-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDifferential Regulation of Effector- and Central-Memory Responses to Infection by IL-12 Revealed by Tracking of Tgd057-Specific CD8+ T Cells
Production of the pro-inflammatory cytokine IL-12 by innate phagocytes drives the differentiation of IFN-γ-producing effector T cells during Toxoplasma gondii infection. However, the role of IL-12 in the regulation of memory CD8+ T cell differentiation and function during murine toxoplasmosis is unclear. To track memory CTL development, we identified a novel H-2Kb-restricted CTL population specific for the Toxoplasma antigen tgd057. Tgd057-specific CTLs were induced by both vaccination and natural peroral infection, and were representative of the polyclonal CTL population. Tgd057-specific primary effector cells required IL-12 for the differentiation of KLRG1+ effector subpopulations and IFN-γ production in response to restimulation with parasite-infected cells, but not to restimulation with cognate peptide. The effect of IL-12 deficiency during the primary response was profoundly imprinted on memory CTLs, which continued to show defects in cell numbers, KLRG1+ effector memory subpopulation differentiation, and IFN-γ recall responses. Importantly, isolated CD62Lhi KLRG1 - CD8+ T cells differentiated in the absence of IL-12 were enhanced in their ability to generate IFN-γ-producing secondary tgd057-specific effector cells. Our data, for the first time, demonstrate the negative impact of IL-12 signaling on the quality of the central memory CTL compartment. Thus, despite the beneficial role of IL-12 in promoting effector differentiation, excessive exposure to IL-12 during CTL priming may limit the development of long-term protective immunity through the decreased fitness of central memory CTL responses.
Published in the journal: . PLoS Pathog 6(3): e32767. doi:10.1371/journal.ppat.1000815
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000815Summary
Production of the pro-inflammatory cytokine IL-12 by innate phagocytes drives the differentiation of IFN-γ-producing effector T cells during Toxoplasma gondii infection. However, the role of IL-12 in the regulation of memory CD8+ T cell differentiation and function during murine toxoplasmosis is unclear. To track memory CTL development, we identified a novel H-2Kb-restricted CTL population specific for the Toxoplasma antigen tgd057. Tgd057-specific CTLs were induced by both vaccination and natural peroral infection, and were representative of the polyclonal CTL population. Tgd057-specific primary effector cells required IL-12 for the differentiation of KLRG1+ effector subpopulations and IFN-γ production in response to restimulation with parasite-infected cells, but not to restimulation with cognate peptide. The effect of IL-12 deficiency during the primary response was profoundly imprinted on memory CTLs, which continued to show defects in cell numbers, KLRG1+ effector memory subpopulation differentiation, and IFN-γ recall responses. Importantly, isolated CD62Lhi KLRG1 - CD8+ T cells differentiated in the absence of IL-12 were enhanced in their ability to generate IFN-γ-producing secondary tgd057-specific effector cells. Our data, for the first time, demonstrate the negative impact of IL-12 signaling on the quality of the central memory CTL compartment. Thus, despite the beneficial role of IL-12 in promoting effector differentiation, excessive exposure to IL-12 during CTL priming may limit the development of long-term protective immunity through the decreased fitness of central memory CTL responses.
Introduction
Infection by the intracellular protozoan parasite Toxoplasma gondii causes severe disease in immunocompromised individuals including neonates, transplant recipients, and people with AIDS [1]. In healthy individuals however, resistance to toxoplasmosis is mediated by the ability of T lymphocytes to produce the essential effector cytokine IFN-γ [2],[3]. Although both innate and adaptive immunity synergize to combat parasitic infection [4],[5], CD8+ T cells are known to be the main mediator of protective immunity [6],[7],[8],[9]. The importance of CTL responses is exemplified by the differential susceptibility of inbred strains of mice to toxoplasmic encephalitis, where resistant strains are protected by CTLs responding to antigens presented by H-2Ld MHC class I products [10]. Unfortunately, the natural Toxoplasma epitopes that drive CTL activation remain incompletely defined and thus encumber our ability to track the development of Ag-specific CTLs.
The Toxoplasma antigens historically identified as CTL targets were defined by the vaccination of mice with purified antigens or DNA expression vectors. The T. gondii surface antigen SAG1 (p30) was the first isolated Ag shown to elicit CTL effector responses [11] and protective immunity in mice [12]. SAG1 DNA vaccination also protected mice from lethal parasite challenge [13]. DNA vaccination using the secreted Toxoplasma proteins GRA1, GRA7, and ROP2 also elicits partially protective immunity [14],[15]. Nonetheless, the precise CTL-specific peptide epitopes derived from these candidate antigens have yet to be identified. Recently, an unbiased epitope discovery screen identified a single H-2Ld-restricted, GRA6 epitope-specific CTL population elicited by T. gondii infection in BALB/c mice [16]. Although these CTLs were argued to be an immunodominant population, another report has emerged describing two H-2Ld-restricted CD8+ T cell populations specific for the GRA4 and ROP7 T. gondii antigens [17]. The latter study utilized caged MHC class I tetramers that allow the exchange of a conditional ligand occupying the MHC molecule for putative antigenic epitopes (pre-selected by a targeted epitope prediction program), and subsequently used large numbers of freshly generated tetramers to screen for Ag-specific CTLs [18]. Since both of these studies involved mice of the H-2d haplotype, and most basic immunological research uses C57BL/6 mice of the H-2b haplotype [19], we chose to screen for putative Toxoplasma CTL epitopes in C57BL/6 mice. Using caged MHC class I tetramers, we identified a single genuine H-2Kb-restricted epitope derived from the protein tgd057.
Having identified a novel population of Toxoplasma-specific CD8+ T cells, we sought to extend our previous findings regarding the role of IL-12 for the differentiation of effector and memory CTLs. T. gondii infection induces a robust type 1 polarized immune response marked by the production of the pro-inflammatory cytokine IL-12 by macrophages and dendritic cells [20]. It is clear that in CD4+ TH1 cells, IL-12 signaling is required for the activation of IFN-γ production [21]. However in CD8+ T cells, the impact of IL-12 signaling on effector and memory responses to viral, bacterial, and parasitic infections has only recently come to light. We have previously shown that among the polyclonal CTL populations induced after T. gondii vaccination, IL-12 is strictly required for IFN-γ production and KLRG1+ effector subpopulation differentiation in vivo [22]. KLRG1 expression in CD8+ T cells is thought to mark replicative senescence in a subset of highly activated CTLs [23],[24],[25]. Furthermore, IL-12 can induce KLRG1 through the activation of T-bet, a master regulator of the type 1 immune program [26]. Therefore, it is thought that primary pathogen challenge induces KLRG1 in IL-12-sensitive CD8+ T cells that, while being highly activated effector cells, are short-lived and do not contribute to secondary memory responses.
As a consequence of the effector program initiated by IL-12 signaling during primary CTL activation, memory precursor CTL development may be negatively impacted. IL-12 deficiency was shown to increase memory CTL precursor frequency in the primary response to L. monocytogenes and LCMV [26],[27], and to impart superior immunity to bacterial re-infection [27]. Unlike bacterial and viral systems where multiple innate cytokines can promote CTL effector differentiation [28],[29], in T. gondii infection, IL-12 appears to singularly control CD8+ T cell differentiation and IFN-γ production [22],[30],[31]. Given the absolute requirement for IFN-γ in CTL protective immunity to T. gondii, it is difficult to assess whether memory responses will be enhanced under conditions of complete and permanent IL-12 deficiency. Therefore, to determine to what extent IL-12 negatively impacts memory response to the parasite, we examined the effects of IL-12 insufficiency during primary CTL activation on subsequent effector and memory responses recalled in the presence of IL-12. Interestingly, the requirement of IL-12 for IFN-γ production during the primary response was imprinted only on effector memory cells during an immediate recall response. However, central memory tgd057-specific CTLs, primed in the absence of IL-12 but recalled in an IL-12 competent environment, were able to repopulate the secondary immune response with IFN-γ producing effector memory cells. Our results indicate that IL-12 has divergent consequences for heterogeneous CD8+ subpopulations. Whereas IL-12 is required for effector memory differentiation of KLRG1+ subsets, it is not required for central memory differentiation, and may even negatively impact TCM function.
Results
Identification of a novel H-2Kb-restricted CD8+ T cell population specific for the Toxoplasma antigen tgd057
Given that the T. gondii secreted proteins GRA1, GRA7, ROP1, ROP2, and the parasite surface protein SAG1 have previously been shown to elicit CTL responses in C57BL/6 mice, we initially screened these proteins for putative CTL epitopes. Their predicted protein sequences were analyzed for peptide epitopes using a program that combines four different prediction routines to choose the octameric or nonameric peptide sequences with the highest potential for binding either H-2Kb or H-2Db molecules, respectively [32]. These putative peptide epitopes (Table S1) were synthesized and individually loaded onto the corresponding MHC class I tetramers [33],[34] using caged MHC class I tetramer technology [18],[35]. Peptide-MHC tetramers were screened for binding to purified CD8+ T cells harvested from spleens of CPS-vaccinated mice. Purified naïve CD8α+ splenocytes were used as a negative control. From this first screen, we were unable to confirm the presence of a genuine CTL epitope (data not shown). Therefore, we screened for peptide epitopes from a second, more extensive set of Toxoplasma proteins using the same method (Tables S2 and S3). Included in the second screen was a large set of secreted proteins (both confirmed and uncharacterized but likely secreted based on sequence) as well as a few non-secreted proteins [17]. This second screen resulted in the discovery of a single genuine CTL epitope – SVLAFRRL – derived from the gene tgd057 (Figure 1A), which encodes a putatively secreted protein of unknown function [36].
Fig. 1. Tgd057 localizes to the parasite's cytoskeleton. 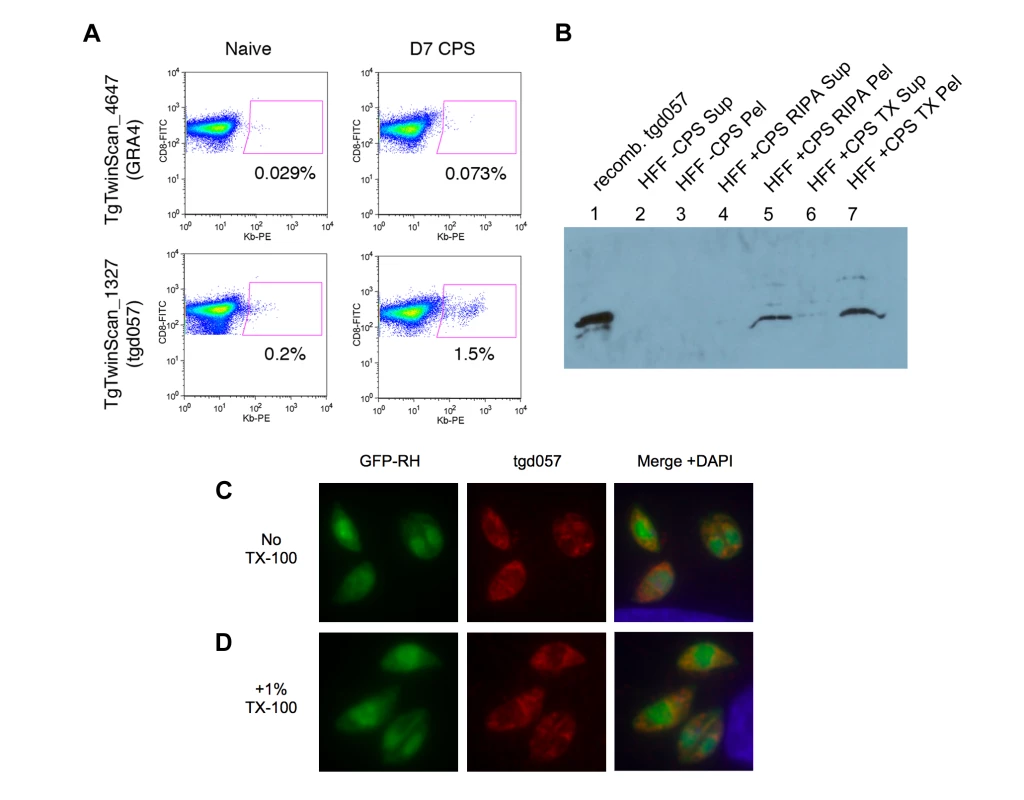
A) Example output data from the screening of putative CTL epitopes derived from GRA4, upper panels, and tgd057, lower panels. The GRA4-epitope tetramer was scored as negative for binding to day 7 CPS-primed CD8α+ T cells, whereas the tgd057-epitope tetramer (Kb/SVLAFRRL) was scored as positive. B) Western blot of CPS parasite cultures using purified anti-tgd057 Abs. Lane 1, 0.15 µg of recombinant tgd057. Lanes 2–3, supernatant and pellet, respectively, of an uninfected HFF culture extracted in mRIPA buffer. Lanes 4–5, supernatant and pellet, respectively, of a CPS-infected HFF culture extracted in mRIPA buffer. Lanes 6–7, supernatant and pellet, respectively, of a CPS-infected HFF culture extracted in 1% Triton X-100 buffer. C and D) Immunofluorescence images of HFFs infected with GFP-RH parasites for 4 hours. C, Cells were fixed and stained with anti-tgd057 Abs, and the nuclear stain DAPI. D, Cells were fixed, treated with 1% Triton X-100 for 20 min, and then stained as in C. From its initial characterization, Wan et al reported that the tgd057 gene sequence encoded a secretion signal peptide (residues 1–21), which would allow for trafficking to the three secretory organelles: micronemes, rhoptries, or dense granules. Since tgd057 contains no other known targeting motif, we predicted that tgd057 would reside in the dense granules, the default pathway for the constitutive secretion of proteins into the parasitophorous vacuole [37]. To establish the localization of tgd057, we first generated polyclonal antisera against recombinant tgd057. Anti-tgd057 antibodies were further purified from the antisera by reactivity to recombinant protein bound to nitrocellulose and extraction from tgd057+ bands. To demonstrate the specificity of our purified, polyclonal anti-tgd057 Abs, we compared their reactivity to recombinant tgd057 protein, uninfected HFF lysates, and CPS-infected HFF lysates. To further characterize tgd057, we performed the protein extraction using two different buffers: modified RIPA and 1% Triton X-100. The anti-tgd057 Abs showed no reaction to either the supernatant or pellet of mRIPA-extracted, uninfected HFF lysates (Figure 1B, lanes 2 and 3). However, there is a clear band in the pellet but not supernatant of the mRIPA-extracted CPS-infected HFF lysate (Figure 1B, lanes 4 and 5), corresponding to a band in the recombinant tgd057 lane (Figure 1B, lane 1). Upon Triton X-100 extraction of the CPS-infected HFF lysate, the majority of tgd057 partitioned to the insoluble fraction. (Figure 1B, lanes 6 and 7). Moreover, multiple minor tgd057 bands with slower mobility were also visible in the mRIPA - and Triton X-100-insoluble CPS-infected HFF lysates, suggesting presence of tgd057 isoforms. Overall, the insolubility of tgd057 in mRIPA and Triton X-100 buffers suggests that tgd057 is associated with membrane insoluble structures such as the parasite's cytoskeleton, although some may be trafficked to membrane soluble structures such as the secretory organelles.
To further characterize the localization of tgd057, immunofluorescence staining was performed on HFFs infected with GFP-expressing RH parasites. As depicted in Figure 1C, the staining pattern is unlike that of the secretory organelles and appears to stain structural elements near the apical complex and also at the posterior end of the parasite. To confirm that tgd057 is resistant to detergent extraction as suggested by Figure 1B, infected HFFs were treated with 1% Triton X-100 to remove membrane-associated proteins prior to staining. In Triton X-100-treated samples, tgd057 staining is clearly retained (Figure 1D).
Tgd057-specific CD8+ T cells are effector cells phenotypically representative of the polyclonal CTL population
Although the precise localization and function of tgd057 remains to be elucidated, it is clear from our screen that tgd057 is available for processing and presentation to CD8+ T cells in vivo. To ascertain the relationship between epitope-specific CD8+ T cells and the polyclonal response to T. gondii infection, the numbers and activation phenotype of tgd057-specific cells were compared to that of the total CD8+ T cell population after vaccination or infection with avirulent parasites. Mice were vaccinated with a single dose of attenuated, CPS-mutant parasites known to elicit protective immunity [38],[39]. CPS vaccination primed CD44hi tgd057-specific CTLs, which constituted 7.66% of the total PEC CD8+ T cells and 2.92% of the total splenic CD8+ T cells on day 8 post-vaccination (Figure 2A and 2B). As we have previously published [22], the polyclonal CTL response is composed of four subpopulations based on the expression of the cell surface activation markers–CD62L and KLRG1. The four subpopulations are termed Fraction (F) I (CD62Lhi KLRG1-), FII (CD62Llo KLRG1-), FIII (CD62Llo KLRG1+), and FIV (CD62Lhi KLRG1+). FI was thought to contain undifferentiated CD8+ T cells due to the lack of granzyme B and IFN-γ expression during the primary effector response. Furthermore, the FI phenotype is shared by the majority of CD8+ T cells in the spleens of naïve mice (Figure 2A). The other three subpopulations induced by Toxoplasma infection contained varying levels of granzyme B+ and IFN-γ+ effector cells, with FIII representing the most effector cell-rich subpopulation [22]. When the PEC tgd057-specific cells were analyzed for CD62L and KLRG1 expression after CPS vaccination, all four subpopulations were represented, including the seemingly undifferentiated FI subset (Figure 2B). In the spleens of these mice, all four subpopulations of tgd057-specific CTLs were also present, although mature FIII phenotype cells were over-represented. To view the immune response to natural Toxoplasma infection, mice were perorally infected with cysts of the avirulent ME49 strain, which not only acutely infects the host, but also establishes chronic infection in the brain and muscle tissue. During the acute phase of ME49 infection, CD44hi tgd057-specific CD8+ T cells were present both in the PECs and in the spleen at a comparable frequency to CPS vaccination (Figure 2C), however ME49 infection induced fewer numbers of tgd057-specific CTLs in these tissues (Figure S1). Heterogeneous effector CTL subpopulations were similarly induced by ME49 infection, but the tgd057-specific CTLs were particularly enriched in FIII phenotype cells, both in the PECs and the spleen. During the chronic phase of ME49 infection, the frequencies and numbers of PEC and splenic CD44hi tgd057-specific CTLs were reduced but still detectable (Figure 2D and S1). In all three tissues sampled, the tgd057-specific CTLs were representative of the total CD8+ T cell population. Interestingly, FIII CTLs remained a major subpopulation both in the spleen and in the periphery, suggesting that KLRG1 positivity can indeed be a stable, long-lasting phenotype. A significant pool of tgd057-specific CTLs was found in the brains of chronically infected mice, a site of spontaneous T. gondii recrudescence, where FII and FIII were the predominant subpopulations (Figure 2D and S1). In general, tgd057-specific CTLs were phenotypically similar to the polyclonal CD8+ T cell population, except during acute infection, where mature effector-phenotype cells were enriched and the naïve-like subpopulation was diminished in the spleen.
Fig. 2. Tgd057-specific CTLs are induced by CPS vaccination and natural ME49 infection. 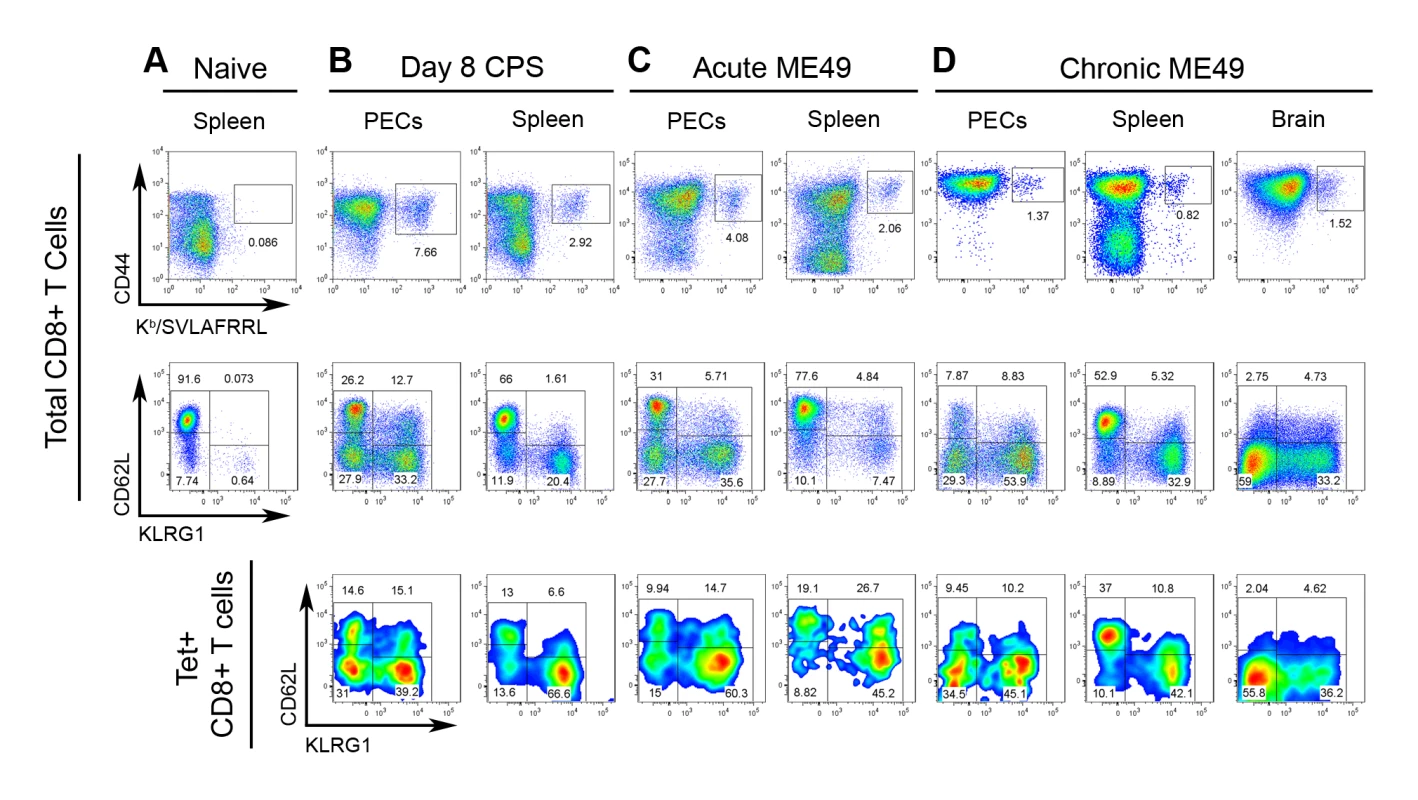
Samples from A) naïve mice and B–D) T. gondii infected mice were stained for CD44 and Kb/SVLAFRRL positivity and the expression of CD62L and KLRG1 in CD8+ T cells ex vivo. Upper row, frequency of CD44hi Kb/SVLAFRRL+ cells of total CD8α+ TCRβ+ cells. Middle row, total CD8α+ cells were analyzed for CD62L and KLRG1 expression. Lower row, Kb/SVLAFRRL+ CD8α+ cells were analyzed for CD62L and KLRG1 expression. A) Spleens were harvested from naïve mice. B) Mice were vaccinated with CPS parasites and PECs and spleens were harvested on day 8 post-vaccination. C) PECs and spleens were harvested on day 7 post-infection of mice perorally infected with 20 ME49 cysts. D) PECs, spleens, and the brain mononuclear cells were analyzed in day 48 cyst-infected mice (as in C). One representative mouse was shown of at least three samples. For the PEC and spleen analysis in A–D, one of at least three independent experiments is shown. For the brain mononuclear cell analysis in D, one of two experiments is shown. Analysis of effector molecule expression between the epitope-specific and total CD8+ T cell populations would provide a direct assessment their relative effector capability. Therefore, granzyme B and IFN-γ production were measured in both tgd057-specific CTLs and the total CD8+ T cell population. Effector cells from day 8 CPS-primed mice were compared to naïve splenocytes and evaluated for granzyme B and IFN-γ co-expression ex vivo and in response to live parasite infection or peptide restimulation (Figure 3). Tgd057-specific CTLs were highly enriched in granzyme B+ effector cells ex vivo; 54% and 49% of the PEC and splenic tgd057-specific CTLs were granzyme B+, respectively, whereas in the total PEC and splenic CD8+ T cells, 29% and 8% were granzyme B+, respectively (Figure 3A). After restimulation with CPS parasites both IFN-γ and granzyme B were upregulated in total PEC and splenic CD8+ T cells, of which the tgd057-specific CTLs remained enriched in frequency of effector molecule-positive cells (Figure 3B). Whole PECs and spleen cultures were also subjected to peptide restimulation to demonstrate the specificity of tgd057-specific CTLs. As shown in Figure 3C, IFN-γ was induced by both PEC and splenic CD8+ T cells in the presence of cognate peptide (SVLAFRRL) but not non-cognate peptide (SIINFEKL). The frequency of tgd057-specific cells and thus Kb/SVLAFRRL+ IFN-γ+ cells was reduced after cognate peptide restimulation, a response we attributed to TCR downregulation, a well-characterized response to strong TCR ligation [40]. Taken together, tgd057-specific CTLs are highly enriched in granzyme B+ effector cells and produce the protective cytokine IFN-γ in response to live parasites and cognate peptide.
Fig. 3. Effector molecule expression among tgd057-specific CTLs. 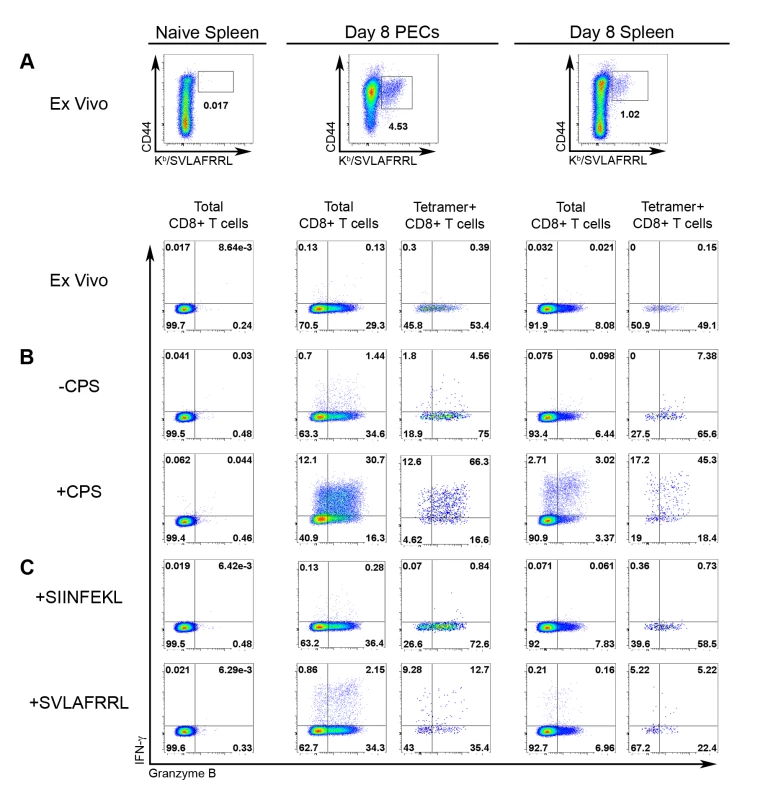
Expression of IFN-γ and granzyme B was analyzed in CD8+ T cells from naïve and day 8 CPS-primed mice. A) The frequencies of tgd057-specific CTLs (CD44hi, Kb/SVLAFRRL+, CD8α+, and TCRβ+ cells) was compared between naïve and primed samples ex vivo, upper row. IFN-γ and granzyme B expression was evaluated between CD8+ T cells from the naïve spleen, and total CD8+ T cells and tgd057-specific (Tetramer+) CD8+ T cells from day 8 CPS-primed PECs and splenocytes, lower row. For each of these populations, IFN-γ and granzyme B expression was analyzed after CPS restimulation (B) and peptide restimulation (C). B) Samples were either unstimulated (upper row) or restimulated (lower row) with CPS parasites. C) Samples were directly incubated with either non-cognate (SIINFEKL, upper row) or cognate (SVLAFRRL, lower row) peptide. FACS plots represent one of three mice. One of three independent experiments is shown. IL-12 is required for effector differentiation in tgd057-specific CTLs
We have previously demonstrated that the innate cytokine IL-12 produced during Toxoplasma vaccination induces CTL IFN-γ production and suggested that IL-12 promotes terminal maturation at the expense of memory precursor subpopulation differentiation [22]. A limitation of our earlier study was that putative parasite-reactive CD8+ T cells were phenotypically defined by activation markers (CD62L downregulation and KLRG1 upregulation) and effector molecule expression (granzyme B and IFN-γ), rather than by parasite-antigen specificity. By definition, we were not able to detect parasite-antigen specific CTLs or CTL precursors that have yet to express activation markers or effector molecules. Utilizing tetramer staining we are now able to resolve the totality of the natural tgd057-specific CTL response, which includes a significant proportion of undifferentiated FI-phenotype cells (Figure 2B–2D). This allows us to address the question of whether IL-12 truly negatively impacts the KLRG1 - putative memory precursor compartment, which encompasses TCM-like FI (CD62Lhi KLRG1-) and TEM-like FII (CD62Llo KLRG1-) precursor cells. To firmly address the requirements of IL-12 during the primary activation of parasite-specific CTLs, tgd057-specific cells were enumerated and phenotyped in WT and Il12a(IL-12p35)−/− CPS-primed mice. On day 7 post-vaccination, the numbers of total and tgd057-specific CTLs were 3-fold lower in PECs of Il12a−/− mice (Figure 4A). In the spleen, the total numbers of CD8+ T cells were unchanged in Il12a−/− mice, although the numbers of tgd057-specific CTLs were slightly lower on average (Figure 4B). Consistent with our previously published results, the distribution of total PEC effector CD8+ subpopulations shifts in IL-12-deficient mice such that the KLRG1 - fractions FI and FII increase in frequency, while the KLRG1+ fractions FIII and FIV become severely diminished in frequency (Figure 4C, left column). Notably, the vast majority of Il12a−/− tgd057-specific CTLs were FI and FII (Figure 4C, right column), mirroring the shift among total PEC CTL populations in the absence of IL-12. This effect was also observed in splenic tgd057-specific CD8+ T cells (Figure 4D). Therefore among both total and tgd057-specific CTLs, pro-inflammatory IL-12 signaling clearly induces the differentiation of the KLRG1+ effector subpopulations FIII and FIV at the expense of the KLRG1 - memory precursor subsets FI and FII.
Fig. 4. Primary CTL activation and differentiation in Il12a−/− mice. 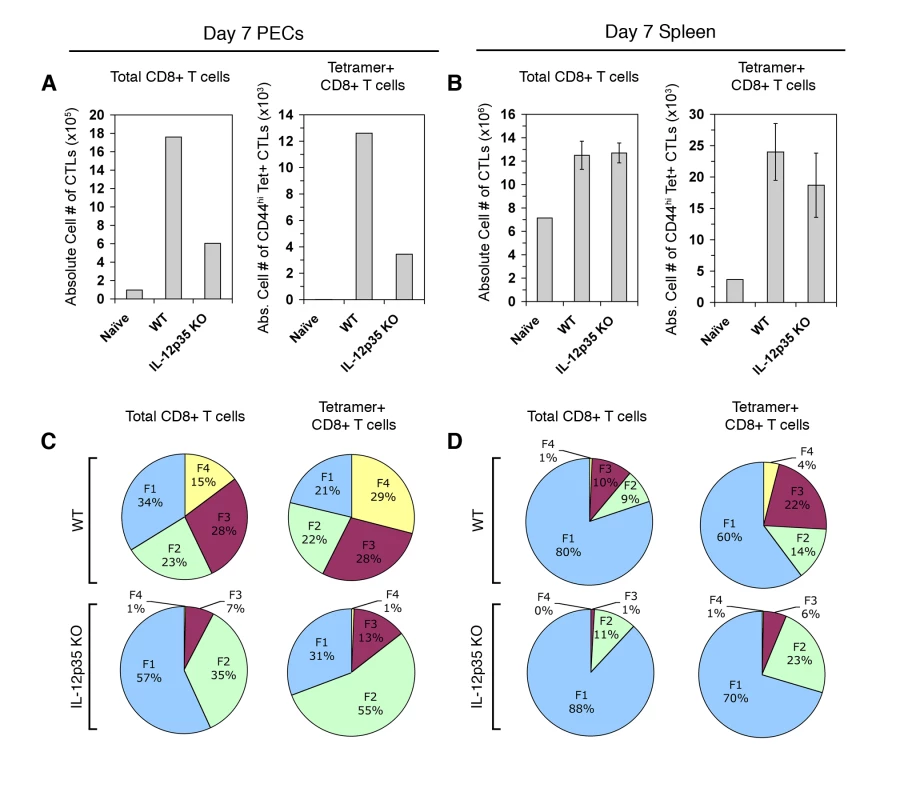
PECs and spleens were analyzed for CTL activation on day 7 post-vaccination in WT and Il12a−/− mice. A and B) Total CD8α+ TCRβ+ cells and CD44hi Kb/SVLAFRRL+ CD8+ T cells were enumerated in PECs (A) and splenocytes (B) of WT naïve, WT primed, and Il12a−/− primed mice. C and D) Subpopulation distribution is shown in total CD8α+ cells and Kb/SVLAFRRL+ CD8α+ cells in PECs (C) and splenocytes (D) of WT and Il12a−/− primed mice. PEC samples were pools of three mice. Bar graphs are shown as mean ± SEM of three mice. Pie charts are shown as mean values for the spleen samples. Data are representative of three independent experiments. As predicted by our previously published results, IFN-γ-producing effector CD8+ T cells fail to differentiate in Il12a−/− PECs and splenocytes, when assayed by ex vivo restimulation with live parasites (Figure 5A and 5B, left panels). When gated on tetramer+ CTLs, it is also clear that Ag-specific CTLs primed in the absence of IL-12 do not produce IFN-γ in response to parasite restimulation (Figure 5A and 5B, right panels). Although the functional defect in CD8+ T cell IFN-γ production due to in vivo IL-12 deficiency is striking, it is still not clear whether this defect is a result of aberrant in vivo differentiation or the lack of IL-12 signaling during ex vivo restimulation. Therefore, day 8 CPS-primed WT and Il12b(IL-12p40)−/− cells were restimulated under different culture conditions to elucidate the individual contributions of parasite antigens and IL-12 signaling for CD8+ T cell IFN-γ production ex vivo (Figure S2). Neutralizing IL-12 signaling during restimulation with anti-IL-12p40 Abs reduced IFN-γ production in WT CTLs but had no affect on Il12b−/− CTLs (green vs. yellow bars). The addition of rIL-12p70 alone stimulated a low percentage of WT CTLs to produce IFN-γ, however, Il12b−/− CTLs were insensitive to exogenous IL-12 signaling (blue vs. purple bars). The addition of CPS parasites plus rIL-12p70 into the restimulation culture not only induced WT CTLs to make more IFN-γ, but also allowed for Il12b−/− CTLs to produce IFN-γ (red bars). However, the frequency of IFN-γ+ cells among Il12b−/− CTLs was much lower relative to the WT CTLs in both the PECs and the spleen. Therefore, exogenous IL-12 signaling can synergize with antigenic stimulation to induce IFN-γ production in Il12b−/− CTLs, however, it cannot fully recover to WT levels, due to the prominent defect in differentiation that occurs with IL-12 deficiency.
Fig. 5. Tgd057-specific CTLs primed in Il12a−/− mice do not produce IFN-γ in response to parasite restimulation. 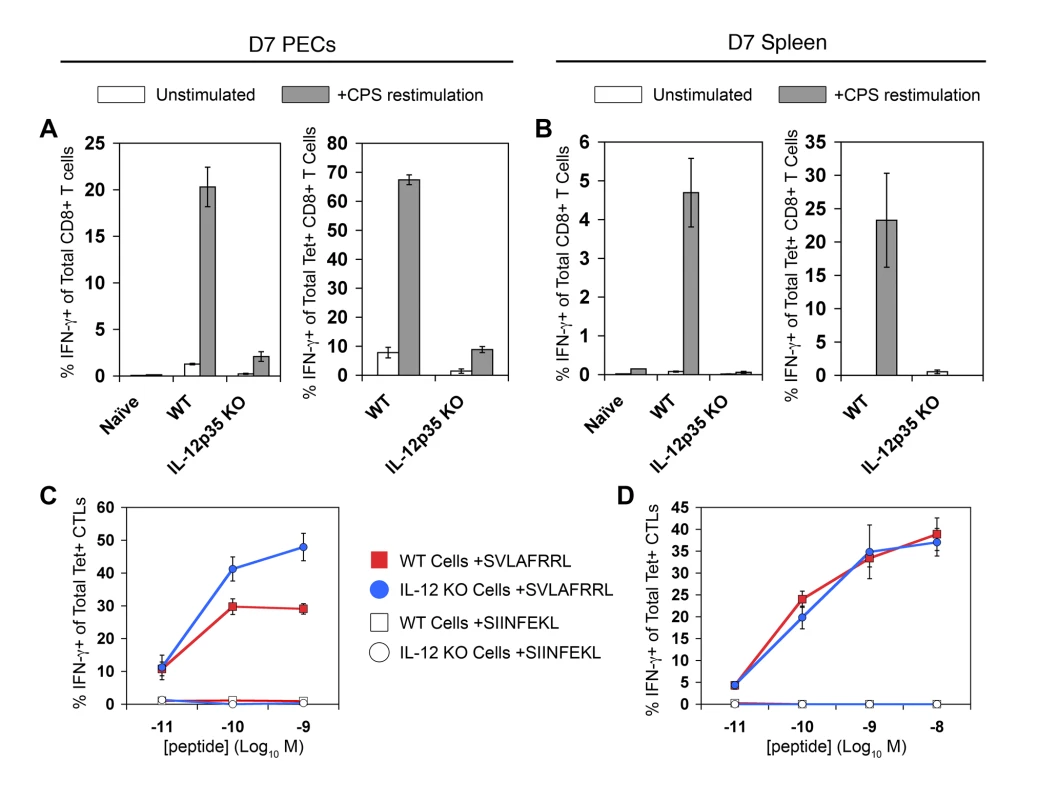
Day 7 CPS-primed PECs and spleens were restimulated ex vivo to assay IFN-γ production. A and B) PECs (A) and splenocytes (B) were restimulated with CPS parasites ex vivo. The frequency of IFN-γ+ cells was determined among total CD8α+ CD3ε+ cells of WT naïve, WT primed, and Il12a−/− primed PECs and spleens, left panels. The frequency of IFN-γ+ cells was determined among total Kb/SVLAFRRL+ CD8α+ CD3ε+ cells of WT naïve, WT primed, and Il12a−/− primed PECs and spleens, right panels. C and D) PECs (C) and splenocytes (D) were directly restimulated with a range of cognate and non-cognate peptide concentrations ex vivo. The frequency of IFN-γ+ cells was determined among total Kb/SVLAFRRL+ CD8α+ CD3ε+ cells of WT primed, and Il12a−/− primed cells. All values are mean ± SEM of three mice. Data are representative of three independent experiments. Because parasite restimulation induces both Ag-dependent T cell activation and cytokine-induced bystander activation, WT and Il12a−/− cells were restimulated with varying concentrations of cognate peptide to determine the ability of tgd057-specific CTLs to respond to TCR ligation only. Surprisingly, both WT and Il12a−/− tgd057-specific CTLs mounted IFN-γ responses to increasing concentrations of cognate peptides (Figure 5C and 5D). The normal IFN-γ responses of Il12a−/− CTLs during peptide restimulation indicate that strong TCR signaling can also overcome the functional defect observed during CPS restimulation. In cultures stimulated with live parasites, it may be that only very low concentrations of SVLAFRRL-peptide are available on antigen presenting cells, thus explaining the strict requirement for pro-inflammatory IL-12 signaling. The difference between microbial and peptide restimulation is an important one and we believe that the IFN-γ responses to whole parasite restimulation better reflects the physiological effector function of CTLs in vivo, as it is well documented that IL-12-deficient mice rapidly succumb to toxoplasmosis due to the lack of T cell IFN-γ production [20],[31].
Effector memory CTL differentiation in IL-12-deficient immune mice
In the murine model of listeriosis, Pearce et al demonstrated that IL-12 deficiency enhances bacterial immunity through the increased generation of memory precursor CTLs (specific for the model antigen OVA) [27]. However, it is not known whether IL-12 has a similar role in memory CTL responses to parasites. Given the differential requirements of IL-12 for effector CTL subset differentiation in the primary response (Figure 4C and 4D), it may be that the enhancement of CD8 memory in IL-12-deficient conditions will be evident as an increase in the frequency of one or more subpopulation(s). By tracking the development and effector function of tgd057-specific CTLs in WT and IL-12-deficient immune mice, we can for the first time elucidate the role of IL-12 in effector/memory differentiation of a natural CTL population induced by parasite vaccination. In agreement with studies in bacterial and viral systems [27],[41], the numbers of PEC tgd057-specific CTLs were greater in Il12a−/− immune-rested mice (day 30 post-vaccination) compared to WT immune-rested mice, despite similar numbers of total CD8+ T cells (Figure 6A). In the spleens of Il12a−/− immune mice, however, the numbers of total CD8+ T cells and tgd057-specific CTLs were reduced on average, but not significantly different (Figure 6B). Tgd057-specific CD8+ T cells of WT immune-rested mice remained heterogeneous, with FI, FII, and FIII subsets present in PECs and the spleen (Figure 6C and 6D, upper row). The KLRG1+ FIII subset represented a major subpopulation of tgd057-specific memory CTLs. This is surprising since KLRG1 positivity is a marker of replicative senescence [24]. Despite the apparent longevity of some KLRG1+ cells, the effect of IL-12 deficiency persisted in memory tgd057-specific CTLs, where the balance of KLRG1 negative and positive subpopulations continued to favor the KLRG1 - subsets in the absence of IL-12 (Figure 6C and 6D). Strikingly, the FI subpopulation remained a major constituent of the PEC tgd057-specific CTLs of Il12a−/− immune mice, whereas this subset was virtually absent from the PEC tgd057-specific CTLs of WT immune mice (Figure 6C), an effect that was not reflected in the total WT and Il12a−/− PEC populations. Hence, IL-12 deficiency during the priming of tgd057-specific CTLs may indeed result in a greater proportion of KLRG1 - memory CTLs.
Fig. 6. IL-12 is required to differentiate IFN-γ-producing effector memory CTLs. 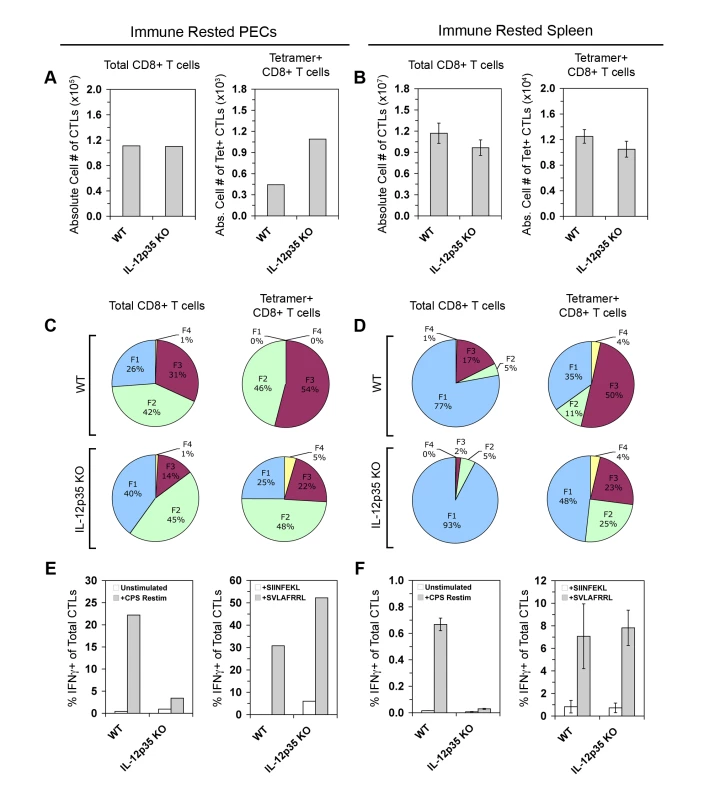
PECs and spleens of immune rested (day 28 post-CPS vaccination) WT and Il12a−/− mice were analyzed for numbers, phenotype, and effector function in memory CTLs. A and B) Total CD8α+ TCRβ+ cells and CD44hi Kb/SVLAFRRL+ CD8+ T cells were enumerated in PECs (A) and spleens (B) of WT and Il12a−/− immune mice. C and D) Subpopulation distributions were shown in total CD8α+ cells and Kb/SVLAFRRL+ CD8α+ cells in PECs (C) and spleens (D) of WT and Il12a−/− immune mice. E and F, left panels) WT and Il12a−/− immune CD8α+ T cells among PECs (E) and from the spleen (F) were analyzed for IFN-γ positivity after restimulation with CPS parasites in vitro. E and F, right panels) WT and Il12a−/− immune Kb/SVLAFRRL+ CD8α+ cells among PECs (E) and from the spleen (F) were analyzed for IFN-γ positivity after restimulation with either non-cognate (SIINFEKL) or cognate peptides (SVLAFRRL) in vitro. PEC samples were pools of four mice. Bar graphs are shown as mean ± SEM of four mice. Pie charts are shown as mean values for the spleen samples. One of two representative experiments is shown. The persistent changes in subpopulation distributions observed in Il12a−/− immune rested mice suggested that there may also be long-lasting functional defects in TEM IFN-γ responses. After ex vivo parasite restimulation, Il12a−/− memory CTLs mounted a weak recall response, whereas WT memory CTLs were efficient IFN-γ producers, especially in peripheral tissue (Figure 6E and 6F, left panels). As previously observed in the primary response, tetramer+ CD8+ T cells from Il12a−/− immune mice were competent IFN-γ producers after cognate peptide restimulation (Figure 6E and 6F, right panels). Therefore the enrichment of KLRG1 - memory CTL precursors in IL-12 deficient conditions may be carried over from the primary response into the memory phase, where the KLRG1 - subpopulations FI and FII account for nearly 75% of all tgd057-specific memory CTLs compared to 46% in WT immune mice. However, IL-12 remains a critical signal for IFN-γ production to parasite restimulation in primary-effector and effector-memory CD8+ T cells.
IL-12 signaling during primary CTL activation is required for immediate recall responses but not for secondary CTL activation in immune mice
Although IL-12 is required for primary-effector and effector-memory differentiation, it is not yet clear how it may affect central memory CD8+ T cells. In primary anti-Toxoplasma effector CTLs, we have previously shown that the FII, FIII, and FIV subpopulations were equally responsive to ex vivo IL-12 signaling via pSTAT4 activation [22]. However, the FI subset was unresponsive to IL-12 signaling, suggesting that the detrimental effects of IL-12 in short-lived effector cell differentiation might spare the FI subset during the primary response. Since these TCM-phenotype FI cells are enriched in Il12a−/− immune mice, TCM function might be normal or even enhanced in memory CTLs differentiated in an IL-12 deficient environment. To test this hypothesis, we transiently neutralized IL-12 in vivo during primary vaccination and queried the ability of the subsequent IL-12-sufficient immune mice to mount an immediate recall response in vivo and to generate effector memory cells 6 days after secondary challenge. Similar to the effects of genetic deletion of IL-12, mice that received anti-IL-12p40 Abs generated reduced numbers of PEC tgd057-specific CTLs on day 8 post-vaccination, which were diminished in KLRG1+ subsets and refractory to CPS restimulation (Figure 7A–7C). The effect of IL-12 neutralization was less severe in the spleen, which may be due to the i.p. route of Ab administration. On day 28 after the initial vaccination, the numbers of tgd057-specific CTLs were increased in PECs but decreased in the spleen of anti-IL-12 treated mice (Figure 7D), similar to Il12a−/− immune rested mice. Furthermore, KLRG1+ subsets, especially FIII, were under-represented in PEC and splenic tgd057-specific CTLs compared to control mice (Figure 7E). To test if effector memory CTLs from IL-12-neutralized immune mice could mount an immediate recall response in IL-12 replete conditions, mice were rechallenged with CPS parasites in vivo. PECs were harvested 12 hours later and directly stained for effector molecule expression. As shown in Figure 7F, recalled control effector memory CTLs expressed granzyme B and IFN-γ whereas those from IL-12 neutralized mice did not. Therefore, the effect of IL-12 deficiency during priming is imprinted on TEM and cannot be ameliorated by the IL-12 sufficient conditions prevailing during the immediate recall response. As a measure of TCM function, which is distinguished by high proliferative potential, IL-12-neutralized immune mice were rechallenged with a high dose of CPS parasites and assayed for secondary CTL activation 6 days later. Upon secondary challenge, the numbers of tgd057-specific CTLs, effector subset distribution, and effector function normalized between control and IL-12-neutralized groups (Figure 7G–7I). Taken together, these data suggest that central memory CD8+ T cells develop normally in the absence of IL-12 during the initial priming events.
Fig. 7. In vivo IL-12 neutralization during vaccination generates defective effector memory but not central memory CTLs. 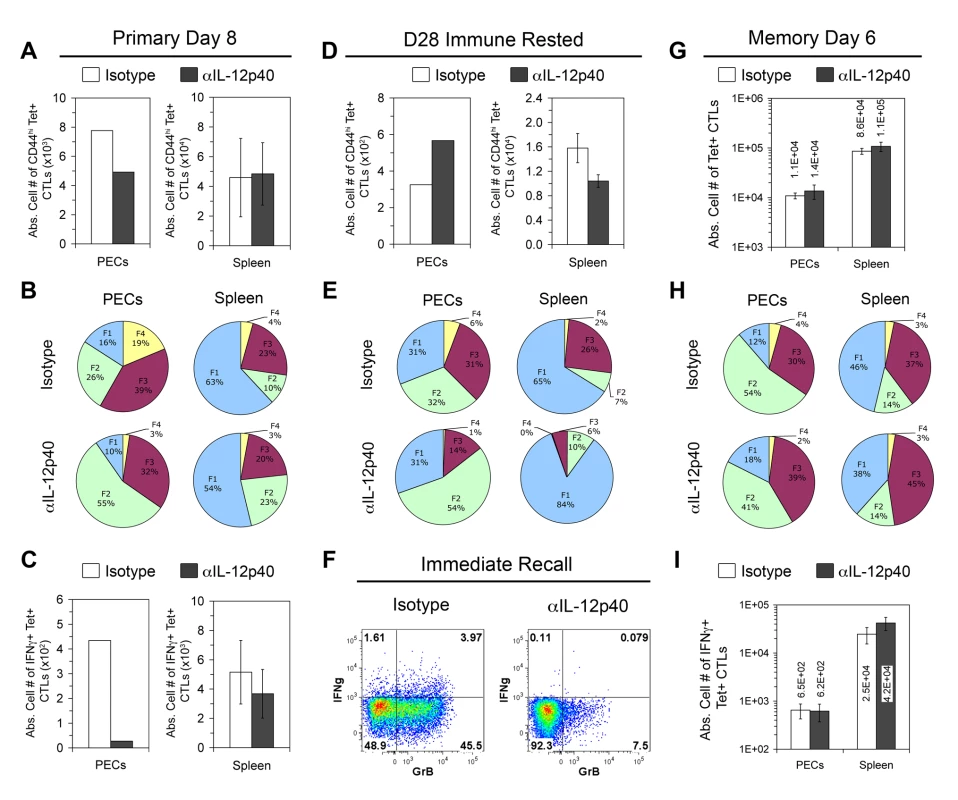
All mice were treated with Abs against IL-12p40 or an isotype control during CPS vaccination. A–C) To confirm the effect of IL-12 neutralization, PECs and spleens were analyzed for the activation of tgd057-specific CTLs on day 8 post-vaccination in IL-12p40 treated (Rx) or isotype Rx mice. CD44hi Kb/SVLAFRRL+ CD8α+ TCRβ+ cells were enumerated in A, and the subpopulation distributions of Kb/SVLAFRRL+ CD8α+ cells were given in B. Samples were restimulated with CPS parasites and the frequency of IFN-γ+ cells in total Kb/SVLAFRRL+ CD8α+ cells is shown in C. D and E) Resting numbers and subpopulation distributions of tgd057-specific CTLs were analyzed in IL-12p40 Rx or isotype Rx immune rested mice as in A and B. F) Immune rested mice were in vivo rechallenged with 2×106 CPS parasites for 12 hrs. PECs from IL-12p40 Rx or isotype Rx rechallenged immune mice were harvested and ex vivo stained for IFN-γ and granzyme B expression in total CD8α+ TCRβ+ cells. FACS plots are from one of three mice per group. G–I) Immune rested mice were in vivo rechallenged with 107 CPS parasites. On day 6 post-rechallenge, PECs and spleens were analyzed for the secondary activation of tgd057-specific CTLs. G and H) Resting numbers and subpopulation distributions of tgd057-specific CTLs were analyzed in IL-12p40 Rx or isotype Rx immune rested mice as in A and B. I) IFN-γ production was assessed in Kb/SVLAFRRL+ CD8α+ cells after CPS restimulation. PEC samples were pools of 3–4 mice. For unpooled samples, bar graphs are shown as mean ± SEM of 3–4 mice and pie charts are mean values of 3–4 mice. One of two representative experiments is shown. IL-12 negatively regulates memory marker-expression among tgd057-specific CTLs
It has become clear that IL-12 deficiency results in the loss of KLRG1+ effector CTLs and hinders the ability of primary and memory cells to respond to parasite rechallenge. While CD8+ T cell numbers and function rely on IL-12, it is not yet clear if there is a qualitative change among memory precursor cells differentiated in the absence of IL-12 during T. gondii infection. Therefore, we probed for qualitative changes in memory phenotype between WT and Il12b−/− CD8+ T cells during primary (day 8) and memory (day 40) phases. CD127 (IL-7Rα) is a memory marker known to be selectively expressed on memory precursor cells [42], which is necessary for their survival [43]. As shown in Figure 8A, there was a greater frequency of splenic tgd057-specific memory precursor cells expressing CD127 from IL-12-deficient mice on day 8 post-vaccination. Moreover, the enhanced CD127 positivity persisted among splenic memory tgd057-specific CTLs on day 40 post-vaccination. As a second measure of memory quality, IL-2 expression, a characteristic of memory-lineage CD8+ T cells [42], was also analyzed. IL-2 positivity has been used in conjunction with TNFα and IFN-γ positivity to define “multifunctional” TH1 cells that are protective against Leishmania major [44], and similarly with CD8+ T cells, the presence of multifunctional CTLs correlates with vaccine efficacy [45]. To measure IL-2 expression via intracellular staining we restimulated splenocytes with the polyclonal T cell activators PMA and ionomycin, and therefore, memory-lineage cells will be both IFN-γ+ and IL-2+. Like CD127, the frequency of IFN-γ+ IL-2+ cells among splenic tgd057-specific CTLs was significantly increased in IL-12-deficient mice on day 8 (Figure 8B). However, there was no significant difference IFN-γ/IL-2-positivity between WT and Il12b−/− memory tgd057-specific CTLs, which is probably due to the ability of all memory CD8+ T cells to produce IL-2. Therefore, there is a qualitative shift among primary tgd057-specific CTLs whereby this population appears more memory-like in the absence of IL-12. Furthermore, the enhanced CD127 positivity among IL-12-deficient CTLs is retained from the primary phase to the memory phase, showing that the effects of IL-12 deficiency can fixed as memory CTLs progressively differentiate over time.
Fig. 8. IL-12 inhibits CD127 and IFN-γ/IL-2 expression among tgd057-specific CTLs. 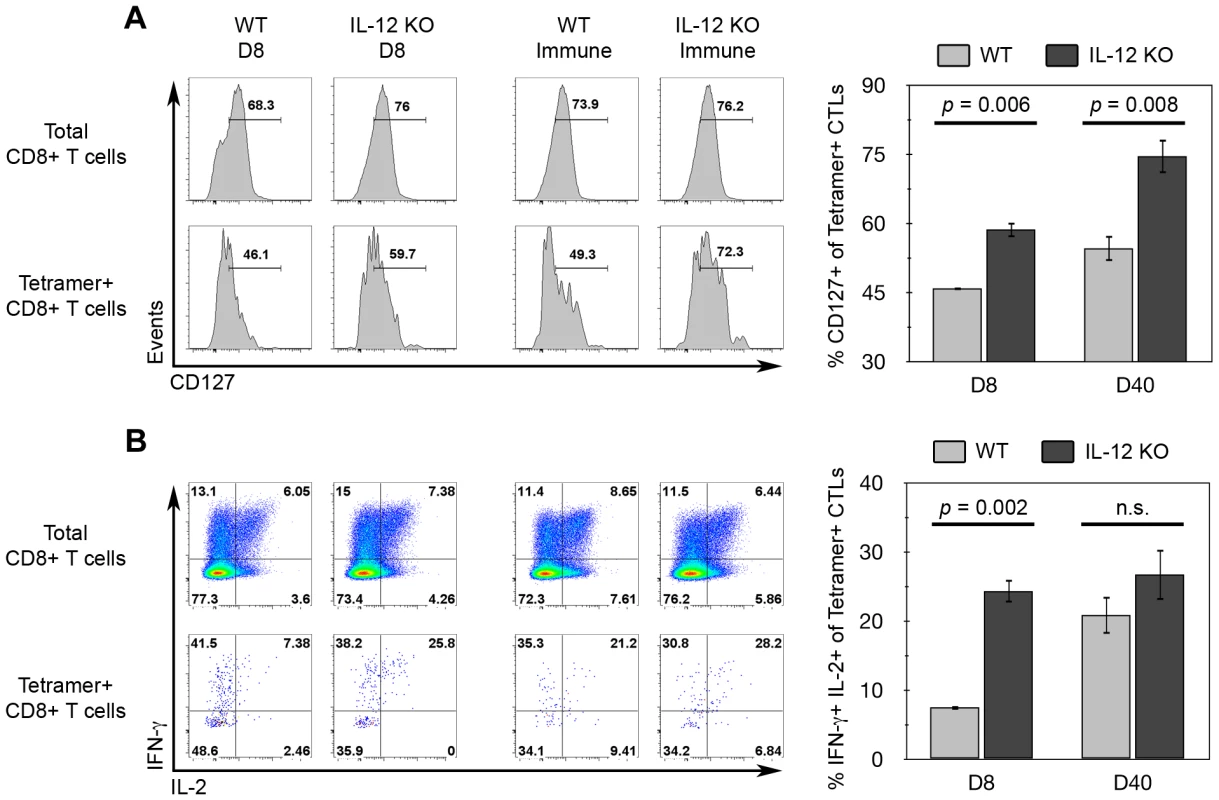
WT and Il12b−/− mice were vaccinated with CPS parasites and spleens were harvested on day 8 (D8) and day 40 (D40, Immune) post-vaccination and analyzed by flow cytometry for memory markers expression among tgd057-specific CTLs. A) Ex vivo analysis of CD127 expression. Left panel, histograms of CD127 expression among either total CD8α+ TCRβ+ cells (total CD8+ T cells) or CD44hi Kb/SVLAFRRL+ CD8α+ TCRβ+ cells (Tetramer+ CD8+ T cells) from each group. Right panel, mean values of the frequency of CD127+ cells gated on Tetramer+ CD8+ T cells. B) IFN-γ and IL-2 expression after ex vivo restimulation with PMA and ionomycin for 4 hours. Left panel, IFN-γ and IL-2 co-expression profiles among either total CD8+ T cells or Tetramer+ CD8+ T cells from each group. Right panel, mean values of the frequency of IFN-γ and IL-2 double-positivity gated on Tetramer+ CD8+ T cells. Bar graphs are shown as mean ± SEM of four mice. n.s., not significant. Central memory CTLs differentiated in the absence of IL-12 are superior generators of secondary effector cells
Transient IL-12-neutralization during T. gondii infection suggested that TCM development does not require IL-12. However, due to the limitations of our experimental approach, it is not yet certain whether the complete absence of IL-12 will alter the functional quality of TCM. To more precisely assay the functional capabilities of central memory CTLs differentiated under IL-12-deficient conditions, CD44hi FI CD8+ splenocytes from WT and Il12a−/− immune mice were sorted and co-transferred into naïve recipient mice. Given the increased precursor frequency of FI+ tgd057-specific memory CTLs in Il12a−/− immune mice (Figure 5C), donor FI+ CTLs were normalized such that the same number of predicted tgd057-specific cells of each genotype were transferred. Recipient, WT immune donor, and Il12a−/− immune donor CTLs were distinguished based on the expression of differential congenic markers (Figure 9A). Chimeric mice were then challenged with CPS parasites and assayed for CTL activation and differentiation 5.5 days later. Despite having transferred the same number of tgd057-specific F1 cells (but 58% fewer total FI CD8+ T cells) from immune Il12a−/− mice, secondary tgd057-specific CTLs derived from Il12a−/− TCM were nearly 10-fold more frequent than those derived from WT TCM among PECs on day 5.5 post-challenge (Figure 9A and 9B). Although PEC tgd057-specific CTLs of donor-origin did not accumulate in great numbers compared to the recipient's response, secondary tgd057-specific CTLs derived from Il12a−/−TCM were present in significantly greater cell numbers compared to those derived from WT TCM (Figure 9C). Ex vivo phenotypic analysis of donor CTL populations revealed that FI TCM generated phenotypically heterogeneous secondary effector CTLs regardless of their origin (Figure 9D). Upon restimulation of PECs from chimeric mice, granzyme B+ and IFN-γ-producing effector memory cells were evident in CTL populations originating from both WT and Il12a−/− TCM donor cells (Figure 9E). Consistent with our data shown in Figure 7, it is clear that the development of central memory FI CTLs does not require IL-12. Remarkably, the resultant central memory CTLs differentiated under IL-12-deficient conditions and recalled in an IL-12 sufficient environment have a qualitative advantage in generating a functionally diverse population of secondary effector CTLs. Taken together with the increased representation of F1 memory CTLs observed after priming in the absence of IL-12 (Figure 6), the remarkable enhancement in the apparent fitness of TCM formed in IL-12 deficient hosts indicates that IL-12 negatively impacts the central memory compartment both quantitatively and qualitatively.
Fig. 9. Central memory CTLs from Il12a−/− mice are functionally competent. 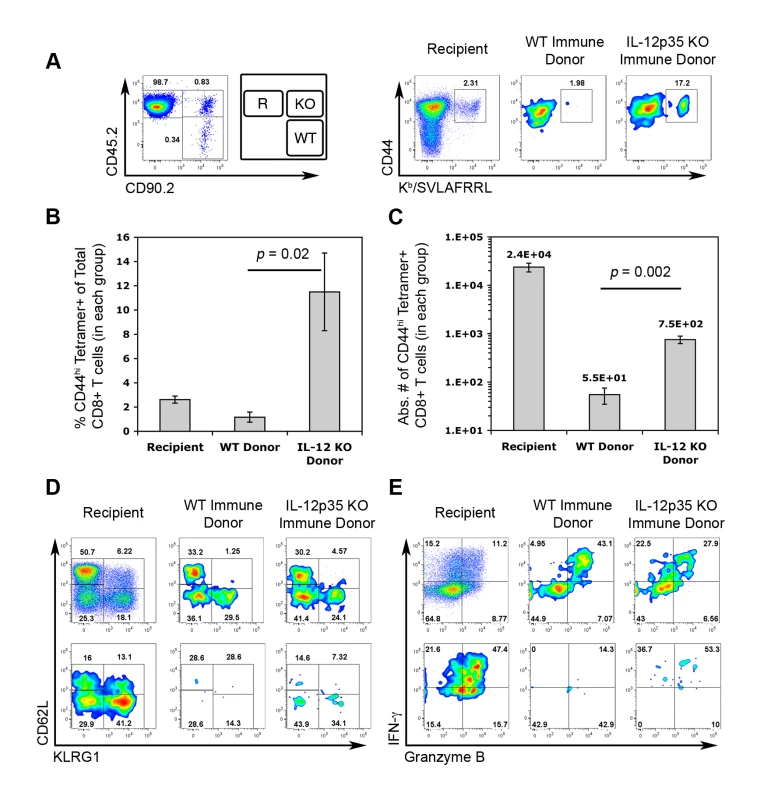
CD44hi FI central memory CD8α+ cells from the spleens of WT and Il12a−/− immune mice were FACS-sorted and then co-transferred into WT naïve mice. We calculated to have transferred ∼1800 FI tgd057-specific memory precursors of each genotype into each recipient mouse. One day post-transfer, chimeric mice were challenged with 3×106 CPS. On day 5.5 post-challenge, PECs were analyzed for the generation tgd057-specific CTLs, subpopulation distributions, and effector function. A, left panel) Donor and recipient cells were distinguished based on the expression of congenic markers. Recipient mice were CD45.2+ and CD90.2-, cells of WT FI TCM origin were CD45.2- and CD90.2+, and cells of Il12a−/− FI TCM origin were CD45.2+ and CD90.2+. The FACS plot shows the relative frequencies of recipient and donor-derived CD8α+ TCRβ+ cells among PECs on day 5.5 post-challenge. A, right panel) The frequency of CD44hi Kb/SVLAFRRL+ CD8α+ T cells among total CD8+ T cells is shown for each gated population in the left panel. B and C) The frequency (B) and absolute cell numbers (C) of CD44hi Kb/SVLAFRRL+ CD8α+ T cells among recipient and donor groups. Bar graphs represent mean values ± SEM of 4 chimeric mice. D) Subpopulation distributions of total CD8+ T cells (upper row) and Kb/SVLAFRRL+ CD8α+ T cells (lower row) among recipient and donor groups. E) Granzyme B and IFN-γ expression in total CD8+ T cells (upper row) and Kb/SVLAFRRL+ CD8α+ cells (lower row) among recipient and donor groups after in vitro CPS restimulation. All FACS plots are from one representative chimeric mouse. One of two independent experiments is shown. Discussion
Using caged MHC class I tetramer screening, we have discovered a new H-2Kb-restricted CTL epitope SVLAFRRL, derived from the T. gondii protein tgd057. Tgd057 joins a growing list of newly identified CTL-targeted Toxoplasma antigens, including GRA6, GRA4, and ROP7. As the CTL epitopes defined for the previously reported three antigens are all H-2Ld-restricted, this study is the first to characterize the CTL response to a natural Toxoplasma antigen in C57BL/6 mice. Because the C57BL/6 mouse strain is the most commonly used genetic background for infectious disease and basic immunology research [19], our discovery contributes an important tool for future research on CD8+ T cell responses during toxoplasmosis. Tgd057 was originally characterized as a highly abundant expressed sequence tag (EST) in T. gondii tachyzoites that is completely unique to this parasite and homologues are not found in other Apicomplexan organisms [36]. Notably, the nucleotide sequence of tgd057 is completely identical amongst Type I, II and III strains of T. gondii (www.toxodb.org/toxo/). Using purified polyclonal anti-tgd057 Abs, we analyzed the localization of tgd057 within the parasite and found that it does not localize to the vacuolar lumen nor the parasitophorous vacuolar membrane, as expected of -secreted proteins. Instead, tgd057 remained largely detergent insoluble, and appears to be associated with the parasite's cytoskeleton. Unlike GRA6 and GRA4, which localize to the intravacuolar network, and ROP7, which traffics to the parasitophorous vacuole membrane, tgd057 staining remained largely within the parasite. It is currently still unclear if tgd057 escapes the parasitophorous vacuole and how processing and presentation of tgd057 occurs. Nevertheless, our results indicate that T. gondii antigens which serve as CTL targets do not necessarily have to be secreted parasite proteins.
Tgd057-specific CTLs were activated during acute infection initiated with either Toxoplasma tachyzoites or bradyzoites, and after vaccination with live, irradiated parasites. Unlike ROP7 - and GRA6-specific CTLs, whose numbers peak at 4–6 weeks post-infection with bradyzoites and correlate with the establishment of chronic infection [16],[17], tgd057-specific CTLs are induced at high frequency on day 7 during acute infection (Figure 2), suggesting that the tgd057 antigen is immediately available for processing in antigen presenting cells. Phenotypically, tgd057-specific effector CTLs were generally representative of the total CD8+ T cell population and contained higher frequencies of cells expressing granzyme B and IFN-γ compared to the polyclonal population. Similar to the immunodominant GRA6-specific CTLs mediating Ld-restricted protective immunity in BALB/c mice which represent 20% of the total CTL population and a presumed majority of the IFN-γ producers [16], tgd057-specific CTLs probably also represent an immunodominant population representing approximately 10% of the total granzyme B+ effector CTL population on day 8 post-vaccination (as shown in Figure 3, where 2.4% (i.e. 53% of 4.53% tetramer+ CTLs are granzyme B+) of CD8+ T cells are both positive for tetramer-binding and granzyme B, whereas 29% of total CD8+ T cells have intracellular granzyme B). Tgd057-specific CTLs (obtained from ES-cloned mice following somatic cell nuclear transfer of individual nuclei from tgd057-tetramer+ CD8+ T cells into ES cells) can mediate significant protective immunity to lethal parasite challenge in adoptive transfer recipients (O. Kirak et al. manuscript submitted), suggesting that tgd057 is a protective CTL target antigen.
In light of previous reports demonstrating enhanced CTL memory responses in the absence of IL-12 during viral and bacterial infection [26],[27], we asked to what extent IL-12 controls memory CTL responses during parasite infection, given that IL-12 drives effector CTL differentiation in the primary response [22]. Using the memory CTL subpopulation distribution to gauge the relative frequencies of TEM (CD62Llo FII and FIII) and TCM (CD62Lhi FI), we observed that IL-12 was required for the KLRG1+ mature FIII TEM subset, but not for the KLRG1 - FI (TCM) and FII (TEM) subsets. Furthermore, in mice transiently depleted of IL-12 during the primary response and in mice genetically deleted of IL-12, the resultant TEM were incapable of mounting IFN-γ recall responses to parasite rechallenge. This indicates that IL-12 signaling during the primary CTL activation initiates a long-lasting effector program, including both IFN-γ production and KLRG1+ FIII subset differentiation. This program is imprinted on TEM and cannot be recovered in the presence of IL-12 during the secondary response. The imprinting effect of IL-12, produced during primary CTL activation, on the effector competence of TEM is consistent with previous in vitro experiments indicating that only a brief window of Ag/costimulation exposure is needed to initiate CTL clonal expansion [46] and that IL-12 stimulation for 30–60 hours in vitro is enough to program optimal effector function in Ag/B7-activated CTLs [47]. To our knowledge, our study is the first to clearly demonstrate effector imprinting by IL-12 in CD8+ effector memory T cells formed in response to microbial infection, an effect that may have been masked in viral and bacterial infection systems, due to the production of multiple and redundant “signal 3” cytokines and to the common practice of using peptide-pulsed rather than naturally-infected antigen presenting cells for restimulation.
The difference in the IFN-γ responses of IL-12-deficient CTLs when restimulated with infected cells versus cognate peptide remains somewhat puzzling. Because the tgd057-peptide dose responsiveness appears to be equivalent in CD8+ T cells developing in the presence or absence of IL-12, it seems unlikely that this difference is simply due to a lower level of antigenic peptide display on infected cells. Whereas the short-term peptide restimulation assay only queried the immediate capability of Ag-specific CTLs to make IFN-γ in response to TCR ligation, the precise contribution of TCR - and cytokine-driven IFN-γ production during the longer parasite restimulation assay is still unclear. Perhaps during the longer period required for the parasite restimulation, a second IL-12-dependent phase of stable IFN-γ production dominates.
It will be interesting to find out whether IL-27 receptor signaling functions along the same pathway as IL-12, given the striking defect in CD8+ T cell responses to T. gondii observed in IL-27R-deficient mice [48]. The role of IL-12 in driving CD8+ T cell effector and effector-memory differentiation which we describe here and elsewhere [22], is strikingly analogous to the established function of IL-12 in enhancing TH1 effector maturation [30],[49] and the recently delineated effect of IL-23 in TH17-mediated inflammation [50]. In all cases, innate signaling does not determine the cytokine phenotype of the T cell response per se. Rather, IL-12/-23's role is to ensure optimal expansion and to drive and maintain the differentiated state of effector cells, such that in their absence, effector cell development is stalled at an earlier differentiation stage.
Although IL-12 plays a positive and protective role in driving effector differentiation of primary-effector and effector-memory T cells responding to T. gondii, our results revealed a negative effect of IL-12 on the TCM compartment. The results presented in Figures 6, 7, 8, and 9 clearly illustrated that IL-12 negative impacts quantitative and qualitative aspects of the central memory response. In Il12a−/− immune rested mice, the reduced frequency of the KLRG1+ FIII subset was accompanied by the emergence of FI as a prominent subset of peripheral memory CTLs (Figure 6B). The prominence of FI in Il12a−/− immune PECs represents a major qualitative shift in the resting pool of peripheral TEM and TCM and suggests that IL-12 drives effector memory differentiation at the cost of TCM development. In addition to the increased incidence of FI cells, our adoptive transfer experiments (Figure 9) revealed a greater proliferative potential of FI-type cells formed in the absence of IL-12, manifesting as increased representation of their descendants during a secondary adaptive immune response. When responding under conditions with sufficient ambient IL-12, FI cells derived from IL-12-deficient mice showed no defect in their ability to generate a heterogenous population of competent effector cells.
Precisely how IL-12 favors the differentiation of effector CD8+ T cells at the expense of central memory T cell or their precursors remains unclear. The mechanism by which IL-12 regulates TCM differentiation may be direct, assuming that at some point during their early development, TCM precursors express IL-12 receptors. Low level or transient IL-12 signaling in TCM precursors might shift the balance of transcription factors that regulate effector versus memory differentiation. Takemoto et al. demonstrated that IL-12 induces T-bet while repressing Eomes in WT effector CTLs [41]. Consequently, IL-12 deficiency during the initial priming may result in greater Eomes expression among central memory precursor CTLs. Eomes expression has been correlated with the enhanced responsiveness of memory CTLs to γc-chain cytokines, as the IL-2/IL-15Rβ chain is a direct target of Eomes [51]. Hence, TCM from IL-12-deficient mice may have enhanced homeostatic proliferation due to IL-15-induced self-renewal. In addition, the recently reported ability of IL-12 to enhance the stability of the immunological synapse [52] may result in a greater frequency of memory precursor T cells differentiating towards a terminally differentiated KLRG1+ effector fate.
Two recent reports highlight a new role for the transcriptional repressor Blimp-1 in the regulation of effector CD8+ T cell terminal differentiation during viral infection. It was demonstrated that CTL differentiation skewed toward TCM development in Blimp-1-deficient mice [53],[54]. As a consequence, these CD8+ T cells lacked KLRG1 expression, effector molecule expression, and cytolytic function - an effect reminiscent of IL-12-deficient CTLs. Interestingly, primary Blimp-1−/− anti-influenza CTLs expressed lower levels of T-bet transcripts and increased levels of Eomes and Bcl6 transcripts [54]. Given that IL-12 signaling also induces T-bet while repressing Eomes expression [41], it seems likely that IL-12 governs the expression of multiple transcription factors, including Blimp-1, for the coordinate induction of the TC1 effector program. However, what the current literature has lacked and what we now show is IL-12's influence extends beyond that of the terminal effector cells, reaching into the memory precursor subpopulation and negatively affecting memory function.
In summary, our results demonstrate that IL-12 signaling has an “antagonistically pleiotropic” effect on the development of effector memory and central memory compartments of the adaptive CD8+ T cell response to an intracellular pathogen. By efficiently promoting effector competence of CD8+ T cells to secrete cytokines and express cytolytic molecules, IL-12 protects the immunologically naive host against acute mortality caused by uncontrolled parasite growth. Nevertheless, this beneficial effect comes at a cost of limiting the reserve pool of memory T cells that can respond to subsequent re-infections. This delicate balance must be considered in the design and implementation of vaccination strategies involving the use of IL-12 and other innate cytokines as immunological adjuvants.
Materials and Methods
Mice and infection
Wild-type (WT) C57BL/6J, Il12a−/− (B6.129S1-Il12atm1Jm/J), Thy1.1+ (B6.PL-Thy1a/CyJ), and CD45.1+ (B6.SJL-Ptprca Pepc/BoyJ) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Il12b−/− mice (C57BL/6NTac-[KO]IL12p40), housed at Taconic Farms (Germantown, NY), were a gift from Dragana Jankovic at the NIAID. All mice were housed under specific pathogen-free conditions at the University of Medicine and Dentistry of NJ (Newark, NJ) and were handled according to UMDNJ Institutional Animal Care and Use Committee guidelines. Sex - and age-matched mice were used in all experiments.
Fresh T. gondii tachyzoites were isolated from infected monolayers of human foreskin fibroblasts (HFFs) in culture. To vaccinate against Toxoplasma, 1×106 live, mutant tachyzoites were i.p. injected into each mouse. These parasites (cps1-1 or CPS) contain a disrupted carbamoyl phosphate synthetase (CPS) II gene, and are avirulent due to the inability to synthesize uracil [38]. In vitro, CPS cultures were grown in DMEM (Invitrogen, Carlsbad, CA) supplemented with 1% FBS, 1% penicillin/streptomycin (P/S), 1% L-glutamine, and 300 µM uracil. To ensure that the CPS parasites do not revert to the virulent parental strain (RH) in vivo, CPS parasites were irradiated (150 Gy) using a 137Cs source before injection. Natural Toxoplasma infection was mimicked by peroral administration of brain cysts containing T. gondii bradyzoites. On the day of infection, cysts were harvested from the brains of C57BL/6 mice chronically infected with the ME49 strain. Then, each mouse was gavaged with brain matter containing 20 cysts. GFP-RH parasites were obtained from American Type Culture Collection.
Epitope prediction and peptide synthesis
Primary amino acid sequence data from selected ORFs was collected from the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov/) and the Toxoplasma gondii Genome resource (www.toxodb.org/toxo/). The first and second screen consisted of 6 and 73 ORFs, respectively, from which 96 and 384 H-2Kb-restricted 8-mer and H-2Db-restricted 9-mer peptides were predicted (Tables S1, S2 and S3) using a consensus epitope prediction program [32],[34]. The peptides identified for screening were synthesized by the biopolymers facility at MIT CCR (Cambridge, MA), whereas the conditional ligands were obtained by manual solid-phase peptide synthesis using commercially available Fmoc-protected amino acids as described earlier [34]. Quality assurance of the synthesis was provided by MALDI-TOF mass spectrometry, after which the crude peptides were lyophilized, taken up in DMSO (10 mg/mL), and kept at -20°C.
Generation of MHC class I tetramers for epitope screening
Refolding of recombinantly expressed H-2Kb and H-2Db complexes holding the SV9-P7* conditional ligand, biotinylation of the soluble MHC class I products and ensuing tetramerization was performed according to published protocols. Peptide exchange was performed in a 96-well plate on ice using longwave UV irradiation to replace the conditional ligand with cognate peptide as described [34].
Generation of anti-tgd057 Abs
Tgd057 cDNA (accession AY313956) was subcloned into the BamH1 site of pQE-60 bacterial expression vector containing a C-terminal 6xHis tag (QIAGEN). The tgd057 expression vector was transduced into E. coli XL1 blue supercompetent cells and tgd057 protein was induced by the addition of 1 mM Isopropyl-b-D-thiogalactoside. After 4 hours of induction recombinant tgd057 protein was harvested from the bacteria, purified by Ni-NTA affinity chromatography, and subsequently dialyzed to remove contaminating molecules, all according to the manufacturer's instructions (QIAGEN). The expression of tgd057 was detected by western blotting with anti-His mAbs and coomassie blue staining. Tgd057 antiserum was commercially generated by immunizing rabbits with 2 doses of 0.5 mg recombinant tgd057 over 2 months (Cocalico Biologicals, Reamstown, PA).
About 180 µg of purified recombinant tgd057 protein was further fractionated by SDS-PAGE. Protein was transferred to PVSF membrane, and then stained with Porcean S for 5 min followed by 2 washes in distilled water with gentle shaking. The tgd057 protein band, dominantly displayed at a MW of about 23 kDa, was cut from the membrane. The membrane strip was blotted with 200 µL anti-tgd057 serum in 20 mL PBS with 3% BSA and 0.05% Tween-20 overnight at 4 degrees C. Anti-tgd057 Abs were stripped by incubating the membrane strip with 5 mL of 100 mM glycine, pH 2.6 for 2 min. The specificity of the purified antibodies was confirmed by comparison with anti-His mAbs in detecting recombinant tgd057 using a western blot.
Western blot
Two flasks of HFFs were inoculated with CPS parasites. Two days later, parasites were harvested just before HFF lysis. Infected HFFs were scraped and lysed by needle passage. After centrifugation, the resultant pellet from one flask (HFF+CPS) was resuspended in 100 µL lysis buffer (Modified RIPA Buffer: 50 mM Tris-HCl, pH 7.4 + 1% NP-40 + 0.25% Na-deoxycholate + 150 mM NaCl + 1 mM EDTA). The pellet from the other flask was vigorously resuspended and incubated in 100 µL 1% Triton X-100 in PBS on ice for 15 min. For the negative control, one flask of uninfected HFFs (HFF-CPS) was harvested and resuspended in 100 µL mRIPA buffer. All lysates were then centrifuged at 10,000 rcf for 15 min. The resultant supernatants were removed and transferred to a new tube. All pellets were resuspended in 100 µL modified RIPA buffer. 20 µL of each sample was separated on 4–12% Ready Gel precast gels (BIO-RAD, Hercules, CA) followed by transfer onto PVSF membrane. The membrane was blotted with anti-tgd057 rabbit Abs at a dilution of 1∶100. Following washing, the blot was incubated with goat anti-rabbit secondary Abs (Invitrogen) for 45 minutes at room temperature. Bands were visualized using LumiGOLD ECL detection kit (SignaGen Laboratories, Ijamsville, MD), followed by exposure to X-ray film.
Immunofluorescence staining and microscopy
HFFs attached to microscope coverslips were infected with parasites in vitro for 30 min at 37°C. Extracellular parasites were subsequently washed out and the infected cells were incubated in DMEM culture medium + 1% FBS, 1% P/S at 37°C. After incubation, the HFFs were fixed (4% formaldehyde in PBS) for 15 min at RT. HFFs were then incubated in blocking buffer (PBS + 3% BSA, 0.2% saponin) for 60 min at RT while shaking. Primary Abs were diluted in blocking buffer and incubated with HFFs for 60 min at RT followed by extensive washing. HFFs were similarly stained with goat anti-rabbit IgG-AlexaFluor-568 secondary Abs (Invitrogen) for 45 min at RT. Coverslips were then mounted on microscope slides with ProLong Gold antifade reagent with DAPI (Invitrogen). Images were acquired on a Zeiss Axiovert 200 M fluorescent microscope and analyzed using AxioVision Rel 4.6 software.
Tissue preparation and in vitro restimulation
Peritoneal exudates cells (PECs) were isolated by peritoneal lavage with RPMI 1640 (Invitrogen) supplemented with 2% FBS, 1% P/S, and 50 µM β-mercaptoethanol. Splenocytes were harvested by physical disruption of the spleen and passage through a 70 µM nylon mesh. The protocol for brain mononuclear cell isolation was kindly provided by Yasuhiro Suzuki. Briefly, brains were perfused with PBS, excised from the mouse and physically disrupted to free the mononuclear cells. Mononuclear cells were isolated by centrifugation in 35% Percoll (GE Healthcare, Piscataway, NJ) solution overlayed on pure FBS. Live cells were counted in a hemacytometer by trypan blue exclusion.
To restimulate the cells, samples were plated in 48-well TC-treated plates in RPMI Complete medium (RPMI 1640 + 10% FBS, 1% P/S, 50 µM β-mercaptoethanol) and inoculated with live CPS parasites (MOI = 0.1). CPS restimulated samples were incubated for 10 hours at 37°C with the addition of GolgiStop (BD Biosciences, San Jose, CA) for the last 4 hours. SVLAFRRL and SIINFEKL peptides were synthesized in house at the UMDNJ Molecular Resource Facility. For peptide restimulations, samples were plated in 96-well TC-treated plates in RPMI Complete medium. Unless otherwise noted, freshly thawed peptides were added at a final concentration of 10−10 M. Peptide restimulated samples were incubated for 6 hours at 37°C with the addition of GolgiStop for the last 4 hours. To induce detectable IL-2 expression by flow cytometry, samples were restimulated with PMA (50 ng/mL) and ionomycin (500 ng/mL) for 6 hours at 37°C with the addition of GolgiStop for the last 4 hours.
Abs and tetramer staining
Cell surface Ab and tetramer staining was performed simultaneously in FACS Buffer (1XPBS + 1% BSA, 0.05% NaN3) for 1 hour on ice. The cells were then fixed in 100 µL BD Cytofix for 20 min on ice. For intracellular staining, Abs were diluted in BD Perm/Wash buffer and samples were stained for 30 min on ice. Fixed samples were resuspended in FACS Buffer and analyzed on either a BD FACS Calibur or BD LSRII flow cytometer. Flow data was analyzed using FlowJo (Tree Star, Ashland, OR). Mouse-specific Abs obtained from BD Biosciences: CD62L (MEL-14), CD45.2 (104). Mouse-specific Abs obtained from eBioscience (San Diego, CA): CD44 (IM7), IFN-γ (XMG1.2), CD3ε (145.2C11), CD8α (53-6.7), CD90.2 (53.2.1), CD127 (A7R34), TCRβ (H57-597), KLRG1 (2F1), IL-2 (JES6-5H4). Anti-human Granzyme B (GB11) was obtained from CALTAG Laboratories (Invitrogen, Carlsbad, CA).
In vivo IL-12p40 neutralization
On day 0, each mouse was i.p. injected with 1 mg of either anti-IL-12p40 mAb (C17.8) or an isotype control (2A3) (BioXCell, West Lebanon, NH). After ∼4 hours mice were vaccinated with irradiated, CPS parasites. On day 3 post-vaccination mice were given another 1 mg dose of mAb.
Sorting and adoptive transfer
CD8+ T cells were negatively selected from immune WT CD45.1 congenic and immune Il12a−/− spleens using magnetic microbeads (Miltenyi Biotec, Auburn, CA). WT and Il12a−/− CD8+ T cells were surface stained for CD44, CD8α, CD62L, and KLRG1. Stained samples were then sorted for CD44hi FI+ (CD62Lhi KLRG1-) cells using a BD FACS Vantage cell sorter. In a separate pre-sorted sample, WT and Il12a−/− immune CD8+ T cells were stained for tetramer positivity within fractions in ex vivo immune splenocytes. The frequency of tetramer+ cells in FI immune CD8+ T cells was used to normalize the numbers of predicted tetramer+ cells in sorted samples. WT and Il12a−/− FI CD8+ T cells were mixed and i.v. injected (retro-orbital) into naïve WT CD90.1 congenic recipient mice.
Supporting Information
Zdroje
1. HillDE
ChirukandothS
DubeyJP
2005 Biology and epidemiology of Toxoplasma gondii in man and animals. Anim Health Res Rev 6 41 61
2. SuzukiY
OrellanaMA
SchreiberRD
RemingtonJS
1988 Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science 240 516 518
3. SuzukiY
ConleyFK
RemingtonJS
1989 Importance of endogenous IFN-gamma for prevention of toxoplasmic encephalitis in mice. J Immunol 143 2045 2050
4. GazzinelliRT
EltoumI
WynnTA
SherA
1993 Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-alpha and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J Immunol 151 3672 3681
5. GazzinelliR
XuY
HienyS
CheeverA
SherA
1992 Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol 149 175 180
6. SuzukiY
RemingtonJS
1988 Dual regulation of resistance against Toxoplasma gondii infection by Lyt-2+ and Lyt-1+, L3T4+ T cells in mice. J Immunol 140 3943 3946
7. GazzinelliRT
HakimFT
HienyS
ShearerGM
SherA
1991 Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol 146 286 292
8. ParkerSJ
RobertsCW
AlexanderJ
1991 CD8+ T cells are the major lymphocyte subpopulation involved in the protective immune response to Toxoplasma gondii in mice. Clin Exp Immunol 84 207 212
9. ShirahataT
YamashitaT
OhtaC
GotoH
NakaneA
1994 CD8+ T lymphocytes are the major cell population involved in the early gamma interferon response and resistance to acute primary Toxoplasma gondii infection in mice. Microbiol Immunol 38 789 796
10. SuzukiY
JohK
KwonOC
YangQ
ConleyFK
1994 MHC class I gene(s) in the D/L region but not the TNF-alpha gene determines development of toxoplasmic encephalitis in mice. J Immunol 153 4649 4654
11. KhanIA
SmithKA
KasperLH
1988 Induction of antigen-specific parasiticidal cytotoxic T cell splenocytes by a major membrane protein (P30) of Toxoplasma gondii. J Immunol 141 3600 3605
12. KhanIA
ElyKH
KasperLH
1991 A purified parasite antigen (p30) mediates CD8+ T cell immunity against fatal Toxoplasma gondii infection in mice. J Immunol 147 3501 3506
13. NielsenHV
LauemollerSL
ChristiansenL
BuusS
FomsgaardA
1999 Complete protection against lethal Toxoplasma gondii infection in mice immunized with a plasmid encoding the SAG1 gene. Infect Immun 67 6358 6363
14. JongertE
de CraeyeS
DewitJ
HuygenK
2007 GRA7 provides protective immunity in cocktail DNA vaccines against Toxoplasma gondii. Parasite Immunol 29 445 453
15. VercammenM
ScorzaT
HuygenK
De BraekeleerJ
DietR
2000 DNA vaccination with genes encoding Toxoplasma gondii antigens GRA1, GRA7, and ROP2 induces partially protective immunity against lethal challenge in mice. Infect Immun 68 38 45
16. BlanchardN
GonzalezF
SchaefferM
JonckerNT
ChengT
2008 Immunodominant, protective response to the parasite Toxoplasma gondii requires antigen processing in the endoplasmic reticulum. Nat Immunol 9 937 944
17. FrickelEM
SahooN
HoppJ
GubbelsMJ
CraverMP
2008 Parasite Stage-Specific Recognition of Endogenous Toxoplasma gondii-Derived CD8(+) T Cell Epitopes. J Infect Dis
18. ToebesM
CoccorisM
BinsA
RodenkoB
GomezR
2006 Design and use of conditional MHC class I ligands. Nat Med 12 246 251
19. RiveraJ
TessarolloL
2008 Genetic background and the dilemma of translating mouse studies to humans. Immunity 28 1 4
20. GazzinelliRT
WysockaM
HayashiS
DenkersEY
HienyS
1994 Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol 153 2533 2543
21. GlimcherLH
MurphyKM
2000 Lineage commitment in the immune system: the T helper lymphocyte grows up. Genes Dev 14 1693 1711
22. WilsonDC
MatthewsS
YapGS
2008 IL-12 signaling drives CD8+ T cell IFN-gamma production and differentiation of KLRG1+ effector subpopulations during Toxoplasma gondii Infection. J Immunol 180 5935 5945
23. ThimmeR
AppayV
KoschellaM
PantherE
RothE
2005 Increased expression of the NK cell receptor KLRG1 by virus-specific CD8 T cells during persistent antigen stimulation. J Virol 79 12112 12116
24. VoehringerD
BlaserC
BrawandP
RauletDH
HankeT
2001 Viral infections induce abundant numbers of senescent CD8 T cells. J Immunol 167 4838 4843
25. VoehringerD
KoschellaM
PircherH
2002 Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1). Blood 100 3698 3702
26. JoshiNS
CuiW
ChandeleA
LeeHK
UrsoDR
2007 Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 27 281 295
27. PearceEL
ShenH
2007 Generation of CD8 T cell memory is regulated by IL-12. J Immunol 179 2074 2081
28. CousensLP
PetersonR
HsuS
DornerA
AltmanJD
1999 Two roads diverged: interferon alpha/beta - and interleukin 12-mediated pathways in promoting T cell interferon gamma responses during viral infection. J Exp Med 189 1315 1328
29. WaySS
Havenar-DaughtonC
KolumamGA
OrgunNN
Murali-KrishnaK
2007 IL-12 and type-I IFN synergize for IFN-gamma production by CD4 T cells, whereas neither are required for IFN-gamma production by CD8 T cells after Listeria monocytogenes infection. J Immunol 178 4498 4505
30. JankovicD
KullbergMC
HienyS
CasparP
CollazoCM
2002 In the absence of IL-12, CD4(+) T cell responses to intracellular pathogens fail to default to a Th2 pattern and are host protective in an IL-10(−/−) setting. Immunity 16 429 439
31. YapG
PesinM
SherA
2000 Cutting edge: IL-12 is required for the maintenance of IFN-gamma production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J Immunol 165 628 631
32. MoutaftsiM
PetersB
PasquettoV
TscharkeDC
SidneyJ
2006 A consensus epitope prediction approach identifies the breadth of murine T(CD8+)-cell responses to vaccinia virus. Nat Biotechnol 24 817 819
33. Gredmark-RussS
CheungEJ
IsaacsonMK
PloeghHL
GrotenbregGM
2008 The CD8 T cell response against Murine gamma herpes virus (MHV-68) is directed towards a broad repertoire of epitopes from both early and late antigens. J Virol
34. GrotenbregGM
RoanNR
GuillenE
MeijersR
WangJH
2008 Discovery of CD8+ T cell epitopes in Chlamydia trachomatis infection through use of caged class I MHC tetramers. Proc Natl Acad Sci U S A 105 3831 3836
35. BakkerAH
HoppesR
LinnemannC
ToebesM
RodenkoB
2008 Conditional MHC class I ligands and peptide exchange technology for the human MHC gene products HLA-A1, -A3, -A11, and -B7. Proc Natl Acad Sci U S A 105 3825 3830
36. WanKL
ChangTL
AjiokaJW
2004 Molecular characterization of tgd057, a novel gene from Toxoplasma gondii. J Biochem Mol Biol 37 474 479
37. JoinerKA
RoosDS
2002 Secretory traffic in the eukaryotic parasite Toxoplasma gondii: less is more. J Cell Biol 157 557 563
38. FoxBA
BzikDJ
2002 De novo pyrimidine biosynthesis is required for virulence of Toxoplasma gondii. Nature 415 926 929
39. ShawMH
FreemanGJ
ScottMF
FoxBA
BzikDJ
2006 Tyk2 negatively regulates adaptive Th1 immunity by mediating IL-10 signaling and promoting IFN-gamma-dependent IL-10 reactivation. J Immunol 176 7263 7271
40. ValituttiS
MullerS
SalioM
LanzavecchiaA
1997 Degradation of T cell receptor (TCR)-CD3-zeta complexes after antigenic stimulation. J Exp Med 185 1859 1864
41. TakemotoN
IntlekoferAM
NorthrupJT
WherryEJ
ReinerSL
2006 Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol 177 7515 7519
42. KaechSM
TanJT
WherryEJ
KoniecznyBT
SurhCD
2003 Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol 4 1191 1198
43. BuentkeE
MathiotA
TolainiM
Di SantoJ
ZamoyskaR
2006 Do CD8 effector cells need IL-7R expression to become resting memory cells? Blood 108 1949 1956
44. DarrahPA
PatelDT
De LucaPM
LindsayRW
DaveyDF
2007 Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med 13 843 850
45. SederRA
DarrahPA
RoedererM
2008 T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol 8 247 258
46. van StipdonkMJ
LemmensEE
SchoenbergerSP
2001 Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol 2 423 429
47. CurtsingerJM
JohnsonCM
MescherMF
2003 CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J Immunol 171 5165 5171
48. MayerKD
MohrsK
ReileyW
WittmerS
KohlmeierJE
2008 Cutting edge: T-bet and IL-27R are critical for in vivo IFN-gamma production by CD8 T cells during infection. J Immunol 180 693 697
49. PakpourN
ZaphC
ScottP
2008 The central memory CD4+ T cell population generated during Leishmania major infection requires IL-12 to produce IFN-gamma. J Immunol 180 8299 8305
50. McGeachyMJ
ChenY
TatoCM
LaurenceA
Joyce-ShaikhB
2009 The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol 10 314 324
51. IntlekoferAM
TakemotoN
WherryEJ
LongworthSA
NorthrupJT
2005 Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol 6 1236 1244
52. MarkiewiczMA
WiseEL
BuchwaldZS
CheneyEE
HansenTH
2009 IL-12 enhances CTL synapse formation and induces self-reactivity. J Immunol 182 1351 1361
53. RutishauserRL
MartinsGA
KalachikovS
ChandeleA
ParishIA
2009 Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity 31 296 308
54. KalliesA
XinA
BelzGT
NuttSL
2009 Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity 31 283 295
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 3- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- All Mold Is Not Alike: The Importance of Intraspecific Diversity in Necrotrophic Plant Pathogens
- Tsetse EP Protein Protects the Fly Midgut from Trypanosome Establishment
- Perforin and IL-2 Upregulation Define Qualitative Differences among Highly Functional Virus-Specific Human CD8 T Cells
- N-Acetylglucosamine Induces White to Opaque Switching, a Mating Prerequisite in
- Origin and Evolution of Sulfadoxine Resistant
- Rapid Evolution of Pandemic Noroviruses of the GII.4 Lineage
- Natural Strain Variation and Antibody Neutralization of Dengue Serotype 3 Viruses
- Fine-Tuning Translation Kinetics Selection as the Driving Force of Codon Usage Bias in the Hepatitis A Virus Capsid
- Structural Basis of Cell Wall Cleavage by a Staphylococcal Autolysin
- Direct Visualization by Cryo-EM of the Mycobacterial Capsular Layer: A Labile Structure Containing ESX-1-Secreted Proteins
- Lipopolysaccharide Is Synthesized via a Novel Pathway with an Evolutionary Connection to Protein -Glycosylation
- MicroRNA Antagonism of the Picornaviral Life Cycle: Alternative Mechanisms of Interference
- Limited Trafficking of a Neurotropic Virus Through Inefficient Retrograde Axonal Transport and the Type I Interferon Response
- Direct Restriction of Virus Release and Incorporation of the Interferon-Induced Protein BST-2 into HIV-1 Particles
- RNAIII Binds to Two Distant Regions of mRNA to Arrest Translation and Promote mRNA Degradation
- Direct TLR2 Signaling Is Critical for NK Cell Activation and Function in Response to Vaccinia Viral Infection
- The Essentials of Protein Import in the Degenerate Mitochondrion of
- Dynamic Imaging of Experimental Induced Hepatic Granulomas Detects Kupffer Cell-Restricted Antigen Presentation to Antigen-Specific CD8 T Cells
- An Accessory to the ‘Trinity’: SR-As Are Essential Pathogen Sensors of Extracellular dsRNA, Mediating Entry and Leading to Subsequent Type I IFN Responses
- Innate Killing of by Macrophages of the Splenic Marginal Zone Requires IRF-7
- Exoerythrocytic Parasites Secrete a Cysteine Protease Inhibitor Involved in Sporozoite Invasion and Capable of Blocking Cell Death of Host Hepatocytes
- Inhibition of Macrophage Migration Inhibitory Factor Ameliorates Ocular -Induced Keratitis
- Membrane Damage Elicits an Immunomodulatory Program in
- Fatal Transmissible Amyloid Encephalopathy: A New Type of Prion Disease Associated with Lack of Prion Protein Membrane Anchoring
- Nucleophosmin Phosphorylation by v-Cyclin-CDK6 Controls KSHV Latency
- A Combination of Independent Transcriptional Regulators Shapes Bacterial Virulence Gene Expression during Infection
- Inhibition of Host Vacuolar H-ATPase Activity by a Effector
- Human Cytomegalovirus Protein pUL117 Targets the Mini-Chromosome Maintenance Complex and Suppresses Cellular DNA Synthesis
- Dispersion as an Important Step in the Biofilm Developmental Cycle
- Kaposi's Sarcoma-Associated Herpesvirus ORF57 Protein Binds and Protects a Nuclear Noncoding RNA from Cellular RNA Decay Pathways
- Differential Regulation of Effector- and Central-Memory Responses to Infection by IL-12 Revealed by Tracking of Tgd057-Specific CD8+ T Cells
- The Human Polyoma JC Virus Agnoprotein Acts as a Viroporin
- Expansion, Maintenance, and Memory in NK and T Cells during Viral Infections: Responding to Pressures for Defense and Regulation
- T Cell-Dependence of Lassa Fever Pathogenesis
- HIV and Mature Dendritic Cells: Trojan Exosomes Riding the Trojan Horse?
- Endocytosis of the Anthrax Toxin Is Mediated by Clathrin, Actin and Unconventional Adaptors
- A Capsid-Encoded PPxY-Motif Facilitates Adenovirus Entry
- Homeostatic Interplay between Bacterial Cell-Cell Signaling and Iron in Virulence
- Serological Profiling of a Protein Microarray Reveals Permanent Host-Pathogen Interplay and Stage-Specific Responses during Candidemia
- YfiBNR Mediates Cyclic di-GMP Dependent Small Colony Variant Formation and Persistence in
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Kaposi's Sarcoma-Associated Herpesvirus ORF57 Protein Binds and Protects a Nuclear Noncoding RNA from Cellular RNA Decay Pathways
- Endocytosis of the Anthrax Toxin Is Mediated by Clathrin, Actin and Unconventional Adaptors
- Perforin and IL-2 Upregulation Define Qualitative Differences among Highly Functional Virus-Specific Human CD8 T Cells
- Inhibition of Macrophage Migration Inhibitory Factor Ameliorates Ocular -Induced Keratitis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



