-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaVaginal Challenge with an SIV-Based Dual Reporter System Reveals That Infection Can Occur throughout the Upper and Lower Female Reproductive Tract
There is currently a great effort world-wide to develop interventions such as vaccines and microbicides to decrease, or hopefully block, HIV transmission. To model the infection of women, the field utilizes the rhesus macaque vaginal transmission model. Understanding the initial events leading to infection after viral challenge of the female reproductive tract (FRT) is crucial for the development of functional prevention strategies. To this end, we developed a novel method for detecting infection in the rhesus macaque FRT after vaginal inoculation. This method utilizes single round replication defective vector that expresses dual reporter proteins, Luciferase and mCherry. Monitoring Luciferase expression allows us to identify the sites of infection within the intact FRT, while fluorescent protein mCherry allows us to visualize the single infected cells. Our studies revealed that virus can access the entire upper and lower reproductive tract. Infection occurs primarily in vaginal and ectocervical tissue, but can spread as far as the ovary and local draining lymph nodes. All classically defined susceptible cell types can be infected with the broadly tropic HIV envelope utilized in this study. Prevention strategies aimed at protecting from HIV infection should consider the entire FRT architecture as potentially susceptible and design interventions accordingly.
Published in the journal: . PLoS Pathog 10(10): e32767. doi:10.1371/journal.ppat.1004440
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004440Summary
There is currently a great effort world-wide to develop interventions such as vaccines and microbicides to decrease, or hopefully block, HIV transmission. To model the infection of women, the field utilizes the rhesus macaque vaginal transmission model. Understanding the initial events leading to infection after viral challenge of the female reproductive tract (FRT) is crucial for the development of functional prevention strategies. To this end, we developed a novel method for detecting infection in the rhesus macaque FRT after vaginal inoculation. This method utilizes single round replication defective vector that expresses dual reporter proteins, Luciferase and mCherry. Monitoring Luciferase expression allows us to identify the sites of infection within the intact FRT, while fluorescent protein mCherry allows us to visualize the single infected cells. Our studies revealed that virus can access the entire upper and lower reproductive tract. Infection occurs primarily in vaginal and ectocervical tissue, but can spread as far as the ovary and local draining lymph nodes. All classically defined susceptible cell types can be infected with the broadly tropic HIV envelope utilized in this study. Prevention strategies aimed at protecting from HIV infection should consider the entire FRT architecture as potentially susceptible and design interventions accordingly.
Introduction
The majority of new female human immunodeficiency virus (HIV) infections are the direct result of vaginal intercourse with an infected male partner [1]–[3]. Previous studies looking at early transmission events have focused on the endocervix within the female reproductive tract (FRT) in part due to the preconception that these are preferential sites for transmission and in part due to the technical and time constraints [4]. Here, we describe methodology that allows us to survey the entire FRT for potential sites of infection and then characterize the initial target cells in a systematic and efficient manner using a rhesus macaque (Macaca mulatta; RM) SIV vaginal transmission model.
Current paradigms imply that a small number of initial transmission events occur, which subsequently rely on the host immune response to infection to fuel viral spread [4], [5]. The evidence that in most infections a single viral variant establishes systemic infection reinforces this model [6]. This is thought to occur in areas in the FRT that are particularly susceptible to viral entry. Multiple studies examining viral acquisition after vaginal exposure in macaque models have concluded that the endocervix and it's less robust single cell columnar epithelial barrier is a primary portal of entry for virions in the FRT [7], [8]. They have also suggested that the transformation zone, where the columnar epithelium of the endocervix merges with the more robust squamous epithelial barriers of the vaginal vault is especially susceptible because of inefficient barrier function where the two different types of epithelium come together. Unfortunately, these studies presented the results in a qualitative manner making it impossible for the reader to know how commonly infection in the endocervix/transformation zone was detected. In these papers it was also suggested that areas in the vaginal vault where squamous epithelial is dysfunctional might also be a site of acquisition in the FRT. Breaks in the vaginal epithelium due to abrasion or ulcerative STIs, or exposure of the more permissive simple columnar epithelium in the ectocervix due to cervical ectopy, can facilitate viral ingress [3]. These cervix-centric studies have led to the field focusing on this as a principle site of transmission and excluding much of the FRT as important to protect [7], [8]. Another implication of this work is that the primary bottleneck for transmission is at the mucosal barrier. Based on these observations it was concluded that preventive modalities, either microbicidal or vaccine based, present in the vaginal vault and endocervix would be sufficient to prevent transmission.
To date, the only clinically demonstrated efficient prevention mechanism is based on oral pre-exposure prophylaxis (PrEP) that results in systemic distribution of antiviral drugs [9]. In contrast, topically applied antiretroviral gels in the FRT have only shown partial or no protection [10], [11]. Poor adherence in efficacy trials has complicated the determination of their efficacy [12], [13]. The disparity may reveal more about the mechanism of viral acquisition than was previously appreciated. Here we consider the possibility that there are more widespread infection events, including the upper FRT and local draining lymph nodes, which have been absent in previous studies on transmission. To this end, we have modified an SIV-based gene delivery vector [14] into a dual reporter system that expresses the mCherry fluorescent protein [15] and enhanced firefly luciferase [16] (Figure 1). This system expresses no viral proteins and thus only undergoes a single round of infection. By examining the entire FRT of animals 2 days after vaginal challenge, we find these initial acquisition events to be widespread.
Fig. 1. Schematic of the lentiviral vector used in this study. 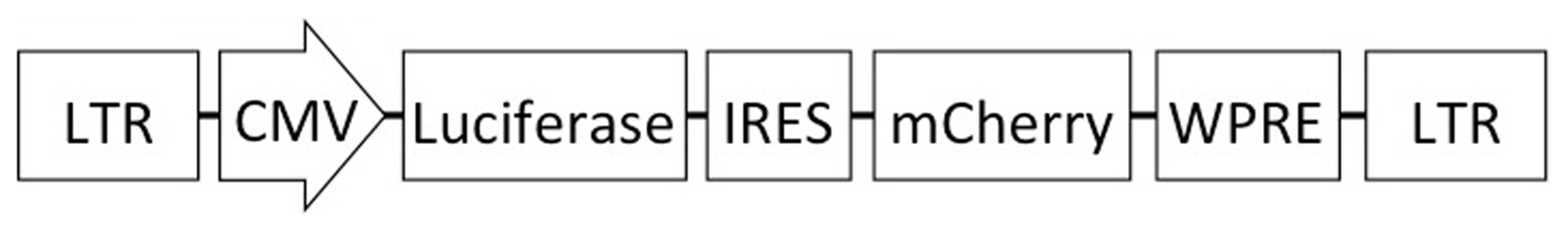
The vector contains an effLuc expression cassette, expression of which is driven by the CMV promoter. An internal ribosomal entry site (IRES) and a mCherry cassette are downstream of luciferase. This vector contains the SIV promoter in the 5′ long-terminal repeat (LTR) for efficient virus production in the context of a Tat-free packaging system, and self-inactivating mutations in the 3′ LTR. Results
Vector design and optimization
The reporter system we use in these studies is designed to examine the localization and phenotype of cells that are susceptible to infection by the initial viral inoculum. We use a non-replicating vector that allows determination of the initial targets of infection, because any transduction events detected must be a derived from the challenge inoculum. Gene delivery through such a vector is commonly described as transduction, rather than infection because it only encompasses the replication steps up to the integration of the reporter genome. The reporter genome is delivered by a SIV-based lentiviral delivery system (Figure 1) which is necessary to avoid species-specific restriction factors like TRIM5α that are known to block HIV infection in rhesus macaques (RM). Use of two reporters provides complementary evidence for successful transduction. The luciferase reporter enables examination of whole tissues to find foci of infection with an in vivo imaging system (IVIS). Luminescence is used as macroscopic guide for detection of tissue sites where individual infected cells can be subsequently identified with fluorescence microscopy of cryosections.
The vector strategy allows different envelopes to be utilized to determine vector tropism. Here we compare HIV-1 envelope (strain JRFL [17]) or VSV-G protein mediated delivery of the vector. JRFL was chosen to be the initial HIV envelope used to develop this system for multiple reasons. It is CCR5 tropic, as is the virus that is sexually transmitted and has a broad tropism, able to infect a wide range of immune cells including T cells, macrophages, and dendritic cells. But perhaps most importantly, it is very efficient at pseudotyping this SIV vector allowing maximal titers of virus to be generated. Using VSV-G envelope enables exploration of barrier function and viral particle distribution throughout the FRT, because VSV-G is capable of transducing nearly all cell types [18]; JRFL envelope identifies sites of HIV susceptible cells.
The reporter particle genome does not express any viral proteins and instead encodes enhanced firefly luciferase [16] and mCherry fluorescent protein [15] from a single transcript. An internal ribosome entry site (IRES) is located upstream of the mCherry open reading frame to insure efficient expression of the downstream protein in this bicistronic message. Expression is driven by the immediate-early CMV promoter to optimize reporter gene transcription. A Woodchuck Hepatitis Virus post-transcriptional regulatory element (WPRE) located at the 3′ end of the message enhances gene expression of the vector [19], [20]. The WPRE is commonly utilized in lentiviral vectors to obtain efficient expression of delivered genes [21].
Reporter virions are generated by transfection of 4 separate plasmids into 293T cells, producing virus particles that undergo all of the early stages of the SIV replication cycle up through integration of the viral genome. The reporter genome is transfected along with the viral protein expression plasmids, expressing all of the proteins necessary to package the reporter genome into a functional, single round replicating virus particle. SIV proteins are expressed using a modified SIV expression plasmid that does not express Env and has deletions of the 5′ and 3′ LTRs [14]. The vector delivering the reporter genes contains the elements necessary for efficient packaging and integration after reverse transcription. Importantly, the packaging construct does not contain any RNA packaging sites ensuring that only the reporter vector genome is packaged into assembling virions. Pseudotyping with a third plasmid for Env expression, either VSV-G or JRFL, determines the cellular tropism and entry mechanism that is utilized by reporter virions. Additionally, an HIV Rev expression plasmid is added to enable nuclear export of reporter genomes, and induce efficient expression of the SIV packaging construct, reporter virus genome, and HIV envelope.
This system enables a single round of replication of a SIV-based virions and allows the identification of cells that have integrated viral genomes, which we utilize here as the threshold for defining an infected cell. Dual reporter expression indicates that the cell was susceptible to entry by the pseudotyped envelope, and permissive to uncoating of the viral capsid, reverse transcription, nuclear import of the viral genome and integration of reporter DNA and expression of fluorescent and luminescent proteins.
To develop our transmission model, we addressed the sensitivity of the ability to detect luciferase signal with an IVIS whole animal imaging system. JRFL pseudotyped dual reporter vector was used to transduce RM activated PBMCs, which were then quantified to show transduction of 0.5 to 2% of cells, varying by experiment. Increasing numbers of transduced cells were then subcutaneously injected into nude mice followed by intraperitoneal administration of luciferin. Luminescent signal was then analyzed an in vivo imaging system (IVIS). This pilot study revealed that we could detect as few as 10 cells in close proximity to an injection site (Figure S1). This pilot study also revealed that we could not detect single transduction events, revealing an important limitation of this system. Therefore, this system can only reveal the localization of small foci of infection where multiple cells are expressing luciferase in the context of tissue.
Macaque female reproductive tract has uneven susceptibility to viral entry
To initiate our development of a transmission model in rhesus macaques, we began with a VSV-G pseudotyped version of the vector, anticipating that the broad tropism would more readily generate a detectable luciferase signal. To increase the possibility of detecting a luciferase signal we utilized a vaginal biopsy, which we anticipated would provide a site of focused transduction and allow detection of signal because of the potential limitation of detection described above. The biopsy site would simulate the effects of microtrauma and generate a place where the vector could bypass mucosal barriers. Six RMs were atraumatically inoculated, vaginally and rectally, with high titer VSV-G pseudotyped reporter virions (TCID50 range: 105.4–106.3). Four of the animals were pretreated with Depo-Provera (DP) to increase the likelihood of finding transmission events [22], while two were normally cycling. 48 hours after inoculation the animals were sacrificed. The entire reproductive tract as well as inguinal and iliac lymph nodes were removed and soaked in d-Luciferin.
The initial analysis for luciferase activity delivered by the VSV-G pseudotyped vector revealed that virions could access viable cells throughout the entire FRT (Figure 2). The background signal from a naïve animal was used to establish the levels of specific luminescent signal (Figure S2). Luciferase activity was detected in the vaginal tissue, ecto - and endocervix, and ovaries. Between animals there was no uniform pattern of infection; although most had multiple foci of transduction present (Table 1). The effect of hormone treatment was not dramatic for the VSV-G pseudotyped vector and there was no evidence that DP treatment made a difference in the degree and localization of luminescence in this small study. The detection of luciferase activity in the ovary was unexpected. Luciferase activity was detected in small focal regions. These regions were dissected from the tissue and subjected to repeated rounds of assay for luciferase activity. Dissected regions that repeatedly demonstrated luciferase activity were frozen for cryosectioning and fluorescence imaging analysis to identify vector-transduced cells based on mCherry expression. In these initial studies we learned that dissecting the smallest possible piece of tissue (2×2 mm2) showing luciferase activity facilitated identification of single cells in thin tissue sections.
Fig. 2. Luciferase reporter expression throughout the female reproductive tract in VSV-G pseudotyped vector inoculated Rhesus Macaques. 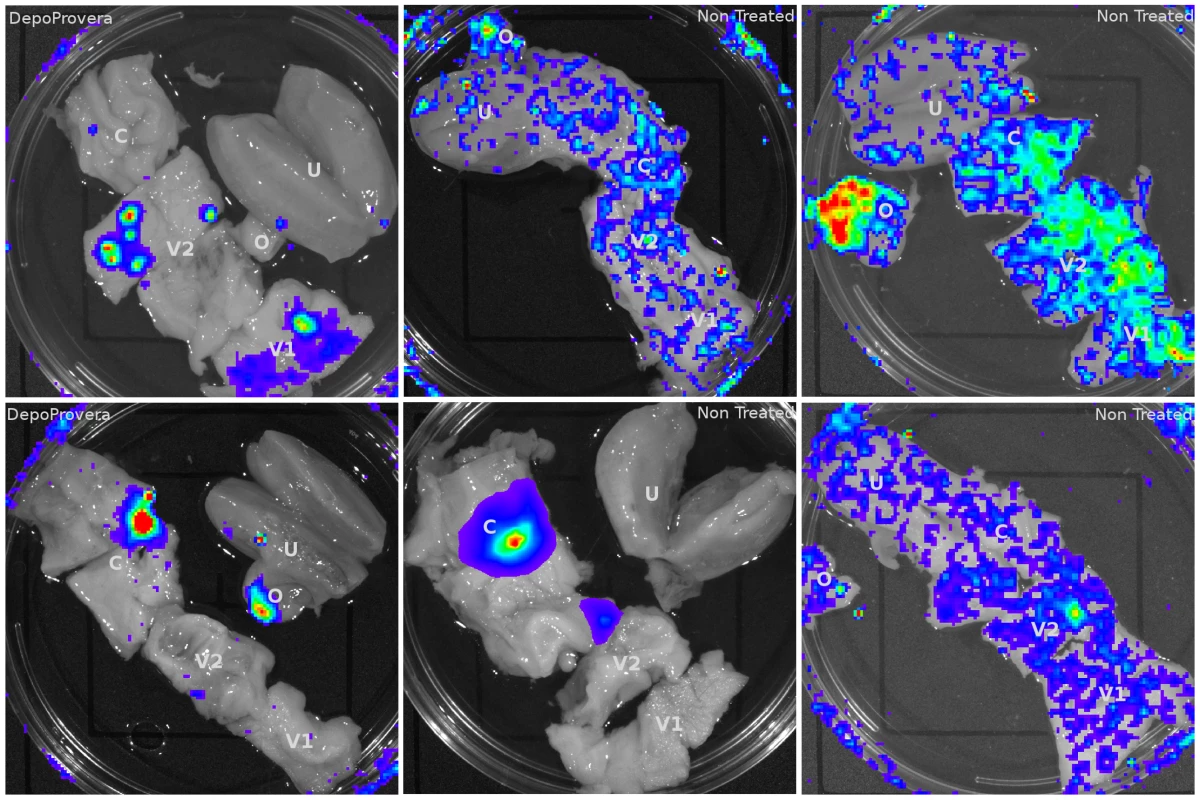
6 animals (4 pretreated with Depo-Provera) were inoculated with VSV-G pseudotyped dual-reporter vectors and sacrificed 48 hours later. Exposure to luciferin reveals reporter expression is present throughout the reproductive tract of Depo-Provera treated and normally cycling monkeys and that expression pattern varies between animals. Tab. 1. Localization of luciferase expression in VSV-G pseudotyped LICh reporter inoculated Rhesus Macaques. 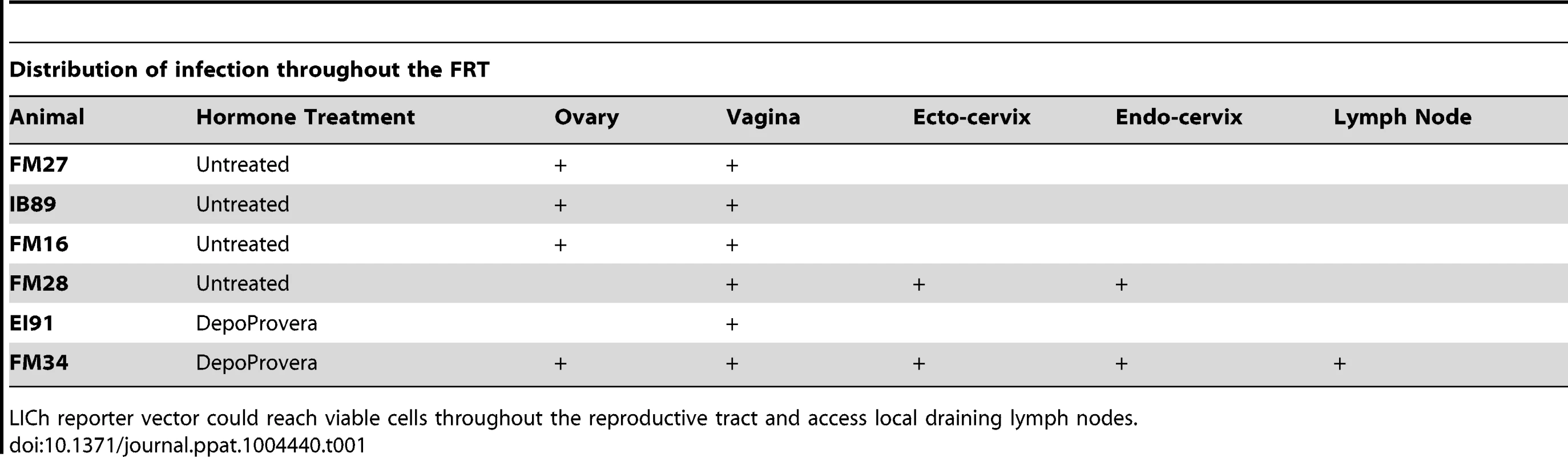
LICh reporter vector could reach viable cells throughout the reproductive tract and access local draining lymph nodes. Expression of mCherry fluorescence revealed that epithelial cells in the ectocervix (Figure 3a, b) and vagina (Figure 3c) appear to be the primary target for VSV-G enveloped virions, although infection events below the basement membrane of squamous epithelium were observed in some cases and associated with a high density of T cells (Figure 3d). Cells that appeared to have strong emission through the mCherry filter set were assessed by two independent measures to confirm the transduction. The primary criteria to validate the presence of infection used spectral imaging, as mCherry has a narrow emission spectra peaking at 610 nm [15], a signature that is not known to exist naturally. Additionally, cryosections were stained for luciferase expression utilizing immunofluorescence with an anti-luciferase antibody, revealing that the mCherry expressing cells were also positive for luciferase expression from the dual reporter vector. To further characterize the signal, we determined fluorescence in the TRITC channel as a negative control. Therefore, cells that scored positive for both mCherry and luciferase expression were analyzed through a 586 nm TRITC filter set and were found to have dim emission, consistent with mCherry having low fluorescent signal at 586 nm. Weak relative emission in orange wavelengths indicates that the fluorescent signal seen is not a part of some broad-spectrum autofluorescent signal that can be detected in tissues and is more applicable to screening large numbers of cells than spectral imaging. Therefore, in many cases we utilized the TRITC signal as a surrogate of spectral imaging. The same criteria held true for confirming the infection of RM PBMCs (Figure S3).
Fig. 3. Infected cells from ectocervical and vaginal tissue of VSV-G enveloped reporter inoculated Rhesus Macaques. 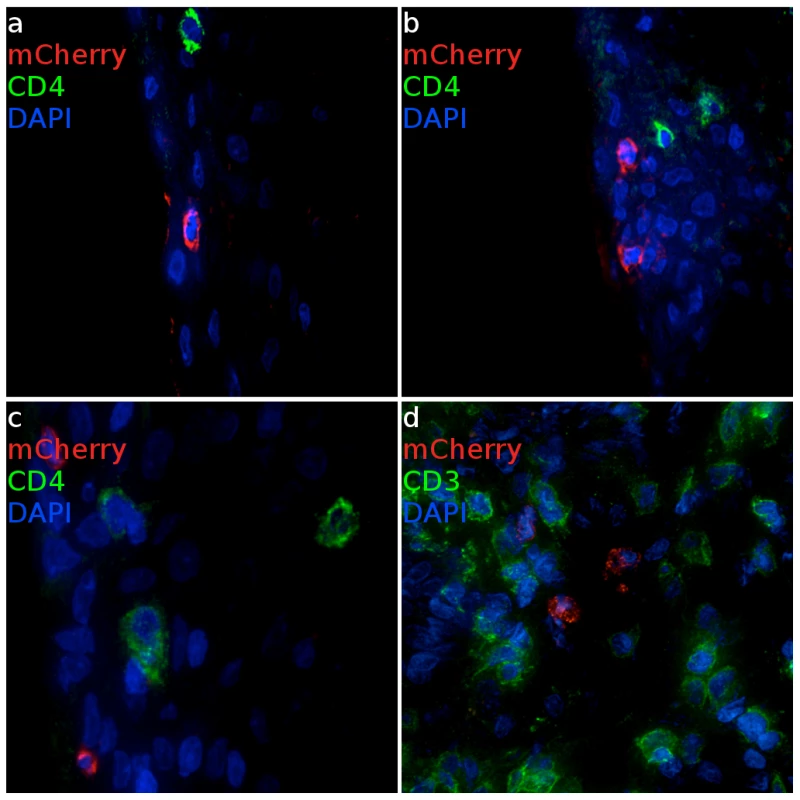
(a, b) Virus primarily infects epithelial cells within the ectocervix. CD4 expressing cells cluster proximal to mCherry expressing cells but are not infected. (Animal code: FM27) (c) Epithelial and (d) sub-epithelial cells in the vagina are also susceptible to infection. Where infection is found, there are abundant numbers of T cells are present. (Animal code: FM28) mCherry signal is shown in red. CD3 or CD4 is shown in green. Nuclei (DAPI) are shown in blue. Scale bars, 30 µm. Through pilot studies with VSV-G pseudotyped dual reporter vector we were able to demonstrate that we can detect vector expressed luciferase signals after vaginal exposure to virus. Importantly, we were able to optimize techniques to unequivocally identify mCherry expression of transduced cells by spectral imaging and luciferase staining in cryosections of small pieces of tissue exhibiting luciferase activity. Through this process we developed criteria to identify the bulk of transduced cells on a fluorescence microscope by 3 criteria: 1) mCherry positive, 2) luciferase positive after antibody staining, and 3) minimal TRITC fluorescence consistent with a signal from mCherry fluorescence rather than broad-spectrum tissue autofluorescence.
HIV Env pseudotyped vector can transduce cells throughout the entire FRT
Having validated the ability of the system to identify sites on infection with VSV-G pseudotyped vector, we next asked if the system would work after pseudotyping with an HIV envelope. We chose to utilize the JRFL CCR5-tropic envelope because of its broad tropism and ability to efficiently generate high titer vector. Concentrated stocks of JRFL Env expressing vector (TCID50 range: 104.8–106.2) were inoculated into eight RMs with vaginal biopsies; four animals were DP treated. During luciferase detection of these animals, dissection into subsequently smaller regions enabled focusing of the luminescent signal into foci (Figure 4a), which were frozen. The change in envelope did not result in noticeably decreased gross luminescent signal or an altered macroscopic pattern of infection. As observed with the VSV-G pseudotyped vector, luciferase activity in the ovary was also detected with the HIV envelope. Detectable levels of luciferase expression were identified within at least one ovary in six out of eight animals (two treated with DP, four untreated, Table 2).
Fig. 4. Identification and phenotyping of infected cells from female reproductive tract. a, 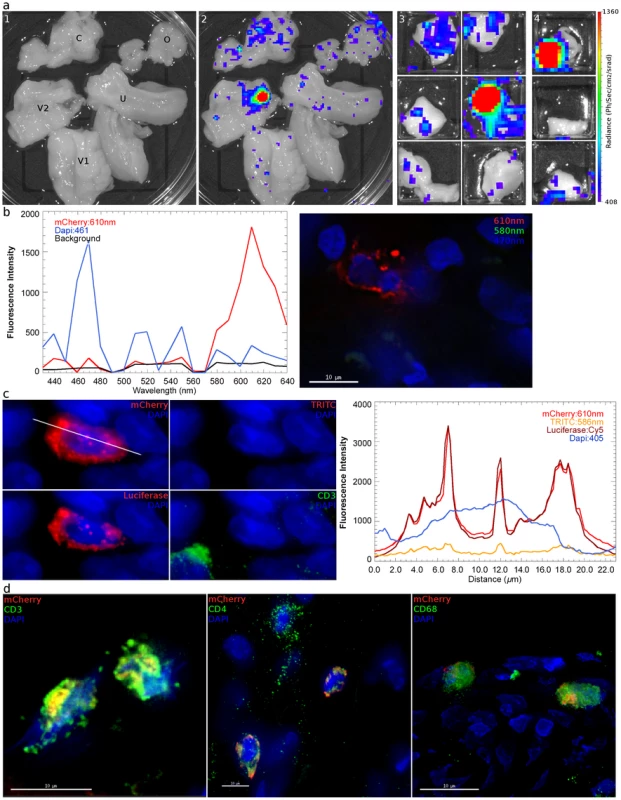
48 hours after inoculation with JRFL pseudotyped LICh vector, the reproductive tract is separated into large sections (panel 1; V1: labia and lower vagina; V2: upper vagina; C: cervix; U: uterus; O: ovary), soaked in d-luciferin to identify luciferase expressing regions with in vivo imaging systems, and dissected into sequentially smaller pieces (panels 2–4) to isolate strongly luminescent regions measuring 2×2 mm2. (animal code: EH99) b, Left panel; The spectral emission profile of a putative infected cell from the tissue identified in a is measured on laser scanning confocal microscope with resonant scanner. Emission profile matches the defined profile of mCherry. Right panel; Pseudo-colored reconstructed image on the right shows the localization of mCherry (red), DAPI (blue) and background signals (green). c, Vaginal tissue is stained for firefly luciferase expression. The combination of mCherry+ (red), TRITC−/low (red), luciferase+ (red) fluorescence is criteria for confirming infection. The right panel shows relative fluorescence intensity of mCherry, TRITC, luciferase, and nucleus is along the line in mCherry image. (animal code: HP79) d, Phenotyping of infected cells by surface marker staining, shown in green, for CD3 (T Cell), CD4 (HIV-1 primary receptor), or CD68 (tissue resident macrophages) shows all conventionally defined HIV susceptible cell types are transduced within the vaginal tissue shown in a. (animal code: EH99). Tab. 2. Localization of luciferase expression and microscopic confirmation of infection in 8 JRFL pseudotyped inoculated Rhesus Macaques. 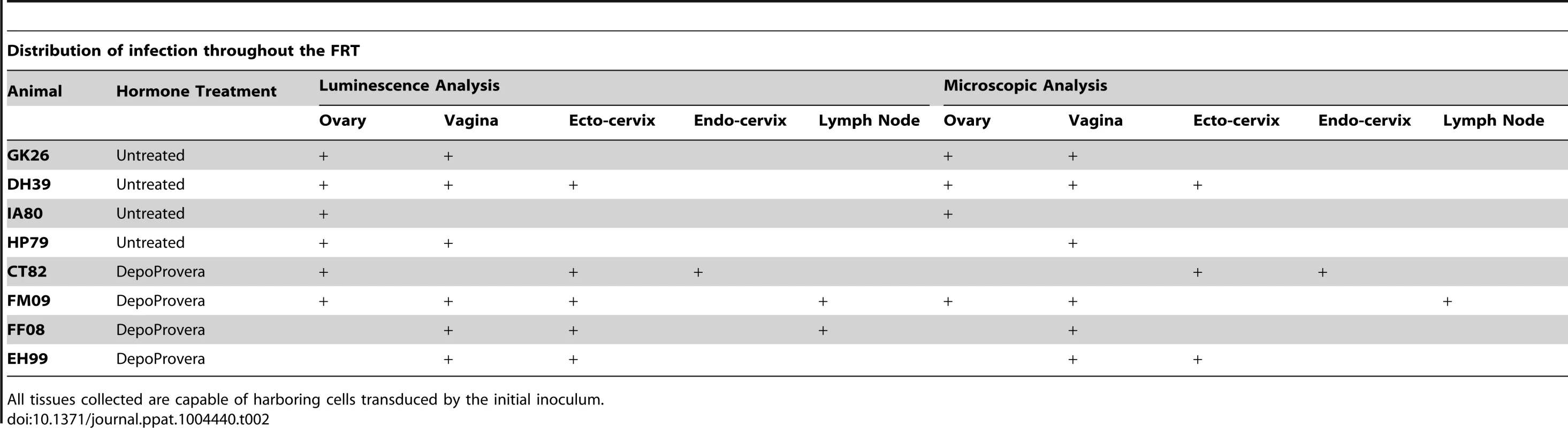
All tissues collected are capable of harboring cells transduced by the initial inoculum. Regions of the FRT expressing luminescent signal after vaginal exposure to JRFL pseudotyped vector were cryosectioned to identify the presence of infected cells by spectral imaging and fluorescence microscopy. The measured emission spectra of infected cells peaked at 609 nm (Figure 4b), confirming expression of the mCherry fluorescent protein in transduced cells. Immunofluorescence staining of tissue sections revealed that the mCherry expressing cells were also positive for luciferase expression. Because spectral imaging could not be applied to all potentially transduced cells, a surrogate of fluorescence spectra was achieved by measuring whether cells express a signal in the TRITC filters, which correspond to 586 nm (Figure 4c, d). In this manner, transduced cells were identified by 3 distinct imaging criteria. Infected cells were found within all JRFL inoculated animals. Tissues where luminescent signal could not be confirmed were typically due to high levels of background autofluorescence that prevented identification of mCherry expression.
We then examined the cellular phenotype of the JRFL pseudotyped reporter transduced cells by staining for CD4 (HIV-1 receptor), CD3 (T cell specific) or CD68 (macrophage specific). We find that both CD4 T cells and macrophages are capable of being infected within the same vaginal domain (Figure 4d). The JRFL pseudotyped vector universally transduced cells expressing CD4, although expression levels vary. This is likely a consequence of the known variability of CD4 expression between monocytic lineages and lymphocytes [23]. Additionally, activation status can also influence CD4 expression levels. CD3 expression was present on the majority of target cells, indicating that T cells were the preferred targets of transduction, even though the JRFL Envelope is defined as macrophage-tropic (Table 3). A similar preference for infection of T cells was observed whether the animals received Depo-Provera treatment or not. Considering transduced cells across all 8 JRFL inoculated animals, T cells were the dominant targets, representing 71% of all transduced cells. A smaller subset of infected cells was found to express CD68 revealing that tissue resident macrophages can also be transduced after vaginal inoculation. Non-specific primary antibody and secondary antibody only controls were used to establish the specificity of each cell marker used for phenotyping (Figure S4).
Tab. 3. Phenotype of SIV infected cells after inoculation JRFL pseudotyped virions. 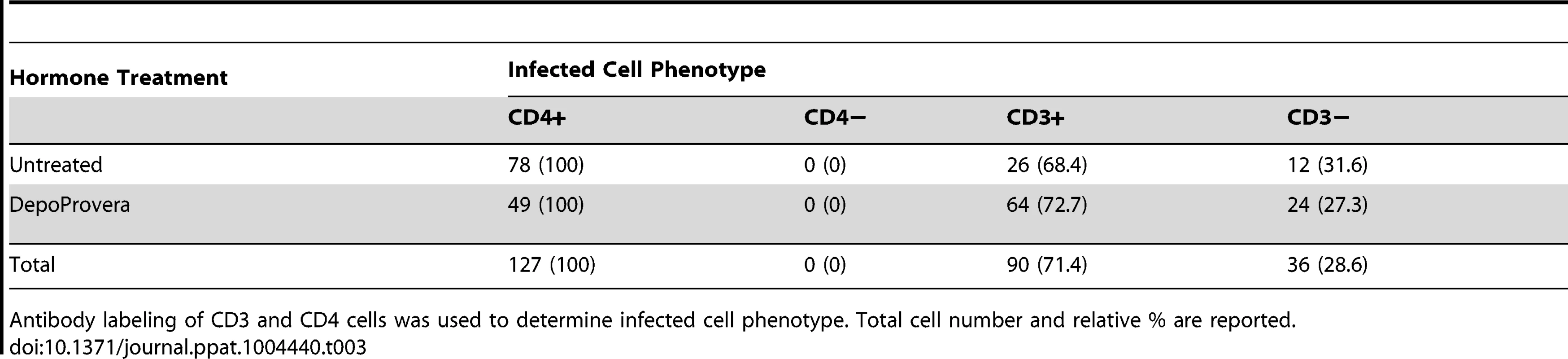
Antibody labeling of CD3 and CD4 cells was used to determine infected cell phenotype. Total cell number and relative % are reported. Throughout the vaginal vault, where viral challenge was administered, transduced cells were consistently located in the lamina propria below the basal epithelium (Figure 5). The ectocervix and vagina were the most common location where infected cells were found, with foci identified in all but one animal challenged with JRFL pseudotyped vector. Luminescent signal and microscopic identification of transduced cells were generally the strongest at biopsy sites, where virions could bypass mucosal barriers. However, biopsy sites represented only a minority of the foci identified within the vaginal vault. In regions where the stratified epithelium was intact, distal from a biopsy, transduction events were found in both T cells (Figure 5, Region 2) and non-T cells (Figure 5, Regions 1, 3). Reporter virions are therefore able to penetrate the squamous epithelium and transduce susceptible cells resident below an unbroken vaginal epithelium. The epithelial thickness and lack of a keratinized epithelium in this animal is consistent with previous descriptions of the RM vaginal epithelium in the luteal phase of the menstrual cycle [24].
Fig. 5. Vaginal tissue with intact epithelium is the most common target for target cell transduction. 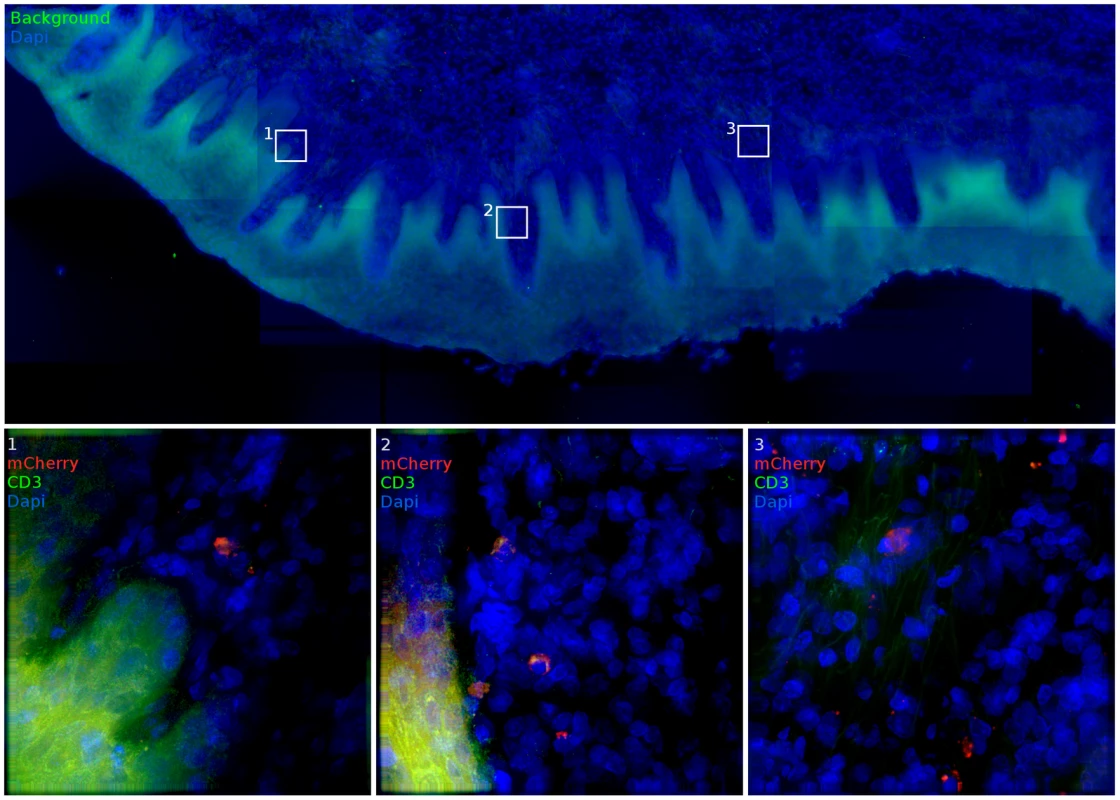
48 hours post JRFL pseudotyped vector administration, the FRT was removed and luminescent foci identified by IVIS analysis (inset). In vaginal domains without biopsy, the stratified epithelium is intact. In these portions of the vaginal vault, infected cells can still be found. mCherry expression (red), CD3 staining (green) and nuclei label (blue) are used to show transduced cells. (animal code: GK26). Further into the FRT, we find infection was also possible within the endocervix, consistent with previous studies that have focused on this site. Only 1 out of the 8 animals had evidence of infection by luminescence analysis of endocervical tissue. However, in animal CT82, a focus containing many transduced cells was identified (Figure 6). The luminescent signal from this tissue was modest, potentially due to mucus produced in the endocervical crypts preventing efficient diffusion of luciferin. It is also possible that foci of infection are rare in the endocervix. Both T cells (Regions 1, 3, 4, 5, 6) and other target cells (Regions 1, 2, 6) could be infected in close proximity. The endocervix had many T cells present, with only a simple columnar epithelium separating them from the luminal surface. The transformation zone, where the epithelial architecture changes from stratified epithelium of ectocervical tissue (Regions 4–6) to simple columnar epithelium of endocervical tissue and the upper FRT (Regions 1–3) did not have a uniquely dense population of target cells transduced relative to other regions identified throughout the FRT where transduction occurred. No breaks in the epithelium were present in the focus of infection within the endocervix.
Fig. 6. Diverse cell types are infected within the cervix. 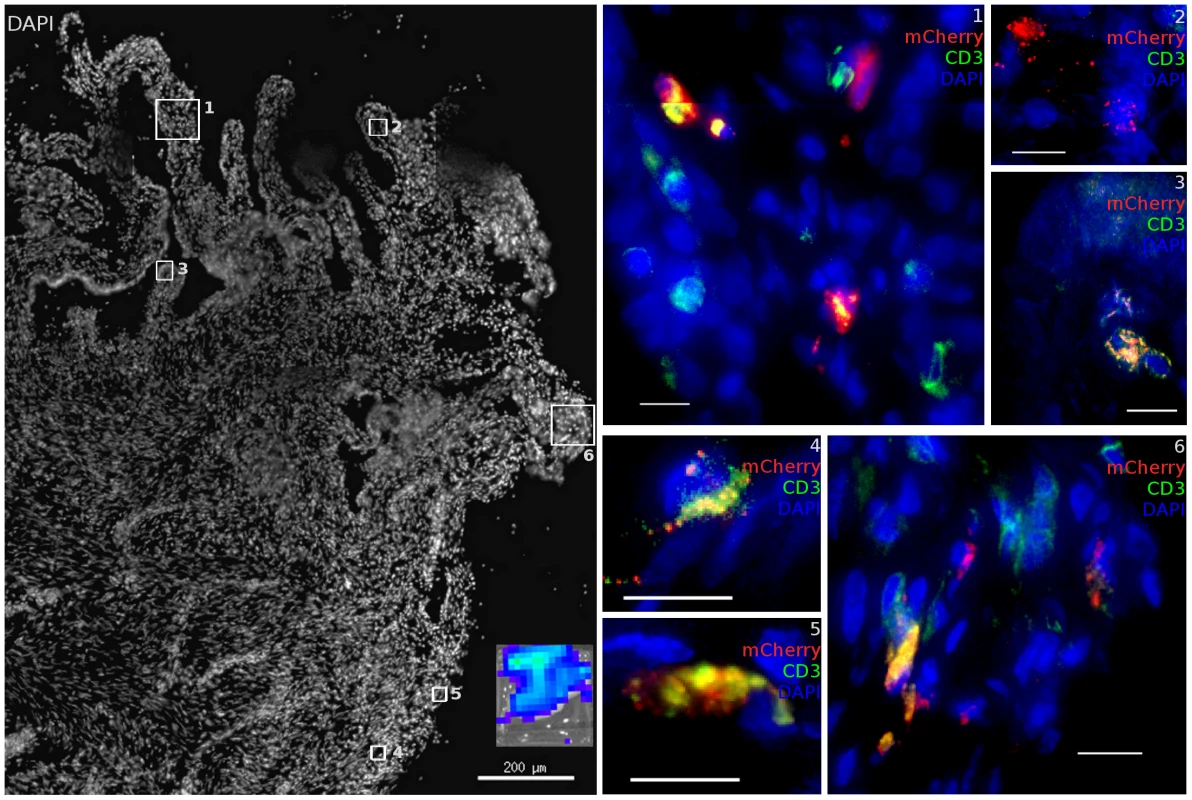
Within the cervix, both endocervical (Region 1–3) and ectocervical (Region 4–6) domains in the area of the transformation zone contain many resident T cells which are susceptible to infection with JRFL pseudotyped LICh vector. Non-T cells are also infected. Inset shows luciferase expression within dissected tissue before freezing. Scale bars measure 10 µm unless otherwise specified. mCherry expression (red), CD3 staining (green) and nuclei label (blue) are used to show transduced cells. (animal code: CT82). Although no luminescent signal was detected in the uterus or oviduct, we did find that virus was able to localize to and transduce cells in the ovary. A luminescent signal was identified in ovarian tissue of 6 out of 8 animals. When ovarian tissue was stained for CD3, 79% of transduced cells found within the ovary are found to be T cells, a further bias towards infection of T cells than is seen on the whole of the FRT. Transduced cells were primarily present in stromal tissue of the cortical zones of the ovary, proximal to developing follicles although not seen in corpus albicans structures. The high density of blood and lymphatic vessels in the helus does not have a corresponding increase in the density of transduction. As seen in Figure 7 regions 1 and 2, close proximity to a blood vessel was not associated with transduced cell localization; the helus and medullary zone rarely had any transduced cells present. More detailed imaging of these cells (Regions 3, 4) shows a typical lymphocyte morphology associated with the infected T cells. Furthermore, RMs did not have to be experiencing a regular menstrual cycle or have recently ovulated for virions to access the ovary, as animal FM09 was treated with Depo-Provera and still had transduced cells identified. 3 of the 4 regularly cycling RMs had transduction events as confirmed by identification of mCherry positive single cells. T cell clustering at the sites where foci of infection, as was found in the lower FRT, did not take place to the same extent in the ovary. Instead, more diffuse distribution of transduction occurred, with transduced cells found over regions spanning the diameter of the ovary. It is not clear if this is due to higher levels of T cell migration or distinct foci of transduction.
Fig. 7. The ovary is susceptible to infection. 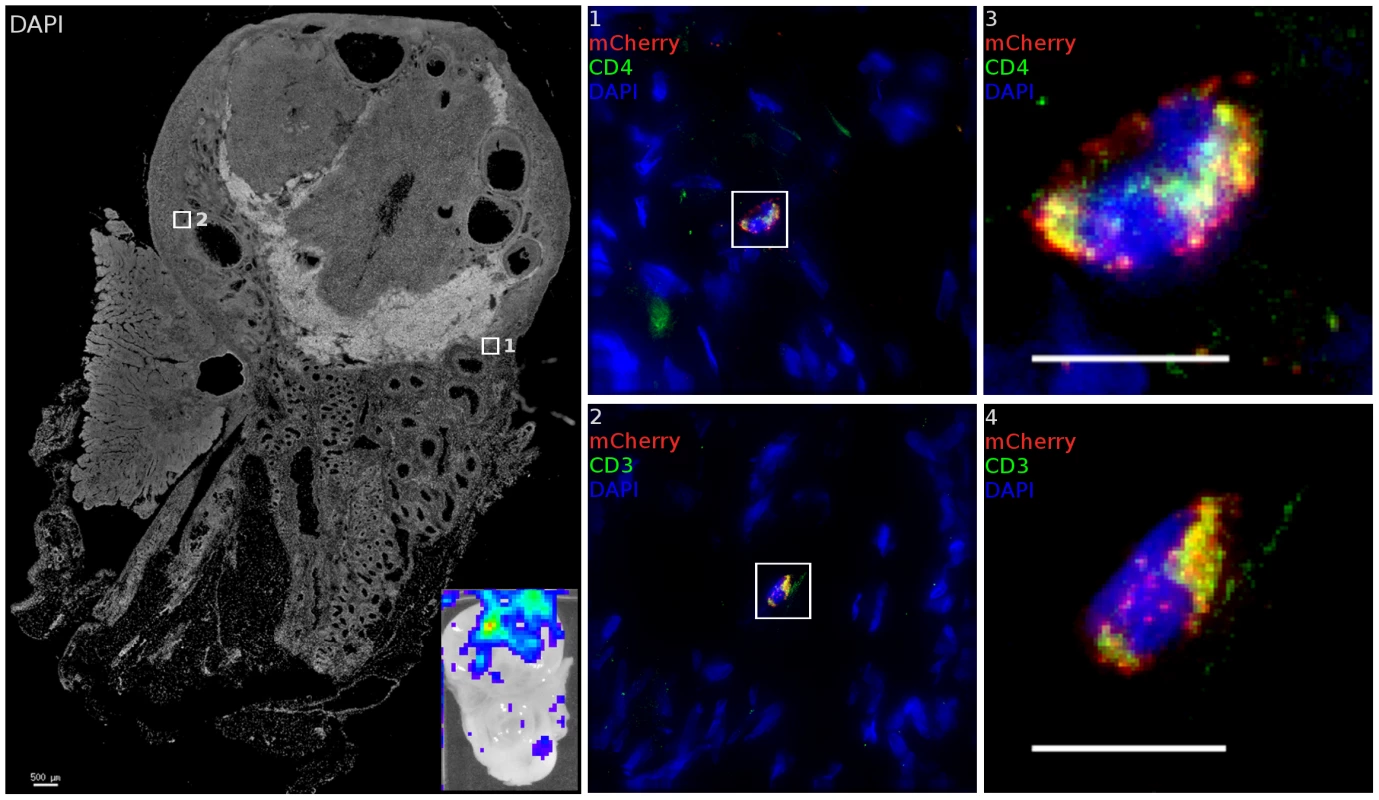
After inoculation with JRFL pseudotyped LICh vector, tissues demonstrating luminescent signal (inset) is stained for luciferase and CD3 or CD4. Inset shows luciferase expression within tissue before freezing. Transduced cells within each region are shown as numbered close-up. Infected cells are found within the cortical zone of the ovary. Infected cells are not found within developing ova. The cell in region 1 and 3 expresses HIV-1 receptor CD4. Staining the adjacent tissue section identifies the transduced cell in region 2 and 4 as being CD3+. (animal code: GK26). Within local draining lymph nodes, only infrequent evidence of infection is found. Two animals were identified with modest luminescent signal in inguinal lymph nodes. Further examination revealed a number of CD4 cells expressing mCherry and luciferase reporters (Figure S5), but high levels of auto-fluorescent background limited the scope of examination.
To validate our findings of mCherry and luciferase reporter expression we utilized nested PCR designed to detect rare transduction events within the tissue of exposed animals. Such PCR based approaches have been previously utilized to find evidence of rare infections events within days of vaginal or oral exposure of SIV [25], [26]. Therefore, we conducted nested PCR on tissue from luciferase positive and negative tissues within the FRT of challenged animals. Tissues from unexposed animals were utilized as a negative control. Tissue was sectioned in the same manner as for fluorescence imaging, and DNA was extracted from the equivalent of 200 µm of tissue. 250 ng aliquots of input genomic DNA, corresponding to approximately 3.8×104 cells, was added per PCR reaction and at least 24 reactions were performed per tissue extract. All final products were separated on agarose gels and visualized by ethidium bromide staining. Amplification of the mCherry gene was confirmed by extracting DNA from the gel and sequencing with the inner set of primers.
Tissues that had a detectable luciferase signal corresponded to those in which there was a positive PCR signal (Figure 8). The ovary of a LICh inoculated animal that did show considerable luminescent signal in that tissue (GK26) contained multiple copies of mCherry DNA. Importantly, genomic DNA extracted from the ovaries of 2 animals not treated with the LICh virus showed no evidence of DNA encoding the mCherry gene. Therefore, the luminescent and mCherry fluorescence signals correlated with the presence of reporter DNA revealing the ability of luciferase signal to reveal sites of transduction. In contrast, DNA from uterine tissue of the same animal which showed no luciferase signal also showed no evidence of transduced DNA as detected by nested PCR. The ability of the luciferase signal to identify DNA positive tissue further validates the two-reporter vector system to identify sites of vector transduction after vaginal exposure in the rhesus macaque model. It is important to note that the PCR method also validates the unanticipated detection of transduced cells within the ovary, while apparently bypassing the uterus.
Fig. 8. Nested PCR of macaque tissues. 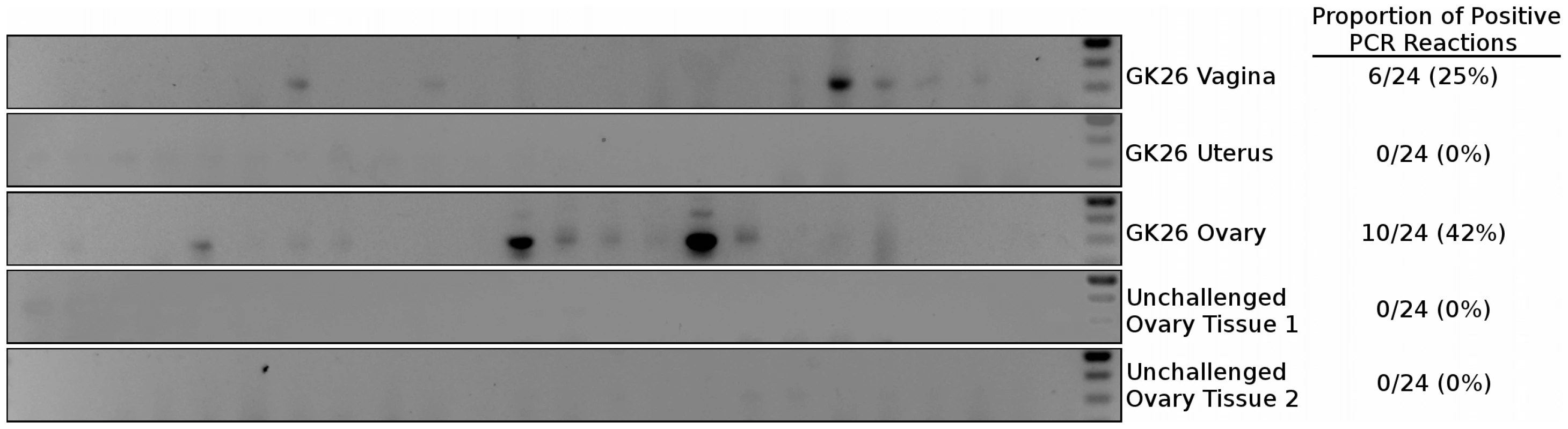
Distribution of LICh reporter DNA-positive tissues in the female reproductive tract after inoculation with JRFL pseudotyped LICh vector. Results are based on the number of mCherry positive reactions, with at least 24 reactions per experiment. Gel shown is representative of three independent DNA extractions and amplifications. To summarize, the regions of the FRT where luciferase and mCherry expressing cells were detected in the JRFL pseudotyped vector exposed RMs, varied between animals and could be identified throughout the FRT and could extend to local draining LNs (Table 2). The majority of animals had more than one site of transduced cells, revealing that there are typically multiple sites where the virus can gain access to potential target cells at the time of exposure in the rhesus macaque model. The primary site of infection was within the vaginal vault with seven of the eight animals having sites of infection identified in the vagina and/or ectocervix. The second most common site of the identification of transduced cells was the ovary. Transduced cells in the ovary were identified in half of the animals. Detection of transduced cells in the endocervix and local draining lymph nodes was rare, both being confirmed in a single animal.
Discussion
The anatomical site and phenotype of the initial cells infected by immunodeficiency viruses within the FRT is heavily debated. Previous studies using similar high virus challenges have suggested that the endocervix is a primary site of infection in a RM vaginal challenge model [8], [26]. However, these studies were limited because they were not able to survey the entire FRT and surrounding lymphatic tissue for sites of infection. In contrast, using the dual reporter system developed here, which allows a survey of the entire FRT, we find a clear preference for infection of the squamous mucosal vaginal and ectocervical barriers of the vaginal vault with a high dose challenge, although only a small fraction of tissue contains transduced cells. When surveying the entire exposed tissue of the FRT two days after inoculation, the infection within the endocervix was rarely detected. The low frequency of transduction events detected in the endocervix might be due to the limitations of the system utilized here. Because of the protective mucus barrier coating the endocervical mucosa, foci of infection may be rare. However, transduction of disperse target cells may take place at a frequency that remains to be determined.
There are disparate models of the initial cell type of infection during male-to-female transmission, with some studies implicating CD4 T cells [26], [27], others implicating Langerhans cells [28], while others report mixed pools of target cells [29], [30]. Using the JRFL envelope, which has broad ability to infect different types of target cells in tissue culture experiments, we detect a preference for the infection of CD4 T cells in the different anatomical sites. Infection of CD4 T cells was detected approximately 70% of the time. However, the vector tropism observed here should be interpreted with caution because JRFL represents a lab adapted strain with a tropism of an HIV derivative isolated from the brain of a chronically infected individual, and may be biased away from the primarily T cell tropic nature of transmitted/founder viruses. Future studies will utilize envelopes from so called transmitted/founder viruses to gain insight into the initial targets of infection in the FRT. However, these initial observations reveal that a variety of immune cell types can be the targets of the initial inoculum of exposure during the transmission event.
While some have suggested that target cells in the upper FRT (uterus) might be sites of transmission [31], the possibility of infection of target cells in the ovary and lymph nodes after exposure has not been considered. It is known that potential target cells are present in the ovary and that hormonal changes associated with the menstrual cycle can modulate both the number and localization of immune cells. The ability of the virus to travel far enough to reach the ovary was unanticipated, but consistent with evidence that materials within the vaginal vault and uterus can migrate to the ovaries. For example, there is a known association between perineal talc use and ovarian cancer risk [32]–[34]. Additionally, retrograde menstruation through the fallopian tubes is commonly reported in females, even in the absence of endometriosis, but never associated with dissemination of pathogens [35], [36]. Likely the mechanism(s) behind this undefined retrograde transport is also influencing virus transport to the ovaries in this model. The ability of the ovary to be the portal of transmission remains to be defined. However, the studies presented here demonstrate that particles from a high titer vaginal inoculation can reach target cells present in this tissue.
The mechanism by which infected cells are present in the lymph node is not apparent; vaginal or rectal inoculation could lead to virions accessing draining lymph nodes. Because the kinetics of expression require a day post-entry for reporters to be detectable, trans-infection of resident T cells by migrating DCs carrying virions to lymph nodes is unlikely, though a possibility at later time points [37]. Migration of infected cells or virions directly entering the lymphatic drainage allows a sufficient time window for detectable reporter expression within lymph nodes after 48 hours.
The distribution of sites transduced by either the VSV-G or JRFL pseudotyped vectors was quite similar. Both were able to reach the ovaries, and typically were observed to breach mucosal barriers in discrete foci. Yet the two different vectors reflected very different and anticipated tropism. This indicates that the distribution of viral particles in the FRT after vaginal exposure is not strongly influenced by envelope tropism. Throughout most of the reproductive tract, the mucosal barriers functioned to exclude virions and prevent infection from occurring, but where the barriers were overcome, there were multiple cells being infected. It is also notable that T cells are abundant where infection occurs, irrespective of the envelope protein (Figure 3). Where mucosa is locally inflamed, such as a site of thinned or broken epithelium, increased numbers of immune cells are recruited [38], [39]. The large numbers of T cells seen where VSV-G pseudotyped virions have infected sub-basal cells are not recruited in response to viral replication, as only a single round of infection occurs. Instead, preexisting target cell clusters associated with functional defects in the mucosal barriers may represent sites of vulnerability.
The phase of the menstrual cycle was not controlled as a part of this study, but could play a significant role in viral acquisition [40], [41]. Animal GK26, which had infection in the vagina (Figure 5) and ovary (Figure 7), had a vaginal epithelial structure representative of the luteal phase. The lack of infection found in the uterus of this animal either by IVIS, or nested PCR is consistent with the lack of CD4+ T cells and macrophages in the uterine endometrium late in the menstrual cycle [38], [42], [43]. However, the possibility of rare transduction events occurring cannot be ruled out as it is not possible to test all uterine tissue for single transduced cells.
These findings on the wide range of locations and cell types susceptible to SIV have fundamental implications for development of prevention strategies. The sites of initial infection are not restricted to specific domains within the FRT tract and are not consistent between RMs. The observed abundant infection within the macaque vaginal vault is consistent with the failure of the diaphragm to protect women from HIV acquisition [44]. Further, we find a high density of target cells where virus enters the epithelium, suggesting that preexisting sites of local inflammation represent hotspots of vulnerability to infection by the initial inoculum. This is consistent with the known association of increased susceptibility to HIV infection with increased local inflammation. To better protect against female acquisition of HIV, it is important that vaccine or antiviral activity be widely distributed throughout the FRT architecture. The development of HIV preventions strategies should consider the upper FRT and resident vulnerable cell types as acquisition targets during male-to-female transmission.
Materials and Methods
Ethics statement
All animal studies were conducted in accordance with protocols approved by Northwestern University and Tulane National Primate Research Center local Institutional Animal Care and Use Committees (IACUC), protocol P0153. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH) and with the recommendations of the Weatherall report; “The use of non-human primates in research”. All procedures were performed under anesthesia using ketamine hydrochloride, and all efforts were made to minimize stress, improve housing conditions, and to provide enrichment opportunities (e.g., objects to manipulate in cage, varied food supplements, foraging and task-oriented feeding methods, interaction with caregivers and research staff). Animals were euthanized by ketamine hydrochloride injection in accordance with the recommendations of the panel on Euthanasia of the American Veterinary Medical Association.
Cell culture and infection
293T cells (American Type Culture Collection) were cultured in Dulbecco's modified Eagle's medium (HyClone) containing 10% fetal bovine serum, 100 Um L−1 penicillin, 100 µg mL−1 streptomycin and 292 µg mL−1 l-glutamine (Gibco). Rhesus macaque peripheral blood mononuclear cells (PBMC) were separated over Ficoll gradient and cultured at 2.5×106 cells mL−1 in RPMI containing 10% fetal bovine serum, 100 U mL−1 penicillin, 100 µg mL−1 streptomycin and 292 µg mL−1 l-glutamine. 24 hours prior to infection with vector, cells were activated with 100 IU ml−1 IL-2 and 5 µg mL−1 Phytohaemagglutinin (PHA). 1000 TCID50 JRFL pseudotyped vector was added to activated PBMC. Infected cells were sorted for mCherry expression on a Beckman Coulter MoFlo system.
Dual reporter vector and virus production
A SIV-based pseudovirus vector system was generated by modification of the SIV3 vector system [14]. The codon-optimized firefly luciferase gene is expressed through a poliovirus internal ribosome entry site (IRES) [16]. mCherry was chosen as it has unique narrow emission spectrum (610 nm) that is not known to exist naturally. The transcription of mCherry and luciferase reporters are driven from the constitutive immediate-early CMV promoter and their expression is stimulated by WPRE to facilitate robust expression. Vector design is shown in Figure 1. Pseudotyped reporter virus is produced by transfection of 293T cells with 4 plasmids complexed with Polyethyleneimine (PEI, Polysciences): reporter described above, SIV3+ packaging vector, REV expression plasmid DM121 and either a VSV-G envelope or JRFL (HIV-1 CCR5 tropic) envelope. Viral supernatants containing pseudotyped virus were collected approximately 48 hours post-transfection, purified through 0.22 µm filters and concentrated over 30% sucrose cushions. Concentrated virus was titred for infectivity (TCID50) on TZM-bl cells as previously described and stored at −80°C until use [45]. TCID50 ranged from 104.8–106.2 for JRFL pseudotyped virions and 105.4–106.3 for VSV-G pseudotyped virions.
Non-human primate studies
In total 14 female rhesus macaques (Macaca mulatta; RM) were used in this study, 5 of which have had one offspring and 8 of which are multiparous. Animals treated with Depo-Provera (DP) were given a single 30 mg dose one month prior to viral inoculation. All animals received a vaginal biopsy in the upper vagina/fornix, and were subsequently atraumatically inoculated vaginally and rectally with virus supernatant. Groups of 6 (2 DP Treated) and 8 RMs (4 DP Treated) were inoculated with VSV-G or JRFL Env expressing virions, respectively. Animals were sacrificed 48 hours after inoculation and their genital tracts were removed, stored in RPMI and shipped on ice overnight for further processing. This timeframe was chosen because it was the shortest time interval in which we could reliably detect reporter expression. Whole reproductive tracts, inguinal, and iliac lymph nodes were washed in PBS, then soaked in 100 mM d-Luciferin (Biosynth) and placed in the IVIS device to examine luciferase expression. Reproductive tract tissue was next divided in 5 regions: labia and lower vagina, upper vagina/fornix, cervix, uterus and ovaries and reimaged. Foci of luminescence detected in the second screen were excised and examined again for luciferase expression. Domains with luciferase detected in the third screen were cut to 2×2 mm2 pieces and frozen in optimal cutting temperature (OCT) compound. All analysis of luminescent signals was performed on Living Image Software.
Nested PCR of mCherry gene
Genomic DNA was isolated from 3 to 5 mg of frozen tissue using the Qaigen DNeasy Blood & Tissue Kit (Qaigen N.V., Valencia, California, USA). In each nested PCR reaction, 250 ng of genomic DNA was utilized to detect a 268-bp DNA fragment of the mCherry reporter gene using an amplification procedure based on previously described methodology [25]. The first round primers were 5′-ATAACATGGCCATCATCAAGGAGT-3′ (forward) and 5′-GTACTGTTCCACGATGGTGTAGTC-3′ (reverse). In the second round, 2 µl of the first round reaction products were amplified with 5′-CCGACTACTTGAAGCTGTCCTT-3′ (forward) and 5′-GTCTTGACCTCAGCGTCGTAGT-3′ (reverse). Amplification was performed using a Bio-Rad iCycler Thermal Cycler system (Bio-Rad Laboratories, Hercules, CA, USA). Each DNA sample was tested in ≥24 replicates. Negative controls were rhesus macaque ovary tissue from animals not exposed to the mCherry containing reporter vector. Second round PCR products were separated on a 1.5% agarose gel and visualized by ethidium bromide staining. Sequences were confirmed by extracting DNA with Qaigen QIAquick Gel Extraction Kit analysis with the second round primers.
Tissue staining and microscopy
Cryosections of tissue (20 micron thickness) were fixed in 1% formaldehyde in PIPES buffer. To decrease background, tissue was treated with 100 mM L-Lysine. Tissue was stained for luciferase expression with polyclonal rabbit anti-firefly (Abcam), pre-labeled with Xenon AlexaFluor-647 (Invitrogen). Phenotyping target cells was achieved by blocking with donkey serum and subsequently staining with either rabbit anti-human CD3 (clone SP7, Abcam) followed by AlexaFluor488 conjugated donkey anti-rabbit Ig (Jackson Immuno), mouse anti-human CD4 (clone OKT4, hybridoma supernatant) followed by AlexaFluor488 conjugated donkey anti-mouse Ig (Invitrogen), or mouse anti-human CD68 (clone EBM11, Dako) followed by AlexaFluor488 conjugated donkey anti-mouse Ig. Spectral imaging of mCherry expression was performed on a Nikon A1R laser scanning confocal microscope equipped with a 60× objective and Nikon Elements Software. 5 color (DAPI, AlexaFluor488, TRITC, mCherry, AlexaFluor647) image stacks containing 20–40 sections in the Z plane in 0.5 µm steps were acquired and deconvolved using softWoRx software (Applied Precision) on a DeltaVision inverted microscope.
Supporting Information
Zdroje
1. Joint United Nations Programme on HIV/AIDS. (2010) Global report: UNAIDS report on the global AIDS epidemic. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS. pp. v.
2. HaynesBF, McElrathMJ (2013) Progress in HIV-1 vaccine development. Curr Opin HIV AIDS 8 : 326–332.
3. ShattockRJ, MooreJP (2003) Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol 1 : 25–34.
4. HaaseAT (2011) Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med 62 : 127–139.
5. KeeleBF, EstesJD (2011) Barriers to mucosal transmission of immunodeficiency viruses. Blood 118 : 839–846.
6. StoneM, KeeleBF, MaZM, BailesE, DutraJ, et al. (2010) A limited number of simian immunodeficiency virus (SIV) env variants are transmitted to rhesus macaques vaginally inoculated with SIVmac251. J Virol 84 : 7083–7095.
7. MillerCJ, LiQ, AbelK, KimEY, MaZM, et al. (2005) Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol 79 : 9217–9227.
8. LiQ, EstesJD, SchlievertPM, DuanL, BrosnahanAJ, et al. (2009) Glycerol monolaurate prevents mucosal SIV transmission. Nature 458 : 1034–1038.
9. GrantRM, LamaJR, AndersonPL, McMahanV, LiuAY, et al. (2010) Preexposure Chemoprophylaxis for HIV Prevention in Men Who Have Sex with Men. New England Journal of Medicine 363 : 2587–2599.
10. Abdool KarimQ, Abdool KarimSS, FrohlichJA, GroblerAC, BaxterC, et al. (2010) Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329 : 1168–1174.
11. J Marrazzo GR, Nair G, et al.. (2013) Pre-exposure prophylaxis for HIV in women: daily oral tenofovir, oral tenofovir/emtricitabine or vaginal tenofovir gel in the VOICE study (MTN 003). Conference on Retroviruses and Opportunistic Infections Goergia World Congress Centre, Atlanta.
12. FriendDR, KiserPF (2013) Assessment of topical microbicides to prevent HIV-1 transmission: Concepts, testing, lessons learned. Antiviral Research 99 : 391–400.
13. Van DammeL, CorneliA, AhmedK, AgotK, LombaardJ, et al. (2012) Preexposure Prophylaxis for HIV Infection among African Women. N Engl J Med
14. NegreD, MangeotPE, DuisitG, BlanchardS, VidalainPO, et al. (2000) Characterization of novel safe lentiviral vectors derived from simian immunodeficiency virus (SIVmac251) that efficiently transduce mature human dendritic cells. Gene Ther 7 : 1613–1623.
15. ShanerNC, CampbellRE, SteinbachPA, GiepmansBN, PalmerAE, et al. (2004) Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol 22 : 1567–1572.
16. RabinovichBA, YeY, EttoT, ChenJQ, LevitskyHI, et al. (2008) Visualizing fewer than 10 mouse T cells with an enhanced firefly luciferase in immunocompetent mouse models of cancer. Proc Natl Acad Sci U S A 105 : 14342–14346.
17. KoyanagiY, MilesS, MitsuyasuRT, MerrillJE, VintersHV, et al. (1987) Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science 236 : 819–822.
18. CroninJ, ZhangXY, ReiserJ (2005) Altering the tropism of lentiviral vectors through pseudotyping. Curr Gene Ther 5 : 387–398.
19. ZuffereyR, DonelloJE, TronoD, HopeTJ (1999) Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J Virol 73 : 2886–2892.
20. DonelloJE, LoebJE, HopeTJ (1998) Woodchuck hepatitis virus contains a tripartite posttranscriptional regulatory element. J Virol 72 : 5085–5092.
21. DurandS, CimarelliA (2011) The inside out of lentiviral vectors. Viruses 3 : 132–159.
22. MarxPA, SpiraAI, GettieA, DaileyPJ, VeazeyRS, et al. (1996) Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med 2 : 1084–1089.
23. LeeB, SharronM, MontanerLJ, WeissmanD, DomsRW (1999) Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc Natl Acad Sci U S A 96 : 5215–5220.
24. PooniaB, WalterL, DufourJ, HarrisonR, MarxPA, et al. (2006) Cyclic changes in the vaginal epithelium of normal rhesus macaques. J Endocrinol 190 : 829–835.
25. MilushJM, KosubD, MarthasM, SchmidtK, ScottF, et al. (2004) Rapid dissemination of SIV following oral inoculation. AIDS 18 : 2371–2380.
26. ZhangZ, SchulerT, ZupancicM, WietgrefeS, StaskusKA, et al. (1999) Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science 286 : 1353–1357.
27. MillerCJ, ShattockRJ (2003) Target cells in vaginal HIV transmission. Microbes Infect 5 : 59–67.
28. SpiraAI, MarxPA, PattersonBK, MahoneyJ, KoupRA, et al. (1996) Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med 183 : 215–225.
29. HladikF, SakchalathornP, BallweberL, LentzG, FialkowM, et al. (2007) Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity 26 : 257–270.
30. PandreaI, ParrishNF, RaehtzK, GaufinT, BarbianHJ, et al. (2012) Mucosal simian immunodeficiency virus transmission in African green monkeys: susceptibility to infection is proportional to target cell availability at mucosal sites. J Virol 86 : 4158–4168.
31. WiraCR, FaheyJV (2008) A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS 22 : 1909–1917.
32. CramerDW, WelchWR, BerkowitzRS, GodleskiJJ (2007) Presence of talc in pelvic lymph nodes of a woman with ovarian cancer and long-term genital exposure to cosmetic talc. Obstetrics and gynecology 110 : 498–501.
33. RosenblattKA, WeissNS, Cushing-HaugenKL, WicklundKG, RossingMA (2011) Genital powder exposure and the risk of epithelial ovarian cancer. Cancer Causes Control 22 : 737–742.
34. CookLS, KambML, WeissNS (1997) Perineal powder exposure and the risk of ovarian cancer. Am J Epidemiol 145 : 459–465.
35. BlumenkrantzMJ, GallagherN, BashoreRA, TenckhoffH (1981) Retrograde menstruation in women undergoing chronic peritoneal dialysis. Obstetrics and gynecology 57 : 667–670.
36. HalmeJ, HammondMG, HulkaJF, RajSG, TalbertLM (1984) Retrograde menstruation in healthy women and in patients with endometriosis. Obstetrics and gynecology 64 : 151–154.
37. MasurierC, SalomonB, GuettariN, PiocheC, LachapelleF, et al. (1998) Dendritic cells route human immunodeficiency virus to lymph nodes after vaginal or intravenous administration to mice. J Virol 72 : 7822–7829.
38. GhanemKG, ShahN, KleinRS, MayerKH, SobelJD, et al. (2005) Influence of sex hormones, HIV status, and concomitant sexually transmitted infection on cervicovaginal inflammation. J Infect Dis 191 : 358–366.
39. RotchfordK, StrumAW, WilkinsonD (2000) Effect of coinfection with STDs and of STD treatment on HIV shedding in genital-tract secretions: systematic review and data synthesis. Sex Transm Dis 27 : 243–248.
40. VishwanathanSA, GuenthnerPC, LinCY, DobardC, SharmaS, et al. (2011) High susceptibility to repeated, low-dose, vaginal SHIV exposure late in the luteal phase of the menstrual cycle of pigtail macaques. J Acquir Immune Defic Syndr 57 : 261–264.
41. KershEN, HenningT, VishwanathanSA, MorrisM, ButlerK, et al. (2014) SHIV susceptibility changes during the menstrual cycle of pigtail macaques. J Med Primatol [epub ahead of print]..
42. AkiyamaM, OkabeH, TakakuraK, FujiyamaY, NodaY (1999) Expression of macrophage inflammatory protein-1alpha (MIP-1alpha) in human endometrium throughout the menstrual cycle. Br J Obstet Gynaecol 106 : 725–730.
43. KallikourdisM, AndersenKG, WelchKA, BetzAG (2007) Alloantigen-enhanced accumulation of CCR5+ ‘effector’ regulatory T cells in the gravid uterus. Proc Natl Acad Sci U S A 104 : 594–599.
44. PadianNS, van der StratenA, RamjeeG, ChipatoT, de BruynG, et al. (2007) Diaphragm and lubricant gel for prevention of HIV acquisition in southern African women: a randomised controlled trial. Lancet 370 : 251–261.
45. WeiX, DeckerJM, LiuH, ZhangZ, AraniRB, et al. (2002) Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother 46 : 1896–1905.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Identification of the Microsporidian as a New Target of the IFNγ-Inducible IRG Resistance SystemČlánek Human Cytomegalovirus Drives Epigenetic Imprinting of the Locus in NKG2C Natural Killer CellsČlánek APOBEC3D and APOBEC3F Potently Promote HIV-1 Diversification and Evolution in Humanized Mouse ModelČlánek Role of Non-conventional T Lymphocytes in Respiratory Infections: The Case of the Pneumococcus
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 10- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Theory and Empiricism in Virulence Evolution
- -Related Fungi and Reptiles: A Fatal Attraction
- Adaptive Prediction As a Strategy in Microbial Infections
- Antimicrobials, Stress and Mutagenesis
- A Novel Function of Human Pumilio Proteins in Cytoplasmic Sensing of Viral Infection
- Social Motility of African Trypanosomes Is a Property of a Distinct Life-Cycle Stage That Occurs Early in Tsetse Fly Transmission
- Autophagy Controls BCG-Induced Trained Immunity and the Response to Intravesical BCG Therapy for Bladder Cancer
- Identification of the Microsporidian as a New Target of the IFNγ-Inducible IRG Resistance System
- mRNA Structural Constraints on EBNA1 Synthesis Impact on Antigen Presentation and Early Priming of CD8 T Cells
- Infection Causes Distinct Epigenetic DNA Methylation Changes in Host Macrophages
- Neutrophil Crawling in Capillaries; A Novel Immune Response to
- Live Attenuated Vaccine Protects against Pulmonary Challenge in Rats and Non-human Primates
- The ESAT-6 Protein of Interacts with Beta-2-Microglobulin (β2M) Affecting Antigen Presentation Function of Macrophage
- Characterization of Uncultivable Bat Influenza Virus Using a Replicative Synthetic Virus
- HIV Acquisition Is Associated with Increased Antimicrobial Peptides and Reduced HIV Neutralizing IgA in the Foreskin Prepuce of Uncircumcised Men
- Uses a Unique Ligand-Binding Mode for Trapping Opines and Acquiring A Competitive Advantage in the Niche Construction on Plant Host
- Involvement of a 1-Cys Peroxiredoxin in Bacterial Virulence
- Ethanol Stimulates WspR-Controlled Biofilm Formation as Part of a Cyclic Relationship Involving Phenazines
- Densovirus Is a Mutualistic Symbiont of a Global Crop Pest () and Protects against a Baculovirus and Bt Biopesticide
- Insights into Intestinal Colonization from Monitoring Fluorescently Labeled Bacteria
- Mycobacterial Antigen Driven Activation of CD14CD16 Monocytes Is a Predictor of Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome
- Lipoprotein LprG Binds Lipoarabinomannan and Determines Its Cell Envelope Localization to Control Phagolysosomal Fusion
- Dampens the DNA Damage Response
- MicroRNAs Suppress NB Domain Genes in Tomato That Confer Resistance to
- Novel Cyclic di-GMP Effectors of the YajQ Protein Family Control Bacterial Virulence
- Vaginal Challenge with an SIV-Based Dual Reporter System Reveals That Infection Can Occur throughout the Upper and Lower Female Reproductive Tract
- Detecting Differential Transmissibilities That Affect the Size of Self-Limited Outbreaks
- One Small Step for a Yeast - Microevolution within Macrophages Renders Hypervirulent Due to a Single Point Mutation
- Expression Profiling during Arabidopsis/Downy Mildew Interaction Reveals a Highly-Expressed Effector That Attenuates Responses to Salicylic Acid
- Human Cytomegalovirus Drives Epigenetic Imprinting of the Locus in NKG2C Natural Killer Cells
- Interaction with Tsg101 Is Necessary for the Efficient Transport and Release of Nucleocapsids in Marburg Virus-Infected Cells
- The N-Terminus of Murine Leukaemia Virus p12 Protein Is Required for Mature Core Stability
- Sterol Biosynthesis Is Required for Heat Resistance but Not Extracellular Survival in
- Allele-Specific Induction of IL-1β Expression by C/EBPβ and PU.1 Contributes to Increased Tuberculosis Susceptibility
- Host Cofactors and Pharmacologic Ligands Share an Essential Interface in HIV-1 Capsid That Is Lost upon Disassembly
- APOBEC3D and APOBEC3F Potently Promote HIV-1 Diversification and Evolution in Humanized Mouse Model
- Structural Basis for the Recognition of Human Cytomegalovirus Glycoprotein B by a Neutralizing Human Antibody
- Systematic Analysis of ZnCys Transcription Factors Required for Development and Pathogenicity by High-Throughput Gene Knockout in the Rice Blast Fungus
- Epstein-Barr Virus Nuclear Antigen 3A Promotes Cellular Proliferation by Repression of the Cyclin-Dependent Kinase Inhibitor p21WAF1/CIP1
- The Host Protein Calprotectin Modulates the Type IV Secretion System via Zinc Sequestration
- Cyclophilin A Associates with Enterovirus-71 Virus Capsid and Plays an Essential Role in Viral Infection as an Uncoating Regulator
- A Novel Alpha Kinase EhAK1 Phosphorylates Actin and Regulates Phagocytosis in
- The pH-Responsive PacC Transcription Factor of Governs Epithelial Entry and Tissue Invasion during Pulmonary Aspergillosis
- Sensing of Immature Particles Produced by Dengue Virus Infected Cells Induces an Antiviral Response by Plasmacytoid Dendritic Cells
- Co-opted Oxysterol-Binding ORP and VAP Proteins Channel Sterols to RNA Virus Replication Sites via Membrane Contact Sites
- Characteristics of Memory B Cells Elicited by a Highly Efficacious HPV Vaccine in Subjects with No Pre-existing Immunity
- HPV16-E7 Expression in Squamous Epithelium Creates a Local Immune Suppressive Environment via CCL2- and CCL5- Mediated Recruitment of Mast Cells
- Dengue Viruses Are Enhanced by Distinct Populations of Serotype Cross-Reactive Antibodies in Human Immune Sera
- CD4 Depletion in SIV-Infected Macaques Results in Macrophage and Microglia Infection with Rapid Turnover of Infected Cells
- A Sialic Acid Binding Site in a Human Picornavirus
- Contact Heterogeneity, Rather Than Transmission Efficiency, Limits the Emergence and Spread of Canine Influenza Virus
- Myosins VIII and XI Play Distinct Roles in Reproduction and Transport of
- HTLV-1 Tax Stabilizes MCL-1 via TRAF6-Dependent K63-Linked Polyubiquitination to Promote Cell Survival and Transformation
- Species Complex: Ecology, Phylogeny, Sexual Reproduction, and Virulence
- A Critical Role for IL-17RB Signaling in HTLV-1 Tax-Induced NF-κB Activation and T-Cell Transformation
- Exosomes from Hepatitis C Infected Patients Transmit HCV Infection and Contain Replication Competent Viral RNA in Complex with Ago2-miR122-HSP90
- Role of Non-conventional T Lymphocytes in Respiratory Infections: The Case of the Pneumococcus
- Kaposi's Sarcoma-Associated Herpesvirus Induces Nrf2 during Infection of Endothelial Cells to Create a Microenvironment Conducive to Infection
- A Relay Network of Extracellular Heme-Binding Proteins Drives Iron Acquisition from Hemoglobin
- Glutamate Secretion and Metabotropic Glutamate Receptor 1 Expression during Kaposi's Sarcoma-Associated Herpesvirus Infection Promotes Cell Proliferation
- Reduces Malaria and Dengue Infection in Vector Mosquitoes and Has Entomopathogenic and Anti-pathogen Activities
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Novel Cyclic di-GMP Effectors of the YajQ Protein Family Control Bacterial Virulence
- MicroRNAs Suppress NB Domain Genes in Tomato That Confer Resistance to
- The ESAT-6 Protein of Interacts with Beta-2-Microglobulin (β2M) Affecting Antigen Presentation Function of Macrophage
- Characterization of Uncultivable Bat Influenza Virus Using a Replicative Synthetic Virus
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



