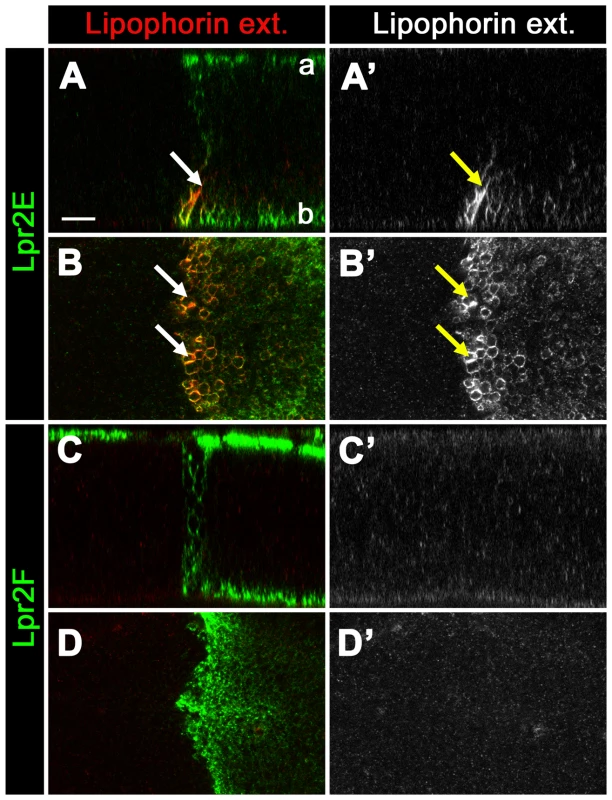
(A–D) Df(3R)lpr1/2 wing imaginal discs overexpressing the lipid uptake-promoting UAS-lpr2E isoform (A–B) or the lipid uptake-defective UAS-lpr2F isoform (C–D) in the posterior compartment driven by en-gal4, shown in green. A modified immunostaining protocol was used to detect extracellular lipophorin (red, also shown in a separate panel in grey). (A, C) Optical cross-sections. Apical (a) and basal (b) sides as indicated. (B, D) Optical basal section. Cells overexpressing Lpr2E displayed increased accumulation of extracellular lipophorins at basolateral membranes near the basal side of the imaginal disc (arrows in A and B). The accumulation was more prominent near the antero-posterior compartment boundary, a region that also displayed higher Lpr2E protein levels. No noticeable changes in extracellular lipophorin were detected in cells overexpressing UAS-lpr2F (C–D). Scale bar: 10 µm. All panels are shown at the same magnification. Discussion
Over the past decade, Drosophila has become a prominent model organism for the study of lipid metabolism. Aspects ranging from the composition and regulation of lipid droplets, to the developmental control of adipose tissue differentiation or to the hormonal regulation of lipid storage and mobilization have revealed multiple parallels between Drosophila and mammals [3], [32]. Moreover, large scale genetic screens in Drosophila have identified novel genes and pathways involved in energy homeostasis, some of which are conserved in mammals [32]–[36]. In this context, we have studied an aspect of lipid metabolism that was largely unknown in Drosophila: the cellular uptake of neutral lipids mediated by the lipophorin receptors.
The Drosophila genome contains two closely related genes (lpr1 and lpr2, Figure 1A) homologous to other described lipophorin receptors in insects like locust, mosquitoes, cockroaches, silkworm, wax moth or bees [14], [37]–[41]. Insect lipophorin receptors were first isolated because of their homology to the mammalian LDLR and subsequently shown to be involved in insect lipid metabolism. In particular, the locust Lipophorin Receptor, by far the best characterized member of the family, was able to induce the endocytic uptake of labeled lipophorins when expressed in mammalian cells [42]. Moreover, it was required for the endocytosis of lipophorins by locust fat body cells [15], [16]. Here, we generated several novel mutations in Drosophila which disrupted lpr1, lpr2 and, in view of a possible functional redundancy between the two receptors, we also generated a deficiency that affects both genes simultaneously (Df(3R)lpr1/2), representing a null mutation for lipophorin receptor function (Figure 1A). Despite the critical role lipophorins play in lipid transport in insects [8], [17] and the embryonic lethal phenotype of a null mutation in the single Drosophila apolipophorin gene [11], complete disruption of both Drosophila lipophorin receptors does not affect the viability of flies. Moreover, we did not detect any significant change in total body TAG content when we compared animals with mutations in lpr1, lpr2 or the double mutant with their isogenic controls (Figure S4A–S4C). Moreover, the fat body cells of mutant and control animals were indistinguishable, containing similar number of lipid droplets and of equivalent sizes (Figure S4G–S4J). The rate of lipid mobilization under starvation conditions was also unaffected in lpr1 and lpr2 mutants (Figure S4D–S4F). Taken together, these results clearly demonstrated that Drosophila lipophorin receptors are not essential for the storage of TAG in the fat body or for its mobilization. Despite this lack of a requirement, we detected lpr1 and lpr2 expression in the adult fat body (Figure 5G and Figure S2C), and lipophorin receptors have been identified in the fat body of other insects [14], [39]–[41], [43], [44]. Thus, the lipophorin receptors probably have functions in the fat body that are unrelated to the uptake of neutral lipids. Significantly, it has recently been reported that lpr2 is involved in immune response in Drosophila as a regulator of the serpin Necrotic metabolism [45]. In addition, it was shown in microarray experiments that lpr2 transcription changes upon immune challenge [46]. Since the fat body is a key immunological organ in the fly [47], it is possible that lpr2 expression in this tissue is related to immunity.
We have shown that lpr1 and lpr2 have a key role in neutral lipid uptake in two Drosophila organs: the imaginal disc and the ovaries. In both cases, they are required to attain high levels of intracellular TAG. lpr1 and lpr2 are expressed in the wing pouch region of wing imaginal discs and mediate the uptake of neutral lipids by these cells (Figure 3). Another protein involved in lipid storage as a component of lipid droplets, the perilipin-like protein Lipid storage droplet-2 (Lsd-2), is also preferentially expressed and required in the wing pouch region for the accumulation of intracellular lipid droplets [26]. Thus, lipid accumulation in this region of the disc appears to be regulated at multiple levels. Unfortunately, the functional relevance of this lipid accumulation is still unknown.
Our results indicate that lpr1 and lpr2 genes are transcribed as multiple isoforms each (Figure 1B) with dramatically different properties. Only those isoforms transcribed from the distal promoters and containing the LA-1 module mediate lipid uptake. Similarly, the lipophorin receptor gene from the mosquito Aedes aegypti has been shown to be translated into fat body and oocyte specific isoforms from two alternative promoters [48]. Several members of the mammalian LDLR family are similarly processed by alternative splicing. Variations in the O-glycosylation region and the LA domains in mammalian VLDLR and ApoER2 have been related to differential sensitivity to proteolytic processing by gamma-secretases [49], [50] and to differential ligand binding [51], [52], respectively. Thus, the multiple lpr1 and lpr2 isoforms might have different ligand binding and/or stability properties, allowing these receptors to be involved in processes as diverse as neutral lipid uptake, regulation of the immune system [45] and regulation of neurite outgrowth [53].
Lipophorin receptors function during oogenesis
During vitellogenesis, the nurse cells and the oocyte grow rapidly accumulating large amounts of yolk proteins and lipids from the hemolymph over approximately 18 hours [21], [54]. Work from the Mahowald lab has shown that Yolkless, an LDLR family protein, mediates the endocytic uptake of yolk proteins in Drosophila [22], [23]. Here we demonstrate that a different receptor type, the lipophorin receptor, is essential for the uptake of neutral lipids during vitellogenesis (Figure 2). This is clearly shown in Df(3R)lpr2 females and in double mutant lpr1−, lpr2−germ-line clones. In both cases, the mutant egg chambers accumulate low levels of neutral lipids (Figure 2J, 2M and Figure S3A). In addition to impaired lipid uptake during vitellogenesis, we observed a second phenotype in Df(3R)lpr1/2 double mutant females, where most of the egg chambers degenerated at mid-oogenesis (Figure 2O and Figure S3B). A simple explanation for this phenotype would be that degeneration was triggered by the low lipid content of Df(3R)lpr1/2 egg chambers. In fact, it is known that multiple challenges like starvation, extreme temperatures or chemical treatments, trigger a mid-oogenesis checkpoint and induce apoptosis at this stage [24]. Significantly, flies with a mutation in the gene midway, which encodes an acyl coenzyme A: diacylglycerol acyltransferase required for the synthesis of TAG, were described to have severely reduced levels of neutral lipids in the germ-line and displayed apoptosis at mid oogenesis, thus paralleling the Df(3R)lpr1/2 phenotype [55]. However, it was difficult to fully attribute degeneration to low lipid levels as we observed some experimental conditions which resulted in egg chambers with very low levels of neutral lipids but that did not undergo degeneration. In particular, in Df(3R)lpr1/2 germ-line clones degeneration was absent even though the neutral lipid content of the egg chambers was low (Figure 2M). Similarly, expression of UAS-lpr1J exclusively in the follicle cells of Df(3R)lpr1/2 females abolished egg chamber degeneration even though neutral lipid accumulation in the nurse cells and oocytes was low (Figure 6E and not shown). These experiments suggest that the lipophorin receptors might have an additional function in the follicle cells which is necessary to avoid egg chamber degeneration. Accordingly, we detected lpr1 expression in the follicular epithelium (Figure 2A, inset). In this direction, it has recently been described that blocking the nutrient sensing TOR pathway in follicle cells induced apoptosis at mid oogenesis [56]. Thus, Lpr1 could be required to maintain elevated levels of TOR activity in follicle cells. In interpreting these results, we should also consider the non-autonomous effects of lipophorin receptors. We have shown that expression of UAS-lpr2E exclusively in the oocyte and nurse cells increases lipid uptake in the follicle cells (Figure 6D), which could potentially impact on their nutritional status and restore their putative anti-apoptotic activity. Conversely, expression of the transgene in the follicle cells might slightly increase lipid uptake by the oocyte and nurse cells, even though we have been unable to detect this effect (Figure 6E), and provide enough lipids to bypass the mid-oogenesis checkpoint. More studies will be required to assess the role of the lipophorin receptors in the follicular epithelium.
A speculative molecular model for lipid uptake mediated by the lipophorin receptors
Drosophila Lpr1 and Lpr2 are bona fide members of the LDLR family, sharing a similar organization of proteins domains with the human LDLR, ApoER2 and VLDLR. The human LDLR is the archetypical endocytic receptor. It is expressed in the liver where it mediates the endocytosis of cholesterol-rich LDL, regulating LDL concentration in serum. Endocytosis of LDL results in the catabolic processing of both, the lipidic and proteic moieties of LDL in lysosomes [13]. Other members of the LDLR family are also well known endocytic receptors with a broad variety of ligands [28], [57]. Drosophila lipophorin receptors can also mediate endocytosis of their ligands. It has recently been reported that Lpr1 is expressed in garland cells and pericardial athrocytes where it is critical for the endocytic clearance of serpin/protease complexes from the hemolymph, thus regulating the innate immune response [45]. Overexpression of Lpr1 and Lpr2 in imaginal discs also induced the endocytosis of lipophorins, which colocalized with endocytic markers [11], [27]. Similarly, the locust lipophorin receptor mediated lipophorin endocytosis in the fat body and in cell culture [14]–[16]. Despite this well documented endocytic activity of LDLRs, our data demonstrates that neutral lipid uptake mediated by Drosophila lipophorin receptors does not require the endocytosis of lipophorin particles. Three lines of evidence support this conclusion: (1) Blocking endocytosis did not affect lipid uptake in the egg chambers (Figure 6G and Figure S6); (2) overexpression of Lpr2E in groups of imaginal disc cells induced lipid uptake both in cells expressing the receptor and in a 1–2 cell diameter region of adjacent cells (Figure 6A, 6B, and Figure S5C, S5D) and (3) expression of Lpr2E in the oocyte and nurse cells promoted lipid uptake in the adjacent, somatic follicular epithelium (Figure 6D). Our results also indicate that Lpr2E is able to locally increase the concentration of lipophorins in the extracellular space (Figure 7A, 7B and Figure S7B, S7D, S7F). Taking into account this data, we propose the following model for lipophorin receptor-mediated neutral lipid uptake: lipophorin receptors interact with lipophorins at the cell surface and promote the extracellular hydrolysis of their DAG core by facilitating the activity of an as-yet-unidentified lipase, associated with the extracellular matrix. The free fatty acids generated during DAG hydrolysis could diffuse a few cell diameters away before being captured by cells, explaining why lipophorin receptors can promote lipid uptake non-autonomously. Significantly, physiological data obtained from studies of flight muscles and oocytes in insects indicated that lipid uptake mostly occurs without the concomitant degradation of the apolipophorin (for reviews, see [54], [58]), which is consistent with our hypothesis. Moreover, a lipophorin-specific lipase activity associated with muscle and oocyte cell membranes was detected [54], [58]. Our model offers a possible explanation to understand why only a subset of lpr1 and lpr2 isoforms mediates lipid uptake, whereby only the lipid-uptake promoting isoforms can stabilize lipophorins in the extracellular matrix (Figure 7 and Figure S7). Alternatively, if lipophorin receptors must interact with both, a lipophorin particle and a lipase to generate a ternary complex and facilitate lipolysis, then the lipid uptake-defective isoforms might lack the ability to interact with the lipase. Identification of such putative lipase(s) will be necessary to test this hypothesis. The proposed model displays a number of resemblances to the lipolytic processing of triglyceride-rich lipoproteins in the microvascular endothelium of adipose tissue, heart and striated muscles in mammals. Circulating triglyceride-rich lipoproteins, chylomicrons from the intestine and VLDL synthesized by the liver, reach the capillary endothelium where they interact with lipoprotein lipase at the luminal surface. Lipoprotein lipase is essential for the lipolytic processing of chylomicrons and VLDL, generating non-esterified fatty acids from the TAG fraction of lipoproteins. The free fatty acids are then transported to the underlying adipocytes and myocytes by specific transporters such as CD-36. Once inside these cells they are re-esterified into newly synthesized TAG stores or enter the β-oxidation cycle (for a recent review, see [59]). Recent data indicated that the extracellular lipolysis of TAG-rich lipoproteins is strongly potentiated by the endothelial protein GPIHBP1. This protein is essential for the transcytosis of lipoprotein lipase from the basolateral to the apical capillary endothelial surface [60]. In addition, it has been suggested that it may facilitate lipolysis by simultaneously interacting with lipoprotein lipase and chylomicrons in the luminal surface of capillaries, providing a molecular platform for lipolysis to occur [61]. In agreement with this essential functions, Gpihbp1-deficient mice manifested severe hyperchylomicronemia [61]. The VLDLR, which is also expressed at the capillary endothelium, seems to participate in the lipolytic processing of TAG-rich lipoproteins in similar ways. The VLDLR can mediate the transcytosis of lipoprotein lipase across cultured endothelial cells [62] and interacts with both, lipoprotein lipase and ApoE containing TAG-rich lipoproteins, potentially tethering them to the endothelium surface and thus promoting the action of lipoprotein lipase (for a review, see [63]). These potential functions were supported by the phenotype of vldlr− mice, which showed delayed clearance of TAG-rich lipoproteins after a meal [64] and increased plasma TAG levels under a high fat diet [65] but normal lipoprotein profiles under regular feeding conditions [66]. Unfortunately, these weak phenotypes have hampered the elucidation of the precise roles that VLDLR plays during the processing of TAG-rich lipoproteins in vivo. We propose that in Drosophila, lipophorin receptors have an activity similar to the bridging role proposed for GPIHBP1 and VLDLR in mammals, bringing lipophorins and a putative lipophorin-specific lipase into close contact on the cell surface and promoting in this way the lipolysis of lipophorins. We speculate that during evolution, a protein related to VLDLR had a critical role in promoting the extracellular hydrolysis of lipoproteins. In insects, this function is carried out by the lipophorin receptors whereas in mammals, GPIHBP1 appears to have taken most of this function, with VLDLR retaining a minor role. Our data supports an ancient function for the LDLR family in promoting the extracellular lipolytic processing of lipoproteins.
Methods
Genetics
Generation of deficiencies in the lpr1-lpr2 genomic region: The three deficiencies used in this work were created by flipase-mediated interchromosomal recombination between the following pairs of FRT-containing transposon insertions: Df(3R)lpr1: P{XP}d03066 and P{XP}d10508 to generate a 19.7 Kb deletion; Df(3R)lpr2: PBac{RB}e00374 and PBac{WH}f03030 to generate a 29.7 Kb deletion; and Df(3R)lpr1/2: PBac{RB}e00374 and P{XP}d10508 to generate a 49,7 Kb deletion [67], [68]. The deficiency breakpoints were checked by PCR using appropriate primers flanking the predicted breakpoints.
To generate Df(3R)lpr1/2 clones in the female germ-line, Df(3R)lpr1/2 was recombined with FRT82B [69] using standard genetic techniques. Females of the genotype: y,w,hs-flp; FRT82B,Df(3R)lpr1/2/FRT82B,ovoD1 were heat-shocked for one hour at 37°C several times during larval stages. The resulting adult females were fed yeast over two days before their ovaries were dissected and processed for immunostaining. To generate rab5 germ-line clones, females of the genotype y,w,hs-flp; rab5,FRT40A/ovoD1,FRT40A were similarly treated. The rab5,FRT40A/CyO stock was obtained from Antoine Guichet [30]. FRT82B,ovoD1 and ovoD1,FRT40A chromosomes were described in [70].
To quantify egg hatching rates, less than a week old adults of the following genotypes: wild-type Oregon R, w;Df(3R)lpr1/TM6 and w;Df(3R)lpr2/TM6 were kept on abundant yeast paste for two days. Homozygous females were selected and allowed to lay eggs in apple plates for periods of 4 hours. About 200 eggs were individually placed in agar plates and incubated at 25°C for 48 hours. The number of empty egg shells and non-eclosed eggs was quantified.
Isolation and characterization of lpr1 and lpr2 isoforms and RT-PCR
Some of the cDNAs corresponding to lpr1 and lpr2 isoforms used in this work were isolated and sequenced by the Berkeley Drosophila Genome Project [19]. In particular, we used the following full length cDNAs: Lpr1D (RE14223), Lpr1H (LD21010), Lpr1J(RE40649), Lpr2F (GH26833) and Lpr2E (LD11117). To identify additional isoforms, total RNA was isolated from whole adult male flies and specific tissues -wing imaginal discs, ovaries, adult brain and adult fat body - using Trizol reagent (Invitrogen). 1 µg RNA for each sample was retrotranscribed using random primer and Transcriptor Reverse Transcriptase (Roche). The obtained cDNA libraries were PCR amplified using four oligo pairs which specifically amplified the complete coding regions corresponding to lpr1 and lpr2 genes transcribed from the proximal and distal promoters. The PCR products were directly cloned into pGEM-T (Promega) or alternatively, used for a second nested PCR reaction before purification and cloning of the products. 33 isolated cDNAs were genotyped by PCR and selected clones were also verified by sequencing to unambiguously define their specific combination of exons. Signal peptides were predicted using the SignalP 3.0 Server [71] and Predisi [20].
RT-PCR was used to examine lpr1 and lpr2 transcription in whole adult flies, larval fat body, wing imaginal discs, ovaries, adult brains and adult fat body. For adult fat body preparation, abdomen carcasses were used which primarily contained fat body. However, contaminating tissues including oenocytes, dorsal vessel, epidermis and muscle were also present in small quantities. Two oligo pairs were used for each gene, which specifically detected exons 2–3 (distal promoter isoforms; lpr1: attcggcaaatgctgcactgc and tgtgatccttgcagtccgcatc, lpr2: accacccagtcagagttaacaac and tgtggtccgggcaatccgagga) and exons 4–5 (proximal promoter isoforms; lpr1: cgaacctctcaaccaaacggat and gccagaacgcgaaaactttgg, lpr2: aagaaacggacgtgtgtgctc and ccaatccgacgactctggag). In addition, oligo pairs for the ribosomal protein gene rp49 were used as control (gaccatccgcccagcatacaggc and gagaacgcaggcgaccgttgg). The oligos were designed to amplify a region encompassing an intron to distinguish cDNA products from genomic DNA products. A rough estimate of relative expression levels in the different tissues was obtained by comparing the PCR products at 20 and 30 cycles to avoid reaching an amplification plateau.
Immunostaining, in situ hybridization, and neutral lipids visualization
To generate α-Lpr1 and α-Lpr2 antibodies, DNA fragments containing the complete Lpr1 or Lpr2 intracellular domains were amplified by PCR from lpr1 cDNA RE38584 and lpr2 cDNA GH26833. The fidelity of the amplification was checked by sequencing and the fragments were cloned in-frame with the 6xHis tag present in the bacterial expression vector pET14b. Protein expression was induced in E. coli. The Lpr1 fragment was purified under denaturing conditions by immobilized metal affinity chromatography (IMAC) and dialyzed to remove urea. The protein precipitated during dialysis and the precipitate was used to immunize guinea pigs. Lpr2 fragment was purified from the soluble fraction by IMAC. Antibodies were raised in guinea pigs by Cocalico biologicals, Inc. Other antibodies used were: rabbit anti-apolipophorin 1∶500 [10]; rat anti-HA 1∶500 (Roche); and the dye DAPI to label nuclei.
For immunostaining, imaginal discs and ovaries were dissected and fixed for 20 minutes with 4% formaldehyde dissolved in PBS (PP) at room temperature, followed by a second fixation in PP plus 0.1% triton X-100. Tissues were extensively washed for 1 hour in PBS containing 0.3% triton X-100 (PBT) and blocked in 1% BSA dissolved in PBT for another hour. After incubation with the primary antibodies overnight at 4°C, tissues were extensively washed three times for a total of one hour in PBT and incubated with the fluorescent secondary antibodies (alexa-fluor conjugates from Invitrogen) for two hours (imaginal discs) or overnight (ovaries). After washing, tissues were mounted in vectashield media.
To detect extracellular lipophorins in imaginal discs, we used an extracellular staining protocol modified from [72]. Imaginal discs were dissected and accumulated in Sf-900 cell culture media (Invitrogen) on ice. They were incubated with α-Lipophorin antibody diluted 1∶100 in cell culture media for 30 minutes with constant rocking and washed four times for a total of 30 minutes with PBS. Incubations and washes were done at 4°C to inhibit antibody endocytosis. Imaginal discs were then fixed with PP and from this point on processed following the standard immunostaining procedure.
Neutral lipids in imaginal discs and ovaries were visualized by nile red staining. Fixed tissues were incubated with 0.002% nile red diluted in PBT for 60 minutes and washed for 10 minutes in the same buffer without the dye. To prepare embryos for microscopy, we avoided the use of organic solvents that would otherwise extract neutral lipids. Thus, after removing the chorion with bleach, we fixed embryos with a heat treatment of 5 seconds at 90°C and devitelinized them by hand using a sharp needle. The embryos were then incubated with the nile red solution as above.
We analyzed the tissue distribution of lpr1 and lpr2 transcription by in situ hybridization using DIG-labeled antisense RNA probes. Some probes were specific to particular isoforms whereas others recognized all isoforms derived from lpr1 or lpr2. They were prepared from the following DNA fragments: (1) All lpr1 isoforms: a 594 base pair DNA fragment including 64 base pairs from the C-terminal coding region and part of the 3′UTR of cDNA RE14223. (2) All lpr2 isoforms: a 581 base pair fragment including 91 base pairs from the C-terminal coding region of cDNA GH26833 and part of its 3′UTR. (3) lpr1 isoforms transcribed from the distal promoter: a 413 base pair fragment derived from exons 1 and 2 of cDNA LD21010. (4) lpr2 isoforms transcribed from the distal promoter: a 500 base pair fragment derived from exon 2 of cDNA LD11117. Since the two lpr genes are highly homologous, probes were designed from the most divergent regions to avoid cross-reactivity. Probes 1 and 2 were used in Figure 3E, 3F and probes 3 and 4 in Figure 2A, 2B. The in situ hybridization protocol was carried out according to [73]. To illustrate immunostaining and in situ data, representative images were selected from each experiment after analyzing a minimum of 10 individual imaginal discs or egg chambers from at least two independent experiments.
Transgene construction and overexpression experiments
A UAS-lpr1D transgene was made by cloning the complete coding sequence of lpr1 cDNA RE14223 [74] into the pUAST plasmid [75]. UAS-lpr1B, UAS-lpr1J, UAS-lpr2F and UAS-lpr2E transgenes were obtained by first amplifying the complete corresponding coding regions by PCR using the following templates: LD21010, RE40649, GH26833 and LD11117 [74] respectively. The resulting fragments were fused to a C-terminal 3xHA tag and transferred to pUAST attB [76] to obtain transgenic flies by the integrase phiC31 based system [76]. This method allows for the integration of the transgenes into the same chromosomal location, minimizing positional effects on transcription. All pUAST attB transgenes used were inserted into the 22A landing site [76]. The pUAST or pUAST attB plasmids do not allow expression in the germ-line. Thus, for expression in oocytes and nurse cells we generated UASp-lpr1J, UASp-lpr2F and UASp-lpr2E using the plasmid pUASP [77]. The inserts are identical to UAS-lpr1J, UAS-lpr2F and UAS-lpr2E including the C-terminal 3xHA tag. To generate the lpr2F-lpr2E chimeras, a NotI site was first inserted after Leu30, located between the signal peptide and the LA-2 domain of lpr2F (GH26833) by directed mutagenesis, generating pAC-lpr2F-NotI. NotI flanked fragments containing LA1 (from Ser186 to Thr232) and LA2 (from Glu233 to Cys272) were generated by PCR using lpr2E cDNA LD11117 as template, subsequently cloned into the NotI site of pAC-lpr2F-NotI and transferred to pUAST attB to generate UAS-lpr2F+LA1 and UAS-lpr2F+LA2 respectively. UAS-lpr2F+LA1+NCN and UAS-lpr2F+NCN were similarly generated by replacing the Asp718 (located at the transcription start site)-NotI region of pAC-lpr2F-NotI vector by fragments containing LA1+NCN (from Met1 to Thr232) and NCN (from Met1 to Ile185) regions of lpr2E cDNA LD11117 obtained by PCR and flanked by Asp718 and NotI sites. In all cases, the limits of the protein domains coincided with exon boundaries. All chimeras also contain a C-terminal 3xHA tag.
En-Gal4, obtained from A. Martínez-Arias, was used to drive expression at the posterior compartment of wing imaginal discs, V32-Gal4, a gift from Daniel St Johnston, to drive expression at the germ-line, and CY2-gal4 [78] to direct expression at the ovarian follicle cells.
Supporting Information
Zdroje
1. KopelmanPG
2000 Obesity as a medical problem. Nature 404 635 643
2. BarshGS
SchwartzMW
2002 Genetic approaches to studying energy balance: perception and integration. Nat Rev Genet 3 589 600
3. BakerKD
ThummelCS
2007 Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metab 6 257 266
4. BharuchaKN
2009 The epicurean fly: using Drosophila melanogaster to study metabolism. Pediatr Res 65 132 137
5. LeopoldP
PerrimonN
2007 Drosophila and the genetics of the internal milieu. Nature 450 186 188
6. SchlegelA
StainierDYR
2007 Lessons from "lower" organisms: what worms, flies, and zebrafish can teach us about human energy metabolism. PLoS Genet 3 e199 doi:10.1371/journal.pgen.0030199
7. VanceD
VanceJ
2002 Biochemistry of lipids, lipoproteins and membranes;
VanceD
VanceJ
Amsterdam Elsevier Science
8. CanavosoLE
JouniZE
KarnasKJ
PenningtonJE
WellsMA
2001 Fat metabolism in insects. Annu Rev Nutr 21 23 46
9. Van der HorstDJ
RoosendaalSD
RodenburgKW
2009 Circulatory lipid transport: lipoprotein assembly and function from an evolutionary perspective. Mol Cell Biochem 326 105 119
10. KuttyRK
KuttyG
KambadurR
DuncanT
KooninEV
1996 Molecular characterization and developmental expression of a retinoid - and fatty acid-binding glycoprotein from Drosophila. A putative lipophorin. J Biol Chem 271 20641 20649
11. CallejoA
CuliJ
GuerreroI
2008 Patched, the receptor of Hedgehog, is a lipoprotein receptor. Proc Natl Acad Sci U S A 105 912 917
12. van der HorstDJ
van HoofD
van MarrewijkWJ
RodenburgKW
2002 Alternative lipid mobilization: the insect shuttle system. Mol Cell Biochem 239 113 119
13. BrownMS
GoldsteinJL
1986 A receptor-mediated pathway for cholesterol homeostasis. Science 232 34 47
14. DantumaNP
PottersM
De WintherMP
TensenCP
KooimanFP
1999 An insect homolog of the vertebrate very low density lipoprotein receptor mediates endocytosis of lipophorins. J Lipid Res 40 973 978
15. Van HoofD
RodenburgKW
van der HorstDJ
2003 Lipophorin receptor-mediated lipoprotein endocytosis in insect fat body cells. J Lipid Res 44 1431 1440
16. Van HoofD
RodenburgKW
Van der HorstDJ
2005 Receptor-mediated endocytosis and intracellular trafficking of lipoproteins and transferrin in insect cells. Insect Biochem Mol Biol 35 117 128
17. RodenburgKW
Van der HorstDJ
2005 Lipoprotein-mediated lipid transport in insects: analogy to the mammalian lipid carrier system and novel concepts for the functioning of LDL receptor family members. Biochim Biophys Acta 1736 10 29
18. SchneiderWJ
NimpfJ
2003 LDL receptor relatives at the crossroad of endocytosis and signaling. Cell Mol Life Sci 60 892 903
19. StapletonM
LiaoG
BroksteinP
HongL
CarninciP
2002 The Drosophila gene collection: identification of putative full-length cDNAs for 70% of D. melanogaster genes. Genome Res 12 1294 1300
20. HillerK
GroteA
ScheerM
MünchR
JahnD
2004 PrediSi: prediction of signal peptides and their cleavage positions. Nucleic Acids Res 32 W375 379
21. SpradlingAC
1993 Developmental genetics of oogenesis.
BateM
AriasAM
The Development of Drosophila melanogaster Cold Spring Harbor Laboratory Press 1, 70
22. SchonbaumCP
LeeS
MahowaldAP
1995 The Drosophila yolkless gene encodes a vitellogenin receptor belonging to the low density lipoprotein receptor superfamily. Proc Natl Acad Sci U S A 92 1485 1489
23. SchonbaumCP
PerrinoJJ
MahowaldAP
2000 Regulation of the vitellogenin receptor during Drosophila melanogaster oogenesis. Mol Biol Cell 11 511 521
24. McCallK
2004 Eggs over easy: cell death in the Drosophila ovary. Dev Biol 274 3 14
25. TerashimaJ
TakakiK
SakuraiS
BownesM
2005 Nutritional status affects 20-hydroxyecdysone concentration and progression of oogenesis in Drosophila melanogaster. J Endocrinol 187 69 79
26. FaunyJD
SilberJ
ZiderA
2005 Drosophila Lipid Storage Droplet 2 gene (Lsd-2) is expressed and controls lipid storage in wing imaginal discs. Dev Dyn 232 725 732
27. KhaliullinaH
PanákováD
EugsterC
RiedelF
CarvalhoM
2009 Patched regulates Smoothened trafficking using lipoprotein-derived lipids. Development 136 4111 4121
28. StricklandDK
GoniasSL
ArgravesWS
2002 Diverse roles for the LDL receptor family. Trends Endocrinol Metab 13 66 74
29. MorrisonHA
DionneH
RustenTE
BrechA
FisherWW
2008 Regulation of early endosomal entry by the Drosophila tumor suppressors Rabenosyn and Vps45. Mol Biol Cell 19 4167 4176
30. CompagnonJ
GervaisL
RomanMS
Chamot-BoeufS
GuichetA
2009 Interplay between Rab5 and PtdIns(4,5)P2 controls early endocytosis in the Drosophila germline. J Cell Sci 122 25 35
31. ZerialM
McBrideH
2001 Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2 107 117
32. PospisilikJA
SchramekD
SchnidarH
CroninSJF
NehmeNT
2010 Drosophila genome-wide obesity screen reveals hedgehog as a determinant of brown versus white adipose cell fate. Cell 140 148 160
33. Al-AnziB
SapinV
WatersC
ZinnK
WymanRJ
2009 Obesity-blocking neurons in Drosophila. Neuron 63 329 341
34. BellerM
SztalrydC
SouthallN
BellM
JäckleH
2008 COPI complex is a regulator of lipid homeostasis. PLoS Biol 6 e292 doi:10.1371/journal.pbio.0060292
35. GronkeS
MildnerA
FellertS
TennagelsN
PetryS
2005 Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila. Cell Metab 1 323 330
36. GuoY
WaltherTC
RaoM
StuurmanN
GoshimaG
2008 Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature 453 657 661
37. Guidugli-LazzariniKR
do NascimentoAM
TanakaED
PiulachsMD
HartfelderK
2008 Expression analysis of putative vitellogenin and lipophorin receptors in honey bee (Apis mellifera L.) queens and workers. J Insect Physiol 54 1138 1147
38. CheonHM
SeoSJ
SunJ
SappingtonTW
RaikhelAS
2001 Molecular characterization of the VLDL receptor homolog mediating binding of lipophorin in oocyte of the mosquito Aedes aegypti. Insect Biochem Mol Biol 31 753 760
39. CiudadL
BellésX
PiulachsM
2007 Structural and RNAi characterization of the German cockroach lipophorin receptor, and the evolutionary relationships of lipoprotein receptors. BMC Mol Biol 8 53
40. GopalapillaiR
Kadono-OkudaK
TsuchidaK
YamamotoK
NohataJ
2006 Lipophorin receptor of Bombyx mori: cDNA cloning, genomic structure, alternative splicing, and isolation of a new isoform. J Lipid Res 47 1005 1013
41. LeeCS
HanJH
KimBS
LeeSM
HwangJS
2003 Wax moth, Galleria mellonella, high density lipophorin receptor: alternative splicing, tissue-specific expression, and developmental regulation. Insect Biochem Mol Biol 33 761 771
42. DantumaNP
PijnenburgMA
DiederenJH
Van der HorstDJ
1997 Developmental down-regulation of receptor-mediated endocytosis of an insect lipoprotein. J Lipid Res 38 254 265
43. Guidugli-LazzariniKR
do NascimentoAM
TanakaED
PiulachsMD
HartfelderK
2008 Expression analysis of putative vitellogenin and lipophorin receptors in honey bee (Apis mellifera L.) queens and workers. Journal of insect physiology 54 1138 1147
44. SeoS-J
CheonH-M
SunJ
SappingtonTW
RaikhelAS
2003 Tissue - and stage-specific expression of two lipophorin receptor variants with seven and eight ligand-binding repeats in the adult mosquito. The Journal of biological chemistry 278 41954 41962
45. SoukupSF
CuliJ
GubbD
2009 Uptake of the necrotic serpin in Drosophila melanogaster via the lipophorin receptor-1. PLoS Genet 5 e1000532 doi:10.1371/journal.pgen.1000532
46. De GregorioE
SpellmanPT
RubinGM
LemaitreB
2001 Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. Proc Natl Acad Sci USA 98 12590 12595
47. FerrandonD
ImlerJL
HetruC
HoffmannJA
2007 The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat Rev Immunol 7 862 874
48. SeoSJ
CheonHM
SunJ
SappingtonTW
RaikhelAS
2003 Tissue - and stage-specific expression of two lipophorin receptor variants with seven and eight ligand-binding repeats in the adult mosquito. J Biol Chem 278 41954 41962
49. MagranéJ
Casaroli-MaranoRP
ReinaM
GåfvelsM
VilaróS
1999 The role of O-linked sugars in determining the very low density lipoprotein receptor stability or release from the cell. FEBS Lett 451 56 62
50. MayP
BockHH
NimpfJ
HerzJ
2003 Differential glycosylation regulates processing of lipoprotein receptors by gamma-secretase. The Journal of biological chemistry 278 37386 37392
51. BrandesC
KahrL
StockingerW
HiesbergerT
SchneiderWJ
2001 Alternative splicing in the ligand binding domain of mouse ApoE receptor-2 produces receptor variants binding reelin but not alpha 2-macroglobulin. J Biol Chem 276 22160 22169
52. SakaiK
TiebelO
LjungbergMC
SullivanM
LeeH-J
2009 A neuronal VLDLR variant lacking the third complement-type repeat exhibits high capacity binding of apoE containing lipoproteins. Brain Res 1276 11 21
53. SeppKJ
HongP
LizarragaSB
LiuJS
MejiaLA
2008 Identification of neural outgrowth genes using genome-wide RNAi. PLoS Genet 4 e1000111 doi:10.1371/journal.pgen.1000111
54. ZieglerR
Van AntwerpenR
2006 Lipid uptake by insect oocytes. Insect Biochem Mol Biol 36 264 272
55. BuszczakM
LuX
SegravesWA
ChangTY
CooleyL
2002 Mutations in the midway gene disrupt a Drosophila acyl coenzyme A: diacylglycerol acyltransferase. Genetics 160 1511 1518
56. ThomsonTC
JohnsonJ
2010 Inducible somatic oocyte destruction in response to rapamycin requires wild-type regulation of follicle cell epithelial polarity. Cell Death Differ 17 1717 1727
57. HussainMM
StricklandDK
BakillahA
1999 The mammalian low-density lipoprotein receptor family. Annu Rev Nutr 19 141 172
58. Van der HorstDJ
1990 Lipid transport function of lipoproteins in flying insects. Biochim Biophys Acta 1047 195 211
59. Dallinga-ThieGM
FranssenR
MooijHL
VisserME
HassingHC
2010 The metabolism of triglyceride-rich lipoproteins revisited: new players, new insight. Atherosclerosis 211 1 8
60. DaviesBS
BeigneuxAP
BarnesRH2nd
TuY
GinP
2010 GPIHBP1 Is Responsible for the Entry of Lipoprotein Lipase into Capillaries. Cell Metab 12 42 52
61. BeigneuxAP
DaviesBS
GinP
WeinsteinMM
FarberE
2007 Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab 5 279 291
62. ObunikeJC
LutzEP
LiZ
PakaL
KatopodisT
2001 Transcytosis of lipoprotein lipase across cultured endothelial cells requires both heparan sulfate proteoglycans and the very low density lipoprotein receptor. The Journal of biological chemistry 276 8934 8941
63. TackenPJ
HofkerMH
HavekesLM
van DijkKW
2001 Living up to a name: the role of the VLDL receptor in lipid metabolism. Curr Opin Lipidol 12 275 279
64. GoudriaanJR
Espirito SantoSM
VosholPJ
TeusinkB
van DijkKW
2004 The VLDL receptor plays a major role in chylomicron metabolism by enhancing LPL-mediated triglyceride hydrolysis. J Lipid Res 45 1475 1481
65. TackenPJ
TeusinkB
JongMC
HaratsD
HavekesLM
2000 LDL receptor deficiency unmasks altered VLDL triglyceride metabolism in VLDL receptor transgenic and knockout mice. J Lipid Res 41 2055 2062
66. FrykmanPK
BrownMS
YamamotoT
GoldsteinJL
HerzJ
1995 Normal plasma lipoproteins and fertility in gene-targeted mice homozygous for a disruption in the gene encoding very low density lipoprotein receptor. Proc Natl Acad Sci U S A 92 8453 8457
67. ParksAL
CookKR
BelvinM
DompeNA
FawcettR
2004 Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat Genet 36 288 292
68. ThibaultST
SingerMA
MiyazakiWY
MilashB
DompeNA
2004 A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat Genet 36 283 287
69. XuT
RubinGM
1993 Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117 1223 1237
70. ChouTB
NollE
PerrimonN
1993 Autosomal P[ovoD1] dominant female-sterile insertions in Drosophila and their use in generating germ-line chimeras. Development 119 1359 1369
71. BendtsenJD
NielsenH
von HeijneG
BrunakS
2004 Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340 783 795
72. StriginiM
CohenSM
2000 Wingless gradient formation in the Drosophila wing. Curr Biol 10 293 300
73. YakobyN
BristowCA
GongD
SchaferX
LembongJ
2008 A combinatorial code for pattern formation in Drosophila oogenesis. Dev Cell 15 725 737
74. StapletonM
CarlsonJ
BroksteinP
YuC
ChampeM
2002 A Drosophila full-length cDNA resource. Genome Biol 3 RESEARCH0080
75. BrandAH
PerrimonN
1993 Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118 401 415
76. BischofJ
MaedaRK
HedigerM
KarchF
BaslerK
2007 An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc Natl Acad Sci U S A 104 3312 3317
77. RorthP
1998 Gal4 in the Drosophila female germline. Mech Dev 78 113 118
78. QueenanAM
GhabrialA
SchupbachT
1997 Ectopic activation of torpedo/Egfr, a Drosophila receptor tyrosine kinase, dorsalizes both the eggshell and the embryo. Development 124 3871 3880
Štítky
Genetika Reprodukční medicína
Článek Break to Make a ConnectionČlánek A New Testing Strategy to Identify Rare Variants with Either Risk or Protective Effect on DiseaseČlánek The Architecture of Gene Regulatory Variation across Multiple Human Tissues: The MuTHER Study
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 2
-
Všechny články tohoto čísla
- Break to Make a Connection
- A New Testing Strategy to Identify Rare Variants with Either Risk or Protective Effect on Disease
- Single-Tissue and Cross-Tissue Heritability of Gene Expression Via Identity-by-Descent in Related or Unrelated Individuals
- Pervasive Adaptive Protein Evolution Apparent in Diversity Patterns around Amino Acid Substitutions in
- The Architecture of Gene Regulatory Variation across Multiple Human Tissues: The MuTHER Study
- MiRNA Control of Vegetative Phase Change in Trees
- New Functions of Ctf18-RFC in Preserving Genome Stability outside Its Role in Sister Chromatid Cohesion
- Genome-Wide Association Studies of the PR Interval in African Americans
- Mapping of the Disease Locus and Identification of As a Candidate Gene in a Canine Model of Primary Open Angle Glaucoma
- Mapping a New Spontaneous Preterm Birth Susceptibility Gene, , Using Linkage, Haplotype Sharing, and Association Analysis
- A Population Genetic Approach to Mapping Neurological Disorder Genes Using Deep Resequencing
- and Genes Modulate the Switch between Attraction and Repulsion during Behavioral Phase Change in the Migratory Locust
- Targeted Sister Chromatid Cohesion by Sir2
- Correlated Evolution of Nearby Residues in Drosophilid Proteins
- Parallel Evolution of a Type IV Secretion System in Radiating Lineages of the Host-Restricted Bacterial Pathogen
- Lipophorin Receptors Mediate the Uptake of Neutral Lipids in Oocytes and Imaginal Disc Cells by an Endocytosis-Independent Mechanism
- Genome-Wide Association Study of Coronary Heart Disease and Its Risk Factors in 8,090 African Americans: The NHLBI CARe Project
- The Evolution of Host Specialization in the Vertebrate Gut Symbiont
- Genome-Wide Association of Familial Late-Onset Alzheimer's Disease Replicates and and Nominates in Interaction with
- Risk Alleles for Systemic Lupus Erythematosus in a Large Case-Control Collection and Associations with Clinical Subphenotypes
- Association between Common Variation at the Locus and Changes in Body Mass Index from Infancy to Late Childhood: The Complex Nature of Genetic Association through Growth and Development
- AID Induces Double-Strand Breaks at Immunoglobulin Switch Regions and Causing Chromosomal Translocations in Yeast THO Mutants
- A Study of CNVs As Trait-Associated Polymorphisms and As Expression Quantitative Trait Loci
- Whole-Genome Comparison Reveals Novel Genetic Elements That Characterize the Genome of Industrial Strains of
- Prevalence of Epistasis in the Evolution of Influenza A Surface Proteins
- Srf1 Is a Novel Regulator of Phospholipase D Activity and Is Essential to Buffer the Toxic Effects of C16:0 Platelet Activating Factor
- Two Frizzled Planar Cell Polarity Signals in the Wing Are Differentially Organized by the Fat/Dachsous Pathway
- Phosphoinositide Regulation of Integrin Trafficking Required for Muscle Attachment and Maintenance
- Pathogenic VCP/TER94 Alleles Are Dominant Actives and Contribute to Neurodegeneration by Altering Cellular ATP Level in a IBMPFD Model
- Meta-Analysis of Genome-Wide Association Studies in Celiac Disease and Rheumatoid Arthritis Identifies Fourteen Non-HLA Shared Loci
- A Genome-Wide Study of DNA Methylation Patterns and Gene Expression Levels in Multiple Human and Chimpanzee Tissues
- Nucleosomes Containing Methylated DNA Stabilize DNA Methyltransferases 3A/3B and Ensure Faithful Epigenetic Inheritance
- Mutations in Zebrafish Result in Adult-Onset Ocular Pathogenesis That Models Myopia and Other Risk Factors for Glaucoma
- [], the Prion Formed by the Chromatin Remodeling Factor Swi1, Is Highly Sensitive to Alterations in Hsp70 Chaperone System Activity
- Characterization of Transcriptome Remodeling during Cambium Formation Identifies and As Opposing Regulators of Secondary Growth
- The Cardiac Transcription Network Modulated by Gata4, Mef2a, Nkx2.5, Srf, Histone Modifications, and MicroRNAs
- Epistatic Interaction Maps Relative to Multiple Metabolic Phenotypes
- Quantitative Models of the Mechanisms That Control Genome-Wide Patterns of Transcription Factor Binding during Early Development
- Genome-Wide Transcript Profiling of Endosperm without Paternal Contribution Identifies Parent-of-Origin–Dependent Regulation of
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Meta-Analysis of Genome-Wide Association Studies in Celiac Disease and Rheumatoid Arthritis Identifies Fourteen Non-HLA Shared Loci
- MiRNA Control of Vegetative Phase Change in Trees
- Risk Alleles for Systemic Lupus Erythematosus in a Large Case-Control Collection and Associations with Clinical Subphenotypes
- The Cardiac Transcription Network Modulated by Gata4, Mef2a, Nkx2.5, Srf, Histone Modifications, and MicroRNAs
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



