-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaUnique Type I Interferon Responses Determine the Functional Fate of Migratory Lung Dendritic Cells during Influenza Virus Infection
Migratory lung dendritic cells (DCs) transport viral antigen from the lungs to the draining mediastinal lymph nodes (MLNs) during influenza virus infection to initiate the adaptive immune response. Two major migratory DC subsets, CD103+ DCs and CD11bhigh DCs participate in this function and it is not clear if these antigen presenting cell (APC) populations become directly infected and if so whether their activity is influenced by the infection. In these experiments we show that both subpopulations can become infected and migrate to the draining MLN but a difference in their response to type I interferon (I-IFN) signaling dictates the capacity of the virus to replicate. CD103+ DCs allow the virus to replicate to significantly higher levels than do the CD11bhigh DCs, and they release infectious virus in the MLNs and when cultured ex-vivo. Virus replication in CD11bhigh DCs is inhibited by I-IFNs, since ablation of the I-IFN receptor (IFNAR) signaling permits virus to replicate vigorously and productively in this subset. Interestingly, CD103+ DCs are less sensitive to I-IFNs upregulating interferon-induced genes to a lesser extent than CD11bhigh DCs. The attenuated IFNAR signaling by CD103+ DCs correlates with their described superior antigen presentation capacity for naïve CD8+ T cells when compared to CD11bhigh DCs. Indeed ablation of IFNAR signaling equalizes the competency of the antigen presenting function for the two subpopulations. Thus, antigen presentation by lung DCs is proportional to virus replication and this is tightly constrained by I-IFN. The “interferon-resistant” CD103+ DCs may have evolved to ensure the presentation of viral antigens to T cells in I-IFN rich environments. Conversely, this trait may be exploitable by viral pathogens as a mechanism for systemic dissemination.
Published in the journal: . PLoS Pathog 7(11): e32767. doi:10.1371/journal.ppat.1002345
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002345Summary
Migratory lung dendritic cells (DCs) transport viral antigen from the lungs to the draining mediastinal lymph nodes (MLNs) during influenza virus infection to initiate the adaptive immune response. Two major migratory DC subsets, CD103+ DCs and CD11bhigh DCs participate in this function and it is not clear if these antigen presenting cell (APC) populations become directly infected and if so whether their activity is influenced by the infection. In these experiments we show that both subpopulations can become infected and migrate to the draining MLN but a difference in their response to type I interferon (I-IFN) signaling dictates the capacity of the virus to replicate. CD103+ DCs allow the virus to replicate to significantly higher levels than do the CD11bhigh DCs, and they release infectious virus in the MLNs and when cultured ex-vivo. Virus replication in CD11bhigh DCs is inhibited by I-IFNs, since ablation of the I-IFN receptor (IFNAR) signaling permits virus to replicate vigorously and productively in this subset. Interestingly, CD103+ DCs are less sensitive to I-IFNs upregulating interferon-induced genes to a lesser extent than CD11bhigh DCs. The attenuated IFNAR signaling by CD103+ DCs correlates with their described superior antigen presentation capacity for naïve CD8+ T cells when compared to CD11bhigh DCs. Indeed ablation of IFNAR signaling equalizes the competency of the antigen presenting function for the two subpopulations. Thus, antigen presentation by lung DCs is proportional to virus replication and this is tightly constrained by I-IFN. The “interferon-resistant” CD103+ DCs may have evolved to ensure the presentation of viral antigens to T cells in I-IFN rich environments. Conversely, this trait may be exploitable by viral pathogens as a mechanism for systemic dissemination.
Introduction
Influenza virus replicates productively in the epithelial cells of the respiratory tract [1], [2]. In close contact to the infected epithelial cells lies a network of specialized antigen presenting cells (APCs) known as dendritic cells (DCs) [3], [4]. Two major subsets of lung DCs known as CD103+ DCs and CD11bhigh DCs can be identified in the steady-state [5], [6], [7], [8]. Following influenza virus infection these cells migrate to the draining mediastinal lymph nodes (MLNs) loaded with viral antigens (Ag) [9], [10], [11], [12] to initiate T cell responses that are critical for virus clearance and recovery from infection [13], [14], [15]. The strategic localization of lung DCs adjacent to the productively infected epithelial cells ensures a supply of viral antigen for presentation to T cells, but also makes DCs an ideal target for virus infection.
Following aerosol infection of mice [9], [16], lung DCs begin to migrate 2 days post-infection (dpi) concomitant with the abrupt production of type I interferons (I-IFNs) and a myriad of other pro-inflammatory cytokines [10], [17]. I-IFNs have potent antiviral activity limiting virus replication in infected cells by inducing the transcription of hundreds of interferon-stimulated genes (ISGs) [18], [19], [20], [21]. The induction of ISGs or the antiviral state by I-IFNs, and other related cytokines such as interferon-lambda, also protect adjacent cells from infection thus restricting unabated spread of the virus in the respiratory tract [22], [23]. I-IFNs have also been shown to function as natural adjuvants for maturing human [24] and mouse DCs in vitro [25] and splenic DCs [26] in vivo. The timing of I-IFN production and lung DC migration are temporally related and it is not known to what extent I-IFNs influence the function of these cells and their interaction with influenza virus.
Here we investigated the role of I-IFN in lung DC function during influenza virus infection by studying the two major migratory lung DC subsets: CD103+ DCs and CD11bhigh DCs. We demonstrate a striking dichotomy in the sensitivity to I-IFN by lung DCs that defines the level of virus replication and Ag presentation to virus-specific naïve CD8+ T cells. Additionally, our findings show evidence that CD103+ DCs permit productive influenza replication and release infectious virus in the draining lymph nodes while CD11bhigh DCs do not.
Results
Influenza viral mRNA transcripts and infectious virus can be demonstrated in the draining MLNs only after 2 days post-infection
Our previous work showed that influenza virus suppressed innate immunity in the lungs of mice for the first 2 days after infection; a period we termed “the stealth phase” [10], [27]. During this privileged time, virus replication proceeds without instigating inflammation or triggering the migration of lung DCs to the MLNs. The end of the stealth phase is characterized by the abrupt and vigorous production of I-IFNs and cytokines and the initiation of Ag-bearing DC migration to the MLNs [10], [28]. The cells arriving in the MLNs were identified by the presence of intracellular viral proteins and no determination was made as to whether they were actually infected or simply carrying viral proteins from the site of infection. To distinguish these two possibilities we isolated MLNs from PR8-infected mice at defined time points after aerosol infection and measured viral mRNA transcripts from all 8 segments of influenza virus. As shown in Figure 1A transcripts of all 8 segments were identified in the MLN of infected mice. The kinetics of the appearance of the viral mRNA in the MLN correlated perfectly with our previous results for Ag-bearing DC migration [10] as it was only detectable starting at 48 hpi, increased steadily, peaked by 96 hpi, and persisted for at least 6 dpi. Homogenates collected from the MLN demonstrated the presence of infectious virus particles at time points that correlated with the appearance of viral mRNA and Ag-bearing DC migration (Figure 1B) [9], [10], [11]. Inspection of CD11c+ cells in the MLNs by immunohistochemistry confirmed viral antigen positive cells only after 48 hpi (Figure 1C).
Fig. 1. Ag-bearing DC migration is associated with localized viral mRNA and virus replication in the MLNs. 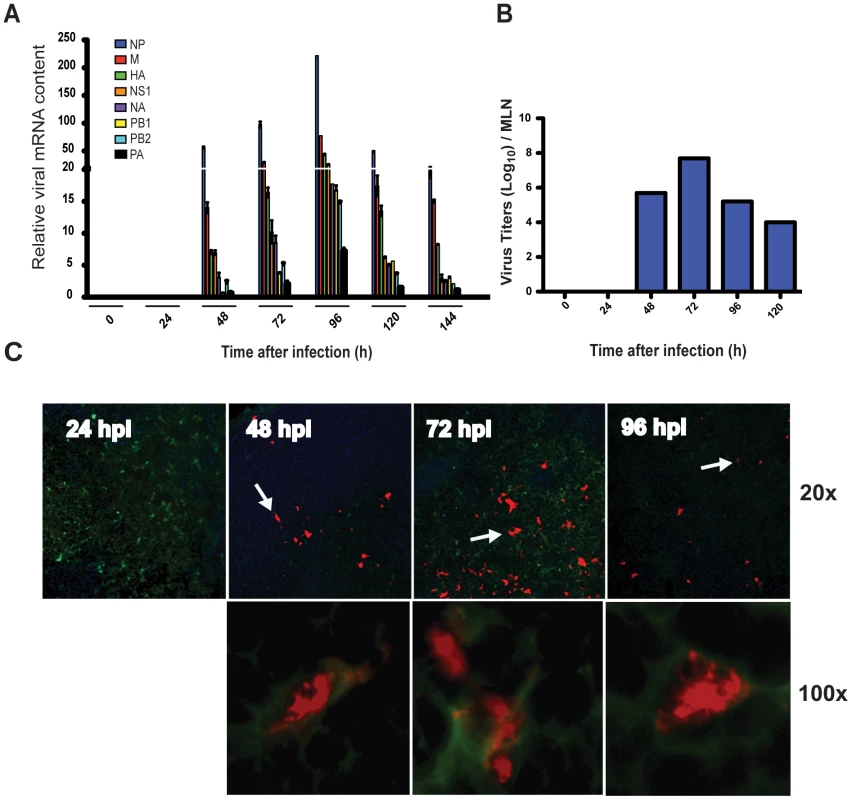
A. Total MLN cDNA obtained from PR8-infected mice, at different time points after infection, was analyzed by qPCR for the 8 segments of influenza viral mRNA (NP (nucleoprotein), HA (hemagglutinin), M (matrix protein), NS1 (non-structural protein 1), NA (neuraminidase), PB1 (polymerase PB1), PB2 (polymerase PB2), and PA (polymerase PA) B. MLNs were isolated from PR8-infected mice and titers of infectious virus were determined. C. Cryostat sections of MLNs isolated from PR8-infected mice were fixed, permeabilized and stained for CD11c (green), NP, M (red) and B220 (blue), and visualized with an immunofluorescence microscope at 20x and 100x magnification. White arrows indicate Ag-bearing CD11c+ cells at 20x magnification that were then zoomed and photographed at 100x. Viral transcripts are carried to the MLNs by migratory cells
Migration of lung DCs is controlled by the chemokine receptor CCR7 [8], [29], [30], and can be blocked by the use of pertussis toxin (PTX) [7]. Intranasal PTX administration to infected mice completely abolished the appearance of viral mRNA in the MLNs (Figure 2A), and significantly reduced the numbers of CCR7+ DCs in the MLNs (Figure 2B) but had no effect on virus replication in the lungs (Figure 2C). These data show that virus reaches the draining MLNs by cell-associated transport rather than by leakage of free virus through efferent lymphatics.
Fig. 2. Blockade of lung migratory cells abolishes viral mRNA production in the MLNs. 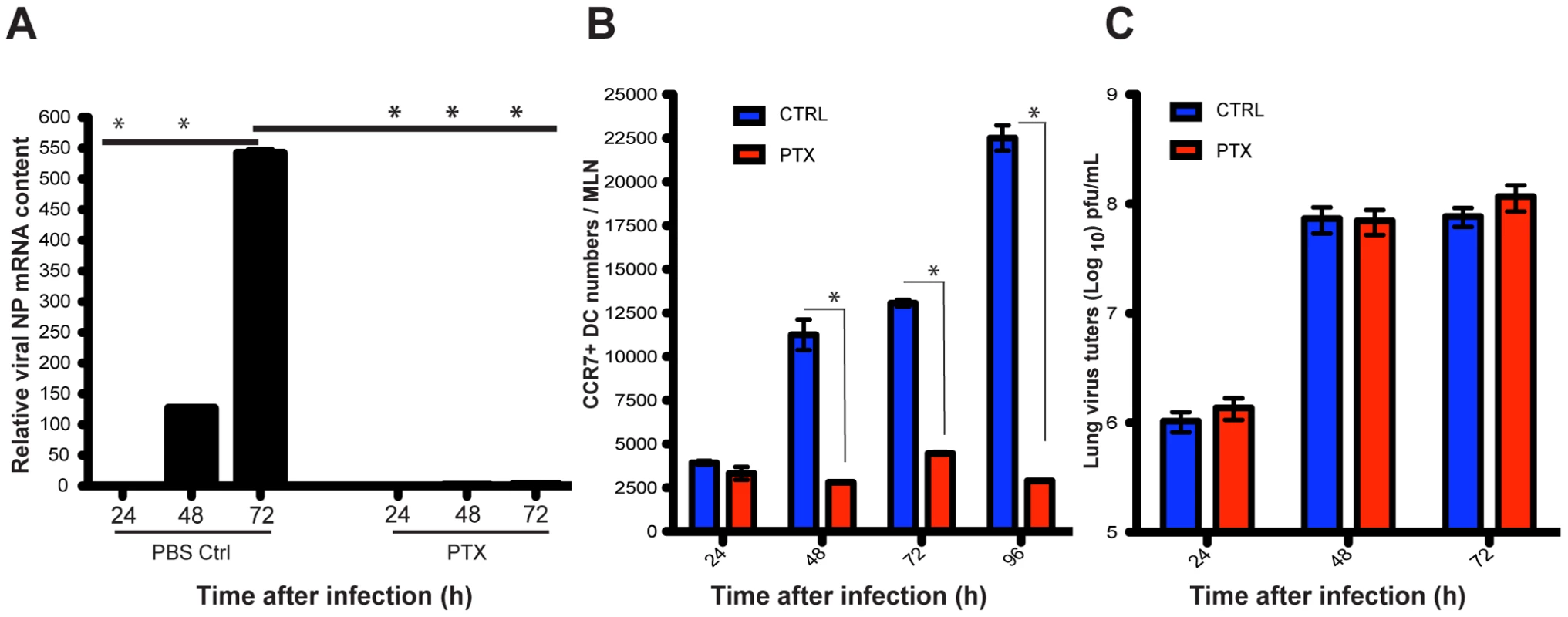
A. Two hours after infection, mice were challenged intranasally with a single dose of pertussis toxin (PTX) or PBS (Ctrl), and thereafter a daily single dose of PTX or PBS was given i.p to each mouse. Total RNA of MLNs was isolated at different time points after infection and viral mRNA (NP segment) was quantitated by qPCR. B. Absolute numbers of CCR7+ DCs in the MLNs during A/PR8 infection of mice were measured. Blue bars represent PBS mock treated mice and red bars mice treated with PTX. C. Lung virus titers of influenza infected mice either challenged with PTX (red bars) or mock challenged with PBS (blue bars). Error bars represent mean +/− SD, * p<0.05. Infectious virus is carried by lung migratory DCs
Both of the major lung DC subsets, CD103+ DCs and CD11bhigh DCs, transport viral Ag to the MLNs [11], [31], but whether it is in the form of infectious virus has not been determined. Figure 3A-C shows a sorting strategy that was used to isolate migratory DCs and other cells from the MLNs of infected animals. Total migratory DCs were isolated and co-cultured (gate V, Figure 3B) with virus permissive MDCK cells in vitro using decreasing numbers of cells. The DC-depleted lymph node cells were similarly cultured with MDCK cells (Figure 3B, gate i-iii pooled together). Infectious virus was isolated from MDCK cells cultured with 1,000 fold less migratory DCs than was observed when DC-depleted lymph node cells were used indicating that DCs were the primary transporters of infectious virus to the MLNs (Figure 3D). Plaque immunostaining of MDCKs infected with supernatant from these co-cultures confirmed the presence of live virus (Figure 3D’).
Fig. 3. Migratory CD103+ DCs are the major cell type carrying infectious virus particles to the MLNs. 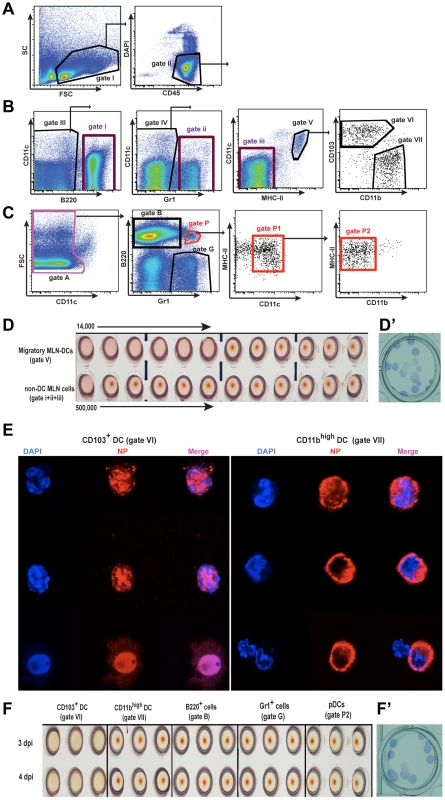
A–C. Isolation of different MLN cell populations from influenza infected mice by cell sorting. A. Live gate for total MLN leukocytes shown as CD45+ DAPI- cells (gate II). B. Sorting strategy to obtain total migratory DCs (gate V), individual CD103+ DCs (gate VI), or CD11bhigh DCs (gate VII), and non-DC MLN cells by pooling gates i, ii, and iii during collection. All these cell populations were selected from the total CD45+ live cell gate I. C. Alternative sorting strategy starting with gate II, to obtain B220+ cells (gate B), Gr1+ cells (gate G), and pDCs (gate P2). D. Total migratory DCs (gate V) comprised of CD103+ DCs and CD11bhigh DCs, or MLN non-DC cells (gates I, ii, and iii pooled) sorted at 3 dpi, were layered over MDCK cells in a 1∶2 serial dilution, starting with 14,000 and 500,000 cells respectively, and were co-cultured in the presence of TPCK-trypsin for 3 days. Supernatants were assayed for infectious virus particles by hemagglutination of RBCs. D'. Supernatants from MLN-DC/MDCK co-cultures were assayed for infectious virus by plaque immunostaining on MDCK cells. E. Immunofluorescence of intracellular viral NP of sorted CD103+DCs and CD11bhigh DCs from infected mice at 3 dpi. F. Individual cell populations, isolated by sorting at day 3 and 4 post-infection, as described above, were injected into 10-day old embryonated eggs, and 2 days later, allantoic fluid was assayed for infectious virus particles by hemagglutination of RBCs. Each cell type at each time point was assayed in triplicate. F’. Allantoic fluid from eggs injected with CD103+ DCs was assayed for infectious virus particles by a plaque immunostaining assay on MDCK cells. CD103+ DCs carry infectious virus from the lungs to MLNs during infection
When individual migratory lung DC subsets were stained for viral NP and visualized by confocal microscopy both CD103+ DCs (gate VI, Figure 3B) and CD11bhigh DCs (gate VII, Figure 3B) were found to have abundant intracellular Ag (Figure 3E). NP co-localized to the nucleus in CD103+ DCs (Figure 3E). In contrast, NP in CD11bhigh DCs surrounded but did not co-localize with the nucleus (Figure 3E). To test which DC subset transferred infectious virus particles to MDCKs in vitro, CD103+ DCs and CD11bhigh DCs isolated from MLNs at 72 or 96 hpi were injected separately into embryonated-chicken eggs (Figure 3F). 40 h later the allantoic fluid was collected and tested for the presence of replicating virus by agglutination of chicken red blood cells (RBCs). Only CD103+ DCs (gate VI, Figure 3B) injected into eggs led to the production of virus particles (Figure 3F), and the presence of infectious virus was confirmed by plaque immunostaining of MDCKs infected with the allantoic fluid from these samples (Figure 3F’). Neither CD11bhigh DCs (gate VII, Figure 3B) nor any other major leukocyte populations (gate B: B220+ cells; gate G: Gr1+ cells; gate P2: pDCs, Figure 3C) caused infectious virus production in embryonated eggs (Figure 3F). Moreover, depletion of Langerin+ CD103+ DCs in PR8-infected Langerin-DTR EGFP mice [32] reduced virus mRNA significantly in the MLNs (Figure 4A), with no apparent compromise of lung virus titers (Figure 4B). Total depletion of CD11c+ cells from infected mice virtually eliminated viral mRNA from the MLN [33] (Figure 4C and 4D).
Fig. 4. Depletion of total DCs or Langerin+ DCs compromises viral load in the MLNs during infection. 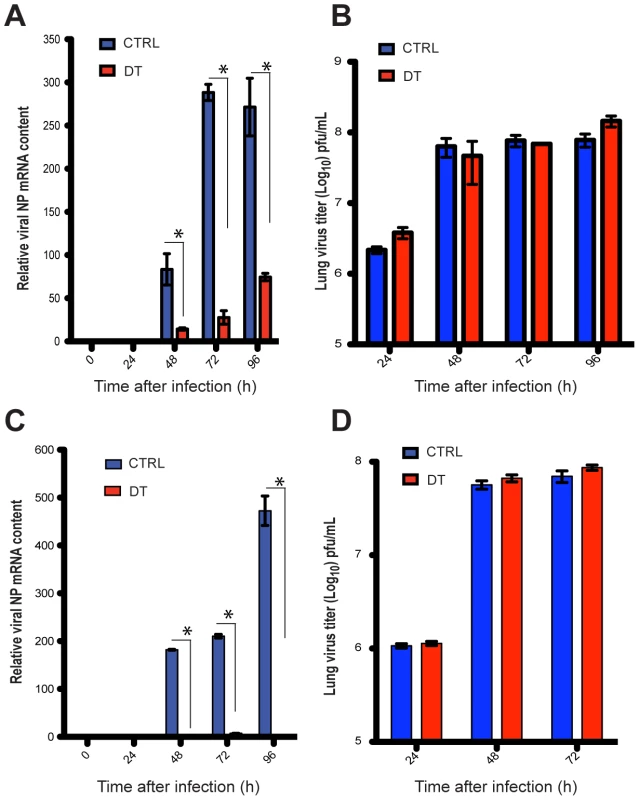
A. Langerin-DTR-EGFP mice were injected i.p with DT daily, starting one day before infection with PR8. MLNs were harvested and NP mRNA was quantitated by qPCR. B. Lung virus titers of DT-treated PR8 infected Langerin-DTR-EGFP mice (red bars) or mock treated (blue bars). C and D. CD11c-DTR-EGFP mice were used instead, performing the same experiments as described in A and B. Error bars represent mean +/- SD, * p<0.05. Multicycle replication of PR8 virus in vitro requires L-1-tosylamido-2-phenylethyl chloromethyl ketone treated-trypsin (TPCK-trypsin) to promote HA cleavage and spread to uninfected cells [34]. We next tested whether virus infection of MDCKs via contact with migratory DCs was dependent on TPCK-trypsin. As shown in Figure S1A, MDCK cells were infected in the absence of trypsin when co-cultured for 2 days with migratory DCs (see black arrows), showing that the transfer of infectious virus to MDCKs was independent of an exogenous added protease. As expected, subsequent robust spread of PR8 virus in MDCK cells was dependent on TPCK-trypsin (Figure S1A). We repeated the experiment with the closely related influenza strain known as WSN virus that is not dependent on TPCK-trypsin for multicycle replication [35], [36]. MDCKs co-cultured with MLN-DCs sorted from WSN infected mice were infected independently of trypsin (Figure S1A). Similar to the ability of CD103+ DCs to transfer infectious virus to embryonated eggs (Figure 3), virus could be transferred to MDCK cells upon co-culture with particular DC subsets isolated from PR8 and WSN infected mice. Specifically, only CD103+ DC but not CD11bhigh DCs caused ex-vivo virus infection of MDCKs, and trypsin was necessary for multicycle replication depending on the type of virus utilized (Figure S1B).
CD103+ DCs have an attenuated IFNAR signaling response that results in enhanced virus replication
As shown here and in previous work, at 2 dpi a rapid inflammatory response develops in the infected lung creating a milieu of proinflammatory cytokines including I-IFNs. IFNAR signaling in hematopoietic cells such as DCs induces the expression of several ISGs that promote the antiviral state [37], [38], [39]. The migration of lung DCs is initiated amidst this inflammatory environment and it was surprising that viral replication in the CD103+ DCs was not inhibited by the antiviral response triggered by I-IFN signaling [10], [40].
Therefore we compared elements associated with IFNAR signaling in steady state CD103+ DCs and CD11bhigh DCs sorted from lungs of naïve mice (sorting strategy, Figure 5A). As shown in Figure 5B, lung CD103+ DCs show a reduced expression of Ifnar1 and Ifnar2 chains, the Jak1 kinase, as well as the transcription factor Stat1, when compared to lung CD11bhigh DCs. These four genes represent key elements of the IFNAR receptor-signaling complex. To determine if the decreased expression of IFNAR signaling components translates into differences in ISG upregulation CD103+ DCs and CD11bhigh DCs were sorted from the MLNs during infection. Both DC subsets found in the MLNs of infected animals showed upregulated ISGs, however wild type CD11bhigh DCs produced higher levels of the ISG gene products than CD103+ DCs, a difference that was abrogated in the absence of IFNAR signaling (Figure 5C). In agreement with a differential IFNAR signaling and ISG response by lung DCs, CD103+ DCs collected from MLNs of infected animals showed greater permissivity to virus replication when compared to CD11bhigh DCs, expressing approximately ten times more viral NP mRNA (Figure 5D). IFNAR deficiency exacerbated the virus burden in CD103+ DCs and increased significantly the viral mRNA content in CD11bhigh DCs (Figure 5D). The difference in virus susceptibility between the CD103+ DCs and CD11bhigh DCs can be observed at the earliest time points in DCs isolated directly from the lungs of infected mice and mirrors the observations in cells collected from the MLN (Figure 5E). These results suggest that IFNAR signaling effectively restricts virus replication in CD11bhigh DCs whereas CD103+ DCs are somewhat refractory to this cytokine. Consistent with these findings, viral mRNA levels in the MLN of infected IFNAR -/- mice were significantly higher than in wild type animals (Figure 1A) indicating that the amount of virus transported to the MLN was higher in IFNAR -/- mice (Figure 5F).
Fig. 5. Unique responses to I-IFNs by lung DC subsets determine virus replication. 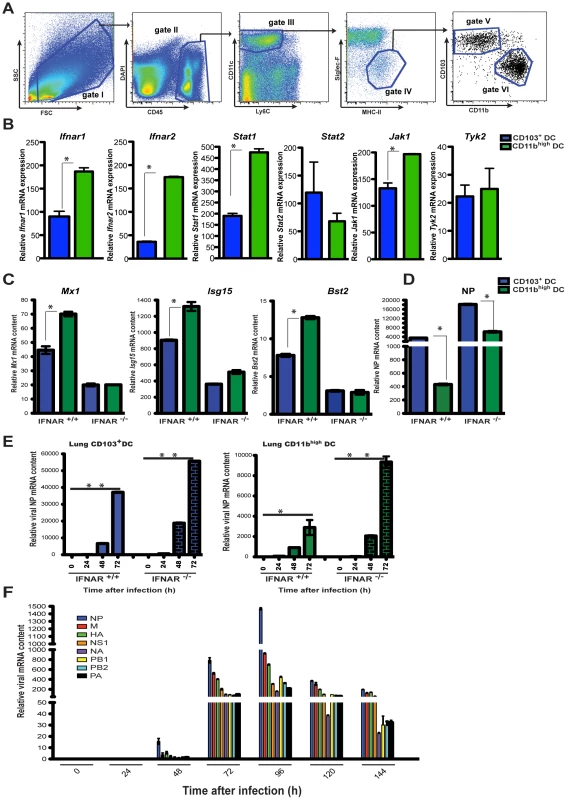
A. Sorting strategy for lung DCs. Lung DCs are defined as CD45+ CD11c+ MHC-II+ Siglec-F- Ly6C- cells (gates I-IV). From gate IV individual DC subsets were further gated into CD103+ DC (gate V) and CD11bhigh DC (gate VI) for separation by cell sorting. B. qPCR analysis of Ifnar genes and signaling related genes in lung CD103+DCs (blue bars) and lung CD11bhigh DCs isolated from IFNAR +/+ naïve mice. C. qPCR analysis of ISG expression by MLN CD103+ DCs (blue bars) or MLN CD11bhigh DCs (green bars) sorted from either IFNAR -/- or IFNAR +/+ PR8-infected mice at 4 dpi. D. Expression of viral NP mRNA was determined by qPCR for individual sorted MLN DC subsets as in (C). E. CD103+ DCs and CD11bhigh DCs isolated from the lungs of IFNAR -/- or IFNAR +/+ mice during the course of PR8 infection were assayed for viral NP mRNA by qPCR. F. Total MLN cDNA obtained from PR8-infected IFNAR -/- mice, at different time points after infection, was analyzed for mRNA of the 8 segments of influenza virus (NP, HA, M, NS1, NA, PB1, PB2, PA) by qPCR. Error bars represent mean +/− SD, * p<0.05. IFNAR signaling determines infection and the division of labor among lung DCs during influenza virus infection
To determine if the increase in viral mRNA seen in the MLNs from IFNAR -/- mice could result from enhanced virus replication from migratory lung DCs, we sorted CD103+ DCs and CD11bhigh DCs from infected IFNAR -/- and IFNAR +/+ mice at 3 and 4 dpi. Individual DC subsets were injected into embryonated eggs and the presence of infectious virus determined 40 h later by hemagglutination of the allantoic fluid. In contrast to what was observed with CD11bhigh DCs from wild type animals, IFNAR -/- CD11bhigh DCs were now permissive for productive infection by influenza virus (Figure 6A).
Fig. 6. IFNAR signaling determines class-I restricted antigen presentation by CD11b high DCs during influenza virus infection. 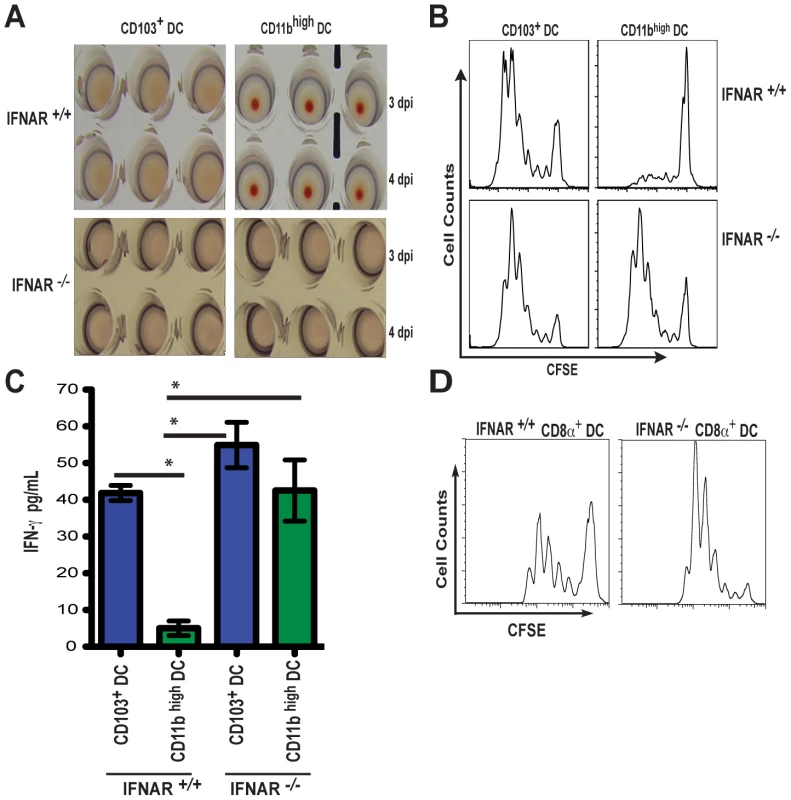
A. Individual MLN DC subsets from IFNAR +/+ or IFNAR -/- mice were isolated by cell sorting at 3 and 4 dpi (described in Figure 3), and injected into 10-day embryonated eggs. 2 days later, allantoic fluid was assayed for infectious virus particles by hemagglutination of RBCs. B. Sorted MLN CD103+ DCs and CD11bhigh DCs from either IFNAR +/+ or IFNAR -/- mice infected with PR8-OT-I were isolated at 4 dpi and co-cultured for 60 h with CFSE-labeled naïve OT-I CD8+ T cells. T cell proliferation was analyzed by flow cytometry. C. IFN-γ production of proliferating OT-I cultures (B) were assayed by ELISA. D. Lymph-node resident CD8α+ DCs isolated at 4 dpi from PR8-OT-I-infected IFNAR +/+ or IFNAR -/- mice were co-cultured with naïve OT-I CD8+ T cells and proliferation was assessed by CFSE dilution. Error bars represent mean +/- SD, * p<0.05. Data from a number of laboratories have confirmed that CD103+ DCs are superior APCs for the priming of naïve CD8+ T cells [11], [12], [31]. Our data indicated a possible relationship between virus replication and potent Ag presentation capacity. Therefore we isolated CD103+ DCs and CD11bhigh DCs from IFNAR +/+ and IFNAR -/- mice infected with PR8-OT-I virus to determine whether suppressed viral replication in CD11bhigh DCs by IFNAR signaling would be the etiological reason behind this difference in Ag presentation.
As anticipated, in vitro proliferation of naïve CD8+ OT-I T cells was superior when co-cultured with IFNAR +/+ CD103+ DCs compared to IFNAR +/+ CD11bhigh DCs (Figure 6B). Strikingly, OT-I T cell proliferation was comparable when both DC subsets were isolated from IFNAR -/- mice (Figure 6B) and the production of interferon-gamma (IFN-γ) from the OT-I T cells was almost equivalent (Figure 6C). As a control, lymph-node resident CD8α+ DCs, known to be potent APCs for CTL priming [12], were sorted from PR8-OT-I infected IFNAR +/+ and IFNAR -/- mice (sorting strategy, Figure S2) and co-cultured with OT-I cells in vitro (Figure 6D). IFNAR +/+ CD8α+ DCs induced OT-I T cell proliferation in vitro but not as robustly as IFNAR +/+ CD103+ DC. In contrast, IFNAR -/- CD8α+ DCs gained an enhanced ability to present Ag in the absence of IFNAR signaling similarly to what is observed for CD11bhigh DCs. These data show that viral replication in a given migratory DC population correlates with priming efficiency of naïve CD8+ T cells and the observed differences in priming are not necessarily an intrinsic feature of each DC subset and can be modulated by I-IFN signaling.
Discussion
The lung serves as a portal for the entry of a myriad of respiratory viruses throughout the lifetime of the host. Induction of cellular T cell immunity requires the obligate interaction of lung DCs with viruses, with the imminent risk of infection. Our data demonstrates that both lung DC subsets are infected with influenza virus in vivo and this event does not impair their migration to the MLNs or their function as APCs. By carefully examining each lung DC subset we found that CD103+ DCs had much greater levels of viral mRNA and produced infectious virus particles, a finding that challenges the widely held belief that influenza virus replicates only in the lung epithelium [1], [2]. On the other hand, CD11bhigh DCs expressed viral mRNA to a much lesser extent and did not support virus growth ex-vivo suggesting that these cells were abortively infected. The dramatic differences in virus susceptibility were striking and led us to investigate whether I-IFNs were uniquely sensed by both DC subsets.
Based on our previous observations, DCs begin to migrate in vivo only when lung inflammation is triggered ∼2 dpi with influenza virus [10] [27]. At this time, the antiviral cytokines, I-IFNs, are readily abundant but their effects on lung DC function were not previously addressed. Importantly, I-IFNs confer only partial protection to leukocytes during respiratory virus infection in vivo [40], and this is further supported by in vitro experiments where DCs and other leukocytes sustain some degree of viral transcription even after treatment with very high concentrations of I-IFNs [41], [42]. Therefore, migrating lung DCs are not necessarily refractory to influenza virus infection, even if they are I-IFN-primed before encountering the virus. In the present study we found that both lung DCs displayed an “interferon signature” (upregulation of ISGs) but the magnitude of the responses was quite different depending on the DC subset analyzed. Indeed, CD103+ DCs were found to be “inherently resistant” to IFNAR signaling with an attenuated ISG response when compared to CD11bhigh DCs. Consequently, our confocal imaging data showed nuclear localization of NP only in CD103+ DCs but not in CD11bhigh DCs suggesting that the latter subset has a tight control over virus replication mediated by stronger I-IFN signaling.
By ablating IFNAR signaling in CD11bhigh DCs we could increase their viral mRNA replication to levels comparable to wild type CD103+ DCs, and it was enough to promote infectious virus production by CD11bhigh DCs. Experimental evidence suggests a divergent hematopoetic origin of lung DC subsets. CD103+ DCs appear to arise from pre-DC precursors while CD11bhigh DCs are monocyte derived [43], [44], [45], [46], and this dichotomy could determine unique IFNAR signaling differences. Currently we are studying in more detail the underlying mechanisms explaining why CD103+ DCs have an attenuated IFNAR signaling and an inherent susceptibility to virus infection. Some of these include, inhibitory genes downstream of the IFNAR signaling pathway [47], [48], silencing of ISGs by epigenetic modifications of chromatin [49], specific expression of microRNAs that favor virus replication or targeting of particular IFNAR signaling components [50], and signaling by suppressor cytokines such as IL-10 [51].
The differential susceptibilities to virus infection by both lung DCs suggested that the relative viral Ag abundance might act as a limiting factor for peptide presentation by mayor histocompatibility class-I (MHC-I) molecules. CD103+ DCs have been characterized as APCs with superior abilities to prime naïve CD8+ T cells relative to CD11bhigh DCs, though both are equally competent at priming naïve CD4+ T cells [11], [31], [52]. The underlying reason for this fundamental difference has been ascribed to a division of labor theory [53](first described for splenic DCs) where unique gene programs define specialized machinery for MHC-II or MHC-I Ag presentation within each DC subset [8]. Here we propose an alternative model for this dichotomy based on the strength of their response to I-IFNs that determines the efficiency in MHC-I Ag presentation by controlling the level of virus replication within each DC subset. The prominent natural susceptibility of CD103+ DCs to virus infection may represent a mechanism that ensures adequate MHC-I Ag presentation in a cytokine milieu dominated by I-IFNs [10], [40], [42] leading to a higher density of peptide-MHC-I complexes on the cell surface facilitated by constant degradation of newly synthesized viral proteins [54], [55], [56], [57]. On the other hand, decreased virus replication in CD11bhigh DCs reduces peptide-MHC-I complex production, but loss of IFNAR signaling boosts viral replication and their ability to prime naïve CD8+ T cells to levels comparable to CD103+ DCs.
In this study, we did not address whether cross-priming [58] represents a major component regulating Ag presentation by CD103+ DCs and CD11bhigh DCs and further studies will be required to address their relationship to direct infection of lung DCs. Intriguingly, lymph node resident-CD8α+ DCs known to be potent APCs for cytotoxic T cells [12] were also boosted by the absence of IFNAR signaling. CD8α+ DCs might benefit from increased numbers of virus-infected DCs (CD103+ DC and CD11bhigh DC) in infected IFNAR -/- mice that may undergo cell death or release viral Ag that upon endocytosis may be used as substrates for crosspriming. Alternatively, I-IFN signaling might be responsible for lymph-node CD8α+ DC crosspresentation in influenza-infected wild-type mice ensuring optimal DC maturation [26] and increased processing of viral antigen from migratory DCs [12]; despite tight control of virus replication by I-IFN.
The hypersensitive IFNAR signaling by CD11bhigh DCs may divert these cells from naïve CTL priming to accomplish other specific tasks in the immune response to influenza virus, which is essentially multifactorial [15], [59]. Some of these functions may include, the production of high affinity IgG antibodies that may depend upon the continuous migration of antigen-bearing CD11bhigh DCs derived from inflammatory monocytes [60], [61], [62], restimulation of effector CD4+ and CD8+ T cells locally in the lungs to trigger cytokine production and cytotoxicity [63], and as potent cytokine and chemokine secretion machines activating and recruiting innate and adaptive effector cell populations to the lungs and lymph nodes [64]. Additionally, the robust I-IFN response may have a protective role in sparing CD11bhigh DCs from death that may explain late antigen presentation during infection to CTLs in the MLNs and lungs, which lingers long after the virus has been cleared [65], [66], [67], [68]. This knowledge about CD11bhigh DCs can potentially be harnessed to improve live DC vaccines in human medicine. The likely human version of CD11bhigh DCs is easily derived from blood monocytes [69], [70] and is commonly used for in vivo immunization protocols [71]. IFNAR signaling suppression in these cells may increase the replication of recombinant attenuated viruses or vectors improving vaccine efficacy for diverse diseases such as cancer [72], [73], [74] and HIV [75], [76], [77], [78].
A danger inherent to the unique sensitivity of CD103+ DCs to infection is that viruses likely evolved to exploit these cells as shuttles and spread to multiple locations. Various viruses of worldwide health concern, such as measles and varicella zoster use the respiratory tract as an entry route from where they disseminate systemically [79], [80]. It would not be surprising, if these viruses hijacked the CD103+ DC equivalent in the human lung as an initial Trojan horse, and upon arrival to the MLNs spread to other susceptible cells that travel out of the lymph nodes into systemic circulation. The same might be happening with particular influenza virus strains in the human respiratory tract such as the highly pathogenic H5N1 virus whose HA contains a polybasic multi-cleavage site [81], and may target CD103+ DCs to spread hematogenously. Our experiments with WSN strain partially demonstrate this phenomenon, since virus could spread from CD103+ DCs ex-vivo and infect MDCKs without the aid of an exogenously added protease such as trypsin. It remains to be determined whether in vivo, CD103+ DCs can promote systemic spread of viruses and whether lymph-node specific proteases aid the cleavage of diverse hemagglutinins of influenza viruses. Altogether, our findings are presented as a model summarized in Figure 7, where the unique sensitivities to IFNAR signaling by individual lung DC subsets have divergent consequences for the adaptive immune response and as well as for viral pathogenesis.
Fig. 7. Model: Unique I-IFNs responses determine the functional fate of migratory lung DCs during respiratory virus infection. 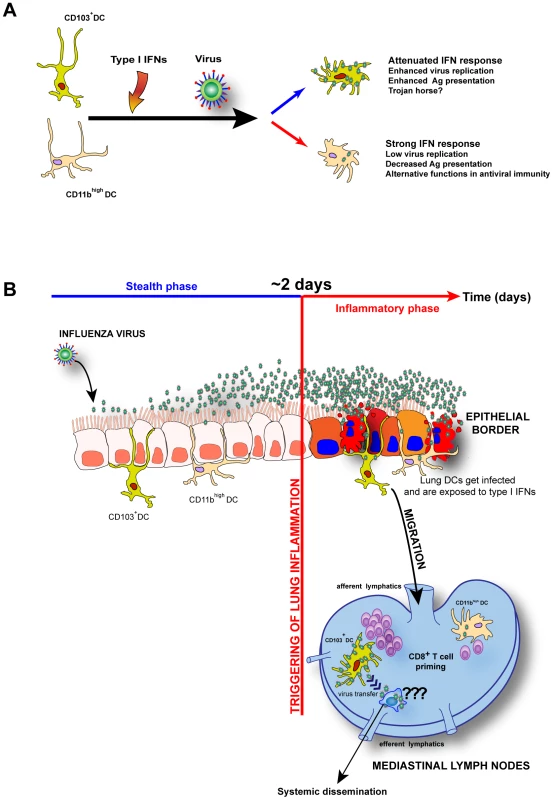
A. CD103+ DCs are naturally resistant to IFNAR signaling and allow virus replication to higher levels leading to enhanced antigen presentation, when compared to CD11bhigh DCs that respond strongly to I-IFNs and impede virus replication and Ag presentation. B. The in vivo implications of virus infection and unique IFNAR signaling by CD103+ DCs and CD11bhigh DCs during influenza virus infection and other respiratory viruses. Both lung DCs begin to migrate to the MLNs when lung inflammation is initiated around 2 days post-infection, an event that is preceded by unabated virus replication without an apparent innate response (stealth phase). IFNAR signaling is not protective in CD103+ DCs and their inherent susceptibility to virus infection may make them function as “Trojan horses” that transfer virus to other leukocytes in the MLNs and upon exit through efferent lymphatics disseminate virus systemically. Materials and Methods
Ethics statement
All animal work was conducted in agreement with approved protocols by the Institutional Animal Care and Use Committee (IACUC) at the Mount Sinai School of Medicine (Protocol Numbers #: 96-308 and 08-0951) and in accordance with guidelines in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The program is fully accredited by the Association for Assessment & Accreditation of Laboratory Animal Care, International (AAALAC).
Mice and viruses
C57BL/6 wild type mice were purchased from Taconic or Jackson laboratories. OT-I transgenic mice [82] were purchased from Jackson Laboratories. OT-I mice were crossed to B6.SJL mice to generate CD45.1+ OT-I mice. C57BL/6 IFNAR -/- mice [83], [84] were kindly provided by Dr. Wilson (Immunology Department, University of Washington, USA). C57BL/6 Langerin diphtheria toxin receptor (DTR) EGFP [32] mice were kindly provided by Dr. Bernard Malissen (INSERM, Lyon, France). C57BL/6 CD11c-DTR EGFP mice [33] were purchased from Jackson laboratories. All mouse colonies were kept under pathogen-free conditions at the Animal Facility of the Mount Sinai School of Medicine.
Influenza virus strains A/PR/8/1934 (H1N1) (PR8), recombinant PR8-OTI, and A/WSN/1933 (H1N1) (WSN) were grown in 10-day embryonated eggs (Charles Rivers, Spafas). PR8-OT-I virus [85] was kindly provided by Dr. Peter Doherty and Dr. Paul Thomas (St. Jude's Research Children's Hospital, Memphis, TN).
Mouse infection
Mice were infected using an Inhalation Exposure System A42X (Glass-Col, USA). Influenza virus was diluted in PBS to obtain a solution of 107.9 virus particles/12 ml. The virus solution was placed inside of a sterile glass nebulizer connected to the infection chamber. Total exposure time with aerosolized virus was 30 min. Under these conditions 100% of animals were infected and showed reproducible lung titers in several experiments at every time point analyzed as described previously [9], [10].
WSN infection of mice was performed by intranasal infection. Briefly, mice were anesthetized with Avertin (Tribromoethanol, Acros Organics) by intraperitoneal injection, and 1,000 plaque forming units (pfu) of WSN virus in 35 µl PBS was applied intranasally to each mouse. Mice were monitored until they were fully awake and placed back into their cages.
Lymph node and lung cell preparations for flow cytometry and fluorescence activated cell sorting (FACs)
MLNs from infected animals were mechanically dissociated and digested in DMEM/1% FCS supplemented with 0.25 mg/ml collagenase (Liberase type III, Roche) for 20 min at 37°C. Collagenase was neutralized by adding sterile PBS containing 2% FCS and 2.5 mM EDTA for 5 min, and then passing the dissociated cell suspension through a 70 µm strainer (BD Biosciences). Single cell suspensions were treated with red-blood cell lysis buffer (BD Biosciences) and resuspended in blocking buffer (PBS containing 2% FCS and 10 µg/ml Fc-receptor block). Lungs from infected mice were perfused with PBS to eliminate excess blood. Lung lobes were gently dissociated using forceps and digested for 50 min at 37°C in DMEM/1% FCS supplemented with collagenase-D (Roche) at 0.25 mg/ml. Single cell suspensions were obtained as described for MLNs.
For DC subset enumeration in the MLNs, lymph node cell suspensions were stained with antibodies against multiple surface antigens: anti-CD11c (clone HL-3), MHC-II (clone 2G9), Gr1 (Ly6C/Ly6G clone RBC865), Ly6C (Al-21), CD103 (clone 2E7), CD11b (clone M1/70), B220 (clone RA3-6B2), CD8α (clone 53-6.7). All antibodies were purchased from BD Bioscience, eBiosciences, and Biolegend. Lymph node DCs were identified as CD11chigh, MHC-II high, B220 negative, and Gr1 negative cells. Individual DC subsets were further gated as CD103+ DC and CD11bhigh DC that were negative for CD8α. Lymph node resident CD8α+ DCs were identified in the MLN by gating on CD11chigh MHC-II intermediate cells as described elsewhere [86]. For enumeration of lung DC subsets, cell suspensions were stained with antibodies against multiple surface antigens: anti-CD11c (clone HL-3), MHC-II (clone 2G9), Gr1 (Ly6C/Ly6G clone RBC865), Siglec-F (clone E50-2440), CD103 (clone 2E7), CD11b (clone M1/70), B220 (clone RA3-6B2), CD8α (clone 53-6.7). Lung DCs were identified as CD11chigh, MHC-II high, Siglec-F negative, B220 negative, and Gr1 negative cells. Individual lung DC subsets were further gated as CD103+ DCs and CD11bhigh DCs. Samples were acquired using a BD LSR-II flow cytometer at the Flow Cytometry Core Facility, Mount Sinai School of Medicine. Dead cells were excluded by DAPI staining. Data was analyzed using FlowJo software (Treestar Corp.)
MLNs and lungs were isolated from infected and control mice as described above under sterile conditions for cell sorting. Total cell suspensions or enriched DC fractions were used to sort individual CD103+ DC, CD11bhigh DC, and CD8α+ DC subsets. Other MLN cell types such as B cells, pDCs and Gr1+ cells were sorted from total cell suspensions. CD11c+ cells were positively selected using anti-CD11c beads (Miltenyi Biotech) or by negative selection utilizing a cocktail of biotinylated mabs, that included anti-Gr1 (clone RB6-8C5), anti-CD19 (clone 6D5), anti-Ter119 (clone Ter-119), anti-CD3 (clone 17A2), and anti-CD49b (clone DX5) followed by incubation with anti-biotin beads (Miltenyi Biotec). In both cases, cells were passed through LS magnetic columns (Miltenyi Biotec) and positive or negative fractions were collected depending on the method employed, then cells were stained as described above and sorted using a BD Aria-II cell sorter (Flow Cytometry Core Facility, Mount Sinai School of Medicine).
Immunohistochemistry of mediastinal lymph nodes
MLNs isolated from PR8 infected mice, were embedded in optimal cutting temperature (O.C.T) medium (Tissue-Tek), frozen over dry ice, and stored at -80° C until further use. 8 µm sections were cut with using a cryostat (Leica), placed over coated microscope slides, and fixed with 4% paraformaldehyde in PBS for 5 min at room temperature. Sections were blocked with PBS containing 2% FCS and 1% mouse serum for 20 min. Then, incubated with purified rat anti-B220 (clone RA3-6B2) and purified hamster anti-CD11c (clone HL3) for 45 min, washed twice with PBS and incubated with goat anti-rat Alexa-647 (Invitrogen) and goat-anti-hamster FITC (Jackson Immunoresearch) polyclonal sera, to visualize B220 (B cell areas) and CD11c (DCs), respectively. Following surface staining, slides were permeabilized with PBS containing Saponin 0.02% for 30 min and then incubated with a mixture of biotinylated antibodies to influenza NP (clone HT103) and M (2E10) proteins. Slides were washed twice and then incubated with Streptavidin-Cy3 (Sigma). Slides were air-dried, and mounted for fluorescence microscopy using Prolong Antifade with DAPI (Invitrogen). Immunofluorescence was performed using a Zeiss Axioplan2 fluorescence microscope. Images were analyzed using ImageJ software.
Determination of lung and lymph node virus titers
Plaque immunostaining assays were performed to quantitate virus titers from infected mice. Lung homogenates from infected mice (1 lung was homogenized in 1.8 ml of PBS containing 0.1% Gelatin) were 10-fold serially diluted. 24 well plates were seeded with Mardy-Darby Canine Kidney (MDCK) cells, 1 day before the infection to achieve confluent monolayers. The plates were washed 3 times with DMEM, and incubated for 1 hr with 100 µl of infected-lung homogenates. After the inoculums were removed, the cells were washed once with DMEM, and 500 µl of overlay media (DMEM-F12 containing 0.6% agar (Oxoid), 0.5% Albumin (MP biomedicals), 0.1% NaHCO3, antibiotics and 1 µg/ml TPCK-trypsin (Worthington) ) was added on top. Infected monolayers were incubated for 40 h at 37°C, and then fixed with 4% paraformaldehyde for 1 hr. Agar overlays were removed gently under running water. Fixed monolayers were washed with PBS twice, and incubated with chicken anti-PR8 polyclonal sera (Charles River) followed by goat HRP-anti-chicken (Jackson Immunoresearch). Plaques were visualized after incubation with the True Blue substrate (KPL) that produces a blue precipitate by an HRP mediated reaction. Plaque forming units (pfu) were counted and virus titers were expressed as pfu/ml.
To determine virus titers in lymph nodes, MLNs were isolated at different time points after infection and homogenized in 500 µl of PBS. Immediately after homogenization, samples were pooled, and 10-fold serial dilutions were injected in triplicates into 10-day embryonated-chicken eggs. 40 h post-inoculation, allantoic fluid was harvested to determine virus particles by hemagglutination of red blood cells (RBCs). Endpoint titers were determined by the method of Reed and Muench [87].
In vitro T cell proliferation assay
A CD8+ T cell isolation kit (Miltenyi Biotec) was used to isolate untouched OT-I CD8+ T cells following manufacturer instructions. OT-I naive T cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) at a final concentration of 2.5 µM. Individual MLN CD11c+ cell populations (CD103+ DCs, CD11bhigh DCs and CD8α+ DCs) were isolated at day 4 post-infection from wild type or IFNAR -/- mice infected with PR8-OT-I and co-cultured in 96 well plates with CFSE-labeled OT-I transgenic T cells at a ratio of 10,000 DCs to 50,000 CFSE-labeled transgenic T cells per well. 60 h later, T cell proliferation was quantitated by CFSE dilution using flow cytometry as described elsewhere [9]. Dead cells were excluded using PI or DAPI staining.
Confocal microscopy of migratory DCs
A suspension of sorted CD103+ DC and CD11bhigh DCs in 100 µl, were placed over microscope poly-lysine coated slides (Shandon) and let to adhere for 2 h at 37°C in an incubator inside a humidified hybridization chamber (Sigma) in order to avoid evaporation of the media. After the incubation time was over, the media was rapidly aspirated (avoiding the cells to dry), and a drop of ∼100 µl of freshly prepared 4% paraformaldehyde in PBS was added to fix the adherent cells at room temperature. After 3 min, the solution was aspirated and replaced with fresh paraformaldehyde 4% (100 µl per sample) and the cells were fixed for additional 15 min. Cells were permeabilized using a solution of Saponin 0.02% dissolved in PBS. Slides were blocked with PBS containing 1% FBS, 1% mouse serum (Jackson Immunoresearch) and Saponin 0.02% for 20 min, and stained for viral NP with biotinilated-HT103 monoclonal antibody for 30 min at room temperature. Followed by 3 washes in Saponin-PBS, secondary staining was performed using Streptavidin-Cy3 (Sigma).
Slides were air-dried and Prolong Antifade with DAPI (Invitrogen) was used as mounting media.
Fluorescence Microscopy was performed on a Zeiss Inverted LSM 510 laser scanning confocal instrument mounted on a Zeiss Axiovert 200M microscope. All images were acquired using a 100x oil objective. Images were analyzed with ImageJ software.
Determination of infectious virus particle production by migratory DCs
MLN DCs from PR8 (day 2 to day 4 post-infection) or WSN infected mice (day 3 post-infection) were sorted by FACs and co-cultured for 3 days with confluent MDCK cells seeded on 96 well plates in DMEM media containing 0.5% Albumin (MP biomedicals), 0.1% NaHCO3 and antibiotics. The culture media was either supplemented or not with TPCK-trypsin (1 µg/ml). Supernatants from these cultures were assayed for the presence of replicating virus by hemagglutination of RBCs to determine whether DCs were capable of transferring infectious virus particles to MDCKs in vitro. Infected MDCK cells were visualized via immunostaining with polyclonal anti-chicken PR8 developed with horseradish peroxidase reaction with True Blue substrate as described for plaque immunostaining assays. Images of infected MDCK cells were acquired using a Nikon Eclipse TS100 microscope light microscopy. Alternatively, sorted DCs or other sorted cells from the MLNs were injected directly into 10-day embryonated eggs. 2 days later, allantoic fluid was isolated and presence of virus was determined by hemagglutination of RBCs. In parallel, allantoic fluid from these experiments was serially diluted and used to infect MDCK cells to determine whether the virus in these preparations was infectious as measured by plaque immunostaining on MDCK cells.
Real-time quantitative PCR (qPCR)
Lungs or lymph nodes (3–5 pooled lymph nodes) from infected mice were homogenized in 3 ml/sample of Trizol Reagent (Invitrogen) and RNA was isolated as indicated by the manufacturer. Total mRNA was converted to cDNA by RT-PCR using oligo-dT reaction (Affinity Script, Stratagene). cDNA was diluted 50 times in water and triplicate reactions were setup in 384-well plates. qPCR reactions based on SYBR green detection, were performed using a Lightcycler equipment (Roche, USA) all reactions were normalized to α-tubulin as previously described [88].
qPCR reactions with sorted DCs required mRNA amplification. The WT-OVATION amplification Kit (Nugen, San Diego, USA) was used for this purpose as described by the supplier.
The list of primers used in this study is the following:
M1/2 forward: 5′-GGGAAGAACACCGATCTTGA-3′; M1/2 reverse: 5′-CGGTGAGCGTGAACACAAAT-3′; NA forward: 5′-CATCTCTTTGTCCCATCCGT-3′; NA reverse: 5′-GTCCTGCATTCCAAGTGAGA-3′; HA forward: 5′-GAGGAGCTGAGGGAGCAAT-3′; HA reverse: 5′-GCCGTTACTCCGTTTGTGTT-3′; PB1 forward: 5′-CCTCCTTACAGCCATGGGA-3′; PB1 reverse: 5′-GTGCTCCAGTTTCGGTGTTT-3′; PB2 forward: 5′-GGATCAGACCGAGTGATGGT-3′; PB2 reverse: 5′-CCATGCTTTAGCCTTTCGACT-3′; PA forward: 5′-CATCAATGAGCAAGGCGAGT-3′; PA forward: 5′-GCCCCTGTAGTGTTGCAAAT-3′; NP forward: 5′-CAGCCTAATCAGACCAAATG-3′; NP reverse: 5′-TACCTGCTTCTCAGTTCAAG-3′;NS1 forward: 5′-TTCACCATTGCCTTCTCTTC-3′;
NS1 reverse: 5′-CCCATTCTCATTACTGCTTC-3′; Mx1 forward: 5′-CAACTGGAATCCTCCTGGAA-3′; Mx1 reverse: 5′-GGCTCTCCTCAGAGGTATCA-3′; Isg15 forward: 5′-GAGCTAGAGCCTGCAGCAAT-3′; Isg15 reverse: 5′-CTTCTGGGCAATCTGCTTCT-3′; Bst2 forward: 5′ - CAAACTCCTGCAACCTGACC-3′;
Bst2 reverse: 5′ - CATTCTCAAGCTCCTTGATGC-3′; Ifnar1 forward: 5′ -GGTTGATCCGTTTATTCCATTC-3′; Ifnar1 reverse: 5′ - CCACATGTTCCCGTCTTGT-3′;
Ifnar2 forward: 5′-CTTCGTGTTTGGTAGTGATGGT-3′; Ifnar2 reverse: 5′-GGGGATGATTTCCAGCCGA -3′; Stat1 forward: 5′-CTTCAGCAGCTGGACTCCAA -3′; Stat1 reverse: 5′ - GGTCGCAAACGAGACATCAT-3′; Stat2 forward: 5′-AAGAGGTGCAGCCCCCACCA-3′; Stat2 reverse: 5′-GCTGCGCCTGTTGGCTCTGA-3′; Jak1 forward: 5′ - TGCAGGAGGGAGCCTGGCAT-3′; Jak1 reverse: 5′-AGCTTGCCCCAGGGGATCGT-3′; Tyk2 forward: 5′-AGCCATCTTGGAAGACAGCAA-3′; Tyk2 reverse: 5′-GACTTTGTGTGCGATGTGGAT-3′; α-tubulin forward: 5′-TGCCTTTGTGCACTGGTATG-3′; α-tubulin reverse: 5′-CTGGAGCAGTTTGACGACAC-3′.
Lung DC migration blockade by pertussis toxin (PTX) during infection
2 h after aerosol infection, mice were anesthetized and 2 µg of PTX dissolved in 35 µl of PBS was delivered intranasally to each mouse. Once a day thereafter, mice received 0.5 µg of PTX i.p to maintain the chemokine receptor blockade. MLNs and lungs of mock and PTX treated mice were collected at day 1, 2, and 3 post-infection. To further confirm ablation of lung DC migration, absolute numbers of CCR7+ CD11c+ cells were determined in infected mice treated with PBS or PTX. Mouse anti-CCR7 antibody (clone 4B12) was used for this experiment.
Diphtheria toxin depletion of DCs during infection
Langerin+ CD103+ DCs were depleted in PR8-infected Langerin-DTR EGFP mice with an intraperitoneal (i.p) injection of 1 µg of diphtheria toxin (DT, Sigma), 1 day before infection. At day 0, mice were infected with aerosolized PR8 virus. Thereafter, a daily dose of 200 ng was administered i.p to each mouse to maintain the DC depletion. Lungs and MLNs were harvested every day for RNA extraction and virus titer determination. For total CD11c+ cell depletion, CD11c-DTR EGFP mice were injected i.p with 4 ng/g (body weight) of DT, 1 day before PR8 infection. Thereafter daily doses of 30 ng of DT were administered i.p up to day 3. Viral message in the MLNs was determined by qPCR as described above. Lung titers were determined by plaque immunostaining assay.
Cytokine ELISA
To determine the concentration of IFN-γ from mixed DC-T cell cultures, an ELISA kit from R&D (UK) was used according to the manufacturer's instructions.
Statistical analysis
Averaged results were expressed as means+/ - standard deviation. A two-tailed Student's t test was used to determine statistical significance of selected samples. P values< 0.05 (95% Confidence) were considered to be significant. Graphs were designed either in Excel or Graph Pad software.
Gene accession list
CD11c (name: Itgax, ID: 16411); CD11b (name: Itgam, ID: 16409); CD103 (name: Itgae, ID: 16407); Ifnar1 (name: Ifnar1, ID: 15975); Ifnar2 (name: Ifnar2, ID: 15976); Stat1 (name: Stat1, ID: 20846); Stat2(name: Stat2, ID: 288774); Jak1(name: Jak1, ID: 16451); Tyk2 (name: Tyk2, ID: 54721); Ly6C (name: Ly6c1, ID: 17067); Ly6G (name: Ly6g, ID: ); CD8α (name: Cd8a, ID: 12525); Isg15 (name: Isg15, ID: 100038882); Mx1 (name: Mx1, ID: 17857); Bst2 (name: Bst2, ID: 69550); IFN-γ (name: Ifng, ID: 15978); Siglec-F (name: Siglec5, ID: 233186); B220 (name: Ptprc, ID: 19264); CD45 (name: Ptprc, ID: 19264); Langerin (name: Cd207, ID: 246278); CCR7 (name: Ccr7, ID: 12775); HA (name: HA, ID: 956529); NP (name: NP, ID: 956531); NS1 (name: NS1, ID: 956533); NA (name: NA, ID: 956530); PA (name: PA, ID: 956535); PB1 (name: PB1, ID: 956534); PB2 (name: PB2, ID: 956536); M ((name: M1, ID: 956527 and name: M2, ID: 956528 ); MHC-II (name: H2-Ab1, ID: 14961 and name: H2-Aa, ID: 14960).
Supporting Information
Zdroje
1. PalesePShawML 2007 Orthomyxoviridae: the viruses and their replication. KnipeDMHowleyPM Fields Virology Philadelphia, PA Lippincott Williams & Wilkins 1647 1689
2. WrightPFNeumannGKawaokaY 2007 In Fields Virology, 5th Edition. DMKnipePMHowley Philadelphia, PA Lippincott Williams & Wilkins 1691 1740
3. BanchereauJSteinmanRM 1998 Dendritic cells and the control of immunity. Nature 392 245 252
4. IwasakiA 2007 Mucosal dendritic cells. Annu Rev Immunol 25 381 418
5. SungSSFuSMRoseCEJrGaskinFJuST 2006 A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol 176 2161 2172
6. JakubzickCBogunovicMBonitoAJKuanELMeradM 2008 Lymph-migrating, tissue-derived dendritic cells are minor constituents within steady-state lymph nodes. J Exp Med 205 2839 2850
7. JakubzickCHelftJKaplanTJRandolphGJ 2008 Optimization of methods to study pulmonary dendritic cell migration reveals distinct capacities of DC subsets to acquire soluble versus particulate antigen. J Immunol Methods 337 121 131
8. del RioMLRodriguez-BarbosaJIKremmerEForsterR 2007 CD103 - and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T cells. J Immunol 178 6861 6866
9. BrimnesMKBonifazLSteinmanRMMoranTM 2003 Influenza virus-induced dendritic cell maturation is associated with the induction of strong T cell immunity to a coadministered, normally nonimmunogenic protein. J Exp Med 198 133 144
10. MoltedoBLopezCBPazosMBeckerMIHermeshT 2009 Cutting edge: stealth influenza virus replication precedes the initiation of adaptive immunity. J Immunol 183 3569 3573
11. GeurtsvanKesselCHWillartMAvan RijtLSMuskensFKoolM 2008 Clearance of influenza virus from the lung depends on migratory langerin+CD11b - but not plasmacytoid dendritic cells. J Exp Med 205 1621 1634
12. BelzGTSmithCMKleinertLReadingPBrooksA 2004 Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc Natl Acad Sci U S A 101 8670 8675
13. ThomasPGKeatingRHulse-PostDJDohertyPC 2006 Cell-mediated protection in influenza infection. Emerg Infect Dis 12 48 54
14. LawrenceCWReamRMBracialeTJ 2005 Frequency, specificity, and sites of expansion of CD8+ T cells during primary pulmonary influenza virus infection. J Immunol 174 5332 5340
15. KohlmeierJEWoodlandDL 2009 Immunity to respiratory viruses. Annu Rev Immunol 27 61 82
16. SchulmanJLKilbourneED 1963 Experimental Transmission of Influenza Virus Infection in Mice. I. The Period of Transmissibility. J Exp Med 118 257 266
17. LienenklausSCornitescuMZietaraNLyszkiewiczMGekaraN 2009 Novel reporter mouse reveals constitutive and inflammatory expression of IFN-beta in vivo. J Immunol 183 3229 3236
18. StarkGRKerrIMWilliamsBRSilvermanRHSchreiberRD 1998 How cells respond to interferons. Annu Rev Biochem 67 227 264
19. de VeerMJHolkoMFrevelMWalkerEDerS 2001 Functional classification of interferon-stimulated genes identified using microarrays. J Leukoc Biol 69 912 920
20. SchogginsJWWilsonSJPanisMMurphyMYJonesCT 2011 A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472 481 485
21. BrassALHuangICBenitaYJohnSPKrishnanMN 2009 The IFITM proteins mediate cellular resistance to influenza A H1N1 virus, West Nile virus, and dengue virus. Cell 139 1243 1254
22. Garcia-SastreADurbinRKZhengHPalesePGertnerR 1998 The role of interferon in influenza virus tissue tropism. J Virol 72 8550 8558
23. MordsteinMKochsGDumoutierLRenauldJCPaludanSR 2008 Interferon-lambda contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses. PLoS Pathog 4 e1000151
24. LuftTPangKCThomasEHertzogPHartDN 1998 Type I IFNs enhance the terminal differentiation of dendritic cells. J Immunol 161 1947 1953
25. GallucciSLolkemaMMatzingerP 1999 Natural adjuvants: endogenous activators of dendritic cells. Nat Med 5 1249 1255
26. LonghiMPTrumpfhellerCIdoyagaJCaskeyMMatosI 2009 Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med 206 1589 1602
27. HermeshTMoltedoBLopezCBMoranTM 2010 Buying Time - The Immune System Determinants of the Incubation Period to Respiratory Viruses. Viruses 2 2541 2558
28. KohlmeierJEWoodlandDL 2010 A call to arms: interferons prepare bone marrow cells to battle peripheral infections. Cell Host Microbe 7 336 337
29. HintzenGOhlLdel RioMLRodriguez-BarbosaJIPabstO 2006 Induction of tolerance to innocuous inhaled antigen relies on a CCR7-dependent dendritic cell-mediated antigen transport to the bronchial lymph node. J Immunol 177 7346 7354
30. ForsterRSchubelABreitfeldDKremmerERenner-MullerI 1999 CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99 23 33
31. KimTSBracialeTJ 2009 Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS One 4 e4204
32. KissenpfennigAHenriSDuboisBLaplace-BuilheCPerrinP 2005 Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity 22 643 654
33. JungSUnutmazDWongPSanoGDe los SantosK 2002 In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity 17 211 220
34. KlenkHDRottROrlichMBlodornJ 1975 Activation of influenza A viruses by trypsin treatment. Virology 68 426 439
35. WagnerRR 1955 A pantropic strain of influenza virus: generalized infection and viremia in the infant mouse. Virology 1 497 515
36. ChoppinPW 1969 Replication of influenza virus in a continuous cell line: high yield of infective virus from cells inoculated at high multiplicity. Virology 39 130 134
37. SamuelCE 2001 Antiviral actions of interferons. Clin Microbiol Rev 14778 809 table of contents
38. SchmidSMordsteinMKochsGGarcia-SastreATenoeverBR 2010 Transcription factor redundancy ensures induction of the antiviral state. J Biol Chem 285 42013 42022
39. YountJSMoltedoBYangYYCharronGMoranTM 2010 Palmitoylome profiling reveals S-palmitoylation-dependent antiviral activity of IFITM3. Nat Chem Biol 6 610 614
40. HermeshTMoltedoBMoranTMLopezCB 2010 Antiviral instruction of bone marrow leukocytes during respiratory viral infections. Cell Host Microbe 7 343 353
41. OsterlundPVeckmanVSirenJKlucherKMHiscottJ 2005 Gene expression and antiviral activity of alpha/beta interferons and interleukin-29 in virus-infected human myeloid dendritic cells. J Virol 79 9608 9617
42. Phipps-YonasHSetoJSealfonSCMoranTMFernandez-SesmaA 2008 Interferon-beta pretreatment of conventional and plasmacytoid human dendritic cells enhances their activation by influenza virus. PLoS Pathog 4 e1000193
43. GinhouxFLiuKHelftJBogunovicMGreterM 2009 The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med 206 3115 3130
44. LiuKVictoraGDSchwickertTAGuermonprezPMeredithMM 2009 In vivo analysis of dendritic cell development and homeostasis. Science 324 392 397
45. LandsmanLVarolCJungS 2007 Distinct differentiation potential of blood monocyte subsets in the lung. J Immunol 178 2000 2007
46. JakubzickCTackeFGinhouxFWagersAJvan RooijenN 2008 Blood monocyte subsets differentially give rise to CD103+ and CD103 - pulmonary dendritic cell populations. J Immunol 180 3019 3027
47. LiuBMinkSWongKASteinNGetmanC 2004 PIAS1 selectively inhibits interferon-inducible genes and is important in innate immunity. Nat Immunol 5 891 898
48. FennerJEStarrRCornishALZhangJGMetcalfD 2006 Suppressor of cytokine signaling 1 regulates the immune response to infection by a unique inhibition of type I interferon activity. Nat Immunol 7 33 39
49. ShilatifardA 2006 Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu Rev Biochem 75 243 269
50. SkalskyRLCullenBR 2010 Viruses, microRNAs, and host interactions. Annu Rev Microbiol 64 123 141
51. ItoSAnsariPSakatsumeMDickensheetsHVazquezN 1999 Interleukin-10 inhibits expression of both interferon alpha - and interferon gamma - induced genes by suppressing tyrosine phosphorylation of STAT1. Blood 93 1456 1463
52. HelftJGinhouxFBogunovicMMeradM 2010 Origin and functional heterogeneity of non-lymphoid tissue dendritic cells in mice. Immunol Rev 234 55 75
53. DudziakDKamphorstAOHeidkampGFBuchholzVRTrumpfhellerC 2007 Differential antigen processing by dendritic cell subsets in vivo. Science 315 107 111
54. RotzschkeOFalkKDeresKSchildHNordaM 1990 Isolation and analysis of naturally processed viral peptides as recognized by cytotoxic T cells. Nature 348 252 254
55. PamerECresswellP 1998 Mechanisms of MHC class I–restricted antigen processing. Annu Rev Immunol 16 323 358
56. RockKLGoldbergAL 1999 Degradation of cell proteins and the generation of MHC class I-presented peptides. Annu Rev Immunol 17 739 779
57. Van BleekGMNathensonSG 1990 Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature 348 213 216
58. CarboneFRBevanMJ 1990 Class I-restricted processing and presentation of exogenous cell-associated antigen in vivo. J Exp Med 171 377 387
59. SchmolkeMGarcia-SastreA 2010 Evasion of innate and adaptive immune responses by influenza A virus. Cell Microbiol 12 873 880
60. SerbinaNVPamerEG 2006 Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol 7 311 317
61. LinKLSuzukiYNakanoHRamsburgEGunnMD 2008 CCR2+ Monocyte-Derived Dendritic Cells and Exudate Macrophages Produce Influenza-Induced Pulmonary Immune Pathology and Mortality. J Immunol 180 2562 2572
62. Le BorgneMEtchartNGoubierALiraSASirardJC 2006 Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell crosspriming in vivo. Immunity 24 191 201
63. HuffordMMKimTSSunJBracialeTJ 2011 Antiviral CD8+ T cell effector activities in situ are regulated by target cell type. J Exp Med 208 167 180
64. BeatySRRoseCEJrSungSS 2007 Diverse and potent chemokine production by lung CD11bhigh dendritic cells in homeostasis and in allergic lung inflammation. J Immunol 178 1882 1895
65. EichelbergerMCWangMLAllanWWebsterRGDohertyPC 1991 Influenza virus RNA in the lung and lymphoid tissue of immunologically intact and CD4-depleted mice. J Gen Virol 72 Pt 7 1695 1698
66. Jelley-GibbsDMBrownDMDibbleJPHaynesLEatonSM 2005 Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J Exp Med 202 697 706
67. ZammitDJTurnerDLKlonowskiKDLefrancoisLCauleyLS 2006 Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity 24 439 449
68. KimTSHuffordMMSunJFuYXBracialeTJ 2010 Antigen persistence and the control of local T cell memory by migrant respiratory dendritic cells after acute virus infection. J Exp Med 207 1161 1172
69. RomaniNGrunerSBrangDKampgenELenzA 1994 Proliferating dendritic cell progenitors in human blood. J Exp Med 180 83 93
70. SallustoFLanzavecchiaA 1994 Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 179 1109 1118
71. SteinmanRM 2010 Some active areas of DC research and their medical potential. Eur J Immunol 40 2085 2088
72. KimSLeeJBLeeGKChangJ 2009 Vaccination with recombinant adenoviruses and dendritic cells expressing prostate-specific antigens is effective in eliciting CTL and suppresses tumor growth in the experimental prostate cancer. Prostate 69 938 948
73. GilboaEViewegJ 2004 Cancer immunotherapy with mRNA-transfected dendritic cells. Immunol Rev 199 251 263
74. DubskyPSaitoHLeogierMDantinCConnollyJE 2007 IL-15-induced human DC efficiently prime melanoma-specific naive CD8+ T cells to differentiate into CTL. Eur J Immunol 37 1678 1690
75. CarneroELiWBorderiaAVMoltedoBMoranT 2009 Optimization of human immunodeficiency virus gag expression by newcastle disease virus vectors for the induction of potent immune responses. J Virol 83 584 597
76. NiuLTerminiJMKanagaveluSKGuptaSRollandMM 2011 Preclinical evaluation of HIV-1 therapeutic ex vivo dendritic cell vaccines expressing consensus Gag antigens and conserved Gag epitopes. Vaccine 29 2110 2119
77. ShankarEMCheKFMessmerDLifsonJDLarssonM 2011 Expression of a Broad Array of Negative Costimulatory Molecules and Blimp-1 in T Cells following Priming by HIV-1 Pulsed Dendritic Cells. Mol Med 17 229 240
78. CobbARobertsLKPaluckaAKMeadHMontesM 2011 Development of a HIV-1 lipopeptide antigen pulsed therapeutic dendritic cell vaccine. J Immunol Methods 365 27 37
79. MossWJGriffinDE 2006 Global measles elimination. Nat Rev Microbiol 4 900 908
80. ArvinAM 1996 Varicella-zoster virus. Clin Microbiol Rev 9 361 381
81. BeigelJHFarrarJHanAMHaydenFGHyerR 2005 Avian influenza A (H5N1) infection in humans. N Engl J Med 353 1374 1385
82. HogquistKAJamesonSCHeathWRHowardJLBevanMJ 1994 T cell receptor antagonist peptides induce positive selection. Cell 76 17 27
83. van den BroekMFMullerUHuangSAguetMZinkernagelRM 1995 Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J Virol 69 4792 4796
84. MullerUSteinhoffUReisLFHemmiSPavlovicJ 1994 Functional role of type I and type II interferons in antiviral defense. Science 264 1918 1921
85. JenkinsMRWebbyRDohertyPCTurnerSJ 2006 Addition of a prominent epitope affects influenza A virus-specific CD8+ T cell immunodominance hierarchies when antigen is limiting. J Immunol 177 2917 2925
86. VermaelenKYCarro-MuinoILambrechtBNPauwelsRA 2001 Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J Exp Med 193 51 60
87. ReedLJMuenchH 1938 A simple method of estimating fifty per cent end points. Am J Hyg 27 493 497
88. YountJSKrausTAHorvathCMMoranTMLopezCB 2006 A novel role for viral-defective interfering particles in enhancing dendritic cell maturation. J Immunol 177 4503 4513
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Microbial Spy Games and Host Response: Roles of a Small Molecule in Communication with Other SpeciesČlánek Sequence-Based Analysis Uncovers an Abundance of Non-Coding RNA in the Total Transcriptome ofČlánek The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 11- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Microbial Spy Games and Host Response: Roles of a Small Molecule in Communication with Other Species
- Simple Rapid Near-Patient Diagnostics for Tuberculosis Remain Elusive—Is a “Treat-to-Test” Strategy More Realistic?
- Ultra-Efficient PrP Amplification Highlights Potentialities and Pitfalls of PMCA Technology
- Assessing Predicted HIV-1 Replicative Capacity in a Clinical Setting
- Inhibition of IL-10 Production by Maternal Antibodies against Group B Streptococcus GAPDH Confers Immunity to Offspring by Favoring Neutrophil Recruitment
- Anti-filarial Activity of Antibiotic Therapy Is Due to Extensive Apoptosis after Depletion from Filarial Nematodes
- West Nile Virus Experimental Evolution and the Trade-off Hypothesis
- Shedding Light on the Elusive Role of Endothelial Cells in Cytomegalovirus Dissemination
- Galactosaminogalactan, a New Immunosuppressive Polysaccharide of
- Fatal Prion Disease in a Mouse Model of Genetic E200K Creutzfeldt-Jakob Disease
- BST2/Tetherin Enhances Entry of Human Cytomegalovirus
- Metagenomic Analysis of Fever, Thrombocytopenia and Leukopenia Syndrome (FTLS) in Henan Province, China: Discovery of a New Bunyavirus
- Neurons are MHC Class I-Dependent Targets for CD8 T Cells upon Neurotropic Viral Infection
- Sap Transporter Mediated Import and Subsequent Degradation of Antimicrobial Peptides in
- A Molecular Mechanism for Bacterial Susceptibility to Zinc
- Genomic Transition to Pathogenicity in Chytrid Fungi
- Evolution of Multidrug Resistance during Infection Involves Mutation of the Essential Two Component Regulator WalKR
- ChemR23 Dampens Lung Inflammation and Enhances Anti-viral Immunity in a Mouse Model of Acute Viral Pneumonia
- SH3 Domain-Mediated Recruitment of Host Cell Amphiphysins by Alphavirus nsP3 Promotes Viral RNA Replication
- A Gammaherpesvirus Cooperates with Interferon-alpha/beta-Induced IRF2 to Halt Viral Replication, Control Reactivation, and Minimize Host Lethality
- Early Secreted Antigen ESAT-6 of Promotes Protective T Helper 17 Cell Responses in a Toll-Like Receptor-2-dependent Manner
- CD4 T Cell Immunity Is Critical for the Control of Simian Varicella Virus Infection in a Nonhuman Primate Model of VZV Infection
- The Role of the P2X Receptor in Infectious Diseases
- Down-Regulation of Shadoo in Prion Infections Traces a Pre-Clinical Event Inversely Related to PrP Accumulation
- Cross-Reactive T Cells Are Involved in Rapid Clearance of 2009 Pandemic H1N1 Influenza Virus in Nonhuman Primates
- Single Molecule Analysis of Replicated DNA Reveals the Usage of Multiple KSHV Genome Regions for Latent Replication
- The Critical Role of Notch Ligand Delta-like 1 in the Pathogenesis of Influenza A Virus (H1N1) Infection
- Sequence-Based Analysis Uncovers an Abundance of Non-Coding RNA in the Total Transcriptome of
- Murine Gamma Herpesvirus 68 Hijacks MAVS and IKKβ to Abrogate NFκB Activation and Antiviral Cytokine Production
- EBV Tegument Protein BNRF1 Disrupts DAXX-ATRX to Activate Viral Early Gene Transcription
- SAG101 Forms a Ternary Complex with EDS1 and PAD4 and Is Required for Resistance Signaling against Turnip Crinkle Virus
- Multiple Candidate Effectors from the Oomycete Pathogen Suppress Host Plant Immunity
- Rab7A Is Required for Efficient Production of Infectious HIV-1
- A TNF-Regulated Recombinatorial Macrophage Immune Receptor Implicated in Granuloma Formation in Tuberculosis
- Novel Anti-bacterial Activities of β-defensin 1 in Human Platelets: Suppression of Pathogen Growth and Signaling of Neutrophil Extracellular Trap Formation
- CD11b, Ly6G Cells Produce Type I Interferon and Exhibit Tissue Protective Properties Following Peripheral Virus Infection
- The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
- A Kinase Chaperones Hepatitis B Virus Capsid Assembly and Captures Capsid Dynamics
- Towards a Structural Comprehension of Bacterial Type VI Secretion Systems: Characterization of the TssJ-TssM Complex of an Pathovar
- Indirect DNA Readout by an H-NS Related Protein: Structure of the DNA Complex of the C-Terminal Domain of Ler
- The Pore-Forming Toxin Listeriolysin O Mediates a Novel Entry Pathway of into Human Hepatocytes
- The Human Herpesvirus-7 (HHV-7) U21 Immunoevasin Subverts NK-Mediated Cytoxicity through Modulation of MICA and MICB
- Avirulence Effector Avr3b is a Secreted NADH and ADP-ribose Pyrophosphorylase that Modulates Plant Immunity
- Murid Herpesvirus-4 Exploits Dendritic Cells to Infect B Cells
- Unique Type I Interferon Responses Determine the Functional Fate of Migratory Lung Dendritic Cells during Influenza Virus Infection
- Evolution of a Species-Specific Determinant within Human CRM1 that Regulates the Post-transcriptional Phases of HIV-1 Replication
- Transcriptome Analysis of Transgenic Mosquitoes with Altered Immunity
- Antibody Evasion by a Gammaherpesvirus O-Glycan Shield
- UDP-glucose 4, 6-dehydratase Activity Plays an Important Role in Maintaining Cell Wall Integrity and Virulence of
- Protease-Resistant Prions Selectively Decrease Shadoo Protein
- A LysM and SH3-Domain Containing Region of the p60 Protein Stimulates Accessory Cells to Promote Activation of Host NK Cells
- Deletion of AIF Ortholog Promotes Chromosome Aneuploidy and Fluconazole-Resistance in a Metacaspase-Independent Manner
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Multiple Candidate Effectors from the Oomycete Pathogen Suppress Host Plant Immunity
- The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
- A TNF-Regulated Recombinatorial Macrophage Immune Receptor Implicated in Granuloma Formation in Tuberculosis
- SH3 Domain-Mediated Recruitment of Host Cell Amphiphysins by Alphavirus nsP3 Promotes Viral RNA Replication
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



