-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEngineered Resistance to
Development in Transgenic
Transposon-mediated transformation was used to produce Anopheles
stephensi that express single-chain antibodies (scFvs) designed to
target the human malaria parasite, Plasmodium falciparum. The
scFvs, m1C3, m4B7, and m2A10, are derived from mouse monoclonal antibodies that
inhibit either ookinete invasion of the midgut or sporozoite invasion of
salivary glands. The scFvs that target the parasite surface, m4B7 and m2A10,
were fused to an Anopheles gambiae antimicrobial peptide,
Cecropin A. Previously-characterized Anopheles cis-acting DNA
regulatory elements were included in the transgenes to coordinate scFv
production with parasite development. Gene amplification and immunoblot analyses
showed promoter-specific increases in transgene expression in blood-fed females.
Transgenic mosquito lines expressing each of the scFv genes had significantly
lower infection levels than controls when challenged with P.
falciparum.
Published in the journal: . PLoS Pathog 7(4): e32767. doi:10.1371/journal.ppat.1002017
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002017Summary
Transposon-mediated transformation was used to produce Anopheles
stephensi that express single-chain antibodies (scFvs) designed to
target the human malaria parasite, Plasmodium falciparum. The
scFvs, m1C3, m4B7, and m2A10, are derived from mouse monoclonal antibodies that
inhibit either ookinete invasion of the midgut or sporozoite invasion of
salivary glands. The scFvs that target the parasite surface, m4B7 and m2A10,
were fused to an Anopheles gambiae antimicrobial peptide,
Cecropin A. Previously-characterized Anopheles cis-acting DNA
regulatory elements were included in the transgenes to coordinate scFv
production with parasite development. Gene amplification and immunoblot analyses
showed promoter-specific increases in transgene expression in blood-fed females.
Transgenic mosquito lines expressing each of the scFv genes had significantly
lower infection levels than controls when challenged with P.
falciparum.Introduction
Plasmodium falciparum, a causative agent of human malaria, is a vector-borne parasite that is responsible for more than 500 million clinical disease cases each year [1]. The selection of insecticide-resistant mosquitoes and drug-resistant parasites necessitates an ongoing search for new disease-control methods. A proposed strategy for interrupting transmission is to replace wild, malaria-susceptible mosquito populations with transgenic, Plasmodium-resistant mosquitoes [2]–[4]. Key components of this approach are effector molecules that inhibit parasite development when expressed from a transgene. The mechanisms by which effector molecules function can vary greatly, as the development of the malaria parasites within mosquitoes involves several transitions of environment, physiology and morphology [5].
When mosquitoes feed on infected humans, they ingest parasites in the form of gametocytes. These produce gametes that fuse to form diploid zygotes that develop into the motile ookinetes. The ookinetes invade and traverse the mosquito midgut epithelium and then rest beneath the basal lamina of the midgut, forming oocysts. Thousands of sporozoites develop within the oocysts before budding out into the circulatory fluid, the hemolymph, and invading the salivary glands. Several effector molecules have been tested for their ability to target the parasite during either early sporogony in the midgut, or late sporogony in the hemolymph or salivary glands [5]–[8]. An effector mechanism based on the mosquito signaling protein Akt is the only one to date shown to inhibit completely P. falciparum development in a transgenic Anopheles mosquito [7].
One effector molecule strategy exploits the finding that monoclonal antibodies (mAbs) that recognize surface-bound or secreted parasite molecules can inhibit pathogen development [9]–[14]. Two mAbs, 4B7 and 1C3, target parasites early in their development within mosquitoes. 4B7 binds P. falciparum surface protein Pfs25, a molecule expressed on the surface of ookinetes, and inhibits parasite development completely when fed to Anopheles mosquitoes in a gametocytemic bloodmeal [9]. In contrast, 1C3 binds a parasite-secreted enzyme, P. falciparum chitinase 1, and inhibits oocyst formation of P. falciparum when incorporated into infectious bloodmeals [10]. A third mAb, 2A10, binds P. falciparum circumsporozoite protein (CSP), and when pre-incubated with sporozoites, greatly decreases their ability to infect cultured hepatocytes [11], [12].
Although the size and complexity of mAbs exclude them from consideration as potential effector molecules, single-chain antibodies (scFvs), which retain the binding specificity of a mAb, are much smaller and can be produced from a single transcription unit [15]. An scFv targeting the P. gallinaceum CSP inhibited sporozoite invasion of salivary glands in Aedes aegypti in both transient assays and transgenic mosquitoes [13], [16]. Anopheles stephensi fed Escherichia coli expressing an anti-P. berghei scFv-immunotoxin were shown to have significantly-reduced oocyst densities when fed on parasite-infected mice [14]. Furthermore, an scFv derived from the 1C3 mAb reduced significantly P. falciparum parasite transmission to mosquitoes [17]. The experiments described in the work presented here test the scFv-based strategy on human malaria parasites in transgenic mosquitoes and support the further development and evaluation of these molecules as disease-control tools.
scFvs based on the 1C3, 4B7 and 2A10 mAbs were expressed in transgenic An. stephensi and their efficacy tested in parasite challenge assays with P. falciparum. Anopheles stephensi was chosen because it is a significant vector of urban malaria transmission in the Indian subcontinent and is an efficient model for transgenic research. To distinguish the novel scFvs developed in this study, we refer to them as “modified” 1C3, 4B7 or 2A10 (m1C3, m4B7, m2A10). For the m4B7 and m2A10 transgenes, the An. gambiae Cecropin A gene (AgCecA) was joined to the scFv gene to form a single open reading frame (ORF). Cecropin A is an antimicrobial peptide that has microbiocidal activity against both gram-negative and gram-positive bacteria, as well as multiple Plasmodium species [18], [19]. This broad activity is due to its ability to form large pores in cell membranes [20]. With the addition of cecropin A, the m4B7 and m2A10 scFvs possess both parasite-binding and antimicrobial activity. The cecropin A peptide was not joined to m1C3 as the target of this scFv is a secreted molecule [17].
Anopheles gambiae carboxypeptidase A (AgCPA [21], [22]) gene regulatory sequences were included in m4B7 and m1C3 transgenes to coordinate their expression with the development of ookinetes. Anopheles stephensi vitellogenin 1 (AsVg1 [23]) regulatory elements were joined to the m2A10 scFv to direct transgene expression in the female fat body. Thus, m2A10 secreted from the fat body into the hemolymph could encounter sporozoites migrating to the salivary gland. When challenged in multiple experiments with P. falciparum infectious gametocyte cultures, scFv-expressing transgenic lines displayed statistically-significant, reduced mean intensities of infection and in most trials lower parasite prevalence when compared to control mosquitoes.
Results
Transgene assembly, transgenesis, and gene copy-number analyses
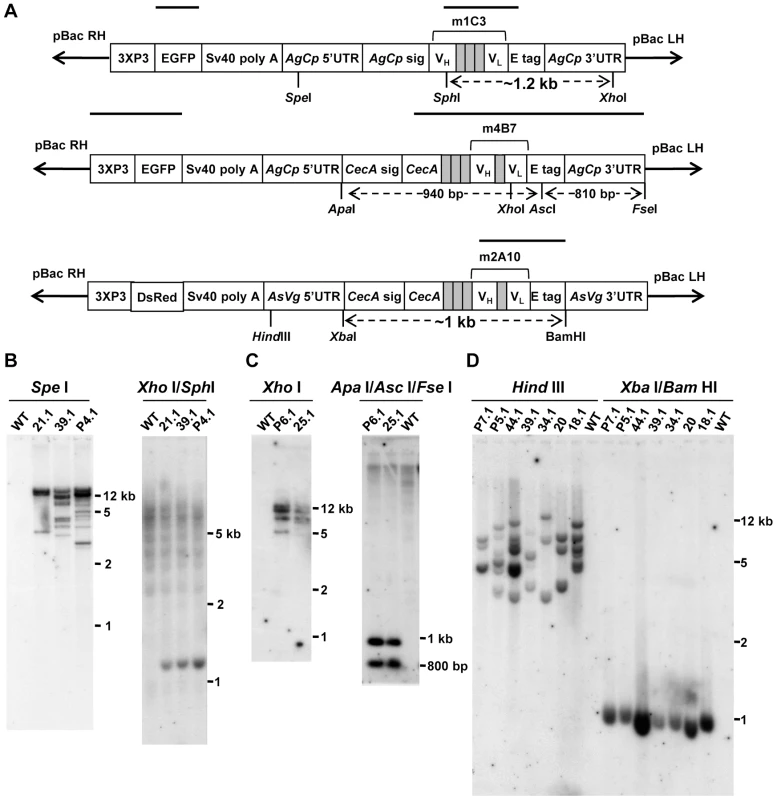
The scFv genes were synthesized commercially to incorporate either the AgCPA signal sequence or the entire AgCecA ORF (Figure 1). Codons corresponding to the amino acids serine, proline, alanine, threonine, and arginine displayed the greatest frequency bias differences between Mus musculus and An. gambiae (Table S1) [24], and these were replaced in the mouse-derived scFv sequences by those favored by the mosquito. DNA sequence encoding a short polypeptide linker (five amino acids) was used to join the heavy - and light-chain variable fragments of m4B7 and m2A10 scFvs and a longer linker (encoding 15 amino acids) joined the two corresponding moieties of m1C3. Long linkers permit intramolecular pairing of variable fragments, while short linkers favor the intermolecular joining of scFv molecules to form multimers containing multiple antigen recognition sites [25]. The m1C3 and m4B7 scFv genes were joined to AgCPA regulatory elements and inserted into a pBac [3xP3-EGFP] plasmid to construct the transformation vectors (Figure 2). Similarly, the m2A10 scFv gene was joined to AsVg1 regulatory elements and inserted into a pBac [3xP3-dsRed] plasmid.
Fig. 1. A model of the modified scFv transgenes. 
The three transformation plasmids pBac [3xP3-EGFP]-m1C3, pBac [3xP3-EGFP]-m4B7 and pBac [3xP3-dsRed]-m2A10 were injected into 980, 615 and 765 embryos, respectively. Three transgenic m1C3 mosquito lines (21.1, 39.1 and P4.1) were established from EGFP-positive families derived from 78 surviving adults. Two transgenic m4B7 mosquito lines (25.1 and P6.1) were established from 89 adults, and seven transgenic m2A10 mosquito lines (18.1, 20, 34.1, 39.1, 44.1, P5.1 and P7.1) were established from 105 adults.
Southern blot analyses were used to verify transgene insertions and to determine the number of integrated constructs in each line (Figure 2). Hybridization of an m1C3 probe to genomic DNA digested with both SphI and XhoI restriction endonucleases produced a diagnostic fragment of ∼1.2 kilobase pairs (kb) in transgenic samples, confirming m1C3 integration. Genomic DNA digested with SpeI and hybridized to an EGFP probe produced multiple fragments in each transgenic sample, indicating that there were at least three, nine, and ten copies in lines 21.1, 39.1, and P4.1, respectively. Genomic DNA digested with ApaI, AscI, and FseI, and hybridized to a probe complementary to the m4B7 gene and the AgCPA 3′UTR produced two diagnostic fragments of 940 and 810 base pairs (bp), verifying transgene insertion. A second blot, comprising XhoI-digested genomic DNA recovered from transgenic mosquitoes and hybridized with a 3XP3 EGFP probe, revealed several fragments in each sample, indicating that at least four copies of the m4B7 transgene were present in each line. Lastly, genomic DNA digested with both XbaI and BamHI and hybridized to an m2A10 probe produced an ∼1 kb diagnostic fragment in each transgenic sample. The same probe hybridized to HindIII-digested genomic DNA bound multiple DNA fragments in each m2A10 sample, indicating the presence of six, three, three, four, six, seven and three copies in transgenic lines 18.1, 20, 34.1, 39.1, 44.1, P5.1, P7.1, respectively. Transgenic lines were maintained by intercrossing at each generation. However, selection pressures on individual transgene insertions, small founding colony sizes and independent assortment likely result in loss over time of some of the insertions.
Characterization of transgene expression
Reverse-transcriptase-PCR (RT-PCR) and Real-time quantitative RT-PCR (RT-qPCR) were used to evaluate the presence and relative abundance of m1C3, m4B7 or m2A10 transcription products in non-blood-fed and blood-fed mosquitoes in all of the established transgenic lines. No significant correlation was seen between transgene copy number and amount of transcription product detected (data not shown). Therefore, the lines m1C3 P4.1, m4B7 25.1 and m2A10 44.1, each of which displayed the highest levels of transcript accumulation in their respective group, were selected for use in all further analyses. Southern blot analyses of the generations of m1C3 P4.1, m4B7 25.1 and m2A10 44.1 used in the challenge assays indicated the presence of eight, four, and four copies of the respective transgenes.
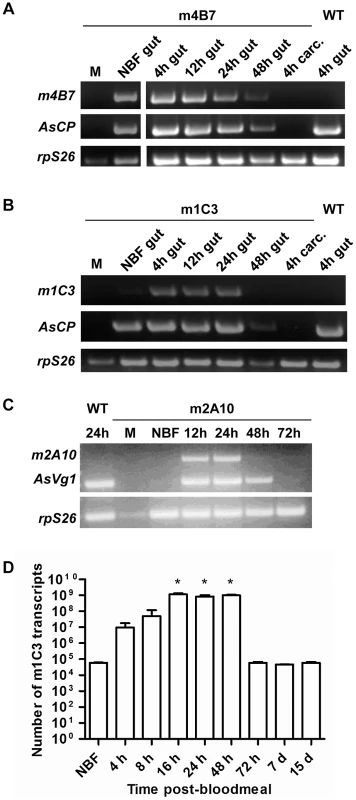
Transgene-specific transcript accumulation profiles detected by RT-PCR were similar in mRNA samples prepared from the dissected midguts of m1C3 P4.1 and m4B7 25.1 females (Figure 3). Both lines showed constitutive accumulation in midguts from non-blood fed mosquitoes. In addition, each line showed accumulation of their respective mRNAs at 4 hours post-bloodmeal (hPBM), and signals were evident at 12 and 24 hPBM. m4B7 transcript also could be detected at low levels at 48 hPBM. No amplification products were produced from mRNA prepared from female carcasses or males of each line. As expected, control reactions using mRNA from midguts dissected at 4 hPBM from wild-type, non-transgenic females were negative. RT-qPCR analysis at multiple post-bloodmeal time points was used as an independent measure of m1C3 P4.1 expression. The highest measured level of m1C3 mRNA, 10,000-fold above the control level, was observed in the 16 hPBM midgut sample (paired T-test, one tailed p-value = 0.005), but similar elevated levels also were seen at 24 and 48 hPBM. At this time, we cannot account for the difference in the RT-PCR and RT-qPCR results at 48 hPBM, although this could result from individual females that responded differentially to the feeding regimen. This difference is not expected to have affected the outcome of the challenge experiments because this scFv targets parasites within the first 24 hPBM.
Immunoblot analyses of m1C3 and m4B7 transgenic mosquitoes were unproductive despite repeated attempts. Although high levels of proteinase inhibitors were used during sample preparation, it is possible that the transgene products were degraded quickly in the strong digestive milieu of the post-feeding midgut lumen.
Transgene transcripts detected in whole transgenic m2A10 44.1 females showed sex - and stage-specificity (Figure 3). No signals were seen in samples derived from mRNA prepared from males and non-blood fed transgenic or control wild-type, non-transgenic females. Specific transcript accumulation was evident in m2A10 44.1 females at 12 and 24 hPBM in an expression pattern similar to that of endogenous AsVg1, but was not as abundant at 48 hPBM.
While expression of the m1C3 and m4B7 scFvs is necessary only during the first 24 hours post-bloodmeal, expression of m2A10 must be sustained over several days, as oocysts can mature asynchronously [26]. Denaturing immunoblot analyses were performed on m2A10 44.1 females sampled over the course of four bloodmeals to evaluate whether protein expression could be induced repeatedly (Figure 4; Figure S1). Anti-E tag antibody specifically detected a polypeptide with an approximate Mr of 32 kiloDaltons (kDa), consistent with the predicted size of m2A10 protein, in transgenic blood-fed females at 24, 48, 72 and 96 hPBM. The continuous presence of m2A10 was detected in females that were given bloodmeals once every five days. Expression of m2A10 also was observed at 12 hPBM in additional immunoblot analyses (data not shown). Immunoblots of hemolymph samples indicated that m2A10 protein was present in the hemolymph of blood-fed transgenic females (Figure 4). Immunoblot analyses of hemolymph samples analyzed in non-denaturing conditions detected m2A10 protein in several multimeric conformations with estimated Mrs of 125, 223, 284, and 485 kDa.
Fig. 4. Bloodmeal-induced expression of m2A10 scFv. 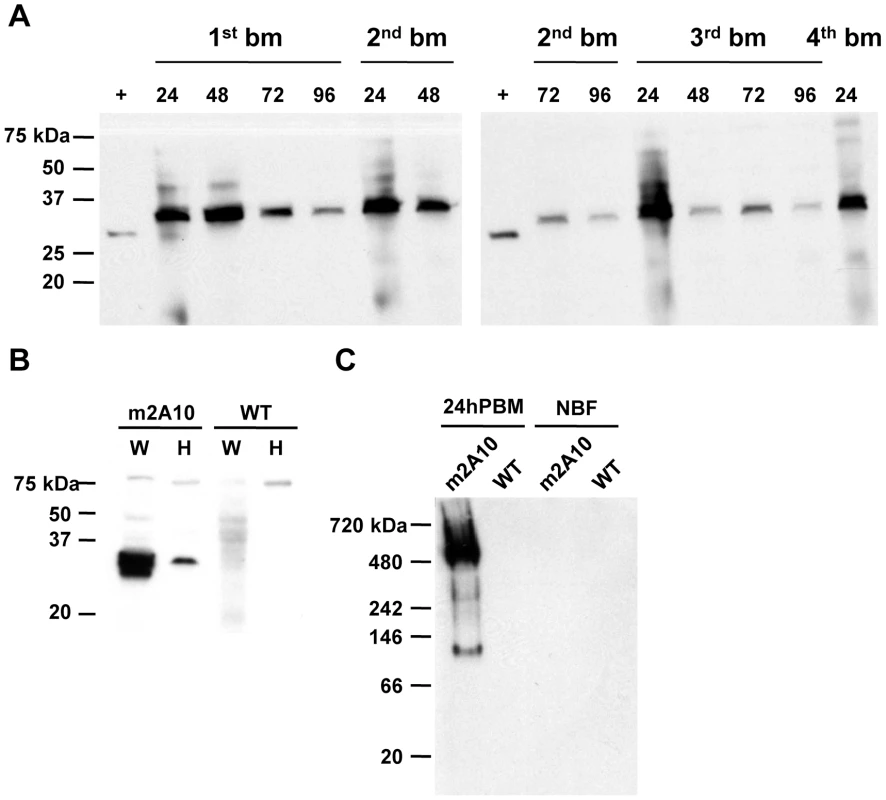
Plasmodium falciparum challenge of transgenic and control mosquitoes
Parasite challenge experiments were performed to test the efficacy of the anti-pathogen effector molecules. Transgenic and control mosquitoes ingested blood containing P. falciparum gametocytes through a membrane-feeding apparatus. Control mosquitoes for most experiments were non-transgenic (wild-type) mosquitoes. In addition, the oocyst prevalence and mean intensities of infection of a group of m2A10 44.1 females were examined for each challenge experiment to determine whether transgenesis alone had an impact on parasite development.
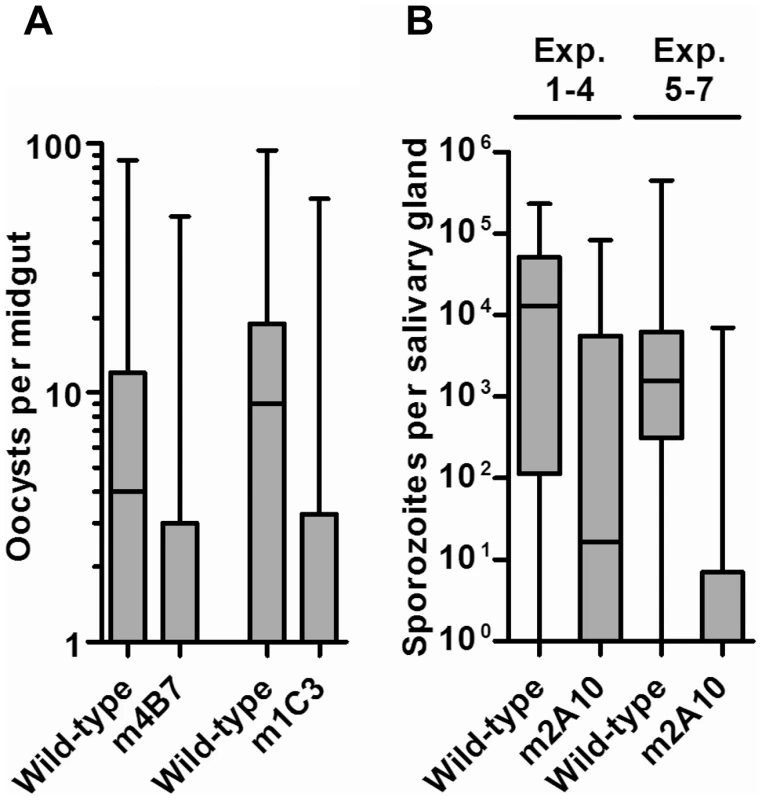
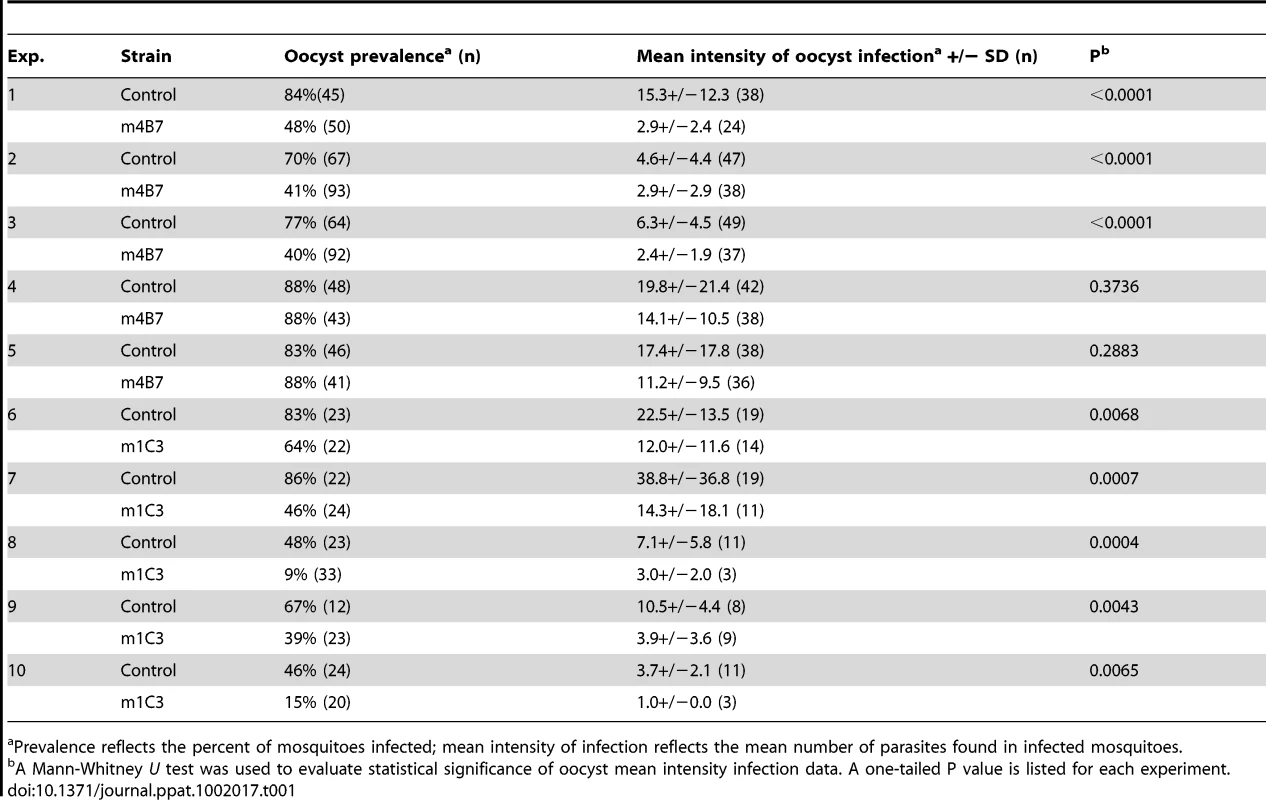
The effect of transgene expression on parasite development for both the m4B7 25.1 and m1C3 P4.1 transgenic lines was measured by comparing the number of oocysts in transgenic and control mosquito midguts at nine days after the infectious bloodmeal (Table 1; Figure 5). The mean intensities of oocyst infection were reduced by 37–81% in three challenge experiments (1, 2 and 3, Table 1) of m4B7 25.1. However, the mean intensities of infection were reduced by only 29–36% in two experiments (4 and 5, Table 1) in which control mosquitoes had greater than 17 oocysts per midgut. Mean intensities of oocyst infection were reduced by 47–73% in mosquitoes expressing m1C3 when compared to controls. Furthermore, with the exception of the high infection-level experiments (4 and 5, Table 1), both m1C3 P4.1 and m4B7 25.1 transgenic mosquitoes had lower prevalence of infections than controls.
Parasite challenge assays of m2A10 44.1 involved dissecting 7–11 mosquitoes of each group 10 days after the infectious bloodmeal to count midgut oocysts and to confirm that both transgenic and control mosquitoes were infected successfully (Table 2). No statistically significant difference in the number of oocysts between transgenic m2A10 44.1 and control mosquitoes was observed (Mann-Whitney U test, one-tailed P value, 0.24<p<0.48). The remaining mosquitoes (n = 8–50) in each group were examined 17–19 days after infection for the presence of sporozoites in the salivary glands (Table 2; Figure 5). All mosquitoes were provided an uninfected bloodmeal every five days to maintain expression of m2A10. Engorged and un-engorged females were not separated after the uninfected bloodmeals in experiments 1, 2, 3 and 4, and a 52–84% reduction in mean intensity of sporozoite infection was observed in transgenic mosquitoes when compared to the controls. To obtain a more precise measurement of the effect of m2A10 expression upon P. falciparum development, an additional three experiments (5, 6 and 7) were performed in which un-engorged females were discarded after each uninfected bloodmeal. A 96–97% reduction of mean intensity of infection was observed in m2A10 44.1 mosquitoes that fed every five days. Furthermore, m2A10 44.1 mosquitoes in experiment 7 had a 14% prevalence of infection compared to 78% observed in the corresponding control.
Tab. 2. 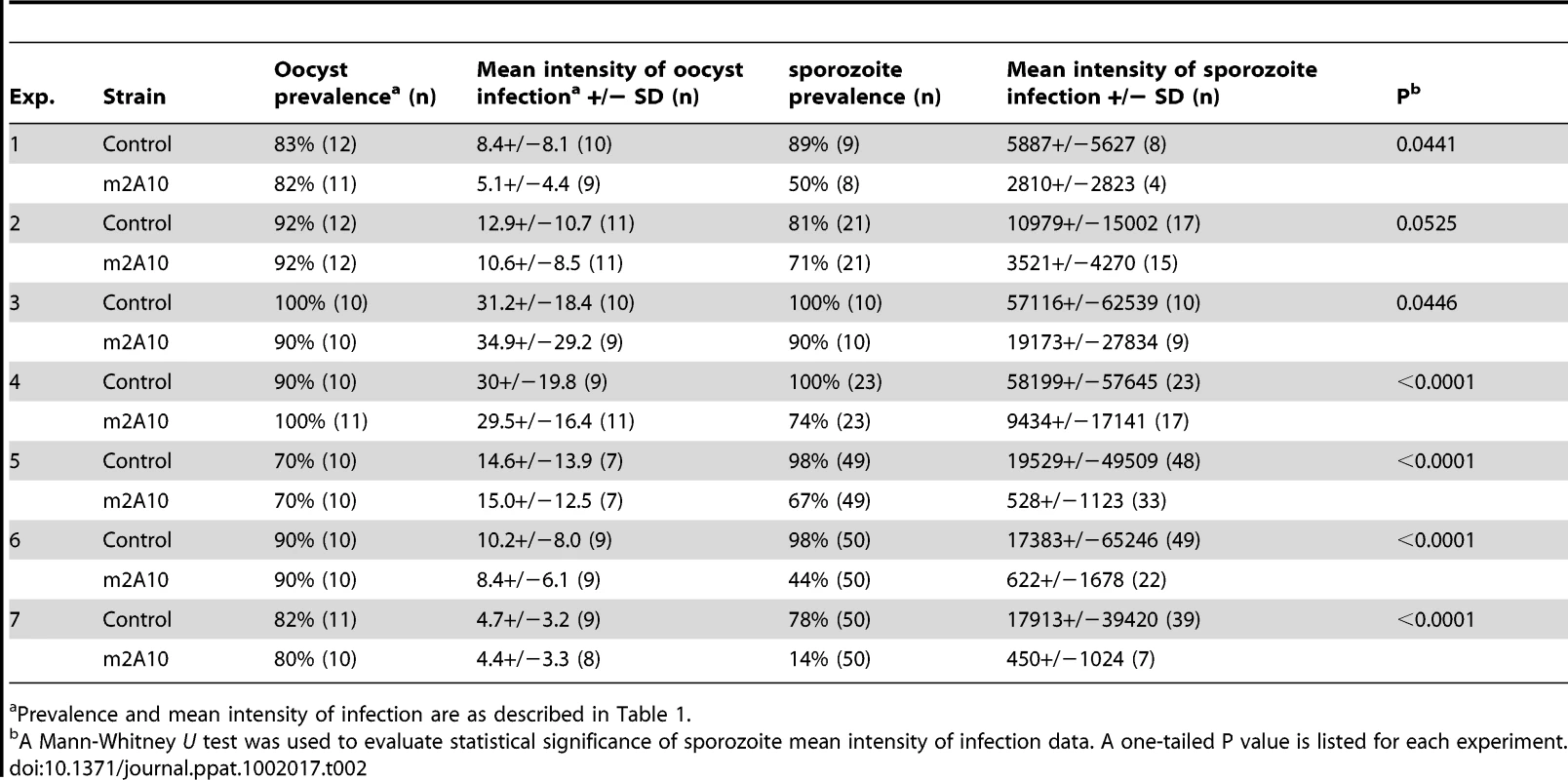
Prevalence and mean intensity of infection are as described in <em class="ref">Table 1</em>. Discussion
Previous evaluations of mosquitoes engineered genetically to express anti-Plasmodium effector genes featured analyses of transgene copy numbers, transgene transcription levels, detection of transgene effector proteins, binding of effector molecules to the target parasite stage and a phenotype of reduced parasite mean intensities of infection and prevalence [7], [8], [16], [22], [27]–[29]. Remarkably, no single study includes all of these data and the emphasis has been on the impact of transgene presence on parasite numbers. Expression of the two midgut-directed scFvs, m1C3 and m4B7, was detected by RT-PCR, but not by immunoblots. The rapid degradation of these scFvs in the midgut environment may have inhibited immunoblot detection. However, the observation that m4B7 25.1 and m1C3 P4.1 transgenic mosquitoes have reduced parasite loads supports the conclusion that these scFvs are expressed in the midgut. Both transgene transcription and translation products were detected in m2A10 44.1 mosquitoes. The finding that the immunoblot analyses of non-denatured m2A10 44.1 samples detected the presence of scFv multimers is consistent with the expectation that the short polypeptide linker joining the VH and VL regions promotes intermolecular scFv interactions. The size of these multimers was similar to the predicted sizes of m2A10 multimers comprising four, seven, nine, and fifteen scFv molecules. Such scFv multimers are reported to have high affinity to target epitopes [25].
Both m1C3 and m4B7 expressed in transgenic lines P4.1 and 25.1, respectively, inhibited parasite development during early sporogony, resulting in significantly reduced mean intensities of oocyst infection in eight of ten challenge experiments. The results of two of the m4B7 25.1 challenge experiments are consistent with the interpretation that there is a threshold level of initial parasite density above which this scFv, at the levels expressed in these transgenic lines, cannot efficiently inhibit ookinete development. The finding that m2A10 44.1 and wild-type control mosquitoes did not differ in midgut infection supports the conclusion that transgene integration alone does not necessarily impair parasite development.
When expression of m2A10 in line 44.1 was induced repeatedly by blood feeding, a highly significant decrease in sporozoite load was observed in transgenic mosquito salivary glands. For this transgenic line, the greatest reduction in prevalence was found in an experiment in which the mean intensity of oocyst infection was low. It is likely that these scFvs would effectively impair P. falciparum transmission in field conditions, as infected wild-caught An. gambiae carry few oocysts. Studies of An. gambiae by Billingsley et al. [30] and Taylor [31] found mean numbers of oocysts per infected mosquito of 1.55 and 3.38, respectively.
Incorporation of multiple transgenes is typical for piggyBac-mediated insertions into An. stephensi [22], [23], [32]. Although it is reasonable to expect that higher transgene copy numbers should yield higher expression levels, no statistically-significant correlations have been reported. We hypothesize that many of the multiple copies have little or no expression as a result of position effects, and that the majority of transgene expression comes from single or small numbers of the transgenes. To mitigate copy-number issues, we have used piggy-Bac-mediated transposition to integrate target sites for φC31 site-specific recombination into multiple locations in the An. stephensi genome and are now testing individual lines for permissiveness for optimum transgene expression [33]. These lines have the added benefit of having been evaluated for the impact on fitness of the introduced exogenous DNA at the specific insertion site, and therefore the effects of anti-pathogen transgene product expression can be measured directly. Furthermore, we are eager to evaluate the phenotype of dual transgenes, for example, those combining m4B7 and 2A10 or m1C3 and m2A10, on parasite mean intensities of infection and prevalence. Additional studies facilitated by this approach could include testing alternate gene regulatory sequences, such as those of the salivary gland-specific anopheline antiplatelet protein or the An. gambiae adult peritrophic matrix protein 1, to measure the effect of different transgene expression patterns upon parasite development [34], [35].
Although the scFvs in lines m1C3 P4.1, m4B725.1, and m2A10 44.1 inhibited parasite development significantly, no transgenic line displayed zero prevalence of infection. It has been demonstrated in an avian malaria model system comprising the vertebrate host, Gallus gallus, the mosquito host, Aedes aegypti, and the parasite, P. gallinaceum, that mosquitoes containing as few as 20 sporozoites in their salivary glands infected chickens during a blood meal [16]. This finding supports the conclusion that a target of zero prevalence is necessary for a transgenic mosquito to be incapable of disease transmission in this system. These results are in contrast to reports of experiments with transgenic mosquitoes and a rodent malaria parasite, P. berghei, in which the effector molecules SM1, PLA2 and CEL-III show a significant inhibition of parasite development (81.6%, 87% and 84.8%, respectively) [22], [27], [28]. Reductions of mean intensities of P. berghei sporozoite infection in salivary glands below ∼400 were sufficient to block transmission. In contrast, experimental infections of humans with P. vivax showed that 10 sporozoites were sufficient to cause malaria [36]. We have opted to take the conservative approach and are attempting to achieve zero prevalence of human parasites in mosquito salivary glands [16].
Two anti-Plasmodium effector molecule strategies have yielded transgenic mosquitoes with zero prevalence: expression of the signaling molecule Akt, and expression of a combination of Cecropin A and Defensin A [7], [29]. The latter study was conducted with the P. gallinaceum/Ae. aegypti/G. gallus model system. The study of Akt demonstrated the feasibility of producing an Anopheles mosquito that is completely resistant to P. falciparum, however this effector molecule may not be an optimal component of a population replacement strategy as these mosquitoes have a significantly reduced lifespan [7]. A synthetic peptide designed to interact with P. yoelii reduced midgut infections of this parasite by 67–87% in An. gambiae but was considerably less efficacious against P. falciparum [8]. Quantitative comparisons of the efficacy of alternative effector molecules are hindered currently by differences in expression that result from variations in transgene location and copy number. Site-specific recombination approaches will allow such evaluations in well-characterized ‘docking-site’ mosquito strains [8], [33].
The finding that m2A10 44.1 mosquitoes display up to 97% decreases in the mean intensity of P. falciparum infection, as well as decreased prevalence of infection, supports the argument that this scFv may be an effective component of a malaria resistance transgene. The m1C3 and m4B7 scFv genes conferred significant reductions in mean intensities of infection, and if expressed in higher quantities, also may be used in the design of a transgenic, parasite-resistant mosquito. Furthermore, expressing the scFv transgenes in additional malaria vectors, in particular, An. gambiae, and challenging these with a variety of P. falciparum isolates would help evaluate whether these effector molecules could be used in multiple transmission areas.
The discovery and characterization of several effector molecules that completely inhibit P. falciparum development will support the engineering of mosquitoes that express multiple effector molecules. Such mosquitoes may have a reduced likelihood of selecting for resistant parasites. Along with vaccines, drugs, and insecticide-treated nets, parasite-resistant transgenic mosquitoes would be a useful component in a malaria-control strategy, especially in regions where existing interventions have been unable to eliminate disease transmission.
Materials and Methods
Mosquito rearing and maintenance
A colony of Anopheles stephensi (gift of M. Jacobs-Lorena, Johns Hopkins University) bred in our insectary for >5 years was used in the experiments. The mosquitoes were maintained in conditions that maximize larval nutrition, and adult size and fitness [37]. These conditions include maintenance of cultures at 27°C with 77% humidity and 12 hr day/night, 30 min dusk/dawn lighting cycle. Larvae were fed a diet of powdered fish food (Tetramin) mixed with yeast. Adults were provided water and raisins ad libitum. Anesthetized chickens, mice, or rabbits were used for blood feeding. Transgenic and wild-type control mosquitoes used in parasite challenge experiments were reared in parallel using standardized insectary procedures.
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Intuitional Animal Care and Use Committee of the University of California, Irvine (NIH Animal Welfare Assurance number: A3416.01 (approved February 20, 2008), Protocol Number: 1998 - 1411 (approved May 21, 2010). The vertebrates used as bloodmeal donors for mosquitoes were anesthetized on a regimen that avoids the build-up of drug tolerance, and all efforts were made to minimize suffering.
scFv sequence modifications
The sequences of the 4B7 and 2A10 variable heavy - and light-chain regions (VH and VL, respectively) were derived from cDNA synthesized from 2A10 and 4B7 hybridoma cell lines (obtained from E. Nardin [New York University], and the Malaria Research and Reference Reagent Resource Center, respectively). 2A10 cDNA was synthesized from total RNA isolated from the hybridoma cell line using primers designed from the known VH and VL sequence [38]. VH and VL cDNA from the 4B7 hybridoma cell line was amplified from its total RNA using the heavy and light primer mixes respectively, provided in the Mouse ScFv Module/Recombinant Phage Antibody System (Amersham Biosciences). The modified scFv genes, including either AgCPA signal sequence or the entire AgCecA ORF, were synthesized commercially (Epoch Biolabs) to allow for incorporation of novel features. The variable regions of 4B7, 2A10, and 1C3 [17] were optimized by replacing the codons corresponding to the amino acids serine, proline, alanine, threonine, and arginine in the mouse-derived sequences with those favored in An. gambiae (Table S1) [24]. For the m4B7 and m2A10 scFvs, the variable regions were joined by sequence encoding a short polypeptide linker, G4S. The VH region of the m2A10 and m4B7 scFv sequences were joined to the AgCecA protein-coding sequence by a long polypeptide linker, (G4S)3 [39]. The variable regions of m1C3 were joined by the same long polypeptide linker. The m2A10 VL was joined to sequence encoding a complete E tag, while the VL of m4B7 and m1C3 were joined to sequence encoding a partial E tag. For m4B7 and m1C3, the remaining E tag coding sequence was joined to the partial E tag at a later cloning step.
Transformation plasmid assembly
The pBacDsRed-AsVg5′-m2A10′-AsVg3′ plasmid was produced in two cloning steps. First, m2A10 sequence from the commercially-synthesized pBSKm2A10 plasmid replaced the CFP gene of pSLfa-AsVg5′-CFP-AsVg3′ [23] using XbaI and BamHI sites. Second, the AsVg5′-m2A10-AsVg3′ sequence was joined to pBacDsRed [37] using AscI sites. A pSLfa-AgCP5′-4B7-AgCP3′ plasmid supplied the AgCP regulatory sequences, as well as a partial E tag sequence, for both the pBacEGFP AgCP5′-m1C3-AgCP3′ and the pBacEGFP AgCP5′-m4B7-AgCP3′ plasmids. The pSLfa-AgCP5′-4B7-AgCP3′ plasmid was cloned in several steps. First, the Mouse ScFv Module/Recombinant Phage Antibody System (Amersham Biosciences) was used to produce a single chain antibody from VH and VL cDNA from the 4B7 cell line. The scFv was cloned into the pCANTAB 5E vector in frame with the E-Tag at the C terminus. BclI sites were added to both ends of 4B7 scFv by amplification using primers 4B7BclF [5′-CGTGATCAGTGAAGCTGGTGGAGTCT-3′] and 4B7BclR [5′-CGTGATCACTATGCGGCACGCGGTT-3′] from the 5′ and 3′ end and cloning into pCR4Blunt-TOPO. The pGEMT[AgCP-SM1] plasmid, containing AgCP (AGAP009593) regulatory regions, was generously provided by Dr. Marcelo Jacobs-Lorena [22]. The BclI-cut 4B7 scFv fragment from TOPO [4B7BclI] was sub-cloned into the Bam HI sites of pGEMT[AgCP-SM1] thereby swapping the SM1 fragment with 4B7. AgCP5′-4B7-AgCP3′ was cloned subsequently into pSLfa1180fa [40] using enzymes SacII and SalI. ApaI and SgrAI were used to replace the 4B7 region of pSLFA-AgCP5′-4B7-AgCP3′ with the commercially-synthesized m4B7 gene. AgCP5′-m4B7-AgCP3′ sequence was then joined to pBacEGFP [40] using a 5′ blunt ligation of AscI and KpnI sites, and a 3′ FseI ligation. To assemble the m1C3 transformation plasmid, the enzymes ApaI and BamHI were used to replace the 4B7 region of pSLfa-AgCP5′-4B7-AgCP3′. AgCP5′-m1C3-AgCP3′ was then joined to pBacEGFP using AscI restriction sites.
Microinjection and southern hybridization analyses
Microinjection of the pBac [3xP3-EGFP]-m1C3, pBac [3xP3-EGFP]-m4B7 or pBac [3xP3-dsRed]-m2A10 plasmids with the piggyBac helper plasmid was performed as described previously, except that 0.1 mM p - nitrophenyl p′-guanidinobenzoate was omitted from isotonic buffer [41]. Each G0 male was mated with 15 virgin females and groups of 5–10 G0 females were mated with 5 males, and G1 progeny were screened as larvae with UV-fluorescence microscopy for the presence of the marker genes. Standard Southern blotting and hybridization techniques were used to detect transgene integration [42]. Genomic DNA was extracted from groups of six transgenic or wild-type control females as described previously, except that DNA pellets were re-suspended in 100 µl of dH2O [43]. The probe used to identify m1C3 integration was amplified from a plasmid thought to contain the EGFP ORF, but which in fact contained the ECFP ORF. These two ORFs share 99% nucleotide sequence identity, so it is likely that the ability of the probe to hybridize to the integrated gene was affected negligibly. The EGFP probe was generated from pMos[3xP3-EGFP] [37] using XbaI and SacI enzymes. The m4B7 probe was generated through a restriction digest of the pBac [3xP3-EGFP]-m4B7 plasmid with both NaeI and FseI. The m2A10 probe was generated through a restriction digest of the pBSK-m2A10 plasmid with both BamHI and BstBI. Probes were labeled with 32P using the Megaprime DNA labeling system (Amersham).
RT-PCR
Total RNA was isolated from whole or dissected mosquitoes using Trizol (Invitrogen). For m2A10 RT-PCR analyses, 10 males or 2–3 whole females were used for each RNA preparation. One microgram of RNA was treated with DNAseI (Promega) for each 50 µl RT-PCR reaction. For m4B7 and m1C3 RT-PCR analyses, 6 males, 4–15 female midguts, or 4 female carcasses were used for each RNA preparation. Two hundred fifty nanograms of RNA were treated with DNAseI for each 12.5 µl RT-PCR reaction. Gene-specific primers and a OneStep RT-PCR Kit (Qiagen) were used for amplification of diagnostic products from m2A10, m4B7, m1C3, AsCPA [32], AsVg1 [23], or An. stephensi ribosomal protein S26 gene [23] transcripts (Table S2). For m2A10 RT-PCR analyses, amplification of diagnostic products from AsVg1 and ribosomal protein S26 gene-specific primers was performed in a single reaction. Diagnostic amplification reactions for the m4B7 25.1 and m1C3 P4.1 lines were initiated with one cycle at 50°C for 30 m, one cycle at 95°C for 15 m, 32 cycles denaturation at 94°C for 30 s, annealing at a reaction-specific temperature (Table S2) for 30 s, and extension at 72°C for 1 m, followed by a final extension at 72°C for 10 m. Diagnostic amplification reactions for the m2A10 44.1 line were performed as described, except that 30 cycles of amplification were completed. For each sample, an additional control RT-PCR reaction tested for the presence of genomic DNA contamination using ribosomal protein S26 gene primers but omitting the reverse transcription step. Multiple biological replicates (≥2) were performed for selected time points for each of the RT-PCR series of experiments.
Real-time quantitative RT-PCR analysis
Female mosquito midguts and carcasses and male midguts were dissected in phosphate-buffered saline (PBS), homogenized in Trizol Reagent (Invitrogen), and total RNA extracted. Midguts were dissected at different time points (4 h, 8 h, 16 h, 24 h, 48 h, 72 h, 7 d, and 15 d) after a bloodmeal. RNA was treated with DNase I (Invitrogen) at 1 U/µg RNA to remove potential genomic DNA contamination. Further purification was performed using a DNA-free kit (Ambion). A total of 0.4 µg of RNA was used for reverse transcription in a reaction volume of 20 µl using ThermoScript RT-PCR System (Invitrogen). Real-time quantitative PCR was performed on an Opticon 2 Real-Time PCR Detection System using the Opticon Monitor software version 3.1 (Bio-Rad laboratories). m1C3 expression was quantified with Platinum SYBR Green qPCR SuperMix UDG with ROX (Invitrogen) using gene-specific primers (Table S2) to amplify a diagnostic fragment 211 bp in length. A series of quantitative standards were generated from serial 10-fold dilutions (a range of 1010-1 molecules) of TOPO-m1C3scFv, in which full-length m1C3 was cloned. Each assay was run in triplicate wells in a 25 µl final reaction volume containing 2.5 µl of Platinum SYBR Green, 400 nM each forward and reverse primer, and 2.5 µl cDNA sample. Each run included negative controls consisting of wild-type control cDNA and water instead of cDNA. The amplification protocol consisted of 2 min at 50°C, 2 min at 95°C, followed by 40 cycles of amplification (94°C for 15 s, 60°C for 45 s, plate read of SYBR Green I fluorescence), after which a melting-curve reaction was conducted from 42°C to 95°C with plate readings every 1°C. GraphPad Prism software was used to calculate statistical significance using paired T-tests.
Immunoblot analysis
Mosquitoes were blood-fed on chickens and homogenized in a protease inhibitor solution made from complete mini (Roche) and Pefabloc SC (Roche). An equal volume of Laemmli sample buffer (Bio-Rad) with 0.1 M dithiothreitol was added. Homogenates were separated on a 12% Tris-HCl polyacrylamide gel in 1×Tris/Glycine/SDS buffer (Bio-Rad), transferred to Immun-Blot PVDF membrane (Bio-Rad), and incubated with goat anti-E tag polyclonal antibody conjugated to horse radish peroxidase (Abcam). ECL Plus Western Blotting Detection Reagents (GE Healthcare) were used to detect bound antibody. Ten females were used for each hemolymph sample preparation. Legs were removed with forceps and the proboscis was cut with a scissor. Individuals were inserted into a pipette tip plugged with glass wool and threaded through a 0.5 ml tube placed in a 1.5 ml collection tube. Centrifugation at 530 g for 10 min at 4°C extracted hemolymph. Each hemolymph sample was mixed with 25 µl 0.15 M NaCl and centrifuged at 2040 g for 5 min at 4°C. Fifteen microliters of the middle fraction of the sample was transferred to a new 1.5 ml tube, to which 10 µl of the protease inhibitor solution was added. For immunoblots with non-denatured samples, native sample buffer (Bio-Rad), 4–15% Tris-HCl polyacrylamide gels (Bio-Rad), 1×Tris/Glycine electrophoresis buffer (Bio-Rad), and native transfer buffer (25 mM Tris, 25 mM Glycine, pH 9.2) were used.
Parasite challenge experiments
Four to six day-old transgenic and wild type female mosquitoes were fed with P. falciparum NF 54 gametocytes using a membrane feeding apparatus. After 15 min of feeding, un-engorged mosquitoes were removed and engorged mosquitoes were maintained in the insectary under standard conditions [37] with daily access to a 10% sucrose solution or water and raisins. Midguts were dissected 9 days after the infectious bloodmeal, stained with 0.1% mercurochrome and the number of oocysts in each preparation counted. Uninfected bloodmeals were provided to transgenic and wild-type control mosquitoes following the membrane feeding. For the m4B7 25.1 experiments, mosquitoes were allowed to feed on the first and second days post-infection. Mosquitoes in the m2A10 experiments were allowed to feed on the 4th, 8th, and 12th days post-infection. Engorged and un-engorged females were not separated after the uninfected bloodmeals in m2A10 experiments 1–4, while un-engorged females were discarded in experiments 5–7. Samples of wild-type control and m2A10 females were dissected for oocyst counts on the 10th day post-infection. The salivary glands of all remaining m2A10 and wild-type control females were dissected 17–19 days post-infection. A hemacytometer was used in m2A10 experiments 1–4 to count salivary gland sporozoites [13]. The samples in experiments 5–7 were dried on 6 mm well slides and stored at −20°C. Sporozoites were stained using SlowFade Gold antifade reagent with DAPI (Invitrogen) and counted with a Zeiss Axioskop using the Axiovision camera and software. Sporozoites were counted using one of three methods, depending on parasite density. Method 1: If the number of sporozoites in each of five fields was counted, and a total of 3 or more sporozoites was found, an average sporozoite/mm2 measurement was calculated. When a field contained greater than 50 parasites, Improvision Volocity software was used to count the number of sporozoite nuclei in the DAPI image (Measurement protocol: 1. Find 2D nuclei: separate touching nuclei with a separation guide of 0.4 µm, reject nuclei with an area of less than 0.2 µm2. 2. Exclude objects by size: exclude objects >10 µm2). Method 2: If the 3 sporozoite requirement of method 1 was not met, fields were examined until 3 sporozoites were counted, and an average sporozoite/mm2 measurement was calculated. Sporozoite/mm2 values were used to calculate the total number of sporozoites present in the 6 mm2 slide well area. Method 3: If a total of 3 sporozoites was not found in up to 25 fields, the entire 6 mm2 slide well area was examined for an exact count. GraphPad Prism software was used to calculate statistical significance using Mann-Whitney U tests.
Accession numbers
The GenBank (http://www.ncbi.nlm.nih.gov/Genbank/) accession numbers for the m1C3, m4B7, and m2A10 genes are HQ315886, HQ315885, and HQ315884, respectively.
Supporting Information
Zdroje
1. SnowRWGuerraCANoorAMMyintHYHaySI
2005
The global distribution of clinical episodes of Plasmodium
falciparum malaria.
Nature
434
214
217
2. JamesAA
2005
Gene drive systems in mosquitoes: rules of the
road.
Trends Parasitol
21
64
67
3. MarshallJMTaylorCE
2009
Malaria control with transgenic mosquitoes.
PLoS Med
6
e20
4. TereniusOMarinottiOSieglaffDJamesAA
2008
Molecular genetic manipulation of vector
mosquitoes.
Cell Host Microbe
4
417
423
5. NirmalaXJamesAA
2003
Engineering Plasmodium-refractory phenotypes in
mosquitoes.
Trends Parasitol
19
384
387
6. RiehleMASrinivasanPMoreiraCKJacobs-LorenaM
2003
Towards genetic manipulation of wild mosquito populations to
combat malaria: advances and challenges.
J Exp Biol
206
3809
3816
7. Corby-HarrisVDrexlerAWatkins de JongLAntonovaYPakpourN
2010
Activation of Akt signaling reduces the prevalence and intensity
of malaria parasite infection and lifespan in Anopheles stephensi
mosquitoes.
PLoS Pathog
6
e1001003
8. MeredithJMBasuSNimmoDDLarget-ThieryIWarrELUnderhillAMcArthurCCCarterVHurdHBourgouinCEgglestonP
2011
Site-Specific Integration and Expression of an Anti-Malarial Gene
in Transgenic Anopheles gambiae Significantly Reduces Plasmodium
Infections.
PLoS One
6
e14587
9. BarrPJGreenKMGibsonHLBathurstICQuakyiIA
1991
Recombinant Pfs25 protein of Plasmodium falciparum elicits
malaria transmission-blocking immunity in experimental
animals.
J Exp Med
174
1203
1208
10. LiFTempletonTJPopovVComerJETsuboiT
2004
Plasmodium ookinete-secreted proteins secreted through a common
micronemal pathway are targets of blocking malaria
transmission.
J Biol Chem
279
26635
26644
11. HollingdaleMRNardinEHTharavanijSSchwartzALNussenzweigRS
1984
Inhibition of entry of Plasmodium falciparum and P. vivax
sporozoites into cultured cells; an in vitro assay of protective
antibodies.
J Immunol
132
909
913
12. BurkotTRDaZWGeysenHMWirtzRASaulA
1991
Fine specificities of monoclonal antibodies against the
Plasmodium falciparum circumsporozoite protein: recognition of both
repetitive and non-repetitive regions.
Parasite Immunol
13
161
170
13. de Lara CapurroMColemanJBeerntsenBTMylesKMOlsonKE
2000
Virus-expressed, recombinant single-chain antibody blocks
sporozoite infection of salivary glands in Plasmodium gallinaceum-infected
Aedes aegypti.
Am J Trop Med Hyg
62
427
433
14. YoshidaSIokaDMatsuokaHEndoHIshiiA
2001
Bacteria expressing single-chain immunotoxin inhibit malaria
parasite development in mosquitoes.
Mol Biochem Parasitol
113
89
96
15. RaagRWhitlowM
1995
Single-chain Fvs.
FASEB J
9
73
80
16. JasinskieneNColemanJAshikyanASalampessyMMarinottiO
2007
Genetic control of malaria parasite transmission: threshold
levels for infection in an avian model system.
Am J Trop Med Hyg
76
1072
1078
17. LiFPatraKPVinetzJM
2005
An anti-Chitinase malaria transmission-blocking single-chain
antibody as an effector molecule for creating a Plasmodium
falciparum-refractory mosquito.
J Infect Dis
192
878
887
18. BomanHGHultmarkD
1987
Cell-free immunity in insects.
Annu Rev Microbiol
41
103
126
19. GwadzRWKaslowDLeeJYMaloyWLZasloffM
1989
Effects of magainins and cecropins on the sporogonic development
of malaria parasites in mosquitoes.
Infect Immun
57
2628
2633
20. ChristensenBFinkJMerrifieldRBMauzerallD
1988
Channel-forming properties of cecropins and related model
compounds incorporated into planar lipid membranes.
Proc Natl Acad Sci U S A
85
5072
5076
21. EdwardsMJLemosFJDonnelly-DomanMJacobs-LorenaM
1997
Rapid induction by a blood meal of a carboxypeptidase gene in the
gut of the mosquito Anopheles gambiae.
Insect Biochem Mol Biol
27
1063
1072
22. ItoJGhoshAMoreiraLAWimmerEAJacobs-LorenaM
2002
Transgenic anopheline mosquitoes impaired in transmission of a
malaria parasite.
Nature
417
452
455
23. NirmalaXMarinottiOSandovalJMPhinSGakharS
2006
Functional characterization of the promoter of the vitellogenin
gene, AsVg1, of the malaria vector, Anopheles stephensi.
Insect Biochem Mol Biol
36
694
700
24. NakamuraYGojoboriTIkemuraT
2000
Codon usage tabulated from international DNA sequence databases:
status for the year 2000.
Nucleic Acids Res
28
292
25. HudsonPJKorttAA
1999
High avidity scFv multimers; diabodies and
triabodies.
J Immunol Methods
231
177
189
26. PonnuduraiTLensenAHvan GemertGJBensinkMPBolmerM
1989
Sporozoite load of mosquitoes infected with Plasmodium
falciparum.
Trans R Soc Trop Med Hyg
83
67
70
27. MoreiraLAItoJGhoshADevenportMZielerH
2002
Bee venom phospholipase inhibits malaria parasite development in
transgenic mosquitoes.
J Biol Chem
277
40839
40843
28. YoshidaSShimadaYKondohDKouzumaYGhoshAK
2007
Hemolytic C-type lectin CEL-III from sea cucumber expressed in
transgenic mosquitoes impairs malaria parasite development.
PLoS Pathog
3
e192
29. KokozaVAhmedAWoon ShinSOkaforNZouZ
2010
Blocking of Plasmodium transmission by cooperative action of
Cecropin A and Defensin A in transgenic Aedes aegypti
mosquitoes.
Proc Natl Acad Sci U S A
107
8111
8116
30. BillingsleyPFMedleyGFCharlwoodDSindenRE
1994
Relationship between prevalence and intensity of Plasmodium
falciparum infection in natural populations of Anopheles
mosquitoes.
Am J Trop Med Hyg
51
260
270
31. TaylorLH
1999
Infection rates in, and the number of Plasmodium falciparum
genotypes carried by Anopheles mosquitoes in Tanzania.
Ann Trop Med Parasitol
93
659
662
32. ChenXMarinottiOWhitmanLJasinskieneNRomansPJamesAA
2007
The Anopheles gambiae vitellogenin gene
(VGT2) promoter directs persistent accumulation of a
reporter gene product in transgenic Anopheles stephensi
following multiple blood meals.
Am J Trop Med Hyg
76
1118
1124
33. AmenyaDABonizzoniMIsaacsATJasinskieneNChenHMarinottiOYanGJamesAA
2010
Comparative fitness assessment of Anopheles
stephensi transgenic lines receptive to site-specific
integration.
Insect Molec Biol
19
263
269
34. YoshidaSWatanabeH
2006
Robust salivary gland-specific transgene expression in Anopheles
stephensi mosquito.
Insect Mol Biol
15
403
410
35. AbrahamEGDonnelly-DomanMFujiokaHGhoshAMoreiraL
2005
Driving midgut-specific expression and secretion of a foreign
protein in transgenic mosquitoes with AgAper1 regulatory
elements.
Insect Mol Biol
14
271
279
36. UngureanuEKillick-KendrickRGarnhamPCBranzeiPRomanescuC
1976
Prepatent periods of a tropical strain of Plasmodium vivax after
inoculations of tenfold dilutions of sporozoites.
Trans R Soc Trop Med Hyg
70
482
483
37. BenedictMQ
1996
Care and maintenance of anopheline mosquito
colonies.
CramptonJMBeardCBLouisC
The Molecular Biology of Insect Disease Vectors: A Methods
Manual
3
12
Chapman and Hall, London
38. AnkerRZavalaFPollokBA
1990
VH and VL region structure of antibodies that recognize the
(NANP)3 dodecapeptide sequence in the circumsporozoite protein of Plasmodium
falciparum.
Eur J Immunol
20
2757
2761
39. ZhengXLZhengAL
2002
Genomic organization and regulation of three cecropin genes in
Anopheles gambiae.
Insect Mol Biol
11
517
525
40. HornCWimmerEA
2000
A versatile vector set for animal transgenesis.
Dev Genes Evol
210
630
637
41. CatterucciaFNolanTLoukerisTGBlassCSavakisC
2000
Stable germline transformation of the malaria mosquito Anopheles
stephensi.
Nature
405
959
962
42. SambrookJFritschEFManiatisT
1989
Molecular Cloning. A Laboratory Manual
Plainview
Cold Spring Harbor Laboratory Press
43. AdelmanZNJasinskieneNVallyKJPeekCTravantyEA
2004
Formation and loss of large, unstable tandem arrays of the
piggyBac transposable element in the yellow fever mosquito, Aedes
aegypti.
Transgenic Res
13
411
425
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Innate Immune Sensing of DNA
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 4- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Low Diversity Variety Multilocus Sequence Types from Thailand Are Consistent with an Ancestral African Origin
- Genetic Assignment Methods for Gaining Insight into the Management of Infectious Disease by Understanding Pathogen, Vector, and Host Movement
- Innate Immune Sensing of DNA
- Engineered Resistance to Development in Transgenic
- -Mediated Detoxification of Reactive Oxygen Species Is Required for Full Virulence in the Rice Blast Fungus
- SLO-1-Channels of Parasitic Nematodes Reconstitute Locomotor Behaviour and Emodepside Sensitivity in Loss of Function Mutants
- Structure-Function Analysis of the LRIM1/APL1C Complex and its Interaction with Complement C3-Like Protein TEP1
- A Effector with Enhanced Inhibitory Activity on the NF-κB Pathway Activates the NLRP3/ASC/Caspase-1 Inflammasome in Macrophages
- The MARCH Family E3 Ubiquitin Ligase K5 Alters Monocyte Metabolism and Proliferation through Receptor Tyrosine Kinase Modulation
- is an Unstable Pathogen Showing Evidence of Significant Genomic Flux
- The Cell Wall Protein CwpV is Antigenically Variable between Strains, but Exhibits Conserved Aggregation-Promoting Function
- Engineering HIV-Resistant Human CD4+ T Cells with CXCR4-Specific Zinc-Finger Nucleases
- SUMO-Interacting Motifs of Human TRIM5α are Important for Antiviral Activity
- Completion of Hepatitis C Virus Replication Cycle in Heterokaryons Excludes Dominant Restrictions in Human Non-liver and Mouse Liver Cell Lines
- : Reservoir Hosts and Tracking the Emergence in Humans and Macaques
- On Being the Right Size: The Impact of Population Size and Stochastic Effects on the Evolution of Drug Resistance in Hospitals and the Community
- Bacterial and Host Determinants of MAL Activation upon EPEC Infection: The Roles of Tir, ABRA, and FLRT3
- Respiratory Syncytial Virus Interferon Antagonist NS1 Protein Suppresses and Skews the Human T Lymphocyte Response
- NF-κB Hyper-Activation by HTLV-1 Tax Induces Cellular Senescence, but Can Be Alleviated by the Viral Anti-Sense Protein HBZ
- Human Cytomegalovirus IE1 Protein Elicits a Type II Interferon-Like Host Cell Response That Depends on Activated STAT1 but Not Interferon-γ
- A New Model to Produce Infectious Hepatitis C Virus without the Replication Requirement
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- NF-κB Hyper-Activation by HTLV-1 Tax Induces Cellular Senescence, but Can Be Alleviated by the Viral Anti-Sense Protein HBZ
- Bacterial and Host Determinants of MAL Activation upon EPEC Infection: The Roles of Tir, ABRA, and FLRT3
- : Reservoir Hosts and Tracking the Emergence in Humans and Macaques
- On Being the Right Size: The Impact of Population Size and Stochastic Effects on the Evolution of Drug Resistance in Hospitals and the Community
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání