-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEarly diagnosis of mild cognitive impairment and mild dementia through basic and instrumental activities of daily living: Development of a new evaluation tool
Elise Cornelis and colleagues describe the development of a new evaluation tool for early diagnosis of mild cognitive impairment and mild dementia through basic and instrumental activities of daily living.
Published in the journal: . PLoS Med 14(3): e32767. doi:10.1371/journal.pmed.1002250
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002250Summary
Elise Cornelis and colleagues describe the development of a new evaluation tool for early diagnosis of mild cognitive impairment and mild dementia through basic and instrumental activities of daily living.
Introduction
Health services are dealing with an increasing number of older patients. Although most seniors are in reasonably good health and living an active life, a considerable number of them are at risk of developing major chronic conditions and mental disorders such as dementia. Worldwide, it is estimated that dementia affects 46.8 million persons, which causes great stress to medical, social, and informal care [1,2]. Several interventions have already proven efficient in reducing caregiver strain, psychological morbidity, and delaying or avoiding admissions in residential care. Since such interventions may be more effective early in the disease course, early diagnosis of dementia is pivotal [3,4]. In this regard, the concept of mild cognitive impairment (MCI) is interesting since it is seen as a transitional zone between normal aging and dementia. However, MCI is a heterogeneous concept in its clinical presentation and its progression to dementia; mainly, amnestic MCI (a-MCI) has high risk of dementia, but some persons remain stable or even revert to normal cognition [5–8]. Boundaries between normal aging, MCI, and mild dementia are vague, and discussion about the MCI criteria and their operationalization is ongoing [6,9]. The differentiation between mild and major neurocognitive disorders (NCD)—referring to the new version of the Diagnostic Statistical Manual of Mental Disorders (DSM-5) [10]—may be a step in a good direction since this entails a stronger emphasis on “independence in activities of daily living (ADL)” [11–14]. The distinction between mild and major NCD is determined by the extent to which cognitive decline interferes with everyday functioning [12,15]. In major NCD or dementia, cognitive impairment influences independence in everyday functioning in a negative way. In mild NCD or MCI, individuals remain autonomous [15,16], although subtle problems may already occur in complex activities [12,17–21]. The process of functional decline shows a typical and distinctive progression [22,23]. Instrumental ADL (i-ADL) such as cooking, shopping, and managing medication will become slightly limited in mild NCD and will require support in major NCD [18,23–26]. Basic ADL (b-ADL), which includes personal hygiene, dressing, and eating, remain stable the longest [27]. Only in major NCD does one need the support of others in performing b-ADL [23,28,29]. Consequently, assessment of ADL is paramount to determine the degree of impairment in everyday functioning and to underpin accurate diagnostic classification in NCD [9,12,15,30]. Besides, ADL disability might increase the risk for incident dementia. In that way, an evaluation of ADL might be useful not just as diagnostic tool but also as an indicator of the risk for future dementia [12,30].
In clinical practice, information about ADL is most commonly ascertained by asking a patient or his or her caregiver to report about the everyday functioning [31]. Report-based ADL scales are usually quick and easy to administer [9,32,33]. Unfortunately, most common report-based ADL tools have limitations for diagnostic purposes. Firstly, they often use scoring systems solely assessing the success or failure of completing a task [17,34]. They do not reflect the process of performing the task, although this could be meaningful for diagnostic purposes, particularly in mild cognitive disorders [35–39]. Secondly, evaluations of ADL often entail gender-dependent tasks, tasks that a person does not perform, or tasks that become subject to family support, which commonly comes into play among an older population. Clear-cut guidelines on how to deal with tasks that a person does not perform are lacking [40]. Thirdly, ADL evaluations have a poor sensitivity to detect mild functional impairments and are mostly unresponsive to detect changes in a person’s ability level [41–44]. The discriminative power of existing tools is insufficient, and psychometric properties are either unavailable or do not meet standards of quality [9,43,45]. Finally, assessment tools do not differentiate between underlying causes of limitations [46]. Nevertheless, in diagnosing NCD in a geriatric population, it is crucial to determine the extent to which cognitive decline is responsible for functional impairment, since comorbidities, physical limitations, or other noncognitive causes of decline in ADL are often seen in old age [12,47].
Over the years, multiple report-based ADL scales have been developed in order to contribute to the early diagnosis of NCD [31]. Tools such as the Functional Activities Questionnaire (FAQ) [48], the Everyday Technology Use Questionnaire (ETUQ) [49], and the Everyday Cognition (ECog) [50] have already targeted some shortcomings of current evaluations by including “new” items specific to everyday technologies and using scoring systems that only take activities into account that are relevant to an individual. These evaluations showed promising results in assessing individuals with NCD [31]. However, they do not solely assess performance in b - or i-ADL but rather evaluate a mixed spectrum of self-care, household, and other activities or assess everyday abilities such as memory, language, or divided attention.
To address the concerns of report-based ADL scales, performance-based scales such as the Assessment of Motor and Process Skills [51,52] and the Naturalistic Action Test [53,54] have been developed. These evaluations examine the process of task performance, detect changes in everyday functioning, and address causation in observable behaviors. However, these assessments also have limitations, such as being more time-consuming and needing a high degree of training of the assessors, which often limit its use in clinical practice [9,31]. Furthermore, most performance-based ADL scales are not freely available and are mostly not yet validated for use in MCI [55].
Currently, the most commonly used tools for assessing b - and i-ADL are respectively the Katz Index [22] and the Lawton Scale [56] [31,57,58]. Although in widespread use, both scales have shortcomings as mentioned above: they have poorly described psychometric properties, the scoring systems are not sensitive enough to detect subtle deficits, and they do not identify causes of limitations in ADL [9,43,58–61]. Many studies have attempted to improve the potential use of the Katz Index and Lawton Scale, including using item response theory methods [34], providing short versions of these scales [62], or by combining both scales in new evaluations [63,64]. However, these improvements could not overcome all mentioned shortcomings.
Therefore, this study set out to develop a new tool to evaluate b - and i-ADL for diagnostic purposes in a geriatric population with NCD. This evaluation is based on the International Classification of Functioning, Disability and Health (ICF) developed by the World Health Organization (WHO) [65]. The ICF provides a framework for describing everyday functioning and advances the understanding and measurement of disability [58]. It is increasingly being applied in clinical practice and research and has gained acceptance as the worldwide framework of assessing human functioning [66,67]. The new evaluation adopted the activities of the Katz Index and Lawton Scale—since they are considered sound as items for describing functioning in b - and i-ADL [68]—and were defined with the ICF terminology. Besides, the new evaluation took over the scoring system of the ICF and added the possibility to determine underlying causes of limitations. This evaluation might be useful in clinical and research settings to evaluate everyday functioning in NCD since it has an advantage over currently used report-based scales by applying the ICF terminology and scoring system. This offers a more standardized evaluation of ADL, which might benefit a more reliable and accurate diagnosis and treatment of NCD. In this study, the construct validity, interrater reliability, and discriminative validity of this new evaluation were determined. We hypothesized that the ICF-based evaluation of b - and i-ADL will have a good construct validity and interrater reliability and will be able to discriminate between cognitively healthy comparisons (HC), MCI, and AD.
Methods
The study protocol was based on the STARD criteria, developed to improve the completeness and transparency of reporting of studies of diagnostic accuracy [69].
Ethics statement
The Ethical Committee of the Universitair Ziekenhuis Brussel approved this study (B.U.N. 143201523678). All data were collected in accordance with the ICH-GCP guidance and the declaration of Helsinki. All participants and informants gave written informed consents.
Participants and procedure
Three groups of community-dwelling older persons (≥65 y) were recruited consecutively through the geriatric day hospital of an academic teaching hospital (UZ Brussel, Belgium): (1) HC, (2) patients with MCI, and (3) with Alzheimer disease (AD). Patients with MCI and AD underwent a procedure for the diagnosis of cognitive disorders that was performed by a multidisciplinary team and is considered as good clinical practice [70]. This procedure consisted of a physical and neurological examination, clinical history taking, and neuropsychological assessment using the Mini-Mental State Examination (MMSE) [71]; Cambrigde Examination for mental disorders of the elderly, cognitive part (CamCog) [72]; memory subscale of the Alzheimer’s Disease Assessment Scale [73]; Visual Association Test (VAT) [74]; Memory Impairment Screen (MIS) [75]; Trail Making Test, parts A and B [76,77]; Frontal Assessment Battery [78]; and Geriatric Depression Scale (GDS-15) [79]. The procedure was completed by an evaluation of ADL using the Katz Index and Lawton Scale, extensive laboratory blood testing, and imaging of the brain by CT or MRI scan. HC were recruited separately from the diagnostic process for MCI and AD. They represent a heterogeneous sample of community-dwelling volunteers and geriatric patients who visited the geriatric day hospital for the diagnosis or treatment of conditions other than cognitive disorders (e.g., osteoporosis). HC were evaluated by the researchers using the same neuropsychological assessment and evaluation of ADL as MCI and AD. For all groups, the number of comorbidities and medication use was inventoried. The number of comorbidities was determined by counting the active diseases listed in the medical records at the moment the participants visited the geriatric day hospital, whether they were being treated pharmaceutically or not. All active diseases were counted in HC or as co-occurring with MCI or AD.
Cognitively healthy persons
Exclusion criteria for the HC (n = 79) were a history of NCD, a score <26/30 on the MMSE, and any self - or informant-based complaint of functional or cognitive deficits, which were suggestive of MCI or AD. Exclusion criteria were a score of <80/105 on the total CamCog score, <18/27 on the memory section of the CamCog score, and <8/12 on the MIS and VAT [80,81].
Patients with MCI
Patients with MCI (n = 73) were diagnosed by clinical consensus of the multidisciplinary team and fulfilled the diagnostic criteria for a-MCI as defined by Petersen [5]. The presence of a major depression was ruled out prior to the diagnosis of MCI.
Patients with AD
Patients with AD (n = 71) fulfilled the National Institute for Neurological and Communicative Disorders and Stroke—Alzheimer’s Disease and Related Disorder Association (NINDS-ADRDA) criteria [82]. Decisions on the diagnosis of AD were also based on the results of the diagnostic procedure and were carefully taken by consensus of the multidisciplinary team. When the presence of a major depression was presumed, this was ruled out prior to the diagnosis of AD.
For all participants, exclusion criteria included any acute pathology, sensory, or communicative impairments that precluded them from participating; history of major psychiatric illness; or any pathology of the central nervous system other than MCI or AD (e.g., stroke, epilepsy). An additional exclusion criterion for patients with MCI or AD was the absence of a reliable informant, in order to control over - or underestimation of functional abilities. Informants were considered as reliable when they were spouses, family, or close friends who were able to provide accurate information about the participant’s daily life. The proxy’s ability to provide accurate information was operationalized by asking each person with MCI or AD if the proxy was someone who knew him or her well and could provide accurate information about his or her daily life.
After the procedure, on the same day, trained occupational therapists carried out the new ICF-based evaluation of everyday functioning in b - and i-ADL. When conducting the new evaluation, the occupational therapists were blinded to the results of the other evaluations and the diagnosis.
The ICF-based evaluation of everyday functioning in b - and i-ADL
This evaluation has been designed as a semistructured interview that takes 10 min to complete. For the HC, self-report was used. For MCI and AD, proxy report was conducted.
Items according to the ICF definitions
According to the linking rules of Cieza et al. (2005) [83], the content of each item included in the Katz Index [22] and the Lawton Scale [56,84] was linked to one or more definitions of the activities component of the ICF (Tables 1 and 2).
Tab. 1. Items of the Katz Index according to the codes and definitions of the ICF. 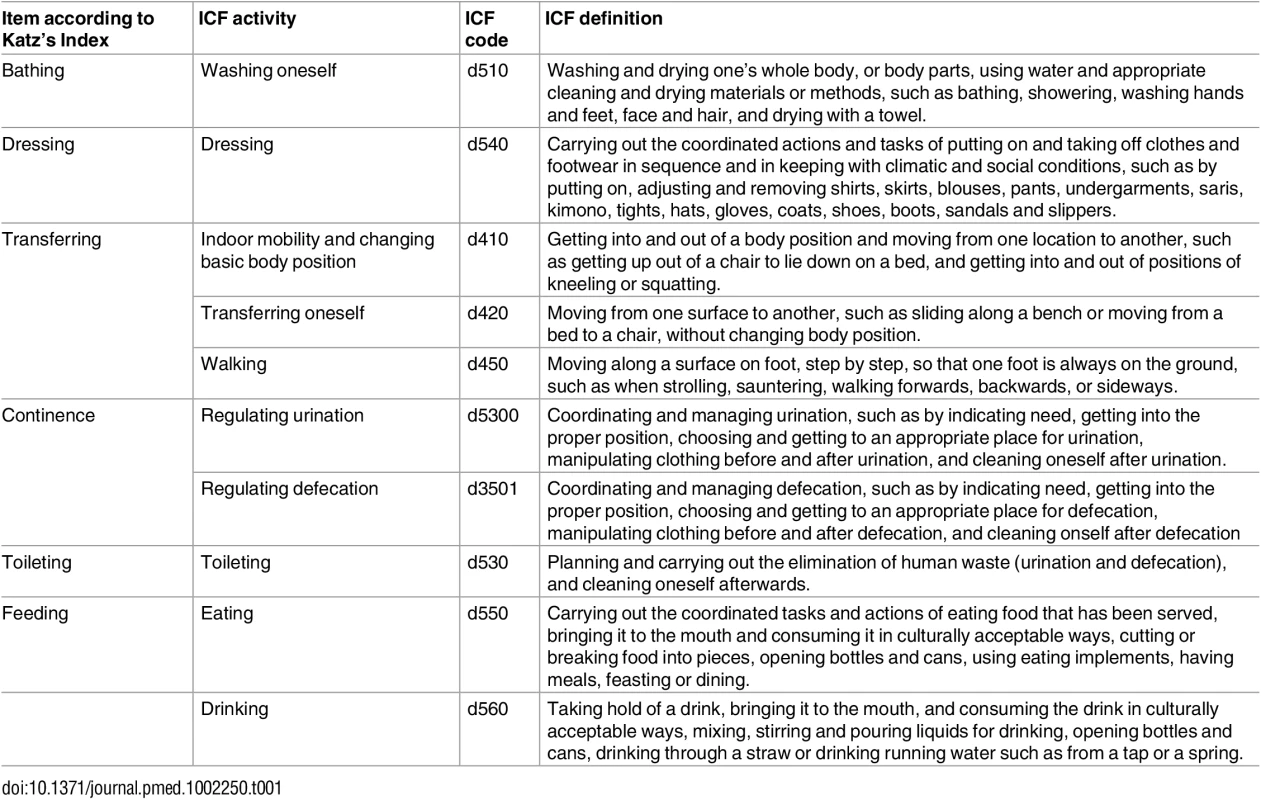
Tab. 2. Items of the Lawton Scale according to the codes and definitions of the ICF. 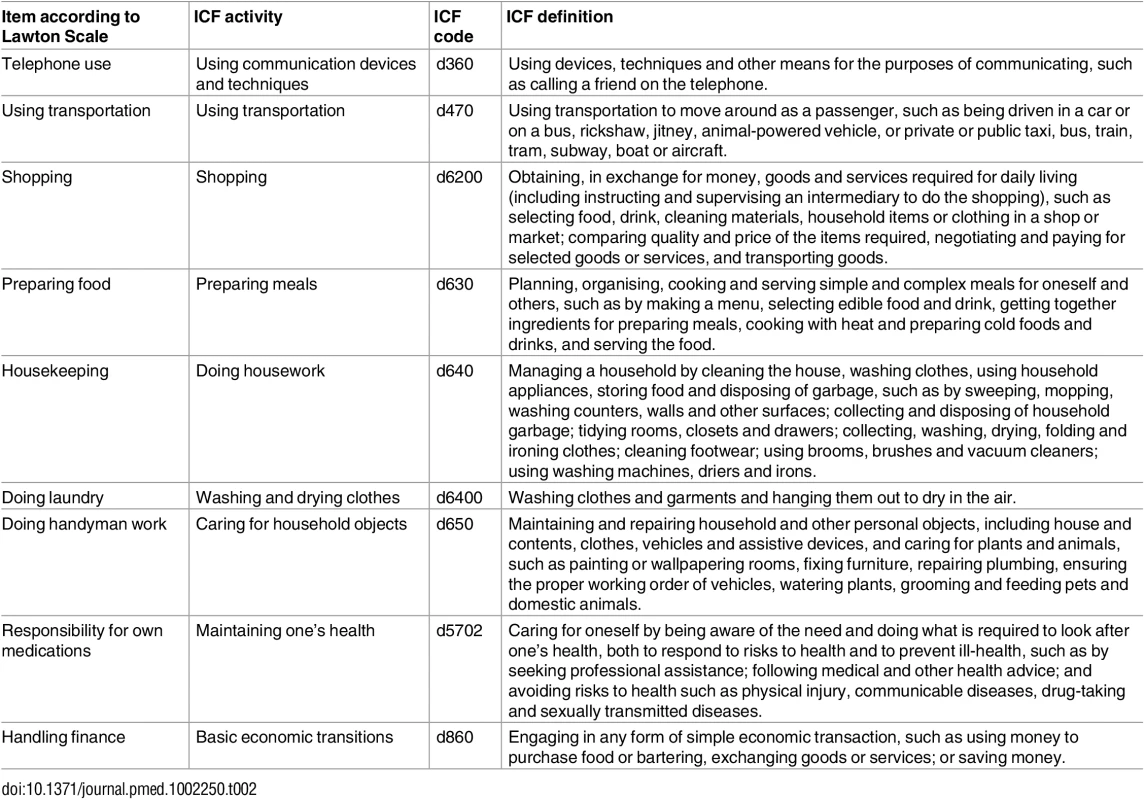
Interview protocol
First, the participant or proxy is asked whether an activity was performed during the past years. It is expected that the interviewer uses the ICF definitions to clarify the content of an activity. Each activity is rated for its relevance, which means that it is currently performed or it was previously performed by the individual. If activities have not been carried out during the past years because they were not relevant for an individual, they are not taken into account. This is mainly important for i-ADL, since these activities may never have been performed before (e.g., gender relevant) and are consequently irrelevant for the individual. For b-ADL, all items are relevant for every individual since—according to the definition of Reuben [85]—these activities are necessary to survive. The sum of relevant activities leads to the Total Number of relevant Activities (TNA). There is no cutoff of how many items are allowed to be not relevant.
Scoring system
The participant or proxy is asked how the activities are currently being performed. Based on the description, the investigator assigns a score. The scoring system adopted the performance qualifiers of the ICF, consisting of a five-point scale ranging from 0 (no difficulty to perform) to 4 (complete difficulty or unable to perform) (Table 3). Each score describes how an activity is performed (ICFScoreAct). The qualifiers were operationalized based on the experience of this research team with the development of the advanced ADL tool (a-ADL tool) [35,86] and on a previous qualitative study [87]. The sum of activities with a limitation (score ≥ 1) leads to the total number of Limited Activities (LimAct).
Tab. 3. Scoring system adopted from the performance qualifiers of the ICF. 
Operationalized by Cornelis et al. [87] and De Vriendt et al. [35,86]. Causes of limitations
If a score of 1 or higher is assigned, the interviewer determines the underlying cause of limitation by asking the participant what causes the limitations. The interviewer probes with the following questions: “Why do you/does (s)he performs this activity differently?” or “What causes the need for help to perform this activity?” In this way, the interviewer interprets the story of the participant and can distinguish cognitive reasons (e.g., global mental functions, memory, attention, etc.), physical reasons (e.g., sensorial functions, mobility, stability, etc.), intrapersonal reasons (e.g., switch in field of interest), social reasons (e.g., loss of partner), and environmental reasons (e.g., car sold, moving to a new place, etc.) of limitations. The assignment of a reason is dichotomous: “yes” when a reason is present and “no” when a reason is absent. It is possible to assign more than one reason of limitation. To clarify how ICF scores can be derived and physical or cognitive causes of limitations can be assigned, some examples are illustrated in Table 4.
Tab. 4. Examples of ICF scores and physical or cognitive reasons of limitations. 
Indices
A “global disability index” (DI) can be calculated for b-ADL (b-ADL-DI) and i-ADL (i-ADL-DI) by taking into account a maximal disability (TNA multiplied by ICFScoreAct 4, which is equal to complete difficulty) and an absolute disability (LimAct multiplied by the severity of each limitation [ICFScoreAct]) (see Fig 1). Furthermore, for each reason of limitation, an index can be calculated. In this study, a “cognitive disability index” (CDI) and a “physical disability index” (PDI) for both b-ADL (b-ADL-CDI and b-ADL-PDI) and i-ADL (i-ADL-CDI and i-ADL-PDI) is computed, considering exclusively activities that are limited because of respectively cognitive and physical limitations (see Fig 1). When limitations are caused by multiple reasons (e.g., using transportation is limited to both physical and cognitive reasons), reasons can be assigned in both indices (e.g., i-ADL-CDI and i-ADL-PDI). All indices are expressed as percentages, with higher scores representing more disability.
Fig. 1. Formulas to calculate the indices of both b- and i-ADL. 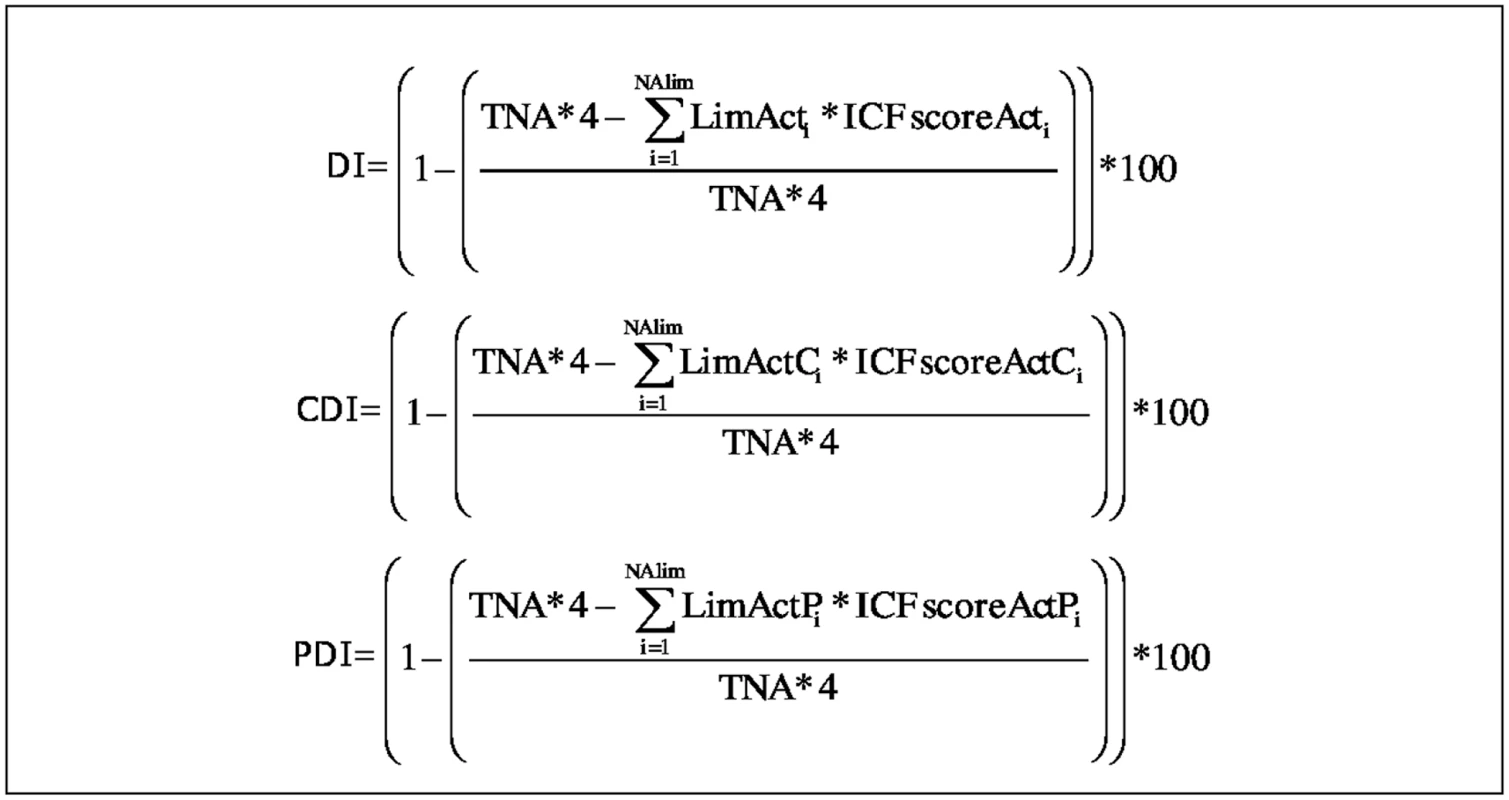
TNA = Total Number of Activities; LimActi = the i-th total number of Limited Activities; LimActCi = the i-th total number of Limited Activities because of a Cognitive reason; LimActPi = the i-th total number of Limited Activities because of a Physical reason; ICFscoreActi = the performance score given to the i-th limited activity; ICFscoreActCi = the performance score given to the i-th limited activity with a Cognitive reason of limitation; ICFscoreActPi = the performance score given to the i-th limited activity with a Physical reason of limitation; TNA*4 = Maximal disability; ∑i=0NAlim LimActi*ICFscoreActi = absolute disability; ∑i=0NAlim LimActCi*ICFscoreActCi = absolute disability because of cognitive reasons; ∑i=0NAlim LimActPi*ICFscoreActPi = absolute disability because of physical reasons. Example (for b-ADL): A person previously performed 6 b-ADL (TNA = 6). ICFScore 0 is assigned to four activities, ICFScore 1 is assigned to one activity because of cognitive problems, and ICFScore 3 is assigned to one activity because of physical factors. This person has two limited activities, and the maximal disability is 24 (TNA*4). His b-ADL-DI is 16.6% (all limited activities are taken into account), the b-ADL-CDI is 4.2% (only the activities limited because of cognitive reasons are taken into account), and the b-ADL-PDI is 12.4% (only the activities limited because of physical reasons are taken into account).
Statistical analyses to determine the clinimetric properties
From the diagnostic procedure, data regarding the MMSE, Katz Index, Lawton Scale, number of comorbidities, and medication use were extracted for this study. There were no missing data. Statistical analyses were performed with IBM SPSS for Mac (version 22.0) (SPSS Inc, Illinois, United States) with an α-level set two sided at p < 0.05 for all analysis. Demographic and clinical characteristics (i.e., age, education, gender, number of used medications, and number of comorbidities) and the MMSE, Katz Index, Lawton Scale were evaluated between groups by one-way ANOVA with Bonferonni post hoc tests or chi-square analysis. The construct validity was checked, in absence of a true golden standard, by determining the new evaluation’s ability to distinguish between HC, MCI, and AD. We hypothesised HC would show less disability than MCI and the latter less than AD, and that the CDI for both b - and i-ADL would differ more than DI and PDI. The indices for b - and i-ADL were compared across the groups using analysis of covariance (ANCOVA) in which age, number of used medications, number of comorbidities, level of education, and gender were included in the model as covariates. Secondly, in checking the construct validity, we calculated correlations between the indices and the MMSE. We hypothesised that (1) the CDI for both b - and i-ADL would show higher correlations with the MMSE than the DI and PDI since the CDI expresses solely deficits caused by cognitive disorders and (2) the i-ADL-CDI would show higher correlations than b-ADL-CDI because performing i-ADL is more vulnerable to cognitive disorders. Correlation analyses were performed using Pearson correlation between the MMSE, Katz Index, Lawton Scale, and the indices. To interpret the correlations, the guideline by Evans (1996) [88] was used: .00–.19 very weak, .20–.39 weak, .40–.59 moderate, .60–.79 strong, and .80–1.0 excellent. The interrater reliability was checked by comparing simultaneous observation of the interview by two independent raters in a sample of 25 participants and was evaluated by computing intraclass correlation coefficients (ICC) in a two-way mixed model and 95% confidence intervals (CI). Lastly, discriminative validity was evaluated by calculating receiver-operating-characteristics (ROC) curves and the AUC with cutoffs, sensitivity, and specificity for the new evaluation of b-ADL and i-ADL and for the Katz Index and Lawton Scale. The ROC curves and AUC for the new evaluation were compared with the Katz Index and Lawton Scale to determine the added diagnostic value of the new evaluation by using the method of DeLong et al. (1988) [89] in MedCalc (version 14.8.1.0) (MedCalc Software, Mariakerke, Belgium).
Results
Participants’ characteristics
All participants reported to have enjoyed the assessment. The interviews lasted between 8 and 15 min for both b - and i-ADL; no adverse events occurred during the diagnostic procedure or the ICF-based evaluation of b - and i-ADL. Table 5 shows the demographic and clinical characteristics of the participants. In comparison to HC, patient groups had less years of education (F(2,220) = 13.7, p < .001) and reported more comorbidities (F(2,220) = 20.1, p < .001) and use of medications (F(2,220) = 15.3, p < .001). Between MCI and AD, no significant differences were found for age, education, medication use, and comorbidities. For MCI and AD, data about their everyday functioning were obtained by spouses (40.3%), children (50.0%), or close friends (9.7%). Almost half of them (48.6%) lived together with the person with MCI or AD. No significant differences between MCI and AD were found for relationship of the proxy (χ2(3) = 4.64, p < .199) and whether or not living together with the proxy (χ2(1) = 1.37, p < .241).
Tab. 5. Participants' characteristics. 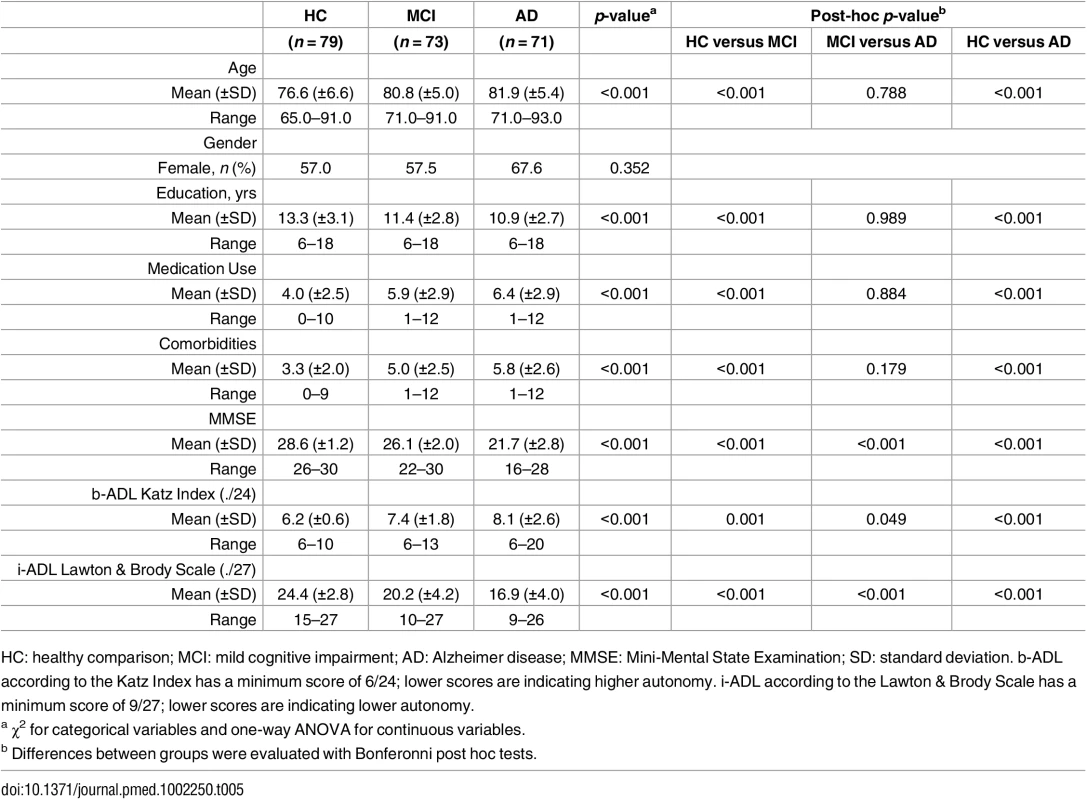
HC: healthy comparison; MCI: mild cognitive impairment; AD: Alzheimer disease; MMSE: Mini-Mental State Examination; SD: standard deviation. b-ADL according to the Katz Index has a minimum score of 6/24; lower scores are indicating higher autonomy. i-ADL according to the Lawton & Brody Scale has a minimum score of 9/27; lower scores are indicating lower autonomy. Construct validity
Indices of everyday functioning in i-ADL
For i-ADL, participants performed an average of eight activities (minimum 4—maximum 9; SD ± 1.2). There were no significant differences between the groups on TACT i-ADL [F(2, 215) = 0.01, p = 0.987]. As illustrated in Table 6, the i-ADL-DI and i-ADL-CDI expressed significantly more severe deficits in AD than in MCI and in MCI than in HC (F(2,215) = 61.5, p < .001; F(2,215) = 88.9, p < .001). The i-ADL-CDI had a moderate correlation with the MMSE (r = –0.588, 95% CI [–0.697 to –0.482]; p < .001). The i-ADL-PDI expressed no significant differences between the groups and had a weak correlation with the MMSE (r = –0.348, 95% CI [–0.473 to –0.224]; p < .001).
Tab. 6. Construct validity—Indices of everyday functioning in b- and i-ADL. 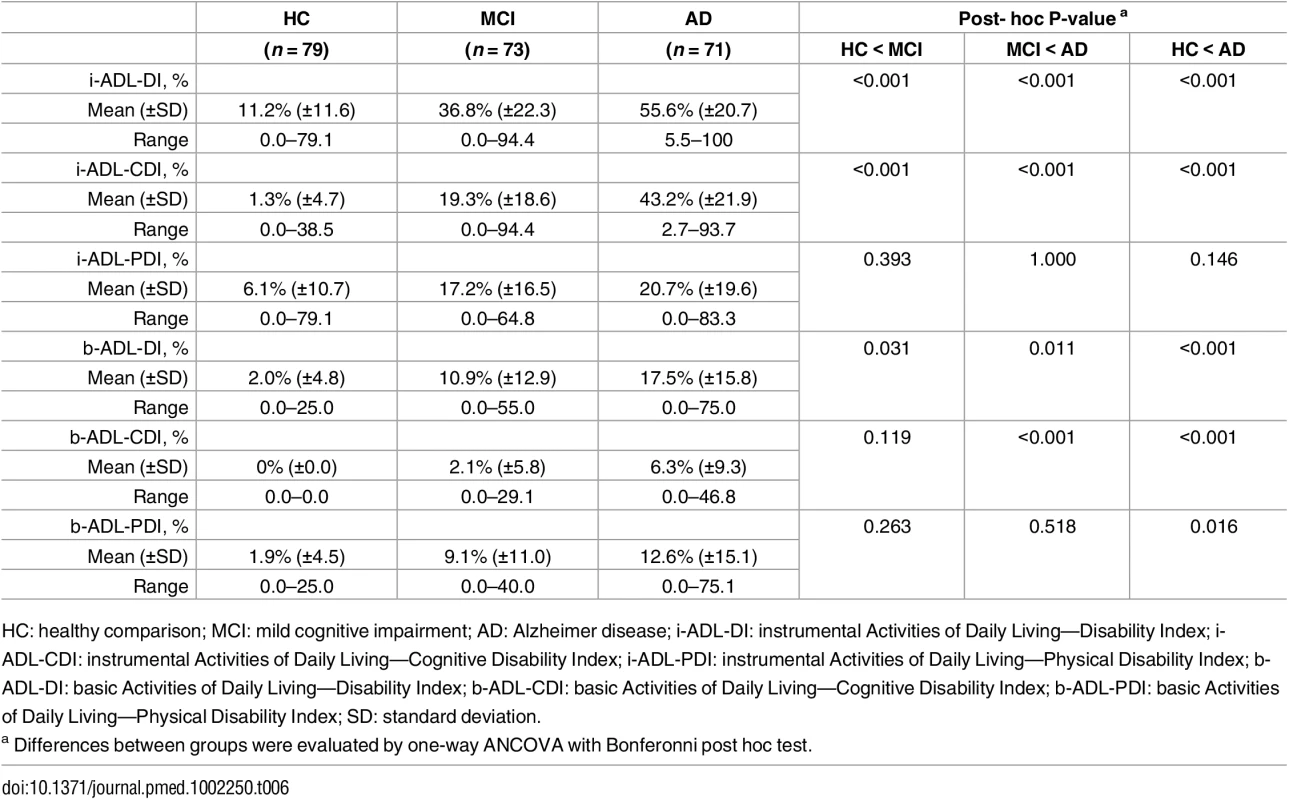
HC: healthy comparison; MCI: mild cognitive impairment; AD: Alzheimer disease; i-ADL-DI: instrumental Activities of Daily Living—Disability Index; i-ADL-CDI: instrumental Activities of Daily Living—Cognitive Disability Index; i-ADL-PDI: instrumental Activities of Daily Living—Physical Disability Index; b-ADL-DI: basic Activities of Daily Living—Disability Index; b-ADL-CDI: basic Activities of Daily Living—Cognitive Disability Index; b-ADL-PDI: basic Activities of Daily Living—Physical Disability Index; SD: standard deviation. Indices of everyday functioning in b-ADL
For b-ADL, participants always performed all activities. Persons with AD and MCI expressed significantly more severe deficits in b-ADL-DI than HC (F(2,215) = 12.6, p = < .05) (Table 6). The b-ADL-CDI showed significantly more severe deficits in AD than HC and MCI (F(2,215) = 17.3, p < .001). The b-ADL-PDI showed significantly more severe deficits in AD than HC (F(2,215) = 3.9, p = .016). No significant differences were found between HC and MCI or MCI and AD. All indices of b-ADL had a weak correlation with the MMSE (ranging from –0.316 to –0.411; all p < .001).
Interrater reliability
The interrater reliability (n = 25) was excellent for b-ADL-DI (ICC = .965, 95% CI [.920–.984]); b-ADL-CDI (ICC = .943, 95% CI [.872–.975]); b-ADL-PDI (ICC = .934, 95% CI [.850–.971]); i-ADL-DI (ICC = .986, 95% CI [.968–.994]); i-ADL-CDI (ICC = .986, 95% CI [.969–.994]); and i-ADL-PDI (ICC = .972, 95% CI [.973–.988]) (all p < .001). No significant differences between raters were observed.
Discriminative validity
Table 7 present the results of the ROC curves for the Katz Index, Lawton Scale, and the indices of the new evaluation.
Tab. 7. Discriminative validity of the b- and i-ADL indices between HC, MCI, and AD. 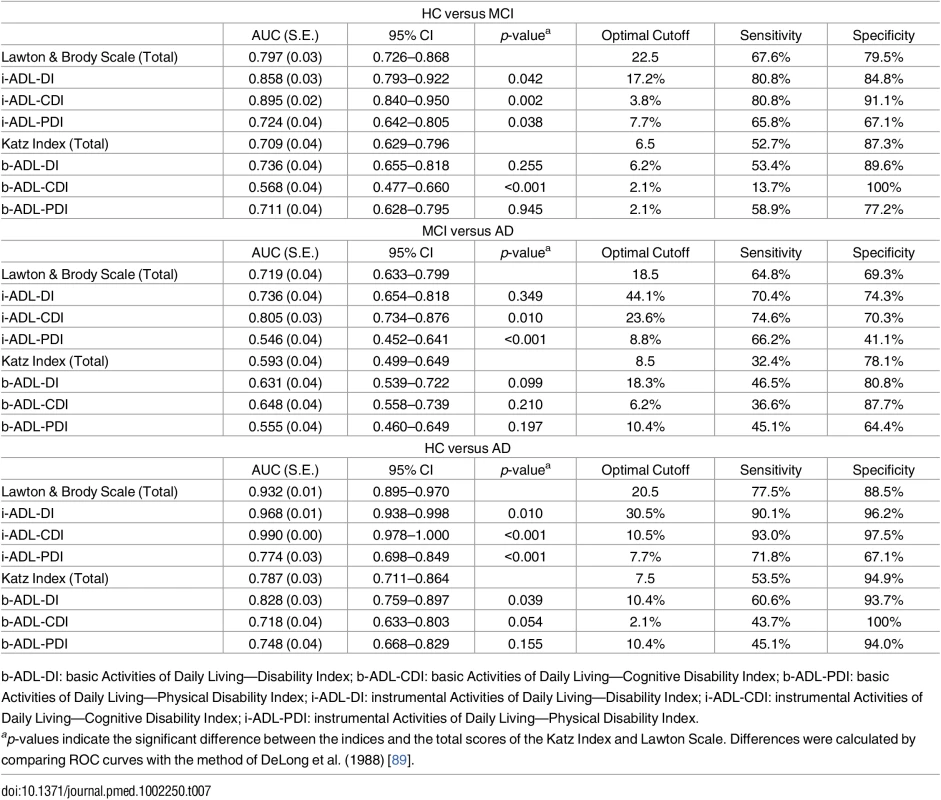
b-ADL-DI: basic Activities of Daily Living—Disability Index; b-ADL-CDI: basic Activities of Daily Living—Cognitive Disability Index; b-ADL-PDI: basic Activities of Daily Living—Physical Disability Index; i-ADL-DI: instrumental Activities of Daily Living—Disability Index; i-ADL-CDI: instrumental Activities of Daily Living—Cognitive Disability Index; i-ADL-PDI: instrumental Activities of Daily Living—Physical Disability Index. Indices of everyday functioning in i-ADL
The AUC of i-ADL-DI ranges from 0.736 to 0.968 and has a significantly better discriminative accuracy than the Lawton Scale for differentiating between HC and MCI and between HC and AD with DeLong’s test (all p < .05). The i-ADL-CDI showed best accuracy, expressed by AUCs ranging from 0.805 to 0.968 and a significantly higher discriminative power than the Lawton Scale with DeLong’s test (all p < .05). The i-ADL-PDI did not show a better accuracy than the Lawton Scale.
Indices of everyday functioning in b-ADL
The AUC of b-ADL-DI showed with DeLong’s test (p < .05) a significantly better discriminative accuracy than the Katz Index in differentiating between HC and AD with an AUC of 0.828. The b-ADL-CDI and b-ADL-PDI showed no better diagnostic accuracy.
Discussion
This study developed and validated an evaluation of everyday functioning in b - and i-ADL by (1) adopting the activities of the Katz Index and Lawton Scale and linking them to the definitions and codes of the ICF, (2) by developing a scoring system based on the performance qualifiers of the ICF, and (3) by adding the possibility to take causes of limitations in performance into account. This new evaluation takes the person as his or her own reference. By doing so, it is possible to compute a set of indices. This study determined the construct validity, discriminative validity, and interrater reliability of this new evaluation in a geriatric population.
The new evaluation showed more accuracy in evaluating b - and i-ADL compared to the Katz Index and Lawton scale and subsequently has the potential to improve diagnostic differentiation between HC, mild NCD (e.g., MCI), and major NCD (e.g., AD). As hypothesised, this evaluation followed the hierarchical continuum of functional decline [19]; b-ADL-DI and i-ADL-DI showed significantly less disability in HC than in MCI and the latter less than in AD. The i-ADL-DI showed more disability than b-ADL-DI and had a significantly better accuracy than the Lawton Scale to differentiate HC from MCI and AD. The b-ADL-DI, in its turn, had a significantly better accuracy than the Katz Index in differentiating HC from AD. Other promising results were seen in the i-ADL-CDI. Although the original Lawton Scale cannot detect mildly affected quality of performance in i-ADL [90], the i-ADL-CDI could detect subtle functional deficits of persons with MCI and AD. The i-ADL-CDI demonstrated a significantly better accuracy than the Lawton Scale and is able to distuinguish between HC, MCI, and AD. This illustrates that it is important to make a distinction in causes, especially in older patients in whom physical limitations are commonly seen and also affect everyday functioning. When considering the diagnosis of NCD, it is of utmost importance to determine to what extent functional limitation is due to cognitive limitations and not due to other causes [17]. The b-ADL-CDI showed, as hypothesized, significantly more severe deficits in AD than in HC and MCI but had no better accuracy than the Katz Index. This can be explained by the fact that performing b-ADL is less vulnerable for cognitive decline and is often largely spared until later stages of the disease (i.e., moderate or severe dementia) [91]. If limitations are observed in MCI or mild AD, it will rather be caused by other reasons such as physical limitations, as illustrated in the b-ADL-PDI. The b-ADL-PDI showed significantly more severe limitations in AD than in HC and had similar accuracy to the Katz Index. Although the differentiation between normal cognition and mild AD is usually not much of a diagnostic dilemma in clinical context, the results of this study clearly state that b-ADL distinguish well when reasons for limitations are taken into account.
Until now, self - and informant-report scales did not show sound psychometrical properties [9,43] and were not considered as the best methods to evaluate everyday functioning since they might over - and underestimate functional ability [32,92]. There is growing evidence that performance-based evaluations might have more advantages over other assessment approaches [9,32,33]. However, only few of them are developed to assess MCI or mild dementia [55,93], and they are mostly too time - and cost-consuming to be administered [9,31]. Two recent performance-based instruments, the Erlangen Test of Activities of Daily Living in Mild Dementia or Mild Cognitive Impairment (ETAM) [55] and the Sydney Test of Activities of Daily Living in Memory Disorders (STAM) [94] have been developed with the aim to assess everyday activities in a time-efficient and reliable way for persons with MCI or mild dementia. Both evaluations show good psychometric characteristics, are easy to administer, and seem to be valuable in clinical practice and research. However, our i-ADL-DI and i-ADL-CDI show similar accurate validity to discriminate between HC, MCI, and AD. So, although this study developed a report-based measure—which may not be as accurate in detecting functional difficulties in persons with mild cognitive decline—the results of this study indicate that the ICF-based evaluation of b - and i-ADL might compete with the recently developed performance-based tools as the standard for classifying functional status and decline [32,33,55,94]. Ongoing research will clarify this and is already assessing the convergent validity between the ICF-based evaluation of b - and i-ADL and a performance-based measure.
Although many studies have already tried to improve the use of the Katz Index and the Lawton Scale, not all improvements were relevant for the diagnosis of cognitive disorders. In this study, we attempted to achieve more clarity, transparency, and nuance by maintaining the activities of the Katz Index and the Lawton Scale but by adopting the terminology and the scoring system of the ICF. A first advantage is that each activity is clearly defined by definitions according to the ICF. In contrast to the content of the original Katz Index and Lawton Scale—which varies depending on setting and circumstances [58,95]—the ICF definitions provide clear descriptions of the content of activities. In this way, no more doubt can arise about the exact content of activities such as, e.g., doing laundry (should ironing also be considered?) or using transportation (should driving a car also be considered?). Furthermore, since the ICF definitions do not impose a manner of performing, this evaluation will remain useful for future generations and might also have advantages across cultures since b - and i-ADL will always be applicable. Secondly, this evaluation only considers activities that are relevant for a person. In contrast with other scales, activities that are gender-dependent or a person has never performed in his or her life are not be taken into account. In this way, each person is considered as his or her own reference and is compared to his or her own previous level of everyday functioning, as suggested by Ganguli (2013) [12]. This might also be considered as an advantage for use in other generations and cultures. Thirdly, by using the detailed ICF qualifiers—ranging from 0 to 4—this evaluation provides a more sensitive scoring system, as recommended by Jekel et al. (2015) [9]. This new evaluation makes it possible to calculate indices and showed an excellent interrater reliability in this study. Lastly, this evaluation has the advantage to discriminate between reasons of limitations. Although other tools such as the FAQ [48], ETUQ [49], and ECog [50] are also valuable instruments in assessing individuals with NCD, they do not make a distinction between reasons of functional decline.
Although the results of our study are promising and may imply a change in the evaluation of everyday functioning in clinical practice, some considerations need to be made. First, a measurement bias might have occurred by using different methods of reporting ADL in HC and patients with MCI and AD. Although a report-based method has the clinical advantages of being easy to obtain, minimally disturbing, and of low cost, proxy and patient-based measures can be biased by mood status, social desirability, diminished awareness, denial, and other cognitive deficits [96,97]. But since informant-reports are generally preferred to self-report in evaluating everyday functioning in clinical practice and research settings, a reliable proxy was questioned about the everyday functioning of participants with MCI and AD in this study [97]. This closely resembles clinical reality, in which health care professionals have to work with the information that is available. Nevertheless, we could not rule out that the informants were not mildly cognitively impaired themselves. However, 50.0% of the informants were children of the persons with MCI and AD and had an estimated age range of 45 to 65 y. Although it is known that children of persons with AD are at high risk of cognitive disorders as they age, it seems unlikely that this would have influenced the results at this point of time. For the HC, only self-report was used because prior research in cognitively healthy older persons suggested that self-report evaluations are generally accurate indicators of ADL for older persons who demonstrate insight into their functional abilities [96,98]. Additionally, a second reflection must be made about the participants in this study. The patients with AD and MCI represent a clinically relevant sample but were significantly older, had more comorbidities, and took more medications than the HC. This suggests that the patient groups were frailer and might have experienced more functional problems. However, not all medications and comorbidities would be expected to contribute equally to functional impairment. Furthermore, this study did not report any measures of current depressive symptoms. The presence of a major depression was ruled out prior to the diagnosis in MCI and AD. However, mild to moderate depressive symptoms are an important comorbidity of cognitive disorders and may have an impact on everyday activities [99–102].
As a result, the contrast between groups might be larger than would be expected in a clear clinical sample. However, in the statistical analysis, our data was controlled for possible confounders such as age, medication use, number of comorbidities, level of education, and gender. Lastly, another consideration is that HC were—apparently—cognitively healthy persons. Yet, it is still possible that mild cognitive problems were present in some of them. However, we used strict cutoffs of MMSE—which can be considered as a valuable instrument for cognitive screening—in order to rule out cognitive deficits.
Based on the results of this study, we argue that this evaluation can contribute to the diagnostic differentiation between cognitively healthy ageing, mild NCD (e.g., MCI), and major NCD (e.g., AD). Particularly, the i-ADL-CDI might be useful. Since it is likely that decline in everyday functioning occurs over time, and this change leads to a conversion from mild to major NCD, further research—a longitudinal prospective follow up study—should address the predictive validity of this evaluation as follow-up assessment [14].
In conclusion, this new ICF-based evaluation for b - and i-ADL addresses important issues in assessing everyday functioning by (1) providing an operationalization of the evaluated activities by ICF codes and definitions, (2) providing a detailed scoring system that is based on the ICF qualifiers, and (3) by making a differentiation in causes of limitations. With validation in longitudinal prospective cohorts, this evaluation might offer a useful addition to the common diagnostic process and be of added value in a multidisciplinary approach with established cognitive and mood measures and biomarkers.
Supporting Information
Zdroje
1. World Health Organization. Mental health and older adults. 2016 [Updated April 2016]. http://www.who.int/mediacentre/factsheets/fs381/en/
2. Prince M, Comas-Herrera A, Knapp M, Guerchet M, Karagiannidou M. World Alzheimer Report 2016: improving healthcare for people living with dementia. Coverage, quality and costs now and in the future. Alzheimer's Disease International. 2016. https://www.alz.co.uk/research/WorldAlzheimerReport2016.pdf
3. Olazaran J, Reisberg B, Clare L, Cruz I, Pena-Casanova J, Del ST, et al. Nonpharmacological therapies in Alzheimer's disease: a systematic review of efficacy. Dementia and geriatric cognitive disorders. 2010;30(2): 161–78. doi: 10.1159/000316119 20838046
4. Prince M, Bryce R, Ferri C. World Alzheimer Report 2011: The benefits of early daignosis and intervention. Alzheimer's Disease International. 2011. https://www.alz.co.uk/research/world-report-2011.
5. Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256(3): 240–6. doi: 10.1111/j.1365-2796.2004.01380.x 15324367
6. Petersen RC, Carraciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L. Mild cognitive impairment: a concept in evolution. Journal of Internal Medicine. 2014;275(3): 214–28. doi: 10.1111/joim.12190 24605806
7. Roberts R, Knopman D. Classification and epidemiology of MCI. Clin Geriatr Med. 2013;29(4):753–72. doi: 10.1016/j.cger.2013.07.003 24094295
8. Albert MS, Dekosky ST, Dickson D, Dubois B, Feldmann HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3): 270–9. doi: 10.1016/j.jalz.2011.03.008 21514249
9. Jekel K, Damian M, Wattmo C, Hausner L, Bullock R, Connelly PJ, Dubois B, et al. Mild cognitive impairment and deficits in instrumental activities of daily living: a systematic review. Alzheimer's Research & Therapy. 2015;7(1): 17.
10. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth edition. Washington, DC: American Psychiatric Association; 2013.
11. Blazer D. Neurocognitive Disorders in DSM-5. The American Journal of Psychiatry. 2013;170(6): 585–7. doi: 10.1176/appi.ajp.2013.13020179 23732964
12. Ganguli M. Can the DSM-5 framework enhance the diagnosis of MCI? American Academy of Neurology. 2013;81(23): 2045–50.
13. Sachdev PS, Blacker D, Blazer DG, Ganguli M, Jeste DV, Paulsen JS, et al. Classifying neurocognitive disorders: the DSM-5 approach. Nature Reviews Neurology. 2014;10 : 634–42. doi: 10.1038/nrneurol.2014.181 25266297
14. Sachs-Ericsson N, Blazer DG. The new DSM-5 diagnosis of mild neurocognitive disorder and its relation to research in mild cognitive impairment. Aging Ment Health. 2015;19(1): 2–12. doi: 10.1080/13607863.2014.920303 24914889
15. Tay L, Lim WS, Chan M, Ali N, Mahanum S, Chew P, et al. New DSM-V Neurocognitive Disorders Criteria and Their Impact on Diagnostic Classifications of Mild Cognitive Impairment and Dementia in a Memory Clinic Setting. Am J Geriatr Psychiatry. 2015;23(8): 768–79. doi: 10.1016/j.jagp.2015.01.004 25728011
16. Geda YE, Nedelska Z. Mild cognitive impairment: a subset of minor neurocognitive disorder? Am J Geriatr Psychiatry. 2012;20(10): 821–6. doi: 10.1097/JGP.0b013e31826abc00 22935926
17. Rodakowski J, Skidmore ER, Reynolds CF, Dew MA, Butters MA, Holm MB, et al. Can performance on daily activities discriminate between older adults with normal cognitive function and those with Mild Cognitive Impairment? JAGS 2014;62(7): 1347–52.
18. Yeh YC, Lin KN, Chen WT, Lin CY, Chen TB, Wang PN. Functional disability profiles in amnestic mild cognitive impairment. Dementia and geriatric cognitive disorders. 2011;31(3): 225–32. doi: 10.1159/000326910 21474931
19. Farias ST, Mungas D, Reed BR, Harvey D, Cahn-Weiner D, Decarli C. MCI is associated with deficits in everyday functioning. Alzheimer disease and associated disorders. 2006;20(4): 217–23. doi: 10.1097/01.wad.0000213849.51495.d9 17132965
20. Giovannetti T, Bettcher BM, Brennan L, Libon DJ, Burke M, Duey K, et al. Characterization of everyday functioning in mild cognitive impairment: a direct assessment approach. Dementia and geriatric cognitive disorders. 2008;25(4): 359–65. doi: 10.1159/000121005 18340108
21. Pedrosa H, De Sa A, Guerreiro M, Maroco J, Simoes MR, Galasko D, de Mendona A. Functional evaluation distinguishes MCI patients from healthy elderly people—the ADCS/MCI/ADL scale. The Journal of Nutrition, Health and Aging. 2010;14(8): 703–9. 20922349
22. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185 : 914–9. 14044222
23. Morris JN, Berg K, Fries BE, Steel K, Howard EP. Scaling functional status within the interRAI suite of assessment instruments. BMC Geriatrics. 2013;13 : 128. doi: 10.1186/1471-2318-13-128 24261417
24. Hesseberg K, Bentzen H, Ranhoff AH, Engedal K, Bergland A. Disability in Instrumentel Activities of Daily Living in Elderly Patients with Mild Cognitive Impairment and Alzheimer's Disease. Dementia and geriatric cognitive disorders. 2013;36(3–4): 146–53. doi: 10.1159/000351010 23900051
25. Lechowski L, de Stampa M, Denis B, Tortat D, Chassagne P, Robert P, et al. Patterns of loss of abilities in instrumental activities of daily living in Alzheimer's disease: the REAL cohort study. Dementia and geriatric cognitive disorders. 2008;25(1): 46–53. doi: 10.1159/000111150 18025829
26. Reppermund S, Brodaty H, Crawford JD, Kochan NA, Draper B, Slavin MJ, et al. Impairment in instrumental activities of daily licing with high cognitive demand is an early marker of mild cognitive impairment: the sydney memory and ageing study. Psychol Med. 2012;43(11): 2437–45.
27. Wade DT. Measurement in Neurological Rehabilitation. Oxford: Oxford University Press; 1992.
28. Njegovan V, Hing MM, Mitchell SL, Molnar FJ. The hierarchy of functional loss associated with cognitive decline in older persons. Journals Gerontol Ser A, Biol Sci Med Sci 2001;56(10): M638–43.
29. Lechowski L, Van Pradelles S, Le Crane M, d'Arailh L, Tortat D, Teillet L, et al. Patterns of loss of basic activities of daily living in Alzheimer Patients: a cross-sectional study of the French REAL cohort. Dementia and geriatric cognitive disorders. 2010;29(1): 46–54. doi: 10.1159/000264632 20110700
30. Fauth EB, Schwartz S, Tschanz J, Ostbye T, Corcoran C, Norton MC. Baseline disability in activiies of daily living predicts dementia risk even after controlling for baseline global cognitive ability and depressive symptoms. Int J Geriatric Psychiatry. 2013;28(6): 597–606.
31. Marshall GA, Amariglio RE, Sperling RA, Rentz DM. Activities of daily living: where do they fit in the diagnosis of Alzheimer's disease? Neurodegener Dis Manag. 2012;2(5):483–491. doi: 10.2217/nmt.12.55 23585777
32. Puente AN, Terry DP, Faraco CC, Brown LC, Miller LS. Functional Impairment in Mild Cognitive Impairment Evidenced Using Performance-Based Measurement. Journal of geriatric Psychiatry and Neurology. 2014;27(4): 253–258. doi: 10.1177/0891988714532016 24763070
33. Wesson J, Cemson L, Brodaty H, Reppermund S. Estimating functional cognition in older adults using observational assessments of task performance in complex everyday activities: A systematic review and evaluation of measurement properties. Neuroscience and biobehavioral reviews. 2016;68 : 335–60. doi: 10.1016/j.neubiorev.2016.05.024 27236042
34. McGrory S, Shenkin SD, Austin EJ, Starr JM. Lawton iADL scale in dementia: can item response theory make it more informative? Age and ageing. 2014;43(4): 491–495. doi: 10.1093/ageing/aft173 24212917
35. De Vriendt P, Mets T, Petrovic M, Gorus E. Discriminative power of the advanced activities of daily living (a-ADL) tool in diagnosis of mild cognitive impairment in an older population. International Psychogeriatrics. 2015;27(9): 1419–27. doi: 10.1017/S1041610215000563 25901578
36. Marson DC, Martin RC, Wadley V, Griffith HR, Snyder S, Goode PS, et al. Clinical interview assessment of financial capacity in older adults with mild cognitive impairment and Alzheimer's disease. Journal of the American Geriatrics Society. 2009;57(5): 806–14. doi: 10.1111/j.1532-5415.2009.02202.x 19453308
37. Steenland NK, Auman CM, Patel PM, Bartell SM, Goldstein FC, Levey AI, et al. Development of a rapid screening instrument for mild cognitive impairment and undiagnosed dementia. Journal of Alzheimer's disease. 2008;15(3): 419–27. 18997295
38. Wadley VG, Okonkwo O, Crowe M, Ross-Meadows LA. Mild cognitive impairment and everyday function: evidence of reduced speed in performing instrumental activities of daily living American Journal of Geriatric Psychiatry 2008;16(5):416–24. doi: 10.1097/JGP.0b013e31816b7303 18448852
39. Wadley VG, Okonkwo O, Crowe M, Vance DE, Elgin JM, Ball KK, et al. Mild cognitive impairment and everyday function: an investigation of driving performance. Journal of geriatric Psychiatry and Neurology. 2009; 22(2): 87–94. doi: 10.1177/0891988708328215 19196629
40. Wattmo C, Wallin AK, Minthon L. Progression of mild Alzheimer's disease: knowledge and prediction models required for future treatment strategies. Alzheimer's Research & Therapy. 2013;5(5):44.
41. Fieo RA, Austin EJ, Starr JM, Deary IJ. Calibrating ADL-IADL scales to improve measurement accuracy and to extend the disability construct into the preclinical range: a systematic review. BMC Geriatr. 2011;11 : 42. doi: 10.1186/1471-2318-11-42 21846335
42. Jette AM. Toward a common language of disablement. J Gerontol A Biol Sci Med Sci. 2009;64 (11):1165–8. doi: 10.1093/gerona/glp093 19617528
43. Sikkes SA, de Lange-de Klerk ES, Pijnenburg YA, Scheltens P, Uitdehaag BM. A systematic review of Instrumental Activities of Daily Living scales in dementia: room for improvement. J Neurol Neurosurg Psychiatry. 2011;80(1):7–12
44. Hancock P, Larner AJ. The diagnosis of dementia: diagnostic accuracy of an instrument measuring activities of daily living in a clinic-based population. Dementia and geriatric cognitive disorders. 2007;23(3): 133–9. doi: 10.1159/000097994 17170525
45. Gold DA. An examination of instrumental activities of daily living assessment in older adults and mild cognitive impairment. Journal of Clinical and Experimental Neuropsychology 2012;34(1): 11–34. doi: 10.1080/13803395.2011.614598 22053873
46. Nygard L. Instrumental activities of daily living: a stepping-stone towards Alzheimer's disease diagnosis in subjects with mild cognitive impairment? Acta Neurol Scand. 2003;179 : 42–6.
47. Rockwood K. The measuring, meaning and importance of activities of daily living (ADLs) as an outcome. International Psychogeriatrics. 2007;19(3): 467–82. doi: 10.1017/S1041610207004966 17359560
48. Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S. Measurement of functional activities in older adults in the community. Journal of Gerontology 1982;37(3): 323–329. 7069156
49. Rosenberg L, Nygård L, Kottorp A. Everyday Technology Usage (ETUQ)–evaluation of the psychometric properties of a new assessment of competence in technology use. Occupational Therapy Journal of Research. 2009;29(2): 52–62.
50. Farias ST, Mungas D, Harvey DJ, Simmons A, Reed BR, DeCarli C. The measurement of everyday cognition (ECog): development and validation of a short form. Alzheimers Dement 2011;7(6):593–601. doi: 10.1016/j.jalz.2011.02.007 22055976
51. Jones KB. Assessment of motor and process skills. Development, standardization, and administration manual, volume 1, edition 7. Colorado: Fort Collins Three Star Press. 2010.
52. Doble SE, Lewis N, Fisk JD, Rockwood K. Test-retest reliability of the assessment of motor and process skills in elderly adults. The Occupational Therapy Journal of Research. 1999;19 : 203–15.
53. Buxbaum LJ. Ideational apraxia and naturalistic action. Cognitive Neuropsychology. 1998;15(6–8):617–43. doi: 10.1080/026432998381032 22448839
54. Schwartz MF, Montgomery MW, Buxbaum LJ, Lee SS, Carew TG, Coslett HB, et al. Naturalistic action impairment in closed head injury. Neuropsychology. 1998;12(1):13–28. 9460731
55. Luttenberger K, Reppermund S, Schmiedeberg-Sohn A, Book S, Graessel E. Validation of the Erlangen Test of Activities of Daily Living in Persons with Mild Dementia or Mild Cogntive Impairment (ETAM). BMC Geriatr. 2016;16 : 111. doi: 10.1186/s12877-016-0271-9 27229937
56. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3): 179–86. 5349366
57. Hartigan I. A compartive review of the Katz ADL and the Barthel Index in assessing the activities of daily living of older people. International journal of older people nursing. 2007;2(3): 204–12. doi: 10.1111/j.1748-3743.2007.00074.x 20925877
58. Yang M, Ding X, Dong B. The measurement of disability in the elderly: a systematic review of self-reported questionnaires. JAMDA. 2014 : 15(2): 150.e1—e9.
59. Burton CL, Strauss E, Bunce D, Hunter MA, Hultsch DF. Functional abilities in older adults with mild cognitive impairment. Gerontology. 2009;55(5): 570–81. doi: 10.1159/000228918 19602873
60. Hancock P, Larner AJ. The diagnosis of dementia: diagnostic accuracy of an instrument measuring activities of daily living in a clinic-based population. Dementia and geriatric cognitive disorders. 2007;23(3): 133–9. doi: 10.1159/000097994 17170525
61. Hsiao JJ, Lu PH, Grill JD, Teng E. Longitudinal Declines in Instrumental Activities of Daily Living in Stable and Progressive Mild Cognitive Impairment. Dementia and geriatric cognitive disorders. 2015;39(1–2): 12–24. doi: 10.1159/000365587 25300404
62. Koskas P, Henry-Feugeas MC, Feugeas JP, Poissonnet A, Pons-Peyneau C, Wolmark Y, et al. The Lawton Instrumental Activities Daily Living/Activities Daily Living Scales: A Sensitive Test to Alzheimer Disease in Community-Dwelling Elderly People? Journal of Geriatric Psychiatry and Neurology. 2014;27(2): 85–93. doi: 10.1177/0891988714522694 24578460
63. Laan W, Zuithoff NP, Drubbel I, Bleijenberg N, Numans ME, de Wit NJ, et al. Validity and reliability of the Katz-15 scale to measure unfavorable health outcomes in community-dwelling older people. Journal of Nutrition Health and Aging. 2014;18(9):848–54.
64. Paula JJ, Bertola L, Avila RT, Assiss Lde O, Albuquerque M, Bicalho MA, et al. Development, validity and reliability of the general activities of daily living scale: a multidimensional measure of activities of daily living for older people. Rev Bras Psiquiatr. 2014;36(2): 143–52. doi: 10.1590/1516-4446-2012-1003 24554276
65. World Health Organization (WHO). International Classification of Functioning, Disability and Health (ICF). Geneva: World Health Organization (WHO), 2001. http://www.who.int/classifications/icf/en/
66. Dahlgren A, Sand A, Larsson A, Karlsson AK, Claesson L. Linking the klein-bell activities of daily living scale to the international classification of functioning, disability and health. J Rehabil Med. 2013;45(4):351–7. doi: 10.2340/16501977-1111 23467910
67. Jelsma J. Use of the international classification of functioning, disability and health: a literature survey. J Rehabil Med. 2009;41(1): 1–12. doi: 10.2340/16501977-0300 19197563
68. Reuben DB, Solomon DH. Assessment in geriatrics. Of caveats and names. J Am Geriatr Soc. 1989;37(6): 570–2. 2654261
69. Bossuyt PM, Reitsma JB, Burns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. British Medical Journal. 2003;326(7379): 41–4. 12511463
70. Mets T. Assessment of dementia in elderly outpatients: a comparative study of European centers and consensus statement. Arch Gerontol Geriatr. 2000; 30(1): 17–24. 15374045
71. Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975; 12(3): 189–98. 1202204
72. Roth M, Tym E, Mountjoy CQ, Huppert FA, Hendrie H, Verma S, et al. CAMDEX. A standardised instrument for the diagnosis of mental disorders in the elderly with special reference to the early detection dementia. Br J Psychiatry. 1986;149 : 698–709. 3790869
73. Mohs RC, Rosen WG, Davis KL. The Alzheimer's disease assessment scale: an instrument for assessing treatment efficacy. Psychopharmacol Bull. 1983;19(3): 448–50. 6635122
74. Lindeboom J, Schmand B, Tulner L, Walstra G, Jonker C. Visual association test to detect early dementia of the Alzheimer type. J Neurol Neurosurg Psychiatry. 2002;73(2): 126–33. doi: 10.1136/jnnp.73.2.126 12122168
75. Buschke H, Kuslansky G, Katz M, Stewart WF, Sliwinski MJ, Eckholdt HM, Lipton RB. Screening for dementia with the Memory Impairment Screen. Neurology. 1995;52(2): 231–238.
76. Corrigan JD, Hinkeldey MS. Relationships between parts A and B of the Trail Making Test. J Clin Psychol. 1987;43(4): 402–9. 3611374
77. Reitan RM. The relation of the trail making test as an indicator to organic brain damage. J Consult Psychol. 1955;19(5): 393–4. 13263471
78. Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a frontal assessment battery at bedside. Neurology. 2000;55(11): 1621–226. 11113214
79. Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiat Res. 1983;17(1):37–49.
80. Dierckx E, Engelborghs S, De Raedt R, De Deyn PP, Ponjaert-Kristoffersen I. Differentiation between mild cognitive impairment, Alzheimer's disease and depressions by means of cued recall. Psychol Med. 2007;37(5): 747–55. doi: 10.1017/S003329170600955X 17164030
81. Gallagher D, Mhaolain AN, Coen R, Walsh C, Kilroy D, Belinski K, et al. Detecting prodromal Alzheimer's disease in mild neuropsychological, cognitive impairment: utility of the CAMCOG and other predictors. Int J Geriatric Psychiatry. 2010;25(12): 1280–7.
82. McKhann G, Drachmann D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA working group under the auspices of department of health and human services task force on Alzheimer's Disease. Neurology. 1984;34(7): 939–44. 6610841
83. Cieza A, Geyh S, Chatterij S, Kostansjek N, Ustun B, Stucki G. ICF linking rules: an update based on lessons learned. J Rehabil Med. 2005;37(4): 212–8. doi: 10.1080/16501970510040263 16024476
84. Lawton MP, Moss M, Fulcomer M, Kleban MH. A research and service oriented multilevel assessment instrument. The journal of Gerontology. 1982;37(1): 91–9. 7053405
85. Reuben DB, Laliberte L, Hiris J, Mor V. A hierarchical exercise scale to measure function at the Advanced Activities of Daily Living (AADL) level. Journal of the American Geriatrics Society. 1990;38(8): 855–61. 2387949
86. De Vriendt P, Gorus E, Cornelis E, Bautmans I, Petrovic M, Mets T. The advanced activities of daily living: a tool allowing the evaluation of subtle functional decline in mild cognitive impairment. The Journal of Nutrition, Health and Aging. 2013;17(1): 64–71. doi: 10.1007/s12603-012-0381-9 23299382
87. Cornelis E, Gorus E, De Vriendt P. Translation of the Katz and Lawton scale to the terminology of the international classification of functioning, disability and health (ICF). Abstracts Wintermeeting Belgische Vereniging voor Gerontologie en Geriatrie 2014. Tijdschrift voor gerontologie en geriatrie. 2014;45(1): 34–65.
88. Evans J. Straightforward statistics for the behavioral sciences. Pacific Grove, CA Brooks/Cole Publishing, 1996.
89. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristics curves: a nonparametric approach. Biometrics. 1988;44(3): 837–45. 3203132
90. Jefferson AL, Byerly LK, Vanderhill S, Lambe S, Wong S, Ozonoff A, et al. Characterization of activities of daily living in individuals with mild cognitive impairment. Am J Geriatr Psychiatry. 2008;16(5): 375–83. doi: 10.1097/JGP.0b013e318162f197 18332397
91. Njegovan V, Hing MM, Mitchell SL, Molnar FJ. The hierarchy of functional loss associated with cognitive decline in older persons. The journals of gerontology Series A Biological sciences and Medical sciences. 2001;56(10): M638–43.
92. Pfeifer L, Drobetz R, Frankhauser S, Mortby ME, Maercker A, Forstmeier S. Caregiver rating bias in mild cognitive impairment and mild Alzheimer's disease: impact of caregiver burden and depression on dyadic rating discrepancy across domains. Int Psychogeriatr. 2013;25(8): 1345–55. doi: 10.1017/S1041610213000562 23651733
93. Moore DJ, Palmer BW, Patterson TL, Jeste DV. A review of performance-based measures of functional living skills. J Psychiatr Res. 2007;41(1–2): 97–118. doi: 10.1016/j.jpsychires.2005.10.008 16360706
94. Reppermund S, Birch RC, Crawford JD, Wesson J, Draper B, Kochan NA, et al. Performance-Based Assessment of Instrumental Activities of Daily Living: Validation of the Sydney Test of Activities of Daily Living in Memory Disorders (STAM). JAMDA. 2016;18(2): 117–122. doi: 10.1016/j.jamda.2016.08.007 27720663
95. Tuokko HA, Smart SM. Functional Everyday Behaviours. In: Pachana NA, Laidlaw K, editors. The oxford handbook of clinical geropsychology. Oxford: Oxford library of psychology 2014.
96. Farias ST, Mungas D, Jagust W. Degree of discrepancy between self and other-reported memory and everyday functioning by cognitive status: dementia, mild cognitive impairment and healthy elders. Int J Geriatric Psychiatry. 2005;20(9):827–34.
97. Rueda AD, Lau KM, Saito N, Harvey D, Risarcher L, Aisen PS, et al. Self-rated and informant-rated everyday function in comparison to objective markers of Alzheimer's disease. Alzheimer's & Dementia. 2015;11(9): 1080–9.
98. Suchy Y, Kraybill ML, Franchow E. Instrumental activities of daily living among community-dwelling older adults: discrepancies between self-report and performance are mediated by cognitive reserve. Journal of Clinical and Experimental Neuropsychology. 2011;33(1): 92–100. doi: 10.1080/13803395.2010.493148 20623400
99. Yen YC, Rebok GW, Gallo JJ, Jones RN, Tennstedt SL. Depressive symptomes impair everyday problem-solving ability through cognitive abilities in late life. Am J Geriatr Psychiatry. 2011;19(2): 142–50. doi: 10.1097/JGP.0b013e3181e89894 20808123
100. de Paula JJ, Diniz BS, Bichalho MA, Rodrigues M, Nicolato R, de Moraes EN, et al. Specific cognitive functions and depressive symptoms as predictors of activities of daily living in older adults with heterogeneous cognitive backgrounds. Front Aging Neurosci. 2015;20(7): 139.
101. Bombin I, Santiango-Ramajo S, Garolera M, Vega-González EM, Cerulla N, Caracuel A, et al. Functional impairment as a defining feature of: amnestic MCI cognitive, emotional, and demographic correlates. Int Psychogeriatr. 2012;24(9):1494–504. doi: 10.1017/S1041610212000622 22717386
102. Zahodne LB, Devanand D, Stern Y. Coupled cognitive and functional change in Alzheimer's disease and the influence of depressive symptoms. J Alzheimers Dis. 2013;34(4): 851–60. doi: 10.3233/JAD-121921 23302654
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 3- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- 2016 Reviewer and Editorial Board Thank You
- , , and mutations in early-onset Alzheimer disease: A genetic screening study of familial and sporadic cases
- Differential associations of plasma lipids with incident dementia and dementia subtypes in the 3C Study: A longitudinal, population-based prospective cohort study
- Mixed pathologies and neural reserve: Implications of complexity for Alzheimer disease drug discovery
- -related risk of mild cognitive impairment and dementia for prevention trials: An analysis of four cohorts
- Neuropathological diagnoses and clinical correlates in older adults in Brazil: A cross-sectional study
- Early diagnosis of mild cognitive impairment and mild dementia through basic and instrumental activities of daily living: Development of a new evaluation tool
- Potentially modifiable lifestyle factors, cognitive reserve, and cognitive function in later life: A cross-sectional study
- Association between fatty acid metabolism in the brain and Alzheimer disease neuropathology and cognitive performance: A nontargeted metabolomic study
- Fine-mapping of the human leukocyte antigen locus as a risk factor for Alzheimer disease: A case–control study
- What’s the “Take Home” from Research on Dementia Trends?
- Cultural representations of dementia
- Dementia and aging populations—A global priority for contextualized research and health policy
- Dementia in the oldest old: Beyond Alzheimer disease
- Rehabilitation for people living with dementia: A practical framework of positive support
- Dementia in low-income and middle-income countries: Different realities mandate tailored solutions
- Challenges and opportunities in understanding dementia and delirium in the acute hospital
- Dementia incidence trend over 1992-2014 in the Netherlands: Analysis of primary care data
- Association between delirium superimposed on dementia and mortality in hospitalized older adults: A prospective cohort study
- Development of an adaptive, personalized, and scalable dementia care program: Early findings from the Care Ecosystem
- Genetic assessment of age-associated Alzheimer disease risk: Development and validation of a polygenic hazard score
- Age-related cognitive decline and associations with sex, education and apolipoprotein E genotype across ethnocultural groups and geographic regions: a collaborative cohort study
- The impact of individual Cognitive Stimulation Therapy (iCST) on cognition, quality of life, caregiver health, and family relationships in dementia: A randomised controlled trial
- Effectiveness of an intervention to facilitate prompt referral to memory clinics in the United Kingdom: Cluster randomised controlled trial
- Subjective and objective cognitive function among older adults with a history of traumatic brain injury: A population-based cohort study
- Association of lifelong exposure to cognitive reserve-enhancing factors with dementia risk: A community-based cohort study
- Multimorbidity and healthcare utilization among home care clients with dementia in Ontario, Canada: A retrospective analysis of a population-based cohort
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Effectiveness of an intervention to facilitate prompt referral to memory clinics in the United Kingdom: Cluster randomised controlled trial
- , , and mutations in early-onset Alzheimer disease: A genetic screening study of familial and sporadic cases
- Challenges and opportunities in understanding dementia and delirium in the acute hospital
- Early diagnosis of mild cognitive impairment and mild dementia through basic and instrumental activities of daily living: Development of a new evaluation tool
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



