-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Pore-Forming Toxin Listeriolysin O Mediates a Novel Entry Pathway of into Human Hepatocytes
Intracellular pathogens have evolved diverse strategies to invade and survive within host cells. Among the most studied facultative intracellular pathogens, Listeria monocytogenes is known to express two invasins-InlA and InlB-that induce bacterial internalization into nonphagocytic cells. The pore-forming toxin listeriolysin O (LLO) facilitates bacterial escape from the internalization vesicle into the cytoplasm, where bacteria divide and undergo cell-to-cell spreading via actin-based motility. In the present study we demonstrate that in addition to InlA and InlB, LLO is required for efficient internalization of L. monocytogenes into human hepatocytes (HepG2). Surprisingly, LLO is an invasion factor sufficient to induce the internalization of noninvasive Listeria innocua or polystyrene beads into host cells in a dose-dependent fashion and at the concentrations produced by L. monocytogenes. To elucidate the mechanisms underlying LLO-induced bacterial entry, we constructed novel LLO derivatives locked at different stages of the toxin assembly on host membranes. We found that LLO-induced bacterial or bead entry only occurs upon LLO pore formation. Scanning electron and fluorescence microscopy studies show that LLO-coated beads stimulate the formation of membrane extensions that ingest the beads into an early endosomal compartment. This LLO-induced internalization pathway is dynamin-and F-actin-dependent, and clathrin-independent. Interestingly, further linking pore formation to bacteria/bead uptake, LLO induces F-actin polymerization in a tyrosine kinase-and pore-dependent fashion. In conclusion, we demonstrate for the first time that a bacterial pathogen perforates the host cell plasma membrane as a strategy to activate the endocytic machinery and gain entry into the host cell.
Published in the journal: . PLoS Pathog 7(11): e32767. doi:10.1371/journal.ppat.1002356
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002356Summary
Intracellular pathogens have evolved diverse strategies to invade and survive within host cells. Among the most studied facultative intracellular pathogens, Listeria monocytogenes is known to express two invasins-InlA and InlB-that induce bacterial internalization into nonphagocytic cells. The pore-forming toxin listeriolysin O (LLO) facilitates bacterial escape from the internalization vesicle into the cytoplasm, where bacteria divide and undergo cell-to-cell spreading via actin-based motility. In the present study we demonstrate that in addition to InlA and InlB, LLO is required for efficient internalization of L. monocytogenes into human hepatocytes (HepG2). Surprisingly, LLO is an invasion factor sufficient to induce the internalization of noninvasive Listeria innocua or polystyrene beads into host cells in a dose-dependent fashion and at the concentrations produced by L. monocytogenes. To elucidate the mechanisms underlying LLO-induced bacterial entry, we constructed novel LLO derivatives locked at different stages of the toxin assembly on host membranes. We found that LLO-induced bacterial or bead entry only occurs upon LLO pore formation. Scanning electron and fluorescence microscopy studies show that LLO-coated beads stimulate the formation of membrane extensions that ingest the beads into an early endosomal compartment. This LLO-induced internalization pathway is dynamin-and F-actin-dependent, and clathrin-independent. Interestingly, further linking pore formation to bacteria/bead uptake, LLO induces F-actin polymerization in a tyrosine kinase-and pore-dependent fashion. In conclusion, we demonstrate for the first time that a bacterial pathogen perforates the host cell plasma membrane as a strategy to activate the endocytic machinery and gain entry into the host cell.
Introduction
Despite the diversity of virulence factors promoting host cell invasion, only two major mechanisms of entry have been observed [1]–[3]. First, invasins on the bacterial cell surface bind to host cell receptors to activate complex signaling cascades that orchestrate the internalization of the bacterium. Second, some bacteria bypass the requirement for a host receptor by utilizing a secretion system that injects effectors into the host cell. The effectors subvert the host signaling machinery to trigger bacterial uptake into macropinosomes [4], [5]. Using Listeria monocytogenes as a model intracellular pathogen, we have analyzed a novel entry pathway that is activated in response to host cell perforation by a pore-forming toxin.
L. monocytogenes is a foodborne pathogen that causes a large spectrum of clinical manifestations ranging from gastroenteritis to life-threatening meningo-encephalitis and sepsis. Susceptible hosts include the elderly and immunocompromised individuals [6]. In pregnant women, the bacterium can cross the maternofetal barrier causing abortion, stillbirth, and neonatal meningitis or sepsis [6]–[8]. To cross the host barriers and infect various organs including the liver [9]–[12], L. monocytogenes expresses multiple virulence factors that induce its entry and survival into various nonphagocytic cells [13]–[16].
Two major genetic loci encode the virulence factors responsible for host cell invasion: the internalin operon and Listeria pathogenicity island 1 (LIPI-1) [17], [18]. The internalin operon encodes internalin (InlA) and InlB that bind to E-cadherin and the hepatocyte growth factor receptor (HGF-Rc/c-Met), respectively [19], [20]. Depending on the receptors expressed by the host cells, L. monocytogenes entry involves one or both internalins [21], [22]. The internalin/host receptor interactions activate signaling cascades within cholesterol-rich microdomains leading to the internalization of the bacterium [23]–[26]. After internalization, the secreted pore-forming toxin listeriolysin O (LLO) and two phospholipases (encoded by LIPI-1) mediate L. monocytogenes escape from the endocytic vesicle into the cytoplasm, where bacteria divide and undergo F-actin based motility to spread from cell to cell [27]–[29].
InlA and InlB were defined as bacterial invasins based upon their critical role in L. monocytogenes invasion of nonphagocytic cells, and the fact that they are sufficient to induce entry of noninvasive bacteria when overexpressed from a plasmid [17], [30]. It is well established that the internalins are critical for host cell invasion; however, they may not be sufficient for inducing efficient bacterial uptake due to their low levels of expression in L. monocytogenes. Additional virulence factors including LLO have been proposed to regulate L. monocytogenes entry into host cells [31]–[35].
LLO is required for L. monocytogenes pathogenesis [36] and belongs to the family of the cholesterol-dependent cytolysins (CDCs) produced by numerous Gram-positive pathogens [37]–[39]. The CDCs are 50–70 kDa proteins synthesized as water soluble monomers that bind to cholesterol in host cell membranes [40]. Three members of the CDCs, intermedilysin, lectinolysin and vaginolysin, have been shown to bind to a host receptor (the complement regulatory molecule CD59) in addition to cholesterol [41]–[43]. Upon binding to host membranes, the CDCs diffuse laterally to form a ring-shaped oligomeric prepore complex. This complex then inserts a large β-barrel pore across the membrane in a cholesterol-dependent fashion [44]. Eukaryotic cells possess sophisticated mechanisms to repair damaged plasma membranes and survive moderate exposure to pore-forming toxins including the CDCs [45]. A growing body of evidence demonstrates that pores formed by pore-forming proteins including perforin, S. aureus alpha hemolysin, and the CDC streptolysin O (SLO), are removed from the plasma membrane through a mechanism that involves membrane internalization [45]–[47]. The ability of CDCs to induce membrane internalization in eukaryotic cells to repair their membrane raised the hypothesis that LLO may affect the internalization of L. monocytogenes. In the present work we explored the link between the formation of pore complexes by LLO and bacterial internalization. Using several experimental approaches, we determined that LLO is a critical invasion factor that perforates the host cell plasma membrane to activate L. monocytogenes internalization into human hepatocytes.
Results
LLO is critical for efficient entry of L. monocytogenes into host cells
The gentamicin survival assay is commonly used to assess the role of virulence factors in the intracellular survival of bacterial pathogens. This assay enumerates viable intracellular bacteria after killing extracellular bacteria with the cell impermeant antibiotic gentamicin. We measured the relative intracellular survival of wild type L. monocytogenes (WT), along with an isogenic LLO-deficient (Δhly) mutant, a double deletion mutant deficient in the expression of InlA and InlB (ΔinlAB), a triple deletion mutant (ΔhlyΔinlAB), and the LLO-complemented mutant (Δhly + pAM401hly and ΔhlyΔinlAB + pAM401hly) strains in HepG2 cells. As presented in Fig. 1A, efficient intracellular survival of L. monocytogenes required the expression of the internalins as well as LLO. In the absence of the three virulence factors, intracellular survival was almost completely abrogated. The defect in intracellular survival of LLO-deficient bacteria (Δhly) was due to the lack of LLO expression, as the LLO-complemented (Δhly + pAM401hly) and WT strains displayed similar intracellular survival.
Fig. 1. LLO is required for efficient entry of L. monocytogenes into HepG2 cells. 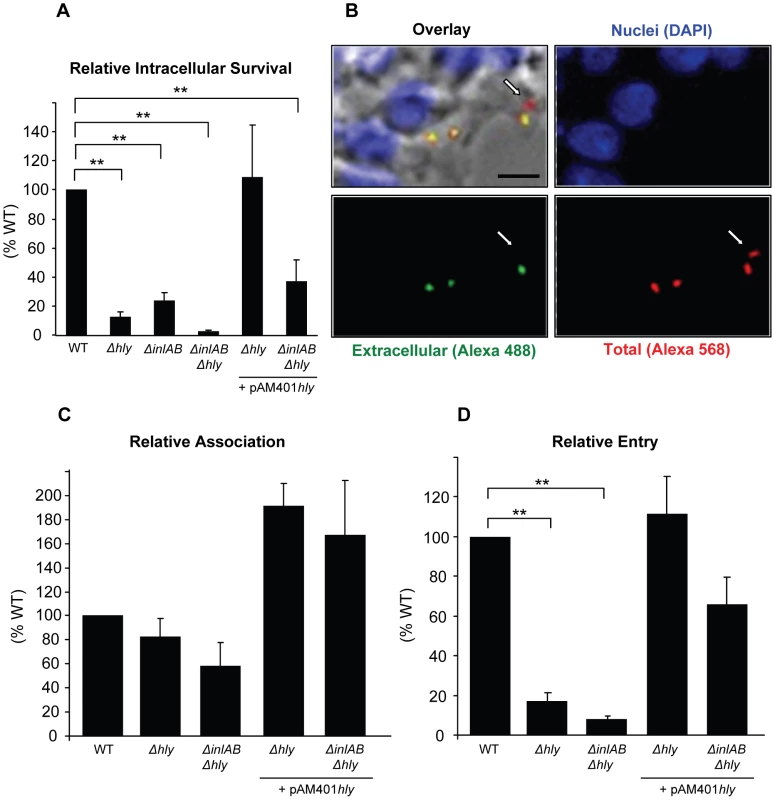
(A) HepG2 cells were infected with isogenic WT (DP10403S), LLO-deficient (Δhly), InlAB-deficient (ΔinlAB), LLO- and InlAB-deficient (ΔhlyΔinlAB), or LLO-complemented (Δhly + pAM401hly; ΔhlyΔinlAB +pAM401hly) bacteria (MOI = 20) for 30 min at 37°C. Gentamicin was added for 1 h and the CFUs were enumerated as described in methods. Results were the mean ± SEM (n≥3) and expressed relative to WT. Statistics indicated here and elsewhere are as follows: * p<0.05; ** p<0.005. (B) to (D) Cells were infected with WT, LLO-deficient (Δhly), LLO- and InlAB-deficient (ΔhlyΔinlAB), or LLO-complemented (Δhly + pAM401hly; ΔhlyΔinlAB + pAM401hly) bacteria (MOI = 20) for 30 min at 37°C. Cells were washed, fixed, and labeled with fluorescent antibodies and DAPI. (B) Representative images of WT extracellular bacteria (green), total bacteria (red), and HepG2 nuclei (blue). The arrow indicates an internalized bacterium. Scale bar = 10 µm. In (C) and (D), results were the mean ± SEM (n≥3) and were expressed relative to WT. The results obtained with the gentamicin assay reflect the efficiencies of several stages of host cell invasion including L. monocytogenes association with host cells, entry into host cells, escape from the internalization vesicle, and intracellular division. LLO is known to promote L. monocytogenes intracellular survival by mediating bacterial escape from the internalization vesicle; therefore, it was difficult to dissociate the role of LLO in escape from its potential role in bacterial association and/or entry using this assay. To specifically assess the role of LLO during the initial stages of the invasion process, we used an automated fluorescence-based assay that measures the efficiencies of bacterial association with and internalization into host cells [48]. We found that LLO and the internalins did not significantly affect bacterial association with HepG2 cells. However, LLO significantly increased bacterial association when overexpressed from a plasmid (Fig. 1C). More importantly, LLO is critical for L. monocytogenes internalization as we observed a marked decrease in entry of the LLO (Δhly) and LLO/internalins (ΔhlyΔinlAB) deficient strains relative to the WT strain (Fig. 1D). The LLO-complemented (Δhly + pAM401hly) and WT strains displayed similar internalization efficiencies (Fig. 1D). We also tested a second strain of L. monocytogenes (LO28) and its isogenic LLO-deficient mutant (LO28 hly::Tn917) and the human epithelial HeLa cells that are commonly used as a model to study the L. monocytogenes intracellular lifecycle. Again, we found that LLO is a key virulence factor that controls bacterial entry into host cells but does not significantly affect bacterial association with host cells (Fig. S1).
Direct and dose-dependent activity of LLO in L. monocytogenes entry into host cells
The lack of LLO in the LLO-deficient strains may indirectly decrease the efficiency of bacterial entry by affecting the expression or regulation of other virulence factors. To verify that the defect in entry was solely due to the absence of LLO, we used LLO-deficient bacteria coated with increasing concentrations of six-His tagged recombinant LLO. LLO was noncovalently adsorbed on the surface of LLO-deficient L. monocytogenes using a previously described protocol [49]. We performed a noncovalent coating because the toxin monomers likely need to dissociate from the bacterial surface to freely diffuse within the host cell membrane and form oligomers and pores. To validate the coating procedure, we measured the fluorescence intensity of bacteria coated with increasing concentrations of an Alexa 488 LLO derivative (Fig. S2). We then measured the entry of LLO-deficient L. monocytogenes coated with increasing concentrations of recombinant LLO and observed that LLO increased bacterial entry into HepG2 cells in a dose-dependent fashion (Fig. 2A). We next determined whether LLO should be localized in the vicinity of the bacteria or whether LLO could act distally to regulate entry. When adding LLO to the culture medium along with the LLO-deficient strain, we observed an increase in the efficiency of bacterial internalization (Fig. 2B). Together, these data show that LLO potentiates internalization of L. monocytogenes into host cells in a dose-dependent fashion by acting locally or from a distance.
Fig. 2. Direct and dose-dependent role of LLO in L. monocytogenes entry. 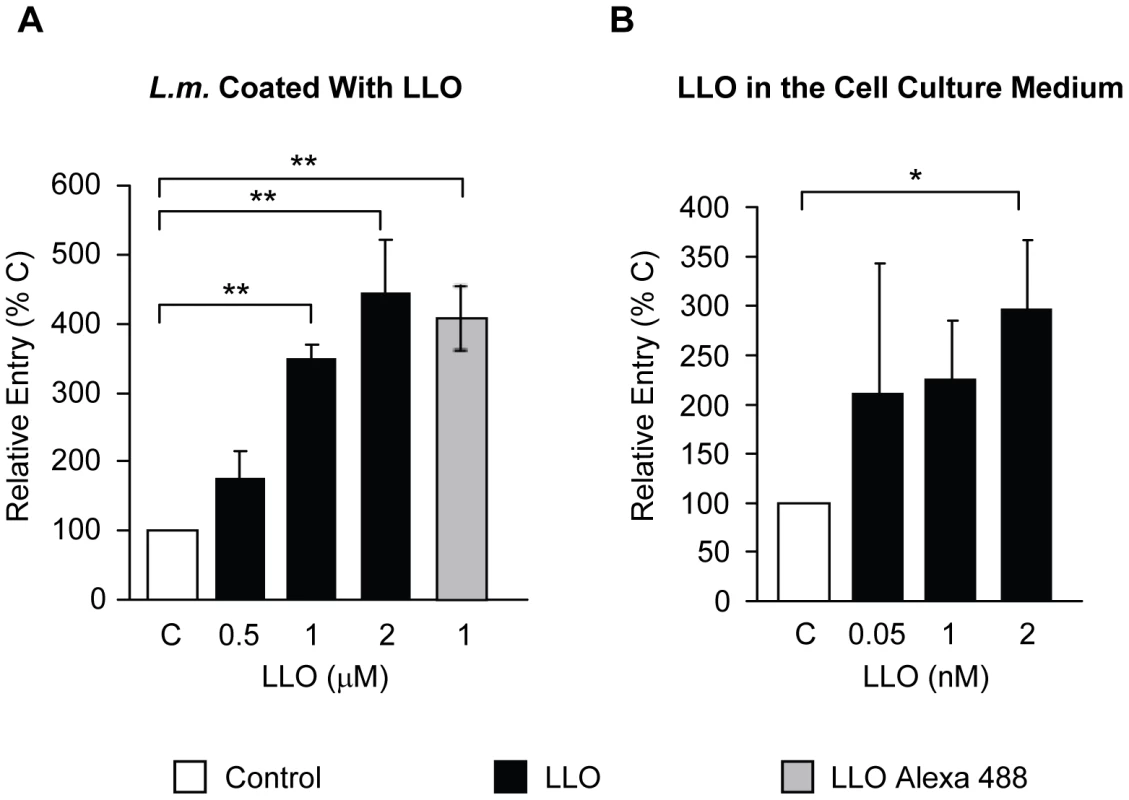
(A) LLO-deficient L. monocytogenes (Δhly, strain DPL2161, L.m.) treated with 1 mM nickel (II) chloride coating buffer in the absence (C) or presence of LLO (black bars) or LLO Alexa 488 (grey bar) were used to infect HepG2 cells at 37°C for 30 min (MOI = 20). (B) HepG2 cells were infected with LLO-deficient L. monocytogenes (Δhly, DPL2161) at 37°C for 30 min (MOI = 20), in the absence (C) or in the presence of LLO added exogenously to the cell culture medium (black bars). In (A) and (B) bacterial entry was measured by fluorescence microscopy. The results were expressed relative to bacteria incubated with host cells in the absence of LLO (C) and were the mean ± SEM (n≥3). LLO is sufficient to induce bacterial and bead entry into host cells
We determined whether LLO is sufficient to induce bacterial entry into host cells or whether it only potentiates the internalins' activity. We used nonpathogenic and noninvasive Listeria innocua that does not express any of the known L. monocytogenes virulence factors [50]. As shown in Fig. 3A, L. innocua noncovalently coated with six-His tagged LLO were able to enter and survive in HepG2 cells. As a second approach, L. innocua were transformed with a plasmid coding for hly (L. innocua phly/prfA*). As presented in Fig. 3B, LLO secreted from L. innocua phly/prfA* was sufficient to induce bacterial entry and survival into HepG2 cells. The efficiency of host cell invasion by L. innocua phly/prfA* was low compared to what was observed with WT L. monocytogenes and LLO-coated L. innocua (Fig. 3A). Because the activity of LLO in bacterial entry is dose-dependent (Fig. 2), we determined whether L. innocua phly/prfA* expresses low levels of LLO compared to WT L. monocytogenes. The hemolytic activity of L. innocua phly/prfA* was approximately 40-fold lower than L. monocytogenes (Fig. 3C). L. innocua phly/prfA* also produced low levels of LLO compared with WT L. monocytogenes (Fig. 3D). These results demonstrate that low concentrations of LLO (below the concentration secreted by L. monocytogenes) are sufficient to induce bacterial entry into host cells. To assess the role of LLO in the absence of any other bacterial factor, we measured the uptake of 1 µm fluorescent polystyrene beads coated with LLO. The beads were first covalently coated with bovine serum albumin (BSA). The BSA-coated beads were then noncovalently coated with LLO [49]. LLO was sufficient to induce internalization of the beads into HepG2 cells in a dose-dependent fashion; whereas, the beads coated with only BSA were not taken up by the cells (Fig. 4A and B). This result clearly showed for the first time that a pore-forming toxin is a bacterial invasion factor. Cell viability was assessed by measuring the release of lactate dehydrogenase (LDH) and by trypan blue exclusion immediately and 24 h following toxin treatment (Fig. S3). These results show that the concentrations of LLO that lead to the optimal bead uptake by host cells do not compromise cellular integrity.
Fig. 3. LLO is sufficient to induce the entry of noninvasive L. innocua into HepG2 cells. 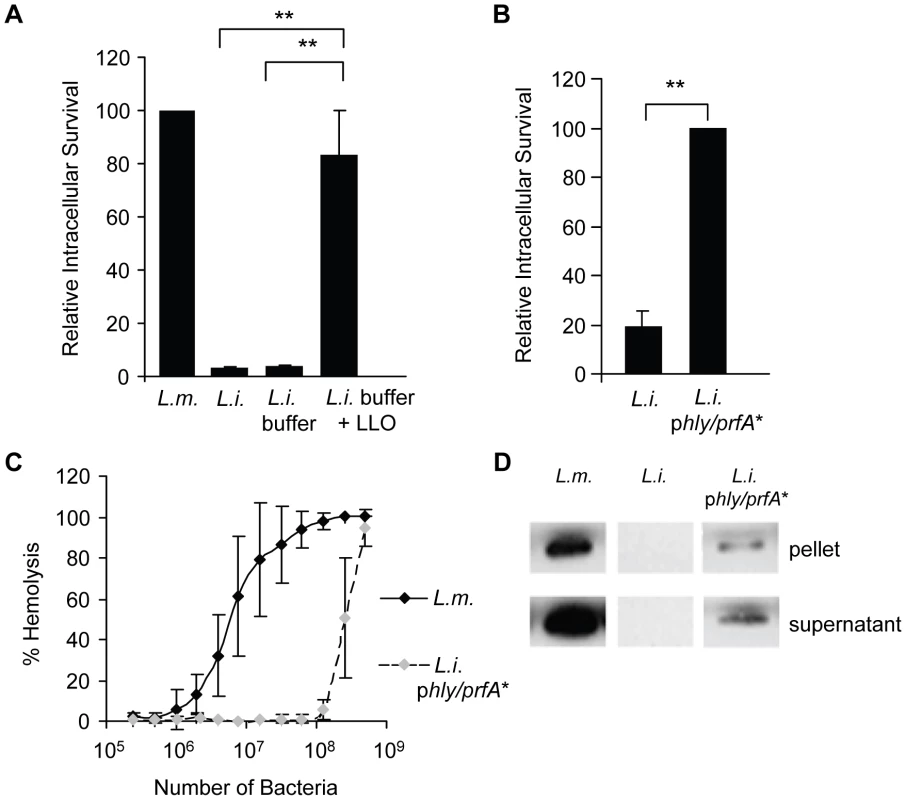
(A) HepG2 cells were infected with WT L. monocytogenes (DP10403S, L.m.), L. innocua (L.i.), or L. innocua treated with 1 mM nickel (II) chloride coating buffer in the absence (L.i. buffer) or presence of 5 µM LLO (L.i. buffer + LLO) at MOI = 20 for 30 min at 37°C. Gentamicin was added for 1 h and the intracellular CFUs were enumerated. Results were the mean ± SEM (n≥3) and expressed relative to L. monocytogenes. (B) HepG2 cells were infected with L. innocua (L.i.) or L. innocua phly/prfA* (L.i. phly/prfA*) for 60 min at 37°C (MOI = 100). Gentamicin was added for 30 min and the intracellular CFUs were enumerated. Results were the mean ± SEM (n≥3) and expressed relative to L. i. phly/prfA*. (C) Hemolytic activities of L. monocytogenes (DP10403S) and L. innocua phly/prfA* measured at 37°C for 30 min, pH 7.4. Results were the mean ± SEM (n≥3). (D) Equivalent amounts of L. monocytogenes and L. innocua lysates and proteins precipitated from their culture supernatants were analyzed by western blotting using an anti-LLO primary antibody. A representative experiment (of 3) is presented. Fig. 4. LLO is sufficient to induce entry of polystyrene beads into HepG2 cells. 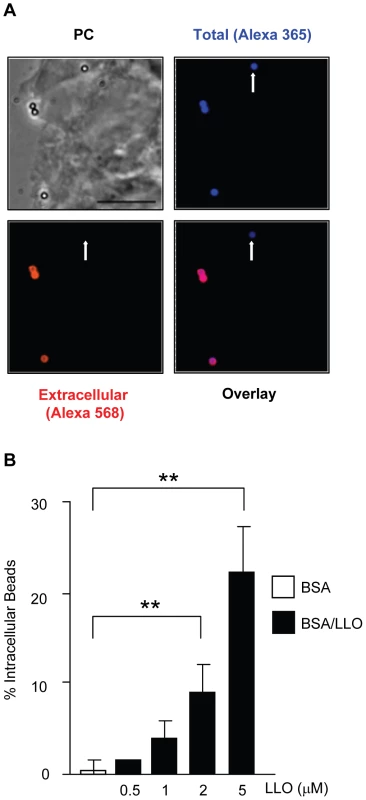
HepG2 cells were incubated with BSA- or BSA/LLO-coated beads for 30 min at 37°C (MOI = 20). Cells were washed, fixed, and extracellular beads were fluorescently labeled with a primary anti-BSA antibody and a secondary fluorescent antibody. (A) Representative fluorescence and phase-contrast (PC) images acquired with a 100 X objective. Scale bar = 10 µm. (B) The mean ± SEM (n≥3) percentage of intracellular beads was determined by counting a minimum of 100 beads in each sample. LLO perforates host cells at physiological temperature and pH
Previous studies have established that pore-formation by LLO is pH sensitive at the host temperature (37°C), with LLO being more active at acidic pH. At 37°C, soluble LLO is inactivated by a pH-triggered unfurling of the domain 3 twin α-helical bundles that prevents further formation of a pore [51]. We hypothesized that the pH and temperature dependent inactivation would not occur if LLO was incubated in the presence of host membranes. Presumably, LLO would bind to the cell membrane where it would assemble into functional pores rather than unfolding in a nonproductive fashion. This hypothesis was based upon the observation that LLO is active when added to a solution of erythrocytes at 37°C and neutral pH (all of our hemolytic assays were carried out at 37°C, pH 7.4). To test this hypothesis, we pre-incubated LLO for 20 min in the absence of host cell membranes (in PBS at 37°C, pH 7.4 or 5.5) before performing the hemolytic analysis at 37°C (pH 7.4 or 5.5). Consistent with the previous observation of Schuerch et al. [51], pre-incubating the toxin at neutral pH and 37°C inactivated LLO; whereas, at acidic pH some activity of the toxin was retained (Fig. 5A). If, however LLO was pre-incubated at 4°C for 20 min before performing the hemolytic assay at 37°C, LLO retained its activity at pH 7.4 and 5.5 (Fig. 5B). These data show that soluble monomers of LLO are inactivated at 37°C and pH 7.4 in the absence of membrane, but in the presence of membranes LLO can rapidly bind to the membrane and form pores.
Fig. 5. Extracellular LLO perforates erythrocytes and HepG2 cells at 37°C, pH 7.4. 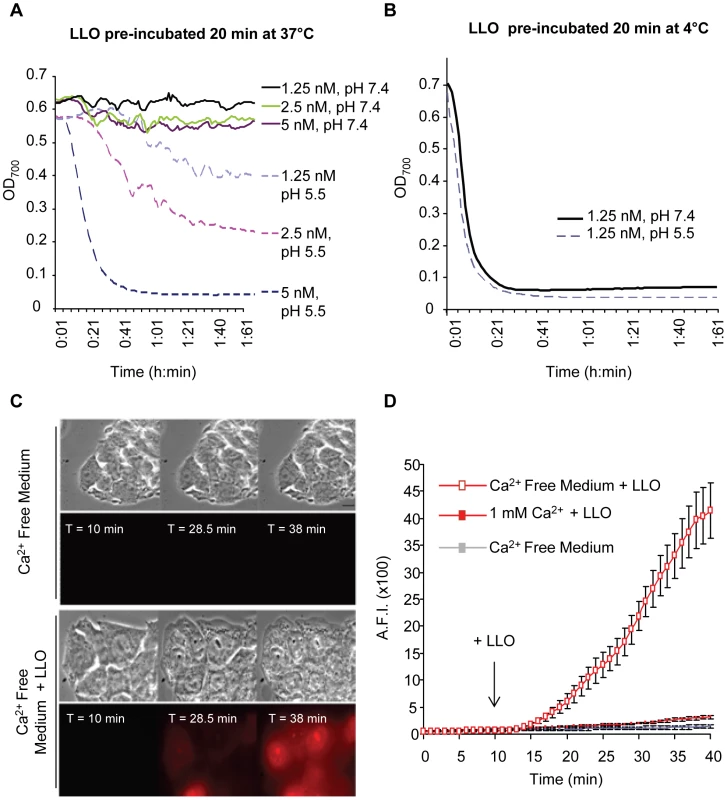
LLO was diluted in PBS pH 7.4 or 5.5 at 4°C in a 96 well plate (20 µl toxin in each well). (A) The plate was incubated at 37°C for 20 min, then, 180 µl of a 37°C suspension of sheep erythrocytes (0.25%) in PBS pH 7.4 or 5.5 was added to each well. (B) The plate was kept on ice for 20 min, then, 180 µl of a 37°C suspension of erythrocytes (0.25%) in PBS pH 7.4 or 5.5 was added to each well. In (A) and (B) following addition of erythrocytes, the plates were incubated at 37°C in a Power Wave 340 spectrophotometer (Bio-Tek) and absorbance (700 nm) was acquired every min [102]. Results show representative experiments (of 3), each performed in duplicate. In (C) and (D), perforation of HepG2 cells was measured by quantitative live cell fluorescence microscopy. HepG2 cells were incubated on the microscope stage at 37°C for 40 min with ethidium homodimer and in the presence or absence of 1 mM calcium. Phase contrast and fluorescence images were recorded at regular time intervals using a 100X objective and LLO was added after 10 min of incubation. Results were expressed as the averagefluorescence intensity (in arbitrary units) in the cells ± SEM of 5 movies for each condition. Scale bar = 10 µm. In response to membrane perforation by pore-forming toxins, host cells reseal their membranes using a repair process that is activated upon the influx of extracellular calcium [45]. To further demonstrate that low concentrations of LLO efficiently perforate HepG2 cells at physiological temperature and pH, we measured host cell perforation in the presence and absence of extracellular calcium. Membrane perforation was quantified by fluorescence imaging using the membrane impermeant dye ethidium homodimer. In solution this dye is weakly fluorescent, but once host cells are perforated, it enters the cells and associates with nucleic acids, which increases its fluorescence quantum yield. As shown in Fig. 5C and D, a baseline level of fluorescence was detected with host cells exposed to ethidium homodimer in calcium-free buffer at 37°C, pH 7.4 (Videos S1 and S2). LLO induces a massive entry of ethidium homodimer into cells incubated in calcium-free buffer (Videos S3 and S4), but not in the presence of 1 mM extracellular calcium. The extensive perforation of host cells was not due to the toxicity of ethidium homodimer, as in its absence LLO induces cell swelling and lysis with similar kinetics (Video S5). In total, these results showed that at 37°C, extracellular LLO efficiently perforates host cells at neutral pH.
Construction and characterization of novel LLO variants unable to form pores
To determine the importance of toxin oligomerization and pore formation in LLO-induced bacterial and bead entry into host cells, we constructed LLO derivatives locked at different stages of the pore-forming mechanism. Studies performed with the CDC perfringolysin O showed that the introduction of two cysteines at specific locations to form an intramolecular disulfide bond inhibits the hemolytic activity of the toxin [52], [53]. Depending on its location, the disulfide bond impedes the conformational remodeling required for formation of oligomers and/or pores, while toxin binding to host membranes is unchanged [52]. Based on these studies, we constructed and characterized LLOmL (monomer-locked) and LLOpL (prepore-locked). LLOmL was expected to bind to host membranes as a monomer unable to rearrange into a prepore complex. LLOpL was expected to bind to host membranes and oligomerize to form a stable prepore complex that cannot undergo the final transition into a pore. Neither mutant exhibited detectable hemolytic activity up to concentrations of 50 µM (higher concentrations were not tested). The loss of activity was due to the formation of the disulfide bonds, as reduction by dithiothreitol (DTT) fully restored the native hemolytic activity of the toxins (Fig. 6A). We next determined the oligomerization state of the toxins associated with erythrocyte membranes. As expected, LLO and LLOpL formed detergent-resistant high molecular weight complexes, whereas LLOmL failed to form such oligomers (Fig. 6B). Importantly, the addition of DTT unlocked LLOmL, as shown by the formation of detergent-resistant high molecular weight oligomers. Finally, the arrangement of the toxins associated with cholesterol-rich lipid layers was analyzed by transmission electron microscopy (Fig. 6C). LLO and LLOpL formed characteristic arc - and ring-shaped oligomers with a diameter of ∼50 nm in the presence and absence of DTT. LLOmL formed short linear assemblies that may be representative of the early stages of toxin oligomerization before formation of the prepore complex. Importantly, LLOmL formed the typical arc - and ring-shaped oligomers when it was reduced by DTT.
Fig. 6. Characterization of the LLO variants. 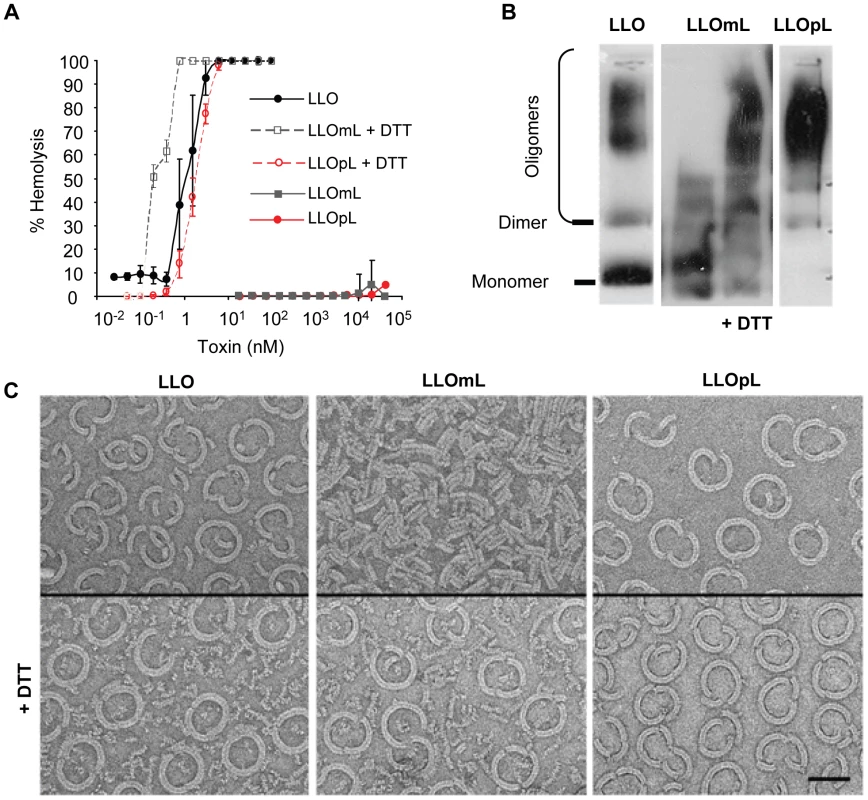
(A) Hemolytic activity of LLO, LLOmL, and LLOpL was measured at pH 7.4, 37°C. DTT = dithiothreitol. (B) LLO, LLOmL, and LLOpL were incubated with erythrocyte ghost membranes, in the presence or absence of DTT, washed and suspended in 1% detergent. The samples were subjected to gradient electrophoresis, in the presence of 1% detergent, and LLO was detected by western blotting using anti-LLO antibodies. (C) Toxins were incubated for 1 h on cholesterol/DOPC lipid layers in the presence or absence of DTT. The samples were processed and analyzed by transmission electron transmission microscopy. Scale bar = 50 nm. LLO-induced bacterial and bead entry requires host cell membrane perforation
We determined the role of LLO oligomerization into prepore and pore complexes in bacterial and bead entry into HepG2 cells. Native LLO induced bacterial and bead internalization, whereas neither LLOmL - nor LLOpL-coated beads were taken up by the cells (Fig.7A and B) even though all three bind to host cells with similar efficiency (Fig. 7C). We also used anti-LLO neutralizing antibodies, which were previously shown to prevent formation of LLO pores [54], to block LLO-mediated uptake of beads. The LLO neutralizing antibodies markedly inhibited LLO hemolytic activity and bead internalization, whereas control anti-LLO antibodies did not (Fig. 7D and E). The prepore to pore conversion of the CDCs is known to require high concentrations of membrane cholesterol [55]. We therefore prevented the formation of membrane pores by depleting host cholesterol using the cholesterol chelating agent methyl β-cyclodextrin (MβCD). Cholesterol depletion completely abrogated LLO-induced host cell perforation and the entry of BSA/LLO-coated beads into HepG2 cells (Fig. 7F and G). To demonstrate that inhibition of bead entry and host cell perforation was specifically due to cholesterol depletion and not to a secondary effect of the MβCD, we show that cholesterol repletion restored bead uptake and membrane perforation by LLO (Fig. 7F and G).
Fig. 7. Formation of pore complexes is required for efficient bacterial and bead entry into HepG2 cells. 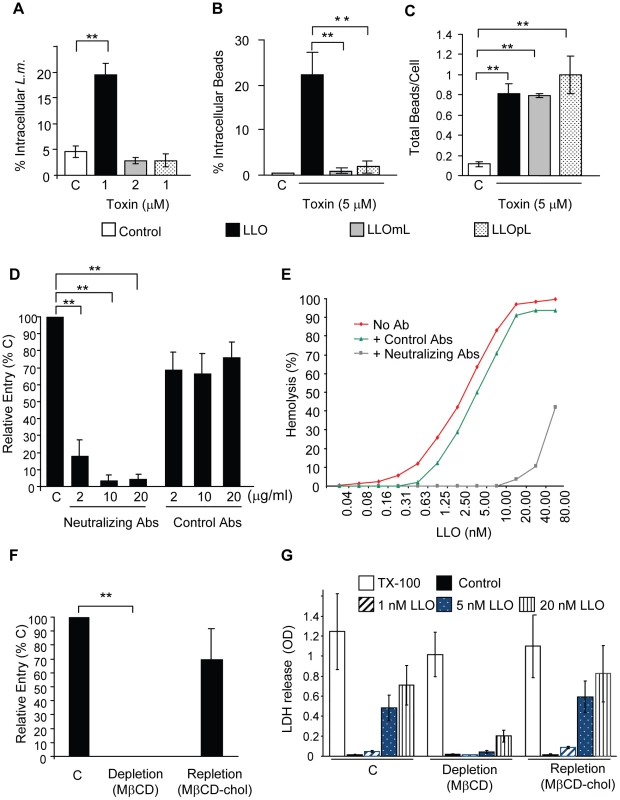
(A) LLO-deficient L. monocytogenes (Δhly, L.m.) were treated with coating buffer in the absence (white bars, C), or presence of LLO, LLOmL, or LLOpL. Cells were infected at 37°C for 30 min (MOI = 20). Samples were washed, fixed, and fluorescently labeled to enumerate bacterial entry by fluorescence microscopy. Results were the mean ± SEM (n≥3). (B) and (C) HepG2 cells were incubated with BSA- (Control; C) or BSA/toxin (LLO, LLOmL, or LLOpL)-coated beads for 30 min at 37°C (MOI = 20). Cells were washed, fixed, and labeled to enumerate bead entry (B) and association (C) by fluorescence microscopy. Results were the mean ± SEM (n≥3). (D) HepG2 cells were incubated with BSA/LLO-coated beads for 30 min at 37°C, in the presence or absence (C) of LLO-neutralizing or control antibodies. Cells were washed, fixed, and fluorescently labeled. Bead entry was measured by fluorescence microscopy and the results, mean ± SEM (n≥3) were expressed relative to the control (C). (E) Representative LLO hemolytic curves in the presence or absence (No Ab) of the neutralizing or control antibodies (10 µg/ml) were performed at pH 7.4, 37°C. (F) Control (C), cholesterol-depleted and -repleted HepG2 cells were incubated with BSA/LLO-coated beads for 30 min at 37°C. Bead entry was measured by fluorescence microscopy and the results, mean ± SEM (n≥3) were expressed relative to the control. (G) Control (C), cholesterol-depleted and -repleted HepG2 cells were incubated with various concentrations of LLO, 0.2% TX-100, or MEM (Control) at 37°C for 30 min. LDH release was measured using the TOX7 assay kit. Results are the mean ± SEM (n≥3). We also observed that BSA/LLO-coated beads formed pores in host cell membranes as detected by propidium iodide incorporation (Fig. S4A and B). Membrane perforation is a key event in LLO-induced entry. Therefore, we hypothesized that a heterologous CDC should also increase L. monocytogenes internalization. LLO-deficient L. monocytogenes were coated with recombinant six-His tagged pneumolysin (PLY), the CDC produced by Streptococcus pneumoniae [56] that exhibited a similar hemolytic activity to LLO (Fig. S5A). Like LLO, PLY was able to mediate L. monocytogenes entry into HepG2 cells (Fig. S5B). These data suggested that the LLO structure did not contain unique features that were required to induce bacterial invasion.
LLO activates a clathrin-independent, but dynamin-dependent internalization pathway
To demonstrate that host cell perforation by LLO leads to the formation of a micron sized internalization vesicle, we analyzed plasma membrane remodeling at the bead entry site. Scanning electron microscopy images showed the formation of plasma membrane extensions entrapping the BSA/LLO-coated beads after 15 min at 37°C. Within 30 min, we observed an increase in the number of beads completely enveloped by the plasma membrane (Fig. 8A), whereas, the formation of membrane extensions was not induced by the BSA-coated beads. To demonstrate that LLO induces entry of BSA/LLO-coated beads within a membrane-bound internalization vesicle, we quantified intracellular beads that colocalize with the early endosomal marker EEA1. After 30 min at 37°C, we observed that a large population of intracellular beads was localized in endosomes to which the EEA1 marker had been recruited (Figs. 8B and C). We further characterized the endocytic molecules involved in this pathway. We measured the entry of BSA/LLO-coated beads in cells transfected with clathrin heavy chain silencing RNA and in cells treated with the clathrin inhibitor chlorpromazine, or with dynasore, a dynamin inhibitor (Fig. 9A and B). The uptake of fluorescent transferrin was measured as a control for the inhibition of clathrin - and dynamin-dependent endocytosis (Fig. 9C). The results show that internalization of BSA/LLO-beads is dynamin-dependent and clathrin-independent.
Fig. 8. LLO-coated beads are internalized into EEA1 positive endosomes. 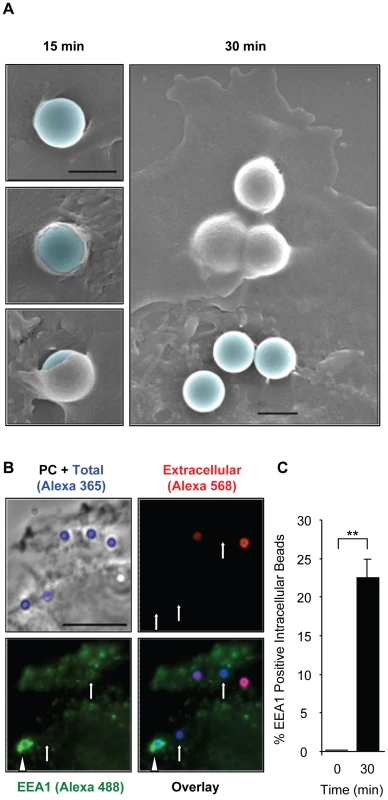
(A) HepG2 cells were incubated at 37°C with BSA/LLO-coated beads for 15 or 30 min, washed, fixed, and processed for scanning electron microscopy analysis. Scale bar = 1 µm. (B) HepG2 cells were incubated for 30 min at 37°C with BSA/LLO-coated beads, washed, fixed, and the extracellular beads were labeled with anti-BSA antibodies and a fluorescent secondary antibody (red). After permeabilization, EEA1 was labeled using anti-EEA1 antibodies and a fluorescent secondary antibody (green). Scale bar = 10 µm. PC = phase contrast. Arrows point out internalized beads and the arrowhead an internalized bead that massively recruited EEA1. (C) Results were expressed as the mean ± SEM (n≥3) percentage of intracellular beads that recruited EEA1. Fig. 9. LLO-coated beads are internalized by a clathrin-independent, dynamin-, F-actin- and tyrosine kinase-dependent pathway. 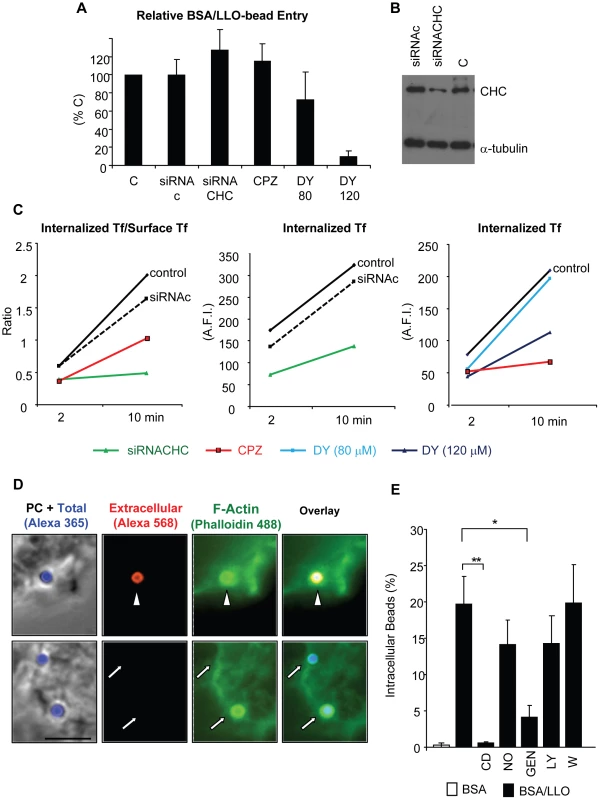
(A) to (C) Clathrin heavy chain was knocked down in HepG2 cells by siRNA (siRNACHC) treatment or was inhibited by pre-incubating the cells for 30 min with 10 µM chlorpromazine (CPZ). Dynamin was inhibited by pre-incubating the cells for 30 min with 120 µM dynasore (DY). CPZ and DY were maintained in the cell culture medium throughout the experiments. (A) HepG2 cells were incubated with BSA/LLO-coated beads for 30 min at 37°C. Cells were washed, fixed, and bead entry was enumerated. Results were the mean ± SEM (n≥3) and were expressed relative to the control (C). (B) Representative western blot analysis of clathrin heavy chain (CHC) in cells treated with scrambled siRNA (siRNAc), specific siRNA (siRNACHC), or control untreated cells (C). The α-tubulin was used as a loading control. (C) Measurement of internalized transferrin (Tf)/Surface associated (Tf) and internalized (Tf) in control untreated cells (C), cells treated with siRNAc or siRNACHC, and cells treated with CPZ or DY. Only internalized (Tf) was measured in DY-treated cells due to DY fluorescence. Representative experiments (of 3) are shown. (D) HepG2 cells were incubated for 30 min with BSA/LLO-coated beads, fixed, and extracellular beads were labeled using anti-BSA antibodies and secondary fluorescent antibodies (red) and F-actin was labeled with fluorescent phalloidin (green). Scale bar = 5 µm, PC = phase contrast. The arrowhead and arrows point out extracellular and internalized beads, respectively. (E) HepG2 cells were pre-incubated in the presence or absence of cytochalasin D (CD), nocodazole (NO), genistein (GEN), LY294002 (LY), or wortmannin (W) at 37°C. Beads were added for 30 min in the presence of the inhibitors. The percentage of internalized beads was enumerated by fluorescence microscopy and the results were the mean ± SEM (n≥3). F-actin remodeling is induced by LLO in a pore-dependent fashion and is required for bead internalization
Internalization of large particles such as bacteria generally requires the rearrangement of subcortical F-actin to form membrane extensions that engulf the particles [57]. Consistent with this idea, the membrane rearrangements observed by scanning electron microscopy (Fig. 8A) were accompanied by the recruitment of F-actin at the bead entry site (Fig. 9D). Furthermore, bead entry was inhibited in cells treated with the F-actin depolymerizing drug cytochalasin D; whereas, microtubule integrity was not required for entry (Fig. 9E). F-actin remodeling involves the activation of the host signaling machinery. As a first approach to determine the transducers involved in entry, we have treated cells with inhibitors of tyrosine kinases (genistein) and phosphoinositide 3-kinases (PI3Ks) (LY294002 and wortmannin). Tyrosine kinases and PI3Ks are key transducers activated upstream from actin polymerization at the L. monocytogenes entry site [13]. We found that only tyrosine kinase(s) activation was critical for LLO-dependent entry (Fig. 9E). We also observed that purified LLO induces membrane ruffling in the 0.5 to 2 nM concentration range (representative Videos S6 and S7). Membrane ruffling started 117±14.3 sec after the addition of 1.2 nM LLO and was optimal for 555±145 sec (calculated from 10 movies). This provided a convenient experimental model to determine whether pore formation and tyrosine kinases were involved in F-actin polymerization induced by LLO. LLO-induced membrane ruffling was F-actin - and tyrosine kinase-dependent as no ruffling was observed in cells stimulated by LLO in the presence of 0.5 µg/ml cytochalasin D or 250 µM genistein (Videos S8 and S9). Importantly, membrane ruffling was only induced by LLO, but not by 0.5 to 50 nM LLOpL (Video S10). These data provide a link between membrane perforation by LLO and the remodeling of the F-actin cytoskeleton.
Discussion
These studies revealed the existence of a novel pathway exploited by L. monocytogenes to gain entry into host cells. This pathway is activated in response to membrane perforation by the pore-forming toxin listeriolysin O (LLO). This is the first demonstration that a pore-forming toxin is able to induce the internalization of a bacterial pathogen into host cells. We have used several approaches to show that LLO is crucial for efficient entry of L. monocyogenes into HepG2 and HeLa cells and have used beads coated with LLO to specifically decipher the molecular machinery underlying this novel pathway.
LLO is known to mediate L. monocytogenes escape from the endocytic vesicle following bacterial internalization into host cells [27]. The present findings demonstrate that LLO is also critical for L. monocytogenes internalization. Moreover, they show that LLO is sufficient to induce bacterial entry. A role for LLO in bacterial entry into nonphagocytic cells was previously proposed [35], whereas other studies did not identify such a role for LLO [58]. All of these studies relied upon the gentamicin survival assay that measures intracellular survival. The gentamicin assay is a powerful method, but it exhibits some limitations. First, it reports several stages of the host cell invasion process and cannot distinguish the role of LLO in bacterial entry from its role in intracellular survival. Second, LLO perforates host cells and likely allows gentamicin entry into the cells. As a result, the intracellular survival of WT L. monocytogenes is underestimated in comparison to a LLO-deficient mutant. In the gentamicin assay performed in the present study, a low MOI (20) was used as higher amounts of bacteria led to substantial entry of gentamicin into the cells (MW 480), as reported by the incorporation of the cell impermeant dye ethidium homodimer (MW 857) (our unpublished data). Therefore, we developed an automated fluorescence-based assay to specifically and accurately measure bacterial association and entry [48]. With this approach, we demonstrate that LLO plays a critical role in bacterial entry, but not in bacterial association with host cells. Similar to LLO, we found that InlA and InlB did not significantly affect bacterial association, but affected bacterial intracellular survival and entry. This result is not surprising due to the abundance of adhesins expressed by L. monocytogenes, as over ten surface adhesins have been identified [31], [59]–[65]. When overexpressed, LLO significantly increased L. monocytogenes association with host cells. This result is in accordance with the observation that LLO promotes Bacillus subtilis attachment to epithelial cells [66]. LLO and other CDCs were also shown to remain partially bound to the bacterial cell wall [67], [68]. Therefore, when overexpressed, the cell wall-associated toxin likely anchors the bacteria to host cells via binding to membrane cholesterol. Indeed, LLO is well known to bind to cholesterol in biological and artificial membranes [40], [69].
Our study focused on elucidating the role of LLO in bacterial entry into host cells. We first investigated whether host cell perforation by LLO was required for activating this entry pathway. Although pore-formation by LLO is pH-sensitive at 37°C, we demonstrated that extracellular LLO perforates host cells at neutral pH. This finding is supported by a previous study that also concluded that LLO is active at neutral and slightly basic pH values [69]. We constructed LLO variants to determine if LLO binding to host membranes, its oligomerization into a prepore complex, and pore formation are required for LLO-induced bacterial entry. The LLO variant unable to undergo the prepore to pore transition demonstrated that the formation of LLO pore complexes is a key event for bacterial and bead internalization into host cells. How does pore-formation by LLO stimulate bacterial or bead uptake? The LLO entry pathway appears to be distinct from the canonical bacterial entry pathways. Bacteria are known to induce their entry by activating host receptors or by injecting effectors into the host cell cytosol [1], [2], [70]. Gram-positive bacteria do not have a type III secretion system, although, they can produce CDC toxins to mediate the translocation of virulence factors [71], [72]. The observation that LLO alone was sufficient to induce bacterial or bead entry ruled out this mechanism. The requirement for membrane perforation does not favor the simple model in which LLO acts by activating a signaling host receptor. Indeed, the LLOpL variant that binds to host cells and is able to rearrange into a prepore complex failed to induce bacterial entry. Also, another pore-forming toxin, PLY, could replace LLO in that function. We do not rule out the existence of a yet unknown receptor for LLO that would be shared by PLY, nevertheless, membrane perforation remains a key trigger for entry.
LLO induces the formation of internalization vesicles that accommodate large particles (bacteria or 1 µm beads) via a cholesterol-, dynamin-, and F-actin-dependent, but clathrin-independent pathway. Several clathrin-independent and dynamin-dependent internalization pathways have been described including lipid raft-dependent pathways [73]. It is important to note that the role of cholesterol in this pathway might be complex, as cholesterol is a structural component of lipid rafts and is critical for LLO binding to host membranes and the formation of LLO pores. However, the observation that LLO associates with and induces the coalescence of lipid raft microdomains favors the hypothesis of a lipid raft-mediated pathway [74]. We found that LLO induces the polymerization of actin at the bead entry site, and that F-actin dynamics and tyrosine kinase activation are required for LLO-mediated bead entry. Using soluble LLO and LLOpL, we observed that membrane perforation by LLO induces F-actin-dependent membrane ruffling. Interestingly, F-actin polymerization within membrane ruffles was tyrosine kinase-dependent. Together, our findings support a model in which host cell perforation by LLO leads to an internalization pathway that involves host tyrosine kinase-dependent stimulation of the actin cytoskeleton and the activity of dynamin. Similar to LLO, PLY promoted bacterial entry into host cells and was shown to induce actin polymerization in neuroblastoma cells [75].
The fact that LLO induces internalization of bacteria in a pore-dependent fashion is reminiscent of the membrane repair pathway observed in eukaryotic cells exposed to pore-forming toxins. In response to the attack by pore-forming proteins, eukaryotic cells undergo membrane endocytosis to remove the pores from their plasma membranes [45], [47]. We identified host cell effectors, F-actin and dynamin, that are required for LLO-induced particle internalization but are dispensable for membrane repair (Fig. S6) [45]. In conclusion, membrane repair is likely a prerequisite for LLO-induced bacteria/bead entry, but the LLO-mediated entry pathway extends beyond or is distinct from the membrane repair pathway.
The mechanisms evolved by L. monocytogenes to gain entry into nonphagocytic cells are complex and involve several invasins such as InlA, InlB, and LLO. The inlA, inlB, and hly genes are controlled by the central regulator of virulence genes, PrfA, and are highly up-regulated in vitro and in vivo during L. monocytogenes infection [76], [77]. Therefore, LLO is expressed together with InlA and InlB to mediate host cell invasion. Depending on the receptors expressed by host cells, InlA and InlB stimulate bacterial entry individually or in concert [13], [21]. LLO likely affects bacterial internalization in a large panel of cells because its major host receptor is cholesterol. We used hepatocytes (HepG2 cells) that express the InlA and InlB receptors, E-cadherin and the HGF-Rc, respectively [78], [79]. We also used HeLa cells that do not express E-cadherin and are only permissive to the InlB entry pathway [30]. Our results showed that LLO played a critical role in L. monocytogenes internalization into both cell lines. Therefore, LLO cooperation with InlB alone or with InlA and InlB is critical for bacterial internalization. Our study on LLO and previous studies on InlA and InlB showed that these molecules are individually sufficient to induce bacterial entry with high efficiency when overexpressed from a plasmid or coated on beads [17], [20], [80]. However, the expression level of these molecules is low in L. monocytogenes and their concerted activity likely ensures efficient bacterial uptake by host cells. The literature shows that most stages in the infectious lifecycle of a pathogenic bacterium involve numerous factors, all working in concert [21], [81], [82]. A recent study demonstrated the importance of the cooperation between InlA and InlB during L. monocytogenes entry into host cells [21]. In this study it was shown that InlA ensured the specificity of bacterial recognition of intestinal cells, whereas InlB increased the InlA internalization rate. Likewise, we speculate that cooperation between LLO, InlA, and InlB occurs during host cell invasion. In this model, the three pathways would contribute simultaneously to the uptake of a given bacteria. Given the identified roles of LLO, InlA, and InlB in stimulating actin polymerization and endocytosis, cooperation between the three invasins likely involves both of these processes. The LLO-dependent entry pathway displays differences and similarities with the InlA and InlB pathways. They differ with respect to the mechanism used to activate the host cells, as membrane perforation is required in the LLO but not in the InlA and InlB pathways. Also, clathrin is involved in the InlA and InlB pathways [83], but not in the LLO pathway. However, the internalization induced by the three invasins shares common effectors such as host tyrosine kinases, dynamin, cholesterol, and F-actin [13]. Determining how host cells integrate the signals simultaneously generated by each invasin and whether the resulting pathway is the sum of the individual pathways or is a new pathway leading to efficient bacterial uptake constitutes an important goal for future research.
Pore-forming toxins are produced by numerous pathogens and may influence their uptake by host cells [84], [85]. In favor of this hypothesis, while this work was under revision, it has been published that the parasite Trypanosoma cruzi invades host cells by exploiting a host cell membrane repair mechanism in response to membrane damage [86]. Among CDC-producing bacteria, the genera Listeria, Arcanobacterium, Bacillus, and Streptococcus include several pathogenic bacteria that have been shown to invade nonphagocytic cells [87]–[92]. Interestingly, the CDC intermedilysin (ILY) is required for internalization of Streptococcus intermedius [88]. However, not all of the CDCs share this property, as a recent study showed that streptolysin O (SLO) inhibits internalization of Group A Streptococcus into keratinocytes [93]. Therefore, a fascinating avenue of research is to elucidate the molecular mechanisms underlying the role of pore-forming toxins in the regulation of host cell invasion by intracellular pathogens.
Materials and Methods
Bacterial strains and plasmids
WT L. monocytogenes (DP10403S), isogenic Δhly (hly is the gene coding for LLO) (DPL2161), and ΔinlAB (DPL4404) deletion mutants were gifts from Dr. Dan Portnoy (U.C. Berkeley, California, USA) [94]–[96]. To construct the triple deletion mutant (Δhly ΔinlAB) we deleted hly in the DPL4404 strain by allelic exchange using the pKSV7 integrational shuttle vector (a gift from Dr. Nancy Freitag, University of Illinois at Chicago, USA) [97]. A ∼1000-bp DNA fragment consisting of the upstream (from bp 962 to 1463) and downstream (from bp 3029 to 3529) sequences flanking the hly open reading frame was amplified from L. monocytogenes chromosomal DNA [96]. The primers (Table S1) were designed to generate restriction sites for EcoRI and PstI. The digested fragment was ligated into pKSV7. The pKSV7 with the 1000 bp fragment was used to perform allelic exchange according to [98]. Nonhemolytic colonies were identified on 5% blood agar plates (Becton Dickinson). The deletion of hly was further ensured by amplification of the chromosomal DNA with the primers used to generate this strain and a second set of primers that amplify the entire hly coding region. WT LO28 L. monocytogenes and the isogenic transposon insertion LO28 hly::Tn917 mutant were gifts from Dr. Pascale Cossart (Pasteur Institute, Paris, France) [99]. L. innocua 33090 was purchased from ATCC. Bacteria were grown overnight at 37° C in brain heart infusion (BHI) (BD Biosciences). For invasion assays, overnight cultures were diluted 1/20 in BHI and grown at 37°C until OD600 = 0.7–0.8. Bacteria were washed three times in phosphate-buffered saline (PBS) and diluted to the indicated multiplicity of infection (MOI) in the cell culture medium without serum. The plasmids pAM401 and pET29b coding for hly were gifts from Dr. D. Portnoy (Jones and Portnoy 1994). The plasmid phly/prfA* coding for hly was a gift from Dr. Svetlana A. Ermolaeva (Gamaleya Research Institute of Epidemiology and Microbiology, Moscow, Russia)[100]. The plasmid pQE-30 coding for ply was kindly provided by Dr. R. K. Tweten [40].
Mammalian cell cultures
Human hepatocyte (HepG2 cells, ATCC HB-8065) and cervical epithelial (HeLa, ATCC CCL-2) cell lines were grown in minimum essential medium (MEM) (+) Earle's salts and L-glutamine (Invitrogen), supplemented with 10% heat inactivated fetal bovine serum (HI-FBS; Lonza), 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 100 U/ml penicillin, and 100 µg/ml streptomycin (Invitrogen). Mammalian cells were maintained at 37°C in 5% CO2 atmosphere. Cells were seeded in 24-well tissue culture plates and grown for 48 h (HepG2; 1×105 cells/well) or 24 h (HeLa; 0.5×105 cells/well) before infection.
Coating of bacteria and polystyrene beads with LLO
Bacteria were coated with six-His-tagged toxin [49]. Briefly, 4×108 bacteria were washed twice and incubated for 10 min on ice in buffer A (20 mM Hepes pH 7.5, 50 mM NaCl, 1 nM nickel chloride). Bacteria were washed and incubated in 200 µl buffer B (20 mM Hepes pH 7.5, 50 mM NaCl) for 10 min with six-His-tagged toxin. Bacteria were then washed once with buffer B and suspended in 200 µl of buffer C (20 mM Hepes pH 7.5, 150 mM NaCl) before infecting HepG2 cells. Carboxylate microspheres (Alexa 350, 1 µm diameter; Molecular Probes) were covalently coated with 5 mg/ml bovine serum albumin (BSA) following the manufacturer's instructions. LLO was noncovalently associated to the surface of the BSA coated beads using the same experimental procedure used to coat bacteria. In each experiment, we verified that host membranes were not damaged by LLO, as this could cause the entry of antibodies used to label extracellular bacteria. Following fixation, cells were labeled with an anti-tubulin antibody (Sigma) and secondary fluorescent antibodies. We observed that in our experimental conditions antibodies could not enter the cells as microtubules were not labeled.
Gentamicin survival assay
HepG2 cells were infected with the L. monocytogenes strains at MOI 20, LLO-coated bacteria at MOI 20, or L. innocua and L. innocua phly/prfA* at MOI 100. The plates were centrifuged at room temperature for 5 min and incubated for 30 or 60 min at 37°C. Cells were washed and incubated with 15 µg/ml (for bacteria grown to OD600 = 0.8) or 100 µg/ml (For L. innocua grown to OD600 = 0.2) gentamicin for 1 h or 30 min, respectively. When measuring the intracellular survival of L. innocua in comparison to L. innocua phly/prfA*, the bacteria were grown to OD = 0.2 because this bacterial density led to the highest secretion levels of LLO (data not shown). Cells were washed three times with PBS and lysed with 0.2% Triton X-100 in H2O. Serial dilutions of cell lysates were immediately performed in PBS and plated on BHI agar. The colony forming units (CFUs) were enumerated after 48 h of incubation at 37°C.
Measurement of bacterial association and entry into host cells
HepG2 cells (or HeLa cells) were infected with bacteria at MOI 20. The plates were centrifuged for 5 min (230 x g) at room temperature and incubated for 30 min at 37°C. Cells were washed with PBS, fixed with PBS/4% paraformaldehyde (PFA) for 15 min at room temperature, and then washed with 0.1 M glycine in PBS and incubated for 1 h in blocking solution (0.1 M glycine, 10% HI-FBS in PBS, pH 7.4)Following fixation and blocking, extracellular bacteria, total bacteria and host cells were labeled as previously described [48]. To quantitate the numbers of bacteria and mammalian cells, 40 sets of images (DAPI, Alexa 488, Alexa 568, phase contrast) were automatically acquired for each experimental condition using a 20 X objective. MetaMorph imaging and analysis software was used to enumerate the total number of bacteria (Nt), extracellular bacteria (Ne), and mammalian cells (Nc) [48]. The percentage of internalization was calculated as (Nt - Ne)/Nt x 100. Bacterial association with host cells was calculated as Nt/Nc. The results were expressed relative to control (% control). For invasion assays in the presence of soluble LLO, LLO was added to the cell culture medium along with L. monocytogenes.
Construction and purification of recombinant toxins
LLO variants were constructed by PCR-based site-directed mutagenesis using pET29b encoding native six-His-tagged LLO as a template. Mutagenic primers (Table S1) were used to construct LLOmL containing the substitutions K344C and I359C; LLOpL containing the substitutions G80C and S213C; and LLO Alexa 488 with the substitutions C484A and D69C. Mutations were introduced by amplifying hly from pET29b using pfu Ultra II fusion polymerase, followed by digestion of methylated template DNA by DpnI (Stratagene). The constructs were transformed in E. coli XL1-Blue and BL21(DE3). Mutations were confirmed by DNA sequence analysis at The Ohio State University Plant-Microbe Genomics Facility. Recombinant six-His-tagged LLO and PLY were purified from E. coli BL21(DE3) as described previously [101]. LLOmL and LLOpL were dialyzed overnight in the absence of reducing agents to allow for disulfide bond formation. Toxins were stored at −80°C in 1 M NaCL, 50 mM phosphate buffer, pH 8. To obtain LLO Alexa 488, LLOC484A/D69C was labeled by chemical coupling to Alexa 488-Maleimide (Molecular Probes) under conditions that lead to a dye to protein molar ratio >0.9, following the manufacturer's instructions. The fluorescent toxin was separated from the unconjugated dye by gel filtration chromatography. All the recombinant toxins purified in this study contain a six His tag.
Hemolytic assays
Sheep erythrocytes (10% suspension; Lampire Biological) were diluted to 0.25% in PBS pH 7.4. Serial dilutions of toxins and erythrocytes were co-incubated in PBS at 37°C for 30 min in 96 well plates. Plates were centrifuged and the absorbance of the supernatant (A540) was measured in a PowerWavex340 spectrophotometer. Erythrocytes incubated with 0.1% Triton X-100 in PBS or PBS alone served to determine the maximum (100%) and minimum (0%) hemolytic activity, respectively. DTT alone had no effect on hemolysis (data no shown). The hemolytic activities of the bacteria were measured using a similar approach except that the indicated amounts of bacteria were added to the wells. The kinetic hemolytic assay in Fig. 5 was performed according to [102]. In this assay a decrease in absorbance reflects the lysis of erythrocytes.
Western blotting analysis of LLO expression by the bacterial strains
Overnight cultures of L. monocytogenes, L. innocua, and L. innocua phly/prfA* were diluted 1/20 in BHI and grown to OD600 = 0.8 (L. monocytogenes) or OD600 = 0.2 (L. innocua, and L. innocua phly/prfA*) in BHI. Bacterial suspensions containing 2.0×108 bacteria were collected and centrifuged. The supernatants were collected for protein precipitation and bacterial pellets were washed and lysed as follows. The supernatants (0.25 ml of L. monocytogenes and 1 ml of L. innocua cultures) were subjected to trichloroacetic acid (TCA) precipitation. One volume cold TCA was added to 4 volumes of supernatant and incubated 1 h on ice. Samples were centrifuged (11,000 g, 10 min, 4°C) and precipitates were washed twice with cold acetone. Bacterial and dried protein pellets were suspended in Laemmli's sample buffer. Bacterial lysates (107 bacteria/well, ∼600 ng/well) and precipitates were subjected to SDS-PAGE and western blotting using rabbit anti-LLO (Abcam) and horseradish peroxidase-conjugated secondary antibodies (Cell Signaling).
HepG2 perforation assay
HepG2 cells (0.5×105) were cultured in glass bottom culture dishes (MatTek; 35 mm petri dish, 10 mm microwell) for 48 h. Cells were washed twice and incubated in the presence or absence of 1 mM CaCl2 in a buffer containing 150 mM NaCl, 1 mM MgCl2, 5 mM KCL, 20 mM Hepes, 10 mM Glucose, 4 µM ethidium homodimer, pH 7.4. Cells were placed on a temperature controlled microscope at 37°C and phase-contrast and fluorescence images were recorded every 10 s using a 100 X objective for 10 min. LLO (0.5 nM) was added and movies were recorded for an additional 28 min. Results were expressed as the average fluorescence intensity measured from at least 5 movies at each time point.
LDH and cell viability measurements
HepG2 cells were incubated with LLO (1, 5, or 20 nM) or BSA/LLO-coated beads for 30 min at 37°C. Following incubation, supernatants were recovered from each sample and centrifuged at 500 x g at 4°C for 5 minutes to pellet any cells released into the supernatant. 10 µl of each supernatant was diluted into 40 µl of cell culture medium without serum in 96 well plates, and assayed for the presence of lactate dehydrogenase with the TOX7 in vitro toxicology assay kit according to the manufacturer's instructions (Sigma). To assess cell viability immediately or 24 h after LLO treatment, HepG2 cells were detached from wells with 0.25% Trypsin-EDTA. Cells were then mixed 1∶1 with 0.4% trypan blue for 3 minutes, after which viable (unstained) and nonviable (stained in blue) cells were enumerated with a hemocytometer.
Toxin oligomerization (NativePAGE)
Erythrocyte ghost membranes (EGM) were prepared as described previously [44] and stored at 4°C in resealing buffer (10 mM phosphate buffer, 5 mM MgCl). EGM (6.75×108) were incubated in PBS with 157 nM LLO on ice for 1 min and were transferred to 37°C for 5 min. When indicated, 4 mM DTT was added to reduce the disulfide bond in LLOmL and LLOpL. Samples were centrifuged at 15,000 x g for 15 min and the supernatant was removed and replaced with an equal volume of PBS. LLO oligomerization was analyzed using the NativePAGE Novex Bis-Tris Gel electrophoresis system. The samples were mixed with NativePAGE sample buffer containing 1% detergent, and run on a 4–16% Bis-Tris gel as described by the manufacturer (Invitrogen). LLO was detected by western blotting.
Toxin oligomerization (transmission electron microscopy)
LLO solutions (750 nM) in buffer (20 mM Hepes, pH 7.0, ±2 mM DTT) were pipetted in Teflon wells as 13 µl droplets and coated with 1 µl of a 0.5 mg/ml lipid mixture containing 50 mol% cholesterol (Avanti) and 50 mol% 1,2 dioleoyl-sn-glycero-3-phosphocholine (Avanti) in chloroform. After incubation in a humid chamber at room temperature for 1 h, the LLO complexes were transferred to carbon support films on electron microscopy (EM) grids and negatively stained with 1% (w/v) uranyl acetate and observed with a FEI Tecnai F20 transmission electron microscope equipped with a Gatan Ultrascan 4K X4K CCD camera.
Invasion assays using polystyrene beads, F-actin and EEA1 labeling
Cells were washed and incubated for 30 min with BSA-, or BSA/LLO-coated beads at a MOI = 20 in MEM. Cells were washed, fixed with PFA and blocked. Extracellular beads were labeled with an anti-BSA rabbit polyclonal antibody (Sigma) followed by a goat anti-rabbit secondary antibody conjugated to Alexa-568. The percentage of internalized beads was determined by fluorescence microscopy based on their unique (Alexa 350, intracellular) or dual fluorescence (Alexa 350 + Alexa 568, extracellular) and expressed as% intracellular beads ( = intracellular beads/total beads * 100). EEA1 was labeled with a primary antibody (Santa Cruz) and a fluorescent (Alexa 488) donkey anti-goat secondary antibody in permeabilized cells. When co-labeling of the BSA beads and EEA1 was performed, a fluorescent donkey anti-rabbit secondary antibody was used to label the primary rabbit anti-BSA antibody. For F-actin labeling, cells were fixed and labeled as described in [102].
Silencing of clathrin heavy chain in HepG2 cells
HepG2 cells were transfected in 24 well cell culture plates with specific human clathrin heavy chain siRNA (Ambion 43908824, Table S1) or with scrambled siRNA (Ambion, 4390843) (50 nM siRNA in 0.5 ml/0.5×105 cell/well) using SiPort neoFx transfection reagent according to the manufacturer instructions (Ambion). After 24 h, cell culture medium was replaced and cells were further incubated for 24 h. Clathrin knock-down efficiency was verified in each experiment by western blotting using primary rabbit anti-clathrin heavy chain (Abcam) and mouse anti-α-tubulin (Sigma) antibodies.
Cell treatment with chemical inhibitors
Cells were pre-incubated with 0.5 µg/ml cytochalasin D (Sigma) for 10 min, 33 µM nocodazole (Sigma) for 60 min, 250 µM Genistein (Sigma) for 60 min, 37 µM LY294002 (EMD chemicals) for 60 min, 1 µM wortmannin (Sigma) for 60 min, 80 or 120 µM dynasore (Sigma) for 30 min, and 10 µM chlorpromazine (Sigma) for 30 min before the addition of coated beads, and the drugs were maintained at the same concentrations in the cell culture medium until the cells were fixed.
Transferrin uptake
HepG2 cells were washed three times in MEM and were serum starved for 2 h. Cells were incubated in MEM at 37°C with 5 µg/ml iron loaded Alexa 568 conjugated tranferrin (Molecular Probes). After 2 and 10 min of incubation, cells were transferred to ice, washed three times with cold medium and acid washed (200 mM NaCl, 50 mM MES, pH5.0) for 5 min. Four washes were performed with a cold buffer containing 150 mM NaCl, 1 mMCaCl2, 1 mM PBS, 5 mM KCl, 20 mM Hepes, pH 7.4. Alexa 488-conjugated transferrin was added (5 µg/ml) for 2 h at 4°C. Cells were then washed and fixed. Phase contrast and fluorescence images of the internalized (Alexa 568-Tf) and cell surface-associated (Alexa 488-Tf) fluorescent transferrin were acquired using a 40X objective. Extracellular and internalized transferrin molecules were measured by quantitative fluorescence microscopy. Briefly, images were first background corrected and the average fluorescence intensities were measured in the cells (about 1000 cells were analyzed for each experimental condition).
Cholesterol depletion and repletion and LLO treatment with neutralizing antibodies
For cholesterol depletion, HepG2 cells were washed twice with MEM (without serum) and incubated at 37°C for 30 min with 5 mM MβCD in MEM. Cells were then washed twice and assayed as described. For cholesterol repletion, cholesterol-depleted cells were washed twice and incubated for 15 min with a solution of 5 mM cholesterol-MβCD in MEM, then washed and assayed as described.
A 1∶1∶1 mixture of three monoclonal anti-LLO neutralizing (H14-3, B8B20-3-2, and A4-8) or control anti-LLO (D21-1-4) antibodies (gift from Dr. P. Cossart, Institute Pasteur, France) was added to purified LLO or to BSA/LLO-coated beads at concentrations of 2, 10, or 20 µg/ml.
Image acquisition
Images were acquired on a motorized inverted epi-fluorescence microscope (Axio Observer D1, Zeiss) equipped with 20 X Plan Neofluar (N.A. = 0.5), 100 X Plan Apo (N.A. = 1.4), and 40X Plan Neofluar (N.A. = 1.3) objectives, a high speed filter changer Lambda DG-4 (300 Watts Xenon Arc bulb, Sutter Instrument Company), an optical emission filter wheel Lambda 10–3 for the fluorescence imaging, and a Smart shutter that controls the illumination for phase contrast imaging (Sutter Instrument Company). The camera (back-illuminated, frame-transfer EMCCD Cascade II 512) was from Photometrics. The filter sets for fluorescence were purchased from Chroma Technology Corporation: DAPI (49000), GFP/FITC/Alexa488 (49002), Cy3/DsRed/Alexa568 (49005). The microscope was controlled by MetaMorph imaging software (Universal Imaging).
Live cell imaging
All movies were acquired on the microscope stage of an inverted fluorescence microscope at 37°C using a 100X objective. Phase contrast and fluorescence images of HepG2 cells were acquired every 10 s for 40 min, LLO was added 10 min after starting recording. In some experiments, cells were incubated in calcium-free buffer as indicated. Movies were accelerated 198 times.
Scanning electron microscopy
Following incubation with BSA/LLO-coated beads, cells were washed in PBS, fixed and processed as described in [23] with the following modifications: Coverslips were sputter coated with gold palladium at 17 mA for 75 sec with a Cressington 108 sputter coater. Samples were examined and photographed with a FEI Nova Nano scanning electron microscope operating at 5 Kv.
Statistics
A minimum of three independent experiments were performed, each in duplicate, unless otherwise indicated. Data were expressed as mean ± Standard Error of the Mean (SEM). P-values were calculated using a standard two-tailed Student's t-test and determined significant if lower than 0.05. In figures, asterisks indicate a significant difference between the indicated experimental conditions (* p<0.05; ** p<0.005).
Accession numbers
The NCBI Reference Sequence (RefSeq) accession numbers for the listeriolysin O gene and protein are as follows: hly (NC_003210.1), LLO ( ZP_05237132).
Supporting Information
Zdroje
1. CossartPSansonettiPJ 2004 Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 304 242 248
2. Pizarro-CerdaJCossartP 2006 Bacterial adhesion and entry into host cells. Cell 124 715 727
3. ValdezYFerreiraRBFinlayBB 2009 Molecular mechanisms of Salmonella virulence and host resistance. Curr Top Microbiol Immunol 337 93 127
4. Lara-TejeroMKatoJWagnerSLiuXGalanJE 2011 A Sorting Platform Determines the Order of Protein Secretion in Bacterial Type III Systems. Science 331 1188 1191
5. GalanJE 2009 Common themes in the design and function of bacterial effectors. Cell Host Microbe 5 571 579
6. Vazquez-BolandJAKuhnMBerchePChakrabortyTDominguez-BernalG 2001 Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev 14 584 640
7. GellinBGBroomeCVBibbWFWeaverREGaventaS 1991 The epidemiology of listeriosis in the United States–1986. Listeriosis Study Group. Am J Epidemiol 133 392 401
8. JacksonKAIwamotoMSwerdlowD 2010 Pregnancy-associated listeriosis. Epidemiol Infect 138 1503 1509
9. LecuitMVandormael-PourninSLefortJHuerreMGounonP 2001 A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science 292 1722 1725
10. PentecostMOttoGTheriotJAAmievaMR 2006 Listeria monocytogenes invades the epithelial junctions at sites of cell extrusion. PLoS Pathog 2 e3
11. DissonOGrayoSHuilletENikitasGLanga-VivesF 2008 Conjugated action of two species-specific invasion proteins for fetoplacental listeriosis. Nature 455 1114 1118
12. GaillardJLJaubertFBercheP 1996 The inlAB locus mediates the entry of Listeria monocytogenes into hepatocytes in vivo. J Exp Med 183 359 369
13. StavruFArchambaudCCossartP 2011 Cell biology and immunology of Listeria monocytogenes infections: novel insights. Immunol Rev 240 160 184
14. SeveauSPizarro-CerdaJCossartP 2007 Molecular mechanisms exploited by Listeria monocytogenes during host cell invasion. Microbes Infect 9 1167 1175
15. Pizarro-CerdaJCossartP 2009 Listeria monocytogenes membrane trafficking and lifestyle: the exception or the rule? Annu Rev Cell Dev Biol 25 649 670
16. IretonK 2007 Entry of the bacterial pathogen Listeria monocytogenes into mammalian cells. Cell Microbiol 9 1365 1375
17. GaillardJLBerchePFrehelCGouinECossartP 1991 Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell 65 1127 1141
18. Vazquez-BolandJADominguez-BernalGGonzalez-ZornBKreftJGoebelW 2001 Pathogenicity islands and virulence evolution in Listeria. Microbes Infect 3 571 584
19. MengaudJOhayonHGounonPMegeRMCossartP 1996 E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 84 923 932
20. ShenYNaujokasMParkMIretonK 2000 InIB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell 103 501 510
21. PentecostMKumaranJGhoshPAmievaMR 2010 Listeria monocytogenes internalin B activates junctional endocytosis to accelerate intestinal invasion. PLoS Pathog 6 e1000900
22. DramsiSBiswasIMaguinEBraunLMastroeniP 1995 Entry of Listeria monocytogenes into hepatocytes requires expression of inIB, a surface protein of the internalin multigene family. Mol Microbiol 16 251 261
23. SeveauSBierneHGirouxSPrevostMCCossartP 2004 Role of lipid rafts in E-cadherin – and HGF-R/Met–mediated entry of Listeria monocytogenes into host cells. J Cell Biol 166 743 753
24. SeveauSThamTNPayrastreBHoppeADSwansonJA 2007 A FRET analysis to unravel the role of cholesterol in Rac1 and PI 3-kinase activation in the InlB/Met signalling pathway. Cell Microbiol 9 790 803
25. VeigaEGuttmanJABonazziMBoucrotEToledo-AranaA 2007 Invasive and adherent bacterial pathogens co-Opt host clathrin for infection. Cell Host Microbe 2 340 351
26. Pizarro-CerdaJBonazziMCossartP 2010 Clathrin-mediated endocytosis: what works for small, also works for big. Bioessays 32 496 504
27. SchnupfPPortnoyDA 2007 Listeriolysin O: a phagosome-specific lysin. Microbes Infect 9 1176 1187
28. TilneyLGPortnoyDA 1989 Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol 109 1597 1608
29. HenryRShaughnessyLLoessnerMJAlberti-SeguiCHigginsDE 2006 Cytolysin-dependent delay of vacuole maturation in macrophages infected with Listeria monocytogenes. Cell Microbiol 8 107 119
30. BraunLOhayonHCossartP 1998 The InIB protein of Listeria monocytogenes is sufficient to promote entry into mammalian cells. Mol Microbiol 27 1077 1087
31. Alvarez-DominguezCVazquez-BolandJACarrasco-MarinELopez-MatoPLeyva-CobianF 1997 Host cell heparan sulfate proteoglycans mediate attachment and entry of Listeria monocytogenes, and the listerial surface protein ActA is involved in heparan sulfate receptor recognition. Infect Immun 65 78 88
32. SuarezMGonzalez-ZornBVegaYChico-CaleroIVazquez-BolandJA 2001 A role for ActA in epithelial cell invasion by Listeria monocytogenes. Cell Microbiol 3 853 864
33. BergmannBRaffelsbauerDKuhnMGoetzMHomS 2002 InlA - but not InlB-mediated internalization of Listeria monocytogenes by non-phagocytic mammalian cells needs the support of other internalins. Mol Microbiol 43 557 570
34. ReisOSousaSCamejoAVilliersVGouinE 2010 LapB, a Novel Listeria monocytogenes LPXTG Surface Adhesin, Required for Entry into Eukaryotic Cells and Virulence. J Infect Dis 202 551 62
35. DramsiSCossartP 2003 Listeriolysin O-mediated calcium influx potentiates entry of Listeria monocytogenes into the human Hep-2 epithelial cell line. Infect Immun 71 3614 3618
36. GaillardJLBerchePSansonettiP 1986 Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect Immun 52 50 55
37. TwetenRK 2005 Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect Immun 73 6199 6209
38. BillingtonSJJostBHSongerJG 2000 Thiol-activated cytolysins: structure, function and role in pathogenesis. FEMS Microbiol Lett 182 197 205
39. MitchellTJAndrewPW 1997 Biological properties of pneumolysin. Microb Drug Resist 3 19 26
40. FarrandAJLaChapelleSHotzeEMJohnsonAETwetenRK 2010 Only two amino acids are essential for cytolytic toxin recognition of cholesterol at the membrane surface. Proc Natl Acad Sci U S A 107 4341 4346
41. GiddingsKSZhaoJSimsPJTwetenRK 2004 Human CD59 is a receptor for the cholesterol-dependent cytolysin intermedilysin. Nat Struct Mol Biol 11 1173 1178
42. GelberSEAguilarJLLewisKLRatnerAJ 2008 Functional and phylogenetic characterization of Vaginolysin, the human-specific cytolysin from Gardnerella vaginalis. J Bacteriol 190 3896 3903
43. WickhamSEHotzeEMFarrandAJPolekhinaGNeroTL 2011 Mapping the intermedilysin-human CD59 receptor interface reveals a deep correspondence with the binding site on CD59 for complement binding proteins C8alpha and C9. J Biol Chem 286 20952 20962
44. ShepardLAShaturskyOJohnsonAETwetenRK 2000 The mechanism of pore assembly for a cholesterol-dependent cytolysin: formation of a large prepore complex precedes the insertion of the transmembrane beta-hairpins. Biochemistry 39 10284 10293
45. IdoneVTamCGossJWToomreDPypaertM 2008 Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J Cell Biol 180 905 914
46. LosFCKaoCYSmithamJMcDonaldKLHaC 2011 RAB-5 - and RAB-11-Dependent Vesicle-Trafficking Pathways Are Required for Plasma Membrane Repair after Attack by Bacterial Pore-Forming Toxin. Cell Host Microbe 9 147 157
47. ThieryJKeefeDSaffarianSMartinvaletDWalchM 2010 Perforin activates clathrin - and dynamin-dependent endocytosis, which is required for plasma membrane repair and delivery of granzyme B for granzyme-mediated apoptosis. Blood 115 1582 1593
48. HaghighatACSeveauS 2010 Quantification of host-microbe interactions by automated fluorescence microscopy. J Immunol Methods 352 186 191
49. GeddeMMHigginsDETilneyLGPortnoyDA 2000 Role of listeriolysin O in cell-to-cell spread of Listeria monocytogenes. Infect Immun 68 999 1003
50. GlaserPFrangeulLBuchrieserCRusniokCAmendA 2001 Comparative genomics of Listeria species. Science 294 849 852
51. SchuerchDWWilson-KubalekEMTwetenRK 2005 Molecular basis of listeriolysin O pH dependence. Proc Natl Acad Sci U S A 102 12537 12542
52. HotzeEMWilson-KubalekEMRossjohnJParkerMWJohnsonAE 2001 Arresting pore formation of a cholesterol-dependent cytolysin by disulfide trapping synchronizes the insertion of the transmembrane beta-sheet from a prepore intermediate. J Biol Chem 276 8261 8268
53. RamachandranRTwetenRKJohnsonAE 2004 Membrane-dependent conformational changes initiate cholesterol-dependent cytolysin oligomerization and intersubunit beta-strand alignment. Nat Struct Mol Biol 11 697 705
54. NatoFReichKLhopitalSRouyreSGeoffroyC 1991 Production and characterization of neutralizing and nonneutralizing monoclonal antibodies against listeriolysin O. Infect Immun 59 4641 4646
55. GiddingsKSJohnsonAETwetenRK 2003 Redefining cholesterol's role in the mechanism of the cholesterol-dependent cytolysins. Proc Natl Acad Sci U S A 100 11315 11320
56. MarriottHMMitchellTJDockrellDH 2008 Pneumolysin: a double-edged sword during the host-pathogen interaction. Curr Mol Med 8 497 509
57. KumariSMgSMayorS 2010 Endocytosis unplugged: multiple ways to enter the cell. Cell Res 20 256 275
58. GrundlingAGonzalezMDHigginsDE 2003 Requirement of the Listeria monocytogenes broad-range phospholipase PC-PLC during infection of human epithelial cells. J Bacteriol 185 6295 6307
59. BurkholderKMBhuniaAK 2010 Listeria monocytogenes uses Listeria adhesion protein (LAP) to promote bacterial transepithelial translocation and induces expression of LAP receptor Hsp60. Infect Immun 78 5062 5073
60. DramsiSBourdichonFCabanesDLecuitMFsihiH 2004 FbpA, a novel multifunctional Listeria monocytogenes virulence factor. Mol Microbiol 53 639 649
61. ReisOSousaSCamejoAVilliersVGouinE 2010 LapB, a novel Listeria monocytogenes LPXTG surface adhesin, required for entry into eukaryotic cells and virulence. J Infect Dis 202 551 562
62. SabetCToledo-AranaAPersonnicNLecuitMDubracS 2008 The Listeria monocytogenes virulence factor InlJ is specifically expressed in vivo and behaves as an adhesin. Infect Immun 76 1368 1378
63. MilohanicEJonquieresRCossartPBerchePGaillardJL 2001 The autolysin Ami contributes to the adhesion of Listeria monocytogenes to eukaryotic cells via its cell wall anchor. Mol Microbiol 39 1212 1224
64. LindenSKBierneHSabetCPngCWFlorinTH 2008 Listeria monocytogenes internalins bind to the human intestinal mucin MUC2. Arch Microbiol 190 101 104
65. CabanesDSousaSCebriaALecuitMGarcia-del PortilloF 2005 Gp96 is a receptor for a novel Listeria monocytogenes virulence factor, Vip, a surface protein. EMBO J 24 2827 2838
66. Krawczyk-BalskaABieleckiJ 2005 Listeria monocytogenes listeriolysin O and phosphatidylinositol-specific phospholipase C affect adherence to epithelial cells. Can J Microbiol 51 745 751
67. BieleckiJ 1994 Association of listeriolysin O with the cell surface of Listeria monocytogenes. Acta Microbiol Pol 43 279 289
68. PriceKECamilliA 2009 Pneumolysin localizes to the cell wall of Streptococcus pneumoniae. J Bacteriol 191 2163 2168
69. BavdekAGekaraNOPriselacDGutierrez AguirreIDarjiA 2007 Sterol and pH interdependence in the binding, oligomerization, and pore formation of Listeriolysin O. Biochemistry 46 4425 4437
70. RottnerKStradalTEWehlandJ 2005 Bacteria-host-cell interactions at the plasma membrane: stories on actin cytoskeleton subversion. Dev Cell 9 3 17
71. MaddenJCRuizNCaparonM 2001 Cytolysin-mediated translocation (CMT): a functional equivalent of type III secretion in gram-positive bacteria. Cell 104 143 152
72. MagassaNChandrasekaranSCaparonMG 2010 Streptococcus pyogenes cytolysin-mediated translocation does not require pore formation by streptolysin O. EMBO Rep 11 400 405
73. MayorSPaganoRE 2007 Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol 8 603 612
74. GekaraNOJacobsTChakrabortyTWeissS 2005 The cholesterol-dependent cytolysin listeriolysin O aggregates rafts via oligomerization. Cell Microbiol 7 1345 1356
75. IlievAIDjannatianJRNauRMitchellTJWoutersFS 2007 Cholesterol-dependent actin remodeling via RhoA and Rac1 activation by the Streptococcus pneumoniae toxin pneumolysin. Proc Natl Acad Sci U S A 104 2897 2902
76. JosephBPrzybillaKStuhlerCSchauerKSlaghuisJ 2006 Identification of Listeria monocytogenes genes contributing to intracellular replication by expression profiling and mutant screening. J Bacteriol 188 556 568
77. CamejoABuchrieserCCouveECarvalhoFReisO 2009 In vivo transcriptional profiling of Listeria monocytogenes and mutagenesis identify new virulence factors involved in infection. PLoS Pathog 5 e1000449
78. SahuSCGainesDWWilliamsKMRaybourneRB 2007 A synthetic polypeptide based on human E-cadherin inhibits invasion of human intestinal and liver cell lines by Listeria monocytogenes. J Med Microbiol 56 1011 1016
79. NagaiTAraoTFurutaKSakaiKKudoK 2011 Sorafenib inhibits the hepatocyte growth factor-mediated epithelial mesenchymal transition in hepatocellular carcinoma. Mol Cancer Ther 10 169 177
80. CossartPPizarro-CerdaJLecuitM 2003 Invasion of mammalian cells by Listeria monocytogenes: functional mimicry to subvert cellular functions. Trends Cell Biol 13 23 31
81. IbarraJASteele-MortimerO 2009 Salmonella–the ultimate insider. Salmonella virulence factors that modulate intracellular survival. Cell Microbiol 11 1579 1586
82. ParsotC 2009 Shigella type III secretion effectors: how, where, when, for what purposes? Curr Opin Microbiol 12 110 116
83. CossartPVeigaE 2008 Non-classical use of clathrin during bacterial infections. J Microsc 231 524 528
84. IacovacheIBischofbergerMvan der GootFG 2010 Structure and assembly of pore-forming proteins. Curr Opin Struct Biol 20 241 246
85. GonzalezMRBischofbergerMPernotLvan der GootFGFrecheB 2008 Bacterial pore-forming toxins: the (w)hole story? Cell Mol Life Sci 65 493 507
86. FernandesMCCortezMFlanneryARTamCMortaraRA 2011 Trypanosoma cruzi subverts the sphingomyelinase-mediated plasma membrane repair pathway for cell invasion. J Exp Med 208 909 921
87. TalbotUMPatonAWPatonJC 1996 Uptake of Streptococcus pneumoniae by respiratory epithelial cells. Infect Immun 64 3772 3777
88. SukenoANagamuneHWhileyRAJafarSIAduse-OpokuJ 2005 Intermedilysin is essential for the invasion of hepatoma HepG2 cells by Streptococcus intermedius. Microbiol Immunol 49 681 694
89. ZhangJRMostovKELammMENannoMShimidaS 2000 The polymeric immunoglobulin receptor translocates pneumococci across human nasopharyngeal epithelial cells. Cell 102 827 837
90. AmmendoliaMGSupertiFBertucciniLChiariniFConteMP 2007 Invasive pathway of Listeria ivanovii in human amnion-derived WISH cells. Int J Immunopathol Pharmacol 20 509 518
91. RussellBHVasanRKeeneDRXuY 2007 Bacillus anthracis internalization by human fibroblasts and epithelial cells. Cell Microbiol 9 1262 1274
92. JostBHBillingtonSJ 2005 Arcanobacterium pyogenes: molecular pathogenesis of an animal opportunist. Antonie Van Leeuwenhoek 88 87 102
93. LogsdonLKHakanssonAPCortesGWesselsMR 2011 Streptolysin O inhibits clathrin-dependent internalization of group a streptococcus. MBio 2 e00332 10
94. PortnoyDAJacksPSHinrichsDJ 1988 Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med 167 1459 1471
95. BakardjievAIStacyBAFisherSJPortnoyDA 2004 Listeriosis in the pregnant guinea pig: a model of vertical transmission. Infect Immun 72 489 497
96. JonesSPortnoyDA 1994 Characterization of Listeria monocytogenes pathogenesis in a strain expressing perfringolysin O in place of listeriolysin O. Infect Immun 62 5608 5613
97. SmithKYoungmanP 1992 Use of a new integrational vector to investigate compartment-specific expression of the Bacillus subtilis spoIIM gene. Biochimie 74 705 711
98. CamilliATilneyLGPortnoyDA 1993 Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol Microbiol 8 143 157
99. CossartPVicenteMFMengaudJBaqueroFPerez-DiazJC 1989 Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect Immun 57 3629 3636
100. PushkarevaVIErmolaevaSA 2010 Listeria monocytogenes virulence factor Listeriolysin O favors bacterial growth in co-culture with the ciliate Tetrahymena pyriformis, causes protozoan encystment and promotes bacterial survival inside cysts. BMC Microbiol 10 26
101. GlomskiIJGeddeMMTsangAWSwansonJAPortnoyDA 2002 The Listeria monocytogenes hemolysin has an acidic pH optimum to compartmentalize activity and prevent damage to infected host cells. J Cell Biol 156 1029 1038
102. ArnettELehrerRIPratikhyaPLuWSeveauS 2010 Defensins enable macrophages to inhibit the intracellular proliferation of Listeria monocytogenes. Cell Microbiol 13 635 51
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Microbial Spy Games and Host Response: Roles of a Small Molecule in Communication with Other SpeciesČlánek Sequence-Based Analysis Uncovers an Abundance of Non-Coding RNA in the Total Transcriptome of
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 11- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Microbial Spy Games and Host Response: Roles of a Small Molecule in Communication with Other Species
- Simple Rapid Near-Patient Diagnostics for Tuberculosis Remain Elusive—Is a “Treat-to-Test” Strategy More Realistic?
- Ultra-Efficient PrP Amplification Highlights Potentialities and Pitfalls of PMCA Technology
- Assessing Predicted HIV-1 Replicative Capacity in a Clinical Setting
- Inhibition of IL-10 Production by Maternal Antibodies against Group B Streptococcus GAPDH Confers Immunity to Offspring by Favoring Neutrophil Recruitment
- Anti-filarial Activity of Antibiotic Therapy Is Due to Extensive Apoptosis after Depletion from Filarial Nematodes
- West Nile Virus Experimental Evolution and the Trade-off Hypothesis
- Shedding Light on the Elusive Role of Endothelial Cells in Cytomegalovirus Dissemination
- Galactosaminogalactan, a New Immunosuppressive Polysaccharide of
- Fatal Prion Disease in a Mouse Model of Genetic E200K Creutzfeldt-Jakob Disease
- BST2/Tetherin Enhances Entry of Human Cytomegalovirus
- Metagenomic Analysis of Fever, Thrombocytopenia and Leukopenia Syndrome (FTLS) in Henan Province, China: Discovery of a New Bunyavirus
- Neurons are MHC Class I-Dependent Targets for CD8 T Cells upon Neurotropic Viral Infection
- Sap Transporter Mediated Import and Subsequent Degradation of Antimicrobial Peptides in
- A Molecular Mechanism for Bacterial Susceptibility to Zinc
- Genomic Transition to Pathogenicity in Chytrid Fungi
- Evolution of Multidrug Resistance during Infection Involves Mutation of the Essential Two Component Regulator WalKR
- ChemR23 Dampens Lung Inflammation and Enhances Anti-viral Immunity in a Mouse Model of Acute Viral Pneumonia
- SH3 Domain-Mediated Recruitment of Host Cell Amphiphysins by Alphavirus nsP3 Promotes Viral RNA Replication
- A Gammaherpesvirus Cooperates with Interferon-alpha/beta-Induced IRF2 to Halt Viral Replication, Control Reactivation, and Minimize Host Lethality
- Early Secreted Antigen ESAT-6 of Promotes Protective T Helper 17 Cell Responses in a Toll-Like Receptor-2-dependent Manner
- CD4 T Cell Immunity Is Critical for the Control of Simian Varicella Virus Infection in a Nonhuman Primate Model of VZV Infection
- The Role of the P2X Receptor in Infectious Diseases
- Down-Regulation of Shadoo in Prion Infections Traces a Pre-Clinical Event Inversely Related to PrP Accumulation
- Cross-Reactive T Cells Are Involved in Rapid Clearance of 2009 Pandemic H1N1 Influenza Virus in Nonhuman Primates
- Single Molecule Analysis of Replicated DNA Reveals the Usage of Multiple KSHV Genome Regions for Latent Replication
- The Critical Role of Notch Ligand Delta-like 1 in the Pathogenesis of Influenza A Virus (H1N1) Infection
- Sequence-Based Analysis Uncovers an Abundance of Non-Coding RNA in the Total Transcriptome of
- Murine Gamma Herpesvirus 68 Hijacks MAVS and IKKβ to Abrogate NFκB Activation and Antiviral Cytokine Production
- EBV Tegument Protein BNRF1 Disrupts DAXX-ATRX to Activate Viral Early Gene Transcription
- SAG101 Forms a Ternary Complex with EDS1 and PAD4 and Is Required for Resistance Signaling against Turnip Crinkle Virus
- Multiple Candidate Effectors from the Oomycete Pathogen Suppress Host Plant Immunity
- Rab7A Is Required for Efficient Production of Infectious HIV-1
- A TNF-Regulated Recombinatorial Macrophage Immune Receptor Implicated in Granuloma Formation in Tuberculosis
- Novel Anti-bacterial Activities of β-defensin 1 in Human Platelets: Suppression of Pathogen Growth and Signaling of Neutrophil Extracellular Trap Formation
- CD11b, Ly6G Cells Produce Type I Interferon and Exhibit Tissue Protective Properties Following Peripheral Virus Infection
- The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
- A Kinase Chaperones Hepatitis B Virus Capsid Assembly and Captures Capsid Dynamics
- Towards a Structural Comprehension of Bacterial Type VI Secretion Systems: Characterization of the TssJ-TssM Complex of an Pathovar
- Indirect DNA Readout by an H-NS Related Protein: Structure of the DNA Complex of the C-Terminal Domain of Ler
- The Pore-Forming Toxin Listeriolysin O Mediates a Novel Entry Pathway of into Human Hepatocytes
- The Human Herpesvirus-7 (HHV-7) U21 Immunoevasin Subverts NK-Mediated Cytoxicity through Modulation of MICA and MICB
- Avirulence Effector Avr3b is a Secreted NADH and ADP-ribose Pyrophosphorylase that Modulates Plant Immunity
- Murid Herpesvirus-4 Exploits Dendritic Cells to Infect B Cells
- Unique Type I Interferon Responses Determine the Functional Fate of Migratory Lung Dendritic Cells during Influenza Virus Infection
- Evolution of a Species-Specific Determinant within Human CRM1 that Regulates the Post-transcriptional Phases of HIV-1 Replication
- Transcriptome Analysis of Transgenic Mosquitoes with Altered Immunity
- Antibody Evasion by a Gammaherpesvirus O-Glycan Shield
- UDP-glucose 4, 6-dehydratase Activity Plays an Important Role in Maintaining Cell Wall Integrity and Virulence of
- Protease-Resistant Prions Selectively Decrease Shadoo Protein
- A LysM and SH3-Domain Containing Region of the p60 Protein Stimulates Accessory Cells to Promote Activation of Host NK Cells
- Deletion of AIF Ortholog Promotes Chromosome Aneuploidy and Fluconazole-Resistance in a Metacaspase-Independent Manner
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Multiple Candidate Effectors from the Oomycete Pathogen Suppress Host Plant Immunity
- The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
- A TNF-Regulated Recombinatorial Macrophage Immune Receptor Implicated in Granuloma Formation in Tuberculosis
- SH3 Domain-Mediated Recruitment of Host Cell Amphiphysins by Alphavirus nsP3 Promotes Viral RNA Replication
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



