-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHIV Controller CD4+ T Cells Respond to Minimal Amounts of Gag Antigen Due to High TCR Avidity
HIV controllers are rare individuals who spontaneously control HIV replication in the absence of antiretroviral treatment. Emerging evidence indicates that HIV control is mediated through very active cellular immune responses, though how such responses can persist over time without immune exhaustion is not yet understood. To investigate the nature of memory CD4+ T cells responsible for long-term anti-HIV responses, we characterized the growth kinetics, Vβ repertoire, and avidity for antigen of patient-derived primary CD4+ T cell lines. Specific cell lines were obtained at a high rate for both HIV controllers (16/17) and efficiently treated patients (19/20) in response to the immunodominant Gag293 peptide. However, lines from controllers showed faster growth kinetics than those of treated patients. After normalizing for growth rates, IFN-γ responses directed against the immunodominant Gag293 peptide showed higher functional avidity in HIV controllers, indicating differentiation into highly efficient effector cells. In contrast, responses to Gag161, Gag263, or CMV peptides did not differ between groups. Gag293-specific CD4+ T cells were characterized by a diverse Vβ repertoire, suggesting that multiple clones contributed to the high avidity CD4+ T cell population in controllers. The high functional avidity of the Gag293-specific response could be explained by a high avidity interaction between the TCR and the peptide-MHC complex, as demonstrated by MHC class II tetramer binding. Thus, HIV controllers harbor a pool of memory CD4+ T cells with the intrinsic ability to recognize minimal amounts of Gag antigen, which may explain how they maintain an active antiviral response in the face of very low viremia.
Published in the journal: . PLoS Pathog 6(2): e32767. doi:10.1371/journal.ppat.1000780
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000780Summary
HIV controllers are rare individuals who spontaneously control HIV replication in the absence of antiretroviral treatment. Emerging evidence indicates that HIV control is mediated through very active cellular immune responses, though how such responses can persist over time without immune exhaustion is not yet understood. To investigate the nature of memory CD4+ T cells responsible for long-term anti-HIV responses, we characterized the growth kinetics, Vβ repertoire, and avidity for antigen of patient-derived primary CD4+ T cell lines. Specific cell lines were obtained at a high rate for both HIV controllers (16/17) and efficiently treated patients (19/20) in response to the immunodominant Gag293 peptide. However, lines from controllers showed faster growth kinetics than those of treated patients. After normalizing for growth rates, IFN-γ responses directed against the immunodominant Gag293 peptide showed higher functional avidity in HIV controllers, indicating differentiation into highly efficient effector cells. In contrast, responses to Gag161, Gag263, or CMV peptides did not differ between groups. Gag293-specific CD4+ T cells were characterized by a diverse Vβ repertoire, suggesting that multiple clones contributed to the high avidity CD4+ T cell population in controllers. The high functional avidity of the Gag293-specific response could be explained by a high avidity interaction between the TCR and the peptide-MHC complex, as demonstrated by MHC class II tetramer binding. Thus, HIV controllers harbor a pool of memory CD4+ T cells with the intrinsic ability to recognize minimal amounts of Gag antigen, which may explain how they maintain an active antiviral response in the face of very low viremia.
Introduction
HIV controllers are rare individuals who spontaneously control HIV replication in the absence of antiretroviral treatment [1],[2]. HIV controllers harbor plasma viral loads that remain undetectable by conventional assays and cell-associated HIV DNA loads that are in the very low range, close to one log below those detected in patients receiving efficient antiretroviral therapy [3]–[5]. HIV controllers show a very low risk of progression to AIDS [3], emphasizing the importance of limited viral dissemination in maintaining a healthy status in the long term.
Emerging evidence indicates that controllers suppress HIV replication through a very active immunological process. HIV controllers harbor effector memory CD8+ T cells capable of rapidly killing infected autologous CD4+ T cells through a cytotoxic mechanism involving the upregulation of perforin and Granzyme B [6],[7]. Signs of immune activation are more prominent in HIV controllers than in efficiently treated patients, and include increased plasma LPS [8], increased expression of T cell activation markers [9], and increased propensity to secrete IFN-γ and MIP-1β upon polyclonal stimulation [10]. Longitudinal studies of efficiently treated patients who achieve undetectable viral load have shown a waning of cellular antiviral responses, which paralleled the progressive decrease in viral burden [11]. In contrast, HIV controllers maintain polyfunctional effector memory T cells with the capacity to secrete multiple cytokines [12]–[14]. How controllers maintain an active antiviral response in the long term in spite of a very low viral burden remains poorly understood.
One element contributing to the persistence of an active immune response may be the quality of the HIV-specific central memory (CM) compartment. CM T cells are thought to be responsible for the long-term maintenance of immune memory, due to their long half-life, high proliferative potential, and capacity to replenish the pool of effector and effector memory (EM) T cells that directly control pathogens [15]–[17] The progressive depletion of the CM CD4+ T cell compartment parallels disease progression in a simian model of AIDS [18]. CM CD4+ T cell functions, such as proliferation and IL-2 secretion, are impaired as early as the primary infection stage in progressive HIV infection [19]–[21], and are only partially recovered in efficiently treated patients [22],[23]. Chronic antigenic stimulation is thought to drive an accelerated differentiation of CM into effector CD4+ T cells, and thus contribute to T cell exhaustion. Importantly, CM CD4+ T cell numbers and functions are preserved in HIV controllers, who appear protected from this accelerated differentiation process [24],[25]. A recent study suggests that inactivation of pro-apoptotic molecules may contribute to the remarkable proliferative capacity of CM CD4+ T cells of HIV controllers, which can exceed that seen in healthy controls after non-specific stimulation [26].
We have previously shown that signs of CD4+ T cell immune activation could be detected in HIV controllers who nevertheless had an intact CM CD4+ T cell compartment, with preserved IL-2 secretion capacity and efficient proliferative responses [10]. How chronic immune activation was induced in controllers, and why it did not generally lead to accelerated CD4+ T cell differentiation and exhaustion remained unclear. To explore these issues, we tested the capacity of Gag-specific memory CD4+ T cell to differentiate in vitro, comparing primary CD4+ T cell lines derived from HIV controllers and efficiently treated patients with equivalent duration of infection. We found that HIV controller harbored a pool of memory CD4+ T cells able to differentiate into effector cells with high functional avidity for an immunodominant Gag epitope. This heightened sensitivity to Gag antigen could be explained by a high avidity interaction between the TCR and the peptide/MHC complex, as measured by class II tetramer binding. The capacity to mount a CD4 recall response in the presence of minimal amounts of Gag antigen may help explain how HIV controllers maintain a continuously activated antiviral response in spite of very low viremia.
Results
Rapid growth of CD4+ T cell lines from HIV controllers
Memory CD4+ T cell responses were compared in patients who spontaneously controlled HIV replication (HIC group, n = 17) and in patients who achieved viral control following successful antiretroviral therapy (HAART group, n = 20). Patients in both groups had viral loads <40 HIV RNA copies/ml plasma. The duration of infection and the CD4+ T cell count did not differ significantly between the two groups (Table 1).
Tab. 1. Virological and immunological characteristics of study subjects. 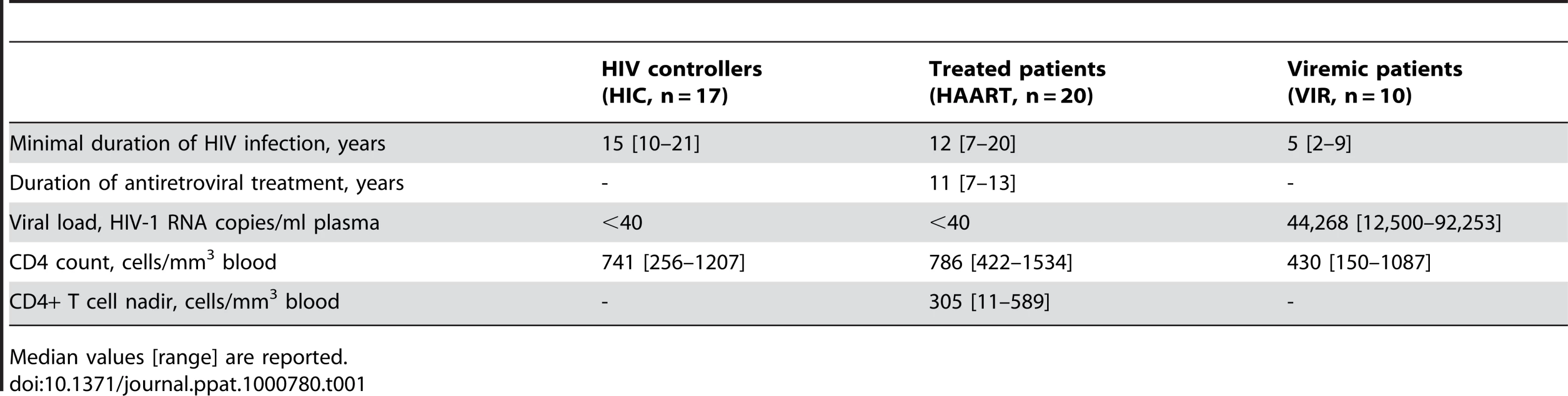
Median values [range] are reported. We analyzed the properties of memory CD4+ T cell precursors by determining their capacity to generate CD4+ T cell lines specific for three immunodominant HIV-1 Gag peptides (Table 2). The peptides were chosen because of their broad immunodominance and their capacity to bind multiple HLA-DRB1 alleles [20], [27]–[30]. The frequency of response was determined by measuring the percentage of patients for whom viable CD4+ T cell lines (defined by a growth ratio >0.7 at day 14) could be obtained after stimulation with a Gag 20-mer peptide. PBMC from HIV-seronegative donors did not yield viable cell lines (not shown). 89 out of 90 cell lines obtained from HIV-seropositive donors proved peptide-specific, as indicated by a positive IFN-γ response measured in ELISPOT assay. The frequency of response to the most immunodominant peptide, Gag293, was remarkably high in both the HIC and HAART groups, with 94% and 95% of responders, respectively (Table 3). Responses to the second peptide, Gag263, were also frequent, with 82% responders in the HIC group and 77% responders in the HAART group. These findings confirmed that several CD4 epitopes in Gag could achieve strong immunodominance in patients with controlled HIV-1 infection. Interestingly, responses to the third peptide, Gag161, were more frequent in the HAART group than in the HIC group, with 91% versus 53% responders, respectively (P<0.05). We did not detect an association between the lack of response to Gag161 and particular HLA-DR genotypes. Ex vivo IFN-γ ELISPOT responses to the 3 Gag peptides were low, as expected for CD4 responses directed to single HIV peptides (Fig. S1). However, it was interesting to note that 7/13 Controllers had a detectable ex vivo response to Gag293 while only 1/13 Controller responded to Gag161 (P<0.05). This finding supported the notion of a higher frequency of Gag293-specific than Gag161-specific CD4+ T cells in Controller patients. Taken together, these observations suggested that the HIV controller status may be associated with a change in the immunodominance pattern of Gag CD4 epitopes.
Tab. 2. Peptides used to generate CD4+ T cell lines. 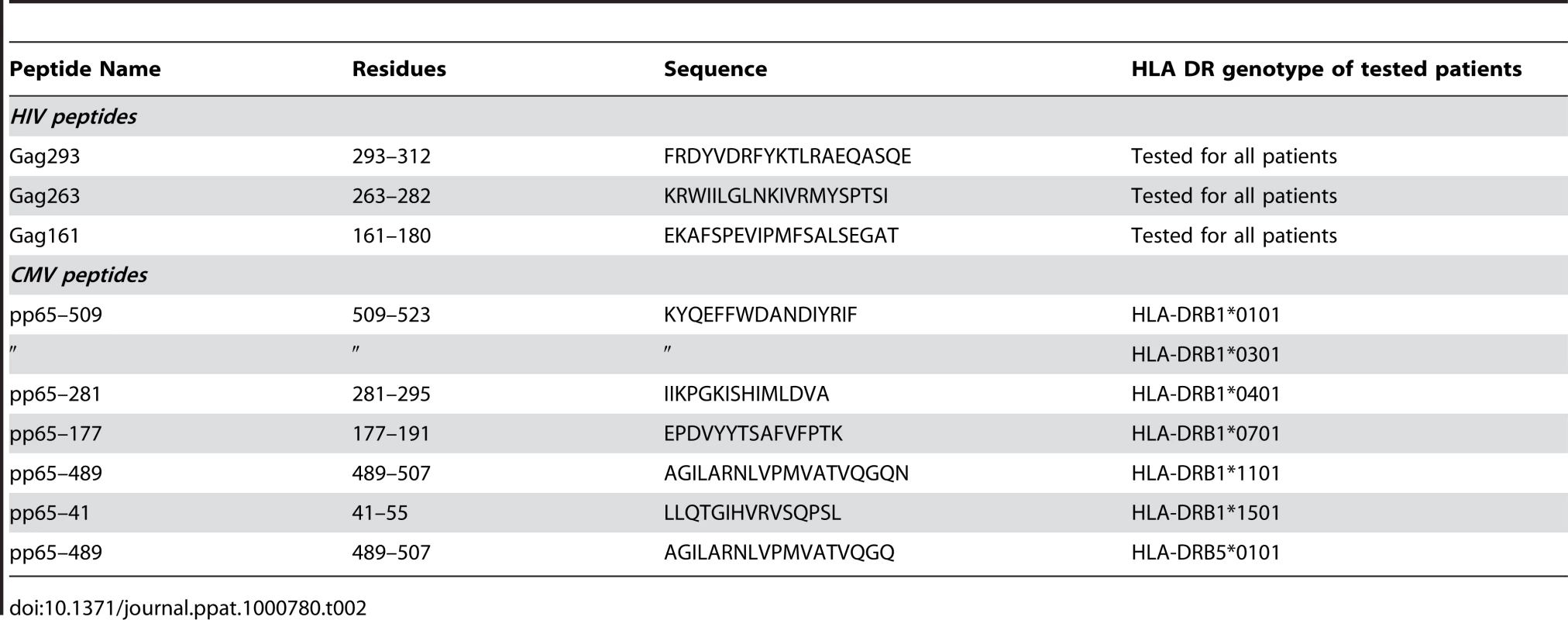
Tab. 3. Frequency of memory CD4+ T cell responses. 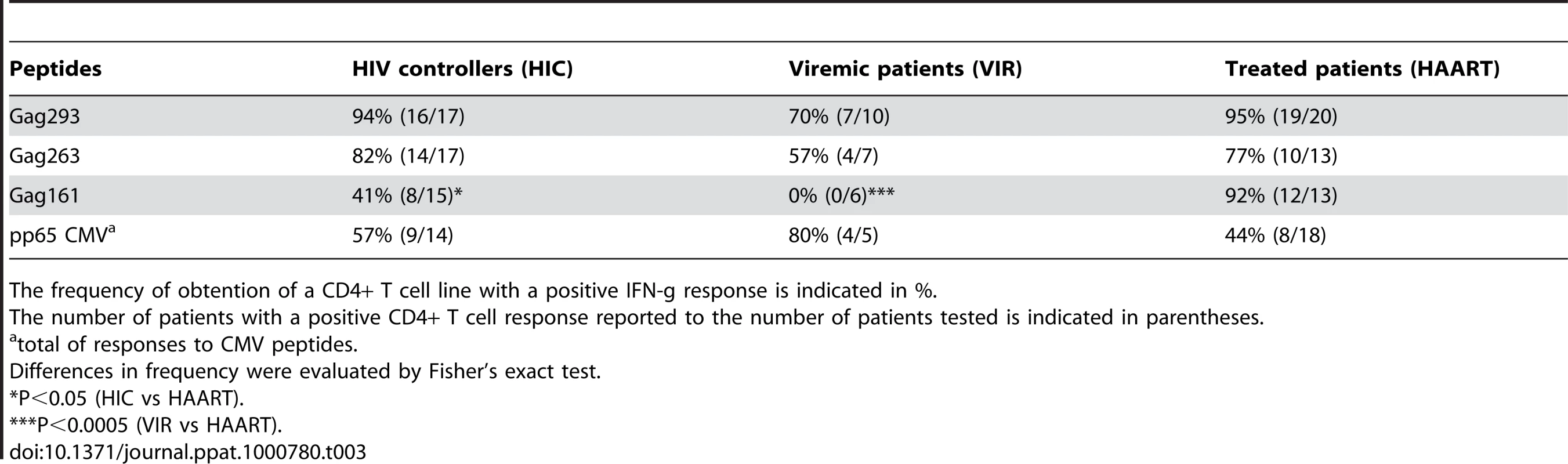
The frequency of obtention of a CD4+ T cell line with a positive IFN-g response is indicated in %. As controls, we generated CD4+ T cell lines specific for the CMV pp65 protein. Since the immunodominance pattern of CMV responses proved more variable than that observed for HIV-1, we optimized the generation of CMV-specific CD4+ T cell lines by choosing the pp65 peptide in function of the HLA DR genotype of the patients (Table 2) [31]–[33]. Using this strategy, close to half of the patients responded to pp65 peptides in both groups, which was consistent with CMV seroprevalence in the studied populations.
CD4+ T cell lines typically showed an initial loss of cells due to apoptosis, followed by growth due to multiplication of HIV - or CMV-specific cells. Measurement of the growth ratio at day 7 showed that viable CD4+ T cells lines from HIV controllers had a faster growth kinetics than those from treated patients, with a significant difference in response to the 3 Gag peptides but not in response to CMV peptides (Fig. 1A). Analysis of CD4+ T cell lines derived from a control group of untreated patients with HIV-1 viremia (VIR) showed limited growth capacity in all cases, consistent with the notion that active HIV-1 replication impaired the proliferative capacity of memory CD4+ T cells [19]. Measurement of growth ratios at day 14 (Fig. 1B) confirmed the rapid amplification of controller CD4+ T cell lines in response to Gag but not to CMV peptides. The clearest differences between the HIC and HAART groups were seen in response to Gag293 (P = 0.003), suggesting that HIV controllers harbored CD4+ T cell precursors with particularly good proliferative capacity in response to this immunodominant Gag peptide.
Fig. 1. Rapid Growth of CD4+ T cell lines from HIV controllers. 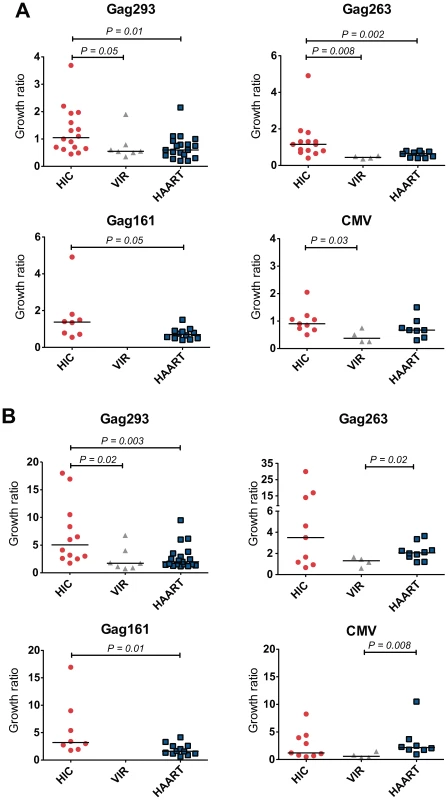
Growth of viable CD4+ T cell lines specific for Gag293, Gag263, Gag161 and CMV peptides was measured in groups of HIV controllers (HIC), viremic patients (VIR), and treated patients (HAART). The growth ratio, corresponding to the number of observed cells divided by the number of input cells, was evaluated at day 7 (A) and day 14 (B) of culture. Each symbol corresponds to one subject. Horizontal bars indicate medians. All statistically significant differences (P<0.05) evaluated by the Mann-Whitney U test are reported on graphs. Efficient differentiation of HIV controller CD4+ T cell precursors
Analysis of IFN-γ production by ELISPOT at day 8 showed a prominent response in HIV controller CD4+ T cell lines (median SFC/106 cells = 7,716), while most cell lines from treated patients remained negative. However, the fact that cell lines from treated patients had not yet entered the exponential growth phase could account for these differences. To compare CD4+ T cell lines at equivalent growth stages, all following measurements were made at doubling time (mean doubling time = 8 days in the HIC group, 13 days in the HAART group, and 14 days in the VIR group). In these conditions, IFN-γ production remained higher in the HIC group compared to the HAART group in response to the immunodominant Gag293 peptide (Fig. 2, P = 0.006). In control experiments, CD4+ T cell depletion abrogated the ELISPOT signal, confirming that IFN-γ was produced by CD4+ T cells, and not by the CD8+ T cells that may have escaped CD8 depletion (Fig. S2).
Fig. 2. Increased IFN-γ production by CD4+ T cell lines from HIV controllers. 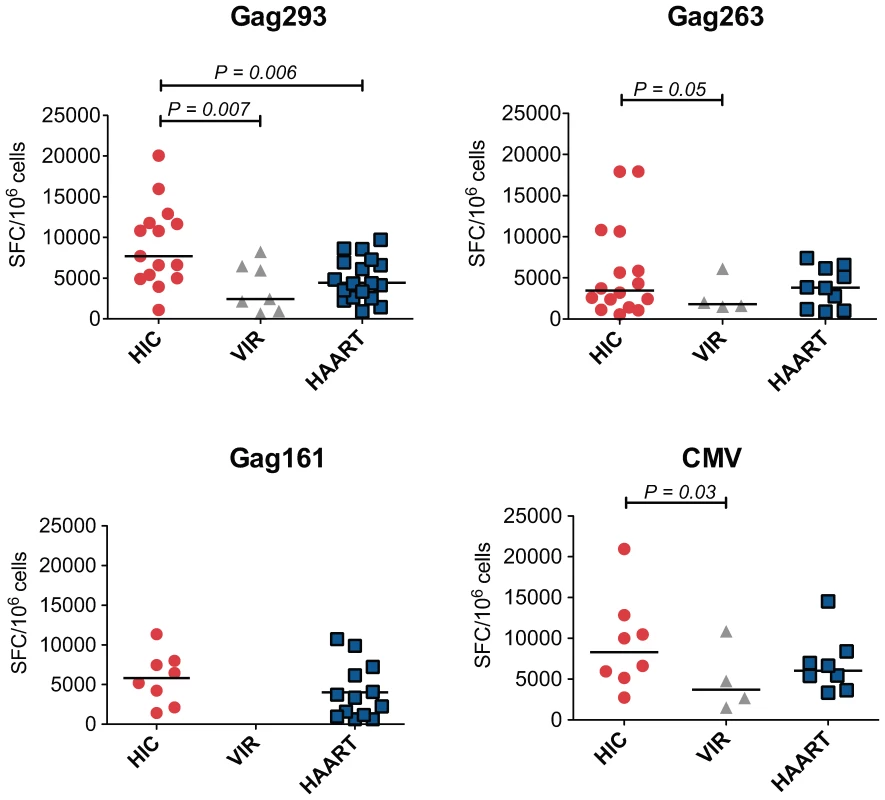
Production of IFN-γ by CD4+ T cell lines specific for Gag293, Gag263, Gag161 and CMV peptides were compared between groups of HIV controllers (HIC), viremic patients (VIR), and treated patients (HAART). IFN-γ production was measured by ELISPOT assay on CD4+ T cell lines restimulated with peptide, and was expressed by the number of spot forming cells (SFC) per million cells. All measurements were made at CD4+ T cell line doubling time. Horizontal bars indicate medians. All statistically significant differences (P<0.05) evaluated by the Mann-Whitney U test are reported on graphs. IFN-γ ELISPOT responses measured at doubling time were higher in the HIC group than in the VIR group for all peptide tested (Fig. 2). However, we and others have previously shown that when responses are measured ex vivo, which gives an evaluation of ongoing effector responses, CD4+ T cells from viremic patients produce as much IFN-γ as those of HIV controllers, while CD4+ T cells from treated patients have low IFN-γ production [10],[11],[22],[24]. The hierarchy of IFN-γ responses measured after proliferation of CD4+ T cell memory precursors is different, and rather reflects the capacity of these precursors to differentiate into cells with effector functions. We conclude that HIV controllers harbor memory CD4+ T cell precursors that can differentiate into efficient cytokine-secreting cells.
Increased functional avidity of memory CD4+ T cells from HIV controllers
To assess the sensitivity of memory CD4+ T cells to antigenic stimulation, we measured IFN-γ production in response to serial peptide dilutions (Fig. 3). We did not observe a significant difference in the dose of Gag293 peptide that induced a half-maximal ELISPOT response (median EC50 = 1.38 10−6 M in HIC vs. 1.55 10−6 M in HAART, P = 0.09). However, we observed that the shapes of the response curves differed, with a marked trailing end in the HIC group, suggesting the presence of a high avidity component within the responding CD4+ T cell population (see representative examples in Fig. 3A and Fig. S3). To extend this observation, we measured the last peptide concentration that gave a positive ELISPOT reading at least 2 fold above background. We verified that measurement of this concentration was reproducible in duplicate experiments (Table S1). Importantly, all measurements were carried out on CD4+ T cell lines at doubling time, to normalize for growth stage.
Fig. 3. Increased functional avidity of memory CD4+ T cells from HIV controllers. 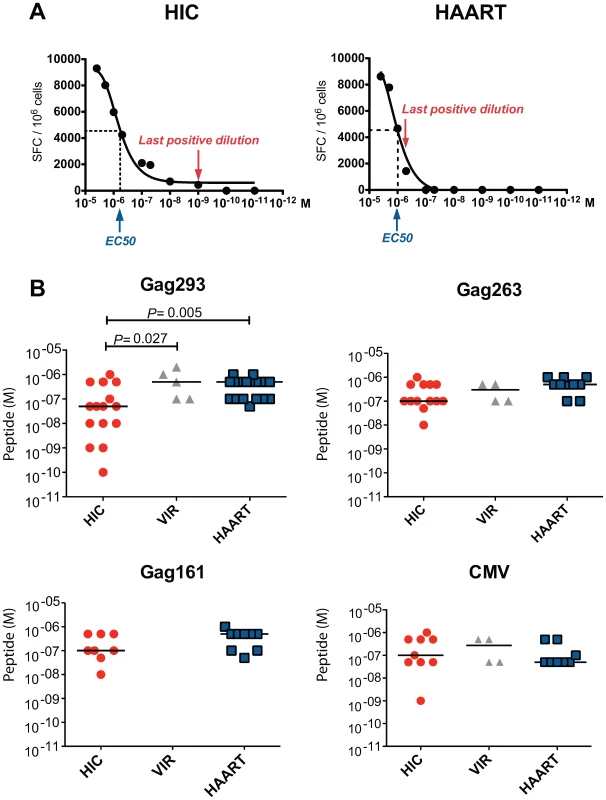
The functional avidity was evaluated by IFN-γ ELISPOT assay in the presence of decreasing peptide concentrations (serial dilutions from 4×10−6 M to 10−11 M). For each peptide dilution, IFN-γ production was expressed as the number of spot forming cells (SFC) per million cells. The functional avidity was defined as the last peptide dilution that gave a positive IFN-γ response at least 2 fold above background level. (A) Representative examples of functional avidity measurement in response to Gag293 for one HIV controller (HIC) and one treated patient (HAART). The peptide dilution at half response (EC50) and the last positive peptide dilution are indicated. (B) Functional avidity of CD4+ T cell lines specific for Gag293, Gag263, Gag161 and CMV peptides were compared between groups of HIV controllers (HIC), viremic patients (VIR), and treated patients (HAART). All measurements were made at CD4+ T cell line doubling time. Horizontal bars indicate medians. All statistically significant differences (P<0.05) evaluated by the Mann-Whitney U test are reported on graphs. This analysis revealed that the functional avidity of CD4+ T cells recognizing the immunodominant Gag293 peptide was higher in HIV controllers (Fig. 3B). In the HIC group, 10 out of 15 patients tested had a positive ELISPOT reading at peptide concentrations ≤5 10−8 M, while in the HAART group only 1 out of 17 patients tested had a positive response at the same concentrations (P = 0.005). In contrast, functional avidities measured for Gag263, Gag161, and CMV peptides did not differ significantly between groups. These results pointed to a particular efficiency of CD4+ T cells specific for the immunodominant Gag293 in HIV controllers. Interestingly, the functional avidity of these cells correlated with the level of the IFN-γ response measured by ELISPOT assay at high peptide dose (R = −0.68, P<0.0001). Thus, CD4+ T cells responses to Gag293 appeared both sensitive and potent in the group of controller patients.
Increased frequency of the HLA-DRB*0701 allele in HIV controllers
Patients were genotyped for the HLA DRB1 gene at 4 digit resolution. Table 4 reports the frequency of the most common HLA DRB1 alleles among the 34 HIV controllers and the 34 efficiently treated patients who were genotyped at initiation of the study. Interestingly, the frequency of the DRB1*0701 allele was 44% in the controller group and 18% in the treated patient group, which yielded a significant difference as measured by Fisher's exact test (P = 0.03). No other DRB1 allele showed significant differences. The frequency of the DRB1*0701 allele in the French population was reported to be 26% [34], close to that seen in the group of treated patients. In contrast, the frequency of DRB1*0701 appeared increased among HIV controllers.
Tab. 4. Phenotypic frequency of HLA DRB1 alleles in study subjects. 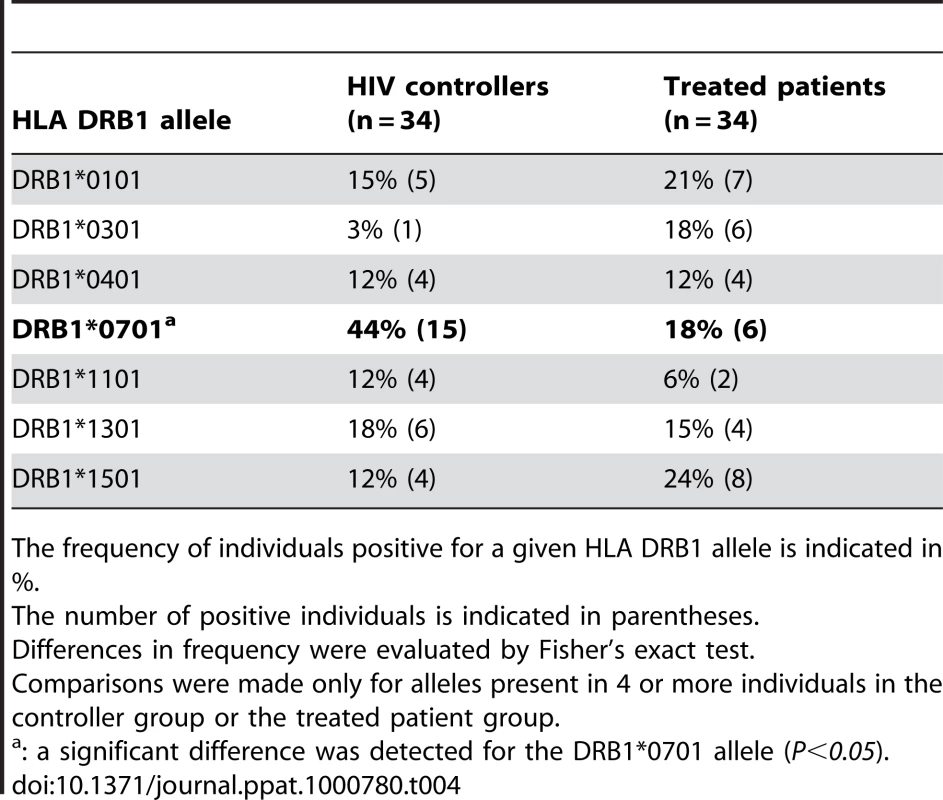
The frequency of individuals positive for a given HLA DRB1 allele is indicated in %. To further explore the possibility that DRB7*0701 conferred an advantage in CD4+ T cell memory function, we compared functional parameters in DRB1*0701 positive versus DRB1*0701-negative individuals included in the study. We did not detect significant differences within the HIC group in terms of growth ratio, IFN-γ response, or functional avidity of CD4+ T cells following Gag293 stimulation. Within the HAART group, DRB1*0701-positive individuals showed lower IFN-γ responses (median SFC/106 cells = 2458, n = 6) than DRB1*0701-negative individuals (median SFC/106 cells = 6116, n = 9, P = 0.02). Taken together, these findings suggest that the DRB1*0701 allele may confer an increased chance to acquire a controller phenotype upon HIV infection, but that once the controller phenotype is established, the presence of the DRB1*0701 allele does not confer further benefit in terms of CD4+ T cell function.
Rapid proliferation of Gag293-specific CD4+ T cells from HIV controllers
To further characterize the nature of Gag293-specific CD4+ T cells, we identified these cells through MHC class II tetramer labeling. Analysis of Gag293-specific CD4+ T cell lines revealed the presence of tetramer-positive (Tet+) cells for all the patients tested, confirming the antigen specificity of the cell lines (representative examples in Fig. 4A and 4B), and the capacity of the Gag293 peptide to bind multiple HLA-DR alleles [27]. At doubling time, the frequency of Tet+ cells was in the order of 1%. (Fig. 4A). The population of tetramer-negative (Tet−) cells may correspond to Gag293-specific cells restricted by an HLA-DR, -DP, or DQ allele distinct from that used in the tetramer, or to cells amplified through bystander effect. At later time points, the population of Tet+ cells could reach up to half of the CD4+ T cells (Fig. 4B), suggesting an efficient amplification of Gag293-specific cells restricted through HLA-DR. Comparison of the percentage of Tet+ cells at doubling time showed no significant difference between the HIC and HAART groups (Fig. 4C), which validated our normalization strategy. Namely, CD4+ T cell lines analyzed at equivalent growth stages contained equivalent numbers of peptide-specific cells, and could thus be usefully compared.
Fig. 4. Characterization of HIV specific CD4+ T cells by MHC class II tetramer staining. 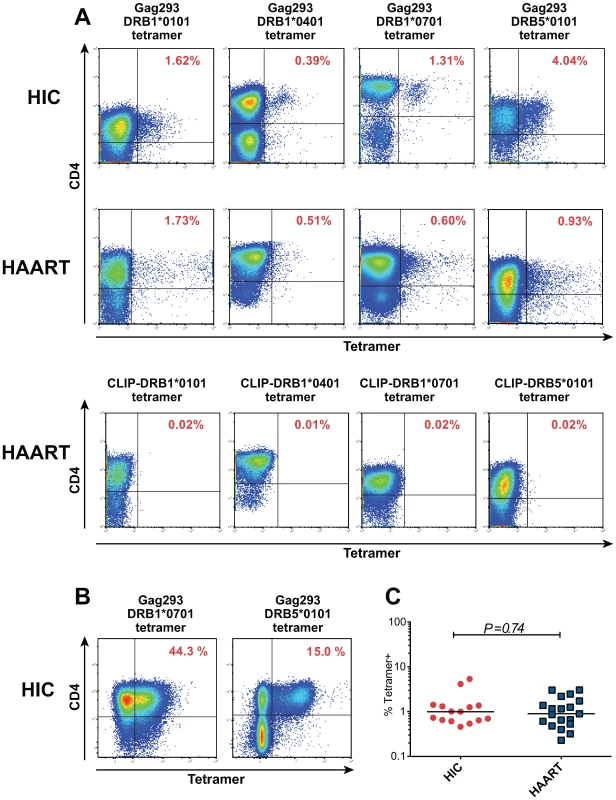
(A) Representative examples of class II tetramer staining for Gag293-specific CD4+ T cells in cell lines at doubling time. Four distinct tetramers corresponding to alleles DRB1*0101, DRB1*0401, DRB1*0701 and DRB5*0101 were loaded with the Gag293 peptide and used to stain CD4+ T cell lines from HIV controllers (HIC) and treated patients (HAART) with matching HLA DR alleles. Negative controls corresponding to cell lines labeled with a DR-matched CLIP-loaded tetramer are shown for the 4 HAART cell lines. (B) Class II tetramer staining for Gag293-specific CD4+ T cells in cell lines from two HIV controllers (HIC) after 18 days of culture, showing the amplification of the tetramer+ population. (C) The percentage of Gag293-specific tetramer+ cells in CD4+ T cell lines at doubling time did not differ significantly between the HIC and HAART groups, as evaluated by the Mann-Whitney U test. To determine the proliferative capacity of Tet+ cells, Gag293-specific CD4+ T cell lines were labeled with CFSE at doubling time and analyzed by flow cytometry 3 days later (Text S1, supplementary methods). The percentage of CD4+ Tet + cells that had divided (CFSElo) was comparably high for the 3 HIC and 3 HAART cell lines analyzed (Fig. S4). The proliferative index, which represents the average number of divisions undergone by the population that divided, showed a trend toward higher values in the HIC cell lines. Interestingly, the difference became more apparent when the number of cells that had undergone 5 divisions or more was computed. In HAART cell lines, 6 to 7% of Tet+ cells had undergone 5 or more divisions, while in HIC cell lines these percentages were of 40%, 35%, and 18%. These data suggested that Tet+ cells from HIV controllers comprised a population endowed with an intrinsically high proliferative capacity and a short generation time.
Diverse repertoire of HIV-specific CD4+ T cells in HIV controllers
The TCR Vβ specificities of Tet+ and Tet − cells within CD4+ T cell lines were determined at doubling time by immunostaining with a panel of anti-Vβ antibodies (Fig. 5A and B). The TCR Vβ repertoire of Gag293-specific CD4+ T cells was diverse in both the HIC and HAART groups and varied depending on the individual. Table 5 lists the Vβ specificities showing an amplification within the Tet+ population, as defined by a ratio Tet+/Tet − ≥4 in a Tet+ population ≥2% of CD4+ T cells. The frequency of amplified Vβ populations is reported in supplementary Table S2. This analysis showed that Vβ1 was amplified in 3 out of 4 cell lines in the HAART group and in 2 out of 4 cell lines in the HIC group. Vβ9 and Vβ13.2 were also amplified in half of cell lines tested. These observations suggest that some Vβ chains may be preferentially selected in response to the Gag293 peptide. However, we did not detect a Vβ signature characteristic of the HIC group. Taken together, these data suggest that multiple clones contribute to the high avidity memory CD4+ T cell population in HIV controllers.
Fig. 5. Gag293-specific CD4+ T cells have a diverse Vβ repertoire. 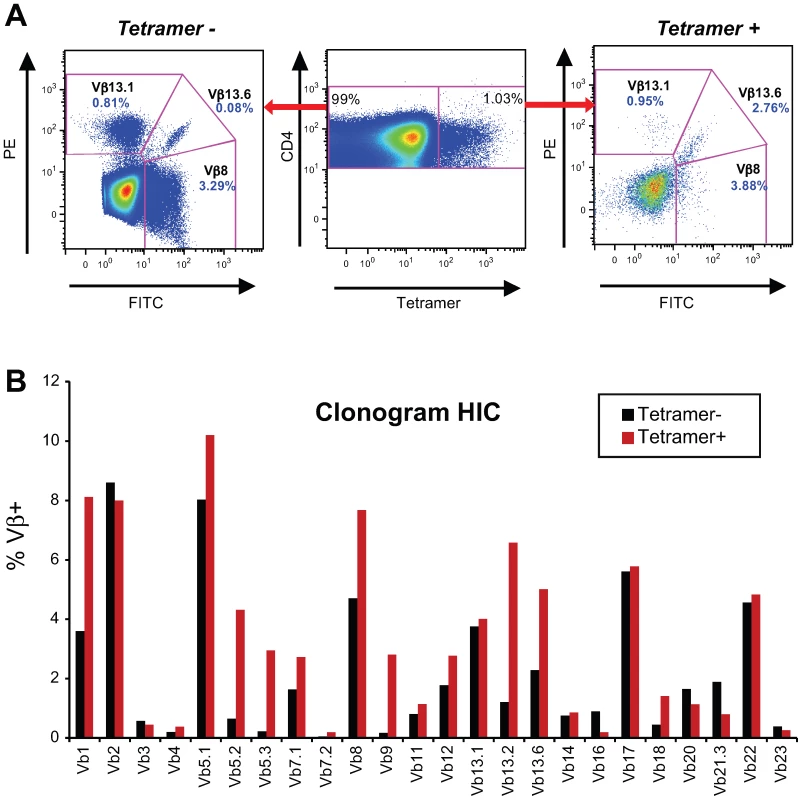
(A) Example of TCR Vβ staining: comparison of three Vβ specificities in the tetramer-negative (left panel) and tetramer-positive (right panel) CD4+ T cell populations obtained for a Gag293-specific cell line from an HIV controller. In this case, Vb13.6 expression is amplified in the tetramer-positive population. (B) Example of Vβ repertoire analysis in an HIV controller CD4+ T cell line at doubling time. The clonogram indicates the percentage of CD4+ T cells labeled with a given Vβ-specific antibody within the tetramer-negative (black bars) and tetramer-positive (red bars) populations. The presence of multiple Vβ specificities within tetramer-positive population indicates that Gag293-specific CD4+ T cells have a diverse Vβ repertoire. Tab. 5. TCR Vβ amplification ratios in Gag293-specific CD4+ T cell lines. 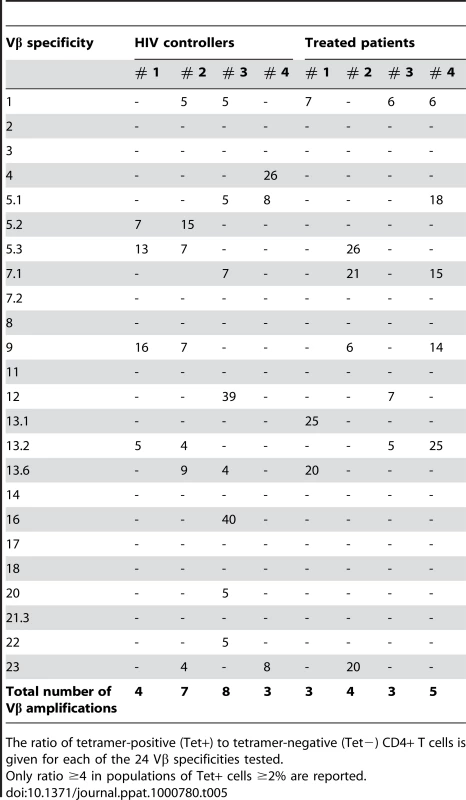
The ratio of tetramer-positive (Tet+) to tetramer-negative (Tet−) CD4+ T cells is given for each of the 24 Vβ specificities tested. Increased TCR avidity of memory CD4+ T cells in HIV controllers
The high functional avidity of controller CD4+ T cells could result from increased avidity of the TCR for the pMHC complex, or from multiple factors that facilitate APC/T cell interactions or effector functions, including expression tuning of costimulatory molecules or efficiency of the IFN-γ secretion system [35]. To explore this issue, we set to directly test the avidity of the TCR/pMHC interaction. The TCR avidity was evaluated by measuring the percentage of Gag293-specific Tet+ CD4+ T cells detected as a function of decreasing class II tetramer concentrations, as described in reference [36] (Fig. 6A). The concentration measured at half-binding (EC50) did not show significant differences between the HIC and HAART groups. However, the shape of the binding curves differed between groups, with a persistence of detectable binding at low tetramer concentrations in the HIC group (Fig. 6B). The TCR avidity was measured by the inverse of the last concentration that gave a Tet+ staining at least 2 fold higher than control CLIP-tetramer staining. Comparison of Gag293-specific CD4+ T cells at doubling time showed that the TCR avidity was significantly higher in the HIC group than in the HAART group (Fig. 6C), indicating a difference in the nature of CD4+ T cell clones responding to the immunodominant Gag293 epitope. Thus, the high functional avidity of HIV controller CD4+ T cells could be explained, at least in part, by an intrinsic property of their TCR.
Fig. 6. Increased TCR avidity of memory CD4+ T cells in HIV controllers. 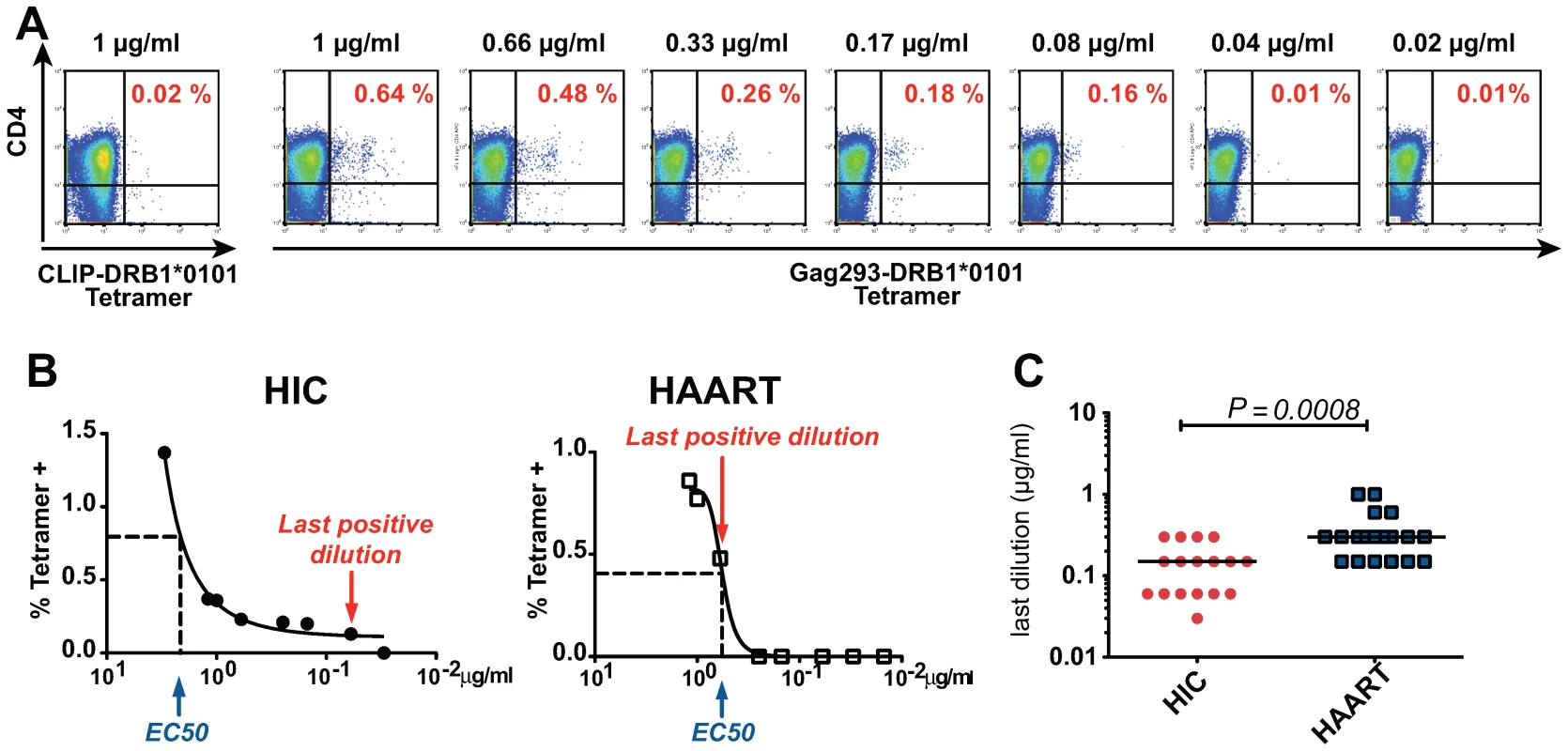
(A) The TCR avidity of Gag293-specific CD4+ T cells was determined by MHC class II tetramer staining in the presence of decreasing tetramer concentrations. An example shows the progressive decrease of Gag293-DRB1*0101 tetramer staining as a function of tetramer concentration in an HIV controller CD4+ T cell line at doubling time. (B) Representative examples of TCR avidity measurement in Gag293-specific CD4+ T cell lines from one HIV controller (HIC) and one treated patient (HAART). The tetramer dilution at half response (EC50) and the last positive tetramer dilution are indicated. (C) The TCR avidity was defined as the inverse of the last tetramer dilution that gave a positive staining at least 2 fold above control CLIP-tetramer staining. The TCR avidity of Gag293-specific CD4+ T cell lines was significantly higher in the controller group (HIC) than in the treated patient group (HAART) as evaluated by the Mann-Whitney U test. Horizontal bars indicate medians. Discussion
This study provides evidence that HIV controllers harbor a pool of high avidity memory CD4+ T cell precursors directed against an immunodominant Gag peptide. Memory CD4+ T cells specific for the Gag293 peptide were endowed with rapid growth potential and, importantly, with IFN-γ secretion capacity, suggesting that they would rapidly generate a pool of CD4+ T cells with effector function upon antigenic stimulation in controller patients. The high functional avidity of Gag293 specific cells points to their capacity of initiating recall responses in the presence of minimal amounts of HIV antigen. The high functional avidity could be explained, at least in part, by a high avidity interaction between the TCR and the cognate Gag293 peptide/MHC complex. Thus, the high sensitivity of controller CD4+ T cells to antigen appeared intrinsic, rather than dependent on the antigen presentation context or the cytokine milieu. The Vβ repertoire of tetramer-positive Gag293-specific cells proved diverse, suggesting that multiple clones contributed to the high avidity CD4 response in HIV controllers. This property may favor the long-term persistence of a high avidity response, since the presence of multiple clones reduces the probability of viral escape or of immune senescence. Taken together, these findings suggest that CD4 recall responses to Gag293 are rapid and efficient in the group of controller patients. We propose that the rapid triggering of recall responses may contribute to viral control. A rapid CD4+ T cell response upon occurrence of “viral blips” will keep the immune system in alert, provide immediate help for CD8+ T cells to exert efficient cytotoxic function, and possibly provide direct antiviral effector function [37]. This rapid recall response may help keep HIV-1 replication under a low threshold, and avoid the progressive undermining of the immune system associated with repeated viral replication episodes [38].
The presence of high avidity CD4+ T cells helps explain how HIV controllers maintain an active T cell response in the face of very low viremia. We and others have previously shown that the level of the specific CD4+ T cell responses in HIV controllers exceeds that seen in efficiently treated patients, even though both groups have very low antigenemia [10],[11],[22],[24]. The triggering of recall responses at very low antigenic load in the controller group may account for this difference. Emerging evidence suggests that the CD8+ T cell response may also be of high avidity in HIV controllers. In particular, individuals harboring the protective HLA-B27 allele frequently develop a high avidity response against the immunodominant KK10 CD8 epitope, the avidity of the response correlating inversely with viral load [39]. The fact that HIV controllers maintain antiviral CD8+ T cells with high cytotoxic potential in spite of their low viral load is also suggestive of a high avidity response [6],[7],[40]. Thus, both the CD4+ and the CD8+ T cell compartments may contribute to the high sensitivity to antigen characterizing antiviral responses in the controller group. Studies in mouse models of chronic viral infections have shown that efficient CD8 responses do not persist in the long term without CD4 help [41]. Therefore, a high avidity CD4 response may be essential in maintaining the quality of the CD8 response in low viremia conditions.
A correlate of a heightened sensitivity to HIV antigens may be a chronic level of immune activation, due to the recall of cellular responses upon each viral replication episode, however limited. Indeed, we have previously reported on signs of ongoing immune activation in the effector memory CD4+ T cell compartment of HIV controllers, as measured by the expression of HLA-DR, the downregulation of the IL-7 receptor, and the secretion of MIP-1β [10]. Other signs of activation include raised levels of LPS in plasma [8] and increased expression of HLA-DR within the HIV-specific CD8+ T cells [6] as compared to efficiently treated patients. These observations confirm the notion that viral control is achieved through an active immunological process. One should note that excessive chronic activation may be deleterious in the long term, as suggested by a trend toward CD4+ T cell decrease in controller patients with the highest degree of immune activation, even in the persistence of undetectable viral load [9]. It will be important in future studies to determine if such individuals show a decrease in T cell functional avidity, which may lead to more prolonged induction of recall responses to achieve viral control, and consequently to prolonged episodes of immune activation. On the other end of the activation spectrum, a few controllers appear to have low HIV-specific CD8 responses and a generally quiescent immune system [42]. This phenotype may result from a particularly successful viral control, with an antigenemia so low that it would not activate the high avidity memory T cell population for long periods of time. It also remains possible that non-T cell based, alternate mechanisms of viral control predominate in these rare individuals.
It was intriguing that HIV controllers responded less frequently to the Gag161 peptide than efficiently treated patients, while the quality of the CD4+ T cell response appeared generally better in the former group. This observation pointed to possible changes in the immunodominance pattern associated with the controller status. Responses to Gag161 may have become subdominant in the controller group due to competition by high avidity CD4+ T cells responding to other epitopes, including that present in the Gag293 peptide. Indeed, high avidity has been shown to sharpen immunodominance in mouse models [43]. The key mechanism appears to be the increased proliferative capacity of high avidity T cells, which progressively fill the memory T cell niche, a phenomenon accounting for the apparent avidity maturation of T cell responses over time [36],[44]. Importantly, in the present study, the duration of HIV-1 infection in the group of efficiently treated patients did not differ significantly from that in the controller group, with median of 12 (7–20) vs. 15 (10–21) years, respectively. Thus, a longer infection time was unlikely to account for the presence of high avidity CD4+ T cells in the controller group.
The CFSE analysis identified a population of Gag293-specific cells with high proliferative capacity in HIV Controller cell lines, which was consistent with the presence of a pool of high avidity CD4+ T cells. The number of divisions undergone by Tet+ cells was heterogeneous, with only a fraction reaching 5 generations and above. This may reflect a range of avidities for the Gag293 antigen, with only a fraction of Tet+ cells being endowed with high avidity and thus high proliferative capacity. This notion is also supported by the shape of the functional avidity curves, which suggests the presence of both high and low avidity populations within the pool of Gag293-specific cells from HIV Controllers. However, the high avidity component was absent in the Gag293-specific CD4+ T cell population from treated patients, independent of the method of analysis (functional avidity, tetramer avidity, or proliferation of Tet+ cells). One should note that the growth ratio of CD4+ T cell lines depended on the intrinsic proliferative capacity of specific cells but also on the frequency of these specific cells at the initiation of culture. We have previously shown that the frequency of p24 Gag-specific cells measured ex vivo by intracellular cytokine staining was approximately 3 fold higher in HIV controllers than in efficiently treated patients [10]. The analysis of ex vivo ELISPOT responses to the Gag293 peptide also showed a trend for higher values in the controller group. Thus, it is likely that both an increased precursor frequency and a higher proliferative capacity contributed to the efficient growth of CD4+ T cell lines from HIV controllers. Both properties may also contribute to the long term persistence of CD4 responses in these patients.
The genetic background may play a role in conferring a better ability to mount high avidity CD4+ T cell responses. The increased frequency of HLA DRB1*0701 in the controller group could suggest a beneficial effect of this allele on the development of anti-HIV CD4 responses. However, since we did not detect an association between the presence of HLA DRB1*0701 and the level or avidity of the CD4 response within the controller group, we speculate that this allele may play a role in initially facilitating viral control, rather than in maintaining high avidity CD4+ T cells. Alternatively, HLA DRB1*0701 may be in linkage disequilibrium with a protective MHC class I allele associated with viral control. A beneficial effect of the HLA DRB1*13 alleles on CD4 responses has also been suggested [13],[20], though we did not detect a significant effect in our study. It will be important to confirm these findings in cohorts of patients powered for large scale genetic studies. An intrinsic advantage in CD4+ T cell growth capacity may also promote efficient CD4 responses in controllers. Van Grevenynghe et al. [26] have reported an increased growth capacity of controller CD4+ T cell lines in response to polyclonal stimulation, as compared to cell lines derived from efficiently treated patients or even from healthy donors. These authors demonstrated a role for the activation of the PI-3 kinase pathway, and the resultant inactivation of the downstream apoptosis inductor FOXO3a, in this particular growth phenotype. We did note a trend for higher growth ratios in controller CD4+ T cell cultures in the presence of CMV peptides, even in the absence of a positive IFN-γ ELISPOT response (not shown). However, an increase in growth propensity only partially accounts for the CD4 response characteristics observed in HIV controllers. High avidity CD4+ T cells were directed against HIV but not CMV, pointing towards a selective advantage in the induction of anti-HIV responses.
The selection of high avidity gag-specific CD4+ T cells may result from a lower exposure to HIV antigens during the acute infection stage, when the repertoire of responding T cells is initially shaped. The few reported cases of acute HIV-1 infection followed by spontaneous viral control support the notion of a lower viral peak in patients who acquire a controller status [45]. Mouse models indicate that low antigen exposure is associated to the development of a high avidity response, since only the high avidity T cells receive sufficient signals through the TCR to proliferate in the long term [44],[46]. Such a scenario may predominate in patients who spontaneously control HIV replication. The presence of high avidity T cells may in turn stabilize the controller status by limiting viral replication episodes.
On the other hand, we cannot rule out that high avidity Gag-specific CD4+ T cells are selected but subsequently lost in progressor patients. Since high avidity CD4+ T cells are the first to respond in the presence of low HIV antigen amounts, they may be the first to get activated in the presence of replicating HIV, and may represent the initial wave of target cells available to the virus. HIV is known to preferentially infect HIV-specific cells [47], and among those it may well preferentially infect the most readily activated population. A recent report suggests that responses to several CD4 epitopes can be detected during the acute infection stage but are subsequently lost in progressor patients, which supports the idea of a rapid culling of the CD4 repertoire [21]. Another reason for the loss of high avidity CD4+ T cells may be senescence due to overstimulation by high antigenic loads in progressor patients. The observation that high avidity CD8 responses can be lost after acute HIV infection supports such a model [48]. An important area of future research will be to elucidate mechanisms that protect high avidity CD4+ T cells from depletion in HIV controllers.
In conclusion, this study provides evidence for the presence of high avidity CD4+ T cells directed against Gag in HIV controllers. It is remarkable that the distinctive properties of HIV-specific T cells in Controllers, including high proliferative potential [14],[24],[49], polyfunctionality [12],[13] and high cytotoxic capacity per cell [6],[7], are all known attributes of high avidity T cells [36],[40],[50],[51]. Thus, high avidity may underlie many of the characteristics of an efficient adaptive immune response against HIV. The presence of high avidity T cells has been associated with control of chronic viral infections in mice [50],[52], monkeys [53], and humans [54]. Since high avidity also confers long-term memory and rapid reactivation in presence of antigen [36],[44],[55], it represents a desirable property to be induced by candidate T cell vaccines against HIV.
Materials and Methods
Patients
HIV controllers (HIC group; n = 17) were recruited through the French “Observatoire National des HIV Controllers” established by ANRS. HIV controllers were defined as HIV-1 infected patients who had been seropositive for >10 years, had received no antiretroviral treatment, and for whom >90% of plasma viral load measurements were <400 copies of HIV RNA/ml. All HIV controllers included in the present study had current viral loads <40 copies/ml. Control groups included: (1) HAART group (n = 20): HIV-1 infected patients successfully treated with antiretroviral therapy for more than 5 years and with a viral load <40 copies of HIV RNA/ml; (2) VIR group (n = 10): viremic patients with viral loads >10,000 copies HIV RNA/ml. Viremic patients had been infected with HIV-1 for more than 1 year and had not received antiretroviral therapy. Patients from the HAART and VIR groups were recruited through the SEROCO-HEMOCO cohort and the Bicêtre hospital.
Ethics statement
The study was promoted by ANRS under number EP36 and approved by the Comité de Protection des Personnes IDF VII under number 05–22. All participants gave written informed consent prior to blood sampling.
Derivation of CD4+ T cell lines
PBMC from HIV infected patients were plated at 2×106 cells per well in 24-well plates in the presence of one HIV-1 Gag or pp65 CMV peptide (10 µM) in RPMI 1640 supplemented with 10% human AB serum, 2 mM L-glutamine, 10 mM HEPES, 100 ug/ml penicillin/streptomycin, 0.5 µM AZT, 5 nM Saquinavir and 5 ng/ml recombinant IL-7 (Cytheris). The peptides used to stimulate the culture were highly purified 20-mers (>99% purity; PolyPeptide Laboratories). Recombinant IL-2 was added after 2 days to a final concentration 100 U/ml. Cell lines were restimulated with IL-2 every 2 days until the end of culture. Starting from day 7, cells were counted every day by trypan blue exclusion to determine the growth ratio (GR: observed number of cells/number of input cells at day 0). The CD8+ T cell population represented a median of 5.9% (range: 0–22.7%) of the CD3+ population. CD8+ T cells were depleted with magnetic beads (IMag particles, BD Biosciences) at doubling time (GR = 2), before performing functional assays. Less than 1% CD8+ T cells remained after CD8 depletion (Fig. S2A).
ELISPOT assay
IFN-γ secretion by CD4+ T cell lines was evaluated by ELISPOT assay as previously described [56]. Briefly, 96-well nitrocellulose plates were coated with 1 µg/ml anti-human IFN-γ capture monoclonal antibody (Mabtech). Cell lines starved off IL-2 for 16 h were plated in duplicate at 30,000 cells/well in coated ELISPOT plates and incubated with 4 µM peptide for 24 h at 37°C. Wells were then washed, incubated with a biotinylated anti-IFN-g detection antibody (Mabtech), followed with alkaline phosphatase-labeled extravidin (Sigma-Aldrich), and with a chromogenic alkaline phosphatase-conjugated substrate. IFN-γ spot-forming cells (SFC) were counted with a Bioreader 4000 system (Bio-Sys). The ELISPOT response was expressed as SFC/106 cells after subtracting background. Wells were counted as positive if the number of SFC was at least two times above background level. Functional avidity assays were carried out on all cell lines with ELISPOT responses >1000 SFC/106 PBMC. ELISPOT responses were measured in response to serial peptide dilutions from 4×10−6 to 10−11 M, and the last dilution that gave a number of SFC at least two times above background was determined.
MHC class II tetramer labeling
At initiation of the study, 34 HIV controllers and 34 treated patients were genotyped for HLA-DRB1. Patients were included in the study if their genotype matched at least one of the 6 HLA-DRB1 alleles available for MHC class II tetramer studies. This panel allowed the analysis of ≥70% of Caucasian patients [34]. PE-labeled tetramers for the DRB1*0101, DRB1*0301, DRB1*1501 and DRB5*0101 alleles were obtained through the NIH Tetramer Facility at Emory University. HLA-DRB1*0401, DRB1*0701, and DRB1*1101 biotinylated monomers were produced in insect cell cutures as previously described [57]–[59]. Monomers were loaded with 0.2 mg/ml peptide by incubation at 37°C for 72 h in the presence of 2.5 mg/ml n-octyl-b-D-glucopyranoside and 1 mM Pefabloc SC (Sigma-Aldrich). Peptide-loaded monomers were tetramerized using APC - or PE-conjugated streptavidin (eBioscience). To each tetramer loaded with the Gag293 peptide corresponded a control tetramer loaded with the CLIP peptide.
The class II tetramer labeling protocol was adapted from [38]. CD4+ T cell lines were incubated with 4 µg/ml class II tetramer for 90 min at 4°C in PBS-1% BSA buffer. Surface marker antibodies CD4-PerCP, CD3-AF750-APC, CD14-FITC (eBioscience), CD8-FITC, CD19-FITC (BD Biosciences), and the Aqua Live/Dead viability dye (Invitrogen) were added for the last 20 min of labeling. The percentage of tetramer-positive (Tet+) cells was measured in the live, CD3+, CD4+, CD8−, CD14−, CD19-gate. Events were acquired on a CyAn flow cytometer (Beckman Coulter, Fullerton, CA) and analyzed using the Flowjo software (Tree Star). Negative controls were obtained by staining with HLA-DR matched tetramers loaded with the CLIP peptide. To determine the avidity of the TCR/pMHC interaction, CD4+ T cell lines were incubated with decreasing concentrations of class II tetramer from 1 to 0.01 µg/ml. The avidity was defined as the inverse of the last concentration that gave a percentage of Tet+ cells at least 2 fold higher than CLIP-tetramer control values.
TCR Vβ repertoire analysis
The TCR Vβ repertoire of HIV-specific CD4+ T cell lines was determined by co-staining cells with an MHC class II tetramer and a panel of Vβ-specific antibodies (IOt-Test Beta Mark TCR Vβ repertoire kit, Beckman Coulter), according to the manufacturer's instructions. The kit covered approximately 70% of human Vβ specificities. The Vβ nomenclature is that of Wei et al. [60]. A Vβ specificity was considered amplified when the Vβ frequency was increased at least 4 fold in the Tet+ compared to the Tet - population. A ratio of 4 was above the range of Vβ variation observed in the ex vivo repertoire of HIV-infected patients [61] and within the range of Vβ expansions induced by superantigens in vitro [62].
Statistical analysis
Data are expressed as medians and range. Analyses were performed with the GraphPad Prism 5.0 software, using nonparametric statistical tests in all cases. Differences in variables between groups were analyzed with the Mann-Whitney U Test. Differences in percentages of response were analyzed with the Fisher's exact test. Correlations were analyzed with Spearman's coefficient R. All significant differences between groups (P<0.05) were reported on data plots.
Supporting Information
Zdroje
1. DeeksSG
WalkerBD
2007 Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity 27 406 416
2. Saez-CirionA
PancinoG
SinetM
VenetA
LambotteO
2007 HIV controllers: how do they tame the virus? Trends Immunol 28 532 540
3. LambotteO
BoufassaF
MadecY
NguyenA
GoujardC
2005 HIV controllers: a homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin Infect Dis 41 1053 1056
4. ViardJP
BurgardM
HubertJB
AaronL
RabianC
2004 Impact of 5 years of maximally successful highly active antiretroviral therapy on CD4 cell count and HIV-1 DNA level. AIDS 18 45 49
5. SajadiMM
HerediaA
LeN
ConstantineNT
RedfieldRR
2007 HIV-1 natural viral suppressors: control of viral replication in the absence of therapy. AIDS 21 517 519
6. Saez-CirionA
LacabaratzC
LambotteO
VermisseP
UrrutiaA
2007 HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar CTL activation phenotype. Proc Natl Acad Sci U S A 104 6776 6781
7. MiguelesSA
OsborneCM
RoyceC
ComptonAA
JoshiRP
2008 Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity 29 1009 1021
8. BrenchleyJM
PriceDA
SchackerTW
AsherTE
SilvestriG
2006 Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 12 1365 1371
9. HuntPW
BrenchleyJ
SinclairE
McCuneJM
RolandM
2008 Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis 197 126 133
10. PotterSJ
LacabaratzC
LambotteO
Perez-PatrigeonS
VingertB
2007 Preserved central memory and activated effector memory CD4+ T-cell subsets in human immunodeficiency virus controllers: an ANRS EP36 study. J Virol 81 13904 13915
11. PitcherCJ
QuittnerC
PetersonDM
ConnorsM
KoupRA
1999 HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med 5 518 525
12. BettsMR
NasonMC
WestSM
De RosaSC
MiguelesSA
2006 HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107 4781 4789
13. FerreAL
HuntPW
CritchfieldJW
YoungDH
MorrisMM
2008 Mucosal immune responses to HIV-1 in elite controllers: A potential correlate of immune control. Blood 113 3978 3989
14. HarariA
DutoitV
CelleraiC
BartPA
Du PasquierRA
2006 Functional signatures of protective antiviral T-cell immunity in human virus infections. Immunol Rev 211 236 254
15. LanzavecchiaA
SallustoF
2005 Understanding the generation and function of memory T cell subsets. Curr Opin Immunol 17 326 332
16. SnyderCM
ChoKS
BonnettEL
van DommelenS
ShellamGR
2008 Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity 29 650 659
17. WoodlandDL
KohlmeierJE
2009 Migration, maintenance and recall of memory T cells in peripheral tissues. Nat Rev Immunol 9 153 161
18. OkoyeA
Meier-SchellersheimM
BrenchleyJM
HagenSI
WalkerJM
2007 Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J Exp Med 204 2171 2185
19. RosenbergES
BillingsleyJM
CaliendoAM
BoswellSL
SaxPE
1997 Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278 1447 1450
20. MalhotraU
HolteS
DuttaS
BerreyMM
DelpitE
2001 Role for HLA class II molecules in HIV-1 suppression and cellular immunity following antiretroviral treatment. J Clin Invest 107 505 517
21. Lubong SabadoR
KavanaghDG
KaufmannDE
FruK
BabcockE
2009 In vitro priming recapitulates in vivo HIV-1 specific T cell responses, revealing rapid loss of virus reactive CD4 T cells in acute HIV-1 infection. PLoS ONE 4 e4256 doi:10.1371/journal.pone.0004256
22. HarariA
PetitpierreS
VallelianF
PantaleoG
2004 Skewed representation of functionally distinct populations of virus-specific CD4 T cells in HIV-1-infected subjects with progressive disease: changes after antiretroviral therapy. Blood 103 966 972
23. BoritzE
PalmerBE
LivingstonB
SetteA
WilsonCC
2003 Diverse repertoire of HIV-1 p24-specific, IFN-gamma-producing CD4+ T cell clones following immune reconstitution on highly active antiretroviral therapy. J Immunol 170 1106 1116
24. YounesSA
Yassine-DiabB
DumontAR
BoulasselMR
GrossmanZ
2003 HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J Exp Med 198 1909 1922
25. EmuB
SinclairE
FavreD
MorettoWJ
HsueP
2005 Phenotypic, functional, and kinetic parameters associated with apparent T-cell control of human immunodeficiency virus replication in individuals with and without antiretroviral treatment. J Virol 79 14169 14178
26. van GrevenyngheJ
ProcopioFA
HeZ
ChomontN
RiouC
2008 Transcription factor FOXO3a controls the persistence of memory CD4(+) T cells during HIV infection. Nat Med 14 266 274
27. KaufmannDE
BaileyPM
SidneyJ
WagnerB
NorrisPJ
2004 Comprehensive analysis of human immunodeficiency virus type 1-specific CD4 responses reveals marked immunodominance of gag and nef and the presence of broadly recognized peptides. J Virol 78 4463 4477
28. YounesSA
TrautmannL
Yassine-DiabB
KalfayanLH
KernaleguenAE
2007 The duration of exposure to HIV modulates the breadth and the magnitude of HIV-specific memory CD4+ T cells. J Immunol 178 788 797
29. FonsecaSG
Coutinho-SilvaA
FonsecaLA
SeguradoAC
MoraesSL
2006 Identification of novel consensus CD4 T-cell epitopes from clade B HIV-1 whole genome that are frequently recognized by HIV-1 infected patients. AIDS 20 2263 2273
30. JonesRB
YueFY
GuXX
HunterDV
MujibS
2009 HIV-1 escapes from IL-2-producing CD4+ T cell responses without high-frequency fixation of mutations. J Virol
31. CasazzaJP
BettsMR
PriceDA
PrecopioML
RuffLE
2006 Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J Exp Med 203 2865 2877
32. Li PiraG
BottoneL
IvaldiF
PelizzoliR
Del GaldoF
2004 Identification of new Th peptides from the cytomegalovirus protein pp65 to design a peptide library for generation of CD4 T cell lines for cellular immunoreconstitution. Int Immunol 16 635 642
33. WiesnerM
ZentzC
HammerMH
CobboldM
KernF
2005 Selection of CMV-specific CD8+ and CD4+ T cells by mini-EBV-transformed B cell lines. Eur J Immunol 35 2110 2121
34. CharronD
1997 HLA: genetic diversity of HLA; functional and medical implications: proceedings of the Twelfth International Histocompatibility Workshop and Conference, held in Saint-Malo and Paris, June 1996: Sèvres; Paris, EDK.
35. KrogerCJ
Alexander-MillerMA
2007 Cutting edge: CD8+ T cell clones possess the potential to differentiate into both high - and low-avidity effector cells. J Immunol 179 748 751
36. WilliamsMA
RavkovEV
BevanMJ
2008 Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity 28 533 545
37. SachaJB
Giraldo-VelaJP
BuechlerMB
MartinsMA
ManessNJ
2009 Gag - and Nef-specific CD4+ T cells recognize and inhibit SIV replication in infected macrophages early after infection. Proc Natl Acad Sci U S A 106 9791 9796
38. SethN
KaufmannD
LaheyT
RosenbergES
WucherpfennigKW
2005 Expansion and contraction of HIV-specific CD4 T cells with short bursts of viremia, but physical loss of the majority of these cells with sustained viral replication. J Immunol 175 6948 6958
39. AlmeidaJR
PriceDA
PapagnoL
ArkoubZA
SauceD
2007 Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J Exp Med 204 2473 2485
40. AlmeidaJR
SauceD
PriceDA
PapagnoL
ShinSY
2009 Antigen sensitivity is a major determinant of CD8+ T-cell polyfunctionality and HIV-suppressive activity. Blood 113 6351 6360
41. CastellinoF
GermainRN
2006 Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu Rev Immunol 24 519 540
42. Saez-CirionA
SinetM
ShinSY
UrrutiaA
VersmisseP
2009 Heterogeneity in HIV suppression by CD8 T cells from HIV controllers: association with Gag-specific CD8 T cell responses. J Immunol 182 7828 7837
43. DzutsevAH
BelyakovIM
IsakovDV
MarguliesDH
BerzofskyJA
2007 Avidity of CD8 T cells sharpens immunodominance. Int Immunol 19 497 507
44. ZehnD
LeeSY
BevanMJ
2009 Complete but curtailed T-cell response to very low-affinity antigen. Nature 458 211 214
45. MadecY
BoufassaF
PorterK
MeyerL
2005 Spontaneous control of viral load and CD4 cell count progression among HIV-1 seroconverters. AIDS 19 2001 2007
46. ReesW
BenderJ
TeagueTK
KedlRM
CrawfordF
1999 An inverse relationship between T cell receptor affinity and antigen dose during CD4(+) T cell responses in vivo and in vitro. Proc Natl Acad of Sci U S A 96 9781 9786
47. DouekDC
BrenchleyJM
BettsMR
AmbrozakDR
HillBJ
2002 HIV preferentially infects HIV-specific CD4+ T cells. Nature 417 95 98
48. LichterfeldM
YuXG
MuiSK
WilliamsKL
TrochaA
2007 Selective depletion of high-avidity human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T cells after early HIV-1 infection. J Virol 81 4199 4214
49. MiguelesSA
LaboricoAC
ShupertWL
SabbaghianMS
RabinR
2002 HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol 3 1061 1068
50. Alexander-MillerMA
2005 High-avidity CD8+ T cells: optimal soldiers in the war against viruses and tumors. Immunol Res 31 13 24
51. ZhaoY
BennettAD
ZhengZ
WangQJ
RobbinsPF
2007 High-affinity TCRs generated by phage display provide CD4+ T cells with the ability to recognize and kill tumor cell lines. J Immunol 179 5845 5854
52. MessaoudiI
Guevara PatinoJA
DyallR
LeMaoultJ
Nikolich-ZugichJ
2002 Direct link between mhc polymorphism, T cell avidity, and diversity in immune defense. Science 298 1797 1800
53. BelyakovIM
KuznetsovVA
KelsallB
KlinmanD
MoniuszkoM
2006 Impact of vaccine-induced mucosal high-avidity CD8+ CTLs in delay of AIDS viral dissemination from mucosa. Blood 107 3258 3264
54. NeveuB
DebeaupuisE
EchasserieauK
le Moullac-VaidyeB
GassinM
2008 Selection of high-avidity CD8 T cells correlates with control of hepatitis C virus infection. Hepatology 48 713 722
55. AhlersJD
BelyakovIM
2009 Memories that last forever: strategies for optimizing vaccine T cell memory. Blood
56. VingertB
AdoteviO
PatinD
JungS
ShrikantP
2006 The Shiga toxin B-subunit targets antigen in vivo to dendritic cells and elicits anti-tumor immunity. Eur J Immunol 36 1124 1135
57. JamesEA
BuiJ
BergerD
HustonL
RotiM
2007 Tetramer-guided epitope mapping reveals broad, individualized repertoires of tetanus toxin-specific CD4+ T cells and suggests HLA-based differences in epitope recognition. Int Immunol 19 1291 1301
58. NovakEJ
LiuAW
NepomGT
KwokWW
1999 MHC class II tetramers identify peptide-specific human CD4(+) T cells proliferating in response to influenza A antigen. J Clin Invest 104 R63 67
59. LemaitreF
ViguierM
ChoMS
FourneauJM
MaillereB
2004 Detection of low-frequency human antigen-specific CD4(+) T cells using MHC class II multimer bead sorting and immunoscope analysis. Eur J Immunol 34 2941 2949
60. WeiS
CharmleyP
RobinsonMA
ConcannonP
1994 The extent of the human germline T-cell receptor V beta gene segment repertoire. Immunogenetics 40 27 36
61. KillianMS
MonteiroJ
MatudJ
HultinLE
HausnerMA
2004 Persistent alterations in the T-cell repertoires of HIV-1-infected and at-risk uninfected men. AIDS 18 161 170
62. ThomasD
DauwalderO
BrunV
BadiouC
FerryT
2009 Staphylococcus aureus superantigens elicit redundant and extensive human Vbeta patterns. Infect Immun 77 2043 2050
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 2- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Pathogen Entrapment by Transglutaminase—A Conserved Early Innate Immune Mechanism
- Broadly Protective Monoclonal Antibodies against H3 Influenza Viruses following Sequential Immunization with Different Hemagglutinins
- Neutrophil-Derived CCL3 Is Essential for the Rapid Recruitment of Dendritic Cells to the Site of Inoculation in Resistant Mice
- Differentiation, Distribution and γδ T Cell-Driven Regulation of IL-22-Producing T Cells in Tuberculosis
- IFN-α-Induced Upregulation of CCR5 Leads to Expanded HIV Tropism In Vivo
- An Extensive Circuitry for Cell Wall Regulation in
- TgMORN1 Is a Key Organizer for the Basal Complex of
- Direct Presentation Is Sufficient for an Efficient Anti-Viral CD8 T Cell Response
- Immunoelectron Microscopic Evidence for Tetherin/BST2 as the Physical Bridge between HIV-1 Virions and the Plasma Membrane
- A New Nuclear Function of the Glycolytic Enzyme Enolase: The Metabolic Regulation of Cytosine-5 Methyltransferase 2 (Dnmt2) Activity
- Genome-Wide mRNA Expression Correlates of Viral Control in CD4+ T-Cells from HIV-1-Infected Individuals
- Structural and Biochemical Characterization of SrcA, a Multi-Cargo Type III Secretion Chaperone in Required for Pathogenic Association with a Host
- A Major Role for the ApiAP2 Protein PfSIP2 in Chromosome End Biology
- HIV Controller CD4+ T Cells Respond to Minimal Amounts of Gag Antigen Due to High TCR Avidity
- Fis Is Essential for Capsule Production in and Regulates Expression of Other Important Virulence Factors
- Vaccinia Protein F12 Has Structural Similarity to Kinesin Light Chain and Contains a Motor Binding Motif Required for Virion Export
- A Novel Pseudopodial Component of the Dendritic Cell Anti-Fungal Response: The Fungipod
- Efficacy of the New Neuraminidase Inhibitor CS-8958 against H5N1 Influenza Viruses
- Long-Lived Antibody and B Cell Memory Responses to the Human Malaria Parasites, and
- IPS-1 Is Essential for the Control of West Nile Virus Infection and Immunity
- Transit through the Flea Vector Induces a Pretransmission Innate Immunity Resistance Phenotype in
- Ats-1 Is Imported into Host Cell Mitochondria and Interferes with Apoptosis Induction
- Six RNA Viruses and Forty-One Hosts: Viral Small RNAs and Modulation of Small RNA Repertoires in Vertebrate and Invertebrate Systems
- The Syk Kinase SmTK4 of Is Involved in the Regulation of Spermatogenesis and Oogenesis
- Optineurin Negatively Regulates the Induction of IFNβ in Response to RNA Virus Infection
- On the Diversity of Malaria Parasites in African Apes and the Origin of from Bonobos
- Five Questions about Viruses and MicroRNAs
- A Broad Distribution of the Alternative Oxidase in Microsporidian Parasites
- Caspase-1 Activation via Rho GTPases: A Common Theme in Mucosal Infections?
- Peptides Presented by HLA-E Molecules Are Targets for Human CD8 T-Cells with Cytotoxic as well as Regulatory Activity
- Interaction of Rim101 and Protein Kinase A Regulates Capsule
- Distinct External Signals Trigger Sequential Release of Apical Organelles during Erythrocyte Invasion by Malaria Parasites
- Exacerbated Innate Host Response to SARS-CoV in Aged Non-Human Primates
- Reverse Genetics in Predicts ARF Cycling Is Essential for Drug Resistance and Virulence
- Universal Features of Post-Transcriptional Gene Regulation Are Critical for Zygote Development
- Highly Differentiated, Resting Gn-Specific Memory CD8 T Cells Persist Years after Infection by Andes Hantavirus
- Arterivirus Nsp1 Modulates the Accumulation of Minus-Strand Templates to Control the Relative Abundance of Viral mRNAs
- Lethal Antibody Enhancement of Dengue Disease in Mice Is Prevented by Fc Modification
- Quantitative Comparison of HTLV-1 and HIV-1 Cell-to-Cell Infection with New Replication Dependent Vectors
- The Disulfide Bonds in Glycoprotein E2 of Hepatitis C Virus Reveal the Tertiary Organization of the Molecule
- IL-1β Processing in Host Defense: Beyond the Inflammasomes
- Kaposi's Sarcoma Associated Herpes Virus (KSHV) Induced COX-2: A Key Factor in Latency, Inflammation, Angiogenesis, Cell Survival and Invasion
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Caspase-1 Activation via Rho GTPases: A Common Theme in Mucosal Infections?
- Kaposi's Sarcoma Associated Herpes Virus (KSHV) Induced COX-2: A Key Factor in Latency, Inflammation, Angiogenesis, Cell Survival and Invasion
- IL-1β Processing in Host Defense: Beyond the Inflammasomes
- Reverse Genetics in Predicts ARF Cycling Is Essential for Drug Resistance and Virulence
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



