-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaLong-Lived Antibody and B Cell Memory Responses to the Human Malaria Parasites, and
Antibodies constitute a critical component of the naturally acquired immunity that develops following frequent exposure to malaria. However, specific antibody titres have been reported to decline rapidly in the absence of reinfection, supporting the widely perceived notion that malaria infections fail to induce durable immunological memory responses. Currently, direct evidence for the presence or absence of immune memory to malaria is limited. In this study, we analysed the longevity of both antibody and B cell memory responses to malaria antigens among individuals who were living in an area of extremely low malaria transmission in northern Thailand, and who were known either to be malaria naïve or to have had a documented clinical attack of P. falciparum and/or P. vivax in the past 6 years. We found that exposure to malaria results in the generation of relatively avid antigen-specific antibodies and the establishment of populations of antigen-specific memory B cells in a significant proportion of malaria-exposed individuals. Both antibody and memory B cell responses to malaria antigens were stably maintained over time in the absence of reinfection. In a number of cases where antigen-specific antibodies were not detected in plasma, stable frequencies of antigen-specific memory B cells were nonetheless observed, suggesting that circulating memory B cells may be maintained independently of long-lived plasma cells. We conclude that infrequent malaria infections are capable of inducing long-lived antibody and memory B cell responses.
Published in the journal: . PLoS Pathog 6(2): e32767. doi:10.1371/journal.ppat.1000770
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000770Summary
Antibodies constitute a critical component of the naturally acquired immunity that develops following frequent exposure to malaria. However, specific antibody titres have been reported to decline rapidly in the absence of reinfection, supporting the widely perceived notion that malaria infections fail to induce durable immunological memory responses. Currently, direct evidence for the presence or absence of immune memory to malaria is limited. In this study, we analysed the longevity of both antibody and B cell memory responses to malaria antigens among individuals who were living in an area of extremely low malaria transmission in northern Thailand, and who were known either to be malaria naïve or to have had a documented clinical attack of P. falciparum and/or P. vivax in the past 6 years. We found that exposure to malaria results in the generation of relatively avid antigen-specific antibodies and the establishment of populations of antigen-specific memory B cells in a significant proportion of malaria-exposed individuals. Both antibody and memory B cell responses to malaria antigens were stably maintained over time in the absence of reinfection. In a number of cases where antigen-specific antibodies were not detected in plasma, stable frequencies of antigen-specific memory B cells were nonetheless observed, suggesting that circulating memory B cells may be maintained independently of long-lived plasma cells. We conclude that infrequent malaria infections are capable of inducing long-lived antibody and memory B cell responses.
Introduction
Malaria, a parasitic disease of humans caused predominantly by two species of Plasmodium, P. falciparum and P. vivax, remains an important cause of mortality and morbidity in many parts of the world. Development of a vaccine against malaria has proven challenging due to the complex nature of the parasite and to the difficulty in correlating naturally-acquired immune responses with clinical immunity. While immunity against some of the severe clinical symptoms may be achieved quite rapidly, following perhaps as few as one or two infections [1], immune effector mechanisms capable of controlling parasite growth develop only after repeated infections over a number of years. Even with repeated infections, protective immunity to malaria is not complete, and asymptomatic infections may exist throughout life. Understanding the causes of this continuing susceptibility to infection and, in particular, understanding the development and maintenance of immunological memory, is essential for rational development of malaria vaccines.
Antibodies are a crucial component of naturally acquired protective immunity against blood stage malaria with roles that may include inhibition of merozoite invasion into new red blood cells (RBCs), blocking cytoadherence of infected RBCs (iRBCs) to endothelial cells, and enhancing phagocytic activity of monocytes and macrophages (reviewed in [2],[3]). It is widely believed that periodic reinfection is required to maintain acquired immunity to malaria and that antimalarial antibodies are short-lived in the absence of reinfection (reviewed in [4]); implying that B cell memory to malaria may be defective or suboptimal. However, the development and persistence of B cell memory following malaria infection has long been a matter of debate (reviewed in [5]). Some studies in animal models have shown that memory B cells do develop and are maintained normally after malaria infection [6],[7]; whereas others have found that malaria infection interferes with the development of memory B cells and long-lived plasma cells [8],[9]. In humans, several studies have demonstrated stable antibody responses to malaria antigens [10],[11],[12], however, short-lived antibody responses have also been observed [13],[14], especially in young children [10],[15]. To date, very few studies have examined the induction and maintenance of malaria-specific memory B cells in humans. Dorfman et al [14] were frequently unable to detect circulating malaria-specific B cells in antibody seropositive children, but it is unclear whether this reflects an absence of such cells or a lack of sensitivity in the assays used to detect them. Conversely, Asito et al [16] observed an increase in both the total CD38+IgD− memory B cell population and the transitional CD10+CD19+ B cell population, following an episode of acute malaria in African children but this study lacked any analysis of the specificity of B cell responses as well as any long term follow up to ascertain the duration of the response.
The aim of this study was to investigate the longevity of the human B cell memory response to malaria in individuals with one or more known malaria infections. To do this, we identified individuals living in an area of very low malaria endemicity in Northern Thailand who were either malaria naïve or who had had recorded (and parasitologically confirmed) clinical episodes of P. falciparum or P vivax infection some years previously and characterised the antibody and memory B cell response to a variety of discrete P. falciparum and P. vivax antigens under conditions of infrequent re-exposure/boosting of the immune response.
Results
Characteristics of the study subjects at recruitment
Malaria-specific humoral immune responses of 93, HIV negative Thai adults were studied (Table 1). Individuals were assigned to one of three groups according to their place of residence and their prior malaria history. Subjects from Chiang Mai were designated “City Naïve” (n = 17). Subjects from Muang Na (Chiang Dao) were designated “Rural with no clinical malaria episode (Rural 1; n = 30)” if they reported no prior episodes of malaria infection and/or if no record of malaria infection was found in the past 6 years.
Tab. 1. Characteristics of study subjects at recruitment. 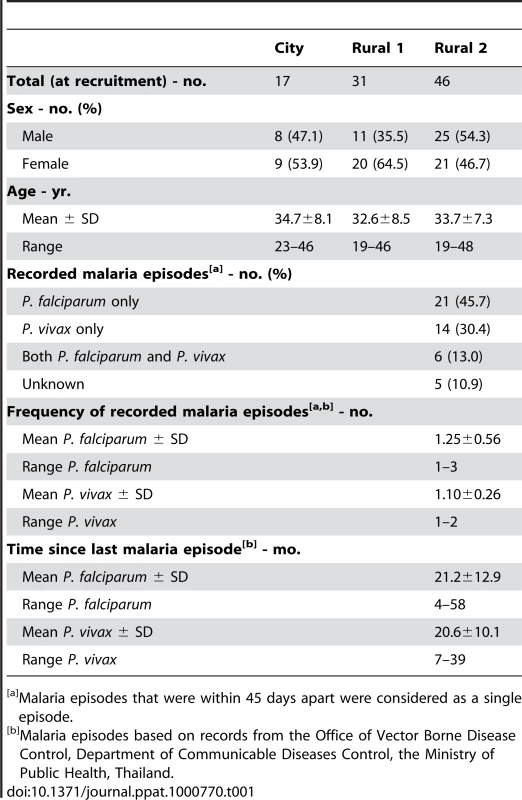
Malaria episodes that were within 45 days apart were considered as a single episode. Muang Na residents who had had one or more fully documented episodes of infection with P. falciparum, P. vivax or both parasite species, as well as those who recalled a previous infection and were seropositive to P. falciparum schizont extract (PfSE) but for whom hospital records could not be found, were designated as “previously malaria infected” (Rural 2; n = 46). In this group, 21 subjects (45.7%) reported at least one episode of infection with P. falciparum, 14 (30.4%) reported at least one episode of infection with P. vivax and 6 (13.0%) reported infection with both species in the past 6 years. The frequency of malaria infections within the six years prior to recruitment varied from 1–3 episodes (mean 1.25±0.56 episodes for P. falciparum and 1.10±0.26 for P. vivax). Five Rural 2 subjects (10.9%) were strongly seropositive to PfSE and recalled prior malaria episodes, but no documentary evidence of these malaria episodes was found. The time since last documented malaria infections prior to recruitment varied from 4–58 (21.2±12.9) months for those known to have been infected with P. falciparum and 7–39 (20.6±10.1) months for those known to have been infected with P. vivax.
Of the 76 rural subjects included at enrolment, 49 (64.5%) were seen again at 3 months, 44 (57.9%) at 6 months and 51 (67.1%) at 12 months. All city individuals were re-sampled 3 months later.
None of the subjects were infected with P. falciparum or P. vivax - as determined by blood film examination and PCR - at any visit. However, one of 76 rural subjects demonstrated a significant increase in antibody titre during the study (but only to one antigen, PfSE) suggesting that this individual may have experienced a recent malaria infection, even though they did not report being ill. The three groups did not differ significantly by age or sex.
Antibody levels against tetanus toxoid (TT) were measured by indirect ELISA. There was no significant difference among the groups in the overall levels of antibodies to TT (Fig. 1A) but only 23.7% of the rural subjects (Rural 1 + Rural 2, n = 76) were seropositive for TT at the time of recruitment. Among the rural individuals (Rural 1 + Rural 2) who had blood collected at recruitment and 12 months later (n = 51), the seropositivity rate for TT (19.6%) did not differ between the two time points (Fig. 1G). The individual data for the rural subjects who were seropositive for TT at the time of recruitment and had blood collected more than one time point (n = 17) are shown in Figure 1M.
Fig. 1. Antibody responses to P. falciparum antigens and tetanus toxoid. 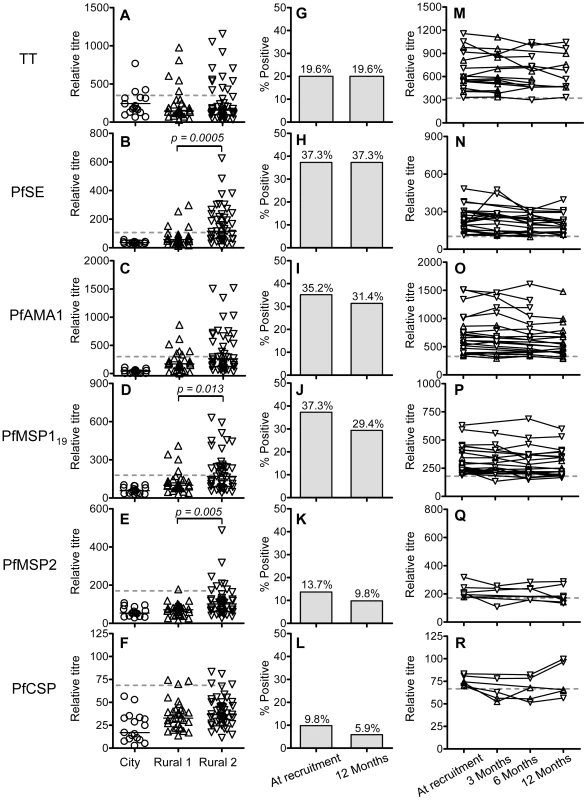
Antibody titres against tetanus toxoid (A) and P. falciparum antigens (B–F) among City naïve (circle), Rural 1 (triangle) or Rural 2 (inverted triangle) subjects at the time of recruitment were determined by indirect ELISA. Each symbol represents the antibody titre of one individual. Solid lines show the median antibody titres in each group. The Mann Whitney U test was used to analyse differences in the levels of antibodies or memory B cells among groups. Figures G–L show the percentages of all rural (i.e. Rural 1 plus Rural 2) subjects who had antibody titres above the cut-off for each antigen at the time of recruitment and 12 months later. Fischer's exact test was used to analyse differences in the proportion of seropositives at recruitment compared to 12 months later but no significant differences were observed. The antibody titres for each seropositive subject over the 12 months of the study are shown in figures M–R. Dotted lines show cut-off values calculated from a mixture model as described in materials and methods. Antibody responses to PfSE
Relative antibody titres to PfSE were measured, at the time of enrolment and at subsequent follow-up. Among the Rural 1 population, 4 (13.3%) individuals had antibody responses above the cut-off, indicating that they had, in fact, been exposed to malaria (Fig. 1B). Among Rural 2 subjects, 25 (54.4%) individuals were seropositive for PfSE. The proportion of seropositives in the Rural 2 group was significantly higher than in the Rural 1 group (p = 0.0003; Fisher's exact test).
There was no difference in the levels of anti-PfSE antibodies between subjects who had been infected with P. falciparum only or infected with P. vivax only (Fig. S1). Overall, the Rural 2 group had levels of anti-PfSE antibodies that were significantly higher than the Rural 1 group (p = 0.0005; Mann Whitney U test). There was no correlation between the levels of anti-PfSE antibodies and age of the subjects or the number of previous malaria episodes they had experienced (data not shown).
Among the rural subjects (Rural 1 + Rural 2) who had blood collected at the beginning and at the end of the study (n = 51), 19 (37.3%) were seropositive for PfSE at the time of recruitment and all of these remained positive 12 months later (Fig. 1H). The individual data for the rural subjects who were seropositive at the time of recruitment and had blood collected at more than one time point (n = 25) are shown in Figure 1N.
Antibody responses to defined P. falciparum antigens
Antibody responses to recombinant malaria antigens PfAMA-1, PfMSP-119, PfMSP-2 and PfCSP were examined by indirect ELISA. PfAMA-1, PfMSP-119 and PfMSP-2 are antigens of blood stage merozoites. PfCSP is the major surface protein on the surface of sporozoites, the infective stage of the malaria parasite. All of these antigens are key P. falciparum vaccine candidates. City naive subjects were seronegative to all P. falciparum antigens tested (Fig. 1C–1F). Among the thirty rural individuals with no known episodes of malaria infection in the past 6 years (Rural 1), 7 (23%), 4 (13%), 1 (3%) and 3 (10%) subjects had positive antibody titres against PfAMA-1, PfMSP-119, PfMSP-2 and PfCSP, respectively (Fig. 1C–1F). Seropositivity to individual malaria antigens, and to PfSE, was not significantly correlated (data not shown) and, overall, 10 (33%) Rural 1 subjects were seropositive to one or more P. falciparum antigens.
The frequencies of antibody responses to PfAMA-1, PfMSP-119, PfMSP-2 and PfCSP in the previously infected (Rural 2) group were 17 (37%), 22 (48%), 7 (15%) and 4 (9%), respectively. Again, seropositivity to individual malaria antigens was not significantly correlated (data not shown) and, overall, 30 (65%) Rural 2 subjects were seropositive to one or more P. falciparum antigens (schizont extract and/or recombinant proteins). The proportion of individuals in the Rural 2 and Rural 1 groups who were seropositive for recombinant malaria antigens was not significantly different, except that a higher proportion of Rural 2 were seropositive for PfMSP-119 (p = 0.003; Fisher's exact test).
The titres of antibodies to PfMSP-119 and PfMSP-2 were significantly higher among Rural 2 subjects than among Rural 1 subjects but among the Rural 2 group the levels of antibodies to individual P. falciparum antigens were not different between previously P. falciparum - and P. vivax - infected subjects (data not shown). Moreover, the titres of antibodies against P. falciparum antigens in some Rural 2 subjects were at least as high as those of the positive control of pooled adult African sera. There was no detectable antibody response to the carrier proteins used in the production of recombinant antigens (data not shown).
Several subjects reporting prior infection only with P. vivax had antibodies to P. falciparum antigens. Of the 14 individuals with recorded P. vivax infections but no recorded P. falciparum infections, 8 (57%) had antibodies to at least one P. falciparum antigen. This is consistent with results of previous studies [17],[18],[19] and may reflect an undiagnosed prior infection with P. falciparum or cross-reactivity of antibodies to the two parasite species [17],[20],[21],[22].
As shown in Fig. 1I–1L, most of the subjects who were seropositive at recruitment and who were tested again 12 months later remained seropositive. The individual data for the rural subjects who were seropositive for the different malaria antigens at the time of recruitment and had blood collected at more than one time point are shown in Fig. 1O–1R. At an individual level, titres of antibodies against PfAMA-1, PfMSP-119 and PfMSP-2 were significantly correlated with titres of anti-PfSE Abs (data not shown). No correlations between age and the antibody titres against individual malaria antigens were found (data not shown).
Antibody responses to P. vivax antigens
We also investigated the antibody responses to P. vivax antigens, PvAMA-1, PvMSP119 and PvDBP by ELISA, all of which are P. vivax blood stage antigens and are key vaccine candidates. City naïve subjects were seronegative to all P. vivax antigens (Fig. 2A–2C). Among Rural 1 subjects, none were seropositive to PvAMA-1 (Fig. 2A), one (3%) had a borderline positive titre to PvMSP-119 (Fig. 2B) and two (6.7%) were seropositive to PvDBP (Fig. 2C). Of the Rural 2 subjects, 5 (11%), 5 (11%) and 3 (6.5%) were seropositive to PvAMA-1, PvMSP-119 and PvDBP, respectively. Overall, 8 (17.4%) Rural 2 subjects were seropositive to one or more P. vivax recombinant antigens. Of the 20 subjects known to have been previously infected with P. vivax (P. vivax only or both P. vivax and P. falciparum), 5 (25%) were seropositive to one or more P. vivax antigens and 15 (75%) were seronegative. The proportion of subjects who were seropositive to P. vivax antigens did not differ significantly between the Rural 1 and Rural 2 groups. Similarly, no significant differences in anti-P. vivax antibody titres were observed between the Rural 1 and Rural 2 groups, although the power of this analysis was poor due to the very low numbers of seropositive subjects. Antibody responses to P. vivax antigens were not different between subjects known to have been infected with P. falciparum - and those known to have been infected with P. vivax (data not shown). Most of the subjects who were seropositive to P. vivax antigens at the time of recruitment and who had samples collected at more than one time point remained seropositive over the course of the study and there was no evidence of declining titres (Fig. 2D–2F).
Fig. 2. Antibody responses to P. vivax antigens. 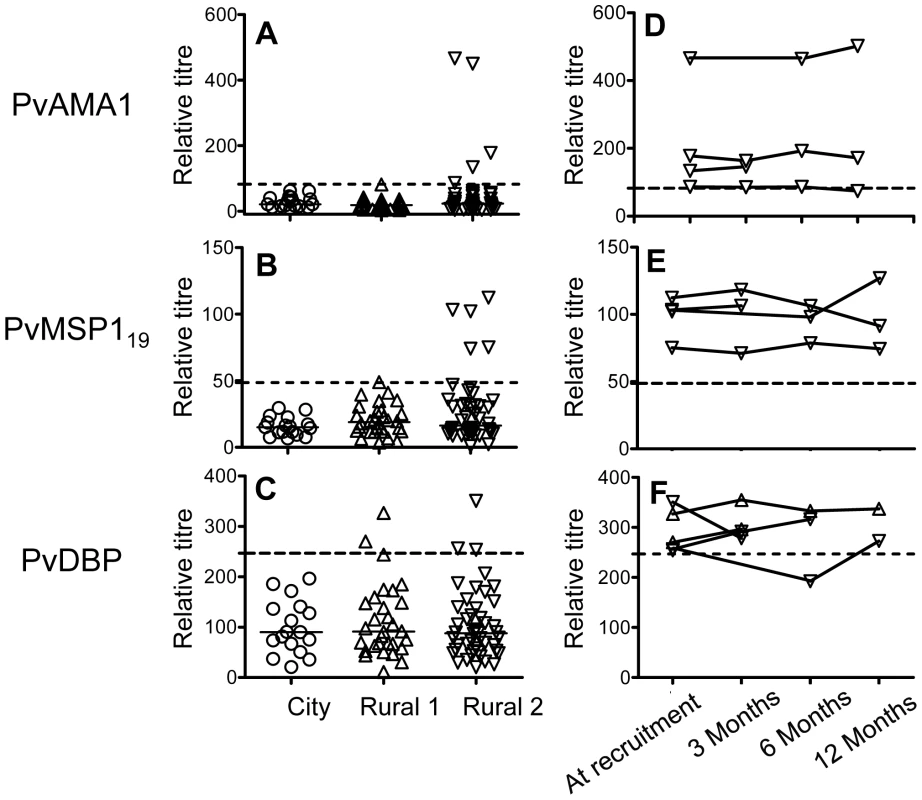
A–C show antibody titres against P. vivax antigens among City naïve (circle), Rural 1 (triangle) or Rural 2 (inverted triangle) subjects at the time of recruitment. Each symbol represents the antibody titre of one individual. Solid lines show the median antibody titres in each group. The titres of antibodies of seropositive rural (i.e. Rural 1 plus Rural 2) subjects at the time of recruitment and at each time point during the 12 months of study are shown in figures D–F. Dotted lines show cut-off values calculated from a mixture model as described in materials and methods. Avidity of antibodies to PfAMA-1 and PfMSP-119
Antibody avidity tends to increase over time as a result of somatic mutation in the immunoglobulin-encoding genes of germinal centre B cells and in response to increasing competition between B cell clones for diminishing amounts of antigen [23],[24]. To determine whether the avidity of the anti-malarial antibody response changed over time, or whether antibody avidity was associated with durability of antibody responses, the avidity indices of anti-PfAMA-1 and anti-PfMSP-119 antibodies were determined for all those individuals (as defined in Figure 1) who were seropositive to one or other antigen at the time of recruitment. Overall, avidity indices for antibodies to both antigens were higher in Rural 2 group than in Rural 1 group (Fig. 3A and 3B), and this difference was statistically significant for antibodies to PfMSP-119. However, there was no detectable change in the avidity of antibodies to either antigen in either group over the 12 months of the study (Fig. 3C–3F).
Fig. 3. Avidity indices for anti-PfAMA-1 and PfMSP-119 antibodies. 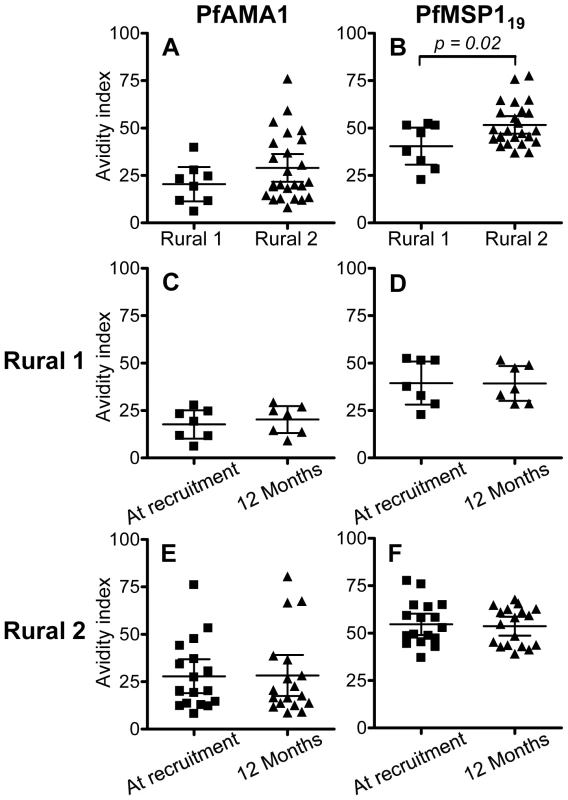
Avidity indices for PfAMA-1 (A) and PfMSP-119 (B) at the time of recruitment were compared between Rural 1 and Rural 2 groups. Avidity indices for antibodies to both antigens were compared at the time of recruitment and at 12 months later for Rural 1 (C and D) and Rural 2 (E and F) subjects. Lines show the mean (95% CI) for each group. An unpaired Student's t-test test was used to analyse differences between rural groups. A paired t-test was used to confirm that there are no differences between indices at time of recruitment and 12 months later. Longevity of antimalarial antibodies
To determine the longevity of the antimalarial antibody responses, we analysed the change in concentrations of antibodies to PfSE, PfAMA-1 and PfMSP-119 in relation to time (in months) since the last documented malaria episode (Figure 4). The half-life of the antibody response was analysed separately for each antigen using data from Rural 2 subjects who were seropositive at the time of enrolment and for whom follow-up samples were obtained (PfSE n = 14; PfAMA1 n = 8; and PfMSP-119 n = 12) in a repeated measurements analysis including multiple data points from the same subjects. Subjects known to be infected with P. vivax but not known to have been infected with P. falciparum were not included in this analysis in order to ensure specificity to P. falciparum.
Fig. 4. Longevity of anti-malarial antibody responses. 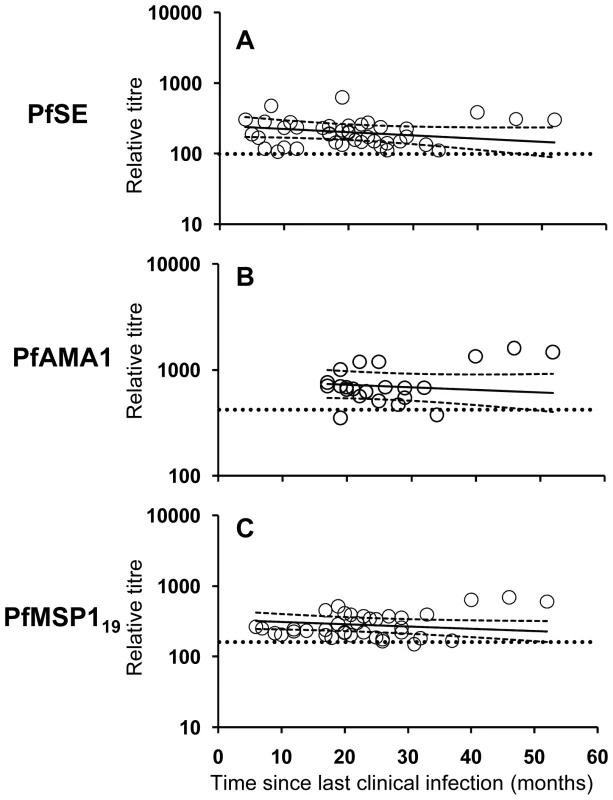
The titres of antibodies specific to PfSE (A), PfAMA-1 (B) and PfMSP-119 (C) in relation to time since last clinical infection in P. falciparum exposed individuals (Rural 2 only) were determined by analyzing longitudinal data with a mixed-effects model. Each symbol represents the antibody level at each time point of one individual. The regression analysis was adjusted for inclusion of multiple data points from the same individual. Solid lines represent best fit regression lines estimating the rates of decline of antibody concentrations over time and the dashed lines represent the 95% CI. Horizontal dotted lines indicate the cut-off as defined in Materials and Methods and Figure 1. Mixed-effects regression models revealed very low rates of decline (converted to years) in anti - PfSE, PfAMA-1 and PfMSP-119 antibody concentrations over time and statistically, these rates could not be distinguished from zero (Table 2). The best estimates of half-lives were: 5.5 years for PfSE, 10.4 years for PfAMA-1 and 7.6 years for PfMSP-119, respectively but, in each case, the 95% CI included infinity. Pooled regression analysis of data for antibodies to PfAMA-1 and PfMSP-119 also yielded a rate of decline that was not statistically significant from zero. Inclusion of anti-PfSE antibody data in the pooled regression analysis resulted in a marginally significant rate of decline equivalent to a half-life of 6.4 years (95% CI = 3.22, 650.48; p = 0.048). These analyses suggest that antibody responses to malaria are stably maintained in this population.
Tab. 2. Longevity of antibody and memory B cell responses to P. falciparum antigens*. 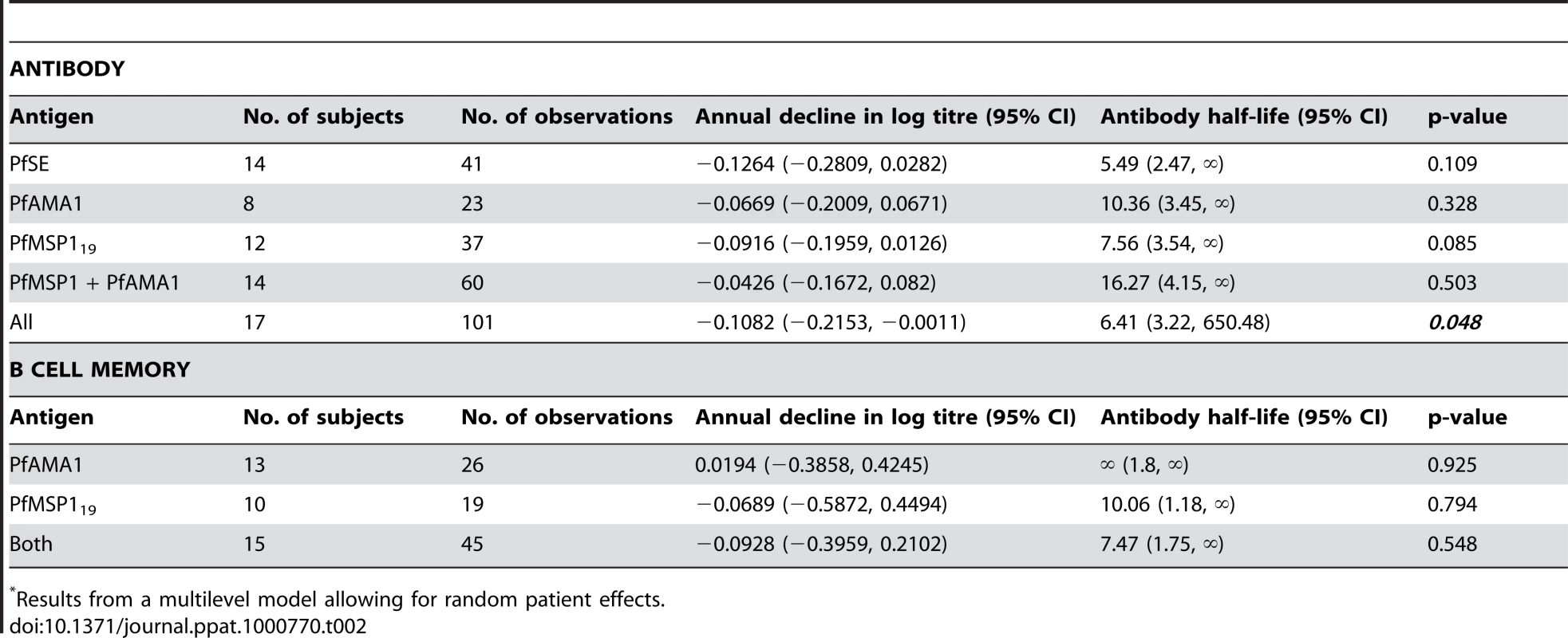
Results from a multilevel model allowing for random patient effects. B cell memory responses to P. falciparum and P. vivax antigens
We next enumerated memory B cells to malaria antigens and to TT using a highly sensitive ELISPOT protocol [25]. The number of subjects available for analysis was limited by availability of cryopreserved PBMCs. Antigen-specific memory B cell frequencies are presented as a percentage of the total number of IgG-secreting cells. Frequencies of TT-specific memory B cells were similar among the three study groups (Fig. 5I). No spots were detected for any individual when cells were tested against the irrelevant control protein (keyhole limpet hemocyanin) and no malaria-specific spots were observed in samples from the City naïve group (data not shown).
Fig. 5. B cell memory responses to malaria antigens and tetanus toxoid. 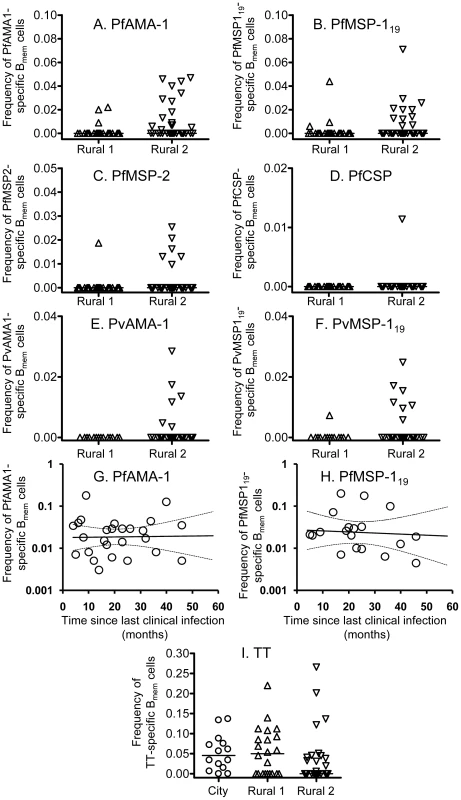
B cell memory responses to P. falciparum antigens (A–D), P. vivax antigens (E and F) and tetanus toxoid (I) at the time of recruitment were determined by ELISPOT assay and are presented as the percentage of all IgG-secreting cells that are specific for each malaria antigen. Each symbol represents the memory B cell numbers for one individual. The longevity of the memory B cell responses specific to PfAMA-1 (G) and PfMSP-119 (H) were determined by analyzing longitudinal data with a mixed-effects model. Solid lines represent best fit regression lines estimating the rates of decline of memory B cell numbers over time and the dashed lines represent the 95% CI. At the time of recruitment, of the 21 Rural 1 subjects whose PBMCs were available, 3 (14.2%), 3 (14.2%), and 1 (4.8%) subjects had memory B cells specific to PfAMA-1 (Fig. 5A), PfMSP-119 (Fig. 5B), and PfMSP-2 (Fig. 5C), respectively. No memory B cells specific to PfCSP were found in the Rural 1 group (Fig. 5D). A much higher proportion of Rural 2 individuals had detectable memory B cells: of the 33 tested, 16 (48%), 11 (33%), 6 (18%) and 1 (3%) gave spots to PfAMA-1, PfMSP-119, PfMSP-2 and PfCSP, respectively. Overall, 19 (58%) Rural 2 subjects had memory B cells to one or more P. falciparum recombinant antigens.
None of the 14 Rural 1 subjects tested had detectable memory B cells against PvAMA-1, and only one individual (7%) had detectable memory B cells to PvMSP-119 (Fig. 5E and 5F). However, among the 26 Rural 2 individuals tested, 6 (23%) and 7 (27%) had memory B cells specific to PvAMA-1 and PvMSP-119, respectively. Nine Rural 2 subjects (35%) had memory B cells specific to one or more P. vivax antigens.
Stability of PfAMA-1 - and PfMSP-119-specific memory B cells
For the Rural 2 individuals, we then characterised the frequency of PfAMA-1 - and PfMSP-119-specific memory B cells in relation to time since their last documented malaria infection, using mixed-effects regression analysis (allowing for repeated measurements from individual subjects) as described above. We found that PfAMA-1 - and PfMSP-119 specific memory B cells were stably maintained over time (Fig. 5G and 5H). The best estimate of the rate of change in AMA-1-specific memory B cell numbers indicated no decline during follow-up, whereas the best estimate for the half-life of MSP-119-specific memory B cells was 10 years (Table 2). Single and pooled regression analysis of data resulted in rates of decline that, statistically, could not be distinguished from zero. Similar observations were made for memory B cells to TT (data not shown). These results indicate that memory B cell responses to malaria antigens are stably maintained in this very low transmission area.
Correlation between antibody titres and memory B cell frequencies
It was immediately evident from the TT data that circulating memory B cells could be detected in many (∼47%) seronegative individuals (Figure 6G). We therefore carried out a systematic analysis of the association between circulating memory B cells and plasma antibody titres at the individual level.
Fig. 6. Correlation between ELISA and ELISPOT responses for each antigen. 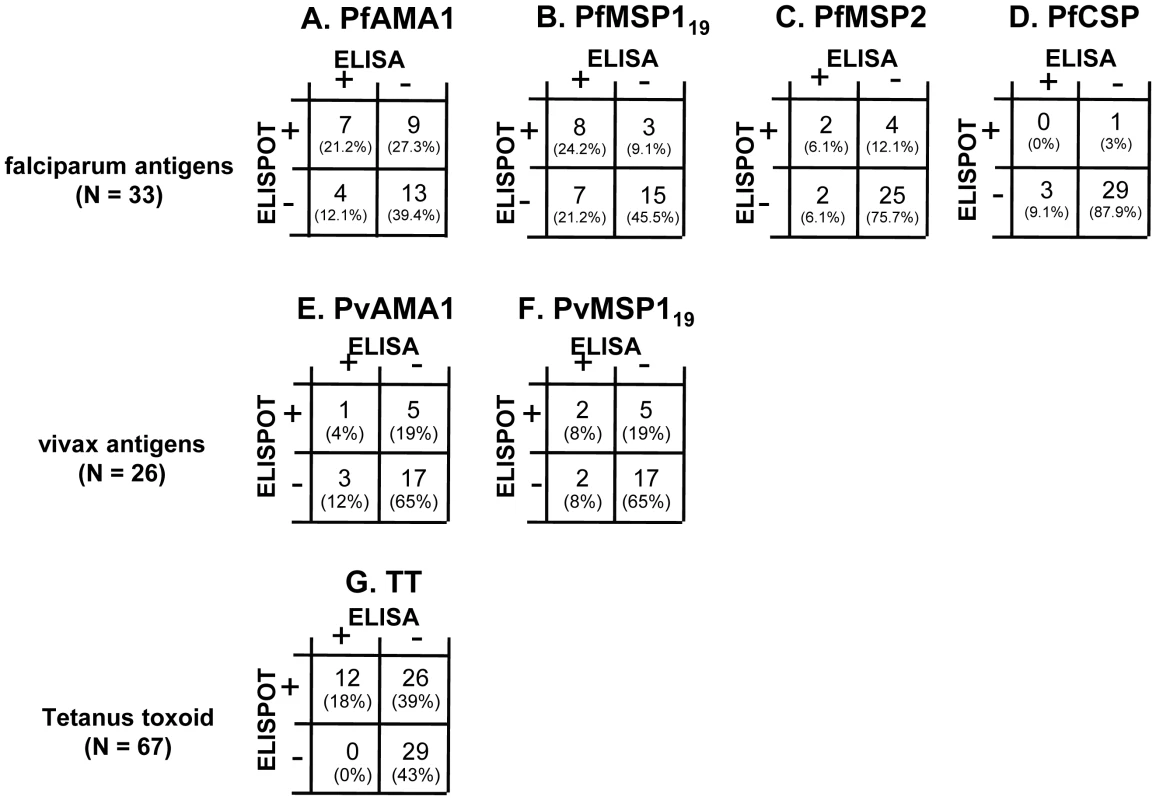
For malaria antigens, data are shown for Rural 2 subjects: 33 tested against P. falciparum antigens (A–D) and 26 tested against P. vivax antigens (E and F). For TT (G), data are shown for all subjects (City naive, Rural 1 and Rural 2) whose PBMC were available (n = 67). The number (and percentages) of subjects who were double positive (top left), ELISA positive but ELISPOT negative (bottom left), ELISA negative but ELISPOT positive (top right), or double negative (bottom right) are shown. No correlation was observed between specific antibody titres and frequencies of memory B cells (data not shown). Among the 33 Rural 2 subjects for whom we had both antibody data and memory B cell responses to P. falciparum antigens at the time of recruitment, 7 (21%), 8 (24%) 2 (6%) and 0 (0%) had both circulating antibody and memory B cells to PfAMA-1, PfMSP-119, PfMSP-2 and PfCSP respectively (Fig. 6A–6D). Four (12%), 7 (21%), 2 (6%) and 3 (9%) individuals were seropositive to the respective antigens but no B cell spots were observed whereas 9 (27%), 3 (9%), 4 (12%), and 1 (3%) were seronegative but had memory B cells to PfAMA-1, PfMSP-119, PfMSP-2 and PfCSP respectively.
Of the 26 Rural 2 subjects for whom we had data on both antibody and memory B cell responses against P. vivax antigens at the time of recruitment, 1 (4%) and 2 (8%) individuals had both antibody and memory B cells against PvAMA-1 and PvMSP-119 respectively (Fig. 6E and 6F), 3 (12%) and 2 (8%) gave positive results in ELISA but not in ELISPOT, and 5 (19%) and 5 (19%) had memory B cells but not antibodies to PvAMA-1 and PvMSP-119, respectively. These results suggest that serum antibody levels alone or memory B cell frequencies alone may not fully represent the humoral immune response to malaria parasites.
Different individuals had different patterns of antibody and memory B cell responses to the various malaria antigens. Table 3 shows the heterogeneity of such responses in all Rural 2 subjects at recruitment. In a number of cases where antigen-specific antibodies were detected, the frequencies of memory B cells were below the limit of detection (Subjects 16, 17 and 29). Likewise, in several subjects where antigen-specific antibodies were not detected, stable frequencies of antigen-specific memory B cells were observed (e.g. Subjects 27, 31 and 33).
Tab. 3. Patterns of antibody and memory B cell responses to malaria antigens and tetanus toxoid in 46 Rural 2 subjects at recruitment. 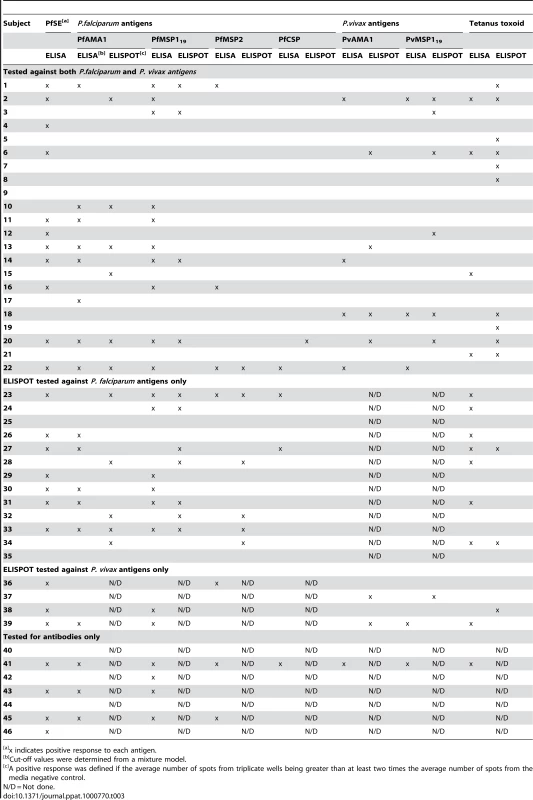
x indicates positive response to each antigen. Discussion
Antibodies are critical in protection against blood stage malaria infection through numerous, diverse mechanisms [2],[3]. In murine malaria infections, B cells are required not only for the production of protective Abs, but also for the development of T cell helper function [26]. However, the development and persistence of B cell memory responses following malaria infection has repeatedly been called into question [27]. It is widely perceived that antibody titres rapidly decline in the absence of re-infection or when individuals leave an endemic area. It is notable that most of the studies reporting short-lived antibody responses have been conducted at or following the time of acute infection, and these infections were terminated by effective antimalarial drug therapy and that these were often observations in children [13]–[16],[28]. It is not clear, from these studies, whether the rapid decline of antibody concentrations observed in children is related to removal of antigen by chemotherapy, by consumption of antibodies and formation of antigen-antibody complexes during parasite clearance or due to limitations in the ability of the bone marrow compartment to support differentiation and/or survival of plasma cells [29],[30]. However, the results of a recently published study in healthy, Gambian children in which antibody titres were found to decline more slowly both in older children and in children with persistent asymptomatic malaria infection suggests that both antigen persistence and immunologic maturity may be important in determining the longevity of the serum antibody responses [15].
One explanation for these observations might be that short-lived antibody responses are the result of induction of short-lived, but not long-lived, plasma cells following acute malaria infection. In murine models of malaria infection, primary P. chabaudi infection leads to expansion of short-lived, immature B220+ splenic plasma cells however secondary infection is accompanied by apparently normal emergence of a larger population of fully mature (Ighi, CD138hi, CD9+, B220−), terminally-differentiated B220 - plasma cells in the bone marrow [31], indicating that memory B cells are efficiently induced by primary infection and are fully able to differentiate into long-lived plasma cells on secondary exposure to antigen. Similar studies have not been reported, to date, in humans but we were able to take advantage of a very particular epidemiological situation in rural Northern Thailand to examine the natural history of the anti-malarial B cell memory response.
In our study area, both P. falciparum and P. vivax are endemic but transmission is kept at extremely low levels by an assiduous malaria surveillance and control programme in which all detected infections are recorded and effectively treated [32]. We have thus been able to recruit a cohort of individuals whose malaria infection history over the previous 6 years are known in considerable detail and have been able to follow these individuals for a period of 12 months to observe both long - and short-term changes in their adaptive immune response to malaria. Furthermore, we were able to recruit a cohort of individuals from the same community with no evidence of malaria infection in the past 6 years , and a cohort of known malaria naives from the city of Chiang Mai, where malaria transmission was eliminated more than 30 years ago (Suwonkerd W; The Ministry of Public Health; personal communication). The very low levels of malaria transmission reported in the Muang Na area [33] are confirmed by our finding that none of the study subjects were found to be infected with malaria parasites (detectable by blood film or PCR) at any point during the study, none of them showed any clinical signs of malaria infection and only one individual showed boosting of antibody responses (and against only 1 malaria antigen) during the study. In addition, anti-malarial antibody responses were not correlated with age, indicating that there is likely to be little or no effective acquired immunity to malaria in this population.
Nevertheless, some of the rural village residents with no record or recollection of malaria infection in the past 6 years (Rural 1) appeared to have experienced malaria infections at some time in their lives as shown by seropositivity to malaria antigens in ELISA and positive B cell ELISPOTs. Given the lack of evidence for acquired protective immunity in this population, and since we found no evidence of asymptomatic malaria infections, it is unlikely that these individuals had experienced undiagnosed malaria infections and thus the presence of antibodies and B cell memory responses in this group suggests that anti-malarial seropositivity can be maintained for many years in the absence of reinfection.
Overall, the prevalence and magnitude of antimalarial antibody and memory B cell responses compared favourably with the anti-tetanus responses. Although the frequencies of tetanus-specific memory B cells tended to be somewhat higher than the frequencies of malaria-specific memory B cells, the prevalence of antibodies to the P. falciparum schizont extract, PfMSP-119 and PfAMA-1 was in fact higher than for tetanus. Thus, despite the fact that the anti-tetanus response is likely induced by a very potent vaccine and boosted by environmental exposure or revaccination (which is routinely given during pregnancy), humoral immune responses to tetanus do not appear to be particularly more robust than those induced by infrequent natural exposure to malaria. Moreover, frequencies of malaria-specific memory B cells in Thai adults were similar to frequencies of diphtheria-specific memory B cells in UK adults (J. Palomero-Gorrindo and J. Hafalla; unpublished data). Therefore, frequencies of malaria-specific memory B cells seem to be of the same order of magnitude as responses to commonly used vaccine antigens [34],[35]. Of note, Buisman et al [35] also found rather higher frequencies of memory B cells to tetanus toxoid than to other antigens.
As the time since last detected malaria infection was known for the Rural 2 group we were able to obtain estimates of the rate of decline of both serum antibodies and circulating memory B cells. Importantly, and in marked contrast to previously published data from African children [14], there was no evidence of any significant decline in either antibody titres or memory B cell responses to PfSE, PfAMA-1 or PfMSP-119 over periods of more than 5 years since the last known malaria infection. One reason for this discrepancy may be that previous studies have characterised the decay of the antibody response in the first few days or weeks after resolution of an acute malaria infection [14],[15] which is likely to capture the initial very rapid decay in antibody titre associated with contraction of the pool of short-lived plasma cells whereas in this study, where the most recent malaria infection occurred many months or years ago, we are capturing the long term “maintenance” phase of the antibody response [36].
Our best estimates of the half-life of this maintenance phase of antibody responses to malaria antigens ranged from ∼5 to ∼16 years, putting them in the same range as the half-lives recently estimated for nonreplicating antigens such as tetanus or diptheria toxoids [37]. However, the 95% confidence intervals for the half-lives of these responses all included infinity, suggesting that antibody responses to malaria may in fact be much more stable than those to nonreplicating antigens and may be maintained in a manner that is more similar to that of antiviral responses [37]. Similar estimates were obtained for the half lives of malaria-specific memory B cell responses – indicating that the circulating memory B cell pool is extremely stable - which is consistent with data from the P. chabaudi mouse model that malaria infection induces long-lived antibody responses as well as memory B cells [31]. However, whilst these analyses are consistent with a long half life for antimalarial antibody and memory cell responses, given the very wide confidence intervals around the our estimates, data from a larger cohort of study subjects is required to obtain definitive half lives for these responses.
However, it is important to note that 11 subjects (24%) who were known to have been infected with malaria had no detectable circulating memory B cells and/or antibodies and less than 50% of subjects tested had either antibodies or circulating memory B cells to PfMSP-2, PfCSP, PvAMA-1 or PvMSP-119. Given that everyone in the study had experienced their most recent malaria infection at least 4 months before recruitment and that the genotypes of their infecting parasites are unknown, we cannot tell whether these seronegative individuals had completely failed to make a humoral response to malaria, whether they had made antibodies to polymorphic epitopes that did not cross-react with the antigens used in our assays or whether they had developed only very short-lived responses. In a previous study, in a higher malaria transmission area, very short-lived antibody responses to malaria were particularly associated with younger individuals who (presumably) had had the fewest number of malaria infections [15] suggesting that the long-lived responses seen in many of our study subjects may develop only after they have experienced a number of malaria infections. Nevertheless, given the very low levels of malaria transmission in our study area, the number of infections required to develop long-live antibody responses is likely to be quite small.
Since the pharmacological half-life of a human IgG molecule is around 21 days [38], long term maintenance of IgG titres indicates either ongoing secretion of antibodies from plasma cells or memory B cell differentiation in response to inflammatory stimuli; there is still no clear consensus on whether persisting specific antigen is required for this process [39] or not [40],[41]. During inflammation, IFN-γ induces plasmablasts to express the chemokine receptor CXCR3, promoting their migration into inflamed tissues [42] and thereby maximising antibody production at sites of infection. Resolution of inflammation leads to loss of survival signals, and these short-lived plasma cells die in situ. However, in the absence of prolonged antigenic stimulation, plasmablasts express another chemokine receptor CXCR4 allowing them to migrate to the bone marrow [43]. It is possible, therefore, that short term fluctuations in serum antibody concentrations may occur in response to infection with memory B cells being stimulated to differentiate into short-lived plasma cells, secrete immunoglobulins and then die. However, in the circumstances of lack of frequent re-exposure to malaria infection (such as in this study), a larger proportion of plasmablasts may differentiate into long-lived plasma cells which maintain the level of antibodies over time. Such a scenario does, of course, beg the question as what would happen to long-lived plasma cells and memory B cells under conditions of repeated or persistent malaria infection.
Immediately after malaria immunisation in humans the frequency of antigen-specific memory B cells is positively correlated with antibody titres [44], suggesting (not surprisingly) that induction of antibody secreting cells and memory B cells is linked. However, the lack of correlation that we observed between antibody titres and memory B cell responses months or years after exposure to malaria antigens confirms recent findings for anti-viral responses [37] and is consistent with accumulating experimental data from animals [45],[46] and data from therapeutic B cell depletion in humans [47],[48] showing that depletion of circulating memory B cells does not affect antibody titres, at least in the short-term. Collectively, these data indicate that although both long-lived plasma cells and memory B cells can be stably maintained the two populations are independently regulated and that activation of circulating memory B cells may not be required for maintenance of serum antibody titres.
High affinity antibodies are expected to play an important role in the humoral immune response. The avidity indices of antibodies against PfAMA-1 and PfMSP-119 did not change during the 12 months of study; this is not surprising since there was no evidence of reinfection of any of the subjects during the follow-up period which might have driven further avidity maturation. However the avidity of anti-PfMSP-119 antibodies was significantly higher among Rural 2 subjects than among Rural 1 individuals, supporting the notion that the Rural 2 population had had more frequent exposure to malaria parasites than the Rural 1 group.
In summary, we conclude that B cell memory responses to malaria are effectively induced and maintained – in a significant proportion of individuals - in areas of low malaria transmission. (This is, of course, an entirely separate issue from whether these particular antibodies confer protective immunity to malaria; whilst there is strong evidence to suggest that malaria-immune individuals have very effective antimalarial antibody responses [49] the antigenic targets of protective antibodies are still very poorly defined. Whilst it is possible that the subjects in this study with long-lived humoral responses to malaria antigens might be protected from reinfection, this issue was not directly addressed in this study). Although it remains possible that persistent and repeated malaria infections in areas of very high endemicity may eventually lead to B cell anergy or clonal exhaustion [50], the fact that individuals in these areas develop high titres of antimalarial antibodies and become resistant to high density malaria infections and clinical symptoms argues against this as a major impediment to the development of effective immune responses. Finally, our results are highly encouraging for vaccine developers since they imply that – once induced – anti-malarial immune responses are likely to be long-lived even in the absence of frequent boosting.
Materials and Methods
Study area and subjects
Study subjects were either long-term adult residents of Muang Na, a village in a low malaria transmission area in the Chiang Dao region of northern Thailand, near the border with Myanmar, or were permanent adult residents of the city of Chiang Mai where malaria transmission does not occur. Ethical approval for the study was obtained from the Research Institute for Health Sciences, Chiang Mai University, from the Ministry of Public Health, Thailand and from the London School of Hygiene and Tropical Medicine, UK. Written informed consent was obtained prior to enrolment in the study.
Subjects were interviewed to ascertain their previous malaria exposure. Residents of Chiang Mai were selected on the basis that they had not travelled to, or lived in, malaria endemic areas. In Muang Na, dates and species (P. falciparum, P. vivax or both) of malaria infections were confirmed from the records of the Office of Vector Borne Disease Control in the Department of Communicable Diseases Control at the Ministry of Public Health, which maintains detailed records of all malaria cases detected by active or passive case detection and during periodic population surveys as described in detail elsewhere [51].
Venous blood was collected in acid citrate dextrose on the day of recruitment, and again 3 months later for City naïve subjects and 3, 6 and 12 months after recruitment for rural subjects. Giemsa-stained blood films were examined for the presence of malaria parasites. Blood samples from each subject were checked for subpatent malaria parasitaemia by PCR. DNA was isolated using FlexiGene DNA extraction kits (Qiagen®) according to the manufacturer's protocol and subjected to nested PCR for P. falciparum and P. vivax as described previously [52].
As HIV infection may have an effect on immunological parameters, all subjects were tested for HIV infection (presence of anti-HIV antibodies by gel particle agglutination assay) at the time of recruitment and at the end of the study (3 months after recruitment for city subjects and 12 months after recruitment for other groups); subjects received pre - and post-test counselling from trained HIV counsellors and HIV-infected individuals were given access to the National Antiretroviral Programme. Data from HIV-infected subjects were excluded from the analysis.
Antigens
P. falciparum circumsporozoite protein (PfCSP) [(NANP)4] and P. falciparum merozoite surface protein-2 (PfMSP-2) were a gift from J.E. Tongren (Centre for Disease Control and Prevention, Atlanta, GA, USA). The 19kDa fragments of P. falciparum and P. vivax MSP-1 (PfMSP-119 and PvMSP-119) were gifts from A. Holder (National Institute of Medical Research, London, UK) and the proteins were expressed as described [53]. P. falciparum apical membrane antigen-1 (PfAMA-1) was a gift from R.F. Anders (LaTrobe University, Victoria, Australia); the equivalent P.vivax antigen (PvAMA-1) was a gift from B. Farber and A. Thomas (Biomedical Primate Research Centre, Rijswik, Netherlands). P. vivax duffy binding protein (PvDBP) was a gift from L.H. Carvalho Centro de Pesquisas René Rachou, Fundação Oswaldo Cruz, Belo Horizonte, MG, Brazil). Since Thai populations are routinely vaccinated with tetanus toxoid (TT), antibody responses to TT were included as a positive control. TT was obtained from the National Institute of Biological Standards and Control (Health Protection Agency, Hertfordshire, UK). Keyhole limpet haemocyanin (KLH) was from Thermos Fisher Scientific (Northumberland, UK).
Continuous cultures of P. falciparum (3D7 strain were maintained in the laboratory [54] and were periodically shown to be free from Mycoplasma contamination by polymerase chain reaction (PCR) (Venor® GeM, Minerva Biolabs). Mature schizonts were obtained by gradient centrifugation over 60% Percoll (Amersham Biosciences), adjusted to a concentration 1×108 schizont-infected red blood cells (iRBC)/ml and exposed to three freeze/thaw cycles to obtain P. falciparum schizont extract (PfSE).
Enzyme-linked immunosorbent assay (ELISA)
Plasma antibody levels were detected by indirect ELISA, as described [55]. Briefly, Immulon 4HB (Dynatech) or Maxisorb (Nunc) plates were coated with antigen (at a concentration equivalent to 105 iRBC/ml for PfSE, 0.5 µg/ml for Plasmodium-derived antigens and TT) in bicarbonate buffer (pH 9.6) overnight at 4°C. Plates were blocked with PBS containing 1% non-fat milk powder. Diluted plasma samples (1∶200 for PfCSP, 1∶1000 for PfSE, PfMSP-119, PfMSP-2, PvMSP-119 and PvDBP; 1∶2000 for PfAMA-1, PvAMA-1 and TT) were incubated in dubplicate. Plates were subsequently developed with anti-human IgG horseradish peroxidase conjugate (Caltag Laboratories, Invitrogen, Paisley, UK) followed by o-phenylenediamine substrate (Sigma). The enzyme reaction was terminated with sulphuric acid (2N) and absorbance was then read at 492 nm on a Spectra MR plate reader (Dynex Technology).
Antibody levels were determined by comparison to a standard curve (derived by serial dilution of a pool of hyperimmune plasma collected in The Gambia, which was given an arbitrary value of 1,000 units/ml of anti-PfSE Abs) on each plate, as described previously [12].
Preparation of peripheral blood mononuclear cells (PBMCs)
PBMCs were separated from citrated blood by gradient centrifugation over Ficoll-Hypaque (Amersham Biosciences). Contaminating erythrocytes were removed by incubating with lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA) at RT for 5 minutes. The cells were washed twice with RPMI, resuspended in 10% human AB serum/RPMI (R10 culture medium), counted, adjusted to the required concentration and cryopreserved in 10% dimethylsulfoxide (DMSO)/foetal calf serum.
Stimulation of cryopreserved PBMC for B cell ELISPOT assay
Cryopreserved PBMCs were quick thawed in a 37°C water bath. The cells were washed twice with warm RPMI, resuspended in R10 culture medium and added at a concentration of 1×106 cells/ml to a 24 well culture plate. The cells were stimulated with medium alone or with a mixture of Phytolacca americana pokeweed mitogen (1/100,000 dilution; a gift from M. Causland and S. Crotty, La Jolla Institute of Allergy and Immunology, CA, USA), 6 µg/ml CpG 2006 (Qiagen/Operon), and 1/10,000 dilution of Staphylococcus Aureus Cowan (SAC) (Sigma), as previously described [25]. The culture plates were incubated in 5% CO2 at 37°C for 5 days.
B cell ELISPOT assay
B cell ELISPOT assays were performed as described previously [25]. Briefly, ELISPOT plates (Millipore) were coated with donkey anti-human IgG (H+L) (Jackson ImmunoResearch), or with 1 µg/ml recombinant malaria proteins overnight at 4°C. After washing once with PBS-T and three times with PBS, 200 µl of 1% bovine serum albumin in RPMI were added to each well and incubated for 2 hours at 37°C, 5% CO2. Cultured PBMCs were recovered from the 24 well culture plates, washed, transferred directly to antigen-coated ELISPOT plates and incubated for 6 hours at 37°C, 5% CO2. After 4 washes with PBS and 4 washes with PBS-T, 100 µl of Biotin-SP-conjugated donkey anti-human IgG (Jackson Immunoresearch) were added to each well and the plates were incubated overnight at 4°C. The plates were washed, 100 µl of alkaline phosphatase-streptavidin (Vector Laboratories) was added and incubated for one hour at room temperature. After three washes with PBS-T and three washes with PBS, 100 µl of 5-bromo-4-chloro-3-indolyl phosphate/ nitro blue tetrazolium - alkaline phosphatase substrate solution (Vector Laboratories) were added to each well and the reaction was allowed to proceed for 8 minutes before being stopped with distilled water. In vitro restimulated PBMCs incubated overnight with an irrelevant protein, KLH, as well as PBMCs cultured without stimulation and then incubated overnight with malaria antigens were used as negative controls. Since no malaria-specific spots were detected in city naïve individuals, this group was not be used to set a cut-off for positivity. Rather, a positive ELISPOT response was defined when spots were observed in 2 or more replicate wells and where the total number of spots in the antigen-coated wells was at least twice the number observed in the negative control wells.
Avidity assay
An enzyme immunoassay for determination of antibodies against malaria antigens was carried out as described above. Following the incubation step of sera with antigens, one duplicate set of sera was treated with 4.0 M guanidine dissociating solution (Guanidine Hydrochloride, Sigma) for 10 minutes prior to washing with PBS-T. Avidity indices were calculated as the ratio of the OD of guanidine -treated wells to the OD of the untreated wells.
Statistical analysis
To determine whether an individual was seropositive for a particular antigen (PfSE, Pf - or Pv-derived antigens, or TT), cut-offs for positive antibody titres were calculated using a mixture model, which assumes that untransformed titres for seropositive and seronegative samples each follow a normal Gaussian distribution [56],[57]. Mann Whitney U test was used to analyse differences in the levels of antibodies or memory B cells among groups (GraphPad Prism software). Fischer's exact test was used to analyse differences in the proportion of positive individuals between Rural 1 and Rural 2 groups, as well as differences in the proportion of seropositives at recruitment compared to 12 months later. Decay rates for antibody titres and memory B cell frequencies were calculated using logarithmically transformed data from subjects who were seropositive or memory B cell positive, respectively, at recruitment. The effect of time since malaria infection was analysed using a log-linear mixed-effects regression model incorporating Gaussian random intercepts. This resulted in an estimate of the decay rate of antibody titres or memory B cell frequencies, assuming a single-exponential decay model. Half-lives were calculated from the estimated decay rate and the boundaries at 95% confidence interval obtained from the mixed-effects model. Where the decay rate is a positive value, the calculated half-life is reported as infinity. All analyses were undertaken using Stata (version 10, Statacorp LP).
Supporting Information
Zdroje
1. GuptaS
SnowRW
DonnellyCA
MarshK
NewboldC
1999 Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat Med 5 340 343
2. WipasaJ
ElliottS
XuH
GoodMF
2002 Immunity to asexual blood stage malaria and vaccine approaches. Immunol Cell Biol 80 401 414
3. BeesonJG
OsierFH
EngwerdaCR
2008 Recent insights into humoral and cellular immune responses against malaria. Trends Parasitol 24 578 584
4. LanghorneJ
NdunguFM
SponaasAM
MarshK
2008 Immunity to malaria: more questions than answers. Nat Immunol 9 725 732
5. StruikSS
RileyEM
2004 Does malaria suffer from lack of memory? Immunol Rev 201 268 290
6. StephensR
AlbanoFR
QuinS
PascalBJ
HarrisonV
2005 Malaria-specific transgenic CD4(+) T cells protect immunodeficient mice from lethal infection and demonstrate requirement for a protective threshold of antibody production for parasite clearance. Blood 106 1676 1684
7. D'Imperio LimaMR
AlvarezJM
FurtadoGC
KipnisTL
CoutinhoA
1996 Ig-isotype patterns of primary and secondary B cell responses to Plasmodium chabaudi chabaudi correlate with IFN-gamma and IL-4 cytokine production with CD45RB expression by CD4+ spleen cells. Scand J Immunol 43 263 270
8. WykesMN
ZhouYH
LiuXQ
GoodMF
2005 Plasmodium yoelii can ablate vaccine-induced long-term protection in mice. J Immunol 175 2510 2516
9. CarvalhoLJ
Ferreira-da-CruzMF
Daniel-RibeiroCT
Pelajo-MachadoM
LenziHL
2007 Germinal center architecture disturbance during Plasmodium berghei ANKA infection in CBA mice. Malar J 6 59
10. TaylorRR
EganA
McGuinnessD
JepsonA
AdairR
1996 Selective recognition of malaria antigens by human serum antibodies is not genetically determined but demonstrates some features of clonal imprinting. Int Immunol 8 905 915
11. UdhayakumarV
KariukiS
KolczackM
GirmaM
RobertsJM
2001 Longitudinal study of natural immune responses to the Plasmodium falciparum apical membrane antigen (AMA-1) in a holoendemic region of malaria in western Kenya: Asembo Bay Cohort Project VIII. Am J Trop Med Hyg 65 100 107
12. DrakeleyCJ
CorranPH
ColemanPG
TongrenJE
McDonaldSL
2005 Estimating medium - and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc Natl Acad Sci U S A 102 5108 5113
13. CavanaghDR
ElhassanIM
RoperC
RobinsonVJ
GihaH
1998 A longitudinal study of type-specific antibody responses to Plasmodium falciparum merozoite surface protein-1 in an area of unstable malaria in Sudan. J Immunol 161 347 359
14. DorfmanJR
BejonP
NdunguFM
LanghorneJ
KortokMM
2005 B cell memory to 3 Plasmodium falciparum blood-stage antigens in a malaria-endemic area. J Infect Dis 191 1623 1630
15. AkpoghenetaOJ
DuahNO
TettehKK
DunyoS
LanarDE
2008 Duration of naturally acquired antibody responses to blood-stage Plasmodium falciparum is age dependent and antigen specific. Infect Immun 76 1748 1755
16. AsitoAS
MoormannAM
KiprotichC
Ng'ang'aZW
Ploutz-SnyderR
2008 Alterations on peripheral B cell subsets following an acute uncomplicated clinical malaria infection in children. Malar J 7 238
17. KumarN
FolgarJP
LubegaP
1992 Recognition of Plasmodium falciparum asexual stage antigens by antibodies in sera from people exposed to Plasmodium vivax. Am J Trop Med Hyg 47 422 428
18. JangpatarapongsaK
SirichaisinthopJ
SattabongkotJ
CuiL
MontgomerySM
2006 Memory T cells protect against Plasmodium vivax infection. Microbes Infect 8 680 686
19. ZeveringY
KhamboonruangC
RungruengthanakitK
TungviboonchaiL
RuengpipattanapanJ
1994 Life-spans of human T-cell responses to determinants from the circumsporozoite proteins of Plasmodium falciparum and Plasmodium vivax. Proc Natl Acad Sci U S A 91 6118 6122
20. IgonetS
Vulliez-Le NormandB
FaureG
RiottotMM
KockenCH
2007 Cross-reactivity studies of an anti-Plasmodium vivax apical membrane antigen 1 monoclonal antibody: binding and structural characterisation. J Mol Biol 366 1523 1537
21. BrachoC
PerezH
1992 An immunocytochemical test for the diagnosis of antibodies to Plasmodium vivax. Parasite Immunol 14 481 487
22. KimYM
HwangHA
YunWS
KimSI
LeeKW
2004 Efficacy of the merozoite surface protein 1 of Plasmodium vivax as an antigen for ELISA to diagnose malaria. Yonsei Med J 45 129 134
23. PhanTG
PausD
ChanTD
TurnerML
NuttSL
2006 High affinity germinal center B cells are actively selected into the plasma cell compartment. J Exp Med 203 2419 2424
24. AdamsCL
MacleodMK
James Milner-WhiteE
AitkenR
GarsideP
2003 Complete analysis of the B-cell response to a protein antigen, from in vivo germinal centre formation to 3-D modelling of affinity maturation. Immunology 108 274 287
25. CrottyS
FelgnerP
DaviesH
GlidewellJ
VillarrealL
2003 Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol 171 4969 4973
26. LanghorneJ
CrossC
SeixasE
LiC
von der WeidT
1998 A role for B cells in the development of T cell helper function in a malaria infection in mice. Proc Natl Acad Sci U S A 95 1730 1734
27. PierceSK
MillerLH
2009 World Malaria Day 2009: what malaria knows about the immune system that immunologists still do not. J Immunol 182 5171 5177
28. KinyanjuiSM
BullP
NewboldCI
MarshK
2003 Kinetics of antibody responses to Plasmodium falciparum-infected erythrocyte variant surface antigens. J Infect Dis 187 667 674
29. PihlgrenM
FriedliM
TougneC
RochatAF
LambertPH
2006 Reduced ability of neonatal and early-life bone marrow stromal cells to support plasmablast survival. J Immunol 176 165 172
30. PihlgrenM
SchallertN
TougneC
BozzottiP
KovarikJ
2001 Delayed and deficient establishment of the long-term bone marrow plasma cell pool during early life. Eur J Immunol 31 939 946
31. StephensR
NdunguFM
LanghorneJ
2009 Germinal centre and marginal zone B cells expand quickly in a second Plasmodium chabaudi malaria infection producing mature plasma cells. Parasite Immunol 31 20 31
32. The Ministry of Public Health, Thailand 2003 Manual for Malaria Control; Bureau of Vector Borne Diseases, editor Bangkok The Agricultural Co-operative Federation of Thailand., Limited
33. Department of Disease Control, The Minstry of Public Health. Report on malaria cases. http://203.157.41.98/malaria/freereoprt/rep.php?free=1&PHPSESSID=368625f72ed8fbe6801903db82d4c152
34. ClutterbuckEA
OhS
HamalubaM
WestcarS
BeverleyPC
2008 Serotype-specific and age-dependent generation of pneumococcal polysaccharide-specific memory B-cell and antibody responses to immunization with a pneumococcal conjugate vaccine. Clin Vaccine Immunol 15 182 193
35. BuismanAM
de RondCG
OzturkK
Ten HulscherHI
van BinnendijkRS
2009 Long-term presence of memory B-cells specific for different vaccine components. Vaccine 28 179 86
36. ManzRA
HauserAE
HiepeF
RadbruchA
2005 Maintenance of serum antibody levels. Annu Rev Immunol 23 367 386
37. AmannaIJ
CarlsonNE
SlifkaMK
2007 Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med 357 1903 1915
38. HopkinsRJ
KramerWG
BlackwelderWC
AshtekarM
HagueL
2004 Safety and pharmacokinetic evaluation of intravenous vaccinia immune globulin in healthy volunteers. Clin Infect Dis 39 759 766
39. BensonMJ
ElguetaR
SchperoW
MolloyM
ZhangW
2009 Distinction of the memory B cell response to cognate antigen versus bystander inflammatory signals. J Exp Med 206 2013 25
40. BernasconiNL
TraggiaiE
LanzavecchiaA
2002 Maintenance of serological memory by polyclonal activation of human memory B cells. Science 298 2199 2202
41. MontesCL
Acosta-RodriguezEV
MerinoMC
BermejoDA
GruppiA
2007 Polyclonal B cell activation in infections: infectious agents' devilry or defense mechanism of the host? J Leukoc Biol 82 1027 1032
42. MuehlinghausG
CiglianoL
HuehnS
PeddinghausA
LeyendeckersH
2005 Regulation of CXCR3 and CXCR4 expression during terminal differentiation of memory B cells into plasma cells. Blood 105 3965 3971
43. TokoyodaK
EgawaT
SugiyamaT
ChoiBI
NagasawaT
2004 Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity 20 707 718
44. CromptonPD
MirceticM
WeissG
BaughmanA
HuangCY
2009 The TLR9 ligand CpG promotes the acquisition of Plasmodium falciparum-specific memory B cells in malaria-naive individuals. J Immunol 182 3318 3326
45. AlwaynIP
XuY
BaskerM
WuC
BuhlerL
2001 Effects of specific anti-B and/or anti-plasma cell immunotherapy on antibody production in baboons: depletion of CD20 - and CD22-positive B cells does not result in significantly decreased production of anti-alphaGal antibody. Xenotransplantation 8 157 171
46. DiLilloDJ
HamaguchiY
UedaY
YangK
UchidaJ
2008 Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J Immunol 180 361 371
47. AnolikJH
BarnardJ
CappioneA
Pugh-BernardAE
FelgarRE
2004 Rituximab improves peripheral B cell abnormalities in human systemic lupus erythematosus. Arthritis Rheum 50 3580 3590
48. VallerskogT
GunnarssonI
WidheM
RisseladaA
KlareskogL
2007 Treatment with rituximab affects both the cellular and the humoral arm of the immune system in patients with SLE. Clin Immunol 122 62 74
49. Bouharoun-TayounH
AttanathP
SabchareonA
ChongsuphajaisiddhiT
DruilheP
1990 Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med 172 1633 1641
50. PierceSK
2009 Understanding B cell activation: from single molecule tracking, through Tolls, to stalking memory in malaria. Immunol Res 43 85 97
51. ThimasarnK
JatapadmaS
VijaykadgaS
SirichaisinthopJ
WongsrichanalaiC
1995 Epidemiology of Malaria in Thailand. J Travel Med 2 59 65
52. SnounouG
ViriyakosolS
ZhuXP
JarraW
PinheiroL
1993 High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol 61 315 320
53. BurghausPA
HolderAA
1994 Expression of the 19-kilodalton carboxy-terminal fragment of the Plasmodium falciparum merozoite surface protein-1 in Escherichia coli as a correctly folded protein. Mol Biochem Parasitol 64 165 169
54. TragerW
JensenJB
1976 Human malaria parasites in continuous culture. Science 193 673 675
55. EganAF
ChappelJA
BurghausPA
MorrisJS
McBrideJS
1995 Serum antibodies from malaria-exposed people recognize conserved epitopes formed by the two epidermal growth factor motifs of MSP1(19), the carboxy-terminal fragment of the major merozoite surface protein of Plasmodium falciparum. Infect Immun 63 456 466
56. VyseAJ
GayNJ
HeskethLM
PebodyR
Morgan-CapnerP
2006 Interpreting serological surveys using mixture models: the seroepidemiology of measles, mumps and rubella in England and Wales at the beginning of the 21st century. Epidemiol Infect 134 1303 1312
57. OhumaEO
OkiroEA
BettA
AbwaoJ
WereS
2009 Evaluation of a measles vaccine campaign by oral-fluid surveys in a rural Kenyan district: interpretation of antibody prevalence data using mixture models. Epidemiol Infect 137 227 233
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek HIV Controller CD4+ T Cells Respond to Minimal Amounts of Gag Antigen Due to High TCR AvidityČlánek Transit through the Flea Vector Induces a Pretransmission Innate Immunity Resistance Phenotype in
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 2- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Pathogen Entrapment by Transglutaminase—A Conserved Early Innate Immune Mechanism
- Broadly Protective Monoclonal Antibodies against H3 Influenza Viruses following Sequential Immunization with Different Hemagglutinins
- Neutrophil-Derived CCL3 Is Essential for the Rapid Recruitment of Dendritic Cells to the Site of Inoculation in Resistant Mice
- Differentiation, Distribution and γδ T Cell-Driven Regulation of IL-22-Producing T Cells in Tuberculosis
- IFN-α-Induced Upregulation of CCR5 Leads to Expanded HIV Tropism In Vivo
- An Extensive Circuitry for Cell Wall Regulation in
- TgMORN1 Is a Key Organizer for the Basal Complex of
- Direct Presentation Is Sufficient for an Efficient Anti-Viral CD8 T Cell Response
- Immunoelectron Microscopic Evidence for Tetherin/BST2 as the Physical Bridge between HIV-1 Virions and the Plasma Membrane
- A New Nuclear Function of the Glycolytic Enzyme Enolase: The Metabolic Regulation of Cytosine-5 Methyltransferase 2 (Dnmt2) Activity
- Genome-Wide mRNA Expression Correlates of Viral Control in CD4+ T-Cells from HIV-1-Infected Individuals
- Structural and Biochemical Characterization of SrcA, a Multi-Cargo Type III Secretion Chaperone in Required for Pathogenic Association with a Host
- A Major Role for the ApiAP2 Protein PfSIP2 in Chromosome End Biology
- HIV Controller CD4+ T Cells Respond to Minimal Amounts of Gag Antigen Due to High TCR Avidity
- Fis Is Essential for Capsule Production in and Regulates Expression of Other Important Virulence Factors
- Vaccinia Protein F12 Has Structural Similarity to Kinesin Light Chain and Contains a Motor Binding Motif Required for Virion Export
- A Novel Pseudopodial Component of the Dendritic Cell Anti-Fungal Response: The Fungipod
- Efficacy of the New Neuraminidase Inhibitor CS-8958 against H5N1 Influenza Viruses
- Long-Lived Antibody and B Cell Memory Responses to the Human Malaria Parasites, and
- IPS-1 Is Essential for the Control of West Nile Virus Infection and Immunity
- Transit through the Flea Vector Induces a Pretransmission Innate Immunity Resistance Phenotype in
- Ats-1 Is Imported into Host Cell Mitochondria and Interferes with Apoptosis Induction
- Six RNA Viruses and Forty-One Hosts: Viral Small RNAs and Modulation of Small RNA Repertoires in Vertebrate and Invertebrate Systems
- The Syk Kinase SmTK4 of Is Involved in the Regulation of Spermatogenesis and Oogenesis
- Optineurin Negatively Regulates the Induction of IFNβ in Response to RNA Virus Infection
- On the Diversity of Malaria Parasites in African Apes and the Origin of from Bonobos
- Five Questions about Viruses and MicroRNAs
- A Broad Distribution of the Alternative Oxidase in Microsporidian Parasites
- Caspase-1 Activation via Rho GTPases: A Common Theme in Mucosal Infections?
- Peptides Presented by HLA-E Molecules Are Targets for Human CD8 T-Cells with Cytotoxic as well as Regulatory Activity
- Interaction of Rim101 and Protein Kinase A Regulates Capsule
- Distinct External Signals Trigger Sequential Release of Apical Organelles during Erythrocyte Invasion by Malaria Parasites
- Exacerbated Innate Host Response to SARS-CoV in Aged Non-Human Primates
- Reverse Genetics in Predicts ARF Cycling Is Essential for Drug Resistance and Virulence
- Universal Features of Post-Transcriptional Gene Regulation Are Critical for Zygote Development
- Highly Differentiated, Resting Gn-Specific Memory CD8 T Cells Persist Years after Infection by Andes Hantavirus
- Arterivirus Nsp1 Modulates the Accumulation of Minus-Strand Templates to Control the Relative Abundance of Viral mRNAs
- Lethal Antibody Enhancement of Dengue Disease in Mice Is Prevented by Fc Modification
- Quantitative Comparison of HTLV-1 and HIV-1 Cell-to-Cell Infection with New Replication Dependent Vectors
- The Disulfide Bonds in Glycoprotein E2 of Hepatitis C Virus Reveal the Tertiary Organization of the Molecule
- IL-1β Processing in Host Defense: Beyond the Inflammasomes
- Kaposi's Sarcoma Associated Herpes Virus (KSHV) Induced COX-2: A Key Factor in Latency, Inflammation, Angiogenesis, Cell Survival and Invasion
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Caspase-1 Activation via Rho GTPases: A Common Theme in Mucosal Infections?
- Kaposi's Sarcoma Associated Herpes Virus (KSHV) Induced COX-2: A Key Factor in Latency, Inflammation, Angiogenesis, Cell Survival and Invasion
- IL-1β Processing in Host Defense: Beyond the Inflammasomes
- Reverse Genetics in Predicts ARF Cycling Is Essential for Drug Resistance and Virulence
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



