-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMiRNA Control of Vegetative Phase Change in Trees
After germination, plants enter juvenile vegetative phase and then transition to an adult vegetative phase before producing reproductive structures. The character and timing of the juvenile-to-adult transition vary widely between species. In annual plants, this transition occurs soon after germination and usually involves relatively minor morphological changes, whereas in trees and other perennial woody plants it occurs after months or years and can involve major changes in shoot architecture. Whether this transition is controlled by the same mechanism in annual and perennial plants is unknown. In the annual forb Arabidopsis thaliana and in maize (Zea mays), vegetative phase change is controlled by the sequential activity of microRNAs miR156 and miR172. miR156 is highly abundant in seedlings and decreases during the juvenile-to-adult transition, while miR172 has an opposite expression pattern. We observed similar changes in the expression of these genes in woody species with highly differentiated, well-characterized juvenile and adult phases (Acacia confusa, Acacia colei, Eucalyptus globulus, Hedera helix, Quercus acutissima), as well as in the tree Populus x canadensis, where vegetative phase change is marked by relatively minor changes in leaf morphology and internode length. Overexpression of miR156 in transgenic P. x canadensis reduced the expression of miR156-targeted SPL genes and miR172, and it drastically prolonged the juvenile phase. Our results indicate that miR156 is an evolutionarily conserved regulator of vegetative phase change in both annual herbaceous plants and perennial trees.
Published in the journal: . PLoS Genet 7(2): e32767. doi:10.1371/journal.pgen.1002012
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002012Summary
After germination, plants enter juvenile vegetative phase and then transition to an adult vegetative phase before producing reproductive structures. The character and timing of the juvenile-to-adult transition vary widely between species. In annual plants, this transition occurs soon after germination and usually involves relatively minor morphological changes, whereas in trees and other perennial woody plants it occurs after months or years and can involve major changes in shoot architecture. Whether this transition is controlled by the same mechanism in annual and perennial plants is unknown. In the annual forb Arabidopsis thaliana and in maize (Zea mays), vegetative phase change is controlled by the sequential activity of microRNAs miR156 and miR172. miR156 is highly abundant in seedlings and decreases during the juvenile-to-adult transition, while miR172 has an opposite expression pattern. We observed similar changes in the expression of these genes in woody species with highly differentiated, well-characterized juvenile and adult phases (Acacia confusa, Acacia colei, Eucalyptus globulus, Hedera helix, Quercus acutissima), as well as in the tree Populus x canadensis, where vegetative phase change is marked by relatively minor changes in leaf morphology and internode length. Overexpression of miR156 in transgenic P. x canadensis reduced the expression of miR156-targeted SPL genes and miR172, and it drastically prolonged the juvenile phase. Our results indicate that miR156 is an evolutionarily conserved regulator of vegetative phase change in both annual herbaceous plants and perennial trees.
Introduction
Plants produce different types of leaves, buds, and internodes at different times in their development. Although many traits vary continuously, other traits are expressed in discontinuous pattern that allows shoot development to be divided into discrete juvenile, adult, and reproductive phases [1]–[5]. These transitions involve changes in many different traits that must be temporally and spatially coordinated if the plant is to survive and reproduce. This problem is particularly important in perennial species, which encounter numerous biotic and abiotic stresses during their long life cycles. Recent studies have begun to reveal the molecular mechanism of these phase transitions in the annual species Arabidopsis and maize, but the molecular mechanism of phase change in perennial woody species is still largely unknown.
In the model annual forb, Arabidopsis thaliana, the major morphological difference between the juvenile and the adult phase of vegetative development is in leaf morphology. Adult leaves have serrations on their leaf margins and trichomes on the abaxial surface, which are lacking in juvenile leaves [6]–[8]. In maize, juvenile leaves lack trichomes but possess epicuticular wax, whereas adult leaves have the opposite traits [4]. These differences are mediated by two miRNAs, miR156 and miR172, both of which target DNA-binding transcription factors. miR156 is highly abundant in seedlings, and decreases during subsequent development, while miR172 has an opposite expression pattern. Overexpression of miR156—which negatively regulates several SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) genes—delays both the juvenile-to-adult and adult-to-reproductive phase transitions. Conversely, increasing the levels of different SPLs can in the most extreme case completely eliminate the juvenile phase [9]–[16]. miR172 targets several transcription factors related to the eponymous APETALA2 (AP2) protein, including TARGET OF EAT1 (TOE1), TOE2, TOE3, SCHLAFMÜTZE (SMZ), and SCHNARCHZAPFEN (SNZ) [17]–[20] in Arabidopsis, and Glossy15 in maize [15]. MIR172b is a direct target of SPL9 in Arabidopsis and its level gradually rises after germination in response to increasing SPL activity. Overexpression of the miR172-regulated genesTOE1 [13] and Glossy15 [15] delays the juvenile-to-adult vegetative transition.
The hierarchical action of miR156 and miR172 and their SPL and AP2 targets in the control of vegetative phase change and flowering is conserved in the annual grasses rice and maize [15], [16], [21]–[25]. It is unknown, however, whether the juvenile-to-adult phase transition in woody perennial plants is controlled by the same factors as it is in annual species. First, the differences between juvenile and adult phases are often much more obvious in shrubs and trees, and are usually more stably expressed in woody plants than in herbaceous species. Second, juvenile and adult vegetative phases are quite brief in herbaceous plants such as A. thaliana and maize, but can last for many years in trees [26]. Here, we show that levels of miR156 and miR172 are closely correlated with the juvenile and adult phases of several woody species that have long been used in studies of vegetative phase change. We also demonstrate that miR156 expression varies with the age and morphology of the shoot in the poplar hybrid Populus x canadensis, and that miR156 overexpression dramatically delays phase change in this tree.
Results
The expression of miR156 and miR172 in woody plants with highly differentiated juvenile and adult phases
In A. thaliana, miR156 and miR172 control both the juvenile-to-adult vegetative transition and the adult-to-reproductive transition. miR156 expression is highest after germination and declines within two weeks, whereas miR172 shows the converse pattern [9], [10], [13], [14], [18]. To explore the possibility that these miRNAs also regulate phase change in woody plants, we examined their expression in several species with distinct, well-characterized juvenile and adult phases.
Juvenile and adult phases of vegetative development were first described in Acacia species native to Australia [27], [28], where these phases are characterized by dramatic differences in leaf morphology. Early in shoot development, these species produces horizontally oriented, bipinnately compound leaves. The transition to the adult phase is marked by the production of phyllodes—vertically-oriented, simple leaves, in which adaxial cell types are present on both surface of the leaf blade [29]. This transition takes place at different nodes in different Acacia species, and is often accompanied by the production of transition leaves in which both leaf types are present in a single leaf (Figure 1B). Juvenile and adult stages of vegetative development are also well differentiated in many species of Eucalyptus, including E. globulus, where juvenile leaves are horizontally oriented, ovate to acuminate in shape, lack a petiole, are covered with epicuticular wax, and produce palisade mesophyll solely on the adaxial surface of the leaf blade; in contrast, adult leaves are vertically oriented, lanceolate, petiolate, waxless, and have palisade mesophyll on both the surfaces of the leaf blade [30] (Figure 1C and 1F). English ivy (Hedera helix) is a classic system for the analysis of vegetative phase change [31]. Juvenile and adult phases of shoot growth in this woody vine differ in leaf shape, phyllotaxis, the orientation of shoot growth, adventitious root production, growth rate, and anthocyanin production [32] (Figure 1D). In the sawtooth oak, Quercus acutissima, juvenile leaves are ovate in shape and have a relatively short petiole, whereas adult leaves have an acute leaf tip and a longer petiole (Figure 1E). Juvenile and adult phases of shoot development in this and other species of oak can also be readily differentiated by their pattern of leaf abscission: adult branches drop their leaves in the Fall, whereas juvenile branches retain their leaves until Spring (Figure 1G).
Fig. 1. The expression of miR156 and its targets is correlated with vegetative phase change in woody plants. 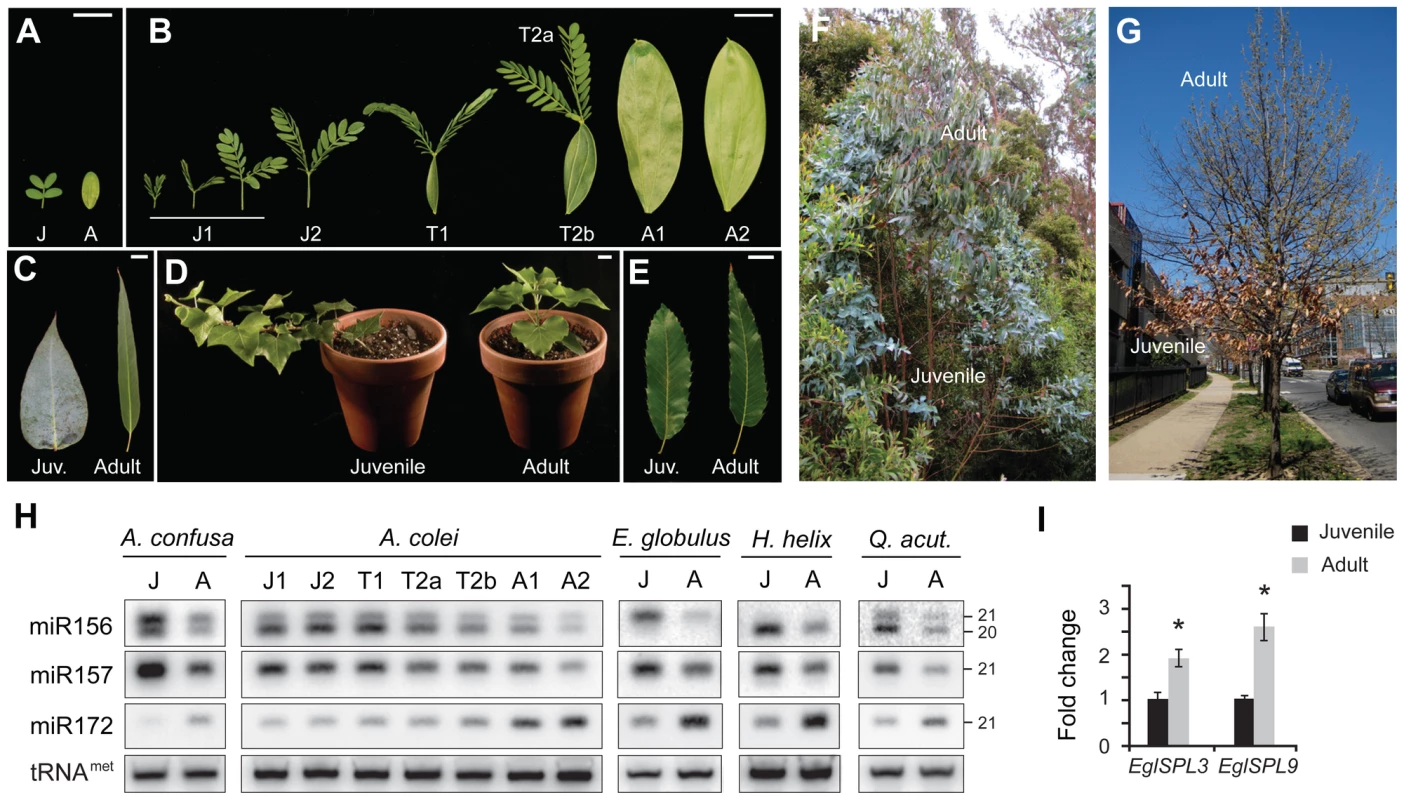
(A) Morphology of first two leaves of A. confusa. (B) Morphology of the first 8 leaves of A. colei. J = juvenile, T = transition, A = adult. (C) Juvenile and adult leaves from a single tree of E. globulus. (D) Juvenile and adult clones of H. helix (English ivy). (E) Juvenile and adult leaves of Q. acutissima. Scale bars indicate 2 cm. (F) One of the E. globulus trees from which the leaves used for expression analysis were harvested. (G) Q. acutissima tree from which the leaves used for expression analysis were harvested. (H) Blots of small RNA isolated from the leaves shown in Figure 1A–1E, hybridized with probes for miR156, miR157 and miR172; tRNAmet was used as a loading control. The H. helix blot represents RNA from shoot apices with leaves 1 cm or less in size. (I) qRT-PCR analyses of the expression of EglSPL3 and EglSPL9 in fully expanded juvenile and adult leaves of E. globulus. Expression was normalized to EglElF4, and then to the average expression in juvenile leaves. Shown are the averages of three technical replicates for samples from three trees (3 technical replicates×3 trees = 9 replicates per sample), ± s. e. m. Asterix = significantly different from juvenile, p<0.01, Student's t test. The levels of miR156 and miR172 were measured by northern blot analyses of the RNAs isolated from fully expanded leaves of A. confusa, A. colei, E. globulus, and Q. acutissima, and shoot apices of H. helix (Figure 1H). Analyses were conducted using juvenile and adult leaves from the same plant in order to control for genetic variation between samples; at least two plants were examined for each species, and RNA levels were quantified by densitometry (Table S1). miR156 was expressed at a significantly higher level in juvenile leaves than in adult leaves, whereas miR172 had the opposite pattern (Student's t test, p<0.0001, n = 12). This relationship was particularly striking in A. confusa and A. colei, where variation in the levels of miR156 and miR172 were correlated with node-to-node changes in leaf shape (Figure 1A, 1B, 1H). The observation that this change in expression occurs at different nodes in these two species (node 2 in A. confusa and node 6 or 7 in A. colei) provides additional evidence that the expression of these miRNAs is associated with vegetative phase change rather than some other feature of shoot development, such as the distance of a leaf from the root system or the overall size of the shoot.
The miR156 probe hybridized to 20 and 21 nt transcripts in A. confusa, A. colei and Q. acutissima, to a single 21 nt transcript in E. globulus, and a single 20 nt transcript in H. helix. Deep sequencing of small RNAs has revealed 20 nt miR156 transcripts in species ranging from moss to flowering plants (www.mirbase.org). Many species also produce a closely-related miRNA that is 21 nt in length and differs from miR156 at three positions (www.mirbase.org); in Arabidopsis, this miRNA has been named miR157 [33]. Hybridization with a probe complementary to miR157 revealed a single 21 nt band in all five species we examined. This miRNA was expressed at the same, or higher, level than miR156, and in the same developmental pattern (Figure 1H). The observation that miR157 probe did not hybridize to a 20 nt fragment in A. confusa, A. colei, H. helix and Q. acutissima, and that the miR156 probe did not hybridize to a 21 nt fragment in H. helix, indicates that these probes do not cross-hybridize; thus, the 21 nt band observed on miR156 blots is unlikely to represent miR157. miR156 transcripts with one additional 3′ or 5′ nucleotide (i.e., 21 nt miR156 transcripts) have been observed by deep sequencing in several plants, including Arabidopsis, rice, and Populus [34]–[36]. It remains to be determined if these size variants are the sole product of specific miR156 loci, or are produced along with 20 nt forms by the imprecise processing of miR156 precursors.
To determine if the variation in miR156 expression is functionally significant, we identified homologs of AtSPL3 and AtSPL9 in the recently completed genome sequence of Eucalyptus grandis (DOE Joint Genome Institute and the Eucalyptus Genome Network; http://www.phytozome.net/eucalyptus.php), and used this sequence information to amplify the related transcripts from adult leaves of E. globulus. Quantitative RT-PCR (qRT-PCR) of juvenile and adult leaves from three different E. globulus trees demonstrated that transcripts of EglSPL3 and EglSPL9 were present at approximately 2-fold higher levels in adult leaves than juvenile leaves (Figure 1I), consistent with the relative abundance of miR156 in these leaves (Figure 1H; Table S1). The expression pattern of these direct targets of miR156 supports the conclusion that miR156 plays an important role in vegetative phase change in E. globulus.
Vegetative phase change in P. x canadensis
As a further test of the hypothesis that that miR156 promotes juvenile development in trees, we took advantage of P. x canadensis cv. Guangzhao Yang, a hybrid of P. deltoides and P. nigra that is readily transformable. All of our studies were performed on clonal shoots regenerated from tissue culture. Although we were unable to examine the morphology of plants grown from seeds, regeneration typically induces rejuvenation in woody plants [37], so it is reasonable to assume that the changes we observed in these regenerated plants mimic the changes that occur in seed-derived plants. This conclusion is supported by the observation that the leaf morphology of one-month old regenerated shoots of the clone used in this study closely resembled the juvenile leaves of P. trichocarpa, as described by [38].
There was a significant difference in the morphology of the leaves of 1-month - and 1-year-old regenerated shoots. 1-month-old plants had small, oval leaves, while the leaves of 1-year-old trees were larger and deltoid in shape (Figure 2A; Table 1). Leaf shape did not change further in older trees. In addition, 1-month-old plants had round petioles with only one vascular bundle, whereas the petioles of 1-year-old trees were flattened in an adaxial-abaxial plane and had three major vascular bundles (Figure 2B). The internodes of 1-month-old plants were also significantly shorter than those of 6-month or 1-year-old trees (Table 1).
Fig. 2. Vegetative phase change and miRNA expression in P. x canadensis. 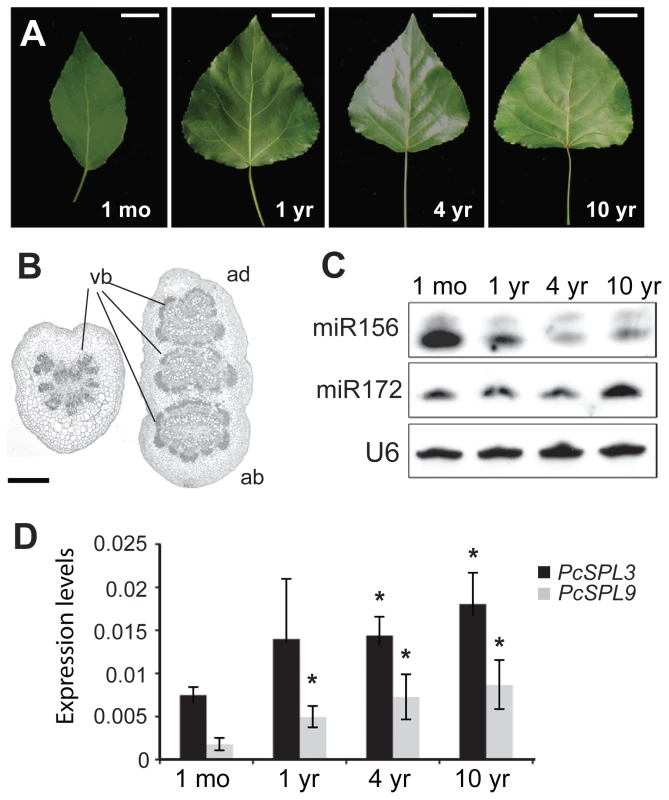
(A) Leaf morphology. Scale bars indicate 2 cm for 1-month-old trees and 4 cm for the rest. (B) Transverse sections of petioles of 1-month- (left) and 1-year-old (right) trees. ab, abaxial. ad, adaxial. vb = vascular bundles. Scale bar indicates 200 µm. (C) Expression of miR156 and miR172, with U6 as loading control. (D) Expression of PcSPL3 and PcSPL9, measured by real-time RT-PCR, and normalized to PcACT. Error bars indicate standard deviation (s.d.). Asterix = significantly different from 1-month-old saplings, p<0.01, Student's t test. Tab. 1. Growth characteristics of 35S::MIR156 P. x canadensis plants. 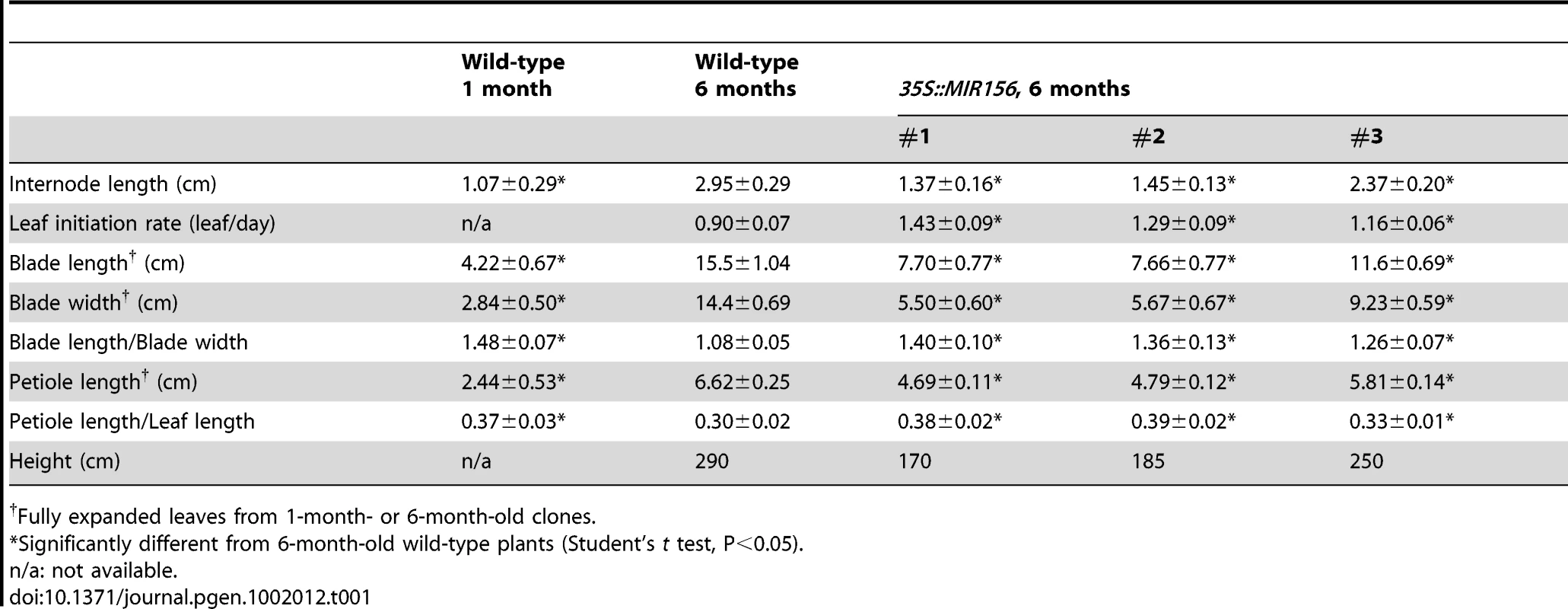
Fully expanded leaves from 1-month- or 6-month-old clones. The expression of miR156 was initially examined in fully expanded leaves from 1-month-, 1-year-, 4-year-, and 10-year-old trees. miR156 was highly expressed in leaves from 1-month-old plants, and was expressed at much lower levels in older trees (Figure 2C). This expression difference is likely to be functionally significant because two miR156 targets, PcSPL3 and PcSPL9, were expressed in the opposite pattern (Figure 2D). The expression of miR172 was similar in 1-month-, 1-year-, and 4-year-old trees, but was elevated in 10-year-old trees (Figure 2C).
To characterize the expression pattern of miR156 and miR172 in more detail, we examined the levels of these miRNAs in 2 cm leaf primordia from the shoot apex of trees of different ages (0.5 m, 2 m, and 4 m tall), and in fully expanded leaves and leaf primordia of branches located at different positions on the primary shoot (Figure 3A). miR156 was most highly expressed in leaf primordia from the primary shoot of 0.5 m and 2 m trees, and was expressed at a lower level in 4-m-tall shoots, while miR172 showed the opposite trend (Figure 3B). Leaves on branches produced at the base of main stem (0.5 m, branch 1) recapitulate the change in leaf shape that occurs during the growth of the main stem. The first leaves on these branches resemble juvenile leaves, and leaf size and shape change gradually until the 10th node, by which time leaves have acquired the size and shape of adult leaves (Figure 3C). Analyses of gene expression in these branch leaves revealed that miR156 was expressed at high levels in basal, juvenile-like leaves, and at lower levels in successively more apical leaves, whereas miR172 was expressed in the opposite pattern (Figure 3D, branch 1). Consistent with their similarity in size and shape, the first few leaves of these basal branches produced approximately as much miR156 as the leaves on the main shoot of 1-month-old plants (compare Figure 2C and Figure 3D).
Fig. 3. Spatial expression pattern of miR156 in P. x canadensis. 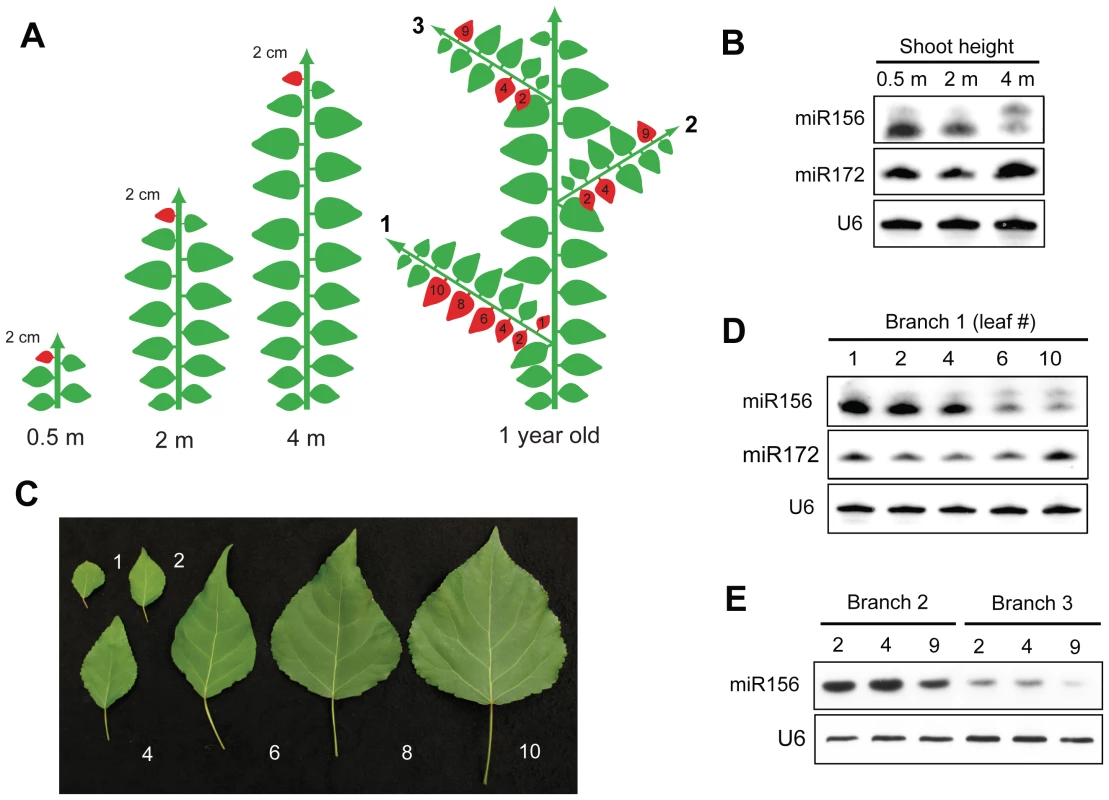
(A) Diagram illustrating the source of the leaf samples analyzed in 3B–3E. 2 cm leaf primordia were harvested from the shoot apex of 0.5 m, 2 m and 4 m tall shoots; fully expanded leaves were harvested from a branch (branch 1) of a 6 month-old tree, and 2 cm leaves or leaf primordia were harvested from branches located 2.5 m (branch 2) and 4 m (branch 3) from the base of a 1-year-old tree. Drawing is not to scale. (B) miRNA expression in 2 cm long leaf primordia from the shoot apices of trees of different heights. (C) Fully-expanded leaves from a single branch of a 6-month-old tree, numbered from the closest position to the trunk. (D) miRNA expression in the leaves illustrated in (C). (E) miR156 expression in 2 cm leaves or leaf primordia on branches located 2.5 m (branch 2), and 4 m (branch 3) from the base of the shoot. The phase identity of a lateral branch typically matches the identity of the primary node from which it originated [1]–[3]. To determine if the expression of miR156 shows a similar pattern, we examined the level of miR156 in leaves from branches located 2.5 m and 4 meters from the base of the shoot. These positions were chosen to correspond to the height of the primary shoots examined in Figure 3B. Consistent with the expression of miR156 in leaf primordia from 2 m and 4 m tall primary shoots (Figure 3B), the leaves of branches located 2.5 m from the base of the shoot (branch 2) had relatively high levels of miR156, whereas leaves on branches located 4 m from the base of the shoot (branch 3) had much lower levels miR156 (Figure 3E). This result provides additional evidence that miR156 regulates vegetative phase change in P. x canadensis.
miR156 delays vegetative phase change in P. x canadensis
To determine if miR156 regulates phase change through its SPL targets, we over-expressed miR156 in P. x canadensis. Ten independent lines were generated; 9 lines had similar phenotypes, and three of these were analyzed in detail. PCR analysis using primers to the 35S promoter confirmed that these 3 lines were indeed transgenic (Figure S1). We confirmed by qRT-PCR that PcSPL3 and PcSPL9 were down-regulated in the most severe line, #1 (Figure 4A). As a control, we regenerated 5 wild-type plants, and plants over-expressing β-GLUCURONIDASE (35S::GUS). 35S::GUS plants were indistinguishable from wild type clones (Figure S2).
Fig. 4. The phenotype of 35S::MIR156 P. x canadensis plants. 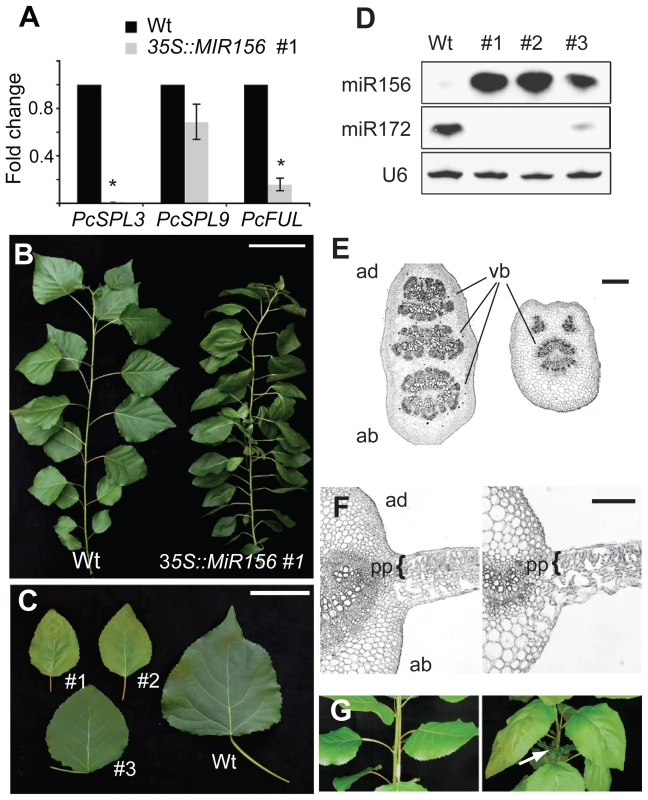
(A) qRT-PCR analysis of the expression of PcSPL3, PcSPL9, and PcFUL in 6-month-old plants, normalized to PcACT. Wild-type expression levels were normalized to 1. Asterix = significantly different from wild type, p<0.01, Student's t test. (B) Single branch from 6-month-old wild-type (WT) and 35S::MIR156 (#1) plants. Scale bar indicates 10 cm. (C) Leaves of 6-month-old wild-type and three independent 35S::MIR156 transgenic lines (#1 to #3). Scale bar indicates 5 cm. (D) Expression of miR156 and miR172. (E) Petiole sections of wild-type (left) and 35S::MIR156 (#1) (right). Scale bar indicates 200 µm. (F) Transverse sections of a major vein and the lamina of 6-month-old fully expanded leaves from wild-type (left) and 35S::MIR156 (#1) (right) plants. pp = palisade parenchyma. Scale bars indicate 50 µm. (G) Primary shoots of 2-month-old wild-type (left) and 35S::MIR156 (#1) (right) plants. Arrows indicate lateral shoots. Scale bars indicate 5 cm. The most obvious phenotype of 35S::MIR156 plants was a change in plant height and leaf shape (Figure 4B and Table 1). Compared to wild-type plants, 35S::MIR156 plants were shorter and produced small, pale-green leaves (Figure 4B and 4C and Table 1). The severity of the phenotype of the three 35S::MIR156 lines (#1–3) was correlated with their miR156 levels, with #1 and #2 having higher miR156 expression and a more severe phenotype than line # 3 (Figure 4C, 4D). At six months of age, 35S::MIR156 plants resembled 1-month-old wild-type plants. Like these juvenile plants, 35S::MIR156 plants had leaves with an oval lamina (compare Figure 4C to Figure 2B; Table 1) and round petioles, containing a single vascular bundle (Figure 4E). Transverse sections of the lamina revealed that 35S::MIR156 plants had only a single layer of palisade mesophyll cells, in contrast to the leaves of 6-month-old wild-type plants, which had two palisade cell layers (Figure 4F). In addition, 35S::MIR156 plants had shorter internodes and a faster rate of leaf initiation than 6-month-old wild type plants, and formed side branches at every node (Table 1 and Figure 4G). These later traits are also characteristic of Arabidopsis and maize plants that over-express miR156 [11], [16], [20], [36], [39]. The changes in leaf and shoot morphology were paralleled by altered expression of SPL genes (Figure 4A) and corresponding changes in the expression of genes that are direct targets of SPL in Arabidopsis [9]–[11], [13], [14]—in particular, a homolog of FRUITFULL (PcFUL) (Figure 4A) and miR172 (Figure 4D).
Discussion
The morphology and physiology of a plant shoot change during its development. The most recognizable example of this is the transition from vegetative to reproductive growth, which is marked by the production of specialized structures, such as flowers or cones. The juvenile-to-adult transition is more difficult to recognize because it is usually accompanied by relatively subtle, species-specific changes. This has created considerable confusion about the nature of vegetative phase change. Because there is no common morphological marker for juvenile and adult phases of vegetative development, it is difficult to know whether temporal variation in particular vegetative traits in different species represent the same, or different, developmental processes.
The identification of miR156 as a regulator of vegetative phase change in Arabidopsis and maize [10], [13], [16], and the results presented here, resolve this long-standing problem. The expression patterns of miR156 and miR172 in woody plants with well-differentiated juvenile and adult phases, and the evidence that over-expressing miR156 delays vegetative phase change in P. x canadensis, strongly suggest that miR156 regulates vegetative phase change in many, if not all, flowering plants. miR156 is present in all major plant taxa, including bryophytes [40], so it would not be surprising if it regulates vegetative phase change throughout the plant kingdom. This result has many important implications. Most importantly, it demonstrates the fundamental similarity between processes that overtly appear to be quite different: it is remarkable that the subtle changes in leaf morphology described as phase change in maize [22] and Arabidopsis [8] correspond to the much more dramatic changes in shoot architecture observed in Acacia, Eucalyptus, or Hedera. There was no a priori evidence that these events actually represent the same developmental process. Our results therefore validate the use of Arabidopsis and maize for the analysis of vegetative phase change, and suggest that the insights gained from these experimentally tractable species are likely to have broad applicability.
‘The evidence that vegetative phase change is mediated by a decrease in the expression of miR156 begs the question of how this decrease is regulated. miR156 plays a critical role in vegetative phase change, but control of this process resides with the factor or factors that control the expression of this miRNA. A recent study of vegetative phase change in Arabidopsis, maize and Nicotiana benthamiana indicates that the decline in miR156 is mediated by a signal produced by leaf primordia; neither the root system nor cotyledons appear to be important for this event [41]. This result suggests that the timing of vegetative phase change could be regulated by leaf number: assuming that all leaves are capable of producing a hypothetical phase change signal, then the switch from juvenile to adult development might occur when leaf number exceeds a certain threshold number. However, this simple model does not account for the tremendous variability in the timing of vegetative phase change in trees. For example, phase change occurs after 1 node in A. confusa (Figure 1A), but 30 or more nodes in A. koa [42]. Similarly, in E. globulus, vegetative phase change occurs between 1 and 5 years after germination [43], [44]. This variability suggests that the juvenile-to-adult transition is only weakly related (if at all) to the overall size of the shoot. Identifying the factors that regulate the expression of miR156 is an important goal for future research.
The results presented here also have important practical implications. Many traits change during shoot development in trees, and the extent to which various traits are controlled by the same or different mechanisms is largely unknown [45], [46], [47]. Correlating changes in the expression of miR156 and miR172 with changes in various heteroblastic traits should make it possible to distinguish traits that are potentially regulated by these miRNAs from traits that are controlled by some other mechanism. It will be particularly interesting to learn if age-related changes in economically important traits—such as adventitious root production—are correlated with changes in miR156 expression, as this may open new avenues for the manipulation of these traits. Using miR156 expression as a marker for vegetative identity also makes possible to study the effects of various factors on phase change in situations in which this is otherwise difficult to do—for example, in species that do not undergo major morphological changes during vegetative development, or in short-term experimental situations that do not permit the development of fully formed leaves or shoots. This will facilitate the integration of information about vegetative phase change across species, and should help to accelerate research on this important but poorly understood developmental process.
Materials and Methods
Plant material and phenotypic analysis
Seeds of A. colei were obtained from the Australian Tree Seed Center (Canberra, Australia), while seeds A. confusa were obtained from the Desert Legume Program of the U. of Arizona (Tucson, AZ). These species were grown in Farfard #52 soil in the U. of Pennsylvania greenhouse, with supplemental illumination to extend the day length to 16 hours. Fully expanded juvenile and adult leaves were harvested from these plants when they were 2 months old. Juvenile and adult shoots of H. helix were harvested from single vines, or clones propagated from single vines. Analyses were conducted with plants growing outdoors in Media, Pennsylvania and the U. of Pennsylvania's Kasky garden, and with shoot apices of juvenile and adult clones grown in a growth chamber in short days (10 hrs light∶ 14 hours dark; 26°C∶21°C day∶night temperature) to prevent flowering (31). Fully expanded juvenile and adult leaves of Q. acutissima were harvested from trees growing on the campus of the U. of Pennsylvania. Juvenile and adult branches of these trees were identified during winter on the basis on the presence (juvenile) or absence (adult) of attached leaves, and newly expanded leaves from these branches were harvested in May, 2010. Juvenile and adult leaves of E. globulus were harvested in October, 2010 from trees of different ages growing at three sites within the Presidio Trust in San Francisco, California.
Leaves of 1-year-, 4-year - and 10-year-old P. x canadensis clones growing within 100 meters of each other at a field site in Shanghai were sampled in June, 2010. Leaves of 1-month - and 6-month-old wild type and transgenic P. x canadensis clones were sampled in the greenhouse in Tübingen. The leaves or leaf primordia from lateral branches were harvested from 1-year-old clones grown in the greenhouse. Fully expanded leaves were detached, measured, and photographed. For leaf anatomy, leaves 1.5 cm in length and petioles were fixed, embedded and sectioned as previously described [39]. The rate of leaf initiation was determined from the number of the leaves produced within one week.
Expression analyses
Leaves or shoot apices from A. confusa. A. colei, H. helix, E. globulus, and Q. acutissima, were frozen in liquid nitrogen, and total RNA was extracted following a protocol modified from [48]. Small RNA was isolated and analyzed using the methods described in [49]. In brief, 1–2 grams of tissue was ground to make fine powder, pre-warmed (at 65°C) RNA extraction buffer (2% CTAB, 2% PVP40, 100 mM Tris-HCl, 25 mM EDTA, 2 M NaCl, 0.5 g/L spermidine, 2% β-mercaptoethanol, pH 8.0) was added, and the mixture was incubated for 20 min at 65°C. RNA was extracted by treating the slurry twice with an equal volume of chloroform/isoamyl alcohol (24∶1), and then precipitated with LiCl at a final concentration of 2.5 M. The pellet was dissolved in STE buffer (1 M NaCl, 10 mM Tris-HCl, 1 mM EDTA, pH 8.0) and extracted one more time with chloroform/isoamyl alcohol. RNA was precipitated with ethanol and used for RNA gel blots, as described in [49]. Densitometry of digitized images of these blots was performed using Image J (http://rsbweb.nih.gov/ij/). E. grandis homologs of AtSPL3 and AtSPL9 were identified by performing tblastn searches of the E. grandis genome (http://www.phytozome.net/eucalyptus.php). PCR primers based on the E. grandis sequence (Table S2) were used to amplify the corresponding genes from cDNA of fully expanded adult leaves of E. globulus, and the resulting PCR products—EglSPL3 (HQ450389), EglSPL9 (HQ450390), and EglEIF4 (HQ450391)—were sequenced. qRT-PCR was performed on RNA isolated by the method described above. Reverse transcription was performed with SuperScript™II reverse transcriptase (Invitrogen) using an oligo(dT) primer. qRT-PCR reactions were performed using the EglSPL3, EglSPL9 and EglEIF4 primers listed in Table S2 and the Power SYBR Green Master Mix (Applied Biosystems). Reactions were monitored and analyzed using StepOneTM Software v2.0.1 (Applied Biosystems), and were normalized to the quantity of EgEIF4. Three technical replicates were performed for samples harvested from three trees, yielding a total of 9 reactions per leaf type.
In the case of P. x canadensis, total RNA was extracted from leaves with Trizol reagent (Invitrogen GmbH, Germany). One µg of total RNA was DNase I-treated and used for cDNA synthesis with oligo(dT) primer and Superscript reverse transcriptase (Invitrogen). qRT-PCR was performed with SYBR-Green PCR Mastermix (Invitrogen) and amplification was real-time monitored on an MJR Opticon Continuous Fluorescence Detection System (Biorad, Hercules, CA) and analyzed using the software provided by the manufacturer. Two biological replicates (each with three technical replicates) were performed. The oligos for PcSPL3, PcSPL9, PcFUL, and PcACT were designed based on the homologous genes of P. trichocarpa [50]: PtSPL3 (XM_002329758), PtSPL9 (XM_002322642.1), PtFUL (XM_002317909.1), and PtACT (XM_002298674) (Table S2).
Transgenic plants
P. x canadensis cv. Guangzhao Yang plants, were grown at 23°C in 16 hours long days. The 35S::MIR156 [11] and p35S::GUS constructs were introduced into Agrobacterium tumefaciens (strain GV3101 [pMP90]) and used for plant transformation. An overnight A. tumefaciens culture was pelleted and resuspended in infection medium (1/2 MS, 45 g/L sucrose, 200 µM acetosyringone). Leaves were infected for 30 min and then transferred to co-culture medium (MS, 0.25 mg/L 6-benzyl aminopurine, 0.25 mg/L kinetin, 0.25 mg/L trans-zeatin, 0.25 mg/L naphthalene acetic acid, 100 µM acetosyringone). After incubation at 24°C for 3 days, leaves were transferred to selective differentiation medium (MS, 0.25 mg/L 6-benzyl aminopurine, 0.25 mg/L kinetin, 0.25 mg/L trans-zeatin, 0.25 mg/L naphthalene acetic acid, 500 mg/L carbenicillin, 50 mg/L kanamycin). Three weeks later, the explants were transferred to selective elongation medium (MS, 0.1 mg/L 6-benzyl aminopurine, 300 mg/L carbenicillin, 100 mg/L kanamycin). This was repeated once. Kanamycin-resistant shoots were transferred into induction medium (MS, 0.2 mg/L indole-3 - butyric acid, 200 mg/L carbenicillin, 50 mg/L kanamycin) for root induction. Wild-type plants were regenerated on plates without kanamycin selection.
Supporting Information
Zdroje
1. BrinkRA 1962 Phase change in higher plants and somatic cell heredity. Quart Rev Biol 37 1 22
2. DoorenbosJ 1965 Juvenile and adult phases in woody plants. Encyl Plant Physiol 15 1222 1235
3. PoethigRS 1990 Phase change and the regulation of shoot morphogenesis in plants. Science 250 923 930
4. PoethigRS 2003 Phase change and the regulation of developmental timing in plants. Science 301 334 336
5. BäurleIDeanC 2006 The timing of developmental transitions in plants. Cell 125 655 664
6. ChienJCSussexIM 1996 Differential regulation of trichome formation on the adaxial and abaxial leaf surfaces by gibberellins and photoperiod in Arabidopsis thaliana (L) Heynh. Plant Physiol 111 1321 1328
7. RöbbelenG 1957 Uber Heterophyllie bei Arabidopsis thaliana (L.) Heynh. Ber Dtsch Bot Ges 70 39 44
8. TelferABollmanKMPoethigRS 1997 Phase change and the regulation of trichome distribution in Arabidopsis thaliana. Development 124 645 654
9. WangJWCzechBWeigelD 2009 miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 138 738 749
10. WuGPoethigRS 2006 Temporal regulation of shoot development in Arabidopsis thaliana by miR156 and its target SPL3. Development 133 3539 3547
11. SchwabRPalatnikJFRiesterMSchommerCSchmidM 2005 Specific effects of microRNAs on the plant transcriptome. Dev Cell 8 517 527
12. GandikotaMBirkenbihlRPHohmannSCardonGHSaedlerH 2007 The miRNA156/157 recognition element in the 3′ UTR of the Arabidopsis SBP box gene SPL3 prevents early flowering by translational inhibition in seedlings. Plant J 49 683 693
13. WuGParkMYConwaySRWangJWWeigelD 2009 The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 138 750 759
14. YamaguchiAWuMFYangLWuGPoethigRS 2009 The microRNA-regulated SBP-Box transcription factor SPL3 is a direct upstream activator of LEAFY, FRUITFULL, and APETALA1. Dev Cell 17 268 278
15. LauterNKampaniACarlsonSGoebelMMooseSP 2005 microRNA172 down-regulates Glossy15 to promote vegetative phase change in maize. Proc Natl Acad Sci USA 102 9412 9417
16. ChuckGCiganAMSaeteurnKHakeS 2007 The heterochronic maize mutant Corngrass1 results from overexpression of a tandem microRNA. Nat Genet 39 544 549
17. SchmidMUhlenhautNHGodardFDemarMBressanR 2003 Dissection of floral induction pathways using global expression analysis. Development 130 6001 6012
18. AukermanMJSakaiH 2003 Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 15 2730 2741
19. ChenX 2004 A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 303 2022 2025
20. MathieuJYantLJMürdterFKüttnerFSchmidM 2009 Repression of flowering by the miR172 target SMZ. PLoS Biol 7 e1000148 doi:10.1371/journal.pbio.1000148
21. EvansMMPassasHJPoethigRS 1994 Heterochronic effects of glossy15 mutations on epidermal cell identity in maize. Development 120 1971 1981
22. PoethigRS 1988 Heterochronic mutations affecting shoot development in maize. Genetics 119 959 973
23. MooseSPSiscoPH 1994 Glossy15 controls the epidermal juvenile-to-adult phase transition in maize. Plant Cell 6 1343 1355
24. XieKWuCXiongL 2006 Genomic organization, differential expression, and interaction of SQUAMOSA promoter-binding-like transcription factors and microRNA156 in rice. Plant Physiol 142 280 293
25. SalviSSponzaGMorganteMTomesDNiuX 2007 Conserved noncoding genomic sequences associated with a flowering-time quantitative trait locus in maize. Proc Natl Acad Sci USA 104 11376 11381
26. LawsonEJPoethigRS 1995 Shoot development in plants: time for a change. Trends Genet 11 263 268
27. HildebrandF 1875 Ueber die Jungendzustände solcher Pflanzen, welche im Alter vom vegetativen Charakter ihrer Verwandten abweichen. Flora 21 321 330
28. GoebelK 1889 Ueber die Jungendzustände der Pflanzen. Flora 72 1 45
29. KaplanDR 1980 Heteroblastic leaf development in Acacia. Morphological and morphogenetic implications. Cellule 73 137 203
30. JamesSABellDT 2001 Leaf morphological and anatomical characteristics of heteroblastic Eucalyptus globulus ssp globulus (Myrtaceae). Aust J Bot 49 259 269
31. WareingPFFrydmanVM 1976 General aspects of phase change, with special reference to Hedera helix L. Acta Hort 56 57 69
32. SteinOLFosketEB 1969 Comparative developmental anatomy of shoots of juvenile and adult Hedera helix. Am J Bot 56 546 551
33. ReinhartBJWeinsteinEGRhoadesMWBartelBBartelDP 2002 MicroRNAs in plants. Genes Dev 16 1616 1626
34. LuCJeongDHKulkarniKPillayMNobutaK 2008 Genome-wide analysis for discovery of rice microRNAs reveals natural antisense microRNAs (nat-miRNAs). Proc Natl Acad Sci U S A 105 4951 4956
35. RajagopalanRVaucheretHTrejoJBartelDP 2006 A diverse and evolutionarily fluid set of microRNAs in Arabidopsis thaliana. Genes Dev 20 3407 3425
36. BarakatAWallPKDiloretoSDepamphilisCWCarlsonJE 2007 Conservation and divergence of microRNAs in Populus. BMC Genomics 8 481
37. GreenwoodMS 1987 Rejuvenation of forest trees. Plant Growth Regul 6 1 12
38. CritchfieldW 1960 Leaf dimorphism in Populus trichocarpa. Am J Bot 47 699 711
39. WangJWSchwabRCzechBMicaEWeigelD 2008 Dual effects of miR156-targeted SPL Genes and CYP78A5/KLUH on plastochron length and organ size in Arabidopsis thaliana. Plant Cell 20 1231 1243
40. AxtellMJBowmanJL 2008 Evolution of plant microRNAs and their targets. Trends Plant Sci 13 343 349
41. YangLConwaySRPoethigRS 2011 Vegetative phase change is mediated by a leaf-derived signal that represses the transcription of miR156. Development 138 245 249
42. DaehlerCCYorkstonMSunWGDudleyN 1999 Genetic variation in morphology and growth characters of Acacia koa in the Hawaiian Islands. Intl J Plant Sci 160 767 773
43. JordanGJPottsBMChalmersPWiltshireRJE 2000 Quantitative genetic evidence that the timing of vegetative phase change in Eucalyptus globulus ssp globulus is an adaptive trait. Aust J Bot 48 561 567
44. JordanGJPottsBMWiltshireRJE 1999 Strong, independent, quantitative genetic control of the timing of vegetative phase change and first flowering in Eucalyptus globulus ssp globulus (Tasmanian Blue Gum). Heredity 83 179 187
45. GreenwoodMS 1995 Juvenility and maturation in conifers - current concepts. Tree Physiol 15 433 438
46. BondBJ 2000 Age-related changes in photosynthesis of woody plants. Trends Plant Sci 5 349 353
47. DayMEGreenwoodMSDiaz-SalaC 2002 Age - and size-related trends in woody plant shoot development: regulatory pathways and evidence for genetic control. Tree Physiol 22 507 513
48. ChangSPuryearJCairneyJ 1993 A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 11 113 116
49. ParkMYWuGGonzalez-SulserAVaucheretHPoethigRS 2005 Nuclear processing and export of microRNAs in Arabidopsis. Proc Natl Acad Sci USA 102 3691 3696
50. TuskanGADiFazioSJanssonSBohlmannJGrigorievI 2006 The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 313 1596 1604
Štítky
Genetika Reprodukční medicína
Článek Break to Make a ConnectionČlánek A New Testing Strategy to Identify Rare Variants with Either Risk or Protective Effect on DiseaseČlánek The Architecture of Gene Regulatory Variation across Multiple Human Tissues: The MuTHER Study
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 2
-
Všechny články tohoto čísla
- Break to Make a Connection
- A New Testing Strategy to Identify Rare Variants with Either Risk or Protective Effect on Disease
- Single-Tissue and Cross-Tissue Heritability of Gene Expression Via Identity-by-Descent in Related or Unrelated Individuals
- Pervasive Adaptive Protein Evolution Apparent in Diversity Patterns around Amino Acid Substitutions in
- The Architecture of Gene Regulatory Variation across Multiple Human Tissues: The MuTHER Study
- MiRNA Control of Vegetative Phase Change in Trees
- New Functions of Ctf18-RFC in Preserving Genome Stability outside Its Role in Sister Chromatid Cohesion
- Genome-Wide Association Studies of the PR Interval in African Americans
- Mapping of the Disease Locus and Identification of As a Candidate Gene in a Canine Model of Primary Open Angle Glaucoma
- Mapping a New Spontaneous Preterm Birth Susceptibility Gene, , Using Linkage, Haplotype Sharing, and Association Analysis
- A Population Genetic Approach to Mapping Neurological Disorder Genes Using Deep Resequencing
- and Genes Modulate the Switch between Attraction and Repulsion during Behavioral Phase Change in the Migratory Locust
- Targeted Sister Chromatid Cohesion by Sir2
- Correlated Evolution of Nearby Residues in Drosophilid Proteins
- Parallel Evolution of a Type IV Secretion System in Radiating Lineages of the Host-Restricted Bacterial Pathogen
- Lipophorin Receptors Mediate the Uptake of Neutral Lipids in Oocytes and Imaginal Disc Cells by an Endocytosis-Independent Mechanism
- Genome-Wide Association Study of Coronary Heart Disease and Its Risk Factors in 8,090 African Americans: The NHLBI CARe Project
- The Evolution of Host Specialization in the Vertebrate Gut Symbiont
- Genome-Wide Association of Familial Late-Onset Alzheimer's Disease Replicates and and Nominates in Interaction with
- Risk Alleles for Systemic Lupus Erythematosus in a Large Case-Control Collection and Associations with Clinical Subphenotypes
- Association between Common Variation at the Locus and Changes in Body Mass Index from Infancy to Late Childhood: The Complex Nature of Genetic Association through Growth and Development
- AID Induces Double-Strand Breaks at Immunoglobulin Switch Regions and Causing Chromosomal Translocations in Yeast THO Mutants
- A Study of CNVs As Trait-Associated Polymorphisms and As Expression Quantitative Trait Loci
- Whole-Genome Comparison Reveals Novel Genetic Elements That Characterize the Genome of Industrial Strains of
- Prevalence of Epistasis in the Evolution of Influenza A Surface Proteins
- Srf1 Is a Novel Regulator of Phospholipase D Activity and Is Essential to Buffer the Toxic Effects of C16:0 Platelet Activating Factor
- Two Frizzled Planar Cell Polarity Signals in the Wing Are Differentially Organized by the Fat/Dachsous Pathway
- Phosphoinositide Regulation of Integrin Trafficking Required for Muscle Attachment and Maintenance
- Pathogenic VCP/TER94 Alleles Are Dominant Actives and Contribute to Neurodegeneration by Altering Cellular ATP Level in a IBMPFD Model
- Meta-Analysis of Genome-Wide Association Studies in Celiac Disease and Rheumatoid Arthritis Identifies Fourteen Non-HLA Shared Loci
- A Genome-Wide Study of DNA Methylation Patterns and Gene Expression Levels in Multiple Human and Chimpanzee Tissues
- Nucleosomes Containing Methylated DNA Stabilize DNA Methyltransferases 3A/3B and Ensure Faithful Epigenetic Inheritance
- Mutations in Zebrafish Result in Adult-Onset Ocular Pathogenesis That Models Myopia and Other Risk Factors for Glaucoma
- [], the Prion Formed by the Chromatin Remodeling Factor Swi1, Is Highly Sensitive to Alterations in Hsp70 Chaperone System Activity
- Characterization of Transcriptome Remodeling during Cambium Formation Identifies and As Opposing Regulators of Secondary Growth
- The Cardiac Transcription Network Modulated by Gata4, Mef2a, Nkx2.5, Srf, Histone Modifications, and MicroRNAs
- Epistatic Interaction Maps Relative to Multiple Metabolic Phenotypes
- Quantitative Models of the Mechanisms That Control Genome-Wide Patterns of Transcription Factor Binding during Early Development
- Genome-Wide Transcript Profiling of Endosperm without Paternal Contribution Identifies Parent-of-Origin–Dependent Regulation of
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Meta-Analysis of Genome-Wide Association Studies in Celiac Disease and Rheumatoid Arthritis Identifies Fourteen Non-HLA Shared Loci
- MiRNA Control of Vegetative Phase Change in Trees
- Risk Alleles for Systemic Lupus Erythematosus in a Large Case-Control Collection and Associations with Clinical Subphenotypes
- The Cardiac Transcription Network Modulated by Gata4, Mef2a, Nkx2.5, Srf, Histone Modifications, and MicroRNAs
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



