-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaIlluminating Targets of Bacterial Secretion
article has not abstract
Published in the journal: . PLoS Pathog 11(8): e32767. doi:10.1371/journal.ppat.1004981
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004981Summary
article has not abstract
Bacterial Secretion
The ability to secrete proteins is important to the pathogenesis of many bacteria. For gram-negative bacteria, the secretion system must deliver cargo through both an inner and outer membrane to reach a potential target. To date, there are six known gram-negative bacterial secretion systems, designated types I–VI secretion. For many highly pathogenic bacteria including Yersinia pestis and Salmonella typhimurium, secretion of protein effectors directly into target host cells is essential for virulence. Proteins secreted via the type III, IV, and VI pathways result in direct transfer of proteins across the host membrane and into the cytosol, and these systems will be the focus of the technology highlighted in this article.
Although the function of effector proteins secreted by these systems varies among different pathogens, common virulence mechanisms are evident. One common function of many secreted virulence factors is the targeting of host cytoskeletal function in order to promote uptake or inhibit phagocytosis. Another is modulating host cell cytotoxicity by inhibiting or promoting cell death in order to suppress innate immune function or to establish a replicative niche. Finally, an important mechanism common to many secreted effectors is the manipulation of host immune signaling. Until recently, fully evaluating the functional targets of bacterial secretion in vivo during infection was extremely difficult.
FRET-Based β-lactamase Substrates as a Molecular Biology Tool
Förster (fluorescence) resonance energy transfer (FRET) has been used extensively as a cell biology tool to monitor the dynamics of intermolecular interactions within cells. FRET employs a donor fluorophore and an acceptor fluorophore in close proximity, and the emission spectra of the donor overlaps with the absorption spectrum of the acceptor. Excitation of the donor fluorophore results in resonance energy transfer to, and emission from, the acceptor. Disruption of the proximity between the two fluorophores results in strong emission from the donor upon excitation. The utility of FRET for measuring inter - and intramolecular interactions has been evident for some time. Using fluorescence as a measure of proximity, fluorophores exhibiting FRET can be incorporated to measure protein-folding dynamics in real time. Further, labeling separate molecules allows for measuring protein–protein interactions, the distance between molecules, and determining protein localization within a cell.
In 1998, Zlokarnik et al. used the gene encoding a common β-lactamase along with a FRET-based substrate to isolate individual cells with defined transcriptional responses from within a population of mammalian cells [1]. Zlokarnik et al. designed and synthesized the membrane-permeant ester CCF2/AM, which consists of a 7-hydroxycoumarin donor fluorophore and a fluorescein acceptor linked by a cephalosporin antibiotic. In the intact molecule, donor excitation at 405 nm (violet light) will result in fluorescein acceptor emission as green light at 520 nm that can be detected using flow cytometry or fluorescence microscopy. In the presence of β-lactamase, the cephalosporin linker is cleaved and the 7-hydroxycoumarin moiety is free from FRET, resulting in emission at 447 nm (blue light). Importantly, CCF2/AM is nonpolar and readily crosses mammalian cell membranes. Once in the cytosol of mammalian cells, the CCF2/AM ester group is hydrolyzed, trapping CCF2 and preventing leakage out of the cell. Thus, activation of a promoter of interest cloned upstream of the β-lactamase gene can be identified by incubating a cell population with CCF2/AM and screening for blue fluorescence.
β-lactamase Hybrids to Monitor Bacterial Secretion In Vivo
In 2004, Charpentier and Oswald demonstrated the utility of β-lactamase–protein hybrids for analyzing bacterial secretion [2]. Briefly, a sequence encoding the common β-lactamase TEM-1 was fused to the C-terminus of known secreted effectors in enteropathogenic Escherichia coli, and the resulting strains were used to infect HeLa cells. HeLa cells were then incubated with CCF2/AM, and translocation of effectors was visualized using fluorescence microscopy, in which cells that had undergone effector translocation exhibited blue fluorescence (Fig 1). In 2005, Marketon et al. extended this technique to identify cells targeted by Y. pestis type III secretion in vivo [3]. Until this point, identifying and measuring the host cells targeted for secretion during infection had been nearly impossible, requiring identification of a relatively small cell population within an entire animal. The authors infected mice with Y. pestis strains carrying translational fusions between secreted effector Yersinia outer proteins (Yops) and the functional region of TEM-1 β-lactamase, and they were able to detect Yop translocation as blue fluorescence during infection. To do this, Marketon et al. harvested the spleens of infected mice and subjected splenocytes to flow cytometry using a panel of fluorescently labeled antibodies to differentiate between T cells, granulocytes and neutrophils, dendritic cells, and macrophages. Using this method, Marketon et al. were able to measure and quantitate Yop translocation in vivo during infection, resulting in the discovery that Y. pestis primarily targets cells of the innate immune system for Yop translocation. Since this initial work, this approach has been used to examine the secretion of bacterial effector proteins for a number of bacteria including enteropathogenic Yersinia [4,5], Salmonella [6], Pseudomonas [7], and Legionella [8] species. Thus, the ability to monitor bacterial secretion in vivo during infection has allowed for direct analysis of host–pathogen interactions during infection and has allowed for an unprecedented look at how pathogenic bacteria target the host to establish and maintain infection.
Fig. 1. Evaluating bacterial secretion using the FRET-based substrate CCF2/AM. 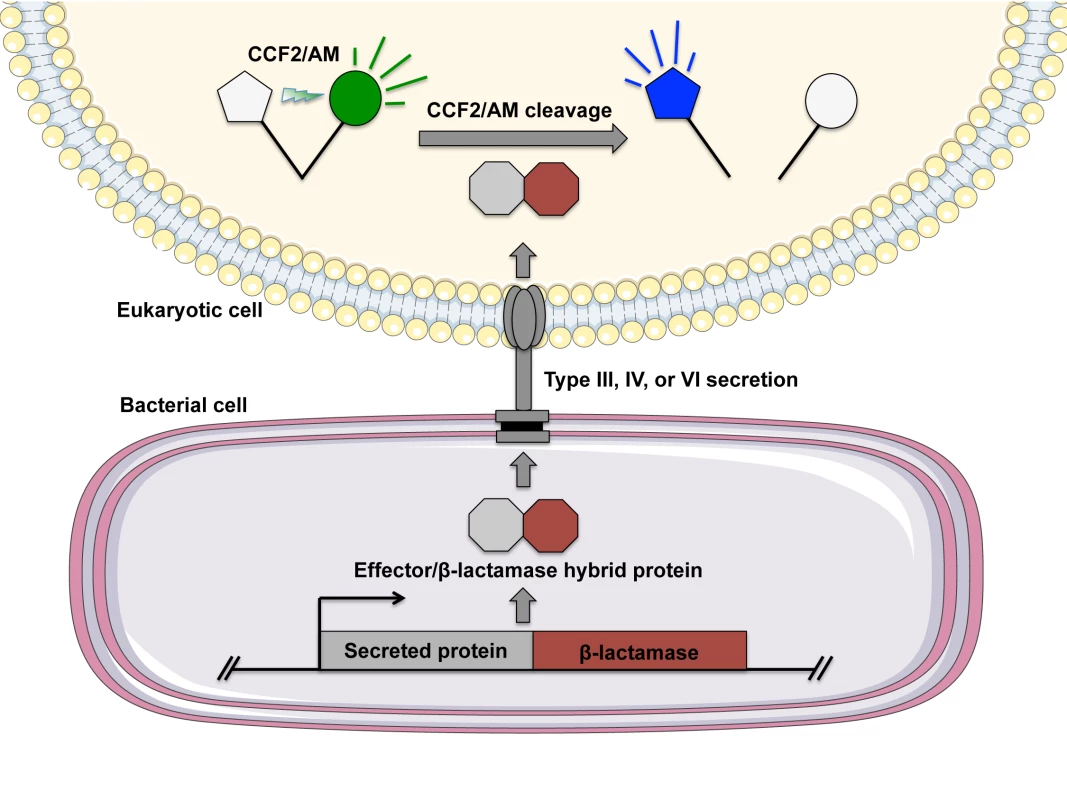
To monitor bacterial secretion, the gene encoding a β-lactamase is cloned in-frame with a portion of the gene encoding a predicted secreted protein to generate a translational fusion. Eukaryotic cells are infected with the bacteria harboring the fusion protein, followed by incubation of infected cells with the fluorescent substrate CCF2/AM (or its analog CCF4/AM). CCF2/AM consists of 7-hydroxycoumarin and fluorescein connected with a cephalosporin linker. Cells can then be examined by flow cytometry, fluorescence microscopy, or using a plate reader in a multiwell format. Under excitation at 405 nm, the 7-hydroxycoumarin and fluorescein moieties of CCF2/AM exhibit FRET, and emission is detected from the acceptor fluorescein as green fluorescence (520 nm). If the target cell has undergone translocation and harbors the bacterial fusion protein, the cephalosporin linker is cleaved by β-lactamase, which disrupts FRET and results in blue fluorescence from 7-hydroxycoumarin at 447 nm. Extending Utility beyond the Identification of Target Cell Types for a More Detailed Evaluation of Secretion
Since the initial in vivo studies, β-lactamase–protein hybrids coupled with FRET substrates have allowed for an increasingly detailed examination of bacterial secretion. Detection techniques have been refined to enable precise quantitation of secretion on a per-cell basis, allowing for the identification of mutants affecting the rate and fidelity of effector secretion [9]. In addition, the lactam ring of CCF2/AM has been modified to create CCF4/AM, which has improved solubility and slightly better FRET and is therefore better suited for screening applications. The fluorescent readout of translocation coupled with mutagenesis of bacterial genes has allowed for the detailed evaluation of translocation kinetics and mechanisms for a number of pathogens, as well as the identification of genes that contribute to effector secretion [10–12]. Of note, the robust fluorescent signal associated with the commonly used FRET substrates lends itself to the design of high throughput assays for the identification of mutants or factors that alter translocation in tissue culture. One potentially important application of this has involved the implementation of assays to identify inhibitors of bacterial secretion for the development of potential therapeutics [13,14]. As the secretion of effectors is essential to the virulence of a number of highly pathogenic gram-negative bacteria, the bacterial secretion machinery and/or secreted effectors may prove effective as new therapeutic targets [15].
Using β-lactamase/FRET Substrates to Probe Host Elements of Pathogenesis
An important application of FRET-substrate–effector β-lactamase hybrids has been for identifying host factors that contribute to effector translocation. This has primarily been accomplished by examining effector translocation in the presence of inhibitors of host function or coupled with the silencing of host genes [16,17]. For example, Newton et al. utilized small interfering RNA (siRNA) silencing to examine the role of specific host Rab GTPases in type IV secretion of effectors for the bacterium Coxiella burnetii [16]. Similarly, Sheahan et al. combined FRET of a Rho GTPase biosensor with flow cytometry in an RNA interference (RNAi) screen to identify host factors involved in type III secretion of Yersinia pseudotuberculosis, revealing a role for the chemokine receptor CCR5 in translocon function [18]. Coupling detection of translocation with methods to deplete specific host cell populations has contributed to the understanding of mechanisms that govern target cell preference in vivo during infection [5,19]. This approach was perhaps most elegantly employed by Durand et al., who used neutrophil depletion studies to show that the presence of professional phagocytes dictated the number of host cells targeted for Yop translocation by the enteric pathogen Y. pseudotuberculosis [5].
A major hurdle to studying the effects of bacterial secretion in vivo is the difficulty of efficiently isolating target cells from infected animals for subsequent analysis. One innovative approach addressed this issue by utilizing the β-lactamase/FRET system to identify and isolate host cells for further study. Using flow cytometry to sort cells from the spleens of mice infected with Y. pseudotuberculosis, Rolán et al. identified cells that had been injected with the effector protein YopH, a potent tyrosine phosphatase, and separated these from cells that had not been injected [20]. These two-cell populations were collected and subsequently analyzed by western blot to identify specific targets with decreased phosphorylation in the presence of YopH. Expanding this analysis to evaluate host responses to effector intoxication in greater detail will likely provide crucial information regarding the direct host–pathogen interactions that occur during infection. Thus, the use of bacterial effector–β-lactamase hybrids along with FRET-based substrates has proven ideal for evaluating host–microbe interactions and has allowed for detailed evaluation of both the bacterial and host factors that contribute to a process essential to virulence of many pathogenic bacteria. In the future, this technique will continue to be highly useful in high-throughput approaches for studying both microbial and host cellular processes that contribute to infection, particularly for the identification of inhibitors that might have therapeutic applications. Further, the coupling of FRET-based substrates with fluorescence-activated cell sorting has great potential for the isolation and analysis of specific target cells from infected organs, providing a high-resolution view of host–pathogen interactions during infection.
Zdroje
1. Zlokarnik G, Negulescu PA, Knapp TE, Mere L, Burres N, Feng L, et al. Quantitation of transcription and clonal selection of single living cells with beta-lactamase as reporter. Science. 1998 Jan 2;279(5347):84–8. 9417030
2. Charpentier X, Oswald E. Identification of the secretion and translocation domain of the enteropathogenic and enterohemorrhagic Escherichia coli effector Cif, using TEM-1 beta-lactamase as a new fluorescence-based reporter. J Bacteriol. 2004 Aug;186(16):5486–95. 15292151
3. Marketon MM, Depaolo RW, DeBord KL, Jabri B, Schneewind O. Plague bacteria target immune cells during infection. Science. 2005 Sep 9;309(5741):1739–41. 16051750
4. Köberle M, Klein-Günther A, Schütz M, Fritz M, Berchtold S, Tolosa E, et al. Yersinia enterocolitica targets cells of the innate and adaptive immune system by injection of Yops in a mouse infection model. PLoS Pathog. 2009 Aug;5(8):e1000551. doi: 10.1371/journal.ppat.1000551 19680448
5. Durand EA, Maldonado-Arocho FJ, Castillo C, Walsh RL, Mecsas J. The presence of professional phagocytes dictates the number of host cells targeted for Yop translocation during infection. Cell Microbiol. 2010 Aug;12(8):1064–82. doi: 10.1111/j.1462-5822.2010.01451.x 20148898
6. Geddes K, Cruz F, Heffron F. Analysis of cells targeted by Salmonella type III secretion in vivo. PLoS Pathog. 2007 Dec;3(12):e196. 18159943
7. Diaz MH, Diaz MH, Hauser AR, Hauser AR. Pseudomonas aeruginosa Cytotoxin ExoU Is Injected into Phagocytic Cells during Acute Pneumonia. Infect Immun. 2010 Mar 16;78(4):1447–56. doi: 10.1128/IAI.01134-09 20100855
8. Copenhaver AM, Casson CN, Nguyen HT, Fung TC, Duda MM, Roy CR, et al. Alveolar macrophages and neutrophils are the primary reservoirs for Legionella pneumophila and mediate cytosolic surveillance of type IV secretion. Infect Immun. 2014 Oct;82(10):4325–36. doi: 10.1128/IAI.01891-14 25092908
9. Dewoody R, Merritt PM, Marketon MM. YopK controls both rate and fidelity of Yop translocation. Mol Microbiol. 2013 Jan;87(2):301–17. doi: 10.1111/mmi.12099 23205707
10. Houppert AS, Kwiatkowski E, Glass EM, DeBord KL, Merritt PM, Schneewind O, et al. Identification of chromosomal genes in Yersinia pestis that influence type III secretion and delivery of Yops into target cells. PLoS ONE. 2012;7(3):e34039. doi: 10.1371/journal.pone.0034039 22479512
11. Paczosa MK, Fisher ML, Maldonado-Arocho FJ, Mecsas J. Yersinia pseudotuberculosis uses Ail and YadA to circumvent neutrophils by directing Yop translocation during lung infection. Cell Microbiol. 2014 Feb;16(2):247–68. doi: 10.1111/cmi.12219 24119087
12. Adams W, Morgan J, Kwuan L, Auerbuch V. Yersinia pseudotuberculosis YopD mutants that genetically separate effector protein translocation from host membrane disruption. Mol Microbiol. 2015 Feb 13.
13. Pan NJ, Brady MJ, Leong JM, Goguen JD. Targeting type III secretion in Yersinia pestis. Antimicrob Agents Chemother. 2009 Feb;53(2):385–92. doi: 10.1128/AAC.00670-08 19015348
14. Harmon DE, Davis AJ, Castillo C, Mecsas J. Identification and characterization of small-molecule inhibitors of Yop translocation in Yersinia pseudotuberculosis. Antimicrob Agents Chemother. 2010 Aug;54(8):3241–54. doi: 10.1128/AAC.00364-10 20498321
15. Marshall NC, Finlay BB. Targeting the type III secretion system to treat bacterial infections. Expert Opin Ther Targets. 2014 Feb;18(2):137–52. doi: 10.1517/14728222.2014.855199 24295327
16. Newton HJ, McDonough JA, Roy CR. Effector protein translocation by the Coxiella burnetii Dot/Icm type IV secretion system requires endocytic maturation of the pathogen-occupied vacuole. PLoS ONE. 2013;8(1):e54566. doi: 10.1371/journal.pone.0054566 23349930
17. Verove J, Bernarde C, Bohn Y-ST, Boulay F, Rabiet M-J, Attree I, et al. Injection of Pseudomonas aeruginosa Exo toxins into host cells can be modulated by host factors at the level of translocon assembly and/or activity. PLoS ONE. 2012;7(1):e30488. doi: 10.1371/journal.pone.0030488 22299042
18. Sheahan K-L, Isberg RR. Identification of Mammalian Proteins That Collaborate with Type III Secretion System Function: Involvement of a Chemokine Receptor in Supporting Translocon Activity. MBio. 2014;6(1).
19. Pechous RD, Sivaraman V, Price PA, Stasulli NM, Goldman WE. Early host cell targets of Yersinia pestis during primary pneumonic plague. PLoS Pathog. 2013;9(10):e1003679. doi: 10.1371/journal.ppat.1003679 24098126
20. Rolán HG, Durand EA, Mecsas J. Identifying Yersinia YopH-targeted signal transduction pathways that impair neutrophil responses during in vivo murine infection. Cell Host Microbe. 2013 Sep 11;14(3):306–17. doi: 10.1016/j.chom.2013.08.013 24034616
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek The Hos2 Histone Deacetylase Controls Virulence through Direct Regulation of Mating-Type GenesČlánek Transgenic Mouse Bioassay: Evidence That Rabbits Are Susceptible to a Variety of Prion IsolatesČlánek -Associated Polyomavirus Uses a Displaced Binding Site on VP1 to Engage Sialylated GlycolipidsČlánek Parsimonious Determination of the Optimal Infectious Dose of a Pathogen for Nonhuman Primate Models
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 8- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- The Long and Winding Road (Apologies to the Beatles)
- The Ebola Virus: From Basic Research to a Global Health Crisis
- Riding the R Train into the Cell
- The Two-Phase Emergence of Non Pandemic HIV-1 Group O in Cameroon
- Tumor Progression Locus 2 Promotes Induction of IFNλ, Interferon Stimulated Genes and Antigen-Specific CD8 T Cell Responses and Protects against Influenza Virus
- Type VI Secretion System Toxins Horizontally Shared between Marine Bacteria
- Incomplete Neutralization and Deviation from Sigmoidal Neutralization Curves for HIV Broadly Neutralizing Monoclonal Antibodies
- E3 Ubiquitin Ligase NEDD4 Promotes Influenza Virus Infection by Decreasing Levels of the Antiviral Protein IFITM3
- The Hos2 Histone Deacetylase Controls Virulence through Direct Regulation of Mating-Type Genes
- Hyperinvasive Meningococci Induce Intra-nuclear Cleavage of the NF-κB Protein p65/RelA by Meningococcal IgA Protease
- Active Transport of Phosphorylated Carbohydrates Promotes Intestinal Colonization and Transmission of a Bacterial Pathogen
- HTLV-1 Tax Stimulates Ubiquitin E3 Ligase, Ring Finger Protein 8, to Assemble Lysine 63-Linked Polyubiquitin Chains for TAK1 and IKK Activation
- Transgenic Mouse Bioassay: Evidence That Rabbits Are Susceptible to a Variety of Prion Isolates
- Widespread Reassortment Shapes the Evolution and Epidemiology of Bluetongue Virus following European Invasion
- Inhibiting the Recruitment of PLCγ1 to Kaposi’s Sarcoma Herpesvirus K15 Protein Reduces the Invasiveness and Angiogenesis of Infected Endothelial Cells
- Goblet Cell Derived RELM-β Recruits CD4 T Cells during Infectious Colitis to Promote Protective Intestinal Epithelial Cell Proliferation
- HLA Class-II Associated HIV Polymorphisms Predict Escape from CD4+ T Cell Responses
- An siRNA Screen Identifies the U2 snRNP Spliceosome as a Host Restriction Factor for Recombinant Adeno-associated Viruses
- Extracellular Adenosine Protects against Lung Infection by Regulating Pulmonary Neutrophil Recruitment
- : Adaptations to the Dixenous Life Cycle Analyzed by Genome Sequencing, Transcriptome Profiling and Co-infection with
- Which Way In? The RalF Arf-GEF Orchestrates Host Cell Invasion
- Intracellular Uropathogenic . Exploits Host Rab35 for Iron Acquisition and Survival within Urinary Bladder Cells
- A Non-enveloped Virus Hijacks Host Disaggregation Machinery to Translocate across the Endoplasmic Reticulum Membrane
- Supporting Role for GTPase Rab27a in Hepatitis C Virus RNA Replication through a Novel miR-122-Mediated Effect
- -Associated Polyomavirus Uses a Displaced Binding Site on VP1 to Engage Sialylated Glycolipids
- The Activation of Effector Avr3b by Plant Cyclophilin is Required for the Nudix Hydrolase Activity of Avr3b
- A Pyranose-2-Phosphate Motif Is Responsible for Both Antibiotic Import and Quorum-Sensing Regulation in
- Double-Edge Sword of Sustained ROCK Activation in Prion Diseases through Neuritogenesis Defects and Prion Accumulation
- The Rsb Phosphoregulatory Network Controls Availability of the Primary Sigma Factor in and Influences the Kinetics of Growth and Development
- Inhibits Virulence through Suppression of Pyochelin and Pyoverdine Biosynthesis
- Illuminating Targets of Bacterial Secretion
- Chemical Signals and Mechanosensing in Bacterial Responses to Their Environment
- Interdisciplinarity and Infectious Diseases: An Ebola Case Study
- Fungi That Infect Insects: Altering Host Behavior and Beyond
- Plasticity and Redundancy in Proteins Important for Invasion
- Are Human Intestinal Eukaryotes Beneficial or Commensals?
- A Novel Virus Causes Scale Drop Disease in
- STAT2 Knockout Syrian Hamsters Support Enhanced Replication and Pathogenicity of Human Adenovirus, Revealing an Important Role of Type I Interferon Response in Viral Control
- Parsimonious Determination of the Optimal Infectious Dose of a Pathogen for Nonhuman Primate Models
- Twenty-Eight Years of Poliovirus Replication in an Immunodeficient Individual: Impact on the Global Polio Eradication Initiative
- AAV-Delivered Antibody Mediates Significant Protective Effects against SIVmac239 Challenge in the Absence of Neutralizing Activity
- Interferon-γ Promotes Inflammation and Development of T-Cell Lymphoma in HTLV-1 bZIP Factor Transgenic Mice
- Transgenic Rabbits Expressing Ovine PrP Are Susceptible to Scrapie
- Mitochondrial Activity and Cyr1 Are Key Regulators of Ras1 Activation of . Virulence Pathways
- Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques
- Serine Phosphorylation of HIV-1 Vpu and Its Binding to Tetherin Regulates Interaction with Clathrin Adaptors
- Inhibition of mTORC1 Enhances the Translation of Chikungunya Proteins the Activation of the MnK/eIF4E Pathway
- Nanoformulations of Rilpivirine for Topical Pericoital and Systemic Coitus-Independent Administration Efficiently Prevent HIV Transmission
- Arming of MAIT Cell Cytolytic Antimicrobial Activity Is Induced by IL-7 and Defective in HIV-1 Infection
- sRNA-Mediated Regulation of P-Fimbriae Phase Variation in Uropathogenic
- Evolutionary and Functional Analysis of Old World Primate TRIM5 Reveals the Ancient Emergence of Primate Lentiviruses and Convergent Evolution Targeting a Conserved Capsid Interface
- Hepcidin and Host Defense against Infectious Diseases
- Type I IFN Induction via Poly-ICLC Protects Mice against Cryptococcosis
- Mucosal B Cells Are Associated with Delayed SIV Acquisition in Vaccinated Female but Not Male Rhesus Macaques Following SIV Rectal Challenge
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques
- Type VI Secretion System Toxins Horizontally Shared between Marine Bacteria
- Illuminating Targets of Bacterial Secretion
- Are Human Intestinal Eukaryotes Beneficial or Commensals?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



