-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaInhibition of Macrophage Migration Inhibitory Factor Ameliorates Ocular -Induced Keratitis
Pseudomonas aeruginosa causes severe sight-threatening corneal infections, with the inflammatory response to the pathogen being the major factor resulting in damage to the cornea that leads to loss of visual acuity. We found that mice deficient for macrophage migration inhibitory factor (MIF), a key regulator of inflammation, had significantly reduced consequences from acute P. aeruginosa keratitis. This improvement in the outcome was manifested as improved bacterial clearance, decreased neutrophil infiltration, and decreased inflammatory responses when P. aeruginosa-infected MIF knock out (KO) mice were compared to infected wild-type mice. Recombinant MIF applied to infected corneas restored the susceptibility of MIF deficient mice to P. aeruginosa-induced disease, demonstrating that MIF is necessary and sufficient to cause significant pathology at this immune privileged site. A MIF inhibitor administered during P. aeruginosa-induced infection ameliorated the disease-associated pathology. MIF regulated epithelial cell responses to infection by enhancing synthesis of proinflammatory mediators in response to P. aeruginosa infection and by promoting bacterial invasion of corneal epithelial cells, a correlate of virulence in the keratitis model. Our results uncover a host factor that elevates inflammation and propagates bacterial cellular invasion, and further suggest that inhibition of MIF during infection may have a beneficial therapeutic effect.
Published in the journal: . PLoS Pathog 6(3): e32767. doi:10.1371/journal.ppat.1000826
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000826Summary
Pseudomonas aeruginosa causes severe sight-threatening corneal infections, with the inflammatory response to the pathogen being the major factor resulting in damage to the cornea that leads to loss of visual acuity. We found that mice deficient for macrophage migration inhibitory factor (MIF), a key regulator of inflammation, had significantly reduced consequences from acute P. aeruginosa keratitis. This improvement in the outcome was manifested as improved bacterial clearance, decreased neutrophil infiltration, and decreased inflammatory responses when P. aeruginosa-infected MIF knock out (KO) mice were compared to infected wild-type mice. Recombinant MIF applied to infected corneas restored the susceptibility of MIF deficient mice to P. aeruginosa-induced disease, demonstrating that MIF is necessary and sufficient to cause significant pathology at this immune privileged site. A MIF inhibitor administered during P. aeruginosa-induced infection ameliorated the disease-associated pathology. MIF regulated epithelial cell responses to infection by enhancing synthesis of proinflammatory mediators in response to P. aeruginosa infection and by promoting bacterial invasion of corneal epithelial cells, a correlate of virulence in the keratitis model. Our results uncover a host factor that elevates inflammation and propagates bacterial cellular invasion, and further suggest that inhibition of MIF during infection may have a beneficial therapeutic effect.
Introduction
Eye trauma and contact lens wear are the main factors that predispose to the development of infectious keratitis associated with vision loss and blindness [1],[2]. The organism most often isolated from contact lens associated corneal ulcers is Pseudomonas aeruginosa [3],[4]. Existing therapies often fail to control the excessive tissue damage that is induced during P. aeruginosa infection [5]. While antibiotic treatment reduces the bacterial burden, tissue damage still occurs as a result of an poorly-controlled local inflammation. Hence, new therapeutic modalities are needed to control the inflammatory response in addition to the antibiotic treatments. We hypothesized that the innate immunity factor–MIF (NP 002406)–could promote the pathogenic consequences of P. aeruginosa infection by potentiating local inflammation, and, if so, could be a suitable drug target for treatment.
MIF is an innate immunity molecule with ubiquitous tissue expression leading to induction of proinflammatory activities. MIF was originally described as a regulator of macrophage responses [6]. It directly or indirectly promotes expression of a large panel of pro-inflammatory cytokines including TNF-α (P01375), IFN-γ (P 01579), IL-1β (NP 000566), IL-2 (AAA 59140), IL-6 (CAG 29292), IL-8 (CAG 46948), MIP-2 (AAF 78449), NO, COX2 (P 00403), products of the arachidonic acid pathway and matrix metalloproteinases [7]. Interestingly, low levels of MIF can override the anti-inflammatory properties of glucocorticoids by reversing the inhibitory effect of glucocorticoid on production of TNF-α, IL-1β, IL-6 and IL-8 [8]. Detailed studies performed in the rat have shown that preformed MIF protein is released into the circulation within 6 hrs of LPS injection [9]. LPS toxicity is exacerbated by co-injection of recombinant MIF (rMIF) with LPS, whereas neutralization of MIF activity reduces the circulating levels of TNF-α by 50% and rescues mice from lethal LPS-induced endotoxic shock [10],[11]. All these properties of the MIF molecule suggest that MIF has a prominent regulatory role related to inflammation, likely with a critical function as an effector molecule that is active early in the course of infection with a pathologic function when continued production exacerbates inflammation, giving rise to attendant tissue pathology.
The contribution of MIF during responses to infections by a variety of pathogens, including bacteria, viruses, and parasites is currently an area of active research. Recent clinical correlative studies have demonstrated increased MIF levels and elevated MIF-dependent proinflammatory cytokines are produced during H1N5 influenza infection, dengue fever, and bacterial urinary tract infections [12],[13],[14]. These results demonstrate an important contribution of MIF to the pathogenesis of viral or bacterial induced inflammation and suggest a possible beneficial role of neutralizing MIF as an adjunctive therapeutic approach to treat the severe forms of disease.
In the eye, the high levels of MIF protein expression and consequent inhibition of cellular migration has supported the conclusion that MIF contributes to the establishment of the eye's immune privilege status due to immunosuppressive activities [15],[16],[17],[18]. While this could be important in the resting state, the strong impact MIF has on induction of inflammation in response to infection suggests a different role for this molecule during active infection. In this study, we tested whether inhibition of MIF in the eye led to a decrease in P. aeruginosa-induced pathology, and if this was associated with decreased PMN infiltration and thus decreased inflammation and corneal pathology. Overall, we found that inhibition of MIF had a pronounced salutary effect on eye pathology emanating from P. aeruginosa keratitis, which we associated with a better ability of PMN from MIF-deficient mice to mediate opsonic killing of this organism, making MIF a promising therapeutic target to control local inflammation in the context of P. aeruginosa corneal infection.
Results
Bacterial burdens after Pseudomonas aeruginosa eye infection are elevated in WT mice compared with MIF KO mice
To determine the effect of MIF on P. aeruginosa-induced keratitis, MIF KO and C57Bl6 WT mice were infected with P. aeruginosa strain 6294 and the bacterial levels in the corneas of mice measured to monitor disease progression. At 24 h after onset of infection there were modest but significantly (P = 0.04) lower bacterial levels in both the extracellular and intracellular samples from the corneas of the infected MIF KO mice when compared to C57Bl6 control animals (Fig. 1A). However, the corneal pathology scores of MIF KO and C57Bl6 were comparable at 24 h.
Fig. 1. MIF KO mice are protected from P. aeruginosa-induced infection. 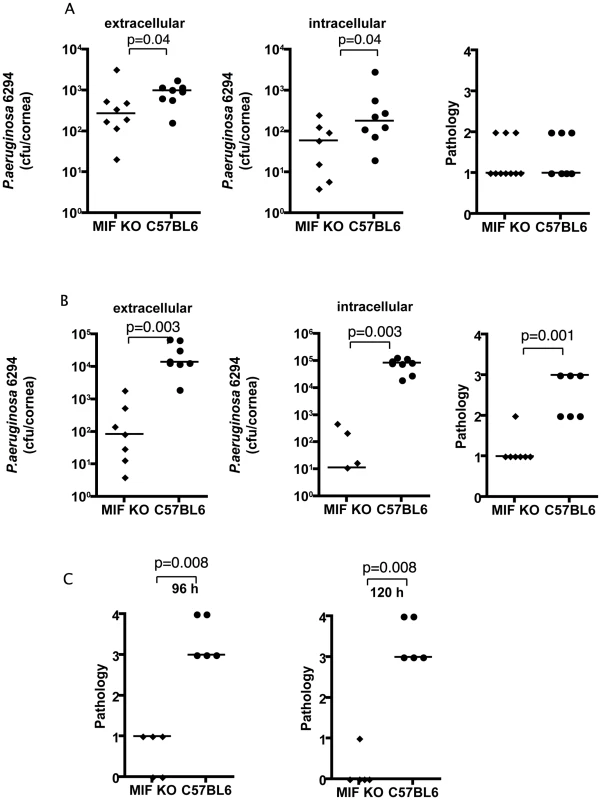
A. Extracellular (left) and intracellular (middle) levels of P. aeruginosa strain 6294 and associated pathology scores (right) determined 24 h following infection with 106 cfu onto eyes of MIF KO or WT C57Bl6 mice. Points indicate values for individual mice, bars the medians. Data are from a representative experiment out of two performed. B. Extracellular (left) and intracellular (middle) cfu of P. aeruginosa strain 6294 at 48 h following infection. Significantly different levels of 6294 were detected both extracellularly (left) (P = 0.0003), intracellularly (middle) (P = 0.0003) as were differences in pathology scores (right; P = 0.001). P values by Mann-Whitney U test. The data are from one representative experiment out of three performed. C. Significantly higher corneal pathology scores induced by P. aeruginosa at 96 h (P = 0.007) and 120 h (P = 0.009) after challenge. Points indicate individual mice, bars the medians. Data are from a representative experiment out of two performed. At 48 h after infection the differences between the MIF KO and C57Bl6 mice increased dramatically: there were about 100-fold fewer bacteria recovered from the corneal cell exterior in the infected MIF KO mice compared to the C57Bl6 controls. Even more dramatically, about 10,000 less intracellular bacteria were recovered from MIF KO mice when compared to C57Bl6 mice (Fig. 1B). Consistent with the reduced bacterial levels, the MIF KO mice infected with P. aeruginosa strain 6294 had a significant decrease in corneal pathology (P = 0.001).
As is known from published data [19], if a P. aeruginosa corneal infection is left to proceed for a longer period of time in C57Bl6 mice, perforation of the cornea occurs. This outcome was observed in an additional group of C57BL6 control mice, but not in MIF KO mice in the C57Bk6 background (Fig. 1C). This reduced pathology in the MIF KO mice was observed across a range of bacterial challenge doses from 1×106 (data not shown) to 1×107 cfu/mouse eye (Fig. 1C). The improved outcomes in MIF KO mice was also obtained with P. aeruginosa strain PAO1 48 h following infection (Figure S1).
MIF deficiency regulates inflammatory responses during P. aeruginosa keratitis
Since MIF regulates the pro-inflammatory responses to LPS challenge in vitro as well as in several in vivo models, we examined how MIF might modulate the molecular responses to P. aeruginosa induced keratitis by measuring local cytokine production based on prior findings as to which mediators are regulated by MIF and a small-scale microarray analysis we performed (data not shown) on infected corneas from WT and MIF KO mice to suggest which cytokines might be candidates for a more thorough investigation. A panel of five cytokines, TNF-α, IL-1β, KC, IL-6, and IL-10, were quantified at the protein level in infected corneas harvested from individual animals. Infected MIF KO mice had significantly lower levels of TNF-α, IL-1β, IL-6, and KC protein in corneal homogenates compared to corneas from infected C57Bl6 mice. There was no difference in the levels of the anti-inflammatory cytokine IL-10 (Fig. 2).
Fig. 2. Inflammatory responses in the corneas of MIF KO and C57Bl6 mice infected with P. aeruginosa strain 6294. 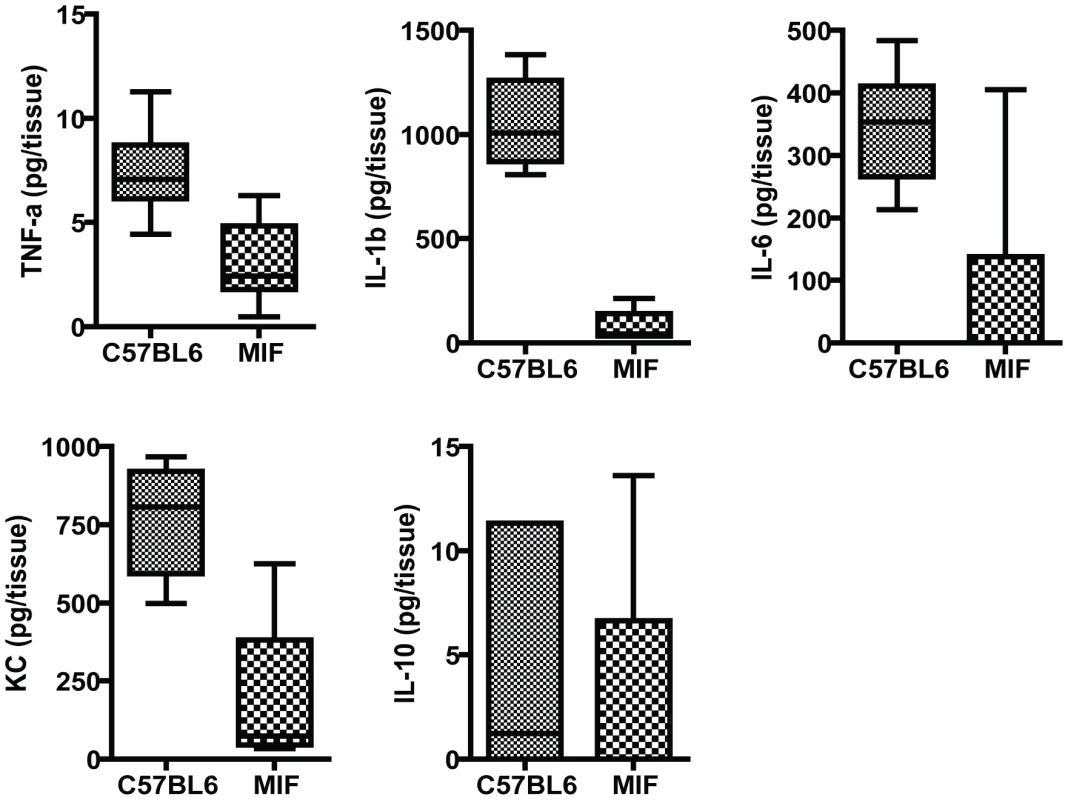
Groups of 7 MIF KO mice and C57Bl6 mice were infected with 1×106 cfu placed onto scratch-injured eyes. Mouse corneas were harvested at 48 h after infection, washed in F12 media, homogenized in PBS containing a mix of protease inhibitors and supplemented with 0.5% Triton to disrupt plasma membranes. The levels of mouse cytokines in corneal lysates were simultaneously measured using a Meso Scale Discovery (MSD) multiplex 7-spot electrochemiluminescence (ECL) assay. The levels of cytokines measured in the different groups were compared with the Mann-Whitney U test. TNF-α, IL-1β, IL-6 and KC were significantly decreased in MIF KO with C57Bl6 mice P = 0.004, P = 0.001, P = 0.01, P = 0.04 respectively. MIF deficiency modulates PMN infiltration to the site of infection in the eye
To determine if differences in inflammatory responses translate into differences in the presence of infiltrating PMNs during infection, histologic observations were carried out. C57Bl/6 mice showed destruction of the epithelial layer of the cornea and elevated infiltration of PMNs in the aqueous chamber. In contrast, in the MIF KO mice, there were less infiltrating PMNs, less edema, and no destruction of the epithelial layer (Fig. 3A and 3B) when either strain 6294 or PAO1 were used to induce infections. The pathology in the C57Bl/6 corneas was consistent with an acute P. aeruginosa infection.
Fig. 3. Histological examination of mouse corneas inoculated with P. aeruginosa 6294 or PAO1 at 48 h post-challenge. 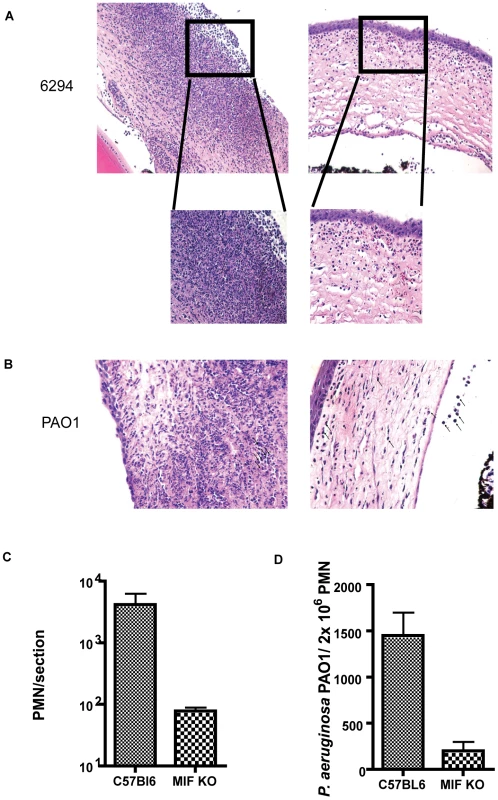
A. Groups of C57Bl6 mice (N = 7) and MIF KO (N = 7) were infected with P. aeruginosa strain 6294 and the eyes harvested at 48 h post-challenge. Tissue was embedded in paraffin, sectioned and stained with H&E. Representative images were collected at 20× magnification. The inset in the magnified area depicts the morphological changes after 48 h of infection using 40× magnification. B. Representative images of H&E tissue sections from C57Bl6 mice and MIF KO infected with P. aeruginosa strain PAO1 (20× magnification). C. PMN recruitment was quantified by counting PMNs per field of vision in three consecutive sections from 3 individual mice. D. Opsonophagocytosis of P. aeruginosa strain PAO1 by PMNs from WT C57Bl6 and MIF deficient mice. Bone marrow derived MIF-deficient and MIF-sufficient PMNs were exposed to P. aeruginosa PAO1 in the presence of complement and murine sera for 3 h at 37C. Upon completion of incubation PMNs were lysed and bacterial levels in the reaction quantified (p = 0.04, P values determined by an unpaired Student t-test). The findings on bacterial levels and PMN infiltration into the P. aeruginosa-infected cornea indicated that MIF promoted PMN infiltration leading to more pathology while also inhibiting the clearance of this microbe from the cornea. To determine a potential cellular basis for the improved outcomes in MIF-deficient mice, we compared the opsonic killing activity of PMNs obtained from MIF-sufficient and MIF-deficient animals. MIF-deficient PMNs phagocytosed P. aeruginosa strain PAO1 more efficiently that MIF-sufficient PMNs, as evident by the decreased number of P. aeruginosa recovered from the PMN/bacteria mixture at the end of the experiment (Fig. 3D).
MIF promotes expression of proinflammatory cytokines in primary corneal epithelial cells
Since it is well documented that human epithelial cells participate in neutrophil recruitment in response to infection, we determined if MIF affects IL-8 synthesis by corneal epithelial cells [20],[21],[22]. Primary human corneal epithelial cells were grown to confluence, treated with either a siRNA to knock-down MIF or with a control (control) siRNA (Fig. 4A). Seventy-two h after the knock-down cells were infected with P. aeruginosa strain 6294 (Fig. 4B). We also evaluated the effect of adding recombinant MIF (rMIF) to cells treated with siRNA for MIF. Tissue culture supernatants were collected and IL-8 levels measured by ELISA. P. aeruginosa infection induced IL-8 expression in control siRNA-treated cells, whereas IL-8 production was much less pronounced in MIF siRNA-treated cells (ANOVA, P<0.01) (Fig. 4B). Treatment with rMIF reconstituted P. aeruginosa-induced IL-8 production in MIF-siRNA treated cells. Protein concentrations in cellular lysates were determined and no differences found, indicating no major changes in cellular numbers occurred during the experiments.
Fig. 4. Corneal epithelial cells produce less IL-8, IL-6, IL-1β after infection with P. aeruginosa strain 6294 when MIF levels are reduced or MIF tautomerase activity inhibited. 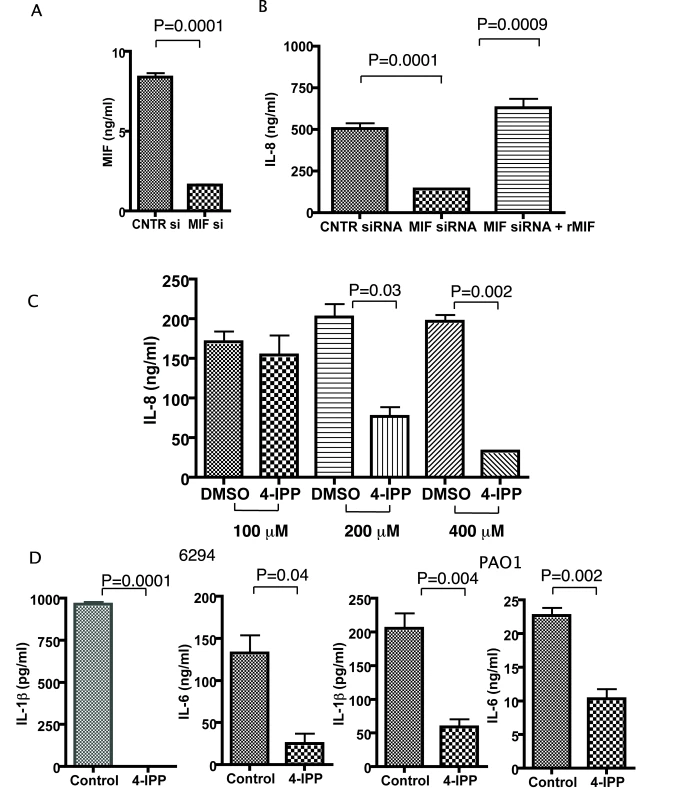
A. 1×106 corneal epithelial cells were seeded in 6-well plates, transfected with control siRNA or MIF siRNA, and MIF levels in the culture supernatants were measured by ELISA. B. 1×106 corneal epithelial cells were infected with P. aeruginosa at an MOI 25 for 60 min after 72 h of initial treatment with either control siRNA or MIF siRNA. Recombinant MIF was added to an additional series of wells, pretreated with MIF siRNA. Tissue culture supernatants were collected 6 h post infection and the levels of IL-8 measured by ELISA. The background IL-8 levels were subtracted. Results are representative of duplicate experiments. C. Corneal epithelial cells were pre-treated with 100, 200 or 400 µM of 4-IPP prior to infection with P. aeruginosa strain 6294. The inhibitor was maintained during the infection and for 6 h post-infection. Supernatants were collected and IL-8 quantified by ELISA. The background levels were subtracted. The results are representative of duplicate experiments. P values determined by an unpaired Student t-test. D. Corneal epithelial cells were treated with 400 µM of 4-IPP prior to infection with P. aeruginosa strain 6294 or PAO1. The inhibitor was maintained during the infection and for 6 h post-infection. Supernatants were collected and IL-1β, IL-6, IL-8 quantified by ELISA. The background levels were subtracted. The results are representative of duplicate experiments. P values determined by an unpaired Student t-test. We next analyzed the effect of a small molecule inhibitor of the MIF tautomerase activity, 4-IPP, on P. aeruginosa-induced inflammation. Published studies have demonstrated that 4-IPP forms covalent complexes with MIF, and, hence, inhibits MIF activity [23]. Treatment with 4-IPP almost completely abolished IL-8 synthesis. This effect of 4-IPP was observed at concentrations of 200 µM and 400 µM (Fig. 4C). Since we were concerned that the 4-IPP treatment could induce cellular apoptosis due to the documented role of MIF in promoting cell survival [24],[25], we next analyzed lysates obtained from non-treated and P. aeruginosa strain 6294 infected cells in the presence or absence of 400 µM 4-IPP and probed for activated caspase 3 as a marker for apoptosis. At 6 h post infection we did not observe activation of caspase 3, however some activation was detected at 24 h post-infection (Figure S2). Hence, we have performed the majority of our infection experiments within 6 h post-infection. When epithelial cells were stimulated by P. aeruginosa strains 6294 or PAO1 both IL-1β and IL-6 secretion was abolished in the presence of the MIF inhibitor (Fig. 4D).
As P. aeruginosa bacteria found in the cornea during infection are mostly intracellular, we determined if MIF modulated the uptake of bacteria by corneal epithelial cells using in vitro invasion assays. Inhibition of MIF by either siRNA treatment or by treatment with small molecule inhibitor 4-IPP, significantly decreased the internalized levels of P. aeruginosa in primary human corneal epithelial cells (Fig. 5). These findings suggest that MIF modifies epithelial responses facilitating bacterial entry in the epithelial cells and thus inhibits the niche that P. aeruginosa uses to survive and escape from PMNs recruited to the eye in response to infection.
Fig. 5. 4-IPP inhibits P. aeruginosa invasion of corneal epithelial cells. 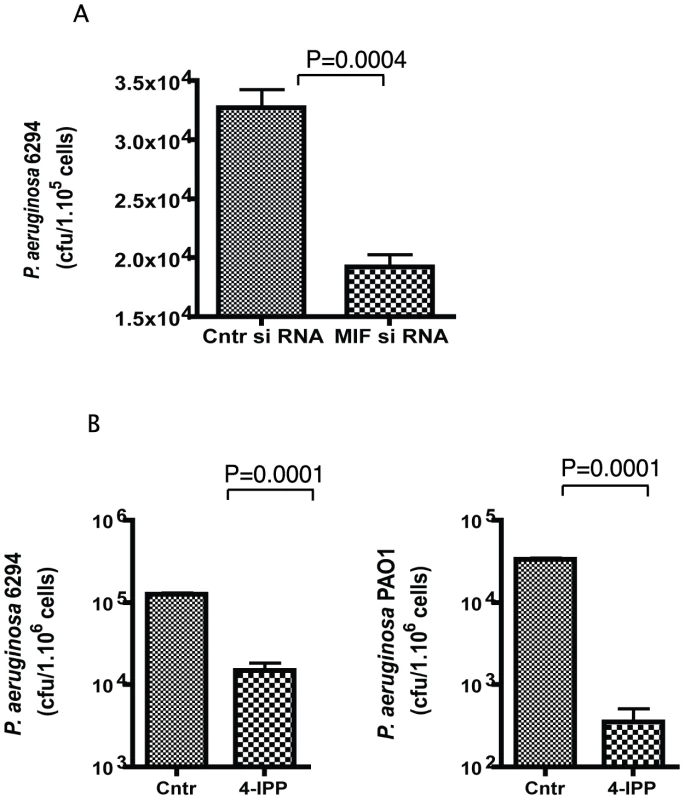
A. Corneal epithelial cells were pre-treated with MIF siRNA or control siRNA for 72 h prior to the experiment. Invasion assays were carried out with P. aeruginosa strain 6294 at a MOI 10 for 3 h. Cells were washed, treated with gentamicin to kill all extracellular bacteria, lysed and plated. The results are representative of triplicate experiments. P values determined by an unpaired Student t-test. B. Corneal epithelial cells were pre-treated with 400 µM of 4-IPP prior to infection with P. aeruginosa strains 6294 or PAO1 and the inhibitor was maintained during the infection. After 3 h, cells were washed, treated with gentamicin, lysed and plated. The results are derived from triplicate experiments. P values determined by an unpaired Student t-test. Recombinant MIF restores the wild-type response to P. aeruginosa-induced acute bacterial keratitis in MIF KO mice
To verify that MIF was the major factor responsible for the reduced infectious complications of P. aeruginosa keratitis in MIF KO mice, reconstitution experiments were performed. One µg of rMIF administered topically onto the eye of infected MIF KO mice restored the wild-type response to infection with P. aeruginosa strain 6294 (Fig. 6). This increased bacterial burden in the reconstituted MIF KO correlated with elevated pathology scores, indicative of increased susceptibility to infection.
Fig. 6. rMIF restores sensitivity to P. aeruginosa-induced disease in MIF KO mice. 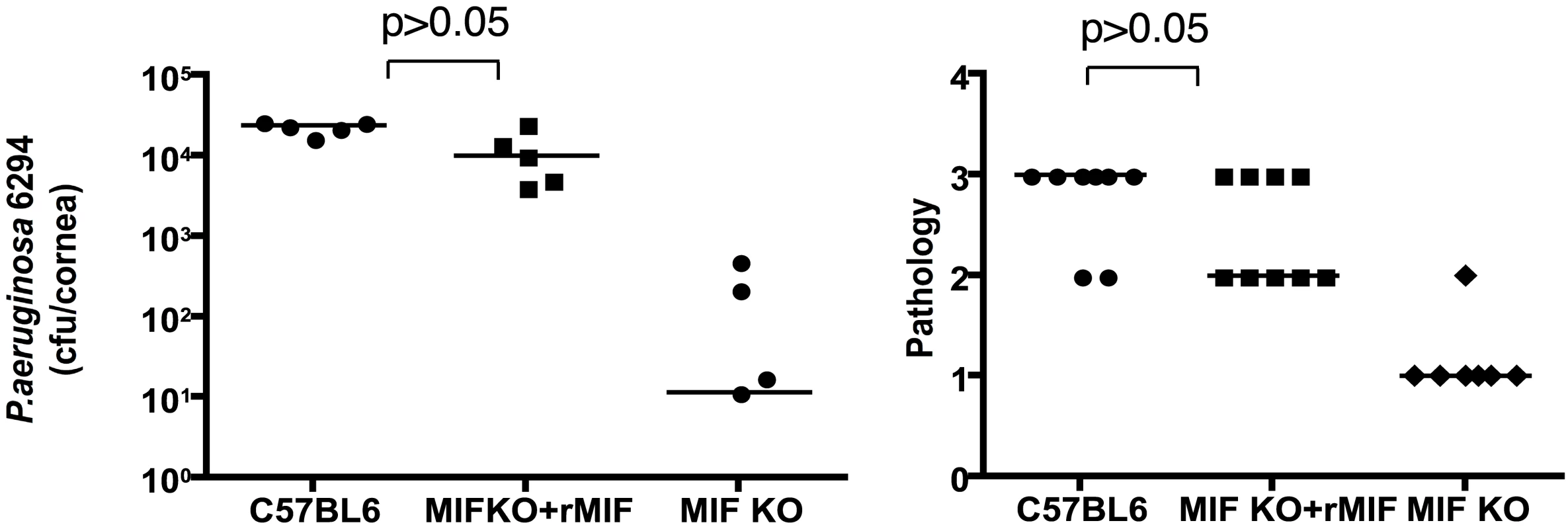
A. Topical treatment of corneas from P. aeruginosa-infected MIF-deficient mice resulted in bacterial levels comparable to that achieved in infected WT C57Bl6 mice (P>0.05 ANOVA and Dunnett's test) while MIF KO mice had reduced levels of P. aeruginosa in the tissue. B. Pathology scores. MIF KO presented with reduced pathology scores (P<0.05, ANOVA and Dunnett's test). Points indicate values from individual mice, bars the medians. The data are from one representative experiment out of two performed. Inhibition of MIF activity by 4-IPP promotes recovery from P. aeruginosa-induced keratitis
To analyze the effect of MIF inhibition during P. aeruginosa-induced keratitis, cohorts of C57Bl6 mice were infected with 5×105 cfu/mouse of P. aeruginosa strain 6294 and treated topically with gentamicin starting 24 h post-infection. A separate cohort of infected C57Bl6 mice received both 4-IPP IP and gentamicin eye-drops on the same schedule. Animals were monitored for 5 days to analyze recovery from infection and the infected corneas were harvested to quantify the intracellular and extracellular bacteria. Mice that received the 4-IPP and gentamicin treatment cleared P. aeruginosa more efficiently than did the group treated only with gentamicin as demonstrated by the significantly decreased extracellular and intracellular cfu recovered from the corneas, and decreased pathology scores (Fig. 7).
Fig. 7. Treatment with 4-IPP promotes recovery from P. aeruginosa-induced corneal infection and pathology. 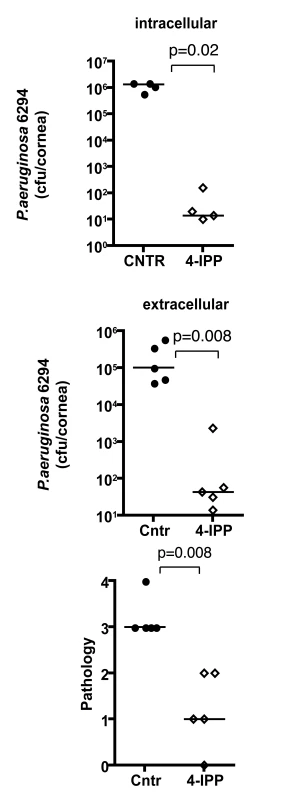
Groups of C57Bl6 mice were treated with either gentamicin alone or gentamicin and 4-IPP for 5 days after infection after which infected corneas were harvested, homogenized, and bacteria quantified. Points indicate values from individual mice, bars the medians. The data are from one representative experiment out of two performed. Discussion
Pseudomonas aeruginosa causes serious corneal disease, associated with acute ocular inflammation. Both bacterial and host factors contribute to the pathogenesis of P. aeruginosa keratitis, indicating that the optimal interventions for this infection will involve both control of bacterial levels and modulation of inflammation to limit pathology while not compromising bacterial clearance. Here, we demonstrated that a master regulator of inflammation–MIF–promoted both pathology associated with P. aeruginosa corneal infection and inhibited bacterial clearance as shown by outcomes in MIF-KO mice which had decreased bacterial burdens in the corneas, decreased inflammation, and decreased neutrophil infiltration compared to MIF-sufficient WT mice. These improved outcomes in MIF-deficient mice could be reversed by treating P. aeruginosa-infected eyes with rMIF and could be mimicked in WT mice treated with the MIF inhibitor 4-IPP. Thus, it appears that one host molecule makes a major contribution to both aspects of disease resulting from P. aeruginosa corneal infection, indicating a high potential for ameliorating the consequences of this infection by therapies that target the activities of MIF.
To define mechanisms associated with the effects of MIF in P. aeruginosa keratitis we used in vitro studies to show that reduction of MIF levels by siRNA treatment or by the use of the tautomerase inhibitor of MIF, 4-IPP, results in decreased production of IL-8, IL-1β, and IL-6 by human corneal epithelial cells after infection. Consistent with this finding, the absence of MIF in vivo also reduced inflammatory responses to P. aeruginosa during experimental murine corneal infection. Furthermore, we also found that PMNs from MIF-deficient mice had a better innate ability to kill P. aeruginosa in an in vitro opsonic-killing assay, indicating a likely cellular basis for the better control of infection in thee animals. Of note, Niederkorn and colleagues [18] found that MIF inhibits perforin release by NK cells and inhibits NK cell motility, indicating a contribution of MIF to the immune-privileged status of the eye. Thus, it appears in the basal state MIF limits cellular activation, but a consequence of this is that the responses to infection are dampened and the increased infiltration of PMN into the infected cornea that is needed to deal with the increased bacterial burdens results in more tissue pathology. A similar improved outcome from P. aeruginosa lung infection was reported for MIF-deficient mice [11].
Successful recovery form acute bacterial infection depends on timely recruitment of neutrophils. PMNs are present in the cornea and aqueous chamber in MIF deficient mice at reduced levels when compared to infected WT control mice, yet, there are sufficient numbers of PMNs recruited in the MIF KO to clear bacteria, as demonstrated by the decreased levels of P. aeruginosa measured at 48 h after the onset of infection. Hence, MIF deficiency both reduces pathology during infection and still allows for sufficient host responses to clear bacteria. Our data suggests that in infected mouse corneas, inflammatory mediators such as KC or MIP2 are initially released by corneal epithelial cells or keratocytes which in turn recruit PMNs to effectively handle bacterial infections in the eye [26], a process that occurs in the infected MIF KO mice leading to resolution of infection. In contrast, in the infected C57Bl6 mice these mediators are produced at higher levels and become part of a classic hypersensitivity response that contributes to tissue damage and elevated pathology [27],[28]. Taken together these data suggest that by modulating the level of inflammation occurring in the eye triggered by P. aeruginosa infection it is possible to promote expression of proinflammatory mediators sufficient to control infection while also preventing inflammation-induced corneal pathology and associated loss of vision.
Materials and Methods
Mice
Ethics Statement: All studies were performed in accordance with the Harvard Medical School Institutional Animal Care and Use Committee guidelines (approval date 10/14/2008; number IACUC 02432).
Breeding pairs of mif knock-out (KO) mice were a kind gift from Dr. C. Gerard (Children's Hospital Boston). Control mice (C57Bl6) were obtained from Taconic Farms. Mice were housed and bred in the Channing Laboratory Animal Care Facilities. Mice were housed and bred in one of the Harvard Medical Area Animal Care Facilities.
Bacterial strains and inocula
P. aeruginosa strains PAO1 and 6294 were used throughout these experiments. Effects from low dose (5×105 cfu/eye) and high dose (1×107 cfu/eye) inocula were evaluated to determine the doses to use in the in vivo infection studies. Generally, the bacterial strains were grown o/n at 37C on Tryptic Soy Broth agar plates prior to experiments.
Infection model
The experimental protocols were approved by the Institutional Animal Care and Use Committee of the Harvard Medical Area Office for Research Subject Protection and were consistent with the Association for Research in Vision and Ophthalmology guidelines for studies in animals. Infections were carried out as described previously [29]. Mice were anesthetized with ketamine and xylazine injections. Three 0.5 cm scratches were made on the cornea and an inoculum of P. aeruginosa delivered in 5 µl onto the eye. Mice remained sedated for about 30 min. For evaluation of corneal pathology, daily scores are recorded by an observer unaware of the experimental status of the animals based on a the following scoring system using a graded scale of 0 to 4 as follows: 0, eye macroscopically identical to the uninfected contra-lateral control eye; 1, faint opacity partially covering the pupil; 2, dense opacity covering the pupil; 3, dense opacity covering the entire anterior segment; and 4, perforation of the cornea, phthisis bulbi (shrinkage of the globe after inflammatory disease), or both. To determine the levels of bacteria in the cornea 24 or 48 h after infection, mice were sacrificed, eyes enucleated and corneas dissected from the ocular surface. To quantify the extracellular levels of P. aeruginosa, corneas were excised and suspended in PBS, vortexed, serial dilutions made and plated on P. aeruginosa selective cetrimide plates. To measure the intracellular levels of P. aeruginosa, the intact corneas were placed in F12 medium containing 5% FBS and 300 µg gentamicin/ml for 60 min, vigorously rinsed to wash away the antibiotic and the tissue then homogenized in 0.05% Triton X100 in 5% FBS-F12, diluted in 5% FBS-F12, and plated for bacterial counts. For treatment, groups of mice were infected with P. aeruginosa strain 6294, treated topically daily for 5 days with 5 µl of a solution of 100 µg gentamicin/ml, along with 50 µl of 1 M 4-IPP injected IP. The 4-IPP was solubilized in DMSO/corn oil [23]. Bacteria were quantified 5 days after infection by homogenizing and plating the corneal tissue.
Reconstitution experiments
Mice were infected with P. aeruginosa strain 6294 and 1 µg recombinant MIF (rMIF). applied topically in 5 µl at the time of infection and subsequently every 6 h after the infection. Corneas were harvested after 48 h of infection, treated with 300 µg gentamicin/ml for 60 min, washed, homogenized with F12 medium containing 5% FBS and 0.05% Triton X100, diluted and plated.
Histopathology examinations
Eyes were enucleated from euthanized mice and fixed in 4% paraformaldehyde then embedded in paraffin. Four µm sections were cut, and stained with hematoxylin-eosin to visualize tissue morphology following previously used techniques [30].
In vitro infection assays
Primary corneal epithelial cells [31] were grown in 6-well plates in either keratinocyte SFM (Invitrogen) medium supplemented with antibiotics, EGF, and pituitary gland extract or in MEM/F12 mix supplemented with EGF, insulin, DMSO, and cholera toxin [31]. Confluent monolayers of corneal cells were treated for 1 h with 4-IPP then infected with different strains of P. aeruginosa (PAO1, 6294) at a MOI of 25 for 1 h. Following treatment for 1 h with 300 µg gentamicin/ml, cells were re-supplemented with growth medium. The keratinocyte SFM growth medium was supplemented with penicillin, streptomycin, EGF, and pituitary gland extract. 6-iodo-6-phenylpirimidine (4-IPP) was used to inhibit MIF activity in vitro and was maintained during the infection and gentamicin treatments. Different concentrations of 4–IPP were used, ranging from 100 µM to 400 µM. The tissue culture supernatants were collected at 6 h and 24 h after infection and total cell lysates were prepared in RIPA buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 1% SDS, and Complete Mini Protease Inhibitor Cocktail (Roche Diagnostics).
Epithelial cell invasion assay
P. aeruginosa strains 6294 or PAO1 were grown to mid-log phase in TSB for 2 h at 37C and used for invasion assays. Bacterial cells were washed and suspended in F12 medium. Human primary corneal epithelial cells were grown to confluence in 6 well plates in F12 medium and infected with the P. aeruginosa strains at a MOI of 25 for 3 h. After the infection, cells were washed with F12, then treated with 300 µg or 400 µg gentamicin/ml for 1 h to kill the extracellular bacteria depending on the activity of the antibiotic. After washing away the antibiotic, cells were lysed in 1% Triton X 100 in MEM and the lysates were diluted then plated on cetrimide plates to enumerate intracellular bacteria.
Cytokine analysis
Commercially available ELISA assays (R&D Systems) were used to determine the levels of cytokines (IL-8, IL-6) produced by P. aeruginosa infected primary human corneal cells. Mouse cytokines were measured using a Meso Scale Discovery (MSD) multiplex 7-spot electrochemiluminescence (ECL) assay and outputs measured by an ultra low noise charge-coupled device (CCD) Imager 2400 (Meso Scale Discovery, Gaithersburg, MD, USA). The cytokines measured included interleukin (IL)-1β, IL-6, IL-12p70, IL-10, IFNγ and the alpha chemokine neutrophil attractant and activator CXCL1/GRO (also known as KC). The MSD ECL platform has been previously validated against cytokine standards recommended by WHO and U.K. National Institute for Biological Standards and Control (NIBSC) and by comparison to traditional ELISA [32].
siRNA experiments
Production of MIF by the primary corneal epithelial cells was reduced by MIF-specific siRNA treatment. Briefly, cells were transfected with MIF-specific or control siRNA (On-target plus SMART pool; Dharmacon) by incubating with a transfection mixture that consists of 50 nM siRNA (Dharmacon) and 6 µl Oligofectamine (Invitrogen) in 1 ml of Opti-MEM (Gibco) for 48 h, after which the cells were maintained with regular growth medium. Epithelial cells were infected with P. aeruginosa (strain 6294 or strain PAOI) 48 h to 72 h after siRNA treatment. A non-siRNA treated control was also included to determine if there is an effect on inflammatory gene expressing from the control siRNA.
Purification of PMNs and opsonophagocytic assays
Murine PMNs were purified from bone marrow by flushing the cells out of the tibias and femurs with 3 ml HBSS (Hanks balanced salt solution) buffer (Invitrogen). Cells were pelleted by centrifugation at 380 g for 10 min at tabletop centrifuge; resuspended in 1 ml HBSS and overlayed on the top of a Histopaque gradient composed of 3 ml Histopaque 1119 (Sigma) and 3 ml Histopaque 1077 (Sigma). Cells were centrigufed for 30 min at 700 g, room temperature, without a brake. Neutrophils were collected from the interface of the Histopague 1077 and 1119, transferred to a fresh tube and washed three times by adding 10 ml og HBSS. Viable cells were enumerated by tryptan blue exclusion technique.
Opsonophagocytic assays were performed by mixing 100 µl of 2.5×106 PMN with 100 µl of 400 fold dilution of P. aeruginosa grown in TSB to reach mid-log phase as estimated by OD 650 reading of 0.4. 100 µl of Rabbit complement sera (Sigma) and 100 µl of freshly harvested mouse sera from either C57Bl6 or MIF KO mice were added to the sample mixture as appropriate. The reaction was incubated for 90 min at 37°C with rotation. After completion of the incubation an aliquot was taken and lyzed with 0.05% Tween 20/TSB. Bacteria were enumerated by plating on P. aeruginosa selective cetrimide plates.
Supporting Information
Zdroje
1. RobertsonDM
PetrollWM
JesterJV
CavanaghHD
2007 The role of contact lens type, oxygen transmission, and care-related solutions in mediating epithelial homeostasis and pseudomonas binding to corneal cells: an overview. Eye Contact Lens 33 394 398; discussion 399–400
2. RobertsonDM
PetrollWM
JesterJV
CavanaghHD
2007 Current concepts: contact lens related Pseudomonas keratitis. Cont Lens Anterior Eye 30 94 107
3. FleiszigSM
EvansDJ
2002 The pathogenesis of bacterial keratitis: studies with Pseudomonas aeruginosa. Clin Exp Optom 85 271 278
4. FleiszigSM
2006 The Glenn A. Fry award lecture 2005. The pathogenesis of contact lens-related keratitis. Optom Vis Sci 83 866 873
5. O'BrienTP
MaguireMG
FinkNE
AlfonsoE
McDonnellP
1995 Efficacy of ofloxacin vs cefazolin and tobramycin in the therapy for bacterial keratitis. Report from the Bacterial Keratitis Study Research Group. Arch Ophthalmol 113 1257 1265
6. CalandraT
RogerT
2003 Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol 3 791 800
7. FlasterH
BernhagenJ
CalandraT
BucalaR
2007 The macrophage migration inhibitory factor-glucocorticoid dyad: regulation of inflammation and immunity. Mol Endocrinol 21 1267 1280
8. CalandraT
BernhagenJ
MetzCN
SpiegelLA
BacherM
1995 MIF as a glucocorticoid-induced modulator of cytokine production. Nature 377 68 71
9. BernhagenJ
CalandraT
CeramiA
BucalaR
1994 Macrophage migration inhibitory factor is a neuroendocrine mediator of endotoxaemia. Trends Microbiol 2 198 201
10. CalandraT
BucalaR
1995 Macrophage migration inhibitory factor: a counter-regulator of glucocorticoid action and critical mediator of septic shock. J Inflamm 47 39 51
11. BozzaM
SatoskarAR
LinG
LuB
HumblesAA
1999 Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J Exp Med 189 341 346
12. Assuncao-MirandaI
AmaralFA
BozzaFA
FagundesCT
SousaLP
2009 Contribution of macrophage migration inhibitory factor to the pathogenesis of dengue virus infection. FASEB J
13. SevketogluE
YilmazA
GedikbasiA
KaryagarS
KiyakA
2009 Urinary macrophage migration inhibitory factor in children with urinary tract infection. Pediatr Nephrol
14. HouXQ
GaoYW
YangST
WangCY
MaZY
2009 Role of macrophage migration inhibitory factor in influenza H5N1 virus pneumonia. Acta Virol 53 225 231
15. MatsudaA
TagawaY
YoshidaK
MatsudaH
NishihiraJ
1997 Expression of macrophage migration inhibitory factor in rat retina and its immunohistochemical localization. J Neuroimmunol 77 85 90
16. MatsudaA
TagawaY
MatsudaH
NishihiraJ
1997 Expression of macrophage migration inhibitory factor in corneal wound healing in rats. Invest Ophthalmol Vis Sci 38 1555 1562
17. MatsudaA
TagawaY
MatsudaH
NishihiraJ
1996 Identification and immunohistochemical localization of macrophage migration inhibitory factor in human cornea. FEBS Lett 385 225 228
18. ApteRS
SinhaD
MayhewE
WistowGJ
NiederkornJY
1998 Cutting edge: role of macrophage migration inhibitory factor in inhibiting NK cell activity and preserving immune privilege. J Immunol 160 5693 5696
19. HazlettLD
2005 Inflammatory response to Pseudomonas aeruginosa keratitis. Ocul Surf 3 S139 141
20. ZhangJ
WuXY
YuFS
2005 Inflammatory responses of corneal epithelial cells to Pseudomonas aeruginosa infection. Curr Eye Res 30 527 534
21. ZhuH
ConibearTC
ThuruthyilSJ
WillcoxMD
2008 Pseudomonas aeruginosa quorum-sensing signal molecules induce IL-8 production by human corneal epithelial cells. Eye Contact Lens 34 179 181
22. MiyazakiD
InoueY
Araki-SasakiK
ShimomuraY
TanoY
1998 Neutrophil chemotaxis induced by corneal epithelial cells after herpes simplex virus type 1 infection. Curr Eye Res 17 687 693
23. WinnerM
MeierJ
ZierowS
RendonBE
CrichlowGV
2008 A novel, macrophage migration inhibitory factor suicide substrate inhibits motility and growth of lung cancer cells. Cancer Res 68 7253 7257
24. LiaoH
BucalaR
MitchellRA
2003 Adhesion-dependent signaling by macrophage migration inhibitory factor (MIF). J Biol Chem 278 76 81
25. LueH
ThieleM
FranzJ
DahlE
SpeckgensS
2007 Macrophage migration inhibitory factor (MIF) promotes cell survival by activation of the Akt pathway and role for CSN5/JAB1 in the control of autocrine MIF activity. Oncogene 26 5046 5059
26. HazlettLD
2002 Pathogenic mechanisms of P. aeruginosa keratitis: a review of the role of T cells, Langerhans cells, PMN, and cytokines. DNA Cell Biol 21 383 390
27. ThakurA
XueM
StapletonF
LloydAR
WakefieldD
2002 Balance of pro - and anti-inflammatory cytokines correlates with outcome of acute experimental Pseudomonas aeruginosa keratitis. Infect Immun 70 2187 2197
28. XueML
ThakurA
WillcoxMD
ZhuH
LloydAR
2003 Role and regulation of CXC-chemokines in acute experimental keratitis. Exp Eye Res 76 221 231
29. PrestonMJ
FleiszigSM
ZaidiTS
GoldbergJB
ShortridgeVD
1995 Rapid and sensitive method for evaluating Pseudomonas aeruginosa virulence factors during corneal infections in mice. Infect Immun 63 3497 3501
30. GadjevaM
WangY
HorwitzBH
2007 NF-kappaB p50 and p65 subunits control intestinal homeostasis. Eur J Immunol 37 2509 2517
31. ZaidiTS
LyczakJ
PrestonM
PierGB
1999 Cystic fibrosis transmembrane conductance regulator-mediated corneal epithelial cell ingestion of Pseudomonas aeruginosa is a key component in the pathogenesis of experimental murine keratitis. Infect Immun 67 1481 1492
32. FichorovaRN
Richardson-HarmanN
AlfanoM
BelecL
CarbonneilC
2008 Biological and technical variables affecting immunoassay recovery of cytokines from human serum and simulated vaginal fluid: a multicenter study. Anal Chem 80 4741 4751
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 3- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- All Mold Is Not Alike: The Importance of Intraspecific Diversity in Necrotrophic Plant Pathogens
- Tsetse EP Protein Protects the Fly Midgut from Trypanosome Establishment
- Perforin and IL-2 Upregulation Define Qualitative Differences among Highly Functional Virus-Specific Human CD8 T Cells
- N-Acetylglucosamine Induces White to Opaque Switching, a Mating Prerequisite in
- Origin and Evolution of Sulfadoxine Resistant
- Rapid Evolution of Pandemic Noroviruses of the GII.4 Lineage
- Natural Strain Variation and Antibody Neutralization of Dengue Serotype 3 Viruses
- Fine-Tuning Translation Kinetics Selection as the Driving Force of Codon Usage Bias in the Hepatitis A Virus Capsid
- Structural Basis of Cell Wall Cleavage by a Staphylococcal Autolysin
- Direct Visualization by Cryo-EM of the Mycobacterial Capsular Layer: A Labile Structure Containing ESX-1-Secreted Proteins
- Lipopolysaccharide Is Synthesized via a Novel Pathway with an Evolutionary Connection to Protein -Glycosylation
- MicroRNA Antagonism of the Picornaviral Life Cycle: Alternative Mechanisms of Interference
- Limited Trafficking of a Neurotropic Virus Through Inefficient Retrograde Axonal Transport and the Type I Interferon Response
- Direct Restriction of Virus Release and Incorporation of the Interferon-Induced Protein BST-2 into HIV-1 Particles
- RNAIII Binds to Two Distant Regions of mRNA to Arrest Translation and Promote mRNA Degradation
- Direct TLR2 Signaling Is Critical for NK Cell Activation and Function in Response to Vaccinia Viral Infection
- The Essentials of Protein Import in the Degenerate Mitochondrion of
- Dynamic Imaging of Experimental Induced Hepatic Granulomas Detects Kupffer Cell-Restricted Antigen Presentation to Antigen-Specific CD8 T Cells
- An Accessory to the ‘Trinity’: SR-As Are Essential Pathogen Sensors of Extracellular dsRNA, Mediating Entry and Leading to Subsequent Type I IFN Responses
- Innate Killing of by Macrophages of the Splenic Marginal Zone Requires IRF-7
- Exoerythrocytic Parasites Secrete a Cysteine Protease Inhibitor Involved in Sporozoite Invasion and Capable of Blocking Cell Death of Host Hepatocytes
- Inhibition of Macrophage Migration Inhibitory Factor Ameliorates Ocular -Induced Keratitis
- Membrane Damage Elicits an Immunomodulatory Program in
- Fatal Transmissible Amyloid Encephalopathy: A New Type of Prion Disease Associated with Lack of Prion Protein Membrane Anchoring
- Nucleophosmin Phosphorylation by v-Cyclin-CDK6 Controls KSHV Latency
- A Combination of Independent Transcriptional Regulators Shapes Bacterial Virulence Gene Expression during Infection
- Inhibition of Host Vacuolar H-ATPase Activity by a Effector
- Human Cytomegalovirus Protein pUL117 Targets the Mini-Chromosome Maintenance Complex and Suppresses Cellular DNA Synthesis
- Dispersion as an Important Step in the Biofilm Developmental Cycle
- Kaposi's Sarcoma-Associated Herpesvirus ORF57 Protein Binds and Protects a Nuclear Noncoding RNA from Cellular RNA Decay Pathways
- Differential Regulation of Effector- and Central-Memory Responses to Infection by IL-12 Revealed by Tracking of Tgd057-Specific CD8+ T Cells
- The Human Polyoma JC Virus Agnoprotein Acts as a Viroporin
- Expansion, Maintenance, and Memory in NK and T Cells during Viral Infections: Responding to Pressures for Defense and Regulation
- T Cell-Dependence of Lassa Fever Pathogenesis
- HIV and Mature Dendritic Cells: Trojan Exosomes Riding the Trojan Horse?
- Endocytosis of the Anthrax Toxin Is Mediated by Clathrin, Actin and Unconventional Adaptors
- A Capsid-Encoded PPxY-Motif Facilitates Adenovirus Entry
- Homeostatic Interplay between Bacterial Cell-Cell Signaling and Iron in Virulence
- Serological Profiling of a Protein Microarray Reveals Permanent Host-Pathogen Interplay and Stage-Specific Responses during Candidemia
- YfiBNR Mediates Cyclic di-GMP Dependent Small Colony Variant Formation and Persistence in
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Kaposi's Sarcoma-Associated Herpesvirus ORF57 Protein Binds and Protects a Nuclear Noncoding RNA from Cellular RNA Decay Pathways
- Endocytosis of the Anthrax Toxin Is Mediated by Clathrin, Actin and Unconventional Adaptors
- Perforin and IL-2 Upregulation Define Qualitative Differences among Highly Functional Virus-Specific Human CD8 T Cells
- Inhibition of Macrophage Migration Inhibitory Factor Ameliorates Ocular -Induced Keratitis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



