-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEvidence That Intracellular Stages of Utilize Amino Sugars as a Major Carbon Source
Intracellular parasites, such as Leishmania spp, must acquire suitable carbon sources from the host cell in order to replicate. Here we present evidence that intracellular amastigote stages of Leishmania exploit amino sugars in the phagolysosome of mammalian macrophages as a source of carbon and energy. L. major parasites are capable of using N-acetylglucosamine and glucosamine as primarily carbon sources and contain key enzymes required for conversion of these sugars to fructose-6-phosphate. The last step in this pathway is catalyzed by glucosamine-6-phosphate deaminase (GND), which was targeted to glycosomes via a canonical C-terminal targeting signal when expressed as a GFP fusion protein. Mutant parasites lacking GND were unable to grow in medium containing amino sugars as sole carbohydrate source and rapidly lost viability, concomitant with the hyper-accumulation of hexosamine-phosphates. Expression of native GND, but not a cytosolic form of GND, in Δgnd parasites restored hexosamine-dependent growth, indicating that toxicity is due to depletion of glycosomal pools of ATP. Non-lethal increases in hexosamine phosphate levels in both Δgnd and wild type parasites was associated with a defect in promastigote metacyclogenesis, suggesting that hexosamine phosphate levels may influence parasite differentiation. Promastigote and amastigote stages of the Δgnd mutant were unable to replicate within macrophages and were either completely cleared or exhibited reduced lesion development in highly susceptible Balb/c mice. Our results suggest that hexosamines are a major class of sugars in the macrophage phagolysosome and that catabolism of scavenged amino sugars is required to sustain essential metabolic pathways and prevent hexosamine toxicity.
Published in the journal: . PLoS Pathog 6(12): e32767. doi:10.1371/journal.ppat.1001245
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001245Summary
Intracellular parasites, such as Leishmania spp, must acquire suitable carbon sources from the host cell in order to replicate. Here we present evidence that intracellular amastigote stages of Leishmania exploit amino sugars in the phagolysosome of mammalian macrophages as a source of carbon and energy. L. major parasites are capable of using N-acetylglucosamine and glucosamine as primarily carbon sources and contain key enzymes required for conversion of these sugars to fructose-6-phosphate. The last step in this pathway is catalyzed by glucosamine-6-phosphate deaminase (GND), which was targeted to glycosomes via a canonical C-terminal targeting signal when expressed as a GFP fusion protein. Mutant parasites lacking GND were unable to grow in medium containing amino sugars as sole carbohydrate source and rapidly lost viability, concomitant with the hyper-accumulation of hexosamine-phosphates. Expression of native GND, but not a cytosolic form of GND, in Δgnd parasites restored hexosamine-dependent growth, indicating that toxicity is due to depletion of glycosomal pools of ATP. Non-lethal increases in hexosamine phosphate levels in both Δgnd and wild type parasites was associated with a defect in promastigote metacyclogenesis, suggesting that hexosamine phosphate levels may influence parasite differentiation. Promastigote and amastigote stages of the Δgnd mutant were unable to replicate within macrophages and were either completely cleared or exhibited reduced lesion development in highly susceptible Balb/c mice. Our results suggest that hexosamines are a major class of sugars in the macrophage phagolysosome and that catabolism of scavenged amino sugars is required to sustain essential metabolic pathways and prevent hexosamine toxicity.
Introduction
A number of microbial pathogens selectively target macrophages and other phagocytic cells during the course of infection of their mammalian hosts [1], [2]. These pathogens can reside within a variety of vacuolar and cytoplasmic compartments from which they must scavenge all of their carbon and nitrogen sources, as well as other essential nutrients [3], [4], [5]. With few exceptions, the biochemical composition of these intracellular niches and the extent to which intracellular pathogens utilize different carbon sources is poorly defined.
Leishmania are sandfly-transmitted protozoan parasites that primarily reside in macrophages throughout infection in their mammalian hosts, causing a spectrum of important diseases in more than 12 million people worldwide [6]. Infection of the mammalian host is initiated by flagellated promastigote stages that develop within the mid - and fore-gut of the sandfly vector. Promastigotes injected into the skin during a sandfly bloodmeal are rapidly phagocytosed by neutrophils and macrophages and delivered to the mature phagolysosome where they differentiate to small, non-motile amastigotes [7]. In animal models, the number of infected macrophages increases rapidly during the early stages of infection, eventually plateauing coincident with the formation of loosely structured granulomatous lesions dominated by infected macrophages [8]. In susceptible animals, lesion development and metastasis of infected macrophages to other tissues can lead to death, while in resistant animals a strong proinflammatory (TH1) response leads to lesion cure without sterile immunity [9].
Recent studies have provided insights into the major carbon sources used by intracellular stages of Leishmania. A L. mexicana mutant lacking three high affinity hexose transporters is unable to establish an infection in macrophages or susceptible mice [10], suggesting that hexose uptake is essential for intracellular growth. However, levels of hexose in the phagolysosome are likely to be limiting for growth as a L. major mutant with a defect in gluconeogenesis is also poorly virulent in macrophages and susceptible mice [11]. These studies suggest that intracellular amastigotes depend on both salvage as well as de novo synthesis of hexoses from the host niche. The phagolysosome of macrophages could potentially contain a range of different sugars as a result of turnover of host glycans, glycoproteins and proteoglycans [12]. We have recently shown that a L. major hexosamine auxotroph is capable of inducing normal lesions in susceptible mice [13], suggesting that the macrophage phagolysosomes contain sufficient levels of the amino sugars, glucosamine (GlcN) or N-acetylglucosamine (GlcNAc), to sustain the minimal hexosamine requirements of intracellular amastigotes. The Leishmania genomes also contain a number of genes that are predicted to comprise a functional N-acetylglucosamine (NAG) catabolic pathway raising the possibility that hexosamine sugars may be an important carbon source in vivo (Fig. 1). This pathway catalyzes the conversion of GlcNAc to fructose-6 phosphate (Fru6P) via successive uptake, phosphorylation, deacetylation and isomerization-deamination reactions [14] (Fig. 1). A number of pathogenic bacteria (i.e. Vibrio cholerae) and fungi (i.e. Candida albicans), but not in the non-pathogenic fungi Saccharomyces cerevisiae [14], [15] have a functional NAG catabolic pathway (Fig. 1). In C. albicans, the NAG catabolic pathway is specifically induced after phagocytosis by macrophages and is important for virulence [16], [17].
Fig. 1. Hexosamine metabolism in Leishmania. 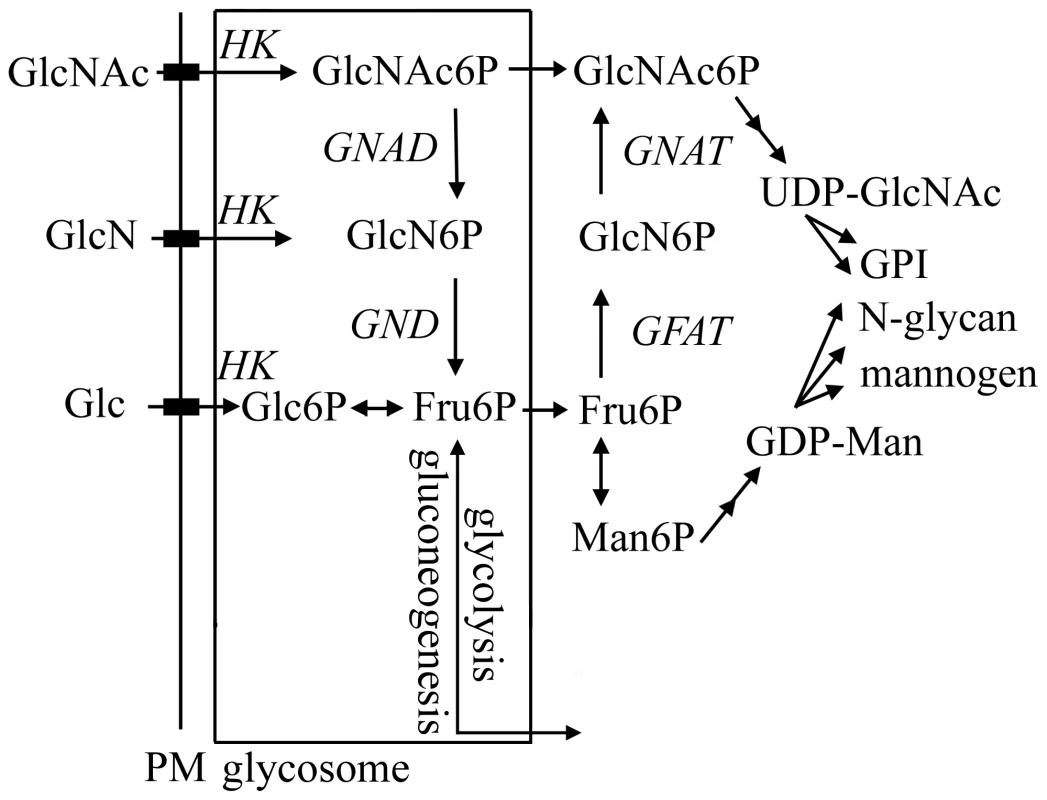
Exogenous hexosamines (GlcN/GlcNAc) are phosphorylated in the glycosome by a hexose kinase (HK) and either transported to the cytosol for conversion to UDP-GlcNAc or catabolized to Fru6P by the activities of N-acetylglucosamine 6-phosphate deacetylase (NAGD) and glucosamine 6-phosphate deaminase (GND). GND generates fructose-6-phosphate (Fru6P), a key intermediate in carbon metabolism and several pathways of glycoconjugate biosynthesis. The de novo synthesis of GlcNAc6P is catalyzed by the cytoplasmic enzymes, glutamine:Fru6P aminotransferase (GFAT) and glucosamine 6-phosphate acetylase (GNAT). In this study we have investigated the role of the putative L. major glucosamine-6 phosphate deaminase (GND) in central carbon metabolism and pathogenicity in vivo. We show that cultured parasite stages are able to utilize GlcNAc and to a lesser extent GlcN as their major carbon source in vitro. We also show that GND is targeted to modified peroxisomes, termed glycosomes and that this localization is essential for growth when hexosamines are the sole carbohydrate source. Finally, we show that GND is important for parasite growth in macrophages and critical for establishment of infection in susceptible animal models. Our findings suggest that intracellular stages of Leishmania are dependent on both scavenged hexosamines and de novo synthesized sugar phosphates to sustain essential pathways of carbohydrate metabolism.
Results
Identification of the NAG-pathway in Leishmania
We have recently shown that L. major can scavenge exogenous GlcN or GlcNAc for the synthesis of essential glycoconjugates [13]. To investigate whether Leishmania utilize exogenous hexosamines as a carbon source, L. major promastigotes were cultivated in defined medium containing either Glc, GlcN or GlcNAc as sole carbohydrate source. L. major promastigotes failed to proliferate in the absence of any sugars although they remain viable for several days (Fig. 2A). Growth was restored when the glucose-free medium was supplemented with Glc, GlcNAc or, to a lesser extent, with GlcN (Fig. 2A), demonstrating that Leishmania expresses a functional NAG catabolic pathway and can utilizes hexosamines as the main carbon source. BLAST searches of the Leishmania genome (tritrypdb.org) revealed the presence of genes encoding a putative GlcNAc6P deacetylase (NAGD, LmjF36.0040) and a GlcN6P deaminase (LmjF32.3260) indicating the presence of a canonical NAG-catabolic pathway (Fig. 1). These genes shared 22% and 41% identical amino acids to C. albicans NagA and GND1, respectively. The genome of the related parasite, T. cruzi also contains homologues for NAGD (Tc00.1047053506507.10) and GND (Tc00.1047053511025.50), while T. brucei contains a homologue for GND (Tb11.01.8520), but not NAGD (Fig. S1). The absence of NAGD in T. brucei is consistent with the recent finding that this parasite can utilize GlcN, but not GlcNAc [13]. None of the trypanosomatid parasites contained identifiable orthologes for hexosamine-specific transporters or kinases, suggesting that the uptake and phosphorylation of hexosamine sugars may be mediated by previously characterized hexose transporters and hexose/gluco-kinase(s) (Fig. 1). Taken together, these data show that Leishmania contains a functional NAG catabolic pathway and that GlcNAc/GlcN catabolism is sufficient to sustain parasite growth.
Fig. 2. GND is required for growth on GlcN or GlcNAc. 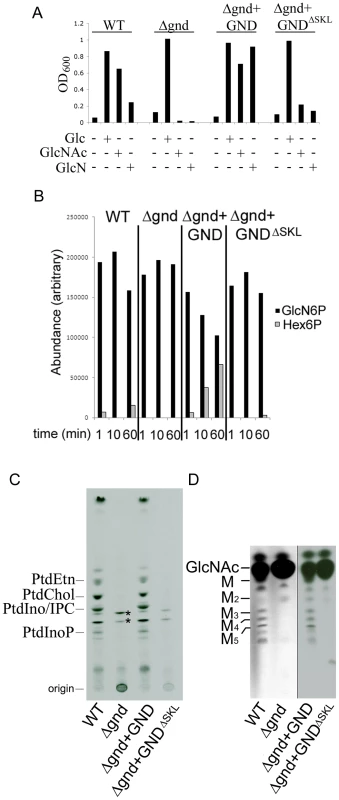
(A) Wild type (WT), Δgnd and complemented strains (Δgnd +GND, Δgnd +GNDΔSKL were suspended in CDM with or without Glc, GlcN or GlcNAc (13 mM). The optical density of cultures (OD600) at day 4 are shown. (B) L. major wild type, Δgnd, Δgnd +GND and Δgnd +GNDΔSKL promastigotes were lysed and GND activity determined by measuring production of hexose-6-phosphates from GlcN6P. (C,D) Wild type, Δgnd and complemented strains were labeled with 3H-GlcN and incorporation of 3H-label into (C) lipids and (D) the carbohydrate reserve polymer mannogen, assessed by HPTLC and fluorography. Major lipid species comprise PtdEtn, PtdCho, PtdIno, inositolphosphoceramide (IPC), PtdInoP and GPI species (asterix); Mannose (M) and mannogen oligomers (Mn). Characterization of Leishmania GND
To further define the role of the NAG catabolic pathway in Leishmania, the two chromosomal alleles of the putative GND gene in L. major were sequentially replaced with nourseothricin and bleomycin resistance cassettes. Several clones were isolated in Glc-rich medium, which contained correct insertion of the resistant cassettes and loss of the GND gene as confirmed by PCR analysis (Fig. S2). In contrast to wild type parasites, Δgnd promastigotes were unable to grow in medium containing GlcNAc or GlcN as the only source of hexose (Fig. 2A). The Δgnd growth phenotype was completely reversed by ectopic expression of GND on the pX-based episome (Fig. 2A). Overexpression of GND in the Δgnd mutant markedly improved growth on GlcN (Fig. 2A), indicating that low levels of GND expression in wild type parasites may account for poor growth on GlcN. Consistent with this conclusion, GND enzyme activity in cell extracts of wild type parasite was very low, but was readily detected in the Δgnd parasite line overexpressing native GND (Fig. 2B). Loss of GND activity in the Δgnd mutant was further confirmed by metabolic labeling with [3H]GlcN and analysis of major end-products of central carbon metabolism. Following labeling of wild type parasites with [3H]GlcN, label was observed in the major cellular phospholipids and the carbohydrate reserve material mannogen (Fig. 2C, D), reflecting the intracellular conversion of GlcN6P to Fru6P and the redistribution of label into other intermediates in central carbon metabolism. In contrast, none of these metabolites were labeled with [3H]GlcN in Δgnd parasites (Fig. 2C,D). The residual labeling of GPI lipids in the mutant reflects the direct incorporation of [3H]-GlcNAc into these glycolipids (Fig. 2C).
The glycosomal localization of GND is required for efficient function
L. major GND is predicted to contain a canonical C-terminal glycosomal targeting signal comprising the tripeptide, SKL. Glycosomal localization was confirmed by expression of a N-terminal GFP chimera of GND in promastigotes. The GFP::GND fusion protein was restricted to punctate structures throughout the parasite cell body co-localizing exactly with the co-expressed glycosomal marker mCherry::FBP fusion protein (Fig. 3A). Significantly, a GFP-fusion protein containing GND without the C-terminal tripeptide SKL (GNDΔSKL) exhibited a cytosolic distribution (Fig. 3B) demonstrating that the SKL motif is essential for the glycosomal targeting of GND. To investigate whether glycosomal targeting of GND is required for activity, Δgnd parasites were transfected with episomes encoding untagged GNDΔSKL. Complementation with GNDΔSKL did not restore normal growth on either GlcN or GlcNAc, although after a lag phase of several days some growth was observed (Fig. 2A). Similarly, metabolic conversion of 3H-GlcN into mannogen or lipids was undetectable in the presence of GNDΔSKL (Fig. 2B, C). The glycosomal localization of GND is therefore required for normal growth on hexosamine sugars.
Fig. 3. Glycosomal targeting of GND. 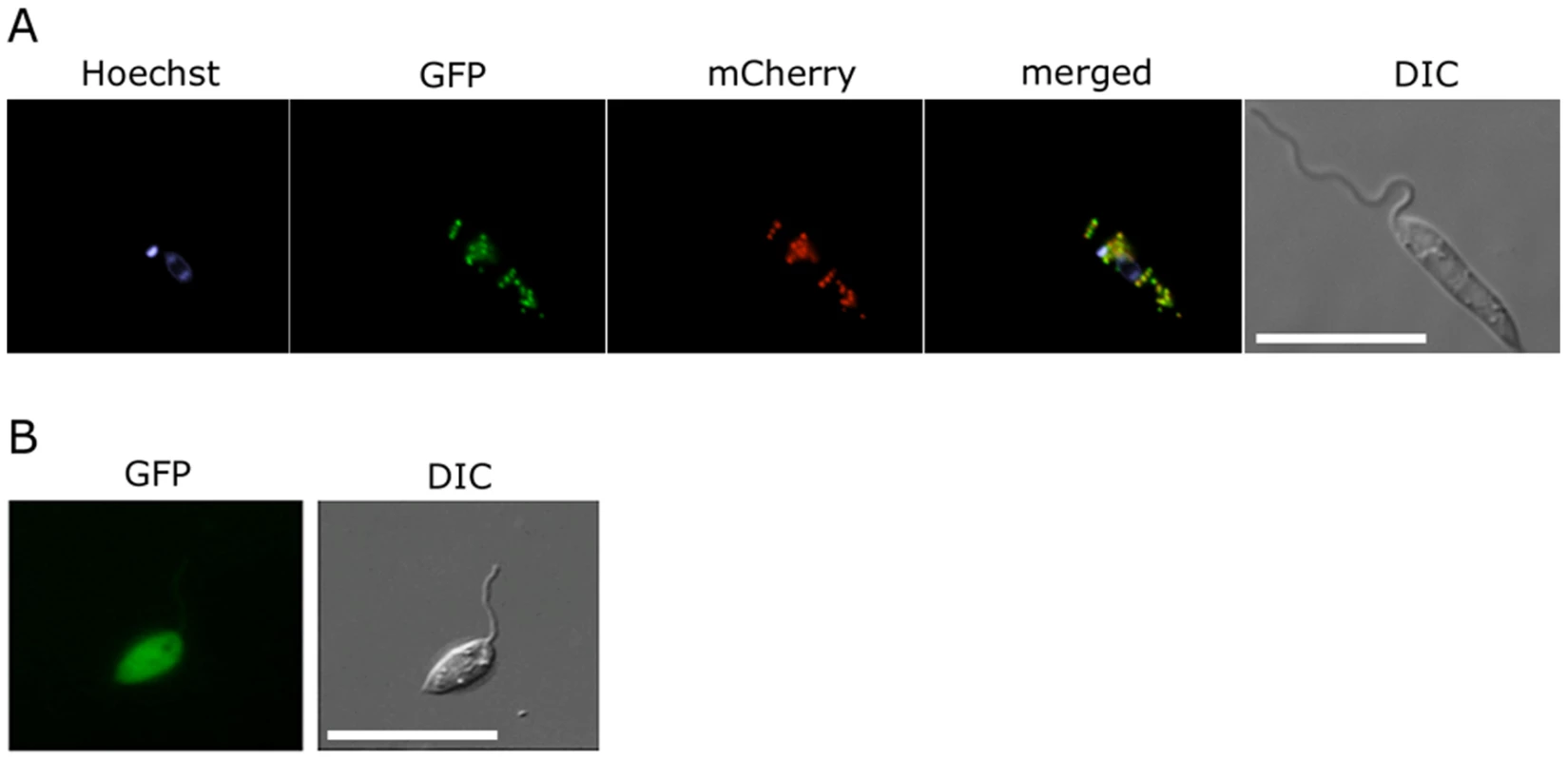
(A) L. major promastigotes were transfected with plasmid encoding GFP-GND and mCherry-FBP (glycosomal marker) protein and the two proteins localized by fluorescence microscopy and Differential interference contrast (DIC) microscopy after labeling with Hoechst dye (nuclear and mtDNA). (B) Localization of the GFP-GND fusion protein lacking the canonical C-terminal tripeptide sequence SKL. Scale bar = 10 µm. Growth on hexosamine sugars is cytotoxic for the L. major Δgnd mutant
While wild type promastigotes require an exogenous carbohydrate source for growth, they retain a high level of viability when completely starved of hexoses/hexosamines for 24hr (Fig. 4A,B). The Δgnd parasites also retain viability when suspended in hexose/hexosamine-free medium, but rapidly lost viability when suspended in medium containing either GlcN or GlcNAc (Fig. 4A,B). Hexosamine toxicity in Δgnd parasites was associated with the hyper-accumulation of GlcNAc6P and GlcN6P (Fig. 5A), most likely reflecting the unregulated phosphorylation of internalized sugars by the glycosomal hexose kinase. Hexosamine toxicity was largely abrogated by addition of alternative carbon sources, such as glucose or glycerol (Fig. 4B), that would allow restoration of ATP levels in the glycosome. To further investigate the consequences of elevated levels of GlcNAc6-P on glycosomal metabolism, wild type and Δgnd parasites were pretreated with GlcNAc, then metabolically labeled with 13C-U-glucose. The de novo synthesis of Glc6P (reflecting glycosomal levels of ATP) was then assessed by measuring the incorporation of 13C into sugar phosphates by gas chromatography mass spectrometry. Wild type parasites rapidly phosphorylated exogenous glucose whether or not they had been pre-cultivated in medium containing GlcNAc (Fig. 5B). In contrast, Δgnd parasites exhibited a marked lag (∼30min) in glucose phosphorylation when pre-incubated in GlcNAc-containing medium, but not when preincubated in Glc/GlcNAc-containing medium (Fig. 5B). These results suggest that hexosamine toxicity arises as a result of the largely unregulated uptake and phosphorylation of exogenous hexosamine sugars by glycosomal hexose kinase, with concomitant depletion of glycosomal pools of ATP. This toxicity is abrogated by the addition of alternative carbon sources that allow net ATP production.
Fig. 4. Exogenous hexosamines become toxic in the absence of GND. 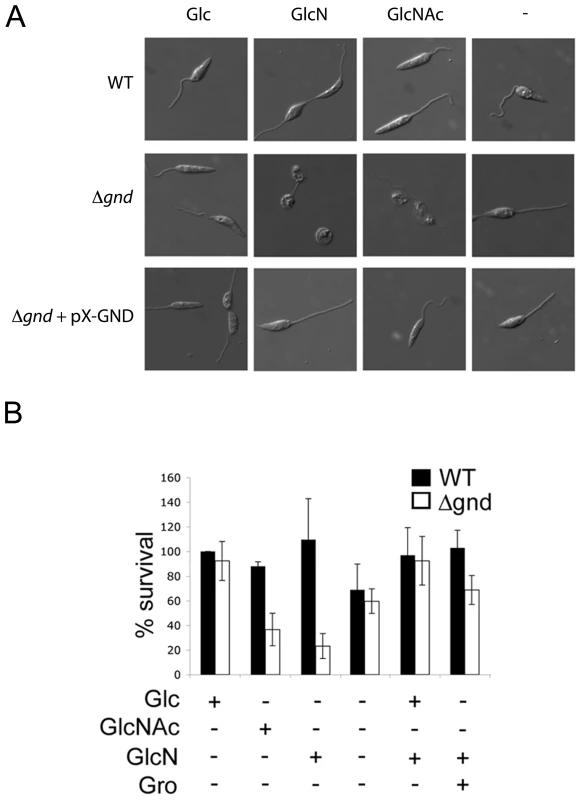
(A) Wild type, Δgnd and Δgnd complemented with full length GND or truncated GND (GNDΔSKL) were cultivated in M199 medium containing either Glc, GlcN, GlcNAc or no hexoses. Parasite morphology was monitored by DIC microscopy after 24 hr. (B) Wild type and Δgnd promastigotes were cultivated in M199 medium with or without indicated sugars for 24 hr. Parasite survival was determined by suspending parasites in complete medium containing glucose and measuring OD600 at day 2. Survival is expressed relative to wild type parasites grown in glucose-supplemented media from three independent experiments. Error bar = SD. Glycerol is abbreviated as Gro. Fig. 5. Accumulation of hexosamine phosphates in GlcNAc-fed Δgnd parasites. 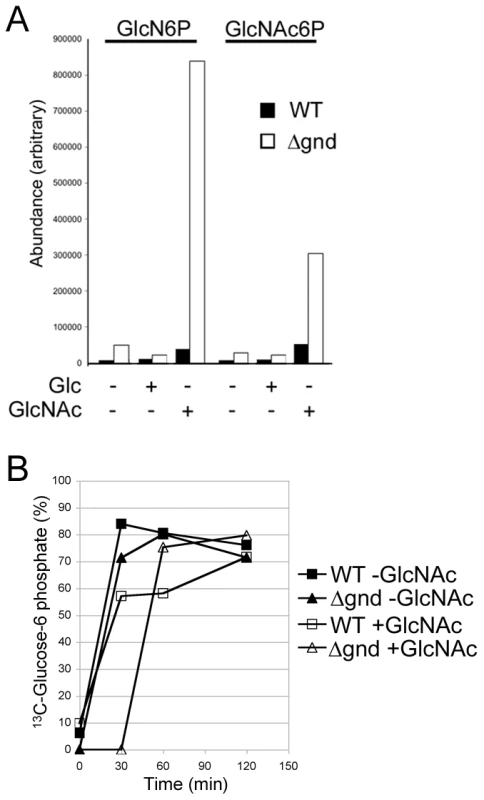
(A) Concentration of intracellular sugar-phosphates in WT and Δgnd parasites after cultivation (4hr) in the presence of Glc or GlcNAc. (B) L. major wild type and Δgnd promastigotes were suspended in hexose-free CDM supplemented with or without 13 mM GlcNAc. After 4 hr, the medium was supplemented with 13 mM [U-13C]-Glc and parasites harvested at the indicated time points. The rate of synthesis of Glc6P (indicated by percent Glc6P labeled with 13C), an indicator of glycolytic flux, was determined by GC-MS. Promastigote differentiation is modulated by hexosamine-phosphate levels
A proportion of L. major promastigotes stages differentiate into a highly infectious metacyclic stage as cultures reach stationary phase growth. Metacyclogenesis can be monitored by measuring the extent to which promastigotes are no longer agglutinated with the peanut agglutinin lectin, reflecting structural changes in the surface lipophosphoglycan [18]. While 9.9 ± 0.9 % of wild type promastigotes became PNA-negative in stationary phase, only 1.6 ± 1 % of Δgnd promastigotes converted to PNA-negative phenotype (Fig. 6A). Metacyclogenesis was largely restored (8 ± 4%) in the Δgnd+GND cell line (Fig. 6A). Inhibition of metacyclogenesis in the Δgnd mutant was associated with a modest increase in intracellular levels of hexosamine-phosphates (5A), presumably reflecting continued de novo synthesis from glucose, combined with reduced catabolism. To further investigate this association, wild type promastigotes were cultivated in medium containing either glucose or GlcNAc as sole carbohydrate. As expected, glucose-fed wild type promastigotes containing normal levels of hexosamine phosphate exhibited a high rate of metacyclogenesis (22 ± 2.8 %). In contrast, GlcNAc-fed promastigotes containing elevated levels of hexosamine phosphate (Fig. 5A) displayed a clear defect in metacyclogenesis (3.6 ± 0.6 %) (Fig. 6B). Changes in the intracellular levels of hexosamine-phosphates may thus regulate key differentiation processes in these parasites.
Fig. 6. Non-lethal accumulation of hexosamine phosphates leads to a defect in metacyclogenesis. 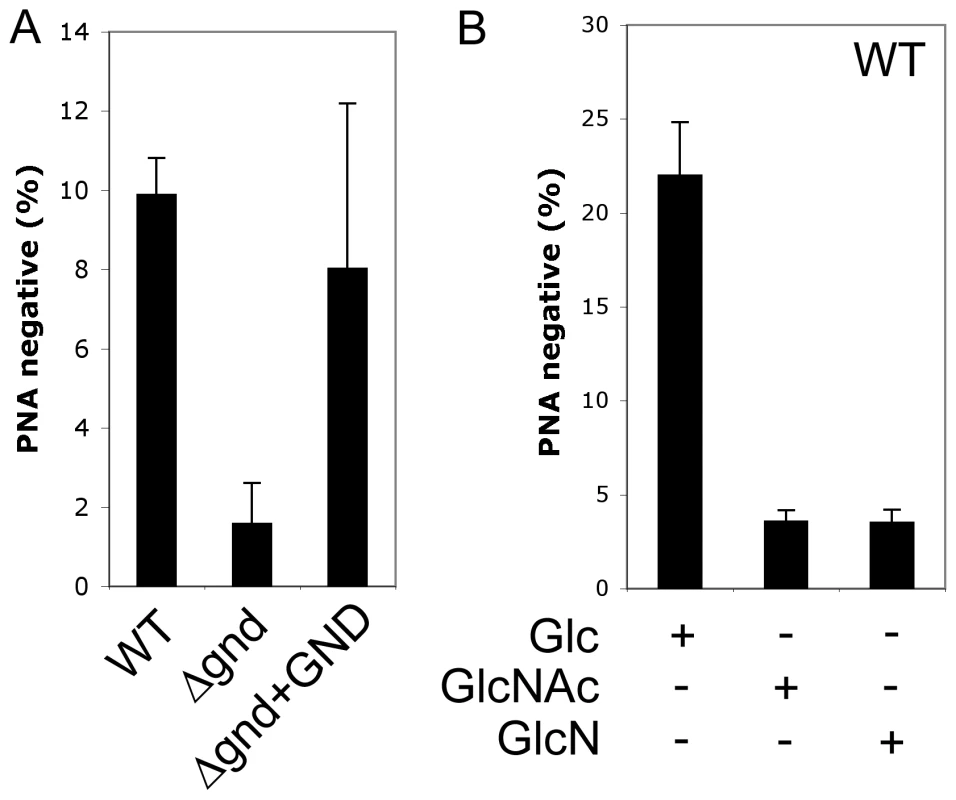
(A) WT, Δgnd and Δgnd+GND promastigotes were cultivated in SDM containing 10% FCS and differentiation to metacyclic stages determined after agglutination with the peanut agglutinin lectin (PNA). Unlike dividing promastigotes, metacyclic promastigotes are not agglutinated by PNA. (B) L. major wild type promastigotes were grown in CDM containing either Glc, GlcNAc or GlcN as the sole carbohydrate and metacyclogenesis assessed by PNA agglutination. Error bars represent SED from three independent experiments. Attenuated virulence of Δgnd parasites in susceptible mice and macrophages
To investigate whether hexosamine metabolism is required for Leishmania infectivity, BALB/c mice were infected subcutaneously with wild type and Δgnd stationary phase promastigotes. Wild type parasites produced large lesions by eight weeks post infection (Fig. 7A,B). In contrast, 50% of the mice infected with Δgnd promastigotes failed to develop any lesions (Fig. 7A,B) and viable parasites could not be recovered from the initial inoculation sites or lymph nodes of these mice (Fig. 7C). The rest of the mice developed small lesions over a significantly longer time period (∼8–12 weeks) than wild type parasites (Fig. 7A,B). The parasite burden in the draining lymph nodes of these mice was 4-fold less than in mice infected with wild type parasites (Fig. 7C). To determine whether reduced infectivity was due to selective loss of virulence in the promastigote stage, naïve mice were infected with lesion-derived wild type and Δgnd amastigotes. As with promastigote infections, Δgnd amastigote infections progressed more slowly than wild type amastigote infections (Fig. 7D). The infectivity of Δgnd was largely restored by ectopic expression of GND (Fig. 6A,B). Unexpectedly, the infectivity of the mutant was also partially restored by ectopic expression of truncated GNDΔSKL (Fig. 7A,B). While expression of this construct did not rescue the growth of mutant promastigote stages on hexosamine, it did allow a low level of growth in culture (Fig. 2A). This GND activity appears to be sufficient to at least partially restore normal virulence in the amastigote stage.
Fig. 7. Attenuated virulence of Δgnd in mice and macrophages. 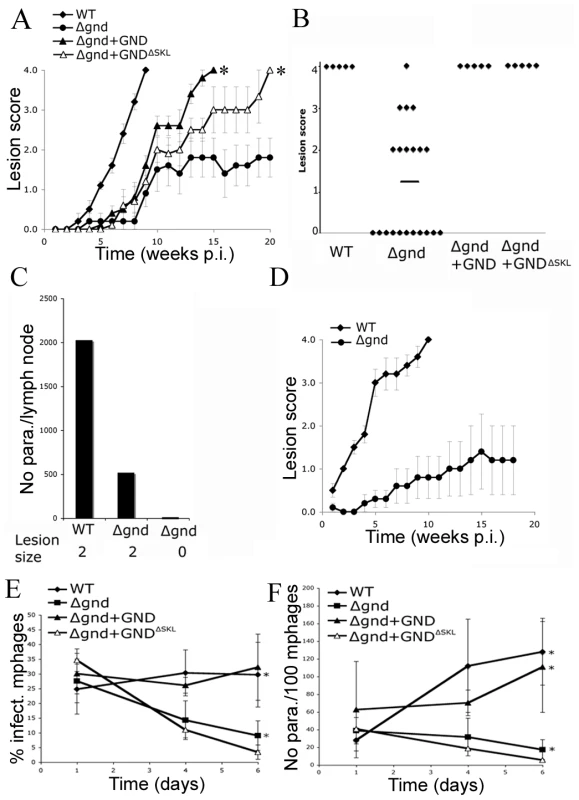
(A) Wild type, Δgnd and complemented Δgnd promastigotes were used to infect BALB/c mice intradermally and lesion formation was scored over time (error bar = SEM, n = 5). * p<0.01 (Student t-test). (B) Lesion scores in mice infected with wild type, Δgnd, and complemented Δgnd promastigotes 20 weeks post-infection. The line represents the average of three independent experiments of 15 mice in total. (C) Lesion burden was determined by the limiting dilution assay from draining lymph nodes (parasite numbers are based on 1 million lymph cells). (D) Lesion-derived amastigotes were used to re-infect naïve BALB/c mice and lesions were scored as in (A). RAW 264.7 macrophages were infected with promastigotes of L. major wild type, Δgnd, Δgnd +GND and Δgnd +GNDΔSKL and (E) percent infected macrophages and (F) intracellular parasite numbers were determined by microscopy at day 1, 4 and 6 p.i. Error bars represent SED from three independent experiments. * p<0.05 (Student t-test). To determine whether the attenuated virulence of Δgnd parasites in mice was associated with a concomitant decreased capacity to survive and grow in macrophages, RAW 264.7 macrophages were infected with wild type and Δgnd stationary promastigotes. Wild type and Δgnd parasites were internalized by infected macrophages with equal efficiency (Fig. 7E). However, Δgnd parasites failed to grow and were effectively cleared by the macrophages within 6 days, while numbers of wild type parasites increased over the same period (Fig. 7E, F). This phenotype was reversed by ectopic expression of full length GND, but not by cytosolic GND (Fig. 7E, F). Similarly to promastigotes, Δgnd lesion-derived amastigotes also failed to replicate in ex vivo infected macrophages (Fig. 8). These data suggest that hexosamine catabolism is particularly critical for amastigote survival in naïve, non-activated macrophages both in vitro and in vivo. Moreover, the fact that lesion-derived Δgnd amastigotes are unable to proliferate within cultured macrophages highlights potential differences in hexosamine levels in lesion and cultured macrophages.
Fig. 8. Survival and growth of Δgnd amastigotes in ex vivo macrophages. 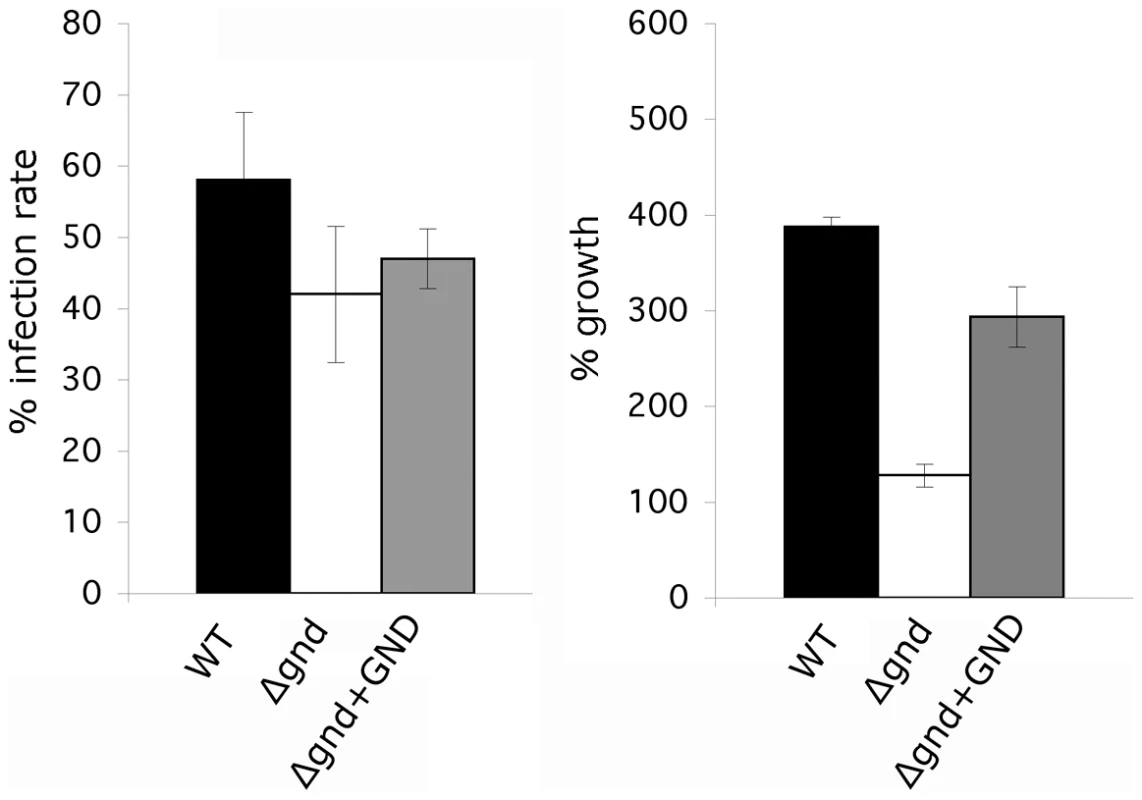
BALB/c mouse peritoneal macrophages were infected with lesion-derived amastigotes of L. major wild type, Δgnd, complemented Δgnd (10∶1 ratio parasites/macrophage). Infected macrophages and intracellular parasite growth (as percentage of number of parasites/100 macrophages compared to day 1) were determined by microscopy at day 4 p.i. (Error bars: SED, n = 3). Discussion
Macrophages and other phagocytic cells appear to restrict glucose levels in endocytic compartments, including mature phagolysosomes, in order to prevent growth of microbes [3], [4]. While many bacterial and fungal pathogens are able to survive in macrophages by exploiting alternative carbon sources, such as lipids and amino acids [19], Leishmania parasites appear unable to proliferate in the absence of a hexose source despite having an active gluconeogenic pathway [11], [20]. In this study we show that catabolism of the amino sugars via the NAG pathway is important for growth in macrophages and establishment of infection in the mammalian host.
Our study has provided the first detailed analysis of hexosamine catabolism in Leishmania. These parasites lack identifiable homologues of other eukaryotic hexosamine sugar transporters or kinases suggesting that GlcN and GlcNAc are internalized by the previously characterized hexose transporters and phosphorylated by glycosomal hexose/gluco-kinases [13]. Glycosomal pools of GlcNAc6P are subsequently catabolized by the combined action of a putative NAGD and the GND characterized in this study (Fig. 1). Leishmania lacking GND were unable to grow in medium containing hexosamines as the sole source of sugar or to catabolize exogenous [3H]-glucosamine, indicating that GND encodes the only GlcN6P deaminase activity in these parasites. Interestingly, much higher concentrations of GlcN than GlcNAc are required to sustain maximal growth rates, despite the fact that both sugars are internalized at similar rates [13]. It is possible that GlcNAc is more efficiently phosphorylated by hexose kinase than GlcN [13], or that GlcNAc and GlcN differentially regulate the activity of GND [21], [22], resulting in different rates of utilization.
Leishmania GND was localized to the peroxisome-like glycosomes that contain all of the enzymes involved in the upper part of glycolysis and the final steps in gluconeogenesis [11], [23], [24], [25]. The glycosomal localization of GND was critical for parasite growth on GlcN/GlcNAc and for preventing hexosamine toxicity. Hexosamine toxicity was associated with the hyper-accumulation of hexosamine-phosphates, cell swelling and eventual lysis. The accumulation of hexosamine phosphates reflects the absence of normal allosteric feedback mechanisms in the glycosomally located hexose kinases [23], [24], [25] and likely leads to the depletion of glycosomal ATP levels and/or osmotic disruption of this organelle. In support of this notion, hexosamine toxicity was prevented by addition of alternative carbon sources, such as glucose and glycerol that are catabolized in the glycosome with net production of ATP. Direct evidence that hexosamine-phosphate accumulation resulted in selective depletion of glycosomal ATP levels was provided by the finding that phosphorylation of 13C-glucose in Δgnd, but not wild type parasites, was delayed following growth in hexosamine-containing medium. Significantly, expression of a cytosolic form of GND in the Δgnd mutant did not prevent hexosamine toxicity. While our previous studies suggest that hexosamine-phosphates can equilibrate across the glycosome membrane [13], this flux appears to be insufficient to prevent the build-up of hexosamine sugars in glycosomes. In addition to preventing hexosamine toxicity, the glycosomal localization of GND may also be required to avoid the futile cycling of hexosamine and hexose-phosphates in the cytosol. Specifically, Leishmania express a cytosolically located glutamine:fructose-6-phosphate amidotransferase that is required for the de novo synthesis of GlcN6P from Fru6P (Fig. 1). Futile cycling of de novo synthesized GlcN6P to Fru6P by cytosolic GND could lead to the depletion of cellular pools of the sugar nucleotide UDP-GlcNAc and reduced synthesis of essential glycoconjugates [13].
Deletion of gnd resulted in an unusual attenuated virulence phenotype in the BALB/c model. In approximately half the infections, mutant parasites were completely cleared, while in the remaining infections, small lesions were induced after a delay of several weeks. This phenotype was reproducible, regardless of whether mutant promastigote or lesion-derived amastigotes were used, indicating that the delayed lesion phenotype was not due to the loss of stage-specific factors or to the evolution of suppressor strains. The poor growth of Δgnd parasites in ex vivo infected macrophages and attenuated virulence in the BALB/c animal strongly suggests that intracellular amastigotes are dependent on the uptake and catabolism of hexosamine sugars for normal growth in the phagolysosome compartment. While previous studies have suggested that both promastigote and amastigote stages need to take up exogenous sugars for normal growth in vitro and in vivo [26], the nature of these sugars has been poorly defined. The uptake and catabolism of amino and neutral sugars is likely to be required to sustain fluxes through essential pathways such as N-glycosylation [13], the pentose phosphate pathway [27], inositol synthesis [28], and catabolism of the major carbohydrate reserve material, mannogen [29], [30]. Interestingly, amastigotes also need to synthesize sugar de novo via the gluconeogenic pathway [11]. The fact that gluconeogenesis is required for both the establishment of infection and induction of lesions suggests that sugar levels in the phagolysosome are generally low at all stages of infection. In the light of these observations it is curious that surviving Δgnd parasites are able to eventually produce small lesions. It is possible that hexosamine sugars constitute the major sugar type in neutrophils and non-activated macrophages encountered during the early stages of infection, while other sugars may become more abundant in macrophages at later stages of infection, providing a more permissive nutrient environment for Δgnd parasites. Alternatively, amastigote requirements for hexose/hexosamine may be higher during early stages of infection, but decrease at later stages, reflecting differences in growth rate [8]. The latter possibility was supported by the finding that complementation of the Δgnd mutant with the cytosolic GNDΔSKL construct led to a significant restoration of virulence in BALB/c mice. While expression of this construct in Δgnd promastigote stages was unable to restore normal growth on amino sugars in vitro, a low rate of growth was observed that may be sufficient to sustain the energy needs of lesion amastigotes. Similarly, we have shown that L. major amastigote GlcN auxotrophs need significantly less amino sugars for viability than promastigotes [13].
While hexosamine/hexose starvation is likely to be a major contributor to the Δgnd loss of virulence phenotype, other factors could contribute to reduced parasite viability in vivo. For example, hexosamine toxicity arising from the hyper-accumulation of GlcN(Ac)6P could occur if the phagolysosomes of infected host cells lacked other carbon sources needed to restore the glycosome energy balance. Even modest increases in the intracellular levels of GlcN6P and GlcNAc6P could alter the physiological state of parasites and virulence in vivo. Intriguingly, sub-lethal increases in hexosamine-phosphates in both Δgnd and wild type parasites inhibited the differentiation of PNA-positive promastigotes to PNA-negative metacyclic promastigotes in stationary phase cultures. Metacyclic promastigotes are preadapted for life in the mammalian host and defects in metacyclogenesis could contribute to loss of virulence of Δgnd promastigote stages. However, lesion-derived Δgnd amastigotes exhibited a similar attenuated virulence phenotype in BALB/c mice, suggesting that other factors are equally or more important. It remains unclear how changes in intracellular pools of hexosamine phosphates could impact on parasite differentiation. Alterations in the cellular levels of hexosamine-phosphates and UDP-GlcNAc have been shown to regulate mammalian growth and differentiation, either by altering the extent to which many cytosolic and nuclear proteins are modified with O-GlcNAc, or by altering the repertoire of surface expressed N-glycans [31], [32]. In the pathogenic fungi, Candida albicans, defects in GlcNAc uptake or catabolism also impact on differentiation (hyphae formation) and virulence [16], [17]. However, neither Candida albicans or Leishmania modify cytosolic proteins with O-GlcNAc or synthesize complex N-glycans [13], [33]. It is possible that the intracellular levels of these metabolites are directly sensed by signaling pathways involved in regulating parasite growth and differentiation. In this respect, it is notable that elevated levels of tetrahydrobiopterin also inhibit metacyclogenesis in L. major [34].
These and previous findings [13] support the conclusion that hexosamines are amongst the most abundant sugars in the Leishmania occupied phagolysosome. Hexosamines are major components in a number of host glycoconjugates that are continuously delivered to macrophage lysosomes. For example, macrophages constitutively internalize a range of extracellular proteoglycans and glycoaminoglycans that are rich in GlcN(Ac) [12], [35]. Other GlcN(Ac)-containing host molecules could be delivered to the parasite vacuole via autophagy or glycan turnover pathways [12], [36]. These glycoconjuates are sequentially degraded by a range of host lysosomal endo and exoglycosidases [35]. Leishmania spp also secrete a chitinase that could be involved in facilitating the degradation of GlcN/GlcNAc-containing glycoconjugates delivered to amastigote-occupied lysosomes [37]. Such as function is supported by the previously unexplained finding that overexpression of the L. mexicana chitinase enhances lesion development in mice [37], [38].
Other trypanosomatid parasites, including the important human pathogen T. cruzi, are predicted to express a NAG catabolic pathway. Mammalian-infective stages of T. cruzi reside within the lysosome and cytoplasm of macrophages and other host cells [39] and may scavenge non-phosphorylated and/or phosphorylated amino sugars in these niches. In contrast, T. brucei contains a putative GND but lacks a putative GlcN6P de-N-acetylase. The absence of a full NAG catabolic pathway in this parasite correlates with a limited capacity to internalize amino sugars [40]. Only GlcN is taken up to any extent and with very low affinity (Km ∼14 mM) [41]. The acquisition of a full NAG catabolic pathway in Leishmania and T. cruzi may thus have preceded a relaxation in the substrate specificity of their hexose transporters, allowing these parasites to colonize new niches within their respective insect and mammalian hosts.
Materials and Methods
Ethics statement
Use of mice was approved by the Institutional Animal Care and Use Committee of the University of Melbourne (ethics number 0811011.1). All animal experiments were performed in accordance with the Australian National Health Medical Research council (Australian code of practice for the care and use of animals for scientific purposes, 7th Edition, 2004, ISBN: 1864962658).
Parasite strains and cell culture
Promastigotes of L. major (MHOM/SU/73/5ASKH) were cultured at 27°C in semi-defined medium-79 (SDM) supplemented with 10% FCS (Gibco, Invitrogen) or in completely defined media (CDM) [11]. For isolation of transfected parasites, the media was supplemented with bleomycin (5 µg/ml; Calbiochem) and/or nourseothricin (70 µg/ml; Werner Inc.) and colonies isolated from SDM-agar (1% Noble Agar, Nunc.) plates.
Generation of untagged and GFP-tagged GND and mCherry-tagged FBP
The L. major GND gene was amplified from L. major wild type genomic DNA with the primers gndF (GCCCCCGGGATGCGG ATCGTGATCTCC) and gndR (GGGGATCCCTACAGCTTCGAATAGGCAC) and cloned into pXG1a, and gfp-gndF (GGCGGCCGCATGCGGATCGTGATCTCC) and gndR for cloning into pXG-GFP+2/. Truncated GND constructs lacking the SKL sequence (GNDΔSKL) were generated by amplification of the L. major GND gene using the primers gndF and gndΔsklR (GGGGATCCCTAATAGGCACGATTCATGT) and cloned as described above. The constructs were verified by diagnostic digests and DNA sequencing. The restriction sites are underlined. For expression of mCherry-tagged FBP, mCherry was PCR amplified from pRSET-mCherry (a generous gift from Roger Tsien) with primers MJM963 (GCGCCCGGGATGGTGAGCAAGGGCGAG) and MJM967 (TTGAGATCTGCTTGTACAGCTC) and cloned into vector pXG-GFP-FBP using SamI and BglII sites. The mCh::FBP chimera was subsequently liberated with SmaI and BamHI and cloned into pXG-SAT.
Analysis of glycolipids and mannogen
Mid log phase promastigotes were washed once in PBS and resuspended in glucose-free RPMI medium (2×108 cell/ml). D-[6-3H]-GlcN (50 µCi/ml 38.3 Ci/mmol; Perkin Elmer) was added and cells incubated for 30 min at 27°C. Parasites were harvested by centrifugation and glycolipids extracted in chloroform:methanol:water (1∶2∶0.8 v/v) [42]. Labeled glyclipids were analysed by high-performance thin-layer chromatography (HPTLC) using Silica Gel 60 aluminium-backed HPTLC sheets (Merck) developed in chloroform:methanol:13 M ammonium:1 M acetic acid:water (180∶140∶9∶9∶23 v/v) and detected with autoradiography after coating with EnHance spray (New England Nuclear) [43]. Mannogen oligomers were extracted and detected by high-pH anion exchange chromatography as previously described [29].
In vitro activity of GND
L. major wild type, Δgnd, Δgnd +GND and Δgnd +GNDΔSKL promastigotes were suspended in hypotonic buffer (1 mM NaHEPES, pH 7.4, 2 mM EGTA, 2 mM DTT, 40 µl/ml PIC) and chilled on ice for 10 min before being lysed by sonication (2×4 sec). Following lysis, samples were centrifuged (25,000 rpm, 0°C, 10 min) to separate organellar and cytosolic fractions. Pellet fractions (3×107 cell equivalents) were suspended in 90 µl assay buffer (50 mM NaHEPES pH 7.4, 2 mM EGTA, 5 mM MgCl2, 0.1 % Triton-X 100, 1 mM DTT, 40 µl/ml PIC), containing 1 mM GlcN6P and incubated at 27°C for 1, 10, 30 or 60 min. The reaction was stopped by addition of chloroform:methanol (1∶2 v/v) and extracts dried under nitrogen before phase partitioning in 1-butanol:water (2∶1 v/v). Polar metabolites in the lower aqueous phase were analysed by liquid chromatography-mass spectrometry using a Agilent QTOF instrument.
Measurment of glycosomal hexose phosphorylation
L. major wild type and Δgnd promastigotes were suspended in CDM with or without 13 mM GlcNAc as sole carbohydrate source and cultivated for 4 hr at 27°C. The medium was supplemented with 13 mM [U-13C]-Glc and parasites harvested at indicated time points. Parasite metabolism was quenched at each time point by immersing the culture flask (one per time point) in an ethanol-dry ice bath resulting in rapid chilling of the culture suspension to 0°C (∼10 sec) without freezing. Aliquots of the chilled parasite suspension (4×107 promastigotes) were removed and centrifuged in a microfuge (12,000× g, 20 sec, 0°C) and the cell pellet washed three times with phosphate buffered saline (0°C) prior to extraction in chloroform:methanol:water (1∶3∶1 v/v) [42]. Water was added to the extract to give a ratio of chloroform:methanol:water (1∶3∶2 v/v), and polar metabolites in the upper aqueous phase derivitized by methoximation and trimethylsilylation [42]. Levels of 13C/12C - Glc6P at each time point were determined by gas chromatography-mass spectrometry as previously described [42].
Fluorescence microscopy
Live L. major promastigotes expressing GFP::GND chimeras and mCherry::FBP were harvested by centrifugation (800× g for 10 min at 25°C), resuspended in PBS containing 8 µg/ml Hoechst (Molecular Probes) for 5 min and overlaid onto poly-L-lysine-coated coverslips. Images were acquired by using a Zeiss Axioplan2 imaging microscope, equipped with Axicam MRm camera and the AXIOVISION 4.3 software (Zeiss) and the montage generated in Photoshop Elements v6 (Adobe).
Macrophage and mouse infections
Infection of RAW 264.7 macrophages with stationary phase, non-opsinized promastigotes (ratio of 10 parasites/macrophage) or lesion-derived amastigotes (ratio one parasite/macrophage) were performed as described recently [13]. Female BALB/c mice (6–8 weeks old) derived from a pathogen-free facility (Bio21 Institute, University of Melbourne). Intradermal injections and isolation of parasites were performed as described else where [13]. Lesions were analyzed and scored as previously described [44] and the parasite burden was determined from total lymph node using the limiting dilution assay [13]. The draining lymph node was harvested and lymph cells were liberated through a mesh sieve. 1×105 cells from each cell suspension were suspended in SDM-79 medium containing 10% FCS and titrated in a 96-well plate, using threefold dilutions. After 7 days in culture at 27°C, the highest dilution containing parasites was determined and the parasite burden per 106 lymph cells was calculated.
Supporting Information
Zdroje
1. IsbergRR
O'ConnorTJ
HeidtmanM
2009
The Legionella pneumophila replication vacuole: making a cosy niche inside host cells.
Nat Rev Microbiol
7
13
24
2. SwansonMS
Fernandez-MoreiraE
2002
A microbial strategy to multiply in macrophages: the pregnant pause.
Traffic
3
170
177
3. SchaibleUE
KaufmannSH
2005
A nutritive view on the host-pathogen interplay.
Trends Microbiol
13
373
380
4. AppelbergR
2006
Macrophage nutriprive antimicrobial mechanisms.
J Leukoc Biol
79
1117
1128
5. NadererT
McConvilleMJ
2008
The Leishmania-macrophage interaction: a metabolic perspective.
Cell Microbiol
10
301
308
6. DaviesCR
KayeP
CroftSL
SundarS
2003
Leishmaniasis: new approaches to disease control.
BMJ
326
377
382
7. PetersNC
EgenJG
SecundinoN
DebrabantA
KimblinN
2008
In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies.
Science
321
970
974
8. BelkaidY
MendezS
LiraR
KadambiN
MilonG
2000
A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity.
J Immunol
165
969
977
9. PetersN
SacksD
2006
Immune privilege in sites of chronic infection: Leishmania and regulatory T cells.
Immunol Rev
213
159
179
10. BurchmoreRJ
Rodriguez-ContrerasD
McBrideK
MerkelP
BarrettMP
2003
Genetic characterization of glucose transporter function in Leishmania mexicana.
Proc Natl Acad Sci U S A
100
3901
3906
11. NadererT
EllisMA
SerneeMF
De SouzaDP
CurtisJ
2006
Virulence of Leishmania major in macrophages and mice requires the gluconeogenic enzyme fructose-1,6-bisphosphatase.
Proc Natl Acad Sci U S A
103
5502
5507
12. WinchesterB
2005
Lysosomal metabolism of glycoproteins.
Glycobiology
15
1R
15R
13. NadererT
WeeE
McConvilleMJ
2008
Role of hexosamine biosynthesis in Leishmania growth and virulence.
Mol Microbiol
69
858
869
14. KumarMJ
JamaluddinMS
NatarajanK
KaurD
DattaA
2000
The inducible N-acetylglucosamine catabolic pathway gene cluster in Candida albicans: discrete N-acetylglucosamine-inducible factors interact at the promoter of NAG1.
Proc Natl Acad Sci U S A
97
14218
14223
15. MeibomKL
LiXB
NielsenAT
WuCY
RosemanS
2004
The Vibrio cholerae chitin utilization program.
Proc Natl Acad Sci U S A
101
2524
2529
16. AlvarezFJ
KonopkaJB
2007
Identification of an N-acetylglucosamine transporter that mediates hyphal induction in Candida albicans.
Mol Biol Cell
18
965
975
17. SinghP
GhoshS
DattaA
2001
Attenuation of virulence and changes in morphology in Candida albicans by disruption of the N-acetylglucosamine catabolic pathway.
Infect Immun
69
7898
7903
18. da SilvaR
SacksDL
1987
Metacyclogenesis is a major determinant of Leishmania promastigote virulence and attenuation.
Infect Immun
55
2802
2806
19. LorenzMC
FinkGR
2002
Life and death in a macrophage: role of the glyoxylate cycle in virulence.
Eukaryot Cell
1
657
662
20. BurchmoreRJ
Rodriguez-ContrerasD
McBrideK
MerkelP
BarrettMP
2003
Genetic characterization of glucose transporter function in Leishmania mexicana.
Proc Natl Acad Sci USA
100
3901
3906
21. Alvarez-AnorveLI
CalcagnoML
PlumbridgeJ
2005
Why does Escherichia coli grow more slowly on glucosamine than on N-acetylglucosamine? Effects of enzyme levels and allosteric activation of GlcN6P deaminase (NagB) on growth rates.
J Bacteriol
187
2974
2982
22. NatarajanK
DattaA
1993
Molecular cloning and analysis of the NAG1 cDNA coding for glucosamine-6-phosphate deaminase from Candida albicans.
J Biol Chem
268
9206
9214
23. HaanstraJR
van TuijlA
KesslerP
ReijndersW
MichelsPA
2008
Compartmentation prevents a lethal turbo-explosion of glycolysis in trypanosomes.
Proc Natl Acad Sci U S A
105
17718
17723
24. FuruyaT
KesslerP
JardimA
SchnauferA
CrudderC
2002
Glucose is toxic to glycosome-deficient trypanosomes.
Proc Natl Acad Sci U S A
99
14177
14182
25. AlbertMA
HaanstraJR
HannaertV
Van RoyJ
OpperdoesFR
2005
Experimental and in silico analyses of glycolytic flux control in bloodstream form Trypanosoma brucei.
J Biol Chem
280
28306
28315
26. Rodriguez-ContrerasD
FengX
KeeneyKM
BouwerHG
LandfearSM
2007
Phenotypic characterization of a glucose transporter null mutant in Leishmania mexicana.
Mol Biochem Parasitol
153
9
18
27. MaugeriDA
CazzuloJJ
BurchmoreRJ
BarrettMP
OgbunudePO
2003
Pentose phosphate metabolism in Leishmania mexicana.
Mol Biochem Parasitol
130
117
125
28. IlgT
2002
Generation of myo-inositol-auxotrophic Leishmania mexicana mutants by targeted replacement of the myo-inositol-1-phosphate synthase gene.
Mol Biochem Parasitol
120
151
156
29. RaltonJE
NadererT
PirainoHL
BashtannykTA
CallaghanJM
2003
Evidence that intracellular β1-2 mannan is a virulence factor in Leishmania parasites.
J Biol Chem
278
40757
40763
30. SerneeMF
RaltonJE
DinevZ
KhairallahGN
O'HairRA
2006
Leishmania β-1,2-mannan is assembled on a mannose-cyclic phosphate primer.
Proc Natl Acad Sci U S A
103
9458
9463
31. DennisJW
NabiIR
DemetriouM
2009
Metabolism, cell surface organization, and disease.
Cell
139
1229
1241
32. ZeidanQ
HartGW
2010
The intersections between O-GlcNAcylation and phosphorylation: implications for multiple signaling pathways.
J Cell Sci
123
13
22
33. BanerjeeS
RobbinsPW
SamuelsonJ
2009
Molecular characterization of nucleocytosolic O-GlcNAc transferases of Giardia lamblia and Cryptosporidium parvum.
Glycobiology
19
331
336
34. CunninghamML
TitusRG
TurcoSJ
BeverleySM
2001
Regulation of differentiation to the infective stage of the protozoan parasite Leishmania major by tetrahydrobiopterin.
Science
292
285
287
35. JiangD
LiangJ
NoblePW
2007
Hyaluronan in tissue injury and repair.
Annu Rev Cell Dev Biol
23
435
461
36. RussellDG
XuS
ChakrabortyP
1992
Intracellular trafficking and the parasitophorous vacuole of Leishmania mexicana-infected macrophages.
J Cell Sci
103
1193
1210
37. JoshiMB
RogersME
ShakarianAM
YamageM
Al-HarthiSA
2005
Molecular characterization, expression, and in vivo analysis of LmexCht1: the chitinase of the human pathogen, Leishmania mexicana.
J Biol Chem
280
3847
3861
38. RogersME
HajmovaM
JoshiMB
SadlovaJ
DwyerDM
2008
Leishmania chitinase facilitates colonization of sand fly vectors and enhances transmission to mice.
Cell Microbiol
10
1363
1372
39. AndradeLO
AndrewsNW
2005
The Trypanosoma cruzi-host-cell interplay: location, invasion, retention.
Nat Rev Microbiol
3
819
823
40. EbikemeCE
PeacockL
CoustouV
RiviereL
BringaudF
2008
N-acetyl D-glucosamine stimulates growth in procyclic forms of Trypanosoma brucei by inducing a metabolic shift.
Parasitology
135
585
594
41. AzemaL
ClaustreS
AlricI
BlonskiC
WillsonM
2004
Interaction of substituted hexose analogues with the Trypanosoma brucei hexose transporter.
Biochem Pharmacol
67
459
467
42. De SouzaDP
SaundersEC
McConvilleMJ
LikicVA
2006
Progressive peak clustering in GC-MS Metabolomic experiments applied to Leishmania parasites.
Bioinformatics
22
1391
1396
43. RaltonJE
McConvilleMJ
1998
Delineation of three pathways of glycosylphosphatidylinositol biosynthesis in Leishmania mexicana - precursors from different pathways are assembled on distinct pools of phosphatidylinositol and undergo fatty acid remodeling.
J Biol Chem
273
4245
4257
44. MitchellGF
HandmanE
1983
Leishmania tropica major in mice: vaccination against cutaneous leishmaniasis in mice of high genetic susceptibility.
Aust J Exp Biol Med Sci
61
11
25
45. OlivaG
FontesMR
GarrattRC
AltamiranoMM
CalcagnoML
1995
Structure and catalytic mechanism of glucosamine 6-phosphate deaminase from Escherichia coli at 2.1 A resolution.
Structure
3
1323
1332
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 12- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Is Adherence to Erythrocytes a Factor in Extrapulmonary Dissemination?
- Identifying the Age Cohort Responsible for Transmission in a Natural Outbreak of
- Blockade of Immunosuppressive Cytokines Restores NK Cell Antiviral Function in Chronic Hepatitis B Virus Infection
- Metaeffector Exploits Host Proteasome to Temporally Regulate Cognate Effector
- The p53-Target Gene Drives Neutrophil-Mediated Protection against Lethal Bacterial Sepsis
- Development of an RNAi Protocol to Investigate Gene Function in the Filarial Nematode,
- Lysine Acetyltransferase GCN5-A Functions in the Cellular Response to Alkaline Stress and Expression of Cyst Genes
- Structural Basis for Apoptosis Inhibition by Epstein-Barr Virus BHRF1
- Molecular Architectures of Trimeric SIV and HIV-1 Envelope Glycoproteins on Intact Viruses: Strain-Dependent Variation in Quaternary Structure
- Interaction of c-Cbl with Myosin IIA Regulates Bleb Associated Macropinocytosis of Kaposi's Sarcoma-Associated Herpesvirus
- Glacial Refugia in Pathogens: European Genetic Structure of Anther Smut Pathogens on and
- Role for Sumoylation in Systemic Inflammation and Immune Homeostasis in Larvae
- Inflammasome Sensor Nlrp1b-Dependent Resistance to Anthrax Is Mediated by Caspase-1, IL-1 Signaling and Neutrophil Recruitment
- Noise Cancellation: Viral Fine Tuning of the Cellular Environment for Its Own Genome Replication
- Infectious Speciation Revisited: Impact of Symbiont-Depletion on Female Fitness and Mating Behavior of
- Eis Regulates Autophagy, Inflammation, and Cell Death through Redox-dependent Signaling
- NleC, a Type III Secretion Protease, Compromises NF-κB Activation by Targeting p65/RelA
- Early Myeloid Dendritic Cell Dysregulation is Predictive of Disease Progression in Simian Immunodeficiency Virus Infection
- HIV Capsid is a Tractable Target for Small Molecule Therapeutic Intervention
- Structural and Functional Studies of Nonstructural Protein 2 of the Hepatitis C Virus Reveal Its Key Role as Organizer of Virion Assembly
- Coming of Age—Sexual Reproduction in Species
- HIV-1 Envelope Subregion Length Variation during Disease Progression
- Compartmentation of Redox Metabolism in Malaria Parasites
- Evidence That Intracellular Stages of Utilize Amino Sugars as a Major Carbon Source
- Rapid End-Point Quantitation of Prion Seeding Activity with Sensitivity Comparable to Bioassays
- Dimeric 2G12 as a Potent Protection against HIV-1
- Hypoxia Induces an Immunodominant Target of Tuberculosis Specific T Cells Absent from Common BCG Vaccines
- CD4 Natural Regulatory T Cells Prevent Experimental Cerebral Malaria via CTLA-4 When Expanded In Vivo
- The Killing of African Trypanosomes by Ethidium Bromide
- H2A.Z Demarcates Intergenic Regions of the Epigenome That Are Dynamically Marked by H3K9ac and H3K4me3
- Large-Scale Field Application of RNAi Technology Reducing Israeli Acute Paralysis Virus Disease in Honey Bees (, Hymenoptera: Apidae)
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HIV-1 Envelope Subregion Length Variation during Disease Progression
- Coming of Age—Sexual Reproduction in Species
- Evidence That Intracellular Stages of Utilize Amino Sugars as a Major Carbon Source
- Compartmentation of Redox Metabolism in Malaria Parasites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



