-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaGlacial Refugia in Pathogens: European Genetic Structure of Anther Smut Pathogens on and
Climate warming is predicted to increase the frequency of invasions by pathogens and to cause the large-scale redistribution of native host species, with dramatic consequences on the health of domesticated and wild populations of plants and animals. The study of historic range shifts in response to climate change, such as during interglacial cycles, can help in the prediction of the routes and dynamics of infectious diseases during the impending ecosystem changes. Here we studied the population structure in Europe of two Microbotryum species causing anther smut disease on the plants Silene latifolia and Silene dioica. Clustering analyses revealed the existence of genetically distinct groups for the pathogen on S. latifolia, providing a clear-cut example of European phylogeography reflecting recolonization from southern refugia after glaciation. The pathogen genetic structure was congruent with the genetic structure of its host species S. latifolia, suggesting dependence of the migration pathway of the anther smut fungus on its host. The fungus, however, appeared to have persisted in more numerous and smaller refugia than its host and to have experienced fewer events of large-scale dispersal. The anther smut pathogen on S. dioica also showed a strong phylogeographic structure that might be related to more northern glacial refugia. Differences in host ecology probably played a role in these differences in the pathogen population structure. Very high selfing rates were inferred in both fungal species, explaining the low levels of admixture between the genetic clusters. The systems studied here indicate that migration patterns caused by climate change can be expected to include pathogen invasions that follow the redistribution of their host species at continental scales, but also that the recolonization by pathogens is not simply a mirror of their hosts, even for obligate biotrophs, and that the ecology of hosts and pathogen mating systems likely affects recolonization patterns.
Published in the journal: . PLoS Pathog 6(12): e32767. doi:10.1371/journal.ppat.1001229
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001229Summary
Climate warming is predicted to increase the frequency of invasions by pathogens and to cause the large-scale redistribution of native host species, with dramatic consequences on the health of domesticated and wild populations of plants and animals. The study of historic range shifts in response to climate change, such as during interglacial cycles, can help in the prediction of the routes and dynamics of infectious diseases during the impending ecosystem changes. Here we studied the population structure in Europe of two Microbotryum species causing anther smut disease on the plants Silene latifolia and Silene dioica. Clustering analyses revealed the existence of genetically distinct groups for the pathogen on S. latifolia, providing a clear-cut example of European phylogeography reflecting recolonization from southern refugia after glaciation. The pathogen genetic structure was congruent with the genetic structure of its host species S. latifolia, suggesting dependence of the migration pathway of the anther smut fungus on its host. The fungus, however, appeared to have persisted in more numerous and smaller refugia than its host and to have experienced fewer events of large-scale dispersal. The anther smut pathogen on S. dioica also showed a strong phylogeographic structure that might be related to more northern glacial refugia. Differences in host ecology probably played a role in these differences in the pathogen population structure. Very high selfing rates were inferred in both fungal species, explaining the low levels of admixture between the genetic clusters. The systems studied here indicate that migration patterns caused by climate change can be expected to include pathogen invasions that follow the redistribution of their host species at continental scales, but also that the recolonization by pathogens is not simply a mirror of their hosts, even for obligate biotrophs, and that the ecology of hosts and pathogen mating systems likely affects recolonization patterns.
Introduction
Understanding the dynamics of emerging infectious diseases and their routes to colonize new geographic regions is a major challenge for ecologists in an effort to prevent negative impacts upon human, domestic, and natural populations. Because pathogens causing emerging diseases have not coevolved with the host or the ecosystem in which they emerged, they may be more likely to pose a threat to biodiversity through biomass loss and extinction of host species than those responsible for endemic diseases [1]. In recent years, concerns about emerging diseases have been increasing in light of the first evidence of a current period of global climate change. Indeed, the increase of average temperatures in many areas of the world is thought to promote the expansion of exotic pathogens [2]. In particular, invasion by fungal pathogens is a major concern in agricultural and biodiversity management, as they infect many crops and wild plants [3], [4]. About 30% of emerging infectious diseases of plants are caused by fungi, and the change of environmental conditions is thought to be the major driver of fungal invasions [1] and disease outbreaks [5]. Fungal disease outbreaks in humans have also been suggested to be linked to climate change [6], [7]. Warmer and wetter conditions favour the growth and transmission of fungal pathogens, and host shifts often occur in conjunction with episodes of global climate change [8]. Based on current trends, emerging infectious diseases caused by fungal pathogens are likely to increase in the near future, with significant severe ecological, economic and social consequences.
One way to predict the invasion routes and dynamics of emerging infectious diseases in response to current climate warming is to study the past migrations of pathogens and their hosts during historic periods of climate changes. During the last glacial maximum, the Arctic ice sheet extended into a large part of Europe and limited the survival of most organisms into Southern Mediterranean refugia (i.e., Iberia, Italy and the Balkans [9], [10]). As the climate warmed and the ice sheet retreated, many species that had persisted inside the glacial refugia experienced massive migrations into the newly available temperate territories [11]. Such processes are expected to lead to a strong, large-scale geographic structure of genetic variation, consistent with what is observed in many widespread European plants (e.g. [12]–[17]). Genetic differences between spreading populations are likely to result from both natural selection and stochastic processes in small populations. Successive founder events during the process of range expansion are expected to lead to a loss of variation and further divergence between lineages derived from different refugia [9], [18]–[21].
Few studies have investigated whether the population structures of pathogens have also been impacted by the glaciations in Europe (but see e.g. [22]–[25]). In the case of host-pathogen systems, comparative phylogeography can also provide insights into host and pathogen co-evolutionary histories and identify causal factors determining their combined distributions [26]–[28]. In fact, pathogen populations are often more differentiated than their hosts, and the study of pathogens can complement or improve our knowledge on the host population genetic structure [29]–[37]. The extent to which the phylogeographic structure of pathogen populations mirrors that of the host depends on the degree of specificity and the obligate nature of pathogenic interaction [38]. A significant co-structure between the populations of the host and the pathogen suggests that the distribution and migration of the host impose a major constraint on the distribution of the pathogen [8]. On the other hand, the absence of congruence in population structure is consistent with independent host and pathogen colonization routes. In addition to pathogen specialization, the hosts' niche breadth and demographic characteristics may affect the persistence of disease and opportunities for host range expansion during large-scale migrations that follow climate change [39]–[41]. Thus, an approach that integrates knowledge of host and pathogen biology is essential to many theoretical and applied issues related to disease emergence in response to climate change.
Microbotryum violaceum sensu lato is a species complex of basidiomycete fungi responsible for anther smut disease in many plants in the Caryophyllaceae. These fungi are obligate pathogens that sterilize their hosts. Infected plants contain fungal teliospores in place of the pollen and female structures do not mature; female plants in dioecious species also develop spore-bearing anthers. Teliospores are transmitted from diseased to healthy plants mostly by insects that normally serve as pollinators. Therefore, the dispersal routes of the host's pollen and of the pathogen's spores are constrained by the same vectors. Plants also disperse by seed, while the fungus is not vertically transmitted, resulting in higher genetic differentiation in the pathogen than in the host [42]. The sibling species encompassed in Microbotryum violaceum sensu lato [43]–[49] show strong host specificity [45], [47], [50]. The most widely studied species are Microbotryum lychnis-dioicae [51] (called MvSl in [46] and hereafter) and M. silenes-dioicae [51] (called MvSd in [46] and hereafter), which infect respectively Silene latifolia (white campion) and S. dioica (red campion). These two closely related host-pathogen systems are interesting models for studying the combined demographic histories of pathogens and their hosts because (i) populations of this pathogen are more differentiated than those of its hosts [42], (ii) the fungus is completely dependent on its hosts and the same vectors disperse the fungus and the host pollen, (iii) these Microbotryum species are highly host specific in the field ([52]), (iv) Silene and Microbotryum species have similar generation times (one per year [53], [54]), (v) there has been no attempt to control the disease because it affects plants without economic interest, and (vi) Silene and Microbotryum are model organisms for a variety of topics in ecology and evolution, with therefore numerous studies available on their life-history and ecology [55].
Recent studies on the phylogeographic history of the two host plants showed genetic evidence of post-glacial recolonization from Mediterranean refugia. In S. latifolia, analyses of chloroplast DNA (cpDNA) polymorphism showed clearly structured haplotype variation in Europe, with haplotypes from Eastern and Western Europe forming divergent groups descended from haplotypes currently distributed in southern Europe, and in particular from the Iberian and Balkan Peninsulas [56]. The phylogeography of S. dioica in Europe has been less well studied, although the pattern of cpDNA polymorphism was also suggestive of post-glacial recolonisation from multiple refugia [57], [58]. A goal of the current study was therefore to determine the extent to which population structure of Microbotryum species parasitizing S. latifolia and S. dioica showed similar patterns of post-glacial history. We also investigated whether life history differences between the two host species constrained the distributions of the pathogens within the host migration pathways. S. latifolia and S. dioica differ with respect to their ecologies, which might strongly impact the genetic structure and diversity of their specialized pathogens. S. latifolia has an extensive range and occurs in most of Europe, as well as in Middle Asia and the Steppe area of south Siberia [59]. This plant is found mainly in open areas, such as hedgerows and in arable fields, therefore often experiencing extinction-recolonisation events in frequently disturbed habitats. In contrast, the distribution of S. dioica covers mainly Central, Northern, and Western Europe [59], but not the Mediterranean regions. This plant is found in meadows, cliffs, moist forest, and mown pastures at higher elevations, preferring colder and more humid habitats than S. latifolia, and experiencing more stable population dynamics [59]–[62]. It has been suggested that these differences in host life history have affected the distribution of genetic diversity at a small geographical scale in the pathogen species, with lower microsatellite variation and higher differentiation among populations in the anther smut fungus parasitizing S. latifolia than in the fungus parasitizing S. dioica [63]. It is likely that the phylogeographic structure of the European populations of the two pathogen species will also be influenced by differences in the dynamics of the host-pathogen systems.
We therefore determined the population structures of the Microbotryum species infecting S. latifolia and S. dioica using microsatellite markers in order to address the following specific questions: (i) Do the phylogeographical structures of the Microbotryum species show signatures of post-glacial recolonisation of Europe, and in particular from Southern refugia? (ii) do the population structures of the two pathogens differ from each other, and if so, are these differences consistent with expectations based upon known ecological differences of their plant hosts? (iii) are the phylogeographic patterns of Microbotryum species comparable at a continental scale to those of their respective hosts?
Results
Genetic polymorphism in Microbotryum lychnidis-dioicae (MvSl) and M. silenes-dioicae (MvSd)
Among Microbotryum samples collected on S. latifolia and on S. doica across Europe (Figure S1), analysis of variation at 11 microsatellite markers revealed that both pathogen species displayed much lower levels of heterozygosity than expected under Hardy-Weinberg Equilibrium (HWE). The dataset included 701 MvSl individuals from 187 localities and 342 MvSd individuals from 68 localities, where hybrids and cross-species disease transmission between MvSl and MvSd identified in a previous study [52] were removed from the dataset. Descriptive statistics on the polymorphism of MvSd and MvSl and on deviations from HWE are shown in Table 1 and additional details are given in Text S1 and Tables S1 and S2. MvSl for instance exhibited only 3% of heterozygous genotypes while 73% were expected under HWE, which is consistent with the high selfing rates previously reported in Microbotryum. One marker, SL19, showed extreme FIS values in MvSl, between −1 and 1, and was almost fixed in the heterozygous state in MvSd (Table 1). Analyses were therefore performed with and without this marker for subsequent analyses, but the results were highly similar.
Tab. 1. Summary statistics on the 11 microsatellite loci in Microbotryum lychnidis-dioicae (MvSl) and Microbotryum silenes-dioicae (MvSd). 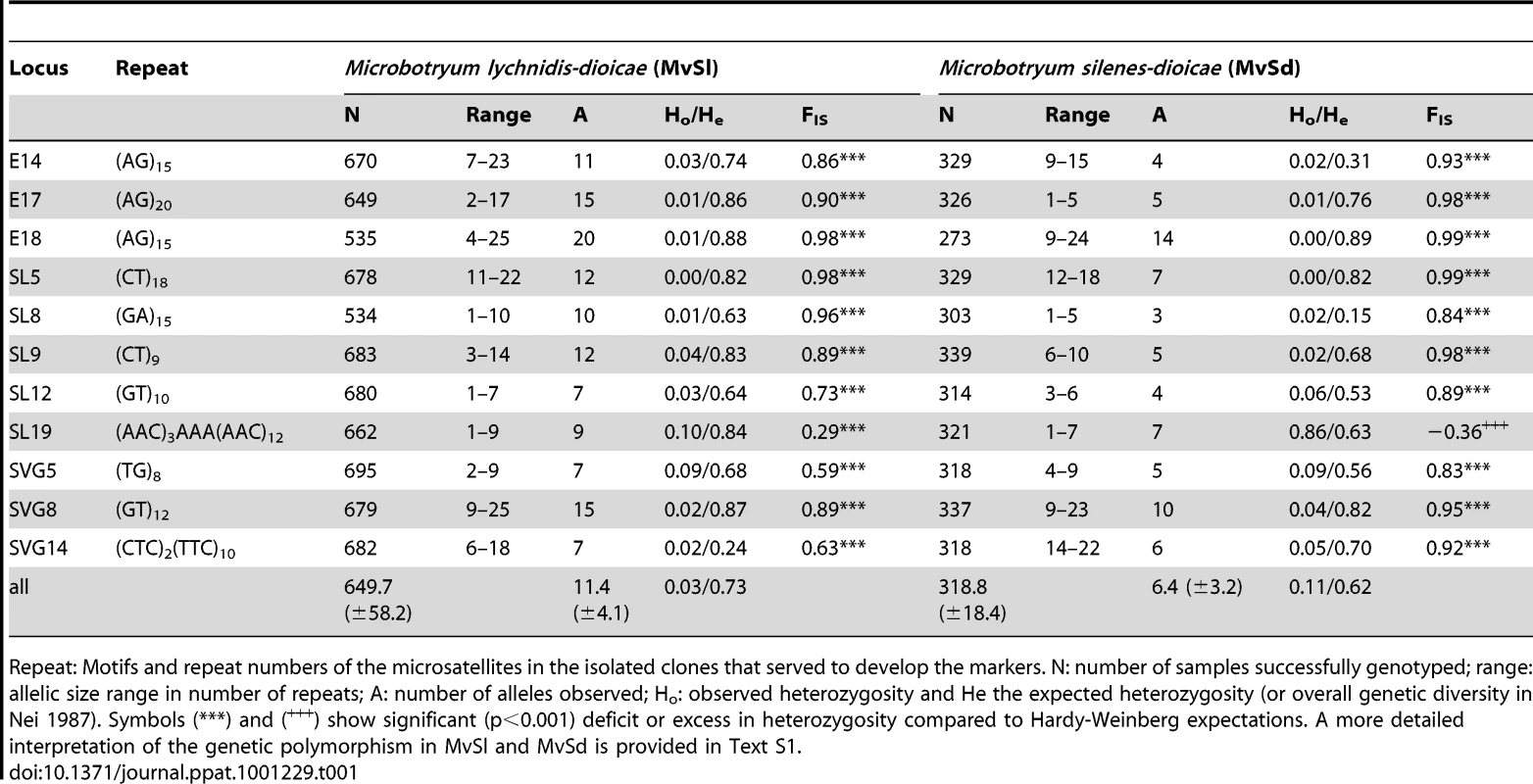
Repeat: Motifs and repeat numbers of the microsatellites in the isolated clones that served to develop the markers. N: number of samples successfully genotyped; range: allelic size range in number of repeats; A: number of alleles observed; Ho: observed heterozygosity and He the expected heterozygosity (or overall genetic diversity in Nei 1987). Symbols (***) and (+++) show significant (p<0.001) deficit or excess in heterozygosity compared to Hardy-Weinberg expectations. A more detailed interpretation of the genetic polymorphism in MvSl and MvSd is provided in Text S1. Genetic structure and genetic diversity in M. lychnidis-dioicae (MvSl)
For MvSl, multiple estimates of the genetic structure at the European scale showed the existence of at least three to five strongly supported clusters, i.e. populations genetically differentiated from each other. We used the model-based Bayesian clustering algorithms implemented in STRUCTURE, InStruct and TESS. The program STRUCTURE assumes a model with K clusters, each of which being characterized by a set of allelic frequencies. Assuming HWE and linkage equilibrium among loci within clusters, the program estimates allelic frequencies in each cluster and the proportion of ancestry from the different clusters in each individual. The program InStruct is an extension of the approach implemented in STRUCTURE, relaxing the assumption of HWE within clusters. InStruct instead jointly estimates selfing rates and individual membership on the basis of selfing rates, and is therefore well suited to selfing organisms such as Microbotryum. TESS is another extension of STRUCTURE, incorporating a spatial component into the clustering algorithm, so that geographically closer individuals are a priori more likely to belong to the same cluster. This may help revealing subtle geographical structure [64]. We attempted to identify the number of clusters (K) that best described the population structure using (1) the probability of the data under the considered value of K, i.e. Ln(Pr(X|K)), and its rate of change when increasing K; and (2) the Deviation Index Criterion (DIC), i.e. a model-complexity penalized measure of how well the model fits the data.
For MvSl, the programs STRUCTURE and InStruct showed that values of DIC decreased and LnP(X|K) increased from K = 1 to 10 (Figures S2a–c), indicating that increasing the number of clusters continuously improved the fit of the model to the data. However, the variation in LnP(X|K) showed a marked break at K = 5 in STRUCTURE analyses, with a much weaker increase of probability with increasing K afterwards (Figures S2a and b). The inclusion of space in the clustering modelling, as implemented in TESS 2.3, resulted in minimal DIC values at K = 5 and K = 6 (Figure S2d). Increasing K above 5 may therefore add little information for understanding large-scale population structure in Europe, although it would likely reveal a genuine population structure, relevant at smaller scale.
The admixture proportion (α) between clusters was low, as shown by the mean α = 0.033±0.000 over all the runs between K = 2 to 15 (10 replicates for each K) in the STRUCTURE analysis. This indicates that most of the genotypes are drawn from a single cluster, with little admixture among clusters (see Figure S3 for K = 5). There is therefore almost complete lack of gene flow among clusters.
Replicates conducted for each of the three algorithms showed dominant and minor modal solutions for membership probabilities (Figure S4). However, the dominant clustering solutions recovered from the three analyses (InStruct, STRUCTURE and TESS) were highly similar (see Figure S3 for K = 5). The three methods were therefore congruent in their inference of the population structure of MvSl in Europe. The differences between the dominant and minor modal solutions most often corresponded to a genetic structure appearing at higher K values (Figure S4). For instance, the Italian cluster was assigned to the Eastern group at K = 2 in the dominant solution, and to the Western group in other simulations.
Figure 1 shows the maps of mean membership probabilities per locality for MvSl genotypes from the InStruct analysis for K = 2 to 5. At K = 2, the analyses revealed a clear West-East partitioning. Simulation of a third cluster separated the Italian genotypes from the Eastern group. At K = 4, the Western cluster was subdivided into two clusters, one with a more northern distribution (blue, called hereafter Northwestern and abbreviated as Nwestern) and the other more to the south (yellow, called hereafter Southwestern and abbreviated as Swestern). At K = 5, the Eastern group splitted into two clusters, one bordering the Balkan peninsula (red, called hereafter the Balkan cluster) and one spreading toward Eastern Europe and Russia (purple, called hereafter the Eastern cluster). When increasing K, further clusters were identified, without evidence of admixture, and corresponding to more local geographical regions: for instance the UK became isolated, and then the most eastern part of Europe (Figure S4).
Fig. 1. Maps of mean membership probabilities per localities from the InStruct analysis for <i>Microbotryum lychnidis-dioicae</i> (MvSl) assuming 2 to 5 clusters. 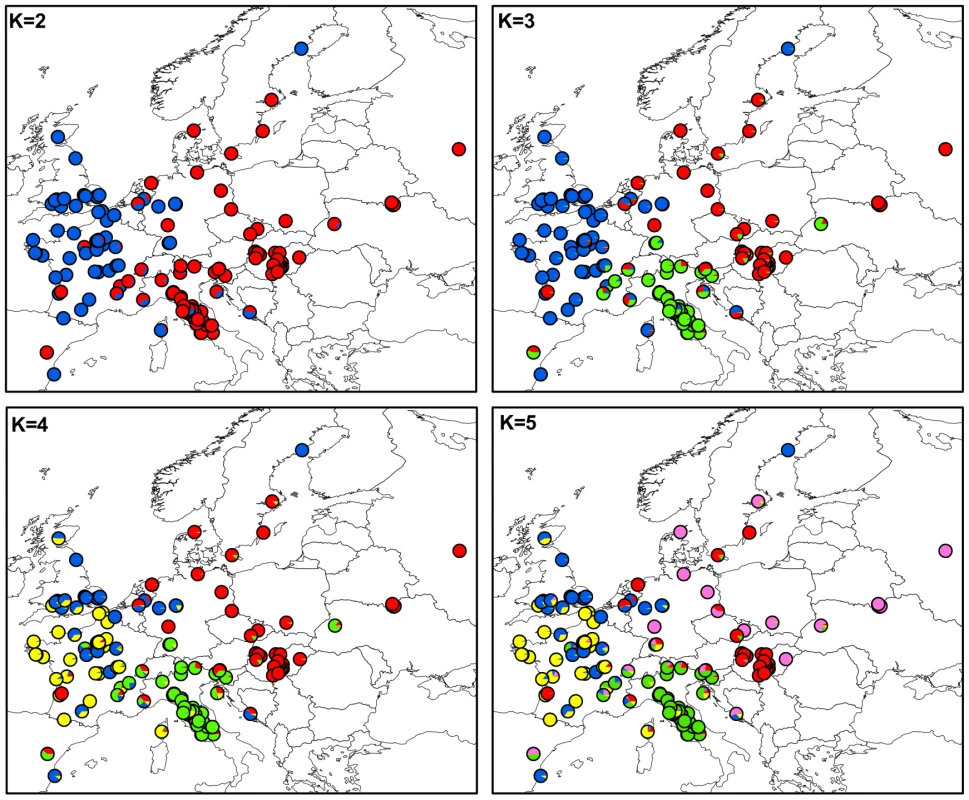
We also applied a Principal Component Analysis (PCA) on the microsatellite allele frequencies, which is a multivariate approach that does not rely on any model assumptions. It instead transforms a number of possibly correlated variables into a smaller number of uncorrelated components, the first principal components accounting for as much variability in the data as possible. The PCA fully recovered the population structure inferred by the three Bayesian clustering methods, as shown by the first four PCs, which explained 35% of the total variance in allelic frequencies (Figures S5 and S6).
The clusters displayed large and significant differences in allelic frequencies (global FST = 0.38, 95% CI: [0.29–0.46], P<0.001 for all pairs of clusters). The Nwestern and Swestern clusters showed the lowest differentiation (FST = 0.24) while the Balkan and Swestern clusters were the most different (FST = 0.47). The clusters also differed significantly with respect to their genetic diversities (Table 2), with the Italian cluster displaying significantly higher gene diversity (He) than the Balkan, Eastern and Nwestern clusters (Wilcoxon Signed Rank (WSR) tests, P = 0.008, P = 0.033, and P = 0.026, respectively, Table 2). The Balkan cluster exhibited a significantly lower allelic richness (number of alleles controlling for differences in sample size) than the Eastern and Italian clusters (P = 0.016 and P = 0.003, respectively), while the others had intermediate values.
Tab. 2. Genetic polymorphism and spatial pattern within each cluster of Microbotryum lychnidis-dioicae (MvSl) and Microbotryum silenes-dioicae (MvSd). 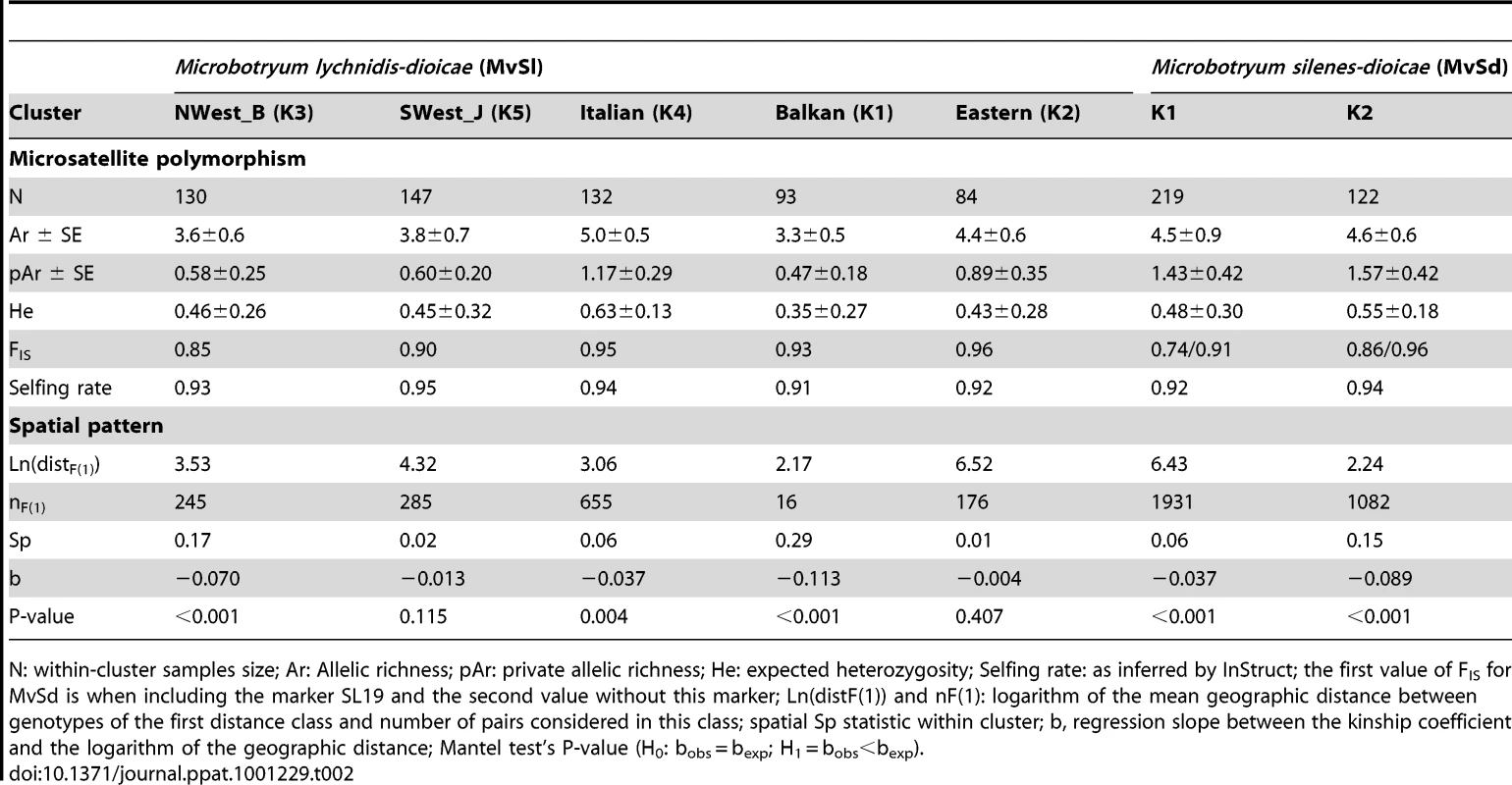
N: within-cluster samples size; Ar: Allelic richness; pAr: private allelic richness; He: expected heterozygosity; Selfing rate: as inferred by InStruct; the first value of FIS for MvSd is when including the marker SL19 and the second value without this marker; Ln(distF(1)) and nF(1): logarithm of the mean geographic distance between genotypes of the first distance class and number of pairs considered in this class; spatial Sp statistic within cluster; b, regression slope between the kinship coefficient and the logarithm of the geographic distance; Mantel test's P-value (H0: bobs = bexp; H1 = bobs<bexp). Within clusters, significant isolation by distance (IBD) was detected in the Balkan, Nwestern, and Italian clusters (Table 2), indicating that genetic differentiation increased with geographic distance in these clusters. No significant IBD was detected within the Swestern and Eastern clusters (Table 2). The level of spatial structure was quantified by the Sp statistic, which accounts for variation in sampling intensities; high values of Sp are indicative of low population density and/or limited dispersal [65]. Sp values were close to 0 within the Italian, Swestern, and Eastern clusters, but were much higher within the Balkan and Nwestern clusters (Table 2).
Within-cluster selfing rates estimated from InStruct analyses were extremely high (s = 0.91±0.03 on average), in agreement with previous studies and with the high FIS values within clusters (Table 2).
A European spatial map of genetic diversity was generated by aggregating geographically close samples together on a grid, considering only grid cells where the sample size was higher than 4. The interpolated values of allelic richness showed that genetic diversity increased in the southward direction, with the highest value observed in the Italian and Iberian peninsulas and the lowest values in northern Europe (Figure 2). Such a latitudinal trend was confirmed by the highly significant negative correlation observed between latitude and allelic richness (r = −0.57, P<0.0001); no significant correlation was found between longitude and allelic richness (r = 0.036, P = 0.892). High genetic diversities were also observed in the northern half of France and along a longitudinal line separating the Eastern and Western parts of Europe (Figure 2).
Fig. 2. Map of allelic richness overall loci. 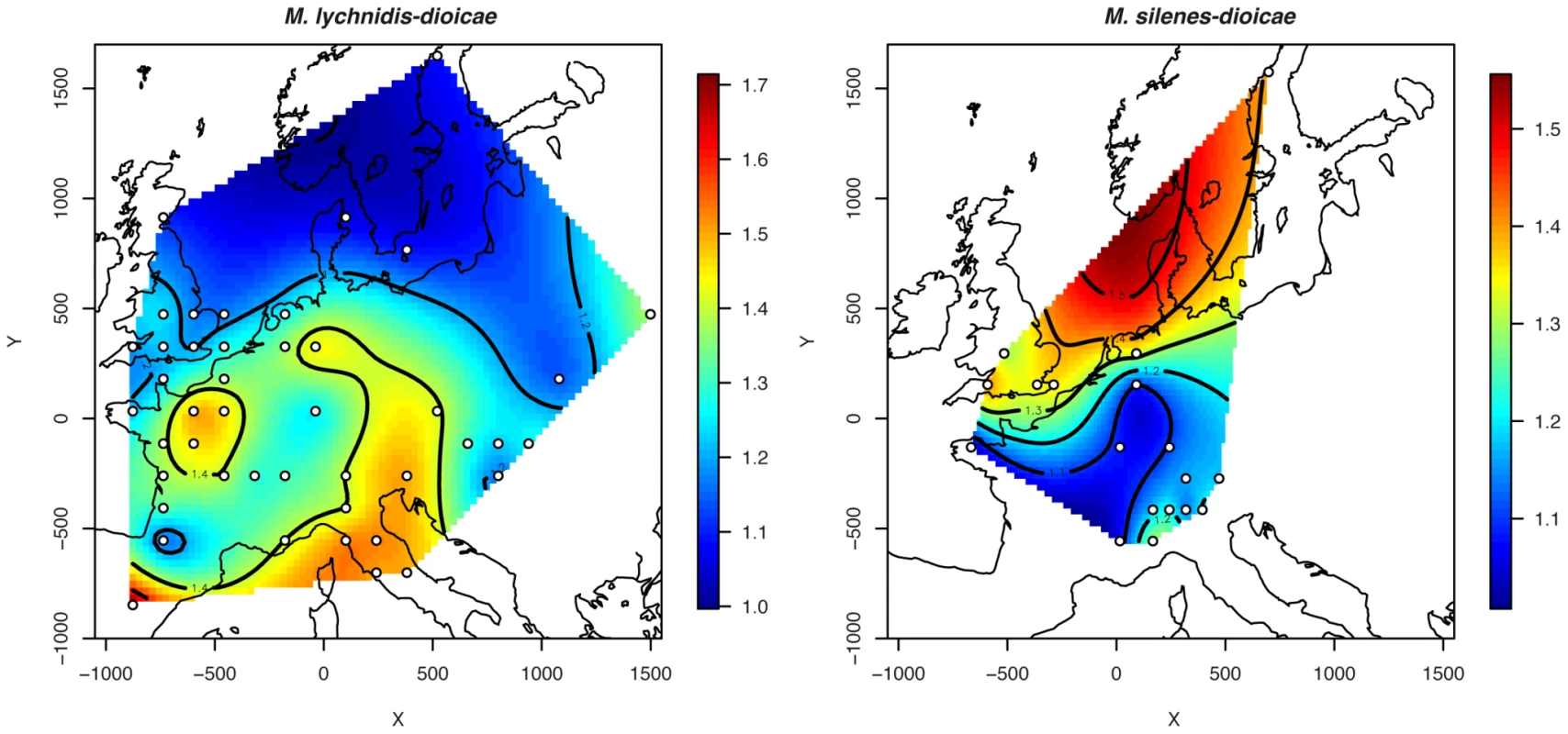
Samples have been aggregated on a grid in order to make this calculation on a minimum sample size of at least 4 genotypes. Localities where the sample size was below this threshold were not considered. We analyzed the relationships among clusters using neighbour-joining population trees, respectively based on Nei's DA distance, shared allele distance DSA, Chord's distance and Goldstein's (δµ)2 distance. As the different trees provided similar topologies, only the tree based on Nei's DA distance is presented (Figure 3). The trees suggested that the Eastern groups would have diverged first, followed by the Italian cluster and then by the Western groups. The Eastern and Western group would then have further split into two clusters each. Rough estimate of separation time between clusters can be deduced from distances between clusters assuming that the divergence between the two species occurred 400,000 yr BP [52] and assuming clocklike evolution of microsatellite markers [66]. The separation of the 5 clusters can thus be roughly estimated to have occurred between 200,000 and 350,000 yr BP (Figure 3).
Fig. 3. Microsatellite distance-based Neighbour-Joining trees on intraspecific clusters for both Microbotryum lychnidis-dioicae (MvSl) and M. silenes-dioicae (MvSd). 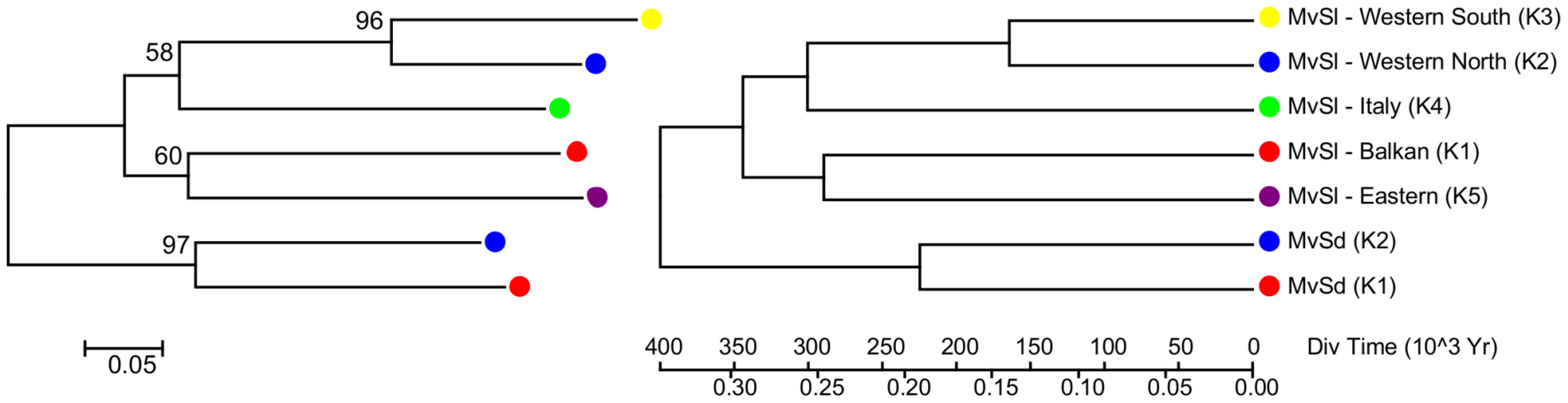
The root of the trees was placed on the branch separating the two species. The bootstrap values above 50% are shown. Genetic structure, genetic diversity and selfing rates in M. silenes-dioicae (MvSd)
In MvSd, multiple estimates of the genetic structure at the European scale provided confidence in existence of several distinct clusters. As for MvSl, the three Bayesian clustering analyses (InStruct, STRUCTURE and TESS) all indicated that DIC decreased and LnP(X|K) increased with increasing K (Figure S7). Again, genotype assignment probabilities were always very high, with very little admixture among clusters (mean α = 0.030±0.002 over the 140 runs from K = 2 to 15 simulated clusters; Figure S8), and were similar for the three algorithms used (data not shown). The spatial distributions of the two clusters identified at K = 2 appeared highly intermingled, with however a slight West-East trend of separation. Further clusters differentiated as K increased, but without any obvious large-scale geographical pattern (Figure 4). Similar genetic partitioning was recovered using PCA (Figure S9). The first PC accounted for 20% of the variance in the allelic frequencies and clearly separated genotypes into the same two groups as those identified using Bayesian clustering approaches at K = 2 (Figure S9). The differences in allelic frequencies between them were high, with a FST value of 0.34 (95% CI: 0.17–0.49). The successive PCs accounted for less than 11% of the total variance in allelic frequencies each and revealed the same clusters of genotypes as those observed in Bayesian clustering analyses (Figure S9).
Fig. 4. Maps of mean membership probabilities per locality from the InStruct analysis for <i>Microbotryum silenes-dioicae</i> (MvSd), assuming 2 to 5 clusters. 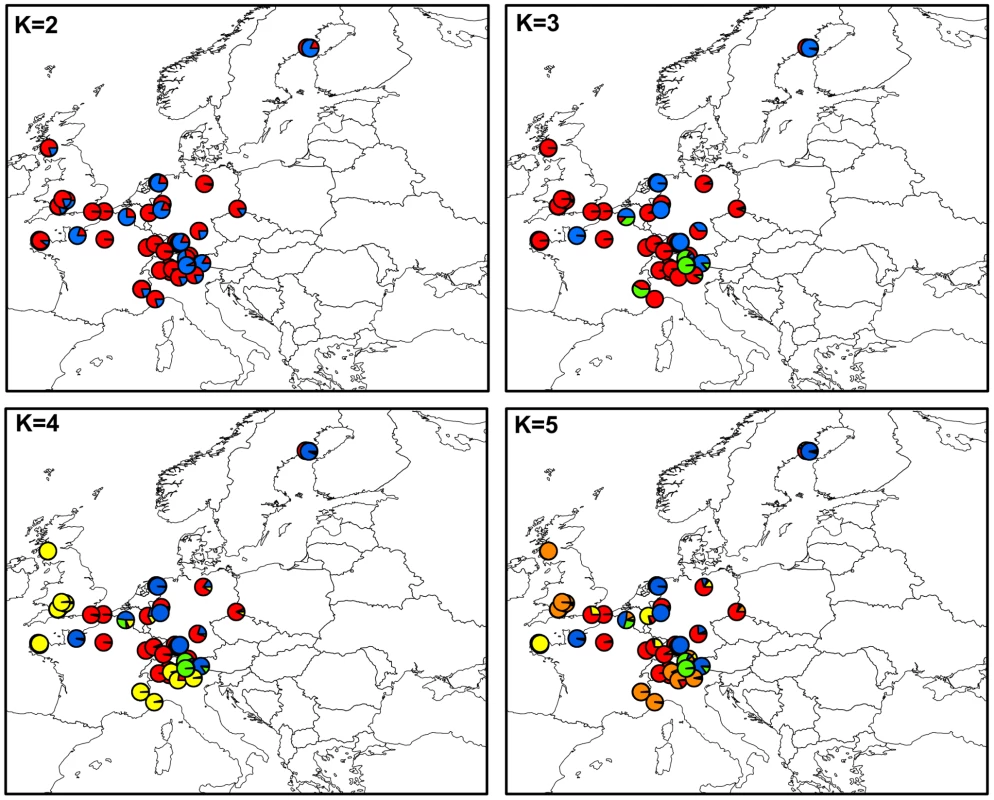
The two clusters identified at K = 2 represented the only structure with a large-scale geographical pattern. The FST values between sites where the sampling was higher or equal to 10 samples showed that a very high level of genetic differentiation between clusters was observed, even in the regions where populations from different clusters were intermingled (Figure S10). Within clusters, there was no significant IBD, i.e. no significant increase in FST with geographic distance (Mantel test, P = 0.418). The two clusters showed a spatial genetic structure of a similar level to that observed in MvSl, with Sp values of 0.06 and 0.15 for the clusters 1 and 2, respectively (Table 2).
Selfing rates within each of the two clusters, inferred from Instruct, were high and similar to those in MvSl (mean selfing rates at K = 2 : 0.93±0.01), consistent with the high FIS values (Table 2). The two clusters of MvSd showed genetic diversities comparable to those observed in MvSl (Table 2), and did not differ significantly from each other with respect to gene diversity (WSR test, P = 0.424) and allelic richness (WSR test, P = 0.594). In contrast to MvSl, spatially interpolated values of allelic richness increased northwards (Figure 2), although the correlation with latitude was significant only at a marginal level (r = 0.43, P = 0.073). The correlation with longitude was not significant (r = −0.008, P = 0.975).
Discussion
Mating system and local population structure
Very high selfing rates were inferred in both Microbotryum species (s = 0.91 for MvSl and s = 0.93 for MvSd), in agreement with the high deficits in heterozygotes (Text S1, Table 1). Microbotryum species are in fact known to have a selfing mating system [67], [68], but the estimations of selfing rates in natural populations inferred here are more precise and even higher than previously thought [67]. These high selfing rates appear to result both from an intrinsic preference for intra-tetrad mating [69] and from lack of outcrossing opportunities when the spores are deposited on a new host plant. The lack of outcrossing opportunity is supported by the observation that selfing rates under choice experiments (when given the opportunity to self or outcross on plants) are lower (ca. 0.70 [70]) than those inferred here in natural populations.
The marker SL19 showed extreme FIS values in MvSl and was almost fixed in the heterozygous state in MvSd. This was not particularly surprising given that Microbotryum species undergo mostly intra-tetrad mating, which can lead to an excess in heterozygosity in regions of the genome near the centromeres and on the sex chromosomes carrying the mating type locus [69], [71]. Because the mating type segregates at the first meiosis division, intra-tetrad mating automatically restores heterozygosity in all regions linked to centromeres and linked to the mating type locus, as they also segregate at the first meiotic division [69], [71].
In addition to selfing, the study of local genetic structure and diversity for both Microbotryum species across Europe revealed patterns consistent with the dynamics of a metapopulation [72]. In particular, we observed very low genetic diversity within demes, and strong differences in allele frequencies (high FST values) between demes (see text S1). Metapopulation dynamics involve frequent extinctions and recolonizations, thus creating strong genetic drift in local populations. In addition, selfing reduces the local effective population size and the frequency of gene exchange between individuals and populations, which reinforces the effects of genetic drift upon allelic frequencies [72], [73]. These results are consistent with previous population genetics and demographic studies conducted at more local scales on Microbotryum species infecting S. latifolia and S. dioica, also showing patterns consistent with metapopulation dynamics [42], [74]–[77].
Genetic structure shaped by multiple glacial refugia
From a biogeographic point of view, Europe is a large peninsula with an East-West orientation, delimited in the south by a strong barrier, the Mediterranean Sea. During glaciation epochs, many species likely went through alternating contractions and expansions of range, involving extinctions of northern populations when the ice-sheet extended southward, and spread of the southern populations from different refugial areas as the glaciation receded. Such colonization processes were likely characterized by recurrent bottlenecks that would have led to lower diversity in the northern populations compared with the southern refugia [21]. The idea that refugia were localized in three areas (Iberia, Italy, Balkans) in Europe is now well-established [10], [11], [15], [17], although increasing evidence suggest that northern and eastern refugia also existed [78]–[84].
Microbotryum lychnidis-dioicae (MvSl)
In MvSl, the strong phylogeographic structure observed at the European scale was composed of at least three genetic clusters with distributions strikingly similar to the major glacial refugia commonly recognized to have existed in Europe for many plant and animal temperate species (e.g., [14]). This pattern suggests that the pathogen likely colonized Northern Europe from at least the three main Mediterranean refugia (Iberian, Italian and Balkan).
The scenario may have been more complex, however, as the Eastern and Western clusters each further split into two groups, with divergence times of the five clusters roughly estimated between 200,000 and 350,000 yr BP. One of the eastern clusters was located north of the Balkans (mainly in Hungary and Czech Republic) and the other from Germany eastward. This pattern is consistent with colonization from distinct refugia located in the Balkans and further East in Eurasia, following a similar scenario as those reported in some animals, such as the bear Ursus actor [10], the vole Myodes glareolus [79], [80], some plant species [78], [85], [86], and also in some pathogens (e.g., [22]–[25]).
The two clusters identified in Western Europe had more diffuse geographic distributions, with a slight longitudinal partitioning. One of the clusters was distributed more towards the West of France while the second was more present in east-central France and in Eastern UK. Such a pattern may be due to the pathogen having survived in distinct regional refugia in Western Europe, from which they would have expanded their range over France and UK. Such a hypothesis is consistent with recent findings that the main glacial refugia in Europe were probably not composed of a single population, but instead could have been structured into several local refugia more or less isolated from one another (see the concept of “refugia within refugia” [87]–[91]).
While the north-south gradient in genetic diversity can be taken as a sign of range expansion from southern glacial refugia, a band of high genetic diversity was observed north of Italy and extending into Germany, as well as a hotspot of diversity in the centre of France. These areas of high of genetic diversity likely come from the colonization history of Europe by the different genetic clusters, establishing suture zones where genetic clusters meet and become intermingled. Such a pattern has been observed previously in a comparative approach of the history of colonization of 22 widespread and co-distributed European trees and shrubs [92], where hotspots of genetic diversity in the colonised ranges were found to be the result of mixed colonization from genetically isolated eastern and western European refugia [92]. The high genetic diversity found in MvSl in the Iberian peninsula also likely results from the co-occurrence of genotypes from four clusters.
Previous studies indicated that the phylogeographic pattern of the host plant S. latifolia similarly showed genetic evidence of post-glacial recolonization from Mediterranean refugia [56]. Analyses of cpDNA haplotypes revealed clear biogeographic structure in Europe, with haplotypes from Eastern and Western Europe forming divergent groups descending from haplotypes currently distributed in Iberian and Balkan Peninsulas [56]. The phylogeographic patterns in the plant S. latifolia and in its anther smut pathogen therefore seem to be congruent. In particular, the Eastern and Western clades identified in the host could correspond to the Eastern and Western genetic clusters in the pathogen MvSl. The pathogen however seems to display a finer genetic structure than its host, with particularly clear genetic evidence of an Italian glacial refugium for the pathogen, but not for the host plant (see figure 4 in [56]). More pronounced geographic structure is in fact expected in anther smut pathogens compared to their hosts, as has been observed at smaller scales [42]. This can be explained by the following observations: 1) the distribution of the pathogen is necessarily embedded within the range of its host, 2) anther smut pathogens are dispersed by the same vectors as the pollen of the plants, without being dispersed by seeds, so that their dispersal ability is lower than that of their host plants [42]. It is therefore likely that MvSl persisted in more fragmented refugia compared with its host. This highlights the potential use of pathogens as proxies for understanding host past migrations and distributions: the finding of distinct clusters in Italy and the Balkan in MvSl reveals that S. latifolia persisted in both these refugia during last glaciations, which was not obvious based solely on our current knowledge of the plant's phylogeograpy. However, statistically explicit comparative analyses linking the host and pathogen genetic polymorphisms, using comparable genetic markers, would be required to draw firm conclusions regarding correlations between the biogeographic structure of S. latifolia and MvSl (e.g. [93]).
Microbotryum silenes-dioicae (MvSd)
The anther smut pathogen MvSd also exhibited a strong genetic structure, albeit with biogeographic patterns more difficult to interpret. The two main clusters had largely intermingled distributions, with an estimated time of divergence of the same order of magnitude as observed for MvSl. The distinct clusters in MvSd could correspond to genetic groups having diverged in distinct southern refugia during the glaciations, similar to MvSl, although the locations of the putative refugia are more difficult to identify. This may be due to the restricted distribution of MvSd, constrained by ecological specificities of the host and disease: the plant S. dioica is very rare in Mediterranean regions, and even more so the disease (we did not find anther smut symptoms on any S. dioica plant in the Pyrenees despite several years of searching). On the other hand, given the more northern current distribution of the plant S. dioica compared to S. latifolia, one can alternatively speculate that its tolerance to cold temperatures [62] might have allowed the host and the disease to remain in more northern refugia, as suggested for other species adapted to cold environments [84]. This could provide an explanation of the marginally significant increase in allelic richness with latitude in MvSd, although we cannot rule out that this pattern resulted from the co-occurrence of a greater number of different clusters in the north.
The phylogeography of the host plant S. dioica based on cpDNA RFLP similarly indicated the existence of genetically distinct groups, more or less longitudinally separated, albeit with large overlap in their ranges [58]. The distribution of the common cpDNA haplotypes was suggested to result from a post-glacial expansion of S. dioica across Europe from multiple southern refugia [57], [58]. However the absence of sampling from Mediterranean peninsulas in prior studies prevents any definitive conclusion regarding the number and location of these refugia. In addition, the geographic distribution of the shared haplotypes in S. dioica and S. latifolia was consistent with a history of hybridization and introgression events, making it difficult to assess whether the present distribution of these haplotypes resulted from the recolonization history of S. dioica or S. latifolia [58].
Lack of gene flow among clusters and lack of IBD
A striking pattern observed in both Microbotryum species was the low level of admixture among genetic clusters (≤3%), suggesting almost complete lack of gene flow, despite the existence of contact zones. Such low levels of gene flow among clusters are likely influenced by the very high selfing rates in Microbotryum. High selfing rates have been invoked to explain reproductive isolation between sympatric Microbotryum species [67], [94], and there could be a similar effect in keeping the genetic clusters distinct within species. The high selfing rates also explain why increasing the number of clusters in Bayesian analyses always increased the explanatory value in describing the population genetic structure, without the appearance of admixed clusters, even for very high K values: this is because each diploid individual mostly reproduces with itself and therefore the smallest ‘panmictic unit’ may indeed be the individual. In selfing species, the genetic structure extends to a much finer scale than in outcrossing species [32], [95]. The lack of gene flow among clusters may result in addition to metapopulation dynamics and rapid expansion during post-glacial recolonization. A theoretical study [96] indeed showed that rapid growth in population size after founding events resulted in gene frequency divergence that is resistant to decay by gene exchange.
Inference for climate change and emerging infectious diseases
Large-scale congruence between the pathogens' phylogeographic patterns and those of their respective hosts indicates that their glacial refugia and migration pathways during recolonization have been similar. While this may be expected for obligate pathogens like Microbotryum species, highly dependent on their hosts for survival and using the same dispersal vectors, we interestingly found that the pathogens likely subsisted during glaciations in a more fragmented distribution, with their genetic diversity divided among a higher number of smaller refugia. Moreover, the extent of large-scale dispersal across Europe after recolonization was less for the pathogen than for its host: in particular, the clusters were much more clumped in MvSl than in S. latifolia, and footprints of refugia appeared in MvSl that were absent in S. latifolia, such as the Italian peninsula. Our findings thus indicate that vector-borne, obligate pathogens may colonize new areas following climate warming with some delay compared to their hosts, and to a lesser extent. The invasive potential of pathogens following climate change is therefore likely to depend on the obligate nature of the interactions with their host and on the dispersal modes, as could be expected.
However, once the original host and its fungal pathogen invade a geographic region, the pathogen poses a risk of emerging as an infectious disease on new host species found in that area. For instance, MvSl was introduced in the United States some time after its host plant S. latifolia, and has remained in a much more restricted geographic area [97]. Cross-species disease transmission was nevertheless documented in the United States to another non-native species, S. vulgaris, that is otherwise free of anther smut disease in the continent [98]. In Europe, we have previously detected rare events of cross-species disease transmission between S. dioica and S. latifolia and of hybridization between MvSl and MvSd that occurred after secondary contact [52]. Host shifts are frequent in fungal pathogens [4], [99], [100] in particular in Microbotryum, where co-phylogenetic analyses showed that speciation events were most often associated with host shifts [47]. This suggests that climate warming may cause emerging infectious diseases, by resulting in contacts between different potential hosts that were allopatric, even when the intrinsic dispersal capacity of the pathogens is limited and their migration pathways are constrained by those of their hosts. Climate warming can also bring into contact differentiated populations from the same species, promoting introgression between previously geographically isolated populations, which can have important and unpredictable evolutionary consequences. We showed in the present study that secondary contact between genetically differentiated clusters happened after the glaciations in MvSl, and that the highly selfing mating system was here important in preventing introgression.
The substantive contrast in phylogeography for the anther smut fungi on S. latifolia and S. dioica, which may be attributed to differences in the hosts' ecology, is also relevant for predicting the fate of infectious diseases following global warming. The redistribution of pathogens under warmer climatic conditions should indeed be highly dependent on the hosts' ecological preferences and adaptive potentials, in particular regarding the temperature and competition in new ecological communities [39]–[41].
Conclusion
In this study, we showed that high selfing rates and metapopulation dynamics in two plant pathogenic fungi had strong impact on their genetic diversity and structure. At the scale of the species' distribution ranges, the population structures in the two fungal species were quite different, likely due to differences in the ecological preferences of the two host-pathogen systems. The broadly distributed S. latifolia and its anther smut pathogen have kept clear genetic footprints of postglacial colonization from the major southern European refugia. The pathogens showed striking evidence for more numerous and localized refugia than their hosts. On the other hand, the ecological preference of the plant S. dioica for wetter and colder habitats [62] probably led to a more restricted and more northern distribution of the plant and its anther smut pathogen, and may have induced a drastic contraction of their distribution ranges with the post-glacial warming and the fragmentation of suitable habitat conditions. The European genetic structures of the anther smut fungi seem to match those of their respective hosts, with even a finer genetic structure, so that the geographic distribution of genetic variation in the pathogens may be useful to draw inferences about host phylogeography.
Beyond the interest of our study for understanding the dynamics of diseases under climate warming and the impact of host life histories on the genetic structure of pathogens, our study illustrates several important points to take into account when performing clustering genetic analyses, which are still often poorly recognized. First, several K values are often interesting to consider in clustering analyses, and it may be non-heuristic to search for a “single optimal” number of clusters. As long as increasing K does not lead to admixed clusters, the new clusters revealed by increasing K probably reveal a genuine genetic structure that may be interesting to investigate. This appears especially true in selfing species, for which the smallest panmictic cluster may be the individual.
Materials and Methods
Teliospore collection and populations
The individuals of Microbotryum analyzed in this study were collected as diploid teliospores from 187 localities on S. latifolia (n = 701) and 68 localities on S. dioica (n = 342) across Europe (Figure S10) and stored in silica gel (see Figure S1 for a detailed description of the sampling). DNA from teliospores of one flower per diseased plant was extracted for genetic analyses. Multiple infections by different genotypes are frequent in the Silene-Microbotryum system, but teliospores within a single flower originate from a single diploid individual [101].
Microsatellite genotyping
DNA was extracted as described in [77]. Teliospores were genotyped using 11 microsatellite markers following the protocol of [77] (Table 1). Among the 11 microsatellite loci used, E14, E17, E18 were described in [102], SL8, SL9, SL12, SL19, SVG5, SVG8, SVG14 described in [103], and SL5 was described in [104].
Data analyses
Sample handling
Hybridization between MvSl and MvSd has been suggested previously [105]. However, we showed recently in a study investigating the divergence process between the two species using the same dataset as in the present work that hybridization was very rare in natural populations [52]. Therefore, we investigated here the genetic polymorphism and population structure in MvSl and MvSd separately, after having removed the few inter-specific hybrids and cross-disease species transmissions from the datasets (see [52] for more details).
Descriptive statistics
The within-species genetic polymorphism at each locus was quantified using the allelic richness (Ar), the observed and unbiased expected heterozygosities (Ho and He), and the fixation index (FIS). These statistics were calculated using FSTAT 2.9.3.2 [106]. Departure from Hardy-Weinberg expectations was tested using exact tests implemented in GENEPOP 4.0 [107], [108].
Linkage disequilibrium (LD) among loci was quantified using the correlation coefficient (r2) calculated in Genetix 4.05 [109]. In order to assess the impact of mating system on the genetic structure, we calculated r2 within sampled localities that contained at least 10 genotypes, i.e. 15 out of 187 localities for MvSl (n = 210, Figure S11) and 13 out of 68 localities for MvSd (n = 197, Figure S12). Genotypic disequilibrium was tested using permutation test implemented in FSTAT 2.9.3.2. The nominal P-value of 0.05 was adjusted for multiple comparisons using a Bonferroni correction (i.e. 6.1×10−5, based on 82,500 permutations).
Population structure
We investigated population structure in MvSl and MvSd using two kinds of analyses: Bayesian model-based clustering and principal component analyses. We applied three variants of Bayesian model-based clustering algorithms. The first one, implemented in STRUCTURE 2.3 [110], [111], partitions multilocus genotypes into clusters while minimizing departure from Hardy Weinberg and linkage equilibrium (HWLE) among loci [110], [111]. The second one, implemented in the InStruct software, is a variant of the previous one specifically suited to selfing organisms [112], such as Microbotryum species (e.g. [69]–[71], [77]). InStruct relaxes the assumption of Hardy-Weinberg equilibrium through the calculation of the expected genotype frequencies on the basis of selfing rates (jointly inferred from the data) rather than as simple products of allele frequencies [112]. The third one implemented in TESS 2.3.1 [64], [113], [114] differs from the STRUCTURE algorithm in including spatially explicit prior distributions describing which sets of individuals are likely to have similar cluster membership [113]. In this approach, clusters correspond to spatially and genetically continuous units separated by small discontinuities that occur where genetic barriers are crossed. The incorporation of a spatial component into the clustering model has the potential to determine if clines provide a sensible description of the underlying pattern of variation [64].
For both STRUCTURE and TESS algorithms, we used a haploid setting because both species of Microbotryum are almost completely homozygous (Table 1). Run conditions for STRUCTURE analyses were as follows: we conducted a series of independent runs with different proposals for the number of clusters (K), testing all values from 1 to 15. Each run used 500,000 iterations after a burn-in of 250,000 iterations, using a model allowing for admixture and correlated allele frequencies. To ensure convergence of the MCMC, we performed 10 independent replicates for each value of K and checked the consistency of results visually and using the procedure implemented in the program CLUMPP 1.1.1 [115]. We used CLUMPP 1.1.1 to account for label switching. We also used CLUMPP 1.1.1 to compute with the Greedy algorithm the symmetric similarity coefficient between pairs of runs (100 random input sequences, G' statistic), in order to identify potential distinct modes among the results of independent replicate runs for each K value and to average individual assignment probabilities (q) over replicated runs showing a similar mode. Barplots were generated by the DISTRUCT program [116]. We attempted to identify the number(s) of clusters (K) that best explained the data using the probability of the data under the considered value of K, i.e. Ln(Pr(X|K)) where X are the data (Ln(D) in [117]) and its rate of change when increasing K.
InStruct run conditions were as follows: we used diploid data to jointly estimate the selfing rate within each clusters and the individual assignment probability (q) to each cluster [112]. We ran 10 independent replicates for each K, testing all values between 1 and 10. Each MCMC chain used 1,000,000 iterations after a burn-in of 200,000 iterations and a thinning interval of 50. The convergence of each run was tested using CLUMPP in a similar way to that described above for the STRUCTURE analysis, and barplots were produced using DISTRUCT. InStruct does not estimate Ln(Pr(X|K)), but it computes instead a Deviation Index Criterion (DIC), i.e. a model-complexity penalized measure of how well the model fits the data [118].
TESS run conditions were as follows: we used the conditional auto-regressive (CAR) Gaussian model of admixture with linear trend surface [64], and set the admixture parameter to α = 1 and the interaction parameter ρ = 0.6 as starting values and subsequently updated. The algorithm was run with a burn-in period of length 20,000 cycles, and estimation was performed using 30,000 additional cycles. We increased the maximal number of clusters from Kmax = 2 to Kmax = 15 (10 replicates for each value). For Kmax = 5, we performed 50 additional longer runs (burn-in 30×103, run-length 100×103, ρ = 0.6, α = 1), and we averaged the estimated admixture coefficients (Q matrix) over the 5 runs with the smallest values of the DIC (DIC = 14 819, s.d. = 9). To account for label switching among runs, we used the software CLUMPP version 1.1, whose greedy algorithm computed a symmetric similarity coefficient equal to 0.85 (100 random input sequences, G' statistic).
Finally, we applied a centred-normed Principal Component Analysis (PCA) on the microsatellite allele frequencies for each species to corroborate the clustering solution inferred by the three Bayesian clustering algorithms using a multivariate approach that does not rely on any model assumption. We conducted the analyses using ADEgenet [119] and ADE4 packages [120] in the R-environment [121].
We represented the relationships among clusters using neighbour-joining population trees, respectively based on Nei's DA distance [122], shared allele distance DSA [123], [124], Chord's distance [125] and Goldstein's (δµ)2 distance, the latter one assuming that microsatellite evolve following a Stepwise Mutation Model (SMM) model [126]. The computation of distance matrix and of bootstrapped distances was performed using PowerMarker [127], and the consensus tree was obtained and plotted using MEGA 4 [128]. As the different trees provided similar topologies, only the tree based on the Da distance of Nei et al. [122] is presented. This distance was found to perform comparatively well in estimation of population trees from microsatellite allele frequency data [129]. The root was placed between the two species.
Spatial pattern in genetic diversity
Allelic richness and private allelic richness of each genetic cluster or combination of clusters identified in the Bayesian clustering analysis were calculating using ADZE 1.0 [130]. The expected heterozygosity (He) was calculated using FSTAT 2.9.3. Variation in allelic richness across space was analysed by aggregating samples according to a grid system whose mesh size was chosen in order to have at least 4 samples per cell (132×139 km). This cut-off value was chosen as a trade-off between the sample size per cell and its spatial distribution. This led for MvSl to 66 cells, out of which 47 contained at least 4 samples, and for MvSd to 31 cells, out of which 19 had at least 4 samples. Spatial interpolation was performed using a thin plate spline method, with a smoothing parameter of λ = 0.005, as implemented in the R package ‘fields’ [121].
Isolation by distance (IBD) analyses
We used pairwise kinship coefficients between individuals (Fij) (Loiselle et al. 1995) to test for isolation by distance, as recommended for highly selfing species [65]. We calculated the average kinship coefficient for different intervals of distance ranges (10, 100, 500, 1500 and 5000 km), the slope of the correlation coefficient between individual kinship coefficients and the logarithm of geographic distance, and the significance of the slope (10,000 permutations of localities) using the software SPAGeDi 1.3 [131]. When the slope was significant, we calculated the ‘Sp’ statistic, which is the ratiowhere is the slope of the correlation coefficient and is the mean Fij between individuals belonging to a first distance interval that includes all pairs of neighbours (in our case, this distance interval corresponds to a local population). Under some assumptions, the Sp statistic is equal to the inverse of 4πDσ2 (with D: effective population density and σ2: average squared axial parent-offspring distance), which matches Wright's neighbourhood size, under the hypothesis of Gaussian dispersal functions. Therefore, high values of the Sp statistic are indicative of low population density or limited dispersal ability.
Supporting Information
Zdroje
1. AndersonPK
CunninghamAA
PatelNG
MoralesFJ
EpsteinPR
2004 Emerging infectious diseases of plants: pathogen pollution, climate change and agrotechnology drivers. Trends Ecol Evol 19 535 544
2. HarvellCD
MitchellCE
WardJR
AltizerS
DobsonAP
2002 Climate warming and disease risks for terrestrial and marine biota. Science 296 2158 2162
3. Desprez-LoustauM
RobinC
BueeM
CourtecuisseR
GarbayeJ
2007 The fungal dimension of biological invasions. Trends Ecol Evol 22 472 480
4. GiraudT
GladieuxP
GavriletsS
2010 Linking the emergence of fungal plant diseases with ecological speciation. Trends Ecol Evol 25 387 395
5. FisherMC
KoenigGL
WhiteTJ
TaylorJW
2000 Pathogenic clones versus environmentally driven population increase: Analysis of an epidemic of the human fungal pathogen Coccidioides immitis. J Clin Microbiol 38 807 813
6. KiddSE
HagenF
TscharkeRL
HuynhM
BartlettKH
2004 A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc Natl Acad Sci USA 101 17258 17263
7. DattaK
BartlettKH
BaerR
ByrnesE
GalanisE
2009 Spread of Cryptococcus gattii into Pacific Northwest Region of the United States. Emerg Infect Dis 15 1185 1191
8. BrooksDR
HobergEP
2007 How will global climate change affect parasite-host assemblages? Trends Parasitol 23 571 574
9. HewittGM
1996 Some genetic consequences of ice ages, and their role in divergence and speciation. Biol J Linn Soc 58 247 276
10. HewittGM
2004 Genetic consequences of climatic oscillations in the Quaternary. Phil Trans R Soc London Biol 359 183 195
11. HewittG
2003 Ice ages: their impact on species distributions and evolution.
RotschildL
ListerA
Evolution on Planet Earth Academic press 339 361
12. KingRA
FerrisC
1998 Chloroplast DNA phylogeography of Alnus glutinosa (L.) Gaertn. Mol Ecol 7 1151 1161
13. TaberletP
FumagalliL
Wust-SaucyAG
CossonJF
1998 Comparative phylogeography and postglacial colonization routes in Europe. Mol Ecol 7 453 464
14. HewittG
2000 The genetic legacy of quaternary ice ages. Nature 405 907 913
15. PalmeAE
VendraminGG
2002 Chloroplast DNA variation, postglacial recolonization and hybridization in hazel, Corylus avellana. Mol Ecol 11 1769 1780
16. PetitRJ
BrewerS
BordacsS
BurgK
CheddadiR
2002 Identification of refugia and post-glacial colonisation routes of European white oaks based on chloroplast DNA and fossil pollen evidence. Forest Ecol Manag 156 49 74
17. FrancoisO
BlumMGB
JakobssonM
RosenbergNA
2008 Demographic history of European populations of Arabidopsis thaliana. PLoS Genet 4 e1000075
18. IbrahimKM
NicholsRA
HewittGM
1996 Spatial patterns of genetic variation generated by different forms of dispersal during range expansion. Heredity 77 282 291
19. PetitRJ
PineauE
DemesureB
BacilieriR
DucoussoA
1997 Chloroplast DNA footprints of postglacial recolonization by oaks. Proc Natl Acad Sci USA 94 9996 10001
20. WidmerA
LexerC
2001 Glacial refugia: sanctuaries for allelic richness, but not for gene diversity. Trends Ecol Evol 16 267 269
21. ExcoffierL
FollM
PetitRJ
2008 Genetic Consequences of Range Expansions. Ann Rev Ecol Syst 40 481 501
22. NieberdingCM
Durette-DessetMC
VanderpoortenA
CasanovaJC
RibasA
2008 Geography and host biogeography matter for understanding the phylogeography of a parasite. Mol Phylogenet Evol 47 538 554
23. RichKA
ThompsonJN
FernandezCC
2008 Diverse historical processes shape deep phylogeographical divergence in the pollinating seed parasite Greya politella. Mol Ecol 17 2430 2448
24. AmicoGC
NickrentDL
2009 Population structure and phylogeography of the mistletoes Tristerix corymbosus and T. aphyllus (Loranthaceae) using chloroplast DNA sequence variation. Am J Bot 96 1571 1580
25. NakaoM
XiaoN
OkamotoM
YanagidaT
SakoY
2009 Geographic pattern of genetic variation in the fox tapeworm Echinococcus multilocularis. Parasitol Int 58 384 389
26. ThrallPH
BurdonJJ
1999 The spatial scale of pathogen dispersal: Consequences for disease dynamics and persistence. Evol Ecol Res 1 681 701
27. GrenfellBT
PybusOG
GogJR
WoodJLN
DalyJM
2004 Unifying the epidemiological and evolutionary dynamics of pathogens. Science 303 327 332
28. MiuraO
TorchinME
KurisAM
HechingerRF
ChibaS
2006 Introduced cryptic species of parasites exhibit different invasion pathways. Proc Natl Acad Sci USA 103 19818 19823
29. ReedDL
SmithVS
HammondSL
RogersAR
ClaytonDH
2004 Genetic analysis of lice supports direct contact between modern and archaic humans. PLoS Biol 2 e340
30. WhitemanNK
ParkerPG
2005 Using parasites to infer host population history: a new rationale for parasite conservation. Animal Conservation 8 175 181
31. WirthT
MeyerA
AchtmanM
2005 Deciphering host migrations and origins by means of their microbes. Mol Ecol 14 3289 3306
32. CriscioneCD
CooperB
BlouinMS
2006 Parasite genotypes identify source populations of migratory fish more accurately than fish genotypes. Ecology 87 823 828
33. NieberdingCM
OlivieriI
2007 Parasites: proxies for host genealogy and ecology? Trends Ecol Evol 22 156 165
34. WhitemanNK
KimballRT
ParkerPG
2007 Co-phylogeography and comparative population genetics of the threatened Galapagos hawk and three ectoparasite species: ecology shapes population histories within parasite communities. Mol Ecol 16 4759 4773
35. BlakesleeAMH
ByersJE
LesserMP
2008 Solving cryptogenic histories using host and parasite molecular genetics: the resolution of Littorina littorea's North American origin. Mol Ecol 17 3684 3696
36. BruyndonckxN
IsabelleH
PhilippeC
GeraldK
2009 Spatio-temporal population genetic structure of the parasitic mite Spinturnix bechstein is shaped by its own demography and the social system of its bat host. Mol Ecol 18 3581 3592
37. BruyndonckxN
BiollazF
DubeyS
GoudetJ
ChristeP
2010 Mites as biological tags of their hosts. Mol Ecol 19 2770 2778
38. BarrettLG
ThrallPH
BurdonJJ
LindeCC
2008 Life history determines genetic structure and evolutionary potential of host-parasite interactions. Trends Ecol Evol 23 678 685
39. HoodME
Mena-AlíJI
GibsonAK
OxelmanB
GiraudT
2010 Distribution of the anther-smut pathogen Microbotryum on species of the Caryophyllaceae. New Phytologist 187 217 229
40. FargetteD
KonateG
FauquetC
MullerE
PeterschmittM
2006 Molecular ecology and emergence of tropical plant viruses. Ann Rev Phytopathol 44 235 260
41. WilsonDJ
FalushD
McVeanG
2005 Germs, genomes and genealogies. Trends Ecol Evol 20 39 45
42. DelmotteF
BucheliE
ShykoffJA
1999 Host and parasite population structure in a natural plant-pathogen system. Heredity 82 300 308
43. LutzM
GokerM
PiatekM
KemlerM
BegerowD
2005 Anther smuts of Caryophyllaceae: molecular characters indicate host-dependent species delimitation. Mycol Prog 4 225 238
44. KemlerM
GokerM
OberwinklerF
BegerowD
2006 Implications of molecular characters for the phylogeny of the Microbotryaceae (Basidiomycota: Urediniomycetes). BMC Evol Biol 6 35
45. Le GacM
HoodME
GiraudT
2007 Evolution of reproductive isolation within a parasitic fungal complex. Evolution 61 1781 1787
46. Le GacM
HoodME
FournierE
GiraudT
2007 Phylogenetic evidence of host-specific cryptic species in the anther smut fungus. Evolution 61 15 26
47. RefrégierG
Le GacM
JabbourF
WidmerA
HoodME
2008 Cophylogeny of the anther smut fungi and their Caryophyllaceous hosts: Prevalence of host shifts and Importance of delimiting parasite species. BMC Evol Biol 8 100
48. SloanD
GiraudT
HoodM
2008 Maximized virulence in a sterilizing pathogen: the anther-smut fungus and its co-evolved hosts. J Evol Biol 21 1544 1554
49. de VienneDM
RefrégierG
HoodM
GuigueA
DevierB
2009 Hybrid sterility and inviability in the parasitic fungal species complex Microbotryum. J Evol Biol 22 683 698
50. de VienneDM
HoodME
GiraudT
2009 Phylogenetic determinants of potential host shifts in fungal pathogens. J Evol Biol 22 2532 2541
51. DenchevC
GiraudT
HoodM
2009 Three new species of anthericolous smut fungi on Caryophyllaceae. Mycologia Balcanica 6 79 84
52. GladieuxP
VerckenE
FontaineM
HoodME
JonotO
2010 Maintenance of fungal pathogen species that are specialized to different hosts: allopatric divergence and introgression through secondary contact. Mol Biol Evol. In press
53. ThrallP
JaroszA
1994 Host-pathogen dynamics in experimental populations of Silene alba and Ustilago violacea. II. Experimental tests on theoretical models. J Ecol 82 561 570
54. ThrallP
JaroszA
1994 Host-pathogen dynamics in experimental populations of Silene alba and Ustilago violacea. I. Ecological and genetic determinants of disease spread. J Ecol 82 549 559
55. BernasconiG
AntonovicsJ
BiereA
CharlesworthD
DelphL
2009 Silene as a model system in ecology and evolution. Heredity 103 5 14
56. TaylorD
KellerS
2007 Historical range expansion determined the phylogenetic diversity introduced during contemporary species invasion. Evolution 61 334 345
57. HathawayL
MalmJU
PrenticeHC
2009 Geographically congruent large-scale patterns of plastid haplotype variation in the European herbs Silene dioica and S. latifolia (Caryophyllaceae). Bot J Linn Soc 161 153 170
58. PrenticeHC
MalmJU
HathawayL
2008 Chloroplast DNA variation in the European herb Silene dioica (red campion): postglacial migration and interspecific introgression. Plant Syst Evol 272 23 37
59. FriedrichH
1979 Caryophyllaceae.
RechingerKH
Illustrierte Flora von Mitteleuropa Hamburg Parey 763 1182
60. BakerHG
1947 Melandrium album (Mill) Garcke. J Ecol 35 274 282
61. BakerHG
1947 Melandrium dioicum (L. Emend) Coss. & Germ. J Ecol 35 283 292
62. KarrenbergS
FavreA
2008 Genetic and ecological differenciation in hybridizing campions Silene dioica and S. latifolia. Evolution 62 763 773
63. BucheliE
GautschiB
ShykoffJA
2001 Differences in population structure of the anther smut fungus Microbotryum violaceum on two closely related host species, Silene latifolia and S. dioica. Mol Ecol 10 285 294
64. DurandE
JayF
GaggiottiOE
FrancoisO
2009 Spatial Inference of Admixture Proportions and Secondary Contact Zones. Mol Biol Evol 26 1963 1973
65. VekemansX
HardyOJ
2004 New insights from fine-scale spatial genetic structure analyses in plant populations. Mol Ecol 13 921 35
66. SunJX
MullikinJC
PattersonN
ReichDE
2009 Microsatellites Are Molecular Clocks That Support Accurate Inferences about History. Mol Biol Evol 26 1017 1027
67. GiraudT
YocktengR
Lopez-VillavicencioM
RefregierG
HoodME
2008 Mating system of the anther smut fungus, Microbotryum violaceum: selfing under heterothallism. Eukaryotic Cell 7 765 775
68. GranbergA
Carlsson-GranerU
ArnqvistP
GilesBE
2008 Variation in breeding system traits within and among populations of Microbotryum violaceum on Silene dioica. Int J Plant Sci 169 293 303
69. HoodME
AntonovicsJ
2000 Intratetrad mating, heterozygosity, and the maintenance of deleterious alleles in Microbotryum violaceum ( = Ustilago violacea). Heredity 85 231 241
70. GiraudT
JonotO
ShykoffJA
2005 Selfing propensity under choice conditions in a parasitic fungus, Microbotryum violaceum, and parameters influencing infection success in artificial inoculations. Int J Plant Sci 166 649 657
71. HoodME
AntonovicsJA
2004 Mating within the meiotic tetrad and the maintenance of genomic heterozygosity. Genetics 166 1751 1759
72. CharlesworthD
2003 Effects of inbreeding on the genetic diversity of populations. Phil Trans R Soc London Biol 358 1051 1070
73. PannellJR
CharlesworthB
2000 Effects of metapopulation processes on measures of genetic diversity. Phil Trans R Soc London Biol 355 1851 1864
74. ThrallPH
AntonovicsJ
1995 Theoretical and empirical studies of metapopulations: population and genetic dynamics of the Silene - Ustilago system. Can J Bot 73 S1249 S1258
75. GilesBE
GoudetJ
1997 Genetic differentiation in Silene dioica metapopulations: Estimation of spatiotemporal effects in a successional plant species. Am Nat 149 507 526
76. AntonovicsJ
2004 Long term study of a plant-pathogen metapopulation.
HanskiI
GaggiottiO
Ecology, Genetics, and Evolution of Metapopulations Amsterdam Academic Press 471 488
77. GiraudT
2004 Patterns of within population dispersal and mating of the fungus Microbotryum violaceum parasitising the plant Silene latifolia. Heredity 93 559 65
78. WillisKJ
van AndelTH
2004 Trees or no trees? The environments of central and eastern Europe during the Last Glaciation. Quat Sci Rev 23 2369 2387
79. DeffontaineV
LiboisR
KotlikP
SommerR
NieberdingC
2005 Beyond the Mediterranean peninsulas: evidence of central European glacial refugia for a temperate forest mammal species, the bank vole (Clethrionomys glareolus). Mol Ecol 14 1727 39
80. KotlikP
DeffontaineV
MascherettiS
ZimaJ
MichauxJ
2006 A northern glacial refugium for bank voles (Clethrionomys glareolus). Proc Natl Acad Sci USA 103 14860 14864
81. BhagwatSA
WillisKJ
2008 Species persistence in northerly glacial refugia of Europe: a matter of chance or biogeographical traits? J Biogeography 35 464 482
82. ProvanJ
BennettK
2008 Phylogeographic insights into cryptic glacial refugia. Trends Ecol Evol 23 564 571
83. BinneyHA
WillisKJ
EdwardsME
BhagwatSA
AndersonPM
2009 The distribution of late-Quaternary woody taxa in northern Eurasia: evidence from a new macrofossil database. Quat Sci Rev 28 2445 2464
84. StewartJR
ListerAM
BarnesI
DalenL
2010 Refugia revisited: individualistic responses of species in space and time. Proc R Soc B 277 661 671
85. WillisKJ
NiklasKJ
2004 The role of Quaternary environmental change in plant macroevolution: the exception or the rule? Phil Trans R Soc London Biol 359 159 172
86. AspockH
2008 Postglacial formation and fluctuations of the biodiversity of Central Europe in the light of climate change. Parasitol Res 103 S7 S10
87. GómezA
LuntD
2007 Refugia within refugia: Patterns of phylogeographic concordance in the Iberian Peninsula.
WeissS
FerrandN
Phylogeography of Southern European Refugia Dordrecht, The Netherlands Springer 155 188
88. UrsenbacherS
SchweigerS
TomovicL
Crnobrnja-IsailovicJ
FumagalliL
2008 Molecular phylogeography of the nose-horned viper (Vipera ammodytes, Linnaeus (1758)): Evidence for high genetic diversity and multiple refugia in the Balkan peninsula. Mol Phylogenet Evol 46 1116 1128
89. DeffontaineV
RenaudS
FontaineM
QuéréJ
LiboisR
2009 A relic bank vole lineage in the French Basque country highlights the biogeographical history of Pyrenean mountains in Europe. Mol Ecol 18 2489 2502
90. GrillA
AmoriG
AloiseG
LisiI
TosiG
2009 Molecular phylogeography of European Sciurus vulgaris: refuge within refugia? Mol Ecol 18 2687 2699
91. PrevisicA
WaltonC
KucinicM
MitrikeskiPT
KerovecM
2009 Pleistocene divergence of Dinaric Drusus endemics (Trichoptera, Limnephilidae) in multiple microrefugia within the Balkan Peninsula. Mol Ecol 18 634 647
92. PetitRJ
AguinagaldeI
de BeaulieuJL
BittkauC
BrewerS
2003 Glacial refugia: Hotspots but not melting pots of genetic diversity. Science 300 1563 1565
93. HickersonMJ
StahlEA
LessiosHA
2006 Test for simultaneous divergence using approximate Bayesian computation. Evolution 60 2435 53
94. GiraudT
RefregierG
de VienneDM
Le GacMA
HoodME
2008 Speciation in fungi. Fungal Genet Biol 45 791 802
95. TatarenkovA
GaoH
MackiewiczM
TaylorDS
TurnerBJ
2007 Strong population structure despite evidence of recent migration in a selfing hermaphroditic vertebrate, the mangrove killifish (Kryptolebias marmoratus). Mol Ecol 16 2701 2711
96. BoileauMG
HebertPDN
SchwartzSS
1992 Non-equilibrium gene frequency divergence: persistent founder effects in natural populations. J Evol Biol 5 25 39
97. AntonovicsJ
HoodME
ThrallPH
AbramsJY
DuthieGM
2003 Herbarium studies on the distribution of anther-smut fungus (Microbotryum violaceum) and Silene species (Caryophyllaceae) in the eastern United States. Am J Bot 90 1522 1531
98. HoodME
AntonovicsJ
HeishmanH
2003 Karyotypic similarity identifies multiple host-shifts of a pathogenic fungus in natural populations. Infect Genet Evol 2 167 172
99. SlippersB
StenlidJ
WingfieldMJ
2005 Emerging pathogens: fungal host jumps following anthropogenic introduction. Trends Ecol Evol 20 420 421
100. TellierA
de VIenneDM
GiraudT
HoodME
RefrégierG
2010 Theory and examples of reciprocal influence between hosts and pathogens, from short-term to long term interactions: coevolution, cospeciation and pathogen speciation following host shifts. In: Host-Pathogen Interactions: Genetics, Immunology and Physiology. 37-77 NY Nova Science Publishers
101. Lopez-VillavicencioM
JonotO
CoanticA
HoodME
EnjalbertJ
2007 Multiple infections by the anther smut pathogen are frequent and involve related strains. PLoS Pathogens 3 e176
102. BucheliE
GautschiB
ShykoffJA
1998 Isolation and characterization of microsatellite loci in the anther smut fungus Microbotryum violaceum. Mol Ecol 7 665 666
103. GiraudT
YocktengR
MartheyS
ChiapelloH
JonotO
2008 Isolation of 60 polymorphic microsatellite loci in EST libraries of four sibling species of the phytopathogenic fungal complex Microbotryum. Mol Ecol Res 8 387 392
104. RefrégierG
HoodME
GiraudT
2010 No evidence of reproductive character displacement between two sister fungal species causing anther smut disease in Silene. Int J Plant Sci 171 847 859
105. van PuttenWF
BiereA
van DammeJM
2005 Host-related genetic differentiation in the anther smut fungus Microbotryum violaceum in sympatric, parapatric and allopatric populations of two host species Silene latifolia and S. dioica. J Evol Biol 18 203 12
106. GoudetJ
2001 FSTAT, a program to estimate and test gene diversities and fixation indices (version 2.9.3). Available from http://www2.unil.ch/popgen/softwares/fstat.htm
107. RaymondM
RoussetF
1995 GENEPOP (Version 1.2): Population genetics software for exact tests and eucumenism. J Hered 86 248 249
108. RoussetF
2008 genepop'007: a complete re-implementation of the genepop software for Windows and Linux. Mol Ecol Res 8 103 106
109. BelkhirK
BorsaP
ChikhiL
RaufasteN
BonhommeF
2001 Genetix 4.02, Logiciel sous Windows TM pour la génétique des populations. Laboratoire Génome, population, interaction, CNRS UMR 5000, Université de Montpellier II (France)
110. PritchardJK
StephensM
DonnellyP
2000 Inference of population structure using multilocus genotype data. Genetics 155 945 959
111. FalushD
StephensM
PritchardJ
2003 Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164 1567 1587
112. GaoH
WilliamsonS
BustamanteCD
2007 A Markov Chain Monte Carlo Approach for Joint Inference of Population Structure and Inbreeding Rates From Multilocus Genotype Data. Genetics 176 1635 1651
113. FrancoisO
AnceletS
GuillotG
2006 Bayesian clustering using hidden Markov random fields in spatial population genetics. Genetics 174 805 816
114. ChenC
DurandE
ForbesF
FrancoisO
2007 Bayesian clustering algorithms ascertaining spatial population structure: a new computer program and a comparison study. Mol Ecol Notes 7 747 756
115. JakobssonM
RosenbergNA
2007 CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 23 1801 6
116. RosenbergN
2004 DISTRUCT: a program for graphical display for population structure. Mol Ecol Notes 4 137 138
117. PritchardJK
WenW
2004 Documentation for Structure software: Version 2
118. SpiegelhalterDJ
BestNG
CarlinBP
LindeAVD
2002 Bayesian measures of model complexity and fit. J Roy Stat Soc B 64 583 639
119. JombartT
2010 Package ‘adegenet’. a R package for the multivariate analysis of genetic markers. http://cran.r-project.org/web/packages/adegenet/index.html
120. ChesselD
DufourA
DrayS
2009 Package ‘ade4’. Analysis of Ecological Data: Exploratory and Euclivian methods in Environmental sciences. http://cran.r-project.org/web/packages/ade4/index.html
121. R Development Core Team 2010 R: A Language and Environment for Statistical Computing
122. NeiM
TajimaF
TatenoY
1983 Accuracy of estimated phylogenetic trees from molecular data. J Mol Evol 19 153 170
123. ChakrabortyR
JinL
1993 Determination of relatedness between individuals using DNA fingerprinting. Hum Biol 65 875 895
124. BowcockAM
Ruiz-linaresA
TomfohrdeJ
MinchE
KiddJR
1994 High-resolution of human evolutionary trees with polymorphic microsatellites. Nature 368 455 457
125. Cavalli-SforzaL
EdwardsA
1967 Phylogenetic analysis: Models and estimation procedures. Am J Hum Genet 19 233 257
126. GoldsteinDB
Ruiz LinaresA
Cavalli-SforzaLL
FeldmanMW
1995 Genetic absolute dating based on microsatellites and the origin of modern humans. Proc Natl Acad Sci USA 92 6723 6727
127. LiuK
MuseSV
2005 PowerMarker: an integrated analysis environment for genetic marker analysis. Bioinformatics 21 2128 2129
128. TamuraK
DudleyJ
NeiM
KumarS
2007 MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24 1596 1599
129. TakezakiN
NeiM
1996 Genetic distances and reconstruction of phylogenetic trees from microsatellite DNA. Genetics 144 389 399
130. SzpiechZA
JakobssonM
RosenbergNA
2008 ADZE: A rarefaction approach for counting alleles private to combinations of populations. Bioinformatics 24 2498 2504
131. HardyOJ
VekemansX
2002 SPAGeDi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol Ecol Notes 2 618 620
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 12- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Is Adherence to Erythrocytes a Factor in Extrapulmonary Dissemination?
- Identifying the Age Cohort Responsible for Transmission in a Natural Outbreak of
- Blockade of Immunosuppressive Cytokines Restores NK Cell Antiviral Function in Chronic Hepatitis B Virus Infection
- Metaeffector Exploits Host Proteasome to Temporally Regulate Cognate Effector
- The p53-Target Gene Drives Neutrophil-Mediated Protection against Lethal Bacterial Sepsis
- Development of an RNAi Protocol to Investigate Gene Function in the Filarial Nematode,
- Lysine Acetyltransferase GCN5-A Functions in the Cellular Response to Alkaline Stress and Expression of Cyst Genes
- Structural Basis for Apoptosis Inhibition by Epstein-Barr Virus BHRF1
- Molecular Architectures of Trimeric SIV and HIV-1 Envelope Glycoproteins on Intact Viruses: Strain-Dependent Variation in Quaternary Structure
- Interaction of c-Cbl with Myosin IIA Regulates Bleb Associated Macropinocytosis of Kaposi's Sarcoma-Associated Herpesvirus
- Glacial Refugia in Pathogens: European Genetic Structure of Anther Smut Pathogens on and
- Role for Sumoylation in Systemic Inflammation and Immune Homeostasis in Larvae
- Inflammasome Sensor Nlrp1b-Dependent Resistance to Anthrax Is Mediated by Caspase-1, IL-1 Signaling and Neutrophil Recruitment
- Noise Cancellation: Viral Fine Tuning of the Cellular Environment for Its Own Genome Replication
- Infectious Speciation Revisited: Impact of Symbiont-Depletion on Female Fitness and Mating Behavior of
- Eis Regulates Autophagy, Inflammation, and Cell Death through Redox-dependent Signaling
- NleC, a Type III Secretion Protease, Compromises NF-κB Activation by Targeting p65/RelA
- Early Myeloid Dendritic Cell Dysregulation is Predictive of Disease Progression in Simian Immunodeficiency Virus Infection
- HIV Capsid is a Tractable Target for Small Molecule Therapeutic Intervention
- Structural and Functional Studies of Nonstructural Protein 2 of the Hepatitis C Virus Reveal Its Key Role as Organizer of Virion Assembly
- Coming of Age—Sexual Reproduction in Species
- HIV-1 Envelope Subregion Length Variation during Disease Progression
- Compartmentation of Redox Metabolism in Malaria Parasites
- Evidence That Intracellular Stages of Utilize Amino Sugars as a Major Carbon Source
- Rapid End-Point Quantitation of Prion Seeding Activity with Sensitivity Comparable to Bioassays
- Dimeric 2G12 as a Potent Protection against HIV-1
- Hypoxia Induces an Immunodominant Target of Tuberculosis Specific T Cells Absent from Common BCG Vaccines
- CD4 Natural Regulatory T Cells Prevent Experimental Cerebral Malaria via CTLA-4 When Expanded In Vivo
- The Killing of African Trypanosomes by Ethidium Bromide
- H2A.Z Demarcates Intergenic Regions of the Epigenome That Are Dynamically Marked by H3K9ac and H3K4me3
- Large-Scale Field Application of RNAi Technology Reducing Israeli Acute Paralysis Virus Disease in Honey Bees (, Hymenoptera: Apidae)
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HIV-1 Envelope Subregion Length Variation during Disease Progression
- Coming of Age—Sexual Reproduction in Species
- Evidence That Intracellular Stages of Utilize Amino Sugars as a Major Carbon Source
- Compartmentation of Redox Metabolism in Malaria Parasites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



