-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaFungi That Infect Insects: Altering Host Behavior and Beyond
article has not abstract
Published in the journal: . PLoS Pathog 11(8): e32767. doi:10.1371/journal.ppat.1005037
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1005037Summary
article has not abstract
Introduction
Approximately 1,000 species of the fungal phyla Microsporidia, Chytridiomycota, Entomophthoromycota (order: Entomophthorales), Basidiomycota, and Ascomycota are known to infect and kill insects [1]. Of these, species such as Beauveria bassiana and Metarhizium robertsii (Ascomycota: Hypocreales) are well-studied models for exploring the mechanisms of fungus–insect physiological interactions, and they are used as biological controls for insect pests [2]. Entomopathogenic Hypocreales are phylogenetically closely related to plant pathogens and endophytes [3], and their sexual stages belong to Cordyceps sensu lato [4]. However, these species are taxonomically diverse and differ from each other considerably in their genomic features [5–8] and, consequently, their host ranges, infection cycles, and life strategies. Fungal infection normally, but not always, results in the death of an insect in situ. The parasitic fungi such as the host-specific pathogen Ophiocordyceps unilateralis sensu lato can control insect brains and manipulate their behavior to reach death locations that are optimal for spore dispersal, the so-called fungal extended phenotype [9]. On the other hand, on top of physiological immune surveillance, insects (especially social insects such as ants and termites) can smell and avoid fungal pathogens, groom each other to clear pathogenic spores, generate a fever response, or die well away from their nestmates, which is termed behavioral or social immunity [10,11]. Either side of these interactions will benefit from behavioral changes in insects that maximize their adaptive fitness. In this paper, both types of insect behavior alternations are reviewed, and the underlying mechanisms are discussed.
The Fungal Infection Cycle and Host Specificity
Entomopathogenic fungi recognize and infect insects through the spore adhesion and formation of appressoria that penetrate the cuticle (Fig 1A). After reaching the hemocoel (body cavity) of an insect, fungal filaments will switch into yeast-like cells that undergo budding for rapid propagation and counteract the immune response of the hosts (Fig 1B). For the infection cycle to complete, dead insects must be either mycosed to produce asexual conidial spores (Fig 1C) or colonized to form a fruiting body (Fig 1D and 1E) to yield sexual spores for the next infection. Alternatively, insect pathogens such as M. robertsii with a broad host range can form a root–rhizosphere relationship to transfer nitrogen from dead insects to a plant and acquire carbon in return [12]; this is a strategy for long-term persistence in the soil when potential hosts are absent.
Fig. 1. Micro- and macrophenotypes related to fungal infection and colonization of insect hosts. 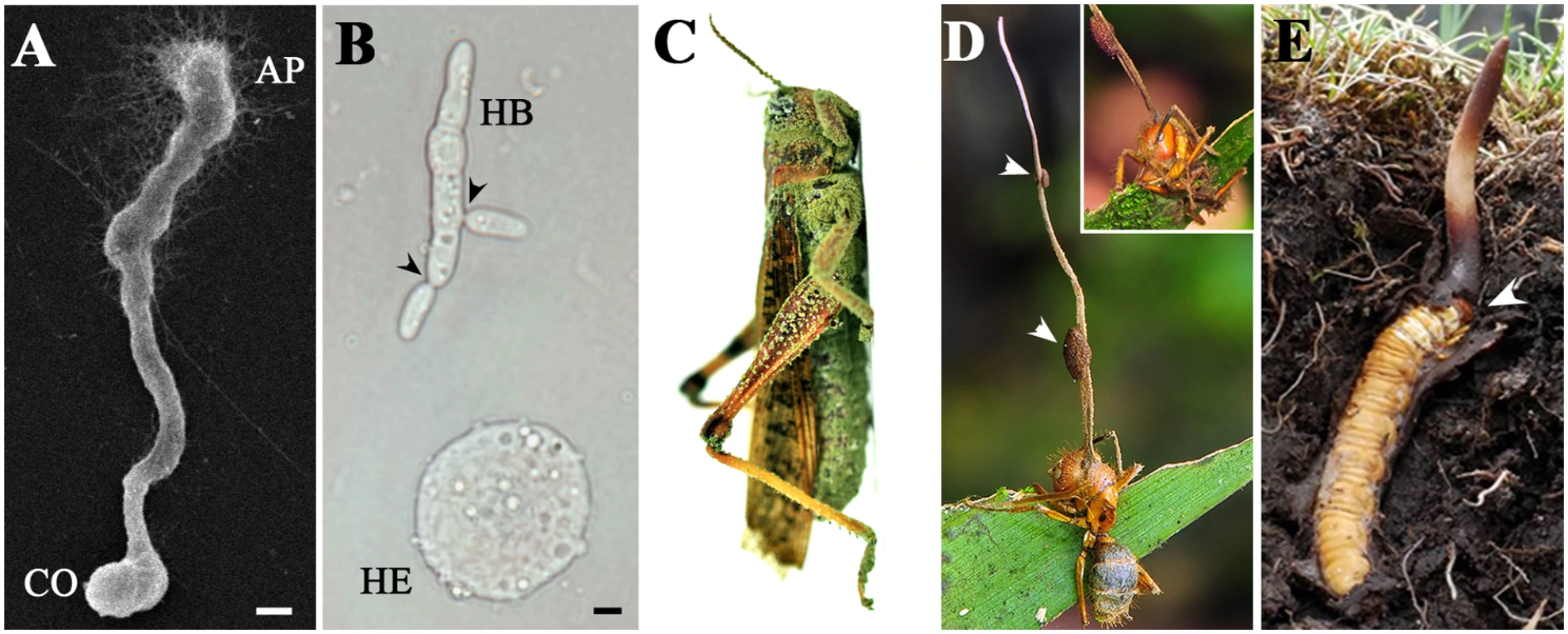
(A) Appressorium (AP) formation concurrent with mucilage production by M. robertsii on an insect cuticle 18 h after inoculation; CO: conidium; bar: 5 μm. (B) Formation of an M. robertsii hyphal body (HB) in a locust hemocoel 36 h after infection. Arrows point to the contraction rings formed for yeast-like budding; HE: hemocyte; bar: 5 μm. (C) Locust cadaver killed and mycosed by the asexual spores of the specific pathogen M. acridum. (D) Zombie ant. This carpenter ant (Camponotus sp.) was killed and colonized by O. unilateralis to form sexual fruiting body (stroma), which erupted from the insect’s head. The insert shows the front of the ant, which was holding tightly to the leaf before its death (courtesy of and copyright by Daniel Winker). Arrows indicate perithecial plates. (E) Caterpillar fungus. A ghost moth (Hepialus sp.) larva infected by O. sinensis remained close to the soil surface with its head up in death, and the stroma erupted from the insect’s head (arrow) (courtesy of and copyright by Daniel Winker). Different species of parasitic fungi have different insect host ranges. Generalist species, such as B. bassiana and M. robertsii, can infect hundreds of insect species of different orders, whereas species such as M. acridum (specific to locusts and grasshoppers) [3,5], Cordyceps militaris (specific to caterpillars) [6], and O. unilateralis sensu lato (specific to formicine ants), only infect a narrow range of insects [13,14]. In particular, O. unilateralis is a species complex that includes different species being highly host specific, which is close to a level of one fungus versus one ant species [13–15]. In terms of fungal host-specificity evolution, the study of Metarhizium species with different host ranges has shown a directional trajectory of speciation from being specialists to becoming generalists, and the process has been coupled with protein family expansions [3]. In particular, the number of divergent G-protein coupled receptors is significantly correlated with host specificity [3,5].
Control of Insect Behavior by Parasitic Fungi
Parasites often manipulate the behavior of their hosts; for example, crickets or grasshoppers commit suicide by drowning when infected by the parasitic hairworm Spinochordodes tellinii [16]. A striking death grip behavior has also been observed in carpenter ants (Camponotus spp.) following infection by the parasitic fungus O. unilateralis [9]. The infected, moribund ants essentially behave like zombies; they walk alone and erratically climb to a certain height in the vegetation (approximately 25 cm above the soil surface). They bite leaf margins in rainforests and twigs in temperate woods and transition from wandering to biting takes place synchronously around noontime (within 11 : 00–14 : 00 h) possibly in association with a solar cue [13,17]. The fruiting body then erupts from their heads (Fig 1D). Likewise, the caterpillar fungus O. sinensis specifically infects the larvae of soil-dwelling ghost moths (Hepialus spp.) and maintains a symbiotic relationship for up to five years [8]. Before mummifying its host, the fungus drives a larva close to the surface of the soil (approximately 1–3 cm), and the fruiting body then grows from the caterpillar’s head (Fig 1E). Infections by obligate Entomophthorales pathogens could also lead to similar “summit” diseases, i.e., the infected insects climbing to an elevated position before death [18]. Such pathogen-controlled behavioral changes benefit the fungi by maximizing spore transmission efficiency to begin the next infection cycle.
Inducement of Insect Behavioral Immunity
In contrast to being passively manipulated, insects can actively avoid and combat parasitic fungal infections through a behavioral or social immune response [19]. This especially occurs in social insects such as honeybees, ants, and termites, which can more easily compromise their relatively fewer antimicrobial peptide genes than nonsocial insects [20,21]. The hygienic behaviors of honeybees, ants, and termites, such as mutual grooming, can be triggered by the odor of Metarhizium fungal spores and provide behavioral resistance to the infection [22,23]. Other studies have indicated that ant allogrooming is coupled with chemical disinfection through the emission of formic acid, which reduces the viability of Metarhizium spores [24]. In termites, a salivary pleiotropic protein containing both a pattern recognition receptor and a β(1,3)-glucanase antimicrobial effector domain is used as a nest-building material to protect colonies against Metarhizium or bacterial infection [25]. Behavioral prophylaxis can also occur through social withdrawal and death in isolation. For example, garden ants (Lasius neglectus) infected by M. anisopliae stay away from the brood chamber, and moribund ants cease social contact with their nestmates and leave their nests hours or days before death [23]. So, in contrast with the zombie ants manipulated by O. unilateralis (Fig 1D), dying away from the colony is an active and altruistic response of fungus-infected ants. Density-dependent prophylaxis also occurs in locusts, but desert locusts (Schistocerca gregaria) actively raise their body temperature to inhibit M. acridum infection [26]. In addition, elevated physiological immune-surveillance has been observed in gregarious locusts, which enabled the locusts to collectively become more resistant to Metarhizium infection than solitary individuals [27].
Remaining Secrets behind the Fungal Manipulation of Insect Behavior
As indicated above, behavioral alterations during fungus–insect interactions are diverse, but generally, host-specific and obligate pathogens manipulate insect behavior while the spores of generalist species can trigger active host behavioral immunity. Particular efforts including comparative proteomics, transcriptomics, and metabolomics have been undertaken to tentatively unravel the molecular mechanisms underlying fungal hijacking diseases [13,28]. However, unlike the single gene of a baculovirus (egt [ecdysteroid UDP-glucosyltransferase]) that can inactivate the gypsy moth molting hormone and thereby promote the summit disease [29] and a protein tyrosine phosphatase (ptp) of baculovirus that functions as a virion structural protein to facilitate virus infection of insect brain tissues to induce enhanced locomotory activity (ELA) [30], the key factor(s) involved in the fungal control of insect behavior is still unknown. Our genome survey indicated that a viral egt-like gene is not present, but a single copy of PTP domain-containing protein-encoding gene exists in the genomes of insect-parasitizing fungi, e.g., MAA_02506 of M. robertsii and BBA_03722 of B. bassiana. It remains to be determined whether the interference with hormone turnover or virus-like PTP-mediated ELA effect occurs during fungal infections. In addition, the genomes of parasitic fungi encode an array of lineage-specific, effector-like polypeptides as well as the gene clusters involved in the biosynthesis of small metabolites [3]. It has been found that the insecticidal cyclopeptide destruxins produced by Metarhizium could be used by the fungus to evade insect immunity, and its ability to produce a toxin is connected with host specificity [31,32]. The findings suggest that fungi could deploy small chemicals to alter insect physiology.
As in mammals, the dopamine (DA; a catecholamine neurotransmitter)-signaling pathway plays a conserved role in the modulation of invertebrate behavior [33]. Coupled with cuticle melanization, the up-regulation of DA-biosynthetic genes in the head of a locust coincides with the behavior change from solitary to gregarious [34]. DA has also been shown to contribute to behavior change and social interactions in ants [35]. An injection of biogenic amines into red wood ants (Formica polyctena) demonstrated that serotonin could stimulate aggressive behavior, while DA administration triggered mandible-opening and biting behaviors directed at foreign insects [36]. As indicated above, the mandibles of zombie ants usually penetrate deeply into plant tissues [9], so it is likely, although unconfirmed, that fungal infection could alter the accumulation of DA and other chemicals in insects, thereby causing precise behavioral manipulation.
Future Directions
Alterations of host behavior during fungus–insect interactions are diverse, intricate, and of great scientific interest. Passive or active behavioral changes in insects are reminiscent of evolutionary adaptations that either promote cross-kingdom control by fungi or altruistic behavior by the hosts. There are still significant gaps in our understanding of the molecular mechanisms underlying behavioral alterations in insects during their interactions with fungi. Due to the taxonomic diversity of both insects and fungi, the molecular machinery involved in insect behavior changes could vary among the interacting species pairs or function on a case-by-case basis. The establishment of an ideal model system (e.g., the zombie ant), the acquisition of genomic information, and the deployment of the knowledge and techniques of the sciences of fungal genetics, secondary metabolism, chemistry, and insect physiology and neurology would help uncover the biological secrets behind changes in insect behavior.
Zdroje
1. Vega FE, Meyling NV, Luangsa-Ard JJ, Blackwell M (2012) Fungal entomopathogens. In: Vega F, Kaya HK, editors. Insect Pathology, 2nd ed. San Diego: Academic Press; pp. 171–220.
2. de Faria MR, Wraight SP (2007) Mycoinsecticides and Mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biol Control 43 : 237–256.
3. Hu X, Xiao G, Zheng P, Shang Y, Su Y, et al. (2014) Trajectory and genomic determinants of fungal-pathogen speciation and host adaptation. Proc Natl Acad Sci U S A 111 : 16796–16801. doi: 10.1073/pnas.1412662111 25368161
4. Sung GH, Hywel-Jones NL, Sung JM, Luangsa-Ard JJ, Shrestha B, et al. (2007) Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud Mycol 57 : 5–59. doi: 10.3114/sim.2007.57.01 18490993
5. Gao Q, Jin K, Ying SH, Zhang Y, Xiao G, et al. (2011) Genome sequencing and comparative transcriptomics of the model entomopathogenic fungi Metarhizium anisopliae and M. acridum. PLoS Genet 7: e1001264. doi: 10.1371/journal.pgen.1001264 21253567
6. Zheng P, Xia Y, Xiao G, Xiong C, Hu X, et al. (2011) Genome sequence of the insect pathogenic fungus Cordyceps militaris, a valued traditional Chinese medicine. Genome Biol 12: R116. doi: 10.1186/gb-2011-12-11-r116 22112802
7. Bushley KE, Raja R, Jaiswal P, Cumbie JS, Nonogaki M, et al. (2013) The genome of Tolypocladium inflatum: evolution, organization, and expression of the cyclosporin biosynthetic gene cluster. PLoS Genet 9: e1003496. doi: 10.1371/journal.pgen.1003496 23818858
8. Hu X, Zhang YJ, Xiao G, Zheng P, Xia Y, et al. (2013) Genome survey uncovers the secrets of sex and lifestyle in caterpillar fungus. Chinese Sci Bull 58 : 2846–2854.
9. Andersen SB, Gerritsma S, Yusah KM, Mayntz D, Hywel-Jones NL, et al. (2009) The life of a dead ant: the expression of an adaptive extended phenotype. Am Nat 174 : 424–433. doi: 10.1086/603640 19627240
10. Heinze J, Walter B (2010) Moribund ants leave their nests to die in social isolation. Curr Biol 20 : 249–252. doi: 10.1016/j.cub.2009.12.031 20116243
11. Yanagawa A, Fujiwara-Tsujii N, Akino T, Yoshimura T, Yanagawa T, et al. (2011) Musty odor of entomopathogens enhances disease-prevention behaviors in the termite Coptotermes formosanus. J Invertebr Pathol 108 : 1–6. doi: 10.1016/j.jip.2011.06.001 21683707
12. Behie SW, Zelisko PM, Bidochka MJ (2012) Endophytic insect-parasitic fungi translocate nitrogen directly from insects to plants. Science 336 : 1576–1577. doi: 10.1126/science.1222289 22723421
13. de Bekker C, Quevillon LE, Smith PB, Fleming KR, Ghosh D, et al. (2014) Species-specific ant brain manipulation by a specialized fungal parasite. BMC Evol Biol 14 : 166. doi: 10.1186/s12862-014-0166-3 25085339
14. Evans HC, Elliot SL, Hughes DP. (2011) Hidden diversity behind the zombie-ant fungus Ophiocordyceps unilateralis: four new species described from carpenter ants in Minas Gerais, Brazil. PLoS One 6: e17024. doi: 10.1371/journal.pone.0017024 21399679
15. Kobmoo N, Mongkolsamrit S, Wutikhun T, Tasanathai K, Khonsanit A, et al. (2015) New species of Ophiocordyceps unilateralis, an ubiquitous pathogen of ants from Thailand. Fungal Biol 119 : 44–52. doi: 10.1016/j.funbio.2014.10.008 25601148
16. Libersat F, Delago A, Gal R (2009) Manipulation of host behavior by parasitic insects and insect parasites. Annu Rev Entomol 54 : 189–207. doi: 10.1146/annurev.ento.54.110807.090556 19067631
17. Hughes DP, Andersen SB, Hywel-Jones NL, Himaman W, Billen J, et al. (2011) Behavioral mechanisms and morphological symptoms of zombie ants dying from fungal infection. BMC Ecol 11 : 13. doi: 10.1186/1472-6785-11-13 21554670
18. Roy HE, Steinkraus DC, Eilenberg J, Hajek AE, Pell JK (2006) Bizarre interactions and endgames: entomopathogenic fungi and their arthropod hosts. Annu Rev Entomol 51 : 331–357. 16332215
19. Cremer S, Armitage SA, Schmid-Hempel P (2007) Social immunity. Curr Biol 17: R693–R702. 17714663
20. Wilson-Rich N, Spivak M, Fefferman NH, Starks PT (2009) Genetic, individual, and group facilitation of disease resistance in insect societies. Annu Rev Entomol 54 : 405–423. doi: 10.1146/annurev.ento.53.103106.093301 18793100
21. Simola DF, Wissler L, Donahue G, Waterhouse RM, Helmkampf M, et al. (2013) Social insect genomes exhibit dramatic evolution in gene composition and regulation while preserving regulatory features linked to sociality. Genome Res 23 : 1235–1247. doi: 10.1101/gr.155408.113 23636946
22. Yanagawa A, Shimizu S (2007) Resistance of the termite, Coptotermes formosanus Shiraki to Metarhizium anisopliae due to grooming. BioControl 52 : 75–85.
23. Ugelvig LV, Cremer S (2007) Social prophylaxis: group interaction promotes collective immunity in ant colonies. Curr Biol 17 : 1967–1971. 17980590
24. Tragust S, Mitteregger B, Barone V, Konrad M, Ugelvig LV, et al. (2013) Ants disinfect fungus-exposed brood by oral uptake and spread of their poison. Curr Biol 23 : 76–82. doi: 10.1016/j.cub.2012.11.034 23246409
25. Bulmer MS, Bachelet I, Raman R, Rosengaus RB, Sasisekharan R (2009) Targeting an antimicrobial effector function in insect immunity as a pest control strategy. Proc Natl Acad Sci U S A 106 : 12652–12657. doi: 10.1073/pnas.0904063106 19506247
26. Blanford S, Thomas M B (1999) Host thermal biology: the key to understanding host–pathogen interactions and microbial pest control? Agric For Entomol 1 : 195–202.
27. Wang Y, Yang P, Cui F, Kang L (2013) Altered immunity in crowded locust reduced fungal (Metarhizium anisopliae) pathogenesis. PLoS Pathog 9: e1003102. doi: 10.1371/journal.ppat.1003102 23326229
28. van Houte S, Ros VI, van Oers MM (2013) Walking with insects: molecular mechanisms behind parasitic manipulation of host behaviour. Mol Ecol 22 : 3458–3475. doi: 10.1111/mec.12307 23742168
29. Hoover K, Grove M, Gardner M, Hughes DP, McNeil J, et al. (2011) A gene for an extended phenotype. Science 333 : 1401. doi: 10.1126/science.1209199 21903803
30. Katsuma S, Koyano Y, Kang W, Kokusho R, Kamita SG, et al. (2012) The baculovirus uses a captured host phosphatase to induce enhanced locomotory activity in host caterpillars. PLoS Pathog 8: e1002644. doi: 10.1371/journal.ppat.1002644 22496662
31. Pedras MS, Irina Zaharia L, Ward DE. (2002) The destruxins: synthesis, biosynthesis, biotransformation, and biological activity. Phytochemistry 59 : 579–596. 11867090
32. Wang B, Kang Q, Lu Y, Bai L, Wang C (2012) Unveiling the biosynthetic puzzle of destruxins in Metarhizium species. Proc Natl Acad Sci U S A 109 : 1287–1292. doi: 10.1073/pnas.1115983109 22232661
33. Perry CJ, Barron AB (2013) Neural mechanisms of reward in insects. Annu Rev Entomol 58 : 543–562. doi: 10.1146/annurev-ento-120811-153631 23020615
34. Wang X, Kang L (2014) Molecular mechanisms of phase change in locusts. Annu Rev Entomol 59 : 225–244. doi: 10.1146/annurev-ento-011613-162019 24160426
35. Wada-Katsumata A, Yamaoka R, Aonuma H (2011) Social interactions influence dopamine and octopamine homeostasis in the brain of the ant Formica japonica. J Exp Biol 214 : 1707–1713. doi: 10.1242/jeb.051565 21525317
36. Szczuka A, Korczyńska J, Wnuk A, Symonowicz B, Szwacka GA, et al. (2013) The effects of serotonin, dopamine, octopamine and tyramine on behavior of workers of the ant Formica polyctena during dyadic aggression tests. Acta Neurobiol Exp 73 : 495–520.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek The Hos2 Histone Deacetylase Controls Virulence through Direct Regulation of Mating-Type GenesČlánek Transgenic Mouse Bioassay: Evidence That Rabbits Are Susceptible to a Variety of Prion IsolatesČlánek -Associated Polyomavirus Uses a Displaced Binding Site on VP1 to Engage Sialylated GlycolipidsČlánek Parsimonious Determination of the Optimal Infectious Dose of a Pathogen for Nonhuman Primate Models
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 8- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- The Long and Winding Road (Apologies to the Beatles)
- The Ebola Virus: From Basic Research to a Global Health Crisis
- Riding the R Train into the Cell
- The Two-Phase Emergence of Non Pandemic HIV-1 Group O in Cameroon
- Tumor Progression Locus 2 Promotes Induction of IFNλ, Interferon Stimulated Genes and Antigen-Specific CD8 T Cell Responses and Protects against Influenza Virus
- Type VI Secretion System Toxins Horizontally Shared between Marine Bacteria
- Incomplete Neutralization and Deviation from Sigmoidal Neutralization Curves for HIV Broadly Neutralizing Monoclonal Antibodies
- E3 Ubiquitin Ligase NEDD4 Promotes Influenza Virus Infection by Decreasing Levels of the Antiviral Protein IFITM3
- The Hos2 Histone Deacetylase Controls Virulence through Direct Regulation of Mating-Type Genes
- Hyperinvasive Meningococci Induce Intra-nuclear Cleavage of the NF-κB Protein p65/RelA by Meningococcal IgA Protease
- Active Transport of Phosphorylated Carbohydrates Promotes Intestinal Colonization and Transmission of a Bacterial Pathogen
- HTLV-1 Tax Stimulates Ubiquitin E3 Ligase, Ring Finger Protein 8, to Assemble Lysine 63-Linked Polyubiquitin Chains for TAK1 and IKK Activation
- Transgenic Mouse Bioassay: Evidence That Rabbits Are Susceptible to a Variety of Prion Isolates
- Widespread Reassortment Shapes the Evolution and Epidemiology of Bluetongue Virus following European Invasion
- Inhibiting the Recruitment of PLCγ1 to Kaposi’s Sarcoma Herpesvirus K15 Protein Reduces the Invasiveness and Angiogenesis of Infected Endothelial Cells
- Goblet Cell Derived RELM-β Recruits CD4 T Cells during Infectious Colitis to Promote Protective Intestinal Epithelial Cell Proliferation
- HLA Class-II Associated HIV Polymorphisms Predict Escape from CD4+ T Cell Responses
- An siRNA Screen Identifies the U2 snRNP Spliceosome as a Host Restriction Factor for Recombinant Adeno-associated Viruses
- Extracellular Adenosine Protects against Lung Infection by Regulating Pulmonary Neutrophil Recruitment
- : Adaptations to the Dixenous Life Cycle Analyzed by Genome Sequencing, Transcriptome Profiling and Co-infection with
- Which Way In? The RalF Arf-GEF Orchestrates Host Cell Invasion
- Intracellular Uropathogenic . Exploits Host Rab35 for Iron Acquisition and Survival within Urinary Bladder Cells
- A Non-enveloped Virus Hijacks Host Disaggregation Machinery to Translocate across the Endoplasmic Reticulum Membrane
- Supporting Role for GTPase Rab27a in Hepatitis C Virus RNA Replication through a Novel miR-122-Mediated Effect
- -Associated Polyomavirus Uses a Displaced Binding Site on VP1 to Engage Sialylated Glycolipids
- The Activation of Effector Avr3b by Plant Cyclophilin is Required for the Nudix Hydrolase Activity of Avr3b
- A Pyranose-2-Phosphate Motif Is Responsible for Both Antibiotic Import and Quorum-Sensing Regulation in
- Double-Edge Sword of Sustained ROCK Activation in Prion Diseases through Neuritogenesis Defects and Prion Accumulation
- The Rsb Phosphoregulatory Network Controls Availability of the Primary Sigma Factor in and Influences the Kinetics of Growth and Development
- Inhibits Virulence through Suppression of Pyochelin and Pyoverdine Biosynthesis
- Illuminating Targets of Bacterial Secretion
- Chemical Signals and Mechanosensing in Bacterial Responses to Their Environment
- Interdisciplinarity and Infectious Diseases: An Ebola Case Study
- Fungi That Infect Insects: Altering Host Behavior and Beyond
- Plasticity and Redundancy in Proteins Important for Invasion
- Are Human Intestinal Eukaryotes Beneficial or Commensals?
- A Novel Virus Causes Scale Drop Disease in
- STAT2 Knockout Syrian Hamsters Support Enhanced Replication and Pathogenicity of Human Adenovirus, Revealing an Important Role of Type I Interferon Response in Viral Control
- Parsimonious Determination of the Optimal Infectious Dose of a Pathogen for Nonhuman Primate Models
- Twenty-Eight Years of Poliovirus Replication in an Immunodeficient Individual: Impact on the Global Polio Eradication Initiative
- AAV-Delivered Antibody Mediates Significant Protective Effects against SIVmac239 Challenge in the Absence of Neutralizing Activity
- Interferon-γ Promotes Inflammation and Development of T-Cell Lymphoma in HTLV-1 bZIP Factor Transgenic Mice
- Transgenic Rabbits Expressing Ovine PrP Are Susceptible to Scrapie
- Mitochondrial Activity and Cyr1 Are Key Regulators of Ras1 Activation of . Virulence Pathways
- Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques
- Serine Phosphorylation of HIV-1 Vpu and Its Binding to Tetherin Regulates Interaction with Clathrin Adaptors
- Inhibition of mTORC1 Enhances the Translation of Chikungunya Proteins the Activation of the MnK/eIF4E Pathway
- Nanoformulations of Rilpivirine for Topical Pericoital and Systemic Coitus-Independent Administration Efficiently Prevent HIV Transmission
- Arming of MAIT Cell Cytolytic Antimicrobial Activity Is Induced by IL-7 and Defective in HIV-1 Infection
- sRNA-Mediated Regulation of P-Fimbriae Phase Variation in Uropathogenic
- Evolutionary and Functional Analysis of Old World Primate TRIM5 Reveals the Ancient Emergence of Primate Lentiviruses and Convergent Evolution Targeting a Conserved Capsid Interface
- Hepcidin and Host Defense against Infectious Diseases
- Type I IFN Induction via Poly-ICLC Protects Mice against Cryptococcosis
- Mucosal B Cells Are Associated with Delayed SIV Acquisition in Vaccinated Female but Not Male Rhesus Macaques Following SIV Rectal Challenge
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques
- Type VI Secretion System Toxins Horizontally Shared between Marine Bacteria
- Illuminating Targets of Bacterial Secretion
- Are Human Intestinal Eukaryotes Beneficial or Commensals?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



