-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMacrophage and T Cell Produced IL-10 Promotes Viral Chronicity
Chronic viral infections lead to CD8+ T cell exhaustion, characterized by impaired cytokine secretion. Presence of the immune-regulatory cytokine IL-10 promotes chronicity of Lymphocytic Choriomeningitis Virus (LCMV) Clone 13 infection, while absence of IL-10/IL-10R signaling early during infection results in viral clearance and higher percentages and numbers of antiviral, cytokine producing T cells. IL-10 is produced by several cell types during LCMV infection but it is currently unclear which cellular sources are responsible for induction of viral chronicity. Here, we demonstrate that although dendritic cells produce IL-10 and overall IL-10 mRNA levels decrease significantly in absence of CD11c+ cells, absence of IL-10 produced by CD11c+ cells failed to improve the LCMV-specific T cell response and control of LCMV infection. Similarly, NK cell specific IL-10 deficiency had no positive impact on the LCMV-specific T cell response or viral control, even though high percentages of NK cells produced IL-10 at early time points after infection. Interestingly, we found markedly improved T cell responses and clearance of normally chronic LCMV Clone 13 infection when either myeloid cells or T cells lacked IL-10 production and mice depleted of monocytes/macrophages or CD4+ T cells exhibited reduced overall levels of IL-10 mRNA. These data suggest that the decision whether LCMV infection becomes chronic or can be cleared critically depends on early CD4+ T cell and monocyte/macrophage produced IL-10.
Published in the journal: . PLoS Pathog 9(11): e32767. doi:10.1371/journal.ppat.1003735
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1003735Summary
Chronic viral infections lead to CD8+ T cell exhaustion, characterized by impaired cytokine secretion. Presence of the immune-regulatory cytokine IL-10 promotes chronicity of Lymphocytic Choriomeningitis Virus (LCMV) Clone 13 infection, while absence of IL-10/IL-10R signaling early during infection results in viral clearance and higher percentages and numbers of antiviral, cytokine producing T cells. IL-10 is produced by several cell types during LCMV infection but it is currently unclear which cellular sources are responsible for induction of viral chronicity. Here, we demonstrate that although dendritic cells produce IL-10 and overall IL-10 mRNA levels decrease significantly in absence of CD11c+ cells, absence of IL-10 produced by CD11c+ cells failed to improve the LCMV-specific T cell response and control of LCMV infection. Similarly, NK cell specific IL-10 deficiency had no positive impact on the LCMV-specific T cell response or viral control, even though high percentages of NK cells produced IL-10 at early time points after infection. Interestingly, we found markedly improved T cell responses and clearance of normally chronic LCMV Clone 13 infection when either myeloid cells or T cells lacked IL-10 production and mice depleted of monocytes/macrophages or CD4+ T cells exhibited reduced overall levels of IL-10 mRNA. These data suggest that the decision whether LCMV infection becomes chronic or can be cleared critically depends on early CD4+ T cell and monocyte/macrophage produced IL-10.
Introduction
The functional down regulation of antiviral T cells, also termed T cell exhaustion, is a major hurdle inhibiting the control or even clearance of chronic infections. T cell exhaustion is characterized by a gradual loss of cytokine producing antiviral CD8+ T cells [1]. The host-derived anti-inflammatory cytokine IL-10 is an important player in driving T cell exhaustion and viral chronicity in LCMV Clone 13 infected mice [2], [3], a commonly used murine model for chronic viral infections. Elevated IL-10 levels were also found to correlate with HIV replication in humans and transition to chronicity during HBV and HCV infection [4], [5], [6]. Since disruption of IL-10 receptor signaling enhances CD8+ T cell effector functions not only after LCMV infection [2], [3], but also in case of HIV-, HBV - or HCV-specific T cells [6], [7], [8], [9], interference with IL-10 signaling is currently proposed as a target for immune-based interventions during chronic viral infections.
IL-10 is expressed during several persistent infections. It may on the one hand favor viral chronicity by suppressing the antiviral immune response, but on the other hand also protect the host from immunopathology [10], [11]. IL-10 acts mainly by modulating the expression of proinflammatory cytokines and chemokines, by modulating the function of antigen presenting cells (i.e. down-regulating for example the expression of MHCI, MHCII, B7-1 and B7-2) and by directly or indirectly suppressing proliferation, functional differentiation and effector activity of antiviral T cells [10], [11]. Suppression of the antiviral immune response through IL-10 is a strategy actively exploited by herpes - and poxviruses which can encode viral IL-10 homologues to weaken the antiviral immune response [12]. Accordingly, rhesus macaques infected with rhesus CMV deficient for rhcmvIL-10 exhibit a T and B cell response of higher quality and quantity [13].
In addition, genetic polymorphisms in the IL-10 promoter are associated with decreased IL-10 production, leading to enhanced control of HCV, HBV, HIV and Epstein Barr Virus (EBV) [14], [15], [16], [17], [18]. Despite the advantages of more effective immune responses in absence of IL-10, this may come at the expense of immunopathology [19], [20], [21]. It is currently unclear which cellular source(s) of IL-10 are decisive in promoting chronicity of high dose LCMV infection. Several cell populations have been reported to produce IL-10 after LCMV infection: LCMV specific CD4+ T cells secrete IL-10 on day 5 post infection [22], IL-10 mRNA is upregulated in dendritic cells (DCs), B cells, macrophages, CD4+ and CD8+ T cells on day 9 post infection [2] and purified CD4+ T cells and DCs produce IL-10 upon LCMV infection of splenocytes in vitro [3]. Furthermore, high percentages of DCs and macrophages, but also B cells, CD4+ and CD8+ T cells produce IL-10 nine and thirty days after Clone 13 infection when assessed in IL-10 reporter (VertX) mice [23]. Significant IL-10 production was found mainly in CD8− DCs and IL-10 serum protein levels were decreased in mice with selective IL-10 deficiency in CD11c+ cells [24]. In contrast, the IL-10 levels remained unaffected in B cell or T cell specific IL-10 knockouts on day 10 post infection [24].
Importantly and surprisingly, functional data evaluating the consequences of cell-specific IL-10 deficiency are lacking. It is conceivable that IL-10 is not primarily acting on a systemic level but in a microenvironment where IL-10 secreting cells and the immediate surrounding target cells are spatially and temporarily connected. Therefore, it is of great importance to identify the biologically relevant IL-10 producing cell populations responsible for impairment of LCMV-specific T cell immunity and consequently viral chronicity.
We show here that even though DCs are a major source of IL-10, specific IL-10 deficiency in DCs neither leads to functional improvement of the LCMV-specific T cell response nor does it prevent viral chronicity. Likewise, even though high percentages of NK cells produced IL-10 early after infection, NK cell specific IL-10 knockout mice were not able to clear LCMV and exhibited exhausted T cells. However, depletion of CD4+ T cells or monocytes/macrophages resulted in decreased overall amounts of IL-10 mRNA and selective IL-10 deficiency in T cells or myeloid cells prevented viral chronicity, identifying CD4+ T cells and monocytes/macrophages as the decisive IL-10 source responsible for suppressing T cell immunity and viral control during high dose LCMV infection.
Results
Kinetics of IL-10 production after high dose infection with LCMV Clone 13
To evaluate the timing when IL-10 determines about persistence or clearance of LCMV, the kinetics of viral titers were compared in C57BL/6 and Il-10−/− mice early after infection (Figure 1A and Figure S1A–E). Interestingly, viral titers were comparable in all tested organs in both mouse strains until day 5/6 post infection. Afterwards, titers gradually decreased in Il-10−/− mice before the virus was cleared around day 10 post infection, whereas the titers remained high in C57BL/6 mice. Next, the question when IL-10R signaling is crucial to establish chronicity was addressed by starting IL-10R blockade at different time points after Clone 13 infection (Figure 1B). Most mice that were treated with αIL-10R antibodies on day 0 and 1 succeeded in clearing LCMV Clone 13, while the groups where αIL-10R treatment was started on day 2 or 3 developed severe immunopathology, which is often observed in situations of concomitant presence of high amount of antigen in presence of high numbers of functional T cells [25], [26]. High viral titers were observed in the group where αIL-10R treatment was started on day 4 post infection, comparable to the untreated group. Similar observations were made in kidney, liver and lung (data not shown). Thus, IL-10 acts during the first two days after infection to prevent immunopathology and to establish viral chronicity.
Fig. 1. Virus titers in presence and absence of IL-10 and IL-10 mRNA expression levels. 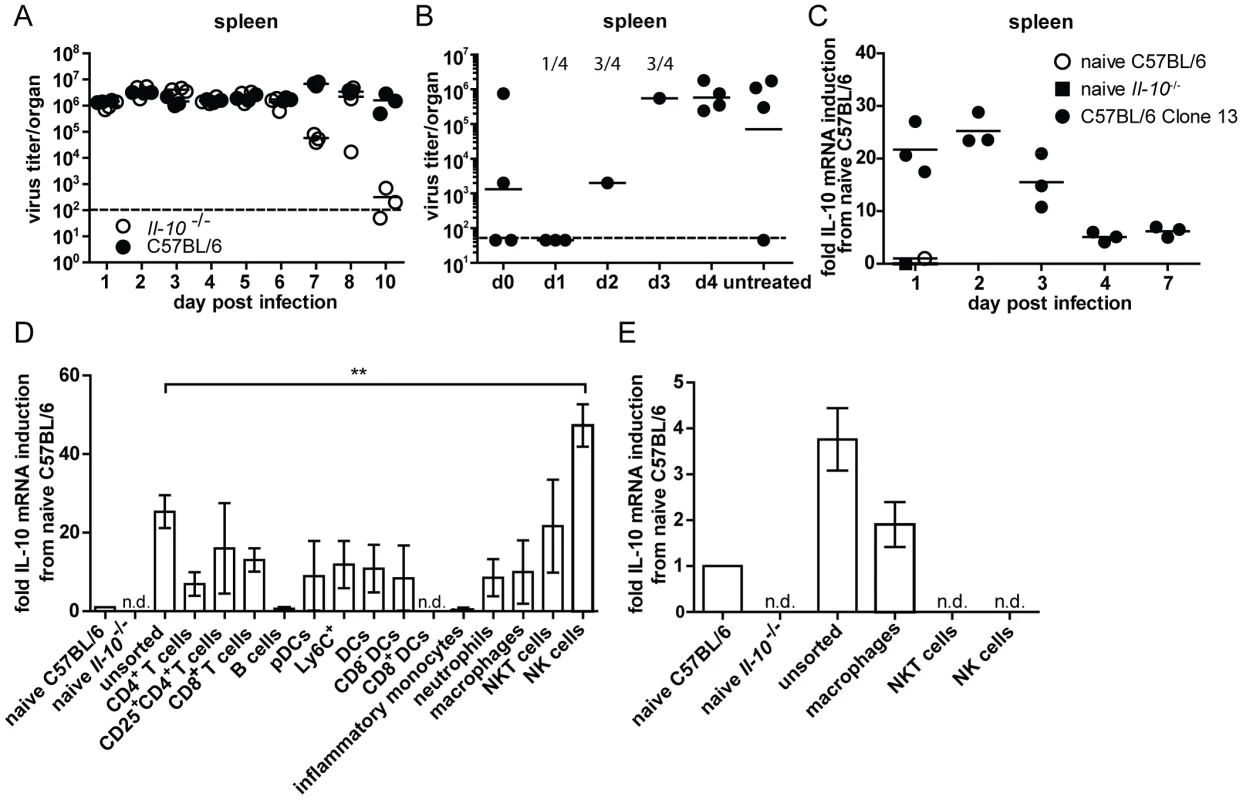
(A) C57BL/6 and Il-10−/− mice were infected with LCMV Clone 13 and virus titers were determined in the spleen at the indicated time points. Several time points were reconfirmed in additional experiments. (B) C57BL/6 mice were infected with Clone 13 and treated with αIL-10R at the indicated time point or left untreated. The treatment was repeated in all groups except the untreated group on day 5 post infection. Virus titers in the spleen were analyzed on day 12 post infection. The numbers indicate the number of mice that had to be killed due to immunopathological disease symptoms during the course of the infection. One representative of two experiments is shown. (C) C57BL/6 mice were infected with Clone 13. RNA was extracted from spleen at the indicated time points. cDNA was subjected to IL-10 qPCR. The relative expression was normalized to β-actin. The fold induction relative to the levels in naïve C57BL/6 is depicted. A naïve Il-10−/− mouse served as negative control. Each symbol represents one mouse. One representative of two experiments is shown. (D+E) Splenocytes from C57BL/6 mice were FACS sorted for the indicated cell populations on (D) day 2 and (E) day 5 post infection with LCMV Clone 13 and IL-10 mRNA levels were measured by qPCR. The relative expression was normalized to β-actin. The fold induction relative to the levels in naïve C57BL/6 is shown. Three samples of pooled splenocytes from two mice were analyzed per experiment. A summary of 10 individual experiments with variable repetitions of specific cell types is shown. n.d.: not detectable. Many cell populations are able to produce IL-10 early after high dose LCMV Clone 13 infection
To evaluate the dynamics of IL-10 production within the first days after LCMV infection, IL-10 mRNA levels were measured with daily intervals for the first 4 days. IL-10 mRNA was maximally induced during the first two days after infection and decreased afterwards in spleen (Figure 1C), lung (Figure S1G) and liver (Figure S1I). In the mesenteric lymph node IL-10 mRNA expression peaked on day 3 post infection (Figure S1H). Quantification of IL-10 mRNA expression revealed that the highest amounts of IL-10 mRNA were expressed on day 2 post infection in the spleen (Figure S1F). This prompted us to concentrate further analysis of IL-10 producing cell populations on spleen of day two post infection. FACS sorting of various cell populations and subsequent measurement of IL-10 mRNA levels by qPCR revealed that CD4+ T cells, CD8+ T cells, CD25+ CD4+ T cells, pDCs, CD8− DCs, neutrophils, macrophages and NKT cells upregulated IL-10 mRNA compared to naïve C57BL/6 splenocytes (Figure 1D). However, only in purified NK cells the level of IL-10 mRNA increased to levels above those present in unsorted splenocytes. On day 5 post infection, IL-10 mRNA could no longer be detected in NK and NKT cells, whereas expression persisted in macrophages (Figure 1E).
Consistent with these data, analyses in TIGER IL-10-GFP reporter mice revealed around 10% of NK cells expressing GFP, while only between 0.5 and 2% of the NKT cells, DCs, CD4+ and CD8+ T cells were GFP+ on day 2 post infection (Figure S2). On day 5 post infection around 6% of the NK cells and 0.4–2% of the NKT cells, DCs, CD4+ and CD8+ T cells were GFP+.
Consecutive waves of IL-10 production by different cell populations
In order to assess cell populations producing high amounts of IL-10 on a population level instead of on a per cell basis, the percentages of different cell populations of the splenocytes were determined on days 2 and 5 post infection. NK cells constituted 0.96±0.23% of the splenocytes on day 2 and 1.81±0.13% on day 5 post infection while NKT cells accounted for 0.11±0.05% on day 2 and 0.2±0.03% on day 5. B cells were present at 28.7±4.3% on day 2 and at 56.6±2.18% on day 5 while CD4+ T cells constituted 7.92±1.47% on day 2 and 8.26±1.35% on day 5 and CD8+ T cells accounted for 4.97±1.26% on day 2 and 11.17±0.87% on day 5. DCs represented 0.75±0.15% on day 2 and 0.92±0.18% on day 5 while F4/80+ macrophages formed 1.43±0.55% on day 2 and 3.42±0.17% on day 5 (data not shown). To integrate the different abundance of IL-10 producing cell populations, C57BL/6 mice were depleted of specific cell populations and overall IL-10 mRNA levels in splenocytes were compared to undepleted control mice on day 2 post infection. Depletion of neutrophils, CD4+ or CD8+ T cells, CD25+ T cells and B cells did not lead to a decrease in the overall IL-10 mRNA levels (Figure 2A and B). Unexpectedly, depletion of NK-like cells did not result in overall decreased IL-10 mRNA levels (Figure 2B) despite the high IL-10 mRNA expression level in purified cells (Figure 1D). In contrast, depletion of CD11c+ cells (DCs, alveolar macrophages and marginal zone macrophages; Figure 2C) led to significantly decreased overall IL-10 mRNA expression. In addition, clodronate liposome meditated depletion of splenic (red pulp, marginal zone and metallophilic [27], but not CD68+ [28]) macrophages led to decreased expression of IL-10 mRNA compared to undepleted controls (Figure 2D). Thus, DCs and monocytes/macrophages account for a significant proportion of IL-10 mRNA on day 2 post infection. Analysis on day 5 post infection revealed that CD8+ T cell depleted mice expressed similar overall levels of IL-10 mRNA as undepleted controls (Figure 2E), while CD4+ T cell depleted, clodronate liposome treated mice and DC specific IL-10 knockout mice (Il-10fl/flxCD11c-Cre+ mice) exhibited reduced levels (Figure 2F–H). We chose to use Il-10fl/flxCD11c-Cre+ mice instead of DT-treated CD11c-DTR+ mice for the analysis of IL-10 mRNA levels on day 5 post infection due to the described toxicity of DT treatment for 5 days [29] and to avoid secondary consequences on viral control which might be caused due to inefficient T cell priming in absence of DCs [30].
Fig. 2. IL-10 mRNA levels on day 2 and 5 post infection after depletion of selective cell populations. 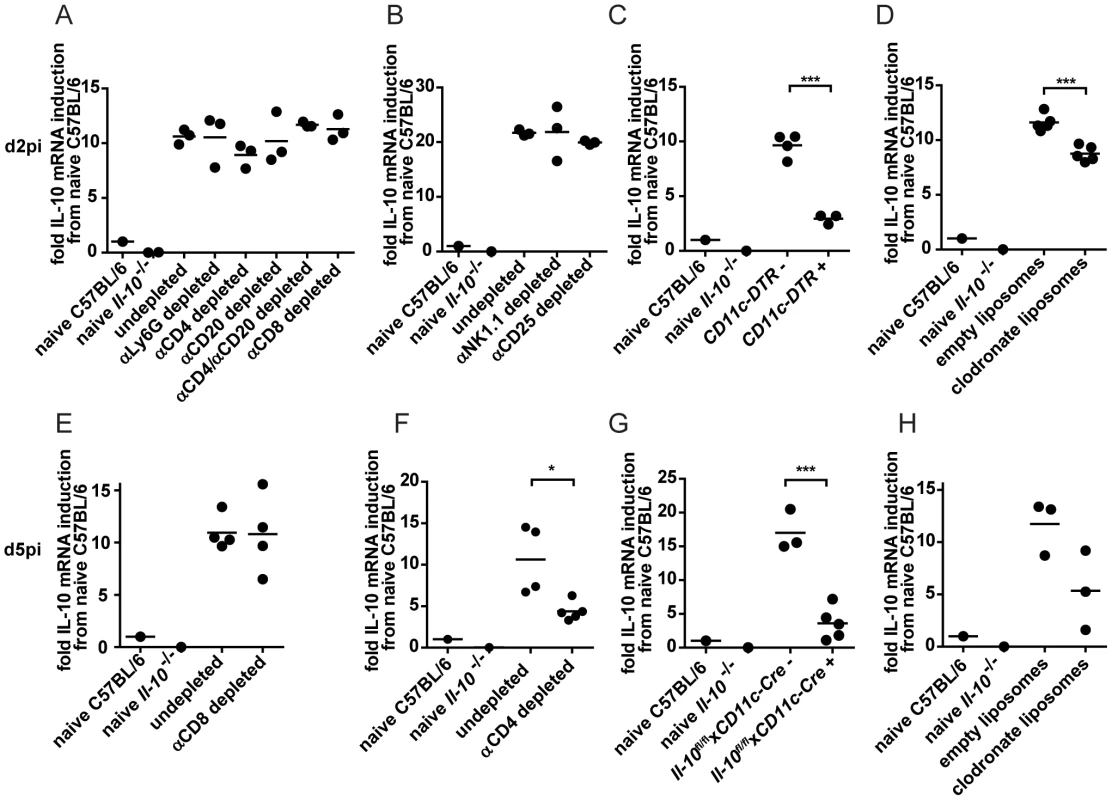
(A–D) IL-10 mRNA levels in the spleen were determined on day 2 post LCMV Clone 13 infection by qPCR. (A) C57BL/6 mice were αLy6G, αCD4, αCD20 or αCD8 depleted or left untreated. (B) C57BL/6 mice were αNK1.1 or αCD25 depleted or left untreated. NK depletion: One representative of three experiments depicted. (C) CD11c-DTR+ and CD11c-DTR− mice were treated with diphtheria toxin. One representative of two experiments is shown. (D) C57BL/6 mice were treated with clodronate containing liposomes or empty control liposomes. One representative of three experiments is shown. (E–H) IL-10 mRNA levels in the spleen were determined on day 5 post infection with LCMV Clone 13 by qPCR. C57BL/6 mice were (E) αCD8 or (F) αCD4 depleted or left untreated. (G) Il-10fl/flxCD11c-Cre+ and Il-10fl/flxCD11c-Cre− mice. (H) C57BL/6 mice were treated with clodronate containing liposomes or empty control liposomes. The relative IL-10 expression was normalized to β-actin. The fold induction relative to the levels in naïve C57BL/6 is shown. A naïve Il-10−/− mouse served as negative control. Each symbol represents one mouse. One representative of two is shown for each experiment. Thus, DCs and monocytes/macrophages account for a first wave of IL-10 production, followed by a second wave of IL-10 production by DCs, CD4+ T cells and monocytes/macrophages on day 5 post infection.
Mast cell, B cell, NK cell and CD11c+ cell produced IL-10 is not required to promote T cell exhaustion and viral chronicity
Even though different cell populations expressed IL-10 at different time points after LCMV infection, it is unclear if IL-10 secreted by any particular cell population is promoting viral chronicity and T cell exhaustion. To assess the effects of cell-type specific IL-10 deficiency on T cell effector functions and the resulting decision between chronicity versus clearance of the viral infection, we made use of conditional IL-10 knockout mice. These mice feature a floxed IL-10 gene and express the Cre recombinase under control of different cell-type specific promoters. While Il-10−/− mice were able to clear LCMV Clone 13 infection by day 12, Il-10fl/flxM5-Cre+ mice, harboring a mast cell specific IL-10 deficiency, were unable to control the infection akin their Cre negative littermate controls and C57BL/6 mice (Figure S3A–D). Single target amplification of the Il-10 locus of single FACS sorted mast cells from Il-10fl/flxM5-Cre+ mice previously confirmed that selectively the deleted version, but never the loxP-flanked product was amplified, while the opposite was true for non-mast cells and Cre-negative control cells [31].
We chose to assess T cell functionality by assessing TNF-α production as a surrogate for polyfunctionality of T cells as it is well established that virtually all TNF-α producing T cells are co-producers of IFN-γ [32], [33] (representative raw data are depicted in Figure S4). We decided to use stimulation with gp33–41 (gp33) as the frequencies of cytokine producing CD8+ T cells are higher than after stimulation with np396–404 and therefore not as prone to unspecific variances close to the detection limit (as the percentage of TNF-α producing T cells is fairly low in exhausted T cells; [34]). TNF-α production by antiviral CD4+ T cells was measured after stimulation with the viral peptide gp61–80 (p13). In line with the fact that mast cell derived IL-10 was dispensable for the development of a chronic infection, the antiviral CD8+ and CD4+ T cells of Il-10fl/flxM5-Cre+ mice showed an exhausted phenotype in lung and spleen (identified by reduced TNF-α production) unlike Il-10−/− mice (Figure S3E–J). In addition, the number of IFN-γ+ CD8+ T cells was not significantly different in Il-10fl/flxM5-Cre+ and Il-10fl/flxM5-Cre− mice in the spleen (Figure S5A). Thus, mast cell specific IL-10 deficiency neither improves T cell mediated cytokine production nor virus control.
Il-10fl/flxCD19-Cre+ mice were previously shown to delete IL-10 specifically in B cells and not or only insignificantly in splenic CD3+ T cells, peritoneal macrophages and tail biopsies [35]. Similar to mast cell specific IL-10 knockout mice, B cell specific IL-10 knockout mice displayed comparable virus titers (Figure 3A–D) and TNF-α production by CD4+ and CD8+ T cells in spleens and lungs to their Cre negative littermates and to C57BL/6 controls (Figure 3E–J; FACS plots of a representative staining are depicted in Figure S4). In addition, the number of IFN-γ+ CD8+ T cells was similar Il-10fl/flxCD19-Cre+ and Il-10fl/flxCD19-Cre− mice in the spleen (Figure S5B; FACS plots of a representative staining are depicted in Figure S4).
Fig. 3. B cell derived IL-10 does not promote viral chronicity and T cell exhaustion. 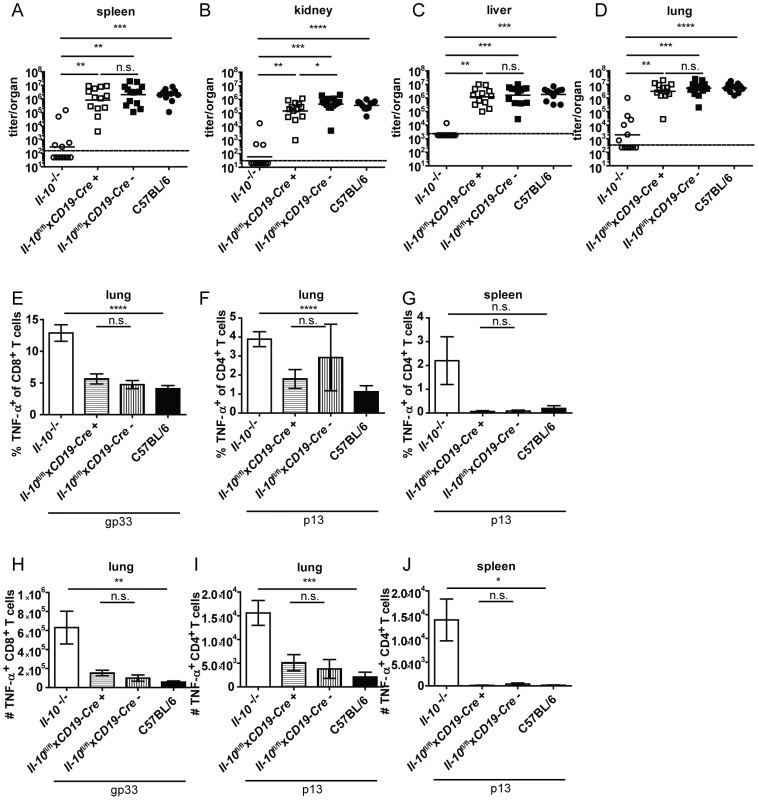
Il-10−/−, Il-10fl/flxCD19-Cre+, Il-10fl/flxCD19-Cre− and C57BL/6 mice were infected with Clone 13. Virus titers were determined in (A) spleen, (B) kidney, (C) liver and (D) lung on day 10 or day 12 post infection. (A–D) Each symbol represents one individual mouse; the solid lines represent the geometric means, the dashed line the detection limit. Statistical significance was determined with the Mann-Whitney test. (E and H) TNF-α production by CD8+ T cells in lung mononuclear cells was determined after 5 hours stimulation with the peptide gp33. TNF-α secretion by CD4+ T cells in (F and I) lung mononuclear cells and (G and J) splenocytes was determined after 5 hours stimulation with the peptide p13. Mean and standard deviation of the (E–G) percentages and (H–J) total numbers are shown. A summary of all mice from three independent experiments with 4–5 mice per group is depicted. Statistical significance was determined with unpaired 2-tailed Student's t test. n.s.: not significant; * p<0.05; ** p<0.01; *** p<0.001; *** p<0.0001. NK cells produced high amounts of IL-10 on a per cell basis on day 2 post infection (Figure 1D). To examine if NK cell derived IL-10 is decisive for Clone 13 infection to become chronic, Il-10fl/flxNcr1-Cre+ mice were derived from Il-10fl/fl and Ncr1-Cre+ mice. This mouse strain deleted the Il-10 gene in 63% of the NK cells (Figure S6A). Il-10fl/flxNcr1-Cre+ mice were infected with Clone 13 and analyzed on day 12 post infection. In agreement with the observation that depletion of NK-like cells did not influence total IL-10 mRNA levels (Figure 2B), Il-10fl/flxNcr1-Cre+ mice displayed high virus titers like their Cre negative littermates and like C57BL/6 mice (Figure 4A–D). In addition, the antiviral CD8+ and CD4+ T cells showed both low percentages and total numbers of TNF-α+ cells and were as exhausted as in the Cre negative littermate controls and as in C57BL/6 mice (Figure 4E–J). Furthermore, the number of IFN-γ+ CD8+ T cells was similar in Il-10fl/flxNcr1-Cre+ and Il-10fl/flxNcr1-Cre− mice in the spleen (Figure S5C). Thus, the high amount of IL-10 mRNA on a per cell basis did not translate into a biologically relevant role of NK cell-derived IL-10 for the decision between chronicity and clearance of LCMV infection.
Fig. 4. NK cell derived IL-10 does not promote viral chronicity and T cell exhaustion. 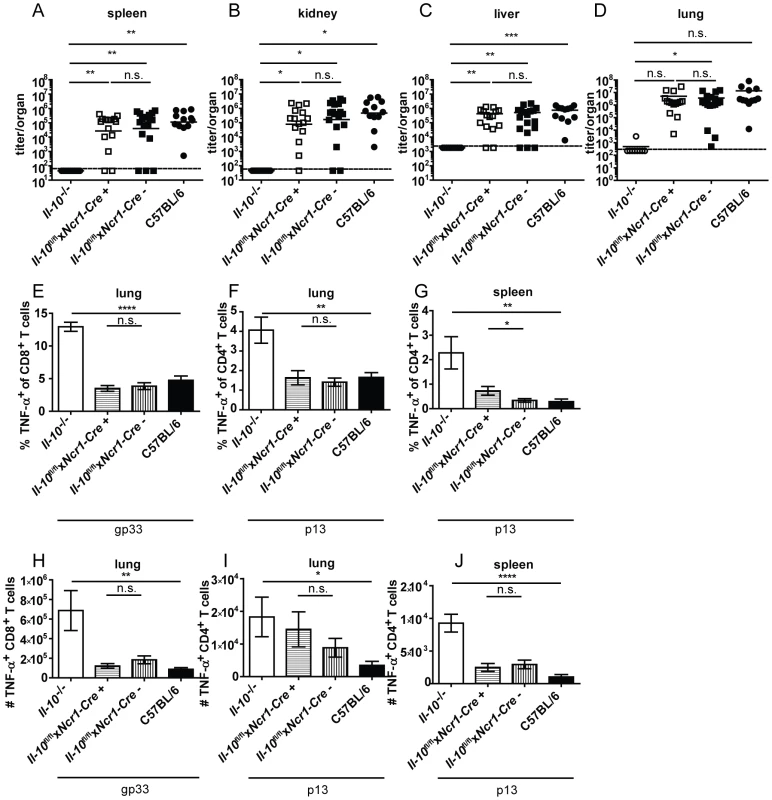
Il-10−/−, Il-10fl/flxNcr1-Cre+, Il-10fl/flxNcr1-Cre− and C57BL/6 mice were infected with Clone 13. Virus titers were determined in (A) spleen, (B) kidney, (C) liver and (D) lung on day 12 post infection. (A–D) Each symbol represents one individual mouse; the solid lines represent the geometric means, the dashed line the detection limit. Statistical significance was determined with the Mann-Whitney test. (E and H) TNF-α production by CD8+ T cells in lung mononuclear cells was determined after 5 hours stimulation with the peptide gp33. TNF-α secretion by CD4+ T cells in (F and I) lung mononuclear cells and (G and J) splenocytes was determined after 5 hours stimulation with the peptide p13. Mean and standard deviation of the (E–G) percentages and (H–J) total numbers are shown. A summary of all mice from three independent experiments with 3–10 mice per group is depicted. Statistical significance was determined with unpaired 2-tailed Student's t test. n.s.: not significant; * p<0.05; ** p<0.01; *** p<0.001; *** p<0.0001. CD8− DCs and pDCs expressed IL-10 mRNA on day 2 post infection (Figure 1D). In accordance with the literature [24], absence of CD11c+ cell-derived IL-10 resulted in strongly decreased overall IL-10 mRNA levels on day 2 and 5 post infection in the spleen (Figure 2C and G). Il-10fl/flxCD11c-Cre+ mice proved to delete the Il-10 gene specifically in DCs (Figure S6B). Unexpectedly, LCMV Clone 13 infection was not controlled in Il-10fl/flxCD11c-Cre+ mice (Figure 5A–D) and resulted in low percentages and total numbers of TNF-α producing antiviral CD8+ and CD4+ T cells comparable to littermate and C57BL/6 controls (Figure 5E–H). Furthermore, the number of IFN-γ+ CD8+ T cells was similar in the spleens of Il-10fl/flxNcr1-Cre+ and Il-10fl/flxNcr1-Cre− mice (Figure S5D). Thus, even though CD11c+ cells contribute substantially to the early overall IL-10 production during LCMV infection (Figure 1D, 2C and G), CD11c+ cell derived IL-10 does not seem to play a role for the outcome of the infection and the quality of the antiviral T cell response.
Fig. 5. CD11c+ cell derived IL-10 does not promote viral chronicity and T cell exhaustion. 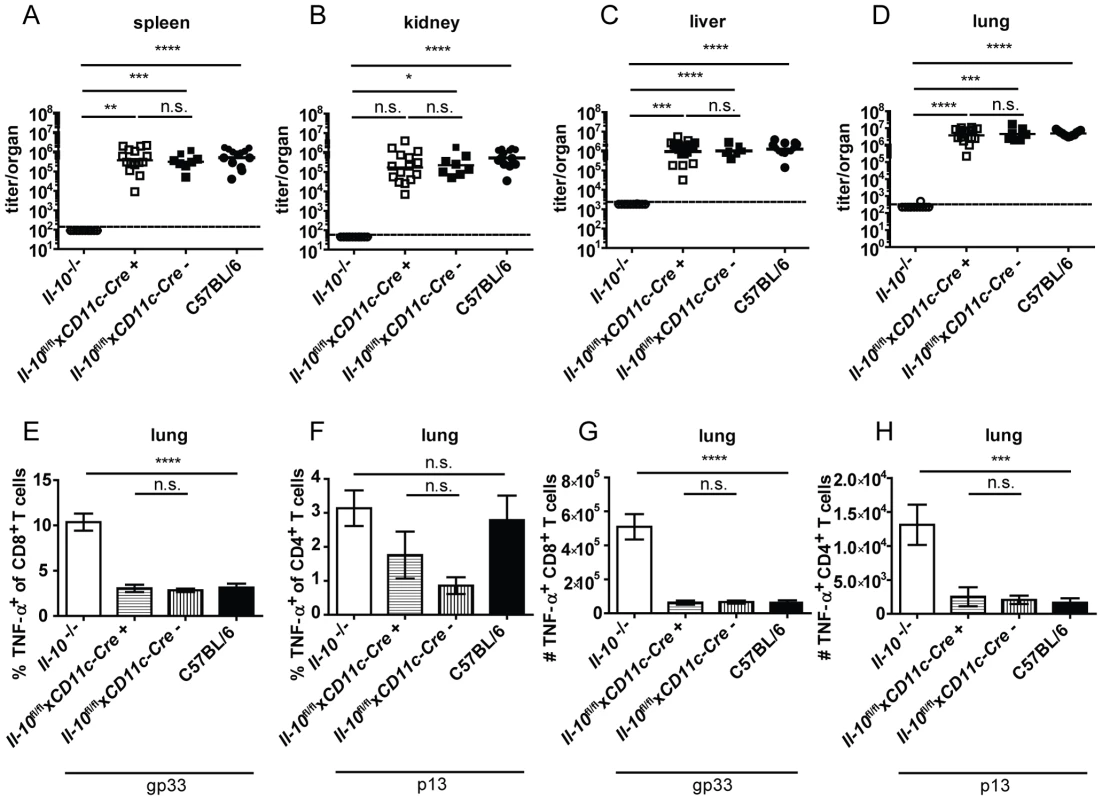
Il-10−/−, Il-10fl/flxCD11c-Cre+, Il-10fl/flxCD11c-Cre− and C57BL/6 mice were infected with Clone 13. Virus titers were determined in (A) spleen, (B) kidney, (C) liver and (D) lung on day 10 or 12 post infection. (A–D) Each symbol represents one individual mouse; the solid lines represent the geometric means, the dashed line the detection limit. Statistical significance was determined with the Mann-Whitney test. (E and G) TNF-α production by CD8+ T cells in lung mononuclear cells was determined after 5 hours stimulation with the peptide gp33. TNF-α secretion by CD4+ T cells in (F and H) lung mononuclear cells was determined after 5 hours stimulation with the peptide p13. Mean and standard deviation of (E and F) the percentages and (G and H) total numbers are shown. A summary of all mice from three independent experiments with 2–8 mice per group is depicted. Statistical significance was determined with unpaired 2-tailed Student's t test. n.s.: not significant; * p<0.05; ** p<0.01; *** p<0.001; *** p<0.0001. Monocyte/macrophage derived IL-10 drives chronicity of LCMV infection
As neutrophils and macrophages produce IL-10 on day 2 and 5 post infection (Figure 1D and E) and as monocyte/macrophage depletion reduced the overall IL-10 mRNA levels (Figure 2D and H), Il-10fl/flxLysM-Cre+ mice, in which selectively myeloid cells lack IL-10 production, were infected with Clone 13. Il-10fl/flxLysM-Cre+ mice were previously shown to delete IL-10 specifically in neutrophils and macrophages and not or only insignificantly in dendritic cells, T cells and B cells [36]. Interestingly, 50% of the Il-10fl/flxLysM-Cre+ mice succeeded in significantly reducing LCMV Clone 13 titers like Il-10−/− mice and unlike the Cre negative littermates and C57BL/6 mice (Figure 6A–D). In line with this, a higher percentage and total number of TNF-α producing T cells was observed in the spleens and lungs of Il-10fl/flxLysM-Cre+ mice which could control the infection compared to Cre negative littermate controls, the Il-10fl/flxLysM-Cre+ mice which could not control the infection and C57BL/6 mice (Figure 6E–J). In addition, the number of IFN-γ+ CD8+ T cells was higher in the Il-10fl/flxLysM-Cre+ which could reduce the virus load than in Il-10fl/flxLysM-Cre− mice in the spleen (Figure S5E). Thus, myeloid cell derived IL-10 contributes significantly to the biologically relevant effects of IL-10 without accounting for the full phenotype. As depletion of monocytes/macrophages decreased the levels of IL-10 mRNA on days 2 and 5 post infection, while αLy6G depletion failed to do so (Figure 2A), it is likely that monocytes/macrophages are the relevant IL-10 source rather than neutrophils.
Fig. 6. Mononuclear cells are a biologically relevant IL-10 source. 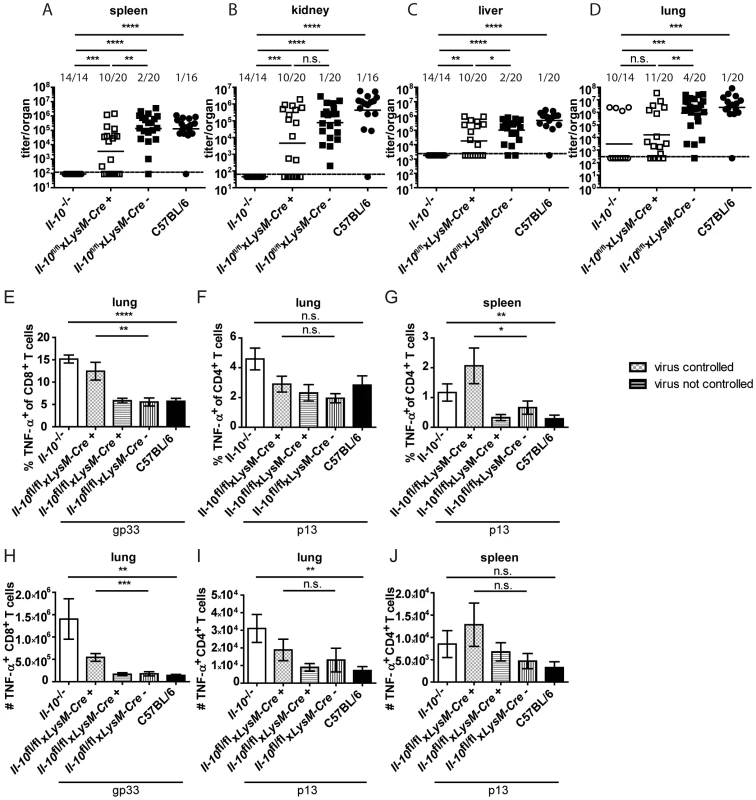
Il-10−/−, Il-10fl/flxLysM-Cre+, Il-10fl/flxLysM-Cre− and C57BL/6 mice were infected with Clone 13. Virus titers were determined in (A) spleen, (B) kidney, (C) liver and (D) lung on day 11 or 12 post infection. Each symbol represents one individual mouse; the solid lines represent the geometric means, the dashed line the detection limit. The numbers indicate how many mice per total mice exhibited virus titers below 103 in spleen and kidney, 5×103 in liver and 104 in lung. Statistical significance was determined with the Mann-Whitney test. (E and H) TNF-α production by CD8+ T cells in lung mononuclear cells was determined after 5 hours stimulation with the peptide gp33. TNF-α secretion by CD4+ T cells in (F and I) lung mononuclear cells and (G and J) splenocytes was determined after 5 hours stimulation with the peptide p13. Mean and standard deviation of the (E–G) percentages and (H–J) total numbers are shown. Statistical significance was determined with unpaired 2-tailed Student's t test. A summary of all mice from four independent experiments with 2–8 mice per group is depicted. n.s.: not significant; * p<0.05; ** p<0.01; *** p<0.001; *** p<0.0001. T cell derived IL-10 drives chronicity of the LCMV infection
Based on the finding that CD4+ and CD8+ T cells produced IL-10 after Clone 13 infection (Figure 1D) and that CD4+ T cell depletion decreased IL-10 mRNA levels on day 5 post infection (Figure 2F), we investigated if T cell derived IL-10 complements macrophage derived IL-10 to exert it's biological effects. Il-10fl/flxCD4-Cre+ mice were shown previously to delete the Il-10 gene specifically in T cells [37]. Interestingly, almost all Il-10fl/flxCD4-Cre+ mice succeeded in clearing Clone 13 within 12 days comparable to Il-10−/− mice and opposed to Cre negative littermate controls and C57BL/6 mice (Figure 7A–D). In line with these data, frequencies and numbers of TNF-α producing T cells were increased in mice with selective IL-10 deficiency in T cells compared to Cre negative littermate controls and C57BL/6 mice (Figure 7E–J). Likewise, the number of IFN-γ+ CD8+ T cells was higher in the spleens of Il-10fl/flxCD4-Cre+ than in Il-10fl/flxCD4-Cre− mice (Figure S5F). However, the percentage and total number of TNF-α producing antiviral CD4+ and CD8+ T cells was lower in Il-10fl/flxCD4-Cre+ mice compared to Il-10−/− mice, underlining the fact that macrophages and potentially also other cell populations contribute to the overall relevant IL-10 production. It should be noted that a slight increase in the viral inoculum led to increased incidence of viral chronicity in Il-10fl/flxCD4-Cre+ mice while most Il-10−/− mice were still able to clear the infection (data not shown), suggesting that IL-10 derived from T cells and myeloid cells act together to exert its full biological activity.
Fig. 7. T cells are a biologically relevant IL-10 source. 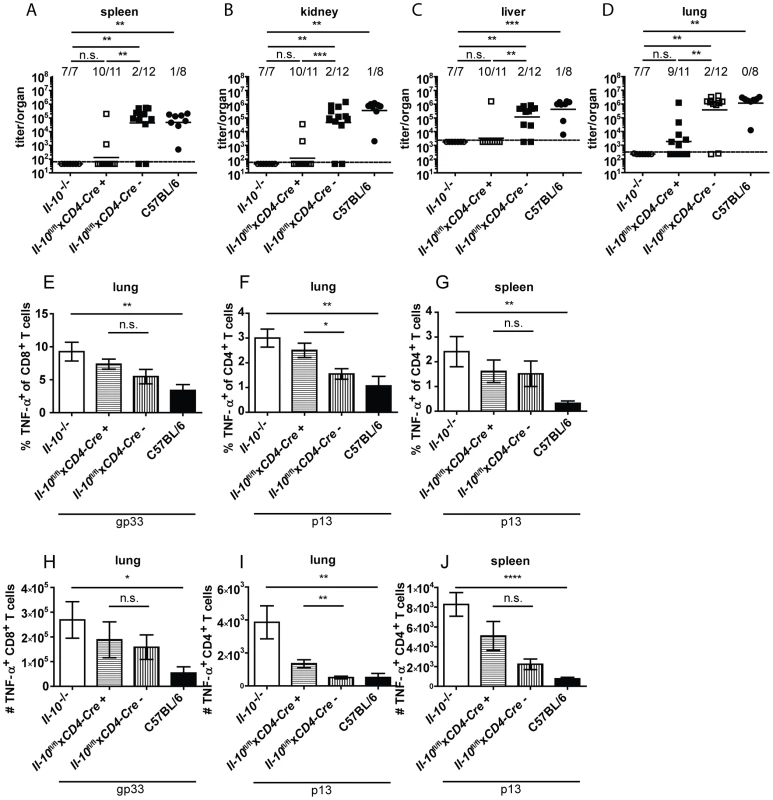
Il-10−/−, Il-10fl/flxCD4-Cre+, Il-10fl/flxCD4-Cre− and C57BL/6 mice were infected with Clone 13. Virus titers were determined in (A) spleen, (B) kidney, (C) liver and (D) lung on day 10 or 12 post infection. Each symbol represents one individual mouse; the solid lines represent the geometric means, the dashed line the detection limit. The numbers indicate how many mice per total mice exhibited virus titers below 103 in spleen and kidney, 5×103 in liver and 104 in lung. Statistical significance was determined with the Mann-Whitney test. (E and H) TNF-α production by CD8+ T cells in lung mononuclear cells was determined after 5 hours stimulation with the peptide gp33. TNF-α secretion by CD4+ T cells in (F and I) lung mononuclear cells and (G and J) splenocytes was determined after 5 hours stimulation with the peptide p13. Mean and standard deviation of the (E–G) percentages and (H–J) total numbers are shown. Statistical significance was determined with unpaired 2-tailed Student's t test. A summary of all mice from two independent experiments with 3–8 mice per group is depicted. * p<0.05; ** p<0.01; *** p<0.001; *** p<0.0001. To further assess which CD4+ T cell subpopulation might be mainly responsible for the IL-10 production, TIGER IL-10 reporter mice were analyzed for GFP expression on day 10 post infection in TFH and TH1 cells using PSGL1 and Ly6C as surrogate markers (with PSGL1+ Ly6C+ CD4+ T cells representing TH1 cells and PSGL1− Ly6C− cells representing TFH cells; Figure S7A–F). GFP+ cells could be detected in both subpopulations. Likewise, GFP expression could be detected in a subpopulation of Neuropilin+ natural Tregs. However, the biggest increase compared to the naïve situation could be detected in Neuropilin− induced Tregs (Figure S7G–I). The finding that induced Tregs as opposed to TH1 cells are probably responsible for the majority of the CD4+ T cell derived IL-10 is supported by the finding that only very few IFN-γ+ CD4+ T cells co-produced IL-10 (Figure S7J–K).
Discussion
Several cell populations are capable of producing IL-10 [10], [38]. DCs, B cells, macrophages, CD4+ and CD8+ T cells were previously shown to produce IL-10 after LCMV infection [2], [3], [22], [24]. However, data on functional consequences of IL-10 deficiency in defined cell types were lacking so far. In agreement with previous studies [2], [3], [22], [24] we demonstrate here that DCs, B cells, macrophages, CD4+ and CD8+ T cells, but also neutrophils, NK cells and NKT cells produce IL-10 in the early phase of high dose LCMV Clone 13 infection. These diverse IL-10 producing cell types potentially all play important and distinct roles in suppressing the host's immune response. However, we clearly show that myeloid and T cell derived IL-10 is critical in promoting viral chronicity and T cell exhaustion, while IL-10 derived from other sources, including DCs, only contributes marginally - if at all - to the suppression of the antiviral immune response. Since depletion of monocytes/macrophages but not neutrophils led to markedly reduced IL-10 mRNA levels, we conclude that monocyte/macrophage derived IL-10 accounts for the reduced virus titer and increased cytokine production by antiviral T cells occurring in the myeloid cell specific IL-10 knockout mice.
IL-10 drives viral persistence in a number of chronic infections. Elevated IL-10 levels were found to correlate with HIV replication in humans and chronicity during HCV and HBV infection [4], [5], [6]. Likewise, disruption of IL-10 receptor signaling enhances CD8+ effector functions of T cells isolated from HIV, HCV and HBV infected individuals [6], [7], [8], [9] like after LCMV infection [2], [3].
The dominant IL-10 producing cell population varies with different infections, likely reflecting differences in pathogen-specific responses and tissue-specific immune regulation. Usually, IL-10 is produced by multiple cell populations [10]. During HIV infection, IL-10 production by T cells, B cells, NK cells and monocytes was reported [4] with monocytes/macrophages being the subset producing highest levels of IL-10 in the peripheral blood [39], [40], [41]. In contrast, B cell derived IL-10 is important during HBV infection [6]. During murine cytomegalovirus (MCMV) infection, IL-10 can be produced by CD4+ T cells, B cells, inflammatory macrophages, dendritic cells and NK cells [19], [35], [42], [43]. The biologically relevant in vivo sources of IL-10 in MCMV infection were identified as CD11c+ cells and to some extent macrophages. IL-10 from these cellular sources led to the suppression of NK/DC cross-talk and consequently to curtailed CD4+ T cell priming, thereby promoting prolonged lytic MCMV [19]. In addition, B cell produced IL-10 was shown to suppress the CD8+ T cell response in the spleen during MCMV infection [35]. During Leishmania major infection anti-IL-10R treatment leads to sterile cure of infection with complete elimination of the parasite. In this infection model, CD4+ and CD8+ T cell derived IL-10 plays an essential role for the establishment of pathogen persistence [44]. Thus, the different importance of certain cell populations serving as IL-10 source might depend on the specific tissue, the cell population affected and the time after infection.
It was recently shown that myeloid suppressor cells inhibit CD8+ T cell proliferation during chronic LCMV infection [45]. However, the fact that in vitro co-cultivated myeloid suppressor cells could still inhibit CD8+ T cell proliferation in presence of an anti-IL-10R antibody [45] renders it rather unlikely that myeloid suppressor cells are a relevant source of IL-10, assuming that IL-10 acts directly on the CD8+ T cells.
CD4+ T cell depletion led to a decrease in the overall IL-10 mRNA levels on day 5 post infection with LCMV while depletion of CD8+ T cells had no effect (Figure 2). Therefore, it is likely that CD4+ T cell derived IL-10 is more important than CD8+ T cell derived IL-10. IL-10 was initially identified as TH2 cytokine [46], but can also be produced by TH1, TH17, TFH, natural and induced Tregs [47]. As αCD25 depletion did not decrease IL-10 mRNA levels on day 2 post infection, natural Tregs do not seem to be relevant IL-10 producers during LCMV infection. These data were supported by the finding that IL-10+ Neuropilin+ natural Tregs did not accumulate upon infection. In contrast to natural Tregs, IL-10-producing Neuropilin− induced Tregs accumulated by day 10 after LCMV infection and likely constitute a subpopulation of CD4+ T cells that is responsible to promote chronicity of the infection, at least on day 10 post infection when LCMV chronicity is already established. Accumulation of induced Tregs during established chronic LCMV infection is in agreement with previous studies [48], [49].
Interestingly, the depletion of monocytes/macrophages decreased IL-10 mRNA levels on days 2 and 5 post infection while CD4+ T cell depletion led to reduced concentrations of IL-10 mRNA only on day 5. This implies a cooperation of macrophages and CD4+ T cells in a way that the macrophages provide a first wave of IL-10 until, in a second wave, different subtypes of CD4+ T cells (TH1, TFH, natural Tregs and induced Tregs with induced Tregs exhibiting the highest percentage of IL-10 producing cells) start to significantly contribute to the overall IL-10 production. Together with the fact that all analyzed CD4+ T cell subpopulations contribute to IL-10 production, it seems as if multiple cell types share the task of IL-10 secretion in a time-dependent manner. It is possible that IL-10 production by monocytes/macrophages is preceded by a first wave of IL-10 production by NK cells. However, absence of NK cell derived IL-10 did not promote control of high dose LCMV infection, suggesting that IL-10 production by monocytes/macrophages plays a more dominant role. It should be noted, however, that the NK cell-specific Il-10 deletion efficacy was only 63% (Figure S6 and [50]), but in combination with the observation that NK cell depletion did not lead to an overall reduction in IL-10 mRNA on day 2 post infection it is rather unlikely that NK cell produced IL-10 plays a decisive role in promoting LCMV chronicity.
The observation that dendritic cells produce IL-10 and that the IL-10 mRNA levels decreased dramatically in absence of CD11c+ cells (Figure 2C) is consistent with the finding that Il-10fl/flxCD11c-Cre mice expressed considerably less IL-10 (Figure 2G and [24]). Importantly and to some extend surprisingly, we extend this observation by showing that IL-10 produced by CD11c+ cells lacks functional consequences with respect to antiviral T cell function or the establishment of viral chronicity (Figure 5).
It is likely that the precise anatomical localization and kinetics of the IL-10 secreting cells in relation to the responding cell is of considerable importance. This would be one possible explanation for the observation that IL-10 produced by CD11c+ cells does not influence the outcome of the infection even though IL-10 promoter activity could be detected in DCs of IL-10 reporter mice and even though depletion of CD11c+ cells dramatically decreased the overall amount of IL-10 mRNA. On the other hand, post-transcriptional regulation of IL-10 may vary in different cell types, such that quantitative differences evidenced by mRNA analyses might not translate to the protein level [51], [52].
One potential caveat of IL-10 mRNA analysis after in vivo depletion of certain cell populations for several days into LCMV infection is that the absence of specific cell types may alter virus titers which might in turn affect the IL-10 production by other cells types. In addition, depletion of CD11c+ cells by DT administration in CD11c-DTR+ mice was shown to cause neutrophilia [53]. So, if neutrophils contributed to IL-10 production significantly, their increased abundance might have masked the decrease in IL-10 mRNA levels in diphtheria toxin treated CD11c-DTR mice.
Our data do not permit a conclusion on whether the more potent antiviral T cell responses observed in absence of T cell or myeloid-derived IL-10 are a cause or a consequence of the concomitantly reduced virus titers, but they underscore an important role of IL-10 in shaping the mutual relationship between prolonged high antigen load and decreased T cell function [26]. It has to be noted that only a defined LCMV inoculum is controlled in the T specific IL-10 knockout mice, while a wider range of inocula is controlled in full IL-10 deficient mice. This is likely one reason why the full IL-10 knockout mice show more robust T cell responses compared to the cell-specific knockout mice, yet these small increases might be sufficient under these experimental settings to allow for virus control.
At present it is unclear on which cell types the T cell or myeloid cell derived IL-10 acts to promote viral chronicity but it is likely that multiple immune cell subsets will be targeted. It was recently shown that splenic DCs treated in vitro with type I IFN produce IL-10 [24] and that anti-IFNR1 blockade led to reduced numbers of IL-10+ PD-L1+ immunoregulatory DCs which in turn stimulated elevated numbers of IFN-γ+ CD4+ T cells and a decrease in viral titers [54]. It is likely that type I IFN also directly induces IL-10 production in macrophages at the early time points after infection. However, as the amounts of IFN decline at later time points [55], this might indicate that CD4+ T cell derived IL-10 is induced by other mechanisms.
In this context we have measured type I IFNs in the serum of C57BL/6 and Il-10−/− mice and we have observed increased concentrations of type I IFN in Il-10−/− mice on day 3 post infection (data not shown). As Type I IFNs are an essential signal 3 in LCMV to drive T cell expansion [56], [57] and effector cell differentiation [58], it is possible that increased peak levels of type I IFNs might contribute to more effective T cell responses which in turn would accelerate virus control. It is also possible that increased levels of type I IFNs in Il-10−/− mice might have a direct effect on LCMV control, but this is rather unlikely as we do not see any changes in viral load in the first 5 days post infection.
As we determined that IL-10 has to act within the first 2 days it is very likely that initially innate immune cells or non-hematopoietic cells are targeted by IL-10. Indeed, we have preliminary data that IL-10 does not directly act on CD4+ or CD8+ T cells and on DCs (data nor shown). The innate target cells could be macrophages themselves (being infected by LCMV) which are modulated by IL-10 such that they would better sustain viral replication, potentially by inhibition of NO production as it was shown for parasite infection [59] or by inhibition of TNF-α and IFN-γ production [60]. We believe that a direct effect of IL-10 on virus production is rather unlikely as we have seen in our in vivo kinetics of viral loads that differences between C57BL/6 and Il-10−/− mice only become apparent after day 6 of infection (Fig. 1).
Alternatively, NK cells might be possible IL-10 target cells as they are mainly situated in the red pulp of the spleen where they might be targeted by macrophage-secreted IL-10. In MCMV infection it has been shown that NK cells are over-activated in absence of IL-10 and are undergoing increased levels of activation-induced cell death [43]. As it has been shown in the LCMV system that activated NK cells can reduce the number of activated virus-specific CD4+ T cells [61], it is conceivable that potentially reduced numbers of NK cells in the absence of IL-10 would translate into better preserved virus-specific CD4+ (and CD8+) T cell responses.
With respect to the secondary requirement of CD4+ T cell derived IL-10 driving viral chronicity we hypothesize that by day 4–5 post infection, activated CD4+ T cells will not only be in secondary lymphoid organs but also in (infected) peripheral tissues where their IL-10 secretion might affect local immune control. Based on our observation that IL-10 does not directly act on T cells and based on additional observations that the per cell killing efficiency of CD8+ T cells (when using optimally peptide-loaded APCs) is not altered in presence or absence of IL-10 (data not shown), we postulate that either the increased frequencies of T cells in peripheral tissues lead to better virus control or that in addition presence of (CD4+ T cell secreted) IL-10 would impact on the antigen presentation on infected cells. IL-10 is known to down-modulate MHC expression and it could thereby reduce sensitivity towards LCMV infected cells.
In summary, our data clearly indicate a non-redundant role of T cell and myeloid cell derived IL-10 in facilitating viral chronicity and T cell exhaustion after LCMV Clone 13 infection as opposed to DCs, which were so far proclaimed to exert this function.
Materials and Methods
Virus and viral peptides
LCMV Clone 13 was propagated and titrated as described [62]. The viral peptides gp33–41 (gp33; KAVYNFATM), np396–404 (np396; FQPQNGQFI) and gp61–80 (p13; LNGPDIYKGVYQFKSVEFD) were purchased from NeoMPS (Strasbourg, France).
Mice
Animal experiments were performed according to the guidelines of the animal experimentation law (SR 455.163; TVV) of the Swiss Federal Government. The protocol was approved by the Cantonal Veterinary Office (animal experimentation number 146/2008 and 127/2011).
Ncr1-Cre mice [50] were crossed to Il-10fl/fl mice [37] yielding Il-10fl/flxNcr1-Cre mice.
C57BL/6 (Janvier Elevage, Le Genest Staint Isle, France), Il-10fl/flxCD4-Cre [37], Il-10fl/flxLysM-Cre [36], Il-10fl/flxCD11c-Cre [24], Il-10fl/flxCD19-Cre [35], Il-10fl/flxM5-Cre [31], Il-10−/− [63], TIGER [64] and CD11c-DTR/GFP [29] mice were kept under specific pathogen-free conditions and were intravenously (i.v.) infected with LCMV Clone 13. Each virus stock was titrated in vivo and injected at doses that led to a chronic infection in C57BL/6 mice and to reduced virus titers by day 10 to 12 in most of the Il-10−/− mice (between 3×105 and 106 ffu per mouse).
Antibodies and in vivo depletion of cell populations
Antibodies used for flow cytometry were purchased from BD Pharmingen (Allschwil, Switzerland), Biolegend (LucernaChem, Luzern, Switzerland) and R&D Systems (Abingdon, England). For the depletion of neutrophils, mice were i.p. injected with 500 µg αLy6G (clone 1A8, BioXCell, West Lebanon, NH, USA) on day -2 and one hour before infection (99.8% reduction in the percentage of Ly6G+ cells). For the depletion of CD4+ T cells, mice were i.p. injected with 200 µg αCD4 (YTS 191.1) on day -2 and one hour before infection (depletion efficacy of 97% while the percentage of CD8+ T cells increased slightly). For the depletion of B cells, mice were i.p. injected with 250 µg αCD20 (18B12; IgG2a; [65]) on day -2 and one hour before infection (depletion efficacy of 98% while the percentage of CD8+ T cells and CD4+ T cells increased compared to mice treated with an isotpye control). For the depletion of CD8+ T cells, mice were i.p. injected with 200 µg αCD8 (YTS 169.4) on day -2 and one hour before infection (depletion efficacy of 99% while the CD4+ T cell population stayed unaffected). Depletion of NK-like cells was performed by i.p. administration of 300 µg αNK1.1 (PK136, BioXCell) on days -3 and -1 (depletion efficacy of 96% while the percentage of CD3+ cells was not affected). For the depletion of regulatory CD4+ T cells mice were i.p. injected with 200 µg of αCD25 (PC-61.5.3, BioXCell) 3 and 1 day before infection (depletion efficacy of 70%). For depletion of CD11c+ cells, CD11c-DTR+ and CD11c-DTR− mice were i.p. treated with 200 ng diphtheria toxin (Sigma-Aldrich, Buchs, Switzerland) on days -2, -1 and 0 (depletion efficacy of 95% while the percentage of B cells was not affected). For depletion of monocytes/macrophages mice were i.p. treated with liposomes containing 1 mg clodronate [27] or equal amounts of empty control liposomes on days -7, -5, -2 and 0 (depletion efficacy of 96% while the percentage of B cells and T cells was not affected). The depleted mice were analyzed on day 2 post infection. When analyzed on day 5 post infection, the depleting agent was continuously administered every second to third day.
Isolation and stimulation of lymphocytes and flow cytometry
Splenocytes and mononuclear cells from lungs were prepared, stimulated and stained as previously described [26]. Phorbol 12-myristate 13-acetate (PMA) and Ionomycin were purchased from Sigma and used at 50 ng/ml and 500 ng/ml, respectively. Multiparameter flow cytometric analysis was performed using a FACS LSRII flow cytometer (BD Biosciences, Allschwil, Switzerland) with FACSDiva software. Analysis was performed using FlowJo software (Tree Star, San Carlos, CA). Sorting was performed using a FACS Aria flow cytometer (BD Biosciences, Allschwil, Switzerland). The sorted cell populations were defined as follows: CD4+ T cells: CD4+ CD25−; CD25+ CD4+ T cells: CD4+ CD25+; CD8+ T cells: CD8+ CD4−; B cells: CD4− CD19+; pDCs: B220+ PDCA-1+ Ly6C+; DCs: CD11c+ I-A/I-E+; CD8− DCs: CD11c+ I-A/I-E+ CD8−; CD8+ DCs: CD11c+ I-A/I-E+ CD8+; inflammatory monocytes: CD11b+ Ly6C+ Ly6G−; neutrophils: CD11b+ Ly6C− Ly6G+; macrophages: CD11bint F4/80+; NKT cells: CD3+ NK1.1+; NK cells: CD3− NK1.1+.
Enrichment of NK cells and DCs and Southern blot
NK cells were isolated from splenocytes on day 7 after i.p. infection with 5×104 pfu MCMV Smith by magnetic negative selection of CD3+ and CD19+ cells and subsequent FACS sorting of CD3− NK1.1+ cells. DCs were isolated from splenocytes on day 7 after s.c. injection with 106 B16-Flt3L cells by magnetic negative selection of CD3+ and CD19+ cells and subsequent FACS sorting of CD11c+ MHCII+ cells. Southern Blot was performed as described [37].
RNA isolation, reverse transcription and IL-10 qPCR
Total RNA was isolated with Trizol (Invitrogen, Basel, Switzerland) according to the manufacturer's instructions. For isolation of RNA from whole organs, the organ was homogenized with a stainless steel bead for 1.5 min at 25 Hz in a Qiagen Tissue Lyser. 1 µg of RNA was reverse transcribed with M-MLV Reverse Transcriptase RNase, H Minus (Promega, Dübendorf, Switzerland). Real-time PCR was performed using a Rotorgene 3000 instrument (Corbett Research, Eight Miles Plains, Australia) to measure SYBR green (Sensi Mix, R&D Systems) incorporation. The following primer sets were used: IL-10 : 5′-ATGCTGCCTGCTCTTACTGACTG-3′ and 5′-CCCAAGTAACCCTTAAATCCTGC-3′ [66]; β-actin: 5′-CCCTGAAGTACCCCATTGAAC-3′ and 5′-CTTTTCACGGTTGGCCTTAG-3′. The amount of IL-10 mRNA was normalized to β-actin RNA levels for each sample and was expressed relative to the levels of uninfected control mice.
Statistical analysis
The two-tailed unpaired t test was applied for statistical analysis when the distribution was Gaussian. Otherwise, the Mann-Whitney test was performed. * p<0.05; ** p<0.01; ***p<0.001; **** p<0.0001; n.s. not significant.
Supporting Information
Zdroje
1. FrebelH, RichterK, OxeniusA (2010) How chronic viral infections impact on antigen-specific T-cell responses. Eur J Immunol 40 : 654–663.
2. BrooksDG, TrifiloMJ, EdelmannKH, TeytonL, McGavernDB, et al. (2006) Interleukin-10 determines viral clearance or persistence in vivo. Nat Med 12 : 1301–1309.
3. EjrnaesM, FilippiCM, MartinicMM, LingEM, TogherLM, et al. (2006) Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med 203 : 2461–2472.
4. BrockmanMA, KwonDS, TigheDP, PavlikDF, RosatoPC, et al. (2009) IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood 114 : 346–356.
5. FlynnJK, DoreGJ, HellardM, YeungB, RawlinsonWD, et al. (2011) Early IL-10 predominant responses are associated with progression to chronic hepatitis C virus infection in injecting drug users. J Viral Hepat 18 : 549–561.
6. DasA, EllisG, PallantC, LopesAR, KhannaP, et al. (2012) IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J Immunol 189 : 3925–3935.
7. ClericiM, WynnTA, BerzofskyJA, BlattSP, HendrixCW, et al. (1994) Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with the human immunodeficiency virus. J Clin Invest 93 : 768–775.
8. LandayAL, ClericiM, HashemiF, KesslerH, BerzofskyJA, et al. (1996) In vitro restoration of T cell immune function in human immunodeficiency virus-positive persons: effects of interleukin (IL)-12 and anti-IL-10. J Infect Dis 173 : 1085–1091.
9. RigopoulouEI, AbbottWG, HaighP, NaoumovNV (2005) Blocking of interleukin-10 receptor–a novel approach to stimulate T-helper cell type 1 responses to hepatitis C virus. Clin Immunol 117 : 57–64.
10. WilsonEB, BrooksDG (2011) The role of IL-10 in regulating immunity to persistent viral infections. Curr Top Microbiol Immunol 350 : 39–65.
11. CouperKN, BlountDG, RileyEM (2008) IL-10: the master regulator of immunity to infection. J Immunol 180 : 5771–5777.
12. SlobedmanB, BarryPA, SpencerJV, AvdicS, AbendrothA (2009) Virus-encoded homologs of cellular interleukin-10 and their control of host immune function. J Virol 83 : 9618–9629.
13. ChangWL, BarryPA (2010) Attenuation of innate immunity by cytomegalovirus IL-10 establishes a long-term deficit of adaptive antiviral immunity. Proc Natl Acad Sci U S A 107 : 22647–22652.
14. PaladinoN, FainboimH, TheilerG, SchroderT, MunozAE, et al. (2006) Gender susceptibility to chronic hepatitis C virus infection associated with interleukin 10 promoter polymorphism. J Virol 80 : 9144–9150.
15. HelminenM, LahdenpohjaN, HurmeM (1999) Polymorphism of the interleukin-10 gene is associated with susceptibility to Epstein-Barr virus infection. J Infect Dis 180 : 496–499.
16. CheongJY, ChoSW, HwangIL, YoonSK, LeeJH, et al. (2006) Association between chronic hepatitis B virus infection and interleukin-10, tumor necrosis factor-alpha gene promoter polymorphisms. J Gastroenterol Hepatol 21 : 1163–1169.
17. ShinHD, ParkBL, KimLH, JungJH, KimJY, et al. (2003) Interleukin 10 haplotype associated with increased risk of hepatocellular carcinoma. Hum Mol Genet 12 : 901–906.
18. ShinHD, WinklerC, StephensJC, BreamJ, YoungH, et al. (2000) Genetic restriction of HIV-1 pathogenesis to AIDS by promoter alleles of IL10. Proc Natl Acad Sci U S A 97 : 14467–14472.
19. MandaricS, WaltonSM, RulickeT, RichterK, Girard-MadouxMJ, et al. (2012) IL-10 suppression of NK/DC crosstalk leads to poor priming of MCMV-specific CD4 T cells and prolonged MCMV persistence. PLoS Pathog 8: e1002846.
20. Tang-FeldmanYJ, LochheadGR, LochheadSR, YuC, PomeroyC (2011) Interleukin-10 repletion suppresses pro-inflammatory cytokines and decreases liver pathology without altering viral replication in murine cytomegalovirus (MCMV)-infected IL-10 knockout mice. Inflamm Res 60 : 233–243.
21. LinMT, HintonDR, ParraB, StohlmanSA, van der VeenRC (1998) The role of IL-10 in mouse hepatitis virus-induced demyelinating encephalomyelitis. Virology 245 : 270–280.
22. BrooksDG, TeytonL, OldstoneMB, McGavernDB (2005) Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J Virol 79 : 10514–10527.
23. WilsonEB, KidaniY, ElsaesserH, BarnardJ, RaffL, et al. (2012) Emergence of distinct multiarmed immunoregulatory antigen-presenting cells during persistent viral infection. Cell Host Microbe 11 : 481–491.
24. NgCT, OldstoneMB (2012) Infected CD8alpha - dendritic cells are the predominant source of IL-10 during establishment of persistent viral infection. Proc Natl Acad Sci U S A 109 : 14116–14121.
25. FrebelH, OxeniusA (2013) The risks of targeting co-inhibitory pathways to modulate pathogen-directed T cell responses. Trends Immunol 34 : 193–199.
26. RichterK, BrockerT, OxeniusA (2012) Antigen amount dictates CD8+ T-cell exhaustion during chronic viral infection irrespective of the type of antigen presenting cell. Eur J Immunol 42 : 2290–2304.
27. SeilerP, AicheleP, OdermattB, HengartnerH, ZinkernagelRM, et al. (1997) Crucial role of marginal zone macrophages and marginal zone metallophils in the clearance of lymphocytic choriomeningitis virus infection. Eur J Immunol 27 : 2626–2633.
28. ZeisbergerSM, OdermattB, MartyC, Zehnder-FjallmanAH, Ballmer-HoferK, et al. (2006) Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer 95 : 272–281.
29. JungS, UnutmazD, WongP, SanoG, De los SantosK, et al. (2002) In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 17 : 211–220.
30. ProbstHC, van den BroekM (2005) Priming of CTLs by lymphocytic choriomeningitis virus depends on dendritic cells. J Immunol 174 : 3920–3924.
31. DudeckA, DudeckJ, ScholtenJ, PetzoldA, SurianarayananS, et al. (2011) Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity 34 : 973–984.
32. CrawfordA, AngelosantoJM, NadwodnyKL, BlackburnSD, WherryEJ (2011) A role for the chemokine RANTES in regulating CD8 T cell responses during chronic viral infection. PLoS Pathog 7: e1002098.
33. Penaloza-MacMasterP, Ur RasheedA, IyerSS, YagitaH, BlazarBR, et al. (2011) Opposing effects of CD70 costimulation during acute and chronic lymphocytic choriomeningitis virus infection of mice. J Virol 85 : 6168–6174.
34. RichterK, PerriardG, OxeniusA (2013) Reversal of chronic to resolved infection by IL-10 blockade is LCMV strain dependent. Eur J Immunol 43 : 649–654.
35. MadanR, DemircikF, SurianarayananS, AllenJL, DivanovicS, et al. (2009) Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J Immunol 183 : 2312–2320.
36. SieweL, Bollati-FogolinM, WickenhauserC, KriegT, MullerW, et al. (2006) Interleukin-10 derived from macrophages and/or neutrophils regulates the inflammatory response to LPS but not the response to CpG DNA. Eur J Immunol 36 : 3248–3255.
37. RoersA, SieweL, StrittmatterE, DeckertM, SchluterD, et al. (2004) T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J Exp Med 200 : 1289–1297.
38. SaraivaM, O'GarraA (2010) The regulation of IL-10 production by immune cells. Nat Rev Immunol 10 : 170–181.
39. HagiwaraE, AbbasiF, MorG, IshigatsuboY, KlinmanDM (1995) Phenotype and frequency of cells secreting IL-2, IL-4, IL-6, IL-10, IFN and TNF-alpha in human peripheral blood. Cytokine 7 : 815–822.
40. KumarA, AngelJB, DaftarianMP, ParatoK, CameronWD, et al. (1998) Differential production of IL-10 by T cells and monocytes of HIV-infected individuals: association of IL-10 production with CD28-mediated immune responsiveness. Clin Exp Immunol 114 : 78–86.
41. SaidEA, DupuyFP, TrautmannL, ZhangY, ShiY, et al. (2010) Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med 16 : 452–459.
42. HumphreysIR, de TrezC, KinkadeA, BenedictCA, CroftM, et al. (2007) Cytomegalovirus exploits IL-10-mediated immune regulation in the salivary glands. J Exp Med 204 : 1217–1225.
43. StaceyMA, MarsdenM, WangEC, WilkinsonGW, HumphreysIR (2011) IL-10 restricts activation-induced death of NK cells during acute murine cytomegalovirus infection. J Immunol 187 : 2944–2952.
44. BelkaidY, HoffmannKF, MendezS, KamhawiS, UdeyMC, et al. (2001) The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J Exp Med 194 : 1497–1506.
45. NorrisBA, UebelhoerLS, NakayaHI, PriceAA, GrakouiA, et al. (2013) Chronic but Not Acute Virus Infection Induces Sustained Expansion of Myeloid Suppressor Cell Numbers that Inhibit Viral-Specific T Cell Immunity. Immunity 38 : 309–321.
46. FiorentinoDF, BondMW, MosmannTR (1989) Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med 170 : 2081–2095.
47. MaynardCL, WeaverCT (2008) Diversity in the contribution of interleukin-10 to T-cell-mediated immune regulation. Immunol Rev 226 : 219–233.
48. PunkosdyGA, BlainM, GlassDD, LozanoMM, O'MaraL, et al. (2011) Regulatory T-cell expansion during chronic viral infection is dependent on endogenous retroviral superantigens. Proc Natl Acad Sci U S A 108 : 3677–3682.
49. SchmitzI, SchneiderC, FrohlichA, FrebelH, ChristD, et al. (2013) IL-21 restricts virus-driven Treg cell expansion in chronic LCMV infection. PLoS Pathog 9: e1003362.
50. EckelhartE, WarschW, ZebedinE, SimmaO, StoiberD, et al. (2011) A novel Ncr1-Cre mouse reveals the essential role of STAT5 for NK-cell survival and development. Blood 117 : 1565–1573.
51. PowellMJ, ThompsonSA, ToneY, WaldmannH, ToneM (2000) Posttranscriptional regulation of IL-10 gene expression through sequences in the 3′-untranslated region. J Immunol 165 : 292–296.
52. MaroofA, BeattieL, ZubairiS, SvenssonM, StagerS, et al. (2008) Posttranscriptional regulation of II10 gene expression allows natural killer cells to express immunoregulatory function. Immunity 29 : 295–305.
53. TittelAP, HeuserC, OhligerC, LlantoC, YonaS, et al. (2012) Functionally relevant neutrophilia in CD11c diphtheria toxin receptor transgenic mice. Nat Methods 9 : 385–390.
54. WilsonEB, YamadaDH, ElsaesserH, HerskovitzJ, DengJ, et al. (2013) Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science 340 : 202–207.
55. TeijaroJR, NgC, LeeAM, SullivanBM, SheehanKC, et al. (2013) Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science 340 : 207–211.
56. AicheleP, UnsoeldH, KoschellaM, SchweierO, KalinkeU, et al. (2006) CD8 T cells specific for lymphocytic choriomeningitis virus require type I IFN receptor for clonal expansion. J Immunol 176 : 4525–4529.
57. KolumamGA, ThomasS, ThompsonLJ, SprentJ, Murali-KrishnaK (2005) Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med 202 : 637–650.
58. WieselM, CrouseJ, BedenikovicG, SutherlandA, JollerN, et al. (2012) Type-I IFN drives the differentiation of short-lived effector CD8+ T cells in vivo. Eur J Immunol 42 : 320–329.
59. GazzinelliRT, OswaldIP, JamesSL, SherA (1992) IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J Immunol 148 : 1792–1796.
60. O'FarrellAM, LiuY, MooreKW, MuiAL (1998) IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3-dependent and -independent pathways. EMBO J 17 : 1006–1018.
61. WaggonerSN, CornbergM, SelinLK, WelshRM (2012) Natural killer cells act as rheostats modulating antiviral T cells. Nature 481 : 394–398.
62. RichterK, AgnelliniP, OxeniusA (2010) On the role of the inhibitory receptor LAG-3 in acute and chronic LCMV infection. Int Immunol 22 : 13–23.
63. KuhnR, LohlerJ, RennickD, RajewskyK, MullerW (1993) Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75 : 263–274.
64. KamanakaM, KimST, WanYY, SutterwalaFS, Lara-TejeroM, et al. (2006) Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity 25 : 941–952.
65. HamelK, DoodesP, CaoY, WangY, MartinsonJ, et al. (2008) Suppression of proteoglycan-induced arthritis by anti-CD20 B Cell depletion therapy is mediated by reduction in autoantibodies and CD4+ T cell reactivity. J Immunol 180 : 4994–5003.
66. NolteMA, Leibundgut-LandmannS, JoffreO, Reis e SousaC (2007) Dendritic cell quiescence during systemic inflammation driven by LPS stimulation of radioresistant cells in vivo. J Exp Med 204 : 1487–1501.
67. RickertRC, RajewskyK, RoesJ (1995) Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature 376 : 352–355.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2013 Číslo 11- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Baculoviruses: Sophisticated Pathogens of Insects
- How Do Viruses Avoid Inhibition by Endogenous Cellular MicroRNAs?
- The Regulation of Trypanosome Gene Expression by RNA-Binding Proteins
- DNA Damage Repair and Bacterial Pathogens
- Disease to Dirt: The Biology of Microbial Amyloids
- Fungal Immune Evasion in a Model Host–Pathogen Interaction: Versus Macrophages
- Infectious Prions Accumulate to High Levels in Non Proliferative C2C12 Myotubes
- The Biology and Taxonomy of Head and Body Lice—Implications for Louse-Borne Disease Prevention
- Antibodies Trap Tissue Migrating Helminth Larvae and Prevent Tissue Damage by Driving IL-4Rα-Independent Alternative Differentiation of Macrophages
- The Effects of Somatic Hypermutation on Neutralization and Binding in the PGT121 Family of Broadly Neutralizing HIV Antibodies
- Natural Selection Promotes Antigenic Evolvability
- Type I Interferon Protects against Pneumococcal Invasive Disease by Inhibiting Bacterial Transmigration across the Lung
- Mode of Parainfluenza Virus Transmission Determines the Dynamics of Primary Infection and Protection from Reinfection
- Type I and Type III Interferons Drive Redundant Amplification Loops to Induce a Transcriptional Signature in Influenza-Infected Airway Epithelia
- Unraveling a Three-Step Spatiotemporal Mechanism of Triggering of Receptor-Induced Nipah Virus Fusion and Cell Entry
- A Novel Membrane Sensor Controls the Localization and ArfGEF Activity of Bacterial RalF
- Macrophage and T Cell Produced IL-10 Promotes Viral Chronicity
- Global Rescue of Defects in HIV-1 Envelope Glycoprotein Incorporation: Implications for Matrix Structure
- Turning Defense into Offense: Defensin Mimetics as Novel Antibiotics Targeting Lipid II
- The Neonatal Fc Receptor (FcRn) Enhances Human Immunodeficiency Virus Type 1 (HIV-1) Transcytosis across Epithelial Cells
- Brd4 Is Displaced from HPV Replication Factories as They Expand and Amplify Viral DNA
- A Viral Genome Landscape of RNA Polyadenylation from KSHV Latent to Lytic Infection
- The Cytotoxic Necrotizing Factor of (CNF) Enhances Inflammation and Yop Delivery during Infection by Activation of Rho GTPases
- The Inflammatory Kinase MAP4K4 Promotes Reactivation of Kaposi's Sarcoma Herpesvirus and Enhances the Invasiveness of Infected Endothelial Cells
- Conservative Sex and the Benefits of Transformation in
- Microbial Endocrinology in the Microbiome-Gut-Brain Axis: How Bacterial Production and Utilization of Neurochemicals Influence Behavior
- Colonization Resistance: Battle of the Bugs or Ménage à Trois with the Host?
- Intracellular Interferons in Fish: A Unique Means to Combat Viral Infection
- SPOC1-Mediated Antiviral Host Cell Response Is Antagonized Early in Human Adenovirus Type 5 Infection
- Involvement of the Cellular Phosphatase DUSP1 in Vaccinia Virus Infection
- Killer Bee Molecules: Antimicrobial Peptides as Effector Molecules to Target Sporogonic Stages of
- A Unique SUMO-2-Interacting Motif within LANA Is Essential for KSHV Latency
- A Role for Host Activation-Induced Cytidine Deaminase in Innate Immune Defense against KSHV
- Haploid Genetic Screens Identify an Essential Role for PLP2 in the Downregulation of Novel Plasma Membrane Targets by Viral E3 Ubiquitin Ligases
- A Small Molecule Glycosaminoglycan Mimetic Blocks Invasion of the Mosquito Midgut
- Identification of the Adenovirus E4orf4 Protein Binding Site on the B55α and Cdc55 Regulatory Subunits of PP2A: Implications for PP2A Function, Tumor Cell Killing and Viral Replication
- Can Non-lytic CD8+ T Cells Drive HIV-1 Escape?
- Deletion of the α-(1,3)-Glucan Synthase Genes Induces a Restructuring of the Conidial Cell Wall Responsible for the Avirulence of
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Baculoviruses: Sophisticated Pathogens of Insects
- Identification of the Adenovirus E4orf4 Protein Binding Site on the B55α and Cdc55 Regulatory Subunits of PP2A: Implications for PP2A Function, Tumor Cell Killing and Viral Replication
- A Unique SUMO-2-Interacting Motif within LANA Is Essential for KSHV Latency
- Natural Selection Promotes Antigenic Evolvability
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



