-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEvades Immune Recognition of Flagellin in Both Mammals and Plants
The building blocks of bacterial flagella, flagellin monomers, are potent stimulators of host innate immune systems. Recognition of flagellin monomers occurs by flagellin-specific pattern-recognition receptors, such as Toll-like receptor 5 (TLR5) in mammals and flagellin-sensitive 2 (FLS2) in plants. Activation of these immune systems via flagellin leads eventually to elimination of the bacterium from the host. In order to prevent immune activation and thus favor survival in the host, bacteria secrete many proteins that hamper such recognition. In our search for Toll like receptor (TLR) antagonists, we screened bacterial supernatants and identified alkaline protease (AprA) of Pseudomonas aeruginosa as a TLR5 signaling inhibitor as evidenced by a marked reduction in IL-8 production and NF-κB activation. AprA effectively degrades the TLR5 ligand monomeric flagellin, while polymeric flagellin (involved in bacterial motility) and TLR5 itself resist degradation. The natural occurring alkaline protease inhibitor AprI of P. aeruginosa blocked flagellin degradation by AprA. P. aeruginosa aprA mutants induced an over 100-fold enhanced activation of TLR5 signaling, because they fail to degrade excess monomeric flagellin in their environment. Interestingly, AprA also prevents flagellin-mediated immune responses (such as growth inhibition and callose deposition) in Arabidopsis thaliana plants. This was due to decreased activation of the receptor FLS2 and clearly demonstrated by delayed stomatal closure with live bacteria in plants. Thus, by degrading the ligand for TLR5 and FLS2, P. aeruginosa escapes recognition by the innate immune systems of both mammals and plants.
Published in the journal: . PLoS Pathog 7(8): e32767. doi:10.1371/journal.ppat.1002206
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002206Summary
The building blocks of bacterial flagella, flagellin monomers, are potent stimulators of host innate immune systems. Recognition of flagellin monomers occurs by flagellin-specific pattern-recognition receptors, such as Toll-like receptor 5 (TLR5) in mammals and flagellin-sensitive 2 (FLS2) in plants. Activation of these immune systems via flagellin leads eventually to elimination of the bacterium from the host. In order to prevent immune activation and thus favor survival in the host, bacteria secrete many proteins that hamper such recognition. In our search for Toll like receptor (TLR) antagonists, we screened bacterial supernatants and identified alkaline protease (AprA) of Pseudomonas aeruginosa as a TLR5 signaling inhibitor as evidenced by a marked reduction in IL-8 production and NF-κB activation. AprA effectively degrades the TLR5 ligand monomeric flagellin, while polymeric flagellin (involved in bacterial motility) and TLR5 itself resist degradation. The natural occurring alkaline protease inhibitor AprI of P. aeruginosa blocked flagellin degradation by AprA. P. aeruginosa aprA mutants induced an over 100-fold enhanced activation of TLR5 signaling, because they fail to degrade excess monomeric flagellin in their environment. Interestingly, AprA also prevents flagellin-mediated immune responses (such as growth inhibition and callose deposition) in Arabidopsis thaliana plants. This was due to decreased activation of the receptor FLS2 and clearly demonstrated by delayed stomatal closure with live bacteria in plants. Thus, by degrading the ligand for TLR5 and FLS2, P. aeruginosa escapes recognition by the innate immune systems of both mammals and plants.
Introduction
The innate immune system detects microorganisms and rapidly responds to invasion by eliminating them. Toll-like receptors (TLRs) recognize various evolutionary conserved structures of microorganisms and play a crucial role in innate immune recognition [1]. Stimulation of these receptors triggers intracellular signaling cascades leading to activation of phagocytes and production of pro-inflammatory cytokines. TLRs are type-1 transmembrane proteins characterized by extracellular leucine-rich-repeat motifs and an intracellular Toll/interleukin-1 receptor domain. Dimerization of TLRs is important for activation and ligand recognition, for example TLR2 recognizes diacylated lipopeptides in combination with TLR1 and triacylated lipopeptides together with TLR6. The most studied TLR member is TLR4, which detects the Gram-negative outer membrane component lipopolysaccharide (LPS). TLR5 senses flagellin [2], which is the major component of the bacterial flagellum.
Flagella consist of a basal body, the flagellar hook and a filament which serves as a propeller [3]. The filament consists of 11 protofilaments composed of several thousand flagellin monomers. Flagellin molecules from various bacteria have a conserved N - and C-terminus and a hypervariable central domain. The conserved regions are important in protofilament formation and motility. TLR5 recognizes a conserved part of flagellin that is buried in the flagellar filament and is only accessible in flagellin monomers [4]. By recognizing this part of flagellin, TLR5 detects almost all flagellated bacteria. Mutation of the TLR5-recognition site generally impairs protofilament assembly and thereby motility and virulence [5]. However, in the human pathogens Campylobacter jejuni and Helicobacter pylori the flagellin is changed in such a way that it is no longer recognized by TLR5, while motility is not affected [6].
Plants have evolved a similar sensing system for flagellin as mammals [7]. In Arabidopsis thaliana, stimulation of the plasma membrane-located receptor FLS2 [8] results in the activation of defense responses, such as callose deposition and the production of pathogenesis-related proteins [7]. Flagellin recognition contributes to the resistance of Arabidopsis to the bacterial pathogen Pseudomonas syringae [9]. Although TLR5 and FLS2 serve a similar function in pathogen recognition, the composition of the receptor, as well as the downstream signaling pathways differ considerably. Furthermore, FLS2 recognizes a different epitope of flagellin than does TLR5. A peptide, called flg22, consisting of 22 amino acids derived from the highly conserved N-terminal region of P. syringae flagellin activates FLS2 even better than purified flagellin [7], [10].
Pseudomonas aeruginosa is a common environmental Gram-negative bacterium, which acts as an opportunistic pathogen in humans and plants. Normally, the human host counteracts this microorganism effectively via the innate immune system [11]. However, immunocompromised patients, severe burn victims and cystic fibrosis patients are sensitive for P. aeruginosa infections. Due to its tendency to colonize surfaces in a biofilm, the bacterium is impervious to therapeutic concentrations of many antibiotics [12].
Detection of P. aeruginosa by TLRs activates the innate immune system and protects the host from infection [13]. Flagellin of P. aeruginosa is a potent TLR5 activator. It is released during bacterial growth, because the long flagellum tail is easily disrupted [14]. The contribution of TLR5 to the inflammatory response of P. aeruginosa may be masked by activation of TLR4 by bacterial LPS [15]. Both receptors cooperate to defend the host from infection: the absence of both TLR4 and TLR5 results in hypersusceptibility for lung infection in mice [16]. Recognition of P. aeruginosa flagellin is important for the efficient clearance of the bacterium in mice [17].
Two extracellular proteases of P. aeruginosa that exert their activity at the invasive stage have been associated with virulence i.e. elastase and alkaline protease. Elastase cleaves collagen, IgG, IgA, and some proteins of the complement system [18]. It also degrades fibronectin to expose receptors for bacterial attachment on the mucosa of the lung [19]. Elastase disrupts the respiratory epithelium and interferes with ciliary function. So far, alkaline protease and elastase together are also reported to cause the inactivation of gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) [20]. A Pseudomonas entomophila strain, that lacks alkaline protease was shown to be less virulent and persistent in a Drosophila infection model [21].
Immune evasion is an important strategy for bacteria to survive in their host. Immune evasion molecules are crucial for this survival, as has been demonstrated for several human and plant pathogens [22], [23], [24], [25]. During infection, monomeric flagellin released from bacterial flagella activates TLR5 signaling. To evade innate immune recognition, some bacteria have evolved strategies in which they manipulate flagellin to impair TLR5 activation [6]. We hypothesized that bacteria secrete proteins that interfere with recognition of TLRs. In our search for immune evasion molecules that act as TLRs antagonists, we identified alkaline protease of P. aeruginosa as a TLR5 signaling inhibitor.
Results
A secreted protein of P. aeruginosa inhibits TLR5 activation
To identify TLR5 inhibitors, we screened several culture supernatants of Gram-positive and Gram-negative bacteria for inhibitory activity on activation of TLR5-transfected HEK cells (HEK/TLR5) containing an NF-κB luciferase reporter. Stimulation of HEK/TLR5 with flagellin triggers the activation of NF-κB and the secretion of Interleukin 8 (IL-8) into culture supernatants. These cells are insensitive for the TLR4 ligand LPS, whereas they respond to monomeric flagellin in the picomolar range (Figure 1A and B). Most bacterial supernatants did not significantly inhibit IL-8 production upon flagellin stimulation. However, the supernatant of P. aeruginosa reduced activation of HEK/TLR5 cells consistently with about 30%. Therefore, this supernatant was chosen to isolate the potential TLR5 inhibitor.
Fig. 1. Fractionated P. aeruginosa supernatant inhibits TLR5 activation. 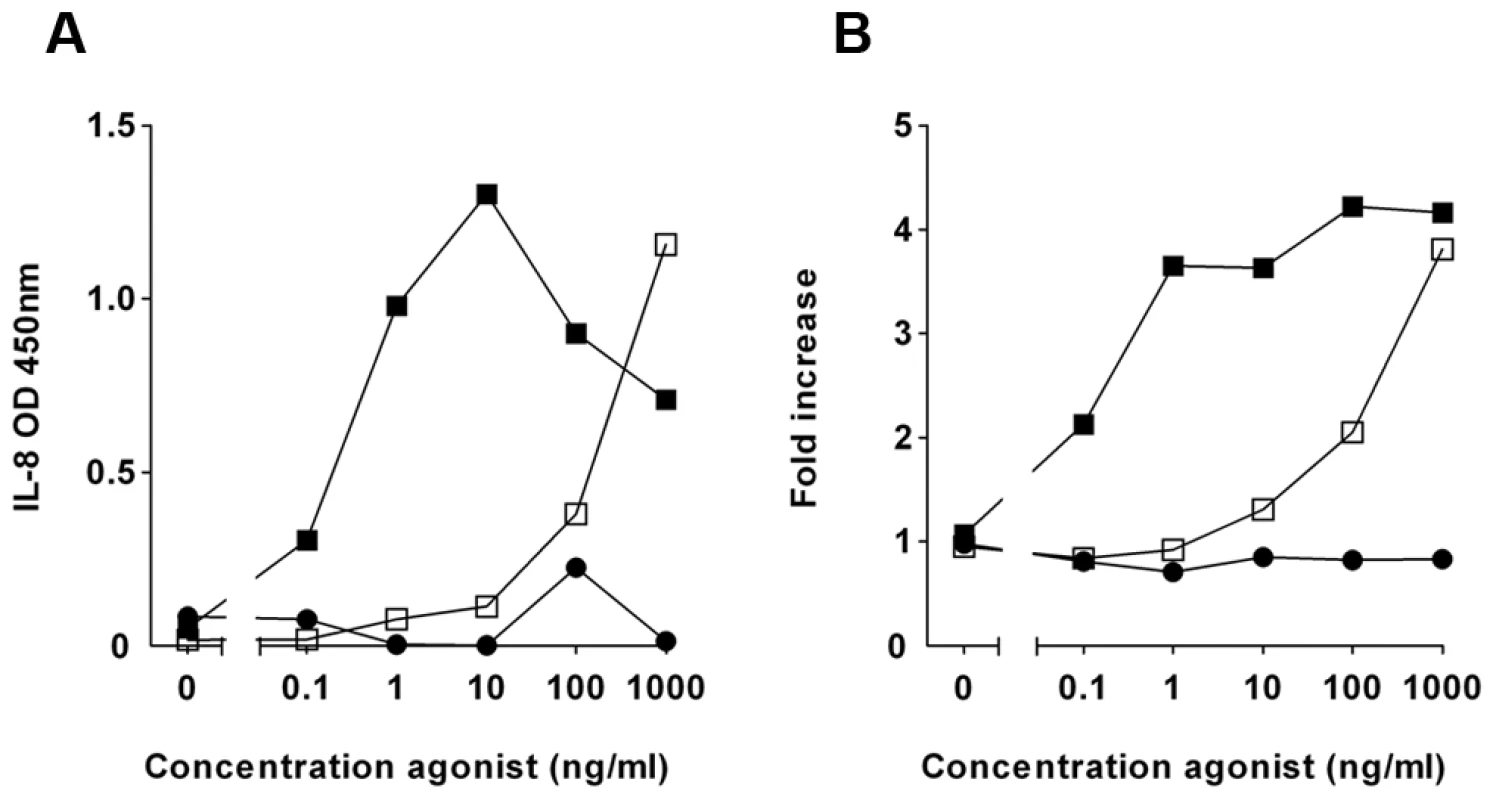
HEK/TLR5 cells were transfected with a NF-κB reporter construct. Cells were incubated with 20-fold diluted elution fraction from a Q sepharose column (inhibitor) for 30 min and subsequently challenged with various concentrations recombinant flagellin of S. Typhimurium (□). Flagellin without inhibitor (▪) and LPS (•). (A) After 6 h the IL-8 concentration in the cell culture supernatant was measured by ELISA. (B) NF-κB activation was determined by measuring luciferase activity in a luminometer and expressed as fold increase of luciferase activity over stimulation with culture medium alone. The presented data are representative for the inhibition of TLR5 signaling that is typically observed with purifications of P. aeruginosa supernatant. To purify the inhibitory compound from P. aeruginosa, we fractionated the supernatant with ion-exchange chromatography. In an agonist dose response experiment, specifically eluted fractions inhibited IL-8 production completely when the HEK/TLR5 cells were stimulated with up to 10 ng/ml flagellin of Salmonella enterica serovar Typhimurium (S. Typhimurium) (Figure 1A). In a parallel independent assay, the same fractions inhibited flagellin-stimulated NF-κB activation (Figure 1B). LPS-stimulation of HEK/TLR4 cells was not affected by the partially purified P. aeruginosa supernatant (Figure S1), which suggests that the inhibitor acts at the receptor level and not at downstream signaling routes. Additional purification of the inhibitory activity by size-exclusion chromatography resulted in one band by SDS-PAGE that correlated with TLR5-inhibiting activity of eluted fractions (Figure S2). We identified, using mass-spectrometry, the protein of interest as alkaline protease.
AprA inhibits TLR5 activation
The gene aprA of P. aeruginosa encodes alkaline protease (designated AprA), which is a 50 kD zinc metalloprotease [26]. AprA is secreted via a type I secretion system, which is encoded by the genes aprD, aprE and aprF [27] (Figure 2A). The gene downstream of aprA, aprI, encodes a highly specific inhibitor of AprA [28]. Some biological substrates of AprA have been described, such as the cytokine IFN-γ [20]. To verify that AprA inhibits TLR5-mediated cell activation, we cloned and expressed aprA with a 6x His-tag or together with the genes encoding the secretion apparatus in Escherichia coli and purified AprA from the medium. The TLR5 inhibitory activity of recombinant His-AprA and secreted recombinant AprA (data not shown) was comparable to that of purified AprA from P. aeruginosa (Figure 1A and 2B). Incubation of HEK/TLR5 cells with recombinant AprA abolished IL-8 production completely even when the cells were stimulated with flagellin concentrations up to 100 ng/ml (Figure 2B). To determine the potency of AprA, different concentrations were tested for inhibition of the flagellin-induced IL-8 production by HEK/TLR5 cells. Complete inhibition of cell activation by 30 ng/ml P. aeruginosa flagellin was observed with 0.3 µg/ml AprA with a half maximal inhibitory concentration of 30 ng/ml (Figure 2C). Flagellin isolated from P. aeruginosa also served as a potent stimulator of HEK/TLR5 cells. As for S. Typhimurium flagellin, AprA completely inhibited the P. aeruginosa flagellin response (Figure 2D). HEK/TLR5 cells are unresponsive to lipopolysaccharide, a contaminant of recombinant proteins isolated from E. coli, in contrast to naturally TLR5 sufficient cells like human neutrophils. To investigate the effect of AprA on TLR5 activation of human neutrophils, we incubated P. aeruginosa flagellin with AprA in the presence of polymyxin B, which neutralizes LPS activity. In addition to HEK/TLR5 cells, treatment of P. aeruginosa flagellin with 1 µg/ml AprA inhibited the IL-8 production of neutrophils completely (Figure 2E). These data demonstrate that AprA from P. aeruginosa is an inhibitor of TLR5-mediated cell activation by different types of flagellin.
Fig. 2. AprA prevents flagellin-induced IL-8 production by HEK/TLR5 cells. 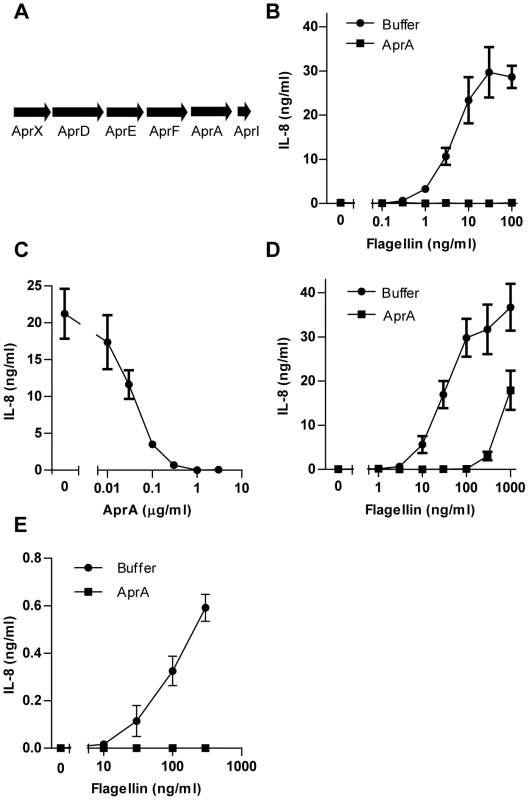
(A) Schematic representation of the gene cluster of aprA, aprI, genes involved in secretion, aprD, aprE, and aprF and aprX on the genome of P. aeruginosa strain PAO126. (B) Different concentrations of recombinant flagellin from S. Typhimurium were treated with 1 µg/ml recombinant His-AprA for 30 min and subsequently added to HEK/TLR5 cells. After 6 h IL-8 was measured in the supernatant by ELISA. (C) His-AprA concentration-dependent inhibition of flagellin-induced HEK/TLR5 cell activation. HEK/TLR5 cells were treated with varying concentrations of AprA and challenged with 30 ng/ml flagellin of P. aeruginosa. (D) Recombinant flagellin of P. aeruginosa was incubated with buffer or 1 µg/ml His-AprA for 30 min and subsequently added to HEK/TLR5 cells for IL-8 release. (E) Recombinant flagellin of P. aeruginosa was incubated with 1 µg/ml recombinant His-AprA in the presence of PMB (10 µg/ml) for 30 min at 37°C, and subsequently added to human neutrophils. After 16 h IL-8 concentration was measured by ELISA. Results represent mean IL-8 concentration ± SEM from three independent experiments. AprA cleaves flagellin
To determine the mechanism of TLR5 inhibition, we investigated the effect of AprA on TLR5 and flagellin separately. To study any direct effect of AprA on TLR5, cells were incubated with the protease for 30 min, washed and subsequently stimulated with flagellin. A washing step between AprA incubation and addition of flagellin preserved TLR5 activation (Figure 3A). This result indicates that TLR5 is not affected by AprA and still properly responds to flagellin.
Fig. 3. AprA of P. aeruginosa cleaves flagellin. 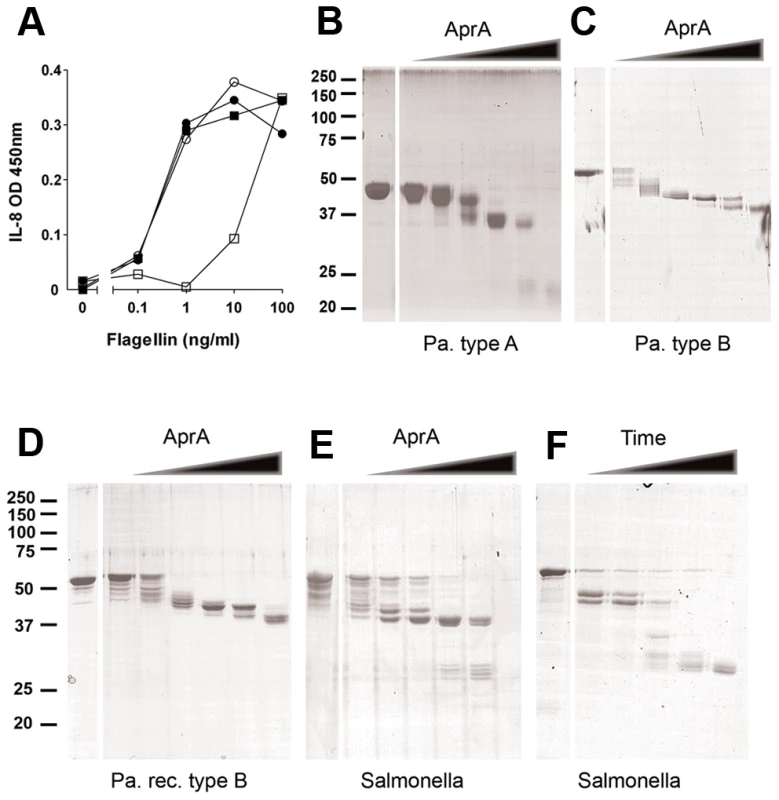
(A) HEK/TLR5 cells were treated for 30 min with AprA purified from P. aeruginosa culture supernatant and directly stimulated (□) or washed (○) before stimulation with varying concentrations flagellin from S. Typhimurium. As control cells were directly stimulated (•) or washed (▪) before addition of flagellin. After 6 h incubation, IL-8 was measured in the supernatant of HEK/TLR5 cells by ELISA. (B–F) Degradation of flagellin by recombinant AprA. Flagellin was mixed with 0, 0.01, 0.03, 0.1, 0.3, 1 and 3 µg/ml AprA for 60 min at 37°C in PBS and protein degradation was analyzed by SDS-PAGE and Coomassie staining. Cleavage of flagellin by AprA was compared for native monomeric (B) flagellin type B isolated from P. aeruginosa strain PAO25 (1 mg/ml), (C) flagellin type A isolated from clinical P. aeruginosa strain (150 µg/ml), (D) recombinant flagellin type B from P. aeruginosa (250 µg/ml) and (E) recombinant flagellin of S. Typhimurium (250 µg/ml). (F) Time-dependent degradation of flagellin by AprA. Flagellin (250 µg/ml) of S. Typhimurium was incubated with AprA for 0, 1, 3, 10, 30 and 60 min at 37°C in PBS. Another possible mechanism to interfere with TLR5 recognition is neutralization or proteolysis of flagellin. To test this hypothesis, isolated flagellin was incubated with AprA and degradation was analyzed by SDS-PAGE. Flagella from P. aeruginosa are composed of either type A or B flagellin, depending on the strain. These two flagellins contain a completely different variable domain, but showed comparable degradation patterns upon incubation with increasing concentrations AprA (Figure 3B and C). An identical cleavage pattern was observed for recombinant P. aeruginosa flagellin type B (Figure 3D). Moreover, AprA cleaved flagellin from another species i.e. S. Typhimurium (Figure 3E). Degradation of flagellin was time-dependent and started within one minute after addition of AprA (Figure 3F). Cleavage of flagellin occurred in multiple steps, dependent on the protease concentration and incubation time. At higher concentrations of AprA, flagellin type A (Figure 3B) and S. Typhimurium (Figure 3E) flagellin were completely degraded, while for P. aeruginosa flagellin type B (Figure 3C and D) a truncated protein of about 37 kD remained visible. The proteolytic activity of AprA is inhibited by 100 mM EDTA [29]. In our experiments EDTA also abolished degradation of flagellin (Figure S3). The optimum pH for AprA is pH 9–10 [18]; however in our experiments flagellin was efficiently degraded under physiological conditions. These findings demonstrate that P. aeruginosa secretes a protease that efficiently degrades flagellin of different species and thereby prevents activation via TLR5.
Flagellar filaments are not degraded by AprA
Flagellin is the most abundant protein of the flagellum, which is essential for bacterial motility and virulence. Secretion of a protease by P. aeruginosa that degrades the flagellum would be disadvantageous for the bacterium. Therefore, we investigated whether AprA also degrades complete flagellar filaments isolated from P. aeruginosa. These filaments consist of polymerized flagellin. Incubation of flagellin polymers with AprA did not result in degradation of flagellin (Figure 4A). However, after depolymerization, the resulting monomeric flagellin was degraded by AprA. Even at higher concentrations AprA did not cleave flagellar filaments as observed for monomeric flagellin. This indicates that AprA inactivates only monomeric flagellin in the surrounding of the bacterium, while the integrity of flagella is preserved.
Fig. 4. Polymeric flagellin resists cleavage by AprA and AprI is an efficient endogenous inhibitor. 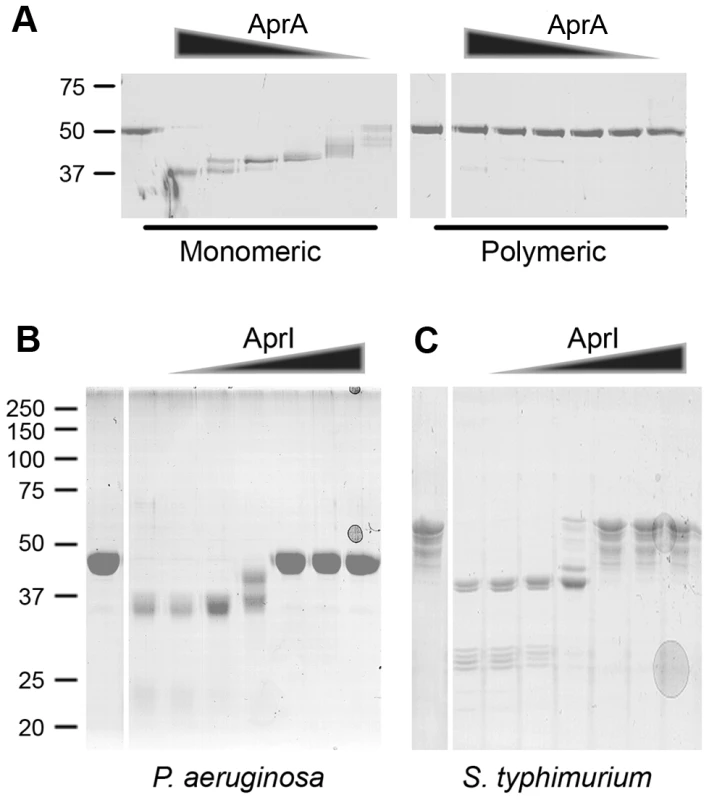
(A) Flagella isolated from PAO1 were treated for 20 min at 70°C to obtain monomeric flagellin. Untreated polymeric flagellin was compared with monomeric flagellin for susceptibility to AprA cleavage. Monomeric and polymeric flagellin was incubated with 0, 3, 1, 0.3, 0.1, 0.03 or 0.01 µg/ml AprA for 60 min at 37°C and analyzed by SDS-PAGE. (B and C) AprA (1 µg/ml) was incubated with 0, 0.03, 0.1, 0.3, 1, 3 or 10 µg/ml AprI and subsequently flagellin of (B) P. aeruginosa or (C) S. Typhimurium was added. Samples were analyzed by SDS-PAGE, untreated flagellin control is shown in the first lane followed by increasing AprI concentrations. AprI blocks AprA-mediated cleavage
Downstream of the aprA gene, aprI is located which, encodes for alkaline protease inhibitor (designated AprI). Kinetic studies revealed a very high affinity of this 11.5 kD protein for AprA [28]. To investigate whether AprI interferes with AprA-mediated cleavage, we cloned and expressed the aprI gene in E. coli as a His-tagged protein and purified it using Ni-affinity chromatography. AprI prevented cleavage of flagellin slightly at equimolar concentrations (Figure 4B and C). Flagellin cleavage was completely blocked at higher AprI concentrations. AprI protected both P. aeruginosa and S. Typhimurium flagellin against AprA-mediated proteolysis. Importantly, incubation of AprA with AprI before addition of flagellin restored activation of HEK/TLR5 cells dose-dependently (Figure S4). In conclusion, AprI of P. aeruginosa is a very potent inhibitor of AprA-mediated cleavage of flagellin.
Pseudomonas aprA mutant activates TLR5
As demonstrated above, purified recombinant AprA effectively blocked flagellin-induced TLR5 activation. To address the importance of this protease in a more natural environment, we compared the TLR5-activating capacity of culture supernatants from P. aeruginosa wild-type (WT) and aprA transposon-insertion mutants. In this setup, we tried to understand the dynamic interaction between the endogenous flagellin and AprA when released simultaneously by growing bacteria. Only the supernatant of the aprA mutant strains triggered TLR5 signaling (Figure 5A). Strikingly the supernatant of the wild-type strain did not initiate IL-8 production at all. This experiment indicates that AprA completely degraded flagellin monomers that were released in the supernatant during overnight growth. As expected, dilution of supernatants of the aprA mutants limited TLR5 activation due to a decrease in flagellin concentration (Figure 5A). To show that the absence of AprA is responsible for the TLR5-activating capacity in the aprA mutant strains, we supplemented the culture medium of these strains with recombinant AprA before inoculation. This resulted in the same lack of activation as for the wild-type strain supernatant (Figure 5B), demonstrating that AprA is essential as well as sufficient for degradation of flagellin. Complementary, addition of AprI to the culture medium abolished AprA-mediated cleavage of flagellin in the wild-type strain resulting in activation of TLR5 to comparable levels of that of the aprA mutant strains (Figure 5C). These results show that the wild-type strain does release flagellin in its environment. To investigate the inflammatory response of naturally TLR4 and TLR5 sufficient cells, we stimulated neutrophils with bacterial supernatant of wild-type and an aprA mutant strain. As for HEK-TLR5 cells, the aprA mutant strain triggered higher IL-8 production in comparison to wild-type P. aeruginosa (Figure 5D). Addition of polymyxin B, to neutralize LPS, slightly inhibited IL-8 production of neutrophils in response to supernatant of wild-type and the aprA mutant strain, without changing the difference in IL-8 production between the two strains. To verify that the aprA mutant strains did not produce AprA, the culture supernatant was probed with a specific antiserum against AprA by western blotting. As expected, the wild-type strain secreted AprA while the two aprA mutant strains did not (Figure 5E). Growth and motility of the mutant strains was comparable with wild-type. Together, these results demonstrate that P. aeruginosa secretes sufficient AprA to neutralize its own monomeric flagellin for detection via TLR5.
Fig. 5. Culture supernatant of aprA mutant strains trigger TLR5. 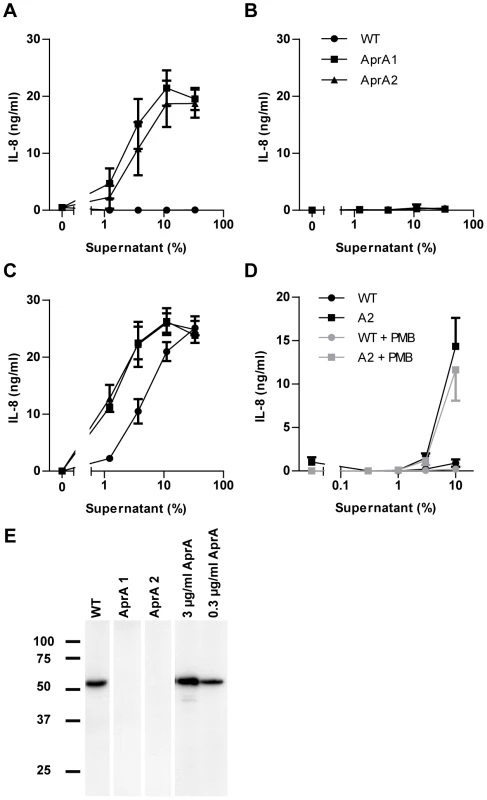
(A) Dilutions of bacterial culture supernatants, collected from overnight grown wild-type (WT), and isogenic aprA mutant strains were used as flagellin source to stimulate HEK/TLR5 cells for IL-8 production. (B) 3 µg/ml recombinant AprA was added to the culture medium before inoculation with WT, aprA1 or aprA2 mutant strains. HEK/TLR5 cells were incubated with dilutions of bacterial culture supernatants and stimulated for 6 h. IL-8 production was measured by ELISA. All three data sets completely overlap in this graph. (C) Wild-type and mutant P. aeruginosa strains were grown in the presence of 10 µg/ml exogenous AprI and dilutions of the culture supernatants were added to HEK/TLR5 cells for IL-8 release. Data are expressed as mean IL-8 concentration ± SD from triplicates. (D) Human neutrophils were stimulated with dilutions of bacterial culture supernatants of wild-type and aprA2 mutant strain. PMB (10 µg/ml) was added prior to stimulation for 30 min at 37°C. After 16 h stimulation, IL-8 concentration in cell supernatant was determined. Results represent mean ± SEM of three independent experiments. (E) Culture supernatant of overnight grown wild-type and aprA mutant strains or recombinant AprA were analyzed for the presence of AprA by immunoblotting. AprA interferes with flagellin recognition by Arabidopsis
Like mammals, plants also possess an innate immune system in which the detection of flagellin monomers results in the activation of effective immune responses [30]. In Arabidopsis, the FLS2 receptor has been demonstrated to specifically interact with a conserved 22-amino acid motif (flg22) of flagellin monomers [31]. Upon interaction of FLS2 with flagellin monomers or flg22, several downstream defense mechanisms are activated, amongst which the deposition of callose polymers [10]. Furthermore, as a result of the activation of energy-costly defense mechanisms, treatment with flagellin or flg22 has a negative effect on Arabidopsis growth [10]. To assess whether AprA activity can also prevent flagellin-induced defense activation in plants, we studied the effect of AprA on flagellin - or flg22-induced callose deposition and growth inhibition of Arabidopsis seedlings. P. aeruginosa flagellin monomers triggered callose deposition (Figure 6A) and affected growth (Figure S6) to a slightly less extent than did flg22, which is in line with earlier observations [30]. Preincubation of flagellin or flg22 with AprA abolished callose deposition (Figure 6A) and restored plant growth to control levels (Figure S5). This result indicates that AprA disrupts the active epitope of flagellin that is normally recognized by FLS2. Moreover, AprA completely neutralized the effects of the peptide flg22, these results suggest that AprA cleaves flagellin at least within this 22-amino acids long conserved motif. Therefore, we examined the mass of protease-treated flg22 and indeed observed clear degradation of the peptide by SELDI-TOF (Figure S6). Addition of AprI prior to AprA treatment of flagellin neutralized the AprA-mediated effects in Arabidopsis, while AprI had no effect when added after AprA treatment of flagellin (Figure 6A, Figure S5).
Fig. 6. AprA prevents recognition of flagellin in Arabidopsis and prevents stomatal closure. 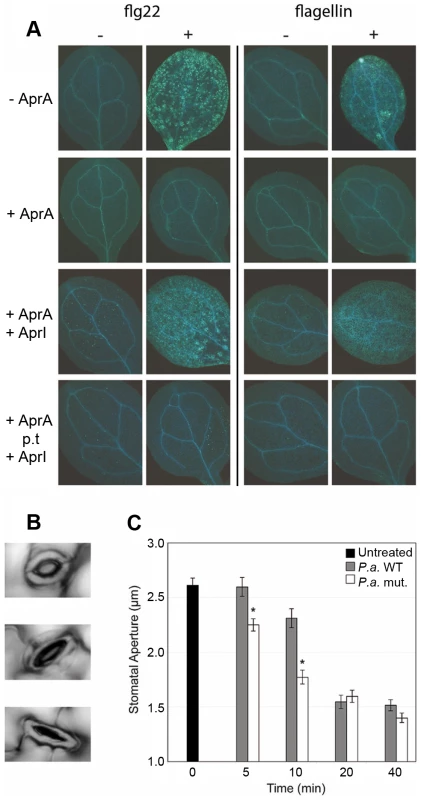
A. thaliana La-er seedlings were incubated with or without 500 nM flg22 or P. aeruginosa flagellin preincubated with 3 µg/ml AprA when indicated. (A). After treatment for 24 h, seedlings were stained for callose deposition by aniline blue and fluorescence was photographed under UV light. In the 3rd row panels AprI was added before AprA treatment and in the bottom panels post AprA treatment (p.t.) of flagellin. (B) Examples of open (top), half open (middle) and closed (bottom) stomata, that were observed during the experiment. (C) Stomatal aperture on leaves of 5-week-old A. thaliana plants up to 40 minutes after treatment with P. aeruginosa PAO1 or isogenic aprA mutant strain (n = 108 to 224). Error bars indicate SEM. Asterisks indicate significant differences (Student's t-test; p<0.001) between WT and AprA mutant treated plants. AprA delays early plant immune responses
The epidermis of plant leaves contains many pores (stomata) of which the aperture is dependent on environmental factors such as humidity and CO2 concentration [32]. Previously, Melotto et al. [33] showed that besides these environmental cues, recognition of bacterial PAMPs, such as flagellin and LPS, trigger plant immune responses that lead to rapid stomatal closure (Figure 6B). In this way, the stomata have an important early defense function that actively prevents bacteria from entering the host [33]. We hypothesized that degradation of flagellin by AprA likely affects the speed of stomatal closure upon bacterial inoculation. Therefore, we monitored the stomatal aperture of A. thaliana leaves after inoculation with wild-type and mutant P. aeruginosa bacteria. Figure 6C shows that both wild-type PAO1 and the aprA mutant strain triggered closure of the stomata within 40 minutes. However, the stomatal aperture decreased significantly faster after treatment with the aprA mutant strain. Hence, we conclude that AprA produced by wild-type bacteria plays an important role in the evasion of host immunity of A. thaliana by hampering closure of the natural pores that are crucial for bacterial invasion.
Discussion
P. aeruginosa produces various proteases that degrade several host proteins and are associated with virulence [20]. Alkaline protease of P. aeruginosa is involved in suppression of the immune response by degradation of cytokines including TNF-α and IFN-γ. Screening for bacterial TLR5 inhibitors resulted in the identification of AprA of P. aeruginosa. In this study, we describe flagellin as an AprA substrate. TLR5 is unaffected by the proteolytic activity of this metalloprotease, however its ligand flagellin is degraded effectively and loses stimulatory activity
The only known ligand for TLR5 is bacterial flagellin, which triggers the production of pro-inflammatory cytokines. Only monomeric flagellin activates TLR5 signaling, while in the polymeric form, which is present in flagella, the TLR5 recognition site is inaccessible. Previous studies showed that the N - and C-terminus of flagellin are located inside the flagellar filament [34]. This highly conserved part of flagellin activates TLR5 and is essential for filament assembly. Here we show that AprA fails to degrade polymeric flagellin and therefore does not affect motility. In this way, P. aeruginosa protects its functional flagellin present in flagella, while it neutralizes TLR5-activating monomeric flagellin (Figure 7). The proteolytic activity of AprA can be inhibited with EDTA [29] and with a natural inhibitor of P. aeruginosa, AprI, which blocks the catalytic site of AprA [26]. We demonstrate that AprI prevents AprA-mediated flagellin cleavage. The physiological role for AprI is unclear. An obvious possibility is that it protects intracellular bacterial proteins from degradation.
Fig. 7. Proposed mechanism for AprA. 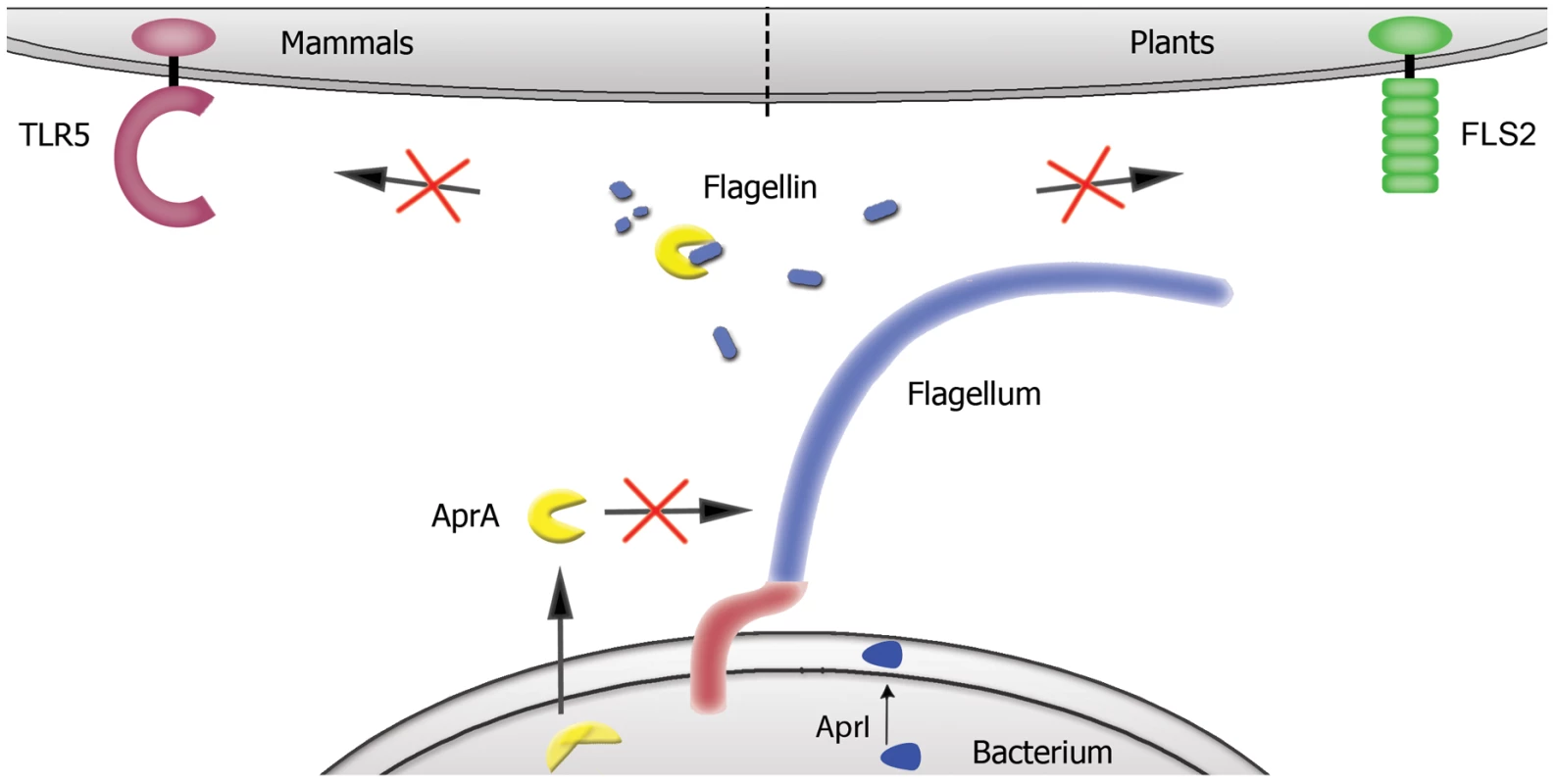
P. aeruginosa secretes AprA, which degrades free monomeric flagellin in the surrounding of the bacterium, whereas polymeric flagellin present in flagella is not affected. In this way flagellin is not recognized by TLR5 and FLS2, and thereby P. aeruginosa escapes activation of the innate immune system in both mammals and plants. AprA is secreted in one step over both membranes and is not present in its active form in the cytoplasm of the bacterium. Bacterial release of monomeric flagellin [14] results in the activation of TLR5. Since picomolar concentrations of flagellin can trigger TLR5, highly efficient degradation is a prerequisite to abolish TLR5 signaling. P. aeruginosa produces sufficient amounts of AprA to degrade its own released flagellin completely, resulting in avoidance of TLR5 activation. Mutant aprA strains of P. aeruginosa lacking AprA illustrate that AprA is responsible for this effect, because supernatants of these strains activate TLR5 signaling. This is best demonstrated in our experiments where we use supernatants of wild-type bacteria that contain endogenous amounts of flagellin and AprA (Figure 5A). No TLR5 activation was observed in these experiments, while the same experiments with mutant strains lacking AprA showed high flagellin-mediated stimulatory capacity. The effect of TLR4 and TLR5 in the host defense against P. aeruginosa was shown to be redundant [13], [16]. However, TLR5 is the only TLR that is significantly higher expressed on neutrophils in the cystic fibrosis lung [35]. In addition, flagellin triggers phagocytosis of P. aeruginosa and oxidative burst by peripheral blood neutrophils. Supernatant of an aprA mutant enhanced the inflammatory response of human neutrophils in comparison to wild-type P. aeruginosa supernatant. This suggests that flagellin-mediated TLR5 activation is important to stimulate an inflammatory response against P. aeruginosa, which is inhibited by AprA. Liehl et al. [21] recently reported that an aprA knockout strain of Pseudomonas entomophila showed decreased virulence in a Drosophila infection model. In their model, P. entomophila AprA is necessary to persist in the host and for pathogenicity. Moreover, AprA protected against the Drosophila immune response. Although no homolog of TLR5 is described in Drosophila, flagellin does trigger the production of antimicrobial peptides [36]. It is tempting to speculate that the immune evasion strategy of P. aeruginosa is also operational in this model. In this scenario, escape of innate immune detection by AprA-mediated degradation of pathogen-derived monomeric flagellin in the environment, avoids the production of antimicrobial peptides by the host, which results in a more persistent infection with P. entomophila.
In plants we observe the same phenomenon. The FLS2 flagellin receptor in Arabidopsis recognizes a different epitope of flagellin then does TLR5. By affecting the ligand instead of targeting the receptor, P. aeruginosa evades recognition of flagellin by both FLS2 and TLR5. The same was true for the flagellin-derived peptide flg22, which stimulates FLS2. Stomatal closure is important to prevent bacteria from entering the plant and recognition of flagellin by FLS2 plays a profound role in this response [37]. P. aeruginosa lacking a functional aprA gene triggers faster closure of stomata in comparison with wild-type. Thus, AprA interferes with this early defense response. Although P. aeruginosa itself is not a true plant pathogen, other Pseudomonas species that infect Arabidopsis like P. syringae also contain an apr operon. AprA and its inhibitor AprI of P. syringae share similarity with AprA and AprI of P. aeruginosa of 71% and 50%, respectively. Hence, evasion of TLR5 and FLS2 recognition as we observed for P. aeruginosa may be a broad mechanism utilized by various bacterial species.
AprA belongs to the superfamily of metzincin metalloproteases and to the family of serralysins [38]. Proteases from Serratia marcescens and Erwinia chrysanthemi belong to the family of serralysins and share high sequence similarity with AprA [39]. These bacteria also possess a highly specific protease inhibitor and a similar secretion system for AprA. In these flagellated bacteria AprA homologs may degrade flagellin in the same way as observed for P. aeruginosa. AprA cleaves flagellin from both P. aeruginosa and S. Typhimurium. Since the cleavage site of AprA is within the conserved domain of flagellin, cleavage of flagellin from other flagellated bacteria can be expected.
Innate immune defense systems recognize evolutionary conserved structures. Evasion of immune receptors is a smart strategy of pathogens to escape activation of the host innate immune response [40], [41]. For a bacterium with a broad host range it is of great advantage to evade activation of the innate immune system by degrading the ligand that is recognized by the host. Here we provide evidence that P. aeruginosa has evolved such a system to circumvent TLR5 and FLS2 activation by flagellin degradation. The consequence is that the benefit for the bacterium, in this case intact flagella and movement should be protected. By secreting AprA, which is specific for monomeric form of flagellin, Pseudomonas has tackled this, without affecting the structure of flagellin in the flagellum. In this way Pseudomonas can evade the activation of pattern-recognition receptors such as TLR5 and FLS2 signaling, creating a window of opportunity to evade the immune system of different hosts and cause disease.
Materials and Methods
Cell culture and bacterial strains
Dulbecco's modified Eagle's medium (DMEM) and Iscoves modified Dulbecco's medium (IMDM) (Invitrogen), fetal bovine serum (FCS) (Gibco), Human embryonic kidney cells transfected with TLR4/CD14/MD-2 (HEK/TLR4) or TLR5 (HEK/TLR5) and Normocin and Blasticidin (all Invivogen) were used for cell and bacterial culture. Human neutrophils from healthy volunteers were isolated as described [40]. IL-8 ELISA kit and high performance ELISA buffer (HPE) were purchased from Sanquin. P. aeruginosa strain PAO25 is a leu arg mutant derivative of strain PAO1 [42]. Clinical isolates of P. aeruginosa, S. Typhimurium, Staphylococcus haemolyticus, Staphylococcus hominis, Staphylococcus warneri, Streptococcus milleri, Enterobacter cloacae, Klebsiella pneumoniae, Escherichia coli, Serratia adorigen, Listeria monocytogenes and Enterobacter cloacae were obtained within the UMC Utrecht and screened for TLR5 antagonists. Competent E. coli TOP10F' and BL21 (DE3) pLys were purchased from Invitrogen and flg22 from Genscript.
HEK/TLR5 assay
HEK/TLR5 cells were maintained in DMEM supplemented with 10% FCS, 10 µg/ml Blasticidin and 100 µg/ml Normocin. Monolayers of HEK/TLR4 and HEK/TLR5 cells were preincubated with 5-fold diluted bacterial supernatant for 30 min and subsequently stimulated with flagellin for 6 h at 37°C. Cell culture supernatant was harvested and stored at −20°C for analysis. Samples were diluted in HPE buffer and IL-8 concentrations were determined by ELISA following manufacturer's protocol, using a standard curve. For some experiments relative IL-8 amounts are expressed as OD at 450 nm.
To measure NF-κB activation, HEK/TLR4 and HEK/TLR5 cells were transiently transfected 2–3 days before stimulation with a NF-κB reporter plasmid pHIV-CAT [43] (AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH). Transfected cells were stimulated with LPS or flagellin for 5 h at 37°C. Cells were lysed with lysis buffer/substrate (Promega) according to manufacturer's protocol and chemiluminescence was measured using a Centro LB 960 microplate luminometer (Berthold). NF-κB activation is expressed as stimulation index, which represents the ratio between stimulated versus control cells.
Neutrophil assay
Flagellin was incubated with 1 µg/ml AprA in the presence of 10 µg/ml polymyxin B (PMB) (Sigma) for 30 min at 37°C. Neutrophils (1.25×105/well) were stimulated with AprA-treated flagellin for 16 h at 37°C. Cell culture supernatant was harvested and stored at −20°C for analysis by IL-8 ELISA.
Isolation and purification of AprA
P. aeruginosa (clinical isolate) was cultured overnight in IMDM under constant agitation and supernatant was collected by centrifugation and filtration. Supernatant was applied on a Q sepharose XL column and eluted with PBS +2 M NaCl pH 7.4 using an Akta FPLC system (GE Healthcare). Active fractions were pooled and concentrated by lyphophilization and resuspended in PBS before gel filtration on a Superdex 75 column (GE Healthcare). Subsequently, active fractions were precipitated with trichloroacetic acid and separated by 12.5% SDS-PAGE gels and stained with silver. Proteins of interest were identified by mass-spectrometry (Alphalyse).
Recombinant AprA was produced in E. coli using two plasmids: i) pAG302 (kindly provided by A. de Groot) contains the aprA and aprI genes under control of the tac promoter on vector pUR6500, a derivative of pMMB67EH containing a kanamycin-resistance cassette; ii) pJF1 (kindly provided by J. Folders) containing the aprD, aprE, and aprF genes under control of the lac promoter on vector pBBR1MCS, which contains a chloramphenicol-resistance gene and is necessary for secretion of AprA. The two plasmids were used to transform E. coli BL21 and clones resistant to chloramphenicol and kanamycin were selected. Protein expression was induced by addition of 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) for 3–4 h at 37°C. Supernatant was collected and diafiltrated against 20 mM phosphate buffer pH 7 using a proflux M-12 system (Millipore). AprA was applied on a Q sepharose XL column and eluted with 20 mM phosphate-buffered 2 M NaCl pH 7. Fractions containing AprA were concentrated using a 3 kD filter (Millipore) and applied on a Superdex 75 column.
For His-tagged AprA, the gene aprA (without the nine residues propeptide) was amplified from genomic PAO1 DNA using a forward and reverse primer with incorporated 5′BamH1 and 3′Not1, respectively. The PCR product was ligated in a modified pET302 vector (Invitrogen) encoding an N-terminal 6x His-tag and transformed in E. coli Top10F' cells (Invitrogen). After sequence verification, the constructs were transformed in E. coli BL21 and His-tagged proteins was purified under denaturing conditions according to manufacturer's instructions using a His trap column (GE Healthcare). Denatured AprA was diluted 50 times in 0.8 M L-arginine +50 mM Tris-HCl pH 9+1 mM CaCl2 at 4°C overnight [44]. After renaturation, AprA was concentrated on a Amicon 30 kD spin column (Millipore) and washed two times with 50 mM HEPES pH 7.8+1 mM CaCl2+0.1 mM ZnCl2. Purity was examined with SDS-PAGE and Coomassie staining.
AprI isolation
The gene aprI without signal sequence of P. aeruginosa strain PAO1 with Xba1 restriction site, 6x His-tag and enterokinase cleavage site fused to the 5′-end and a 3′ EcoR1 restriction site was synthesized by BaseClear. The aprI construct was ligated into a pRSETB vector and used to transform E. coli Top10F' cells. Protein expression was performed in E. coli BL21 and alkaline protease inhibitor was purified under denaturing conditions according to manufacturer's instructions using a His trap FF column (GE Healthcare). Purified denatured protein was diluted 10-fold in PBS and concentrated on a His trap column. Purity of AprI was assessed by SDS-PAGE and the protein was dialyzed against PBS.
Recombinant flagellin isolation
Constructs for recombinant S. Typhimurium and P. aeruginosa flagellin were generated by an overhang extension polymerase chain reaction (PCR) as described previously [40]. Briefly, genes fliC of S. Typhimurium (clinical isolate) and flagellin type B of P. aeruginosa strain PAO25 were cloned directly downstream of the 6x His-tag and enterokinase cleavage site of the pRSETB vector (Invitrogen). PCRs were performed using VentR DNA polymerase (New England Bio Labs). After verification of the sequence the vector was used to transform E. coli BL21 and the protein expression was performed as described for AprA. His-tagged flagellin was isolated by lysing bacteria with cellytic B according to manufacturer's instructions (Sigma) supplemented with DNAse/RNAse and protease inhibitor cocktail (Roche) followed by purification with a His trap FF column. The protein was eluted with 0.5 M imidazole in PBS and dialyzed against PBS. Purity was assessed by SDS-PAGE with Coomassie staining.
Isolation of native flagellar filaments from P. aeruginosa
P. aeruginosa strain PAO25 (flagellin type B) and a clinical isolate (flagellin type A) were grown overnight in Luria-Bertani broth and bacteria were pelleted by centrifugation. Pellets were resuspended in PBS and flagella were sheared from bacteria by blending, followed by centrifugation at 8,000 g for 15 min to pellet the bacteria. Flagella were collected from the supernatant by centrifugation at 100,000 g for 60 min. The pellets obtained were resuspended in PBS and purity was examined by SDS-PAGE. Isolated flagella were heated at 70°C for 20 min for depolymerization.
P. aeruginosa transposon mutants
P. aeruginosa mutants aprA1 (3969) and aprA2 (16254) were obtained from the P. aeruginosa PAO1 transposon mutant library [45] (University of Washington). Transposon insertions in the aprA mutant strains were confirmed by PCR. Bacteria were grown overnight in IMDM and supernatant was harvested by centrifugation and filtration. Where indicated, 3 µg/ml recombinant AprA or 10 µg/ml AprI was added to the culture medium before inoculation with the strains. HEK/TLR5 cells were stimulated with dilutions of these overnight culture supernatants and IL-8 release was measured by ELISA. For neutrophil stimulation bacterial supernatant was incubated with 10 µg/ml PMB for 30 min at 37°C. Neutrophils were stimulated with untreated and PMB-treated bacterial supernatant for 16 h at 37°C. Cell culture supernatant was harvested and IL-8 concentration was determined by ELISA.
Flagellin and flg22 degradation
Recombinant AprA, AprI and flagellin of P. aeruginosa or S. Typhimurium in PBS were incubated for 1 h (unless specified otherwise) at 37°C. Cleavage products were analyzed by SDS-PAGE and stained with Coomassie. AprA was inhibited by preincubation with 100 mM EDTA or AprI before addition of flagellin. Flg22 was incubated with 1 µg/ml AprA for 1 h at 37°C. The sample was spotted on a NP-20 array (Biorad) and analyzed using a ProteinChip SELDI reader (Biorad).
AprA detection
The presence of AprA in supernatants of P. aeruginosa mutants was checked by Western blotting using a polyclonal rabbit AprA antiserum (generously provided by R. Voulhoux). Recombinant AprA or bacterial supernatant was separated with SDS-PAGE. Proteins were transferred to an Immobilon-P membrane (Millipore) and blocked with 4% skimmed milk in PBS + 0.05% Tween. Subsequently blots were incubated with 1/500 diluted rabbit AprA antiserum followed by HRP-conjugated goat-anti-rabbit IgG (Biorad) and bands were visualized by enhanced chemiluminescence (Amersham).
Arabidopsis callose deposition assay
Seeds of A. thaliana ecotype Landsberg erecta (La-er) were vapor face sterilized and sown on Murashige-Skoog (MS; Sigma) medium containing 0.6% Plant Agar (Duchefa) and 1% (w/v) sucrose (Sigma). After a two-day vernalization period at 4°C, plates were transferred to growth chambers with an 8 h day (200 µEm−2.sec−1 at 24°C) and 16 h night (20°C) cycle for seven days. Three seedlings were transferred to a single well containing 1 ml MS containing 1% (w/v) sucrose and the components required for the treatments as indicated in the text. After 24 h the medium was replaced by 1 ml 96% EtOH followed by incubation overnight for removal of chlorophyll. The next day, decolorized seedlings were washed in 0.07 M phosphate buffer (pH 9) and subsequently incubated with the same buffer containing 0.01% aniline blue (water blue; Merck). Samples were placed in the dark for a period of 20 h at RT. Microscopic slides were prepared in a matrix of fresh aniline blue. Observations were performed with a fluorescence microscope (Olympus Ax70 with Olympus U-RFL-T) with UV filter (bandpass 340 to 380 nm, long-path 425 nm) [46].
Arabidopsis growth assay
Ten vapor-face sterilized seeds of A. thaliana (La-er) were transferred to a well of 24-well plates containing 1 ml MS medium with 1% (w/v) sucrose and treatment-specific components. Seeds were vernalized by putting the 24-well plates at 4°C for two days. Subsequently, plates were transferred to growth chambers with an 8-h day (200 µEm−2.sec−1 at 24°C) and 16 h night (20°C) cycle. Differences in growth rate were monitored after 7–10 days by photography.
Stomatal closure assay
Wild-type A. thaliana Col-0 plants were grown for 5 weeks in an autoclaved mixture of sand/potting soil in a growth chamber with a 9 h day (200 µE m−2 s−1 and 24°C) and 15 h night (20°C) cycle and 70% relative humidity as described [47]. Wild-type P. aeruginosa PAO1 aprA mutant strains were grown overnight in liquid Kings Medium B at 28°C. The leaves of 5-week-old plants were dipped for 2 seconds in a solution of 10 mM MgSO4 and 0.015% (v/v) Silwet containing 2.5·107 cfu/ml of P. aeruginosa bacteria. The epidermis of two leaves were peeled off before treatment (t = 0) and 5, 10, 20 and 40 min after treatment and immediately observed under a Zeiss Axioskop2 microscope (400x). Pictures were taken at 10–15 random regions of the leaves. Stomatal aperture was then measured using the software package ImageJ.
Accession numbers
Swiss-prot accession numbers: P. aeruginosa AprA (Q03023), AprI (Q03026), flagellin type B (P72151) and flagellin from S. Typhimurium (P06179).
Supporting Information
Zdroje
1. AkiraSUematsuSTakeuchiO 2006 Pathogen recognition and innate immunity. Cell 124 783 801
2. HayashiFSmithKDOzinskyAHawnTRYiEC 2001 The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410 1099 1103
3. ChevanceFFHughesKT 2008 Coordinating assembly of a bacterial macromolecular machine. Nat Rev Microbiol 6 455 465
4. SmithKDAndersen-NissenEHayashiFStrobeKBergmanMA 2003 Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immunol 4 1247 1253
5. Andersen-NissenESmithKDBonneauRStrongRKAderemA 2007 A conserved surface on Toll-like receptor 5 recognizes bacterial flagellin. J Exp Med 204 393 403
6. Andersen-NissenESmithKDStrobeKLBarrettSLCooksonBT 2005 Evasion of Toll-like receptor 5 by flagellated bacteria. Proc Natl Acad Sci U S A 102 9247 9252
7. Gomez-GomezLBollerT 2002 Flagellin perception: a paradigm for innate immunity. Trends Plant Sci 7 251 256
8. RobatzekSChinchillaDBollerT 2006 Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev 20 537 542
9. ZipfelCRobatzekSNavarroLOakeleyEJJonesJD 2004 Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428 764 767
10. Gomez-GomezLFelixGBollerT 1999 A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. Plant J 18 277 284
11. LyczakJBCannonCLPierGB 2000 Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect 2 1051 1060
12. CostertonJWStewartPSGreenbergEP 1999 Bacterial biofilms: a common cause of persistent infections. Science 284 1318 1322
13. SkerrettSJLiggittHDHajjarAMWilsonCB 2004 Cutting edge: myeloid differentiation factor 88 is essential for pulmonary host defense against Pseudomonas aeruginosa but not Staphylococcus aureus. J Immunol 172 3377 3381
14. NouwensASWillcoxMDWalshBJCordwellSJ 2002 Proteomic comparison of membrane and extracellular proteins from invasive (PAO1) and cytotoxic (6206) strains of Pseudomonas aeruginosa. Proteomics 2 1325 1346
15. FeuilletVMedjaneSMondorIDemariaOPagniPP 2006 Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc Natl Acad Sci U S A 103 12487 12492
16. RamphalRBalloyVJyotJVermaASi-TaharM 2008 Control of Pseudomonas aeruginosa in the lung requires the recognition of either lipopolysaccharide or flagellin. J Immunol 181 586 592
17. BalloyVVermaAKuraviSSi-TaharMChignardM 2007 The role of flagellin versus motility in acute lung disease caused by Pseudomonas aeruginosa. J Infect Dis 196 289 296
18. MatsumotoK 2004 Role of bacterial proteases in pseudomonal and serratial keratitis. Biol Chem 385 1007 1016
19. AzghaniAOKondepudiAYJohnsonAR 1992 Interaction of Pseudomonas aeruginosa with human lung fibroblasts: role of bacterial elastase. Am J Respir Cell Mol Biol 6 652 657
20. ParmelyMGaleAClabaughMHorvatRZhouWW 1990 Proteolytic inactivation of cytokines by Pseudomonas aeruginosa. Infect Immun 58 3009 3014
21. LiehlPBlightMVodovarNBoccardFLemaitreB 2006 Prevalence of local immune response against oral infection in a Drosophila/Pseudomonas infection model. PLoS Pathog 2 e56
22. RooijakkersSHvan KesselKPvan StrijpJA 2005 Staphylococcal innate immune evasion. Trends Microbiol 13 596 601
23. FosterTJ 2005 Immune evasion by staphylococci. Nat Rev Microbiol 3 948 958
24. ChavakisTPreissnerKTHerrmannM 2007 The anti-inflammatory activities of Staphylococcus aureus. Trends Immunol 28 408 418
25. de JongeRvan EsseHPKombrinkAShinyaTDesakiY 2010 Conserved fungal LysM effector Ecp6 prevents chitin-triggered immunity in plants. Science 329 953 955
26. HegeTFeltzerREGrayRDBaumannU 2001 Crystal structure of a complex between Pseudomonas aeruginosa alkaline protease and its cognate inhibitor: inhibition by a zinc-NH2 coordinative bond. J Biol Chem 276 35087 35092
27. StoverCKPhamXQErwinALMizoguchiSDWarrenerP 2000 Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406 959 964
28. FeltzerREGrayRDDeanWLPierceWMJr 2000 Alkaline proteinase inhibitor of Pseudomonas aeruginosa. Interaction of native and N-terminally truncated inhibitor proteins with Pseudomonas metalloproteinases. J Biol Chem 275 21002 21009
29. CaballeroARMoreauJMEngelLSMarquartMEHillJM 2001 Pseudomonas aeruginosa protease IV enzyme assays and comparison to other Pseudomonas proteases. Anal Biochem 290 330 337
30. FelixGDuranJDVolkoSBollerT 1999 Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 18 265 276
31. Gomez-GomezLBauerZBollerT 2001 Both the extracellular leucine-rich repeat domain and the kinase activity of FSL2 are required for flagellin binding and signaling in Arabidopsis. Plant Cell 13 1155 1163
32. FanLMZhaoZAssmannSM 2004 Guard cells: a dynamic signaling model. Curr Opin Plant Biol 7 537 546
33. MelottoMUnderwoodWKoczanJNomuraKHeSY 2006 Plant stomata function in innate immunity against bacterial invasion. Cell 126 969 980
34. YonekuraKMaki-YonekuraSNambaK 2003 Complete atomic model of the bacterial flagellar filament by electron cryomicroscopy. Nature 424 643 650
35. KollerBKapplerMLatzinPGaggarASchreinerM 2008 TLR expression on neutrophils at the pulmonary site of infection: TLR1/TLR2-mediated up-regulation of TLR5 expression in cystic fibrosis lung disease. J Immunol 181 2753 2763
36. SamakovlisCAslingBBomanHGGateffEHultmarkD 1992 In vitro induction of cecropin genes—an immune response in a Drosophila blood cell line. Biochem Biophys Res Commun 188 1169 1175
37. ZengWHeSY 2010 A prominent role of the flagellin receptor FLAGELLIN-SENSING2 in mediating stomatal response to Pseudomonas syringae pv tomato DC3000 in Arabidopsis. Plant Physiol 153 1188 1198
38. StockerWGramsFBaumannUReinemerPGomis-RuthFX 1995 The metzincins—topological and sequential relations between the astacins, adamalysins, serralysins, and matrixins (collagenases) define a superfamily of zinc-peptidases. Protein Sci 4 823 840
39. GuzzoJDuongFWandersmanCMurgierMLazdunskiA 1991 The secretion genes of Pseudomonas aeruginosa alkaline protease are functionally related to those of Erwinia chrysanthemi proteases and Escherichia coli alpha-haemolysin. Mol Microbiol 5 447 453
40. BestebroerJPoppelierMJUlfmanLHLentingPJDenisCV 2007 Staphylococcal superantigen-like 5 binds PSGL-1 and inhibits P-selectin-mediated neutrophil rolling. Blood 109 2936 2943
41. de HaasCJVeldkampKEPeschelAWeerkampFVan WamelWJ 2004 Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J Exp Med 199 687 695
42. HaasDHollowayBW 1976 R factor variants with enhanced sex factor activity in Pseudomonas aeruginosa. Mol Gen Genet 144 243 251
43. NabelGBaltimoreD 1987 An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature 326 711 713
44. WalasekPHonekJF 2005 Nonnatural amino acid incorporation into the methionine 214 position of the metzincin Pseudomonas aeruginosa alkaline protease. BMC Biochem 6 21
45. JacobsMAAlwoodAThaipisuttikulISpencerDHaugenE 2003 Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 100 14339 14344
46. PozoMJVan Der EntSVan LoonLCPieterseCM 2008 Transcription factor MYC2 is involved in priming for enhanced defense during rhizobacteria-induced systemic resistance in Arabidopsis thaliana. New Phytol 180 511 523
47. Van der EntSVerhagenBWVan DoornRBakkerDVerlaanMG 2008 MYB72 is required in early signaling steps of rhizobacteria-induced systemic resistance in Arabidopsis. Plant Physiol 146 1293 1304
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Crystal Structure of Reovirus Attachment Protein σ1 in Complex with Sialylated OligosaccharidesČlánek A Protein Thermometer Controls Temperature-Dependent Transcription of Flagellar Motility Genes inČlánek Modulation of NKp30- and NKp46-Mediated Natural Killer Cell Responses by Poxviral Hemagglutinin
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 8- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Phenotypic Screens, Chemical Genomics, and Antimalarial Lead Discovery
- Characterisation of Regulatory T Cells in Nasal Associated Lymphoid Tissue in Children: Relationships with Pneumococcal Colonization
- Crystal Structure of Reovirus Attachment Protein σ1 in Complex with Sialylated Oligosaccharides
- Absence of Cross-Presenting Cells in the Salivary Gland and Viral Immune Evasion Confine Cytomegalovirus Immune Control to Effector CD4 T Cells
- Transcriptomic Analysis of Host Immune and Cell Death Responses Associated with the Influenza A Virus PB1-F2 Protein
- A Quorum Sensing Regulated Small Volatile Molecule Reduces Acute Virulence and Promotes Chronic Infection Phenotypes
- Autocrine Regulation of Pulmonary Inflammation by Effector T-Cell Derived IL-10 during Infection with Respiratory Syncytial Virus
- A Protein Thermometer Controls Temperature-Dependent Transcription of Flagellar Motility Genes in
- Association of Human TLR1 and TLR6 Deficiency with Altered Immune Responses to BCG Vaccination in South African Infants
- Histo-Blood Group Antigens Act as Attachment Factors of Rabbit Hemorrhagic Disease Virus Infection in a Virus Strain-Dependent Manner
- MrkH, a Novel c-di-GMP-Dependent Transcriptional Activator, Controls Biofilm Formation by Regulating Type 3 Fimbriae Expression
- Beta-HPV 5 and 8 E6 Promote p300 Degradation by Blocking AKT/p300 Association
- Modulation of NKp30- and NKp46-Mediated Natural Killer Cell Responses by Poxviral Hemagglutinin
- Transportin 3 Promotes a Nuclear Maturation Step Required for Efficient HIV-1 Integration
- Coordination of KSHV Latent and Lytic Gene Control by CTCF-Cohesin Mediated Chromosome Conformation
- A Novel Persistence Associated EBV miRNA Expression Profile Is Disrupted in Neoplasia
- The Plant Pathogen pv. Is Genetically Monomorphic and under Strong Selection to Evade Tomato Immunity
- IL-10 Blocks the Development of Resistance to Re-Infection with
- Anti-Apoptotic Machinery Protects the Necrotrophic Fungus from Host-Induced Apoptotic-Like Cell Death during Plant Infection
- Crystal Structure of PrgI-SipD: Insight into a Secretion Competent State of the Type Three Secretion System Needle Tip and its Interaction with Host Ligands
- Evades Immune Recognition of Flagellin in Both Mammals and Plants
- Tumor Cell Marker PVRL4 (Nectin 4) Is an Epithelial Cell Receptor for Measles Virus
- Provides Insights into the Evolution of the Salmonellae
- B Cell Repertoire Analysis Identifies New Antigenic Domains on Glycoprotein B of Human Cytomegalovirus which Are Target of Neutralizing Antibodies
- Thy1 Nk Cells from Vaccinia Virus-Primed Mice Confer Protection against Vaccinia Virus Challenge in the Absence of Adaptive Lymphocytes
- The Cytokine Network of Acute HIV Infection: A Promising Target for Vaccines and Therapy to Reduce Viral Set-Point?
- Dendritic Cell Status Modulates the Outcome of HIV-Related B Cell Disease Progression
- Differential Contribution of PB1-F2 to the Virulence of Highly Pathogenic H5N1 Influenza A Virus in Mammalian and Avian Species
- A Communal Bacterial Adhesin Anchors Biofilm and Bystander Cells to Surfaces
- Two Group A Streptococcal Peptide Pheromones Act through Opposing Rgg Regulators to Control Biofilm Development
- Activation of HIV Transcription by the Viral Tat Protein Requires a Demethylation Step Mediated by Lysine-specific Demethylase 1 (LSD1/KDM1)
- Unique Evolution of the UPR Pathway with a Novel bZIP Transcription Factor, Hxl1, for Controlling Pathogenicity of
- Disruption of PML Nuclear Bodies Is Mediated by ORF61 SUMO-Interacting Motifs and Required for Varicella-Zoster Virus Pathogenesis in Skin
- Flagellar Motility Is Not Directly Required to Maintain Attachment to Surfaces
- Viral Infection Induces Expression of Novel Phased MicroRNAs from Conserved Cellular MicroRNA Precursors
- Functional Cure of SIVagm Infection in Rhesus Macaques Results in Complete Recovery of CD4 T Cells and Is Reverted by CD8 Cell Depletion
- Recruitment of the Major Vault Protein by InlK: A Strategy to Avoid Autophagy
- The Steroid Catabolic Pathway of the Intracellular Pathogen Is Important for Pathogenesis and a Target for Vaccine Development
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Tumor Cell Marker PVRL4 (Nectin 4) Is an Epithelial Cell Receptor for Measles Virus
- Two Group A Streptococcal Peptide Pheromones Act through Opposing Rgg Regulators to Control Biofilm Development
- Differential Contribution of PB1-F2 to the Virulence of Highly Pathogenic H5N1 Influenza A Virus in Mammalian and Avian Species
- Recruitment of the Major Vault Protein by InlK: A Strategy to Avoid Autophagy
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



