-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaUniversal Features of Post-Transcriptional Gene Regulation Are Critical for Zygote Development
A universal feature of metazoan sexual development is the generation of oocyte P granules that withhold certain mRNA species from translation to provide coding potential for proteins during early post-fertilization development. Stabilisation of translationally quiescent mRNA pools in female Plasmodium gametocytes depends on the RNA helicase DOZI, but the molecular machinery involved in the silencing of transcripts in these protozoans is unknown. Using affinity purification coupled with mass-spectrometric analysis we identify a messenger ribonucleoprotein (mRNP) from Plasmodium berghei gametocytes defined by DOZI and the Sm-like factor CITH (homolog of worm CAR-I and fly Trailer Hitch). This mRNP includes 16 major factors, including proteins with homologies to components of metazoan P granules and archaeal proteins. Containing translationally silent transcripts, this mRNP integrates eIF4E and poly(A)-binding protein but excludes P body RNA degradation factors and translation-initiation promoting eIF4G. Gene deletion mutants of 2 core components of this mRNP (DOZI and CITH) are fertilization-competent, but zygotes fail to develop into ookinetes in a female gametocyte-mutant fashion. Through RNA-immunoprecipitation and global expression profiling of CITH-KO mutants we highlight CITH as a crucial repressor of maternally supplied mRNAs. Our data define Plasmodium P granules as an ancient mRNP whose protein core has remained evolutionarily conserved from single-cell organisms to germ cells of multi-cellular animals and stores translationally silent mRNAs that are critical for early post-fertilization development during the initial stages of mosquito infection. Therefore, translational repression may offer avenues as a target for the generation of transmission blocking strategies and contribute to limiting the spread of malaria.
Published in the journal: . PLoS Pathog 6(2): e32767. doi:10.1371/journal.ppat.1000767
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000767Summary
A universal feature of metazoan sexual development is the generation of oocyte P granules that withhold certain mRNA species from translation to provide coding potential for proteins during early post-fertilization development. Stabilisation of translationally quiescent mRNA pools in female Plasmodium gametocytes depends on the RNA helicase DOZI, but the molecular machinery involved in the silencing of transcripts in these protozoans is unknown. Using affinity purification coupled with mass-spectrometric analysis we identify a messenger ribonucleoprotein (mRNP) from Plasmodium berghei gametocytes defined by DOZI and the Sm-like factor CITH (homolog of worm CAR-I and fly Trailer Hitch). This mRNP includes 16 major factors, including proteins with homologies to components of metazoan P granules and archaeal proteins. Containing translationally silent transcripts, this mRNP integrates eIF4E and poly(A)-binding protein but excludes P body RNA degradation factors and translation-initiation promoting eIF4G. Gene deletion mutants of 2 core components of this mRNP (DOZI and CITH) are fertilization-competent, but zygotes fail to develop into ookinetes in a female gametocyte-mutant fashion. Through RNA-immunoprecipitation and global expression profiling of CITH-KO mutants we highlight CITH as a crucial repressor of maternally supplied mRNAs. Our data define Plasmodium P granules as an ancient mRNP whose protein core has remained evolutionarily conserved from single-cell organisms to germ cells of multi-cellular animals and stores translationally silent mRNAs that are critical for early post-fertilization development during the initial stages of mosquito infection. Therefore, translational repression may offer avenues as a target for the generation of transmission blocking strategies and contribute to limiting the spread of malaria.
Introduction
Early post-fertilization development in multi-cellular organisms relies on mRNAs supplied in the oocyte in translationally silent P body related storage particles known as P granules. Translation of these maternal mRNA pools depends on fertilization and occurs prior to maternal to zygote transition when transcription from the zygotic genome is initiated [1],[2]. Many P granule components are known [3]–[7] but there is a long-standing question to what constitutes the evolutionarily conserved and essential protein core that controls related events in unicellular eukaryotes during sexual reproduction. In the protozoan Plasmodium, formation of a diploid zygote during sexual development coincides with, and is essential for parasite transmission from the human to the mosquito host. Plasmodium are haploid throughout most of their life cycle and sexual development in malaria parasites is initiated with the generation of sexual precursor cells, or gametocytes, in the blood of the mammalian host. These mature, haploid male or female forms present distinct proteomic profiles [8] in the absence of sex chromosomes. In the mosquito midgut fertilization yields a diploid zygote that undergoes meiosis without cell division resulting in a tetraploid cell that within 18 hours transforms into the motile ookinete able to truly infect the mosquito. Zygote to ookinete transformation relies on the translational activation of stored, silent mRNAs probably deposited in mRNPs of unknown composition in the female gametocyte [9]. Translationally quiescent mRNAs are found in the cytoplasm of female gametocytes [9]–[12], where long-term maintenance and stabilisation depends on the conserved DEAD-box RNA helicase DOZI [9], a homolog of Saccharomyces cerevisiae (yeast) Dhh1p, Drosophila melanogaster (fly) Me31b, Caenorhabditis elegans (worm) CGH-1 and vertebrate members Xenopus laevis P54 and human RCK/P54. In the absence of DOZI, Plasmodium berghei zygotes fail to develop into ookinetes, most likely due to a failure to form mRNPs that store and stabilise silenced transcripts. Collectively these destabilized mRNAs encode proteins that are essential for zygote to ookinete transformation during the initial phase of mosquito infection and include adhesins and factors known to be necessary for ookinete motility and traversal through mosquito midgut cells [9]. Translational silencing of certain mRNA species is mediated by a U-rich RNA motif present in the 5′ or 3′ untranslated regions of the implicated mRNAs [13] which also have been shown to specifically silence transgene expression [14].
We provide here the most in-depth characterisation of the protein composition of a P granule to date and demonstrate that the Plasmodium particle has a protein core with widespread phylogenetic conservation containing proteins known to form equivalent particles in metazoan oocytes. In addition novel protein components are demonstrated that, although highly conserved, to our knowledge have not been associated with mRNP formation. Functional characterisation of two of the conserved core components revealed distinct phenotypes implying that functionally distinct sub-populations of silenced mRNPs exist.
Results
DOZI (CGH-1/Me31b) and CITH (CAR-I/Trailer hitch) define a protozoan, maternal P granule
The construction and characterization of a recombinant parasite line that expresses DOZI::GFP from a modified dozi allele has been previously reported [9]. Through immunoprecipitation (IP) of DOZI::GFP followed by RNA analysis of IP eluates by Northern and RT-PCR analysis we have previously shown a clear physical association of this DEAD-box RNA helicase with mRNAs known to be translationally silenced in mature, female gametocytes [9]. To define the molecular nature of this putative complex we sought to identify proteins that co-operate with DOZI in the assembly and maintenance of translationally repressed mRNAs. In two independent IP experiments targeting DOZI::GFP a complex from Plasmodium berghei gametocytes was purified and analyzed by LC-MS/MS yielding a group of DOZI interaction partners (Figure 1A-B,D; Table S1); one of the co-eluted proteins, PB000768.03.0, showed strong homology with worm CAR-I and fly Trailer Hitch but also Xenopus Rap55; these proteins co-localize with their respective DOZI homologues CGH-1 and Me31b to germ cell and P granules [3]–[7] – the Plasmodium protein contains both the conserved LSM14 domain and the extended FDF motif (Figures 1D, S1) known to compete with the enhancer of mRNA decapping EDC3 for binding to DDX6 helicases [15], and is therefore designated CITH (CAR-I/Trailer Hitch Homolog; Figure S1). To corroborate the DOZI pull down results, a reciprocal IP (Figure 1C) was performed using lysates from gametocytes of a transgenic P. berghei line expressing only C-terminally GFP-tagged CITH (Figure S2). Mass-spectrometric analysis of the CITH::GFP pull down resulted in the identification of the same 16 core factors (Figures 1D and Table S1). A linear regression analysis revealed no bias towards high molecular weight or abundant proteins (Figure S3); 7 of the 16 proteins were previously found to be sexual stage specific in P. berghei (PB000695.03.0, PB000120.01.0, PB001107.03.0, PB000768.03.0, PB000603.01.0, PB000647.02.0, PB000124.01.0) [8].
Fig. 1. Identification of a multiprotein complex engaged in storage of translationally silent mRNAs in female Plasmodium gametocytes. 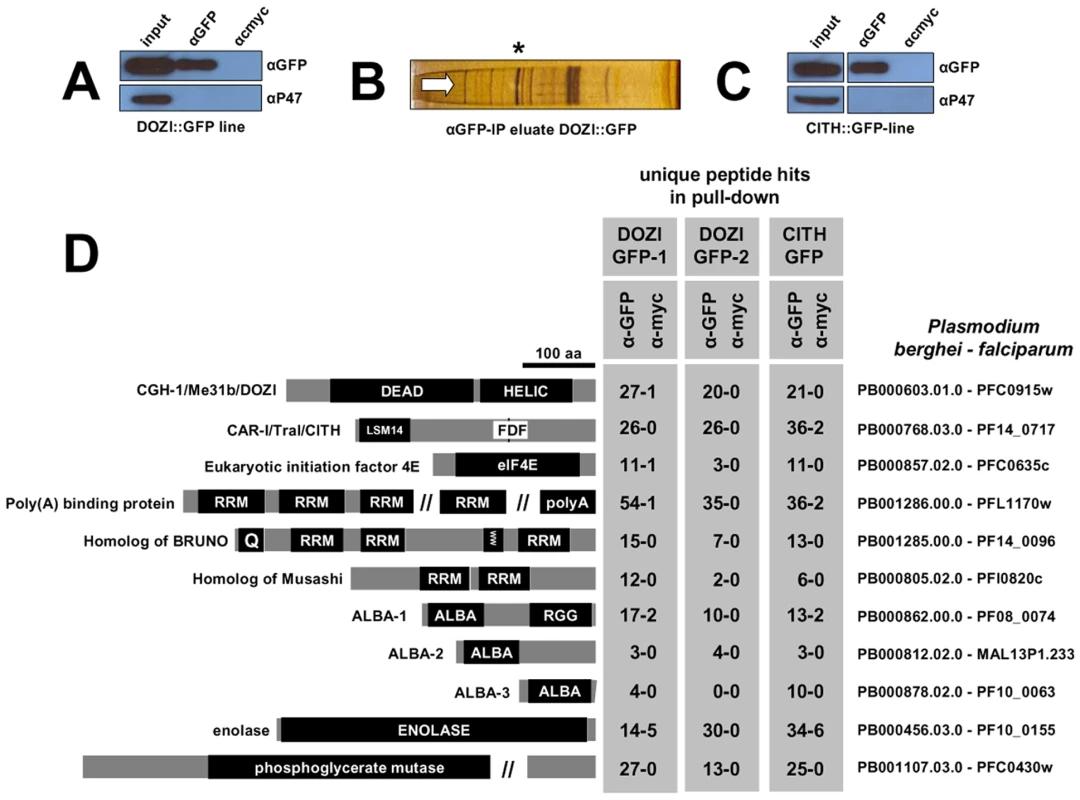
A and C Western blot analysis of DOZI and CITH immunoprecipitation (IP) eluates show specific isolation of the respective GFP fusion proteins. P47, a female gametocyte specific protein, did not co-IP and is present only in input fractions. Equivalent amounts were loaded. B Silver staining of the DOZI::GFP IP eluate separated on a 12% SDS-PAGE. The asterisk indicates the position/size of the DOZI::GFP fusion protein. D Reciprocal IP targeting C-terminal GFP-fusion proteins of DOZI and CITH resulted in the pull down of a set of proteins that were identified using LC-MS/MS. Shown are all proteins with conserved motifs (drawn to scale) and – grey underlaid – the number of unique peptide hits/protein in specific anti-GFP and control IPs. Alignments of these factors and additional Plasmodium-specific factors are shown in Table S1, Figures S1, S4, S5, S6, S7, S8, S9 and S10. Homology was defined on amino acid level, as well as domain presence and architecture. The analysis of the DOZI and CITH pull down eluates gives an unprecedented depth of characterisation of the protein component of a P granule (Figure 1D). Among the DOZI and CITH-associated proteins identified with a high level of confidence are the Plasmodium homologs of the 5′ cap binding protein eIF4E (PB000857.02.0, Figure S4) and poly(A) binding protein (PABP; PB001286.00.0, Figure S5). Both are commonly found in mammalian stress granules [16] and PABP protects mRNAs from de-adenylation and degradation. In addition we identified orthologs of proteins that function as translational regulators in metazoans; one protein with strong homology to the ELAV/BRUNO-family and a second with weak homology to Musashi: the Plasmodium proteins are Homolog of Drosophila BRUNO (HoBo, PB001285.00.0, Figure S6), and Homolog of Musashi with two RNA recognition motifs (HoMu, PB000805.02.0 Figure S7). Drosophila BRUNO targets mRNAs such as oskar containing the 3′ UTR BRUNO response element for silencing [17], while Musashi is a translational regulator found to compete with eIF4G for PABP-binding in neural stem cells [18]. For the first time we identify in maternal mRNPs Alba domain proteins (Acetylation Lowers Binding Affinity); the entire complement of P. berghei Alba domain proteins (Alba-1, PB000862.00.0; Alba-2, PB000812.02.0 and Alba-3, PB000878.02.0) (Figure 1D) co-IPs with DOZI and CITH. These proteins are small, with predicted molecular weights of 27, 23 and 12 kDa, respectively with a single, N-terminal Alba domain (Figure S8). Alba-1 contains multiple RGG-box RNA binding domains at the C-terminus, a characteristic of plant and protozoan proteins. Phylogenetic analyses places Alba-1 and Alba-2 into the MDP2/Rpp25 superfamily, whereas Alba-3 belongs to the POP7/Rpp20 group (Figures 2 and S8) [19]. Interestingly, within the Apicomplexa only the genus Plasmodium appears to have 2 members within the MDP2 group. Two enzymes potentially associated with glycolysis were identified, i.e. a member of the phosphoglycerate mutase (PGAM) family (PB001107.03.0, Figure S9) and enolase (PB000456.03.0, Figure S10). Lastly, 5 abundant proteins show no or little homology to proteins outside Plasmodium spp.; they are PB000695.03.0, PB000120.01.0, PB000647.02.0, PB000124.01.0 and PB000642.01.0.
Fig. 2. Phylogenetic position of Plasmodium Alba-domain proteins. 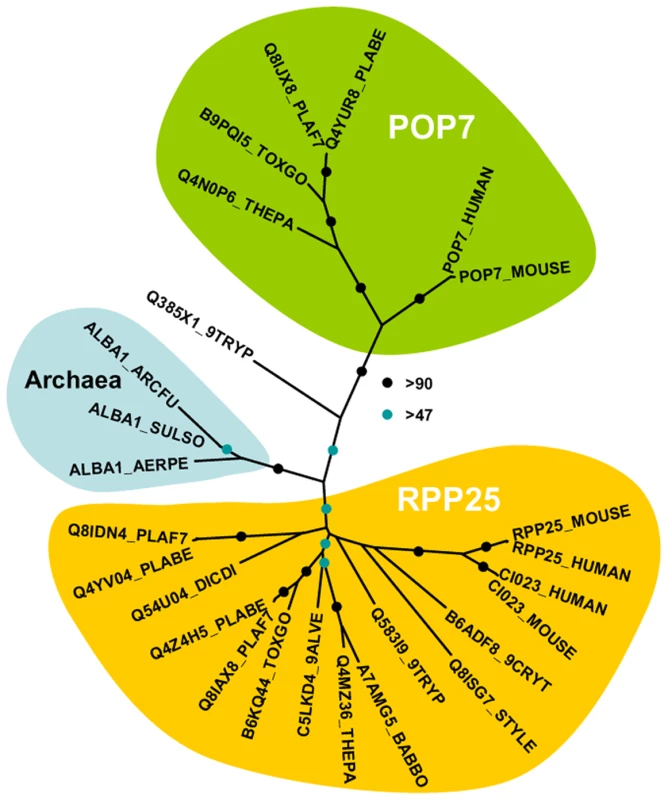
We generated a multiple sequence alignment of the conserved region of a range of Alba-domain proteins (PFAM PF01918). The phylogenetic tree was generated with PHYML. Bootstrap values (100 replicates) are based on neighbor joining and maximum likelihood analyses. Accession numbers are from uniprot; species abbreviations are for Perkinsus marinus (9ALVE), Cryptosporidium muris (9CRYT), Trypanosoma brucei (9TRYP), Aeropyrum pernix (AERPE), Archaeoglobus fulgidus (ARCFU), Babesia bovis (BABBO), Dictyostelium discoideum (DICDI), Plasmodium berghei (PLABE), Plasmodium falciparum (PLAF7), Stylonychia lemnae (STYLE), Sulfolobus solfataricus (SULSO), Theileria parva (THEPA) and Toxoplasma gondii (TOXGO). DOZI and CITH complexes contain translationally repressed mRNAs
Consistent with the similarities in protein content of the DOZI and CITH IPs, the same silenced mRNA species associated with DOZI [9] were also found to co-elute with CITH by Northern analysis and RT-PCR (Figures 3A and B) but not transcripts known to be translated in gametocytes. The RNA-IP experiments indicate that CITH together with DOZI resides in a stable, translationally quiescent P body-like structure. The bulk of DOZI and CITH protein is present in female gametocytes as shown by immunofluorescence and proteome analysis of asexual stage and purified gametocytes [8] and expression of both proteins persists throughout ookinete development [8],[13]. The P. falciparum DOZI ortholog (PFC0915w) has also been detected in sporozoites [20]. We consistently observe large CITH::GFP granules in live gametocyte preparations (Figure 3C); in addition the protein overlaps partially with two of the best characterised maternally silenced mRNAs, p25 and p28, in cytoplasmic foci with a speckled appearance typical for such mRNPs (Figure 3D).
Fig. 3. CITH co-localizes with translationally repressed mRNAs. 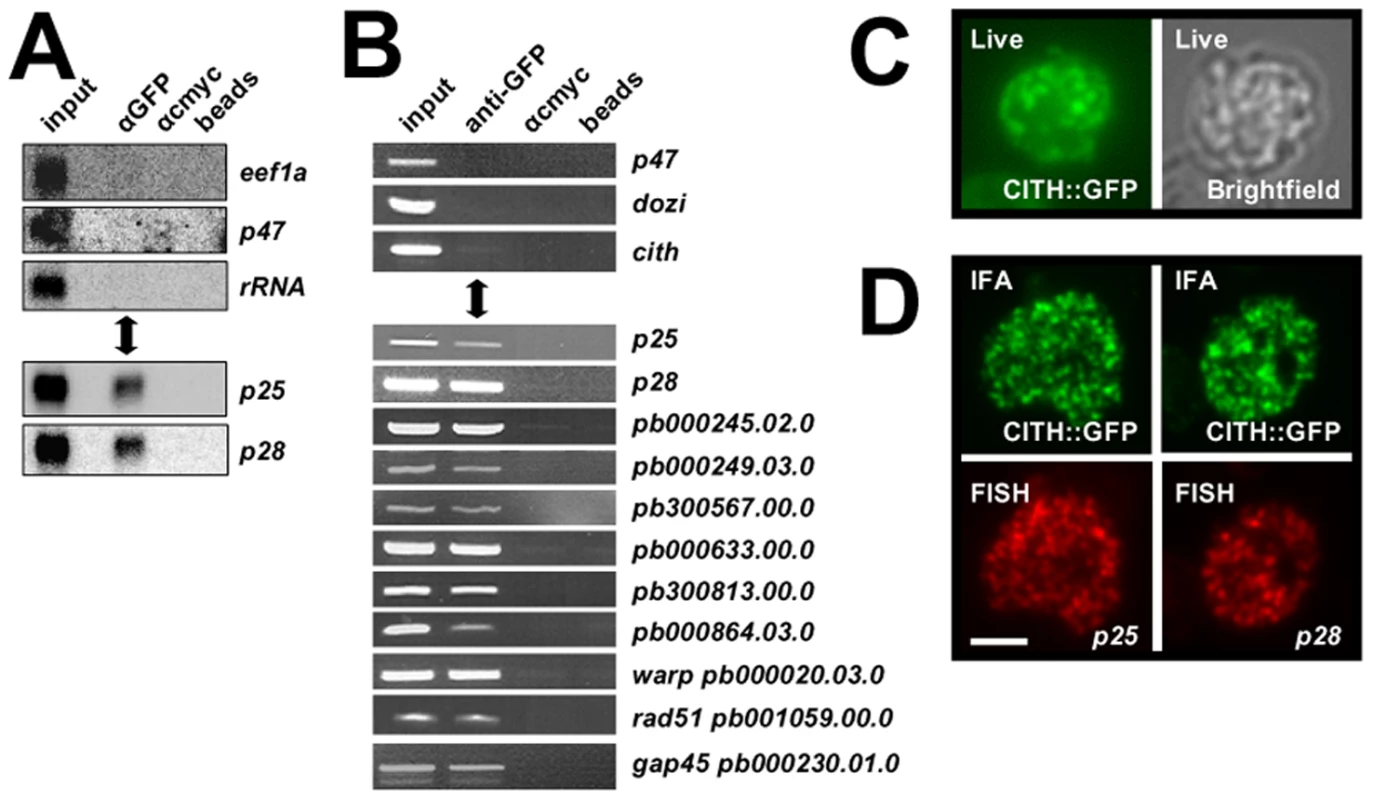
A Northern analysis of CITH::GFP IP eluates show specific co-elution of translationally repressed mRNAs p25 and p28, but not transcripts known to be translated (p47, eef1a) or ribosomal RNA. Equivalent amounts of eluates and input were loaded. B RT-PCR analyses show specific enrichment of transcripts known to be translationally repressed in the anti-GFP pull down; transcripts of expressed proteins (P47, DOZI, CITH) are absent. C The CITH::GFP fusion protein is present in characteristic foci in the cytoplasm of live parasites. D Fluorescent in situ hybridization combined with immunofluorescence analysis shows overlapping signals for p25 and p28 mRNAs and CITH::GFP. Scale bar = 4 µm. DOZI and CITH gene deletion mutants are fertile but abort zygote to ookinete transformation
The similarities of mRNA and major protein contents of DOZI and CITH IPs indicate that they are largely a component of the same mRNP responsible for post-transcriptional regulation of gene expression at the level of translation initially defined by DOZI. As zygotes lacking DOZI fail to progress through meiosis and are unable to transform into ookinetes [9] we wanted to identify any possible effects on zygote to ookinete transformation in the absence of CITH. Mutant parasite lines that lack pbcith (Δpbcith) (Figure S11) showed normal asexual blood stage development and wild type production of gametocytes and gametes but failed to generate ookinetes (data not shown). To analyse in greater detail possible fertilization and meiosis defects we generated Δpbcith and Δpbdozi lines [934cl1(Figure S12) and line 927cl1 (Figure S13)], respectively] in a reporter line with red fluorescent protein (RFP) expression exclusive to female gametocytes, that persists throughout ookinete development (Figures 4A-C and S14; see also Protocol S1 and www.pberghei.eu). In addition to RFP under the control of the female-specific promoter of gene pb000504.02.0, GFP is driven by the male-specific promoter of gene pb000791.03.0. Both transgenes are stably introduced into the 230p locus on chromosome 2. Therefore, stage specific RFP expression permits identification of female gametes and zygotes after fertilization for FACS-analysis of their DNA contents by Hoechst staining. Such analyses made 4 hours after activation, when the zygote normally has completed meiosis, are able to reveal cell ploidy and are therefore a quantitative indicator of fertilization success and zygote development from the diploid to the tetraploid state (Figures 4D and E). These studies confirmed that the Δpbdozi line fertilises normally when compared to wild type; the male and female nuclei fuse but fail to complete meiotic replication and remain diploid (Figure 4F). Surprisingly, Δpbcith mutants present a different phenotype where they also fertilise normally yet progress through meiotic DNA replication to establish tetraploidy (Figure 4G). However, further development of the spherical zygote into the motile, banana-shaped ookinete is aborted soon after zygote stage I/II, before gross morphological changes become apparent [21]. Consequently, neither gene deletion mutants are able to transform into ookinetes (Table 1A). Standard cross-fertilization assays [8] in which gametes of Δpbcith were crossed with either fertile male (parasite line 137.1, Δp47) or female gametes (parasite line 370.1, Δp48/45) demonstrated that male gametes are unaffected by the absence of CITH – the block in development of the zygote is due to the absence of the protein provided by female gametes resulting in sterility (Table 1B). Therefore, despite the clear similarities in proteins associated with DOZI and CITH, their maternal origin and essential role in zygote to ookinete transformation, the specific effects on early zygote development are different.
Fig. 4. Zygotes formed by Δpbcith and Δpbdozi gene deletion mutants abort zygote to ookinete transformation. 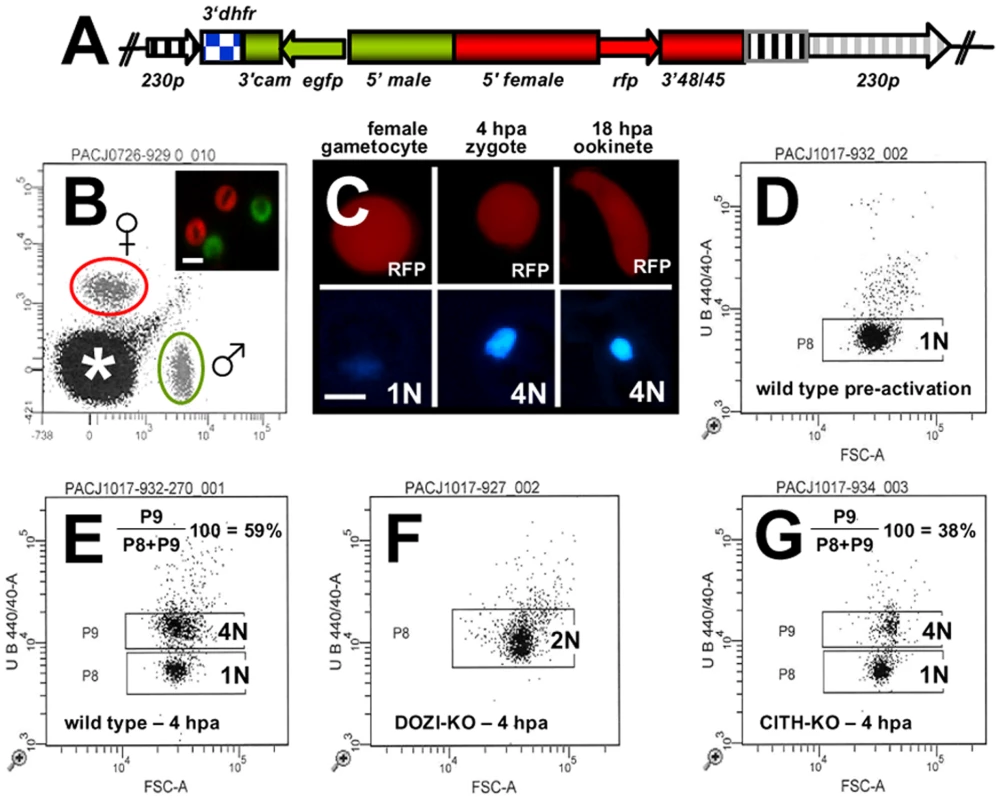
A Schematic representation of the vector used to introduce the gfp/rfp male/female expression cassette into the p230p locus. B Identification of populations of RFP+ female gametocytes and GFP+ males by FACS in blood infected with parasites of line 820cl1m1cl1. Gametocyte populations are clearly separated from the population of red blood cells and red blood cells infected with the asexual bloods stages (asterisk). The inset shows male (GFP+) and female gametocytes (RFP+). Scale bar = 4 µm. C RFP and Hoechst 33258 staining of female gametocyte, zygote and ookinete of line 820cl1m1cl. Zygotes and ookinetes were collected at 4 hours post activation (hpa) and 18 hpa, respectively. Note the increased Hoechst fluorescence intensity of the nuclei of zygotes and ookinetes as a result of fertilization and meiotic DNA replication, resulting in tetraploid nuclei. Scale bar = 2 µm. D and E FACS analysis of Hoechst fluorescence intensity of wild type female gametes (D) and zygotes (E). In (D) haploid female gametes are shown before fertilization and (E) shows unfertilized females (1N) and tetraploid zygotes (4N) collected at 4 hpa. F Δpbdozi females are fertilized as shown by the doubling in DNA content, but do not achieve tetraploidy. G Females of Δpbcith mutants develop into tetraploid forms. Tab. 1. Ookinete formation in wild type and mutant parasite lines. 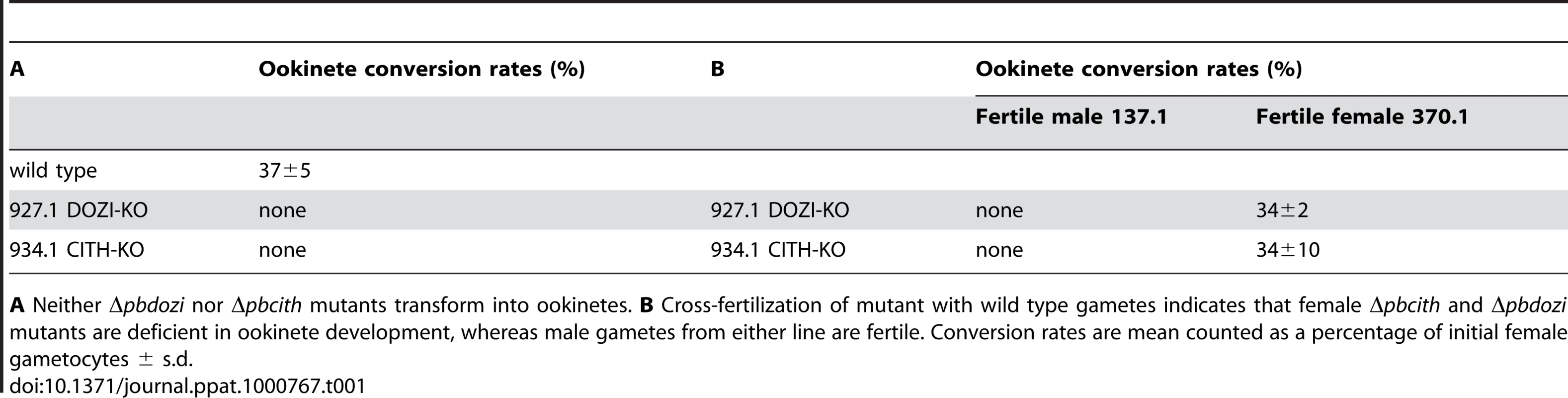
A Neither Δpbdozi nor Δpbcith mutants transform into ookinetes. B Cross-fertilization of mutant with wild type gametes indicates that female Δpbcith and Δpbdozi mutants are deficient in ookinete development, whereas male gametes from either line are fertile. Conversion rates are mean counted as a percentage of initial female gametocytes ± s.d. Maternal lethal effects of CITH and DOZI
In Δpbdozi gametocytes the expression levels of 370 transcripts (6% of all Plasmodium genes) were more than 2-fold reduced when compared to wild type gametocytes [9]. In order to identify if similar molecular effects contribute to the observed developmental defect in the pbcith mutant parasite, we performed a small Northern survey of abundant but translationally repressed mRNAs, among them the hallmark gene p28. Using RNA isolated from gametocytes, p28 together with 3 additional transcripts appeared less abundant in the CITH KO parasites, indicating a destabilising effect on these mRNAs in the absence of CITH (Figure 5A), thus prompting us to perform a global transcriptome profiling of gametocyte RNA and identify whether mRNA destabilisation is a global phenomenon. Microarray hybridisation of Δpbcith mutants revealed that the expression levels of 232 transcripts were significantly changed, with 183 mRNAs more than 2-fold down regulated (DR) representing 50% of the Δpbdozi number (Table S2). As in Δpbdozi, several transcripts (46) were unexpectedly up-regulated (UR) in the absence of CITH. In total, 82% of the protein products of all differentially expressed transcripts are absent from the gametocyte proteome [8] indicating that these transcripts are stabilised and silenced in a CITH dependent manner. 127 mRNAs were common to the DOZI and CITH data sets (Figure 5B and Table S3) although neither the degree of a given individual transcript nor the rank order was consistent between the two mutants (R2 = 0.25; Pearson r = 0.50, Figure S15A). 117 are DR in both KOs, 3 were UR, whereas 7 transcripts are inversely modulated. Gene Ontology (GO) enrichment analysis (Figure S15B) revealed no bias most likely due to incomplete and therefore high number of hypothetical annotations (89 genes). However, 21 proteins are predicted to contain a signal peptide and 24 contain one or more trans-membrane domains suggesting cell surface localisation; among those are known adhesins – factors that function in host-cell receptor interactions and promote successful invasion of the midgut epithelium resulting in infection of the mosquito – and include p25, p28, warp, p36, and members of the pb-fam-5/cpw-wpc and lap families including ccp2 and lap5. In addition 5 alveolins (membrane sac proteins), inner membrane complex 1b protein, gliding motility associated protein gap45, 3 protein kinases, a member of the ap2/erf family of transcription factors (api-o) that initiates transcription of ookinete-specific genes [22] and rad51 are DR; finally, so are the 9 mRNAs previously shown to share a cis-acting RNA motif that confers silencing in female gametocytes [13],[14]. In total, only 8% of the common differentially expressed genes are present in the Plasmodium gametocyte proteome [8] suggesting that CITH and DOZI co-operate in the protection from degradation of translationally quiescent, maternally supplied mRNAs.
Fig. 5. CITH and DOZI gene deletion mutant gametocytes suffer substantial mRNA loss. 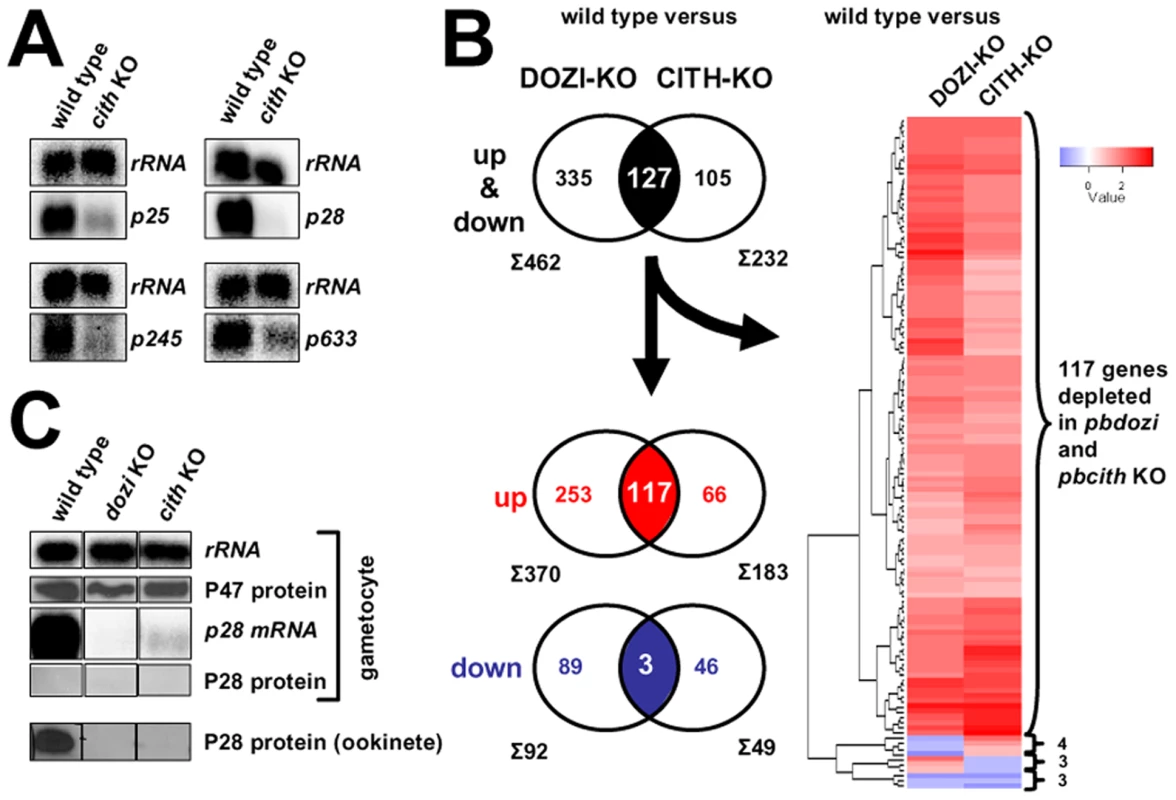
A Northern blot analysis of four translationally repressed transcripts in wild type and Δpbcith mutants shows a clear de-stabilizing effect in the absence of CITH in gametocytes. B Venn diagrams of all 2-fold differentially expressed genes common to DOZI and CITH KO parasites. Hierarchical cluster analysis of the differentially expressed genes common to both lists. C Absence of DOZI or CITH does not result in the precocious translation of p28 mRNA into protein in female gametocytes. Are there DOZI and CITH-specific mRNPs?
While a large number of genes are co-regulated by DOZI and CITH, the differences in the repertoire of DR mRNAs between Δpbdozi and Δpbcith gametocytes may indicate the presence of mRNPs with distinct mRNA content that is reflected in the observed developmental defects. A number of meiosis-associated transcripts were exclusively depleted in the Δpbdozi mutant which arrests before completion of meiosis (Table S4); these include the RNA-binding protein mei2 (pb001281.02.0) and the chromosome segregation myosin-ATPase (pb300220.00.0). Three additional AP2/ERF transcription factors (pb00974.00.0, pb001077.01.0, pb300561.00.0) [23],[24] as well as 6 mRNAs encoding Zn-finger domain proteins are significantly destabilized, and their protein products are likely to play a role in the activation of the zygotic genome.
Failure to establish the P granule results in p28 destabilization but not translation
p28 is one of the first and best characterized translationally repressed mRNAs. Its protein product, which is displayed on the surface of the ookinete, plays an important role during mosquito midgut invasion making it a promising candidate for transmission blocking intervention [25]. DOZI and CITH gene deletion mutants fail to stabilize p28 and this failure could potentially lead to the precocious translation of P28 protein in blood stage gametocytes. Therefore we wanted to know the fate of p28 mRNA in DOZI and CITH gene deletion mutants. As shown in Figure 5C, absence of either factor does not result in P28 protein translation indicating that the mRNA is most likely degraded when not stored, and unable to resume translation.
Discussion
Zygote to ookinete transformation occurs over an 18-hour period in the female mosquito midgut which is a hostile environment actively engaged in the systematic destruction of cellular material in order to provide nutrition for mosquito egg production and maturation. It is known that animal oocytes store mRNA in order to bypass the need for transcription during early embryogenesis before activation of the newly formed zygotic genome [1],[2],[26]; whilst this requirement holds true for the parasite, Plasmodium zygotes undergo meiosis within 4 hours of fertilization, which is followed by the timely formation of the ookinete – a motile parasite form able to actively escape the hostile mosquito midgut environment by penetrating the surrounding epithelium; these different developmental requirements may influence the composition of the P granule.
Maternal mRNA storage depends on a protein core conserved from unicellular organisms to germ cells of metazoans
The LC-MS/MS analysis of the DOZI and CITH-associated proteins revealed 16 common, major protein factors. They could be grouped into a number of different classes based on predicted activity: 1. Proteins with homology to constituents of metazoan P granules; these proteins (DOZI, CITH, eIF4E, HoBo/BRUNO, and HoMu/Musashi) have been demonstrated to be present in mRNPs from various organisms although never in a single mRNP as presented in this study. The presence of PABP in Plasmodium P granules and metazoan germ cell granules [4],[7] may indicate an intrinsic readiness of the particle to present repressed transcripts to the ribosome in response to the identified need for speed at the same time protecting mRNAs from degradation. 2. Alba domain containing proteins have not been identified in association with mRNPs before. In Archaea they are known to bind RNA, principally ribosomal RNA [27], and eukaryotic POP7 and Rpp25-related proteins take part in tRNA and rRNA processing, while ciliate MDP2 is a factor in macronuclear development [28],[29]. In the Archaea Alba regulates transcription through chromatin organisation where DNA binding affinity is controlled by the sirtuin SIR2 and Pat [30],[31]. In Plasmodium SIR2 regulates the expression of sub-telomerically located genes of multigene families encoding variant antigens [32]–[37]. However, the association of Alba proteins with factors that regulate translational repression (TR) might indicate that sirtuin de-acetylases and their counterpart acetylases also have a post-transcriptional role in the control of gene expression in Plasmodium. 3. Two proteins associated with glycolysis were identified independently in DOZI and CITH IP-eluates, enolase and a member of the PGAM family. The role in TR implied by their association with the DOZI/CITH mRNP found in Plasmodium and possible moonlighting functions are currently obscure and requires further attention. However, it is well established that enolase has roles in biological processes besides its role in glycolysis, including transcription, heat shock, autoimmunity and it may also serve as a plasminogen receptor and function in the bacterial degradasome [38]. PGAM family members are known to participate in complexes including those which repress gene expression at the level of transcription [39].
P body RNA degradation factors are absent from Plasmodium maternal P granules
CGH-1/Me31b/Dhh1 are present in diverse, functionally distinct P body families; these include maternal P [5]–[7] and stress granules [16], but also co-localisation with RNA de-capping factors such as DCP1/2 [40] and presence in a miRNA-induced silencing complex [41] has been shown. It is significant that in this study and in contrast to the identification of proteins that are present in P bodies and stress granules, no factors were identified that constitute the core of P bodies during RNA degradation [42] and that interact with DOZI. Plasmodium homologs of most proteins with exonuclease activity and involved in mRNA de-adenylation and decapping (e.g. XRN1, Lsm1-7, DCP1 and 2, UPF1-3) are readily identified in the annotated genome (Table S5). Yet they were absent from the IP eluates, confirming that gametocyte mRNPs defined by DOZI and CITH contain stable, translationally repressed transcripts awaiting re-activation and translation following fertilization. This emphasizes that in the context of the gametocyte the activity of DOZI is predominantly one of mRNA storage and not degradation. It is intriguing that specific and overlapping, but also non-identical mRNA populations are destabilized in the gametocyte in the absence of DOZI and/or CITH. Our data support the existence of different forms of P granules that are defined by the destabilized mRNA populations in the CITH and DOZI depleted mutants and the observed different developmental defects of these mutants.
Repression acts on diverse mRNA populations
Our experiments detailing the destabilising effect on a substantial mRNA population of the gametocyte show that silencing influences diverse processes during zygote to ookinete formation. For example, the newly formed zygote is provided with coding potential for proteins known and likely to be involved in ookinete development, for instance in the activation of the zygotic genome; the presence of AP2/ERF transcription factors (TF) and DNA Zinc-finger binding domains in the down regulated set of genes indicate that these factors are already supplied in the female gametocyte. One of these TF (API-O) promotes transcription of genes during ookinete development and is present in DOZI-defined mRNPs [22]. Secondly, 25% of the commonly down regulated mRNAs encode proteins with known and predicted surface localisation. In the case of P25 and P28 it is well established that they facilitate the escape of the parasite from the hostile mosquito midgut milieu [43]; transcription of these mRNAs in blood stage gametocytes and subsequent retention in silent mRNPs provides rapid access of these transcripts to ribosomes and therefore the production of these essential proteins. However, storage in P granules may also contribute to immune evasion mechanisms in the mammalian host. Antibodies to P25 and P28 are promising transmission blocking vaccines – their presence in a mosquito blood meal substantially reduces the ability of the parasite to infect the mosquito vector [44],[45]. We have shown here and previously [9] that prevention of complex formation in gametocytes in CITH and DOZI KO mutants induces degradation and not translation of p25 and p28 mRNAs, while female gametocytes are fully translation competent as shown in numerous GFP transgene experiments [8],[46],[47]. In addition gfp translation can be abrogated when tethered to the 3′ UTR of p28 [46]. It is therefore tempting to speculate that the parasite has evolved a fail-safe mechanism that results in the degradation of mRNAs meant to be silenced in case the transcript fails to be stored in P granules.
The maternal P granule is evolutionarily ancient
Our experiments corroborate that protozoans, like female germ cells of higher eukaryotes, rely on the storage of mRNA in the female gamete during sexual reproduction, specifically during early post-fertilization development. DOZI and CITH in Plasmodium are bona fide translational repressors that contribute to successful ookinete development in and infection of the mosquito vector by storing a substantial mRNA population in pre-fertilization, female gametocytes. In worm oocytes CGH-1 granules associate with roughly 6% of all known expressed genes [4] compared with approximately 7% down regulated mRNAs in Plasmodium gametocytes. Although the protected mRNA species are not conserved in the 2 organisms, the fundamental DOZI/CGH-1-dependent protection of transcripts is. The normal generation of ookinetes from crossings of CITH and DOZI-KO male gametes wild type female gametes also show that the observed sterile developmental phenotype is entirely a maternal effect previously identified for Drosophila Trailer hitch where mutant female flies are sterile and present defects in egg laying [7],[48].
Conclusions
Translational repression (TR) is an important mechanism of post-transcriptional gene regulation that in metazoan germ-line but also somatic cells generates spatial and temporal protein diversity that is independent from transcriptional control and protein targeting signals. Our data demonstrate that such mRNPs in the protozoan Plasmodium rely on an evolutionarily conserved and ancient protein core that secures mRNP integrity and future translatability of stored mRNAs in a DOZI and CITH-dependent manner. The relatively tractable nature of the P. berghei malaria model will allow a detailed dissection of the role of conserved and species-specific proteins in TR. Furthermore, the novel involvement of Alba proteins in TR and the coupling of post-transcriptional modifications to signalling as an effector of TR may yet prove to be informative of control of TR in general.
Materials and Methods
Ethics statement for animal experimentation
All studies in which animals are involved were performed according to the regulations of the Dutch “Experiments on Animals Act” and European regulations (EU directive no. 86/609 regarding the Protection of Animals used for Experimental and Other Scientific Purposes) and approved by the Animal Experiments Committee of the LUMC (ADEC; established under section 18 of the “Experiments on Animals Act” and registered at the Dutch Inspectorate for Health Protection and Veterinary Public Health, which is part of the Ministry of Health, Welfare and Sport).
Generation of Plasmodium berghei mutants expressing C-terminally GFP-tagged CITH and DOZI
The mutant parasite line that expresses a C-terminally GFP-tagged version of DOZI (683cl4) has been described [9]. CITH::GFP parasites [line 909cl1 (Figure S2)] were generated in the parent reference line of the ANKA strain cl15cy1 with a GFP-tagging vector pL1200 containing a single genomic targeting region for single cross-over homologous recombination generated by PCR with primers 2831-EcoRI and 2832-NotI. Mutant parasites express only the GFP-tagged gene. Targeting regions, primers used and genotype analysis are shown in Table S6. Please also refer to www.pberghei.eu and Table S7 for mutant P. berghei parasites lines used in this study.
Immunoprecipitation (IP) experiments and mass-spectrometric analysis
IP of DOZI::GFP and CITH::GFP complexes was performed on whole cell lysates from purified gametocytes as described in Supplementary Online Material of reference [9] using monoclonal anti-GFP antibodies (Roche) and control anti-cmyc antibodies (SIGMA). Processing of eluates and mass-spectrometric analysis by LTQ-FT are described in Protocol S1. Total RNA from IP eluates was extracted with TRIzol and used in Northern blot analysis and RT-PCR. Primers used are shown in Table S6. Western blot analysis of IP eluates was performed using monoclonal anti-GFP antibodies (Roche) and anti-P47 [49] as described [9].
Generation of mutants deficient in expressing CITH and DOZI
pbcith (pb000768.03.0) was targeted for genetic disruption by standard double-crossover homologous recombination with vectors containing the Toxoplasma gondii (tg) dhfr/ts selection cassette flanked by targeting sequences of the corresponding ORF (Figure S11). Targeting regions were generated by PCR with primers 2773-Asp718I and 2774-HindIII, and 2775-EcoRI and 2776-NotI. Transfection and selection of mutant parasites was performed using genetic modification technology developed for P. berghei [50]. Correct integration of plasmids and disruption of the genes was verified by Southern analysis of separated chromosomes and diagnostic PCRs, and Northern analysis. Targeting regions, primers used and gels are shown in Figure S12. pbcith was disrupted in three independent experiments (856, 893, 934); lines 856 and 893 were generated in a wild type reference line of the ANKA strain (507cl1) that constitutively expresses a gfp transgene under the control of the eef1a promoter, stably integrated into the pb230p locus without use of a drug-selectable marker [50]. Line 934 was generated in a second wild type reference line (line 820cl1m1cl1; Figure S12) which contains gfp and rfp transgenes under the control of male (pb000791.03.0) and female (pb000504.02.0) specific promoters, respectively, stably integrated into the pb230p locus without the use of a drug selectable marker (see Protocol S1 Generation of a reporter P. berghei line that expresses RFP in female gametocytes, gametes and zygotes for further details of the generation of this line). Cloned parasite lines of transfection 856 and 934 were obtained by limiting dilution and used for further analysis of the phenotype (Figure S12). Parasite lines in which pbdozi has been disrupted in the ANKA strain have been described. In addition, we disrupted for this study pbdozi in the reference line 820cl1m1cl1 (927cl1; Figure S13) using the same DNA construct as described [9].
Generation of a reporter P. berghei line (820cl1m1cl1) that expresses RFP in female gametocytes, gametes and zygotes
Generation of a reporter P. berghei line (820cl1m1cl1) is described in detail in Protocol S1 and Figure S14.
In vitro (cross) fertilization and ookinete maturation assays
The fertility of wild type and mutant gamete populations was analysed by standard in vitro fertilization and ookinete maturation assays [8],[49] from highly pure gametocyte populations [51]. Fertility (ookinete conversion) of gametes is defined as the percentage of female gametes that develop into mature ookinetes determined by counting female gametes and mature ookinetes in Giemsa stained blood smears 16–18 hours after gametocyte activation. Fertility of individual sexes (macro - and micro-gametes) was determined by in vitro cross-fertilization studies in which gametes are cross-fertilised with gametes of lines that produce only fertile male (270cl1) or only fertile female gametes (137cl1) [8],[9],[49]. All assays were done in triplicate on multiple occasions in independent experiments.
Analysis of fertilization and meiosis by FACS
Fertilization and meiosis in wild type and mutant lines was inferred from their DNA content (or ploidy) determined by FACS measurement of fluorescence intensity of cells stained with the DNA-specific dye Hoechst-33258. For these experiments we used the mutant lines generated in the parent line 820cl1m1cl (see Protocol S1) that expresses RFP in the female gametocyte/gamete and continues into the zygote and ookinete. Stage specific RFP expression allows selection of female gametes and zygote stages in the process of FACS-analysis of the DNA content of cells. Activation of gametocytes was performed in in vitro (cross) fertilization and ookinete maturation assays as described above. At 4 hours post activation (hpa) cells were stained for one hour at room temperature with Hoechst-33258 (10 µM) and analysed at room temperature by FACS using a LSR-II flow cytometer (Becton Dickinson) with the following filters and settings: UB 440/40 (Hoechst)|400 (parameter)|5000 (threshold); BE 575/26 (RFP)|500|5000; BF 530/30 (GFP)|500|5000; FSC|250|2000; SSC|200|5000. Cells for Hoechst analysis were selected on size by gating on FSC and SSC. Per sample 10.000–500.000 cells were analyzed (medium flow speed, sample pressure: medium) and all measurements were performed on triplicate cultures. Female cells were selected for Hoechst-33258 fluorescence intensity based on their RFP expression (Figure 4). To determine the Hoechst-fluorescence intensity from the populations of unfertilized female gametes and zygotes gates were set as shown in the Figures. Data processing and analysis was performed using the program FlowJo (www.flowjo.com).
Microarray analysis of CITH KO parasite lines
Hybridisation with total RNA from wild type and CITH KO mutants were done in biological triplicates on glass slides from Agilent Technologies (www.agilent.com) containing sixty-mer oligonucleotides for the 5283 predicted P. berghei transcripts as described in Supplementary Online Material of reference [9]. Transcripts were tested for differential abundance through competitive hybridization of WT vs. CITH-KO labeled RNAs. Significance of expression was determined using TIGR MIDAS and MeV software and a LOWESS normalization method (p value <.05). Genes found differentially expressed in both wild type vs. DOZI-KO and wild type vs. CITH-KO and with a fold change cut-off of 2, were clustered using a Euclidean distance matrix of log2 ratio of genes for each condition. The heat map was drawn using the gplots package of R/Bioconductor [52] with up-regulated genes in the wild type parasites in red and down regulated genes in the wild type parasites in blue.
Oligonucleotide primers
For primers used in the generation of plasmid vectors, templates for probes for Northern and Southern blots, RT-PCR, please refer to Table S6.
Parasite lines used and generated in this study
Please refer to Table S7 and www.pberghei.eu.
Supporting Information
Zdroje
1. StitzelML
SeydouxG
2007 Regulation of the oocyte-to-zygote transition. Science 316 407 408
2. LiangHL
NienCY
LiuHY
MetzsteinMM
KirovN
2008 The zinc-finger protein Zelda is a key activator of the early zygotic genome in Drosophila. Nature 456 400 403
3. AudhyaA
HyndmanF
McLeodIX
MaddoxAS
YatesJR3rd
2005 A complex containing the Sm protein CAR-1 and the RNA helicase CGH-1 is required for embryonic cytokinesis in Caenorhabditis elegans. J Cell Biol 171 267 279
4. BoagPR
AtalayA
RobidaS
ReinkeV
BlackwellTK
2008 Protection of specific maternal messenger RNAs by the P body protein CGH-1 (Dhh1/RCK) during Caenorhabditis elegans oogenesis. J Cell Biol 182 543 557
5. BoagPR
NakamuraA
BlackwellTK
2005 A conserved RNA-protein complex component involved in physiological germline apoptosis regulation in C. elegans. Development 132 4975 4986
6. SquirrellJM
EggersZT
LuedkeN
SaariB
GrimsonA
2006 CAR-1, a protein that localizes with the mRNA decapping component DCAP-1, is required for cytokinesis and ER organization in Caenorhabditis elegans embryos. Mol Biol Cell 17 336 344
7. WilhelmJE
BuszczakM
SaylesS
2005 Efficient protein trafficking requires trailer hitch, a component of a ribonucleoprotein complex localized to the ER in Drosophila. Dev Cell 9 675 685
8. KhanSM
Franke-FayardB
MairGR
LasonderE
JanseCJ
2005 Proteome analysis of separated male and female gametocytes reveals novel sex-specific Plasmodium biology. Cell 121 675 687
9. MairGR
BraksJA
GarverLS
WiegantJC
HallN
2006 Regulation of sexual development of Plasmodium by translational repression. Science 313 667 669
10. ShawMK
ThompsonJ
SindenRE
1996 Localization of ribosomal RNA and Pbs21-mRNA in the sexual stages of Plasmodium berghei using electron microscope in situ hybridization. Eur J Cell Biol 71 270 276
11. ThompsonJ
SindenRE
1994 In situ detection of Pbs21 mRNA during sexual development of Plasmodium berghei. Mol Biochem Parasitol 68 189 196
12. VervenneRA
DirksRW
RamesarJ
WatersAP
JanseCJ
1994 Differential expression in blood stages of the gene coding for the 21-kilodalton surface protein of ookinetes of Plasmodium berghei as detected by RNA in situ hybridisation. Mol Biochem Parasitol 68 259 266
13. HallN
KarrasM
RaineJD
CarltonJM
KooijTW
2005 A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science 307 82 86
14. BraksJA
MairGR
Franke-FayardB
JanseCJ
WatersAP
2008 A conserved U-rich RNA region implicated in regulation of translation in Plasmodium female gametocytes. Nucleic Acids Res 36 1176 1186
15. TritschlerF
EulalioA
HelmsS
SchmidtS
ColesM
2008 Similar modes of interaction enable Trailer Hitch and EDC3 to associate with DCP1 and Me31B in distinct protein complexes. Mol Cell Biol 28 6695 6708
16. AndersonP
KedershaN
2008 Stress granules: the Tao of RNA triage. Trends Biochem Sci 33 141 150
17. ChekulaevaM
HentzeMW
EphrussiA
2006 Bruno acts as a dual repressor of oskar translation, promoting mRNA oligomerization and formation of silencing particles. Cell 124 521 533
18. KawaharaH
ImaiT
ImatakaH
TsujimotoM
MatsumotoK
2008 Neural RNA-binding protein Musashi1 inhibits translation initiation by competing with eIF4G for PABP. J Cell Biol 181 639 653
19. AravindL
IyerLM
AnantharamanV
2003 The two faces of Alba: the evolutionary connection between proteins participating in chromatin structure and RNA metabolism. Genome Biol 4 R64
20. LasonderE
JanseCJ
van GemertGJ
MairGR
VermuntAM
2008 Proteomic profiling of Plasmodium sporozoite maturation identifies new proteins essential for parasite development and infectivity. PLoS Pathog 4 e1000195 doi:10.1371/journal.ppat.1000195
21. JanseCJ
MonsB
RouwenhorstRJ
Van der KloosterPF
OverdulveJP
1985 In vitro formation of ookinetes and functional maturity of Plasmodium berghei gametocytes. Parasitology 91 (Pt1) 19 29
22. YudaM
IwanagaS
ShigenobuS
MairGR
JanseCJ
2009 Identification of a transcription factor in the mosquito-invasive stage of malaria parasites. Mol Microbiol 71 1402 1414
23. BalajiS
BabuMM
IyerLM
AravindL
2005 Discovery of the principal specific transcription factors of Apicomplexa and their implication for the evolution of the AP2-integrase DNA binding domains. Nucleic Acids Res 33 3994 4006
24. De SilvaEK
GehrkeAR
OlszewskiK
LeonI
ChahalJS
2008 Specific DNA-binding by apicomplexan AP2 transcription factors. Proc Natl Acad Sci U S A 105 8393 8398
25. PatonMG
BarkerGC
MatsuokaH
RamesarJ
JanseCJ
1993 Structure and expression of a post-transcriptionally regulated malaria gene encoding a surface protein from the sexual stages of Plasmodium berghei. Mol Biochem Parasitol 59 263 275
26. De RenzisS
ElementoO
TavazoieS
WieschausEF
2007 Unmasking activation of the zygotic genome using chromosomal deletions in the Drosophila embryo. PLoS Biol 5 e117 doi:10.1371/journal.pbio.0050117
27. GuoR
XueH
HuangL
2003 Ssh10b, a conserved thermophilic archaeal protein, binds RNA in vivo. Mol Microbiol 50 1605 1615
28. FetzerCP
HoganDJ
LippsHJ
2002 A PIWI homolog is one of the proteins expressed exclusively during macronuclear development in the ciliate Stylonychia lemnae. Nucleic Acids Res 30 4380 4386
29. StolcV
KatzA
AltmanS
1998 Rpp2, an essential protein subunit of nuclear RNase P, is required for processing of precursor tRNAs and 35S precursor rRNA in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 95 6716 6721
30. BellSD
BottingCH
WardleworthBN
JacksonSP
WhiteMF
2002 The interaction of Alba, a conserved archaeal chromatin protein, with Sir2 and its regulation by acetylation. Science 296 148 151
31. MarshVL
Peak-ChewSY
BellSD
2005 Sir2 and the acetyltransferase, Pat, regulate the archaeal chromatin protein, Alba. J Biol Chem 280 21122 21128
32. ChakrabartySP
SaikumariYK
BopannaMP
BalaramH
2008 Biochemical characterization of Plasmodium falciparum Sir2, a NAD+-dependent deacetylase. Mol Biochem Parasitol 158 139 151
33. FrenchJB
CenY
SauveAA
2008 Plasmodium falciparum Sir2 is an NAD+-dependent deacetylase and an acetyllysine-dependent and acetyllysine-independent NAD+ glycohydrolase. Biochemistry 47 10227 10239
34. Mancio-SilvaL
Rojas-MezaAP
VargasM
ScherfA
Hernandez-RivasR
2008 Differential association of Orc1 and Sir2 proteins to telomeric domains in Plasmodium falciparum. J Cell Sci 121 2046 2053
35. MerrickCJ
DuraisinghMT
2007 Plasmodium falciparum Sir2: an unusual sirtuin with dual histone deacetylase and ADP-ribosyltransferase activity. Eukaryot Cell 6 2081 2091
36. PrustyD
MehraP
SrivastavaS
ShivangeAV
GuptaA
2008 Nicotinamide inhibits Plasmodium falciparum Sir2 activity in vitro and parasite growth. FEMS Microbiol Lett 282 266 272
37. TonkinCJ
CarretCK
DuraisinghMT
VossTS
RalphSA
2009 Sir2 paralogues cooperate to regulate virulence genes and antigenic variation in Plasmodium falciparum. PLoS Biol 7 e84 doi:10.1371/journal.pbio.1000084
38. ChandranV
LuisiBF
2006 Recognition of enolase in the Escherichia coli RNA degradosome. J Mol Biol 358 8 15
39. LoSC
HanninkM
2008 PGAM5 tethers a ternary complex containing Keap1 and Nrf2 to mitochondria. Exp Cell Res 314 1789 1803
40. ShethU
ParkerR
2003 Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science 300 805 808
41. HammellCM
LubinI
BoagPR
BlackwellTK
AmbrosV
2009 nhl-2 Modulates microRNA activity in Caenorhabditis elegans. Cell 136 926 938
42. ParkerR
ShethU
2007 P bodies and the control of mRNA translation and degradation. Mol Cell 25 635 646
43. TomasAM
MargosG
DimopoulosG
van LinLH
de Koning-WardTF
2001 P25 and P28 proteins of the malaria ookinete surface have multiple and partially redundant functions. Embo J 20 3975 3983
44. RamjaneeS
RobertsonJS
Franke-FayardB
SinhaR
WatersAP
2007 The use of transgenic Plasmodium berghei expressing the Plasmodium vivax antigen P25 to determine the transmission-blocking activity of sera from malaria vaccine trials. Vaccine 25 886 894
45. SaxenaAK
WuY
GarbocziDN
2007 Plasmodium p25 and p28 surface proteins: potential transmission-blocking vaccines. Eukaryot Cell 6 1260 1265
46. BraksJA
Franke-FayardB
KroezeH
JanseCJ
WatersAP
2006 Development and application of a positive-negative selectable marker system for use in reverse genetics in Plasmodium. Nucleic Acids Res 34 e39
47. LavazecC
MoreiraCK
MairGR
WatersAP
JanseCJ
2009 Analysis of mutant Plasmodium berghei parasites lacking expression of multiple PbCCp genes. Mol Biochem Parasitol 163 1 7
48. SneeMJ
MacdonaldPM
2009 Bicaudal C and trailer hitch have similar roles in gurken mRNA localization and cytoskeletal organization. Dev Biol 328 434 444
49. van DijkMR
JanseCJ
ThompsonJ
WatersAP
BraksJA
2001 A central role for P48/45 in malaria parasite male gamete fertility. Cell 104 153 164
50. JanseCJ
Franke-FayardB
MairGR
RamesarJ
ThielC
2006 High efficiency transfection of Plasmodium berghei facilitates novel selection procedures. Mol Biochem Parasitol 145 60 70
51. BeetsmaAL
van de WielTJ
SauerweinRW
ElingWM
1998 Plasmodium berghei ANKA: purification of large numbers of infectious gametocytes. Exp Parasitol 88 69 72
52. GentlemanRC
CareyVJ
BatesDM
BolstadB
DettlingM
2004 Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5 R80
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 2- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Pathogen Entrapment by Transglutaminase—A Conserved Early Innate Immune Mechanism
- Broadly Protective Monoclonal Antibodies against H3 Influenza Viruses following Sequential Immunization with Different Hemagglutinins
- Neutrophil-Derived CCL3 Is Essential for the Rapid Recruitment of Dendritic Cells to the Site of Inoculation in Resistant Mice
- Differentiation, Distribution and γδ T Cell-Driven Regulation of IL-22-Producing T Cells in Tuberculosis
- IFN-α-Induced Upregulation of CCR5 Leads to Expanded HIV Tropism In Vivo
- An Extensive Circuitry for Cell Wall Regulation in
- TgMORN1 Is a Key Organizer for the Basal Complex of
- Direct Presentation Is Sufficient for an Efficient Anti-Viral CD8 T Cell Response
- Immunoelectron Microscopic Evidence for Tetherin/BST2 as the Physical Bridge between HIV-1 Virions and the Plasma Membrane
- A New Nuclear Function of the Glycolytic Enzyme Enolase: The Metabolic Regulation of Cytosine-5 Methyltransferase 2 (Dnmt2) Activity
- Genome-Wide mRNA Expression Correlates of Viral Control in CD4+ T-Cells from HIV-1-Infected Individuals
- Structural and Biochemical Characterization of SrcA, a Multi-Cargo Type III Secretion Chaperone in Required for Pathogenic Association with a Host
- A Major Role for the ApiAP2 Protein PfSIP2 in Chromosome End Biology
- HIV Controller CD4+ T Cells Respond to Minimal Amounts of Gag Antigen Due to High TCR Avidity
- Fis Is Essential for Capsule Production in and Regulates Expression of Other Important Virulence Factors
- Vaccinia Protein F12 Has Structural Similarity to Kinesin Light Chain and Contains a Motor Binding Motif Required for Virion Export
- A Novel Pseudopodial Component of the Dendritic Cell Anti-Fungal Response: The Fungipod
- Efficacy of the New Neuraminidase Inhibitor CS-8958 against H5N1 Influenza Viruses
- Long-Lived Antibody and B Cell Memory Responses to the Human Malaria Parasites, and
- IPS-1 Is Essential for the Control of West Nile Virus Infection and Immunity
- Transit through the Flea Vector Induces a Pretransmission Innate Immunity Resistance Phenotype in
- Ats-1 Is Imported into Host Cell Mitochondria and Interferes with Apoptosis Induction
- Six RNA Viruses and Forty-One Hosts: Viral Small RNAs and Modulation of Small RNA Repertoires in Vertebrate and Invertebrate Systems
- The Syk Kinase SmTK4 of Is Involved in the Regulation of Spermatogenesis and Oogenesis
- Optineurin Negatively Regulates the Induction of IFNβ in Response to RNA Virus Infection
- On the Diversity of Malaria Parasites in African Apes and the Origin of from Bonobos
- Five Questions about Viruses and MicroRNAs
- A Broad Distribution of the Alternative Oxidase in Microsporidian Parasites
- Caspase-1 Activation via Rho GTPases: A Common Theme in Mucosal Infections?
- Peptides Presented by HLA-E Molecules Are Targets for Human CD8 T-Cells with Cytotoxic as well as Regulatory Activity
- Interaction of Rim101 and Protein Kinase A Regulates Capsule
- Distinct External Signals Trigger Sequential Release of Apical Organelles during Erythrocyte Invasion by Malaria Parasites
- Exacerbated Innate Host Response to SARS-CoV in Aged Non-Human Primates
- Reverse Genetics in Predicts ARF Cycling Is Essential for Drug Resistance and Virulence
- Universal Features of Post-Transcriptional Gene Regulation Are Critical for Zygote Development
- Highly Differentiated, Resting Gn-Specific Memory CD8 T Cells Persist Years after Infection by Andes Hantavirus
- Arterivirus Nsp1 Modulates the Accumulation of Minus-Strand Templates to Control the Relative Abundance of Viral mRNAs
- Lethal Antibody Enhancement of Dengue Disease in Mice Is Prevented by Fc Modification
- Quantitative Comparison of HTLV-1 and HIV-1 Cell-to-Cell Infection with New Replication Dependent Vectors
- The Disulfide Bonds in Glycoprotein E2 of Hepatitis C Virus Reveal the Tertiary Organization of the Molecule
- IL-1β Processing in Host Defense: Beyond the Inflammasomes
- Kaposi's Sarcoma Associated Herpes Virus (KSHV) Induced COX-2: A Key Factor in Latency, Inflammation, Angiogenesis, Cell Survival and Invasion
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Caspase-1 Activation via Rho GTPases: A Common Theme in Mucosal Infections?
- Kaposi's Sarcoma Associated Herpes Virus (KSHV) Induced COX-2: A Key Factor in Latency, Inflammation, Angiogenesis, Cell Survival and Invasion
- IL-1β Processing in Host Defense: Beyond the Inflammasomes
- Reverse Genetics in Predicts ARF Cycling Is Essential for Drug Resistance and Virulence
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



