-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
The influenza A virus RNA polymerase is a heterotrimeric complex responsible for viral genome transcription and replication in the nucleus of infected cells. We recently carried out a proteomic analysis of purified polymerase expressed in human cells and identified a number of polymerase-associated cellular proteins. Here we characterise the role of one such host factors, SFPQ/PSF, during virus infection. Down-regulation of SFPQ/PSF by silencing with two independent siRNAs reduced the virus yield by 2–5 log in low-multiplicity infections, while the replication of unrelated viruses as VSV or Adenovirus was almost unaffected. As the SFPQ/PSF protein is frequently associated to NonO/p54, we tested the potential implication of the latter in influenza virus replication. However, down-regulation of NonO/p54 by silencing with two independent siRNAs did not affect virus yields. Down-regulation of SFPQ/PSF by siRNA silencing led to a reduction and delay of influenza virus gene expression. Immunofluorescence analyses showed a good correlation between SFPQ/PSF and NP levels in infected cells. Analysis of virus RNA accumulation in silenced cells showed that production of mRNA, cRNA and vRNA is reduced by more than 5-fold but splicing is not affected. Likewise, the accumulation of viral mRNA in cicloheximide-treated cells was reduced by 3-fold. In contrast, down-regulation of SFPQ/PSF in a recombinant virus replicon system indicated that, while the accumulation of viral mRNA is reduced by 5-fold, vRNA levels are slightly increased. In vitro transcription of recombinant RNPs generated in SFPQ/PSF-silenced cells indicated a 4–5-fold reduction in polyadenylation but no alteration in cap snatching. These results indicate that SFPQ/PSF is a host factor essential for influenza virus transcription that increases the efficiency of viral mRNA polyadenylation and open the possibility to develop new antivirals targeting the accumulation of primary transcripts, a very early step during infection.
Published in the journal: . PLoS Pathog 7(11): e32767. doi:10.1371/journal.ppat.1002397
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002397Summary
The influenza A virus RNA polymerase is a heterotrimeric complex responsible for viral genome transcription and replication in the nucleus of infected cells. We recently carried out a proteomic analysis of purified polymerase expressed in human cells and identified a number of polymerase-associated cellular proteins. Here we characterise the role of one such host factors, SFPQ/PSF, during virus infection. Down-regulation of SFPQ/PSF by silencing with two independent siRNAs reduced the virus yield by 2–5 log in low-multiplicity infections, while the replication of unrelated viruses as VSV or Adenovirus was almost unaffected. As the SFPQ/PSF protein is frequently associated to NonO/p54, we tested the potential implication of the latter in influenza virus replication. However, down-regulation of NonO/p54 by silencing with two independent siRNAs did not affect virus yields. Down-regulation of SFPQ/PSF by siRNA silencing led to a reduction and delay of influenza virus gene expression. Immunofluorescence analyses showed a good correlation between SFPQ/PSF and NP levels in infected cells. Analysis of virus RNA accumulation in silenced cells showed that production of mRNA, cRNA and vRNA is reduced by more than 5-fold but splicing is not affected. Likewise, the accumulation of viral mRNA in cicloheximide-treated cells was reduced by 3-fold. In contrast, down-regulation of SFPQ/PSF in a recombinant virus replicon system indicated that, while the accumulation of viral mRNA is reduced by 5-fold, vRNA levels are slightly increased. In vitro transcription of recombinant RNPs generated in SFPQ/PSF-silenced cells indicated a 4–5-fold reduction in polyadenylation but no alteration in cap snatching. These results indicate that SFPQ/PSF is a host factor essential for influenza virus transcription that increases the efficiency of viral mRNA polyadenylation and open the possibility to develop new antivirals targeting the accumulation of primary transcripts, a very early step during infection.
Introduction
The influenza A viruses belong to the family Orthomyxoviridae and contain a segmented, single-stranded RNA genome of negative polarity (for a review see [1]. Each of the genomic RNA segments is encapsidated in a ribonucleoprotein particle (RNP) containing the polymerase complex and a number of nucleoprotein (NP) monomers, depending on their size [2], [3]. Contrary to many other RNA viruses, the influenza virus RNPs are transcribed and replicated in the nucleus of infected cells. The enzyme responsible for these activities is the viral polymerase, a heterotrimer that comprises the PB1, PB2 and PA subunits [4]–[6]. The PB1 subunit acts as polymerase [7], [8] while PB2 and PA are responsible for cap-binding and cap-snatching, respectively [9]–[12]. The heterotrimer has a compact structure [2], [13]–[15] and is required for both transcription and replication [7], [16]–[19]. The polymerase complex can be found associated to the RNP structure or in a soluble form [20], the latter being able to oligomerise in vivo [21], [22]. Along the years, a number of human cell factors have been described as interactors of influenza virus polymerase and in some specific cases their role in virus replication has been studied [23]–[36].
In one such studies, we identified the human SFPQ/PSF factor as associated in vivo to influenza virus polymerase by proteomic analysis of purified complexes [34]. Human SFPQ/PSF is a nuclear multifunctional protein that has been implicated in a series of steps in the human gene expression pathway (for a review, see [37]. It was first described as associated to the polypyrimidine tract-binding protein (PTB) [38] and contains regions rich in arginine/glycine and proline/glutamine close to its N-terminus as well as two RRMs located more C-terminal. SFPQ/PSF can be found as a heterodimer with p54nrb/NonO, a protein that is highly homologous to the SFPQ/PSF C-terminal half. The SFPQ/PSF-p54nrb/NonO heterodimer co-purifies with DNA topoisomerase and interacts with RAD1 recombinase, leading to the stimulation of nucleic acid strand transfer and the cleavage/religation steps [39]–[43]. In addition, several reports have shown that SFPQ/PSF and/or p54nrb/NonO can regulate cellular transcription in a variety of genes (reviewed in [37]. In agreement with the SFPQ/PSF association with PTB, its binding has been reported to elements in the splicing machinery, like the U4/U6-U5 tri-snRNP and many other splicing factors [44]–[46] and the RNA pol II CTD [47], probably in a RRM-dependent manner [48]. Consistent with these interactions, SFPQ/PSF has been shown to stimulate the splicing of mRNAs transcribed by strong transcriptional activators and to control alternative splicing [49], [50]. In spite of the above mentioned associations, most of SFPQ/PSF-p54nrb/NonO can be found in the “nuclear matrix” fraction [51], consistent with the proposed role for the heterodimer in the specific retention in the nuclear matrix of RNA which has been A>I hyperedited [37], [52].
In addition to these many potential functions assigned to SFPQ/PSF or the SFPQ/PSF-p54nrb/NonO heterodimer, the former has been shown to bind specifically to a defined stem-loop in hepatitis delta RNA [53] and the 3′-end of HCV genome [54], while the latter inhibits the transport and expression of HIV mRNAs containing the instability region (INS) [55]. Here we have analysed the role of SFPQ/PSF in the influenza virus infection. Silencing the SFPQ/PSF gene, but not p54nrb/NonO, strongly reduced virus multiplication. The accumulation of viral genomic vRNA and mRNA as well as viral proteins was reduced, probably as a consequence of the inhibition of primary and secondary transcription, but normal splicing of virus mRNA was observed. In vitro transcription of recombinant RNPs generated in SFPQ-silenced cells resulted in reduced levels of viral poly A+ RNA. These results are consistent with a role for SFPQ/PSF during the polyadenylation step in the synthesis of viral mRNAs from the parental RNP templates, the earliest nuclear step in virus replication cycle, as well as during secondary transcription.
Results/Discussion
A proteomic analysis of the intracellular complexes formed by recombinant influenza virus polymerase revealed a series of human proteins that were stably associated to the viral enzyme [34]. One of such associated factors was SFPQ/PSF, a multifunctional nuclear protein involved in transcription, post-transcriptional processing of mRNAs and DNA rearrangements [37]. To study the role of SFPQ/PSF in influenza virus replication we first analysed the expression and localisation of the protein along the infection cycle. Cultures of A549 cells were infected with VIC influenza virus at high multiplicity and total cell extracts were prepared at various times thereafter. The accumulation of SFPQ/PSF was determined by Western-blot using specific antibodies and is shown in Figure 1A. No changes in the level of expression of the protein were observed when compared to GAPDH as a standard, whereas the progressive accumulation of virus proteins was apparent (see NP marker in Figure 1A). Similar experiments were obtained when the WSN virus strain was used (data not shown). The localisation of SFPQ/PSF was analysed by confocal immunofluorescence and is presented in Figure 1B. The protein was found in a punctuate pattern within the nucleus, slightly more intense in the nuclear periphery and excluded from the nucleoli. No significant change could be observed in such distribution along virus infection, although a small increase in cytoplasmic staining was apparent at late times (Figure 1B). Similar results were obtained using the HeLa cell line and the WSN virus strain (data not shown).
Fig. 1. Accumulation and localisation of SFPQ/PSF in influenza virus infected cells. 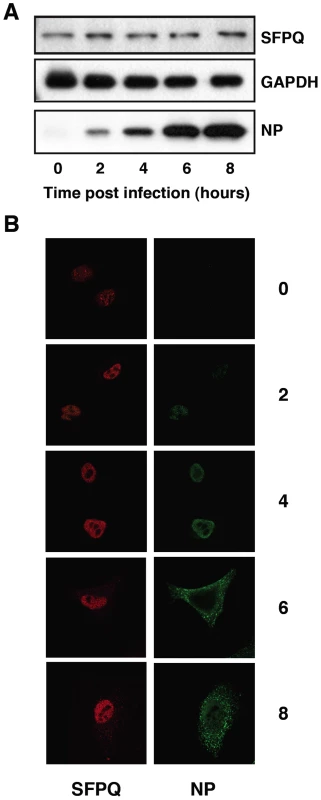
Cultures of human A549 cells were infected with the VIC strain of influenza virus at 3 pfu/cell. (A) At the times after infection indicated (hours), extracts were prepared and analysed by Western-blot with the antibodies indicated to the right. (B) At the same times, cultures were fixed and processed for immunofluorescence using antibodies specific for SFPQ/PSF and NP, as indicated. SFPQ/PSF is specifically required for efficient influenza virus infection
In previous studies we analysed the localisation of SFPQ/PSF and NP by confocal immunofluorescence of cells infected with VIC influenza virus. A clear co-localisation was observed at 4–6 hours post-infection (hpi), particularly prominent at the periphery of the cell nucleus [34]. To further analyse this association in infected cells we carried out co-immunoprecipitation experiments. Cultures of A549 cells were infected at high moi with the VIC strain of influenza virus and at various times after infection cell extracts were prepared and used for immunoprecipitation with anti-SFPQ/PSF or control antibodies. The immunoprecipitates were analysed by Western-blotting with antibodies specific for SFPQ/PSF and NP. The results are presented in Figure 2 and a clear co-immunoprecipitation of NP was observed with the SFPQ/PSF-specific antibodies. In view of these results, the relevance of SFPQ/PSF for influenza virus multiplication was then studied by gene silencing. Cultures of A549 cells were transfected with SFPQ/PSF-specific siRNA, or a scrambled unspecific siRNA as a control, and then infected with VIC influenza virus at low multiplicity. Samples were withdrawn from the supernatant medium at various times after infection and the virus titre was determined by plaque-assay on MDCK cells. The results of kinetics experiments run in triplicate are presented in Figure 3A. A protracted virus growth kinetics was apparent in the SFPQ/PSF–silenced cultures as compared to cultures transfected with control siRNA and the final titre was reduced by 2–3 log units in various experiments similar to that presented in Figure 3A. The level of SFPQ/PSF down-regulation was verified at various times after siRNA transfection and virus infection and is presented in Figure 3D. These results suggested that SFPQ/PSF plays an important role during influenza virus infection. To verify that the virus growth inhibition is really due to SFPQ/PSF down-regulation and not to an spurious off-target effect of the particular siRNA used, similar experiments were performed with an independent SFPQ/PSF-specific siRNA and the results are presented in Figure 3B and 3C. Furthermore, two additional virus strains were used in these experiments, WSN and a VIC/WSN recombinant virus. Again, a strong reduction in the yield of virus production was obtained, thereby confirming that SFPQ/PSF in an important human host factor for the multiplication of influenza virus.
Fig. 2. Association of viral ribonucleoproteins with SFPQ/PSF. 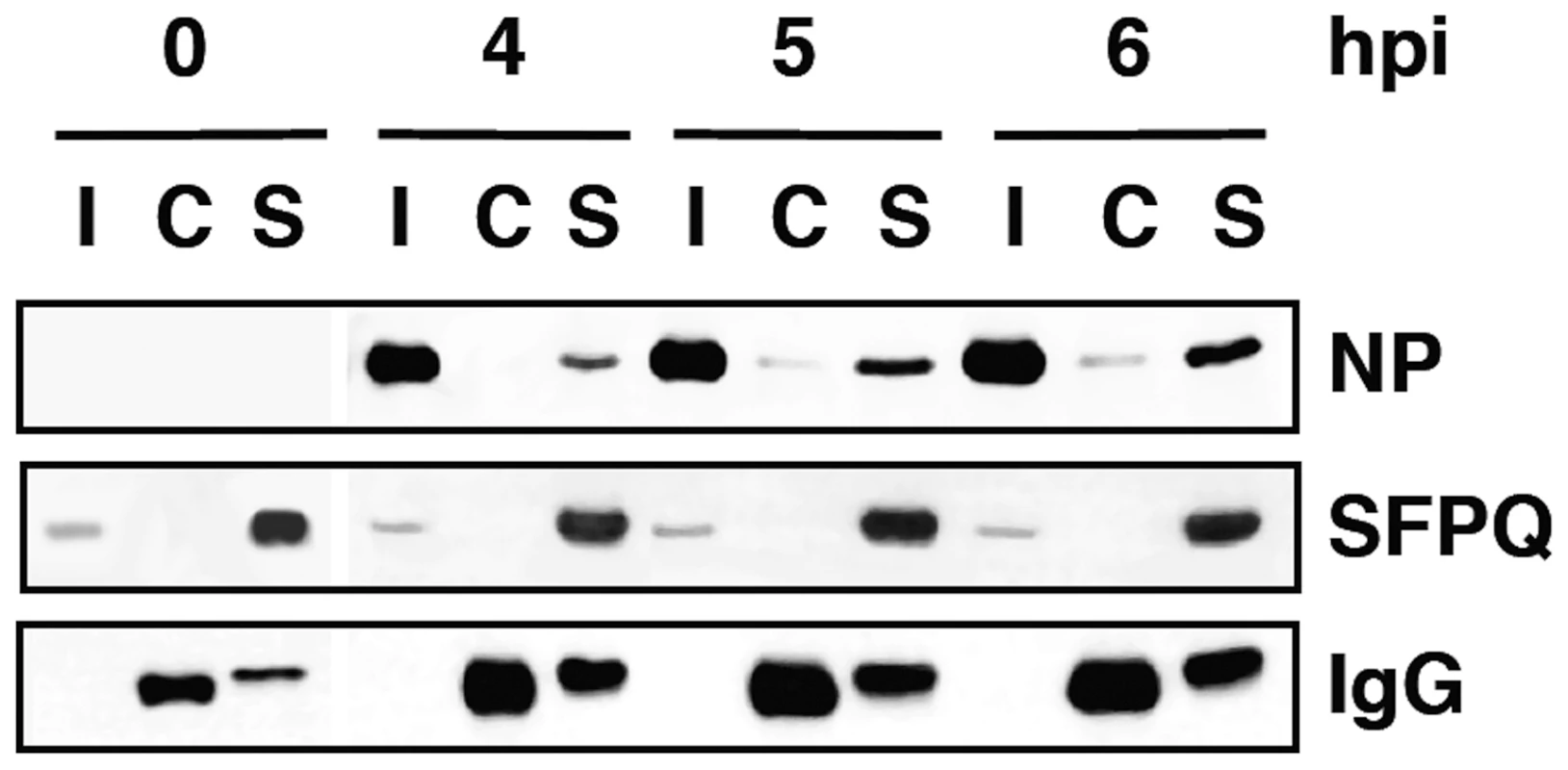
Cultures of human A549 cells were infected with the VIC strain of influenza virus at 3 pfu/cell. Total cell extracts were prepared at the times after infection indicated (hpi) and immunoprecipitated with anti-SFPQ/PSF or control antibodies. The immunoprecipitates were analysed by Western-blot using antibodies specific for SFPQ/PSF or NP, as indicated. I: Input extract. C: Control antibody. S: SFPQ/PSF specific antibody. Fig. 3. Kinetics of influenza virus multiplication in SFPQ/PSF-silenced cells. 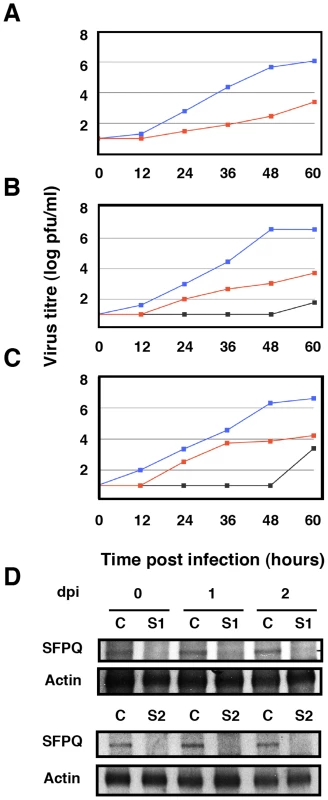
Cultures of human A549 cells were transfected with SFPQ/PSF-specific or control siRNAs as described under Materials and Methods and then infected with influenza virus at a moi of 0.001 pfu/cell. Aliquots of the supernatant media were withdrawn at the times indicated and used for virus titration by plaque-assay. The results presented are representative of two/three independent experiments. (A) Cultures were silenced with control siRNA (blue) or siRNA-1 (red) and infected with the VIC strain. (B) Cultures were silenced with control siRNA (blue), siRNA-1 (red) or siRNA-2 (grey) and infected with a recombinant virus containing the M, HA and NA segment of WSN in the background of VIC strain. (C) Cultures were silenced with control siRNA (blue), siRNA-1 (red) or siRNA-2 (grey) and infected with WSN strain. (D) At the times after infection indicated (days), the accumulation of SFPQ/PSF was determined in the cultures transfected with control (C) or SFPQ/PSF siRNAs (S1 or S2, as indicated) by Western-blot using anti SFPQ/PSF antibodies. As SFPQ/PSF is a multifunctional protein involved in many steps of cellular transcription and post-transcriptional RNA processing [37], it is conceivable that its down-regulation indirectly leads to reductions in influenza virus multiplication. For instance, it would be conceivable that SFPQ/PSF down-regulation inhibits cellular transcription and/or splicing to a level that makes influenza virus unable to replicate, as it depends on these processes for its own transcription and gene expression. Such a possibility would seem unlikely, as the general pattern of cellular protein synthesis is not altered by SFPQ/PSF silencing (see below), but we carried out controls to ascertain the specificity of SFPQ/PSF requirement for influenza virus multiplication. The multiplication of two additional viruses was studied in SFPQ/PSF-silenced cells: Vesicular stomatitis virus (VSV), as an additional example of negative-stranded RNA virus, and Adenovirus 5 (Ad5), as a nuclear virus that is strongly dependent on the cellular transcriptional and splicing machineries. Cultures of A549 cells were SFPQ/PSF - or control-silenced and infected with either VSV or Ad5 and the virus accumulated in the culture supernatant (VSV) or the infected cells (Ad5) was determined by plaque-assay on BHK21 (VSV) or HEK293T cells (Ad5). As presented in Figure 4A and 4B, the multiplication of neither virus was affected by the down-regulation of SFPQ/PSF, indicating that this human protein is a host factor specifically important for influenza virus multiplication.
Fig. 4. Kinetics of VSV and Ad5 multiplication in SFPQ/PSF-silenced cells. 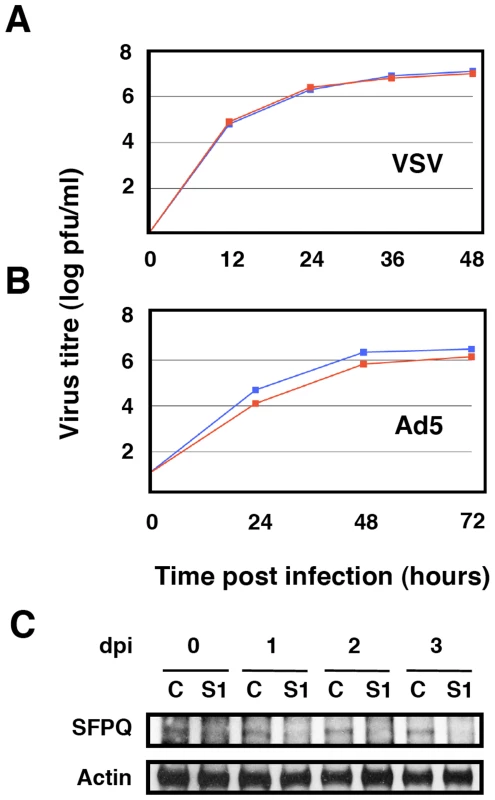
Cultures of human A549 cells were transfected with SFPQ/PSF-specific siRNA-1 (S1) or control (C) siRNAs, infected with VSV or Ad5 as described under Materials and Methods and virus titration was performed with samples obtained at the times indicated. The results presented are representative of two/three independent experiments. (A) Cultures were silenced with control siRNA (blue) or siRNA-1 (red) and infected with VSV at a moi of 0.001 pfu/cell. (B) Cultures were silenced with control siRNA (blue) or siRNA-1 (red) and infected with Ad5 at a moi of 2 pfu/cell. (C) At the times after infection indicated (days), the accumulation of SFPQ/PSF was determined in the cultures transfected with control (C) or SFPQ/PSF siRNAs (S1) by Western-blot using anti SFPQ/PSF antibodies. Since it has been shown that SFPQ/PSF associates to p54nrb/NonO (see above), it was important to ascertain whether influenza virus requires SFPQ/PSF or the SFPQ/PSF-p54nrb/NonO heterodimer for proper multiplication. Hence, we analysed the multiplication of influenza virus in A549 cells after silencing p54nrb/NonO by transfection of a p54nrb/NonO-specific siRNA. Importantly, silencing of p54nrb/NonO did not alter the accumulation of SFPQ/PSF or vice versa (data not shown). As indicated in Figure 5, no reduction in virus yield was observed when using the VIC virus strain (Figure 5A) and a small reduction in WSN virus amplification was observed by down-regulation of p54nrb/NonO, much more limited than that observed when silencing SFPQ/PSF (Figure 5B), in spite of an almost complete block of p54nrb/NonO expression (Figure 5C). Therefore, we conclude that it is SFPQ/PSF by itself what influenza virus requires carrying out a normal infection cycle.
Fig. 5. Kinetics of influenza virus multiplication in NonO-silenced cells. 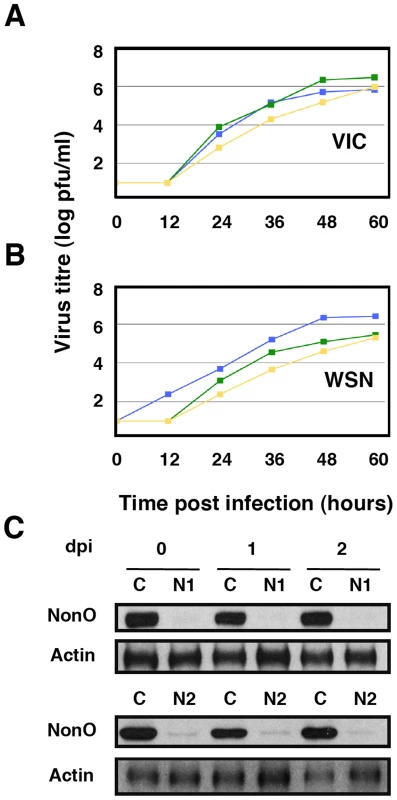
Cultures of human A549 cells were transfected with NonO-specific (N1 or N2, as indicated) or control (C) siRNAs as described under Materials and Methods and then infected with influenza virus at a moi of 0.001 pfu/cell. Aliquots of the supernatant media were withdrawn at the times indicated and used for virus titration by plaque-assay. The results presented are representative of two/three independent experiments. (A) Cultures were silenced with control siRNA (blue), siRNA N1 (green) or siRNA N2 (yellow) and infected with the VIC strain. (B) Cultures were silenced as above and infected with WSN virus. (C) At the times after infection indicated (days), the accumulation of NonO/p54 was determined in the cultures transfected with control (C) or SFPQ/PSF siRNAs (N1 or N2, as indicated) by Western-blot using anti NonO/p54 antibodies. Down-regulation of SFPQ/PSF reduces virus RNA replication and gene expression
Once the relevance of SFPQ/PSF for influenza virus infection was verified, we analysed the role of this host factor in the virus cycle. First, the synthesis of viral proteins was studied in SFPQ/PSF - and control-silenced A549 cells. Cultures of SFPQ/PSF - or control-silenced A549 cells were infected at high multiplicity and pulse-labelled with 35S-met-cys at various times after infection. The labelled proteins were analysed by polyacrylamide gel electrophoresis and autoradiography and the results are presented in Figure 6. The synthesis of the major virus proteins was reduced and delayed in SFPQ/PSF-silenced cells, indicating that SFPQ/PSF down-regulation leads to a general reduction and delay in virus gene expression. However, it is worth mentioning that only a slight change was observed in the level and pattern of cellular protein synthesis upon SFPQ/PSF silencing (compare lanes 0 in siCRTL and S1 panels in Figure 6). Similar results were obtained when the accumulation of viral proteins was determined by Western-blot (Figure S1).
Fig. 6. Kinetics of influenza virus protein synthesis in SFPQ/PSF-silenced cells. 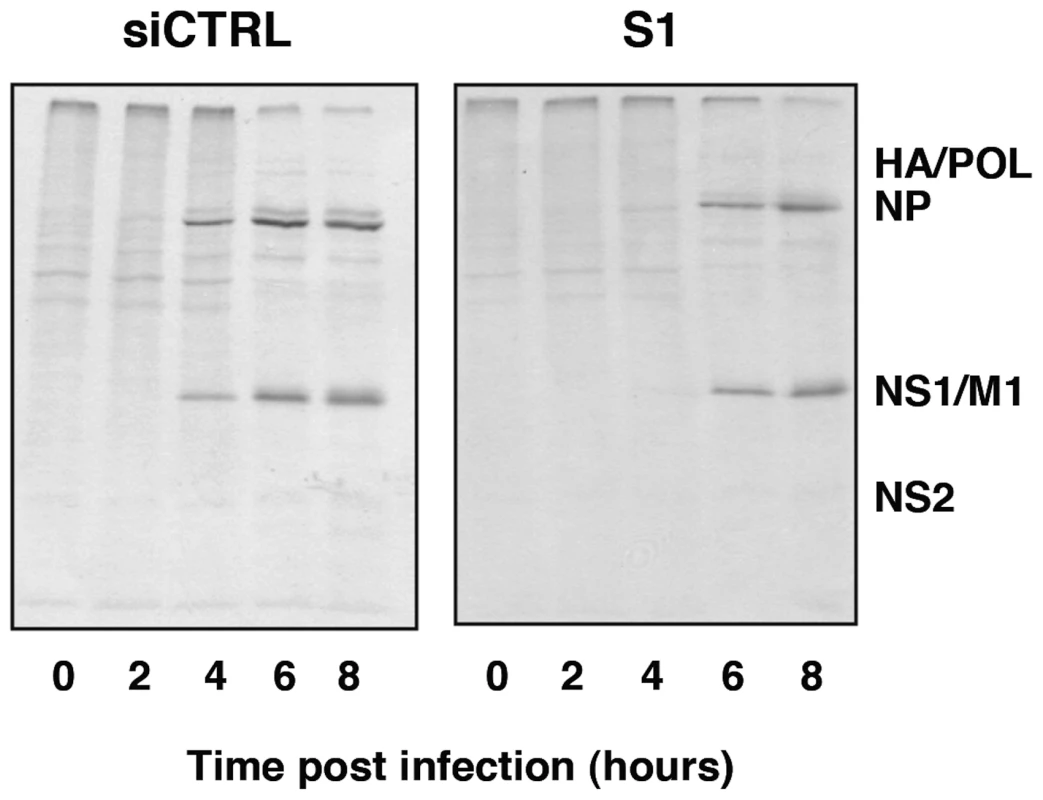
Cultures of human A549 cells were transfected with SFPQ/PSF-specific siRNA-1 (S1) or control siRNA (siCTRL) as described under Materials and Methods and then infected with VIC virus at a moi of 3 pfu/cell. At the times in hours indicated the cultures were pulse-labelled with 35S-met-cys and total protein extracts were prepared. The samples were analysed by polyacrylamide gel electrophoresis and autoradiography. The mobility of some of the virus-specific proteins is indicated to the right. To analyse further the phenotype of the infection cycle in SFPQ/PSF-silenced cells, the localisation of progeny RNPs was studied by confocal immunofluorescence with anti-NP antibodies. The results are presented in Figure 7. As expected, the level of SFPQ/PSF was decreased in SFPQ/PSF-silenced cells and a general reduction in NP signal was observed, as compared with infected, control-silenced cells (Figure 7A). Only cells with a level of SFPQ/PSF similar to that of control-silenced cells showed an accumulation of NP comparable to control infected cells, although the localisation was not normal (Figure 7A; arrow). Significantly, a linear correlation was observed between the immunofluorescence signals of SFPQ/PSF and NP in random fields of control - or SFPQ/PSF-silenced cells (Figure 7B). Thus, the low levels of virus protein synthesis (Figure 6) and the small virus production (Figure 3) observed in SFPQ/PSF-silenced cultures could be simply the consequence of the infection of a small number of cells not completely silenced upon transfection of SFPQ/PSF-specific siRNA.
Fig. 7. Cytological analysis of influenza virus infection of SFPQ/PSF-silenced cells. 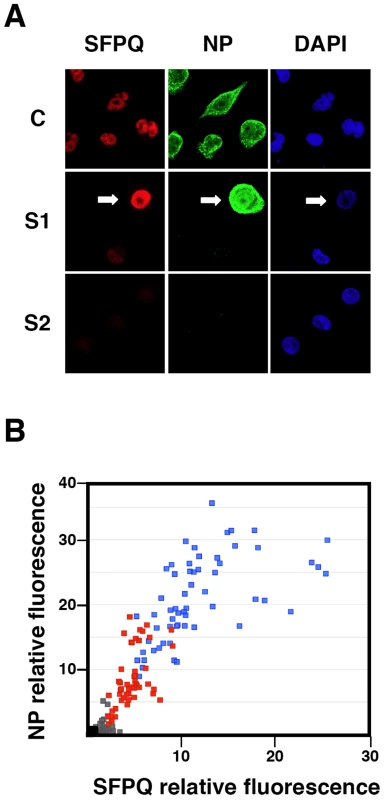
Cultures of human A549 cells were transfected with control siRNA (C) or SFPQ/PSF-specific siRNAs (S1 or S2 as indicated), as described under Materials and Methods, and then infected with VIC virus at a moi of 3 pfu/cell. The cultures were fixed at 6 hpi and processed for immunofluorescence using antibodies specific for SFPQ/PSF and NP. (A) The images presented are representative confocal sections showing the presence of nuclei (DAPI –blue-), SFPQ/PSF (red) and NP (green). (B) Graphical representation of the NP (vertical axis) and SFPQ/PSF (horizontal axis) signals obtained for random 1024x1024 images of each sample. Blue: Control-silenced cultures. Red: SFPQ/PSF-silenced cultures (S1). Grey: SFPQ/PSF-silenced cultures (S2). The black dot indicates the background signals obtained for cultures stained with the secondary antibodies only. Viral transcription, but not splicing, is affected in SFPQ/PSF silenced cells
The reduction of virus protein synthesis under conditions of SFPQ/PSF down-regulation might be due to defects in genome amplification, virus transcription, splicing or translation of viral mRNA. As SFPQ/PSF has been described as a splicing factor, we first analysed whether its down-regulation would alter the splicing of virus mRNAs. The amounts of NS1 and NS2 mRNAs were determined in control and SFPQ/PSF silenced infected cells by a RT-qPCR procedure using TaqMan probes (Table S1). The proportion of NS1 versus total NS mRNA was indistinguishable in both experimental conditions (ratio control/silenced cells 1.016+/−0.035; n = 7 experiments). Next, the levels of accumulation of vRNA, cRNA and mRNA were determined in SFPQ/PSF-silenced and control-silenced cells. Total cell RNA was isolated at various times after high-multiplicity infections and the amounts of virus-specific RNAs corresponding to the NP, NS and M virus segments were determined by hybridisation with specific probes. The results of a representative experiment are presented in Figure 8. In control-silenced cells, the kinetics of accumulation of virus RNAs showed a pattern analogous to that previously described [56], [57], while in SFPQ/PSF-silenced cells a protracted kinetics was observed and 4-5 fold reductions in maximal accumulations of vRNA, cRNA and mRNA were determined. These results indicated that SFPQ/PSF is required for virus RNA replication but could not tell whether this was a direct effect and whether SFPQ/PSF also played a role in virus transcription. To clarify these questions, the accumulations of primary virus transcripts were determined after infection of SFPQ/PSF-silenced and control-silenced cells. The cells were infected at high multiplicity in the presence of cycloheximide to avoid the synthesis of viral proteins and hence the replication of viral RNA [58]. The accumulation of total NS transcripts was determined by RT-qPCR and demonstrated that silencing SFPQ/PSF leads to a 3-fold reduction in primary transcription (Figure 9A). To verify the inhibition of virus multiplication, virus mRNA was determined in infected cells treated or not with cycloheximide. The results are presented in Figure 9B and document around 50-fold reduction upon treatment with the drug.
Fig. 8. Accumulation of positive- and negative-polarity RNAs during the influenza virus infection of SFPQ/PSF-silenced cells. 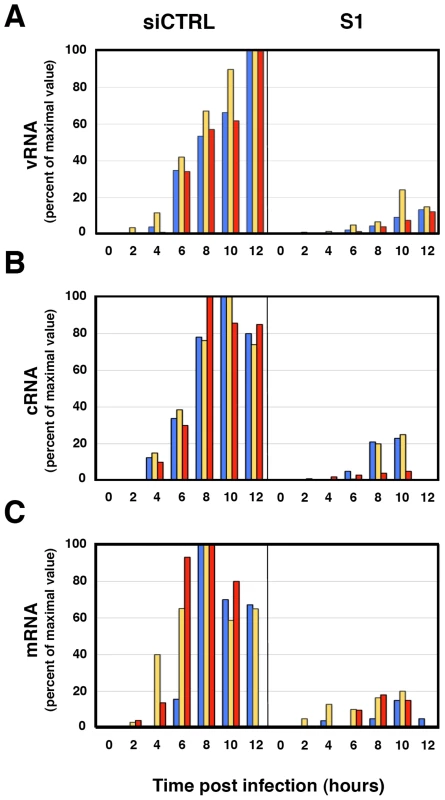
Cultures of human A549 cells were transfected with SFPQ/PSF-specific (S1) or control siRNAs (siCTRL) as described under Materials and Methods and then infected with influenza virus at a moi of 3 pfu/cell. Total cell RNA was isolated at the times indicated and used for determination of vRNA (A) cRNA (B) or mRNA (C) by hybridisation with probes specific for NP (blue), NS (yellow) and M (red). The data is presented as percent of maximal values and is representative of three independent kinetics experiments. Fig. 9. Accumulation of mRNA during the influenza virus infection of SFPQ/PSF-silenced cells treated with cycloheximide. 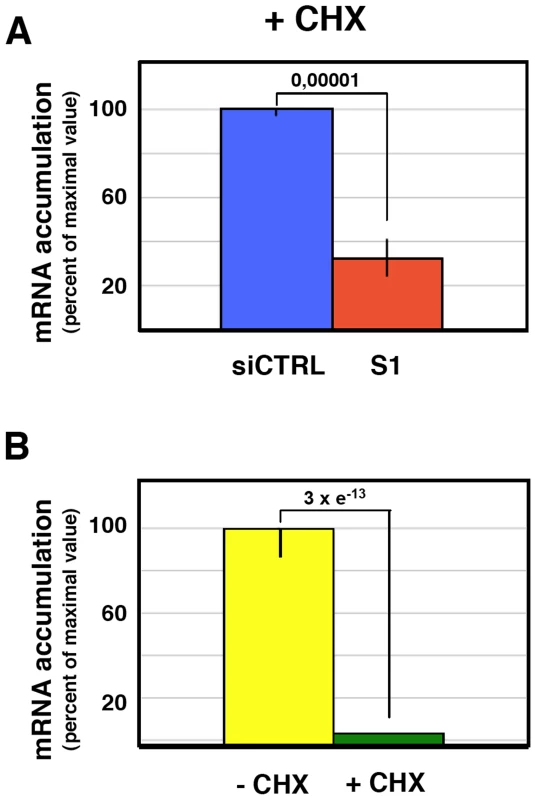
Cultures of human A549 cells were transfected with SFPQ/PSF-specific (S1) or control siRNAs (siCTRL) as described under Materials and Methods and then infected with influenza virus at a moi of 3 pfu/cell for 6 hours in the presence or absence of cycloheximide (100 µg/ml). The accumulation of viral RNA was determined by RT-qPCR using probes specific for the NS segment. Significance was determined by the Student's t test. (A) The accumulation of viral RNA in control and SFPQ/PSF-silenced cells treated with cycloheximide is presented as average and standard deviation of 6 determinations. (B) The accumulation of mRNA in infected cells treated or not with cycloheximide is presented as average and standard deviation of 6 determinations. These results suggest that SFPQ/PSF might simply be required for virus primary transcription and the observed reduction in vRNA accumulation would be an indirect consequence, since viral protein expression is essential for viral RNA replication [58]. To test whether virus RNA replication is directly inhibited by SFPQ/PSF down-regulation, in addition to the observed inhibition of primary transcription, we made use of a recombinant replicon system to analyse RNA replication and secondary transcription. In this system, no primary transcription is required for RNA replication to take place, as the virus proteins are provided by plasmid expression in trans. Human HEK293T cells were transfected with SFPQ-specific or control siRNAs and later transfected with plasmids encoding the virus polymerase subunits, NP and a virus genomic plasmid encoding the cat gene in negative polarity. The down-regulation of SFPQ/PSF was ascertained by Western-blot (Figure 10D) and the CAT protein accumulation was determined as a reporter of total replicon activity. The results indicated that silencing SFPQ/PSF lead to a 5-fold reduction in CAT expression (Figure 10A). To determine whether this reduction was due to alterations in viral RNA replication, transcription or both, total cell RNA was isolated and used to determine negative-polarity and positive-polarity RNA accumulations by hybridisation with specific probes. As shown in Figure 10B, a 5-fold reduction in viral transcription was apparent, whereas a two-fold increase was observed between the accumulations of vRNA in control or SFPQ/PSF-silenced cells (Figure 10C). These results indicated that SFPQ/PSF is required for viral transcription, but not for virus RNA replication and suggest that, in the absence of SFPQ/PSF, the viral RNPs are preferentially devoted to RNA replication instead of transcribing their template.
Fig. 10. Accumulation of positive- and negative-polarity RNA in a recombinant replicon system. 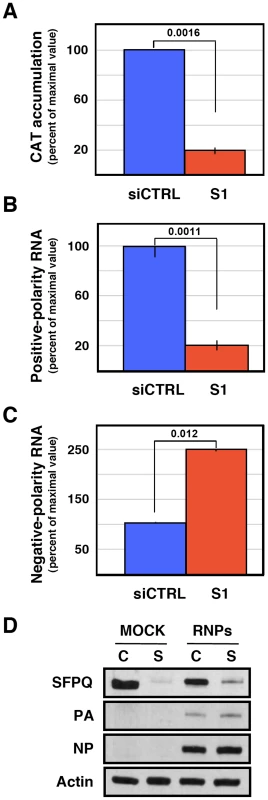
Cultures of HEK293T cells were transfected with SFPQ/PSF-specific (S1) or control siRNAs (siCTRL) as described under Materials and Methods. When the expression of SFPQ/PSF was known to be down-regulated, the cultures were further transfected with plasmids expressing the polymerase subunits and the NP, as well as with a genomic plasmid expressing a pseudoviral gene containing the cat gene in negative polarity. As control, the cultures were transfected with empty plasmids. At 24 hours post plasmid transfection total cell extracts were prepared and total cell RNA was isolated. Significance was determined by the Student's t test. (A) The accumulation of CAT protein is indicated as percent of maximal values. (B) The accumulation of positive-polarity RNA was determined by hybridisation with CAT-specific probes and is presented as percent of maximal values. (C) The accumulation of negative-polarity RNA was determined by hybridisation with CAT-specific probes and is presented as percent of maximal values. The values presented represent the average and standard deviation of 3 independent transfection experiments. (D) The silencing of SFPQ was controlled by Western-blot, using actin as loading control. The level of expression of PA and NP was ascertained by Western-blot. Depletion of SFPQ/PSF leads to diminished production of viral polyadenylated RNA in vitro
One possible mechanism to explain the effects of SFPQ/PSF down-regulation on influenza virus transcription would imply that the interaction of SFPQ/PSF with the viral polymerase present in the RNP increases the affinity of the enzyme for the cap structure. The apparent Kd for the interaction of the isolated PB2 cap-binding domain with 7mGTP is around 170 µM [10], in good agreement with the inhibition data reported for cap-RNA crosslinking to influenza virus RNPs [59], whereas the affinity of binding of eIF4E or CBC to cap-analogues is much higher [60], [61]. This is in contradistinction to the elution profiles of eIF4F and influenza polymerase-template complexes on a 7mGTP-Sepharose resin [14] which show a stronger binding of the latter. Hence, it is conceivable that some cellular factor(s), for instance SFPQ/PSF, interact with viral polymerase and enhances its affinity for binding to cap. To test such possibility we generated recombinant RNPs by transfection of HEK293T cells, which had previously been either control - or SFPQ/PSF-silenced. Total cell extracts of these cells were used for in vitro transcription using ß-globin mRNA as cap-donor. A wide concentration range of cap-donor was employed to test for a potential difference in cap-donor dose response when the reaction was performed in the presence or absence of SFPQ/PSF protein. The results are presented in Figure 11 and no change in the profile of virus RNA synthesis was apparent when SFPQ/PSF was silenced (Figure 11B). However, a clear change in the size distribution of RNA products was observed depending on the downregulation of SFPQ/PSF and irrespective of the cap-donor concentration (Figure 11A). In the presence of SFPQ/PSF the RNA profile was reminiscent of a polyadenylated virus mRNA, as the transcript size was slightly larger than the template (used in the gel as a marker) while downregulation of SFPQ/PSF led to a variety of RNA sizes, always smaller than the template. To further characterise the transcripts generated with SFPQ/PSF-silenced samples they were separated into poly A+ and poly A− fractions and analysed by denaturing polyacrylamide gel electrophoresis. The results are presented in Figure 12. A consistent reduction in the amount of polyadenylated RNA was observed when SFPQ/PSF was downregulated, with a corresponding increased in the poly A− fraction (Figure 12A). Quantification of 5 experiments indicated that the total amount of transcript was not affected by SFPQ/PSF downregulation but the fraction of poly A+ viral mRNA was reduced about 4–5 fold (Figure 12 B). Similar results were obtained when other SFPQ/PSF-specific siRNA was used (see Figure S2). A considerable fraction of the viral poly A− transcrips showed sizes smaller than the template, consistent with RNA degradation or premature termination but, interestingly, the profile of these poly A− transcripts was identical for control - or SFPQ/PSF-silenced samples, suggesting that the reduction in poly A+ transcripts is not due to a defect in transcript elongation but most probably to a deficiency in the polyadenylation step.
Fig. 11. Cap-donor dose-response for in vitro transcription of recombinant RNPs. 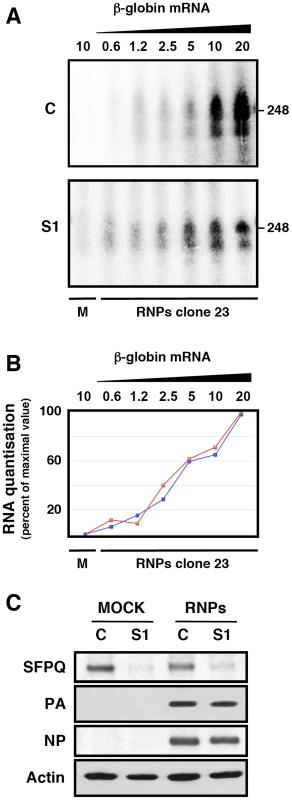
Cultures of HEK293T cells were transfected with SFPQ/PSF-specific (S1) or control siRNAs (siCTRL) as described under Materials and Methods. When the expression of SFPQ/PSF was known to be down-regulated, the cultures were further transfected with plasmids expressing the polymerase subunits and the NP, as well as with a genomic plasmid expressing a deleted NS RNA segment (clone 23) [2] in negative polarity. Extracts of these cultures were used for in vitro transcription using increasing amounts of ß-globin mRNA as a cap donor and the transcripts were analysed by denaturing polyacrylamide gel electrophoresis. (A) Denaturing polyacrylamide gel electrophoresis of in vitro transcripts. M denotes a mock-reconstitution of recombinant RNPs in which pcDNA3 plasmid was transfected. The concentrations (µg/ml) of ß-globin mRNA used as cap-donor are indicated to the top. The mobility of a genome-size marker of 248 nt is indicated to the right. (B) The quantisation of the results presented in A is presented as percent of maximal value in each series. Blue line: Control-silenced extract. Red line: SFPQ/PSF-silenced extract. (C) The down-regulation of SFPQ/PSF by silencing was verified by Western-blot with specific antibodies. The level of expression of PA and NP was ascertained by Western-blot. Fig. 12. Dependence of SFPQ/PSF for the in vitro polyadenylation of transcripts. 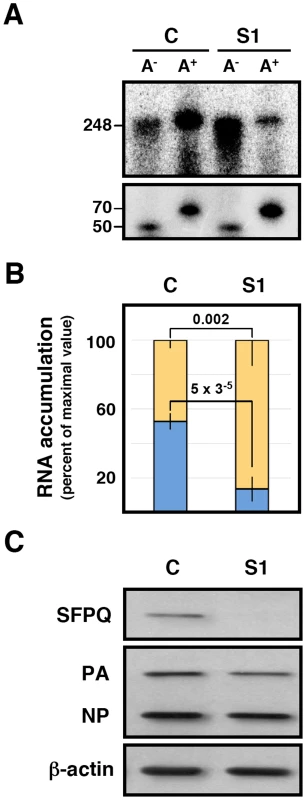
Recombinant RNPs were generated in control-silenced (C) or SFPQ/PSF-silenced (S1) HEK293T cells and in vitro transcription was performed as indicated in the legend to Figure 11. The transcripts were separated into poly A+ and poly A− fractions, using a non-polyadenylated oligonucleotide (50 nt) and a polyadenylated oligonucleotide (70 nt) as recovery probes, and the fractionated transcripts were analysed by electrophoresis on denaturing polyacrylamide gels. (A) Representative results of five independent experiments. The positions of a 248 nt marker identical to the clone 23 genome, as well as the recovery probes are indicated to the left. (B) Quantification of the proportion of the poly A+ (blue) and poly A− (yellow) transcripts after normalisation for recovery. The data represent averages and standard deviations of five experiments. Significance was determined by the Student's t test. (C) The silencing of SFPQ was controlled by Western-blot, using actin as loading control. The level of expression of PA and NP was ascertained by Western-blot. Concluding remarks
The results presented here show that SFPQ/PSF is specifically required for influenza virus multiplication and indicate that this cellular factor is essential for the transcription of viral RNPs during both primary and secondary mRNA synthesis. Furthermore, the results shown suggest that SFPQ/PSF plays a role during the polyadenylation step in virus transcription. With the evidence presented, the following picture could be envisaged upon SFPQ down-regulation in infected cells: The primary viral mRNAs lacking poly A would be unstable and hence their accumulation diminished as compared to infections performed in normal cells. In addition, normal recycling of the transcribing polymerase could be affected. As a consequence of these effects, viral RNA replication would be indirectly inhibited, since little polymerase and NP would be synthesised. Moreover, a similar inhibition could be predicted for secondary transcription of the small amount of viral progeny RNA, with the final result of very low viral gene expression and virus production.
At present it is not clear how SFPQ/PSF participates in the polyadenylation step of viral transcription. The available evidence indicates that polyadenylation of viral mRNAs is carried out by the polymerase by reiterative copy of the oligo U signal located close to the 5′-terminus of the vRNA template [62]–[65] and is mechanistically distinct from the cellular cleavage and polyadenylation process. The SFPQ/PSF protein is an RNA-binding protein that has been described as essential for the formation of the spliceosome and can be cross-linked to the pre-mRNA in the spliceosome [46]. Furthermore, purified SFPQ/PSF can be specifically cross-linked to poly U, but not to poly C, A or G, showing the same sequence specificity than PTB [38]. Therefore, it is tempting to speculate that SFPQ/PSF could interact both with the viral polymerase [34] and with the viral polyadenylation signal within the RNP to promote polymerase stuttering at the site. Further experiments will be required to test this and other possible alternatives.
Down-regulation of SFPQ/PSF leads to a dramatic decrease in the yield of virus infection and hence it could be considered as a new target for antiviral design, a particularly interesting one as it is involved in a very early stage of the infection. Silencing of mouse SFPQ/PSF leads to chromosome instability [66] and we have verified that down-regulation of SFPQ/PSF in some human cells strongly reduces their growth kinetics (unpublished results). Therefore, one should aim at blocking the association of SFPQ/PSF with virus polymerase/RNP for the design of potential new antivirals.
Materials and Methods
Biological materials
The HEK 293T cell line [67] was obtained from J.C. de la Torre and the A549 human cell line [68] was obtained from J.A. Melero. The MDCK and BHK21 cell lines were purchased from ATCC. Cell culture was carried out as described [69]. The influenza virus strains A/Victoria/3/75 (H3N2) (VIC), WSN (H1N1) and a recombinant of both strains (VIC/WSN) was used in these experiments. Titrations were done in MDCK cells as described [70]. Vesicular stomatitis virus (VSV) was provided by R. Alfonso and titrated in BHK21 cells. Adenovirus 5 was provided by P. Fortes. Virus stocks were prepared and titrated in HEK 293T cells as described [71].
Plasmids pCMVPB1, pCMVPB2, pCMVPA and pCMVNP, expressing the influenza virus polymerase subunits and the NP have been described [57]. Plasmid pHHCAT, that transcribes a virus-like cat gene in negative polarity, was provided by A. Rodríguez. The monoclonal antibodies specific for PA have been described [72], [73]. A monoclonal antibody specific for the N-terminal region of M1 and M2 proteins [74] was provided by A. García-Sastre. Antisera specific for PB1 and NP were generated by immunisation of rabbits with recombinant proteins [2], [14], [31]. A monoclonal antibody specific for SFPQ/PSF (ab11825) and polyclonal sera specific for GAPDH (ab9485) and actin (ab8226) were purchased to Abcam. The secondary antibodies used for Western-blot and immunofluorescence was purchased to Sigma and Invitrogen, respectively. Total mouse IgG, with no known specificity, was used as immunoprecipitation control.
Protein analyses
Protein samples were separated by polyacrylamide-SDS gel electrophoresis and transferred to Immobilon filters. Western-blotting was carried out essentially as described [75]: The membranes were saturated with 3% BSA for 1 h and then incubated with the primary antibodies for 1 h at room temperature. The filters were washed with PBS containing 0.25% Tween 20 and incubated with the appropriate secondary antibody conjugated to horseradish peroxidase. After further washing as above the filters were developed by enhanced chemiluminiscence. For immunoprecipitations, extracts from mock-infected or infected cells were incubated with goat-antimouse-agarose beads (A6531, Sigma) loaded with a monoclonal antibody specific for SFPQ/PSF or an equal amount of total mouse IgG as negative control. After extensive washing with a buffer containing 50 mM Tris HCl pH 7,5, 150 mM NaCl, 0,5% NP-40, 1,5 mM MgCl2, 1 mM DTT, 1 u/µl HPRI and protease inhibitors containing EDTA, the beads were extracted by boiling with Laemmli loading buffer and the samples were analysed by Western-blot with antibodies specific for SFPQ/PSF and NP. For immunofluorescence, cells were fixed with 3% paraformaldehyde. The cultures were permeabilised with 0.5% Triton X100 and processed for indirect immunofluorescence as described before [31]. Images were collected on a Leica SP5 confocal microscope (Leica Microsystems) and processed with the LAS AF Software (Leica Microsystems). For quantisation of cellular staining with anti-SFPQ/PSF and NP antibodies, the average intensities of 50 random images (1024×1024 pixels) of each preparation were determined using the LAS AF Software. The procedures for protein labelling in vivo have been described [76]. Cultures were washed, incubated for 1 h in a DMEM medium lacking met-cys and labelled with 35S-met-cys to a final concentration of 200 µCi/ml. After incubation for 1 h, total extracts were prepared in Laemmli sample buffer and processed by polyacrylamide gel electrophoresis and autoradiography. Quantisation of CAT protein in total cell extracts was done by ELISA (GE Healthcare).
RNA analyses
The amplification in vivo of recombinant RNPs was performed essentially as described [77]. In brief, cultures of HEK293T cells (2.5 106 cells) were transfected with a mixture of plasmids expressing the polymerase subunits (pCMVPB1, 12.5 ng; pCMVPB2, 12.5 ng; pCMVPA, 2.5 ng), NP (pCMVNP, 500 ng) and a genomic plasmid expressing a vRNA-like cat gene (pHHCAT, 500 ng), using the calcium phosphate technique [78]. At 24 hours post-transfection, total cell extracts were prepared for CAT determination or total cell RNA was extracted. For RNA extraction cell pellets were resuspended in 1 ml of TRIZOL reagent (Invitrogen) and the RNA was purified as recommended by the manufacturer. The RNA was digested with RNAse-free DNAse (1 u/µg) for 1 h at 37°C, extracted with phenol-chloroform-isoamylalcohol and precipitated with ethanol. For alternative splicing studies, poly A+ RNA was isolated by two rounds of chromatography on oligo-dT-cellulose as described previously [79]. The purified RNA was resuspended in nuclease-free water and the absorbance was measured at 260 nm (NanoDrop ND-1000).
For dot-blot hybridisation, aliquots of purified RNAs were denatured for 15 min at 55°C in 10SSC, 7.5% formaldehyde and fixed on nylon filters by UV cross-linking. As controls, total yeast RNA or various amounts of plasmids containing cDNAs of the full-length virus genomic segments or the corresponding viral mRNAs, were fixed on the hybridisation filters. Hybridisation was carried out overnight at 40°C in 6xSSC, 0.5% SDS, 5× Denhardt's mixture 26–47% formamide, depending on the probe, and 100 µg/ml single-stranded DNA. After washing with 0.5xSSC-0.5% SDS at 40°C, the filters were quantified in a phosphorimager. As probes, synthetic oligonucleotides specific to detect the various RNA species of each RNA segment analysed were used (Table S1). They were labelled with gamma-32P-ATP and polynucleotide kinase. Additionally, specific riboprobes were used to specifically detect cRNAs. They were transcribed by T3 RNA polymerase using as templates synthetic DNAs containing T3 promoters fused to the viral sequences (Table S1).
For siRNA transfection, cultured A549 cells were incubated independently with 5 nM of siSFPQ 1 (107613), siSFPQ 2 (15923), specific for SFPQ, or siNonO 1 (s9614), siNonO 2 (s9612), specific for NonO, or an irrelevant siRNA (AM4611) from Ambion, using Lipofectamine (Invitrogen) as recommended by the manufacturer. Transfection was carried out twice on consecutive days to increase the silencing efficiency before infection.
Quantification of virus-specific RNAs for splicing and primary transcription analyses was carried out by RT-qPCR as follows: The RT reaction was performed by addition of 100 ng of RNA resuspended in 10 µl of nuclease-free water and 10 µl of Reaction Mix 2x (Applied Biosystems) as recommended by the manufacturer. From each 20-µl reaction, 2 µl of cDNA was transferred directly to 96-well PCR plates and 12,5 µl of TaqMan universal master mixture (Applied Biosystems) and 1,25 µl of Custom TaqMan assay (designed by Applied BioSystems) were added. PCR was carried out in a PRISM 7000 Sequence detection system (Applied Biosystems), with 1 cycle of 50°C for 2 min followed by 1 cycle of 95°C for 10 min, 40 cycles of 95°C for 15 s and 60°C for 1 min. The cycle threshold (Ct) was determined with analytical software (SDS; Applied Biosystems). Serial dilutions of cDNA were used to ensure amplification in the lineal range. To construct calibration curves for quantification, we generated PCR products whose sequences were identical to the spliced or unspliced mRNAs of NS segment. The sequences of TaqMan probes and primers are presented in Table S1. For in vitro transcription, extracts from control - or SFPQ/PSF-silenced HEK293T cells in which a mini-RNP was reconstituted [2] were incubated for 60 min at 30°C in 20 µl reactions containing 50 mM Tris.HCl, 100 mM KCl, 2 mM MgCl2, 1 mM DTT, 1 mM each of ATP, CTP and UTP, 1 µM alpha-32P-GTP (0.5 mCi/µmol), 10 µg/ml actinomycin D, 1 u/µl HPRI, pH 8.0 and µg/ml (or various amounts, depending on the experiment) ß-globin mRNA. The reaction products were phenol-extracted, ethanol-precipitated and analysed by electrophoresis on 6% polyacrylamide-7 M urea gels. The purified in vitro transcripts were separated into poly A+ and poly A− fractions by chromatography on oligo dT-cellulose as described [80]. To monitor the recovery of RNAs during extraction and fractionation two labelled synthetic oligonucleotides were added, a 50 nt oligonucleotide lacking poly T sequences and a 70 nt long containing a 39T tract at its 3′-terminus.
Supporting Information
Zdroje
1. PalesePShawM 2007 Orthomyxoviridae: the viruses and their replication. KnipeDMHowleyPM Fields Virology 5th edition Philadelphia Lippincott Williams & Wilkins 1647 1689
2. ColomaRValpuestaJMArranzRCarrascosaJLOrtinJ 2009 The structure of a biologically active influenza virus ribonucleoprotein complex. PLoS Pathog 5 e1000491
3. OrtegaJMartín-BenitoJZürcherTValpuestaJMCarrascosaJL 2000 Ultrastructural and functional analyses of of recombinant influenza virus ribonucleoproteins suggest dimerization of nucleoprotein during virus amplification. J Virol 74 156 163
4. EltonDDigardPTileyLOrtínJ 2005 Structure and function of the influenza virus RNP. KawaokaY Current Topics in Influenza Virology Norfolk Horizon Scientific Press 1 92
5. NeumannGBrownleeGGFodorEKawaokaY 2004 Orthomyxovirus replication, transcription, and polyadenylation. Curr Top Microbiol Immunol 283 121 143
6. Resa-InfantePJorbaNColomaROrtínJ 2011 The influenza virus RNA synthesis machine: Advances in its structure and function. RNA Biology 8 1 9
7. BiswasSKNayakDP 1994 Mutational analysis of the conserved motifs of influenza A virus polymerase basic protein 1. J Virol 68 1819 1826
8. PochOSauvagetIDelarueMTordoN 1990 Identification of four conserved motifs among the RNA-dependent polymerase encoding elements. EMBO J 8 3867 3874
9. DiasABouvierDCrepinTMcCarthyAAHartDJ 2009 The cap-snatching endonuclease of influenza virus polymerase resides in the PA subunit. Nature 458 914 918
10. GuilligayDTarendeauFResa-InfantePColomaRCrepinT 2008 The structural basis for cap binding by influenza virus polymerase subunit PB2. Nat Struct Mol Biol 15 500 506
11. UlmanenIBroniBAKrugRM 1981 The role of two of the influenza virus core P proteins in recognizing cap 1 structures (m7GpppNm) on RNAs and in initiating viral RNA transcription. Proc Natl Acad Sci U S A 78 7355 7359
12. YuanPBartlamMLouZChenSZhouJ 2009 Crystal structure of an avian influenza polymerase PA(N) reveals an endonuclease active site. Nature 458 909 913
13. AreaEMartín-BenitoJGastaminzaPTorreiraEValpuestaJM 2004 Three-dimensional structure of the influenza virus RNA polymerase: localization of subunit domains. Proc Natl Acad Sci U S A 101 308 313
14. Resa-InfantePRecuero-ChecaMAZamarreñoNLlorcaOOrtínJ 2010 Structural and functional characterisation of an influenza virus RNA polymerase-genomic RNA complex. J Virol 84 10477 10487
15. TorreiraESchoehnGFernandezYJorbaNRuigrokRW 2007 Three-dimensional model for the isolated recombinant influenza virus polymerase heterotrimer. Nucleic Acids Res 35 3774 3783
16. FechterPMingayLSharpsJChambersAFodorE 2003 Two aromatic residues in the PB2 subunit of influenza A RNA polymerase are crucial for cap binding. J Biol Chem 278 20381 20388
17. FodorECrowMMingayLJDengTSharpsJ 2002 A single amino acid mutation in the PA subunit of the influenza virus RNA polymerase inhibits endonucleolytic cleavage of capped RNAs. J Virol 76 8989 9001
18. GastaminzaPPeralesBFalcónAMOrtínJ 2003 Influenza virus mutants in the N-terminal region of PB2 protein are affected in virus RNA replication but not transcription. J Virol 76 5098 5108
19. PeralesBOrtínJ 1997 The influenza A virus PB2 polymerase subunit is required for the replication of viral RNA. J Virol 71 1381 1385
20. DetjenBMSt AngeloCKatzeMGKrugRM 1987 The three influenza virus polymerase (P) proteins not associated with viral nucleocapsids in the infected cell are in the form of a complex. J Virol 61 16 22
21. HuetSAvilovSFerbitzLDaigleNCusackS 2009 Nuclear import and assembly of the influenza A virus RNA polymerase studied in live cells by Fluorescence Cross Correlation Spectroscopy. J Virol 84 1254 1264
22. JorbaNAreaEOrtinJ 2008 Oligomerization of the influenza virus polymerase complex in vivo. J Gen Virol 89 520 524
23. EngelhardtOGSmithMFodorE 2005 Association of the influenza A virus RNA-dependent RNA polymerase with cellular RNA polymerase II. J Virol 79 5812 5818
24. GabrielGHerwigAKlenkHD 2008 Interaction of Polymerase Subunit PB2 and NP with Importin alpha1 Is a Determinant of Host Range of Influenza A Virus. PLoS Pathog 4 e11
25. HondaA 2008 Role of host protein Ebp1 in influenza virus growth: intracellular localization of Ebp1 in virus-infected and uninfected cells. J Biotechnol 133 208 212
26. HondaAOkamotoTIshihamaA 2007 Host factor Ebp1: selective inhibitor of influenza virus transcriptase. Genes Cells 12 133 142
27. HuarteMSanz-EzquerroJJRoncalFOrtinJNietoA 2001 PA subunit from influenza virus polymerase complex interacts with a cellular protein with homology to a family of transcriptional activators. J Virol 75 8597 8604
28. MayerDMolawiKMartinez-SobridoLGhanemAThomasS 2007 Identification of Cellular Interaction Partners of the Influenza Virus Ribonucleoprotein Complex and Polymerase Complex Using Proteomic-Based Approaches. J Proteome Res 6 672 682
29. MomoseFHandaHNagataK 1996 Identification of host factors that regulate the influenza virus RNA polymerase activity. Biochimie 78 1103 1108
30. MomoseFNaitoTYanoKSugimotoSMorikawaY 2002 Identification of Hsp90 as a stimulatory host factor involved in influenza virus RNA synthesis. J Biol Chem 277 45306 45314
31. Resa-InfantePJorbaNZamarrenoNFernandezYJuarezS 2008 The host-dependent interaction of alpha-importins with influenza PB2 polymerase subunit is required for virus RNA replication. PLoS One 3 e3904
32. ShimizuKHandaHNakadaSNagataK 1994 Regulation of influenza virus RNA polymerase activity by cellular and viral factors. Nucleic Acids Res 22 5047 5053
33. KawaguchiANagataK 2007 De novo replication of the influenza virus RNA genome is regulated by DNA replicative helicase, MCM. Embo J 26 4566 4575
34. JorbaNJuarezSTorreiraEGastaminzaPZamarrenoN 2008 Analysis of the interaction of influenza virus polymerase complex with human cell factors. Proteomics 8 2077 2088
35. LiGZhangJTongXLiuWYeX 2011 Heat shock protein 70 inhibits the activity of influenza a virus ribonucleoprotein and blocks the replication of virus in vitro and in vivo. PLoS One 6 e16546
36. KawaguchiAMomoseFNagataK 2011 Replication-coupled and host factor-mediated encapsidation of the influenza virus genome by viral nucleoprotein. J Virol 85 6197 6204
37. Shav-TalYZiporiD 2002 PSF and p54(nrb)/NonO—multi-functional nuclear proteins. FEBS Lett 531 109 114
38. PattonJGPorroEBGalceranJTempstPNadal-GinardB 1993 Cloning and characterization of PSF, a novel pre-mRNA splicing factor. Genes Dev 7 393 406
39. AkhmedovATLopezBS 2000 Human 100-kDa homologous DNA-pairing protein is the splicing factor PSF and promotes DNA strand invasion. Nucleic Acids Res 28 3022 3030
40. BladenCLUdayakumarDTakedaYDynanWS 2005 Identification of the polypyrimidine tract binding protein-associated splicing factor.p54(nrb) complex as a candidate DNA double-strand break rejoining factor. J Biol Chem 280 5205 5210
41. MorozumiYTakizawaYTakakuMKurumizakaH 2009 Human PSF binds to RAD51 and modulates its homologous-pairing and strand-exchange activities. Nucleic Acids Res 37 4296 4307
42. StraubTGruePUhseALisbyMKnudsenBR 1998 The RNA-splicing factor PSF/p54 controls DNA-topoisomerase I activity by a direct interaction. J Biol Chem 273 26261 26264
43. StraubTKnudsenBRBoegeF 2000 PSF/p54(nrb) stimulates “jumping” of DNA topoisomerase I between separate DNA helices. Biochemistry 39 7552 7558
44. PengRHawkinsILinkAJPattonJG 2006 The splicing factor PSF is part of a large complex that assembles in the absence of pre-mRNA and contains all five snRNPs. RNA Biol 3 69 76
45. PengRDyeBTPerezIBarnardDCThompsonAB 2002 PSF and p54nrb bind a conserved stem in U5 snRNA. Rna 8 1334 1347
46. GozaniOPattonJGReedR 1994 A novel set of spliceosome-associated proteins and the essential splicing factor PSF bind stably to pre-mRNA prior to catalytic step II of the splicing reaction. Embo J 13 3356 3367
47. EmiliAShalesMMcCrackenSXieWTuckerPW 2002 Splicing and transcription-associated proteins PSF and p54nrb/nonO bind to the RNA polymerase II CTD. Rna 8 1102 1111
48. DyeBTPattonJG 2001 An RNA recognition motif (RRM) is required for the localization of PTB-associated splicing factor (PSF) to subnuclear speckles. Exp Cell Res 263 131 144
49. MeltonAAJacksonJWangJLynchKW 2007 Combinatorial control of signal-induced exon repression by hnRNP L and PSF. Mol Cell Biol 27 6972 6984
50. RosoninaEIpJYCalarcoJABakowskiMAEmiliA 2005 Role for PSF in mediating transcriptional activator-dependent stimulation of pre-mRNA processing in vivo. Mol Cell Biol 25 6734 6746
51. MarkoMLeichterMPatrinou-GeorgoulaMGuialisA 2010 hnRNP M interacts with PSF and p54(nrb) and co-localizes within defined nuclear structures. Exp Cell Res 316 390 400
52. ZhangZCarmichaelGG 2001 The fate of dsRNA in the nucleus: a p54(nrb)-containing complex mediates the nuclear retention of promiscuously A-to-I edited RNAs. Cell 106 465 475
53. Greco-StewartVSThibaultCSPelchatM 2006 Binding of the polypyrimidine tract-binding protein-associated splicing factor (PSF) to the hepatitis delta virus RNA. Virology 356 35 44
54. HarrisDZhangZChaubeyBPandeyVN 2006 Identification of cellular factors associated with the 3′-nontranslated region of the hepatitis C virus genome. Mol Cell Proteomics 5 1006 1018
55. ZolotukhinASMichalowskiDBearJSmulevitchSVTraishAM 2003 PSF acts through the human immunodeficiency virus type 1 mRNA instability elements to regulate virus expression. Mol Cell Biol 23 6618 6630
56. ChaseGDengTFodorELeungBWMayerD 2008 Hsp90 inhibitors reduce influenza virus replication in cell culture. Virology 377 431 439
57. FalcónAMMariónRMZürcherTGómezPPortelaA 2004 Defective RNA replication and late gene expression in temperature-sensitive (A/Victoria/3/75) influenza viruses expressing deleted forms of NS1 protein. J Virol 78 3880 3888
58. HayAJLomnicziBBellamyARSkehelJJ 1977 Transcription of the influenza virus genome. Virology 83 337 355
59. HookerLSullyRHandaBOnoNKoyanoH 2003 Quantitative analysis of influenza virus RNP interaction with RNA cap structures and comparison to human cap binding protein eIF4E. Biochemistry 42 6234 6240
60. NiedzwieckaAMarcotrigianoJStepinskiJJankowska-AnyszkaMWyslouch-CieszynskaA 2002 Biophysical studies of eIF4E cap-binding protein: recognition of mRNA 5′ cap structure and synthetic fragments of eIF4G and 4E-BP1 proteins. J Mol Biol 319 615 635
61. WorchRNiedzwieckaAStepinskiJMazzaCJankowska-AnyszkaM 2005 Specificity of recognition of mRNA 5′ cap by human nuclear cap-binding complex. Rna 11 1355 1363
62. LiXPaleseP 1994 Characterization of the polyadenilation signal of influenza virus RNA. J Virol 68 1245 1249
63. LuoGXLuytjesWEnamiMPaleseP 1991 The polyadenylation signal of influenza virus RNA involves a stretch of uridines followed by the RNA duplex of the panhandle structure. J Virol 65 2861 2867
64. PoonLLFodorEBrownleeGG 2000 Polyuridylated mRNA synthesized by a recombinant influenza virus is defective in nuclear export. J Virol 74 418 427
65. RobertsonJSSchubertMLazzariniRA 1981 Polyadenylation sites for influenza mRNA. J Virol 38 157 163
66. RajeshCBakerDKPierceAJPittmanDL 2010 The splicing-factor related protein SFPQ/PSF interacts with RAD51D and is necessary for homology-directed repair and sister chromatid cohesion. Nucleic Acids Res 38
67. DuBridgeRBTangPHsiaHCLeongPMMillerJH 1987 Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol Cell Biol 7 379 387
68. GiardDJAaronsonSATodaroGJArnsteinPKerseyJH 1973 In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst 51 1417 1423
69. OrtínJNájeraRLópezCDávilaMDomingoE 1980 Genetic variability of Hong Kong (H3N2) influenza viruses: spontaneous mutations and their location in the viral genome. Gene 11 319 331
70. TobitaKSugiuraAEnomotoCFuruyamaM 1975 Plaque-assay and primary isolation of influenza A viruses in an established line of canine kidney cells (MDCK) in the presence of trypsin. Med Microbiol Immunol 162 9 14
71. AparicioORazquinNZaratieguiMNarvaizaIFortesP 2006 Adenovirus virus-associated RNA is processed to functional interfering RNAs involved in virus production. J Virol 80 1376 1384
72. BárcenaJOchoaMde la LunaSMeleroJANietoA 1994 Monoclonal antibodies against influenza virus PB2 and NP polypeptides interfere with the initiation step of viral mRNA synthesis in vitro. J Virol 68 6900 6909
73. OchoaMBárcenaJde la LunaSMeleroJADouglasAR 1995 Epitope mapping of cross-reactive monoclonal antibodies specific for the influenza A virus PA and PB2 polypeptides. Virus Res 37 305 315
74. SalvatoreMBaslerCFParisienJPHorvarthCMBourmakinaS 2002 Effects of Influenza A virus NS1 protein on protein expression: the NS1 protein enhances translation and is not required for shutoff of host protein synthesis. J Virol 76 1206 1212
75. MariónRMZürcherTde la LunaSOrtínJ 1997 Influenza virus NS1 protein interacts with viral transcription-replication complexes in vivo. J Gen Virol 78 2447 2451
76. ZürcherTMariónRMOrtínJ 2000 The protein synthesis shut-off induced by influenza virus infection is independent of PKR activity. J Virol 74 8781 8784
77. JorbaNColomaROrtinJ 2009 Genetic trans-complementation establishes a new model for influenza virus RNA transcription and replication. PLoS Pathog 5 e1000462
78. WiglerMPellicerASilversteinSAxelRUrlaubG 1979 DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A 76 1373 1376
79. ValcárcelJPortelaAOrtínJ 1991 Regulated M1 mRNA splicing in influenza virus-infected cells. J Gen Virol 72 1301 1308
80. PeralesBde la LunaSPalaciosIOrtínJ 1996 Mutational analysis identifies functional domains in the Influenza A PB2 polymerase subunit. J Virol 70 1678 1686
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Microbial Spy Games and Host Response: Roles of a Small Molecule in Communication with Other SpeciesČlánek Sequence-Based Analysis Uncovers an Abundance of Non-Coding RNA in the Total Transcriptome of
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 11- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Microbial Spy Games and Host Response: Roles of a Small Molecule in Communication with Other Species
- Simple Rapid Near-Patient Diagnostics for Tuberculosis Remain Elusive—Is a “Treat-to-Test” Strategy More Realistic?
- Ultra-Efficient PrP Amplification Highlights Potentialities and Pitfalls of PMCA Technology
- Assessing Predicted HIV-1 Replicative Capacity in a Clinical Setting
- Inhibition of IL-10 Production by Maternal Antibodies against Group B Streptococcus GAPDH Confers Immunity to Offspring by Favoring Neutrophil Recruitment
- Anti-filarial Activity of Antibiotic Therapy Is Due to Extensive Apoptosis after Depletion from Filarial Nematodes
- West Nile Virus Experimental Evolution and the Trade-off Hypothesis
- Shedding Light on the Elusive Role of Endothelial Cells in Cytomegalovirus Dissemination
- Galactosaminogalactan, a New Immunosuppressive Polysaccharide of
- Fatal Prion Disease in a Mouse Model of Genetic E200K Creutzfeldt-Jakob Disease
- BST2/Tetherin Enhances Entry of Human Cytomegalovirus
- Metagenomic Analysis of Fever, Thrombocytopenia and Leukopenia Syndrome (FTLS) in Henan Province, China: Discovery of a New Bunyavirus
- Neurons are MHC Class I-Dependent Targets for CD8 T Cells upon Neurotropic Viral Infection
- Sap Transporter Mediated Import and Subsequent Degradation of Antimicrobial Peptides in
- A Molecular Mechanism for Bacterial Susceptibility to Zinc
- Genomic Transition to Pathogenicity in Chytrid Fungi
- Evolution of Multidrug Resistance during Infection Involves Mutation of the Essential Two Component Regulator WalKR
- ChemR23 Dampens Lung Inflammation and Enhances Anti-viral Immunity in a Mouse Model of Acute Viral Pneumonia
- SH3 Domain-Mediated Recruitment of Host Cell Amphiphysins by Alphavirus nsP3 Promotes Viral RNA Replication
- A Gammaherpesvirus Cooperates with Interferon-alpha/beta-Induced IRF2 to Halt Viral Replication, Control Reactivation, and Minimize Host Lethality
- Early Secreted Antigen ESAT-6 of Promotes Protective T Helper 17 Cell Responses in a Toll-Like Receptor-2-dependent Manner
- CD4 T Cell Immunity Is Critical for the Control of Simian Varicella Virus Infection in a Nonhuman Primate Model of VZV Infection
- The Role of the P2X Receptor in Infectious Diseases
- Down-Regulation of Shadoo in Prion Infections Traces a Pre-Clinical Event Inversely Related to PrP Accumulation
- Cross-Reactive T Cells Are Involved in Rapid Clearance of 2009 Pandemic H1N1 Influenza Virus in Nonhuman Primates
- Single Molecule Analysis of Replicated DNA Reveals the Usage of Multiple KSHV Genome Regions for Latent Replication
- The Critical Role of Notch Ligand Delta-like 1 in the Pathogenesis of Influenza A Virus (H1N1) Infection
- Sequence-Based Analysis Uncovers an Abundance of Non-Coding RNA in the Total Transcriptome of
- Murine Gamma Herpesvirus 68 Hijacks MAVS and IKKβ to Abrogate NFκB Activation and Antiviral Cytokine Production
- EBV Tegument Protein BNRF1 Disrupts DAXX-ATRX to Activate Viral Early Gene Transcription
- SAG101 Forms a Ternary Complex with EDS1 and PAD4 and Is Required for Resistance Signaling against Turnip Crinkle Virus
- Multiple Candidate Effectors from the Oomycete Pathogen Suppress Host Plant Immunity
- Rab7A Is Required for Efficient Production of Infectious HIV-1
- A TNF-Regulated Recombinatorial Macrophage Immune Receptor Implicated in Granuloma Formation in Tuberculosis
- Novel Anti-bacterial Activities of β-defensin 1 in Human Platelets: Suppression of Pathogen Growth and Signaling of Neutrophil Extracellular Trap Formation
- CD11b, Ly6G Cells Produce Type I Interferon and Exhibit Tissue Protective Properties Following Peripheral Virus Infection
- The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
- A Kinase Chaperones Hepatitis B Virus Capsid Assembly and Captures Capsid Dynamics
- Towards a Structural Comprehension of Bacterial Type VI Secretion Systems: Characterization of the TssJ-TssM Complex of an Pathovar
- Indirect DNA Readout by an H-NS Related Protein: Structure of the DNA Complex of the C-Terminal Domain of Ler
- The Pore-Forming Toxin Listeriolysin O Mediates a Novel Entry Pathway of into Human Hepatocytes
- The Human Herpesvirus-7 (HHV-7) U21 Immunoevasin Subverts NK-Mediated Cytoxicity through Modulation of MICA and MICB
- Avirulence Effector Avr3b is a Secreted NADH and ADP-ribose Pyrophosphorylase that Modulates Plant Immunity
- Murid Herpesvirus-4 Exploits Dendritic Cells to Infect B Cells
- Unique Type I Interferon Responses Determine the Functional Fate of Migratory Lung Dendritic Cells during Influenza Virus Infection
- Evolution of a Species-Specific Determinant within Human CRM1 that Regulates the Post-transcriptional Phases of HIV-1 Replication
- Transcriptome Analysis of Transgenic Mosquitoes with Altered Immunity
- Antibody Evasion by a Gammaherpesvirus O-Glycan Shield
- UDP-glucose 4, 6-dehydratase Activity Plays an Important Role in Maintaining Cell Wall Integrity and Virulence of
- Protease-Resistant Prions Selectively Decrease Shadoo Protein
- A LysM and SH3-Domain Containing Region of the p60 Protein Stimulates Accessory Cells to Promote Activation of Host NK Cells
- Deletion of AIF Ortholog Promotes Chromosome Aneuploidy and Fluconazole-Resistance in a Metacaspase-Independent Manner
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Multiple Candidate Effectors from the Oomycete Pathogen Suppress Host Plant Immunity
- The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
- A TNF-Regulated Recombinatorial Macrophage Immune Receptor Implicated in Granuloma Formation in Tuberculosis
- SH3 Domain-Mediated Recruitment of Host Cell Amphiphysins by Alphavirus nsP3 Promotes Viral RNA Replication
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



