-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaBlockade of Immunosuppressive Cytokines Restores NK Cell Antiviral Function in Chronic Hepatitis B Virus Infection
NK cells are enriched in the liver, constituting around a third of intrahepatic lymphocytes. We have previously demonstrated that they upregulate the death ligand TRAIL in patients with chronic hepatitis B virus infection (CHB), allowing them to kill hepatocytes bearing TRAIL receptors. In this study we investigated whether, in addition to their pathogenic role, NK cells have antiviral potential in CHB. We characterised NK cell subsets and effector function in 64 patients with CHB compared to 31 healthy controls. We found that, in contrast to their upregulated TRAIL expression and maintenance of cytolytic function, NK cells had a markedly impaired capacity to produce IFN-γ in CHB. This functional dichotomy of NK cells could be recapitulated in vitro by exposure to the immunosuppressive cytokine IL-10, which was induced in patients with active CHB. IL-10 selectively suppressed NK cell IFN-γ production without altering cytotoxicity or death ligand expression. Potent antiviral therapy reduced TRAIL-expressing CD56bright NK cells, consistent with the reduction in liver inflammation it induced; however, it was not able to normalise IL-10 levels or the capacity of NK cells to produce the antiviral cytokine IFN-γ. Blockade of IL-10 +/ − TGF-β restored the capacity of NK cells from both the periphery and liver of patients with CHB to produce IFN-γ, thereby enhancing their non-cytolytic antiviral capacity. In conclusion, NK cells may be driven to a state of partial functional tolerance by the immunosuppressive cytokine environment in CHB. Their defective capacity to produce the antiviral cytokine IFN-γ persists in patients on antiviral therapy but can be corrected in vitro by IL-10+/ − TGF-β blockade.
Published in the journal: . PLoS Pathog 6(12): e32767. doi:10.1371/journal.ppat.1001227
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001227Summary
NK cells are enriched in the liver, constituting around a third of intrahepatic lymphocytes. We have previously demonstrated that they upregulate the death ligand TRAIL in patients with chronic hepatitis B virus infection (CHB), allowing them to kill hepatocytes bearing TRAIL receptors. In this study we investigated whether, in addition to their pathogenic role, NK cells have antiviral potential in CHB. We characterised NK cell subsets and effector function in 64 patients with CHB compared to 31 healthy controls. We found that, in contrast to their upregulated TRAIL expression and maintenance of cytolytic function, NK cells had a markedly impaired capacity to produce IFN-γ in CHB. This functional dichotomy of NK cells could be recapitulated in vitro by exposure to the immunosuppressive cytokine IL-10, which was induced in patients with active CHB. IL-10 selectively suppressed NK cell IFN-γ production without altering cytotoxicity or death ligand expression. Potent antiviral therapy reduced TRAIL-expressing CD56bright NK cells, consistent with the reduction in liver inflammation it induced; however, it was not able to normalise IL-10 levels or the capacity of NK cells to produce the antiviral cytokine IFN-γ. Blockade of IL-10 +/ − TGF-β restored the capacity of NK cells from both the periphery and liver of patients with CHB to produce IFN-γ, thereby enhancing their non-cytolytic antiviral capacity. In conclusion, NK cells may be driven to a state of partial functional tolerance by the immunosuppressive cytokine environment in CHB. Their defective capacity to produce the antiviral cytokine IFN-γ persists in patients on antiviral therapy but can be corrected in vitro by IL-10+/ − TGF-β blockade.
Introduction
NK cells constitute a major cellular arm of the innate immune system and, as such, have been viewed as most relevant in the setting of the initial response to an acute infection. However, they may also be appropriately or inappropriately activated to exert effector function when persistent infection and its pathological sequelae become established. Their role may be particularly important in patients with CHB, in whom the virus-specific CD8 T cell arm of protection is markedly diminished and dysfunctional [1], [2].
NK cells are greatly enriched in the liver, the site of HBV replication[3], [4]. We have previously demonstrated an increase in activated CD56bright NK cells in the livers of patients undergoing flares of eAg-negative CHB. This subset can be induced to express TNF-related apoptosis-inducing ligand (TRAIL), which is able to kill hepatocytes that have upregulated death-inducing TRAIL receptors, thereby contributing to liver inflammation in CHB[4]. The CD56bright subset can also be a potent source of cytokines such as IFN-γ[5], [6], a key cytokine shaping adaptive immunity and the delicate balance between protective and pathogenic responses. IFN-γ can clear HBV-infected hepatocytes through non-cytolytic mechanisms[7], [8]. NK cell-derived IFN-γ could therefore constitute a vital antiviral mechanism in the liver, where hepatocytes are relatively resistant to the cytolytic mechanisms of perforin and granzyme production[9].
The intensity and quality of NK cell effector function is determined by the balance of activatory and inhibitory signals through their array of receptors (NK-R), in addition to the influences exerted by the cytokine microenvironment. The TRAIL pathway of NK cell-mediated hepatocyte killing can be driven by the cytokines IFN-α and IL-8, induced during flares of CHB[4]. Similarly, NK cells in HCV infection can be polarised towards cytolysis and expression of TRAIL as a result of exposure to endogenous[10] or therapeutic[11] IFN-α. Conversely, intrahepatic NK cell function can be down-regulated by the immunosuppressive cytokine IL-10 produced by Kupffer cells[12]. In addition, a role for IL-17 in curtailing NK cell function was recently demonstrated in disseminated vaccinia virus infection of mice with pre-existing dermatitis[13]. In this study we have investigated cytokine-driven modulation of IFN-γ production by NK cells in patients with CHB and explored the potential to restore their non-cytolytic antiviral function.
Results
Expansion of the CD56bright subset of NK cells in CHB
To explore NK cell effector potential in the setting of persistent HBV infection, we first analysed the frequency of CD56bright(CD16dim/neg) and CD56dim(CD16pos) NK cell subsets in 64 patients with CHB compared to 31 healthy age-matched controls (Table 1). The proportion of circulating CD56bright NK cells was significantly increased in patients with CHB (representative FACS plots Fig1a, summary data Fig1b), with a tendency to further increases in those with liver inflammation (Fig1b). There was a trend for the percent of circulating NK cells to decrease in CHB (Fig 1c) but the absolute number of circulating CD56bright NK cells was still significantly increased (p<0.05 data not shown).
Fig. 1. NK cell frequency and altered subset distribution in the periphery and intrahepatic compartment. 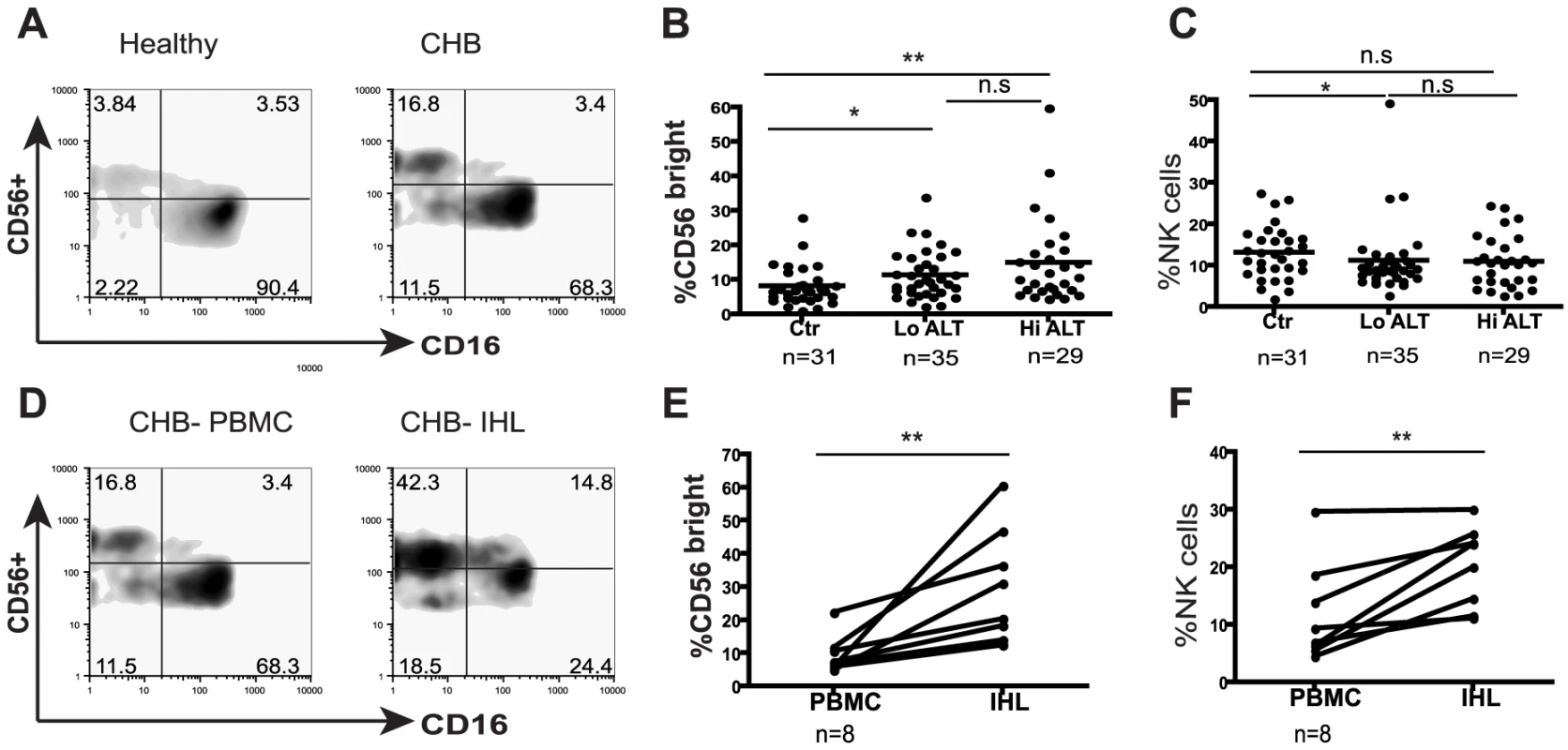
(A) Representative density plots gated on CD3- PBMC and co-stained for CD56 and CD16 to identify NK cells from a healthy control and a CHB patient. (B) Summary data of the proportions of CD56bright subset in the periphery of CHB patients with low ALT (n = 35, ALT <50IU/L, median 34) compared to high ALT (n = 29, median ALT 112) and healthy controls (n = 31). (C) Frequency of circulating NK cells in CHB patients with low ALT and high ALT and healthy controls. (D) Density plots of NK cells from peripheral blood and intrahepatic lymphocytes from a representative CHB patient. (E) Paired cumulative results of peripheral and intrahepatic CD56bright NK cells frequencies from 8 patients with CHB. (F) NK cell frequency in peripheral blood and intrahepatic compartment from 8 patients with CHB with paired samples. The non-parametric Mann-Whitney U test was used to compare data between groups and the Wilcoxon signed rank test was used between paired variables. *p<0.05 or ** p<0.01 designates values that differ significantly between groups. Ctr = healthy controls. Tab. 1. Characteristics of study population. 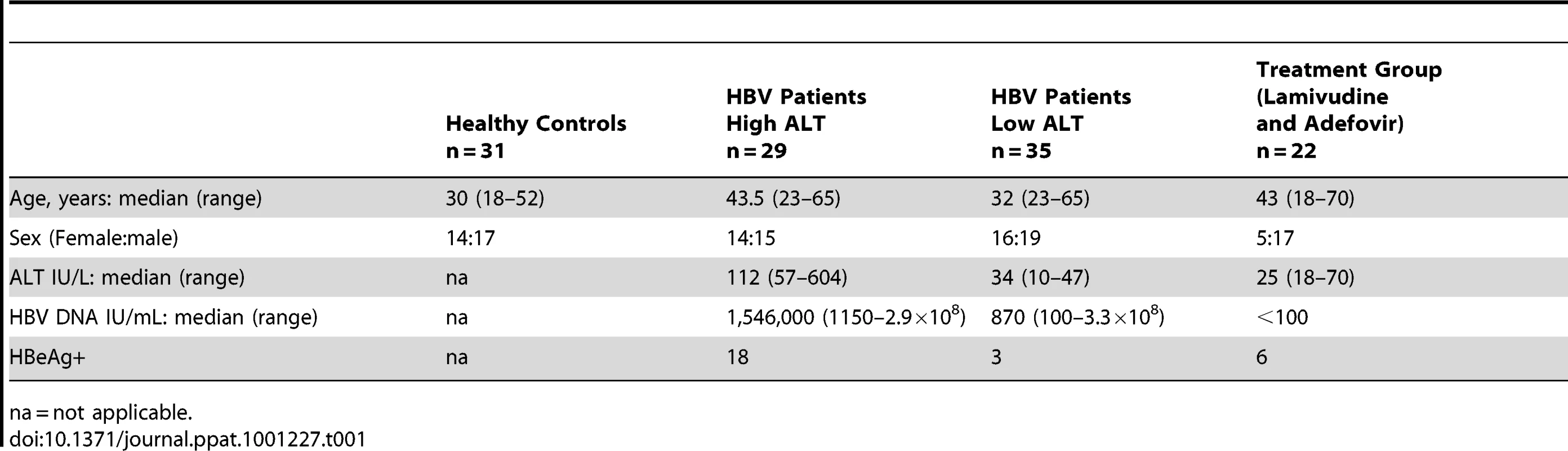
na = not applicable. To determine whether there was a further enrichment of this immunoregulatory CD56bright NK cell subset at the site of viral replication, we compared the proportions in intrahepatic and circulating lymphocytes. In all eight patients with CHB from whom paired samples were available, the percent of CD56bright of total NK cells was higher in the intrahepatic compared to peripheral compartment (Fig1d,e). Since NK cells make up a significantly greater proportion of intrahepatic than circulating lymphocytes in these patients (Fig 1f), this corresponds to a substantial enrichment of CD56bright NK cells in the liver.
Impaired non-cytolytic antiviral potential of NK cells in CHB
We have previously shown that the CD56bright subset of NK cells can mediate hepatocyte apoptosis through expression of the death ligand TRAIL in flares of eAg-negative CHB[4]. In this cohort of patients we confirmed an increase in TRAIL expression (largely on the CD56bright subset, Fig 2a representative plots) in patients with either eAg+ or eAg - CHB who had evidence of liver inflammation (Fig2a summary data).
Fig. 2. Skewed NK cell effector function in CHB is only partially corrected during therapy. 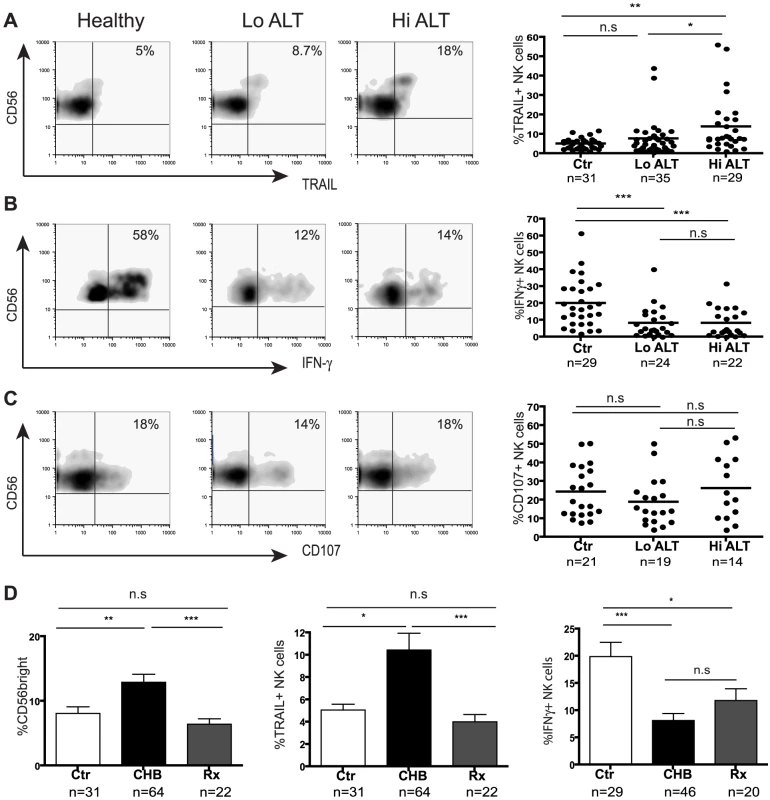
(Panels A–C) Representative density plots from a healthy control and HBV patients with low ALT (ALT <50 IU/L, median 33) and raised ALT (ALT>50 IU/L, median 112) and summary data for TRAIL expression, IFN-γ production and CD107 expression. (D) Summary bar charts of CD56bright proportions, NK cell TRAIL expression and NK cell IFN-γ production from healthy, CHB and patients on antiviral therapy. Results are expressed as mean ± SEM. Rx = treated patients. *p<0.05, **p<0.01, ***p<0.001 by Mann-Whitney test. The CD56bright subset of NK cells can also be a potent source of IFN-γ[14], a cytokine that has direct non-cytolytic antiviral effects on HBV replication [7], [8] and can promote adaptive immune responses[6]. Despite the enrichment of CD56bright NK cells in CHB, we found that they had an impaired capacity to produce IFN-γ (representative plots, Fig2b). There was a significant reduction in production of IFN-γ by NK cells from 46 patients with CHB compared to 29 healthy controls (Fig2b). This reduction was seen irrespective of disease activity (liver inflammation Fig2b, viral load or eAg status, data not shown) or method of NK cell stimulation (IL-12/IL-18 (Fig2b), IL-12/IL-15, K562 with IL-12/IL-18 or PMA/ionomycin, data not shown). Both the CD56bright subset and the CD56dim subset (that has recently been recognised to also make a contribution to cytokine production[15]) showed significantly impaired IFN-γ production (FigS1a). Similarly, CD56bright and CD56dim NK cells in CHB showed a trend to produce less TNF-α, despite the strong stimulus required to reliably elicit this cytokine (FigS1b). Simultaneous assessment of IFN-γ and TNF-a production showed a significant reduction in dual producing NK cells in CHB (FigS1c).
To assess NK cell cytolytic potential, we determined their capacity to degranulate as evidenced by CD107 expression following stimulation with K562 target cells and cytokines. There was no significant difference in NK cell degranulation potential in 33 patients with CHB compared to 21 controls (Fig2c). Differential analysis by NK cell subset or by patient disease status did not show any differences (data not shown). NK cells in CHB were therefore biased towards cytolytic and death-ligand mediated effector functions and defective IFN-γ production.
To determine the potential of potent antiviral treatment to correct this bias in NK cell effector function, we studied a group of 22 patients with HBV viraemia well-suppressed on a combination of Lamivudine and Adefovir. Upon viral suppression and normalisation of liver inflammatory markers, there was no significant change in the percent of NK cells (FigS2a), but the proportion of CD56bright NK cells decreased to levels observed in healthy controls (Fig2d); in line with this, NK cell TRAIL expression reduced to baseline levels (Fig2d). However NK cell IFN-γ production was only partially augmented upon antiviral treatment (mainly CD56dim subset, FigS2b) and remained significantly lower than that in healthy controls (Fig2d).
IL-10 is induced in CHB and recapitulates the NK cell defect in IFN-γ production
Effector function of NK cells is tightly regulated by the cytokine milieu and their production of IFN-γ can be inhibited by immunosuppressive cytokines such as IL-10[12], [16] and IL-17[13]. The levels of IL-17A were not elevated in sera from patients with CHB compared to controls (Fig3a). In contrast, circulating concentrations of IL-10 were significantly increased in patients with active HBV disease (Fig3b,c by CBA, confirmed by ELISA, data not shown), correlating with viral load (r = 0.48, p = 0.002) and ALT (r = 0.37, p = 0.03). IL-10 levels showed a trend to decrease on antiviral treatment but remained significantly higher than in controls (Fig3c), consistent with the limited restoration of NK cell IFN-γ production in these patients.
Fig. 3. IL-10 is elevated in CHB and suppresses NK cell IFN-γ production. 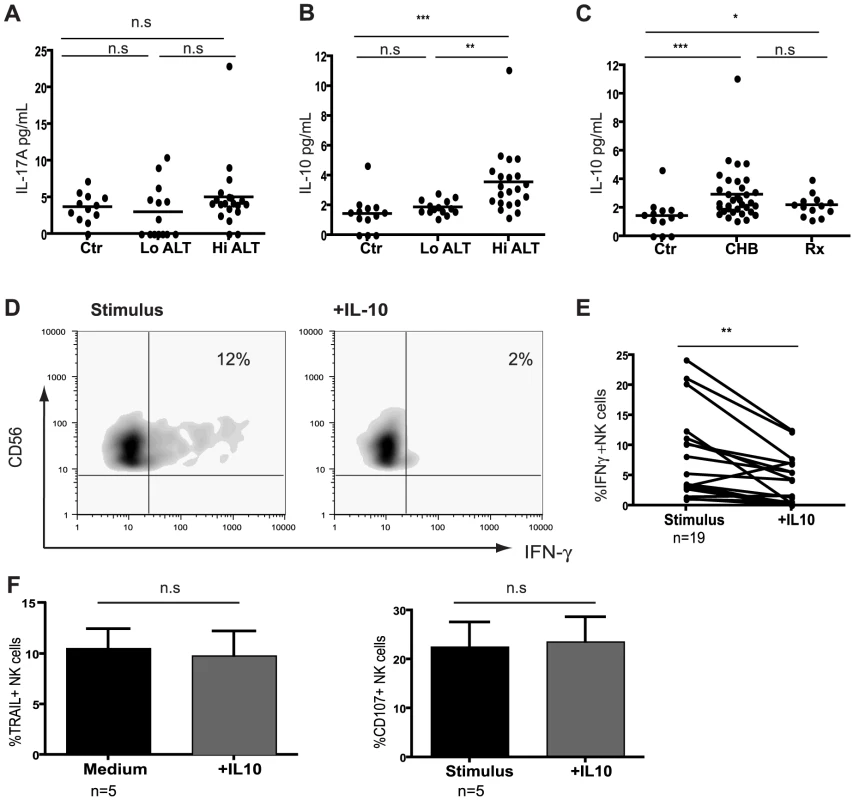
(A) Levels of cytokines IL-17A and (B) IL-10 determined using Cytometric Bead Arrays flex sets using sera from 13 healthy controls, 14 low ALT (median ALT 35, all eAg-) and 21 high ALT patients (median ALT 115, 13eAg-). (C) Cumulative IL-10 results including therapy group (n = 13, median ALT 25). (D) Representative density plots of the effect of exogenous IL-10 on IFN-γ production by NK cells from a CHB patient and (E) paired cumulative results from 19 CHB patients. (F) Summary bar charts of the effect of exogenous IL-10 on the expression of TRAIL and CD107 in 5 CHB patients. Results are paired and expressed as mean ± SEM. Stimulus = IL12+IL18. Significance determined by the Mann-Whitney test for comparison between groups and the Wilcoxon signed rank test for paired data, *p<0.05, **p<0.01, ***p<0.001. To test whether IL-10 could induce the defect in NK cell IFN-γ production seen in CHB, we re-assessed NK cell effector function with or without the addition of exogenous IL-10. IL-10 significantly suppressed NK-cell derived IFN-γ (Fig3d), particularly in those patients in whom it was not already substantially reduced (Fig3e, and in healthy controls, data not shown). By contrast, IL-10 had no effect on cytolytic ability or TRAIL phenotype (Fig3f) and did not affect the percent of NK cells (FigS3a). The ability of IFN-α to further induce NK cell TRAIL expression in vitro[4] was also not abrogated by IL-10 (data not shown). The effect of IL-10 was consistent but more modest on purified NK cells (FigS3b), suggesting that some of its suppressive activity on NK cells is mediated indirectly via other constituents such as APCs. The contrasting effects of IL-10 on TRAIL and IFN-γ expression represented differential regulation of these effector functions in the same NK cells rather than the emergence of two distinct subsets. The small population of TRAIL-expressing NK cells present in healthy donors were at least as able to produce IFN-γ as the rest of the NK cell population (FigS3c). The addition of exogenous IL-10 suppressed IFN-γ in NK cells regardless of their TRAIL expression (FigS3c). In line with this, gating on the expanded population of TRAIL-expressing NK cells found in CHB demonstrated that their IFN-γ-producing capacity was no more reduced than that of the non-TRAIL-expressing fraction (FigS3d).
Restoration of NK cell IFN-γ production upon blockade of immunosuppressive cytokines
Since IL-10 was induced in CHB and exogenous IL-10 was able to mimic the selective suppression of NK cell effector function, we next investigated the potential to restore NK cell IFN-γ production by IL-10 blockade. Addition of antiIL10/IL10-R blocking mAbs restored the ability of both CD56bright and CD56dim NK cells from patients with active CHB to produce IFN-γ (mean 2.5 fold increase, Fig4a,b,d). The majority of patients without biochemical evidence of liver inflammation (and with low viral loads) did not respond to this strategy (Fig4c,d), in line with their lower levels of circulating IL-10 (Fig3b). A subset of those patients failing to respond to IL-10 blockade did show recovery of NK cell IFN-γ production following blockade of both IL-10 and TGFβ, another immunosuppressive cytokine known to be able to inhibit NK cell production (Fig4e,f).
Fig. 4. IL-10 blockade alone or in combination with TGFβRII blocking restores NK cell IFN-γ production. 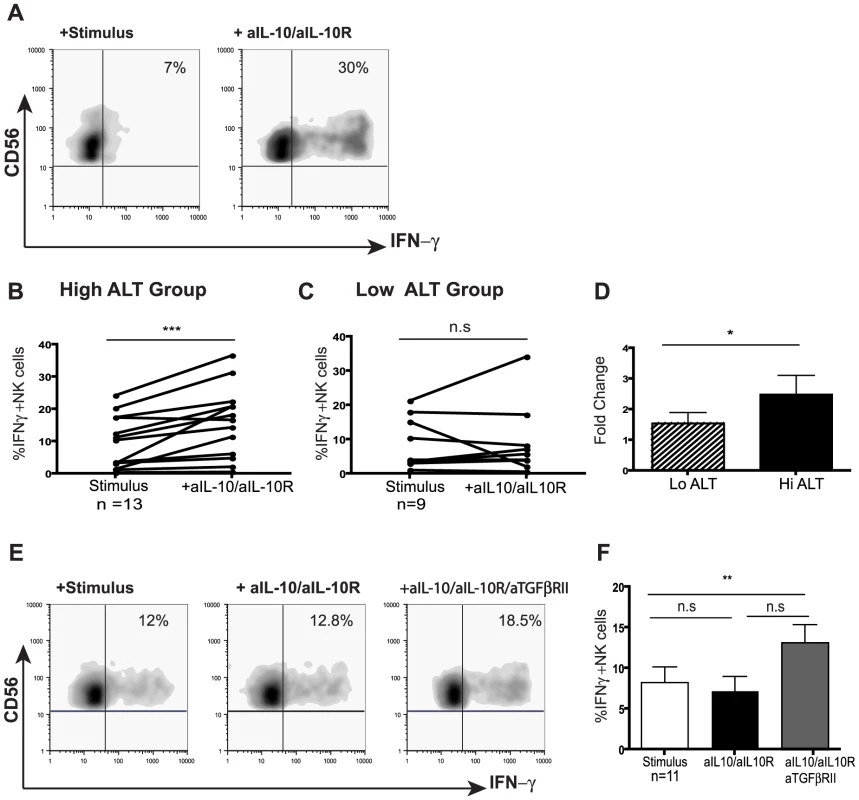
(A) Representative density plot from a CHB patient of peripheral NK cell IFN-γ production in the presence of anti-IL-10 and anti-IL10 receptor blocking mAb. (B) Paired summary data from CHB patients with either active disease (High ALT median 104, n = 13) or (C) inactive disease (Low ALT median 33, n = 9). (D) Fold change in IFN-γ produced by total NK cells following IL-10 blockade in both groups of patients. (E, F) Representative density dot plots from a CHB patient and summary bar chart of paired results from 11 patients (n = 11 median ALT 42) of NK cell IFN-γ production following IL-10 blockade alone or in combination with anti-TGFβRII blocking antibodies. Stimulus = IL12+IL18. Significance determined by the Mann-Whitney test for comparison between groups and the Wilcoxon signed rank test for paired data, *p<0.05, **p<0.01. To investigate whether the suppression of NK cell IFN-γ was maintained at the site of HBV replication, paired liver and blood samples from eight patients with CHB were examined (Table 2). CD56bright NK cell IFN-γ production showed a trend to be even lower in the liver than the periphery of patients with CHB (FigS4a). Levels of intrahepatic NK cell IFN-γ production did not significantly correlate with levels of ALT (FigS4b), viral load or liver histology in this small sample of patients, only one of whom had histological evidence of significant liver inflammation (Table 2). Due to limited cell numbers, individual cytokine blockade could not be performed but dual IL-10/TGFβRII blockade reconstituted the proportion of NK cells able to produce IFN-γ (%positive, Fig5a) and increased their level of IFN-γ production (MFI, Fig5b). The fold increase in the capacity of CD56bright NK cells to secrete IFN-γ upon IL-10/TGFβ blockade was greater in the liver than the periphery (Fig5a,b).
Fig. 5. Blockade of IL10/TGF enhances intrahepatic NK cell IFN-γ production. 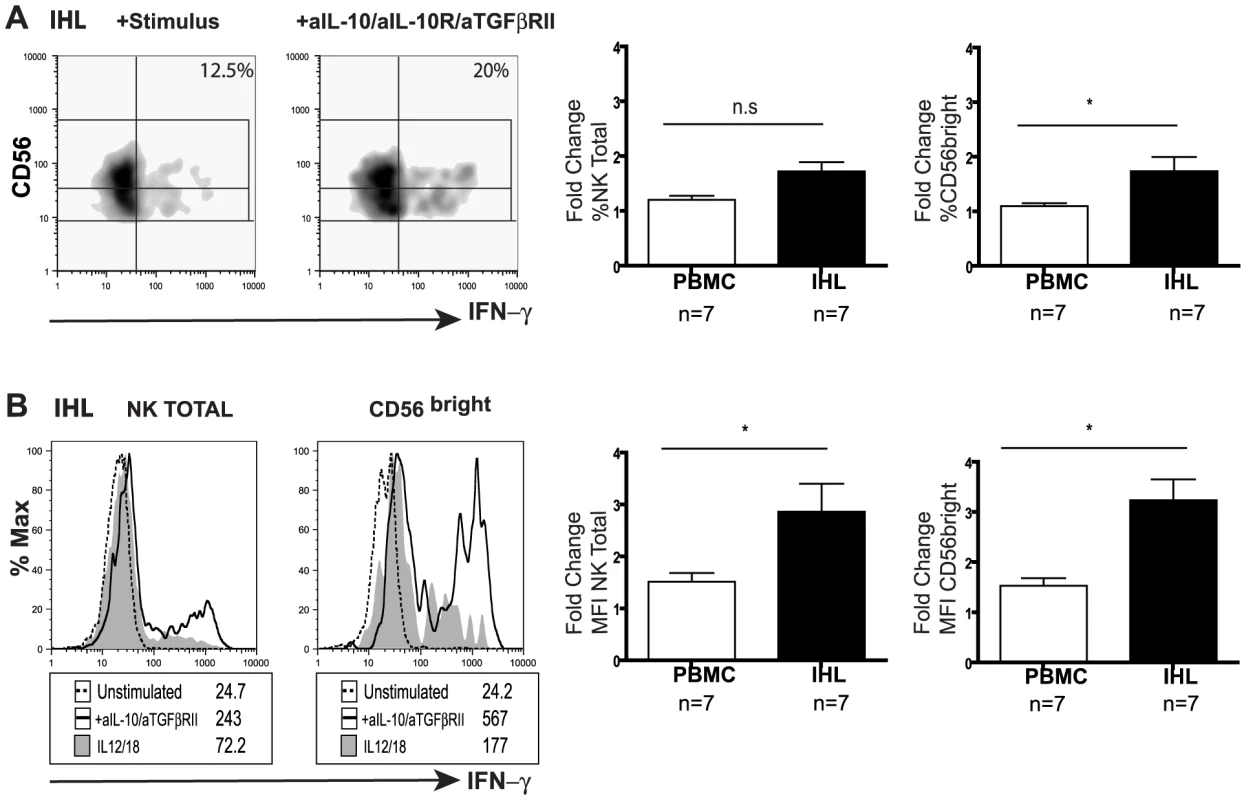
(A) Representative density plots and (B) histograms for total intrahepatic NK cell and CD56bright subset IFN-γ production upon blockade with anti-lL-10, anti-IL10 receptor and anti-TGFβRII blocking antibodies. Paired summary bar charts of fold change increase in the percentage and mean fluorescence intensity (MFI) of NK total and CD56bright IFN-γ+ cells in the periphery and intrahepatic compartment of 7 CHB (median ALT 56). Results are expressed as mean ± SEM. Stimulus = IL12+IL18. *p<0.05 by Wilcoxon signed rank test. Tab. 2. Patient characteristics with available liver biopsy specimens. 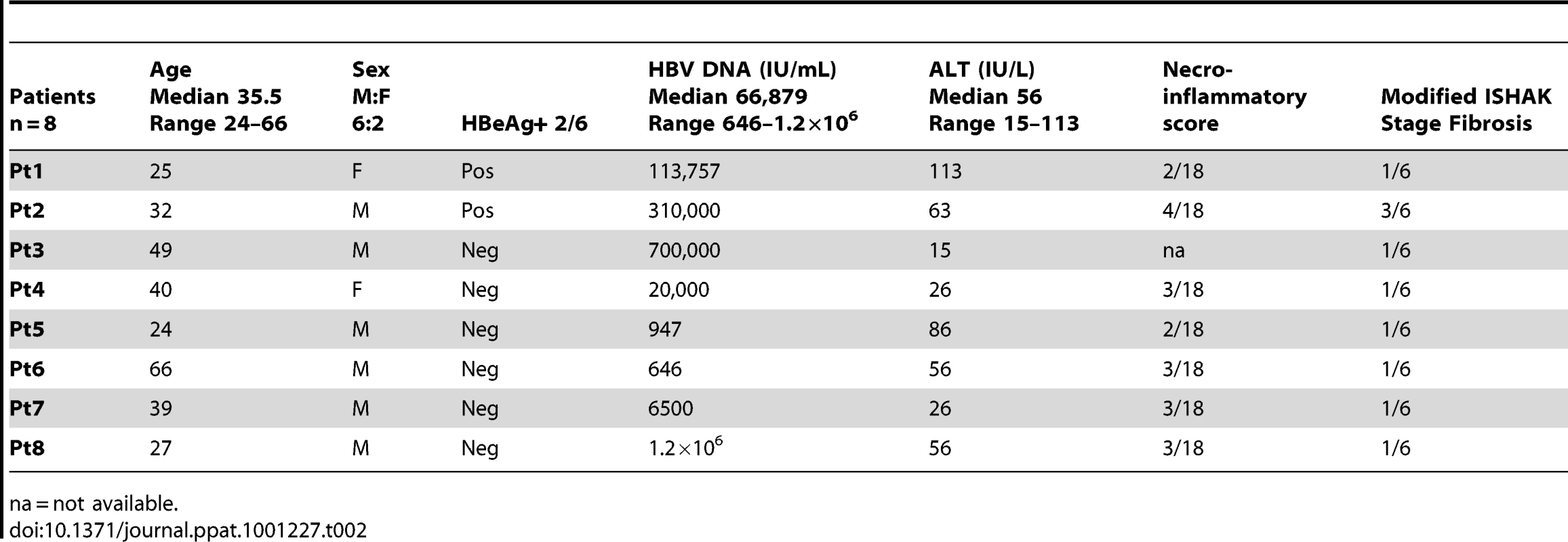
na = not available. Discussion
Accumulating evidence points to a contribution of NK cells in the battle to control persistent intracellular pathogens[6], [17], [18]. Although NK cells have been considered part of the innate immune response, recent data have suggested that they can possess properties previously ascribed to the adaptive arm, including the capacity to develop memory and tolerance[19], [20], [21]. In this study we show that NK cells can develop selective defects in antiviral function in the setting of chronic infection and inflammation, reminiscent of the hierarchical loss of effector function manifested by exhausted T cells[22].
Just as T cell defects have been attributed to excessive antigenic stimulation, functional impairment of NK cells has been ascribed to excessive stimulatory signals through the activating receptor NKG2D, resulting in its down-modulation[19], [20]. This is a plausible mechanism in CHB since data from transgenic mice suggest that HBV can upregulate the intrahepatic expression of NKG2D ligands[23]. However, a recent study and our unpublished data do not support this mechanism, showing no down-regulation of NKG2D or consistent changes in other NK cell receptors that could account for the NK cell impairment seen in CHB[24]. Instead, our data suggest that the selective NK cell functional defects seen in this infection may be attributable to the immunosuppressive cytokine milieu.
Our analysis of NK cell effector potential in a large cohort of patients with CHB revealed preservation of cytolytic capacity and an increase in TRAIL-bearing CD56bright NK cells. Despite this increase in the subset of NK cells that are usually the most potent source of cytokines[14], there was a decrease in the overall NK cell capacity to produce IFN-γ. Such divergence of effector function is in line with the recent finding that cytokines are trafficked and secreted via completely different pathways to cytotoxic granules in NK cells[25]. Consistent with these distinct trafficking pathways, separate signalling pathways have been shown to control the release of cytokines and cytotoxic granules in NK cells[26], [27]. Unique molecular switches are starting to be identified that couple NK cell receptor signalling with the generation of cytokines rather than cytotoxic functions[28], [29]. It is therefore conceivable that a pathway specific to NK cell cytokine production is dysregulated in patients with CHB.
The immunosuppressive cytokine IL-10 has been shown to specifically impair NK cell IFN-γ production[30], in contrast with IL-17 and excessive NKG2D signalling, both of which result in down-modulation of all NK cell effector functions[13], [20]. The liver is an immunotolerant organ, predisposed to the production of immunosuppressive cytokines; down-regulation of intrahepatic NK cell IFN-γ production has been linked to the local release of IL-10 by Kupffer cells[12], [31]. We found that exposure of NK cells to IL-10 in vitro was able to recapitulate the selective reduction in IFN-γ production noted in patients with CHB. Furthermore, its blockade was able to restore the capacity of NK cells from patients with active HBV infection to produce IFN-γ. IL-10 was not able to inhibit cytotoxic degranulation and could not overcome the capacity of IFN-α to induce TRAIL, in line with the maintenance of these pathogenic functions of NK cells in CHB. IL-10 was consistently modestly elevated in the serum of patients with CHB, but would be expected to be at higher concentrations at the site of infection in the liver and in close proximity to the cells from which it is released. NK cells themselves can produce IL-10[14], [32] to allow auto-suppression, but in the HBV-infected liver there are a number of other candidate cellular sources and there is likely to be a complex regulatory network involved in maintaining its production, as recently described in HIV infection[33].
We recently reported a transient induction of IL-10 in early acute HBV infection that was temporally associated with a transient suppression of the capacity of NK cells to produce IFN-γ, coincident with the increase in viraemia and production of viral antigens[16]. In our cohort of patients with CHB it was difficult to distinguish the influence of viraemia or liver inflammation, since both were increased in patients with elevated levels of IL-10. Future study of a group of patients with high viral load but normal ALT (immunotolerant phase) could help to dissect the role of these factors. The fact that NK cell IFN-γ production and IL-10 levels were not significantly normalised by potent antiviral therapy suggests that the continued secretion of high levels of HBV proteins in these patients may play a role. In patients with low level CHB without evidence of liver inflammation, IL-10 was not elevated and its blockade alone could not rescue NK function, which instead required additional TGF-β blockade. TGF-β is another immunosuppressive cytokine that characterises the tolerising liver environment and has been shown to be increased in CHB[34]. TGF-β has been shown to be an alternative key regulator of the capacity of human NK cells to produce IFN-γ, suppressing IFN-γ and T-bet via Smad2/3/4[35].
The collective action of TGF-β and IL-10 may represent an important feedback mechanism to limit exuberant immune responses and tissue immunopathology in a vital organ like the liver. However, in the context of chronic infections, elevated levels may attenuate immune responses sufficiently to contribute to the failure of resolution of infection. A role for IL-10 in persistent viral infection has been highlighted recently by studies showing that blockade of the IL-10 receptor is associated with resolution of LCMV infection[36], [37]. Genetic studies have also highlighted the importance of IL-10 in the antiviral response to HBV; polymorphisms of the IL-10 promoter resulting in elevated IL-10 production are associated with viral persistence, increased disease severity and progression[38], [39].
Our data suggest that immunosupressive cytokines may polarise NK cells in CHB, having no effect on their expression of death ligands and cytolytic granules but inhibiting IFN-γ production. NK cells expressing death ligands like TRAIL would only be able to have a direct antiviral effect at the expense of liver damage. The decline in liver inflammation seen on antiviral treatment is compatible with the reduction in TRAIL-expressing CD56bright NK cells that we noted in this setting. However, potent antiviral therapy was unable to significantly restore the capacity of NK cells to produce IFN-γ, which would therefore retain an impaired capacity for non-cytolytic clearance of HBV from hepatocytes and boosting of adaptive immune responses. Our findings raise the possibility of immunotherapeutic targeting of IL-10 and TGF-β in CHB, with the caveat that these cytokines govern a critical balance between impeding pathogen clearance and restraining immunopathology.
Materials and Methods
Ethics statement
Clinical assessment and blood sampling were performed during routine hepatitis clinics, with written informed consent and local ethical board approval of the Royal Free Hospital, the Royal London Hospital and Camden Primary Care Ethics Review Board.
Patients and healthy subjects
All patients were anti-Hepatitis C - and anti-Human Immunodeficiency Virus-antibody negative and treatment naïve with the exception of a sub-group of 22 patients suppressed on a combination of Lamivudine and Adefovir. Patient characteristics are included in Table 1. Paired peripheral blood and liver biopsy specimens (surplus to diagnostic requirements) were obtained from 8 CHB-infected patients (Table 2).
Isolation and storage of PBMC and Intrahepatic lymphocyte isolation
Peripheral blood mononuclear cells (PBMC) were isolated by gradient centrifugation on Ficoll-Hypaque and frozen or immediately studied as described later. Sera were collected and frozen for later use. Intrahepatic lymphocytes were isolated as previously described[4].
Extracellular staining and flow cytometric analysis
For phenotypic analysis, PBMC isolated from HBV patients and healthy donors were stained with fluorochrome-conjugated antibodies to CD3-Cy5.5/PerCP, CD56-FITC, CD16-APC, and TRAIL-PE or isotype matched controls (BD Biosciences, Cowley, U.K.). In selected experiments TRAIL expression was determined following overnight incubation with 50 ng/mL of rhIL-10 (eBioscience). PBMC were acquired on a FACS Calibur flow cytometer (Becton Dickinson) and analysed using Flowjo analysis software (Treestar).
Cytokine production by intracellular staining
As previously described[16], PBMC were incubated with 50 ng/mL of rhIL-12 (Miltenyi) and rhIL-18 (R&D Systems, Abingdon, U.K.) for 21 hours at 37°C. 1mM monensin (Sigma-Aldrich, Gillingham, U.K.) was added for the final 3 hours. Cells were fixed and permeabilised followed by intracellular staining for IFN-γ-PE (R&D systems). Where indicated the same experiments were performed in the presence of rhIL-10 (50ng/mL), or blocking antibodies to anti-IL10 (5 µg/mL) (eBioscience) and anti-IL-10R (10 µg/mL) alone or in combination with antiTGFβRII (10 µg/mL) (BD Biosciences). NK IFN-γ production was determined by subtracting baseline IFN-γ production from that observed after cytokine or antibody treatment. NK cells from PBMC of a randomly selected group of patients were isolated (>96% purity and viability) (Miltenyi Biotec, Germany, NK isolation kit) to assess the effect of exogenous IL-10 on IFN-γ production.
For TNF-α production, PBMC were stimulated with phorbol myristate acetate (PMA) (3 ng/mL) and ionomycin (100 ng/mL) (Sigma) for 3 hours; 1mM monensin (Sigma-Aldrich, Gillingham, U.K.) was added for the final 2 hours. Cells were then stained with the same antibody combination used for phenotyping prior to permealisation and intracellular staining for TNF-α. In selected experiments NK cell TNF-α and IFN-γ co-expression was assessed following PMA/I stimulation.
CD107 degranulation assay
As previously described[16], PBMC were incubated with K562 cells (5∶1 E:T ratio) for 3 hours at 37°C following overnight stimulation with a combination of rhIL-12/rhIL-18 or medium alone in the presence or absence of rh-IL10. CD107a-PE antibody (BD Biosciences, Cowley, U.K.) was added at the time of stimulation with target cells and 1mM monensin was added during the last two hours of the incubation prior to staining and acquisition.
Determination of serum cytokine concentrations by Cytometric Bead Array (CBA)
CBA flex-sets were used for the determination of IL-10, IL-17 (BD Biosciences, Cowley, U.K) according to manufacturers’ protocols for serum samples.
Statistical analysis
Statistical significance was performed between paired samples using the Wilcoxon signed rank test and between HBV patients and healthy controls using the Mann-Whitney U test. Correlations between variables were evaluated with the Spearman rank correlation test. P<0.05 was considered to be significant for all tests.
Supporting Information
Zdroje
1. MainiMK
BoniC
LeeCK
LarrubiaJR
ReignatS
2000 The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med 191 1269 1280
2. MainiMK
SchurichA
2010 The molecular basis of the failed immune response in chronic HBV: therapeutic implications. J Hepatol 52 616 619
3. DohertyDG
NorrisS
Madrigal-EstebasL
McEnteeG
TraynorO
1999 The human liver contains multiple populations of NK cells, T cells, and CD3+CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J Immunol 163 2314 2321
4. DunnC
BrunettoM
ReynoldsG
ChristophidesT
KennedyPT
2007 Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J Exp Med 204 667 680
5. CooperMA
FehnigerTA
CaligiuriMA
2001 The biology of human natural killer-cell subsets. Trends Immunol 22 633 640
6. VivierE
TomaselloE
BaratinM
WalzerT
UgoliniS
2008 Functions of natural killer cells. Nat Immunol 9 503 510
7. GuidottiLG
IshikawaT
HobbsMV
MatzkeB
SchreiberR
1996 Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity 4 25 36
8. KakimiK
GuidottiLG
KoezukaY
ChisariFV
2000 Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med 192 921 930
9. TayCH
WelshRM
1997 Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J Virol 71 267 275
10. AhlenstielG
TiterenceRH
KohC
EdlichB
FeldJJ
Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alfa-dependent manner. Gastroenterology 138 325 335 e321-322
11. StegmannKA
BjorkstromNK
VeberH
CiesekS
RieseP
1885 1897
12. TuZ
BozorgzadehA
PierceRH
KurtisJ
CrispeIN
2008 TLR-dependent cross talk between human Kupffer cells and NK cells. J Exp Med 205 233 244
13. KawakamiY
TomimoriY
YumotoK
HasegawaS
AndoT
2009 Inhibition of NK cell activity by IL-17 allows vaccinia virus to induce severe skin lesions in a mouse model of eczema vaccinatum. J Exp Med 206 1219 1225
14. CooperMA
FehnigerTA
TurnerSC
ChenKS
GhaheriBA
2001 Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood 97 3146 3151
15. FauriatC
LongEO
LjunggrenHG
Bryceson YT Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood 115 2167 2176
16. DunnC
PeppaD
KhannaP
NebbiaG
JonesM
2009 Temporal analysis of early immune responses in patients with acute hepatitis B virus infection. Gastroenterology 137 1289 1300
17. AlterG
MartinMP
TeigenN
CarrWH
SuscovichTJ
2007 Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J Exp Med 204 3027 3036
18. KhakooSI
ThioCL
MartinMP
BrooksCR
GaoX
2004 HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science 305 872 874
19. CoudertJD
ZimmerJ
TomaselloE
CebecauerM
ColonnaM
2005 Altered NKG2D function in NK cells induced by chronic exposure to NKG2D ligand-expressing tumor cells. Blood 106 1711 1717
20. OppenheimDE
RobertsSJ
ClarkeSL
FillerR
LewisJM
2005 Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol 6 928 937
21. SunJ
MadanR
KarpCL
BracialeTJ
2009 Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat Med 15 277 284
22. WherryEJ
BlattmanJN
Murali-KrishnaK
Van Der MostR
AhmedR
2003 Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol 77 4911 4927
23. ChenY
WeiH
SunR
DongZ
ZhangJ
2007 Increased susceptibility to liver injury in hepatitis B virus transgenic mice involves NKG2D-ligand interaction and natural killer cells. Hepatology 46 706 715
24. OlivieroB
VarchettaS
PaudiceE
MicheloneG
ZaramellaM
2009 Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology 137 1151 1160, 1160 e1151-1157
25. ReefmanE
KayJG
WoodSM
OffenhauserC
BrownDL
Cytokine secretion is distinct from secretion of cytotoxic granules in NK cells J Immunol 184 4852 4862
26. CarauxA
KimN
BellSE
ZompiS
RansonT
2006 Phospholipase C-gamma2 is essential for NK cell cytotoxicity and innate immunity to malignant and virally infected cells. Blood 107 994 1002
27. KimN
SaudemontA
WebbL
CampsM
RuckleT
2007 The p110delta catalytic isoform of PI3K is a key player in NK-cell development and cytokine secretion. Blood 110 3202 3208
28. GuoH
SamarakoonA
VanhaesebroeckB
MalarkannanS
2008 The p110 delta of PI3K plays a critical role in NK cell terminal maturation and cytokine/chemokine generation. J Exp Med 205 2419 2435
29. MalarkannanS
RegunathanJ
ChuH
KutlesaS
ChenY
2007 Bcl10 plays a divergent role in NK cell-mediated cytotoxicity and cytokine generation. J Immunol 179 3752 3762
30. TrippCS
WolfSF
UnanueER
1993 Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci U S A 90 3725 3729
31. LassenMG
LukensJR
DolinaJS
BrownMG
Intrahepatic IL-10 maintains NKG2A+Ly49 − liver NK cells in a functionally hyporesponsive state J Immunol 184 2693 2701
32. MaroofA
BeattieL
ZubairiS
SvenssonM
StagerS
2008 Posttranscriptional regulation of II10 gene expression allows natural killer cells to express immunoregulatory function. Immunity 29 295 305
33. BrockmanMA
KwonDS
TigheDP
PavlikDF
RosatoPC
2009 IL-10 is upregulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood 114 346 356
34. FlisiakR
Al-KadasiH
JaroszewiczJ
ProkopowiczD
FlisiakI
2004 Effect of lamivudine treatment on plasma levels of transforming growth factor beta1, tissue inhibitor of metalloproteinases-1 and metalloproteinase-1 in patients with chronic hepatitis B. World J Gastroenterol 10 2661 2665
35. YuJ
WeiM
BecknellB
TrottaR
LiuS
2006 Pro - and antiinflammatory cytokine signaling: reciprocal antagonism regulates interferon-gamma production by human natural killer cells. Immunity 24 575 590
36. BrooksDG
TrifiloMJ
EdelmannKH
TeytonL
McGavernDB
2006 Interleukin-10 determines viral clearance or persistence in vivo. Nat Med 12 1301 1309
37. EjrnaesM
FilippiCM
MartinicMM
LingEM
TogherLM
2006 Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med 203 2461 2472
38. CheongJY
ChoSW
HwangIL
YoonSK
LeeJH
2006 Association between chronic hepatitis B virus infection and interleukin-10, tumor necrosis factor-alpha gene promoter polymorphisms. J Gastroenterol Hepatol 21 1163 1169
39. MiyazoeS
HamasakiK
NakataK
KajiyaY
KitajimaK
2002 Influence of interleukin-10 gene promoter polymorphisms on disease progression in patients chronically infected with hepatitis B virus. Am J Gastroenterol 97 2086 2092
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 12- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Is Adherence to Erythrocytes a Factor in Extrapulmonary Dissemination?
- Identifying the Age Cohort Responsible for Transmission in a Natural Outbreak of
- Blockade of Immunosuppressive Cytokines Restores NK Cell Antiviral Function in Chronic Hepatitis B Virus Infection
- Metaeffector Exploits Host Proteasome to Temporally Regulate Cognate Effector
- The p53-Target Gene Drives Neutrophil-Mediated Protection against Lethal Bacterial Sepsis
- Development of an RNAi Protocol to Investigate Gene Function in the Filarial Nematode,
- Lysine Acetyltransferase GCN5-A Functions in the Cellular Response to Alkaline Stress and Expression of Cyst Genes
- Structural Basis for Apoptosis Inhibition by Epstein-Barr Virus BHRF1
- Molecular Architectures of Trimeric SIV and HIV-1 Envelope Glycoproteins on Intact Viruses: Strain-Dependent Variation in Quaternary Structure
- Interaction of c-Cbl with Myosin IIA Regulates Bleb Associated Macropinocytosis of Kaposi's Sarcoma-Associated Herpesvirus
- Glacial Refugia in Pathogens: European Genetic Structure of Anther Smut Pathogens on and
- Role for Sumoylation in Systemic Inflammation and Immune Homeostasis in Larvae
- Inflammasome Sensor Nlrp1b-Dependent Resistance to Anthrax Is Mediated by Caspase-1, IL-1 Signaling and Neutrophil Recruitment
- Noise Cancellation: Viral Fine Tuning of the Cellular Environment for Its Own Genome Replication
- Infectious Speciation Revisited: Impact of Symbiont-Depletion on Female Fitness and Mating Behavior of
- Eis Regulates Autophagy, Inflammation, and Cell Death through Redox-dependent Signaling
- NleC, a Type III Secretion Protease, Compromises NF-κB Activation by Targeting p65/RelA
- Early Myeloid Dendritic Cell Dysregulation is Predictive of Disease Progression in Simian Immunodeficiency Virus Infection
- HIV Capsid is a Tractable Target for Small Molecule Therapeutic Intervention
- Structural and Functional Studies of Nonstructural Protein 2 of the Hepatitis C Virus Reveal Its Key Role as Organizer of Virion Assembly
- Coming of Age—Sexual Reproduction in Species
- HIV-1 Envelope Subregion Length Variation during Disease Progression
- Compartmentation of Redox Metabolism in Malaria Parasites
- Evidence That Intracellular Stages of Utilize Amino Sugars as a Major Carbon Source
- Rapid End-Point Quantitation of Prion Seeding Activity with Sensitivity Comparable to Bioassays
- Dimeric 2G12 as a Potent Protection against HIV-1
- Hypoxia Induces an Immunodominant Target of Tuberculosis Specific T Cells Absent from Common BCG Vaccines
- CD4 Natural Regulatory T Cells Prevent Experimental Cerebral Malaria via CTLA-4 When Expanded In Vivo
- The Killing of African Trypanosomes by Ethidium Bromide
- H2A.Z Demarcates Intergenic Regions of the Epigenome That Are Dynamically Marked by H3K9ac and H3K4me3
- Large-Scale Field Application of RNAi Technology Reducing Israeli Acute Paralysis Virus Disease in Honey Bees (, Hymenoptera: Apidae)
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HIV-1 Envelope Subregion Length Variation during Disease Progression
- Coming of Age—Sexual Reproduction in Species
- Evidence That Intracellular Stages of Utilize Amino Sugars as a Major Carbon Source
- Compartmentation of Redox Metabolism in Malaria Parasites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



