-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaInteraction of Rim101 and Protein Kinase A Regulates Capsule
Cryptococcus neoformans is a prevalent human fungal pathogen that must survive within various tissues in order to establish a human infection. We have identified the C. neoformans Rim101 transcription factor, a highly conserved pH-response regulator in many fungal species. The rim101Δ mutant strain displays growth defects similar to other fungal species in the presence of alkaline pH, increased salt concentrations, and iron limitation. However, the rim101Δ strain is also characterized by a striking defect in capsule, an important virulence-associated phenotype. This capsular defect is likely due to alterations in polysaccharide attachment to the cell surface, not in polysaccharide biosynthesis. In contrast to many other C. neoformans capsule-defective strains, the rim101Δ mutant is hypervirulent in animal models of cryptococcosis. Whereas Rim101 activation in other fungal species occurs through the conserved Rim pathway, we demonstrate that C. neoformans Rim101 is also activated by the cAMP/PKA pathway. We report here that C. neoformans uses PKA and the Rim pathway to regulate the localization, activation, and processing of the Rim101 transcription factor. We also demonstrate specific host-relevant activating conditions for Rim101 cleavage, showing that C. neoformans has co-opted conserved signaling pathways to respond to the specific niche within the infected host. These results establish a novel mechanism for Rim101 activation and the integration of two conserved signaling cascades in response to host environmental conditions.
Published in the journal: . PLoS Pathog 6(2): e32767. doi:10.1371/journal.ppat.1000776
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000776Summary
Cryptococcus neoformans is a prevalent human fungal pathogen that must survive within various tissues in order to establish a human infection. We have identified the C. neoformans Rim101 transcription factor, a highly conserved pH-response regulator in many fungal species. The rim101Δ mutant strain displays growth defects similar to other fungal species in the presence of alkaline pH, increased salt concentrations, and iron limitation. However, the rim101Δ strain is also characterized by a striking defect in capsule, an important virulence-associated phenotype. This capsular defect is likely due to alterations in polysaccharide attachment to the cell surface, not in polysaccharide biosynthesis. In contrast to many other C. neoformans capsule-defective strains, the rim101Δ mutant is hypervirulent in animal models of cryptococcosis. Whereas Rim101 activation in other fungal species occurs through the conserved Rim pathway, we demonstrate that C. neoformans Rim101 is also activated by the cAMP/PKA pathway. We report here that C. neoformans uses PKA and the Rim pathway to regulate the localization, activation, and processing of the Rim101 transcription factor. We also demonstrate specific host-relevant activating conditions for Rim101 cleavage, showing that C. neoformans has co-opted conserved signaling pathways to respond to the specific niche within the infected host. These results establish a novel mechanism for Rim101 activation and the integration of two conserved signaling cascades in response to host environmental conditions.
Introduction
All cells, including pathogenic microorganisms, must be able to sense and respond to changes in their environment. As these cells enter a human host, they need to protect themselves from the immune system and rapidly adapt to human physiologic conditions, such as low nutrient availability, varying pH, and mammalian concentrations of carbon dioxide [1]. Therefore, they must coordinate multiple signaling pathways in order to control appropriate cellular responses.
One of the most common environmental stresses for pathogenic fungi is a change in the extracellular pH. Alterations in pH can affect a large number of cellular processes including membrane and cell wall stability, morphogenesis, protein stability and function, and nutrient uptake [2]–[8]. Many of these responses to pH are regulated by the Rim101 transcription factor and its homologues (PacC in filamentous fungi). Additionally, many pathogenic fungi respond to the neutral or slightly alkaline pH of the host by inducing virulence-associated phenotypes [2], [9]–[14]. Therefore, in diverse fungi such as Aspergillus and Candida species, mutants defective in pH sensing/response no longer induce phenotypes associated with virulence in pathogenic species. For example, C. albicans rim101Δ/Δ mutants do not undergo pH-dependent dimorphic switching, do not appropriately increase uptake of iron, and do not secrete the proteases and phosphatases necessary for invasion of host tissues [3], [5], [15]–[19]. A. nidulans pacC (rim101) mutants display decreased growth, decreased secondary metabolite production, and defective invasive growth [9], [14], [20]–[22]. Although A. nidulans is non-pathogenic, these cellular processes have been associated with virulence in other Aspergillus species.
In addition to the direct effects of ambient pH on cell integrity and various metabolic processes, pH changes also affect nutrient uptake. For example, under alkaline conditions, the availability of free iron is greatly reduced as the iron equilibrium shifts from the bioavailable ferrous form to the insoluble ferric form. Studying iron flux is an important new horizon in fungal pathogenesis, as the human host keeps free iron levels at extremely low concentrations (10−18M) through constitutively expressing iron-binding proteins such as transferrin and lactoferrin. In this way, the host protects against invading microorganisms. Fungal pathogens unable to increase iron uptake in this iron-limited host environment often have severe defects in virulence [5], [23]–[26]. The pH-responsive Rim101 transcription factor is involved in the regulation of iron homeostasis, directly binding to the promoters of genes encoding high affinity iron uptake proteins: iron transporters, iron permeases and siderophore transporters [20],[23],[27].
Cryptococcus neoformans is an opportunistic human fungal pathogen. Unlike the distantly related pathogens Candida albicans or Aspergillus fumigatus, C. neoformans grows within a very narrow range of pH values in the host. It grows well at the human physiological pH of the blood and cerebrospinal fluid (pH 7.4) as well as in acidic environments such as the phagolysosome of the macrophage (pH 5) [28],[29]. However, unlike C. albicans and A. fumigatus, C. neoformans demonstrates a severe growth defect above pH 8. Despite this increased sensitivity to alkaline pH, there is still evidence that C. neoformans responds to the slightly alkaline pH of the infected host blood by inducing virulence-associated phenotypes. Capsule production, a major virulence determinate, is optimal at human physiological pH [28], [30]–[32].
On a molecular level, C. neoformans capsule synthesis is transcriptionally regulated by elements of the cAMP/PKA pathway. Strains with mutations in core cAMP signaling elements (such as the Gpa1 Gα protein, adenylyl cyclase Cac1, or the PKA catalytic subunit Pka1) display defective expression of capsule, and these mutant strains are attenuated for virulence in animal models of cryptococcosis. When the regulatory subunit of PKA is mutated in the pkr1Δ strain, the cells have constitutive activation of PKA signaling and display high production of capsule, even in non-inducing conditions [33]–[38]. Other inducing conditions for capsule include iron deprivation, nutrient limitation, and the presence of serum. The mechanisms by which these environmental signals are sensed and subsequently transduced to specific intracellular signaling pathways are not yet known. To explore the interaction of the inducing environmental signals, signal transduction pathways, and downstream effectors controlling capsule synthesis, we have begun to characterize specific transcription factors that are predicted to be targeted by the cAMP pathway, and which also directly control capsule gene expression. By examining the biological function and regulation of the C. neoformans Rim101 homolog transcription factor, we have determined a new pathway for C. neoformans regulation of capsule production. We have also defined a novel interaction between the highly conserved Rim and PKA signaling pathways.
Results
Identifying PKA-regulated transcription factors
To identify potential transcriptional regulators of C. neoformans capsule that are also directly phosphorylated by PKA, we used a bioinformatic survey of the annotated C. neoformans genome. We first searched the available annotation (GO terms, gene names, homology designators) for proteins likely to be involved in transcriptional regulation: transcription factors, DNA binding motifs, zinc finger domains, and other transcriptional regulators. Given the incomplete annotation of the genome, we accepted that many transcriptional regulators might be initially misidentified or excluded by this approach.
Among this subset of proteins, we searched the predicted protein sequence for consensus sequences for PKA phosphorylation (R/K R/K X S/T), to identify potential direct targets of the Protein Kinase A enzyme. One of these proteins is a homologue of Rim101/PacC, a conserved fungal C2H2 transcription factor (GenBank ID CNH00970). The C. neoformans Rim101 protein contains a RRASSL motif at aa730, and has highest homology to Aspergillus nidulans PacC in the C2H2 domain. Analysis of the protein for conserved domains revealed that the only significant Pfam-A match is the zinc finger C2H2 domain (aa133–155).
Disruption of RIM101
To characterize the biological role of this C. neoformans transcriptional regulator, we disrupted the C. neoformans RIM101 gene by homologous recombination. Southern blot analysis confirmed that the rim101::nat mutant allele precisely replaced the native gene in the rim101Δ deletion strain (TOC2) (Table 1) without additional ectopic integration events (data not shown). In addition to this mutant strain in which the entire RIM101 open reading frame was deleted, we created an independent rim101Δ mutant with a partial gene deletion. All phenotypes between these strains were identical, and the TOC2 strain was therefore chosen as the representative rim101Δ mutant strain. To ensure that any phenotypes observed in the rim101Δ mutant strain were due to disruption of the RIM101 gene, we created a rim101Δ+RIM101 complemented strain (TOC4) by integrating a wild-type copy of the RIM101 gene into the genome of the rim101Δ mutant strain.
Tab. 1. List of strains used in this study. 
The rim101Δ mutant strain grew at a similar rate as wild-type on rich media (YPD) and minimal media (YNB) at 30°C, 37°C and 39°C. We found no defect in melanin production on Niger-seed medium. We also examined the mutant strain for resistance to hydrogen peroxide and paraquat by disc diffusion assays and established that the zone of inhibition for the rim101Δ mutant strain was similar to that of wild-type, showing no additional sensitivity to reactive oxygen species.
Rim101 is required for capsule induction
The rim101Δ mutant strain has a major defect in polysaccharide capsule, an important virulence factor in C. neoformans. We incubated wild-type and rim101Δ mutant strains in a capsule inducing medium, Dulbecco's modified Eagle's medium (DMEM) containing 25 mM NaHCO3, for 72 hrs at 37°C and 5% CO2. Incubation in this media usually leads to large polysaccharide capsules surrounding each cell which can be quantitatively measured by analyzing the percent packed cell volume (Cryptocrit analysis) [31]. The rim101Δ mutant strain exhibits markedly reduced capsule around the cell (3.6% packed cell volume) compared to the wild-type strain (6.2% packed cell volume). The rim101Δ mutant capsule-defective phenotype was not noted in previous reports of the C. neoformans Rim101 protein [39]; therefore, we confirmed our observation in several ways. We examined several independent rim101Δ mutants, including partial and complete gene deletions, which all displayed similar capsule defects in the inducing conditions. These differences in capsule were microscopically visualized by the exclusion of India ink (Figure 1A). Reintroduction of the wild-type allele fully complemented the capsule phenotype (6.8% packed cell volume).
Fig. 1. C. neoformans Rim101 is required for capsule attachment. 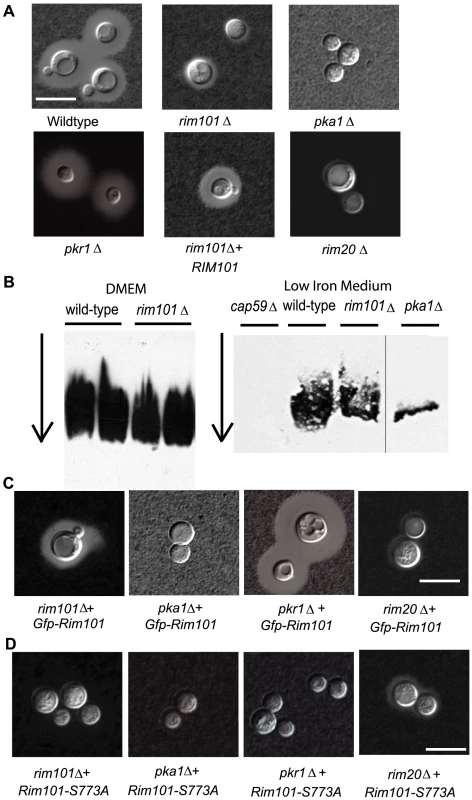
A. rim101Δ mutants have a capsule defect. Cells were incubated in capsule inducing conditions for 3 days. Capsule was assessed by staining with India ink and visualizing the zone of exclusion at 63× magnification (scale bar = 10 m). B. The rim101Δ mutant sheds equivalent capsule to wild-type. Electrophoretic mobility and quantity of shed polysaccharide was assessed by a blotting technique of culture medium filtrate, using an anti-GXM antibody to probe for capsule as previously described [47]. Cells were incubated in Dulbecco's modified Eagle's medium or low iron medium for 1 week before filtering. Arrow indicates direction of electrophoresis. C. Gfp-tagged Rim101 is functional. Cells were incubated in capsule inducing conditions for 3 days. Capsule was assessed by staining with India ink and visualizing the zone of exclusion at 63× magnification (scale bar = 10 m). D. Rim101-S773A does not complement capsule. Cells were incubated in capsule inducing conditions for 3 days. Capsule was assessed by staining with India ink and visualizing the zone of exclusion at 63× magnification (scale bar = 10 m). To confirm the role of Rim101 and the Rim pathway in C. neoformans capsule production, we searched the C. neoformans genome for conserved elements of the Rim pathway, and we identified the RIM20 gene. Rim20 is a scaffold protein required for Rim101 cleavage/activation in other fungal species [6], [40]–[45]. When we mutated this gene, we observed a similar capsule defect in the rim20Δ mutant compared to the rim101Δ mutant strain (Figure 1A).
C. neoformans capsule is secreted out of the cell and subsequently bound to the cell wall [46]. To determine whether rim101Δ mutant strains produce and secrete capsular polysaccharide, we used a previously described gel electrophoresis technique to quantify this polymer [47]. C. neoformans cells were incubated in capsule-inducing DMEM for 1 week, after which polysaccharide that was shed into the medium was analyzed for relative abundance and size by reactivity against an anti-GXM antibody (mAb18B7). We noted a similar amount of secreted polysaccharide in the rim101Δ mutant as wild-type (Figure 1B) suggesting that there is no significant GXM synthesis defect in the rim101Δ strain. We repeated this assay using low iron medium [31] as the capsule inducing condition instead of Dulbecco's modified Eagle's medium, and we again observed a similar amount of secreted capsule in wild-type and rim101Δ mutant strains (Figure 1B). As previously described, we used a cap59 mutant strain that is unable to secrete capsule as a negative control [48] and detected no capsule by this assay in this strain. We also demonstrated the quantitative nature of this assay by analyzing the pka1Δ mutant strain, in which there is a previously documented decrease in capsule production compared to wild-type [36] (Figure 1B). Therefore, the hypocapsular phenotype of the rim101Δ mutant strain in the presence of intact polysaccharide production suggests a defect in capsule attachment. This result is similar to the phenotype of the C. neoformans ags1Δ mutant strain which can synthesize and secrete capsule but cannot bind it [49],[50].
CnRim101 retains conserved physiological roles with Rim101/PacC from other fungal species
In fungi as diverse as Aspergillus, Saccharomyces, Candida, and Ustilago species, the Rim101/PacC proteins control multiple pH-related phenotypes, including regulating iron homeostasis; maintaining membrane and cell wall-associated proteins; and secreting proteases, secondary metabolites, and phosphatases [2],[5],[14],[51]. Many of these factors are important in the virulence of pathogenic fungi.
We tested the C. neoformans rim101Δ mutant strain to determine if this protein retains conserved physiological roles with Rim101/PacC proteins in other fungal species. On alkaline media, the rim101Δ mutant strain exhibits a severe growth defect compared to the wild-type and the reconstituted strains at alkaline pH above 7.6 (p<0.01) (Figure 2A). Similar to other rim101-defective fungal strains, the C. neoformans rim101Δ mutant also displayed sensitivity to media containing 200 mM LiCl or 1.5M NaCl (Figure 2B). To confirm that this growth defect was due to specific sensitivity to ionic stress as opposed to general sensitivity to osmotic stress, we tested the cells for growth on media containing 2.5M sorbitol and detected no difference in growth between the rim101Δ mutant strain and wild-type. We also determined no defects in response to cell wall stress for the rim101Δ mutant strain during growth on calcofluor white, Congo red, or 0.05% SDS. These data suggests that, unlike in other fungal species, there are no major defects in cell wall integrity in the C. neoformans rim101Δ mutant strain.
Fig. 2. Rim101 retains conserved phenotypes from other fungal species. 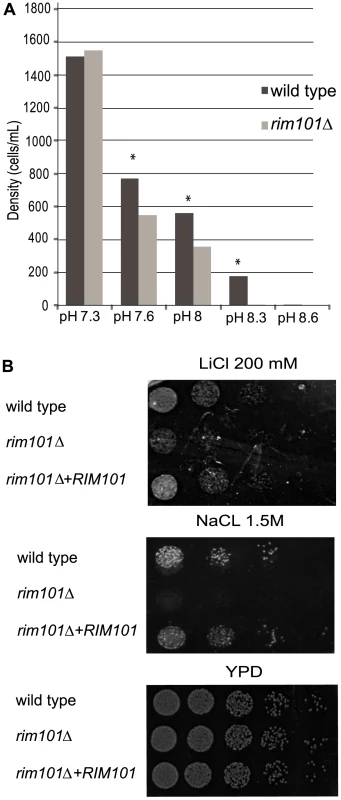
A. The rim101Δ mutant is sensitive to alkaline pH. Cells were incubated in buffered YNB, and growth was determined by cell counts after 72 hours. B. The rim101Δ mutant is sensitive to salt stress. 1×105 cells from each strain were serially diluted (5-fold dilution) onto YPD plates containing 1.5M NaCl or 200 mM LiCl. The plates were incubated at 30°C for 3 days. Cells were plated onto YPD plates for 48 hours as a control. Rim101 localization is regulated by PKA and Rim20
In other fungal species, the Rim101 protein is activated by cleavage and subsequently localized to the nucleus, as expected for a transcription factor [52]. To test whether C. neoformans Rim101 is cleaved and localized to the nucleus, we created a Gfp-Rim101 fusion protein. We fused the green fluorescent protein gene to the N-terminus of the RIM101 gene, expressing the new transgene under control of a constitutive histone promoter. Introduction of this plasmid (pTO2) by biolistic transformation into the rim101Δ mutant strains fully complemented the rim101Δ mutant capsule phenotype, indicating that the Gfp-Rim101 fusion protein is functional (Figure 1C).
Unlike A. nidulans, in which localization is dependent on activation, C. neoformans Rim101 is nuclear under all conditions tested. Using epifluorescent microscopy, we observed a nuclear pattern of localization for C. neoformans Gfp-Rim101 after 24 hours growth in various conditions, including YNB buffered at pH 8, Dulbecco's modified Eagle's medium, YNB (pH 5.4), and YPD (Figure 3). In contrast, the Gfp-Rim101 protein in the pka1Δ and rim20Δ mutant backgrounds localized to both the nucleus and the cytoplasm under the same growth conditions, suggesting that PKA activity and Rim20-mediated cleavage are both important for complete nuclear localization (Figure 3). In addition, overexpression of the Gfp-tagged Rim101 protein was not able to suppress the pka1Δ or the rim20Δ mutant capsule phenotype (Figure 1C).
Fig. 3. Rim101 localization in mutant backgrounds. 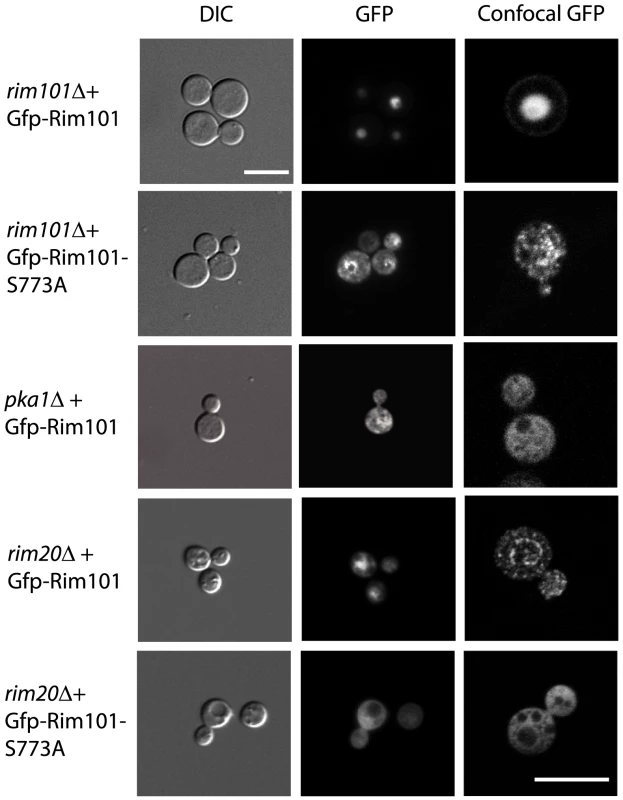
Rim101 localization is dependent on PKA and Rim20. The pattern of Gfp-Rim101 localization in the indicated strains was visualized by DIC and fluorescent microscopy at 63× magnification and by confocal microscopy at 100× magnification. To further explore the potential association of PKA and Rim101, we mutated the single Rim101 consensus sequence for PKA phosphorylation by changing serine 773 to an alanine, creating the Rim101-S773A mutant protein encoded in plasmid pTO3. When examining the localization of the Gfp-Rim101-S773A protein, we observed both nuclear and cytoplasmic fluorescence, similar to the localization of wild-type Gfp-Rim101 expressed in the pka1Δ and rim20Δ mutant backgrounds. Introduction of the Gfp-Rim101-S773A protein into the rim20Δ background also resulted in both nuclear and cytoplasmic localization of Rim10. Additionally, the Rim101-S773A mutant protein in the rim101Δ mutant background did not complement the capsule phenotype (Figure 1D). This observation, coupled with our documentation that some Gfp-Rim101-S773A protein is localizing to the nucleus, indicates that serine 773 is necessary for full function of the Rim101 protein. When we transformed the Rim101-S773A mutant protein into the pkr1Δ mutant strain, in which PKA signaling and capsule production are constitutively active, multiple independent transformants had markedly attenuated capsule, even though the wild-type RIM101 gene was still present in these strains (Figure 1D). In contrast, introduction of the plasmid containing the wild-type Rim101 fused to Gfp into the pkr1Δ strain had no effect on capsule. This suggests that the Rim101-S773A mutant protein is acting in a dominant negative manner on C. neoformans capsule.
Rim101 cleavage is mediated by PKA and Rim20
To examine the interaction of PKA and the C. neoformans Rim pathway, we used western blotting techniques to compare Rim101 protein processing in multiple strain backgrounds. Using an anti-Gfp monoclonal antibody for detection, we identified bands corresponding to the Gfp-Rim101 fusion protein from cell lysates of cultures incubated in YPD to mid-log phase (Figure 4A). The protein we detected when both Pka1 and Rim20 were wild-type had a molecular weight of approximately 120kD (Figure 4A). In contrast, expression of the identical Gfp-Rim101 fusion protein in the pka1 or rim20Δ mutant backgrounds resulted in a protein band of approximately 140kD, which is the predicted size of the full length fusion protein. The Gfp-Rim101-S773A protein also migrated with a reduced electrophoretic mobility in the rim101Δ or rim20Δ backgrounds, resulting in an approximately 140kD band. The 120kD processed form of Gfp-Rim101 was dependent on both PKA and Rim20. Treatment with lambda phosphatases did not alter the mobility of any of these bands (data not shown). We also observed multiple smaller bands in the pka1Δ and rim20Δ mutant backgrounds. These may represent degradation products, suggesting that both Pka1 and Rim20 are necessary to prevent aberrant proteosomal involvement [53]. In addition, the strains with a 140kD Gfp-Rim101 protein were the same strains that had both cytoplasmic and nuclear patterns of Gfp fluorescence. Only the 120kD processed form had predominantly nuclear localization (Figure 3).
Fig. 4. Western blot analysis of Rim101 in rim101 and pka1Δ mutant backgrounds. 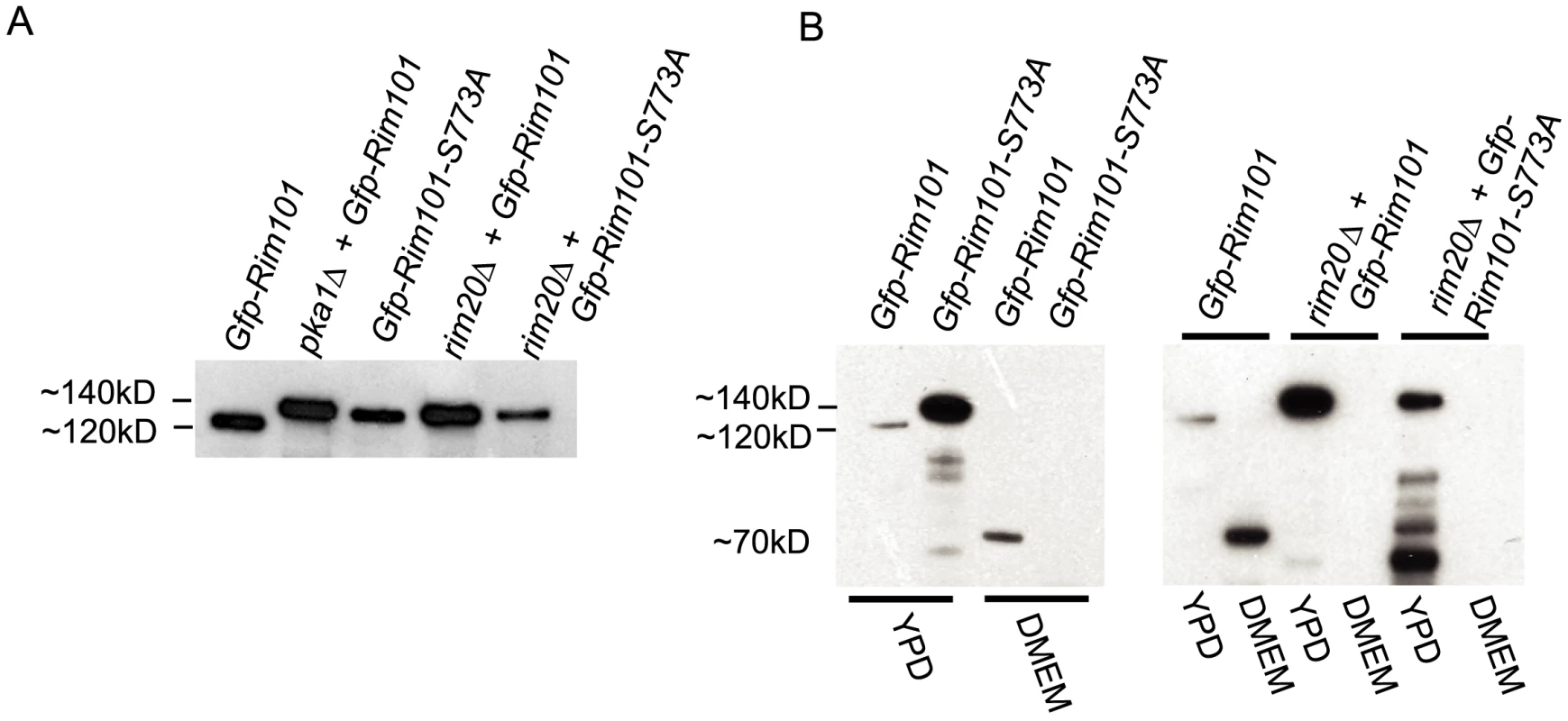
A. Rim101cleavage is dependent on PKA and Rim20. Immunoprecipitated Gfp-Rim101 from rim101Δ+ Gfp-rim101, rim101Δ+ Gfp-rim101-S773A, pka1Δ+ Gfp-rim10, rim20Δ+ Gfp-rim101, and rim20Δ+ Gfp-rim101-S773A strains was immunoblotted using anti-GFP antibody. B. Rim101 is cleaved after induction in capsule media. Gfp-Rim101 was immunoprecipitated from rim101Δ, rim101Δ+ rim101-S773A, rim20Δ+ Gfp-rim101, and rim20Δ+ Gfp-rim101-S773A cell lysates after incubation in either YPD medium or the capsule-inducing medium DMEM at 30°C to mid-log phase. Samples were run on Bis-Tris gels and immunoblotted using an anti-GFP antibody. A. nidulans PacC undergoes two successive cleavage events that regulate its function as an alkaline-responsive transcription factor [42],[43],[53],[54]. To examine whether C. neoformans Rim101 also undergoes a second cleavage event under activating conditions, we incubated the rim101Δ +Gfp-RIM101 strain to mid-log phase in either YPD or capsule inducing media. When incubated in DMEM, we detected an additional band at approximately 70kD, corresponding to a potentially cleaved N-terminal fragment of the Rim101 protein (Figure 4B). This band was not present when PKA, Rim20, or the PKA phosphorylation consensus sequence were mutated, or under non-inducing conditions, demonstrating that PKA phosphorylation and Rim20 are necessary for this further cleavage of Rim101 under inducing conditions.
Rim101 downstream targets—capsule and iron
To define the downstream targets of the Rim101 transcription factor, we performed comparative transcriptional profiling between the rim101Δ mutant strain and wild-type using whole genome microarrays. We confirmed these results for several genes using quantitative real-time PCR (data not shown). These results indicate that Rim101 controls the transcription of many genes involved in several categories of cellular function (Table 2).
Tab. 2. Subset of Rim101-dependent gene expression in capsule-inducing conditions. 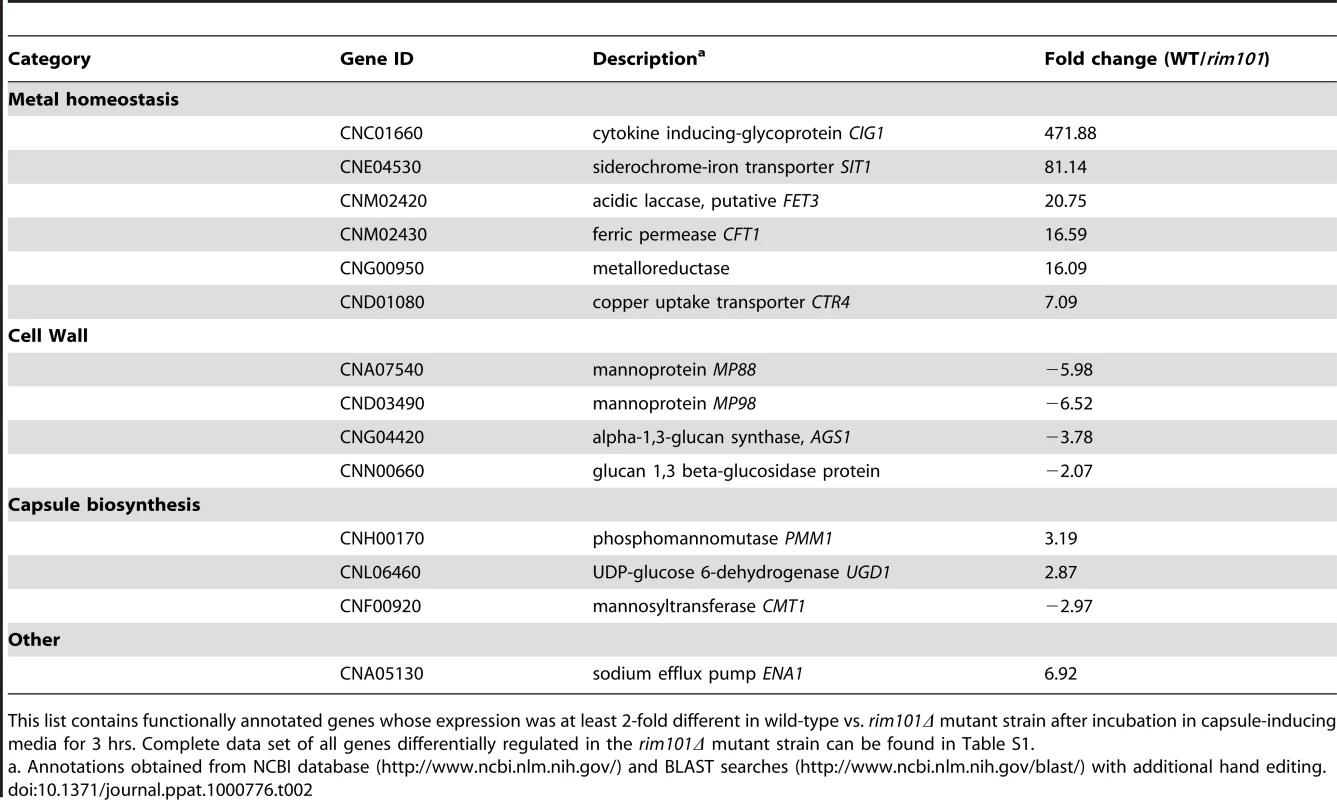
This list contains functionally annotated genes whose expression was at least 2-fold different in wild-type vs. rim101Δ mutant strain after incubation in capsule-inducing media for 3 hrs. Complete data set of all genes differentially regulated in the rim101Δ mutant strain can be found in Table S1. Under capsule-inducing conditions, we were able to document differential expression for a limited number of genes that may be involved in capsule biosynthesis. We observed a 2.9-fold greater expression of UGD1 in the wild-type compared to the rim101Δ mutant strain. UGD1 encodes a UDP-glucose dehydrogenase that is necessary for UPD-glucuronic acid synthesis and thus capsule biosynthesis [55],[56]. A mannosyltransferase (Cmt1) was 2.97-fold greater expressed in the rim101Δ mutant strain than the wild-type. Mannosyltransferases such as Cmt1 have been implicated in the biosynthesis of GXM [57]. In addition, we observed a 3.2-fold decrease in expression of a phosphomannomutase gene (PMM) in the rim101Δ mutant strain. PMM is involved in the biosynthesis of GDP-mannose, another nucleotide sugar essential for capsule production, and is transcriptionally regulated by PKA [24], [58]–[61]. The data also show a small number of other nucleotide sugar-related genes that are differentially expressed and may be involved in capsule production. The fact that many highly inducible capsule genes are not transcriptionally regulated by Rim101 is consistent with our observation that the capsule defect is due to adherence, not production.
Transcriptional profiling also suggested that Rim101 controls the expression of several genes involved in iron or metal homeostasis, including the iron transporter gene CFT1, siderophore importer gene SIT1, and copper transporter gene CTR4 [24], [60]–[62]. In addition, we documented Rim101-dependent expression of homologues of S. cerevisiae iron permeases (FRP1) and reductases (FET3), which are known to be regulated by PKA and by the Cryptococcus transcription factor Cir1 [60],[61],[63],[64]. To demonstrate that decreased expression of these genes was biologically relevant, we incubated the rim101Δ mutant strain in low iron medium, and we observed a distinct growth defect compared to wild-type or the reconstituted RIM101 strain (Figure 5A). C. neoformans strains typically induce capsule in response to growth in this medium. Although the rim101Δ mutant strain grew slowly in low iron media, it eventually reached saturation phase. However, even when grown to saturation, the rim101Δ mutant strain did not exhibit capsule in low iron media (data not shown). Further analysis of these iron homeostasis genes revealed that the promoters of all of these iron regulating genes contain the potential Rim101 consensus binding sequence GCCAAG or the diverged sequence CCAAGAA, recognized by the S. cerevisiae, C. albicans and A. nidulans Rim101 orthologs [7],[27],[65]. These results indicate that C. neoformans Rim101 retains conserved roles in regulating iron homeostasis and import.
Fig. 5. Rim101 in low iron media. 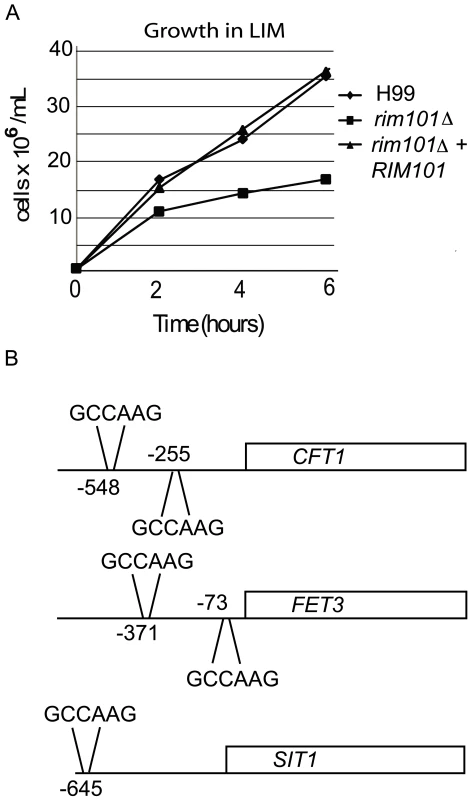
A. Rim101 is required for growth in low-iron medium. Strains were incubated in low-iron medium and growth was quantified by monitoring the absorbance of the culture at 600 nm. B. The 5′-untranslated regions of C. neoformans genes involved in iron regulation were evaluated for the presence of putative Rim101 binding sites (GCCAAG or CCAAGAA). Rim101 and virulence
The ability to produce capsule is important for the pathogenicity of C. neoformans, and other capsule-deficient strains are severely attenuated for virulence in animal models of cryptococcosis [33], [35], [66]–[68]. In addition, the ability to obtain iron from the host and to grow in low iron conditions is important for microbial survival in the host [60],[63],[69]. We therefore hypothesized that the hypocapsular rim101Δ mutant would be avirulent in animal models of cryptococcosis. However, a recent manuscript in which the investigators tested virulence properties in a large collection of C. neoformans mutants suggested that the rim101Δ mutant strain might be more virulent than wild-type [39]. We therefore tested the role of Rim101 in C. neoformans pathogenicity. Female A/Jcr mice (10 per strain) were inoculated intranasally with 5×105 CFU of the wildtype, rim101Δ mutant, or rim101Δ+RIM101 complemented strains. Mice were monitored for survival and sacrificed at predetermined clinical endpoints predicting mortality (Figure 6A). Infection with either the wild-type or the rim101Δ+RIM101 complemented strain resulted in complete mortality 18 and 19 days after infection respectively; there was no statistically significant difference between the survival of these two groups (p = 0.13). Mice infected with the rim101Δ mutant strain succumbed to the infection 16 days post-infection; this represents a statistically significant decrease in survival compared to animals infected with the wild-type strain (p<0.002). We repeated the infection and sacrificed mice on day 2, day 9 and day 14 to determine fungal burden in the lungs, spleen, and brain. In all organs, we found no statistically significant difference in rates of dissemination among the 3 inoculated strains.
Fig. 6. Virulence analysis of the rim101Δ mutant strain. 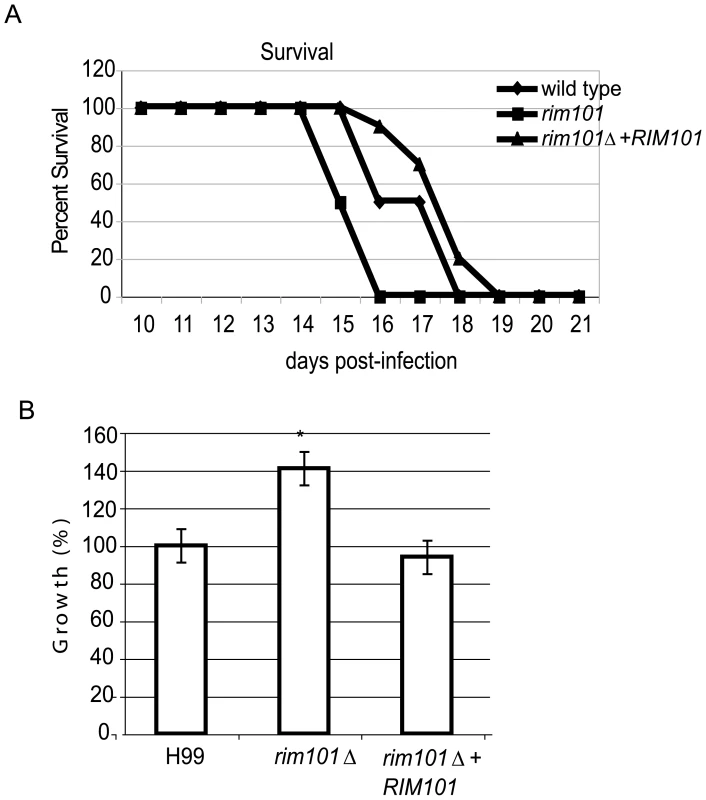
A. rim101Δ mutant strains are hypervirulent in the murine inhalation model of cryptococcosis. AJc/r mice were inoculated intranasally with 5×105cryptococcal cells and monitored for survival. B. rim101Δ mutant cells survive better than wild-type within macrophages. The rim101Δ mutant and isogenic wild-type strains were co-incubated with J744.1 macrophage-like cells previously activated by INF-gamma and LPS. Extracellular yeast cells were removed after one hour of co-incubation. After 24 hours of co-culture, the macrophages were lysed with SDS, and surviving yeast cells were quantitatively cultured. To precisely control for the number of added cells, the colony-forming units from each strain were normalized to that of wild-type cells. Data points represent the average of triplicate samples +/− standard error. The subtle but reproducible increased virulence of the rim101Δ mutant cells in the inhalation model of C. neoformans infection may be due to enhanced survival in the acidic environment of the alveolar macrophage. We specifically tested intracellular survival of the wild-type, rim101Δ mutant, and rim101Δ+RIM101 complemented strains. As described previously, we co-cultured C. neoformans strains with IFNγ - and LPS-activated J774.1 macrophage-like cells [70]. There was no significant difference in the phagocytosis index of these strains by the macrophages, signifying that the altered capsule in the rim101Δ mutant did not affect fungal cell uptake into macrophages [71]. In contrast, the rim101Δ mutant cells demonstrated increased intracellular survival (p<0.004) within macrophages when normalized against the wild-type (Figure 6B).
Discussion
Microbial pathogens use varied adaptive mechanisms to survive the harsh conditions of the infected host. Cryptococcus neoformans creates a polysaccharide capsule in response to host conditions such as low iron and high CO2 concentrations [1],[24]. The C. neoformans genome contains a number of genes involved in the biosynthesis of this capsule, and many of these genes are highly transcriptionally regulated, at least partially in response to the PKA pathway [38]. This led us to screen through the genome for transcription factors that are potentially regulated by PKA, and we previously found that the Nrg1 protein regulates capsule. Deletion of the NRG1 gene resulted in a partial capsule reduction, and mutation of the putative PKA phosphorylation consensus sequence prevented full capsule induction. However, not all of the transcriptionally regulated capsule genes appeared to be targets for Nrg1, and many of the nrg1Δ mutant phenotypes were not as severe as mutations in the more upstream components of the cAMP pathway [37]. Therefore, we hypothesized that several transcriptional regulators would control capsule gene induction in response to the PKA pathway. Using a combination of bioinformatic and phenotypic screening, we identified the C. neoformans Rim101 protein as another potential novel PKA-dependent transcriptional regulator of capsule genes.
We hypothesized that the C. neoformans Rim101 protein may be a target of direct PKA phosphorylation due to the presence of a consensus sequence for PKA phosphorylation at amino acid positions 730–736. In contrast, the previously described C. albicans and S. cerevisiae Rim101 proteins do not contain potential PKA phosphorylation consensus sequences. However, there are multiple ways in which PKA can regulate downstream targets, including indirect activation of upstream regulatory proteins as well as by occupying the chromatin of the target genes [72]. Our bioinformatic approach, therefore, does not identify all of the targets of PKA, but does allow us to potentially identify direct targets of PKA phosphorylation.
To determine the relationship between Rim101 and PKA, we used complementary genetic, biochemical, and protein localization experiments. Our results suggest that PKA and Rim20 are necessary for maintenance of Rim101 nuclear localization by altering the cleavage of this transcription factor. Rim20 has been previously implicated in the first cleavage of Rim101, by binding to PEST domains, which are also present in C. neoformans Rim101 [43],[45]. In contrast to the predominantly nuclear localization of Rim101 in wild-type cells, we observed both nuclear and cytoplasmic localization of this protein in the pka1 and rim20 mutant strain backgrounds. We also observed both nuclear and cytoplasmic localization of the Rim101-S773A mutant protein with a putative PKA phosphorylation consensus sequence mutation. In addition, the GFP-tagged Rim101 protein in all of these strains had decreased electrophoretic mobility when compared to the rim101Δ+Gfp-RIM101 strain. The larger band is not due to hyperphosphorylation as this mobility shift was not reversed by treatment with phosphatase. Together, these data indicate that both the cAMP/PKA pathway and the Rim pathway are involved in C. neoformans Rim101 processing and cellular localization.
In Aspergillus and Candida, PacC/Rim101 is activated by two cleavage events, first mediated Rim20 and Rim13 and second by the proteosome [43],[45],[53]. We demonstrate that C. neoformans Rim101 activation may also occur in response to two protein cleavage events, as the Rim101 protein is further cleaved from the 120kD form to a 70kD form in capsule inducing conditions. This further cleavage was not observed when PKA1 or RIM20 were disrupted, suggesting that the initial cleavage to 120 kD is necessary for further processing and activation of Rim101. The multiple smaller bands/laddering observed when PKA1 or RIM20 are disrupted may indicate altered proteosome-mediated processing events, suggesting that both Pka1 and Rim20 are necessary to cause appropriate proteosomal involvement and maintain the balance between processing and degradation. This is consistent with data from A. nidulans, where PacC is first converted by PalB and PalA under alkaline conditions to a 53kD intermediate which exposes the second processing site to the proteosome [53]. Hervas-Aguilar et al. also demonstrated that phosphorylation can accumulate on the 72 and 53kD PacC intermediates during alkaline conditions and affect processing. This is consistent with our results that PKA is involved in regulating processing of Rim101 in C. neoformans, although there is large divergence in the C-terminal and in the potential signaling motifs between these orthologous proteins in these distantly related species. Interestingly, capsule-inducing conditions are not alkaline and thus are not a traditional activating condition for Rim101 proteins. Therefore, CnRim101 may have acquired novel activating conditions in order to respond to the specific host conditions experienced by cryptococcal cells in vivo.
When we examined the targets of Rim101 transcriptional activation, we found that many Rim101 downstream targets and responses from other pathogenic fungi, such as C. albicans, are conserved in C. neoformans. We demonstrated that CnRim101 is important for growth under alkaline conditions in vitro. Using comparative transcriptional profiling, we determined that ENA1, a known downstream target of Rim101 in other fungal species, showed decreased expression in the rim101Δ mutant strain (Table 2). The promoter of the ENA1 gene also had a conserved predicted Rim101 binding sequence, suggesting that it might be a direct target of Rim101 in C. neoformans, unlike in S. cerevisiae, where Rim101 regulates ENA1 through Nrg1 [37]. Idnurm et al. showed that Ena1 is required for C. neoformans survival under alkaline conditions, and that appropriate response to alkaline conditions is necessary for virulence of C. neoformans [73]. Therefore, decreased expression of ENA1 in the rim101Δ mutant strain may explain the defect in alkaline growth of the rim101Δ mutant.
Extracellular pH is involved in regulating iron uptake genes through the Rim101 pathway in C. albicans and S. cerevisiae [3], [5]–[7],[15],[23],[25]. The relationship between iron homeostasis and Rim101 is also conserved in C. neoformans. In order to determine the mechanism for the rim101Δ mutant strain sensitivity to low iron, we compared the transcriptional profile between wild-type and the rim101Δ mutant strain after incubation in capsule-inducing conditions. Our microarray analysis concluded that a number of iron homeostasis genes are differentially regulated between the rim101Δ mutant and the wild-type and we confirmed these alterations in gene expression using quantitative real-time PCR. When we examined the putative promoter regions of the candidate genes, we discovered potential Rim101 consensus binding sequences in CFT1, FET3, and SIT1 among others, suggesting these genes are direct targets of Rim101. Similarly, in C. albicans, Rim101 binds directly to the promoter region of the ferric reductase genes FRE1 and FRP1 to cause increased transcription under iron-limited environments [23].
In C. neoformans, iron uptake is regulated by two pathways: PKA and Cir1. Transcriptional profiling showed that many iron genes, such as the iron permease Cft1 and reductase Cfo1 are differentially regulated by PKA [24],[26],[60],[61]. We have demonstrated that Rim101 is regulated by PKA, thus providing a mechanism for PKA regulation of these iron genes. However, in our transcriptional profiling, we did not demonstrate any difference in expression of Cir1 in the rim101Δ mutant strain, further suggesting that there are two pathways that regulate iron homeostasis. In C. albicans, two signaling pathways regulate iron homeostasis in response to different forms of iron limitation. In C. albicans, the ferric reductase gene FRP1 is differentially regulated by Rim101 and by CBF transcription factors in response to different forms of iron limitation [23]. It is possible that C. neoformans has a similar set of transcription factors to regulate the expression of these iron homeostasis genes under different iron-limiting environments, and that the cell uses both Cir1 and Rim101 to regulate the expression of Cft1 under different environmental stimuli and iron source limitations.
Despite the decreased surface capsule observed in the rim101Δ mutant cells when stained with India ink, this strain was still able to secrete glucuronoxylomannan (GXM) at a similar size and concentration as wild type when the cells were grown in capsule inducing conditions. This data does not preclude other differences in structure and modifications to the GXM in the mutant strain. In accordance with the amount of secreted polysaccharide from the rim101Δ mutant strain, our transcriptional profiling revealed that few capsule biosynthesis genes are transcriptionally regulated by Rim101. In the rim101Δ mutant strain we observed decreased expression of UDP-glucose dehydrogenase Ugd1, mannosyltransferase Cmt1, and phosphomannomutase [55]–[58]. Unlike the iron uptake genes, these capsule biosynthesis genes do not have conserved Rim101 binding sites in the promoter regions, suggesting that these are not direct targets of Rim101. Therefore, our data indicates that CnRim101 is required for the transcriptional activation of some genes involved in capsule biosynthesis; however, the most important effects of Rim101 on capsule are likely due to changes in polysaccharide binding to the cell surface. We hypothesize that Rim101 regulates capsule by altering the expression of genes responsible for anchoring capsule to the cell wall, rather than acting as a direct regulator of these capsule biosynthesis genes.
Unexpectedly for an acapsular strain, the rim101Δ mutant displayed no attenuation in virulence in the mouse inhalation model of cryptococcosis. This confirms prior broad screening experiments of C. neoformans mutants to identify genes required for survival within mice [39]. In these studies, the rim101Δ strain was slightly more virulent than wild-type, as we demonstrated here. Follow-up experiments determining fungal load in the brain, lung, and spleen showed no defects in dissemination. When Rim101 is mutated in Candida, the resulting strains are avirulent as Rim101 regulates processes necessary for fungal virulence [3],[14],[19]. In a fungal pathogen of plants, Fusarium oxysporum, a rim101Δ mutant strain is more virulent than wild-type due to the derepression of acid response genes conferring a survival advantage in the acidic host environment of the tomato [2]. Similarly, our data indicates that the C. neoformans rim101Δ mutant grows better than wild-type within the acidic phagolysosome of the activated macrophage [29],[74]. Perhaps the derepression of acid responsive genes in the rim101Δ mutant could explain the increased growth within the acidic phagolysosome and thus within the lungs of the infected host. Another explanation for the retained virulence of the rim101Δ mutant strain is that the capsular polysaccharide may be shed into the surrounding tissues. This capsular material has well defined immunosuppressant effects. Capsular polysaccharide has even recently been used as an experimental therapy for autoimmune diseases such as rheumatoid arthritis [75]. Therefore, the retained virulence may be attributed to the profound immunomodulatory effects of strains that produce and secrete large amounts of capsule. Also, not all capsule-defective C. neoformans strains are hypovirulent in model systems. The acapsular ags1Δ mutant is fully virulent in the nematode model of cryptococcosis, although sensitive to temperature and thus avirulent in the mouse [49],[50]. The virulence of these strains suggests that capsule may be playing an important role in suppressing the immune system, even when not bound to the cell as an anti-phagocytic mechanism.
It is also possible that the hypocapsular rim101Δ mutant may present an altered cell surface for immune recognition, exposing different antigens resulting in a substantively different immune response than for an encapsulated WT strain. In this model, the increased virulence might result from alterations of the exposed C. neoformans surface antigens leading to over-stimulation of the immune system, such as seen in the response to β-glucan in the C. albicans cell wall [76],[77]. In our microarray data we observed increased expression of MP88 and MP98, two immuno-dominant mannoproteins, in the rim101Δ mutant strain, further supporting a model of an altered antigen surface on the fungus as a result of absent Rim101 activity [78],[79]. MP88 has also been documented as having increased expression in a pka1Δ mutant strain, which may be due to decreased Rim101 activity [60]. A more detailed evaluation of the nature of this cellular infiltration into the infected lungs will help define the varied immune response to different C. neoformans strains.
In summary, we have demonstrated that the C. neoformans Rim101 transcription factor retains conserved functions with orthologous proteins from other fungal species, such as regulation of pH response, cell wall formation, and iron homeostasis. However, the phenotypic output resulting from a C. neoformans Rim101 mutation supports the hypothesis that this conserved protein has been co-opted for unique, species-specific function. In contrast to other fungal species such as Candida or Aspergillus that have adapted to the neutral/alkaline pH of the host lungs and use Rim101 as an inducing signal for virulence, C. neoformans may be better adapted for acidic microenvironments in the host, such as the macrophage phagolysosome. Moreover, our experiments demonstrating PKA regulation of CnRim101 further suggests that conserved signaling elements can be regulated in novel ways to allow adaptation of microorganisms to specific niches in the environment of the infected host.
Materials and Methods
Strains and media
Cryptococcus neoformans strains used in this study are listed in Table 1. All C. neoformans strains were created in the H99 strain background unless otherwise stated. Strains were maintained on YPD (1% yeast extract, 2% peptone, 2% glucose), a standard yeast medium. Selective media contained nourseothricin (100 mg/L Werner BioAgents, Jena-Cospeda, Germany) or neomycin (G418) (200 mg/L Clontech, Takara-Bio Inc.). Capsule inducing medium (Dulbecco's modified Eagle's media with 25 mM NaHCO3) was prepared as previously described [31]. YNB media (0.67% yeast nitrogen base without amino acids, 2% glucose) was prepared as previously described [33]. Alkaline pH media were created by buffering YNB with 25 mM NaMOPS and adjusting to target pH with NaOH. Resistance to hydrogen peroxide was tested by disc diffusion as described previously [28]. Niger seed agar was prepared from 70 g Niger seed extract (Niger seed pulverized and boiled for 15 min and filtered through cheesecloth) and 4% Bacto agar as previously described [33].
Molecular biology techniques
Standard techniques for Southern hybridization were performed as described [80]. C. neoformans genomic DNA for Southern blot analysis was prepared using CTAB phenol-chloroform extraction as described [81].
Gene disruption
The wild-type RIM101 gene (NCBI GeneID CNH0097) was mutated using biolistic transformation and homologous recombination with a rim101::nat mutant allele in which the entire RIM101 coding region was precisely replaced with the nourseothrycin resistance gene (nat) [82],[83]. The rim101::nat mutant allele was created using PCR overlap extension as described [84]. Several rim101Δ mutants from independent transformation events displayed identical phenotypes in vitro; therefore one strain (TOC2) was chosen as the rim101 strain for the presented experiments. Putative deletion strains were confirmed by PCR and Southern blot analysis. A second rim101Δ strain was made by creating an identical rim101Δ disruption construct first created by Liu et. al [39] and biolistic transforming it into the H99 strain background. This strain has a partial deletion of the RIM101 gene. Putative mutant strains were confirmed by PCR and Southern blot analysis, and phenotypically compared to the full TOC2 deletion strain.
To reconstitute the wild-type allele, the RIM101 locus was amplified from the H99 wild-type strain using primers (5′ CTGTATCCTTCACTTGAGGC 3′) and (5′ AGCTGTGCGTATCCAATAAT 3′). The neomycin resistance allele was also amplified separately using the M13 forward and reverse primers and both alleles were transformed using biolistic transformation into strain TOC2 to make the reconstituted strain TOC4 as described previously [85]. The reconstituted strain was tested by PCR for presence of the wild-type allele, and examined for reversion of the mutant phenotypes.
The wild-type RIM20 allele (NCBI GeneID CNG00250) was similarly mutated with a rim20::nat mutant allele created by PCR overlap extension. Several rim20Δ mutants from independent transformation events displayed identical phenotypes in vitro; therefore one strain (TOC14) was chosen as the rim20 strain for the presented experiments. This strain was confirmed using PCR and Southern analysis.
Protein localization/GFP fusion
We created a green fluorescent protein (Gfp)-Rim101 fusion protein to examine the subcellular localization of Rim101. A histone H3 promoter-GFP fusion [86] was cloned into the neomycin-resistance containing plasmid pJAF, utilizing BamHI and EcoRI to make the resulting plasmid, pCN50. The coding region and the terminator sequence of RIM101 was amplified using primers modified with BamHI sites (5′-AGTTAGGATCCATGGCTTACCCAATTCTCCC-3′ and 5′-ACTGATGGATCCGAGGAAAGCGTCAAGGATATG-3′). The RIM101 gene was then cloned into the pCN50 plasmid at the BamHI site to create the plasmid pTO2 in which the Gfp-Rim101 fusion protein is constitutively expressed under the His3 promoter. pTO2 was then biolistically transformed into C. neoformans strain TOC2, JKH7, CDC7, and TOC17 as previously described to create strains TOC10, TOC12, TOC13 and TOC21 respectively. pTO2 was mutated into pTO3 using PCR-mediated site-directed mutagenesis to change serine 773 to alanine, using primers 5′-GAGAGTGATGCCGCACGTCGATACTGTCCTG-3′ and 5′ - AGTTAAGATCTATGGCTTACCCAATTCTCCC-3′. pTO3 was then biolistically transformed into TOC2, CDC7 and TOC17 to create strains TOC18, TOC20 and TOC22 respectively.
Microscopy
Bright field, differential interference microscopy (DIC) and fluorescent images were captured with a Zeiss Axio Imager.A1 fluorescent microscope equipped with an AxioCam mrM digital camera. Confocal images were captured using a Zeiss LSM inverted confocal microscope with the Argon/2 488 laser at×100 magnification. To visualize capsule, cells were grown in inducing conditions (described above), then stained with India ink on glass slides. Images were collected at ×63 magnification. To visualize GFP, cells were washed three times in PBS, and images were collected at ×63 magnification, using 488 nm wavelength for fluorescence. The strains exhibited significant artifactual fluorescence signal when fixed and DAPI stained, therefore all fluorescence images were taken without fixing, precluding DAPI staining.
Capsule blotting
Estimation of shed capsule polysaccharide size and amount was performed using a technique described by Yoneda and Doering [47]. Conditioned media was made by growing strains in Dulbecco's modified Eagle's medium or low iron medium for 1 week at 30°C with shaking. The cells were incubated at 70°C for 15 minutes to denature enzymes, then pelleted for 3 minutes at 1500 rpm. The resulting supernatant was sterile filtered using a 0.2u filter. 15 uL of conditioned media was mixed with 6x loading dye and run on an agarose gel at 25V for 15 h. The polysaccharides were then transferred to a nylon membrane using Southern blotting techniques. The membrane was air-dried and blocked using Tris-Buffered Saline-Tween-20 (TBS-t) with 5% milk. To detect the polysaccharide, the membrane was incubated with monoclonal antibody 18b7 (1/1000 dilution) [87], washed with TBS-t, then incubated with an anti-mouse peroxidase-conjugated secondary antibody (1/25,000 dilution, Jackson Labs) and detected using SuperSignal West Fempto Maximum Sensitivity Substrate (ThermoScientific).
Protein extraction, immunoprecipitation and western blot analysis
Protein extracts were obtained using a method previously described [88]. Briefly, cells were incubated to an optical density at 600 nm of 1 in YPD and capsule-inducing conditions. Twenty-milliliter samples of growing cells were pelleted and resuspended in 0.5 mL of lysis buffer containing 2x protease inhibitors (Complete, Mini, EDTA-free; Roche) and 2x phosphatase inhibitors (PhosStop; Roche). To lyse the cells, the supernatant was removed and the cells were lysed by bead beating (0.5 mL of 3uM glass beads in a Mini-BeadBeater-16 (BioSpec), 4 cycles for 30 s each). Following lysis, the samples were immunoprecipitated using 1.6µg anti-GFP antibody (Roche) for 1 hour, then rotated with 80µL protein G Sepharose (Thermo Scientific) for 1 hour. After washing, the samples were eluted by the addition of 40µL 5x Laemmli sample buffer and boiling. Western blots were performed as described previously, using NuPAGE Tris-Acetate gels or NuPAGE Bis-Tris gels to separate the samples. To detect the GFP-labeled proteins, the blots were incubated with anti-GFP primary antibody (1/5,000 dilution) and an anti-mouse peroxidase-conjugated secondary antibody (1/25,000 dilution, Jackson Labs). As a control for non-specific immunoprecipitation, samples were tested in a mock IP (in which no antibody was added) and probed with the anti-GFP antibody, and no bands were observed in this control experiment.
RNA and cDNA preparation
Strains were incubated to mid-logarithmic phase in YPD then washed three times with sterile water before incubation in capsule-inducing media for 3 hours. Cells were washed three times before centrifugation and freezing on dry ice and lyophilizing. RNA was prepared from lyophilized samples using the RNeasy kit (Qiagen). cDNA for real time-PCR was generated using RETROscript (Ambion) using oligo-dT primer. Quantitative real-time PCR was performed as previously described, using the constitutive GPD1 gene to normalize the samples [37].
Microarray and data analysis
The microarray used in this study was developed by the Cryptococcus Community Microarray Consortium with financial support from individual researchers and the Burroughs Wellcome Fund (http://genome.wustl.edu/services/microarray/cryptococcus_neoformans). RNA labeling and hybridization were performed by the Duke University Microarray Core Facility according to their established protocols for custom spotted arrays [37],[89]. Data was analyzed using JMP genomics (SAS institute, Cary NC) and initial background subtraction was performed. We used ANOVA normalization and FDR analysis to calculate differences between treatment effects for pairs of inducing conditions. Genes were considered for further evaluation if they showed log2-transformed fold changes with a p-value <0.02.
Virulence and data analysis
The virulence of the C. neoformans strains was assessed using a murine inhalation model of cryptococosis as described previously [70]. 10 female A/Jcr mice were inoculated intranasally with 5×105 C. neoformans cells of wild-type (H99), rim101Δmutant (TOC2), or rim101+RIM101 complemented strain (TOC4). Mice were monitored daily for signs of infection and were sacrificed at predetermined clinical endpoints predicting mortality.
The statistical significance between the survival curves of all animals infected with each strain was evaluated using the log-rank test (JMP software, SAS institute, Cary NC). Cell counts were analyzed using Student's t-test. All studies were performed in compliance with the institutional guidelines for animal experimentation.
Survival within alveolar macrophage-like J744.1 cells was tested by aliquoting 50 ul of 1×105 macrophage cells/mL into wells in a 96-well plate. The cells were activated by adding LPS and INFγ and incubating overnight. 50 ul of 2×106cells/mL of each cryptococcal strain were added to the wells and co-incubated for 1 hour after which the excess cells were removed and fresh media was added. The engulfed cells were incubated for 24 hours then disrupted with 0.5% SDS for 5 minutes to lyse the macrophages. The media was removed, and the well was washed 2× with 100 uL PBS. The washes were combined and diluted at 1∶100 before plating. Plates were incubated for 2 days before colony counts [70].
Supporting Information
Zdroje
1. VartivarianSE
AnaissieEJ
CowartRE
SpriggHA
TinglerMJ
1993 Regulation of cryptococcal capsular polysaccharide by iron. J Infect Dis 167 186 190
2. Arechiga-CarvajalE
Ruiz-HerreraJ
2005 The RIM101/pacC Homologue from the Basidiomycete Ustilago maydis Is Functional in Multiple pH-Sensitive Phenomena. Eukaryotic Cell 4 999 1008
3. BensenE
MartinSJ
LiM
BermanJ
DAD
2004 Transcriptional profiling in Candida albicans reveals new adaptive responses to extracellular pH and functions for Rim101p. Mol Microbiology 54 1335 1351
4. CastrejonF
GomezA
SanzM
DuranA
RonceroC
2006 The RIM101 Pathway Contributes to Yeast Cell Wall Assembly and Its Function Becomes Essential in the Absence of Mitogen-Activated Protein Kinase Slt2p. Eukaryotic Cell 5 507 517
5. DavisDA
2009 How human pathogenic fungi sense and adapt to pH: the link to virulence. Current Opinion in Microbiology 12 365 370
6. DavisD
WilsonRB
MitchellAP
2000 RIM101-Dependent and -Independent Pathways Govern pH Responses in Candida albicans. Mol Cell Biol 20 971 978
7. LambTM
MitchellAP
2003 The Transcription Factor Rim101p Governs Ion Tolerance and Cell Differentiation by Direct Repression of the Regulatory Genes NRG1 and SMP1 in Saccharomyces cerevisiae. Mol Cell Biol 23 677 686
8. LambT
XuW
DiamondA
MitchellAP
2001 Alkaline Response Genes of Saccharomyces cerevisiae and Their Relationship to the RIM101 Pathway. J Biol Chem 276 1850 1856
9. CaddickMX
BrownleeAG
ArstHN
1986 Regulation of gene expression by pH of the growth medium in Aspergillus nidulans. Molecular and General Genetics MGG 203 346 353
10. CornetM
RichardML
GaillardinC
2009 The homologue of the Saccharomyces cerevisiae RIM9 gene is required for ambient pH signalling in Candida albicans. Research in Microbiology 160 219 223
11. CornetM
BidardF
SchwarzP
Da CostaG
Blanchin-RolandS
DromerF
GaillardinC
2005 Deletions of Endocytic Components VPS28 and VPS32 Affect Growth at Alkaline pH and Virulence through both RIM101-Dependent and RIM101-Independent Pathways in Candida albicans. Infect Immun 73 7977 7987
12. SuSSY
MitchellAP
1993 Molecular characterization of the yeast meiotic regulatory gene RIM1. Nucl Acids Res 21 3789 3797
13. SuSSY
MitchellAP
1993 Identification of Functionally Related Genes That Stimulate Early Meiotic Gene Expression in Yeast. Genetics 133 67 77
14. BignellE
Negrete-UrtasunS
CalcagnoAM
HaynesK
ArstHNJr
2005 The Aspergillus pH-responsive transcription factor PacC regulates virulence. Molecular Microbiology 55 1072 1084
15. BaekY-U
MartinSJ
DavisDA
2006 Evidence for Novel pH-Dependent Regulation of Candida albicans Rim101, a Direct Transcriptional Repressor of the Cell Wall β-Glycosidase Phr2. Eukaryotic Cell 5 1550 1559
16. LiuH
2001 Transcriptional control of dimorphism in Candida albicans. Current Opinion in Microbiology 4 728 735
17. KullasAL
SamuelJMartin
DanaDavis
2007 Adaptation to environmental pH: integrating the Rim101 and calcineurin signal transduction pathways. Molecular Microbiology 66 858 871
18. NobileCJ
SolisN
MyersCL
FayAJ
DeneaultJ-S
2008 Candida albicans transcription factor Rim101 mediates pathogenic interactions through cell wall functions. Cellular Microbiology 10 2180 2196
19. VillarCC
KashlevaH
NobileCJ
MitchellAP
Dongari-BagtzoglouA
2007 Mucosal Tissue Invasion by Candida albicans Is Associated with E-Cadherin Degradation, Mediated by Transcription Factor Rim101p and Protease Sap5p. Infect Immun 75 2126 2135
20. EisendleM
ObereggerH
ButtingerR
IllmerP
HaasH
2004 Biosynthesis and Uptake of Siderophores Is Controlled by the PacC-Mediated Ambient-pH Regulatory System in Aspergillus nidulans. Eukaryotic Cell 3 561 563
21. EspesoEA
TilburnJ
ArstHNJr
PenalvaMA
1993 pH regulation is a major determinant in expression of a fungal penicillin biosynthetic gene. EMBO J 12 3947 3956
22. PeñalvaMA
TilburnJ
BignellE
ArstHNJr
2008 Ambient pH gene regulation in fungi: making connections. Trends in Microbiology 16 291 300
23. BaekY
LiM
DavisD
2008 Candida albicans Ferric Reductases Are Differentially Regulated in Response to Distinct Forms of Iron Limitation by the Rim101 and CBF Transcription Factors. Eukaryotic Cell 7 1168 1179
24. JungWH
KronstadJW
2008 Iron and fungal pathogenesis: a case study with Cryptococcus neoformans. Cellular Microbiology 10 277 284
25. LanC-Y
RodarteG
MurilloLA
JonesT
DavisRW
2004 Regulatory networks affected by iron availability in Candida albicans. Molecular Microbiology 53 1451 1469
26. TangenKL
JungWH
ShamAP
LianT
KronstadJW
2007 The iron - and cAMP-regulated gene SIT1 influences ferrioxamine B utilization, melanization and cell wall structure in Cryptococcus neoformans. Microbiology 153 29 41
27. RamonAM
FonziWA
2003 Diverged Binding Specificity of Rim101p, the Candida albicans Ortholog of PacC. Eukaryotic Cell 2 718 728
28. CoxGM
HarrisonTS
McDadeHC
TabordaCP
HeinrichG
2003 Superoxide dismutase influences the virulence of Cryptococcus neoformans by affecting growth within macrophages. Infect Immun 71 173 180
29. NybergK
JohanssonU
JohanssonA
CamnerP
1992 Phagolysosomal pH in alveolar macrophages. Environ Health Perspect 97 149 152
30. MogensenEG
JanbonG
ChaloupkaJ
SteegbornC
FuMS
2006 Cryptococcus neoformans Senses CO2 through the Carbonic Anhydrase Can2 and the Adenylyl Cyclase Cac1. Eukaryotic Cell 5 103 111
31. GrangerDL
PerfectJR
DurackDT
1985 Virulence of Cryptococcus neoformans. Regulation of capsule synthesis by carbon dioxide. J Clin Invest 76 508 516
32. BahnY-S
KojimaK
CoxGM
HeitmanJ
2006 A Unique Fungal Two-Component System Regulates Stress Responses, Drug Sensitivity, Sexual Development, and Virulence of Cryptococcus neoformans. Mol Biol Cell 17 3122 3135
33. AlspaughJ
PerfectJ
HeitmanJ
1997 Cryptococcus neoformans mating and virulence are regulated by the G-protein alpha subunit GPA1 and cAMP. Genes Dev 11 3206 3217
34. AlspaughJ
PerfectJR
HeitmanJ
1998 Signal Transduction Pathways Regulating Differentiation and Pathogenicity of Cryptococcus neoformans. Fungal Genetics and Biology 25 1 14
35. AlspaughJ
Pukkila-WorleyR
HarashimaT
CavalloLM
FunnellD
CoxGM
PerfectJR
KronstadJW
HeitmanJ
2002 Adenylyl Cyclase Functions Downstream of the Gα Protein Gpa1 and Controls Mating and Pathogenicity of Cryptococcus neoformans. Eukaryotic Cell 1 75 84
36. D'SouzaCA
AlspaughJA
YueC
HarashimaT
CoxGM
2001 Cyclic AMP-Dependent Protein Kinase Controls Virulence of the Fungal Pathogen Cryptococcus neoformans. Mol Cell Biol 21 3179 3191
37. CramerKL
GerraldQD
NicholsCB
PriceMS
AlspaughJA
2006 Transcription Factor Nrg1 Mediates Capsule Formation, Stress Response, and Pathogenesis in Cryptococcus neoformans. Eukaryotic Cell 5 1147 1156
38. Pukkila-WorleyR
GerraldQD
KrausPR
BoilyM-J
DavisMJ
2005 Transcriptional Network of Multiple Capsule and Melanin Genes Governed by the Cryptococcus neoformans Cyclic AMP Cascade. Eukaryotic Cell 4 190 201
39. LiuOW
ChunCD
ChowED
ChenC
MadhaniHD
2008 Systematic Genetic Analysis of Virulence in the Human Fungal Pathogen Cryptococcus neoformans. 135 174 188
40. BowersK
LottridgeJ
HelliwellSB
GoldthwaiteLM
LuzioJP
2004 Protein -Protein Interactions of ESCRT Complexes in the Yeast Saccharomyces cerevisiae. Traffic 5 194 210
41. Blanchin-RolandS
Da CostaG
GaillardinC
2008 Ambient pH signalling in the yeast Yarrowia lipolytica involves YlRim23p/PalC, which interacts with Snf7p/Vps32p, but does not require the long C terminus of YlRim9p/PalI. Microbiology 154 1668 1676
42. DiezE
AlvaroJ
EspesoEA
RainbowL
SuarezT
2002 Activation of the Aspergillus PacC zinc finger transcription factor requires two proteolytic steps. EMBO J 21 1350 1359
43. PenasMM
Hervas-AguilarA
Munera-HuertasT
ReoyoE
PenalvaMA
2007 Further Characterization of the Signaling Proteolysis Step in the Aspergillus nidulans pH Signal Transduction Pathway. Eukaryotic Cell 6 960 970
44. VincentO
RainbowL
TilburnJ
ArstHNJr
PenalvaMA
2003 YPXL/I Is a Protein Interaction Motif Recognized by Aspergillus PalA and Its Human Homologue, AIP1/Alix. Mol Cell Biol 23 1647 1655
45. XuW
MitchellAP
2001 Yeast PalA/AIP1/Alix Homolog Rim20p Associates with a PEST-Like Region and Is Required for Its Proteolytic Cleavage. J Bacteriol 183 6917 6923
46. YonedaA
DoeringTL
2006 A Eukaryotic Capsular Polysaccharide Is Synthesized Intracellularly and Secreted via Exocytosis. Mol Biol Cell 17 5131 5140
47. YonedaA
DoeringTL
2008 Regulation of Cryptococcus neoformans Capsule Size Is Mediated at the Polymer Level. Eukaryotic Cell 7 546 549
48. Garcia-RiveraJ
ChangYC
Kwon-ChungKJ
CasadevallA
2004 Cryptococcus neoformans CAP59 (or Cap59p) Is Involved in the Extracellular Trafficking of Capsular Glucuronoxylomannan. Eukaryotic Cell 3 385 392
49. ReeseAJ
DoeringTL
2003 Cell wall α1,3-glucan is required to anchor the Cryptococcus neoformans capsule. Molecular Microbiology 50 1401 1409
50. ReeseAJ
YonedaA
BregerJA
LiuABH
GriffithCL
2007 Loss of cell wall α (1-3) glucan affects Cryptococcus neoformans from ultrastructure to virulence. Molecular Microbiology 63 1385 1398
51. DavisD
2003 Adaptation to environmental pH in Candida albicans and its relation to pathogenesis. Current Genetics 44 1 7
52. MingotJM
EspesoEA
DiezE
PenalvaMA
2001 Ambient pH Signaling Regulates Nuclear Localization of the Aspergillus nidulans PacC Transcription Factor. Mol Cell Biol 21 1688 1699
53. Hervis-AguilarA
RodriguezJM
TilburnJ
ArstHN
PenalvaMA
2007 Evidence for the Direct Involvement of the Proteasome in the Proteolytic Processing of the Aspergillus nidulans Zinc Finger Transcription Factor PacC. Journal of Biological Chemistry 282 34735 34747
54. OrejasM
EspesoEA
TilburnJ
SarkarS
ArstHN
1995 Activation of the Aspergillus PacC transcription factor in response to alkaline ambient pH requires proteolysis of the carboxy-terminal moiety. Genes & Development 9 1622 1632
55. GriffithCL
KluttsJS
ZhangL
LeverySB
DoeringTL
2004 UDP-glucose Dehydrogenase Plays Multiple Roles in the Biology of the Pathogenic Fungus Cryptococcus neoformans. J Biol Chem 279 51669 51676
56. MoyrandF
JanbonG
2004 UGD1, Encoding the Cryptococcus neoformans UDP-Glucose Dehydrogenase, Is Essential for Growth at 37°C and for Capsule Biosynthesis. Eukaryotic Cell 3 1601 1608
57. SommerU
LiuH
DoeringTL
2003 An α-1,3-mannosyltransferase of Cryptococcus neoformans. J Biol Chem M307223200
58. CottrellTR
GriffithCL
LiuH
NenningerAA
DoeringTL
2007 The Pathogenic Fungus Cryptococcus neoformans Expresses Two Functional GDP-Mannose Transporters with Distinct Expression Patterns and Roles in Capsule Synthesis. Eukaryotic Cell 6 776 785
59. WillsEA
RobertsIS
Del PoetaM
JohannaRivera
CasadevallA
2001 Identification and characterization of the Cryptococcus neoformans phosphomannose isomerase-encoding gene, MAN1, and its impact on pathogenicity. Molecular Microbiology 40 610 620
60. HuG
SteenBR
LianT
ShamAP
TamN
2007 Transcriptional Regulation by Protein Kinase A in Cryptococcus neoformans. PLoS Pathog 3 e42 doi:10.1371/journal.ppat.0030042
61. LianT
MeganISimmer
CletusAD'Souza
BarbaraRSteen
ScottDZuyderduyn
StevenJMJones
MarcoAMarra
JamesWKronstad
2005 Iron-regulated transcription and capsule formation in the fungal pathogen Cryptococcus neoformans. Molecular Microbiology 55 1452 1472
62. WatermanSR
HachamM
HuG
ZhuX
ParkYD
2007 Role of a CUF1/CTR4 copper regulatory axis in the virulence of Cryptococcus neoformans. J Clin Invest 117 794 802
63. JungWH
ShamA
WhiteR
KronstadJW
2006 Iron Regulation of the Major Virulence Factors in the AIDS-Associated Pathogen Cryptococcus neoformans. PLoS Biol 4 e410 doi:10.1371/journal.pbio.0040410
64. JungWH
ShamA
LianT
SinghA
KosmanDJ
2008 Iron Source Preference and Regulation of Iron Uptake in Cryptococcus neoformans. PLoS Pathog 4 e45 doi:10.1371/journal.ppat.0040045
65. TilburnJ
SarkarS
WiddickDA
EspesoEA
OrejasM
1995 The Aspergillus PacC zinc finger transcription factor mediates regulation of both acid - and alkaline-expressed genes by ambient pH. EMBO J 14 779 790
66. ChangYC
Kwon-ChungKJ
1998 Isolation of the Third Capsule-Associated Gene, CAP60, Required for Virulence in Cryptococcus neoformans. Infect Immun 66 2230 2236
67. ChangYC
Kwon-ChungKJ
1999 Isolation, characterization, and localization of a capsule-associated gene, CAP10, of Cryptococcus neoformans. J Bacteriol 181 5636 5643
68. ChangYC
PenoyerLA
Kwon-ChungKJ
1996 The second capsule gene of Cryptococcus neoformans, CAP64, is essential for virulence. Infect Immun 64 1977 1983
69. WeinbergED
1999 The Role of Iron In Protozoan and Fungal Infectious Diseases. Journal of Eurkaryotic Microbiology 46 231 238
70. CoxGM
MukherjeeJ
ColeGT
CasadevallA
PerfectJR
2000 Urease as a Virulence Factor in Experimental Cryptococcosis. Infect Immun 68 443 448
71. BulmerG
SansMD
1968 Cryptococcus neoformans III. Inhibition of Phagocytosis. Journal of Bacteriology 95 5 8
72. PokholokDK
ZeitlingerJ
HannettNM
ReynoldsDB
YoungRA
2006 Activated Signal Transduction Kinases Frequently Occupy Target Genes. Science 313 533 536
73. IdnurmA
WaltonFJ
FloydA
ReedyJL
HeitmanJ
2009 Identification of ENA1 as a Virulence Gene of the Human Pathogenic Fungus Cryptococcus neoformans through Signature-Tagged Insertional Mutagenesis. Eukaryotic Cell 8 315 326
74. NybergK
JohanssonU
RundquistI
CamnerP
1989 Estimation of pH in individual alveolar macrophage phagolysosomes. Exp Lung Res 15 499 510
75. MonariC
BevilacquaS
PiccioniM
PericoliniE
PeritoS
2009 A Microbial Polysaccharide Reduces the Severity of Rheumatoid Arthritis by Influencing Th17 Differentiation and Proinflammatory Cytokines Production. J Immunol 183 191 200
76. GowN
NeteaM
MunroC
FerwerdaG
BatesS
2007 Immune Recognition of Candida albicans β-glucan by Dectin 1. The Journal of Infectious Diseases 196 1565 1571
77. NeteaMG
BrownGD
KullbergBJ
GowNAR
2008 An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Micro 6 67 78
78. HuangC
NongS-h
MansourMK
SpechtCA
LevitzSM
2002 Purification and Characterization of a Second Immunoreactive Mannoprotein from Cryptococcus neoformans That Stimulates T-Cell Responses. Infect Immun 70 5485 5493
79. LevitzSM
NongS-h
MansourMK
HuangC
SpechtCA
2001 Molecular characterization of a mannoprotein with homology to chitin deacetylases that stimulates T cell responses to Cryptococcus neoformans. Proceedings of the National Academy of Sciences of the United States of America 98 10422 10427
80. SambrookJ
FritschEF
ManiatisT
1989 Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY Cold Spring Harbor Laboratory Press
81. PitkinJW
PanaccioneDG
WaltonJD
1996 A putative cyclic peptide efflux pump encoded by the TOXA gene of the plant-pathogenic fungus Cochliobolus carbonum. Microbiology 142 1557 1565
82. McDadeHC
CoxGM
2001 A new dominant selectable marker for use in Cryptococcus neoformans. Med Mycol 39 151 154
83. ToffalettiDL
RudeTH
JohnstonSA
DurackDT
PerfectJR
1993 Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J Bacteriol 175 1405 1411
84. FraserJA
SubaranRL
NicholsCB
HeitmanJ
2003 Recapitulation of the Sexual Cycle of the Primary Fungal Pathogen Cryptococcus neoformans var. gattii: Implications for an Outbreak on Vancouver Island, Canada. Eukaryotic Cell 2 1036 1045
85. GoinsCL
GerikKJ
LodgeJK
2006 Improvements to gene deletion in the fungal pathogen Cryptococcus neoformans: Absence of Ku proteins increases homologous recombination, and co-transformation of independent DNA molecules allows rapid complementation of deletion phenotypes. Fungal Genetics and Biology 43 531 544
86. IdnurmA
GilesSS
PerfectJR
HeitmanJ
2007 Peroxisome Function Regulates Growth on Glucose in the Basidiomycete Fungus Cryptococcus neoformans. Eukaryotic Cell 6 60 72
87. CasadevallA
CleareW
FeldmesserM
Glatman-FreedmanA
GoldmanDL
1998 Characterization of a Murine Monoclonal Antibody to Cryptococcus neoformans Polysaccharide That Is a Candidate for Human Therapeutic Studies. Antimicrob Agents Chemother 42 1437 1446
88. NicholsCB
FerreyraJ
BallouER
AlspaughJA
2009 Subcellular Localization Directs Signaling Specificity of the Cryptococcus neoformans Ras1 Protein. Eukaryotic Cell 8 181 189
89. PriceMS
NicholsCB
AlspaughJA
2008 The Cryptococcus neoformans Rho-GDP Dissociation Inhibitor Mediates Intracellular Survival and Virulence. Infect Immun 76 5729 5737
90. PerfectJR
LangS
DurackDT
1980 Chronic cryptococcal meningitis: a new experimental model in rabbits. Am J Pathol 101 177 194
91. HicksJK
BahnY-S
HeitmanJ
2005 Pde1 Phosphodiesterase Modulates Cyclic AMP Levels through a Protein Kinase A-Mediated Negative Feedback Loop in Cryptococcus neoformans. Eukaryotic Cell 4 1971 1981
92. ChangYC
Kwon-ChungKJ
1994 Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol Cell Biol 14 4912 4919
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek HIV Controller CD4+ T Cells Respond to Minimal Amounts of Gag Antigen Due to High TCR AvidityČlánek Transit through the Flea Vector Induces a Pretransmission Innate Immunity Resistance Phenotype in
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 2- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Pathogen Entrapment by Transglutaminase—A Conserved Early Innate Immune Mechanism
- Broadly Protective Monoclonal Antibodies against H3 Influenza Viruses following Sequential Immunization with Different Hemagglutinins
- Neutrophil-Derived CCL3 Is Essential for the Rapid Recruitment of Dendritic Cells to the Site of Inoculation in Resistant Mice
- Differentiation, Distribution and γδ T Cell-Driven Regulation of IL-22-Producing T Cells in Tuberculosis
- IFN-α-Induced Upregulation of CCR5 Leads to Expanded HIV Tropism In Vivo
- An Extensive Circuitry for Cell Wall Regulation in
- TgMORN1 Is a Key Organizer for the Basal Complex of
- Direct Presentation Is Sufficient for an Efficient Anti-Viral CD8 T Cell Response
- Immunoelectron Microscopic Evidence for Tetherin/BST2 as the Physical Bridge between HIV-1 Virions and the Plasma Membrane
- A New Nuclear Function of the Glycolytic Enzyme Enolase: The Metabolic Regulation of Cytosine-5 Methyltransferase 2 (Dnmt2) Activity
- Genome-Wide mRNA Expression Correlates of Viral Control in CD4+ T-Cells from HIV-1-Infected Individuals
- Structural and Biochemical Characterization of SrcA, a Multi-Cargo Type III Secretion Chaperone in Required for Pathogenic Association with a Host
- A Major Role for the ApiAP2 Protein PfSIP2 in Chromosome End Biology
- HIV Controller CD4+ T Cells Respond to Minimal Amounts of Gag Antigen Due to High TCR Avidity
- Fis Is Essential for Capsule Production in and Regulates Expression of Other Important Virulence Factors
- Vaccinia Protein F12 Has Structural Similarity to Kinesin Light Chain and Contains a Motor Binding Motif Required for Virion Export
- A Novel Pseudopodial Component of the Dendritic Cell Anti-Fungal Response: The Fungipod
- Efficacy of the New Neuraminidase Inhibitor CS-8958 against H5N1 Influenza Viruses
- Long-Lived Antibody and B Cell Memory Responses to the Human Malaria Parasites, and
- IPS-1 Is Essential for the Control of West Nile Virus Infection and Immunity
- Transit through the Flea Vector Induces a Pretransmission Innate Immunity Resistance Phenotype in
- Ats-1 Is Imported into Host Cell Mitochondria and Interferes with Apoptosis Induction
- Six RNA Viruses and Forty-One Hosts: Viral Small RNAs and Modulation of Small RNA Repertoires in Vertebrate and Invertebrate Systems
- The Syk Kinase SmTK4 of Is Involved in the Regulation of Spermatogenesis and Oogenesis
- Optineurin Negatively Regulates the Induction of IFNβ in Response to RNA Virus Infection
- On the Diversity of Malaria Parasites in African Apes and the Origin of from Bonobos
- Five Questions about Viruses and MicroRNAs
- A Broad Distribution of the Alternative Oxidase in Microsporidian Parasites
- Caspase-1 Activation via Rho GTPases: A Common Theme in Mucosal Infections?
- Peptides Presented by HLA-E Molecules Are Targets for Human CD8 T-Cells with Cytotoxic as well as Regulatory Activity
- Interaction of Rim101 and Protein Kinase A Regulates Capsule
- Distinct External Signals Trigger Sequential Release of Apical Organelles during Erythrocyte Invasion by Malaria Parasites
- Exacerbated Innate Host Response to SARS-CoV in Aged Non-Human Primates
- Reverse Genetics in Predicts ARF Cycling Is Essential for Drug Resistance and Virulence
- Universal Features of Post-Transcriptional Gene Regulation Are Critical for Zygote Development
- Highly Differentiated, Resting Gn-Specific Memory CD8 T Cells Persist Years after Infection by Andes Hantavirus
- Arterivirus Nsp1 Modulates the Accumulation of Minus-Strand Templates to Control the Relative Abundance of Viral mRNAs
- Lethal Antibody Enhancement of Dengue Disease in Mice Is Prevented by Fc Modification
- Quantitative Comparison of HTLV-1 and HIV-1 Cell-to-Cell Infection with New Replication Dependent Vectors
- The Disulfide Bonds in Glycoprotein E2 of Hepatitis C Virus Reveal the Tertiary Organization of the Molecule
- IL-1β Processing in Host Defense: Beyond the Inflammasomes
- Kaposi's Sarcoma Associated Herpes Virus (KSHV) Induced COX-2: A Key Factor in Latency, Inflammation, Angiogenesis, Cell Survival and Invasion
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Caspase-1 Activation via Rho GTPases: A Common Theme in Mucosal Infections?
- Kaposi's Sarcoma Associated Herpes Virus (KSHV) Induced COX-2: A Key Factor in Latency, Inflammation, Angiogenesis, Cell Survival and Invasion
- IL-1β Processing in Host Defense: Beyond the Inflammasomes
- Reverse Genetics in Predicts ARF Cycling Is Essential for Drug Resistance and Virulence
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



